Pelvic Ring Fractures
and nonoperative treatment of injuries to the pelvic ring have only
recently evolved (and continue to evolve) to include early open
reduction and internal fixation to restore normal anatomic
relationships. Prior to the 1980s, there was a significant paucity of
both clinical and scientific information regarding the biomechanics of
the bony and ligamentous structures, the techniques for stabilization
of pelvic ring disruptions, and the functional outcomes of patients who
survived these injuries. Indeed, as recently as the 1970s, many pelvic
ring disruptions were treated with nonoperative techniques, consisting
of skeletal traction and pelvic slings to prevent excessive cephalad
migration of the hemipelvis. The usual axiom in the infancy of
treatment for pelvic injuries was that if a patient survived a pelvic
fracture, they generally did well.
numbers of these patients began to characterize injury patterns,
associated injuries, and causes of mortality. Additionally, they began
to document the high incidence of poor functional outcome and chronic
pain in patients with displaced vertically unstable pelvic fractures
treated nonoperatively. A brief historical overview on the treatment of
pelvic fracture-dislocations will help place into perspective the
current techniques and philosophies used today as well as the
directions that we find ourselves headed moving into the future of
managing pelvic trauma.
in 1948, we see one of the first reports making a clear distinction
between bony and ligamentous injuries with respect to functional
outcome. In his series of 50 patients, those with pure sacroiliac (SI)
dislocations faired worse (measured as ability to return to work) than
those with fractures of the ilium or sacrum. In addition to the
posterior ring injury pattern, Holdsworth also noticed that functional
outcome appeared to be related to the anatomicality of the reduction
(that is, those patients with anatomic or near anatomic reductions
appeared to do better functionally). These views were echoed by Raf132 in 1966 and later by Dujardin39
in 1998 in their analysis of functional outcomes some 50 years later.
Raf’s relatively detailed analysis also reported on the high incidence
of
chronic pain and limp (33%), neurologic injury, and bowel and bladder
dysfunction in patients with unstable or displaced high-energy pelvic
fracture-dislocations.
to the earlier literature regarding the classification, treatment, and
outcome of high-energy traumatic pelvic fracture-dislocations. In a
series of publications70,167
on a large series of patients (over 400) through the early 1970s, they
further documented the high morbidity and mortality associated with
displaced vertically unstable pelvic fractures. Functional outcome and
pain were related to both the pattern of posterior ring injury
(dislocation versus fracture) as well as the final position of healing.
Although a minority of the overall patient population, the so-called
“double-vertical” or “Malgaigne” injury pattern (vertical injury
through both the posterior and anterior aspect of the pelvic ring) had
a mortality rate of 7%. In other early publications91,136 on large series of patients during the same era, mortality rates were reportedly as high as 18%.
understanding developed in the previously cited publications, an effort
was made to better manage the musculoskeletal as well as associated
visceral injuries to improve the early and late mortality and morbidity
in patients with severe pelvic injuries. Slatis168 and Riska139
in concurrent but separate publications documented modest improvement
in both mortality and late morbidity in patients with displaced
vertically unstable pelvic fractures treated with an external fixation
frame at the time of injury.
fixators for treating patients with pelvic fractures increased
markedly. However, with ever-increasing critical analyses on more
well-defined subgroups of patients, it became clear that external
fixation alone could be sufficient for certain anterior or lateral
compression injuries,86,192 but it was inadequate in controlling displaced unstable posterior pelvic ring injuries.77
the management of severe unstable pelvic ring injuries provided the
impetus to develop a better understanding of the biomechanics of the
pelvic ring and internal stabilization devices/techniques such that
improved treatment algorithms could be used to improve the functional
outcomes and significant morbidity and mortality associated with these
injuries. This chapter will focus on the modern clinical literature
discussing the emergent, surgical, and postoperative management of
traumatic injuries to the pelvic ring, and provide an overview of
recent novel techniques utilizing surgical navigation, spinal-pelvic,
and percutaneous fixation techniques.
descending order) motorcycle crashes, auto-pedestrian collisions,
falls, and motor vehicle crashes (MVCs) and crush injuries.1,36
Trauma registries and data banks have noted a concomitant increase in
the incidence of pelvic fracture with the increasing incidence of
high-velocity MVCs.36,72,138
Side impact and vehicle incompatibility (small versus large vehicles)
are the main risk factors for pelvic fracture morbidity and mortality.146 Not surprisingly, based on these mechanisms, the predominant epidemiologic marker is a man at an average age of 33 years.102
Because of the enormous forces that are required to disrupt the pelvic
ring, traumatic pelvic fractures and the accompanying visceral and soft
tissue injuries are associated with diverse outcomes and an assortment
of morbidity and mortality rates ranging from 10% to 50%.57,91,108,136,139,140
professionals regarding mechanism of injury, patient presentation, and
physical examination should raise the suspicion for pelvic injuries.
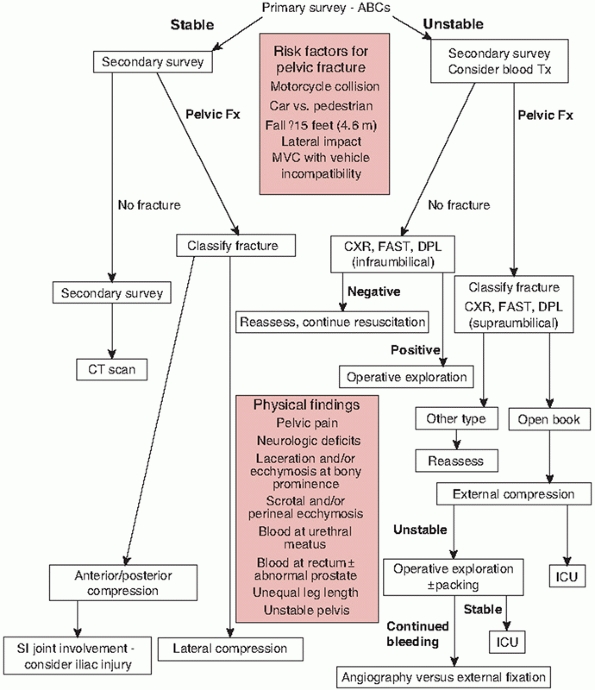
Established standard prehospital transport protocols and emergency
department management algorithms (Fig. 44-1) have documented benefit and validity and should be followed to improve patient survivability and decrease morbidity.19,41,93
Appropriate immobilization of the cervical, thoracic, and lumbar spine
as well as injured extremities, airway protection, and initial
circulatory support with expedient transport to a definitive care
facility are the main goals.
intraabdominal, soft tissue, pelvic, and extremity hemorrhage and
requires a coordinated multidisciplinary team approach.83
Once the primary survey is completed and a secure airway with adequate
oxygenation established, sources of hemorrhage must be identified and
controlled. The sequence of assessments outlined in the Advanced Trauma
Life Support course and handbook is helpful for organizing an approach
to managing potentially unstable trauma patients.3
Hemodynamic trends are helpful and the time required, in the trauma
resuscitation area, to make a decision as to hemodynamic stability is a
worthwhile investment. Initial presentation with hemodynamic stability
should not be taken as a pass on further vigilance. Patients with
pelvic fractures after high-energy trauma require close observation in
an intensive care unit or monitored environment for the first 24 to 36
hours as they can deteriorate rapidly from internal hemorrhage.
can help to identify sources of internal hemorrhage and are part of the
routine initial screening radiologic evaluation of any trauma patient.3
For patients presenting with hemodynamic instability and hypotension
(shock) after blunt abdominal/pelvic trauma despite adequate attempts
at initial resuscitation (crystalloid, colloid, and allogeneic blood
transfusion), exploratory laparotomy is indicated to rule out abdominal
hemorrhage. However, for those patients demonstrating appropriate
response to fluid resuscitation without shock, further investigation
for ongoing internal hemorrhage has typically used the diagnostic
peritoneal lavage (DPL) performed in the trauma bay. In patients with
known or suspected pelvic fracture, the DPL should be performed above
the umbilicus to avoid false-positive results from pelvic hematoma.
Modern evaluation of a patient with blunt abdominal and pelvic trauma,
however, routinely involves computed tomography (CT) scanning of the
chest, abdomen, and pelvis, examining for free fluid within the
peritoneal and preperitoneal cavities. In the majority of institutions
within North America today, DPL is reserved for those situations of
blunt abdominal trauma where free fluid is noted on the CT scan without
evidence of solid organ injury.
(FAST) has been purported to detect abdominal hemorrhage and, when
positive in a hemodynamically unstable patient, is an indication for
laparotomy. This hand-held sonographic device is noninvasive, imparts
no radiation to the patient, and is available for both trauma/critical
surgeons as well as trained emergency physicians. Literature supporting
its use claims that this screening test has the ability to detect small
amounts of free fluid within the abdominal cavity, and it has become
part of the primary survey in most trauma centers within the United
States. However, because of the lower sensitivity and negative
predictive value as well as significant operator variability, recent
literature reviews of the technique do not definitively advocate its
use as a supplant to DPL and screening CT scan.62,81,185
 |
|
FIGURE 44-1
Algorithm for managing hypotension and hemorrhage from pelvic fracture. (Adapted from Flint L, Meredith W, Schwab C, et al., eds. Trauma: Contemporary Principles and Therapy. Philadelphia: Lippincott Williams & Wilkins, 2008, Chapter 49 Pelvic Fractures, page 529, Fig. 6.) |
pelvis to cause fractures and dislocations, associated injuries to
other organ systems are common. An early decision for exploration of
the chest or abdomen when thoracic or abdominal bleeding are suspected
based on radiographic or clinical findings is central to lowering
mortality in multiply injured patients with pelvic fractures. While
multiple tests such as radiographs, screening CT scan, DPL, and FAST
are available, it is critical to remember that overall the decision to
proceed to the operating room for thoracotomy, laparotomy, or pelvic
packing is based primarily on the clinical presentation of the patient.
Ongoing hypotension or shock, continuous bleeding from a chest tube,
and a tense rigid abdomen are indicators that immediate operative care
is needed to stabilize the patient and prevent ongoing blood loss.
injuries, particularly those that may be contributing to ongoing blood
loss. Once radiographic examination and CT scan have ruled out thoracic
and abdominal hemorrhage, persistent blood loss and hypotension are
assumed to be pelvic in origin until proven otherwise. Historically,
the clinical examination would assess for gross instability of the
pelvis by compression and manipulation. Anteroposterior (AP) and
lateral compression on the iliac wings may elicit pain or frank
rotational instability or there may even be a palpable gap or
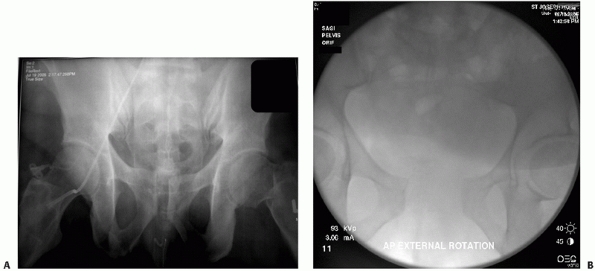
separation of the symphysis. External rotation and shortening of the
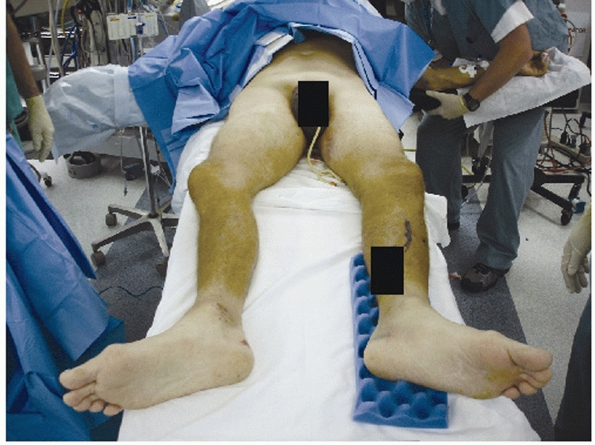
lower extremity may indicate an “open-book” and/or vertical shear
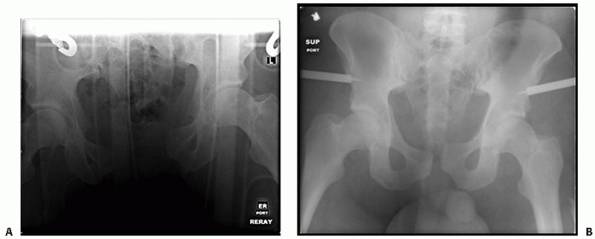
injury (Fig. 44-2). Gonzales et al. documented
that clinical examination in conscious patients who could comply with
the physical examination yielded a sensitivity of 90% for the diagnosis
of pelvic fracture.59 However,
subtle instability is difficult to detect, this can be exceedingly
uncomfortable for the patient, and aggressive manipulation may
destabilize pelvic clot and exacerbate bleeding.
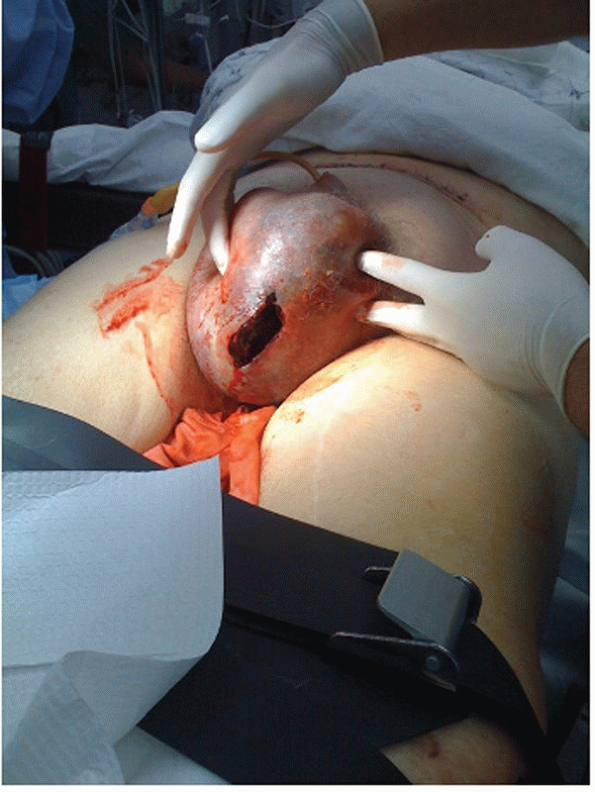
from the rectum, vagina, or urethra (Fig. 44-3).
Importantly, rectal and/or vaginal examination is mandatory to rule out
mucosal tears, which may communicate with the pelvic fracture,
indicating an open and contaminated injury. An abnormally high prostate
position in males on rectal examination is suggestive of a urethral
tear.
 |
|
FIGURE 44-2 Clinical photograph showing external rotation and leg length discrepancy in a patient with an open book pelvic fracture.
|
findings that may be present in patients that have a pelvic fracture.
Obtaining an accurate neurologic examination is often difficult
secondary to the patient’s variable mental status. However, rapid
examination of a few major areas is important because the sciatic nerve
and branches of the lumbosacral plexus lie in close proximity to common
fracture-dislocation sites. Rectal examination for tone and
bulbocavernosus reflex is easy to perform and can be accomplished in
the obtunded or noncompliant patient to rule out cauda equina syndrome.
The bulbocavernosus reflex in the female is elicited by gently tugging
on the Foley catheter. Peripheral nerve examination is possible for
distal motor groups at the ankle and foot, but proximal muscle weakness
is difficult to identify secondary to pain.
 |
|
FIGURE 44-3 Clinical photograph showing scrotal ecchymosis and hematoma in a patient with a pelvic fracture.
|
|
TABLE 44-1 Pelvic Fracture Clinical Examination
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
associated with injuries to the pelvic ring and account for the
majority of deaths directly related to the pelvic fracture.130 Huittinen and Slatis,71
in a classic contribution to the field of pelvic fracture care, showed
with postmortem angiography that pelvic fracture hemorrhage results
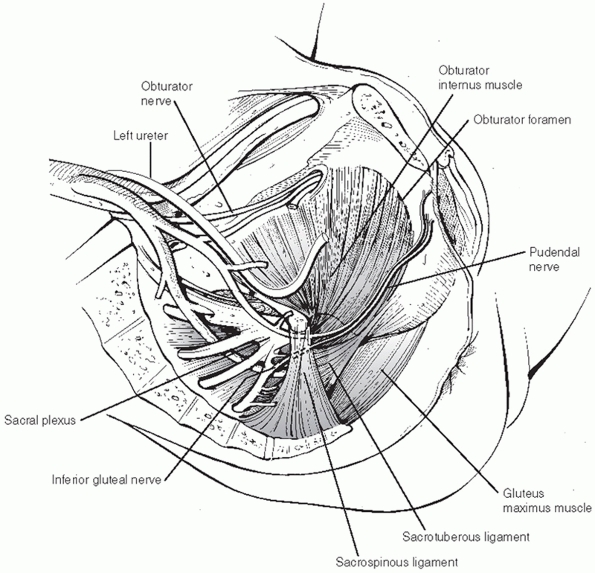
most frequently from the venous structures. Recall that the venous and
arterial structures that traverse the pelvic cavity are intimately
associated and supported by the ligamentous elements that stabilize the
pelvic ring (Fig. 44-4). They also posited that a substantial proportion of bleeding arises from fracture edges. However, a recent study by Elzik43
demonstrated that changes in hematocrit and blood pressure with
presumed blood loss were equal in patients with pure dislocations and
those with fractures.
venous damage and bleeding as these thin-walled vessels tend to be more
numerous and fragile. Pelvic venous hemorrhage stops in the majority of
patients secondary to tamponade from increasing tissue pressure within
the pelvic retroperitoneal space. However, the pelvic cavity and
connection with the retroperitoneal space is large, and considerable
volumes of blood can be lost, even after closure of open-book-type
injuries.63 Additionally, since
arterial bleeding can overcome the tamponade effect of the
retroperitoneal tissues, single or multiple arterial lacerations are
more likely to be present in patients who die of pelvic fracture
hemorrhage.9
|
TABLE 44-2 Myotomes of the Lower Extremity
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
 |
|
FIGURE 44-4 Picture showing relationship of vascular structures to ligamentous structures within pelvic cavity.
|
internal iliac system, with the superior gluteal and pudendal arteries
being the most commonly identified source.40
is inadequate in determining the risk of ongoing hemorrhage, as it does
not accurately represent the displacement that occurred at the time of
impact. Metz103 documented an equal
number of arterial bleeding sites seen on angiography in patients with
anterior-posterior compression and lateral compression injury patterns.
suspicion for pelvic fracture hemorrhage, severe bleeding can occur in
essentially all fracture patterns and the entire clinical picture
(associated injuries, blood pressure, and heart rate) should dictate
resuscitation maneuvers.9
have been ruled out and pelvic fracture bleeding is suspected, patients
receive blood transfusions as needed to manage hemodynamic instability.
Persistent pelvic hemorrhage and hemodynamic instability despite
appropriate and adequate fluid resuscitation is usually the result of
coagulopathy and/or pelvic arterial bleeding and requires emergent
intervention to prevent ongoing blood loss, shock, and death.48 In an excellent epidemiologic review from the German Trauma Registry over a 10-year period, Hauschild66
found that mortality directly attributable to pelvic injury and
hemorrhage was 7%. This was significantly lower than the 11% mortality
found in an earlier period prior to initiation of a pelvic hemorrhage
management protocol. Such interventions and protocols may include any
or all of the following: application of a pelvic binder, pelvic
external fixation, pelvic angiographic embolization, and/or pelvic
packing. As of the writing of this chapter, there is still significant
controversy and institutional variability in terms of which of these
methods is used and in what order. It is important to reiterate,
however, that the institution dealing with these types of patients and
injuries have a fixed protocol within the emergency department to deal
with these scenarios in an efficient and timely fashion as they arise.
closure of pelvic diastasis (open-book-type pelvic injuries) with
reduction of pelvic volume improves the prospect of tamponade of pelvic
bleeding and controlling hemorrhage.48,55,63,101,120
Additionally, stabilization of the fracture (whether temporarily or
definitively) so that motion is minimized is intuitively appealing as a
means of controlling hemorrhage via clot formation. External
compression devices such as inflatable garments, pelvic binders, and
external fixators have been used to achieve these goals. These devices
potentially serve three functions: (1) they return blood from the lower
extremities to the central vascular system (autotransfusion), (2) they
have the ability to close open-book-type injuries, reducing pelvic
volume resulting in a tamponade
effect within the retroperitoneal space, and (3) they stabilize the pelvic ring permitting clot formation.100
 |
|
FIGURE 44-5
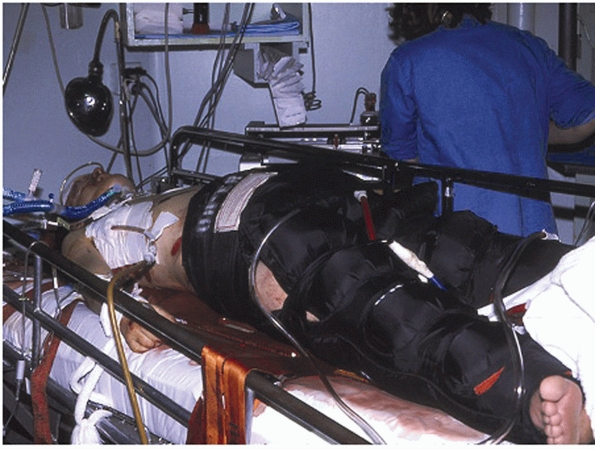
Clinical picture showing the use of the pneumatic antishock garment in a hypotensive patient with a pelvic fracture. (Courtesy of Edward Carrillo, MD.) |
 |
|
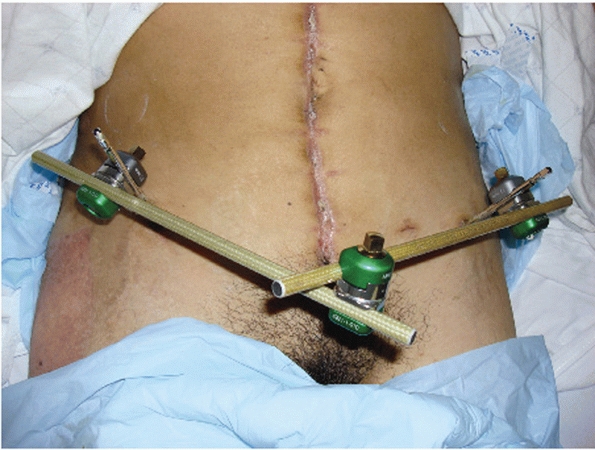
FIGURE 44-6 Clinical photograph showing an anterior external fixator placed low enough to provide access to the abdomen for laparotomy.
|
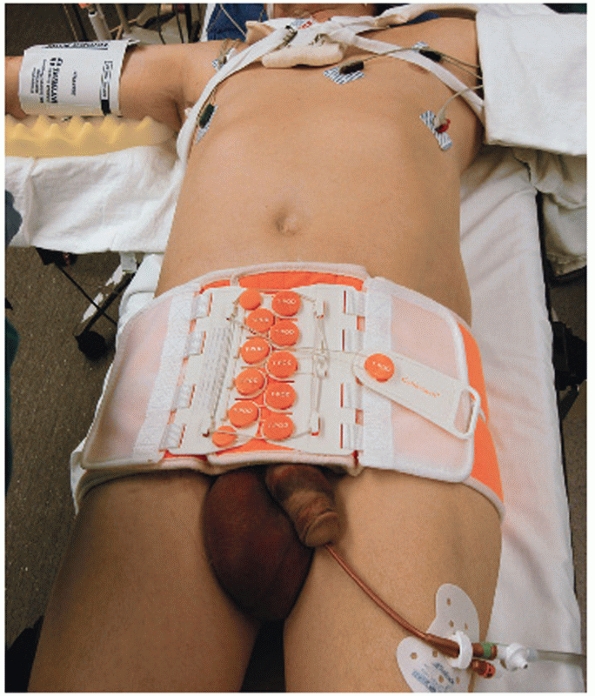
garment and military antishock trousers supported these concepts for
the management of bleeding due to pelvic fracture (Fig. 44-5).
