VASCULARIZED BONE GRAFTS
Hand, Wrist, Elbow, Shoulder, and Microvascular Surgery, Department of
Orthopaedic Surgery, Southwestern Medical School, Dallas, Texas 75235.
vascularized bone grafts has continued to be refined. Offering rapid
bony union, increased stiffness and strength, less resorption, and more
rapid hypertrophy than conventional bone grafts, they have distinct
advantages in selected cases. They do, however, require specialized
microsurgical skills and careful preoperative planning. With sources
including the fibula, iliac crest, rib, radial styloid, and indications
ranging from nonunion, tumor reconstruction, congenital pseudarthrosis,
and radial club hand (52) to traumatic and infectious defects, the opportunities that can be afforded by vascularized bone grafts are vast and varied.
first experiments on free tissue transposition in dogs in 1971 when
they isolated a rib on the internal mammary pedicle and transposed it
to the jaw (48). This was followed, again in the canine, with a free vascularized rib graft in 1973 by McCullough and Fredrickson (37).
With the advent of microvascular techniques and development of
microsurgical instruments, anastomosis of vessels as small as 1 mm was
successfully accomplished by Buncke in 1965 in a rhesus monkey (11).
Improvement on these techniques opened the door for the transfer of
composite tissue. The first free skin flap was reported by Daniel and
Taylor in 1973, involving the transfer of an iliofemoral island flap
based on the superficial circumflex iliac artery to the right lower
extremity (15). This was followed by a report
in 1975 of the first free bone graft by Taylor et al., who transferred
a fibula to a tibial defect (53). Buncke et al. (10) successfully transferred the first osteocutaneous flap utilizing a rib to the tibia in 1977 to treat a tibial
pseudarthrosis, and this was followed shortly by Taylor’s report of the
first such graft using groin skin and iliac bone in 1978 (56).
-
To provide restoration of skeletal continuity
-
To achieve union rapidly, thus avoiding the slow process of creeping substitution as required for conventional grafts
-
To provide viable soft tissue coverage early
-
To restore and maintain the anatomy of the limb
-
To restore limb function including motion and strength
and perform the procedures with the least morbidity and operative time
necessary. For three-quarters of a century, conventional
corticocancellous bone grafting, which has been shown to involve
necrosis of the graft followed by a long process of revascularization,
osteogenesis, and remodeling, has been the sole option in treatment of
segmental bone defects. The living bone graft circumvents this
prolonged process. With these goals in mind, the next step is the
assessment of the patient and his or her expectations, the underlying
pathology, and what is available to use as graft tissue. All of these
points have to be considered in individualizing the treatment plan for
each patient. Once this is determined, the surgical team can move to
the next step in planning a vascularized bone graft.
to planning a vascularized bone graft. Patient age and underlying
medical condition play an important role in determining which patients
are candidates. Although there is no age limit for vascularized bone
grafts, elderly patients with underlying medical problems may present
an unacceptably high surgical risk. Carefully weigh the risks of a long
surgical procedure and requirement for prolonged limb protection
against patient expectations. Those patients with evidence of
peripheral vascular disease may also present risks that predispose to
failure. Patients with multiple previous attempts at treatment may have
altered local anatomy and limited availability for tissue coverage.
Patients who smoke have been shown to have delayed bone healing. Those
with hypercoagulable conditions or clotting abnormalities may also
require special consideration.
infection, the condition of the recipient vessels in the defect must be
carefully considered, as they may be at increased risk for failure of
the graft because of poor arterial inflow and venous outflow. These
procedures are often long and tedious, and the extensive dissection and
long operative time required may exacerbate a previously quiescent
infection and may increase the risk of graft failure.
for vascularized transfer. First, the donor bone must be of sufficient
size to fill the defect. Free vascularized grafts offer advantages over
conventional grafts in cases of defects greater than 6 to 8 cm. Lesser
defects may not require as extensive a procedure, and a pedicled
vascular graft may suit such a case well. Free vascularized iliac crest
grafts are useful for defects no longer than 10 cm because of both the
curvature and the structural characteristics of the bone. The nutrient
vessels must be of adequate size for successful microvascular
anastomoses. Arteriograms may aid in determining the availability and
status of vessels. Last, donor site morbidity must be minimized.
continue to expand. Well established are the uses of such grafts in
patients with large defects secondary to trauma or after resection of
locally aggressive or malignant bone tumors. Refractory nonunions,
resection for osteomyelitis, and congenital pseudarthrosis of the tibia
or forearm are other situations in which living grafts can be most
useful. More recently, the use of vascularized bone grafts in the
treatment of avascular necrosis of the femoral head has been reported
with some mixed results. Avascular necrosis of the scaphoid (Priser’s
disease) and of the lunate (Kienbock’s disease) has also been treated
with vascularized bone grafts in hopes of arresting the underlying
disease process.
in both upper and lower extremities. Perhaps the most frequent
indication for vascularized bone grafts is the posttraumatic and often
massive bone loss associated with severe trauma. Autogenous cancellous
bone graft methods are not well suited for large defects of bone
greater than 6 to 8 cm, as resorption of the graft may occur, and it
does not provide structural support. In addition, scarring
and
relative avascularity of the recipient bed may not be conducive to
graft incorporation. Defects in the tibia are most common; however,
defects of the femur, ankle, and radius have also been treated with
vascular bone grafts. An important concern in this setting is the
condition of the recipient site, as often the bed has significant
scarring. The arterial inflow to the area may be tenuous as well
because of the “zone of injury,” which may extend a significant
distance from the apparent bony defect. The fibular graft is most often
utilized, as it nicely fits the longitudinal defect and is of
sufficient length for most reconstructive settings. The rib graft was
the first reported graft used clinically, but its usefulness in
orthopaedics is somewhat limited because of its curved and malleable
nature. It also has the potential for higher donor site morbidity if
careful harvesting technique is not followed. The iliac crest is also
useful; however, again the curvature of the bone often limits its
application for defects less than 10 cm, and donor site morbidity is
not insignificant.
