Bone and Soft Tissue Reconstruction
One – General Principles: Basics > Principles of Treatment > 14 –
Bone and Soft Tissue Reconstruction
are difficult to treat and often require a multidisciplinary approach
for wound management. These wounds place a significant financial burden
on the patient and society because of prolonged patient disability.
Despite the great diversity among the individuals who sustain open
fractures, a majority of these patients are typically young active
adults who tend to be injured in automobile or motorcycle accidents or
while engaged in sporting activities.86
of the bone as well as the soft tissue injuries. Advances in
microsurgical
techniques
and our knowledge of the vascular anatomy of the extremities have led
to novel advances in wound coverage that can allow for rapid coverage
of these wounds and replacement of injured bone, nerve, and muscle. In
this chapter, we will review a multidisciplinary approach for the
management of bone and soft tissue defects, which includes a
combination of orthopaedic surgery and plastic surgery expertise, in
addition to providing the reader with a variety of reconstructive
options for upper and lower extremity open fracture management.
the time of Hippocrates, who described crude attempts at external
fixation for the purpose of examination and treatment of open fractures.4
During the ancient Egyptian period, documents demonstrate that complex
fractures were treated expectantly, and open fractures were considered
a mortal injury.8 It was not until
the sixteenth century, when Ambroise Paré, the French barber-surgeon,
revolutionized surgical management of open extremity injuries by
developing amputation techniques which used tourniquet control of blood
loss, as well as hemostatic clamps and vascular ligatures.227
It is only in the eighteenth century, that Percival Pott, a renowned
surgeon and educator of the English, introduced the option of limb
salvage after open fracture through the use of fracture reduction and
wound management.
reconstruction, external and internal fixation and antimicrobial agents
have minimized the chances of mortality after an isolated open
extremity fracture. Continuing advances in the field of microsurgery,
including refinement and development of free tissue transfers, and
pedicled and local flaps, as well as a better understanding of wound
pathophysiology have improved the surgeons ability to obtain rapid
wound coverage, allowing patients to return to ambulation and the
workforce; however, many challenges remain and the patient may still
succumb to local infection or other soft tissue or bony complications
requiring amputation after major limb trauma.
treatment of bone and soft tissue reconstruction, we have a
responsibility to carefully evaluate our results in a sound
evidence-based fashion. Comparisons of different types of treatment,
and careful evaluation of their outcome, complications, and
cost/benefit analysis are essential to providing the optimal treatment
for these injuries.
and the management of the soft tissue is often as important as the
treatment of the fracture itself.389
Historically, the outcome of the treatment of open fractures was
typically determined by the soft tissue defect. In 1966, Carpenter
stated that “If the soft tissues overlying the tibia are not preserved,
any hope of primary healing of the underlying fracture is lost forever.”58
Although Carpenter was referring to the tibia, the importance the soft
tissue envelope to bone healing is real and applicable throughout the
body. If soft tissue reconstruction is successful in these injuries,
the bone often becomes the problematic area, and the final outcome
depends on the extent of bone devascularization and contamination.168
prevented the orthopaedic surgeon from adequately débriding the soft
tissues. This has resulted in the “expectant” management of the soft
tissues, an approach that unfortunately still prevails in some
surgeons’ minds today. Waiting for devitalized tissue to “declare
itself” prolongs definitive fracture management, increases the risk of
infection, and attenuates the inflammatory response. Pedicled flaps and
free tissue transfers are capable of covering large soft tissue
defects, thus allowing the surgeon the freedom to perform a wide and
thorough débridement. Early collaboration and communication with
surgeons skilled in these techniques is crucial for successful outcomes.
of these injuries?” Although each surgical subspecialty may feel that
they are the most appropriate ones to care for these injuries, it is
important to understand that optimal collaboration is often the best
means to treat these highly complex wounds. Even if the primary
treating orthopaedic surgeon is not trained in microsurgical
techniques, knowledge of the prerequisites, timing, and availability of
soft tissue and bone reconstruction will affect the initial treatment
plan.151 A surgeon or group of
surgeons highly familiar with the soft tissue as well as bony anatomy,
in addition to having microsurgical skills, is optimally the best
suited to address these injuries.
management begins with application of Advanced Trauma Life Support
(ATLS) protocols.1 Once the basics
of ATLS are satisfied, a complete assessment of each wound can be made.
Understanding the mechanism of injury and the patient’s unique medical
and social history are imperative. When possible, a discussion of the
possible reconstructive options should be discussed with the patient
and family.
is stabilized, the surgeon begins the detailed evaluation of the open
fracture and the associated soft tissue deficit. It is imperative to
determine the time of injury, the mechanism of injury, fracture
configuration, associated systemic injuries or medical conditions, the
degree of soft tissue injury, the vascular, sensory, and motor status
of the extremity, and the patient’s occupational and leisure time
activities. All these factors ultimately influence the decision for
limb salvage versus amputation, and must be addressed. Prevention of
further injury is paramount. A careful motor, sensory, and vascular
examination can determine if a compartment syndrome or dysvascular limb
is present. Identification and documentation of nerve injuries and
associated injuries as part of the secondary ATLS survey is also
performed.
wound pattern and any contamination documented. Photographic
documentation can be helpful when available. Wounds should not be
explored in the emergency department setting, but rather, exploration
should be performed whenever possible in the sterile conditions of an
operating room. With polytrauma patients, where the work-up of other
injuries takes priority over treatment of the open fracture/soft tissue
injuries, careful packing
of
the wound with a sponge moistened with saline and diluted antiseptic
solution (Betadine or chlorhexidine) prevents desiccation of the
exposed bone and soft tissues until they can be addressed formally in
the operating suite.
injuries have followed the initial attempts of Cauchoix and associates,
who were mainly interested in the size of the skin defect, degree of
soft tissue crush injury, and complexity of the fracture.59
Rittmann et al. divided the severity into three groups, focused on the
amount of necrotic and contaminated material found in the wounds in
addition to the degree of neurovascular injury.328,329
open fractures and described a classification system that is still in
use today with various modifications by multiple authors.154,155,156,157,159,160
In their classic paper, Guistilo and Anderson devised a three-tier
classification system: Type 1 fractures have a clean wound smaller than
1 cm in size; type 2 wounds have a soft tissue injury larger than 1 cm
and without extensive soft tissue damage; and type 3 wounds are severe
soft tissue lacerations with segmental or severely comminuted fractures
in high energy trauma.156,157
In 1982, Gustilo and Mendoza reviewed their expanded experience of 1400
open fractures and noted that there were five factors associated with
final outcome: the degree of soft tissue injury, the adequacy of
débridement, appropriate use of antibiotics, fracture stability, and
early soft tissue coverage.158 In
1984, Gustilo et al. identified differences within the type III
fractures and further subdivided that group into three subgroups based
on the soft tissue injuries. The type IIIA injuries are those with
large soft tissue injuries or flaps that still had adequate soft tissue
coverage of bone. This group also includes fractures with severe
comminution or segmental fractures regardless of the size of the soft
tissue damage. The type IIIB fractures are those with extensive soft
tissue loss and much devascularized bone with massive contamination,
and type IIIC fractures are those associated with an arterial injury (Table 14-1).
lacerations, abrasions, contusion, degloving injuries, and burns. In
addition, soft tissue damage can occur in absence of frank skin
laceration and can result in tissue damage that is even more extensive
than that seen in open fractures.389,390,391
Closed injuries that are associated with skin contusions, deep
abrasions, burns, or frank separation of the dermal layer from the
subcutaneous tissues have been classified by Tscherne (Table 14-2).389,390,391
Although not critically validated, this classification system has
heightened our awareness of the importance of soft tissue injuries
associated with closed fractures.
|
TABLE 14-1 Gustilo and Anderson Classification of Open Fractures of the Tibia156,157,158
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
TABLE 14-2 Tscherne et al.389,390,391 Classification of Soft Tissue Injury Associated with Closed Fractures
|
||||||||
|---|---|---|---|---|---|---|---|---|
|
the severity of the underlying soft tissue injury. Penetrating injury
will cause local and immediate surrounding tissue trauma; therefore,
the surgical débridement required will typically be limited to the
surrounding region of penetration. Blunt force resulting from motor
vehicle crashes or falls will lead to more extensive soft tissue trauma
and possible associated neurovascular injury with increased muscle
contusion, devascularization, and necrosis. A ringer injury or press
injury typically carry a poorer prognosis because of the amount of
associated tissue damage. Electrical injuries associated with fracture
may appear innocuous but will always be associated with significant
underlying soft tissue damage.
the surgeon must have some understanding of the normal wound healing
process. Surgically induced wounds heal in several stages. The wound
passes through phases of coagulation, inflammation, matrix synthesis
and deposition, angiogenesis, fibroplasia, epithelialization,
contraction, and remodeling. These processes have been divided into
three main stages: inflammation, fibroplasia, and maturation.
Interruption in any of these stages can lead to wound healing
complications.201
cellular responses to clear the wound of debris and devitalized tissue.
Increased capillary permeability and leukocyte infiltration occur
secondary to inflammatory mediators and vasoactive substances.
Inflammatory cells clean the wound of harmful bacteria and devitalized
tissue. Fibronectin and hyaluronate deposition from fibroblasts in the
first 24 to 48 hours provides scaffolding for further fibroblast
migration.118,368
first 2 to 3 days as large populations of fibroblasts migrate to the
wound. Secretion of a variety of substances is necessary for wound
healing and includes large quantities of glycosaminoglycans and
collagen. Collagen levels rise for approximately 3 weeks corresponding
to increasing wound tensile strength. After 3 weeks the rate of
degradation equals the rate of deposition. Angiogenesis
is an important aspect of the fibroblast proliferation phase, as it helps to support new cells in the healing wound.
to 2 years. It is characterized by collagen remodeling and wound
strengthening. Collagen is the principal building block of connective
tissue and is found in at least 13 different types. Early wounds are
comprised of a majority of type III collagen. As the wound matures,
type III collagen is replaced by type I collagen. Collagen
cross-linking improves tensile strength. There is a rapid increase in
strength of the wound by 6 weeks as the wound reaches 70% of the
strength of normal tissue. The wound then gradually plateaus to 80% of
normal strength, but never returns to preinjury levels.201
migrate through a sequence of mobilization, migration, mitosis, and
cellular differentiation of epithelial cells. Wound contraction starts
at about 1 week. It is facilitated by the transformation of certain
fibroblasts into myofibroblasts containing α-smooth muscle actin. These
cells adhere to the wound margins as well as to each other and effect
contraction of the wound. These stages are imperative for proper wound
healing, as interruption of these processes results in chronic wound
complications.118,368
heal through a process of “secondary wound healing,” which is dominated
by wound contraction and re-epithelialization. If infection, ischemia,
or ongoing trauma inhibit the wound from completing the
re-epithelialization process, the wound will then enter into a
protracted inflammatory state.268 In
these chronic wounds the wound environment is predominated by
neutrophils with the increase production of proteolytic enzymes.101
In most situations a chronic wound must be converted to a clean acute
wound through the process of surgical débridement for healing to occur.
Surgical débridement re-establishes a normal healing environment,
allowing the wound to heal through primary or secondary intention.
injury there are several principles one should keep in mind to expedite
patient care and maximize patient outcome (Table 14-3).
understanding the mechanism of injury, one must determine whether a
compartment syndrome129 may be an
issue or if ongoing vascular compromise is present. Any salvage of the
extremity is dependent on the prevention of further injury or the
neutralization of ongoing injury.
aggressive tumor-like débridement of all necrotic and nonviable tissue,
including bone, is essential.133
This is often considered the most important single step in the
management of soft tissue trauma and will be further discussed later in
this chapter. Often reconstructive plans impede adequate soft tissue
débridement, as the surgeon is afraid to lose further soft tissues,
which would make the reconstruction more complicated or difficult.
|
TABLE 14-3 The Eight General Principles of Management of Soft Tissue Injuries Associated with Fractures
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
been accomplished, bone stability should be achieved. Bone stability
can be achieved with external fixation, internal fixation, or a
combination of both. In highly contaminated wounds or wounds that have
poor soft tissue coverage, external fixation is often preferred. In
wounds that are adequately débrided with good soft tissue coverage of
the bone, internal fixation can be used.
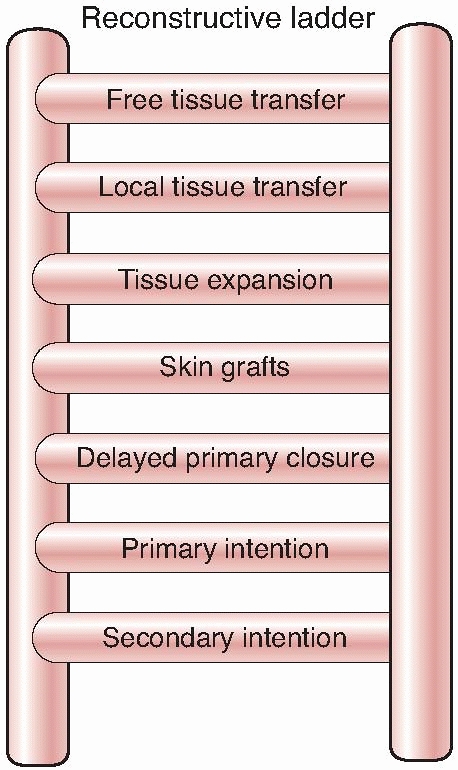
When soft tissue coverage is considered, a surgeon should evaluate the
simplest type of procedure needed to achieve wound coverage, and
increase in complexity as needed. The reconstructive ladder progresses
as follows: primary closure, skin grafting, local cutaneous flaps,
fasciocutaneous transposition flaps, island fascial or fasciocutaneous
flaps, local or distant one-stage muscle or myocutaneous transposition,
distant temporary pedicle flaps, and microvascular free tissue
transfer. When evaluating the wound for possible coverage options, it
is imperative to consider patient factors; defect genesis; the
location, size, and depth of the defect; exposed structures; structures
needing reconstruction; the degree of contamination; and the quality of
the surrounding tissues.
Although the data presented by Godina is compelling, achieving wound
coverage within 72 hours can be difficult secondary to both hospital
system issues (operating room and surgeon availability) and patient
factors. With advances in wound management with vacuum-assisted closure
devices and antibiotic bead pouches, wound coverage can occur later
than the 72 hours initially recommended without untoward complications.133
 |
|
FIGURE 14-1 The reconstructive ladder.
|
secondary reconstructive needs are often ignored. It is important to
determine these needs before the soft tissue coverage and initial
reconstructive procedure. If nerve grafts need to be placed in the
future, the vascular pedicle of the free flap should be placed as far
away from the nerve graft sites whenever possible. If future bone
grafting (vascularized or conventional) or tendon work needs to be
performed, planning of the location of the free flap or pedicled flap
needs to occur to prevent future injury to the flaps vascular supply of
the flap, potentially compromising its survival or soft tissue coverage.
tissue reconstruction should be considered. Composite reconstruction
refers to the use of flaps that contain more than one type of tissue.
Such an example is an osteocutaneous flap, such as a free fibular
graft, which may contain bone, skin, and muscle. This piece of
composite tissue can then be used to replace segmental defects of the
tibia and replace any overlying skin loss at the same time. The concept
of replacing like tissue with like tissue when possible can be applied
to upper extremity injuries as well. As a general rule when there is a
need for bone, muscle, and skin, one should always consider the
possibility of reconstructing the defect with a composite flap.
reasonable solution in selected situations. Although technically
feasible, some heroic efforts to reconstruct parts can lead to
prolonged recovery times with loss of gainful employment, psychological
problems, and increased morbidity for the patient.
much and should seek assistance and advice. This is the most humbling
of the principles, but can be one of the most important. Collaboration
with other surgeons may be extremely helpful in difficult cases. A
different perspective can often drastically change the patient’s
outcome.
severe soft tissue injury are typically polytrauma patients with
multiple organ systems affected. As such, their fractures and soft
tissue injuries must be considered in the context of the polytrauma,
recognizing the patient as a whole. Care of these patients and their
injuries progresses in three phases: acute stabilization,
reconstruction, and rehabilitation. The acute phase includes wound
débridement, fracture stabilization, soft tissue reconstruction, and
initiating muscle function and joint mobility. The reconstructive phase
addresses indirect sequelae of injury, such as nonunions, infections,
and malunions. Finally the rehabilitative phase focuses on returning
the patient to society.
after the ATLS protocols. The injuries are stabilized, wounds are
débrided as necessary, antimicrobial measures are taken, and early soft
tissue coverage is achieved. Local infection control must be tailored
to the clinical situation once adequate tissue cultures have been
obtained. There is great debate as to the appropriateness of broad
spectrum antibiotic therapy in cases of open fractures and protracted
wound closure involving multiple débridements.80,172,215,308
Level 1 data support the use of a short course of a first-generation
cephalosporin beginning immediately after injury, in conjunction with
rapid débridement and fracture management.172
management of any traumatic wound. Adequate débridement requires the
complete removal of foreign material and devitalized tissue. Inadequate
débridement can promote wound infection, delay surgical healing, and
attenuate the inflammatory response.
take place as soon as possible after the injury, under general
anesthesia in the operating room. Débridement performed in the
emergency room or on the ward is often inadequate, as it is limited by
inadequate lighting and patient analgesia. Débridement in the operating
room also allows the surgeon to have on hand the appropriate surgical
tools for the removal of devitalized bone and soft tissue and to obtain
hemostasis.
“zone of injury,” which refers to the area throughout which trauma has
occurred. The extent of the zone of injury is not always apparent on
initial assessment, particularly in degloving, crush, and electrical
injuries. If one cannot assure complete excision of all necrotic
tissue, soft tissue and bony reconstruction should be postponed and a
second débridement planned within 24 hours. The need for fasciotomy
should be considered at the first débridement. Injuries sustained in an
agricultural setting or in industrial machinery are subject to heavier
and deeper contamination. Mechanical roller injuries involving
crushing,
avulsion, or degloving will also result in more severe tissue damage
and have a worse prognosis than blunt trauma or guillotine type
injuries.46,157,160,215
Such injuries should routinely undergo serial débridements over the
course of 48 hours to ensure that all devitalized tissue is removed
before soft tissue reconstruction.
to remove additional loose debris and decrease bacterial contamination.
Several different solutions are available for irrigation. Antibiotic
solutions (bacitracin, neomycin, and polymyxin) and detergents (Castile
soap, benzalkonium chloride) are used by many surgeons in an attempt to
minimize infection rates. Although wound irrigation with antibiotic
solutions have been effective in some experimental studies,103,333
there is still a lack of convincing clinical data that it provides a
benefit over soap lavage alone. Anglen recently conducted a prospective
randomized study of 458 open fractures and concluded that irrigation of
open fracture wounds with antibiotic solution offers no advantages over
the use of a nonsterile soap solution.10
Anglen et al. also conducted a series of 10 detailed experiments to
investigate the efficacy of soap solution over normal saline irrigation.11,12 Soap and other detergents work by disrupting the bonds formed between microorganisms and the tissues.50,383 Anglen found that soap solution removed significantly more bacteria from these wounds than saline irrigation alone.12
Irrigation of the wound should be performed with more than 4 liters of
fluid, ideally under high-pressure flow, as this technique has been
shown to remove significantly more bacteria, debris, and clot and
lessen the rate of wound infection when compared with small volume low
pressure irrigation.11,330
bacterial counts, should still be used prudently. When using high
pressure irrigation, one should avoid driving foreign material further
into the wound bed, hydrodissection of uninjured areas, and tissue
insufflation.135,274
High pressure irrigation should be utilized judiciously in the hand, as
the water jet can injure or avulse nerves or digital vessels. In such
cases, copious amounts of gravity fed irrigation in conjunction with
careful débridement will suffice.
variable pressure throughout the débridement process. Devices such as
the Versa jet Hydro surgery System (Smith and Nephew, USA) use a
controlled fluid jet that allows for precise débridement over tendons
in addition to gross débridement of acute and chronic wounds.56,224,351
In a prospective trial this device has been shown to decrease operative
times and allow for increased precision during the débridement process.146
surgeon’s concerns over wound closure. If the surgeon is at all
concerned about wound closure, early consultation with a plastic
surgeon or other wound management specialist should be carried out to
allow for a multidisciplinary approach to wound management. Such
collaborations will allay concerns and allow for an aggressive initial
débridement minimizing late wound complications.
adequate débridement. With each débridement the surgeon’s goal should
be to remove all foreign and necrotic tissue. Wound débridement and
careful wound evaluation should take place as soon as possible after
the injury, under general anesthesia in the operating room. The
débridement process starts with a careful wound scrub using a surgical
brush and sterile soap or iodine solution, followed by irrigation with
4 to 8 L of sterile saline, ideally heated to 37°C to avoid excessive
cooling of the patient. If there is excessive bleeding, a tourniquet
may be inflated before the irrigation process.
be identified, marked, and protected before sharp débridement of the
nonviable soft tissues. Major motor nerves should never be débrided,
but rather dissected from necrotic tissue and preserved. Free bony
fragments that are completely denuded of soft tissue attachments, and
therefore avascular, should be removed from the wound. Avulsed parts
can often be used as a source of “spare-parts” for wounds requiring
skin grafting or flap closure, and this should always be considered
before discarding them.43 After débridement, the final assessment of tissue viability must be made with the tourniquet deflated.
the wound edges should be removed. Clotted venules are a sign of skin
devitalization and they should be débrided with the surrounding skin
and soft tissue. Healthy muscle is bright red and shiny and will
contract when grasped with the forceps. If there is any question
regarding muscle viability it may be stimulated with the
electrocautery; if there is no evidence of contraction, it should be
débrided.
devitalized tissue, immediate reconstruction can be considered. Clean
surgical instruments, ideally on a separate operating tray, should be
used for any immediate reconstructive procedure, as it has been shown
that instruments used for débridement can carry a bacterial
concentration in excess of 103 organisms.20
If one cannot assure complete excision of all necrotic tissue,
reconstruction should be postponed and a second débridement planned
within 24 hours. Débridements should continue at 24- to 48-hour
intervals until the wound is clean and ready for reconstruction.
to progress through the normal stages of healing and remains arrested
in the inflammatory stage.20,201
In traumatic cases, such wounds exist because of an infection
associated with a retained sequestrum, hardware, or other foreign
material. To allow these wounds to heal, all necrotic and infected
material must be removed before any attempt at soft tissue
reconstruction. Thus, one must turn the chronic wound into an acute
wound through the process of thorough débridement. The one caveat to
this recommendation is the removal of hardware that is providing
critical and stable fracture fixation. If the application of an
external fixator is not possible, hardware can be maintained within an
infected field until more definitive fixation is possible or bony
healing has occurred, providing systemic antibiotics have been
administered and the hardware is covered with well-vascularized tissue.55,325
structures are often hidden within scar and granulation tissue.
Débridement must be extended beyond the zone of injury, into normal
tissue, to ensure complete resection of all contaminated tissue. Use of
a tourniquet early in the case is important to best visualize and avoid
injury to vital structures such as nerves and blood vessels. The
tourniquet should be released before closure
or dressing application to confirm the removal of all devascularized tissue.
superficial tissues to deep, from the margins to the center of the
wound. Every effort is made to preserve nerves and blood vessels
crossing the zone of injury. If nerves must be transected, they should
be tagged with dyed monofilament suture and documented in the operative
records so that they may be more easily identified during later wound
débridements or reconstructive efforts. Tissue from the wound should
always be sent for bacterial cultures as well as pathologic analysis to
rule out the possibility of osteomyelitis, or vasculitis.20
of some debate. Normal saline wet to dry dressings have been the most
common form of wound dressing after surgical débridement. They help to
prevent soft tissue desiccation, they obliterate dead space, and the
dressing changes provide an opportunity for continuous surveillance of
the wound, in addition to providing excellent mechanical wound
débridement. One disadvantage is patient discomfort with dressing
changes, which may be alleviated by moistening the gauze before
removal. Their use is labor intensive. For contaminated wounds,
immediately after injury, Dakin’s solution or Betadine solution may be
used judiciously. Dakin’s solution is bacteriostatic and Betadine is
bacteriocidal. Their use is controversial, especially if used for more
than 3 days, because of their negative effects on wound healing and
soft tissue toxicity.24,229
In cases of established infection, the application of topical
antibiotics such as silver sulfadiazine, Sulfamylon (mafenide acetate),
and silver nitrate has been shown to reduce bacterial counts.125,235 For Pseudomonas
infections, 0.25% acetic acid may be used to reduce surface bacterial
counts. Consultation with an infectious disease specialist is
recommended in such cases.
that they ensure consistent monitoring of the wound site. This is in
contrast specifically to the use of a vacuum-assisted closure (VAC)
device, in which the sponge is commonly not changed for 2 to 4 days,
thus preventing wound surveillance by the surgeon who will be
performing the reconstruction.
gels, semipermeable films, or even antibiotic impregnated ointments may
be used in cases in which there has been avulsion of the dermal surface
but without damage to the underlying muscle. The dressings may take the
form of a hydrogel, antibiotic impregnated gauze, or simple a
semipermeable film. Semipermeable films and semiocclusive hydrogels are
impermeable to water and bacteria but permeable to oxygen and water
vapor. Occlusive hydrocolloids are impermeable to even water vapor and
oxygen. Thus these dressings are not as useful in wounds that require
mechanical débridement or wounds that are exudative because of
accumulation of fluid under them.
always be covered with a nonadherent gauze or hydrogel dressing to
protect them until soft tissue coverage can be obtained. Nerve repairs
and blood vessel repairs should be covered with local soft tissue,
immediately after repair, to allow for a moist healing environment, as
opposed to gauze dressings.
going to be performed immediately, whether because of concomitant
life-threatening injuries or other medical issues, a negative pressure
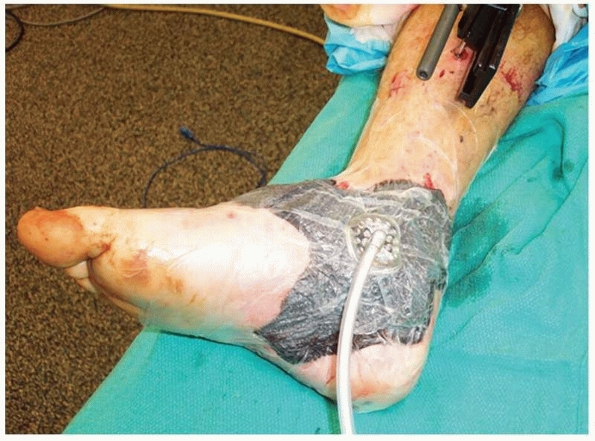
dressing can be used until definitive closure. A wound VAC
(Vacuum-Assisted Closure) can help to remove surrounding edema,
decrease local metalloproteinases and other inhibitors of wound healing
while promoting angiogenesis.17,293
sponge, in some cases impregnated with silver for more contaminated
cases, sealed by an adhesive drape and attached to suction. All pores
in the sponge communicate so that negative pressure applied to the
sponge by the suction is applied equally and completely to the entire
wound surface. The effects of the VAC on the wound are multiple. The
application of negative pressure causes the sponge to collapse toward
its center. Traction forces are thus applied to the wound perimeter
pulling the wound edges together progressively making the wound
smaller. The VAC sponge should be cut to fit inside the wound to
maximize these traction forces on the wound edges. The sponge should
not overlap intact skin, as skin maceration may occur. In addition, the
VAC removes wound edema, and it appears to increase circulation and
decrease bacterial counts (Figure 14-2).293
has been associated with a decreased requirement for skin grafting,
free tissue transfers and flap coverage.94,121
Herscovici reported on 21 patients, 16 of whom had lower extremity
wounds because of high-energy trauma. At the time of initial
presentation, all wounds “would have required flap coverage”; however,
after an average of 19 days of VAC treatment, 12 of the wounds no
longer required flap procedures to achieve wound coverage.180
tendons and nerves, as continuous suction can produce desiccation and
injury to these structures. When neurovascular structures are exposed,
local tissue or flap coverage should be performed in an urgent or
emergent manner to prevent desiccation. If wounds remain contaminated
despite surgical débridement, wet to dry dressing changes can be
performed every 8 hours until the next scheduled surgical débridement
or until the wound is clean enough to accept a VAC device.
 |
|
FIGURE 14-2 A vacuum-assisted closure device properly placed on a wound after débridement.
|
infections was incorporated into general practice with the development
of joint arthroplasty in Europe in the 1970s. Buchholz et al. reported
in a sentinel paper that penicillin, erythromycin, and gentamicin mixed
into the polymethyl methacrylate cement used to secure prostheses to
bone was found to provide high local concentrations of antibiotics for
extended periods of time.45 Since
its original description local antibiotic therapy, through the use of
antibiotic-impregnated cement, has been used for prophylaxis in cases
of open fractures and to treat chronic osteomyelitis. In 1979, Klemm
created gentamicin-impregnated beads and used them to occupy dead space
after débridement of infected bone. Klemm reported his experience in
more than 100 patients, achieving an infection cure rate of 91.4%.225
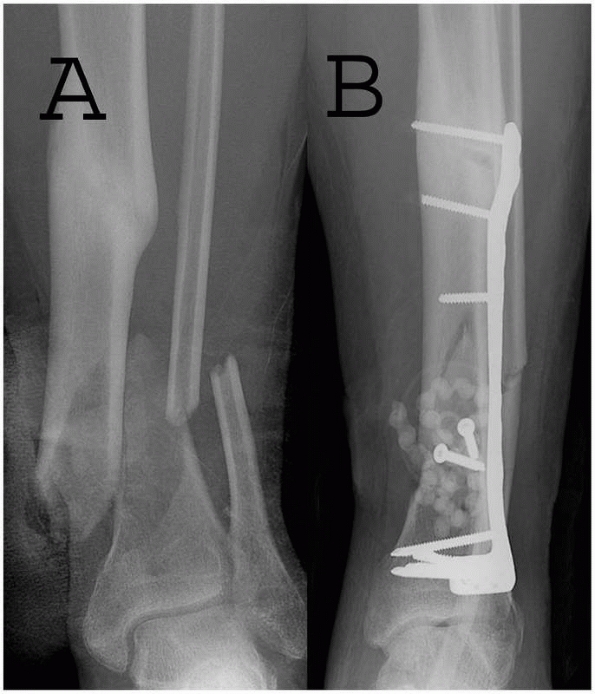
spacers. Beads are generally prepared in the operating room from
commercially available polymethyl methacrylate (Figure 14-3).
