PREOPERATIVE PLANNING AND PERIOPERATIVE MANAGEMENT
– SURGICAL PRINCIPLES AND TECHNIQUES > CHAPTER 5 – PREOPERATIVE
PLANNING AND PERIOPERATIVE MANAGEMENT
patient’s satisfaction and functional outcome. Not only must the
procedure be performed technically well, but also all the associated
factors, from preoperative evaluation to postoperative therapy and
follow-up, must be efficient and well coordinated.
the patient’s medical and social situation to minimize the risks of
perioperative complications and problems. Preoperative clearance may
require consultation with a medical specialist or anesthesiologist. It
may be necessary to coordinate autologous blood donations.
Additionally, it may be necessary to make discharge plans, which could
involve home care or transfer to a rehabilitation or skilled nursing
facility.
frequency and duration of inpatient hospitalization decline. Patient
education prepares patient and family for the anticipated surgery and
the postoperative recovery period. Patients’ understanding of their
role in this process is important to a successful outcome.
perform efficiently without sacrificing quality. With preoperative
planning, the surgeon thinks through the operation ahead of time, which
allows the operation to run more smoothly and quickly and provides
important time-management benefits.
demands increased preoperative preparation. For example, there are now
a multitude of implants available for surgeons to choose from.
Preoperative preparation enables the surgeon to select the optimal
implant for a given patient and become familiar with its use, and to be
sure that all necessary equipment is available for the procedure. Being
well prepared includes having backup plans to handle any contingencies
that might arise.
Careful perioperative management can help minimize their occurrence,
thus increasing the patient’s chances for a successful outcome. It can
also provide a solid medical-legal defense should any serious
complication arise.
consultation may be necessary to manage chronic medical conditions that
may be exacerbated by surgery. Orthopaedists can anticipate treating a
larger proportion of elderly patients, many of whom have multiple
chronic medical problems and decreased functional reserves.
can help ensure that all possible conditions are covered. Written
questionnaires, some of which can be computer coded, are also
available. If written forms are used to collect medical data, review
them carefully with the patient to ensure that the history is accurate
and complete. Make certain that the patient understands the
terminology; for example, a patient who has had several
“heart
attacks” may answer no to questions about myocardial infarctions
because he is unfamiliar with the form’s medical terminology.
 |
|
Table 5.1. Outline for Review of Current and Past Medical History
|
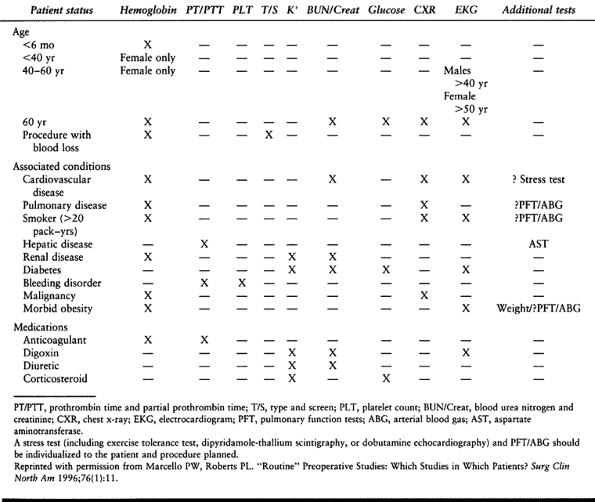
medical conditions, to screen for asymptomatic conditions, and to
identify patients who are at increased risk of adverse postoperative
outcomes (152). Performing standard testing
(e.g., electrocardiogram, chest x-ray, blood chemistries) on all
preoperative patients has been shown to be not only unnecessarily
costly, but also of limited benefit in identifying patients with
potential medical problems. Instead, current practice calls for
individualized evaluation and presurgical testing based on each
patient’s medical history, family history, age, and physical
examination (Table 5.2) (72,90).
Although the value of routine screening tests in predicting
postoperative complications has also been shown to be extremely low (72),
abnormalities in electrocardiograms, chest x-rays, and nutritional
status have been shown to be associated with postoperative
complications in a general surgical population (152).
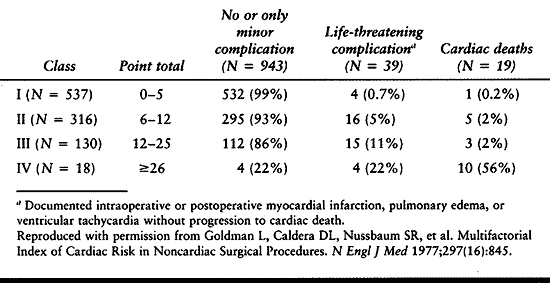
Velanovich found a strong association between postsurgical
complications and the American Society of Anesthesiologists’
classification used to assess patients’ anesthetic risk (Table 5.3) (152).
 |
|
Table 5.2. Recommended Preoperative Testing for Elective Surgery
|
 |
|
Table 5.3. The American Society of Anesthesiologists’ Clinical Classification System
|
patient populations, has a significant impact on perioperative
morbidity and mortality. Question patients specifically
about
chest pain and shortness of breath on moderate exertion, in addition to
any history of myocardial infarction. Physical examination findings
that may suggest congestive heart failure include jugular venous
distention, rales, or an S3 gallop.
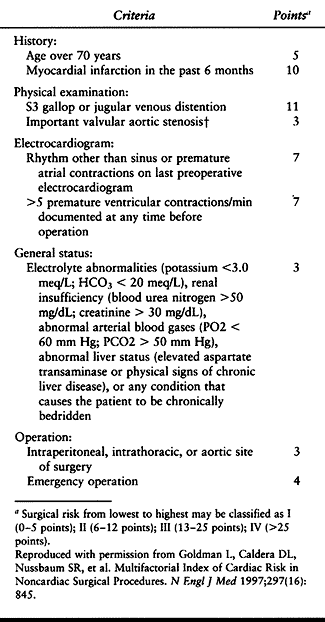
Patients with unstable angina, severe aortic stenosis, or a 6-month
history of myocardial infarction are at highest surgical risk. Medical
evaluation of patients with cardiac disease may call for preoperative
echocardiograms and stress tests, which usually require advance
scheduling.
 |
|
Table 5.4. Calculation of Multifactorial Cardiac Risk Index
|
 |
|
Table 5.5. Cardiac Risk Index
|
to increase surgical risk, but it may be associated with other risk
factors, such as cardiac disease, that can contribute to perioperative
complications. Patients can continue to take most antihypertension
medications during the perioperative period. Carefully monitor the
volume status and electrolyte balance of patients taking diuretics.
those who are morbidly obese, and those with asthma and chronic
pulmonary disease (99). Patients with significant hypercapnia (elevated CO2) and an FeV1 (forced expiratory volume in 1 sec) of less than 1 liter are at greatest risk of pulmonary complications (6).
Evaluation of postoperative arterial blood gas (ABG) results can be
difficult in these patients when there is a question of possible
pulmonary embolism. A baseline preoperative ABG is helpful if patients
become symptomatic after surgery.
stops smoking in the weeks preceding surgery. Preoperative instruction
in coughing and deep breathing exercises is also helpful. Use of
narcotic analgesics, which can depress the respiratory system, should
be minimized when
possible
to decrease postoperative pulmonary complications. The use of
continuous epidural anesthesia may be beneficial for patients with
pulmonary disease undergoing lower extremity surgery.
volumes, common in the postsurgical patient, may result in significant
problems for patients with underlying pulmonary disease. Use incentive
spirometry, and administer inhaled bronchodilating agents as soon as
any sign of bronchospasm develops.
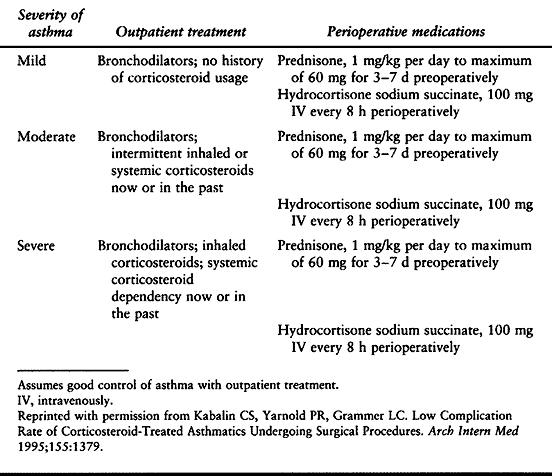
of bronchodilators and inhaled corticosteroids. In some cases,
intravenous corticosteroids may also be indicated. Guidelines have been
set for the preoperative medication of asthmatic patients based on the
severity of their disease (Table 5.6) (71).
 |
|
Table 5.6. Recommended Preoperative Medication for Surgery in Asthmatic Patients, Stratified by Asthma Severity
|
Volume status, blood pressure, and electrolyte abnormalities must be
carefully managed. These patients are at risk for developing
hyperkalemia leading to cardiac arrhythmias. Patients with end-stage
renal disease may have uremic platelet dysfunction leading to increased
bleeding and a higher risk of infection. Dosages of medications
excreted by the kidneys need to be adjusted in these patients. Avoid
the use of nephrotoxic medications, which might cause further renal
damage.
severely volume depleted and hypotensive. The postsurgical risk is
particularly great in patients with limited reserve, such as the
elderly and those with diabetes, congestive heart failure, or
preexisting renal disease. Rhabdomyolysis leading to acute renal
failure is a risk in trauma patients who sustain a significant crush
injury or extremity ischemia. Laboratory findings include myoglobin in
the urine and elevated skeletal muscle creatine kinase. Treatment
includes alkalinization of the urine with sodium bicarbonate,
intravenous furosemide (Lasix), and intravenous mannitol. Carefully
monitor electrolytes because these patients may become hyperkalemic.
patients with liver disease increases with the severity of their
disease. Patients who have evidence of hepatic decompensation
(hypoalbuminemia, coagulopathy, ascites, encephalopathy) are at the
greatest risk. In patients with cirrhosis, the risk correlates with
Child’s classification. Elective surgery is contraindicated in patients
with decompensated cirrhosis (Child’s class C), acute alcoholic
hepatitis, and acute viral hepatitis. Patients with chronic hepatitis
without evidence of cirrhosis or hepatic decompensation generally do
not have an increased complication risk (42).
system in patients with liver disease, since they may also have
impaired hemostasis. Closely monitor the fluid status, electrolyte
balance, and blood pressure of these patients, and avoid using
potentially hepatotoxic medications. Disturbances in hepatic function
may lead to prolonged action of anesthetic agents that are metabolized
in the liver. Sedatives, narcotic analgesics, and intravenous induction
agents should be used with caution, as they may cause a prolonged
depression of consciousness and precipitate hepatic encephalopathy if
used in standard doses. Resistance to curare-like neuromuscular
blocking agents
may require large dosages for effectiveness, leading to problems with anesthetic reversal after surgery.
A patient may have severe cardiac disease with a history of silent
myocardial infarctions without being aware of it. Patients with
diabetes mellitus also have increased risk for postoperative infections
and problems with wound healing. Most experts recommend that surgery be
done on diabetic patients early in the day to simplify insulin
administration and glucose monitoring (64).
diabetes are usually managed after surgery with fingerstick glucose
levels every 6 hours and a sliding-scale insulin dosage for blood
sugars greater than 250 mg/dl. It may be necessary to hold long-acting
oral hypoglycemics preoperatively to decrease the risk of hypoglycemia.
diabetes must be carefully monitored after surgery for development of
ketoacidosis. Type I diabetics require both insulin and glucose while
fasting. Provide intravenous fluids containing dextrose while they are
fasting and either sliding-scale subcutaneous or continuous intravenous
insulin. Patients with type II adult onset diabetes are not at risk for
ketoacidosis. They may be managed by either sliding-scale subcutaneous
or continuous intravenous insulin (45). While
many treatment regimens have been suggested for insulin-dependent
patients, on the morning of surgery we generally hold the patient’s
regular insulin and give one half of the usual dose of long-acting
insulin, while administering an intravenous infusion of 5% dextrose.
with steroids may require supplemental steroid doses for stress during
the perioperative period. Patients who have been treated with steroids
for a year prior to surgery may have suppression of the
hypothalamic-pituitary-adrenal axis. Normally, the adrenal glands
respond to surgical stress by producing additional corticosteroids,
equivalent to 300–400 mg of hydrocortisone per day, for the first 2
days after surgery. Patients whose adrenal response is
suppressed are unable to produce the necessary corticosteroids; without supplementation, they may develop an adrenal crisis.
more can be assumed to have adrenal suppression and require
perioperative steroids. These patients should receive hydrocortisone
hemisuccinate, 100 mg intravenously every 6–8 hours, beginning
preoperatively and continuing for 72 hours after surgery. Other
patients who may be at risk include those who have been on higher doses
of prednisone (20 mg daily) at some point during the year before
surgery, and even some patients who take chronic daily doses of less
than 7.5 mg. These patients should undergo a cosintropin (synthetic
adrenocorticotropic hormone) stimulation test to measure their adrenal
response. If a patient’s serum cortisol level rises above 20 mg/dl at 1
hour after injection of 25 units of cosintropin, then the adrenal axis
is not suppressed, and perioperative steroid supplementation is not
required (6) .
severe disease of the cervical spine. Obtain lateral flexion and
extension radiographs of the cervical spine to rule out instability,
which could result in neurologic injury during intubation or operative
positioning.
immunodeficiency virus (HIV) have extended the length of time before
infected patients advance to acquired immunodeficiency syndrome (AIDS).
It is expected that an increasing number of asymptomatic HIV-positive
patients will require orthopaedic surgical procedures. Issues
concerning the risk of transmission to the surgeon are addressed later
in this chapter (see the section on Surgeon’s Safety).
expressed concern that the suppressive effect of anesthesia and
surgical trauma on cellular immunity could be detrimental to the
immunologic competence of patients with HIV infection. Initial concerns
about potential disease progression as a result of surgery were based
mainly on emotional preconceptions and anecdotal misconceptions (129).
increased postoperative infection rate in HIV-positive patients. In
general, the risk of postoperative infection in patients with HIV who
have not advanced to AIDS is not higher than that in the general
population (88). The risk of surgical
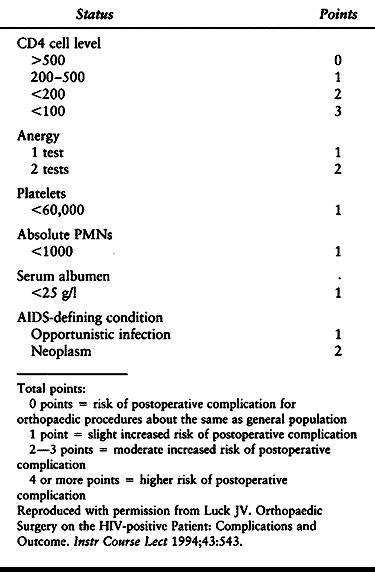
complications increases with the progression of disease. Factors
reported to have the greatest correlation with outcome risk are a
history of opportunistic infection, a CD4 level less than 200, a serum
albumin less than 25 g/l, and cutaneous anergy (88). In a review of orthopaedic surgery in the HIV-positive patient, Luck (88) provides a surgical-risk rating system (Table 5.7).
Attempts to correlate surgical outcome with CD4 lymphocyte counts alone
has provided conflicting results; it is now generally accepted that CD4
counts alone are not a predictor of poor surgical outcome.
 |
|
Table 5.7. Surgical Risk-rating System for HIV-positive Patients
|
healing complications has been reported to be increased in patients
with AIDS, but it is generally not increased in patients who are HIV
positive without AIDS. In hemophilic patients, the incidence of late
infection of prosthetic joints has been reported to be increased in
patients who are also HIV positive, especially when their CD4 count is
less than 200 (119,148).
The incidence of late prosthetic joint infections in patients with
hemophilia was higher than in the general population prior to the HIV
pandemic. The infection risk may be even higher in the HIV-positive
hemophilic patient, but current studies have not shown a
statistically
significant increase. At this time, there are no data to show that this
risk applies to the HIV-positive nonhemophilic patient with a
prosthetic joint (88a).
patients with advanced-stage AIDS who must undergo surgery. Patients
who have an unacceptably low white blood cell count (absolute
polymorphonuclear leukocyte count < 1,000) may be treated with
granulocyte-stimulating factor. Measles, mumps, and rubella vaccine
should be updated 2 weeks before surgery in patients who are anergic (88).
