Anatomy and Approaches
-
Anterior cervical discectomy
-
Corpectomy
-
Spinal cord decompression
-
Bone grafting
-
Instrumentation procedures
the carotid pulses (decreased or bruits) and thyroid gland (for
thyromegaly, which can inhibit exposure). If previous anterior surgery
was performed, the patient must be evaluated carefully for a recurrent
laryngeal nerve palsy (using direct laryngoscopy) if the surgeon
decides to approach the contralateral side. Appropriate imaging studies
should confirm the exact location of the pathology to be approached.
to extend the neck slightly and drop the shoulders posteriorly. The
head is rotated about 10 degrees to 15 degrees to the contralateral
side. The medial border of the sternocleidomastoid (SCM) is palpated
and marked. This represents the location of the longitudinal incision,
if so desired, which is useful for multilevel, extensile approaches. If
one-level or two-level surgery is planned, a transverse incision can be
used and is centered over the desired vertebral level, which results in
a more cosmetically acceptable scar. Surface landmarks are used to
decide on the incision location, as follows:
-
Hyoid bone—C3 body
-
Thyroid cartilage—C4-5 disc space
-
Cricoid cartilage—C6 body
the platysma is identified. It is divided in line with the incision. A
plane deep to the platysma is developed to ensure adequate
visualization of the medial fascial border of the SCM. The trachea is
palpated medially through the overlying strap muscles, which include
the sternohyoid and sternothyroid. Dissection, through an investing
fascial layer, between the strap muscles and the SCM is developed
bluntly.
pulse), continue blunt dissection in a posteromedial direction toward
the spine. The anterior aspect of the spine can be palpated through the
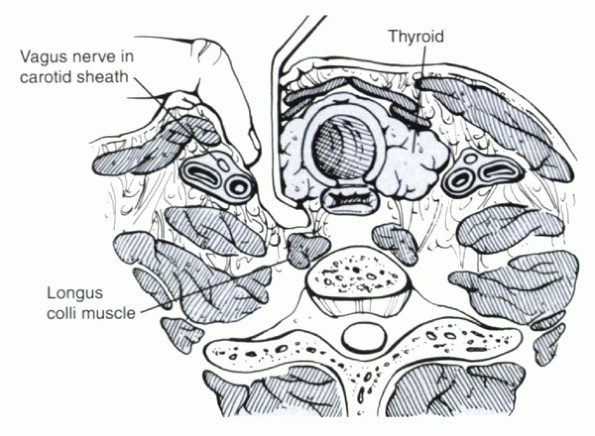
alar and prevertebral fascia within this interval (Fig. 25.1-1). The trachea and esophagus are
retracted gently to the contralateral side, allowing visualization of
the alar fascia, which overlies the prevertebral fascia. These fascial
layers are cut to expose the anterior surface of the spine. A peanut
dissector now can be used to sweep the tissue from the midline to
reveal the anterior longitudinal ligament, longus colli, and underlying
disc spaces. Identification of the disc spaces in the arthritic spine
may be impeded by large protruding osteophytes. In general, the disc
spaces are located at peaks, whereas the midvertebral bodies are in the
“valleys.” The correct level can be confirmed with a lateral radiograph
by marking the disc space with a spinal needle.
 |
|
Figure 25.1-1
Superficially, an interval between the sternocleidomastoid and midline strap muscles is developed. Deeper, dissection proceeds between the carotid sheath (laterally) and the trachea and esophagus (medially). |
elevated subperiosteally off the anterior cervical body and disc space;
this is stopped laterally as soon as the vertebral body starts to turn
posteriorly. The vertebral arteries can be injured with more
posterolateral exposure. Next, a deepbladed, self-retaining retractor
can be inserted deep to the longus colli with toothed blades on the
lateral side and smooth blades on the medial side.
of a drain, the wound is irrigated copiously with normal saline, and
the esophagus is inspected for possible injury. Indigo carmine (or blue
food coloring) can be infused orally into the esophagus to show subtle
tears. The skin can be closed with a running 4-0 subcuticular stitch.
extubated safely in the operating room. After longer procedures,
extubation may be delayed until airway swelling has resolved. The need
and type of postoperative immobilization are determined by the
stability of the spine. Prophylactic antibiotics are administered for
24 to 48 hours. Drains are removed when the output is less than 10 mL
per 8-hour period.
superior laryngeal nerve injury, occurs in about 4% to 5% of cases. The
risk of RLN palsy is extremely low with surgery performed above C5. The
RLN is thought to be at more risk in the following situations:
-
Right-sided versus left-sided surgery (slight, probably insignificant)
-
Exposure below C5 (significant)
-
Revision surgery (10% of cases)
within the carotid sheath and has less medial-lateral variability than
on the right side. Although the left RLN descends in a fairly
consistent manner to loop under the arch of the aorta, the right RLN
does not extend as far distal before looping around the subclavian
artery back toward the larynx. Despite these anatomic differences, a
study showed no difference in the incidence of RLN palsy between
right-sided and left-sided exposure.
sympathetic plexus, which lies on top of the longus colli muscle and is
at most risk at C6. It is diagnosed clinically by the presence of:
-
Ptosis (drooping eyelid)
-
Meiosis (papillary constriction)
-
Anhydrosis (dry eye)
wound infection. The risk of infection after uncomplicated anterior
cervical spine surgery is less than 1%.
be achieved through a transoral approach. It is used most often to
excise or débride infections and tumors (i.e., odontoidectomy). It
allows access to the anterior aspects of C1-2 articulations,
dens,
and, with some extension, the anterior occipitocervical junction and
upper body of C3. The approach is contraindicated in the presence of
active oral infection or maxillomandibular pathology, which would
inhibit adequate exposure.
treated preoperatively. The mouth should be examined for loose teeth
and the integrity of the posterior oropharyngeal membranes. Jaw
mobility should be assessed because it can be a limiting factor to
visualization. Macroglossia, which occurs with some syndromic
conditions, can make lingual retraction difficult and may necessitate
midline division (the so-called tongue-splitting approach) for adequate
exposure. Preoperative sagittal magnetic resonance imaging or computed
tomography reconstructions can be used to determine the location of the
hard palate and glossal muscles, which influence the extent of
attainable exposure.
intubation is preferred because nasotracheal intubation leaves the tube
crossing the surgical field in front of the oropharynx. Slight
Trendelenburg position can help prevent migration of irrigant and
debris into the patient’s airway during the procedure, in addition to
an adequate seal around the endotracheal tube cuff balloon. Antibiotic
prophylaxis should include gram-negative coverage.
retractor should be used to keep the mouth open and the tongue
depressed inferiorly. The incision can be determined by landmarks and
intraoperative radiographs. The C2-3 disc space is palpable near the
inferior aspect of the oral cavity; the anterior C1 ring often can be
palpated superiorly. If in doubt, a spinal needle can be inserted and a
lateral radiograph obtained.
constrictor muscles, buccopharyngeal fascia, and prevertebral fascia)
are incised as one layer down to the bone of the vertebrae. Then the
fascia is stripped subperiosteally laterally as a unit with a
periosteal elevator until the lateral masses of the C1-2 joints are
exposed. The soft palate can be retracted cranially to expose the
anterior atlantooccipital membrane (the occipital insertion of the
anterior longitudinal ligament, which stems from the superior C1 ring
to the foramen magnum) and the apical ligament (which projects from the
odontoid process to the foramen magnum). Inferior retraction of the
tongue may give access to the C3 body and the C3-4 disc space. Exposure
of these structures is provided better by other approaches, however.
irrigated copiously with saline-antibiotic solution. Closure should be
watertight because this is thought to be a major factor in decreasing
the incidence of postoperative infection using this approach.
aspiration after extubation. The patient routinely remains intubated
for at least 24 hours until oral swelling has resolved. Early
tracheostomy might be considered if prolonged intubation is likely.
field. The infection risk is relatively high. Early reports, published
before the routine use of perioperative antibiotics, documented
infection rates of 66%. Larger contemporary series documented rates of
0% to 3%, similar to other cervical approaches. Specific antibiotic
prophylaxis, multilayer closure, and avoidance of spinal implants are
thought to be responsible for the lower infection rates.
tongue, and injury to the gingiva are other potential complications.
