Musculoskeletal Tissues and the Musculoskeletal System
Orthopaedics and the Musculoskeletal System > 1 – Musculoskeletal
Tissues and the Musculoskeletal System
tissues that form the musculoskeletal system: bone, cartilage, dense
fibrous tissue, and muscle. These tissues differ in vascularity,
innervation, mechanical and biological properties, and composition, but
they share a common origin from mesenchymal cells. Understanding of
diseases and injuries of the musculoskeletal system and their treatment
depends on knowledge of these tissues. Failure to consider the
biological and mechanical properties of the tissues, tissue changes
caused by disease or injury, or the responses of the tissues to
persistent changes in use can lead to misinterpretation of diagnostic
information, suboptimal treatment decisions, and undesirable
results
of treatment. Furthermore, future advances in the diagnosis and
treatment of musculoskeletal problems will depend on increased
knowledge of the cell and matrix biology of the musculoskeletal tissues.
diseases and injuries, this chapter reviews the structure and
composition of the musculoskeletal tissues and the organization of the
musculoskeletal system. The first section summarizes the distinctive
characteristics of connective tissues including mesenchymal cells and
the matrices they synthesize. The next sections review the structure,
composition, and properties of bone; periosteum; the dense fibrous
tissues (tendon, ligament, and joint capsule); tendon, ligament, and
joint capsule insertions into bone; articular cartilage; growth
cartilage; meniscus; synovium; muscle-tendon junction; and
intervertebral disc. Although skeletal muscle is not a connective
tissue, it is included in this chapter, because it forms a critical
part of the musculoskeletal system. The last section discusses the
formation and development of the musculoskeletal system.
histologists and pathologists led them to view connective tissue as a
continuous basic tissue, or connecting substance, that extended
throughout the body and assumed specialized forms including cartilage,
periosteum, bone, tendon, fibrous septa, and fascia in different
locations without altering the basic character of the tissue. The
definition of basic connective tissue structure proposed by Virchow,
“the greater part of the tissue is composed of intercellular substance,
in which, at certain intervals cells are embedded,” remains unchanged.
musculoskeletal system, but all tissues and organ systems of
multicellular organisms depend on connective tissue for mechanical
support. The parenchymal cells of liver, kidney, and brain could not
maintain the organization of these tissues or the tissue functions
without their structural connective tissue framework. Normal function
of the respiratory and cardiovascular systems depends on the repetitive
mechanical performance of the connective tissues that form the airways
and the blood vessels.
the supporting structure and joints of the musculoskeletal system
(bone, cartilage, dense fibrous tissue ligaments, tendons, and joint
capsules) have primarily mechanical functions. Because of their obvious
mechanical roles, and the prominence of their matrix component relative
to their cellular component, these tissues are often regarded as
homogeneous and inert. Yet, even in the mature skeleton they remain
metabolically active, the cells and matrices are degraded and replaced,
and the tissues respond to hormonal, metabolic, and mechanical stimuli.
functional classes of tissues appear: epithelium and mesenchyme. The
skeletal connective tissues originate from a subdivision of the
mesenchyme. Mesenchyme (Greek mesos, middle and enchyma, infusion)
refers to the location of mesenchyme between the epithelial layers of
endoderm and ectoderm. Epithelial tissues may develop from endoderm,
ectoderm, and mesoderm, but mesenchyme appears to develop only from
mesoderm.
relationship of the cells to the matrix distinguish epithelia from
mesenchyme. Epithelial cells form sheets or layers of cells. They
establish close relationships with adjacent cells, frequently binding
their membranes together with specialized cell junctions and devoting a
large portion of their membranes to contact with other cells.
Epithelial tissues generally have a sparse extracellular matrix, and a
specialized form of matrix, basement membrane, that frequently serves
as the bed for epithelial cells and separates them from mesenchymal
tissue. Mesenchymal cells do not generally form sheets or layers. In
the mesenchyme forming the skeletal connective tissues, cells rarely
establish extensive contact with other cells and they surround
themselves with an abundant extracellular matrix consisting of a
macromolecular framework synthesized by the cells and water that fills
the macromolecular framework. The cell membranes bind to specific
macromolecules within the matrix, and although the cells may appear
fixed in place by the surrounding matrix, they can migrate through the
matrix.
through the tissue, they have the potential to divide rapidly and
differentiate into specialized musculoskeletal tissue cells including
the cells of
cartilage,
bone, dense fibrous tissues, and muscle. Systemic factors including
nutrition and hormonal balance combined with local factors in the cell
environment such as the composition of the matrix, concentrations of
oxygen, cytokines and nutrients, pH, and mechanical forces influence
mesenchymal cell proliferation and differentiation. These systemic and
local factors interact with the genomic potential of the cell to
determine the progression from undifferentiated stem cells to highly
differentiated cells like chondrocytes and osteocytes.
series of stages with transition from one stage to the next dependent
on signals from the local environment. The variety of forms these
mesenchymal cells can assume include blood, fat, and muscle cells as
well as the specialized connective tissue cells, fibroblasts,
chondrocytes, osteoblasts, and osteocytes. Cell differentiation creates
persistent, but not necessarily permanent changes in the cells. In
general, the differentiated cell form persists through many generations
of the cell. Some cells, like chondrocytes, rarely divide but maintain
their differentiated form for their entire life. During cell
differentiation, the cells not only change their form but also change
the types of molecules they synthesize and thus the composition and
organization of the matrix that surrounds them.
undifferentiated mesenchymal cells persist. With increasing age they
may lose some of their capacity for proliferation and differentiation,
but they can still respond to appropriate signals by migrating,
proliferating, and differentiating into the mature cells of bone,
cartilage, and dense fibrous tissue including osteoblasts, osteocytes,
chondrocytes, and fibroblasts.
elaborate highly organized frameworks of organic macromolecules filled
with water. Light microscopic examination of these matrices shows
fibrils embedded in an amorphous ground substance. Biochemical
examination shows that the fibrils consist of multiple types of
collagen and elastin, the ground substance consists primarily of water
and proteoglycans, and the matrix contains another class of
macromolecules called noncollagenous proteins. In addition to an
organic matrix, bone has an inorganic matrix that consists primarily of
calcium phosphate.
and tensile strength, but the tissues vary in collagen concentration
and organization, and in the types of collagens that form part of their
organic matrix. All collagens function as structural proteins in the
extracellular matrix and a significant portion of each collagen
molecule consists of a triple helix formed from three amino acid chains
(Figure 1-1). This helical structure gives the molecules stiffness and strength.
different types of collagen. Differences in molecular topology and
polymeric form divide the 13 known collagen types into three classes:
class I (fibrillar collagens), class II (basement membrane collagens),
and class III (short chain collagens). A specific type of collagen may
vary slightly among tissues. For example, bone type I collagen, which
normally mineralizes, appears to differ in structure and composition
from tendon type I collagen, which does not mineralize under normal
conditions.
electron microscopy in all connective tissues. The five collagens in
this group—types I, II, III, V, and XI—have triple helical domains
consisting of about 1,000 amino acid residues in each of three
polypeptide chains. Type I collagen forms the principal matrix
macromolecule of skin, bone, meniscus, annulus fibrosis, tendon,
ligament, joint capsule, and all other dense fibrous tissues. Figure 1-2
shows the assembly of type I collagen microfibrils. Type II collagen
forms the banded fibrils found in hyaline cartilage, the nucleus
pulposus of the intervertebral disc, and the vitreous humor of the eye.
The “minor” fibrillar collagens, types V and XI, also contribute to the
matrices of the connective tissues. Type V forms part of the matrix in
tissues containing type I collagen, usually about 3% of the amount of
type I. Type XI forms part of the type II collagen fibrils. Type III
collagen occurs in association with type I collagen in most tissues
other than bone and appears in repair tissue.
critical parts of basement membranes. Type IV contributes the major
structural component of basement membranes. Type VII acts as an
anchoring filament in epithelial basement membranes, and type VIII
forms part of endothelial basement membranes.
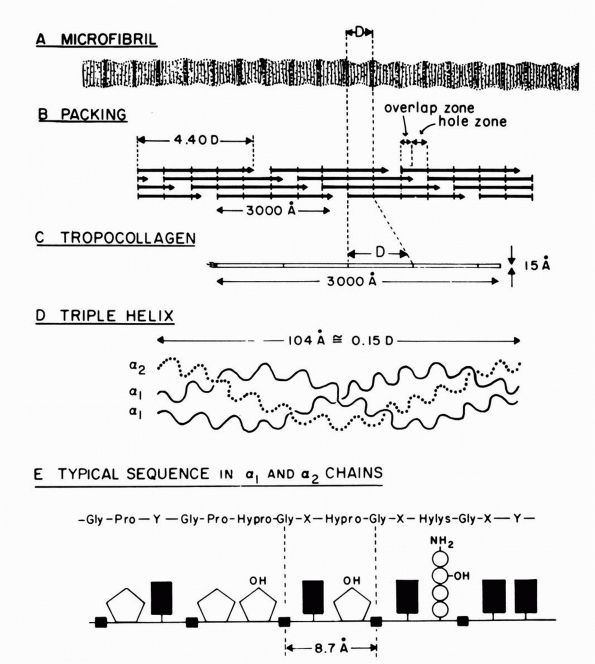 |
|
FIGURE 1-1. Type I collagen. (A)
A stained microfibril of collagen exhibiting characteristic crossstriations with a regular repeat period (D) of approximately 680 Å. (B) A two-dimensional representation of the packing arrangement of tropocollagen macromolecules in the microfibril. (C) Each tropocollagen molecule has large numbers of darkly staining bands, and five of these, which are separated by a regular distance of 680 Å, account for the repeat period (D) in the microfibril. The H2N-terminal and probably the HOOC-terminal ends of the molecule are atypical and nonhelical in structure and are called “telopeptides.” (D) Each tropocollagen molecule consists of three polypeptides, two with identical amino acid sequences (α1 chains) and one with a slightly different amino acid sequence (α2 chain). Each α chain is coiled in a tight left-handed helix with a pitch of 9.5 Å, and the three chains are coiled around each other in a right-handed “super-helix” with a pitch of about 104 Å. (E) Gly occurs in every third position throughout most of the polypeptide chains, and large amounts of Pro and Hypro occur in the other two positions. X and Y represent any amino acid other than Gly, Pro, Hypro Lys, or Hys. (Grant ME, Prockop DJ. The biosynthesis of collagen. N Eng J Med 1972;286:194, 242, 291) (see color image) |
IX and X—remain less understood than the forms and functions of the
other classes of collagens. Type VI collagen appears in small amounts
in many tissues. When examined by electron microscopy, it appears as
filamentous banded aggregates. These aggregates often appear in the
matrix immediately surrounding cells. Type IX collagen forms covalent
bonds with type II collagen molecules and thus contributes to the
extracellular matrix of hyaline cartilage. It may influence type II
collagen fibril diameter
and
the organization of the hyaline cartilage matrix. Type X collagen
occurs in the calcified cartilage region of the physis, articular
cartilage, and bone fracture callus. Although its limited distribution
suggests that it may have an important role in chondrocyte enlargement
or cartilage mineralization, its functions remain unknown. The forms
and functions of types XII and XIII collagens likewise remain unknown.
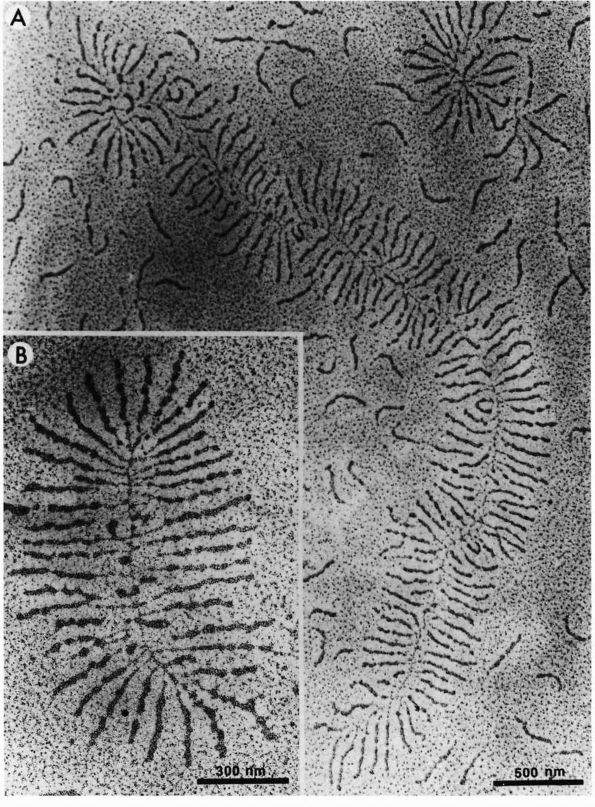 |
|
FIGURE 1-2. Electron micrographs showing the structure of proteoglycan aggregates. (A) A large aggregate. The central filament is hyaluronic acid and the projecting side arms are proteoglycan monomers. (B) A smaller proteoglycan aggregate.
|
elastin fibrils lack the cross-banding pattern seen in electron
microscopic studies of fibrillar collagens (Figure 1-3)
and differ from collagens in amino acid composition, confirmation of
the amino acid chains, and mechanical properties. In addition, elastin
also forms lamellae or sheet-like structures. Unlike collagen, elastin
can undergo some deformation without rupturing or tearing. Following
deformation it returns to its original size and shape. Amino acid
chains of elastin contain two amino acids not found in collagens
(desmosine and isodesmosine), and the elastin amino acid chains form
random coils, unlike the highly ordered triple helices of collagens.
The random coil confirmation of the amino acid chains makes it possible
for elastin fibers and sheets to undergo some deformation without
molecular damage and then resume their original shape and size.
cartilage or bone, and it contributes only a small amount to the
extracellular matrices most of
other
connective tissues. Trace amounts appear in the intervertebral disc and
meniscus. Many ligaments also have some elastin, usually less than 5%,
but a few ligaments, such as the nuchal ligament and the ligamentum
flavum, have high elastin concentrations, up to 75%.
 |
|
FIGURE 1-3.
Electron micrograph showing cross-banded collagen fibrils lying parallel to an elastic fiber consisting of multiple dark microfibrils and regions of amorphous elastin. |
of the cartilage, intervertebral disc, dense fibrous tissue, bone, and
muscle matrices. These musculoskeletal tissues vary considerably in the
concentration and possibly the function of proteoglycans. The highest
concentrations of proteoglycans occur in hyaline cartilages and nucleus
pulposus. In these tissues the concentration of proteoglycans may
approach 30 to 40% of the tissue dry weight, and these molecules
significantly influence fluid flow through the matrix and help give the
tissues stiffness to compression and resilience. They may have similar
space-filling and mechanical roles in the other tissues, but the much
lower concentrations in these tissues (in the dense fibrous tissues and
bone, they contribute at most a few percent of the dry weight) make
their effect on the tissue mechanical properties proportionately less.
Muscle also contains specific types of proteoglycans, but they form
only a small fraction of the tissue.
monomers), the basic units of proteoglycan molecules, consist of
polysaccharide chains covalently bound to protein. Most types of
proteoglycans contain relatively little protein, about 5% or less.
Glycosaminoglycans, a special class of polysaccharide consisting of
repeating disaccharide units containing a derivative of either
glucosamine or galactosamine and carrying one or two negative charges,
form the principal part of proteoglycan molecules (Figure 1-4).
Connective tissue glycosaminoglycans include hyaluronic acid,
chondroitin 4 sulfate, chondroitin 6 sulfate, dermatan sulfate, and
keratan sulfate.
multiple covalently bound oligosaccharides and longer chondroitin and
keratan sulfate chains. Each glycosaminoglycan chain creates a string
of negative charges that bind water and cations in solution. Because of
this property, aggrecans can expand to fill a large domain in solution.
In most musculoskeletal tissues, an intact collagen fibril network
limits the swelling of the proteoglycans, but loss or degradation of
the collagen fibril network will allow a tissue that contains a high
concentration of large proteoglycans to swell, increasing the water
concentration, and the permeability of the tissue.
hyaluronic acid filament with multiple attached aggrecans and link
proteins, may reach a length of more than 10,000 nanometers with more
than 300 aggrecans (Figure 1-2). Link proteins
stabilize the association between monomers and hyaluronic acid and may
have a role in directing the assembly of aggregates in the matrix.
Aggregates appear to help
anchor
aggrecans within the matrix, preventing their displacement and thereby
organizing and stabilizing the macromolecular framework. They also help
determine the permeability of the matrix and thus the flow of water
through the matrix.
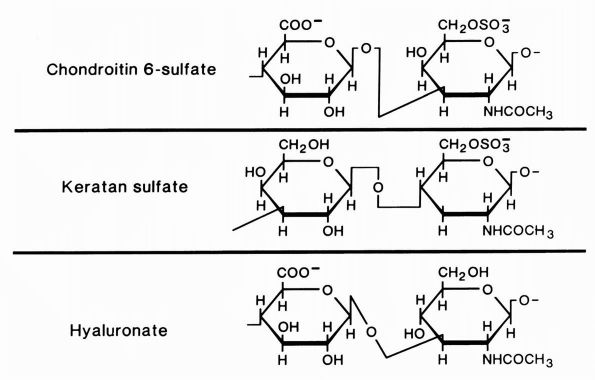 |
|
FIGURE 1-4.
Diagrammatic representation of the structures of the glycosaminoglycans, chondroitin sulfate, keratan sulfate, and hyaluronate. Chondroitin sulfate has two negative charges per disaccharide and the others have one. (From Buckwalter JA, Cooper RR. The cells and matrices of skeletal connective tissues. Chapter 1. In: Albright JA, Brand RA, eds. The Scientific Basis of Orthopaedics. Norwalk, CT: Appleton & Lange, 1987:23) |
chondroitin sulfate and dermatan sulfate. They consist of one or two
glycosaminoglycan chains covalently bound to a protein core. They form
specific associations with collagen fibrils and may influence matrix
organization and the ability of cells to bind to the matrix collagen
fibrils.
glycoproteins than about the collagens, elastin, or proteoglycans.
Although they form part of the macromolecular framework of
musculoskeletal tissues, few noncollagenous proteins have been
identified and their functions have not been well defined. Most of them
consist primarily of protein with small numbers of attached
monosaccharides and oliosaccharides. They appear to have roles in the
organization and maintenance of the macromolecular structure of the
matrix and in establishing and maintaining the relationships between
the cells and the other matrix macromolecules.
musculoskeletal tissue matrices include link protein, fibronectin, and
tenascin. Link protein helps organize and stabilize the extracellular
matrix through its effect on proteoglycan aggregation. Soluble
fibronectin occurs in many body fluids including plasma, urine,
amniotic fluid, and cerebral spinal fluid. Insoluble or cellular
fibronectin appears in most musculoskeletal tissue matrices.
Fibronectins examined by electron microscopy appear as fine filaments
or granules. They may coat the surface of fibrillar collagens and
associate with cell membranes. Tenascin, another matrix glycoprotein,
occurs in perichondrium, periosteum, tendon, and muscle-tendon junction.
on rapid controlled mineralization (deposition of relatively insoluble
mineral within the organic matrix) of some organic matrices and
prevention of mineralization in others, yet the conditions that control
and promote normal and pathologic mineralization of musculoskeletal
tissues remain poorly understood. Bone, the growth plate cartilage
longitudinal septa, and a thin zone of articular cartilage organic
matrices all mineralize normally. The organic matrices of other
cartilage regions and dense fibrous tissues mineralize in association
with certain diseases including chondrocalcinosis, and muscle and some
dense fibrous tissues mineralize following some injuries. The
deposition of mineral in the organic matrix radically changes the
properties of the tissue. It increases stiffness and compressive
strength of bone, but pathologic mineralization of cartilage and dense
fibrous tissue may accelerate or be associated with degenerative
changes in these tissues.
light weight gives vertebrates their mobility, dexterity, and strength.
