Principles of Orthopaedic Pharmacology
pharmacologic intervention. This chapter will summarize the basic
science principles behind the most commonly used drugs in orthopaedic
practice.
causing a specific infection matched with the spectrum of the
antibacterial is essential to maximize the therapeutic effect while
minimizing resistance. Therefore, in general, antibac-terials are most
effective when they are directed against a single pathogen for a short
period of time and potentially harmful when directed against multiple
pathogens for a long period of time.
-
Inhibition of cell wall synthesis: penicillins, cephalospo-rins, imipenem, vancomycin
-
Penicillins (amoxicillin, piperacillin) are inactivated by β-lactamase and are thus ineffective against Staphylococcus aureus.
-
Semisynthetic penicillins (nafcillin, oxacillin, dicloxacillin) are active against β-lactamase—producing S. aureus.
-
Cephalosporins: cephalothin, cephalexin, cefazolin, cephamandole, ceftriaxone
-
Semisynthetic penicillins and
cephalosporins are more effective than vancomycin against infections
caused by methicillin-sensitive strains of S. aureus.
-
-
Inhibition of protein synthesis: doxycycline, azithromycin, erythromycin, clindamycin, gentamicin
-
Clindamycin is active against anaerobes and most gram-positive cocci except enterococci, but it selects for Clostridium difficile.
-
Aminoglycosides are used against gram-negative bacilli; they are associated with renal and auditory toxicity.
-
-
Interference with DNA metabolism: quinolones, metro-nidazole
-
Quinolones are primarily active against gram-negative bacteria.
-
Metronidazole is effective against all
anaerobes and many parasites by exploiting their inability to
metabolize oxygen free radicals.
-
-
Antimetabolites: trimethoprim, sulfonamides
-
Mimic essential nutrients to block replication
-
Combination of trimethoprim and the sulfonamide sulfamethoxazole blocks folic acid synthesis with a wide spectrum of activity.
-
-
Surgical prophylaxis
-
In general, antibacterial prophylaxis is indicated in
-
procedures with a high inherent infection
rate or when the prevalence of infection is low but such an infection
would have catastrophic results. -
For most elective orthopaedic procedures, the potentially offending pathogens are actually relatively limited: S. aureus, Staphylococcus epidermidis, aerobic streptococci, and anaerobes.
-
For maximum benefit, the antimicrobial should be administered within 1 hour preceding the incision.
P.388 -
-
Open fractures
-
Antimicrobials should be given as soon as possible.
-
Risk of infection in open fractures depends on the severity of soft tissue injury.
-
S. aureus and gram-negative bacilli are the most common pathogens causing infections after open fractures.
-
Certain environmental exposures require specific coverage:
-
Farm-related exposures: penicillin for Clostridium perfringens
-
Soil: clindamycin or metronidazole for anaerobic microorganisms
-
Fresh water: third-generation cephalosporin or quinolone for Pseudomonas species
-
-
-
Osteomyelitis
-
β-lactam agents, aminoglycosides, and doxycycline are able to achieve serum-level concentrations in acutely infected bone.
-
Clindamycin is able to treat organisms such as S. aureus that have the ability to evade antimicrobials following macrophage ingestion.
-
diagnosed each year in the United States; of these, 200,000 to 600,000
(10% to 30%) propagate to pulmonary emboli (PE). Many pharmacologic
agents used for DVT prophylaxis and treatment intervene at steps along
the clotting cascade with the goal of preventing fibrin formation, the
end product of the cascade (Fig. 18-1).
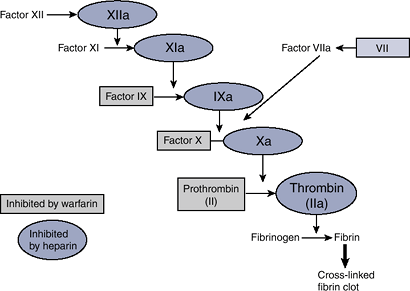 |
|
Figure 18-1 The coagulation cascade.
|
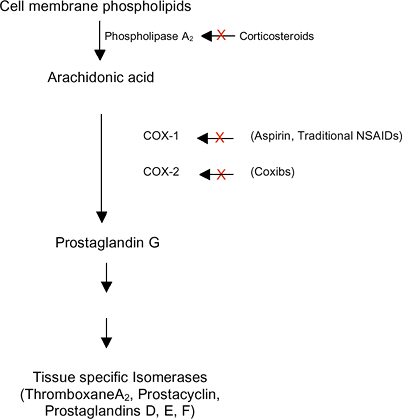 |
|
Figure 18-2 Effectors of inflammation on the arachidonic acid metabolic pathway.
|
-
Aspirin
-
Irreversibly binds cyclooxygenase (COX-1 and COX-2), thereby blocking arachidonic acid from forming prostaglandins (Fig. 18-2)
-
In platelets, aspirin suppresses thromboxane A2 production; thromboxane A2 triggers platelet aggregation via phospholipase C.
