Lumbar Microdiscectomy
discectomy techniques for the treatment of low back pain from nerve
root compression related to disc trauma or degenerative disc disease.
Approximately 300,000 microdiscectomy procedures of the lumbar spine
are performed annually. The incidence of males undergoing lumbar
microdiscectomy can be up to twice that of females, in part because of
the higher load burden on the male lumbar spine.
surgical microscope to treat lumbar disc disease, first applied over 25
years ago. Benefits include further reduced trauma to spinal muscles;
decreased manipulation of nerve structures; smaller closure scars; and
diminished morbidity, anesthesia, and operative time.
basis or with one overnight hospital stay. The procedure has proved to
be an effective surgery, with 85% to 95% of patients receiving
significant pain relief or abatement, depending on the series. There is
a reoperation rate of approximately 5% to 10%. Because the integrity of
all the muscles, ligaments, and joints is maintained, the mechanical
aspect of the lower back, as well as the function, is kept intact.
vertebrae (L1 to L5) and the first sacral vertebra (S1); the latter
vertebra is fused to the sacrum. As with all spinal vertebrae, lumbar
vertebrae are separated by an intervertebral fibrocartilage disc. The
name of each disc is derived from the two adjacent vertebral bodies.
Therefore, the disc between L4 and L5 is the L4-5 disc. The most caudal
intervertebral disc of the lumbar spine is the L5-S1 disc. The
posterior bony elements of vertebrae include paired pedicles,
transverse processes, inferior and superior articular processes, and
laminae and a spinous process. The laminae are continuous with the
articular processes, which join with the articular processes of the
respective bodies, above and below, to form the bilateral facet joints.
The laminae unite posteriorly to form the spinous process. At any
vertebral level, the combination of the intervertebral disc and pair of
facet joints is often referred to as the trijoint complex. The space
between laminae is the interlaminar space; throughout the spine, these
spaces are connected by the relatively elastic ligamentum flavum
(yellow ligament) (Fig. 17-1). The kinematics
of disc movement are defined by the relationships of the disc, of the
facet joints, and of the strong ligaments, with their attachments to
the posterior elements.
made possible by strong ligamentous support. The anterior longitudinal
ligament (ALL), a broad ligament that extends from the occipital bone
to the sacrum, unites the anterior aspects of the lumbar vertebrae. The
posterior longitudinal ligament (PLL) begins at the axis and continues
the entire length of the spine. It joins the posterior aspect of the
vertebral bodies widening slightly at each intervertebral space to
directly reinforce the respective discs.
distal termination of the spinal cord, known as the conus
medullaris/cauda equina complex, which travels within the spinal canal
and is demarcated similar to the cord in other spinal regions:
anteriorly by the posterior vertebral bodies, laterally by the
pedicles, and posteriorly by the laminae and facet joints. At the
inferior aspect of the body of L1, the spinal cord with its lumbar and
sacral nerve roots form the cauda equina complex. A nonnervous thread,
the filum terminale, continues to the base of the coccyx, essentially
attaching the distal cord to the coccygeal ligament. The dural sac
containing the lumbar and sacral nerve roots provides bilateral exits
through bony neural foramina that are formed by superior and inferior
notches on the adjacent pedicles or, more distally, within the lateral
walls of the sacrum. Each nerve root derives its name from the pedicle
above. Therefore, the L3 roots exit underneath the L3 pedicles.
(unilaterally) offcenter of the midline and compresses one root.
Typically, the lower nerve root is affected; for example, in an L4-5
disc
herniation,
the traversing L5 nerve root is compressed. The L4 root would generally
be spared compression, as it exits beneath the higher L4 pedicle, just
above the herniating L4-5 disc. Similarly, a typical L5-S1 herniation
compresses the S1 nerve root.
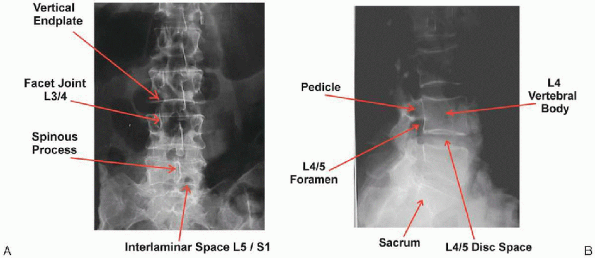 |
|
FIGURE 17-1. Anteroposterior (A) and lateral (B) x-ray film views of lumbar vertebrae.
|
trauma from normal use and with age-related conditions (arthritis) and
progressive disorders (spondylolisthesis, scoliosis). Initial disc
changes may present as peripheral tears of the annular fibers,
delamination of the outer layers (annulus), or nuclear degeneration
with diminished water-holding properties. Disc bulging without
herniation can also occur. With progressive trauma and diminishment of
the functional integrity of the trijoint complex, affected vertebrae
may become unstable.
circumferential) typically occur in conjunction with nuclear
degeneration, resulting in loss of disc height. Similar bony and
cartilage degenerative changes in the facet joints result in joint
subluxation and capsular laxity. Herniation of a disc can occur either
early or late in this degenerative process. Further disc resorption
with narrowing of the disc space and development of disc fibrosis and
osteophyte formation ensues, along with the development of spinal
stenosis.
herniation is to differentiate a true herniation from a bulging disc.
