PRIMARY ARTHROPLASTY OF THE SHOULDER
– JOINT RECONSTRUCTION, ARTHRITIS, AND ARTHROPLASTY > Upper
Extremity > CHAPTER 101 – PRIMARY ARTHROPLASTY OF THE SHOULDER
(a) the stable and painless articulation between the glenoid and the
humeral head, (b) a subdeltoid space that is free of restricting
structures, and (c) the integrated action of the acromioclavicular,
sternoclavicular, and scapulothoracic articulations. The critical
contribution of the soft tissues surrounding the glenohumeral joint
cannot be overemphasized. The glenoid surface is only slightly curved,
and its surface area is about 25% that of the humeral head. Thus, the
humeral head rests against the glenoid surface, relying on the
surrounding capsule and musculotendinous units for stability as well as
mobility. Soft-tissue abnormalities such as scarring, contracture,
laxity, muscle atrophy, neurologic disease, or rotator cuff
tendinopathy severely compromise normal shoulder function.
structures of the shoulder must be recognized in considering the role,
indications, technique, and potential benefits and risks of shoulder
arthroplasty.
shoulder arthroplasty is but a part of a process that also includes
proper patient selection; evaluation of sternoclavicular,
acromioclavicular, and scapulothoracic relations; evaluation of the
integrity and function of the rotator cuff, laxity or contracture of
the capsule and glenohumeral ligaments, and the power of the deltoid
muscle; and the ability of the surgeon and patient to interact and
cooperate in the rehabilitation essential for successful arthroplasty.
If these variables can be handled successfully, pain relief following
total shoulder replacement is predictable (10,38,51).
However, although passive motion may be achieved through technically
satisfactory attention to the many intraoperative variables, active
motion will depend on the
strength of the muscles powering the arthroplasty. Thus, adequate rehabilitation aimed at both motion and strength is essential.
glenohumeral joint have identifying features that can present unique
problems of bone and soft-tissue reconstruction. It is important to
recognize some of these features, as they may provide clues to
intraoperative pitfalls. As an example, primary osteoarthritis presents
the problem of asymmetric, posterior wear on the glenoid. Rheumatoid
arthritis may display associated acromioclavicular disease, lower
extremity disorders, elbow and wrist involvement, rotator cuff disease,
osteopenia, and bone destruction, each of which will affect operative
technique, postoperative rehabilitation, and the result. Posttraumatic
arthritis often exhibits scarring and soft-tissue contracture,
malunited tuberosities, or nerve injury. In rotator-cuff tear
arthropathy, excessive wear into the acromioclavicular joint, acromion,
and glenoid, combined with severe soft-tissue deficits, make
reconstruction uniquely challenging (39). In
addition, as the characteristics of each arthropathy influence the
long-term results, patients may be better informed preoperatively by a
surgeon who recognizes the differences between and stages of
glenohumeral arthritic disease.
technique to apply to all patients who require total shoulder
replacement. In this chapter, a general guide to the technique of
primary replacement arthroplasty is followed by a discussion of some of
the features of each arthropathy and its associated surgical
reconstruction.
constraint. Most modern systems are modeled after the classic
unconstrained arthroplasty, initially described by Neer (38).
The popularity of this prosthesis stems in part from the fact that its
insertion requires the removal of only that part of the humeral head
and glenoid normally covered with articular cartilage. This minimizes
bone removal, allowing duplication of normal anatomy by the implant and
leaving some bone stock for salvage through arthrodesis if necessary.
The Neer design relies on soft-tissue integrity to stabilize and move
the implant because it replicates normal anatomy. Thus, the surgeon
must be able to anticipate and treat capsular laxity or contracture and
must be able to reconstruct a deficient rotator cuff.
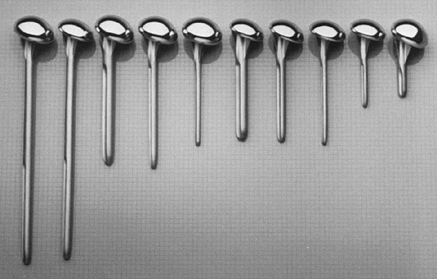
with two head thicknesses (15 and 22 mm), each with the same radius of
curvature (Fig. 101.1). There are three
different stem diameters (6.3, 9.5, and 12.7 mm) for each head
thickness. The most commonly used stem lengths are 125 and 150 mm. An
extra-long stem (252 mm) is used in tumor reconstruction or to treat an
associated humeral shaft fracture. An extra-short stem (63 mm) is
useful in some patients with juvenile rheumatoid arthritis or
epiphyseal dysplasia (Fig. 101.2). The proximal
humerus prosthesis may be used with or without bone cement for total
shoulder arthroplasty. Hemiarthroplasty for traumatic reconstruction
generally requires cement fixation to allow the implant to sit proud of
the humeral shaft, thus restoring length and avoiding inferior
subluxation of the head. Because its radius of curvature approximates
the normal humeral head, the implant can be used without the glenoid
component if the glenoid is free of disease, as in acute fractures and
some cases of osteonecrosis (22).
 |
|
Figure 101.1.
The Neer II proximal humerus. There are two head sizes, each with the same radius of curvature. The holes in the fin are for securing the tuberosities in fractures or after tuberosity osteotomy. |
 |
|
Figure 101.2.
The Neer II design has three different stem diameters for each head size. The variable stem lengths allow adjustments in unique situations such as tumor reconstruction or juvenile rheumatoid arthritis. |
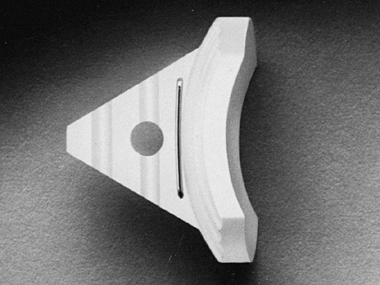
of the average adult glenoid and is made of high-density polyethylene.
It has a keel that must be inserted into the neck of the scapula, and a
radiographic marker
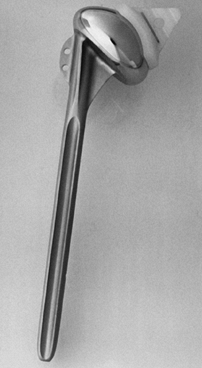
wire (Fig. 101.3). Its radius of curvature matches that of the proximal humeral component (Fig. 101.4).
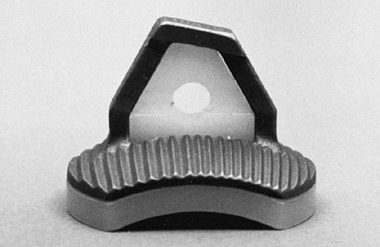
More recently, a metal-backed glenoid was designed to minimize the
stress on the polyethylene glenoid in hopes of avoiding implant
breakage or prosthetic deformation in a high-demand patient (Fig. 101.5). The metal-backed implant is rarely used, as the all polyethylene components are used routinely.
 |
|
Figure 101.3. The standard polyethylene glenoid.
|
 |
|
Figure 101.4. The radius of curvature of the glenoid matches that of the proximal humeral component.
|
 |
|
Figure 101.5. The metal-backed glenoid component.
|
available. The modular designs are attractive in terms of ease of
revision surgery, tensioning of soft tissues, inventory, and fracture
treatment (26). The Biomet (Warsaw, IN) modular
shoulder has four outer head sizes that each have three or four
possible neck lengths. These heads can be used with stems ranging from
6 to 15 mm in diameter and 7 to 19 cm in length. The Kirschner
Integrated Shoulder System (Biomet) adds modularity to the Neer design,
and the Atlas further increases options by adding humeral modularity (Fig. 101.6).
 |
|
Figure 101.6. Components of the Atlas Modular Total Shoulder System.
|
reconstruction of soft tissue for stability and motion, other
prosthetic designs provide more inherent stability if the rotator cuff
is deficient. Among the constrained designs are the
Stanmore and the Michael Reese models (45).
The trispherical prosthesis (Gristina), although constrained, is
intended to allow more motion through a double ball-and-socket design.
Constrained designs may not permit the forces across the joint to be
shared with the surrounding soft tissue, and complications in some
series have been high. In some constrained implant designs, the amount
of bone resection has been greater than in a nonconstrained implant. As
has been the experience with constrained implants in the lower
extremity, loosening appears to be a greater problem. Although these
designs may be considered in situations where there is no functioning
rotator cuff, we felt that they are generally not indicated (46). Their use has been abandoned by most shoulder surgeons.
shoulder is pain from an arthritic glenohumeral joint that is
unresponsive to nonoperative treatment such as rest, anti-inflammatory
medications, or an occasional intra-articular injection. It is critical
to identify the pain as coming from the glenohumeral joint and not from
adjacent structures. Some patients may have mild radiographic arthritic
changes, although their predominant pain comes from acromioclavicular
joint disease, cervical spine disease, or impingement syndrome. A
radiographic finding of mild arthritis in a patient with a recurrent
dislocation may suggest that dislocation arthropathy is the source of
pain (47),
although the pain may originate from continued instability or hardware
protrusion into the joint. A patient with rheumatoid arthritis may
benefit from acromioclavicular joint resection or bursectomy if the
radiographic changes are not severe. In addition, there is a
precollapse phase before the development of cuff tear arthropathy when
acromioplasty and cuff repair, rather than shoulder replacement, are
better advised.
replacement, because the gain in active motion with arthroplasty may be
less predictable than improvement in pain. Active motion depends to a
great degree on the surgeon’s ability to reconstruct and rehabilitate
the musculotendinous units moving the implant, which may or may not be
limited by the disease. Rheumatoid patients may, in fact, have limited
active motion because of associated rotator cuff weakness or rheumatoid
myopathy, which implant insertion will not correct. A patient with cuff
tear arthropathy may have pain relief following arthroplasty without
improved active motion, because the cuff may be irreversibly weakened
due to the length of time before treatment or the quality of residual
tissue (39). Nevertheless, in some patients
(notably those with ankylosing spondylitis or other rheumatic
diseases), restricted motion may cause hardship with daily living
activities or put undue stress on more distal joints. In this
situation, it is reasonable to attempt to gain motion with prosthetic
replacement.
nature of the disease process. Humeral head replacement alone is
indicated in the following situations:
-
Four-part fractures, where it is
anticipated that the articular segment is completely devoid of
soft-tissue attachment and blood supply, making avascular necrosis
likely (Fig. 101.7)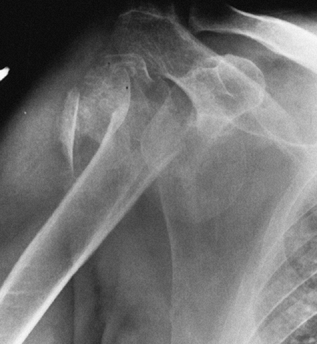 Figure 101.7.
Figure 101.7.
A four-part fracture dislocation. The humeral head is devoid of
soft-tissue attachment, late collapse is likely, and prosthetic
replacement is the surgical treatment of choice. -
A head-splitting fracture with destruction of the articular surface
-
Recurrent acute or chronic dislocations,
where the articular impression fracture of the humeral head is 40% or
more of the articular surface -
Osteonecrosis, if the disease has caused collapse or deformity of the humeral head but there is not yet disease of the glenoid
-
Some forms of primary osteoarthritis, if the glenoid still has some cartilage remaining, and the glenoid surface is congruent
-
Severe disease processes where the
glenoid bone stock available for insertion of an implant is limited,
such as in some forms of rheumatoid arthritis or revision surgery -
Elderly patients with nonunion of the surgical neck (Fig. 101.8)
![]() Figure 101.8.
Figure 101.8.
Painful nonunion of surgical neck in a 78-year-old woman. Prosthetic
replacement and tuberosity repair were selected because of the
patient’s age and osteopenia of the humeral head. -
Very young patients, if glenoid cartilage loss is not severe
-
Some types of tumor reconstruction
-
Some patients with rotator-cuff tear arthropathy
resurfacing arthroplasty of the glenoid. A recent study reported on the
short-term follow-up of hemiarthroplasty combined with biologic
resurfacing of the glenoid with either capsule or autogenous fascia
lata in patients with an average age of 48 years (9).
