Lumbar Spinal Laminectomy
canal, most often caused by degenerative changes, that produces
compression of the neural elements. The narrowing may involve a single
or multiple motion segments. Symptoms created by lumbar stenosis
usually have an insidious onset and progress slowly. Vague complaints
of low backache and stiffness are typical and are related to
degenerative disc disease; in addition there are symptoms of
osteoarthritis that are often poorly localized discomfort in the
lumbosacral region. This pain, when associated with changes in position
and with lifting or bending may be indicative of underlying spinal
instability associated with degenerative scoliosis or
spondylolisthesis. Back symptoms tend to worsen with activity
(mechanical pain) and improve with rest. There are two categories of
leg pain symptoms associated with lumbar spinal stenosis. One type of
stenosis presents as unilateral leg pain along with numbness, burning,
and paresthesias radiating in a dermatomal distribution. This radicular
type of presentation is present in less than 20% of symptomatic
stenosis patients and is more often seen with severe foraminal or
lateral recess stenosis. Neurogenic claudication is defined as the
onset of lower extremity pain, paresthesias, or weakness on walking.
Symptoms are typically bilateral and consist of an aching, cramping, or
burning sensation in the legs. The discomfort starts in the buttocks
and often progresses distally to the thighs, calves, and feet. These
symptoms are classically exacerbated by standing and by exercising in
an erect or extended posture and are relieved by sitting and forward
flexion. Patients often assume a hunched or “simian” posture when
walking. This phenomenon can be explained by the fact that the size of
the spinal canal varies with different postures. Physical examination
most commonly reveals a normal neurologic examination. Lumbar extension
may exacerbate lower extremity symptoms. Range of motion (ROM)
evaluation of the hips and knees should always be performed to rule out
degenerative joint disease as a cause of leg pain. Groin pain is not
characteristic of lumbar spinal stenosis and should alert the examiner
to the possibility of hip arthritis or dysfunction of the sacroiliac
joint. An abdominal examination should be performed to rule out an
aortic aneurysm. Palpation of distal pulses is necessary to evaluate
the possibility of peripheral vascular disease.
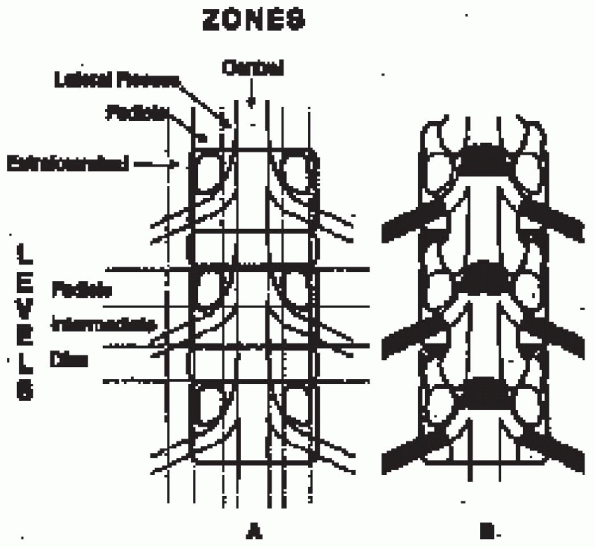
and the intervertebral nerve root foramen, can potentially compromise
the neural elements. The cauda equina is bound anteriorly by the disc,
the posterior longitudinal ligament (PLL), and the vertebral body. The
pedicles, together with the lateral margins of the ligamentum flavum,
create the lateral margins; the laminae, facet joints, and ligamentum
flavum form the posterior margins. The spinal nerve-root canal lies
within the lateral recess zone, beginning with the origin of the nerve
root sleeve at the disc level and ending as the nerve root passes along
the inferomedial border of the pedicle at the cephalad aspect of the
intermediate level. The intervertebral foramen lies within the pedicle
zone. The superior portion of the foramen is located at the
intermediate level, and the inferior portion is located at the disc
level. The intraspinal pathway of the nerve root and the spinal nerve
is formed by the lateral recess zone at the disc level, the pedicle
level as it extends to the cephalad aspect of the intermediate level,
and the intervertebral foramen at the intermediate level. These three
areas correspond to the entrance zone, the midzone, and the exit zone (Fig. 18-1).
either cause or anatomy. The original etiologic classification,
distinguishes congenital or developmental from acquired or degenerative
spinal stenosis. Congenital lumbar spinal stenosis is seen in patients
of normal stature with congenital short pedicles or in those with a
bone dysplasia, such as in achondroplastic dwarfs. Other rare disorders
that may manifest congenital stenosis include hypochondroplasia,
diastrophic dwarfism, Morquio’s syndrome, hereditary exostosis, and
cheirolumbar dysotosis. These individuals often first experience
symptoms in their 30s and 40s. Acquired stenosis is most likely
degenerative, usually starting in the sixth or seventh decade of life.
used to identify specific areas of narrowing of the spinal canal and
are particularly helpful as guides for operative decompression. The
anatomy of the spinal canal at each vertebral segment can be understood
better by dividing the canal into a series of transverse regions (three
levels from cephalad to caudad) and sagittal regions (three zones from
midline laterally). From cephalad to caudad, the three transverse
levels are the pedicle level, the intermediate (vertebral body) level,
and the disc level. The pedicle level extends from the
superior
to the inferior cortical margin of the pedicle. The intermediate level
begins at the inferior border of the pedicle and extends caudally to
the inferior end plate of the vertebrae. The disc level begins at the
inferior end plate and extends caudally to the superior border of the
next pedicle. From the midline laterally, the three sagittal zones are
the central zone, the lateral-recess zone, and the pedicle zone. The
central zone is the area between the normal lateral borders of the
noncompressed dural sac. The lateral recess zone is the area between
the lateral border of the dural sac medially and a longitudinal line
connecting the medial edges of the pedicles laterally. The pedicle zone
is the area between the medial and lateral borders of the pedicle.
 |
|
FIGURE 18-1.