As discs degenerate during the normal aging process they lose water
content and disc height. Naturally the lateral wall of the disc bulge
outward in a symmetrical fashion much as the sides of a tire low on air
bulge outward as the rim gets closer to the ground. A true herniation
is not merely a symmetrical bulging but rather an asymmetrical protrusion
of disc material outward from the normal circumference of the disc. The
asymmetry of the protrusion is the key differentiation factor between
bulge and herniation.
focuses on the manner in which the disc herniates. Herniations are (a)
contained (annulus or the PLL prevents the nuclear material from
escaping), (b) extruded (nuclear material has escaped through the
annulus or the PLL but remains in contact with the nucleus pulposus),
or (c) sequestered (one or several separate pieces of nuclear material
have escaped the annulus and are separated from the remainder of the
nucleus pulposus, usually migrating further upward or downward within
the canal).
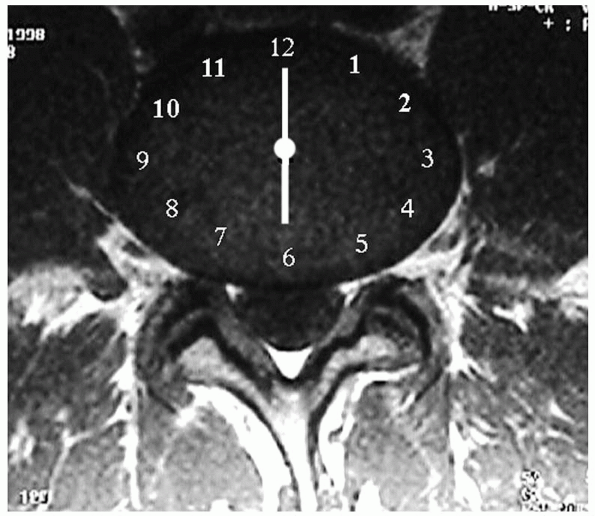 |
|
FIGURE 17-2.
Magnetic resonance imaging. 6-o’clock position, central disc; 5:30 or 6:30, posterolateral disc; 5-o’clock or 7-o’clock position, intraforaminal disc; 3:30 to 4:30, extraforaminal disc; 7:30 to 8:30, far-lateral disc. |
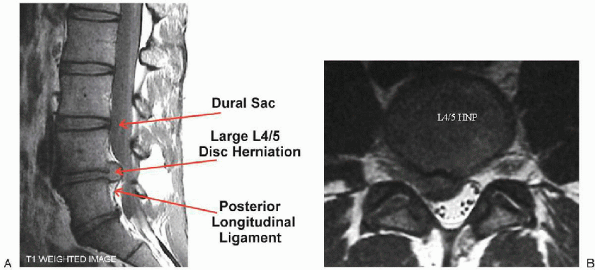 |
|
FIGURE 17-3. Magnetic resonance imaging views of a large L4-5 disc herniation, sagittal (A) and axial (B).
|
Herniations are central, posterolateral, intraforaminal, or
extraforaminal. Each type has a specific position on a clock face that
is visually “templated” over the cross-sectional view of a disc (Fig. 17-2).
The 12-o’clock position is the disc’s anterior midline; similarly, the
6-o’clock position is the posterior midline. The apex of a central
herniation occurs at the 6-o’clock position, and the apex of
posterolateral herniations are at the 5:30 (left) or 6:30 (right)
position, depending on if the herniation occurs on the left or right.
The apex of intraforaminal herniations are centered at the 5-o’clock or
7-o’clock position, and the apex of extraforaminal disc herniations are
positioned between 3:30 and 4:30 on the left or 7:30 and 8:30 on the
right. The latter type is also termed far-lateral herniations and is
notable for affecting the upper root. For example, a far-lateral L4-5
herniation affects the upper L4 root after it exits from under the L4
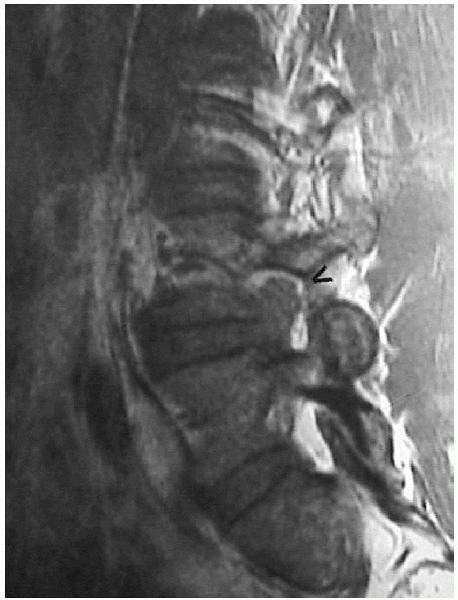
pedicle, rather than affecting the more medial L5 root (Figs. 17-3, 17-4 and 17-5).
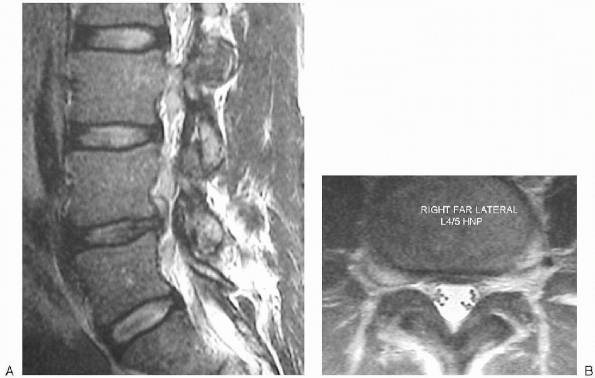 |
|
FIGURE 17-4. Magnetic resonance images of large right L4-5 extraforaminal disc herniation, sagittal (A) and axial (B).
