Anterior Shoulder Repair
encountered problem in orthopaedic surgery. Although it most commonly
occurs in young individuals (younger than 30 years), it can be
encountered in older patients, including the geriatric population. The
operative management of recurrent anterior instability has evolved over
the past 40 years. A significant number of well-described techniques
have been used to treat this problem, ranging from bony procedures to
soft tissue procedures to a combination of both approaches. More
recently, it has become widely recognized that the treatment of
recurrent anterior glenohumeral instability should be directed at the
underlying pathoanatomy. In most patients, this involves the anterior
capsule and the capsulolabral attachments. In this chapter, we discuss
the operative management of recurrent anterior glenohumeral instability
using this pathoanatomic approach.
is now recognized that the essential lesion most likely represents a
combination of findings that includes the capsule and the capsulolabral
complex. Therefore, knowledge of the anatomy of the glenohumeral joint is critically important in the performance of this procedure.
socket joint; however, it is actually a “ball on a dish.” The relative
flatness of the glenoid provides very little inherent constraint during
glenohumeral motion. Unlike the hip joint in which the femoral head is
well contained within the acetabulum, the humeral head has a much
greater freedom of motion on the relatively flat glenoid. These
relationships explain the wide range of motion possible at the
glenohumeral joint and why the glenohumeral joint is the joint that
most commonly becomes unstable.
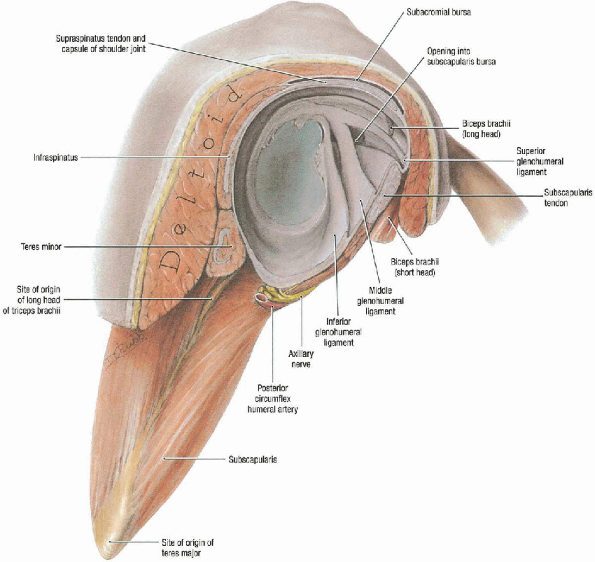
the fibrocartilaginous labrum forming a peripheral rim for the glenoid
that increases both the surface area and its concavity (Fig. 4-1).
The glenohumeral joint capsule attaches to the edge of the glenoid
anteriorly, inferiorly, and posteriorly. Superiorly, it is contiguous
with the underside of the rotator cuff and inserts just medial to the
biceps labral complex.
glenohumeral ligaments. These are thickenings of the capsule that form
the superior, middle, and inferior glenohumeral ligaments. The inferior
glenohumeral ligament is the most important anterior stabilizer,
particularly in the abducted and externally rotated position. The
middle glenohumeral ligament is also an important anterior stabilizer.
The superior glenohumeral ligament is less consistent and provides more
of a restraint for inferior translation.
and the anteroinferior glenoid provides the important soft tissue
restraint that maintains the humeral head in a reduced position on the
glenoid in the abducted and externally rotated position. Disruption or
compromise of these soft tissue stabilizers allows abnormal anterior
translation resulting in instability. When this occurs because of a
traumatic event, it most commonly results in disruption of the
capsulolabral attachment to the anterior glenoid. This is often
referred to as a Bankart lesion. When this disruption occurs, it may
also involve a small avulsion fracture of bone that can be visualized
radiographically. Avulsions occur less commonly than pure soft tissue
disruption. When the capsulolabral attachment is disrupted, recurrent
episodes of instability are more likely to occur. With each episode of
instability, there is some additional damage to the anterior capsule.
This can result in stretching or plastic deformation of the capsule
(glenohumeral ligaments), further compromising the anterior
stabilizers. Therefore, because the primary etiology of recurrent
anterior instability is disruption of the anterior capsulolabral
stabilizing mechanism, operative management should be directed at
restoring the integrity of this essential support. Although recurrent
anterior dislocations may also produce impression fractures of the
posterolateral portion of the humeral head or anterior glenoid wear, in
most cases anterior capsulolabral reconstruction will be successful in
correcting the problem. In addition, there are specific situations in
which the bony changes must be addressed. These instances are discussed
later in the chapter.
degree, frequency, etiology, and duration. The direction of dislocation
can be anterior, posterior, inferior, or multidirectional. The degree
of instability can represent subluxation
or
dislocation. Frequency can be a single episode or recurrent episodes.
The etiology can be traumatic or atraumatic. Traumatic episodes
generally result from a specific significant injury. An atraumatic
etiology generally indicates that instability develops in the absence
of a specific injury. The atraumatic group can be further classified as
voluntary or involuntary. Voluntary atraumatic instability occurs when
an individual consciously attempts to cause the subluxation or
dislocation. Involuntary episodes occur without specific voluntary
actions by the individual. The duration of instability can be acute or
chronic. Acute dislocations are generally considered to be those that
are recognized
within
24 hours of the occurrence. A dislocation that has been present for
more than 24 hours is considered chronic. This aspect of classification
has not been universally agreed on. Some consider any dislocation less
than 3 weeks in duration as acute, and all beyond 3 weeks as chronic.
Other classifications have considered acute to be less than 24 hours,
subacute between 1 day and 3 weeks and chronic beyond 3 weeks. In this
specific chapter, we specifically focus on the treatment of recurrent
anterior glenohumeral instability in which the initial episode occurred
as a result of a traumatic event and recurrent episodes occurred
involuntarily.
 |
|
FIGURE 4-1.
