FAILED AND REVISION CERVICAL SPINE SURGERY
VIII – THE SPINE > Disc Injury and Degeneration > CHAPTER 148 –
FAILED AND REVISION CERVICAL SPINE SURGERY
population of chronic, failed cervical spine surgery patients is
smaller than for the lumbar spine. However, cervical spine surgery is
becoming more common. Fusion procedures increased 70% between the
periods of 1979–1981 and 1988–1990 (11). With
this increase, more complications will be encountered and the need for
revisions will rise. This chapter will focus on pseudarthrosis,
residual compression, postlaminectomy kyphosis, hardware failure, and
progressive or adjacent segment degeneration.
unresolved psychosocial problems are less likely to achieve a
satisfactory outcome from primary cervical spine surgery. Unrealistic
expectations and poor compliance with postoperative care also reduce
the chances for successful post-surgical outcome. Nutrition, smoking,
diabetes, and steroid use all have implications in wound healing, bony
fusion, and recovery.
primary cervical surgery and will make revision surgery even more
difficult. When failure is the result of, or compounded by, any of
these factors, the issues must be addressed before surgical revision is
likely to succeed.
errors in diagnosis, surgical timing, and intended procedure. Common
errors of surgical judgment include choosing the wrong approach,
selecting improper fusion levels, or recommending improper
postoperative care. These errors will be considered in terms of their
outcome: deformity, pseudarthrosis, or inadequate decompression.
Perioperative events, including infection, dural leak and
pseudomeningocele, and hematoma are not uncommon causes of clinical
failure and must be evaluated before planning a revision procedure.
fusion procedures, but it is not always the cause of postoperative neck
pain. Anterior cervical discectomy (ACD), without fusion, relieves
radicular and axial neck pain in many patients. The success of ACD
highlights the fact that nonunion is not always painful (26).
Nonetheless, the most common cause of axial pain or radicular symptoms
after anterior cervical discectomy and fusion (ACDF) is still
pseudarthrosis (36,41). Published reviews of ACDF outcomes report pseudarthrosis in up to 26% of patients (13,22,32,36).
Patients at increased risk for pseudarthrosis after ACDF include those
undergoing multilevel, individual fusions and those fused with
allograft (20,48).
-
Continued or worsening axial pain 6 months after the index procedure
-
Complete radiolucency at the host–graft interface
-
Vertebral body motion of >2 mm on flexion–extension films
interface between the graft and the vertebral body below. Only 9% of
patients with pseudarthrosis felt better after their initial surgery;
27% felt the same, and 64% felt worse.
cervical fusion is relatively sparse. Posterior fusions do not enjoy
the biological advantage of grafting under compression, but overall,
fusion rates are felt to be high. Reported pseudarthrosis rates
following traditional wiring techniques range from 0% to 50% (16),
but it can be expected in 10% of patients. Outcomes after rigid
posterior plating are reported with rates of 0% to 1.4% pseudarthrosis (16).
cause of failure in both anterior and posterior cervical spine
procedures (29). The diffuse nature of
degenerative changes in the cervical spine sometimes requires more
global or comprehensive procedures than the surgeon is initially
willing to entertain. Residual compression after an index spine
procedure may result from any of the following:
-
Failure to perform a complete decompression at the injured/involved level
-
Failure to decompress adjacent involved levels
-
Migration of graft or fixation materials into the canal or foramen
-
Wrong-level surgery
posterior longitudinal ligament (PLL) may be a significant source of
residual compression (44). Some surgeons feel
that posterior osteophytes will resorb after successful fusion.
Therefore, they avoid PLL resection and the dangers of operating near
the spinal cord. However, the extent of osteophyte resorption is
controversial. Recent studies have noted little remodeling or
resorption after solid ACDF (32,38).
Some authors have advocated more complete decompression in this area
through PLL resection and direct visualization with an operating
microscope or loupes (19).
fracture may impair neurologic recovery. While stabilization alone
affords some protection of neurologic tissues, adequate decompression
of bony and soft-tissue elements increases neurologic recovery (31).
angulation of the cervical spine occurring after posterior
decompression (21). Wide decompression
necessarily sacrifices all or part of the facet and eliminates the
attachments for the posterior spinal musculature. Bilateral facetectomy
of more than 25% of the facet increases cervical motion in all planes
and should prompt posterior fusion to prevent deformity (27,28).
After extensive laminectomy, glacial progression toward scoliosis or
kyphosis occurs in 30% to 50% of younger patients, and fusion is
indicated (39).
-
Young age (into third decade)
-
Preoperative deformity [particularly S-shaped or kyphotic deformities (25)]
-
Removal of more than four laminae
-
Destruction of facets
-
Tumors
-
Removal of C-2 posterior elements (major semispinalis insertion)
-
Paralysis with paraspinal muscle weakness
-
Anterior instability following fracture
status of the cervical spine and its relationship to the construct
employed, the likelihood of failure of most modern instrumentation is
small. Fixation failure reflects the types of failure already described:
-
Persistent pseudarthrosis results in fatigue failure or failure at the host–hardware interface.
-
Underestimated or unrecognized biomechanical loads cause acute failure.
-
Aggressive postoperative mobilization, particularly in osteopenic bone, causes acute or progressive hardware loosening.
-
Noncompliant patients can accelerate all these processes, particularly when smoking retards the healing process.
-
Progressive destruction by tumor or infection further destabilizes the spine.