If possible the beads and spacers should be covered with local tissue.
In wounds with extensive soft tissue damage, closure may not be
possible at the time of débridement and coverage may be achieved with
an adhesive wound film such as Op-Site. This “bead pouch” should be
replaced every 48 to 72 hours under sterile conditions. Final wound
closure may then be achieved with primary closure or, in cases with
more extensive soft tissue damage, skin grafts and/or flap coverage (Figure 14-4). Alternatively, a spacer may be needed in cases of bone loss to keep an extremity out to length.
tobramycin, and vancomycin. Mixing of more than one antibiotic into
bone cement has been shown to have a synergistic effect. Penner et al.
demonstrated higher elution rates in vitro when tobramycin and
vancomycin were tested together as compared with either one tested
alone in saline baths.310 Thus, not
only does a combination of different antibiotics increase the
antimicrobial spectrum, it could also lead to increased concentrations
of antibiotics in the tissues. Currently, efficient methods of local
antibiotic delivery with biodegradable substrates are being
investigated.261
 |
|
FIGURE 14-3 Use of PMMA antibiotic beads in an open tibia fracture. A. Preoperative and (B)
postoperative views of an open distal tibia fracture with bone loss. The fracture was treated with open reduction, and antibiotic beads were placed. The PMMA beads provide a method for antibiotic delivery as well as dead-space management, but necessitate a future operation for removal. |
polymethylmethacrylate (PMMA), consisting of a powdered polymer mixed
with a liquid monomer to form a solid structure. Currently the after
antibiotic-laden PMMA bone cement products are approved by the United
States Food and Drug Administration (FDA): Simplex P, which contains 1
g tobramycin (Stryker Howmedica Osteonics, Mahwah, NJ); Palacos G,
which contains 0.85 g gentamicin (Zimmer, Warsaw, IN); SmartSet GHV and
SmartSet MHV, which contain 1 g gentamicin (DePuy Orthopaedics, Inc.,
Warsaw, IN); and the PROSTALAC prosthesis (DePuy Orthopaedics, Inc.).
Premixed antibiotic PMMA beads are available and widely used in Europe
under the name Septopal (Biomet Merck, Dordrecht, The Netherlands) but
are not currently approved in the United States.
for control or prevention of soft tissue infections have not been
clearly shown to provide a benefit over intravenous antibiotic therapy
in some studies.281 In addition,
concern has been raised over the development of antibiotic resistance
because of the prolonged release of antibiotic at subtherapeutic levels.295
PMMA has the additional undesirable quality of systemic toxicity from
the absorbed monomer, a factor shown to cause acute intraoperative
hypotension in its use in arthroplasty. Although this has not been a
significant clinical problem in the depot delivery of drugs, the
theoretical risk remains. Finally, although antibiotic-laden cement
serves as an adequate substance for dead-space management, it does not
participate in the bone healing process.
intermittent inhalation of 100% oxygen in specialized chambers at
pressures greater than that at sea level (>1 atmosphere absolute,
ATA). Typical protocols recommended by the Undersea and Hyperbaric
Medical Society (UHMS) for treating wounds expose the patient to
pressures of 2 to 2.5 ATA lasting 90 to 120 minutes per session for
approximately 40 treatments. The arterial partial pressure of oxygen
rises to approximately 1500 mmHg under these hyperbaric conditions;
oxygen tensions can approach 500 mmHg in soft tissue and 200 mmHg in
bone.213
soft tissue and bone can enhance the healing of bone stems from lines
of evidence similar to those that exist in the many other conditions
for which HBOT has been applied. Traumatized and osteomyelitic limbs
and bone structures have been shown to be hypoxic, with a partial
pressure of 20 to 25 mmHg in animal models, and thus oxygen content can
be dramatically raised under hyperbaric conditions.260
In the presence of infection, the phagocytic and bactericidal abilities
of leukocytes parallel the oxygen tension in the tissue. The hypoxic
conditions in the diseased bone reduce the ability of neutrophils to
generate the reactive oxygen species necessary to kill bacteria, and
hyperbaric
oxygen (HBO) can enhance this bactericidal activity.27
The processes of collagen synthesis and osteogenesis are also inhibited
in a hypoxic state, and studies have suggested that improved oxygen
tension can normalize, if not enhance, these functions. 226 Other
efforts have provided evidence of hyperbaric oxygen inducing
angiogenesis, suppressing anaerobic organisms, and enhancing antibiotic
activity.27
 |
|
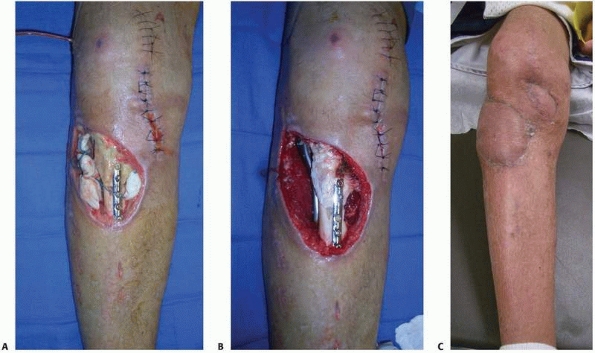
FIGURE 14-4 A.
A Grade IIIB contaminated tibial plateau fracture in a 45-year-old smoker with diabetes was treated with an antibiotic bead pouch. B. After serial wound débridements a healthy, uninfected bed was ready for coverage with medial and lateral head gastrocnemius muscle flaps and skin graft. C. Postoperative appearance. |
2003 for studies on the use of HBOT in fracture healing and nonunion
treatment identified 68 references. The review found no level I
evidence to support the use of HBOT in acute fracture healing.31
A recent review performed for the Center of Medicare and Medicaid
Services to assess the use of HBOT in treating hypoxic wounds found 57
studies examining the subject published between 1998 and 2001.405
In this review Wang et al. concluded that these studies as a group
suggested HBOT had potential beneficial adjunctive effects for
conditions such as chronic nonhealing diabetic wounds, compromised skin
grafts, osteoradionecrosis, soft tissue radionecrosis, gas gangrene,
and chronic osteomyelitis. One nonrandomized controlled trial and one
case series specifically studying chronic osteomyelitis was identified,
but these studies were found to be inconsistent in their reported
results.115 Nevertheless, it is
notable that in the United States, Medicare currently provides coverage
for those patients receiving HBOT as adjunctive therapy for chronic
osteomyelitis that is not responding to standard medical and surgical
treatment. The definitive value of HBOT remains to be determined
through prospective randomized trials.
setting is often debated, and different authors have advocated
different time scales including immediate (emergency) closure,254 early closure (before 5 days),141 and delayed closure (6 to 21 days).100
In our opinion the requirements for wound closure should be no
different when dealing with primary closure, pedicled flaps, or free
tissue transfer; wounds must be free of necrotic tissue and infection.
There is experimental and clinical evidence that quantitative
bacteriology immediately before wound closure correlates with the
likelihood of subsequent infection.62,84
Breidenbach et al. evaluated 50 free tissue transfers carried out for
complex wound closure in the extremities to determine predictors of
subsequent infection, and found that quantitative cultures had the
highest positive predictive value (89%), negative predictive value
(95%), sensitivity, and specificity.42
Mechanism of injury, type and degree of contamination, wound location
and systemic factors such as diabetes, corticosteroid use,
immunosuppression, advanced age, and malnutrition also affect the
likelihood of clinical infection.201
used for extremity reconstruction. In that study, he was able to reduce
the postoperative infection rate in patients with open fractures to
1.5% in a subset of patients undergoing reconstruction within 72 hours.141
Many subsequent studies support these data, and when free tissue
transfer is to be used, reconstruction within 5 days of the injury is a
commonly adopted guideline. This approach has been extrapolated into
the general practice of
trauma
reconstruction. “Emergency” free flap reconstruction in the upper limb
(within 24 hours of injury) potentially can allow for earlier
rehabilitation and a quicker resolution of the inflammatory response
after trauma. Several authors have reported successful series of
emergency free flaps in the upper extremity.66,254,297
Nevertheless, no prospective comparative studies have examined the
benefits of very early versus later coverage with regard to outcome or
functionality. In contrast, studies have shown that flap
reconstructions performed beyond the frequently quoted critical
interval of 72 hours with or without temporary vacuum-assisted closure
coverage yields results similar to those of immediate reconstruction
within the first 3 days.211,370
injured lower extremity be done in four distinct phases: (i) emergency
evaluation, orthopaedic stabilization, and débridement of obviously
devitalized structures and tissues; (ii) wound management with serial
débridement; (iii) soft tissue coverage; and (iv) delayed bone
reconstruction.427 Soft tissue
coverage and bone reconstruction may be performed simultaneously using
osteocutaneous flaps. In summation, a wound should be closed when it is
clean. The quicker the wound is made clean the sooner reconstruction
may occur. If the surgeon is sure all necrotic material has been
removed from the wound, then reconstruction should proceed.
must make two critical decisions early within the reconstructive
process; the first is to determine if it is technically possible to
save the injured extremity, and the second is to determine whether
salvaging the limb is in the best interest of the patient. An
insensate, painful, or chronically unstable leg may provide no benefit
over a prosthesis. Many factors have historically come into play when
making these decisions, such as patient age, comorbid injuries, and
preinjury ambulation status. Several algorithms have been designed to
aid the surgeon in this decision-making process.152,238
an adult, sciatic nerve transaction in an adult, and irreparable
vascular injury. Relative indications for amputation are
life-threatening multisystem trauma, a warm ischemia time of greater
than 6 hours, an insensate plantar foot, a crushed foot with fracture
comminution, extensive bone loss, and multiple joint disruption with
multilevel injury, advanced peripheral vascular disease, and
rehabilitation concerns.187,203,275
the salvaged extremities is often poorer than after treatment with
early amputation and prosthetic fitting.89,117,126,170
The Lower Extremity Assessment Project (LEAP) was a multicenter
prospective study of severe lower extremity trauma in the United States
civilian population designed to answer this question. The investigators
collected prospective outcome data on patients with Gustilo grade III B
and grade IIIC open fractures. Patient outcomes were evaluated through
the use of the Sickness Impact Profile, which is a self-reported health
status questionnaire. At 2 and 7 years after injury, patients who
underwent amputation had functional outcomes that were similar to those
who underwent reconstruction. Predictors of poor outcome after
reconstruction included a low education level, nonwhite race, poverty,
lack of private health insurance, smoking status, poor social support
network, and involvement in disability compensation litigation.
Approximately 50% of the patients in each group were able to return to
work at 2 years.39,257
sensation within the injured extremity has no bearing on long-term
outcome. Patients with an insensate extremity at the time of
presentation did not demonstrate significantly worse outcomes at 2
years when compared with patients who presented with a sensate foot.
Approximately 55% of those with absent or abnormal sensation had
recovered normal plantar sensation at 2 years after injury. This study
suggests that initial plantar sensation is not a prognostic factor for
long-term plantar sensation and should not be used a component of our
limb salvage decision algorithm.40
outcome is more significantly affected by the patient’s economic,
social, and personal resources than by the bony injury or level of
amputation. Further research is still required to optimize triage
decisions to avoid failed reconstructive attempts and examine
psychosocial variables, which can be modified to improve outcomes.52,336
If the patient is still adamant about limb salvage and understands the
long-term potential for future surgery, we still remain aggressive in
our attempts to salvage the severely injured extremity.
this chapter. However, ideal candidates for lower extremity
replantation are young, healthy patients with a guillotine-type
amputation at a very distal level.131
Unfortunately, lower extremity wounds are commonly more severe than
upper extremity injuries; lower extremity amputations are often
associated with compounding factors such as contamination, crushing,
multiple level injury, and the requirement for severe shortening, all
if which mitigate against replantation. Before an amputated limb is
discarded, however, the salvage of uninjured soft tissues should be
considered with the goal of maintaining maximum limb length and
functioning joints, because this will minimize energy expenditure
during ambulation. For example, the glabrous sole of the foot can
provide durable stump coverage and an intact ankle joint can be rotated
to simulate a missing knee joint.210,439 Often these salvaged parts may be transferred without microsurgery if their sensory and vascular supply remains intact.
setting remains controversial. When wounds are associated with
fractures in the acute setting, provisional stabilization should be
attempted to maintain soft tissue space, optimize pain control, and
minimize bone shortening (Figure 14-5). In
blast injuries, large amounts of debris are forced into the wounds with
tremendous energy and the level of contamination is typically higher
than that seen in most blunt open trauma. In this setting, there is
significant potential for widespread osteomyelitis when intramedullary
fixation is selected; therefore, external fixation is preferred. In
blunt trauma cases despite the degree of soft tissue injury, there is a
trend toward immediate and definitive internal fixation, the unreamed
nail being the preferred implant for tibial
fractures.161,231
Intramedullary nailing or plate fixation is only applicable when the
fracture is covered or will soon be covered by an adequate soft tissue
envelope.143
 |
|
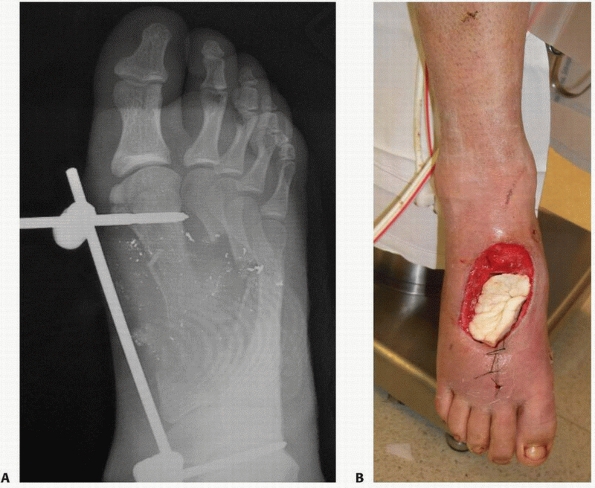
FIGURE 14-5 A.
A 17-year-old man sustained a buckshot injury to the foot resulting in severe comminution of the first, second, and third metatarsals with bone loss. Initial stabilization was achieved with an external fixator. B. A tobramycin and vancomycin polymethylmethacrylate (PMMA) antibiotic spacer was used to eliminate the dead space and keep the foot out to length before using a free fibular osteocutaneous flap transfer to reconstruct the injured foot. |
has yet to be determined through prospective randomized trials. Bach’s
prospective randomized study of 59 patients with Grade II or III tibial
fractures found both plating and external fixation to produce good
results, but plating was associated with a higher complication rate.22
Historically, external fixation, especially with the use of transfixion
pins, has been associated with frequent complications such as pin tract
infection and nonunion.79
with the use of temporary screws, Kirschner wires, and external
fixation until the time of soft tissue coverage. Once there is adequate
soft tissue coverage, the fixation may be change to definitive internal
fixation or plating.37,362
When the wound has entered a subacute, colonized phase, internal
fixation, especially intramedullary nail fixation, becomes hazardous,
predisposing to infection.53
Changing from external to internal fixation should always be timed
properly and adequate soft tissue coverage should be present.427
salvage has been made, bony fixation and wound coverage may proceed.
Wound coverage may be obtained by multiple means, including primary
closure, local flaps advancement, and free tissue transfer. As our
experience and success with free tissue transfer has increased,
surgeons have moved away from the classic reconstructive ladder and now
opt to reconstruct defects with more complex procedures if they can
provide a more rapid and complete reconstructive solution.267 The most common reconstructive techniques will now be discussed in detail.
superficial epidermal and dermal elements of the skin to a new location
where the graft is capable of re-establishing blood flow. Skin grafts
may be taken as split thickness (including only part of the dermis) or
full thickness (including all of the dermis).273 Full-thickness grafts have greater primary
contracture rates (the amount the graft rolls or shrinks initially once
it is harvested) because of a higher percentage of elastin retained
within the graft; however, full-thickness grafts are less likely to
contract secondarily (after healing has
occurred) because of greater preservation of the deep dermal
architecture when compared with split-thickness grafts.348,401 Return of sensation is also superior when compared with split-thickness grafts.7
thus undergo less primary contracture but have greater secondary
contracture rates. Because of high secondary contracture rates,
split-thickness grafts should be avoided over joints (Figure 14-6). Split-thickness grafts are more likely to take over compromised beds as compared with full thickness grafts.85
The split-thickness graft donor site heals through a process of
reepithelization and contraction as keratinocytes migrate out of
retained hair follicles within the donor site.25,347
survive and will not do well in an area of frank infection or on tendon
devoid of paratenon, bone, or cartilage. In wounds in which these
structures predominate, local, regional, or free tissue transfers are
required for successful wound closure. In addition, skin grafting
should be avoided in areas that may require secondary
surgery
for bone or nerve grafting. The greatest risks for graft failure
include infection, shearing, motion at the graft site, seroma or
hematoma accumulation beneath the graft, and finally poor wound bed
vascularity.273
 |
|
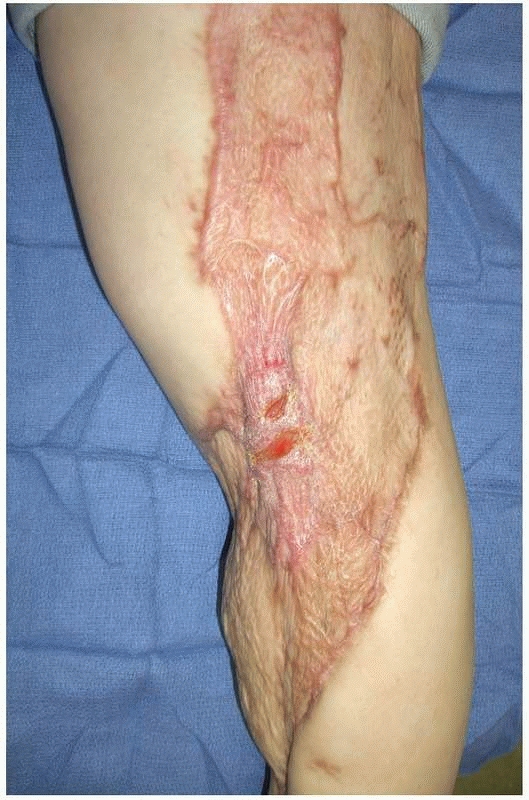
FIGURE 14-6
Late effects of skin grafting over the popliteal fossa. Although the wound is healed, the split-thickness skin graft has not provided durable coverage and is subject to chronic breakdown with knee extension. |
process called serum imbibition. During this stage of healing, the
graft obtains nutrients from the underlying wound bed through a
diffusion process. This commonly occurs in the first 24 to 48 hours.
After this point the skin graft undergoes revascularization through an
ingrowth of capillary buds primarily from the wound bed.30,82,83 Clinically most grafts are adherent to the wound bed by the fourth to fifth postoperative day.
clean before attempts at skin grafting. Infection is one of the leading
causes of skin graft failure. Because skin grafts are completely
dependent on the wound bed they are transplanted to for nutrition, they
possess no intrinsic ability to resolve infection.232
the graft to be harvested. Most frequently we harvest the skin grafts
from the upper thigh of the involved limb so that scarring may be
concealed under clothing and only one limb is operated on. The anterior
and lateral aspect of the limb is preferred so that the patient is not
lying on the donor site when in a supine position and the contralateral
limb does not abrade the donor site. With careful planning it is almost
never necessary to reposition a patient after skin graft harvest.
-
Ensure that the blade is inserted
correctly in the dermatome device. Set and check dermatome thickness
(usually 0.010 to 0.15 in) with a No. 15 scalpel blade. The thin,
beveled edge of this knife blade is about 0.10 in., whereas the
thickest portion of the blade is 0.015 in. thick. -
Clean the donor site to remove any
material that will cause the dermatome to stick and apply copious
amounts of mineral oil to the donor skin and the dermatome. -
Apply counter-traction to the skin in
front of and behind the dermatome blade. Dermatomes, particularly when
fitted with larger guards, function much more effectively on flat
surfaces. -
The harvested graft is passed through a graft mesher on a dermal carrier (Figure 14-7).
This is done to allow for drainage through the graft, make grafts more
conformable to the underlying wound bed, and increase the surface area
of the graft. -
The perimeter of the skin graft is then
fixed to the wound bed with either staples or absorbable sutures.
Motion is minimized at the graft site with the use of a tie over
bolster or a VAC sponge. A tie over bolster employs silk sutures placed
circumferentially around the skin graft and left long to tie over
mineral oil-soaked cotton balls wrapped in a nonadherent gauze placed
firmly over the graft. The extremity is usually splinted to avoid
unnecessary shear or trauma to the skin graft site.
accumulate below the graft, the dressings may be removed at 24 hours,
otherwise grafts at our institution are left covered for 5 days and
then inspected. If the skin graft site develops increasing drainage
from the wound site or a foul odor or the patient develops increasing
pain or fever the skin graft is inspected immediately to rule out
infection.
as Adaptic (Johnson and Johnson, New Brunswick, NJ) or Tegaderm (3M,
St. Paul, MN) as long as care is taken to dry the surrounding skin
around the donor site before applying the dressing. The advantage of
Tegaderm is decreased pain at the donor site; however, very commonly
fluid and serum accumulate under the dressing necessitating puncture
and drainage of the dressing if there is a suspicion of infection.102
Xeroform (Sherwood Medical Industries, Ltd., Markham, Ontario, Canada)
for large donor sites is more beneficial and will dry into
an
eschar when exposed to air. Once dry, it is painless, although the site
remains sensitive until the eschar is formed over several days.347
 |
|
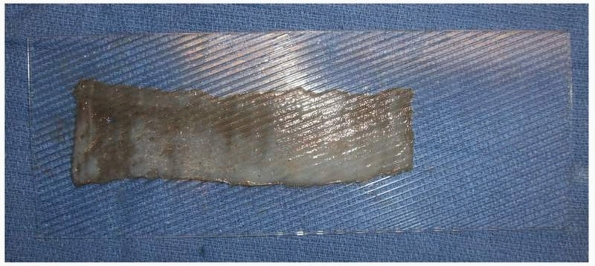
FIGURE 14-7 A split-thickness skin graft with the dermal side up on a dermal carrier that meshes graft at a ratio of 1:1.5.
|
to another. The flap may be based on a random or axial blood supply.
Random flaps have no named or defined blood supply. They are raised in
a subdermal or subfascial plane and rely on the subdermal vascular
plexus of the skin for circulation. To ensure adequate circulation,
random flaps should be limited to a length no greater than 2.5 times
the width of their base, which is the uncut border of the flap. This
ratio may be even more limited in poorly perfused extremities. Varied
random pattern flaps include z-plasty, four flap z-plasty, rhomboid
flap, banner flap, V-Y advancement flaps, and rotational flaps.
free tissue transfers. The flaps can contain more than one type of
tissue. Fasciocutaneous flaps contain skin and the underlying fascia,
musculocutaneous flaps contain skin, fascia, and muscle, and
osteocutaneous flaps contain bone, fascia, and skin.
A muscle for free tissue transfer must be able to survive on one
vascular pedicle that is dominant and that will support the entire
muscle mass. The classification (with examples) is as follows:
control a 10-fold higher bacterial count than fasciocutaneous flaps,
and improve antibiotic delivery to the wound site.54
Although the potential antimicrobial advantages of muscle flaps have
also been demonstrated clinically, a recent study by Yazar et al.
comparing lower limb wounds reconstructed with free fasciocutaneous or
free muscle flaps in a total of 177 cases showed no difference in
outcomes or infection rates.429 This highlights the important role of adequate débridement, regardless of the type of flap used.
has historically been accomplished with the use of pedicled, local or
distant rotational flaps. However, when defects are very large or
encompass multiple structures including nerve, bone, or muscle, the use
of composite free tissue transfer provides a reliable and single stage
means of reconstruction.
extremity includes the transfer of additional vascularized tissue to
the injured area, the ability to carry vascularized nerve, bone, skin,
and muscle to the injured area in one procedure, and the avoidance of
any additional functional deficits to the injured limb that may be
incurred with the use of a local or pedicled flap. Free flaps are not
tethered at one end, as is the case for pedicled flaps, and this allows
for more freedom in flap positioning and insetting. More recently
developed fasciocutaneous and perforator flaps also allow for primary
closure of donor sites with minimal sacrifice of donor site muscle.
