Evaluation of Bone Tumors
– Evaluation and Management of Musculoskeletal Oncology Problems > 1
– Evaluation of Bone Tumors
and arriving at a specific diagnosis can elude even the most
experienced orthopaedic oncologist. However, by using a systematic
approach to the evaluation of these tumors, including consideration of
the pathogenesis, often a narrow enough differential diagnosis may be
arrived at by clinical and radiological means to allow specific
treatment (often observation) or to guide the subsequent evaluation and
care. This chapter discusses pathogenesis and an approach to evaluation
of bone tumors.
-
The vast majority of bone tumors arise de
novo with no identifiable predisposing precursor lesion or associated
agent. However, well-defined clinical precursor lesions have been
established (Box 1-1). -
Established etiologic agents for osteosarcoma are given in Box 1-2.
-
Implicated but unproven causes:
-
Chromium, nickel, cobalt, aluminum, titanium, methyl-methacrylate, polyethylene
-
Trauma
-
-
Incidence of bone sarcomas
-
0.2% of all cancers
-
0.8 new cases per 100,000 population per year in North America and Europe
-
-
Most common bone sarcomas
-
#1: Osteosarcoma
-
#2: Chondrosarcoma
-
#3: Ewing sarcoma
-
-
Age distribution is bimodal
-
First peak: Second decade of life
-
Osteosarcoma predominates at this age
-
Ewing sarcoma #2
-
-
Second peak: >mt60 years old
-
Chondrosarcoma predominates
-
Secondary osteosarcomas #2
-
Paget’s osteosarcoma
-
Postradiation osteosarcoma
-
-
-
-
Unknown for most bone tumors
-
Best described for congenital and inherited syndromes (Table 1-1)
of cell or tissue involved. Hence, bone tumors are divided into
osseous, cartilaginous, cystic, myogenic, lipogenic, epithelial,
neural, notochordal, fibrous, fibrohistiocytic, giant cell, round cell,
and vascular categories as well as those of undefined neoplastic
nature. The organization of the subsequent chapters follows this
classification scheme.
.
history, physical examination, and radiological evaluation is to at
least classify the lesion into one of three categories of biological
behavior: latent, active, or aggressive (Table 1-2).
The last category includes both aggressive benign lesions and
malignancies. In some cases, a specific diagnosis may be attainable,
but if not, this classification provides some guide to the need for
diagnostic evaluation and treatment.
one of four clinical scenarios: (1) incidentally noted bone lesions,
(2) painless bony masses, (3) painful bone lesions, and (4) pathologic
fractures. The differential diagnosis and approach are guided by which
of these scenarios applies. Hence, the clinical features are divided
into those four categories.
occurs commonly in a number of ways, such as when a minor injury leads
to an x-ray that reveals a bone lesion, shoulder pain from impingement
leads to discovery of a proximal humeral bone lesion, or a bone scan
done for an entirely different purpose lights up unexpectedly at the
site of a previously unrecognized bone lesion not associated with the
original problem.
-
Common bone lesions discovered incidentally
-
Adults: enchondroma
-
Pediatrics: nonossifying fibroma
-
-
Injury leading to discovery
-
Did pain precede the injury? Pain
preceding the injury may indicate an active or aggressive lesion rather
than the typical latent lesion discovered incidentally. -
Is pain resolving since injury? Complete
resolution of the injury-related pain supports the incidental nature of
the bone lesion.
-
-
Pain leading to discovery. The key is to
determine whether the pain is more likely to be from a common cause of
pain at the affected anatomic site or from the bone lesion itself.-
Shoulder: Elicit exacerbating features such as overhead activity as an indicator of impingement. Consider subacromial injection.
-
Knee: Elicit exacerbating features such
as going up and down stairs and sitting for long periods with knees
flexed as indications of patellar-femoral discomfort. Consider
intra-articular injection.
-
-
Bone scan leading to discovery
-
Has there ever been pain or local
tenderness at the site of the bone lesion? Some patients may have had a
mild aching pain at the site of a low-grade malignancy that they never
sought medical attention for in the past.
-
-
Bone tenderness (tumor) versus joint line tenderness (intra-articular pathology) is an important distinguishing feature.
-
Signs of the possible joint problem causing pain (if any) should be elicited.
-
Blood work: No role unless infection
suspected (obtain complete blood count >obCBC>cb with
differential, erythrocyte sedimentation rate >obESR>cb,
C-reactive protein >obCRP>cb) -
If suspected cartilage neoplasm, consider computed tomography (CT) or magnetic resonance imaging (MRI)
-
CT scan: Delineates endosteal scalloping, periosteal reaction best
P.5Table 1.1 Congenital And Inherited Syndromes Predisposing to Bone SarcomasSyndrome Inheritance Pattern Typical Tumors/Findings Malignancy Implicated Genes Pathophysiology Ollier’s disease and Maffucci syndrome Mostly sporadic
Some families suggest ADMultiple enchondromas (hemangiomas in Maffucci syndrome) Chondro-sarcoma in Ollier’s disease; multiple malignancies in Maffucci syndrome Unknown Unknown McCune-Albright syndrome (MAS), polyostotic fibrous dysplasia, monostotic fibrous dysplasia Sporadic Multiple lesions of fibrous dysplasia (endocrine abnormalities and coast of Maine pigmented skin lesions with MAS) GNAS1 (guanine nucleotide-binding protein, α-stimulating activity polypeptide 1) Extent of disease dependent upon how large of a cell mass is affected by somatic mutation during embryogenesis Multiple osteochondromas 50% sporadic
50% ADOsteochondromas Chondrosarcoma EXT1, EXT2 (exostosin) Mutant exostosin glycoproteins alter heparin sulfate proteoglycans, affecting FGF and Ihh signaling Retinoblastoma syndrome 60% sporadic
40% ADRetinoblastoma Osteosarcoma
Other cancersRB1 Mutant RB1 tumor suppressor gene allows tumor development Rothmund-Thomson syndrome AR Poikiloderma
Short stature
Sparseness of eyebrows/lashes
Juvenile cataracts
Sunlight sensitivity
Hypogonadism
Defective dentition
Nail problemsOsteosarcoma
Cutaneous epitheliomas
Gastric adenocarcinomas
FibrosarcomasRECQL4 Mutant helicase gene Bloom syndrome Telangiectatic erythema resembling SLE
Dwarfism
Thin, triangular faceOsteosarcoma
Other cancersBME Mutant RECQ helicase gene Li-Fraumeni syndrome <45 with sarcoma and
first-degree relative <45 with any cancer and another first- or
second-degree relative in same lineage with any cancer at any ageOsteosarcoma
Soft tissue sarcoma
Breast cancer
Brain tumors
Acute leukemia
Adrenal cortical cancers
Gonadal giant cell tumorsTP53 gene (71%) Mutant p53 tumor suppressor gene P.6 -
-
MRI scan: Helps differentiate features of hyaline cartilage
-
Lobular organization pattern, bright with T2 weighting, dark with T1 weighting
-
-
If fibrous dysplasia or enchondroma considered, consider total body bone scan to establish solitary or polyostotic process
-
Enchondroma versus Ollier’s disease/enchondromatosis
-
Monostotic versus polyostotic fibrous dysplasia or McCune-Albright syndrome
-
-
If presumptive diagnosis of latent lesion
is established on plain radiographs, key is serial clinical and
radiographic follow-up.-
Common serial follow-up plan: 3 months, 6 months, 1 year, and 2 years after discovery
-
If lesion behaves more aggressively than expected, biopsy and appropriate surgical treatment may be necessary.