Aspiration of debris and secretions can occur if the endotracheal
balloon cuff seal is lost during or after surgery. Early extubation
(<24 hours) may lead to acute respiratory distress, necessitating
emergent reintubation that may risk neurologic compromise in an
unstable spine.
approach to the cervicothoracic junction can be used for anterior
corpectomy, discectomy, or osteotomy for fractures, herniated discs,
infection, or deformities. Because the upper thoracic vertebrae are
accessed with inferior retraction of the arch of the aorta, congenital
anomalies of the great vessels may be a relative contraindication.
whereas T4 lesions may be approached better through a high thoracotomy.
Sagittal magnetic resonance imaging can show the axial relationship of
the manubrium, sternum, and clavicle to the upper thoracic and lower
cervical vertebrae. An unusually high manubrium in relation to the
spine can preclude adequate exposure of the desired levels, making a
transsternal approach preferable.
incision is made from midsternum to 1 cm above the sternal notch. The
incision is carried laterally, usually to the left side, along a line
about 1 cm proximal to the midpoint of the clavicle.
dissection along their anterior surfaces. The two heads of the SCM are
visualized inserting deep to the bones. The
tendons
are released from their bone attachments, and their ends are tagged and
retracted superiorly. Next, the sternohyoid and sternothyroid tendons
are visualized, dissected, tagged, transected as far distally as
possible, and reflected proximally. A small area of fatty tissue
beneath the sternal notch is visualized and removed using a peanut
dissector.
section of manubrium are resected carefully. The contralateral
sternoclavicular joint should be left intact. Deep to the periosteal
layer is the subclavian vein and the thymus. The spine is accessed
proximally using the standard anterior retropharyngeal approach. The
interval between the carotid sheath and the trachea/esophagus is
continued carefully caudally by blunt dissection along the anterior
surface of the spine. A narrow Deaver-type retractor is placed at the
inferior aspect of the wound to retract the subclavian vein and arch of
the aorta. At the completion of the procedure, the wound is irrigated,
and the strap and SCM muscles are reapproximated to the remaining
periosteal sleeve.
because it often crosses the operative field in the lower cervical
spine. The thoracic duct is at greater risk with left-sided approaches.
It lies lateral to the carotid sheath, deep to the subclavian artery
and vein, and should be suture ligated proximally and distally to
prevent chylothorax if injured.
used to expose from the occiput to the sacrum. It is indicated for a
variety of diagnoses and is useful for the following:
 |
|
Figure 25.1-2
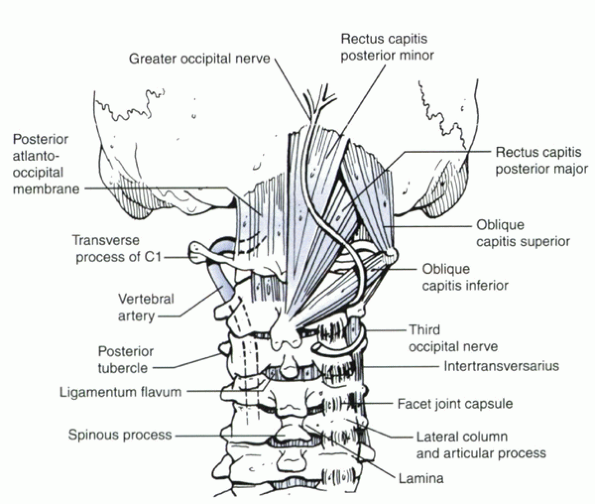
Dissection beyond 1.5 cm from the midline of the C1 ring risks injury to the vertebral artery. Lateral dissection or retraction at the level of the external occipital protuberance risks injury to the greater occipital nerve (sensation to the back of the head). Care must be taken to maintain instruments at the subperiosteal level. |
-
Insertion of instrumentation and fusion
-
Decompressive procedures, such as laminectomy or laminoplasty
removing anterior neurocompressive pathology, such as midline herniated
discs and abscesses.
before surgery. An awake, fiberoptic intubation is recommended if the
spine is unstable.
Dissection is carried through the subcutaneous tissue to the trapezius
fascia. The fascia is incised, and dissection proceeds through the
relatively avascular plane within the ligamentum nuchae. With the tips
of the spinous processes exposed, dissection is taken laterally to
maintain the interspinous ligaments. One of the spinous processes can
be marked with a clamp to confirm intraoperative radiographic
identification of the correct level. Subperiosteal dissection is
carried over the spinous processes and laminae, elevating the
paraspinal muscles off the bone. Care is taken to leave the facet
capsule intact until, or if, exposure and decortication for fusion has
been decided at that level.
injury to the vertebral artery (Fig. 25.1-2).
Lateral dissection or retraction at the level of the external occipital
protuberance risks injury to the greater occipital nerve (sensation to
the back of the head). Care must be taken to maintain instruments at
the subperiosteal level. The wound is irrigated with copious amounts of
warmed normal saline solution. If desired, a medium-sized drain can be
placed deep to the fascia layer. Closure of all layers (muscle, fascia,
subcutaneous tissue, and skin) is routine.
decided based on cervical stability. External methods may not be needed
if rigid internal fixation was implanted. Prophylactic antibiotic
coverage is continued for 24 to 48 hours after surgery. Drain outputs
tend to be higher than after anterior cervical procedures. They are
removed when outputs are less than 30 to 40 mL per 8-hour shift but
should not remain more than 48 hours.
WJ, Sweeney CA, Connolly PJ. Recurrent laryngeal nerve injury with
anterior cervical spine surgery: risk with laterality of surgical
approach. Spine 2001;26:1337-1342.
NA, Lu J, Yang H, et al. Vulnerability of the sympathetic trunk during
the anterior approach to the lower cervical spine. Spine
2000;13:1603-1606.
PC, Bohlman HH, Riley LH, et al. The anterior retropharyngeal approach
to the upper part of the cervical spine. J Bone Joint Surg
1987;69A:1371-1383.
JL, Koriwchak MJ, Winkle M, et al. Vocal fold paralysis following the
anterior approach to the cervical spine. Ann Otol Rhinol Laryngol
1996;105:85-91.
spine is required to address spinal pathology, the vertebral levels
that require exposure determine the options for surgical access. The
anterior approach should be considered for spinal pathology involving
the anterior and middle columns of the spine in Denis’ three-column
model. The mechanical advantages achieved with anterior reconstruction
of the load-bearing capabilities of the spine are significant.
Indications for surgery via the anterior approach mostly fall into the
following six categories:
-
Trauma
-
Infection
-
Tumor
-
Degenerative disc disease
-
Iatrogenic causes
-
Deformity
thoracoscopy. For the lumbar spine, we describe retroperitoneal-flank
incision, retroperitoneal-medial incision, transabdominal, and
laparoscopic/endoscopic approaches.
This approach provides the best exposure to the midthoracic vertebral
bodies; the view at the cephalad and caudal extremes of the thoracic
segments is more limited. The relatively narrow thoracic inlet and
scapula limit the view in the cephalad direction. The diaphragm limits
the view of the caudal vertebral bodies, unless the diaphragm is
detached as part of the dissection (see later under approach to
thoracolumbar junction). This approach can provide excellent anterior
exposure of the thecal sac, multiple vertebral bodies are exposed
easily, and instrumentation across multiple levels can be performed
readily. Only the contralateral pedicles and posterior elements are
inaccessible in this approach. The main disadvantage of this approach
is the increased pulmonary and chest wall morbidity of thoracotomy
compared with thoracoscopy or posterolateral, extrapulmonary
approaches. Another drawback is that a posterior approach is required
to address additional posterior spinal pathology and for placement of
posterior instrumentation.
approached from the left or right side, many surgeons advocate the use
of a left-sided approach because of the relative ease and safety of
mobilizing and retracting on the aorta versus the vena cava or the
azygos venous system. For upper thoracic lesions (T2-6), a right-sided
thoracotomy is preferred because the left side of the upper thoracic
spine is less accessible owing to the location of the aortic arch and
great vessels. For lower thoracic and thoracolumbar lesions (T7-L2), a
left-sided thoracotomy is preferred because it is technically easier to
mobilize the aorta, and liver retraction is avoided. Within these
generalizations, the side of the approach should allow maximal exposure
of the pathology being treated.
patient is placed in the full lateral decubitus position with the side
to be operated in the up position. The thoracic spine now is oriented
perpendicular to the floor to facilitate safer spinal cord
decompression and hardware placement into vertebral bodies. For
approaches to the upper thoracic to midthoracic spine (T2-8), the use
of a double-lumen endotracheal tube allows for selective lung
ventilation, maximizing lung deflation on the operated side to
facilitate spine exposure. For lesions below T8, a double-lumen tube
usually is not necessary. Fluoroscopy or plain radiographs are taken to
mark the appropriate vertebral level and to plan the incision. The rib
level may be identified by palpating and counting ribs from distal to
proximal.
axillary line, below the angle of the scapula, to the border of the
paraspinous muscles posteriorly for exposure of upper thoracic lesions.
