Metastatic Tumors
challenging problem for spinal surgeons. Advances in imaging and spinal
instrumentation have enhanced the surgeon’s ability to perform complex
decompression and stabilization procedures. The lack of a validated set
of criteria to predict bone instability and neurologic compromise has
made it difficult, however, for the surgeon to decide whether
aggressive surgical intervention or nonoperative care is best for the
individual patient. This chapter presents current information on the
evaluation and treatment of metastatic disease of the spine.
metastases. In North America, approximately 18,000 new patients
annually present with spinal cord compression from metastatic diseases.
After a review of nearly 2800 cases from various clinical series,
Mclain and Weinstein reported that nearly 60% of all metastases to the
spine were secondary to breast, lung, or prostate carcinomas; myeloma;
or lymphoma (Table 12-1). The high frequency of
metastatic involvement of the spine is not completely understood.
Batson’s paravertebral venous plexus may play an important role. This
plexus of thin-walled, valveless vessels most likely accounts for the
predilection of metastatic lesions for specific areas of the spine.
Metastases from breast and lung carcinomas more commonly occur in the
thoracic spine, and prostate carcinoma metastases are more common in
the lumbar spine.
patients with metastatic disease of the spine. The pain is gradual in
onset, but progressive. It often is unrelenting, nonmechanical pain
that worsens at night. Early in the disease course, the pain is usually
axial. As the disease progresses, neural compression can lead to
complaints of radicular pain.
process, and they frequently prompt patients to seek medical attention.
Motor weakness has been reported as the presenting symptom in 76% of
patients, including 17% who were paralyzed; 50% of the patients noted
numbness or paresthesias. Radicular pain often accurately localized the
tumor to within one or two vertebral levels. Isolated bowel and bladder
dysfunction are uncommon presenting complaints but, when present, must
be assessed with magnetic resonance imaging (MRI) or computed
tomography (CT)myelography to determine if neurologic compression is
the cause. Patients who present with progressive neurologic signs or
symptoms must undergo urgent evaluation. Severe deficits or rapidly
progressive deficits portend a much poorer prognosis.
weight loss, anorexia, fatigue, hemoptysis, hematuria, hematochezia,
hematemesis, and tobacco use. The presence of a mass in the neck,
axilla, breast, abdomen, or groin and a previous personal or family
history of malignancy should be sought.
examination, should be performed on all patients suspected of having
metastatic disease of the spine. The examination should focus on areas
in which most primary tumors occur, including the breasts, chest,
prostate, abdomen, and lymphatic system. A detailed neurologic
examination also must be performed and documented for future comparison
if neurologic deterioration occurs. Localized spinal tenderness often
is present over the area involved with tumor. Multilevel tenderness may
represent multilevel disease.
metastatic disease of the spine include plain radiography, bone scan,
CT, CT-myelography, and MRI. Each modality has advantages and
limitations. Plain radiography is helpful in assessing overall spinal
alignment and spinal instability.
Small
metastatic lesions may be overlooked, however, because 30% to 50% of
the trabecular bone must be destroyed before radiographic evidence of
bone destruction is apparent. A technetium-99m bone scan is a useful
test for identifying metastatic lesions with an osteoblastic response.
False-negative scans do occur, however, most commonly with tumors
having a minimal osteoblastic response, such as multiple myeloma. CT is
helpful in defining bony integrity, which aids in the assessment of
impending spinal instability and preoperative planning. CT-myelography
is useful for patients who cannot undergo MRI for evaluation of neural
element compression. Limitations of this technique include possible
rapid neurologic deterioration caused by cerebrospinal fluid pressure
shifts and the potential to miss additional sites of neural compression
in the presence of a complete block.
|
TABLE 12-1 PRIMARY TUMOR IN SPINAL METASTASES
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
metastatic disease of the spine. Bone involvement, soft tissue
extension, and neural element compression can be assessed in the same
study. A sensitivity of 93% and a specificity of 97% for MRI have been
reported in the assessment of metastatic disease. Gadolinium-enhanced
MRI is not helpful in most cases of vertebral metastases.
