Arthroscopy of the Elbow
advance in the treatment of elbow disorders in recent years. It is now
being performed by an ever-increasing number of surgeons for a wide
variety of conditions (2,4,5,9,11,16,17,19,24,28,30,32,35).
It is useful both for diagnosis and treatment, but the techniques are
demanding, and potentially devastating neurovascular injuries are a
concern (4,9,17,28,34).
As elbow arthroscopy assumes a greater role in the diagnosis and
management of elbow problems, definite indications are still emerging.
Here we present the emerging techniques. Great caution must be
exercised in expanding one’s competency.
history and physical examination and routine investigation. The
indications for diagnostic arthroscopy in general are (a) undiagnosed
pain with abnormalities on clinical or radiographic examination, (b)
suspected loose body, (c) snapping (or clicking, locking, clunking,
etc.), (d) the need to obtain a biopsy, (e) contracture of spontaneous
onset, (f) evaluation of valgus instability in overhead athletes, and
(g) suspicion of an intraarticular benign tumor such as an osteoid
osteoma. Patients with pain but no abnormalities on careful clinical
examination, radiographs, or other investigations rarely are diagnosed
by arthroscopy. Absence of physical or other findings is a relative
contraindication, unless it is being performed to prove that no
intraarticular pathology exists.
arthroscopy were (a) removal of loose bodies, (b) synovectomy, (c)
debridement of the joint surface or adhesions, and (d) excision of
posterior osteophytes causing impingement, as occurs in athletes and in
early osteoarthritis. More recently, release of contractures and
osteocapsular arthroplasty for osteoarthritis have become increasingly
common. With release of contractures one must consider the risk of
neurovascular injury. The ability to distend the capsule, which is so
essential for displacement of the nerves away from the portals, is
greatly reduced in stiff elbows (8). Endoscopy has also been used to treat olecranon bursitis (25) and lateral epicondylitis (14).
and physical examination. Standard anteroposterior (AP) and lateral
radiographs are usually sufficient, although oblique views are
sometimes helpful. Magnetic resonance imaging (MRI) and computed
tomography (CT) scans offer little help and are rarely indicated.
Arthrography is not necessary.
whether or not the ulnar nerve subluxates or dislocates anteriorly. If
it does, as is the case in 16% of the population, it is at risk for
injury when the anterior medial portal is established.
mechanical symptoms, including locking, catching, or snapping, and are
often seen in association with degenerative changes such as osteophytes
on the olecranon and coronoid or osteochondritis dissecans. They do not
cause flexion contractures; those patients almost always have
associated posterior-impinging osteophytes on the olecranon and in the
olecranon fossa, as part of an early degenerative process.
routinely, and oblique views are sometimes helpful. Unfortunately, as
many as 30% of loose bodies are not detected on plain radiographs (24).
Thus it is wise to evaluate the entire elbow thoroughly at the time of
arthroscopy so that none are missed. Patients undergoing arthroscopy
for removal of anterior loose bodies should all be arthroscoped
posteriorly as well. Especially in degenerative conditions, one will
often find loose bodies “that are not loose” (i.e., that are stuck in
the soft tissues and only minimally mobile).
the elbow. The proximity of the neurovascular structures demands
accurate portal placement. Although many portals have been described,
there are nine primary working portals about the elbow. A thorough
knowledge of anatomy and of the approximate location of these portals
is essential to avoid complications.
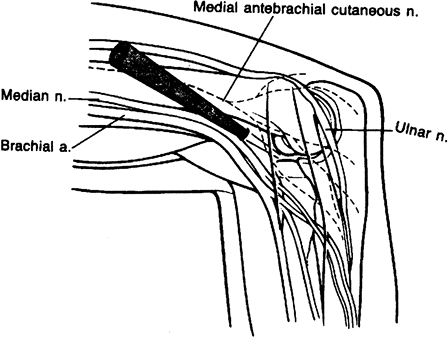
proximal anteromedial portal as described by Poehling et al. is favored
by most prone arthroscopists (30). It allows
full access to the anterior aspect of the elbow joint with minimal
risks of neurovascular structures. The essential at-risk structure in
utilizing these portals is the ulnar nerve. It is therefore mandatory
that one stay anterior to the intermuscular septum in establishing this
portal. It is usually located 2 cm above the medial epicondyle and
approximately 1 to 2 cm anterior to the intermuscular septum (Fig. 2-1).
