THE DISTAL RADIOULNAR JOINT
of Hartford Orthopaedic, Plastic & Hand Surgeons, Inc., Hartford,
Connecticut 06106, was a technical contributor to this chapter.
important part of the wrist joint, providing several key functions to
the articulation between the forearm and hand. Most important, the DRUJ
acts in concert with the proximal radioulnar joint to allow the wrist
and hand to rotate from pronation to supination and back again. The
radioulnar joint appeared in evolution 36 million years ago (7),
and the unique ability to transfer rotational force to the grasping
hand is considered to have played a decisive role in human evolution. A
forearm that can rotate while the hand grasps is a necessity in using
spears, axes, and knives and in carrying infants and baskets,
activities that allowed hominids to evolve from food gatherers to food
producers.
radioulnar joint can be difficult, and their management challenging.
Although a wide variety of surgical procedures have been described to
address DRUJ pathology, the best approach to these disorders remains
controversial. This
chapter
summarizes current knowledge about the distal radioulnar joint and
suggests treatment strategies for specific conditions. Before one
considers the pathologic conditions that affect the DRUJ and options
for their treatment, however, it is necessary to understand the anatomy
and biomechanics of the joint (7,58).
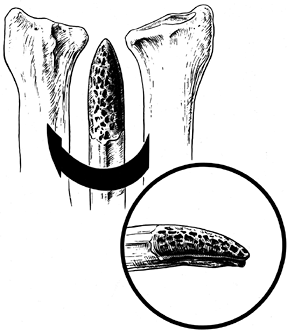
The sigmoid notch of the distal radius is concave and hemicylindrical
with three distinct margins: a sharp dorsal margin, a smoother palmar
margin, and a distal margin, which is the junction between the lunate
facet and the sigmoid notch (Fig. 43.1). The
opposed ulnar head is also hemicylindrical but has a smaller radius of
curvature than the sigmoid notch. Because of the mismatch in radii,
there is some inherent laxity in the normal joint, and translatory
motion parallel to the plane of the articular surfaces normally occurs
during rotation of the hand. When the forearm is in neutral rotation,
it is possible to translate the ulnar head passively approximately 3 mm
dorsal and 5 mm palmar against the stabilized radius (79).
In the extremes of forearm rotation, less than 10% of the ulnar head
may be in contact with the notch. This relationship is important to
consider when the normal shape and relative lengths of the radius and
ulna are changed, either surgically, developmentally, or traumatically.
 |
|
Figure 43.1.
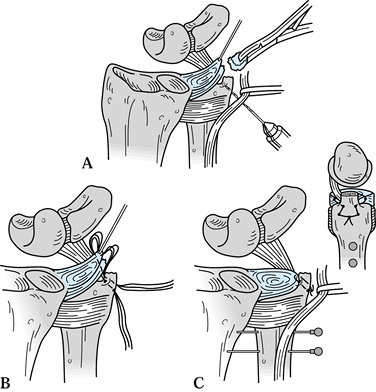
Bony anatomy of the distal radius showing the confluence of the lunate facet and the sigmoid notch. Redrawn from an illustration by Elizabeth Roselius © 1993, with permission. |
bony anatomy of the DRUJ. It is a continuation of the subcutaneous
ridge of the ulnar shaft and stands as a strut on the end of the ulna
to stabilize the ulnar soft tissues of the wrist. The sheath of the
extensor carpi ulnaris, the ulnocarpal ligaments, and the triangular
fibrocartilage all attach to the distal ulna and help maintain the
congruency of the DRUJ; most of these attachments are at the base of
the ulnar styloid (44,45,59,76,77,100).
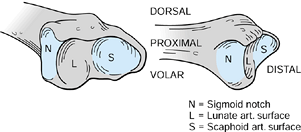
DRUJ have been the focus of many studies. One of the most important
structures is the triangular fibrocartilage complex (TFCC), a term
coined by Palmer and Werner (Fig. 43.2) (77).
It arises from the ulnar aspect of the lunate fossa of the radius and
courses ulnarward to insert into the base of the ulnar styloid. It also
flows distally, where it is joined by fibers arising from the ulnar
aspect of the ulnar styloid and inserts distally into the triquetrum,
hamate, and base of the fifth metacarpal. The prestyloid recess is a
constant perforation found just distal to the level of the ulnar
styloid and should not be confused with a traumatic disruption.
 |
|
Figure 43.2.
Illustration of the triangular fibrocartilage and ulnotriquetral and ulnolunate ligaments, components of the triangular fibrocartilage complex (TFCC). S = scaphoid, L = lunate, T = triquetrum, P = pisiform. Redrawn from an illustration by Elizabeth Roselius © 1993, with permission. |
fibrocartilage (TFC) proper. The periphery of the TFC is thickest,
usually measuring 5 mm, and is the portion best suited to bear tensile
loads. The rim is well vascularized and therefore has good healing
potential (62). Tearing of the TFC along its
peripheral margin allows DRUJ displacement and instability. In
contrast, the central portion of the TFC is thin (usually only 1–2 mm
thick) and has a disorganized, random collagen arrangement and little
vascularity. This portion is better suited for force transmission and
compressive loading.
congenital perforations of the TFC. That has implications for treatment
because it would be important to know if a lesion discovered during
evaluation of a patient’s wrist were congenital or traumatic. Weigl and
Spira (108) examined the TFC of 84 cadaver
wrists with ages ranging from 1 day to 91 years and found that a
congenital fenestration was sometimes present on the border between the
radial and middle third of the disc. The frequency of the congenital
lesions was not listed, but the authors found
that 42% of specimens of all ages had fenestrations, usually without degenerative changes. Tan et al. (99)
studied 120 fetal and infant wrists and found perforations in 27 (23%).
Mikic found no perforations in untraumatized wrists younger than 20
years of age, including 38 fetal hands. Palmer and Werner found that
53% of their specimens had TFCC perforations, but all had associated
erosions of the cartilage of the lunate and distal ulna. In most cases,
the history and physical exam help clarify the etiology of the lesion.
considered part of the TFCC. They share a common origin from the region
of the ulnar styloid base and fan out past the triangular
fibrocartilage to insert on the triquetrum and lunate, respectively.
The ligaments are important stabilizers of the ulnar corner of the
wrist and resist palmar–ulnar displacement of the carpus, particularly
in power grip. In rheumatoid arthritis the ligaments may become
attenuated and allow such displacement (109), a
problem that must be considered in planning treatment of DRUJ problems
in patients with rheumatoid arthritis, as discussed below.
capsule and the dorsal and palmar radioulnar ligaments. The DRUJ
capsule is uniformly thin and contributes little in the way of
stability. The characteristics and function of the dorsal and palmar
radioulnar ligaments have been the subject of debate. Some authors (17,78,108) consider them nothing more than thickenings of the periphery of the TFCC. Other investigators (53)
have studied them in cadaver models and have tried to assign specific
biomechanical roles to them. The roles the ligaments may play in DRUJ
biomechanics are discussed in the next session.
pronator quadratus and the extensor carpi ulnaris. The pronator
quadratus has a superficial head, which is a prime mover for forearm
pronation, and a deep head, which helps stabilize the DRUJ (92).
The pronator quadratus actively stabilizes the joint by coapting the
ulnar head in the sigmoid notch, particularly in pronation, and
passively stabilizes the joint by viscoelastic forces in supination (17,50,78).
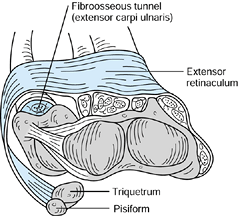
demonstrated how the ECU is maintained in its position over the dorsal
distal ulna by a separate fibroosseous tunnel deep to and separate from
the extensor retinaculum (Fig. 43.3). The
extensor retinaculum swings around and over the medial border of the
ulna to insert primarily on the pisiform and triquetrum. This separate
arrangement allows unrestricted rotation of the radius and ulna.
Studies based on cadaver dissections have shown that an intact ECU and
fibroosseous tunnel partially stabilize the DRUJ even after the TFC and
other ligaments are sectioned (91).
 |
|
Figure 43.3.
The extensor carpi ulnaris lies in a fibroosseous tunnel separate from the extensor retinaculum; it is more superficial and passes around the medial border of the ulna to attach to the pisiform and triquetrum. Redrawn from an illustration by Elizabeth Roselius © 1993, with permission. |
the forearm around which the radius moves. If there is a dislocation,
it is technically the radius that is displaced dorsally or palmarly
with respect to the ulna. In the orthopaedic literature and common
usage, however, it is the convention to describe the position of the
distal ulna with respect to the radius in describing DRUJ dislocations
and instability. The conventional nomenclature is used in this chapter.
translational components of motion and does not have a single center of
rotation. Many laboratory studies have been done to study the forces
and structures that normally stabilize the joint as well as pathologic
changes that occur after DRUJ injuries. In a recent study, Kihara et
al. (53) used a cadaver model to assess the
roles four structures play in stabilizing the DRUJ in different
positions of forearm rotation: (a) the dorsal radioulnar ligaments; (b)
the palmar radioulnar ligaments of the TFCC; (c) the pronator quadratus
and the distal portion of the interosseous membrane underlying the
muscle; and (d) the entire interosseous membrane. The authors showed
that all four structures contribute to the stability of the DRUJ.
Dislocation, and often diastasis, could occur only if all four
structures were divided. It was not possible to define the roles of the
dorsal radioulnar ligaments or palmar radioulnar ligaments while the
interosseous membrane was intact.
effects of the dorsal and palmar radioulnar ligaments are dependent on
forearm rotation. Schuind et al. (88) and others (32,108) have demonstrated that the palmar radioulnar
ligaments (RUL) become maximally tightened and thus stabilize the DRUJ
in forearm supination, whereas the dorsal RUL becomes maximally
tightened and stabilizes the DRUJ in forearm pronation (Fig. 43.4). Paradoxically, af Eckenstam and Hagert (5)
concluded the opposite: sectioning studies on five cadavers showed that
stability in supination was maintained by the dorsal fibers, and
stability in pronation was maintained by the palmar fibers. A study by
Adams and Holley (2) measured strain on the
surface of the TFC articular disc and calculated the strain at the
dorsal and palmar margins of the disc. In supination, strain increased
dorsally; in pronation, strain increased palmarly. This apparent
paradox can be rectified if one realizes that, in pronation, the dorsal
radioulnar ligament tightens and tends to displace the ulna dorsally (Fig. 43.5).
Left unchecked, this dynamic tensioning ultimately would lead to
subluxation and dislocation of the joint. It is the palmar radioulnar
ligaments that check that force and keep the joint reduced. If the
interosseous membrane is disrupted and the palmar radioulnar ligament
is sectioned, the DRUJ dislocates in pronation. The opposite is true in
supination.
