CHAPTER 57 –
Cole & Sekiya: Surgical Techniques of the Shoulder, Elbow and Knee in Sports Medicine, 1st ed.
Copyright ©
2008 Saunders, An Imprint of Elsevier
CHAPTER 57 – Autologous Chondrocyte Implantation in the Knee
Scott D. Gillogly, MD
Articular cartilage injury is extremely common, and until recently, methods for treatment of the cartilaginous lesions did not produce good long-term results. The technique of autologous chondrocyte implantation (ACI), first reported by Peterson, Brittberg, and colleagues in 1994, has gained a major role in the treatment of large full-thickness chondral injuries.[4] Results are now available with up to 12 years of followup, and more than 80% of the patients have had improvement with relatively minor complications. [1] [15] In this technique, a small biopsy specimen of healthy chondral tissue obtained arthroscopically undergoes in vitro chondrocyte amplification in cell culture, returning autologous chondrocyte cells available for implantation into the defect at the second stage of the repair procedure. The goal in use of autologous chondrocytes is to produce a repair tissue that more closely resembles the morphologic characteristics of the type II hyaline cartilage (>90%), thus restoring the durability and natural function of the knee joint. [4] [5]
The first step in determining the appropriate treatment of a suspected chondral defect is to obtain an adequate history. Even in the face of a known cartilage defect, it must be determined whether the symptoms are originating from the defect or arising from some as yet unrecognized pathologic process that would not benefit from ACI. This is particularly relevant to patients who have undergone prior repair techniques, such as marrow stimulation, for the cartilage defect. Whether the symptoms are arising from the previously treated lesion, from a new source, or perhaps as a result of an incomplete rehabilitation program must be discerned.
Patients with condylar lesions typically have pain with weight bearing or increased loading, complaints of mechanical symptoms, swelling, or point tenderness in the area of the defect. The presence of a trochlear or patellar lesion will register similar complaints, but with exacerbation of symptoms by stairs or getting in and out of a chair or car and with anterior knee pain. Patellar subluxation symptoms are often present as well.
Finally, characteristics of the individual patient must be considered in the complete treatment and followup planning. Information is often available through previous operative reports and intraoperative photographs. Taking advantage of any available information will help in determining the suitability of the defect for ACI.
Physical examination provides the critical step in evaluation and assessment of the patient’s suitability for ACI. A thorough evaluation of the lower extremity, including observation of gait and a hip and possibly an ankle examination, is warranted, as is a complete evaluation of the knee. A knee ligamentous examination will establish stability. Should there be any question regarding the ligamentous examination, magnetic resonance imaging can assist in confirming the clinical findings. Evaluation on physical examination of meniscal function includes provocative meniscal tests, radiographic assessment, and possibly magnetic resonance imaging evaluation.
For adequate evaluation of a patient for ACI, it is essential that weight-bearing anteroposterior and 45-degree posteroanterior and patellar alignment radiographs be obtained. [7] [10] This allows evaluation of the alignment of the tibiofemoral and patellofemoral portions of the knee and gives an indication of any underlying bone involvement associated with the defect. A long-leg limb alignment radiograph view can assess the mechanical axis and determine the potential need for realignment. As mentioned, magnetic resonance imaging can then be used to assess both the ligament and the meniscal status as well as to define the degree of subchondral bone involvement. Increased signal and edema in the subchondral bone of a chronic nature may indicate persistent overload of the involved compartment, making realignment considerations more likely to be necessary in addition to ACI. Bone loss of more than 7 to 8 mm in depth requires bone grafting before or at the time of cell implantation. Whereas magnetic resonance imaging is helpful to evaluate subchondral bone loss and the soft tissues of the knee, it presently does not have adequate sensitivity or specificity, as performed in the community, to evaluate the extent of chondral injury or subtle chondromalacia changes.[10]
Indications and Contraindications
ACI is indicated for symptomatic, full-thickness chondral lesions and osteochondritis dissecans lesions of the femoral condyles and trochlear groove in physiologically young patients who can be compliant with the rehabilitation protocol. Results of treatment of chondral injuries of the patella and tibia with ACI have not been as consistently good as those of the femoral condyles and trochlea, although with realignment and appropriate patellar tracking, the results are more favorable. ACI is not indicated for treatment of advanced osteoarthritis or in the presence of bipolar sclerotic bone-on-bone lesions. ACI is also contraindicated in active inflammatory arthritis or infection.[10]
In summary, the prerequisites for a successful outcome with ACI (in addition to a focal chondral defect) include appropriate bone alignment, ligamentous stability, meniscal function, adequate motion and muscle strength, and compliance of the patient, without significant bone arthritic changes.