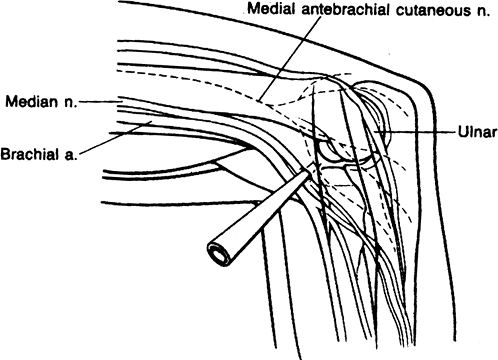
approximately 2 cm anterior and 2 cm distal to the medial epicondyle.
The median nerve, brachial artery, and ulnar nerve are all at risk in
utilizing this portal (1). It also allows full access to the anterior aspect of the elbow (Fig. 2-2).
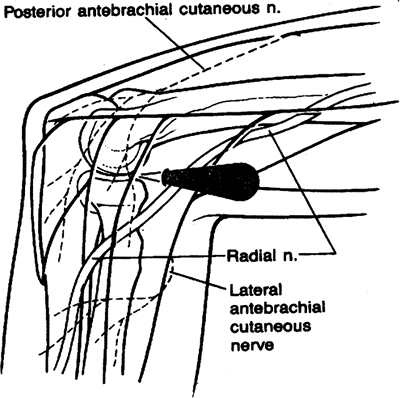
arthroscopy of the elbow. The standard anterolateral portal is usually
described as being 1 cm distal and 3 cm anterior to the lateral
epicondyle. This portal, initially described by Andrews and Carson,
courses in close proximity to the posterior interosseous nerve (Fig. 2-3) (2).
 |
|
Figure 2-1. Proximal anteromedial portal.
|
 |
|
Figure 2-2. Standard anteromedial portal.
|
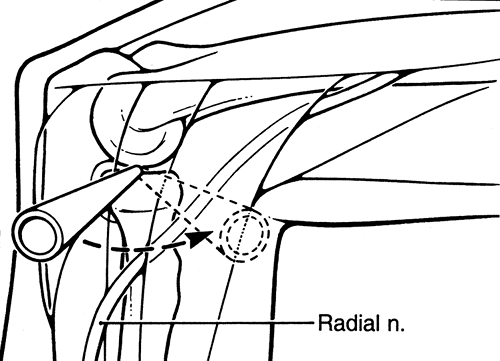
cm directly anterior to the lateral epicondyle. Although this portal
provides less risk to the radial nerve, it still may come in close
proximity to the radial nerve. This portal is useful in debriding the
anterior aspect of the radial head during radial head excision (Fig. 2-4).
authors as a diagnostic portal. Described separately by Day and by
Field et al., this portal provides a maximum safety buffer by utilizing
the brachialis muscle as a cushion to protect the radial nerve (7,33).
It is useful primarily in diagnostic anterior compartment arthroscopy
of the elbow and in lateral epicondylectomy and capsular release
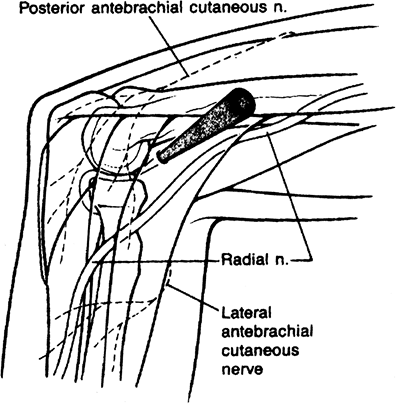
procedures (Fig. 2-5).
anterior compartment arthroscopy as well. Although somewhat difficult
to enter, an incision is made directly over the radial
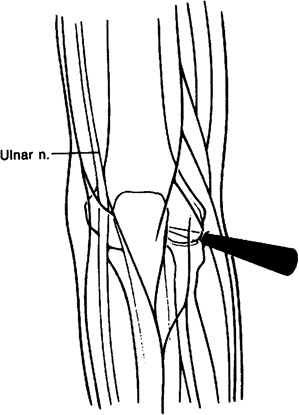
capitellar articulation and the soft tissues are immobilized to allow the cannula to enter the anterior aspect of the elbow (Fig. 2-6).
 |
|
Figure 2-3. Standard anterolateral portal.
|
 |
|
Figure 2-4. Standard anterior midlateral portal.
|
 |
|
Figure 2-5. Proximal anterolateral portal.
|
 |
|
Figure 2-6. Midlateral portal.
