PYOGENIC AND GRANULOMATOUS INFECTIONS OF THE SPINE
VIII – THE SPINE > Tumors and Infections > CHAPTER 150 – PYOGENIC
AND GRANULOMATOUS INFECTIONS OF THE SPINE
about the treatment of infections of the spine. Tuberculosis patients
with deformity and paralysis forced us to address this devastating
process with aggressive surgical and medical treatment. Even with the
decrease of tuberculosis in developed countries, the principles of
treating infections, pyogenic or granulomatous, have been influenced by
the experience of treating tuberculosis.
discitis in children, to osteomyelitis in adults, to postsurgical
infections. The infection usually affects the vertebral body and disc
and, less commonly, the posterior elements, except in cases of
postsurgical infection (Fig. 150.1). The lumbar
spine is the most common location of infection, followed by the
thoracic spine; cervical spine infection is least common (132). The least common sites for spinal osteomyelitis are the occiput, atlas, and axis, with only a few isolated cases reported (178).
 |
|
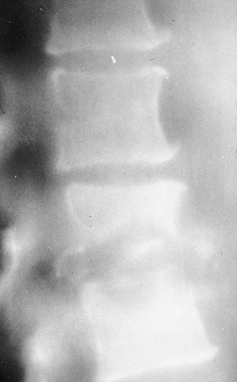
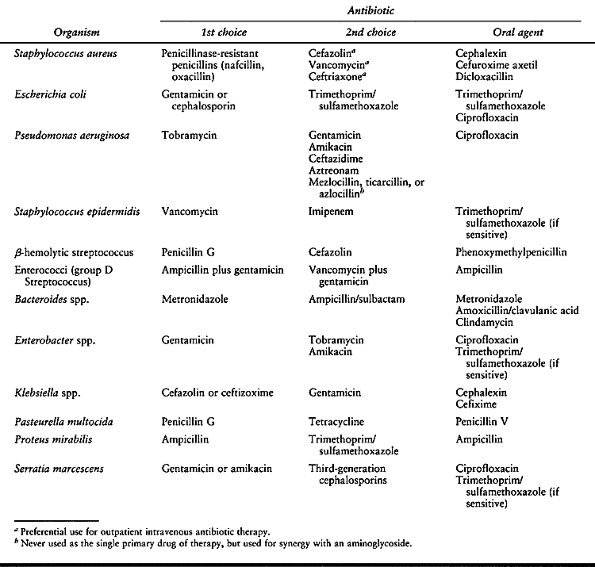
Figure 150.1.
Hematogenous osteomyelitis most commonly invades first the anterior portion of the vertebral body, just adjacent to the endplate. Radiographic changes take time to appear and the usual picture is that of simultaneous involvement of two adjacent endplates with narrowing of the intervertebral disc space. This tomogram of the lumbar spine shows endplate destruction of the lower vertebral combine with loss of a good deal of the body of the upper vertebra. |
incidence in younger male intravenous drug abusers has been noted.
Pyogenic spinal osteomyelitis is usually monomicrobial, unless it is
secondary to a systemic disease, in which case a polymicrobial
infection is more common. There has been an increase in gram-negative
infections compared with the more common gram-positive infections (19). The increased infection rates from gram-negative organisms may be due to wide use of broad-spectrum antibiotics.
being the most common, readily infect the vertebral bodies and discs,
with more than 50% of tuberculosis infections of bone occurring in the
spine. The onset is insidious, with destruction of the vertebral
bodies, discs, and ligaments if the disease progresses unchecked by
medical and surgical treatment. As structural stability is destroyed,
kyphosis combined with inflammatory debris and necrotic material can
cause progressive paraplegia. Therefore, in the treatment of spinal
infections, it is critical to make the diagnosis early so that
antibiotic therapy or surgical debridement and fusion can be done
before bony collapse and neurologic compromise occur.
of organisms for both discitis and vertebral osteomyelitis. Other
etiologies include surgery, direct spread from a pulmonary abscess,
penetrating trauma, and soft-tissue deficits, such as a decubitus
ulcer. Batson (10) demonstrated venous return
from the pelvis into the venous plexus of the vertebral column. He
theorized that the paravertebral venous reservoir could allow continued
venous return and mixing in the setting of changing abdominal and
intrathoracic pressures. In his view, these interconnecting venous
systems provided an explanation for the presence of vertebral
metastases in the absence of lung metastases.
the importance of Batson’s venous plexus, demonstrating by injection
studies an arterial system of nutrient vessels that supplies the
vertebral bodies under physiologic arterial pressures. They found that
the richly vascular metaphyseal bone near the anterior longitudinal
ligament correlates with the most common site of infections.
metaphyseal cancellous infarction caused by a septic embolus. The
vascular anatomy of the spine, which changes as a child matures,
provides the most likely explanation for the differences in spinal
infections in children and adults, as well as for the characteristic
locations of infections in the vertebral unit. The interosseous
arteries in children are anastomotic; therefore, occlusion of a single
nutrient artery leads to destruction of only a small portion of bone
because of collateral flow. In adults, a larger portion of bone is
destroyed because the interosseous arteries are end arteries, and
septic thrombus spreads into peripheral interosseous arteries. The disc
is avascular and is attacked by infection equally in all ages.
reported an incidence of this infection of 1.9 per 10,000 admissions
per year. Spinal epidural abscess tends to occur in an older, more
medically debilitated population and to be monomicrobial, despite its
frequent occurrence in a more medically complex environment. The most
common organism is S. aureus. Epidural
abscess may be due to direct seeding from invasive procedures, such as
spinal anesthesia or epidural steroid injection, may form adjacent to
an area of osteomyelitis, or, less commonly, may occur from spontaneous
hematogenous spread (2,27,107). The distribution
of this infection parallels the distribution of vertebral
osteomyelitis: It is more common in the lumbar spine and less common in
the thoracic and cervical segments (41).
 |
|
Figure 150.2.
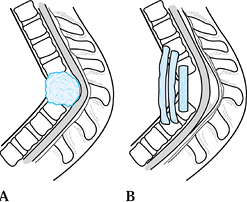
Epidural abscess formation occurs in about 15% of vertebral infections. MRI shows an epidural abscess compressing the thecal sac. A: Lateral view. B: Transverse section. |
following abdominal stab wounds and, rarely, gunshot wounds, the
National Spinal Cord Injury Model System reported no cases of spinal
infection in a series of 90 patients, despite a 20% incidence of
alimentary perforation (64,65,118,164).
mellitus; chronic steroid use; drug and alcohol abuse; rheumatoid
arthritis; urinary, respiratory, or abdominal sepsis; previous surgery;
dental infection or extraction; urinary tract manipulation; and any
type of spinal needle procedure: acupuncture, spinal anesthesia,
epidural catheters, or steroid injections (10,22,27,63,103,112,118,124,145,148,165,166).
Increasing age may be an independent risk factor, with the increasing
incidence of gram-negative or anaerobic infections in elderly patients,
often in the absence of any concomitant risk factors (23).
disease are prominent risk factors for granulomatous infection.
Tuberculosis is found most commonly in underdeveloped nations. Big
cities in Western countries still have cases of tuberculosis in higher
risk patients such as the homeless, immigrants, alcoholics, and other
immunocompromised individuals such as those with human immunodeficiency
virus infection (97). Other granulomatous
infections have geographic risk factors, such as coccidioidomycosis in
the San Joaquin Valley of California or histoplasmosis in the central
United States.
tuberculosis is the result of hematogenous dissemination from a primary
infected visceral focus. The primary focus can be active or quiescent,
apparent or obscure, and located in the lung, lymphatic system, kidney,
or other viscus. In a typical lesion, the tuberculous bacilli find
their way to the paradiscal area of two contiguous vertebrae, which
supports the concept that the spread is via the arterial blood supply.
Anterior extension of the lesion, with involvement of multiple
vertebral bodies, is caused by extension of the abscess beneath the
periosteum and anterior longitudinal ligament. The anterior and
posterior longitudinal ligaments and periosteum are stripped from the
vertebral
bodies,
which results in loss of periosteal blood supply and destruction of the
anterolateral surfaces of several contiguous vertebrae.
Periosteal stripping combined with arterial occlusion due to
endarteritis causes ischemic infarction leading in turn to necrosis of
the involved bone. The body of the vertebra is thus softened and yields
to compressive forces. The intervertebral disc is not involved
primarily because it is avascular. However, involvement of the
paradiscal regions of the vertebra compromises disc nutrition. A disc
may then be invaded by the infectious process and destroyed.
Radiographically, it is typical to see more than one vertebra involved
(average, 3.4 vertebrae) (71). The most common
finding is narrowing of the disc space and vertebral osteolysis. In
more advanced disease, a paravertebral shadow is produced by extension
of the tuberculous granulation tissue and formation of an abscess in
the paravertebral region (Fig. 150.3); later, vertebral collapse and angulation of the spine occur.