Many of the medications used to suppress HIV and to provide prophylaxis
against AIDS-related opportunistic infections suppress bone-marrow
function (88). As a result, AIDS patients may have chronic anemia that may benefit from treatment with erythropoietin (62). Patients with thrombocytopenia (platelet count < 60,000) should receive a platelet transfusion immediately before surgery.
effect on the healing of wounds and fractures, as well as on
immunocompetence (138). Protein-calorie
malnutrition is associated with depression of multiple aspects of the
immune system response, including decreases in polymorphonuclear
phagocytosis and bactericidal activity, circulating opsonins,
complement levels, cell-mediated immunity, lymphocyte blastogenic
responses to mitogen, number of T lymphocytes, function of T-helper
cells, new antibody synthesis, and secretory IgA levels (1).
surgical intervention; they will likely benefit from increased
postoperative nutritional support. Trauma and elective surgery both
markedly increase a malnourished individual’s nutritional needs.
In addition, nicotine is suspected of increasing the risk of
postoperative wound infections. Studies have recommended that tobacco
use be discontinued from a half day to a week prior to surgery, and for
at least a week after surgery, to minimize nicotine’s adverse effects
on wound healing (80).
result of alcohol abuse, and the problem extends to patients in all
communities. While it may be easy to identify the inebriated trauma
patient, it may be far more difficult to identify alcohol abuse in a
businessperson seeking a routine total joint arthroplasty.
Approximately 10% of chronic alcoholics who abruptly discontinue
drinking may develop significant alcohol withdrawal, possibly
associated with a hyperkinetic state, hallucinations, and seizures.
Consider treating all patients with suspected alcohol abuse with
benzodiazepines, thiamine, and multivitamins. Advanced liver disease in
chronic alcoholics may lead to bleeding disorders. Obtain a prothrombin
time to screen for coagulation disorders, a platelet count to rule out
thrombocytopenia, and a bleeding time to rule out platelet dysfunction.
Withdrawal from cocaine is not medically dangerous; the half-life of
cocaine is approximately 90 min, allowing most surgery to be delayed
until after the period of acute intoxication. Cocaine abuse has been
associated with several cardiac complications, and chronic abuse may
accelerate coronary atherosclerosis, interstitial myocardial fibrosis,
and congestive heart failure. Chronic abuse may also lead to recurrent
pneumonia, pulmonary barotrauma, diffuse alveolar hemorrhage, and
asthma (140).
illicit drug use. Patients with chronic preoperative pain may have
developed dependence on opioids, a class of drugs that includes heroin,
morphine, codeine, oxycodone, and meperidine. Withdrawal symptoms may
develop as the patient’s postoperative analgesia is reduced.
To make the diagnosis of opioid dependence, the physician must observe at least three signs and symptoms of opioid withdrawal (Table 5.8).
Treatment of opioid withdrawal may require methadone, a long-acting
opioid, from which the patient may be weaned in an outpatient drug
dependency program. Clonipine 0.1–0.3 mg may also be helpful in
reducing the signs of opioid withdrawal, especially in patients with
hypertension (140).
 |
|
Table 5.8. Signs and Symptoms in Opioid Withdrawal
|
oxazepam, diazepam, and chlordiazepoxide) is extremely common and may
lead to physiologic dependence and withdrawal. Signs and symptoms of
withdrawal may include lethargy, ataxia, irritability, dysphoria,
fatigue, tremor, and, rarely, seizure. Place patients who develop
withdrawal symptoms on a longer-acting benzodiazepine (diazepam,
chlordiazepoxide), which can be slowly tapered over 6–12 weeks.
Propranolol, 20 mg every 6 hours, can be used for the treatment of
hypertension, tachycardia, and anxiety that may occur during
benzodiazepine withdrawal (140).
become the focus of intense interest largely in response to concerns
about posttransfusion disease and its consequences. Transfusion of
donated blood can be minimized through the use of predonation
autologous blood and through perioperative blood salvage (8).
requires an audit of the appropriateness of all transfusions as part of
its hospital accreditation process. Failure to obtain informed consent
and inappropriate or unnecessary transfusion have even become the focus
for plaintiff’s attorneys in cases of posttransfusion AIDS. The
patient’s physician, not the blood bank, has been the target of
litigation in these cases (96).
emergency or elective cases involving large amounts of blood loss. As
the physicians responsible for prescribing transfusions, they must
therefore be thoroughly familiar with the indications for specific
blood components as well as the dangers of blood product transfusion.
of fractionated whole blood) is based on the concept that patients are
best treated by administration of only the specific blood product they
require. The use of pooled blood products from multiple donors has been
reduced to minimize the risk of disease transmission.
most of the plasma from a donor unit of whole blood. A unit of PRBC
contains the same amount of hemoglobin as a unit of whole blood, but
its hematocrit, depending on the preservative solution, is at least
doubled (to 70%). Transfusion of PRBC rather than whole blood allows
the recipient to receive the equivalent number of red cells without
unnecessary expansion of the plasma volume. The use of PRBC allows
blood banks to use supernatant cells and plasma to prepare other
components. Neither whole blood nor PRBC is sterilized, so any
plasma-or cell-associated organisms not detected by donor screening may
be transmitted to the recipient by transfusion of these products.
delivery, particularly delivery to vital organs, is inadequate. A
threshold for transfusion based on a given hemoglobin level cannot be
set, since oxygen delivery depends on several factors, including
hemoglobin level, inspired oxygen concentration, cardiac output, and
pulmonary gas exchange. Increased cardiac output in a young, healthy
patient may easily compensate for a low hemoglobin level, but an older
patient with cardiac disease or one who is taking beta-blocker
medication may be unable to increase cardiac output and will require
transfusion.
requires a PRBC transfusion include the patient’s physiologic state,
underlying medical condition, ability to compensate for diminished
oxygen-carrying capacity, oxygen requirements of vital organs, and
ongoing and anticipated blood loss (5).
plasma separated from one unit of whole blood obtained from a single
donor. It contains normal plasma levels of labile (factors V and VIII)
and stable clotting factors, albumin, and gamma globulin. ABO
compatibility testing is mandatory for all recipients, and Rh
compatibility testing is recommended for women of child-bearing age, as
the small number of red cells that may be present in an Rh-positive
unit could sensitize the Rh-negative patient. FFP is indicated for
patients with plasma coagulation factor deficiencies, including those
who are undergoing a massive transfusion and those with a heritable
coagulation factor deficiency or coagulopathy secondary to severe liver
disease. FFP is also indicated when immediate correction of
anticoagulation due to warfarin is required, for replacement
transfusion in plasmapheresis, for immunodeficient patients, and for
patients with thrombotic thrombocytopenic purpura. The approximate
volume of each unit of FFP is 200–175 cc. Larger-volume jumbo units,
approximately 400–600 cc, are obtained from a single donor using
apheresis. FFP should be administered immediately after thawing, with
the dose guided by coagulation testing.
The maximal effect of FFP declines within 2 to 4 hours after administration.
solvent/detergent plasma. This component is prepared from pools of
2,500 donor units, and it is treated with a solvent, tri-(N-butyl)
phosphate, and a detergent, triton X-100, to inactivate lipid-enveloped
viruses, including hepatitis B, hepatitis C, and HIV. Its drawbacks
relate to the pooling process, which increases patient exposure to
non-lipid-enveloped viruses such as hepatitis A and parvovirus B19.
Solvent/detergent plasma is indicated for the rapid reversal of
warfarin anticoagulation and therapeutic plasma exchange for
microangiopathic hemolytic anemia. Its approximate volume is 200 cc and
it must be used within 24 hours of thawing.
precipitate that forms during slow thawing of FFP at refrigerator
temperature. It contains fibrinogen, fibronectin, and factor VIII. One
unit of cryoprecipitate contains approximately 10 ml of plasma and an
average of 200 mg of fibrinogen, or 80–120 units of factor VIII
activity. Cryoprecipitate is especially useful as a source of
fibrinogen in consumptive coagulopathies. It is also indicated for the
treatment of patients with hypofibrinogenemia and those with mild
hemophilia A. Patients with severe classic hemophilia are treated with
factor VIII concentrate. In view of the changing availability of stored
coagulation products and the increased risk associated with the
administration of some of these products, we recommend consultation
with a hematologist for the treatment of any complex coagulopathy.
or in packs that contain a pool of platelets from about six to ten
donors. Administration of pooled platelets poses a greater infection
risk than does that of single-donor units. Pooled platelets are more
readily available, however, since they are easily obtained as a
component of whole blood donations. A more complex apheresis process
must be used to obtain a sufficient quantity of platelets from a single
donor.
permit oxygen exchange, and they must be preserved at room temperature
with constant agitation. Properly stored, they can be used for up to 5
days after collection. Platelet concentrates should be typed for ABO,
and because they are contaminated with red cells they should be tested
for the Rh antigen. One unit of platelets separated from a single unit
of whole blood contains at least 5.5×1010 platelets and usually raises the platelet count of a 70 kg patient by about 7,500×109
platelets per liter. The increase in posttransfusion platelet count may
be far less than anticipated for a variety of reasons, including fever,
splenomegaly, rapid hemorrhage, disseminated intravascular coagulation,
and refractoriness due to formation of anti-HLA antibodies. It must be
appreciated that the best test for platelet effectiveness is cessation
of bleeding.
results from thrombocytopenia. Massive transfusion—at least 20 units of
blood in a 24-hour interval—may result in generalized oozing, with
platelet counts lower than 80×109/liter, suggesting the need
for platelet transfusion. The administration of platelets in this
setting should be based on documented platelet counts. Do not
administer platelets prophylactically except in the setting of
preoperative platelet deficiency—a platelet count of less than 10×109/liter.
Patients who are actively bleeding, whose bleeding time is greater than
twice normal, and whose platelet count is less than 50×109/liter should receive platelets. The preoperative platelet count of a surgical candidate should be at least 60–80×109/liter.
safe blood supply, the recognition of new transfusion-related
complications, and the inability to totally eradicate previously
recognized complications, reaffirms the admonition that blood should be
transfused only when absolutely necessary (11).
Most of the complications associated with transfusion of blood and
blood products are related to an immune response to incompatible units,
to an adverse physiologic response to transfusion, or to infectious
disease transmission (Table 5.9).
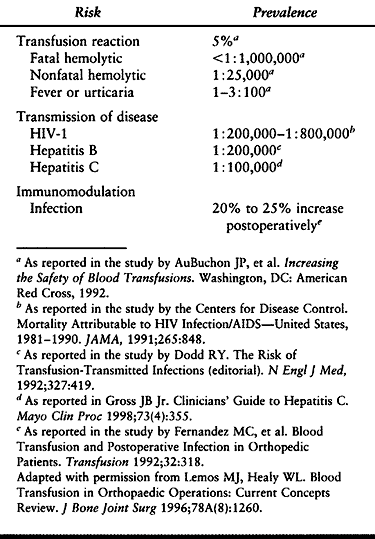 |
|
Table 5.9. Estimated Risk of Allogenic Blood Transfusion
|
meticulous guidelines for patient identification, blood collection and
labeling, pretransfusion testing, and transfusion administration and
monitoring. These policies are necessary to decrease the risk of human
error; a fatal transfusion reaction can occur from administration of an
improperly labeled specimen, confusion of specimens in the laboratory,
or transfusion of blood to the wrong patient.
blood transfusions are nonhemolytic transfusion reactions, which occur
in as many as 2% to 5% of all transfusions (11).
Although they may cause discomfort to the transfusion recipient, these
reactions are usually not serious. Febrile reactions, the most common
of these complications, most often result from the recipient’s antibody
response to leukocyte antigens in the donor blood. Chills, fever,
headache, myalgia, nausea, and, occasionally, severe rigors may occur
during the transfusion or up to several hours after it has been
completed. Treatment of febrile reactions is supportive and rarely
requires cessation of the transfusion. Approximately 15% of patients
who have
such
a reaction will react similarly with future transfusions.
Leukocyte-removal filters diminish the likelihood of febrile reactions,
but use of such filters is expensive and retards blood flow. For these
reasons, they should be reserved for those patients who have had at
least two adverse reactions.
consisting only of slight urticaria. Like febrile reactions, they
usually occur toward the end of the transfusion of erythrocytes.
Allergic transfusion reactions, thought to be related to the presence
of foreign protein in the transfused blood, can cause urticaria
associated with itching, shaking chills, fever, and erythema. The more
serious findings of laryngeal edema and bronchospasm (anaphylaxis)
fortunately are much less frequent, occurring in less than 1% of such
reactions (34). For most patients, treatment is
supportive and the reaction may be anticipated to subside spontaneously
within several hours of the transfusion. The risk of an allergic
response is increased for individuals with hay fever, atopy, or asthma.
These patients may benefit from pretreatment with diphenhydramine HCl
or possibly with hydrocortisone. Patients who have previously had
multiple severe allergic reactions to transfusions are at high risk of
developing further such reactions. These reactions may be ameliorated
by using washed components.
high morbidity and mortality rates associated with them mandates a
thorough knowledge of their manifestations and treatment. Estimates of
the risk of hemolytic transfusion reactions range from 1:4,000 to
1:25,000 (83,100,115).
Of the fatal reactions, nearly half have arisen from administrative and
clerical errors, including mislabeled samples and incorrect patient
identification. Hemolytic transfusion reactions occur with small
quantities of transfused erythrocytes, usually less than 50 cc (10).
The classic signs and symptoms include chills, fever, chest pain, and
flank pain. Less commonly, patients experience nausea, hemoglobinuria,
shock, and a subjective sensation of impending death.
symptoms. In that situation, the patient’s only manifestation may be
the oozing that occurs because of the consumption of clotting factors.
The consequences of hemolytic transfusion reactions are serious and may
be fatal, arising mainly from the effects of intravascular hemolysis on
the renal and coagulation systems. Immediately stop the transfusion and
return the unused blood and a sample of the patient’s own blood to the
blood bank for recrossmatching.
presence of hemoglobinemia. Visually compare the postreaction serum
with the pretransfusion specimen in the blood bank. Verify that the
identity of the unit and the requisition correspond to the patient,
confirming that the patient has received the intended unit of blood.
Perform a direct antiglobulin test on the patient’s red cells. Other
tests indicated when hemolytic reaction is strongly suspected include
retyping the transfused blood, and obtaining serum bilirubin and
urinary urobilinogen. Hemoglobin concentration, platelet count, partial
thromboplastin time, serum fibrinogen level, and serum potassium levels
may be necessary in patients with overt hemolysis. If hyperkalemia due
to hemolysis is suspected, an electrocardiogram will confirm the
diagnosis before the clinical laboratory determination.
hemolytic reaction initially involves hydration with generous
administration of fluids and diuretics. Monitor urinary output with an
indwelling catheter and maintain output at 75–100 ml/hour. Use
intravenous fluids and pressors, if necessary, to prevent hypotension,
thereby ensuring
an
adequate renal blood flow. If there is no diuresis, indicating renal
failure, cease attempts at hydration. Intravenous furosemide may be
necessary to maintain adequate renal perfusion. Contact consulting
services immediately; consider transferring the patient to an intensive
care unit.
dramatic events than acute hemolytic transfusion reactions. A delayed
reaction is characterized by initial survival of transfused
erythrocytes, followed by hemolysis within 1 to 7 days (116,139).
The reaction results from an anamnestic response of an antibody that
was formed after a previous sensitizing event, but that is no longer
detectable in pretransfusion testing. The patient usually manifests
only anemia, or no increase in hemoglobin in response to the
transfusion, but he may also have chills, fever, and jaundice. Renal
failure is extremely rare.
often than acute reactions and may frequently be missed. In one series,
one third of the patients with delayed hemolytic transfusion reactions
were asymptomatic and were diagnosed only when a second transfusion was
ordered (100). If you are considering returning
to surgery a postoperative patient in whom there is continued occult
blood loss from a traumatic or surgical source, first rule out delayed
hemolytic transfusion reaction as a possibility. Unnecessary surgery
can be avoided if the diagnosis of delayed hemolytic transfusion
reaction is made.
refers to transfusion of more than 10 units of blood in a 24-hour
period. Hemostatic defects, hypothermia, and metabolic abnormalities
have been reported in association with the transfusion of such volumes
of blood. Anticipating these problems and taking appropriate measures
to treat or prevent their development can be life saving.
may result from dilutional thrombocytopenia, disseminated intravascular
coagulation, low levels of factors V and VIII, or the occurrence of a
hemolytic transfusion reaction. Dilutional thrombocytopenia is the most
likely cause of a hemorrhagic diathesis in a patient who has received a
massive transfusion, since the transfused stored blood lacks functional
platelets. Patients who manifest acute thrombocytopenia develop a
hemorrhagic diathesis at a much higher platelet count than do patients
with chronic thrombocytopenia, and a platelet count of 100×109/l or less is a reasonably accurate guide to predicting a bleeding problem from dilutional thrombocytopenia.
with massive transfusions include deficiencies of factors V and VIII.