However, these garments have to be partially or completely removed to
allow access to the abdomen in patients who require abdominal
exploration. Additionally, reports of compartment syndrome and skin
necrosis following the use of the devices have been published along
with randomized and cadaveric studies that demonstrated equal efficacy
with simple external frames or aggressive resuscitative protocols.15,54,100
These difficulties and limitations ultimately restricted the utility of
these devices to use in the field for transportation of multitrauma
hypotensive patients.
pioneered by European trauma surgeons accelerated interest in early
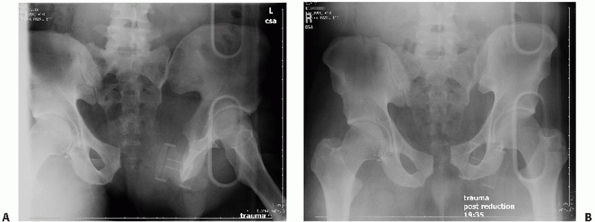
application of pelvic external fixation for control of bleeding.48,77
The two types of pelvic external fixation frames commonly used today
are anterior external fixators, which are applied percutaneously to the
innominate bones, and pelvic “C” clamps, which are applied
percutaneously to the posterior iliac fossa. Both devices can be
positioned in such a way to allow access to the abdomen for laparotomy
if needed.
 |
|
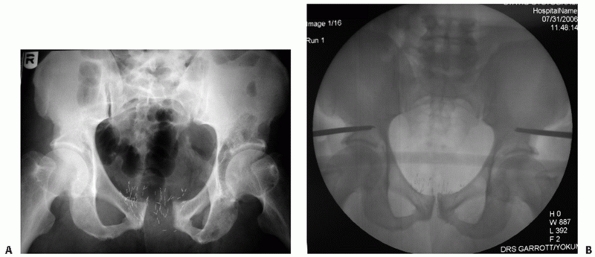
FIGURE 44-7 Anteroposterior radiograph of the pelvis before (A) and after (B) application of external fixator.
|
These frames require a sufficiently intact posterior ring that is able
to provide a hinge or fulcrum to internally rotate the hemipelvis
around. There has been some debate over optimal pin position for
anterior pelvic external fixation frames, with pins being positioned
either above the acetabulum49 or into the iliac crest, and this will be discussed in greater detail below.78,101,186
translates the entire hemipelvis medially. The pins are placed onto the
external iliac fossa posteriorly and as such, do not rely on an intact
posterior ring for a hinge to perform their function (Figs. 44-8 and 44-9).45,128
In situations where a patient’s physiologic status does not permit
definitive open reduction and internal fixation of the pelvic ring, and
there is a complete injury of the posterior ring, this clamp is
superior in controlling the posterior ring injury than a traditional
iliac crest or supra-acetabular anterior external fixator. The C-clamps
can be used
for
treating an anterior pelvic ring injury as well. However, as with
anterior external fixators, the use of external circumferential binders
has largely replaced C-clamps for this injury in the acute setting, and
the C-clamps are too large and bulky to be considered useful for
definitive treatment of the anterior injury in situations where open
reduction and internal fixation (ORIF) is contraindicated.
 |
|
FIGURE 44-8 Clinical photograph showing a pelvic C-clamp on a patient with a pelvic fracture.
|
soon after the patient arrives at the definitive care facility. Some
orthopaedic surgeons feel strongly that application in the operating
room (rather than in the resuscitation area) using sterile technique
and image intensifier control is necessary for safe placement of
external fixators. Pin site complications such as persistent
serosanguinous oozing, infection, aseptic pin loosening, and skin
necrosis are frequent occurrences,96 particularly for critically ill patients in the intensive care unit (ICU). Miller104
found that a significant proportion of patients with a bleeding pelvic
fracture and hemodynamic instability have unacceptable delays in
resuscitation with attempts at external fixation. Additionally, there
have been reports of pelvic visceral injury following application of
the pelvic C-clamps. Because of these and other concerns, application
of external fixators for persistent hemodynamic instability in the
resuscitation area or trauma bay has not been universally accepted and
in fact is being practiced with decreasing frequency.
or pelvic binder (T-Pod Bio-cybernetics International, La Verne, CA)
reduces pelvic volume and can be applied safely and quickly in the
trauma bay by caregivers who are not familiar with external fixation
application (Figs. 44-10 and 44-11).14,35
The binder or sheet is applied in a straight transverse fashion with
the primary force vector being delivered to the greater trochanters. If
other injuries permit, internal rotation and adduction of the lower
extremities aids in reducing external rotation deformities of the
pelvic ring as well.
 |
|
FIGURE 44-9 Anteroposterior radiograph of the pelvis before (A) and after (B) application of pelvic clamp.
|
 |
|
FIGURE 44-10 Clinical photograph showing application of a pelvic binder in a patient with an open book and vertical shear pelvic injury.
|
external compression devices is the development of pressure ulceration
and full thickness skin necrosis and slough. In critically ill patients
with prolonged ICU stays, the potential for major skin and soft tissue
problems is significant. A concern that has been voiced by some
orthopaedic traumatologists and critical care personnel is
overcorrection or worsening of the deformity, particularly in lateral
compression injuries (which tend to be the most frequent and rarely
necessitate the use of a binder) where the pelvis is already internally
rotated, with subsequent injury to the pelvic visceral or vascular
structures. Fracture of the superior and inferior ramus is a frequent
injury found in an internal rotation or lateral compression injury, and
the potential exists for a spike of ramus to be driven further
across the midline into the bladder or blood vessels (Fig. 44-12).
While this concern is theoretically plausible and has prevented the use
of this device in the field, there are currently no reports that such
damage has occurred as result of application of a pelvic binder.
 |
|
FIGURE 44-11 AP radiograph of the pelvis before (A) and after (B) application of the pelvic binder.
|
hemodynamic instability despite pelvic compression, appropriate
resuscitation, and exclusion of thoracic, abdominal, and extremity
hemorrhage, laparotomy with pelvic packing or angiography should be
considered.45,64
For patients greater than 60 years of age, arterial bleeding in
conjunction with pelvic fracture is more likely and consideration
should be given to angiographic embolization first, provided that these
services are immediately accessible.80
These basic tenets are well outlined in the practice management
guidelines promulgated by the Eastern Association for the Surgery of
Trauma.41
and should be based on initial response to resuscitation as well as
volume of hemorrhage and the absence or presence of a contrast blush on
CT scanning.10 Delay in proceeding
with angiography for application of external fixators can compromise
resuscitation efforts in a significant proportion of patients.49,64,104
Necrosis of pelvic viscera and sexual dysfunction is a tangible risk
with bilateral and, in rare instances, unilateral internal iliac
arterial occlusion occurs.134 Soft
tissue necrosis (particularly gluteal) in patients who have undergone
definitive pelvic fixation via large extensile exposures following
angiographic embolization of the internal iliac artery has been noted.189 The risk of adverse reactions (allergic and renal) to modern contrast media is approximately 2% to 3%.111
 |
|
FIGURE 44-12 Radiograph of a lateral compression injury with ramus fracture crossing the midline and potentially impinging on the bladder.
|
preferable to taking a desperately ill, unstable patient to the
angiography suite.45 Over the last decade, this technique has gained increasing attention,30,51,183
particularly with the increasing reports of complications associated
with aggressive arterial embolization as noted earlier. Indeed, some
authors118 cite lower ultimate blood
loss and decreased mortality compared to pelvic arterial embolization.
However, although pelvic packing has gained considerable momentum and
popularity in some centers around Europe and Asia, it has not been
widely accepted in North America and, therefore, requires a fairly
specialized facility with readily available surgical teams (both
general and orthopaedic) familiar with this technique. It is not
recommended for the inexperienced orthopaedic surgeon.
considered a maneuver for skeletal stabilization and comfort, many
surgeons believe that it helps in the early resuscitative phase as well
by stabilizing fracture fragments, allowing clot formation, and
providing tamponade by increasing tension on the iliopsoas and gluteal
musculature.
 |
|
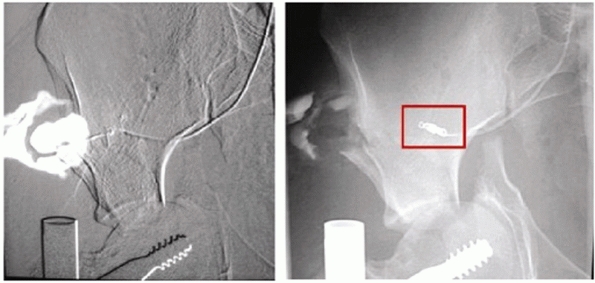
FIGURE 44-13 Fluoroscopic images showing extravasation (left) of contrast in a superior gluteal artery injury, which is then controlled with angiographic coiling (right). (Courtesy of Prof. Dr. J Reuger.)
|
blunt trauma that result in pelvic fracture, these patients often
present with multisystem injury that plays a pivotal role in the
patient’s ultimate survival as well as functional outcome. Not
infrequently, a patient with pelvic fractures from blunt trauma will
also have associated head, chest, and abdominal injury as well as other
extremity fractures. Biffl8 and Demetriades36
have reported on the most frequently encountered associated injuries in
patients with traumatic pelvic fractures: 63% of patients had chest
injury (pulmonary contusion, rib fracture, hemopneumothorax), 50% had
other long bone fractures, 40% had head or brain injury, 40% had
evidence of solid organ injury (spleen or liver), and 25% had some sort
of spinal fracture. Intestinal injury was reported to occur in up to
14% in the study by Demetriades.36
For these reasons, it is clearly evident that the patient with a
traumatic pelvic fracture requires a systematic well-organized
multidisciplinary approach to effectively and efficiently manage the
multiple injuries that are frequently encountered with this patient
population.
in pelvic fractures with a reported incidence as high as 15% to 20%
(males, 21%; females, 8%). Bladder and urethral trauma occur with
relatively equal frequency (approximately 7%).4,22,23,46,109
Because of the significant force required to rupture a hollow viscus
within the pelvis, associated mortality can be as high 22% to 34% when
pelvic fractures are accompanied by a bladder rupture.23,92
The clinical finding most consistently observed after bladder/urethral
injury is gross hematuria, which is present in approximately 95% of
patients; however, it should be suspected when greater than 30 red
blood cells are noted on a urinalysis.21,47 The majority of bladder ruptures are extraperitoneal.
potentially an issue, contrast urethrography and cystography is
performed in hemodynamically stable patients. Initially, a contrast
urethrogram is obtained by inflating the Foley catheter balloon in the
penile urethra with 2 to 3 mL of saline and instilling 10 to 15 mL of
water-soluble contrast material and obtaining an AP view of the pelvis.
Failure to pass contrast into the bladder indicates urethral
disruption. If no urethral disruption is found and the catheter passes
easily into the bladder then a further 300 mL of water-soluble contrast
is instilled into the bladder and the cystogram (either plain
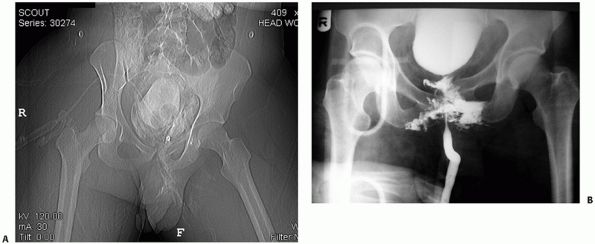
radiograph or CT scan) is performed.46,122 The radiographic appearance of a bladder rupture and urethral disruption are demonstrated in Fig. 44-14.
 |
|
FIGURE 44-14 Radiographic appearance of (A) bladder rupture and (B) urethral disruption.
|
to manage the associated genitourinary injuries. Extraperitoneal
bladder rupture and urethral injuries can be treated nonoperatively
with Foley catheter drainage. However, if ORIF of the anterior pelvis
is necessary, then simultaneous extraperitoneal bladder repair and ORIF
of the anterior pelvic ring can be performed at the same surgical
sitting through the same incision. If for some reason extraperitoneal
bladder repair is contraindicated, then strong consideration should be
given to external fixation as being the definitive treatment for the
anterior pelvic injury. Intraperitoneal bladder rupture is treated with
exploratory laparotomy and suture repair. Suprapubic catheterization is
not usually necessary but, when indicated, placement of the catheter
must take into account the potential for contamination of anterior
internal fixation. The urologist and the orthopaedic trauma surgeon
need to communicate closely to allow ideal placement of suprapubic
catheters, timing of bladder/urethral repair, and locations of surgical
incisions that avoid contaminating the orthopaedic operative field.119
between a fracture fragment and the external environment or a pelvic
visceral cavity. Possible routes of contamination can occur by direct
protrusion through the skin and soft tissues, or penetration into the
vagina or rectum. As documented in the reports by Brenneman16 and Raffa,133
these injuries are observed in 4% to 5% of patients with pelvic
fracture and significantly increase the incidence of deep pelvic and
soft tissue infection, osteomyelitis, mortality, and long-term
disability.140 In fact, very early reports on open pelvic fractures reported mortality
rates as high as 40% to 50%.124,133
Skin lacerations communicating with pelvic fracture sites may be found
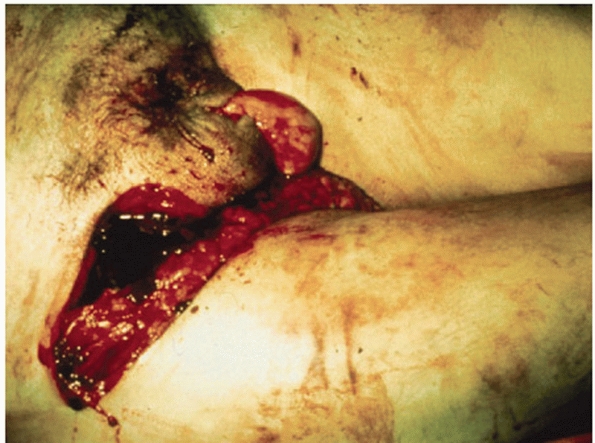
along the course of the iliac crest, the groin, and the perineum (Fig. 44-15).
During the acute evaluation of genitourinary injury in the patient with
a pelvic fracture, a careful digital rectal and, if appropriate,
vaginal examination is mandatory. Damage to the anal sphincter with a
perineal laceration suggests the need for diverting colostomy.
Similarly, when fecal soiling of an open perineal wound that
communicates with the fracture is possible, diversion and distal
washout is performed to reduce the chance of pelvic wound infection.137,194
Plans for definitive repair of the pelvic fracture need to be
understood and communicated before the decision to perform fecal
diversion is made so optimal placement of the ostomy can be ensured.
Diverting colostomy should be performed within the first 6 to 8 hours
following injury to reduce the incidence of sepsis and death.194
 |
|
FIGURE 44-15 Clinical photograph of perineal laceration communicating with a pelvic fracture.
|
contamination (an open iliac crest fracture for instance), then
standard open fracture treatment with serial irrigation and
débridement, intravenous antibiotic therapy, and drainage/packing are
all that are necessary. Similarly, an open fracture that penetrates the
vaginal vault can be managed with serial débridements, packing, and
closure of the vaginal wound over a drain by the gynecologic service
when clean.
initial trauma series screening of the cervical spine and chest. With
enough experience, many of the injuries to the posterior pelvic ring
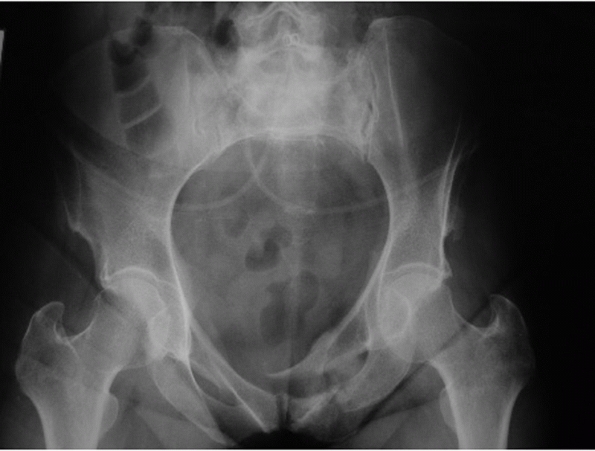
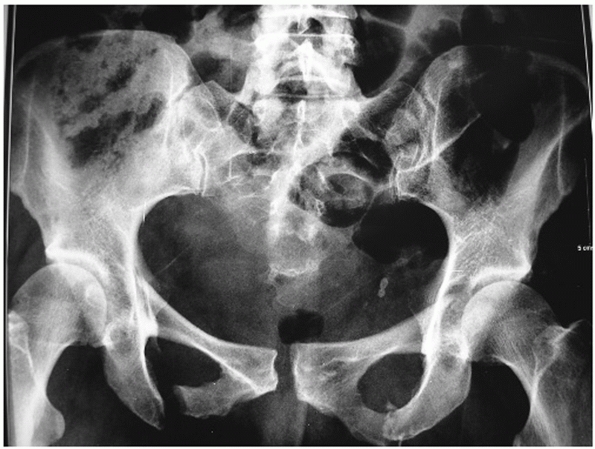
can be diagnosed with this single projection. A good AP radiograph
should have the pubic symphysis colinear with the sacral spinous
processes (Fig. 44-16). Use of the lumbar
spinous processes is also possible but the observer needs to be aware
that if the patient’s torso is turned or twisted, they will not line up
even if a true AP of the pelvis is obtained. A nicely centered AP film
allows side to side comparison of bony landmarks to aid in diagnosis of
subtle displacements of the sacrum or SI joint. The cortical density of
the pelvic brim and iliopectineal line should be traced back to its
intersection with the lateral margin of the sacral ala. This
intersection should be at the same level (usually the superior margin
of the S2 foramen) bilaterally. Asymmetry in the SI joint space and the
appearance of the sacral foramina should alert the surgeon to the
possibility of an SI joint dislocation or sacral fracture. Fractures of
the L5 transverse process may indicate a vertical shear injury that has
avulsed the transverse process via the iliolumbar ligament. Symphyseal
diastasis or displaced rami fractures should alert the examiner to
additional injuries in the posterior ring even though they may not be
readily apparent on first glance.
 |
|
FIGURE 44-16 AP radiograph of the pelvic ring.
|
is taken with the x-ray beam directed caudally approximately 45 degrees
to the radiographic film. A true inlet view of the pelvis, however, may
require variations on this degree of angulation because of the normal
variations in sagittal plane pelvic obliquity and sacral inclination.
This view simulates a direct view into the pelvis from above along its
longitudinal axis. The inlet view is helpful in imaging external or
internal rotation of the hemipelvis, opening of the SI joint, an
impaction fracture of the sacral ala, and AP translation in the plane
of the sacrum of the hemipelvis (see later).
is obtained by directing the x-ray beam approximately 45 degrees
cephalad to the radiographic film. This view simulates looking at the
sacrum and SI joints directly en face. The outlet view is helpful in
imaging cephalad “vertical” shift of the hemipelvis again, in the plane
of the sacrum, and sacral fractures relative to the foramina.
Additionally, many pelvic ring injuries with an unstable hemipelvis
exhibit a flexion or extension deformity as a result of the muscle
forces acting on the hemipelvis. This can be noted on any of the three
projections but is particularly evident on the outlet view of the
pelvic ring. The point of rotation is typically the posterior ring;
therefore, sagittal plane rotational deformity (flexion or extension)
will be seen as one pubic body riding higher or lower than the
contralateral pubic body.
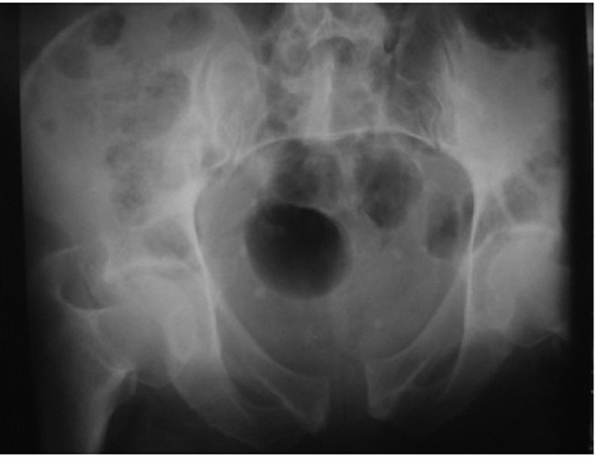 |
|
FIGURE 44-17 Inlet projection of the pelvic ring.
|
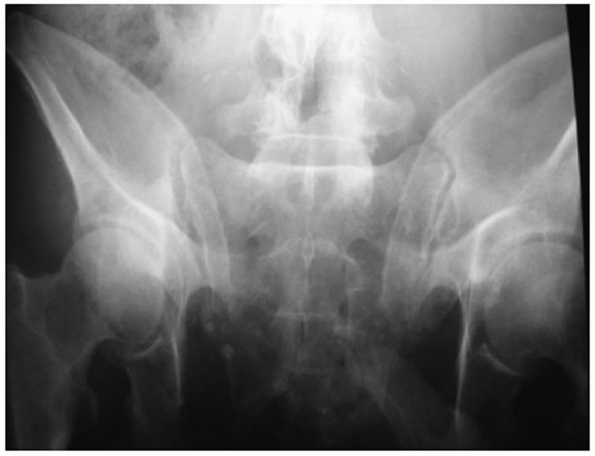 |
|
FIGURE 44-18 Outlet projection of the pelvic ring.
|
Therefore, a given amount of translation or displacement seen on the
inlet or outlet view is in fact the sum of displacement vectors in both
the coronal and axial planes. For example, “posterior” shift seen on
the inlet projection is in fact a combination of both posterior and
cephalad translation relative to the longitudinal axis of the patient.
injury. As the pelvis is a ring structure, any disruption in one
location (no matter how seemingly insignificant) must (by virtue of
ring structure mechanics) be accompanied by disruption in another
location. Two- to 3-mm axial sections (or 3 mm of vertical travel per
360-degree rotation of the gantry in a spiral CT) are recommended to
disclose the majority of significant injuries and allow for
good-quality three-dimensional reconstructions.
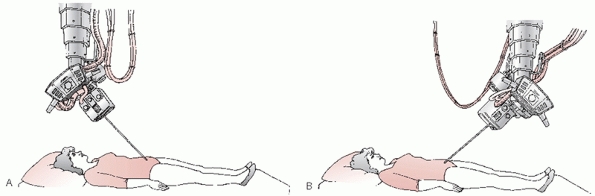 |
|
FIGURE 44-19 Schematic representing the direction of the incident x-ray beam for (A) inlet projection and (B) outlet projection of the pelvic ring.
|
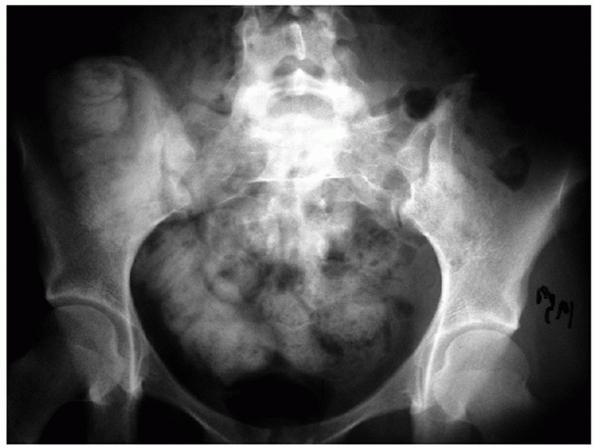 |
|
FIGURE 44-20
AP radiograph of the pelvis that appears relatively normal, but on closer inspection displays the paradoxical inlet view of the upper sacrum and AP view of the distal sacrum. |
appearance of the sacrum on the AP projection. A paradoxical inlet view
of the upper sacrum and outlet or AP view of the distal sacrum
necessitates a lateral pelvic or sacral radiograph and CT scan with
sagittal reconstructions to rule out an occult sacral
fracture-dislocation or U-shaped sacral fracture or spinal-pelvic
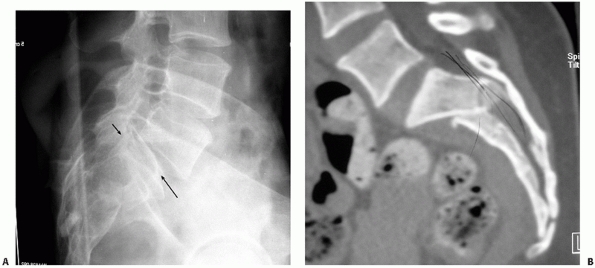
dissociation (Figs. 44-20 and 44-21).154
pelvic fractures and dislocations require some form of classification
scheme for epidemiologic and reporting purposes. And, like
classification schemes for other musculoskeletal injuries, those for
the pelvis are numerous and at least somewhat controversial. Perhaps
the first attempt at characterization of any pelvic fracture was that
by Malgaigne94 in 1859 (without the
benefit of radiographs) of the so-called “double vertical fracture”—a
pelvic ring injury represented by fractures through the inferior and
superior ramus anteriorly, and a fracture or dislocation through the
posterior ring. Based on their work through the 1950s and
1960s, Peltier121 and Huittinen70
published in separate reports the first attempts at classifying pelvic
ring injuries based on the mechanisms and structures injured and
attempted to correlate this with the complications and outcomes that
they observed. However, it was not until the ground-breaking work by
Pennal123 that was later expanded on and modified by Tile181 (Table 44-3)
that we see our first comprehensive attempts to classify pelvic
injuries based on mechanism and involvement of the structural elements
of the pelvis.
 |
|
FIGURE 44-21 (A) Lateral sacral radiograph and (B) CT scan (sagittal reconstruction) of the same patient in Figure 44-20 demonstrating sacral fracture-dislocation with spinal pelvic dissociation.
|
and Association for the Study of Internal Fixation group is more
comprehensive and serves primarily to provide a widely accepted and
standardized classification system for data collection and reporting.
