Idiopathic Scoliosis
severe musculoskeletal disorder of unknown etiology whose diagnosis and
treatment have been central to the development of orthopedic surgery as
a specialty. In its milder forms, scoliosis may produce only a change
in the shape of the trunk, but when severe can be markedly disfiguring
besides leading to cardiac and pulmonary compromise (Figure 18.1).
The goal of this chapter is to present the key elements in diagnosis,
natural history, and treatment of both early-onset and adolescent
idiopathic scoliosis (AIS).
therefore the term idiopathic remains appropriate. Scoliosis can also
be classified based on associated conditions because it occurs in many
neuromuscular disorders (cerebral palsy, muscular dystrophy, and
others) as well as in association with generalized diseases and
syndromes (neurofibromatosis, Marfan syndrome, bone dysplasia).
Congenital scoliosis, caused by failure in vertebral formation or
segmentation, causes a more mechanically understandable type of
scoliosis.
 |
|
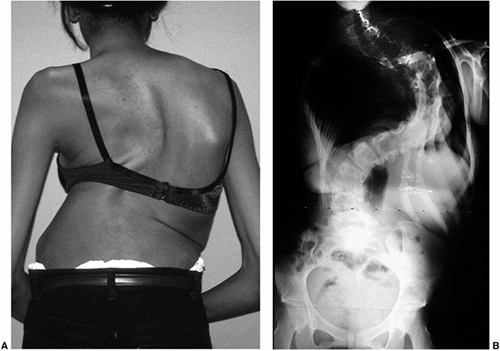
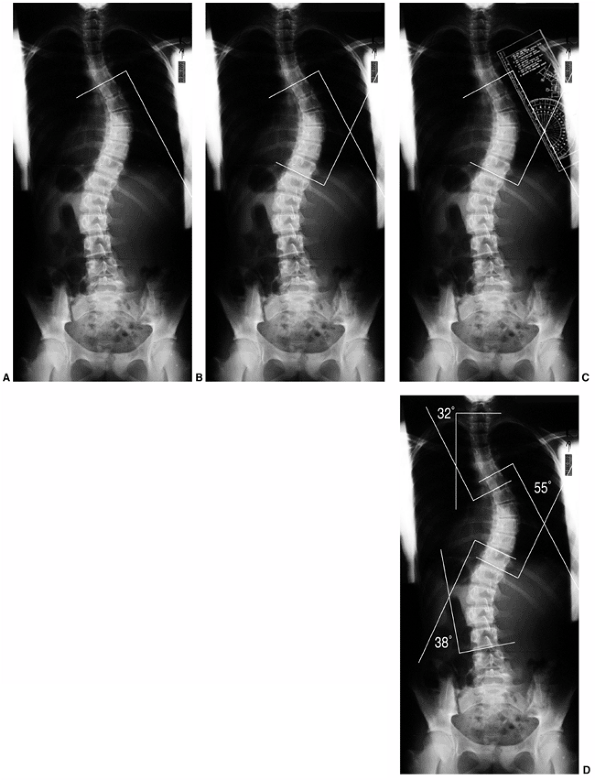
Figure 18.1 A:
This 16-year-old girl with severe scoliosis refused early treatment and had severe progression. Her clinical examination demonstrated marked trunk and rib deformity, and she had reduced pulmonary function. B: The posteroanterior radiograph demonstrates a right thoracic curvature of 125 degrees. With proper diagnosis and early treatment, deformity such as this should be completely avoidable in AIS. |
neuromuscular, syndrome related, congenital) largely dictates its
natural history, including the risk for and rate of curve progression,
as well as the effect the curve will have on cardiopulmonary function,
mobility, and appearance. Additionally, the age at onset plays heavily
on the natural history, with childhood forms being more serious than
the classic adolescent-onset scoliosis seen in women. Although
scoliosis includes both sagittal-plane and torsional malalignment of
the spinal column, the deformity is most readily noted as frontal plane
deformity. A better understanding of the three-dimensional nature of
scoliosis has led to many recent advances in its treatment.
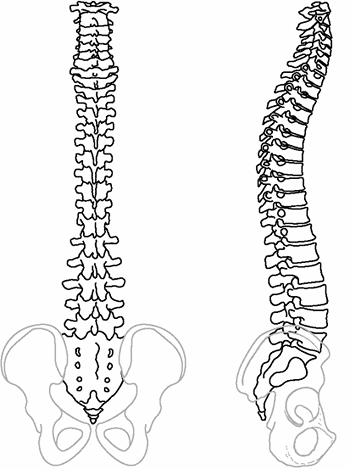
has sagittal plane contours including thoracic kyphosis averaging 30 to
35 degrees (range 10 to 50 degrees, T5-T12) and lumbar lordosis
averaging 50 to 60 degrees (range 35 to 80 degrees, T12-S1) (Figure 18.2) (1,2,3). The scoliotic spine deviates from midline in the frontal plane and rotates maximally at the apex of the curve (4,5).
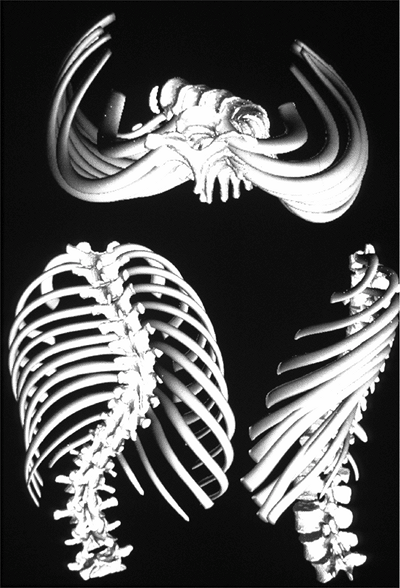
The vertebral rotation at the apex of the curve, through the attached
ribs, produces the typical chest wall prominence (Adams sign) that
allows early diagnosis (6,7) (Figure 18.3).
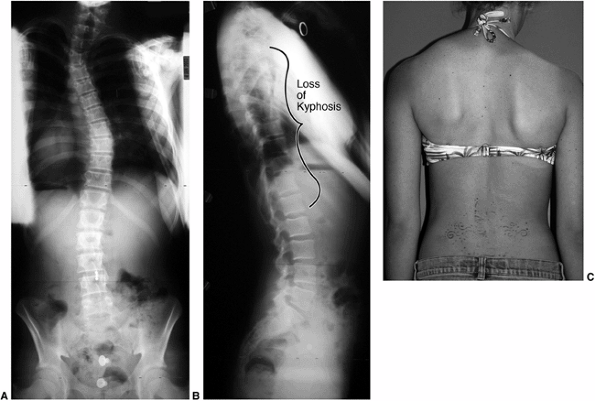
scoliosis was also kyphotic (increased roundback). It is now understood
that the apparent “hump” on the back is caused by rib prominence
secondary to the rotational deformity of the vertebrae and rib cage,
and that most thoracic idiopathic scoliosis is associated with a decrease in normal thoracic kyphosis (8,9,10,11). Dickson et al. (10,11) have added to Somerville’s postulate (12)
that an early evolution to lordosis in the normally kyphotic thoracic
spine leads to a rotational buckling of the spinal column (Fig. 18.4).
This is not to say that thoracic scoliosis is always hypokyphotic
because many congenital, neuromuscular (and a few idiopathic) cases
have a true kyphotic component.
 |
|
Figure 18.2
These posteroanterior and lateral schematic drawings of the normal cervicothoracic and lumbosacral spine demonstrate the normal alignment including cervical lordosis, thoracic kyphosis, and lumbosacral lordosis. |
Therefore, in most cases the typical right thoracic curve is associated
with a frontal plane deviation with the apex of the curve shifted to
the right, a sagittal plane alteration generally of local hypokyphosis
at the apex, and a transverse plane rotation with the right side
rotated posteriorly.
deformity in both discs and vertebrae. Wedging develops in both
structures, and changes in vertebral body shape are thought to follow
the Hueter-Volkmann principles of bone growth (14),
that is, reduced growth in regions of excessive compression as might
occur in the concavity of a scoliotic spine. This results in asymmetric
growth and/or remodeling of the vertebral bodies, pedicles, laminae,
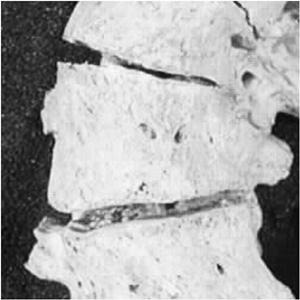
and facet joints, as well as of the transverse and spinous processes (Fig. 18.5). Reduced concave growth accentuates the deformity, increases the compressive forces, and perpetuates the process.
unknown, substantial research has been performed, and many theories
have been proposed. These range from genetic factors to disorders of
bone, muscle, and disc, as well as growth abnormalities and factors
related to the central nervous system.
of scoliosis in the family members of affected individuals, thereby
confirming the existence of a genetic component to the etiology of
scoliosis (15,16,17,18,19). Risenborough and Wynne-Davies found scoliosis in 11.1% of first degree relatives of 207 patients with idiopathic scoliosis (19). These familial studies suggest a polygenic inheritance pattern, rather than a clear autosomal or sex-linked trait.
confirmation of genetic etiologic factors. In monozygotic (identical)
twins the frequency of scoliosis in both twins (when one is affected)
is higher, ranging from 73% to 92%, compared to dizygotic twins, in
which the frequency ranges from 36% to 63% (20,21).
Genetic studies of families in which multiple family members are
affected have suggested several sites within the genome that appear to
be linked to scoliosis (22,23,24); however, the genes and gene products responsible for the development of idiopathic
scoliosis remain unknown. Specific candidate genes, however, have been
ruled out (types I and II collagen, fibrillin, and elastin) (25,26). A common pathway explaining exactly how scoliosis develops remains to be elucidated on the basis of genetic findings.
 |
|
Figure 18.3
A three-dimensional reconstruction of the scoliotic spine and trunk demonstrates the three-plane deformity of the spine and attached ribs. The torsional deformity is maximal at the apex of the curvature. (Courtesy of St. Justine Hospital, Montreal, Canada.) |
scoliosis is centered in each of the structural tissues of the spine
(bone, muscle, ligament/disc). There are known conditions in which each
of these tissues is pathologic and associated with scoliosis. For
example, fibrous dysplasia (bone–collagen abnormality) resulting in
dysplastic, misshapen vertebrae (27), muscle
disorders such as Duchenne muscular dystrophy leading to a collapsing
scoliosis, and soft tissue–collagen disorders such as Marfan syndrome
are clearly associated with the development of scoliosis.
are not thought to be related to idiopathic scoliosis, collagen
cross-linking deficiencies have been proposed (28), but have not been confirmed in biopsy specimens (29).
It seems plausible, however, that subtle deficiencies in any of the
tissues of the spine could result in a predilection for collapse of the
spine and idiopathic scoliosis progression (30). Several researchers (31,32,33) have suggested that AIS
may be related to osteopenia. They found that the mineral density of
the vertebral bone is lower in girls with scoliosis who were aged 12 to
14 years, compared to matched controls; the mineral density is lower
not only in the vertebrae but also in the proximal femur (31,34). However, the rationale for how osteopenia relates to the pathogenesis of scoliosis remains undefined.
 |
|
Figure 18.4 A: This posteroanterior radiograph demonstrates the appearance of a double thoracic scoliosis curve pattern. B:
The lateral radiograph demonstrates the relatively straight sagittal profile of the thoracic spine with loss of normal thoracic kyphosis. This is a common feature of adolescent idiopathic scoliosis. C: The clinical appearance of this patient demonstrates a prominent scapula. However, this is not caused by kyphosis but by the rotational deformity of the ribs, which secondarily makes the right scapula more prominent. Additionally, a left upper thoracic trapezial fullness can be appreciated in this patient, caused by the left upper thoracic curvature. |
an attractive etiologic theory because scoliosis development and
progression are temporally related to the time of rapid adolescent
growth (35,36).
Differential growth rates between the right and left side of the spine
could generate an asymmetry that would be accentuated with asymmetric
biomechanical loading and the Hueter-Volkmann effect (37,38,39,40).
have postulated that the etiology of scoliosis relates to altered
growth primarily in the sagittal plane with the development of relative
thoracic lordosis. If the condition is severe enough, the spine rotates
laterally to maintain global sagittal balance. The increased length of
the anterior spine is effectively “shortened” by rotation or buckling (45)
of the apical segment laterally. This theory accounts for all three
planes of deformity. In addition, computer-generated finite element
modeling of anterior spinal overgrowth has been able to replicate the
typical three-dimensional deformity of scoliosis (46).
Studies of the growth mechanism of the anterior and posterior aspects
of the vertebral elements suggest a different mechanism of growth in
each (endochondral growth anteriorly and intramembranous growth
posteriorly) (47). Interestingly, it has been
documented that thoracic kyphosis tends to decrease in normal children
during the normal adolescent growth spurt (48).
Therefore, irregularities in the changing sagittal shape of the spine
during the rapid period of adolescent growth may be important in the
development of scoliosis.
 |
|
Figure 18.5
This anterior view of a human scoliotic specimen demonstrates the substantial wedging of the apical vertebra. These changes in shape of the vertebra are thought to be a result of altered growth, according to the Hueter-Volkmann law. This appears to be a component of the progression seen in idiopathic scoliosis during rapid phases of growth. (Courtesy of Stefan Parent, MD) |
also appear to be related to the development of scoliosis. The growth
and maturational changes that occur during the transition from
childhood to adolescence are complex and highly regulated. Growth
hormone and estrogen are known to be involved in pubertal changes, and
their roles in scoliosis development have been widely studied (62,63,64,65).
Although the relation between scoliosis progression and skeletal growth
is well recognized, the proposed alterations in the regulation of
growth that could be responsible for scoliosis are not yet defined.
may result in scoliosis, and the role of the central nervous system in
idiopathic scoliosis has been studied in detail (66,67,68,69,70,71,72,73). Goldberg et al. (71)
noted greater asymmetry of the cerebral cortices in patients with
scoliosis. Also, abnormalities in equilibrium and vestibular function
have been noted in patients with scoliosis (66,74,75,76,77,78,79); however, it is difficult to be sure whether these findings are primary or secondary (80). Woods et al. (79)
have suggested a neurologic etiology of scoliosis based on the
surprising finding that hearing-impaired children seem to have a lower
incidence of scoliosis. Syringomyelia is associated with an increased
incidence of scoliosis (81,82,83),
possibly due to direct pressure on the sensory or motor tracts of the
spinal cord. Alternatively, there may be no relation to the dilation of
the central canal, but instead brain stem irritation from an associated
Chiari malformation or enlargement of the fourth ventricle of the brain
could be the cause.
pineal gland may be related to scoliosis. This theory is based on
research involving pinealectomy in chickens. The procedure was found to
result in a high incidence of severe scoliosis in the birds (84,85,86). In these studies, post-pinealectomy melatonin deficiency presumably led to scoliosis in the chickens (87).
Melatonin receptors are located in the brainstem and spinal cord dorsal
gray matter, areas associated with postural control. Results of
subsequent studies of human melatonin levels have been conflicting and
inconclusive. Machida et al. found a lower-than-normal melatonin
concentration in the serum of patients with progressive scoliosis
compared to the serum of those with stable curves (88). In contrast, Hilibrand et al. (89) and Fagan et al. (90)
found no difference in urine melatonin levels between patients with
scoliosis and age-matched normal control subjects. In addition, Bagnall
et al. (91) found no difference in serum
melatonin levels of patients with scoliosis. Confounding these studies
is a recent report of melatonin signaling dysfunction in osteoblasts
from patients with scoliosis. Melatonin typically inhibits adenyl
cyclase (cAMP) activity; however, this was not the case in bone-forming
cells of patients with scoliosis (92). It is unclear whether this relates to the generalized osteopenia seen in patients with AIS (34).
Therefore, there is as yet no confirmation that melatonin deficiency in
humans is associated with scoliosis, as is seen in chickens.
as well as ultrastructural changes in the sarcolemma at the
myotendinous junction, supporting the concept of a primary muscle
disorder (94). However, as in the findings
relating to equilibrium, it is difficult to determine a causal
relation; the findings in muscle could be secondary, reflecting the
response of the muscles to asymmetric spine loading (95).
has also been suggested to exist in higher levels in patients with
progressive scoliosis (96). Platelets are
contractile in nature and have features similar to those of muscle;
both are known to be dependent on calcium signaling. Therefore, a
possible relation between calmodulin and muscular dysfunction is
postulated; however, calmodulin is ubiquitous in the body and appears
to be related to many other important intracellular processes including
regulation of DNA synthesis, growth, and development (97).
At the very least, this raises the question of whether the increased
calmodulin levels measured in patients with progressive scoliosis
merely represent the more rapid growth of these patients (which in
itself is known to be a risk factor for curve progression).
naturally unstable construct, made of multiple mobile segments. As Stagnara (98)
has noted, one should not be surprised that a minor disturbance in
either the structure, support system, or growth of the spine could lead
to scoliosis, particularly in a complex structure whose “normal” state
includes multiple curves (sagittal plane) based on an oblique
foundation (the sacrum). There are likely several causes of idiopathic
scoliosis, and active research continues in an attempt to find a
unifying theory as to its development.
and several useful classification systems have been developed. The
Terminology Committee of the Scoliosis Research Society (SRS) gives a
detailed technical description of curve location (this is not the same
as the curve pattern descriptions developed for the purpose of planning
surgical correction—see “Surgical Correction of Idiopathic Scoliosis” section).
defined by the location of the apical vertebrae as noted in the
following list:
-
Cervical: apex between C2 and C6
-
Cervicothoracic: apex between C7 and T1
-
Thoracic: apex between T2 and T11
-
Thoracolumbar: apex between T12 and L1
-
Lumbar: apex between L2 and L4
-
Lumbosacral: apex at L5 or below
its center and is the most laterally deviated disk or vertebra of the
curve. Usually a single vertebra can be defined, but in other cases a
pair of vertebrae are at the apex (in this case the “apical disc” is
used to define the level of the apex). The apical vertebra(e) are also
the most horizontal. Therefore, a patient with an apical vertebra at T8
is said to have a thoracic curve, whereas an apex at T12 is considered
a thoracolumbar scoliosis. The end vertebrae
of a curve define the proximal and distal extent of a curve and are
determined by locating the vertebrae most tilted from the horizontal
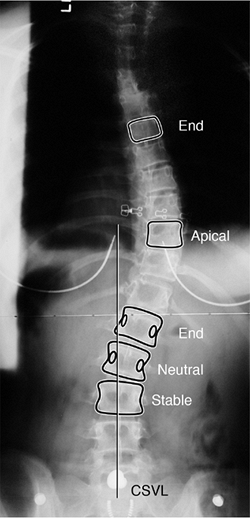
(these vertebrae are used for making the Cobb measurement). The central sacral vertical line
(CSVL), a vertical line that bisects the sacrum, is used for assessing
the balance of the spine in relation to its base (the pelvis). The stable vertebra is the most cephalad vertebra distal to the major curve that is bisected by the CSVL. The neutral
vertebra is the most cephalad vertebra that remains without transverse
plane rotation. This is most easily recognized as a symmetric
appearance of the pedicles (Fig. 18.6).
-
Infantile (0 to 3 years)
-
Juvenile (4 to 10 years)
-
Adolescent (11 years to 17 years)
-
Adult (≥18 years)
history of the disorder; early-onset cases are more likely to be
progressive. The onset of scoliosis before the adolescent growth spurt
is more likely to have an underlying spinal cord abnormality as the
cause of the deformity. The incidence of such an abnormality is
approximately 20% in the juvenile and infantile groups (99,100).
 |
|
Figure 18.6
Posteroanterior radiograph demonstrating the important vertebra and landmarks which define this curvature. The two end vertebrae of the thoracic curve are at T6 and L1, with the apex or apical vertebra at T10. The end vertebrae define the ones most tilted from the horizontal, and are used for measuring the Cobb angle of the curvature. The neutral vertebra is the most cephalad vertebra that has neutrally rotated pedicles, whereas the stable vertebra is the most proximal one that remains bisected by the central sacral vertical line (CSVL). The CSVL is drawn vertically from the midsacrum. These landmarks become important in ultimately defining a curvature, as well as in determining the levels for surgical treatment. |
major curve is usually the first to develop and is the curve of
greatest magnitude. However, at times, two or even three curves of
equal severity exist, which make the determination of a major versus a
minor curve difficult. Minor or compensatory curves develop after
formation of the primary curve as a means of balancing the head and
trunk over the pelvis. Similar compensation occurs in the sagittal
plane where the typical lordotic thoracic curve may end both cranially
and caudally with a junctional kyphosis. It is important to recognize
focal and global alterations in the sagittal plane when planning
surgical correction.
curves being the more rigid ones (not correcting well when bent to the
side). The degree of curve rigidity, which differentiates a structural
from a nonstructural curve, has been debated, although Lenke et al. (101)
have proposed a limit of 25 degrees on side-bending radiographs as the
value above which a curve is considered to be structural.
etiology as idiopathic (or idiopathic-like), neuromuscular,
syndrome-related, or congenital. It is important to consider a patient
presenting with scoliosis as a patient presenting with a sign (i.e.,
scoliosis) rather than a diagnosis—scoliosis. Although most scoliosis
(approximately 80%) is idiopathic, the remaining cases are associated
with a wide variety of disorders in which scoliosis is often the
presenting complaint.
The scoliosis associated with these conditions will be discussed in
other chapters of this text. Neuromuscular disorders of either
neuropathic or myopathic etiology make up a large proportion of the
nonidiopathic causes of scoliosis in childhood. Intra- or extraspinal
tumors or abnormalities must also be considered as possible causes of
scoliosis. Congenital scoliosis and kyphosis as well may lead to
progressive spine deformity. An awareness of each potentially
associated condition helps when analyzing the various proposed
etiologic factors in idiopathic scoliosis. More importantly, the
diagnosis of idiopathic scoliosis requires the exclusion of these other
conditions.
with scoliosis requires the physician to assess the patient for all of
the conditions (listed in Table 18.1) that are
associated with scoliosis. Most adolescents presenting with scoliosis
will in fact be diagnosed with the idiopathic variety of the disorder;
however, a careful history and physical examination are required in
order to be certain no other causes exist. The history should therefore
focus on neurologic symptoms, the family history, and pain.
evaluation of scoliosis is the key to arriving at the correct
diagnosis. For example, in North America a screening examination
(either in school or at a routine primary care visit) often leads to
referral. While recording the patient’s history, the physician should
include questions about family history of scoliosis, the patient’s
recent growth, and the physical changes of puberty (breast budding,
axillary/pubic hair, onset of menses in girls, and voice change in
boys). When compared to the rates of occurrence in the general
population, scoliosis occurs 3 times more frequently in a child whose
parent is similarly affected and 7 times more frequently if a sibling
is affected (17). Additionally, if the
patient’s parents or sibling has been treated for scoliosis, that may
suggest a greater likelihood of progression in the patient. A record of
increases in height over the prior few years is important in predicting
remaining spinal growth and the risk for curve progression (102,103).
This information may be available from the primary care physician, or
sometimes from measurements on the wall/door in the family’s home.
Breast development and the onset of menses are important maturational
time points in females (104,105).
The past surgical history is important in identifying scoliosis
associated with congenital heart disease and/or a prior thoracotomy.
The family history as well as a review of body systems should identify
disorders known to be associated with scoliosis (Table 18.1).
because most patients with idiopathic scoliosis have little or no
discomfort. After scoliosis has been diagnosed (in a screening
setting), patients often develop “pain” that continues until the
diagnosis and prognosis have been clarified by an orthopedic consultant
who can provide reassurance. Despite the common belief among physicians
that mild idiopathic scoliosis is never painful, a recent study
suggests that adolescents with mild curves often report discomfort of a
mild fatigue variety. Ramirez et al. (106)
noted back pain in 23% of 2442 patients with “idiopathic” scoliosis.