These factors gradually decrease to 15% and 50% of normal,
respectively, after 21 days of storage (97),
and they are therefore in lower concentrations in stored blood than in
fresh blood or plasma. Hemostasis is unaffected even though levels of
factors V and VIII are reduced to as low as 10%–20% of normal;
therefore, their deficit is an unlikely primary cause of bleeding
during massive blood transfusion. Nevertheless, these deficiencies may
intensify bleeding from other causes (98).
Disseminated intravascular coagulation is fairly uncommon and is
associated with a high rate of mortality. Discussion of this entity is
beyond the scope of this chapter.
be considered when a hemorrhagic diathesis develops following blood
transfusion. A massive transfusion may demand such speed in blood
processing that the risk of human error increases significantly.
has been stored at 4°C, can cause rapid lowering of the core body
temperature by as much as 10–15°. Hypothermia causes shivering, which
increases oxygen consumption dramatically and may increase ventricular
irritability and even induce cardiac arrest if the hypothermia is
profound. Blood may be warmed toward body temperature, but it should
not be warmed above 37°C. Warming must be done with a special device
that warms the blood during its passage through the transfusion set.
The warming system must be equipped with a visible thermometer and,
ideally, with a warning system to prevent overheating. Blood will
hemolyze if heated above 45°C.
hyperkalemia, and reduction in the level of ionized calcium, have also
been associated with the transfusion of massive volumes of stored
blood. Monitor patients closely for these problems and try to prevent
them by appropriate treatment.
effects on the immune system of large homologous blood transfusions.
The concern is an increased risk of postoperative infections following
allogeneic blood transfusions (36,40,79,101).
Other investigators have questioned how large an effect, if any,
allogeneic blood transfusions have on the postoperative infection rate (151).
blood transfusions to impair the immunosuppressive response, decreasing
the patient’s ability to combat cancer recurrence or metastatic
disease. In one study of 155 patients with nonmetastatic osteosarcoma
treated with amputation and adjuvant chemotherapy, perioperative blood
transfusion was associated with a significant decrease in disease-free
state and overall decreased survival (27).
history review, exclusion of anonymous donors, and improved serologic
screening methods. Despite these improvements, risk of disease
transmission remains a concern with any blood transfusion. The greatest
threat to the safety of the blood supply is blood donation by
seronegative donors who are infectious but who have not yet undergone
seroconversion (131).
detection tests, will improve the safety of the blood supply, it
remains unlikely that any test or combination of tests will ever
decrease the risk for disease transmission to zero (131).
surface antigen (HBsAg) has greatly reduced the incidence of
transfusion-related hepatitis B. Infection risk is estimated to range
from 1 in 200,000 transfusions to 1 in 800,000 (83).
Ten percent of patients infected with HBV develop chronic hepatitis,
and less than 1% develop fulminant hepatitis. Acute HBV infection is
self-limited in approximately 90% of cases.
is a serious health threat. It is estimated that as many as 4 million
Americans (1.5% of the population) are infected with HCV. While acute
HBV infection is self-limited in 90% to 95% of cases, 85% of patients
with acute hepatitis C develop chronic infection (58).
Chronic HCV infection can lead to cirrhosis, liver failure, and
hepatocellular carcinoma. In the United States, HCV accounts for
approximately 20% of cases of acute hepatitis, 70% of chronic
hepatitis, and 30% of end-stage liver disease (66).
HCV infection is the disease that most often necessitates liver
transplantation. Approximately 10,000 people die each year from HCV
infection, and the number is expected to triple in the next 10–20 years.
characterized. The clinical course of HCV infection is variable, with
fewer than 20% of patients developing symptoms of infection. Since most
infections are asymptomatic, patients do not know that they are
infected. Chronic HCV infection is typically a very slow process in
which symptoms may not be noted for decades after infection. One
investigator estimates that cirrhosis occurs in at least 20% of
patients within 20 years of infection (103).
The risk of hepatocellular carcinoma increases after the development of
cirrhosis, with a reported 5-year risk of 7% and a 10-year risk of 14% (39).
transmission of HBV and HCV has occurred far more often than
transmission of HIV. Patients who received blood transfusions prior to
the introduction of screening tests for HCV in 1990 are at risk for
having contracted chronic HCV infection. Widespread testing and
educational efforts have been launched to identify this group of
patients who are at risk. Patients who received blood transfusions
prior to July 1992, when more sensitive screening tests were
introduced, should be tested for exposure to hepatitis C virus. A
nationwide “targeted look-back” has begun in the United States, in an
attempt to find patients who received blood from donors who
subsequently tested positive for hepatitis C. The risk of HCV infection
prior to implementation of anti-HCV testing in mid 1990 was
conservatively estimated at 1% per unit of transfused blood. These
estimates predict that during that time, 40,000 people acquired HCV
infection yearly from blood transfusions (3). With the current screening methods, the risk of HCV transmission is about 0.001% per unit of blood transfused (58).
the risk of receiving a seropositive unit of a blood component was
approximately 0.04% (2). By November 1995, the
number of cases of transfusion-associated cases of AIDS diagnosed and
reported in the United States was 7,700 (24). Many previously asymptomatic patients continue to be diagnosed each year from transfusions administered prior to 1986.
however, the number of transfusion-associated AIDS cases that have
occurred from screened transfusions fell to less than 20 cases per year
from 1986 to 1991 (132). Concern over potential
transmission of the HIV virus has resulted in new laws requiring
consent for blood transfusion, alterations in transfusion policies, and
use of alternative techniques to avoid homologous blood transfusion.
transfusion-associated AIDS are in place. Elimination of the
“high-risk” blood donor through questioning or through self-exclusion
has proven partially effective. As a result, the prevalence of
HIV-positive donors was reduced by nearly two thirds from 1985 to 1987 (113). A second and more effective adjunctive mechanism was the implementation of anti-HIV testing (153).
Used for screening since 1985, this test is more than 99% sensitive.
All positive results detected by repeated enzyme immunoassay must be
confirmed by western blot testing. Since the advent of screening
methods, the risk of transfusion-transmitted HIV infection has been
estimated to be 1 in 493,000 (95% confidence interval, 202,000 to
2,778,000) (131)—about one-fifth the risk of HCV transmission by blood transfusion.
of the infection and the development of detectable antibodies, there
remains a small risk of HIV transmission from screened blood donors.
Refinements in the screening assays have narrowed this period to
approximately 22
days (131).
The polymerase chain reaction technique can be used to identify viral
nucleic DNA or RNA before an antibody response develops and may further
reduce the risk of disease transmission (154).
directed donation programs, in the use of autologous blood, and in
avoidance of blood transfusion unless specifically indicated. Patients
with hemophilia are far more likely to have developed
transfusion-associated AIDS than any other patient group in a typical
orthopaedic surgery practice. Previously, factor VIII and factor IX
concentrate used in the treatment of hemophilia was derived in
commercial lots from 10,000 to 30,000 donors, resulting in an extremely
high risk of disease transmission. The risk of new HIV infection in
these patients is directly related to the volume of clotting factor and
blood component used, and it has decreased markedly since the
introduction of heat-treated concentrates prepared from plasma screened
for HIV antibody. Development of factor VIII concentrate derived from
recombinant techniques has significantly reduced the risk of
transfusion-associated AIDS in this patient population.
also been reported as a result of blood transfusion. HTLV-I infection
has been associated with adult T + -cell leukemia and HTLV-I–associated
myelopathy (HAM), also termed tropical spastic paraparesis (127).
The incubation period between infection and manifestations of these
diseases can be extremely long. This retrovirus is most common on the
island of Kyushu, Japan, and in parts of the Caribbean basin. Only a
small percentage of the population in the United States is believed to
be HTLV-I seropositive (approximately 0.025% of donors tested).
Nevertheless, blood-donor screening for antibody to HTLV-I was
initiated in 1989 due to these disease associations (20).
Seroconversion for antibody to HTLV-I is reported to have occurred in
one patient, who developed an associated myelopathy following multiple
trauma and subsequent transfusion with many units of banked blood
products. The report of this seroconversion underscores the potential
value of screening blood donors for antibody to this virus (32).
transmitted via blood transfusion, although it is likely that many of
the cases of posttransfusion CMV infection are due to reactivation of
existing infection—possibly because of immunosuppression in the
patient—rather than to primary infection (156).
The prevalence of CMV infection, endemic in the United States,
increases with age, so that as many as 70% of blood donors over age 60
are infected by CMV. Posttransfusion CMV infection is not known to be a
significant clinical problem in the immunocompetent transfusion
recipient, and blood is not routinely tested for this agent.
donors with a negative test for IgG anti-CMV include AIDS patients who
are CMV-seronegative, CMV-negative bone marrow transplantation
recipients, and solid-organ transplant recipients who receive organs
from CMV -seronegative donors (146). Since CMV
is carried in the transfused leukocyte, leukocyte-removal filters can
be used to further reduce the risk of transfusion-transmitted CMV to
these patients. Concern also exists over the possible transmission of
prions, which cause spongiform encephalopathies such as variant
Creutzfeldt-Jakob disease, in which there is rapidly progressive
dementia and motor dysfunction (22). In March
1997, the World Health Organization concluded that there has been no
proven or even probable transmission of Creutzfeldt-Jakob disease
through blood products (13).
infectious diseases could be transmitted by blood transfusion, few pose
clinical problems. Blood donor testing and the use of screening to
exclude donors who have been exposed to malaria, syphilis,
toxoplasmosis, yersiniosis, salmonellosis, typhus, trypanosomiasis,
herpesvirus infections, brucellosis, Colorado tick fever,
leishmaniasis, Epstein-Barr virus, and filariasis minimize the
potential risk of transmission of such diseases through donated blood.
transfusion cannot be completely eliminated, even with the best
screening methods. The goal of reducing the risks associated with
transfusions, therefore, is directly related to reducing the number of
transfusions required by surgical patients. The methods available for
minimizing blood transfusions include meticulous hemostasis in the
operative field, hypotensive anesthesia, hemodilution, use of prebanked
autologous blood, intraoperative and postoperative blood salvage, and
acceptance of a lower hematocrit “transfusion trigger.” Concern about
transmission of human immunodeficiency virus has heightened interest in
these methods for orthopaedic surgeons and their patients and has made
blood conservation an important part of every surgeon’s practice.
surgeon and the anesthesiologist. The most effective way to reduce the
need for blood transfusion is to control the amount of blood lost
during surgery. Methods under the surgeon’s control include careful
operative exposure through avascular tissue planes; good hemostasis
using an
electrocautery
and ligatures; short operating time; and the judicious use of collagen
pads, thrombin powder, sterile bone wax, and tourniquets (83).
anesthesia can effectively reduce intraoperative blood loss in
orthopaedic surgical patients, particularly during spine surgery and
total hip arthroplasty surgery (91,105).
A series of 24 Jehovah’s Witness patients had a 30% reduction in
intraoperative blood loss with the use of hypotensive anesthesia,
compared with the blood loss for the previous arthroplasty on their
contralateral hip under normotensive anesthesia (105).
Nelson et al. recommend the combined use of narcotic and inhalant
anesthesias to achieve the goal of a stable hypotensive level
throughout the surgical procedure. They further recommend positioning
the patient to reduce engorgement of blood vessels at the surgical
site, adhering closely to the use of local measures to minimize blood
loss (107). These recommendations deserve close attention.
need for homologous blood transfusion is hemodilution. Whole blood
collected from the patient immediately before surgery is simultaneously
replaced with colloid or crystalloid to maintain a constant circulating
blood volume. Since the blood lost during surgery is diluted and has a
lower hematocrit, fewer red cells are lost. The whole blood that was
collected preoperatively is transfused after the major blood loss has
ceased, or before then if necessary because of hypoxemia, hypotension,
or tachycardia. The hemodilution technique has been recommended for any
elective surgery procedure in which a 1,000 cc blood loss is
anticipated (108).
After a patient is determined to be medically eligible for autologous
transfusion and informed consent has been obtained, blood is collected
and stored. During the donation period, place patients on oral iron
supplementation. The rate of donation of blood is limited primarily by
the donor’s ability to regenerate and maintain an adequate
red-blood-cell mass. Multiple serial autologous donations may be
obtained as long as the donor’s hemoglobin level is at least 11 g/dl,
or the hematocrit is at least 33%. These levels are considerably lower
than those required for voluntary homologous donation.
Current studies are evaluating the role of erythropoietin to increase
erythropoiesis in patients undergoing autologous blood donation (see
below). The only requirement in addition to those of standard blood
banking techniques is special identification and segregation of the
donor’s blood to ensure that it is appropriately and safely redirected
to the donor.
with serious risks, including disseminated intravascular coagulation,
fatal air embolism, hemoglobinemia, and hemolytic nephrotoxicity (16,78).
Recent improvements in the design of the autotransfusing device have
eliminated many of the problems that had been associated with
intraoperative autologous transfusion. Intraoperatively, lost blood is
suctioned into an enclosed system in which the blood cells are
separated from the debris and serum through a centrifugation process.
The autologous red cells are washed, suspended in a saline solution,
and then reinfused. As much as 50%–60% of the shed red blood cells may
be salvaged by this technique.
when intraoperative autologous transfusion is used than when it is not
used, and the combined use of preoperatively deposited and
intraoperatively collected autologous blood can often eliminate the
need for homologous transfusion (57). The
technique has been shown to be effective in orthopaedic patients
undergoing spine surgery, revision total joint arthroplasty, and
acetabular fracture surgery (31,57,159).
Indications for its use include patients in whom the estimated
intrasurgical blood loss is expected to be more than 900–1,500 cc (44,150). Potential complications of this technique include cell hemolysis and air embolism (150).
Postoperative salvage consists of reinfusing unwashed, filtered
salvaged blood from surgical drains during the early postoperative
period (first 4–6 hours) when drainage is at its greatest. Unwashed
shed blood is deficient in coagulation factors and platelets and may
contain free hemoglobin and fibrin degradation products from hemolysis
and lysis of clots, in addition to fat particles, bone fragments,
methylmethacrylate monomer, and vasoactive mediators.
hyperthermia when the shed blood is reinfused. Postoperative autologous
transfusion with unwashed shed blood should be limited to two units,
and reinfusion should occur within 6 hours after the start of the
collection (83). Relative contraindications for the postoperative salvage of shed blood include tumor and infection (83).
well proven. A better appreciation of the risks associated with the
administration of blood products, however, has brought about the need
to reevaluate the “transfusion trigger” for perioperative blood
administration. Studies have demonstrated that patients can safely
undergo anesthesia and surgery with preoperative values as low as 8
g/dl (142). Some patients with chronic anemia, such as those with chronic renal failure, tolerate hemoglobin levels less that 7 g/dl (102).
should be performed, consider the physiologic parameters unique to the
individual patient rather than arbitrary hemoglobin or hematocrit
levels. The decision to transfuse should take into account the duration
of the anemia, the intravascular volume, the extent of the operation,
the probability of massive blood loss, and the presence of coexisting
medical morbidity, such as impaired pulmonary function, inadequate
cardiac output, myocardial ischemia, or cerebrovascular or peripheral
vascular disease (102).
catheters, and continuous electrocardiography, as well as frequent
sampling for hemoglobin levels, has proven beneficial in determining
when to transfuse (84). Instead of scheduling invasive monitoring for every patient, Nelson et al. (107)
have recommended dividing patients into high-risk and low-risk
categories on the basis of clinical judgment. They suggest monitoring
hypovolemia in low-risk patients by following routine vital signs. It
may be successfully managed with the infusion of crystalloid solutions
if the hemoglobin is above a level of approximately 7 g/dl or the
hematocrit is above 20%.
require not only more invasive monitoring but also, as a rule, higher
hemoglobin and hematocrit levels than do low-risk patients. In these
high-risk patients, the relationship between anemia and signs such as
tachycardia, tachypnea, and low mixed venous oxygen saturation should
be evaluated more rigorously, and transfusion of PRBC is recommended
until the physiologic signs of anemia are reversed (107).
response to hypoxemia and hemorrhagic stress. It binds to receptors in
the bone marrow, stimulating the production of red blood cells (83).
Recombinant human erythropoietin has a wide variety of potential
clinical applications. It is approved for the treatment of anemia due
to several conditions, including chronic renal failure and malignancy,
and in HIV-positive patients undergoing treatment with zidovudine. Its
potential uses in orthopaedic surgery are currently being explored (19).
as an adjunct during preoperative autologous blood donations for
elective surgery (55). Often, the number of
units that can be donated is limited as the patient becomes anemic.