-
In most cases, the lesion will remain
unchanged (e.g., enchondroma) or go on to heal (e.g., nonossifying
fibroma), and after 2 years of follow-up, most asymptomatic lesions may
be considered stable.
-
-
Biopsy is usually unnecessary.
-
Osteochondroma (most common by far)
-
Low-grade malignancies with associated soft tissue mass
-
Parosteal osteosarcoma
-
Periosteal chondrosarcoma
-
Secondary chondrosarcoma arising in osteochondroma
-
-
Age
-
Pediatric patient: Osteochondroma grows during skeletal immaturity
-
Adult patients: Osteochondromas should not grow once growth has ceased. Consider other diagnoses.
-
-
Pain
-
Osteochondroma typically painless unless pressure on other structures
-
Pain may be associated with low-grade malignancies.
-
-
Family history of osteochondromatosis
-
Half of cases are spontaneous mutations,
so many patients will have negative family history despite
autosomal-dominant (AD) mode of transmission.
-
-
Bone versus soft tissue origin
-
Soft tissue masses close to bone may mimic surface bone lesions (myositis ossificans).
-
-
Signs of multiple osteochondromatosis
-
Other bony masses
-
Short stature
-
-
Findings that might warrant surgical excision of osteochondroma
-
Local tenderness, limited motion, nerve compression
-
-
Blood work: No role unless infection suspected (obtain CBC with differential, ESR, CRP)
-
Total body bone scan: establish monostotic versus polyostotic processP.7
-
Solitary osteochondroma versus osteochondromatosis
-
-
If distinction between benign and malignant lesions necessary, consider CT scan.
-
Myositis ossificans shows peripherally mature mineralization.
-
Osteochondroma shows continuity of cortical and medullary bone from stalk to adjacent bone.
-
Parosteal osteosarcoma gives a “stuck-on”
appearance, often with a very thin radiolucency between the lesion and
the underlying bone.
-
-
If osteochondroma or parosteal osteosarcoma suspected, MRI scan should be obtained.
-
For osteochondroma: assess thickness of cartilage cap, especially in adults, to differentiate from peripheral chondrosarcoma.
-
Cap thickness >mt2 cm is consistent with a chondrosarcoma.
-
Cap thickness 1 to 2 cm in adults is concerning for chondrosarcoma.
-
-
For parosteal osteosarcoma, MRI determines both soft tissue extent and involvement of underlying medullary cavity.
-
-
If parosteal osteosarcoma is a diagnostic
consideration or if the diagnosis cannot be established with imaging
studies, a biopsy is needed.
-
Pediatric patients (<20)
-
Painful active lesions
-
Osteoid osteoma
-
-
Painful aggressive benign lesions
-
Osteoblastoma
-
Aneurysmal bone cyst
-
Langerhans cell histiocytosis
-
-
Painful malignant lesions
-
Osteosarcoma
-
Ewing sarcoma
-
-
-
Young adult patients (20 to 40)
-
Giant cell tumor of bone
-
Lymphoma
-
Ewing sarcoma (5 to 30 years)
-
-
Adult patients (>mt40)
-
Metastatic carcinoma
-
Myeloma
-
Lymphoma
-
Chondrosarcoma and other sarcomas
-
-
If osteoid osteoma suggested, seek
typical pain pattern (worse at night, relieved by nonsteroidal
anti-inflammatory agents >obNSAIDs>cb). -
Pain at rest in addition to mechanical pain suggests tumor-related pain.
-
Tumor-related causes of rest pain
-
Bone destruction
-
Intraosseous pressure
-
Proteinases
-
Inflammatory cytokine response
-
-
Tumor-related causes of activity-related pain
-
Advanced bone destruction
-
Extra-osseous soft tissue pressure
-
-
-
If malignancy suspected, check for systemic symptoms, more common with disseminated malignancies.
-
Fevers, chills, unintentional weight loss, anorexia, fatigue
-
-
In adults, elicit risk factors for and signs of systemic malignancies:
-
Family history of breast cancer
-
Prostate symptoms for prostate cancer
-
Smoking history for lung cancer
-
Hematuria for renal cancer
-
Thyroid masses/symptoms for thyroid cancer
-
Complete review of systems
-
-
Adults (also see section on metastatic disease)
-
Potential sources of primary carcinoma (especially breast, prostate, lung, kidney, thyroid)
-
Other evidence of metastatic disease (lymph nodes, soft tissues)
-
-
All: Site of primary symptoms
-
Blood work
-
Pediatrics: Infection is always a consideration, so CBC with differential, ESR, and CRP are needed.