Only 9% of those with pain were subsequently found to have an
underlying pathologic condition to explain it (diagnoses such as
spondylolysis/spondylolisthesis, Scheuermann kyphosis, syringomyelia,
herniated disc, tethered cord, and intraspinal tumor).
|
TABLE 18.1 SCOLIOSIS RESEARCH SOCIETY’S DIAGNOSES BY WHICH SCOLIOSIS CAN BE CLASSIFIED
|
|
|---|---|
|
complaint of back pain should make one question, “Is this truly an
idiopathic curve?” A child or adolescent who presents with severe
back pain and is subsequently found to have scoliosis requires a very
carefully taken history, a physical examination, and a radiographic
study [a bone scan and/or magnetic residence imaging (MRI) study may be
required] because an underlying etiologic cause is more likely (106,107,108).
However, the clinician must distinguish between “severe pain”
(requiring further workup) and the mild fatigue pain (as described
earlier), reported by Ramirez et al. and Fairbank et al (106,109).
During adolescence, activity-related musculoskeletal low back pain
occurs at a frequency greater than in childhood but less than in
adulthood (110,111).
presence of neurologic symptoms and signs are the most useful findings
in identifying nonidiopathic scoliosis. In younger patients (less than
10 years) with an unrecognized neurologic cause, actual neurologic
findings are often absent on physical examination, and the spinal
curvature itself must be considered as the initial sign of a neural
axis abnormality (99,100,112,113,114).
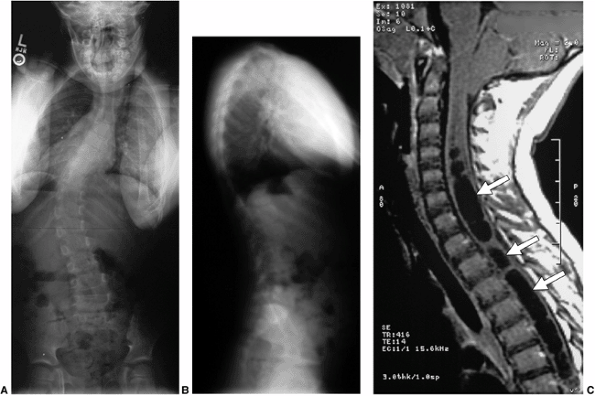
The most common intraspinal abnormality found in this age group is
syringomyelia (dilation of the central spinal canal) with an associated
Chiari malformation (brain stem below the level of the foramen magnum) (Fig. 18.7).
nonidiopathic type of scoliosis. Neurologic symptoms such as weakness,
sensory changes (upper and lower extremities), and balance/gait
disturbance suggest intraspinal pathology (syringomyelia, tethered
cord, tumor, etc.) as the cause of
spinal curvature (107,113,114).
The neurologic history should therefore focus on information about the
patient’s difficulties with grasping, walking, running, and stair
climbing. A history of radiating pain, numbness, tingling in the limbs,
and difficulties with bowel or bladder control should also be sought.
 |
|
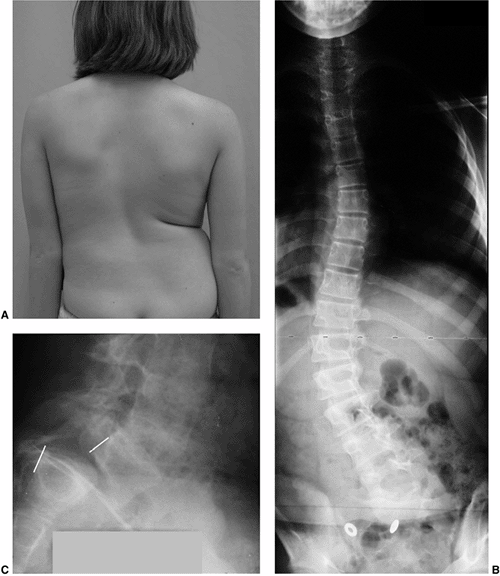
Figure 18.7 A: Posteroanterior radiograph of a juvenile patient with a left thoracic curve. B:
The lateral radiograph demonstrates relatively normal or even slightly increased thoracic kyphosis. Because the patient is in the juvenile age-group and the curve is left-side with an increased rather than decreased thoracic kyphosis, a spinal magnetic resonance imaging (MRI) was ordered. C: MRI of the midsagittal section of the cervicothoracic spine demonstrates a large syringomyelia (arrows) with significant dilatation of the central spinal canal. The syringomyelia was treated with suboccipital decompression. |
includes evaluation of trunk shape, trunk balance, the neurologic
system, limb length, skin markings, and skeletal abnormalities.
Assessment of pubertal development includes assessment of the stages of
breast development and the presence of axillary/pubic hair (Tanner
stages). This can be done discreetly without fully undressing the
patient. Girls can be asked to wear a two-piece swimsuit for the
physical examination (the instruction regarding this can be given at
the time of fixing the appointment). This reduces the patient’s anxiety
and apprehension, yet allows assessment of breast and overall
development.
inspected for asymmetry of shoulder height, scapular position, and
shape of the waist viewed from both front and rear. Potential pelvic
tilt (an indicator of limb length difference) is determined by
palpating the iliac crests and posterior inferior iliac spines
bilaterally in the standing patient with both hips and knees fully
extended. Lateral translation of the head can be measured in
centimeters of deviation from the gluteal cleft by dropping a plumb
line from C7. Deviation of the chest cage (trunk shift) should also be
assessed because patients can have full head compensation (return of
the head and neck back to midline) yet have marked lateralization of
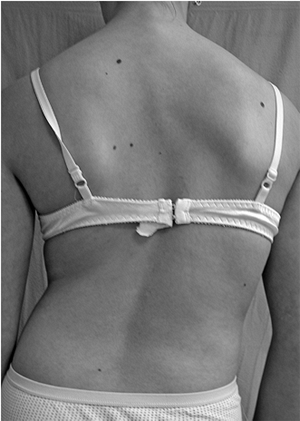
the trunk (Fig. 18.8).
has the patient bend forward at the waist with the knees straight and
palms together. This examination should be performed from behind (to
assess lumbar and midthoracic rotation) and from the front (to assess
upper thoracic rotation), as well as from the side (to assess
kyphosis). Any
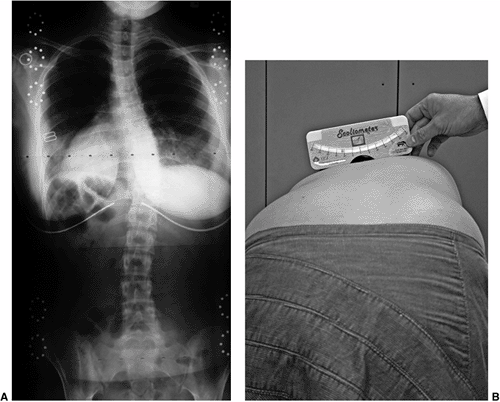
asymmetry of the upper thoracic, midthoracic, thoracolumbar, and lumbar regions should be quantitated with a scoliometer (116) [to determine the angle of trunk rotation(ATR)] or by measuring the height of the prominence in centimeters (Fig. 18.9). This prominence reflects the rotational deformity of the spine associated with scoliosis (117,118).
Although there is not always an exact correlation, in general an ATR of
5 to 7 degrees is associated with a radiographic Cobb angle measurement
of 15 to 20 degrees. [This is only an approximate
guideline—occasionally patients may have little trunk rotation and yet
have significant radiographic scoliosis, and vice versa (119).]
 |
|
Figure 18.8
Careful examination of the back is required in order to identify the physical features of scoliosis. These include asymmetry of the scapulae, shift of the trunk, and asymmetry of the waistline, as well as asymmetry in the level of the shoulders. |
decreased range during forward/side bending may be caused by pain,
lumbar muscle spasm, and/or hamstring tightness; any of these should
suggest underlying pathology. These findings plus abnormalities in
straight-leg-raise testing suggest irritation of the lumbar roots
caused by spondylolysis, disc herniation, infection, neoplasm, or other
factors.
motor strength in the major muscle groups of all four extremities, and
sensation. Watching the patient’s gait, toe-and-heel walk, tandem walk,
deep squat, and single-leg hop allows rapid assessment of balance and
motor strength. The presence of a cavus deformity of the feet,
especially if it is unilateral, suggests an abnormality of the
neurologic system/spinal cord. Testing for reflexes should include deep
tendon reflexes of the upper and lower extremities as well as the
Babinksi test for long tract signs. Abdominal reflexes are obtained by
lightly stroking the abdominal wall with a blunt instrument (key, end
of reflex hammer) adjacent to the umbilicus with the patient supine and
relaxed. The expected brisk and symmetrical unilateral contraction of
the abdominal musculature pulling the umbilicus toward the side being
stroked indicates normalcy. When the reflex is persistently abnormal
(reflex absent on one side and present on the other), intraspinal
disorders, particularly syringomyelia, should be considered. The upper
extremity examination should not be ignored because cervical-level
pathology (particularly syringomyelia) presents here (81,120).
examination include inspection of the skin (both on the back and
elsewhere) for cutaneous evidence of an associated disease. Café au
lait spots and/or axillary freckles suggest possible neurofibromatosis,
whereas dimpling or a hairy patch in the lumbosacral area may suggest
an underlying spinal dysraphism. Excessive laxity of skin or joints may
be related to a connective tissue disorder such as Marfan syndrome or
Ehlers-Danlos syndrome.
position if pelvic tilt is noted during the standing examination. A
spinal curvature which results from a limb-length difference is usually
compensatory and serves to rebalance the trunk over the pelvis. A short
right leg results in a compensatory right lumbar curve. There is no
rotational deformity of the spine with these curves, and in the lumbar
region the prominence noted on the forward bend test is on the concave
side of the curve (the long leg makes the iliac crest and lumbar spine
more prominent on that side). This is the opposite of what is seen in
true lumbar scoliosis, where the rotational prominence noted on the
bending test is found on the side of the curve convexity. The presence
of the bending test rotational prominence on the “wrong” side in a
lumbar curve is almost always diagnostic of spinal asymmetry caused by
limb-length discrepancy rather than true scoliosis. The prominence
disappears if the pelvis is leveled with an appropriately sized block
underneath the short leg.
upright (standing) posteroanterior projection of the entire spine
exposed on a single cassette. In an adolescent, because of the larger
body size, this requires a three-foot length film for visualizing the
entire spine as well as the head and
pelvis
on a single radiograph. Many radiology units do not have long
cassettes, and so a chest-film-sized cassette can be substituted
instead, with the film centered on the area of maximal deformity
(usually the thorax). If a lumbar curve is present, a separate film
must be taken. Clearly, it would be better for the child to be referred
to a center that uses long cassettes, allowing a single film.
 |
|
Figure 18.9 A: A 28-degree right thoracic scoliosis as seen on the posteroanterior radiograph. B:
The Adams forward bend test demonstrated an 11-degree scoliometer measurement, indicating a corresponding measure for the angle of trunk rotation associated with this scoliosis. The forward bend test remains one of the most reliable means of detecting early scoliosis, other than a radiograph. Scoliometer measurements greater than 7 degrees generally warrant a screening posteroanterior radiograph. |
because diagnostic and treatment standards developed over the years are
based on films in the upright posture. In very young patients, or in
those with severe neuromuscular involvement, radiographs taken in the
sitting or even supine position may be the only ones possible. The
magnitude of the curve is greater when the patient is upright (compared
to supine), and this is of particular importance in infantile and
congenital curves when radiographs are taken before and after walking
age. “Curve progression” may mistakenly be noted with the first
upright-position radiograph as compared to prior supine views, when in
fact one has simply documented that gravity causes a curve to be more
severe.
x-ray screening of a thoracic curve unless back pain or sagittal
deformity are noted. It is important to assess the sagittal alignment
(noted globally and regionally) once the diagnosis of scoliosis has
been confirmed. For this radiograph, the sagittal balance varies with
the method of arm positioning (the arms must be flexed for the spine to
be clearly visualized). With the arms held straight forward, the trunk
shifts posteriorly, and therefore the best position for viewing relaxed
standing is with the arms flexed as little as possible to clear the
spine (121) (Fig. 18.10).
A lateral view of the lumbosacral junction is often performed in lumbar
scoliosis to assess for spondylolysis/spondylolisthesis as a possible
cause (Fig. 18.11).
radiation exposure of sensitive tissue (e.g., breast, thyroid, ovaries,
and bone marrow) include taking only the required number of x-rays,
utilizing rare earth radiographic enhancing screens with fast film, and
a posterior-to-anterior exposure (122,123,124).
The lifetime risk for developing breast or thyroid cancer has been
suggested to increase by 1% to 2% in patients who are exposed to
multiple x-rays during the course of treatment of scoliosis; however,
these data relate to the 1960s and 1970s, before new radiation-reducing
techniques
became
available. The greatest reduction in breast and thyroid exposure is
associated with the posteroanterior exposure (compared to the
anteroposterior); this reduces breast/ thyroid exposure three- to
sevenfold (124).
Anteroposterior projection can shield the breasts; this is, however,
not recommended because this projection increases thyroid exposure
(shielding the thyroid obstructs the view of the upper spine). Doctors
counsel their patients by assuring them that during the radiographic
procedure, the exposure to the x-rays required to treat the disorder
correctly will be minimal and that the benefit of undergoing the
procedure outweighs the risk of not knowing the type and severity of
the scoliosis.
 |
|
Figure 18.10
Standing-position radiographs of the spine are typically obtained on three-foot cassettes. The lateral radiograph, when required in patients undergoing active treatment, should be obtained with as little disruption to the normal sagittal balance as possible. The position demonstrated in this figure allows the arms to be flexed slightly forward to clear the spine, yet produces very little shift of the trunk posteriorly, and therefore closely approximates normal standing posture. |
lateral-bend radiographs (to assess curve flexibility) as well as a
standing-position lateral view are required. Radiographs of
side-bending allow one to determine the degree of curve flexibility,
and to decide what levels to include in the instrumented and fused
segments. Controversy remains regarding the best method of obtaining AP
films of side-bending. Supine-position side-bending views (patient
maximally bent to the right and left) are standard at many
institutions, whereas others believe that a standing-position bend film
is a better indicator, particularly in the lumbar spine. Lateral
bending over a bolster provides somewhat greater correction and has
been proposed as a more accurate predictor of the correction obtainable
with the more powerful modern surgical instrumentation methods (125,126) (Fig. 18.12). In curves greater than 60 to 70 degrees, longitudinal traction films may also be helpful in evaluating curve flexibility (127,128).
There is no universal standard for how to obtain radiographs of
bending. Additionally, there is little agreement on how to make use of
the information gained. Flexible minor curves may be spared arthrodesis
in many cases, and this flexibility information has been utilized (yet
not necessarily standardized) in surgical decision making. More severe
cases of scoliosis, that is, curves that do not straighten to less than
50 to 60 degrees, have been suggested as benefiting from an anterior
release procedure prior to posterior instrumentation.
rib prominence rather than in the posteroanterior direction, provides a
more accurate picture of large curves that have a large rotational
component. From this angle, the true magnitude of the scoliosis can be
measured more accurately (129).
by looking for soft tissue abnormalities, congenital bony abnormalities
(wedge vertebrae, etc.), and then by assessing curvature (coronal plane
deviation). Bone assessment includes looking for wedged or
hemivertebrae (Fig. 18.13) and bar formation
bridging a disc space as well as midline irregularities such as spina
bifida or a bony spike suggesting diastematomyelia. The pedicles should
be inspected in order to verify that they are present bilaterally and
that the interpedicular distance is not abnormally increased, which
would suggest an intraspinal mass (130,131).
Absent pedicles or vertebral body lucency are associated with lytic
processes, such as tumor or infection. If a curve is noted, the
symmetry and levelness of the pelvis are analyzed. A limb-length
discrepancy can be estimated by determining height differences between
iliac wing and hip joint, assuming the patient had hips and knees fully
extended when the film was exposed.
allows quantification of the curve. A protractor (that is not bent or
warped) with single-degree demarcations allows for more accurate
measurements. The caudal and cranial end vertebrae to be measured are
the vertebrae that are the most tilted, with the degree of tilt between
these two vertebrae defining the Cobb angle (in a normal spine this
angle is 0 degrees). One should outline the superior end plate of the
cranial end vertebra and the inferior end plate of the caudal end
vertebra, construct a perpendicular to each of these lines, and then
measure the angle at which the lines cross. When more than one curve
exists, a Cobb angle measurement
should be made for each curve (Fig. 18.14).
When comparing serial radiographs of the same patient, the end vertebra
chosen should generally remain constant; however, adjustments may be
required over time to allow for brace-influenced change or other curve
pattern changes.
 |
|
Figure 18.11 A:
This 10-year-old girl presented with symptoms of increasing trunk decompensation, as well as low back pain and posterior thigh discomfort. She has an obvious trunk shift to the left, suggesting scoliosis. The posteroanterior rather than anteroposterior view is preferred because there is reduced radiation exposure. B: The standing-position posteroanterior radiograph confirms a 43-degree left lumbar scoliosis. C: Standing-position lateral view focused at the L5-S1 level demonstrates severe spondylolisthesis. Most of this patient’s lumbar deformity is related to an asymmetric forward slipping of L5 on S1, with rotational deformity translated to the lumbar spine above. Following correction of her spondylolisthesis with fusion from L4 to the sacrum, her scoliosis reduced to less than 15 degrees. |
(approximately 5 degrees for any curve measurement) should be
understood by the surgeon and the anxious parents (and patient) (133).
Therefore, a 6-degree difference is accepted by most surgeons as the
criterion for determining curve progression in idiopathic scoliosis. A
useful maneuver for both the neophyte and the expert surgeon is to view
the current radiographic film, the prior visit film, and the original
film side by side. A good scoliosis clinic should have the capacity for
allowing simultaneous viewing of at least 3 films displayed in a row.
Before making a Cobb
measurement
one should use the “eyeball method” to see whether the radiographic
curve appears to be getting worse. Patients and their parents often
want to make this assessment with the physician. This type of exercise
puts the Cobb measurement in perspective (and sometimes humbles the
physician with regard to accuracy of interpretation and reproducibility
of measurements).
 |
|
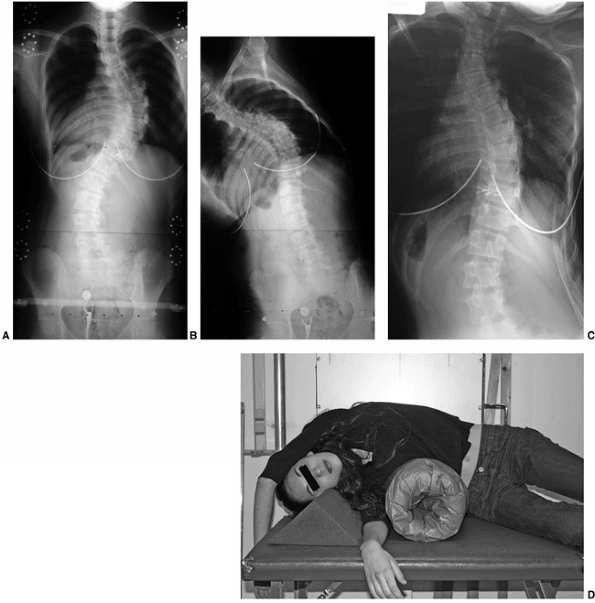
Figure 18.12 A:
This standing-position preoperative posteroanterior radiograph demonstrates right thoracic scoliosis with moderate left lumbar scoliosis. B: The flexibility of the left upper thoracic and left lumbar curves was assessed via the left-side-bending radiograph. C: The flexibility of the right thoracic curve was evaluated using the bolster side-bending technique. D: The bolster side-bending film is taken with the trunk laterally flexed on a bolster positioned under the ribs that correspond to the apex of the deformity. |
demonstrated on radiographic film by asymmetry of the pedicles and a
shift of the spinous processes toward the concavity. Two methods are
available for quantifying this rotation, one suggested by Nash and Moe (134) and the other by Perdriolle (135).
Vertebral rotation is not routinely measured clinically, however, and
both methods have substantial inaccuracies, which limit their
usefulness (136).
order to estimate remaining spinal growth, an important predictor of
risk for curve progression. The most widely used method in patients
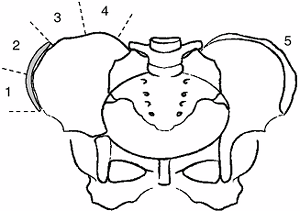
with scoliosis, although probably the least reliable, is that of Risser
(137), who noted that the iliac crest apophysis
ossifies in a predictable fashion from lateral to medial, and that its
fusion to the body of the ilium mirrors the fusion of the vertebral
ring apophysis,
signifying
completion of spinal growth. The lateral-to-medial ossification of the
iliac crest apophysis occurs over a period of 18 to 24 months, finally
capping the entire iliac wing. Risser classified the extent of
apophyseal ossification in stages, ranging from Risser 0, indicating
absence of ossification in the apophysis, to Risser V, indicating
fusion of the fully ossified apophysis to the ilium (spinal growth
complete) (138). Risser I through IV are assigned to the intermediate levels of maturity as seen in Figure 18.15.
 |
|
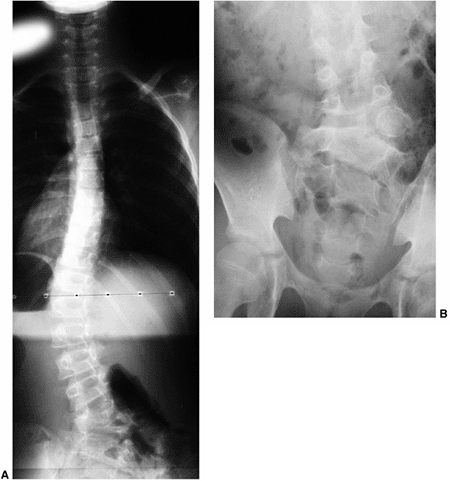
Figure 18.13 A:
This adolescent patient presented with spinal deformity. The standing-position posteroanterior radiograph demonstrates an obvious left thoracolumbar deformity. On careful examination, an abnormality at the lumbosacral junction is suggested. B: A cone-down radiograph of the lumbosacral junction demonstrates a clear hemivertebra. This congenital malformation is the primary deformity, and the thoracolumbar deformity above is a compensatory curve. It is certainly important to recognize this, because treatment of the thoracolumbar curve would lead to marked decompensation to the left. |
anteroposterior radiographs, which place the iliac apophysis close on
the x-ray film. This is in contrast to the common current practice of
posteroanterior projections and may explain some of the difficulties in
reading this sign (139). Despite the common
reporting of the Risser sign as a measure of maturity, the appearance
of the iliac apophysis generally occurs after the most important period
of rapid growth (103,105). Little and Sussman (140) have suggested that the Risser sign is no more accurate at predicting scoliosis progression than chronologic age.
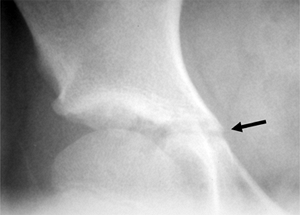
also provides a landmark for assessing growth potential. The triradiate
growth cartilage usually closes before the iliac apophysis appears
(Risser 0), at about the time of maximal spinal growth (141,142) (Fig. 18.16).
Skeletal age can also be measured using the Greulich and Pyle atlas to
compare hand radiographs against illustrated standards, although these
readings become less accurate (large standard deviations) in the
juvenile age-group (143). For additional discussion of assessing skeletal growth, see Chapter 2.
beyond plain radiography. Specialized imaging methods that can be used
to evaluate cases with unusual features include MRI, computerized
tomography (CT), and bone scintigraphy, each with specific indications
and advantages.
replaced myelography in the study of the neural elements in spine
disorders. An exception is the patient who has had prior placement of
stainless steel hardware (making MRI visualization nearly impossible)
and who continues
to have symptoms or develops new ones that have to be studied.
 |
|
Figure 18.14 A:
Measurement of the Cobb angle. The end vertebrae of each curve must be selected before any measurement can be made. The end vertebrae of the curve are those which are most tilted from the horizontal. B: The endplates of the superior and inferior end vertebrae of the thoracic curve are marked on this figure. Perpendicular lines are constructed. C: The angle between the two lines is measured with a protractor and defined as the Cobb angle measure of the scoliosis. D: This method is used for quantifying the magnitude of scoliosis at each of the three regions: upper thoracic, main thoracic, and lumbar. |
 |
|
Figure 18.15
Risser sign. The iliac apophysis ossifies in a predictable manner beginning laterally and progressing medially. The capping of the iliac wing is correlated with slowing and completion of spinal growth, generally occurring over a period of 18 to 24 months. |
Left thoracic curves have been shown to have an increased association
with spinal cord anomalies and may be an indication for MRI study (144,148).