Generally speaking, classification systems are most valuable in
grouping injuries for reporting results and outcomes; however, some
authors believe that they can predict resuscitative requirements and
outcome to a certain degree.32 It is
important to bear in mind that static radiographic imaging will
disclose neither the full extent of instability nor the resuscitative
needs of the patient in all instances and appropriate treatment will be
based on accurate assessment of the resultant injury, instability
pattern, and condition of the patient.
|
TABLE 44-3 Tile Classification
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
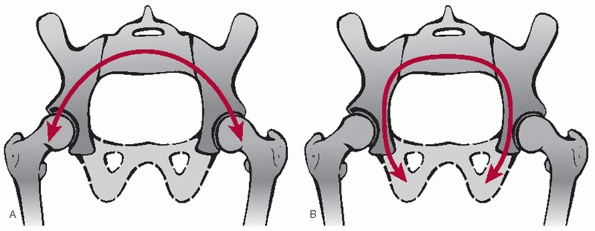
lateral compression (LC) injuries are the most commonly encountered. At
the time of impact, the hemipelvis on the side of impact is pushed
(internally rotated) into the contralateral pelvis. The injury pattern
usually includes rami fractures anteriorly with either a sacral
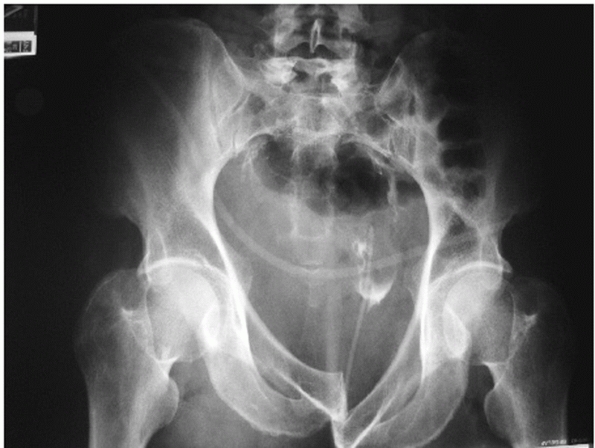
impaction and/or iliac wing fracture posteriorly; however, symphyseal
disruptions (the so-called “locked symphysis”) are also possible. This
type of injury is rotationally unstable (in the axial or horizontal
plane) and vertically stable (little tendency for cephalad
displacement) with some degree of sagittal plane rotation (flexion) of
the hemipelvis. Young and Burgess further subdivided the LCs based on
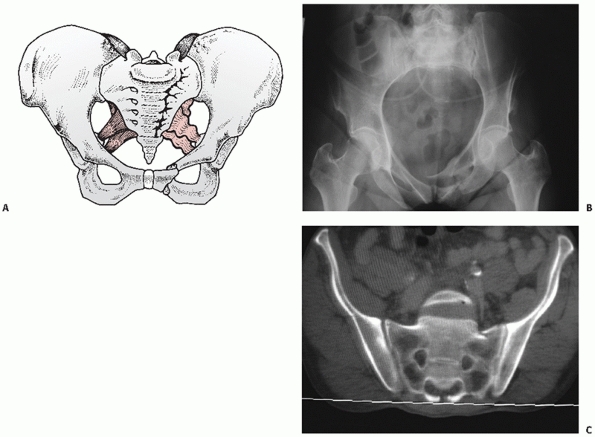
the posterior injury pattern with LCI representing a sacral impaction
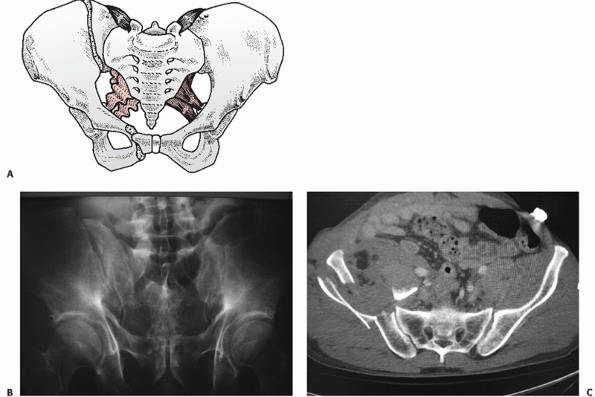
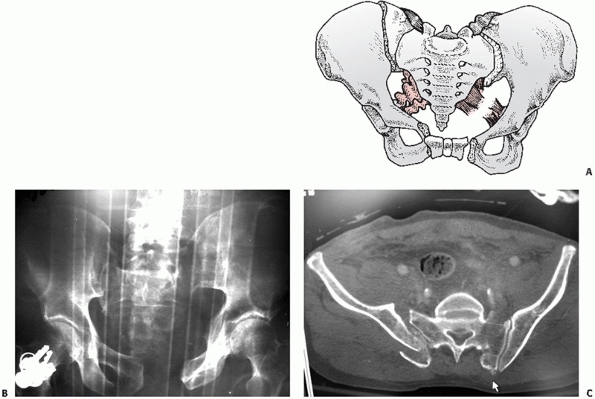
fracture (Figs. 44-22 and 44-23), LCII representing an iliac wing fracture (Fig. 44-24),
and LCIII representing the “wind-swept pelvis” with either an LCI or
LCII on the side of impact, and an external rotation or “open-book”
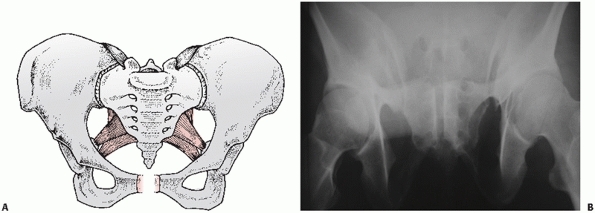
type injury involving the contralateral hemipelvis (Fig. 44-25).
|
TABLE 44-4 Young and Burgess Classification
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
 |
|
FIGURE 44-22 (A) Schematic and (B) inlet radiograph with (C) CT scan demonstrating a typical LCI injury with a sacral impaction fracture posteriorly and rami fractures anteriorly.
|
 |
|
FIGURE 44-23 AP radiograph demonstrating the less common “locked symphysis” associated with an LCI injury.
|
pelvis. Upon impact, external rotation of one or both hemipelves
occurs, with the fulcrum or point of rotation being the sacroiliac
joints. The first point of failure is the symphysis pubis. As
increasing external rotation and abduction forces are applied, there is
sequential failure of the sacrotuberous (ST), sacrospinous (SSp), and
anterior sacroiliac (ASI) ligaments under tension. Based on early
cadaveric biomechanical studies, ST, SSp, and ASI ligament disruption
is assumed to have occurred when there is more than 2.5 cm of
symphyseal diastasis. With further external rotation, the
intra-articular and posterior SI ligaments ultimately fail, resulting
in a completely unstable SI joint. At this stage, all of the supporting
ligamentous structures of the pelvic ring, including the pelvic floor
and perineal musculature, have been disrupted.67,190 The Young and Burgess subclassification196
of the APC injury patterns is largely based on the observations of
these cadaveric studies: with the APC I injury representing slight
widening of the symphysis and anterior SI joint but intact ST, SSp, and
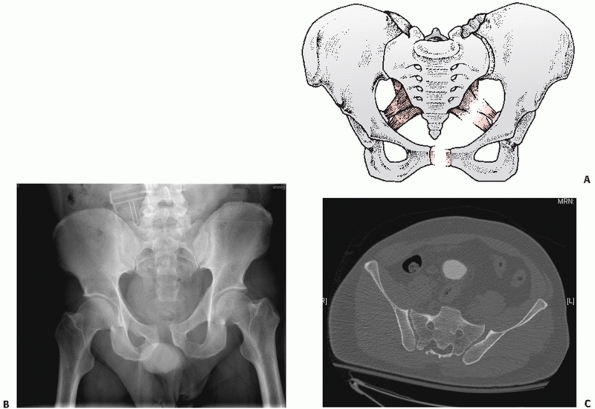
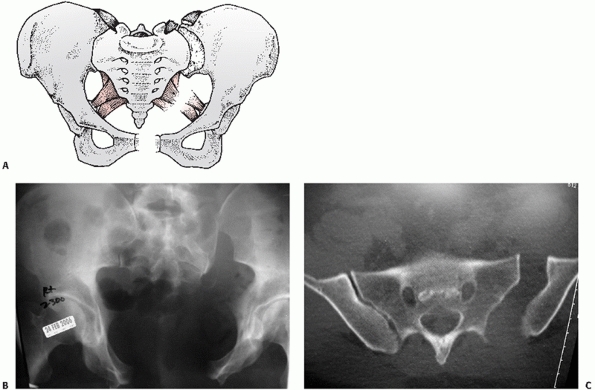
posterior sacroiliac (PSI) ligaments (Fig. 44-26), the APC II representing failure of the ST and SSp ligaments but not the PSI ligaments (Fig. 44-27), and the APC III representing total ligamentous disruption of the SI joint and pelvic ring (Fig. 44-28).
difficult to classify some of these injuries into a specific subgroup
since the AP, inlet, and outlet radiographs as well as the CT scan at
the time of presentation are merely snapshots in time after the initial
trauma and displacement. At the moment of impact, the
unstable
portion of the pelvis may in fact have been in a completely different
position than where it has come to rest in the trauma bay. For this
reason, it may at times be necessary to perform an examination under
anesthetic with fluoroscopy to disclose the complete spectrum of
ligamentous instability of the pelvic ring and a single static film
with minimal displacement cannot always be taken for granted as a
stable pelvic ring (Fig. 44-29).
For example, it can be very difficult to distinguish between an APC I
and APC II on injury films, and there are situations where an APC II
injury still has some posterior SI ligament intact but they are
sufficiently attenuated or injured that, for all intents and purposes,
the SI joint behaves more like an APC III injury.
 |
|
FIGURE 44-24 (A) Schematic and (B) AP radiograph with (C) CT scan of a typical LCII injury associated with an iliac wing fracture.
|
 |
|
FIGURE 44-25 (A) Schematic and (B) AP radiograph with (C) CT scan of a typical LCIII injury.
|
 |
|
FIGURE 44-26 (A) Schematic and (B) outlet radiograph of a typical APC I injury.
|
 |
|
FIGURE 44-27 (A) Schematic and (B) AP radiograph with (C) CT scan of a typical APC II injury.
|
vector is directed cephalad with the most common mechanism being a fall
from height. The iliac crest of the injured hemipelvis is seen to be
riding cephalad compared to the contralateral side, often with a
fracture of the ipsilateral transverse process of L5, which has been
avulsed with the iliolumbar ligament (Fig. 44-30). CM injuries typically involve multiple force vectors and combinations of two or more of the mechanisms described earlier (Fig. 44-31).
 |
|
FIGURE 44-28 (A) Schematic and (B) AP radiograph with (C) CT scan of a typical APC III injury.
|
information and categorizing injury patterns when reporting results of
study populations, they have little utility in planning surgical
approach, operative fixation, or functional outcome for a particular
patient. In the setting of treatment planning, it is perhaps more
useful to subdivide the pelvic injury into the anterior injury and the
more important posterior injury.
 |
|
FIGURE 44-29 AP radiograph (A) before and (B) after stress radiographs under anesthetic showing occult instability of the pelvic ring.
|
of symphyseal disruptions (ligamentous injury), pubic body, or rami
fractures (bony injury). Rarely, the anterior ring injury can present
itself as an acetabular fracture. Posterior pelvic ring injuries can be
characterized similarly. Working from medial to lateral, posterior
injuries include sacral fractures, SI joint dislocations, SI
fracture-dislocations (crescent fractures), and
iliac
wing fractures. The combination and extent of anterior and posterior
injury, in concert with the physiologic state of the patient and
condition of soft tissue envelope, will dictate the final
reconstructive and rehabilitative plan.
 |
|
FIGURE 44-30 (A) Schematic and (B) AP radiograph with (C) CT scan of a typical VS injury.
|
 |
|
FIGURE 44-31 (A) Schematic and (B) AP radiograph with (C) CT scan of a typical CM injury.
|
 |
|
FIGURE 44-32 Axial CT scan demonstrating a zone 1 sacral fracture lateral to the neural foramen.
|
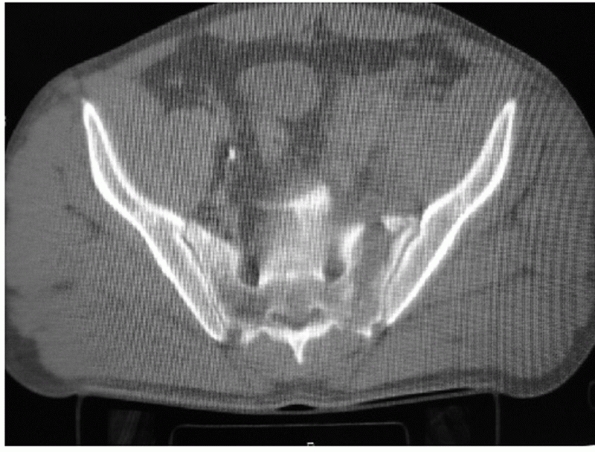
based on the fracture pattern and location of the injury. The
classification by Denis37 is the
most commonly used, and it categorizes the fractures based on fracture
line orientation and location. Traumatic sacral fractures occur in
approximately 30% of all pelvic ring injuries, but as high as 75% in
some cases if small LCI injuries are included. Zone 1 fractures are
vertical or oblique and pass lateral to the sacral foramina (Fig. 44-32).
They comprise 50% of sacral fractures and result in neurologic deficit
in 6% of cases. Zone 2 fractures are vertical or oblique and traverse
one or more of the sacral foramina (Fig. 44-33).
They comprise 36% of sacral factures and result in neurologic deficit
in 30% of cases. Zones 1 and 2 sacral fractures compromise pelvic ring
stability but not spinal stability unless the fracture line extends
proximally to disrupt the L5/S1 articulation as described by Isler.73
can be horizontal, vertical, or oblique but they are all medial to the
sacral foramina and enter the sacral spinal canal. They comprise 16% of
sacral fractures and carry a 60% risk of neurologic injury both to
individual nerve roots and the cauda equina. Zone 3 fractures can
result in either pelvic ring or spinal instability depending on the
fracture pattern. Vertical midline “splits,” as described by Moed,105 are associated with APC type instabilities of the pelvic ring (Fig. 44-34).
Horizontal fracture patterns do not affect pelvic ring stability but
may affect spinal stability depending on the location relative to the
SI joints.
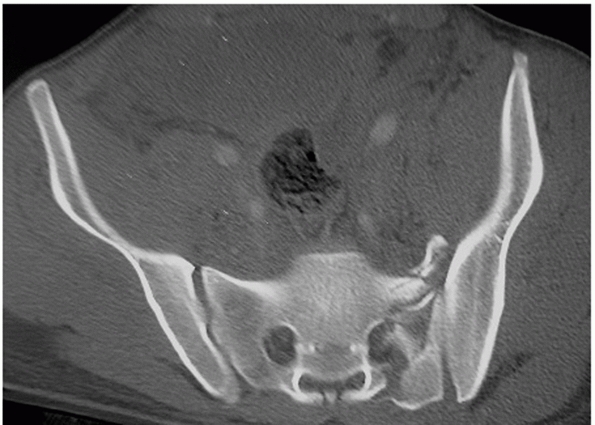 |
|
FIGURE 44-33 Axial CT scan demonstrating a zone 2 sacral fracture within the neural foramen.
|
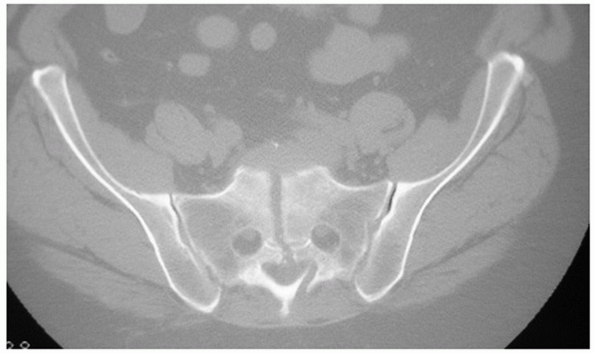 |
|
FIGURE 44-34 Axial CT scan demonstrating a zone 3 vertical sacral fracture medial to the neural foramen.
|
are stable injuries that carry a risk for injury to the spinal nerves
and cauda equina secondary to canal occlusion from fracture fragments.
Those fractures that are located below the level of the SI joints
generally involve sacral roots S3 and below. Horizontal fractures at
the level of the SI joints are usually associated with bilateral
vertical fracture lines (usually transforaminal) creating a U- or an
H-shape fracture pattern.85,95,166,167 The various fracture configurations have been described by Denis,37 Roy-Camille,147 and Strange-Vognsen.178
Unlike the other sacral fractures (zones 1 and 2) that are the result
of vertical shear and/or internal and external rotation injury to the
pelvic ring, these injuries are the result of rapid acute hyperflexion
of the pelvis and lumbosacral junction. This is an unstable injury
pattern that results in spinal-pelvic dissociation (i.e., no mechanical
continuity between the spine and pelvis). These injuries frequently
occur through the vestigial disc space between the sacral vertebral
bodies and lead to kyphotic deformation and impaction with compromise
of the sacral spinal canal (Fig. 44-35).
(innominate bones) which are joined anteriorly at the pubic symphysis,
and the sacrum posteriorly, which functions to connect the axial
skeleton (the spine) to the lower extremities via the innominate bones (Fig. 44-36).
The posterior pelvis and sacrum are a peculiar structure/entity in that
they serve a dual function: specifically, they act as the caudal
segment of the axial skeleton and the primary keystone structure
dictating the stability of the pelvic ring. The spinal-pelvic junction,
which directly involves
the
two SI joints, and the L5/S1 disc and facet joints, is a complex region
of anatomy and poorly understood biomechanics. Surgical and nonsurgical
treatment of injuries to the posterior pelvic ring and sacrum must,
therefore, take into account the peculiarities of the subspecialties of
spinal surgery and orthopaedic traumatology. This, in part, contributes
to both the varied and poor outcomes after pelvic fracture as well as
the difficulties in treating these injuries.
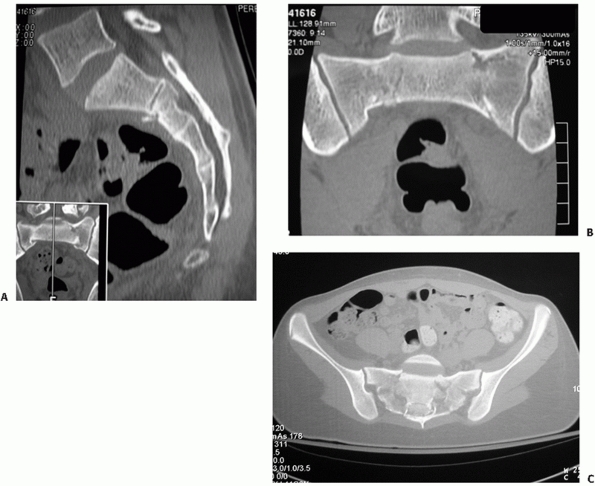 |
|
FIGURE 44-35 (A) Sagittal, (B) coronal, and (C) axial reconstructions of a U-shaped sacral fracture.
|
is the culmination of three embryonic pelvic anlages—the ilium, the
ischium, and the pubis—which fuse together at the triradiate cartilage
and the future acetabulum. The ilium functions as a weigh-station in
the transfer of forces from the axial skeleton to the lower
extremities. The structural quality of the bone in the ilium reflects
this concept of force transfer, as the bone is strongest and thickest
in one column that runs from the ischial tuberosity to the SI joint
(sitting force transfer), and a second column of that runs from the
dome of the acetabulum to the SI joint (standing force transfer) (Fig. 44-37).
Both of these columns of bone traverse the sciatic buttress, which is
an extremely dense region of bone at the superior aspect of the greater
sciatic notch.
It articulates with the most distal lumbar vertebra via the L5/S1 disc
and facet joints, as well as both innominate bones via the SI joints.
The sacrum is convex posterior and concave anterior, with variable
degrees of kyphosis inherent in this curvature. Because the sacrum is
formed by the fusion of vertebral bodies, it is subject to segmentation
anomalies (such as sacralization of L5 or lumbarization of S1), which
may be complete (bilateral) or incomplete (unilateral) (Fig. 44-39).
Five sacral nerve roots exit the five sacral foramina to join the
lumbosacral and sacral plexi, which supply the perineal structures and
lower extremities. The primary named neurologic structures exiting the
pelvis via the greater sciatic notch are the sciatic nerve, the
superior and inferior gluteal nerve and artery, and the internal
pudendal nerve and artery. The obturator nerve and artery exit the
pelvis via the obturator foramen.
virtue of the respective anatomic geometry of the sacral ala and the
medial surface of the ilium. Thus, the SI joints, and in fact the
entire pelvic ring, rely primarily on their supporting ligamentous
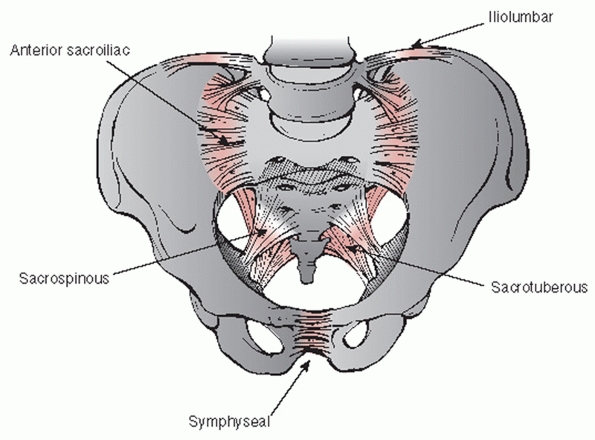
structures to maintain normal anatomic relationships. The primary
stabilizing ligamentous structures of the posterior pelvic ring are the
anterior, intra-articular, and posterior SI ligaments, the
sacrotuberous ligaments, and the sacrospinous ligaments (Fig. 44-40).
The primary supporting ligamentous structures of the anterior pelvic
ring are the symphyseal ligaments, which supply less than 15% of the
total stability to the pelvic ring.190
Sectioning of these ligaments will result in only mild opening of the
symphysis to a maximum of 2.5 cm, and no further external rotation or
vertical instability because of the intact ST, SSp, and SI ligaments.
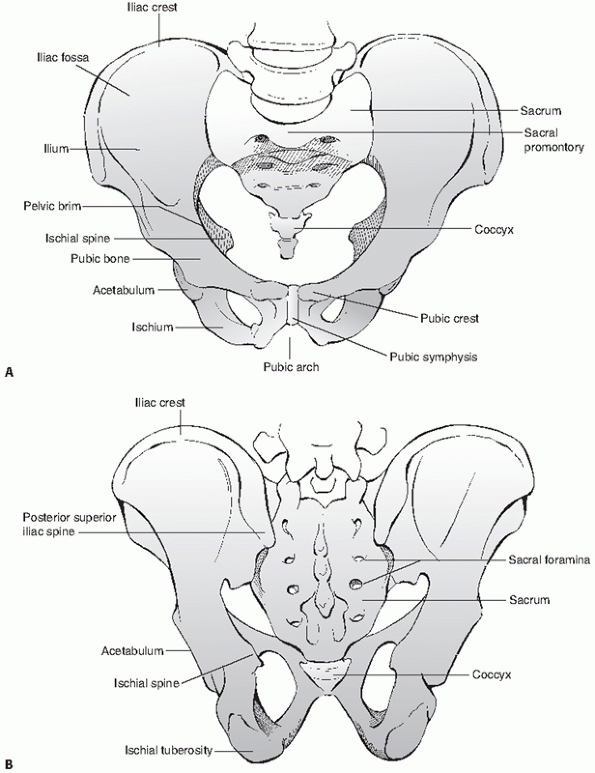 |
|
FIGURE 44-36 Schematic showing bony architecture of the pelvic ring (A) from anterior and (B) from posterior.
|
 |
|
FIGURE 44-37 Schematic demonstrating lines of force transfer during (A) standing and (B) sitting.
|
 |
|
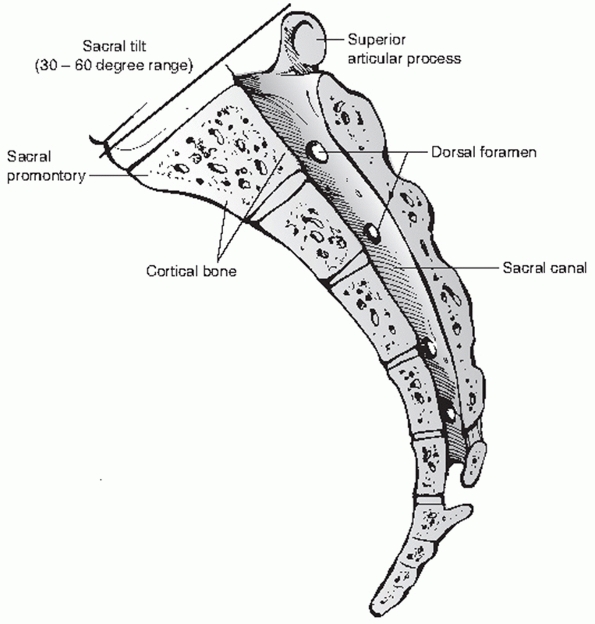
FIGURE 44-38 Lateral midsagittal view of the sacrum.
|
SI joints, and acetabulae, the forces that the symphysis and SI joints
are exposed to are very different depending on what type of loading the
pelvis is subjected to. During bilateral stance, the symphysis and
inferior SI joint are under tension, and the superior SI joint is under
compression. During single leg stance, however, the symphysis is under
compression and vertical shear stresses, while the SI joint is under
tension superiorly and compression inferiorly.190
Behavior of the symphysis is also dependent on what ligaments are
intact posteriorly. When the symphyseal ligaments are disrupted but the
sacrotuberous and sacrospinous ligaments are intact, the tendency is
for symphysis to close with weight bearing. This is noted clinically
when an APC I pelvic injury is treated nonoperatively and the initial
injury diastasis lessens over time with patient mobilization.
 |
|
FIGURE 44-39 Three-dimensional reconstruction of a pelvis demonstrating (A) unilateral (right) and (B) bilateral sacralization of L5.
|
 |
|
FIGURE 44-40 Schematic drawing demonstrating the supporting ligamentous structures of the pelvic ring.
|
pelvic ring, these ligaments provide support for the many vascular,
neural, and visceral structures contained within the pelvis. The major
trunks of the iliac arterial system pass near the SI joints ventrally (Fig. 44-41)
and exit the pelvis via the greater and lesser sciatic notches and
obturator foramen. Disruption of these ligaments and bony structures
increases the risk of arterial and venous injury (usually the anterior
and posterior divisions of the internal iliac arteries and venous
plexus) and severe hemorrhage.
posterior to the pubic symphysis and the rectum is immediately anterior
to the sacrum (Fig. 44-42). Because of the
intimate association of these and other pelvic visceral structures to
the bony pelvis, visceral injury is not uncommon owing to the
significant force transfer required to produce a pelvic fracture.