differentiating benign versus malignant compression fractures have
shown mixed results. Complete bone marrow replacement by tumor,
resulting in a low T1-weighted signal, has been described in 88% of
metastatic fractures. In 77% of benign fractures, normal marrow
elements were preserved on T1weighted images. These findings helped to
delineate benign versus malignant fractures. In contrast, other studies
have shown a high false-positive rate when MRI was used to detect
malignant compression fractures. Early in fracture healing, the
presence of fracture hematoma, marrow signal changes, and paraspinal
mass effect can lead to the misinterpretation of benign lesions as
malignant. On the basis of this information, a reasonable approach for
a neurologically intact patient presenting with an acute compression
fracture is to provide symptomatic relief with bracing and analgesics,
then regular follow-up. Patients with benign compression fractures
usually improve markedly in 2 to 3 months. Patients with malignant
compression fractures have progressive symptoms and should undergo
work-up at that time.
be initiated, the diagnosis must be confirmed. This is accomplished
most readily by confirming the presence of a primary lesion through a
metastatic work-up. This work-up includes CT scan of the chest,
abdomen, and pelvis; chest radiograph; bone scan; and appropriate
laboratory studies, including serum protein electrophoresis. When a
primary lesion is identified, the presence of a metastatic lesion can
be confirmed via needle or open biopsy. In the case of multi-focal
lesions, a biopsy specimen of the most accessible lesion should be
obtained. When the diagnosis of metastatic disease has been confirmed,
it is not necessary to obtain more biopsy specimens of the spine for
subsequent lesions if the imaging studies and time interval are
consistent with metastasis.
palliative, not curative. The goal of treatment is to maximize the
patient’s quality of life by providing pain relief and maintaining or
restoring neurologic function. In most cases, this goal is reached
through nonoperative means, primarily by chemotherapy and radiotherapy.
For patients who do not respond to nonoperative treatment and are
appropriate candidates, surgical intervention offers an opportunity to
maintain or restore quality of life.
guide treatment of patients with metastatic disease. The Harrington
scheme divided patients with spinal metastases into five categories
based on the extent of neurologic compromise and bone destruction:
-
Class I: Patients have minimal neurologic involvement.
-
Class II: Patients have involvement of bone without collapse or instability and minimal neurologic involvement.
-
Class III: Patients have major neurologic impairment without spinal instability.
-
Class IV:
Patients have vertebral collapse with pain attributable to mechanical
causes or instability but without severe neurologic compromise. -
Class V: Patients have vertebral collapse or instability with major neurologic impairment.
with chemotherapy or hormonal manipulation. If these modalities are
unsuccessful, local radiotherapy is recommended. Class III patients
usually respond to radiotherapy alone. If neurologic compromise is
acute, the addition of corticosteroid treatment should be considered.
Surgery is reserved for radioresistant tumors and progressive tumors
after radiotherapy. Surgical treatment is recommended for class IV and
V patients.
determine the prognosis and life expectancy of patients with metastatic
spinal tumors. This system assigns a point value to six
characteristics, and the total determines a prognostic score. Treatment
recommendations are based on this prognostic score. The six
characteristics are:
-
General health condition (poor, 0 points; moderate, 1 point; good, 2 points)
-
Number of extraspinal bone metastases (≥3, 0 points; 1 to 2, 1 point; 0, 2 points)
-
Number of vertebral metastases (≥3, 0 points; 2, 1 point; 1, 2 points)
-
Metastases to the major internal organs (unremovable, 0 points; removable, 1 point; no metastases, 2 points)
-
Primary site of cancer (lung, stomach, 0
points; kidney, liver, uterus, others, unidentified, 1 point; thyroid,
prostate, breast, rectum, 2 points) -
Spinal cord palsy (complete, 0 points; incomplete, 1 point; none, 2 points)
patients with a total score of 9 or higher survived an average of 12
months or longer, patients with a score of 8 or lower survived 12
months or less, and patients with a score of 5 or lower survived 3
months or less. On the basis of this information, it was recommended
that patients with a score of 9 or higher undergo an excisional
procedure, and a palliative operation was indicated for patients with
scores of 5 points or less.