The glenoid labrum is a fibrocartilaginous ring that expands the surface area of the glenoid and provides a deepening affect. The glenohumeral joint capsule is characterized by thickenings anteriorly and posteriorly that correspond to the glenohumeral ligaments. The important ligaments anteriorly are the superior, middle, and inferior glenohumeral ligaments. Lateral view. |
anterior glenohumeral instability is indicated in patients with
episodes of instability that significantly interfere with their ability
to perform their daily activities. The degree of disability
associated with this condition varies from patient to patient. Some
patients have episodes that only occur as a result of specific athletic
activities, whereas others have episodes that occur with everyday
activities that involve using the extremity overhead. Some patients
describe episodes of instability during sleep. Each patient will
describe the degree of disability associated with this condition. Some
patients accept a more significant limitation of their activities in an
effort to avoid surgery. The decision to proceed with surgery should be
based on an in-depth discussion between the patient and the surgeon,
which addresses the nature of the problem, the natural history of
glenohumeral instability, the patient’s lifestyle and activity needs,
and operative management particularly with respect to anticipated
outcomes and potential benefits and risks. With this information, the
patient is able to make an informed decision.
are candidates for operative management is the frequency and ease with
which episodes occur. Patients who have had three episodes of
instability occurring only with athletic activity (each of which was
separated by 2 or 3 years) are clearly less disabled by the condition
than someone who has had six episodes of instability over the past 6
months, all of which occurred with everyday activities that include
dressing and sleeping. The role of nonoperative management is generally
limited. In patients with recurrent anterior instability of traumatic
origin an exercise program is unlikely to resolve the problem or
significantly reduce the frequency of episodes of instability. Some
patients may prefer to limit their activities to avoid surgery. This
may be possible with patients in whom the episodes occur during
specific athletic activities, but it is certainly not feasible for
those experiencing episodes with activities of daily living. This
distribution further indicates the importance of a careful evaluation
of each individual patient to determine the appropriate candidates for
operative management.
contraindicated in patients who exhibit a voluntary component to the
instability because these patients often have underlying psychologic
problems that would preclude a successful outcome. In addition,
patients who will not be compliant with postoperative management,
specifically the duration of immobilization or a gradual return to
activities and participation in the rehabilitation program, should also
not be considered as candidates for operative management. In these
subgroups of patients, the likelihood of an unsuccessful outcome is
significant; thus, operative management should be avoided.
confirmation of the diagnosis. This is accomplished with a careful
history and physical examination. Standard radiographs should be
obtained to identify findings that suggest recurrent anterior
instability, including Hill Sachs impression fractures, anterior
glenoid wear, and calcification about the anterior glenoid. If a
patient has had radiographs obtained in the emergency room before
closed reduction, these should be obtained and reviewed to confirm the
direction of instability. In patients for whom these x-rays are not
available, we prefer to obtain a magnetic resonance imaging (MRI) to
document changes in the anterior capsulolabral complex. An MRI also
identifies bony changes consistent with anterior instability. If
standard radiographs and/or an MRI suggest significant anterior glenoid
wear, a computed tomography (CT) scan should be obtained to further
delineate these changes. Although it is uncommon to require anterior
glenoid bone grafting, this possibility should be identified
preoperatively.
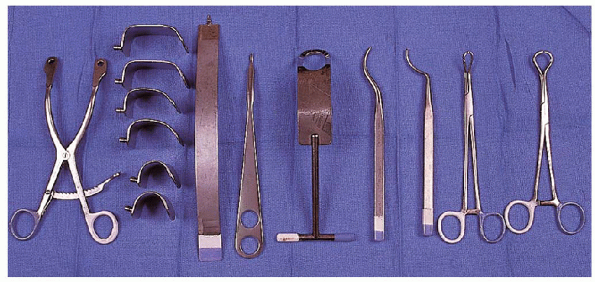
extensive. There are specific instruments that allow the preparation of
the bone tunnels to be performed more easily. Currently, there are a
variety of suture anchors available to reattach the anterior capsule to
the glenoid if this technique is preferred. However, the technique we
describe uses sutures placed through bone tunnels in the anterior
glenoid to reattach the capsule.
A modular retractor with blades of variable depth is preferable to
allow for the varying amounts of soft tissue that may be encountered.
Exposure of the glenoid is facilitated with use of a short-spike
levering type retractor. This facilitates retraction of the anterior
capsule during preparation of the bone
tunnels.
Use of a small Hohmann retractor may also be helpful; similarly, a
Fukuda humeral head retractor enhances exposure of the glenoid
articular surface. Preparation of the bone tunnels is accomplished more
easily with use of an offset awl and a sharp tenaculum. These are
designed for this procedure and are available from different instrument
manufacturers. Passage of the sutures through the bone tunnels is
facilitated by using needles of varying sizes to accommodate the
curvature of the bone tunnel. Although cutting needles are recommended
for this portion of the procedure, we generally prefer noncutting
needles for passage of the sutures through the soft tissues.
 |
|
FIGURE 4-2. Equipment used during anterior shoulder repair include (from left to right):
self-retaining retractor with blades of variable depth, a single spike retractor for the anterior glenoid, a small spike levering retractor, a humeral head retractor, offset awls of different sizes, and reaming tenacula of different sizes. |
regional or general anesthesia. The vast majority of these procedures
in our institution are performed under regional anesthesia. This
approach is well-received by patients and has the benefit of providing
early postoperative pain relief. However, the
use of general anesthesia does allow for the examination of both
shoulders intraoperatively. This may be the preferred approach when
examination of the contralateral shoulder is important to confirm the
diagnosis. However, the goal should always be to enter the operating
room with a confirmed diagnosis and an established operative plan based
on careful preoperative evaluation. After induction of
anesthesia, the first step is the physical examination. The involved
shoulder should be carefully and systematically assessed for range of
motion and humeral head translation. Passive range of motion should be
documented, including forward elevation: external rotation with the
elbow both at the side and in 90 degrees abduction and internal
rotation with the elbow at the side and in 90 degrees of abduction.