Although the preliminary results are promising, longer follow-up with a
greater number of patients is required before this procedure can be
advocated for routine use.
solution for glenohumeral arthritis if attention is paid to the details
of surgery and rehabilitation. Following are the most common
indications for total shoulder replacement:
-
Primary osteoarthritis with destruction of both humeral head and glenoid (Fig. 101.9)
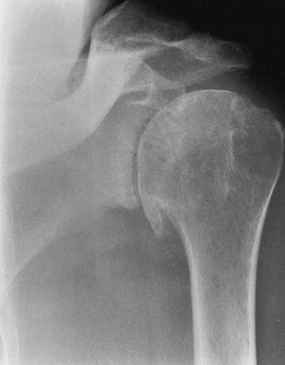 Figure 101.9.
Figure 101.9.
AP radiograph of primary glenohumeral osteoarthritis, recognizable by
the large, inferiorly protruding osteophyte and subchondral cyst
formation. -
Osteonecrosis in which the incongruity of the humeral head has also destroyed the glenoid
-
Rheumatoid arthritis, which usually affects the glenoid and humeral head (Fig. 101.10)
![]() Figure 101.10. Rheumatoid arthritis of the glenohumeral joint.
Figure 101.10. Rheumatoid arthritis of the glenohumeral joint. -
Posttraumatic arthritis in which joint incongruity or malunion has destroyed the glenoid (Fig. 101.11)
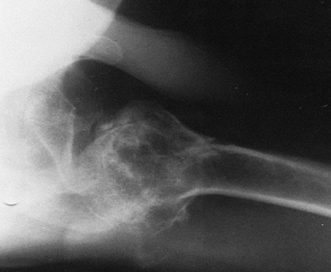 Figure 101.11.
Figure 101.11.
Axillary radiograph in patient with posttraumatic arthritis. There is
joint destruction, humeral head subluxation, and tuberosity malunion. -
Rotator-cuff tear arthropathy if the cuff is unreconstructible (Fig. 101.12)
![]() Figure 101.12.
Figure 101.12.
Rotator-cuff tear arthropathy. There is severe superior migration of
the humeral head, and wear into the acromion and the acromioclavicular
joint. Severe glenohumeral arthritis is evident. -
Severe arthritis of dislocations, the result of repeated
P.2635
instability of the humeral head or capsulorrhaphy arthropathy (Fig. 101.13)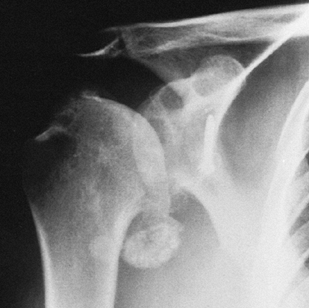 Figure 101.13. Arthritis of recurrent dislocations. The patient had a previous Bristow procedure.
Figure 101.13. Arthritis of recurrent dislocations. The patient had a previous Bristow procedure. -
Arthritis due to old infection that is quiescent or treated
-
Failed prosthetic replacement, humeral head resection, or arthrodesis
-
Active or recent infection
-
Paralysis of both the deltoid muscle and rotator cuff muscles
-
Neuropathic arthropathy
reconstructed rotator cuff tendons and deltoid for power, injury to the
axillary and suprascapular nerves or other extensive paralytic
processes will so weaken and destabilize the replacement that
arthrodesis may be preferable. A Charcot joint is usually a
contraindication, although in some neuropathic joints, stiffness and
pain predominate and implantation is worth consideration.
presence of a rotator cuff tear if the tissue is inadequate for repair
or if the muscle degeneration as documented on magnetic resonance
imaging (MRI) is too severe to allow the cuff to centralize the head in
the glenoid. Inadequate centralization of the humeral head in the
presence of a glenoid component may lead to asymmetric loading of the
glenoid, which can result in premature loosening (16).
If the humeral head is fixed superiorly, it is also unlikely that cuff
repair will be successful in the setting of total shoulder
arthroplasty. In our experience, however, glenoid resurfacing allows
more predictable pain relief. Therefore, we will consider
reconstruction of massive rotator cuff tears using residual tendon,
tendon transfers, or grafting if clinically indicated. Patient
considerations also play a role in this decision, as does the technical
difficulty of reconstructing a massive tear.
although some patients have such severe erosion and bone loss of the
glenoid that insertion of a glenoid component may be impossible, and
hemiarthroplasty of the humerus alone can be used.
arthroplasty is the patient’s inability or unwillingness to cooperate
with the extensive rehabilitation necessary for adequate functioning of
the implant.
consider alternative methods of treatment in the patient who cannot
avoid impact-loading types of physical activity. In the patient with a
prior infection, as with other arthroplasties, the joint must be free
of infection before implantation.
The Mayo Clinic experience with this total shoulder arthroplasty has
recently shown the survivorship (defined as no further surgery) to be
93% at 10 years and 87% after 15 years (51). The diagnoses were mainly
osteoarthritis and rheumatoid arthritis, with a lesser proportion of
posttraumatic arthritis. Other series have also reported good pain
relief in patients following total shoulder arthroplasty. In general,
pain relief following shoulder replacement is independent of function.
Function tends to be related to the diagnosis; soft-tissue adequacy is
an important factor for success. Total shoulder arthroplasty in younger
patients is not as successful as in the elderly (50). Also, revision surgery generally produces inferior results (44,49).
cervical spine disease, acromioclavicular disease, and nerve and muscle
deficits. Failure to recognize associated diseases before surgery can
preclude a good result.
radiographically with anteroposterior (AP), lateral, and axillary
views. An AP view may reveal the degree of osteophyte formation of the
humeral head (Fig. 101.9), the amount of
superior head migration, the status of the acromioclavicular joint, the
presence of a subacromial spur, the thickness and size of the
intramedullary canal of the humerus, and deformity or hardware in the
humeral shaft. In the posttraumatic shoulder, it may indicate the
position of the tuberosities and humeral head relative to the shaft.
The lateral radiograph may indicate the amount of anterior or posterior
subluxation of the humerus and the position of the tuberosities.
amount and position of glenoid wear, the extent of medial migration,
and the position of the humeral head (Fig. 101.11, Fig. 101.14).
If there is asymmetric wear of the glenoid anteriorly or posteriorly,
plans may include bone grafting at surgery. A computed tomography (CT)
scan may be extremely helpful in assessing the position of the
tuberosities and the amount of glenoid wear (Fig. 101.15).
An axillary view may provide sufficient information regarding the
glenoid orientation; however, a CT scan provides a clearer picture and
may be useful for surgeons with less experience in shoulder
arthroplasty. To avoid component malposition, it is crucial that
posterior wear of the glenoid be identified prior to surgery, because
glenoid version is difficult to assess intraoperatively.
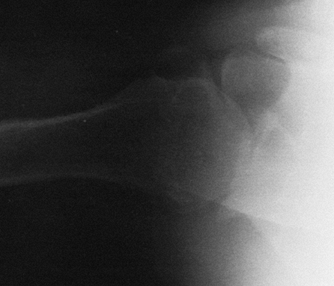 |
|
Figure 101.14. Axillary radiograph showing the loss of joint space and degree of wear of the glenoid in a patient with primary osteoarthritis.
|
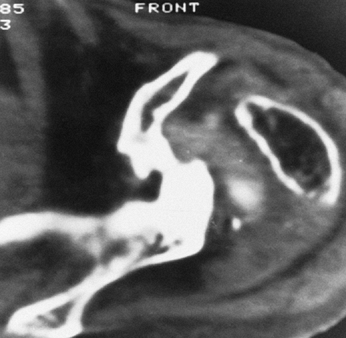 |
|
Figure 101.15.
CT scan in a patient with severe destructive rheumatoid arthritis. The humeral head has been completely destroyed, and there is marked bone loss of the glenoid. |
joint based on the history and physical examination, a complete blood
count, erythrocyte sedimentation rate, and joint aspiration, as well as
technetium and gallium scans, may be helpful in determining the current
status of the infection.
surgery resolve any medical conditions that could lead to problems at
the time of operation. In addition, a physical therapist should meet
with the patient preoperatively to plan and explain the therapy program.
patient, and anesthesiologist. General anesthesia or interscalene block
are the two most commonly used.
-
Position the patient to avoid
hyperextension of the neck. Fasten the head securely with tape to
either a horseshoe head-rest or the operating table itself. This
ensures that extremity movement during surgery will not dislodge the
endotracheal tube. Place the patient in a semisitting or beach-chair
position, with the hips flexed to 30°. Move the patient close to the
table edge to permit hyperextension of his arm when the humeral
component is inserted (Fig. 101.16). Because
support of the arm will permit more effective posterior retraction of
the humerus, secure to the operating table an arm board that can easily
be moved into or out of the operating field. This aids exposure and
insertion of the glenoid (Fig. 101.17) (17). Figure 101.16.
Figure 101.16.
Positioning the patient for total shoulder arthroplasty should enable
the arm to be hyperextended for humeral component insertion or for
rotator cuff mobilization.![]() Figure 101.17. Glenoid exposure is aided by posterior humeral retraction, made easier by the support of an arm board.
Figure 101.17. Glenoid exposure is aided by posterior humeral retraction, made easier by the support of an arm board. -
Place a towel under the medial border of
the body of the scapula to stabilize it and ease exposure of the
glenoid. Then secure the rest of the torso. Use adherent drapes to
outline the area to be prepared and to keep the patient’s hair from
entering the operating field. Prepare the skin of the upper extremity
from the base of the neck to the fingertips. If an assistant holds the
wrist during scrubbing of the patient, the arm can be moved into
abduction or adduction for ease of skin preparation. The skin
preparation includes the lower third of the neck superiorly, the middle
of the chest anteriorly, the midscapula posteriorly, and the chest wall
at the level of the lower border of the scapula. Secure the drape with
towel clips or skin staples, and place iodine-impregnated adhesive
plastic draping on the entire operative field. -
Within 1 hour before the incision, give 1 g intravenous cephalosporin antibiotic.
replacement. The long deltopectoral approach with anterior deltoid
detachment from the clavicle and acromion is no longer often used and
has been supplanted by an approach that does not detach the deltoid
from either the acromion or the clavicle. Sometimes detachment of the
anterior deltoid may be necessary for exposure or mobilization of the
tuberosities, anterior acromioplasty, or bone grafting of the glenoid.
However, it is preferable to maintain the integrity of the deltoid
origin to avoid the complication of deltoid detachment. We recommend a
series of sequential releases to aid in exposure. Release the deltoid
insertion over 1 to 2 cm in a subperiosteal fashion. A portion of the
pectoralis major insertion may also be released, which should allow
ample exposure. If greater exposure is needed, release the lateral
portion of the conjoint tendon. In rare situations, it may be necessary
to make a separate posterior approach, in addition to an anterior
approach, for mobilization of tuberosities; for mobilization of
scarred, retracted, rotator cuff; for glenoid bone grafting; or, in the
case of a longstanding fixed posterior dislocation, to remove the
humeral head from the posterior glenoid.
-
If a long deltopectoral incision has been
selected, infiltrate the skin and subcutaneous area with a 1:500,000
concentration of epinephrine. Avoid the axillary skin folds in the
incision. Begin the incision at the clavicle, between the coracoid
process and the acromioclavicular joint, and extend it distally to the
lateral insertion of the deltoid muscle (about 17 cm) (Fig. 101.18). Place skin retractors and obtain hemostasis. Figure 101.18.
Figure 101.18.
Deltopectoral approach. Coracoid, acromion, and clavicle have been
marked. The skin incision extends from the clavicle at a point between
the coracoid process and acromioclavicular joint to the deltoid
insertion. -
Incise the fascia over the deltoid and
pectoralis. Develop the plane between the subcutaneous tissue and
deltoid laterally and the pectoralis medially. -
Find the deltopectoral interval by identifying the “fat” over the cephalic vein in the infraclavicular triangle (Fig. 101.19). If the cephalic vein is not easily identified,
P.2638
the coracoid process may provide proximal identification of the
deltopectoral interval, and the tendon of the pectoralis can be used as
a guide to the deltopectoral interval distally. The cephalic vein is
routinely ligated and removed.![]() Figure 101.19. Identify the cephalic vein, the guide to the deltopectoral interval. It can be preserved, or ligated and excised.