 |
|
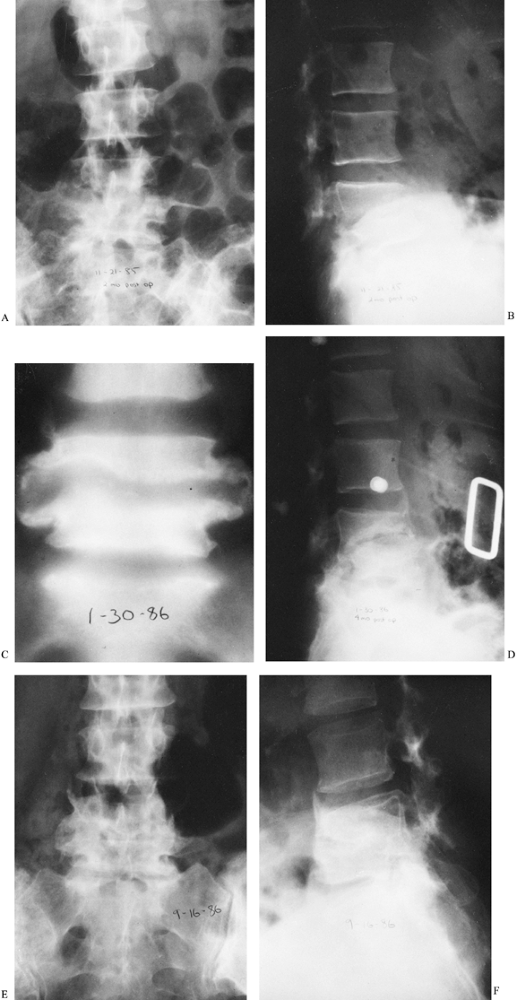
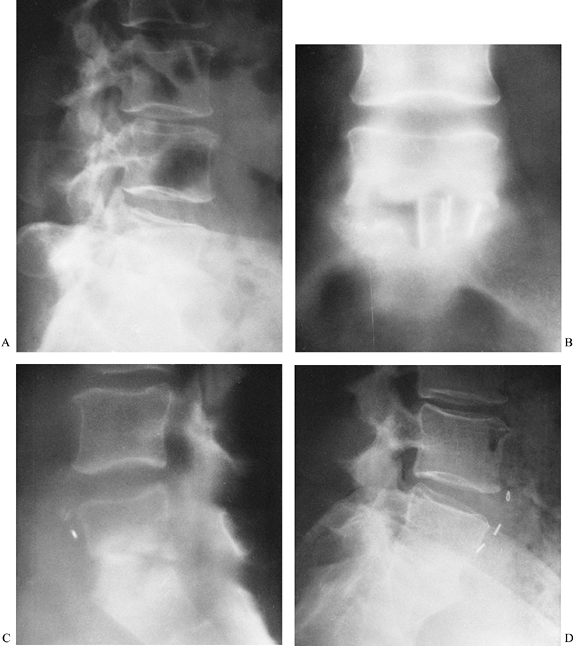
Figure 150.3. A,B:
This patient with osteomyelitis has complete loss of the disc space with partial destruction of the contiguous vertebral bodies. The lateral x-ray (B) shows kyphotic angulation of L-4 in relation to L-5. This 70-year-old woman was having severe pain and muscle spasm. The physical examination revealed loss of L-5 nerve root function on the right. C: The patient elected nonoperative care. After a needle biopsy revealed the causative organism, she was treated with antibiotics and a body jacket. At 4 months, a tomogram of the involved level shows osteophyte formation that is starting to bridge the disc space. D: A lateral x-ray taken at 4 months reveals correction of the kyphotic angulation. After 2 months of bed rest, a body jacket was applied and molded in hyperextension. E,F: Radiographs taken after 1 year show fusion of L-4 to L-5. She returned to work as a farm wife, pain free and with resolution of the foot drop due to the L-5 root lesion. This patient shows that nonoperative treatment can be successful. |
history and physical examination, a complete blood cell count and
erythrocyte sedimentation rate (ESR), venous blood cultures if
temperature spikes are noted, nuclear medicine imaging (technetium Tc
99m or gallium Ga 65), plain radiographs, and magnetic resonance
imaging (MRI) if symptoms are present for more than 1 month. Computed
tomography (CT) may be useful for delineating bony destruction and can
be used to guide needle biopsy. Lateral tomograms may also be indicated
for preoperative evaluation, particularly to delineate bony destruction
in the thoracic spine.
This delay may be due to a lack of any distinctive early physical or
radiographic findings or to a failure to look for spinal infection.
Dramatic regional pain that is worsened by motion or compression is the
most common symptom. The pain persists despite bed rest and is
classically exacerbated with motion. Pain, particularly at night, may
not be relieved with analgesics. Fever is not a consistent finding.
Anorexia and weight loss have been noted, and although the presentation
may be acute, the most typical presentation is subacute or chronic.
Chills, night sweats, hemoptysis,
or chronic bronchial cough are also suggestive of infection.
severe paraspinal muscle spasm associated with marked tenderness to
palpation. A pseudoscoliosis due to spasm may be present. Loss of
spinal motion is typical. Patients tend to splint and guard in an
attempt to decrease pain; they may be unwilling to bear weight,
particularly children. There may also be a mass and a concomitant
deformity visible in the area of infection. Neurologic findings may
vary from meningeal signs to mild weakness and, finally, paraplegia.
One of the earliest findings of spinal cord involvement from
tuberculosis is sustained clonus in the ankle.
of vertebral osteomyelitis is an elevated ESR, usually greater than 40
mm/h (Westergren method). However, Schofferman et al. (135)
reported normal values for the ESR in seven of nine patients with
occult infections by indolent organisms, such as diphtheroid or
coagulase-negative staphylococci. Serial ESR readings are valuable for
following a patient’s response to intravenous antibiotic therapy (121).
C-reactive protein (CRP) is an acute-phase protein synthesized by
hepatocytes. An elevated CRP level is seen in various conditions,
including infection, inflammation, and malignancy, as a response to
tissue injury. Healthy individuals show only trace amounts of CRP. CRP
levels rise after surgery but also drop quickly thereafter. An elevated
CRP is more helpful for determining postoperative infection during the
immediate postoperative period because the ESR can remain elevated at
that time (96,151).
unreliable. In a series of 38 patients with documented spinal
infection, the average WBC count was only slightly increased over the
usual high-normal value of 10,000 cells/mm3 (162).
vertebral osteomyelitis, particularly if the blood culture is obtained
during a febrile episode (47,123).
Negative culture results are common, however. The tuberculin purified
protein derivative (PPD) test is usually positive in patients with
tuberculosis. Before administering a PPD test, do an anergy battery to
detect immune compromise.
 |
|
Table 150.1. Unusual Organisms Causing Pyogenic Vertebral Osteomyelitis
|
exotic pathogens, and often with multiple organisms. Oral flora is
commonly cultivated from intravenous drug abusers and patients with
dental abscesses or extensive oral surgery. Resistant strains of
bacteria are of particular concern in chronically ill or hospitalized
patients.
osteomyelitis tend to differentiate it from tuberculous involvement of
the spine, which classically shows relative sparing of the disc space.
With pyogenic vertebral osteomyelitis, the earliest x-ray finding is
usually disc-space narrowing, which is noted at about 2–3 weeks after
the onset of infection.
Disc-space narrowing is followed by endplate erosion, then by progressive vertebral body destruction (Fig. 150.3) (121).
endplate sclerosis, with increased density noted in the subchondral
bone secondary to deposition of new bone on the existing trabeculae and
new subperiosteal bone formation (162). This
subchondral sclerosis may be preceded by a period of relative
radiolucency at about 6 weeks postinfection. The process of increasing
postinfection sclerosis will then proceed and can ultimately lead to
spinal fusion at about 6 to 24 months.
radiographs may not reveal disc-space narrowing until 24–36 months
after the onset of the disease process. There may be loss of vertebral
density, but reactive new bone is rarely seen and fusion is rarely
noted. With tuberculous vertebral infection, a common finding is a
large paravertebral soft-tissue mass with calcifications, which is
often noted on plain radiographs and CT. This is relatively
pathognomonic for tuberculosis. Vertebral pyogenic osteomyelitis, as
well as vertebral discitis, tends to be more common in the lumbar
spine, less common in the thoracic spine, and least common in the
cervical spine. In contrast, tubercular spondylitis is most common in
the thoracic spine and at the thoracolumbar junction.
plain radiographic findings. They can be particularly helpful in
imaging the thoracic spine and cervicothoracic junction (Fig. 150.4).
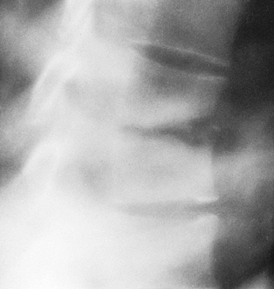 |
|
Figure 150.4.
Lateral tomogram illustrates endplate destruction in this thoracic infection. Without special studies such as tomography, these lesions can be difficult to visualize. |
However, in infected patients, myelography carries the risk of possible
intrathecal spread of the infection. Bone density can be followed with
CT and may give some clue as to whether the infection is progressing or
resolving. Increasing bone density has been noted after successful
treatment of vertebral body osteomyelitis with antibiotics (83).
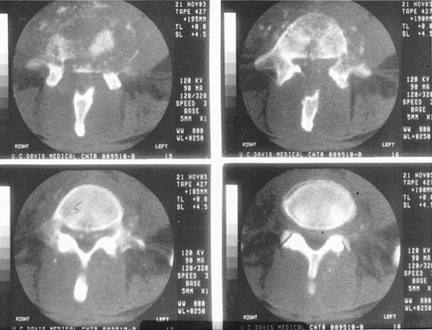 |
|
Figure 150.5.
CT of an L4-5 infection gives a better picture of the actual bony loss. The cut in the upper right hand corner shows virtually no anterior bony support; the spine is expected to be unstable. This is the same patient described in Figure 150.3. The vertebral angulation can be appreciated on the original lateral x-ray (Fig. 150.3B). |
patients with active vertebral osteomyelitis, but false-positive
results have been reported, particularly in the elderly population (3,134). Gallium scan has also been used to image pyogenic vertebral osteomyelitis. Haase et al. (62)
described a butterfly appearance of pyogenic vertebral osteomyelitis on
gallium scan; the butterfly shape, reflecting soft-tissue uptake,
appears on either side of the spine on an anteroposterior view.
Bruschwein (21a), reporting on a review of 100
consecutive patients with spinal infections studied with gallium
scanning, found a sensitivity of 89%, a specificity of 85%, and an
accuracy of 86% (9). Indium-labeled leukocyte imaging of the spine has an accuracy of only 31% (167,174).
sensitivity of 96%, a specificity of 92%, and an accuracy of 94% for
MRI in diagnosing spinal infections. In a study of 27 patients with
pyogenic vertebral osteomyelitis, MRI accurately detected abnormalities
in all patients; radiography did so only in 48%, CT in 65%, technetium
bone scan in 71%, and gallium scan in 86%. The most consistent finding
on MRI was increased signal intensity, particularly on T2-weighted
images (Fig. 150.6).
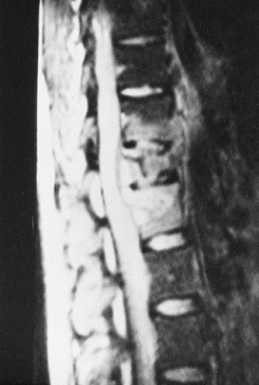 |
|
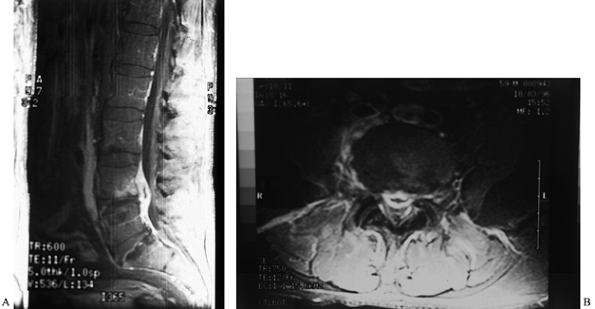
Figure 150.6.
MRI provides a means for earlier diagnosis of vertebral osteomyelitis. This T2-weighted image shows increased signal uptake in the involved vertebral bodies. In addition, it yields information about the amount of soft-tissue and spinal cord involvement. |
disc-space narrowing, low signal intensity in the marrow of at least
two adjacent vertebrae, subligamentous or epidural soft-tissue masses,
and erosion of cortical bone (152). T2-weighted
images demonstrate narrowed discs with variable signal changes,
abnormal high signal intensity in the marrow of at least two adjacent
vertebrae, high-signal subligamentous or epidural masses, and cortical
bony erosion. MRI demonstrates disc sparing in patients with
tuberculous spondylitis, as well as the extraosseous soft-tissue
extensions.
distinguishing epidural abscesses from the adjacent compressed thecal
sac, as well as identifying a paraspinal mass most likely to yield a
positive percutaneous biopsy (125,142).
