TUMORS AND INFECTIONS OF THE CERVICAL SPINE
VIII – THE SPINE > Tumors and Infections > CHAPTER 151 – TUMORS
AND INFECTIONS OF THE CERVICAL SPINE
Clinical Instructor, University of California–San Diego School of
Medicine, Department of Orthopaedics, San Diego Center for Spinal
Disorders, San Diego, California, 92123.
Professor, University of California—San Diego School of Medicine,
Department of Orthopaedics, San Diego Center for Spinal Disorders, San
Diego, California, 92123.
destructive lesions of the cervical spine. For cervical tumors, the
indications and techniques for biopsy are reviewed, followed by the
exposure and reconstruction of destructive lesions. Tumor
classification and details on the various tumors are presented in Chapter 126, Chapter 127, Chapter 128, Chapter 129 and Chapter 130. Many of the general principles of spinal tumors are covered in Chapter 152,
Tumors of the Thoracic and Lumbar Spine. This chapter focuses solely on
the management of these tumors in the cervical spine. Similarly, the
general principles of management of bone infection are covered in Chapter 132 and Chapter 133, and the details of the various types of infections are covered in Chapter 150.
In this chapter, we focus on the management of cervical infections. The
evaluation of osteomyelitis and epidural abscess are reviewed, followed
by a discussion of the outcome of different treatments.
spine, the surgeon should first determine the oncologic stage and
evaluate for local and systemic disease. The Enneking system of staging
for musculoskeletal neoplasms (see Chapter 126) has been adapted to neoplasms of the axial skeleton (4).
As with extremity neoplasms, spinal tumors are staged according to
histologic grade, compartmental location, and the presence or absence
of metastases. There is a major difference between surgical staging of
spinal neoplasms compared with neoplasms of long bones; the
Weinstein-Boriani-Biagini (WBB) classification addresses the unique
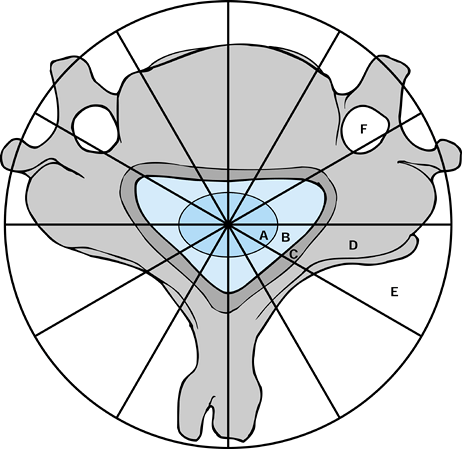
anatomy of the spine (25). In the transverse
plane, the vertebra is divided into 12 zones, numbered 1 to 12
clockwise starting on the left half of the spinous process. The layers
are further divided from paravertebral extraosseous to dura, denoted as
layers A through E, with a further layer F for the vertebral artery
canal (Fig. 151.1). Recording the spinal
segments involved defines the longitudinal extent of tumor involvement.
A simpler method of categorizing the level of involvement
is
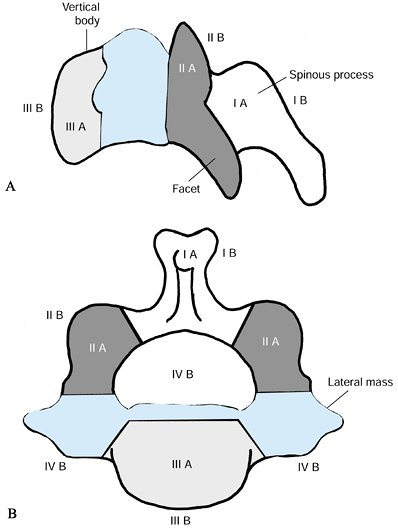
to divide the vertebral body into four zones, I through IV. Tumor
extension is designated as A, B, and C for interosseous, extraosseous,
and distant tumor spread, respectively (Fig. 151.2).
This classification is helpful because the zones of tumor involvement
correspond to the surgical approach: tumors involving zones I and II
are usually resected and, if necessary, stabilized posteriorly. Zone
III lesions are usually approached anteriorly. Zone IV lesions that
require a complete or en bloc excision
must be managed through a combined anterior and posterior approach.
Zone IIIB lesions should be carefully analyzed preoperatively to
anticipate possible invasion of or adherence to critical neural
elements, esophagus, or trachea. The general workup of tumors of the
cervical spine and the different tumor types are covered in Chapter 152.
 |
|
Figure 151.1.
Weinstein-Boriani-Biagini classification–surgical staging of spinal neoplasms. There is a major difference between surgical staging of spinal neoplasms compared with neoplasms of long bones. A: Intradural. B: Epidural. C: Involving bony canal. D: Intraosseous. E: Paravertebral, extraosseous. F: Vertebral artery canal. |
 |
|
Figure 151.2.
Cervical spine tumor staging system, modified from the thoracolumbar staging system of Weinstein and McLain. The vertebral body is divided into zones I to IV. Zones I and II: lamina, facets, and pedicle. Zone III: vertebral body. Zone IV: epidural space, dural contents, and posterior vertebral body and annulus. A: Interosseous. B: Extraosseous. C: Designates distant metastatic spread, not shown. (Modified from McLain RF, Weinstein JN. Tumors of the Spine. Seminars in Spine Surgery 1990;2:157.) |
patients with primary spinal malignancy correlate significantly with
tumor type and the extent of the initial surgical procedure. Hart et
al. (25) and Boriani et al. (4)
showed decreased recurrence rates of giant cell tumor and chordoma,
respectively, using the WBB and the Enneking surgical staging systems
with en bloc excision performed at a
tertiary referral center. Although they have not been specifically
studied, it is reasonable to extrapolate the results obtained in other
areas of the spine to cervical tumors.
different from other regions, however. The reported frequency of
cervical metastatic disease is much less than thoracic or lumbar
metastases. Also, the presentation of cervical metastatic disease
differs from metastatic disease at the thoracic and lumbar levels (49).
A review of the natural history shows the average life expectancy to be
14.7 months after cervical metastatic disease is diagnosed (52).
Pain with cervical metastatic disease is more frequent (93%), whereas
neurologic deficit is less frequent than when disease is present at the
thoracic or lumbar levels (5–14% cervical versus 50% thora co-lumbar) (52,53).
