CHAPTER 30 –
Cole & Sekiya: Surgical Techniques of the Shoulder, Elbow and Knee in Sports Medicine, 1st ed.
Copyright ©
2008 Saunders, An Imprint of Elsevier
CHAPTER 30 – Nonarthroplasty Options for Glenohumeral Arthritis: Meniscal Allograft Resurfacing
Brian J. Cole, MD, MBA
Glenohumeral arthritis in young patients poses a particularly difficult treatment challenge. Nevertheless, independent of the patient’s age, nonoperative treatment is often initially effective. Accordingly, nonsteroidal anti-inflammatory medications, intraarticular injections of cortisone or commercially available hyaluronate solutions,[*] appropriate activity modification, and physical therapy represent first-line therapy for patients with symptomatic glenohumeral degenerative joint disease.
Given the inherent problems with prosthetic glenoid resurfacing in this population (i.e., concerns about early glenoid loosening) and the difficulties with revision surgery posed by limited glenoid bone stock, prosthetic glenoid resurfacing is problematic in young, active patients. Arthroscopic débridement with capsular release often represents initial surgical therapy for this demanding population but can fail to provide long-term satisfactory results. [4] [15] Furthermore, traditional hemiarthroplasty and humeral head resurfacing offer reasonable treatment options for select young individuals with unipolar disease, such as osteonecrosis of the humeral head, but may not result in significant pain relief or functional improvement for those with bipolar processes.
In this challenging population, a growing number of techniques have evolved to address bipolar glenohumeral disease with biologic resurfacing of the glenoid. Several biologic interpositional options have been proposed with limited success, including use of the anterior capsule, Achilles tendon allograft, meniscal allograft, and, more recently, various soft tissue rotator cuff augmentation devices. [1] [3] [5] [10] [16] This chapter presents one such technique in which glenoid resurfacing is accomplished by an allograft meniscus combined with conventional humeral prosthetic replacement. The technique of glenoid resurfacing with a meniscal allograft tissue is well described. [5] [16] The premise behind the use of a human meniscus is that it provides a conforming biomechanical construct of fibrocartilaginous tissue with peripheral “liftoff,” theoretically decreasing contact forces across an otherwise arthritic glenohumeral joint.[5]
*
Note that use of commercially available hyaluronate solutions in the glenohumeral joint is not an indication approved by the Food and Drug Administration.
Patients often present with a history of previous surgical intervention. It is therefore essential to obtain all pertinent operative reports and intraoperative records detailing types of implants used. A typical history might include the following[12]:
| • | Labral repair by use of a headed, bioabsorbable tack | |
| • | Labral repair by use of multiple bioabsorbable, knotless anchors[6] | |
| • | Labral repair by use of multiple metallic suture anchors | |
| • | Use of “thermal capsulorrhaphy” technique[11] | |
| • | Use of postoperative intraarticular continuous pain catheters with local anesthetic agents | |
| • | Postoperative course after labral repair in which the patient does well during the initial 6 to 9 months, then develops increasing shoulder pain, particularly at rest and at night with associated loss of glenohumeral motion | |
| • | Open, nonanatomic stabilization, such as a Magnuson-Stack or Putti-Platt procedure | |
| • | Traumatic anterior or posterior instability with subsequent pain after adequate rehabilitation or operative stabilization | |
| • | History of proximal humerus fracture with or without open reduction and internal fixation |
Typical findings include the following:
Particular attention should be paid to the status of the axillary nerve after previous surgical intervention or traumatic instability.