Gadolinium contrast also helps distinguish active infection from an
infection that has adequately responded to antibiotic therapy (125).
examinations, the differential diagnosis will usually include
infection, primary neoplasm, and metastatic involvement of the spine.
Tissue from a biopsy is required to differentiate these entities. If
the diagnosis is not in question but the causative organism has not
been identified, aspiration or biopsy for culture is still required.
For the cervical spine, we recommend an anterior approach with a formal
operative exposure to avoid the high risk of inadvertently perforating
neck structures with a biopsy needle if a percutaneous technique is
used. Biopsy samples of the posterior cervical elements may be obtained
percutaneously, although formal open exposure facilitates
visualization. In the thoracic and lumbar spine, we recommend a
posterior CT-guide needle biopsy technique. If additional tissue is
required, a Craig needle biopsy can be performed under regional or
general anesthesia (Fig. 150.7).
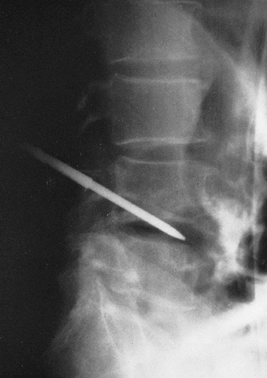 |
|
Figure 150.7.
A radiograph demonstrates a Craig needle in the L4-5 disc space. Usually, a needle of this size can be used percutaneously in the lumbar spine. In the cervical spine, open techniques are safer. In the thoracic spine, a CT-guided needle biopsy or an open procedure is safer. |
results are increased if a patient has not been treated with
antibiotics before the biopsy. If a patient’s condition permits,
discontinue antibiotic therapy for 2 weeks and then proceed with biopsy
and culture. Although the clinical presentation and the corroborating
radiographic evidence and histology from a biopsy can confirm the
presence of infection, culture results are required to prescribe a
specific antibiotic regimen. Histology is adequate to diagnose
tuberculosis and most fungal infections.
intravenous antibiotics, rest, and spinal immobilization. In pediatric
discitis, this is the treatment of choice. Generally, a regimen of 6
weeks of an intravenous antibiotic followed by 6 weeks of an oral
antibiotic is suggested (Table 150.2). Some recommend continuing antibiotic treatment until 3 months after the ESR has returned to normal (163).
This presumes that a patient is responding well to treatment and that
the response is followed by monitoring the ESR weekly, WBC counts daily
(initially) and then every third day, daily temperature reading, and
complaints of pain. A Hickman catheter or other long-term indwelling
intravenous catheter allows outpatient administration of intravenous
antibiotics. We prefer to discontinue antibiotics when a patient is
afebrile, pain has nearly resolved, and the ESR is normal. When back
pain improves, we allow patients to ambulate in a custom-made
thoracolumbar sacral orthosis or, occasionally, in an off-the-shelf
brace.
 |
|
Table 150.2. Intravenous Antibiotics of Choice for Some of the More Common Organisms Causing Pyogenic Infections of the Spine
|
antibiotic produce no abatement of fever or decrease in the ESR or WBC
count, then consider a thorough anterior debridement and strut grafting
(Fig. 150.8). Other indications for surgical intervention include the following:
 |
|
Figure 150.8. A: Lateral radiograph shows disc-space narrowing of L5-S1 due to Escherichia coli infection. Antibiotics failed to lower the ESR, and the patient continued to have pain. Anteroposterior (B) and lateral (C)
radiographs show the site after surgical debridement and strut grafting. Two bicortical iliac grafts fill the debrided area of the vertebral bodies. D: One year after surgery, the L5–S1 disc space is fused. The patient has no back pain symptoms. |
anterior column, which results in kyphosis, are best treated by
anterior debridement and mechanical reconstruction with strut grafting (26,46,48,100,105,110).
Although several bones may be used for strut grafting, including rib
and fibula, tricortical iliac crest autograft is preferred. The
cortical portion provides immediate stability, and early union is
enhanced by the cancellous component. Before debridement, we recommend
a 2-week course of an intravenous antibiotic, if possible, to decrease
purulence and surrounding inflammation (153).
The infection decreases the mechanical strength of the anterior and
middle bony columns. A laminectomy then destabilizes the remaining
(bony–ligamentous) posterior column, adding to instability and
increasing the potential for progressive kyphosis. Laminectomy may be
appropriate in treating an isolated epidural abscess (29).
In this setting, early aggressive laminectomy may be the treatment of
choice, permitting decompression and evacuation of the epidural
abscess, in conjunction with appropriate antibiotic therapy (68,130).
reported good success with combined same-day simultaneous and
sequential anterior decompression and posterior spinal instrumentation.
In addition, Hopf et al. (74) recommended anterior debridement
and anterior instrumentation as a single-stage procedure. Rath et al. (127)
demonstrated success with posterolateral debridement of the infection
and posterior instrumentation and autograft, which represents an
alternative way to treat osteomyelitis and disc-space infection.
Percutaneous drainage combined with percutaneous placement of pedicle
screws for an external fixator has also been used by Jeanneret and
Mageral (78). Fusion with bone grafting and
instrumentation should be added if a large laminectomy involving
multiple segments is performed. This is especially true in children, in
whom spinal growth will lead to a progressive kyphotic deformity if
fusion is not performed.
infection is usually successful when performed under the coverage of
intravenous antibiotics. The decision to proceed with this major
surgery in the face of infection is based on what is best for a patient
in the context of a surgeon’s abilities and available support services.
Anterior debridement and strut grafting followed by posterior
instrumentation and grafting work best in our hands and others (59).
If one or more vertebral bodies are to be resected to gain adequate
anterior decompression and strut grafting, we recommend subsequent
posterior stabilization with instrumentation to provide immediate
mechanical stability and protection for the neural elements. A spanning
segmented instrumentation construct is appropriate (Fig. 150.9).
 |
|
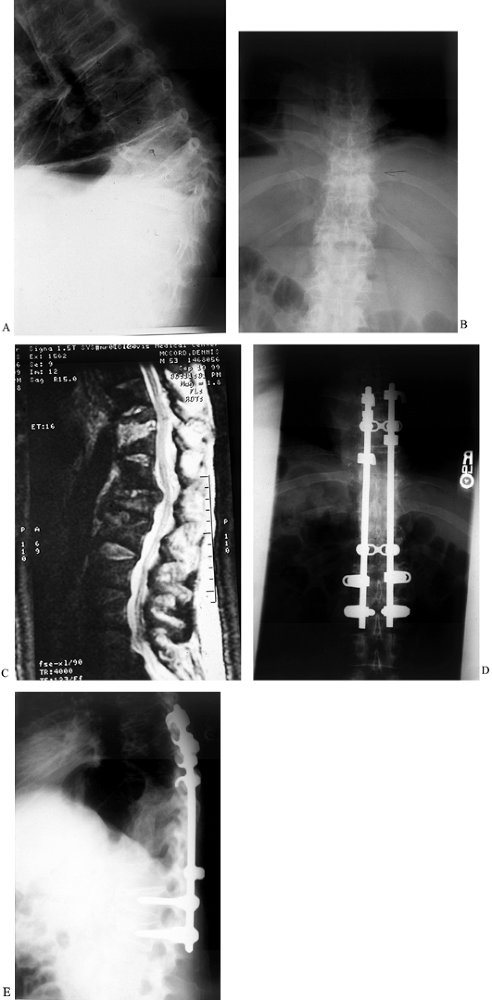
Figure 150.9.
A 52-year-old white man had a history of weight loss, fevers, chills, and progressive loss of lower extremity function over 8 months. He presented to the emergency department with weakness in both lower extremities. He had an obvious kyphotic deformity with pain at the apex of the kyphosis as seen on lateral (A) and AP (B) radiographs, and MRI (C). Anterior debridement of T-10 and T-11 vertebral bodies was performed, with subsequent anterior strut grafting with autogenous iliac crest bone graft. Posterior segmental instrumentation and fusion were performed the same day as seen on AP(D) and lateral (E) radiographs. Intraoperative cultures revealed S. aureus, and the patient underwent long-term intravenous antibiotic treatment. He recovered and walked without any assistive devices. |
encouraging patients to spend time in the fresh air, and by relying on
patients’ natural recuperative powers (67,80).
Abscesses were drained when necessary, and the vertebral column was
debrided if the patient became paraplegic. In 1895, Menard (113)
decompressed an abscess surrounding the spinal cord and was delighted
to find that the patient recovered neurologically. This led him and
other surgeons to decompress the spinal cord through a variety of
posterolateral and anterolateral approaches (17,139). The combination of rest, fusion, and debridement allowed the paraspinal abscesses to regress spontaneously (81). Some patients, however, demonstrated progressive bony destruction, paralysis, and spread of the disease.
spinal tuberculosis. With drugs as the only treatment, patients could
be cured not only of active disease but also of paralysis (170).
Operative treatment was reserved for failure of drug therapy,
recrudescence of disease, Pott’s paraplegia that did not resolve after
4–6 weeks of treatment, progressive or worsening paraplegia, or the
development of spinal cord involvement or other complications. In 94%
of patients without neurologic compromise, clinical healing of the
lesion occurred without surgery (154). However,
with neurologic involvement, only 38% recovered completely with drug
treatment alone, while 69% had complete recovery after surgical
decompression.
of treatment before and after the development of antituberculous drugs
and with and without surgical fusion. Of 227 adult patients treated
without antibiotics or surgery, bony ankylosis occurred within an
average of 5.7 years. Children treated without antibiotics or surgery
took even longer to stabilize, requiring on average 9 years to bony
ankylosis. Patients treated nonoperatively with antibiotics experienced
fusion in 4.9 years. Martin’s impression was that antibiotics and
surgical fusion produced a more stable spine within a shorter time; he
reported a 96.2% fusion rate.
found that antibiotics improved patients’ general condition and made
the surgery safer. It did not, in his opinion, prevent paraplegia or
promote recovery from it. He found that 24 (48%) of 50 patients
recovered with antibiotics alone, while 60% of patients who underwent
surgical decompression recovered. He suggested that an early surgery
might prevent or abort the onset of paralysis.
patients with Pott’s paraplegia, one half of whom were operated on and
the other one half treated conservatively. He found better results in
the surgically treated patients, with 28 (93%) of 30 patients showing
improvement and many being completely cured of any neurologic
compromise. Many patients were relieved of their painful flexor spasms.
studied patients treated with or without surgery, with emphasis on the
fusion rate. In the nonsurgical group, 50% had autofusion at an average
of 15.2 months. The surgical group had a fusion rate of 92%.
older than 60 years of age in Poland could tolerate and benefit from
surgical fusion. Of 133 patients, 61 had conservative treatment, while
72 underwent surgical decompression with or without fusion. Only 13 of
the conservatively treated patients returned to their regular
lifestyle; none could return to work as a farmer or laborer. Of the
surgically treated patients, 41 (57%) of 72 had complete clinical and
radiographic recovery; 21 patients returned to agricultural work.
been highly effective in controlling bony tuberculosis and even curing
it. The newer drugs have been more effective in preventing recurrence
of disease due to resistant organisms. However, for at least the first
6 months of chemotherapy, further bony destruction and collapse may
occur, causing increased vertebral angulation and cord compression.