The prevalence of upper cervical neurologic deficit is lower than that
of the lower cervical spine, possibly related to the wider canal in the
upper region.
be diagnosed solely on the basis of the radiologic workup of plain
radiographs and computed tomography (CT) scans. By recognizing the
benign or inactive lesions that, by their self-limiting nature, do not
require biopsy, an unnecessary procedure can be avoided. Also, in cases
such as spinal metastases from a known primary carcinoma, no biopsy is
necessary. A similar situation involves the patient with multiple
myeloma and spinal involvement with impending or actual cord
compression. In patients with multiple myeloma, the laboratory tests
provide the diagnosis. Because the myelomas are sensitive to radiation
therapy, proceeding directly with radiation rather than performing a
biopsy or other surgery is most appropriate.
is uncertain, one must make a definitive diagnosis with a complete
workup and a biopsy or aspiration. It is important to realize, however,
that planning the biopsy should be the last step, after appropriate
staging and other workup. The benefit of performing the biopsy at the
end of the staging and evaluation are
-
Unnecessary biopsy can be avoided (e.g., multiple myeloma)
-
Other more accessible sites may be found for biopsy (e.g., a metastatic lesion)
-
Prebiopsy embolization can be performed, if necessary (e.g., renal cell metastasis or other vascular lesion).
procedure with possible complications that can change the course of
treatment and significantly alter the treatment outcome (40). The
orthopaedist who is responsible for the definitive treatment and
subsequent care should be responsible for performing the initial biopsy. The recommendations regarding performance of biopsies, either open or percutaneous, are
-
Place the biopsy tract where it will be
fully removed at the time of definitive treatment. This may not be
possible during anterior biopsies. -
Ensure minimal tissue contamination by avoiding excessive dissection of tissue planes.
-
Obtain adequate tissue for diagnosis (may require confirmation by frozen section).
-
Maintain adequate hemostasis.
-
Use drains only through the wound or in
proximity so that the track is removed with the definitive resection.
The drain can provide a track along which malignant cells can pass,
which may increase the margin necessary for subsequent resection.
of whether an aspiration, needle biopsy, or open biopsy is performed.
Coexistent infection and tumor have been reported (16);
therefore, we recommend routine microbiologic culture. Biopsy
techniques include percutaneous (aspiration, fine-needle, or
large-bore), open incisional biopsy, or excisional biopsy. When normal
marrow elements are present, aspiration effectively rules out
metastatic malignancy. Needle aspiration and fine-needle biopsy,
however, provide only a small specimen and, therefore, introduce a
sampling error. Simple aspiration or fine-needle biopsy should be used
predominantly in the cervical spine to rule out infection, confirm
suspected metastatic disease, or diagnose recurrence of a known lesion.
Because aspiration biopsy may fail to provide definitive diagnosis in
up to 20% of cases (6), large-needle or open biopsy is often necessary.
under CT guidance. If a benign lesion such as osteoid osteoma or
osteoblastoma is suspected, however, open biopsy followed by excision
often provides the easiest solution. We also do not favor routine
needle or trocar biopsy of suspected aneurysmal bone cysts. Most of
these lesions, when well-demonstrated radiologically, are typical and
do not require biopsy. Some lesions can have a clinical and
radiographic appearance similar to that of a malignant tumor and, in
fact, may also contain malignant portions; therefore, a gross
pathologic specimen may be needed for diagnosis. Additionally, the
small amount of tissue obtained with needle or trocar biopsy often
creates confusion in the diagnosis or may not include the pre-existing
lesion (50).
Open biopsy with curettage or excision, or both, on the other hand,
provides the entire lesion for pathologic examination. Furthermore,
there have been reports that extradural bleeding of aneurysmal bone
cysts of the spine following needle biopsy cause neurologic deficit (26).
Finally, because open excisional biopsy can cure the condition, there
seems to be little justification for needle or trocar biopsy, which may
be negative and possibly risky (1).
or anterolaterally following the standard surgical approach (see the
discussion of surgical approaches, later). It is often not possible to
entirely excise the biopsy tract with anterolateral and lateral
biopsies due to the multiple tissue planes in the anterior neck and the
presence of the carotid sheath. Avoid transverse posterior skin
incisions. Plan the incision so that it will not compromise subsequent
surgical procedures.
disease, divide the cervical spine into three regions: the upper
cervical vertebral bodies (C1–C3), the lower cervical vertebral bodies
(C4–C7), and the posterior elements and posterior epidural space.
Biopsies of the C1–C3 vertebral bodies can be performed through a
transoral approach, with the patient under general nasotracheal
anesthesia, or through a high lateral approach. The C4–C7 vertebral
bodies can be accessed through a lateral or anterolateral approach (see
the discussion of surgical approaches, later) or with open biopsy.
Closed biopsy techniques of the cervical spine are technically
demanding and often fraught with neurologic and vascular complications.
If metastasis is suspected and the lesion is extensive enough to
require surgical stabilization, we recommend an open biopsy to confirm
the diagnosis by immediate frozen section, followed with a definitive
surgical procedure. A fine-needle biopsy is possible under CT guidance,
especially if surgical reconstruction is not deemed necessary.
concurrent goals of relief of pain, and the maintenance of spinal
stability and neurologic integrity.
-
Differentiate “latent” or “active” (i.e.,
stage 1 or 2 based on the Musculoskeletal Tumor Society surgical
staging system) from “aggressive” or stage 3 lesions. -
Treat stage 1 or 2 lesions with intralesional curettage.
-
Precisely localize the tumor.
-
Protect structural and neurologic integrity.
-
In children, perform a posterior arthrodesis if performing a laminectomy.
-
-
Treat stage 3 lesions with marginal or en bloc excision.
-
Be prepared for excessive bleeding.
-
Control bony bleeding with liberal application of bone wax and Gelfoam.
-
Preoperative embolization may be helpful to avoid excessive bleeding, especially in the case of aneurysmal bone cysts.
-
Be prepared to ligate or bypass the vertebral artery if necessary.
-
Perform preoperative angiography to assess collateral blood flow.
-
Consider packing the wound and embolizing the tumor or the vertebral artery if uncontrolled bleeding is encountered.
-
-
-
If the anterior part of the vertebra is severely involved, perform a posterior stabilization first to establish stability.
osteoblastoma, are common in children and have a predilection for the
posterior elements. For characteristic osteoid osteoma and small
osteoblastomas, excisional biopsy and intralesional curettage is
sufficient treatment. The key to operative management is precise
localization of the osteoid osteoma before surgery. If the lesion is
located in the lamina, remove the posterior cortex of the lamina using
a power drill to expose the nidus. While removing the nidus, take care
not to damage the dura, because the anterior cortex is usually thin.
soft tissue as well as the vertebral body and, similar to giant cell
tumors and aneurysmal bone cysts (ABCs), have the potential for local
recurrence. Because of the size and expansile nature of these tumors,
surgical excision is more radical and often leads to spinal
instability, necessitating spinal fusion. “Active” stage 2 lesions are
positive on a bone scan and have a well-marginated sclerotic border.