These changes may reflect local biomechanical alterations that result
from an existing fusion (adjacent segment degeneration) or may simply
reflect the natural history of that patient’s cervical spondylosis. As
such, this degeneration may take the form of recurrent stenosis,
recurrent disc herniation, or degenerative disc disease. Up to 89% of
ACDF patients report symptomatic degeneration at long-term follow-up (17,43).
Anecdotal evidence suggests that fusions ending at C-5 or C-6 are
particularly associated with recurrent axial neck pain, perhaps because
of the increased segmental motion at these levels (40).
arthrodesis, rule out inadequate decompression first. Then evaluate
radicular or myelopathic symptoms for evidence of foraminal or canal
stenosis or recurrent disc herniation. In patients with predominantly
axial pain, assessment of the cervical discs above and below the fusion
mass remains controversial. Some authors recommend cervical discography
with anterior fusion for concordant pain (33).
-
Residual axial pain
-
Recurrent or residual myelopathy
-
Recurrent or residual radiculopathy
-
Development or progression of deformity
 |
|
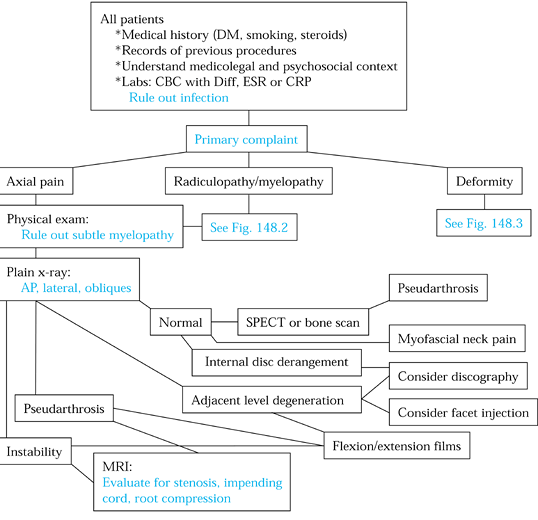
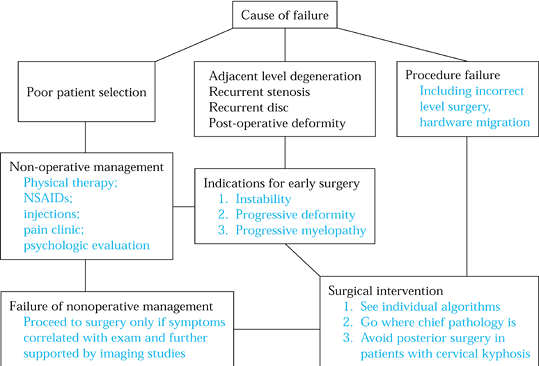
Figure 148.1. Evaluation algorithm for patients with failed cervical spine surgery
|
 |
|
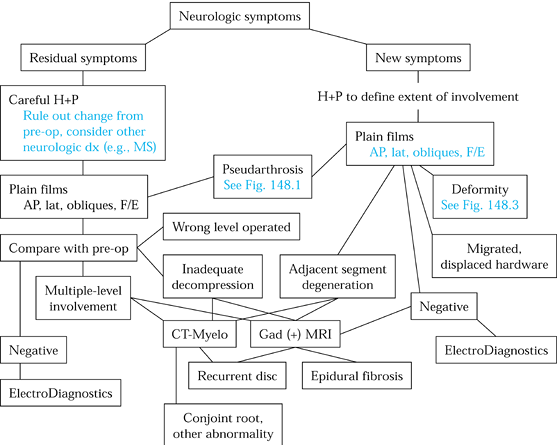
Figure 148.2. Evaluation algorithm for radiculopathy and myelopathy.
|
 |
|
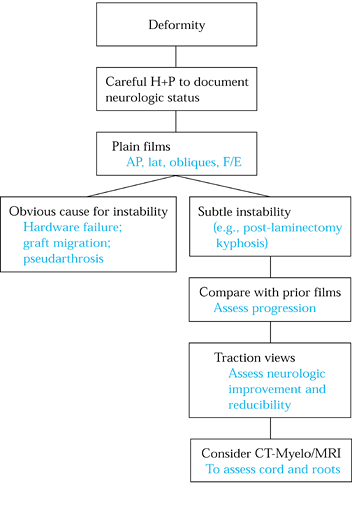
Figure 148.3. Evaluation algorithm for postsurgical deformity.
|
-
Axial neck pain
-
Recurrent radiculopathy or myelopathy from regrowth of posterior osteophytes
-
Deformity from failure of intended fusion after wide posterior decompression
-
Neural compression after cervical spine
surgery, most commonly related to inadequate decompression, recurrent
disc herniation, or recurrent stenosis -
Radicular pain (radiculitis), one of the
earliest symptoms of neural compression; often relieved by distraction
and increased with axial loading -
Weakness and sensory changes, presenting in a radicular pattern (radiculopathy) with increasing compression
-
In advanced cases, lower motor neuron
findings, including weakness and diminished reflexes, at the level of
compression; below the compression, upper motor neuron signs, including
hyperreflexia and spasticity -
Ataxia, clumsiness, diffuse lower
extremity weakness, and bowel and bladder problems (i.e., myelopathy)
after cervical spine surgery, often related to the following:Large central disc (less common)Severe, unaddressed osteophytosis (with normal or stenotic canal)Hardware displacementPostoperative deformity
history of prior posterior cervical spine surgery and an often subtle,
progressive pattern of pain and neurologic change (18):
-
Neck pain (75%)
-
Severe neck deformity (30%)
-
Progressive myelopathy (90%)
-
Radiculopathy (50%)
of tracheal or esophageal impingement. Sudden changes in alignment,
neurologic status, or pain often indicate failure of hardware. Patients
may be aware of a “pop” or acute onset of instability. However, slow
pull-out of lateral mass plates, for example, can present with slowly
increasing deformity, such as is seen in postlaminectomy kyphosis.
treatment decisions. First, obtain complete records of previous
procedures and determine the following:
-
What types of postoperative immobilization were employed?
