The Elbow
-
The sequelae of medial traction and lateral compression from valgus stress, including:
-
Medial collateral ligament (MCL) injury
-
Common flexor tendon pathology
-
Medial traction spurs
-
Ulnar neuropathy
-
Osteochondritis dissecans
-
-
Lateral collateral ligament injury
-
Lateral epicondylitis
-
Posttraumatic osseous abnormalities such as:
-
Radiographically occult fractures
-
Stress fractures
-
Bone contusions
-
Apophyseal avulsions
-
-
Cartilaginous extension of fractures in children (a condition difficult to evaluate with computed tomography [CT])
-
Intra-articular loose bodies and capsular pathology, especially if fluid or contrast material is present within the elbow joint
-
Biceps and triceps tendon injuries
-
Entrapment neuropathies
-
Bursitis
-
Arthropathies
-
Soft-tissue masses about the elbow
-
It is important to locate the joint as close to isocenter as possible either using a prone position or an open magnet.
-
Axial images must include the radial tuberosity.
-
Sagittal images should have a larger field of view to evaluate a possibly retracted biceps tendon.
GRE sequences provide useful supplemental information for identifying loose bodies within the elbow. GRE volume sequences allow acquisition of multiple very thin axial images, which may subsequently be reformatted in any plane. In general, GRE sequences should be avoided after elbow surgery because magnetic susceptibility artifacts associated with micrometallic debris may obscure important findings and may also be mistaken for loose bodies. Artifact surrounding orthopaedic hardware is most prominent on GRE sequences because of the lack of a 180° refocusing pulse. It is least prominent on FSE sequences due to the presence of multiple 180° pulses. FSE T2-weighted sequences may be used to obtain higher-resolution images in the same amount of time as conventional spin-echo sequences or to reduce the overall time of the examination. The ability to shorten the examination with FSE is useful when scanning claustrophobic patients or patients who become uncomfortable in the prone position with the arm overhead.
 |
|
FIGURE 9.1 ● Routine images obtained on a 3 T system show examples of PD-weighted (A) and FS PD FSE (B) axial images and an FS PD FSE sagittal image (C) in a patient with rupture of the distal biceps tendon.
|
intravenously or directly into the elbow joint as a dilute solution. Intravenous gadolinium may provide additional information in the assessment of neoplastic or inflammatory processes about the elbow. Articular injection of saline or dilute gadolinium may be useful in patients without a joint effusion to detect loose bodies, to determine if the capsule is disrupted, or to determine if an osteochondral fracture fragment is stable.
 |
|
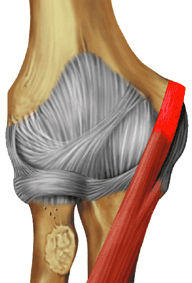
FIGURE 9.2 ● Biceps brachii The biceps brachii is composed of two heads and is biarticulate, spanning the shoulder and elbow joints. The distal biceps brachii tendon inserts on the radial tuberosity, with superficial fibers contributing to the bicipital aponeurosis that blends with the antebrachial fascia. Most biceps brachii tears occur proximally involving the long head; however, distal avulsions are not uncommonly seen and are secondary to sudden or prolonged flexion against a heavy load. The biceps brachii acts as a flexor of both the shoulder and elbow joints. When the elbow is flexed, the biceps brachii also acts as a powerful supinator due to its slightly medial insertion on the rotating proximal radius.
|
 |
|
FIGURE 9.3 ● Brachialis The brachialis contributes more during isometric elbow flexion, whereas the biceps brachii is more active during dynamic elbow flexion. Isolated rupture of the brachialis muscle is a rare injury. Strains typically occur at the musculocutaneous junction.
|
 |
|
FIGURE 9.4 ● Triceps brachii The triceps brachii is composed of three heads. The long head of the triceps brachii is biarticulate, spanning both the shoulder and elbow joints. In addition to adduction of the shoulder, the triceps brachii acts to extend both the shoulder and elbow joints. The distal triceps tendon inserts on the olecranon, where avulsions can occur, typically following a fall on the outstretched upper extremity resulting in deceleration stress on an already contracted triceps. Midsubstance tendon and musculocutaneous injuries of the triceps brachii are less common.
|
 |
|
FIGURE 9.5 ● Pronator Teres The pronator teres acts synergistically with the pronator quadratus to pronate the forearm. The median nerve has variable anatomy with respect to the pronator teres. Most commonly the median nerve runs between the humeral and ulnar heads of the pronator teres, but it also can travel deep to both heads as well as perforate the humeral head. Pronator syndrome results from compression of the median nerve as it courses through the pronator teres and is manifested clinically by pain in the wrist and forearm and weakness of the thenar muscles.
|
 |
|
FIGURE 9.6 ● Flexor Carpi Radialis The flexor carpi radialis lies radial to the palmaris longus and ulnar to the pronator teres throughout its course. It contributes to flexion and abduction of the wrist. Along with the pronator teres it is the most common tendon involved in medial epicondylitis. Distal flexor carpi radialis tendon rupture, usually occurring after a fall on the outstretched hand, can clinically mimic scaphoid fractures.
|
 |
|
FIGURE 9.7 ● Palmaris longus The palmaris longus is present in approximately 85% of the population and functions to flex the wrist and tighten the palmar aponeurosis. It does not have a tendon sheath but has a paratenon. It is the most commonly used tendon graft of the hand, often used for repair of the elbow MCL in throwing athletes.
|
 |
|
FIGURE 9.8 ● Flexor carpi ulnaris The flexor carpi ulnaris flexes and adducts the hand. It is an important dynamic stabilizer of the pisotriquetral joint and contributes superficial fibers to the pisohamate ligament. As it is superficial and just medial to the ulnar nerve, it serves as a marker when ulnar nerve block is performed.
|
 |
|
FIGURE 9.9 ● Flexor digitorum superficialis The flexor digitorum superficialis tendons flex the middle phalanges of each finger and using the pulley system as a fulcrum contribute to flexion of the fingers at the metacarpophalangeal joint. The deep fibers of the flexor digitorum superficialis origin are closely apposed with the anterior bundle of the medial collateral ligament at the elbow, which is why edema and hemorrhage in the flexor digitorum superficialis are commonly seen in the setting of MCL tears. In the forearm, the median nerve lies just deep to the arch of the flexor digitorum superficialis muscle, and this is an area of potential nerve compression. The flexor digitorum superficialis divides into four musculotendinous units in the distal forearm and the tendons travel though the carpal tunnel before dividing again at the level of the proximal phalanges.
|
 |
|
FIGURE 9.10 ● Flexor digitorum profundus The flexor digitorum profundus tendons flex the distal phalanges at the distal interphalangeal joints and assist in flexion of the wrist and proximal phalanges. The flexor digitorum profundus divides into four musculotendinous units in the distal forearm, and the tendons travel though the carpal tunnel deep to the flexor digitorum superficialis tendons. Distal avulsions of a flexor digitorum profundus tendon, or “jersey finger,” can occur when an athlete gets a finger caught in an opposing player's jersey.
|
 |
|
FIGURE 9.11 ● Flexor pollicis longus The flexor pollicis longus flexes the thumb. Compression of the anterior interosseous nerve can lead to denervation of the flexor pollicis longus muscle, which may be isolated or concomitant with flexor digitorum profundus and pronator quadratus denervation.
|
 |
|
FIGURE 9.12 ● Brachioradialis The brachioradialis is a strong elbow flexor when the forearm is in a neutral position between supination and pronation. In forearm pronation, the brachioradialis tends to supinate as it flexes. In forearm supination, the brachioradialis tends to pronate as it flexes.
|
 |
|
FIGURE 9.13 ● Extensor carpi radialis longus The extensor carpi radialis longus extends and abducts the wrist. If extensor carpi ulnaris function is lost due to posterior interosseous nerve palsy, the extensor carpi radialis causes radial deviation because normally the attachment of the extensor carpi ulnaris to the ulnar aspect of the fifth metacarpal functions to neutralize the abduction movement applied by the extensor carpi radialis longus.
|
 |
|
FIGURE 9.14 ● Extensor carpi radialis brevis The extensor carpi radialis brevis, which provides neutral extension of the wrist, is the most common tendon involved in lateral epicondylitis. Distal ruptures of the extensor carpi radialis brevis significantly affect wrist extension.
|
 |
|
FIGURE 9.15 ● Extensor digitorum The extensor digitorum extends the medial four digits at the metacarpophalangeal joints and contributes to wrist extension. The proximal tendon, as part of the common extensor origin, is often involved in lateral epicondylitis. The extensor digitorum tendons are connected at the level of the metacarpal bones by fibrous bands called juncturae tendinum. Boutonnière deformity results from disruption of the central slip component of the extensor tendon at its insertion into the middle phalanx.
|
 |
|
FIGURE 9.16 ● Extensor digitI Minimi The extensor digiti minimi extends the proximal phalanx of the little finger at the metacarpophalangeal joint and contributes to wrist extension. Because the extensor digiti minimi tendon lies just superficial to the radioulnar articulation, it is often the first tendon to be involved in rheumatoid arthritis.
|
 |
|
FIGURE 9.17 ● Extensor carpi ulnaris The extensor carpi ulnaris tendon extends and adducts the wrist. It is commonly affected in tendinosis and tenosynovitis as it passes through the groove on the distal ulna. Subluxation of the extensor carpi ulnaris can also occur at this location related to disruption or insufficiency of the ligament that covers the tendon in this groove. The extensor carpi ulnaris tendon subsheath is a component of the triangular fibrocartilage complex.
|
 |
|
FIGURE 9.18 ● Anconeus The anconeus is located posterolateral to the elbow and functions to tighten the joint capsule and is a weak extensor of the elbow. In about 10% of the population an anomalous muscle, the anconeus epitrochlearis, arises from the medial border of the olecranon and the adjacent triceps inserting into the medial epicondyle. The anconeus epitrochlearis is thus located posteromedial to the elbow and can cause compression of the ulnar nerve in the cubital tunnel.
|
 |
|
FIGURE 9.19 ● Supinator The supinator is the primary supinator of the forearm when the elbow is extended. When the elbow is flexed, the supinator and the biceps brachii work synergistically to supinate the forearm. The deep branch of the radial nerve (i.e., the posterior interosseous nerve) travels between the humeral and ulnar origins of the supinator as it courses down the forearm, posterolateral to the proximal radius. The supinator is a potential site of entrapment of this nerve.
|
-
Axial scans are best for evaluating the neurovascular structures.
-
Sagittal scans show the bony articular relationship to best advantage.
-
There are normal “bare areas” at the ulnar trochlear notch and the capitellum; these should not be mistaken for pathology.
-
Coronal scans are best for evaluating common tendon origins and collateral ligaments.
-
The anterior bundle of the MCL, extending from the inferior margin of the medial epicondyle to the medial anterior margin of the coronoid process, is especially well demarcated, as is the lateral ulnar collateral ligament (LUCL), seen extending along the posterolateral aspect of the proximal radius to insert laterally on the tubercle of the supinator crest of the ulna.
-
The anterior articulation between the trochlea and the coronoid, the proximal radioulnar joint, and the articulation between the radius and the capitellum are all well demonstrated.
-
The rough nonarticular area at the posterior margin of the capitellum should not be mistaken for an osteochondral defect on coronal images through the posterior aspect of the radial head.
-
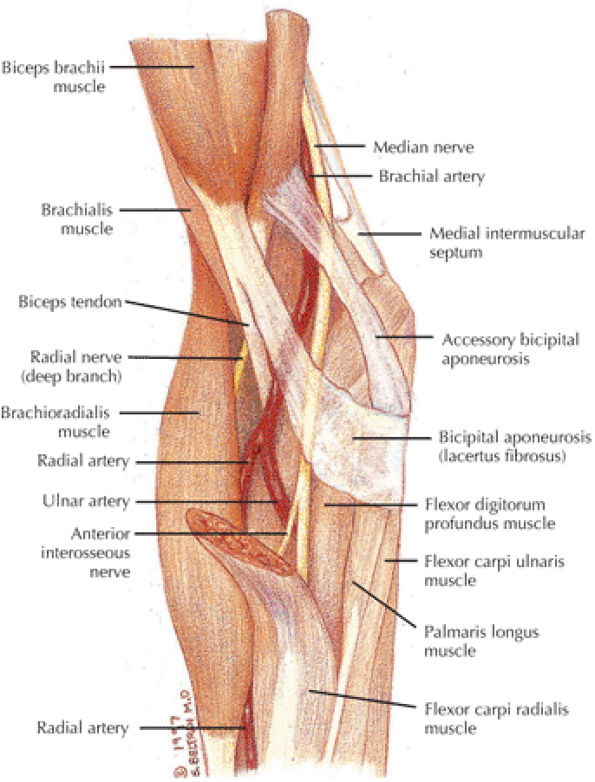
The biceps and brachialis muscles are clearly depicted anteriorly, and the biceps tendon can be followed to the radial tuberosity and the brachialis tendon to the ulnar tuberosity.
-
The bicipital aponeurosis, also known as the lacertus fibrosus, appears as a thin black line that extends from the myotendinous junction of the biceps to the fascia overlying the flexor-pronator muscle group medially.
-
The median nerve and the brachial artery and veins lie just deep to the lacertus fibrosus, at the level of the medial epicondyle.
-
The hypointense common flexor and common extensor tendons can be seen arising from the medial and lateral epicondyles, respectively.
-
The radial nerve is located between the brachialis and brachioradialis muscles, and the deep branch can be followed distally as it passes between the deep and the superficial heads of the supinator muscle to form the posterior interosseus nerve (an important site of potential impingement).
-
The annular ligament is a thin hypointense structure that lies just superficial to the articular cartilage of the radial head, which demonstrates intermediate signal intensity.
-
The insertion of the LUCL can be seen at the lateral margin of the ulna at the level of the radial neck.
-
The insertion of the anterior bundle of the MCL can be seen at the medial margin of the coronoid process, just anterior and lateral to the ulnar nerve.
-
Posteriorly, the triceps tendon can be followed to the olecranon.
-
The anconeus muscle is well seen posterolaterally.
-
The ulnar nerve and accompanying posterior ulnar recurrent artery and veins are seen posteromedially, deep to the cubital tunnel retinaculum at the level of the medial epicondyle. The ulnar nerve can be followed distally as it passes deep to the humeral and ulnar heads of the flexor carpi ulnaris muscle.
-
The proximal radioulnar joint and the posterior compartment articulation between the olecranon and the olecranon fossa are also well seen.