Figure 101.19. Identify the cephalic vein, the guide to the deltopectoral interval. It can be preserved, or ligated and excised. -
Bluntly dissect the deltoid free from
adherent underlying bursa or rotator cuff. This is facilitated if the
deltoid is relaxed by slightly abducting the arm to find the plane
between the deltoid and the bursa. Bluntly dissect between the
pectoralis and the clavipectoral fascia overlying the muscles attaching
to the coracoid. -
Then retract the deltoid and pectoralis
with blunt Richardson retractors. Gentle abduction of 20° to 25°
relaxes the deltoid muscle for ease of retraction. In some pathologic
conditions (rotator-cuff tear arthropathy, rheumatoid arthritis), the
rotator cuff may at first glance appear indistinguishable from the
bursa. Avoid excising what is thought to be inflamed, thickened bursa
until it can be clearly distinguished from the tendinous portion of the
rotator cuff. -
Incise the clavipectoral fascia
superiorly until the coracoacromial ligament is identified. Then free
the subscapularis bluntly from the conjoint tendon of the arm muscles,
which should remain attached to the coracoid process to protect the
brachial plexus and the musculocutaneous nerve from injury by
retraction. Cauterize the acromial branch of the thoracoacromial
artery, and under most circumstances divide the coracoacromial ligament
(Fig. 101.20). Release of the coracoacromial
ligament facilitates exposure and allows more room for the
subscapularis repair at the end of the procedure. However, if the
rotator cuff is deficient, do not release this ligament because it may
diminish anterosuperior stability of the humeral head, increasing the
risk of dislocation.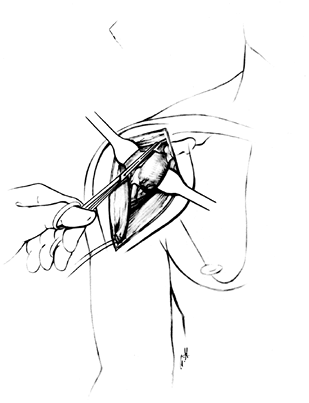 Figure 101.20.
Figure 101.20.
Retraction may be rendered less traumatic by release of a small amount
of the deltoid insertion. Retract the pectoralis major and muscles
attached to the coracoid. Excise the coracoacromial ligament. See the
text for important additional details. -
Free the subacromial bursa from any
tissue to which it adheres, such as the undersurface of the acromion,
before excision. This is more accessible if an assistant places slight
traction on the operated arm. Place a blunt retractor, instrument, or
finger between the rotator cuff and the bursa so the bursa can be well
defined before its removal. -
Assess the integrity of the rotator cuff.
Abduction and external rotation enables identification of the
subscapularis and assessment of its thickness and integrity.
Hyperextension and internal rotation of the shoulder bring the
supraspinatus, infraspinatus, and teres minor tendons into the
operative field. It is critical to identify the upper and lower margins
of the subscapularis tendon. Identify the upper margin by tracing the
subscapularis tendon from the coracoid process to the lesser tuberosity
or by the rotator interval between the subscapularis and supraspinatus
tendons. Identify the lower margin of the subscapularis by the anterior
humeral circumflex vessels. -
Then externally rotate the humerus and
cauterize the anterior humeral circumflex vessels. Maintaining the
humerus in internal rotation during division of the subscapularis will
jeopardize the axillary nerve, whereas external rotation and a little
flexion help protect the axillary nerve. -
Now evaluate the anterior structures
(which were evaluated in the office by examination prior to surgery)
under anesthetic. Extensive subscapularis release will allow sufficient
external rotation in most cases. However, if the patient is unable to
externally rotate to neutral in the operating room under anesthetic,
lengthen the capsule and subscapularis tendon. If the shoulder is tight
anteriorly, divide the subscapularis tendon and capsule laterally, near
the bicipital groove, and mobilize them as a single flap (Fig. 101.21).
At the conclusion of the procedure, fix the subscapularis and capsule
to bone at a more medial location using suture anchors. Repair the
medial limb of the subscapularis tendon to the lateral limb of the
capsule, effectively lengthening the anterior structures of the
shoulder. Alternatively, perform a Z-lengthening of the capsule to the subscapularis.![]() Figure 101.21. Divide the subscapularis tendon and capsule.
Figure 101.21. Divide the subscapularis tendon and capsule. -
If there is no significant limitation to
external rotation, divide the subscapularis tendon 1.5 cm medial to its
insertion on the lesser tuberosity adjacent to the bicipital groove. Be
certain to divide the subscapularis in its entirety by placing a curved
clamp deep to the substance of the subscapularis to help with
identification of the most superior and inferior margins during
division (Fig. 101.22, Fig. 101.23).
Detaching the subscapularis too close to its insertion on the lesser
tuberosity leaves tissue that is inadequate for proper closure. When
incising the subscapularis superiorly, divide only this tendon; do not
inadvertently divide the biceps tendon in the interval between the
subscapularis and supraspinatus. In many instances, the capsule and
subscapularis can be divided together, unless preoperative evaluation
has determined that tight anterior structures require subscapularis
lengthening, as discussed previously.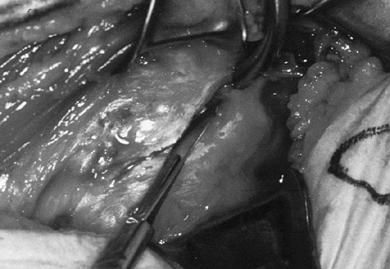 Figure 101.22. Divide the subscapularis from superior to inferior in its entirety.
Figure 101.22. Divide the subscapularis from superior to inferior in its entirety.![]() Figure 101.23.
Figure 101.23.
Division of the subscapularis and capsule provides access to the joint.
Avoid the biceps tendon at the superior margin. Cauterize the anterior
humeral circumflex vessels at the inferior margin. -
Place several nonabsorbable sutures in the proximal subscapularis tendon for identification, retraction, and reattachment (Fig. 101.24).
It is usually unnecessary to detach the muscles attached to the
coracoid or to osteotomize the coracoid process. This adds exposure if
P.2640
needed,
but take care to protect the musculocutaneous nerve. Mobilize the
subscapularis superiorly by sectioning the soft tissue superficial to
the biceps tendon in the direction of the base of the coracoid process.
The subscapularis may be adherent to the coracoid process. Divide these
adhesions for effective subscapularis mobilization and retraction.
Although the anterior capsule is divided in its entirety, and even
excised if abnormally thick, keep the most inferior capsule intact to
protect the axillary nerve, which is at risk from traction and the heat
generated during cement polymerization.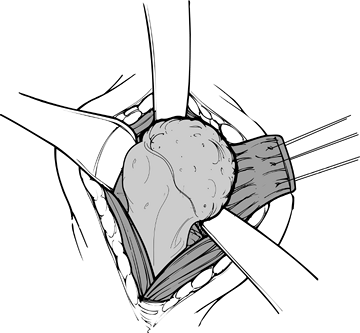 Figure 101.24.
Figure 101.24.
Place stay sutures in the subscapularis for retraction. The
subscapularis is the only muscle divided during the procedure, allowing
dislocation of the humeral head before osteotomy. -
Dislocate the humeral head by gently
extending and externally rotating the arm and placing a blunt elevator
between the humeral head and the glenoid. Take care with osteopenic
bone, as in rheumatoid arthritis, because the shaft can be fractured
during dislocation of the head. The humeral head is now ready for
osteotomy (Fig. 101.25).![]() Figure 101.25.
Figure 101.25.
Dislocate the humeral head, place the retractors superiorly and
inferiorly, and identify the marginal osteophytes. (From Craig EV.
Total Shoulder Replacement for Primary Osteoarthritis and
Osteonecrosis. In: Craig EV, ed. The Shoulder. Master Techniques in Orthopaedic Surgery. Philadelphia: Lippincott Williams & Wilkins, 1997:18, with permission.) -
Before osteotomy of the humeral head, assess for osteophytes (Fig. 101.26),
particularly inferiorly, which are common with osteoarthritis. These
can mislead you to remove excess humeral neck and jeopardize the
axillary nerve. Identification of the circumferential osteophytes
enables more accurate identification of the amount of humeral head
normally covered by articular cartilage. To remove osteophytes,
position flat retractors between the humeral head and the inferior
capsule and between the humeral head and superior rotator cuff. Remove
the osteophytes with an osteotome or rongeur.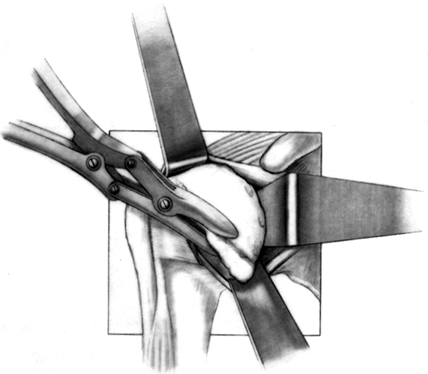 Figure 101.26.
Figure 101.26.
Remove the most prominent marginal osteophytes. Any residual
osteophytes can be excised after the trial stem is inserted. (From
Craig EV. Total Shoulder Replacement for Primary Osteoarthritis and
Osteonecrosis. In: Craig EV, ed. The Shoulder. Master Techniques in Orthopaedic Surgery. Philadelphia: Lippincott Williams & Wilkins, 1997:18, with permission.) -
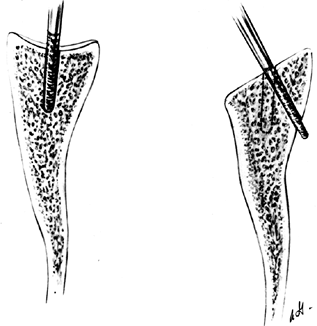
The articular surface of the humerus is usually in 30°
P.2641P.2642
to 40° of retroversion relative to the transverse axis of the elbow.
Gauge this by flexing the elbow to 90° and externally rotating the arm
30° to 40°, as required. Hold the trial implant against the humeral
head to determine the osteotomy site and the appropriate retroversion
of the osteotomy (Fig. 101.27).
The usual amount of retroversion should be increased in patients with
anterior instability, and decreased in patients with posterior
instability. Numerous implants have guides to make the cut more
accurate, including the intramedullary guide of the Atlas implant.![]() Figure 101.27. Use the trial humeral component to mark the osteotomy site.
Figure 101.27. Use the trial humeral component to mark the osteotomy site. -
If a resection jig is not used, mark the
angle of the osteotomy site with cautery and osteotomize the humeral
head with an oscillating or sharp osteotome saw. The three important
components of the cut to judge are the neck–shaft angle, the degree of
retroversion, and the superior–inferior depth. -
Begin the osteotomy just inside the
supraspinatus insertion, at the sulcus between the articular cartilage
and greater tuberosity (Fig. 101.28). A surprisingly small amount of bone is removed with the humeral head osteotomy (Fig. 101.29). Save the resected humeral head because it is an excellent source of bone graft.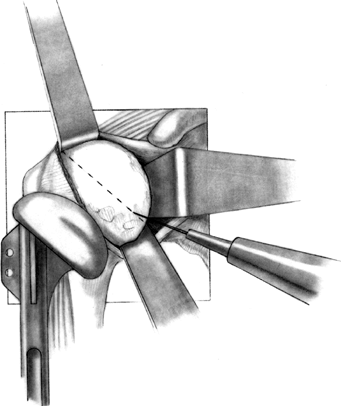 Figure 101.28.
Figure 101.28.
Once the correct angle has been marked using the trial component as a
guide, use an oscillating saw or sharp osteotome to make the cut, while
protecting the soft tissues. If the osteotomy is to be made freehand,
without a resection guide, a trial prosthesis may be utilized to
identify the osteotomy site. (From Craig EV. Total Shoulder Replacement
for Primary Osteoarthritis and Osteonecrosis. In: Craig EV, ed. The Shoulder. Master Techniques in Orthopaedic Surgery. Philadelphia: Lippincott Williams & Wilkins, 1997:20, with permission.)![]() Figure 101.29. Assemble the humeral head resection guide directly onto the T-handled
Figure 101.29. Assemble the humeral head resection guide directly onto the T-handled
reamer. The holes on the cutting jig are for pin placement, to secure
the cutting jig to the humerus. Place a flexible rotator cuff probe
beneath the supraspinatus and the biceps tendon to ensure the proper
exit site for the osteotomy cut. (From Craig EV. Total Shoulder
Replacement for Primary Osteoarthritis and Osteonecrosis. In: Craig EV,
ed. The Shoulder. Master Techniques in Orthopaedic Surgery. Philadelphia: Lippincott Williams & Wilkins, 1997:22, with permission.) -
Locate the canal of the humerus with a curet or gouge, and then sequentially prepare it by using graduated T-handled reamers (Fig. 101.30). Protect the soft tissues during reaming.