Coronal plane of spine demonstrating neural pathway zones. Lateral recess, entrance zone; pedicle (foraminal), midzone; extraforaminal, exit zone. Spivak JM. Degenerative lumbar spinal stenosis. J Bone Joint Surg Am 1998;80(7):1054, with permission. |
 |
|
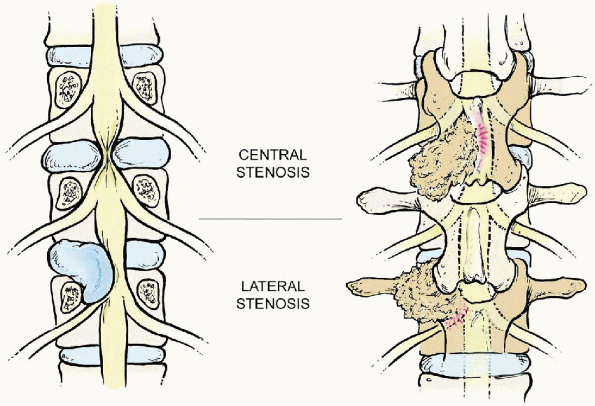
FIGURE 18-2. Central stenosis (top, left and right)
usually develops at the disc level from a bulging disc with facet joint overgrowth from the inferior articular process of the cephalad vertebrae and thickening and redundancy of the ligamentum flavum. Lateral stenosis (bottom, left and right) includes both the lateral recess and foraminal stenosis resulting from overgrowth from the superior articular process of the caudad vertebra and other degenerative changes similar to those of central stenosis. Lateral recess stenosis affects the spinal nerve root at the disc level. |
Central spinal stenosis commonly occurs at the disc level, as a result
of a bulging disc, facet joint overgrowth (mainly from the inferior
articular process of the cephalad vertebrae), and thickening and
redundancy of the ligamentum flavum. Lateral stenosis includes both
lateral recess and foraminal stenosis. Lateral recess stenosis, which
occurs as a result of the degenerative changes similar to those
associated with central spinal stenosis,
affects
the spinal nerve root canal at the disc level and the superior aspect
of the pedicle level. Lateral recess stenosis is uncommon at the
inferior aspect of the pedicle but may exist with hypertrophic
granulation tissue from the posteriorly located pars interarticularis
in patients with spondylolytic defects. Foraminal stenosis is most
common at the disc level. Thus, it usually begins in the inferior
portion of the intervertebral level. It is at this level that the
exiting nerve root may be compressed by disc material, an osteophyte
from the inferior aspect of the cephalad vertebrae, or from the
superior articular process of the caudad vertebrae.
for the treatment of symptomatic lumbar stenosis because it was thought
that this disorder was always progressive. However, more recent
information has suggested a more favorable natural history. The disease
has been shown to be relatively stable for 70% of patients with
symptomatic spinal stenosis. Long-term follow-up has indicated a 15%
incidence of worsening of symptoms and a 15% incidence of improvement
of symptoms.
and rapid catastrophic neurologic deterioration is very rare, the
decision to perform an operation should be made after failure of
nonoperative treatment, such as physical therapy, epidurals, and oral
medication. The presence of a nonprogressive neurologic deficit has
been shown to poorly correlate with physical function and thus is not
an absolute surgical indication. Progression of a functionally
disabling neurologic deficit or cauda equina syndrome, although rarely
associated with lumbar spinal stenosis, are two indications for urgent
operative intervention. Surgery should be reserved for those patients
who have evidence of severe spinal stenosis on imaging studies and have
a preponderance of lower extremity symptoms. Patient expectations
should be thoroughly discussed before surgery. The goals of surgery are
to decrease pain and improve function.
for general anesthesia: electrocardiogram (ECG), complete blood count
(CBC), erythrocyte sedimentation rate (ESR), liver and renal function
studies, and urinalysis. Any preexisting medical condition should be
followed and worked up. The preoperative imaging studies carry great
importance in determining the anatomic site of compression and the
proper extent of the decompression.
spinal stenosis is suspected often demonstrates multilevel spondylosis.
This may or may not be associated with stenosis of the spinal canal.
Degenerative spondylolisthesis and degenerative lumbar scoliosis are
radiographic findings suggestive of lumbar spinal stenosis.
Anteroposterior and lateral radiographs should be taken in the standing
position; dynamic flexion and extension radiographs taken in the
recumbent position are useful for determining whether there is abnormal
motion, or instability, at the affected level. For those patients who
have associated degenerative scoliosis, weight-bearing anteroposterior
and lateral radiographs taken on a long plate are particularly helpful
for assessing curve magnitude as well as the overall balance of the
entire spine in the coronal and sagittal planes (Fig. 18-3).
single test for establishing the diagnosis of lumbar spinal stenosis.
It provides excellent osseous detail, especially in the region of the
lateral recess. Limitations include ionizing radiation, inferior soft
tissue visualization, and inability to visualize the conus medullaris (Fig. 18-4).