Some orthopaedic patients also have medical conditions resulting in
mild chronic anemia. Many patients’ preoperative hematocrits are
lowered following autologous donation, and 10% to 20% still require
allogeneic blood transfusions (54). Anemia (a
hematocrit of 39% or less) at the time of the first autologous donation
is reported to be the most important indicator that a patient will
require allogeneic blood transfusions in addition to autologous units (56).
no clinical benefit in patients without preexisting anemia (hematocrit
> 39%) undergoing preoperative autologous blood donation, as these
patients were able to stimulate sufficient erythropoiesis through their
endogenous erythropoietin response (54). In
patients undergoing autologous blood donations who have preexisting
anemia, the role of recombinant human erythropoietin remains
controversial. Recombinant human erythropoietin has been shown to be
effective in decreasing the need for allogeneic blood transfusions in
hip arthroplasty patients who did not donate autologous blood (21,38).
blood transfusions, or directed donations, as an alternative to
standard homologous blood transfusions. This transfusion practice,
unlike autologous blood transfusion, is controversial. There is no
evidence to suggest that it is any safer than homologous blood
transfusion, and under some circumstances (e.g., graft-versus-host
disease in surgical patients who have received blood from first-degree
relatives), it may be extremely deleterious (51,65,109,147).
In addition, there is concern that individuals asked to donate for
family members may feel compelled to violate the usual voluntary
self-exclusion criteria. Our blood bank provides directed donor service
for patients who desire it solely for reasons of public relations
rather than for any medical reason.
and its importance has grown as surgery has become more technically
complex. Surgeons who think through the operative procedure in advance
can easily alter the sequence of steps to provide the optimal outcome,
a luxury not often available in the middle of surgery. Preoperative
surgical plans have most commonly been used for complex acute and
reconstructive trauma, osteotomies, total joint arthroplasty, and other
technically challenging procedures (92).
The benefits of preoperative planning can also be applied to simple, straightforward cases.
planned procedure and a written series of steps and reminders. Mast et
al. have described the “surgical tactic” as an outline of sequential
steps used in the operating room that will bring about the desired end
result (93). While a well-thought-out
preoperative plan is not a guarantee of success, it may reduce the
potential for intraoperative error and allow the operation to proceed
smoothly from step to step. It is important to share these plans with
the anesthesiologist and operating room staff, so that they will be
able to anticipate all the needs of both surgeon and patient.
planning helps ensure that all necessary equipment is requested and
available, and that all support personnel have been brought on board.
As the steps are planned, the required equipment is listed.
be better suited for different situations. In some cases, a combination
of methods may be used to obtain the best possible preoperative plan.
preoperative planning kits, which include templates for all the AO
fracture implants, are an excellent resource for planning operative
fracture care. The textbook Planning and Reduction Technique in Fracture Surgery by Mast, Jakob, and Ganz (93)
provides numerous case examples with preoperative plans and surgical
techniques for fracture reduction and fixation. Obtaining adequate,
good-quality x-ray films is a major hurdle in preoperative planning in
trauma patients. Radiographs obtained without overlying plaster splints
are best for imaging individual fracture fragments. Traction views are
frequently beneficial in correcting overlap or malrotation of
fragments. Traction views may be especially helpful in the
supracondylar femur and distal humerus fractures, where muscle pull
often malrotates the distal fragments.
body habitus of the patient and the length of the beam from the
cassette. The amount of magnification can vary from 6% to 36% with a
40-inch exposure distance, and from 3% to 17.5% with a 72-inch exposure
distance (144). Most templates are magnified by
10% to 15% to account for the radiographic magnification. Special
radiographic magnification markers can be applied to the skin at the
level of the bone to determine the individual magnification, but this
degree of accuracy may not always be necessary. Exact measurements can
also be obtained from computed tomography.
greatest deformity usually exists in a plane other than either the AP
or the lateral view. Mathematical formulas can be used to determine the
maximal deformity (117,123). A properly planned oblique osteotomy can provide multiplanar correction of a malunion (69,125,128).
Plain radiographs also do not account for rotational deformities that
may be present. Rotational deformities may be evaluated by physical
examination, and if necessary more precise evaluation can be obtained
by axial computed tomography (CT) scans. (See Chapter 32 for more details.)
case of fractures involving the shaft of relatively straight bones.
Follow these steps for a simple fracture:
-
Obtain a radiograph of the fracture (Fig. 5.1A).
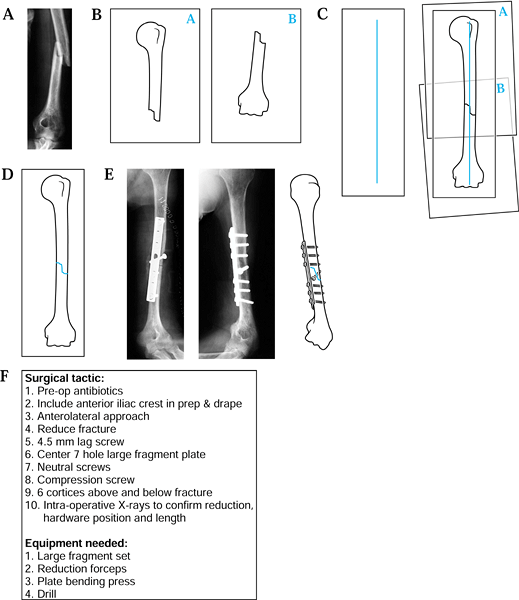
![]() Figure 5.1. Preoperative planning for humeral shaft fracture using the direct overlay method. A: Radiograph of fracture. B: Tracings of each fracture. C: Reduction of the fracture. D: Tracing of the “reduced” fracture. E: Addition of appropriate screws and plates. F: The surgical tactic.
Figure 5.1. Preoperative planning for humeral shaft fracture using the direct overlay method. A: Radiograph of fracture. B: Tracings of each fracture. C: Reduction of the fracture. D: Tracing of the “reduced” fracture. E: Addition of appropriate screws and plates. F: The surgical tactic. -
On separate pages, trace the proximal fragment, the distal fragments, and any intervening comminution or butterfly fragments (Fig. 5.1B).
-
Draw a vertical line on a separate piece of paper.
-
“Reduce” the proximal and distal fragments by aligning their long axes with the vertical line (Fig. 5.1C).
-
“Reduce” any comminution or butterfly fragments into a best-fit position.
-
Make a final composite drawing of the reduced fracture (Fig. 5.1D).
-
Using selected templates, trace the appropriate fixation implant (Fig. 5.1E).
-
Write out the surgical tactic, listing all sequential steps required to achieve the planned result (Fig. 5.1F).
fragment, simple fracture patterns may be drawn onto a single sheet of
tracing paper by rotating the paper into correct alignment as each
fragment is drawn. Alternatively,
cut out the individual fracture fragments, creating a jigsaw puzzle of fracture fragments.
into place—when the fragment is malrotated and its radiographic
projection does not match either the true AP or lateral view. In these
cases, make an approximation of the size and shape of the fracture
fragments, which may sometimes be inferred from the space left over
after other fragments are drawn in their “reduced” positions.
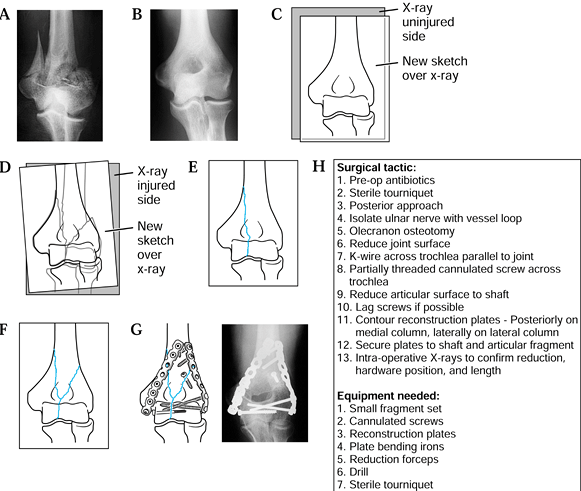 |
|
Figure 5.2. Preoperative planning for supracondylar elbow fracture using the reverse image method. A: Radiograph of the fractured supracondylar humerus with intracondylar extension. B: Reversed radiograph of the uninjured side. C: Traced outline of reversed radiograph. D,E: Individual fracture lines. F: The final drawing. G: Addition of appropriate screws and plates. H: The surgical tactic.
|
-
Obtain radiographs of the injured and uninjured extremities (Fig. 5.2A, Fig. 5.2B).
-
Reverse the radiograph of the uninjured or unaffected extremity and trace its outline (Fig. 5.2B, Fig. 5.2C).
-
Place the tracing over the radiograph of
the injured or affected extremity, and align the shafts or articular
surfaces (align whichever appear more similar). -
Trace the fracture lines, adjusting the paper until all major fracture lines have been traced (Fig. 5.2D, Fig. 5.2E and Fig. 5.2F).
-
Using selected templates, trace the appropriate fixation implant (Fig. 5.2G).
-
Write out the surgical tactic, listing all sequential steps required to achieve the planned result (Fig. 5.2H).
the mechanical and anatomic axes of the lower limb may be utilized to
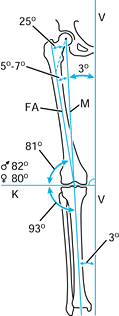
create a preoperative plan (Fig. 5.3).
 |
|
Figure 5.3. Normal anatomic and mechanical axis relationships of the lower extremity. The mechanical axis (M)
goes from the center of the femoral head to the center of the ankle. The angle between the mechanical axis and the anatomic axis of the femur (FA) is normally between 5° and 7°. The axis of the knee joint (K) is perpendicular to the vertical axis of the body (V). (Redrawn with permission from Müller ME. Intertrochanteric Osteotomy: Indication, Preoperative Planning, Technique. In: Schatzker J, ed. The Intertrochanteric Osteotomy. Berlin, Springer-Verlag, 1984.) |
to do preoperative planning for fractures involving the distal femur or
proximal tibia. This template provides not only the normal axis lines
of the knee joint but also the anatomic axes of the femoral and tibial
shafts (Fig. 5.4).
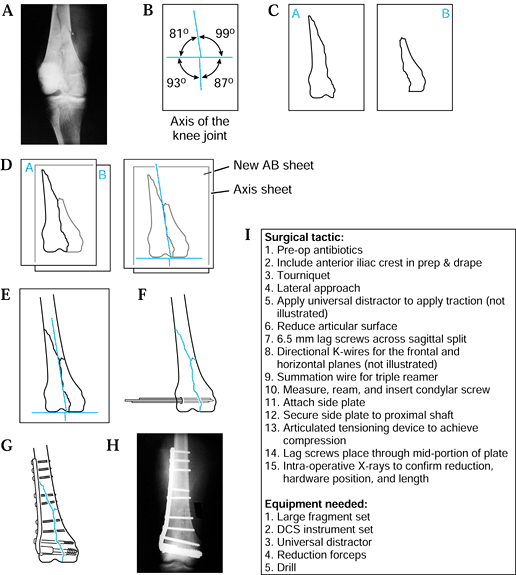 |
|
Figure 5.4. Preoperative planning for supracondylar femur fracture using the joint axis template. A: Radiograph of fracture. B: Knee joint axis template. C: Tracing of individual fracture fragments. D: Reduction of the two most distal fracture fragments. E: Reduction of the metaphyseal and shaft component. F:
Use a template to draw initial fixation of the intercondylar split; show insertion of the two 5.5 mm cancellous screws. Insert a guidewire parallel to the anterior femoral condyles and distal femoral condyles. After reaming, insert the dynamic condylar lag screw (DCS). G,H: Use templates to draw in side plate and screws of the appropriate length. Indicate the use of the articulated tensioning device if necessary to aid the reduction and achieve interfragmentary compression. I: The surgical tactic. |
-
Obtain a radiograph of the fracture (Fig. 5.4A).
-
Trace each of the fracture fragments on an individual sheet of paper (Fig. 5.4C).
-
“Reduce” and align the articular fracture fragments along the horizontal axis of the knee joint template (Fig. 5.4D).
-
“Reduce” the metaphyseal and diaphyseal
fragments with the articular fragments, aligning the diaphyseal
fragment along the shaft axis line (Fig. 5.4E). -
Using selected templates, trace the appropriate fixation implant (Fig. 5.4F, Fig. 5.4G).
-
Write out the surgical tactic, listing all sequential steps required to achieve the planned result (Fig. 5.4I).
preoperative planning for osteotomies, stating that, “Nowhere in
surgery is preoperative meditation and careful planning of more value” (18). Numerous different types of osteotomies have been developed, which are described in Chapter 26, Chapter 27, Chapter 28, Chapter 29, Chapter 30, Chapter 31 and Chapter 32 and Chapter 104.
Careful planning of an osteotomy may permit multiple simultaneous
corrections, including length, rotation, angulation, and displacement.
An understanding of the normal mechanical axis and the anatomic axis of
the lower limb is an essential part of any planned osteotomy of the
lower limb (Fig. 5.3). Standing long-cassette radiographs may be necessary to plan the degree of correction required.
sequentially executed. When a blade plate is required, the preoperative
plan must identify the location where the cortical window for the
seating chisel will be placed, and its direction and depth. Because it
is difficult to control small proximal or distal fragments, in many
cases the path for the blade is best cut before you make any of the
osteotomy cuts. The osteotomy becomes the final step prior to the
planned fixation. Intraoperatively, confirm restoration of alignment
with fluoroscopy, using an electrocautery cord as a radiopaque “plumb”
line. Center one end of the cord over the femoral head and the other
over the ankle joint,
reproducing
the new mechanical axis. Then visualize the location where the
mechanical axis (electrocautery cord) crosses the knee joint and
compare it with the preoperative plans.
planned osteotomy site can also serve as markers for rotational or
angular corrections. If a blade plate is to be used, K-wires may also
be used as alignment guides for placement of the seating chisel. You
can also score the cortical bone to judge rotational corrections. Figure 5.4 is an example of this technique applied to the distal femur.
arthroplasty implants for preoperative planning. For total hip
arthroplasty, these templates allow the surgeon to plan the size and
position for the implants. Variables that should be considered include
the location of the hip center, the neck cut, the implant position, and
the anticipated implant neck length. Different implants provide varying
amounts of femoral offset, a factor that may influence abductor muscle
function. Further details of preoperative planning for total joint
arthroplasty are provided in Chapter 101, Chapter 102 and Chapter 103, Chapter 105, Chapter 106, Chapter 108 and Chapter 109.
the preoperative planning of osteotomies and total joint replacement.
These systems require digitization of the radiographs. Once all the
data have been acquired, they offer the benefit of allowing the surgeon
to easily try different degrees of correction (143). In some cases, three-dimensional CT may be helpful to better visualize certain deformities or complex fractures (74).
Reconstruction models can also be made from CT data to assist in the
planning of extremely complex correction involving deformed bones or
joints (112). (See Chapter 17.)
and informed decision making, appropriate indications for surgery,
complete preoperative planning, technically precise surgery, gentle
soft-tissue technique, and a well-designed and well-executed
postoperative rehabilitation program. Good results from a surgery
depend on many seemingly insignificant aspects of surgical technique
that lead to precision and efficiency and minimize complications.
the operation with an attitude of confidence and with the assurance
that they will be able to handle any contingencies or unexpected
complications. Establish a businesslike attitude in the operating room.
Lighthearted conversation and background music in the operating room
are appropriate in some circumstances but must not distract from the
conduct of the surgery. Distractions slow down the operating team and
may lead to inadvertent breaks in surgical technique and other errors.
operating room staff because of perceived deficiencies is inappropriate
and unproductive. The operating team will perform most effectively and
most efficiently if you maintain a calm and dignified demeanor
throughout the case and if the procedure is approached in a methodical
and logical fashion.
personnel before the surgery is always beneficial. You are ultimately
responsible for the availability and condition of instruments and
implants. Although this responsibility is customarily delegated to the
nursing staff, in whole or in part, it is prudent to review with the
scrub nurse the instrument and implant setup immediately before the
case. It is helpful to talk through the planned surgery quickly with
the operating team before the surgical preparation to be certain that
everybody understands what is planned, all instruments are present and
functional, and appropriate implants are available.
anesthesiologist, who bases the decision on the requirements of the
surgery, patient safety, and patient desires (see Chapter 7).