-
The usual site of osteochondritis dissecans—along the anterior aspect of the capitellum—is also well seen on axial images.
|
FIGURE 9.20 ● Normal coronal anatomy. (A) A fracture of the olecranon or a muscle strain or tear involving the muscle belly of the triceps may clinically mimic signs and symptoms of a distal triceps tendon tear. (B) The normal distal triceps tendon fibers are interspersed with intermediate to high bands of linear signal, which should not be mistaken for tears or tendinosis. (C) Loose bodies not uncommonly lodge in the posterior aspect of the joint, particularly within the olecranon fossa. (D) The lateral ulnar collateral ligament (LUCL) originates on the posterior lateral aspect of the lateral epicondyle and courses posteriorly and distally around the radial head and neck to insert on the lateral ulna. The LUCL forms a cradle around the radial head and neck and prevents posterior dislocation of the radial head. On this image, the oblique course of the distal LUCL posterior to the radial neck is visualized, and the LUCL is seen inserting on the lateral ulnar diametaphysis. (E) In cases of suspected fracture, T1-weighted images are useful in visualizing the low-signal fracture line, particularly with fractures of the radial head and coronoid process. (F) The proximal portion of the LUCL is visualized on this image, with its origin from the posterolateral aspect of the lateral epicondyle. Most tears of the LUCL occur at the origin. (G) In addition to the ulnohumeral and radiohumeral articulations, there is a third elbow articulation between the medial aspect of the radial head and the lateral aspect of the coronoid, which is covered with cartilage and susceptible to chondromalacia. (H) The distal insertion fibers of the anterior bundle of the MCL are continuous with the sublime tubercle, and any fluid interposed between the distal fibers and the medial aspect of the sublime tubercle is interpreted as a deep articular-sided partial tear with partial stripping of the distal ligament from the sublime tubercle, an appearance called the T sign. (I) Osteochondritis dissecans commonly affects the capitellum, particularly in adolescents 13 to 16 years of age. A distinct entity affecting the capitellum is Panner's disease, which is posttraumatic avascular necrosis of the capitellum; it affects a younger age group (children 5 to 11 years). (J) The radial collateral ligament (RCL) is located just anterior to the LUCL, and its fibers originate on the lateral aspect of the lateral epicondyle and insert on the lateral aspect of the annular ligament surrounding the radial head. RCL tears often accompany LUCL tears, or, in cases of posttraumatic lateral-sided blowout, the RCL, LUCL, and common extensor tendon can all be pulled off the lateral epicondyle as a single unit. (K) Fractures of the coronoid process are generally caused by hyperextension or posterior subluxation/dislocation. As a result, coronoid process fractures are frequently accompanied by fractures of the radial head and distal humerus. (L) A ruptured, retracted distal biceps tendon may manifest as fluid signal and hemorrhage at the radial tuberosity insertion site, with no tendon visualized. Viewing successive coronal images anterior to the radial tuberosity, the torn and retracted end of the distal biceps may be located and the distance of retraction measured.
|
|
FIGURE 9.21 ● Normal axial anatomy. (A) The coronoid fossa is a depression on the anterior aspect of the distal humerus that receives the coronoid during flexion, whereas the olecranon fossa is a depression on the posterior aspect of the distal humerus that receives the olecranon during extension. The coronoid fossa and olecranon fossa are common locations in which loose bodies may lodge. (B) It is not uncommon for the ulnar nerve to appear somewhat bright on MR images. This appearance may be seen in cases of ulnar neuritis but is not specific for the diagnosis. Other findings that increase the specificity for ulnar neuritis include enlargement of the ulnar nerve, thickening of the cubital retinaculum, inflammation around the ulnar nerve, and a space-occupying lesion within the cubital tunnel or an anconeus epitrochlearis. C) The cubital tunnel, formed by the posterior aspect of the medial epicondyle and the medial aspect of the olecranon, is covered by the cubital tunnel retinaculum. Scarring and thickening of the cubital tunnel retinaculum can be associated with ulnar neuritis. Also, in 11% of the population, the cubital tunnel retinaculum is replaced by the anconeus epitrochlearis muscle, which may cause ulnar nerve impingement within the cubital tunnel. (D) The common extensor tendon and common flexor tendon origins are visualized as curvilinear foci of dark tendon signal along the anterior margins of the lateral epicondyle and medial epicondyle, respectively. Tears of these tendon origins are suggested when fluid signal is visualized along the lateral or medial epicondyle in place of the normal dark tendon signal. (E) The radial nerve and its branches innervate the muscles along the anterior, radial, and posterior aspect of the elbow, including (clockwise around the elbow) the brachialis, brachioradialis, extensor carpi radialis longus, extensor carpi radialis brevis, extensor digitorum, extensor carpi ulnaris, anconeus, and triceps muscles. Evidence for denervation of these muscles suggests abnormality of the radial nerve or its branches. (F) The RCL and LUCL are visualized on this axial image as linear dark signal just deep to the common extensor tendon, with a plane of synovium separating the lateral collateral ligament complex from the common extensor tendon. Fluid signal deep to the common extensor tendon replacing the anterior fibers of the lateral collateral ligament complex represents tears of the RCL, whereas fluid signal replacing the posterior fibers suggests tears of the LUCL. (G) The ulnar nerve and its branches innervate the muscles along the posteromedial aspect of the elbow, including (clockwise around the elbow) the flexor digitorum profundus and flexor carpi ulnaris. Evidence for denervation of these muscles suggests abnormality of the ulnar nerve. (H) The median nerve is visualized on this axial image anteromedially coursing between the pronator teres and brachialis muscles. The radial nerve is visualized anterolaterally coursing just deep to the brachioradialis muscle. Bright signal in the radial and median nerve suggesting neuritis is not as commonly visualized. However, denervation of the muscles supplied by the nerves, as evidenced by high signal or atrophy of the muscles, suggests neuritis. (I) The median nerve and its branches innervate the muscles along the anteromedial aspect of the elbow, including (clockwise around the elbow) the flexor digitorum superficialis, palmaris longus, flexor carpi radialis, and pronator teres muscle. Evidence for denervation of these muscles suggests abnormality of the median nerve. (J) The LUCL is visualized on this axial image inserting distally on a ridge along the lateral aspect of the ulna known as Gerdy's tubercle. Although most tears of the LUCL occur at its proximal origin, the entire course of the LUCL from origin to insertion should be examined in the axial, coronal, and sagittal planes. (K) The radial tuberosity is the bony insertion site for the distal biceps tendon. Bony hypertrophy and bone marrow edema in the radial tuberosity are indirect signs of tendinosis and/or tearing of the distal biceps tendon. (L) A fibrous band of aponeurotic tissue called the lacertus fibrosis attaches to the distal biceps tendon. In the setting of an insertional distal biceps tendon tear, if the lacertus fibrosis is intact, the distal biceps tendon demonstrates little if any retraction. However, if the lacertus fibrosis is torn, the amount of distal biceps tendon retraction may be quite severe.
|
-
Laterally, the components of the common extensor tendon can be followed to the lateral epicondyle.
-
Medially, the components of the common flexor tendon can be seen extending proximally to the medial epicondyle.
-
The intermediate-signal-intensity ulnar nerve is seen along the posterior margin of the medial epicondyle.
-
Near the midline, the attachment of the triceps muscle and tendon to the olecranon can be seen.
-
Normal obliteration of the subcutaneous fat is seen posterior to the olecranon at the site of the superficial olecranon bursa.
-
The posterior and anterior fat pads can be seen along the margins of the distal humerus.
-
The joint capsule appears as a thin, hypointense structure just superficial to the fat pads.
-
The brachialis muscle lies just superficial to the anterior joint capsule and can be followed distally to its insertion on the ulnar tuberosity.
-
The biceps can be followed distally to its insertion on the radial tuberosity. The adjacent low-signal-intensity brachial and ulnar arteries should not be mistaken for the biceps tendon on sagittal images.
-
The articulation of the radius and the capitellum and the articulation between the trochlea and the trochlear notch are clearly demonstrated.
-
The normal bare area of the ulna in the midportion of the trochlear notch should not be confused with an osteochondral defect. Similarly, the rough nonarticular area at the posterior margin of the capitellum should not be confused with an impaction fracture or osteochondral defect.
-
MCL
-
Common flexor tendon
-
Proximal flexor muscles
-
Ulnar nerve
-
Trochlea and coronoid (and overlying cartilage)
-
Medial epicondyle
-
Radial collateral ligament (RCL)
-
LUCL
-
Common extensor tendon
-
Proximal extensor muscles
-
Radius and capitellum (and overlying cartilage)
-
Biceps tendon
-
Brachialis tendon and muscle
-
Radial and median nerves
-
Ulnohumeral joint (with the coronoid process as the key stabilizer)
-
MCL (primarily the anterior bundle)
-
LUCL
-
Radial head
-
Common flexor and extensor tendons
and is one of the main stabilizers of the elbow. The posterior bundle of the MCL is thought to be less functionally important and occasionally is difficult to visualize in the coronal plane.
|
FIGURE 9.22 ● Normal sagittal anatomy. (A) The supinator muscle, visualized on this sagittal image, is a landmark for the division of the radial nerve into superficial and deep branches. The deep branch pierces the supinator muscle to become the posterior interosseous nerve. The tendinous origin of the supinator muscle (known as the arcade of Frohse) is a common location for posterior interosseous nerve entrapment. (B) This sagittal image demonstrates the lateral collateral ligament complex and the common extensor tendon as a triangle of dark signal with its base fanning out over the radial head. The apex of the triangle represents the proximal origin of the common extensor tendon. The anterior aspect of the base of the triangle represents the RCL, whereas the posterior aspect of the base represents the LUCL. Fluid signal within any part of this triangle of dark signal usually suggests a tear to the corresponding ligament or common extensor tendon. (C) Posterior dislocation of the radius may result in “kissing” contusions or fractures at the anterior aspect of the radial head and posterior aspect of the capitellum, at the point where the two bones impact one another during a posterior dislocation event. (D) This sagittal image demonstrates the course of the proximal to mid-lateral ulnar collateral ligament taking a turn around the posterolateral aspect of the radial head, forming a cradle that prevents the radial head from posterior dislocation. (E) Reactive bone marrow edema and hypertrophic changes in the radial tuberosity commonly accompany insertional distal biceps tendon pathology. (F) Sagittal and coronal images demonstrate the articular cartilage surfaces of the radial head, capitellum, and trochlea. In the setting of any significant chondromalacia, the remainder of the elbow joint should be scrutinized for loose bodies. (G) If fractures of the olecranon are displaced by less than 2 mm, the fracture can be treated conservatively. Fractures with greater than 2 mm of displacement are usually treated by ORIF. (H) The normal trochlear groove is located in the central portion of the articular surface of the proximal ulna and represents a bare area devoid of cartilage. This normal pseudodefect should not be mistaken for a chondral defect on sagittal images. (I) If fractures of the coronoid process involve less than 50%, the fracture may be treated conservatively. However, if more than 50% of the coronoid is involved, or if there are other fractures or valgus instability, surgical treatment is preferred. (J) The coronoid fossa and olecranon fossa are areas of bone thinning on the anterior and posterior aspect of the distal humerus, respectively. The coronoid fossa receives the coronoid on flexion, and the olecranon fossa receives the olecranon on extension. These fossae are common locations for loose bodies. In addition, hypertrophic bone can form in the olecranon and coronoid fossae due to degenerative arthritis, and the hypertrophic bone can limit flexion and extension. (K) Medial epicondylitis is 10 times less common than lateral epicondylitis. (L) The ulnar nerve courses posterior to the medial epicondyle and is visualized on this sagittal image. This is the most common location for ulnar neuritis, and findings on sagittal images can confirm abnormalities of the ulnar nerve visualized in the axial plane. (M) Little Leaguer's elbow describes avulsion injuries of the medial epicondyle, due to an acute traumatic event or chronic repetitive microtrauma. (N) Fluid signal seen on sagittal images within or replacing the dark common flexor tendon origin at the medial epicondyle is suggestive of a tear of the common flexor tendon.
|
 |
|
FIGURE 9.23 Medial Collateral Ligament.
|
LUCL posterior to the proximal radius is seen as it courses toward and attaches to the supinator crest on the ulna. Suspected tears and sprains of the RCL and LUCL are confirmed and further characterized in the axial and sagittal planes. Tears of the LUCL predispose to recurrent radial head dislocations. In cases of significant elbow trauma leading to dislocation, it is not uncommon for the RCL, LUCL, and common extensor tendon origin to completely tear off of the lateral epicondyle together as a single ligamentous/tendinous unit.
 |
|
FIGURE 9.24 Common Flexor Tendon.
|
 |
|
FIGURE 9.25 Ulnohumeral Articulation.
|
 |
|
FIGURE 9.26 Ulnar Nerve.
|
on coronal images should not be misinterpreted as a cartilage defect. Another common cartilage pseudolesion occurs at the lateral aspect of the capitellum, where a normal groove devoid of cartilage between the capitellum and lateral epicondyle may mimic an osteochondral defect. These cartilage surfaces must be examined carefully for the presence of chondral degeneration and associated stress-related subchondral edema and cystic change. Chondral degeneration is most commonly visualized on the opposing surfaces of the medial radial head and adjacent crest at the lateral margin of the trochlea. Osteochondral lesions of the capitellum commonly occur in teenage and young adult throwing athletes, whereas Panner's disease (capitellar osteochondrosis) occurs in younger children. Fractures of the radial head and neck, not uncommonly occult on plain films, are easily visualized on MR images.
 |
|
FIGURE 9.27 Lateral Collateral Ligament Complex.
|
 |
|
FIGURE 9.28 Common Extensor Tendon.
|
 |
|
FIGURE 9.29 Radiohumeral Articulation.
|
 |
|
FIGURE 9.30 Biceps and Brachialis.
|
of the elbow reveals the origins of the RCL and LUCL anteriorly along the lateral epicondyle and the origin of the common extensor tendon just posterior to the RCL and LUCL. The flexor and extensor muscles are inspected for strains, tears, or denervation injury. Proximal-to-distal images in the anterior compartment allow the biceps and brachialis tendons to be followed to their insertions. The course of the median and radial nerves is also examined anteriorly. In the posterior aspect of the joint the triceps tendon is evaluated for tears, strain, or tendinosis.
 |
|
FIGURE 9.31 Triceps Tendon.
|
identified along the anteromedial-most aspect of the medial epicondyle and its course can be followed distally to where it fans out into its various components making up the proximal flexor muscle group. The individual flexor muscles are also delineated and strains, tears, or denervation injuries are identified and localized.
 |
|
FIGURE 9.32 Medial Collateral Ligament.
|
 |
|
FIGURE 9.33 Common Flexor Tendon.
|
 |
|
FIGURE 9.34 Ulnar Nerve.
|
 |
|
FIGURE 9.35 Ulnohumeral Joint.