With current microsurgical techniques free flap loss rates range
between 1% and 4% for elective free tissue reconstruction.23,216
The upper extremity is particularly suited for free tissue transfer as
the majority of recipient blood vessels utilized for anastomosis are
located close to the skin, and are of relatively large caliber.
the primary coverage of large traumatic wounds with exposed bone,
joint, and tendons or hardware; (ii) the coverage of complex composite
defects requiring bone and soft tissue replacement; (iii) the coverage
of soft tissue deficits resulting from the release of contractures or
scarring from previous trauma; and (iv) the coverage of extensive burns
or electrical injuries.230,246,247,337,346
transfer within the upper and lower extremities, and in many cases free
tissue transfer may be the only option for limb salvage after severe
soft tissue loss. Despite this, relative contraindications to free
tissue transfer include a history of a hypercoagulable state, a history
of a recent upper extremity deep venous thrombosis, and evidence of
ongoing infection within the traumatic defect. Other contraindications
would include an inadequate recipient vessel for flap anastomosis.
Disregarding technical error, the status of the recipient vessel used
for flap anastomosis may play the greatest role in flap failure;
recipient vessels within the zone of injury are prone to postoperative
and intraoperative thrombosis. Recipient vessels for microvascular
transfer ideally should be located out of the zone of injury,
radiation, or infection. Petechial staining of the adventitia, a
ribbon-like appearance of the recipient vessels, and poor flow at the
time of arteriotomy are all suggestive of vessel injury, and
alternative vessels should be chosen as recipient vessels for
microvascular anastomosis. In rare cases, arterial-venous fistulas may
be created proximally within the upper extremity or axilla using the
cephalic or saphenous vein. These fistulas can be brought into the zone
of injury and divided to provide adequate inflow and outflow for a free
tissue transfer.249 Commonly used
recipient vessels in the upper extremity are the thoracodorsal,
thoracoacromial, circumflex scapular, transverse cervical, brachial,
circumflex humeral, superior ulnar collateral, radial collateral,
ulnar, radial, and digital vessels.414
Common recipient vessels in the lower extremity are the superficial
femoral, the popliteal, the posterior tibial, and the anterior tibial
arteries.248
functional requirements and the surgeon’s experience. Muscle flaps are
useful for large three-dimensional defects when soft-tissue bulk is
necessary; however, direct coverage of tendons with muscle flaps
encourages dense adhesions limiting postoperative tendon excursion. In
general, fascial or fasciocutaneous flaps are more useful for coverage
of exposed tendons and areas in which a gliding tissue plane needs to
be preserved.
an algorithm that is based on the location of the defect. The
gastrocnemius
muscle
flap has been used to cover defects around the knee and proximal tibia;
the soleus muscle flap has been used to cover defects within the middle
third of the tibia, and free flaps have been reserved to cover defects
overlying the lower third of the tibia and ankle. Nonetheless, with
continuing advancements in microsurgery, there are now several reliable
fasciocutaneous flaps and free flaps that may be used for proximal and
distal defects in addition to the standard options. An overview of the
standard options will be provided with a subsequent explanation of
newer approaches for soft tissue coverage.
|
TABLE 14-4 Reconstructive Options for the Lower Extremity
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
flap coverage. The bone in this area is covered with enough soft tissue
that most defects can be covered with skin grafts. Should the size of
the defect prohibit primary closure or skin grafting, the rectus
abdominus or the rectus femoris muscle flap may be used in a pedicled
fashion to cover most defects in this region. The anterolateral thigh
(ALT) flap and tensor fascia lata muscle can also be used to cover
wounds surrounding the femur and the greater trochanter.
is a versatile flap harvested from the anterolateral region of the
thigh. It is most often used as a free flap for lower third injuries in
the leg or for reconstruction in the upper extremities, but it may also
be pedicled to cover defects in the groin and thigh. Its blood supply
is through the descending branch of the lateral femoral circumflex
artery. Several branches of this vessel supply the overlying skin.
These skin vessels are either septocutaneous or they take a course
through the vastus lateralis muscle before supplying the skin.237
Inclusion of the lateral femoral cutaneous nerve allows for the flap to
become sensate. The length of the pedicle is approximately 8 cm, but it
can have a longer effective length when the skin paddle is designed so
that the perforator is eccentrically located. The flap is easy to
design and can be as large as 40 × 20 cm. (Figure 14-8).
The skin is relatively pliable and the flap can be thinned to a great
degree without compromising the blood supply. This flap can also be
used as a flow-through flap that maintains distal blood supply in the
extremity,15 which is particularly useful in extremities that have compromise of one or more vessels.132,266
tissue components such as muscle (vastus lateralis or rectus femoris),
fascia, and skin in a variety of combinations.70
It has disadvantages such as a color mismatch (when reconstructing
defects in distant locations), and the presence of hair in some
patients. When large defects are reconstructed, skin grafts are
required at the donor site. Donor site morbidity is minimal when the
donor site is closed primarily and some residual functional deficit is
sometimes noted when a large skin graft is required.236
If necessary the flap can be thinned down to a 5-mm thickness. This
allows for an aesthetically appealing reconstruction while providing a
tendon gliding surface when necessary.
adipofascial flap for areas with adequate skin but a lack of soft
tissue. This type of flap can then be buried or skin grafted. When
reconstructing lower extremity defects, the flap is designed with a
variation in tissue types tailored to the recipient site requirement.
Certain areas such as the foot and ankle will require thin cutaneous
flaps, whereas other areas will require more tissue bulk. For defects
closer to the thigh such as the groin or knee, a pedicled flap can be
elevated with the pedicle based proximally or distally. A distally
based pedicled anterolateral thigh flap is based on retrograde blood
flow from the descending branch of the lateral femoral circumflex
artery with the pivot point greater
than 2 cm above the knee. Longer pedicle length can be achieved by designing the flap more proximally on the upper thigh.
 |
|
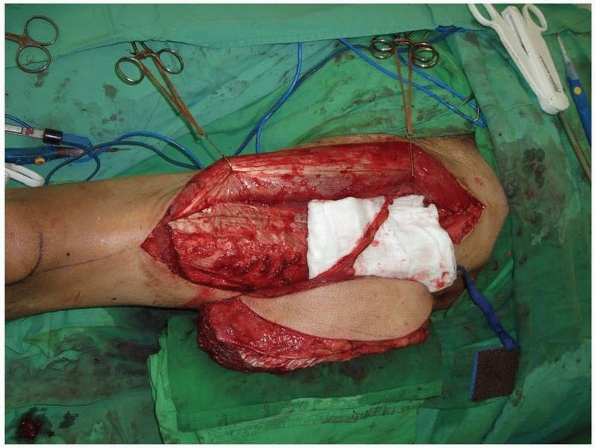
FIGURE 14-8 Anterolateral thigh flap with a vastus lateralis muscle component prior to pedicle division.
|
Areas such as the foot and ankle, which require a pliable thin flap for
defect coverage, can be covered with a cutaneous flap. A strip of
fascia lata can be incorporated to reconstruct tendon defects such as
an Achilles tendon. Harvested as a myocutaneous flap, it can be used to
cover amputation stump defects. A strip of fascia lata can be
incorporated with the flap and used for tendon reconstruction.63 For areas with exposed bone or extensive soft tissue loss, the cutaneous portion is often adequate for reconstruction;185 however, if necessary, a myocutaneous flap can be used.
covered with the medial or lateral gastrocnemius muscle flap. These
muscles may be used in conjunction with each other for large defects.
The medial head of the gastrocnemius will cover the inferior thigh, knee, and proximal tibia
and is more frequently used than the lateral head as it is larger in
size. The lateral head may also be used alone or in combination with
the medial head for coverage of lateral knee defects and lateral distal thigh
wounds. The tendinous inferior margin of the gastrocnemius muscle may
be used to augment the repair of an injured quadriceps tendon. For
coverage of extremely large defects, or in situations in which
compromise of the gastrocnemius muscles will hinder ambulation, a free
flap can be used for proximal third coverage. Other non-microsurgical
options for proximal third coverage include the reverse ALT flap.
the superficial posterior compartment and its function is to flex the
knee and plantarflex the foot. It has two heads, which lie superficial
to the soleus. It is dispensable only if the soleus muscle is intact.
Its blood supply is the medial and lateral sural arteries, which are
branches from the popliteal artery. This is a type I muscle and the
pedicle length is 6 cm. Ideally, only one head of the gastrocnemius is
needed for a reconstruction around the knee; however, both heads may be
used, depending on the reconstructive requirements. Each head is
considered a separate unit for the purpose of flap design. The medial
head is longer and its muscular fibers extend more inferiorly. The
distal soleus tendon unites with the gastrocnemius to form the Achilles
tendon. For defects at the level of the midportion of the tibia, the
gastrocnemius may not provide adequate coverage and the soleus muscle
is preferred for coverage.
flap include active infection and/or significant disruption of the soft
tissue and/or vascular pedicle. Additional contraindications for the
flap include any procedure or injury that may have traumatized or
injured the sural artery, such as a previous repair of a popliteal
arterial laceration or repair of a popliteal aneurysm. Occasionally,
severe compartment syndromes may render the muscle fibrotic and
unusable for transfer.
gastrocnemius can support a skin paddle, a paddle is not commonly used
because of its unreliability and the limitation in size of the skin.
The medial gastrocnemius is dissected through a posterior midline
incision. The sural nerve and lesser saphenous vein are two key
landmarks that are seen superficial to the muscle belly and preserved.
The muscle fascia is split, and the junction between the two heads is
incised (Figure 14-9). Blunt dissection in the
plane between the gastrocnemius and the soleus is gently done with the
finger. The superficial dissection is then performed, and the muscle is
transected distally with a cuff of tendon attached for use in fixation
to the wound edge. The tunnel through which the muscle is passed should
be of adequate size so as to not constrict the blood supply of the
flap. To expand the muscle area, the fascia may be incised, with
careful attention being paid to not injure the underlying muscle. The
flap may be used as an advancement flap to cover part of an amputation
stump or upper tibial defects, or as a cross leg flap.
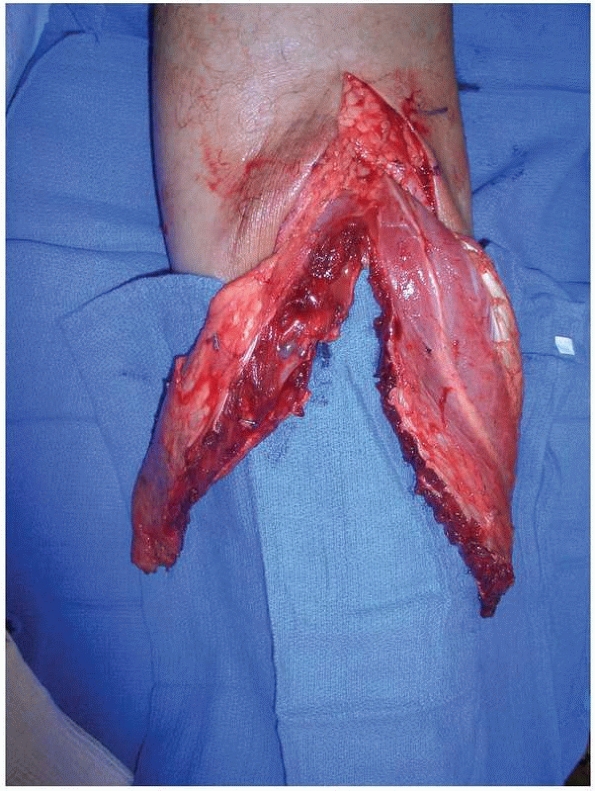 |
|
FIGURE 14-9 Gastrocnemius muscle flap after division of the medial and lateral heads along the raphe.
|
for reconstruction of middle third tibia defects; however we use this
flap sparingly, and often opt for free tissue coverage in this area,
especially if there is comminution of the bone.71
There are several factors that may prohibit the successful transfer of
the soleus muscle: (i) the size of the defect, (ii) the status of the
muscle, and (iii) the status of the surrounding tissue and bone.240 The standard soleus flap can cover most defects under 75 cm2.
Large defects occupying the majority of the middle third and lower
third of the leg are best covered with a free tissue transfer. The
soleus can be used in conjunction with the medial or lateral
gastrocnemius muscles for larger defects spanning the upper aspect of
the leg, but doing so will compromise active plantar flexion.
it can often be traumatized after comminuted fractures of the tibia and
fibula. Often, during initial wound evaluation and débridement, the
muscle can be inspected through the soft tissue defect. If the muscle
is extensively lacerated by fracture fragments or contains a
significant amount of intramuscular hematoma, one should use another
flap for soft tissue coverage. In addition, any associated injury to
the popliteal, peroneal, or posterior tibial arteries can adversely
affect the survival of the soleus muscle.240
pedicles from the posterior tibial, popliteal, and peroneal arteries
and minor segmental pedicles from the posterior tibial artery. The
muscle originates from the posterior surface of the tibia, the
interosseous membrane, and the proximal fibula. It lies in the
superficial posterior compartment deep to the plantaris muscle and
distally joins the gastrocnemius muscle as the conjoined, Achilles
tendon. It is a bipennate muscle with the medial and lateral muscle
bellies each receiving an independent neurovascular supply; this allows
the lateral and medial portions to be mobilized independently while
preserving some function of the remaining soleus muscle. The medial
head originates from the tibia and receives the majority of its blood
supply from the posterior tibial artery. The lateral head originates
from the fibula and receives the majority of its blood supply from the
peroneal artery. Typically the soleus muscle is used as a proximally
based flap (Figure 14-10). Dividing the muscle
longitudinally at the level of the septum allows for the elevation of
medial and lateral hemisoleus flaps; however, the proximal dissection
is typically more tedious because the distinction between the two heads
is often not clear.
receives segmental arterial perforators from the posterior tibial
artery. These distal perforators may be absent in up to 26% of
patients; in these cases distal perfusion to the muscle is provided by
axial blood flow from more proximal perforators. The diameter and
position of these distal perforators is variable but, if present and of
large enough caliber, they can allow for a portion of the muscle to be
harvested in a reverse fashion (Figures 14-11).
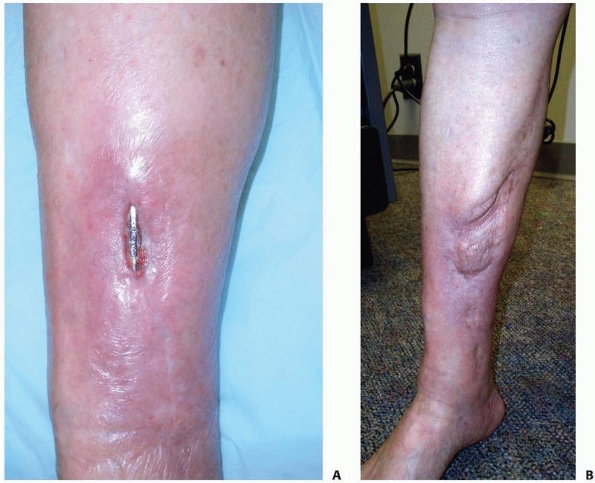 |
|
FIGURE 14-10
A medial hemisoleus flap was used to cover this healed infected tibia fracture in a 75-year-old diabetic woman after the hardware was removed. A. Preoperative image. B. Postoperative view at 6 months. The infection is resolved, and the patient is ambulating without difficulty. |
adherent to the underlying muscle bed. Weight bearing status is
determined by the stability of the underlying fractures. In a study by
Hallock of 29 soleus flaps, 24 were used for coverage of high energy
impact defects. All of the soleus flaps in this study were based on a
proximal pedicle. The complication rate was low (13.8%) and there were
no cases of total flap loss.166 Similar results were found by both Pu and Tobin, when using a proximally based flap.316,385
for lower third tibia coverage; however, other nonmicrosurgical options
for soft tissue closure of ankle defects include the reversed soleus
flap (described in the preceding) and the sural artery flap.
is perfused by reverse flow through the anastomosis between the
superficial sural artery and the lowermost perforator of the peroneal
artery. This flap has been used for the successful coverage of defects
of the posterior and inferior surface of the heel, the Achilles tendon,
the middle and distal one third of the leg, the dorsum of the foot, and
the medial and lateral malleoli. The flap is contraindicated in
patients with destruction of the vascular pedicle or the lowermost
perforator of the peroneal artery. Sacrifice of the sural nerve results
in hyposensitivity of the lateral border of the foot and a higher rate
of complications may be anticipated
in
patients with comorbid conditions such as peripheral vascular disease,
diabetes mellitus, and venous insufficiency. In this patient population
a delay procedure may be performed to increase its survival rate.
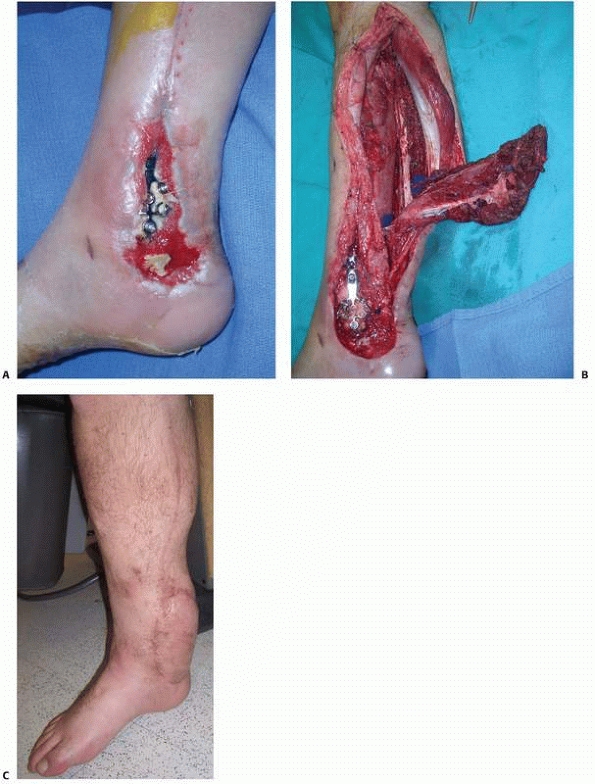 |
|
FIGURE 14-11 A. An infected Gustillo IIIB distal tibia-fibular fracture after open reduction and internal fixation. B.
Intraoperative view shows the raised, distally based right hemisoleus flap. Blue markers indicate the distally based perforating vessels from the posterior tibial artery. C. One year after surgery, the wound is healed. |
harvest all in an effort to decrease its main problem of vascular
congestion. Our technique is as follows:
-
The patient is placed in the prone position
-
Markings are an axial line drawn from the
superior aspect of the lateral malleolus to the midpoint of the
popliteal flexion crease. The skin island, at least 2 to 3 cm. distal
to the popliteal flexion crease, is centered on this axis and planned
according to defect size. -
The pivot point of the subcutaneous
pedicle is at least three finger breadths (4 to 6 cm) superiorly from
the superiormost aspect of the lateral malleolus. -
The subcutaneous pedicle should be at
least 3 to 4 cm. wide and the skin raised over the subcutaneous pedicle
must be very thin so as to not injure the pedicle. -
Dissection is performed in a plane deep
to the fascia with an incision isolating the skin paddle and dividing
the proximal blood supply if a delay is not planned (Figure 14-12). -
The distally based sural flap is then
transferred to the recipient site usually with division of the
intervening skin bridge to alleviate any possible compression of the
subcutaneous pedicle. -
Skin grafts are used liberally over the flap pedicle and at the donor site of the flap.
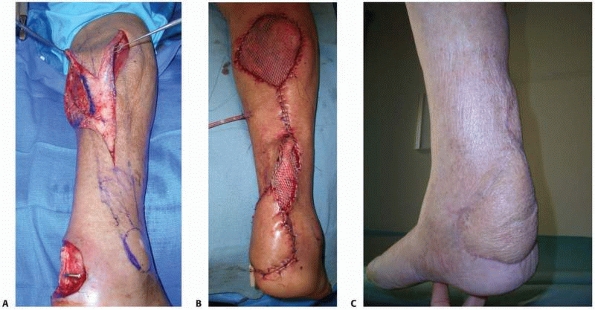 |
|
FIGURE 14-12 A.
Intraoperative view of a distally based sural flap being raised for coverage of a calcaneal wound. Note the superior skin bridge has not been divided. B. Intraoperative view of the distally based sural flap at the time of flap inset with division of the skin bridge over the pedicle and liberal use of skin grafts over the pedicle of the flap and the donor site. C. View of the healed distally based sural flap 16 months postoperatively. |
of congestion or compression of the vascular pedicle. This can be
achieved either by an adequately elevated position of the leg and/or by
the use of conventional splints with a gap over the flap; however, some
authors prefer the use of external fixation devices that serve to not
only immobilize the limb, but also obviate the need for tight
compressive dressings. External fixation also allows the treatment of
concomitant fractures and prevents the development of an equinus
deformity as well as facilitating elevation of the limb.
well as venous flow must be performed regularly during the first
several postoperative days. Capillary refill should be tested every
several hours in the postoperative period so that interventions may be
made to prevent flap loss. A revision procedure should be performed if
any signs of poor arterial perfusion or venous congestion are
identified. The administration of anticoagulants in the postoperative
period after pedicled flap reconstruction remains controversial;
however, we commonly will use anticoagulation therapy such as heparin
and/or aspirin in mildly congested flaps reserving leech therapy for
those with more significant congestion.69,280
If serious compromise of the flap is evident, the flap may be laid back
in the donor site bed as a last resort, in essence creating a delayed
flap.
approximately 4 to 5 days after the procedure with a gradual dangling
protocol of the limb to assess tissue tolerance. If signs of
significant edema or venous compromise are evident in the flap tissue
after 10 to 15 minutes of dangling the extremity, bed rest with
elevation should be prolonged for several more days until the flap can
tolerate the dependent position. Donor site morbidity is generally low,
with the most common finding being sural nerve neuroma and scarring.
Neuromas that are painful and significantly distressing to the patient
may be resected and the nerve stump buried in the gastrocnemius muscle.
split-thickness skin graft if there is no exposed tendon or bone. Local
toe fillet flaps are capable of covering smaller distal defects.
Transmetatarsal amputation may be considered if there is extensive
concomitant injury to the toes. In such cases, the plantar surface can
be advanced to cover the remaining dorsal defect. When tendon or bone
is exposed, free tissue transfer provides the most reliable means of
durable coverage and preserves the remaining foot function. Smaller
defects can be treated with the sural artery flap.
option for heel coverage is the medial plantar artery flap, which
provides sensate coverage of heel defects without the need for
microsurgery. This flap is based on the medial plantar artery. The
sural artery flap can also be extended to cover moderate sized heel
defects (Figure 14-13).
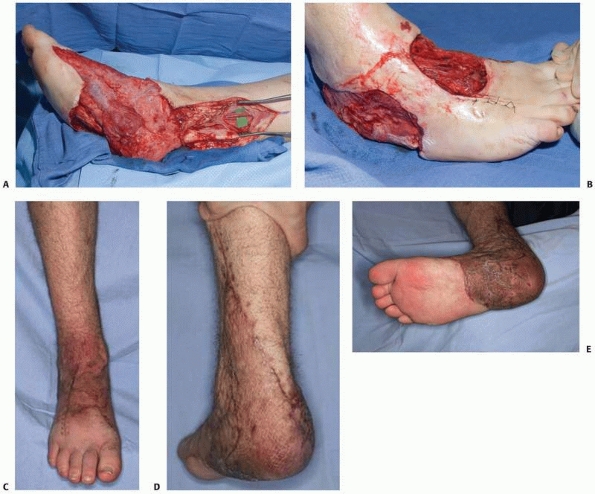 |
|
FIGURE 14-13 Small- to moderate-sized foot and ankle defects can be covered with the sural artery flap. A. This 55-year-old man has developed wound breakdown following ankle fracture fixation. B. The sural artery flap is harvested and pedicled to cover the defect. C. The donor site is skin grafted. D,E. The flap allows for thin pliable coverage of the area and will eventually allow for normal shoe wear.
|
that the latissimus dorsi muscle, gracilis muscle, anterolateral thigh
and scapular flaps to be the most versatile for lower extremity
coverage. For osteocutaneous defects, we most frequently use a free
fibular flap or a fibular flap in combination with a latissimus dorsi
muscle flap. For moderate-sized ankle defects the gracilis muscle is
our flap of choice as it produces minimal donor site morbidity and can
be contoured nicely to the malleolar region and will not interfere with
normal shoe wear.
a very reliable muscle for coverage of soft tissue defects for the
chest, shoulder, and elbow. The muscle provides a workhorse flap for
extremity coverage and is based on the thoracodorsal artery as the
major pedicle and on branches of the intercostals and lumbar arteries
as secondary segmental vessels. It is a Type IV muscle and has a
pedicle length of 8 to 12 cm, which can be obtained by dissecting the
thoracodorsal vessels proximally toward the axillary artery and vein.
Its innervation is the thoracodorsal nerve, which is a direct branch of
the brachial plexus, and it enters the muscle 10 cm from the apex of
the axilla. It is important to identify the anterior border of the
muscle preoperatively by having the patient contract the muscle with
the hand supported on the hip in a standing position. Marking of the
skin over the posterior superior iliac spine and the scapular tip is
helpful also.
large skin and soft tissue defect that cannot be managed with local
flaps. Contraindications for flap use include previous injury, or in
some cases, axillary lymphadenectomy. Breast cancer surgery, in
particular, axillary node dissection, may injure the nerve or arterial
supply, rendering the muscle fibrotic and inadequate for transfer.
muscle flap but also a musculocutaneous flap. A muscle flap covered
with a split-thickness skin graft is often less bulky and
can seal deep defects (Figure 14-14),
whereas musculocutaneous flaps give better aesthetic reconstruction
because the skin paddle can conform to the skin texture of the
surrounding tissues, particularly when it is used as a pedicled flap.
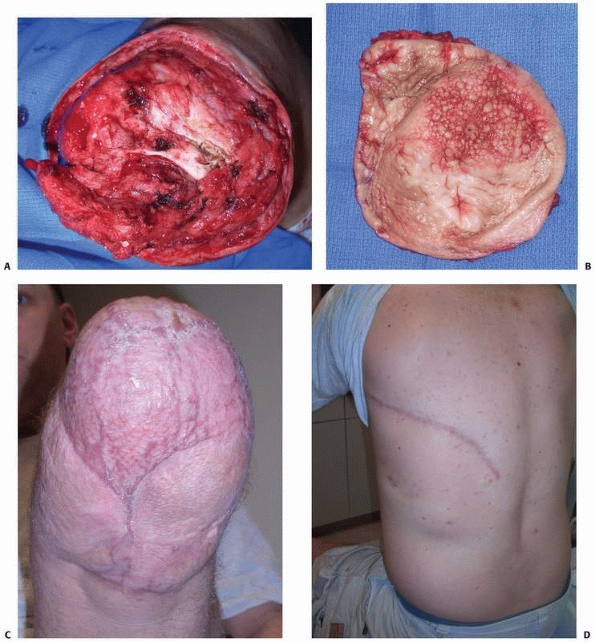 |
|
FIGURE 14-14 A. A below-knee amputation stump following resection of poor quality skin. B.
Thorough débridement of osteomyelitis of the distal tibia. The stump was then covered with a latissimus dorsi muscle flap and split thickness skin graft. C. At 6 months postoperatively, the stump has been resurfaced nicely. D. The latissimus dorsi flap donor site at 6 months. |
general anesthesia is placed in a lateral decubitus position with an
axillary roll. Dissection is most easily accomplished beginning from
the anterior border of the muscle, and this method allows early pedicle
identification. If a skin paddle is chosen, then it may be oriented
along the muscle fibers with care being taken to center the paddle on
the muscle belly. If no skin paddle is selected, the skin flaps are
elevated commonly parallel to the muscle fibers to expose the muscle
origins and insertions. The muscle is released from the lumbosacral
fascia and iliac crest. The pedicle is identified and the serratus
branch is then divided so that the muscle may be reflected toward the
axilla. Pedicle dissection should be performed with loupe magnification
(2.5× or greater). If performing a functional muscle transfer, marking
sutures should be placed along the long axis of the muscle to allow
adequate tension adjustment at the recipient
site.