Translation of the humeral head on the glenoid in anterior, posterior,
and inferior directions should be assessed. The testing position should
include those that predispose to anterior, posterior, and inferior
instability. Range of motion and translation should always be compared
with the uninvolved shoulder.
 |
|
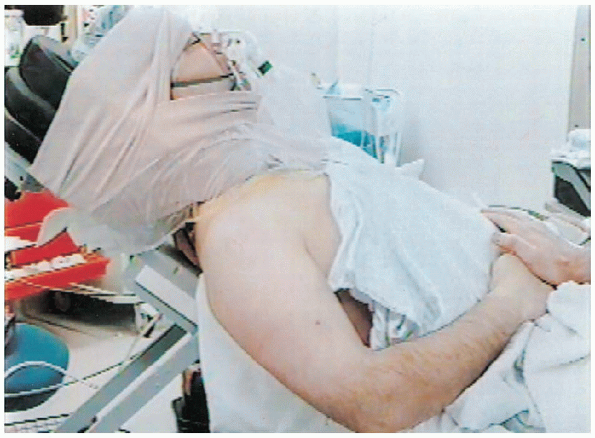
FIGURE 4-3. A patient is positioned in 30 to 40 degrees of elevation. The operative extremity is positioned to allow free mobility.
|
patient is positioned for the surgical procedure. The use of a specific
positioning device that allows varying amounts of elevation of the head
and trunk is helpful. When arthroscopy is performed before the anterior
shoulder repair, we use the sitting position. Pillows should be placed
under the knees to prevent sliding. Following completion of the
arthroscopic component, the position should be changed to 30 to 40
degrees of elevation for the anterior shoulder repair (Fig. 4-3).
The shoulder is shaved just before preparation of the skin. The area of
shaving should include the anterior aspect of the shoulder and the
axilla. The preparation includes the shoulder and the entire upper
extremity, beginning at the base of the neck and including the shoulder
girdle. Draping is performed to provide a secure sterile field that
incorporates the anterior and superior aspects of the shoulder.
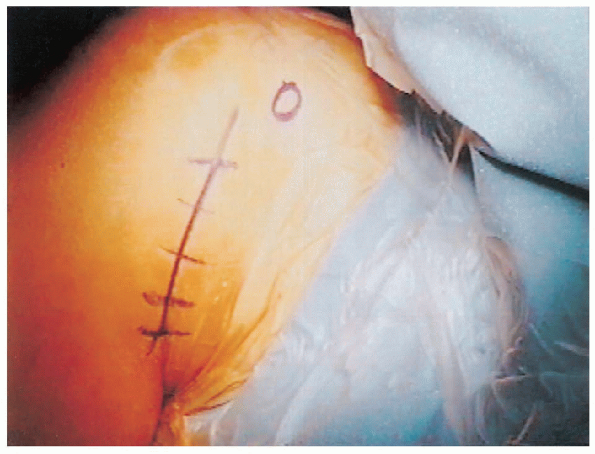
the anterior aspect of the shoulder are identified. A surgical marking
pen is used to mark the coracoid process, the lateral clavicle, the
acromioclavicular joint, and the anterior and lateral portions of the
acromion. An anterior inferior incision is used in line with the
axillary skin fold (Fig. 4-4). The incision is
marked by placing the arm in an adducted position next to the chest
wall. This allows identification of skin lines for improved cosmesis.
The incision extends from the anterior aspect of the axilla superiorly
toward the coracoid process. An incision of varying length can be used
based on the size of the patient. Smaller incisions can be used in
thin, less muscular patients, whereas a longer incision is necessary in
larger more heavily muscled patients. Although some
have
advocated use of an anterior/inferior axillary incision for cosmetic
reasons, we prefer a more anterior incision to facilitate exposure.
Although the appearance of the incision is related, in part, to its
length and location, the method of skin closure and the variable
tendency for the incision to spread are more important factors.
 |
|
FIGURE 4-4.
The anterior axillary incision extends from the axillary skin fold to the area just lateral to the coracoid process. Length of the incision vary from 5 to 7 cm depending on the patient’s size. |
subcutaneous flaps are developed medially, laterally, superiorly (up to
the coracoid process), and inferiorly. This allows identification of
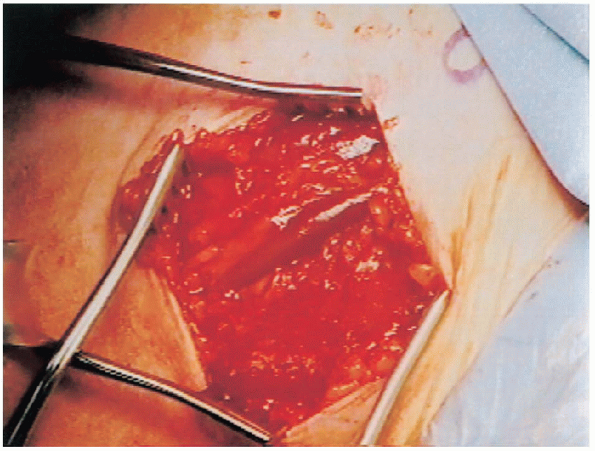
the deltopectoral interval. The deltopectoral interval is identified by the fat stripe covering the cephalic vein (Fig. 4-5).
If difficulty is encountered identifying the interval, attention should
be turned to the proximal portion of the exposure in the area of the
coracoid process. The interval is formed just distal to the coracoid
process and is often easier to identify in this area. The deltopectoral
interval should be carefully developed proximally and distally. The
cephalic vein is more commonly retracted laterally with the deltoid
because there are a number of branches that enter the vein from the
deltoid. Occasionally, medial retraction of the cephalic vein with the
pectoralis major is used based on anatomy of the interval. The interval
is developed proximally up to the coracoid and distally to the
pectoralis major insertion. The subdeltoid space is mobilized and a
self-retaining retractor is used to retract the deltoid and the
pectoralis major for exposure of the deeper tissues. The next layer is
the clavipectoral fascia, which is divided longitudinally just lateral
to the conjoined tendon muscles. This allows the conjoined tendon
muscles to be mobilized and retracted medially. The release of the
superior 1 cm of the pectoralis major tendon insertion can facilitate
exposure in large, muscular patients. This must be done carefully and
should be repaired at the completion of the procedure.
 |
|
FIGURE 4-5.