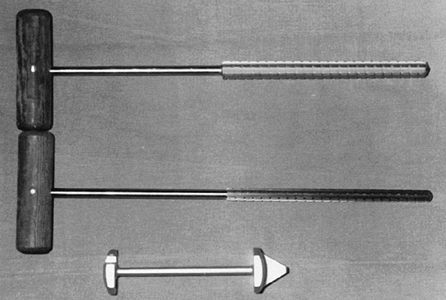 Figure 101.30. Use T-handled reamers to prepare the canal of the humerus (top). A glenoid guide (bottom) may be used for localization of the glenoid slot.
Figure 101.30. Use T-handled reamers to prepare the canal of the humerus (top). A glenoid guide (bottom) may be used for localization of the glenoid slot. -
Next, insert the trial humerus prosthesis
into the intramedullary canal in about 25° to 35° of retroversion,
which can be estimated by positioning the fin on the humeral component
lateral and posterior to the bicipital
P.2643P.2644
groove.
Sometimes it may be necessary to create a small vertical trough for the
fin. During the insertion of the humeral prosthesis, keep the arm
hyperextended off the side of the table and protect the biceps tendon
and supraspinatus with retractors. If the humerus is correctly oriented
in the appropriate amount of retroversion, the articular surface of the
implant should face directly toward the glenoid with the arm in neutral
rotation. Seat the trial implant with a mallet and impactor, then trim
remaining osteophytes or protruding bone from around the implant. The
depth of the insertion should permit the top of the humeral head to
extend slightly above the most superior portion of the greater
tuberosity. In addition, proper depth should permit closure of the
rotator cuff over the implant. As described earlier, subscapularis
lengthening may be required for complete closure of the anterior soft
tissues. -
Selection of the appropriate humeral head
implant is critical. Determine the correct head size from the size of
the humeral head that has been removed, as well as from an assessment
of whether the rotator cuff can be repaired around the implant. In
general, a large head size provides a longer lever arm and the
potential for more power, whereas a smaller head size makes it easier
to close the rotator cuff around the prosthesis. Use the largest stem
that fits in the intramedullary canal of the humerus. However, withhold
final selection of the humeral component until the glenoid has been
resurfaced and the ability to close the rotator cuff evaluated. -
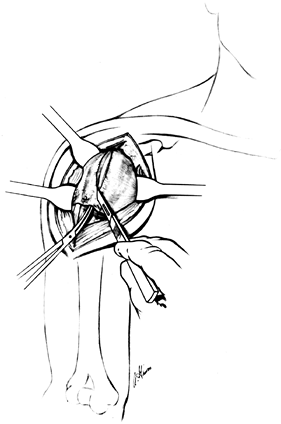
Remove the trial prosthesis and use the
arm board to position the shoulder for inspection of the joint and
preparation of the glenoid. Explore the joint, remove loose bodies, and
completely excise the synovium. Completely excise the anterior and
posterior labrum. Inspect the glenoid to determine whether it will
require resurfacing and whether the quality of bone is sufficient to
accept a component. Posterior wear of the glenoid can be difficult to
estimate in the operating room and should have been assessed
preoperatively on an axillary view of the shoulder or a CT scan.
Thoroughly inspect the rotator cuff and biceps tendon. Evaluate the
acromioclavicular joint for arthritis and for inferiorly protruding
osteophytes, which may cause mechanical impingement. Also, inspect the
undersurface of the acromion for an overhanging subacromial spur, which
may compromise the result. If necessary, perform an anterior
acromioplasty and resect the distal clavicle. If there is a tear in the
rotator cuff, mobilization for repair is less difficult once the
humeral head has been removed. For even further exposure of a massively
torn, retracted rotator cuff, excise the distal clavicle. -
Note that the humerus is prepared but the
permanent implant is not inserted prior to glenoid preparation and
insertion. We recommend the routine use of a cemented glenoid
prosthesis. Prior to implanting a cementless glenoid component, the
risks and benefits as well as the rationale for implant selection
should clearly have been explained to the patient. -
Adequate muscle relaxation is essential
to expose the glenoid. It is usually unnecessary to trim the glenoid
osteophytes, although they may distort the normal glenoid anatomy and
make orientation of the glenoid slot difficult to judge. For ease of
insertion of the glenoid, adjust the arm board to bring the elbow level
with the shoulder and support the elbow on operative draping or towels
so that the humerus will not fall into hyperextension and obscure the
glenoid. Carefully retract the proximal humerus posteriorly with the
attached rotator cuff; this is facilitated by placing the ring
retractor behind the posterior glenoid (Fig. 101.31).
With a flat retractor, expose the inferior glenoid by retracting or
excising some of the inferior capsular insertion. A second flat
retractor may be needed anteriorly to retract some remnants of the
anterior capsule (Fig. 101.31). Remove the
remaining glenoid cartilage with a curet, taking care to preserve the
subchondral bone. Because the base of the coracoid contains cancellous
bone useful for anchoring the glenoid component, it is usually
necessary to remove the soft tissue from the area between the superior
glenoid and the base of the coracoid. During removal of the soft tissue
to expose the base of the coracoid, do not divide the long head of the
biceps.![]() Figure 101.31.
Figure 101.31.
Place a ring retractor behind the posterior rim of the glenoid,
exposing the glenoid fossa and retracting the humeral shaft. (From
Craig EV. Total Shoulder Replacement for Primary Osteoarthritis and
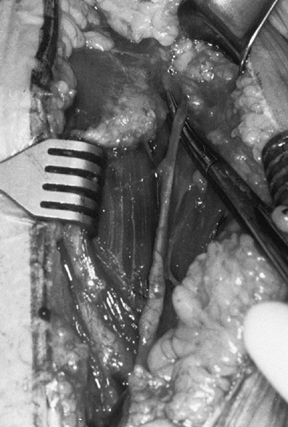
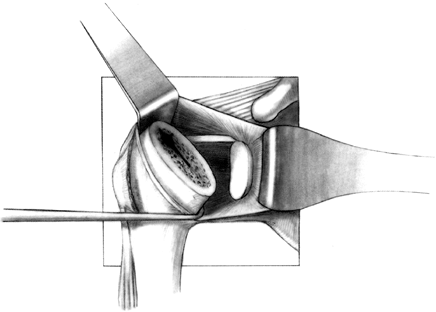
Osteonecrosis. In: Craig EV, ed. The Shoulder. Master Techniques in Orthopaedic Surgery. Philadelphia: Lippincott Williams & Wilkins, 1997:27, with permission.) -
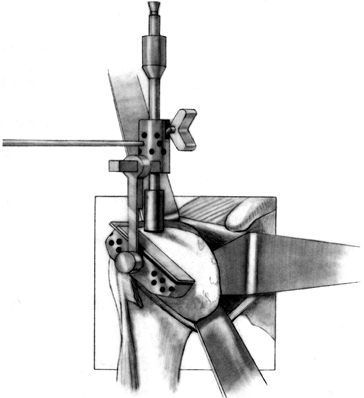
Prepare the glenoid for the keel or peg
of the component. The slot for a keel extends along a line from a point
immediately below the base of the coracoid to just above the
infraglenoid tubercle, and it includes the cancellous bone at the base
of the coracoid process (Fig. 101.32). Drill
holes at the most superior and inferior aspects of the anticipated
slot, and connect them with a burr or drill. Do not widen the glenoid
slot excessively, or the component will toggle and secure seating will
be difficult. The slot corresponds exactly to the size of the keel.
Orient the slot so that it lies directly in the cancellous bone of the
glenoid neck. This orientation is often difficult, particularly if
excessive wear has occurred anteriorly or posteriorly. With the
anterior capsule detached, palpate the anterior glenoid neck to aid
proper orientation while the slot is deepened. If excessive asymmetric
wear has occurred on the glenoid, orienting the slot and deepening it
perpendicular to the flat surface of exposed glenoid can result in
perforation of the cortex (Fig. 101.33).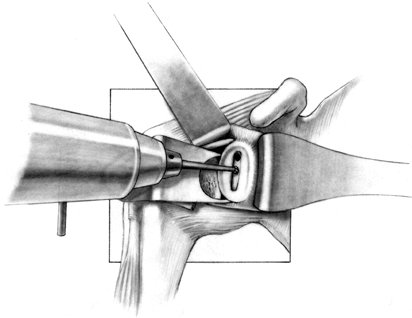 Figure 101.32.
Figure 101.32.
In this arthroplasty system, create a slot from superior to inferior to
house the glenoid keel. Widen this slot only to the normal glenoid
component width, but it may be undermined superiorly and inferiorly for
better and more secure cement penetration. (From Craig EV. Total
Shoulder Replacement for Primary Osteoarthritis and Osteonecrosis. In:
Craig EV, ed.The Shoulder. Master Techniques in Orthopaedic Surgery. Philadelphia: Lippincott Williams & Wilkins, 1997:29, with permission.)![]() Figure 101.33.
Figure 101.33.
The glenoid slot must be made along the exact axis of the glenoid in
available cancellous bone. With excessive asymmetric glenoid wear,
there is danger of cortical perforation if the drill bit is oriented
perpendicular to the exposed glenoid rather than perpendicular to the
glenoid neck. -
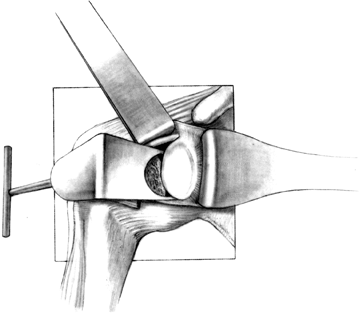
With the initial drill hole and
superficial slot made, determine the orientation of the glenoid neck
with a narrow curet. The arm must often be rotated internally or
externally for optimal glenoid exposure. When this slot has been
prepared, and the proper orientation of the glenoid neck has been
found, undermine the slot superiorly into the cancellous portion of the
base of the coracoid and inferiorly into the inferior glenoid neck (Fig. 101.34). A glenoid guide may be used for this part of the procedure (Fig. 101.35).
Remove the remaining cartilage and subchondral bone with your
preference of a burr or a reamer. The implant must be supported by
subchondral bone.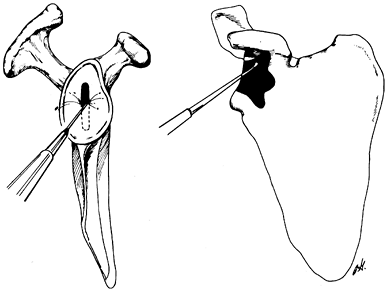 Figure 101.34.
Figure 101.34.
In preparing the glenoid slot, undermine superiorly into the base of
the coracoid and inferiorly into the cancellous portion of the glenoid
neck.![]() Figure 101.35.
Figure 101.35.
Use the glenoid contouring device to prepare the exposed glenoid face
to match precisely the posterior surface of the glenoid prosthesis. The
keel attachment to the contouring device ensures contouring of only
that amount of glenoid resurfaced by the prosthesis. The contouring
device moves back and forth along the exposed extension of the
stem-keel instrument. (From Craig EV. Total Shoulder Replacement for
Primary Osteoarthritis and Osteonecrosis. In: Craig EV, ed.The Shoulder. Master Techniques in Orthopaedic Surgery. Philadelphia: Lippincott Williams & Wilkins, 1997:31, with permission.) -
If, during the preparation of the keel,
the posterior or anterior cortex is perforated, pack the defect with
cancellous bone before final glenoid insertion. Then use a burr to
smooth ridges on the glenoid and ensure solid seating of the prosthesis
on the glenoid. Inadequate removal of the peripheral synovium is a
common error, particularly at the anterior inferior and posterior
inferior aspects of the glenoid. -
Drill several holes in the remaining subchondral bone for bone cement penetration, and implant the trial glenoid (Fig. 101.36, Fig. 101.37 and Fig. 101.38).
Eliminate any rocking. Several pitfalls exist in seating the trial
glenoid that may result in prominence, rocking, or poor fit of the
glenoid component (Table 101.1).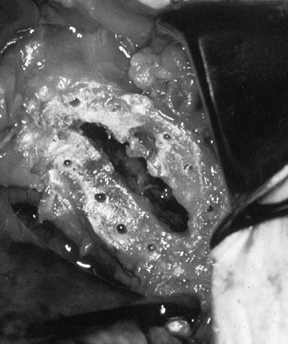 Figure 101.36. Make multiple small drill holes in the subchondral bone to help anchor the cement.
