Benign Tumors of Bone
Editors: Tornetta, Paul; Einhorn, Thomas A.; Damron, Timothy A.
Title: Oncology and Basic Science, 7th Edition
Copyright ©2008 Lippincott Williams & Wilkins
> Table of Contents > Section I
– Evaluation and Management of Musculoskeletal Oncology Problems > 4
– Treatment Principles > 4.2 – Benign Tumors of Bone
– Evaluation and Management of Musculoskeletal Oncology Problems > 4
– Treatment Principles > 4.2 – Benign Tumors of Bone
4.2
Benign Tumors of Bone
Cynthia M. Kelly
Musculoskeletal tumors are, in general, uncommon
problems, and malignant tumors of bone are considered rare.
Comparatively speaking, benign tumors of bone are more commonly seen by
orthopaedic surgeons and primary care practitioners. Benign tumors of
bone may exhibit various biologic and clinical behaviors that require a
broad spectrum of treatment options. Management of these lesions is
best handled by an orthopaedic surgeon and, in certain instances, by a
fellowship-trained orthopaedic oncologist. Many benign tumors of bone
are incidental findings, noted during radiographic evaluation of an
extremity for an unrelated complaint such as a sports injury or other
trauma. It is important to recognize lesions that are most likely
benign processes that can be observed versus those that need to be
treated surgically. In rare instances benign lesions have the potential
to undergo malignant transformation. It is therefore important for the
physician to understand these variations in biological behavior in
order to care for patients appropriately.
problems, and malignant tumors of bone are considered rare.
Comparatively speaking, benign tumors of bone are more commonly seen by
orthopaedic surgeons and primary care practitioners. Benign tumors of
bone may exhibit various biologic and clinical behaviors that require a
broad spectrum of treatment options. Management of these lesions is
best handled by an orthopaedic surgeon and, in certain instances, by a
fellowship-trained orthopaedic oncologist. Many benign tumors of bone
are incidental findings, noted during radiographic evaluation of an
extremity for an unrelated complaint such as a sports injury or other
trauma. It is important to recognize lesions that are most likely
benign processes that can be observed versus those that need to be
treated surgically. In rare instances benign lesions have the potential
to undergo malignant transformation. It is therefore important for the
physician to understand these variations in biological behavior in
order to care for patients appropriately.
Pathogenesis
Etiology
-
Most bone tumors, either benign or malignant, have no identifiable etiology.
-
Few benign bone lesions are associated with preexisting conditions or have a hereditary or familial pattern.
Pathophysiology
-
Unknown for most benign tumors of bone and is best described for congenital and inherited syndromes (Table 4.2-1)
Classification
-
Classification terms for benign bone
tumors are based on their biological behavior, tissue of origin, or
syndrome names generally associated with polyostotic diseases. -
Based on biologic behavior
-
Stage of the lesion
-
Latent, active, and aggressive (see Box 4.2-1; see also Chapter 1, Evaluation of Bone Tumors)
-
-
Based on tissue of origin
-
World Health Organization classifies tumors into general categories based on the type of neoplastic tissue within the lesion (Table 4.2-2).
-
Other lesions of bone that mimic tumors (Box 4.2-2)
-
-
Syndromes associated with polyostotic disease (Table 4.2-3)
Diagnosis
History and Physical Examination
-
History: including presenting complaint and past medical history
-
Patients with benign bone lesions generally present with one of four scenarios:
-
Painless bone mass
-
Incidental radiographic finding
-
Painful bone lesion
-
Pathologic fracture
-
-
An important early factor in evaluation of a patient with a benign tumor of bone is: “Is the lesion associated with pain?”
-
For those with pain, consideration should be given to the patient’s pain complaints and exacerbating activities.
-
For painless lesions, the history should focus on the means of discovery.
-
Clinical Features
-
Painless bone mass
-
Painless bone masses are usually noted as a bump on the affected bone and may be solitary or multiple.