|
 |
|
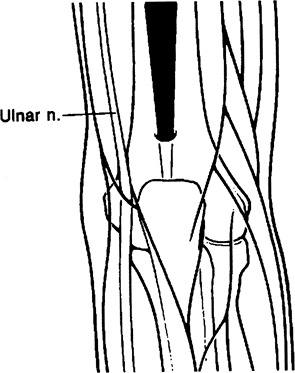
Figure 2-7. Posterior central portal.
|
in posterior elbow compartment arthroscopy. The straight posterior or
posterocentral portal is located approximately 3 cm above the tip of
the olecranon (Fig. 2-7). This is primarily
used in visualizing the medial and lateral compartments as well as the
olecranon fossa. It is also used for instrumentation in ulnohumeral
arthroplasty, olecranon spur excision, and debridement of medial gutter
and elevation of the triceps in arthrofibrotic patients.
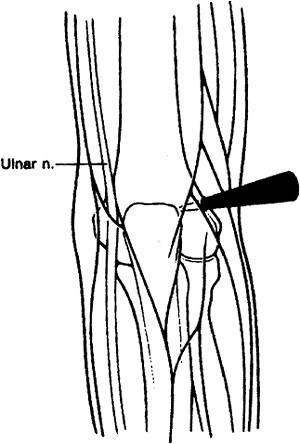
anywhere from the level of the tip of the olecranon to 3 cm proximal to
this area in the posterolateral gutter. It is established outside the
triceps tendon and is useful in debriding the olecranon fossa and in
visualizing the fossa during ulnohumeral arthroplasty. This is also
useful in debriding the lateral gutter and in visualizing both the tip
of the olecranon and the posterolateral gutter (Fig. 2-8).
the posterolateral gutter at the level of the radiocapitellar
articulation. Also known as the soft-spot portal (Fig. 2-9),
it allows complete access to the posterolateral gutter and is useful in
debriding posterolateral plica, osteochondritis dissecans of the
capitellum, and in excising the radial head.
indication for elbow arthroscopy. Success rates have been consistently
reported in the 90% range or better (4,18,20,22,27).
 |
|
Figure 2-8. Proximal posterolateral portal.
|
 |
|
Figure 2-9. Distal posterolateral portal.
|
 |
|
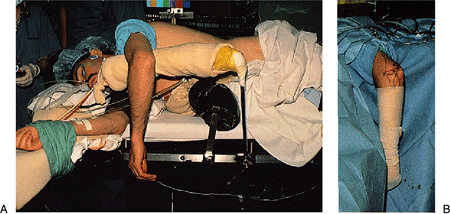
Figure 2-10. A:
The patient is placed in the lateral decubitus position with the arm resting on a padded bolster. The arm hangs free with the elbow flexed to 90 degrees. B: The forearm and hand are wrapped with an elastic bandage. This combination of forearm wrapping and tourniquet application limits the intraoperative swelling to the region of the elbow and permits rapid diffusion of any accumulated fluid from the elbow region. |
Loose bodies are removed with various-sized graspers that have teeth.
Those that are smooth on their outside surface, without irregular
surfaces or corners, work best, as they do not catch on the soft
tissues as they exit the elbow. Always grasp loose bodies so that they
can
be pulled out longitudinally, rather than obliquely or transversely,
which often requires that they be rotated into position for the
grasper. Grasp them very firmly. Rotate them fully so as to confirm
they are not still attached to soft tissue prior to extraction. Observe
the fragment until it exits the capsule so that it can be recovered if
lost from the jaws of the grasper. Check each one after extraction, to
confirm a fragment has not broken off in the soft tissues. Rotate the
loose body in the soft tissues to “work it out.” Large loose bodies in
the anterior elbow can be pushed out with the sheath of the scope
(uncouple and back the scope itself out of the sheath a few millimeters
to avoid damaging the lens) while pulling it with the grasper. Finally,
don’t hesitate to enlarge the portal somewhat rather than risk losing
the fragment in the soft tissues.