Bone has an elaborate vascular supply and several specific types of
bone cells that form and resorb the bone matrix. Like the other
musculoskeletal tissues, bone consists of mesenchymal cells and an
extracellular matrix, but unlike the other tissues bone matrix
mineralizes.
They vary in size from the ear ossicles to the long bones of the leg.
The variety of shapes allows them to be classified into three groups:
long bones, short bones, and flat bones. Long bones like the femur,
tibia, or humerus have an expanded metaphysis and epiphysis at either
end with thick walled tubular diaphysis. The thick cortical walls of
the diaphysis become thinner and increase in diameter as they form the
metaphysis, and articular cartilage covers the epiphyses where they
form synovial joints. The metacarpals, metatarsals, and phalanges, like
the larger limb bones, have the form of long bones. Short bones, like
the tarsals, carpals, and centra of the vertebrae, have approximately
the same length in all directions. Flat or tabular bones have one
dimension that is much shorter than the other two, like the scapula or
wing of the ilium.
tissue assumes two forms: the outer cortical or compact bone and the
inner cancellous or trabecular bone (Figure 1-5).
Cortical bone forms about 80% of the skeleton and surrounds the thin
bars or plates of cancellous bone with compact lamellae. In long bones,
dense cortical bone forms the cylindrical diaphysis that surrounds a
marrow cavity containing little or no trabecular bone. In the
metaphyses of long bones, the cortical bone thins and trabecular bone
fills the medullary cavity. Short and flat bones usually have thinner
cortices than the diaphyses of long bones and contain cancellous bone.
Cancellous and cortical bone modify their structure in response to
persistent changes in loading, hormonal influences, and other factors.
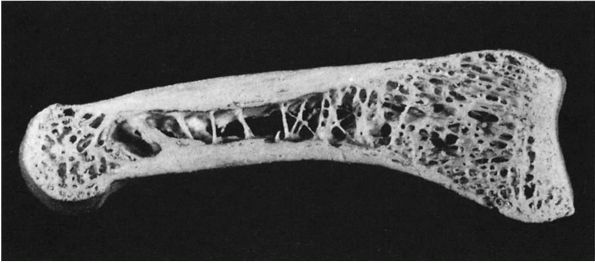 |
|
FIGURE 1-5.
Longitudinal section of a human phalanx. Outer lamellae of cortical bone surround the inner cancellous bone. The metaphyses contain more cancellous bone than the diaphysis and the thick cortical bone of the diaphysis becomes thinner in the metaphysis. Larger bones like the femur follow the same structural pattern. (From Buckwalter JA, Cooper RR. Bone structure and function. In: AAOS Instructional Course Lectures, XXXVI, Park Ridge, IL: American Academy of Orthopaedic Surgeons, 1987:27-48) |
organization, equal size blocks of cortical and cancellous bone have
different mechanical properties. The two types of bone have the same
composition, but cortical bone has much greater density. Because the
compression strength of bone is proportional to the square of the
density, cortical bone has compressive strength that may be in order of
magnitude greater than that of cancellous bone. Differences in the
organization and orientation of cortical and cancellous bone matrices
may also make a difference in their mechanical properties.
form seams of unmineralized bone organic matrix, called osteoid, on the
surface of mineralized bone matrix. Normally, osteoid mineralizes soon
after it appears. Therefore, normal bone contains only small amounts of
unmineralized matrix.
For this reason, failure to mineralize bone matrix during growth or
during normal turnover of bone matrix in mature individuals produces
weaker bone. Individuals with impaired mineralization of bone matrix
may develop skeletal deformities or fractures. In children, the
clinical condition associated with impaired mineralization, rickets,
predisposes the patient to skeletal deformity. In adults, the clinical
condition associated with impaired mineralization, osteomalacia,
predisposes the patient to fractures.
fiber, or primary) bone and lamellar (mature, secondary) bone. Woven
bone forms the embryonic skeleton and the new bone formed in the
metaphyseal parts of growth plates. Mature bone replaces this woven
bone as the skeleton develops and during skeletal growth. Small amounts
of woven bone may persist after skeletal maturity as part of tendon and
ligament insertions, the suture margins of cranial bones, and the ear
ossicles. With these exceptions, woven bone rarely appears in the
normal human skeleton after 4 or 5 years of age, although it is the
first bone formed in many healing fractures at any age and it also
appears during the rapid turnover and formation of bone associated with
metabolic, neoplastic, and infectious or inflammatory diseases.
and the rate of bone formation. Cells rapidly form the irregular,
almost random, collagen fibril matrix of woven bone. The appearance of
the irregular arrangement of collagen fibrils gives woven bone its
name. It contains approximately four times as many osteocytes per unit
volume of lamellar bone, and they vary in size, orientation, and
distribution. The mineralization of the woven bone matrix also follows
an irregular pattern with mineral deposits varying in size and their
relationship to collagen fibrils. In contrast, cells form lamellar bone
more slowly and the cell density is less. The collagen fibrils of
lamellar bone vary less in diameter and lie in tightly aligned parallel
sheets forming distinct lamellae 4 to 12 microns thick with an almost
uniform distribution of mineral throughout the matrix.
high cell and water content, and the irregular mineralization, the
mechanical properties of woven bone differ from those of lamellar bone.
It is more flexible, more easily deformed, and weaker than mature
lamellar bone. For this reason, the immature skeleton and healing
fractures have less stiffness and strength than the mature skeleton or
a fracture remodeled with lamellar bone.
coordinated actions of different types of bone cells. The morphology,
function, and characteristics of bone cells separate them into four
groups: undifferentiated or osteoprogenitor cells, osteoblasts,
osteocytes, and osteoclasts.
with single nuclei, few organelles, and irregular forms, remain in an
undifferentiated state until stimulated to proliferate or differentiate
into osteoblasts. They usually reside in the canals of bone, the
endosteum, and the periosteum, although cells that can differentiate
into osteoblasts also exist in tissues other than bone.
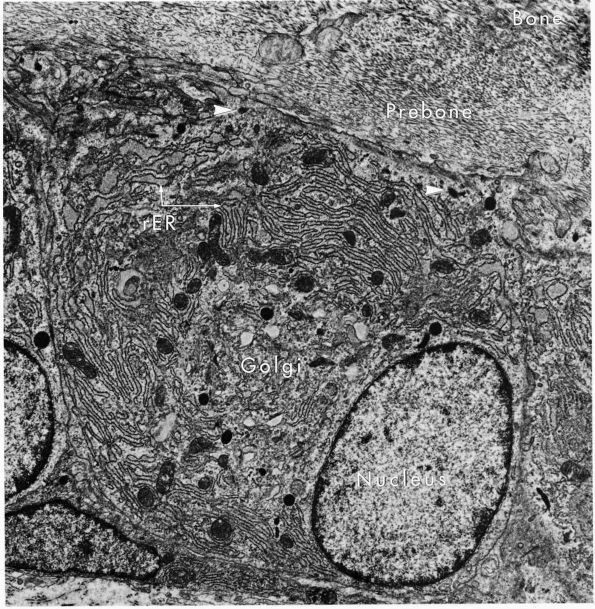
eccentric, nucleus, contain large volumes of synthetic organelles:
endoplasmic reticulum and Golgi membranes (Figure 1-6).
They lie on bone surfaces where, when stimulated, they form new bone
organic matrix and participate in controlling matrix mineralization.
When active, they assume a round, oval, or polyhedral form and a seam
of new osteoid separates them from mineralized matrix. Their
cytoplasmic processes extend through the osteoid to contact osteocytes
within mineralized matrix. Once they are actively engaged in
synthesizing new matrix, they can follow one of two courses. They can
decrease their synthetic activity, remain on the bone surface, and
assume the flatter form of a bone surface lining cell or they can
surround themselves with matrix and become osteocytes.
mature skeleton. Combined with the periosteal and endosteal cells, they
cover the bone matrix surfaces. Their long cytoplasmic processes extend
from their oval- or lensshaped bodies to contact other osteocytes
within the bone matrix or the cell processes of osteoblasts, forming a
network of cells that extends from the bone surfaces throughout the
bone matrix (Figures 1-7 and 1-8).
The cell membranes of the osteocytes and their cell processes cover
more than 90% of the total surface area of mature bone matrix. This
arrangement gives them access to almost all the mineralized matrix
surface area and may be critical in the cell mediated exchange of
mineral that takes place between bone fluid and the blood. In
particular, they may help maintain the composition of bone fluid and
the body’s mineral balance.
fill much of their cytoplasm with mitochondria to supply the energy
required for
these
cells to resorb bone. They usually lie directly against the bone matrix
on endosteal, periosteal, and Haversian system bone surfaces (Figure 1-9),
but unlike osteocytes, and presumably osteoblasts, they can move from
one site of bone resorption to another. Osteoclasts appear to form by
fusion of multiple bone-marrow-derived mononuclear cells. When they
have finished their bone resorbing activity, they may divide to reform
multiple mononuclear cells.
 |
|
FIGURE 1-6.
Electron micrograph of an osteoblast from demineralized rat alveolar bone showing the arrangement of the organelles. Numerous collagen fibrils, which these cells secrete, are present in the adjacent prebone or osteon and bone (upper right). The procollagen, which is the precursor of the collagen fibrils, is carried within secretory granules (arrowheads) originating from the Golgi saccules. Procollagen is released into the prebone by fusion of the secretory granule with the apical plasma membrane of the cell. (×12,000) (From Melvyn Weinstock; Ham AW, Cormack DH. Histology, 8th Ed. Philadelphia: JB Lippincott, 1979) |
the complex folding of their cytoplasmic membrane where it lies against
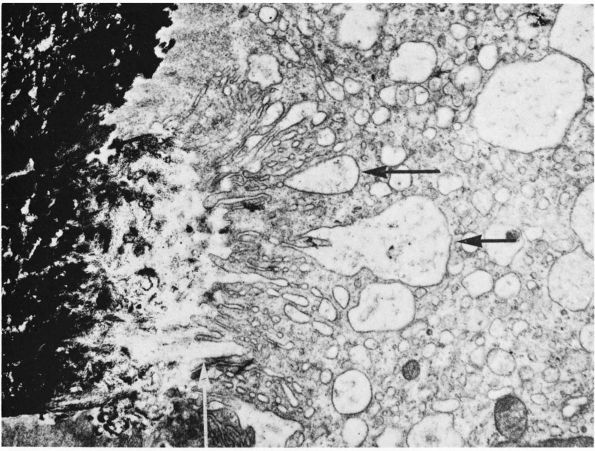
the bone matrix at sites of bone resorption (Figure 1-10).
This ruffled or brushed border appears to play a critical role in bone
resorption, possibly by increasing the surface area of the cell
relative to the bone and creating a sharply localized environment that
rapidly degrades bone matrix. The fluid between the brush border and
the bone matrix probably has a high concentration of hydrogen ions and
proteolytic enzymes: the acidic environment could demineralize bone
matrix, and the enzymes could degrade the
organic
bone matrix. In cancellous bone, osteoclasts resorbing the bone surface
create a characteristic depression called a Howship’s lacuna. In
cortical bone, several osteoclasts lead the osteonal cutting cones that
remodel dense cortical bone.
 |
|
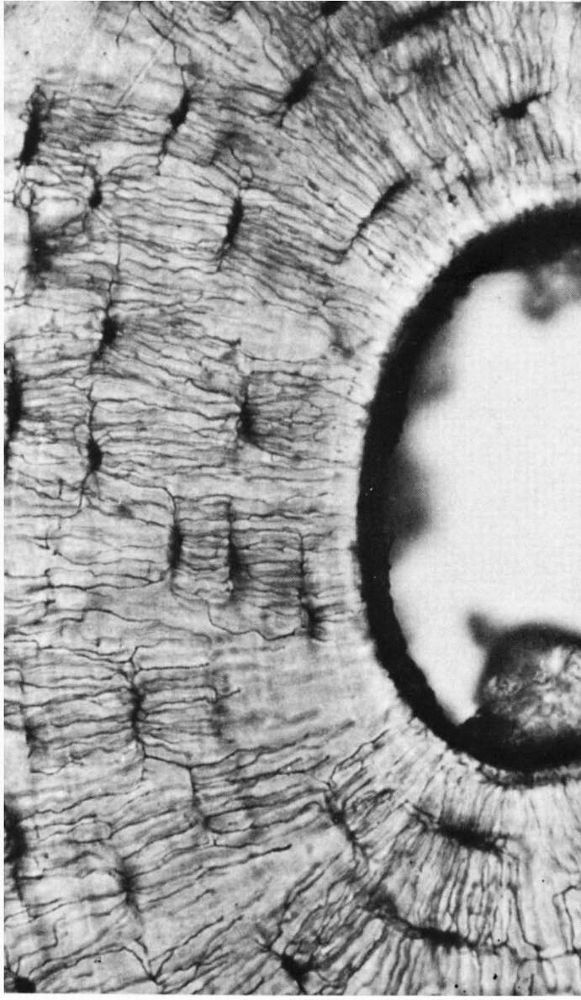
FIGURE 1-7.
A photomicrograph of a ground bone section. The lacunae in which the osteocytes reside are dark flattened oval structures. The fine lines connecting these are canaliculi. The canaliculi extend to the empty canal on the right. In life this contained blood vessels that supplied tissue fluid to the canaliculi. (Preparation by H. Whittaker; Ham AW, Cormack DH. Histology, 8th Ed. Philadelphia: JB Lippincott, 1979) |
inorganic mineral, and the matrix fluid. The inorganic matrix component
contributes approximately 70% of wet bone weight, although it may
contribute up to 80%. The organic macromolecules contribute about 20%
of the bone wet weight and water contributes 8 to 10%. The organic
matrix gives bone its form and provides its tensile strength; the
mineral component gives bone strength in compression.
 |
|
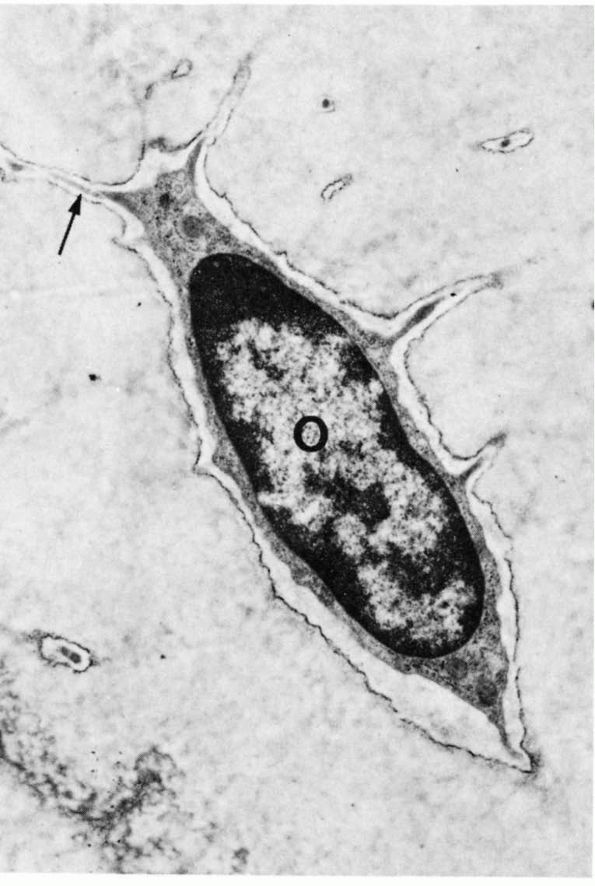
FIGURE 1-8.
Low-power electron micrograph of an osteocyte and its processes in a section of decalcified bone. The nucleus (0) and an arrow point to a process in a canaliculus. Two processes in canaliculi cut in cross section can be seen one near the upper right corner and the other toward the lower left corner. (From S. C. Luk and G. T. Simon; Ham AW, Cormack DH. Histology, 8th Ed. Philadelphia: JB Lippincott, 1979) |
matrix show the contributions of the inorganic and organic matrix
components to the mechanical properties of bone. Removal of either
component leaves bone with its original form and shape, but
demineralized bone, like a tendon or ligament, has great flexibility. A
demineralized long bone, such as the fibula, can be twisted or bent
without fracture. In contrast, removal of the organic matrix makes bone
brittle. Only a slight deformation will crack the inorganic matrix and
a sharp blow will shatter it.
fibrous tissues like tendon, ligament, annulus fibrosis, meniscus, and
joint capsule. Type I collagen contributes over 90% of the organic
matrix. The other 10% includes small proteoglycans, many of
noncollagenous proteins including osteonectin and small amounts of type
V collagen and possibly other collagens.
 |
|
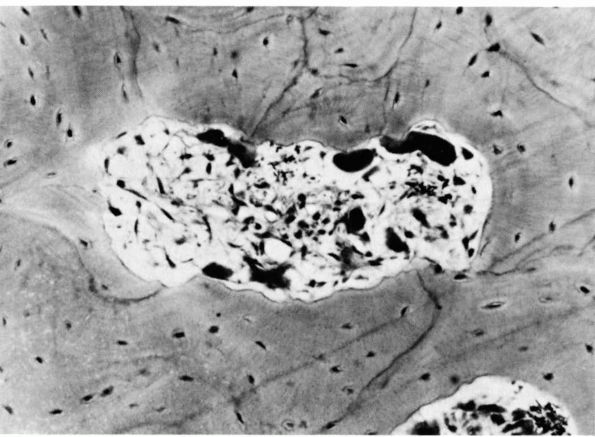
FIGURE 1-9.
Photomicrograph of a cross section of the shaft of a bone showing a resorption cavity in cross or somewhat oblique section. The large dark cells are osteoclasts; their activity explains the etched-out borders of the cavity. (Ham AW, Cormack DH. Histology, 8th Ed. Philadelphia: JB Lippincott, 1979) |
 |
|
FIGURE 1-10.
Electron micrograph of a section of a bone surface undergoing resorption. Calcified bone appears black at the left. The main part of the picture is occupied by the cytoplasm of an osteoclast. Extending from the top to the bottom, in the middle of the picture, is the ruffled border of the osteoclast; this consists of complex folds and projections that abut on the bone at the left. Between the ruffled border of the osteoclast and the heavily calcified bone is an area where the calcium content is much less, which suggests that the osteoclast is dissolving or otherwise removing mineral from this area. Black granules of mineral can be seen in some of the large vesicles that are indicated by horizontal arrows, and that probably form because of the bottom of crypts being pinched off. In the original print a collagenic microfibril showing typical periodicity could be seen at this site indicated by the vertical arrow. (×20,000) (From B. Boothroyd and N. M. Hancox; Ham AW, Cormack DH. Histology, 8th Ed. Philadelphia: JB Lippincott, 1979) |
the bone organic matrix. Compared to bone organic matrix, osteoid
contains more noncollagenous macromolecules and water. Once
mineralization occurs, the organic matrix remains stable until
resorbed. Abnormalities of the organic matrix can weaken bone. For
example, many patients with osteogenesis imperfecta have disturbances
of synthesis, secretion, or assembly of the collagen component of the
bone organic matrix that increase bone fragility.