-
Grade of malignancy (slow growth, 1 point; moderate growth, 2 points; rapid growth, 4 points)
-
Visceral metastases (no metastasis, 0 points; treatable, 2 points; untreatable, 4 points)
-
Bone metastases (solitary or isolated, 1 point; multiple, 2 points)
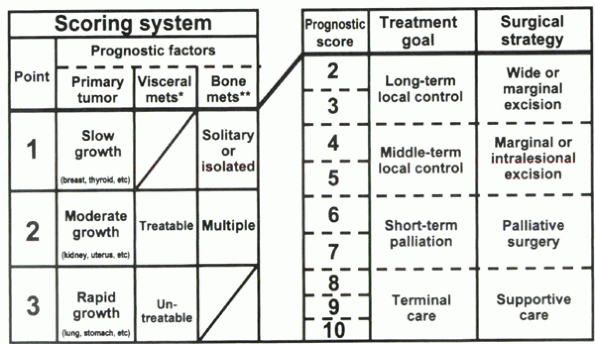 |
|
Figure 12-1 Surgical strategy for spinal metastases (mets). (From Tomita et al.)
|
score between 2 and 10. For patients with a score of 2 or 3 points, the
treatment goal is long-term local control, and a wide or marginal
excision is recommended. For patients with a score of 4 or 5 points,
marginal or intralesional excision is recommended for medium-term local
control. For patients with a score of 6 or 7 points, the treatment goal
is short-term palliation, and palliative surgery is recommended. A
score of 8 to 10 points indicates nonoperative supportive care. In a
series of 61 patients, successful local control was achieved in 43
(83%) of 52 surgically treated patients.
For patients with an acceptable prognosis for survival (>3 to 6
months) and satisfactory medical status, surgical indications include
progressive neurologic deficit secondary to neurologic compression from
a radioresistent tumor; spinal instability; severe intractable pain
unrelieved by nonoperative measures; radioresistant tumor that is
enlarging, causing intractable pain or impending instability; and the
need for a definitive histologic diagnosis. Patients with inadequate
bone stock, multiple areas of epidural compression, or life expectancy
less than 3 months generally are not considered for operative treatment.
spinal instability in patients with metastatic disease of the spine
must be determined. No validated system exists for
making
this determination, however. Nevertheless, multiple criteria have been
proposed to determine the presence of spinal instability.
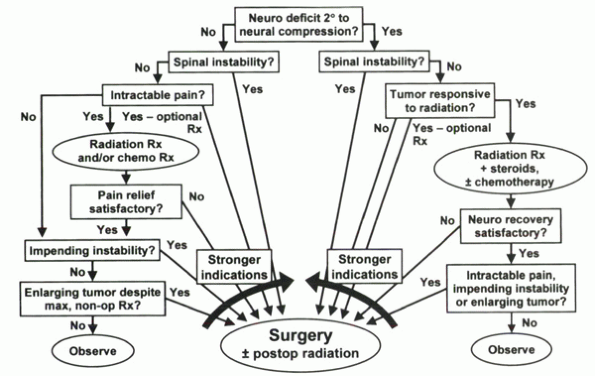 |
|
Algorithm 12-1 Treatment algorithm for metastatic tumors of the spine.
|
assessment of spinal instability as it relates to spinal trauma. White
and Panjabi defined stability as “the ability of the spine, under
physiologic loads, to prevent initial or additional neurologic damage,
severe intractable pain, and gross deformity.” Radiographic criteria
are used widely in the form of checklists for the diagnosis of spinal
instability after cervical spine trauma. These criteria have not been
validated for instability caused by neoplasms.
-
Anterior column—includes the anterior longitudinal ligament and the anterior half of the vertebral body, disk, and annulus
-
Middle column—includes the posterior half of the vertebral body, disk, and annulus and the posterior longitudinal ligament
-
Posterior column—includes the posterior elements and their associated ligaments and facet capsules
of spinal stability after trauma, their usefulness in patients with
metastatic disease of the spine has been questioned.
In
an attempt to design a set of criteria specific for instability in
spinal tumors, Kostuik and Errico proposed a six-column system (Fig. 12-2).