 |
|
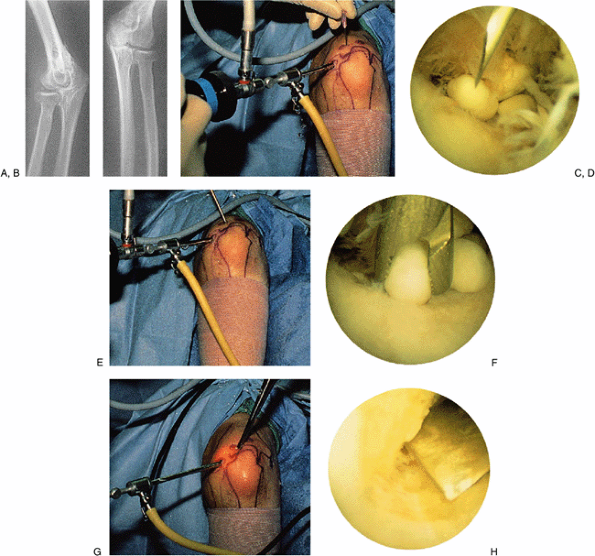
Figure 2-11. A typical case example illustrating many of the techniques used in elbow arthroscopy. A,B: Radiographs of a patient with synovial osteochondromatosis. C,D:
Technique for posterior compartment surgery and establishing the appropriate position of the portal. The arthroscope is directed to the posterior compartment, where loose bodies are seen. A needle is introduced in the precise position that one estimates to be ideal for the portal to be established. E,F: After establishing the portal with a knife under direct vision, a grasping instrument is inserted to remove the loose body. The remaining loose bodies are similarly removed. G,H: The area of synovial osteochondromatosis that is adherent to the posterior aspect of the humerus at the edge of the olecranon fossa is osteotomized with a small, curved osteotome under direct vision. This is quicker and more efficient than using a bur or a small biting instrument, which tends to make the risk rather tedious. If the osteotomized piece is large, it can be fragmented for removal. |
radial head excision, excision of olecranon or coronoid spurs,
arthroscopic ulnohumeral arthroplasty, or olecranon fenestration and in
lateral epicondylitis.
 |
|
Figure 2-12. A: Arthritic radial head. B: Anterior debridement of an arthritic radial head. C: Coplaning through a posterior soft portal of the radial head.
|
proximal medial portal and the midlateral portal is used for
instrumentation (Fig. 2-12A). The anterior aspect of the radial head is resected to prevent penetration of the anterior capsule. Penetration of the anterior capsule in this area will result in damage to the posterior interosseous nerve.
It is essential that during arthroscopic radial head excision the
anterolateral capsule not be violated by the shaver; otherwise,
significant neurologic complications will occur. Once a majority of the
anterior aspect of the radial head has been excised (Fig. 2-12B),
the inflow is placed in this anterolateral portal and a soft-spot
portal is utilized to bring the shaver from the posterior into the
anterior compartment. This shaver is then used to plane the posterior
aspect of the radial head until it is even with the resected anterior
margin (Fig. 2-12C). In cases of
radiocapitellar impingement this planing is continued for distances of
approximately 6 mm. In cases in which there is proximal radial ulnar
joint involvement, this is continued until the proximal radial ulnar
joint is completely free of the remaining proximal radial head.
operation. The very idea of attempting a total arthroscopic synovectomy
may provoke anxiety. First, the radial nerve lies right against, or
within a few millimeters of, the anterolateral joint capsule (Fig. 2-13).
Second, the ulnar nerve lies adjacent to the capsule in the
posteromedial gutter. Add to these perils the fact that diffuse
proliferative synovitis such as that seen in rheumatoid arthritis can
obliterate one’s view of the joint.
area that does not place structures at risk (e.g., the olecranon
fossa). The initial work can be done with poor visualization if both
the scope and the shaver are in the fossa. Then work toward the medial
gutter. Small pituitary rongeurs are useful for beginning to clear the
gutter. The synovium is grasped without fully closing the jaws, so as
not to pull out the capsule or ulnar nerve. As the view enlarges, a 3.5
shaver can be used, with the side cutting opening facing away from the
nerve (toward the scope) and gravity outflow without suction. In the
anterior elbow, we use a third portal, such as the proximal
anterolateral portal, to place a retractor. A Howarth blunt periosteal
elevator that is easy to place and broad enough to be effective in
retracting the anterior capsule is inserted. Start working with the
shaver against the distal humerus and
progress
from proximal to distal and from medial to lateral. Again, by having
the shaver connected only to gravity outflow and with the opening
facing away from the capsule (Fig. 2-14),
one can sweep across the capsule and remove the synovium without
perforating the capsule. Capsular release is usually required not just
to restore motion, but to eliminate the pain at the limits of motion.