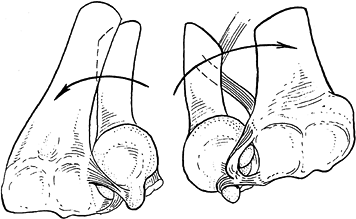 |
|
Figure 43.4.
When the wrist is pronated, as on the left, the dorsal radioulnar ligament is taut, seating the ulnar head against the dorsal rim of the sigmoid notch. In supination, on the right, the palmar radioulnar ligament displaces the ulnar head against the palmar radial rim. Reprinted with permission (59). |
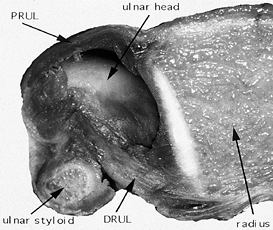 |
|
Figure 43.5.
Cross section through the DRUJ in a cadaver specimen. The central portion of the triangular fibrocartilage has been removed to expose the ulnar head. When the forearm is pronated, as shown here, the dorsal radioulnar ligament (DRUL) is tighter than the palmar radioulnar ligament (PRUL). |
distal radius change the biomechanics of the TFC complex. In another
study by Kihara et al. (52), dorsally angulated
fractures of the distal radius were simulated in a cadaver model. The
authors found that incongruency of the DRUJ occurred with increasing
dorsal tilt of the distal radius, most pronounced with a change of more
than 20° from the initial anatomic alignment. Also, with dorsal
angulation, the intact interosseous membrane tightened and thus limited
forearm rotation. The authors confirmed that DRUJ dislocation occurred
only if both the TFCC and the interosseous membrane were sectioned.
components of the DRUJ also have been characterized. Compression and
axial loading across the wrist are primarily transmitted to the distal
radius, but the force is partially transmitted through the TFC to the
ulnar head. Palmer et al. (78) showed that in a
forearm with ulnar neutral variance, 80% of the static load is borne by
the radius and 20% by the ulna. As ulnar length increases from -2.5 mm
to + 2.5 mm, the load borne by the ulna increases from 4% to 42%.
Removal of the TFCC decreased the load borne by the distal ulna by
approximately 12%.
regional structural differences within the triangular fibrocartilage
may explain the quality and location of the TFCC tears seen clinically.
Chidgey et al. (24,25)
found that the central disc of the TFC has undulating sheets of
crisscrossed, poorly vascularized collagen fibers and that the origin
of this disc from the radius is reinforced by collagen bundles
projecting out from the radius for 1 to 2 mm. This corresponded with
the clinical observation that central disc tears identified
arthroscopically in 48 patients usually were oriented parallel to and 1
to 2 mm away from the radial origin of the TFCC at the junction between
the short, radially oriented fibers and the remainder of the disc. It
is postulated that high shear forces occur in this transition zone and
account for the tear patterns seen. Tears were notably absent from the
ulnar side of the central disc.
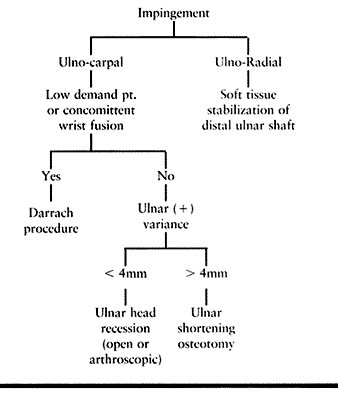
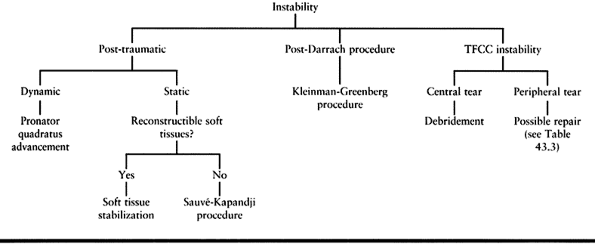
but most conditions affecting the DRUJ fall into one of three categories: impingement, instability, or incongruity (Table 43.1).
 |
|
Table 43.1. Classification of Distal Radioulnar Joint Disorders
|
wrist pain made worse with ulnar wrist deviation. In ulnar impaction
syndrome, ulnar deviation causes the lunate and triquetrum to abut
against the ulnar head and TFCC. Radiographs show a long ulna relative
to the radius and occasionally sclerotic or cystic changes in the ulnar
head, lunate, or triquetrum. Tears of the lunotriquetral ligament and
TFCC are common in this condition. Ulnar impingement syndrome is a
special variant of impingement disorder. In that case, the distal ulnar
shaft contacts the distal radius abnormally (usually the result of
prior surgery) to cause pain, made worse with medial-lateral
compression of the distal forearm and ulnar wrist deviation. A number
of conditions may predispose a patient to an impingement syndrome (Table 43.1).
relate to bony changes or soft tissue injuries or both. Fractures of
the distal radius or distal ulna can alter the bony alignment of the
DRUJ (52). Nonunion or malunion of these fractures may result in pain, weakness, and lost motion in addition to instability.
recently because of the importance of TFCC stability. Ulnar styloid
fractures commonly occur together with fractures of the distal radius
and are often overlooked while emphasis is placed on treating the
radius fracture. Ulnar styloid fractures can be troublesome and
painful, however, and can be a mark of TFCC instability. Symptomatic
nonunions of the styloid do occur. Hauck et al. (44)
recently classified these as type 1 with a stable DRUJ and type 2 with
an unstable DRUJ. Type 1 fractures occur through the tip of the styloid
and do not cause TFCC instability in and of themselves. Should
nonunions of this type occur, they can be treated successfully by
excision of the styloid fragment. Type 2 nonunions occur through the
base of the styloid, are much larger fragments, and imply TFCC
instability if displaced.
in association with instability of the DRUJ. The most common tear
occurs within the articular disc of the TFC near its attachment to the
radius. This tear does not seem to cause joint instability; however,
the tear itself may be unstable and symptomatic (1,3,25,73,83). Despite the recognition of specific types of TFC lesions (Table 43.2) (74),
the exact mechanisms of injury remain unclear. Adams et al. postulated
that TFCC tears result from a distraction force during a violent axial
load of the forearm; however, their cadaver study did not demonstrate
the types of TFCC tears often seen clinically (3).
Tears probably result from a combination of compression across the
wrist, which traps the disc in the ulnocarpal joint, together with DRUJ
distraction or twisting, which creates enough shear force to tear the
disc. The regional differences in collagen arrangement also may play a
role.
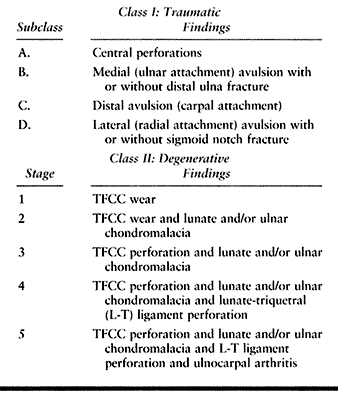 |
|
Table 43.2. Palmer’s Classification of TFCC Lesions
|
conditions. Osteoarthritis is usually posttraumatic but is occasionally
primary. In either case, the DRUJ becomes painful, and there is
progressive loss of motion of the wrist and forearm and decreased grip
strength (65). Rheumatoid arthritis also commonly affects the DRUJ (8,109).
Incongruity may result from trauma such as radius fractures that extend
into the DRUJ. Whatever the cause, when the distal ulna and sigmoid
notch of the radius have incongruous surfaces, the result may be
similar; symptoms include pain, swelling, lost motion, and catching or
clicking at the DRUJ with forearm rotation.
functions to the articulation between the forearm and hand–wrist unit.
The goal in treating any derangement of the DRUJ is to restore a
pain-free, stable junction between the forearm and the rest of the
wrist. That junction should allow forearm rotation, which occurs as the
radius rotates around the distal ulna, and should restore or maintain
as much stability as possible to the ulnar side of the wrist. Treatment
options chosen for individual patients should be based on an
understanding of the underlying pathology and specific pathoanatomic
disorders (Table 43.3, Table 43.4 and Table 43.5).
Three critical questions should be addressed and answered before any
treatment is selected: (a) What is the duration of the disorder (acute
or chronic)? (b) What is the principal cause of the disorder:
ligamentous, cartilaginous, bony, or a combination thereof? And (c)
what is the condition of the DRUJ articular surfaces: normal,
incongruous, or arthritic?
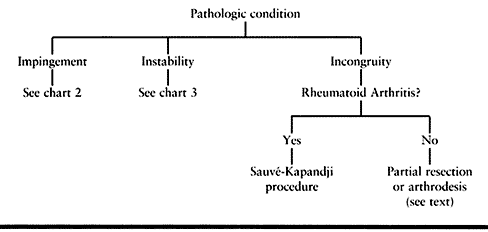 |
|
Table 43.3. DRUJ Pathology General Treatment Algorithm
|
 |
|
Table 43.4. Impingement Lesions Treatment Algorithm
|
 |
|
Table 43.5. Instability Lesions Treatment Algorithm
|
affecting the DRUJ with a detailed, accurate history. Knowing the
mechanism of injury or symptom production can be helpful. For example,
pain with ulnar wrist deviation that began insidiously or with chronic
overuse suggests an impingement problem. Patients with traumatic
lesions of the TFCC generally give a history of falling on the
outstretched hand, which causes hyperextension of the wrist, or a
history of the onset of ulnar-sided wrist pain after lifting a heavy
object (80). A torque injury with axial load
can also cause DRUJ instability. Specific activities that load the
wrist during pronation and supination such as using a screwdriver or
using a key may exacerbate the pain from a TFCC lesion. Patients often
describe a sensation of catching or snapping in the wrist when there
are problems in the DRUJ.
wrist and forearm ranges of motion, both active and passive. A rigid
endpoint with loss of motion suggests a bony problem such as fracture
malunion, whereas a soft endpoint with limited motion implies scarring
or soft tissue contractures. Look for subluxation of the ECU by
watching the tendon while the patient actively supinates and pronates
the forearm. Provocative maneuvers and applied stresses can be helpful.
Pain with ulnar wrist deviation suggests an ulnar impaction syndrome. A
TFCC tear should be suspected if the patient’s pain is reproduced when
torque is applied to the hand while axial load is applied across the
wrist and the forearm is stabilized. Inflammatory changes of the DRUJ
usually are associated with generalized conditions such as rheumatoid
arthritis (18). Note the presence of any obvious deformity and unilateral grip weakness.
is a history of a specific traumatic event, include two views that show
both the elbow and wrist because a dislocation of either the proximal
or distal radioulnar joints may accompany any forearm fracture (Fig. 43.6).