Good results with ACI, as with any method of cartilage repair, should not be expected if coexisting knee disease is not addressed. In the senior author’s (S. D. G.) experience, performance of one additional procedure at the time of ACI is generally preferred; otherwise, staging is more prudent. Of the author’s initial 285 patients undergoing ACI, 60.1% underwent a concomitant procedure. In order of decreasing frequency, these included anteromedialization of the tibial tubercle, high tibial osteotomy or distal femoral osteotomy, anterior cruciate ligament reconstruction, and meniscal transplantation. An additional 11.5% underwent a staged procedure, typically bone grafting of an osteochondral defect or hemicallotasis osteotomy.[7]
ACI is typically performed under general anesthesia. In general, we prefer to allow the patient and the anesthesiologist to decide on the specific technique. The patient is placed on the operating table in the supine position. The involved lower extremity is positioned so that the knee may be placed into maximum flexion, if necessary, and rests with the foot on a sandbag or other positioning device so the knee is at 90 degrees of flexion. Prophylactic antibiotics are routinely administered.
After preparation and draping, a midline incision is generally recommended, followed by a medial or lateral parapatellar arthrotomy, exposing the corresponding chondral injury for condyle defects. For patellar defects, the patella is generally reflected superiorly through a tibial tubercle osteotomy that is commonly done for purposes of patellofemoral realignment. As with any surgical procedure, good exposure is critical for performance of the intended technique and good outcome. The approach must allow the surgeon access to properly suture the periosteal patch to the chondral defect. The end result should never be compromised for the sake of an ill-advised concern to keep the approach small.
Surgical Landmarks, Incisions, and Portals
| • | Tibial tubercle | |
| • | Inferior, lateral, and medial poles of the patella | |
| • | Patellar tendon | |
| • | Lateral and medial femoral condyle | |
| • | Tibia plateau |
An examination of the knee under general anesthesia is beneficial in revealing pathologic changes, such as ligamentous laxity, in the relaxed patient that may have previously gone undetected.
Specific Steps (
Box 57-1
)
ACI is a staged procedure. An arthroscopic chondral biopsy specimen is first obtained and sent for culture; then, in the second stage, the actual implantation of the cultured chondrocytes is performed. Whereas the first step is intended for the chondral biopsy, it also serves as a determination of the suitability of the chondral lesion for ACI. At this time, the size and location of the defect, the depth of the defect, the status of the surrounding articular cartilage and underlying bone, and the status of the opposing chondral surfaces are definitively evaluated. Containment of the defect is assessed, and other pathologic processes that might require treatment for optimal ACI results are determined. In general, the defects treated by this technique are larger than 2 cm2; the average size in the authors’ series has been well over 5.6 cm2. [5] [7] [9]
| Surgical Steps [5] [6] [9] | |||||||||||||||||||||||||||
Stage 1: chondral biopsy
|
A standard arthroscopic approach to the knee is used. The knee must be thoroughly evaluated in all three compartments. Any coexisting pathologic process must be noted and addressed for optimal outcome. Examination under anesthesia again confirms previous clinical assessments.
If a chondral lesion is considered appropriate for ACI, a biopsy specimen is obtained. The most common site is the superomedial edge of the medial femoral condyle or the superolateral edge of the lateral femoral condyle that is non–weight bearing and nonarticulating with the tibia or patella. The other area from which a biopsy specimen is frequently obtained is the lateral intercondylar notch of the medial femoral condyle, particularly if a notchplasty has been performed. An arthroscopic gouge or ring curet is used to obtain several slivers of full-thickness cartilage, each approximately the size of a pencil eraser (i.e., 5 mm by 10 mm). After the slivers are removed from the knee, they are placed in the biopsy medium or shipping vial in sterile fashion. Two or three slivers of cartilage will provide chondrocytes for culturing a 12-fold increase in autologous cells.
Stage 2: Chodrocyte Implantation
A standard medial or lateral parapatellar incision and arthrotomy are used for exposure. As with any surgical procedure, exposure is essential. If the lesion is on the central portion of the condyle, a mini-arthrotomy can be used. For larger, difficult to reach chondral injuries, a medial or lateral incision with a medial or lateral parapatellar arthrotomy and eversion of the patella may be necessary, especially when the lesion is on the posterior portion of the lateral condyle. We favor a subvastus approach rather than cutting the quadriceps tendon, when feasible.