|
 |
|
FIGURE 9.36 RCL and LUCL.
|
 |
|
FIGURE 9.37 Common Extensor Tendon.
|
 |
|
FIGURE 9.38 Radiohumeral Articulation.
|
brachialis eventually inserting medially on the proximal ulna and the biceps inserting on the proximal radius.
 |
|
FIGURE 9.39 Normal Biceps and Brachialis Tendon.
|
 |
|
FIGURE 9.40 Median and Radial Nerve.
|
on sagittal images, while triangulating on the tendon simultaneously in the coronal and axial planes. The anterior bundle of the MCL is not as well seen on sagittal images but can occasionally be visualized on images just deep to the common flexor tendon. Strain or tears of the flexor muscles distal to the common flexor tendon insertion are also evaluated on sagittal images.
 |
|
FIGURE 9.41 Triceps Tendon.
|
 |
|
FIGURE 9.42 Common Flexor Tendon and Ulnar Nerve.
|
of the LUCL and RCL overlap somewhat on MR images, and occasionally it is difficult to completely distinguish them. The course of the LUCL, as it runs behind the radial head to insert on the supinator crest of the ulna, can be followed on the next three or four successive medial images. The sagittal plane is helpful to confirm or further characterize tears of the lateral ligaments and tendons that are suspected on images obtained in other planes.
 |
|
FIGURE 9.43 Ulnohumeral Articulation.
|
band of the medial collateral ligament (Fig. 9.48B). There is mild tendinosis of the common flexor tendon (Fig. 9.48C). There is mild tendinosis of the common extensor tendon. There is a sprain of the proximal origin of the lateral ulnar collateral ligament with bone marrow edema in the lateral aspect of the capitellum (Fig. 9.48D). There is muscle edema corresponding to the flexor digitorum superficialis muscle, indicating a muscle strain (Fig. 9.48E). There is also mild edema demonstrated in the extensor digitorum muscle (Fig. 9.48F).
 |
|
FIGURE 9.44 Common Extensor Tendon and Lateral Ligament Complex.
|
 |
|
FIGURE 9.45 Radiohumeral Articulation.
|
 |
|
FIGURE 9.46 Biceps and Brachialis.
|
-
Partial undersurface tear of the distal aspect of the anterior band medial collateral ligament. There is a small focus of decreased signal intensity associated with this, which may represent a small osseous avulsion. There is a sprain of the proximal fibers of the anterior band of the medial collateral ligament.
-
There is a sprain of the origin of the lateral ulnar collateral ligament associated with subchondral marrow edema of the lateral aspect of the capitellum.
-
Grade 1 to 2 muscle strain of the brachialis. Mild grade 1 strain of the extensor digitorum muscle and also grade 1 strain of the flexor digitorum superficialis muscle medially.
-
Large hemorrhagic joint effusion with elevation of anterior and posterior fat pads
-
Intact brachialis and biceps tendon distally
 |
|
FIGURE 9.47 Triceps Tendon.
|
 |
|
FIGURE 9.48 Sample Elbow Case.
|
-
The elbow is a tri-arthrodial ginglymus joint.
-
The medial and lateral humeral condyles are the origin of the common flexor and extensor groups of the forearm.
-
The coronoid process is the key to the bony stability of the joint.
-
Posterolateral stability depends on the LUCL.
-
The normal but complex maturation of the elbow ossification centers can be mistaken for pathology.
with the trochlear notch of the ulna in a hinge fashion, allowing flexion and extension of the elbow joint (Fig. 9.49).
 |
|
FIGURE 9.49 ● The bones that form the elbow and proximal radioulnar joints. The radial head articulates with the radial notch of the ulna and the capitellum. The trochlea articulates with the trochlear notch of the ulna. The trochlear notch is composed of the olecranon proximally and the coronoid distally.
|
proximal to the capitellum anteriorly and receives the radial head during flexion (see Fig. 9.50). The coronoid fossa lies just proximal to the trochlea and receives the coronoid process during flexion. Posteriorly, the olecranon fossa receives the tip of the olecranon during extension (Fig. 9.51). The olecranon and coronoid fossae are separated by a thin membrane of bone in about 90% of individuals. A supracondylar process of the humerus is found about 6 cm proximal to the medial epicondyle in 1% to 3% of individuals.16 The supracondylar process may fracture and contribute to median and ulnar nerve entrapment.
 |
|
FIGURE 9.50 ● (A) Coronal section anatomy demonstrating the ulnohumeral joint and the radiocapitellar and the proximal radioulnar joint. (B) The anterior aspect of the extended elbow joint. The anterior capsule has been opened to expose the anterior articular surface. The capitellum and trochlea are separated by the trochleocapitellar groove that articulates with the medial rim of the radial head. The radial fossa of the humerus lies just proximal to the capitellum and receives the radial head during full flexion. The coronoid fossa lies just proximal to the trochlea and receives the coronoid process during full flexion. The anterior bundle of the MCL is seen extending from the anteroinferior aspect of the medial epicondyle to the medial margin of the coronoid. (C) Arthroscopic view demonstrating trochlear groove coronoid and radial head.
|
 |
|
FIGURE 9.51 ● (A) The lateral epicondyle serves as the origin of the lateral collateral ligament complex and the supinator-extensor muscle mass. The prominent medial epicondyle serves as the origin of the MCL and the flexor-pronator muscle mass. (B) The posterior aspect of the flexed elbow joint. The capsule has been opened to reveal the olecranon fossa, which receives the olecranon during full extension. The posterior bundle of the MCL is seen extending from the posteroinferior aspect of the medial epicondyle to the medial margin of the olecranon.
|
the elbow joint. It is for this reason that any fracture of the coronoid process (which forms the anterior lip of the notch) has significant implications for the future stability and motion of the joint. The ulnohumeral articulation allows the hinge-like flexion and extension of the elbow. The trochlear notch is divided longitudinally into medial and lateral facets by a guiding ridge. In most individuals, the trochlear notch is also divided transversely by a bare area that is not covered by a continuous surface of articular cartilage (Fig. 9.52). Therefore, in most individuals there are four articular surfaces of the trochlear notch that articulate with the trochlea of the humerus. A fifth articular surface, known as the radial notch (Fig. 9.53), arises just distal to the coronoid laterally and articulates with the radial head. This radial notch, also known as the lesser sigmoid notch, consists of an arc of about 70° and is oriented perpendicular to the greater sigmoid notch.
 |
|
FIGURE 9.52 ● A) An anterior view of the proximal ulna with the humerus and radius removed shows the articular surfaces of the radial notch and the trochlear notch. The annular ligament extends from the anterior and posterior margins of the radial notch. A bare area that is normally devoid of articular cartilage extends transversely across the midportion of the trochlear notch. (B) The cortical notches create pseudodefects of the trochlear groove on sagittal MR images. These pseudodefects correspond to the medial and lateral edges of the waist of the trochlear groove. The transverse trochlear ridge is nonarticular and may be visualized as a focal convexity along the concave trochlear groove as viewed in the sagittal plane. (C) Pseudodefect seen as a focal defect in the articular surface of the trochlear groove on a T1-weighted sagittal MR arthrogram. The absence of articular cartilage corresponds to normal small cortical notches at the medial and lateral aspects of the waist of the trochlear groove (on either side of the transverse trochlear ridge). The transverse trochlear ridge is nonarticular and represents a bare area. (C reprinted with permission from
Stoller DW. MRI, Arthroscopy, and Surgical Anatomy of the Joints. Philadelphia: Lippincott-Raven, 1999.
) |
 |
|
FIGURE 9.53 ● Lateral perspective of the proximal ulna. The radial notch (lesser semilunar notch) articulates with the radial head and is located lateral to the coronoid process. The LUCL attaches to the supinator crest or crista supinatoris. A transversely oriented, nonarticular trochlear ridge can be visualized on sagittal MR images and varies in prominence.
|
at the proximal radioulnar joint. The anterolateral third of the radial circumference is normally devoid of articular cartilage and lacks strong subchondral bone. It is this portion of the radial head that is most commonly fractured. The radial tuberosity (see Fig. 9.56), at the distal margin of the radial neck, consists of an anterior surface for the bicipitoradial bursa and a posterior aspect where the biceps tendon attaches. The bicipitoradial bursa separates the biceps tendon from the radial tuberosity during full pronation. The pseudodefect of the capitellum (Fig. 9.57) is seen at the radial head-capitellar articulation on posterior coronal and on sagittal images. This is the result of the normal interruption of the posterior capitellar articular surface at the junction with the lateral epicondyle.
 |
|
FIGURE 9.54 ● An oblique longitudinal section of the extended elbow and the proximal radioulnar joint shows the articular surfaces and relations of the joints. The triceps muscle and tendon are seen attaching to the olecranon. The posterior intracapsular fat pad is seen within the olecranon fossa.
|
 |
|
FIGURE 9.55 ● (A) Arthroscopic view demonstrating radial head, annular ligament, and capitellum. (B) T1-weighted axial MR arthrogram showing the anterior and posterior attachments of the annular ligament at the level of the proximal radioulnar joint. (B reprinted with permission from
Stoller DW. MRI, Arthroscopy, and Surgical Anatomy of the Joints. Philadelphia: Lippincott-Raven, 1999.
) |
the lateral epicondyle. The lateral epicondyle appears at about 10 or 11 years of age and fuses at approximately age 14. It initially appears as a thin, vertically oriented sliver that may be mistaken for an avulsion fracture.
 |
|
FIGURE 9.56 ● The cylindrical radial head has a concave surface that articulates with the capitellum. The radial tuberosity is located at the distal medial aspect of the radial neck and serves as the insertion site for the biceps tendon. The radial neck shaft angle (approximately 15°) is formed in the direction opposite to the radial tuberosity.
|
 |
|
FIGURE 9.57 ● (A) The normal pseudodefect of the capitellum is seen at the level of the radial head capitellar joint. Posterior to the lateral capitellar articular margin is a normal groove at the junction of the capitellum and the distal humerus (lateral epicondyle). (B) MR arthrogram. The pseudodefect may be accentuated in extension, during which the lateral aspect of the radial head projects lateral and posterior to the articular surface of the capitellum. The normal groove between the capitellum and the lateral epicondyle produces the pseudodefect on sagittal MR images. (Reprinted with permission from
Stoller DW. MRI, Arthroscopy, and Surgical Anatomy of the Joints. Philadelphia: Lippincott-Raven, 1999.
) (C) Prominent pseudodefect on a posterior coronal FS PD FSE image. |
 |
|
FIGURE 9.58 ● Epiphyseal maturation. (A) Ossification of the capitellum on a T1-weighted coronal image of the left elbow in a 5-year-old child. There is also early ossification (arrows) of the radial head (RH). The trochlea (T) remains unossified. (B) Ossification of the capitellum on a T1-weighted coronal image of the right elbow in a 2-year-old child. The radial head, trochlea, and medial epicondyle (M) remain unossified. The cartilaginous templates of the epiphyses are well seen.
|
of the coronoid process (see Fig. 9.50B). The anterior bundle, which is clearly displayed on coronal images (Fig. 9.61), provides the primary constraint to valgus stress and commonly is damaged in throwing athletes (Fig. 9.62).17,18,19 However, the anterior band has a variable insertion that should not be mistaken for pathology.20
 |
|
FIGURE 9.59 ● (A) Arthroscopic view of intraarticular plica. Lateral plica (fold or fringe) may be symptomatic with a presentation of pain, inflammation, and snapping. (B) Interior exposure of the elbow joint shows the articular surfaces of the capitellum, trochlea, radial head, radial notch, and trochlear notch. A bare area that is normally devoid of articular cartilage extends transversely across the midportion of the trochlear notch. (B reprinted with permission from
Stoller DW. MRI, Arthroscopy, and Surgical Anatomy of the Joints. Philadelphia: Lippincott-Raven, 1999.
) |
 |
|
FIGURE 9.60 ● The medial collateral ligament complex includes the anterior and posterior bands and the transverse ligament (oblique band). The posterior bundle and the transverse ligament lie at the deep margin of the ulnar nerve and make up the floor of the cubital tunnel. The functionally important anterior band (bundle) extends from the inferior aspect of the medial epicondyle to the medial aspect of the coronoid process. The anterior, posterior, and transverse bundles of the medial collateral ligament are shown. (A) Sagittal color illustration. (B, C) FS T1-weighted sagittal MR arthrograms (the image in B is more medial than that in C). (B, C reprinted with permission from
Stoller DW. MRI, Arthroscopy, and Surgical Anatomy of the Joints. Philadelphia: Lippincott-Raven, 1999.
) |
 |
|
FIGURE 9.61 ● (A) MR arthrography. A T1-weighted FS coronal image obtained after intra-articular injection of dilute gadolinium reveals the normal anterior bundle of the MCL attaching to the medial margin of the coronoid process (small black arrows). The inferior margin of the annular ligament (open arrows) is seen at the inferior margin of the radial head. Contrast extends through a defect in the lateral capsule, secondary to detachment of the RCL and the extensor carpi radialis brevis tendon from the anterior aspect of the lateral epicondyle (curved arrow). The anterior (B) and posterior (C) bundles of the medial collateral ligament complex are shown on T1-weighted coronal MR arthrograms. (B, C reprinted with permission from
Stoller DW. MRI, Arthroscopy, and Surgical Anatomy of the Joints. Philadelphia: Lippincott-Raven, 1999.
) |
 |
|
FIGURE 9.62 ● Primary ligamentous stabilizers of the elbow joint. A T1-weighted coronal image reveals a midsubstance rupture of the anterior bundle of the MCL (open arrow). The normal LUCL (arrowheads) is also well seen extending along the posterolateral aspect of the radial head from the lateral epicondyle to the lateral aspect of the ulna. The anterior bundle of the MCL is the primary restraint to valgus stress, whereas the LUCL is the primary restraint to varus stress and posterolateral rotatory stress.
|
-
RCL (Fig. 9.64)
-
Annular ligament (see Fig. 9.64)
-
Variably present accessory lateral collateral ligament
-
LUCL (see Fig. 9.64, Fig. 9.65)
-
The anterior compartment contains the biceps and brachialis muscles (Fig. 9.66), which are evaluated best on sagittal and axial images. The brachialis extends along the anterior joint capsule and inserts on the ulnar tuberosity. The biceps lies superficial to the brachialis and inserts on the radial tuberosity. At the level of the joint line, the biceps is visualized only as a small anteriorly placed tendon.
-
The posterior compartment contains the triceps (see Fig. 9.66) and anconeus (Fig. 9.67) muscles, and they are evaluated best on sagittal and axial images. The triceps inserts on the proximal aspect of the olecranon. The anconeus arises from the posterior aspect of the lateral epicondyle and inserts more distally on the olecranon. The anconeus provides dynamic support to the lateral collateral ligament in resisting varus stress.