 |
|
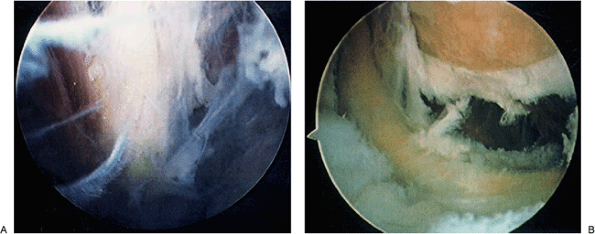
Figure 2-13. Visualization of the radial (A) and ulnar nerves (B) reveal their proximity to the capsule and vulnerability to injury.
|
 |
|
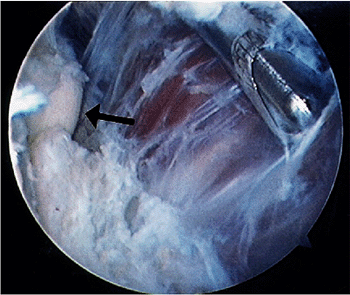
Figure 2-14. Removal of tissue from the capsule is performed without suction. Arrow identifies the radial head.
|
sutured, the tourniquet and elastic bandage are removed, and the
swelling around the elbow is manually compressed to decrease it. Moving
the elbow passively through an arc of motion several times assists in
this regard.
to damage the triceps tendon or to involve the ulnar nerve. The
arthroscope is placed in the superior posterolateral portal and
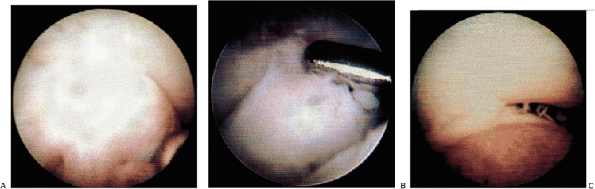
the shaver in the posterior central portal. The spur is evaluated and the margin of excision is delineated (Fig. 2-15A). The shaver is then used to resect the spur using a medial-to-lateral direction of movement (Fig. 2-15B).
It is important that the suction be left off when working along the
medial gutter to prevent injury to the ulnar nerve. Bony excision is
continued until the spur and deformity are excised (Fig. 2-15C). It is essential that extensive resection of the olecranon tip be avoided to prevent worsening onset of instability symptoms.
 |
|
Figure 2-15. A: Olecranon spur. B: Shaver in place. C: Spur excised.
|
Initially pioneered by Outerbridge and Kashiwago and refined by Morrey,
this procedure has been adapted to arthroscopy with successful results (6).
In this procedure, the arthroscope is placed in the proximal
posterolateral portal and the instrumentation is placed in the
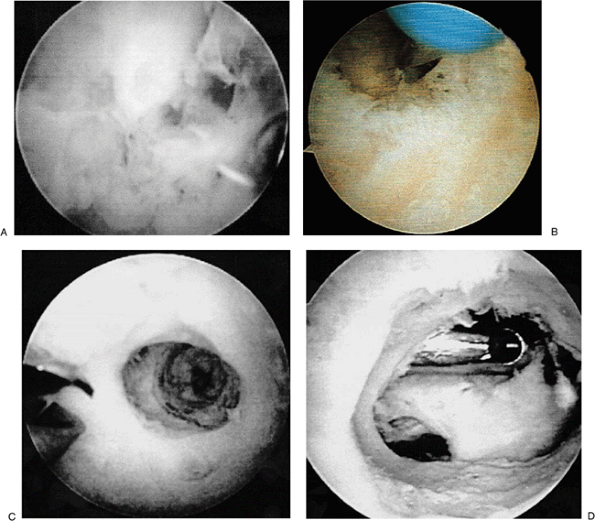
posterior central or straight posterior portal. The olecranon fossa is
evaluated (Fig. 2-16A) and a pilot hole is made in the center of the olecranon fossa (Fig. 2-16B).
This pilot hole delineates the adequate orientation of the excision as
well as the thickness of the olecranon fossa. The shaver is then
introduced into this pilot drill hole for a distance of approximately 2
cm or until the tip of the coronoid and the tip of the olecranon recede
on flexion and extension, respectively (Fig. 2-16C).
