ANTERIOR APPROACH TO SCOLIOSIS
characterized by coronal, sagittal, and horizontal plane deviation.
Although posterior spinal fusion and instrumentation can be used for
most patients, there are many situations in which the anterior approach
is necessary. With recent advances in thoracoscopic techniques, the
indications for anterior surgery may be expanded (46). The deformity surgeon must, therefore, be aware of the anterior surgical options available.
anterior surgery for the treatment of spinal conditions started with
Hodgson, who reported the results of anterior spinal decompression and
fusion for tuberculosis of the spine in 1956 (13,14). Dwyer expanded the technique developed by Hodgson to approach the convex side of a scoliosis curve (9,10). During the past 30 years, spinal surgeons have become facile with the anterior or combined anterior and posterior approaches.
cases of idiopathic, congenital, neuromuscular, and adult scoliosis.
The neuromuscular curves include those caused by cerebral palsy,
myelomeningocoele (MM), muscular dystrophy, polio, trauma, Friedrich’s
ataxia, and syringomyelia. The combination of curve magnitude,
rigidity, spasticity, and paralysis frequently necessitates combined
anterior and posterior spinal fusion.
for surgery are at times controversial in severely involved children,
and complications are frequent. Patients with impending skin compromise
due to pelvic obliquity or kyphosis, poor sitting balance with muscular
fatigue and pain, loss of upper extremity function due to using their
arms to support and elevate the trunk, and progressive deterioration of
pulmonary function are strong indications for surgery (39).
Attempts to delay surgery with bracing until adequate trunk height is
obtained are a common dilemma in the preadolescents with significant
deformity. Absolute Cobb angular measurements for which surgery is
indicated range from 40° to 60°. Issues regarding age, medical
comorbidity, and family acceptance make rigid guidelines difficult to
implement.
indicated, same-day sequential surgery usually is performed. The
anterior approach allows more correction by excising the discs and
ligaments (5), increases fusion rates, prevents crankshafting in immature spines (8,20,21), decompresses the spinal cord, and provides exposure for internal thoracoplasty (40). This is followed by posterior instrumentation and fusion. Powell et al. (36)
compared the results between staged and same-day surgery. They found
less blood loss, shorter hospital stays, and reduced cost with same-day
surgery. Ferguson et al. (11) reviewed the
results of same-day surgery in patients with neuromuscular scoliosis.
The overall complication rate was reduced from 124% to 88%. The number
of patients without any complications in this high-risk group increased
from 35% to 63%.
changed since the second edition of this book. The Dwyer and Zielke
instrumentations are no longer used in our practice. Anterior
instrumentation is used now for structural support as well as for
deformity correction. In select cases of idiopathic scoliosis, anterior
instrumentation may be all that is necessary. Valuable motion segments
may be preserved in the lumbar spine when fusing with anterior
instrumentation.
anterior procedure is a thorough history and physical examination. For
all cases, obtain an appropriate medical consultation prior to surgical
intervention. A complete examination of the chest, abdomen, back, and
lower extremities is important. Assess spinal balance, as this aides in
determining fusion level. For example, a lumbar scoliosis with an
associated rigid thoracic kyphosis must be fused across both curves to
prevent decompensation and progression of the thoracic deformity.
muscle strength, rapid curve progression, increasing motor weakness, or
cavus foot deformity. Evaluate with magnetic resonance imaging (MRI) to
look for an intraspinal lesion or tethered cord (37).
Do a myelogram only in cases that are difficult to interpret. An
assessment of pelvic obliquity and hip joint contracture is vital if
the fusion is to be carried into the lumbosacral region. At times, a
release of hip flexion contracture may be required to decrease
excessive lumbar lordosis prior to spine surgery.
sitting 14×36 films in the posteroanterior and lateral planes. Supine
side bending films determine the curve flexibility and assist in
determining the fusion endpoint. Image the lumbosacral junction to
detect an occult spondylolisthesis. Preoperative MRI is not necessary
for idiopathic adolescent (48) and adult deformity.
determine which group of patients will require respiratory assistance
in the perioperative period. Forced vital capacity (FVC) values less
than 40% of predicted values, and small children with recurrent upper
respiratory infections should be evaluated closely (16).
In idiopathic scoliosis, little effect on the FVC occurs until curves
approach 60° to 100°. However, patients with spinal muscular atrophy or
muscular dystrophy have marked reductions in FVC to less than 50% with
curves of only 30° (39).
evoked potential exams to establish a baseline with which comparison
can be made intraoperatively. Theoretically, detecting early subtle
changes in evoked potential can enhance the safety of the procedure.
multiple disciplines. A careful history should elicit any urologic,
cardiac, musculoskeletal, or neurologic anomalies. Approximately one
third of these patients have urologic abnormalities, and 10% possess
cardiac malformations (24). Evaluation of the
genitourinary system with an intravenous pyelogram or ultrasound can
detect anomalies, which may need treatment prior to spinal surgery.
of staged anterior and posterior surgery noted that all infectious
complications but one occurred in malnourished patients. A serum
albumin over 3.5 g/dl and total lymphocyte count of more than 1,500/µl
are acceptable. The normalization of nutritional parameters following
combined spinal surgery correlates with the length of fusion according
to Lenke et al. (22). Shorter fusions required
approximately 6 weeks, and longer fusions about 12 weeks before
nutritional parameters normalized when perioperative hyperalimentation
was not used.
undergoing combined anterior and posterior procedures. We place a
central line at surgery and continue hyperalimentation until the
postoperative ileus resolves and the patient’s appetite returns. We
have found a decrease in hospital stay and perioperative morbidity with
this regimen.
Autologous donation does not compromise nutritional status, nor does it
increase complication rates or the need for postoperative transfusions.
The blood lost during surgery in these patients is more dilute, since
their hematocrit at the time of surgery is lower. Homologous blood
donation causes immunosuppression and places the patient at risk for
transmission of viral diseases.
used in our institution, there are recent studies that question its
utility (41,42 and 43)
in selected populations. There are significant costs for equipment and
technicians, and the salvaged blood has a low hematocrit. There is no
definitive evidence that IAT reduces the need for postoperative
transfusion in select populations. However, for our combined procedures
to correct scoliosis and kyphosis, IAT is used. We feel that this
decreases the amount of homologous blood required for the surgery.
but for certain lumbar and thoracolumbar curves we use the anterior
approach. Anterior instrumentation systems provide better correction of
rotation, as forces are exerted directly through the vertebral body and
disc spaces. When an anterior approach is selected, fewer levels are
fused, which may prevent the future development of low-back pain. The
Dwyer system was one of the first used this way (9,10).