Magnetic resonance imaging (MRI) provides superior visualization of the
soft tissue elements of the spinal canal and is especially useful for
the evaluation of abnormalities of the intervertebral disc. Its
diagnostic accuracy is superior to those of myelography and plain CT,
and it is as accurate and sensitive as myelography followed by CT. The
combination of axial and sagittal images allows for complete evaluation
of the central spinal canal and the neural foramen. Regions of very low
signal intensity on T2-weighted images caused by sclerotic osteophytes
can lead to overestimation of the amount of true osseous stenosis. MRI
of the scoliotic lumbar spine often is suboptimal because axial images
are often not made in the proper plane parallel to the involved disc
spaces. The combination of high-quality MRI and plain CT can provide
complete evaluation of the spinal canal and often obviates the need for
preoperative myelography (Fig. 18-5).
way to obtain a complete anatomic evaluation of compression of the
neural elements within the lumbar spine. Lateral myelograms made with
the spine in flexion and extension often demonstrate a dynamic
component of the stenosis that may be due to segmental instability (in
flexion) or encroachment on the spinal canal by bulging discs and
ligamentum flavum (in extension). Its main disadvantage includes its
invasive nature and insensitivity to foraminal disease. Recent studies
have suggested that MRI and CT myelography provide complementary
information. However, postmyelographic CT is superior to MRI as a
single study for the preoperative planning of decompression for lumbar
spinal stenosis.
(EMG), nerve conduction velocities (NCVs), and somatosensory evoked
potentials (SSEPs), are not considered part of the routine assessment
for establishing the diagnosis of lumbar spinal stenosis. It is not
uncommon to have normal electromyograms and NCVs in a case of classic
lumbar spinal stenosis. However, the more typical pattern in
symptomatic
patients
is that of a polyradiculopathy, often with bilateral involvement of
multiple levels. The NCV, which measures the speed at which the nerve
impulse travels, is particularly useful in differentiating peripheral
neuropathy (prolonged) from radiculopathy (normal).
 |
|
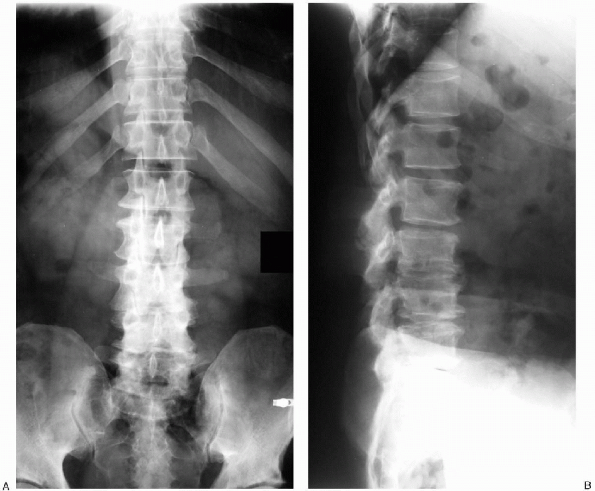
FIGURE 18-3. A: Coronal radiograph demonstrating sclerosis of the facet joints and vertebral body osteophyte formation. B:
Lateral radiograph revealing typical findings associated with advanced lumbar spondylosis: loss of lumbar lordosis and multilevel intervertebral disc space narrowing. |
 |
|
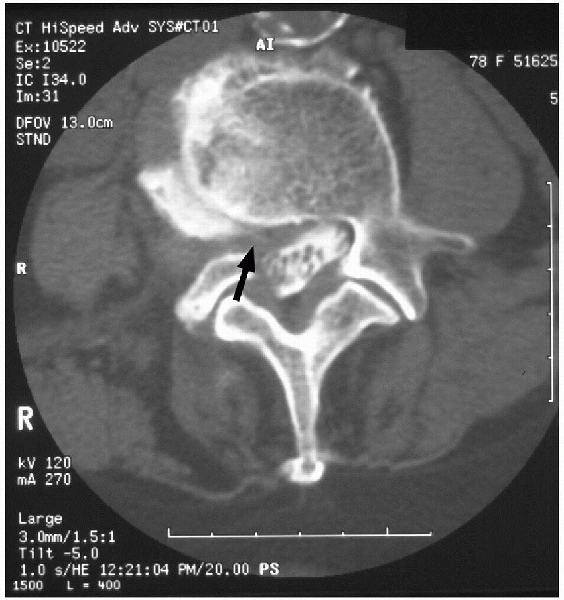
FIGURE 18-4. Axial computed tomography demonstrating high-grade right lateral recess stenosis.
|
 |
|
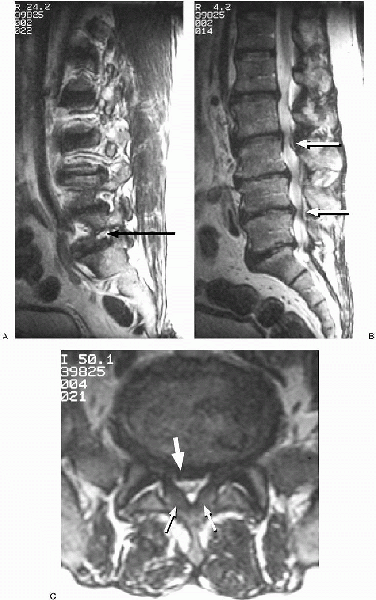
FIGURE 18-5. T2-weighted magnetic resonance images. A: Parasagittal L5-S1 foraminal stenosis (arrow) with L5 nerve root compression. B: Myelogram-like effect of T2-weighted sagittal image demonstrating multiple levels of severe central canal stenosis (arrows). C: Axial image revealing redundant ligamentum flavum (small arrows), and a significant bulging of the disc (large arrow) posteriorly, creating a severe degree of central and lateral recess stenosis.