Note that a large fragment of disc material has migrated upward from the disc space and is compressing the L4 root against the inferior aspect of the pedicle. Compared to the L5-S1 foramen below almost the entire foramen of L4-5 is occupied by disc material (arrow). |
 |
|
FIGURE 17-5. Magnetic resonance image of foraminal herniation.
|
progressive leg pain or weakness and extremity numbness) or cauda
equina syndrome (including bowel and bladder dysfunction) is a
contraindication for a trial of conservative therapy. Most patients
improve following adequate conservative care lasting 2 to 3 months.
Although the definition and course of conservative therapy can differ
across physicians and patients, in general, it may include an initial
24- to 72-hour period of bed rest, nonsteroidal antiinflammatory drugs
(NSAIDs) and other pharmacologic agents, physical therapy, and a trial
of oral steroids or a consideration of epidural steroid injections. Patients
who either fail to improve or plateau at a level of symptoms that are
unacceptable to them may wish to consider a surgical option.
Classically, the leg pain radiates below the knee in a single
dermatomal pattern. The patient typically demonstrates positive root
tension signs with the straight-leg raising test or a cross straight
leg raise. If a neurologic deficit is present, it should correlate with
the clinical presentation and the radiographic findings. Magnetic
resonance imaging (MRI) is the imaging procedure of choice. Plain
radiographs are helpful in ruling out concurrent diagnoses such as
spondylolysis or spondylolisthesis. In some cases, flexion and
extension radiographs may be ordered to rule out instability. Although
the absolute indications for surgery are diagnosis of a severe
neurologic deficit or cauda equina syndrome, most surgical patients are
indicated for failure to improve after adequate conservative therapy.
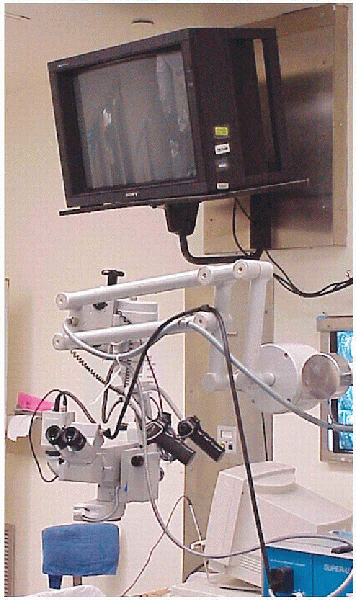
magnification and illumination for performing a microdiscectomy and is
my preference (Fig. 17-6). Some surgeons prefer
the use of the headlamp with loupe magnification systems. Microscopes
offer the additional advantage of a “built-in” option to connect the
scope to a video camera to record the surgery and/or a monitor for
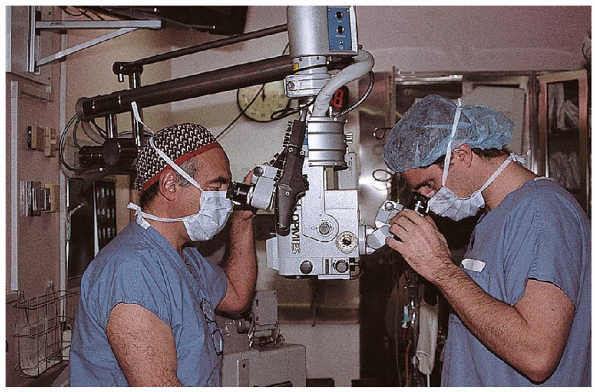
observation and teaching purposes. Using a microscope, the first
assistant and surgeon have identical views of the field, which
facilitates coordination in the surgical technique (Fig. 17-7) and resident education.
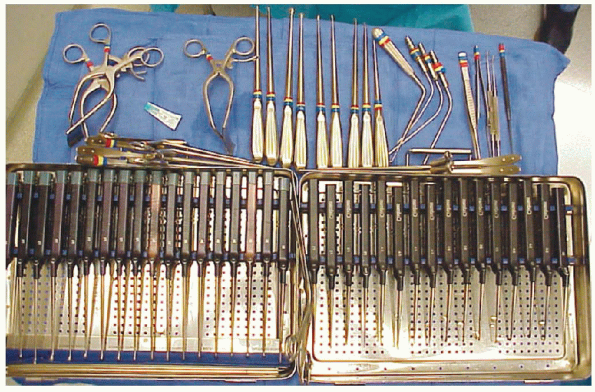
microretractor, a collection of small Kerrisons, pituitaries, and
curettes, although surgeons often have other personal preferences for
instruments to use through the small incision (Fig. 17-8). Small
microretractors have a tendency to tilt, lateral side up, in small
wounds. To correct this tendency, one can hook a chain with a weight
over the lateral blade of the retractor system and hang it off the edge
of the table. A good resource for a hook, chain, and weight is a hip
self-retractor system (see the section on surgical procedure for
further detail).
 |
|
FIGURE 17-6. Operating microscope, camera, and TV monitor.
|
 |
|
FIGURE 17-7. The operating scope permits the surgeon and first assistant to have virtually the same view of the operative field.
|
Amsco surgical table with the Andrews attachment is a frequent choice
of surgeons. This particular table has a heavy base that makes it
possible to position the patient eccentrically and maintain a stable
operative field. Alternatively, some surgeons use large gel rolls or a
Jackson table.