-
Clinically, aspirin has little if any role in prophylaxis of venous thromboembolism.
-
-
Warfarin
-
Warfarin is a vitamin K antagonist of vitamin K oxidoreductase (VKOR), the enzyme responsible for the activation of vitamin K.
-
Coagulation factors II, VII, IX, and X require vitamin K for activation.
-
In the absence of active vitamin K, inactive clotting factors accumulate, leading ultimately to decreased fibrin formation.
-
The anticoagulant effect is measured by the prothrombin time (PT) and the international normalized ratio (INR) target of 2 to 3.
-
-
Heparin
-
Heparin binds antithrombin (AT), exposing AT’s active site and increasing the affinity of AT for active clotting factors.
-
Clinically, heparin has unpredictable pharmacokinetics.
-
Heparin-induced thrombocytopenia (HIT) is
a condition in which patients become thrombocytopenic and prothrombotic
simultaneously. -
Fixed low-dose subcutaneous heparin (i.e., 5,000 U) has limited efficacy for venous thromboembolism.
-
Effects of heparin can be reversed with intravenous protamine.
P.389 -
-
Low-molecular-weight heparins (LMWHs)
-
Exert effect by the same mechanism that heparin does
-
Pharmacokinetic profile is very
predictable and therefore monitoring is largely unnecessary except in
renal insufficiency and weight extremes. -
Appears to be as efficacious as warfarin in orthopaedic patients
-
Dalteparin, enoxaparin, tinzaparin, and nadroparin are approved by the U.S. Food and Drug Administration (FDA).
-
No antidote exists, but protamine can neutralize up to 60% of effect.
-
-
Factor Xa inhibitor (Fondaparinux)
-
Chemically unrelated to heparin and LMWH, but has similar mechanism
-
No known antidote
-
some inflammatory component. Pharmacological agents to manage the
symptoms of inflammatory conditions are the most frequently prescribed
medicines in the United States.
prostanoids, effector molecules that regulate pain and inflammation.
These prostanoids are produced by the metabolism of arachidonic acid by
the enzyme cyclooxygenase (COX (Fig. 18-2)).
Two isoforms of the enzyme have been described: COX-1, a constitutively
expressed regulator of many physiologic functions, and COX-2, an
inducible form activated during pro-inflammatory conditions.
-
Traditional NSAIDs
-
Possess great variability in their potency and duration of action secondary to varying COX-1 or -2 specificity
-
Clinically, the efficacy of NSAIDs for numerous acute and chronic conditions is well established.
-
Gastrointestinal side effects, namely gastroduodenal ulcers, remain a significant problem.
-
-
COX-2 inhibitors (coxibs)
-
Have the potential advantage of
preferentially suppressing COX-2, which is upregulated during
inflammation, without disturbing the normal homeo-static functions of
COX-1 -
Efficacy of coxibs is comparable to that of traditional NSAIDs.
-
Gastrointestinal effects are lower with coxibs use compared to traditional NSAIDs.
-
Unlike traditional NSAIDs, COX-2 inhibitors do not have a significant effect on the endogenous production of thromboxane A2,
potentially triggering a significant increase in the risk for
thrombotic cardiovascular events. These findings led to the withdrawal
of valdecoxib and rofecoxib from the U.S. market and issuance of a
“black box” warning for celecoxib.Table 18-1 Commonly Used Nonsteroidal Anti-Inflammatory DrugsGeneric Name Trade Name Salicylates Aspirin — Diflunisal Dolobid Salsalate Disalcid Traditional NSAIDs Arylalkanoic acids Diclofenac Voltaren Indomethacin Indomethacin Sulindac Clinoril 2-Arylpropionic acids (profens) Flurbiprofen Ansaid Ibuprofen Motrin Ketoprofen Orudis Ketorolac Toradol Naproxen Naprosyn Oxaprozin Daypro N-Arylanthranilic acids (fenamic acids) Meclofenamate Meclomen Oxicams Piroxicam
MeloxicamFeldene
MobicAcetic acids Etodolac
TolmetinLodine
TolectinCOX-2 inhibitors (coxibs) Celecoxib Celebrex
-
-
Salicylate
-
The mechanism of action of salicylates is summarized by the description of aspirin in the section on anticoagulation.
-
steroid hormones produced by the adrenal glands. They encompass both
glucocorticoids (regulators of inflammation and metabolism) and
mineralocorticoids (mediators of electrolytes).