The lower pseudarthrosis rates and improved sagittal contour fueled the
transition to solid rod systems such as Isola, the Texas Scottish Rite
Hospital system (TSRH), and Kaneda.
thoracolumbar curve of 60° or less, with a flexible thoracic deformity
that reduces to less than 20° on supine bending films. The number of
levels to be fused depends on whether the curve apex is at the disc or
the vertebral body level. When the apex lies at the disc, two levels
above and below are fused. If the apex is at the body, then one body
above and below the apical vertebra is fused.
correction of coronal deformity when comparing their results using the
Dwyer, Zielke, and TSRH systems. The TSRH system produced less
segmental kyphosis. There was only one pseudarthrosis in their series
of 18 cases with TSRH. This was successfully salvaged with a posterior
fusion.
there are many common features. The curves tend to occur early,
progress rapidly, involve the entire spine, and result in pelvic
obliquity, and they progress in adulthood (39). A combined anterior and posterior procedure is often utilized with fusion from T2 to the pelvis.
flexibility and leveling of the pelvis on side-bending films. When the
pelvis becomes level on side-bending films, an anterior release is not
necessary. However, when lumbar rigidity precludes correction to less
than 30°, the anterior discectomy and bone grafting is performed prior
to the posterior procedure.
and posterior instrumentation (Dwyer anterior and Luque-Galveston
posterior) to anterior release with segmental fixation posteriorly with
either Isola (DePuy Acromed, Warsaw, IN) or CD Horizon (Sofamor Danek,
Memphis, TN) instrumentation. This change emphasizes the role of the
anterior procedure in mobilizing a rigid deformity, establishing lumbar
lordosis, and improving fusion rates and overall sagittal balance.
Recently, we have utilized titanium cages anteriorly to improve
sagittal contour prior to the posterior procedure. This prevents loss
of lordosis from graft settling that may be seen with the use of
autograft or allograft alone. Pelvic fixation varies with the patients’
anatomy, but an iliac post is most commonly used. The iliac post,
placed between the inner and outer tables of the ilium, increases the
rigidity of the fixation.
approaching 100% in thoracic-level patients. Nearly 60% of L4 level
patients have scoliosis. The overall goal of surgery is to achieve a
vertical torso centered over a level pelvis. Therefore, most patients
require a fusion from T2 to the sacrum (Fig. 155.1).
 |
|
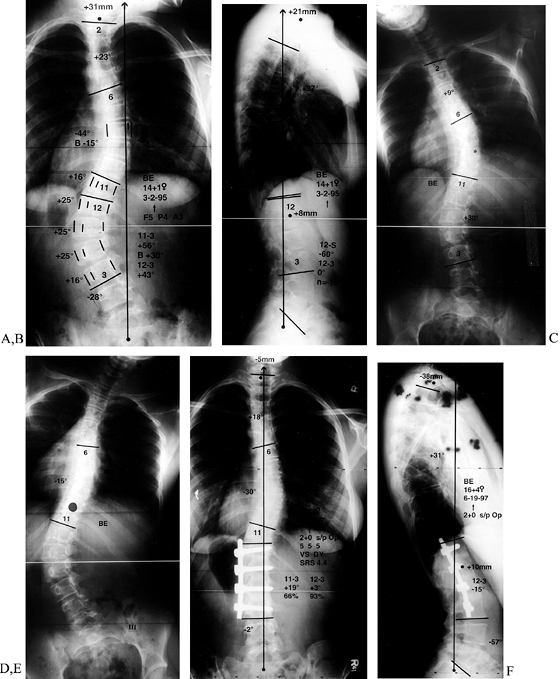
Figure 155.1. A:
Preoperative AP view of a child with myelomeningocoele and a curve of 58°. A combined anterior release and posterior fusion with Cotrel-Dubousset rods, pedicle screws, and Luque wires was utilized. Postoperative lateral (B) and AP (C) views show excellent correction of coronal and sagittal deformities. A solid fusion is evident at 1-year follow-up. (Courtesy of John Sarwark.) |
instrumentation and fusions with the Dwyer technique with a
supplemental posterior fusion to the sacrum with Luque instrumentation.
This technique allowed excellent coronal and sagittal correction. We
had only one pseudarthrosis with this technique and it was associated
with persistent infection necessitating early removal of the posterior
hardware.
followed by posterior Isola instrumentation with iliac post fixation in
the pelvis. The fusion rates have remained acceptable with this
modification. The real benefit has been the improved sagittal balance
obtained from the anterior mobilization followed by posterior deformity
correction and fusion. Segmental fixation posteriorly combines hooks,
sublaminar wires, and pedicle screws.
The kyphosis caused by some anterior instrumentation is only
exacerbated in this population. Anterior instrumentation also reduces
the sagittal correction obtained by posterior techniques.
cerebral palsy (CP) patients. As the magnitude of neurologic
involvement increases, the severity of the curve increases (27,38,39).
Ambulatory patients with CP have approximately half the rate of
significant deformity that nonambulators have. Curve progression into
adulthood is known to occur; therefore, maintenance of ambulation
through aggressive physical therapy and close follow-up is necessary.
The risks and benefits of surgery should be thoroughly discussed with
the parents. When there is any prospect of functional benefit to the
patient, surgery is recommended. It is difficult to judge the true
benefit for those with severe cognitive impairment, seizure disorder,
and malnutrition.
Therefore, fusion is required from the upper thoracic spine to the
pelvis. The anterior procedure releases the rigid structures, which
enhances posterior correction and fusion. If pelvic obliquity is not
significant (i.e., it corrects on side-bending films), then fusion may
be carried down to L4 or L5.
whether the hemivertebra is segmented, semisegmented, or incarcerated.