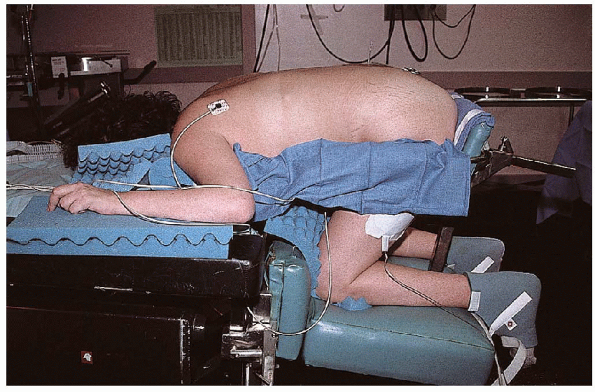
prone on the surgical table in the knee-chest position. Placed thus,
the lumbar spine flexes slightly and the interlaminar spaces widen a
bit (Fig. 17-9). The knee-chest position allows the patient’s abdomen to hang free, which decreases intraabdominal pressure. Placing
the abdomen in a pressure-free state improves the prone patient’s
ventilation and also significantly lessens epidural bleeding during
surgery. Less epidural bleeding improves the surgeon’s vision of the
surgical field and contributes to a more controlled procedure and
benefits the patient in reduced blood loss.
 |
|
FIGURE 17-8.
These sterile operating room trays hold the typical assortment of instruments required to perform microdiscectomy. Upper left are William’s retractors, a wide assortment of curettes, Kerrisons (usually angled with narrow shoes), and various nerve root retractors and “feelers” for peridural work. |
 |
|
FIGURE 17-9. Patient placed in the knee-chest position, using the Andrews attachment to a standard Amsco surgical table.
|
importance in spinal surgery. When the incision is as small as 1 to 2
inches, localization takes on added critical significance. One must
precisely locate the correct interlaminar space to avoid wrong level
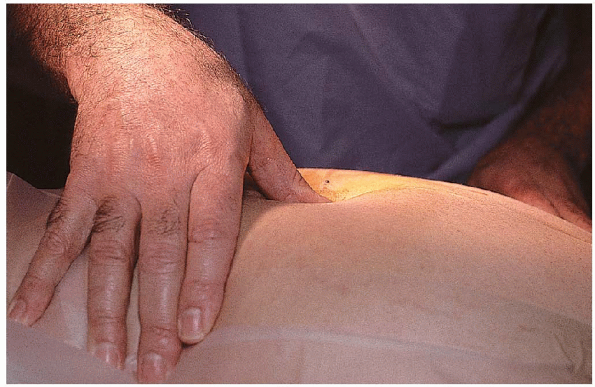
surgery. Localization begins with palpation of the interspinous spaces (Fig. 17-10).
The L4-5 interspace can usually be located on a line visualized
perpendicular to the spine and at the level of the top of the iliac
crests. The vertebral levels and interlaminar space can then be
confirmed with a lateral intraoperative radiograph taken with a sterile
needle placed about 1 inch from the midline but opposite to the planned
laminotomy. On palpation,
the L5 spinous process is the first mobile
process, working cranially from the fixed sacral processes.
Furthermore, the L5-S1 interspace is usually larger than the L4-5
interspace.
 |
|
FIGURE 17-10. Surgeon palpating the interspinous spaces.
|
 |
|
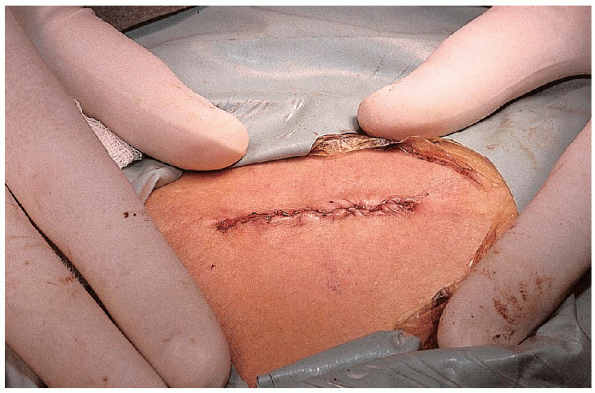
FIGURE 17-11. The incision, the length of which may vary from 1 to 2 inches, depending on the size of the patient.
|
patient and should be carefully considered. Most often, a 1.25-inch
incision is sufficient and well tolerated (Fig. 17-11). However,
if the incision is too small for a patient, excessive retraction of the
skin edges in an attempt “to make the field work” can adversely impact
wound healing. It also produces unnecessary frustration for the surgeon
in maneuvering instruments and tissues and attempting to visualize
structures.
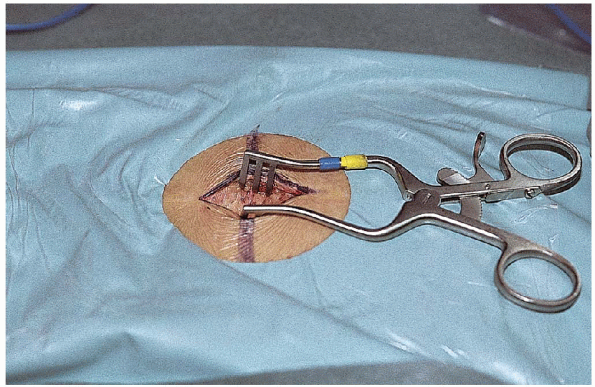
fascial incision must be done off the midline to maintain the integrity
of the spinous ligament. A completely intact ligament is mandatory,
because all spine retractor systems employ the interspinous ligament
complex for countertraction while retracting the paravertebral muscles (Fig. 17-12). If
the medial blade of the retractor system slips between the spinous
processes and disrupts the interspinous ligament, adequate
countertraction is not available, and the exposure is reduced. The
surgeon must enlarge the incision or use another type of retractor,
such as a Taylor retractor. As with small
microretractors, Taylor retractors have a tendency to tilt, lateral
side up, in small wounds. Some surgeons tie the Taylor retractor down
to the base of the operating room table. Alternatively, one can hook a
chain with a weight over the Taylor retractor.