It has also been suggested that all male patients should have a
screening MRI, although no studies exist to substantiate this.
Indications are not clear for routine MRI prior to corrective surgery
in patients with typical idiopathic scoliosis (for whom clinical
neurologic examination has shown normal results) (149). Several prospective studies have been completed (150,151,152)
of routine MRI screening for preoperative assessment (spine and brain)
of all patients with idiopathic scoliosis. There is no evidence that an
MRI is helpful in an otherwise normal adolescent with scoliosis.
Clearly, however, patients with an abnormality in the neurologic
examination (144) or with cutaneous findings
(suggestive of dysraphism or neurofibromatosis) should have an MRI
study of the spine and/or brain. Additionally, Ouellet et al. (153)
have suggested that a hyperkyphotic sagittal alignment of the thoracic
spine should raise suspicion of a syringomyelia and trigger an MRI
study. Severe angular and rotational deformities may be difficult to
analyze with an MRI because the spinal canal deviates into and out of
the planar cuts of the sagittal and coronal images. CT myelography that
produces a dye column may be better for revealing stenosis or an
intraspinal filling defect in extremely severe cases of scoliosis.
 |
|
Figure 18.16 The triradiate cartilage of the acetabulum is seen here (arrow).
The closure of this growth cartilage signifies completion of the most rapid phase of adolescent growth. However, at least 2 years of growth may be remaining following closure of the triradiate cartilage. |
no obvious cause may require a bone scan and/or MRI to evaluate for
possible tumor, infection, or spondylolysis. The bone scan is an
excellent screening test for studying the patient with scoliosis who is
experiencing pain. The test allows one to screen for conditions ranging
from osteoid osteoma to hydronephrosis. A single-photon emission
computed tomography (SPECT) type of bone scan (computerized tomographic
enhancement) is very useful in identifying spondylolysis and its
varying presentations (unilateral, bilateral, cold scan, hot scan,
etc.). If an area of increased activity is noted on the bone scan,
additional imaging (either MR or CT) may be required. An MRI may also
be used as a screening tool for patients who are in pain, although
cortical lesions (spondylolysis) may be harder to identify, and benign
osteoid osteomas have been overinterpreted as malignancies. Our
preference remains to screen painful backs with a SPECT scan and those
with atypical curves or neurologic signs with an MRI. The screening MRI
should include the entire length from the brain stem/posterior fossa to
the sacrum. Individual MRI sequences for the brain, cervical, thoracic,
and lumbar regions are not required. A limited number of images,
primarily in the sagittal and coronal planes, are sufficient to
identify a tumor, Arnold-Chiari malformation, syringomyelia, or
tethered cord. All aspects of the evaluation of a patient with
scoliosis (history, physical examination, imaging studies) should be
focused first on identifying possible nonidiopathic causes of the
deformity, and only secondarily on characterizing the specific features
of the curve. If one assumes an idiopathic etiology, an underlying
spinal cord abnormality or associated syndrome will be very difficult
to identify. One cannot recognize what one does not look for.
patients with spinal deformity and, because its etiology is unknown,
this diagnosis is one of exclusion made only after a careful evaluation
has ruled out other causes of
scoliosis.
The natural history of nonidiopathic scoliosis along with the
appropriate choice of treatment (and associated risks of treatment) may
deviate substantially from that of idiopathic scoliosis. The history,
examination, and imaging studies should be focused both on evaluating
the severity of the deformity and on identifying its cause. Clinical
features and treatment of idiopathic scoliosis also vary according to
the age-group to which the patient belongs (infantile, juvenile,
adolescent). These are summarized in the subsequent text.
greater than 10 degrees) in the childhood and adolescent population has
been reported as ranging from 0.5 to 3 per 100 (154,155,156,157,158,159,160). The reported prevalence of larger curves (greater than 30 degrees) ranges from 1.5 to 3 per 1000 (161,162) (Table 18.2). Therefore, small to moderate curves are the more common ones, and severe (life-threatening) curves are rare.
demonstrates a strong predominance of adolescent scoliosis, with a
series from Boston showing 0.5% infantile, 10.5% juvenile, and 89%
adolescent incidence (19). The natural history for each group varies substantially.
divided into three groups according to the age of onset (infantile,
juvenile, adolescent), there is a movement to simplify this to
“early-onset scoliosis” (before age 10 years) and “late-onset
scoliosis” (typical adolescent scoliosis) (157). Dickson and Weinstein (163) and Weinstein et al. (164)
believe that only early-onset scoliosis has the potential for evolution
into severe thoracic deformity with cardiac and pulmonary compromise.
This simpler classification is reasonable, although the traditional
three age-group division defined by the SRS remains the standard in
North America.
|
TABLE 18.2 PREVALENCE OF SCOLIOSIS
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
More recent reports, however, suggest a decrease in the frequency of
infantile cases, more closely paralleling the North American experience
(167).
The series from Britain suggests that the vast majority (up to 90%) of
these curves are self-limiting and resolve spontaneously (169);
however, the few that are progressive can be difficult to manage, often
resulting in lasting deformity and pulmonary impairment (171) (Fig. 18.17).
who, in a study of 135 patients with IIS, determined certain
radiographic prognostic parameters: (a) rib vertebral angle difference
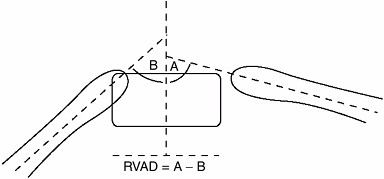
(RVAD) and (b) phase of the rib head. The difference in the obliquity
between the two ribs attaching to the apical vertebra (right versus
left) is known as the RVAD. The RVAD is
the most commonly utilized measure and is determined at the apical
vertebra on an anteroposterior radiograph. The ribs in the concavity of
progressive infantile scoliosis are relatively horizontal, whereas
those on the convex side are more vertically aligned (Fig. 18.18).
Eighty-three percent of Mehta’s reported cases resolved when the RVAD
was less than 20 degrees, compared to 84% progressing when the RVAD was
greater than 20 degrees (172,173).
approximately 8% to 16% of childhood idiopathic scoliosis (174,175,176),
and in many respects represents a transitional group between the
infantile and adolescent groups. Curves with onset in this age group
are often progressive, with potential for severe trunk deformity and
eventual cardiac and pulmonary compromise. Many patients who present in
adolescence (previously undiagnosed and untreated) with severe thoracic
curves requiring immediate surgery had the onset of their curves in the
juvenile age period, making the differentiation between juvenile and
adolescent grouping problematic.
 |
|
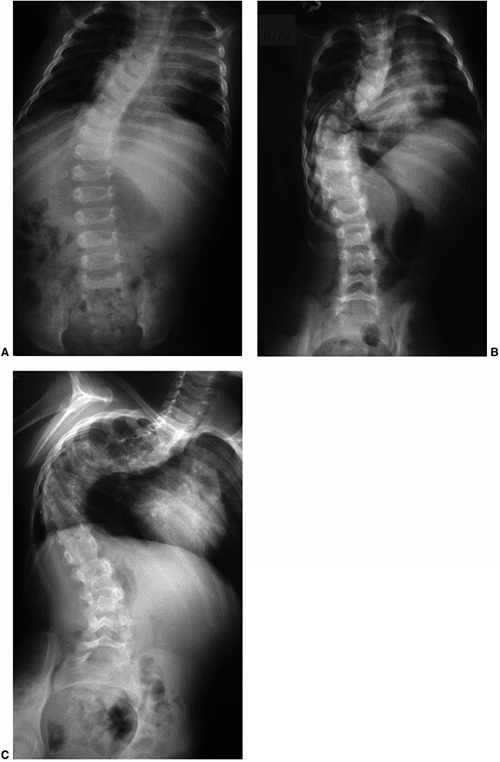
Figure 18.17 A:
This 4-month-old baby boy presented with a left thoracic scoliosis. A magnetic residence imaging (MRI) study was performed and found to be normal. He was therefore diagnosed with infantile idiopathic scoliosis. The rib vertebral angle difference was greater than 20 degrees, suggesting likely progression. B: At 3 years 2 months of age, after substantial progression and multiple attempts at casting, his curve has continued to increase with substantial chest wall deformity. C: At 9 years 2 months his curve has progressed to a severe degree. This was despite attempts at bracing, as well as subcutaneous growth rodding. His growth rods required removal after several years because of infection. This demonstrates the challenges of treating progressive infantile idiopathic scoliosis. |
 |
|
Figure 18.18
In infantile idiopathic scoliosis the rib vertebral angle difference (RVAD) helps in predicting curve progression. The RVAD is constructed by first determining the angle of the right and left ribs at the apical vertebral level of the deformity. The slope of the ribs relative to the transverse plane is measured for each rib. The difference in the angle between the right and left sides is the RVAD. A difference of more than 20 degrees suggests a high likelihood of a progressive form of infantile idiopathic scoliosis, according to Mehta. |
In a series of 109 patients evaluated by Robinson and McMaster, the
boys presented at a mean age of 5 years 8 months compared to a mean age
of 7 years 2 months for the girls. The ratio of girls to boys was 1:1.6
for those younger than 6 years and 2.7:1 for those older than 6 years
at presentation. Additionally, there were equal numbers of right- and
left-side curves in the younger group (less than 6 years) with a
preponderance of right-side curves (3.9:1) in the patients older than 6
years (174). When curves reach 30 degrees they are nearly always progressive if left untreated (178).
The rate of progression is 1 to 3 degrees per year before the age of 10
years, and this increases sharply to 4.5 to 11 degrees per year after
that age (174). This is particularly true of
thoracic curves which, despite treatment with braces, require
arthrodesis in more than 90% of the patients (174,178).
The surgical treatment of JIS is similar to that for AIS; however,
anterior growth ablation (fusion) in addition to posterior
instrumentation and fusion is more commonly indicated to prevent
“crankshaft” rotational growth following posterior fusion (see
subsequent text). In very young patients, instrumentation involving a
system that can be periodically lengthened is sometimes used
(instrumentation without fusion or fusion only at proximal and distal
hook sites).
theoretically develop a curve after the age of 10 years, corresponding
to the rapid growth phase of adolescence. Again, the separation of
adolescent and juvenile curves is somewhat arbitrary because an
11-year-old girl who presents with a 70 degree scoliosis almost
certainly had the onset of scoliosis in the juvenile age period. As
noted previously, the data indicate that the prevalence of curves of 10
degrees or greater ranges between 0.5% and 3%. This data has been
collected from a variety of sources including screening chest x-rays
and school screening programs (Table 18.2).
Roughly 2% of adolescents have a scoliosis of 10 degrees or greater,
but only 5% of these cases experience progression of the curve to
greater than 30 degrees. The ratio of boys to girls is equal among
patients with minor curves, but girls predominate as the curve
magnitude increases, with the ratio reaching 1:8 among those requiring
treatment (179).
will not is critical in deciding which patients need treatment. The
parameters that are significant in assessing the risk for scoliosis
progression include gender, remaining skeletal growth, curve location,
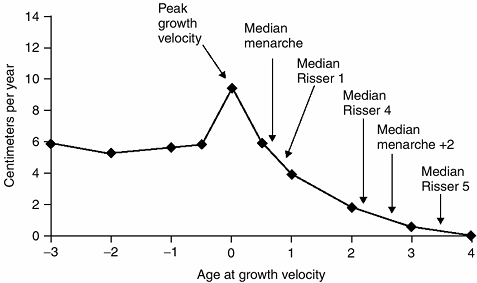
and curve magnitude. Scoliosis progression is most rapid during peak
skeletal growth (early infancy and adolescence). The peak growth
velocity of adolescence averages 8 to 10 cm of overall height gain per
year (35,142), with half of this growth coming from the trunk (spine) (180) (Fig. 18.19).
Several determinants are useful in predicting the remaining growth. The
age of the patient is one such obvious determinant. However,
substantial variations in skeletal growth are seen among patients of
the same chronologic age; therefore, bone age is a more consistent
indicator (181). Menarchal status helps
determine the growth spurt in girls (the onset of menses generally
follows approximately 12 months after the most rapid stage of skeletal
growth). For additional information on growth, see Chapter 2.
inaccuracies noted in the preceding text, has been used for assessing
the risk for curve progression. When the Risser sign is 1 or less the
risk for progression is up to 60% to 70%, whereas if the patient is
Risser 3, the risk is reduced to less than 10% (36,182).
of maturity (menarcheal status, Risser sign) are quite variable and
appear just after the adolescent growth spurt. If there is no accurate
record of prior growth performance, it is impossible to tell whether a
premenarcheal, Risser 0 patient is approaching, in the midst of, or
past the time of most rapid growth and consequent risk for scoliosis
progression. Recently, closure of the triradiate cartilage of the
acetabulum has been identified as a radiographic sign which more
closely approximates the time of peak growth velocity (142).
 |
|
Figure 18.19
During the adolescent growth spurt, the rate of increase in height rises from approximately 6 cm per year to as much as 10 cm per year. The age at peak height velocity or the time of most rapid growth occurs before the onset of menses or appearance of the Risser sign. It is during this phase of growth that scoliosis progression is most likely. (From Little DG, Song KM, Katz D, et al. Relationship of peak height velocity to other maturity indicators in idiopathic scoliosis in girls. J Bone Joint Surg Am 2000;82:685–693, with permission.) |
important variable for predicting the probability of progression.
Curves with an apex above T-12 are more likely to progress than
isolated lumbar curves (182). Curve magnitude at initial diagnosis also appears to be a factor associated with progression (36,183) (Fig. 18.20).
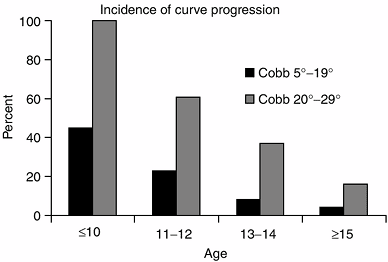
In a series of skeletally immature patients (Risser 0 or 1), curve
progression occurred in 22% of cases with a curve at initial diagnosis
of 5 to 19 degrees, compared with 68% incidence of curve progression
when the initial curve was 20 to 29 degrees (36). The rate of curve progression increased to 90% when the initial curve was 30 to 59 degrees (184).
should be understood when considering treatment in childhood and
adolescence. The risk of curve progression is greatest during the rapid
phases of growth as discussed in the preceding text; however, not all
curves stabilize after growth stops. In the long-term studies performed
at the University of Iowa, more than two thirds of the patients
experienced curve progression even after skeletal maturity. Thoracic
curves of less than 30 degrees tended not to progress, with the most
marked progression occurring in curves that were between 50 to 75
degrees at the completion of growth (continuing to progress at a rate
of approximately 1 degree per year). Lumbar curves generally progressed
if they were greater than 30 degrees at skeletal maturity (185,186).
Several studies provide insights into what the future holds for
affected individuals. Early studies of patients with untreated
scoliosis whose cases were followed for up to 50 years reported a
mortality rate twice that expected in the general population, with
cardiopulmonary problems being cited as the most common cause of death (187,188). Disability and back pain were common among the patients (188,189).
Unfortunately, the etiology of the scoliosis in these studies was mixed
(idiopathic, congenital, neuromuscular), and the severity of the
scoliosis was not known in the cases of many of the patients, making
correlations to those with idiopathic scoliosis impossible.
 |
|
Figure 18.20
The incidence of scoliosis curve progression is greatest for younger ages and for larger curves. (From Lonstein JE, Carlson JM. The prediction of curve progression in untreated idiopathic scoliosis during growth. J Bone Joint Surg Am 1984;66:1061–1071, with permission.) |
were included, the increased mortality rate reported previously has not
been confirmed (164,190).
Mortality from cor pulmonale and right heart failure was seen only in
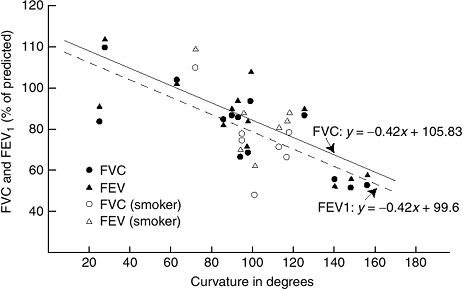
severe thoracic curves (greater than 90 to 100 degrees) (190,191).
Forced vital capacity (FVC) and forced expiratory volume in one second
(FEV1) decrease linearly, with approximately a 20% reduction in
predicted values in those with curves of 100° (190) (Fig. 18.21).
The associated deformity of the chest cavity causes restrictive lung
disease. Thoracic lordosis also decreases lung volume and increases the
deleterious effects of scoliosis on pulmonary function (194).
studies have demonstrated slightly higher rates of back pain compared to control groups (190,195,196,197). The 1476 patients with AIS surveyed in Montreal had more frequent and more severe back pain than did 1755 control subjects (195). Disability rates have been higher in some series (188,195) and similar in others (190).
After 50-year follow-up, 65% of patients with late-onset idiopathic
scoliosis reported chronic back pain compared with 35% of the controls (164).
 |
|
Figure 18.21
Pulmonary function as it relates to curve severity in both non-smoking and smoking patients. As can be seen, severe scoliosis is associated with reduced pulmonary function in both groups. (From Weinstein SL, Zavala DC, Ponseti IV. Idiopathic scoliosis: long-term follow-up and prognosis in untreated patients. J Bone Joint Surg Am 1981;63:702–712, with permission.) |
individual and with the cultural setting. Nowadays, many patients are
seriously concerned about the appearance of their backs, and seek
medical treatment to correct their deformities (198).
Some studies report that the rate of marriage is lower among women with
scoliosis; this implies a psychosocial impact of the deformity (187,188).
Many modern parents are unwilling to accept significant deformity of
any type in their child, whether it is dental, dermatologic, or
orthopaedic, particularly if there is a reasonable and safe way to
correct the condition. However, as safe as scoliosis surgery has
become, it carries with it finite risks and lasting consequences, most
notably loss of spinal motion within the treated segments. Balancing
these risks against current and/or future deformity challenges the
decision-making skills of the treating surgeon.
countries to detect scoliosis at an early stage. The goal is to detect
childhood scoliosis early enough to allow brace treatment rather than
in its late stages when surgical correction and fusion would be needed (199,200,201).
Screening programs for any disease are indicated if effective early
treatment methods exist, and if the disorder is frequent enough to
justify the cost. Although screening programs for scoliosis are
widespread in North America, the variable sensitivity and specificity
of the screening exam and the borderline efficacy of brace treatment
have caused some to suggest that school screening is not justified (163,200,202,203,204).
performed on school children between the fifth and sixth grades (age 10
to 12 years) (205). The Adams forward bend test is employed in combination with scoliometer (116) measurement of the maximum ATR (Fig. 18.9). A referral and radiograph are recommended when the ATR is greater than 7 degrees (93,94,179,180).
The 7 degrees ATR standard detects nearly all curves greater than 30
degrees, but leads to a large number of patient referrals (2 to 3 per
100 children screened) (117,206)
for radiographs in adolescents who have only spinal asymmetry (Cobb
<10 degrees) or mild scoliosis (Cobb <25 degrees) not needing
treatment. Overall, in school screening programs, the incidence of
curves of Cobb angle greater than 10 degrees is approximately 3%, and
of curves greater than 25 degrees, about 0.3%.
ATR with a scoliometer have been shown to have reasonable intraexaminer
agreement (119,207,208,209). Although there is an overall linear correlation between the ATR and Cobb angles (119,210),
precise prediction of the magnitude of deformity is not possible
without a radiograph. The positive predictive value (the probability of
having scoliosis with an abnormal screening test) of the forward bend
test is highly variable (118,160,202,203,211), and is thought by many to be too low for use in current scoliosis screening practices.
over-referral), many experts believe that the emphasis placed on
screening for early diagnosis has greatly increased awareness of
scoliosis, not only in the lay public but also among primary care
physicians. It appears that the combination of increased awareness plus
the efficacy of screening programs has reduced the number of patients
who do not see a physician until they have a marked deformity (199,201,212,213). This theory remains controversial,
and others have presented longitudinal data (following the institution of a screening program) that contradicts this opinion (161,203,214).
scoliosis depends on understanding the natural history of the untreated
condition and comparing it with the outcomes of treatment. As in many
other pediatric conditions, the short-term outcomes of treatment are
reasonably well known; however, the long-term results are less well
defined. Because of this lack of knowledge, there is continuing
controversy regarding treatment choices in any individual patient.
curves of less than 20 degrees, and only a few curves progress enough
to require treatment, most patients are simply monitored. Idiopathic
curves of less than 25 degrees should be monitored every 4 to 12 months
(depending on the age and growth rate of the patient) with clinical and
radiographic examination. Those in the rapid phases of growth are seen
at more frequent intervals (every 4 to 6 months). Curves greater than
30 degrees should be monitored for progression after skeletal maturity,
with radiographs obtained approximately every 5 years. Curve
progression in the mature patient (when it occurs) is slow enough
(approximately 1 degree per year) that more frequent follow-up is not
indicated.
Scoliosis braces of many different styles and corrective mechanical
principles have been developed, the common goal being to modify spinal
growth by applying an external force (218).
Because brace treatment depends on spinal growth modulation, treatment
is prescribed only for patients with substantial spinal growth
remaining (Risser 2 to 3 or less). The upper limit of curve magnitude
that is amenable to brace treatment is approximately 45 degrees. Most
studies have confirmed that, even in the most cooperative patients, the
final result of brace treatment is merely maintenance of the curve at
the degree of severity present at the onset of bracing. Correction may
occur while the brace is used, but when the brace is discontinued the
curve will generally settle to its pretreatment level of severity (219,220,221).
Patients with scoliosis and their parents should be advised of this
limitation, because most of them have the mistaken belief that the
brace will provide permanent correction as is seen with orthodontic
bracing, a common point of reference for the lay person. In more severe
curves, with trunk deformity already present, correct information may
cause some patients and their parents to select surgical correction
rather than brace maintenance.
and adolescents with a curve between 25 degrees and 45 degrees. Most
surgeons insist that curve progression of more than 5 degrees be
documented before using bracing in curves of less than 30 degrees.
These indications may need to be altered depending on the clinical
circumstance; for example, a 10-year-old premenarcheal girl with a
curve which has increased from 14 to 22 degrees in the prior 6 months
should probably be braced before reaching 25 degrees. Early bracing may
also be considered when a strong positive family history for
progressive scoliosis exists (for instance, if the mother or a sibling
has required treatment for scoliosis).
Milwaukee Children’s Hospital in the 1940s, became the standard against
which other designs were compared (222). This brace remained popular into the 1980s (223)
and even now remains an option in selected situations. The original
design provided longitudinal traction between the skull and pelvis with
lateral translational forces directed through pads on the chest wall (Fig. 18.22).
The brace uprights can be adjusted in length to accommodate growth—a
feature that is useful in the treatment of infantile and juvenile
patients. In addition, it remains one of the few designs that has the
potential to maintain upper thoracic curves.
have replaced the Milwaukee brace in most centers, because of more
ready acceptance by the patients. Because no upright or neckpiece is
used, the brace is less conspicuous, a feature that is highly important
to the adolescent. The wearing of a visible scoliosis brace is seen as
a stigma by many teenage patients, and consequently produces a negative
self-image (228,229).
Despite improvements in the appearance of the brace (worn under the
clothes, no visible neckpiece), many teenagers will not cooperate with
brace wear. Reasons for failure include pain (230),
poor fit, discomfort from the heat, family environment, and concerns
about self-esteem. The brace must be acceptable to the patient if it is
to be worn and has any chance of limiting the curve progression (Fig. 18.24).
produces a trunk bend that is so severe that it precludes walking. This
brace is therefore prescribed only for nighttime wear, and is best
suited for single curves that are more distal (thoracolumbar and lumbar
curves).