The fully segmented variety is the most common and produces the
greatest deformity. In growing patients, use an anterior and posterior
approach to perform a hemiepiphysiodesis, where obliteration of the
convex endplates will halt spinal growth on the convexity and allow
concave growth to correct the deformity (47). Thompson et al. (45)
reported their results of convex epiphysiodesis for hemivertebra. The
operative objectives were to prevent the development of severe
deformity and allow growth of the remaining concave epiphysis to
correct scoliosis. They found the procedure to be safe, and it yielded
some correction in 76% of their patients and slowed progression in the
remaining patients. The greatest correction was obtained in the lumbar
spine of the youngest patients in their series.
technique has been described for lumbosacral hemivertebra with severe
coronal plane imbalance. Callahan et al. (3)
reported the results of hemivertebral excision in 10 patients. Nine of
10 were excised in a single stage from T12 to L3. The procedure was
safe and effective with a curve correction of 67% obtained. Greatest
correction was obtained in the younger patients (under 4 years old).
scoliosis is the high rate of complications, particularly
pseudarthrosis. In a review of 62 adult cases, a 16.7% incidence of
pseudarthrosis was present among patients undergoing posterior spinal
fusion with Harrington rod instrumentation (34).
treat all thoracolumbar or lumbar structural curves greater than 65° by
combined anterior discectomy and fusion, followed by posterior spinal
fusion with Cotrel-Dubousset (CD) instrumentation. This aggressive
approach is indicated because these curves are stiff and there is
little correction with posterior instrumentation and fusion alone. The
anterior discectomy and fusion, followed by posterior instrumentation
and fusion, results in a better correction of the curve and a more
balanced spine, and it enhances the fusion rate (Fig. 155.2).
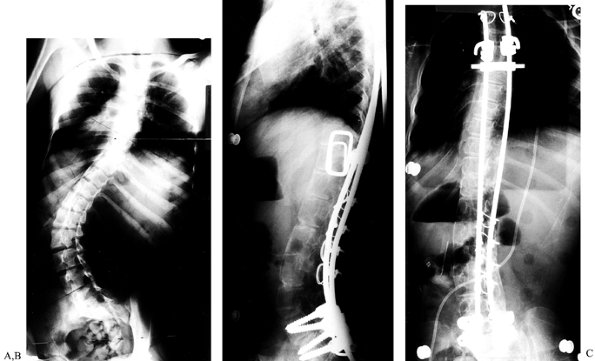
 |
|
Figure 155.2. A: A 26-year-old woman with an increasing throracolumbar curve and low back pain. B: A lateral x-ray reveals a junctional kyphosis from T11 to L1. C:
The postoperative AP x-ray shows correction of the curve to 20° with titanium cages filled with autogenous bone placed between T11 and L3. D: The lateral x-ray demonstrates the restoration of the normal lordosis between T11 and L3. |
either the thoracolumbar or the lumbar area, an anterior approach using
anterior CD instrumentation is performed. If a curve has an associated
kyphosis of the thoracolumbar or lumbar spine, we have used titanium
cages to correct sagittal alignment. The cage size is selected to
restore each disc space to a more lordotic posture.
-
Place the patient in the lateral
decubitus position with the table in a flexed position. Move the upper
arm forward to rotate the scapula away from the posterior portion of
the vertebral column. Place an axillary roll between the patient and
the table to minimize pressure on the brachial plexus during the
procedure (Fig. 155.3).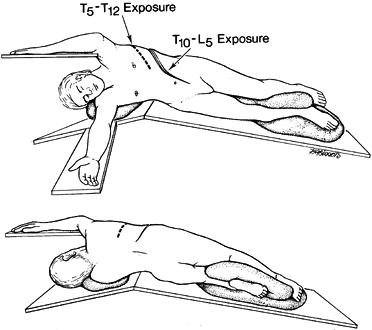 Figure 155.3. A:
Figure 155.3. A:
Anterior view of a patient in the lateral decubitus position. A roll is
placed under the axilla to minimize the compression of the axillary
artery. The dotted line represents the
skin incision for exposure of T5 to T12. For both exposures, the table
is flexed to accentuate the spinal curvature. B: The posterior view of the patient showing the position of the arms and the posterior extent of the incisions. -
Expose the anterior spine by one of two
standard approaches: Approach the thoracic spine through the bed of the
convex fifth rib, which provides good visualization of T5 to T12 (Fig. 155.3);
or approach the thoracolumbar spine through the bed of the tenth rib.
Extend this incision anteriorly to the lateral border of the rectus
sheath. The incisional length varies, depending on the number of levels
exposed.
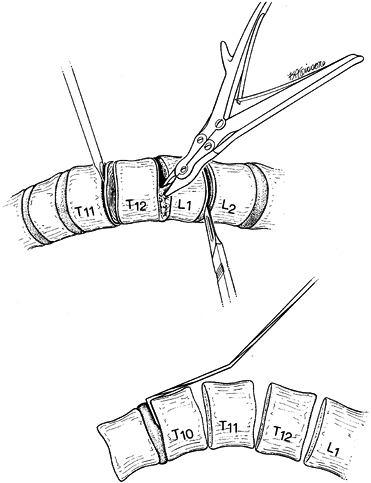
thoracolumbar approach is the rib cephalad to the upper-end vertebra in
the fusion. For example, if the upper vertebra to be instrumented or
fused is T10, then the ninth rib is resected. Once the rib is excised,
the pleura is incised to expose the vertebral column. A double lumen
endotracheal tube combined with moist laparotomy sponges and a
well-contoured malleable retractor allows excellent retraction of the
thoracic contents.
-
The thoracolumbar approach enters the retroperitoneal space on the convex side of the curve.
-
After removing the appropriate rib, split
the costal cartilage along the longitudinal axis. Identify the yellow
fatty tissue of the retroperitoneal space immediately beneath the split
costal cartilage. Enter the plane by blunt, finger dissection. -
Sweep the peritoneum off the undersurface
of the diaphragm and the anterior surface of the psoas muscle to expose
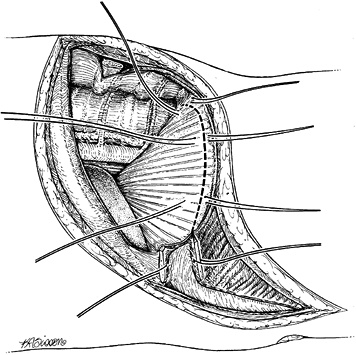
the spine. Incise the diaphragm along its peripheral margin from
anterior to posterior (Fig. 155.4). Leave 1.5
cm of diaphragm as an edge for reattachment at the conclusion of the
procedure. Place stay sutures at 3 cm intervals. If the diaphragm is
cut too close to the periphery, muscular contraction may make closure
difficult.![]() Figure 155.4. The costal cartilage has been split and marking sutures are placed on both cut ends. The dotted line
Figure 155.4. The costal cartilage has been split and marking sutures are placed on both cut ends. The dotted line
demonstrates the detachment of the peripheral portion of the diaphragm.