The muscle can be split longitudinally into halves based on the medial
and lateral branches of the thoracodorsal artery, which bifurcate upon
entering the muscle.
insertion of the muscle at the humerus is divided. Additional distal
coverage, if it is being used as a pedicled flap for extremity
reconstruction, can be obtained by releasing its insertion from the
intertubercular groove of the humerus as well. When the latissimus
dorsi flap is transferred as a free flap, the vascular pedicle is
divided at the juncture with the axillary artery and vein to obtain the
maximum pedicle length. Large suction drains should be left beneath the
skin flaps and in the axilla to avoid postoperative hematoma or seroma
problems. Frequently there is difficulty elevating the flap
simultaneously with donor site preparation, particularly when it is
used for upper extremity reconstruction. In addition, if used as a
musculocutaneous flap it may be excessively thick in obese patients.
harvest of a latissimus dorsi flap. Seromas can be relieved with
frequent aspiration and compressive garments. Scarring over the donor
site is inevitable, and endoscopic harvest can be entertained to
minimize subsequent scarring.251
Total flap necrosis is rare when used as a pedicled flap; however,
partial flap necrosis because of an inconsistent blood supply to the
lower third of the muscle is not uncommon. Bleeding at the distal edge
of the flap should be checked when it is elevated. Kinking and tension
on the pedicle can cause a disturbance of flap circulation and must be
recognized immediately.
harvested with the patient in the supine position. This vertically
oriented, Type III muscle (two dominant vascular pedicles), extends
between the costal margin and the pubic region and is enclosed by the
anterior and posterior rectus sheaths. The superior blood supply is
from the superior epigastric artery, which is a continuation of the
internal mammary artery. Distally the blood supply is from the inferior
epigastric artery, which is a branch of the external iliac artery. The
pedicle length is 5 to 7 cm superiorly and 8 to 10 cm inferiorly.
Because of the larger size of the inferior epigastric artery and the
venae comitantes, it is more commonly used as a free tissue transfer.
Large defects of the thigh, where local soft tissues are either not
sufficient in area or unusable because of radiation, for example, may
be covered with pedicled rectus muscle only or myocutaneous flaps (Figure 14-15).
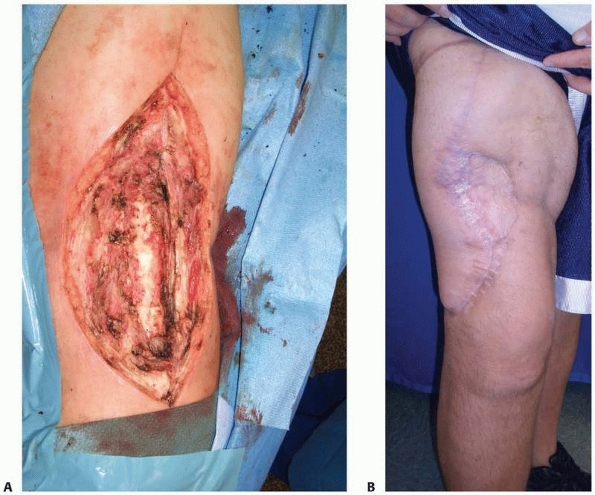 |
|
FIGURE 14-15 A.
After resection of a recurrent liposarcoma in a radiated field in the thigh, there is extensive exposure of the femur and the scarred wound bed. B. Six months after a pedicled vertical rectus abdominis myocutaneous flap for soft tissue coverage. Anteriorly, a skin graft was used over a portion of the flap to minimize size of the skin paddle and facilitate abdominal wall closure. |
twelfth intercostals nerves that enter the deep surface of the muscle
at its middle to lateral aspects. The size of the muscle is up to 25 ×
6 cm2. The skin territory that can be harvested is 21 × 14 cm2
and its blood supply is based on musculocutaneous perforators. The
donor site created via the fascial incision in the anterior rectus
sheath to access a muscle-only flap may be closed primarily with a
running or interrupted absorbable or nonabsorbable suture on a tapered
needle. When harvesting a myocutaneous flap, a portion of the anterior
rectus sheath is taken with the flap and a mesh or biologic implant may
be used to reinforce the closure of the abdominal wall fascia to
prevent hernia or bulge. Drains are commonly used under the raised skin
flaps after muscle harvest. An abdominal wall binder may be used to aid
in postoperative recovery in cases of a free tissue transfer. Although
the rectus abdominis muscle is considered a work horse flap, its
popularity has decreased slightly because of the lower donor site
morbidity that other muscle and nonmuscle flaps have to offer.
donor site for free tissue transfer to cover foot and ankle soft tissue
defects.322
Typically, the ipsilateral limb is chosen so that only one limb is
immobilized. The blood supply to the muscle is from the medial femoral
circumflex artery, which originates from the profunda femoris artery.
The major pedicle can be identified 8 to 10 cm inferior to the pubic
tubercle. The flap also has a minor arterial pedicle, which enters the
muscle at the level of the midthigh. This artery originates from the
superficial femoral artery. The muscle receives its innervation from
the anterior branch of the obturator nerve. This branch can be
harvested with the muscle if there are requirements for a functional
muscle transfer.
it lies between the adductor longus medially and the semitendinous
muscle inferiorly. It lies superficial to the adductor magnus. The
gracilis may be confused with the sartorius and is differentiated from
the sartorius and semimembranous by identification of its
musculotendinous portion: At the level of the medial femoral condyle
the gracilis consists of muscle and tendon, whereas the semimembranous
is entirely composed of tendon and the sartorius is entirely muscular.
magnification and determined to be adequate for a microvascular
anastomosis, the secondary pedicle is divided. The origin of the muscle
and the branch of the obturator nerve are then divided. The muscle is
left to perfuse on its major pedicle until the recipient vessels have
been prepared for microvascular anastomosis. Before muscle transfer a
final definitive débridement of the defect site is performed and the
flap transfer is performed. (Figure 14-16).
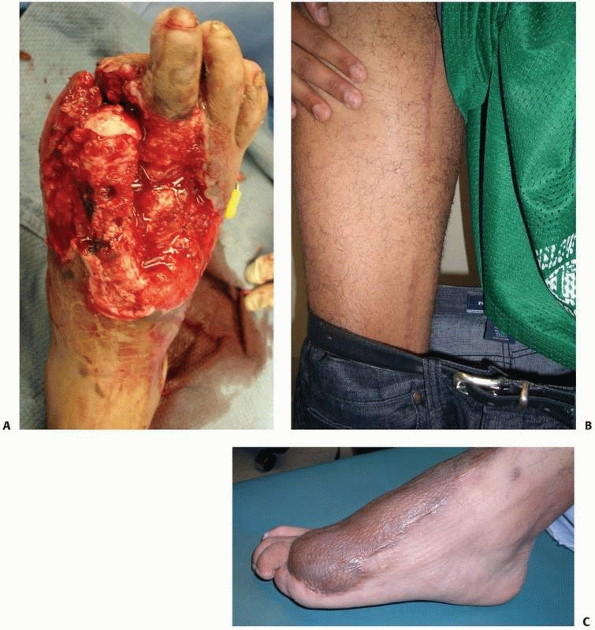 |
|
FIGURE 14-16 A. A gunshot wound to the dorsum of the foot with severe comminution of the metatarsals and loss of the hallux and second toe. B. The donor site after gracilis muscle harvest using two incisions and preserving a central skin bridge. C. One and a half years after gracilis muscle transfer for coverage of the dorsal foot wound.
|
reconstructive armamentarium over the last 3 decades, and the options
for most soft tissue defects are now extensive. As innovations in flap
design continue to develop, reconstructive algorithms move away from
the traditional reconstructive ladder toward delivering composite flaps
to provide the best possible reconstructive solution.63
Although free flaps are often the preferred method of reconstruction,
all surgeons should be familiar with other options for upper limb
reconstruction. The major factors determining flap choice are location,
size, and tissue involvement.181
vital structures such as tendons devoid of paratenon, nerves, vessels,
bone, and hardware requires flap coverage. Although a split skin graft (Figure 14-17) will
typically survive when grafted onto major nerves, vessels, periosteum,
and the paratenon large areas of exposed bone need durable coverage,
nerves and vessels need robust protection, and the preservation of
tendon excursion requires that the overlying tissue is not firmly
adherent to the paratenon. Flap coverage is also indicated in
situations in which there is a need for the restoration of sensation.
|
TABLE 14-5 Reconstructive Options for the Upper Extremity
|
||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
be either pedicled or free. The two most common pedicled
fasciocutaneous flaps used in the upper extremity are the radial
forearm flap and the posterior interosseous flap. Both can be used in
an anterograde or retrograde fashion and can provide thin pliable
coverage for most defects involving the dorsum of the hand, the palm, the forearm, and the elbow.
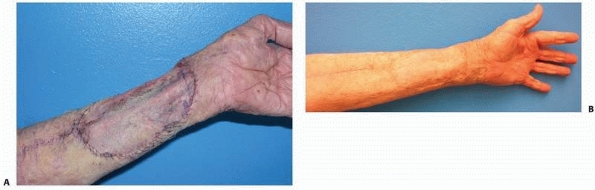 |
|
FIGURE 14-17 A split-thickness skin graft on the patient’s forearm shown (A) 1 week and (B) 8 months after surgery.
|
flap, or as a free flap from the contralateral limb. The radial artery
gives off fasciocutaneous perforators along its length, to supply the
radial two thirds of the forearm skin, and it also gives branches to
the distal half of the radius. A flap measuring up to 15 × 35 cm can be
raised as a fascial flap, a fasciocutaneous flap, or an osteocutaneous
flap, in an antegrade or retrograde manner.212 Palmaris longus, flexor carpi radialis, and the lateral and medial antebrachial nerves can also be included.124,422
The pivot point can be as proximal as the origin of the radial artery,
approximately 1 to 4 cm distal to the intercondylar line, or as distal
as the wrist crease, allowing it to be used for defects anywhere from
the elbow to the dorsum of the hand. The versatility of this flap and
the relative ease in flap elevation have made it a workhorse flap for
forearm and hand reconstruction.
be confirmed before the flap is elevated. The patient should have a
normal Allen’s test to ensure safe flap harvest. If there is concern
about the patency of the ulnar artery during the surgical procedure,
the radial artery can be temporarily clamped, before flap division, and
perfusion to the hand can be examined. The importance of checking
collateral circulation to the hand when raising the flap from the
already traumatized forearm cannot be overemphasized (Figure 14-18).
flap was designed in an attempt to find alternatives to the radial
forearm flap. The posterior interosseous artery, which this flap is
based on, arises from the common interosseous artery in the antecubital
fossa and passes dorsally through the interosseous membrane. The
descending branch runs in the septum between the extensor carpi ulnaris
and extensor digiti minimi, giving rise to several fasciocutaneous
perforators to the skin. A fasciocutaneous flap up to 8 cm in width and
12 cm in length can be raised centered over a line drawn from the
lateral epicondyle to the distal radioulnar joint.
which also runs in this septum, gives off its branches to the extensor
digitorum communis and extensor carpi ulnaris at this level, and these
branches are prone to injury when dissecting the flap. The posterior
interosseus flap can also be used in a retrograde manner, making use of
the collateral flow to the posterior interosseus artery through its
distal anastomosis with the anterior interosseous artery. The principal
advantages of this flap are as
an
alternative when the radial or ulnar artery has already been damaged or
sacrificed, and when a thin pliable skin flap is needed.13,272
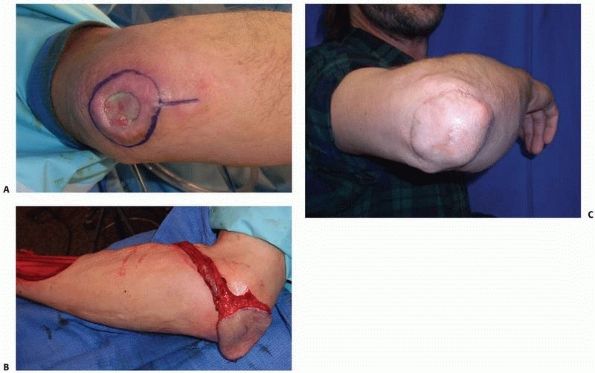 |
|
FIGURE 14-18 A. A 34-year-old man with chronic wound of the elbow with osteomyelitis of the olecranon, after a fall from a bike. B. Intraoperative view of radial forearm pedicled flap for elbow reconstruction. C. Appearance at 6 months postoperatively.
|
extremity. If joints are to be crossed, fasciocutaneous flaps are much
preferred as muscle flaps can undergo atrophy and restrict flexion and
extension across joints. Some fasciocutaneous free flaps, such as the
lateral arm flap and scapular flap, have limitations in size and
overall thickness. If a larger skin island is needed, tissue expansion
can be performed before flap transfer.
of fasciocutaneous tissue based on the circumflex scapular branch of
the subscapular artery.374 This is
an excellent choice for coverage of large wounds of the forearm. The
dissection is relatively easy, and a flap of up to 10 × 25 cm (scapular
flap) or 15 × 30 cm (parascapular flap) can be raised and used to
reconstruct large defects of the forearm. For defects involving the
radius or ulna, the scapular flap can be harvested as an osteocutaneous
flap by incorporating the lateral part of the scapula, with very little
extra morbidity.396 Coverage can be
extended even further by combining this flap with the latissimus dorsi
or serratus anterior muscle flaps on one pedicle.135,421 Donor sites of up to 7 to 8 cm width can usually be closed directly.
to the scapular flap but is based on the descending branch of the
circumflex scapular artery. Similarly, it provides a large area of
pliable and relatively thin tissue for forearm coverage, but the donor
site usually requires skin grafting.
discussed. It has recently seen a huge gain in popularity as a free
flap, with some authors proclaiming it as the ideal soft tissue flap.
Its vascular pedicle is reliably at least 8 cm long and can be
lengthened up to 20 cm, the flap is easy to design and can be made up
to 40 × 20 cm large. It can be thinned to 3 to 5 mm without
compromising its vascularity.222,319
Some subcutaneous fat can be included to minimize tendon adhesion in
the forearm and hand. Wei reviewed a series of 672 flaps with a success
rate of over 98%.408 Its many
advantages make it a versatile flap that has been reliably used in
upper limb reconstructions including defects of the forearm and elbow (Figure 14-19).23,63,132,200,406
arm flap is a fasciocutaneous flap perfused by the septal perforators
of the posterior radial collateral artery, the terminal branch of the
profunda brachii. An area of thin, pliable skin can be harvested up to
20 × 14 cm; however, only donor sites of 6 cm width or less can be
closed primarily. This flap has a very short vascular leash, and its
use as a pedicle flap is therefore limited, with most surgeons
preferring to use it as a free flap. The radial recurrent artery
provides the retrograde flow when this flap is used as a reversed flap.
The reverse lateral arm flap has been used successfully for olecranon
and antecubital coverage. As a
free
flap, the lateral arm flap is extremely versatile, capable of carrying
bone (the humerus) and nerve (the posterior antebrachial nerve).
Historically this has been a workhorse flap for upper extremity
reconstruction.18,298,392
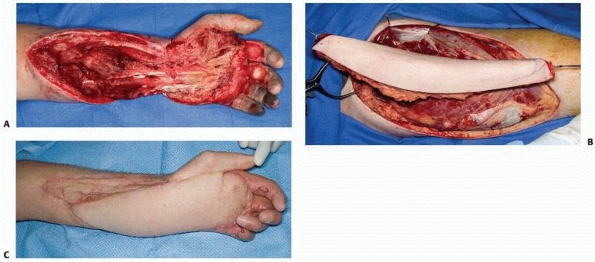 |
|
FIGURE 14-19 A. A 48-year-old man had a roller press injury resulting in loss of most of the palmar and forearm skin. B. Coverage was obtained with use of an anterolateral thigh flap. C. The flap appearance at 3 months, at the time of secondary flexor tendon tenolysis surgery.
|
forearm, elbow, and humerus are the latissimus dorsi muscle, rectus
abdominis muscle, serratus anterior muscle, and gracilis muscle. The
choice of muscle flap depends on the size of the defect, donor site
availability, and donor site morbidity.
and has a long pedicle (8 to 11 cm), making it one of the most
versatile flaps for reconstructing large defects in the upper
extremity. Additionally, in the majority of patients, the thoracodorsal
trunk has two major divisions, allowing the surgeon to harvest only a
portion of the muscle if a narrower flap is needed. Conversely, if a
broader flap is required, the serratus muscle (±vascularized rib) can
be raised with the flap, taking care to preserve its arterial supply,
which arises as a branch from the thoracodorsal artery.
muscle to re-establish lost biceps or triceps function. It can be used
in a pedicled fashion for coverage of the elbow, but should not be used
for elbow defects extending distal to the olecranon. For such defects
the radial forearm flap has been found to provide more reliable
coverage.72,200
Functional morbidity of the donor site is variable, with conflicting
reports in the literature. If it is anticipated that a resulting degree
of shoulder weakness (adduction, as in patients who crutch-walker and
those with paraplegia) would have a major impact on the patient, then
an alternative flap should be considered.
can be harvested with or without the underlying ribs, based on the
thoracodorsal pedicle. This flap is a relatively thin, broad sheet of
muscle but can be very versatile when combined with components of rib
or the latissimus dorsi muscle.
pedicle (5 to 7 cm) arising from the deep inferior epigastric vessels,
and can be used for coverage in most situations encountered in forearm
trauma. Its main disadvantage is the abdominal wall hernia, which can
sometimes occur at the donor site, especially if the fascia is
harvested.
requiring muscle coverage. The dominant pedicle is the medial femoral
circumflex artery arising from the profunda femoris, and is usually
approximately 6 to 7 cm in length. The muscle is unipennate, and has an
excursion of approximately 10 cm. The main advantage of this muscle
flap in the forearm is its use as a functional motor unit as discussed
previously.
microsurgery was the groin flap. This flap is based on the superficial
femoral circumflex artery, which arises from the femoral artery along
with the superficial inferior epigastric artery in the femoral triangle.290,364
The flap has shown great versatility. It may include the lateral
cutaneous branch of the femoral nerve if a neurotized flap is required.207
The flap may be combined with the abdominohypogastric flap for large
defects or it may be expanded before transfer. If bone is required, a
portion of the iliac crest may be harvested.120,323 The flap may also be split longitudinally to cover defects on both aspects of the hand.363
especially if the wound is well healed at the flap’s distal margin. Any
compromise to the arm’s vascularity, such as preoperative radiation or
electrical injury may prolong the period of revascularization. If there
is doubt about the vascularity of the flap prior to division, the
pedicle may be occluded with a tourniquet.420 The disadvantage of the groin flap is the mandatory period of hand
immobilization before pedicle division. This can result in hand, elbow,
and shoulder stiffness. Despite this the groin flap still remains a
reliable means of providing soft tissue coverage for large hand wounds
without the need for microvascular experience (Figure 14-20).290
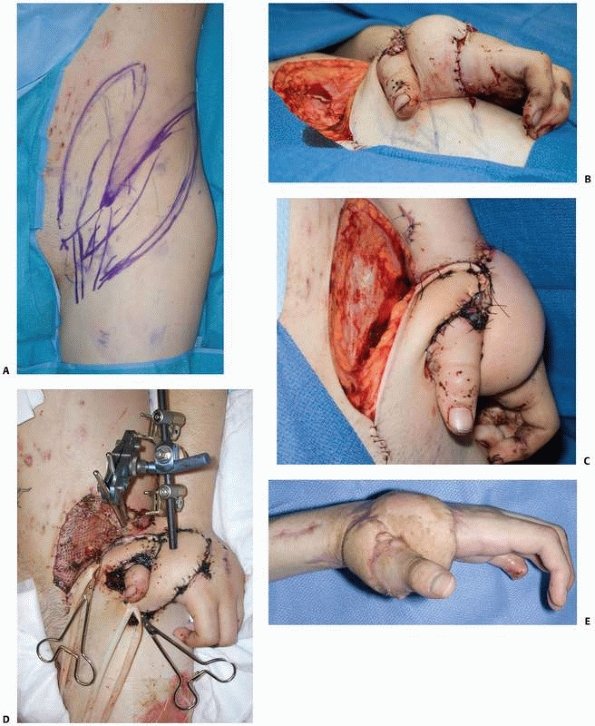 |
|
FIGURE 14-20 A.
The groin flap can provide versatile coverage of the hand. Here a groin flap was designed with two separate skin paddles to cover both the palmar (B) and dorsal (C) surfaces of the hand. D. The hand was temporarily stabilized to the groin with the use of an external fixator. E. The appearance of the hand following flap division and insetting. |
tissue reconstructions of the forearm are the anterolateral thigh flap
and the scapular flap. If bone is required, the fibular osteocutaneous
flap is a good match for the radius or ulna. Flaps based on the
subscapular or thoracodorsal system, taken with rib are also very
versatile for the reconstruction of smaller bony defects.*
rectus abdominus flaps result in functional loss and donor site
morbidity including, particularly in the abdomen, the potential for
hernia formation. In addition, in the coverage of joint surfaces,
muscle
flaps tend to undergo fibrosis and atrophy over time, which may limit
joint excursion, particularly when they are placed over the elbow or
the dorsum of the hand. Muscle is still indicated for those
circumstances involving osteomyelitis or soft tissue contamination.
completion of the case, as 5% to 25% of transferred flaps require
re-exploration for microcirculatory compromise, which can be caused by
arterial or venous thrombosis.47,64,217 Free flaps salvage rates after thrombosis range from 42% to 85%.64,216,233 Early recognition of vascular compromise has been shown to provide the best chance of successful flap salvage.64,182,350
advanced from clinical observation to implantable Doppler probes. The
best method for monitoring has yet to be established. Clinical
observation of the nonburied free flap remains the gold standard to
which monitoring systems are generally compared.105
Monitoring devices should ideally be sensitive enough to supersede
clinical evidence of vascular thrombosis but specific enough to avoid
unnecessary re-exploration. Here we review the current literature
regarding monitoring methods and protocols.
assessing capillary refill, temperature, swelling, and flap color. Its
use is confined to monitoring surface skin flaps and is less reliable
in the monitoring of muscle flaps and buried flaps.105
Capillary refill can be assessed by simply applying deep pressure to
the transferred tissue and releasing using one’s finger or the flat end
of a surgical instrument and then releasing the pressure and evaluating
capillary refill time, which is commonly 2 to 3 seconds. With
increasing disturbance of the blood supply, a livid bluish
discoloration (shortened capillary refill less than 2 seconds) or
paleness of the flap (delayed capillary refill greater than 3 seconds)
appears in a venous congested or ischemic flap, respectively. When
these criteria cannot be reliably assessed or for confirmation, one may
then use the pin prick test.
observation to be effective, the person performing flap evaluation
needs to be educated with regard to the signs of flap failure. Nursing
units and young residents require annual in-service education to
improve their diagnostic acumen, as these two groups are in constant
flux in most medical centers.
cutaneous component. The test is performed by puncturing the cutaneous
paddle of the flap with a 24- or 25-gauge needle. The puncture should
not be too deep into the tissue, and it should be in a portion of the
flap that is not in close proximity to the vascular pedicle and
microanastomosis. An indicator of flap viability is a stream of
continuous bright red blood upon puncture. A congested flap will
produce a continuous stream of dark venous blood. Care should be taken
not to perform this test too frequently, particularly in patients on
anticoagulants, because repeated puncture trauma may lead to a bruised
flap, which may hinder further evaluation of the tissue. This test is
certainly the least expensive of the various methods of flap monitoring.
temperature of the flap and the adjacent skin is associated with
arterial compromise and a difference of between 1°C and 2°C is more
indicative of venous compromise. A simple liquid crystal temperature
probe may be placed on the flap tissue with a second probe placed on
the adjacent normal skin. Temperature changes in flaps such as toe
flaps placed on an extremity will be more accurate than flaps placed on
the trunk, where the flap temperature may be a direct reflection of the
body part on which it is placed.
technique widely accepted as the method of choice, but the
ultrasonography with handheld Doppler (5 to 8 MHz) is the most common
technique in use.204,365,399
Its most important limitation is differentiating between the recipient
vessels and the flap’s vascular pedicle because of their potential
close proximity. A clinician may detect the Doppler signal of the
recipient vessels instead of the signal from the flap’s vascular
pedicle, which may mislead the observer into believing that the flap’s
pedicle is patent when in reality a thrombosis has occurred. This
limitation may be overcome by performing a Doppler ultrasound
examination of an arterial signal within the flap tissue
intraoperatively and then simultaneously compressing the donor (flap)
artery to ensure that this is a true artery within the flap. Upon
compression of the donor (flap) artery there should be loss of the
arterial ultrasonic signal within the flap.
method of determining the status of the vein of a flap. The Doppler
signal of a vein is detected intraoperatively after flap
revascularization and a suture may be placed to mark it. The venous
sound is at times difficult to detect but when heard is a clear
indication that the vein is patent. When a venous signal is detected,
the flap is compressed and a “venous augment” sound should be heard.
across a microvascular anastomosis and is an effective tool to monitor
flap perfusion and improve salvage rates, especially in buried flaps.92
Initial research demonstrated a 3% false-positive rate, which led to
unnecessary re-explorations, and a 5% falsenegative rate when the probe
was placed on the artery.378 Further, up to a 5-hour delay was found between a venous obstruction and the loss of the arterial signal in large muscle flaps.213
Best results occur if an implantable probe is placed on the vein
instead of the artery allowing the detection of venous obstruction
immediately followed by detection of arterial thrombosis.
that transmit two separate wavelengths of visible red (660 nm) and
infrared (940 nm) light and a photodiode receiver. It can distinguish
the
difference in light absorption between oxyhemoglobin and reduced
hemoglobin and thereby measure oxygen saturation. By the way of
photoplethysmography the oximeter can identify pulsatile flow and will
therefore provide a continuous display of both the pulse rate and
arterial saturation. This is an excellent monitor for replanted and
revascularized digits and toe-to-hand transfers.145
mm below the surface of the flap, and this light is reflected by the
red blood cells moving within the capillaries enclosed within a 1-mm3
volume of tissue. The frequency shift between the transmitted and
reflected light is directly proportional to the velocity of capillary
blood flow. This flow value provides an objective measurement of flap
perfusion. Laser Doppler interpretation requires experience, as values
differ depending on tissue type and patient. Furthermore, perfusion
readings may fluctuate for any given patient because of physiologic
microcirculation variation or artifacts. Therefore, the observer must
monitor the trend rather than the absolute values. This method is
limited to monitoring cutaneous circulatory phenomena, as the probe
only penetrates 1.5 mm into the flap. Estimated sensitivity and
specificity values have been reported at 93% and 94%, respectively, and
this technique has been found to be superior to thermometry when used
alone for the evaluation of replantations.186
performed has been debated, but most series recommending a minimum of
hourly monitoring for the first 24 to 48 hours after surgery.184,234 The majority (greater than 80%) of vascular complications occur within the first 48 to 72 hours postoperatively.217 Postoperative venous thrombosis is the most common vascular complication.234 With these factors in mind our current recommendations for flap monitoring include:
-
Placement of an implantable venous Doppler probe when feasible.
-
Flap inspection every hour by experienced nursing staff for first 48 hours, moving to every 2 hours for the next 24 hours.