In the multiply traumatized patient, this is especially critical
because other injuries may mask physical findings that would indicate
injuries of the proximal or distal forearm joints. Standard
posteroanterior (PA) and lateral views of the wrist can be used to rule
out bony problems separate from the DRUJ; however, obtain ulnar
variance view radiographs before any procedure on the DRUJ. The ulnar
variance lateral view (Fig. 43.7A) is taken
with the arm at the side (0° shoulder abduction), the elbow flexed 90°,
the forearm in neutral rotation, and the wrist in neutral alignment (36). The ulnar variance PA view (Fig. 43.7B) is obtained with
the patient’s shoulder abducted 90°, the elbow flexed 90°, and the
forearm in neutral rotation. For this view, there is disagreement about
whether the wrist should be in ulnar deviation (36)
to show the lunate centered over the lunate fossa of the radius or
neutral deviation to show the lunate centered over the DRUJ (75).
However, it should be evident that wrist position will not affect the
relative lengths of the ulna and radius, and most patients are more
relaxed with their wrist in neutral rather than extreme ulnar
deviation. I prefer that patients keep their wrists in neutral for this
x-ray.
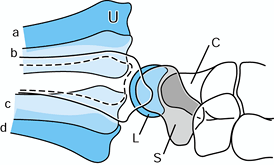 |
|
Figure 43.6. Illustration of lateral radiographs of the wrist in neutral rotation with dorsal dislocation (a), dorsal subluxation (b), palmar subluxation (c), and palmar dislocation (d) of the ulna (U) on the radiocarpal mass. Complete superimposition of the proximal pole of the scaphoid (S) and the lunate (L) is seen. The radial styloid process is centered over the proximal part of the carpus. C, capitate. Redrawn from an illustration by Elizabeth Roselius © 1993, with permission.
|
 |
|
Figure 43.7. Forearm positioning for ulnar variance view x-rays: lateral view (A) and PA view (B).
|
evaluating the DRUJ, particularly when there is a deformity related to
the joint with associated pain and lost motion (22,26,54,66,67,90,91,108). Take three sets of scans, one each with the forearm in pronation, supination, and neutral rotation (Fig. 43.8),
and compare to the uninjured wrist to assess joint instability
accurately. The scan with the forearm in pronation is most sensitive
for detecting palmar subluxation, whereas the neutral image is best for
detecting dorsal subluxation and DRUJ diastasis. The supination view is
best for confirming the degree of reduction or subluxation of the DRUJ (20).
On any single CT image, one can gain clues about the position of the
ulna relative to the radius by drawing a line through the dorsal radial
and ulnar borders of the radius and a second line through the palmar
radial and ulnar corners of the radius. An ulna that is adequately
reduced must lie between those lines (66,67).
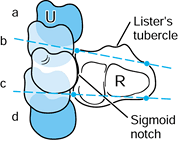 |
|
Figure 43.8.
Illustration of CT scans of a transverse section through the distal radioulnar joint. The sigmoid notch and Lister’s tubercle of the radius are seen. The positions of the ulna (U) with (a) dorsal dislocation, (b) dorsal subluxation, (c) palmar subluxation, and (d) palmar dislocation are demonstrated. A line drawn through the dorsal ulnar and radial borders of the radius (R) shows the limit of dorsal congruity of the DRUJ, and a line drawn through the palmar ulnar and radial borders of the radius shows the limit of palmar congruity. Redrawn from an illustration by Elizabeth Roselius © 1993, with permission. |
useful as better imaging coils and software are being developed and as
investigators improve their abilities to interpret the images and
correlate them with specific abnormalities (18,101).
Arthrography remains a useful tool for evaluating the DRUJ, especially
TFCC tears, and dynamic images of the arthrogram are most helpful. The
images can be stored on videotape and studied in detail to detect
subtle abnormalities and localize ligament and cartilage tears
precisely.
warranted for patients with minor disorders of the DRUJ. Strains of the
radioulnar or other ulnar-sided wrist ligaments may respond to rest,
ice after activity, oral antiinflammatory medications, and splints to
hold the wrist in
neutral
position. Tendonitis of the ECU may be treated with a steroid injection
in the ECU tendon sheath, and then the wrist should be immobilized in
pronation for 2 to 4 weeks, with the splint extending above the elbow.
amenable to nonoperative treatment. Instability may be dorsal, palmar,
or multidirectional. Dorsal dislocations are most common and may occur
in conjunction with forearm fractures, such as the Galeazzi
fracture-dislocation and its variants, or soft-tissue lesions without
associated fractures (21,29).
Simple, easily reducible dorsal dislocations can be treated by
immobilizing the forearm and wrist in 20° supination in a rigid splint
or cast for 4 to 6 weeks. Palmar dislocations are rare, usually
chronic, and often result from rotatory malunions in the forearm (29).
In that setting, immobilization usually fails. If the DRUJ has
multidirectional instability, it suggests a severe injury of the joint
structures and TFCC, and nonoperative management is difficult.
treating disorders of the DRUJ. Tailor treatment choices to meet the
goals and occupational and avocational demands of individual patients.
For example, a resection arthroplasty may work well for a sedentary,
low-demand elderly patient but may be a failure for a young laborer. It
is helpful to consider treatment options in categories (Table 43.3, Table 43.4 and Table 43.5).
Soft-tissue procedures are aimed at repairing, debriding, or replacing
injured or destroyed soft tissues. Arthroplasties and arthrodeses are
indicated to treat problems of bony malalignment, arthritis, and
irreparable soft tissue injuries.
procedures. In the setting of an ulnar positive variant and ulnocarpal
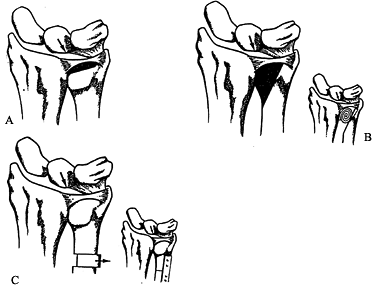
impaction syndrome, the ulnar column of the wrist must be unloaded (Fig. 43.9). This can be done by resecting a 2- to 4-mm wafer of distal ulna as described by Feldon et al. (38).
The procedure retains the integrity of the TFCC to the base of the
styloid process as well as the integrity of the DRUJ. It requires only
a limited arthrotomy and allows direct inspection of the affected area.
It is contraindicated in patients with DRUJ arthritis or more than 4 mm
ulnar positive variance. More recently, this has been done
arthroscopically, using a burr to remove the wafer of distal ulna (110).
 |
|
Figure 43.9. Partial resection arthroplasty options. A: Feldon “wafer” resection. B: Bowers HIT resection. C: Ulnar shortening osteotomy. Redrawn from an illustration by Elizabeth Roselius © 1993, with permission.
|
variance, an ulnar shortening osteotomy is preferred. Several
techniques have been described to accomplish this, and all work well (17,31,82,87,107).
The integrity of the TFCC must be known before ulnar shortening. Assess
indirectly by an arthrogram or, at the time of surgery, either
arthroscopically or by open technique (Fig. 43.10).
If the TFCC is torn or destabilized, it should be either repaired or
debrided, depending on the characteristics and location of the tear, at
the same time as the shortening is done.
 |
|
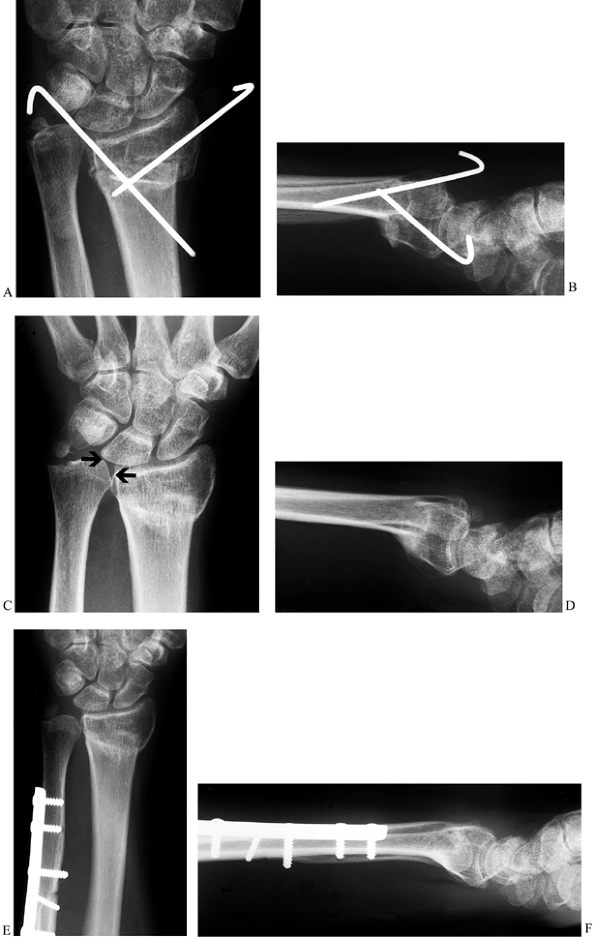
Figure 43.10. Ulnar impaction syndrome. Posteroanterior (A) and lateral (B) radiographs showing attempted K-wire fixation of a radius fracture. This resulted in a malunion of the radius (C,D) and ulnar impaction syndrome from the ulnar positive variance (arrows, C), and the patient was referred for further treatment. An ulnar shortening osteotomy was performed (E,F),
combined with arthroscopic debridement of a TFCC tear. The stable fibrous nonunion at the base of the ulnar styloid was left alone. The patient improved significantly and was pain-free at last follow-up. |
option for treating impingement disorders. This procedure is often
credited to Darrach, who popularized it, although he was not the first
to describe it (30). In a review of 24 cases of distal ulna resection, Dingman (34) discovered the best results were seen in those patients in whom the ulnar styloid process was left in situ
to keep the TFCC intact, and he suggested that the resection should be
subperiosteal because those patients in whom some bony regeneration
occurred also had better results. Hartz and Beckenbaugh (43)
reported the long-term results of the Darrach procedure in a group of
62 patients with posttraumatic DRUJ pathology. All patients improved.
The authors found that the amount of ulna resected and whether or not
the dissection was subperiosteal or regrowth of the distal ulna
occurred had no effect on clinical outcome.
 |
|
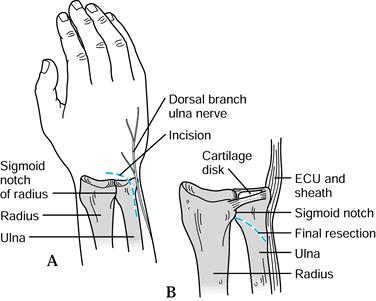
Figure 43.11. The Darrach procedure. A: The skin incision; take care to avoid the dorsal cutaneous branch of the ulnar nerve. B:
The DRUJ and TFCC are shown from a dorsal view. The distal ulna is resected at the radioulnar articulation just proximal to the sigmoid notch. The osteotomy angles obliquely proximalward to avoid a dorsal ulnar prominence. ECU, extensor carpi ulnaris. Redrawn from an illustration by Elizabeth Roselius © 1993, with permission. |
-
Make a dorsal ulnar incision over the
distal ulna. Identify the dorsal cutaneous branch of the ulnar nerve
and protect it throughout the case. -
Develop the interval between the extensor
digiti quinti and the superficial extensor retinaculum and divide the
retinaculum longitudinally, preserving it for subsequent closure.