-
The medial and lateral compartment muscles are seen best on coronal and axial images:
-
The medial compartment structures (Fig. 9.68) include the pronator teres and the flexors of the hand and wrist that arise from the medial epicondyle as the common flexor tendon. The common flexor tendon provides dynamic support to the MCL in resisting valgus stress.
-
The lateral compartment structures (Figs. 9.69 and 9.70) include the supinator, the brachioradialis, the extensor carpi radialis longus, as well as the extensors of the hand and wrist that arise from the lateral epicondyle as the common extensor tendon.
-
 |
|
FIGURE 9.63 ● (A) The lateral ligament complex consists of the RCL, the annular ligament, a variably present accessory lateral collateral ligament, and the LUCL. (B) Components of the lateral collateral ligament complex. The LUCL origin from the lateral epicondyle is close to the axis of rotation of the elbow, permitting the ligament to remain taut throughout the range of elbow motion. Gross dissection identifies the anterior (C), posterior (D), and transverse (E) bundles of the MCL. (C–E reprinted with permission from
Stoller DW. MRI, Arthroscopy, and Surgical Anatomy of the Joints. Philadelphia: Lippincott-Raven, 1999.
) |
 |
|
FIGURE 9.64 ● Normal lateral collateral ligament complex structures on T1-weighted sagittal (A) and coronal FS PD FSE (B) images. (A reprinted with permission from
Stoller DW. MRI, Arthroscopy, and Surgical Anatomy of the Joints. Philadelphia: Lippincott-Raven, 1999
) |
 |
|
FIGURE 9.65 ● (A) Coronal FS PD FSE image of LUCL origin. (B) The LUCL, which originates more posteriorly than the RCL proper, courses superficial to the annular ligament and attaches onto the supinator crest of the ulna.
|
 |
|
FIGURE 9.66 ● Sagittal section through the ulnohumeral joint.
|
 |
|
FIGURE 9.67 ● Sagittal section of the articulation of the radial head and capitellar joint.
|
 |
|
FIGURE 9.68 ● The medial compartment structures include the pronator teres and the flexors of the hand and wrist, which arise from the medial epicondyle as the common flexor tendon. The common flexor tendon provides dynamic support to the medial collateral ligament complex in resisting valgus stress. (Reprinted with permission from
Stoller DW. MRI, Arthroscopy, and Surgical Anatomy of the Joints. Philadelphia: Lippincott-Raven, 1999.
) |
 |
|
FIGURE 9.69 ● The extensor carpi radialis brevis, the extensor digitorum, the extensor digiti minimi, and the extensor carpi ulnaris arise from the lateral epicondyle as the common extensor tendon. The anconeus and supinator muscles are also shown. The anconeus arises from the posterior aspect of the lateral epicondyle and inserts more distally on the olecranon. The anconeus provides dynamic support to the lateral collateral ligament in resisting varus stress. (Reprinted with permission from
Stoller DW. MRI, Arthroscopy, and Surgical Anatomy of the Joints. Philadelphia: Lippincott-Raven,1999.
) |
 |
|
FIGURE 9.70 ● The muscles of the elbow are divided into anterior, posterior, medial, and lateral compartments. The anterior compartment contains the biceps and brachialis muscles. The posterior compartment contains the triceps and anconeus muscles. The medial compartment contains the pronator teres and the flexors of the hand and wrist, which originate on the medial epicondyle. The lateral compartment contains the supinator, the brachioradialis, the extensor radialis longus, and the extensors of the hand and wrist, which originate on the lateral epicondyle. (Reprinted with permission from
Stoller DW. MRI, Arthroscopy, and Surgical Anatomy of the Joints. Philadelphia: Lippincott-Raven, 1999.
) |
-
The anterior band of the MCL is the primary restraint to valgus stress.
-
MCL rupture frequently occurs with posterior dislocation.
-
The MR “T sign” is indicative of a partial tear at the sublime tubercle, which may be occult to surgical inspection.
-
Strain of the flexor digitorum superficialis frequently accompanies an MCL injury.
-
Lateral compartment bone bruises strongly suggest MCL disruption.
 |
|
FIGURE 9.71 ● Brachial artery and anastomosis about the elbow. The brachial artery courses in the anterior compartment of the arm and gives off collateral arterial branches proximal to the elbow joint. The brachial artery bifurcates into the radial and ulnar arteries at the level of the radial head.
|
 |
|
FIGURE 9.72 ● (A) The anterior bundle of the MCL is the primary restraint to valgus stress. The anterior bundle can be subdivided into an anterior and posterior band, not to be confused with the posterior bundle. The anterior portion of the anterior bundle tightens during extension and the posterior portion tightens during flexion. Medial perspective sagittal color illustration. (B) The anterior bundle consists of parallel collagen bundles in two layers. One layer is between two synovial layers of the joint capsule. A second layer is superficial to the joint capsule and blends with the deep surface of the flexor mass. Coronal color illustration with medial epicondyle sectioned. (C) Origin and course of the anterior bundle on a coronal MR arthrogram.
|
 |
|
FIGURE 9.73 ● (A) In the throwing arm, valgus stress results in compressive forces on the lateral aspect of the elbow and tension forces are produced over the medial aspect of the elbow. (B) A chronically thickened anterior bundle of the MCL in a symptomatic pitcher with medial complex instability. Coronal T1-weighted image.
|
the ligament is incised to inspect the torn capsular fibers.31,32 MR imaging, therefore, is particularly important in localizing these partial tears, which are treated with repair or reconstruction. Detection of partial detachment of the deep undersurface fibers of the anterior bundle (Fig. 9.81) may require intra-articular contrast and MR or CT arthrography.33 The capsular fibers of the anterior bundle of the MCL normally insert on the medial margin of the coronoid process at the sublime tubercle. Undersurface partial tears of the anterior bundle are characterized by distal extension of fluid or contrast along the medial margin of the coronoid, producing the so-called T sign.33 FS images may show marrow edema due to a stress response at the humeral origin or the coronoid attachment. It is important to differentiate partial tears from an anatomically variant distal MCL insertion (Fig. 9.82).
 |
|
FIGURE 9.74 ● MCL rupture. (A) T1-weighted and (B) STIR coronal images, as well as (C) a PD axial image, reveal a midsubstance rupture of the anterior bundle of the MCL (arrows). A strain of the adjacent flexor digitorum superficialis muscle is also noted.
|
 |
|
FIGURE 9.75 ● Proximal anterior bundle tear on coronal (A) and sagittal (B) FS PD FSE images.
|
 |
|
FIGURE 9.76 ● Distal avulsion of the anterior bundle of the MCL (A) and disruption of the posterior bundle of the MCL (B) on coronal FS PD FSE images.
|
 |
|
FIGURE 9.77 ● Proximal anterior bundle avulsion and heterotopic ossification on T1-weighted coronal image.
|
 |
|
FIGURE 9.78 ● (A) The MCL, the radiocapitellar joint, and the olecranon resist forces acting across the elbow joint. In the overhead athlete the olecranon is subjected to medial shearing forces with valgus stress. Valgus laxity (with resulting valgus extension overload) is associated with osteophyte formation and loose bodies. (B) Valgus stress with tear of the anterior bundle of the MCL and subchondral capitellar and lateral epicondylar edema. Coronal FS PD FSE image.
|
-
Heterotopic ossification indicated by increased fat signal of bone marrow in the ligament (Fig. 9.83) or hypointensity in sclerotic ossification
-
Increased signal within the ligament, usually in the anterior bundle on FS PD FSE images (Fig. 9.84)
-
A chronically thickened ligament (anterior bundle), hyperintense on T1- or PD-weighted images and hypointense on FS PD FSE images (Fig. 9.85)
-
Epicondylar avulsion, including visualization of corticated structures, the donor site on the humerus, surrounding edema and Little Leaguer's elbow, and a hyperintense sublime tubercle from stress response avulsive stress
-
Stress response or fracture at the humeral origin site or attachment site on ulnar coronoid
-
Synovitis, intermediate to hyperintense on FS PD FSE images
-
A traction spur (hyperintense on T1- and PD-weighted images if it contains marrow and hypointense if it is calcified)
-
Associated lateral impaction injury, on T1- and PD-weighted images indicated by hypointense (if edematous) capitellum, hypointense subchondral cysts (in chronic injury), and hypointense subchondral sclerosis, hypointense loose bodies (especially in osteoarthritis)
-
Possible visualization of discontinuity of fibers in a complete tear
-
Possible hypertrophy of sublime tubercle
-
Hyperintense associated flexor-pronator (FPG) strain or tear on FS PD FSE images (Figs. 9.86 and 9.87)
 |
|
FIGURE 9.79 ● Acute rupture of the distal anterior bundle associated with a brachialis muscle strain. (A) Sagittal color illustration, medial perspective. (B) Coronal FS PD FSE image. (C) Axial FS PD FSE image.
|
 |
|
FIGURE 9.80 ● Assessment of acute MCL injury with involvement of both anterior and posterior bundles. There are proximal tears of both the anterior and posterior bundles. In the acceleration phase of overhead throwing, the increased valgus stress to the elbow joint is associated with injury to the MCL. At 90° of flexion the MCL provides more than 50% of the resistance to valgus stress. (A) Coronal FS PD FSE image. (B) Axial FS PD FSE image.C) Sagittal FS PD FSE image. (D) Sagittal color illustration.
|
 |
|
FIGURE 9.81 ● Tear of the deep portion of the anterior bundle from its ulnar insertion. The T sign is characterized by the extension of fluid or contrast between the distal anterior bundle undersurface and the adjacent bone. Coronal FS PD FSE image.
|
 |
|
FIGURE 9.82 ● (A) Distal attachment of the anterior bundle MCL to the coronoid process without extension of contrast. This may be seen as a normal variant or secondary to degenerative changes in the ligament. Coronal FS T1-weighted MR arthrogram. (B) Normal flush sublime tubercle attachment of the anterior bundle shown for comparison. Coronal FS T1-weighted MR arthrogram.
|
 |
|
FIGURE 9.83 ● Chronic anterior bundle MCL proximal tear with heterotopic ossification within the substance of the ligament. (A) Coronal T1-weighted FSE image. (B) Axial T1-weighted FSE image.
|
 |
|
FIGURE 9.84 ● Hyperintensity in a partial tear of the anterior bundle and anterior fibers of the posterior bundle of the MCL. (A, B) Coronal FSE PD FSE images. (C) Sagittal FS PD FSE image.
|
 |
|
FIGURE 9.85 ● Acute or chronic injury with an incompetent thickened anterior bundle of the MCL. Note the thin posterior bundle morphology in comparison. (A) Coronal FS PD FSE image. (B) Axial FS PD FSE image.
|
 |
|
FIGURE 9.86 ● Flexor digitorum superficialis (A) and pronator teres muscle (B) strain in association with acute proximal tear of the anterior bundle of the MCL. Coronal FS PD FSE images.
|
 |
|
FIGURE 9.87 ● Flexor digitorum superficialis strain in association with an anterior bundle tear of the MCL. Coronal FS PD FSE image.
|
-
Extravasation of contrast in complete ruptures
-
Extension of contrast around the sublime tubercle in distal deep partial tears
-
“T sign” on coronal images in distal partial tears that spare the superficial fibers (caused by fluid tracking between the torn fibers and the medial aspect of the coronoid process)
 |
|
FIGURE 9.88 ● Traditionally MCL avulsions were treated by reattaching the ligament to bone through drill holes and midsubstance ruptures were treated with primary repair. Current recommendations now suggest MCL reconstruction for both acute and chronic injuries. MCL reconstruction uses a graft (e.g., palmaris longus) through tunnels in the ulna at the sublime tubercle and in the humeral epicondyle. Divergent drill holes in the medial epicondyle originate from the isometric point of the MCL. Coronal T1-weighted image of MCL graft reconstruction.
|
-
This is a midlife overuse injury.
-
There is a frequent association with ulnar neuritis.
-
Medial epicondylitis is a degenerative disorder, similar to supraspinatus and patellar tendinosis, and there is no inflammatory response.
 |
|
FIGURE 9.89 ● Muscle strain and partial tear of the MCL. T2*-weighted sagittal (A) and coronal (B) images reveal increased signal within the flexor digitorum superficialis muscle (large arrow), as well as partial tearing of the anterior bundle of the MCL from the coronoid (open arrow). The common flexor tendon (curved arrow) is intact. (C) Sagittal color illustration, medial perspective. In the evaluation of medial epicondylitis, especially in the throwing athlete, the MCL should be assessed for instability secondary to excessive valgus forces.
|
 |
|
FIGURE 9.90 ● (A) Microtear of the humeral head origin of the pronator teres with tendon degeneration and associated inflammatory synovitis. Angiofibroblastic hyperplasia is characterized by gray and friable pathologic tissue. (B, C) Medial epicondylitis with tendinosis of the common flexor tendon and pronator teres muscle strain. (B) Coronal T1-weighted image. (C) Coronal FS PD FSE image.
|
 |
|
FIGURE 9.91 ● (A) Findings in medial epicondylitis findings include macroscopic partial or complete tearing of the flexor-pronator origin and tears of the MCL. Overuse tendinopathy of the flexor-pronator group is due to chronic valgus stress and is therefore often associated with throwing sports. (B–D) Tendinosis with partial tendon tearing and muscle strain affecting the flexor-pronator musculature. Associated anterior bundle pathology is also demonstrated. Muscle strain may contribute to increased transmission of forces to the medial collateral ligament. (B, C) Coronal FS PD FSE images. (D) Axial FS PD FSE image.
|
-
Intermediate-signal-intensity tendon with or without reactive epicondylar edema (hypointense on T1- or PD-weighted images and hyperintense on FS PD FSE images)
-
Partial MCL tear
-
Synovitis, intermediate to hyperintense on FS PD FSE images
-
Thickened tendon (see Fig. 9.92)
-
Discontinuous fibers possibly seen in tendon tear, hyperintense MCL on FS PD FSE images if the tear is acute (see Fig. 9.93)
-
Flexor-pronator group (FPG) swelling and edema
-
Associated lateral impaction injury (see Fig. 9.93B) with subchondral cysts (in chronic injury), subchondral sclerosis, and loose bodies (especially in osteoarthritis)
-
Associated ulnar neuritis, indicated by hyperintensity on FS PD FSE images and thickening of the nerve, usually within the cubital tunnel
 |
|
FIGURE 9.92 ● Common flexor tendinosis characterized by tendon degeneration. Coronal FS PD FSE image.
|
 |
|
FIGURE 9.93 ● (A) Complete tear of flexor-pronator origin from the medial epicondyle. Medial epicondylitis is the result of a repetitive stress or overuse of the flexor-pronator musculature. Chronic repetitive concentric and eccentric contractile loading of the flexor-pronator group results in degenerative musculotendinous changes, including tendinous failure. The pronator teres and the flexor carpi radialis muscles are frequently involved in this degenerative process. Larger diffuse tears with tendon rupture may also involve the palmaris longus, flexor digitorum superficialis, and flexor carpi ulnaris. Coronal color graphic. (B, C) Flexor digitorum superficialis tendon tear with pronator teres strain. Associated laxity in the torn distal anterior bundle is demonstrated. (B) Coronal FS PD FSE image. (C) Axial FS PD FSE image.
|
 |
|
FIGURE 9.94 ● Little Leaguer's elbow is an extension overload injury (valgus stress) with medial epicondylar avulsion. The childhood injury pattern is microtrauma to the apophysis and ossification center of the medial epicondyle. The adolescent pattern is an avulsion of the medial epicondyle and possible nonunion injury.
|
-
A zone of separation through the epicondylar region with or without epicondylar edema
-
Presence of a discontinuous MCL, indicating a tear
-
Variable signal within the fragment, depending on whether there is sclerosis or edema
-
Marrow edema in the parent bone (e.g., humerus)
-
Fluid signal in MCL tears (tears may be complete or incomplete)
 |
|
FIGURE 9.95 ● Little Leaguer's elbow in a 10-year-old. In a type 1 medial epicondylar avulsion the entire apophysis separates and rotates. In a type 2 avulsion there is a smaller “flake” avulsion. Coronal FS PD FSE image.
|
-
Although common in athletes, lateral epicondylitis more frequently occurs in the general population.