|
-
Frazier suction with stylet (8, 10, 12 French)
-
Cobb elevators
-
Cobb curettes
-
Stille-Horseley bone cutter
-
Leksell rongeur (narrow, 3 mm; medium, 5 mm; large, 8 mm)
-
Assortment of Kerrison rongeurs
-
Deep Gelpi retractor or McCullough retractors
-
Pituitary rongeur
-
Penfield (no. 2, no. 3, no. 4)
-
Ball dissector
-
Woodson elevator and spatula
-
Scoville nerve root retractor
-
Bone wax
-
Gelfoam soaked in thrombin
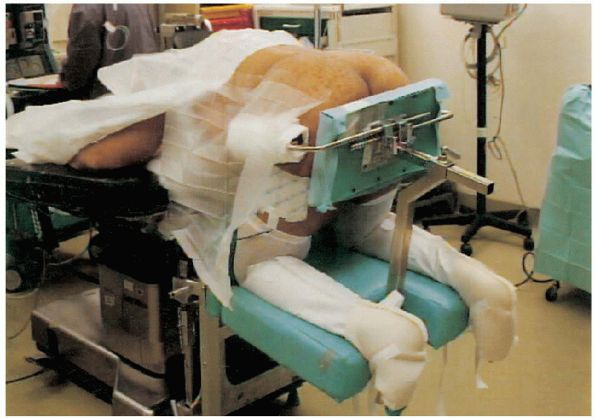
positioned prone, either on a Jackson table or a four-poster frame, or
in a knee-chest position (on the Andrews frame) with the abdomen
hanging free (Fig. 18-7). We prefer the abdomen
to be free of compression, thus lowering central venous and inferior
vena cava pressures and diminishing intraoperative blood loss. The
Jackson table allows for hip extension, thus maximizing lumbar
lordosis. This is particularly effective when internal fixation is
planned. All pressure points should be checked and padded well. The
facial structures particularly the eyes should be free of pressure.
Compression stockings and intermittent pneumatic compression boots are
routinely used to decrease the incidence of deep venous thrombosis
(DVT). Bladder catheterization should be employed for cases lasting
longer than 2 hours or when excessive blood loss is anticipated.
Perioperative antibiotics should be administered in the form of one
preoperative dose in the holding area and postoperative doses over 24
hours.
 |
|
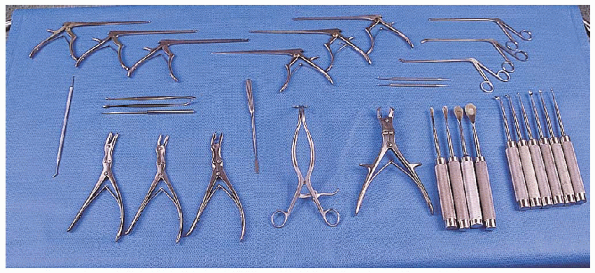
FIGURE 18-6. Equipment: Kerrison rongeurs (6), pituitary rongeurs (3) (top row); Woodson elevator and spatula, Penfields (no. 2, no. 3, no. 4), Scoville nerve root retractor, ball dissectors (2) (middle row);
Leksell rongeurs (narrow, 3 mm; medium, 5 mm; large, 8 mm), deep Gelpi retractor, Stille-Horseley bone cutter, Cobb elevators, Cobb curettes (bottom row). |
 |
|
FIGURE 18-7. Patient is prone and placed in a knee-chest position with the abdomen hanging free.
|
landmarks used to design an appropriate midline skin incision are the
top of the iliac crest (usually L4-5) and the lowest interspinous
region palpated (usually L5-S1) (Fig. 18-8). One should consider using an operating microscope or loupe magnification with headlight illumination to
facilitate the decompression. Placement of a needle followed by a lateral radiograph is helpful to localize the correct level.  A standard midline incision (Fig. 18-9)
A standard midline incision (Fig. 18-9)
is then made, and the underlying fascia is identified. The midline
fascia is then split with an electrocautery, and the paraspinal
musculature is stripped in a subperiosteal manner with Cobb elevators (Fig. 18-10).  The paraspinal gutters are then packed with gauze sponges for hemostasis (Fig. 18-11). After the sponges are removed, self-retaining cerebellar retractors are placed (Fig. 18-12).
The paraspinal gutters are then packed with gauze sponges for hemostasis (Fig. 18-11). After the sponges are removed, self-retaining cerebellar retractors are placed (Fig. 18-12).
 |
|
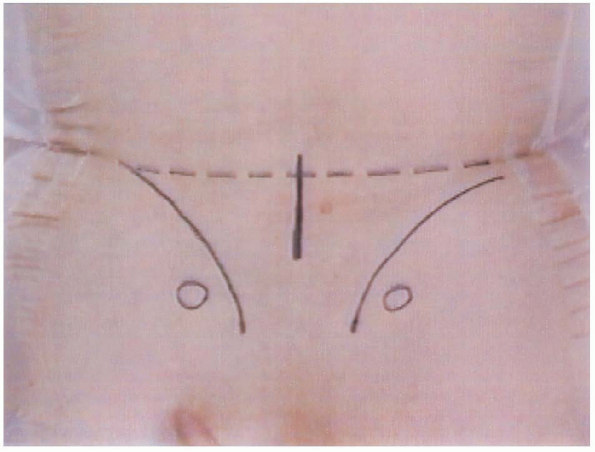
FIGURE 18-8.