Magnetic resonance imaging without contrast enhancement is performed to assess for the following:
| • | Presence of joint effusion | |
| • | Presence of loose bodies | |
| • | Synovitis | |
| • | Capsular thickening | |
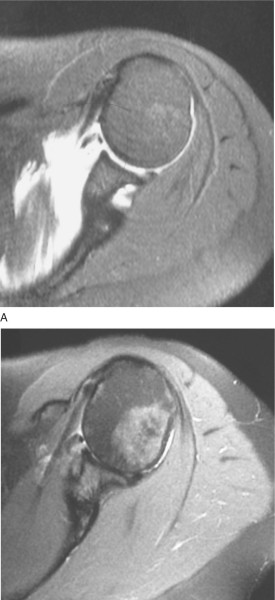
| • | Status of glenohumeral cartilage ( Fig. 30-1 ) |
|
| • | Status of the rotator cuff | |
| • | Evidence of inflammatory reaction to bioabsorbable implants | |
| • | Glenoid version and evidence of posterior wear pattern |
|
|
|
|
Figure 30-1 |
Computed tomography with multiplanar and three-dimensional reconstructions is performed to assess for the following:
Indications and Contraindications
The ideal candidate for biologic resurfacing is relatively young (younger than 50 years) and active and has well-preserved deltoid and rotator cuff musculature. Indications include the following:
Relative contraindications include the following:
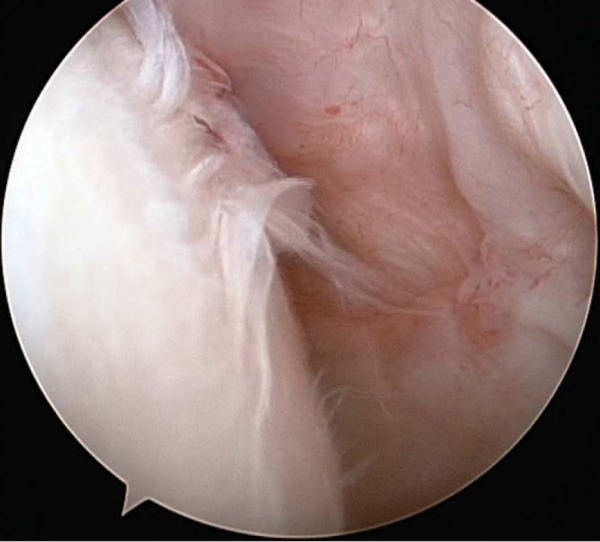
Arthroscopic débridement permits evaluation of the humeral and glenoid joint surfaces, removal of chondral loose bodies, and synovectomy when indicated. Selective capsular release, in the authors’ opinion, should always precede more significant operative treatment (
Fig. 30-2
).
|
|
|
|
Figure 30-2 at the time of diagnostic arthroscopy and débridement, demonstrating grade III-IV changes across the humeral head as well as glenohumeral synovitis. |
At times, particularly in the setting of the shoulder that has had multiple operations, it can be difficult to determine whether the glenohumeral joint is the sole or primary pain generator on the basis of history, physical examination findings, and imaging studies. In these cases, a fluoroscopically guided glenohumeral injection of local anesthetic is potentially diagnostic of the cause of the patient’s pain. If significant pain relief is not received from the injection, the patient may not respond well to a resurfacing procedure. Furthermore, it is particularly important in a young population that the patient accept the limited-goals nature of the proposed procedure, that is, the intent is to provide reasonable pain relief and functional range of motion, not to restore normal shoulder function. The patient should understand that such a procedure may represent a temporary or “bridging” solution to the problem and that revision to conventional total shoulder arthroplasty may be indicated in the future. Most important, although the procedure may improve the patient’s symptoms, albeit to a lesser degree compared with glenohumeral replacement, it does not prevent or compromise this later revision to conventional total shoulder arthroplasty.
Allograft Sizing, Processing, and Preservation
The smallest available lateral meniscal allograft should be requested for glenoid resurfacing. The anterior and posterior horns of the lateral meniscus are situated relatively close to one another, giving it a smaller radius of curvature and making it a better geometric match for the glenoid than the medial meniscus. Fresh-frozen allografts that have tested free of viral and bacterial contamination are preferred. Theoretically, a contralateral lateral meniscus can be chosen and positioned such that the indentation for the popliteus tendon is superior near the biceps insertion. However, specific differences in surgical technique have not been well elucidated, and thus the surgeon’s preferences often prevail in determining exact graft position relative to the glenoid anatomy. Recipients should be educated about the small but finite risk of disease transmission with implantation of any allograft tissue. This risk is strongly mitigated through diligent donor screening policies and polymerase chain reaction testing for viral contaminants.