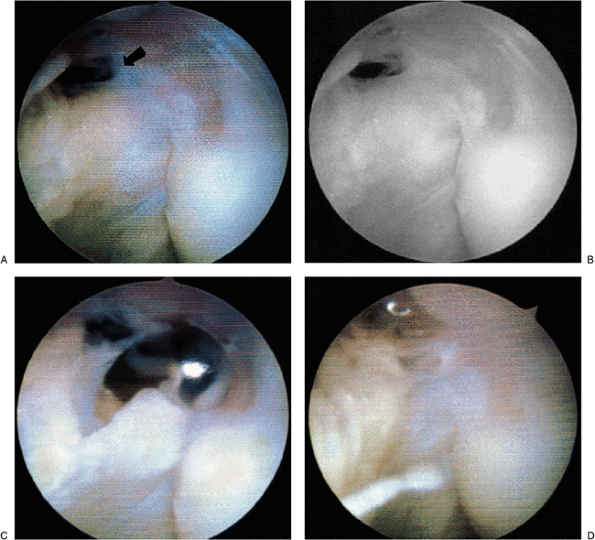
management of lateral epicondylitis. In these patients, concomitant
damage is also often noted to the capsule and to the posterolateral
aspect of the elbow. In this initial view, the fenestrations of the
capsule as a result of the chronic inflammation of lateral
epicondylitis may be noted (Fig. 2-17A). The
straight lateral portal is utilized to excise the capsule, allowing
visualization of extensor carpi radialis brevis tendon and the
contained Nirschl lesion (Fig. 2-17B). The shaver is then used to resect this lesion and abrade the bone (Fig. 2-17C).
Occasionally, a cautery device may be used to further elevate this area
and allow better access to the lateral epicondyle. Any concomitant
calcification should be removed as well. Once the entire degenerative
lesion has been excised and the epicondyle is abraded, the arthroscopic
portion of the procedure is completed (Fig. 2-17D).
 |
|
Figure 2-16. A: Olecranon fossa. B: Cannulated drill placed in olecranon fossa. C: Pilot drill hole. D: Completed ulnohumeral arthroplasty; shaver introduced through the anterior portal.
|
This procedure involves four components: (a) removal of all loose
bodies, including those that are not loose but are stuck in the
synovium; (b) removal of all osteophytes in the ulnohumeral
articulation, including those on the olecranon, coronoid, and medial
trochlea and in the three fossae—olecranon, coronoid, and radial; (c)
total synovectomy; and (d) anterior and posterior capsulectomy, with
posteromedial and posterolateral capsulectomy for those patients
lacking significant degrees of flexion. In patients
with
preexistent ulnar neuritis or neuropathy and those with severe loss of
flexion, the ulnar nerve is transposed subcutaneously as well (Fig. 2-18).
 |
|
Figure 2-17. A: Initial view of the lateral capsule in a patient with lateral epicondylitis. Note rent in capsule (arrow). B:
The angiofibrotic dysplasia or degenerative lesion of the tendon of the extensor carpi radialis brevis tendon or so-called Nirschl lesion as visualized arthroscopically. C: Excision of the lesion with a shaver placement during excision. D: Completed excision and abrasion of the lateral epicondyle. |
skill and experience. The respective loose body and synovectomy
components of the operation are as described earlier. The osteophyte
removal is accomplished with a combination of instruments, principally
burrs. Osteotomes can be useful, but removal of the osteotomized
fragment can be tedious because of sharp edges and soft-tissue
attachments. The shaver (rather than burr) can be used once the bone
has been cut into and trabecular bone has been exposed. A shaver is
less likely than is a burr to wrap up soft tissue, which puts nerves at
risk.
 |
|
Figure 2-18. Open exposure of the ulnar nerve; translocation performed after the scope procedure.
|
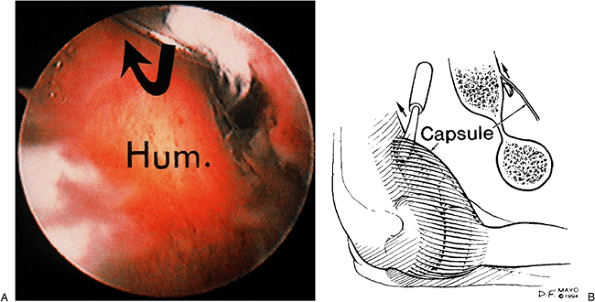
sequential stages. Blunt stripping of the capsule off the humerus can
be performed with a periosteal elevator (Fig. 2-19).
It is probably associated with minimal risk. However, it does not seem
to be as effective as capsulotomy or capsulectomy. This is best and
most safely performed with a hand instrument such as a wide duck-billed
basket punch biopsy (Fig. 2-20). The anterior
capsule is most safely cut across its midsection, starting from medial
and working toward the lateral side. The plane of dissection between
the capsule/scar and brachialis is more obvious medially than
laterally. Finally, capsulectomy can be performed after the previous
two stages have been completed. This is best performed with a shaver
using no suction, but with the outflow on the shaver simply left open
to let drainage fall to the floor. One should progress from proximal
medial to distal medial, then from proximal lateral to distal lateral.