For midthoracic to lower thoracic lesions, the incision generally is
made over the rib one to two levels above the vertebral level of
interest. For thoracolumbar lesions, the incision is made over the 10th
or 11th rib.
For upper thoracic exposures, part of the trapezius and rhomboid
muscles are divided, and the scapula is retracted anteriorly and
superiorly to facilitate deep exposure. Subperiosteal dissection of the
rib is performed, and care is taken to avoid injury to the
neurovascular bundle in the subcostal groove on the inferior surface of
the rib. The rib is resected from the costochondral junction anteriorly
to the angle of the rib posteriorly, about 3 to 4 cm from the rib head.
With intrapleural exposures, the chest is entered through the rib bed
periosteum, the underlying endothoracic chest wall fascia, and the
parietal pleura. The ribs are spread using a self-retaining thoracotomy
retractor (i.e., Finochietto retractor). The lung is deflated, and deep
retractors are placed to expose the vertebral bodies.
are covered by a glistening parietal pleura. The intervertebral discs
protrude prominently (the “mounds”), and the vertebral bodies between
are relatively concave (the “valleys”). After appropriate levels are
confirmed radiographically and anatomically by counting ribs, a pleural
flap is elevated to expose the spine. The segmental vessels are
ligated, if necessary, at the midpoint of the vertebral body. The
anastomotic vascular arcade in the region of the proximal neural
foramen should be preserved to decrease the risk of ischemic thoracic
spinal cord complications. The vascular watershed area of the thoracic
spinal cord is located between T5-9. The artery of Adamkiewicz, the
largest of the thoracic radicular feeder arteries, usually is found on
the left side at the level of T10, but this anatomy is variable. The
sympathetic trunk lies at the level of the costovertebral articulation,
lateral to the prominent rib head. The intended operation on the
thoracic spine is carried out.
junction to expose the lower thoracic spine to the upper lumbar spine,
the skin incision is made in the region of the 10th or 11th rib. This
incision is carried more obliquely and ventrally, depending on the
caudal exposure required. The 10th or 11th rib is exposed and resected,
and the lower chest cavity is entered in a standard fashion. The costal
cartilage is split longitudinally, allowing access to the preperitoneal
fat layer that lies caudal to the diaphragm and is contiguous with the
rostral aspect of the retroperitoneal space (Fig. 25.2-2).
The costal cartilage is tagged with suture for repair during wound
closure. The peritoneum is cleared from the undersurface of the
diaphragm using a sponge stick, avoiding entry into the peritoneal
cavity. Rents in the peritoneum should be repaired with absorbable
suture. The diaphragm is kept under tension, and a circumferential
incision is made along its peripheral attachment to the costal margin,
leaving a generous 2-cm cuff of diaphragm muscle for later repair. The
diaphragm muscle can be marked with suture to facilitate repair during
closure. The spleen, kidney, and stomach are retracted medially using a
broad, padded, malleable retractor. The left crus of the diaphragm is
tagged with suture and cut at its attachment to the anterior
longitudinal ligament at L1 and L2. The vertebral bodies of the
thoracolumbar junction are visualized. The great vessels are protected
carefully with malleable retractors. Segmental vessels are identified
and ligated at the level of the midvertebral body. The psoas muscle is
retracted posteriorly. Then the intended operation on the spine is
carried out. A right-sided approach to the thoracolumbar junction may
be required in unusual cases (e.g., tumorous involvement of the
right-side L1 vertebral body and pedicle). The right-sided approach is
similar to the left-sided approach except that care should be taken to
retract the liver and great venous structures on the right side. After
the spinal procedure is completed, the diaphragm should be reattached
accurately to the cuff of diaphragm on the costal margin. The crus
should be reattached to the anterior longitudinal ligament. The costal
cartilage is repaired.
the chest cavity, directed superiorly toward the apex, and brought out
below the incision. The lung is reinflated. The adjacent ribs are
reapproximated and secured using heavy absorbable suture. The rib bed
is reapproximated to reestablish a pleural seal. Superficial muscles
and soft tissue are closed in anatomic layers.
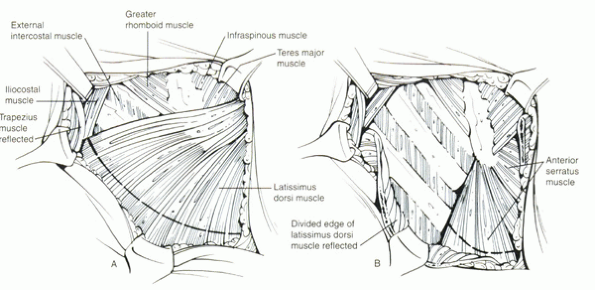 |
|
Figure 25.2-1
The first step of the transthoracic approach is incision of the skin and subcutaneous fascia. This exposes the underlying muscles (A), which, for most exposures, is the latissimus dorsi. This muscle is divided in line with the incision to expose the underlying ribs and intercostal muscles (B). |
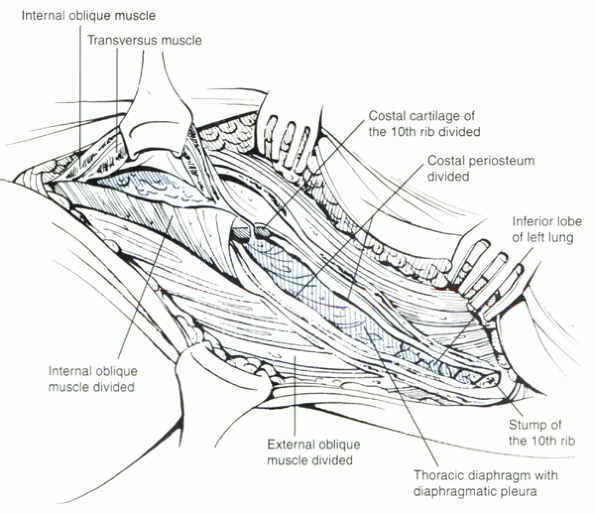 |
|
Figure 25.2-2 The 10th rib has been resected. After resection of the costal cartilage, exposure of the thoracolumbar junction is afforded.
|
transthoracic approaches. Atelectasis, pneumonia, pleural effusions,
and airway obstruction are possible complications. Chest wall
discomfort is significant after thoracotomy, and 10% of patients
experience chronic chest wall pain. Congestive heart failure and
pulmonary edema can occur, related to excessive fluid replacement in
susceptible patients.
involving corrective osteotomy. Anterior spinal artery syndromes have
been reported after scoliosis correction or circumferential spinal
osteotomy.
risk of surgery because two major body cavities are exposed. In
addition to specific complications related to thoracotomy, injury to
the spleen, kidney, or ureter may occur during surgical dissection or
retraction. Ileus may develop postoperatively. Injury to the
sympathetic chain may produce asymmetric warmth of the involved
extremity, but this rarely is a problem for the patient. Careful
closure of the peritoneum and diaphragm prevents herniation of visceral
structures. Combined thoracoabdominal exposure should be reserved for
patients with satisfactory cardiopulmonary reserves who are likely to
tolerate the surgical risks.
and physical therapists are important for these patients. Rapid
mobilization prevents pulmonary complications and decreases the risk of
postoperative thromboembolic complications.