Expose the distal ulna through a longitudinal subperiosteal dissection.
Leave the interosseous membrane and pronator quadratus undisturbed. -
The site of distal ulnar resection should
be at the level of the radioulnar articulation; angle it 30° obliquely
and proximally to avoid leaving a sharp dorsoulnar corner. The resected
segment should be 4 to 6 mm on the radial border and 8 mm on the ulnar
border. -
Dissect out and remove the distal segment, leaving the ligaments and triangular fibrocartilage intact.
-
Close the periosteum meticulously and plicate the dorsal retinaculum. Drains are unnecessary.
-
For the first 10 days postoperatively,
use an above-elbow bulky dressing reinforced with plaster splints with
the forearm maintained in neutral rotation. For the following 2 weeks,
the patient should splint the wrist intermittently as symptoms allow
and then begin progressive range-of-motion exercises.
problems with the procedure. Failures have been attributed to excessive
bony resection, distal ulnar instability with insufficient soft-tissue
structures to tether the remaining ulna, extensor tendon rupture, ulnar
deviation of the wrist, weakness, and wrist pain (10,11,13,33,39,43,60,70,71,103,105).
shortened distal ulna. The most common cause of that is a prior
resection, but it may result from a growth disturbance of the distal
ulnar physis (10). In such cases, the shortened
ulna may impinge on the distal radius, causing a painful, disabling
pseudarticulation. Bell et al. (10) first
reported the condition in a series of 11 cases, 10 of which resulted
from excision of the distal ulna after injury to the wrist. Further
resection of the ulna is contraindicated in ulnar impingement syndrome.
Instead, the ulnar stump should be stabilized by some means. Bell et
al. recommended creation of a tenodesis using the ECU in the method of
Goldner and Hayes (see below).
radioulnar stability via soft-tissue reconstructions using a sling of
fascia lata or a slip of the overlying ECU and retinaculum (17).
No large or long-term studies demonstrate any one specific technique to
be superior over another. The techniques commonly used are described
below.
 |
|
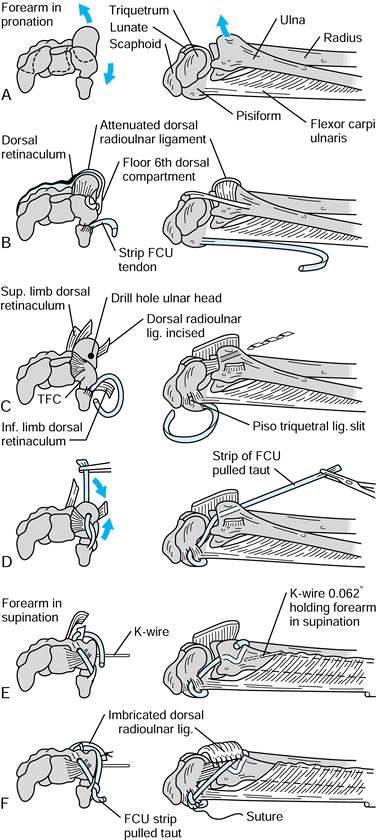
Figure 43.12.
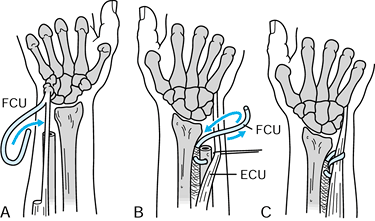
The technique of ulnotriquetral augmentation tenodesis for stabilization of the DRUJ, as described by Hui and Linscheid. A: Starting pathologic position (ulnar head sits dorsal). B: Harvested strip of FCU. C: Drill hole in ulnar head. D: FCU strip passed through drill hole. E: K-wire to reduce DRUJ. F: FCU pulled taut and sutured (see text for details). FCU, flexor carpi ulnaris; TFC, triangular fibrocartilage. Redrawn from an illustration by Elizabeth Roselius © 1993, with permission. |
-
Make a slightly curving incision from the
ulnar border of the small finger metacarpal to the middorsal forearm.
Expose the extensor retinaculum and reflect it to its insertion into
the pisiform. -
Incise the capsule radial to the ECU
tendon and reflect it medially. Make a drill hole through the ulnar
head angled from proximal-dorsal to distal-palmar (Fig. 43.12A, Fig. 43.12B). -
Expose the FCU through a separate
longitudinal incision on the palmar aspect of the wrist. Split it
longitudinally as far proximally as possible and then divide it
proximally, leaving the medial half of the insertion intact distally (Fig. 43.12C). -
Make a hole in the pisotriquetral capsule
and pass the tendon slip intracapsularly, through the drill hole in the
distal ulna and through an enlargement of the prestyloid recess of the
TFC (Fig. 43.12D). -
Supinate the forearm to reduce the ulnar
head and transfix it to the distal radius with a K-wire. Pull the FCU
tendon slip taught, double it back to the radioulnar capsule, and
suture it securely to the pisotriquetral ligament (Fig. 43.12E). -
Imbricate the dorsal radioulnar ligament, replace the ECU in its sheath, and imbricate the extensor retinaculum (Fig. 43.12F).
-
Postoperatively, immobilize the patient’s
wrist and forearm in a splint extending above the elbow for 6 weeks and
then remove the K-wire and splint and institute physical therapy. After
3 months, the patient should begin vigorous exercises to regain
pronation.
The authors state that their operation is applicable both to the
patient with an intact ulnar head and to the one with continuing
instability of the ulnar stump after ulnar head excision.
 |
|
Figure 43.13.
The distal ulna can be stablized with a sling created from the flexor carpi ulnaris (FCU), as described by Tsai and Stilwell. A: Palmar view: harvested strip of FCU. B: Dorsal view: FCU strip passed through distal ulna. C: Dorsal view: FCU passed around extensor carpi ulnaris (ECU) and secured to interosseus membrane. Redrawn from an illustration by Elizabeth Roselius © 1993, with permission. |
-
Make an incision along the course of the
FCU tendon. Expose the tendon from the musculotendinous junction to the
pisiform and dissect out a strip from proximal to distal (Fig. 43.13A). -
Release Guyon’s canal to avoid ulnar nerve compression with subsequent rerouting and tightening of the tendon.
-
Make another incision dorsally, exposing
the ulnar head or distal shaft in the case of previous ulnar head
excision. Drill a hole from the medullary canal opening obliquely and
proximally, emerging on the dorsal aspect of the ulna. Expose the
interosseous membrane close to the ulna distally (Fig. 43.13B). -
Leave the FCU tendon slip attached to the
pisiform and pass the free end dorsally, deep to the ulnar nerve and
artery, into the medullary canal of the ulna and out the dorsal
cortical hole. -
Make parallel incisions in the
interosseous membrane perpendicular to the long axis of the forearm and
pass the end of the slip of the FCU through these incisions, from
proximal to distal. -
Supinate the forearm and pass the
remaining tendon slip around the ECU to keep the soft-tissue leash
positioned around the ECU; this helps keep the tendon stabilized
dorsally on the distal ulna. Tighten the tendon slip, and suture it to
the interosseous membrane.Postoperatively, maintain the forearm in supination for
6 weeks and then begin controlled motion and night splinting for an
additional 2 to 4 weeks. The patient should avoid heavy stress on the
ligament for 3 months after surgery (Fig. 43.13C).
to create new “ligaments” out of adjacent tissue to stabilize the DRUJ.
Blatt and Ashworth (15) designed a procedure to
create a new distal-palmar to proximal-dorsal ligamentous structure
using a distally based flap of palmar wrist capsule. Breen and Jupiter
described another variation of a soft-tissue procedure designed to
treat an unstable ulnar shaft after a Darrach procedure (19).
They use strips of both the FCU and ECU. The ECU is passed from dorsal
to palmar through a drill hole in the ulna and the FCU is pulled in the
opposite direction, and then both tendon slips are pulled taught and
sutured to each other with the forearm in supination.
that a new ligament can be constructed to passively stabilize the ulna.
Although there are insufficient data and large enough series to
conclude that any one technique is definitely better than the others,
it has been suggested (23) that the
Hui-Linscheid procedure may be better than the Tsai-Stilwell procedure
because the intraarticular course of the FCU in the latter results in
more stiffness and loss of pronation. Another group of procedures that
has been described relies on active muscle force to dynamically
stabilize the distal ulna. These procedures are more applicable to an
unstable distal ulna after the Darrach procedure than to a distal
radioulnar joint that is unstable but still has an ulnar head.
 |
|
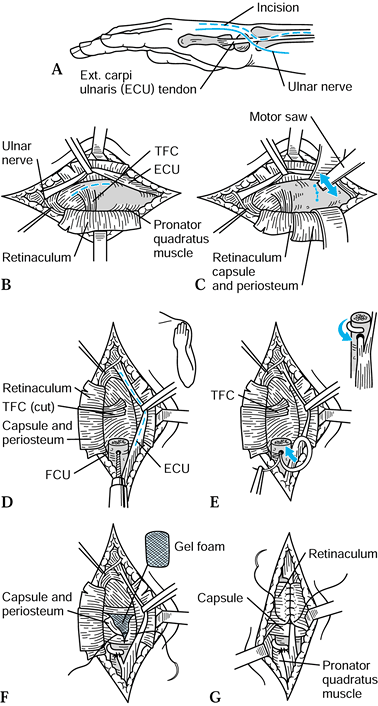
Figure 43.14. Goldner-Hayes technique of stabilizing the distal ulna using half of the extensor carpi ulnaris. A: Incision. B: The dorsal retinaculum is reflected to expose the TFC and distal ulna. C: If not done previously, the ulnar head is removed as shown. D:
Dorsal view, following ulnar head resection; a drill hole is made in the distal ulna in preparation for passing one half the ECU to be harvested (dotted line). E: The harvested ECU is passed through the tunnel in the distal ulna, wrapped 180° around the ulnar shaft, and sutured to itself (inset). F: Gel foam may be placed over the cut surface of the distal ulna (optional), and then the capsule and periosteum are closed. G: The retinaculum is carefully closed over the remaining ECU tendon. Redrawn from an illustration by Elizabeth Roselius © 1993, with permission. |
-
Make a longitudinal incision dorsally
over the distal radioulnar joint. Identify and isolate the dorsal
cutaneous branch of the ulnar nerve. -
Identify the ECU tendon and retract it,
and identify and retract the extensor digitorum communis tendon to the
little finger. Elevate the periosteum off the distal ulna by sharp
dissection. -
If the ulnar head has been resected
previously, shape the end of the ulna with a rongeur so that the dorsal
edge is rounded, and trim the radial aspect so that there will be no
impingement on the tendons to the little finger. -
Split the ECU from its insertion distally
to a point 1 cm proximal to the newly shaped end of the ulna; the strip
should be about one third of the width of the tendon and taken from the
ulnar side of the tendon. -
While the forearm is held in supination,
drill a 3-mm hole in the proximal ulna. The hole should enter the
dorsal ulna 5 mm proximal to the end of the ulna and exit the medullary
canal. Enlarge the tunnel slightly with curets if necessary. -
Pass the freed segment of the ECU through
the hole from a dorsal to palmar position. Direct the tendon to the
ulnar side of the remaining proximal ulna and suture it back on itself
where the tendon entered the bone. This creates a cuff of tendon
directly over the end of the ulna, which stabilizes the remaining ECU
and tethers it toward the radial side of the ulna. Wrap the remaining
tendon 180° around the ulnar stump. It should not be wrapped
circumferentially, as this would cause impingement with forearm
rotation. -
Close the soft tissues in routine fashion.
for 2 weeks and then begin gradual forearm rotation exercises. Protect
the wrist for 3 months with a removable splint at night and
intermittent splinting during the day. Goldner noted that snapping and
popping of the soft tissues occasionally persist for 4 to 6 months
postoperatively. Maximum improvement may take 1 year.
 |
|
Figure 43.15.