Figure 101.36. Make multiple small drill holes in the subchondral bone to help anchor the cement.![]() Figure 101.37.
Figure 101.37.
The trial glenoid is about to be inserted. The trial components for the
standard and metal-backed glenoids are of different colors.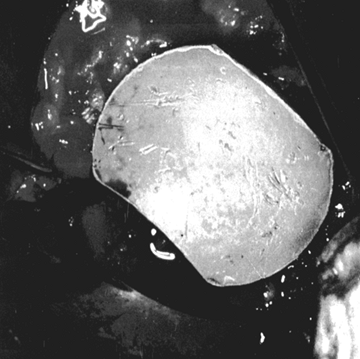 Figure 101.38.
Figure 101.38.
The inserted trial glenoid. This must be seated securely on subchondral
bone. There must not be any anterior and posterior rocking.![]() Table 101.1. Pitfalls in Seating the Glenoid Component
Table 101.1. Pitfalls in Seating the Glenoid Component -
As with other arthroplasties that use
bone cement to anchor components, cement technique is critical.
Irrigate the wound, using the water pick with antibiotic solution to
rid the glenoid of clot and fibrinous debris. Before cementing the
glenoid component, remove all blood and bone fragments from the depths
of the glenoid slot. To ensure dry bone surfaces, dry the slot with
surgical sponges, and use hydrogen peroxide irrigation,
P.2648
staggered cement insertion if necessary, or even hypotensive anesthesia if a dry field cannot be obtained. -
Use a syringe to ensure penetration of
cement into the depths of the prepared glenoid. Insert the glenoid
component by hand and hold it until the cement hardens. Remove excess
cement, particularly from the posterior recess. Anterior cement may
bind the subscapularis and impede tendon closure. Be aware that the
cement often adheres to the ring retractor and may make removal
difficult. -
Once the glenoid prosthesis has been
inserted and cement polymerization has occurred, check the prosthesis
for security of fixation (Fig. 101.39). Once
the glenoid is cemented and the ring retractor is removed, be careful
when delivering the proximal humerus into the wound that the greater
tuberosity does not lever against the newly cemented glenoid. It is
helpful to place a bone hook in the neck of the humerus, pulling
laterally while the arm is gently rotated externally (Fig. 101.40).
Ensure adequate clearance of the greater tuberosity by palpation. Check
the joint for loose fragments of methacrylate as well as any fragments
that may protrude beyond the edge of the glenoid component. Remove them
carefully with an osteotome or rongeur.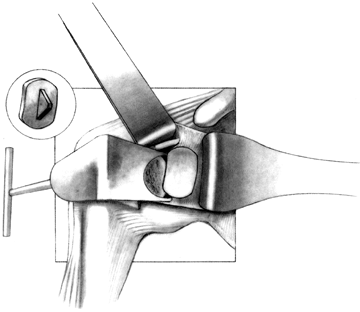 Figure 101.39.
Figure 101.39.
Cement a standard polyethylene glenoid component into place. (From
Craig EV. Total Shoulder Replacement for Primary Osteoarthritis and
Osteonecrosis. In: Craig EV, ed.The Shoulder. Master Techniques in Orthopaedic Surgery. Philadelphia: Lippincott Williams & Wilkins, 1997:45, with permission.)![]() Figure 101.40.
Figure 101.40.
With the glenoid implantation completed, expose the humeral osteotomy
site. Lateral traction with a bone hook during the external rotation
diminishes the likelihood that the greater tuber- osity will impinge
and lever on the posterior glenoid component. (From Craig EV. Total
Shoulder Replacement for Primary Osteoarthritis and Osteonecrosis. In:
Craig EV, ed.The Shoulder. Master Techniques in Orthopaedic Surgery. Philadelphia: Lippincott Williams & Wilkins, 1997:34, with permission.) -
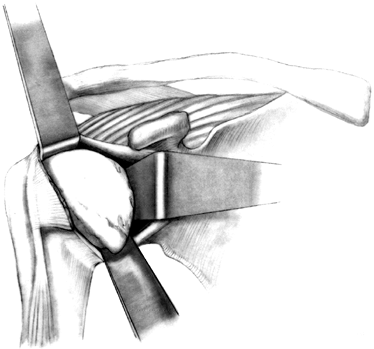
Soft-tissue balancing is critical for
motion, stability, and prosthetic longevity. Before inserting the
humeral head, make a final check to ensure that the soft tissue is
completely mobilized, especially if the rotator cuff has been torn and
retracted. The biceps tendon has a tendency
P.2649
to
get caught beneath the humeral head as the component is being inserted,
so gently retract it. Check the trial humeral head once again to make
sure that the orientation is correct. The humeral component should face
directly toward the glenoid with the arm in neutral rotation (Fig. 101.41).
The top of the humeral head should be superior to the top of the
greater tuberosity, to prevent impingement of the greater tuberosity.
The fin of the prosthesis should be just lateral to the bicipital
groove (Fig. 101.42).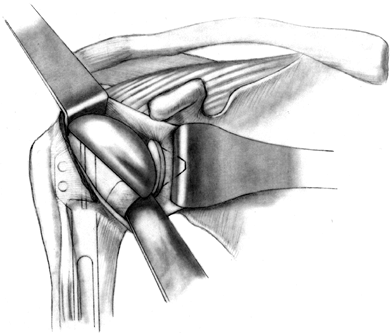 Figure 101.41.
Figure 101.41.
Reduce the humeral head, and test motion and stability. (From Craig EV.
Total Shoulder Replacement for Primary Osteoarthritis and
Osteonecrosis. In: Craig EV, ed.The Shoulder. Master Techniques in Orthopaedic Surgery. Philadelphia: Lippincott Williams & Wilkins, 1997:38, with permission.)![]() Figure 101.42.
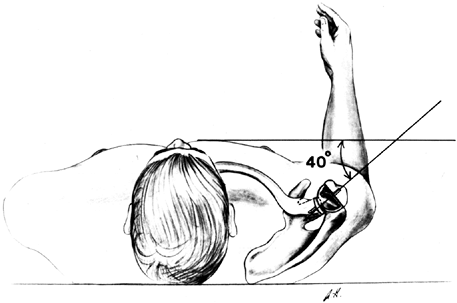
Figure 101.42.
Insert the prosthesis in 40° of retroversion from the transverse axis
of the elbow. With proper retroversion, the fin of the prosthesis is
usually lateral to the bicipital groove, and with the arm in neutral
rotation, the center of the humeral component articulates directly with
the glenoid. -
Check the height of the humeral component
so that soft tissues can be closed. There are potential problems
associated with leaving the humeral prosthesis too proud. It may not
articulate properly with the glenoid, it may abut the undersurface of
the acromion, or closure of the subscapularis tendon may not be
possible. The dangers of seating the prosthesis too low are that the
prominent greater tuberosity may impinge, the prosthesis may be
unstable, or the myofascial sleeve may have inadequate deltoid tension
to allow maximum power postoperatively. Check this with the humerus
reduced, putting traction on the arm in a longitudinal direction. The
prosthesis should not be seated so deeply that the humerus subluxates
inferiorly with traction. -
Whether to cement the humeral component is a matter of surgical judgment (17).
If the humeral component has a secure press fit, and the intact
tuberosities prevent rotation, then the component may be used without
cement. This is particularly appealing in young patients, in whom the
avoidance of methylmethacrylate may be preferred. In addition, if
glenoid revision becomes necessary, the uncemented humeral component
can be an advantage when removing the prosthesis. The humeral component
should be cemented in older patients, in patients with rheumatoid
arthritis, in osteoporotic bone, or if doubt exists about rotational
stability. If cementing, irrigate the canal and dry with a sponge.
Place a sponge temporarily in the glenoid to prevent excess
methacrylate from extruding into the glenoid as the humeral component
is inserted. A bone plug may be used to contain the cement in the
humeral canal. -
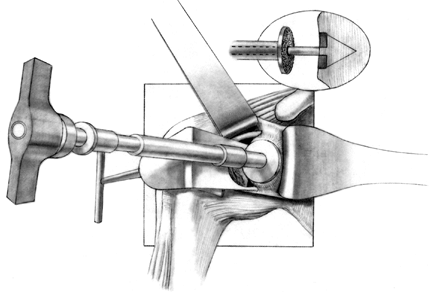
Component orientation may be difficult to
maintain during insertion of the humeral head. Inadvertent malrotation
may be prevented by temporarily placing a small, thin curet or
Steinmann pin in a hole of the fin of the prosthesis to ensure the
exact amount of retroversion until seating of the humeral component is
near completion (when using systems in which an inserter that maintains
precise version is not available) (Fig. 101.43).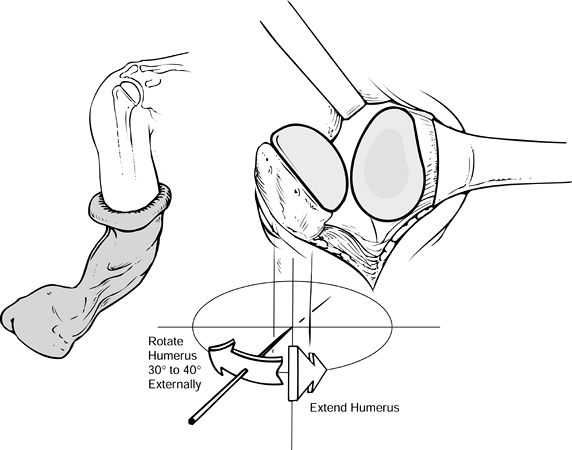 Figure 101.43.
Figure 101.43.
When inserting the humeral component, maintain proper orientation, with
the arm extended and externally rotated the desired amount (usually
40°). -
Reduce the humeral head and bring the
subscapularis to its point of division for closure. Test the shoulder
for stability in forward elevation, external rotation, and internal
rotation. It is particularly important after rotator cuff closure, and
before wound closure, to bring the arm into external rotation and
forward elevation. Thus, the exact ranges of motion sought in therapy
can be observed directly, and any tension on the suture lines can be
identified. Soft-tissue contracture must be corrected at surgery. If
motion is not obtained intraoperatively, postoperative rehabilitation
is not likely to restore it. -
Close the subscapularis with a nonabsorbable suture (Fig. 101.44).
In some instances, if there is severe contraction, once the joint has
been resurfaced it may be difficult to reapproximate the subscapularis.
Subscapularis release will often allow direct repair; however, in
certain cases, subscapularis tendon lengthening may be performed, as
described earlier in this chapter.![]() Figure 101.44. Suture the only detached muscle, the subscapularis, to the site of its detachment on the humerus.
Figure 101.44. Suture the only detached muscle, the subscapularis, to the site of its detachment on the humerus. -
Place a Hemovac drain between the deltoid and the rotator cuff, and bring it out through a site separate from the skin incision.
-
Close the deltopectoral interval with an
absorbable suture, and close the wound in layers. Apply a sterile
dressing and support the shoulder with a postoperative sling and wrap,
with the arm in neutral rotation (Fig. 101.45).
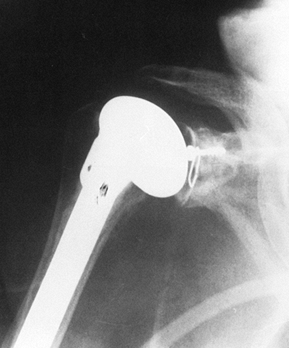
P.2651P.2652
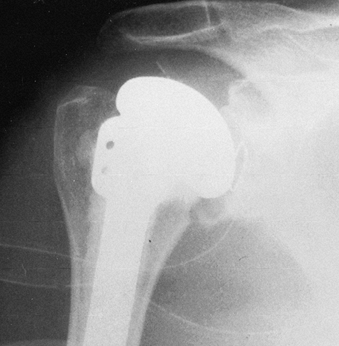
Take final radiographs to document the position of the prosthesis (Fig. 101.46).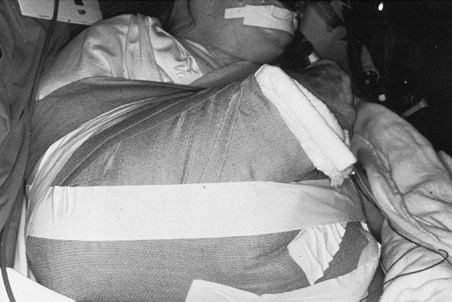 Figure 101.45. Place the arm in a sling and elastic bandage, with the arm in neutral rotation.