-
Discontinuation of flap monitoring after day 4, unless there are extenuating circumstances.
type of anticoagulation regimen after free tissue transfer, and in
pedicled flap reconstruction the frequency of use is dependent on its
vascular supply.90,140
Unfortunately, there is no consensus on anticoagulation therapy after
free tissue transfer, and a full discussion of all pertinent studies
pertaining to postoperative anticoagulation is beyond the scope of this
chapter. It is sufficient to say that scientific findings are often
clouded by anecdotal experience. The three most common anticoagulants
in use are aspirin, heparin, and dextran.
pathway, decreases the production of thromboxane and prostacyclin, both
of which are powerful platelet aggregators. Aspirin’s effectiveness in
decreasing macrovascular graft occlusion has been clearly demonstrated
in several studies.2,21
The effective dose of aspirin to inhibit thromboxane while preserving
some of the provasodilatory effects of prostacyclin function is
relatively low, within the range of 50 to 100 mg per day.78,81,412
Despite its use postoperatively to prevent thrombosis, aspirin’s most
beneficial effect may be when it is given several hours before surgery.
The administration of aspirin 10 hours before surgery has been shown to
result in a significant increase in vessel patency and a decrease in
platelet aggregation.339
Large prospective randomized human trials are not yet in existence.
Hematoma formation with the potential for flap loss has been linked to
full systemic postoperative anticoagulation. In Pugh’s retrospective
study, the incidence of hematoma formation after lower-leg
reconstruction and systemic anticoagulation with heparin was 66%.317
The use of subcutaneous heparin or low molecular weight heparin (LMWH)
is warranted for prevention of deep venous thrombosis, while also
providing a benefit with regard to vessel patency. Khouri et al. found,
in the largest multicenter prospective free flap tissue study, that
only postoperatively administered subcutaneous heparin had a
statistically significant effect on the prevention of postoperative
free flap thrombosis.217
aspirin has been shown to produce no increase in the rate of
postoperative hematoma formation.69
In our opinion, this combination of drugs provides a safe and
economical means of providing thrombosis prophylaxis for routine free
flap procedures. This combination therapy also provides the benefits of
coronary protection and deep venous thrombosis prophylaxis.77
Subcutaneous heparin does not require monitoring of coagulation
factors, and both medications may be given without intravenous access.
LMWH also provides the benefits of higher bioavailability, a longer
plasma half-life, and a steady dose-response curve and it causes fewer
cases of hematoma formation and thrombocytopenia when compared with
unfractionated heparin.19
improving patency rates in the immediate preoperative period when given
as a single preoperative bolus;340,440 however, the effectiveness of prolonged administration is debatable.315,334,338
An increasing number of reports have noted significant morbidity
associated with the use of dextran and have questioned its use in
routine microsurgical cases.171,174
Complications from dextran administration can include renal failure,
congestive heart failure, myocardial infarction, pulmonary edema,
pleural effusion, and pneumonia.
prospective randomized multicenter trials will be necessary to
definitively decide which anticoagulation therapy is the most effective
in preventing postoperative flap thrombosis. Until
that time we feel that a combination of low-dose aspirin and
subcutaneous or low-molecular-weight heparin provide adequate flap
protection with minimal associated morbidity and little additional cost.216
will improve flap survival and prevent morbidity and mortality. From a
cardiac standpoint, surgical patients with coronary artery disease or
risk factors for coronary artery disease who undergo tissue transfer
surgery should undergo an appropriate evaluation by their cardiologist
or internist before surgical intervention. The administration of
β-blockade with Atenolol has been shown to have reduced cardiovascular
complications and mortality for up to 2 years in this patient
population.265 Hyperglycemia
associated with relative insulin resistance or diabetes has been
reported to increase the incidence of complications in the surgical
patient.119,287
For this patient population, intensive insulin therapy to maintain
blood sugar levels between 80 and 110 mg/dL has been shown to
substantially reduce morbidity and mortality from 8% to 4.6%.400
hydration in the perioperative period, and commonly a Foley catheter
will be used to record and maintain a urine output of at least 50
cc/hour. In our institution, patients commonly are given nothing by
mouth until the morning after surgery in the event reoperation is
necessary. Hematocrit levels are kept at greater than 30% in patients
with coronary artery disease and greater than 25% in those without it.
microsurgery, flap failure will occur. Flap failure can be partial or
complete. It is important to recognize the cause of flap failure so it
may be reversed or prevented in the next reconstructive attempt.
Arterial insufficiency leading to flap complications can be recognized
by decreased capillary refill, pallor, reduced temperature, and the
absence of bleeding on pin prick testing. The complication can result
from arterial spasm, vessel plaque, torsion of the pedicle, pressure on
the flap, technical error with injury to the pedicle, a flap harvested
that is too large for its blood supply, or small vessel disease
secondary to diabetes or smoking. If pharmacologic agents do not
relieve spasm at the level of arterial inflow, the vessel anastomosis
should be redone.
flap has a violaceous color and brisk capillary refill, and dark blood
is seen after pin prick. Venous obstruction can occur as a result of
flap edema, hematoma, tight closure over the pedicle, or pedicle
torsion. Venous compromise will lead to microvascular thrombi, which
will then compromise arterial flow if not promptly addressed.
Conservative treatment in the acute phase, besides pharmacologic
therapy as discussed, may include drainage of an underlying hematoma
with suture release to decrease the pressure. Leeches may also be
helpful if sufficient venous outflow cannot be established despite a
patent venous anastomosis. The leeches work by biting the venous
congested tissue and extracting blood via direct suction and injecting hirudin medicinalis, a potent anticoagulant present in their saliva. Aeromonas hydrophilia
is an important microbe present in the leech, and prophylactic
antibiotics (usually a second- or thirdgeneration cephalosporin or an
aminoglycoside or fluoroquinolone) must be given when patients are
undergoing leech therapy.91,276,413
Because of blood loss from the therapy, it is also important to check
serial hemoglobin levels and have the patient typed and crossmatched
for blood transfusion at all times.
serve as a source of infection in an already compromised limb. The
timing of removal is dependent on the recipient bed on which it was
inset. Scarred, radiated, or dysvascular wound beds only provide
minimal blood supply to the overlying flap tissue; therefore, upon flap
compromise more flap tissue is lost.343,411 If a second free flap is considered, obvious errors that led to the original flap compromise need to be recognized and avoided.
to the physician. The severity of the disease is staged depending upon
the infection’s particular features, including its etiology,
pathogenesis, the extent of bone involvement, its duration, and host
factors particular to the individual patient (infant, child, adult, or
immunocompromised). Long bone osteomyelitis may be either hematogenous
or caused by a contiguous spread of infection. A single pathogenic
organism is almost always recovered from the bone in hematogenous
osteomyelitis; Staphylococcus aureus is
the most common organism isolated. A variety of multidrug resistant
organisms (MDROs) continues to be a source of concern in arresting
infection. The primary weapons to treat these infections are
culture-specific antibiotics, aggressive débridement, muscle flaps, and
bone grafts.
chronic), pathogenesis (trauma, contiguous spread, hematogenous, or
surgical), site, extent, or type of patient. Although several
classifications of osteomyelitis have been described by different
authors, the two most widely used in the medical literature and
clinical practice are the classification systems by Waldvogel et al.404 and Cierny et al.74
Under the Waldvogel system, osteomyelitis is first described according
to duration as either acute or chronic. Secondly, the disease is
classified according to source of infection, as hematogenous when it
originates from a bacteremia, or as contiguous focus when it originates
from an infection in a nearby tissue. A final category of the
classification is vascular insufficiency. One of the limitations of the
Waldvogel classification system is that it does not consider infection
originating from direct penetration of microorganisms into the bone, as
may occur after trauma or surgery. In addition, it is an etiologic
classification system that does not readily lend itself to guiding
surgical or antibiotic therapy. Because of the wide variability in the
etiology of osteomyelitis, a classification based upon the pathogenesis
of the disease, such as that of the Waldvogel system, has limited value
in clinical practice.
It is a clinical classification based on anatomic, clinical, and
radiographic features. It characterizes osteomyelitis as being in one
of four anatomic stages. Stage 1, or medullary, osteomyelitis is
confined to the medullary cavity of the bone. Stage 2, or superficial,
osteomyelitis involves only the cortical bone and most often originates
from a direct inoculation or a contiguous focus infection. Stage 3, or
localized, osteomyelitis usually involves both cortical and medullary
bone. In this stage the bone remains stable, and the infectious process
does not
involve
the entire bone diameter. Stage 4, or diffuse, osteomyelitis involves
the entire thickness of the bone, with loss of stability, as in an
infected nonunion. The Cierny-Mader system adds a second dimension,
characterizing the host as either A, B, or C. The A hosts are patients
without systemic or local compromising factors. B hosts are affected by
one or more compromising factors. C hosts are patients so severely
compromised that the radical treatment necessary would have an
unacceptable risk/benefit ratio.
|
TABLE 14-6 Cierny-Mader Osteomyelitis Staging System
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
combination of diverse imaging techniques for an accurate diagnosis.
Conventional radiography should always be the first imaging modality to
start with, as it provides an overview of the anatomy and the
pathologic conditions of the bone and soft tissues of the region of
interest. Sonography is most useful in the diagnosis of fluid
collections, periosteal involvement, and surrounding soft tissue
abnormalities and may provide guidance for diagnostic or therapeutic
aspiration, drainage, or tissue biopsy. Computed tomography (CT) can be
a useful method to detect early osseous erosion and document the
presence of a sequestrum, foreign body, or gas formation, but generally
it is less sensitive than other modalities for the detection of bone
infection. Magnetic resonance imaging (MRI) is the most sensitive and
specific imaging modality for the detection of osteomyelitis. It
provides superb anatomic detail and more accurate information regarding
the extent of the infectious process and soft tissues involved. Nuclear
medicine imaging is particularly useful in identifying multifocal
osseous involvement.
directed on the basis of cultures or deep bone biopsy, and antibiotic
sensitivity tests.75 After cultures
have been obtained, a parenteral course of antibiotics is begun to
cover clinically suspected pathogens. When the organism is identified,
a specific antibiotic or antibiotics are selected through sensitivity
testing. If immediate débridement surgery is required before cultures
can be obtained, broad-spectrum antibiotics may be initiated
empirically, and the regimen modified when the results of cultures and
sensitivity tests are known. Initial antibiotic therapy for long-bone
osteomyelitis may consist of either nafcillin or clindamycin (or
vancomycin when methicillin-resistant S. epidermidis or S. aureus [MRSA], or Enterococcus
sp. are suspected) and ciprofloxacin (except with children, in whom an
aminoglycoside should be used). Levofloxacin has been used, but serum
levels often fall below minimum inhibitory concentrations, and at
present dosing has failed in both a human and animal osteomyelitis
study.150,360
Bone requires 3 to 4 weeks to revascularize after débridement surgery,
and thus antibiotics are used to treat live infected bone and protect
it as it undergoes revascularization. After the last major débridement,
the patient is treated with 4 to 6 weeks of antimicrobial therapy, and
outpatient intravenous therapy may be used.76,262
stage of the infection. With Cierny-Mader Stage 1 osteomyelitis,
children may be treated with antibiotic therapy alone, because their
bones are highly vascular and respond effectively to antibiotics. In
adults, a Stage 1 infection is more refractory to therapy and usually
is treated with both antibiotics and surgery. With Stage 2 infections,
the patient may be treated with a 2-week course of antibiotics after
superficial débridement and soft tissue coverage. In these cases, the
arrest rate is approximately 80%. In Stages 3 and 4, the patient is
treated with 4 to 6 weeks of antimicrobial therapy dated from the last
major débridement. At this stage of the disease, most antibiotic
regimens will fail without adequate débridement regardless of the
duration of therapy. Even after necrotic tissue has been débrided, the
remaining bed of tissue must be considered contaminated.
regardless of whether it is conventional or automatic, cannot test all
antimicrobial agents against all microorganisms and detect all patterns
of resistance.324 The strategy of
using multiple susceptibility testing methods continues to evolve,
particularly with regard to Gram-negative organisms. A current issue is
the susceptibility testing of the polymyxin class of antimicrobial
agents (colistin or polymyxin B and polymyxin B) for the therapy of
infections caused by multidrug-resistant isolates of Pseudomonas aeruginosa and Acinetobacter spp.369
Guidelines for testing Gram-negative control strains with polymyxin B
and colistin have been established for broth microdilution.206
Currently, however, guidelines do not exist for disc-diffusion testing
of polymyxins. Synergy testing represents a shift in the approach by
microbiology laboratories driven by the growing challenge of
multiresistant bacteria and the need to correlate in vitro tests with
in vivo outcomes.
testing is wholly lacking with respect to biofilm. Clinical
microbiologists test antimicrobial efficacy against planktonic and
nonbiofilm agar based growth cultures. Therefore, the biofilm phenotype
and the associated 50- to 500-fold increase in antibiotic resistance
(compared with their planktonic counterparts) are often ignored. The
result is that antimicrobial therapy is prescribed against a nonbiofilm
infection and what is effective against planktonic infections is not
always the same as what is effective against a biofilm infection.
challenging. Adequate surgical débridement decreases the bacterial
load, removes necrotic tissues, and gives a chance for the host immune
system and antibiotics to arrest the infection. Adequate débridement
may leave a large bony defect or dead space. Appropriate management of
dead space is essential to arrest the disease and maintain the bone’s
integrity. The objective of dead space management is the replacement of
dead bone and scar tissue with durable vascularized tissue. Complete
wound closure should be attained whenever possible, and local tissue
flaps or free flaps
may be used to fill dead space.262
An alternative technique is to place cancellous bone grafts beneath
local or transferred tissues until structural integrity is improved.
This technique requires careful preoperative planning to conserve the
patient’s limited cancellous bone reserves. Open cancellous grafts
without soft tissue coverage are useful when free tissue transfer is
not an option and local tissue flaps are inadequate.
Antibiotic-impregnated acrylic beads or antibiotic-loaded cement also
may be used to sterilize and temporarily maintain dead space.214
The beads are usually removed after 2 to 4 weeks and replaced with a
cancellous bone graft. The antibiotics used in the beads are most often
vancomycin, tobramycin, and gentamicin.259
Chronic osteomyelitis of bone with nonunion or bone defects is
traditionally treated by a two-stage procedure involving initial
débridement and antibiotic delivery, with initial external fixation,
and then definitive internal fixation. Antibiotic cementcoated
interlocking intramedullary nails can help convert two-stage processes
into a single-stage procedure and can be used in patients who are not
ideal candidates for external fixation, as well as in patients who do
not want to have an external fixator applied.
which showed that muscle flaps are able to control a 10-fold higher
bacterial count than fasciocutaneous flaps and also improve antibiotic
delivery to the wound site. What is poorly understood is that this
study compared random pattern fasciocutaneous flaps to axial pattern
muscle flaps. Therefore this is a flawed comparison because the
vascular supply is completely different. In a recent study by Yazar et
al. comparing lower limb wounds reconstructed with free fasciocutaneous
or free muscle flaps in a total of 177 cases, there was no difference
in outcomes or infection rates.429 This highlights the important role of adequate débridement, regardless of the type of flap used.
or a vascularized bone graft. Vascularized bone grafts have become an
important tool for the reconstructive surgeon dealing with the
management of long bone defects or difficult nonunions. They are
especially important in the setting of osteomyelitis treatment because
they combine the advantages of viable cancellous autografts with the
stability of cortical analogues, while leaving the nutrient blood
supply intact. Indications for their use include skeletal defects more
than 6 cm in length,252 defects
associated with a poor soft tissue envelope, and in cases in which
smaller sized nonvascularized bone grafts have failed to incorporate.
Vascularized bone grafts incorporate into the recipient site through a
different process than avascular grafts. The vascularized grafts bypass
the process of creeping substitution, which involves necrosis of the
graft, resorption, and new bone formation. They maintain their mass,
architecture, and biomechanical strength. Furthermore, the transferred
vascularized bone has the ability to hypertrophy, especially in the
lower extremity, because of increased mechanical loading. Available
donor bones to be used for vascularized bone grafts include the fibula,
ilium, scapula, and rib.430
reconstruction options: distraction osteogenesis (Ilizarov technique),
nonvascularized bone grafting, or vascularized bone grafting. The
specific technique employed is dependent on the size of the defect, the
quality of the soft tissue envelope, and the location of the defect.
who in the western Siberian city of Kurgan, discovered that normal
tissue could be generated under carefully applied tension.193,194,195,196
The effect of the tension-stress effect on bone resulted in
neovascularization, increased metabolic activity, and cellular
proliferation, similar to but not identical to normal enchondral
ossification at the physis. The resulting fibrous tissue in between the
distracted bone segments ossifies in an orderly fashion, resulting in
structurally sound bone. The soft tissues concomitantly grow linearly
in response to the applied tension.199
that are held in place by fine wires that are crossed and secured to
the ring. The wires are tensioned to between 60 and 130 kg. A series of
rings are constructed and bridged together with threaded posts, each
with a distraction or compression device that can be adjusted every
several hours to effect compression or distraction. Many modifications
to this system have been described.
be filled with one of two methods: by acutely shortening the bone and
then gradually lengthening it to restore the original bone length, or
transporting bone either proximal or distal to the bone defect to
gradually fill in the defect.98,99,147,148
device, a free tissue transfer can be performed to address complex
lower extremity bone injury with significant soft tissue defects. In
the acute setting, the external ring fixator or modifications of the
fine wire fixators can be applied as the primary management of the
fracture. When these devices are applied it is imperative that if soft
tissue coverage is required, discussions between the microsurgeon and
orthopaedic surgeon occur early in the care of the injured extremity.
The fixation can also be used as a means of transporting bone to fill
in a segmental defect or a nonunion in chronic cases.
allograft tissues. They are ideal for small defects and voids and can
be obtained from a number of anatomic locations and are typically
cancellous or corticocancellous in composition. Autografts are superior
in general to allograft material. For most bone defects of less than 6
cm with a well vascularized bed, adequate soft tissue coverage and
absence of infection, a conventional cancellous or corticocancellous
bone graft is generally recommended.34
The most common areas for nonvascularized autograft bone harvest
include the iliac crest (anterior or posterior), distal radius, and
olecranon.44 Cancellous bone has greater inductive capacity than cortical bone and should be used unless mechanical stability is required.
with resorption and replacement of the necrotic bone graft.28
Creeping substitution results in rapid revascularization in small
cancellous grafts, but is slow and incomplete in cortical bone. As much
as 40% to 50% of lamellar bone remains necrotic, and the
revascularization process that does occur causes significant mechanical
weakening because of bone resorption at 6 to 12 months.34,49,366
Allografts, like autografts, must also be replaced by living bone. They
are replaced more slowly and less completely, and they invoke a local
and systemic immune response that diminishes the stimulus of new bone
formation. This effect may be diminished by freezing, freeze drying,
irradiating or decalcifying the graft, or eliminated with the use of
immunosuppressive drugs.* Structural nonvascularized grafts
of all types have substantial problems with fatigue fracture, even
years after the surgical procedure. Successful grafting requires a
well-vascularized bed, adequate immobilization, and protection from
excessive stress by rigid internal fixation.112
of vascularized bone remain alive and dynamic in its new site. Because
of its preserved circulation, cell viability is greater than in
conventional grafts,16,32 obviating the need of the gradual creeping substitution of living bone into a “dead” bone.28,34,49 During healing, extensive osteopenia is not seen with vascularized bone grafts as it is in conventional bone grafts.88 Vascularized grafts have improved strength, healing and stress response as compared with nonvascularized bone grafts.† The incidence of stress fracture is lower than in massive structural autografts or allografts.112,169,312,367,410 Finally, union is more rapid, and bone hypertrophy in response to applied stress may occur with time.130 Bone healing is more likely in difficult circumstances including scarred or irradiated beds, or in an avascular bone bed.127,128
circulation, and mechanical properties, vascularized grafts have other
significant advantages over conventional grafts. These include the
possibility to restore longitudinal growth by inclusion of the growth
plate,41,388,436 revascularize necrotic bone,‡ improve local blood flow in scarred soft tissue beds,314,384
and reconstruct composite tissue loss in one procedure by the inclusion
of skin, muscle, tendon, nerve, and other tissues with the bone graft.
information reported in the preceding, it would seem that vascularized
autografts would be ideal for grafting under most circumstances. Their
use as free tissue transfers is technically demanding, however, and
pedicle grafts are often more limited in dimension and pedicle length
and hence indications. Prolonged operative times and extensive
dissection increase the risk of complications, and donor site morbidity
may be significant. Therefore, for bone defects less than 6 to 8 cm
with normal soft tissues, conventional techniques remain the method of
choice under many circumstances.
graft are its largely cancellous nature and the large amount of soft
tissue that may be raised with the bone as a combined
osteomusculocutaneous flap. In such flaps, a more reliable skin flap
may be obtained with inclusion of both superficial and deep circumflex
iliac vessels. The advantages of the osteocutaneous flap include the
ability to: (i) supply vascularized bone to what is frequently a poor
recipient bed for a bone graft, (ii) reconstruct both soft tissue and
bony defects simultaneously, and (iii) be used in facilities without a
capability for microvascular surgery when used as a pedicle flap for
the upper extremity.323 It may also be used for smaller defects.
in segmental bone defects larger than 6 to 8 centimeters due to tumor
resection,* traumatic bone loss,† osteomyelitis, or infected nonunion.‡
in cases in which “biologic failure” of bone healing is likely or has
already occurred.288 Examples
include persistent nonunion after conventional treatment, poorly
vascularized bone and/or its soft tissue bed because of scarring,
infection or irradiation, and congenital pseudarthrosis.9,270,301,312,397,409
tissue loss requiring complex reconstruction, joint arthrodesis in
exceptional circumstances, and the need for longitudinal growth with
physeal transfer.
vascularized bone graft because its structure and shape are appropriate
for diaphyseal reconstruction (Figure 14-21). A
long, straight segment of 26 to 30 cm in length can be harvested, and
osteosynthesis can be securely obtained to the recipient bone. The
blood supply to the fibula, as to other long bones, is derived normally
from a nutrient artery via radially oriented branches that penetrate
the cortex and anastomose with the periosteal vessels. The resulting
blood flow is centrifugal from medulla to cortex. This arrangement is
the norm for the fibula, which has a single nutrient vessel entering
its middle third from the peroneal artery. Additional periosteal
branches from the peroneal and anterior tibial artery also supply the
diaphysis.138 The proximal epiphysis
is supplied by an arcade of vessels, of which the lateral inferior
genicular vessels are the most important.388 This vessel must be anastomosed if physeal growth is desired after transfer of the fibular head.41,388
fasciocutaneous skin paddle of up to 10 × 20 cm. This is possible
because of a series of fasciocutaneous or myocutaneous perforators from
the peroneal artery that typically pierce the soleus muscle adjacent to
the lateral intermuscular septum.228,434
The location of the perforators may be determined in the operating room
prior to skin incision with the use of a Doppler ultrasound
stethoscope. Osteomuscular flaps including the flexor hallucis longu
or, portions of the soleus or peroneal muscles may also be raised using
the same peroneal artery pedicle.29,67,68 The peroneal pedicle has a length of 6 to 8 cm, and an arterial diameter of 1.5 to 3.0 mm.
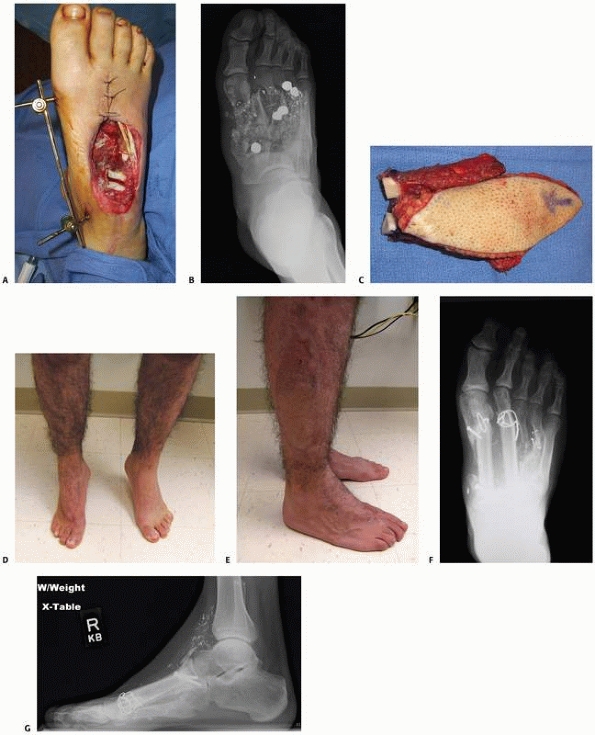 |
|
FIGURE 14-21 A,B. Following a gunshot wound to the foot, there was loss of the first and second metatarsals and an extensive dorsal foot wound. C. Intraoperative view of the fibular osteocutaneous flap. D-G.
Clinical photographs and x-rays 16 months following the free fibular osteocutaneous flap for first and second metatarsal ray reconstruction. The patient was able to ambulate without difficulty and to play soccer. |
the upper and lower extremities with the use of the free fibula flap in
cases of osteomyelitis,407 pathologic fracture,128 segmental bone loss of the femur,407 tibia,416 radius and ulna,5,423 humerus,6 and pelvis (Figure 14-22).289 The bone is capable of hypertrophying over time through a process of fracture and callous healing.407
In addition single or multiple osteotomies may be made in the bone as
long as one preserves a periosteal sleeve and the nutrient vessel. This
then allows for double fibular strut reconstruction in cases of
segmental bony injuries.65,205,407
through a lateral approach with the patient in the supine or lateral
position. Preoperative vascular studies, although controversial in the
literature, have been very useful to us in preoperative planning in
cases of posttraumatic reconstruction and in patients with peripheral
vascular disease.104,256
We obtain a CT angiogram in all patients in preparation for free
fibular transfer. Unlike a formal angiogram, a CT angiogram adds no
additional morbidity while providing information on inflow and outflow
vessels in both legs. In 10% of the population the peroneal artery is
the dominant arterial supply to the leg, and is referred to as the
peroneal arteria magna; in such cases the contralateral leg should be
considered for graft harvest.104,256
the fibula in a line running from the fibular head to the lateral
malleolus. We have found it helpful to always include a skin paddle in
the flap design; it facilitates closure as well as postoperative flap
monitoring. Inclusion of a cuff of soleus muscle or flexor hallucis
longus muscle (FHL) can improve the reliability of the skin paddle if
skin perforators are small. Dissection is initiated between the plane
of the soleus and peroneal muscles. Once the fibula is visualized
laterally the peroneal nerve is identified and protected as dissection
then continues superficial to the periosteum in a medial direction (Figure 14-23).
The interosseous membrane is incised. The bone is then divided
proximally and distally with the use of a Gigli saw or sagittal saw,
taking care to protect the surrounding neurovascular structures. Six
centimeters of the distal fibula must remain intact to stabilize the
ankle. In the skeletally immature patients we always perform a
syndesmosis at the lateral malleolus after fibula harvest.291
Six centimeters of fibula bone is also preserved proximally (below the
head of the fibula) to preserve the stability of the knee. This is
achieved by maintaining the attachment of the tibia to the fibula, and
the attachments of the biceps femoris muscle and the fibular collateral
ligament to the head of the fibula. The proximal part of the fibula
hosts parts of the origins of the peroneus longus, the extensor
digitorum longus, the extensor hallucis longus, the soleus, and the
tibialis posterior muscles. Minimizing dissection at the level of the
fibular head will also help to avoid injury to the peroneal nerve.
identified distally deep to the tibialis posterior muscle and just
dorsal to the FHL. The artery is divided and ligated distally.