It is important, however, for you to advise the anesthesiologist on the
requirements for the operation to be performed. In the upper extremity,
regional or local anesthesia frequently is used. General anesthesia is
used when the operation is too long for regional or local anesthesia or
when extremely delicate surgery, such as neurovascular anastomosis,
requires the patient to be very still.
however, regional anesthesia by blocking multiple peripheral nerves is
used infrequently because multiple blocks are required and usually do
not provide sufficient anesthesia for the use of a tourniquet. If there
is no contraindication,
epidural
anesthesia works extremely well for lower extremity surgery,
particularly when performed in an ambulatory surgery unit, because the
anesthetic does not have the residual effects of general anesthesia.
Epidural anesthesia may also be used in conjunction with general
anesthesia. The pain relief provided by the epidural permits a
“lighter” general anesthesia and is useful postoperatively for pain
management. Epidural anesthesia may be combined with hypotensive
anesthesia in appropriately selected patients, resulting in less blood
loss, fewer transfusions, and a decreased rate of deep vein thrombosis (86,134).
Plan for the prophylaxis of perioperative deep venous thrombosis before
selecting epidural anesthesia, to avoid the risk of the patient’s
developing an epidural hematoma when low-molecular-weight heparin is
being concurrently administered (68,162).
Spinal anesthesia is equally effective but can not be readministered
easily if the surgical procedure takes longer than estimated. It can
provide a higher level of anesthesia, but the risk of complications
such as headaches is slightly increased.
reconstructive surgery, pelvic surgery, and spine surgery—hypotensive
anesthesia, when not contraindicated, is extremely useful to minimize
it. Use of hypotensive anesthesia requires a careful preoperative
workup of the patient; therefore, it is important to consult with the
anesthesiologist early.
surgery remains controversial. In our hospital and in most institutions
in North America, prophylactic antibiotics are used when wound exposure
time is expected to be more than 2 hours, or when major orthopaedic
implants will be used. The antibiotic used most commonly for
prophylaxis in orthopaedic surgery is cefazolin (104).
cephalosporin that provides broad-spectrum gram-positive coverage; it
has a high peak serum level and a long half-life. We generally give 1 g
cefazolin sodium intravenously with the preoperative medications,
although some authors recommend an initial dose of 2 g to achieve a
higher antibiotic level in bone (157). If you plan to use a tourniquet, administer the antibiotic at least 5 minutes before it is inflated (12).
patient, infuse it slowly over at least 1 hour to avoid anaphylactoid
reactions that can occur from rapid infusion. When there is a question
of possible infection, hold preoperative antibiotics until
intraoperative cultures are obtained. For operative procedures of
longer duration, administer an additional intraoperative dose of
antibiotics at approximately 4 hours.
continue antibiotics postoperatively, at 1 g every 8 hours for 24
hours; then discontinue. Some surgeons prefer to administer
prophylactic antibiotics for a longer period of time, but there is no
concrete evidence showing that longer duration is beneficial (106).
Other antibiotics may be required if there has been major prior
surgery, previous infection, or contamination. When the wound is large
or has been exposed for more than 2 hours, we thoroughly irrigate it
with a low-pressure mechanical irrigator with sterile normal saline,
followed by bacitracin solution, 25,000 units per liter of saline.
in total joint surgery. Methylmethacrylate beads impregnated with
antibiotics have been advocated to treat open fractures and bone
infections. These applications are discussed in more detail in Chapter 12, Chapter 105, Chapter 106, Chapter 132, Chapter 133, and Chapter 135.
The majority of postoperative wound infections are caused by airborne
contamination. The second most common source of organisms is the
patient, and the third is the surgical team. An operating room of
modern design with positive-pressure air flow is important. Maintain
good discipline in the operating room to minimize unnecessary
conversation, traffic, and movement. In particular, personnel moving in
and out of the operating room must avoid traveling directly through the
doors leading into the adjacent hallways; when possible, they should
pass through the substerile room that is present in most surgical
suites.
room personnel wear hoods to cover exposed hair. Masks must direct air
flow from the mouth and nose, away from the operative field. All
operating personnel must wear freshly laundered surgical scrub suits,
which include pants that are secured at the ankle to minimize fallout.
Wear double surgical gloves or similar coverage during the drape and
surgical procedures. Some surgeons recommend replacing outer gloves
following draping. After draping, one pair of gloves is satisfactory
for strictly soft-tissue surgery when implants are not used, although
most orthopaedic surgeons routinely double-glove. Gowns must be
impervious to water and must cover the back.
hours and involving implants or large surgical exposures, a special
low-bacteria environment using either horizontal or vertical laminar
air flow or ultraviolet lamps may be preferable. If horizontal laminar
air flow is used, take care, especially in total knee arthroplasty, not
to impede the air flow onto the operative field. Turbulent or impeded
air flow may actually increase the rate of contamination.
Specialized positions are used for surgery to the cervical spine,
shoulder, and low back (see specific chapters). The orthopaedic table
is used to position patients for internal fixation of hip fractures,
femur fractures, fractures of the tibia, and other special situations.
anesthesiologist and the surgeon. Particularly when the patient will
undergo a prolonged procedure, take care to avoid putting pressure on
neurovascular structures, muscle compartments, and bony prominences.
Position the patient to allow free expansion of the thoracic cavity and
movement of the diaphragm. Place gel or foam pads in areas of high
pressure concentration to avoid injury to the skin.
-
Place the patient on her back on a
well-padded, standard operating table, and insert pads under the
occiput and heels. For lower extremity surgery, place upper extremities
on padded arm boards and gently secure them. Avoid placing tension on
the brachial plexus and axillary artery by abducting the arms no higher
than 90°, and preferably no more than 60°. Insert disposable foam elbow
pads under the elbows. -
When access to the thorax or shoulders is
necessary, use sheets to tuck the arms alongside the trunk on the
operating table. For upper extremity surgery or other surgery not
involving the lower extremities, place a pillow under the calves to
keep the knees and hips slightly flexed. Place disposable foam pads on
the heels. Avoid crossing the legs.
-
Place the patient prone. Position
bolsters alongside the ventral thorax and abdomen to permit expansion
of the thorax and downward movement of the diaphragm. Make the bolsters
by rolling blankets and taping them into rolls approximately 4 inches
in diameter. The bolsters contact the anterior shoulder, avoiding the
axilla, and end slightly below the anterosuperior iliac spine. Avoid
placing pressure on the lateral femoral cutaneous nerve just medial to
the anterosuperior iliac spine. -
Place pads beneath both knees. Place a
pillow transversely under both legs to elevate the feet off the
mattress and to protect the legs. For the upper extremities, the same
rules for positioning in the supine position apply in the prone
position. Specialized frames, positions, and devices are used for spine
surgery in the prone position.
-
Place the patient in the lateral
decubitus position. Stabilize the trunk with a bean bag or
appropriately positioned kidney rests placed beneath the mattress.
Provide additional support for small patients, using folded sheets or
towels. Position kidney rests to support the patient at the pelvis,
unless they will interfere with the surgical approach. -
To avoid placing pressure on the shoulder
and axillary neurovascular bundle on the downside, place a lateral
chest roll made with a rolled bath towel or a cloth-covered liter
saline bag transversely supporting the thorax just below the axilla.
Unless surgery is to be performed on the upper extremities, gain
additional stability by placing upper extremities on an airplane splint
secured to the operating table. It is necessary to use elbow pads and
appropriate padding to protect the extremities. -
If the lower extremities are not involved
in the operative procedure, place a blanket beneath the lower leg for
padding and a pillow between the legs to cushion them. Place blankets
over the upper thigh and secure them with the strap from the operating
table to further stabilize the patient. Carefully pad above and below
the peroneal nerve on the lower leg to avoid placing pressure on it. -
If the surgery involves the upper leg,
such as in total hip arthroplasty, surround the lower leg with layers
of sheets. Build the sheets up to the level of the upper leg to provide
a smooth platform for the surgery and to protect the lower leg. Do not
tape across the chest wall; taping may produce excessive downward
pressure and interfere with respiration.
varies considerably according to the table type and the operative
procedure. The general principles of positioning a patient for surgery
of an intertrochanteric hip fracture, however, apply in most cases.
-
Place the patient in the supine position
on the padded table top, using the general principles already described
for a regular table. Use a radiolucent perineal post; pad it, and
position it to one side of the genitalia. -
Place the lower extremity designated for surgery into traction on a foot piece (Fig. 5.5).
If the manufacturer has not provided a special foot holder, use
appropriate padding and proper wrap to protect the soft tissues and
heel, particularly in elderly patients. Secure the foot to prevent
intrasurgical loss of traction.![]() Figure 5.5. A: Supine position on the Chick-Langren Orthopaedic table for internal fixation of a right hip fracture. B:
Figure 5.5. A: Supine position on the Chick-Langren Orthopaedic table for internal fixation of a right hip fracture. B:
Positioning for internal fixation of a right hip using bilateral lower
extremity traction. We prefer this position to that shown in (A),
because it stabilizes the pelvis better and permits more effective
traction for reduction of the fracture. (Courtesy of Chick Orthopaedic,
Chick Medical Products, Greenwood, SC.) -
Place the other lower extremity in the
lithotomy position in a leg holder and abduct it to allow clearance for
the C-arm fluoroscope. We have found it most effective to place the
healthy leg in traction as well. Be careful when placing patients in
the lithotomy position to avoid placing excess pressure on the
popliteal fossa. Limit the length of time in this position to avoid
complications of compartment syndrome (35,137). -
Use bilateral traction to centralize the pelvis on the perineal post and avoid pelvic rotation. Bilateral traction
P.98P.99
permits more effective fracture reduction that can be better
maintained. It also facilitates radiography. Bilateral traction
presents problems only when a patient has a hip adduction contracture.
Abduction to 45° is usually necessary for positioning of the C-arm
fluoroscope. Occasionally, in very comminuted intertrochanteric or
subtrochanteric fractures, it is necessary to support the shaft of the
femur or provide an upward push on the trochanters. Some operating
tables have attachments for this purpose. -
Alternatively, place a well-padded crutch
beneath the point at which upward pressure is desired and push it into
place. The rubber foot of the crutch prevents it from shifting, and the
oblique position of the crutch usually prevents it from interfering
with the swing of the C-arm. Position the C-arm fluoroscope so that it
swings freely from the AP to the lateral position without impinging on
the patient. Test the swing before draping the patient.
-
The torso has a tendency to roll into the
supine position. To prevent this, gently lock the elbow into full
extension and secure the forearm to an airplane splint with broad
adhesive tape (Fig. 5.6). Protect the ulnar
nerve. Use a vertical pelvic post with a well-padded perineal post
positioned so that the patient’s anterosuperior iliac spine lies
adjacent to and slightly above the pelvic post. This position
stabilizes the pelvis and permits adequate flexion of the hip.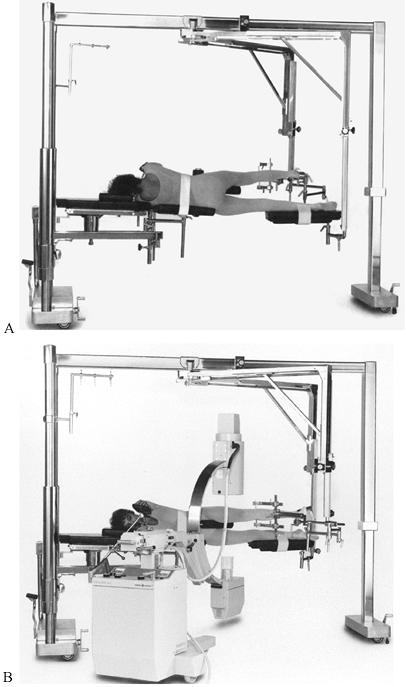 Figure 5.6. A:
Figure 5.6. A:
Posterior view of a patient in the lateral decubitus position for
closed intramedullary nailing of the right femur. If the right upper
extremity is secured to the arm board with the elbow in extension, the
torso is stable and the cross-body strap is not necessary. The affected
limb is in skeletal traction with a pin in the proximal tibia. If a
large-threaded Steinmann pin is used, the auxiliary foot support is not
necessary in most cases. The unaffected limb rests on a well-padded
foot board. Extend the board proximal to the knee to avoid injury to
the common peroneal nerve, pad the leg, and secure the leg to the board
with muslin or 3-inch tape. B: On this
frontal view, note that the pelvic post is positioned just caudal to
the anterosuperior iliac spines (it is behind the C-arm). The perineal
post can be used to support a short proximal fragment. With a stout
Steinmann pin in the tibia, the foot support is not necessary.
(Courtesy of Chick Orthopaedic, Chick Medical Products, Greenwood, SC.) -
Secure the uppermost lower extremity that
is designated for surgery (usually for closed intramedullary nailing)
using tibial pin skeletal traction to the tibial pin-holding device on
the orthopaedic table. Not all orthopaedic tables provide this
extremely useful device. -
Before placing the patient in the lateral
position, insert a large-threaded Steinmann pin transversely through
the tibia at or slightly below the tibial tubercle (in adults). Pad the
skin around the pin with sterile dressings, and secure it to the
traction device. -
Place the extremity in traction, and
position it as necessary for the procedure. Secure the downside lower
extremity to a well-padded leg board that attaches to the orthopaedic
table. Securely wrap the extremity to the leg board, which must extend
proximal to the knee. Take care to avoid pressure on the peroneal
nerve. Additional details on the position used for closed
intramedullary nailing of the femur are provided in Chapter 11 and Chapter 20.
surgery; it provides a bloodless field, which is essential for most
surgery of the hand and foot. There are, however, potential dangers
associated with the use of the tourniquet, and you must be familiar
with its proper use. Use a constant-pressure-regulated tourniquet that
is inflated with nitrogen. We apply it only to the upper arm and
proximal thigh (finger and toe tourniquets fashioned from Penrose
drains are described in the chapters on hand surgery). Even with
modern, reliable pneumatic tourniquets, it is possible to apply
excessive pressure. Use a calibration gauge to check the accuracy of
tourniquet pressure readings before each use.
for various sizes of extremities. The pressure pattern produced by a
tourniquet is cone shaped, diminishing in width as the depth of the
extremity is reached. Therefore, wide tourniquets are necessary for
large extremities; do not use narrow tourniquets on large limbs (60).
distally as it is inflated; in certain procedures in the distal arm and
thigh, therefore, it is necessary to secure the tourniquet in a
proximal position to allow adequate room for the surgery.
-
Place three strips of 2-inch tape on the
anterior, lateral, and posterior surfaces of the upper thigh or upper
arm. After the tourniquet is in place, fold them upward over the
tourniquet and secure them with circumferential tape around the
tourniquet (Fig. 5.7).![]() Figure 5.7. Tourniquet in place on the upper thigh, wrapped and secured to the thigh to prevent tourniquet migration.
Figure 5.7. Tourniquet in place on the upper thigh, wrapped and secured to the thigh to prevent tourniquet migration. -
Before applying the tourniquet, pad the
extremity with several smoothly applied layers of soft cast-padding
material to prevent wrinkling and pinching of the skin as the
tourniquet inflates. Apply the tourniquet snugly, and tie it securely.
Improper application may result in “unwrapping” of the tourniquet
during the surgery, and the result can be soft-tissue or neurovascular
injury. -
Failure of the tourniquet during surgery
is a great inconvenience and in some cases also a significant
interference. Check all components of the tourniquet system before use.
Keep prep solutions and chemicals away from the tourniquet and padding
materials. Before preparing the skin, cover the tourniquet with an
adhesive plastic towel drape. Sterile disposable tourniquets are
available for application after a sterile field is established. -
In most cases, exsanguinate the limb
before inflating the tourniquet by wrapping the limb with an Esmarch or
rubber bandage from distal to proximal. Esmarch bandages, once used as
tourniquets, are no longer recommended because their pressure cannot be
monitored, and damage to neurovascular structures can occur. -
Do not exsanguinate the limb if infection or tumor is present.
-
Appropriate tourniquet inflation pressure
is just enough to prevent arterial blood flow into the limb—no higher.