The deltopectoral interval is marked by a fat stripe that separates the two muscles. The cephalic vein lies within the interval. |
 |
|
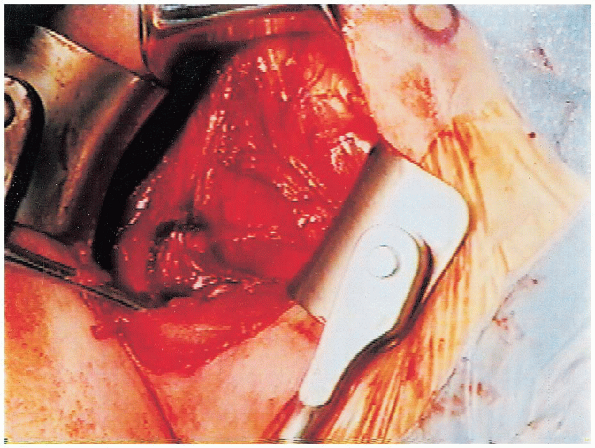
FIGURE 4-6. The vessels that mark the inferior aspect of the subscapularis tendon are cauterized to reduce bleeding.
|
humerus are then identified. These include the bicipital groove, the
lesser tuberosity with the insertion of the subscapularis tendon, and
the rotator interval. The veins that are located at the inferior aspect
of the subscapularis tendon are also identified and cauterized to
prevent bleeding as the dissection progresses (Fig. 4-6).

A no. 15 blade on a long handle is preferred for this dissection. An
incision is made into the subscapularis tendon, approximately 1 cm
medial to its insertion into the lesser tuberosity.
The
incision is made in line with the humeral shaft, beginning superiorly
at the rotator interval and progressing distally to the inferior aspect
of the subscapularis. The most inferior aspect of the incision should
curve slightly medially. The
scalpel blade is beveled medially to carefully divide the tendon fibers
and facilitate entrance into the interval between the tendon and the
capsule. Note that the tendon fibers are striated, whereas the
capsular tissue is not. This distinction is an important component of
the dissection. When striated fibers are no longer visible, the
capsular layer has been exposed. If the dissection enters the deeper tissues too quickly, the capsule may be perforated.
As the tendon is elevated off the capsule, the dissection progresses
medially to the musculotendinous junction. With elevation of the
tendon, the muscle can be more easily separated from the capsule with a
periosteal elevator. It is important to continue the dissection to the
rotator interval superiorly and to the inferior portion of the
subscapularis to expose the entire anterior and inferior capsule. At
the inferior portion, there is less tendon and more muscle tissue.
Bleeding may, therefore, occur and should be controlled by
cauterization. The medial edge of the subscapularis tendon is tagged
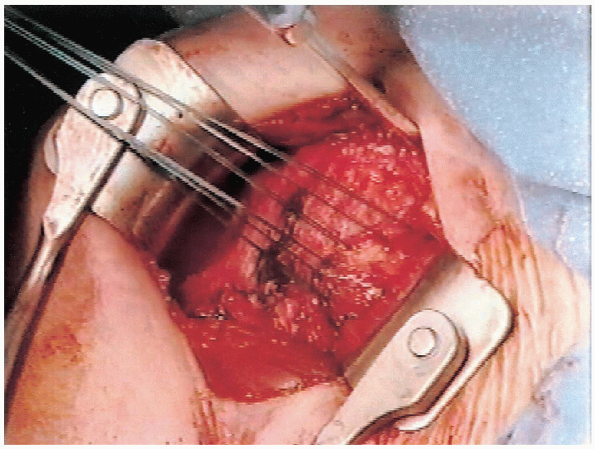
with no. 1 Mersilene sutures (Fig. 4-7). The
subscapularis tendon and muscle is dissected off the underlying capsule
medially until the anterior glenoid neck is palpable. The subscapularis
tendon is then placed behind the self-retaining retractor. The capsular
layer is now exposed. Two specific aspects of the capsule should be
evaluated.
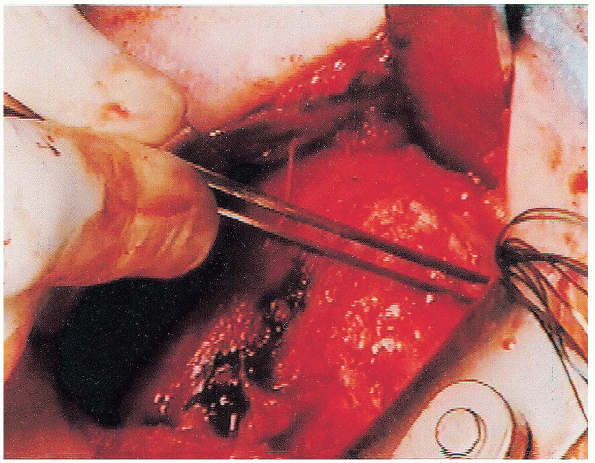
This fenestration varies from a small lateral opening to a large
opening that extends to the anterosuperior corner of the glenoid. The
fenestration should be repaired using no. 1 Mersilene in simple
interrupted sutures.

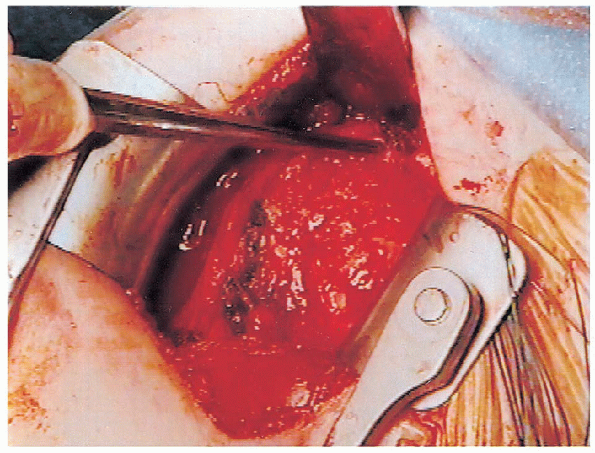
This portion of the repair imbricates the area of the superior
glenohumeral ligament to the most anterior portion of the rotator cuff (Fig. 4-9).