-
What was the condition of the bone?
-
Was allograft or autograft employed?
-
Was the PLL resected?
Patients with prior cervical spine surgery are likely to demonstrate
complex abnormalities. Perform a detailed and stepwise assessment of
sensory, motor, and reflex abnormalities, as well as a careful search
for evidence of myelopathy to define new or residual neurologic
deficits. Note bowel or bladder changes, gait disturbance, and the
unilaterality or bilaterality of symptoms. The following may be a
useful checklist:
-
Recognize possible aberrant innervation patterns (23).
-
Map complex sensory deficits on the skin with a skin marker.
-
Chart complex motor and reflex changes.
-
Distinguish cervical problems from those of shoulder, cardiac, cranial, or peripheral origin.
-
Assess the location and healing of the prior incision(s).
-
Assess cervical range of motion (ROM) and neck and body posture.
-
Note areas of tenderness and spasticity of paravertebral and anterior strap muscles.
views with prior studies to assess progression of spondylosis, fusion,
deformity, or hardware migration. New degeneration most often presents
with narrowing of adjacent disc heights and increase in osteophyte
formation. Space available for the cord, from the posterior vertebral
body to its lamina, is normally over 17 mm; an AP diameter of less than
13 mm suggests spinal cord compression (12).
Oblique views assess the fusion mass, intervertebral foramina,
pedicles, and facets, and they show persistent compression due to
uncovertebral joint spurring.
suspected, flexion–extension lateral films are useful. Dynamic x-rays
are also helpful in evaluating new instability secondary to
adjacent-level degenerative disc disease. In a patient with
postoperative deformity, 5 pounds of axial traction may be used to
determine reducibility (15).
complaints and physical exam. For example, pseudarthrosis is often
difficult to identify. As in the lumbar spine, shingling of the bone
mass may obscure the fusion defect (5).
Therefore, clinical criteria (intractable neck pain with or without
radicular symptoms) must be correlated with radiographic criteria (6). Radiographic signs of pseudarthrosis include the following (6):
-
Gross motion at previous fusion site on dynamic radiographs
-
Persistence of disc-space lucency
-
Graft displacement or failure to incorporate
-
No dissolution of endplates
-
Hardware failure
Single photon emission computed tomography (SPECT) scanning employs a
tomographic camera to remove three-dimensional superimposition from
scintigraphic images, thereby improving image contrast and offering
more complete spatial information than conventional bone scans. It is
increasingly being used to demonstrate increased focal uptake at sites
of pseudarthrosis (37).
compression indirectly through contour of the dural sac. While
myelography is invasive, it is helpful in the presence of spinal
deformity. Further, it offers information on conjoint nerve roots and
other pathologies not readily appreciated with magnetic resonance
imaging (MRI) (23). When spinal instrumentation is in place, myelography may be the only way to visualize neural structures.
and intrinsic changes in the cord. However, major abnormalities are
commonly encountered in asymptomatic patients (1).
In postoperative patients, gadolinium enhancement reveals recurrent
discs as nonenhancing space-occupying lesions, which scar enhances.
with undetermined axial pain, reporting 70% good to excellent results
with anterior fusion after concordant discography (41). However, others cite higher failure rates, including one report of 54% fair to poor results (8).
These authors cite a 13% complication rate with cervical discography,
including one case of acute epidural abscess resulting in quadriplegia.
Discography is likely to remain controversial for some time.
determine painful levels. Resolution or mitigation of pain indicates
specific root-level pathology and the likelihood of surgical relief of
symptoms following surgery.
patients requiring primary treatment. Early operative treatment should
be considered in any patient demonstrating the following:
-
Progressive motor or gait impairment
-
Persistent disabling pain and weakness (3 months)
-
Progressive deformity
-
Instability
-
Static neurologic deficits with significant axial or radicular pain
consistent findings in both clinical examination and imaging studies.
Pseudarthrosis alone, for example, is not an indication for revision
surgery. Nonoperative treatment should be fully explored in the
majority of “failed neck” patients.
is useful even in those patients for whom later surgery is felt to be
unavoidable. During this period, develop rapport with the patient,
document compliance with prescribed treatment, maximize medical status,
and optimize nutritional and smoking status. In certain patients, urine
nicotine levels may be obtained.
 |
|
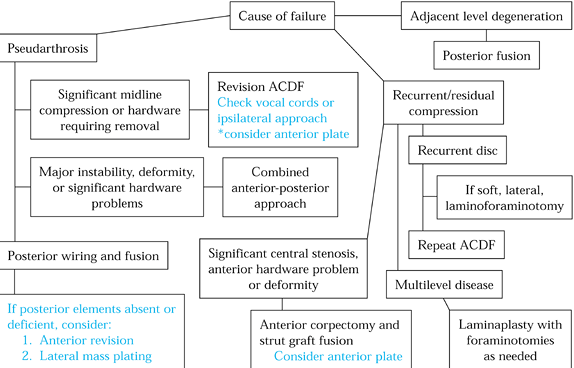
Figure 148.4. Algorithm for treatment of patients with failed anterior surgery.
|
-
Prior decompression may have rendered the spine unstable.