thoracic spine was introduced by Regan in the early 1990s (Mack et al,
1993). Since then, the field of endoscopic spine surgery has been
expanding at a rapid rate. The main benefit of these “minimal incision”
techniques is to lessen the “approach-related” trauma associated with
the classic open surgical approaches. Because endoscopy requires only
small incisions, the level of postoperative pain and length of hospital
stay are less than with traditional open procedures. Patients
experience less chronic chest wall discomfort and improved cosmesis
with the endoscopic technique.
surgery (VATS) is the steep learning curve. The basic anatomy and
dissection techniques are familiar to most spine surgeons. Significant
adaptation of thoracoscopic surgical techniques using longer tools,
restricted access, and new methods of perceiving, visualizing,
illuminating, and magnifying the operative site create distinct
technical challenges, however. Refinement of surgical techniques and
instruments has expanded the use of VATS in spine care to include many
procedures that previously could be performed only by open approaches (Table 25.2-1).
required for VATS. This procedure is contraindicated in patients who
cannot tolerate one-lung ventilation. Another contraindication to VATS
is pleural adhesions. Extensive scarring from a previous operation at
the site of spinal pathology also may preclude thoracoscopic techniques.
the left side, depending on the location and eccentricity of the spinal
lesion. Thoracoscopically, exposure from T1-2 to the T12-L1 interspace
is possible. A right-sided approach is preferred when possible because
more spinal surface area is available behind the azygos vein than
behind the aorta. If exposure is needed from T10-12, a left-sided
approach is preferred because the liver causes the right diaphragm to
ride higher, limiting visibility to the spine.
the patient is positioned in the lateral decubitus position as
described for a transthoracic approach. The surgeon should be prepared
to convert to open thoracotomy if thoracoscopic methods fail. Video
monitors are placed on both sides of the patient so that the surgeon
and assistants can see the thoracoscopic image.
-
1-cm rigid rod lens endoscope (0-degree and 30-degree angle field of view)
-
FRED antifog solution
-
Harmonic scalpel (high-frequency coagulator)
-
Suction/irrigation apparatus
-
Endoscopic fan retractor
operating on the thoracic spine, which is 14 to 30 cm from the surface
of the skin, are required, including the following:
-
Cobb elevator
-
Rib dissector
-
Rib cutter
-
Pituitary rongeurs
-
Graspers
-
Nerve hooks
-
Osteotomes
-
Microscissors
-
DeBakey type forceps
-
Right-angle tissue forceps
-
Peanut dissectors
-
Bone graft impactors
one area of the thoracic spine. The first portal is made in the sixth
or seventh intercostal space in the anterior axillary line. The portals
are created to minimize the risk of injury. First, the lung is deflated
on the operated side. The skin incision is made directly over the
intercostal entry sites. Blunt finger dissection directly on top of the
rib prevents injury to the intercostal neurovascular bundle. A blunt
puncture of the parietal pleura allows finger feel and dissection to
ensure that the lung is not adherent at the portal site. Then a
flexible trocar is introduced, and a 1-cm diameter
rigid-rod
endoscope with a 0-degree or 30-degree angled lens is inserted for
visualization. A flexible trocar decreases the risk of postoperative
portal pain compared with rigid trocars. Additional portals are placed
strategically for retraction of lung tissue, for placement of working
instruments, and for placement of implants. Portals are placed far
enough apart to prevent “fencing” of the instruments, and the portal
placement should triangulate over the area of the spine being worked on
(Fig. 25.2-3).
|
TABLE 25.2-1 INDICATIONS FOR THORACOSCOPIC SURGERY OF THE SPINE
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||
illustration, important structures are noted as in a right-sided
approach. The azygos vein and pulsatile aorta lie anteriorly. The
second rib can be visualized in the apex of the lung. The “mounds” of
the spine represent the intervertebral discs, and the “valleys”
represent the vertebral bodies. The segmental vessels lie in the
valleys. After confirmation of the appropriate spinal disc level using
a marking needle and radiography, the pleura is incised longitudinally.
If necessary, the segmental vessels may be ligated away from the
neuroforamen with the harmonic scalpel. The sympathetic chain lies
lateral to the prominent rib head. The rib head directs the surgeon to
the disc space, and 2 cm of the rib head is removed by dissecting it
free from the stout costovertebral and costotransverse ligaments. (The
T9 rib head leads to the T8-9 disc space, and so on.) The superior
portion of the pedicle can be removed with a high-speed drill or
Kerrison rongeur. Stabilization of working instruments against the
chest wall promotes safety. The lateral aspect of the thecal sac is
visualized so that the disc herniation can be removed safely.
Absolutely clear visualization must be maintained during decompression
of the spinal cord. After discectomy, the rib head provides adequate
bone for interbody fusion, with or without instrumentation.
desired. A small chest tube is placed under direct vision in the
posterior chest cavity directed toward the apex. Lung reinflation is
observed to check for air leaks. Portals are removed, and the wounds
are closed in layers to prevent air leak. The chest tube is connected
to water seal or suction. A postoperative chest radiograph is taken to
rule out pneumothorax and to check graft/hardware placement.
the anatomy. Any structure of the mediastinum and thorax is at risk.
Cardiac arrhythmias are prevented by avoiding use
of
monopolar cautery near the heart. Fan retractors can lacerate the lung
if not used carefully. Neurologic complications and spinal cord
injuries can occur. The surgeon always must be prepared to obtain
hemostasis if a great vessel injury should occur: A 4 × 4 sponge stick
and a thoracotomy tray should be immediately available. One must never
“plunge” with any instruments to avoid catastrophic great vessel injury.
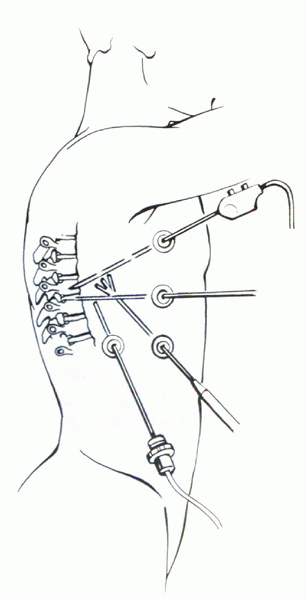 |
|
Figure 25.2-3
During thoracoscopic spine surgery, the portals should be placed far enough apart to prevent “fencing” of the instruments, while allowing triangulation over the operative area. |
-
Pneumothorax
-
Hemothorax
-
Chylothorax
-
Atelectasis
-
Pneumonia
-
Neurologic injury
-
Intercostal neuralgia
-
Infection
-
Spinal instability
-
Fixation-related problems
aggressive pulmonary physiotherapy is required to decrease the risk of
serious pulmonary complications.
midlumbar spine (L2-5). A left-sided approach is preferred because the
thin-walled inferior vena cava usually does not tolerate the degree of
manipulation that the aorta does. In addition, liver retraction is
avoided with a left-sided approach. It provides broad exposure of the
lumbar spine with less risk to the viscera and great vessels compared
with the transperitoneal approach. This approach provides unilateral
exposure of the lumbar spine. When bilateral exposure of the lumbar
spine is required, a supine-position medial incision retroperitoneal
approach or a supine-position transperitoneal approach is used.