 |
|
FIGURE 17-12. The Williams retractor in position.
|
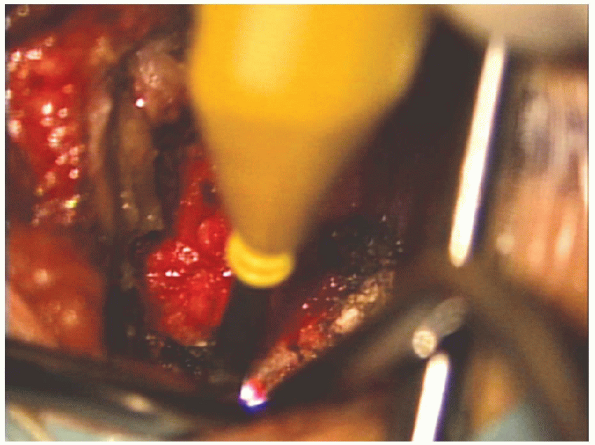 |
|
FIGURE 17-13. Electrocautery is used to clear the soft tissue from the lamina.
|
the interlaminar space is exposed and the soft tissues are removed with
a pituitary and electrocautery, the operating scope is brought into
position (Figs. 17-13 and 17-14).

Using the operative scope, a laminotomy is done next to expose the
canal and the spinal nerves. An enlarged view of the laminotomy can be
achieved by minor adjustments: upward, downward, medial, and lateral
tilting of the scope (Fig. 17-15). Once
the dural tube is exposed, remaining ligament and lamina can be safely
removed using Kerrison rongeurs. The blunt posterior aspect of the
Kerrisons should always be positioned in contact with the dura when
performing these tasks; in that way, one again minimizes the
possibility of accidentally injuring the dura with the cutting edges of
the instrument.
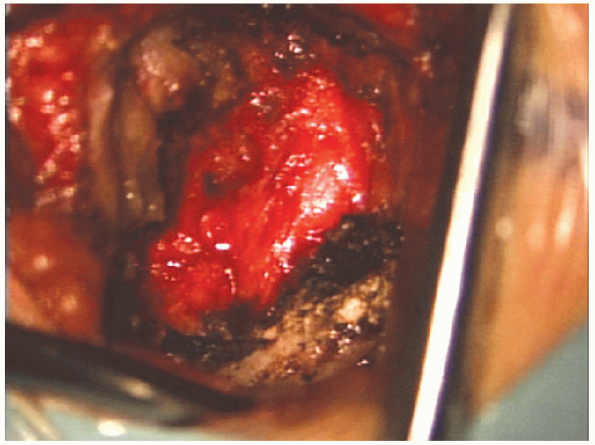 |
|
FIGURE 17-14. Superior lamina is cleared of soft tissue.
|
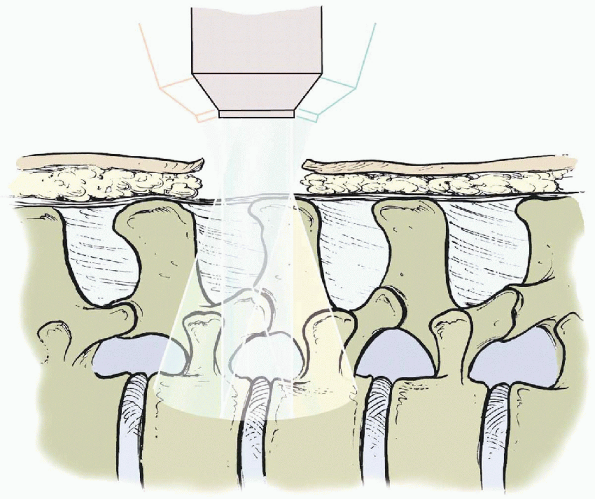 |
|
FIGURE 17-15.
Graphic demonstrating operating microscope illumination. The microscope can be tilted upward, downward, medially, and laterally, offering a significantly larger view of the field than one might expect through such a small incision. |
surgeon must gently retract the exiting nerve root medially to find the
disc space and the offending herniation. The surgeon must appreciate
that the position of the disc varies relative to the position of the
interlaminar space at different levels of the spine. For example, the
L5-S1 disc lies directly beneath a relatively large L5-S1 interlaminar
space; the surgeon may not need to remove any lamina to proceed or only
a very small amount. However, with each more cephalad intervertebral
disc space, the disc is incrementally higher relative to the
interlaminar space, increasing the amounts of lamina that must be
removed to access the disc.
L5-S1 allows for easy entry into the spinal canal with a small scalpel
blade and forceps. By lifting the ligamentum flavum upward, the surgeon
minimizes an incidental durotomy. Once the dura is exposed, further
removal of ligamentum flavum and lamina can be performed with Kerrison
rongeurs.
the L4-5 level and higher, the interlaminar space is not sufficiently
large to allow for the opening of the ligamentum with a scalpel.
Because the ligamentum inserts underneath the superior lamina, removal
of the inferior edge of the superior lamina enlarges the interspace (Fig. 17-16). This can be performed with a Kerrison or a burr (Fig. 17-17).