General anesthesia with endotracheal airway is preferred. Depending on the experience of the anesthesiology staff, regional anesthesia in the form of an interscalene block can serve as a useful adjunct, both for minimizing the need for intraoperative inhaled anesthetics and for reducing the need for postoperative, narcotic pain medication.
The patient is placed in a well-padded, modified beach chair position. The medial border of the scapula on the operative side is roughly aligned with the edge of the operating table; a small, rolled towel can be useful to protract the scapula further, facilitating intraoperative access to the face of the glenoid. The patient is positioned such that full glenohumeral extension and some degree of glenohumeral adduction is possible. An articulated arm-holding device, such as the McConnell arm holder (McConnell Orthopedic Manufacturing Company, Greenville, Texas), can be a useful adjunct.
Surgical Landmarks, Incisions, and Portals
| • | Coracoid process | |
| • | Acromion | |
| • | Deltoid insertion | |
| • | Axillary crease |
| • | Deltopectoral approach |
| • | Cephalic vein | |
| • | Musculocutaneous nerve | |
| • | Axillary nerve |
Examination Under Anesthesia and Diagnostic Arthroscopy
Passive range of motion and stability are evaluated carefully and documented.
Specific Steps (
Box 30-1
)
Surface landmarks—coracoid process, acromion, deltoid insertion—are marked on the skin. An 8- to 10-cm incision is made from a point immediately lateral to the lateral tip of the coracoid process in the direction of the deltoid insertion. The fat stripe indicating the interval between the medial border of the anterior deltoid and the superior border of the pectoralis major is located. The cephalic vein is identified, preserved, and taken laterally with the deltoid. Medial mobilization of the cephalic vein may be preferred to avoid inadvertent laceration as many of the surgical steps are performed medially with prolonged posterior displacement of the proximal humerus. The blades of a self-retaining retractor, such as a Kolbel retractor, are placed beneath the deltoid and pectoralis major. A Hohman retractor is placed superior to the coracoacromial ligament. The tip of the coracoid process is visualized, and the “red stripe” that marks the lateral border of the conjoined tendon is identified. By use of blunt dissection, a plane is developed deep to the conjoined tendon. The musculocutaneous nerve is palpated at its entry into the deep surface of the conjoined tendon. If the entry point is distal, then one of the blades of the self-retaining retractor may be safely placed deep to the conjoined tendon. Otherwise, gentle, intermittent manual retraction is used to avoid traction injury to the nerve.
2. Lesser Tuberosity Osteotomy
The arm is forward flexed and internally rotated. The long head of the biceps tendon is transected in its groove and sutured to the superior aspect of the pectoralis major insertion. The remnant of the long head of the biceps tendon is used as a guide to open the rotator interval and is then transected at its junction with the superior labrum. A 1-inch flat osteotome is then used to osteotomize the lesser tuberosity. A 5-mm-thick wafer of bone is taken, permitting reflection of the subscapularis (
Fig. 30-3
). Traction sutures are placed around the tuberosity fragment to assist with release. The axillary nerve is identified by palpation. If there is any doubt about its location, gentle dissection can be used along the inferior border of the subscapularis to visualize the nerve directly. A “360-degree release” of the subscapularis tendon is then performed, with release of the coracohumeral ligament superiorly, release of the anterior capsular reflection posteriorly, and release of subdeltoid and subcoracoid adhesions. The final element of the release—the inferior glenohumeral capsule—is addressed after humeral preparation.
|
|
|
|
Figure 30-3 |
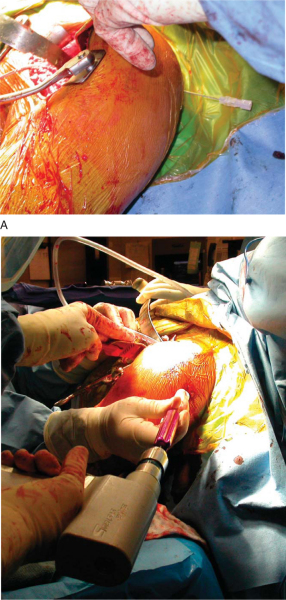
The humerus is maintained in a position of adduction and neutral flexion or extension as the capsular attachment to the humerus is released and the humerus is progressively externally rotated. Osteophytes are removed from the humerus, permitting identification of the anatomic neck. A freehand cut is made to match the native version and inclination of the proximal humerus, and a third-generation system that permits precise recapitulation of the native anatomy is used. As an alternative to the traditional stemmed component, a humeral head resurfacing implant can be used, permitting even greater preservation of proximal humeral bone stock but making access to the glenoid more challenging in some cases.