-
Most common example: osteochondroma
-
-
Incidental radiographic finding
-
A common scenario is that a patient
injures an area, an x-ray is obtained, and an asymptomatic bony lesion
is noted. These incidentally identified lesions generally are benign,
and important radiographic features to be considered in these
situations will be discussed below. -
Most common example: nonossifying fibroma (fibrous cortical defect)
-
-
Painful bone lesion
-
When a patient presents with pain as the
primary complaint and an underlying tumor of bone is identified, a more
thorough evaluation is warranted to rule out an aggressive benign tumor
that is associated with structural compromise of the bone or even a
malignancy. -
Differential diagnosis in this situation may be extensive.
-
-
Pathologic fractureP.55
-
Most children with a pathologic fracture
through an underlying benign tumor of bone can be managed
conservatively initially to allow for fracture healing, and then a
biopsy may be performed in a delayed fashion if appropriate. -
Key question in determining whether there is cause for concern: Was pain present preceding the time of the fracture?
-
Preceding pain: Possible aggressive benign or malignant lesion: may warrant biopsy~hsurgical intervention
-
No preceding pain: Very likely a benign, inactive lesion
-
Common examples: nonossifying fibroma, simple bone cyst
-
Box 4.2-1 Staging of Benign Bone Lesions*
-
Stage 1 (latent)
-
Nonossifying fibroma
-
Enchondroma
-
Unicameral bone cyst
-
Osteochondroma
-
Osteoid osteoma
-
Fibrous dysplasia
-
Eosinophilic granuloma (Langerhans cell histiocytosis)
-
Stage 2 (active)
-
Enchondroma
-
Osteochondroma
-
Osteoid osteoma
-
Osteoblastoma
-
Giant cell tumor
-
Chondromyxoid fibroma
-
Fibrous dysplasia
-
Eosinophilic granuloma (Langerhans cell histiocytosis)
-
Aneurysmal bone cyst
-
Unicameral bone cyst
-
Osteofibrous dysplasia
-
Juxtacortical chondroma
-
Chondroblastoma
-
Stage 3 (aggressive)
-
Giant cell tumor
-
Osteoblastoma
-
Chondroblastoma
-
Aneurysmal bone cyst
*Many types of benign bone lesions may be classified in more than one stage based on their biologic behavior.
|
Table 4.2-1 Benign Bone Lesions With Consistent Genetic Defects And/Or Hereditary Or Familial Inheritance Patterns
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Physical Examination
-
General physical examination
-
Look for signs of multifocal disease
(café-au-lait spots, axillary freckling, multiple bony masses, soft
tissue myxomas, soft tissue hemangiomas, short stature, limb
deformities), generalized lymphadenopathy. -
Examine for possible sites of referred pain.
-
-
Focused examination concentrating on the area of concern
-
Specific findings to assess are the
presence of a visible mass, limb length discrepancy, overlying skin
changes, increased tactile temperature, presence of a soft tissue mass,
tenderness on palpation of the area, compromised joint range of motion,
surrounding muscle atrophy, neurovascular status of the limb and any
lymphadenopathy.
-
Radiographic Evaluation
-
X-ray films are a necessary part of the work-up of any patient with a musculoskeletal complaint.
-
At a minimum, two orthogonal view x-rays of the affected area should be obtained, an AP and a lateral view.
-
Oblique views may also be of assistance.
-
Example: avulsive cortical irregularity (periosteal desmoid), which always occurs at the posteromedial distal femoral metaphysis
-
-
-
Compare to old radiographs, when available, in order to establish natural history.P.56Table 4.2-2 World Health Organization Benign Bone Tumor Categories
Category Examples Cartilage tumors Chondroma (enchondroma, periosteal chondroma, multiple chondromatosis, osteochondroma, chondroblastoma, chondromyxoid fibroma) Osteogenic tumors Osteoma, osteoid osteoma, osteoblastoma Fibrogenic tumors Desmoplastic fibroma Fibrohistiocytic tumors Benign fibrous histiocytoma Giant cell tumors Giant cell tumor, osteoclastoma Vascular tumors Hemangioma Smooth muscle tumors Leiomyoma Lipogenic tumors Lipoma Neural tumors Neurilemoma Miscellaneous lesions Aneurysmal bone cyst, simple
cyst, fibrous dysplasia, osteofibrous dysplasia, Langerhans cell
histiocytosis, Erdheim-Chester disease, chest wall hamartomaJoint lesions Synovial chondromatosis -
In the case of an inactive lesion that is
an incidental radiographic finding, observation and serial x-rays are
often all that is warranted. This regimen affords the opportunity to
observe the natural history of the process.