The repair can be expected to provide some degree of resistance to
inferior translation in patients with a significant component of
inferior laxity. The degree of capsular laxity should also be assessed.
With the arm in neutral rotation, the capsule can often appear very
redundant anteriorly and inferiorly. This is particularly true in
patients with excessive degrees of external rotation. This finding
provides a preliminary indication of the degree of capsular shift that
may be necessary to achieve stability.
 |
|
FIGURE 4-7. The medial edge of the subscapularis tendon is tagged with sutures and retracted medially to expose the underlying capsule.
|
 |
|
FIGURE 4-8.
The rotator interval fenestration is identified superiorly. It can be variable in size, although currently it is small as shown here. |
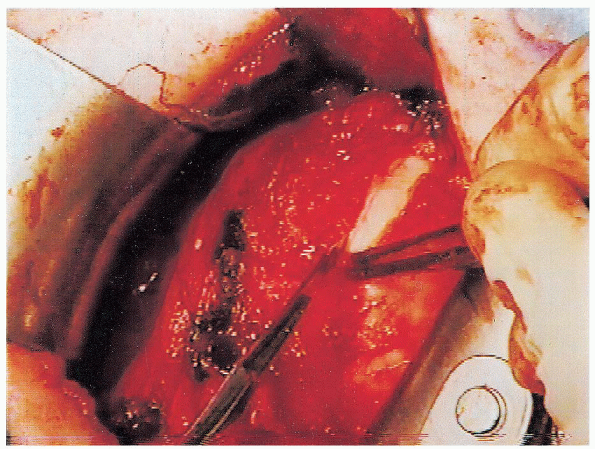
The capsulotomy extends superiorly from the rotator interval to the
anteroinferior aspect of the humeral neck. The medial edge of the
capsule is tagged with two no. 1 Mersilene sutures that are placed near
its midportion. A Fukuda humeral
head
retractor is then inserted. This allows assessment of the anterior
glenoid and the integrity of the capsulolabral insertion. The
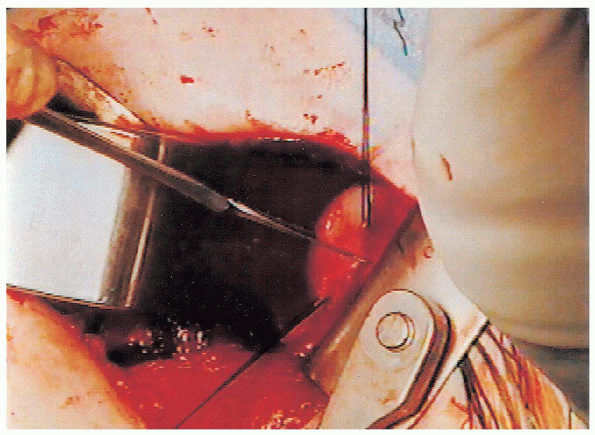
midportion of the glenoid is identified and a horizontal capsulotomy is
performed (Fig. 4-11).
The glenoid articular surface and the anterior glenoid can now be well
visualized. With the attached sutures, the superior and inferior leaves
of the capsule can be retracted superiorly and inferiorly,
respectively. The capsulolabral insertion is then evaluated. Different
findings are often encountered. In some patients, the capsulolabral
complex is intact. This is usually encountered in patients in whom the
underlying pathoanatomy is significant laxity and redundancy. These
patients require only the inferior capsular shift portion of the
procedure. In some patients, the capsulolabral complex is completely
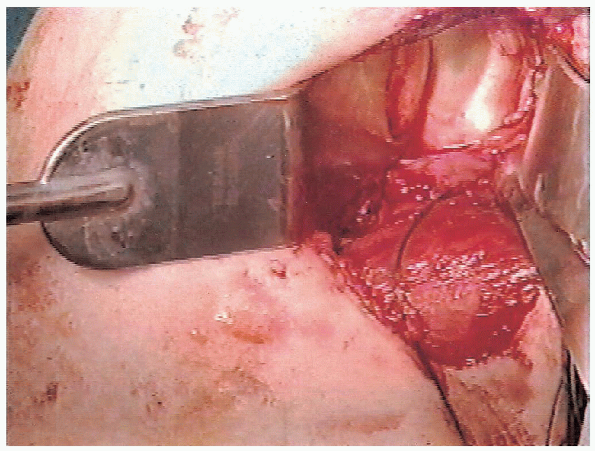
stripped off from its insertion into the anterior glenoid (Fig. 4-12).
The anterior labrum may be absent or torn and detached from the
capsule. These patients require reattachment of the anterior capsule to
the anterior edge of the glenoid, with an inferior capsular shift that
varies based on the amount of inferior capsular redundancy. Some
patients appear to have an intact capsular insertion located medial to
the anterior glenoid margin. In these cases, the anterior labrum is
usually absent and the capsular attachment is generally tenuous. An
elevator should be used to elevate the capsular attachment so that it
can be advanced to the anterior glenoid margin and reattached in a more
anatomic position.
 |
|
FIGURE 4-9. Closure of the rotator interval defect is performed with nonabsorbable interrupted sutures.
|
 |
|
FIGURE 4-10. The vertical capsulotomy is performed 1 cm medial to the location of the subscapularis tenotomy.
|
 |
|
FIGURE 4-11. The horizontal capsulotomy is directed to the midpoint of the glenoid.
|
 |
|
FIGURE 4-12.
With the humeral head retractor and the glenoid retractor in position, the capsulolabral detachment is evident with clear visualization of the anterior glenoid. |
reattachment is an essential portion of the procedure. An anterior
glenoid neck retractor is used to expose the area of reattachment.