The spine is divided into three columns as just described, then
subdivided further into left and right halves. The spine is considered
unstable if at least three of the six columns are destroyed or if more
than 20 degrees of angulation is present.
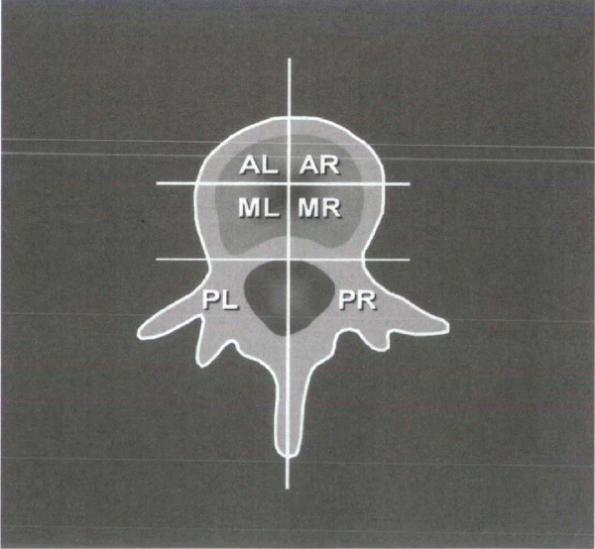 |
|
Figure 12-2 Six-column system for evaluation of stability in spine tumors. (From Kostuik
JP, Errico JN. Differential diagnosis and surgical treatment of metastastic spine tumors. In: Frymoyer JW, ed. The adult spine: principles and practice. Vol 1. New York: Raven Press, 1991:861-888.) |
Asdourian et al to describe the stages of vertebral deformity in
metastatic vertebral breast cancer. This information was used to define
spinal instability and impending instability (Fig. 12-3). Four stages of vertebral deformity were recognized:
-
Stage I: A portion (IA), or all (IB), of the vertebral body marrow is replaced by tumor.
-
Stage II:
Vertebral body collapse may occur at either one end plate (IIA) or both
end plates (IIB). With progressive collapse, a bone fragment from the
posterior vertebral body, termed the delta sign, may cause spinal canal encroachment. -
Stage III:
With end-stage collapse, resulting in either a kyphotic deformity
(IIIA) or symmetric collapse (IIIB), the delta sign is consistently
present. -
Stage IV: Subluxation creates a translational deformity.
impending axial instability, and type II or type III deformity
indicates axial instability. Impending translational instability is
defined by type II or type III vertebral deformity with accompanying
metastatic involvement of the posterior elements. Translational
instability is present with type IV vertebral deformity. Treatment
guidelines for metastatic vertebral breast cancer were proposed on the
basis of these data. For impending axial instability, radiotherapy,
chemotherapy, or hormonal manipulation was recommended. If spinal canal
compromise was present, surgical decompression was considered if the
tumor was radioresistant. When axial instability was present, with or
without spinal canal compromise, surgical stabilization was
recommended. An anterior approach was advised for single-level
involvement and a posterior approach for multilevel involvement.
Impending translational instability, with or without canal compromise,
was thought to indicate surgery with either an anterior-alone or a
combined anterior-posterior approach. For translational instability, a
posterior approach was recommended for stabilization, and a
posterolateral or anterior approach was added for decompression.
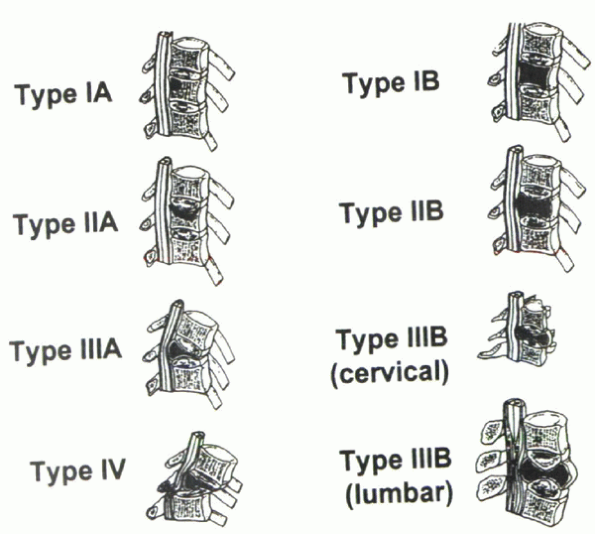 |
|
Figure 12-3 Stage of vertebral deformity. (From Asdourian et al.)