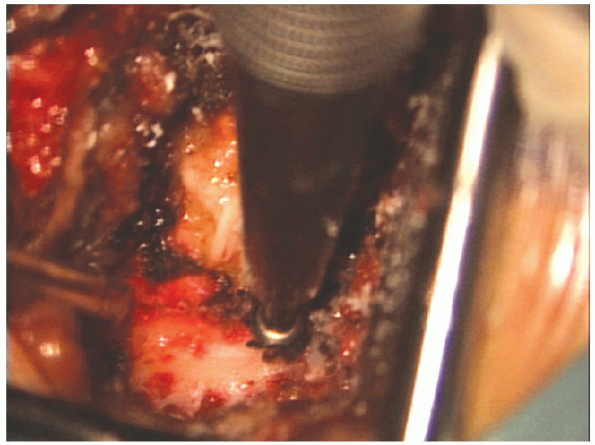
 Bleeding from the cancellous bone of lamina can be controlled with the application of bone wax (Fig. 17-18).
Bleeding from the cancellous bone of lamina can be controlled with the application of bone wax (Fig. 17-18).
Entry into the spinal canal is accomplished by enlarging the laminotomy
above the insertion of the ligamentum flavum with a Kerrison
 on the undersurface of the lamina (Figs. 17-19, 17-20 and 17-21), or alternatively once again employing
on the undersurface of the lamina (Figs. 17-19, 17-20 and 17-21), or alternatively once again employinga scalpel (Fig. 17-22).  The
The
lateral edge of the laminotomy may safely remove up to 50% of the
medial aspect of the facet joint, exposing the underlying nerve root (Fig. 17-23).  Removal of greater than 50% of the medial facet may lead to postoperative facet fracture with resultant pain and/or instability.
Removal of greater than 50% of the medial facet may lead to postoperative facet fracture with resultant pain and/or instability.
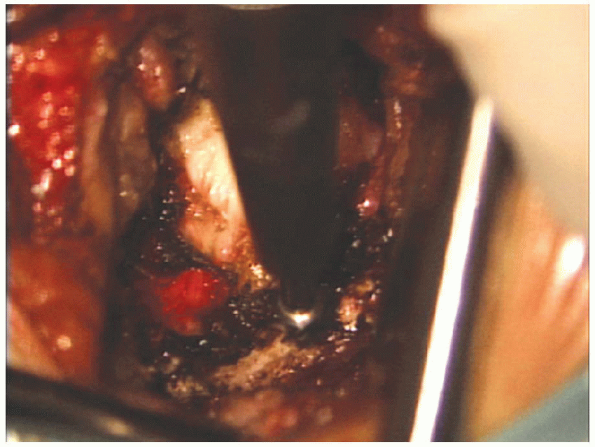 |
|
FIGURE 17-16. The burr is used to thin out the inferior aspect of the superior lamina.
|
 |
|
FIGURE 17-17. The lamina is thinned with the burr, down toward the underlying ligamentum flavum.
|
 |
|
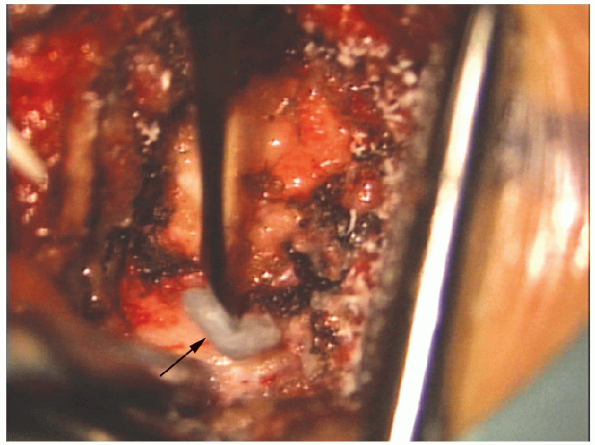
FIGURE 17-18. Bone wax (arrow) on a small elevator can be used to control bleeding from cancellous bone.
|
 |
|
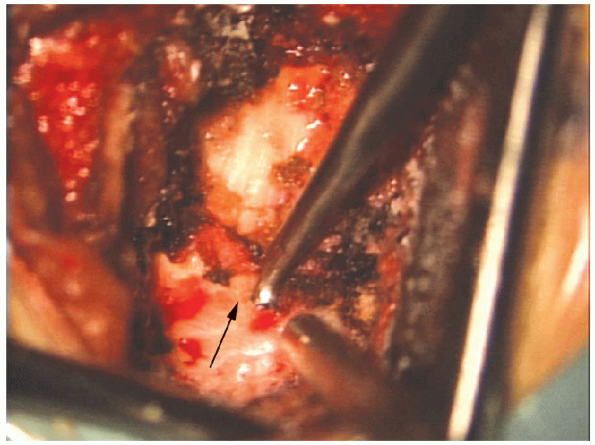
FIGURE 17-19. An upgoing curette (arrow) is used to separate the underlying ligamentum flavum from the undersurface of the lamina.
|
 |
|
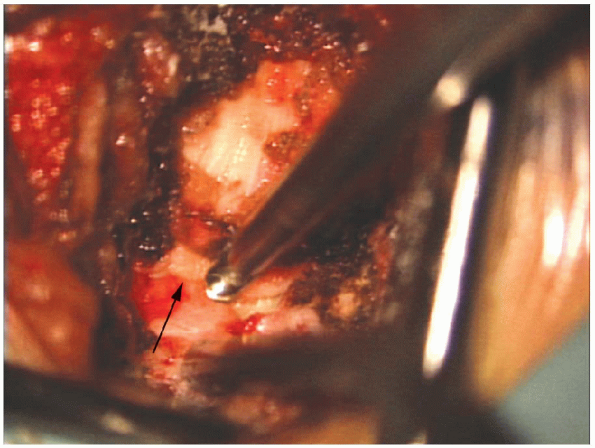
FIGURE 17-20. A Kerrison rongeur (arrow) removes the remaining thinned out lamina.
|
used to retract the nerve root medially, positioning it medial to the
herniation where it can be protected using a right-angle nerve
retractor (Fig. 17-24). Traction
must be relaxed on the protected “medialized” nerve root by the first
assistant at every opportunity, such as when the surgeon has briefly
removed instruments from the field. Placing the nerve root under
tension may cause a temporary intraneural ischemia, with a potential of
injury. Intermittent relaxation of the root likely lessens any
potential damage occurring from the ischemia by shortening the ischemic
state and allowing recovery in between periods of stretch and tension.