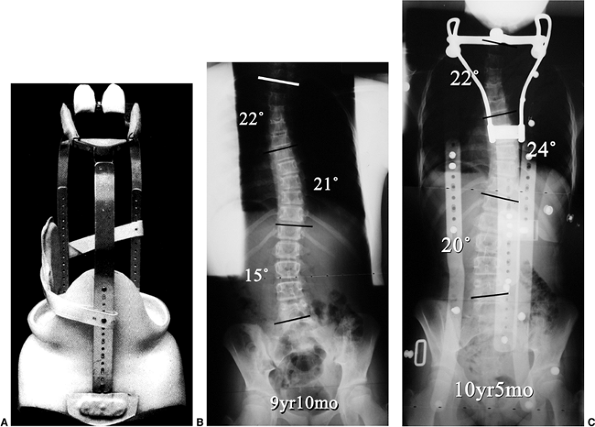 |
|
Figure 18.22 A: Milwaukee brace. B:
posteroanterior radiograph of a 9-year 10-month-old girl with juvenile-onset scoliosis that progressed and was treated with a Milwaukee brace. C: The subsequent in-brace radiograph demonstrates the superstructure. This brace was chosen primarily to help address the upper thoracic deformity. |
caused by the constant corrective molding of the trunk and spine during
growth. As such, full-time (23 hours per day) brace wear was first
advised by Blount and continues to be recommended by many who prescribe
scoliosis braces. About 15 years ago, certain centers began to treat
patients for only 16 hours per day, allowing the child to go to school
without the brace, hoping that the abbreviated schedule would lead to
greater compliance by the patient (226,232).
This schedule is popular with many surgeons (and patients). However,
one must question the biomechanical validity of the concept, because
the preponderance of brace wear is at night when the curve is least
severe (patient supine) whereas during daytime hours, when the curve is
at its greatest (patient upright, forces of gravity at work), it is
left uncorrected.
Natural History Committee found a dose-dependent relation between the
number of in-brace hours per day and success in preventing curve
progression (233), thereby suggesting that
ensuring more hours of brace wear per day provides more effective
correction. This contradicted a study with the Wilmington brace that
did not demonstrate a difference in efficacy between part-time and
full-time bracing (226). The recommendation for
full-time brace wear is also in contrast to the Charleston nighttime
bending brace philosophy, which attempts to produce hypercorrection. In
a series reported by Price et al. the average curve correction while in
the brace was 73% (234), and they postulated that less time per day of wear was required because of the marked correction while in the brace.
has been presumed for many years, yet controlled treatment trials with
and without bracing have not been completed until recently (215,235).
Earlier studies reporting high success rates for brace treatment were
subsequently noted to have included many patients who were at low risk
for progression. Lonstein and Winter evaluated 1020 patients treated
with a Milwaukee brace, over half of whom were at
substantial
risk for progression, and for whom the natural history was known. In
the group with an initial curve between 20 and 29 degrees and at high
risk for progression, the brace was found to be effective compared to
natural history data pertaining to the untreated state (223). This is in contrast to the findings of Noonan et al. (236), who have recently called into question the efficacy of the Milwaukee brace.
 |
|
Figure 18.23 A: Boston brace. B:
This posteroanterior radiograph demonstrates a right thoracic left lumbar curve pattern in an adolescent patient with remaining growth. C: The in-brace radiograph demonstrates a reduction of both the thoracic and lumbar curves. |
not randomized) study of bracing by the SRS were published. Results
were compared in 286 patients, aged 10 to 15 years, with an initial
curve of 25 to 35 degrees: 129 were observed but received no treatment,
111 were treated with an underarm brace, and 46 were treated with
nighttime electrical stimulation (46 patients). Curve progression at
the end of bracing (skeletal maturity) was limited to less than 5
degrees in 74% of those treated with a brace, compared with 34% in
those who received no treatment. The group treated with electrical
stimulation had a success rate of only 33% (215). Although critics (163)
site flaws in this and other studies, most centers have accepted the
results and continue to advise brace treatment for progressive curves
in skeletally immature adolescents. A properly controlled randomized
trial of bracing remains to be done.
mixed, with differing inclusion criteria making direct comparisons
impossible (232,233).
However, in two recent studies a full-time underarm brace was more
successful than a nighttime Charleston bending brace both in preventing
curve progression and in preventing surgery (216,237).
Both of these studies were retrospective with potential biases, and no
prospective comparison of various braces has been performed to confirm
these conclusions. In a series from Dallas, the nighttime Charleston
brace equaled the efficacy of a Boston brace for single lumbar and
thoracolumbar curves (216).
brace should be maximized, with careful fitting and adjustment of the
pads by an experienced orthotist with a specialized knowledge of
scoliosis treatment. Efficacy is increased when the in-brace
radiographic Cobb angle is reduced by 20% or more compared to the
prebrace Cobb measurement (236).
curves provides the standard of care in most of the developed world,
the scientific basis for brace efficacy is not powerful. When to
intervene remains a difficult decision to make. This is because
although very mild curves are easier
to
control with a brace, wholesale adoption of this policy would lead to
many children being braced unnecessarily. Waiting until the curve
reaches 30 degrees allows one to brace the smallest number of patients,
yet the margin between a curve of 30 degrees (brace instituted) and 40
to 45 degrees (surgery indicated) is distressingly small. Finally, many
adolescents strongly resist brace wear and such resistance is
understandable (238,239),
particularly in teenage boys with scoliosis who might have to wear a
brace for as long as 5 years (skeletal maturity is often reached only
at age ≥18 years). Karol (240) recently reported a bracing failure rate of 74% in boys. Each of these factors
makes orthotic (brace) treatment of scoliosis a continuing challenge.
 |
|
Figure 18.24 A:
This premenarcheal adolescent girl with open triradiate cartilage presented with this radiograph. There had been documented progression, and she was placed into a thoracic-lumbar spinal orthosis (TLSO). B: In-brace correction was excellent. However, her compliance was poor. One year later, her curve had progressed substantially (C), and continued progression is shown (D, E). Continued bracing through the adolescent growth spurt is certainly a substantial challenge for many patients and clearly not successful in all patients. |
 |
|
Figure 18.25 A: Charleston nighttime bending brace. B: This thoracolumbar curve is one that appears amenable to treatment with a nighttime bending brace. C: The in-brace radiograph demonstrates nearly complete correction of the thoracolumbar curvature.
|
corrective force, and a molded body cast may be indicated. In the years
before the advent of posterior instrumentation, this was the primary
means of obtaining curve correction prior to fusion. Today it is used
to aid in immobilizing patients who are too small for rigid
instrumentation prior to fusion, or in those with severe deformities
who are too young to undergo fusion. Progressive IIS is currently the
most common indication for a well-molded corrective body cast. In these
young patients, rib deformity associated with the cast pressure is
almost certain to occur. However, this approach may delay the repeated
surgeries that will follow application of a growing rod instrumentation
system in such a patient. The complications associated with growth rod
constructs make them a method of last resort. Risser type casting is a
good choice in patients with progressive infantile idiopathic
scoliosis, either before moving to a brace or after a bracing failure
has occurred (Fig. 18.26).
include improved spinal alignment/balance and prevention of subsequent
curve progression. Corrective instrumentation plus arthrodesis (fusion)
provides the best method for achieving lasting correction; it can be
used either anteriorly or posteriorly to restore spinal alignment as
well as to provide postoperative stabilization while the fusion mass
progressively solidifies. All current methods rely on the development
of a stable bony fusion mass to maintain the correction over time, with
the rods serving as internal struts while fusion proceeds. Application
of a rod system without a bony fusion predictably leads to eventual rod
fracture and loss of correction; instrumentation without fusion (as in
growing rod constructs) can be useful as a temporizing method.
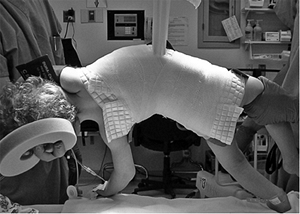 |
|
Figure 18.26
A body cast is often required in the treatment of progressive infantile idiopathic scoliosis. This demonstrates a method of applying a bending force by suspending the trunk with muslin before rolling a Goretex-lined fiberglass cast. |
based on curve magnitude, clinical deformity, risk for progression,
skeletal maturity, and curve pattern. In general, thoracic curves of
Cobb angle greater than 40 to 50 degrees in skeletally immature
patients should be surgically corrected, whereas surgical correction is
reserved for curves of 50 degrees or more in mature patients (in whom
there is a lower risk of progression). These Cobb angle ranges are
meant as guidelines rather than absolute indications and are based on
the natural history of untreated scoliosis in immature and mature
patients. Factors other than Cobb angle should be strongly considered
when deciding between operative and nonoperative treatment. Trunk
deformity (rotation) and trunk balance are important factors in
deciding when to advise surgical correction. A patient with a lumbar
curve of 35 degrees may have such a severe lateral trunk shift that
surgical correction is indicated (Fig. 18.27).
The curve pattern has a great impact on the deformity of the trunk
associated with the scoliosis, with long, single curves producing a
more noticeably unbalanced trunk as compared to double or triple curves
(Fig. 18.28).
implied that both immediate and long-term outcomes will be improved as
compared to nonoperative treatment. The short-term results of surgical
treatment are well known, with a variety of surgical techniques having
been studied for most curve types. Midterm (5 to 10 years) outcomes of
modern corrective surgery are becoming available (241,242,243)
but, like all advancing technologies, the surgical methods tend to
change faster than the results can be collected. The 20-year follow-up
study by Dickson et al. (244) of Harrington’s
surgically treated patients showed good long-term results. Although the
methods of Harrington (single distraction rod) have been replaced in
many parts of the world, the fusion achieved in patients with scoliosis
in that era led to lasting stabilization in most cases (244).
to correct the deformity and to immobilize the spine while the fusion
progressed. The addition of Harrington’s instrumentation improved
scoliosis correction and greatly reduced the incidence of
pseudarthrosis following scoliosis surgery.
rod with a hook at either end and a threaded compression rod attached
to the transverse processes on the convex side of the curve. Coronal
plane improvement provided by the Harrington distraction rod was often
gained at the expense of decreased thoracic kyphosis and flattening of
the lumbar spine (so-called flat-back deformity), as noted in the sagittal plane (Fig. 18.29).
Subsequent modifications of the Harrington concept included the use of
sublaminar wires or a square-ended rod to allow rod contouring (in an
attempt to maintain thoracic kyphosis and lumbar lordosis) while
preventing rod rotation with distraction (247).
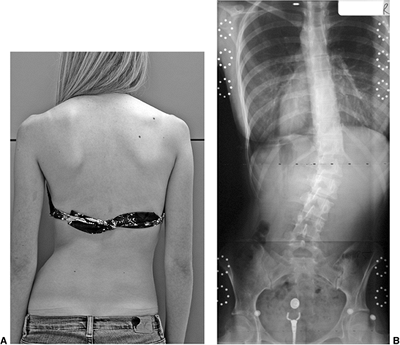 |
|
Figure 18.27 A:
This photography demonstrates relatively substantial trunk decompensation to the right associated with thoracolumbar scoliosis. B: A relatively great length of the spine is involved in the deformity, and this may in some way be responsible for the fairly sizable trunk decompensation despite a Cobb angle measurement of 35 degrees. Other thoracolumbar deformities of similar Cobb angle magnitude are often associated with much less clinical deformity. This highlights the shortcomings of using only the Cobb angle measure in determining appropriate treatment. |
greatly advanced by Moe’s clarification that most scoliosis (with
apparent double curves) requiring surgical correction did not
require fusion of the lumbar curve. He noted that the lumbar curve is
very often secondary and does not require fusion (the so-called King-Moe type 2 curve). Application of the King-Moe curve classification will be emphasized later in this chapter (248).
introduced a multi-hook system, which allowed distraction and
compression on the same rod. Sagittal plane contouring of the rods and
segmental hook fixation improved curve correction and postoperative
stability. Many other segmental fixation posterior instrumentation
systems utilizing similar concepts are now available for surgical
correction of scoliosis. Current options for attachment to the
posterior spine include hooks (for attachment to the transverse
processes, laminae, and pedicles), sublaminar wires (Luque), and
pedicle screws. Pedicle screw attachment is highly effective; however,
there is still some controversy about their use, especially in the
thoracic spine where the size of the pedicles through the concave apex
can be quite small (250,251).
the goal is to obtain fusion, and one must first perform careful
subperiosteal exposure of the spine as well as meticulous facet
excision. The spine must also be decorticated prior to adding bone
graft. The complexity of modern instrumentation sometimes causes
surgeons to pay too little attention to the details required for
obtaining a successful fusion.
 |
|
Figure 18.28
This classic series of photographs from J.I.P. James demonstrates the clinical appearance of four patients, each with a 70 degree magnitude scoliotic curve, although with different curve patterns. The clinical deformity is greater in single curves, particularly in the thoracic spine. (From Montgomery F, Willner S. The natural history of idiopathic scoliosis. Incidence of treatment in 15 cohorts of children born between 1963 and 1977. Spine 1997;22:772–774, with permission.) |
instrumentation systems. Frontal plane realignment of the spine can be
accomplished by translating the vertebra to the concave side rod. This
translational movement may be performed by connecting the concave rod,
precontoured to the desired sagittal profile, to each fixation site
along the spine and then rotating the rod into the sagittal plane. This
rod rotation maneuver, popularized by Dubousset, remains an effective
method for translating the apex of the curve into a more normal
position. Another method for translating the apex in space involves
locking the concave rod into the position of anticipated correction and
then sequentially (with hooks or screws) or incrementally (with
sublaminar wires) drawing the spine to the rod (Fig. 18.30). Compression and distraction forces are then added in order to enhance both frontal and sagittal plane correction.
(usually left-side) rod in the thoracic spine increases thoracic
kyphosis and decreases a right thoracic scoliosis. Both results are
generally desired, given the frequent loss of normal thoracic kyphosis
noted in idiopathic scoliosis. Similarly, compression
applied on the same left-side rod in the lumbar spine (of a double
curve) corrects scoliosis and allows restoration/maintenance of lumbar
lordosis. In most posterior instrumentation systems that are used in
correcting a right thoracic curve, the left-side (concave) thoracic rod
that is placed first provides most of the deformity correction. The
second rod, placed on the right side, primarily adds stability and
resistance to fatigue failure. Using these same principles for the
correction of a kyphotic thoracic scoliosis, the convex (right-side)
rod is placed first. The correction of both planes of deformity
(scoliosis and kyphosis) is addressed with compression toward the apex.
With these force application strategies, the logical creation of hook
patterns becomes possible (Fig. 18.31).
Thoracic lordoscoliosis requires concave distraction centered on the
apex of the curve, whereas thoracic kyphoscoliosis is addressed with
convex compression.
(particularly at the apex of the curve) has traditionally not been
specifically addressed by translation, rod derotation, compression, and
distraction techniques (252). To some degree,
differential rod contouring in the sagittal plane between the convex
and concave side rods can apply some derotational forces to the apical
vertebrae. The concave (left) sides of the apical vertebrae need to be
displaced (rotated) more posteriorly than the convex (right) sides of
these vertebrae in order to produce derotation in the transverse plane.
Therefore, contouring more kyphosis in the
left rod than in the right rod leads to this effect (Fig. 18.32).
With pedicle screw fixation throughout the thoracic curve, moments may
be applied to the vertebrae in order to provide the corrective torque
to reduce this plane of deformity. Monoaxial screws in the apical
region are rotated to the right (clockwise if viewed from the feet)
while the ends of the curve are rotated in the opposite direction (Fig. 18.33).
In most modern systems, the two rods are cross-linked to improve
stability, although there is some question of whether this is needed
when segmental screws are utilized.
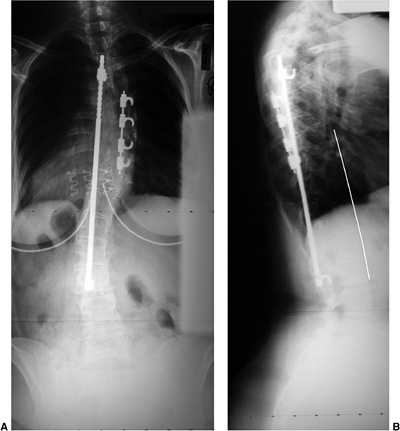 |
|
Figure 18.29 A: Harrington distraction rod is seen on the left, combined with a smaller threaded compression rod on the right. B:
Distraction into the lumbar spine associated with this system unfortunately often leads to a reduction in lumbar lordosis, creating a flat-back deformity. |
warrant a combined anterior and posterior approach in idiopathic
scoliosis. Standard recommendations have been for patients with large
(greater than 75 degrees) rigid curves (bend correction less than 50
degrees) and those at risk for postfusion crankshaft deformity. Curve
flexibility is increased by anterior disc excision, allowing greater
correction with posterior instrumentation. The bone graft used
anteriorly leads to a very stable fusion (anterior and posterior). The
procedure involves anterior disc excision with release of the anterior
longitudinal ligament, removal of the annulus fibrosis and nucleus
pulposus, excision of the vertebral endplate cartilage, and
occasionally (in severe cases) excision of the rib head at the
costovertebral joint (Fig. 18.34).
is caused when anterior spinal growth continues despite successful
posterior fusion, resulting in worsening rotational deformity (Fig. 18.35).
The problem occurs only in skeletally immature patients (Risser 0,
triradiate cartilage open). Measuring crankshaft growth is difficult,
although it has been defined as an increase in the Cobb angle of more
than 10 degrees, or an increase in apical rotation despite successful
posterior fusion. This largely axial rotational deformity is difficult
to measure with routine radiography. It does appear that an anterior
fusion arrests the anterior growth center and limits the development of
this late deformity in young patients who are at risk (257).
is the increased area for arthrodesis (vertebral endplates), presumably
reducing the risk of pseudarthrosis. The thoracoscopic approach
provides a means of accomplishing anterior disc excision and fusion
with minimally invasive
methods (258,259,260), thereby reducing the approach-related morbidity of a thoracotomy (259,261,262).
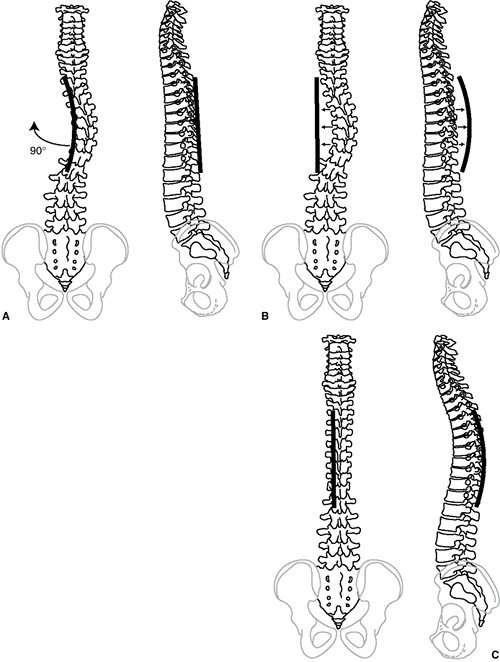 |
|
Figure 18.30 A:
Thoracic scoliosis correction in this illustration is accomplished by placing the pre-contoured rod into the scoliosis and rotating the rod 90 degrees to correct both the coronal and sagittal deformities. B: In this method the spine is approximated to the rod, maintaining the rod in the appropriately contoured and aligned position. C: Either of these two methods allows correction of the scoliosis with restoration of thoracic kyphosis. |
anterior corrective instrumentation with screw fixation into vertebral
bodies and a single or double anterolateral rod construct can be
considered for some curve patterns. The first anterior instrumentation
systems introduced by Dwyer (263) and then by Zielke and Berthet (264)
were both flexible compression systems that generated kyphosis within
the instrumented segments. This production of kyphosis may be desirable
in the scoliotic thoracic spine (that has become relatively lordotic),
but is generally undesirable in the lumbar region.
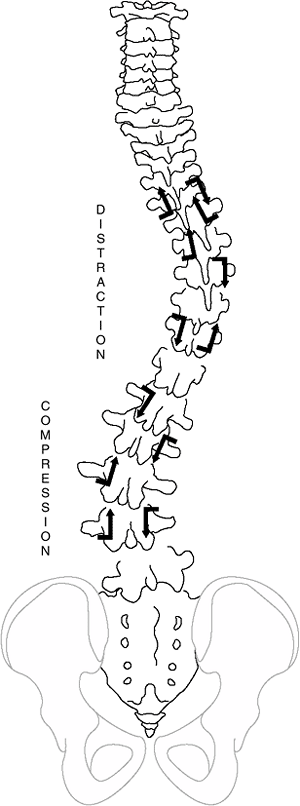 |
|
Figure 18.31
This hook pattern represents the commonly used method for achieving correction of a right thoracic left lumbar scoliosis curve pattern. The left-side rod would be placed first, with distraction in the thoracic spine being an appropriate force to correct both scoliosis and restore thoracic kyphosis, whereas compression in the lumbar spine is used for correcting the scoliosis as well as for maintaining lumbar lordosis. The right-side rod is placed secondarily for additional stabilization. This sequence applies in most cases in which the thoracic spine has lost normal thoracic kyphosis. In patients with increased kyphosis, a right-side thoracic compression rod should ideally be placed first. |
developed in an attempt to improve the sagittal alignment, particularly
when treating thoracolumbar and lumbar curves. Scoliotic curve patterns
that are amenable to corrective anterior instrumentation and fusion
generally include those with a single structural deformity (thoracic,
thoracolumbar or lumbar curves, Lenke 1A–C, 5C). The Lenke
classification is presented in the next section.
 |
|
Figure 18.32 A:
Translation of the thoracic apex to the corrected position, either by rod derotation or translation of the vertebra to a stationary rod, does not necessarily correct vertebral rotation. B: To address the rotational deformity of the apical vertebra, it is often desirable to use differential rod contouring between the left side and right side rods. The left side rod, contoured into a greater degree of kyphosis, provides a posteriorly directed translational force on the left while a slightly under-contoured sagittal alignment on the right side rod provides an opposite direction of anterior translation reducing apical vertebral rotation. |
Direct access to the vertebral bodies and intervertebral discs is
possible through an open anterior thoracoabdominal approach. Anterior
disc excision creates mobility (272), which
enhances correction in the frontal and axial planes, but decreases
sagittal plane lordosis. Special attention to the sagittal plane is
required when anterior compression instrumentation is used distal to
the thoracolumbar junction so as to avoid production of an iatrogenic
flat-back deformity (caused by loss of requisite lordosis). Structural
interbody support in the form of a structural bone graft or an
interbody “cage” has been advocated as a means of maintaining sagittal
alignment (269,273).
Double-rod, double-screw anterior systems have also been introduced as
a means of providing additional control of the sagittal plane (271,274) and overall construct stability (275) (Fig. 18.36).
In most cases, anterior instrumentation can achieve similar or greater
correction than posterior instrumentation for the same curve, often
with fewer levels requiring instrumentation (276,277).
 |
|
Figure 18.33
Use of pedicle screws offers a more powerful means of correcting axial plane vertebral rotation. Fixed-head pedicle screws placed segmentally can be manipulated around the concave rod to provide a means of apical derotation. |
scoliosis suggest similar advantages (shorter fusion, greater
restoration of kyphosis) (276,278).
The mid-upper thoracic vertebrae are less robust than those of the
thoracolumbar junction, and it is difficult for the screw to maintain
purchase there. This procedure typically increases thoracic kyphosis
because of disc excision and anterior compression between vertebral
body screws. Anterior instrumentation techniques have traditionally
been performed through a thoracotomy, but minimally invasive techniques
have been developed for the management of thoracic scoliosis (262,279,280).
Through four to six lateral chest wall incisions (1 to 2 cm long),
thoracic discectomy, bone grafting, and segmental instrumentation
provide anterior scoliosis correction without the chest wall morbidity
of a thoracotomy. The advantages of this approach over a traditional
anterior exposure have been demonstrated with regard to recovery of
pulmonary function and of shoulder movement and strength (262). This technically challenging operation requires more time than open surgery (281),
and calls for great skill and experience on the part of the surgeon.