grafts in the setting of tumor resection and reconstruction. Patients
with locally aggressive or malignant tumors of bone often require en bloc resections that leave a large defect requiring reconstruction. Weiland et al. (61,62)
have reported on a large series in which they used free fibula graft
for the reconstruction of defects after massive tumor resections.
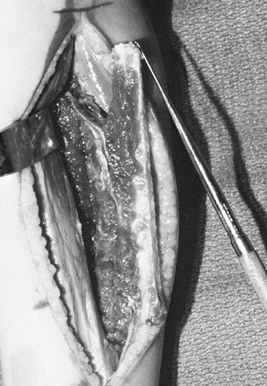
swelling, and increased deformity of his wrist and forearm over several
months’ duration (Fig. 36.1).
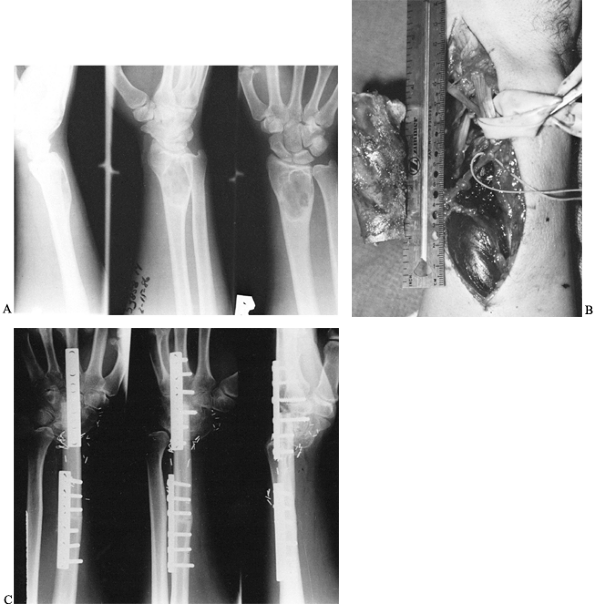 |
|
Figure 36.1. A: Radiographs of a 20-year-old man demonstrate a lytic lesion in the distal radius that has thinned and expanded the cortex. B: Intraoperative photograph demonstrating a 7-cm en bloc resection of the distal radius from a dorsal approach. C: This 4-month postoperative radiograph demonstrates sound union proximally and distally.
|
A low-grade chondrosarcoma was treated by wide local excision, leaving
a defect measuring 14 cm in the proximal femur. The defect was
reconstructed using a vascularized fibula doweled into the recipient
femur and external fixation to stabilize the graft. The graft healed
uneventfully, but the patient later required a valgus intertrochanteric
osteotomy to correct a resultant varus deformity of the proximal femur.
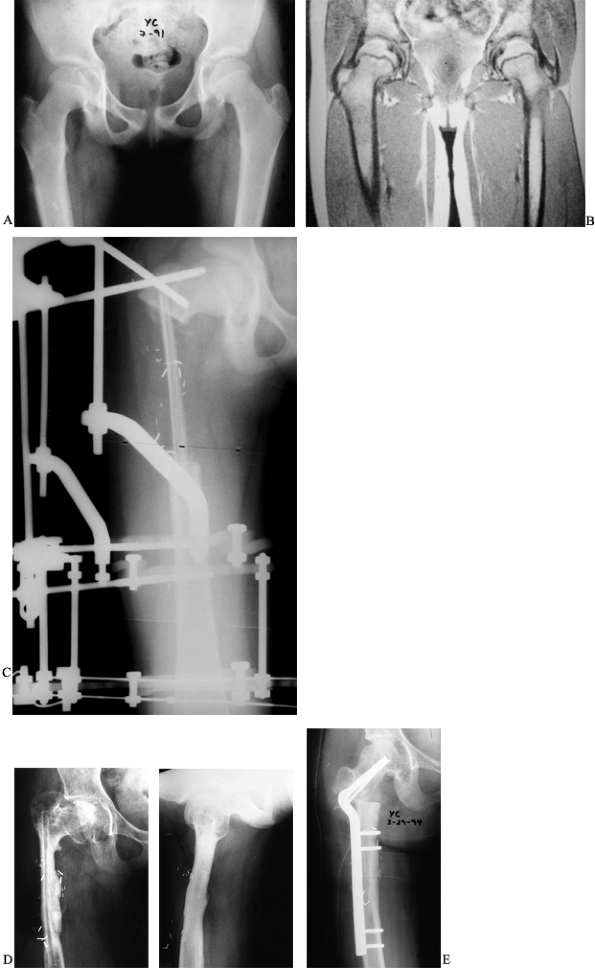 |
|
Figure 36.2. A: Radiographs of a 10-year-old girl demonstrate a lucency and periosteal reaction in the right proximal femur. B: An MRI of the femur further delineates the lesion in the proximal shaft. C:
Postoperative radiograph revealing the fibular graft and the external fixator in place. Note the doweling of the graft into the femur. D: Anteroposterior and lateral radiographs 3 years postoperatively reveal incorporation and hypertrophy of the graft but a varus deformity of the femoral neck. E: A 50° intertrochanteric valgus corrective osteotomy performed to improve neck shaft angle. |
osteomyelitis can pose management and treatment problems for many
reasons. The resection of soft tissue and bone is often too massive to
obtain adequate debridement of the infected area. Often the patient has
undergone multiple procedures including several irrigations and
debridements. As many as 18 procedures were reported in a recent study (5)
to have preceded presentation for the definitive grafting procedure.