|
establish criteria for impending spinal instability, 100 thoracic and
lumbar vertebrae with osteolytic lesions were analyzed radiologically
by Taneichi et al. The data yielded a set of criteria to determine
impending vertebral collapse in metastatic disease. For the thoracic
spine (T1-10), the criteria are 50% to 60% involvement of the vertebral
body with no destruction of other structures or 25% to 30% body
involvement with costovertebral joint destruction. In the thoracolumbar
and lumbar spine (T10-L5), the criteria are 35% to 40% involvement of
the vertebral body or 20% to 25% body involvement with posterior
element destruction.
instability in patients with metastatic disease, all of the available
classification systems should be considered. None of these systems has
been validated for use in metastatic disease, however, and all have
limitations. These systems aid in guiding treatment, but other factors
also should be considered when trying to determine the appropriate
treatment option, including the biology of the specific tumor type, the
radiosensitivity of the tumor, the response of the bone (blastic versus
lytic metastasis), the patient’s ability to withstand an operative
procedure, the severity of pain, the quality of the host bone, and,
above all, the wishes of the individual patient.
system for primary neoplasms also can be applied to metastatic lesions.
In this system, the vertebral body is divided into zones of involvement
to determine the optimal surgical approach. The tumor is classified by
dividing each vertebra into 12 triangular zones (numbered 1 to 12 in
clockwise order) and into five layers from the paravertebral
extraosseous region to the intrathecal region (Fig. 12-4).
The longitudinal extent of tumor involvement is obtained by recording
the spinal segments involved. This system has been used to characterize
primary bone tumors. The principles of the classification system,
describing the anatomic location and providing information for surgical
planning and communication, are applicable to metastatic spinal disease.
clinical series of patients treated surgically for cord compression due
to metastatic tumors has been tabulated by Mclain and Weinstein. The
overall trend indicated improved results with anterior decompression,
but the location of epidural compression should dictate the approach
for decompression. After tumor excision, the spine must be
reconstructed. In patients who have a limited life expectancy or in
whom postoperative radiotherapy is planned, methyl methacrylate is a
good choice for anterior reconstruction because of its excellent
resistance to compressive loads and immediate stabilizing effect.
Posterior reconstruction with methyl methacrylate is discouraged
because of its poor ability to resist tensile forces. When patient life
expectancy is expected to be measured in years, the use of allograft or
autograft bone is preferred because, without the support of a bony
arthrodesis, methyl methacrylate is likely to fail with time.
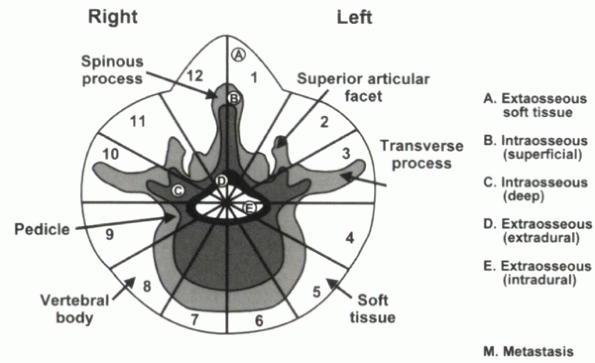 |
|
Figure 12-4 Weinstein, Boriani, and Biagnini surgical staging system.