-
-
Consider proximal x-ray evaluation of an
area that can be associated with a referred pain pattern (e.g., hip
pathology presenting as knee pain, spine pathology presenting as hip
pain). -
Size of the lesion on initial evaluation is generally of limited help in establishing a diagnosis.
-
Systematic review of the x-ray is essential.
-
The soft tissues should be evaluated for mineralization or other significant findings.
-
The bone then should be evaluated with attention to the following (also see Chapter 1):
-
Location of the lesion
-
What the lesion is doing to the bone
-
What the bone is doing in response to the lesion
-
Any other characteristic findings that may suggest a given diagnosis, especially matrix mineralization
-
-
Box 4.2-2 Lesions That Simulate Bone Tumors
|
Specific Radiographic Findings (also see Table 1-6)
-
Dense sclerotic margin around the tumor is a characteristic sign of a benign tumor of bone. (Fig. 4.2-1).
-
Mature periosteal reaction.
If the periosteum has had an opportunity to react to the expansion of
the bone by forming mature bone, a benign process is favored. -
Lack of sclerotic rim suggests more rapid growth of the underlying lesion (Fig. 4.2-2).
-
Soft tissue extension:
Any extension of the lesion into the surrounding soft tissues is an
ominous sign and suggests a rapidly growing process—a malignancy, an
infection, or an aggressive benign process. -
Onion-skinning, sequential periosteal elevation, is also indicative of a rapidly growing process; it can be seen in both benign and malignant tumors.
-
Codman’s triangles are a result of rapid periosteal elevation indicative of a rapidly growing process such as an infection or malignancy.P.57Table 4.2-3 Polyostotic Diseases
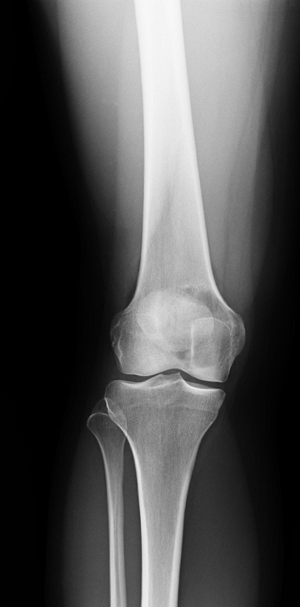
Disease Associated Conditions Polyostotic fibrous dysplasia Endocrine abnormalities in McCune-Albright syndrome or soft tissue myxomas in Mazabraud syndrome Eosinophilic granuloma (Langerhans cell histiocytosis) Hand-Schuller-Christian disease and Letterer-Siwe disease Enchondromatosis (Ollier’s disease) Vascular abnormalities (hemangiomas) in Maffucci syndrome Chronic recurrent multifocal osteomyelitis (CRMO) Multiple nonossifying fibromas Type I peripheral neurofibromatosis or Jaffe-Campanacci syndrome 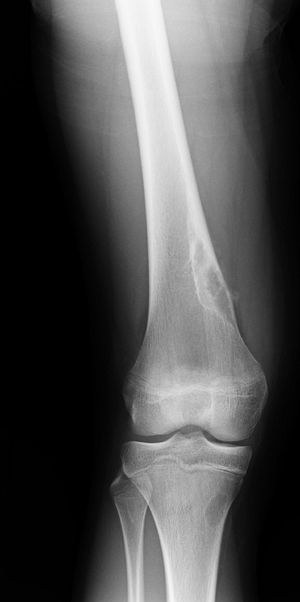 Figure 4.2-1 Benign nonossifiying fibroma of femur. Note sclerotic margins.