This leaves a significantly scarred and relatively avascular soft
tissue bed to work with. The integrity of the surrounding vessels could
also be potentially compromised, leading to high risk of graft failure.
Furthermore, the potential of recurrence of the infection leading to
almost certain graft failure is a foremost concern.
that resulted in a comminuted open fracture of the proximal tibia
complicated by osteomyelitis (Fig. 36.3).
Following debridement and application of an external fixator, the
patient was left with a 13-cm bone defect, with interval healing after
a vascular latissimus dorsi flap. A vascularized fibular graft was
performed. The patient subsequently fractured at the proximal juncture
site, however, and later required an open reduction, internal fixation,
and plating before complete healing occurred.
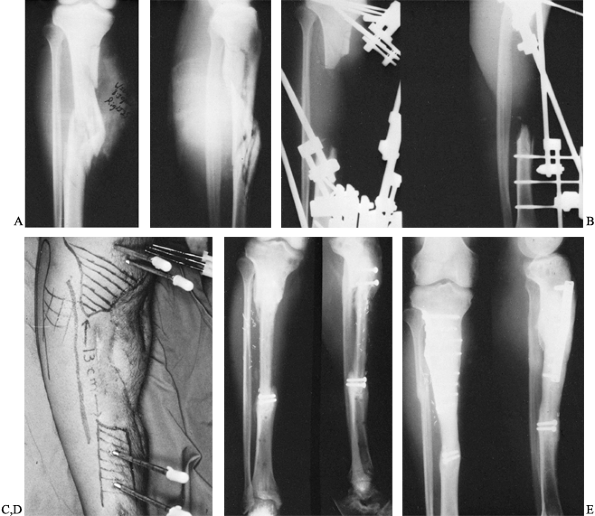 |
|
Figure 36.3. A: Radiograph of a patient who sustained a bumper-type injury that resulted in a comminuted open fracture of the proximal tibia. B: Radiographic appearance following radical debridement demonstrates a 13-cm bone defect. C: Clinical appearance of leg following free soft-tissue transfer and coverage. D: A 6-month postoperative radiograph demonstrates sound union of vascularized fibular graft proximally and distally. E:
Radiograph of patient 3 months following a repeated internal fixation performed because of a stress fracture of the proximal juncture site. |
the well-known sites are the tibia and scaphoid. The tibia is often
subjected to open injuries, and the blood supply to the area can be
easily interrupted. The anatomy of the blood supply to the scaphoid
lends itself to compromise, especially with proximal pole fractures.
These situations may not often require the massive size grafts as seen
in the previously discussed conditions; however, they still require a
nutrient blood supply in which osteocytes and osteoblasts can survive,
thus facilitating healing of the bone without the usual replacement of
the graft with creeping substitution. In the case of scaphoid
nonunions, a local pedicled vascularized bone graft will often be
adequate to treat a nonunion. Zaidemberg et al. (72)
described a vascularized graft to the scaphoid based on the radial
styloid that offers promising results. Several other authors have
reported on vascularized metacarpal bone grafts based on the branches
of the radial and ulnar arteries.
challenging problems facing the orthopaedic surgeon today. Conventional
bone-grafting methods have failed to successfully treat these patients.
Chen, Weiland, Hagan, and Bunke have all reported promising results
using vascularized free fibular grafts in the treatment of this
difficult problem. Congenial pseudarthrosis of the forearm is uncommon.
Sellers (45) reported only 23 cases after a
review of the literature. Conventional treatment has been fraught with
poor results and recurrent nonunion. Allieu (1) and
Sellers (45) both report improved results with the use of free vascularized graft for this condition.
presented with a congenital pseudarthrosis of the right tibia and
fibula that had defied several previous attempts at bone grafting and
immobilization (Fig. 36.4). An extraperiosteal
dissection and excision of the pseudarthrosis of the tibia and fibula
were performed, resulting in an 8-cm tibial defect. A buttress plate
was used to secure the graft to the tibia proximally and distally. Six
months postoperatively there was good incorporation of the fibula graft
proximally as well as distally. The patient subsequently required a
contralateral epiphysiodesis to equalize a leg length discrepancy and
an osteotomy of the tibia to correct valgus bowing.