This last region is the site of greatest risk of nerve injury.
 |
|
Figure 2-19. A,B: The capsule is stripped from the distal humerus by blunt pressure, if possible.
|
 |
|
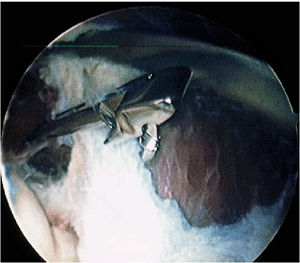
Figure 2-20. Capsulectomy with a blunt basket forceps.
|
more complex osteocapsular procedures, one should proceed in a logical
manner. First, establish the view and place one or two retractors into
the joint. Second, clean up the joint by performing a synovectomy.
Debride the capsule of any loose scar tissue so that it has the
appearance and texture of a structure. Remove the osteophytes and clean
up the bone debris. Strip the capsule off the humerus if not already
done. (It is useful to do this earlier if the joint is quite tight.)
Cut the capsule with the duckbill basket biopsy punch. Excise the
capsule with the shaver. Then incise and resect the capsule just
anterior to the medial and lateral collateral ligaments. By following
this stepwise sequence, one can progress as far as one’s skill permits
with the least likelihood of complications.
the patient is instructed to start using the elbow as tolerated. It is
kept elevated when not in use for the first day to decrease swelling.
If the procedure was performed for improving motion or for the
treatment of arthritis, an indwelling catheter is inserted for brachial
plexus block anesthetic if the neurologic examination is normal in the
recovery room, and the patient is started on a full range of motion on
a continuous passive motion (CPM) machine the same day. All
circumferential dressings must be removed to avoid skin damage during
CPM. Only an elastic sleeve is used to hold the absorbent dressing in
place.
a similar postoperative course. Soft dressings are placed on the arm,
and the patient is asked to begin immediate motion. In cases of
stiffness, CPM can be utilized for the first 3 to 10 days.
extension exercises are started at 1 week, and resistive exercises
begin at 2 to 3 weeks. If necessary, formal strengthening is initiated
by 3 to 4 weeks postoperatively. The patient is allowed to resume
normal activities as tolerated usually 3 to 12 weeks after surgery.
influenced by the procedure; that is, the correct final diagnosis was
(a) changed from that of the preoperative diagnosis, which was proved
to be incorrect, (b) was established when the diagnosis could not be
made preoperatively, or (c) was expanded or confirmed when the
preoperative diagnosis was incomplete or uncertain. The procedure was
said to be of therapeutic benefit to the patient if it was (a)
completely successful and obviated the need for any further surgery,
(b) partially successful in that the patient was clinically improved
and needed no further surgery, or (c) adjunctive in that an important
part of the operation was performed arthroscopically and the
arthroscopy directed the surgical intervention in an important manner.
Of the 71 consecutive arthroscopies in that series, approximately
three-fourths of the patients who undergo arthroscopy of the elbow
benefit (24).
The distribution according to type of benefits was as follows: 31%
diagnostic benefit, 24% both diagnostic and therapeutic benefit, and
17% therapeutic benefit only.
radiographic findings but for whom the diagnosis remains obscure or
unknown can be diagnosed by arthroscopy in most cases. Those with
suspected loose bodies usually are confirmed to have cartilaginous
loose bodies or some other mechanical cause for their symptoms.
Undiagnosed painful snapping of the elbow can be associated with loose
bodies, radiohumeral plicae, posttraumatic arthritis, primary
degenerative arthritis, dense soft-tissue adhesions (e.g., following
radial head excision), and posterolateral rotatory instability.
Patients with spontaneous onset of contracture typically are found to
have a form of inflammatory arthritis, or osteoid osteoma, as in a
series of our own cases (unpublished data). Valgus instability can be
diagnosed with the arthroscopic valgus stress test described by Andrews
(3,6).
arthroscopy has been considered to be removal of loose bodies, with
greater than 90% success rates reported (4,22,24).