Figure 4.2-1 Benign nonossifiying fibroma of femur. Note sclerotic margins.![]() Figure 4.2-2 Lytic epiphyseal and metaphyseal lesion of distal femur without sclerotic margins, consistent with giant cell tumor of bone.
Figure 4.2-2 Lytic epiphyseal and metaphyseal lesion of distal femur without sclerotic margins, consistent with giant cell tumor of bone. -
Location of the lesion within the bone is important in the formulation of the differential diagnosis.
-
Majority of lesions occur within the metaphysis.
-
Neoplasms that arise in the epiphysis
are giant cell tumor, chondroblastoma, and clear cell chondrosarcoma.
In addition, juxta-articular cysts such as those from pigmented
villonodular synovitis and degenerative geodes are often located in the
epiphysis of adult bones. Aneurysmal bone cysts rarely occur in the
epiphysis. Brodie’s abscesses often are located in the epiphysis of
children, and tuberculosis may cause epiphyseal “kissing cysts” in
adults (Fig. 1-14 and Box 1-5). -
Benign-appearing diaphyseal lesions tend to be eosinophilic granuloma, fibrous dysplasia, “inactive” positioned simple bone cysts, and osteomyelitis.
-
P.58
Other Imaging Studies
-
Technetium-99m bone scan may be helpful in identifying other sites of skeletal involvement.
-
Computed tomography (CT) scans
are generally helpful in assessing specific bone lesions such as
osteoid osteoma (to identify the nidus) and enchondroma (to determine
the extent of endosteal scalloping). -
Magnetic resonance imaging (MRI)
is more useful to delineate the extent of any associated soft tissue
masses and the intraosseous extent of the process. It also helps to
distinguish between tissues of similar densities but different
histologies, unlike CT scanning, which primarily evaluates tissue
densities. In certain situations, specific findings may be helpful:-
Fluid–fluid levels are associated with aneurysmal bone cyst.
-
Perilesional edema is often seen with
osteomyelitis, osteoid osteoma, eosinophilic granuloma (Langerhans cell
histiocytosis), and chondroblastoma and may be extensive. -
Peripheral enhancement alone (without
central enhancement) is seen with simple cysts, degenerative geodes, or
intraosseous synovial cysts. -
Fat, especially surrounding a central area of ossification (the cockade sign), is diagnostic of intraosseous lipoma.
-
Treatment
Biopsy (also see Chapter 3, Biopsy of Musculoskeletal Tumors)
-
A biopsy should be the last step in the evaluation of a benign bone tumor and is to be performed only after careful planning.
-
There is always the remote possibility
that what is considered to be a benign tumor may ultimately be a
malignancy, and therefore a properly performed biopsy is essential for
future limb salvage options. -
It is recommended that the surgeon who
would perform the ultimate limb-sparing surgical procedure also be the
one to perform the biopsy so that the biopsy track may be placed in the
appropriate location for a definitive orthopaedic surgery.-
If a radiologist is to perform the biopsy, guidance for needle placement is recommended from the orthopedist.
-
-
Biopsies are performed to confirm the diagnosis as suspected by the orthopaedic surgeon.
-
Adequate tissue from representative areas of the tumor is necessary for the pathologist to render the appropriate diagnosis.
-
A multidisciplinary approach to the
biopsy is also helpful for the pathologist, who then has an
understanding of the physical examination and x-ray findings with which
to correlate the histologic appearance of the lesion.
-
Technique
-
There are several ways in which to biopsy a bone tumor: skinny needle, core needle, and open incisional or excisional biopsy
-
Benign bone lesions usually do not afford
easy access for needle biopsy, as the surrounding bone is usually
intact and patients are often young, so the difficulty of entering the
bone is compounded by the inability of the patient to tolerate any
procedure performed under a local anesthetic. -
Skinny needles may be appropriate tools for inaccessible lesions (especially the spine and pelvis).
-
Core-needle biopsy may be appropriate for a bone lesion with soft tissue extension in an adolescent or adult patient.