This procedure is currently performed only at a few centers (Fig. 18.37).
correction of idiopathic scoliosis (anterior versus posterior
instrumentation) depends on the curve pattern, sagittal deformity, age
of the patient, and experience of the surgeon. The selection of the
fusion levels for any given patient also depends on several factors and
remains a topic of much controversy. The fusion should be as short as
possible, minimizing the changes in spinal mobility, yet be long enough
to ensure optimal correction and lasting spinal balance (both coronal
and sagittal).
young children with curves that progress relentlessly despite
aggressive cast and/or brace treatment, includes a subcutaneously
positioned distraction construct that spans the deformity (282).
At the proximal and distal hook sites, a limited fusion is performed to
reduce the incidence of hook dislodgment. In the intervening segments,
the spine is not stripped subperiosteally
because exposure alone may lead to spontaneous fusion in a young child
(this is the rationale for the concept of a “subcutaneous” rod).
Sequential distraction is performed every 6 to 12 months during growth
until no further correction can be obtained (Fig. 18.38). The height gained with these procedures is usually modest and complications are common (282) [hook dislodgement, spontaneous fusion (283),
infection]; therefore, these systems are presently used in children
younger than 5 to 6 years of age, for whom there are few other options.
Eventually an instrumentation and fusion are performed. A short convex
hemiepiphyseodesis (anterior and posterior) over the apical levels may
also be included in the subcutaneous rod technique (253,284).
However, this leaves very few unfused segments for growth. Another
option for correction without fusion has recently received limited
approval from the U.S. Food and Drug Administration (FDA). The vertical
expandable prosthetic titanium rib (VEPTR) attaches to the ribs,
usually on the concave side of the deformity. Periodic distraction of
the chest/ribs offers another means of controlling severe early-onset
scoliosis that is associated with reduced chest volume. The clinical
experience with the device in such cases remains limited (285).
distinct curve patterns, the most common being a right thoracic curve.
One must understand these curve patterns in order to surgically correct
scoliosis. The most widely used classification, developed by Moe as
reported by King et al. (King-Moe classification) (Fig. 18.39),
was designed primarily to decide when to instrument the thoracic curve
alone (in patients with apparent double curves) and when to instrument
both the thoracic and lumbar curves. Despite the common use of this
classification when planning surgery, the system was not designed
as a comprehensive classification of idiopathic scoliosis curve patterns.
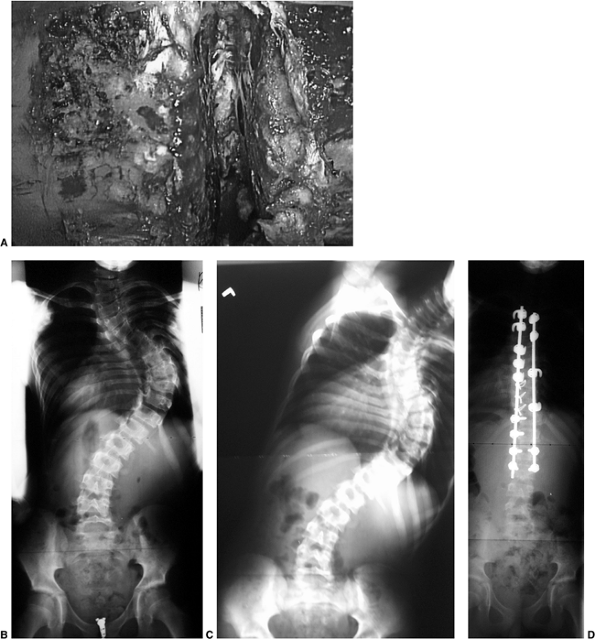 |
|
Figure 18.34 A:
This is a clinical photograph of anterior thoracic disc excision. It is a useful technique for increasing curve flexibility as well as removing additional anterior vertebral body growth potential. B: This juvenile patient with severe right thoracic scoliosis was treated with thoracoscopic anterior spinal release and posterior instrumentation. C: The rigidity of the right thoracic curve is seen on this radiograph of side-bending, with the curve remaining greater than 70 degrees. D: Following anterior release and posterior segmental instrumentation, near-total correction was possible. The indications for an anterior procedure in this case were the curve rigidity and the crankshaft potential. |
 |
|
Figure 18.35 A: This 8-year, 9-month-old girl presented with a 60 degree right thoracic scoliosis. B: She underwent an isolated posterior instrumentation and fusion, which reduced the deformity to 22 degrees. C:
Because of continued anterior spinal growth, a crankshaft deformity developed with curve progression. The trunk decompensated, and increased thoracic prominence developed despite a successful posterior fusion. Two years postoperatively the curve had progressed to 43 degrees. |
curves, are not included in the King-Moe classification. Others have
designed classification systems that are more comprehensive (286,287).
The system proposed by Lenke et al. considers both frontal and sagittal
plane deformity and is designed to guide surgical treatment decision
making for all curve patterns. This classification system is a triad
system including a description of the curve type (1,2,3,4,5,6), lumbar curve size (A, B, C), and sagittal plane alignment (hyperkyphotic, normal, hypokyphotic) (Fig. 18.40).
The perceived utility of the Lenke et al. system was its comprehensive,
biplanar nature and, most importantly, its ability to predict regions
of the spine that theoretically require fusion. Specific criteria for
establishing “structural” minor curves were set forth. Although not
perfect in predicting which curves experienced surgeons will choose to
instrument, the classification system correlated approximately 85% of
the time with what was actually done by surgeons (288,289).
scoliosis curve pattern, occurring in roughly one half of, operative
cases. The surgical approach may be either anterior or posterior. The
extent of fusion is generally limited to the thoracic curve, although
the lowest instrumented vertebra is chosen largely on the basis of the
features of the lumbar deformity. Using the Lenke system of classifying
the
lumbar
curve, 1A curves can be safely instrumented distally to 1 (or 2)
level(s) proximal to the stable vertebra (vertebra bisected by the
CSVL) when treated posteriorly, and to the lower end vertebra when
instrumented anteriorly (Fig. 18.41).
The 1B curve pattern is amenable to either approach (anterior or
posterior), with the lower instrumented vertebra being usually the
stable vertebra between the thoracic and lumbar curves (around T12) (Fig. 18.42).
Lenke 1C curves present with a substantial lumbar curve, with the
lumbar apical vertebra completely deviated lateral to the CSVL. The
surgical treatment approach in this type of curve in the King-Moe
classification (type II) has been associated with substantial
controversy through the years regarding the appropriateness of sparing
lumbar motion with an isolated (selective) thoracic fusion. In most
cases of Lenke 1C curves, a selective thoracic fusion can be performed
while maintaining frontal plane balance. The larger the thoracic
deformity (Cobb angle, apical translation, rotation) compared to the
lumbar curve, the more likely the success of selective thoracic
instrumentation. Lenke, Bridwell, and others (290–292) have suggested
that selective thoracic fusion of the King II, Lenke 1C curve pattern
should be considered only where the thoracic deformity is 25% greater
than the lumbar deformity, measured as explained in the preceding text (Fig. 18.43).
However, the clinical appearance of the patient should be the ultimate
determinant. A dominant lumbar rotational prominence, even if highly
flexible, likely requires inclusion in the fusion (Fig. 18.44). If the lumbar curve is greater than 45 to 50 degrees, vigorous correction of only the thoracic curve with
a posterior instrumentation system may result in postoperative truncal decompensation to the left (293,294).
If selective fusion is decided upon in such patients, correction of the
thoracic curve should be modest in order to minimize the chance of
residual trunk imbalance (292). If selective
fusion is chosen, the lower instrumented vertebra is the stable
vertebra below the thoracic curve (approximately T12). The hook
patterns and methods of force application for posterior instrumentation
have been described in the preceding text. Additionally, the
instrumentation
should
not end inferiorly just above a “junctional kyphosis” (as noted on the
lateral view). The junction between the thoracic and lumbar curves
(approximately T12) may be focally kyphotic in the sagittal plane. A
selective fusion of the thoracic spine in such a case (with concave
distraction) will exaggerate the kyphosis at the thoracolumbar
junction. When the lateral view demonstrates junctional kyphosis, the
corrective instrumentation should extend distal to the kyphosis (but
also beyond any lumbar scoliosis apex). The 1B and 1C thoracic curves
can also be corrected with anterior instrumentation. The advantages
include a potentially shorter fusion and ease of recreating normal
kyphosis in the hypokyphotic thoracic spine. The levels selected for
fusion in anterior instrumentation to correct thoracic scoliosis
include all vertebrae within the measured Cobb angle. Because the
anterior instrumentation approach on the thoracic spine is kyphogenic
(adding approximately only 10 degrees of kyphosis on account of disc
excision and compression), this approach should be avoided when the
T5-T12 sagittal Cobb angle is greater than 35 to 40 degrees
preoperatively (Fig. 18.45).
 |
|
Figure 18.36 A:
This schematic demonstrates the single-rod anterior construct used in thoracic scoliosis correction. Note the structural grafting of the lower two levels. B: This dual-rod construct is generally preferred for management of thoracolumbar scoliosis. Structural grafting may also be required in this construct to aid in maintaining lumbar lordosis. This construct offers greater construct rigidity compared to the single-rod constructs. |
 |
|
Figure 18.37
Thoracoscopic approach to anterior scoliosis correction utilizes several small incisions and portals to access the anterior thoracic spine for performance of discectomy as well as screw and rod placement. |
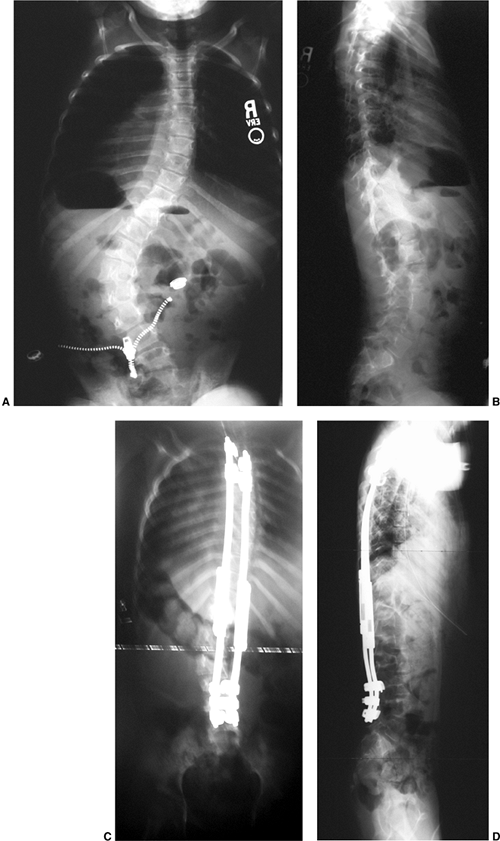 |
|
Figure 18.38 A: This juvenile patient presented with progressive lumbar scoliosis. The curve progressed despite attempts at bracing. B: The lateral radiograph demonstrates reasonable sagittal alignment. C: This patient underwent growing dual-rod instrumentation with fusion performed only at the proximal and distal hook sites. D: The sagittal profile is maintained and the growth connectors allow periodic lengthening with growth.
|
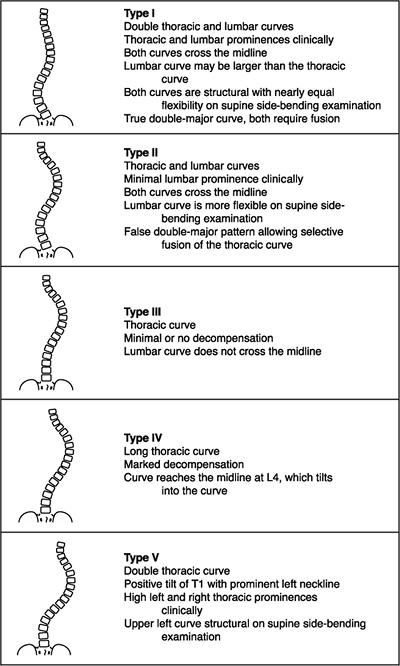 |
|
Figure 18.39
The features of the King-Moe classification. (From King HA, Moe JH, Bradford DS, et al. The selection of fusion levels in thoracic idiopathic scoliosis. J Bone Joint Surg Am 1983;65:1302–1313, with permission.) |
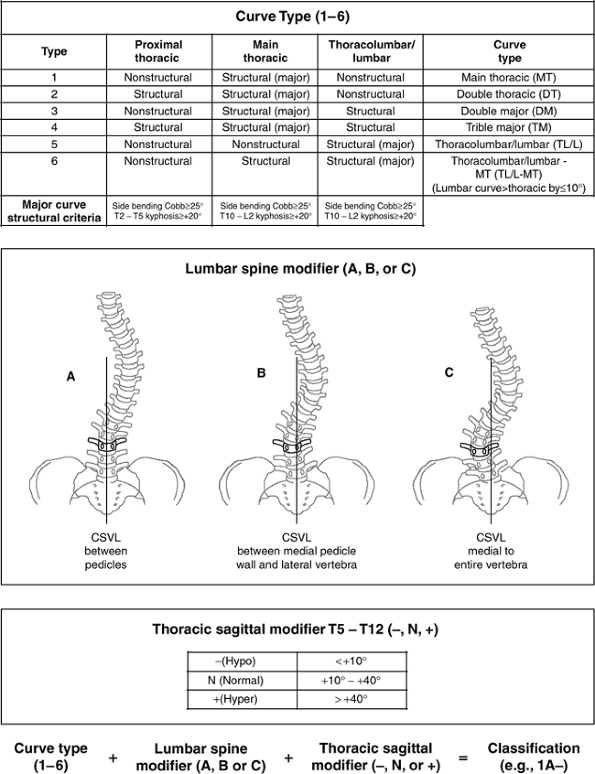 |
|
Figure 18.40
The Lenke et al. (1997) classification. This comprehensive classification system for scoliosis is a triad system that involves curve type, lumbar spine modifier, and thoracic sagittal modifier. |
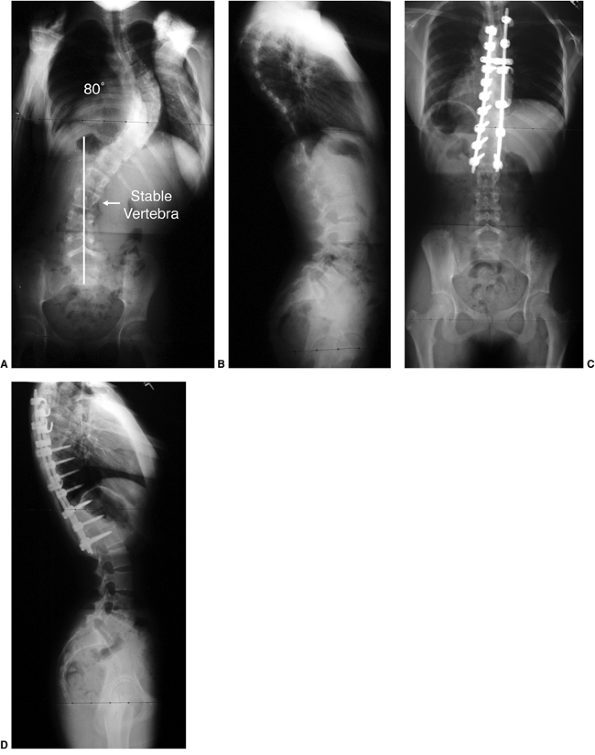 |
|
Figure 18.41 A: This Lenke 1A curve has a stable vertebra at L2. B: Lateral radiograph demonstrates hypokyphosis. C, D:
Posterior instrumentation was performed, stopping distally at L1, one level proximal to the stable vertebra. The distal and apical segments of the construct were stabilized with pedicle screws. |
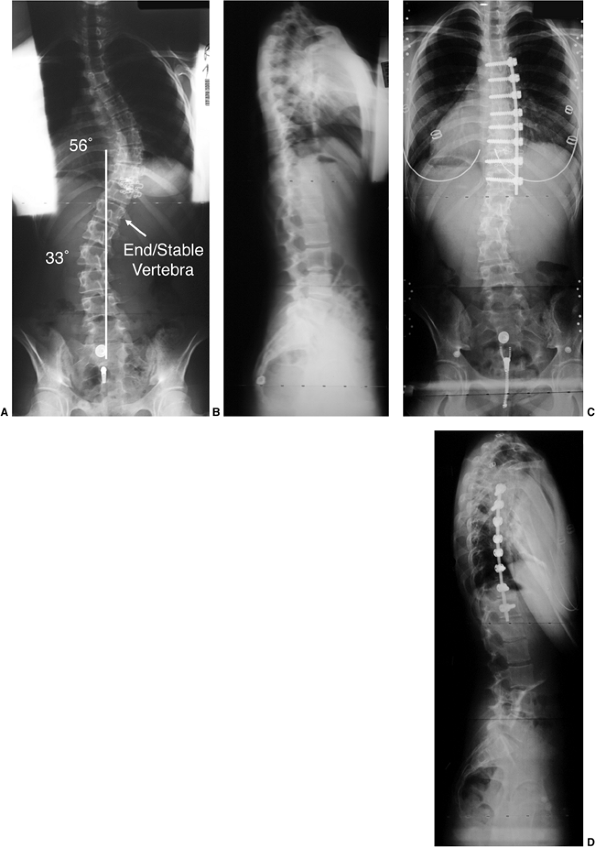 |
|
Figure 18.42 A:
This posteroanterior radiograph demonstrates a Lenke 1B scoliosis. The lumbar curve was almost completely corrected to 15 degrees on side bending. B: Typical thoracic hypokyphosis is noted. C, D: Postoperative radiographs of this patient following thoracoscopic anterior instrumented correction of only the thoracic curve, ending at the end vertebrae proximally and distally. |
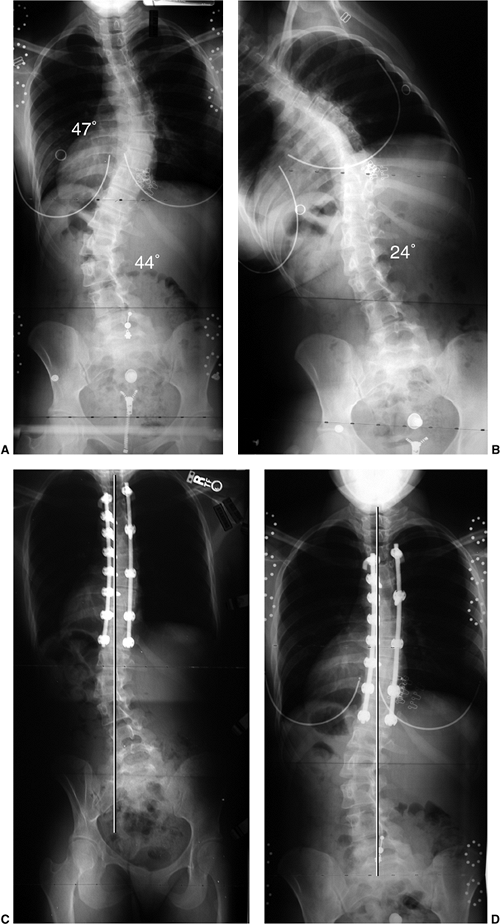 |
|
Figure 18.43 A:
This Lenke 1C curve demonstrates a lumbar curve which corrects to just less than 25 degrees. The similarity between the thoracic and lumbar curves, despite this lumbar curve flexibility, makes a selective thoracic fusion more likely to result in decompensation if aggressively corrected. B: Lumbar side-bending correction to 24 degrees. C: The patient underwent selective thoracic correction with pedicle screw fixation. In the early postoperative period, she was substantially decompensated to the left. D: Six months following surgery her trunk shift had resolved and she remains well balanced without instrumentation or fusion of her lumbar spine. |
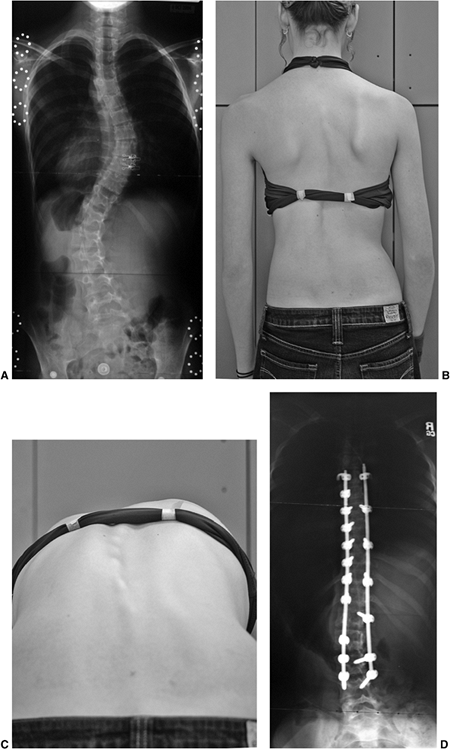 |
|
Figure 18.44 A:
This posteroanterior radiograph demonstrates relatively well-balanced right thoracic and left lumbar curves. The thoracic curve measures slightly larger, and the lumbar curve on side bending corrects to 20 degrees. This classifies as a Lenke 1C curve. B: The clinical appearance of this patient demonstrates nearly equal deformity of the thoracic and lumbar regions. C: On forward bending, however, the lumbar prominence (ATR 19 degrees) was larger than the thoracic prominence (ATR 9 degrees), confirming the greater rotational deformity present in the lumbar spine. D: Based largely on the greater lumbar rotation, this patient was not felt to be a candidate for selective thoracic fusion, and underwent instrumentation of both thoracic and lumbar curves. |
structural thoracic curves (right main thoracic and left upper
thoracic) and is recognized by the presence of an elevated left
shoulder. An isolated right thoracic curve is typically associated with
an elevated right shoulder. If the left shoulder is higher, an upper
thoracic curve to the left should be suspected. A left upper thoracic
curve that is relatively rigid (remains greater than 20 to 25 degrees
on side-bending film) generally requires instrumentation beginning
proximally at T1 or T2 (295) (Fig. 18.46).
If the double pattern is not recognized and the right thoracic curve
alone is straightened, the left shoulder elevation is often worse
following the surgery (296,297,298).
The sagittal alignment of the upper thoracic region also requires
careful assessment. This is an area that (as in the thoracolumbar
spine) may also be found to be hyperkyphotic. If this region just
proximal to the thoracic curve is locally kyphotic (>20 degrees
T2–T5), the instrumentation should extend proximally to cover this area
in an attempt to avoid aggravating a proximal junctional kyphosis (Fig. 18.47).
presents in association with a right thoracic curve may vary
substantially in both magnitude (Cobb angle) and severity of rotation.
Either the thoracic or lumbar curve may dominate such a double-major
curve pattern, although the thoracic curve is more often the primary
one. In deciding on surgical treatment, one must determine which of the
curves requires instrumentation and fusion (thoracic, lumbar, or both).
When the thoracic curve is larger and/or more rigid than the lumbar
curve (King II), selective fusion of only the thoracic curve should be
considered (248,290).
There are situations, however, when the lumbar curve is large enough to
require fusion in order to achieve a well-balanced spine after
correction. This is always the case when the lumbar curve is dominant.
curves is required, the approach is posterior, and the distal extent is
usually to the L-3 or L-4 level. Ideally the distal extent of the
fusion should be as proximal as possible in order to preserve lumbar
motion segments, yet long enough to avoid creating trunk imbalance.
Choosing between L-3 and L-4 can be difficult. The most predictable
spinal balance occurs when the fusion/instrumentation extends distally
to the stable vertebra (the vertebra best bisected by the CSVL). In
patients with limited remaining growth and a left side lumbar curve,
fusion to L-3 may be considered if there is minimal axial rotation of
L-3 as noted on the side-bend film to the left, and if the L-3 level is
above the pelvis with side bending to the right (L3 parallel to top of
sacrum). When posterior instrumentation is to be done, laminar hook or
pedicle screw fixation can be used for attaching to lumbar vertebrae.
It is clear, however, that pedicle screws provide greater control and
correction of a lumbar curve. Again, the surgical goal is to achieve
improved spinal alignment with global truncal balance with both C-7 and
the trunk well centered over the pelvis in both the sagittal and
coronal planes (Fig. 18.48).
have a significant thoracic component and may be convex to the left or
right (the left being the more common). In such cases, isolated fusion
of a lumbar or thoracolumbar curve is appropriate and can be
accomplished by either anterior or posterior methods. Correction with
posterior hook constructs has not been as successful as anterior
instrumentation in achieving derotation of the lumbar curve. Limited
anterior instrumentation of the apical 3 or 4 vertebrae has been
proposed by Bernstein and Hall (277) with satisfactory early outcomes in most patients. Longer anterior constructs (299) that include all the measured Cobb angle levels, some with the use of bone graft or a structural cage in the disc space (269,300), have become popular in the treatment of these curves (Fig. 18.49).