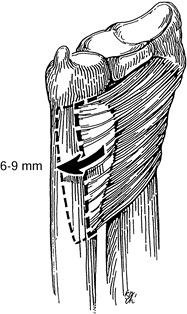
The pronator quadratus advancement recommended by Johnson to treat dynamic instability of the DRUJ, seen from a palmar perspective. Redrawn from an illustration by Elizabeth Roselius © 1993, with permission. |
-
Make a dorsoulnar incision over the distal forearm. Identify and protect the dorsal cutaneous branch of the ulnar nerve.
-
Identify the tendon of the ECU but do not unroof its sheath.
-
Identify the pronator quadratus muscle
distally and remove its origin from the ulna, working distally to
proximally and including as much periosteum as possible with the
tendon. By passively pronating the forearm, the muscle can be relaxed
enough to allow for easy advancement dorsally. -
Advance the muscle 6 to 9 mm dorsally and secure it to the tendon sheath of the ECU with interrupted, nonabsorbable sutures.
-
Rotate the forearm back to neutral
position and cross-pin the radius and ulna with two 0.062-in. Kirschner
wires (K-wires). Maintain the forearm in neutral for 4 weeks and then
remove the pins and let the patient begin a supervised exercise
program, including gentle range of motion and strengthening.
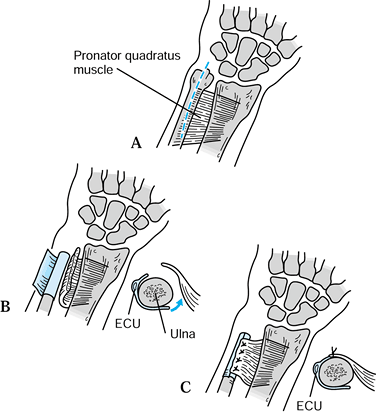
described a technique for transferring the pronator quadratus dorsally
through the interosseous space to stabilize the distal ulna (Fig. 43.16).
They suggested that this be used in combination with the Darrach
procedure at the time of the original surgery or to treat Darrach
procedure failures.
 |
|
Figure 43.16. Illustration of the pronator quadratus transfer described by Ruby et al. A: Incision of skin and periosteum. B: Periosteal flap is created, and pronator quadratus is delivered through the interosseous space. C:
Pronator is sutured to the periosteal flap to stabilize the distal ulna. Redrawn from an illustration by Elizabeth Roselius © 1993, with permission. |
designed to solve the problem that combines the principles of
stabilizing the ulnar stump with a strip of the ECU (as in the Goldner
and Hayes procedure) with transfer of the pronator quadratus origin [as
in the transfer described by Ruby et al. (85)]. Their original report described use of the procedure in six patients, and it was successful in all.
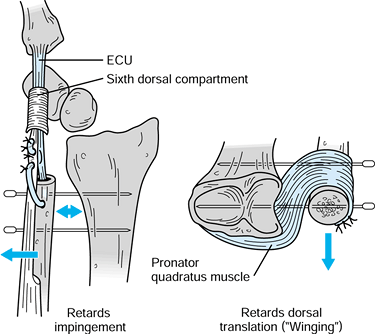 |
|
Figure 43.17.
Kleinman-Greenberg procedure for treating distal ulna instability resulting from a failed Darrach resection. The ECU tenodesis retards radioulnar impingement, and the pronator quadratus transfer limits dorsal translation. Temporary percutaneous pinning of the ulna to the radius adds additional stability. Redrawn with permission (56). |
-
Make a curvilinear dorsoulnar approach to
the ulnar stump at the wrist level, extending the incision proximally
along the distal ulnar diaphysis. -
Approach the ulna through the interval
between the ECU and FCU, isolating the end of the previous Darrach
resection. Smooth the contour of the remaining distal ulna if necessary. -
Dissect the pronator quadratus free of
its palmar-medial insertion on the ulna and mobilize it radially to
allow subsequent transfer from a palmar to a dorsal direction through
the interosseous membrane. -
Use a burr to ream the intramedullary
canal for later intramedullary passage of the ECU tendon. Harvest a
slip of the ECU from proximal to distal, leaving its distal insertion
intact. Make an exit hole 1.5 cm proximal to the end of the ulna. Pass
the distally based 50% of the ECU through the canal and out the
cortical drill hole in preparation for tenodesis. -
Drill two divergent K-wires (0.062 in.)
percutaneously through the ulnar border into the radius using a lamina
spreader to maintain the desired interosseous distance. The wires
should diverge to prevent postoperative migration of the radius and
ulna. -
Transfer the previously harvested
pronator quadratus through the interosseous space and secure it to the
dorsal-medial ulna. Tension the ECU to support the radioulnar space
with the wrist in 10° ulnar deviation and suture it in place to
complete the tenodesis. -
Postoperatively, immobilize the wrist and
forearm in a bulky dressing reinforced with plaster splints extending
above the elbow for 2 weeks, followed by a cylinder cast for 4 weeks.
Remove the K-wires at 6 weeks, and then begin range-of-motion exercises.
dual goals of restoring radioulnar stability while still allowing
forearm rotation. Chronic instability that is deemed too severe for a
soft-tissue reconstruction or instability that persists despite other
attempts at management is an indication for a salvage operation using a
bony procedure. Arthrodesis of the distal radioulnar joint combined
with a surgically created pseudarthrosis site proximally in the ulna is
now commonly referred to as the Sauvé-Kapandji procedure. In many cases
of painful instability of the DRUJ, it may be the most predictable
treatment option.
described their procedure for the DRUJ in an article that was published
in French in 1936. They were not the first to describe such a procedure
(9,57) but have often
been credited with its inception, and the association between their
names and the procedure as it is now performed is deeply entrenched in
the literature. In their description, Sauvé and Kapandji recommended
resection of a 3-cm segment of distal ulna just proximal to the DRUJ,
followed by decortication of the opposing joint surfaces and fixation
with a single screw. They also advocated interposition of the pronator
quadratus into the gap in the ulna to prevent reossification of the
pseudarthrosis site.
continues to evolve. For example, the use of a single screw across the
arthrodesis site as suggested by Sauvé and Kapandji has been supported
by some authors (64,84,111),
but a single screw does not provide rotational control of the distal
ulnar segment. Therefore, other authors recommended the use of two
screws (51) or two K-wires (96) to stabilize the distal fragment. Various uses have been proposed for the bone resected from the ulna. Goncalves (42) used it as a cortical strut wedged between the radius and ulna. Blanco and Blanco (14)
used a 20 mm × 6 mm peg fashioned from the excised ulnar segment
together with two K-wires to stabilize the arthrodesis construct.
Sauvé-Kapandji procedure have remained unchanged: one must stabilize
the DRUJ, restore forearm rotation, prevent reformation of bone across
the resected segment, and avoid painful instability of the residual
ulnar shaft.
-
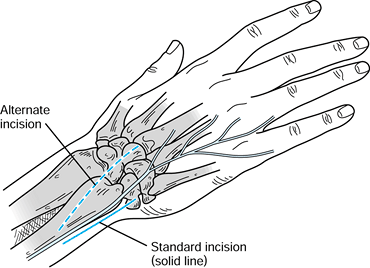
Make a straight longitudinal incision, 6 to 8 cm long, along the ulnar border of the distal forearm (Fig. 43.18).
Bluntly dissect and identify the dorsal cutaneous branch of the ulnar
nerve; isolate and protect this nerve throughout the procedure. An
alternative incision may be used for selected patients in whom
additional procedures
P.1401
are
planned at the same sitting. For example, in patients with rheumatoid
arthritis, the Sauvé-Kapandji procedure may be combined with another
soft-tissue procedure such as a dorsal wrist synovectomy,
tenosynovectomy, or tendon transfer to treat extensor tendon ruptures
that result from the caput ulnae syndrome. In such cases, start the
incision more dorsally to facilitate exposure for the additional
procedure, and then extended it proximally and obliquely to expose the
distal ulna. Again, identify and protect the dorsal cutaneous branch of
the ulnar nerve, which usually crosses the operative field.![]() Figure 43.18. The Sauvé-Kapandji procedure. The standard (solid line) and alternate (dotted line)
Figure 43.18. The Sauvé-Kapandji procedure. The standard (solid line) and alternate (dotted line)
incisions, emphasizing the position of the dorsal cutaneous branch of
the ulnar nerve. The alternate incision courses more obliquely and
dorsally than the standard incision and can be used if additional
procedures are planned such as extensor tenosynovectomies in patients
with rheumatoid arthritis. (Illustration by Birk Cox. Reprinted with
permission from Slater RR, Szabo RM. The Sauvé-Kapandji Procedure. Tech Hand Upper Extremity Surg 1998;3:148.) -
Expose the distal 4 to 6 cm of the ulna
extraperiosteally through the interval between the ECU and FCU. Next,
select the appropriate level for an osteotomy of the ulnar diaphysis (Fig. 43.19).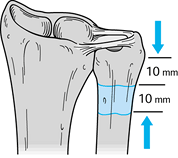 Figure 43.19.
Figure 43.19.