These lesions can be treated by curettage and have a low local
recurrence rate (5% to 10%) (3). The
“aggressive” stage 3 lesions are surrounded by a large pseudocapsule,
which can be observed on a contrast-enhanced CT scan. Intralesional
curettage has been associated with a 20% rate of local recurrence (36). Although en bloc excision is the treatment of choice, owing to anatomic restraints in
the cervical spine, selected stage 3 benign tumors can be treated by
incisional biopsy and frozen section confirmation of tumor type,
followed at the same surgery with marginal excision.
cervical spine is a challenge and, owing to the increased surgical
risks, should be reserved for the aggressive stage 3 lesion or
recurrent tumors.
-
Dissect the tumor outside its wall, leaving some of the soft tissue attached to the thin wall.
-
If the tumor is next to the dura, dissect the wall with a Freer elevator, taking care not to compress the spinal cord.
-
Be prepared to ligate or bypass the vertebral artery, if necessary.
-
If a significant part of the esophagus is
encased in tumor, an esophagectomy and gastric pull-up or colon
interposition is indicated (8).
in the case of ABCs but is generally discouraged because of the
potential for cord damage, induced sarcoma, and growth retardation (5).
Low-dose radiotherapy may be considered for the well-circumscribed
recurrent ABC lesion. Embolization may be effective for decreasing
vascularity and making surgical resection and decompression less
morbid, and may eliminate symptoms from expansive hemangioma.
consider reconstructing the potentially destabilized spine. The extent
of this destabilization depends on the age of the patient as well as
the amount of posterior element resection. In children, laminectomy
frequently results in secondary kyphosis that is difficult to correct.
Therefore, in skeletally immature children, perform a posterior
arthrodesis traversing the extent of the laminectomy (37).
In the adult, when resection of any part of the lateral mass or pedicle
is necessary, simultaneously arthrodese and instrument the affected
levels using the remaining posterior spinal elements. Harvest an
autologous graft through a separate incision, using a separate setup to
avoid cross-contamination of the donor site.
predicated on the tumor type and the extent of local and systemic
spread. Avoid surgery for primary malignant spinal tumors unless there
is a good chance the surgery can offer significant palliation or a
cure. Marginal or intralesional resection of the tumor, followed by
radiation therapy, is an appropriate palliative approach to an
intermediate-grade osteosarcoma with soft-tissue involvement. A wide
resection for a low-grade chondrosarcoma in the vertebral body
represents an attempt to cure by surgery alone.
myeloma should necessarily be somewhat different owing to their
different prognoses, although they are a continuum of the same disease
and most cases of solitary plasmacytoma progress to multiple myeloma.
Radiation is the initial treatment of choice in either case.
Prophylactic laminectomy and stabilization before radiotherapy can be
used if cord compromise or spinal instability is present. In the rare
instance of a cervical solitary plasmacytoma, prognosis is enhanced by
surgical excision reducing the tumor burden. In such cases, perform an
intralesional excision and stabilization, followed by radiation therapy.
local recurrence rates and the difficulty in accessing this lesion in
the cervical spine. Unlike sacral chordomas, in which sacral nerve
roots can be sacrificed, cervical chordomas often involve the clivus
and upper cervical spine, in which, at best, only decompression and
marginal excision is possible. Often, the only option for surgical
treatment is posterior stabilization and fusion, followed by anterior
intralesional or marginal excision and decompression.
common tumors in the cervical spine with a predilection for the
anterior column. In the cervical spine, the weight-bearing axis falls
at or posterior to the vertebral body, and the articular processes
support the weight of the skull. For this reason, destruction of the
vertebral body results in some loss of vertebral body height, but
kyphotic deformity is uncommon. Instability is also an uncommon.
Destruction of the lateral masses as well as the vertebral body must
occur to permit rotatory instability. Except for extensive lysis in one
or more contiguous bodies, or the involvement of the spinous process of
C-2, where the nuchal fascia inserts, metastatic involvement of the
upper cervical spine rarely results in kyphosis or true flexion
instability (49).
neurologic deficit is much lower owing to the space available for the
cord. The development of neurologic deficit here is usually due to
extension of the tumor rather than to angular kyphosis. The sudden
onset or rapid progression of neurologic deficit is usually due to a
vascular accident rather than vertebral collapse and usually has a poor
prognosis. Reporting on all locations of spinal tumors, Harrington (23)
noted 62% of initially paraplegic patients regained enough neurologic
function to ambulate after surgical intervention, but patients with
rapid paraplegia exhibited a poor prognosis for recovery.
expectancy, type of tumor, location of tumor (accessibility),
radiosensitivity, degree of instability, and neurologic status of the
patient. Because the primary goal is to improve the patient’s quality
of life, thoroughly consider the patient’s
personal
preference and family situation. Metastasis of lung carcinoma has a 7-
to 9-month mean survival time, whereas breast carcinoma has a survival
exceeding 30 months. Consider embolization of tumors with hemorrhagic
tendencies, such as renal and thyroid. Treat radiosensitive tumors such
as lymphoma, myeloma, and prostate with nonoperative management:
Radiate with doses up to 4,000 cGy as long as there is no instability,
neurologic threat, or significant deformity. Doses in excess of 5,000
cGy may cause acute or chronic radiation myelitis. Radiation therapy is
compatible with internal fixation devices and methacrylate but may
cause failure of supporting bone graft struts. Radiation therapy alone
is rarely effective in relieving a well-established neural deficit,
especially in the presence of a collapsed vertebral body and bony
impingement.
there is severe pain, instability, or impending kyphotic collapse, or
when the tumor is known to be radioresistant. Tumors with greater than
50% involvement of the vertebral body and greater than 50% destruction
of the ipsilateral middle and posterior columns require prophylactic
surgical stabilization. Kyphotic deformity and amount of subluxation
should also be considered, but the assessment of instability is still
somewhat subjective. The goal of surgery is to prevent neurologic
compromise, but severe neurologic deficit is not a contraindication for
surgery.
-
Treat patients with tumors in the posterior C-2 arch with early radiation therapy so that progressive kyphosis does not develop.