Beanbags provide support for the torso and hips. The break in the table
should be used to widen the space between the iliac crest and ribs. The
right leg is kept straight, and the left hip is flexed to relax the
ipsilateral iliopsoas muscle to facilitate retraction. Intraoperative
radiographs are used to localize the appropriate vertebral levels and
to plan the location of the skin incision.
serratus are incised. The external oblique fascia is identified, and
the muscle is opened in line with the skin incision to the lateral
border of the rectus fascia. The internal oblique and transversus
abdominis muscles are opened. Deeply the iliocostal muscle is divided
partially posteriorly. The transversalis fascia is incised to enter the
plane of the retroperitoneal space. Carefully the peritoneum is
dissected free from the abdominal wall. Dissection of the medially
located muscle layers and fascia is more difficult, and peritoneal
tears can occur. Tears in the peritoneum are repaired. Posteriorly the
peritoneum is dissected free from the inferior pole of the left kidney
and from the posterior abdominal wall. A plane between the
retroperitoneal organs (e.g., kidney and ureter) and the quadratus
lumborum/psoas muscles is developed with blunt dissection. The
retroperitoneal organs and peritoneal sac are retracted medially and
toward the dependent side using a self-retaining, broad-based,
table-mounted retraction system (Bookwalter or Omni self-retaining
retractor system). Fluoroscopy or plain radiograph is used to confirm
the appropriate spinal level. The psoas muscle is mobilized posteriorly
using a Cobb elevator to expose the spine. Care is taken to avoid
aggressively mobilizing, retracting, or even transecting the psoas
muscle and to avoid damaging critical neural structures—the lumbosacral
plexus within the psoas muscle, the genitofemoral nerve (sensation to
the scrotum and inner thigh) on the ventral surface of the muscle, and
the sympathetic trunk along the medial border of the muscle. Avoiding
excessive use of monopolar cautery in this area is important to avoid
injury to the sympathetic trunk. Segmental vessels are identified as
the neural foramina are approached. If necessary, these vessels are
ligated midway between the parent vessel and the foramen to minimize
vascular compromise of the neuronal elements. After the segmental
vessels are secured, the aorta and iliac vessels are mobilized with a
Cobb elevator to expose the ventrolateral surface of the vertebral
bodies. The intended operation on the spine is performed. When closing,
all peritoneal and retroperitoneal contents are allowed to fall back
into normal anatomic position. Abdominal muscle layers are closed
anatomically. Skin is closed in the standard fashion.
incision is curved proximally to the end of the 11th or 12th rib. After
the retroperitoneum is entered, upper lumbar exposure is achieved by
retracting the lower pole of the kidney medially and superiorly. After
the ureter is mobilized, the left diaphragmatic crus, which extends to
the second vertebral body, is taken down.
with the retroperitoneal approach. In revision approaches to the
retroperitoneum, preoperative ipsilateral ureteral stenting can help
the surgeon identify and avoid ureter injury during dissection. Because
one or more major anterior abdominal wall muscles are divided, wound
dehiscence, herniation, and hematoma can occur. Unsightly bulging of
the abdominal musculature can result postoperatively. The surgeon
should be prepared for vascular anomaly, and instruments for dissection
and repair of vascular structures always should be available when
performing retroperitoneal or transperitoneal approaches. All bowel
perforations must be oversewn, usually with the help of a general
surgeon. Ureteral injury or delayed ureteral fibrosis can result from
excessive manipulation or traction.
is found most commonly at L5-S1, L4-5, and L3-4 levels, this exposure
commonly is required to perform anterior lumbar interbody fusion
(ALIF). Insertion of an artificial disc implant (currently undergoing
U.S. Food and Drug Administration clinical trials) requires a direct
anterior disc exposure that can be provided by this approach. The
muscles of the abdominal wall can be spared from transection with this
approach. A medial incision also allows for conversion to a
transperitoneal approach if necessary. A left-sided approach is
described.
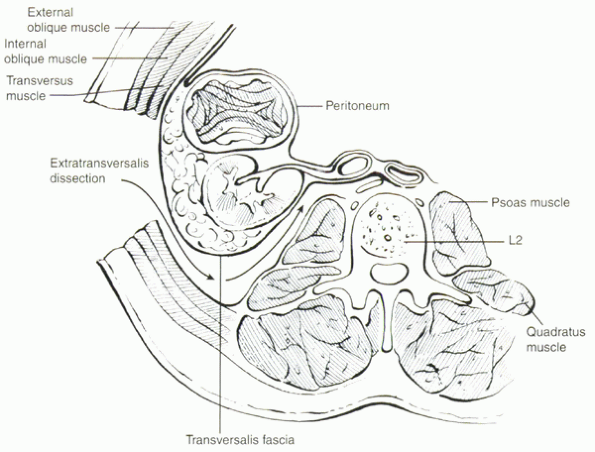 |
|
Figure 25.2-4 The plane of dissection during a retroperitoneal approach to the lumbar spine.
|
elevated (either by a bolster or by gently angling the operating table
to a Trendelenburg position) to displace the abdominal contents
rostrally. Intraoperative fluoroscopy is used to visualize the
appropriate lumbar segment in the anteroposterior and lateral
projections. This fluoroscopic localization helps to confirm the
location of the skin incision and to determine the angle of approach to
a particular disc space on the lateral view. A pulse oximeter can be
placed on the left great toe to monitor blood flow to the left lower
extremity to adjust retractor tension when deep retractors are used to
retract the great vessels during deep surgical dissection. A midline,
left-sided paramedian, or Pfannenstiel incision can be used. A midline
incision along the linea alba decreases the risk of rectus muscle
denervation as the rectus muscle is approached medially and is
retracted laterally. An incision above the umbilicus allows exposure of
L4 and above; an incision below the umbilicus allows for exposure of L4
to S1. An approach to L5-S1 generally requires an incision to extend to
just above the superior aspect of the symphysis pubis. The rectus
sheath is entered, and the left rectus muscle is retracted. The
transversalis fascia is exposed, and the plane of retroperitoneal
approach is entered. Electrocautery is used to cauterize deep
epigastric vessels during blunt retroperitoneal dissection. The
peritoneal sac is dissected from the abdominal wall using a sponge
stick. The inferior part of the posterior rectus sheath is incised to
allow better exposure of deeper structures. A table-mounted,
self-retaining retractor system (Omni) is used. As the ventral spine is
approached, the discs have a convex morphology, and the vertebral
bodies have a concave morphology.
aortic bifurcation. Injury to the superior hypogastric plexus, which
lies directly anterior to the L5 vertebral body and L5-S1 disc space,
produces retrograde ejaculation in male patients. The prevertebral
tissues, including the superior hypogastric plexus, are displaced en
bloc by blunt dissection over the disc space to decrease the risk of
hypogastric plexus injury. Use of bipolar electrocautery or vascular
clips in this area, instead of monopolar cautery, also reduces the risk
of hypogastric plexus injury. The iliac vessels are retracted laterally
to expose the L5-S1 disc space. The middle sacral artery and vein, if
present, can be ligated using clips or cauterized using bipolar
cautery. The intended procedure is performed on the L5-S1 disc.
by mobilizing the great vessels to the right. Lumbar segmental vessels
are ligated. The iliolumbar vein must be isolated and doubly ligated to
mobilize effectively the great venous structures to the right
sufficiently to expose
past the midline to the right side of the disc (Fig. 25.2-5).
Lymphatic channels may be entered during this dissection with generally
little clinical consequence. Atraumatic retractors are placed to expose
the disc space, retracting vascular structures to the right. The
intended operation is the performed on the L4-5 or L3-4 or both disc
spaces. For closure, the retractors are removed, and the peritoneal sac
is allowed to fall back into anatomic position. The abdominal wall
fascia is closed securely to prevent hernia formation. The posterior
rectus sheath can be left unrepaired. Subcutaneous tissue and skin are
closed in layers. Concurrent to the retroperitoneal exposure, a second
surgeon can harvest anterior iliac crest bone, if required, through a
separate incision.