This is particularly true if a significant amount of kyphotic
angulation is already present when drug therapy is started. Without
chemotherapy, there will be progressive thinning of the intervertebral
space, atrophy of osseous tissue, and decalcification, which can
persist for an average of 2–3 years (61). In
properly selected patients, medical treatment is adequate and can be
expected to yield a relatively high rate (up to 79% in one series) of
solid bony fusion (88).
hour before or 2 hours after a meal; children, 10–20 mg/kg per day (not
to exceed 600 mg)
12 months. A four-drug regimen for 12 months or a three-drug regimen
for 18 months is appropriate for eradicating difficult infections (115).
Initiate antibiotics at least 2 weeks before surgery and, if possible,
continue them postoperatively. In cases of acute paraplegia requiring
emergency decompression, begin antibiotics before spinal surgery.
most orthopaedic surgeons continue to favor surgical debridement.
Hodgson et al. (71) proposed that debridement be done as soon as possible after the diagnosis is established for the following reasons:
confirmed that tuberculosis can penetrate the covering of the spinal
cord (dura), causing irreversible paraplegia; therefore, they felt an
urgent need for surgical drainage to prevent this complication. In
their hands, early anterior debridement and fusion of the spine
resulted in 4% mortality, but the fusion rate was 93% and 26 of 35
patients with paraplegia recovered complete function. There was a close
correlation between the duration of neurologic symptoms before
operation and the time required to recover from paraplegia (69). In tuberculosis, bone grafting is safe, even in the presence of drainage (4,5).
column is reliable and effective in treating neurologically compromised
patients, posterior laminectomy is not effective and may lead to
neurologic deterioration (15). Infectious
destruction is usually anterior, causing the involved vertebral body to
collapse and angulate into kyphosis. Laminectomy destabilizes the spine
further and aggravates this progression into kyphosis. Hence, there is
no question as to the superiority of the anterior approach (8,45).
noted that anterior tuberculous disease was almost always more
widespread than demonstrated on radiographs. Even late decompression,
when symptoms have been present for an extended period, can produce
neurologic recovery. Bone grafting leads to an acceptable risk of
fusion in both children and adults (4,79,85).
studied anterior fusion, anterior debridement, and combined anterior
and posterior fusion in children followed for at least 10 years.
Anterior fusion alone had the worst prognosis in terms of progression
of kyphosis. Combined anterior and posterior fusion decreased the
incidence of kyphosis. On the other hand, Upadhyay et al. (156,157,158,159 and 160)
showed that a short anterior spinal arthrodesis done at a early age was
not associated with progression of deformity during growth and
development from disproportionate posterior spinal growth. Therefore,
they did not recommend a posterior fusion to stop posterior growth.
They also reported that patients who had reduction of kyphosis at the
time of the fusion showed a difference only at the sixth month of
follow-up, compared with patients who had only debridement. At final
follow-up, however, they found no difference in kyphosis between
the
groups and stressed the importance of achieving complete reduction and
fusion to prevent kyphosis. In summary, our preferred approach to the
treatment of children is a combined anterior and posterior fusion when
multiple levels of radical debridement are required; we use the
anterior approach only when only a short fusion is necessary.
debridement and anterior fusion, simple debridement, and medical
treatment alone, the Medical Research Council showed radical
debridement and fusion to be superior in the following ways (31,32,50,150):
the simple-debridement group, whereas it actually decreased in the
radical-debridement and fusion group.
has had the experience or stated the case so eloquently as the group
from Hong Kong (25,51,70,71,79,85,89,90,144,147,169,175,176). While the surgery is technically demanding and risky, the alternative seems to be worse. Yau and Hodgson (175)
reported on the penetration of the lung by vertebral abscesses and on
irreversible paraplegia from tuberculous infection passing through the
dura and directly involving the spinal cord (61,69).
Adding internal fixation to the treatment of tuberculosis of the spine
diminishes the incidence of kyphosis and pain, reduces the incidence of
bedsores, pulmonary infections, and recurrence rates and shortens
hospital stay (73). Moon et al. (116,117)
described similar findings in which anterior debridement and posterior
fixation provided early fusion, prevented progression of kyphosis, and
achieved correction of kyphosis. Other authors have suggested using
anterior instrumentation routinely at the time of debridement (74,93).
Therefore, if proper facilities and expertise for surgical drainage and
grafting of the infected vertebral column are available, surgical
fusion and stabilization are indicated (14,25,28,57,87,101,116,129,160).
Nonoperative treatment with antibiotics and orthotic support continues
to be an option for patients without significant destruction and
kyphosis (125).
been used for pyogenic and granulomatous infections, we have not
routinely used it. Anterior instrumentation may be used in these
situations if immediate stability is needed or posterior
instrumentation cannot be performed in a timely manner.
candidates for surgery. Spinal osteotomy, halo–pelvic distraction, and
anterior and posterior surgery have been used to correct these
deformities (176). Although the complication
rate associated with halo–pelvic traction and the multiple surgical
procedures was high, the average amount of correction was 28.3% in 30
patients; more important, further progression of the deformity was
halted. Halo–pelvic traction is still a viable technique and sometimes
safer than immediate correction with anterior and posterior
osteotomies. In these cases, a modest correction of the spinal
deformity balanced with prevention of further progression is the goal.
refined the staging of atlantoaxial tuberculosis, describing three
stages with progressive bone destruction. In stage I, there is minimal
bony destruction, and a transoral biopsy and decompression can be used
to surgically treat the infection, followed by halo orthosis. In stage
II, there is minimal bony destruction, but anterior displacement of C-1
and C-2 is present. For this, he advised transoral biopsy and
decompression followed by reduction with a halo orthosis and a
posterior surgical fusion of C1–2. In stage III, marked bony
destruction with displacement of C-1 and C-2 occurs. For this stage, an
anterior decompression is performed, followed by halo-traction
reduction and posterior fusion from the occiput to C-2 or C-3. We
endorse this step-by-step approach as a practical way to approach this
difficult problem.
found a high incidence of neurologic compromise and recommended
anterior decompression and fusion in all cases of tuberculosis of the
cervical spine (see Chapter 151). Other
authors have reported similarly on lower cervical spine tuberculosis,
noting a high incidence of neurologic compromise requiring surgical
treatment with bone grafting and anterior plating combined with
antibiotic treatment (102,104).
granulomatous diseases is destruction of the anterior column. This is
the area that usually needs to be drained or, if weakened by bony
destruction, supported by grafting. In most cases, posterior procedures
are supplemental to the anterior operation. For Pott’s paraplegia, the
preferred procedure is anterolateral decompression. Arthrodesis of the
spine is usually necessary to support the weakened anterior column.
Strut grafting is difficult through a costotransversectomy, so
anterolateral decompression, debridement, and fusion are best
accomplished through this approach. Posterior decompression further
weakens the spinal column and can cause further collapse and neurologic
deterioration (106). We prefer anterior decompression and strut grafting followed by a posterior stabilization procedure (Fig. 150.10). We debride infected and weakened bone and reconstruct the anterior column with rigid bone strut
grafting, which we then augment by posterior instrumentation using a neutralization construct and fusion.
 |
|
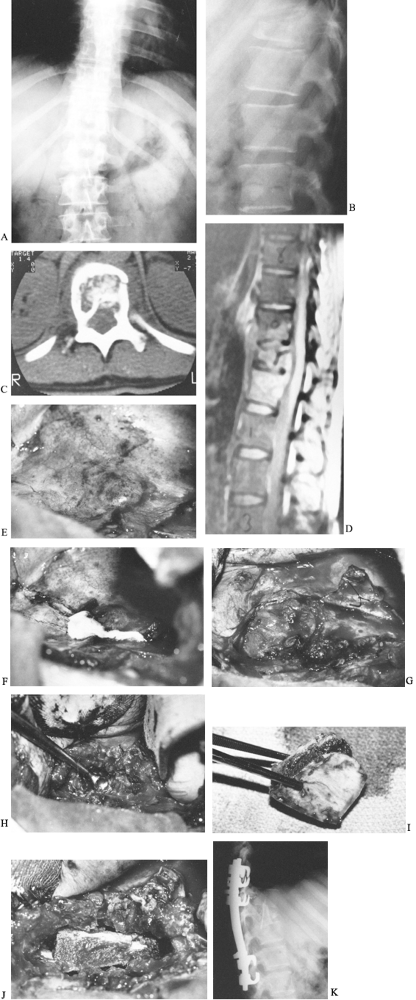
Figure 150.10. Anteroposterior (A) and lateral (B)
radiographs from a 30-year-old baker who had back pain after stepping into a hole. He had an ESR of 120 mm/h, a positive PPD with 10 mm of induration, and a negative coccidioidin titer. The lateral film demonstrates loss of height of the T-11 body and endplate destruction. C: CT demonstrates destruction of the vertebral body with extension posteriorly into the neural canal. D: MRI reveals the amount of vertebral destruction and compression of the spinal cord. Because of the amount of vertebral body involvement, it was elected to debride and strut-graft this lesion anteriorly and to fuse and stabilize the spine with instrumentation posteriorly. E: An anterolateral thoracic approach through the tenth rib was used to expose the T-11 vertebra. This photograph demonstrates the abscess over the vertebral body expanding the parietal pleura. The aorta lies just anterior to the spinal column. F: The abscess is incised along the posterolateral border of the spinal column parallel to it. Gross purulence exudes from the incision. G: A second incision is made perpendicular to the first from posterior to anterior, forming a T, and the corners can be elevated off the vertebral body and anchored anteriorly with skip sutures. Later, these flaps can then be used as closure over the grafted area. The segmental vessels to the vertebral bodies have been ligated. H: The necrotic bone and abscess material are removed by curettage or drilling with a high-speed burr. The involved bone, disc, and other debris are removed until good bleeding bone is located at each end of the lesion. If decompression of the spinal cord or cauda equina is needed, it is done at this time. The amount of material removed can be impressive. I: Bicortical bone can be removed from the ilium. This should be done with a separate draping and surgical setup so as not to contaminate the graft site. The graft is measured before it is cut to ensure an adequate length to strut the defect. J: The strut is impacted into the defect created by the debridement. The table, which had previously been flexed to provide access to the chest, is now straightened, locking the graft in place. If an acute kyphosis is present, additional strut grafts may be needed to bridge it completely. These are placed more anteriorly and should also be implanted into the bony portion of the vertebra. K: A postoperative lateral radiograph shows the iliac strut graft to span the infected level extending into the vertebrae above and below. Cotrel-Dubousset instrumentation extends an additional level above and below the T-11 vertebra. A posterior fusion supplements this instrumentation. The patient was ambulatory and taking tuberculosis medication when he left the hospital 2 weeks after the second surgery. He will continue his medication for 1 year after surgery. |
extremities, but cases of spinal involvement have been reported.