Dissection proceeds proximally to the peroneal-posterior tibial
arterial bifurcation. Here the artery is ligated distal to the
junction, preserving the posterior tibial artery. The surgeon should
always verify the position of the tibial nerve and posterior tibial
artery before ligation of the peroneal vessels.
meshed skin graft is always used to cover the donor site; a tight
primary closure can increase the risk of compartment syndrome within
the donor leg. Meticulous closure of the donor site, with particular
attention to the flexor hallucis longus muscle, is critical to
decreasing donor limb morbidity. Patients are typically able to resume
pain free weight-bearing ambulation 4 to 6 weeks after fibular harvest.
performed with care, as inadvertent screw placement can injure or
avulse the pedicle or nutrient vessels. Plates applied to the surface
of the fibula should use unicortical screws and ideally the plate and
screws should be placed on the lateral surface of the fibula, away from
the vascular pedicle. The periosteum at the bone/plate interface should
not be stripped and only minimal periosteal stripping should be
performed at the points of screw insertion. The bone to bone contact
between the fibula and recipient site can be maximized by creating step
cuts, or the fibula can be telescoped into the recipient bone when the
size is appropriate, such as the femur or humerus. Spanning plates are
ideal as they allow for firm fixation above and below the intercalated
fibula, yet allow for unicortical purchase of the fibula for
stabilization.381
supply from the superficial circumflex iliac artery and deep circumflex
iliac artery (DCIA).36 Of the two, the DCIA system is most important.345
Musculocutaneous perforators penetrating the abdominal wall 1 cm
proximal to the iliac crest provide its nutrition. In the experience of
several authors, the skin paddle has been less reliable than a standard
groin flap, particularly if slightly rotated in relation to the
underlying bone.36,138,344
Its size, when based on the DCIA, is quite variable, ranging from 7 ×
10 to 15 × 30 cm. The entire iliac bone, however, is well supplied by
the DCIA via multiple perforating arteries at the points of muscle
attachment.302 It remains the
pedicle of choice for osteocutaneous flaps, although double-pedicle
flaps have been described using both the superficial and deep
circumferential iliac vessels and may be desirable.228
practical limit of 10 cm in length as a vascularized graft because of
its curved shape. It is relatively less suited for diaphyseal
reconstruction than the fibula, as remodeling to tolerate weightbearing
is prolonged.367 Further, osteosynthesis is difficult and weak.
been demonstrated experimentally to produce predictable new bone
formation, provided they have adequate vascularity.223,379
Bone formation after free vascularized transfer of periosteum may be
enhanced by enclosing a cancellous bone graft in a periosteal wrap.332 A variety of donor sites have been identified, including clavicle, fibula, ilium, humerus, tibia and femur, among others.*
In the upper extremity, thin corticoperiosteal grafts and small
periosteal bone grafts harvested from the supracondylar region of the
femur have proved to be of great use, based on either the descending
genicular or medial superior genicular artery and vein (Figure 14-24).357
This graft is elastic and can be readily conformed to the shape of
small tubular bones. It has been successfully used for clavicle,
humerus, and forearm applications,
including pathologic fractures from radiation necrosis and other recalcitrant nonunions.106
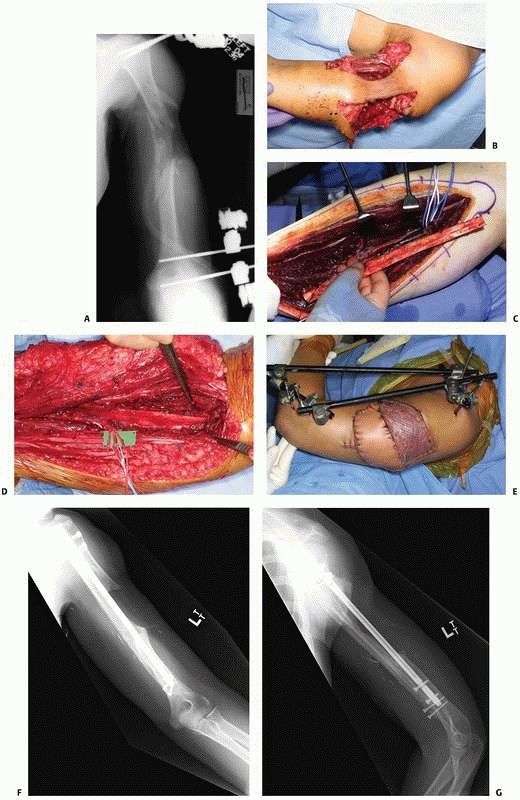 |
|
FIGURE 14-22 A,B.
A 44-year-old woman sustained a gunshot wound to the left humerus resulting in a large entrance and exit wound (greater than 15 cm each) with segmental bone loss of the humerus exceeding 10 cm in length. The fractures were temporarily stabilized with external fixation and the soft tissue defect addressed with an ipsilateral free latissimus dorsi flap. C-E. Once the soft tissues were stabilized, a free vascularized fibula was used to bridge the bony defect after an intramedullary nail had been placed. F,G. The fibula was incorporated into the proximal and distal ends of the humerus by 3 months, resulting in a salvaged and very functional upper extremity. |
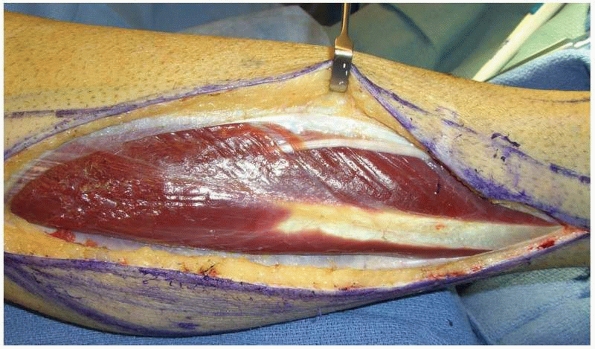 |
|
FIGURE 14-23
The superficial peroneal nerve is shown in the lateral compartment of the right leg during free fibula osteoseptocutaneous flap harvest. |
The posterior rib graft, which includes its nutrient artery, requires
ligation of the dorsal branch of the posterior intercostal artery.302
Because this vessel supplies the spinal cord, the potential for causing
paraplegia exists. Further, dissection is difficult and usually
requires a thoracotomy.
flap with vascularized bone graft on a single pedicle have multiple
advantages, including the ability to have a vascularized bone graft and
then cover it with healthy muscle. One such vascularized bone graft and
muscle flap composite graft is the rib, serratus anterior, and
latissimus dorsi flap.219,252,294,309,380,430
Based on the thoracodorsal vessel and its branches to the serratus and
latissimus, up to two nonadjacent ribs can be harvested with the
overlying serratus muscle which provides the vascularity to the bone. A
significant length of rib can be harvested and by making a corticotomy
on its concave side, the curved rib can be straightened to be applied
to a long bone or long bone defect (Figure 14-25). Hypertrophy of the ribs, in comparison to a fibular graft, does occur with time.
acute nerve injuries comes from the works of Seddon, which stemmed from
the treatment of World War II patients.349
Seddon introduced a simple classification of traumatic nerve injuries:
neurapraxia, which was minimal injury with localized ischemic
demyelination of the nerve; axonotmesis, characterized by interruption
of the axons and their myelin sheath with the endoneurial tubes
remaining intact; and neurotmesis, which is a completely severed nerve
or one that is so seriously disorganized that spontaneous regeneration
is impossible. Sunderlund in 1951 proposed a five-level classification
that related to the internal structure of the nerve; however, it relied
on pathologic examination of the nerve, which is quite impractical in
the trauma setting.377
type of injury to it (neurapraxic, axonotmetic, neurotmetic), the time
from the injury, the soft tissue bed quality, the defect size if the
nerve is transected, and associated nerve/muscle injuries. In acute
fractures with nerve injury, there is tremendous debate on whether to
explore or observe. Depending on the energy of the trauma, the
decisions may vary. In particular, debate continues regarding treatment
of radial nerve injury associated with distal humeral fractures (see Chapter 34).*
Generally in acute closed fractures, observation of the nerve injury
with serial examination for 3 to 6 months should be undertaken. If no
recovery is seen, electrodiagnostic testing should be considered as
early as 6 weeks postinjury. If no improvement is observed by 4 to 6
months, exploration with interposition nerve grafting or alternatively
nerve transfers should be considered.
high degree of trauma (i.e., not a sharp laceration) with associated
soft tissue injuries, the nerve ends should be tagged and the soft
tissue and bone injury addressed. The greatest challenge is the
determination of the zone of injury of the nerve.† If acute
nerve grafting is to be performed, resecting the injured portion is
imperative. Unfortunately, intraoperative assessment by histologic
section, touch, or visualization of the injured nerve cannot inform us
of the zone of injury. Delay of a few weeks allows the development of
intraneural fibrosis, and allows tactile and pathologic visualization
of the zone of injury; however, the scarred tissues make the surgical
reconstruction more difficult. If acute soft tissue reconstruction is
performed, and a delayed nerve reconstruction is planned, the surgeon
should consider placing the nerve to be reconstructed in a location
where it can be easily accessed. Finally, if more than 6 to 12 months
pass between injury and reconstruction, tendon transfers or free
functioning
muscle transfers should be considered as there is a time dependent,
irreversible degradation of the motor endplate that occurs after motor
nerve injury.358
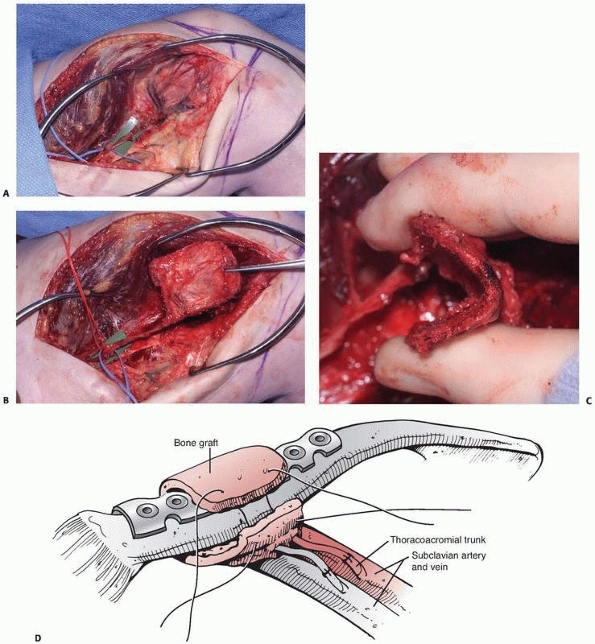 |
|
FIGURE 14-24
Medial femoral condyle corticoperiosteal grafts can be used to span shorter defects or be used to wrap around difficult fractures to provide a vascularized bone graft option. After elevating the vastus medialis, the medial femoral condyle is exposed to demonstrate a ring of periosteal vessels based on the descending genicular or medial superior genicular artery and vein (A), a corticocancellous graft is elevated (B). C,D. The graft is quite flexible and can be molded around bones at the recipient site. |
avulsions have varied widely over the past 50 years, and the results of
treatment have been reported as fair to dismal. After World War II, the
standard approach was surgical reconstruction by shoulder fusion, elbow
bone block, and finger tenodesis.177 In the 1960s transhumeral (above elbow) amputation combined with shoulder fusion in slight abduction and flexion was advocated.122
Yeoman and Seddon noted the tendency for injured patients to become
“one-handed” within 2 years of injury, which led to a dramatic
reduction in successful outcomes regardless of the treatment approach.430
Their retrospective study revealed no good results from the primitive
surgical reconstruction of that era, but predominantly good and fair
outcomes when amputation plus shoulder fusion were performed within 24
months of injury. They also noted that the loss of glenohumeral motion
caused by brachial plexus injuries limited the effectiveness of
body-powered prostheses and that manual laborers seemed to accept hook
prostheses much more readily than did office workers with similar
injuries. Although these observations remain valid today, there have
been advances in brachial plexus reconstruction that have yielded
outcomes superior to the historical
results.
A better understanding of the pathophysiology of nerve injury and
repair, as well as the recent advances in microsurgical techniques,
have allowed reliable restoration of elbow flexion and shoulder
abduction in addition to useful prehension of the hand in some cases.
The specific treatment of these injuries is beyond the scope of this
chapter; however, there are multiple modalities, including nerve
grafting, nerve repair, nerve transfers, tendon transfers, and free
tissue transfers that can be used to improve function and outcome.26,57,292,358
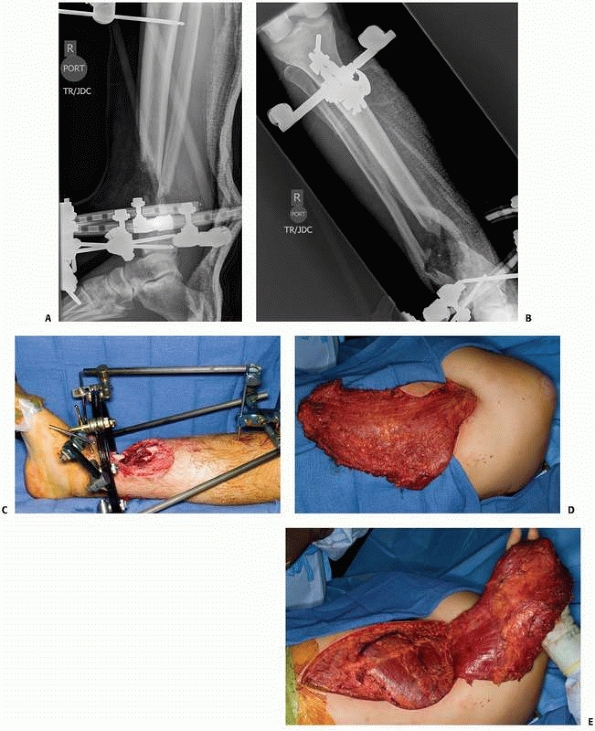 |
|
FIGURE 14-25 A-C.
This 19-year-old man sustained open distal tibia and fibula fractures with significant bone and soft tissue loss. His injury was first stabilized with an external fixator. The zone of injury of the open fracture extended far beyond the margins of the wound, and with segmental bone loss, skin loss, and extensive soft tissue injury, the decision for a composite rib, serratus, and latissimus flap was made. The latissimus was raised with a cutaneous paddle (D) and after elevation, the serratus and its branch of the thoracodorsal artery and vein were identified over the fourth and sixth ribs (E). (continues) |
of improving the aesthetic outcome of the procedure is higher than
others. Clearly, when reconstructing a facial defect, this becomes a
high focus of the reconstruction. With advancements in the
understanding of flap anatomy, major advancements have been seen in the
aesthetic refinements that can be achieved when reconstructing defects
in the extremities.318
Improvements in aesthetic outcomes come at two stages in the
reconstruction. The first is during the initial reconstructive
procedure, when a flap is chosen to meet the functional needs at the
recipient site and to achieve a reasonable aesthetic outcome. Choosing
a flap that has qualities that match the recipient site, such as color,
thickness, and pliability is important. Primary flap thinning can be
performed in the operating room to achieve the best aesthetic outcome
in the initial setting. The second stage is carried out with the use of
additional surgical procedures to refine flap shape; several months
after the initial procedure, secondary procedures may be performed,
such as flap debulking through direct excision or liposuction419 or even using the arthroscopic shaver device,382 flap advancement, and serial excision of the flap.355
Workhorse flaps in reconstructive surgery that are thin include the
radial forearm flap, the lateral arm flap, and many of the discussed
perforator flaps. Additionally, depending on the needs at the recipient
site, fascia flaps covered with skin grafts often produce aesthetically
and functionally good results. These flaps include the temporoparietal
fascia flap,331 the posterior rectus sheath flap,342 the lateral arm fascia flap,375 as well as the anterolateral thigh fascia flap.188
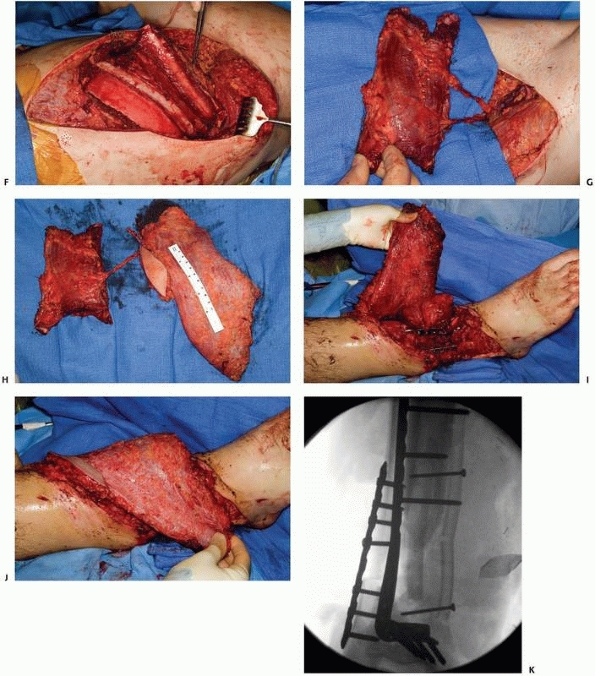 |
|
FIGURE 14-25 (continued) F,G.
The sixth and fourth ribs were elevated extraperiosteally, leaving the serratus attachments intact. The entire flap is shown in (H). I-K. The ribs were inset into the bone defect. (continues) |
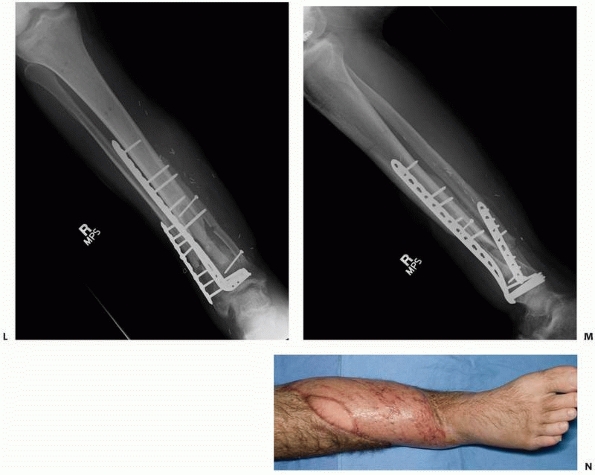 |
|
FIGURE 14-25 (continued) L-N. Seven months after reconstruction, the flap has contoured nicely and there was consolidation of the ribs to the tibia.
|
donor site morbidity has been the advent of perforator flap surgery.
Whereas the muscle was always thought to be a necessary carrier of the
blood supply in musculocutaneous flaps, perforator flaps are performed
by harvesting the skin and subcutaneous tissue, with a variety of
tissue components, while preserving the muscle at the donor site. The
skin and subcutaneous tissue are elevated and a large perforator is
found. This perforator is then dissected from the surrounding muscle
and traced to the mother vessel. The flap is harvested while the muscle
is left intact. The remaining muscle is supplied through its secondary
blood supply, and innervation of the muscle is maintained by preserving
the nerves in the region. Functionally, the patient experiences minimal
donor site morbidly. Because the blood supply to the flap is through a
perforator that is clearly visualized and its anatomic basis has been
studied through anatomic dissections,398
the surrounding flap can then be trimmed to thin the flap and provide a
nicely contoured flap during the initial reconstructive procedure.63,95 Preoperative planning with the aid of CT and ultrasound to identify perforating blood vessels can improve surgical success.38,60,139,163
Once the surgeon’s skill in microsurgical techniques and flap
dissection has reached a high level, one can perform microdissection of
a perforator, which allows a detailed visualization of the arterial
anatomy of the flap, eventually allowing for aggressive and accurate
thinning of the flap.113,220,221,424
epigastric perforator flap based on the deep inferior epigastric
artery, the anterolateral thigh flap, the thoracodorsal artery
perforator flap,14 and the gastrocnemius perforator flap.162
As previously discussed, the most commonly used perforator flaps is the
anterolateral thigh perforator flap because of its versatility and the
ability to include a variety of structures as well as the ability to
thin and tailor the flap to fit the defect.433
reconstructive surgery is tissue expansion. This tool has been used in
both upper and lower extremity reconstruction.164,165 The flap is expanded before harvest and transfer to the defect site.167,387
Alternatively tissue expanders can be used to expand the tissue
surrounding a defect to provide additional tissue to help in
reconstruction, minimizing flap requirements. After acute
reconstruction, if the patient is unhappy with the shape or color of
the flap, tissue expanders can be placed around the flap, under the
normal skin of the extremity, and once expansion is complete the flap
can be excised, with the native expanded local skin used to cover the
resultant defect. Tissue expansion is associated with complications
including infection and implant extrusion, and is usually not
recommended in cases of acute reconstruction of contaminated wounds.
the benefit of which has not been fully used at this point. Endoscopic
technique now allows for the successful harvested of flaps such as the
latissimus dorsi,279 the rectus abdominis,253 the gracilis, the temporoparietal fascia flap,73 and others. It is also used in harvesting vein grafts and nerve grafts which are often used in reconstructive surgery.250
Comparative studies between open and endoscopically assisted muscle
harvest have found patients to have less donor site pain and shorter
scar lengths after endoscopic harvest.251
over the past 15 years; materials are now available which can provide a
scaffold for the ingrowth of fibroblasts and blood vessels over
avascular or minimal vascularized structures such as tendon and bone.
Integra dermal regeneration template (Integra Life sciences,
Plainsboro, NJ) was initially developed in the late 1980s as a means of
facilitating burn wound management.51,425,426
More recently, the indications for this material have been extended to
include the treatment of acute and chronic traumatic wounds (Figure 14-26).175,283,403
collagen glycosaminoglycan biodegradable matrix and a superficial
semipermeable silicone layer. The deep layer allows for the ingrowth of
native fibroblasts. Fibroblasts can form a “neodermis” upon the
collagen scaffold, which is similar in appearance to normal dermis.282,371
This neodermis can then support a thin split-thickness skin graft. The
silicone layer prevents desiccation during the ingrowth period and it
is removed before application of a skin graft. Major contraindications
to the use of this material include ongoing infection and open
fractures exposed within the wound.
with skin grafting in 16 combat-related soft tissue wounds. The average
wound size was 87 cm2. Eleven wounds
contained exposed tendon and five wounds had exposed bone devoid of
overlying periosteum. Integra application was combined with overlying
VAC therapy for an average of 19 days before the application of a
split-thickness skin graft to the wounds. Treatment was successful in
83% cases. Failure was associated with the application over cortical
bone.175
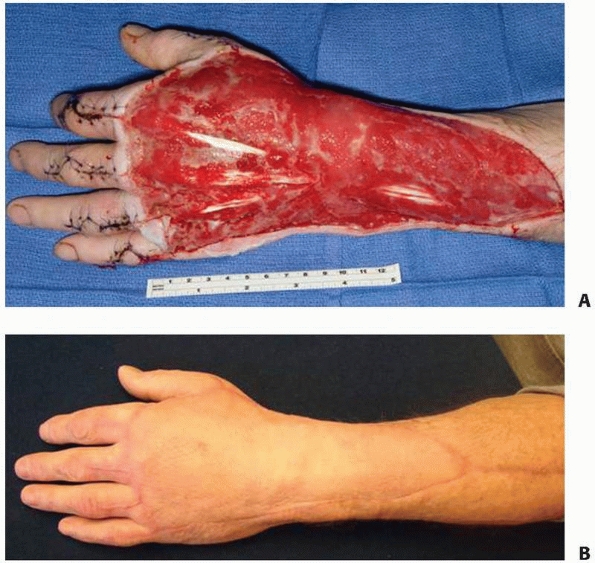 |
|
FIGURE 14-26 The application of Integra can allow for skin grafting to be performed over wounds previously requiring flap coverage. A.
This 32-year-old man sustained an extensive degloving injury to the dorsum of his hand. Integra was applied over the exposed tendons and then VAC therapy was instituted for 14 days. B. After the establishment of a neodermis, the Integra was covered with a split-thickness skin graft providing for stable and functional coverage of the hand. |
with the use of fibrin glue for fixation of the Integra and subsequent
VAC therapy. In a retrospective review, Jeschke and colleagues found
the use of fibrin glue and VAC therapy improved the “take rate” of spit
thickness skin grafts from 78% to 98% and shortened the time to skin
grafting. Overall hospital stay was also decreased.202
Despite this trend, the surgeon should exercise restraint in trying to
apply these technologies to exposed bone, tendon and large defects.
Pedicled flaps and free tissue transfer provide reliable solutions to
even the largest soft tissue defects, and should be considered the
standard of care until formal comparative outcome studies are available
to assess functional outcome and long-term consequences of these newer
reconstructive technologies.
overview of randomised trials of antiplatelet therapy—III: Reduction in
venous thrombosis and pulmonary embolism by antiplatelet prophylaxis
among surgical and medical patients. Antiplatelet Trialists’
Collaboration. BMJ 1994:308(6923): 235-246.
M, Rydholm A, Holmberg J, et al. Reconstruction with a free
vascularized fibular graft for malignant bone tumor. Acta Orthop Scand
1988;59(4):430-437.
R, Delcroix L, Innocenti M, et al. Reconstruction of large
posttraumatic skeletal defects of the forearm by vascularized free
fibular graft. Microsurgery 2004;24: 423-429.
R, Delcroix L, Innocenti M, et al. Free fibula flap for humerus
segmental reconstruction: report on 13 cases. Chirurgia Degli Organi di
Movimento 2008;91(1):21-26.
PA, Shaw WW. The evolution of the surgical management of severe lower
extremity trauma. Clin Plast Surg 1986;13(4):549-569.
JO. Comparison of soap and antibiotic solutions for irrigation of
lower-limb open fracture wounds. A prospective, randomized study. J
Bone Joint Surg Am 2005; 87(7):1415-1422.
JO, Gainor BJ, Simpson WA, et al. The use of detergent irrigation for
musculoskeletal wounds. Int Orthop 2003;27(1):40-46.
C, Grilli D, Dominikow D, et al. Posterior interosseous reverse forearm
flap: experience with 80 consecutive cases. Plast Reconstr Surg
1993;92:285-293.
C, Grilli D, Siebert J. Latissimus dorsi musculocutaneous flap without
muscle. Plast Reconstr Surg 1995;96(7):1608-1614.
M, Nagase Y, Mae O, Namba Y. Reconstruction of posttraumatic defects of
the foot by flow-through anterolateral or anteromedial thigh flaps with
preservation of posterior tibial vessels. Ann Plast Surg
1997;38(6):598-603.
M, Wood M, Cooney WR. Revascularized segmental diaphyseal bone
transfers in the canine. An analysis of viability. J Reconstr Microsurg
1984;1(1):11-19.
LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound
control and treatment: clinical experience. Ann Plast Surg 1997;38:563.
ZM, Kersnic M, Smith RW, et al. Free lateral arm osteocutaneous
neurosensory flap for thumb reconstruction. J Hand Surg (Br)
1991;16:395-399.
CE, Janis JE, Steinberg J, et al. Clinical approach to wounds:
débridement and wound bed preparation including the use of dressings
and wound-healing adjuvants. Plast Reconstr Surg 2006;117:72-109s.
AW, Hansen ST. Plates versus external fixation in severe open tibial
shaft fractures. A randomized trial. Clin Orthop Relat Res
1989;241:89-94.
AB, Ott R, Laub DR. Failure of healing of split skin donor sites in
anhidrotic ectodermal dysplasia. Plast Reconstr Surg 1979;64:97-100.
KA, Steinmann SP, Shin AY, et al. Gracilis free muscle transfer for
restoration of function after complete brachial plexus avulsion.