Preoperative bony landmarks. Circles are posterior superior iliac spines, curved lines are the iliac crests, and the center vertical line represents the incision. |
 |
|
FIGURE 18-9. Incision.
|
 along with a partial facetectomy to decompress the lateral recesses and foramina, bilaterally. One should begin the decompression in the least stenotic area, progressing to the more stenotic areas (Fig. 18-18). In addition, a central trough should be created first (Fig. 18-19),
along with a partial facetectomy to decompress the lateral recesses and foramina, bilaterally. One should begin the decompression in the least stenotic area, progressing to the more stenotic areas (Fig. 18-18). In addition, a central trough should be created first (Fig. 18-19),  followed by lateral decompression of the recesses and foramen (Fig. 18-20).
followed by lateral decompression of the recesses and foramen (Fig. 18-20).
Compressed nerve roots are then directly visualized and decompressed
from their origin at the thecal sac and throughout their course as they
exit the neural foramina (Fig. 18-21).

 |
|
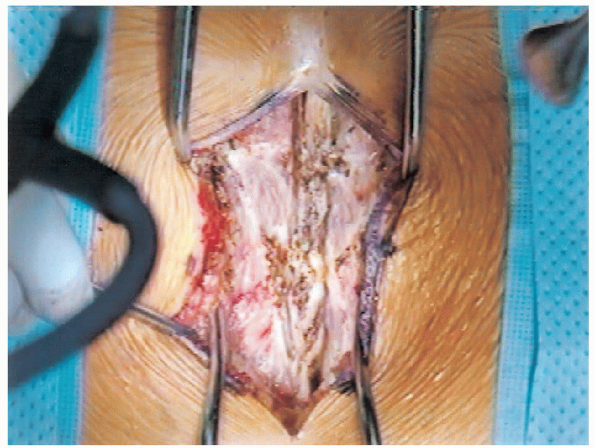
FIGURE 18-10. Opening of fascia and exposure of spinous processes.
|
 |
|
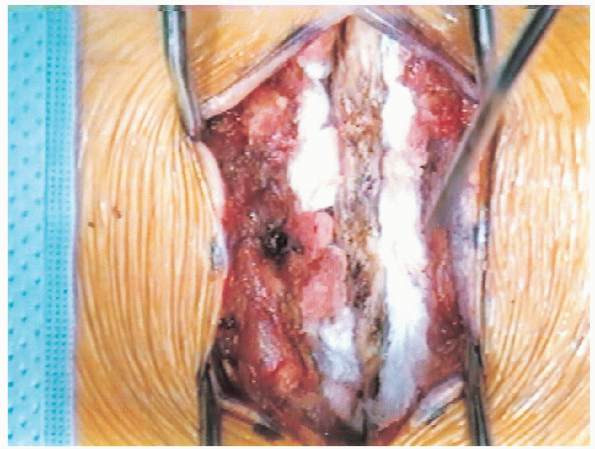
FIGURE 18-11. Exposure of spinous processes, laminae, and facet joints. Blunt dissection with Cobb elevators and packing with gauze.
|
performed in line with the nerve roots as they move through the
foramen, thereby avoiding amputation of the roots. As each level is decompressed, one should consider packing the neural structures with Gelfoam soaked in thrombin (Fig. 18-22) along with paddies to maintain adequate hemostasis (Fig. 18-23). The electrocautery should be set at a low
level when working in the spinal canal. A sharp 0.25-inch osteotome is
particularly effective for performing a partial facetectomy in the
presence of severe stenosis. The discs should be
directly inspected following a laminectomy to exclude significant
anterior compression secondary to herniation. Small disc-osteophyte
complexes should be ignored; however, larger soft herniations may require excision.
Alternative techniques of decompression for the treatment of
degenerative central and lateral recess stenosis have been designed in
an attempt to preserve more of the osteoligamentous arch, theoretically
diminishing the problem of postoperative instability. These techniques
include a beveled laminectomy with angular resection of only the
anterior portion of the lateral aspect of the lamina, selective single
or multiple unilateral or bilateral laminotomy (fenestration
procedure), multiple partial laminectomy, and lumbar laminoplasty. The
problem of regrowth of bone with clinically important recurrent
stenosis may be more frequent in association with methods of
decompression involving limited resection of bone. Incidental
durotomy should be primarily repaired when encountered. Nonbraided 6-0
nonabsorbable suture is our suture of choice. Placing
the patient in a Trendelenburg position, while instructing the
anesthesiologist to hold an inspiration to prevent movement, is helpful
when repairing the defect. Fibrin glue or tissue seal
is particularly useful for small dural defects not reparable. If
persistent postoperative cerebrospinal fluid leakage is anticipated,
one should consider placing a subarachnoid diverting drain for 2 to 3
days postoperatively. When closing the wound, consider a medium-size
Hemovac drain to prevent postoperative compressive hematoma formation.
Drains should be avoided with persistent leakage of cerebrospinal fluid
to avoid an arachnoid-cutaneous fistula. Avoid
overzealous decompression around the area of the pars interarticularis
to prevent intraoperative or postoperative iatrogenic pars fractures.
 |
|
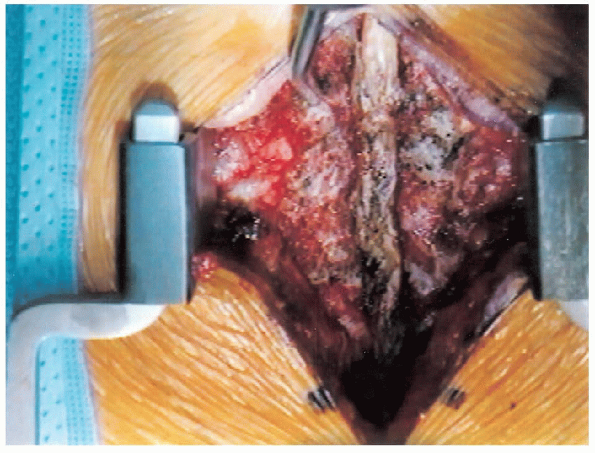
FIGURE 18-12. Spinous processes, laminae, and facet joints are exposed.