-
The extensor carpi radialis brevis is the tendon most often affected.
-
Signal changes caused by steroid injection should not be confused with edema.
-
MR is the best imaging modality for identifying high-grade lesions that are unlikely to respond to conservative therapy.
and partially avulsed (Fig. 9.99) from the lateral epicondyle.49 Scar tissue, formed in response to the partial avulsion, is susceptible to further tearing with repeated trauma. Histologic studies of surgical specimens of patients with lateral epicondylitis show angiofibroblastic tendinosis with a lack of inflammation, suggesting that the abnormal signal seen on MR images is secondary to tendon degeneration and repair rather than to inflammation.34,43 Signal alteration in the region of a local steroid injection should not be confused with primary muscle pathology on MR imaging.
 |
|
FIGURE 9.96 ● Flake avulsion of the medial epicondyle in a 14-year-old baseball pitcher. (A) Axial PD FSE image. (B) Axial FS PD FSE image.
|
 |
|
FIGURE 9.97 ● (A) Anatomic relationship of the extensor carpi radialis longus, extensor carpi radialis brevis, brachioradialis, and extensor digitorum communis to the underlying radiocapitellar joint. Common extensor tendinosis on coronal T1-weighted (B) and FS PD FSE (C) MR arthrograms.
|
 |
|
FIGURE 9.98 ● (A) Partial tear with granulation tissue (angiofibroblastic tendinosis) at the origin of the extensor carpi radialis brevis. There is also tendinosis of the extensor digitorum communis. Lateral color graphic. (B) Coronal FS PD FSE image with partial tear of the extensor carpi radialis brevis. This is a degenerative and not an inflammatory process.
|
Tendinosis and tearing typically involve the extensor carpi radialis brevis portion of the common extensor tendon anteriorly. In complete tears, there is a fluid-filled gap separating the tendon from its adjacent bony attachment site.
 |
|
FIGURE 9.99 ● Partial tearing of the common extensor tendon. MR arthrography with injection of dilute gadolinium into the elbow joint. There is contrast delineating high-grade tearing of the extensor carpi radialis brevis from the lateral epicondyle (arrows) on this FS T1-weighted coronal image.
|
-
Increased thickening and signal intensity changes of the common extensor tendon origin at the lateral epicondyle (see Fig. 9.99)
-
Loose bodies
-
Edema and/or fracture lines after trauma
-
Synovitis and a thickened synovium
-
Variable disruption of fibers
-
Macroscopic tear of the extensor carpi radialis brevis, with or without tears of the extensor digitorum communis (Fig. 9.100)
-
Strains (Fig. 9.101) and tears (Fig. 9.102) of the LUCL in advanced cases
-
Associated extensor muscle belly strain (Fig. 9.103)
-
Osteochondral injuries, including fracture/trabecular injury in posterior dislocation, capitellar injury, humeral trochlear injury, injury of the coronoid process, and injury of the radial head/neck
-
Grade I: reversible tendon changes
-
Grade II: nonreversible changes
-
Grade III: tendon rupture
-
Grade IV: additional fibrosis and calcification
-
LUCL disruption is not the only essential lesion in posterolateral rotatory instability (PLRI).
-
Both the RCL and the LUCL play a significant role in preventing PLRI of the elbow.
-
Proximal tears of the RCL and LUCL result in symptomatic PLRI.
-
The ulna-humerus-radius articulation
-
The MCL complex
-
The lateral collateral ligament complex
-
The capsule
 |
|
FIGURE 9.100 ● In lateral epicondylitis, angiofibroblastic tendinosis (disorganized, immature collagen formation with immature fibroblastic and vascular elements) is secondary to eccentric or concentric overloading of the extensor muscle mass. The extensor digitorum communis may also be involved in addition to the preferentially affected extensor carpi radialis brevis. (A) Sagittal color illustration of lateral epicondylitis. (B) Coronal T1-weighted image. C) Coronal FS PD FSE image. (D) Sagittal T1-weighted image.
|
 |
|
FIGURE 9.101 ● Common extensor tendon tear with discontinuity of the radial collateral ligament and degeneration of the proximal origin of the LUCL on coronal (A) and sagittal (B) T1-weighted MR arthrograms.
|
 |
|
FIGURE 9.102 ● (A) A complete, massive tear of the common extensor tendon and lateral collateral ligament complex except for the annular ligament. Lateral epicondylitis is a degenerative tendinopathy. The term “epicondylitis” is misleading as inflammation is present only in the initial stages of the disease. Angiofibroblastic tendinosis is present with partial to complete tearing of the extensor carpi radialis brevis tendon. Lateral color illustration. (B) Massive tear of the common extensor tendon and underlying lateral collateral ligament. MR arthrography with injection of dilute gadolinium into the elbow joint. There is contrast extending through a large defect in the lateral capsule and common extensor tendon (arrows) on this FS T1-weighted coronal image.
|
 |
|
FIGURE 9.103 ● (A) Extensor carpi radialis brevis muscle overloading is seen with a grade 1 muscle strain distal to the elbow joint on an axial FS PD FSE image. (B, C) Torn LUCL in a patient whose symptoms of instability and pain worsened after extensor tendon release. T1-weighted (B) and FS T1-weighted (C) coronal images obtained after intravenous injection of gadolinium reveal complete absence of the common extensor tendon and LUCL adjacent to the lateral epicondyle (curved arrow). Micrometallic artifact is noted from prior surgical release (open arrow).
|
 |
|
FIGURE 9.104 ● Arthroscopic view of a capsular rent or linear tear in association with lateral epicondylitis.
|
-
Consider posterior interosseous nerve impingement.
-
Lateral synovial plicae may be symptomatic.
-
This injury can be missed in children because of spontaneous reduction.
-
Associated fractures of the coronoid, radial head, and humeral condyles may occur.
-
Instability starts posterolaterally but may progress medially to involve the entire joint.
-
The primary lesion of instability is no longer considered isolated insufficiency of the LUCL. The RCL and LUCL together represent the main restraint to PLRI.
-
MCL tearing occurs late.
-
Clinical evaluation is difficult.
than in adults.72,73 Many dislocations in children go unrecognized because there is spontaneous reduction and the only finding is a swollen tender elbow.57 MR imaging in such cases usually shows an effusion as well as a contusion or strain of the brachialis muscle. Bone contusions may be seen at the posterior margin of the capitellum as well as at the radial head and coronoid process (Fig. 9.111).
 |
|
FIGURE 9.105 ● Coronal T1-weighted MR arthrogram (A) and a coronal T1-weighted FSE image (B) showing the intact LUCL. Elbow dislocation is the most common cause of lateral collateral ligament complex insufficiency. Sports-related (usually contact sports) injuries of the lateral collateral ligament complex occur secondary to a collision or fall on an outstretched hand. Acute varus stress may also cause lateral collateral ligament complex disruption but is less common than elbow dislocation.
|
 |
|
FIGURE 9.106 ● (A) Tear of the LUCL of the lateral collateral ligament complex of the elbow. The other components of the lateral collateral ligament complex include the radial collateral ligament, the annular ligament, and the accessory lateral collateral ligament. Lateral instability of the elbow ranges from mild laxity to recurrent dislocation. (B) LUCL insufficiency with high-grade partial tear of the common extensor tendon and partial tear at the origin of the LUCL with resultant ligamentous laxity. Coronal FS PD FSE image. C) In posterolateral rotatory instability (PLRI), the proximal ulna and radial head externally rotate about the distal humerus when the forearm is positioned in supination and slight flexion. On this sagittal T1-weighted MR image, the radial head is translating posterior to the capitellum.
|
 |
|
FIGURE 9.107 ● Tear of both the RCL (A) and LUCL (B) proximally. Coronal FS PD FSE images.
|
 |
|
FIGURE 9.108 ● External rotation of the ulna on the humerus plus posterolateral radius-humerus subluxation is a common pathway to elbow dislocation. The pathoanatomy of injury, as demonstrated on this coronal FS PD FSE image, involves a circle of soft-tissue disruption from the LUCL to the anterior bundle of the MCL.
|
 |
|
FIGURE 9.109 ● Radiohumeral meniscus. (A) An FS PD FSE coronal image shows a prominent meniscus-like structure (arrow) that extends into the lateral margin of the radiohumeral joint. (B) A thin lateral synovial fringe is seen in a different patient on an FS T1-weighted coronal image obtained after intra-articular injection of gadolinium. (C) The lateral synovial fringe or radiocapitellar meniscus is not a true fibrocartilaginous meniscus. Located posterolaterally, the synovial fringe may become inflamed or thickened. Symptoms of thickening and inflammation may mimic tennis elbow.
|
 |
|
FIGURE 9.110 ● Anterior dislocation associated with a direct blow to the posterior aspect of a flexed elbow in a child. The olecranon is forced anterior to the distal humerus. The radial head is shown anterior to the capitellum on a T1-weighted sagittal image (A) and an axial FS PD FSE image (B).
|
and may then dislocate. This mechanism involves hypersupination, valgus stress, and axial compressive loading of the elbow, all of which may occur during a fallK on the outstretched hand.
 |
|
FIGURE 9.111 ● (A) Contusions and fractures associated with elbow dislocations may involve the radial head, the coronoid process, and occasionally the humeral epicondyles. The “terrible triad” complex dislocation that occurs is associated with the lateral collateral ligament complex and capsular tear involves a radial head fracture, a coronoid fracture, and an MCL tear. (B, C) Elbow dislocation demonstrating anterior radial head fracture and posterior capitellar contusion in stage 1 posterolateral instability. (B) Sagittal PD FSE image. (C) Sagittal FS PD FSE image.
|
-
Stage 1: There is posterolateral subluxation of the ulna and radius relative to the humerus, with disruption of the LUCL. Ruptures of both the LUCL and RCL, however, are considered essential for the development of PLRI. If the RCL and annular ligament are intact (Fig. 9.113), however, the LUCL may be injured without instability. Thus, a positive pivot shift may require the entire lateral collateral ligament complex to be disrupted (Fig. 9.114).
-
Stage 2: There is incomplete dislocation, so that the coronoid appears perched on the trochlea. There is further disruption of the lateral ligamentous structures in stage 2 as well as tearing of the anterior and posterior joint capsule (see Fig. 9.114).
-
Stage 3: There is complete posterior dislocation with progressive disruption of the MCL complex. Stage 3 is further subdivided into two categories:
-
Stage 3A: Disruption of the posterior bundle of the MCL only; the elbow is stable to valgus stress (Fig. 9.115)
-
Stage 3B: Disruption of the anterior bundle of the MCL so that the elbow is unstable in all directions (Fig. 9.116)
-
-
Increased marrow signal in areas of edema
-
High-signal-intensity joint effusions with or without periarticular leakage with capsular rupture
-
Disruption of the MCL anatomy with high-signal tears
-
High-signal-intensity stress lesions at the bony attachments of torn ligaments
-
Edema/disruption of neurovascular structures
-
Disruption of the LCL (RCL/LUCL) anatomy with high-signal-intensity tears
-
Associated medial epicondylar avulsion (in children) with hyperintense surrounding edema
-
Associated fractures include “kissing” fractures of the radial head and capitellum and coronoid fractures.
-
Associated muscle strains (Fig. 9.118)
laxity, posterolateral instability, heterotopic ossification, flexion contractures, and median and ulnar nerve palsies. Volkmann's ischemic contracture and brachial artery injury may also complicate posterior elbow dislocation.
 |
|
FIGURE 9.112 ● The circle of Horii representing the progression of structures injured in elbow dislocation. The lateral ligamentous complex is torn first, followed by the anterior and posterior capsule, with the dislocation completed by disruption of the MCL. The MCL is the final structure to be injured and may be intact in the presence of posterolateral instability. (A) Coronal color illustration with anterior perspective. (B) Superior view of the trochlear notch, radial head, collateral ligaments, and capsule with the humerus removed.
|
 |
|
FIGURE 9.113 ● Disruption of the LUCL and common extensor tendon associated with a posterior dislocation. If the annular ligament and RCL are intact, the LUCL may be torn without resultant instability.
|
 |
|
FIGURE 9.114 ● (A, B) Coronal FS PD FSE images showing rupture of the RCL proper with abnormal laxity of the LUCL in posterolateral instability (PLRI). Both the radial collateral ligament and the LUCL have key roles in preventing PLRI of the elbow. (C) Lateral color illustration of “perched elbow” in PLRI. In stage 1 PLRI there is posterolateral subluxation of the ulna and radius relative to the humerus in association with a tear of the LUCL. In stage 2 PLRI the coronoid process is perched on the trochlea. In stage 3A there is complete dislocation with tear of the posterior bundle of the MCL. In stage 3B PRLI there is a complete dislocation with tear of the entire medial collateral ligament complex with the elbow unstable in all directions. (D, E) Stage 2 instability in a 13-year-old child with a history of instability and recurrent elbow dislocation.
|
 |
|
FIGURE 9.115 ● Stage 3A posterolateral instability with complete dislocation and tearing of the entire lateral collateral ligament complex, including the annular ligament. The posterior bundle of the MCL was torn but the anterior bundle was still intact. (A, B) Coronal PD FSE images. (C) Sagittal FS PD FSE image.
|
 |
|
FIGURE 9.116 ● Complete posterior dislocation with disruption of the entire medial collateral ligament complex in stage 3B posterolateral instability. (A) Sagittal color illustration. (B) Coronal FS PD FSE image. (C) Sagittal FS PD FSE image.
|
 |
|
FIGURE 9.117 ● When the cubital tunnel retinaculum is torn, the ulnar nerve may undergo subluxation. The ulnar nerve is more commonly compromised than the anterior interosseous branch of the median nerve in posterior dislocations. Neurapraxia may occur in up to 20% of cases and primarily involves the ulnar nerve. Axial FS PD FSE image.
|
 |
|
FIGURE 9.118 ● Pronator teres strain in association with a posterior dislocation. (A) Sagittal FS PD FSE image. (B) Axial FS PD FSE image.
|
-
The coronoid is the keystone of bony stability of the elbow.