-
Otherwise, open incisional bone biopsy is the workhorse tool for benign bone lesions.
-
Excisional biopsies are performed when
the entire lesion is to be removed and are practical when limb function
will not be impaired over an incisional biopsy (e.g., osteochondroma).
-
-
“Culture all tumors and biopsy all
infections.” Osteomyelitis is the great imposter, often mimicking the
presentation and appearance of bone tumors.
Surgical Management
General Principles
-
Treatment options for benign tumors of bone are matched to the aggressiveness of the lesion.
-
Specific treatment considerations may apply to individual tumor types and the stage of the lesion (see Box 4.2-1).
-
-
Other variables affecting treatment choices
-
Clinical picture, radiographic evaluation, physician’s judgment and experience, patient and/or family goals/desires
-
Stage 1 Lesions
-
Treatment options for Stage 1 lesions
-
Observation with serial radiographs
-
Curettage or excision
-
Curettage and grafting with autograft, allograft, or bone graft substitute products
-
-
Stage 1 lesions identified as an incidental finding: Observation is appropriate in most cases.
-
Serial radiographic and clinical evaluation
-
The potential for these lesions to remain
stable or resolve on their own is greater than for a lesion associated
with symptoms or a more active radiographic appearance.
-
-
Stage 1 lesions in high-stress areas
(proximal femur) and those that are symptomatic or cause structural
compromise to the bone should be considered for surgical intervention.-
Generally, curettage and grafting with or without stabilization is elected in these cases.
-
Need for stabilization is determined by
the presence or absence of fracture, anatomic site, risk of subsequent
fracture, activity level, and ability to comply with postoperative
restrictions.
-
P.59
Stage 2 Lesions
-
Concerns: Stage 2 lesions often pose a
greater risk of pathologic fracture based on the location and size of
the lesion as well as activity and age of the patient. -
Standard of care: Generally treated with
an intralesional procedure (with the exception of juxtacortical
chondroma), with the defect filled with bone graft, bone graft
substitute materials, or cementation -
Specific exceptions
-
Juxtacortical chondroma: Recurrence rate is less following en bloc excision.
-
Osteoid osteoma: Radiofrequency ablation
has generally supplanted curettage: medical management with
nonsteroidal anti-inflammatory agents is also an option.
-
Stage 3 Lesions
-
Concerns: Stage 3 lesions continue their
growth unless treated. Their biological behavior is more aggressive and
they therefore have a greater risk of pathologic fracture, local
recurrence, and in some cases (giant cell tumors and chondroblastomas)
a risk of metastatic disease. -
Standard of care: Generally treated with
an extended curettage with or without adjuvant agents (phenol, laser,
liquid nitrogen) and with or without stabilization-
Defect may be filled with bone graft or bone graft substitute products, bone cement, or structural grafts.
-
Indications for stabilization same as for stage 1 and 2 lesions (above)
-
-
Exceptions
-
Expendable bone or multiply recurrent tumors with extensive bone destruction: wide en bloc excision
-
In the case of wide excision,
reconstruction commences in a similar fashion to that following removal
of a sarcoma, including a megaprosthesis or allograft prosthetic
composite reconstruction (see Chapter 4.3, Malignant Bone Lesions).
-
Reconstruction
-
Removal of large, active, or aggressive
lesions may result in a large structural defect that warrants
additional consideration for reconstructive options.-
None of the commercially available bone
graft substitute materials are approved by the Food and Drug
Administration for reconstruction of structural defects in bone. -
A combination of an osteoinductive agent,
an osteoconductive product, and a source of osteoprogenitor cells (bone
marrow aspirate) recreates the elements contained in an autograft. -
Autograft bone may be in limited supply
for treatment of large defects, and either allograft or bone graft
substitute products are often used as supplement or alone in these
situations. -
Methylmethacrylate bone cement has been
useful in reconstruction of major structural defects in the bone and
also in the subchondral and periarticular areas, particularly in the
treatment of giant cell tumors of the bone.-
Advantages
-
Immediate structural stability
-
Exothermic reaction may kill cells at periphery.