Indeed, Cydulka,31 Demetriades,36 and Poole130 have documented that associated neurologic, thoracic, and abdominal visceral
injuries are more predictive than the pelvic fracture itself in determining mortality and functional outcome.
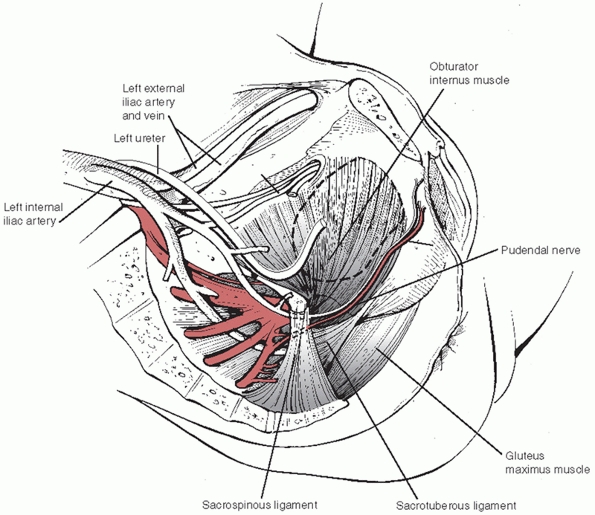 |
|
FIGURE 44-41 Schematic drawing demonstrating the close relationship between the vascular and ligamentous structures of the pelvic cavity.
|
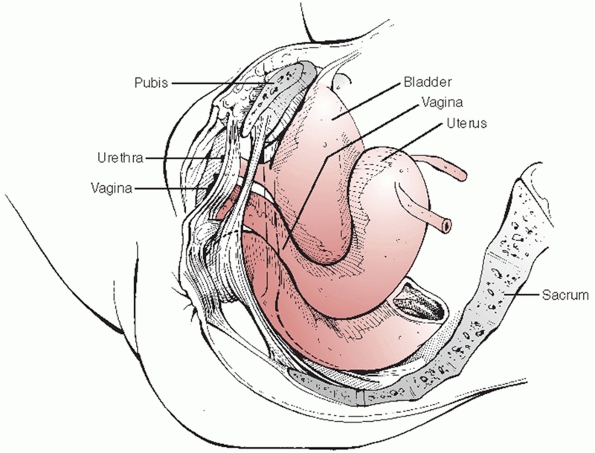 |
|
FIGURE 44-42
Schematic drawing demonstrating the close relationship of the pelvic viscera (bladder, vagina, urethra, and rectum) to bony structures of the pelvis. |
general anesthetic on a radiolucent table that will permit
intraoperative “C-arm” fluoroscopic imaging (such as an OSI flat-top
table, Orthopaedic Systems Inc., Berlin, NJ). Placing a small bump or
rolled sheet/blanket in the small of the back to accentuate the lumbar
lordosis will tilt the pelvis (extension), bringing the pubic bodies
and symphysis more into profile. This can help with drill angulation
during screw placement.
catheter is preferred. If a suprapubic catheter is needed, have the
urologist or trauma surgeon place it from well above the umbilicus to
avoid contaminating the operative field and dissection. If the patient
has an external fixator in place, the bars and clamps should be removed
prior to preparation with sterile solution. It can be useful to keep
pre-existing external fixator pins in situ, particularly for wide
disruptions of the symphysis, as they can aid in approximating the
pubic bodies and manipulating the pelvis for reduction. However, the
pin sites should be excluded from the surgical field with sterile gauze
and a small occlusive dressing.
from the umbilicus to the top of the perineum in the vertical direction
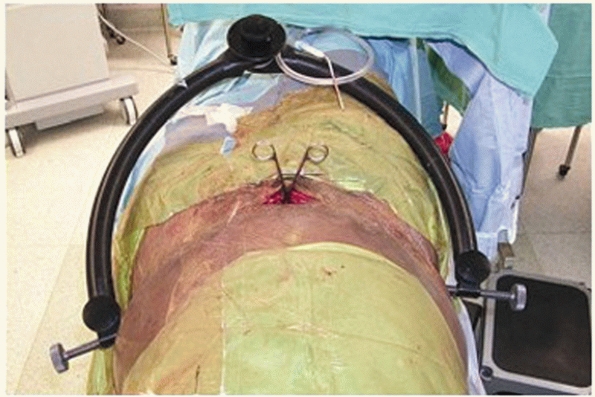
with the lateral borders of the exposure out past the iliac crests (Fig. 44-43). If a suprapubic catheter has been placed, be sure to prep out or cover the catheter site with an occlusive dressing.
abdominal skin with the perineum (“bikini line”). The skin incision for
the majority of individuals need be no more than 7 or 8 cm. Once the
anterior abdominal (rectus) fascia is reached, the linea alba is
exposed with a longitudinal dissection for approximately 10 cm. Care
must be taken at this point to avoid lateral dissection in the
subcutaneous fatty tissue as this puts the spermatic cord and round
ligament with attendant sensorimotor nerves at risk for injury. In
cases of severe symphysis diastasis and high-energy disruptions, one
will often encounter a tear in the rectus fascia at its attachment to
the pubis on one or both sides. This should be repaired with
nonabsorbable suture to prevent subsequent herniae formation.
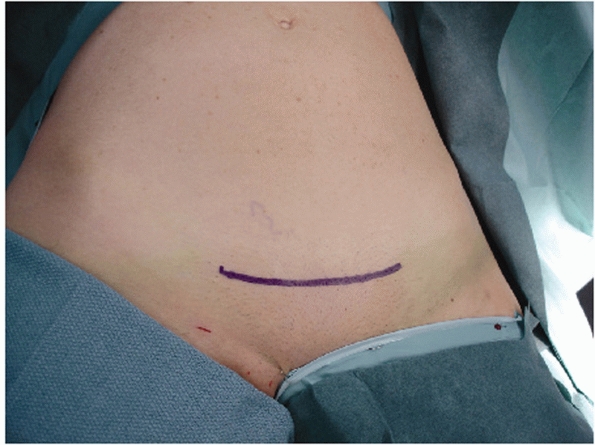 |
|
FIGURE 44-43 Clinical photograph showing positioning, draping, and incision for surgical approach to the anterior pelvic ring.
|
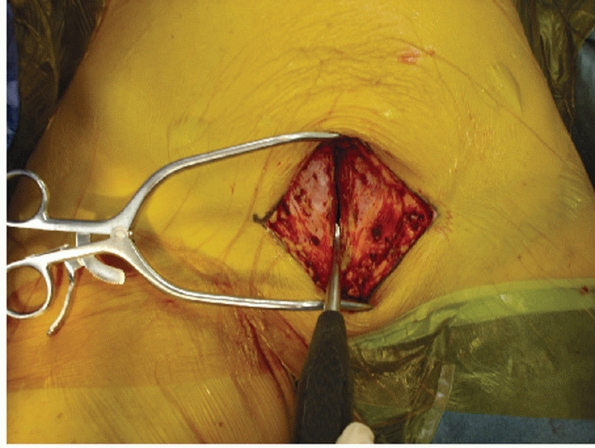 |
|
FIGURE 44-44 Clinical photograph showing vertical splitting of the rectus fascia along the linea alba.
|
along the linea alba from approximately 5 cm above the pubic bodies to
a point approximately 1 to 2 cm below the top of the pubic bodies
anteriorly (Fig. 44-44). Excessive dissection
along the anterior aspect of the pubic body, releasing the rectus
attachment and the suspensory ligament of the penis or clitoris, should
be avoided. The intermuscular plane between the two bodies of the
rectus is followed until the transversalis fascia is reached
posteriorly. In almost all cases, there will be a small rent in the
fascia where the surgeon is able to place a finger for blunt
dissection. The retropubic space of Retzius between the bladder and the
pubis is then developed in blunt fashion separating the bladder away
from the posterior aspect of the pubic bodies. The easiest spot to get
into the retropubic space without inadvertently entering the peritoneal
cavity is directly behind the pubic body at the insertion of the
transversalis fascia.
catheter). Ensure that the bladder has been drained successfully and is
not distended. The bladder is protected with a malleable retractor for
gentle retraction. Take care to avoid excessive inferior pressure with
the malleable retractor as this can cause injury to the urethra and
bladder neck. Place a self-retaining retractor to spread the two sides
of the rectus abdominis. The hematoma is drained and the retropubic
space is irrigated.
until the superior medial aspect of the obturator foramen is
accessible. It is not necessary to subperiosteally dissect to the
foramen; it is sufficient to leave the rectus fascia and periosteum
attached. Next, the dissection carries along the superior aspect of the
pubic body releasing the rectus in subperiosteal fashion until the
pubic tubercle is reached bilaterally. At this point, the surgeon
should have adequate exposure for reduction and fixation of a
symphyseal dislocation (Fig. 44-45).
dissection along the superior pubic ramus will be necessary. Flexion of
the hip with a knee roll or triangle relaxes the external iliac and
femoral neurovascular bundle helping to minimize the risk for injury. A
narrow Dever retractor is placed under the rectus and neurovascular
bundle to improve visualization. The periosteum is incised sharply and
peeled back with an elevator. At the level
of
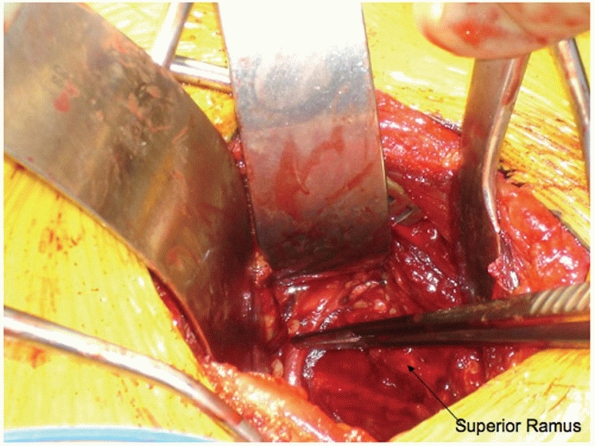
the iliopectineal fascia, the surgeon must look for the anastomotic
connection between the external iliac and obturator vessels (so-called
corona mortis); it needs to be isolated and ligated to allow further
dissection up to the pubic root and supra-acetabular bone of the inner
table of the ilium (Fig. 44-46). From this midline rectus incision and approach (originally described by Stoppa176
for the repair of inguinal herniae), the surgeon can repair all
injuries of the anterior pelvic ring from the pubic symphysis to the
pubic root.
 |
|
FIGURE 44-45
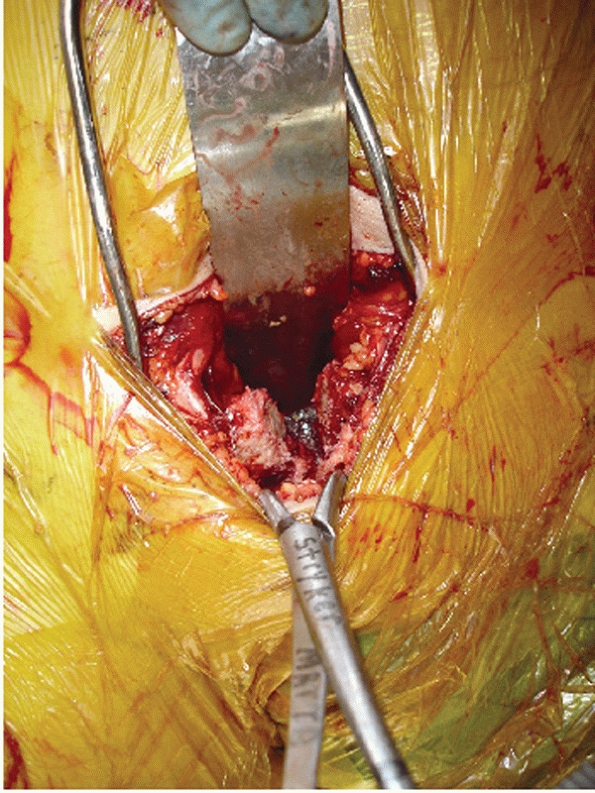
Clinical photograph showing exposure of symphysis with protection of the bladder by a malleable retractor and reduction of the symphysis with a pointed reduction clamp. |
It is particularly hazardous if a suprapubic catheter is in place, as
this will allow a direct path of communication for bacteria between the
catheter and fixation.
patient is positioned in the prone position on a radiolucent operative
table. Preferably, the surgeon should have the ability to apply
longitudinal traction to the unstable hemipelvis. This can be
accomplished by placing the contralateral extremity into a traction
boot without applying traction. The affected side is then placed into
traction. The standard perineal post used for hip fractures, however,
is counterproductive for these cases. The perineal post is designed for
traction across the hip joint by using the ischial tuberosity and pubis
as the stable point of resistance. With an unstable vertical shear
hemipelvis, distal translation with traction cannot occur because the
ischium will abut the perineal post and be blocked from further caudal
translation.
 |
|
FIGURE 44-46 Clinical photograph demonstrating an external iliac to obturator vascular anastomosis (corona mortis).
|
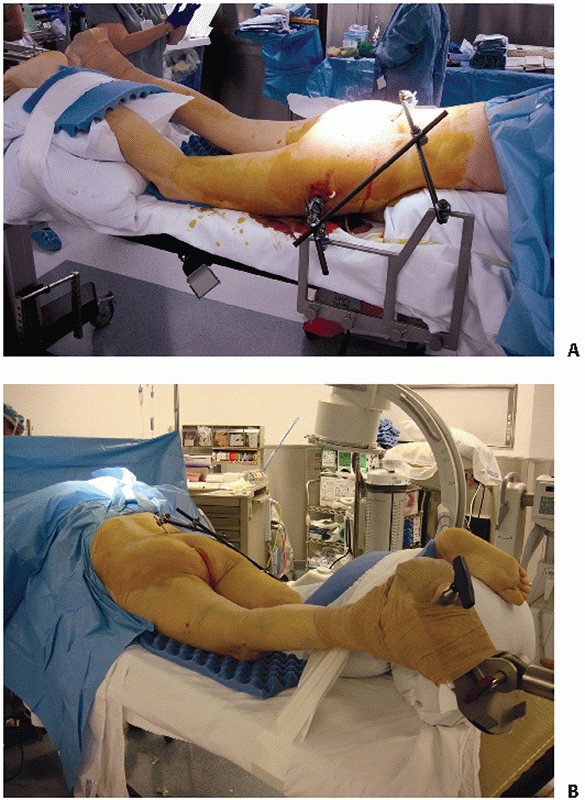
need for significant translation with traction is expected, then the
surgeon should consider a rigid stabilization of the pelvis to the
table permitting aggressive longitudinal traction. In such cases, the
contralateral hemipelvis can be rigidly attached to the operative table
with an external fixator and two Schanz screws placed into the
posterior superior iliac spine (PSIS) and proximal femur as described
by Matta99 and Lefaivre88 (Fig. 44-47).
adequate ventilation are very important, particularly for patients with
chest trauma. It is preferable to use longitudinal chest rolls that
come short of the pelvis, allowing the pelvis to hang freely. This
allows the hip and pelvis to extend, which helps reduce some of the
flexion deformity of the hemipelvis often seen with SI dislocations.
Additionally, having the lower trunk resting on the anterior sacroiliac
spine (ASIS) may impede the surgeon’s ability to manipulate the
fracture and cause some posterior translation of a particularly
unstable hemipelvis.
 |
|
FIGURE 44-47 (A)
Intraoperative photograph demonstrating prone patient positioning and application of table-skeletal fixation to stabilize the pelvis and (B) apply traction. Note that the pelvis is hanging freely off the end of the chest rolls. |
flank on the affected side. The field should continue to include the
buttock and upper thigh, with the natal cleft and contralateral buttock
excluded from the field. The incision is vertical and paramedian,
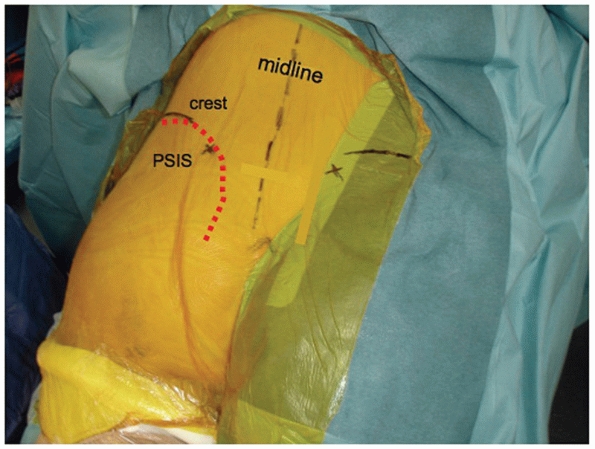
centered directly over the involved SI joint (Fig. 44-48).
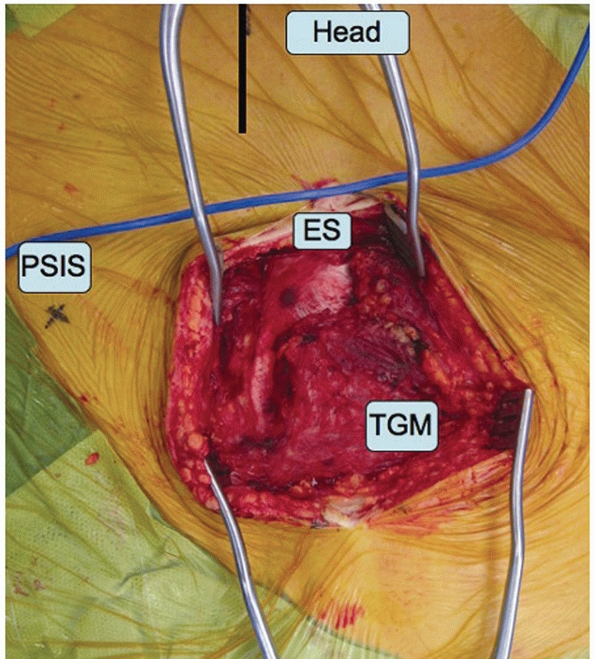
skin incision. The tissues that bridge the SI joint posteriorly in the
intact state include the lumbodorsal fascia (LDF), the transverse
fibers of the gluteus maximus (TGM), the paraspinal erector spinae
muscles (ES), the iliolumbar ligament (ILL), and the posterior SI
ligaments (PSILs). With SI joint dislocations, some or all of these
fascial, muscular, and ligamentous layers may be completely disrupted,
and no further dissection is needed.
SI joint posteriorly, the TGM needs to be mobilized. Recall that these
muscular fibers arise from the spinous processes of the sacrum. The
muscle fibers of the TGM overlap the underlying ES at a 90-degree angle
so care must be taken to avoid transecting the ES when elevating the
TGM from the spinous processes (Fig. 44-49).
The TGM attachment to the sacral spinous processes is released and the
TGM is reflected laterally and inferiorly to expose the inferior aspect
of the SI joint. Occasionally, some of the LDF will need to be released
from the posterior iliac crest, allowing for dissection up over the
superior aspect of the SI joint and sacral ala to permit digital
palpation as an assessment of reduction of the anterosuperior aspect of
the SI joint.
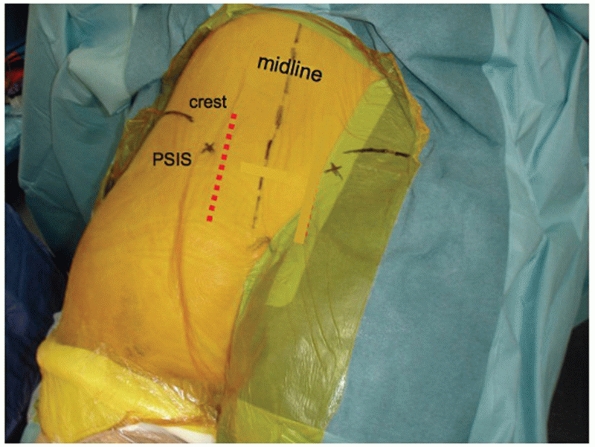
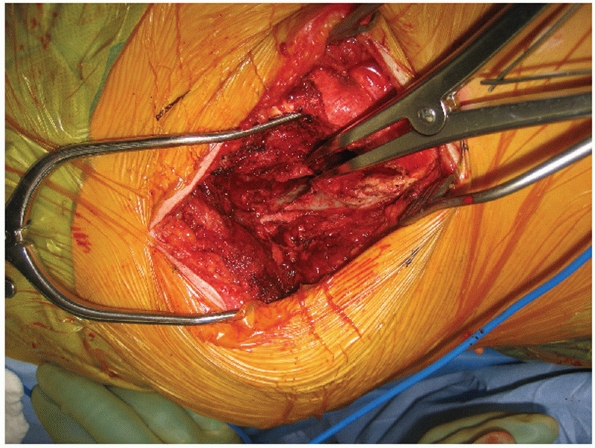
to evacuate a significant amount of blood clot and hematoma from the
joint. On occasion, loose fragments of denuded articular cartilage will
require removal, but as a general rule, routine removal of articular
surfaces for SI joint fusion is not performed in the acute setting
unless there is significant (near total) cartilaginous destruction to
begin with. During removal of blood clot and debris, specific attention
must be paid to the superior gluteal vessels and the internal iliac
vascular system. Removal of clot may result in direct iatrogenic
vascular injury or restart arterial bleeding that was initially
controlled by tamponade and vasospasm (Fig. 44-50).
 |
|
FIGURE 44-48 Palpable landmarks and location of incision to approach the SI joint for open reduction.
|
 |
|
FIGURE 44-49 Intraoperative photograph demonstrating elevation of the TGM away from their attachments to the sacral spinous processes.
|
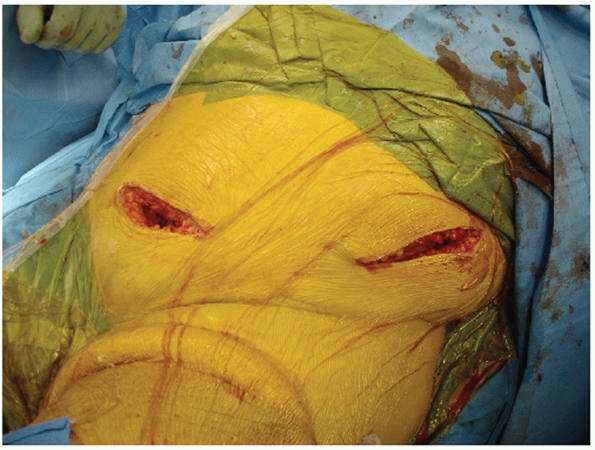
the patient in the supine position, an incision is made along the iliac
crest as one would make for the upper limb of a Smith-Peterson approach
(Fig. 44-51). The incision should start 1 or 2
cm posterior to the ASIS to avoid injuring the lateral femoral
cutaneous nerve. When elevating and releasing the external oblique
abdominal muscle, recall that the muscle fibers come over the crest to
insert just below the crest on the lateral table of the ilium. To
properly release this muscle, the surgeon needs to find the interval
between the external oblique tendon and the tensor fascia and gluteals
as they have a common insertion point. An incision is made in the
intermuscular plane between these two muscles and the external oblique
is elevated subperiosteally over the crest into the internal iliac
fossa along with the iliacus muscle. Fairly large nutrient vessels to
the iliac wing are encountered when elevating iliacus; these nutrient
foramina can be occluded with bone wax to control the continuous venous
oozing.