Depending on the size of the glenoid, it may be helpful to place a
small Hohmann type retractor inferiorly and superiorly for additional
exposure. The capsulolabral attachment is usually intact inferiorly at
about the 6-o’clock position. When a left shoulder is being treated,
the capsular detachment extends up to the 11-o’clock position. When a
right shoulder is being treated, the capsular detachment extends to the
1-o’clock position. The goal of this portion of the procedure is to
reattach the capsule to its anatomic position at the anterior margin of
the glenoid to restore the soft tissue buttress to prevent anterior
translation.
suture anchors are used, it is important that they be placed directly
at the junction of the anterior glenoid neck and the glenoid articular
surface. This allows the capsule to be brought into its anatomic position. It is important not to place the anchors further medially because this results in a nonanatomic capsulolabral reattachment. This chapter focuses on the technique for preparation of bone tunnels.
 We have found that the most straightforward approach is to use an offset awl combined
We have found that the most straightforward approach is to use an offset awl combinedwith a sharp tenaculum. These instruments can be found in the Bankart
repair instrument sets that are available from different instrument
manufacturers. The bone tunnel should be placed through the anterior
glenoid articular surface and through the glenoid neck. Three bone tunnels are usually necessary to reattach the detached area of capsule.
These are placed at the 5-o’clock, 3-o’clock, and 2-o’clock positions
for a right shoulder and the 7-o’clock, 9-o’clock, and 10-o’clock
positions for the left shoulder. Because restoration of the
anterior/inferior capsular reattachment is critically important for
success of the repair, placement of bone tunnels in this area is
particularly important. The offset awl initiates the bone tunnel at the
glenoid articular surface, approximately 3 to 4 mm from the edge of the
bone. It is directed perpendicular to the glenoid and advanced through
the subchondral bone. The awl is then placed on the anterior glenoid
neck directly opposite the location on the glenoid articular surface.
The awl is inserted perpendicular to the bone surface and directed
toward the deepest insertion point of the previously prepared hole. It is essential to maintain a sufficient bony bridge to prevent inadvertent fracture.
At this point, the tenaculum is used. One end of the tenaculum is
placed through the entry point in the glenoid articular surface and the
other is placed through the glenoid neck entry point. The tenaculum is
then tightened and carefully rotated in a medial and lateral direction
to form one continuous tunnel. There will be some initial resistance as
the tenaculum is rotated, which will gradually decrease as the tunnel
is completed and enlarged. Excessive resistance
may be an indication that the tenaculum is not in the proper position
or that the prepared holes are too far apart to allow easy conversion
to a tunnel. If this occurs, proper placement of the tenaculum
should first be confirmed. If this is not the problem, the awl should
be reinserted to deepen and enlarge the holes. When the bone tunnels
are complete, a no. 2 Mersilene suture, on a no. 5 cutting needle, is
passed through the bone tunnel. In some patients, needles can be more
easily passed through the articular surface, whereas in other patients
they may be more easily passed through the entry point on the glenoid
neck. When the tip of the needle is visualized
exiting the bone, it should be carefully advanced by following the
curvature of the needle. This avoids excessive force on the bone
tunnel and decreases the risk of fracture. The sutures are passed
through the remaining bone tunnels in a similar fashion (Fig. 4-14).
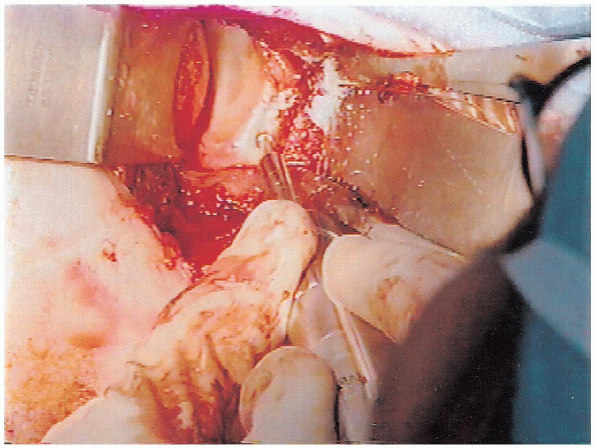 |
|
FIGURE 4-13. A motorized burr is used to decorticate the glenoid neck in the area for the capsular reattachment.
|
 The anterior glenoid neck retractor is repositioned into an extracapsular position. The
The anterior glenoid neck retractor is repositioned into an extracapsular position. Thesutures previously placed in the superior and inferior leaves of the
capsule should be used to advance the capsule laterally thereby
avoiding passing the sutures in an undesirable lateral position; doing
so could result in excessive tightening. The Fukuda humeral head
retractor remains in place while a Darrach elevator is placed below the
inferior capsule to protect the axillary nerve as the needle is passed.
The most inferior suture is then placed in a horizontal mattress
fashion through the anterior/inferior capsule. As the sutures are
passed, the capsule is advanced laterally and superiorly. The sutures
are placed at the glenoid margin so that the reattachment provides the
desired soft tissue buttress. The middle and superior sutures are
passed in a similar fashion, with care taken to advance the capsule
laterally and superiorly (Fig. 4-15). The most inferior suture is tied first. Care
must be taken to ensure that the suture is tight enough to bring the
capsule into direct and secure contact with the glenoid rim. The
middle and superior sutures are then tied. At this point, the capsular
reattachment should be assessed. A small elevator should be used to
confirm that the capsulolabral detachment has been repaired and is
securely in position. The Fukuda humeral head retractor is then removed
and the self-retaining retractor is reinserted—retracting the deltoid
laterally
and the subscapularis, conjoined tendon muscles, and pectoralis major medially.