-
For destruction of the lateral mass of
C-1, perform an occiput to C-3 fusion with adjunctive radiation therapy
because rotatory instability is common (49). -
For destruction of the dens with
instability, perform a C1–C2 fusion. If cord compression is impending,
remove the arch of C-1 and perform an occiput to C-3 fusion. -
In the lower cervical spine, consider
early combined anterior and posterior stabilization owing to the
difficulty of fixation and the increased stress at the cervicothoracic
junction.
stage and extent. Determine what anatomic structures may need to be
sacrificed to perform the resection. Sacrifice of one vertebral artery
can be tolerated if cure is a reasonable goal, but obtain a
preoperative angiogram to assess collateral flow. If the cervical
esophagus or a significant part of the thoracic esophagus is involved
by tumor, total esophagectomy and gastric pull-up or colon
interposition can be performed (8).
have shown a high rate of pain relief (94% to 95%), motor recovery (64%
to 92%), and ambulation (87%). The results of surgery are usually
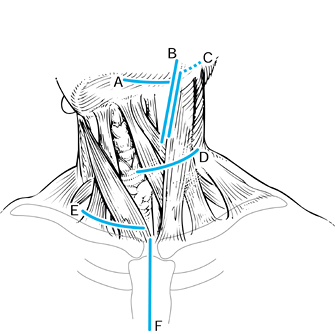
maintained until the terminal stage, with local recurrences in 30% (2,48). In an elderly population with a mean age of 73, a mortality rate of 16% was reported within 7 days after surgery (60).
deep paths to the cervical spine: transpharyngeal, lateral and
posterior to the carotid sheath, or anterior to the carotid sheath.
 |
|
Figure 151.3. Incisions for the anterior approaches to the spine. A: Submandibular approach, described by McAfee et al. (43). This approach is retropharyngeal and prevascular (medial to carotid sheath). The incision is 1 cm below the jaw. B:
Extension of the prevascular approach. This approach is a cephalad extension of the standard anterolateral approach to the midcervical spine, and when it is combined with the submandibular approach, it allows direct exposure from the tip of the clivus and can be extended caudal to the lower cervical spine. C: Retrovascular, lateral approach (lateral and posterior to carotid sheath), popularized by Whitesides and Kelly (64). This approach allows direct exposure of one side or the other only at the level of the C1–C2 articulation. D: Standard anterolateral approach to the midcervical spine, medial to the carotid sheath. E: Exposure to lower cervical spine, which may require clavicular osteotomy for cervicothoracic exposure. F: Sternal splitting approach to the cervicothoracic spine. |
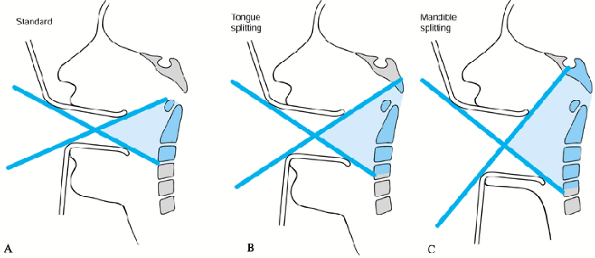
transoral approach. Routine tracheostomy is necessary only if a tongue
or mandibule-splitting approach is used. See Chapter 138 for a detailed description. The standard transoral approach can be used for exposure of C1–C2 (Fig. 151.4A), and this can be enlarged by the tongue-splitting (Fig. 151.4B) or transmandibular (Fig. 151.4C) approach, for decompression from the level of the clivus to C-4 (31).
If division of the soft palate becomes necessary (only during extensile
approaches), it is incised on one side of the midline to avoid the
uvula. This approach allows for tumor resection, but the risk of sepsis
makes placing implants impractical.
 |
|
Figure 151.4. Transpharyngeal approaches. A: Standard transoral approach: can expose tip of odontoid to C-2. B: Tongue-splitting approach: can expose tip of clivus to C-3. C: Mandible-splitting approach: can expose clivus to C-4.
|
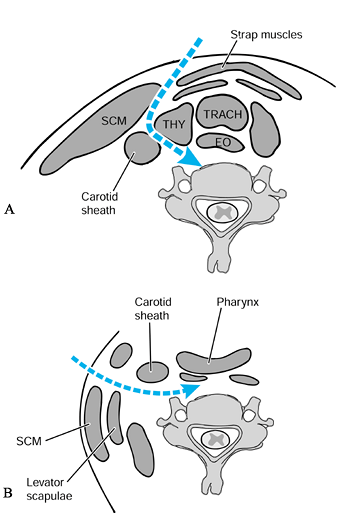
through the submandibular approach, which is prevascular and
retropharyngeal (incision: Fig. 151.3A; deep dissection: Fig. 151.5A). This approach is a cephalad extension of the standard approach to the midcervical spine (34,43).
 |
|
Figure 151.5. Standard anterior approaches to the spine. A: Anterolaterally: medial to the carotid sheath. B: Lateral: lateral to the carotid sheath.
|
-
Make a transverse incision 1 cm below the
jaw line, which can be extended into a longitudinal incision if more
extensile exposure is needed (Fig. 151.3A and Fig. 151.3B). -
Recruit the assistance of a head and neck
surgeon familiar with radical neck dissections to decrease the
incidence of complications. -
Develop the interval medial to the
sternocleidomastoid by preserving the mandibular branch of the facial
nerve and by dividing the submandibular salivary gland and the vascular
leashes of the superior thyroid, lingual, and facial arteries. -
Keep the patient intubated at least overnight due to the upper pharyngeal edema common with this approach.
cervical spine. The visualization is similar to that obtained by the
transmucosal route. This approach allows decompression up to the clivus
and reconstruction of the anterior column with strut graft and internal
fixation. We prefer this prevascular extraoral approach to the upper
cervical spine owing to the excellent extensile exposure and decreased
risk of infection compared with those of the transpharyngeal approach,
especially if instrumentation is used. There is a reported 40%
incidence of mostly transient palsies of the marginal mandibular branch
of the facial nerve (34).
Owing to the restriction in mobilizing the carotid sheath, this
approach allows direct exposure of one side or the other only at
the
level of the C1–C2 articulation and, therefore, is best suited for
fusion and even instrumentation but not decompression. Lans (34)
reported on 10 cases of C1–C2 arthrodesis through this bilateral,
lateral approach first described by Barbour, with instrumentation using
bilateral transarticular screws.
is usually the surgical treatment of choice for lesions from C-3 to
C-7. Rarely, if a lateral biopsy was obtained and the goal of surgery
was a wide margin with excision of the biopsy tract, the exposure would
need to begin from the lateral approach and proceed posterior to the
neurovascular bundle (22).
may be limited by the diameter of the thoracic inlet, the height of the
clavicles and manubrium anteriorly, and the extent of cervicothoracic
kyphosis. Obtain a preoperative radiograph to compare the upper margin
of the clavicles and manubrium with the level of the vertebral body. If
clearance of the clavicle and manubrium is not possible, we prefer the
sternal splitting approach (62) to clavicular osteotomy (33).