Diagnosis depends on identification of acid-fast bacilli, as granuloma
formation is not necessarily a feature of the disease (106,133,137).
In the presence of a persistent inflammatory process, ask about a
history of contact with shellfish and other sea life, gardening, or
trauma. Surgical excision of the infected focus and antibiotic
administration are the mainstays of therapy (54,133).
the most infectious of all fungi capable of producing systemic disease.
The localized form is usually benign, but the disseminated form is
progressive and potentially lethal. Of those with disseminated disease,
20% have osseous lesions. The fungus is endemic to the southwestern
United States, Central America, and parts of South America. It is
particularly prevalent in central California, where it carries the name
San Joaquin Valley fever (171). Although the
disease occurs in all ages, it is most prevalent in individuals 25–55
years of age, and dissemination is greater in men. Disseminated disease
is 10 times more common in blacks than whites and is of even greater
hazard to Filipinos (171).
and are spread hematogenously. If respiratory symptoms and fever
develop in a patient in an endemic region and last longer than 1 month,
disseminated disease should be suspected. In an endemic area, 50% to
84% of the population will have a positive coccidioidin skin test. It
takes 3–6 weeks for an exposed patient to test positive. Because of
anergy, the test is unreliable when systemic disease is present. A
serologic complement fixation titer of 1:64 or higher is thought to be
diagnostic of disseminated disease (154).
Often, multiple spinal lesions are found. Although the discs are
spared, paraspinal masses are seen with contiguous rib involvement (35). Treatment with amphotericin B or fluconazole (Diflucan) is recommended (39,133). Indications for surgical procedures are similar to those recommended for tuberculosis.
 |
|
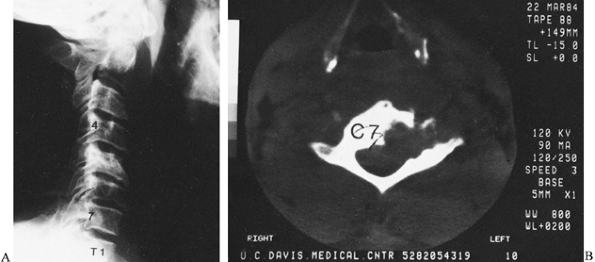
Figure 150.11.
Coccidioidomycosis can occur anywhere in the spine. In this case, the body and left lateral mass of the C-7 vertebra are destroyed. It is not as clearly seen on the lateral radiograph (A), but CT reveals the amount of vertebral destruction (B). During anterior debridement and strut grafting, the vertebral artery was identified. This 40-year-old man preoperatively received large amounts of amphotericin b with little effect on the bone disease. |
that is respiratory in origin but capable of dissemination. It is
endemic in the southeastern and midwestern United States. Men are
affected nine times more often than women; all ages may be affected,
although there is a higher frequency in the third and fourth decades.
infection, but as it disseminates hematogenously, generalized symptoms
of fever, night sweats, anorexia, and weight loss develop. Skin tests
are often negative early in the disease, but a culture of the skin or
lesion will reveal budding yeast cells. Serologic tests may show a high
titer only to Histoplasma capsulatum.
and it has a greater tendency than coccidioidomycosis for fistula
formation and erosion into joints. The disc cartilage is usually
involved early, and large paravertebral masses involving ribs may be
seen. Hilar adenopathy may be noted on the chest radiograph, as in
tuberculosis (56). Treatment for blastomycosis
is oral ketoconazole (Nizoral) or itraconazole (Sporanox), but
amphotericin B may be needed in immunocompromised patients. Indications
for surgery and the procedures are similar to those for tuberculosis.
is a chronic systemic fungal disease originating in the respiratory
tract. It may affect all ages, but is most prevalent between 40 and 60
years of age and is twice as common in men. Cryptococcosis is commonly
seen in patients with leukemia, Hodgkin’s disease, or sarcoidosis in
whom central nervous system findings develop. The pulmonary disease is
rarely symptomatic. Spread is by the hematogenous route and often
results in a cryptococcal meningitis, with 10% of disseminated cases
involving bone. The bony lesions are heralded by pain, swelling, and
progressive loss of spine motion (34).
lesions may reveal the organism. India ink capsule stain is helpful,
especially in spinal fluid specimens. Cryptococcal antibodies may be
measured, and some authors believe that their presence indicates a good
prognosis. Radiographically, the findings are indistinguishable from
coccidioidomycosis (34).
treated medically with amphotericin B or fluconazole plus flucytosine
(Ancobon) (79). Guidelines for surgery are similar to those for tuberculosis.
skin wound contamination from infected animal tissues. Infection via
inoculation of the conjunctiva has been demonstrated, and there is some
evidence that inhalation of aerosols containing bacteria can lead to
the disease (11,12).
Men are affected more often than women, probably because of a higher
rate of occupational exposure. Initial symptoms may include fever,
sweats, weakness, weight loss, headache, myalgia, lymphadenopathy, and
hepatosplenomegaly. Late complications are multisystemic and may
include septic arthritis, central nervous system involvement,
osteomyelitis, and spine involvement (172). Of patients with spinal involvement, about 12% will have spinal cord compromise.
bacteremic episode and from involved lymph nodes or granulomas later in
the course of the disease. The organisms are dangerous to laboratory
personnel, and any suspected materials should be clearly identified (172). The Brucella
agglutination test is quite reliable, and about 97% of infected
patients will become positive within 3 weeks of exposure. Brucellosis
is a reportable disease.
in the course of the disease and are similar to but less severe than
those seen in tuberculosis (11,172).
A paravertebral abscess usually is not present, as in tuberculosis, and
the spinal involvement usually is in the lumbar area (11,172).
therapy, usually with doxycycline and rifampin, for at least 6 weeks.
Surgical intervention is usually limited to a biopsy to obtain a tissue
diagnosis. Occasionally, stabilization of the spine or decompression of
the cord may be necessary. The indications and techniques are identical
to those proposed for treatment of the tuberculous spine. Brucellosis
is a completely curable infection. The primary pitfall is a delay in
diagnosis of more than 1 month, which can lead to multisystem
involvement with severe sequelae (11,12,172).
It is most common in immunosuppressed patients but has also been
reported in cases of postoperative disc-space infection and after
invasive monitoring (17,21,76,77,143,146).
The radiographic appearance is not pathognomonic but is distinctive,
generally demonstrating dense reactive new bone, a small lytic region,
and the absence of sequestration. Histologic diagnosis is usually made
with a potassium hydroxide preparation. Recommended treatment is
amphotericin B alone or together with itraconazole; rifampin may be
added (42,94). Surgery is recommended in accordance with the previously outlined guidelines for tuberculosis (40,88,133,136).
 |
|
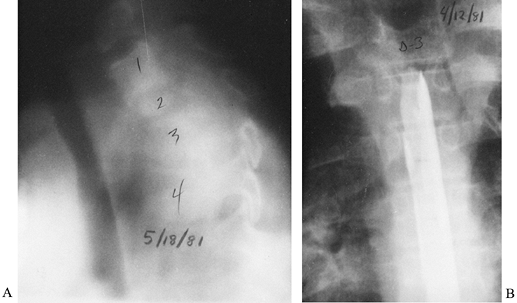
Figure 150.12.
Aspergillosis can infect the spine in immunocompromised patients, including those with acquired immunodeficiency syndrome (AIDS) or AIDS-related complex, intravenous drug abusers, or patients receiving cancer chemotherapy. This and other unusual organisms (e.g., Candida) are appearing with increasing frequency. This young man had aspergillosis that destroyed the T-2 and T-4 vertebral bodies with acute collapse and angulation. A: Tomography demonstrates the amount of destruction and angular deformity. B: Myelography reveals a blockage of the spinal cord canal created by granulation tissue and the deformity. The patient was paraplegic and required anterior debridement and posterior stabilization. There was complete recovery of neurologic function after anterior decompression. |
becoming increasingly common, primarily in the settings of immune
compromise or secondary overgrowth following antibiotic usage (44,52,55,67,82,120,146).
Systemic candidiasis may be diagnosed with positive sputum, urine, or
blood cultures. Medical treatment is amphotericin B or fluconazole, and
surgery is indicated within the guidelines described for tuberculosis.
However, the cause is an anaerobic, gram-positive, branching,
filamentous bacterium. In actinomycosis, granulomatous suppurative
lesions form and often develop sinus tracts, particularly in the head
and neck region (79,136). The infection can be treated medically with penicillin (133). The need for surgery is determined in accordance with the previously delineated recommendations for spinal stabilization.
complication. Postoperative infection following disc penetration is
most likely due to direct inoculation (37,38,122,177).
Therefore, the routine use of prophylactic antibiotics is recommended
whenever the intervertebral disc is entered (including procedures such
as minor discography) or other spinal surgery is undertaken (122).
Infection rates increase with the extent or complexity of spinal
surgery. The lowest rates (0.7% to 0.8%) are reported with
intervertebral disc surgery without fusion (37).
For patients undergoing spine fusion without instrumentation, the rates
range from 0.9% to 6%. The highest rates are reported with the use of
spinal instrumentation (0.5% to 15%); the average is about 8% (149).
microsurgical techniques, probably because of decreased soft-tissue
injury (37). Patients with discitis will
usually be pain-free for 1–2 weeks after surgery, and then
progressively increasing low-back pain develops. This may be
accompanied by temperature elevation, an increased ESR, elevated CRP
and WBC counts, increasingly tender paravertebral musculature,
increasing muscle spasm, and decreased lumbosacral range of motion. The
neurologic status usually does not change from the immediate
postoperative examination. Plain radiographs are rarely definitive in
this period, but may demonstrate disc-space
narrowing
and evidence of endplate involvement by 2–3 weeks after the onset of
infection. Nucleotide bone scan will be positive because of the
surgery, and so are not useful in differentiating infection. If the
presentation is delayed, MRI may demonstrate the characteristic
findings of a spinal infection (Fig. 150.13).