Figure 101.45. Place the arm in a sling and elastic bandage, with the arm in neutral rotation.![]() Figure 101.46. Radiographic appearance of the total shoulder arthroplasty.
Figure 101.46. Radiographic appearance of the total shoulder arthroplasty.
depending on the conditions encountered intraoperatively. For example,
in some patients with rheumatoid arthritis or severe cuff tear
arthropathy, an abduction brace may be necessary if the quality of the
soft tissue is poor. Patients who have had fixed posterior dislocation
and have excessive posterior capsular laxity may need to be kept in an
external rotation brace to allow the soft tissues to tighten.
total shoulder replacement arthroplasty, each case must be
individualized, both for intraoperative technique and postoperative
rehabilitation. The subsequent sections deal with features unique to
each diagnostic category of the disease process.
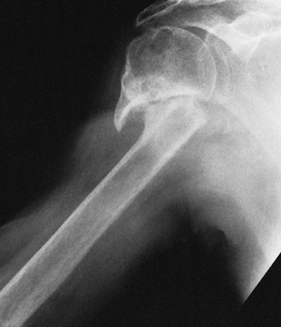
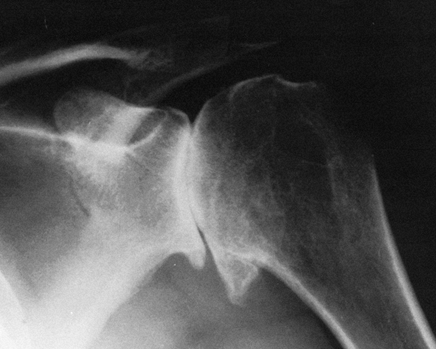
radiographically by the large circumferential osteophyte (seen on the
AP radiograph as an inferior protrusion), the sclerosis on both sides
of the joint, and subchondral cyst formation in the humeral head. The
axillary view often reveals asymmetrical glenoid wear (erosion
posteriorly) with apparent posterior subluxation. It is thus critical
to evaluate the shoulder before surgery with at least AP and axillary
views (Fig. 101.47, Fig. 101.48 and Fig. 101.49). A CT scan may be useful in assessing the amount of asymmetric glenoid wear.
 |
|
Figure 101.47.
Primary osteoarthritis of the shoulder. A large inferior osteophyte, subchondral cyst formation, and sclerosis on both sides of the joint. |
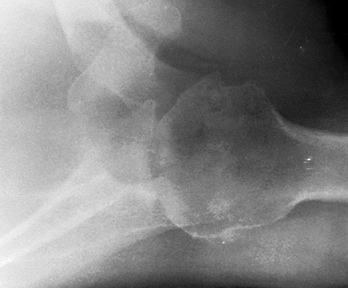 |
|
Figure 101.48. Primary osteoarthritis. An axillary view reveals the extent of joint space narrowing.
|
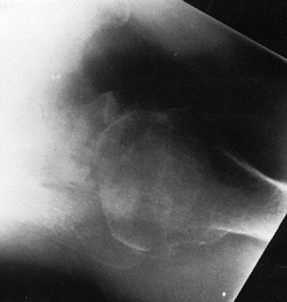 |
|
Figure 101.49.
Primary osteoarthritis. An axillary view reveals asymmetric glenoid wear, as the posterior glenoid is worn more deeply than the anterior glenoid, and showing apparent posterior subluxation of the humeral head typical of this disease. |
candidate for total shoulder replacement because the rotator cuff is
almost always normal (12,37).
However, several features of primary osteoarthritis may make
replacement of the shoulder technically difficult. Loose bodies occur
frequently and must be carefully sought intraoperatively. The inferior
osteophyte may be quite large and overhang the proximal metaphysis (15).
As previously cautioned, during division of the subscapularis tendon,
take extreme care to protect the axillary nerve, which courses close to
the
subscapularis and capsule and is in jeopardy when trimming the
osteophyte. The “macro head” with the large circumferential osteophyte
can give the impression that a large amount of articular surface must
be resected. The unwary surgeon may remove excessive bone while
osteotomizing the head. To guard against this, it is usually helpful to
trim the obvious osteophytic excrescences first. The remaining humeral
head will more accurately reflect exactly how much bone must be
removed. Remove only the area of humeral head normally occupied by
articular cartilage. This makes it appealing to use a trial implant to
mark the area of osteotomy before beginning head resection.
facilitated by global capsulotomy or capsulectomy. Preparing the
glenoid in osteoarthritis may be technically difficult because of
uneven glenoid wear. Perforation of the cortex may occur while
preparing the glenoid slot if this factor is not considered. Determine
the correct orientation of the slot by palpation of the neck of the
glenoid or by use of a straight blunt instrument along the anterior
glenoid neck. If uneven wear exists, the implant may not rest properly
on the subchondral bone, and further reconstruction of the glenoid
should be considered. This is usually accomplished by reaming or
trimming the glenoid with a burr or rongeur to allow secure prosthetic
seating. Although the excessively worn side can be built up with
cement, this is unwise, because the excess cement mantle may crack and
loosen. If the wear is more severe, bone grafting of the excessively
worn side can be accomplished using the humeral head (Fig. 101.50), although this is rarely required (40).
 |
|
Figure 101.50. Total shoulder replacement with glenoid bone grafting for asymmetric glenoid wear. The graft is fixed with a navicular screw.
|
disease of the acromioclavicular joint or an associated overhanging
anterior acromion. If so, an acromioclavicular joint resection or
anterior acromioplasty may be required. It is uncommon to have a
rotator cuff tear with osteoarthritis, but if this does exist, repair
it at the time of the arthroplasty.
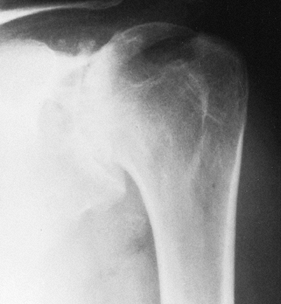
from the severe, unpredictable destruction of both bone and soft
tissue, which is the hallmark of the disease (Fig. 101.51, Fig. 101.52, Fig. 101.53 and Fig. 101.54). Severe medial and superior wear of the
glenoid, cuff defects of varying sizes, poor soft-tissue quality,
associated acromioclavicular joint disease, osteoporosis, and severe
bone loss are among the factors that present technical difficulties (29,36,52).
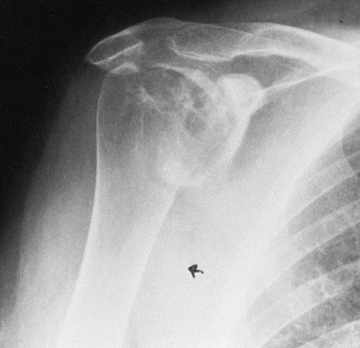 |
|
Figure 101.51.
Rheumatoid arthritis of the shoulder. Superior migration of the humeral head, cyst formation, marginal erosion, and severe joint destruction. |
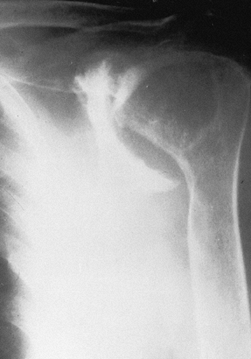 |
|
Figure 101.52. Rheumatoid arthritis with severe superior and medial destruction of the glenoid.
|
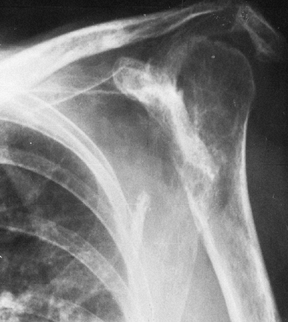 |
|
Figure 101.53.
In this patient with rheumatoid arthritis, there is a fracture of the acromion from superior migration, wear into the acromioclavicular joint, and severe medial migration of the humerus with glenoid bone loss. |
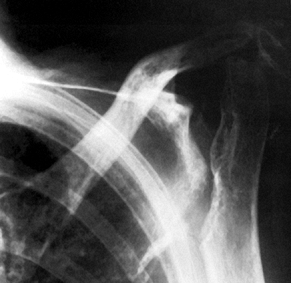 |
|
Figure 101.54.
Rheumatoid arthritis with severe bone loss in the proximal humerus. The amount of glenoid bone loss precluded insertion of a glenoid component, and a proximal humeral component alone was used. |
-
Evaluate the acromioclavicular joint
preoperatively. If radiographic acromioclavicular arthritis exists, if
there is tenderness of the acromioclavicular joint, or if there is bone
loss from the distal clavicle, excise the distal clavicle. -
Because there is usually dramatic
subacromial bursal disease, carefully excise the bursa. The bursa in
rheumatoid arthritis may be confused with the rotator cuff tendons, so
the limits must be defined during excision. Extensive synovectomy is
almost always needed for exposure. -
In addition to joint involvement,
rheumatoid arthritis myopathy may weaken the normal humeral
head–depressor effect of the rotator cuff, causing the humeral head to
ride upward, even in the absence of a cuff tear. This can sometimes
cause secondary impingement and may require an acromioplasty. Myopathy
may also limit postoperative strength, despite satisfactory passive
range of motion. -
Superior head migration may occur from
rotator cuff weakness, a feature often accentuated by the use of
crutches or a cane. In this situation, leave the coracoacromial
ligament intact to prevent anterosuperior head migration. -
The rotator cuff is torn in about 30% of
rheumatoid arthritis patients. Such tears are usually not massive and
should be repaired at the time of surgery, with direct suture into bone
by the usual methods of cuff closure. More often, the rotator cuff is
thin and of poor quality. -
Severe osteopenia can weaken bone and
result in humerus fracture during initial head dislocation, and
tuberosity fracture at the time of humeral head osteotomy or during
retraction of the humerus for glenoid insertion. The glenoid can also
be fractured if the retractor is used too forcibly. -
In rheumatoid arthritis, always cement
the humeral component because the severe osteopenia will often not
permit a secure press fit. -
The glenoid may be severely eroded deep into the neck of the scapula. To assess this, a preoperative CT scan is helpful.
-
If the glenoid bone loss is extensive,
and bone stock does not allow glenoid component insertion, the humeral
head may be used alone as a hemiarthroplasty. Postoperative
rehabilitation often requires modification in the rheumatoid patient
because of contralateral arm, ipsilateral elbow, or wrist and hand
disease.
With a nonconstrained arthroplasty, the head may ride up, impact the
superior rim of the glenoid, and asymmetrically load the glenoid. There
is concern that this may lead to stresses on the component and
potential implant failure (16). For this
reason, some authors have argued that if the rotator cuff cannot be
reconstructed with functioning tissue, consideration should be given to
a humeral head prosthesis alone without a glenoid component (16). The ideal treatment for this most difficult group of patients remains uncertain.
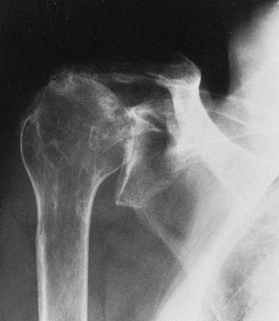
severe destructive glenohumeral arthritis; superior migration of the
humeral head because of a nonfunctioning cuff; severe superior and
medial wear into the glenoid, the coracoid, the acromioclavicular
joint, and the acromion; rounding of the greater tuberosity from
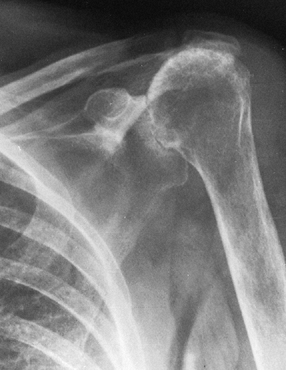
mechanical impingement; and variable collapse of the humeral head (Fig. 101.55) (39).
There may be an unfused acromial epiphysis. Cuff tear arthropathy
combines the difficulty of excessive and longstanding major soft-tissue
defect with severe glenohumeral destruction. MRI is useful to evaluate
muscle wasting and fatty infiltration of the cuff musculature. For most
massive defects, if there is fatty infiltration of the cuff muscles,
the restoration of cuff continuity may not be associated with increased
muscle power. However,
the
static superior tenodesis effect may contribute to increased active
range of motion or function. Also take patient age and function into
account in the decision-making process. The following are technical
modifications for cuff tear arthropathy:
 |
|
Figure 101.55.