-
Adults (also see Chapter 8, Metastatic Disease)
-
CBC, ESR, serum calcium and phosphorus,
alkaline phosphatase, lactate dehydrogenase, serum protein
electrophoresis, prostate-specific antigen, urinalysis
-
-
-
Bone scan
-
Assesses for multifocal disease in potentially polyostotic pediatric processes and in metastatic or multifocal adult tumors
-
Identifies potential sites of referred pain
-
Identifies intense uptake within osteoid osteomas, which is needed to support this presumptive clinicoradiographic diagnosis
-
Stages for potential bone metastases in suspected primary sarcomas
-
-
CT scan of primary site
-
Identifies scalloping and cortical destruction better than MRI
-
Useful for cartilage tumors and giant cell tumors
-
May be used to complement MRI
-
-
MRI of primary site
-
Best identifies intramedullary and extramedullary extent and relationship to surrounding structures
-
Should be done prior to biopsy
-
-
CT of chest
-
Primary staging study for sarcomas
-
Lung metastases most common site of metastases from sarcomas
-
-
Part of metastatic work-up for adults with aggressive bone lesions and suspected metastatic disease (also see Chapter 8)
-
-
CT of abdomen and pelvis
-
Allows identification of renal cell carcinoma prior to biopsy
-
Mammography
-
Part of metastatic work-up in adult women with aggressive bone lesions and suspected metastatic disease (also see Chapter 8)
-
-
Biopsy: After completion of all imaging studies, biopsy is necessary in most painful bone lesions.
-
Most common bone lesions associated with pathologic fractures in pediatric patients
-
Benign tumors (Figs. 1-1 and 1-2)
-
Unicameral bone cyst
-
Nonossifying fibroma
-
Fibrous dysplasia
-
-
Malignant tumors (Figs. 1-3 and 1-4)
-
Osteosarcoma
-
Ewing sarcoma
-
-
-
Most common bone lesions associated with pathologic fractures in adults
-
Young adults
-
Giant cell tumor (Fig. 1-5)
-
Lymphoma
-
-
Adults >mt40
-
Metastatic carcinoma
-
Myeloma
-
Lymphoma (Figs. 1-6 and 1-7)
-
-
-
Key question: Did the patient have pain at the site leading up to the time of the fracture?
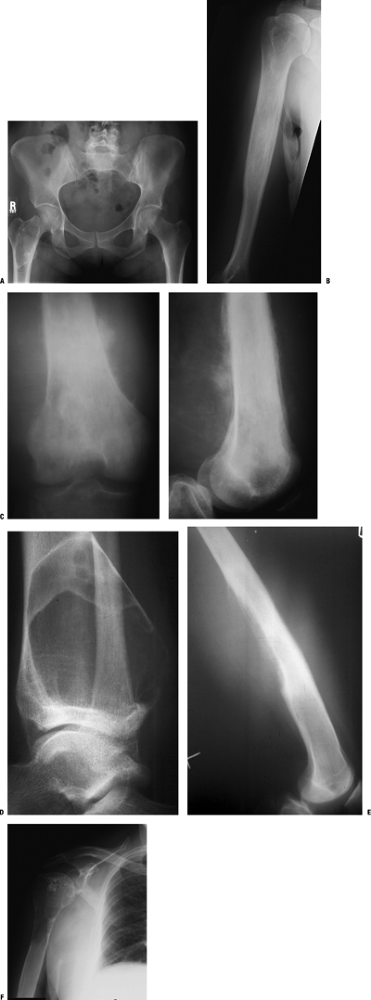
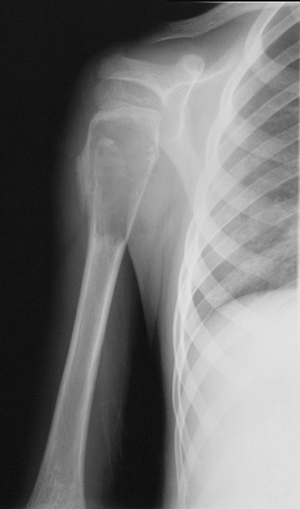 Figure 1-1 Pathologic fracture through unicameral bone cyst typically occurs without preceding pain.
Figure 1-1 Pathologic fracture through unicameral bone cyst typically occurs without preceding pain.![]() Figure 1-2 Pathologic fracture through nonossifying fibroma typically occurs without preceding pain.
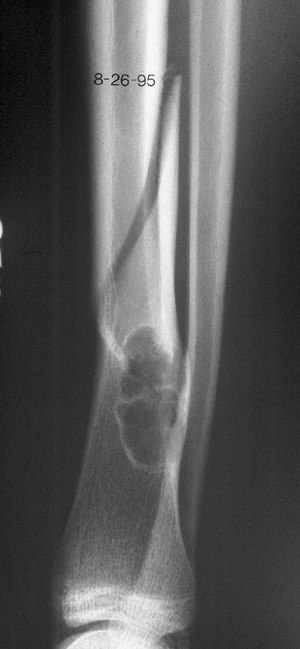
Figure 1-2 Pathologic fracture through nonossifying fibroma typically occurs without preceding pain.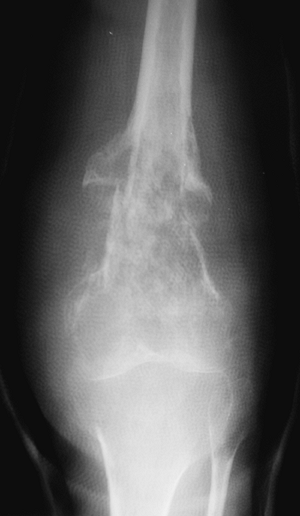 Figure 1-3 Pathologic fracture through an osteosarcoma is usually preceded by progressively worsening pain.P.9
Figure 1-3 Pathologic fracture through an osteosarcoma is usually preceded by progressively worsening pain.P.9![]() Figure 1-4 Pathologic fracture through aggressive lesions such as this Ewing sarcoma is usually preceded by pain.