The Sauvé-Kapandji procedure. Illustration of the ideal ulnar resection
leaving approximately 1 cm of ulna distal to the osteotomy to allow
fixation with two screws and resecting a 10- to 12-mm cuff of distal
ulnar shaft. (Illustration by Birk Cox. Reprinted with permission from
Slater RR, Szabo RM. The Sauvé-Kapandji Procedure. Tech Hand Upper Extremity Surg 1998;3:148.) -
Cut the bone just proximal to the flare
of the ulnar head, which will leave enough of a distal ulnar segment to
accommodate two fixation screws. It is helpful to use fluoroscopy to
confirm that the proposed osteotomy site is appropriate. Make a second
cut proximal and parallel to the first and remove a 10- to 12-mm
segment of ulna. Save the removed bone for subsequent grafting into the
DRUJ arthrodesis site. -
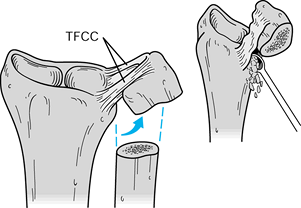
Next, expose the distal radioulnar joint.
Make a dorsoulnar capsulotomy just radial to the ECU tendon.
Alternatively, stay palmar to the ECU tendon and expose the joint by
grasping the distal ulnar segment with a towel clip and reflecting it
distally and medially, using the TFCC as a hinge (Fig. 43.20).
Denude both the ulnar head and sigmoid fossa of the radius of all
remaining cartilage and subchondral bone to create flush surfaces of
cancellous bone on each side of the arthrodesis site.![]() Figure 43.20.
Figure 43.20.
The Sauvé-Kapandji procedure. The DRUJ can be exposed through a
straight medial approach, reflecting the distal ulnar segment distally
and medially, hinging it on the TFCC as illustrated. The joint then can
be decorticated and prepared for fusion (inset). (Illustration by Birk Cox. Reprinted with permission from Slater RR, Szabo RM. The Sauvé-Kapandji Procedure. Tech Hand Upper Extremity Surg 1998;3:148.) -
Cannulated screws are ideal for fixation
of the arthrodesis site and offer several advantages over K-wires and
solid screws. Using cannulated screws over guide wires allows accurate
screw placement and facilitates the alignment of the cortices of the
distal ulna and radius. There is no need to remove hardware.
Rehabilitation can begin sooner because of secure fixation. K-wires can
be problematic because buried pins can irritate cutaneous nerves, and
percutaneous pins can cause wound problems; both are avoided if screws
are used. -
After selecting the desired fixation
device and preparing the joint surfaces for fusion, the next step is to
establish ulnar neutral variance. Move the ulnar head proximally or
distally as required to bring its distal surface parallel with the
distal radius surface (Fig. 43.21); confirm correct position fluoroscopically.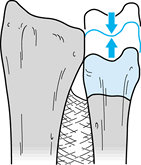 Figure 43.21.
Figure 43.21.
The Sauvé-Kapandji procedure. Establish ulnar neutral variance by
moving the distal ulnar segment proximally or distally as necessary to
align the articular surface of the ulnar head even with the articular
surface of the distal radius. Fluoroscopy is helpful for this step.
(Illustration by Birk Cox. Reprinted with permission from Slater RR,
Szabo RM. The Sauvé-Kapandji Procedure. Tech Hand Upper Extremity Surg 1998;3:148.) -
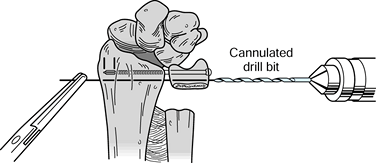
Select two appropriate guide wires from the chosen screw set and drill them into place to stabilize the ulnar
P.1402
head in proper position. Pass one wire through the radioulnar joint a
few millimeters proximal to the subchondral bone; place the second wire
proximal enough to allow room for seating of both screw heads without
impingement on each other (Fig. 43.22).
Confirm correct placement of the guide wires with fluoroscopy. It is
important to do this while holding the forearm in neutral rotation.
This is best accomplished with the patient’s elbow resting on the
operating table while the forearm is supported perpendicular to the
table in neutral rotation. Avoid the tendency to rest the forearm in
pronation on the operating table.![]() Figure 43.22.
Figure 43.22.
The Sauvé-Kapandji procedure. Drill two guide wires for the selected
cannulated screws parallel across the distal ulnar segment into the
distal radius and across to the far cortex of the radius, and use a
cannulated measuring guide to determine the appropriate screw lengths.
After the screw lengths have been determined, advance the guide wires
through the skin and hold them with a clamp to prevent displacement
while the guide wires are overdrilled and the screws placed.
(Illustration by Birk Cox. Reprinted with permission from Slater RR,
Szabo RM. The Sauvé-Kapandji Procedure. Tech Hand Upper Extremity Surg 1998;3:148.) -
Advance the distal wire to the far
(radial) cortex of the radius and measure for screw length. The
proximal screw provides rotational control and needs only tricortical
fixation; therefore, it should be 5 mm shorter than the distal screw.
It also can be smaller in diameter if desired, which helps minimize the
risk of fracturing the narrow ulnar diaphysis. After the guide wires
are in proper alignment and the screw lengths have been measured,
advance the wires through the skin to the radial side of the forearm
with a mallet and grasp them with a clamp to avoid loss of position
during the next steps (Fig. 43.22). Using a mallet minimizes the chance of wrapping up a radial sensory nerve branch with the power drive. -
Next, overdrill the guide wires with a
cannulated drill bit. A tap is not usually necessary, but use it if the
cortical bone is particularly dense. Before insertion of the screws,
pack the cancellous bone harvested previously from the excised ulnar
segment into the arthrodesis site as a graft (Fig. 43.23).
In cases with severe bone loss, insert the resected ulnar piece as a
corticocancellous bone graft into the DRUJ space. Then insert the
selected screws over the guide wires while manually compressing the
ulnar head against the radius. Tighten the distal screw first to avoid
compressing the radial and ulnar shafts together and levering the ulnar
head out of position.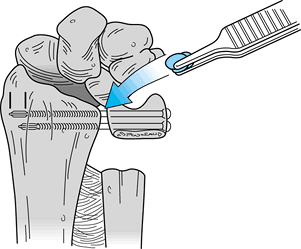 Figure 43.23.
Figure 43.23.
The Sauvé-Kapandji procedure. Cancellous bone from the excised ulnar
segment can be packed into the arthrodesis site before the screws are
fully seated. The final position of the screws is illustrated.
(Illustration by Birk Cox. Reprinted with permission from Slater RR,
Szabo RM. The Sauvé-Kapandji Procedure. Tech Hand Upper Extremity Surg 1998;3:148.) -
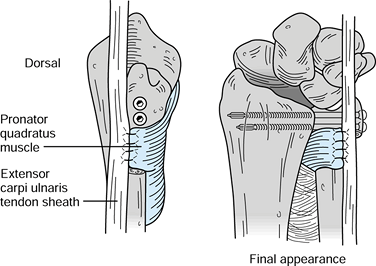
Make a final adjustment of the proximal
ulnar shaft length with another osteotomy (if necessary) so that there
is a gap of 10 to 12 mm between the proximal and distal ulnar segments.
Suture the fascia of the underlying pronator quadratus into the
resultant gap to prevent reossification across the pseudarthrosis site (Fig. 43.24).
This also helps stabilize the stump of the ulnar shaft. Repair the
extensor compartments if they were disrupted, for example, to perform
tenosynovectomies. Close the skin in routine fashion and obtain final
radiographs.![]() Figure 43.24.
Figure 43.24.
The Sauvé-Kapandji procedure. Suture the pronator quadratus in place in
the gap where the ulnar segment was excised (left). The final
appearance of the construct is illustrated (right). (Illustration by
Birk Cox. Reprinted with permission from Slater RR, Szabo RM. The
Sauvé-Kapandji Procedure. Tech Hand Upper Extremity Surg 1998;3:148.)
diagnosed with increasing frequency as a cause of ulnar-sided wrist
pain. Disruption of the TFC may be associated with instability of the
distal radioulnar joint, or the lesions themselves may be unstable and
symptomatic without causing instability of the entire joint. Treatment
depends on location and characteristics of the injury (Table 43.6). Tears at the periphery of the TFC, along the well-vascularized medial insertion, are amenable to repair. Increasingly,
surgeons are making the repairs arthroscopically, as described in Chapter 75, but the procedure also may be done by open technique, as originally described.
 |
|
Table 43.6. Treatment of TFCC Tears
|
 |
|
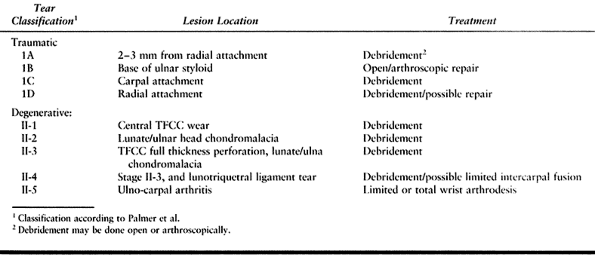
Figure 43.25. Open repair of peripheral TFCC tears. A:
Chronic granulation tissue is debrided from the site of injury, a cancellous bony trough is made at the base of the fovea, and drill holes are made across the ulnar cortex into the trough. B: 3-0 nonabsorbable sutures are placed through the prepared edge of the TFCC and passed through the drill holes. C: The distal ulna is percutaneously pinned to the radius with K-wires, and then the sutures are tied securely to reconstitute normal tension in the TFCC. Reprinted with permission (46). |
-
Make a dorsal skin incision paralleling
the distal 3 cm of ulna and extending 3 cm distally and radially. Raise
a radially based long retinacular flap to later support the ECU tendon
dorsal to the flexion-extension axis of rotation. Retract the ECU
tendon ulnarly and enter the ulnocarpal joint via a capsulotomy between
the fifth and sixth dorsal compartments. Carefully avoid any further
injury to the TFCC. -
Identify the traumatically separated TFCC
and debride any granulation tissue from the fovea. Create a trough at
the base of the fovea by curettage to cancellous bone. -
Place the forearm in neutral rotation and
percutaneously pin the distal ulna and radius with two parallel
0.062-in. K-wires to maintain this alignment. Drill parallel small
holes through the distal ulna, exiting at the base of fovea. -
Place 3-0 nonabsorbable sutures through
the prepared edge of the TFCC and pass them through the drill holes.
