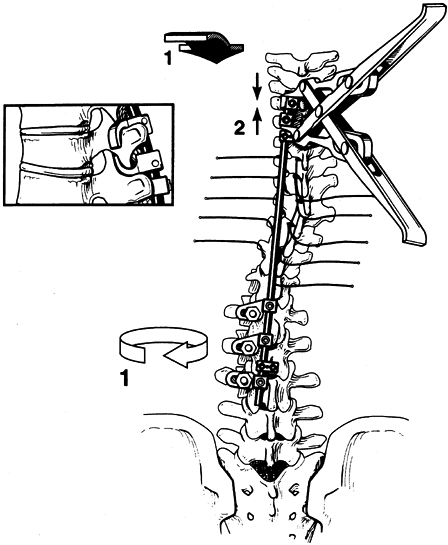
POSTERIOR SURGERY FOR SCOLIOSIS
stable, balanced spine tend to be overshadowed by the marketing of new
implant systems. With the development of each new system comes the
promise of “perfect results with no complications.” The belief that
more hardware is better has shifted our focus away from the concepts of
precise planning and selection of fusion levels, meticulous operative
technique, and careful postoperative management. New implant systems
can and should be added to our armamentarium of scoliosis management,
but scientific study is mandatory, and the tried and proven principles
must not be forgotten.
Establish the exact diagnosis in each patient whenever possible. An
understanding of the natural history and the type of curve pattern that
might develop is necessary to determine the appropriate approach for
the treatment of idiopathic scoliosis; a patient with congenital
scoliosis requires still another approach during evaluation and
treatment.
 |
|
Table 156.1. Etiology of Scoliosis: Conditions for Which Posterior Techniques Are Useful
|
on the underlying diagnosis, curve magnitude and progression, the
patient’s age and health, and the surgeon’s skill and judgment. All
variables must be carefully considered
before
rendering a surgical decision. In general, there are four indications
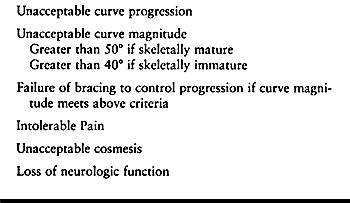
for surgical treatment in idiopathic or congenital scoliosis: severe
curves, unacceptable curve progression, pain, and unacceptable cosmesis
(Table 156.2).
 |
|
Table 156.2. Indications for Surgery for Idiopathic Scoliosis
|
reported on the role that thoracic lordosis plays in decreasing
pulmonary function and recommended screening and treatment in severe
cases. In patients with thoracic lordosis, surgery may be necessary
even though the coronal curve may not appear large enough to warrant it.
shown that curves greater than 50° tend to progress even after skeletal
maturity. There is a correlation between age, Risser sign, and curve
magnitude as prognostic indications for progression (34,54).
In young patients without evidence of menarche, a Risser sign of 1 or
less, and a curve of 40° or more, orthotics management is less
effective and surgery might be indicated. On the other hand, there are
times when combined thoracic and lumbar curves exceed 45° to 50° in
magnitude but show no loss of pulmonary function, are cosmetically
acceptable, and remain stable. These curves might be best managed by
observation. All patients are different, so determine treatment on an
individual basis.
investigation to rule out organic causes must be undertaken. Adults
with untreated curves, especially large curves, may have disabling pain
that can be relieved by surgical treatment (30,43,48,51,57).
for surgical treatment. Severe trunk decompensation or rotation may be
a reason for surgery, but that decision must be made by the patient and
family. Scoliosis surgery is a major undertaking: Careful thought must
be given if the only indication is cosmesis. Rib deformity may not be
corrected by correcting the curve, and improving the deformity always
leaves a surgical scar.
In patients who are marginal ambulators or wheelchair bound,
progressive spinal curvatures may compromise ambulation or sitting
balance. In wheelchair-bound patients, listing to the side because of a
decompensated spine necessitates increased use of the upper extremities
to achieve support and balance, which severely impairs overall
function. In patients with poor sensation, progressive scoliosis tends
to create pelvic obliquity, with subsequent asymmetric pressure-loading
on the buttocks and eventual skin breakdown.
 |
|
Table 156.3. Indications for Surgery for Neuromuscular Scoliosis
|
integral to the overall success of spinal surgery. Attention to detail
at this phase is essential for a smooth perioperative course. Document
a careful history and physical examination of the patient. The
neurologic evaluation is especially important in patients with
congenital or neurologic conditions. Any abnormal findings will
necessitate further evaluation with electromyography, myelogram,
computed tomography (CT), or magnetic resonance imaging (MRI). In the
physical examination of the spine, note rotational prominences, the
amount of decompensation of the spine, and any evidence of defects or
cutaneous abnormalities along the midline. Cutaneous abnormalities such
as hair patches, nevi, dermal sinuses, and lipomas are frequently
associated with intraspinal pathology (19,27,61). Myelography or MRI may be indicated to rule out disease in or about the spinal cord.