Neurosurg Focus 2004;15:16(5): E8.
E, Sullivan T, Berg E. Animal model for evaluating bone repair with and
without adjunctive hyperbaric oxygen therapy (HBO): comparing dose
schedules. J Invest Surg 1990;3(4):387-392.
S, Velander E, Frank HA, Lambert PB. Survival of arteries in
experimental full thickness skin autografts. Transplantation
1964;2:167-174.
MH, Stanford R, Turner R. Hyperbaric oxygen therapy for promoting
fracture healing and treating fracture non-union. Cochrane Database
Syst Rev 2005;(1): CD004712.
A, Weiland AJ, Dorfman A. The effect of prolonged ischemia time on
osteocyte and osteoblast survival in composite bone grafts
revascularized by microvascular anastomoses. Plast Reconstr Surg
1982;69(1):19-29.
A, Weiland AJ, Dorfman H. Free vascularized bone grafts: factors
affecting their survival and ability to heal to recipient bone defects.
Plastic Reconst Surg 1982; 69(1):19-29.
AT, Wood MB, Sheetz KK. Arthrodesis of the ankle with a free
vascularized autogenous bone graft. Reconstruction of segmental loss of
bone secondary to osteomyelitis, tumor, or trauma. J Bone Joint Surg Am
1995;77(12):1867-1875.
K, Danai T. The iliac bone or osteocutaneous transplant pedicled to the
deep circumflex iliac artery. I. Anatomical and technical
considerations. J Maxillofac Surg 1983;11(5):195-200.
M, Bastian L, Krettek C, et al. Surgical options for the treatment of
severe tibial pilon fractures: a study of three techniques. J Orthop
Trauma 2001;15:153-160.
PN, Ali SR. Planning of perforator flaps. In: Bolondeel PN, Morris SF,
Hallock GG, Neligan PC, eds. Perforator Flaps: Anatomy, Technique, and
Clinical Application. 1 ed. St. Louis: QMP, 2006, pp. 109-114.
MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of
reconstruction or amputation of leg threatening injuries. NEJM
2002;347:1924-1931.
MJ, McCarthy ML, Jones AJ, et al. The insensate foot following severe
lower extremity trauma: an indication for amputation. J Bone Joint Surg
(Am) 2005; 87(2601-2608).
WC, Trager S. Quantitative culture technique and infection in complex
wounds of the extremities closed with free flaps. Plast Reconstr Surg
1995;95(5): 860-865.
RJ, Cohen MS, Berzins A, Sumner DR. Bone graft harvesting from the
distal radius, olecranon, and iliac crest: a quantitative analysis. J
Hand Surg [Am] 2001;26(1): 135-141.
RA Jr, Neumeister MW. Outcomes after mutilating hand injuries: review
of the literature and recommendations for assessment. Hand Clin
2003;19(1):193-204.
DT, Cordeiro PG, Hu QY, et al. Free flap re-exploration: indications
treatment and outcomes in 1193 free flaps. Plast Reconstr Surg
2007;119:2092-2100.
HJ, Furnas DW, Gordon L, Achauer BM. Free osteocutaneous flap from a
rib to the tibia. Plast Reconstr Surg 1977;59(6):799-804.
T, Christensen GD, Anglen JO, et al. Sequential irrigation with common
detergents: a promising new method for decontaminating orthopedic
wounds. Am J Orthop 1999;28(3):156-160.
JF, Yannas IV, Quinby WC, et al. Successful use of a physiologically
acceptable artifical skin in the treatment of extensiveburn injury. Ann
Surg 1981;194:413-427.
JW, Jacobs CL, Swiontkowski MF, et al. Complex limb salvage of early
amputaiton for severe lower-limb injury: a meta-analysis of
observational studies. J Orthop Trauma 2006;21:70-76.
W, Chang N, Mathes SJ. Comparison of the effect of bacterial
inoculation in musculocutaneous and fasciocutaneous flaps. Plast
Reconstr Surg 1986;77(5): 785-794.
JW, Kohanzadeh S, Tynan M, Evans GR. Free flap reconstruction for
infection of ankle fracture hardware: case report and review of the
literature. Surg Infect (Larchmt) 2006;7:315-322.
WJ, Beggs DJ, DeFede JL, et al. A prospective randomised controlled
clinical trial comparing hydrosurgery débridement with conventional
surgical débridement in lower extremity ulcers. Int Wound J
2008;5:288-294.
N, Mathes SJ. Comparison of the effect of bacterial inoculation in
musculocutaneous and random-pattern flaps. Plast Reconstr Surg
1982;70(1):1-10.
HC, Tang YB, Mardini S, Tsai BW. Reconstruction of the hand and upper
limb with free flaps based on musculocutaneous perforators.
Microsurgery 2004;24(4): 270-280.
KT, Mardini S, Chuang DC, et al. Timing of presentation of the first
signs of vascular compromise dictates the salvage outcome of free flap
transfers. Plast Reconstr Surg 2007;120(1):187-195.
MC, Chang MC, Chen CM, Chen TH. Double-strut free vascularized fibular
grafting for reconstruction of the lower extremities. Injury
2003;34:763-769.
SH, Wei FC, Chen HC, et al. Emergency free-flap transfer for
reconstruction of acute complex extremity wounds. Plast Reconstr Surg
1992;89(5):882-888; discussion 9-90.
ZW, Chen LE, Zhang GJ, Yu HL. Treatment of tibial defect with
vascularized osteocutaneous pedicled transfer of fibula. J Reconstr
Microsurg 1986;2(3):199-203, 5.
W, Varvares MA, Hadlock T, et al. Effects of aspirin and low-dose
heparin in head and neck reconstruction using microvascular free flaps.
Laryngoscope 2005;115(6): 973-976.
EK, Ulusal B, Ulusal A, et al. Using the descending branch of the
lateral femoral circumflex vessel as a source of two independent flaps.
Plast Reconstr Surg 2006; 117(6):2059-2063.
U, Moran S, Karacor Z. Soft tissue coverage and outcome of Gustillo
Grade IIIB Midshaft tibia fractures: a 15-year experience. Plast
Reconstr Surg 2008;122:479-485.
UH, Moran SL, Li S, Khan S. Soft tissue coverage of the elbow: an
outcome analysis and reconstructive algorithm. Plast Reconstr Surg
2007;11:1852-1857.
KC, Cederna PS. Endoscopic harvest of temporoparietal fascial free
flaps for coverage of hand wounds. J Hand Surg 2002;27A(3):525-533.
G, Mader JT. The surgical Treatment of Adult Osteomyelitis. In: CMC E,
ed. London: Churchill Livingstone, 1983, pp. 15-35.
GP, Reisch JS. Prevention of venous thromboembolism in general surgical
patients: results of meta-analysis. Ann Surg 1998;208:227.
RJ, Mayo G, Price P, Fitzgerald GA. Suppression of thromboxane A2 but
not of systemic prostacyclin by controlled-release aspirin. NEJM
1991;325:1137.
JD, Ansel LJ, Schwartzberg RA. A sequential protocol for managment of
severe open tibial fractures. Clin Orthop 1995;315:84-103.
JM, Rapaport FT. The vascularization of skin autografts and homografts:
an experimental study in man. Ann Surg 1956;143:306-315.
JM, Smahel J, Ballantyne DL, Harper AD. Inosculation of vessels of skin
graft and host bed: a fortuitous encounter. Br J Plast Surg
1975:274-282.
WP 3rd, Fitzgerald RH Jr, Dobyns JH, Washington JA 2nd. Quantitative
wound cultures in upper extremity trauma. J Trauma 1982;22(2):112-117.
BVM. The effect of graft thickness, donor site and graft bed on graft
shrinkage in the hooded rat. Br J Plast Surg 1969;22:125-133.
CB, McCarthy JG. Comparison of residual osseous mass between
vascularized and nonvascularized onlay bone transfers. Plast Reconstr
Surg 1983;72(5):672-675.
AB, Best AK, Schemitsch EH, et al. Salvage after severe lower-extremity
trauma: are the outcomes worth the means? Plast Reconstr Surg
1999;103(4):1212-1220.
Chalain TMB. Exploring the use of the medicinal leech: a clinical
risk-benefit analysis. J Reconstr Microsurg 1996;12:165-172.
la Torre J, Hedden W, Grant JH 3rd, et al. Retrospective review of the
internal Doppler probe for intra- and postoperative microvascular
surveillance. J Reconstr Microsurg 2003;19(5):287-290.
AJ, Argenta LC, Marks MW, et al. The use of the vacuum-assisted closure
therapy for treatment of lower-extremity wound with exposed bone. Plast
Reconstr Surg 2001;108:1184.
Piñal F, García-Bernal FJ, Studer A, et al. Super-thinned iliac flap
for major defects on the elbow and wrist flexion creases. J Hand Surg
[Am] 2008;33(10):1899-1904.
AL. Management of peripheral nerve problems in the upper and lower
extremity using quantitative sensory testing. Hand Clin
1999;15(4):697-715, x.
GK, Kontos S, Katsenis D, Dalas A. Treatment of high-energy tibial
plateau fractures by the Ilizarov circular fixator. J Bone Joint Surg
Br 1996;78(5):710-717.
GK, Kontos S, Lyritsis E. Use of the Ilizarov technique for treatment
of nonunion of the tibia associated with infection. J Bone Joint Surg
Am 1995;77(6):835-846.
CA, Olivier WA, Baux G, et al. Microvascular free-tissue transfer for
traumatic defects of the upper extremity: a 25-year experience. J
Reconstr Microsurg 2003;19(7): 455-462.
MI, Peters CR, Sherer J. Use of semipermeable polyurethane membrane as
a dressing for split skin graft donor sites. Plast Reconstr Surg
1979;64:112-114.
DR, Wilson FC. Topical antibiotic irrigation in the prophylaxis of
operative wound infections in orthopedic surgery. Orthop Clin North Am
1991;22(3):419-426.
JJ, Cordeiro PG. The current role of preoperative arteriography in free
fibula flaps. Plast Reconstr Surg 1998;102:1083-1088.
JJ, Cordeiro PG, Hidalgo DA. Efficacy of conventional monitoring
techniques in free tissue transfer: an 11 year experience in 750
consecutive cases. Plast Reconstr Surg 1999;104(1):97.
K, Tominaga S, Shibata T. Bone grafts with microvascular anastomoses of
vascular pedicles: an experimental study in dogs. J Bone Joint Surg Am
1977;59(6):809-815.
R, Ponzer S, Tornkvist H, et al. The Holstein-Lewis humeral shaft
fracture: aspects of radial nerve injury, primary treatment, and
outcome. J Orthop Trauma 2008; 22(10):693-697.
R, Ponzer S, Tornkvist H, et al. Primary radial nerve palsy in patients
with acute humeral shaft fractures. J Orthop Trauma 2008;22(6):408-414.
SG, Rizzo M. Management of radial nerve injury associated with humeral
shaft fractures: an evidence-based approach. J Reconstr Microsurg
2008;24(8):569-573.
WF, Eady JL, Burchardt H. Autogenous cortical bone grafts in the
reconstruction of segmental skeletal defects. J Bone Joint Surg Am
1980;62(7):1039-1058.
S, Kim D, Jones NF. Microdissection thinning of a pedicled deep
inferior epigastric perforator flap for burn scar contracture of the
groin case report. J Reconstr Microsurg 2005;21(7):447-450; discussion
51-52.
JL, Pisarello J, Brighton CT, et al. Adjunctive hyperbaric oxygen in
the treatment of chronic refractory osteomyelitis. J Trauma
1987;27:763-768.
MJ. The function of below-knee amputee versus the patient with salvaged
grade III tibial fracture. Clin Orthop 1994;301:227-232.
R Jr, Bassett J, Glover JL, et al. Complications of coronary artery
surgery in diabetic patients. Am Surg 1992;57:551-557.
F, May JW, Smith RJ. Composite groin flap with iliac bone for primary
thumb reconstruction. Case report. J Bone Joint Surg Am 1976;58:130-132.
W, Lang E, Klinzl L. Vacuum-assisted wound closure after
dermatofasciotomy of the lower extremity [in German]. Unfallchirurg
1996;99:283.
RJ, Swiontkowski MF, Bach AW, et al. Radial nerve palsy caused by open
humeral shaft fractures. J Hand Surg [Am] 1993;18(1):121-124.
G, van Genechten F, Merle N, et al. A compound radial artery forearm
flap in hand surgery: an original modification of the Chinese forearm
flap. Br J Plast Surg 1984;37(2):139-148.
TJ. Improving re-employment rates after limb salvage of acute severe
tibial fractures by microvascular soft-tissue reconstruction. Plast
Reconstr Surg 1994;93: 1028-1034.
JB, Moran SL, Bishop AT, et al. Free vascularized fibular graft salvage
of complications of long-bone allograft after tumor reconstruction. J
Bone Joint Surg Am 2008;90(1):93-100.
JB, Moran SL, Bishop AT, et al. Vascularized fibula flap onlay for
salvage of pathologic fracture of the long bones. Plast Reconstr Surg
2008;121(6):2001-2009.
A, Suda H. Experimental study and clinical observations on hypertrophy
of vascularized bone grafts. Microsurgery 1994;15(10):726-732.
TM, Wei FC, Lin CH. Clinical experience of 1284 free anterolateral
thigh flaps. Handchir Mikrochir Plast Chir 2002;34(4):239-244.
S, McCormick F, Chou R, Wandel AG. War wounds: lessons learned from
Operation Iraqi Freedom. Plast Reconstr Surg 2008;122(1):146-153.
G, Sherman R, Levin LS. Decision making in reconstructive surgery of
the upper extremity. New York: Springer-Verlag; 1999.
R, Wood MB, Sim FH, Shives TC. Vascularized bone transfer for limb
salvage and reconstruction after resection of aggressive bone lesions.
J Reconstr Microsurg 1987;3(3):183-18.
RE, Geisweid A, Feller AM. The value of preoperative Doppler sonography
for planning free perforator flaps. Plast Reconstr Surg
2000;105:2381-2386.
A, Gerder M, Casale P, et al. Fourteen hundred fifty-seven years of
microsurgical experience. Plast Reconstr Surg 1997;100(2):355-363.
S, Majumder S, Batchelor AGB, et al. Fix and flap: the radical
orthopedic and plastic treatment of severe open fractures of the tibia.
J Bone Joint Surg (Br) 2000;82B: 959-966.
MF, Randolph MA, Schofield BH, et al. Immunologic and ultrastructural
changes during early rejection of vascularized bone allografts. Plast
Reconstr Surg 1991; 88(5):860-868.
B, Paulus DA, Caffee HH. Pulse oximetry for vascular monitoring in
upper extremity replacement surgery. J Hand Surg [Am] 1986;11A:687.
G, Delogu D, Esposito G, et al. Versajet hydrosurgery versus classic
escharectomy for Brun débridement: a prospective randomized trial. J
Burn Care Res 2007;28: 720-724.
SA, Jackson JM, Wall DM, et al. Management of segmental defects by the
Ilizarov intercalary bone transport method. Clin Orthop Relat Res
1992;(280):136-142.
BM, Masem M, May JW. Therapeutic value of intravenous heparin in
microvascular surgery: an experimental vascular thrombosis study. Plast
Reconstr Surg 1988; 82:463.
RN, Newman MT, Shariaty S, et al. Ciprofloxacin, lomefloxacin, or
levofloxacin as treatment for chronic osteomyelitis. Antimicrob Agents
Chemother 2000;44(1): 164-166.
TL, Beatty ME. Soft tissue coverage for lower-extremity trauma: current
practice and techniques. A review. J Orthop Trauma 1988;2(2):158-173.
RT, Gould RJ, Peclet M, et al. the mangled extremity syndrome (M.E.S.):
a severity grading system for multisystem injury of the extremity. J
Trauma 1985;25: 1147-1150.
F, Ding BF. Vascularized free fibula transfer in the treatment of bone
tumours. Report of three cases. Arch Orthop Trauma Surg
1981;98(3):209-215.
RB, Anderson JT. JSBS classics. Prevention of infection in the
treatment of 1025 open fractures of long bones. Retrospective and
prospective analyses. J Bone Joint Surg Am 2002;84-A(4):682.
RB, Anderson JT. Prevention of infection in the treatment of 1025 open
fractures of long bones: retrospective and prospective analyses. J Bone
Joint Surg Am 1976; 58(4):453-458.
RB, Mendoza RM. Results of treatment of 1400 open fractures. In:
Gustilo RB, editor. Management of Open Fractures and Their
Complications. Philadelphia: Saunders, 1982; pp. 202-208.
N, Krettek C, Schandelmaier P, et al. A new solid unreamed tibial nail
for shaft fractures with severe soft tissue injury. Injury
1993;24:49-54.
CK, Kirk WC, Pederson WC. Osmotic complications of low-molecular-weight
dextran therapy in free flap surgery. Microsurgery 1992;13:36.
CJ, Adams CA, Eachempati SR. Surgical Infection Society Guideline:
prophylactic antibiotic use in open fractures: an evidence-based
guideline. Surg Infect 2006;7: 379-405.
MW, Bamberger HB. Humeral shaft fractures and radial nerve palsy: to
explore or not to explore…that is the question. Am J Orthop
2008;37(8):415-419.
KD, Wechsler ME, Schwartzstein RM, et al. The adult respiratory
distress syndrome after dextran infusion as an antithrombotic agent in
free TRAM flap breast reconstruction. Plast Reconstr Surg 1999;103:1706.
MD, Potter BK, Evans KN, et al. Bioartifical dermal substitute: a
preliminary report on its use for the management of complex
combat-related soft tissue wounds. J Orthop Trauma 2007;21:394-399.
VR, Pearl RM. The irreplaceable free flap: Part I. Skeletal
reconstruction by microvascular free bone transfer. Ann Plast Surg
1983;10(1):36-42.
VR, Pearl RM. The irreplaceable free flap: part II. Skeletal
reconstruction by microvascular free bone transfer. Ann Plast Surg
1983;10(1):43-54.
D, Sanders RW, Scaduto JM, et al. Vacuum-assisted wound closure (VAC
therapy) for management of patients with high-energy soft tissue
injuries. J Orthop Trauma 2003;17:683-688.
F, Ninkovic M, Ninkovic M. Rational flap selection and timing for
coverage of complex upper extremity trauma. J Plast Reconstr Aesthet
Surg 2006;60:760-768.
DA, Jones CS. The role of emergent exploration in free tissue transfer:
a review of 150 consecutive cases. Plast Reconstr Surg 1990;86:492-498.
JP, Shin HW, Kim JJ, et al. The use of anterolateral thigh perforator
flaps in chronic osteomyelitis of the lower extremity. Plast Reconstr
Surg 2005;115(1): 142-147.
SER, van Adrichem LNA, Mulder HD, et al. Comparison of laser Doppler
flowmetry and thermometry in the postoperative monitoring of
replantations. J Hand Surg 1995;20:88-93.
H, Poole GV, Hansen, KJ, et al. Salvage of lower extremities following
combined orthopaedic and vascular trauma: a predictive salvage index.
Clin Am 1987;53: 205-208.
CH, Yang CC, Kuo YR, et al. Free anterolateral thigh adipofascial
perforator flap. Plast Reconstr Surg 2003;112(4):976-982.
KC, Zhang F, Lineaweaver WC, et al. Serratus anterior-rib composite
flap: anatomic studies and clinical application to hand reconstruction.
Ann Plast Surg 1999;42(2): 132-136.
H, Sailer R, Daniaux H, Pechlaner S. Revascularization of a partially
necrotic talus with a vascularized bone graft from the iliac crest.
Arch Orthop Trauma Surg 1989;108(1):27-29.
K, Tomita K, Hashimoto F, et al. Long-term follow-up of vascularized
bone grafts for the reconstruction of tibial nonunion: evaluation with
computed tomographic scanning. J Trauma 1992;32(6):693-697.
GA. The tension-stress effect on the genesis and growth of tissues.
Part I. The influence of stability of fixation and soft-tissue
preservation. Clin Orthop Relat Res 1989;Jan(238):249-281.
GA. The tension-stress effect on the genesis and growth of tissues:
Part II. The influence of the rate and frequency of distraction. Clin
Orthop Relat Res 1989;Feb(239): 263-285.
GA, Ledyaev VI. The replacement of long tubular bone defects by
lengthening distraction osteotomy of one of the fragments. 1969. Clin
Orthop Relat Res 1992; Jul(280):7-10.
PC, Randolph MA, Paskert JP, et al. Vascularized bone allografts: in
vitro assessment of cell-mediated and humoral responses. Plast Reconstr
Surg 1991;87(2): 315-325.
E, Peretti G, Bellocci Met al. Histology and ultrastructure of
arteries, veins, and peripheral nerves during limb lengthening. Clin
Orthop Relat Res 1994;Nov(308): 54-62.
MH, Moran SL. Why wounds fail to heal. In: Moran SL, Cooney WP,
editors. Soft Tissue Surgery. Baltimore: Lippincott Williams &
Wilkins, 2008; pp. 1-10.
MG, Rose C, Angele P, et al. Development of new reconstructive
techniques: Use of Integra in combination with fibrin glue and
negative-pressure therapy for reconstruction of acute and chronic
wounds. Plast Reconstr Surg 2004;113:525-530.
K, Daines M, Hower T, et al. Objective criteria accurately predict
amputation following lower extremity trauma. J Trauma 1990;30:568-573.
BM, Greenhalgh RM. The use of the ultrasound Doppler flowmeter in
reconstructive microvascular surgery. Br J Plast Surg 1985;36:245.
NF, Swartz WM, Mears DC, et al. The “double barrel” free vascularized
fibular bone graft. Plast Reconstr Surg 1988;81(3):378-385.
RN, Anderegg TR, Swenson JM. Quality control guidelines for testing
gram-negative control strains with polymyxin B and colistin (polymyxin
E) by standardized methods. J Clin Microbiol 2005;43(2):925-927.
JB, Bour CJ, May JW Jr. The reconstruction of defects in the femoral
shaft with vascularized transfers of fibular bone. J Bone Joint Surg Am
1987;69(3):365-374.
JB, Gerhard HJ, Guerrero J, et al. Treatment of segmental defects of
the radius with use of the vascularized osteoseptocutaneous fibular
autogenous graft. J Bone Joint Surg Am 1997;79(4):542-550.
YL, Nigriny J, Chang J. The timing of microsurgical reconstruction in
lower extremity trauma. Microsurgery 2008;28(8):632-634.
MR, Jones NF. The reverse radial forearm flap for soft tissue
reconstruction of the wrist and hand. Tech Hand Up Extrem Surg
2005;9(1):47-51.
M, Tamura H, Nagayoshi I, et al. Hyperbaric oxygen therapy in
orthopedic conditions. Undersea Hyperb Med 2004;31(1):155-162.
ME, Rapp RP, Smith KM. Antibiotic beads and osteomyelitis: here today,
what’s coming tomorrow? Orthopedics 2006;29(7):599-603.
M, Botte MJ, Hoyt DB, et al. Outcomes in open tibial fractures:
relationship in delay in treatment and infection. J Trauma 2003;55:951.
RK, Cooley BC, Kunselman AR, et al. A prospective study of
microvascular free-flap surgery and outcome. Plast Reconstr Surg
1998;102:711-721.
RK, Cooley BC, Kunselman AR, et al. A prospective study of
microvascular free-flap surgery and outcome. Plast Reconstr Surg
1998;102(3):711-721.
DH, Kam AC, Chandika P, et al. Surgical management and outcome in
patients with radial nerve lesions. J Neurosurg 2001;95(4):573-583.
PD, Blackwell KE. Latissimus-serratus-rib free flap for oromandibular
and maxillary reconstruction. Arch Otolaryngol Head Neck Surg
2007;133(8):791-795.
N, Saito M, Sumiya Y, Itoh N. Reconstruction of hand skin defects by
microdissected mini anterolateral thigh perforator flaps. J Plast
Reconstr Aesthet Surg 2008; 61(9):1073-1077.
N, Saitoh M, Okamura T, et al. Concept and anatomical basis of
microdissected tailoring method for free flap transfer. Plast Reconstr
Surg 2009;123(1):152-162.
N, Satoh K. Consideration of a thin flap as an entity and clinical
applications of the thin anterolateral thigh flap. Plast Reconstr Surg
1996;97(5):985-992.
MB, Hunter S, Heimbach DM, et al. The VersaJet water dissector: a new
tool for tangential excision. Burn Care Rehab 2005;26:483-487.
K. Gentamicin-PMMA-beads in treating bone and soft tissue infections
(Author’s trans.). Zentralbl Chir 1979;104(14):934-942.
DR, Silver IA, Hunt TK. Regulation of wound-healing angiogenesis:
effect of oxygen gradients and inspired oxygen concentration. Surgery
1981;90(2):262-270.
MS. Early limb salvage: open tibia fractures of Ambroise Pare
(1510-1590) and Percival Pott (1714-1789). World J Surg
1997;21(1):116-122.
I, Higaki H, Soeda S. Combined vascularized fibula and peroneal
compositeflap transfer for severe heat-press injury of the forearm.
Plast Reconstr Surg 1991; 88(2):338-341.
RA, Gilles C. Effects of sodium hypochlorite (Dakin’s solution) on
cells of the wound module. Arch Surg 1988;123(4):420-423.
T, Bickert B, Germann G, et al. Outcome assessment after reconstruction
of complex defects of the forearm and hand with osteocutaneous free
flaps. Plast Reconstr Surg 2006;118(2):443-454; discussion 55-56.
S, Schusterman MA, Reece GP, et al. Timing of pedicle thrombosis and
flap loss after free-tissue transfer. Plast Reconstr Surg
1996;98:1230-1233.
SS, Schusterman MA, Reece GP, et al. Timing of pedicle thrombosis and
flap loss after free-tissue transfer. Plast Reconstr Surg
1996;98(7):1230-1233.
JO, Robson MC, Heggers JP, et al. Comparison of silver sulfadiazine,
povidone-iodine, and physiological saline in the treatment of chronic
pressure ulcers. J Am Geriatr Soc 1981;29:232.
YR, Jeng SF, Kuo MH, et al. Free anterolateral thigh flap for extremity
reconstruction: clinical experience and functional assessment of donor
site. Plast Reconstr Surg 2001;107(7):1766-1771.
YR, Seng-Feng J, Kuo FM, et al. Versatility of the free anterolateral
thigh flap for reconstruction of soft-tissue defects: review of 140
cases. Ann Plast Surg 2002;48(2): 161-166.
LB, Barfred T. Radial nerve palsy after simple fracture of the humerus.
Scand J Plast Reconstr Surg Hand Surg 2000;34(4):363-366.
SC, Moran SL. The pedicled soleus muscle flap for coverage of the
middle an distal third of the tibia. In: Moran SL, Cooney WPI, eds.
Soft Tissue Surgery. Philadelphia: Lippincott Williams & Wilkins,
2008, pp. 345-360.
PC. Femoral head reconstruction and revascularization. Treatment for
ischemic necrosis. Clin Orthop Relat Res 1996;Feb(323):139-145.
PC, Hung LK. Bone reconstruction after giant-cell tumor resection at
the proximal end of the humerus with vascularized iliac crest graft. A
report of three cases. Clin Orthop Relat Res 1989;Oct(247):101-105.
LS, Erdmann D. Primary and secondary microvascular reconstruction of
the upper extremity. Hand Clin 2001;17(3):447-455, ix.
LS, Goldner RD, Urbaniak JR, et al. Management of severe
musculoskeletal injuries of the upper extremity. J Orthop Trauma
1990;4:432-440.