-
Coronoid fractures are commonly associated with elbow dislocation.
-
Fractures are graded I to III. Grade III has a poor prognosis.
evaluated when a coronoid process fracture is identified on MR examination.
 |
|
FIGURE 9.119 ● (A) Lateral color illustration and (B) Sagittal FS PD FSE image of a coronoid process fracture. (B) A coronoid (Regan/Morrey) fracture is a distraction injury due to bony avulsion of the brachialis insertion by posterior dislocation of the ulna. The etiology is related to a fall on an outstretched arm and hyperextension of the elbow.
|
 |
|
FIGURE 9.120 ● (A) Sagittal color illustration shows that a coronoid process fracture occurs as a shear injury from trochlear contact during posterior dislocation. (B, C) Coexisting coronoid and radial head fractures and MCL injury as part of the “terrible triad” are associated with a posterior dislocation event. Sagittal FS PD FSE images.
|
-
Type I fractures: Type I fractures are small shear fractures that do not destabilize the joint. They should be recognized, however, as an indicator of posterior elbow dislocation/subluxation injury that may be associated with significant soft-tissue disruption.
-
Type II fractures: Type II fractures involve less than 50% of the coronoid. Fixation is necessary if the joint remains dislocated or subluxed, but type II fractures may otherwise be managed conservatively with early mobilization.
-
Type III fractures: Type III fractures involve more than 50% of the coronoid and have a poor prognosis.
-
Fractures of the radial head are the most common elbow fracture in adults.
-
The typical location is the anterolateral margin of the head.
-
In association with distal radioulnar joint dislocation, the injury is called the Essex-Lopresti fracture.
-
If radial head excision is planned, it is essential to check MCL integrity.
-
Grade I: fracture with less than 2 mm of displacement (Fig. 9.121)
-
Grade II: fracture with more than 2 mm of displacement
-
Grade III: comminuted fracture (Fig. 9.122)
-
Grade IV: comminuted and dislocated fracture
 |
|
FIGURE 9.121 ● (A) Radial head fracture as a result of an applied axial load from a fall on an outstretched hand. Grade I fractures of the radial head are nondisplaced or minimally displaced (less than 2 mm of offset). Type II are displaced but reconstructible. Type III fractures are severely comminuted and unreconstructible. Fractures that involve less than 30% of the radial head are stable unless they are associated with more than 2 mm of incongruity. (B, C) Radial head fracture. T1-weighted coronal (B) and axial (C) images reveal a minimally displaced, comminuted fracture of the radial head. The ligaments about the elbow are normal.
|
-
Fracture of the capitellum is rare.
-
It is important to differentiate a subtle fracture from a capitellar pseudodefect.
-
Type I injuries (Hahn-Steinthal fractures): coronal fractures without trochlear involvement
-
Type II injuries (Kocher-Lorenz fracture): coronal fractures with a sleeve avulsion of the articular cartilage (Fig. 9.123)
-
Type III injuries (Broberg-Morrey fracture): comminuted type I injuries
-
Type IV injuries (McKee fracture): type III injuries with trochlear involvement
 |
|
FIGURE 9.122 ● Radial head fracture with comminution. These fractures may be associated with posterior elbow dislocation. The anterolateral aspect of the radial head is more vulnerable to injury because of a relative lack of subchondral bone.
|
-
Olecranon fractures are frequently high-energy injuries, so a diligent check for collateral damage should be made.
-
Injuries in adolescence may result in an os supratrochleare dorsale.
-
The relationship to the ulnar trochlear notch determines fracture stability.
 |
|
FIGURE 9.123 ● Type II Kocher-Lorenz fracture with a superficial layer of subchondral bone and attached articular cartilage. This type of fracture may be difficult to identify on conventional radiographs. Lateral color illustration.
|
 |
|
FIGURE 9.124 ● (A) Transverse fracture originating in the middle third of the olecranon fossa. (B) Coronoid fractures are associated with olecranon fractures in transolecranon fracture dislocations. Sagittal FS PD FSE image.
|
-
In children the medial epicondyle may be displaced into the joint.
-
The trochlear ossification center should not be mistaken for a displaced medial epicondyle.
-
If the medial epicondyle is displaced, the status and position of the ulnar nerve should be carefully assessed.
-
Involvement of the trochlear ridge determines the stability of the fracture.
point of the elbow in flexion, with impact on the olecranon, is another mechanism of injury. A varus stress or axial load may also cause a fracture through the trochlea that extends to the medial condyle, although this mechanism is seen more typically in adults. If the injury involves only the epicondyle it may be displaced inferiorly, due to the pull of the attached flexor muscles (Fig. 9.127). T1- or PD-weighted images demonstrate marrow fat signal intensity of the avulsed medial epicondyle in the older child and adolescent pattern of injury (Fig. 9.128).
 |
|
FIGURE 9.125 ● Transverse olecranon stress fractures are related to triceps traction with an extension force. Olecranon stress fractures are associated with pain during the acceleration phase of throwing. (A) Sagittal T1-weighted image. (B) Sagittal FS PD FSE image.
|
If this diagnosis is missed and the injury is treated nonsurgically, a poor functional result can be anticipated.95 MR imaging, rather than arthrography, can be used to exclude involvement of the unossified trochlear cartilage. This type of injury is most common in adolescents, accounting for 10% of all elbow fractures.
 |
|
FIGURE 9.126 ● Medial epicondylar fracture involving the ossification center of the medial epicondyle in a 7-year-old. These injuries are often associated with repetitive throwing activity. Acute valgus stress is a less common mechanism of injury. Coronal PD FSE image.
|
 |
|
FIGURE 9.127 ● Avulsion fracture of the medial epicondyle in a 5-year-old boy with medial elbow pain after a fall on his outstretched arm. Radiographs (not shown) revealed partial ossification of the capitellum, radial head, and medial epicondyle with apparent distal displacement of the medial epicondyle compared with the uninjured elbow. T1-weighted (A) and STIR (B) coronal images reveal distal avulsion of the medial epicondylar apophysis (arrows) that is just beginning to ossify. The fracture (curved arrows) is well delineated on the STIR sequence. C, capitellum.
|
 |
|
FIGURE 9.128 ● Medial epicondylar avulsion fractures are the most common fracture in the juvenile or adolescent throwing athlete. The mechanism of medial epicondylar apophyseal failure is medial traction from an acute valgus stress associated with forceful mass muscle contraction of the flexor-pronator group muscles. (A) Coronal T1-weighted image. (B) Axial FS PD FSE image.
|
-
Type I: The lateral trochlear ridge is intact and mediolateral stability is preserved (Salter-Harris type II fracture).
-
Type II: The fracture originates in the capitotrochlear sulcus and the lateral trochlear ridge is part of the fracture. These fractures are unstable (Salter-Harris type IV fracture).
-
Type I: Type I fractures are incomplete and nondisplaced.
-
Type II: The fracture is through the epiphysis.
-
Type III: The lateral condyle rotates or is displaced.
-
This is the most common pediatric elbow injury.
-
The median nerve and brachial artery are at risk.
-
Type I: Type I fractures, which account for 30% of cases, are nondisplaced.
-
Type II: Type II fractures, which account for 24% of cases, are displaced but the posterior cortex is intact.
-
Type III: Type III fractures, which account for 45% of cases, are displaced posterior fractures with complete cortical disruption.
 |
|
FIGURE 9.129 ● Nondisplaced supracondylar fracture as a simple extra-articular fracture of the distal humerus. (A) Coronal color graphic. (B) Coronal FS PD FSE image.
|
-
This is the most common physeal injury and is usually a Salter-Harris type IV.
-
May be invisible on radiographs or CT
-
A missed fracture may result in significant loss of function or deformity.
-
Type I: The lateral trochlear ridge is intact and mediolateral stability is preserved (Salter-Harris type II fracture).
-
Type II: The fracture line is medial to the trochlear groove and the lateral trochlear ridge is part of the fragment. These are usually unstable (Salter-Harris type IV) fractures, but stability may be preserved by an articular cartilage hinge even in the presence of a complete physeal injury.
-
Type 1: Fracture is incomplete and nondisplaced.
-
Type 2: Fracture extends through the epiphysis and the fragment is laterally displaced.
-
Type 3: The lateral condyle rotates or is completely displaced.
MR imaging with GRE technique may be useful in depicting physeal bars and growth arrest after growth plate injuries.103
-
Capitellar Osteochondritis Dissecans
-
Osteochondritis dissecans frequently leads to arthrosis.
-
The lesion typically occurs anteriorly and should not be confused with capitellar pseudodefect, which occurs posteriorly.
-
Contrast-enhanced sagittal images are used to characterize and stage the lesion.
 |
|
FIGURE 9.130 ● Radiographically subtle elbow fracture. T1-weighted coronal image reveals a Salter-Harris type II fracture (arrows) with mild lateral displacement of the lateral humeral condyle (curved arrow). The fracture does not extend into the capitellum (C). This 5-year-old child did well with closed reduction. R, unossified radial head cartilage; T, unossified trochlear cartilage.
|
the posterolateral aspect of the radial head and the capitellum is a normal feature of the extended elbow joint. However, this further creates the illusion of an osteochondral defect as the cartilage of the radial head opposes the rough nonarticular portion of the humerus.
 |
|
FIGURE 9.131 ● (A) Osteochondritis dissecans is seen with chronic valgus stress and lateral impaction in gymnasts and adolescent pitchers. The end arteries of the capitellum are vulnerable to necrosis and there are increased rotatory and axial loading forces in the throwing athlete. Radiocapitellar compressive and shearing forces occur during the acceleration and deceleration phases of overhead throwing. (C–D) Osteochondritis dissecans with complete fragment separation as a loose body in the olecranon fossa. (B) Coronal T1-weighted image. (C) Coronal FS PD FSE image. (D) Axial FS PD FSE image.
|
 |
|
FIGURE 9.132 ● Unstable lesions tend to be greater than 1 cm and are associated with a loose fragment or hyperintense fluid interface insinuating beneath or undermining the fragment. (A) Sagittal FS PD FSE image. (B, C) Separate case of detached osteochondral fragment in an unstable osteochondritis dissecans lesion. (B) Sagittal T1-weighted image. (C) Sagittal FS PD FSE image.
|
 |
|
FIGURE 9.133 ● Pseudodefect of the capitellum should not be mistaken for an osteochondral defect or osteochondritis dissecans. There is a normal transition between the capitellum and lateral epicondyle. (A) Sagittal T1-weighted MR arthrogram. (B) Coronal T1-weighted MR arthrogram.
|
-
Grade 1: The hyaline cartilage covering the lesion remains intact.
-
Grade 2: There is an enhancing zone of separation, but these are considered stable lesions.
-
Grade 3: Fluid is seen in the zone of separation. These lesions are unstable.
-
Grade 4: There is a loosened and displaced fragment of bone.
-
Panner's disease occurs in childhood as opposed to osteochondritis dissecans, which typically occurs in adolescence.
-
The condition is similar to Legg-Calvé-Perthes disease of the hip in that patients may recover with little or no deformity.
-
The elbow is a common site for loose bodies.
-
Air bubbles may simulate loose bodies but are identified by susceptibility artifact on GRE sequences.
-
Loose bodies are most commonly found anteriorly or lodged in the trochlear notch.
notch of the ulna), the midportion of which contains a variably sized bare area that is normally devoid of articular cartilage. A deep groove with a strip of synovium and fat extends transversely across the trochlear notch at the junction of the olecranon and the coronoid portions of the proximal ulna (Fig. 9.140). Loose bodies may lodge in this transverse groove or at the margins of this groove, where the ulna is normally somewhat constricted. This normal waist or constriction in the midportion of the trochlear notch should not be mistaken for an osteochondral defect (see Fig. 9.140). A thin transverse bony ridge may also extend across this portion of the trochlear notch, which lacks articular cartilage and should not be mistaken for an intra-articular osteophyte or a loose body on a single sagittal MR image.123
 |
|
FIGURE 9.134 ● Panner's disease is seen in a younger patient population (5- to 11-year-old patients) compared to osteochondritis dissecans. Loose body formation and residual deformity are not usually seen in Panner's disease. Panner's disease is associated with avascular necrosis secondary to trauma.
|
-
Low-signal round structures with variable internal high signal equivalent to fat (marrow) and adjacent moderate-signal synovial thickening on T1-weighted images
-
A high-signal joint effusion, low-signal loose bodies and synovial folds or hypertrophy, and intermediate-signal fragment on FS PD FSE images
-
Increased signal within loose bodies on STIR images
-
May appear slightly larger than their actual size on T2* GRE sequences because of magnetic susceptibility effects that are normally dampened by the 180° refocusing pulse on spin-echo images
to inject several small air bubbles into the joint that may mimic loose bodies on MR images. Air bubbles can be recognized by a characteristic margin of high signal adjacent to the signal void (due to a magnetic susceptibility artifact), which is not found along the margins of a real loose body. Multiple foci of magnetic susceptibility artifact have a similar appearance and may also be seen at the site of micrometallic deposition associated with prior surgery. These foci of magnetic susceptibility artifact are most prominent on GRE T2*-weighted images.
 |
|
FIGURE 9.135 ● Panner's disease (osteochondrosis) in a 9-year-old girl. (A) Sclerosis within the capitellar ossification center is seen on this AP radiograph. (B) A T1-weighted coronal image reveals decreased signal throughout the capitellum (arrow). (C) A T1-weighted axial image reveals decreased signal throughout the capitellum (large arrow). The overlying articular cartilage is intact (small arrows). C, capitellum.