|
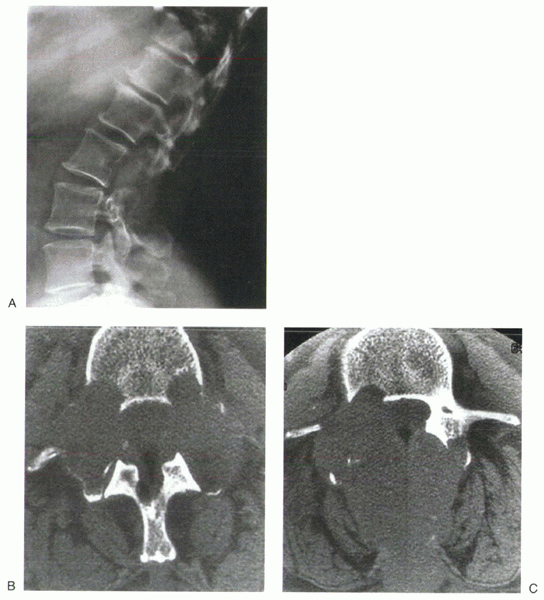 |
|
Figure 12-5 (A) Lateral radiograph of the lumbar spine shows lytic destruction of the posterior elements of L2 and L3. (B) CT scan shows bilateral pedicular involvement at L2. (C) CT scan shows right pedicular involvement at L3. (D) Sagittal MRI of the lumbar spine shows neural element compression. (E) Axial MRI shows spinal canal encroachment by metastatic lesion. (F) Posteroanterior and lateral radiographs after decompression and stabilization. (G) Posteroanterior and lateral radiographs show lytic destruction of L2 and L3 vertebral bodies. (H) Sagittal MRI shows tumor involvement of L2 and L3 vertebral bodies with neural compression. (I) Posteroanterior and lateral radiographs after L2 and L3 corpectomy and strut grafting.
|
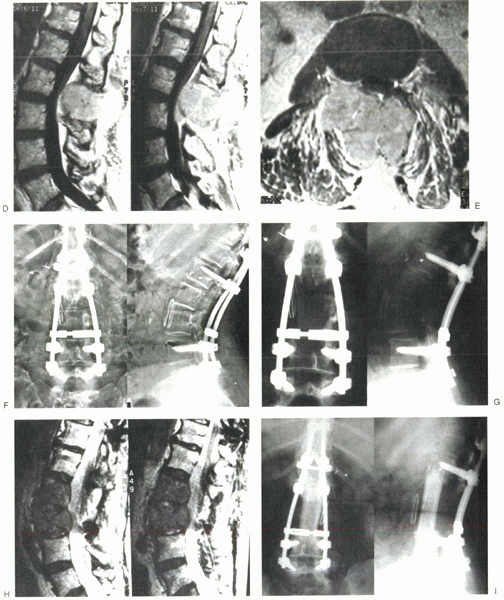 |
|
Figure 12-5 (continued)
|
attention for back pain with an associated midline mass in the upper
lumbar spine. Her history revealed a recent 15lb weight loss and
worsening back pain over the last month with no previous malignancy.
Physical examination confirmed posterior midline tenderness over the
mass and mild right-sided iliopsoas weakness. Radiographic evaluation
of the spinal lesion was performed; the lateral plain film, CT, and MRI
images are shown in Figure 12-5A-C, D-E.
A work-up to identify a primary malignancy revealed a right kidney
mass. Biopsy of the posterior spinal mass confirmed the diagnosis of
metastatic renal cell carcinoma. Further metastatic work-up revealed no
other lesions. The treatment decision at this time was to proceed with
radiotherapy in an attempt to decrease tumor size and improve pain
control. Despite maximal radiotherapy, the patient’s symptoms
progressed. Operative decompression and stabilization were planned.
Before surgery, the patient developed a lower extremity deep venous
thrombosis requiring filter placement.
embolization followed by L2-3 decompression with intralesional tumor
excision and posterior spinal fusion from T10-L5 with instrumentation (Fig. 12-5F).
The postoperative course was complicated by wound dehiscence that
responded to operative débridement and dressing changes. She otherwise
did well postoperatively with considerable improvement in pain.
back pain. Radiography and MRI revealed progression of disease into the
L2 and L3 vertebral bodies (Fig. 12-5G, H).