Release of the inferior capsule completes the 360-degree release of the subscapularis and permits the posterior translation of the proximal humerus that is necessary for broad access to the glenoid. The traction sutures placed through the lesser tuberosity are pulled in a superior direction to put the inferior border of the subscapularis under tension. A plane is developed between the lower muscle border of the subscapularis and the anterior inferior capsule. This maneuver permits clear visualization along both the superior and inferior surfaces of the inferior glenohumeral capsule, allowing it to be released in a safe, controlled manner, with minimal risk to the axillary nerve. For additional safety, the release is performed at the capsulolabral junction, and a finger is maintained superior and posterior to the nerve. The inferior capsule typically is released until the triceps origin can be visualized.
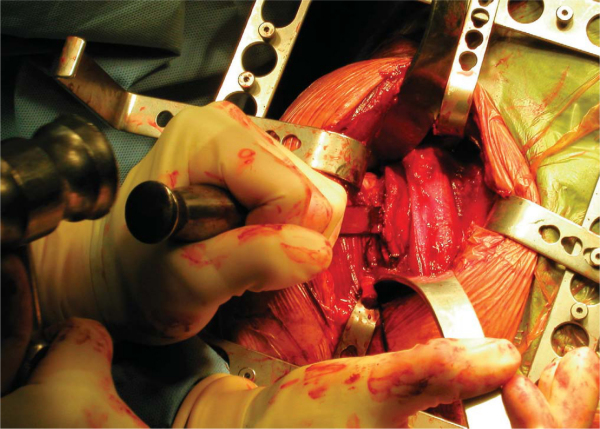
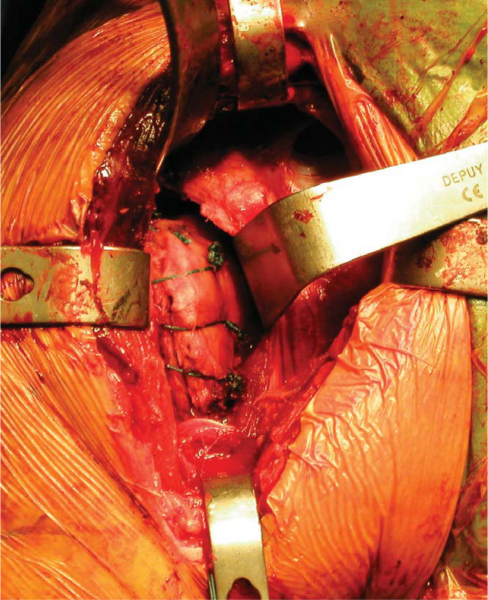
5. Glenoid Preparation and Meniscal Allograft Implantation
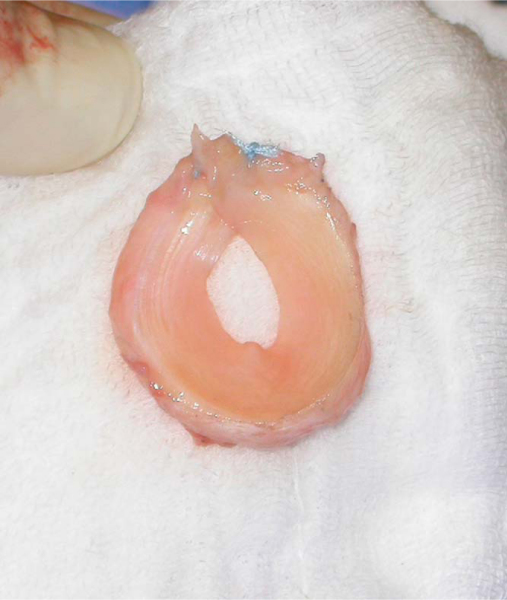
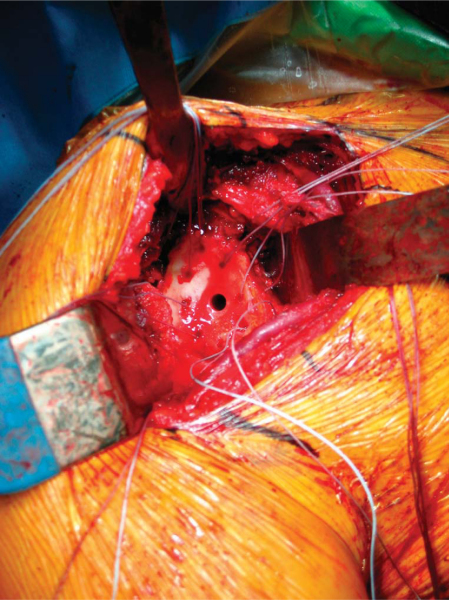
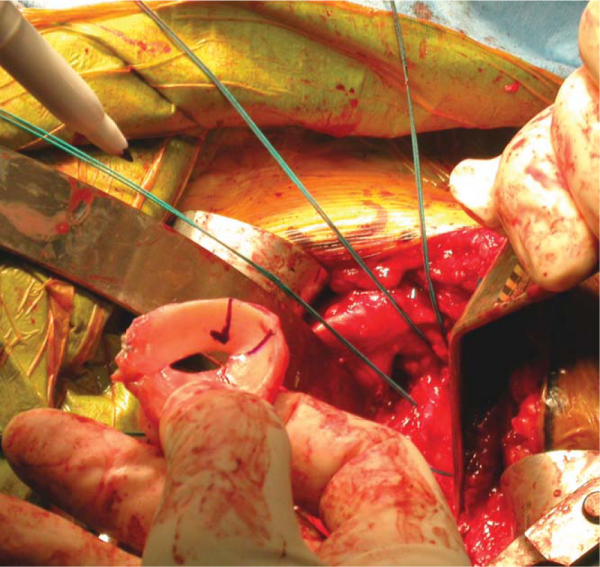
A Fukuda or similar type of retractor is then placed behind the posterior rim of the glenoid, and the humerus is placed in a position of slight external rotation, relaxing the posterior capsule and facilitating