The diaphragm is detached 0.75 inch from the periphery to facilitate
closure. -
Reflect the pleura and ligate the
intercostal vessels in the midportion of the vertebral body to complete
the thoracic exposure (Fig. 155.5). Carefully
elevate the vessels off of the vertebral body, and double-ligate with
suture, then retract the vascular elements from the working field.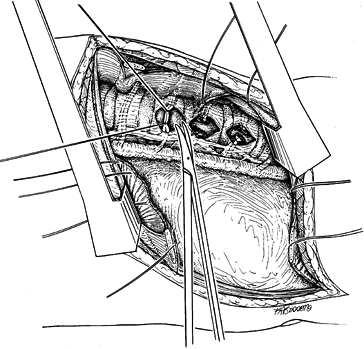 Figure 155.5. The segmental vessels are ligated at the midportion of the vertebral body.
Figure 155.5. The segmental vessels are ligated at the midportion of the vertebral body.
longitudinal arterial trunk of the spinal cord and also the posterior
lateral longitudinal arterial trunks of the cord. Spinal cord blood
supply is not compromised by ligating multiple intercostal vessels as
long as this anastomosis is preserved (7).
-
Detach the crura of the diaphragm to
expose the thoracolumbar spine at L1-2. Detach the origin of the psoas
at the L1 vertebral body or reflect it anteriorly. For patients with
large psoas muscles, the muscle may be split along its longitudinal
axis to expose the spine.
critical that the lumbar segmental vessels arising from the iliac
artery and vein be ligated in the middle of the vertebral body. There
is also a lumbar segmental vessel from the iliac artery and vein that
passes into the pelvis. Identify this vessel carefully so that the
iliac artery and vein can be mobilized away from the spine. Inadvertent
transection of this vessel is difficult to manage because the vessel
retracts into the pelvis. This can lead to extensive hemorrhage.
-
Once the spine is completely exposed,
place retractors to protect the vascular structures. Place a Chandler
elevator on the opposite side of the disc space to be resected. -
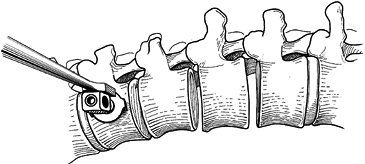
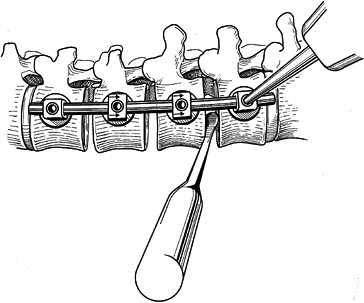
Resect the disc back to the posterior longitudinal ligament with a knife and rongeurs (Fig. 155.6).
Use a headlight and loupes during this phase of the procedure.
Meticulous technique is needed to avoid inadvertently penetrating the
ligament, which is closely adherent to the dura and spinal cord.
Exposing a broader surface area improves the fusion rate.![]() Figure 155.6. A:
Figure 155.6. A:
After exposure of the spinal column, the intervertebral disc is removed
thoroughly by means of sharp dissection with a knife and rongeur. The
vertebral endplates are removed by using a fine osteotome. B:
After the disc has been removed at all levels, the staple starter is
placed on the superior border of the uppermost vertebra that is to be
fused.
-
If the posterior longitudinal ligament is
inadvertently transected, brisk bleeding may be encountered from the
epidural venous plexus. Obtain control by gently packing with
thrombin-soaked Gelfoam pledgets. Small dural tears may be sealed in a
similar fashion. It is the second-listed author’s experience that this
local treatment plus curve correction will cause the tear to seal off.
Larger tears require closure. Facilitate exposure of the tear by
removal of a portion of the vertebral body, and use 6-0 nylon sutures
to repair the dural tear. -
On completion of the discectomy, excise
the vertebral endplates using a fine osteotome. Take care to remove
only the endplate. If too much of the body is removed, the purchase of
any form of instrumentation on the vertebra will be compromised.
the surgeon multiple options for anterior fixation. The anterior
surgical options include discectomy and fusion with autograft or
threaded/solid rod systems. Personal experience, anatomy and location
of the curve, number of levels, patient age, and cost will dictate the
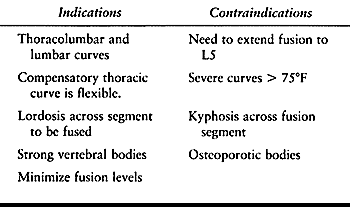
specific approach. The indications and contraindications for anterior
instrumentation are outlined in Table 155.1.
Additional consideration should be given for anterior instrumentation
when prominent posterior hardware and crankshaft phenomena are a
concern.
 |
|
Table 155.1. Indications and Contraindications for Anterior Instrumentation
|
determined the same way regardless of the instrumentation used. The
apex of the curve of concern is determined from the standing
radiograph. It is the most laterally displaced portion of the Cobb
curve from a line joining the center of the end vertebral bodies as
measured by the Cobb method. If the regional apex is a vertebra,
further apparent as the single most rotated vertebra, the apex vertebra
plus one vertebra above and one below are included in this
instrumentation construct. As the scoliosis reaches 55° or greater, it
is generally necessary to add two vertebrae above and two vertebrae
below the end vertebra. If the regional apex is a disc, further
apparent because of similar adjacent vertebral rotation, then two
vertebrae above and two vertebrae below are added. In addition, the
first caudal disc space that shows reversal of coronal plane angulation
on a convex bending radiograph can usually be excluded.
techniques for insertion of a threaded rod system [Zielke (Osteotech,
Inc., Eatontown, NJ)] and two solid rod systems (DePuy Motech and
Isola).
 |
|
Figure 155.7. The threaded Zielke rod with associated locking nuts. (Courtesy of Daniel Benson, M.D.)
|
-
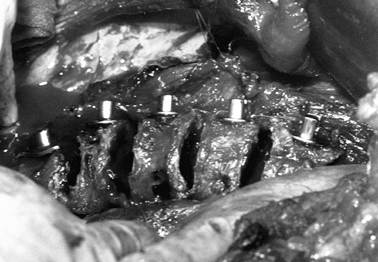
Insert the instrumentation after exposure
of the anterior spine has been completed. Place appropriate-size screws
across the vertebral bodies (Fig. 155.8) by
measuring the width of the vertebral body, and selecting the
appropriate-length screw to allow several threads to protrude from the
opposite cortex. Place the screw as posterior as possible in the
vertebral body.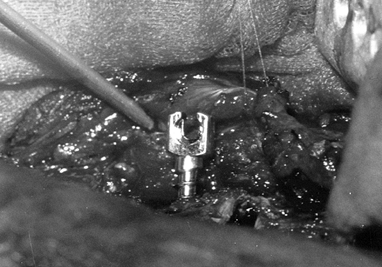 Figure 155.8. The slotted screw head for the insertion of the Zielke rod. (Courtesy of Daniel Benson, M.D.)