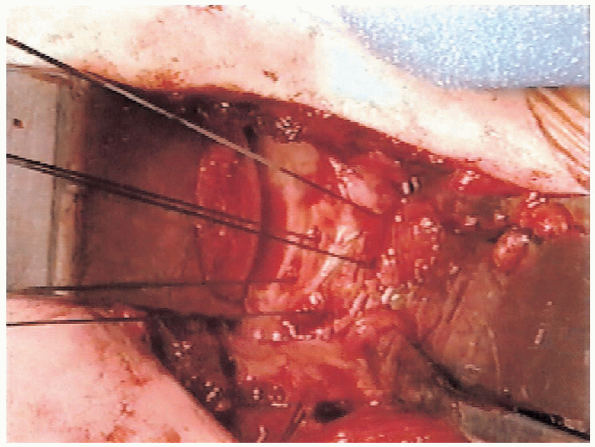 |
|
FIGURE 4-14. Three sutures have been placed through the bone tunnels that were prepared with the offset awl and reaming tenaculum.
|
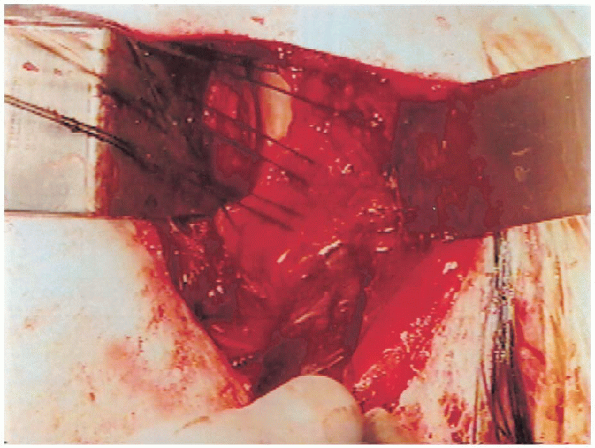 |
|
FIGURE 4-15. The sutures have been passed through the capsule as three horizontal mattress sutures.
|
The amount of shift depends on (a) the size of the inferior capsular
pouch, (b) the presence or absence of a capsulolabral detachment, (c)
the degree of external rotation present preoperatively, and (d) the
presence of underlying ligamentous laxity.
provide additional capsular support anteriorly and inferiorly but
should not result in excessive capsular tightening that would restrict
range of motion. To accomplish the inferior capsular shift, the
anterior/inferior capsule is detached from its humeral neck insertion
in an anterior to posterior direction, usually to the 6-o’clock
position. If additional capsular advancement is needed, the capsule can
be detached more posteriorly. A burr is then used to decorticate the
anterior/inferior glenoid neck to enhance healing of the capsule after
the advancement (Fig. 4-16). The inferior
capsular flap can be repaired to the remaining lateral capsule.
However, inferiorly, it comes into contact with the glenoid neck, and
the decortication facilitates healing. The inferior capsular flap is
advanced in a superior and lateral direction (Fig. 4-17).
It can usually be advanced to the most superior aspect of the original
capsulotomy near or at the rotator interval. It is reattached to the
lateral capsule with no. 2 Mersilene in simple interrupted sutures.
is assessed. This includes external rotation with the elbow at the
side. Although the range of motion is less than preoperative, the
decrease should only be 10 to 15 degrees when a capsulolabral
reattachment has been performed. When the underlying pathoanatomy is
capsular laxity and redundancy, a more significant decrease in external
rotation is desired. However, there should always be at least 30
degrees of external rotation present. Abduction in the coronal plane is
then assessed, making certain it is at least 90 degrees and the repair
is secure throughout this range.
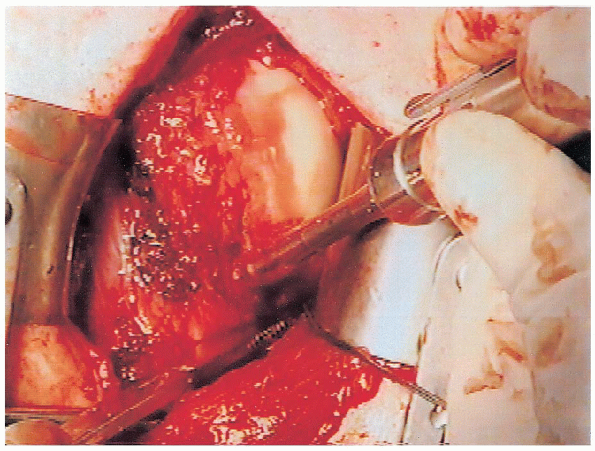 |
|
FIGURE 4-16. The motorized burr is used to decorticate the anterior-inferior humeral neck to facilitate capsular reattachment.
|
rotation should also be evaluated. There should be at least 80 degrees
of external rotation in this position without excessive stress on the
repair. In patients with significant capsular laxity, we accept 10 to
15 degrees less because of the amount of soft tissue stretching that is
anticipated to occur postoperatively. However, in patients who are
involved in overhead throwing, 90 degrees of external rotation in the
position of 90 degrees of abduction is desirable.
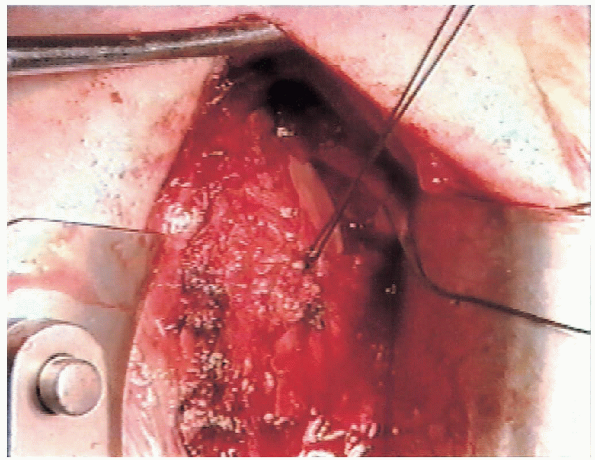
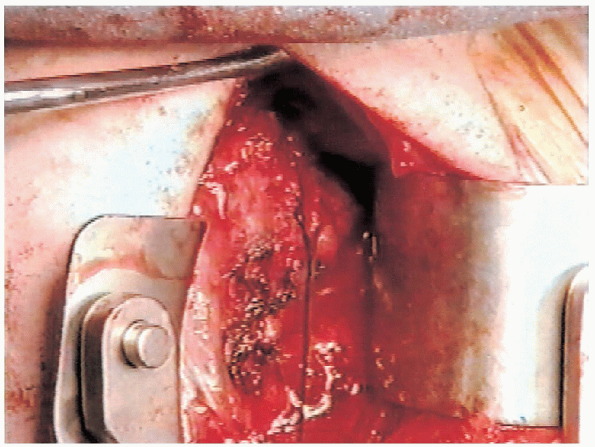
flap, attention is turned to the superior flap. The superior flap is
advanced inferiorly and laterally (Fig. 4-18). Inferior advancement is more important than lateral advancement.