glenoid exposure. An anterior glenoid retractor is placed into the subscapularis recess, and a Hohman retractor is placed along the superior edge of the glenoid. The labrum is débrided. The glenoid face is reamed lightly. A microfracture awl is used to make multiple perforations across the glenoid, spaced 5 mm apart. The lateral meniscal allograft is removed from its bony attachment, and the ends of the meniscus are sutured over one another with a heavy, nonabsorbable suture (
Fig. 30-4
). The degree of overlap is judged to allow the size of the prepared meniscus to approximate the size of the recipient glenoid. A Bankart awl or small drill bit is used to prepare the glenoid for transosseous sutures. A total of eight heavy, nonabsorbable sutures are used, starting at 12-o’clock and progressing in 90-minute intervals (
Fig. 30-5
). The meniscal allograft is marked similarly (
Fig. 30-6
). Posterior sutures can be difficult to place in transos seous fashion, and suture anchors can be used instead, placed in percutaneous fashion as one would do during an arthroscopic posterior labral repair (
Fig. 30-7
). By use of a small trocar-tipped or cutting needle, these sutures are now passed through the meniscal allograft, which is then advanced down the sutures and onto the face of the glenoid (
Fig. 30-8
). Sutures are tied in balanced fashion (12-o’clock, then 6-o’clock, and so on).