The sternal splitting approach is very familiar to cardiothoracic
surgeons (only the superior portion of the sternum needs to be split)
and can be easily extended into the neck. This allows an extensile
approach to the cervicothoracic junction, and the exposure can be
further enhanced by ligation of the brachiocephalic vein. Such ligation
produces significant edema in the upper extremity.
can be replaced in several ways. In patients with benign lesions, or
lesions with which there is long life expectancy, we prefer to use
autograft or allograft struts to replace the anterior defect, followed
by anterior instrumentation. If massive resection of the vertebral
bodies is necessary at multiple levels, we perform posterior
stabilization before anterior resection and reconstruction. The
different scenarios for upper cervical, midcervical, and lower cervical
reconstructions are shown in Figure 151.6, Figure 151.7 and Figure 151.8.
 |
|
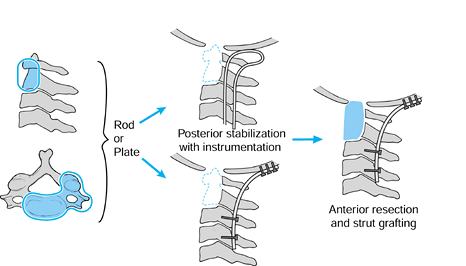
Figure 151.6.
Scenario for upper cervical reconstruction. For extensive destruction of C1–C2, perform a posterior stabilization first with instrumentation, with next-stage anterior resection and strut grafting as needed. |
 |
|
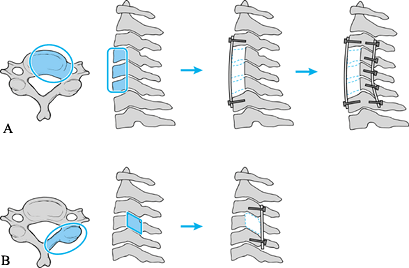
Figure 151.7. Scenario for midcervical reconstruction. A:
For extensive anterior destruction and fixed kyphosis in the midcervical spine, perform anterior decompression, strut grafting, and instrumentation, followed by posterior instrumentation. Perform the posterior instrumentation can be performed first if there is no significant deformity or if there is severe instability. B: For posterior destruction of the facets, perform a posterior-only reconstruction with plating or wiring. |
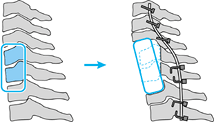 |
|
Figure 151.8.
Scenario for cervicothoracic reconstruction. For extensive cervicothoracic destruction, perform a posterior stabilization followed by anterior reconstruction. |
stage 3 lesions, some advocate use of methylmethacrylate (PMMA)
anteriorly, with or without posterior arthrodesis or plating (36).
Similar to its use in the extremities, PMMA has the advantage of
immediate stability, local control due to the heat of polymerization,
and rapid recognition of early recurrence. Despite the advantages of
PMMA, we recommend reconstruction with autograft or allograft to
provide a biologic reconstruction in these patients who usually have a
long life expectancy. The benefits of a biologic reconstruction must be
weighed against the risk of failure if radiation is used. Use of
titanium or carbon fiber cages is controversial, and recognition of
early recurrence with use of titanium is more difficult. Postoperative
radiation therapy may be used if resection is incomplete, and in these
cases, we would recommend use of PMMA.
be less than 6 to 12 months, we use PMMA combined with metal implants
to give immediate stable fixation (25).
Radiation therapy can be used with PMMA without fear of impacting the
healing of bone graft. Stabilization of the spine with PMMA can be
fraught with major complications, however (42).
Take care to avoid spinal cord injury, which can be caused by direct
mass effect or by heat generated from the exothermic reaction of cement
solidification.
-
Protect the cord with Gelfoam, wire mesh, silicon sheets, or various plastics positioned anterior to the dura.
-
As the cement begins to harden, irrigate
with cooled saline to reduce its temperature. When multiple level
corpectomies are performed, most authors agree that anterior plating is
best (24,35). -
Do not use PMMA alone for anterior
fixation. The average length of time to failure of PMMA fixation
anteriorly used alone was 194 days (42). -
Perform posterior fixation for cases of
multiple level corpectomies. In cases of massive anterior instability
without rigid kyphosis, we recommend posterior fixation before anterior
resection. Owing to different anatomy, the upper, mid-, and lower
cervical spine require different strategies for stabilization and
fixation (Fig. 151.6, Fig. 151.7 and Fig. 151.8, respectively).
cervical spine or the cervicothoracic junction owing to the
difficulties inherent in anterior approach and stabilization in these
areas. Laminectomy alone is contraindicated in the presence of anterior
compression and kyphosis. If the posterior elements can be left intact,
then standard wiring techniques can be used. In the case of a
laminectomy, lateral mass plates may be used for stabilization. Special
techniques are available for occipitocervical and cervicothoracic
instrumentation.
-
Use of a contoured rod with wires for occipital and cervical laminar fixation (Fig. 151.9).
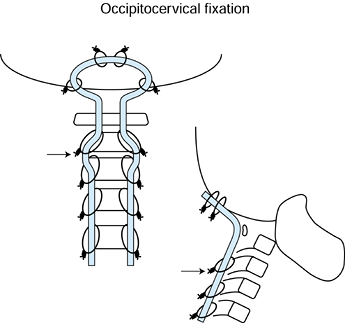 Figure 151.9.
Figure 151.9.
Preferred method for occipitocervical instrumentation. Use a contoured
rod as shown. Wiring to the occiput is performed through four drill
holes through the occiput and passage of the two superior-most wires.
The next set of wires are inserted from two occipital drill holes
through the foramen magnum. A total of six occipital drill holes are
necessary. After wiring of the lamina, the loop resists settling
(arrow). -
Plating with use of lateral mass screws in the cervical spine and screws in the occiput
-
Combination of the above-mentioned techniques
-
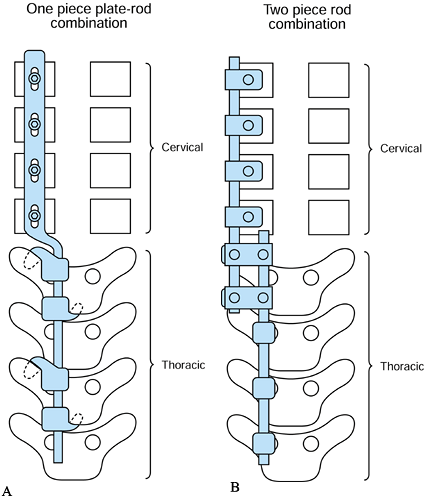
Options for cervicothoracic instrumentation are
-
Use of rods and wires if posterior elements are intact.