The definitive procedure for accurate diagnosis of postoperative
discitis is CT-guide neddle biopsy followed by culture of the organism
from the disc space (Fig. 150.14).
 |
|
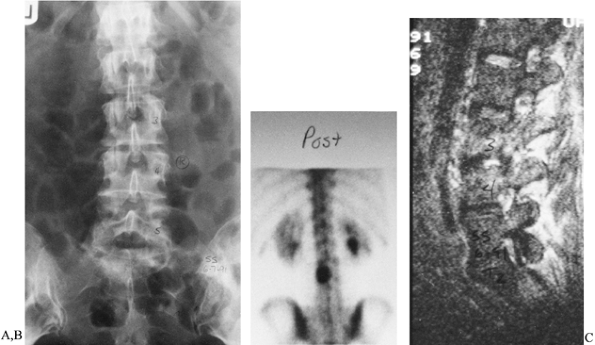
Figure 150.13. A:
Back pain and spasms developed in this patient 8 months after a percutaneous discectomy of the L3-4 disc. Percussion at the level of the discectomy produced severe, localized pain. The ESR was 120 mm/h. This anteroposterior radiograph shows only slight disc-space narrowing. B: Tc 99m bone scan demonstrates increased uptake at the level of the previous discectomy. This is much too intense to be due to postoperative changes. C: T2-weighted MRI shows increased signal uptake in the L3-4 disc space, indicative of infection. A Craig needle biopsy confirmed the diagnosis of disc-space infection; S. aureus was cultured. The patient recovered after 6 weeks of immobilization in a body jacket and intravenous antibiotic treatment. |
 |
|
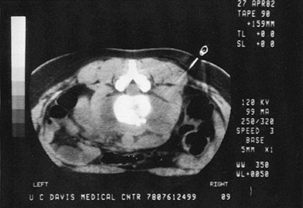
Figure 150.14. CT shows a needle being directed into a paravertebral abscess surrounding an infected vertebra.
|
can be given; most patients can be successfully treated with an
intravenous antibiotic, bed rest or immobilization in a brace, and
serial monitoring of the ESR and CRP level. We follow the guidelines
for surgery discussed previously for primary infection of the disc
space if medical treatment fails.
resulted in a classification of patients with spinal instrumentation
and postoperative infections into three groups on the basis of a
clinical staging system for adult osteomyelitis developed by Cierny.
Group 1 patients had a single-organism infection, either superficial or
deep; group 2 had multiple-organism deep infections; and group 3 had
multiple organisms with myonecrosis. Host response was also ranked into
three classes: Class A included patients with normal systemic defenses
and normal metabolic capabilities and vascularity; class B patients
demonstrated local or multiple systemic diseases, including cigarette
smoking; and class C patients were immunocompromised or severely
malnourished.
single irrigation and debridement, and closure over suction drainage
tubes without the use of inflow irrigation (43).
Group 2 patients required an average of three irrigations and
debridements and had a higher percentage of resolution of the infection
when a closed inflow–outflow suction irrigation system was used (53).
Group 3 patients were difficult to manage and tended to have a poor
outcome. Patients with diminished host defenses (classes B and C)
demonstrated an increased risk for postoperative wound infection.
stability during treatment. Bone graft, if loose, grossly infected, and
surrounded by purulence, should be debrided; otherwise the bone graft
can be left in place.
It is not entirely clear whether this drainage and infection are due to
primary inoculation with low-virulence organisms or prominence of
implants causing formation of bursal sacs that become infected
secondarily. Propionibacterium species, S. epidermidis, and Micrococcus
species have been found to be the organisms most responsible for
causing these late infections. Treatment requires removal of the
implants, debridement, and intravenous antibiotics.
infections is to be aggressive in irrigating and debriding such wounds,
rather than treating them with antibiotics alone. Multiple debridements
may be necessary to achieve formation of granulation tissue before
primary closure can
be
performed. Wounds that have gross purulence or failed closure may be
managed with dressing changes and healing by secondary intention. At
times, latissimus dorsi or rotational flaps may be needed to obtain
coverage. In our experience, suction–irrigation and multiple
debridements have worked well in treating most infections
postoperatively. Antibiotic beads placed at the time of each
debridement have also shown promise in terms of adding a concentrated
antibiotic locally to the infection.
Patients complain of local pain, tenderness over the spine, generalized
malaise, and fever. The symptoms may be highly variable in
immunocompromised patients. Heusner (66)
described four phases of neurologic involvement from epidural abscess.
In early phases I and II, there is localized pain with the development
of radicular pain and early neurologic changes, such as diminished
reflexes. In phase III, progressive neurologic symptoms occur,
including evidence of upper motor neuron impairment such as
hyperflexia. Motor weakness may eventually develop, with impaired bowel
and bladder function. Finally, in phase IV, complete paralysis
develops. Epidural abscesses may occur from either metastatic seeding
or direct extension (20,66).
Spinal cord dysfunction is probably due to a combination of mechanical
compression and anterior spinal artery thrombosis, which can cause
ischemia and direct infection of the cord (20,66).
Multiple reports have described many conditions that can lead to an
epidural abscess, including intravenous drug abuse, lumbar puncture,
and urinary tract and upper respiratory tract infections (9,13,36,92). Unfortunately, the diagnosis is frequently delayed (9).
The ESR will be elevated, and blood cultures may be positive. MRI is
the best tool for diagnosing epidural abscess. Radionuclide studies
(technetium or gallium scan) may not be helpful.
the epidural space and abscess in the subdural space, which is rare.
Only 45% of the patients with an epidural abscess are infected by S. aureus (41). Gram-negative rods, anaerobes, mycobacteria, and fungi are responsible for the remaining 55%.
antibiotics immediately. A penicillinase-resistant penicillin or
vancomycin will provide coverage for S. aureus, and an aminoglycoside for other suspected organisms, until the Gram stain and culture results are available (84).
Posterior compressive lesions should be treated with surgical drainage
by laminectomy, with maintenance of mechanical stability by
preservation of the facets. The wound may be closed over drains or
packed open in cases in which there is gross purulence. If an epidural
abscess occurs anteriorly, particularly with a disc-space infection
with extension into the epidural space, anterior debridement and
decompression of the anterior epidural space are necessary.
antibiotics alone without surgery has been reported, the risk of
progression of neurologic compromise is high (16,30,98).
The current treatment of choice for patients with cord compromise is
surgical debridement and antibiotics. In patients without neurologic
involvement who are poor surgical candidates, antibiotics may be used
initially, with monitoring of neurologic status. Surgery may be
necessary if significant neurologic findings develop.
may present as masses that can be palpated externally. Pyogenic
abscesses rarely reach this extent without proving lethal. In the
lumbar spine, an abscess will generally follow the course of the psoas
muscle, although it can also appear as a paravertebral mass. An abscess
that dissects along the psoas may extend below Poupart’s ligament and
present on the anteromedial surface of the thigh (adductor region) or
in the gluteal region. Occasionally, an abscess will appear over the
crest of the ilium in Petit’s triangle.
-
To drain a paravertebral mass
posteriorly, make an incision 4–8 cm lateral to the vertebral spinous
processes in a line parallel to the spine. -
Use a Cobb elevator or even finger
dissection to bluntly dissect around the erector spinae muscles until
the transverse processes of the vertebrae are reached. -
Usually, the abscess is entered
immediately. If not, puncture the thoracolumbar fascia that separates
the quadratus lumborum muscle from the erector group. -
Locate the abscess by working under the transverse process, and then debride and drain it (173). Send purulent material and tissue for Gram stain, culture, sensitivities, and histology.
-
After drainage, close the tissues in
layers over a drain, or pack the wound open. This is determined by the
local conditions and surgeon preference (71,85).
drained posterolaterally through Petit’s triangle or anteriorly beneath
Poupart’s ligament. Petit’s triangle is bordered by the lateral margin
of the latissimus dorsi muscle, the medial border of the external
oblique abdominal muscle, and inferiorly by the crest of the ilium.
-
Make an incision 2.5 cm above the crest
of the ilium and parallel to it. Begin the incision lateral to the
erector spinae muscle group. -
Bluntly dissect through the internal oblique abdominal muscle to gain access to the abscess cavity.
-
The incision may be also made directly
over the iliac crest, in which case detach the internal and external
oblique abdominal muscles from the ilium and expose its inner surface. -
Palpate the abscess extraperitoneally, and then open and drain it.
-
Manage the wound as described above.
-
Make an incision from the anterosuperior
iliac spine extending distally and medially for about 6 cm roughly
parallel to the inguinal ligament. -
Identify the sartorius muscle, and carry
the dissection medial to it to the level of the anteroinferior iliac
spine. Protect the femoral nerve, artery, and vein, which lie just
medial to this dissection. -
Identify the abscess on the medial surface of the wing of the ilium under Poupart’s ligament (173). If the psoas abscess presents medially in the adductor region of the thigh, drain it through a Ludloff approach.
-
For the Ludloff approach, make a
longitudinal incision on the medial aspect of the thigh, starting 2–3
cm below the pubic tubercle. Develop the interval between the gracilis
and adductor longus muscles. -
Develop a plane between the adductor
longus and brevis muscles anteriorly and the gracilis and adductor
magnus muscles posteriorly. -
Protect the posterior branch of the obturator nerve and the neurovascular bundle to the gracilis.
-
The psoas muscle, attaching to the lesser trochanter, and the floor of the hip joint are located in the base of the wound.
-
Drain the abscess through this wound (106).
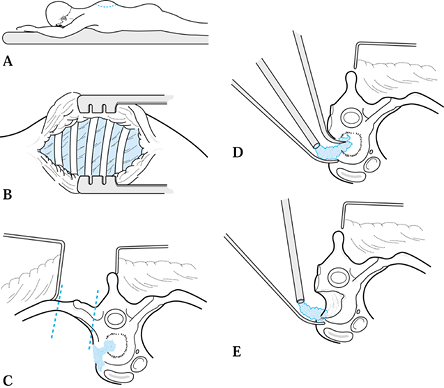 |
|
Figure 150.15. A:
Incision for a costotransversectomy (as described by Capner). With the patient in the prone position, make a curved incision 10 cm above the lesion, curving 7.5 cm laterally and ending 10 cm below. B: Reflect the skin and fascial layers medially as a flap. C: Remove a portion of the rib and transverse process of the vertebra over the abscess cavity. Expose the ribs and transverse processes of several vertebrae. D: The lateral surface of the abscess can be elevated free by working anterior and lateral to the vertebral body, usually without entering the pleural cavity. Curet and debride the abscess as well as possible. E: Decompression of the canal is possible if the pedicle and posterior surface of the vertebral body are removed. All debris is curetted and suctioned out of the cavity. It is difficult to insert a strut graft with this approach. |
-
Make a midline spinal incision extending over two or three spinous processes.
-
Reflect the muscle and soft tissues away from the spinous processes and the vertebral laminae on the side of the abscess.
-
Widely expose the middle transverse process, and resect it at its base.