|
 |
|
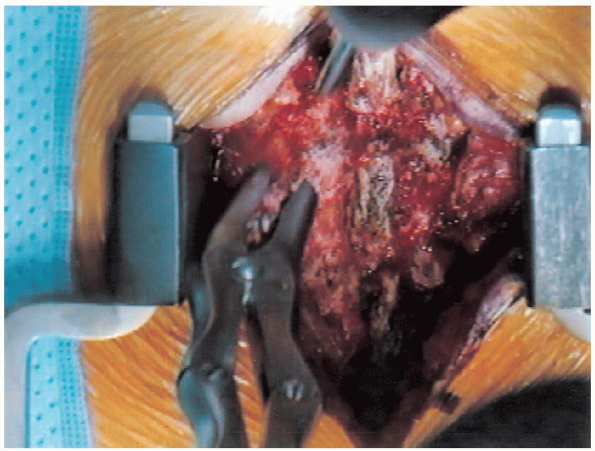
FIGURE 18-13. Spinous process resection.
|
 |
|
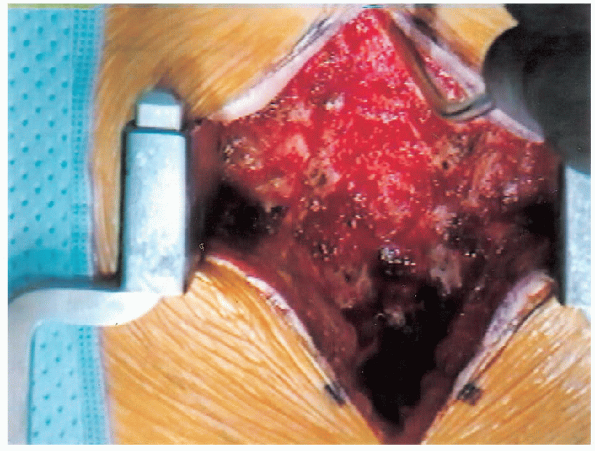
FIGURE 18-14. Spine following resection of spinous processes.
|
 |
|
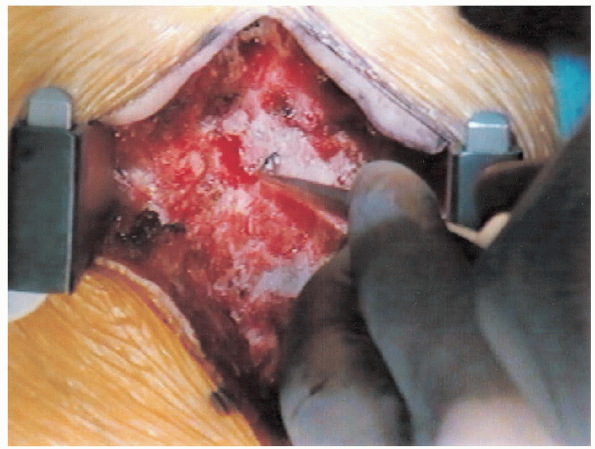
FIGURE 18-15. Finding the edge of the lamina with an “upgoing” curette.
|
 |
|
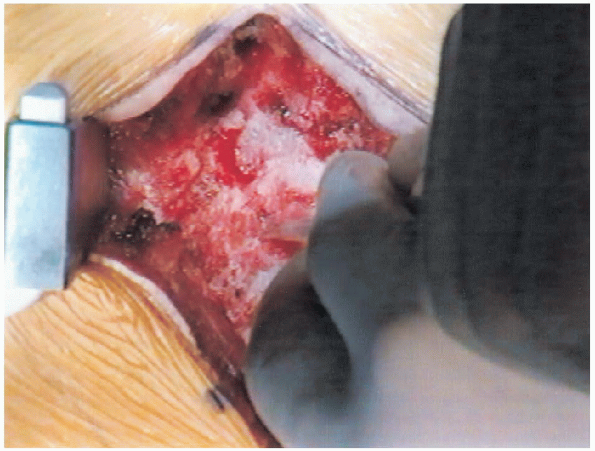
FIGURE 18-16. Curette introduced sublaminally.
|
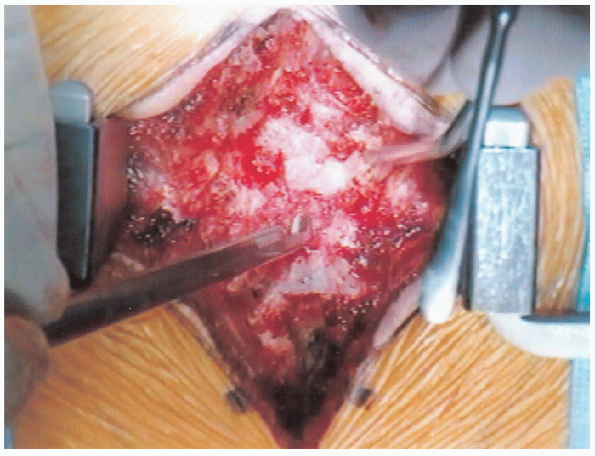 |
|
FIGURE 18-17. Beginning the laminectomy.