-
Use of a one-piece plate and rod
combination with screws in the cervical lateral masses and hooks in the
thoracic spine (Acromed/DePuy/Johnson and Johnson, Rahnam, MA) (Fig. 151.10A).![]() Figure 151.10. Options for cervicothoracic fixation. A: Use of a one-piece plate and rod combination. Lateral mass screws in the cervical spine and hooks in the thoracic spine. B:
Figure 151.10. Options for cervicothoracic fixation. A: Use of a one-piece plate and rod combination. Lateral mass screws in the cervical spine and hooks in the thoracic spine. B:
Use of two different diameter rods—the cervical rod affixed to the
cervical spine via lateral mass screws, and the thoracic rod affixed
via thoracic screws or hooks. Use rod-to-rod connectors to attach the
two rods together. -
Use of a two-piece cervical plate with
cervical lateral mass screws and thoracic rod using thoracic hooks or
pedicle screw (Danek, Memphis, TN, or Acromed/DePuy/Johnson and
Johnson, Rahnam, MA) -
Use of two different diameter rods
spanning the cervical and thoracic spine segments, affixed separately
to cervical lateral mass screws and thoracic screws or hooks and
connected by rod-to-rod connectors (Synthes, Paoli, PA) or
(Acromed/DePuy/Johnson and Johnson, Rahnam, MA) (Fig. 151.10B).
should be condemned unless it is used to augment the lateral mass
screws. We do not recommend augmentation of lateral mass screws with
PMMA if the anterior cortex has been breached due to risk of vertebral
artery injury. In cases in which the patient’s life expectancy is more
than 2 years (plasmacytoma or breast carcinoma), or in cases in which
global instability exists where anterior stabilization alone is
insufficient, a combined anterior and posterior approach should be used.
for a general discussion of infections of the spine. Hematogenous spine
infections are less common in the cervical spine than in the thoracic
or lumbar spines, but they do have a predilection for the anterior
compartment of the spine. The most common complaint of patients with
cervical infections is neck pain (18). Because
of the nonspecific nature of cervical infections, delayed diagnosis is
common. Radionuclide studies can detect spinal infection before plain
films can and have the advantage of revealing other foci of infection,
which occur in 4% of cases (46). Gallium scans
have been shown to detect infection earlier in the course of the
disease than technetium scans, and they are more useful for following
the response to treatment (47). Gallium scans
become normal during the resolution of the infection, whereas
technetium scans remain positive for many months after the disease has
resolved. Single-photon emission computed tomography (SPECT) allows the
advantage of three-dimensional localization with higher sensitivity as
compared with planar technetium scintigraphy or gallium scintigraphy (17). Indium scans are not helpful owing to their low sensitivity (17%) (63).
MRI is the modality of choice. MRI has 96% sensitivity, 93%
specificity, and 94% accuracy in detecting vertebral osteomyelitis and
becomes positive at about the same time as the gallium scan (46). Eismont (14)
noted some degree of neurologic deficit in 80% of patients with
cervical osteomyelitis, and the MRI can also help identify the site and
extent of compression.
by bacteriologic or histologic examination of tissue or an aspirate.
The only circumstances in which a diagnosis can be made without tissue
biopsy are in pediatric discitis (rare in the cervical spine) and when
there is a positive blood culture with a patient who has typical signs
and symptoms of spinal infection.
-
Establish diagnosis
-
Relieve pain
-
Prevent or reverse neurologic deficit
-
Establish or maintain spinal stability
-
Eradicate infection
external immobilization and appropriate antibiotics if there is no
abscess formation, neurologic deficit, or vertebral collapse and
instability. Associated conditions that compromise wound healing or
immune response should be managed aggressively. Bring diabetes or other
systemic diseases under control, and address proper nutrition and
reversal of hypoxia and metabolic deficits. Compared with thoracic and
lumbar spine infections, infections of the cervical spine have a higher
risk of complications and surgical treatment is often required in
addition to antibiotic therapy.
-
Choose the antibiotic according to culture and sensitivity results.
-
Withhold antibiotics in cases in which a biopsy is done, in case a second biopsy is required.
-
In patients who have systemic toxicity or
neurologic deficit, start maximum-dose broad-spectrum bacteriocidal
antibiotics as soon as biopsy is obtained. -
If the patient does not respond
clinically to antibiotics, or the sedimentation rate does not decrease
to one half or two thirds by completion of treatment, perform a repeat
biopsy. -
Immobilize patients for pain control and to prevent deformity or deterioration of neurologic status.
-
The need for tissue and bacteriologic diagnosis
-
To drain an abscess that is clinically significant (fevers or sepsis)
-
Cases refractory to nonoperative treatment
-
Presence of neurologic deficit
-
Prevention or correction of spinal deformity or instability
osteomyelitis, if surgery is deemed to be necessary, a solely anterior
surgical approach with discectomy and debridement of pus and strut
grafting from healthy bone above to below is sufficient. Laminectomy is
contraindicated except for the rare case of associated posterior
epidural abscess. If there is evidence of epidural extension, excise
the posterior longitudinal ligament to ensure decompression and removal
of infected tissue.
in the presence of active infection has been shown to be safe and
effective (44). Iliac bone is preferable to
that of the fibula because it has more cancellous bone.
Revascularization of cortical graft may not be complete even after 1
year (57). Experience has shown that
instrumentation and even allograft may also be placed anteriorly in
situations of active infection, as long as adequate debridement has
been performed back to healthy, bleeding bone (55,57). Dietze et al. (13)
reported no recurrence of cervical infection with a 37-month follow-up
after debridement and use of allograft and instrumentation, but they
presented information on only five patients. In cases of significant
kyphosis, or to avoid halo immobilization, the anterior strut graft can
be safely followed by second-stage posterior instrumentation.
addressed with standard surgical approaches. The occipitocervical
junction is difficult to treat owing to anatomic and mechanical
constraints. Upper cervical osteomyelitis is rare but generally
requires fusion because of associated instability. Stabilization of the
upper cervical spine should be performed in cases of instability as
defined by traction or flexion-extension radiographs, odontoid and
transverse ligament resection or destruction, or clivus and odontoid
resection or destruction in the presence of basilar invagination. The
principle of debridement to healthy bone still applies. For high
cervical infections that require drainage owing to abscess formation or
cord compression, we recommend a transoral drainage and posterior
stabilization. Many authors recommend posterior stabilization due to
the nonsterile environment of the posterior pharynx and devitalized
bone (21,39). Zigler et al. (65)
described five patients with pyogenic osteomyelitis of the
occipitocervical region treated by operation and antibiotics. The
options used were anterior debridement and occipitocervical fusion,
transoral drainage, posterior occipitocervical fusion, and posterior
C1–C2 fusion; and all five patients recovered. The surgical procedure
must be individualized in each case according to the degree of bony
destruction and instability.