-
Corticosteroids influence all types of inflammatory events by a complex signaling pathway that has been fairly well elucidated:
-
The mediating receptor belongs to the
family of cyto-solic steroid—hormone receptors. Once bound, the
steroid-receptor complex migrates to the nucleus, -
where it binds the promotor region of target genes called glucocorticoid-responsive elements (GRE).Table 18-2 Commonly Used Injectable Corticosteroids
Solubility Generic Name Example Trade Name Most soluble Betamethasone sodium phosphate Celestone Soluble Dexamethasone sodium phosphate
Prednisolone sodium phosphateDecadron
HydeltrasolSlightly soluble Prednisolone tebutate
Triamcinolone diacetate
Methylprednisolone acetateHydeltra-T.B.A.
Aristospan Forte
Depo-MedrolRelatively insoluble Dexamethasone acetate
Hydrocortisone acetate
Hydrocortisone
Prednisolone acetate
Triamcinolone acetonide
Triamcinolone hexacetonideDecadron-LA
HydrocortisonePredalone
Kenalog
AristospanCombination Betamethasone sodium phosphate-betamethasone acetate Celestone Soluspan -
Active GREs solicit other proteins to
structurally modify the chromatin, thereby leading to altered
transcription of target genes. An example is the gene that codes for
annexin I (also called lipocortin-1).-
Annexin I is an anti-inflammatory protein that binds to cell membranes and physically interacts with phospholipase A2, blocking its action on ar-achidonic acid and the subsequent production of inflammatory mediating prostaglandins (see Fig. 18-2).
-
P.390 -
-
Commonly used synthetic corticosteroids are listed in Table 18-2. Firm guidelines regarding choice and dose for intra-articular, intrabursal, and intra-tendon sheath injection are lacking.
-
Most conditions are treated with a combination of short-acting (water-soluble) and long-acting (water-insoluble) preparations.
-
A minimum of 4 weeks between intra-articular injections is usually recommended.
-
Associated adverse effects include
corticosteroid ar-thropathy, ligament and tendon ruptures, infection,
adrenal suppression, skin atrophy, and hyperpig-mentation.
-
skeletal homeostasis that lead to impairment of normal bone formation,
mineralization, and remodeling. Examples of such conditions include
osteoporosis, Paget’s disease, rickets, and osteomalacia. A number of
antiresorptive agents are available to treat the orthopaedic
manifestations of these diseases.
-
Bisphosphonates
-
Synthetic analogs of inorganic pyrophosphate in which the P-O-P bond has been replaced with a non-hydrolyzable P-C-P bond.
-
Bone is preferentially targeted because
the diphos-phate configuration provides a three-dimensional structure
for binding divalent ions such as Ca2+. -
The drug binds the mineral phase of bone
exposed during bone resorption and is ingested by the endocytic
activity of the osteoclast. -
The mechanism of action by which the
bisphosphonate inactivates the osteoclast is divided into two
pharmacologic groups: nitrogen-containing and non—nitrogen-containing.-
Non—nitrogen-containing bisphosphonates:
metabolized to nonhydrolyzable analogs of ATP; osteoclastic
intracellular accumulation of these cytotoxic ATP analogs is thought to
induce apoptosis. -
Nitrogen-containing bisphosphonates:
exert their effectiveness via the mevalonate pathway, which is
responsible for the production of the cholesterol precursors farnesyl
diphosphate and geranylgeranyl diphosphate (GGPP). Farnesyl diphosphate
and GGPP are necessary for the production of proteins that are
synthesized by a process called protein prenylation. One such protein,
guanosine triphosphatase, plays a regulatory role in osteoclast
morphology, including membrane ruffling and protein trafficking.
-
-
FDA-approved bisphosphonates: etidronate,
tiludro-nate, alendronate, pamidronate, risedronate, iban-dronate, and
zoledronic acid (Table 18-3)
-
-
Calcitonin
-
Single-chain 32-amino-acid polypeptide hormone synthesized by the parafollicular cells of the thyroid gland.
-
Inhibits bone resorption by inducing changes in the osteoclasts’ cytoskeleton by disrupting actin rings,
-
the structures responsible for the formation of bone resorption pits, or Howship’s lacunae.
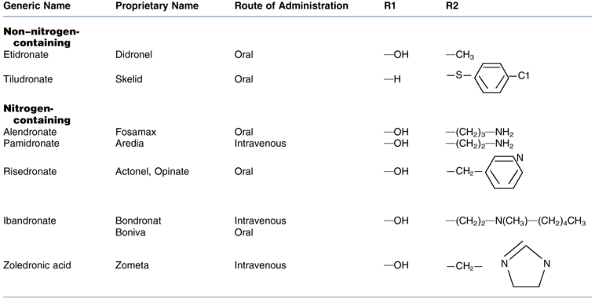 Table 18-3 Fda-Approved Bisphosphonates
Table 18-3 Fda-Approved Bisphosphonates -
Salmon calcitonin (calcitonin extracted
from salmon) is more potent than human calcitonin, though the two forms
differ by just one amino acid. -
Immunogenicity can develop with long-term salmon calcitonin use.