Pedicle screw fixation has allowed better control and correction of
these curves when corrected with posterior instrumentation (301).
curves in all three locations: left upper thoracic, right thoracic, and
left lumbar/thoracolumbar. Although it may not be necessary to
surgically treat all three curves, each should be considered
individually to determine if they are structural (relatively rigid) and
treated accordingly. In these triple curves, the lower thoracic curve,
if dominant, should certainly be included in the arthrodesis. The
inclusion criteria for the upper thoracic and lumbar curves are the
same as
those
used for the corresponding double curve patterns. For example,
elevation of the left shoulder, in an upper thoracic curve that remains
greater than 20 to 25 degrees on side bending, should be considered for
inclusion within the levels of fusion. Similarly, the lumbar curve
should be instrumented when its Cobb angle magnitude is equal to or
greater than the thoracic curve. The greater the magnitude, rotation,
and rigidity of the lumbar curve, the more likely it is that the lumbar
curve requires fusion (290,292).
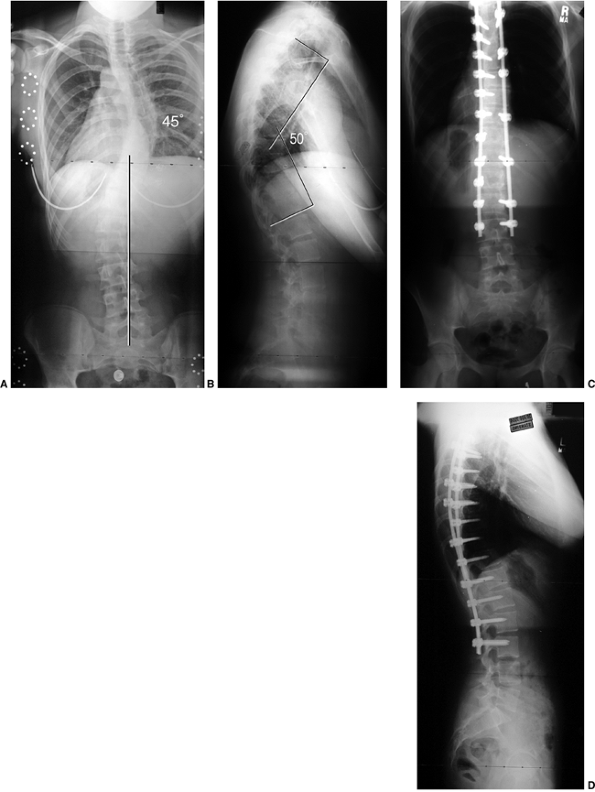 |
|
Figure 18.45 A: This posteroanterior radiograph demonstrates a Lenke 1B + deformity. B: The lateral radiograph, however, demonstrates hyperkyphosis, making this a curve pattern. C:
Because of the substantial kyphosis associated with this deformity, the posterior instrumentation was extended proximally and distal to the end vertebra and stable vertebra. D: In this case the sagittal plane determined the proximal and distal levels for instrumentation so as to safely correct both the hyperkyphosis and the thoracic and mild lumbar scoliosis. In order to avoid stopping at the apex of the lumbar curve, the fusion was extended to L-3 distally. |
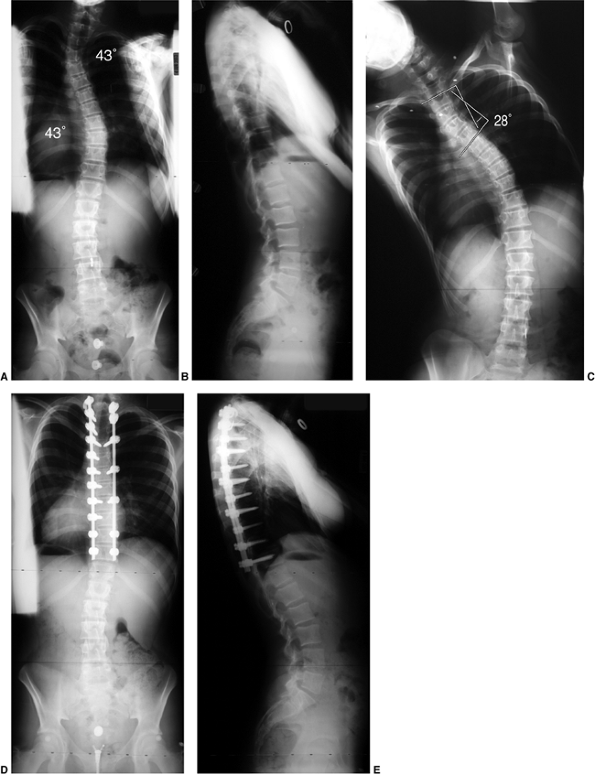 |
|
Figure 18.46 A: Posteroanterior view of double thoracic scoliosis (Lenke 2B). B: Lateral view demonstrating loss of thoracic kyphosis. C: On side bending, the upper thoracic curve remains at 28 degrees. D, E:
Because of the rigidity of the upper thoracic curve and elevation of the left shoulder preoperatively, both the upper thoracic and main thoracic curves underwent instrumented fusion. |
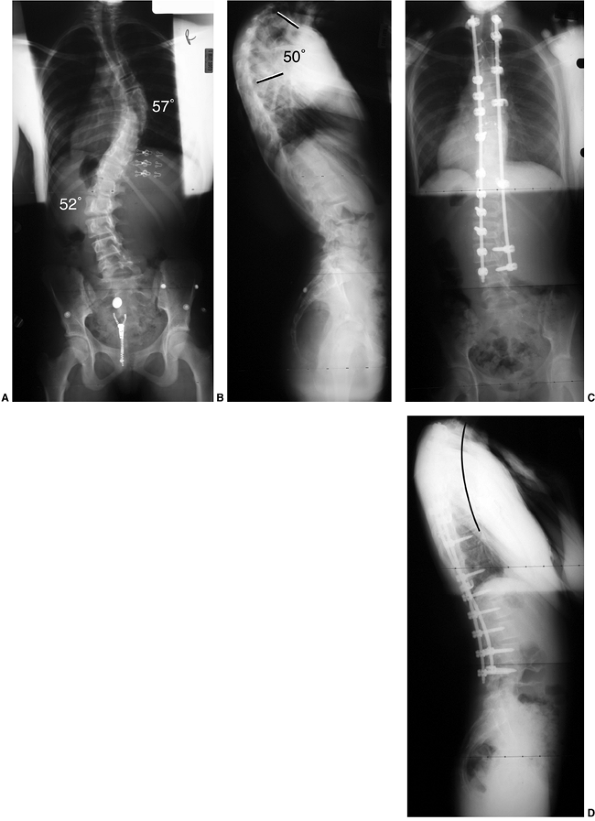 |
|
Figure 18.47 A, B: These radiographs demonstrate right thoracic, left lumbar scoliosis with substantial upper thoracic kyphosis. C, D: In this case the instrumentation was extended proximally to correct the kyphotic aspect of the deformity.
|
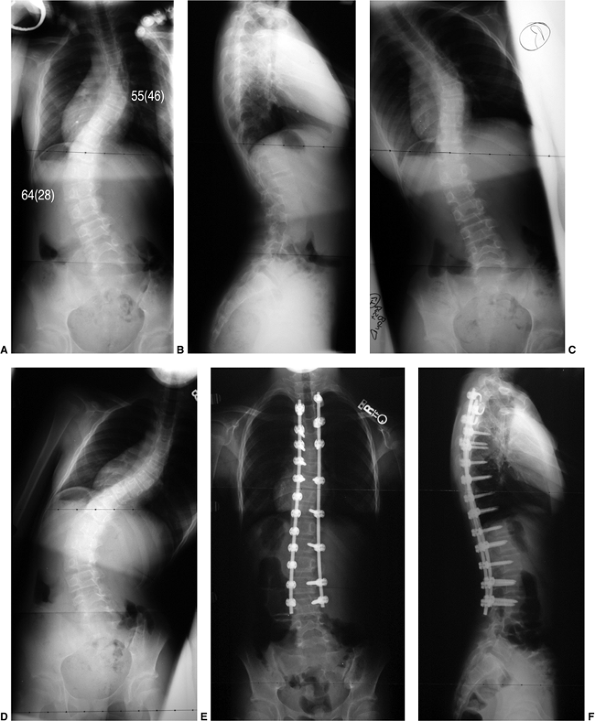 |
|
Figure 18.48 A, B: This double-major scoliosis has Lenke 6C curve pattern. C: Side-bending radiograph to the left demonstrates correction of the lumbar curve to 28 degrees. D: Side-bending to the right demonstrates correction of the thoracic curve to 46 degrees. E, F:
On the basis of the structural features of the right thoracic and left lumbar curves, instrumented correction was performed from T3 to L3. |
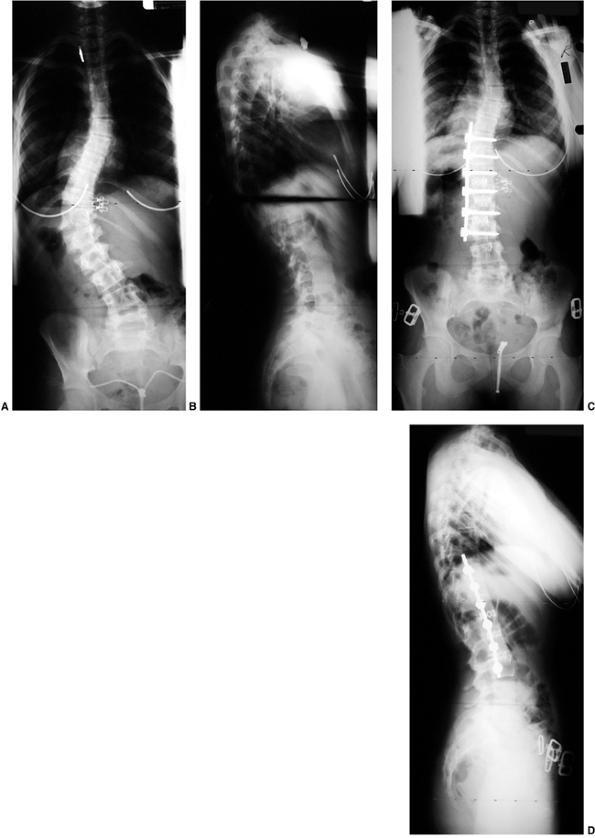 |
|
Figure 18.49 A, B: Posteroanterior and lateral radiographs demonstrate a Lenke 5C thoracolumbar scoliosis. C, D:
This deformity was addressed by single anterior rod correction through a thoracoabdominal approach. Interbody structural support consisted of cages at the lower three levels to prevent loss of lumbar lordosis. |
AIS [Harrington instrumentation in the 1960s, Cotrel-Dubousset (CD)
instrumentation in the 1980s] are fairly recent, very long-term outcome
data are not yet available. Spinal instrumentation techniques continue
to undergo modification and improvement almost faster than outcome
studies can be performed. The SRS has recently developed an outcomes
assessment system that will allow more precise analysis of functional
outcomes in patients with scoliosis (302,303,304).
treated with Harrington instrumentation and fusion. An average coronal
plane improvement of the Cobb angle by 14% to 48% has been reported (252,305).
The analysis of long-term functioning after long posterior fusions has
focused on the incidence of late-onset low back pain. Conflicting
results have been reported regarding the prevalence of pain and the
correlation of pain with the caudal level of instrumentation. Some
researchers (306,307) found no increase in pain or any correlation with the caudal level of arthrodesis, whereas others (196,308)
did identify an increased incidence of back pain an average of 21 to 23
years after Harrington instrumentation, as compared with a control
population. Cochran et al. (309) noted an
increased frequency of pain in patients fused at L-4 or L-5 compared to
those fused at L-3 or above, but several other studies have not been
able to correlate the levels of fusion with subsequent back pain (196,308,310,311,312).
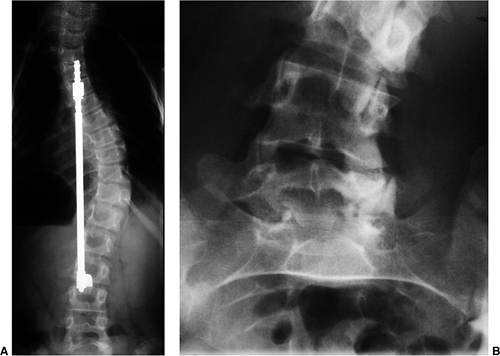 |
|
Figure 18.50 A: Radiographs following Harrington instrumentation at L-3 level. B:
Subsequent radiograph 20 years later demonstrated increased deformity in the lower lumbar spine with facet sclerosis and degeneration at L3-L4, L4-L5, and L5-S1 despite instrumentation at L3. |
potential for low back pain with more caudal levels of instrumentation
and fusion, it seems intuitive that one should minimize the caudal
extent of a fusion. Winter et al. (313)
demonstrated a significant loss of spinal motion when arthrodeses
included L-4 compared to arthrodeses fused only inferiorly to upper- or
mid lumbar levels. Fusion to more caudal levels has also been
associated with higher rates of radiographically visible degenerative
changes in the unfused distal levels (spondylolisthesis, lateral
listhesis, disc narrowing, and facet sclerosis) (310,314) (Fig. 18.50).
fusion, alignment in both the coronal and sagittal planes of the
unfused segments should be optimized. Loss of lumbar lordosis
(flat-back syndrome) results in substantial functional deficits. These
are related to positive sagittal balance that has been associated with
distraction instrumentation across the lumbar spine (312,315,316,317).
It remains unclear whether the long-term functional results are better
if the spine is fused to L4, with maintenance of lordosis and improved
coronal position, rather than to L2 or L3, with slightly greater
residual deformity of the remaining caudal levels. The relation of
alignment and degenerative changes to the development of low back pain
requires additional follow-up and analysis (318).
(5- to 15-year follow-up) results of CD instrumentation suggest
improved coronal and sagittal plane correction, compared to Harrington
instrumentation (320). An average correction of 41% to 61% can be expected when considering all curve types (305,321,322).
The segmental hook constructs have provided clear improvement (compared
to Harrington instrumentation) in postoperative sagittal alignment (252,323),
although little improvement in the axial rotation deformity has been
seen. This is despite the early expectation that systems such as the CD
type would substantially derotate the spine by an intraoperative
rotational maneuver of the rod as the instrumentation is applied (252,324,325,326,327).
Postoperative immobilization has been minimal at most centers using
multisegmental instrumentation systems (as compared to Harrington
instrumentation); on the other hand, the incidence of pseudarthrosis
and loss of correction has been less with the more stable
multisegmental systems (242,252,320,322).
decompensation to the left was commonly noted in King type II curves
when selective thoracic fusion was performed (291,294,328). Several techniques have been suggested to avoid this difficulty (290,293).
The most important is to avoid overcorrection of the thoracic spine
beyond a degree that the unfused lumbar curve can adapt to (100).
Also, hook reversal at the lower-most hook of the concave rod (most
inferior hook upgoing), or the use of pedicle screws, allows initiation
of coronal plane balance and lumbar lordosis, minimizing the risk for
trunk decompensation (291,292,294).
results after an average follow-up of 5 years of a consecutive series
of 185 patients with idiopathic scoliosis treated with Isola
instrumentation. Correction of the largest curve averaged 65% while rib
hump rotation decreased 39%. These results are generally better than
those reported with original CD constructs. Features of the Isola
construct that likely contributed to these improved radiographic
outcomes are the segmental fixation, particularly in the apical
segments, in addition to stable fixation at the ends of the construct.
Other hybrid systems with largely segmental fixation have resulted in
comparable degrees of deformity correction (322,329,330).
hooks has led to improved degrees of correction. This has been most
clearly demonstrated with the application of screw fixation in lumbar
scoliosis that is a component of a double-major curve (331,332). Dislodgement of distal hooks with consequent loss of correction, although not common, does occur with some frequency (252) (Fig. 18.51).
Similar loss of distal fixation is much less common when pedicle screws
are utilized. It has been suggested that, in addition to achieving
greater correction and fixation stability, distal pedicle screws may
obviate a distal level of fusion in many cases. Although no clinical
data exist to confirm a reduction in distal junctional
degeneration/malalignment, distal pedicle screw insertion requires much
less dissection/muscle injury at the caudal end of the construct than
does fitting an upgoing hook (Fig. 18.52).
and lumbar spine has a growing number of proponents throughout North
America (Fig. 18.53). In 1995 Suk et al. reported greater correction of thoracic deformity with segmental pedicle screws than with hooks (329), and other surgeons have also adopted this approach with similar outcomes (322).
In these reports the percentage correction with thoracic hook
constructs ranged from 50% to 55% compared to the results with screw
fixation, which were 72% to 76%.
of the goals of surgical treatment; however, as mentioned in the
preceding text, problems with lateral truncal decompression appear to
increase for certain curve patterns (Lenke 1C, King II) when selective
thoracic fusion is performed. Balance of trunk must be maintained in
the context of the correction achieved in the instrumented segments,
and the (sometimes unpredictable) spontaneous correction of the
uninstrumented segments. In fact, the impressive outcomes of segmental
thoracic pedicle screw correction have led Suk et al. to suggest
expanding the fusion into the proximal thoracic spine (up to T2) in
many patients so as to avoid shoulder imbalance (elevation of the left
shoulder) postoperatively (even when none existed preoperatively) (329).
additional advantages of pedicle screws have been suggested (although
still largely unproven). These include: greater transverse plane (rib
hump) correction, thereby reducing the need for thoracoplasy; more
powerful correction, thereby obviating the need for anterior release in
curves up to 90 to 100 degrees; and correction and fixation such that
crankshaft growth can be prevented with the posterior procedure alone.
Although surgeons have reported less frequent use of anterior
release/fusion since adopting segmental pedicle screw constructs, there
are few comparative data to prove these points (322,333). On the other
hand, the real as well as the perceived risks of using thoracic pedicle
screws in the surgical treatment of scoliosis have slowed their usage.
The room for error is less in the thoracic spine compared to the lumbar
region, and the consequences of misplacement substantially greater
(spinal cord versus lumbar root) (Fig. 18.54).
Several techniques for thoracic pedicle screw insertion have been
reported with very low rates of neurologic injury/screw revision. Like
all surgical techniques, a complete understanding of the local anatomy
and proper training by experienced educators is required to perform
this surgery safely and successfully.
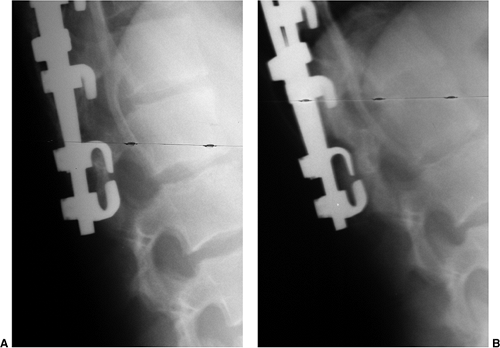 |
|
Figure 18.51 A: Laminar hook fixation is common in the lower constructs for thoracic and lumbar curves. B:
They can, however, on rare occasion, dislodge with fracture of the lamina. Pedicle screw fixation offers a method of salvaging such a situation, and of reducing the incidence of such failure to begin with. |
 |
|
Figure 18.52
This figure demonstrates the distal extent of a pedicle screw versus an up-going laminar hook. As can be seen, the dissection required to place a pedicle screw does not require exposure distal to the midspinous process. An up-going laminar hook, on the other hand, risks disruption of the adjacent facet capsule as well as interspinous ligaments. These, in addition to its greater stability, are the advantages of distal pedicle screw fixation. |
been primarily used for the correction of lumbar and thoracolumbar
scoliosis. The percentage of frontal plane correction with Dwyer,
Zielke, Texas Scottish Rite Hospital (TSRH), or Kaneda instrumentation
has been reported as being between 67% and 98% (265,266,267,268,269,277,334). Some surgeons have noticed a loss of sagittal plane lumbar lordosis when anterior compressive systems are used (335,336).
Even solid ¼ rod systems, such as the TSRH system, have not been able
to entirely preserve normal lordosis when used without anterior
interbody structural support (265). Sweet et
al. have reported that sagittal alignment in the lumbar spine can be
maintained if interbody structural support is added (269);
however, even with this interbody support, if a pseudarthrosis
develops, eventual rod breakage and collapse into increased scoliosis
and kyphosis will occur. Two-rod anterior systems provide another
option, with better construct stability (274,299); however, reported follow-up remains limited (Fig. 18.55).
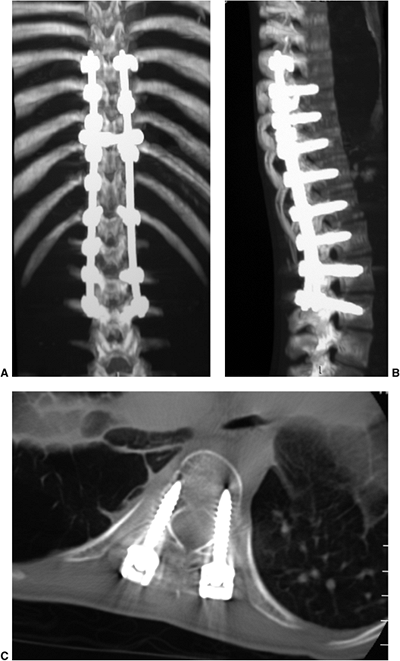 |
|
Figure 18.53 A,B:
These postoperative computed tomography (CT) reconstructions following pedicle screw fixation demonstrate complete realignment in both frontal and sagittal planes. C: The transverse images demonstrate the relatively narrow path which these pedicle screws must negotiate. |
spine are beginning to be reported. Betz et al. made a nonrandomized
comparison of anterior versus posterior thoracic instrumentation for
thoracic idiopathic scoliosis. The anterior 3.2-mm or 4.0-mm threaded
rods resulted in coronal plane scoliosis correction comparable to that
achieved by posterior hook systems, but were associated with a 31%
incidence of rod breakage. The distal level of fusion in the anterior
fusion group of patients was, on average, two segments more proximal
than in those in the posterior group (276).
Lenke et al. have also reported a greater spontaneous improvement of
the uninstrumented portion of the lumbar spine when the thoracic
instrumentation was anterior and (shorter) as compared to posterior, in
treating thoracic curves that have a compensatory lumbar curve pattern.
At the 2-year follow-up, the thoracic curves
were
corrected 58% and the lumbar curves (uninstrumented) 56% in the
anterior instrumentation group, compared to 38% and 37%, respectively,
in the posterior instrumentation group (278).
The rate of pseudarthrosis and rod breakage appears to be greatly
reduced after the introduction of solid (nonthreaded) rods for thoracic
curves, although proximal screw pull-out can be more problematic
because the stiffness of the rod is increased.
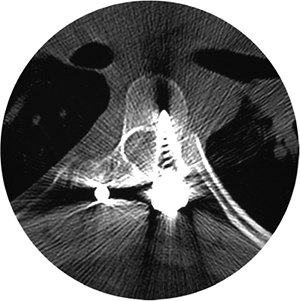 |
|
Figure 18.54
This pedicle screw was misdirected, reaching the medial aspect of the spinal canal. There were no neurologic deficits associated with this; however, screw removal was performed. This certainly demonstrates the potential risks to the spinal cord associated with malpositioned thoracic pedicle screws. |
although over the last 20 years these procedures have become much safer
due to advances in anesthesia, blood loss management, instrumentation
systems, and neurologic monitoring.
instrumentation methods, coupled with better surgical skills, spinal
cord monitoring, and methods to minimize blood loss, have altered the
way one advises patients regarding possible complications following
scoliosis surgery. In the past the surgeon focused on blood loss, hook
dislodgment, infections, and paraplegia or other neurologic deficits.
Although all potential complications must be mentioned, in the current
era patients are more likely to experience problems such as
postoperative trunk imbalance or a need for subsequent implant removal.