-
Prior fusions create significant lever arms that must be considered in any subsequent construct.
-
Surgical soft-tissue injury from the prior surgery interferes with blood supply, healing potential, and local biomechanics.
allograft use in both index and revision cervical spine procedures, the
decreased vascularity and altered biomechanics of the revision
situation are an indication for autograft bone (14).
The additional stabilization offered by posterior wiring encourages the
anterior fusion mass to consolidate. This approach remains the gold
standard today, with 94% to 100% union rates reported (4,13,20,34) (Fig. 148.5).
 |
|
Figure 148.5. Treatment algorithm for failed anterior surgery.
|
undertaken to compare anterior and posterior treatment of anterior
pseudarthrosis (4,22,29).
Interestingly, while over 80% of patients treated posteriorly felt
better than before surgery, only 70% of those treated circumferentially
reported symptomatic improvement. These results argue for a posterior
approach unless specific hardware concerns require an anterior approach.
-
Use a standard, midline approach to the involved level.
-
Several wiring methods may be employed.
If the spinous processes are intact, a triple wire technique provides
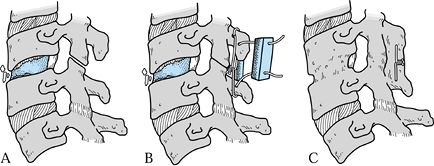
good resistance to torsional and flexion forces (Fig. 148.6).![]() Figure 148.6. Posterior stabilization for failed anterior cervical discectomy and fusion. A: Graft collapse and pseudarthrosis result in focal kyphosis, fragmentation, and instability. B:
Figure 148.6. Posterior stabilization for failed anterior cervical discectomy and fusion. A: Graft collapse and pseudarthrosis result in focal kyphosis, fragmentation, and instability. B:
Posterior instrumentation and fusion are carried out without disturbing
the anterior graft. The simplest approach is a triple wire technique
using autograft bone. Approximate the spinous processes using an
18-gauge interspinous wire applied in a modified Rodger’s technique.
Apply the split autograft to either side of the intact spinous
processes and compress the “sandwich” with a pair of 22-gauge wires. C: Posterior wiring will most often cause the anterior graft to consolidate at the same time the posterior fusion is healing. -
Use lateral mass plates for multisegmental fusions as well.
-
Postoperative immobilization includes a Philadelphia collar for 6–12 weeks.
is often required in cases of residual radiculopathy, or failed or
migrated hardware. Although repeat anterior surgery was felt to have
lower success rates than posterior surgery, recent authors, citing
modern anterior osteosynthesis techniques, report increased fusion
rates with excellent and good results in 83% (9).
rates of wound problems, the biomechanical advantages of anterior
column grafting, and the ability to explore the decompression site and
to remove any remaining osteophytes. Anterior repair is being
increasingly recommended for patients with collapse of a previous
anterior graft in conjunction with pseudarthrosis.
avoid operating through the scarring on the previously approached side.
Contralateral exposure should not be carried out until laryngoscopic
evaluation of the vocal cords has confirmed that both cords are
functioning normally. This approach limits the risk of sequential
injury to both recurrent laryngeal nerves.
-
Use a transverse incision to approach the cervical spine in the standard fashion (see Chapter 138).
-
Locate the failed fusion intraoperatively by x-ray.
-
Next, place distraction pins into the superior and inferior vertebral bodies.
-
Once slight distraction is applied,
excise residual graft, scar, and fibrous tissue with curets, rongeurs,
and a high-speed burr. -
Carefully contour the superior and inferior endplates with the burr.
-
Carefully measure the disc space for height, depth, and width.
-
Fashion the graft to fit the distracted disc space by cutting it 2–4 mm longer than the interspace is high.
-
The graft should be at least 5 mm thick to prevent resorption and restore disc space height.
-
To prevent microfracture, cut the iliac crest with a saw rather than osteotomes.
-
Carefully remeasure the graft depth. In
smaller patients, a graft depth of 12–14 mm may cause cord impingement.
Contour the graft so that it can be countersunk 2 mm below the anterior
cortex without impinging on the back rim of the vertebral body.
osteosynthesis. We use a unicortical fixation system with screws that
lock to the plate.
-
Measure the sagittal depth of the vertebral body and preset the drill depth.
-
When drilling, be aware of the endplate
angle to avoid penetrating the intervertebral disc space above or below
the intended body. -
Immediately mobilize the patient in a Philadelphia collar (usually worn for 6–12 weeks).
fairly straightforward. If significant compression remains anteriorly,
the approach must be anterior. If significant compression remains
posteriorly, the approach must be posterior. Similarly, posterior
hardware problems are addressed posteriorly, and so on.
spine disease is indicated in patients with less than three levels of
disease. Occasionally, large central osteophytes will militate against
posterior decompression in even multilevel spondylosis.
procedure for recurrent or residual radiculopathy or myelopathy most
often involves anterior corpectomy with strut graft fusion (Fig. 148.7).