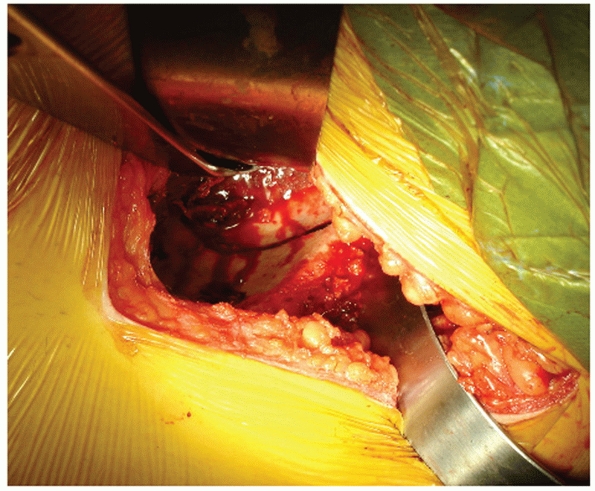
psoas over the pelvic brim into the true pelvis. A blunt Hohmann or
Malleable retractor can be placed over the brim to keep the abdominal
and pelvic contents away. The SI joint dislocation is usually
encountered at this point. Continue careful dissection medially onto
the sacral ala. The L5 nerve root is at risk during this portion of the
dissection as it is located approximately 1 cm medial to the SI joint
in direct contact with the alar bone. Maintaining a subperiosteal plane
will help prevent injury to the nerve. Additionally, direct finger
palpation can find the nerve and push it out of the way as the surgeon
dissects onto
the
ala. Once adequate exposure of the ala is obtained, the surgeon can
drive the point of a sharp Hohmann retractor into the ala under direct
vision. This allows good visualization of the superior and anterior
portion of the SI joint for clamp placement, reduction, and application
of fixation (Fig. 44-52).
 |
|
FIGURE 44-50 Intraoperative photograph demonstrating exposure of the SI joint with lamina spreader.
|
same as for SI dislocations. Exposure of the posterior aspect of the
sacrum for purposes of reduction is most easily performed through a
midline incision. Care must be taken to make note of any occult spina
bifida of the sacrum, which can occur in up to 15% of patients.153
The paraspinal muscles can be elevated subperiosteally from the spinous
processes, over the sacrum, to the posterior inferior iliac spine
(PIIS) and PSIS of the ilium. Care must be taken to avoid dropping into
the lateral aspect of the spinal canal through laminar fractures and
defects. One must also avoid releasing and destroying the posterior SI
ligaments, which, in the case of sacral fractures, are intact.
 |
|
FIGURE 44-51 Intraoperative photograph showing incision along anterior iliac crest for exposure of the SI joint from anterior.
|
for intertransverse fusions of the spine) can be used. By developing
the distal intermuscular plane between the multifidus and longissimus
paraspinal muscles, the sacral fracture can be exposed as well. This
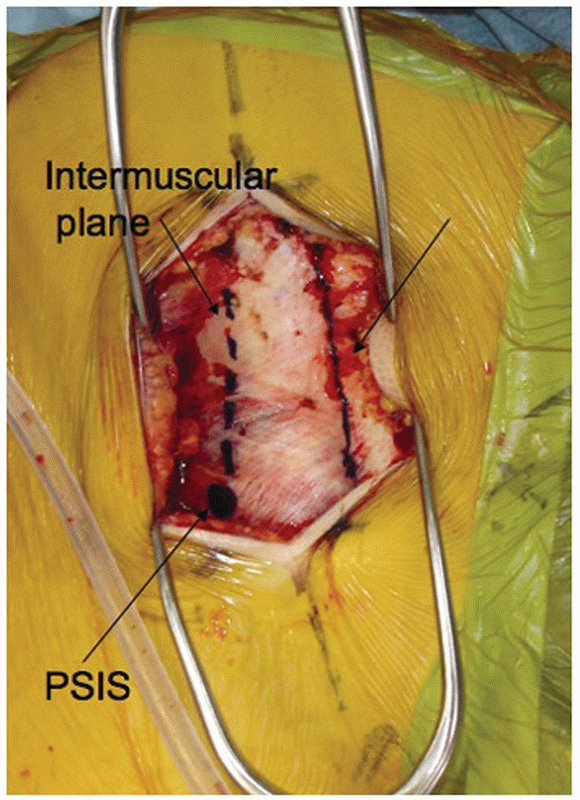
intermuscular plane comes down to the pelvis just above the PSIS. The
paraspinal muscles are then elevated away from the crest and posterior
aspect of the sacrum. This approach is especially helpful when the
surgeon has elected to use a spinal pelvic fixation construct, because
this intermuscular plane will lead directly from the PSIS to the
insertion point for pedicle screws at L5 (Fig. 44-53).
iliac wing fractures largely depends on the location of the fracture.
Posterior and medial fractures can be managed with the same incision
and surgical approach that is used for SI dislocations. However, as the
fracture line exits the crest more laterally and anteriorly, a
curvilinear incision along the crest will need to be made, and on
occasion approached with the patient supine using the upper limb of a
Smith-Peterson approach (Fig. 44-54).
 |
|
FIGURE 44-52
Intraoperative photograph showing exposure of the anterior aspect of the SI joint with a Hohmann retractor placed in the sacral ala to protect the L5 nerve root. |
 |
|
FIGURE 44-53
Intraoperative photograph demonstrating the lumbodorsal fascia and intermuscular plane for the paramedian approach to the sacrum. |
approach involves elevating the gluteal muscles away from the external
iliac fossa from the crest down to the greater sciatic notch. Again,
the surgeon needs to be mindful of the TGM as mentioned earlier.
However, in this case, the TGM is more likely to be transected from the
traumatic injury. Careful dissection of the TGM is needed to reflect
the entire muscle laterally and distally to expose the whole outer
table of the ilium. It is important to see the entirety of the fracture
because a seemingly anatomic reduction at the crest can still be
associated with significant displacement at the notch. Additionally,
the strongest most reliable bone for fragment manipulation and fixation
is the sciatic buttress just above the notch.
 |
|
FIGURE 44-54 Skin incision for surgical approach to the iliac wing and crest from posterior.
|
to the superior gluteal neurovascular bundle, and the cluneal sensory
nerves should be protected to avoid injury and painful neuroma
formation when dissecting along the crest.
Smith-Peterson is used to release the external oblique muscle from the
crest and then elevate the iliacus from the inner table of the ilium as
described above for anterior approaches to the SI joint.
-
With complete instability of the
posterior ring (i.e., posterior SI ligamentous disruption or
nonimpacted displaced sacral fractures), anterior fixation alone is
inadequate for maintaining reduction and restoring stability to the
pelvic ring. -
With complete instability of the
posterior ring and cephalad displacement, any posterior fixation should
be supplemented with some form of anterior stabilization (ORIF or
external fixation).175 While the pubic symphysis supplies only 10% to 15% of the stability to the intact pelvic ring,67,190 it is critical in restoring the normal loading response and stability to the unstable hemipelvis.151 -
The posterior injury is regarded as the
more critical and in need of accurate reduction with stable fixation.
Although there are exceptions, reduction and stabilization generally
proceeds from the posterior pelvic ring to the anterior pelvic ring.88,89
-
Symphyseal dislocations demonstrating
greater than 2.5 cm of diastasis on either static or dynamic
(examination under anesthetic) imaging -
Augment posterior fixation in vertically displaced unstable pelvic ring injuries
-
Locked symphysis
-
Pain and inability to mobilize
include anterior external fixators or internal fixation with plate and
screws. Available data from biomechanical studies67,175
have shown no significant difference between anterior external or
internal fixation of the pelvis for controlling external rotation of
hemipelvis. However, anterior internal fixation is superior to external
fixation in resisting vertical displacement of the hemipelvis.
Additionally, there is significant improvement in pelvic ring stability
when posterior fixation is augmented with some form of anterior
fixation in vertical shear injury patterns.67,151
commonly used in the treatment of the anterior pelvic ring injury.
Indeed, their ease of placement and minimally invasive application in
the treatment of both symphyseal disruptions and rami fractures lends
to their utility.54 However, reports
of pin site complications, interference with access to the abdomen,
cumbersome nature, inability to accurately fine tune and maintain
reductions, and entrapment of the bladder have limited their use in modern pelvic reconstructions.6,53,96
Currently, the primary role of anterior pelvic external fixation is
stabilization of the anterior ring when ORIF is precluded by an open
contaminated anterior abdominal laparotomy incision or bladder rupture,
and other situations that may contraindicate ORIF.
consideration in choosing the form of stabilization for the anterior
pelvis is delivery of a fetus and the potential need for cesarean
section in females of childbearing age. Provided that adequate
reduction can be maintained, anterior external fixation may be
preferable in these patients so that when the pelvis is healed, the
fixator can be removed and there will be no residual fixation
inhibiting relaxation of the pelvic ring at the time of delivery.
exist for the placement of anterior pelvic external fixation: multipin
iliac crest frames and two-pin supra-acetabular frames. The classic
iliac crest frame involves placement of two or three 5-mm partially
threaded Schanz pins (with 5-mm pins being significantly stronger than
4-mm pins)18 into the iliac wing
from the iliac crest. Larger incisions that tend to be problematic with
abdominal swelling in massively resuscitated patients are required for
the application of this frame. Additionally, while the iliac crest is
sufficiently thick to accommodate the Schanz pin, the remainder of the
wing toward the pelvic brim thins to just a few millimeters, frequently
resulting in cortical perforation (Fig. 44-55).
Generally speaking, the technique for placement of these pins requires
dissection into the internal iliac fossa for finger palpation to help
guide the surgeon to the correct trajectory and minimize cutout or
perforation.
external fixator uses small incisions for pins that are placed into the
bone above the acetabulum and directed toward the PIIS into the robust
region of the sciatic buttress.50,125
In addition to being placed below the level of the abdomen, this
two-pin external fixator applies a more appropriate vector for closure
of an “open-book” injury, avoiding the abduction that is occasionally
noted with the traditional iliac crest frame (Fig. 44-56).38
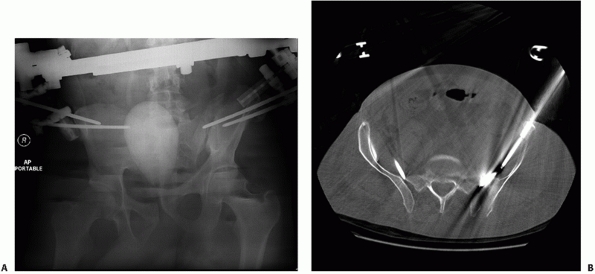 |
|
FIGURE 44-55 (A) AP radiograph and (B) axial CT scan demonstrating perforation and cutout of external fixation pins placed in the crest.
|
 |
|
FIGURE 44-56 AP radiograph demonstrating pin location for supra-acetabular external fixator pin placement.
|
requires the use of intraoperative fluoroscopy. The incision is
approximately two fingerbreadths below the ASIS. The C-arm is brought
over the top to give an obturator oblique view of the acetabulum with
some tilt of the intensifier to give a partial outlet view as well
(so-called obturator-outlet). The C-arm is adjusted until the
“teardrop” is visualized. This radiographic structure represents the
inner and outer tables of the ilium and the top the greater sciatic
notch inferiorly. The center of the teardrop represents the column of
bone extending from the anterior inferior iliac spine (AIIS) to the
PIIS via the sciatic buttress (Fig. 44-57).
needing only a single pin on each side and being placed into thick,
good-quality bone above the acetabulum. This large corridor
of
bone can be accessed with pins reaching all the way back to the PIIS,
giving some element of control of the posterior ring. The traditional
iliac crest pins and frames often required two or three pins placed
bilaterally into the thin bone of the iliac wing78,101,186 with frequent perforation into the internal iliac fossa, and poor fixation (see Fig. 44-55).
Recent biomechanical studies have demonstrated that the
supra-acetabular frame was equal in stiffness to the iliac crest frame
for resisting flexion and extension; however, it was stiffer in
resisting in internal and external rotation.5,79,129
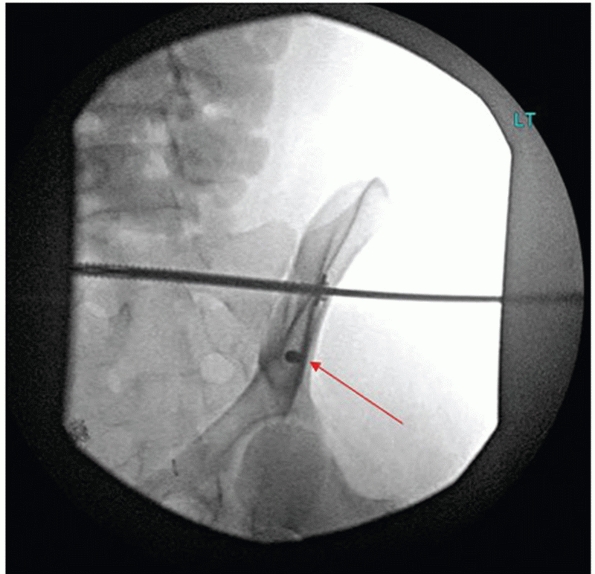 |
|
FIGURE 44-57 Intraoperative obturator outlet fluoroscopic view showing teardrop for supra-acetabular pin placement.
|
Symphyseal Disruptions. Prior to surgical preparation of the operative
site, internally rotate the lower extremities, tape them together, and
wrap a sheet around the patient at the level of the greater
trochanters. These maneuvers will help in reduction of widely displaced
symphyseal injuries. Once the pubic bodies are exposed (see earlier
surgical approach to the anterior pelvic ring), they can be
approximated using large reduction forceps such as a Weber clamp and
manipulation with the external fixator pins if they are present. The
tines of the clamp do not need to be placed into the obturator foramen
as this requires excessive dissection and adequate purchase can be
obtained in the lateral aspect of the pubic body (Fig. 44-58).
superiorly; therefore, reduction clamps should be applied anteriorly to
avoid interfering with the placement of internal fixation.
posterior ring instability, the unstable hemipelvis demonstrates a
tendency to rotate externally and superiorly (flexion) and translate
posteriorly. If there is significant sagittal plane rotational
deformity and posterior translation, applying traction to the affected
extremity is often all that may be needed to correct this.
Alternatively, a large pelvic reduction clamp such as a Farabeuf or
Jungbleuth can be secured to the anterior aspects of the pubic bodies
with 4.5-mm cortical screws to perform the same maneuver (Fig. 44-59).
The use of these clamps is particularly useful in situations where
multiplanar manipulation (AP translation and flexion-extension) is
required to reduce the anterior pelvic ring.
 |
|
FIGURE 44-58 Photograph demonstrating placement of tines for reduction of symphyseal dislocations.
|
degrees of posterior SI ligament injury. Whereas some symphyseal
disruptions will close anatomically with simple internal rotation of
the unstable hemipelvis, other injuries result in flexion (sagittal
plane rotation) and even some anterior to posterior translation that
require multiplanar reduction maneuvers. For the more unstable injury
patterns, the Farabeuf or Jungbleuth reduction clamps are preferred
since they are more effective at controlling and reducing
internal/external rotation, flexion/extension, and translational
deformity.
aspects of the pelvic ring, appropriate imaging using all three of the
AP, inlet, and outlet views is necessary.
applied. As of the writing of this chapter, there are no scientific
data to support the use of one plate/screw configuration over another.
Some authors believe that two-hole plates are advantageous because they
allow some degree of “physiologic” motion to occur at the symphysis.191
However, one study has shown a higher rate of fixation failure and
pelvic malunion with two-hole plating, recommending a plating
configuration that has at least two points of fixation on either side
of the symphysis (Fig.44-60).152
Dual-plating and multiplanar plates have not been found to offer
improved stability over single uniplanar symphyseal plates in
biomechanical studies.161
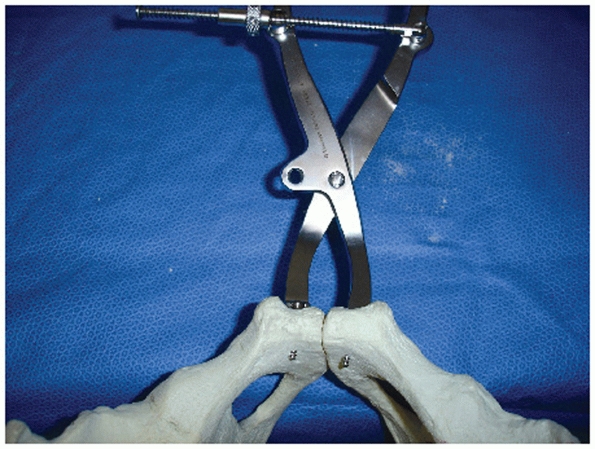 |
|
FIGURE 44-59 Photograph demonstrating placement of a Jungbleuth clamp for multiplanar reduction of the pubic symphysis.
|
 |
|
FIGURE 44-60 (A) Inlet and (B)
outlet radiographs demonstrating loss of fixation and malunion with two-hole symphyseal plate. Note the sagittal plane flexion of the unstable left hemipelvis. |
superior symphyseal plating, the use of 3.5-mm or 4.5-mm plates
(reconstruction or not) with 3.5-mm, 4.5-mm, or 6.5-mm screws is at
this point completely arbitrary. The use of a locked screw/plate
construct to augment fixation is another option in osteoporotic bone,
but there are no published clinical or biomechanical studies endorsing
this technique. The author’s preference is a four- or six-hole
symphyseal specific plate with two 4.5-mm cortical screws in each pubic
bone (Fig. 44-61).
to the superior aspect of the pubic bodies, with at least two holes on
either side of the symphysis. The two screws on either side of, and
closest to the symphysis, are applied first using compression technique
(i.e., lateral eccentric drilling). The screw trajectory should be to
obtain the longest screw possible exiting the inferior cortex of the
pubic body to obtain bicortical purchase. A finger placed along the
posterior aspect of the symphysis helps the surgeon to estimate the
trajectory. A malleable retractor should be placed between the bladder
and pubis to protect the bladder. Drill with the 3.2-mm drill bit,
measure, and place a fully threaded 4.5-mm screw. Securing the first
two screws will usually correct any flexion-extension, but longitudinal
traction also helps to reduce this deformity. With only one screw on
each side of the symphysis, fine-tuning adjustments correcting anterior
to posterior translation can still be made with a reduction clamp. One
of the tines of the reduction clamp is placed posterior to the pubic
body of the unstable hemipelvis, while the second tine is placed
anterior to the contralateral pubic body. A twisting or rotational
maneuver will translate the posterior displaced pubic body anteriorly.
When the symphysis is reduced in the axial plane, the final two 4.5-mm
screws can be placed to further stabilize the reduction.
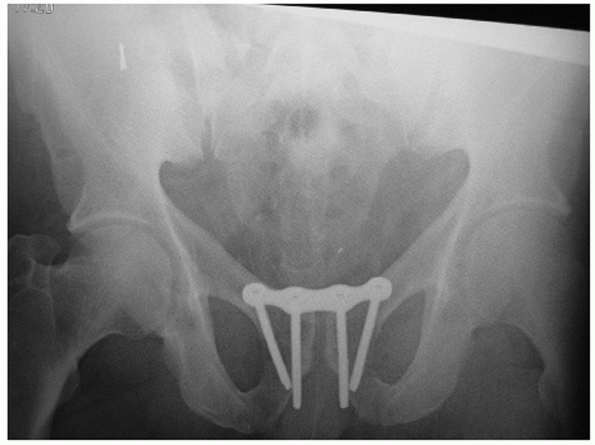 |
|
FIGURE 44-61 AP radiograph of a reduced pubic symphysis stabilized with a four-hole symphyseal plate and four 4.5-mm cortical screws.
|
the screws should be directed at such an angle to obtain bicortical
purchase with both screws in the pubic body. Take care to avoid
penetrating the posterior cortex of the pubic body with the screws as
they can irritate and/or perforate the bladder. This can be verified
with digital palpation of the posterior pubic body and an appropriate
inlet radiographic view with the image intensifier.
purchase, the retropubic space is irrigated and drained. The drain is
brought through one of the rectus muscles, away from the incision. The
rectus fascia and muscle are repaired in a single-layer closure with
full-thickness interrupted figure-of-eight nonabsorbable suture.
injuries, rami fractures are a bony injury with surrounding periosteum.
The inguinal ligament plays an important stabilizing role in these
injuries as well; rami fractures medial to the insertion of the
inguinal ligament behave akin to a symphyseal disruption, while those
lateral to the insertion benefit from the tension band of the inguinal
ligament and are not unstable or prone to wide separation unless there
is significant soft tissue disruption. Some anterior ring stability is
provided by an intact periosteal sleeve
in
minimally displaced fractures. Additionally, bony injuries with viable
periosteum have much greater potential for rapid and predictable
healing. Because of these factors, many rami fractures can be treated
nonoperatively. However, for widely displaced fracture-dislocations
with considerable soft tissue damage and instability (particularly
vertical shear injuries), ORIF of the ramus fracture may be indicated
to augment posterior fixation and improve on the reduction of the
pelvic ring.
for symphyseal disruptions; however, internal fixation with plating
does not carry the same long-term obstetrical or hardware failure
sequelae since the fixation does not cross the mobile symphysis.
Reduction of a widely displaced superior ramus fracture can be more
problematic than the pubic symphysis since it is difficult to place the
tine of a reduction clamp lateral to a ramus fracture. In these
instances, indirect reduction techniques frequently need to be used.
midline approach is to secure a contoured pelvic reconstruction plate
lateral to the fracture in the pubic root or supra-acetabular bone.
Several confirmatory radiographs are needed to ensure that the screw is
not in the hip joint. The tines of a reduction clamp are then secured
to the medial fracture fragment and the most medial hole in the plate.
Closure of the clamp pulls on the plate and lateral fracture fragment,
reducing the fracture. The reduction often needs fine-tuning with an
additional reduction forceps to bring the ramus up to the plate. Screws
are placed through the medial aspect of the plate into the pubic body
to maintain the reduction.
described and may allow percutaneous application of internal fixation
for stabilization of a ramus fracture.141,173
However, a closed or careful percutaneous reduction with a bone hook
must be possible to allow passage of the screw through the narrow
corridor provided by the superior ramus. It is also a good option for
fixation after formal open reduction of the ramus if there is minimal
comminution (Fig. 44-62).
largely on the location of the fracture. The ramus can be divided into
three zones173 (medial, middle, and
lateral) that can aid in the decision-making process. Those fractures
that are located medially in the superior ramus may be treated with a
retrograde ramus screw, while those fractures that are located more
laterally near the pubic root are better treated with an anterior
column screw. The use of intraoperative fluoroscopy and drilltip
guidewires for cannulated screws greatly facilitates this technique.
The obturator oblique, inlet, and outlet views are all important in
verifying that the guidewire and screw have not perforated the ramus
cortex or exited the fracture site because the external iliac vascular
structures lay directly on the superior ramus.
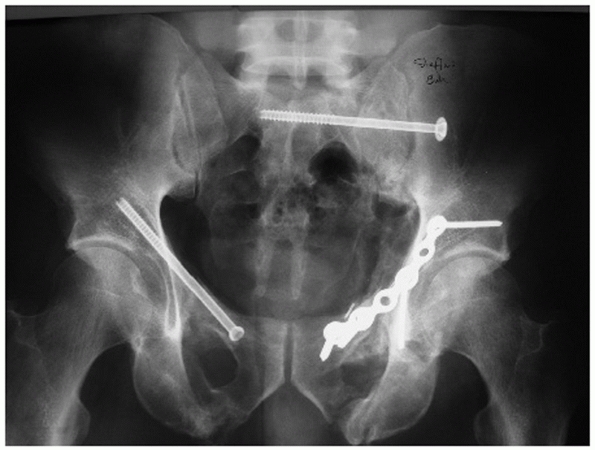 |
|
FIGURE 44-62
AP radiograph demonstrating the use of a retrograde ramus screw (right) and a pelvic reconstruction plate (left) in the treatment of bilateral rami fractures. |
-
Displaced iliac wing fractures that enter and exit both the crest and greater sciatic notch or sacroiliac joint
-
Disruption of the posterior SI ligaments resulting in multiplanar instability of the SI joint
-
Nonimpacted, comminuted, displaced sacral fractures
-
Any posterior ring injury that has demonstrated or has the propensity for cephalad (vertical) displacement
-
U-shaped sacral fractures with spinal-pelvic dissociation
plate or lag screw along the crest supplemented with a second
reconstruction plate or lag screw at the level of the pelvic brim
(anterior approach) or sciatic buttress (posterior approach) will
usually suffice in neutralizing deforming forces until healing has
occurred (Fig. 44-63).163,164,179
Iliac wing fractures are more often associated with open wounds than
other pelvic ring injuries and may be associated with entrapped bowel.24,44
Careful examination of the wound and CT scan is imperative. Early
reconstruction of the ilium and serial débridements with open packing
and delayed closure is the recommended approach for treatment of these
injuries.
joint, resulting in partial dislocation of the SI joint and disruption
of some or all of the SI ligaments.12,13
The treatment of these so-called “crescent” fractures needs to be
approached on a caseto-case basis—with the size of the crescent
fragment being the primary variable. The crescent fragment is a
variable-sized fragment of bone that contains the PSIS and PIIS and
remains attached to the sacrum via the posterior and (at times) the
intra-articular SI ligaments. The anterior SI ligaments are disrupted
and displace along with the iliac wing.
the SI joint and have a large crescent fragment with uninjured
posterior and intra-articular ligaments; the injury and treatment
parallel an iliac wing fracture. Stable fixation of the iliac wing to
this crescent fragment with intact ligaments will provide adequate and
stable fixation to maintain reduction of the posterior ring until
healing occurs, and no SI screws should be needed (Fig. 44-64).
the crescent fragment becomes smaller and there is increasing damage to
the posterior and intra-articular ligaments; the injury and treatment
begin to parallel an SI joint dislocation. While the crescent fragment
may be reduced back to the iliac wing as a
guide
to reduction, achieving and maintaining reduction will require
placement of clamps similar to SI joint reductions (see later) and one
or more SI screws to achieve adequate stability (Fig. 44-65).
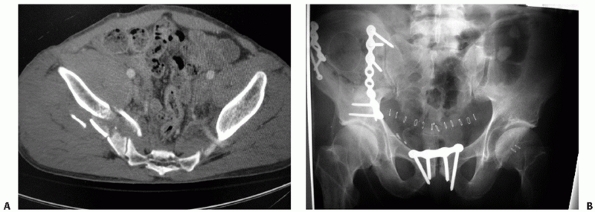 |
|
FIGURE 44-63 (A) Axial CT scan and (B) postoperative AP radiograph demonstrating an iliac wing fracture treated with ORIF.
|
approached from posterior as described earlier. Reduction of the iliac
wing to the crescent fragment using the Weber pelvic reduction clamp is
generally the first order of business since this fragment is
anatomically located. It is important to expose the posterior ilium all
the way down to the greater notch so that (a) the inferior margin of
the fracture can be visualized, (b) reduction clamps can be placed
through the notch to reduce the anterior aspect of the SI joint (see
later), and (c) plate fixation can be placed along the sciatic buttress.
reconstruction plate along the iliac crest with supplemental lag screws
from the PIIS into the sciatic buttress just above the greater notch.