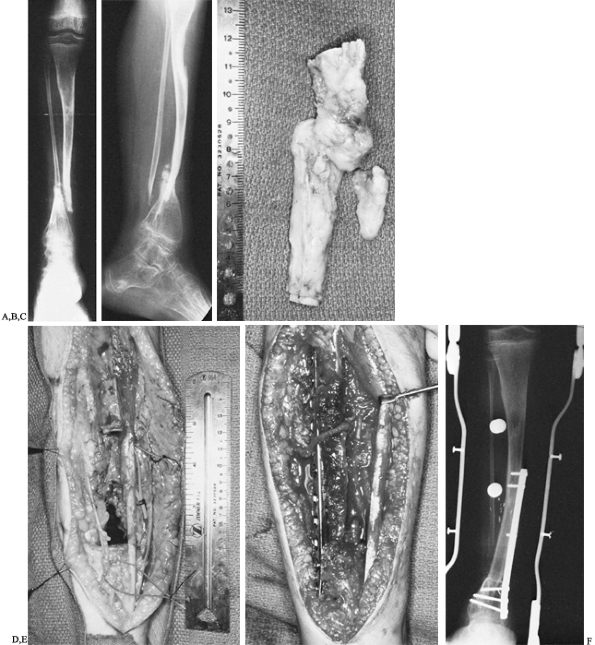 |
|
Figure 36.4. A: Anteroposterior radiograph of an 11-year-old boy with von Recklinghausen’s disease and congenital pseudarthrosis of the tibia. B:
Lateral radiograph demonstrating a proximally as well as distally bowed tibia along with marked osteoporosis of the distal tibia and foot. C: Intraoperative photograph of resected specimen, including tibial and fibular pseudarthrosis. D: Intraoperative photograph demonstrating bony defect. Vessel loops are noted around the anterior neurovascular bundle as well as the saphenous vein. E: Intraoperative photograph demonstrating buttress plate. The vascular pedicle can be seen transversing the proximal portion of the plate into the graft. F: Radiograph of leg 6 months postoperatively, demonstrating sound union of the vascularized graft. However, tibial bowing was noted. |
necrosis of the femoral head have been published. This difficult
problem often presents in the younger patient as a result of trauma,
steroid use, or alcohol use or may be idiopathic. This group of
patients is often too young for hip arthroplasty but is severely
debilitated secondary to pain and stiffness. A vascularized bone graft
may offer them a chance to retain their native hip as long as possible
while restoring some of their quality of life. Both iliac crest and
fibula have been reported in the literature as possible sites of donor
bone graft. Results have been mixed, with some authors reporting good
results and others saying that the results are no better than with
simple core decompression. Scaphoid and lunate AVN have also been
treated with vascularized bone grafts with promising results.
in microvascular techniques and be able to perform multiple types of
microvascular anastomoses that may be required in scarred or otherwise
traumatized tissue. Assess the patient, the defect, and the possible
donor sites as described elsewhere in this chapter well before the
procedure. Obtain preoperative arteriograms of both the donor and
recipient site to evaluate any vascular abnormalities that could
preclude a successful graft transfer. Be aware that a normal
arteriogram may not provide a true assessment of the arterial inflow.
Scarred blood vessels or those easily prone to spasm may appear normal
on an arteriogram. The surgeon will often be required to make
intraoperative judgments of vascular viability and should be able to
recognize
vessel damage that would interfere with successful microvascular anastomoses.
intimately related to the undersurface of the bone, is the predominant
blood supply to the fibula, supplying a nutrient artery and segmental
musculoperiosteal vessels (53). Commonly an
arteriogram of a normal leg exhibits a proximal anterior tibial artery
takeoff followed by a bifurcation of posterior tibial and peroneal
arteries. Occasionally, a separate peroneal artery does not exist,
which may preclude use of the fibula as a donor.
-
Although not essential, a two-team
approach can save considerable operative time. The surgical technique
for the donor fibula is rather constant, with the patient supine and
the hip and the knee flexed. -
Use a lateral approach to the fibula, extending from the neck of the fibula in a distal direction.
-
Identify the interval between the peroneus longus and the soleus muscles (Fig. 36.5). Incise the deep fascia the entire length of the incision.
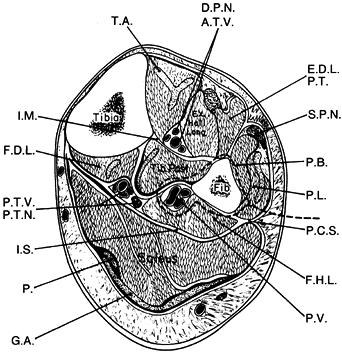 Figure 36.5. Cross section of the middle third of the lower extremity, outlining the lateral approach for harvest of the fibula (dotted line). T.A., tibialis anterior; D.P.N., deep peroneal nerve; A.T.V., anterior tibialis vessels; E.D.L., extensor digitorum longus; P.T., peroneus tertius, S.P.N., superficial peroneal nerve; P.B., peroneus brevis; P.L., peroneus longus; P.C.S., posterior crural septum; F.H.L., flexor hallucis longus; P.V., peroneal vessels; G.A., gastrocnemius aponeurosis; P., plantaris; I.S., intermuscular septum; P.T.V., posterior tibial vessels, P.T.N., posterior tibial nerve; F.D.L., flexor digitorium longus; I.M., interosseous membrane. (From Weiland AJ. Vascularized Bone Transfers. In Murray JA, ed. AAOS Instructional Course Lectures, Vol. 33. St. Louis: CV Mosby, 1984:448.)
Figure 36.5. Cross section of the middle third of the lower extremity, outlining the lateral approach for harvest of the fibula (dotted line). T.A., tibialis anterior; D.P.N., deep peroneal nerve; A.T.V., anterior tibialis vessels; E.D.L., extensor digitorum longus; P.T., peroneus tertius, S.P.N., superficial peroneal nerve; P.B., peroneus brevis; P.L., peroneus longus; P.C.S., posterior crural septum; F.H.L., flexor hallucis longus; P.V., peroneal vessels; G.A., gastrocnemius aponeurosis; P., plantaris; I.S., intermuscular septum; P.T.V., posterior tibial vessels, P.T.N., posterior tibial nerve; F.D.L., flexor digitorium longus; I.M., interosseous membrane. (From Weiland AJ. Vascularized Bone Transfers. In Murray JA, ed. AAOS Instructional Course Lectures, Vol. 33. St. Louis: CV Mosby, 1984:448.) -
Expose the lateral border of the fibula.