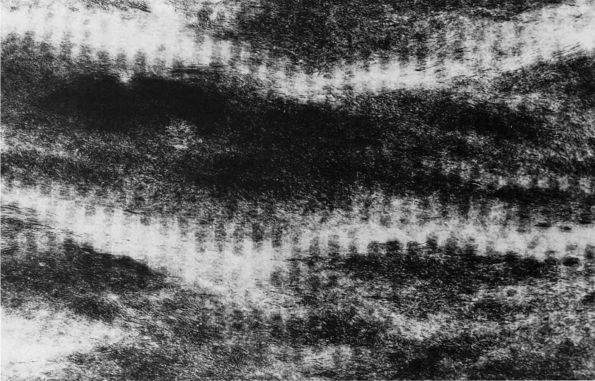
transformation of osteoid into mineralized bone matrix remain unclear,
but morphologic studies show that soon after osteoblasts produce
osteoid, mineral appears within the bone type I collagen fibrils and
then extends through the matrix without altering the organization of
the collagen fibrils (Figure 1-11) or affecting
osteocytes within the mineralized matrix. Mineralization of the bone
matrix not only increases the stiffness and strength of bone, it
provides a reservoir for minerals needed for normal function of other
tissues and organ systems. The bone matrix contains about 99% of the
body’s calcium, 80% of the phosphate, and large proportions of the
sodium, magnesium, and carbonate.
 |
|
FIGURE 1-11.
Electron micrograph of an undecalcified unstained section of embryonic chick bone. The ordered disposition of the dense mineral phase along the axial direction of the collagen fibrils is evident. Note also that the mineral phase is in lateral register as well. (×110,000) (Glimcher MJ. A basic architectural principle in the organization of mineralized tissues. Clin Orthop 1968:61:16) |
calcium phosphate species that range from relatively soluble complexes
to insoluble crystalline hydroxyapatite. As bone matures, the inorganic
matrix becomes primarily crystalline hydroxyapatite, although sodium,
magnesium, citrate, and fluoride may also be present. Because the
degree of mineralization increases with maturation, the material
properties of bone change as well. In particular, with increasing
mineralization, bone stiffness increases. This change helps explain why
children’s and adult’s bones may differ in their patterns of fracture.
When subjected to excessive load, normal adult bone usually breaks
rather than deforming permanently. In contrast, children’s bones may
bow or buckle rather than break.
osteoblasts replace it. The reason for this physiologic turnover of
bone tissue has not been established, but
it
may have a role in maintaining the structural integrity of the bone
tissue. To preserve normal bone mass and mechanical properties,
osteoblastic bone formation must balance osteoclastic bone resorption.
A variety of stimuli can alter this balance. For example, repetitive
loading of the skeleton can increase bone formation relative to bone
resorption and thereby increase bone mass and strength. Immobilization
decreases bone formation relative to bone resorption, thereby
decreasing bone mass and strength.
maximum value about 10 years after completion of skeletal growth,
remains stable for a variable period, and then begins to decrease,
progressively weakening the skeleton. The reasons for the age-related
loss of bone mass and the mechanisms that normally coordinate and
control bone cell function remain poorly understood, but investigations
of bone turnover show that both systemic and local factors help control
osteoclast and osteoblast function.
resorption and bone formation include nutrition, exercise, and hormonal
activity, especially parathyroid hormone. Additional factors include
Vitamin D and its metabolites, thyroid hormone, growth hormone,
insulin, estrogens, testosterone, and calcitonin. Dietary
abnormalities, lack of physical activity, and some disturbances of
hormone balance are the most common known causes of clinically
significant systemic increases in bone resorption relative to bone
formation. Protein deficiency impairs bone formation during bone growth
and remodeling. Prolonged lack of exercise will decrease bone mass.
Vitamin D deficiency and abnormalities of Vitamin D metabolism produce
rickets or osteomalacia. Excessive parathyroid hormone increases bone
turnover and decreases bone mass; excessive thyroid hormones can have
similar effects. Exogenous corticosteroids decrease the synthetic
activity of osteoblasts and may interfere with the ability of
undifferentiated cells to assume the form of osteoblasts and adversely
affect calcium balance. As a result, patients receiving corticosteroids
for prolonged periods may develop severe osteopenia and multiple
pathologic fractures. Estrogen also influences bone cell function. In
many women, bone mass begins to decrease rapidly after menopause and
then continues to decrease rapidly for 5 to 10 years. Estrogen
replacement can slow or reverse this rapid loss.
oxygen tension, pH, local ion concentrations, interactions between
cells, local concentrations of nutrients and metabolites, mechanical
and electrical signals, and interaction between cells and matrix
molecules can influence the balance between bone loss and bone
formation. Localized mechanical loading of bone has particular
significance. Immobilization or decreased loading of a limb causes a
relatively rapid loss of bone mass whereas repetitive increased loading
can increase bone density.
molecules that influence multiple cell functions, can influence bone
cell function and may help couple bone resorption and formation.
Cytokines produced by neoplasms may increase bone formation or, more
frequently, increase bone resorption. Interleukin-1, a cytokine
produced by monocytes, stimulates osteoclast formation and osteoclastic
bone resorption. It may contribute to the loss of bone in conditions
like rheumatoid arthritis. Transforming growth factor β,
a cytokine present in bone matrix, may be one of the factors that
balances bone resorption and bone formation. Osteoclastic resorption of
bone matrix may release or activate transforming growth factor β. Activated transforming growth factor β then may inhibit osteoclastic activity and stimulate osteoblasts to form bone.
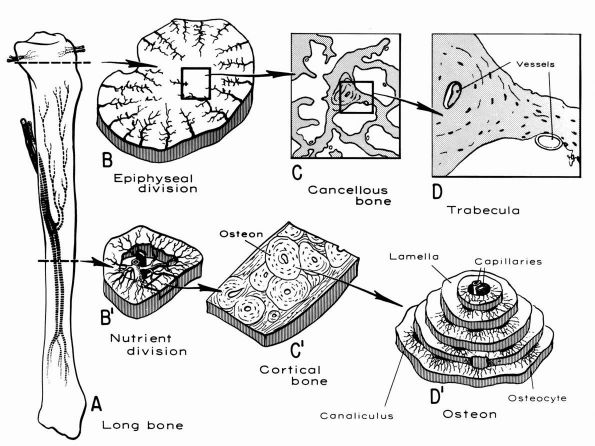
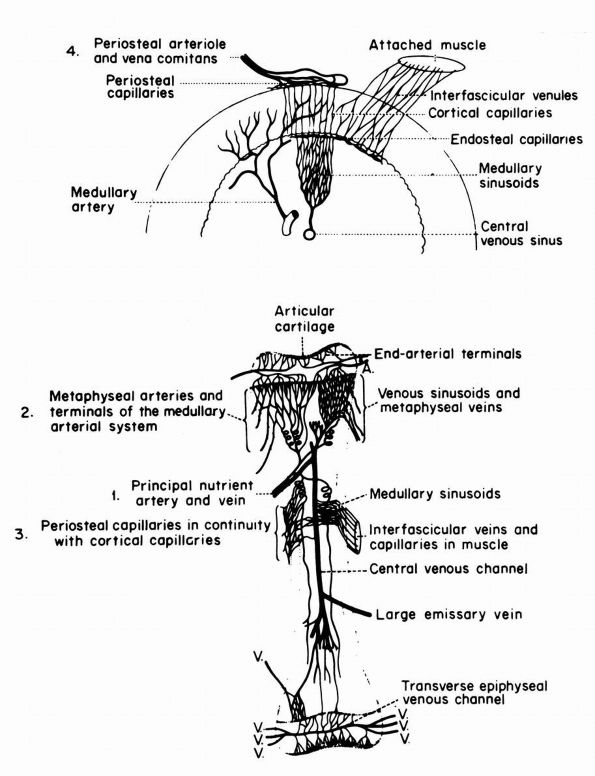
blood supply: nutrient arteries, epiphyseal and metaphyseal penetrating
arteries, and periosteal arteries (Figure 1-13).
The nutrient arteries pass through the diaphyseal cortex and branch
proximally and distally forming the medullary arterial system that
supplies the diaphysis. The proximal and distal branches of the
nutrient arteries join multiple fine branches of periosteal and
metaphyseal arteries that contribute to the medullary vascular system.
Under
normal circumstances this medullary vascular system supplies most of
periosteum covered bone, therefore the primary direction of blood flow
through the cortex is centrifugal. In regions of dense fascial
insertions into bone, such as muscle insertions or interosseous
membrane insertions, periosteal or insertion site vessels usually
supply the outer third of the bone cortex.
 |
|
FIGURE 1-12. Distribution of nutrient blood supply to the diaphyseal and epiphyseal regions of a long bone. (A) Basic pattern of nutrient circulation to a long bone (human tibia). (B) Pattern of circulation in epiphyseal-metaphyseal region. Arteries perforate thin cortical shell to enter cancellous bone. (C) Structure of cancellous bone. (D) A trabeculae of bone. Capillaries abut against thin trabecula. In thicker trabecula, an osteon can be seen. (B′)
Cross section of mid diaphysis. Here there is a single nutrient artery and vein. Lateral branches arise from the artery to supply the cortical bone. (C′) Cortical bone. Osteons and interstitial bone between osteons. (D′) Diagrammatic concept of a single osteon canaliculi of the osteocytes are canals in which the processes of the osteocytes are located. It is by way of these canaliculi that nutrition if derived from the vessels in the Haversian canal (Kelly PJ, Peterson LFA. The blood supply of bone. Heart Bull 1963;12:96) |
cross the growth plate, and epiphyses depend on penetrating epiphyseal
vessels for their blood supply. With closure of the physis,
interosseous anastomoses develop between the penetrating epiphyseal
arteries and the medullary arteries, but these anastomoses rarely
provide sufficient blood flow to support the epiphyseal bone cells
without the contribution of the epiphyseal vessels. For this reason,
even after closure of the physis, the blood supply to many epiphyses is
vulnerable to interruption. This is a particular problem in the region
of the femoral head where a dislocation of the hip or damage to the
epiphyseal penetrating vessels can cause necrosis and eventually
collapse of the femoral head.
canals of bone and particularly in association with blood vessels.
Presumably, these nerves have the primary function of controlling bone
blood flow. Specialized complex nerve endings have not been described
within bone tissue.
 |
|
FIGURE 1-13.
Blood supply of a long bone. Three basic blood supplies are shown: (1) nutrient; (2) metaphyseal, which anastomoses with epiphyseal after epiphyseal closure; and (3) periosteal. The numerous metaphyseal arteries arise from periarticular networks and anastomose with terminal branches of ascending and descending medullary arteries. Periosteal capillaries emerge from the cortex (efferent blood flow). (4) A periosteal arteriole feeds capillaries that provide afferent blood flow to a limited outer layer of cortex (Rhinelander FW. Circulation of bone. In: Bourne GH (ed). The Biochemistry and Physiology of Bone, 2nd Ed. New York: Academic Press, 1972: 2) |
insertions of tendons, ligaments, joint capsules, and interosseous
membranes, a tough thin membranous fibrous tissue, the periosteum,
covers the external surface of bone. It allows some ligaments, tendons,
and joint capsules to attach to bone and also provides a source of
cells that can form new bone or cartilage.
The outer layer consists of a dense fibrous tissue matrix and
fibroblast-like cells. Tendon, ligament, and joint capsule insertions
that do not penetrate directly into bone attach to this layer. In some
regions, the dense fibrous tissue insertions form a continuous sheet or
membrane of tissue with the periosteum. The inner osteogenic, or
cambium, layer contains cells capable of forming cartilage and bone.
periosteum of infants and children readily forms new bone. It shows
this capacity when osteomyelitis or trauma destroys the diaphysis of a
young individual’s bone and the periosteum regenerates a new diaphysis.
With increasing age periosteum becomes thinner and less vascular and
its ability to form new bone declines. The cells of the deeper layer
become flattened and quiescent, although they continue to form new bone
that increases bone diameter and they still have the potential to form
bone or cartilage in response to injury.
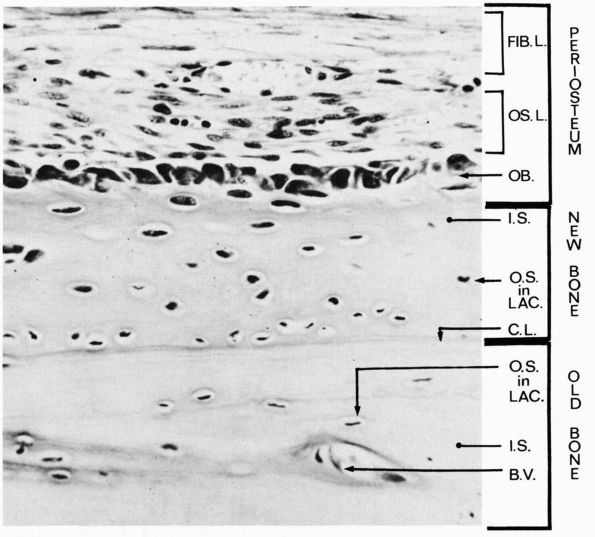 |
|
FIGURE 1-14.
A longitudinal section of a rabbit’s rib close to a fracture that had been healing for a short time. During this time the osteogenic cells of the periosteum have proliferated and some have differentiated into osteoblasts, which have laid down a layer of new bone on the original bone that was fractured. Three layers are labeled at the right: periosteum, new bone, and old bone. Within the periosteum the fibrous layer is labeled FIB.L., the osteogenic layer, OS.L., and the layer of osteoblasts, OB. Within the layer of new bone the intercellular substance is labeled I.S., and osteocyte in a lacuna is labeled O.S. in LAC., and the cementing line between the new bone and the old is labeled C.L. Within the old bone intercellular substance is labeled I.S., an osteocyte in a lacuna, O.S. in LAC., and a blood vessel in a canal is labeled B.V. (Ham AW, Cormack DH. Histology, 8th Ed. Philadelphia: JB Lippincott, 1979) |
outer fibrous layer of the periosteum. At intervals these periosteal
blood vessels anastomose with the vessels of the overlying muscle.
Branches of the vessels on the surface of the periosteum penetrate the
fibrous layer and contribute to the vascular system of the deeper layer
of the periosteum and to the blood vessels that penetrate bone to join
the medullary vascular system.
surface and often accompany periosteal blood vessels. Presumably, they
help regulate periosteal blood flow.
tissues—tendon, ligament, and joint capsule—have a major role in
providing the stability and mobility of the musculoskeletal system.
These tissues differ in shape and location, and vary slightly in
structure, composition, and function, but they have in common their
insertion into bone and their ability to resist large tensile loads
with minimal deformation. Tendons transmit the muscle forces to bone
that produce joint movement; ligaments and joint capsules stabilize
joints and the relationships between adjacent bones while allowing and
guiding joint movement. Diseases or injuries that affect these tissues
can destabilize joints or lead to loss of muscle function. Contractures
of these tissues limit muscle and joint motion and contribute to
skeletal deformity.
tough yet flexible and pliant fibrous sheets, bands, and cords that
consist of highly oriented dense fibrous tissue. The high degree of
matrix organization and density of the matrix (reflecting a high
concentration of collagen) (Figure 1-15) distinguish these tissues from irregular dense fibrous tissues and loose fibrous tissues.
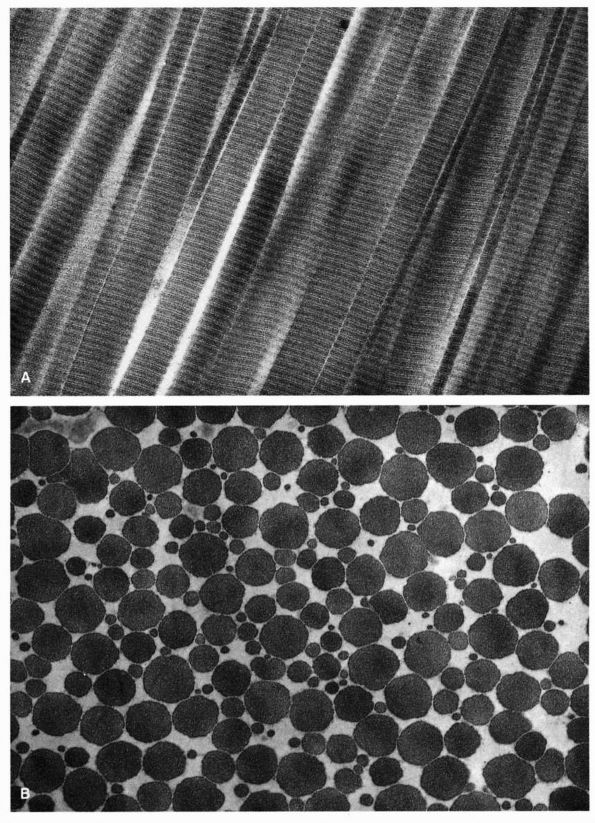 |
|
FIGURE 1-15. Electron micrographs of the type I collagen fibrils of ligament. (A) A longitudinal section shows the densely packed highly oriented collagen microfibrils. (B) A transverse section also shows the densely packed collagen microfibrils.
|
strings that form the tendons of the lumbrical muscles to the large
fibrous cords that form the Achilles tendons, but in any shape or size
they unite muscle with bone and transmit the force of muscle
contraction to bone. They consist of three parts: the substance of the
tendon itself, the muscle-tendon junction, and the bone insertion (Figure 1-16).
Connective tissues surrounding tendons allow low friction gliding and
access for blood vessels to the tendon substance. Many tendons have a
well-developed mesotendon, a structure that attaches the tendon to the
surrounding connective tissue and consists of loose elastic connective
tissue that can stretch and recoil with the tendon and provide a blood
supply to the tendon substance. In certain locations, the surrounding
connective tissue forms sheaths that enclose the tendon, and
specialized pulleys of dense fibrous tissue that influence the line of
tendon action.
and dense linear arrays of collagen fibrils, form the tendon substance
and give tendons their fibrous appearance. The endotendon—a less dense
connective tissue containing fibroblasts, blood vessels, nerves, and
lymphatics—surrounds individual tendon fascicles. The separation of
tendon fascicles by endotendon may allow small gliding movements
between adjacent tendon bundles. The endotendon tissue continues to
form the epitenon, a thin layer of connective
tissue
that covers the surface of the tendon. Where the tendon joins the
muscle, the fibrous tissue of the epitenon continues as the thin
fibrous covering of the attached muscle called the epimysium.
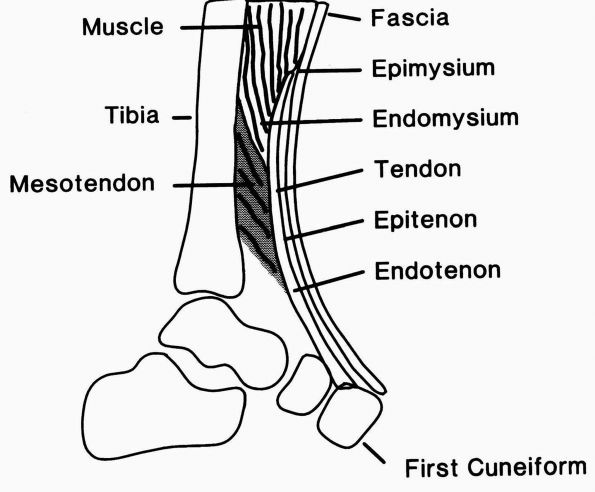 |
|
FIGURE 1-16.