Figure 155.8. The slotted screw head for the insertion of the Zielke rod. (Courtesy of Daniel Benson, M.D.) -
Following appropriate placement of the screws (Fig. 155.9), place a threaded rod in the screw heads, beginning at the apex; working proximally and distally, compress the convexity (Fig. 155.10).
![]() Figure 155.9. The spine has been exposed and all the Zielke screws are in place. (Courtesy of Daniel Benson, M.D.)
Figure 155.9. The spine has been exposed and all the Zielke screws are in place. (Courtesy of Daniel Benson, M.D.)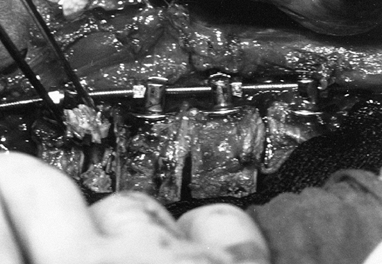 Figure 155.10.
Figure 155.10.
The compression force has been applied to the convex side of the curve,
and resected rib for the graft is placed into the intervertebral disc
space. (Courtesy of Daniel Benson, M.D.) -
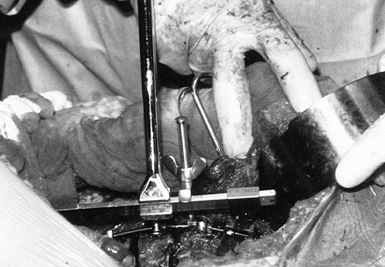
Prior to any significant compression across the apex, place the derotator bar (Fig. 155.11).
This consists of a metal bar that is fitted to the ends of the rod. and
a tension screw that is placed between the apex of the rod and the bar.
Tightening the tension screw affords some increase in lordosis of the
spine, which may be further increased by rotating the bar (Fig. 155.12). This is seen intraoperatively by an opening anteriorly of the disc spaces.![]() Figure 155.11. The derotation bar has been applied to the Zielke rod. (Courtesy of Daniel Benson, M.D.)
Figure 155.11. The derotation bar has been applied to the Zielke rod. (Courtesy of Daniel Benson, M.D.) Figure 155.12.
Figure 155.12.
The Zielke rod has been inserted and all hex nuts tightened. This
provides the curve correction. (Courtesy of Daniel Benson, M.D.) -
Place rib graft anteriorly to facilitate
fusion and maintain adequate lumbar lordosis. Then achieve compression
by tightening the hex nuts.
coronal correction of scoliosis. The rate of curve correction varies
between 60% and 80%, with adolescents achieving the higher rate of
correction. In an early report that looked at the correction of this
instrumentation, 19 patients were evaluated with an average success
rate of 77% for the thoracolumbar curve and 92% correction in the
lumbar curve (49). These results have been duplicated in many studies (15,30,35).
In a report that stratified results obtained by Zielke, Dwyer, and
Harrington rod instrumentation, the Zielke appeared to have the highest
rate of correction (26).
adolescents. Kaneda reported a correction rate of 59%, with all
developing a pseudarthrosis. The literature suggests that loss of
correction occurs between surgical intervention and later follow-up. In
one report, 18 patients who had an initial mean correction of 82% in
the lower curve found the rate dropped to 63% at follow-up. The same
trend
was found in the correction rates associated with the upper curve, with
a loss from 33% to 18% at follow-up. Rotational correction was also
reduced from 66% to 56%. This loss of correction is likely the result
of a lack of stiffness of the threaded rod.
with an average loss of lordosis of 8° to 10° over the instrumented
segments. Many studies (17,23,32)
have documented the occurrence of this increased kyphosis, but none
have shown it to affect the surgical outcome. This, however, can be
reduced with appropriate anterior column support by structural grafts
such as femoral rings, and threaded or titanium cages.
-
Place the patient in the lateral
decubitus position and make a single skin incision paralleling the
eighth rib. Mobilize and detach the serratus anterior muscle. Make an
incision in the intercostal space at T4 or T5 for instrumentation of
the T5 vertebra. Make a second incision between the intercostal space
three or four ribs distally (i.e., to T8 or T9) for instrumentation of
T12 (Fig. 155.13).![]() Figure 155.13.
Figure 155.13.
Incisions made at the intercostal space at T4 or T5, and between the
intercostal spaces at approximately three to four ribs distally.
(Redrawn from Moss Miami 4.0 mm Anterior System Surgical Technique:
Anterior Treatment of Thoracic Idiopathic Scoliosis. DePuy Motech,
Warsaw, Indiana, 1997.) -
If the preoperative analysis suggests
that a thoracoplasty is necessary for cosmetic reasons, perform it at
this stage. A rib approximately 2 cm in length is removed as far
posteriorly as possible between the two intercostal space entrances. If
there is a very significant rib hump, then the entire posterior 2 cm of
rib is removed from each of the vertebrae to be instrumented. Rib
resections better mobilize the chest wall, making the discectomies and
instrumentation less difficult to perform. -
Facilitate exposure of the disc space,
especially near the proximal vertebrae, by removing the rib head
covering the disc space. Insert the vertebral body staples in
approximately the same anatomic position in each vertebral body.
Position these as far posteriorly as possible to prevent penetration of
the spinal canal with the staple prongs. -
In the most proximal vertebrae, insert
the most proximal staple so that the screw can be placed eccentrically
into the vertebral body. Insert the screw from posterior
P.4022
to
anterior, as with the other screws. This superior eccentric placement
adds strength by putting the screw threads against the superior
endplate of the vertebral body. Place an identical staple in the most
inferior vertebral body so that the screw is in the inferior aspect of
that vertebral body (Fig. 155.14).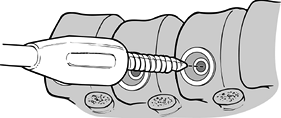 Figure 155.14.
Figure 155.14.
Proximal screw placed in inferior aspect of vertebral body. (Redrawn
from Moss Miami 4.0 mm Anterior System Surgical Technique: Anterior
Treatment of Thoracic Idiopathic Scoliosis. DePuy Motech, Warsaw,
Indiana, 1997.) -
Use an awl to make a hole for the
insertion of the screws. When the screws are positioned, it is
important that they penetrate the concave side of the vertebral body. -
Perform bone grafting before rod
insertion. Begin distally, and wedge the disc space open and pack the
interspaces. It is important to pack the discs between T10 and L2
firmly with bone graft. A structural type of anterior support may be
needed to help ensure maintenance of the sagittal correction. When
grafting the apical vertebrae, consider the original sagittal contour.