|
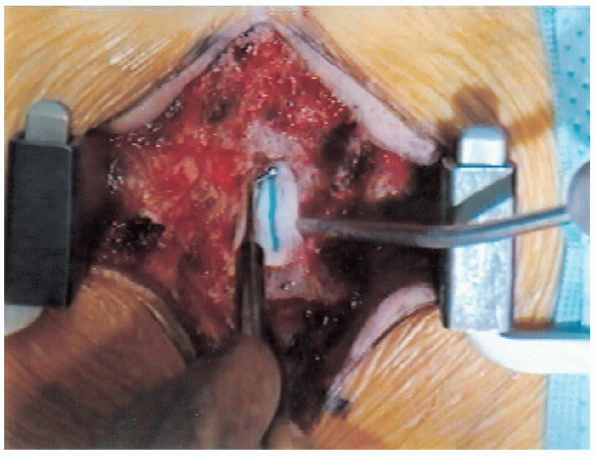 |
|
FIGURE 18-18. Introducing patty between the lamina and dural sac using a ball-point probe.
|
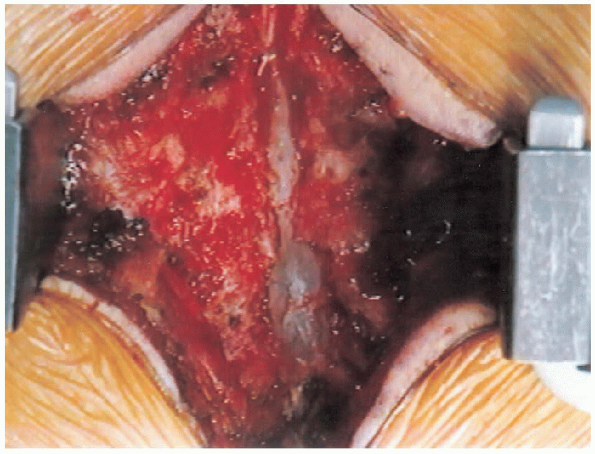 |
|
FIGURE 18-19. Central trough laminectomy completed.
|
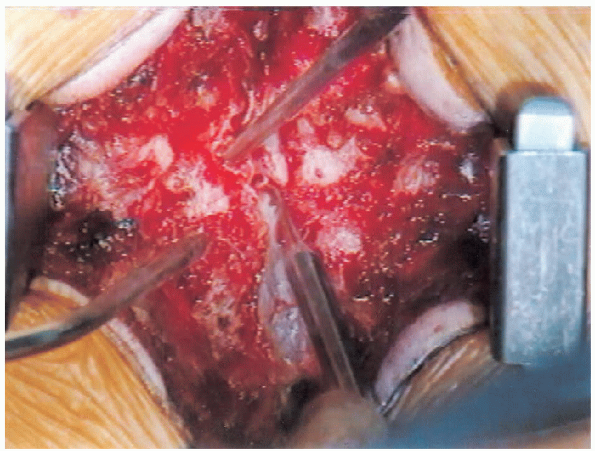 |
|
FIGURE 18-20. Lateral recess decompression initiation.
|
 |
|
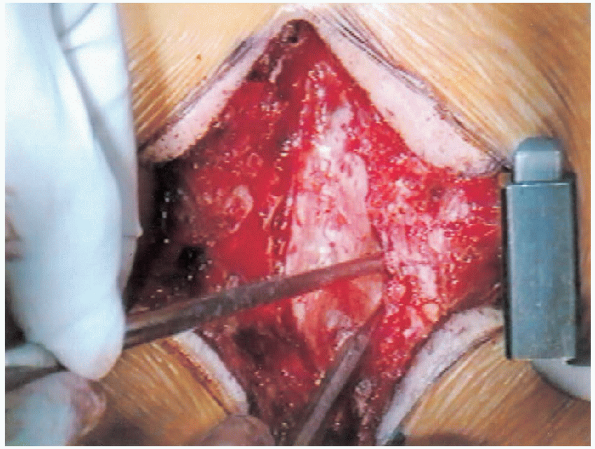
FIGURE 18-21.
Widening of the laminectomy while keeping a close watch to avoid injuring the pars interarticularis. Care is taken to avoid too much resection of the medial facet. |
 |
|
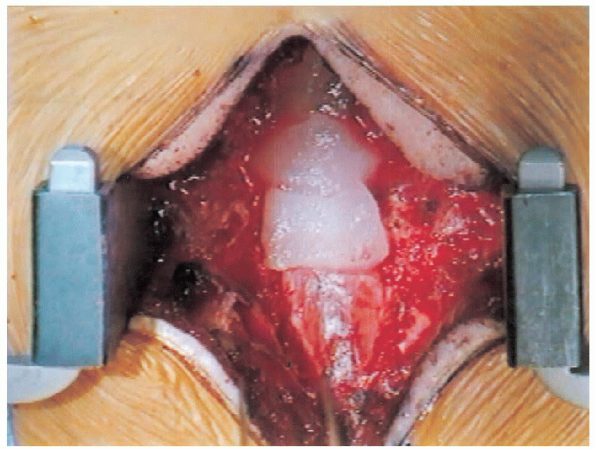
FIGURE 18-22. Covering the dura with Gelfoam for hemostasis.
|
 |
|
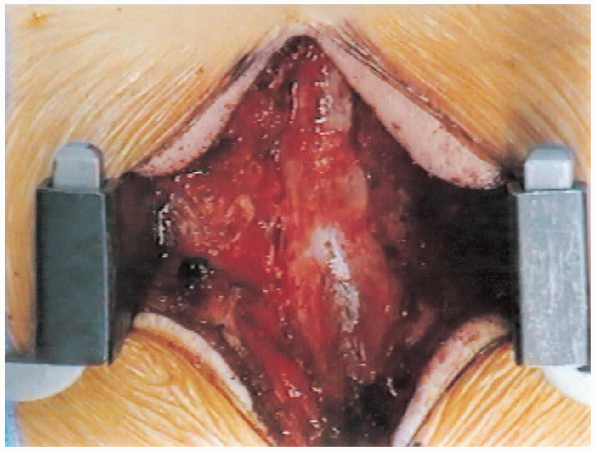
FIGURE 18-23. Completion of laminectomy.