|
|
|
|
Figure 30-4 |
|
|
|
|
Figure 30-5 |
|
|
|
|
Figure 30-6 |
|
|
|
|
Figure 30-7 |
|
|
|
|
Figure 30-8 |
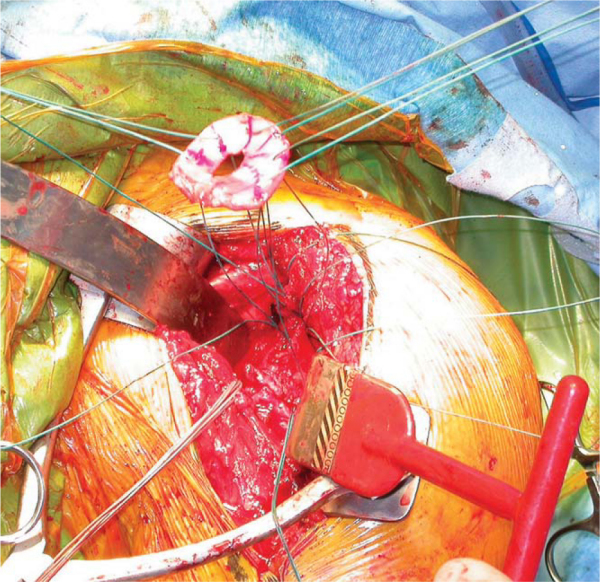
6. Humeral Implant, Lesser Tuberosity Repair, and Closure
The humeral head protector is removed and replaced with the humeral trial. The subscapularis is reduced to its insertion, and soft tissue balance is assessed. The trial is then removed, and a series of four holes are drilled through the bicipital groove along the lateral edge of the lesser tuberosity footprint. A series of three holes are drilled medial to the footprint. Four No. 5 nonabsorbable sutures are then placed through the holes in horizontal fashion. The superior-most of these sutures will pass through a lateral drill hole and then around the neck of the implant rather than through a matching medial drill hole. The humeral implant is then inserted. A small amount of cement can be used along the proximal aspect of the implant, but given the future possibility of conversion to total shoulder arthroplasty, the humeral stem is not cemented in its entirety. The previously placed No. 5 sutures are then passed around the lesser tuberosity wafer and tied down securely (
Fig. 30-9
). The humerus is internally and externally rotated to assess the security of the tuberosity repair. There should be no observable motion at the repair site. If desired, the rotator interval can be closed with the humerus in maximal external rotation. Motion, stability, and soft tissue balance are assessed once again. A drain is not used routinely. The deltopectoral interval is closed loosely with 2-0 Ethibond (Ethicon, Inc., Somerville, NJ) nonabsorbable sutures. Colored sutures can serve as a useful landmark in the event of future revision to total shoulder arthroplasty.
|
|
|
|
Figure 30-9 |
| • | At 7 to 10 days for wound check and radiographs (true anteroposterior view in plane of scapula and outlet view) |
| • | Pendulum exercises are begun. | |
| • | Lesser tuberosity osteotomy precautions: No active internal rotation, backward extension, or passive external rotation beyond the safe limit determined intraoperatively is allowed. | |
| • | A simple sling is worn for weeks 0 to 4. | |
| • | Active use of the elbow, wrist, and hand is encouraged. | |
| • | Active-assisted range of motion is permitted up to 120 degrees of forward flexion, 75 degrees of abduction. |
| • | Active-assisted range of motion is progressed to active range of motion for internal rotation and backward extension as tolerated. | |
| • | Active-assisted range of motion is progressed to active range of motion in forward flexion, abduction, and external rotation as tolerated. | |
| • | Begin gentle deltoid and rotator cuff isometrics, with the exception of internal rotation. | |
| • | Start periscapular strengthening exercises. |
| • | Progress strengthening, including resisted internal rotation. | |
| • | Perform gentle end-range stretching. |
| PEARLS AND PITFALLS | ||||||||||||||||||
|
Complications are similar to those of total shoulder arthroplasty:
| • | Injury to the axillary nerve | |
| • | Injury to the musculocutaneous nerve | |
| • | Failure of the lesser tuberosity repair | |
| • | Infection | |
| • | Incomplete relief of pain | |
| • | Failure of the meniscus-glenoid interface |
Published and presented results of biologic resurfacing procedures are limited, and those available reflect only short- and intermediate-term outcomes (
Table 30-1
).
| Author | Procedure | Followup | Outcome |
|---|---|---|---|
| Burkhead and Hutton[3] (1995) | Humeral hemiarthroplasty | 34 months | Range of motion in forward flexion improved from 81 to 137 degrees |
| Biologic resurfacing of glenoid with either fascia lata autograft or anterior capsule | Neer’s full exercise rating system: 8 excellent, 2 satisfactory results | ||
| Krishnan et al[10] (2004) | Humeral hemiarthroplasty | 3 years | ASES score improved from 25 to 75 |
| Biologic resurfacing of glenoid and acromion with Achilles tendon allograft | Range of motion in forward flexion improved from 35 to 95 degrees | ||
| Argo et al[1] (2005) | Arthroscopic interposition grafting with porcine xenograft | 13 months | VAS for pain dropped from 8 to 2 |
| Range of motion in forward flexion improved from 90 to 150 degrees | |||
| Range of motion in abduction improved from 70 to 120 degrees |
1.