The likelihood of posterior instability represents a strong indication
for anterior plate osteosynthesis in conjunction with well-fashioned
struts. In patients with single-level and radicular findings, standard
ACDF is recommended. Plate fixation may be helpful in these patients as
well (7). Preoperative flexion–extension radiographs will better define the stability of the cervical spine.
 |
|
Figure 148.7. Treatment of residual cord compression after inadequate decompression. A:
In patients who have undergone previous but inadequate surgical treatment, the original compressive lesion is densely consolidated, and access may be hindered by surgical scarring, old hardware, and, often, a well-integrated strut graft. B: Remove the strut graft with rongeurs and a high-speed burr, back to the posterior vertebral cortex. Study the preoperative CT scan to determine whether there is a distinct interval between the back of the graft and the residual vertebral body. C: Use a burr and microcurets to thin the posterior cortex in the lateral recess of the side with least compression. D: Enter the canal with a small curet or elevator directed toward the neural foramen and away from the cord. Thin the compressive mass with the burr, and use a diamond tip burr to thin the lateral recess to eggshell thickness. E: Use a small elevator or curet to reflect the residual bone fragments directly away from the canal and spinal cord. Dural adhesions may be divided with a fine Penfield elevator or microcuret. |
-
Incise the annulus and remove adjacent discs with a pituitary rongeur and a small, angled curet.
-
Remove the anterior portion of the vertebral body with rongeurs.
-
Carefully thin the posterior cortices with a burr.
-
Use a micro-Kerrison rongeur or curets to decompress the cord.
-
Fashion a strut graft with iliac crest (two or fewer levels) or use allograft or autograft fibula.
-
Contour and apply a plate.
-
Use standard closure and postoperative immobilization (as described previously).
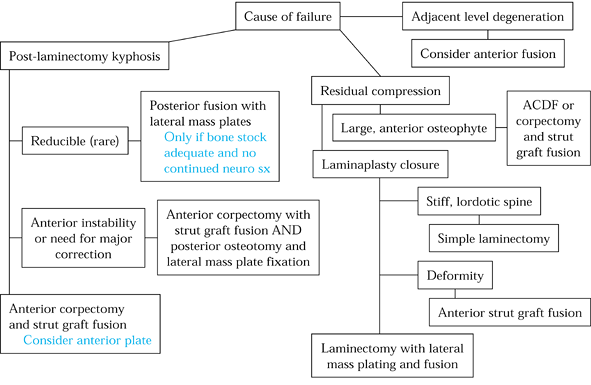
described. They include anterior corpectomy and fusion, posterior
fusion with lateral mass plates, and circumferential procedures (Fig. 148.8).
 |
|
Figure 148.8. Algorithm for treatment of failed posterior cervical spine surgery.
|
preoperative traction. In most cases, moderate correction of the
deformity is achieved and fusion is planned to the next normal level
above and below the deformity (24,35).
inadequate bone stock, and corrective osteotomy through this approach
is generally ineffective (2). Anterior cervical
corpectomy with strut graft fusion and plating has become the favored
approach in all cases without new posterior pathology (35,42,47).
This approach is particularly beneficial in patients with significant
anterior instability as well. Generally, the circumferential approach
allows for greater correction of the sagittal plane deformity. While
reasoning of this surgical approach is sound, the added risks of a
second operation make its use less compelling in the majority of
postlaminectomy kyphosis patients (10,35).
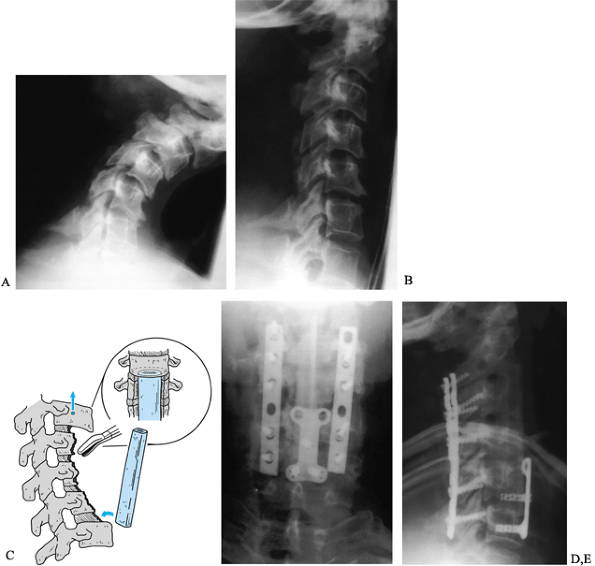 |
|
Figure 148.9. Postlaminectomy kyphosis. A:
This patient presented with progressive pain and deformity 3 months after multilevel cervical laminectomy. The deformity was passively correctable, to a degree, and the patient was neurologically intact. B: After 48 hours in halo traction, significant correction was obtained and surgical treatment was initiated. C: A staged anteroposterior reconstruction was planned. A five-level anterior vertebrectomy was performed initially, reconstructed with an autograft fibular strut. D,E: Five days later, we performed a posterior instrumentation and fusion using lateral mass plates. The slight increase in cervical lordosis produced by the plates caused the anterior strut to loosen and shift. During the same surgical procedure, the graft was repositioned and a short anterior plate placed to buttress the distal pole of the graft. Fusion was successful and the excellent sagittal correction was fully maintained. Figures D & E show anteroposterior and lateral views 1 year after surgery. |
that anterior corpectomy and plate yielded a mean correction of only
16° of kyphosis, but that 95% of these patients reported improvement of
their presenting complaints. The authors report
limited
correction of the deformity due to fusions of the remaining levels at
the facet and posterior element levels. Moreover, the goals of
stabilization and prevention of further progression having been met,
10% reported complete relief of their symptoms and 55% noted
substantial improvement.