Currently, it has become our impression that treatment of
osteoarthritis by osteocapsular arthroplasty, which includes excision
of loose bodies and osteophytes from the olecranon and coronoid, as
well as from each respective fossa, and capsular release may be one of
the most gratifying procedures, as it is usually so predictably
effective and beneficial in terms of both pain relief and restoration
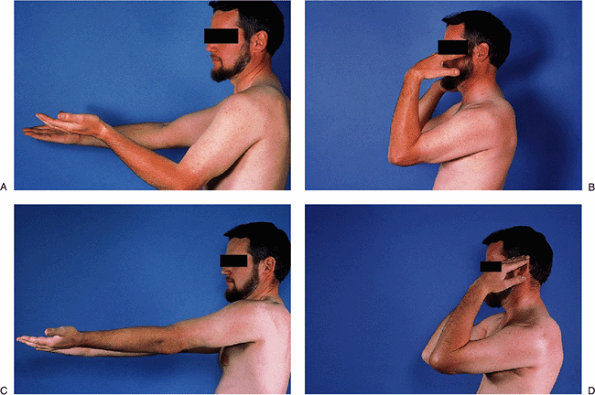
of motion (Fig. 2-21).
osteoarthritis and loose bodies to benefit from arthroscopic removal of
osteophytes and loose bodies (31). They
performed a fenestration of the distal humerus through the olecranon
fossa to the coronoid fossa. They did not notice any improvement in
elbow range of motion, presumably because they did not release the
capsular contractures.
This is due to the fact that not until recently did we become
comfortable with the technical challenges and execution of the
procedure. Satisfactory pain relief is obtained in about 75% to 90% of
cases. Range of motion is improved, particularly if one is careful to
remove the contracted capsule and scar tissue around the elbow.
Theoretically, the risk of late deterioration to increased
biomechanical loading of the ulnohumeral articulation will occur if the
radial head is excised. The radial head should probably be left in
unless the preceding indications are present, as its role in stability
would be greater in a rheumatoid elbow, which has already suffered bone
loss and soft-tissue damage.
They had a 93% early success, which declined to 57% by 3.5 years, but
concluded that the decline might have been due to limitations of the
arthroscopic technique. Those procedures were not total synovectomies
and did not include capsular releases. Current experience in our
institution suggests that both of these factors are important. Further
follow-up will be necessary to ascertain the long-term benefit. Also,
the role of arthroscopic synovectomy in more advanced stages of disease
and joint destruction remains to be determined. Our experience
indicates that a percentage of patients will benefit regardless of the
stage of disease.
elbow is being performed more frequently now. It has been shown by
several authors to be effective (12,29), but complications
such as nerve transection have been reported (10,12).
Although the safety of this procedure remains to be confirmed, it seems
likely that the decreased morbidity and increased surgical access to
remove all contracted tissue may bring this procedure into the mainstay
of treatment of the stiff elbow (21). The relative importance of capsulotomy versus capsulectomy is yet to be clarified.
 |
|
Figure 2-21. A–D: Pre- and postoperative motion after arthroscopic debridement.
|
-
Persistent drainage from the portals.
-
Deep infection.
-
Minor contractures, usually related to the nature of the underlying condition (such as inflammatory arthritis).
-
Transient palsies caused by extravasation
of local anesthetic, direct blunt trauma, compression by the tourniquet
or forearm wrapping, or the use of the indwelling catheter
postoperatively.
any permanent nerve or vascular injuries, the risk of injury to these
structures is real, and transections of all three major nerves have
been reported. The anterolateral and the anteromedial portals are most
likely
to be associated with nerve injury because of the proximities of the
radial, posterior interosseous, ulnar, and median nerves to these
portals (2,16,17,28).
These injuries are best avoided by careful technique and constant
vigilance. The distances between these nerves and all the portals are
increased substantially by flexing the elbow to 90 degrees and
distending the joint with saline (28).
Displacement of the nerves anteriorly away from the portals is
accomplished by capsular distention with 15 to 25 mL of saline, but the
average intracapsular capacity of stiff elbows is only 6 mL (Fig. 2-22).
 |
|
Figure 2-22.
Intraarticular pressure (IAP) versus infusion volume: comparison of stiff and normal elbows. The capacity is greatly reduced in the stiff elbow. |
T, Berggren M, Adolfsson L. Complete transection of the median and
radial nerves during arthroscopic release of post-traumatic elbow
contracture. Arthroscopy 1999;15:784–787.
J, Neff R, Shall L. Compression neuropathy of the radial nerve as a
complication of elbow arthroscopy: a case report and review of the
literature. Arthroscopy 1988;4:284–286.