Approach the fibula in a proximal-to-distal direction and elevate, with
extraperiosteal dissection, the peroneus longus and brevis off the
anterior border of the fibula. -
Divide the anterior crural septum along
the length of the graft and identify and protect the deep peroneal
nerve and anterior tibial artery and vein as the extensor group of
muscles is dissected from the interosseous membrane. -
Divide the posterior crural septum the
entire length of the graft and reflect the soleus as well as flexor
hallucis muscles off the posterior border of the fibula. Preserve the
nerve to the flexor hallucis longus while performing this dissection.
Continue this dissection until the peroneal vessels are encountered. -
The nutrient artery is found in the
middle half of the fibula, usually just proximal to the midpoint. The
periosteal vessels pass circumferentially around the fibula. Preserve
them with extraperiosteal dissection. The venous drainage is via paired
venae comitantes of the peroneal artery and closely parallels it. -
Measure the length of the fibular graft
needed and mark it, taking care to preserve at least the distal 6 cm of
fibula to maintain stability of the ankle mortise. In children less
than 10 years of age, we perform a synostosis between the fibula and
tibia to prevent proximal migration of the distal fibula, which can
cause valgus ankle deformity. -
Perform the proximal and distal
osteotomies with a Gigli or power saw, taking care to place a bone
retractor between the peroneal vessels and the fibula. Retract the bone
graft posteriorly and laterally and divide the interosseous membrane
along the entire length of the bone graft. -
Retract the graft anteriorly. Dissect the
tibialis posterior muscle off the posterior aspect of the middle third
of the fibula. At this point, the fibula graft will be isolated on the
peroneal neurovascular bundle (Fig. 36.6).
Dissect the pedicle proximally until the bifurcation of the posterior
tibial artery and peroneal artery is identified. Before dividing the
pedicle, deflate the tourniquet and allow circulation to the fibula for
10 to 15 min.![]() Figure 36.6.
Figure 36.6.
The osteotomized fibula after the interosseous membrane has been
divided. The vascular pedicle of the peroneal artery and vein is
clearly visible in the proximal extent of the wound. -
The structure most at risk proximally is the peroneal nerve and its branches, which must be protected at all times.
-
The technique for preparing the recipient
site varies depending on the clinical condition being treated. In
posttraumatic cases, focus initial attention at identification
P.1218
and
protection of neurovascular structures. Failure to isolate a healthy
level of recipient vessels is the most common cause for failure of free
tissue transfer. The zone of injury often far exceeds the limits of the
bone defect in posttraumatic and infected cases. -
Resect necrotic or nonviable bone ends.
-
Rigidly fix the fibular graft into the
defect. A variety of techniques may be used, including doweling of the
end of the fibula inside the tibia, end-to-end apposition, or
bayonet-type fixation. Regardless of the position of the fibular graft,
rigid fixation is needed with external and/or internal fixation. Do not
use medullary fixation through the vascularized fibular graft to avoid
disrupting its blood supply. In most instances, pack cancellous bone
graft in and around the juncture points of the recipient and donor bone
to promote more rapid union. -
Perform the microvascular anastomoses.
Usually one artery and one vein are anastomosed. Whenever possible,
perform end-to-side arterial anastomosis so as not to compromise distal
circulation to the extremity. End-to-end vein anastomosis is standard
practice. The caliber of the recipient vein must be equal to or larger
than that of the peroneal vein so that venous hypertension does not
occur. Some authors recommend two venous anastomoses to assure adequate
venous drainage. The recipient vein may be from either the superficial
venous system or venae comitantes, as long as it is of sufficient
caliber and scar-free. -
The fibular graft, because it is devoid
of larger amounts of soft tissue or muscle, seems particularly tolerant
of ischemia lasting up to 6 h. Do not rush attempts at achieving secure
bony fixation to limit ischemia time, as secure fixation is of utmost
importance. -
Adequate circulation is assessed by a
patent and functioning anastomosis as well as by bleeding at the edges
of the muscle cuff on the fibular graft. Before wound closure,
ascertain that the vascular pedicle is not redundant, twisted, or
kinked. It is preferable to resect and tailor a functioning anastomosis
that is looped or kinked because of the potential danger of thrombosis. -
Although there is no universal agreement
on the use of anticoagulants, some surgeons find it helpful to give
intraoperative heparin before removing the vascular clamps from large
lower extremity vessels. -
Pack cancellous bone chips harvested from
the iliac crest about the proximal and distal juncture sites, insert
deep drains, and close the skin. -
Close the donor site defect in two
layers, the subcutaneous layer and the skin. Do not close the fascia,
as this may lead to a compartment syndrome. Postoperatively, continue
low-dose aspirin therapy and instruct patients not to smoke.
monitoring the vascularity to the bone graft in a noninvasive fashion.