This pressure depends on the age of the patient, the status of the
vasculature, the blood pressure, and the size of the extremity (135). Reid, Camp, and Jacob (122)
recommend a pressure of 50–75 mm Hg above systolic blood pressure
(i.e., 135 to 225 mm Hg) in the upper extremity. Pressures of 100–150
mm Hg over systolic blood pressure (i.e., 175 to 305 mm Hg) are usually
necessary in the lower extremity. Inflate tourniquets quickly to
prevent venostasis, which may fill the venous tree before occlusion of
arterial blood flow.
by the limb without residual effects varies considerably from patient
to patient and depends on the condition of the limb and the pathology
being treated. In general, tourniquet times should not exceed 2 hours
and are best kept at 1½ hours or less. In many procedures, it is
possible to complete the exposure and obtain good control of potential
bleeding vessels within 1–1½ hours of tourniquet time. At that point,
the tourniquet often can be deflated and not require reinflation for
the remainder of the procedure.
results in some temporary posttourniquet problems. The most common
complaints are partial anesthesia and tingling, and hypersensitivity in
the limb for 1 to 2 days following surgery. Rarely, muscle weakness
accompanies these symptoms. With tourniquet times of 2 hours or less,
true palsies almost never occur; if they do, they are usually the
result of pressure on a peripheral nerve, rather than ischemic changes
from tourniquet use.
advisable. Do this only when the tourniquet is absolutely essential and
when the advantages justify the risks. If the tourniquet is in place
for 1 hour, deflate it for at least 20 minutes to restore normal
oxygenation, pH, and chemistry of the limb. Tourniquet time may be
extended to 3 hours if the tourniquet is deflated for at least 20
minutes at the end of each hour. Deflation for 20 minutes every hour,
however, usually interferes with the operative procedure, so it is
wiser to proceed with the tourniquet for 2 hours and then discontinue
its use. When the tourniquet is deflated, it sometimes causes a
venous-tourniquet effect and must be removed from the limb during the
operative procedure.
to the extremity has been restored, especially when more than a single
operative procedure is involved. In such cases, a tourniquet could
potentially remain inflated and in place long beyond the accepted
limits. It is also critically important to identify adequate peripheral
pulses when vascular damage could potentially occur, such as during a
total knee replacement. Diagnosis of the vascular injury while the
patient is still in the operating room may save hours.
limb before surgery, and to remove nail polish. Warn them to avoid
activities for 3 weeks before surgery that could cause minor scratches
and abrasions to the affected extremity. For clean individuals, special
bathing before surgery is not necessary. Shaving in advance of the
actual time of the surgery is contraindicated because of the risk of
accidental nicking of the skin. Razor cuts more than a few hours old
may harbor bacteria. Shaving and prepping in most hospitals are
performed in the operating room immediately before the surgery,
preferably in a preoperative preparation room to avoid contaminating
the operating suite with hair. If shaving must be done in the operating
room, do it wet to avoid aerosolizing hair, which may contaminate the
surgical instruments. Because shaving increases bacterial production
from sweat and sebaceous glands, shave only the immediate area of the
surgical incision. It is usually not necessary to shave the entire
extremity.
preparation of the skin for surgery are variable and remain
controversial. The most commonly used skin-preparation solutions are
halogen agents that contain organic iodides such as povidone-iodine
soap and solution. Most surgeons prefer to defat the skin and remove
loose skin debris with a 5- to 10-minute scrub with soap using sterile
sponges and gloves.
-
Take care to avoid dripping excessive
soap or solution, which can cause burns of sensitive mucosal membranes,
onto the genitalia. Also avoid dripping it onto the operating table. If
you use alcohol, take extreme care to prevent it from pooling beneath
the operative field and posing a fire hazard during use of
electrocautery. -
Wash and dry the limb or surgical site
with sterile towels. Then apply povidone-iodine solution either from a
prepackaged spray bottle, which is safest from the standpoint of
contamination, or with sponge sticks, working from the site outward.
Let this solution dry in place, because its antibacterial action
continues for a substantial period of time during the surgery. If the
patient has a history of sensitivity to iodine, other choices are
chlorhexidine gluconate and hexachlorophene [such as pHisoHex (Sanofi
Winthrop, New York)].
the extremity on a sterile sheet or have an assistant support it using
sterile gloves or towels. This maneuver is exceedingly difficult with
heavy extremities that are unstable because of fractures. Take care to
avoid contamination, particularly proximally.
We present general guidelines for satisfactory draping and three
typical draping routines: one for the upper or lower extremity, one for
the shoulder, and one for the hip. They use the standard sheets
available in an average general surgery pack without specialized split
and extremity sheets as well.
patient satisfactorily, ideal technique requires three people. One
assistant supports the extremity and maintains its sterility as it is
being draped, and the other two obtain the draping materials from the
back table and deploy them. Perform draping after the scrub nurse,
surgeon, and assistant are fully gloved and gowned. Drape with double
gloves, because inadvertent contamination can occur. Strip off the
outer set of gloves after the drape and put on a new second set for the
procedure.
-
Place a cover sheet over the end of the
table to protect the surgeon and assistants from accidentally
contaminating their gowns by brushing against the nonsterile operating
table. Roll a double-layered tubular stocking or waterproof cover over
the extremity (Fig. 5.8). Use a folded, sterile
towel to cover the tourniquet. Secure it snugly around the thigh or the
arm just distal to the tourniquet with one or two towel clips.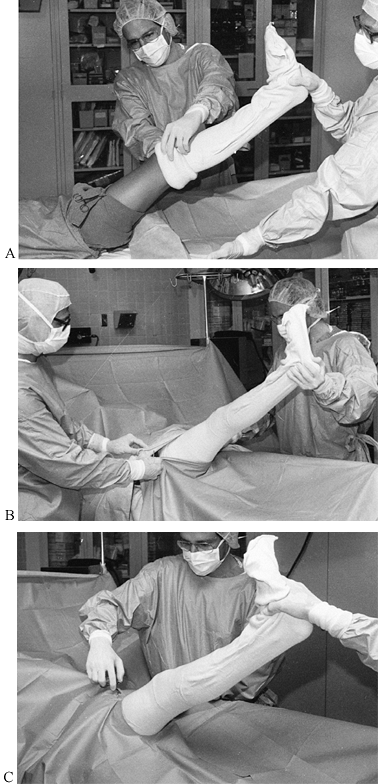 Figure 5.8. A: Draping the lower and upper extremity with two cover sheets, sterile towel, and tubular stockinet. B: Placement of cuffed sheets. C: Extremity extended through the hole of a laparotomy sheet. (Courtesy of David Templeman, M.D.)
Figure 5.8. A: Draping the lower and upper extremity with two cover sheets, sterile towel, and tubular stockinet. B: Placement of cuffed sheets. C: Extremity extended through the hole of a laparotomy sheet. (Courtesy of David Templeman, M.D.) -
Roll the stockinet into place, up to or
over the tourniquet, to prevent accidental contamination of sterile
gloves and gown. In addition, if incisions are carried far proximally,
the towel prevents accidental exposure of unprepared skin. Place a
second half-sheet with the proximal end folded downward 6 inches
beneath the extremity, taking care not to contaminate the folded edge.
Lay the limb down on this sheet. Place a second half-sheet with its
distal edge folded double for a distance of approximately 1 foot over
the proximal portion of the limb, matching the folded edge of the
underlying half-sheet. Secure these two sheets snugly together and to
the rolled stockinet with four towel clips (Fig. 5.8B). -
Next, place the limb through the hole in
a large laparotomy or extremity sheet. If a laparotomy sheet is used,
secure it snugly around the limb with two towel clips just below the
previously applied two half-sheets. Leave no exposed underlying
half-sheets (Fig. 5.8C).
disposable paper sheets. This draping routine—the equivalent of more
than four standard cloth sheets plus a waterproof layer—provides
optimal protection against contamination. When the drape is completed,
incise the stockinet with a pair of scissors and fold it back to expose
an adequate area for the surgery. We cover the skin with a
povidone-impregnated adhesive plastic sheet. If you do not wish to use
plastic incision drapes, suture or staple skin towels to the skin after
the incision is made. If a povidone-impregnated adhesive plastic skin
drape is used, defat the skin with alcohol before applying the drape to
ensure optimal adhesion. Adhesive drapes that remain in place provide
nearly ideal protection from the surrounding skin.
contamination. If the plastic drape loosens during the procedure, we
recommend removing and replacing it with standard skin towel drapes.
Carry the preparation and drape up to the tourniquet, so that the
entire limb is available if necessary.
-
For surgery on the upper extremity distal to the shoulder, drape as for the lower extremity; use a hand table if desired.
-
Roll a tubular stockinet to the elbow.
Secure a sheet into the axilla and across the chest. Cover the proximal
extent of the shoulder with a second sheet posteriorly and a third
anteriorly, which meet on the lateral neck. Secure these drapes with an
adhesive drape and then pass the limb through an extremity or
laparotomy sheet, and secure it with staples and another adhesive drape
(Fig. 5.9).![]() Figure 5.9.
Figure 5.9.
This shoulder drape is completed. Note that the sheets are attached
high on the neck and wide on the chest wall to provide full exposure.
decubitus position in nearly an identical manner. Modify as necessary
to be certain that the perineum is draped out of the operative field to
provide freedom of movement of the limb, which is necessary for hip
arthroplasty. Do not use a tourniquet.
-
Position the initial barrier sheet. Roll
a tubular or water-impervious stockinet over the extremity to the mid
thigh. Bring the first half-sheet into position, and secure it with
staples to the medial surface of the thigh, approximately 8 inches
distal to the perineum. Bring it carefully proximal along the anterior
and posterior aspects of the thigh and onto the torso. -
Secure this drape to the skin with large
skin staples. Towel clips can be used as well, but they tear through
paper drapes and interfere with radiography. Carry both the skin
preparation and drape well proximally onto the rib cage. -
Put the second sheet in place, and use
skin staples to secure it at the uppermost margin of the operative
field, proximal to the iliac crest. Fold the lower sheet into the
groin. Further secure these sheets with application of a
povidone-impregnated adhesive plastic drape (Fig. 5.10A).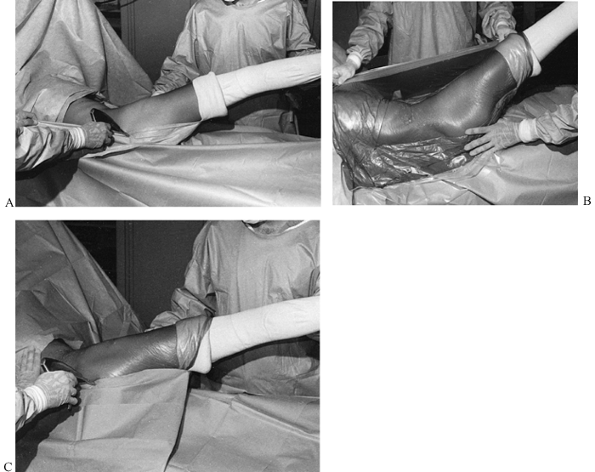 Figure 5.10. A: Draping in the lateral decubitus position for hip surgery. B: Exposed skin is covered with a large povidone-impregnated plastic drape. C: The extremity is brought through the hole in a laparotomy sheet.
Figure 5.10. A: Draping in the lateral decubitus position for hip surgery. B: Exposed skin is covered with a large povidone-impregnated plastic drape. C: The extremity is brought through the hole in a laparotomy sheet. -
Place the extremity through the hole in a
laparotomy sheet, and secure it to the medial thigh in the same
location as the previous sheets. Enlarge the hole in the drape with a
pair of scissors; bring it proximally over the extremity, and secure it
to the patient with skin staples. Secure this drape with a large
povidone-impregnated plastic sheet. With this draping arrangement, a
number of impervious layers are placed around the operative site. The
limb is free to move because the sheets are not brought directly into
the groin, and the groin is well draped out of the field (Fig. 5.10B).
We have found this fairly lightweight drape suitable for all
reconstructive surgery around the hip and pelvis. Some surgeons prefer
to add additional impervious layers, particularly if prolonged surgery
involving considerable blood loss is expected. Special draping sets
using combinations of adhesive-backed U-drapes work very well also, but
are usually somewhat expensive. -
Bring the extremity through the holes in a laparotomy sheet cut to fit the exposure desired (Fig. 5.10C).
Staple it in place and secure it with a second plastic drape. This
method avoids draping into the groin and gives ample sheet in the groin
area for movement of the extremity.
the orthopaedic fracture table is similar to that just described for
the hip. Square off the operative site with sterile sheets secured to
the skin with staples and an adhesive drape (Fig. 5.11A).
Place the final drape, a laparotomy sheet, on the hip. The extremity is
not draped free. Appropriate draping in both procedures allows the
C-arm to move beneath the drapes, preserving the sterile field (Fig. 5.11B).
With the C-arm in the lateral position, place the drapes so that they
hang 2 inches or so off the floor. Note that the entire hip and thigh
are available for the operative procedure. Drape only the C-arm
receiving head; do not drape the arm itself. Draping hinders movement
and nearly always results in contamination. For hip fractures, a
vertical adherent clear plastic drape works very well when full access
to the extremity is not necessary.
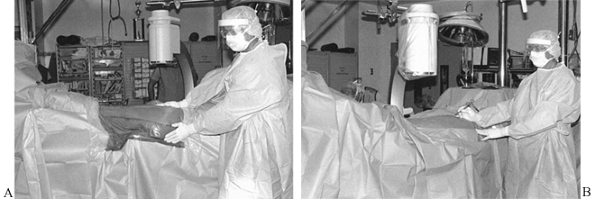 |
|
Figure 5.11. A: Drape for intramedullary nailing of the femur. B: Lateral decubitus position on a fracture table.
|
First cover the vertical posts and other table parts that might be
contacted during surgery. Place a large drape sheet above the knee and
secure it circumferentially with staples. Apply a similar drape around
the ankle. Leave enough room for distal cross-screw insertion. Then
create a “mesenteric” drape with a laparotomy sheet. Secure it with an
adhesive plastic skin
drape. The entire leg is left free, and the mesentery covers the C-arm in the lateral position.
 |
|
Figure 5.12. Drape for intramedullary nailing of the tibia on a fracture table.
|
technique with good hemostasis is essential for good results. Straight
skin incisions generally provide the best exposure, the least risk of
skin necrosis, and the best cosmetic results. Incisions can be
longitudinal between the joints and over the extensor surface of
joints. Over the flexor surface of joints, incisions must be
transverse. When it is necessary to extend incisions that cross the
flexor surface of joints, make curvilinear ones. In the torso, follow
Langer’s lines (see Chapter 1, Chapter 2 and Chapter 3). Hoppenfeld and deBoer’s text, Surgical Exposures in Orthopaedics, provides numerous detailed drawings and descriptions of various surgical approaches as well (67).
with a pair of scissors or cutting current. For easy closure and best
cosmetic results, make incisions at right angles to the skin. It is
often difficult in orthopaedic procedures when dealing with very
irregular surfaces to make such incisions. Take care to remain at right
angles to the skin even as the incision curves across the surface of
the limb.
with a sharp knife. Avoid blunt spreading with scissors or
electrocautery, which adds to soft-tissue damage. In certain areas
where subcutaneous and muscle hemorrhage is a particular problem, as in
the spine, electrocautery is used more frequently with minimal
morbidity. Rapid, precise dissection with the scalpel where tissues
must be cut, and blunt dissection with the finger or an elevator where
tissue planes can be split bluntly, is the most atraumatic and rapid
technique. Dissection with the scalpel, particularly in the region of
important neurovascular bundles, requires precise knowledge of anatomy.
Neurovascular bundles can be safely dissected out with a #15 blade if
the surgeon knows the anatomy and works from proximal to distal to
avoid cutting into the axillae of nerve and vascular branches. The good
surgeon treading in an anatomic region that she visits infrequently
will greatly improve her surgical technique by thoroughly reviewing the
anatomy and surgical approach before surgery.
practice to use a curved Mayo scissors to split the fascia lata and
expose the posterior border of the tensor fascia lata muscle, but this
is totally unnecessary. The surgeon wastes time in switching from the
knife to the scissors and back again. Most scissors are too dull and
result in slow, traumatic surgery. The knife is precise and quick. In
certain situations, the spread-and-cut technique with scissors is
permissible. It must be recognized, however, that using the scissors
for spreading and cutting is usually slow and traumatic. It is most
effective in dissecting out neurovascular structures where heavy
scarring is present, and in finding the digital neurovascular bundles
in the hand and foot.
layer by layer as the dissection proceeds. Isolate small vessels, clamp
with the tip of a forceps, and coagulate. Ligate larger vessels with an
appropriate-size suture. Major arteries and veins in the proximal
portions of the limb generally require double ligature, usually with a
suture ligature in the arteries, using appropriate-size, nonabsorbable
suture material. In the subcutaneous fat, it is sometimes quicker
to
control hemorrhage with light pressure from a sponge or laparotomy tape
and then touch the bleeding points with the electrocautery.
-
Prolonged and overvigorous retraction is damaging. Use self-retaining retractors sparingly and with minimal pressure.
-
Retract only tissues that need to be retracted, and avoid the skin edges.
-
Broad blades or tines cause less injury than narrow ones.
-
Do not use self-retaining retractors on
neurovascular bundles. Hand-held retractors can also cause nerve injury
if used with excessive force. -
Be certain to place the retractors in the correct position, and avoid placing pressure on sensitive structures.