Argo D, Savoie FH, Field LD. Arthroscopic treatment of glenohumeral arthritis with interpositional grafting. Presented at Arthroscopy Association of North America, 24th annual meeting; Vancouver, BC, Canada; 2005.
2.
Athwal G, Shridharani S, O’Driscoll SW: Osteolysis and arthropathy of the shoulder after use of bioabsorbable knotless suture anchors.
J Bone Joint Surg Am 2006; 88:1840-1845.
3.
Burkhead Jr WZ, Hutton KS: Biologic resurfacing of the glenoid with hemiarthroplasty of the shoulder.
J Shoulder Elbow Surg 1995; 4:263-270.
4.
Cameron BD, Galatz LM, Ramsey ML, et al: Non-prosthetic management of grade IV osteochondral lesions of the glenohumeral joint.
J Shoulder Elbow Surg 2002; 11:25-32.
5.
Creighton RA, Cole BJ, Nicholson GP, et al: Effect of lateral meniscal allograft on shoulder articular contact areas and pressures.
J Shoulder Elbow Surg Nov 8, 2006;Epub
6.
Freehill MQ, Harms DJ, Huber SM, et al: Poly-l-lactic acid tack synovitis after arthroscopic stabilization of the shoulder.
Am J Sports Med 2003; 31:643-647.
7.
Gerber C, Lambert SM: Allograft reconstruction of segmental defects of the humeral head for the treatment of chronic locked posterior dislocation of the shoulder.
J Bone Joint Surg Am 1996; 78:376-382.
8.
Hamada S, Hamada M, Nishiue S, Doi T: Osteochondritis dissecans of the humeral head.
Arthroscopy 1992; 8:132-137.
9.
Johnson DL, Warner JJ: Osteochondritis dissecans of the humeral head: treatment with a matched osteochondral allograft.
J Shoulder Elbow Surg 1997; 6:160-163.
10.
Krishnan SG, Burkhead Jr WZ, Nowinski RJ: Humeral hemiarthroplasty with biologic resurfacing of the glenoid and acromion for rotator cuff tear arthropathy.
Tech Shoulder Elbow Surg 2004; 5:51-59.
11.
Levine WN, Clark Jr AM, D’Alessandro DF, Yamaguchi K: Chondrolysis following arthroscopic thermal capsulorrhaphy to treat shoulder instability. A report of two cases.
J Bone Joint Surg Am 2005; 87:616-621.
12.
Petty DH, Jazrawi LM, Estrada LS, Andrews JR: Glenohumeral chondrolysis after shoulder arthroscopy: case reports and review of the literature.
Am J Sports Med 2004; 32:509-515.
13.
Scheibel M, Bartl C, Magosch P, et al: Osteochondral autologous transplantation for the treatment of full-thickness articular cartilage defects of the shoulder.
J Bone Joint Surg Br 2004; 86:991-997.
14.
Siebold R, Lichtenberg S, Habermeyer P: Combination of microfracture and periostal-flap for the treatment of focal full thickness articular cartilage lesions of the shoulder: a prospective study.
Knee Surg Sports Traumatol Arthrosc 2003; 11:183-189.
15.
Weinstein DM, Bucchieri JS, Pollock RG, et al: Arthroscopic débridement of the shoulder for osteoarthritis.
Arthroscopy 2000; 16:471-476.
16.
Yamaguchi K, Ball C, Galatz L. Meniscal allograft interposition arthroplasty of the arthritic shoulder: early results and a review of the technique. Presented at the 18th open meeting of the American Shoulder and Elbow Surgeons; Dallas, Texas; 2002.