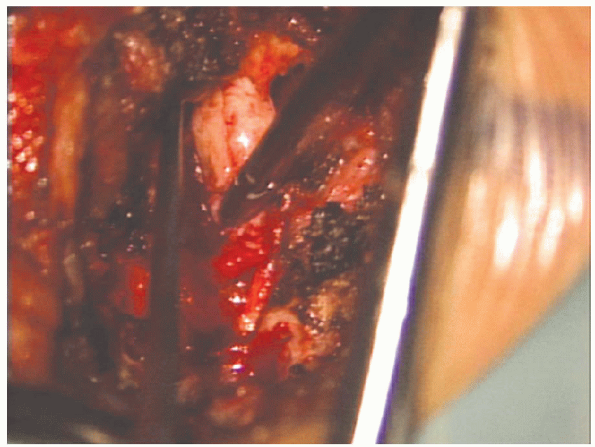
and removal of the herniated portion of the disc material (Fig. 17-25). A
single vertical incision is preferable to a rectangular annulotomy. A
thin, healed annular scar is probably a stronger barrier to recurrent
disc herniation than a broad fibrous scar over a large annulotomy.
The pituitaries and curettes allow for local, aggressive removal of
disc material from directly under the nerve root. Aggressive “radical”
discectomy of the entire disc space with scraping of the endplates is
not indicated. There is no evidence that this prevents recurrence or
promotes a spontaneous interbody fusion over the course of time.
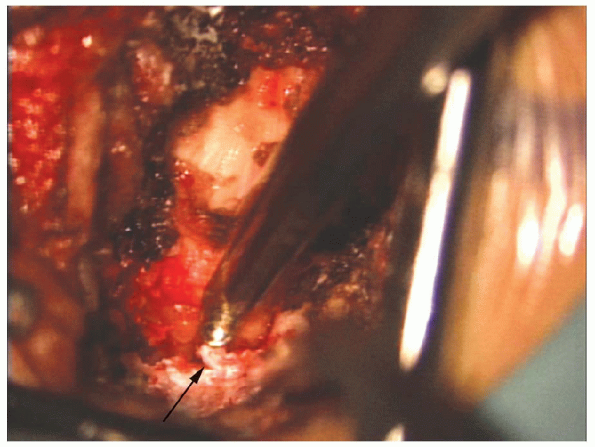 |
|
FIGURE 17-21. The Kerrison (arrow) removes the lamina above the insertion of the ligamentum flavum exposing the epidural fat in the spinal canal.
|
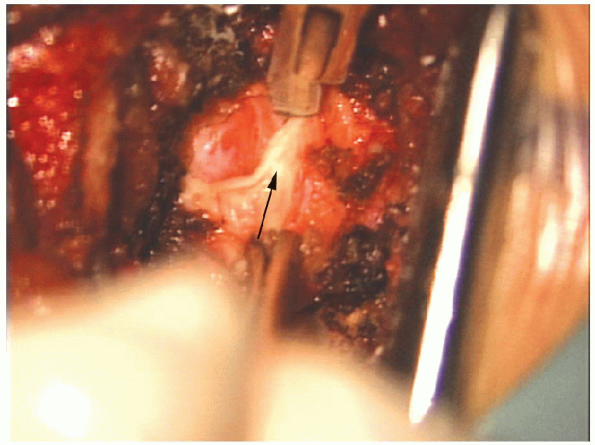 |
|
FIGURE 17-22. A no. 15 scalpel blade (arrow) can be used to cut the ligamentum flavum aiding removal.
|
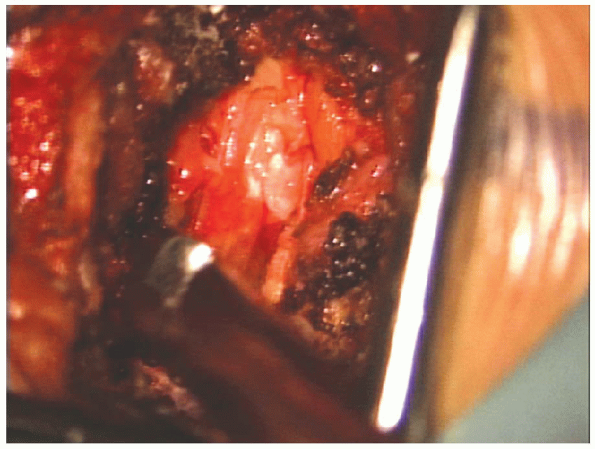 |
|
FIGURE 17-23. The Kerrison sequentially removes approximately the lateral half of the ligamentum flavum.