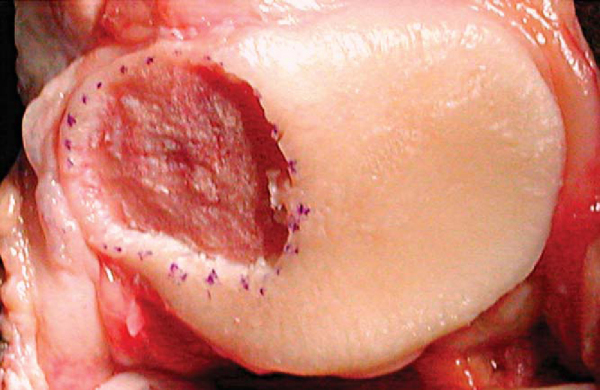
During débridement of the defect, all damaged and unhealthy-appearing cartilage, calcified cartilage, and fibrocartilage must be removed. Any thinned, fissured, or damaged surrounding cartilage needs to be débrided to an edge leaving healthy, firm articular cartilage. Bleeding may introduce stem cells and fibroblasts into the defect and therefore must be controlled. The goal of adequate débridement of the defect is to have a dry bed with clean subchondral bone and a healthy, sharply demarcated cartilage border at the periphery. This is best accomplished by scoring the periphery of the defect with a No. 15 scalpel blade and using curets to remove the damaged tissue (
Fig. 57-1
). The best method for obtaining the correct size for the periosteal graft is to cut a template from sterile paper (glove wrapping). The template is oversized by 1 to 1. 5 mm around the circumference because the harvested periosteum tends to contract (
Fig. 57-2
).
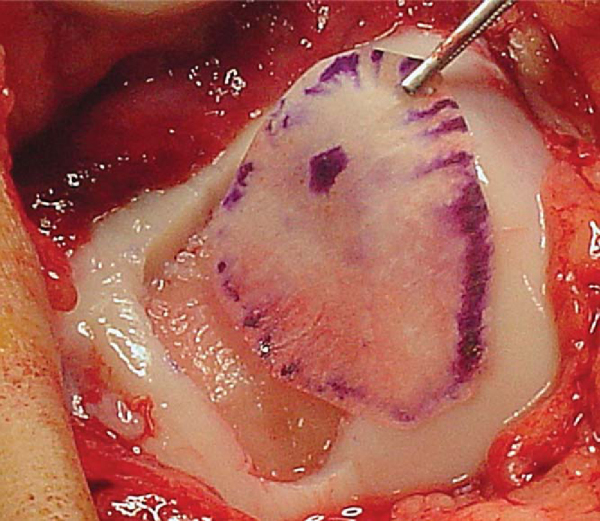
The periosteum harvest is from the proximal medial tibia, two fingerbreadths distal to the pes anserinus and medial collateral ligament insertion on the subcutaneous border. An incision is made just anterior to the posterior border of the tibia. All fat and fascia layers should be removed from the periosteum by both sharp and blunt dissection with a moist sponge. Leaving the thin fascia layer on the periosteum is one of the most common mistakes made with harvesting of the periosteal graft. The template is then placed over the exposed periosteum, and a scalpel (No. 15 blade) is used to sharply demarcate the periosteal graft (
Fig. 57-3
). A sharp curved periosteal elevator is used to gently elevate the periosteum off the bone.
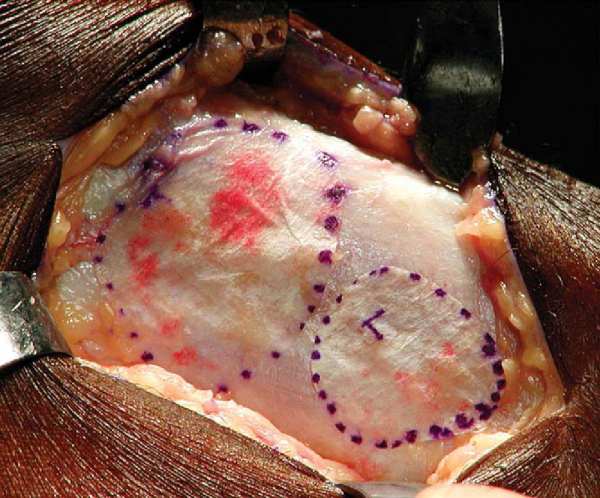
The periosteal graft is then aligned over the defect in the orientation matching the template, with the cambium layer facing the defect. The periosteum is then sutured to the cartilage rim with multiple 6-0 Vicryl interrupted sutures spaced every 2 to 3 mm (
Fig. 57-4
). If the defect is uncontained—meaning that there is not a circumferential rim of healthy cartilage through which sutures can be passed—suture anchors may be used to attach the periosteal graft on the uncontained side (
Fig. 57-5
). The knots should be tied on the periosteal side, not over the surface of the cartilage, thus minimizing any friction or toggling that could cause loosening of the knots. Redundant periosteal graft can be trimmed as the graft is being secured, ensuring that even tension is maintained on the graft. A small opening is maintained on one edge of the graft to allow injection of chondrocyte cells.
|
|
|
|
Figure 57-5 |
5. Sealing the Periosteal Graft
The watertight integrity of the secured graft can be tested by an 18-gauge catheter and a saline-filled tuberculin syringe placed deep to the periosteum through the small opening. Additional sutures can be placed as necessary to ensure a watertight seal. The suture line at the periosteal graft edge is then sealed with fibrin glue with one of the commercially available preparations (
Fig. 57-6
).