-
Begin the anterior approach with the
patient supine. Tape the shoulders caudally and turn the head slightly
away from the operative side. Place a bolster between the shoulders to
provide mild hyperextension. A bolster may not be useful in patients
with a fixed-flexion deformity due to the wedging of the vertebral
bodies. -
A transverse incision may be used in most
cases up to five levels. In longer procedures, an extensile incision
along the anterior border of the sternocleidomastoid muscle can be used
instead. A standard, left-sided approach is recommended. -
Use blunt, finger dissection to dissect the platysma from overlying and underlying tissues.
-
Carefully develop the intervals between the tissue planes. This will increase the extensibility of the incision.
-
The omohyoid may cross the surgical
field, depending on the level of dissection. Division for increased
exposure is rarely required.
This scar may exert a tethering effect. Debridement of the scar will
allow increased exposure of the anterior bodies. Overly aggressive
lateral dissection threatens the sympathetic chain.
-
To achieve correction of the deformity, completely excise the intercalary discs to the level of the PLL.
-
Use a rongeur to remove large portions of the intervening bodies.
-
Next, use a dental burr to remove bone laterally and posteriorly to the posterior cortex of the body.
-
Use fine Kerrisons to remove the cortical wafer from the PLL.
-
Once adequate decompression and
visualization have been achieved, obtain radiographs to assess
correction. Adjust the alignment and traction to achieve lordosis. -
Next, fashion an allograft or autograft
fibular strut in the Whitecloud-LaRocca method with an H-shape. For
less than two or three levels, iliac crest struts may be used. While
crest graft offers a faster incorporation time, fibular graft is
stronger in compression. -
Prior to insertion, undercut the
endplates of the cranial and caudal vertebra with the burr. This allows
the graft to be pushed into position cranially and, with a concurrent
extension force on the neck, impacted into the caudal vertebra. -
Release the extension moment provided by traction to lock the graft into place.
-
Anterior plate osteosynthesis is
recommended. In long constructs, a short anterior plate can be applied
at the caudal vertebra to buttress the inferior pole of the graft. -
Screws are not placed into the graft
because of the risk of graft fracture. A heavy suture may be passed
around the graft and the plate to keep the graft from shifting
posteriorly.
patient is turned and prepared for posterior instrumentation and
fusion. While this procedure is not necessary in two- or three-level
fusions, we routinely add posterior fusion to reconstructions of four
or more levels, and to those with poor bone quality, translational
instability, or risk factors for pseudarthrosis.
-
Expose the posterior cervical spine in
the usual fashion, taking care to document levels and protect levels
not instrumented anteriorly. -
Use a burr to decorticate the laminae and facets, and remove articular cartilage from the facets with a small curet.
-
Use a 2.0 mm drill to prepare pilot holes for lateral mass plating.
-
Contour the lateral mass plate to neutralize
the alignment obtained during the anterior procedure. Increasing
lordosis at this point will tend to loosen the anterior construct. -
Graft the facet joints and apply the plates over the graft.
-
Close in layers and apply a sterile dressing.
immobilization for 12 weeks may be useful in patients with poor bone,
previous failures, or multiple-level procedures. These patients may
also benefit from a posterior stabilization procedure, often with
lateral mass plates.
surgical intervention. In some cases, external immobilization can be
used until fusion occurs. However, when failed hardware results in
neurologic or soft-tissue compression, instability, or progressive
deformity, surgical treatment is offered.
be approached anteriorly. Failed posterior hardware may be approached
posteriorly. However, in cases of poor bone quality, deformity, or
three-column destabilization, circumferential stabilization procedures
are recommended.
The inflammation and tissue reaction around the old hardware will make
the exposure difficult and will distort tissue planes. Inadvertent
entry into the carotid sheath, esophagus, or thoracic duct can result
in potentially lethal complications.
addressed. In some cases, postoperative halo immobilization suffices.
Typically, however, revision internal fixation is employed in
conjunction with postoperative immobilization (7).
replaced with lateral mass plates. Sublaminar wires are more
problematic, and they may be retained or removed. In cases where dense
scar makes dissection difficult, the broken wire may be retightened
around the intact lamina and left in place. If monofilament wire must
be removed, contour the end to be pulled through the canal so that it
can be pulled out smoothly. Place a Woodson or Penfield elevator
between the wire and the thecal sack and extract the wire by winding
the free end up with a needle-holder. Cut braided wires close to the
lamina to remove as much of the frayed portion as possible before
trying to remove them.
related to recurrent compression. Recurrence of stenosis or disc
herniation often reflects the same global nature of cervical
spondylosis seen in patients with recurrent axial symptoms. Whether or
not surgical intervention accelerates degenerative changes at
neighboring levels, principles regarding their treatment remain the
same.
ACDF of the new level or posterior keyhole foraminotomy. There are
proponents of each approach. In the patient with a previously operated
neck, the same principles apply, with the caveat that repeat anterior
surgery requires special attention to the status of the recurrent
laryngeal nerves and vulnerable soft-tissue structures of the neck (as
previously discussed).
result of soft disc pathology at one or two levels, keyhole
foraminotomy may be indicated. Most often, this is seen in patients
with a prior ACDF and an incompletely excised disc, or with recurrent
soft disc pathology at a neighboring level.
avoidance of fusion-related problems and the need for only minimal
laminar resection to expose the lateral edge of the dura. Disadvantages
include its failure to decompress the cord and stabilize the spine.