Treat by irrigation, debridement, and administration of
culture-specific intravenous antibiotics. If more than 50% of the facet
joint is resected during debridement (very rare), then perform a
fusion. Autogenous bone graft placed in a thoroughly debrided bed will
usually result in a successful arthrodesis because of the abundant
blood supply. We recommend a halo brace for immobilization after
debridement of posterior infection and bone grafting. Stabilization
with bone screws and plate or wire techniques is possible in spite of
the
infection, but the use of posterior cervical instrumentation in the
presence of active infection is controversial. The length of time of
administration of postoperative intravenous antibiotics will depend on
the causative organism, but they should be given for at least 6 weeks;
however, 4-week courses have been reported with good results (12).
fusion compared with thoracic and lumbar infections. Almost all cases
of cervical infection that can be treated nonoperatively fuse
spontaneously (45), as compared with only 50% of all patients with thoracic and lumbar vertebral osteomyelitis treated nonoperatively (19). Human immunodeficiency virus (HIV) status (27) and intravenous drug abuse (59)
do not appear to affect the neurologic outcome of patients with spinal
infections adversely. Infants with vertebral osteomyelitis (15), elderly patients, and those with underlying disease (58)
have a high recurrence rate and a poorer prognosis. Factors that
predispose to paralysis include increased age, a subaxial level of
infection, and a concomitant disease (diabetes or rheumatoid arthritis)
(14). Relapse of infection occurs in up to 25%
of cases but is much less common when antibiotics are administered for
more than 4 weeks (14,58).
the prognosis for patients with paralysis from cervical spine infection
is better with an anterior surgical procedure than with the posterior
approach; three of seven patients deteriorated and four remained
unchanged after laminectomy. Stone et al. (61)
reported that all surgical patients with myelopathy and radiculopathy
achieved solid fusion, and at final follow-up, they were ambulatory and
neurologically intact. When doubt exists regarding the reversibility of
a spinal cord lesion, perform a decompression. Recovery from paralysis
has been noted in patients who underwent decompression as late as 5
months after the onset of weakness (47).
a series of 25 epidural infections, the cervical spine was noted to be
involved in 13. Three were multifocal (cervical and thoracic) (54).
Cervical epidural abscess may occur via hematogenous spread from a
remote location or from a contiguous focus of vertebral osteomyelitis,
or by direct inoculation at the time of operation or injection.
sixth and seventh decade of life, and there is a high incidence in
patients who are intravenous drug abusers, so comorbid conditions may
impair immunocompetence (29). Even though most
epidural abscesses are seen after invasive processes that violate the
epidural space, there are reports of multifocal abscesses when systemic
infection is the cause of the abscess (7).
Some areas are filled with fat and veins, and others are in direct
contact with bone or ligament, creating individual metameric segments.
In the cervical spine, except for a space dorsal to the origin of the
spinal nerves, the epidural space is mostly a potential space.
Individual metamers are septated, preventing free communication between
the anterior and posterior epidural space (30). Because the majority of epidural abscesses from hematogenous spread are located posteriorly (29),
they do not involve the anterior epidural space or circumferentially
surround the thecal sac. Conversely, postsurgical cases or cases
associated with discitis or vertebral osteomyelitis not only involve
the anterior epidural space but may be circumferential because of the
common postsurgical disruption of normal anatomic septations (41).
modality of choice. False-negative results may occur with nonenhanced
MRI, especially with extensive abscesses that do not have a discrete
proximal or distal extent (20). Concomitant
meningitis may also cause signal changes in the abscess that may be
similar to infected cerebral spinal fluid, resulting in a
false-negative magnetic resonance imaging MRI scan (51).
Myelography and CT are sensitive for confirming the presence and extent
of an extradural compressive lesion but should be avoided if epidural
abscess is suspected because dural puncture risks spreading the
infection to the intrathecal compartment. There is also a small but
real risk of causing an acute neurologic deterioration owing to
resultant spinal coning if lumbar puncture is performed caudal to a
spinal block (41). Owing to the frequently
rapid evolution of the disease process and associated illnesses, the
mortality rate is as high as 20% (29). In cases
in which MRI is not readily available, CT with myelography should be
performed because, despite the increased risk, confirmation of the
diagnosis should not be delayed.
clinical condition of the patient. Medical management is often
successful in the treatment of lumbar epidural abscess, but cervical
epidural abscess presents an increased risk to the spinal cord.
Indications for nonoperative treatment of spinal epidural abscess are
-
Poor surgical candidates due to severe concomitant medical problems
-
Patients with abscess involving a
considerable length of the spinal canal (cervical to lumbar) and who
have epiduritis but a normal neurologic examination -
Patients with normal spinal cord or cauda equina function
-
Patients with complete paralysis for more than 3 days (38)
antibiotics. Close monitoring with hospitalization and numerous MRIs is
needed. The disadvantages of medical treatment are that neurologic
deterioration can be precipitous, and once present, the deficits may be
irreversible. We recommend aggressive surgical management of cervical
epidural abscesses. When surgical drainage is performed and
osteomyelitis is not present, shorter courses of intravenous
antibiotics (less than 4 weeks) have been successful (11).
laminectomy is the most effective approach for decompression. The
extent of the exposure is determined by the operative findings. If
purulent material is found (acute infection), a limited approach can be
used (see case example, Fig. 151.11). In
chronic infection, dense granulation tissue is present; decompress the
full extent of the abscess. Stabilization is usually not necessary.
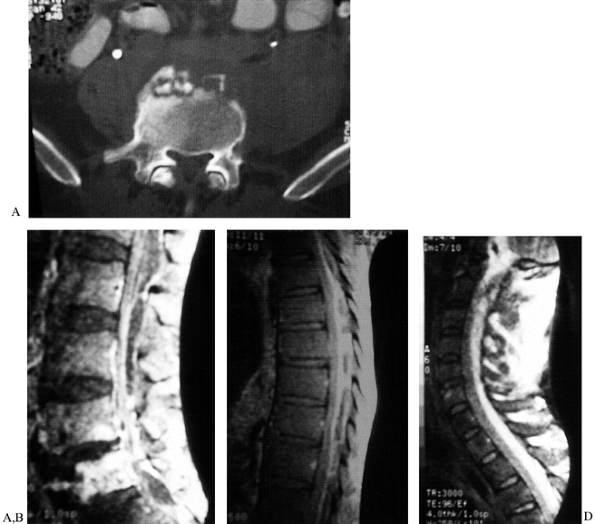 |
|
Figure 151.11.