-
Reflect the periosteum from the
contiguous rib, and resect the medial portion of the rib by dividing it
5 cm lateral to the tip of the transverse process. Do not enter the
pleural cavity. -
Remove more than one transverse process and rib, if necessary, to completely debride the abscess.
-
The neurovascular bundles between the ribs must be dissected free, ligated, and sacrificed (24).
approach using a semicircular incision lateral to the spine that starts
superior to the kyphotic deformity and ends inferior to it.
-
Elevate the skin flap and muscles medially to expose the medial 8 cm of three or more ribs and their transverse processes.
-
Subperiosteally resect the rib judged to be in the center of the abscess, being careful to stay outside the pleura.
-
Remove at least 7 cm of the medial rib in an adult, freeing the medial end with a periosteal elevator.
-
When the rib is teased free, pus should pour out of the gap created.
-
Explore and debride the abscess cavity.
Remove the necrotic material and any sequestered bone, and thoroughly
irrigate the cavity.
granulomatous infection, many authors recommend immediate arthrodesis
of the spine. This requires a more extensive approach than for simple
drainage. When neurologic compromise is present, the spinal cord or
cauda equina may have to be decompressed by removing additional bone or
soft tissue. This is usually the result of bony collapse with the
development of an acute gibbus.
-
Remove the debris, pus, sequestered bone,
and disc, using curets and pituitary rongeurs. Some of this material
can be removed with a large sucker tip. -
Remove the tissue across the entire breadth of the vertebral body.
-
Remove diseased bone or areas where graft will be inserted, using double-action rongeurs, a drill, or an osteotome.
-
Expose the spinal canal for the entire
length of the diseased area, decompressing the neural elements.
Granulation tissue, fibrous tissue, or the posterior longitudinal
ligament may require sharp incision to expose the dura mater. -
Remove the disc at each end of the cavity to expose the endplates of the vertebrae above and below.
-
Scrape the cartilage off the endplates, revealing bleeding cancellous bone.
-
Place the strut graft into the endplates,
keying the grafts into mortises made with a drill or curet to prevent
dislodgement. The strut graft should correct the deformity as much as
possible and hold the vertebrae apart. -
The strut grafts should be strong yet
osteogenic in nature; autologous cortical or bicortical iliac crest
graft is ideal, but the area to be grafted may be too large for the
iliac grafts available. Longer struts can be obtained from the fibula
or ribs. These should be supplemented with iliac bone because the
fibula is strong but mostly cortical bone and the rib is osteogenic but
relatively weak and will fail if stressed. -
The best source of bone is the patient’s
own ilium, but cadaver bank bone is a good second alternative,
especially when a long segment of bone is needed. -
In the thoracic and lumbar spine, we
usually supplement anterior struts with a second-stage posterior
instrumentation and fusion. With this technique, there is less chance
of graft dislodgement anteriorly. Anterior instrumentation may also be
used successfully if immediate stability is needed and posterior
fixation cannot be done in a timely manner.
-
Place the patient in a left lateral
position on a regular operating table with the right shoulder flexed to
120° and placed on an arm rest. -
Stand on the spinal side of the patient, tilting the table toward you to afford better visualization.
-
Approach the upper thoracic spine through
a right thoracotomy, using the bed of the third rib. If significant
kyphosis is present, a costotransversectomy might be better.-
Make a curved incision around the medial
and inferior aspects of the scapula. After dividing the parascapular
muscles, retract the scapula forward and upward. -
Excise the third rib, and enter the
pleural cavity through its bed. To improve visualization, cut the
insertion of the scalenus posterior muscle and remove the second rib.
The level is decided by following the rib head into the vertebral body.
The third rib head articulates with the junction between the T-2 and
T-3 vertebral bodies (71).
-
sternum-splitting operation may be indicated. The procedure is an
extension of the exposure for the cervicothoracic junction.
-
Extend the incision in the midline down the sternum to the xiphoid process.
-
Clear the anterior mediastinal tissues by
blunt dissection behind the manubrium, working distally from the
suprasternal notch. Work proximally from the xiphoid process in the
same manner. -
Divide the sternum with an oscillating
saw, and retract the two halves laterally. With this approach, the
vessels and midline structures can be retracted more widely. -
Mobilize the recurrent laryngeal nerve so
that it will lie obliquely across the operative field. Protect it
during the procedure with a moist sponge to prevent paralysis of the
vocal cords. -
Identify the vertebral artery behind the
carotid sheath. The artery passes upward and laterally to enter the
foramen in the C-6 vertebra. -
Approach the spine from the right because
the innominate artery on that side takes off from the aorta at a lower
level than the left subclavian vessels. In addition, the left
innominate vein runs obliquely and distally to join the right
innominate vein. The thoracic duct is also avoided. -
Anterior access to the distal cervical and proximal thoracic vertebrae is fairly good when the vessels are retracted.
-
After decompression and any stabilization
are complete, insert a suction drain, and close the sternum with
stainless-steel wire or staples. If the pleura was opened, drain the
chest with a large chest tube attached to underwater suction for at
least 48 hours (85).
the right side. In early disease without kyphosis, the right side is
best because fewer important structures are present. In more severe or
chronic disease when kyphosis is present, the left side is preferable
because the vena cava or aorta can become incorporated in the abscess
wall. The side of the larger abscess or lung penetration may also
determine the side of approach.
-
Use a bean bag to hold the patient in the
lateral decubitus position, and flex the table at the level of the
lesion to facilitate exposure. -
Make an incision along the rib to be
excised. It should be two levels higher than the lesion. Additional
ribs can be removed for better exposure. Divide muscle layers in line
with the incision, and resect the rib subperiosteally. -
Enter the pleural cavity and divide the
adhesions (if present), freeing the lung as completely as possible. If
thick adhesions between the lung and the abscess are present, portions
of the lung inevitably will be left adherent to the abscess cavity in
order to mobilize the lung and gain exposure. Open the lung abscess,
and remove any caseous material (60). Close the cavity with absorbable suture. A thoracic surgeon often performs this portion of the procedure. -
After the parietal pleura covering the
abscess is exposed, mobilize the aorta so that an interval is developed
between the two. Ligate and cut the segmental intercostal vessels
traversing this segment. If severe kyphosis exists, the aorta will be
acutely angulated and the segmentals bunched together at the apex of
the curve. Take great care in developing the plane between the
vertebral body and aorta: Adhesions can compromise the integrity of the
aortic wall. -
After the aorta is mobilized and
protected, open the abscess with a T-shaped incision. Make the
transverse portion of the T on the anterior portion of the vertebral
body parallel to the aorta. Retract the triangular flaps created by the
T incision, and attach them by stay sutures to the muscles at the wound
edges. Work from proximal and distal to the mid portion of the
kyphosis, particularly if cord compression is present. -
Radically excise all bony sequestra, sequestered disc, granulation tissue, and avascular bone.
-
The posterior longitudinal ligament forms
the posterior limit of the abscess cavity and is just anterior to the
spinal cord. Carefully, incise the ligament and remove it with
pituitary rongeurs. Allow the dura to slide or prolapse forward into
the area vacated by the debridement. -
Debride the bone until bleeding
cancellous bone surfaces are exposed. Cut or drill slots into the
exposed cancellous bone of the end vertebrae. -
Apply posterior pressure on the kyphosis
to open the interval so that it can be measured with a caliper. Place
several ribs or an iliac bicortical graft into the slots, and gently
impact the graft into place. -
Release the posterior pressure on the
spine, and the grafts will be firmly held in compression. It is wise to
supplement the impacted bone with additional struts, although these
will not be under compression. Usually, five to six ribs or two
bicortical iliac crest grafts can be fitted into the thoracic spine (71,85). -
Complete closure of the abscess cavity
flaps is unnecessary. In Hong Kong, streptomycin, 1 g, and isoniazid,
200 mg, are placed in the abscess cavity before closure (Fig. 150.10 and Fig. 150.16) (71).![]() Figure 150.16. A:
Figure 150.16. A:
An anterior thoracolumbar approach is best for thoracic or lumbar
involvement. With the patient in the lateral decubitus position, make
an incision over the rib just superior to the apex of the kyphosis. The
rib can be removed for use as a strut graft. Remove the tuberculous
debris, devascularized disc, and avascular vertebral body by curettage
or use of a power burr. B: If the cord is
compressed, completely expose it anteriorly to remove any pressure.
Place strut grafts of bicortical ilium and rib or fibula to bridge the
angular kyphosis from good vertebrae to good vertebrae. Sometimes,
correction of the kyphosis can be obtained during the grafting
procedure. This anterior fusion will need posterior instrumentation to
stabilize the spine and keep the anterior strut grafts from displacing.
graft, usually a second-stage posterior procedure is planned to provide
immediate stability, to support the strut anteriorly, and to prevent
later collapse and angulation. In the cervical spine, we use a
posterior wiring technique; in the thoracolumbar spine, we most often
use segmental instrumentation. In both cases, we combine the
instrumentation with a posterolateral spinal fusion. In the cervical
spine, the levels of the instrumentation extend from the first good
vertebra superiorly to the first good vertebra inferiorly. In the
thoracolumbar spine, it is important to include vertebrae at least two
levels above and below the resected vertebral bodies.
external immobilization. Use a halo orthosis vest to control motion in
the cervical spine and a polypropylene fitted body jacket for the
thoracolumbar area. Continue external
immobilization
for 3 months in the cervical spine and up to 6 months in the
thoracolumbar spine. The patient may get out of bed as soon as the
external immobilization device is applied and may ambulate, if not
paraplegic, as early as tolerated.
incorporation and consolidation of the fusion. The vertebral column is
also monitored for any angulation or vertebral collapse during the
later stages of recovery. Good nutrition and antibiotics complete the
treatment program.
procedures. This is usually of no special concern and can be ignored,
unless there is a significant air leak. This will generally scar and
seal in time. Use a chest tube if a pneumothorax develops.
decompression. Always try to close the rent. If closure is
unsuccessful, a spinal fluid fistula may result. These eventually heal
but may persist for up to 3–4 months.
will produce Horner’s syndrome, which usually is only temporary. In the
lumbar area, the extremity of the operated side will be warmer. This
too will usually resolve with time.
ureter can be cut accidentally. If so, this must be recognized and a
reanastomosis performed at once. If you suspect preoperatively that the
ureter might be involved in the abscess, place a stent into the ureter
on the side being operated on. This will make the ureter easier to
palpate and therefore less likely to be cut. A stent with a fiberoptic
light can even allow the ureter to be visualized among the debris and
bleeding as the abscess is incised and cleaned out.
vessel is cut or torn during mobilization of the great vessels. This is
most likely to occur at the lumbosacral junction, where the main
structure preventing access and mobilization of the common iliac
vessels is the iliolumbar vein. Locate this vessel before it is torn.