If the starting sagittal profile is lordotic or hypokyphotic, only a
small amount of graft can be inserted into the disc at the apex of the
curve, to allow kyphosis to occur during compression with
instrumentation. -
Measure the appropriate length of the rod from the proximal to the distal screw. Add ¼ inch (i.e., 1/8
inch for each end), and cut the rod. Bend the rod to parallel the
anatomically correct kyphosis–lordosis of the area of the spine needing
instrumentation. Insert the rod so that the kyphosis is used to
accommodate the scoliosis that is still present. It is important to
remember to straighten out the table if it has been bent to facilitate
the thoracotomy and disc exposure. When applying the nuts, it is easier
to start at the top three nuts and then work toward the distal end. -
After inserting the inner setscrews and
outer nuts, the rods should be loose. The spine can be corrected by
rotating the rod into a normal sagittal plane (Fig. 155.15).
Correction can be facilitated by the anesthesiologist pulling on the
lower arm and by the surgeon pressing on the apex of the curvature.
With the rod rotated into an anatomically correct position, tighten
both the inner and outer apical nuts. At this point, a decision has to
be made about the anticipated coronal correction that is needed. If
there is a structural upper thoracic curve, only a modest correction
can be obtained in the main thoracic curve equal to the correction seen
on the bending films of the upper thoracic curve. If there is no
residual structural curve, then near-complete reduction of the curve
can be obtained.![]() Figure 155.15.
Figure 155.15.
Rotating the rod into a normal sagittal plane. (Redrawn from Moss Miami
4.0 mm Anterior System Surgical Technique: Anterior Treatment of
Thoracic Idiopathic Scoliosis. DePuy Motech, Warsaw, Indiana, 1997.) -
Obtain correction by using a compressor
on each screw. Gradually compress each screw toward the apex. This
needs to be done slowly and sequentially. After it has been completed,
perform the final tightening in both the inner setscrews and the outer
nuts. It is important to be cautious during this final tightening,
especially in the end vertebrae. Torquing the screw could translate the
screw out of the vertebral body. It is recommended that you use the rod
stabilizer device that goes over the
P.4023
rod and provides neutralizing forces while tightening the inner setscrews and outer nuts (Fig. 155.16).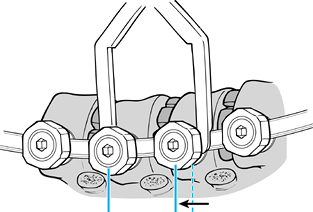 Figure 155.16.
Figure 155.16.
Screws are gradually compressed toward the apex. (Redrawn from Moss
Miami 4.0 mm Anterior System Surgical Technique: Anterior Treatment of
Thoracic Idiopathic Scoliosis. DePuy Motech, Warsaw, Indiana, 1997.)
obtained with this instrumentation. Preoperative side-bending films of
a thoracic curve show excellent flexibility. The postoperative
anteroposterior (AP) and lateral films show near-complete correction.
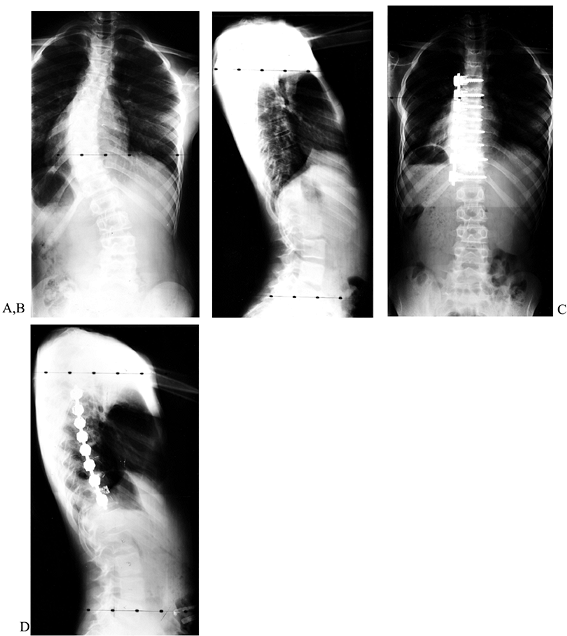 |
|
Figure 155.17. The preoperative AP (A) and lateral (B) views of a child with a thoracic scoliosis. The postoperative films (C,D)
show near complete correction of the coronal and sagittal deformities. The cage is used to prevent kyphosis at the thoracolumbar junction. |
-
Expose the anterior spine as previously described.
-
Place the end screws first from posterior
to anterior, horizontal to the frontal plane of the vertebral body and
paralleling the apex. Start the hole with an awl and continue with the
5.5 mm tap. Tap the vertebra until
P.4024
the tip just exits the far side of the cortex (Fig. 155.18).
Insert a ballpoint probe to make sure that the far side of the cortex
has been exited. Tap the first third of the hole with a 7 mm tap and
insert a 7 mm closed-top screw with washer (Fig. 155.19).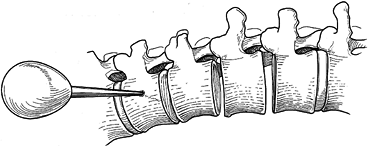 Figure 155.18.
Figure 155.18.
ollowing a thorough 360° discectomy in which the posterior longitudinal
ligament is exposed, placement of the upper vertebral screw site is
begun with an awl, which is positioned at the furthest lateral waist of
the vertebral body. (Redrawn from Asher MA. Surgical
Technique for Anterior Segmental Instrumentation of Thoracolumbar and
Lumbar Scoliosis Using the Anterior Isola Spinal System. AcroMed Corp., Cleveland, OH, 1996.)![]() Figure 155.19. Insertion of a 7 mm closed-top screw with washer. A staple may be used if desired. (Redrawn from Asher MA. Surgical
Figure 155.19. Insertion of a 7 mm closed-top screw with washer. A staple may be used if desired. (Redrawn from Asher MA. Surgical
Technique for Anterior Segmental Instrumentation of Thoracolumbar and
Lumbar Scoliosis Using the Anterior Isola Spinal System. AcroMed Corp., Cleveland, OH, 1996.) -
Use a staple, and place it prior to the
insertion of the screw. Insert the screw to maximum torque. The screw
should protrude through the far cortex by at least one to two threads. -
Repeat the same process at the lower-end
vertebra. After the superior and inferior vertebrae have been
instrumented with the staple and screws, cut the rod and contour it so
that it fits through both the superior and inferior screws (Fig. 155.20).