Diagrammatic representation of the tibialis anterior tendon showing the muscle-tendon junction, the tendon substance, and the bone insertion. The epimysium and endomysium of muscle and the epitenon and endotenon of tendon form continuous structures. The epimysium consists of a fibrous envelope that surrounds the muscle, and the endomysium consists of the fine sheaths that surround individual muscle fibers. Permysium refers to the fibrous sheath enveloping primary bundles of muscle fibers. Loose connective tissue surrounds the tendon proximally where it pursues a straight course, but a sheath forms distally where it changes direction. (From Buckwalter JA, Maynard JA, Vailis AC. Skeletal fibrous tissues: tendon, joint capsule, and ligament. Chapter 14. In: Albright JA, Brand RA, eds. The Scientific Basis of Orthopaedics. Norwalk, CT: Appleton & Lange, 1987:388) |
force of muscle contraction to the tendon. The attachment of muscle to
tendon occurs through continuation of the collagen fibrils of the
fibrous tissue layers of muscle (epimysium, perimysium, and endomysium)
into the collagen fibrils of the tendon and through elaborate
interdigitation of the muscle cell membrane with the collagen fibrils
of the tendon. This interdigitation of muscle cell and tendon has the
appearance of interlocking fingers when examined by electron
microscopy, and provides a strong bond between the muscle cell and the
tendon collagen. Collagen fibrils do not enter the muscle cells but lie
next to their basement membranes. The muscle cell plasma membrane
thickens at the muscle-tendon junction and muscle myofilaments extend
directly to it.
forces to move joints and tendon nutrition, depend on the peritendonous
connective tissue structures sometimes called peritenon. These
structures range from loose connective tissue to elaborate well-defined
mesotendons, sheaths, and pulleys.
tissue usually consists of loose areolar tissue. In some locations,
this tissue must stretch several centimeters and then recoil without
tearing or disrupting the tendon blood supply. It consists of an
interlacing meshwork of thin collagen fibrils and elastic fibers filled
with abundant soft, almost fluid ground substance.
attachment and their bone insertion, often as they cross or near a
joint, the surrounding connective tissue may form a bursa or a discrete
tendon sheath. These structures allow low friction movement between the
tendon and adjacent bone, joint capsule, tendon, ligament, fibrous
tissue retinacula, or fibrous tissue pulleys. Tendon bursae and sheaths
consist of flattened synovial-lined sacks that usually cover only a
portion of the tendon circumference. Tendon sheaths and bursae resemble
synovial joints in that they consist of cavities lined with
synovial-like cells, they contain synovial-like fluid, and they
facilitate low friction gliding between two surfaces. Mesotenons
generally attach to one surface of a tendon within a tendon sheath and
provide the blood supply to this portion of the tendon.
fascial slings lie over the outer surface of some regions of tendon
sheaths. These firm fibrous structures direct the line of tendon
movement and prevent displacement or bowstringing of the tendon that
would decrease the efficiency of the muscle-tendon unit. For example,
the dense fibrous tissue (extensor tendon retinacula) of the wrist keep
the wrist and digital extensor tendons from displacing dorsally when
they extend the fingers and dorsiflex the wrist. The flexor tendons of
the fingers and thumb pass through a more elaborate series of pulleys
and sheaths that make efficient finger flexion possible.
functions, and in some regions ligament and capsule form a continuous
structure. Like tendons, both of them consist primarily of
high-oriented,
densely
packed collagen fibrils. Unlike tendons, they more often assume the
form of layered sheets or lamellae. Both ligament and capsule attach to
adjacent bones and cross synovial joints, yet allow at least some
motion between the bones. Ligaments have the primary function of
restraining abnormal motion between adjacent bones. Joint capsules also
restrain abnormal joint motion or displacement of articular surfaces,
but usually to a lesser extent. Both capsule and ligament consist of a
proximal bone insertion, ligament, or capsular substance and a distal
bone insertion, and both contain nerves that may sense joint motion and
displacement.
joints. A synovial membrane lines the interior of the joint capsule and
loose areolar connective tissue covers the exterior. This loose tissue
often contains plexes of small blood vessels that supply the capsule.
Nerves and blood vessels from this loose connective tissue penetrate
the fibrous capsule to supply the capsule and outer later of synovium.
Each end of the capsule attaches in a continuous line around the
articular surface of the bones forming the joint, usually near the
periphery of the articular cartilage surface. Tendons and ligaments
reinforce some regions of joint capsules. For example, the glenohumeral
ligaments form part of the glenohumeral joint capsule and the expansion
of the semimembranosus tendon contributes to the posterior oblique
ligament of the knee and part of the knee joint capsule.
location and bony attachments (for example, the anterior glenohumeral
ligament or the anterior talofibular ligament) or by their relationship
to other ligaments (the medial collateral ligament of the knee or the
posterior cruciate ligament of the knee). Unlike joint capsules,
ligaments vary in their anatomic relationship to synovial joints. This
variability separates ligaments into three types: intra-articular or
intracapsular ligaments, articular or capsular ligaments, and
extra-articular or extracapsular ligaments. Intra-articular ligaments,
including the cruciate ligaments of the knee, have the form of distinct
separate structures. In contrast, capsular ligaments, like the
glenohumeral ligaments, appear as thickenings of joint capsules.
Extra-articular ligaments, like the coracoacromial ligament, lie at a
distance from a synovial joint. Despite these differences in
relationship to joints, the function of the three ligament types
remains that of stabilizing adjacent bones or restraining abnormal
joint motion.
slightly in cell and matrix composition; but they all contain the same
basic cell types, share similar patterns of vascular supply and
innervation, and have the same primary matrix macromolecule, type I
collagen.
ligament, and joint capsule. The endothelial cells of blood vessels,
and in some locations nerve cell processes, exist within tendon,
ligament, and joint capsule, but they form only a small part of the
tissue. The fibroblasts surround themselves with a dense fibrous tissue
matrix and throughout life continue to maintain the matrix. They vary
in shape, activity, and density among ligaments, tendons, and joint
capsules and among regions of the same structure. Most dense fibrous
tissue fibroblasts have long small diameter cell processes that extend
between collagen fibrils throughout the matrix. Generally, younger
tissues have a higher cell density and cells with a larger cytoplasmic
volume and intracellular density of endoplasmic reticulum. With
increasing age, the cell density usually decreases and the cells appear
to become less active.
of most dense fibrous tissues, and the matrix macromolecules contribute
the other 40%. Because most dense fibrous tissue cells lie at some
distance from blood vessels, these cells must depend on diffusion of
nutrients and metabolites through the tissue fluid. In addition, the
interaction of the tissue fluid and the matrix macromolecules
influences the mechanical properties of the tissue.
proteins combine to form the macromolecular framework of the dense
fibrous tissues. Collagens, the major component of the dense fibrous
tissue molecular framework, contribute 70 to 80% of the dry weight of
many dense fibrous tissues. Type I collagen commonly forms more than
90% of the tissue collagen. Type III collagen also occurs within the
dense fibrous tissues; in some tissues it forms about 10% of the total
collagen, and other collagen types may also be present in small
amounts. Most dense fibrous tissues have some elastin, less than 5% of
their dry weight, but some
ligaments,
in particular the nuchal ligament and ligamentum flavum, have much
higher elastin concentrations, up to 75% of the tissue dry weight.
Proteoglycans usually contribute less than 1% of the dry weight of
dense fibrous tissues, but may have important roles in organizing the
extracellular matrix and interacting with the tissue fluid. Most dense
fibrous tissues appear to contain both large aggregating proteoglycans
and small nonaggregating proteoglycans. The large proteoglycans
presumably occupy the interfibrillar regions of the matrix and the
small proteoglycans lie directly on or near the surface of collagen
fibrils. Noncollagenous proteins also form a critical part of the dense
fibrous tissue matrix even though they contribute only a few percent to
the dry weight of most of the tissues. Fibronectin occurs in all dense
fibrous tissues; other noncollagenous proteins also contribute to the
structure of these tissues, but their composition, structure, and
function have not been well defined.
capsules attach the flexible dense fibrous tissue securely to rigid
bone, yet they allow motion between the bone and the dense fibrous
tissue without damage to the dense fibrous tissue. Despite their small
size, insertions have a more complex and variable structure than the
substance of the tissue; and they have different mechanical properties.
They vary in size, strength, and the angle of their collagen fiber
bundles relative to the bone and in the proportion of their collagen
fibers that penetrate directly into bone. Based on differences in the
angle between the collagen fibers of the dense fibrous tissue structure
and the bone and on the proportion of collagen fibrils that penetrate
directly into bone, dense fibrous tissue insertions can be separated
into two types: direct insertions (insertions where many of the
collagen fibrils pass directly into bone) and indirect or periosteal
insertion (insertions where only a few of the collagen fibrils pass
directly into bone) (Figure 1-17).
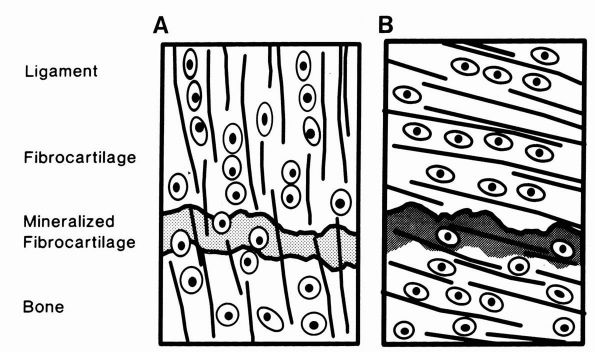 |
|
FIGURE 1-17. Diagrammatic representations of direct and indirect dense fibrous tissue insertions into bone. (A)
In direct insertions, a high proportion of the collagen fibers pass directly into bone, and the fibrocartilage and mineralized fibrocartilage zones are well developed. (B) In indirect insertions, a high proportion of the collagen fibers pass into the periosteum and the fibrocartilage and mineralized fibrocartilage zones often are not well developed. (From Buckwalter JA, Maynard JA, Vailis AC. Skeletal fibrous tissues: tendon, joint capsule, and ligament. Chapter 14. In: Albright JA, Brand RA, eds. The Scientific Basis of Orthopaedics. Norwalk, CT: Appleton & Lange, 1987: 391) |
collateral ligament of the knee into the femur, consist of sharply
defined regions where the ligament joins the bone; only a thin layer of
the substance of the ligament, tendon, or capsule joins the fibrous
layer of the periosteum. Most of the collagen fibrils at the insertion
pass directly from the substance of the tendon, ligament, or joint
capsule into the bone cortex, usually entering at a right angle to the
bone surface. These fibrils then mingle with the collagen fibrils of
the organic matrix of bone creating a strong bond between the tendon,
ligament, or capsule and the bone matrix. Where dense fibrous tissue
structures approach the bone surface at oblique angles, the collagen
fibrils may make a sharp turn to enter the bone at a right angle.
through four zones of increasing stiffness: the substance of the dense
fibrous tissue structure, fibrocartilage, mineralized fibrocartilage,
and bone.
the fibroblasts of the tendon, joint capsule, or ligament and more
spherical. A sharp border of unmineralized matrix separates the zone of
fibrocartilage from the mineralized fibrocartilage zone.
of the medial collateral ligament of the knee into the tibia, usually
cover more bone surface area than direct insertions because a larger
proportion of their collagen fibrils join the periosteum. Like direct
insertions, indirect insertions have superficial and deep collagen
fibrils, but most of their collagen fibrils form the superficial layer
that joins the fibrous layer of the periosteum. The deep collagen
fibrils enter the bone cortex, but they generally do not pass through
sharply defined zones of mineralized and unmineralized fibrocartilage.
of blood vessels extending throughout their substance. Generally, these
vascular systems follow the longitudinal pattern of the collagenous
matrix, but they may have multiple anastomoses between parallel
vessels. Some blood vessels in tendon, ligament, and joint capsule
insertions enter the bone.
vessels, like those found in periosteum and bone, dense fibrous tissues
have specialized nerve endings that lie on the surface or within the
substance of the tissue. Presumably, the nerve fibers in the dense
fibrous tissues function as pain receptors, vasomotor efferents, and
mechanoreceptors sensitive to stretching or distortion. The
mechanoreceptors presumably sense joint position, muscle tension, and
loads applied to ligaments, capsules, and tendons. In tendons, they can
adjust muscle tension. In ligaments and capsules, they may have a role
in initiating protective reflexes that oppose potentially damaging
joint movements.
mechanical functions in synovial joints including load bearing, shock
absorption, and participation in joint lubrication. They may also
contribute to joint stability. Menisci and meniscus-like structures
consist of dense fibrous tissue, or fibrocartilage, and project from
the margins of synovial joints to interpose themselves between
articular cartilage surfaces. They include the knee menisci (two
C-shaped menisci that lie on the tibial plateaus and form part of the
knee joint), the articular discs of the sternoclavicular and
acromioclavicular joints, the triangular fibrocartilage that binds the
distal ends of the ulna and radius together and forms part of the wrist
joint, and the labra found in some joints like the hip and shoulder.
Because the structures other than the knee menisci have not been
extensively studied, this section refers only to the knee menisci.
orientation and cell morphology vary from the surface to the deeper
central regions. The superficial regions that lie against articular
cartilage usually consist of a mesh of fine fibrils. Immediately deep
to these fine fibrils, small diameter collagen fibrils with a radial
orientation relative to the body of the meniscus form a thicker
subsurface layer. The flattened ellipsoid shaped cells of this layer
orient their maximum diameter roughly parallel to the articular
surface. In the deeper central or middle region, the bulk of the
meniscus is made up of large diameter collagen fibril bundles that
surround larger cells with a more spherical shape. The deeper collagen
fibril bundles follow the curve of the menisci and smaller radially
oriented fibril bundles weave among the circumferential fibril bundles.
The circumferential arrangement of the large collagen bundles gives the
menisci great tensile strength for loads applied parallel to the
orientation of the fibers. The radial fibers may resist the development
and propagation of longitudinal tears between the larger
circumferential collagen fiber bundles.
cells, lack cell-to-cell contacts and attach their membranes to
specific matrix macromolecules.
They
have the primary function of maintaining the meniscal matrix. Most of
them lie at a distance from blood vessels, so like chondrocytes, they
rely on diffusion through the matrix for transport of nutrients and
metabolites.
meniscus. As in the other musculoskeletal tissues, the interactions
between the matrix fluid and the macromolecular framework significantly
influence the mechanical properties of menisci.
to 40% of the meniscus wet weight and consists of collagens,
noncollagenous proteins, proteoglycans, and elastin. The collagens give
menisci their form and tensile strength and contribute approximately
75% of the dry weight of the tissue. Type I collagen makes up more than
90% of the total tissue collagen. Type II collagen, type V collagen,
and type VI collagen each contribute 1 to 2% of the total tissue
collagen. Noncollagenous proteins, including link protein, fibronectin,
and other noncollagenous proteins, contribute 8 to 13% of the dry
weight. Large aggregating proteoglycans and smaller nonaggregating
proteoglycans together contribute about 2% of meniscal dry weight.
Presumably they have functions like the proteoglycans found in other
dense fibrous tissues. Elastin forms less than 1% of the tissue dry
weight. This small amount of elastin probably does not significantly
influence the organization of the matrix or the mechanical properties.
blood supply, at least in their more peripheral regions. Branches from
the geniculate arteries form a capillary plexus along the peripheral
borders of the knee menisci. Small radial branches project from the
circumferential parameniscal vessels into the meniscal substance. These
vessels penetrate into 10 to 30% of the width of the medial meniscus
and 10 to 25% of the width of the lateral meniscus, leaving the cells
of the inner portions of the menisci dependent on diffusion of
nutrients and metabolites.
and other meniscal-like structures. Although these nerves enter the
more superficial regions of some parts of the tissue, they generally do
not penetrate into the central regions. The functions of these nerve
endings have not been clearly defined, but they may contribute to joint
proprioception.
joints and the bursae and sheaths of tendons. In synovial joints,
synovium attaches directly around the margins of the articular
cartilage. It covers the inner surfaces of the joint capsule, bone
surfaces, and intra-articular ligaments and tendons. Normally it does
not extend over articular cartilage, intra-articular discs, or menisci.
When examined from inside a joint, most of the synovial membrane has a
smooth even surface, but some regions may have small projections or
villi, and in others the synovium may form folds or fringes that
project into the joint cavity. Fat lying outside the synovial membrane
contributes to the ability of synovium to fill potential spaces in the
joint cavity.
intimal layer and a peripheral subintimal layer that lies on joint
capsule or periarticular fat. Both layers vary in thickness among
joints and among different regions of the same joint.
of synovial cells in an amorphous matrix. The cells vary considerably
in shape from flattened ellipsoids, to elongated cells, to polyhedral
or spherical cells, and they may have cell processes. The synovial
cells do not form a continuous layer; in some regions they leave gaps
between cell membranes that fill with extracellular matrices. Unlike
epithelial cells, the superficial synovial cells do not lie on a
basement membrane.
identified. Type A cells have surface filopodia, plasma membrane
invaginations, and vesicles. They contain mitochondria, lysosomes,
cytoplasmic filaments, and Golgi membranes. B cells lack most of these
characteristics, but contain a high concentration of endoplasmic
reticulum. Some investigators have proposed that A and B cells have
different
primary
functions: they suggest that A cells produce the hyaluronic acid that
serves as part of the synovial fluid and have phagocytic capability,
and that B cells synthesize proteins, including enzymes. Cells with
features of both A and B cells appear commonly; and the two cell types
may not represent distinct phenotypes, but morphologic variants of the
same cell.
layer from the joint capsule or the other synovial-covered tissues
including bone, tendon, and ligament. The cells of the subintima
include blood vessel endothelial cells, fibroblasts, macrophages, mast
cells, and fat cells. The subintimal matrix may have a loose areolar
form or a denser matrix with a higher concentration of collagen and
elastin.
extensive network of small blood vessels. The network forms from
vessels that pass through the joint capsule. Where synovium covers
bone, tendon, or ligament, the subintimal blood vessels form
anastomoses with blood vessels from the underlying tissue.
of a sparse population of mesenchymal cells embedded within an abundant
extracellular matrix. The cells contribute about 5% of the total tissue
volume, and the matrix contributes approximately 95%. The roughly
spherical shape of most cartilage cells, or chondrocytes; the unique
composition of the matrix they synthesize, assemble, and maintain; and
the lack of blood vessels and nerves distinguish cartilage from dense
fibrous tissues and bone.
the body, mechanical properties, and gross and microscopic appearance
differentiate three types of adult human cartilage: hyaline, fibrous,
and elastic cartilage. Elastic cartilage forms the auricle of the
external ear, a portion of the epiglottis, and some of the laryngeal or
bronchiolar cartilages; it does not form part of the musculoskeletal
system. Fibrous cartilage (also considered a form of dense fibrous
tissue) forms part of the intervertebral discs, pubic symphysis, and
tendon, ligament, and joint capsule insertions. Menisci consist of a
specialized form of fibrous cartilage. The most widespread form of
cartilage, hyaline cartilage, forms most of the skeleton before it is
removed and replaced by bone through the process of enchondral
ossification. It also forms the physeal cartilages that produce
longitudinal bone growth until skeletal growth ceases; in adults it
persists as the nasal, laryngeal, bronchiolar, articular, and costal
cartilages. This section discusses the structure of two specialized
forms of hyaline cartilage that have important roles in the
musculoskeletal system: articular cartilage and growth cartilage.
knee, depends on the unique mechanical properties of the articular
cartilage that forms their bearing surfaces. It distributes loads,
thereby minimizing stresses on subchondral bone. When loaded, it
deforms and when unloaded, it regains it original shape. It provides a
surface with almost unequalled gliding properties and has remarkable
durability.