A case of epidural abscess formation leading to severe cervical spinal cord impingement. A 45-year-old homeless patient with increasing low back pain for months, fevers, and chills. The patient failed to seek treatment and finally presented with severe progressive neurologic deficit and respiratory difficulties. A: Lumbar spine CT scan showing anterior sequestra. B: Lumbar MRI showing vertebral osteomyelitis and massive epidural abscess. C: Thoracic MRI showing massive posterior epidural abscess involving at least 50% of spinal canal. D: Cervical MRI showing massive posterior epidural abscess with almost 75% involvement of the spinal canal at the level of C-2. The probable start of the infection was lumbar discitis and vertebral osteomyelitis that progressed to epidural extension and eventual marked spinal cord compression. Treatment was limited cervical laminectomy. After the cervical laminectomy, the patient was placed in Trendelenberg position, with massive drainage of the thoracic and lumbar pus. Postoperatively, the patient had significant but partial neurologic recovery. The patient was lost to follow-up. |
discitis or osteomyelitis and should be approached anteriorly (29).
If the patient has discitis, osteomyelitis, and instability, anterior
debridement and reconstruction can be carried out without formal
excision of the granulation tissue formed by the abscess. Incise the
posterior longitudinal ligament to allow evacuation of purulent
material. If cord compression is symptomatic, complete exposure of the
granulating abscess is necessary to allow excision of this dense,
tenacious material from the thecal sack. Extreme caution is advised.
-
Remove necrotic disc and endplate
material, and resect diseased bone back to healthy, bleeding vertebral
bone. If only the endplate and less than half of the body remain at any
level, resect the remnant and extend to the next disc space. -
Use a micropituitary and small curet to
fenestrate the posterior longitudinal ligament (PLL) laterally. Use
small Kerrison rongeur to expand the window, and resect a portion of
posterior vertebral rim. -
If there are no signs of cord
compression, drain any purulent material, gently irrigate, and proceed
with stabilization. If there are signs of cord compromise, carefully
resect the posterior vertebral cortex and PLL over the length of the
lesion to provide full decompression. -
If the thickened granulation tissue is to be removed from the surface of the cord, magnification is necessary.
-
Carefully develop the interval between the abscess and thecal sack with Roton dissectors and a nerve hook.
-
Monitor spinal cord function constantly.
-
Once the mass is debulked and cultures
obtained, irrigate with antibiotic solution and stabilize the resected
segment with an autograft strut. -
Stabilize in a halo. Do not use implants in the presence of deep infection.
cervical epidural abscess is not as favorable as that for thoracic and
lumbar infections. The mortality rate with cervical abscess was
reported to be as high as 38% despite aggressive treatment, and the
neurologic deficits were more severe and refractory to treatment (20). Diabetes, HIV infection (20,33), and vertebral osteomyelitis (28)
are associated conditions that carry a poor prognosis. Reporting on
predominantly cervical and cervicothoracic epidural abscesses, Redekop
and Del Maestro (54) reported a 20% mortality
rate, and only 56% retained or recovered ambulation. They attribute the
high morbidity and mortality rates to delay in diagnosis and treatment,
which has been shown to be a factor in all epidural abscesses.
Reporting on all locations of epidural abscesses, no patients with
paralysis for longer than 36 hours recovered significant neurologic
function (22,28,29),
and only 40% of patients who initially had less than antigravity
strength were eventually ambulatory and continent despite surgical
intervention within 36 hours (56). If rapid
acute progressive paraplegia occurs within the first 12 hours,
prognosis is poor, presumably secondary to spinal cord infarction
rather than mechanical compression (33).
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
HJ, Castelli MJ, Reyes CV, Gattuso P. Fine-needle Aspiration Biopsy of
Vertebral Body Lesions: Cytologic, Pathologic, and Clinical
Correlations of 57 Cases. Diagn Cytopathol 1994;11:348.
KD. The Use of Methylmethacrylate for Vertebral Body Replacement and
Anterior Stabilization of Pathological Fracture Dislocations of the
Spine due to Metastatic Disease. J Bone Joint Surg 1981;63A:36.
RA, Boriani S, Biagini R, et al. A System for Surgical Staging and
Management of Spine Tumors. A Clinical Outcome Study of Giant Cell
Tumors of the Spine. Spine 1997;22:1773.
RF, Hunt CD, Krieger AJ, Vaid C. HIV Status Does Not Affect
Microbiologic Spectrum or Neurologic Outcome in Spinal Infections. Surg Neurol 1994;42: 417.
AM, Boriani S. Benign Tumors of the Cervical Spine, 3rd ed. The
Cervical Spine Research Society Editorial Committee. Philadelphia:
Lippincott-Raven, 1998:621.
D, Lesion F, Viaud C, et al. Decreased Morbidity from Acute Bacterial
Spinal Epidural Abscesses Using Computed Tomography and Nonsurgical
Treatment in Selected Patients. Ann Neurol 1985;17:350.
PC, Bohlman HH, Ducker T, Eismont FJ. Failure of Stabilization of the
Spine with Methacrylate. A Retrospective Analysis of Twenty-four Cases.
J Bone Joint Surg [Am] 1986;68:1145.
HD, Litvinoff J. Pyogenic Cervical Osteomyelitis. Chondro-osteomyelitis
of the Cervical Spine Frequently Associated with Parenteral Drug Use. Arch Neurol 1976;33:571.
FL, Montgomerie JZ. Vertebral Osteomyelitis in Intravenous Drug
Abusers: Report of Three Cases and Review of the Literature. Rev Infect Dis 1980;2:196.
V, van Krieken FM, Bao SD, et al. Microsurgery of the Cervical Spine in
Elderly Patients. Part 2: Surgery of Malignant Tumorous Disease. Acta Neurochir (Wien) 1994;131:241.
JL, Cybulski GR, Rodriguez J, et al. Anterior Cervical Debridement and
Strut-grafting for Osteomyelitis of the Cervical Spine. J Neurosurg 1989;70:879.
JE, Bohlman HH, Robinson RA, et al. Pyogenic Osteomyelitis of the
Occiput, the Atlas, and the Axis: A Report of Five Cases. J Bone Joint Surg [Am] 1987;69:1069.