Overlying skin islands may be taken with the fibula; however, the
presence or absence of the cutaneous circulation may not accurately
reflect the osseous circulation. Bone scans have been used in the
laboratory and clinically but are useful only for the first week after
surgery and are impractical as a sequential monitoring system. If a
scintiscan performed within the first week is negative, patent
microvascular anastomosis is unlikely (65).
weight bearing for at least 2.5 months or until incorporation and
callus around the juncture sites are seen. In the adult, lower
extremity graft incorporation usually occurs in 4 to 6 months, but it
may be wise to protect the graft site with an orthosis throughout the
first year.
vascularized fibular grafting, should perform cadaveric dissections
before surgery to refamiliarize themselves with the relationships of
neurovascular structures to the interosseous membranes. With attention
to meticulous technique, a very low incidence of donor-site morbidity
can be expected.
circumflex iliac artery (SCIA), this graft has undergone changes. In an
elegant study combining cadaver dissection, angiography, and clinical
trial, Taylor showed that the most reliable pedicle for the
osteocutaneous iliac crest graft is the deep circumflex iliac artery,
as it supplies a greater portion of the bone as well as an elliptical
area of skin over the iliac crest and the iliacus, transversus, and
internal oblique muscles as compared to the superficial system (54).
In planning this graft, the incisions, orientation of the bone, and the
choice of donor side should be carefully planned in advance. Use the
ipsilateral crest in the tibia if the recipient artery is to be the
anterior tibial; however, use the contralateral crest for the recipient
posterior tibial artery.
iliac artery (DCIA), can be harvested with or without a skin flap. If a
skin graft is taken, place the incision in the long axis along the
upper border of the anterior part of the iliac crest. The upper limit
of area of skin that can be harvested is not known currently. Areas
averaging 18 × 7.7 cm have been taken and closed primarily (55).
Skin medial to the anterior superior iliac spine (ASIS) relies more on
its blood supply from the anastomoses between the DCIA and the SCIA,
and this means there may be temporary sluggish flow to this skin, but
expect viability.
-
Place the patient supine on the table
with a small bump under the donor hip to help elevate the crest and aid
in harvesting. If skin is to be harvested with the graft, mark the area
on the skin. -
Make an incision from the femoral artery
to a point about 10 cm posterior to the anterior superior iliac spine.
A transinguinal approach to the vascular pedicle is preferred. -
Expose the external oblique muscle and
incise it in line with its fibers, approximately 3 cm superior to the
iliac crest. This edge of the muscle includes perforator branches of
the DCIA. Then curve the incision toward the ASIS and parallel to the
inguinal ligament to enter the inguinal canal. Next, identify the
spermatic cord or round ligament and retract upward and medially. The
external iliac artery can be palpated through the transversalis fascia. -
Incise the fascia and identify the DCIA
and vein. They are most easily identified where they converge toward
each other about 1 to 2 cm lateral to the external iliac artery. Trace
the DCIA vessels laterally, dividing the transversalis fascia, internal
oblique, and transversus abdominis from the inguinal ligament. As the
ASIS is approached, it may become easier to identify the ascending
branch lateral to the ASIS and then trace it medially to its origin
from the SCIA by incising the internal oblique muscle 3 cm above and
behind the ASIS. -
To isolate the bone, incise the
transversus muscle parallel to the iliac crest, leaving a minimal rim
of muscle on the bone. Incise the transversalis fascia, retract the
extraperitoneal fat, and expose the line between the transversalis and
the iliacus fascia. Incise the iliacus 1 cm medial to this line, thus
exposing the periosteum of the iliac fossa. Bluntly dissect the iliacus
muscle away from the remainder of the bone. -
Incise the lower skin border and cut the
attachment of the tensor fasciae latae and glutei muscles sharply from
the bone. Divide the inguinal ligament and the origin of the sartorius
muscle just medial to the ASIS. -
Osteotomize the bone as measured to
isolate the flap on its vascular pedicle. Allow the flap to sit for 20
min to assure viability before sectioning the pedicle. -
The lateral cutaneous nerve of the thigh
will be encountered in close relation to the DCIA. The nerve may have
to be sectioned to remove the graft, but attempt to preserve it. -
At the lateral margin of the iliac crest,
use an oscillating saw to cut through the crest, creating a graft 2.5
cm in depth (wide). Then continue the cut medially with the
osteocutaneous flap finally isolated on its vascular pedicle,
consisting of the deep circumflex iliac artery and vein. -
Using sutures widely placed at the edges
of the graft, carefully preserve the attachment of the overlying skin
and subcutaneous tissue to the iliac crest to avoid accidental shearing
of the small nutrient vessels supplying the skin. -
Because of the curvature of the ilium,
grafts longer then 10 cm usually cannot be obtained. A portion of the
iliacus must be retained on the inner table in order to preserve the
nutrient blood supply to the bone. Close the donor site primarily.
Harvest cancellous graft for use at the junction sites before closing
the donor site. The hip may be flexed about 30° by flexing the table to
facilitate closure of the defect. -
To avoid abdominal herniation, suture the
iliacus fascia and muscle to the transversalis fascia and muscle. Next,
suture the internal and external oblique muscles to the glutei, the
fascia lata, and its muscle. Repair the inguinal canal and reattach the
inguinal ligament laterally. -
Internally fix the graft into the
recipient site; there is healthy bone on either end of the defect.
Plates can often easily be used to fix the graft but should not lie on
top of the nutrient artery. Fixation into the graft itself should be
limited and avoid the nutrient artery and pedicle. Two plates can be
used at either end or T-plates if a metaphysis is involved. Use no more
than two screws in the graft. The use of Kirschner wires and screws
alone has been reported (51) but is often associated with loss of position at the junction site. -
If adequate bone stock is not available for internal fixation, use external fixation (19,27,31).