Cuff tear arthropathy of the glenohumeral joint. There is loss of the acromiohumeral interval; deep wear into the acromion, acromioclavicular joint, and glenoid; and rounding of the greater tuberosity from mechanical impingement wear. This is an extremely difficult reconstruction because of the extensive bone and soft-tissue loss. |
-
Preserve the coracoacromial ligament to prevent anterosuperior head migration (14).
-
Do not perform glenoid replacement for
end-stage cuff tear arthropathy, because if the cuff is unable to
center the head, a “rocking horse” effect may occur, leading to
loosening (16). -
Good results have been reported with hemiarthroplasty for arthrosis associated with massive cuff tears (1,53).
massive tear of the rotator cuff. With attention to meticulous surgical
planning and details, with patience, and with knowledge of alternative
means for coverage, the rotator cuff may be reconstructed around the
unconstrained implant (14). This reconstruction is technically demanding. The following technical steps can help provide soft-tissue coverage:
-
Before surgery, prepare the thigh for possible use of a fascia lata graft.
-
Position the arm near the side of the
table so that hyperextension and internal rotation will expose the
infraspinatus and teres minor, which have retracted posteriorly.
External rotation and slight abduction will expose the subscapularis
anteriorly. -
Before excising the subacromial bursa on
entering the deltopectoral interval, use a blunt elevator to clear the
soft tissue from the undersurface of the acromion to free the adherent
rotator cuff. -
Placing sutures in the rotator cuff
(rather than clamps) will enable traction to be placed on the cuff for
mobilization, while preserving tissue integrity, and will permit
tension on the sutures to assess tendon mobility. -
Before cementing the humeral component
into place, place drill holes in the greater and lesser tuberosities
and pass sutures through them if cuff reconstruction is planned. -
If a larger head is used for stability,
rotator cuff closure may be difficult. Thus, a smaller head may be a
better choice in patients with cuff tears because available soft tissue
will be more easily approximated to the greater tuberosity.
tendon, which retracts and is scarred to the base of the coracoid and
undersurface of the acromion. The tear then extends to the
infraspinatus and teres minor. The tendon of the infraspinatus is
pulled inferiorly by the teres minor and is adherent to the posterior
inferior humeral head. The following sequential steps in soft-tissue
mobilization for later implant coverage are necessary:
-
Release the multiple adhesions between
the rotator cuff, bursa, and deltoid bluntly by finding the plane
between the bursa and the cuff posteriorly, and by rotating the humerus
as adhesions are bluntly or sharply divided. -
Perform the humeral head osteotomy first, after which cuff mobilization is easier (Fig. 101.56).
![]() Figure 101.56. In cuff tear arthropathy, mobilize the rotator cuff after humeral head osteotomy and before glenoid component insertion.
Figure 101.56. In cuff tear arthropathy, mobilize the rotator cuff after humeral head osteotomy and before glenoid component insertion. -
Retrieve the infraspinatus tendon (which
has been pulled inferiorly by a portion of intact teres minor) by
pulling cephalad as adhesions are freed. -
The supraspinatus tendon usually retracts
posteriorly, but a portion, pulled by intact subscapularis, may adhere
to the base of the coracoid process. Retrieve this by bluntly freeing
it from the base of the coracoid process. Although you can safely
incise along the lateral edge of the coracoid process to free or
retrieve scarred tendon, dissection medial to the coracoid process
jeopardizes both the musculocutaneous and suprascapular nerves (Fig. 101.57).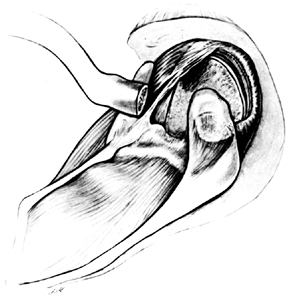 Figure 101.57.
Figure 101.57.
Cuff tear arthropathy. Early distal clavicle excision aids in exposure
of the retracted supraspinatus, which is often adherent to both the
base of the coracoid and the undersurface of the acromion. The torn
infraspinatus is pulled inferiorly by tension from some intact teres
minor fibers. -
The subscapularis is often bound by dense
adhesions to the inferior base of the coracoid. Release these adhesions
to increase subscapularis mobility. -
The subscapularis can be transferred to
make up for an extensive superior defect. Identify the interval between
the subscapularis and the capsule. Separate the subscapularis from this
underlying capsule. Leave the inferior portion of the subscapularis
intact to help act as a humeral head depressor and rotate the superior
four fifths of the subscapularis superiorly for coverage or suture it
into the biceps tendon (Fig. 101.58).![]() Figure 101.58.
Figure 101.58.
With severe cuff deficiency, an intact biceps may be moved posteriorly
to help provide superior or posterior soft-tissue coverage, and it can
be used as a stent into which the mobilized spinatus tendons can be
sutured. The upper four fifths of the subscapularis can be moved
superiorly for added superior coverage. -
Mobilize the teres minor and
infraspinatus tendons from within the joint by making a posterior
capsular incision close to the glenoid. This may enable these two
tendons, retracted and scarred to the posterior capsule, to be
retrieved. -
Dissect the conjoined tendon of the short
head of biceps and coracobrachialis from the coracoid process. Flexing
the elbow may provide some coverage of the gap in the supraspinatus
tendon, although this will not reach to the supraglenoid tubercle.
During this mobilization, avoid traction on the musculocutaneous nerve.
Usually, little additional coverage is obtained by this maneuver. -
With severe posterior and inferior
retraction of the cuff, it may adhere to the posterior glenoid neck,
making retrieval extremely difficult. Occasionally, a separate
posterior incision may be needed to help mobilize the cuff from this
retracted position. -
If there is insufficient tendon to
provide coverage after complete mobilization, consider fascia lata
grafting, freeze-dried cadaver grafting (such as Achilles tendon
allograft), or other methods of additional coverage for large tendon
deficits.
setting, a posterior shoulder dislocation may lead to the loss of a
significant portion of the articular surface. If the involvement is
greater than 40%, consider hemiarthroplasty, depending on whether the
patient is a suitable candidate. Gerber and Lambert (18) described the use of osteoarticular allografts for this condition with a satisfactory outcome.
This can be recognized radiographically by osteophyte formation on the
humeral head and the presence of a Hill-Sachs lesion on axillary view.
There may also be anteroinferior wear of the glenoid from recurrent
anterior translation.
The use of screws and staples about the shoulder has decreased, which
has lessened the incidence of this severe complication (42).
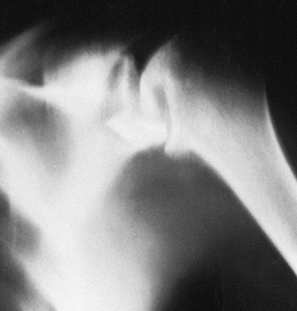 |
|
Figure 101.59.
Tomogram of the glenohumeral joint in a patient who had a surgical procedure for recurrent dislocation. A staple has penetrated the glenohumeral joint and destroyed the humeral head. |
These patients occasionally require total shoulder arthroplasty. If a
shoulder replacement is planned for a patient with this condition,
prepare for a subscapularis release and lengthening (Fig. 101.60, Fig. 101.61), as described earlier in this chapter.
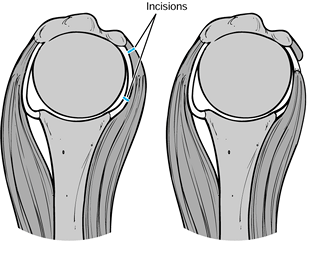 |
|
Figure 101.60.
Subscapularis capsular lengthening. Incise the subscapularis 1.5 cm from its insertion, identify the interval between subscapularis and capsule, and incise the capsule near the glenoid. Then suture the subscapularis to the lateral capsular flap, effectively adding length to the subscapularis. |
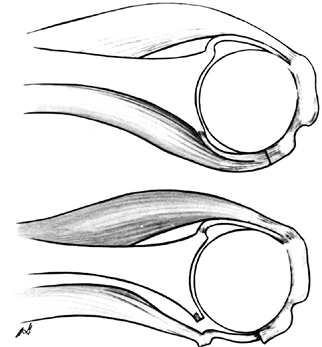 |
|
Figure 101.61.
The reverse undercutting method. If the subscapularis and capsule have been incised together at the beginning of the procedure, divide the capsule near the glenoid attachment at the conclusion of the arthroplasty, and cut the capsular–subscapularis interval in a reverse manner toward the lesser tuberosity insertion. Then bring the free end of the capsule outward and suture it to the original site of the subscapularis division, effectively lengthening the subscapularis and permitting more external rotation. |
arthroplasty. Malunion or nonunion of tuberosities, nerve injuries, and
shortening of the subscapularis muscle commonly occur. The humeral head
has often collapsed and healed in excessive retroversion, anteversion,
varus, or valgus relative to the shaft and tuberosities. Prior internal
fixation may have to be moved. Identification of the position of the
tuberosities relative to the head may be extremely difficult and should
be determined preoperatively, either by plain radiograph or CT scan
with or without three-dimensional reconstruction. If the relative
position of the tuberosities to the shaft is not severely distorted,
maintaining the greater tuberosity and attached rotator cuff in
continuity with the shaft makes rehabilitation less complicated. If
there is severe displacement or malunion, however, the tuberosities
must be osteotomized and repositioned. This adds the problems of
fixation and union to the other technical challenges.
deltoid insertion subperiosteally, the pectoralis major tendon, and the
conjoint tendon, in that order, as required. Consider direct
visualization of the axillary and musculocutaneous nerves if previous
trauma or prior surgery has distorted the anatomy sufficiently to make
identification of these nerves uncertain.
(head-splitting or large impression fractures), the extent of cartilage
destruction may make preservation of the humeral head impractical (21).
In four-part displaced fractures, the degree of displacement has almost
certainly disrupted blood supply to the remaining shell of the humeral
head because of tenuous soft-tissue attachments. Considering that later
total shoulder replacement for posttraumatic arthrosis is difficult and
unpredictable, because of distorted anatomy, malunited tuberosities,
nerve injury, and bone loss, primary arthroplasty is recommended to
treat the acute fracture (21). This single
operation is usually more successful than a failed open reduction and
internal fixation followed by arthroplasty. The technique for proximal
humeral replacement alone in acute fractures follows.
-
While the patient is being prepared in
the operating room, avoid excessive abduction because neurovascular
injury can occur from the sharp, bony fragments. -
Make a long deltopectoral incision from
the clavicle to the lateral deltoid insertion and develop the
deltopectoral interval. The clavipectoral fascia is often disrupted,
but if it is not, incise it to the level of the coracoacromial
ligament. Retract the coracoid muscles and pectoralis medially, and the
deltoid laterally. -
Locate the biceps tendon; it is a
reliable guide to location and identification of the tuberosities. It
also provides a guide for the amount of soft-tissue tension after
insertion of the prosthesis. -
Develop the interval between the
supraspinatus tendon and the subscapularis by dissecting the biceps
tendon toward its origin. Then remove the fragment or fragments of
humeral head and evacuate the hematoma. With anterior
fracture–dislocations, the humeral head may be in close proximity to
the axillary artery or brachial plexus. Consider an angiogram in
elderly patients if there are signs of vascular compromise. Carefully
use a tap from the large fragment set as a “cork-screw” to assist in
removal of the head if it is difficult to access. Prepare the medullary
canal as in standard total shoulder replacement. -
Because rotational stability is usually
lost once the tuberosities have fractured, cement the humeral
component, reattaching the tuberosities to the prosthetic fin. Before
cementing the prosthesis in place, drill holes in the humeral shaft and
the tuberosities, and pass #5 nonabsorbable sutures for later
reattachment of the tuberosities. Do not excise the tuberosities; bone
healing is essential to ensure continuity of the attached tendons. -
Cement the prosthesis in 30° to 40° of
retroversion. The prosthesis must be placed proud enough that
tuberosities can be sutured around the fin of the implant, and tension
on the soft-tissue myofascial sleeve is preserved. This usually amounts
to approximately 1–2 cm, although the distance from the humeral shaft
should be assessed in each individual case. Seating the prosthesis too
low on the humeral shaft will make the soft tissues slack, resulting in
a weakened deltoid and an unstable prosthesis. In addition, tuberosity
prominence can result in mechanical impingement. A slack biceps at the
time of the humeral trial is often an indication that the prosthesis
has been seated too deeply into the humerus. Reattach the tuberosities
with #5 nonabsorbable suture. Secure the tuberosities to one another,
to the fin of the implant, and to the humeral shaft segment itself.