|
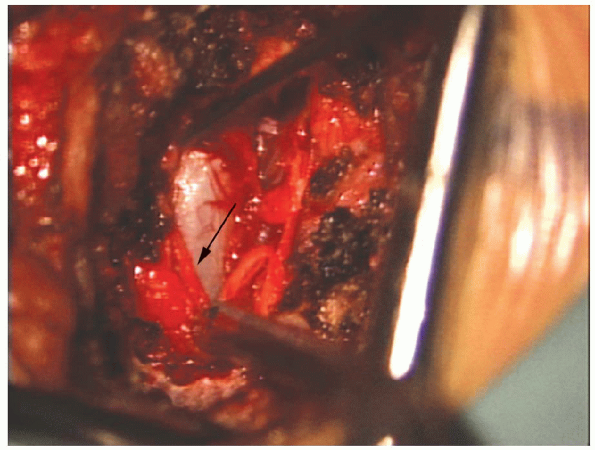 |
|
FIGURE 17-24. The dural tube is exposed under the overlying epidural fat (arrow).
|
 |
|
FIGURE 17-25. A pituitary rongeur is used to remove disc material from the disc space.
|
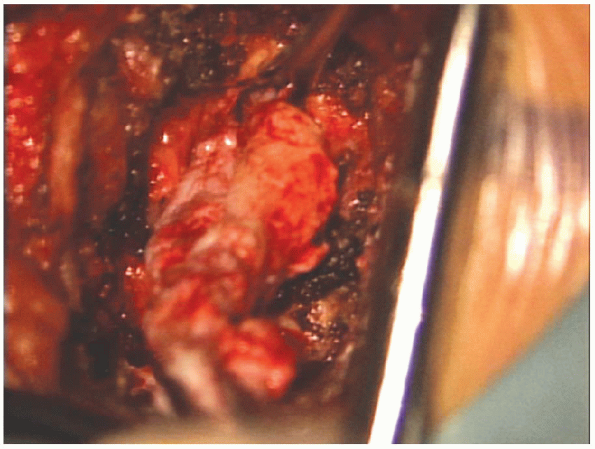
an adequate amount of disc material has been removed from the actual
disc space, the surgeon must examine the spinal canal for additional
extruded fragments that may have migrated. A thorough review of
preoperative MRI scans at this time is essential. Removal
of all extruded disc fragments is highly dependent on (a)
interpretation of the preoperative MRI scans, (b) sufficient lamina
removal, and (c) correlation of intraoperative findings with
preoperative images (Fig. 17-26).
 It is this correlation that helps minimize
It is this correlation that helps minimizethe risk of retained fragments. Illumination and magnification of the microscope greatly minimizes the risk.
 |
|
FIGURE 17-26. Large extruded fragment being removed from under the nerve root.
|
 |
|
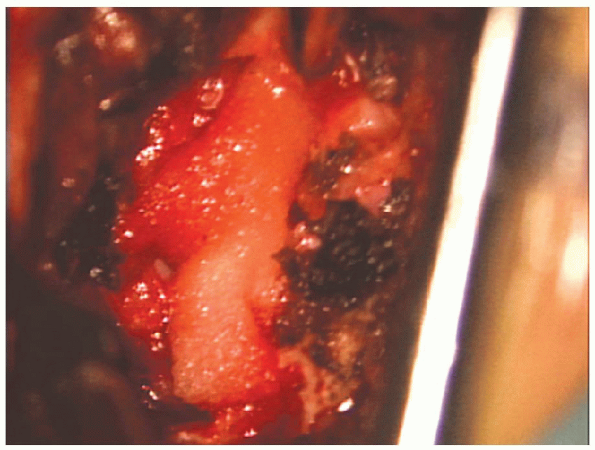
FIGURE 17-27. Gelfoam is placed over the nerve root.
|
removal of the herniated material is complete and the surgeon is
satisfied with the nerve root decompression, a fat graft or Gelfoam is
used to cover the laminotomy (Fig. 17-27).

Currently, there is no consensus on the risks and benefits of
postoperative scar barriers. Bupivacaine (Marcaine) may be used to
infiltrate wound edges to reduce postoperative pain at the incision
site just before closure. The fascia is closed
before injecting the skin with bupivacaine. If bupivacaine drips onto
the nerve root it may cause numbness of the affected root that will be
difficult to differentiate from a neural deficit. The surgical
wound is closed in a standard manner: a subcuticular stitch with
Steri-Strips and a sterile dressing. The dressed incision is protected
by a sterile eye patch, which is usually large enough to cover the
small incision. At the time of closure, ketorolac (Toradol), a
nonnarcotic injectable analgesic, may be given to the patient and
continued postoperatively for comfort to ease early mobilization and
prevent the side effects of narcotic medications.
include incidental durotomy, infection, bowel and bladder dysfunction,
and nerve root injury. Although incidental durotomy is best avoided
during disc surgery, adequate intraoperative repair has not been shown
to adversely affect long-term outcome.
outpatient surgery or on the first postoperative morning) is beneficial
to the patient. Pain right after discharge is usually best managed with
NSAIDs. Only if necessary, mild narcotic pain medication such as
hydrocodone bitartrate and acetaminophen (Vicodin) or propoxyphene and
acetaminophen (Darvocet) can be prescribed. I prefer to use a
nonaddictive medication or restrict narcotic analgesics to only several
weeks for mild to moderate pain relief. After that, over-the-counter
medications are appropriate.
check. At this time, a formal physical therapy program is prescribed to
enhance patient recovery. They are custom designed to include exercise
to any weak lower extremity muscle groups secondary to neurologic
deficits. General trunk strengthening and aerobic exercise is
encouraged.
Lumbar disc surgery: results of the prospective lumbar discectomy study
of the joint section on disorders of the spine and peripheral nerves of
the American Association of Neurological Surgeons and the Congress of
Neurological Surgeons. Neurosurgery 1991; 29: 301-308.