Due to the size, complexity, and modularity of the implants, they are
more likely to produce muscle irritation, bursitis, or even a late
low-grade infection in the bursitic area (337,338,339).
scoliosis surgery is not well documented in a large series, although
data from the membership of the SRS is collected annually. In 1995 the
reported incidence of neurologic injury after surgery was 1% in all
types of scoliosis surgery in all ages. One patient of the 1643
patients undergoing surgery for idiopathic scoliosis developed a
complete spinal cord injury. The incidence of partial spinal cord and
nerve root injury was 10 of 1643 for the idiopathic scoliosis group
compared to 7 out of 211 for the congenital scoliosis group. More than
half of these patients had subsequent recovery of normal neurologic
function. In a series of 1090 patients undergoing spinal deformity
correction reported by Bridwell et al., four patients developed a
neurologic deficit postoperatively. However, only one of these patients
had idiopathic scoliosis, and the total number of idiopathic scoliosis
cases is not reported (340).
classified as: a result of direct trauma (contusion) to the cord;
excessive traction to the neural elements caused by corrective
instrumentation; and vascular insufficiency to the cord. The blood
supply to the spinal cord is segmental and enters through the neural
foramina. There has been some controversy as to the risk of vascular
insufficiency to the cord associated with ligation of the anterior
segmental blood vessels in anterior spine surgery. Winter et al.
reported 1197 cases in which segmental vessels were divided with no
neurologic sequelae noted (341). There have,
however, been other reports suggesting a possible vascular cause of
spinal cord dysfunction postoperatively after segmental vessel ligation
(342). Those at greatest risk appear to be patients with congenital malformations and hyperkyphosis (342,343,344).
If an anterior procedure requires division of the segmental vessels,
they should be ligated in the midvertebral body area rather than near
the neural foramen. In high-risk cases (congenital, kyphosis, revision
surgery) temporary clamping of the vessels with concomitant spinal cord
monitoring has been suggested by some as a means of detecting a
potentially critical source of spinal cord blood supply (343).
minimize operative blood loss in surgery for scoliosis; however, the
mean arterial pressure must be maintained at a safe level in order to
ensure adequate blood flow to the spinal cord. In extremely complex
corrections (kyphosis, osteotomies, revision surgery) where the risk
for cord ischemia is greater, the surgeon may elect to keep the blood
pressure higher, even though blood loss will be greater, to ensure cord
perfusion (342).
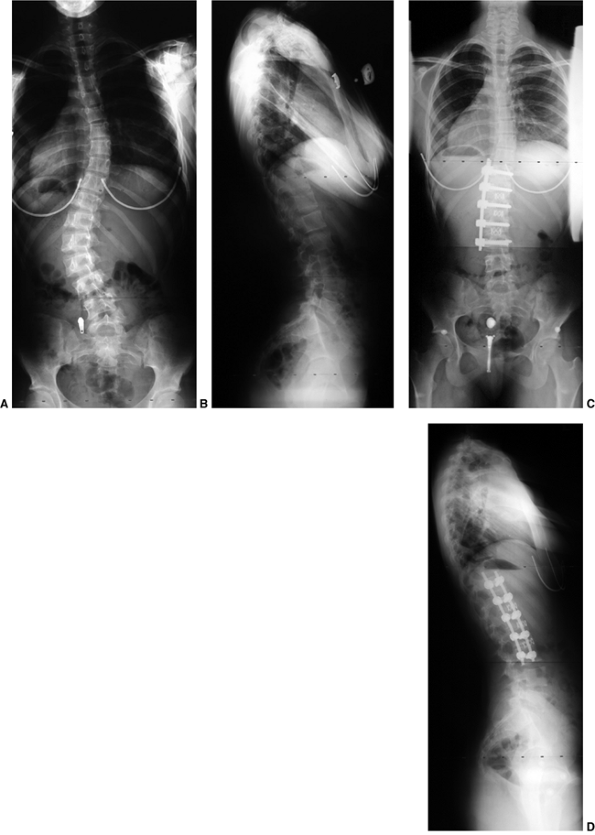 |
|
Figure 18.55 A,B: This demonstrates a typical left thoracolumbar scoliosis. C,D:
Our preferred treatment in such cases is a dual-rod anterior construct, two screws in each vertebra with a two-hole vertebral staple that provides excellent postoperative stability. Interbody support is utilized to maintain lumbar lordosis while compression between the screws is performed to correct the scoliosis. No bracing is required following such instrumentation. |
was the first widely used method for monitoring spinal cord function
after deformity correction. This technique includes decreasing the
level of anesthesia intraoperatively to a level that allows the patient
to follow commands. The patient is instructed to move his or her
feet/toes, confirming the competency of the spinal cord motor tracts (345).
almost standard in surgical correction of spine deformity. Monitoring
of both sensory and motor pathways is possible; however, from a
technical standpoint, sensory monitoring is simpler and more widely
accepted. Somatosensory evoked potentials (SSEPs) are obtained by
stimulating distally (legs) and measuring the response proximally
(brain), and have been very reliable in detecting changes in spinal
cord function, giving the surgeon relatively rapid feedback about any
effect that the deformity correction procedure may be having on
neurologic function (346). The lag time between
the insult to the spinal cord and the resulting monitoring changes may
be 10 to 20 minutes. Changes in motor pathway monitoring (neurogenic
motor evoked potentials, cortical motor evoked potentials, and Hoffmann
reflexes) generally result in a more rapid loss of signal when injury
occurs. Other factors besides injury to the cord have been found to
affect spinal cord monitoring, resulting in false-positive indications
of injury. These include: hypotension, hypothermia, dislodgment of the
monitoring leads, and other technical malfunctions in the system. If
changes are noted, these factors should be evaluated and corrected, and
if the monitoring abnormalities persist, a wake-up test should be
conducted to confirm the findings. Loosening of the implants to remove
any corrective forces, or complete removal of the implants, should be
performed as soon as a deficit is confirmed. Institution of the
methylprednisolone steroid spinal cord injury protocol (347)
also seems warranted, although the efficacy in this specific group of
patients with spinal cord injury has not been carefully studied.
requiring transfusion. This requires appropriate anticipation by the
surgeon based on the type of deformity and the extent of the planned
surgery. Preoperative autologous donation may be the most reliable way
to avoid exposure to allogenic blood products, although this is not
possible in all patients (reasons include: small size of patient,
psychological stress of donating, long distance to the blood bank,
preoperative anemia, congenital heart disease, expense). Alternatives
to minimize allogenic blood exposure include intraoperative blood
salvage, preoperative erythropoietin administration (348), intraoperative hemodilution (349), and (used with the precautions noted in preceding text) controlled hypotensive anesthesia (350).
Additionally, medications that enhance clotting, such as Amicar and
aprotinin, have also been investigated and may be useful when high
blood loss is anticipated.
respiratory compromise, wound infection, and delayed neurologic injury.
The incidence of respiratory complications in idiopathic scoliosis is
approximately 1%, whereas wound infection occurs in approximately 2% of
cases (childhood and adult surgery). Delayed neurologic injury has been
recognized with increasing frequency, and the importance of careful
neurologic monitoring of lower extremity function for the 48 hours
following corrective surgery must be emphasized. Cases have been
reported which confirmed that the patient had intact neurologic
function after the surgery but suffered loss of motor and sensory
function in the days following surgery (340,351,352).
The etiology of delayed-onset paraplegia is unclear. It may be
vascular, resulting from postoperative hypotension, or mechanical,
resulting from a compressive hematoma.
or late in the postoperative period. Despite greatly improved implant
systems, there is still a potential for early failure of the
bone–hardware interface by either implant dislodgment or bony fracture.
If a pseudarthrosis develops, late implant failure can be anticipated.
The time period before implant failure sets in depends to some degree
on the size and number of the rods used. In the presence of a
pseudarthrosis, small single-rod systems may be fatigued and fracture
in less than a year compared to a double-rod system, which may not fail
for up to 5 years. Rod fracture is diagnostic of pseudarthrosis;
however, pseudarthrosis and rod failure may not be associated with any
clinical problems. If pain or curve progression is noted, revision
surgery may be necessary.
need for late hardware removal because of their prominence (in contrast
to the experience with Harrington rods). Another problem with the new
systems has been the development of delayed infection and/or metal
reaction (337,338,339).
The cause of these problems likely relates to the bulkiness of the
systems and their modularity. Loosening of any one component of the
complex system can lead to formation of a bursa, which can eventually
become infected.
been anticipated preoperatively. The problems and causes of trunk
decompensation have been discussed in the preceding text. The
correction of spinal deformities should
result
in a harmonious transition from the instrumented to the noninstrumented
regions of the spine. Abrupt changes in sagittal or coronal alignment
may result in “junctional” problems. Levels adjacent to a fused segment
of spine are subjected to increased mechanical stresses, and this is
likely increased if malalignment exists. The use of new multisegmental,
powerfully corrective instrumentation has the potential for increasing
the incidence of postsurgical trunk imbalance (Fig. 18.56).
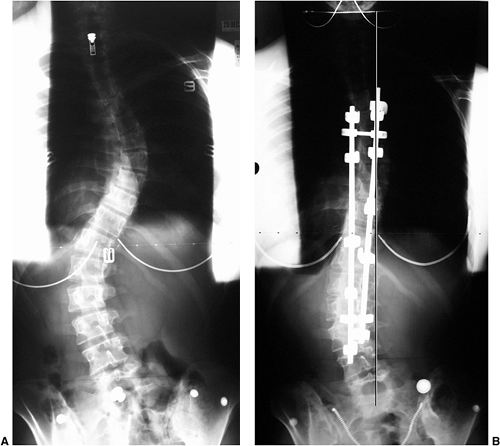 |
|
Figure 18.56 A: This right thoracic left lumbar scoliosis underwent posterior instrumentation and fusion to L-3. B:
Postoperatively there was modest correction of the lumbar curve with remaining obliquity of the lowest instrumented vertebra and increased decompensation to the left. The stable vertebra in this case was L-5 and fusion with a hook system likely should have included L-4. Even in cases of more aggressive pedicle screw fixation, stopping at L-3 may result in disc wedging below the instrumented segment and/or decompensation. This type of decision making remains a challenge for all who are involved in scoliosis treatment, and there are pros and cons associated with the decision to stop one level short of the stable vertebra, or play it safe in the short term and fuse an additional segment. |
individual biases; some are founded on facts, some on intuition, and
some on past experiences/mistakes. Our choices for a given patient’s
treatment have evolved and are continually being assessed. The
recommendations that follow reflect our current approaches to bracing
and surgical treatment of AIS.
potential to modify the natural history of some cases of scoliosis.
However, we freely acknowledge the challenges of such therapy for many
teenaged patients. We offer a thoracic-lumbar spinal orthosis (TLSO) to
female patients whom we believe to be at risk for progression, but we
differ on the ideal wearing time (PON suggests 22 hours per day, DRW
suggests16 hours per day in order to avoid wearing at school). Rarely
do we prescribe a TLSO for a male teenager with scoliosis. In general,
a custom-made underarm TLSO is the type of brace we use; however, a
nighttime bending brace is considered (and often preferred by the
patient) when a thoracolumbar curve is dominant.
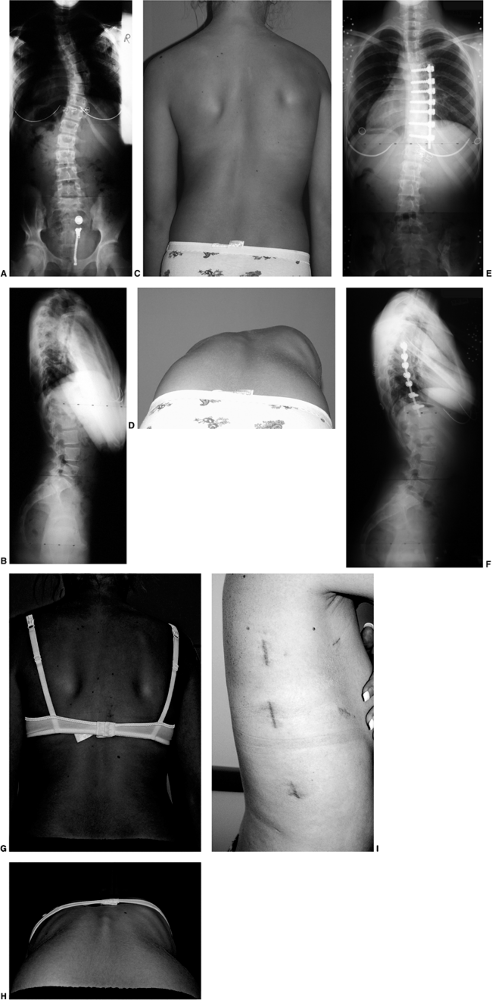 |
|
Figure 18.57 A,B:
This 11-year-old girl with right thoracic scoliosis and slight loss of thoracic kyphosis is an ideal candidate for selective thoracic fusion. In this case it was performed thoracoscopically. C,D: The clinical appearance of this patient demonstrates modest right thoracic prominence. E,F: Three years after thoracoscopic anterior instrumentation and fusion, her curve has been corrected, and solid anterior fusion is visualized best on the lateral view. Her sagittal plane deformity has been improved as well. G, H, I: The clinical appearance of this patient demonstrates re-balancing of the trunk and nearly complete correction of her thoracic rotational deformity. This approach has the advantage of sparing the long posterior muscle stripping exposure. |
makes generalizations regarding preferred methods difficult. In
general, most cases are treated with posterior instrumentation, and we
make every effort to spare the lumbar spine. In addition, the benefits
of thoracic pedicle screws suggested in the literature are borne out in
our experience, and their use has been adopted where feasible.
recommendations. All patients with more than one structural curve are
instrumented posteriorly. Single thoracic curves (Lenke 1A–C), however,
are considered for thoracoscopic anterior instrumentation if several
specific criteria are met: weight 40 to 60 kg, Cobb angle less than 75
degrees, thoracic kyphosis less than 35 degrees, and patient being
willing to wear a TLSO for 3 months postoperatively. This minimally
invasive approach to the correction of scoliosis has not been widely
accepted throughout the world; however, our experience with this
technique has yielded correction comparable to posterior constructs (Fig. 18.57).
Patients treated with this approach are required to wear a
postoperative brace for 3 months, and this fact alone has led many to
opt for a posterior correction instead (no brace needed). Our
comparison of thoracoscopic correction with open anterior
instrumentation clearly demonstrates a more rapid recovery with regard
to pain and pulmonary and
shoulder
function when the former method is used. However, the benefit of
thoracoscopy as compared to an open posterior approach appears
primarily related to the reduced number of instrumented levels.
Thoracoscopy is challenging and demands substantial learning experience
for both the surgeon and the anesthesiologist. Yet, those in various
parts of the world who have mastered the technique are paving the way
for others in the minimally invasive treatment of scoliosis, which we
believe continues to hold promise.
instrumentation remains our preferred technique is thoracolumbar
scoliosis. A relatively short fusion, ending distally at L-3 in most
cases, has yielded consistent results. The newer instrumentation
systems offer dual-rod anterior constructs that are far more rigid and
fatigue-resistant than single-rod constructs. We prefer such
instrumentation when vertebral body size will permit two screws.
Following a thorough discectomy, interbody support is placed in order
to “set” the degree of desired lordosis. This is somewhat determined by
the pelvic anatomy and inclination of the sacral endplate relative to
the femoral heads (pelvic incidence). Patients with a larger pelvic
incidence require a greater degree of lumbar lordosis. Scoliosis
correction is accomplished by compression of the more posterior of
the two rods (inserted first). The second rod merely adds stability (Fig. 18.55). When two rods are used anteriorly, no postoperative bracing is utilized.
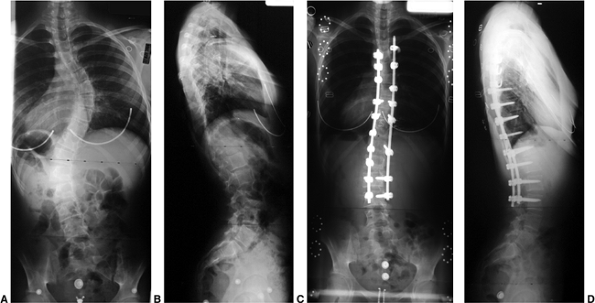 |
|
Figure 18.58 Our preferred method for treatment of double/major scoliosis (A, B) typically involves segmental pedicle screw instrumentation. C, D:
However, in cases where the pedicles cannot be safely negotiated through the apex or in the upper thoracic region, pedicle hooks are utilized on the left-side rod. It is nearly always possible to insert pedicle screws distal to the T-10 level. The key to maximizing correction in many of these cases relates to aggressive soft tissue releases and segmental fixation. |
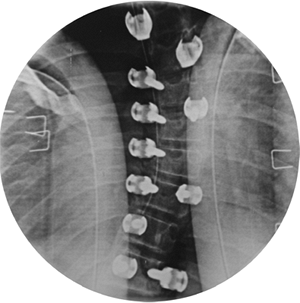 |
|
Figure 18.59
This intraoperative intensifier view demonstrates the appearance of pedicle screws placed in the thoracic apex. There is a gradual transition in the rotation of the left-sided apical screws, maximal at the apex. The lateral view should also confirm appropriate screw length. |
 |
|
Figure 18.60 A:
Photograph demonstrating our preferred method for harvesting iliac crest bone grafts—through a 5 to 6-cm transverse incision below the waistline. The skin is mobilized proximally to harvest adequate bone. B: This allows the incision to be hidden by even relatively low-riding clothing and two-piece bathing suits. |
exceptional cases, posterior instrumentation is preferred. This
includes all cases requiring instrumentation of more than one curve, as
well as large thoracic curves (greater than 75 degrees), hyperkyphotic
thoracic curves, and those who prefer the “tried and true” proven
posterior method. Posterior fixation options are much greater, and
conversion from one implant type to another can be done on the fly with
little downside effect. The first choice of implants is generally a
pedicle screw. Beginning the instrumentation distally and moving
proximally, pedicle screw fixation is almost always possible below the
thoracic apex. Even within the concave apex of the thoracic spine,
interosseous screw fixation is often possible; however, if a
significant breach in the pedicle occurs, conversion to a pedicle hook
(up-going above the apex) is simple and reliable (Fig. 18.58).
Segmental fixation throughout the concave thoracic curve is sought in
order to maximize correction. Pedicle screw fixation on the convex side
may be less critical for maximal correction, yet these pedicle screws
are often easier and safer to place than those on the concavity. We
utilize EMG pedicle screw stimulation to assess cortical integrity of
the pedicle and remove or replace screws with values less than 4 to 6
mAmps. Radiographic confirmation of screw position is also assessed
with the image intensifier intraoperatively (Fig. 18.59).
fashion, they offer a powerful means of correction of the deformity.
This is possible not only in the coronal and sagittal planes but also
in the transverse (axial) plane. Segmental transverse plane derotation
allows direct correction of the rotational deformity and results in
improved rib hump correction.
crest autograft. However, with the use of pedicle screw fixation, the
lamina and facet joints are free of obstructing instrumentation, and
the augmentation of local bone with allograft when combined with
aggressive midline decortication seems adequate in most cases. For
thoracoscopic anterior instrumentation cases, however, autogenous iliac
crest graft remains our choice. Careful harvest through a low
transverse incision leaves a scar hidden by even current teenage
fashions (Fig. 18.60).
open anterior, thoracoscopic anterior) each have a specific role for
our patients depending on the factors described in the preceding text.
In addition to these, “the surgeon factor” should also be considered.
That is, the surgeon should do what he/she does best. There is no
question that the posterior approach is universally applicable to all
AIS curve patterns and many use the anterior approach only for release
of rigid curves and crankshaft prevention. This is completely
acceptable and appropriate. The treatment decisions involved in
scoliosis correction can be complicated, and a constant evolution of
surgical techniques (both anterior and posterior) make dogmatic
declarations regarding treatment inappropriate.
M, Bridwell KH. Segmental analysis of the sagittal plane alignment of
the normal thoracic and lumbar spines and thoracolumbar junction. Spine 1989;14:717–721.
R, Lenke LG, Keeney JA, et al. Comparison of standing sagittal spinal
alignment in asymptomatic adolescents and adults. Spine 1998;23:211–215.
R, Le Borgne P, Dansereau J, et al. Idiopathic scoliosis in three
dimensions: a succession of two-dimensional deformities? Spine 2001;26:2719–2726.
MA, Cook LT. The transverse plane evolution of the most common
adolescent idiopathic scoliosis deformities. A cross-sectional study of
181 patients. Spine 1995;20:1386–1391.
M, Minami S, Kitahara H. et al. Idiopathic scoliosis in twins studied
by DNA fingerprinting: the incidence and type of scoliosis. J Bone Joint Surg Br 1998;80:212–217.
LB, Mangino M, De Serio S, et al. Assignment of a locus for autosomal
dominant idiopathic scoliosis (IS) to human chromosome 17p11. Hum Genet 2002;111:401–404.
NH, Mims B, Child A, et al. Genetic analysis of structural elastic
fiber and collagen genes in familial adolescent idiopathic scoliosis. J Orthop Res 1996;14:994–999.
JC, Qin L, Cheung CS, et al. Generalized low areal and volumetric bone
mineral density in adolescent idiopathic scoliosis. J Bone Miner Res 2000;15:1587–1595.
M, Pecina M, Prebeg Z. A longitudinal study of growth velocity and
development of secondary gender characteristics versus onset of
idiopathic scoliosis. Clin Orthop 1991; 270:278–282.
IA, Laible JP. Three-dimensional osseo-ligamentous model of the thorax
representing initiation of scoliosis by asymmetric growth. J Biomech 1990;23:589–595.
IA, Spence H, Aronsson DD, et al. Mechanical modulation of vertebral
body growth. Implications for scoliosis progression. Spine 1996;21:1162–1167.
X, Chau WW, Chan YL, et al. Relative anterior spinal overgrowth in
adolescent idiopathic scoliosis. Results of disproportionate
endochondral-membranous bone growth. J Bone Joint Surg Br 2003;85:1026–1031.
R, Labelle H, Forest F, et al. Morphologic discrimination among healthy
subjects and patients with progressive and nonprogressive adolescent
idiopathic scoliosis. Spine 1998;23: 1109–1115.
SS, Hsu LC, Ho EK, et al. Disproportionate body growth in girls with
adolescent idiopathic scoliosis. A longitudinal study. Spine 1991;16:S343–S347.
M, Minami S, Nakata Y, et al. Association between estrogen receptor
gene polymorphisms and curve severity of idiopathic scoliosis. Spine 2002;27:2357–2362.
CJ, Dowling FE, Fogarty EE, et al. Adolescent idiopathic scoliosis and
cerebral asymmetry. An examination of a nonspinal perceptual system. Spine 1995;20:1685–1691.
M, Pecak F, Trontelj JV, et al. Postural control in scoliosis. A
statokinesimetric study in patients with scoliosis due to neuromuscular
disorders and in patients with idiopathic scoliosis. Acta Orthop Scand 1981;52:59–63.
R, Mixon J, Fisher A et al., Scoliosis Research Society. Idiopathic
scoliosis and the central nervous system: a motor control problem. The
Harrington lecture, 1983. Spine 1985; 10:1–14.
LA, Haller RJ, Hansen PD, et al. Decreased incidence of scoliosis in
hearing-impaired children. Implications for a neurologic basis for
idiopathic scoliosis. Spine 1995;20: 776–780.
HG, Sakka SA, Powell MP, et al. Absent superficial abdominal reflexes
in children with scoliosis. An early indicator of syringomyelia. J Bone Joint Surg Br 1995;77:762–767.
X, Jiang H, Raso J, et al. Characterization of the scoliosis that
develops after pinealectomy in the chicken and comparison with
adolescent idiopathic scoliosis in humans. Spine 1997;22:2626–2635.
M, Dubousset J, Imamura Y, et al. Role of melatonin deficiency in the
development of scoliosis in pinealectomised chickens. J Bone Joint Surg Br 1995;77:134–138.
AB, Kennaway DJ, Sutherland AD. Total 24-hour melatonin secretion in
adolescent idiopathic scoliosis. A case-control study. Spine 1998;23:41–46.