Tighten the sutures to bring the torn edge of the TFCC back to the
fovea and tie them securely while trying to reconstitute the normal
tension in the cartilage disc. -
Repair the dorsal capsule with inverted
sutures and pass the previously designed flap of extensor retinaculum
deep to the ECU and secure it distally to prevent ulnar subluxation of
the tendon. That further enhances DRUJ stability as well. -
Postoperatively, immobilize the extremity
in a plaster-reinforced bulky dressing extending above the elbow for 10
to 14 days, followed by an above-elbow cast for 4 weeks. Remove the
K-wires after 4 weeks and immobilize the wrist in a below-elbow cast
for an additional 6 weeks. Begin vigorous rehabilitation of the forearm
and wrist at 12 weeks.
reported that 8 of 11 patients with follow-up greater than 1 year after
this procedure returned to normal activities without pain. Measured
grip strength averaged 87% of that of the uninjured hand, and measured
wrist and forearm motion averaged between 96% and 99% of the
contralateral side. Similar results have been reported by other authors
after open and arthroscopic TFCC repairs (27,102).
palmar instability of the DRUJ. It has been my experience that the
usual cause of this problem is a malunion of one or both forearm bones,
and treatment with soft-tissue procedures is ineffective. If palmar
instability is found (usually aggravated by supination), conduct a
careful radiographic assessment of the forearm with comparative views
of the normal extremity. Corrective osteotomy (angular, rotational, or
both) is required to eliminate the instability.
variety of surgical options have been proposed for treatment. Swanson
recommended resection of the distal ulna and replacement with a
silicone cap (94). Complications from silicone
synovitis, particulate debris, and prosthesis failure led to the
abandonment of this procedure and development of different techniques.
Other forms of resection arthroplasty have gained more popularity.
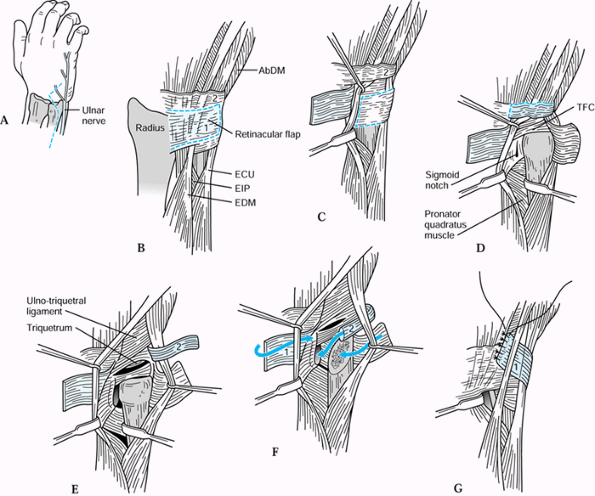
is that the portion of the distal ulna that articulates with the
sigmoid notch of the distal radius is excised while the distal ulnar
shaft and styloid process are preserved (Fig. 43.26).
This preserves the triangular fibrocartilage complex, removes the
damaged portion of the ulnar head, and maintains stability of the
distal ulna.
 |
|
Figure 43.26. Bowers’ hemiresection interposition technique (HIT) of DRUJ arthroplasty. A: Incision, emphasizing the location of the dorsal cutaneous branch of the ulnar nerve. B: Outline of the dorsal retinacular flaps, proximal (1) and distal (2). C: The proximal flap has been reflected showing the proposed flap in the DRUJ capsule (dotted line). D: The capsular flap has been reflected to expose the ulnar head and DRUJ. E: The second retinacular flap (2) has been reflected to expose the TFC and its carpal face. F: The ulnar head has been resected, and the capsular and retinacular flaps are set for repositioning. G: The first retinacular flap (1) is used to stabilize the ECU tendon. EIP, extensor indicis proprius, EDM, extensor digiti minimi. Redrawn from an illustration by Elizabeth Roselius © 1993, with permission.
|
-
With the forearm in full pronation, open
the interval between the extensor digiti quinti and ECU. Identify and
protect the dorsal cutaneous branch of the ulnar nerve (Fig. 43.26A). -
Develop two flaps of the extensor
retinaculum. The proximal flap, about one half the width of the
retinaculum, is based radially; the distal flap is based ulnarly.
Radial retraction of the extensor digiti quinti at this level places
the surgeon immediately dorsal to the sigmoid notch of the radius (Fig. 43.26B, Fig. 43.26C). -
Open the capsule of the DRUJ close to the
radius, allowing enough tissue to repair later. The entire triangular
fibrocartilage can be seen through this interval. Remove the convexity
of the ulnar head. The resection should preserve the shaft and ulnar
styloid relationship (Fig. 43.26F). -
Suture the ulnarly based capsular flap
over the raw bone of the distal ulna, with the sutures entering the
palmar wrist capsule. Rotate the forearm to verify that there is no
bony contact between the radius and ulna (Fig. 43.26G). -
If there is inadequate tissue to prevent
radioulnar contact, place an “anchovy” of tendon from the palmaris
longus tendon in the defect previously occupied by the ulnar head. If
there is ulnar positive variance and impaction of the remaining ulnar
styloid with the carpus on ulnar deviation of the wrist, the HIT may
need to be combined with ulnar shortening to prevent translation and
impingement of the residual ulna and ulnar styloid. -
After proper bony alignment is obtained,
suture the distal, ulnarly based retinacular flap to the dorsal
capsular structure remaining on the sigmoid notch. Pass the proximal,
radially based flap around the ECU and suture it to the distal aspect
of the retinaculum of the fourth compartment. -
Postoperatively, immobilize the forearm in neutral rotation for 3 weeks and then begin rehabilitation.
that the distal ulna resection be performed with the intention of
sculpting the distal ulna to carefully “match” the slope and shape of
the distal radius throughout the arc of forearm pronation and
supination. As Watson describes it, the operation should be performed
as follows.
-
Place the patient on the operating table
with the affected extremity fully pronated on a hand table. The surgeon
sits cephalad to the arm. Under tourniquet control, make a longitudinal
incision dorsoulnarly over the distal ulna. Open the proximal edge of
the extensor retinaculum over the ulnar head. -
Use Lexell rongeurs to begin the
resection of the distal ulna approximately 5 to 6 cm proximal to the
end of the ulna between the extensor digitorum communis and ECU. Resect
the distal ulna in a long, sloping manner resembling an eccentrically
sharpened pencil, not unlike the shape of a finger with the remaining
ulnar cortex, representing the finger “nail,” facing ulnarward (Fig. 43.27).
Rotate the forearm into full pronation and shape the ulna to match the
surface and shape of the radius where they oppose each other.
Similarly, rotate the forearm into full supination and shape the ulna
to match the opposed surface of the radius. Inspect the flare of the
radius at the sigmoid fossa. The dorsal rim of the fossa is usually
more prominent than the palmar flare. If the flare protrudes ulnarward,
it may impinge on a dorsal riding ulna. In that case, trim the radius
to avoid that problem.![]() Figure 43.27. Illustration of the matched ulna resection arthroplasty of the DRUJ. The distal ulna is shaped to resemble a finger (inset)
Figure 43.27. Illustration of the matched ulna resection arthroplasty of the DRUJ. The distal ulna is shaped to resemble a finger (inset)
with the finger “nail” facing ulnarward and contoured so there is no
impingement against the distal radius through a full arc of forearm
rotation. -
After removal of the ulnar head, the
ulnar styloid will migrate radially and may impinge on the carpals.
Check an x-ray and be sure the distalmost ulna lies at the level of the
articular surface of the radius. Resection of the ulnar styloid is
almost always necessary to achieve this. Then palpate between the
radius and ulna to assure there are matching surfaces throughout the
arc of forearm rotation. No interposition of soft tissue is necessary.
The deep fascia of the ECU sheath may remain attached to the periosteum
of the ulna, but this is not necessary. The raw bone of the distal ulna
will become securely adherent to the ulnar sling mechanism without
sutures or fixation. -
Occasionally the slope of the sulcus of
the radius is reversed. When seen on a PA radiograph, the proximal
portion of the radius sulcus joint protrudes more ulnarward than the
distal portion; that is, the slope of the radius sulcus joint may be
from proximal ulnar to distal radial. When the joint is shaped in this
fashion, the proximal portion of the sulcus should be removed with a
rongeur so that the radius and ulnar surfaces are again parallel to one
another. -
Close the skin in routine fashion and
apply a bulky hand dressing with a plaster splint that extends to the
elbow but not above. The below-elbow dressing allows immediate forearm
pronation and supination. Seven to 10 days postoperatively, patients
should begin full mobilization out of the splint.
the Bowers and Watson procedures. In a series in patients with
posttraumatic DRUJ disorders reported by Saffar et al. (86),
pain relief, wrist motion, and grip strength improved more after the
Sauvé-Kapandji procedure than after the Bowers or Watson procedures. My
own experience and observations are similar, and therefore I prefer the
Sauvé-Kapandji procedure. It provides more predictable results in a
variety of settings, including osteoarthritis. A recent study by Minami
et al. (65) reported 15 patients with
osteoarthritis of the DRUJ treated in this fashion, and all achieved
good results. They suggested that this should not be done if the TFCC
is intact or repairable but gave no supporting data for this statement.
I do not consider an intact TFCC a contraindication to the
Sauvé-Kapandji procedure. In fact, it seems logical to leave the distal
ulna as a buttress for the intact TFCC. Taleisnik (97) considers retention of the distal ulna to help stabilize the TFCC, an advantage of the procedure.
make an effort to distinguish the pain and instability of the DRUJ from
radiocarpal and midcarpal joint symptoms. This is done by careful
palpation, ballottement, and compression of the specific areas and a
comparison of the degree of symptoms elicited by forearm rotation
versus wrist flexion and extension. Despite advanced radiographic
findings of radiocarpal and/or midcarpal arthritis, many arthiritis
patients’ complaints of “wrist pain” can be satisfied by addressing the
DRUJ pathology.
the hand and wrist in RA tend to cause palmar and ulnar displacement of
the wrist, resulting in decreased mobility, strength, and function.
Commonly, resection of the distal end of the ulna, the Darrach
procedure, has been recommended for patients with RA and ulnar-sided
wrist pain (48,69,81).
However, removal of the distal ulna accelerates further palmar and
ulnar translocation and ultimately dislocation of the carpus. This
complication has been seen in patients with rheumatoid arthritis
treated with the Darrach procedure (13,104). Black et al. (13)
showed this to occur in 5 of 34 cases reviewed. Six other cases
developed spontaneous radiocarpal ankylosis that stabilized the wrist
and prevented the ulnar-palmar drift of the carpus.
arthroplasties and the alternative of wrist arthrodesis, the
Sauvé-Kapandji procedure is an excellent choice for patients with
rheumatoid arthritis and may obviate the need for further surgery on
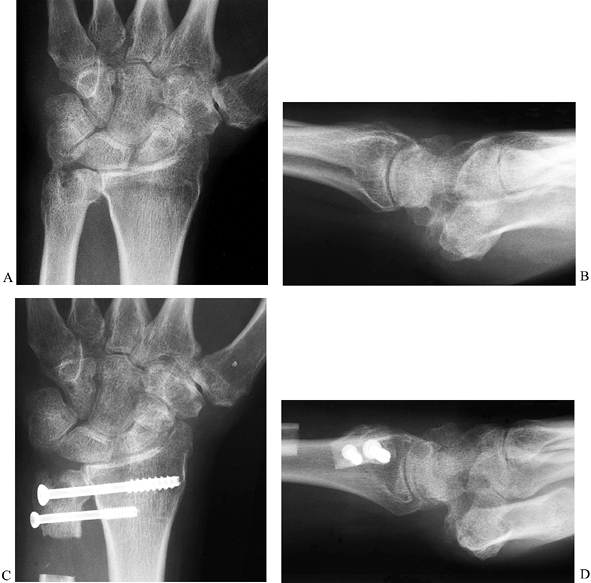
the radiocarpal joint (Fig. 43.28). With the
Sauvé-Kapandji procedure, retention of the distal ulna provides a bony
support for the ulnar side of the wrist and preserves the soft-tissue
attachments of the ulnocarpal complex. The resulting stability at the
wrist in rheumatoid patients may help prevent more distal joint
deformity (104). The ulnar osteotomy allows as
much shortening as necessary to match the length of the radius while
retaining forearm rotation.
 |
|
Figure 43.28.