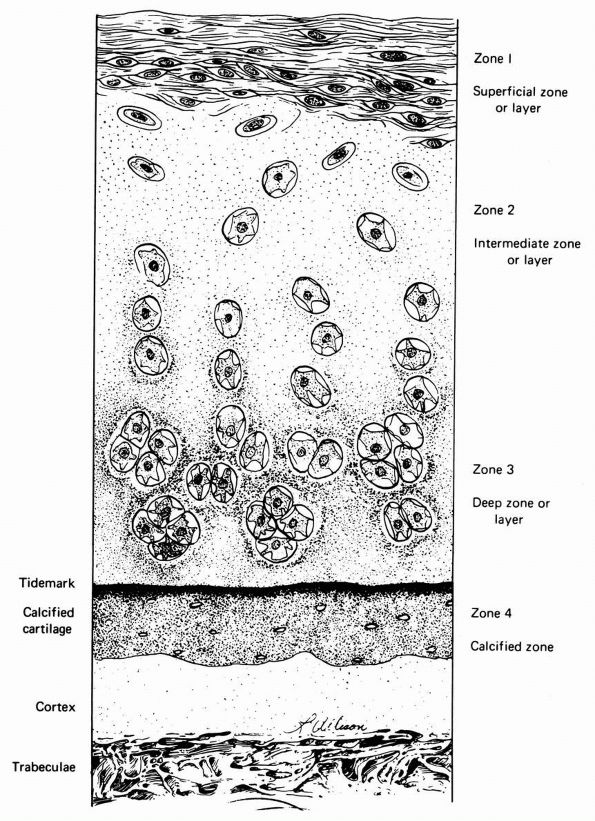
cartilage has an elaborate internal organization. This organization can
be described by dividing articular cartilage into four successive zones
beginning at the joint surface: the superficial or gliding zone, the
intermediate, middle or transitional zone, the deep or radial zone, and
the calcified cartilage zone (Figure 1-18 and Color Figure 1-1).
Within zones, differences in matrix composition and organization
distinguish three regions or compartments: the pericellular region, the
territorial region, and the interterritorial region.
concentration, collagen fibril orientation and cell alignment, and
morphology.
zone, forms the joint surface. A thin cell-free layer of matrix,
containing primarily fine fibrils, lies directly next to the synovial
cavity. Deep to this layer, elongated flattened chondrocytes surrounded
by a larger volume of matrices per cell align their major axes parallel
to
the articular surface. The collagen fibrils of this zone lie roughly parallel to the articular surface.
 |
|
FIGURE 1-18. Diagrammatic representation of the organization of articular cartilage into zones.
|
volume of the superficial zone. Its more spherical cells contain
greater volumes of endoplasmic reticulum, Golgi membranes,
mitochondria, and glycogen. The larger interterritorial matrix collagen
fibrils of the transitional zone have a more random orientation than
those of the gliding zone.
spherical cells of the transitional zone but tend to align themselves
in columns. This zone has the largest collagen fibrils, the highest
proteoglycan content, and the lowest water content.
cartilage from the stiffer subchondral bone. Collagen fibrils penetrate
from the deep zone of cartilage directly through calcified cartilage
into bone, thereby anchoring articular cartilage to subchondral bone.
chondrocytes, collagen content, collagen fibril diameter, collagen
fibril orientation, and proteoglycan and noncollagenous protein content
and organization.
matrix, consists of a thin layer of matrix containing little or no
fibrillar collagen. It appears to attach directly to the chondrocyte
cell membranes and probably contains noncollagenous proteins that help chondrocytes bind themselves to the matrix.
pericellular matrix and sometimes pairs or clusters of chondrocytes and
their pericellular matrices. Thin collagen fibrils in the territorial
matrix near the cells appear to bind to the pericellular matrix. At a
distance from the cell they spread and intersect at various angles,
forming a basket like structure around the cells.
compartment, has collagen fibrils of greater diameter than the
territorial matrix. The organization and orientation of these
interterritorial collagen matrix fibrils change as they pass from the
articular surface to the deep region of the cartilage. In the most
superficial zone they lie primarily parallel to the joint surface, in
the transition zone they assume a more random orientation, and in the
deep zone they tend to lie perpendicular to the joint surface.
makes it possible for them to produce precisely directed longitudinal
bone growth. The growth cartilages increase their volume and therefore
bone length by synthesizing new matrices and by cell swelling. The
organization of the growth cartilage matrix and the surrounding fibrous
tissue directs the increasing volume of cells and matrices, so that it
produces longitudinal bone growth. In the region of the growth
cartilage nearest to the metaphysis, the longitudinal cartilage septae
of the growth cartilage mineralize, and in the metaphysis, osteoblasts
cover the mineralized cartilage bars with new woven bone. Osteoclasts
then resorb the woven bone and calcified cartilage and osteoblasts form
lamellar bone to complete the replacement of cartilage by mature bone.
and a regional organization similar to that found in articular
cartilage. The matrix regions, like those of articular cartilage,
consist of the pericellular, territorial, and interterritorial
compartments. The layered or zonal organization differs considerably
from that of articular cartilage. The identified growth cartilage
layers or zones consist of reserve or resting, proliferative, and
hypertrophic or maturing zones.
epiphyseal pole of the growth plate, show relatively little evidence of
metabolic activity. The functions of these small cells have not been
clearly established, but they may serve as stem cells for the
proliferative zone.
synthesize a new matrix, and assume a highly oriented flattened
disclike shape. In rapidly growing bones they create long columns of
highly ordered cells that resemble stacks of plates.
proliferative cells begin to enlarge, creating the hypertrophic zone.
From the proliferative zone to the last part of the hypertrophic zone
they increase their volume fivefold to tenfold, contributing
significantly to bone growth, and assume a spherical or polygonal
shape. As the cells enlarge, their matrix changes: the territorial
matrix volume per cell increases significantly as its collagen
concentration decreases and mineral appears in the interterritorial
matrix.
fibrils of the growth cartilage changes among zones. In the reserve
zone and upper region of the proliferative zone, the fibrils have
little apparent orientation, but in the middle and lower regions of the
proliferative zone and throughout the hypertrophic zone, these
interterritorial matrix collagen fibrils lie parallel to the long axis
of the bone forming longitudinal columns or septae that surround the
chondrocyte columns and their territorial and pericellular matrices.
provisional calcification, the longitudinal septae mineralize. The
enlarged chondrocytes condense and metaphyseal capillary sprouts
penetrate the territorial matrix and cell lacunae, bringing osteoblasts
that begin to form new bone on the calcified cartilage septae.
(Figures 1-21 and 1-22),
and no nerve cell processes or blood vessels. Like many other
mesenchymal cells, chondrocytes surround themselves with the matrix
they synthesize, attach themselves to the matrix macromolecules, and do
not form contacts with other cells.
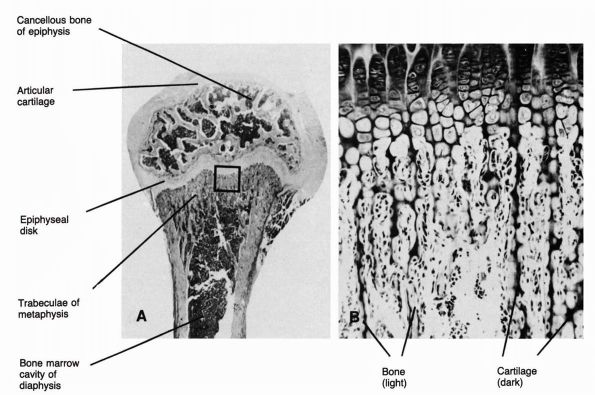 |
|
FIGURE 1-19. Micrographs showing the structure of a long bone physis. (A)
A low-power photomicrograph of a longitudinal section cut through the end of a long bone of a growing rat. At this stage of development osteogenesis has spread out from the epiphyseal center of ossification so that only the articular cartilage above and the epiphyseal plate below remain cartilaginous. On the diaphyseal side of the epiphyseal plate are the metaphyseal trabeculae, which (B) consist of cartilage cores on which bone has been deposited. (Ham AW, Cormack DH. Histology, 8th Ed. Philadelphia: JB Lippincott, 1979) |
does not end with the synthesis and assembly of the matrix. The normal
degradation of matrix macromolecules, especially proteoglycans, forces
the cells to synthesize new matrix components to preserve the tissue.
In addition, the matrix acts as a mechanical signal transducer for the
chondrocytes. Deformation of the matrix generates signals, transmitted
through the matrix, that cause the cells to alter their synthetic
activity. For example, absence of joint loading and motion causes
deterioration of the articular cartilage matrix, possibly because of
the lack of mechanical signals to stimulate normal chondrocyte function.
Depending on the type of cartilage and its age, water contributes 60 to
80% of the wet weight of cartilage. The volume concentration,
organization, and behavior of the tissue fluid depend on its
interaction with the structural macromolecules. These molecules
contribute 20 to 40% of the wet weight of cartilage and include
collagens, proteoglycans, and noncollagenous proteins. Hyaline
cartilage does not contain elastin. In most hyaline cartilages,
collagens contribute about 50% of the tissue dry weight, proteoglycans
contribute 30 to 35%,
and
noncollagenous proteins contribute about 15 to 20%. The collagens form
the fibrillar meshwork that gives cartilage its tensile strength and
form. The proteoglycans and noncollagenous proteins bind to the
collagen network or become mechanically entrapped within it, and the
chondrocytes attach themselves to the matrix macromolecules.
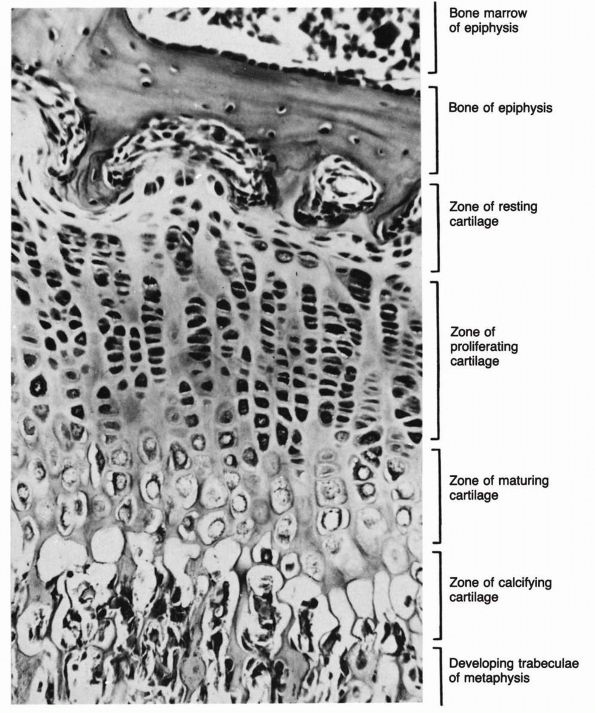 |
|
FIGURE 1-20.
High-power photomicrograph of longitudinal section cut through upper end of tibia of a guinea pig. Note different zones of cells in the epiphyseal plate. (Ham AW, Cormack DH. Histology, 8th Ed. Philadelphia: JB Lippincott, 1979) |
identified by electron microscopy and account for 90 to 95% of total
hyaline cartilage collagen. Hyaline cartilage also contains at least
two other collagens, types IX and XI, and may contain trace amounts of
other collagens. The mineralizing regions of articular cartilage and
growth plate cartilage also contain small amounts of type X collagen.
other musculoskeletal tissue except for the nucleus pulposus. Hyaline
cartilage has a high concentration of large aggregating proteoglycans
that give the tissue its unique material properties in compression. It
also contains smaller nonaggregating proteoglycans like those found in
dense fibrous tissues. The large aggregates help control the fluid flow
through the matrix.
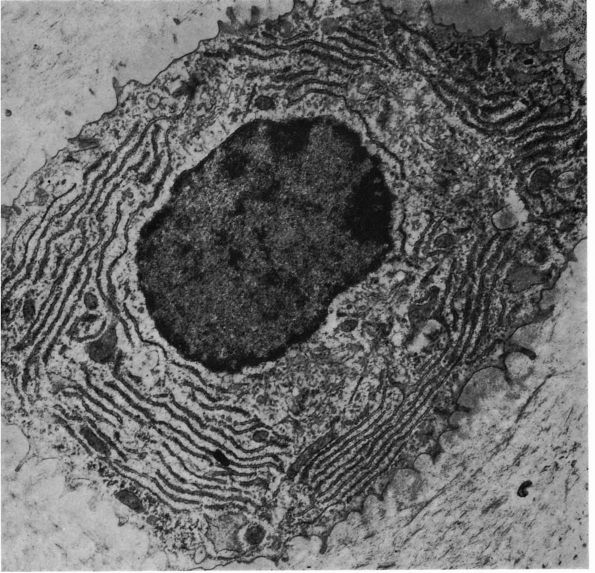 |
|
FIGURE 1-21.
Ultrastructural characteristics of a chondrocyte. The cytoplasm reveals well-developed rough-surfaced vesicles of endoplasmic reticulum and a well-developed Golgi apparatus. The cytoplasmic processes extend off into the intercellular substance (cytoplasmic footlets). As the chondrocyte becomes older and synthesis of protein is less, the rough-surfaced vesicles and Golgi apparatus become less prominent, and glycogen and lipid material accumulate in the cytoplasm. (Ham AW. Histology, 7th Ed. Philadelphia: JB Lippincott, 1974) |
hyaline cartilage. Link proteins stabilize and increase the size of
proteoglycan aggregates. Chondronectin and anchorin CII may have a role
in matrix organization and in establishing and maintaining the
relationships between the chondrocytes and the matrix macromolecules.
mechanical properties of the intervertebral discs. These 23 specialized
connective tissue structures unite adjacent vertebral bodies from the
second and third cervical vertebrae to the fifth lumbar
vertebrae
and sacrum. They vary in size from the small diameter thin discs in the
cervical region to the large diameter thick discs of the lower lumbar
region. Throughout the spine they contribute to stability while
allowing movement between vertebrae and absorbing compressive loads.
With age the gross and microscopic appearance, cell content and matrix
composition of disc tissues change more than any other tissues.
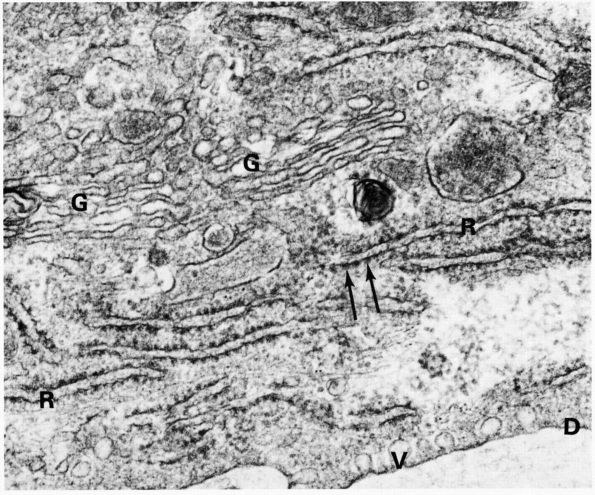 |
|
FIGURE 1-22. Part of a chondrocyte showing paired membranes of rough endoplasmic reticulum (R) with ribosome granules (arrows). The rough endoplasmic reticulum (R) with ribosome granules (arrows).
The rough endoplasmic reticulum contrasts with the smooth membranes of the Golgi apparatus (G). Micropinocytotic vesicles (V). Limiting cell membrane (D). Transmission electron micrograph ×37,500. Glutaraldehyde. (Meachim G. The matrix. In Freeman MA. Adult Articular Cartilage, 2nd Ed. London: Pitman, 1973:1) |
hyaline cartilage endplate, annulus fibrosis, transition zone, and
nucleus pulposus. These tissues vary in structure, composition, cell
populations, and mechanical properties.
inferior surfaces of each disc and separate the other disc tissues from
the vertebral bone. Grossly and microscopically they closely resemble
other hyaline cartilages. The dense collagen fibers of the annulus
fibrosis pass through the outer edges of the endplates into the
vertebral bodies. With age the cartilage plates mineralize and
eventually cannot be identified as distinct structures.
collagen fibers formed into two components: the outer circumferential
rings of collagen lamellae or layers (Figure 1-23) and an inner larger fibrocartilaginous component. The collagen
lamellae that form the outer concentric rings of the annulus have a
high degree of orientation: collagen fibrils within a layer lie
parallel to each other; collagen fibrils within adjacent layers lie at
40 to 70° angles to the collagen fibers within layers on either side.
In the fibrocartilaginous component of the annulus, collagen fibrils
run concentrically and vertically but lack the high degree of
orientation found in the outer concentric rings.
 |
|
FIGURE 1-23. Electron micrograph of the cut surface of a human annulus fibrosus. Notice the dense layers of collagen fibers.
|
pulposus and annulus fibrosus have a sharp boundary where the
gelatinous nucleus pulposus lies directly against the fibrous annulus.
With growth, a transition zone appears that lies between the
fibrocartilaginous component of the annulus fibrosis and the nucleus
pulposus.
 |
|
FIGURE 1-24.
Electron micrograph showing the collagen fibers of a human nucleus pulposus. Notice loose arrangement of the fibers compared with the annulus fibrosus. |
nucleus pulposus tissue has a translucent appearance. It contains
relatively few collagen fibers, and they lack any apparent orientation (Figure 1-24). With age, the nucleus pulposus becomes more fibrous making it increasingly difficult to separate
the nucleus pulposus from the transition zone and the fibrocartilaginous part of the annulus fibrosus.
matrix macromolecules. The composition of the hyaline cartilage plates
has not been extensively studied, but it appears to resemble other
hyaline cartilages. The annulus, like other dense fibrous tissues, has
water concentration of 60 to 70% throughout life. In contrast, the
water concentration of the nucleus pulposus declines from about 80 to
90% at birth to about 70% in adults.
notochordal cells and connective tissue cells. Notochordal cells form
the nucleus pulposus in the fetus. With growth, development, and aging
they gradually disappear, and the cells found in the nucleus of older
individuals have the appearance of connective tissue cells, often
resembling chondrocytes. The connective tissue cells of the outer
annulus have the appearance of fibroblasts, like those found in other
dense fibrous tissues. The connective tissue cells of the inner regions
of the annulus and other disc components have a more spherical form,
like chondrocytes. The cells of the cartilage endplate tissue have the
shape and organelles of hyaline cartilage chondrocytes.
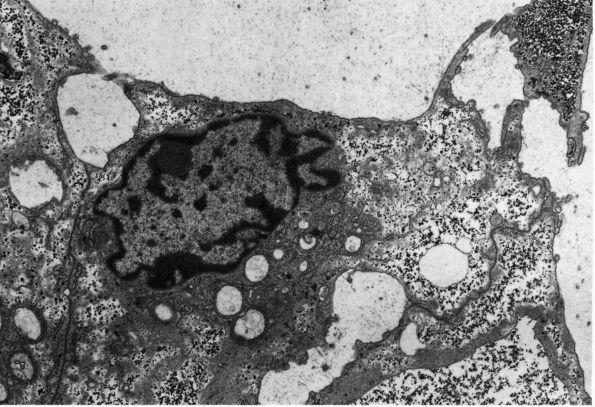 |
|
FIGURE 1-25.