Excessive lateral advancement restricts external rotation, which is not desirable.
The superior flap is secured to the lateral capsular tissue with no. 2
Mersilene using simple interrupted sutures. The area of overlap of the
superior and inferior flaps should be imbricated with one or two
horizontal mattress sutures passed first through the inferior flap and
then through the overlying superior flap. Placement of these sutures
reinforces the repair and provides additional anterior stability. When
the repair of the superior flap is completed, range of motion should
once again be assessed in the same testing sequence described
previously. Range of motion should not be significantly changed from the testing performed after repair of the inferior flap.
 |
|
FIGURE 4-17. The inferior flap is then advanced superiorly and secured to the lateral capsule.
|
 |
|
FIGURE 4-18. The superior capsular flap is advanced inferiorly and secured to the lateral capsule.
|
portion. An anatomic repair is performed to avoid any additional
limitation of external rotation. Although older repair techniques have
focused on advancement of the subscapularis as an important method of
presenting recurrent instability, the repair we describe focuses on
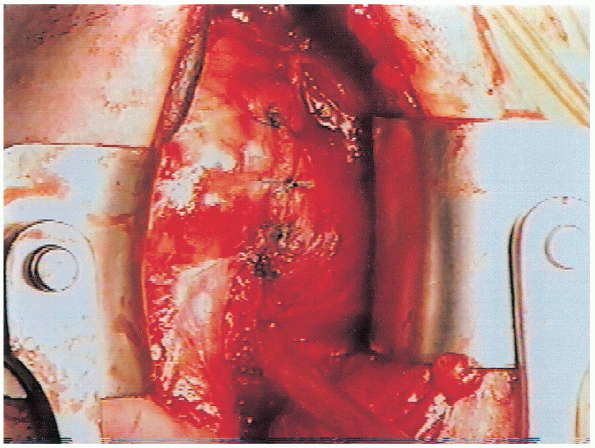
correction of the pathoanatomy. Therefore, the subscapularis, which is
not an etiologic factor, should be repaired anatomically using no. 1
Mersilene in simple interrupted sutures (Fig. 4-19). It is very important that a secure subscapularis repair be performed to avoid the risk of disruption postoperatively.
When the subscapularis repair is completed, external rotation should,
once again, be assessed with the elbow at the side to confirm that
there is no additional limitation.
deltopectoral interval using absorbable sutures, subcutaneous tissue
closure, and skin closure. We prefer to close the skin with a running
subcuticular nonabsorbable suture (no. 3-0 Prolene) that can be removed
postoperatively, because it provides improved cosmesis. Steri-Strips
are applied with a sterile dressing. The operative extremity is then
placed in a standard sling.
 |
|
FIGURE 4-19.
The subscapularis tendon is repaired directly to its remaining lateral portion. Shortening of the subscapularis should be avoided. |
duration of sling immobilization is based on consideration of different
factors that include the age of the patient, the degree of underlying
ligamentous laxity, whether a capsulolabral reattachment was performed,
and the security of the repair. In general, a somewhat longer
period of immobilization is preferred for younger patients, those with
significant underlying ligamentous laxity, and those in whom a
capsulolabral detachment was not performed; conversely, a shorter
period of immobilization is used for older patients, those without
underlying ligamentous laxity, and those in whom a capsulolabral
reattachment was performed.
Although shoulder range of motion is not performed, the patient is
instructed in active range of motion of the elbow, wrist, and hand. In
addition, isometric deltoid and external rotation exercises are
performed. Isometric internal rotation exercises are not performed
because of the subscapularis detachment and repair. These exercises are
continued during the period of immobilization. When the sling is
discontinued, an active range of motion program is initiated, focusing
on forward elevation, external rotation, and internal rotation behind
the back. Passive stretching exercises are not performed initially;
instead, we rely on the patient’s active exercise to regain range of
motion. If recovery of range of motion is slower than desired, gentle
stretching may be performed 6 to 8 weeks postoperatively. When active
range of motion is started, isometric internal rotation exercises are
added. Resistive strengthening exercises are begun when the patient has
progressed in regaining active range of motion.
This
generally occurs 6 to 8 weeks postoperatively. Patients are monitored
for recovery of active range of motion that includes forward elevation
and external rotation with the arm at the side and at 90 degrees of
abduction and internal rotation behind the back. Strength recovery is
also monitored as the patients are gradually progressed through
isometric, isotonic, and then isokinetic-type exercises. Strengthening
is performed below the shoulder level for the first 4 to 6 months
postoperatively. Strengthening overhead can then be safely added.
and encouraged to return to their everyday activities as soon as
possible. They are allowed to jog 6 to 8 weeks postoperatively. Aerobic
exercise is permitted before that time but is limited to an exercise
bicycle as long as the operative extremity is not used to grasp the
handle bars. Six months following the surgery, patients can return to
overhead noncontact athletic activity. This specifically includes
swimming, tennis, and other overhead racket sports. Basketball and
baseball activities can be resumed 6 to 9 months following the surgery.
Full activity basketball, which involves a significant amount of
contact, should be resumed closer to 9 months following surgery.
Patients can return to full unrestricted activity 9 to 12 months
following the surgery. This includes football, snow skiing, and water
skiing. Some modifications can be made for sports based on the position
played and whether the dominant or nondominant extremity is involved.
An approach to the repair of avulsion of the glenohumeral ligaments in
the management of traumatic anterior glenohumeral instability. J Bone Joint Surg Am 1989; 71A: 506-513.