P.2660
While the tuberosities are being sutured together, hold them securely to the fin with a towel clip. -
If there is severe comminution or bone
loss, consider adding bone graft at this time. Close the interval
between the supraspinatus tendon and the subscapularis with
nonabsorbable suture, copiously irrigate the wound, and place suction
drains deep to the deltoid muscle. Then reapproximate the deltopectoral
interval.
rehabilitation after inserting a prosthesis with intact rotator cuff
and bone. The tuberosities must heal before active muscle contraction
is begun to avoid displacement. Nevertheless, begin passive or
assistive exercises in 5 or 6 days. Delay assistive external rotation
for 2 weeks to allow some healing of the tuberosities.
arthroplasty. Injuries to the brachial plexus and the vascular
structures are of particular concern. The reported incidence of
neurologic complications following total shoulder replacement in the
largest reported series was 4% (34). However, of these, only 1% had deficits that interfered with rehabilitation, and most neurologic deficits resolved.
As is the case with hip and knee arthroplasty, increased infection
rates are seen in patients with host-related risk factors such as
diabetes mellitus, rheumatoid arthritis revision surgery, and previous
infection (36,54).
occur during manipulation of the limb, reaming of the intramedullary
canal, broaching of the canal, and insertion of the prosthesis (54). Treat intraoperative humeral fractures with cerclage wire and a long-stem prosthesis (19)
or dynamic compression plating. Intraoperative fracture of the glenoid
is less common. Treatment of this complication involves the use of bone
graft or a revision implant with a wedge to accommodate the defect (54).
One study of nine periprosthetic fractures suggested that long oblique
and spiral fractures be treated nonoperatively, whereas transverse and
short oblique fractures be treated with surgery (55).
Instability can be anterior, posterior, inferior, or superior. Anterior
instability is most commonly due to subscapularis rupture. Less
commonly, this pattern of instability can be due to increased
anteversion of the humeral or glenoid components (41).
Subscapularis tendon rupture, if diagnosed early, can be repaired
directly. Late diagnosis often leads to an irreparable situation,
requiring pectoralis major tendon transfer or Achilles tendon allograft
to reconstruct the anterior soft tissues.
instability. However, posterior glenoid wear, which is common in
degenerative arthrosis, may lead to a retroverted glenoid component if
this osseous deformity is not noted in the preoperative planning and
accounted for at the time of surgery. Soft-tissue imbalance can be
dealt with by lengthening the tight anterior structures, tightening the
loose posterior structures, revising a malpositioned glenoid or humeral
component, or using a larger humeral head implant (27,41).
is inserted too low, usually following reconstruction for a complex
proximal humeral fracture (41). The humeral
component should always be cemented proud on the humeral shaft in
hemiarthroplasty for four-part fracture. In addition, intraoperative
attention to soft-tissue tension, particularly of the biceps tendon,
can help avoid this problem.
shoulder instability to treat following an arthroplasty. It is usually
caused by a rotator cuff tear. Deficiency of the coracoacromial
ligament will worsen the problem and lead to anterosuperior migration
of the implant. Therefore, the coracoacromial ligament should be left
intact if an arthroplasty is performed in a patient whose cuff tear is
so large that the depressant activity of the rotator cuff is lost.
Also, the rotator cuff should be carefully reconstructed at the time of
arthroplasty if any deficiency exists (41).
component, which suggests loosening, has been shown to occur in 30% to
100% of cases (2,40,46,51). However, the majority of these implants are not clinically symptomatic (48).
Lucent lines around humeral components are less common than in the
glenoid; however, symptomatic loosening of humeral components is rare
in most series (2,40,51,54).
patient assessment. There has been an increase in the emphasis on
outcomes research as expenditures for health care have increased and
the justification for resource allocation has become paramount (30). Although detailed review of this topic is beyond the scope of this chapter, some important
concepts with respect to outcome evaluation should be mentioned.
results of surgery with assessments such as radiographs and physical
examination. These measures do not take into account the patients’
perspectives. There are many questionnaires available to measure
patient outcomes for individuals with disorders of the shoulder. These
instruments have been tested for reliability, validity, and
responsiveness to clinically significant change (3,4).
Scales that are specific to the shoulder and upper limb and that can
therefore be used for the assessment of patients before and after total
shoulder arthroplasty include the simple shoulder test (32), the shoulder rating questionnaire (31), and the DASH (an acronym for disabilities of the arm, shoulder, and hand) (25).
The latter tool is specific to the entire upper limb, allowing
comparisons of patients with various disorders, whereas the former
instruments are specific exclusively to the shoulder joint.
used to measure overall health. These instruments have a broader
perspective, including emotional, social, mental, and physical health,
and do not restrict attribution to a particular disorder. Generic
health status measures are valuable because they allow comparisons
across conditions and treatments. The disadvantage of these
questionnaires, however, is that they may not measure clinically
important change because an isolated problem may not be reflected in
this global measure (5,23). The specific measures are more responsive to change in the phenomenon of interest and may be more relevant (20,43).
questionnaires should be used to evaluate patients undergoing total
shoulder arthroplasty. Investigators should avoid nonstandardized
outcome assessment tools in the evaluation of patients who have
undergone total shoulder replacement, because this complicates
comparison across different groups (3,4).
Measures of impairment, such as diagnostic imaging and physical
examination, complement health-related quality-of-life instruments and
remain an important part of patient outcome assessment.
The unique character of the shoulder, where stability and function
depend on the surrounding soft tissues, makes it essential that the
postoperative focus be on the care of the soft tissues and in
particular on the rotator cuff.
goals of the surgeon and the patient, and the resolution of the
intraoperative variables. It can be broadly divided into early and late
phases. In the early period, the immediate aim is to maintain the
motion achieved in the operating room following bone and soft-tissue
reconstruction by preventing adhesions in both the subacromial and the
glenohumeral joint spaces. This requires protection of repaired or
reconstructed soft tissues.
rehabilitation. After intraoperatively assessing implant stability and
tension in repaired tissue, the surgeon makes the decisions concerning
the desired pace toward the short-term goal of improved range of
motion. Although passive range of motion can be established through
effective bone and soft-tissue surgery, the degree of active range of
motion depends critically on the muscle power of the rotator cuff and
deltoid muscles. While the early phase of rehabilitation concentrates
on establishing range of motion, it is important to add strengthening
exercises later for muscle rehabilitation. The timing and progress of
rehabilitation must be individualized.
rehabilitation program that is safe, effective, and, most important,
easy to understand, so the patient can reliably continue it after
discharge. In the first phase after surgery, range of motion is
established by a series of exercises aimed at restoring forward
elevation in the plane of the scapula, external rotation, and internal
rotation. The exercises are all patient-assisted, although more
recently Neer emphasized that passive motion can be an effective means
of establishing range of motion. A typical rehabilitation program is
performed five times daily for 15–20 minutes each session.
Rehabilitation after total shoulder arthroplasty is more intensive than
after hip or knee replacement, and it is the key to success.
gravity-assisted pendulum exercise by bending at the waist and making
circles with the operated arm. This is followed by assistive forward
elevation, standing and using an overhead pulley, with the unoperated
arm acting to raise and lower the operated arm. External rotation is
performed with the patient supine, the arm at the side, and the elbow
flexed to 90°, with the arm pushed into external rotation by a stick or
cane. Internal rotation is initiated by stretching both arms into
extension and cephalad toward the scapula.
motion comfortably in the early postoperative period, provided there is
adequate bone and soft-tissue reconstruction (11).
Patients who exercise with continuous passive motion achieve adequate
motion more rapidly and can be safely discharged earlier from the
hospital without compromising soft-tissue and bony repair. Whether
continuous passive motion itself is important or whether it acts merely
to deliver passive motion remains to be seen.
replacement to minimize postoperative adhesions. A mechanical
(continuous passive motion) device can be used,
a
physical therapist can be involved, or assistive exercises can be
performed by the patient. It is important that progress be monitored
closely, as many patients lack the confidence or understanding to carry
on with the program by themselves. Where stability of the implant or
the quality of the soft-tissue repair is such that early motion is
unsafe, modify the basic program just described.
exercises. It appears most important to concentrate on the anterior and
middle deltoid and the rotator cuff, especially the infraspinatus and
teres minor. Therapy for this area is often initiated as isometric
exercises, with the later addition of active resistive exercises. One
effective means of adding progressive resistive exercises is with an
elastic exercise band, a dental dam, or surgical tubing, a portable and
inexpensive method that may effectively be used at home.
continues for at least 1 year, with more resistive exercises added as
strength improves.
rehabilitation program for patients with osteoarthritis, in whom the
deltoid and rotator cuff are normal and the only muscle detached and
repaired is the subscapularis. In patients with a severe soft-tissue
deficit, such as cuff tear arthropathy, and in some patients with
rheumatoid arthritis, the program might be modified as shown in Table 101.3.
 |
|
Table 101.2. Total Shoulder Rehabilitation Program after Arthroplasty for the Osteoarthritic Patient
|
 |
|
Table 101.3. Modified Rehabilitation Program for Soft-Tissue Reconstruction
|
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
CT, Jackins S, Matsen FA II. Prosthetic Replacement of the Shoulder for
the Treatment of Defects in the Rotator Cuff and the Surface of the
Glenohumeral Joint [Published Erratum Appears in J Bone Joint Surg Am 1993;75:1112]. J Bone Joint Surg Am 1993;75:485.
DE, Hogg-Johnson S, Bombardier C. Evaluating Changes in Health Status:
Reliability and Responsiveness of Five Generic Health Status Measures
in Workers with Musculoskeletal Disorders. J Clin Epidemiol 1997;50:79.
C, Melfi CA, Paul J, et al. Comparison of a Generic and a
Disease-specific Measure of Pain and Physical Function after Knee
Replacement Surgery. Med Care 1995;33:AS131.
JL, Barrett WP, Jackins SE, Matsen FA II. Glenoid Loosening in Total
Shoulder Arthroplasty. Association with Rotator Cuff Deficiency. J Arthroplasty 1988;3:39.
C, Lambert SM. Allograft Reconstruction of Segmental Defects of the
Humeral Head for the Treatment of Chronic Locked Posterior Dislocation
of the Shoulder. J Bone Joint Surg Am 1996;78:376.
G, Melfi C, Paul J, et al. Comparison of a Generic (SF-36) and a
Disease Specific (WOMAC) (Western Ontario and McMaster Universities
Osteoarthritis Index) Instrument in the Measurement of Outcomes after
Knee Replacement Surgery. J Rheumatol 1995;22:1193.
PL, Amadio PC, Bombardier C. Development of an Upper Extremity Outcome
Measure: The DASH (Disabilities of the Arm, Shoulder and Hand)
[Corrected]. The Upper Extremity Collaborative Group (UECG) [Published
Erratum Appears in Am J Ind Med 1996;30:372]. Am J Ind Med 1996;29:602.
JC, Warren RF, Cohen SB, et al. A Self-Administered Questionnaire for
Assessment of Symptoms and Function of the Shoulder [Comments]. J Bone Joint Surg Am 1997;79:738.
SM, Harryman DTI, Matsen FA I. A Practical Tool for Evaluation of
Function: The Simple Shoulder Test. In: Matsen FA I, Fu FH, eds.The Shoulder: A Balance of Mobility and Stability. Rosemont, IL: American Academy of Orthopaedic Surgery, 1993.
PB, Hawkins RJ, Fowler PJ, Miniaci A. Release of the Subscapularis for
Internal Rotation Contracture and Pain after Anterior Repair for
Recurrent Anterior Dislocation of the Shoulder. J Bone Joint Surg Am 1992;74:734.
SW, Evans DC. Long-term Results of Staple Capsulorrhaphy for Anterior
Instability of the Shoulder [Erratum Appears in J Bone Joint Surg Am 1993;75:791]. J Bone Joint Surg Am 1993;75:249.
JW, Cofield RH, Rowland CM. Neer Hemiarthroplasty and Neer Total
Shoulder Arthroplasty in Patients Fifty Years Old or Less. Long-term
Results. J Bone Joint Surg Am 1998;80:464.