Electron micrograph of a human nucleus pulposus notochordal cells. Notice that, unlike connective tissue cells, the membranes of notochordal cells bind to the membranes of adjacent cells. |
These cells contain endoplasmic reticulum, Golgi membranes,
mitochondria, glycogen, and bundles of microfilaments. Unlike
mesenchymal cells, notochordal cells form multiple specialized
junctions with other cells and their membranes form elaborate
interdigitations with those of other cells. During skeletal growth, the
notochordal cell clusters or cords disappear and the glycogen content
of the cells declines. In some regions they retain cell-to-cell contact
by elongated cell processes. With aging, the density of notochordal
cells declines further, and definite notochordal cells have not been
identified in the human nucleus pulposus after age 32.
axes parallel to the collagen fibrils. In the inner fibrocartilaginous
region of the annulus more of the cells assume a spherical form like
those seen in other fibrocartilages (Figure 1-26).
In the nucleus pulposus connective tissue cells have oval nuclei and
slightly elongated form, but most have a more spherical shape. The
proportion of viable nucleus pulposus cells declines with age, but even
in discs from elderly people some viable connective tissue cells
survive.
elastin, proteoglycans, and noncollagenous proteins. The concentrations
and specific types of these molecules vary among the disc tissues.
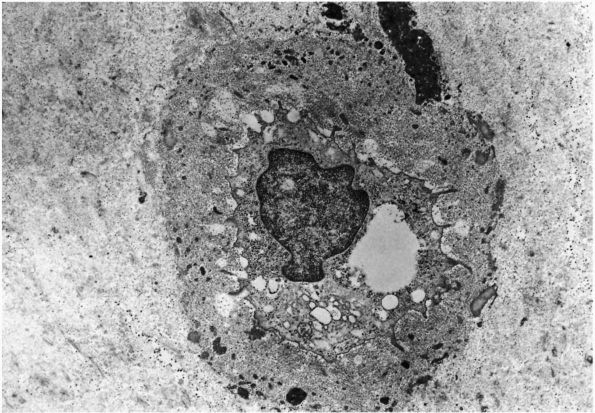 |
|
FIGURE 1-26.
Electron micrograph of a human connective tissue cell from the inner region of the annulus fibrosus. Notice that this cell lacks any contact with other cells and has formed a distinct pericellular matrix. |
collagens, give the disc its form and tensile strength. The
concentration of collagen decreases progressively from the outer
annulus to the most central portion of the nucleus. In the adult disc
the contribution of collagen to the tissue dry weight declines from 60
to 70% in the outer rim of the annulus to 10 to 20% in the most central
part of the nucleus.
collagens. The fibrillar collagens found in the annulus fibrosus
include type I, type II, type III, and type V. The concentration of
type I collagen declines from about 80% of the total collagen in the
outer rim of the annulus to 0 in the transition zone and nucleus. The
concentration of type II collagen follows the opposite pattern. It
increases from 0 to 80% of the total collagen from the outer rim of the
annulus to the transition zone and nucleus. Type III collagen occurs in
trace amounts and type V collagen contributes about 3% of the total
collagen of the annulus fibrosus. The short chain collagens found in
annulus fibrosus include type VI and IX. Type IX collagen forms only
about 1% of annulus fibrosus collagen, but type VI occurs in unusually
high concentrations; it forms about 10% of total annulus fibrosus
collagen.
the matrix of the nucleus pulposus. It contains at least three
fibril-forming collagens: type II, type III, and type XI. Type II is
the predominant collagen contributing about 80% of the total nucleus
pulposus collagen. Type III occurs in trace amounts, and type XI
contributes about 3% of the total collagen of the nucleus. Nucleus
pulposus short chain collagens include type VI and type IX. As in the
annulus fibrosus type IX forms only about 1% of the total collagen, but
type VI contributes even more to the nucleus than to the annulus,
forming about 14 to 20% of the total collagen. The reason for the
unusually high concentration of type VI collagen in the annulus and
nucleus remains unknown. This short chain collagen forms banded
fibrillar aggregates of longitudinal fibrils about 5 nm in diameter
with alternating transverse bands consisting of two 15 nm wide dark
bands separated by a 15 nm wide lucent strip. These fibrillar
aggregates occur most frequently in the matrix immediately surrounding
cells. They may have a role in resisting tensile loads on the matrix or
may provide a loose fibrillar network that helps organize other matrix
molecules.
significant part of the disc macromolecular framework and help give
disc stiffness to compression and resiliency. Their concentration
increases from the periphery of the disc to the center. They contribute
about 10 to 20% of the dry weight of the outer annulus and as much as
50% of the dry weight of the central nucleus.
differ from those found in hyaline cartilages like the disc cartilage
endplates, articular cartilage, or epiphyseal or growth plate
cartilage.
The
proteoglycan populations found in annulus fibrosus and nucleus pulposus
include fewer aggregates, smaller aggregates, and smaller more variable
aggrecans. Many disc proteoglycan aggregates consist of star-shaped
clusters of monomers rather than long aggregates. Aggregated and
nonaggregated disc proteoglycan monomers have shorter protein core
filaments, vary more in size, and have less chondroitin sulfate and
more keratan sulfate than those found in hyaline cartilages. The high
concentration of these small variable monomers in the nucleus pulposus
suggests that fragments of degraded proteoglycans accumulate in the
central regions of the disc.
nucleus pulposus. In the annulus, elastin appears as fusiform or
cylindrical fibers lying parallel to the collagen fibrils. In the
nucleus, elastin assumes irregular lobular shapes without apparent
relationship to collagen fibrils. The contribution of elastin to disc
mechanical properties remains unclear.
proteins appear to help organize and stabilize the extracellular matrix
of intervertebral disc and may facilitate adhesion of cell membranes to
other matrix macromolecules. The concentration of disc noncollagenous
proteins increases with age. In the annulus, the proportion of tissue
dry weight contributed by noncollagenous proteins increases from 5 to
25% with increasing age and in the nucleus it increases from 20% of the
dry weight to 45% of the dry weight. Some of the increasing
concentration of noncollagenous proteins may be due to accumulation of
degraded molecules. Electron microscopic studies have identified dense
granular sheaths around disc collagen fibers. These sheaths increase in
volume with increasing age, suggesting that they represent collections
of noncollagenous proteins.
surface on the annulus fibrosus, and occasional small vessels penetrate
a short distance into the outer layers of the annulus. The intraosseous
blood vessels of the vertebrae contact but do not penetrate the
cartilage endplates. This arrangement of blood vessels leaves the
central disc as the largest avascular structure in the body. The cells
must rely on diffusion of nutrients and metabolites through a large
volume of matrix. Mineralization of the endplates with increasing age
may further compromise diffusion of nutrients to the central regions of
the disc.
surface of the annulus and some of these nerve cell processes penetrate
the outer most collagen lamellae. Despite multiple investigations of
disc innervation, no nerves have been identified deep to the outer
annular layers. The cartilage plates also have perivascular nerves.
tissues, especially the nucleus pulposus. The four tissue components of
the disc are easily identified in the newborn. A clear gelatinous
nucleus fills almost half the disc and consists almost entirely of
notochordal tissue. A narrow transition zone and larger ring of annulus
fibrocartilage surround the nucleus. The outermost layers of the
annulus encircle the annular fibrocartilage. As the disc grows and
matures during childhood the nucleus gradually becomes opaque. The
decreasing water concentration makes it dense, firm, and difficult to
separate from the fibrocartilage and transition zones. The annular
fibrocartilage ring increases in size as the proportion of the disc
occupied by the nucleus decreases. In the elderly, the components of
the disc inside the outer annular layers consist almost entirely of
fibrocartilage, and clefts and fissures appear in the central parts of
the disc.
cells fill most of the fetal nucleus pulposus. With growth and
maturation, their numerical density progressively decreases and cells
with the morphologic features of connective tissue cells appear in the
nucleus pulposus. In adults, few if any notochordal cells remain and
the percent of necrotic cells in the nucleus increases from about 2% in
the fetus to about 50% in adults. In adults and the elderly a dense
pericellular matrix forms around most cells in the nucleus pulposus and
the inner annulus fibrosus, possibly because of accumulation of cell
products and metabolites. In the elderly less than 20% of the nucleus
cells remain viable. The causes of these age changes remain unclear.
They may result from decreased nutrition of central disc cells due to
increased disc volume, decreased diffusion
of
nutrients into the disc following mineralization of the cartilage
endplates, and accumulation of cell products and metabolites in the
matrix.
motion or stabilize joints against resistance depends not only on the
composition and structure of the muscle cells, but on the muscle nerves
and blood vessels, the organization of the tissue, and the attachment
of muscle cells to tendons. Unlike the musculoskeletal connective
tissues, muscle consists primarily of cells contained within a small
volume of highly organized matrix (Figure 1-27).
The matrix contains collagens, elastin, proteoglycans, and
noncollagenous proteins that maintain the structure of the tissue
including the muscle nerves and blood vessels.
 |
|
FIGURE 1-27. (A)
Skeletal muscle, longitudinal section. Note that the fibers do not branch or anastomose. Each fiber has many flattened, slender sarcolemmal nuclei lying peripherally beneath the membrane and oriented parallel with the long axis of the fiber. In muscle disease these nuclei come to occupy a central position. Large numbers of axially disposed myofibrils are contained within the fiber. (×670) (B) Striated muscle, cross section. An enlargement of a muscle fiber is depicted. The myofibrils are separated by sarcoplasm. The matrix consists of the endomysium, perimysium, and epimysium. |
bundles called fascicles. Aggregates of fascicles combined with their
extracellular matrix form named muscles. Although the extracellular
matrix makes up only a small fraction of muscle volume (Figure 1-28),
it is critical for normal muscle function, maintenance of muscle
structure, and healing. A basal lamina containing collagens,
noncollagenous proteins, and muscle-specific proteoglycans surrounds
each myofiber. The basal lamina together with the surrounding
irregularly arranged fine collagen fibers form the endomysium. A
thicker matrix sheath composed primarily of collagen fibers and elastic
fibers (the perimysium) covers muscle fasciculi. The epimysium, a
denser peripheral sheath of connective tissue, covers the entire muscle
and usually joins with the fascia overlying the muscle and with the
muscle-tendon junction.
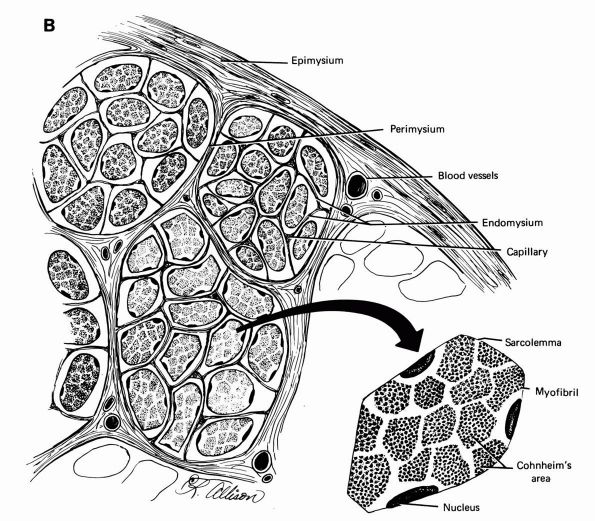 |
|
FIGURE 1-27. (Continued)
|
cells of blood vessels, and nerve cell processes, but most of the
tissue consists of large highly differentiated muscle cells. Each
muscle cell, or myofiber, contains multiple nuclei, a unique form of
endoplasmic reticulum called the sacroplasmic reticulum and contractile
protein filaments (actin and myosin) organized into cylindrical
organelles called myofibrils (Figure 1-28).
When viewed by the electron microscope, actin filaments appear as thin
filaments and myosin filaments appear as thick filaments (Figures 1-29 and 1-30).
Each myofibril consists of regular repeating units, consisting
primarily of actin and myosin, called sarcomeres. These myofibrils
often extend through the entire length of the cell and fill most of the
cell volume. Flattened vesicles of sarcoplasmic reticulum surround the
myofibrils and a series of invaginations of the cell membrane, called
transverse tubules, extend from the cell surface to lie next to each
myofibril and the membranes of the sarcoplasmic reticulum (Figure 1-31). The interfibrillar sarcoplasm contains mitochondria, lysosomes, and ribosomes.
cells called myoblasts. Myofibers cannot proliferate; therefore,
increase in muscle mass occurs through cell growth. The myofibers can
grow in length by fusion with myoblasts, thereby increasing the number
of nuclei. They can grow in diameter by increasing the size and number
of myofibers. In mature individuals, some myoblasts survive. They
remain next to mature myofibers, and following muscle injury they can
proliferate and fuse to form new muscle cells.
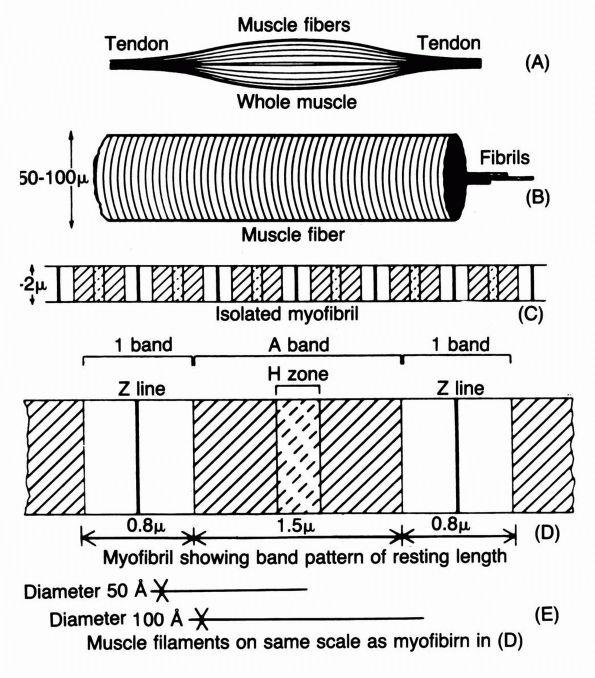 |
|
FIGURE 1-28. The dimensions and arrangement of the myofibrils in a muscle. The whole muscle (A) is made up of fibers (B) that contain cross-striated myofibrils (C, D). Myofibrils consist primarily of actin and myosin filaments (E)
that overlap and interdigitate in a stereospecific manner. (Huxley HE. The molecular basis of contraction. In: Bourne GH, ed. The Structure and Function of Muscle. New York: Academic Press, 1972) |
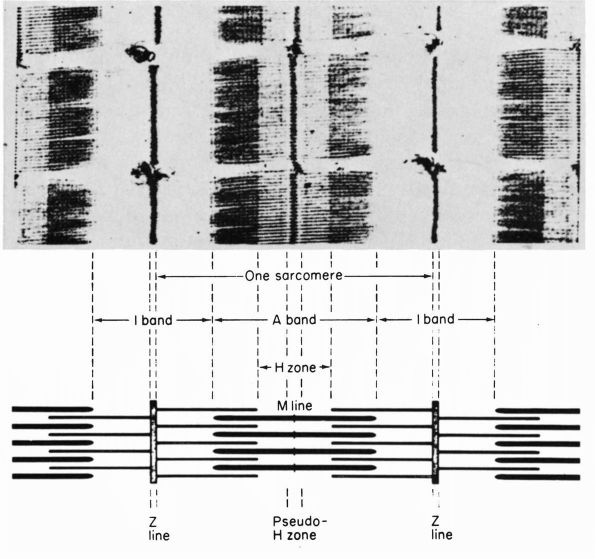 |
|
FIGURE 1-29. Longitudinal section of frog sartorius muscle (top)
together with a diagram showing the overlap of filaments that gives rise to the band pattern. The A band is most dense in its lateral zones where the thick and thin filaments overlap. The central zone of the A band (the H zone) is less dense, since it contains thick filaments only. The I bands are less dense still because they contain only thin filaments. The sarcomere length here is about 2.5 u. (Huxley HE. The molecular basis of contraction. In: Bourne GH, ed. The Structure and Function of Muscle. New York: Academic Press, 1972) |
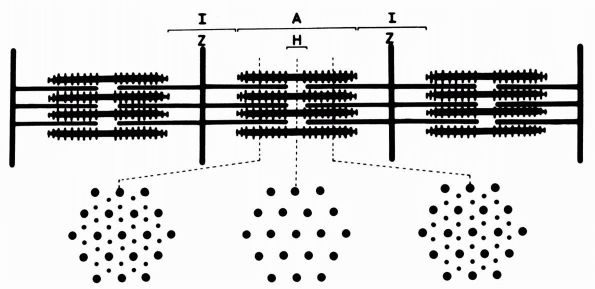 |
|
FIGURE 1-30.
Structure of striated muscle, showing overlapping arrays of actin- and myosincontaining filaments, the latter with projecting cross-bridges on them. To facilitate showing the relationships, the diagram is drawn with considerable longitudinal foreshortening, with filament diameters and side-spacings as shown. The filament lengths should be about five times the lengths shown. (Huxley HE. The molecular basis of contraction. In: Bourne GH, ed. The Structure and Function of Muscle. New York: Academic Press, 1971) |
studying the small volume of matrix found in muscle, the composition
and organization of the muscle matrix remains poorly understood. The
muscle basal lamina contain laminin (a noncollagenous protein that
forms part of basement membranes), types IV and V collagen, and heparin
sulfate proteoglycan. The outer connective tissue envelopes of muscle
consist primarily of type I collagen fibrils.
passing between muscle fasciculi within the muscle tissue matrix. They
then enter the muscle fascicles to form rich capillary networks around
individual myofibrils.
contraction require elaborate innervation. Nerves that sense muscle
tension terminate on intrafusal fibers and Golgi tendon organs. Three
types of motor neurons supply myofibers: (1) α motor neurons innervating extrafusal myofibers, (2) β motor neurons innervating both extrafusal and intrafusal myofibers, and (3) γ motor neurons innervating intrafusal myofibers.
motor neuron generally innervates more than one myofiber. A motor unit
consists of the motor neuron and the muscle fibers it innervates. Motor
nerves attach to myofibers through neuromuscular junctions that
transmit signals from the motor nerve terminas to limited regions of
the myofibers. Neuromuscular junctions consist of five principal parts:
(1) the nerve terminal filled with thousands of vesicles containing
neurotransmitter, (2) a Schwann cell overlying the nerve terminal, (3)
a synaptic space lined with basement membrane, (4) the postsynaptic
membrane containing receptors for the neurotransmitter, and (5)
postjunctional sarcoplasm necessary for the structural and metabolic
support of the postsynaptic membrane. Release of neurotransmitter from
the motor nerve terminal into the synaptic space creates an action
potential in the muscle cell membrane. The action potential in the
muscle cell membrane extends into folds in the cell membrane to the
sarcoplasmic reticulum. The sarcoplasmic reticulum releases calcium
ions, and the rapid increase in intracellular calcium triggers
simultaneous contraction of myofibers throughout the cell.