|
lumbar spinal stenosis has been the subject of many debates in the
recent literature. Following routine decompression in the absence of
spinal deformity or instability, concomitant spinal arthrodesis
generally does not provide additional clinical benefit. Instability
created by resection of the facet joints at the time of operation may
be an indication for arthrodesis to prevent postoperative instability
and pain. It is generally accepted that stability will be maintained if
greater than 50% of each facet joint is left intact. However,
biomechanical evidence has suggested that instability may occur after
unilateral total facetectomy, even if the remaining facet has been left
completely intact.
lumbar spinal stenosis with associated degenerative spondylolisthesis
has also been extensively studied. Most studies indicated that there is
an improvement in clinical outcome in these patients when an
arthrodesis is performed at the time of initial decompression.
Investigators have discovered that there is a higher chance of
reformation of spinal stenosis and instability following laminectomy
without fusion in patients with spondylolisthesis. Preoperative
radiographic and anatomic risk factors associated with the
postoperative development or progression of spondylolisthesis at the
level of the fourth and fifth lumbar vertebrae include a well
maintained disc height, the absence of degenerative osteophytes, and a
smaller, sagittally oriented facet joint.
association with degenerative scoliosis is not as clear cut a picture
as that of degenerative spondylolisthesis. Not all patients with
surgically significant spinal stenosis within a lumbar scoliosis
require a simultaneous posterolateral fusion procedure. Certain factors
should be assessed preoperatively. The flexibility of the curve should
be evaluated by side-bending radiographs. A flexible curve that lacks
osteophytes may require arthrodesis to avoid postoperative progression
following laminectomy. In some instances, a single symptomatic nerve
root can be isolated by means of selective diagnostic injections,
allowing for a more limited decompression. A documented history of
curve progression should also prompt concomitant arthrodesis. In
addition, arthrodesis with curve correction is often necessitated with
radiculopathy within the concavity of the curve. Also, a lateral
listhesis is an indication of instability and should be fused. Lastly,
a patient with significant loss of lordosis and sagittal imbalance may
prompt an arthrodesis to prevent further postoperative kyphosis and
increasing back pain.
concerns. When combined with a laminectomy, “radical” disc excision may
lead to iatrogenic spondylolisthesis because it potentially
destabilizes the anterior column. A unilateral discectomy performed in
addition to a total laminectomy should not lead to postoperative
instability when the spine is stable preoperatively. However, if
bilateral discectomy is performed along with laminectomy, consideration
should be given to concomitant arthrodesis to prevent postoperative
instability.
spinal stenosis remains controversial. Earlier studies suggested
superior clinical results and improved fusion rates when
instrumentation was employed. Rigid constructs have been associated
with a better clinical result than semirigid constructs, which allow
more motion between the fixation screws and the rod or plate. More
recent studies suggest that adding instrumentation may improve the
fusion rate, although this seldom correlates with clinical outcome. In
the face of a degenerative scoliosis, after removal of compressive bone
and ligamentous tissue, additional decompression can be accomplished by
realignment of the spine with use of segmental distraction via
instrumentation along the concavity of the curve. This is done after
setting of the convex rod first, with mild compression between screws
to preserve or improve lumbar lordosis. For the rare presentation of a
painful, collapsing, degenerative scoliosis with back pain only, there
may be a role for corrective instrumentation and fusion alone.
They are encouraged to remain out of bed, and ambulation training is
begun on the first postoperative day. Occasionally, a lumbosacral
corset is prescribed for excessive back pain, although it is not part
of the routine recommendation. A patient who had a durotomy is advised
to remain flat on the back for 24 to 48 hours to prevent headache and
leakage of cerebrospinal fluid. Most patients are discharged home on
the second postoperative day.
stenosis have been generally good. However, more recent studies suggest
that the long-term outcome may not be as good as originally thought.
The initial favorable clinical outcome
seems
to deteriorate over time. Factors associated with a poorer outcome
include multiple comorbidities, single-level decompressions, a
predominance of low back pain, diabetes with neuropathy, osteoarthrosis
of the hip, and preoperative degenerative scoliosis. There is a 5%
incidence of symptomatic degeneration at levels adjacent to the
decompression. Recurrent symptoms may be caused by recurrence of
stenosis at a level that was previously decompressed, progression of
stenosis at an adjacent level, or mechanical back pain with instability.
for lumbar spinal stenosis also can affect the function of the bladder.
The results of cystomanography and electromyography do not change after
decompression, but the postvoiding residual volume often improves in
those patients in whom it had been elevated preoperatively.
Degenerative lumbar spondylolisthesis with spinal stenosis: a
prospective, randomized study comparing decompressive laminectomy and
arthrodesis with and without spinal instrumentation. Spine 1997; 22: 2807-2812.
Degenerative lumbar spondylolisthesis with spinal stenosis: a
prospective study comparing decompression with decompression and
intertransverse process arthrodesis. J Bone Joint Surg Am 1991; 73A: 802-808.
The effect of nerve-root injections on the need for operative treatment
of lumbar radicular pain. A prospective, controlled, double-blind
study. J Bone Joint Surg Am 2000; 82A: 1589-1593.