Add about 1 cm to each side of the rod to ensure that the rod passes
completely through the end screws. After the rod has been cut to length
and contoured, it is used as a guide for placement of the intermediate
screws.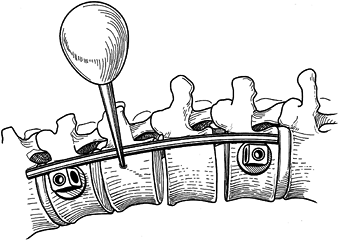 Figure 155.20.
Figure 155.20.
Rod is cut and contoured. Intermediate screws should be placed very
slightly posterior on the waist of the body. (Redrawn from Asher MA. Surgical
Technique for Anterior Segmental Instrumentation of Thoracolumbar and
Lumbar Scoliosis Using the Anterior Isola Spinal System. AcroMed Corp., Cleveland, OH, 1996.) -
Insert open-ended screws and staples at
the intervening levels. Insert the rod into the superior and inferior
screws and drop into the intermediate screws (Fig. 155.21).
Place caps on the intermediate screws. After placing the caps, rotate
the rod approximately 180° to obtain both a coronal and a sagittal
correction (Fig. 155.22).![]() Figure 155.21.
Figure 155.21.
The middle screws are 7.0 mm in diameter and should protrude through
the far cortex of the body by one to three turns. (Redrawn from Asher
MA. Surgical Technique for Anterior Segmental
Instrumentation of Thoracolumbar and Lumbar Scoliosis Using the
Anterior Isola Spinal System. AcroMed Corp., Cleveland, OH, 1996.)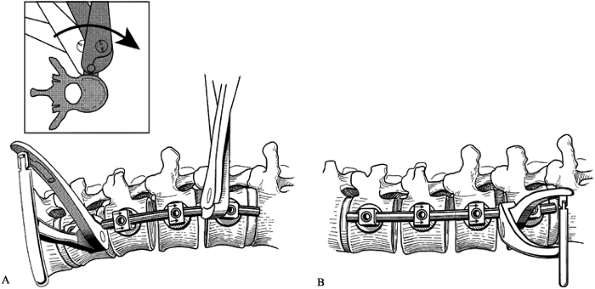 Figure 155.22. A: The rod is rotated to place the sagittal plane contour of the rod (B) in the true sagittal plane. (Redrawn from Asher MA. Surgical
Figure 155.22. A: The rod is rotated to place the sagittal plane contour of the rod (B) in the true sagittal plane. (Redrawn from Asher MA. Surgical
Technique for Anterior Segmental Instrumentation of Thoracolumbar and
Lumbar Scoliosis Using the Anterior Isola Spinal System. AcroMed Corp., Cleveland, OH, 1996.) -
Secure the rod by tightening one of the
intermediate setscrews. After the rod has been rotated, a Cobb elevator
can be placed into the vertebral space to pry open the previously
concave side of the curve (Fig. 155.23). At
this point, some distraction may actually be applied between the screw
connector bodies to further open the space. Completely fill the disc
spaces with bone graft.![]() Figure 155.23. Disc space is restored utilizing a Cobb elevator. (Redrawn from Asher MA. Surgical
Figure 155.23. Disc space is restored utilizing a Cobb elevator. (Redrawn from Asher MA. Surgical
Technique for Anterior Segmental Instrumentation of Thoracolumbar and
Lumbar Scoliosis Using the Anterior Isola Spinal System. AcroMed Corp., Cleveland, OH, 1996.) -
Compress the vertebral screws in order to
compress the disc spaces. This is done sequentially, starting from the
top to the second screw (Fig. 155.24). The second
P.4025P.4026P.4027
compression occurs between the bottom and the next upper screw. The final compression is applied across the apical vertebra.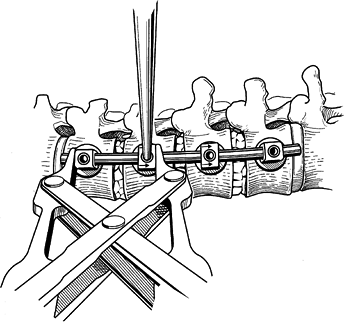 Figure 155.24. Disc spaces are compressed to provide anterior column load sharing. (Redrawn from Asher MA. Surgical
Figure 155.24. Disc spaces are compressed to provide anterior column load sharing. (Redrawn from Asher MA. Surgical
Technique for Anterior Segmental Instrumentation of Thoracolumbar and
Lumbar Scoliosis Using the Anterior Isola Spinal System. AcroMed Corp., Cleveland, OH, 1996.) -
Close the wound over a chest tube as previously described. A sample case is illustrated in Fig. 155.25.
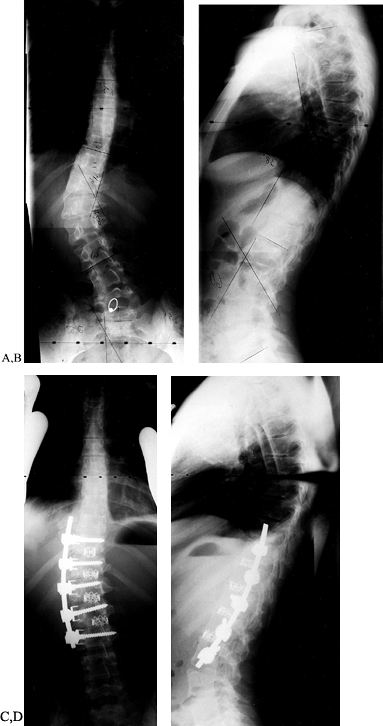
![]() Figure 155.25. This 14-year-old girl has left thoracolumbar major and right thoracic compensatory scoliosis. Standing frontal plane (A) and sagittal plane (B)
Figure 155.25. This 14-year-old girl has left thoracolumbar major and right thoracic compensatory scoliosis. Standing frontal plane (A) and sagittal plane (B)
radiographs show imbalance to the left in the coronal plane, and in the
sagittal plane flattening of the upper lumbar spine. On the left bend
x-ray, there is not a clear opening of the disc space above the lower
Cobb (C). On the right bend (D), the upper scoliosis corrects from 44° to 15°. Two-year postoperative radiographs (E,F)show
restoration of coronal balance and a well-healed fusion with normal
sagittal plane alignment. The standing postoperative posteroanterior
radiograph (E) shows instrumentation from
T12 to L3, the upper end vertebra level having been left out to allow
for compensation. The sagittal plane angulation has been restored (F).
postoperatively. Carefully monitor hemodynamic parameters such as
hemoglobin and hematocrit, platelets, blood coagulation, electrolytes
including calcium and magnesium, and urinary output. Record chest tube
and drain outputs. Obtain daily chest radiographs until the chest tube
is discontinued, usually by the third postoperative day. The goal of
respiratory care is to prevent atelectasis and wean the patient from
the ventilator safely and as soon as possible.
patient may ambulate when the chest tube is removed. Apply a
custom-molded underarm thoracolumbar spine orthosis (TLSO) to all
patients; this is worn full time for 6 months. Strongly stress walking
to improve aerobic conditioning and an overall sense of well-being.