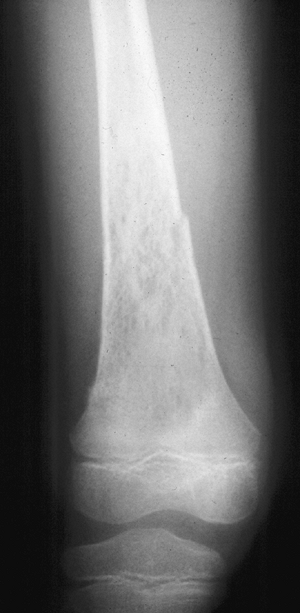
Figure 1-4 Pathologic fracture through aggressive lesions such as this Ewing sarcoma is usually preceded by pain.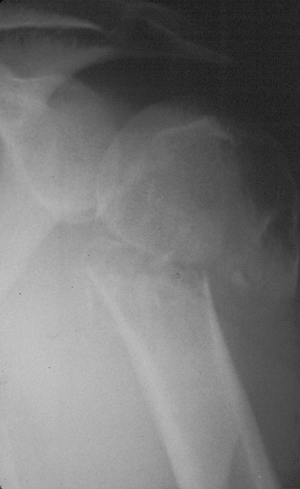 Figure 1-5 Pathologic fractures through giant cell tumors are usually preceded by pain, as was the case here.
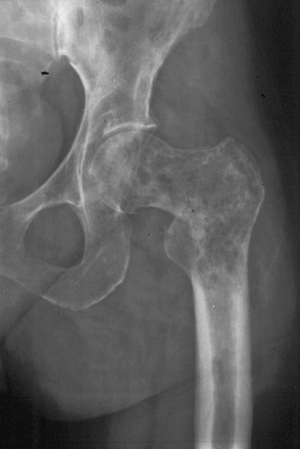
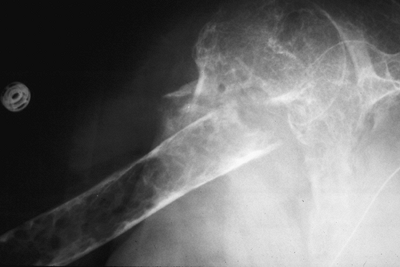
Figure 1-5 Pathologic fractures through giant cell tumors are usually preceded by pain, as was the case here.![]() Figure 1-6 Pathologic fractures through lesions created by metastatic carcinoma are often preceded by pain.
Figure 1-6 Pathologic fractures through lesions created by metastatic carcinoma are often preceded by pain.-
“No” implies a latent lesion without
progressive destruction. Most of these lesions can be diagnosed by
radiograph alone without biopsy. This situation is most common in the
pediatric population. Many of these fractures may be allowed to heal
before addressing the lesion itself, unless there is the potential for
deformity. -
“Yes” implies a potentially aggressive or
malignant lesion. These lesions usually require a biopsy to establish
the diagnosis. This situation includes most malignant primary bone
tumors and metastatic lesions.
-
-
Site of pathologic fracture: Open? Neurovascular status intact distally?
-
General examination to elicit source of metastatic disease and other evidence of disseminated malignancy (see section above on painful bone lesions)
-
Radiographic differential diagnosis by age (Table 1-3)
-
Location
 |
|
Figure 1-7 Pathologic fractures through multiple myeloma lesions are often preceded by pain.
|
|
Table 1.3 Radiographic Differential Diagnosis by Age
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
-
Epiphyseal, metaphyseal, and diaphyseal locations in long bones (Box 1-3)
-
Epiphyseal sites are an uncommon site of bone lesions and are affected by a relatively short list of potential lesions.
-
Similarly, the diaphysis is a less common site for bone lesions.
-
By contrast, the metaphysis is the most common site of long bone involvement, and most lesions can affect the metaphysis.
-
-
Surface bone lesions
-
Specific osseous and cartilaginous lesions arise from the surface of the bone or are intracortical.
-
A CT scan is often helpful to better define the relationship of these lesions to the bone cortex (Table 1-4 and Fig. 1-8).Table 1.4 Differential Diagnosis of Surface and Intracortical Eccentric Bone Lesions
Osseous Lesions Cartilaginous Lesions Surface bone lesions Parosteal osteosarcoma
Periosteal osteosarcoma
High-grade peripheral osteosarcomaOsteochondroma
Periosteal chondroma
Periosteal chondrosarcomaIntracortical lesions Osteoid osteoma Periosteal chondroma
Chondromyxoid fibroma
Nonossifying fibroma -
Axioms of tumor location
-
Most common bone lesion in the phalanges: enchondromas
-
Two tumors with a very strong predilection for the tibia: osteofibrous dysplasia and adantinoma
-
Most common locations for aggressive
benign and primary malignant bone tumors in children and young adults
involve areas of rapid longitudinal growth: distal femur, proximal
tibia, proximal femur, proximal humerus. -
Chondrosarcomas have a predilection for the proximal peripheral skeleton: pelvis, scapula, proximal humerus, femur.
-
Chordoma usually occurs in sacrum or base of skull.
-
Three most common bone tumors in patients >mt40: metastases, metastases, metastases!
-
Most metastases occur proximal to knees
and elbows: those distal to knees and elbows are usually due to lung or
renal metastases or melanoma. -
Common anterior spine lesions: metastases, hemangiomas of bone, Langerhans cell histiocytosis, osteomyelitis, giant cell tumor
-
Common posterior spine lesions: aneurysmal bone cyst, osteoid osteoma, osteoblastoma
-
Bone lesions that skip across joints to
involve adjacent bones are frequently vascular: angiosarcoma and
Gorham’s disease (disappearing bone disease).
-
-
Three key questions
-
What is the lesion doing to bone? This
question can also be asked as, “What is the border of the lesion?”
Three types of borders are defined by the width of the zone of
transition with normal bone. Each type of border leads to a
differential diagnosis of latent, active, or aggressive bone lesions (Table 1-5 and Figs. 1-9 and 1-10). -
How is the bone responding to the lesion (Table 1-6 and Figs. 1-11 and 1-12)?
-
What matrix, if any, makes up the lesion?
-
Matrix refers to the pattern of mineralization within a lesion that is not purely radiolucent.
-
The specific pattern of mineralization helps point to a specific category of bone lesions (Table 1-7 and Fig. 1-13).