As the crescent fragment becomes smaller, this injury approaches an SI
dislocation, and consideration must be given to supplemental fixation
with SI screws or plates.
anterior or posterior; however, it is often easier to reduce the SI
joint and apply reduction clamps from the posterior approach.106
The difficulty noted with either approach is that the majority of the
reduction is performed indirectly and not under direct visualization.
From posterior, only the inferior aspect of the joint is visualized,
and the remainder of the anterior reduction must be assessed by finger
palpation through the greater notch and fluoroscopy. From anterior,
only the superior aspect of the ala is visualized. Finger palpation and
clamp placement to assess and reduce the inferior and posterior aspect
of the joint are much more difficult.
 |
|
FIGURE 44-64 (A) Axial CT scan and (B)
postoperative radiograph demonstrating a large crescent fragment with little to no involvement of the SI joint treated with ORIF of the iliac wing only. |
requires a good knowledge of the three-dimensional anatomy of the
sacrum, ilium, and SI joint. Although some AP translation and
lateral-medial translation of the hemipelvis may be necessary to reduce
the SI joint anatomically, longitudinal traction is the single most
important maneuver to use, and it is important to position the patient
on the table in such a way that adequate longitudinal traction can be
applied.
intraoperatively with direct visualization, digital palpation, and the
image intensifier. Confirmation with direct visualization is performed
by matching the inferior portion of the ilium at the most medial aspect
of the greater sciatic notch with its recess in the lateral
aspect of the sacrum. Radiographically, ensure that the iliac crests and ischial tuberosities are at the same level bilaterally.
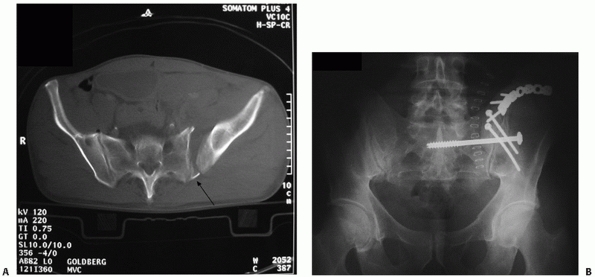 |
|
FIGURE 44-65 (A) Axial CT scan and (B)
postoperative radiograph demonstrating a small crescent fragment with significant involvement of the SI joint treated with ORIF and a supplemental SI screw. |
to the sacrum and adequate length has been achieved, it may be that the
weight of the patient resting on the ASIS is resulting in posterior
translation of the unstable hemipelvis. For this reason, it is
important to let the pelvis hang freely to avoid inadvertent posterior
translation and prevention of anterior translation during reduction
maneuvers. Additionally, having the patient rest prone on the ASIS can
also induce an external rotation deforming force on the pelvic ring,
which may be difficult to overcome with posterior reduction maneuvers.
placement of two reduction clamps to medially translate and internally
rotate the hemipelvis. Large pointed reduction clamps (such as the
Weber clamp or offset pelvic reduction clamps) are used. The first
reduction clamp is used inferiorly. One tine is placed over the sacral
spinous process or into the posterior cortex of the sacrum inferiorly,
and the other is placed into the cortical bone of the medial aspect of
the greater sciatic notch on the outer table of the ilium. As the clamp
is closed, the inferior SI joint is reduced (Fig. 44-66).
performed by placing a second offset reduction clamp through the
greater sciatic notch. This tine is placed with the help of the index
finger of the other hand palpating the anterior surface of the sacral
ala to localize the sacral foramina and nerve roots. The tine can then
be safely placed onto the lateral ala. The posterior tine is placed
onto the outer table of the ilium and the anterior aspect of the SI
joint can then be reduced (Fig. 44-67).
Reduction of the joint is then assessed by further palpation and
intraoperative fluoroscopy. Final confirmation with inlet, outlet, and
AP radiographs will help disclose subtle rotational deformities not
appreciated by direct visualization or palpation.
but this technique is more difficult. One tine is placed carefully over
the top of the joint onto the sacral ala anteriorly (avoid the L5 nerve
root), and the second tine is placed just lateral to the PSIS. While
this clamp is being closed to reduce the superior SI joint, an internal
rotation maneuver is used to help close down the anterior aspect of the
SI joint. This internal rotation force can be accomplished by
internally rotating the affected extremity or by placing a threaded
Schanz pin into the ilium to serve as a “joystick.”
views (AP, inlet, and outlet views of the pelvis) to assess reduction
of the joint and disclose any residual subtle rotational and
translational deformities. If the joint is satisfactorily reduced,
rigid fixation with one or two SI lag screws is performed. If the
reduction has been performed using the anterior approach, the surgeon
has the option to stabilize the reduction using an anterior SI plate
with or without a supplemental SI screw.165 In previous biomechanical studies, no difference in strength has been found between SI screws and anterior SI plating.28
 |
|
FIGURE 44-66 Photograph demonstrating reduction of the SI joint from the posterior approach using inferior clamp placement.
|
 |
|
FIGURE 44-67
Photographs demonstrating reduction of the SI joint from the posterior approach using clamp placement through the greater notch onto the anterior aspect of the ala (A: from posterior; B: from anterior). |
exists regarding the order of fixation when an SI joint dislocation is
accompanied by symphyseal dislocation anteriorly. Letournel’s89,90
“Golden Rule” has classically stated that since anatomic reduction of
the weight bearing arch posteriorly is the primary goal in
stabilization of the pelvic ring, it should be reduced first followed
by ORIF of the anterior ring. In general, and for wide displacements,
this is true. A poorly performed initial reduction and fixation of the
anterior ring can and will preclude or markedly impair the surgeon’s
ability to then anatomically reduce the more critical posterior ring.
That being said, in the modern of age of percutaneous SI screw fixation
that can be placed from either the prone or supine position, there are
instances where initial ORIF of the anterior ring will facilitate and
permit closed manipulative reduction and percutaneous screw fixation of
the SI joint. Or, at the most, necessitate opening the lateral window
anteriorly to fine-tune the reduction of the SI joint from anterior. In
these cases, it is prudent to reduce and maintain the reduction of the
anterior aspect of the pelvis with a clamp initially (i.e., no internal
fixation). Then reassess the position of the posterior ring and if
possible reduce and stabilize with an SI screw. It is important to
avoid rigid internal fixation anteriorly prior to reduction and
fixation posteriorly. Once the screw has been placed posteriorly, the
fixation can then be placed anteriorly.
The gluteal muscles and lumbodorsal fascia are reattached to their
respective insertions and origins with nonabsorbable monofilament
suture in figure-of-eight interrupted fashion. Routine subcutaneous and
skin closure follows. Staples are not recommended for the skin, as they
tend to be irritating for the patient.
surgeon, excellent intraoperative fluoroscopic imaging, and thorough
knowledge of pelvic anatomy and radiographic and surface landmarks are
necessary to prevent complications with the use of this technique.2,20,26,42,142,143,180 Although biomechanical studies28,60,159,195
have not shown significant superiority of either SI screw fixation,
transiliac fixation, or anterior SI plating, SI screws can be applied
in either the prone or supine position and in open or percutaneous
situations of severe soft tissue damage when closed reduction is
possible. For these reasons, SI screws are the most commonly used form
of fixation for the unstable SI joint, the other forms of fixation
being reserved for salvage situations.76,160
the safe and accurate placement of SI screws: the lateral sacrum, the
pelvic inlet, and the pelvic outlet.197 Of the three projections, the lateral142
view of the sacrum potentially gives the surgeon the most information
on one single view. However, there is considerable debate among
experienced pelvic surgeons as to which views are truly necessary. In
truth and reality, however, a wise and prudent surgeon should be
familiar with the radiographic landmarks on all three views such that
he or she will be able to safely place an SI screw if one or more of
the views is suboptimal secondary to patient size, bowel gas, or the
technical expertise of the x-ray technologist.
the surgeon can visualize the anterior aspect of the sacral canal
(posterior aspect of the S1 body), the iliac cortical density (which
corresponds to the sacral ala and L5 nerve root), the anterior cortex
of the sacral promontory, and the vestigial disc space between S1 and
S2 (which corresponds to the S1 foramen and nerve root). On occasion
with good-quality radiographs in a thin patient, the upper sacral nerve
root tunnel can also be visualized as it courses from the spinal canal
to the foramen. The inlet projection (Fig. 44-69) is helpful for orienting the path of the screw toward the anterior aspect of the promontory, and the outlet projection (Fig. 44-70) ensures that the screw is above the S1 foramen heading toward the superior endplate of S1.
 |
|
FIGURE 44-68 Lateral projection of the sacrum demonstrating the important radiographic landmarks for placement of an iliosacral screw.
|
increase in popularity and utility with spinal pedicle screws and total
joint arthroplasty, they have not enjoyed the same popularity with
pelvic surgery. This is, in a part, due to the inherent difficulties in
fiducial positioning and obtaining image quality sufficient for
tracking and navigation. However, there have been a number of
preliminary reports examining the placement of SI screws in cadaveric
pelves demonstrating improved or equal accuracy with a substantial
decrease in radiation exposure to the surgeon.27,34,69,170
Currently, these systems, whether based on optical or electromagnetic
guidance, still add considerable time to the procedure and should not
be used in place of experience and knowledge of three-dimensional
pelvic anatomy.
trajectory for an SI dislocation should be perpendicular to the plane
of the SI joint: from posterior to anterior and from inferior to
superior as the screw travels medially. With any potential
displacement, the unstable hemipelvis will use the SI screw as a rail.
If the screw trajectory is directed cephalad from lateral to medial,
the tendency will be to direct the hemipelvis medially (point of
increasing stability) as it travels superiorly. If the screw’s
trajectory is caudal from lateral to medial, the hemipelvis will be
directed laterally (point of decreased stability) as it tends to
displace superiorly.
trajectory of the SI screw (see later) is necessary since an oblique
trajectory from posterior to anterior will cause a subtle anterior
translation as well as medial translation on tightening of the screw.
This translational effect can be used to the surgeon’s advantage to
reduce minor residual deformities, provided that the screw trajectory
is properly planned and still lies within the “safe corridor” for SI
screw placement.
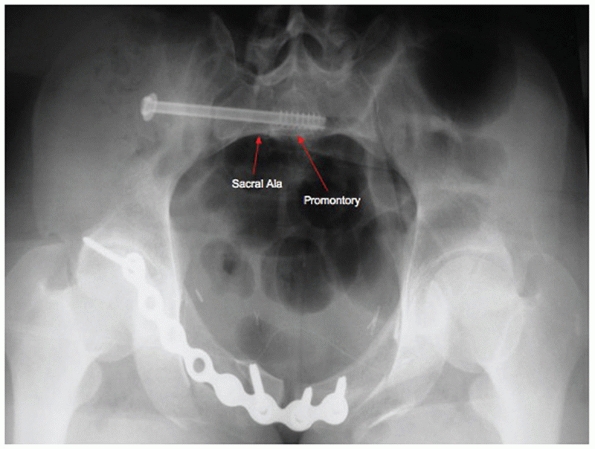 |
|
FIGURE 44-69
Inlet projection of the posterior pelvis demonstrating the important radiographic landmarks for placement of an iliosacral screw (anterior cortex of the promontory and ala. |
 |
|
FIGURE 44-70
Outlet projection of the pelvis demonstrating the important radiographic landmarks for placement of an iliosacral screw (superior endplate of SI and the S1 foramen). |
6.5 mm, 7.3 mm, or 8.0 mm have all been used to stabilize SI joint
dislocations. There are no studies citing the superiority of one
diameter over the other. A drill-tipped guidewire rather than a
threaded-tipped guidewire is preferred by many pelvic surgeons because
it is easier to redirect should the need arise. Starting with the
lateral projection, the guidewire is placed below the iliac-cortical
density (ICD) at the posterior cortex of the sacral body. Once this
position is secure, the outlet projection is used to direct the
guidewire in a cephalad direction above the S1 foramen toward the
superior endplate. The inlet projection is used to ensure that the
guidewire’s trajectory is directed toward the anterior aspect of the S1
body (sacral promontory).
sacral foramen and the lateral view is repeated to ensure that the
guidewire is still below the iliac cortical density (and L5 nerve
root). If the guidewire is below the ICD, it is advanced into the
anterior aspect of the S1 vertebral body and promontory to a point just
past the midline. Placement of the guidewire into the contralateral ala
is suboptimal as this places the contralateral L5 nerve root at risk
and screw purchase is poor because of the decreased bone density in the
sacral ala. Therefore, long thread lengths with the tip of the screw in
the sacral body and promontory offer the greatest resistance to pull
out.20,82
Recall that the first sacral foramen courses from posterior-superior to
anteriorinferior. The SI screw should be directed as close to the
superior endplate and anterior aspect of S1 to stay as far from the S1
foramen as possible.149 Washers are
recommended to prevent the screw head from penetrating the thin cortex
of the outer table of the ilium, thus compromising fixation and making
later removal difficult.
purchase of the first SI screw is thought to be suboptimal or
considerable instability of the pelvic ring exists.107 Biomechanical studies have shown increased stability with a second screw, with a second
screw into the S2 vertebral body being superior to a second screw into S1.151,188
CT scan is necessary to plan the trajectory of the SI screw. The
geometry and diameter of the safe corridor for placement of SI screws20,113
can differ significantly among patients and need to be taken into
consideration to avoid injury to major neurovascular structures, which
are but millimeters away in some cases.180
Segmentation defects of the lumbosacral junction and sacral dysmorphism
with partial or complete sacralization of L5 or lumbarization of S1 are
not uncommon and need to be appreciated prior to surgical stabilization
(see Fig. 44-39). A good general rule of thumb
is that the iliac crest is “always” at the level of the L4-5 disc
space. It is important to recognize segmentation defects because the
“safe corridor” for SI screw placement can be significantly altered by
the anomalous anatomy. For example, a sacralized L5 often exhibits a
deep “ala” with a more anteriorly directed trajectory. If these
differences in anatomy are not appreciated preoperatively, the
potential for iatrogenic nerve injury exists.
As more surgeons have become familiar with placing SI screws
percutaneously in either the prone or supine position, this use of
anterior plate stabilization alone has decreased. Currently, anterior
SI plating is most commonly used to augment an SI screw after an
anterior reduction of the SI joint has been performed. One or two
two-hole plates are placed to span the SI joint with one screw in the
posterior ilium and the other in the lateral sacral ala. Care must be
taken to gently push the L5 nerve root medially with a finger prior to
placing a retractor and reduction clamp onto the sacral ala. The
difficulty with this technique then lies in the fact that it can be
difficult to place the fixation with the clamp holding the reduction
anteriorly (Figs. 44-71 and 44-72).165
Transiliac bars or plates spanning the sacrum are rarely used today in
cases of failed SI screws or revision scenarios with poor fixation.28,60,159,195
regarded as a pelvic ring injury, spinal injury, or both. Additionally,
while most sacral fractures do not result in injury or instability of
the SI joints, there can be variable degrees of injury and instability
of the L5/S1 facet joint with the cephalad extension of the fracture.73
Longitudinal or vertical sacral fractures result in disruption of the
posterior pelvic ring. U-shaped sacral fractures (bilateral
longitudinal transforaminal fractures joined by a transverse fracture
line) result in disruption of the spinal-pelvic junction—and only
rarely the pelvic ring. A final point to bear in mind is that sacral
fractures can result in various degrees of neurologic deficit: ranging
from unilateral peripheral nerve root injuries in the case of vertical
shear fracture patterns37 or cauda equina lesions in the case of transverse or U-shaped fractures.56,157
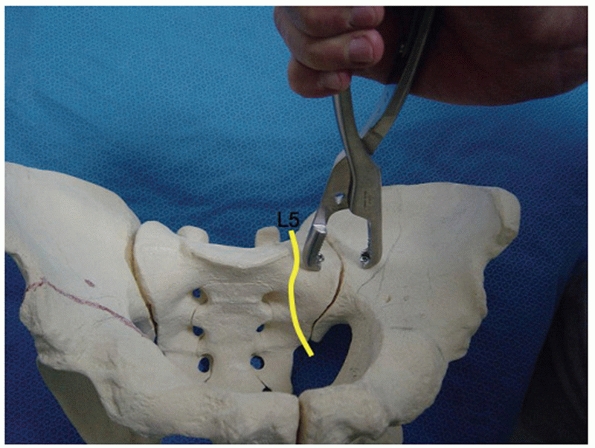 |
|
FIGURE 44-71
Photograph demonstrating reduction technique for the SI joint from the anterior approach. The clamp is lateral to the L5 nerve root. |
 |
|
FIGURE 44-72 Radiograph demonstrating anterior plating of the SI joint.
|
sacral fractures is the unique nature of the sacrum as an anatomic and
biomechanic structure. The sacrum functions both as the keystone
articular segment for the posterior pelvic ring as well as the final
articulating junction between the axial skeleton (spine) and the pelvic
ring. All force transfer from the torso to the lower extremities occurs
through the perisacral articulations (sacroiliac and L5/S1 facet
joints). These complex anatomic and biomechanic characteristics coupled
with the intimate relationships of neurologic structures and associated
injuries can make treating sacral fractures a difficult task. Reduction
and stabilization techniques and decision algorithms largely depend on
which of the above components are disrupted—for example, is there a
neurologic injury (and if so, what type), and is the surgeon dealing
with instability of the pelvic ring, the lumbosacral junction, or both?
-
Anterior and posterior pelvic ring
disruption with vertical or cephalad instability in the case of a
vertical shear sacral fracture -
Nonimpacted and/or comminuted sacral alar fractures with external rotation deformity of the hemipelvis
-
U-shaped sacral fractures with spinal pelvic dissociation, cauda equina syndrome, or excessive sacral kyphosis
-
Impacted sacral fractures from lateral
compression injuries with excessive internal rotation and pelvic
deformity that, when the anterior ring is reduced, the posterior ring
becomes disimpacted and unstable. (However, the majority of
longitudinal sacral fractures that arise from lateral compression
P.1451injuries and result in impaction of the sacral ala, are inherently stable, and will not require any operative stabilization.)
fractures, like SI joint dislocations, relies heavily on longitudinal
traction. Appropriate length or position of the hemipelvis is best
judged radiographically on the AP view of the pelvis by checking that
the iliac crests, acetabular domes, and ischial tuberosities are at the
same levels bilaterally.
of the sacrum to close any residual fracture gap is accomplished with
reduction clamps. If the fracture gap is minimal and no rotational or
translational deformities exist, an SI lag screw can be used to reduce
the deformity. Care must be taken during reduction and stabilization of
comminuted alar and transforaminal sacral fractures to avoid
inadvertent L5 or sacral nerve root injury; the patient should be
counseled as to the potential for this to occur in severe fractures.
However, it is imperative that any fracture gap is reduced and closed
to improve stability135 and avoid a nonunion (Fig. 44-73).150
table. It is important to have the patient resting on chest rolls
without any pressure on the ASIS or pelvis to prevent posterior
translation and impairment of reduction.149
Hyperextension of the lumbosacral junction with the hip extended helps
to reduce the flexion deformity of the unstable hemipelvis that is
often seen with these injuries. Longitudinal traction applied through
the femoral traction pin is helpful in reducing both cephalad
displacement and flexion. Where possible, keeping the knee flexed helps
to relax tension on the sciatic nerve, but excessive knee flexion will
interfere with obtaining adequate inlet-outlet views with the image
intensifier intraoperatively. If significant cephalad displacement
exists and/or the surgery is being performed on a delayed basis, then
more forceful traction may be needed. In order to prevent pulling the
patient down to the foot of the operating room table and tilting of the
pelvis, the surgeon can rigidly fix the patient to the operating room
table using table-skeletal fixation as described separately by Matta99 and Lefaivre.88
often accomplishes the majority of the sacral fracture reduction. After
the traction has been applied, but prior to surgical preparation, the
intensifier is brought into the operating room to gauge the reduction
and a decision is made as to whether a formal open reduction will be
needed as well. Because of fracture comminution and plastic deformation
of the alar cortex, it can sometimes be difficult to estimate the
adequacy of reduction of a sacral fracture. With good-quality AP,
inlet, and outlet radiographs, the surgeon should assure himself or
herself that the iliac crests, acetabular domes, and ischial
tuberosities are at the same level bilaterally, that the distance from
the sacral spinous process to the PSIS is the same bilaterally, that
the profile of the ischial spine is the same bilaterally, and that the
contour of the pelvic brim from the midline of the sacrum to the pubis
is symmetrical bilaterally. Anatomic reduction (or as close as
possible) is important for improving fracture stability and increasing
the diameter of the safe corridor for SI screw placement.135
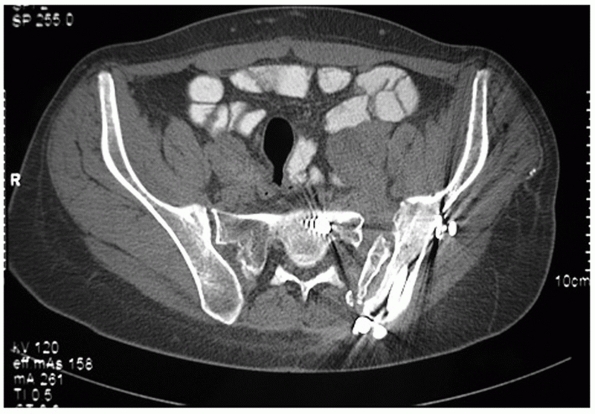 |
|
FIGURE 44-73 Axial CT scan demonstrating residual fracture gap and nonunion of a sacral fracture.
|
approached from posterior as described earlier. An important point to
keep in mind when performing open reduction of the sacrum is to
preoperatively verify either the presence or absence of an occult spina
bifida.153 Hasty dissection into the
midline or careless placement of a clamp with a spina bifida occulta
can result in iatrogenic injury to the nerves in the sacral spinal
canal.
by placing the tines of a pointed reduction clamp (Weber) onto the PSIS
and sacral spinous process. Residual anterior or posterior displacement
is most easily reduced by placing a threaded Schanz pin into the PSIS
or PIIS and manually translating the hemipelvis. It is best to do this
prior to closing the fracture gap so that the hemipelvis can be easily
manipulated. Residual internal or external rotation can be reduced by
using this same Schanz pin as a “joystick” to rotate the hemipelvis in
the desired direction. Alternatively, the lower extremity can be
internally or externally rotated to achieve the same result (Fig. 44-74).
fractures differs slightly from the technique used for stabilization of
SI joint dislocations. First and foremost, the screw trajectory should
be as perpendicular as possible to the fracture plane to achieve
optimal reduction and stability; often, this necessitates that the
screw be placed straight across from lateral to medial, parallel to the
S1 endplate and promontory on the outlet and inlet views respectively.
Second, some authors advocate the use of a “transsacral” screw gaining
purchase in the contralateral ilium (Fig. 44-75).7,33
Theoretically, this screw would have increased resistance to cephalad
displacement because the point of rotation (tip of the screw) lies on
the opposite side of the pelvis. Any cephalad migration and rotation of
the screw is impeded by the superior endplate of S1 and contralateral
ala. There are no published biomechanical studies that have confirmed
this assertion. Careful scrutiny of the preoperative CT scan is
necessary in determining whether the patient’s anatomy will permit the
passage of such a screw. For the majority of sacral fractures, one or
two SI or transsacral screws with or without supplemental anterior
fixation will be adequate for maintaining reduction until healing has
occurred. As of the writing of this chapter, the literature is not
clear on the additional benefit provided by a second SI screw.162,188
However, if a second screw can be safely placed into either S1 or S2,
most surgeons would agree to proceed with this supplemental technique
in the case of a displaced, unstable sacral fracture.
as triangular osteosynthesis to augment the SI screw fixation. These constructs, initially reported on by Kach and Trentz,75 have been shown to be superior both biomechanically156 and clinically to SI screws alone for maintaining reduction in comminuted sacral fractures150,155 (Fig. 44-78).
 |
|
FIGURE 44-74 Intraoperative photographs demonstrating reduction maneuvers for treatment of a sacral fracture using (A) a Schanz pin as a joystick and (B) rotation of the extremity.
|
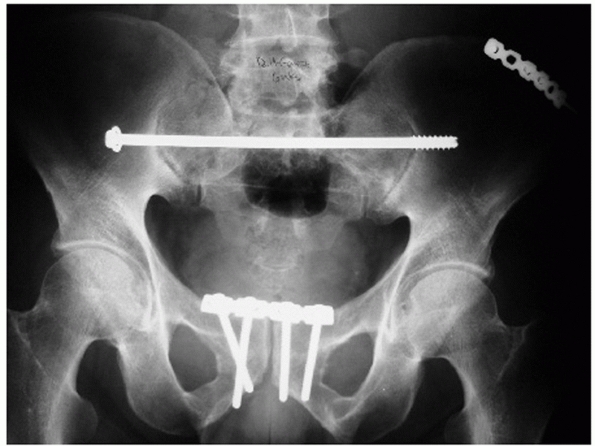 |
|
FIGURE 44-75 Postoperative radiograph demonstrating a transsacral screw for treatment of a sacral fracture.
|
 |
|
FIGURE 44-76 Coronal reconstruction CT scan demonstrating L5/S1 facet joint disruption associated with a vertical shear sacral fracture.
|
(also known as lumbopelvic fixation or triangular osteosynthesis)
essentially involves bypassing the sacral fracture with fixation so
that the lines of force transmission from the spine to the ilium travel
through the fixation instead of the sacrum (Fig. 44-79).
as the spinal point of fixation. If the L5 transverse process is
broken, some, or all, of the lateral pedicle may be disrupted and the
surgeon may choose L4. Alternatively, if there is minimal comminution
laterally and posteriorly on the sacrum, the surgeon may choose the S1
pedicle as the spinal point of fixation. The access point for the L5
pedicle is found immediately lateral to the L4-5 facet joint; take care
to not disrupt this joint capsule as this may lead to facet joint
degeneration and mechanical low back pain. The entry point for the
screw is at the junction of the transverse process with the lateral
wall of the facet. AP, lateral, and oblique views are used to guide the
pedicle screw into the body of L5. If the orthopaedic trauma surgeon is
not familiar with this technique, spinal surgical assistance should be
sought.