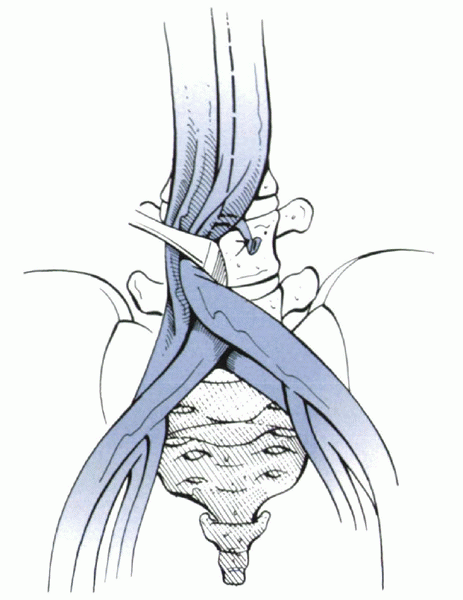 |
|
Figure 25.2-5
The level of the bifurcation of the great vessels can vary, but it usually is located at or near the L5 vertebral body. Direct anterior exposure of the L4-5 disc space usually requires lateral retraction. Ligation of the iliolumbar vein facilitates this maneuver. Exposure of the L5-S1 disc space usually can be performed by working in between the bifurcation. |
similar potential complications. Vascular and bowel injuries are more
likely to occur with transperitoneal approaches, whereas ureteral
injury occurs more commonly with retroperitoneal approaches. Both
approaches are associated with sterility in men secondary to retrograde
ejaculation due to inadvertent injury to the superior hypogastric
plexus.
bilateral exposure of the L5-S1 region. It also can be used for
exposure of L4-5, but this can be problematic because of the location
of the aortic bifurcation. The location of the aortic bifurcation can
be determined preoperatively by studying the vessel pattern anterior to
the lower lumbar spine as seen on magnetic resonance imaging. L3-4 can
be approached by mobilizing the great vessels to the right after
ligation of segmental lumbar vessels and the ascending lumbar vein. The
transperitoneal approach is contraindicated in the presence of spinal
infection.
and broad-spectrum antibiotics are used preoperatively. The patient is
placed in the supine position with the sacrum angled and elevated
upward on the operating room table. The Trendelenburg position allows
the abdominal contents to shift into the upper abdomen. Skin incision
caudal to the umbilicus provides exposure of L5-S1. A midline or
transverse (Pfannenstiel’s) incision may be used. Fluoroscopy is used
to identify the level of the spinal lesion. A small incision of less
than 6 cm can be used for exposure of one or two disc spaces, and this
“mini-open” approach is adequate for ALIF procedures. The incision is
carried down in the midline through the subcutaneous tissues, fascia,
and peritoneum. After the abdominal contents are retracted rostrally
with a table-mounted, self-retaining retractor, the posterior
peritoneum is exposed. The pelvic portion of the colon and its
mesentery are retracted to the left, and the ureters are identified and
protected. The retroperitoneal space is entered by incising the
posterior peritoneum in the midline and extending the peritoneal
opening caudally past the aortic bifurcation by following the course of
the right common iliac artery to its bifurcation at the external and
internal iliac arteries. The right ureter is identified and avoided as
it crosses over the right external iliac artery.
palpated easily and visualized below the aortic bifurcation. The
prevertebral tissues, including the hypogastric plexus and the middle
sacral artery, are identified and mobilized laterally. When a large
left common iliac vein hinders access to the body of L5 and the sacral
promontory, it must be mobilized properly and protected before the disc
space is incised. The left iliac vein occasionally can appear as a
flat, white, bloodless ribbon across the L5-S1 disc space. Retrograde
ejaculation is one of the significant complications directly resulting
from insult to the hypogastric plexus; monopolar cautery should be
avoided to reduce risk of damage to the hypogastric plexus. Retractors
are placed to expose the L5-S1 disc space (Fig. 25.2-6).
lumbar levels is possible with significant vascular dissection and
mobilization; the retroperitoneal route usually is a more appropriate
choice for accessing the upper lumbar and midlumbar spine. At the
conclusion of the spinal procedure, the posterior peritoneum is closed
with 3-0 polyglactin 910 (Vicryl) suture, the bowel and omentum are
returned to their normal anatomic position, and the abdominal fascia
and anterior peritoneum are closed with interrupted sutures. The
subcutaneous tissue and skin are closed. Postoperatively an ileus is
expected.
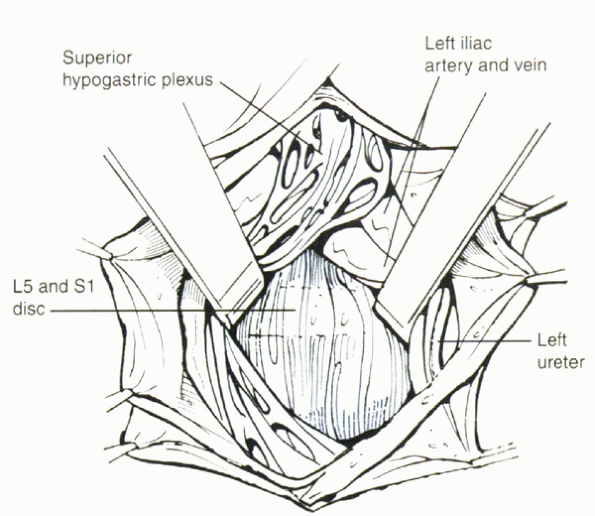 |
|
Figure 25.2-6
Using a transperitoneal approach, the prevertebral tissues, including the hypogastric plexus and the middle sacral artery, are identified and mobilized laterally. When a large left common iliac vein hinders access to the body of L5 and the sacral promontory, it must be mobilized properly and protected before the disc space is incised. The left iliac vein occasionally can appear as a flat, white, bloodless ribbon across the L5-S1 disc space. |
“approachrelated” trauma compared with open approaches. A laparoscopic
transperitoneal approach can be used to perform ALIF in the lower
lumbar spine and lumbosacral junction. Laparoscopic techniques require
additional equipment and special operating room setup and are
associated with a steep learning curve. Laparoscopic ALIF can be used
as a “stand-alone” procedure, or it can be combined with posterior
spinal fusion with instrumentation using minimally invasive or open
techniques. Similar to open techniques, injuries to deep anatomic
structures potentially can occur.
an optimal view of the C-arm monitor and the video monitor. The patient
is placed supine on a radiolucent table in the Trendelenburg position
to displace the abdominal contents rostrally out of the pelvis. Pillows
are placed under the patient’s hips to accentuate lordosis at the
lumbosacral junction and beneath the knees to prevent hyperextension. A
nasogastric tube and Foley catheter are placed to decompress the
stomach and bladder.
four incisions are used: Two paramedian incisions provide conduits for
the working forceps; an umbilical incision provides access for the
viewing camera; and the incision for the interbody working channel and
devices is centered over the midline suprapubic region and measures 2
to 3 cm in width (Fig. 25.2-7). The viewing
camera is placed through the umbilical portal and is held by a robotic
arm (AESOP; Computer Motion Inc, Goleta, CA). Sealing trocars are used
to allow carbon dioxide insufflation (15 mL/min at 10 mm Hg pressure).
sacral promontory is identified by palpation and by fluoroscopy, and
the midline is confirmed by fluoroscopy. The peritoneum is opened
sharply. In male patients, unipolar cautery should be avoided, and a
blunt Kittner dissector is used with a gentle sweeping motion to
mobilize the presacral sympathetic plexus. This maneuver may help to
decrease the incidence of retrograde ejaculation, which is a known
complication associated with anterior approaches in the lumbosacral
region. In female patients, unipolar cautery can be used to expose the
anterior face of the vertebral body and disc space.
artery and vein, which are ligated and divided. These vessels do not
predict the midline of the vertebral body. The anterior curvature of
the lumbosacral junction is palpated, as are the left and right lateral
convexities in conjunction with fluoroscopy to help delineate the
midline. The left iliac vein protrudes more anteriorly and may require
more retraction. When the disc space is well exposed with protective
retractors in place, ALIF with grafting can be performed. After
completion of the ALIF, the peritoneum is closed using clip or running
suture ligation. The abdominal wall incisions are closed with
interrupted absorbable suture.
retroperitoneal flank approach to the upper lumbar and midlumbar discs.
The long flank incision is replaced by four smaller endoscopy
incisions. The patient is placed in the fully lateral position with the
appropriate disc space localized with a C-arm.
The
initial viewing portal is established directly over this disc space,
and an optical trocar is inserted and used to dissect through the
retroperitoneal space down to the level of the psoas muscle. The
retroperitoneal space is dilated and maintained with carbon dioxide
insufflation. Three additional working/visualization portals are
established. The anterior limit of the retroperitoneaum is delineated
by a line between the anterior tip of the 11th rib and the anterior
superior iliac spine (Fig. 25.2-8).
A marking needle is used to confirm the trajectory into the pathologic
disc space. The disc space is reached by splitting the fibers of the
psoas longitudinally or by retracting the psoas muscle posteriorly.