-
-
Bone scans are invaluable in determining
whether a bone lesion is solitary or one of a larger number of bone
lesions throughout the skeleton (Table 1-8). -
Skeletal surveys may be necessary in
conditions where the bone scan may be “cold,” such as myeloma and
Langerhans cell histiocytosis.
-
Common differential diagnoses
-
Holes in bone: FEGNOMASHIC or FOGMACHINE (Box 1-4)
-
Epiphyseal lesions. Lesions of the
epiphysis may either be isolated to one side of the joint or involve
both sides. The differential diagnosis differs for these two general
groups, although for those lesions
P.12P.13P.14P.15P.16P.17P.18P.19P.20P.21P.22P.23
that
may involve both sides of the joint, an isolated lesion early on in the
disease process may involve only one side. PGCAT is a convenient
mnemonic to recall the most common lesions in both of these categories,
but it does not distinguish between lesions that are always solitary
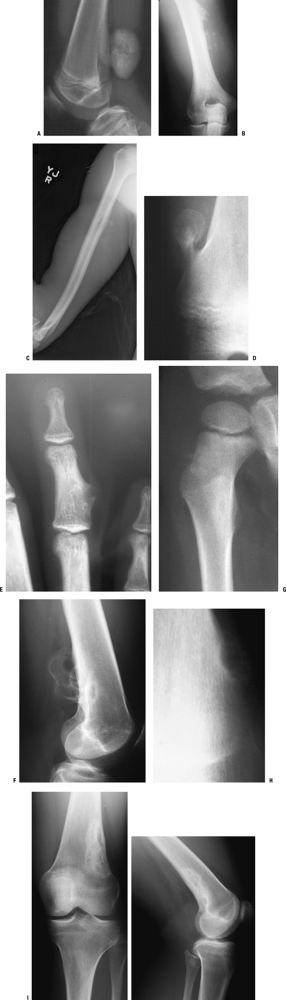
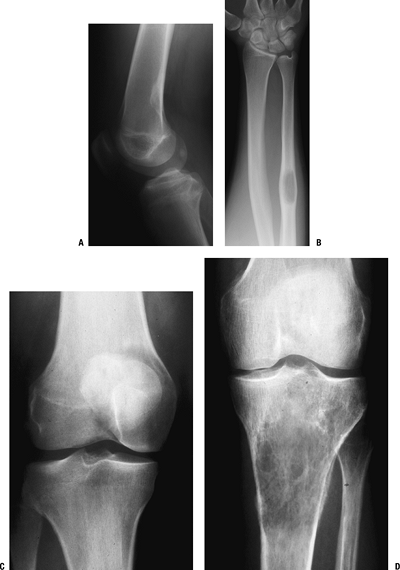
and those that may involve one or both sides of the joint (Figs. 1-14 and 1-15 and Box 1-5).![]() Figure 1-8 Common surface and intracortical eccentric bone lesions are displayed radiographically. (A) Parosteal osteosarcoma, (B) periosteal osteosarcoma, (C) high-grade surface osteosarcoma, (D) osteochondroma, (E) periosteal chondroma, (F) periosteal chondrosarcoma, (G) osteoid osteoma, (H) chondromyxoid fibroma, (I) nonossifying fibroma.Table 1.5 Types of Borders That Define Bone Lesions
Figure 1-8 Common surface and intracortical eccentric bone lesions are displayed radiographically. (A) Parosteal osteosarcoma, (B) periosteal osteosarcoma, (C) high-grade surface osteosarcoma, (D) osteochondroma, (E) periosteal chondroma, (F) periosteal chondrosarcoma, (G) osteoid osteoma, (H) chondromyxoid fibroma, (I) nonossifying fibroma.Table 1.5 Types of Borders That Define Bone LesionsBorder Type of Transition Width of Transition Type of Bone Lesion Common Examples Geographic Narrow Pencil-thin line Latent benign vs. active Nonossifying fibroma (fibrous cortical defect)
Chondromyxoid fibroma
Unicameral bone cystMoth-eaten Intermediate Millimeters Active benign Aneurysmal bone cyst*
Chondroblastoma*
Giant cell tumor*Permeative Broad Centimeters Aggressive benign vs. malignant Aneurysmal bone cyst*
Chondroblastoma*
Giant cell tumor*
Sarcoma
Metastatic carcinoma
Lymphoma*Some
tumors, such as aneurysmal bone cyst, chondroblastoma, and giant cell
tumor, may have a spectrum of clinical behavior patterns.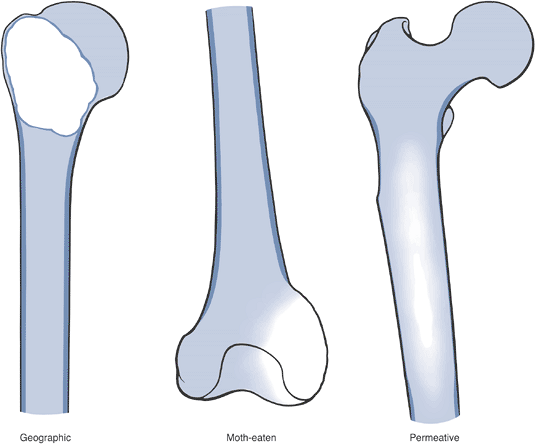 Figure 1-9
Figure 1-9
Border types. The interface at the edge of any given bone lesion on
plain radiographs may be classified as geographic, moth-eaten, or
permeative. A geographic border, as illustrated by the proximal humeral
tumor on the left, has a very narrow zone of transition (able to be
traced with a pencil-thin line) between normal and abnormal bone and
usually represents an indolent benign process, such as a unicameral
bone cyst. A moth-eaten border, as illustrated by the distal femoral
tumor in the center, has a slightly blurred zone of transition
(typically a few millimeters) and may reflect a more active process,
such as a giant cell tumor of bone. A permeative border, as illustrated
by the proximal femoral diaphyseal tumor on the right, is typified by a
broad zone of transition (usually 1 cm or greater) and usually
represents a more aggressive process, such as a sarcoma or metastatic
carcinoma. These border types are only one piece of the radiographic
interpretation, and they cannot be relied upon wholly to determine
biologic activity.![]() Figure 1-10 Bone lesions with a spectrum of borders. (A) Nonossifying fibroma with a geographic border. (B) Fibrous dysplasia with a geographic to slightly moth-eaten border. (C) Giant cell tumor of bone with a moth-eaten border. (D) Lytic osteosarcoma with a permeative border.Table 1.6 Radiographic Types of Responses to Bone Lesions
Figure 1-10 Bone lesions with a spectrum of borders. (A) Nonossifying fibroma with a geographic border. (B) Fibrous dysplasia with a geographic to slightly moth-eaten border. (C) Giant cell tumor of bone with a moth-eaten border. (D) Lytic osteosarcoma with a permeative border.Table 1.6 Radiographic Types of Responses to Bone LesionsTypes of Response Definition Classic Examples Marginal sclerosis Dense peripheral medullary lamellar bone margin to inactive, active, or very slowly expansile aggressive lesions Nonossifying fibroma