Almost any external fixator can be used, and the choice is dependent on
the anatomic site, bone quality, and functional demands. Ilizarov-type
frames offer multiplanar control and variation as to configuration,
whereas the others offer ease of application. It has been used in many
defects involving transfer of free vascularized grafts including the
humerus, forearm, hand, femur, tibia, and foot for defects ranging from
trauma and tumor to infection and congenital deformities (19). -
Regardless of the frame used, it must not
block access to the microvascular anastomoses. The pins or wires can be
carefully passed through the graft, always protecting the nutrient
artery and pedicle. Pack cancellous graft around the junction sites and
close the wound over drains. -
Healing of the graft can be expected in 8 to 12 weeks, roughly the same as healing rate for fractures at the same site.
that the posterior intercostal vessels give rise to the principal
nutrient vessel of the ribs, whereas the anterior intercostal arteries
supply mainly the periosteum of the rib. The graft, therefore, is based
on the posterior vessels (10). The graft can survive on the anterior pedicle, but the bone may show some necrosis.
-
Measure the pattern of the defect and
trace out the pattern on the chest. Make a posterior transverse
incision between two ribs (10). -
Identify the intercostal vessels
posteriorly at the beginning of the dissection and develop the flap
margins of the underlying rib in an anterior direction. -
Expose the rib and cut a segment sized to
the bony defect. Dissect the vessels back to the pedicle and carefully
ligate them. Then transpose the graft to the defect and secure it.
Close the donor defect primarily over drains.
only when the intramedullary blood supply is maintained via the
nutrient artery, which is a branch of the posterior intercostal artery.
Thus, the rib must be isolated on its nutrient artery. This requires a
complicated posterior dissection within 3 cm of the costovertebral
joint rather than a simple lateral segmental excision. This location
will often obligate ligation of the adjacent dorsal branch of the
intercostal artery, which risks transection of the artery of
Adamkiewicz. This vessel arises between T-7 and L-1 and is the
principal supply to the thoracolumbar segment of the spinal cord. Loss
of this can result in permanent paraplegia. Preoperative angiography is
recommended; however, the sixth rib should be selected when
arteriography is not possible.
to be difficult to treat. Conventional methods of treatment including
bone grafting and internal fixation have resulted in reports of
successful union in 90% of patients (14,22,36,44,47,72).
The period of immobilization is long, however, and the 10% of cases
that are refractory despite conventional procedures present especially
difficult treatment dilemmas. The vascularized bone graft offers the
advantages of decreased times of immobilization and a high union rate
for these refractory nonunions (12,32,33,72).
Zaidemberg has most recently described a technique using a vascularized
graft from the distal dorsoradial aspect of the radius based on a
consistent retrograde branch from the radial artery that has shown good
results and is technically straightforward (72).
-
Perform a dorsal approach to the wrist by
making an oblique incision on the radiodorsal aspect of the wrist. Take
care to avoid injury to the dorsal sensory branch of the radial nerve. -
Divide the extensor retinaculum. Retract
the first dorsal compartment containing the extensor pollicis brevis
and abductor pollicis longus tendons palmarly. Reflect the extensor
carpi radialis longus and finger extensor tendons ulnarly. The
longitudinal course of the vessel is easily identified overlying the
distal radius. Design a vascularized bone graft centering this
perfusing periosteal blood vessel in the bone to be harvested. -
Visualize the nonunion of the scaphoid
and freshen the sclerotic bone ends. Reduce the fracture. If adequate
reduction of the scaphoid fracture cannot be achieved, then a combined
palmar approach may be necessary to reduce the scaphoid fracture before
bone grafting. -
Fashion a trough 15 to 20 mm in length running parallel to the long axis of the scaphoid.
-
Harvest a bone graft corresponding in
size to the cavity created in the scaphoid from the distal radius
underlying the vascular pedicle. The bone graft is then easily
transposed to the recipient scaphoid site. Secure the bone graft with
Kirschner wires. -
If additional cancellous bone graft is
needed, it can be harvested through the same distal radius site. A
monitoring island of skin can be raised with the bone for postoperative
documentation of vascularity if desired, as the periosteal vessel does
continue into the skin overlying the bone. -
Close and apply a long arm cast with
thumb spica for 1 month, followed by a short arm cast with thumb spica
for 2 weeks. At 6 weeks, use radiographs to assess union. When stable
bony union is certain, begin range-of-motion exercises.
other surgery, such as infection, wound problems, and others. Use good
surgical technique to minimize these.
occur when a fibula is harvested as a result of dissection of its
origin off of the fibula, damage to its nerve, or postoperative
scarring. Avoid loss of FHL function and peroneal nerve palsy by
identifying and protecting these structures when necessary and avoiding
unnecessary dissection into the muscles surrounding the fibula. When
the fibula is harvested, a major vessel to the leg is sacrificed, but
this rarely leads to any problems.
cutaneous nerve to the thigh may need to be sacrificed. Advise patients
about this ahead of time. Iliac grafts may be bulky. Minimize bulk by
placing the grafts in the defect in the coronal plane. Secondary
debulking may be necessary.