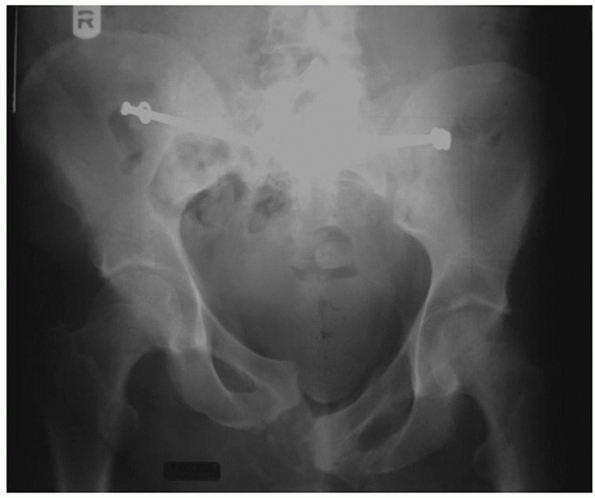 |
|
FIGURE 44-77 Failure of iliosacral screws and malunion of a comminuted sacral fracture.
|
 |
|
FIGURE 44-78 Postoperative AP radiograph demonstrating sacral fracture treated with a triangular osteosynthesis construct.
|
being just distal to the PSIS. Since this fixation can be prominent at
the PSIS or PIIS, an attempt should be made to recess the screw just
under the overhang of the posterior crest, in the cleft between the
ilium and sacrum without taking down the posterior SI ligaments.148,155
A 3.5-mm drill bit is used to perforate the outer cortex and enter the
medullary cavity between the inner and outer tables of the iliac wing.
Preoperative axial images of the contralateral side should be studied
to estimate the correct angle, which is usually between 10 and 20
degrees off the vertical in a line aiming for the AIIS or, the greater
tuberosity as an external reference point. The trajectory should lie
just above the sciatic buttress (as seen on the iliac oblique view of
the ipsilateral hemipelvis) and in a radiographic teardrop representing
the cross section of the iliac wing (as seen on the obturator oblique
view of the ipsilateral hemipelvis) (Fig. 44-80).
It is best to drill a short distance and let the screw find its own way
down the medullary cavity between the inner and outer tables. The iliac
screw is then connected to the previously placed pedicle screw with a
5-mm rod and the appropriate screw-rod clamps. It is important that
reduction first be obtained and the SI screw positioned with closure of
any fracture gap prior to locking down the spinal pelvic construct as
it will prevent any further fracture manipulation.
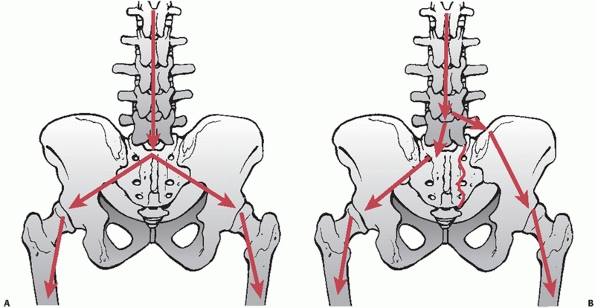 |
|
FIGURE 44-79 Force transmission in (A) a normal intact spine and pelvis, and (B) in a patient with a sacral fracture treated with spinal pelvic fixation.
|
constructs are that they provide improved stability with a lower rate
of hardware failure and loss of reduction. Patients are able to fully
weight bear within a few weeks of surgery.148,150,155
However, the surgeon needs to be familiar with placing pedicle screws
or obtain assistance for such, and there are a number of potential
reported complications and technical aspects that need to be considered
when using these techniques. Prominent and symptomatic fixation,
delayed union and nonunion, and lumbosacral scoliosis are the most
commonly encountered problems unless careful attention is paid during
reduction and implantation. Spinal pelvic constructs usually need to be
removed after the fracture is healed in 6 to 9 months.150
circumstances with longitudinal sacral fractures: (i) the patient
presents with sacral radiculopathy and the preoperative CT scan
demonstrates a fragment of bone in the nerve root foramen or (ii) the
patient is neurologically intact but the CT scan shows a fragment of
bone in the foramen that may injure the nerve root with the reduction
maneuver (Fig. 44-81). If sacral nerve root decompression is necessary, it can be performed directly
through the fracture—a sacral laminectomy is not required. Careful
spreading of the fracture with a laminar spreader and removal of clot
will expose the sacral nerve roots and offending fragment of bone,
which can be gently removed with a pituitary rongeur (Fig. 44-82).
 |
|
FIGURE 44-80 Obturator outlet and iliac oblique views demonstrating the path of the iliac screw.
|
fracture is essentially a sacral fracture dislocation. It tends to
occur through the vestigial disc space and result in kyphosis and
posterior translation of the upper sacral (and lumbar) spinal segments (Fig. 44-83).147,178
These fractures can be easily overlooked on the standard AP pelvis and
even axial CT scans, but they are quite evident on the sagittal CT
reconstruction. These injuries generally do not result in pelvic ring
instability, as the SI joints and distal surrounding sacrum are intact;
they are more commonly associated with spinal instability. They can,
however, be associated with cauda equina syndrome and should be treated
by an experienced spinal surgeon. Although some trauma surgeons have
advocated bilateral SI screw fixation alone for impacted, stable
injuries without significant kyphosis or neurologic deficit,114
these injuries commonly require reduction, decompression, and some form
of bilateral posterior lumbopelvic fixation to control kyphotic
deformity and allow early mobilization of the patient (Fig. 44-84).157
sacral laminectomy and removal of bone in the anterior aspect of the
sacral spinal canal are usually required to decompress the sacral nerve
roots (Fig. 44-85). Reduction may or may not be
required. If the fracture is impacted and kyphosis is not significant,
in situ stabilization may be all that is needed.114
However, if the kyphosis and sacral inclination are excessive, then
reduction can be performed by first placing the spinal pelvic construct
and distracting across the rods to disimpact the fracture.
Hyperextending the lumbosacral spine, pelvis, and hips can help to
correct the kyphosis along with an osteotome placed along the fracture
line to the front of the sacrum acting as a lever. The spinal pelvic
construct is then locked into position. No SI screws are needed. L5 to
sacral fusion may or may not be performed depending on the integrity of
the L5/S1 facet joints.157
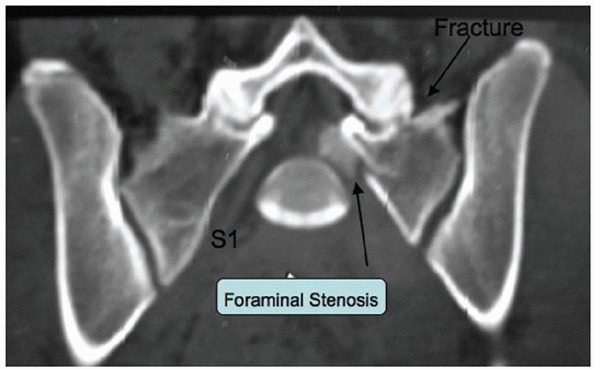 |
|
FIGURE 44-81 CT scan demonstrating S1 intraforaminal bone fragment.
|
discontinued when drainage is less than 25 mL per 8-hour shift. Sutures
are generally left in place for 2 to 3 weeks. Postoperative ileus is
common and needs to be managed with careful advancement of dietary
intake. Diet should not be advanced unless flatus and bowel sounds have
returned. Aggressive postoperative bowel regimens are necessary and
should be part of the postoperative protocol.
 |
|
FIGURE 44-82 Intraoperative photograph demonstrating sacral nerve root decompression through the fracture.
|
 |
|
FIGURE 44-83 (A) Axial and (B)
sagittal reconstruction CT scan demonstrating spinal pelvic dissociation from a sacral fracture dislocation at S1/S2. Note complete occlusion of the canal. |
While there is agreement that these injuries increase the rate of wound
and soft tissue complications, there is only anecdotal experience to
guide management. Some surgeons believe that thorough drainage,
débridement, and vacuum-assisted closure with quiescence of the wound
should precede formal ORIF, while others believe that percutaneous
drainage and lavage are all that are required.65,84,184
If the lesion is large enough in diameter, complete full-thickness
necrosis and slough may result since the degloved skin and fat are now
receiving their entire blood supply from collateral arterioles in the
skin from the periphery of the lesion, with exposed bone and fixation
necessitating débridement and flap coverage. Considerable acumen and
experience are required to pass judgment on the safest time to reduce
the fracture, place fixation, and appropriately manage these wounds in
concert with a plastic surgeon.
 |
|
FIGURE 44-84 (A) AP and (B)
lateral postoperative pelvic radiographs demonstrating reduction of sacral kyphosis and stabilization with bilateral spinal-pelvic construct. |
that will dictate their ability to mobilize. However, all other factors
being equal, patients should be mobilized to a chair the day following
surgery, and then begin gaiting with physical therapy the next day.
This helps with pulmonary toilet and bowel/bladder function.
Any
pelvic ring injury that is associated with complete disruption of the
posterior ring should be mobilized with touchdown weight bearing for 10
to 12 weeks (fractures shorter and dislocations longer), at which point
full weight bearing may be permitted. Incomplete posterior ring
disruptions such as stable impacted lateral compression injuries or APC
1 and 2 injuries can, for the most part, be allowed full weight bearing
immediately postoperatively. However, careful radiographic follow-up is
necessary within a week of mobilization to ensure that greater occult
instability has not been missed. Generally speaking, patients with
stable impacted sacral fractures will declare themselves in bed by
being able to move and roll easily. Patients who have significant pain
and inability to even log roll in bed likely have a more unstable
pelvis that may require at the least a fluoroscopic stress examination
under anesthetic and possibly fixation.
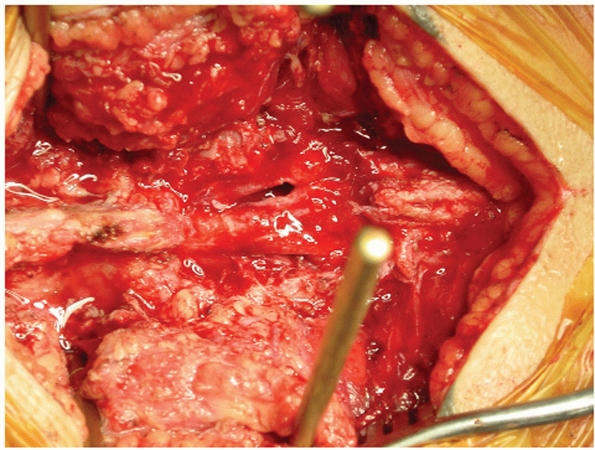 |
|
FIGURE 44-85
Intraoperative photograph demonstrating decompression of the cauda equina in a patient with cauda equina syndrome secondary to a sacral fracture dislocation. |
controversial topic that unfortunately has no scientifically
established standard of care, yet remains a major source of litigation
and debate.17,169 From the available data, it appears that some form of prophylaxis should be started within 12 to 24 hours of pelvic fracture.52,171,172 This can be in the form of low-molecular-weight heparins or unfractionated heparins given subcutaneously.174
 |
|
FIGURE 44-86 (A) Preoperative and (B) intraoperative photograph demonstrating fluctuance, ecchymosis, and degloving cavity with a large Morel-Lavallee lesion.
|
anticoagulation because of sensitivity or associated head, chest, or
abdominal trauma, consideration should be given to placement of an
inferior vena cava filter (IVCF). Modern IVCF placement is associated
with a low complication rate131 and
usually involves placement of removable filters that should be removed
within 3 months depending on the manufacturer and clinical situation.
However, few patients have the filter removed, and the incidence of
postphlebitic syndrome can be as high as 70%. IVC filters, while
reducing the incidence of pulmonary embolism (PE), have not been shown
to improve survival or affect the incidence of fatal PE.131
the calves or feet have been shown to help decrease the incidence of
venous thrombus formation and should be used in all patients whose
condition tolerates their application.171
Screening with Duplex Doppler, contrast venography, and magnetic
resonance venography has not been shown to be efficacious in patients
with pelvic fractures.11,177
has no scientific basis. The risk factors that need to be considered in
the decision to send a patient home on anticoagulation are ambulatory
status, age older than 40 years, risk factors for deep venous
thrombosis (DVT)/PE, body mass index greater than 30 kg/m2,
female sex, birth control pills or hormone replacement therapy,
adenocarcinoma or malignancy, prior history of DVT/PE, and smoking.52
If a patient is young, healthy, and ambulatory with no other
independent risk factors for DVT/PE, then consideration can be made for
no discharge anticoagulation provided that appropriate in-hospital
anticoagulation has been used and after careful discussion with the
patient and documentation in the medical chart. All other patients
should be discharged on some form of anticoagulation
(low-molecular-weight heparin or warfarin) for 4 to 6 weeks. Aspirin
and elastic stockings are considered inadequate discharge
thromboprophylaxis.52
scenarios, with some occurring as minimally displaced stable isolated
injuries in young healthy patients with good bone quality and others
occurring as part of a multiple trauma where numerous organ systems are
involved in physiologically unstable patients. These injuries can
result in only minimal nuisance to the patient for a few weeks or
potentially life-threatening situations that permanently affect the
patient with either complications or neurologic, vascular, or visceral
injury. In addition, surgery for pelvic fracture reduction and fixation
can be associated with large blood losses and fluid shifts, periods of
hypotension, and prolonged anesthetics and operative times, all of
which contribute significantly to the increased potential for
complications.
potentially life threatening and needs to be dealt with expeditiously.
Injury to vessels such as the superior gluteal artery can occur with
fracture reduction and fixation placement of the posterior pelvic ring.
Unless the vessel is easily identified and separated from the superior
gluteal nerve, the wound and vessel should be packed and closed and
angiographic embolization performed to address the offending vessel.
Constant uncontrollable venous bleeding secondary to coagulopathy,
hypothermia, or massive transfusion can lead to significant blood loss
as well and should not be overlooked. The tendency is for the surgeon
to become focused on achieving a difficult reduction whilst ignoring
the patient’s physiologic status and blood loss. It is imperative that
the surgeon and anesthesiologist stay in close communication throughout
the case regarding body temperature, blood loss volume, urine output,
and blood gas results. If the patient’s physiologic status deteriorates
before the pelvis is reduced and stabilized, the wound needs to be
packed and closed, and the patient transferred to the intensive care
unit for resuscitation and stabilization with plans to return to the
operating room in the next few days to finish the job. Continuing to
work on a deteriorating patient or accept a quick but suboptimal
reduction and fixation is, in most cases, unacceptable.
is an extremely anxiety-provoking scenario, particularly after a
difficult reduction has finally been achieved. Poor anterior pelvic
fixation (with extensive comminution or osteoporosis) can be augmented
with either multiplanar plates, locked plate/screw constructs, or a
supplemental anterior pelvic external fixator. There are no data in the
current literature to guide the surgeon in this respect, and he or she
must depend on their clinical acumen to decide which supplement would
be best indicated for that particular scenario. Dealing with inadequate
posterior pelvic fixation, again, usually entails the addition of
supplemental fixation techniques to the construct. One SI screw can be
supplemented with a second and even third SI screw into S1 and S2.107
A poor-quality purchase in S1 promontory may necessitate taking the
screw all the way across into the contralateral ilium (a transsacral
screw).7 If fracture comminution and
sacral anatomy preclude multiple SI screw placements, then a spinal
pelvic construct may be needed. As a final effort, transiliac bars or
plates can be used to augment poor posterior pelvic ring fixation.60
dealt with quickly to avoid deep pelvic abscess formation. If the
patient presents with prolonged (greater than 3 to 5 days) wound
drainage and/or erythema, fever, and elevated white blood cell count in
the early postoperative period (first 2 to 3 weeks), then a return trip
to the operating room is necessary for wound débridement and irrigation
to prevent osteomyelitis and infected internal fixation. Depending on
the intraoperative findings, multiple repeat débridements may be
necessary, followed by culture-specific intravenous antibiotics for up
to 6 weeks (after consultation with infectious disease specialists).
of the healing process and the prospect of an established, deep
infection or abscess around the fixation and bone is present, then
careful workup and management are necessary to eradicate the problem.
Preoperative bloodwork for white blood cell counts, erythrocyte
sedimentation rate, and C-reactive protein levels, while not
necessarily useful for ruling infection in or out, is useful for
following the patient’s response to surgical and medical therapies. A
preoperative CT scan of the pelvis and abdomen with contrast is helpful
to assess for abscess formation, location, and depth as well as the
extent of fracture healing. As a general rule, it is not considered a
good idea to treat a deep pelvic infection around internal fixation
with percutaneous drainage and antibiotic therapy alone unless the
patient is too physiologically frail because of age and/or comorbidity
to withstand an open procedure. Depending on the extent of infection
and fixation/bone involvement, the surgeon should consider multiple
débridements, removal of fixation (if the fracture is healed), and
placement of antibiotic beads.
be difficult to manage. In some instances, the patient’s preoperative
mental status or associated injuries preclude a thorough physical
examination. In these cases, it is important to document only what is
known and not write a cursory “neurovascular intact” in passing on the
chart. Displaced posterior pelvic fractures and dislocations are not
infrequently associated with subtle neurologic injuries that may not
manifest until sometime in the postoperative period. If a patient is
known to be neurologically intact preoperatively and then demonstrates
a deficit postoperatively, then a postoperative CT scan is needed to
assess the situation. MRI is generally not useful secondary to the
image distortion created from the internal fixation. At times, a
postmyelogram CT scan will be needed to fully assess the nerve roots.
must be gauged to assess the need for reoperation and nerve root
decompression. A patient who presents with radiculitis only and no
motor or sensory loss can be managed nonoperatively with observation
and medications to control neurogenic pain such as steroids or
gabapentin. However, if radicular pain does not decrease over the next
2 to 3 weeks, then the fixation should be revised or the bone fragment
removed. If a new motor deficit is diagnosed postoperatively and either
fixation or bone fragment appears to be responsible on CT scan, then
reoperation should be the next course of action if the patient’s
condition permits. New neurologic deficits without radiographic
evidence of nerve root impingement are managed with observation, pain
medicines, physical therapy, splinting, and tendon transfers as needed
depending on the severity of the deficit and muscle groups involved.
 |
|
FIGURE 44-87 Postoperative CT scan showing intraforaminal iliosacral screw on the S1 nerve root.
|
Pelvic obliquity, sitting imbalance, and leg length discrepancy and low
back pain with disability are the usual sequelae. Internal rotation and
adduction of the hemipelvis can create significant problems such as
painful sitting, dyspareunia, and constipation for patients as well.97
For these reasons, acting on loss of reduction early is essential in
preventing late deformity and disability; frequent follow-up
radiographs are important in the early postoperative period when the
patient begins to mobilize.
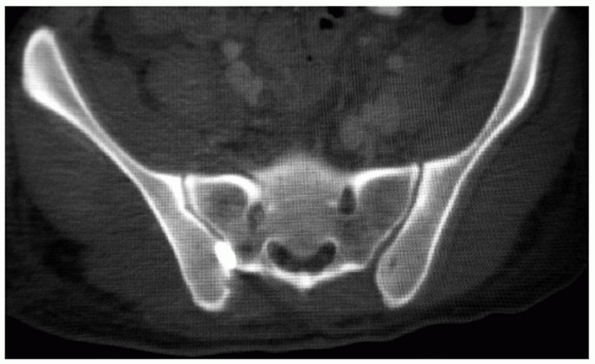 |
|
FIGURE 44-88 Postoperative CT scan showing overclosure of the S1 foramen after reduction of a sacral fracture.
|
routinely include early anatomic reduction and internal fixation within
the last two decades. Prior to the 1980s, very little was understood
regarding the biomechanics and contributions to stability of the
various pelvic bony and ligamentous structures. As recently as the
1970s, many pelvic ring disruptions were treated with nonoperative
techniques—generally skeletal traction and pelvic slings to prevent
excessive cephalad migration of the hemipelvis.181
However, many clinicians have documented the high incidence of poor
functional outcome and chronic pain in patients with vertically
unstable pelvic fractures treated nonoperatively.68,74,87,98,158
The results of these and other studies provided the impetus to pursue
operative means to achieve and maintain anatomic reduction.
external fixators alone, which were found to be nearly totally
ineffective at controlling vertical and posterior displacement of the
posterior aspect of the ring.78
Clinical outcome studies with the use of external fixators subsequently
found that results were not improved over nonoperative management and,
in fact, traction and pelvic sling alone may have been more successful
at treating this unstable injury.98
stabilization of the pelvic fracture and mobilization of the patient as
well as numerous reports citing improved outcome with anatomic
reduction of the posterior ring continued to provide the impetus to
develop more rigid and stable posterior fixation constructs.39,58,87,144
pelvic ring has been shown to provide further biomechanical stability
in the case of the vertically unstable hemipelvis. Whether this is in
the form of an external fixator or symphyseal plate seems to be of
little significance biomechanically. However, more orthopaedic trauma
surgeons are moving toward routine use of symphyseal plates that can be
placed early, do not give rise to the attendant pin-site complications
seen with external fixators, and seem to have improved biomechanics and
clinical outcome.151,152,175,187
support the position that the long-term functional results are improved
if reduction with less than 1 cm of combined displacement of the
posterior ring is obtained, especially with pure dislocations of the SI
complex. Fractures of the posterior ring tend to fare better than
dislocations, presumably because bone healing can restore initial
strength and stability.25 SI
dislocations rely purely on ligamentous healing and scar formation and
these patients tend to fare worse in terms of short- and long-term
problems with pain and ambulation compared with patients with other
fracture patterns. Of interest, however, patients with pure
dislocations of the SI joint that healed with a solid bony ankylosis
did not demonstrate an improved outcome over those that did not
ankylose the joint.110
anatomic), more recent clinical outcome studies have shown that, with
modern fixation techniques, a substantial proportion of patients
continue to have poor outcomes with chronic posterior pelvic pain.25,31,39,74,110,112,127
Males frequently complain of erectile dysfunction and females
frequently complain of dyspareunia, urinary difficulty, and
pregnancy-related issues with an increase in the need for cesarean
section.29,92,134
-
Trauma patients often have a borderline socioeconomic status with poor social and financial support groups
-
Extensive soft tissue damage and associated long bone and extremity fractures
-
Associated neurologic, visceral, and urogenital injuries with dyspareunia, sexual dysfunction, and incontinence
-
Prolonged recovery and rehabilitation time with loss of job, home, and family
significantly higher mortality in the elderly: 12.3% versus 2.3% in the
younger age groups, despite equal need for transfusion and similar
injury patterns. This is largely due to the decreased physiologic
reserve and higher incidence of cardiovascular disease.115
Outcome studies after fixation of pelvic injuries are difficult to
interpret because of poor follow-up, heterogeneity of the injury
pattern, associated visceral and neurologic injury, and the lack of a
reliable outcome measure for pelvic ring injuries.32
Debate continues regarding the definition of adequate reduction of the
pelvic ring and how malreduction affects functional outcome, if at all.39,110,112,182
Less than 50% of patients with severe pelvic fractures return to their
previous level of function and work status, and the majority of
patients sustain persistent impairment in both the physical and mental
components of the Short Form-36 questionnaire.116,127,187
exist among the small number of surgeons who treat these injuries on a
regular basis. There is little disagreement regarding the optimal
timing and form of anterior or posterior fixation, with the vast
majority of surgeons agreeing that a “close as possible” reduction
performed “as soon as possible” with multihole plates anteriorly and SI
screws posteriorly is optimal.
screws for an unstable posterior pelvic ring injury as discussed, for
both SI joint dislocations and sacral fractures. However, the literary
evidence does not come down convincingly on one side or the other, with
there being biomechanical studies stating some or no benefit to
additional screws for both injuries.107,126,151,162,188,195 At this point, it is purely anecdotal and surgeon preference is based on individual expertise and experiences.
performing an anterior versus a posterior reduction in the case of
complete instability of the hemipelvis. As noted, the classic teaching
for this scenario (generally a complete SI dislocation associated with
a symphyseal disruption) has been to perform open reduction and
fixation of the more biomechanically critical posterior ring injury
first, followed by open reduction and fixation of the anterior ring
injury.89,90
The advocates of an initial posterior reduction base their rationale on
(a) that an anatomic reduction of the posterior ring is necessary for a
good outcome and (b) that an anatomic reduction of the posterior ring
will be impeded by a (mal)reduced and rigidly fixed anterior ring.