Endoscopic curets and pituitary rongeurs are used to perform discectomy
and scraping of the end plates. A single lateral fusion cage is
inserted with autograft harvested from the ipsilateral iliac crest. The
final position of the cage is confirmed with the C-arm in
anteroposterior and lateral projections. Hemostasis is obtained with
cautery-tipped graspers or with the harmonic scalpel. The endoscopic
instruments are removed, and the small fascial incisions are closed
with absorbable stitch. Skin and subcutaneous tissue are closed in the
usual manner.
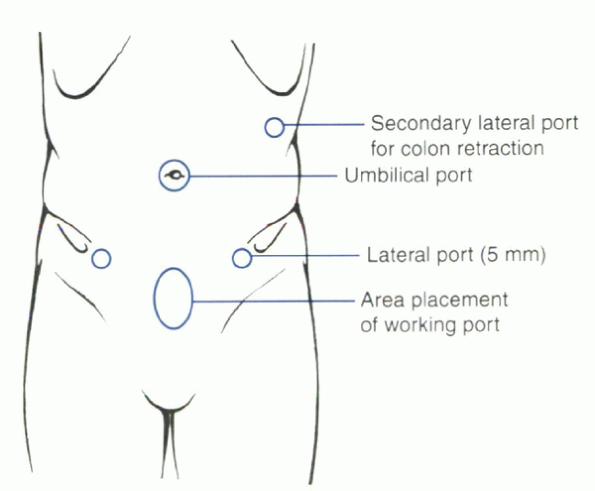 |
|
Figure 25.2-7 Location of portals for laparoscopic lumbar surgery.
|
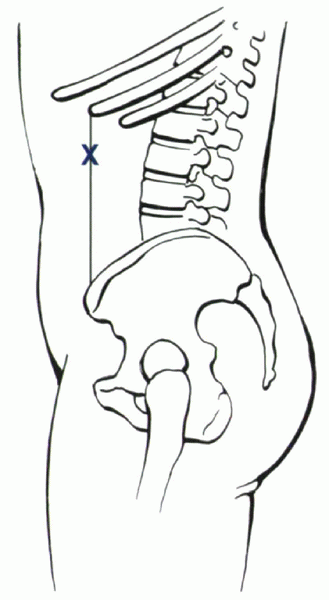 |
|
Figure 25.2-8
The initial skin incision for endoscopic lumbar surgery. The anterior limit of the retroperitoneum is delineated by a line between the anterior tip of the 11th rib and the anterior superior iliac spine. |
performed by using a specialized retractor system with custom deep
blades when the retroperitoneal cavity has been established by gas
insufflation, balloon dissection, or manual dissection. A gasless
retroperitoneal endoscopic approach to L5-S1 can be performed with the
patient in a supine position. The retroperitoneal cavity is established
via a midline infraumbilical incision and an endoscopic portal in the
right or left flank. A specialized retractor system is used to expose
the L5-S1 disc space.
MK, Green C. A review of the management of lumbar fractures with focus
on surgical decision-making and techniques. Contemp Neurosurg
1999;21:1-5.
RM, Murphy MJ, Southwick WO. Surgical approaches to the spine. In:
Rothman RH, Simeone FA, eds. The spine. Philadelphia: WB Saunders,
1999:1537-1571.
K, Taneichi H, Abumi K, et al. Anterior decompression and stabilization
with the Kaneda device for thoracolumbar burst fractures associated
with neurological deficits. J Bone Joint Surg 1997; 79A:69.
TG, Cloyd D. laparoscopic lumbar discectomy: description of
transperitoneal and retroperitoneal techniques. Neurol Clin North Am
1996;7:77-85.
D, Paolucci V, Zdeblick A. Combined endoscopic retroperitoneal approach
to the lumbar spine using microsurgical endoscopy. In: Zdeblick TA, ed.
Anterior approaches to the spine. St. Louis: Quality Medical
Publishing, 1999:219-241.
authors and do not reflect the official policy or position of the U.S.
Government, the Department of Defense, or the Department of the Air
Force.
lumbar pathology. It allows easy access to multiple levels for
decompression, instrumentation, and arthrodesis. It is a versatile
exposure through which many procedures can be performed, such as:
-
Microdiscectomy
-
Laminotomy/laminectomy/laminoplasty
-
Facet fusion
-
Intertransverse fusion
-
Posterior lumbar interbody fusion
-
Instrumentation
isolated anterior procedure without posterior exposure may be
preferred. Previous midline surgery, such as laminectomy, may be
considered a relative contraindication because reexposure increases the
risk of durotomy, but a second midline approach often remains the best
option for revision surgery.
of the facet joints and transverse processes without midline exposure
or significant retraction of the paraspinal muscles. This approach is
ideal for patients undergoing posterolateral fusions (with or without
instrumentation) who do not need midline decompression. It is
especially useful in avoiding the midline of patients with previous
laminectomy who do not require revision decompression because direct
access to the fusion bed is permitted without dissection around midline
scar tissue. Finally, a paramedian approach is the ideal exposure for
excision of far-lateral disc herniations.
access to the anterior column; however, posterolateral approaches, such
as costotransversectomy and lateral extracavitary exposures, can allow
excellent visualization of the ipsilateral pedicle and thecal sac to
the midline, while avoiding a formal thoracotomy. Indications include
tumor, trauma, degenerative conditions, and infections involving the
ventral or ventrolateral aspect of the spinal column or thecal sac.
three-dimensional perspective of the pathologic lesion, including the
important relationships between the bone, neural, and surrounding
visceral structures. Computed tomography of the bone and magnetic
resonance imaging in the axial, coronal, and sagittal planes help
create the three-dimensional image in the surgeon’s mind. With this
image and the knowledge of normal anatomic relationships, the surgeon
can rehearse the operation in his or her mind in a stepwise fashion,
predicting areas of difficulty. This rehearsing also helps predict if a
selected approach would give adequate exposure to address the pathology
completely.
abnormal anatomy. The number of non-rib-bearing lumbar vertebrae is
noted. Lumbarized or sacralized distal segments are noted. The
numbering of vertebrae on the plain films must coincide with the
numbering on ancillary studies. Surgeons tend to start counting the
lumbar vertebrae caudally, backward from the last mobile segment,
whereas radiologists number starting at the first non-rib-bearing
vertebrae. This difference may result in a discrepancy between the
numbering of the surgeon and radiologist if a transitional vertebra is
present. Additionally, preoperative radiographs can identify spina
bifida occulta and bifid sacral areas that lack protective bone
overlying the dura and neural elements.
antibiotics and deep venous thrombosis prophylaxis with thigh-high
stockings and sequential compression devices. Provisions are made for
electrophysiologic monitoring if deformity is to be corrected. With few
exceptions, patients are placed in a prone position on an operative
frame allowing the abdomen to hang free, diminishing the amount of
epidural venous engorgement and reducing operative blood loss. For
uninstrumented procedures, the patient may be placed on a Wilson frame
to expand the interlaminar spaces. We prefer to use the Jackson table
with the hips extended for instrumented lumbar procedures to prevent
loss of lumbar lordosis. We do not place patients in the lateral
decubitus or
three-quarter
prone position for posterior or posterolateral approaches. These
positions can be disorienting and make it difficult for the surgeon and
assistant to have adequate visualization.
with the arms abducted not more than 90 degrees to avoid brachial
plexus stretch injury. If the procedure involves the upper thoracic
spine, the arms are tucked at the patient’s side. The arms are padded
adequately with special attention paid to the ulnar groove, avoiding
peripheral compressive neuropathy. The head is placed in a neutral
position, avoiding extension or pressure on the orbits. For longer
procedures or procedures involving the cervicothoracic junction, the
head is secured with three-point fixation.
landmarks. The bone landmarks help orient the surgeon and limit the
dissection necessary during the initial exposure. The patient is
prepared and draped widely, allowing proximal or distal extension if
necessary. The area also should include the iliac crest if bone graft
is to be harvested. Intraoperative radiography and fluoroscopy are key
to identifying definitively the appropriate operative level.
similar, allowing for either unilateral or bilateral access to the
posterior spinal canal at any vertebral level. The incision is marked
in the midline and infiltrated with epinephrine to aid in hemostasis.