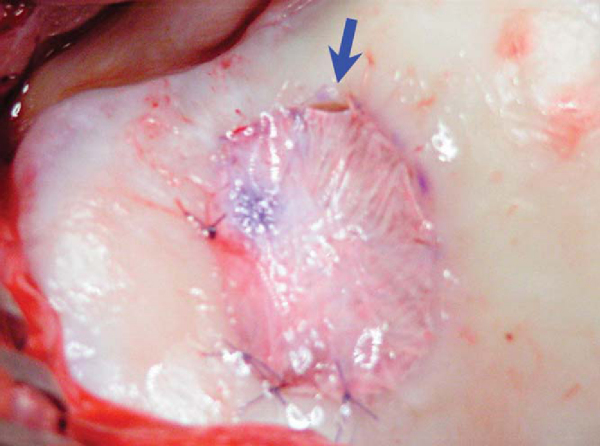
6. Implantation of Chondrocytes
The sterile cells are aspirated from the shipping vial into a tuberculin syringe by sterile technique. The autologous chondrocytes are then introduced through the tuberculin syringe with an 18-gauge plastic angiocatheter and injected under the periosteal graft. The injection site is then closed with one or two additional sutures and sealed with fibrin glue. No additional manipulation of the joint should follow the implantation (
Fig. 57-7
).
The arthrotomy and wound are then closed in a layered fashion, and a soft sterile dressing and knee immobilizer are applied to the knee. A drain is not typically used so as not to place any suction on the graft. A subcutaneous drain may be placed after the joint is closed, if necessary.
Combined Procedures[7]
| • | Ligament reconstruction should be done first; then proceed with the ACI (this protects the periosteal graft). | |
| • | ACI rehabilitation program is the overriding protocol postoperatively. |
| • | Autologous bone graft is placed in débrided defect bed. | |
| • | ACI is performed 4 to 6 months after the bone graft. |
Rehabilitation after ACI is based on the maturation process of the chondrocytes, the size of the defect, and the location of the defect. [7] [9] The concept of a slow, gradual maturation of the repair tissue is crucial to understanding the rehabilitation after ACI.[9] The hyaline-like repair tissue must be both protected and stimulated to allow the maturation and remodeling of the tissue in the proper manner. In multiple procedures, ACI should remain the determining step in rehabilitation while the principles of early motion and progressive joint loading are maintained.
Complications[13]
| • | Arthrofibrosis (2%) | |
| • | Graft failure or delamination (1. 4%) | |
| • | Periosteal overgrowth (1. 3%-17%) | |
| • | Mechanical symptoms (1%) | |
| • | Infection |
See
Table 57-1
.
| Author | Followup | Outcome |
|---|---|---|
| Peterson et al[16] (2000) | 2 to 9 years | 23 of 25 simple condyle defects: successful |
| 16 of 18 (89%) patients with osteochondritis dissecans: good–excellent | ||
| Bahuaud et al[2] 1998) | 2 years | 84% good–excellent |
| (Minas et al[14] (1998) | 6 years | 87% of 235 with good results |
| Bentley et al[3] (2003) | 19 months | 88% good results for ACI versus 69% after mosaicplasty |
| Gillogly and Hamby[8] (2001) | 5 years | 91% of 112 with good–excellent results |
| Knutsen et al[11] (2002) | 2 years | 40 patients with ACI versus 40 patients with microfracture: similar results |
Since the first 23 cases reported in 1994, ACI has been performed in more than 8000 patients throughout the world with more than 12 years of followup (
Fig. 57-8
). [1] [5] [12] [15] [16] [17] These results show a significant trend toward objective and subjective satisfaction in patients and their treating physicians. The mechanical durability of ACI cartilage also appears to be significantly greater compared with fibrocartilage regeneration techniques as evaluated by second-look arthroscopy and duration of satisfactory results. [15] [16] In all, ACI holds significant promise in the armamentarium of the orthopedic surgeon in treating cartilage injuries.
Peterson et al[15] have also published long-term biomechanical durability data, showing a 96% durability factor with the first 62 consecutive patients treated at 2 years and then again at 7.5 years.