If it cannot be safely moved, ligate and cut it. If the vena cava or
aorta is injured, do an immediate vascular repair. Usually, a stitch or
two will control the problem, but occasionally a friable vena cava will
be impossible to repair and will need to be ligated.
thoracolumbar or lumbar area, a paralytic ileus is common. Patients
should not be fed until bowel sounds are actively present, and they
have noticed some flatus. Even oral fluids, if given too early, can
aggravate the ileus and prolong final recovery.
a thoracotomy. The most common is atelectasis, which will respond to
deep breathing, coughing, and use of the inspirometer. However, if left
untreated, this can develop into pneumonia. If a chest tube is
improperly placed or if it becomes blocked, pleural effusion or
hemothorax can occur with lung collapse and subsequent pneumonia.
neurologic status after anterior decompression. This is probably the
result of excessive trauma to the already jeopardized spinal cord,
particularly if the paraparesis is of long duration and the surgical
approach is difficult. It is likely to resolve because the spinal cord
in Pott’s paraplegia is resilient and can withstand a great deal of
trauma without permanent damage. On the other hand, if worsening
of
paraplegia is caused by spasm of or injury to the anterior spinal
artery, then the prognosis for recovery is bleak. There is no good way
to know which condition is causing the problem, and treatment for
either condition is not very effective. If arterial spasm is suspected,
administer intravenous methylprednisolone. Image the strut-grafted area
with radiography or CT, or both, to ensure that a graft has not slipped
posteriorly into the spinal cord. If this is suspected, reoperate and
reinsert the graft into a firm bony bed so that it will not impinge on
the cord. Hematoma or abscess is unlikely to cause compression of the
spinal cord, because most of the abscess has been removed and the area
around the debridement cannot contain the hematoma.
infection is not being sufficiently controlled by the antibiotic. This
is usually evident 6–12 weeks after surgery, and culture and
sensitivity reports will help in modifying the drug regimen. Usually,
the grafts need not be replaced, but if destruction is severe,
reoperation should be considered.
fracture. Fractures occur 12–18 months after surgery, when the bone is
weakened by resorption and replacement by new bone. These resemble
stress fractures and will heal with immobilization and rest. Fracture
of the graft is best recognized with tomography in the sagittal
(lateral) plane.
this is thought to be secondary to persistent infection, it must be
dealt with medically. When further spinal collapse and increase in
kyphosis occur, restabilization of the vertebral column is required. If
the disease is judged to be quiescent, the kyphotic angle is stable,
and the patient is asymptomatic, a nonunion can be ignored. If symptoms
do occur, a revision posterolateral fusion with instrumentation can be
performed.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
M, Powell O, Isaacs G, et al. Hematogenous Pyogenic Vertebral
Osteomyelitis: Diagnostic Value of Radionuclide Bone Imaging. J Nucl Med 1986;27:1680.
M, Olabe J, Bacci F. Infection of the Intervertebral Disc Space after
Placement of a Percutaneous Lumboperitoneal Shunt for Benign
Intracranial Hypertension. Neurosurgery 1990;26:1005.
Controlled Trial of Ambulant Out-patient Treatment and In-patient Rest
in Bed in the Management of Tuberculosis of the Spine in Young Korean
Patients on Standard Chemotherapy: A Study in Masan, Korea. First
Report of the Medical Research Council Working Party on Tuberculosis of
the Spine. J Bone Joint Surg Br 1973;55:678.
Controlled Trial of Anterior Spinal Fusion and Debridement in the
Surgical Management of Tuberculosis of the Spine in Patients on
Standard Chemotherapy: A Study in Hong Kong. Fourth Report of the
Medical Research Council Working Party on Tuberculosis of the Spine. J Brit Surg 1974;61:853.
M, Dinnenberg S, Greendyke W, Hopkins R. Roentgenographic Features of
Osseous Coccidioidomycosis and Differential Diagnosis. J Bone Joint Surg Am 1971;53:1157.
W. Infection of the Intervertebral Space Following Conventional and
Microsurgical Operation on the Herniated Lumbar Intervertebral Disc: A
Controlled Clinical Trail. Acta Neurochir (Wien) 1986;82:43.
J, Lehrer R, Stiehm E. Severe Candida Infections: Clinical Perspective,
Immune Defense Mechanisms and Current Concepts of Therapy. Ann Intern Med 1978;89:91.
S, Chan D, Woodward H. Treatment of Hematogenous Pyogenic Vertebral
Osteomyelitis with Anterior Debridement and Primary Bone Grafting. Spine 1989;14:284.
C, Guyot D, Fata J, Salciccioli G. Cervical Osteomyelitis Due to
Intravenous Heroin Use: Radiologic Findings in 14 Patients. AJR 1990;155:333.
Assessments of Controlled Trials of Ambulatory Treatment, Debridement
and Anterior Spinal Fusion in the Management of Tuberculosis of the
Spine: Studies in Bulawayo (Rhodesia) and Hong Kong. Sixth Report of
the Medical Research Council. J Bone Joint Surg Br 1978;60:163.
S, Shu L, Yau A, Hodgson A. Progressive Kyphosis Following Solid
Anterior Spine Fusion in Children with Tuberculosis of the Spine. J Bone Joint Surg Am 1975;57:1104.
D, Duffy K, Dawson E, Delamarter A. Lumbar Osteomyelitis and Epidural
and Paraspinous Abscesses: Case Report of an Unusual Source of
Contamination from a Gunshot Wound to the Abdomen. Spine 1991;16:380.
A, Stock F, Fang H, Ong G. Anterior Spinal Fusion: The Operative
Approach and Pathological Findings in 412 Patients with Pott’s Disease
of the Spine. Br J Surg 1960;48:172.
B, Mageral F. Treatment of Osteomyelitis of the Spine Using
Percutaneous Suction/Irrigation and Percutaneous External Spinal
Fixation. J Spinal Disord 1994;7:185.
R, Hillman J, Southwick W. The Importance of Direct Attack upon Lesions
of the Vertebral Bodies, Particularly in Pott’s Disease. J Bone Joint Surg Am 1953;35:17.
F, Islam C, Korkusuz Z. Prevention of Postoperative Late Kyphosis in
Pott’s Disease by Anterior Decompression and Intervertebral Grafting. World J Surg 1997;21:524.
P, Repanti M, Katsardis T, Stamatakis M. Anterior Decompression and
Fusion for Aspergillus Osteomyelitis of the Lumbar Spine Associated
with Paraparesis. Spine 1994;19:2715.
E, Schluger N, Bonk S, Rom W. Spinal Tuberculosis in Patients with
Human Immunodeficiency Virus Infection: Clinical Presentation, Therapy
and Outcome. Tuber Lung Dis 1996;77:329.
D, Lesoin F, Viaud C, et al. Decreased Morbidity from Acute Bacterial
Spinal Epidural Abscesses Using Computed Tomography and Nonsurgical
Treatment in Selected Patients. Ann Neurol 1985;17:350.
J, Kaplan L, Liebergall M, Floman Y. Serratia Osteomyelitis Causing
Neurological Deterioration after Spine Fracture: A Report of Two Cases.
J Bone Joint Surg Br 1989;71:256.
HJ, McCormick C, Carey M. Pyogenic Cervical Osteomyelitis Presenting as
a Massive Prevertebral Abscess in a Patient with Rheumatoid Arthritis. Am J Med 1988;84:363.
M, Woo Y, Lee K, et al. Posterior Instrumentation and Anterior
Interbody Fusion for Tuberculous Kyphosis of Dorsal and Lumbar Spines. Spine 1995;20:1910.
J. Anatomic Basis for the Pathogenesis and Radiologic Features of
Vertebral Osteomyelitis and its Differentiation from Childhood
Discitis: A Microarteriographic Investigation. Acta Radiol Diagn 1985;26:137.
S, Neff U, Schneider O, Richter H. Neurosurgical Management of Thoracic
and Lumbar Vertebral Osteomyelitis and Discitis in Adults: A Review of
43 Consecutive Surgically Treated Patients. Neurosurgery 1996;38:926.
O, Rand N, Kaplan L, et al. Sequential or Simultaneous, Same-day
Anterior Decompression and Posterior Stabilization in the Management of
Vertebral Osteomyelitis of the Lumbar Spine. Spine 1998;23:1885.
F, Mikolich D, Mates S. Technetium (Tc-99m) Diphosphonate Bone Scan:
False-normal Findings in Elderly Patients with Hematogenous Vertebral
Osteomyelitis. Arch Intern Med 1987;147:2024.
K, Kothe R, Leong J, Wehling P. Growth Changes of Solidly Fused
Kyphotic Bloc after Surgery for Tuberculosis: Comparison of Four
Procedures. Spine 1997;22:1150.
J, Cotler H, Sasso R, et al. Postoperative Infections in Spinal
Implants: Classification and Analysis—A Multicenter Study. Spine 1991;16:981.
10-year Assessment of a Controlled Trial Comparing Debridement and
Anterior Fusion in the Management of Tuberculosis of the Spine in
Patients in Standard Chemotherapy in Hong Kong. Eighth Report of the
Medical Research Council. J Bone Joint Surg Br 1982;64:393.
S, Saji M, Sell P, et al. The Effect of Age on the Change in Deformity
after Anterior Debridement Surgery for Tuberculosis of the Spine. Spine 1996;21:2356.
S, Saji M, Sell P, et al. Longitudinal Changes in Spinal Deformity
after Anterior Spinal Surgery for Tuberculosis of the Spine in Adults:
A Comparative Analysis between Radical and Debridement Surgery. Spine 1994;19:542.
S, Saji M, Sell P, et al. Spinal Deformity after Childhood Surgery for
Tuberculosis of the Spine: A Comparison of Radical Surgery and
Debridement. J Bone Joint Surg Br 1994;76:91.
S, Saji M, Sell P, Yau A. The Effect of Age on the Change in Deformity
after Radical Resection and Anterior Arthrodesis for Tuberculosis of
the Spine. J Bone Joint Surg Am 1994;76:701.
S, Sell P, Saji M, et al. Surgical Management of Spinal Tuberculosis in
Adults: Hong Kong Operation Compared with Debridement Surgery for
Short- and Long-term Outcome of Deformity. Clin Orthop 1994;302:173.
R, Adkins R. The Effects of Removal of Bullet Fragments Retained in the
Spinal Canal: A Collaborative Study by the National Spinal Cord Injury
Model System. Spine 1991;16:934.
D, Van Dam B, Abreu S. Preoperative Indium-labeled White Blood Cell
Scintigraphy in Suspected Osteomyelitis of the Axial Skeleton. Spine 1988;13:1168.
A, Hsu L, O’Brien J, Hodgson A. Tuberculous Kyphosis: Correction with
Spinal Osteotomy, Halo Pelvic Distraction, and Anterior and Posterior
Fusion. J Bone Joint Surg Am 1974;56:1419.