-
Both injectable and intranasal
preparations are FDA approved for the treatment of symptoms associated
with Paget’s disease and osteoporosis.
P.391 -
-
Parathyroid hormone (PTH)
-
Affects both osteoblastic bone formation and osteoclastic bone resorption in a coupled fashion
-
Duration and dosing control the net
metabolic effect: daily PTH injections increase bone mass, but
continuous infusions lead to bone resorption. -
Biological activity of PTH is found
within amino acids 1 to 34 at the N-terminal and is mediated by second
messenger cAMP following binding to G protein—coupled receptors on
osteoblasts. -
Bound PTH stimulates osteoblasts to produce RANKL and IL-6, both of which lead to osteoclast proliferation and maturation.
-
Clinically indicated for patients with osteoporosis that has failed to respond to other therapies.
-
Contraindicated in patients at increased
risk for osteogenic sarcoma (e.g., skeletally immature, Paget’s
disease, previous irradiation).
-
actual skeletal tissue as opposed to scar tissue. This repair process
very closely imitates embryonic bone development. Interest in the
enhancement of fracture repair has lead to an increased understanding
of the various growth factors involved in bone regeneration, most
notably the bone morphogenetic proteins (BMPs).
-
BMPs
-
Growth factors capable of inducing undifferentiated mesenchymal cells into osteoblasts leading to new bone formation.
-
16 isoforms have been sequenced: BMP-1 to BMP-16.
-
All BMPs (with the exception of BMP-1)
belong to the transforming growth factor-β (TGF-β) super-family; the
TGF-β family of proteins is intricately involved in the regulation of
cellular growth and differentiation. -
BMPs act by binding serine/threonine
kinase receptors, leading to activation of the SMAD transcription
factor that in turn activates or suppresses a target gene. -
Currently two recombinant BMPs are FDAapproved: rh-BMP-7 (also called OP1) and rh-BMP-2.
-
attract and maintain water molecules. Proteoglycans are composed of
glycosaminoglycans (keratan sulfate, dermatan sulfate, chondroitin
sulfate) bound to a central core protein. In degenerative joint
conditions, the relative proteoglycan production by chondrocytes is
decreased. Theoretically, replacing
proteoglycans
might retard the degenerative process, and to that end a number of
molecules have been investigated for this purpose. The published
results from clinical trials have failed to show convincing efficacy,
possibly due to limited bioavailability of the active moieties.
-
Glucosamine
-
Glucosamine (C6H14NO5) is a naturally occurring amino sugar derived from chitin (extracted from shells of crabs, lobsters, and shrimp).
-
Glucosamine serves as a precursor for
glycoproteins, including glycosaminoglycans. Specifically, it composes
half of the disaccharide units found in keratan sulfate and hyaluronic
acid. -
In an animal model, exogenous glucosamine has been shown to rebuild cartilage by enhancing cartilage proteoglycan synthesis.
-
An anti-inflammatory contribution has also been described.
-
Not FDA approved for the treatment of osteoarthritis, but rather is labeled as a dietary supplement
-
Clinically, glucosamine seems to improve
pain and function scores in patients with osteoarthritis, though the
data are quite variable. Its role as a disease-modifying agent has yet
to be substantiated.
-
-
Chondroitin sulfate
-
Chondroitin sulfate is the major component of ag-grecan, the large aggregating type of proteoglycan.
-
Derived from shark or beef cartilage or bovine trachea
-
Often supplied in combination with
glucosamine * Addition of chondroitin sulfate to cultured human
chondrocytes demonstrates increased proteoglycan production and
decreased collagen breakdown. -
Mechanism of action remains
controversial, as the bioavailability of oral chondroitin sulfate has
not been adequately demonstrated. -
Clinically, most studies support its efficacy in relieving the symptoms associated with osteoarthritis.
-
-
Hyaluronic acid (HA)
-
HA is a stabilizing polysaccharide
secreted by type B synovial cells. It is composed of repeating
disaccharide units of acetylglucosamine and glucuronic acid and is
capable of binding many aggrecan molecules. -
Normal concentration of HA in a healthy
knee is 2 to 4 mg/mL. Arthritic knees produce about half of the normal
concentration of HA, with decreased molecular size. -
Exact mechanism of action of HA is
unknown. Anti-inflammatory, direct analgesic, and physical alterations
of synovial fluid have all been described. -
Currently two preparations are FDA approved: Hyalgan and Synvisc.
-
Extracted from rooster combs
-
W, Pineo G, Heit J, et al. Prevention of venous thromboembolism: The
Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126;338S-400S.
K, Arden N, Doherty M, et al. EULAE Recommendations 2003: an
evidenced-based approach to the management of knee osteoarthritis:
Report of a Task Force of the Standing Committee for International
Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003;62:1145–1155.