for multiple clinical situations where obtaining bone union has
traditionally been difficult. However, the ability to perform such a
procedure and the practical nature of doing this are two different
situations. It is our opinion that the vascularized fibular graft is
most useful for defects of the tibial and for avascular necrosis of the
femoral head. The use of iliac crest grafts is somewhat more limited
because of the more curved nature of the bone and the morbidity of the
donor site. It should be considered if the fibular graft is not an
option for a patient because of trauma or previous harvesting. Rib
grafts have very limited use in orthopaedic surgery and are used only
as a last resort to the other graft sites. Their curved nature makes
them very amenable to use in the mandible. The dorsal/radial graft is
useful for nonunions of the scaphoid, but we would recommend its use
only for selected proximal pole nonunions and only after failed open
reduction, internal fixation, and standard bone grafting, as it is more
involved and requires greater dissection on the dorsal aspect of the
wrist.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
Y, Gomis R, Yoshimura M, et al. Congenital Pseudarthrosis of the
Forearm. Two Cases Treated by Free Vascularized Fibular Graft. J Hand Surg 1981;6:475.
A, Weiland AJ, Dorfman HD. Free Vascularized Bone Grafts: Factors
Affecting Their Survival and Ability to Heal to Recipient Bone Defects.
Plast Reconstruct Surg 1982;69:19.
A, Weiland AJ, Östrup LT, Dorfman H. Microvascular Free Bone Transfer
with Revascularization of the Medullary and Periosteal Circulation or
the Periosteal Circulation Alone. J Bone Joint Surg 1982;64A:73.
A, Weiland AJ, Östrup LT. Bone Scintigraphy in Evaluating the Viability
of Composite Bone Grafts Revascularized by Microvascular Anastomosis,
Conventional Autogenous Bone Grafts and Free Nonrevascularized
Periosteal Grafts. J Bone Joint Surg 1982;64A:799.
SE, Heller JG, Petersilge CA, et al. Tibial Stress Fracture after a
Graft has been Obtained from the Fibula: A Report of Five Cases. J Bone Joint Surg 1996;78A:1248.
IG, Golubev VG, Goncharenko IV, et al. Transfer of Free Vascularized
Bone and Skin Bone Autografts: Experiences on the Application of
External Fixation Apparatus. J Reconstruct Microsurg 1990;6:1.
IG, Golubev VG, Goncharenko IV, et al. Transfer of Free Vascularized
Bone and Skin-Bone Autografts: Experiences in the Application of
External Fixation Apparatus. J Reconstruct Microsurg 1990;6:1.
Y, Iwata H, Torii S, et al. Vascularized Pedicle Bone-grafting for
Nontraumatic Avascular Necrosis of the Femoral Head: A 5 to 11 Year
Follow-up. Arch Orthop Trauma Surg 1997;116:251.
GA. The Tension-Stress Effect on the Genesis and Growth of Tissues.
Part I. The Influence of Stability of Fixation and Soft Tissue
Preservation. Clin Orthop 1989;238:249.
H, Torii S, Hasegawa Y, et al. Section III: Basic Science and
Pathology: Indications and Results of Vascularized Pedicle Iliac Bone
Graft in Avascular Necrosis of the Femoral Head. Clin Orthop 1993;295:281.
JB, Gerhard HJ, Guerrero J, et al. Treatment of Segmental Defects of
the Radius with Use of the Vascularized Osteoseptocutaneous Fibular
Autogenous Graft. J Bone Joint Surg 1997;79A:542.
JN, Mimoun M, Boabighi A, Baux S. Vascularized Bone Graft Pedicled on
the Volar Carpal Artery for Non-union of the Scaphoid. J Hand Surg 1987;12B:203.
T, Hillmann A, Wuisman P, Winkelmann W. Reconstruction of Tibia by
Ipsilateral Vascularized Fibula and Allograft: 12 Cases with Malignant
Bone Tumors. Acta Orthop Scand 1997;68:298.
HH, Rickard TA, Zemel NP, Ashworth CR. Treatment of Ununited Fractures
of the Scaphoid by Iliac Bone Grafts and Kirschner-Wire Fixation. J Bone Joint Surg 1988;70:982.
H, Gori Y, Tatsumi Y, et al. An Experience of Vascularized Fibula Head
Transplantation in a Child with Radial Club Hand (in Japanese). Seikeigeka 1981;32:1645.
GI, Townsend P, Corlett R. Superiority of the Deep Circumflex Iliac
Vessels as the Supply for Free Groin Flaps: Experimental Work. Plast Reconstruct Surg 1979;64:595.
GI, Townsend P, Corlett R. Superiority of the Deep Circumflex Iliac
Vessels as the Supply for Free Groin Flaps: Clinical Work. Plast Reconstruct Surg 1979;64:745.
AJ, Berggren A, Jones L. The Acute Effects of Blocking Medullary Blood
Supply on Regional Cortical Blood Flow in Canine Ribs as Measured by
the Hydrogen Washout Technique. Clin Orthop 1982;165:265.
AJ, Phillips TW, Randolph MA. Bone Grafts: A Radiologic, Histologic,
and Biomechanical Model Comparing Autografts, Allografts, and Free
Vascularized Bone Grafts. Plast Reconstruct Surg 1984;74:368.
AJ, Weiss APC, Moore JR, Tolo VT. Vascularized Fibular Grafts in the
Treatment of Congenital Pseudarthrosis of the Tibia. J Bone Joint Surg 1990;72A:654.
A, Isiklar ZU, Tuncay C, Tandogan R. Treatment of Scaphoid Nonunions
with a Vascularized Bone Graft Based on the First Dorsal Metacarpal
Artery. J Hand Surg 1997;22B:425;1997.