Fibrous dysplasiaCortical thickening Sometimes massive cortical response to very slow-growing lesions Osteoid osteoma
OsteomyelitisOrderly periosteal new bone Regular laminar periosteal response without accompanying aggressive features Stress fracture Endosteal expansion Combination of orderly periosteal bone formation with endosteal erosion (scalloping) Low- to intermediate-grade chondrosarcoma Periosteal neocortical response Rapid expansion of bone and destruction of overlying cortex remains contained by periosteum and thin neocortex Aneurysmal bone cyst
Giant cell tumor of bonePoorly organized new bone Inability of periosteal response to contain rapid destruction
Soft tissue mass extends beyond the intact cortexOsteosarcoma: Codman’s triangle
Ewing sarcoma: sunburst appearance, onion-skinningAdapted from Levesque J, Marx R, Bell RS, et al. A Clinical Guide to Primary Bone Tumors. Philadelphia: Williams & Wilkins, 1998. 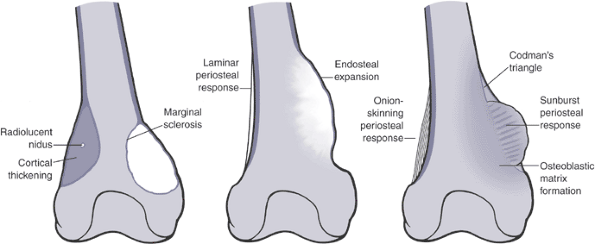 Figure 1-11
Figure 1-11
Varying types of bony response are seen in association with different
tumors of bone. On the left, a distal femur shows typical benign types
of bone response. The lateral bone lesion, with a central nidus
surrounded by a dense cortical thickening or hyperostosis, is typical
for an osteoid osteoma. On the same femur, there is also a medial
lesion with a reactive sclerotic rim and slight endosteal expansion.
This appearance in the distal femoral metaphysis of a child is typical
for a non-ossifying fibroma. In the center femur, there is an
aneurysmal dilatation or ballooning of the cortex with an eggshell-thin
rim of peripheral bone that is typical of an aneurysmal bone cyst. On
the right femur, aggressive types of periosteal reaction are shown,
including onion-skinning periosteal reaction on the lateral distal
femoral metaphysis and a medial sunburst osteoblastic response with
bordering Codman’s triangles proximally and distally. These aggressive
types of periosteal response may be seen independently or together.
Although onion-skinning is often associated with Ewing sarcoma,
sunburst response with osteosarcoma, and Codman’s triangle with
osteosarcoma, each type of response may be seen in other conditions as
well, including osteomyelitis, Langerhans cell histiocytosis, lymphoma,
and metastatic carcinoma, among others.![]() Figure 1-12 Bone lesions with a spectrum of responses in the surrounding bone. (A) A proximal femoral fibrous dysplasia lesion shows marginal sclerosis. (B) A humeral diaphyseal region of cortical thickening is seen here in association with an area of chronic osteomyelitis. (C)
Figure 1-12 Bone lesions with a spectrum of responses in the surrounding bone. (A) A proximal femoral fibrous dysplasia lesion shows marginal sclerosis. (B) A humeral diaphyseal region of cortical thickening is seen here in association with an area of chronic osteomyelitis. (C)
Poorly organized new bone formation demonstrating both Codman’s
triangle and a sunburst appearance is shown here in an osteosarcoma. (D) Periosteal neocortical response is seen in the posterior cortex of the distal tibia due to this aneurysmal bone cyst. (E) Onion-skinning periosteal reaction with Codman’s triangle and an associated soft tissue mass is shown in this Ewing sarcoma. (F) Endosteal expansion and erosions are seen with this low-grade chondrosarcoma.Table 1.7 Patterns of Mineralization Within Bone LesionsDescription Typical Lesions Other Distinguishing Features Radiolucent (lytic) Absence of matrix Numerous (nonspecific) Numerous Mineralized Calcified Punctate rings and arcs Hyaline cartilage lesions*
Enchondroma
ChondrosarcomaGeographic
Permeative zone of transition
Endosteal erosions
Periosteal reactionOssified More organized pattern of bone formation Osseous lesions
Osteoid osteoma
Osteoblastoma
Osteosarcoma
Lesions with marked sclerotic response
Breast carcinoma metastases
Prostate carcinoma metastases
LymphomaReactive peripheral bone, central nidus
Scant to densely sclerotic lesions
Scant to densely sclerotic lesionsPermeative destructive lesions
Variable sclerotic response in surrounding boneBlurring of trabeculae Fibrous dysplasia Geographic long lesion in long bone
Ground glass*
Immature cartilage lesions, such as chondroblastoma and periosteal
chondroma, do not show the degree of mineralization characteristic of