Proponents of an initial anterior reduction do not necessarily disagree
with assertion “a” or “b” but believe that reducing the anterior ring
first and applying other indirect reduction maneuvers, such as
traction, will help with reduction of the posterior ring, permitting
closed manipulative indirect reduction of the posterior ring and
percutaneous fixation. In reality, both camps are correct in their
respective philosophies. Some patients will have soft tissue wounds
and/or a precarious physiologic status that will preclude a formal
posterior open reduction and so the “anterior first” strategy will need
to be used to impose less surgical morbidity on the patient. The bottom
line is that the surgeon needs to use the techniques with which he or
she can most often reliably achieve the best reduction with the least
morbidity to that particular patient.
among pelvic trauma surgeons that will change significantly in the next
decade surrounds the need for open anatomic reduction versus closed
near-anatomic reductions with percutaneous fixation. As detailed,
existing outcome studies often group together many injury patterns, and
the multitude of associated injuries to the neurovascular and visceral
structures makes these studies very difficult to interpret—particularly
when quality of reduction is taken as one of the variables. Many
surgeons believe that a closed or minimally invasive reduction that
minimizes prolonged surgical morbidity is preferential to a prolonged
open surgical reduction with increased blood loss and risk of
complications. What we as pelvic surgeons do not have the luxury of is
a comparative outcome study with subsets of patients who are matched
for fracture pattern and associated injuries. Likely as we move into
the next decade, this will become the greatest area of advancement in
pelvic trauma surgery with the evolution, improved confidence, and
accuracy with navigational techniques.
JE, Davis GG, Alexander CB, et al. Pelvic trauma in rapidly fatal motor
vehicle accidents. J Orthop Trauma 2003;17:406-410.
DT, Jones CB, Routt ML Jr. Superior gluteal artery injury during
iliosacral screw placement. J Orthop Trauma 1999;13(3):220-227.
College of Surgeons Committee on Trauma. Advanced Trauma Life Support
for Doctors. Sixth Edition, 1997. Chicago, IL: Author.
MT, Arebi S, Le TT, et al. Orthogonal pin construct versus parallel
uniplanar pin constructs for pelvic external fixation: a biomechanical
assessment of stiffness and strength. J Orthop Trauma 2009;23:100-105.
CS, Ali A, Helfet DL. Bladder incarceration in a traumatic symphysis
pubis diastasis treated with external fixation: a case report and
review of the literature. J Orthop Trauma 1998;12:64-67.
PE, Antoniades J, Matta JM. Trans-sacral fixation for failed posterior
fixation of the pelvic ring. Arch Orthop Trauma Surg 2006;126(1):49-52.
Epub 2005 Nov 26.
WL, Smith WR, Moore EE, et al. Evolution of a multidisciplinary
clinical pathway for the management of unstable patients with pelvic
fractures. Ann Surg 2001;233: 843-850.
CC, Cummings P, Jurkovich GJ, et al. Predicting major hemorrhage in
patients with pelvic fracture. J Trauma 2006;61(2):346-352.
CC, Jurkovick GJ, Linnau KF, et al. Assessment of volume of hemorrhage
and outcome from pelvic fracture. Arch Surg 2003;138:504-509.
DS, Starr AJ, Reinert CM, et al. The effect of screening for deep vein
thrombosis on the prevalence of pulmonary embolism in patients with
fractures of the pelvis or acetabulum: a review of 973 patients. J
Orthop Trauma 2005;19(2):92-95.
J Jr, Koval KJ, Helfet DL. Operative stabilization of fracture
dislocations of the sacroiliac joint. Clin Orthop Rel Res 1996;141-146.
J Jr, Koval KJ, Helfet DL. The crescent fracture: a posterior fracture
dislocation of the sacroiliac joint. J Orthop Trauma 1996;10(3):165-170.
S, Browner BD, Cox EF. MAS trousers improperly applied causing a
compartment syndrome in the lower extremity. J Trauma 1982;22:598-599.
LD, Zolfaghari D, Kennedy E, et al. Incidence and prophylaxis of deep
vein thrombosis in a high risk trauma population. Am J Surg
1996;172(1):13-14.
TD, Stone JP, Schuster JH, et al. External fixation of unstable pelvic
ring fractures: comparative rigidity of some current frame
configurations. Med Biol Eng Comput 1982;20(6):727-733.
JP. The injury severity scale of road traffic casualties in relation to
time of death, hospital treatment time, and disability. Accident Anal
Prev 1975;7:249.
DA, Scheid DK, Maar DC, et al. Safe placement of S1 and S2 iliosacral
screws: the “vestibule” concept. J Orthop Trauma 2000;14(4):264-269.
PR, McAninch JW. Major bladder trauma: mechanisms of injury and a
unified method of diagnosis and repair. J Urol 1984;132(2):254-257.
JD, Blum DA, Ansel LJ. Outcome after fixation of unstable posterior
pelvic ring injuries. Clin Orthop Relat Res 1996;329:160-179.
C, Coons D, Aschenbrenner J. Risks to the superior gluteal
neurovascular bundle during percutaneous iliosacral screw insertion: an
anatomical cadaver study. J Orthop Trauma 2005;19(2):96-101.
C, Coons D, Tornetta P, et al. Standard multi-planar fluoroscopy versus
a fluoroscopically based navigation system for the percutaneous
insertion of iliosacral screws: a cadaver model. J Orthop Trauma
2005;19(4):254-258.
CP, van der Meulen MCH, Goodman SB. Biomechanical comparison of
posterior internal fixation techniques for unstable pelvic fractures. J
Orthop Trauma 1996;10(8):517-522.
CE, Bosse MJ, McCarthy ML, et al. Effect of trauma and pelvic fracture
on female genitourinary, sexual, and reproductive function. J Orthop
Trauma 1997;11(2): 73-81.
CC, Osborn PM, Moore EE, et al. Preperitoneal pelvic packing for
hemodynamically unstable pelvic fractures: a paradigm shift. J Trauma
2007;62(4):834-839; discussion 839-842.
RK, Parreira JG, Coimbra R, et al. The role of associated injuries on
outcome of blunt trauma patients sustaining pelvic fractures. Injury
2000;31:677-682.
SA, Burgess AR, Siegel JH, et al. Pelvic fracture in multiple trauma:
Classification by mechanism is the key to pattern of organ injury,
resuscitative requirements, and outcome. J Trauma 1989;29(7):981-1002.
CS, Prayson MJ, Shuler TE, et al. Trans-sacral versus modified pelvic
landmarks for percutaneous iliosacral screw placement—a computed
tomographic analysis and cadaveric study. Am J Orthop 2000;29(9
suppl):16-21.
AC, Stott PM, Boden RA. The accuracy of computer-assisted percutaneous
iliosacral screw placement. Clin Orthop Relat Res 2007;463:179-186.
NA, Wixted JJ, Drew J, et al. Use of the trauma pelvic orthotic device
(T-POD) for provisional stabilisation of anterior-posterior compression
type pelvic fractures: a cadaveric study. Injury 2008;39:903-906. Epub
2008 Jun 30.
D, Karaiskakis M, Toutouzas K, et al. Pelvic fractures: epidemiology
and predictors of associated abdominal injuries and outcomes. J Am Coll
Surg 2002;195: 1-10.
F, Davis S, Compfort T. Sacral fractures, an important problem.
Retrospective analysis of 236 cases. Clin Orthop Relat Res
1988;227:67-81.
FH, Hossenbaccus M, Duparc F, et al. Long-term functional prognosis of
posterior injuries in high-energy pelvic disruption. J Orthop Trauma
1998;12: 145-150.
GS, Vrahas MS. Review of the pathophysiology and acute management of
haemorrhage in pelvic fracture. Injury 2006;602-613. Epub 2005 Nov 23.
Association for the Surgery of Trauma. Practice management guidelines
for hemorrhage in pelvic fracture. Available at:
http://www.east.org/tpg/pelvis.pdf. Accessed September 22, 2009.
NA, Xu R, Biyani A, et al. Morphologic considerations of the first
sacral pedicle for iliosacral screw placement. Spine 1997;22:841-846.
ME, Dirschl DR, Dahners LE. Hemorrhage in pelvic fractures does not
correlate with fracture length. J Trauma 2008;65:436-441.
KH. Lap belt iliac wing fracture: a predictor of bowel injury in
children. Pediatr Radiol 2002;32:892-895. Epub 2002 Jul 26.
W, Keel M, Eid K, et al. Control of severe hemorrhage using C-clamp and
pelvic packing in multiply injured patients with pelvic ring
disruption. J Orthop Trauma 2001;15:468-474.
A, Pohlemann T, Krettek C. A simple supra-acetabular external fixation
for pelvic ring fractures. Oper Orthop Traumatol 2005;17:296-312.
H, Luo CF, Chen J, et al. Control of severe pelvic fracture hemorrhage
with pelvic packing. Chin J Traumatol 2008;11:120-122.
WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the
Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.
Chest 2004; 126(3 suppl):338S-400S.
JJ, Morey AF. Bladder entrapment after external fixation of traumatic
pubic diastasis: importance of follow-up computed tomography in
establishing prompt diagnosis. Mil Med 2000;165:492-493.
AJ, Wilber JH, Lieberman JM, et al. The effect of laparotomy and
external fixator stabilization on pelvic volume in an unstable pelvic
injury. J Trauma 1995;38: 396-400; discussion 400-401.
A, Phillips T, Sclafani SJ, et al. Early open reduction and internal
fixation of the disrupted pelvic ring. J Trauma 1986;26:325-333.
RP, Fried PQ, Bukhalo M. The utility of clinical examination in
screening for pelvic fractures in blunt trauma. J Am Coll Surg
2002;194:121-125.
JT, Varga E, Woodside T, et al. The strength of iliosacral lag screws
and trans-iliac bars in the fixation of vertically unstable pelvic
injuries with sacral fractures. Injury 1996;27:561-564.
DR, Starr AJ, Reinert CM, et al. Vertically unstable pelvic fractures
fixed with percutaneous iliosacral screws: does posterior injury
pattern predict fixation failure? J Orthop Trauma 2003;17:399-405.
XL, Pullinger R. Are diagnostic peritoneal lavage or focused abdominal
sonography for trauma safe screening investigations for hemodynamically
stable patients after blunt abdominal trauma? A review of the
literature. J Trauma 2007;62:779-784.
MR, Vrahas MS, Thomas KA. Pressure-volume characteristics of the intact
and disrupted pelvic retroperitoneum. J Trauma 1998;44:454-459.
DJ, Olson SA, Matta JM. Diagnosis and management of closed internal
degloving injuries associated with pelvic and acetabular fractures: the
Morel-Lavallée lesion. J Trauma 1997;42:1046-1051.
O, Strohm PC, Culemann U, et al. Mortality in patients with pelvic
fractures: results from the German pelvic injury register. J Trauma
2008;64:449-455.
TC, Tile M. The effects of ligament sectioning and internal fixation of
bending stiffness of the pelvic ring. In Proceedings of the 13th
international Conference on Biomechanics. Perth, Australia, December,
1991.
T, Pohlemann T, Tarte S, et al. Computer-assisted fracture reduction of
pelvic ring fractures: an in vitro study. Clin Orthop Relat Res
2002;231-239.
VM, Slätis P. Fractures of the pelvis. Trauma mechanism, types of
injury and principles of treatment. Acta Chir Scand 1972;138:563-569.
V-M, Slatis P. Postmortem angiography and dissection of the hypogastric
artery in pelvic fractures. Surgery 1973;73:454-462.
K, Sharkey PW, Stephen DJ, et al. The increasing incidence of severe
pelvic injury in motor vehicle collisions. Injury 2004;35:759-765.
S, Halici M, Tuncel M, et al. Functional outcome of open reduction and
internal fixation for completely unstable pelvic ring fractures (type
C): a report of 40 cases. J Orthop Trauma 2003;17:555-562.
K, Trentz O. Distraction spondylodesis of the sacrum in “vertical shear
lesions” of the pelvis. Unfallchirurg 1994;97:28-38.
JF, Werier J, Blachut P, et al. Early fixation of the vertically
unstable pelvis: the role of iliosacral screw fixation of the posterior
lesion. J Orthop Trauma 1999; 107-113.
WY, Hearn TC, Seleem O, et al. Effect of pin location on stability of
pelvic external fixation. Clin Orthop Relat Res 1999;237-244.
BJ, Velmahos GC, Chan LS, et al. Angiographic embolization for pelvic
fractures in older patients. Arch Surg 2004;139:728-733.
AW, Sirois M, Laupland KB, et al. Prospective evaluation of hand-held
focused abdominal sonography for trauma (FAST) in blunt abdominal
trauma. Can J Surg 2005;48:453-460.
W, Hearn T, Tile M, et al. The effect of thread length and location on
extraction strengths of iliosacral lag screws. Injury 1994;25:5-9.
L, Trentz O. The use of vacuum assisted closure (VAC) in soft tissue
injuries after high energy pelvic trauma. Langenbecks Arch Surg
2007;392:601-609. Epub 2006 Sep 16.
BF, Levine MI, McNeish LM. Bilateral fracture-dislocation of the
sacrum. A case report. J Bone Joint Surg Am 1986;68A:1099-1101.
O, Karlsson J, Berg U, et al. Unstable fractures of the pelvis treated
with a trapezoid compression frame. Acta Orthop Scand 1984;55:325-329.
BA, Gentilello LM, Tarver AA, et al. Improved outcome with early
fixation of skeletally unstable pelvic fractures. J Trauma 1991;28-31.
KA, Starr AJ, Reinert CM. Reduction of displaced pelvic ring
disruptions using a pelvic reduction frame. J Orthop Trauma
2009;23:299-308.
E. Surgical fixation of displaced pelvic fractures and dislocations of
the symphysis pubis (excluding acetabular fractures) [in French]. Rev
Chir Orthop Reparatrice Appar Mot 1981;67:771-782.
MA, Mason JT, Luna GK, et al. Risk factors for urethral injury in men
with traumatic pelvic fractures. J Urol 1988;140:506-507.
R, Walton D, Dickinson D, et al. Pelvic disruptions in the
poly-traumatized patient: A management protocol. Clin Orthop Relat Res
1980;151:22-30.
WT, Khan SN, James CL, et al. Complications of temporary and definitive
external fixation of pelvic ring injuries. Injury 2005;36:599-604.
KL, Bickell W, Pepe PE, et al. Prospective randomized Evaluation of
Anti-shock MAST in post-traumatic hypotension. J Trauma 1986;26:779-786.
CM, Hak DJ, Goulet JA, et al. Pelvic fracture patterns and their
corresponding sources of hemorrhage. Orthop Clin N Am 2004;35:431-437.
PR, Moore PS, Mansell E, et al. External fixation or arteriogram in
bleeding pelvic fracture: initial therapy guided by markers of arterial
hemorrhage. J Trauma 2003;54:437-443.
BR, Karges DE. Techniques for reduction and fixation of pelvic ring
disruptions through the posterior approach. Clin Orthop Rel Res
1996;102-114.
BR, Geer BL. S2 iliosacral screw fixation for disruptions of the
posterior pelvic ring: a report of 49 cases. J Orthop Trauma
2006;378-383.
BH, Sagi HC. Minimum 1-year follow-up for patients with vertical shear
sacroiliac joint dislocations treated with iliosacral screws: does
joint ankylosis or anatomical reduction contribute to functional
outcome? J Orthop Trauma 2008;22:293-298.
S, Kalra MK, Torres WE, et al. Adverse reactions to intravenous
iodinated contrast media: an update. Curr Probl Diagn Radiol
2006;35:164-169.
JV, Trenhaile SW, Miranda MA, et al. Vertical shear injuries: is there
a relationship between residual displacement and functional outcome? J
Trauma 1999;46: 1024-1030.
FK, Malkani AL, Haikal L, et al. Cross-sectional geometry of the sacral
ala for safe insertion of iliosacral lag screws: a computed tomography
model. J Orthop Trauma 2000;14:31-35.
SE, Jones CB, Harding SP, et al. Percutaneous stabilization of U-shaped
sacral fractures using iliosacral screws: technique and early results.
J Orthop Trauma 2001; 15:238-246.
DP, Luchette FA, Pereira SJ, et al. Pelvic fracture in the elderly is
associated with increased mortality. Surgery 2002;132:710-714.
CW, Twaddle B, Agel J, et al. Outcome after pelvic ring fractures:
evaluation using the Medical Outcomes Short-Form SF-36. Injury
1996;27:635-641.
Trauma Association. Compendium for the Classification of Fractures and
Dislocations. J Orthop Trauma 1996;10(suppl):66-75.
PM, Smith WR, Moore EE, et al. Direct retroperitoneal pelvic packing
versus pelvic angiography: a comparison of two management protocols for
hemodynamically unstable pelvic fractures. Injury 2009;40:54-60. Epub
2008 Nov 30.
JK, Benson GS, Corriere JN. Diagnosis and initial management of
urological injuries associated with 200 consecutive pelvic fractures. J
Urol 1983;130:712-714.
MY, Parisky YR, Cornwell EE, et al. CT cystography versus conventional
cystography in evaluation of bladder injury. AJR Am J Roentgenol
1999;173:1269-1272.
KA, Kahler DM. Supra-acetabular placement of external fixator pins: a
safe and expedient method of providing the injured pelvis with
stability. Am J Orthop 2005;34:148-151.
T, Angst M, Schneider E, et al. Fixation of transforaminal sacrum
fractures: a biomechanical study. J Orthop Trauma 1993;7:107-117.
KJ, Joosse P, Van Dijke GA, et al. External fixation of the pelvic
ring: an experimental study on the role of pin diameter, pin position,
and para-symphyseal fixator pins. Acta Orthop 2007;78:648-653.
Study Group. Eight-year follow-up of patients with permanent vena cava
filters in the prevention of pulmonary embolism: the PREPIC (Prevention
du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study.
Circulation 2005;112: 416-422. Epub 2005 Jul 11.
JI, Velmahos GC, Best CR, et al. Male sexual function after bilateral
iliac artery embolization for pelvic fracture. J Trauma 2004;56:734-739.
MC, Bono CM, Litkouhi B, et al. The effect of sacral fracture
mal-reduction on the safe placement of iliosacral screws. J Orthop
Trauma 2003;17:88-94.
M, Otte D, Gänsslen A, et al. Injuries of the pelvic ring in road
traffic accidents: a medical and technical analysis. Injury
2001;32:123-128.
ML Jr, Simonian PT, Grujic L. The retrograde medullary superior pubic
ramus screw for the treatment of anterior pelvic ring disruptions: a
new technique. J Orthop Trauma 1995;9:35-44.
ML, Simonian PT, Agnew SG, et al. Radiographic recognition of the
sacral alar slope for optimal placement of iliosacral screws: A
cadaveric and clinical study. J Orthop Trauma 1996;10:171-177.
ML, Simonian PT, Mills WJ. Ilio-sacral screw fixation: Early
complications of the percutaneous technique. J Orthop Trauma
1997;11:584-589.
ML Jr, Falicov A, Woodhouse E, et al. Circumferential pelvic anti-shock
sheeting: a temporary resuscitation aid. J Orthop Trauma 2006;20(1
suppl):S3-S6.
SA, Sochor MS, Staples KS, et al. Pelvic ring fractures: implications
of vehicle design, crash type, and occupant characteristics. Surgery
2004;136:842-847.
R, Saillant G, Gagna G, et al. Transverse fracture of the upper sacrum.
Suicidal jumper’s fracture. Spine 1984;10:838-845.
HC. Technical aspects and recommended treatment algorithms in
triangular osteosynthesis and spinopelvic fixation for vertical shear
transforaminal sacral fractures. J Orthop Trauma 2009;23:354-360.
HC, Lindvall EM. Inadvertent intra-foraminal iliosacral screw placement
despite apparent appropriate positioning on intra-operative
fluoroscopy. J Orthop Trauma 2005;19:130-133.
HC, Militano U, Caron T, et al. A comprehensive analysis with minimum
one-year follow-up of vertically unstable trans-foraminal sacral
fractures treated with spinal-pelvic fixation. J Orthop Trauma
2009;23:313-319; discussion 319-321.
HC, Ordway NT, DiPasquale T. Biomechanical analysis of fixation for
vertically unstable sacro-iliac dislocations with iliosacral screws and
symphyseal plating. J Orthop Trauma 2004;18:135-139.
HC, Papp S. Comparative and clinical and radiographic outcome of
two-hole and multi-hole symphyseal plating. J Orthop Trauma 2008;1-5.
ER, Ebrahim NA, Rusin JJ, et al. Limitations of radiography and
computed tomography in the diagnosis of transverse sacral fracture from
a high fall. Clin Orthop Relat Res 1991;122-126.
TA, Josten C, Muhr G. Triangular osteosynthesis of vertically unstable
sacrum fractures: a new concept allowing early weight-bearing. J Orthop
Trauma 1998; 12:307-314.
TA, Ledoux WR, Chapman JR, et al. Triangular osteosynthesis and
iliosacral screw fixation for unstable sacral fractures: a cadaveric
and biomechanical evaluation under cyclic loads. J Orthop Trauma
2003;17:22-31.
TA, Bellabarba C, Nork SE, et al. Decompression and lumbo-pelvic
fixation for sacral fracture-dislocations with spino-pelvic
dissociation. J Orthop Trauma 2006; 20:447-457.
JA, Mino DE, Werner FW, et al. Posterior stabilization of pelvic
fractures by use of threaded compression rods. Case reports and
mechanical testing. Clin Orthop Relat Res 1985;192:240-254.
TE, Boone DC, Gruen GS, et al. Percutaneous iliosacral screw fixation:
Early treatment for unstable pelvic ring disruptions. J Trauma
1995;38:453-458.
PT, Schwappach JR, Routt ML, et al. Evaluation of new plate designs for
symphysis pubis internal fixation. J Trauma 1996;41:498-502.
PT, Routt ML, Harrington RM, et al. Internal fixation of the
transforaminal sacral fracture. Clin Orthop Relat Res 1996;323:202-209.
PT, Routt ML Jr, Harrington RM, et al. The unstable iliac fracture: a
biomechanical evaluation of internal fixation. Injury 1997;28:469-475.
LA, Waddell JP, Leighton RK, et al. Anterior approach and stabilization
of the disrupted sacroiliac joint. J Trauma 1987;27:1332-1339.
P, Karaharju ED. External fixation of unstable pelvic fractures.
Experiences in 22 patients treated with a trapezoid compression frame.
Clin Orthop Relat Res 1980; 151;73.
GP, Lefaivre KA, Nicolaou S, et al. A systematic review of
thromboprophylaxis for pelvic and acetabular fractures. J Orthop Trauma
2009;23:379-384.
HE, Yuan PS, Sasso R, et al. An evaluation of image-guided technologies
in the placement of percutaneous iliosacral screws. Spine
2006;31:234-238.
JP, Riley RS, McClenney MD, et al. Mechanical prophylaxis against
deep-vein thrombosis after pelvic and acetabular fractures. J Bone
Joint Surg Am 2001;83A: 1047-1051.
JP, Singhania AK, Lopez-Ben RR, et al. Deep-vein thrombosis in
high-energy skeletal trauma despite thromboprophylaxis. J Bone Joint
Surg Br 2005;87B:965-968.
AJ, Nakatani T, Reinert CM, et al. Superior pubic ramus fractures fixed
with percutaneous screws: what predicts fixation failure? J Orthop
Trauma 2008;22:81-87.
N, Dodenhoff RM, Ward AJ, et al. Thromboprophylaxis in pelvic and
acetabular trauma surgery. The role of early treatment with
low-molecular-weight heparin. J Bone Joint Surg Br 2005;87B:209-212.
GW, Gabel GT, Noble PC, et al. Anterior and posterior internal fixation
of vertical shear fractures of the pelvis. J Orthop Res 1991;9:237-245.
RE, Rives JL, Warlaumont CR, et al. The use of Dacron in the repair of
hernias of the groin. Surg Clin North Am 1984;64:269-285.
MD, Morgan SJ, Bosse MJ, et al. Prospective comparison of
contrast-enhanced computed tomography versus magnetic resonance
venography in the detection of occult deep pelvic vein thrombosis in
patients with pelvic and acetabular fractures. J Orthop Trauma
2002;16:613-621.
D, Schmidt A, Freese J, et al. Proximity of iliosacral screws to
neurovascular structures after internal fixation. Clin Orthop Relat Res
1996;194-198.
P III, Matta JM. Outcome of operatively treated unstable posterior
pelvic ring disruptions. Clin Orthop Relat Res 1996;329:186-193.
A, Madsen JE, Skaga NO, et al. Extraperitoneal pelvic packing: a
salvage procedure to control massive traumatic pelvic hemorrhage. J
Trauma 2007;62: 843-852.
CL, Fung HT, Chung KL, et al. Focused abdominal sonography for trauma
in the emergency department for blunt abdominal trauma. Int J Emerg Med
2008;1:183-187. Epub 2008 Sep 26.
den Bosch EW, Van der Kleyn, Hogervorst M, et al. Functional outcome of
internal fixation for pelvic ring fractures. J Trauma 1999;47:365-371.
Zwienen CM, van den Bosch EW, Snijders CJ, et al. Biomechanical
comparison of sacroiliac screw techniques for unstable pelvic ring
fractures. J Orthop Trauma 2004; 18:589-595.
GC, Toutouzas KG, Vassiliu P, et al. A prospective study on the safety
and efficacy of angiographic embolization for pelvic and visceral
injuries. J Trauma 2002; 53:303-308.
LX, Gristina AG, Wilson JR, et al. Two-hole plate fixation for
traumatic symphysis pubis diastasis. J Trauma 1988;28:813-817.
JJ, Hanson GW, Tullos HS. Unstable fractures of the pelvis treated by
external fixation. J Bone Joint Surg Am 1982;64A:1010-1020.
K, Scalise J, Olsen SA, et al. Biomechanical comparison of posterior
pelvic ring fixation. J Orthop Trauma 2003;17:481-487.
JWR, Burgess AR, Brumback RJ, et al. Pelvic fractures: value of plain
radiography in early assessment and management. Radiology
1986;160:445-451.