-
Provides clear radiographic interface with surrounding normal bone, facilitating recognition of local recurrence
-
-
-
Results and Outcomes
-
Results of treatment for benign tumors of
bone are generally favorable, barring any complications of a surgical
procedure or pathologic fracture through a lesion. -
Local recurrence rates vary according to the underlying diagnosis.
-
Many assessment tools are available for
functional outcome, including the Musculoskeletal Tumor Society Rating
Scale, The Hospital for Special Surgery Knee and Harris Hip scores, as
well as the Health Satisfaction Questionnaire (SF-36).
Postoperative Management and Follow-Up
-
Variables important in determining postoperative management (including activity, weight bearing)
-
Surgical procedure performed, upper
versus lower extremity, specific location, size of defect, type of
reconstruction, presence or absence of stabilization device, underlying
disease process, medical comorbidities, and compliance
-
-
Stage 3 (aggressive) lesions merit a
closer follow-up, much like a sarcoma, particularly for the first 2
years postoperatively, to evaluate for local recurrence and metastatic
foci of disease. -
Benign bone lesions with a high risk of local recurrence
-
Giant cell tumor of bone: near 50%
recurrence if treated with simple curettage and bone grafting and as
low as 3% to 17% when adjuvant agents, extended curettage, and
cementation are used -
Aneurysmal bone cysts: Risk of recurrence
for secondary aneurysmal bone cyst depends on the accompanying lesion.
Primary aneurysmal bone cysts have a local recurrence rate as high as
32%. -
Chondroblastomas: 5% local recurrence risk
-
-
Benign bone tumors with risk of pulmonary metastases: giant cell tumor, chondroblastoma
P.60
Suggested Reading
Aboulafia AJ, Temple HT, Scully SP. Surgical treatment of benign bone tumors. AAOS Instr Course Lect 2002;51:441–450.
American Academy of Orthopaedic Surgeons Instructional Lecture Series, Chapters 45–48. Volume 51, 2002.
Enneking
WF, Dunham W, Gebhardt MC, et al. A system for the functional
evaluation of reconstructive procedures after surgical treatment of
tumors of the musculoskeletal system. Clin Orthop 1993;(286):241–246.
WF, Dunham W, Gebhardt MC, et al. A system for the functional
evaluation of reconstructive procedures after surgical treatment of
tumors of the musculoskeletal system. Clin Orthop 1993;(286):241–246.
Gitelis S, Wilkins RM, Conrad E. Benign bone tumors. J Bone Joint Surg [Am] 1995;77:1756–1782.
Harris
WH. Traumatic arthritis of the hip after dislocation and acetabular
fractures: treatment by mold arthroplasty. An end-result study using a
new method of result evaluation. J Bone Joint Surg [Am] 1969;51:737–755.
WH. Traumatic arthritis of the hip after dislocation and acetabular
fractures: treatment by mold arthroplasty. An end-result study using a
new method of result evaluation. J Bone Joint Surg [Am] 1969;51:737–755.
Insall JN, Dorr LD, Scott RD, et al. Rationale of the Knee Society Clinical Rating System. Clin Orthop 1989;(248):13–14.
Lewis M. Musculoskeletal Oncology: A Multidisciplinary Approach. Philadelphia: WB Saunders, 1992:1–73.
Menendez LR. Musculoskeletal tumors. Orthopaedic Knowledge Update. Rosemont, IL: AAOS, 2002:113–117.
Mirra JM. Bone Tumors: Clinical, Radiographic and Pathologic Correlations. Philadelphia: Lea & Febiger, 1989.
Schajowicz F, Ackerman LV, Sissons HA. Histologic typing of bone tumors. In International Histologic Classification of Tumors, vol. 6. Geneva: World Health Organization, 1972.
Ware JE Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30(6):473–483.
Wetzel LH, Levine E, Murphey MD. A comparison of MR imaging and CT in the evaluation of musculoskeletal masses. Radiographics 1987;7:851–874.