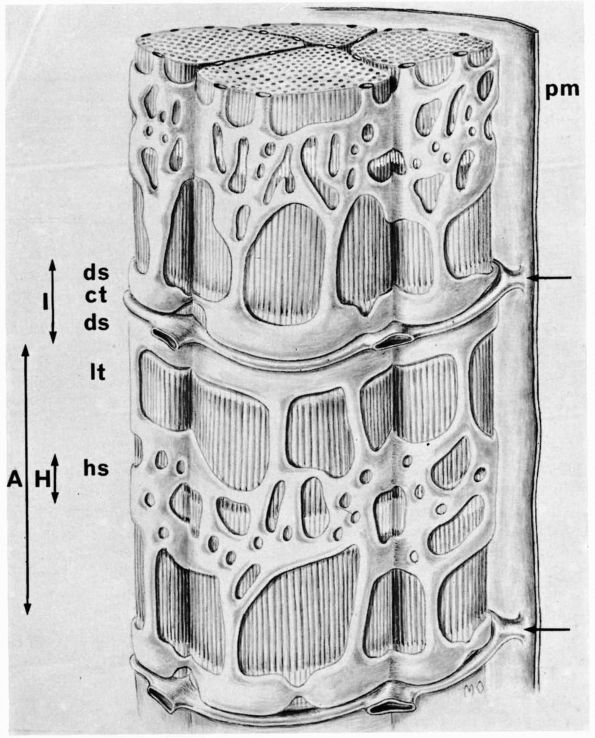 |
|
FIGURE 1-31.
Three-dimensional drawing of parts of four myofibrils to illustrate (1) the sarcolemma (labeled pm on the right), (2) the transverse tubules that extend into the substance of the fiber from the sarcolemma (from points indicated by arrows on the right), and (3) the sarcoplasmic reticulum, which is interposed, and so lies between myofibrils, over their I, A, and H portions (labels for the latter on left). The transverse tubules are delicate tubules that are invaginations of the sarcolemma; hence their walls are composed of cell membrane, and their lumens open onto the outer surface of the sarcolemma. Transverse tubules (in the frog) enter the fiber at the level of Z line (indicated by arrows on right side), and each one branches as it extends across the fiber so as to surround myofibrils whose Z lines are in register with the site where it entered, as is shown by following the two tubules in this illustration, from right to left. The sarcoplasmic reticulum consists of cisternae and channels of smooth-surfaced endoplasmic reticulum that lie between and so surround myofibrils. In the region of the I band, the extent of which is indicated at the left of the illustration, the cisternae, known as terminal cisternae, are large and flattened, but they may be more or less distended (ds); they lie to either side of the transverse tubule (ct). The cisternae, by means of channels that run longitudinally (lt) over the A band to the region of the H zone, connect with a network of more or less flattened sacs called the H sacs (hs). The site of the H zone in the A band is indicated at the left of the illustration. (From C. P. Leblond; Ham AW. Histology, 7th Ed. Philadelphia: JB Lippincott, 1974) |
system depend on the formation and growth of the specific skeletal
connective tissues and muscle, and the integration of these tissues
into the system that provides the stability and mobility of the body.
Cartilage, muscle, bone, and dense fibrous tissue form, grow, and
become organized into the musculoskeletal system during early prenatal
life so that by 6 months the final form of the musculoskeletal system
is easily recognized (Figure 1-32).
Disturbances of these processes cause congenital abnormalities
including failure of segmentation of vertebrae, congenital dislocations
of the hip, clubfeet, and absence of part or all of a limb. Following
birth the musculoskeletal system continues to grow and modify its form
until the physes close.
 |
|
FIGURE 1-32. Skeletal development of a 6-month-old fetus.
|
embryo takes shape, developing the head, the trunk, and protrusions
called limb buds. Between the ectoderm and the entoderm lies the
diffuse, loose, cellular mesenchyme, which differentiates into the
musculoskeletal connective tissues and skeletal muscle. The first
recognizable musculoskeletal structures appear as dense concentrations
of mesenchymal cells that take the shape of the bones.
enlarge, become more compact, and differentiate into a sheet of cells
recognized as precartilage. Then, matrix is laid down between the cells
(Figures 1-33 and 1-34). Cartilage increases in thickness by growth, both internally and externally (Figure 1-35).
Internal growth occurs by multiplication of cartilage cells and
production of new matrix. Peripheral growth occurs from the investing
sheath (the perichondrium), whose inner cells are transformed into
chondrocytes. Figure 1-33 shows the development of cartilage and skeletal muscle from mesenchyme.
forms either from mesenchymal membranes (e.g., facial and cranial
bones) or from cartilage (e.g., the long bones of the limbs). Although
the bone is identical in each instance, in the latter type the
cartilage must first be removed before bone can be laid down.
forms the original model of the facial and the cranial bones. At one or
more central points of the membrane, intramembranous ossification
begins (Figure 1-36). These ossification
centers are characterized by the appearance of osteoblasts that lay
down a meshwork of bony trabeculae spread radially in all directions.
The mesenchyme at the periphery differentiates into a fibrous sheath
(the periosteum), the undersurface of which differentiates into
osteoblasts, which in turn deposit parallel plates of compact bone (the
lamellae). This is periosteal ossification, by which the inner and the
outer tables of the skull are formed. Trabeculae are arranged mainly
along lines of greatest stress.
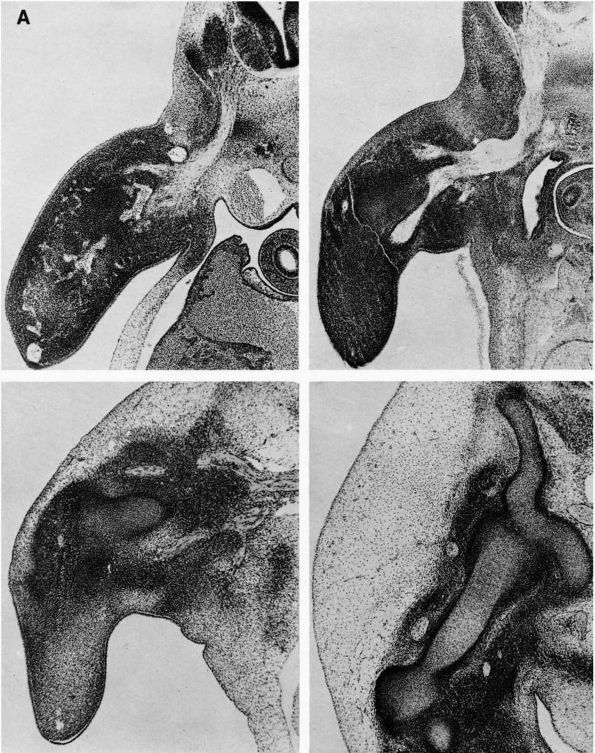 |
|
FIGURE 1-33. (A)
Origin of a limb bud. Development of the upper limb bud and histogenesis of the humerus are shown. The limb bud originates as a small elevation of the body wall and at first consists of a condensed mass of proliferating mesoblastic cells. Within a few days, a central condensation of mesoblasts occurs. This is the skeletomuscle condensation, so-called because separate muscle and skeleton cannot be identified. At the same time, a broad sheet of branching nerve trunks from the cord and the spinal ganglia stream into the base of the arm bud (top, left). A few days later, muscular and skeletal condensations become distinct, and massive nerve trunks enter the center of the muscle condensations (top, right). In the next section, definite muscle groups can be identified (bottom, left) containing conspicuous nerve trunks, and major branches of the brachial plexus are obvious. At the same time, the central part of the skeletal condensation is being transformed into cartilage (this tissue appears lighter in color). The cartilage of different bones is deposited separately in the last section (bottom, right) so that these central cartilaginous cores within the skeletal bed acquire the shape of the bones of which they are forerunners. These sections represent developmental periods of about 2 days each. (B) These diagram drawings correspond to these sections in A. (From the Carnegie Institution of Washington, Streeter GL. Developmental horizons in human embryos (Pub 583), Contrib Embryol 1949;33:149.) |
 |
|
FIGURE 1-33. (Continued)
|
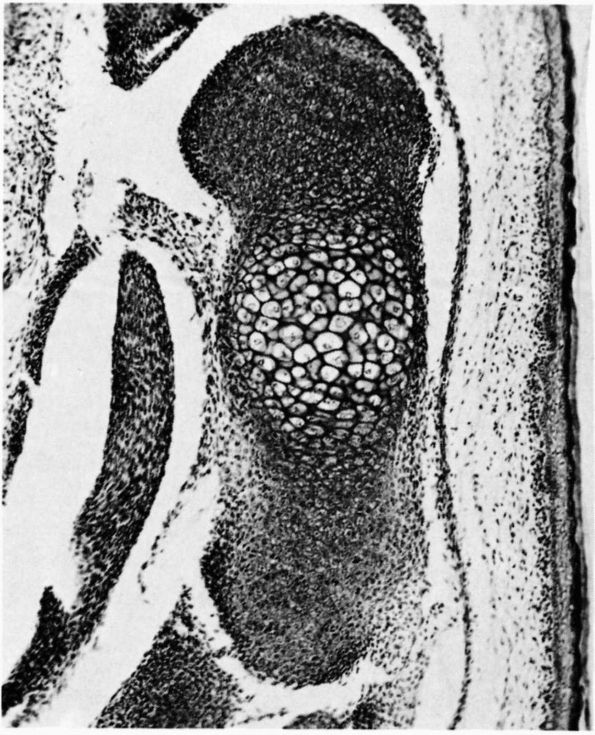 |
|
FIGURE 1-34.
Early fetal anlage of a long bone. It is composed of mesenchyme at the center of which the cells become rounded and assume the appearance of chondrocytes. Later, the peripheral mesenchyme will give rise to vascular tissue that will invade the calcified cartilage and replace the latter with bone. |
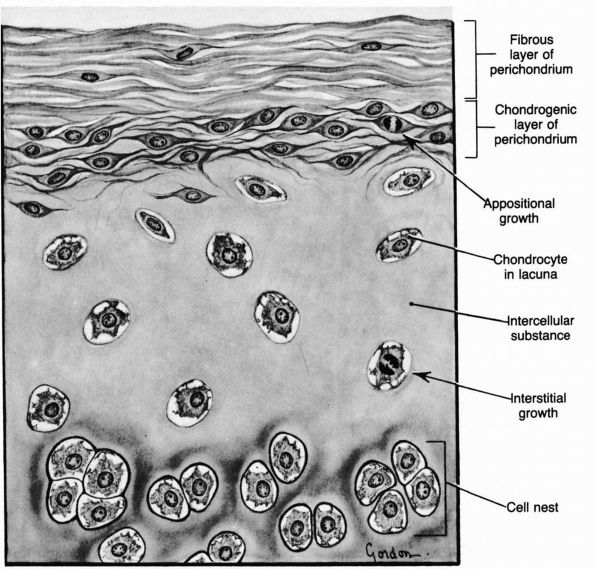 |
|
FIGURE 1-35.
Appositional and interstitial cartilage growth. Cartilage grows from the perichondrium when fibroblast-like cells enlarge into chondroblasts and become encapsulated. They multiply, form groups, and synthesize new matrix producing appositional growth. Cells within the tissue also proliferate and synthesize matrix producing interstitial growth. (Ham AW. Histology, 7th Ed. Philadelphia: JB Lippincott, 1974) |
destruction of cartilage and its replacement by bone. Two processes are
involved: ossification centrally within the cartilage, or endochondral
ossification, and ossification peripherally beneath the perichondrium
(or periosteum), or perichondrial or periosteal ossification.
enlarge and become arranged radially as the matrix mineralizes.
Invading from blood vessels the perichondrium (Figures 1-37 and 1-38) bring osteoblasts that deposit new bone that replaces the cartilage.
inner layer of perichondrium (now more appropriately named the
periosteum) lay down parallel layers of compact bone. The cartilaginous
physes form at the end of each long bone and produce enchondral bone
throughout skeletal growth. Periosteal ossification contributes to
growth thickness of the structure.
which allow little movement, and diarthroses, which allow low friction
movement.
(the suture or syndesmosis), cartilage (the synchondrosis), or bone (the synostosis).
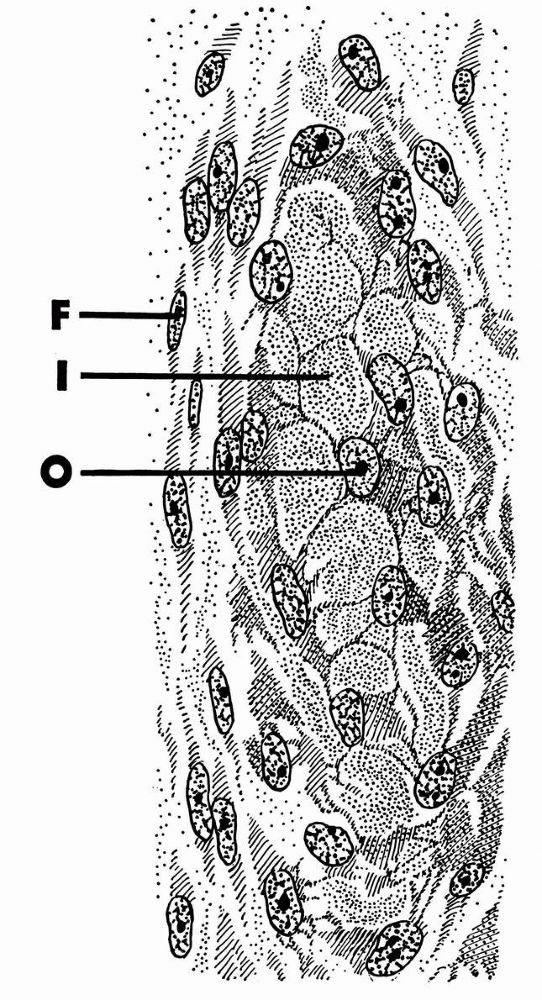 |
|
FIGURE 1-36.
Intramembranous bone formation. Fibroblasts (F). Homogeneous interstitial bone substance (I), collagenous fibrils no longer visible. Connective tissue cells (O) that have developed processes to become osteoblasts and later osteocytes. |
in the mesenchyme. The joint capsule and ligaments form from the dense
external tissue, which is continuous with the periosteum. The cells on
the inner surface of the capsule flatten into the synovial membrane.
Menisci and meniscus-like structures form from mesenchyme that projects
into the joint cavity.
body. Mesenchymal tissue (designated as sclerotomes) migrate toward the
notochord and come to lie in paired segmental masses alongside the
notochord. Intersegmental arteries separate each sclerotomic
mesenchymal mass from similar masses before and behind. Each sclerotome
then differentiates into a caudal compact portion and a cranial less
dense half. The denser caudal half then unites with the looser cranial
half of the succeeding sclerotome to form the substance of the vertebra
(Figure 1-39). Both the condensed and the
looser portions grow about the notochord to form the body of the
vertebra. From the denser (now cranial) half, dorsal extensions pass
around the neural tube to form the vertebral arch, and paired
ventrolateral outgrowths form the costal processes or forerunners of
the ribs. The mesenchymal tissue in the intervertebral fissure gives
rise to the intervertebral disc. The nucleus pulposus in the disc
constitutes the remnant of the notochord. The two parts of sclerotomes,
in joining, enclose the intersegmental artery, which therefore passes
through the center of the vertebral body. In the seventh embryonic
week, centers of chondrification appear, two in the vertebral body and
one in each half of the vertebral arch. These four centers enlarge and
fuse into a complete cartilaginous vertebra. Vertebral ossification
starts in the tenth week. A single center in the body and one in each
half of the arch appears, but union is not completed until several
years after birth (Figure 1-40).
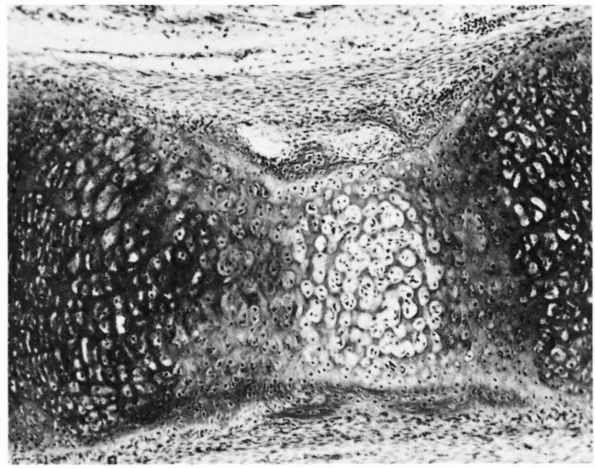 |
|
FIGURE 1-37.
Ossification of a fetal cartilaginous long bone. The cartilage cells at the center of the calcified cartilage have become enlarged, and the matrix is sparse. A bone collar has formed about this level and is gradually replacing the cartilage. |
enchondral ossification at the cephalad and the caudad epiphyseal
plates. About the rim of the superior and the inferior surfaces, a
prominent ring of cartilage exists to which is attached the fibers of
the longitudinal ligament of the spine (Figure 1-41).
It does not participate in growth. Gradually, it develops secondary
ossification centers that are triangular in cross section but actually
skirt the rim of the body. Eventually, this center appears as a line
parallel with the upper and the lower surfaces of the body and
resembles a plate (Figure 1-42). The term plate
is reserved for the growth cartilage that intervenes between the ring
and the main body of bone. The secondary centers fuse with the main
body by the age of 17. The central artery can be seen up to 6 years of
age, after which it is obliterated (Figure 1-43). It may persist beyond this time in certain conditions (e.g., Scheuermann’s disease).
 |
|
FIGURE 1-38. Development of a typical long bone. (a) Cartilage model. (b) Periosteal bone collar appears. (c) Center of calcifying cartilage. (d) Further development of calcified cartilage. (e) Vascular mesenchyme enters, resorbs calcified cartilage, and new bone is laid down toward either extremity of the model. (f) Endochondral ossification is further advanced, bone increased in length. (g) Blood vessels and mesenchyme enter upper epiphyseal cartilage. (h) Development of epiphyseal ossification center. (i) Ossification center develops in lower epiphysis. (j and k)
The lower and then the upper epiphyseal cartilage plates disappear, bone ceases to grow in length, a continuous bone marrow cavity traverses the entire length of the bone, and blood vessels of diaphysis, metaphysis, and epiphysis intercommunicate. (Redrawn from Maximow AA, Bloom W. A Textbook of Histology. Philadelphia: WB Saunders, 1968) |
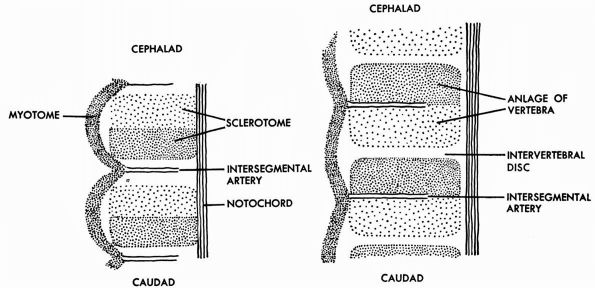 |
|
FIGURE 1-39. Early stages of differentiation of vertebrae. (Redrawn from Arey LB. Developmental Anatomy. Philadelphia: WB Saunders, 1974)
|
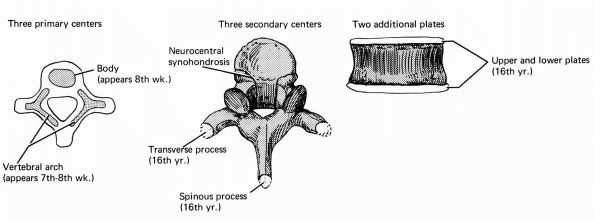 |
|
FIGURE 1-40.
Development of vertebra. Three primary ossification centers develop in utero; three secondary centers appear during the growth period and, with epiphyseal plates, become completely ossified as growth is completed. (After Goss CM. Gray’s Anatomy, 28th Ed. Philadelphia: Lea and Febiger, 1966) |
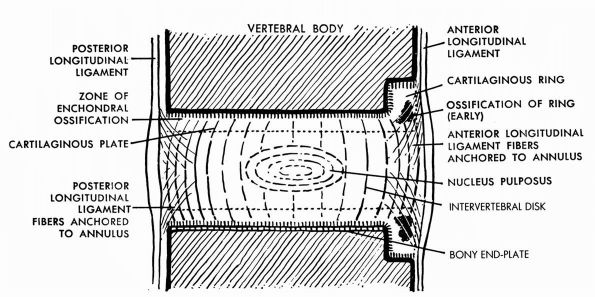 |
|
FIGURE 1-41.