mature hyaline lesions.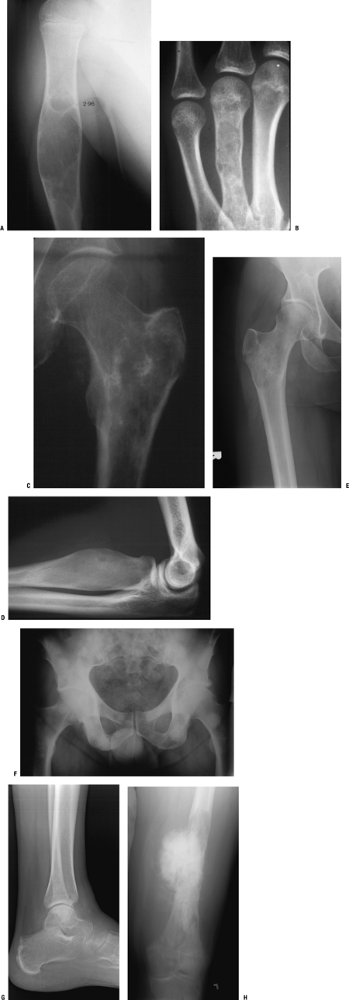 Figure 1-13 Bone lesions with a spectrum of types of matrix mineralization. (A) Absence of matrix mineralization is seen in a lucent lesion from a unicameral bone cyst. (B) The typical rings and arcs of hyaline cartilage matrix mineralization are seen in this metacarpal enchondroma. (C) The same type of hyaline cartilage matrix mineralization is apparent in this chondrosarcoma. (D) Ground glass appearance is typical of fibrous dysplasia, as in this lesion of the proximal radius. (E)
Figure 1-13 Bone lesions with a spectrum of types of matrix mineralization. (A) Absence of matrix mineralization is seen in a lucent lesion from a unicameral bone cyst. (B) The typical rings and arcs of hyaline cartilage matrix mineralization are seen in this metacarpal enchondroma. (C) The same type of hyaline cartilage matrix mineralization is apparent in this chondrosarcoma. (D) Ground glass appearance is typical of fibrous dysplasia, as in this lesion of the proximal radius. (E)
A mixed sclerotic and partially lytic lesion of the proximal femur,
which is frequently seen in the setting of metastatic breast cancer. (F) Densely sclerotic metastases are the most common appearance in bony metastatic prostate carcinoma. (G)
Lytic metastases are the most common appearance for metastatic lung
cancer, which is also the most common primary source for metastases
distal to the elbows and knees, as shown in this tarsal navicular
metastatic deposit from lung carcinoma. (H) A cumulus cloud pattern of matrix mineralization is most characteristic of osteosarcoma.Table 1.8 Multifocal Bone LesionsPediatrics Adults Metabolic bone diseases Multifocal Paget’s disease of bone Infections Chronic recurrent multifocal osteomyelitis Malignant neoplasms Metastases (neuroblastoma, rhabdomyosarcoma, osteosarcoma)
Multifocal osteosarcomaMetastases
MyelomaEndocrinopathies Hyperparathyroidism (brown tumors) Vascular conditions Disappearing bone disease (Gorham’s disease)
Skeletal/extraskeletal lymphangiomatosis
Angiosarcoma
Multifocal bone infarctsCongenital diseases Osteochondromatosis (multiple hereditary exostoses) Developmental skeletal dysplasias Ollier’s disease (multiple enchondromatosis)
Maffucci syndrome (multiple enchondromatosis with hemangiomas)
Polyostotic fibrous dysplasia
McCune-Albright syndrome (polyostotic fibrous dysplasia with endocrinopathy)Other diseases Langerhans cell histiocytosis Erdheim-Chester disease -
As a subset of epiphyseal lesions,
juxta-articular lesions may involve one or both sides of the joint and
typically represent non-neoplastic arthropathies.-
Inflammatory arthropathies
-
Infectious arthropathy
-
Crystal-induced arthropathy (gout, pseudogout)
-
Pigmented villonodular synovitis
-
Synovial chondromatosis
-
Intraosseous ganglions
-
Degenerative cysts (geodes)
-
Isolated epiphyseal lesions (as a subset
of the larger group of epiphyseal lesions) are neoplastic conditions
that involve only one side of the joint. -
Aneurysmal bone cysts
-
Chondroblastoma (confined to epiphysis; pediatrics or adults)
-
Giant cell tumor (meta-epiphyseal; mostly adults)
-
Clear cell chondrosarcoma (adults)
-
Spine lesions
-
Other than metastatic disease, which
often begins its destruction in the pedicle, and hence may enlarge to
affect either the anterior or posterior elements or both, there is a
sharp delineation between the processes that affect the vertebral body
from those that affect the posterior elements. Infections and pediatric
malignancies, for instance, nearly always affect the anterior spine,
and the benign tumors that characteristically involve the two anatomic
regions do not overlap (Table 1-9).P.24![]() Figure 1-14
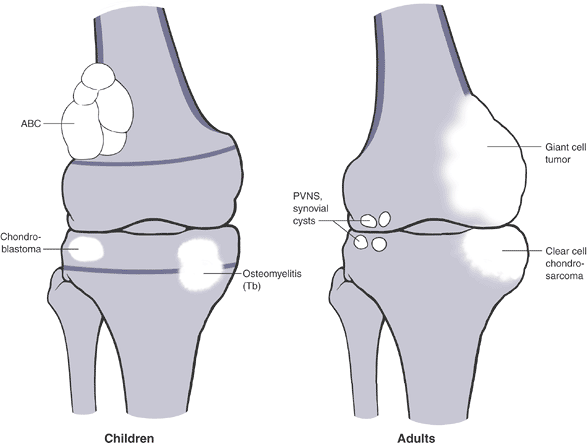
Figure 1-14
PGCAT lesions. Epiphyseal lesions have a relatively narrow differential
diagnosis that can be recalled using the mnemonic PGCAT, which includes
such common epiphyseal lesions as PVNS (pigmented villonodular
synovitis), GCT (giant cell tumor of bone), chondroblastoma, ABC
(aneurysmal bone cyst), and Tb (tuberculosis). In the figure, the femur
on the left, with open growth plates shown, illustrates common
pediatric epiphyseal lesions. Chondroblastoma is represented by the
purely epiphyseal radiolucency without sclerotic borders. Aneurysmal
bone cyst is represented by the septated, expansile lesion centered in
the meta-epiphysis. Osteomyelitis, of which tuberculosis is only a
representative example, may manifest itself as a metaphyseal
radiolucency eroding across into the epiphysis, as shown on the
pediatric figure. The femur on the right, without open growth plates,
illustrates the common adult epiphyseal lesions. Synovial processes, of
which PVNS is only a representative example, are relatively common.