Radiographic appearance of the wrist of a patient with rheumatoid arthritis before and after the Sauvé-Kapandji procedure. The preoperative PA (A) and lateral (B) and postoperative PA (C) and lateral (D) views are shown. |
patients with rheumatoid arthritis have been generally good. In one
series, 21 wrists in 17 patients were followed for an average of 39
months following the Sauvé-Kapandji procedure (104).
All patients reported an increased ability to use their wrists in the
functions of daily living after surgery when compared to before surgery
and were pleased with the results. At an average of 39 months’
follow-up, forearm pronation averaged 78°, and supination 86°. X-rays
showed that ulnar and palmar translocations of the carpus were
prevented.
is so severe that no radiocarpal motion is left, or the wrist is
already dislocated. In that setting, a wrist arthrodesis or replacement
arthroplasty is indicated. Then there is no benefit to the
Sauvé-Kapandji procedure, and the Darrach procedure can be used to
treat the DRUJ.
described two cases of comminuted fractures of the radial head
associated with dislocations of the DRUJ. He speculated that the
mechanism of injury was a violent longitudinal compression force along
the long axis of the forearm that resulted in disruption of the DRUJ
and the interosseous membrane and fracture of the radial head.
Recognizing the extent of the injury to the forearm, Essex-Lopresti
recommended not excising the radial head, or, if that were necessary,
implanting a prosthesis in place of the radial head to hold the radius
out to length while the interosseous membrane healed.
have shown that injuries of the Essex-Lopresti type can occur in
conjunction with a variety of forearm fractures. The essential features
of the injury are a disrupted DRUJ, a radial head fracture, and an
associated forearm fracture. It is important to recognize this injury
pattern so that it can be treated appropriately. Every effort should be
made to preserve the radial head and perform open reduction with
internal fixation of the fracture if a stable closed reduction is not
obtainable. If the radial head must be excised for some reason, a
prosthesis may be put in its place temporarily. A definitive procedure
may be done later. One option for the symptomatic, established
Essex-Lopresti lesion is replacement of the fragmented radial head or
prosthesis with an allograft radial head. A preliminary report of that
procedure in five patients followed for 2 to 7 years described good or
excellent results in all patients (95).
described his experience with 18 cases. Since then, Galeazzi’s name has
come to be associated with a fracture of the distal radius in
combination with a dislocated DRUJ. It is estimated (68)
that these injuries account for nearly 7% of all fractures of the
forearm in adults. The mechanism of injury is usually a fall on the
outstretched hand with the forearm pronated. With the hand fixed to the
ground, continued rotation of the patient’s body causes hyperpronation,
and the resultant forces cause the radial shaft to fracture. The radius
then shortens, producing a disruption of the TFCC or an avulsion
fracture of the ulnar styloid, which destabilizes the DRUJ.
generally results in persistent symptoms, including recurrent or
persistent dislocations of the DRUJ (63).
Therefore, Galeazzi fracture-dislocations should be treated operatively
whenever possible to maximize the chance for a good outcome. The
fracture should be fixed rigidly, generally by open reduction and
plating. The DRUJ should then be reduced. It should snap into place
with a solid endpoint and is usually most stable in supination.
into subluxation or dislocation, it suggests soft tissue interposed in
the joint. Most often, the ECU is the offending tissue. It may displace
around the ulnar border of the dorsally dislocated ulna (6).
Other structures that have been reported to block reduction include the
extensor digitorum tendons, the extensor digiti minimi, the flexor
pollicis longus, and the median nerve (12,93).
In those cases where the joint cannot be reduced, make an incision over
the dorsal aspect of the DRUJ, reflect the displaced soft tissues out
of the way, and repair the sheath of the ECU
to
keep it centered over the distal ulna. Postoperatively, immobilize the
forearm in approximately 20° to 30° supination for the first 4 weeks,
followed by rehabilitation to gradually restore forearm rotation.
forearm may be the result of prior trauma to the forearm, interosseous
membrane, or DRUJ. In selected cases, capsulotomy of the DRUJ may
improve forearm rotation. This is particularly true in those patients
who had a previous fracture of the distal radius with involvement of
the DRUJ and are left with the sequelae of a contracted joint capsule.
In a report of 18 patients, af Eckenstam noted 15 patients had improved
forearm rotation following DRUJ capsulotomy (4). Other authors have made similar observations (17).
A dorsal approach and capsulotomy should be used for patients with loss
of pronation, and a palmar approach and release should be used for
patients with loss of supination (5,55).
way to diagnose and treat lesions of those joints, particularly the
triangular fibrocartilage complex. Indications for this surgery are
becoming better established. It is beyond the scope of this chapter,
however, to address this in detail, and the reader is referred to Chapter 75.
successful treatment strategy for the multiple disorders of the distal
radioulnar joint, one must first localize the pathology and be able to
categorize it based on a good understanding of the anatomy and
biomechanics of the region. For impingement problems, particularly
ulnocarpal impingement, restoration of the length relationships between
ulna and radius is required, frequently in the form of ulnar
shortening. Instability often can be treated with an appropriate
soft-tissue procedure. When the quality and condition of the soft
tissues are inadequate, arthrodesis of the DRUJ in the form of the
Sauvé-Kapandji procedure solves the instability problem. Injuries to
the TFCC are an important source of problems related to the DRUJ. They
should be assessed and treated either openly or arthroscopically, as
the surgeon’s capabilities allow. Incongruity of the DRUJ requires a
bony procedure in the form of a resection arthroplasty or distal ulnar
excision (rarely), or the Sauvé-Kapandji procedure.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
M. The Caput Ulnae Syndrome in Rheumatoid Arthritis: A Study of the
Morphology, Abnormal Anatomy, and Clinical Picture. Acta Rheumatol Scand [Suppl] 1963;5:1.
P, Belsky MR, Terrono AL. Partial (“Wafer”) Distal Ulna Resection for
Triangular Fibrocartilage Tears and/or Ulnar Impaction Syndrome
(abstract). J Hand Surg 1990;15A:826.
JL, Hayes MG. Stabilization of the Remaining Ulna Using One-Half of the
Extensor Carpi Ulnaris Tendon after Reconstruction of the Distal Ulna. Orthop Trans 1979;3:330.
FC, Linscheid RL. Ulnotriquetral Augmentation Tenodesis: A
Reconstructive Procedure for Dorsal Subluxation of the Distal
Radioulnar Joint. J Hand Surg 1982;7:230.
RK, Shrewsbury MM. The Pronator Quadratus in Motions and in
Stabilization of the Radius and Ulna at the Distal Radioulnar Joint. J Hand Surg 1976;1:205.
H, Palmer AK, Werner FW, et al. The Effect of Dorsally Angulated Distal
Radius Fractures on Distal Radioulnar Joint Congruency and Forearm
Rotation. J Hand Surg 1996;21A:40.
GJ, McMurtry RY, Rubenstein JD, Ogston NG. Computerized Tomography of
the Distal Radioulnar Joint: Correlation with Ligamentous Pathology in
a Cadaveric Model. J Hand Surg 1986;11A:711.
WB, Graham TJ. The Distal Radioulnar Joint Capsule: Clinical Anatomy
and Role in Postraumatic Limitation of Forearm Rotation. J Hand Surg 1998;23A:588.
DE, Palmer AK, Levinsohn EM. The Role of Radiography and Computerized
Tomography in the Diagnosis of Subluxation and Dislocation of the
Distal Radioulnar Joint. J Hand Surg 1983;8:23.
DE, Palmer AK, Levinsohn EM. Radiography and Computerized Tomography in
the Diagnosis of Incongruity of the Distal Radio-Ulnar Joint. A
Prospective Study. J Bone Joint Surg 1985;67A:247.
MA, Groll SR, Klug M, Windler D. Stress Computed Tomography Analysis of
the Distal Radioulnar Joint: A Diagnostic Tool for Determining
Translational Motion. J Hand Surg 1991;16A:75.
LK, Ferenz CC, Dell PC. The Pronator Quadratus Interposition Transfer:
An Adjunct to Resection Arthroplasty of the Distal Radioulnar Joint. J Hand Surg 1996;21A:60.
P, Barbato B, Werther JR, Lenerle JP. Operative Treatment of
Posttraumatic Distal Radio-Ulnar Joint Disorders. Comparative Study of
Clinical Results between Four Procedures. (abstract). In Proceedings of the American Society for Surgery of the Hand, Nashville, TN, 1996.
L, Kapandji M. Nouvelle Technique de Traitment Chirurgical des
Luxations Recidivantes Isolées de l’Extremité Inferiéure du Cubitus. J Chir 1936;47:589.
JM, Khuri SM. Entrapment of the Median Nerve and Flexor Pollicis Longus
in an Epiphyseal Fracture-Dislocation of the Distal Radioulnar Joint: A
Case Report. J Hand Surg 1984;9A:711.
AB. Implant Arthroplasty for Disabilities of the Distal Radioulnar
Joint. Use of a Silicone Rubber Capping Implant Following Resection of
the Ulnar Head. Orthop Clin North Am 1973;4:373.
RM, Hotchkiss RN, Slater RR. The Use of Frozen-Allograft Radial Head
Replacement for Treatment of Established Symptomatic Proximal
Translation of the Radius: Preliminary Experience in Five Cases. J Hand Surg 1997;22A:269.
SM, Miller RJ, McCanci SE, Meyers SP. Lesions of the Triangular
Fibrocartilage Complex: MR Findings with a Three Dimensional
Gradient-Recalled-Echo Sequence. Radiology 1996;199:227.
TM, Stilwell JH. Repair of Chronic Subluxation of the Distal Radioulnar
Joint (Ulnar Dorsal) Using Flexor Carpi Ulnaris Tendon. J Hand Surg 1984;9B:289.
HK, Brown RE. Ulnar Impingement Syndrome after Darrach Procedure:
Treatment by Advancement Lengthening Osteotomy of the Ulna. J Hand Surg 1989;14A:302.