Sagittal section through adjacent vertebral bodies during ossification of the cartilaginous ring. (Redrawn from Schmid P. Zur Entstehung der Adolezentenkyphose. Dtsch Med Wochenschr 1949;74:798) |
in the atlas. The body differentiates typically but soon is taken over
by the axis serving as a
peg-like
extension (dens) of the latter, about which the atlas rotates. The
atlas is left as a ring. The sacral and the coccygeal vertebrae
represent vertebra with reduced vertebral arches. The sacral vertebrae
eventually fuse into a single mass. The coccygeal vertebrae exist as
rudimentary structures. The entire spine at birth displays one
continuous curve convex posteriorly. As the erect posture is assumed
after the first year, secondary forward curves develop the cervical and
the lumbar regions. Finally, the lordosis in the cervical and the
dorsal regions is balanced by the kyphosis in the thoracic and the
sacral regions.
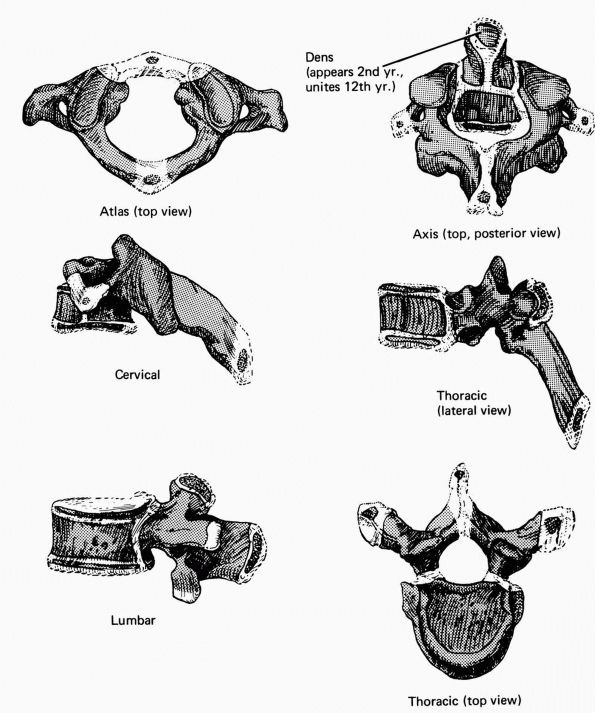 |
|
FIGURE 1-42. Ossification of the vertebrae.
|
vertebra is replaced by a joint for the head of the rib. The center of
ossification appears at the tangle of the rib. However, the distal ends
of the long ribs always remain cartilaginous. In the neck the ribs are
represented by their tubercles, which are fused with the transverse
processes and their heads fused with
the
bodies; between these processes is an interval, the transverse foramen,
through which the vertebral arteries course. When the costal processes
are overdeveloped in the cervical region, a supernumerary rib is
formed, which may lead to compression of nerve structures.
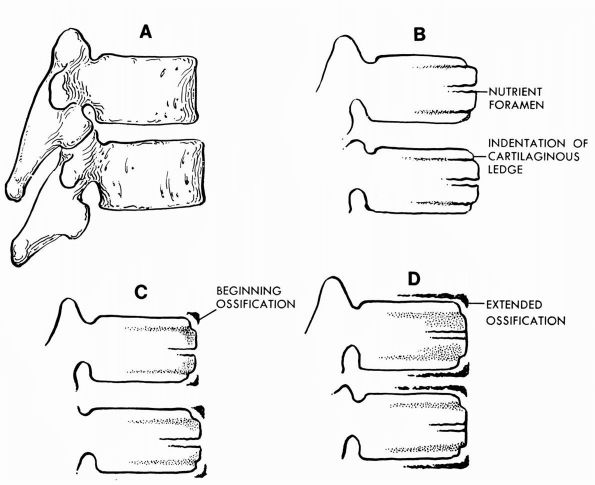 |
|
FIGURE 1-43. The appearance of normal thoracic vertebrae in children at various ages. (A) Normal thoracic vertebrae, fully developed, lateral view. (B) Lateral roentgenographic view of vertebral bodies in children under 6 years of age. (C) Lateral view in children, 6 to 9 years of age. Ossification starting in the cartilaginous ring is most visible anteriorly. (D)
At 9 to 15 years of age. Ossification is more extensive in the ring and progresses posteriorly. The nutrient foramen normally is obliterated after 6 years of age. |
ventrolaterally placed mesenchyme, which initially have no connection
with the ribs or which each other.
anterior bony centrum by cartilaginous bridges. The bony arch is
completed by the second year. Junction between arch and body occurs
between the third and sixth years.
unsegmented somatic mesenchyme. Definite masses are formed at the sites
of the future pectoral and pelvic girdles and limb buds. The sequence
of bone development through cartilaginous and osseous stages follows.
ossify. Before ossification, a peculiar tissue resembling both
membranous and cartilaginous tissue makes it difficult to classify the
origin. Two primary centers of ossification appear.
ossification and several epiphyseal centers that appear later. An early
primary center forms the body and the spine. The other, after birth,
gives rise to the coracoid process.
single primary center in the diaphysis and an epiphyseal center at each
end. Additional epiphyseal centers are constant at the lower end of the
humerus. Each carpal bone ossifies from a single center. The
metacarpals ossify from a single primary center and an epiphyseal
center (Figures 1-44 and 1-45).
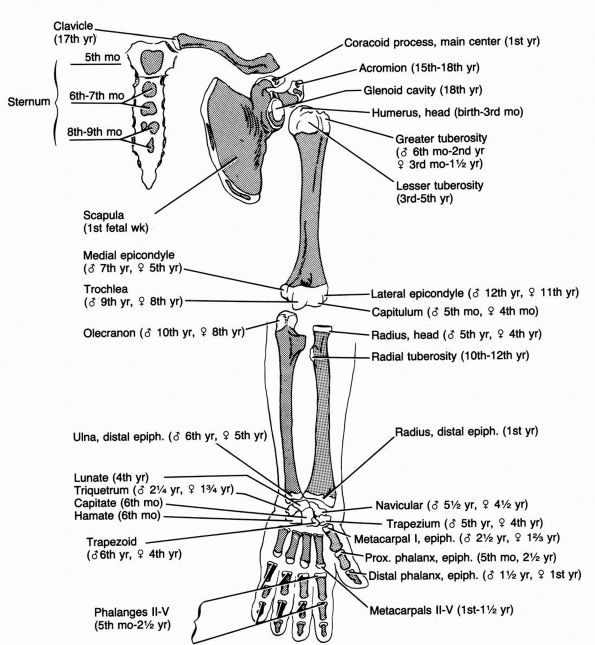 |
|
FIGURE 1-44.
The appearance at birth of the upper extremity. The ages at which the ossification of the epiphyses appear are shown with the differentiation between male and female indicated. |
perpendicular to the vertebral column. Later, it rotates to a position
parallel with the vertebral column and in relation to the first three
sacral vertebrae. Three main centers of ossification appear for the
ilium, the ischium, and the pubis. The three elements join at a
cup-shaped depression, the acetabulum, the articulation for the head of
the femur.
tarsus, the metatarsals, and the phalanges corresponds to that of the
bones of the upper extremity (Figures 1-46 and 1-47).
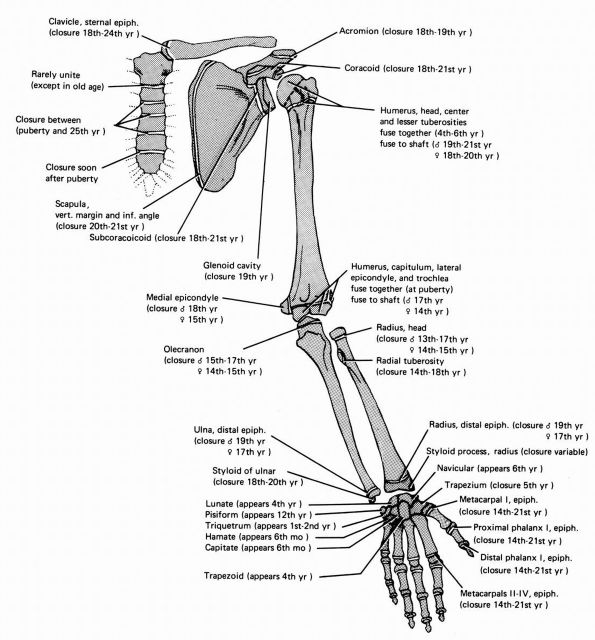 |
|
FIGURE 1-45. Stages of advancing ossification and epiphyseal closure of the upper extremity.
|
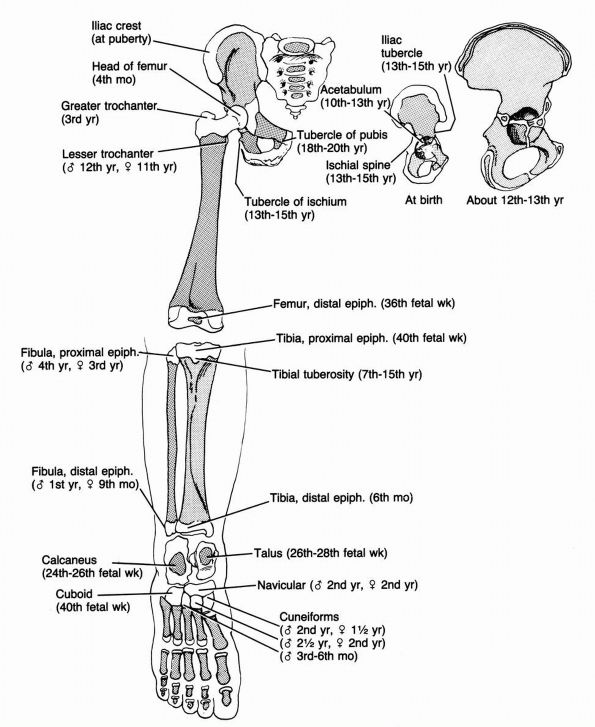 |
|
FIGURE 1-46.
The appearance at birth of the lower extremity. The ages at which ossification of the epiphyses appear are shown with the differentiation between male and female indicated. |
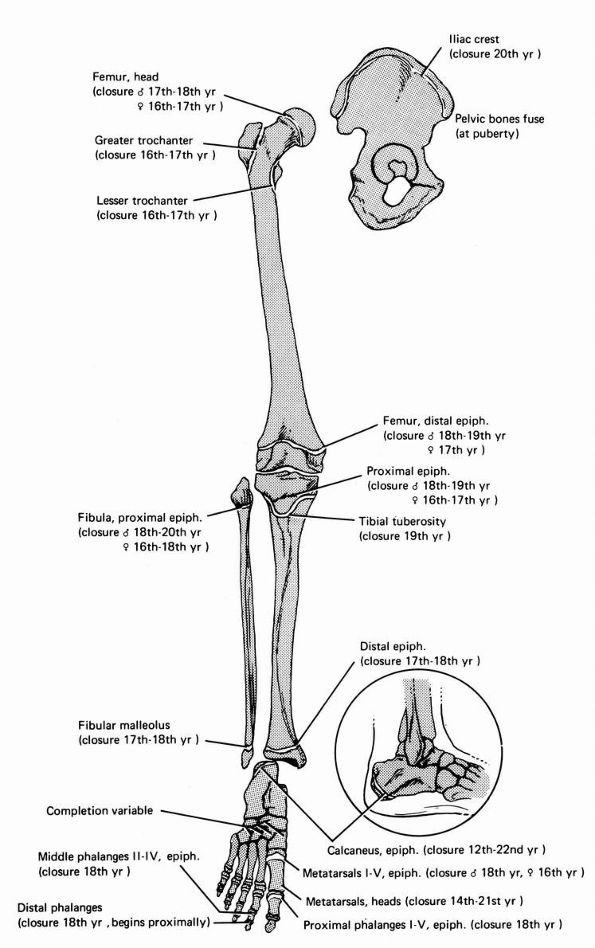 |
|
FIGURE 1-47. The stage of advancing ossification and epiphyseal closure of the lower extremity.
|
connective tissues (bone, cartilage, and dense fibrous tissues) and
muscle formed into functional units that make possible movements that
vary from the finely controlled motions of the upper limbs necessary to
play musical instruments, to the powerful motions of all four
extremities and the spine necessary to lift a heavy weights from the
ground, to the rapid coordinated repetitive motions required for
success in many sports. Mesenchymal cells form all musculoskeletal
tissues, but specific tissues differ in structure, composition,
mechanical properties, innervation, and blood supply. The skeletal
connective tissues (bone, cartilage, and dense fibrous tissue) consist
of sparsely distributed cells surrounded by a large volume of
extracellular organic matrix synthesized by the cells. Their matrix
molecular frameworks consist of collagens, proteoglycans, and
noncollagenous proteins. Each tissue has a unique combination of these
three classes of macromolecules, and in some tissues, like meniscus,
intervertebral disc, and ligament, elastin also forms part of the
organic matrix. In addition to their organic matrix, bone and some
cartilage regions have an inorganic matrix consisting of relatively
insoluble mineral deposited within the organic matrix. These
differences in matrix composition give the skeletal connective tissues
different mechanical properties. Hyaline cartilage has the stiffness in
compression, the ability to distribute loads and the durability to form
the low friction gliding surfaces of synovial joints. The dense fibrous
tissues have the tensile strength and flexibility to serve as tendons
and ligaments, and bone has the stiffness and strength to provide the
rigid support necessary for normal function of the other tissues.
Hyaline cartilage lacks nerves. Bone contains perivascular nerves and
some regions of the dense fibrous tissues have nerves that may sense
tissue deformation as well as perivascular nerves, but none of the
connective tissue cells have direct innervation. Hyaline cartilage, the
intervertebral disc (except for the outermost layers of the annulus
fibrosus), and some regions of dense fibrous tissue lack blood vessels.
Unlike the skeletal connective tissues, skeletal muscle consists
primarily of cells with only a small volume of extracellular matrices,
the tissue has a network of blood vessels that reaches every cell, a
motor nerve innervates each muscle cell, and the cells generate the
force necessary to produce movement.
understanding orthopaedic diseases, musculoskeletal function and
dysfunction cannot be understood in terms of individual tissues alone.
The mobility, strength, and stability of the body depend on the
organization and integration of these tissues into functional units.
Voluntary movement requires skeletal muscle contraction, but the
mechanisms by which muscle contraction produces joint motion, or the
failure of muscle contraction to produce joint motion, cannot be
understood by considering muscle cells alone. Muscles move or stabilize
synovial joints and the spine by transmitting the force generated by
individual muscle cells to tendons through elaborate muscle-tendon
junctions. Many tendons pass through sheaths and retinacula that make
it possible to effectively transmit the force generated by muscle to
bone through distinct insertions into bone or periosteum. The force
then acts on synovial joints consisting of articular cartilage,
menisci, bone, joint capsule, synovium and ligaments, or the spine. The
spine includes these tissues with the addition of the intervertebral
disc. Ultimately muscle contraction causes joint motion or stabilizes
joints against resistance, but synovial joint or spine function can
only be understood by considering how the multiple musculoskeletal
tissues perform simultaneously as parts of a functional unit. Likewise
understanding orthopaedic diseases depends on considering how
disturbances in the structure or function of one or more tissues alters
musculoskeletal function measured in terms of motion, strength, and
stability.
SP, McDevitt CA. The Meniscus: structure, function, repair and
replacement. In: Buckwalter JA, Einhorn T, Simon S, eds. Orthopaedic
Basic Science—Biology and Biomechanics of the Musculoskeletal System.
Rosemont, IL: American Academy of Orthopaedic Surgeons, 2000:531-545. This book chapter provides an overview of meniscal structure, composition, and function.
MPG, Boskey A, Kaufman JJ, Einhorn TA. Form and function of bone. In:
Buckwalter JA, Einhorn T, Simon S, eds. Orthopaedic Basic
Science—Biology and Biomechanics of the Musculoskeletal System.
Rosemont, IL: American Academy of Orthopaedic Surgeons, 2000:319-370. This book chapter discusses current understanding of bone structure and function.
JA, Boden SD, Eyre DR, Mow VC, Weidenbaum M. Intervertebral disc
structure, composition and function. In: Buckwalter JA, Einhorn T,
Simon S, eds. Orthopaedic Basic Science—Biology and Biomechanics of the
Musculoskeletal System. Rosemont, IL: American Academy of Orthopaedic
Surgeons, 2000:547-556. This book chapter provides a detailed overview of human intervertebral disc structure, composition, and function.
JA, Ehrlich MG, Sandell LJ, Trippel SB (eds). Skeletal Growth and
Development: Clinical Issues and Basic Science Advances. Rosemont, IL:
American Academy of Orthopaedic Surgeons, 1998. This
book discusses current understanding of skeletal growth and
development, including the clinical presentation of selected disorders
of skeletal growth and development.
JA, Glimcher MJ, Cooper RR, Recker R. Bone biology. part I. structure,
blood supply, cells, matrix and mineralization. J Bone Joint Surg
1995;77A:1256-1275.
JA, Glimcher MJ, Cooper RR, Recker R. Bone biology. part II. formation,
form, modeling and remodeling. J Bone Joint Surg 1995;77A:1276-1289. These two articles discuss the structure, composition, and biology of bone.
JA, Mankin HJ. Articular cartilage I. Tissue design and
chondrocyte-matrix interactions. J Bone Joint Surg 1997;79A:600-611. This article presents an overview of articular cartilage structure and biology.
WE, Best TM. Anatomy, physiology and mechanics of skeletal muscle. In:
Buckwalter JA, Einhorn T, Simon S, eds. Orthopaedic Basic
Science—Biology and Biomechanics of the Musculoskeletal System.
Rosemont, IL: American Academy of Orthopaedic Surgeons, 2000:683-716. This chapter provides a review of skeletal muscle structure and function.
JP, Goldstein S, Kuhn J, Lipiello L, Kaplan FS, Zaleske DJ. The
Formation and growth of skeletal tissue. In: Buckwalter JA, Einhorn T,
Simon S, eds. Orthopaedic Basic Science—The Biology and Biomechanics of
the Musculoskeletal System. Rosemont, IL: American Academy of
Orthopaedic Surgeons, 2000:77-109. A book chapter that reviews the formation, growth, remodeling, and maturation of skeletal tissues.
HJ, Mow VC, Buckwalter JA. Articular cartilage structure, composition
and function. In: Buckwalter JA, Einhorn T, Simon S, eds. Orthopaedic
Basic Science—Biology and Biomechanics of the Musculoskeletal System.
Rosemont, IL: American Academy of Orthopaedic Surgeons, 2000:443-470. This book chapter discusses articular cartilage structure and biology.
SL-Y, An KN, Frank CB, Livesay GA, Ma CB, Zeminski J, Wayne JS, Meyers
BS. Anatomy, biology and biomechanics of tendon and ligament. In:
Buckwalter JA, Einhorn T, Simon S. eds. Orthopaedic Basic
Science—Biology and Biomechanics of the Musculoskeletal System.
Rosemont, IL: American Academy of Orthopaedic Surgeons, 2000:581-616. This book chapter reviews the structure, function, and biology of tendons and ligaments.