Laminoforaminotomy is contraindicated in patients with cervical
kyphosis or major anterior cord compression (17).
continues as to the best method to treat multilevel recurrent or
residual stenosis. Laminaplasty is recommended for recurrent stenosis
above and below a previously, anteriorly fused level, or in the case of
disease affecting more than three levels (28). Others report success with vertebrectomy and strut graft fusion techniques (42).
the incidence of instability associated with multiple-level
laminectomies. However, decompression may be incomplete if foraminal
stenosis is not addressed. Contraindications include cervical deformity
(especially kyphosis) and major anterior cord compression.
recommended for patients with recurrent or residual stenosis after
laminectomy and in those patients who are not candidates for posterior
decompressive laminaplasty.
predominantly axial pain, the possibility of adjacent-segment disc
degeneration must be considered. Advocates of cervical discography
recommend anterior fusion for concordant pain (41).
-
With the patient prone, obtain precise
radiographic localization prior to skin incision. Employ a standard
midline approach with careful, unilateral exposure to the lateral
aspect of the facet capsule. -
Thin the lateral portion of the lamina and the medial portion of the facet with a burr.
-
Carefully develop the interval between the medial facet capsule and the ligamentum flavum with a fine curved curet or Penfield.
-
Remove the medial 25% of the facet with
Kerrison rongeurs. Remove the volar facet capsule to visualize the root
and the lateral margin of the thecal sack. -
Expand the laminotomy over the junction of the root and dura taking care to preserve over 50% of the facet.
-
If a contained herniation is found, incise the PLL to retrieve the fragment.
-
Apply a collar for comfort for the first
2–3 weeks after surgery. If more than 50% of both facets, or more than
75% of one facet, is removed, consider fusion with lateral mass plates.
posterior elements and lateral masses of the involved cervical spine,
as well as exposure of at least one normal level above and below the
compression. The surgeon must elevate laminae at the margins of
stenosis as well as those directly over the narrowed segment.
-
Through a standard, midline approach, carefully elevate the paraspinal musculature to the lateral edges of the facets.
-
Use a high-speed burr to cut longitudinal bone troughs through the lamina–facet junction.
-
Use a fine Kerrison punch to complete the
trough on the side of primary compression (i.e., at the greatest
narrowing or most significant radiculopathy). -
Excise the ligamentum flavum and
interspinous ligaments at the proximal and distal junctions of the
laminaplasty segment with the normal spine. -
Apply gentle, sustained pressure on the
spinous processes and upward on the open laminotomy to elevate the
laminar flap on its intact cortical “hinge.” Allow the intact lamina to
deform plastically, being careful not to snap it off by applying too
much force. -
Release dural adhesions to the lamina with a Penfield elevator.
-
The lamina flap is then hinged wide open
on the intact lamina in a staged fashion, moving cranially from the
inferiormost hinged lamina. An elevation of 10–15 mm on the open side
increases the sagittal diameter of the canal by 4–5 mm.
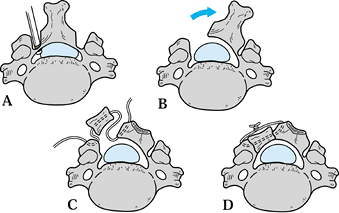 |
|
Figure 148.10. Trap-door laminoplasty for extensive stenosis or adjacent-level degeneration. A:
Cut longitudinal troughs through the involved laminae using a high-speed burr. Troughs should be centered over the medial edge of the facet so that penetration exposes the lateral recess and not the cord. Complete the defect on the side of most compression using a small Kerrison. B: Thin the opposite lamina down to the volar cortex, then gradually elevate the opened side up and away from the facet, allowing time for the intact hinge to deform plastically. If the hinge is too stiff, thin it further. C: Use the spinous processes or an allograft bone strut to hold the laminoplasty open. Groove both ends of the graft to capture the cut edges of the lamina and the lateral mass. Use a small drill to make holes at the margins of both, and pass a heavy suture through the drill holes and lengthwise through the graft. D: Position the strut and allow the lamina to close down slightly to capture the strut. Tie the heavy suture over the outside of the graft to prevent it from migrating into the canal or dislodging and allowing the laminaplasty to close. |
-
Cut three grafts to length (12–14 mm).
-
Cut a trough into each end of the graft.
-
One graft is inserted between the lamina and lateral mass at C-3, C-5, and C-7.
-
To avoid inadvertent fusion, care must be taken to avoid bone graft touching another level.
-
To keep the graft from displacing, pass a
heavy suture longitudinally through the cancellous bone of each spinous
process or rib allograft. Pass the suture through a small drill hole in
the laminar margin and through the medial edge of the facet, and tie it
tightly to compress the graft strut between the lamina and the facet
margin. The secured strut will keep the flap from closing. -
Supplemental foraminotomies may then be carried out to treat radiculopathy, as needed.
-
Close the posterior wound over drains.
Reattach the deep layer to the spinous processes at the margins of the
laminaplasty. Immobilize in a Philadelphia collar for 6–12 weeks. Some
authors recommend a CT scan at 8 weeks to check healing on the hinge
side and ensure canal patency. Once healing is complete, physical
therapy emphasizing cervical extension exercises is recommended.
prior cervical spine procedure. Significant deficits in extensor muscle
strength and flexibility are common. Therefore, a supervised isometric
strengthening and ROM program is indicated prior to returning to
unlimited activity. At 12 weeks, or when x-rays demonstrate adequate
bony union, prescribe a twice-daily regimen of 10 minutes of neck
muscle ROM and isometric strengthening. Weekly physical therapy
supervision to monitor progress and add incremental exercises until
functional strength and ROM are regained may be recommended.