We prefer a straight midline incision as opposed to transverse, “T,”
hockey-stick, or omega incisions because all the latter stray off
midline, increasing the chance of skin necrosis or poor wound healing.
The skin and subcutaneous tissues are incised with a scalpel. Weitlaner
or cerebellar self-retaining retractors are placed. The muscle fascia
is incised in the midline with electrocautery. It is important to
adhere to the midline in a subperiosteal plane because dissection
within the muscle needlessly increases bleeding. Although there are
many ways to perform the subperiosteal dissection, we prefer to use
electrocautery and either a periosteal elevator or a sturdy suction tip
for retraction. In the presence of bone destruction by tumor, trauma,
infection, or intrinsic bone disease, blunt dissection with a
periosteal elevator may result in catastrophic neural injury.
Difficulty also may occur in patients with previous laminectomy. Risks
are minimized by identifying areas of normal anatomy above and below
the laminectomy defect, which act as a guide to identifying the dural
plane, then dissecting the scar off the dura.
facet capsules. Injury to the facet capsule cephalad to a fusion can
increase the risk of subsequent adjacent-level instability. It often is
easier for the contralateral surgeon to perform the dissection lateral
to the facet and along the transverse process in the lumbar spine or
the ribs in the thoracic spine. With lateral dissection, it is
important to identify and coagulate the arteries on the lateral aspect
of the pars interarticularis and the lateral aspect of the facet joint.
As dissection is deepened, bigger self-retaining retractors are placed.
We use angled Gelpi retractors because of their small radiographic
profile and their small surface area, which minimizes muscle trauma and
ischemia.
harvesting of posterior iliac crest bone graft. The midline
thoracodorsal fascia is reapproximated temporarily with towel clips. A
plane is developed just superficial to the thoracodorsal fascia out to
the posterior superior iliac spine, then along the iliac crest
laterally. The thoracodorsal fascia inserts onto the iliac crest from
above, and the fascia of the gluteus maximus and medius insert onto the
iliac crest from below. The dissection is made on the curve of the
iliac crest to minimize blood loss. Then the gluteus can be elevated
subperiosteally off the posterior surface of the iliac crest allowing
harvesting of corticocancellous bone graft. Alternatively a separate
vertical incision is made overlying the iliac crest. An oblique
incision along the crest should be avoided because this runs
perpendicular to and endangers the superior cluneal nerves, resulting
in diminished sensation over the posterior buttocks and the potential
formation of painful neuromas.
to the lumbar spine. This approach may be performed with a midline or a
paramedian skin incision. In either case, a fascial incision is placed
approximately two to three fingerbreadths (approximately 3 to 4 cm)
lateral to the midline. The key to this exposure is to identify the
intermuscular plane between the multifidus and longissimus muscles.
This provides an avascular plane that directly leads to the junction of
the lateral aspect of the facet and the medial transverse process.
There is frequently a small line of indentation in the fascia
identifying this plane. After opening the fascia, the index finger is
used to dissect through the muscle mass down to the facet joints and
transverse processes. While palpating for the right plane, it is easy
to mistake the facet for the tip of the transverse process because the
transverse process is much deeper than expected. After placement of a
self-retaining retractor, the transverse process and lateral pars
interarticularis are stripped of muscular attachments.
approach far-lateral disc herniations. Smaller unilateral paramedian
skin and fascial incisions are made. Then the muscle-splitting
dissection is performed and self-retaining retractor placed. By
incising and reflecting the intertransversarii muscle, the offending
far-lateral disc and exiting nerve root are visualized. Extreme care
should be used when dissecting around the dorsal root ganglion to avoid
postoperative neuropathy.
is a transverse incision over the level of the far-lateral disc
herniation. Dissection between the erector spinae and the ventral
quadratus lumborum exposes the transverse process. At this point, it is
imperative to use a table-mounted retractor to reflect the erector
spinae muscles medially and
dorsally. The resulting angle of exposure is similar to that with the muscle-splitting approach.
|
TABLE 25.3-1 SPECIFIC TECHNIQUES
|
||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
posterolateral approaches and is described first. A midline skin
incision long enough to expose three levels above and three levels
below the planned decompression is needed to allow for lateral muscle
retraction. Usually this approach is supplemented with posterior
instrumentation so that bilateral subperiosteal muscle takedown is
performed out to the tip of the transverse processes. We do not
advocate transverse section of the paraspinal muscles because this
weakens them.
two or three levels above and below the pathologic level before any
decompression. A temporary rod is connected to the pedicle screws
contralateral to the planned approach. This rod allows for temporary
stabilization and avoids any movement that may jeopardize the spinal
cord as the spine is progressively and often circumferentially
destabilized. Dissecting from medial to lateral under the paraspinal
muscles exposes 3 to 5 cm of each rib. By starting at the midline, the
dissection can proceed deep to the trapezius and rhomboid muscles in
the upper thoracic spine and directly onto the ribs.
is removed by incising the costotransverse ligaments, freeing the
transverse process from the underlying rib. Usually the rib at the
level of the lesion and one level below are removed for adequate
exposure. The neurovascular bundle is protected as the ribs are
dissected free with a Doyen dissector. The ribs are cut 3 to 5 cm
lateral to the junction of the rib and the transverse process. The
radiate ligament is incised, freeing the rib from the vertebral body.
The freed rib is removed and saved for bone graft. The pleura is
dissected bluntly off the anterolateral vertebral bodies.
neural foramen. The intercostal artery and nerve root at the pathologic
level are identified, doubly ligated, and divided. This should not be
done bilaterally to avoid spinal cord devascularization. It is
important to ligate the nerve proximal to the dorsal root ganglion to
avoid painful neuroma formation.
After
exposure, decompression can be performed anteriorly and posteriorly.
The vertebral body can be reconstructed and bilateral posterior
instrumentation placed as needed.
costotransversectomy to allow farther lateral approach to the vertebral
body. A midline, paramedian, or hockey-stick incision is made exposing
at least three levels above and below the pathology. A subcutaneous
flap is developed exposing the midline and lateral edge of the
paraspinous muscles. The thoracodorsal fascia is incised in a “T” or
paramedian manner. In the thoracic spine, the upper back musculature
(trapezius, latissimus dorsi, and rhomboids) is flapped from the
midline or split along their fibers to access the underlying dorsal rib
cage lateral to the paraspinous muscles.
medial off the dorsal rib cage or the quadratus lumborum in the lumbar
spine. If the erector spinae is extremely bulky, a vertical
muscle-splitting incision eliminates excessive muscle retraction, but
it provides a less ventral view of the dural sac. The paraspinal
muscles are retracted to the contralateral side. If instrumentation is
planned, the paraspinal muscles also are dissected off the midline
structures (spinous process and lamina) to allow placement of posterior
instrumentation.
three ribs are resected, and the procedure proceeds as with
costotransversectomy described previously. In the lumbar spine, the
nerve roots cannot be sacrificed owing to their contribution to lower
extremity function. This fact along with the large bulk of the
paraspinous muscles in the lumbar spine significantly complicates
exposure below the L2 level.
it is mandatory to perform a neurologic exam as the patient is waking
up in the operating room, followed by a more complete exam after the
effects of anesthesia have worn off. Patients are mobilized as quickly
as possible to improve pulmonary toilet and to diminish the risk of
deep venous thrombosis. The need for a postoperative orthosis is
dictated by the amount of instability due to the pathologic process and
the amount of surgical destabilization. It is imperative after the
posterolateral approaches to check a chest radiograph to evaluate for
pneumothorax.
|
TABLE 25.3-2 MORBIDITY OF POSTERIOR THORACIC AND LUMBAR APPROACHES
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
morbidity associated with posterior thoracic and lumbar approaches.
Many of these complications are similar to complications seen with any
other surgical procedure and need no further explanation.
RG, Dietze DD, Millan MM, Peace D. Lateral parascapular extrapleural
approach to the upper thoracic spine. J Neurosurg 1991;75:349-355.
SJ, Holst R, Hemmy DC, Sances A. Lateral extracavitary approach to
traumatic lesions of the thoracic and lumbar spine. J Neurosurg
1976;45:628-637.