SL, Baumeister S. Lower extremity. In: Wei FC, Mardini S, eds. Flaps
and Reconstructive Surgery. London: Elsevier, 1009, pp. 63-70.
CH, Mardini S, et al. Sixty-five clinical cases of free tissue transfer
using long arteriovenous fistulas or vein grafts. J Trauma
2004;56:1107-1117.
CH, Mardini S, Levin SL, et al. Endoscopically assisted sural nerve
harvest for upper extremity posttraumatic nerve defects an evaluation
of functional outcomes. Plast Reconstr Surg 2007;119(2):616-626.
CH, Wei FC, Levin LS, Chen MC. Donor-site morbidity comparison between
endoscopically assisted and traditional harvest of free latissimus
dorsi muscle flap. Plast Reconstr Surg 1999;104:1070-1078.
CH, Wei FC, Levin LS, et al. Free composite serratus anterior and rib
flaps for tibial composite bone and soft-tissue defect. Plast Reconstr
Surg 1997;99(6):1656-1665.
CH, Wei FC, Lin YT, Su CI. Endoscopically assisted fascia-saving
harvest of rectus abdominis. Plast Reconstr Surg 2001;108(3):713-718.
B, Belangero WD, Castro de Medeiros R. Fractures of the distal third of
the humerus with palsy of the radial nerve: management using
minimally-invasive percutaneous plate osteosynthesis. J Bone Joint Surg
Br 2006;88(12):1625-1628.
BS, Wei FC, Ng SH, et al. Routine donor leg angiography before
vascularized free fibula transplantation is not necessary: a
prospective study of 120 clinical cases. Plast Reconstr Surg
1999;103:121-127.
EJ, Bosse MJ. Factors influencing outcome following limb threatening
lower limb trauma: lessons learned from the Lower Extremity Assessment
Project (LEAP). J Am Acad Orthop Surg 2006;14:S205-210.
SE, Novak CB. Nerve transfers. New options for reconstruction following
nerve injury. Hand Clin 1999;15(4):643-666, ix.
J, Calhoun JH. Adult Long Bone Osteomyelitis. In: Mader JHC, ed.
Musculoskeletal Infections. New York: Marcel Dekker, 2003, pp. 149-182.
JT, Brown GL, Wells CH, et al. A mechanism for the amelioration by
hyperbaric oxygen of experimental staphylococcal osteomyelitis in
rabbits. J Infect Dis 1980; 142(6):915-922.
JT, Calhoun JH, Cobos J. In vitro evaluation of antibiotic diffusion
from antibiotic-impregnated biodegradable beads and
polymethylmethacrylate beads. Antimicrob Agents Chemother
1997;41(2):415-418.
JT, Ortiz M, Calhoun JH. Update on the diagnosis and management of
osteomyelitis. Clin Podiatr Med Surg 1996;13(4):701-724.
KN, Quarles LD, Seaber AV, et al. An experimental canine model of
osteonecrosis: characterization of the repair process. J Orthop Res
1993;11(3):350-357.
KN, Zalavras CG, Soucacos PN, et al. Free vascularized fibular grafts
for reconstruction of skeletal defects. J Am Acad Orthop Surg
2004;12(5):360-369.
DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and
cardiovascular morbidity after noncardiac surgery. NEJM
1996;335(23):1713-1720.
S, Lin LC, Moran SL, et al. Anterolateral thigh flap. In: Wei FC ed.
Flaps and Reconstructive Surgery. London: Elsevier, 2009, pp. 93-101.
BA, Schultz GS. Interaction of cytokines, proteases and growth factors
in acute and chronic wounds. Wound Rep Reg 1996;4:411-420.
SJ, Nahai F. Classification of the vascular anatomy of muscles:
experimental and clinical correlation. Plast Reconstr Surg
1981;67:177-187.
C. Comment on “Scaphoid nonunion: treatment with a pedicled
vascularized bone graft based on the 1,2 intercompartmental
supraretinacular branch of the radial artery”. J Hand Surg [Br]
2003;28(3):281-282; author reply 2.
KU, Bishop AT, Berger RA, editors. Vascularized bone grafting for
Kienbock’s disease: method and results of retrograde-flow metaphyseal
grafts and comparison with cortical graft sites (SS-03). 51st Annual
Meeting of the American Society for Surgery of the Hand; 1996;
Nashville, TN.
N, Barbieri CH, Cortez M. The posterior interosseous forearm island
flap for skin defects in the hand and elbow. A prospective study of 51
cases. J Hand Surg 1996;21B:237-243.
PL, Nanos G. Initial evaluation and management of complex traumatic
wounds. In: Moran SL, Cooney WP, editors. Soft Tissue Surgery.
Philadelphia: Lippincott Williams & Wilkins, 2009, pp. 11-37.
MG, Heckman JD, Corley FG. Severe open fracutres of the lower
extremity: a retrospective evaluation of the Mangled Extremity Severity
Score (MESS). J Orthop Trauma 1994;8:81-87.
JP, Olive D. [Conservative treatment of malignant bone tumors by
vascularized bone grafts]. Presse Med 1983;12(15):960-961.
A, Kaneda K, Itoga H. Treatment of infected segmental defect of long
bone with vascularized bone transfer. J Reconstr Microsurg
1992;8(2):75-82.
T, Jackson IT, Elmazar H, et al. The effect of low-molecular-weight
heparin in the survival of a rabbit congested skin flap. Plast Reconstr
Surg 2002;109(6): 1994-1999.
HD, Gravel C, Chapman MW, et al. Comparison of antibiotic beads and
intravenous antibiotics in open fractures. Clin Orthop 2000;372:254-261.
NS, Staiano JJ, Ojeh NO, et al. Reconstructive surgery with a dermal
regeneration template: clinical and histologic study. Plast Reconstr
Surg 2001;108:93-103.
JA, Defranzo AJ, Hadaegh A, et al. Acceleration of Integra
incorporation in complex tissue defects with subatmospheric pressure.
Plast Reconstr Surg 2004;113: 1339-1346.
JB, Mazur JM, Zehr D, et al. A biomechanical comparison of vascularized
and conventional autogenous bone grafts. Plast Reconstr Surg
1984;73(3):382-386.
JR, Weiland AJ, Daniel RK. Use of free vascularized bone grafts in the
treatment of bone tumors. Clin Orthop Relat Res 1983;May(175):37-44.
SL, Bakri K, Mardini S, et al. The use of vascularized fibular grafts
for the reconstruction of spinal and sacral defects. Microsurgery
2009;29:393-400.
SL, Johnson CH. Skin and soft tissue: pedicled flaps. In: Berger RA,
Weiss AP, eds. Hand Surgery. Philadelphia: Lippincott Williams &
Wilkins, 2004, pp. 1131-1160.
SL, Shin AY, Bishop AT. the use of massive bone allograft with
intramedullary free fibular flap for limb salvage in a pediatric and
adolescent population. Plast Reconstr Surg 2006;118:413-419.
SL, Steinmann SP, Shin AY. Adult brachial plexus injuries: mechanism,
patterns of injury, and physical diagnosis. Hand Clin 2005;21(1):13-24.
MJ, Argenta, LC, Shelton-Brown EL, et al. Vacuum-assisted closure: a
new method for wound control and treatment: animal studies and basic
foundation. Ann Plast 1997;38(6):553-562.
D, Alford EL, Wigoda P, Cohen V. Free composite myo-osseous flap with
serratus anterior and rib: indications in head and neck reconstruction.
Head Neck 1998;20(2):106-112.
D, van de Belt H, van Horn JR, et al. Residual gentamicin-release from
antibiotic-loaded polymethylmethacrylate beads after 5 years of
implantation. Biomaterials 2003; 24(10):1829-1831.
DP, Sykes PJ. The versatility of the free fibula flap in the management
of traumatic long bone defects. Injury 1991;22(4):275-281.
M, Deetjen H, Ohler K, Anderl H. Emergency free tissue transfer for
severe upper extremity injuries. J Hand Surg [Br] 1995;20(1):53-58.
FR, Dell PC, McAndrew MP, et al. Vascularized autografts for
reconstruction of skeletal defects following lower extremity trauma. A
review. Clin Orthop Relat Res 1989;Jun(243):65-70.
T, Tsukada S, Obara K, et al. Free vascularized fibular graft for
replacement of the radius after excision of giant cell tumor: case
report. J Microsurg 1981;3(1):48-53.
DM, Eilert RE, Waldstein G. Congenital pseudarthrosis of the ulna: a
report of two cases and a review of the literature. J Pediatr Orthop
1985;5(4):463-467.
O, Coskunfirat OK, Ozgentas HE. The use of free anterolateral thigh
flap for reconstructing soft tissue defects of the lower extremities.
Ann Plast Surg 2004;53(5): 455-461.
JE, Rodriguez ED, Bludbond-Langer R, et al. The anterolateral thigh
flap is highly effective for reconstruction of complex lower extremity
trauma. J Trauma 2007;62(1): 162-165.
BM, Maros E, Pribaz JJ, et al. Lower extremity trauma: trends in the
management of soft-tissue reconstruction of open tibia-fibula
fractures. Plast Reconstr Surg 2006; 117:1315-1322.
JP, Yaremchuk MJ, Randolph MA, et al. Prolonging survival in
vascularized bone allograft transplantation: developing specific immune
unresponsiveness. J Reconstr Microsurg 1987;3(3):253-263.
JP, Yaremchuk MJ, Randolph MA, et al. The role of cyclosporin in
prolonging survival in vascularized bone allografts. Plast Reconstr
Surg 1987;80(2):240-247.
M, Wilkins J, Moore TM. Considerations in reducing the infection rate
in open tibial fractures. Clin Orthop 1983;178:36-41.
CN, Davies HT, Cole RP, et al. Combined latissimus dorsi-serratus
anterior/rib composite free flap in mandibular reconstruction. Int J
Oral Maxillofac Surg 1992; 21(2):92-96.
MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and
tobramycin combined in acrylic bone-cement. J Arthroplasty
1996;11(8):939-944.
CV, Masquelet AC, Romana MC, et al. Periosteal flaps: anatomical bases
of sites of elevation. Surg Radiol Anat 1990;12(1):3-7.
RW, Levack B, Satku K, Patradul A. Free vascularised fibular graft in
the treatment of congenital pseudarthrosis of the tibia. J Bone Joint
Surg Br 1985;67(1):64-70.
RW, Vajara R, Satku K. Free vascularized bone transplants in
problematic nonunions of fractures. J Trauma 1983;23(4):341-349.
J, Truppa K, Bilos ZJ, et al. Replantation and revascularization of the
digits in a community microsurgical practice. J Reconstr Microsurg
1997;13:163.
LLQ. Medial hemisoleus muscle flap: a reliable flap for soft tissue
reconstruction of the middle third tibial wound. Wound Int
2006;91:194-200.
CM, Dennis RHI, Massac EA. Evaluation of intraoperative anticoagulants
in microvascular free-flap surgery. J Natl Med Assoc 1996;88:655.
C, Schwabegger AH, Gardetto A, et al. Aesthetic refinements in
reconstructive microsurgery of the lower leg. J Reconstr Microsurg
2004;20(2):123-131.
M, Birch R, Eastwood DM. Clinical outcome of nerve injuries associated
with supracondylar fractures of the humerus in children: the experience
of a specialist referral centre. J Bone Joint Surg Br 2006;88(1):90-94.
MR, Bishop AT, Wood MB. Arthrodesis of the knee with a vascularized
fibular rotatory graft. J Bone Joint Surg Am 1995;77(5):751-759.
RJ, Robertson BC, Chang B. Limb salvage in the lower extremity using
free gracilis muscle reconstruction. Plast Reconstr Surg
2000;106:1507-1513.
JF, Winters R, Puckett Cl. The use of the osteocutaneous groin flap in
gunshot wounds of the hand. J Hand Surg [Am] 1984;9:12-17.
E, Zurakowski D, Vrahas M. Acute infections after fracture repair:
management with hardware in place. Clin Orthop Relat Res
2008;466:466-472.
K, Solonen KA, Lindholm TS. Results of treatment of aseptic necrosis of
the femoral head with vascularized bone graft. Ital J Orthop Traumatol
1989;15(2): 145-153.
D, Chin K, Jupiter JB. Radial nerve palsy associated with high-energy
humeral shaft fractures. J Hand Surg [Am] 2004;29(1):144-147.
RA, Quellette EA, Mendietta CG, et al. Free temporoparietal fascial
flap for coverage of a large palmar forearm wound after hand
replantation. J Reconstr Microsurg 2001;17(6):421-423.
MC, Masquelet AC. Vascularized periosteum associated with cancellous
bone graft: an experimental study. Plast Reconstr Surg
1990;85(4):587-592.
BD, Wilson FC, Funderburk CH. The use of bacitracin irrigation to
prevent infection in postoperative skeletal wounds. An experimental
study. J Bone Joint Surg Am 1989 Mar;71(3):427-430.
DM, Chu B, Bern S, May JW. The effect of dextran on microvascular
thrombosis in an experimental rabbit model. Plast Reconstr Surg
1993;92:511.
M, Wulker N, Wirth CJ. Reconstruction of the lateral ligaments of the
ankle using a regional periosteal flap. J Bone Joint Surg Br
1997;79(3):446-451.
D, Kim HM, Chung KC. A systemic review of outcomes and complications of
reconstruction and amputation for type IIIB and IIIC fractures of the
tibia. Plast Reconstr Surg 2008;122:1796-1805.
M, Daigle JP. Early free tissue transfer for extremity reconstruction
following high-voltage electrical burn injuries. J Reconstr Microsurg
2008;24:259-266.
L, Knudsen F, Dougan P. The effect of dextran 40 on patency following
severe trauma in small arteries and veins. Br J Plast Surg 1995;48:121.
L, Wiesland JB, Dougan P, et al. Effects of low and ultralow oral doses
of acetylsalicylic acid in microvascular surgery. An experimental study
in rabbits. Scand J Plast Reconstr Surg Hand Surg 1991;25:203.
L, Wieslander JB, Dougan P, et al. Studies of the antithrombotic
effects of dextran 40 following microarterial trauma. Br J Plast Surg
1991;44:15.
CJ, Orlando GS, Herceg S, et al. Pfannenstiel incision as an
alternative approach for harvesting the rectus abdominis muscle for
free-tissue transfer. Plast Reconstr Surg 2000;105(4):1330-1333.
CJ, Orlando GS, Serletti JM. Clinical applications of the posterior
rectus sheathperitoneal free flap. Plast Reconstr Surg
2000;106(2):321-326.
CJ, Smith A, Kim S, et al. Effects of late loss of arterial inflow on
free flap survival. J Reconstr Microsurg 2002;18(7):579-584.
AH, Anzel SH, Salyer WA. Transfer of vascularized grafts of iliac bone
to the extremities. J Bone Joint Surg Am 1987;69(9):1319-1327.
R, Mayou BJ. A new vascularized bone graft transferred by microvascular
anastomosis as a free flap. Br J Surg. 1979;66(11):787-788.
M, Ofer N, Germann G, et al. Microvascular reconstruction in burn and
electrical burn injuries of the severely traumatized upper extremity.
Plast Reconstr Surg 2007;119:605-615.
C, Greenhalgh DG, Warden GD. A comparison of full-thickness versus
split-thickness autografts for the coverage of deep palm burns in the
very young pediatric patient. J Burn Care Rehabil 1993;14:20-33.
JM, Moran SL, Orlando GS, et al. Urokinase protocol for free-flap
salvage following prolonged venous thrombosis. Plast Reconstr Surg
1998;102:1947-1953.
DM, Sherman CE, Moran SL. Hydrosurgical tangential excision of partial
thickness hand burns. Plast Reconstr Surg 2008;122:96e-97e.
JW, Field GA, Goldberg VM, et al. Fate of vascularized and
nonvascularized autografts. Clin Orthop Relat Res
1985;Jul-Aug(197):32-43.
JW, Field GA, Wilber RG, Goldberg VM. Experimental vascularized bone
grafts: histopathologic correlations with postoperative bone scan: the
risk of false-positive results. J Orthop Res 1987;5(3):311-319.
YC, Harwood P, Grotz MR, et al. Radial nerve palsy associated with
fractures of the shaft of the humerus: a systematic review. J Bone
Joint Surg Br 2005;87(12): 1647-1652.
KK, Bishop AT, Berger RA. The arterial blood supply of the distal
radius and its potential use in vascularized pedicled bone grafts. J
Hand Surg 1995;20A:902-914.
M, Doi K, Kuwata N, et al. Experimental study on vascularized bone
allografts for reconstruction of massive bone defects. Microsurgery
1994;15(9):663-670.
ME, Calhoun JH, Mader JT. Comparative evaluation of oral levofloxacin
and parenteral nafcillin in the treatment of experimental
methicillin-susceptible Staphylococcus aureus osteomyelitis in rabbits. J Antimicrob Chemother 2001;48(2):253-258.
DG, Dheerendra SK, Bari A, et al. Management of clinical radial nerve
palsy with closed fracture shaft of humerus—a postal questionnaire
survey. Surgeon 2008; 6(2):76-78.
M, Sanders R, DiPasquale T, et al. A staged protocol for soft tissue
management in the treatment of complex pilon fractures. J Orthop Trauma
2004;18:s32-38.
GA, Yaremchuk MJ, Manson PN. Doppler ultrasound surface monitoring of
both arterial and venous flow in clinical free tissue transfers. J
Reconstr Microsurg 1986;3:39.
KA, Rindell K, Paavilainen T. Vascularized pedicled bone graft into the
femoral head—treatment of aseptic necrosis of the femoral head. Arch
Orthop Trauma Surg 1990;109(3):160-163.
AE, Gohritz A. Delayed flap coverage of open extremity fractures after
previous vacuum-assisted closure (VAC) therapy—worse or worth? J Plast
Reconstr Aesthet Surg 2008;62(5):675-683.
R, McPherson M, Longaker MT. Histologic study of artificial skin used
in the treatment of full-thickness thermal injury. J Burn Care Rehabil
1990;11:7-13.
W, Hierner R, Dielert E, et al. The iliac crest region: donor site for
vascularized bone periosteal and soft tissue flaps. Ann Plast Surg
1991;26(1):105-109.
AN, Sanger JR, Matloub HS. Lateral arm fascial flap microarterial
anatomy and potential J Reconstr Microsurg 2000;16(4):279-286.
T, Bishop AT, Muramatsu K. Role of conventional and vascularized bone
grafts in scaphoid nonunion with avascular necrosis: a canine
experimental study [in proc cit]. J Hand Surg 2000;25A(5):849-859
[MEDLINE record in process].
T, Harii K, Nakatsuka T, et al. Vascularized periosteal grafts: an
experimental study using two different forms of tibial periosteum in
rabbits. Plast Reconstr Surg 1986;78(4):489-497.
S, Ohtsuka M, Tsukie T. Use of the latissimus dorsi and the serratus
anterior muscles as a combined flap. Ann Plast Surg 1988;20(4):333-339.
CH. Reconstruction of the bones and joints of the upper extremity by
vascularized free fibular graft: report of 46 cases. J Reconstr
Microsurg 1992;8:285-292.
NC, Cigna E, Varkey P, et al. Debulking of free myocutaneous flaps for
head and neck reconstruction using an arthroscopic shaver. Int J Oral
Maxillofac Surg 2007; 36(5):450-452.
BB, Conroy BP, Malicky ES, et al. Benzalkonium chloride. A potential
disinfecting irrigation solution for orthopaedic wounds. Clin Orthop
Relat Res 1998;Jan(346): 255-261.
FC. A new method perforator-based tissue expansion for a preexpanded
free cutaneous perforator flap. Burns 2003;29(8):845-848.
TM, Ludwig L, Tonkin M. Vascularized fibular epiphyseal transfer. A
clinical study. Clin Orthop Relat Res 1986;Sep(210):228-234.
H. The management of open fractures. In: Tscherne H, Gotzen L, eds.
Fractures with Soft Tissue Injuries. New York: Springer Verlag, 1984,
pp. 10-32.
H, Oestern HJ. Die Klassifizierung des Weichteilschadens bei offenen
und geschlossenen Frakturen. Unfallheilkunde 1982;85:111-115.
TC, Wang KC, Fang CM, et al. Reverse pedicle lateral arm flap for
reconstruction of posterior soft-tissue defects of the elbow. Ann Plast
Surg 1997;38:635-641.
Y, Sugioka Y. Effects of vascularized bone graft on surrounding
necrotic bone: an experimental study. J Reconstr Microsurg
1990;6(2):101-107; discussion 9, 11.
SW, Wei FC, Shih CH. Management of femoral diaphyseal infected nonunion
with antibiotic beads, local therapy, external skeletal fixation, and
staged bone grafting. J Trauma 1999;46:97-103.
JR, Coogan PG, Gunneson EB, et al. Treatment of osteonecrosis of the
femoral head with free vascularized fibular grafting. A long-term
follow-up study of one hundred and three hips. J Bone Joint Surg Am
1995;77(5):681-694.
ML, Bridger AG, Zur KB, Genden EM. The scapular osteofasciocutaneous
flap: a 12-year experience. Arch Otolaryngol Head Neck Surg
2001;127(7):862-869.
F, Iketani M, Hirukawa M, et al. Treatment of congenital
pseudoarthrosis of the tibia by a free vascularized fibular graft: case
report. J Microsurg 1981;3(1):40-47.
AC, Lu F, Mizuno H, et al. Defining vascular supply and territory of
thinned perforator flaps: Part I. Anterolateral thigh perforator flap.
Plast Reconstr Surg 2006; 118(1):288-289.
Berg JS, Rudolph R. Immunohistochemistry of fibronectin and actin in
ungrafted wound and wounds covered with full-thickness and
split-thickness skin grafts. Plast Reconstr Surg 1993;91:684-692.
P, Abid A, Darodes P, et al. Integra artificial skin in the management
of severe tissue defects, including bone exposure, in injured children.
J Pediatr Orthop 2005; 14:381-384.
FA, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features,
therapeutic considerations, and unusual aspects. 1970;282(6):316-322.
C, Schwaitzberg S, Berliner E, et al. Hyperbaric oxygen for treating
wounds: a systematic review of the literature. Arch Surg
2003;138:272-279.
HT, Erdmann D, Fletcher JW, et al. Anterolateral thigh flap technique
in hand and upper extremity reconstruction. Tech Hand Up Extrem Surg
2004;8(4):257-261.
FC, El-Gammal TA, Lin CH, Ueng WN. Free fibular osteoseptocutaneous
graft for reconstruction of segmental femoral shaft defects. J Trauma
1997;43:784-792.
FC, Jain V, Celik N, et al. Have we found an ideal soft-tissue flap? An
experience with 672 anterolateral thigh flaps. Plast Reconstr Surg
2002;109(7):2219-2226; discussion 27-30.
AJ, Moore JR, Daniel RK. Vascularized bone autografts. Experience with
41 cases. Clin Orthop Relat Res 1983;Apr(174):87-95.
BB, Pett SB, Alonso D, et al. Differential inhibition by aspirin of
vascular and platelet prostaglandin synthesis in atherosclerotic
patients. NEJM 1983;308(14): 800-805.
MR, O’Hare PM, Sanders R, et al. The medicinal leech and its use in
plastic surgery: a possible cause of infection. Br J Plast Surg
1983;36:240-244.
JM, Guo L. Upper extremity. In: Wei FC, Mardini S, eds. Flaps and
Reconstructive Surgery. London: Elsevier, 2009, pp. 51-61.
MB. Free vascularized bone transfers for nonunions, segmental gaps, and
following tumor resection. Orthopedics 1986;9(6):810-816.
MB. Upper extremity reconstruction by vascularized bone transfers:
results and complications. J Hand Surg [Am] 1987;12(3):422-427.
MB, Cooney WP 3rd, Irons GB Jr. Skeletal reconstruction by vascularized
bone transfer: indications and results. Mayo Clin Proc
1985;60(11):729-734.
WA, Shestak KC, Newton ED, et al. Liposuction-assisted revision and
recontouring of free microvascular tissue transfers. Aesthetic Plast
Surg 1993;17(2): 103-107.
WC, Chang YP, So YC, et al. The combined use of flaps based on the
subscapular vascular system for limb reconstruction. Br J Plast Surg
1997;50(2):73-80.
H, Inada Y, Shono M, et al. Radical forearm flap with vascularized
tendons for hand reconstruction. Plast Reconstr Surg 1996;98(2):328-333.
H, Tamai S, Ono H, et al. Free vascularized fibular grafts in surgery
of the upper limb. J Reconstr Microsurg 1999;15:515-521.
WG, Chiang YC, Wei FC, et al. Thin anterolateral thigh perforator flap
using a modified perforator microdissection technique and its clinical
application for foot resurfacing. Plast Reconstr Surg
2006;117(3):1004-1008.
IV, Burke JF, Gordon PL, et al. Design of an artificial skin. II.
Control of chemical composition. J Biomed Mater Res 1980;14:107-131.
MJ. Acute management of severe soft-tissue damage accompanying open
fractures of the lower extremity. Clin Plast Surg 1986;13:621-629.
MJ, Nettelblad H, Randolph MA, et al. Vascularized bone allograft
transplantation in a genetically defined rat model. Plast Reconstr Surg
1985;75(3):355-362.
S, Lin CH, Lin YT, et al. Outcome comparison between free muscle and
free fasciocutaneous flaps for reconstruction of distal third and ankle
traumatic open tibial fractures. Plast Reconstr Surg
2006;117(7):2468-2475; discussion 76-77.
S, Lin CH, Wei FC. One-stage reconstruction of composite bone and
soft-tissue defects in traumatic lower extremities. Plast Reconstr Surg
2004;114(6):1457-1466.
S, Giderolu K, Aköz T. Anterolateral thigh flap: ideal free flap choice
for lower extremity soft-tissue reconstruction. J Reconstr Microsurg
2003;19(4):225-233.
S, Gidero¢lu K, Aköz T. Anterolateral thigh flap ideal free flap choice
for lower extremity soft-tissue reconstruction. J Reconstr Microsurg
2003;19(4):225-233.
M, Shimamura K, Iwai Y, et al. Free vascularized fibular transplant. A
new method for monitoring circulation of the grafted fibula. J Bone
Joint Surg Am 1983; 65(9):1295-1301.
Y, Kondoh T. Treatment of chylous fistula with fibrin glue and
clavicular periosteal flap. Br J Oral Maxillofac Surg
2002;40(2):138-139.
K. [Experimental study of vascularized fibular grafting including the
epiphyseal growth plate—autogenous orthotopic grafting]. Nippon
Seikeigeka Gakkai Zasshi 1984;58(8):813-828.
AX, Chen ZG, Yu GR. [Repair of humeral fracture and non-union with
transfer of vascularized periosteal flap]. Zhongguo Xiu Fu Chong Jian
Wai Ke Za Zhi 2001;15(5): 310-311.
BM, Wieslander JB. Dextrin’s antithrombotic properties in small
arteries are not altered by low-molecular-weight heparin or the
fibrinolytic inhibitor tranexamic acid: an experimental study.
Microsurgery 1993;14:289.
F, Liu J, Zhong G. [Applied anatomy of osteo-periosteal flap pedicled
with superior malleolar branch of anterior tibial artery]. Zhongguo Xiu
Fu Chong Jian Wai Ke Za Zhi 1997;11(5):312-314.
EM, Wood MB, Brown ML. Vascularized bone transfer: evaluation of
viability by postoperative bone scan. J Reconstr Microsurg
1985;2(1):13-19.