|
 |
|
FIGURE 9.136 ● Panner's disease in a 10-year-old girl. T1-weighted coronal (A) and GRE T2*-weighted sagittal (B) images show irregular ossification of the anteromedial aspect of the capitellum (arrows). A STIR axial image (C) shows a smooth articular surface in this portion of the capitellum (arrowheads). There is insufficient contrast between the signal intensity of fluid and the unossified epiphyseal cartilage on the T1-weighted (A) and the T2*-weighted (B) scans to determine if the surface of the cartilage is intact. C, capitellum.
|
 |
|
FIGURE 9.137 ● Loose bodies form from a nidus of cartilage or an osteochondral fragment. The loose body may be unattached or pedunculated with a tether of synovium. (A) Sagittal color illustration. (B) Sagittal PD-weighted image. (C) Sagittal FS PD FSE image.
|
 |
|
FIGURE 9.138 ● Loose bodies. The loose body in the anterior compartment (curved arrow) is apparently attached to the synovium since it does not appear to respect gravity and sink posteriorly toward the humerus. A posterior compartment loose body (open arrow) is also noted on this GRE T2*-weighted axial image.
|
 |
|
FIGURE 9.139 ● Loose body and synovitis. PD-weighted sagittal image shows a small loose body in the central aspect of the trochlear notch of the ulna (curved white arrow). Curvilinear foci of synovial thickening (small white arrows) should not be mistaken for loose bodies.
|
 |
|
FIGURE 9.140 ● A variably sized nonarticular groove is seen in the midportion of the trochlear notch and should not be mistaken for an osteochondral defect of the ulna. Loose bodies may lodge in this groove, which is normally devoid of articular cartilage. Sagittal T1-weighted MR arthrogram.
|
 |
|
FIGURE 9.141 ● Air bubbles inadvertently injected at MR arthrography. An FS T1-weighted coronal image performed after dilute gadolinium was injected into the elbow joint. Multiple small air bubbles are noted anteriorly that should not be mistaken for loose bodies (small black arrows). Fewer air bubbles, as a consequence of a diagnostic aspiration or therapeutic injection, may be more confusing than this case, especially if the radiologist is unaware that such a procedure was performed. C, capitellum.
|
-
Thought to be an accessory bone
-
May enlarge and cause symptoms
-
MR shows a deep olecranon fossa, which can help differentiate it from loose bodies.
-
The elbow is a common location of idiopathic synovial osteochondromatosis.
-
It is typically a condition of middle-aged men.
-
Nerve entrapment may be associated with this lesion.
-
Early-stage disease may demonstrate normal articular cartilage.
-
Nonossified nodules may mimic fluid on MR. A careful inspection for “septations” must be made.
swelling with progressive limitation of motion.134 Gross distention of the joint space may occasionally cause entrapment of various nerves about the elbow.135,136,137,138 Malignant degeneration has been rarely reported.57
 |
|
FIGURE 9.142 ● Os supratrochleare dorsale is an accessory ossicle located within the olecranon fossa. The os supratrochleare dorsale may be symptomatic and associated with pain and loss of elbow extension. It is typically between 1 and 2 cm in size. (A) Coronal color graphic with sagittal inset. (B) Sagittal T1-weighted image. (C) Sagittal FS PD FSE image.
|
 |
|
FIGURE 9.143 ● Symptomatic os supratrochleare dorsale and synovitis. PD- (A) and T2-weighted (B) sagittal images reveal a large intraarticular ossicle (large white arrows). There is enlargement and remodeling of the olecranon fossa (small black arrows), probably caused by gradual enlargement of the adjacent os supratrochleare dorsale. Foci of synovial thickening (small white arrows) should not be mistaken for loose bodies.
|
-
In the initial phase, there is active intrasynovial disease without loose body formation.
-
In the transition phase, there is both active intrasynovial proliferation and multiple loose bodies, which may or may not be ossified.
-
In the final phase, the process may apparently become quiescent with multiple osteochondral loose bodies and no active intrasynovial disease.
 |
|
FIGURE 9.144 ● (A) Synovial osteochondromatosis on a sagittal color section. (B, C) Diffuse primary synovial osteochondromatosis. T1-weighted sagittal (B) and T2-weighted axial (C) images reveal multiple osteochondral loose bodies (arrows) throughout the elbow joint. Thirty loose bodies were arthroscopically removed.
|
-
This disorder is becoming increasingly common, especially in middle-aged men.
-
Retraction occurs only if the lacertus fibrosus is torn.
-
Pre-tear tendinosis may be accompanied by significant biceps bursal effusion.
-
FS PD FSE axial and sagittal scans are the most useful sequences.
-
There should be sagittal coverage to 10 cm proximal to the joint line to look for a retracted tendon.
-
Consider use of the FABS position.
tendon. The primary function of the biceps brachii is flexion of the elbow and supination of the forearm. Flexion of the elbow is assisted by the brachialis, which lies just posterior to the biceps. Supination of the forearm is assisted by the supinator muscle.
 |
|
FIGURE 9.145 ● Normal distal biceps tendon. The biceps tendon (arrows) undergoes a 90° rotation as it extends approximately 7 cm from the myotendinous junction to the radial tuberosity (RT). GRE T2*-weighted sagittal image.
|
 |
|
FIGURE 9.146 ● Sagittal orientation of the distal course of the biceps on an axial T1-weighted MR arthrogram.
|
(Fig. 9.150). Irregularity of the radial tuberosity and chronic inflammation of the adjacent radial bicipital bursa may also contribute.159,160,161 There is a zone of relatively poor blood supply within the distal biceps tendon, approximately 10 mm from its insertion on the radial tuberosity.152 This hypovascular zone may be impinged between the radius and the ulna during pronation. CT and MR studies of pronation in asymptomatic volunteers showed that the space between the radius and ulna progressively narrows (by 50%) during pronation. On average, this space is approximately 8 mm in supination, 6 mm in neutral position, and 4 mm in pronation.152 Repetitive impingement during pronation, coupled with an intrinsically poor blood supply to the distal biceps tendon, may result in a failed healing response and degenerative tendinosis. Enlargement of the degenerated tendon, as well as irregularity and hypertrophy of the radial tuberosity, may lead to inflammation of the adjacent bursa. Each of these factors may contribute to worsening impingement between the radial tuberosity and the ulna, leading to further degeneration of the distal biceps tendon. This process may ultimately result in complete tendon rupture or, less commonly, partial tendon rupture or bursitis.
 |
|
FIGURE 9.147 ● Lacertus fibrosus tear associated with a brachialis muscle strain. The lacertus fibrosus is usually injured in conjunction with biceps tendon ruptures. (A) Axial FS PD FSE image. (B) Sagittal FS PD FSE image.
|
 |
|
FIGURE 9.148 ● (A) Coronal color illustration of a distal biceps tendon rupture from the radial tuberosity. The intact lacertus fibrosus limits proximal retraction of the tendon. The lacertus fibrosus originates from the medial aspect of the biceps muscle belly and inserts onto the dorsal aspect of the ulna. (B, C) Biceps tendon rupture at the radial tuberosity. (B) Sagittal FS PD FSE image. (C) Coronal FS PD FSE image.
|
rupture may both cause irritation of the adjacent median nerve, further complicating the clinical findings.162 An inflamed bursa may occur in inflammatory arthropathies, especially rheumatoid arthritis. A rheumatoid bursa may also contain rice bodies.
 |
|
FIGURE 9.149 ● High-grade partial tear of the distal biceps tendon. PD- (A) and T2-weighted (B) sagittal images reveal a lax and redundant-appearing biceps tendon (curved arrows) with surrounding edema. (C) A T2-weighted axial image reveals a thin strand of the biceps tendon (curved arrow) that remains attached to the radial tuberosity. Increased signal delineates rupture of the lacertus fibrosus (small black arrows).
|
 |
|
FIGURE 9.150 ● Intact biceps tendon associated with intrasubstance tendon degeneration with mild tendon heterogeneity and adjacent fluid. Axial FS PD FSE image.
|
-
Fluid surrounding the tendon, which is thickened and may or may not be retracted (Fig. 9.152)
-
A retracted biceps tendon (see 9.152)
-
A frayed and hyperintense distal tendon end if there is preexisting degeneration
-
Hypertrophy of the radial tuberosity
-
A fluid-filled bicipital bursa
-
Discontinuous signal intensity within the tendon, indicating a tear
-
Hemorrhage adjacent to the torn tendon
 |
|
FIGURE 9.151 ● Bicipital radial bursitis may present as an antecubital mass or as pain associated with supination. Associated abnormalities include biceps tendinopathy tears and radial tuberosity hypertrophy. (A) Coronal color illustration. (B) Axial FS PD FSE image.
|
 |
|
FIGURE 9.152 ● Proximal biceps tendon retraction with tendon redundancy. (A, B) Axial FS PD FSE images. (C) Sagittal FS PD FSE image.
|
 |
|
FIGURE 9.153 ● The brachialis is susceptible to hemorrhage where it crosses the elbow joint as a muscle belly and not as tendinous tissue. Myositis ossificans with scar formation is a potential complication of brachialis muscle strains. Sagittal T1-weighted (A) and FS PD FSE (B) images.
|
 |
|
FIGURE 9.154 ● Distal biceps tendon repair on (A) a coronal color illustration, (B) an axial PD-weighted image, and (C) an axial FS PD FSE image. Complications of operative repair include radial nerve palsy, posterior interosseous nerve palsy, heterotopic ossification, radioulnar synostosis, and elbow flexion contractures.
|
-
A very rare injury
-
Partial tears involve the central third of the tendon.
-
Injury is frequently associated with olecranon bursitis.
-
A missed triceps tear may result in severe functional disability.
-
Associated Salter-Harris type II fracture of olecranon should be sought in adolescents.
-
With MR it is possible to differentiate partial-from full-thickness tears.
-
Long head, which arises from the infraglenoid tubercle of the scapula
-
Lateral head, which arises from the lateral and posterior aspect of the humerus
-
Medial head, which arises further distally, from the medial and posterior aspect of the humerus
 |
|
FIGURE 9.155 ● Triceps tendon rupture occurs with deceleration stress on a contracted triceps muscle or eccentric contraction against resistance. Triceps tears occur in motorcycle accidents and football, soccer, and rugby injuries. Systemic disease, including hyperparathyroidism, and steroid use are associated etiologies. (A) Coronal color illustration. (B) Sagittal FS PD FSE image. (C) Axial PD FSE image.
|
 |
|
FIGURE 9.156 ● Direct trauma with myotendinous strain of the triceps on sagittal (A) and axial (B) FS PD FSE images.
|
 |
|
FIGURE 9.157 ● Tendinosis represents collagen degeneration without the influx of inflammatory cells. (A) Coronal posterior view color illustration. (B) Sagittal T1-weighted image. (C) Sagittal FS PD FSE image.
|
-
Discontinuous signal intensity in the tendon
-
Tendon retraction (see Figs. 9.155 and 9.158)
-
Tendinosis, with a variably thickened tendon
-
Fluid gap, highlighting avulsion of the tendo-osseous attachment (with or without an osseous fleck or fragment) or a musculotendinous tear
-
Bursal fluid and bursitis
-
Soft-tissue fluid and/or edema
-
Fluid signal intensity in an anterior-to-posterior surface defect through the tendon (in partial tears)
-
Reactive hyperemia in the olecranon (see Fig. 9.158)
-
Hyperemic bone trabecular injury/fracture, especially in trauma
-
Intramuscular hemorrhage in a myotendinous junction or muscle tear (see Fig. 9.156)
 |
|
FIGURE 9.158 ● Triceps tendon rupture from the insertion site in the olecranon with posttraumatic olecranon bursitis and olecranon osseous marrow edema. Sagittal FS PD FSE image.
|
-
The flexor carpi ulnaris aponeurosis tenses during flexion and reduces the volume of the cubital tunnel.
-
The cubital tunnel retinaculum (Osborne ligament) may be anomalous or thickened and compress the nerve.
-
An anomalous muscle, the anconeus epitrochlearis, may cause a mass effect.
-
MCL and medial epicondylar pathology are frequent causes of cubital tunnel syndrome.
-
Pre-joint compression may occur at the arcade of Struthers.
-
Increased size and signal of the nerve on axial FS PD FSE scans indicate ulnar neuropathy, but engorged veins must be ruled out.
band) is normally a thin fibrous structure that extends from the medial epicondyle to the olecranon.201,205 Anatomic variations of the cubital tunnel retinaculum may contribute to ulnar neuropathy (Fig. 9.162).123,203,206,207 These variations, and the appearance of the ulnar nerve itself, can be identified with MR imaging. A thickened cubital tunnel retinaculum (sometimes called the Osborne lesion) (Fig. 9.163) results in dynamic compression of the ulnar nerve during elbow flexion and can be found in 22% of the population.57 In 11% of the population, the cubital tunnel retinaculum may be replaced by an anomalous muscle, the anconeus epitrochlearis (Fig. 9.164), which results in static compression of the ulnar nerve (Fig. 9.165).208,209,210 Even more rarely an enlarged medial head of the triceps may be the compressive agent.211 In 10% of the population, the cubital tunnel retinaculum may be absent, allowing anterior dislocation of the nerve over the medial epicondyle during flexion, with subsequent friction neuritis (Fig. 9.166).201,212
 |
|
FIGURE 9.159 ● Ulnar nerve at the level of the medial epicondyle and cubital tunnel retinaculum on an axial T1-weighted MR arthrogram.
|
 |
|
FIGURE 9.160 ● The flexor carpi ulnaris aponeurosis or arcuate ligament distal to the medial epicondyle and thus distal to the cubital tunnel retinaculum. Axial T1-weighted MR arthrogram.
|
 |
|
FIGURE 9.161 ● Narrowing of the cubital tunnel with flexion and secondary compression of the ulnar nerve. Axial color section in extension (A) and flexion (B).
|
 |
|
FIGURE 9.162 ● Compression of the ulnar nerve at the level of the cubital tunnel retinaculum. Posterior view color coronal illustration.
|
-
Displacement and flattening of the nerve adjacent to a mass
-
Swelling and enlargement of the nerve proximal or distal to a mass
-
Infiltration of the perineural fat
-
Intraneural increased signal intensity on FS PD FSE and FS images (Fig. 9.168)200
-
May result from anatomic variations or enlargement of the bicipital bursa
-
Anterior interosseous nerve involvement results in weakness of the thumb and index finger.