-
Advise the assistant to be diligent in maintaining the proper position and pressure.
clamps. Be careful not to crush the tissue with the forceps; instead,
use only one tine of the forceps to retract the tissue (Fig. 5.13A).
During suturing, for example, rather than pinching the tissue, it is
better to pass the needle between the tines of the forceps, which are
used to support the tissues (Fig. 5.13B). This technique avoids skin trauma and places the forceps in an ideal position to grasp the end of the suture needle.
 |
|
Figure 5.13. Forceps technique.
|
necessary. Do not use it unless you have to. Do not repeatedly handle
tendons or neurovascular bundles. When necessary, place neurovascular
bundles in an appropriate-size Penrose drain loop, which is then used
for gentle retraction. Surgical exposures that exploit the intervals
between muscles are less traumatic than those that split muscles.
Minimize wound exposure to limit contamination and keep tissues moist.
Cover the portions of the wound not in the active surgical field with
saline-moistened sponges. In some instances, particularly in prolonged
procedures where a portion of the wound is not being used, it may be
appropriate to use temporary retaining sutures to hold the tissues
approximated, or staples in the skin. When a second wound is necessary,
such in as bone-grafting procedures, close the ancillary incisions as
soon as possible rather than leaving the wound open until you close the
main wound.
as in an open fracture before closure. Excise all damaged and nonviable
muscle. Copiously irrigate with sterile saline using a mechanical
pulsatile lavage-type irrigator.
avoid exposing it to excessive heat, which is necrogenic. To preserve
vascular supply, expose only the portion of the bone absolutely
essential to the operation. In the mid diaphysis, the periosteum and
contiguous muscle envelope supply little blood to the cortex;
therefore, subperiosteal dissection is permissible. Preserve the
nutrient artery, perforating vessels, and ligament and tendon
insertions, if possible. Bone fragments with only minimal muscle
attachments are probably devascularized, but if they are anatomically
and securely fixed they may become revascularized.
results from overheating of the bone by cutting and drilling
instruments, especially if they are dull and run at excessive speeds.
Keep all cutting and drilling tools sharp, and cool them with saline
during use. Replace drill points and saw blades frequently. Sharp hand
instruments are essential for precise, skillful, and rapid carpentry of
bone. Avoid stress-risers in bone, which may later lead to pathologic
fracture. Holes or windows in bone, particularly in the mid diaphysis,
are significant stress-risers. Holes smaller than 5 mm in diameter
present few problems, but holes more than 10 mm in diameter, or more
than 30% of the diameter of the bone, lower the strength of bone,
particularly in torsion. Make windows as smooth and round as possible.
Terminate cuts in bone at a drill hole to dissipate the stress-riser
effect.
a problem. We prefer bone wax for hemostasis. Soften the wax by
kneading it, and apply it to the bleeding area with the finger
protected by a laparotomy tape. Remove all excess wax.
bleeding bone surfaces, suction-drainage is frequently utilized,
although some authors question the necessity of routine drainage (14,30,82,124).
Many drains and methods are available. Take care when placing suction
drains directly on raw, bleeding bone surfaces or in intramedullary
canals to avoid exsanguination. Pass the trocar through the soft
tissues at an angle. This technique may better allow the drainage
tract, which exits the various fascial barriers at separate locations,
to seal. It prevents the development of a persistent draining tract.
Direct connection to wall suction is generally contraindicated because
of the risk of excessive bleeding. Commercially available,
self-activated suction reservoirs are safest. On occasion, it may be
prudent to delay activation of suction until several hours after the
completion of surgery to allow formation of a clot on bone surfaces.
then advance the drain to the premarked point, making sure that the
drain is free. Then close the remaining wound. If a drain is
inadvertently sewn in, postoperative removal may be possible; attach a
5-pound weight to break the offending suture. Inspect all drains upon
removal to ensure that breakage has not occurred.
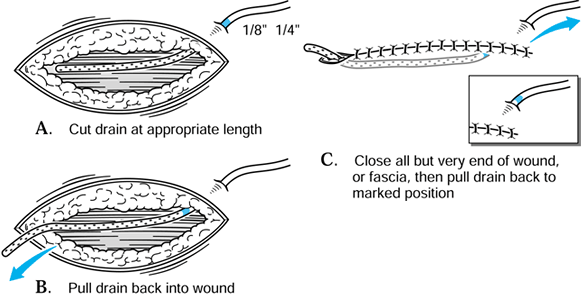 |
|
Figure 5.14. Technique to ensure drain is not inadvertently sewn into the wound.
|
dressings and moving the patient from the operating table, suture the
drain to the skin or carefully tape it to the extremity. In addition to
providing egress for blood and serum, drains provide a route for
organisms to enter the wound. Remove drains as soon as bleeding and
drainage from the wound have ceased, in most cases by 48 hours after
surgery. To avoid contaminating the wound, do not irrigate plugged
drains. Use large enough drains to prevent plugging by clots.
wounds before closure. In small wounds for which exposure time has been
short, bulb or syringe-type irrigation is adequate. When wounds have
been exposed for more than 1 hour and involve large surface areas,
copious irrigation with a mechanical pulsatile lavage irrigator is
recommended. The addition of antibiotics or sterile castile soap to the
irrigation solution may be helpful in removing surface bacteria (7,34).
Other authors have reported detrimental effects of aggressive pulsatile
lavage on the soft tissues and early fracture healing (33,89).
generally watertight. Use the smallest possible suture that provides
adequate strength. In orthopaedics, running sutures for closure are
generally contraindicated, because use of the limb may result in
failure of these sutures and wound dehiscence. Interrupted sutures
provide a stronger closure and allow fine adjustment of tension to
avoid tissue necrosis.
or larger, for the repair of ligaments, tendon insertions (except in
the hand and foot and in children, where smaller or different types of
sutures may be indicated), fascia, and fibrous joint capsules. Braided
sutures are easier to handle and are less likely to slip than
monofilament. Use monofilament sutures when the risk of infection is
high, because they are associated with a lower rate of infection than
braided sutures.
the quadriceps origin using figure-eight sutures. Figure-eight sutures
are helpful when tension is high or sutures tend to pull through the
tissues. Close the deep fascia of the thigh with 0 or 2-0 interrupted
sutures. Close the subcutaneous tissues with inverted, interrupted, 3-0
polyglactin sutures. When cosmetic issues are not of great concern and
speed is important, we close the skin with staples. We generally remove
staples 7 to 14 days postoperatively and replace them with skin tapes.
Interrupted sutures, preferably using a monofilament synthetic,
nonabsorbable material, are an alternative to staples. Avoid
strangulation of the subdermal vascular plexus by approximating the
skin edges without tension.
extremity wounds. Place sutures at right angles to the skin and 3–4 mm
from the edge. Simple, interrupted sutures are best. We avoid mattress
sutures, if possible, because they devascularize skin edges more than
simple sutures. The suture described by Allgöwer et al. is useful where
the viability of the skin is marginal (Fig. 5.15).
The subcuticular stitch is placed on the most precarious side of the
wound. Use Gillie’s suture on the corners of flaps. For most skin
closures, we prefer a running subcuticular suture of either 3-0 or 4-0
absorbable polyglactin, and in some cases nylon or proline, which is
later removed. Augment this closure with skin tapes placed so that
there is no tension. When significant postoperative swelling is
expected, do not use skin tapes. Avoid benzoin adhesive under skin
tapes because of the risk of allergic reaction.
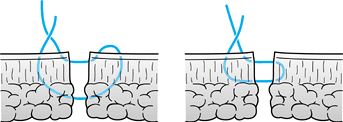 |
|
Figure 5.15.
Allgöwer stitch for closing skin with marginal vascular supply. (Redrawn with permission from Müeller ME, Allgöwer M, Schneider R, Willenegger H. Manual of Internal Fixation. New York: Springer-Verlag, 1979.) |
dressing that we call a Robert Jones dressing. Place a sterile,
nonadhesive dressing on the wound. Do not use nonabsorbent dressings,
which do not allow passage of blood and serum, because accumulation of
blood and serum around the incision can cause inflammation. We prefer
gauze impregnated with 3% bismuth tribromophenate (Xeroform) in a
petrolatum blend, with some of the petrolatum scraped off. Over the
gauze, place sterile gauze sponges followed by abdominal pads,
depending on the amount of postoperative blood and fluid expected from
the wound. Encase the entire extremity in bulk, sterile cotton that is
rolled to produce a snug dressing approximately 2–3 inches thick.
plaster splints to maintain the limb in the desired position. Finally,
overwrap with a bias-cut stockinet. This dressing removes fluids from
the operative site by capillary action. It provides firm support to the
limb to limit swelling, yet there is ample volume to permit swelling of the limb without pressure or neurovascular compromise. The outer hard dressing positions the limb appropriately.
thigh, hip, trunk, or shoulder. In these locations, hold the gauze
sponges and abdominal pads in place with tape or a burn stocking. Use
tape with care in orthopaedics, because considerable postoperative
swelling often occurs. Strong adhesive tape, such as paper tape,
applied tightly may shear the skin and cause blisters. Dress patients
who have a history of tape sensitivity with hypoallergenic tape or no
tape at all. An elastic bandage (Ace-wrap) applied in spica fashion
around hip incisions holds the dressing in place and also provides
gentle compression.
pillows, as the heat generated as the plaster sets may be severe enough
to cause a superficial skin burn. Splints should initially remain
uncovered and elevated on cloth blankets or towels until the heat is
dispersed.
surgical team can elevate the limb, position any necessary traction
devices, or reapply thromboembolic stockings or sequential compression
devices. Delay can be minimized if the surgical team performs these
tasks rather than waiting for written postoperative orders to be
followed. Additionally, the surgeon can then be sure that the
appropriate devices and position are used.
emotional issue. Inadvertent percutaneous self-injury may be anxiety
provoking, especially if the patient is perceived to be in a high-risk
category or known to be infected with a transmissible disease. Having
accurate information about the potential for disease transmission is
the most effective way to minimize anxiety.
the safety of the surgical team, especially for orthopaedic surgeons,
who are frequently exposed to sharp bone fragments and use power tools
that aerosolize particles. The actual risk of AIDS transmission is low;
the risk of transmission of other infectious diseases, namely hepatitis
B and C, is much greater. While adequate prophylaxis
to
hepatitis B may be obtained through vaccination, the operating team
must take precautions to minimize their risk of contracting hepatitis C
and other infectious organisms. Universal precautions have been
developed to minimize the surgical team’s risk of intraoperative
infection exposure (47).
hepatitis B (HBV) a preventable disease. Because of the high risk of
percutaneous injury, all operating room personnel should receive
hepatitis B vaccine. HBV is much more contagious than other viruses;
the risk of transmission of infection has been estimated to be 30%
following a single percutaneous exposure to blood from a patient whose
HBV disease is in the most infectious period (136).
has effected a dramatic decrease in the transmission of HBV to health
care workers through blood exposure. In 1993, an estimated 1,450 health
care workers became infected with HBV though exposure to blood and body
fluids, a 90% decrease from the number infected in 1985. Between 5% and
10% of those infected with HBV develop chronic hepatitis and are at
risk for developing cirrhosis and hepatocellular carcinoma. An
estimated 100 to 200 health care workers have died annually during the
1990s as a result of complications related to chronic HBV infection (25).
Among the orthopaedic surgeons surveyed at the 1991 meeting of the
American Academy of Orthopaedic Surgeons, 67% had received the
hepatitis B vaccination. The incidence of vaccination was inversely
proportional to the age of the surgeon. Ninety percent of surgeons
between 20 and 29 years old had been vaccinated, while only 35% of
surgeons 60 years or older had been vaccinated. Thirteen percent of
individuals showed evidence of infection with HBV and less than 1%
showed infection with hepatitis C. The prevalence of both infections
increased with age. Only 3% of those in their third decade of life were
infected with HBV, while 27% of surgeons 60 years or older showed
serologic evidence of HBV infection (133).
Further periodic serologic testing is not currently recommended once
adequate antibody response has been confirmed following primary
vaccination (25).
large percentage of the population, is a potential risk for practicing
orthopaedic surgeons since no effective vaccine exists. The risk of
infection from needle sticks in HCV is intermediate between that of HIV
and HBV (103). The risk to health care workers from a random needle stick has been estimated to be about 0.1% (58).
Several studies have reported that the risk of seroconversion in health
care workers sustaining a percutaneous exposure to blood from
anti-HCV-positive patients ranges from 0% to 6% (41,77,81).
There appears to be a higher risk from hollow-bore needle sticks than
from the more common suture needle sticks or the other sharp objects
surgeons are exposed to. In one study, four seroconversions were seen
following 331 hollow-bore needle sticks, while no seroconversions were
reported following 105 injuries with suture needles or other sharp
objects (118).
hepatitis C be tested after exposure and again 6–9 months later to
detect hepatitis C antibodies (48). If hepatitis C infection is diagnosed, liver function studies should be performed to evaluate for chronic hepatitis.
recommended following exposure to non-A, non-B hepatitis (now known as
hepatitis C). The immune globulin, which came from pooled plasma (some
from donors infected with HCV), was felt to confer passive immunity.
Since 1992, however, patients who are hepatitis C positive have been
excluded as plasma donors. Current data do not support the use of
immune globulin for prophylaxis against hepatitis C.
short term in the treatment of chronic HCV infection. Treatment is
recommended for patients who are at greatest risk of progression to
end-stage cirrhosis. This group includes patients with a persistently
elevated alanine aminotransferase, presence of HCV RNA in the blood,
and a liver biopsy showing either portal or bridging fibrosis and at
least a moderate degree of inflammation and necrosis (103). Combination of the antiviral medication ribavirin with alpha interferon has shown promise in early studies (121).
an orthopaedic surgeon is unknown. While the risk of seroconversion
after a single percutaneous exposure may be low, great concern remains
about the cumulative risk to orthopaedic surgeons, who often sustain
numerous percutaneous injuries over the course of a lifetime. The
cumulative risk has been estimated to be as high as l% to 4% (160).
represent an overestimate for several reasons. The risk of HIV
transmission is affected by the degree of viremia in the patient, the
volume of the exposure, and the seroprevalence in the patient
population, which varies greatly by geographical region. An estimated
5% to 10% of acute trauma patients in certain urban areas may be HIV
positive (88).
It is believed that the greatest risk of transmission is from a
percutaneous injury, and that aerosolized particles, mucous membrane
exposure, and exposure to intact skin present a low risk of disease
transmission. Percutaneous injury with a hollow-bore needle is believed
more likely to result in HIV transmission than injury by a solid suture
needle, which is more commonly the experience of orthopaedic surgeons (46).
solid-core suture needle injury. Needle type, size, and depth of
penetration have been shown to be independent predictors for the volume
of blood transferred in a percutaneous needle stick. The volume of
blood transmitted is reduced by at least 50% when the needle passes
through a glove made of any kind of material (94).
The probability of transmission of infections may be more associated
with the patient’s viral titer than with the amount of the inoculum (114).
Prospective studies of health care workers have estimated that the
average risk of HIV transmission after a percutaneous exposure to
HIV-infected blood is approximately 0.3% (95% confidence interval, 0.2%
to 0.5%) (15).
Prevention had received 52 reports of documented U.S. health care
workers who had seroconversion following occupational HIV exposure.
Possible occupational HIV transmission has been reported in 114 cases
in which the health care worker had no other known risk factor but
there was no specific documented exposure (26).
A seroprevalence survey involving 48% of the orthopaedic surgeons
attending the 1991 annual meeting of the American Academy of
Orthopaedic Surgeons found only two HIV-positive individuals (0.06%),
and both of these surgeons reported additional nonoccupational risk
factors (23).
infection before systemic infection occurs. During this time,
postexposure antiretroviral intervention may modify viral replication.
The U.S. Public Health Service has established recommendations for
postexposure prophylaxis based on the risk of infection following the
exposure and the infectivity of the exposure source (26) (Fig. 5.16).
These recommendations are likely to continue to evolve as new
retroviral agents or combination of agents are introduced and as HIV
strains develop drug resistance. The risks of toxicity and side effects
from prophylaxis will need to be continually weighed against the
relatively low risk of disease transmission following an occupational
exposure.