KM, Raso VJ, Hill DL, et al. Melatonin levels in idiopathic scoliosis.
Diurnal and nocturnal serum melatonin levels in girls with adolescent
idiopathic scoliosis. Spine 1996;21:1974–1978.
S, Tredwell SJ, Day B, et al. An ultrastructural study of multifidus
muscle in progressive idiopathic scoliosis. Changes resulting from a
sarcolemmal defect at the myotendinous junction. J Neurol Sci 1980;46:13–31.
C, Bjork R, Ortengren R, et al. Electromyography of the paravertebral
muscles in idiopathic scoliosis. Measurements of amplitude and spectral
changes under load. Acta Orthop Scand 1984;55:304–309.
K, Lowe T, Lawellin D, et al. Levels of platelet calmodulin for the
prediction of progression and severity of adolescent idiopathic
scoliosis. J Bone Joint Surg Am 1994;76:1186–1192.
GP, Reed WC, Deacon DH, et al. Growth factor modulated
calmodulin-binding protein stimulates nuclear DNA synthesis in
hemopoietic progenitor cells. Biochemistry 1994;33:6605–6610.
P, Lenke LG, Bridwell KH. Incidence of neural axis abnormalities in
infantile and juvenile patients with spinal deformity. Is a magnetic
resonance image screening necessary? Spine 1998;23:206–210.
MB, Lenke LG, Szymanski DA, et al. Prevalence of neural axis
abnormalities in patients with infantile idiopathic scoliosis. J Bone Joint Surg Am 2002;84-A:2230–2234.
LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new
classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am 2001;83-A:1169–1181.
DG, Song KM, Katz D, et al. Relationship of peak height velocity to
other maturity indicators in idiopathic scoliosis in girls. J Bone Joint Surg Am 2000;82:685–693.
JC, Pynsent PB, Van Poortvliet JA, et al. Influence of anthropometric
factors and joint laxity in the incidence of adolescent back pain. Spine 1984;9:461–464.
K, King JD, Nelson MD. Routine use of magnetic resonance imaging in
idiopathic scoliosis patients less than eleven years of age. Spine 1992;17:S109–S116.
TW, Mazur JM, Cummings RJ. An evaluation of the Adams forward bend test
and the scoliometer in a scoliosis school screening setting. J Pediatr Orthop 1995;15:535–538.
MC, Stanford CF, Mahar AT, et al. Standing lateral radiographic
positioning does not represent customary standing balance. Spine 2003;28:1176–1182.
AR, Goldberg MS, Hanley JA, et al. Projecting the lifetime risk of
cancer from exposure to diagnostic ionizing radiation for adolescent
idiopathic scoliosis. Health Phys 1994;66:621–633.
AR, Goldberg MS, Mayo NE, et al. Reducing the lifetime risk of cancer
from spinal radiographs among people with adolescent idiopathic
scoliosis. Spine 1996;21:1540–1547.
KD, Cheung KM, Lu DS, et al. Assessment of scoliosis correction in
relation to flexibility using the fulcrum bending correction index. Spine 1998;23:2303–2307.
JJ, Winter RB, Lonstein JE. Comparison of the use of supine bending and
traction radiographs in the selection of the fusion area in adolescent
idiopathic scoliosis. Spine 1996;21: 2469–2473.
S, Passuti N, Delecrin J. Interpretation and utility of traction
radiography in scoliosis surgery. Analysis of patients treated with
Cotrel-Dubousset instrumentation. Spine 1997;22: 2542–2546.
JL. The lumbar spinal canal in children. Part II: the interpedicular
distance and its relation to the sagittal diameter and transverse
pedicular width. Eur J Radiol 1981;1:312–321.
JO, Herring JA, Browne RH. Posterior arthrodesis and instrumentation in
the immature (Risser-grade-0) spine in idiopathic scoliosis. J Bone Joint Surg Am 1995;77:39–45.
EA, Hennrikus WL, Schwend RM, et al. A prospective evaluation of
idiopathic left thoracic scoliosis with magnetic resonance imaging. J Pediatr Orthop 1996;16:354–358.
JC, Guo X, Sher AH, et al. Correlation between curve severity,
somatosensory evoked potentials, and magnetic resonance imaging in
adolescent idiopathic scoliosis. Spine 1999; 24:1679–1684.
T, Fras C, Burke S, et al. Clinical value of routine preoperative
magnetic resonance imaging in adolescent idiopathic scoliosis. A
prospective study of three hundred and twenty-seven patients. J Bone Joint Surg Am 2001;83-A:577–579.
MF, Lenke LG, Bridwell KH, et al. Preoperative spinal canal
investigation in adolescent idiopathic scoliosis curves > or = 70
degrees Spine 1994;19:1606–1610.
JA, LaPlaza J, Erickson MA, et al. Sagittal plane deformity in the
thoracic spine: a clue to the presence of syringomyelia as a cause of
scoliosis. Spine 2003;28:2147–2151.
AR, Eisberg HB. The incidence of scoliosis in the state of Delaware. A
study of 50,000 minifilms of the chest made during a survey for
tuberculosis. J Bone Joint Surg Am 1955;37:1243–1249.
AJ, Howel D, Millner PA, et al. Late-onset idiopathic scoliosis in
children six to fourteen years old. A cross-sectional prevalence study.
J Bone Joint Surg Am 1996;78:1330–1336.
F, Willner S. The natural history of idiopathic scoliosis. Incidence of
treatment in 15 cohorts of children born between 1963 and 1977. Spine 1997;22:772–774.
SL, Dolan LA, Spratt KF, et al. Health and function of patients with
untreated idiopathic scoliosis: a 50-year natural history study. JAMA 2003;289:559–567.
JIP. Idiopathic scoliosis: the prognosis, diagnosis, and operative
indications related to curve patterns and the age of onset. J Bone Joint Surg Br 1954;36:36–49.
JH, Kettleson DN. Idiopathic scoliosis. Analysis of curve patterns and
the preliminary results of Milwaukee-brace treatment in one hundred
sixty-nine patients. J Bone Joint Surg Am 1970;52:1509–1533.
M, Hwang S-C, Green WT. Growth of the normal trunk in boys and girls
during the second decade of life. Related to age, maturity, and
ossification of the iliac epiphyses. J Bone Joint Surg Am 1965;47:1554–1564.
LE, Nachemson AL. Prediction of progression of the curve in girls who
have adolescent idiopathic scoliosis of moderate severity. Logistic
regression analysis based on data from the Brace Study of the Scoliosis
Research Society. J Bone Joint Surg Am 1995;77:823–827.
A, Lonstein J, Weinstein S. Report of the Prevalence and Natural
History Committee of the Scoliosis Research Society. Annual Meeting of
the Scoliosis Research Society, Denver, CO, 1982.
K, Larsson S, Nachemson A, et al. A long term follow-up of patients
with untreated scoliosis. A study of mortality, cause of death, and
symptoms. Annual Meeting of the Scoliosis Research Society,
Minneapolis, MN, 1991.
SS, Mullaji AB, Luk KD, et al. Relation of spinal and thoracic cage
deformities and their flexibilities with altered pulmonary functions in
adolescent idiopathic scoliosis. Spine 1995;20:2415–2420.
JH, Erwin WD, Rossi D. Harrington instrumentation and arthrodesis for
idiopathic scoliosis. A twenty-one-year follow-up. J Bone Joint Surg Am 1990;72:678–683.
PN, Soucacos PK, Zacharis KC, et al. School-screening for scoliosis. A
prospective epidemiological study in northwestern and central Greece. J Bone Joint Surg Am 1997;79:1498–1503.
JE, van der Meer R, Hageman MA, et al. The benefits of school screening
for scoliosis in the central part of The Netherlands. Eur Spine J 1996;5:374–379.
CJ, Dowling FE, Fogarty EE, et al. School scoliosis screening and the
United States Preventive Services Task Force. An examination of
long-term results. Spine 1995;20:1368–1374.
P, Kreitz BG, Cassidy JD, et al. A study of the diagnostic accuracy and
reliability of the scoliometer and Adam’s forward bend test. Spine 1998;23:796–802.
G. Threshold values for supine and standing Cobb angles and rib hump
measurements: prognostic factors for scoliosis. Eur Spine J 1996;5:79–84.
AL, Peterson LE. Effectiveness of treatment with a brace in girls who
have adolescent idiopathic scoliosis. A prospective, controlled study
based on data from the Brace Study of the Scoliosis Research Society. J Bone Joint Surg Am 1995;77:815–822.
DE, Richards BS, Browne RH, et al. A comparison between the Boston
brace and the Charleston bending brace in adolescent idiopathic
scoliosis. Spine 1997;22:1302–1312.
U, Normelli H, Aaro S, et al. Long-term results of Boston brace
treatment on vertebral rotation in idiopathic scoliosis. Spine 1993;18:432–435.
JE, Winter RB. The Milwaukee brace for the treatment of adolescent
idiopathic scoliosis. A review of one thousand and twenty patients. J Bone Joint Surg Am 1994;76:1207–1221.
HG, Hall JE, Stanish W. The Boston brace system for the treatment of
low thoracic and lumbar scoliosis by the use of a girdle without
superstructure. Clin Orthop 1977;29:87–92.
NJ, Bowen JR. Adolescent idiopathic scoliosis: treatment with the
Wilmington brace. A comparison of full-time and part-time use. J Bone Joint Surg Am 1996;78:1056–1062.
WE Jr, Green NE, Pierre CB, et al. Stress and coping with scoliosis:
psychological effects on adolescents and their families. J Pediatr Orthop 1989;9:257–261.
CT, Scott DS, Reed FR Jr, et al. Nighttime bracing for adolescent
idiopathic scoliosis with the Charleston bending brace: long-term
follow-up. J Pediatr Orthop 1997;17:703–707.
DE, Bernstein SM, Riddick MF, et al. A meta-analysis of the efficacy of
non-operative treatments for idiopathic scoliosis [see Comments]. J Bone Joint Surg Am 1997;79:664–674.
CT, Scott DS, Reed FE Jr, et al. Nighttime bracing for adolescent
idiopathic scoliosis with the Charleston bending brace. Preliminary
report. Spine 1990;15:1294–1299.
R, Flynn J, Ramirez N, et al. Effectiveness of TLSO bracing in the
conservative treatment of idiopathic scoliosis. J Pediatr Orthop 1995;15:176–181.
FC, Bunch WH, Barnett PM. Psychological factors in failure to wear the
Milwaukee brace for treatment of idiopathic scoliosis. Clin Orthop 1977;126:62–66.
LG, Bridwell KH, Blanke K, et al. Radiographic results of arthrodesis
with Cotrel-Dubousset instrumentation for the treatment of adolescent
idiopathic scoliosis. A five to ten-year follow-up study. J Bone Joint Surg Am 1998;80:807–814.
M, Lai SM, Burton D, et al. Safety and efficacy of Isola
instrumentation and arthrodesis for adolescent idiopathic scoliosis:
two- to 12-year follow-up. Spine 2004;29:2013–2023.
I, Remes V, Yrjonen T, et al. Harrington and Cotrel-Dubousset
instrumentation in adolescent idiopathic scoliosis. Long-term
functional and radiographic outcomes. J Bone Joint Surg Am 2003;85-A:2303–2309.
RF, Lonstein JE, Winter RB, et al. Curve progression in Risser stage 0
or 1 patients after posterior spinal fusion for idiopathic scoliosis. J Pediatr Orthop 1997;17:718–725.
AS, Richards BS. Preventing the crankshaft phenomenon by combining
anterior fusion with posterior instrumentation. Does it work?. Spine 1995;20:1392–1398.
JJ, Mack MJ, Picetti GD III. A technical report on video-assisted
thoracoscopy in thoracic spinal surgery. Preliminary description. Spine 1995;20:831–837.
PO, Wenger DR, Mubarak SJ, et al. Anterior release and fusion in
pediatric spinal deformity. A comparison of early outcome and cost of
thoracoscopic and open thoracotomy approaches. Spine 1997;22:1398–1406.
PO, Marks M, Faro F, et al. Use of video-assisted thoracoscopic surgery
to reduce perioperative morbidity in scoliosis surgery. Spine 2003;28:S249–S254.
SI, Lee CK, Chung SS. Comparison of Zielke ventral derotation system
and Cotrel-Dubousset instrumentation in the treatment of idiopathic
lumbar and thoracolumbar scoliosis. Spine 1994;19:419–429.
KD, Leong JC, Reyes L, et al. The comparative results of treatment in
idiopathic thoracolumbar and lumbar scoliosis using the Harrington,
Dwyer, and Zielke instrumentations. Spine 1989;14:275–280.
FA, Lenke LG, Bridwell KH, et al. Maintaining lumbar lordosis with
anterior single solid-rod instrumentation in thoracolumbar and lumbar
adolescent idiopathic scoliosis. Spine 1999;24:1655–1662.
AE, Baumann R, Brown H, et al. Selective anterior fusion of
thoracolumbar/lumbar curves in adolescents: when can the associated
thoracic curve be left unfused? Spine 2003;28:706–713.
K, Shono Y, Satoh S, et al. Anterior correction of thoracic scoliosis
with Kaneda anterior spinal system. A preliminary report. Spine 1997;22:1358–1368.
TR, Bergman M, O’Brien M, et al. The effect of the three columns of the
spine on the instantaneous axis of rotation in flexion and extension. Spine 1991;16:S312–S318.
CG, Eysel P, Dubousset J. Operative treatment of scoliosis with
Cotrel-Dubousset-Hopf instrumentation. New anterior spinal device. Spine 1997;22:618–627; discussion.
KB, Kim C, Newton PO. Spinal lordosis with marked opisthotonus
secondary to dystonia musculorum deformans: case report with surgical
management. Spine 2001;26:2283–2288.
RR, Harms J, Clements DH, et al. Comparison of anterior and posterior
instrumentation for correction of adolescent thoracic idiopathic
scoliosis. Spine 1999;24:225–239.
LG, Betz RR, Bridwell KH, et al. Spontaneous lumbar curve coronal
correction after selective anterior or posterior thoracic fusion in
adolescent idiopathic scoliosis. Spine 1999;24: 1663–1671.
H, Hee H, Yu Z, et al. Results of thoracoscopic instrumented fusion
versus conventional posterior instrumented fusion in adolescent
idiopathic scoliosis undergoing selective thoracic fusion. Spine 2004;29:2031–2038.
JH, Kharrat K, Winter RB, et al. Harrington instrumentation without
fusion plus external orthotic support for the treatment of difficult
curvature problems in young children. Clin Orthop 1984;185:35–45.
DS, Iqbal MJ, Thompson AG, et al. Convex spinal epiphysiodesis in the
management of progressive infantile idiopathic scoliosis. Spine 1996;21:1884–1888.
RW, Murrell GA, Motley G, et al. A logical coronal pattern
classification of 2,000 consecutive idiopathic scoliosis cases based on
the Scoliosis Research Society–defined apical vertebra. Spine 1998;23:1380–1391.
LG, Betz RR, Harms J, et al. A new comprehensive classification system
of adolescent idiopathic scoliosis. Presented at 32nd Annual Meeting of
the SRS, 1997.
LG, Betz RR, Clements D, et al. Curve prevalence of a new
classification of operative adolescent idiopathic scoliosis: does
classification correlate with treatment? Spine 2002;27: 604–611.
PO, Faro FD, Lenke LG, et al. Factors involved in the decision to
perform a selective versus nonselective fusion of Lenke 1B and 1C
(King-Moe II) curves in adolescent idiopathic scoliosis. Spine 2003;28:S217–S223.
LG, Bridwell KH, Baldus C, et al. Preventing decompensation in King
type II curves treated with Cotrel-Dubousset instrumentation. Strict
guidelines for selective thoracic fusion. Spine 1992;17:S274–S281.
KH, McAllister JW, Betz RR, et al. Coronal decompensation produced by
Cotrel-Dubousset “derotation” maneuver for idiopathic right thoracic
scoliosis. Spine 1991;16:769–777.
BS, Birch JG, Herring JA, et al. Frontal plane and sagittal plane
balance following Cotrel-Dubousset instrumentation for idiopathic
scoliosis. Spine 1989;14:733–737.
LG, Bridwell KH, O’Brien MF, et al. Recognition and treatment of the
proximal thoracic curve in adolescent idiopathic scoliosis treated with
Cotrel-Dubousset instrumentation. Spine 1994;19:1589–1597.
TR, Lenke LG, Graham EJ, et al. Correlation of radiographic, clinical,
and patient assessment of shoulder balance following fusion versus
nonfusion of the proximal thoracic curve in adolescent idiopathic
scoliosis. Spine 2002;27:2013–2020.
TR, Lenke LG, Won DS, et al. Spontaneous proximal thoracic curve
correction after isolated fusion of the main thoracic curve in
adolescent idiopathic scoliosis. Spine 2001;26:1966–1975.
K, Shono Y, Satoh S, et al. New anterior instrumentation for the
management of thoracolumbar and lumbar scoliosis. Application of the
Kaneda two-rod system. Spine 1996;21: 1250–1261.
FA, Lenke LG, Bridwell KH, et al. Prospective radiographic and clinical
outcomes and complications of single solid rod instrumented anterior
spinal fusion in adolescent idiopathic scoliosis. Spine 2001;26:1956–1965.
H, Geck M, Clark C. The posterior approach for lumbar and thoracolumbar
adolescent idiopathic scoliosis: posterior shortening and pedicle
screws. Spine 2004;29:269–276.
TR, Merola A, Zipnick RI, et al. Meta-analysis of surgical outcome in
adolescent idiopathic scoliosis. A 35-year English literature review of
11,000 patients. Spine 1995;20:1575–1584.
AA, Haher TR, Brkaric M, et al. A multicenter study of the outcomes of
the surgical treatment of adolescent idiopathic scoliosis using the
Scoliosis Research Society (SRS) outcome instrument. Spine 2002;27:2046–2051.
TR, Gorup JM, Shin TM, et al. Results of the Scoliosis Research Society
instrument for evaluation of surgical outcome in adolescent idiopathic
scoliosis. A multicenter study of 244 patients. Spine 1999;24:1435–1440.
AJ, Nachemson AL. Back pain and function 23 years after fusion for
adolescent idiopathic scoliosis: a case-control study—part II. Spine 2003;28:E373–E383.
T, Irstam L, Nachemson A. Long-term anatomic and functional changes in
patients with adolescent idiopathic scoliosis treated by Harrington rod
fusion. Spine 1983;8:576–584.
PJ, Von Schroeder HP, Johnson GE, et al. Adolescent idiopathic
scoliosis. Long-term effect of instrumentation extending to the lumbar
spine. J Bone Joint Surg Am 1995;77:1210–1216.
B, Mayo NE, Goldberg MS, et al. The Ste-Justine Adolescent Idiopathic
Scoliosis Cohort Study. Part IV: surgical correction and back pain. Spine 1994;19:1582–1588.
AJ, Nachemson AL. Radiologic findings and curve progression 22 years
after treatment for adolescent idiopathic scoliosis: comparison of
brace and surgical treatment with matching control group of straight
individuals. Spine 2001;26:516–525.
RB, Carr P, Mattson H. A study of functional spinal motion in women
after instrumentation and fusion for deformity or trauma. Spine 1997;22:1760–1764.
I, Remes V, Yrjonen T, et al. Comparison of long-term functional and
radiologic outcomes after Harrington instrumentation and spondylodesis
in adolescent idiopathic scoliosis: a review of 78 patients. Spine 2002;27:176–180.
V, Boachie-Adjei O, Backus SI, et al. Characterization of gait function
in patients with postsurgical sagittal (flatback) deformity: a
prospective study of 21 patients. Spine 2002;27:2328–2337.
FS, Fernandez-Baillo N, Arauz de Robles S, et al. The low lumbar spine
below Cotrel-Dubousset instrumentation: long-term findings. Spine 2000;25:2333–2341.
T, Grob D, Scheier H, et al. Cotrel-Dubousset and Harrington
instrumentation in idiopathic scoliosis: a comparison of long-term
results. Eur Spine J 1995;4:280–283.
V, Helenius I, Schlenzka D, et al. Cotrel-Dubousset (CD) or Universal
Spine System (USS) instrumentation in adolescent idiopathic scoliosis
(AIS): comparison of midterm clinical, functional, and radiologic
outcomes. Spine 2004;29:2024–2030.
YJ, Lenke LG, Cho SK, et al. Comparative analysis of pedicle screw
versus hook instrumentation in posterior spinal fusion of adolescent
idiopathic scoliosis. Spine 2004;29:2040–2048.
KH, Betz R, Capelli AM, et al. Sagittal plane analysis in idiopathic
scoliosis patients treated with Cotrel-Dubousset instrumentation. Spine 1990;15:921–926.
BJ, Tredwell SJ, Jang SB, et al. Effects of three-dimensional
assessment on surgical correction and on hook strategies in multi-hook
instrumentation for adolescent idiopathic scoliosis. Spine 1998;23:201–205.
H, Dansereau J, Bellefleur C, et al. Perioperative three-dimensional
correction of idiopathic scoliosis with the Cotrel-Dubousset procedure.
Spine 1995;20:1406–1409.
DD, Stokes IA, Ronchetti PJ, et al. Surgical correction of vertebral
axial rotation in adolescent idiopathic scoliosis: prediction by
lateral bending films. J Spinal Disord 1996;9:214–219.
U, Transfeldt EE, Hedlund R. The segmental effect of Cotrel-Dubousset
instrumentation on vertebral rotation, rib hump and the thoracic cage
in idiopathic scoliosis. Eur Spine J 1996;5:387–393.
IT, Tuzuner M, Akalin S, et al. Spinal imbalance and decompensation
problems in patients treated with Cotrel-Dubousset instrumentation. Eur Spine J 1996;5:380–386.
LG, Betz RR, Bridwell KH, et al. Intraobserver and interobserver
reliability of the classification of thoracic adolescent idiopathic
scoliosis. J Bone Joint Surg Am 1998;80:1097–1106.
SJ, Schuette AM, Emans JB. Lumbar pedicle screws versus hooks. Results
in double major curves in adolescent idiopathic scoliosis. Spine 1997;22:1369–1379.
CL, Lenke LG, Bridwell KH, et al. The use of pedicle screw fixation to
improve correction in the lumbar spine of patients with idiopathic
scoliosis. Is it warranted? Spine 1996;21:1241–1249.
V, Papin P, Marchesi D, et al. Adolescent idiopathic thoracic
scoliosis: apical correction with specialized pedicle hooks. Eur Spine J 1999;8:266–271.
R, Galland O, Mechin H, et al. The Dwyer procedure in the treatment of
idiopathic scoliosis. A 10-year follow-up review of 21 patients. Spine 1990;15:75–80.
TG, Peters JD. Anterior spinal fusion with Zielke instrumentation for
idiopathic scoliosis. A frontal and sagittal curve analysis in 36
patients. Spine 1993;18:423–426.
KH, Lenke LG, Baldus C, et al. Major intraoperative neurologic deficits
in pediatric and adult spinal deformity patients. Incidence and
etiology at one institution. Spine 1998; 23:324–331.
DM, Marrero G, King J, et al. Avoiding paraplegia during anterior
spinal surgery. The role of somatosensory evoked potential monitoring
with temporary occlusion of segmental spinal arteries. Spine 1991;16:S365–S370.
SD, Johnson JR, Shields CB, et al. Correlation of motor-evoked
potentials, somatosensory-evoked potentials, and the wake-up test in a
case of kyphoscoliosis. J Spinal Disord 1993;6:194–198.
JE, Levine CR, Sudhir KG. Intraoperative awakening to monitor spinal
cord function during Harrington instrumentation and spine fusion.
Description of procedure and report of three cases. J Bone Joint Surg Am 1978;60:533–536.
MG, Stazzone EJ, Gelijns AC, et al. The effectiveness of preoperative
erythropoietin in averting allogenic blood transfusion among children
undergoing scoliosis surgery. J Pediatr Orthop B 1998;7:203–209.
D, Jedeikin R, Metser U, et al. Acute normovolemic haemodilution and
idiopathic scoliosis surgery: effects on homologous blood requirements.
Anaesth Intensive Care 1993; 21:429–431.