along the course of the median nerve. If the anterior interosseous nerve is involved, there is weakness or paralysis of the flexor pollicis longus and the flexor profundus to the index and middle fingers.229 This implies a compression between 5 and 8 cm distal to the lateral epicondyle, where the anterior interosseous nerve divides from the superficial branch of the median nerve. The clinical picture can be complicated by the presence of the Martin-Gruber anastomosis. This variation, occurring in 15% of the population, involves a communication between the median and ulnar nerves in the forearm.230,231 Thus, median nerve compression at the elbow can result in weakness of the intrinsic hand muscles. Localized compression of the median nerve results in the pronator syndrome, which presents with pain and paresthesia in the proximal volar forearm.228,232
 |
|
FIGURE 9.163 ● Thickened cubital tunnel retinaculum in a patient with ulnar neuritis. (A) A T1-weighted axial image reveals the ulnar nerve (black arrow) deep to a thickened cubital tunnel retinaculum (white arrows). (B, C) T1-weighted sagittal images show the course of the ulnar nerve (small arrows) passing beneath the thickened cubital tunnel retinaculum (white arrows) at the level of the medial epicondyle. Proximally, the ulnar nerve is just posterior to the medial intermuscular septum (curved arrow). The ulnar nerve then passes between the two heads of the flexor carpi ulnaris.
|
 |
|
FIGURE 9.164 ● The anconeus epitrochlearis muscle (also called the fourth head of the triceps muscle) decreases the cubital tunnel volume and, combined with aggravation from prolonged elbow flexion, may produce ulnar nerve compression neuropathy. The muscle may also produce a vascular steal phenomenon. Axial T1-weighted MR arthrogram.
|
 |
|
FIGURE 9.165 ● The accessory or anomalous anconeus epitrochlearis muscle replaces the cubital tunnel retinaculum and may cause compression ulnar neuropathy. (A) Color coronal illustration. (B) Axial T1-weighted FSE image. (C) Axial FS PD FSE image. (D) Posterior coronal T1-weighted FSE image.
|
 |
|
FIGURE 9.166 ● (A) Subluxation of the ulnar nerve with friction neuritis on a posterior perspective color coronal illustration. (B, C) Subluxation of the ulnar nerve after cubital tunnel release. The ulnar nerve demonstrates persistent hyperintensity in this symptomatic patient. (B) Axial PD FSE image. (C) Axial FS PD FSE image.
|
 |
|
FIGURE 9.167 ● Traction on the ulnar nerve at the shoulder and wrist and compression of the ulnar nerve at the elbow and forearm in the throwing arm. In the cocking position the flexor carpi ulnaris generates compressive force, increasing pressure in the cubital tunnel up to six times greater than the resting pressure.
|
 |
|
FIGURE 9.168 ● Edematous indurated ulnar nerve. There may be a variable number of inflammatory cells associated with the compression neuritis. Chronic compression is associated with pain, muscle weakness, and/or atrophy. (A) Axial FS PD FSE image. (B) Coronal FS PD FSE image. (C, D) In a separate case there is a shorter segment of ulnar neuritis with a thickened edematous ulnar nerve. (C) Axial FS PD FSE image. (D) Coronal FS PD FSE image.
|
 |
|
FIGURE 9.169 ● Residual ulnar nerve hyperintensity after anterior subcutaneous transfer on axial PD-weighted (A) and FS PD FSE (B) images.
|
 |
|
FIGURE 9.170 ● Normal ulnar nerve signal intensity after an anterior submuscular transfer on axial PD-weighted (A) and FS PD FSE (B) images. The cubital tunnel retinaculum has been divided and the ulnar nerve has been transferred deep to the pronator teres muscle anteriorly. The aponeurosis between the humeral and ulnar heads of the flexor carpi ulnaris is released distally to provide further decompression of the nerve.
|
-
Denervation hyperintensity on FS PD FSE images (Fig. 9.171)
-
Fatty atrophy of affected muscles in chronic cases
-
Distal humerus fracture (supracondylar)
-
Swelling of the flexor pollicis longus and profundus flexor
-
Varying degrees of hemorrhage
-
Fibrosis (streaky decreased signal intensity on all pulse sequences) in the entrapment region
 |
|
FIGURE 9.171 ● (A) Color illustration demonstrating an inflamed median nerve as it courses between the two heads of pronator teres before passing deep to the flexor digitorum superficialis. (B) Axial FSE T2-weighted image demonstrates abnormal increased signal consistent with denervation involving the flexor digitorum superficialis, the flexor pollicis longus, and the flexor digitorum profundus. Signal changes correspond to compression of median nerve branches in the region of the pronator teres, including the anterior interosseous nerve and the muscular branch to flexor digitorum superficialis.
|
-
Precubital compression produces combined wrist weakness and sensory loss.
-
Cubital compression produces pain only.
-
Postcubital compression results in digital weakness.
-
Precubital entrapment usually occurs at a fibrous arch of the long head of the triceps,233 although it may also be caused by a humeral diaphyseal fracture (Holstein-Lewis fracture) (Fig. 9.173).234 In nerve entrapment at this location the presentation is reduced power of forearm supination/flexion and weak wrist extension. There is also a loss of sensation in the dorsal and radial aspects of the wrist.
-
Postcubital entrapment is more common and involves the posterior interosseous branch of the nerve, resulting in posterior interosseous nerve syndrome (PINS). Entrapment at this location presents as a loss of extension of the metacarpophalangeal joints and radial deviation of the wrist with extension. The usual cause of PINS is impingement related to thickening of the tendinous origin of the supinator, the arcade of Frohse.235,236,237,238 Parosteal lipomas arising from the proximal radius239 and ganglion cysts arising from the anterior margin of the elbow joint240,241,242,243 may also compress the radial nerve. PINS may be subdivided into two types depending on whether the medial or lateral subdivision of the nerve is involved. In medial branch compression, the extensor carpi ulnaris, the extensor digiti minimi, and the extensor digitorum communis are affected. In lateral branch compression, weakness affects the abductor pollicis longus, the extensor pollicis brevis, the extensor pollicis longus, and the extensor indicis.
-
Cubital causes of entrapment, including lateral epicondylitis or soft-tissue masses from the elbow, result in the radial tunnel syndrome, which presents with vague forearm pain but no loss of power.235,244,245 The most common compression site is the arcade of Frohse.
-
Most common site of bursitis in the body
-
Associated triceps tendinopathy is common.
-
Systemic causes are important in older patients.
-
There is associated infection in 20% of cases. Infection is best detected with contrast-enhanced imaging.
from direct spread from abrasions and cellulitis. MR imaging is useful for the identification of osteomyelitis, which sometimes develops in the underlying olecranon; however, this is thought to be uncommon. Septic olecranon bursitis can be excluded in the absence of bursal or local soft-tissue enhancement after gadolinium administration.257
 |
|
FIGURE 9.172 ● (A) Color illustration demonstrating the superficial and deep branches of the radial nerve. The deep branch of the radial nerve is inflamed as it courses posteriorly between the ulnar and humeral (not seen) origins of the supinator muscle. (B) Axial and (C) sagittal FS PD FSE images demonstrate abnormal increased signal within the anconeus, extensor carpi ulnaris, and extensor digitorum muscles consistent with denervation. Note the absence of perifascial edema, which is typically seen in the setting of muscular injury or strain.
|
 |
|
FIGURE 9.173 ● Holstein-Lewis fracture of the distal third of the humerus may cause radial nerve palsy. The radial nerve is tethered by the intermuscular septum as it emerges from the spiral groove and may be susceptible to injury, especially if there is lateral displacement of the distal fracture fragment. Sagittal FS PD FSE image.
|
-
Typically seen in male manual laborers
-
The coronoid and olecranon processes are usually involved.
-
Overuse associated with athletics affects the ulnohumeral joint.
-
Age-related changes affect the radiohumeral joint.
-
In the throwing athlete, chondromalacia and chondral defects are more commonly seen in the posterolateral aspect of the trochlear notch.263
-
In older individuals, articular cartilage loss is more common in the radiohumeral articulation rather than in the ulnohumeral joint (see Fig. 9.176).264
-
Early chondral degeneration characteristically involves opposing surfaces at the medial margin of the radiohumeral articulation. The medial rim of the radial head and the adjacent crest at the lateral margin of the trochlea are the usual sites of early articular cartilage loss.264
-
With progressive degeneration of the articular cartilage, there is usually involvement of the entire radial head and the anteroinferior aspect of the capitellum (Fig. 9.177).
-
Deep fissuring or full-thickness defects of the articular cartilage, often resulting in abnormalities of the exposed subchondral cancellous bone
-
Advanced articular cartilage loss
-
Associated marginal spurring
-
Loose body formation (Fig. 9.178)
-
Subchondral cysts
-
Associated edema
-
Synovitis
 |
|
FIGURE 9.174 ● Olecranon bursitis is associated with triceps tendinopathy and tears. Inflammation of the bursa may be secondary to acute or repetitive trauma or systemic disease. Traumatic bursitis not uncommonly occurs with football injuries associated with playing on artificial turf. (A) Posterior coronal color illustration. (B) Sagittal T1-weighted image. (C) Sagittal FS PD FSE image.
|
 |
|
FIGURE 9.175 ● Large subcutaneous olecranon bursa overlying the olecranon process. The posterior margin of the bursa is convex. (A) Axial T1-weighted image. (B) Axial FS PD FSE image.
|
 |
|
FIGURE 9.176 ● (A) Arthrosis with radial head and ulnar chondral fragmentation and subchondral sclerosis in early osteoarthritis. Coronal color illustration with coronal section inset. Coronal T1-weighted (B) and FS PD FSE (C) images demonstrate subchondral capitellar erosions and sclerosis and subtle ulnar-sided osteophytic spurring.
|
 |
|
FIGURE 9.177 ● Advanced degeneration of the radiohumeral articulation (A) and prominent spurring of the ulnohumeral articulation. (B) Sagittal T1-weighted images.
|
-
The elbow is affected in 20% to 50% of patients.
-
Synovial hypertrophy may cause neural entrapment.
-
Bone cysts occur away from load-bearing areas.
-
Gadolinium enhancement shows the extent of synovial hypertrophy and can be used to monitor treatment.
-
On conventional T1-weighted images, thickened synovium is greater in signal intensity than fluid and demonstrates decreased signal intensity on T2-weighted images.
-
Contrast enhancement (actively inflamed synovium enhances after intravenous administration of gadolinium) may be necessary to differentiate fluid from acute synovitis in some cases.269
-
Measurement of gadolinium uptake rates into the thickened synovium can help to assess the aggressiveness of the disease and also to monitor response to therapy.
-
MR arthrography, with either saline or dilute gadolinium, is also useful for the identification of synovitis.
appearance, however, is more characteristic of the recurrent intra-articular hemorrhage and hemosiderin-laden synovium commonly seen in hemophilia and pigmented villonodular synovitis.270 Similar low-signal-intensity nodular synovial lesions may also be seen in hemodialysis-related amyloid arthropathy, a complication of long-term hemodialysis caused by the deposition of a unique form of amyloid derived from circulating beta2-microglobulin.
 |
|
FIGURE 9.178 ● (A) Sagittal color illustration of osteoarthritis. (B, C) Acquired loose bodies can be classified as related to osteochondritis dissecans, degenerative arthritis, or synovial proliferation (synovial chondromatosis). (B) Sagittal FS PD FSE image. (C) Arthroscopic image.
|
-
Nodules on the extensor aspect of the elbow
-
Swollen, entrapped nerves
-
Muscle changes resulting from nerve compression (high signal on FS sequences)
-
Characterized by low-signal synovial hypertrophy
-
Usually monarticular and erodes the joint surface
-
Conditions mimicking pigmented villonodular synovitis include rheumatoid, hemophiliac, and amyloid arthropathies.
 |
|
FIGURE 9.179 ● Rheumatoid involvement of the elbow with enhancement of inflamed synovium (pannus), cyst formation, and a bare area of ulnar erosion adjacent to the chondral surface. Subchondral marrow edema is indicated. (A) Sagittal color section. (B) Coronal FS PD FSE image. (C) Coronal contrast-enhanced FS T1-weighted image.
|
-
May cause epitrochlear adenopathy, which needs to be differentiated from hematoma or sarcoma
-
MR characteristics include identification of the fatty hilum of an enlarged node or visualization of a chain of nodes.
-
A nonspecific mass of low to intermediate signal intensity on T1-weighted images and intermediate to high signal intensity on FS PD FSE images
-
Gadolinium administration produces enhancement of the mass and surrounding lymphedema. Central fluid that does not enhance with gadolinium may be present if there is central necrosis and liquefaction.288
-
Suppuration of lymph nodes occurs in about 15% of cases, and nodes typically range in size from 1 to 5 cm.280 Lymphedema and infiltration of the surrounding fat due to cellulitis are characteristic.288
-
The mass is located at the site of epitrochlear lymph nodes, adjacent to the medial neurovascular bundle and just proximal to the elbow. There may be associated axillary adenopathy.
 |
|
FIGURE 9.180 ● (A) Differential considerations for a benign soft-tissue lesion include a benign lipoma with uniform fat signal intensity located deep to the brachioradialis muscle and radial to the flexor-pronator muscles. Sagittal T1-weighted image. (B) Intramuscular hemangioma involving the pronator teres. Axial FS PD FSE image.
|
 |
|
FIGURE 9.181 ● Cat-scratch disease with enlarged medial subcutaneous epitrochlear lymph nodes superior to the medial epicondyle. A central area of fat represents the hilum of the enlarged lymph node. There is lymphatic infiltration and edema of adjacent subcutaneous tissue as part of the inflammatory process. (A) Sagittal PD FSE image. (B) Sagittal FS PD FSE image.
|
 |
|
FIGURE 9.182 ● Different presentations of osteomyelitis about the elbow. (A) Infected olecranon bursitis with adjacent olecranon cortical destruction. Color sagittal graphic. (B) Intramedullary abscess. Color sagittal graphic. (C) Sequestrum or necrotic fragment in chronic osteomyelitis. Color sagittal graphic. (D) Septic joint with debris. Initial focus of olecranon osteomyelitis marrow edema demonstrated in an immature skeleton. Acute hematogenous osteomyelitis is more common in the pediatric patient and chronic posttraumatic osteomyelitis is seen in the adult population. Sagittal FS PD FSE image.
|
-
The olecranon is the most common site of bone infection in the elbow.
-
MR may provide an early diagnosis in children.
-
Significant marrow edema, hypointense on T1-weighted and hyperintense on FS PD FSE images
-
Reactive joint effusion
-
Signal intensity changes in bone marrow in children
-
Variable bone destruction
-
Soft-tissue abscesses and sinus tracts, including a Brodie abscess with a sclerotic rim
-
Cellulitis
-
Myositis
-
Low-signal sequestrum (dead, necrotic bone seen in chronic disease) on both axial T1- and T2-weighted (including FS PD FSE) sequences
-
Hypointense involucrum (periosteal reaction) on all sequences