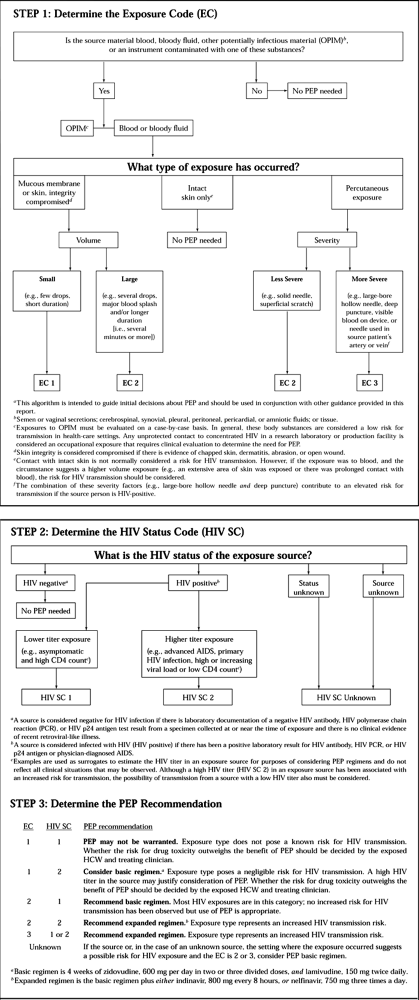 |
|
Figure 5.16. Determining the need for HIV postexposure prophylaxis (PEP) after an occupational exposurea.
(Reprinted with permission from Centers for Disease Control. Public Health Service Guidelines for the Management of Health Care Worker Exposure to HIV and Recommendations for Postexposure Prophylaxis. MMWR Morb Mortal Wkly Rep 1998;47:1. |
blood during surgery were potentially preventable by the use of
additional barrier precautions, such as face shields and
fluid-resistant gowns (111). Although there
have been no documented cases of transmission, the possibility has been
raised that HIV could be transmitted via aerosolized particles produced
by power tools (70). Use face shields to prevent this kind of contamination (76)
and splash shields to minimize the spread of the aerosolized fluids
from mechanical pulsatile lavage. Double gloving, initially popularized
in total joint arthroplasty as a way of minimizing the risk of the
surgeon’s contaminating the patient (95), has
become important in protecting the surgeon. Latex glove perforation
occurs frequently. The use of double gloves has been shown to be
effective in minimizing the risk of perforation. Double gloving may
result in a 60% to 80% decrease in inner glove perforation and visible
blood contamination (49). Some authorities
recommend inspecting and routinely changing latex gloves at least every
2 hours during long-duration procedures (87,126). One author has recommended that gloves be changed at least every 25 minutes (114). Cloth, Kevlar, and stainless-steel-reinforced gloves provide additional protection from perforation (63,87,126,145).
Despite the use of these reinforced protective gloves, perforation of
the inner gloves nevertheless occurs and may go unrecognized. Fluid
sensors have been developed to alert surgeons when a glove has been
penetrated.
gown may become saturated with blood or fluid. The use of a
self-contained air exchange system may also offer protection, but the
benefits it confers may be outweighed by communication difficulties it
causes that could lead to accidental injury (28).
Alternatively, an impervious urology apron worn under the surgical gown
effectively prevents contamination of the trunk and lower extremities (49).
Knee-length rubber boots or impervious shoe covers are recommended for
cases in which the potential for blood loss is significant, or during
arthroscopy.
recommended changing surgical methods to reduce the risk of injury: Be
aware and cautious, use pop-off needles, avoid having more than one
person suture at a time, announce the passage of sharp instruments and
pass them in a kidney basin or other device. Take care to avoid digital
palpation of fracture sites or sharp bone surfaces. Cover pins and
wires with protective caps. Periodically inspect gloves and gowns (4).
-
Wash the affected area immediately.
-
Bleed the wound.
-
Apply 70% isopropyl alcohol on the wound directly.
-
Report the incident.
operation performed and the condition of the patient. Several general
principles apply to the majority of patients, however. Elevate limbs
postoperatively to improve venous and lymphatic return, but not so high
that arterial input is compromised. In general, the ideal level is
where the most distal portion of the extremity is 10 cm above the
heart. Elevation on pillows is usually more comfortable for the patient
and less hazardous than slings. Immobilize only joints that must be
immobilized. Unless contraindicated, all mobile parts must be moved
after surgery, either by active, active-assistive, or passive exercises.
to the toilet, and subsequently for ambulation is important. With
today’s surgical techniques, enforced bed rest after surgery is
unusual. If a patient is on enforced bed rest, it is important to
institute an in-bed physical therapy program to maintain joint range of
motion, muscle strength, and, if possible, aerobic conditioning. Not
only does such a regimen play a major role in mobilizing the patient
later, but it also provides great psychological benefit.
in orthopaedic surgery. Recent studies have highlighted the
pervasiveness of this problem among several orthopaedic patient
populations (50,130,158).
Without prophylaxis, a deep venous thrombosis may occur in 40% to 60%
of patients undergoing total hip or knee arthroplasty. Proximal deep
venous thrombosis may occur in 15% to 20%, and a fatal pulmonary
embolism may occur in 0.5% to 2% (85). Risk
factors that have been identified for thromboembolic disease include
age (becoming clinically important by age 40 and increasing
thereafter); prolonged immobility or paralysis; history of prior
thromboembolism; cancer; major surgery (particularly operations on the
pelvis and lower extremities); obesity; varicose veins; congestive
heart failure; myocardial infarction; stroke; fractures of pelvis, hip,
or leg; and possibly high-dose estrogen use (29).
and authorities recommend both mechanical and pharmacologic methods.
Intraoperative anesthetic techniques including hypotensive and epidural
anesthesia have been shown to reduce the risk of thromboembolism (86,134).
Mechanical methods include use of compression stockings and various
pneumatic compression devices. Pharmacologic methods include dextran,
aspirin, warfarin, heparin, and low-molecular-weight heparin.
are asymptomatic, remains difficult. Methods for diagnosis of deep
venous thrombosis include impedance plethysmography, fibrinogen I-125
scanning, venography, and duplex ultrasound. Diagnostic methods for
pulmonary embolism are ventilation-perfusion scanning and pulmonary
angiography. There is controversy over the ideal diagnostic testing
modality because of variation in sensitivity and specificity of the
different tests. They also vary in their ability to image different
parts of the venous systems, and in the invasive risk they pose to the
patient.
controversy also exists over whether all thrombi should be treated,
what method of treatment should be used, and how long treatment should
last. Low-molecular-weight heparin, which does not require laboratory
monitoring, is increasingly the treatment of choice in the outpatient
management of deep venous thrombosis (155). The
numerous investigations and recommendations on this important subject
are beyond the scope of this chapter. While many therapeutic methods
have been shown to reduce the risk of thromboembolism, no single method
is completely effective or applicable to all situations. Metaanalysis
of recent randomized clinical trials has shown low-molecular-weight
heparin to be superior to warfarin in prevention of deep venous
thrombosis, but its use is associated with a greater number of minor
bleeding complications (110). Each surgeon must
assess the varying risk that thromboembolism presents to each
individual patient, selecting the prophylactic method that appears to
provide the greatest risk reduction when balanced against any potential
risk of the treatment itself.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
GC, Auchincloss JM, Johnson DP, Newman JH. The Timing of Tourniquet
Application in Relation to Prophylactic Antibiotics Administration. J Bone Joint Surg 1988;70B:322.
J, Flanagan P. Blood Transfusion Risk: Protecting against the Unknown:
Worries over Variant CJD Should Not Detract from Work on Other, Better
Known, Risks (editorial). BMJ 1998;316:717.
Orthopaedic Perioperative Erythropoietin Study Group. Effectiveness of
Perioperative Recombinant Human Erythropoietin in Elective Hip
Replacement. Lancet 1993;341:1227.
for Disease Control. Immunization of Health-care Workers:
Recommendations of the Advisory Committee on Immunization Practices
(ACIP) and the Hospital Infection Control Practices Advisory Committee
(HICPAC). MMWR Morb Mortal Wkly Rep 1997;46(RR-18):1.
for Disease Control. Public Health Service Guidelines for the
Management of Health-care Worker Exposures to HIV and Recommendations
for Postexposure Prophylaxis. MMWR Morb Mortal Wkly Rep 1998;47(RR-7):1.
R, Cazzola A, Bacci G, et al. Effect of Perioperative Transfusions on
Survival in Osteosarcoma Treated by Multimodal Therapy. Cancer 1989;64:1727.
L, Reynolds MR, Esterhai JL Jr. Hazards to the Orthopaedic Trauma
Surgeon: Occupations Exposure to HIV and Viral Hepatitis (A Review
Article). J Orthop Trauma 1996;10:289.
GP, Anderson FA Jr, Heit J, et al. Prevention of Venous
Thromboembolism: Fourth ACCP Consensus Conference on Antithrombotic
Therapy. Chest 1995;108(suppl. 4):312S.
DR, Duff GP, Dahners LE, et al. High Pressure Pulsatile Lavage
Irrigation of Intraarticular Fractures: Effects on Fracture Healing. J Orthop Trauma 1998;12:460.
RW, Schutzer SF, Deafenbaugh MK, Bartosh RA. Compartment Syndrome
Complicating Use of the Hemi-lithotomy Position during Femoral Nailing:
A Report of Two Cases. J Bone Joint Surg 1989;71:1556.
PM, Ritter MA, Abels RI. The Effects of Recombinant Human
Erythropoietin on Perioperative Transfusion Requirements in Patients
Having a Major Orthopaedic Operation. J Bone Joint Surg 1996;78A:62.
G, Giustina G, Degos F, et al. Morbidity and Mortality in Compensated
Cirrhosis Type C: A Retrospective Follow-up Study of 384 Patients. Gastroenterology 1997;112:463.
F, Rinaldi R, Cattelan AM, et al. Low Prevalence of Antibodies to
Hepatitis C Virus in Hospital Employees (letter to the editor). Infection 1992;20:295.
DM, Lombardi AV, Mallory TH, et al. An Evaluation of the Efficacy of
Postoperative Blood Salvage after Total Joint Arthroplasty: A
Prospective Randomized Trial. J Arthroplasty 1991;1:109.
LT, Rudnick S, Price TH, et al. Increased Preoperative Collection of
Autologous Blood with Recombinant Human Erythropoietin Therapy. N Engl J Med 1989;321:1163.
LT, Vizmeg K, Sobecks R, et al. Prevalence and Classification of Anemia
in Elective Orthopaedic Surgery Patients: Implications for Blood
Conservation Programs. Vox Sang 1992;63:90.
SC, Margolis HS. Hepatitis B Immunization: Vaccine Types, Efficacy, and
Indications for Immunization. In: Remington JS, Swartz MN, eds. Current Topics in Infectious Disease. Boston: Blackwell Scientific, 1992:282.
AR, McClure AG, Skyhar MJ, et al. Local Compression Patterns beneath
Pneumatic Tourniquets Applied to Arms and Thighs of Human Cadavers. J Orthop Res 1987;5:247.
D Jr, DiPasquale T, Sanders R. Comparison of Cloth Gloves Used in
Orthopaedic Surgery: A Clinical and Experimental Evaluation. J Orthop Trauma 1998;12:106.
JF, Messer A. Evaluation of the Role of Coronal and Sagittal Axial CT
Scan Reconstruction for the Imaging of Acetabular Fractures. Clin Orthop 1994;305:152.
G. Early Clinical Experience with a Disposable Unit for the
Intraoperative Salvage and Reinfusion of Blood Loss (Intraoperative
Autotransfusion). Am J Surg 1970;120:718.
GR, Richardson M, Bosse MJ, et al. Efficacy of Surgical Wounds Drainage
in Orthopaedic Trauma Patients: A Randomized Prospective Trial. J Orthop Trauma 1998;12:348.
JR, Huo MM, Hanmway J, et al. The Prevalence of Deep Venous Thrombosis
after Total Hip Arthroplasty with Hypotensive Epidural Anesthesia. J Bone Joint Surg 1994;76A:341.
S, Steinberg EL, Gruen OA, et al. Outer Gloves in Orthopaedic
Procedures: A Polyester/Stainless Steel Wire Weave Glove Liner Compared
with Latex. J Orthop Trauma 1998;12:101.
JV Jr., Logan LR, Benson DR, Glasser DB. Human Immodeficiency Virus
Infection: Complications and Outcomes of Orthopaedic Surgery. J Amer Acad Orthop Surg 1996;4:297.
M, Petty W, Hendeles L. Effect of Irrigation and Tourniquet Application
on Aminoglycoside Antibiotic Concentrations in Bone. J Orthop Res 1988;6:311.
S, Woolwine JD, Gerberding JL. Efficacy of Gloves in Reducing Blood
Volumes Transferred during Simulated Needlestick Injury. J Infect Dis 1993;168:1589.
SF, Berg EW, Saunders EA. Efficacy of Double-gloving as a Barrier to
Microbial Contamination during Total Joint Arthroplasty. J Bone Joint Surg 1981;63A:811.
P, Heal JM, Blumberg N. Infection or Suspected Infection after Hip
Replacement Surgery with Autologous or Homologous Blood Transfusions. Transfusion 1991;31:212.
PM, Bourke DL, Walsh PC. A Randomized Trial of Perioperative
Hemodilution versus Transfusion of Preoperatively Deposited Autologous
Blood in Elective Surgery. Transfusion 1992;32:226.
AJ, Koppenhagen K, Kirchof B, et al. Efficacy and Safety of Low
Molecular Weight Heparin, Unfractionated Heparin and Warfarin for
Thrombo-embolism Prophylaxis in Orthopaedic Surgery: A Meta-analysis of
Randomised Clinic Trials. Haemostasis 1997;27:75.
A, Cesarelli M, DiSalle F, et al. Preliminary Notes on the Conversion
of Three-dimensional Images of Bone into CAD Structures for the
Pre-operative Planning of Intertrochanteric Osteotomies. Med Biol Eng Comput 1993;31:529.
MS, Abel MD, Moore SB. Transfusion-related Acute Lung Injury Associated
with Passive Transfer of Anti-leukocyte Antibodies. Am Rev Respir Dis 1983;128:185.
RM, Vaughan JJ, Von Fraunhofer JA, et al. A Method of Determining the
Angular Malalignments of the Knee and Ankle Joints Resulting from a
Tibial Malunion. Clin Orthop 1987;223:213.
V, Petrosillo N, Ippolito G, Italian Study Group on Occupational Risk
of HIV and Other Bloodborne Infections. Risk of Hepatitis C
Seroconversion after Occupational Exposure in Health Care Workers. Am J Infect Control 1995;23:273.
MV, Crossett LS, Herndon JH. Postoperative Infection Following
Orthopaedic Surgery in Human Immunodeficiency Virus-infected
Hemophiliacs with CD4 Counts < or = 200/mm3. J Arthroplasty 1995;10:716.
BJ, Sangeorzan BP, Hansen ST Jr, Judd RP. Mathematically Directed
Single-cut Osteotomy for Correction of Tibial Malunion. J Orthop Trauma 1989;3:267.
G, Wirnsberger GH, Obernosterer A, Babinski K. Thromboembolic
Complications after Arthroscopic Knee Surgery: Incidence and Risk
Factors in 101 Patients. Acta Orthop Scand 1998;69:144.
RM, Ward JW, Buehler JW. Trends in Transfusion-associated Acquired
Immune Deficiency Syndrome in the United States, 1982 to 1991. Transfusion 1993;33:890.
CN, Tokars JI, Chamberland ME, American Academy of Orthopaedic Surgeons
Serosurvey Study Committee. Use of the Hepatitis-B Vaccine and
Infection with Hepatitis B and C among Orthopaedic Surgeons. J Bone Joint Surg 1996;78A:1791.
M, Shamiss A, Orgad S, et al. The Role of Blood from HLA-homozygous
Donors in Fatal Transfusion-associated Graft-versus-Host Disease after
Open Heart Surgery. N Engl J Med 1989;321:25.
V. The Value of Routine Preoperative Laboratory Testing in Predicting
Postoperative Complications: A Multivariate Analysis. Surgery 1991;109:236.
JW, Grindon AJ, Feorino PM, et al. Laboratory and Epidemiologic
Evaluation of an Enzyme Immunoassay for Antibodies to HTLV III. JAMA 1986;256:356.
JW, Holmberg SD, Allen JR, et al. Transmission of Human
Immunodeficiency Virus (HIV) by Blood Transfusions Screened as Negative
for HIV Antibody. N Engl J Med 1988;318:473.
JS, Hulstyn MJ, Fadale PD, et al. Incidence of Deep Vein Thrombosis
after Arthroscopic Knee Surgery: A Prospective Study. J Arthroscopic Rel Res 1995;11:701.
ST, Marsh JS, Tanner JB. Transfusion of Previously Deposited Autologous
Blood for Patients Undergoing Hip Replacement Surgery. J Bone Joint Surg 1987;69A:325.