Formal physical therapy is not required except for gait training.
instrumentation systems, and vertebral fracture can occur. Apply
corrective force slowly over time to allow the viscoelastic
characteristics of the spine to work. Patients with osteoporosis
require even more care while tensioning instrumentation. Advanced
osteoporosis is a contraindication to anterior instrumentation.
Bicortical fixation significantly improves fixation strength and should
be attempted at each level. Many systems utilize screw–staple or
screw–washer combinations that maximize stability. When pullout does
occur, it is usually at the proximal or distal end of the instrumented
levels. Augmentation with methylmethacrylate can be utilized, or the
level may need to be bypassed.
major when they significantly alter the expected course of recovery, or
minor when there is no delay in expected recovery. McDonnell et al. (29)
reviewed complications of anterior spine procedures. They noted that
pulmonary (37%) and thoracostomy tube (14%) complications were the most
common of the major complications. Genitourinary complications were
present in 11.6% of all patients and made up 42% of the minor
complications.
complications. The neuromuscular, adult, and congenital scoliosis
groups had overall complication rates of 52%, 41%, and 36%,
respectively (29). Patient age is also a significant factor contributing to perioperative morbidity. McDonnell et al. (29) found that the complications increased with patient age and were highest in the 61- to 85-year age group.
hypothermia can produce a severe coagulopathy. Careful monitoring of
only the hemoglobin during surgery is not adequate. The prothrombin and
partial thromboplastin times and platelet counts must also be monitored
in these procedures. The administration of fresh frozen plasma and
platelets may at times be required. Arrangements with the hematology
lab to provide a quick turnaround on coagulation profiles can be
critically important.
ligation of multiple segmental vessels is rare. Take care to avoid the
foraminal region where the vascular anastomosis of the cord lies. The
aorta and vena cava need to be carefully protected. Reported rates of
injury in 447 cases of anterior spine procedures are low, with
McDonnell et al. (29) reporting no great vessel injuries in 447 cases.
deformity. Animal studies have confirmed that spinal cord injury can
result from distraction or compression of the spine. While mechanical
disruption does not typically occur, infarcts or hemorrhages can result
from aggressive deformity correction. Bridwell et al. (2)
reported their incidence of major neurologic deficits after adult and
pediatric deformity surgery: Four cases were identified in 1,153
patients. All these cases were similar in that anterior and posterior
procedures were performed on the same day for large deformity with the
ligation of segmental vessels on one side, and intraoperative or
perioperative hypotension placing the mean arterial pressure below 50
mm Hg for at least 15 minutes.
misdirected vertebral body screws. Taking care to place the screws
parallel to the posterior longitudinal ligament can avoid canal
penetration. If misdirection occurs, revise the screw or bypass the
level.
anemia, and prolonged operative time. They noted that “the lowest
intraoperative blood pressure averaged 77 mm Hg, with an average of 260
minutes spent below 75% of the baseline preoperative pressure.” The
cause of blindness was ischemic optic neuropathy in 19 and retinal
artery occlusion in seven. Improvement was obtained in only five
patients who had partial loss of vision initially. Mayfield tongs may
be of benefit in reducing facial pressure when the patient is placed
prone for posterior surgery.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
KH, Lenke LC, Baldus C, Blanke K. Major Intraoperative Neurologic
Deficits in Pediatric and Adult Spinal Deformity Patients—Incidence and
Etiology at One Institution. Proceedings of the Scoliosis Research Society, 1996.
RL, Hansen MM, Nicholas DA, Allen BL. Same-Day vs. Staged
Anterior-Posterior Spinal Surgery in a Neuromuscular Scoliosis
Population: The Evaluation of Medical Complications. J Pediatr Orthop 1996;16:293.
JP, Maurais GR, Richardson WJ, et al. Combined Single Stage Anterior
and Posterior Osteotomy for Correction of Iatrogenic Lumbar Kyphosis. Spine 1988;13:257.
CS, Nachemson AL. The Crankshaft Phenomenon after Posterior Harrington
Fusion in Skeletally Immature Patients with Thoracic or Thoracolumbar
Idiopathic Scoliosis Followed to Maturity. Spine 1997;22:58.
KD, Leong JC, Reyes FL, Hsu LC. The Comparative Results of Treatment in
Idiopathic Thoracolumbar and Lumbar Scoliosis Using the Harrington,
Dwyer and Zielke Instrumentation. Spine 1989;14:275.
BR, Tolo VT, McAfee PC, Burest P. Nutritional Deficiencies after Staged
Anterior and Posterior Spinal Reconstructive Surgery. Clin Orthop 1988;234:5.
MM, Kroon D, Tredwell SJ, Wadsworth LD. The Role of Autologous Blood
Transfusion in Adolescents Undergoing Spinal Surgery. Spine 1995;20:532.
ET, Krengel WF, King HA, Lagrone MO. Comparison of Same-Day Sequential
Anterior and Posterior Spinal Fusion with Delayed Two-Stage Anterior
and Posterior Spinal Fusion. Spine 1994;19:1256.
TA, Dickson JH, Erwin WE. Efficacy and Cost Considerations of
Intraoperative Autologous Transfusion in Spinal Fusion for Idiopathic
Scoliosis with Predeposited Blood. Spine 1996;21:848.
MB, Georgopoulos G, Eilert RE. Intraoperative Blood Salvage in Children
and Young Adults Undergoing Spinal Surgery with Predeposited Autologous
Blood: Efficacy and Cost Effectiveness. J Pediatr Orthop 1993;13:777.
AG, Marks DS, Sayampanathan S, Piggott H. Long-Term Results of Combined
Anterior and Posterior Convex Epiphsiodesis for Congenital Scoliosis
Due to Hemivertebrae. Spine 1995;20:1380.
RB, Lonstein JE, Heithoff KB, Kirkham JA. Magnetic Resonance Imaging
Evaluation of the Adolescent Patient with Idiopathic Scoliosis before
Spinal Instrumentation and Fusion. Spine 1997;22:855.