Degenerative geodes and intraosseous ganglions are more common examples
of such processes. Giant cell tumor of bone has its epicenter in the
metaphysis but classically erodes down to subchondral bone within the
epiphysis.P.25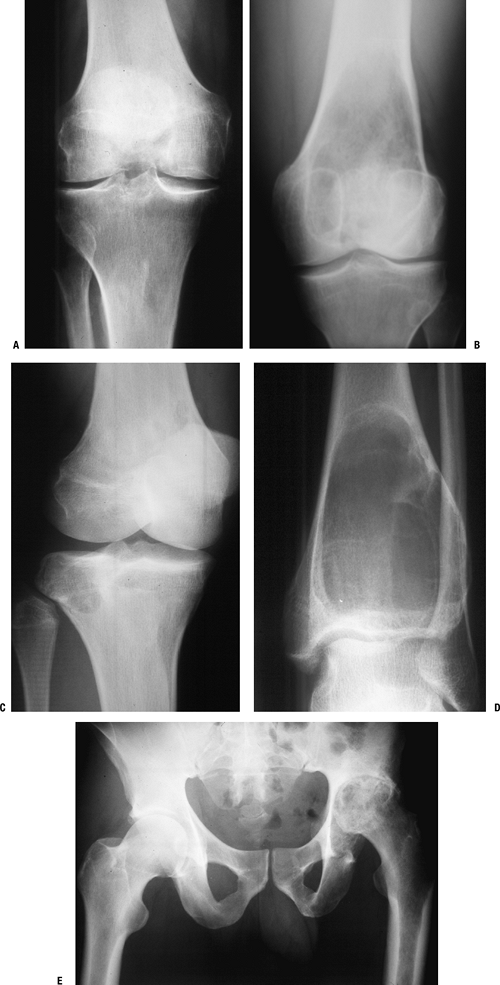 Figure 1-15 Bone lesions that frequently occur in the epiphyseal region. (A) Pigmented villonodular synovitis may show juxta-articular erosions. (B) Giant cell tumor of bone frequently extends from the metaphyseal region into the epiphysis and down to subchondral bone. (C) Chondroblastoma has its epicenter within the epiphysis and extends into the metaphysis occasionally. (D)
Figure 1-15 Bone lesions that frequently occur in the epiphyseal region. (A) Pigmented villonodular synovitis may show juxta-articular erosions. (B) Giant cell tumor of bone frequently extends from the metaphyseal region into the epiphysis and down to subchondral bone. (C) Chondroblastoma has its epicenter within the epiphysis and extends into the metaphysis occasionally. (D)
Most commonly, when aneurysmal bone cyst is seen in the epiphysis, it
occurs as a secondary lesion within a chondroblastoma or giant cell
tumor, although the lesion shown was a primary aneurysmal bone cyst
extending into the epiphysis.
P.26
(E) Infections, such as tuberculosis, can
cause juxta-articular cysts in an epiphyseal location. This
immunosuppressed patient had tuberculous left hip arthritis.Table 1.9 Differential Diagnosis for Spine LesionsPediatrics Adults Anterior elements (vertebral body) Infections Bacterial infection (discitis)
Tuberculosis (Pott’s disease)Benign tumors Histiocytosis Hemangioma
Giant cell tumorMalignancies Ewing sarcoma Metastases
MyelomaLymphoma Posterior elements Benign tumors Aneurysmal bone cyst
Osteoid osteoma
Osteoblastoma
OsteochondromaMalignancies Metastases P.27Table 1.10 Musculoskeletal Tumor Society Staging SystemStage Anatomic Extent Histologic Grade* Presence of Metastases Treatment 0 — Benign None Curettage or marginal excision IA Intraosseous Low None Wide resection of involved bone IB Extraosseous extension Low None Wide resection of involved bone and soft tissue IIA Intraosseous High None Wide resection of involved bone
ChemotherapyIIB Extraosseous High None Wide resection of involved bone and soft tissue
ChemotherapyIII Either Either low or high Present Wide resection of involved bone and soft tissue and metastases
Chemotherapy*Low-grade
tumors by definition have <15% chance of metastasis; high-grade
tumors have >15% chance of metastasis. Ewing sarcoma is high grade
by definition, and its treatment differs in that radiotherapy is an
alternative to wide resection for nonexpendable, nonreconstructible
bone sites.
-
-
Sacral lesions
-
Dural cysts
-
Giant cell tumors
-
Aneurysmal bone cysts
-
Chordoma
-
Chondrosarcoma
-
Metastases
-
Mineralized bone lesions
-
Central lesions
-
Enchondroma (popcorn; rings and arcs)
-
Bone infarct (smoke up the chimney)
-
Fibrous dysplasia (ground glass)
-
Intraosseous lipoma (cockade sign)
-
Osteoblastoma
-
Chondrosarcoma
-
Osteosarcoma, high-grade conventional
-
Osteosarcoma, low-grade central
-
Metastatic carcinoma (especially prostate, breast, less commonly lung)
-
Lymphoma (variably)
-
Eccentric lesions
-
Osteochondroma (stalk, cortical continuity)
-
Periosteal chondroma (heaped-up borders)
-
Myositis ossificans (peripheral ossification)
-
Periosteal chondrosarcoma
-
Parosteal osteosarcoma (stuck on cortex)
-
-
-
treatment. Staging describes both the extent of the tumor and the
potential for development of metastatic disease. Staging in general
involves determining the specific diagnosis, histologic grade, local
extent and/or size of tumor, and the presence (and type) or absence of
metastatic disease. Various staging systems exist, but that of the
Musculoskeletal Tumor Society is commonly employed (Table 1-10).
-
Recommended tests
-
Suspected bone sarcoma
-
CT chest (#1 site of sarcoma metastases is lung)
-
Bone scan (#2 site of sarcoma metastases is bone)
-
-
Suspected metastatic carcinoma (see Chapter 8)
-