The hypovascular scar bed predisposes to infection or failure of
healing. Some authors have reported complication rates for anterior
surgery following failed posterior procedures similar to those reported
in index anterior procedures (42,46,47). However, hardware problems are more likely in osteopenic bone and when normal anatomic landmarks are distorted.
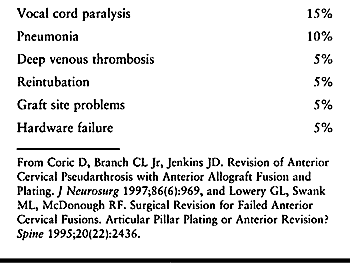 |
|
Table 148.1. Complications of Revision Anterior Surgery
|
increases the possibility of transient sore throat or swallowing
difficulty. Further, dissection through scar requires meticulous
attention to detail and great caution. Perforation of the esophagus is
a life-threatening injury, and only one third are recognized at the
time of surgery. Mortality for injuries recognized early is 15%. If
recognized late, mortality rises to 30% (30,36).
Although the ipsilateral operative approach requires dissection through
scar, the contralateral approach should not be undertaken without
confirming the function of both vocal cords through laryngoscopy.
increased in the revision situation as well. Horner’s syndrome from
injury to the sympathetic chain and overretraction of the longus colli
is ostensibly more likely if the longus muscles are encased in scar, or
the dissection difficult.
cervical spine surgery are wound infection (1.2%) and failure of
healing. Correction of identifiable nutritional deficiencies may
decrease these problems. The incidence of dural tear also rises when
dissecting through posterior scar (16).
scheme: 7, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
HH, Dabb B. Anterior and Posterior Cervical Osteotomy. In: Bradford DS,
ed. Master Techniques in Orthopaedic Surgery: The Spine. Philadelphia:
Lippincott-Raven, 1997:75.
HH, Emery SE, Goodfellow DB, et al. Robinson Anterior Cervical
Discectomy and Arthrodesis for Cervical Radiculopathy. Long-term
Follow-up of One Hundred and Twenty-two Patients. J Bone Joint Surg Am 1993;75:1298.
AE, Khalil MA, Sassard WR, Newman BP. Repair of Symptomatic
Pseudarthrosis of Anterior Cervical Fusion. Posterior versus Anterior
Repair. Spine 1992;17:1137.
MD, Malanin TI, Davis PB. A Roentgenographic Evaluation of Frozen
Allografts vs Autografts in Anterior Cervical Spine Fusions. Clin Orthop 1976;199:231.
PJ, Esses SJ, Kostuik JP. Anterior Cervical Fusion: Outcome Analysis of
Patients Fused With and Without Anterior Cervical Plates. J Spinal Disord 1996;9:202.
GR, Douglas RA, Meyer PR Jr, et al. Complications in Three-column
Cervical Spine Injuries Requiring Anterior-posterior Stabilization. Spine 1992;17:253.
JC, White JI, LaRocca H. Fusion Rates in Multilevel Cervical
Spondylosis Comparing Allograft Fibula with Autograft Fibula in 126
Patients. Spine 1991;16(suppl 10):S561.
HN, Kurz LT, Overholt DP. Surgical Management of Cervical Soft Disc
Herniation. A Comparison between Anterior and Posterior Approaches. Spine 1990;15:1026.
JA, Hanson GW, Rose JR, et al. Anterior Discectomy, Microscopic
Decompression and Fusion. A Treatment for Cervical Spondylotic
Radiculopathy. J Spinal Disord 1989;2:43.
GL, Swank ML, McDonough RF. Surgical Revision for Failed Anterior
Cervical Fusions. Articular Pillar Plating or Anterior Revision? Spine 1995;20:2436.
K, Tada K, Matsuda T, et al. Posterior Extensive Simultaneous
Multisegment Decompression with Posterior Lateral Fusion for Cervical
Myelopathy with Cervical Instability and Kyphotic and/or S-shaped
Deformities. Spine 1989;14:1160.
GP, Visarius H, Nolte LP, Herkowitz HN. A Biomechanical Comparison of
Cervical Laminaplasty and Cervical Laminectomy with Progressive
Facetectomy. Spine 1993;18:1995.
KP, Smith MD, Gundry CR, Pollei SR. Cervical Discogenic Pain.
Prospective Correlation of Magnetic Resonance Imaging and Discography
in Asymptomatic Subjects and Pain Sufferers. Spine 1994;21:300.
K, Okamoto A, Kamikozuru M, et al. An Analysis of Failures in Primary
Cervical Anterior Spinal Cord Decompression and Fusion. J Spinal Disord 1993;6:277.
FH, Svien HJ, Bickel WH, et al. Swan-neck Deformity following Extensive
Cervical Laminectomy. A Review of Twenty-one Cases. J Bone Joint Surg Am 1974;56:564.
WJ, Collier BD, Flatley TJ, et al. Painful Pseudarthrosis following
Lumbar Spinal Fusion. Detection by Combined SPECT and Planar Bone
Scintigraphy. Skeletal Radiol 1987;16:136.
JM, Clifton AG, Whitiar P. Appearance of Posterior Osteophyte After
Sound Anterior Interbody Fusion in the Cervical Spine. A High
Definition CT Study. Neuroradiology 1993;35:227.
W, Thuomas KA, Hedlund R, et al. Degenerative Changes following
Anterior Cervical Discectomy and Fusion Evaluated by Fast Spin-echo MR
Imaging. Acta Radiol 1996;37:614.