Because radiotherapy was maximized previously, treatment options were
limited and consisted of either permanent bracing or surgery. The
patient chose to proceed with operative intervention, and in March
1999, she underwent an L2 and L3 corpectomy with strut grafting after
preoperative embolization (Fig. 12-5I). She did well postoperatively, with considerable improvement in back pain until her death 1.5 years later.
management of spinal metastasis. Initially the patient was thought to
be in Harrington’s class III, and radiotherapy was administered.
Because renal cell cancer is not sensitive to radiotherapy, it would
have been reasonable to perform surgery before radiotherapy. She did
not respond to radiotherapy, and her symptoms progressed. Her Tokuhashi
score showed that she was an acceptable candidate for surgery, and she
underwent a posterior decompression and fusion. The results of anterior
decompression and fusion are better than posterior procedures, but the
surgery must be done from the side that is involved. Preoperative
embolization is required in cases of renal cell metastasis to decrease
intraoperative bleeding. A wound infection developed postoperatively.
This complication occurs in approximately 35% of cases in which a
posterior procedure is done after radiotherapy and is one of the
reasons to consider early surgery in selected cases before radiation.
for 1 year until the disease spread into the anterior aspect of the
spine and caused compression of the cauda equina. Her Tokuhashi score
was still acceptable, and after another embolization, she underwent a
decompression and stabilization procedure. This time the surgery was
performed from the front because the disease was located anteriorly.
She did well for another 1.5 years until she died. One could argue that
when she initially presented to us, her Tomita score was only 3, and
because the tumor type is not radiosensitive, we should have considered
an en bloc spondylectomy to decrease the risk of local recurrence. In
her case, however, the CT scan in Figure 12-5B
shows that it would not have been possible to perform an en bloc
spondylectomy with clear margins because of the extent of the tumor.
The surgical classification schemes are helpful in determining the
appropriate approach and likelihood of success when an aggressive
treatment option is considered.
spine is a multidisciplinary effort that should follow a logical,
orderly protocol such as the one proposed in Algorithm 12-1.
The goal should be clearly defined. Patients considered for possible
surgical intervention should have a reasonable life expectancy and have
failed to attain treatment goals through nonoperative measures. If
surgical intervention is chosen, the approach should be based on the
location of epidural compression, presence of instability, or both.
Stabilization can be achieved with methyl methacrylate, bone graft, and
instrumentation, either alone or in combination. Above all, treatment
should be tailored to the individual patient, always adhering to the
goal of maximizing the patient’s overall quality of life.
WA, Provencher MT, Weinstein JN. Classification of spinal metastases.
In: Heiner JP, Kinsella TJ, Zdeblick TA, eds. Management of metastatic
disease to the musculoskeletal system. St. Louis: Quality Medical
Publishing, 2002:371.
A, Lobato RD, Rivas JJ, et al. Spinal metastatic disease: analysis of
factors determining functional prognosis and the choice of treatment.
Neurosurgery 1984;15:820-827.
GA, Gillespie PJ, Grebbell ES. The radiologic demonstration of osseous
metastases: experimental observations. Clin Radiol 1967;18:158-162.
RW, Kim JH, Posner JB. Epidural spinal cord compression from metastatic
tumor: diagnosis and treatment. Ann Neurol 1978;3:40-51.
KD. Anterior decompression and stabilization of the spine as a
treatment for vertebral collapse and spinal cord compression from
metastatic malignancy. Clin Orthop 1988;233:177-197.
JP, Errico JN. Differential diagnosis and surgical treatment of
metastastic spine tumors. In: Frymoyer JW, ed. The adult spine:
principles and practice. Vol 1. New York: Raven Press, 1991:861-888.
KC, Poon PY. Sensitivity and specificity of MRI in detecting malignant
spinal cord compression and in distinguishing malignant from benign
compression fractures of vertebrae. Magn Res Imaging 1988;6:547-556.
LH, Frassica DA, Kostuik JP, Frassica FJ. Metastatic disease to the
spine: diagnosis and treatment. Instr Course Lect 2000;49:471-477.
Y, Matsuzaki H, Toriyama S, et al. Scoring system for the preoperative
evaluation of metastatic spine tumor prognosis. Spine 1990;15:1110-1113.