advance for evaluating the spinal column and its contents. This
noninvasive tool has been valuable in diagnosing syringomyelia and
other spinal cord anomalies. However, the three-dimensional nature of
scoliosis can make clear imaging difficult. A close working
relationship with the radiologist is necessary so that specific
problems may be more carefully evaluated. (See Chapter 4 on imaging modalities.)
findings are present on the physical examination, when spinal
dysraphism is expected, and when MRI has proven inadequate or
equivocal. Water-soluble dyes are preferred whenever, possible because
they can be used in conjunction with CT and are useful in conditions in
which the neurologic deficits are not consistent with the known
diagnosis.
posteroanterior and lateral radiographs of the spine. Measure the curve
by the Cobb method (12). Look for rotation and
any evidence of congenital anomalies, tumors, or bony abnormalities.
The Nash and Moe classification of rotation is useful in determining
structural curvatures and selecting fusion levels (29).
The lateral radiograph shows curvatures in the sagittal plane and the
possible coexistence of lumbosacral spondylolisthesis. Supine right and
left side-bending films demonstrate curve flexibility and give a good
indication of the amount of correction that can be anticipated from
surgery (29). In severe deformities, bending
films help in deciding whether to perform anterior procedures. For
patients with severe rigid curves, it may be desirable to consider
anterior releases or wedge resection before the posterior procedure.
(See Chapter 155 for the anterior approach to
scoliosis.) This allows better mobility of the spine and may decrease
the pseudarthrosis rate. When excessive kyphosis or lordosis is noted,
take lateral flexion and extension views. In patients with paralytic
scoliosis, a supine view in maximum traction including the pelvis is
useful to determine the amount of curve flexibility and what type of
correction of pelvic obliquity might be expected.
on the clinical setting. In adolescent patients with scoliosis and back
pain, perform technetium polyphosphate bone imaging to rule out tumor
or inflammatory causes.
Keim and Reina (28) have reported osteoid osteoma as a cause of painful scoliosis.
urinalysis, and electrolyte values in patients who are otherwise
healthy. Any major medical problem might necessitate further studies.
In patients with neuromuscular conditions or with severe scoliosis,
obtain a preoperative electrocardiogram. Electrocardiography is also
indicated in adults undergoing corrective spinal surgery. In patients
with known cardiomyopathy, congenital heart disease, or cor pulmonale,
obtain anesthesia, pulmonary, and cardiology consultations before
surgery. For patients with severe debilitating health problems, careful
preoperative evaluation and management by an appropriate team of
physicians, surgeons, and paraprofessionals ensures an easier and
smoother postoperative course.
For most patients, however, the decreased pulmonary function does not
play a major role in the perioperative course. Therefore, routine
preoperative pulmonary function studies are unnecessary.
indicated in patients with cor pulmonale, severe curves, thoracic
lordosis, neuromuscular conditions, or a history of pulmonary problems.
A 40% or greater loss of vital capacity and maximum breathing capacity
increases the risk of postoperative complications (60).
In patients with recurrent pneumonia and bronchitis secondary to
scoliosis, a complicated postoperative course must be anticipated;
pulmonary studies are helpful in these patients.
with severe lung disease or cor pulmonale. This trial of traction will
establish the operability of a patient’s curve. Any increase in cardiac
or pulmonary compromise in halo traction is a contraindication for
surgery (9).
modalities to make the curve more mobile (or limber) or to obtain
preliminary correction has been advocated in the past. There are now
few indications for these methods because they add little if any
correction to the spine. With rare exception, they have limited
applicability.
important aspects of the preoperative planning. An inadequate length of
fusion will lead to progression of the curve above or below the fusion
(lengthening or “adding on” to the curve). This may eventually lead to
loss of spine balance. A fusion that is too long, however,
unnecessarily immobilizes the spine. A careful review of curve patterns
and preoperative side-bending radiographs is valuable in defining the
primary and compensatory curves. In general, the primary curve should
be fused and the compensatory curves should be left unfused. For
idiopathic scoliosis, fusion levels should include all vertebrae that
are measured as a part of the primary curve (9,11,16,47).
scoliosis, understanding the curve pattern will help avoid a common
pitfall: not incorporating the sacrum into the fusion. An oblique
sacrum and pelvis must be considered as part of the curvature and
should be included in the instrumentation and fusion.
combined thoracic and lumbar idiopathic scoliosis. It is generally
accepted that the fusion should include all vertebrae that are a part
of the measured curve. In thoracic scoliosis, Harrington (24,25)
suggests fusion from one level above the curve to two levels below, as
long as the inferior level falls within the “stable zone.” Moe (44,45,47) and Goldstein (20,21 and 22)
stressed the importance of extending the fusion from the neutrally
rotated vertebra superiorly to the neutral vertebra inferiorly. Moe
advocates thoracic fusion for combined thoracic and lumbar curves when
the lumbar curve is more flexible on preoperative side-bending
radiographs.
reported the long-term results of the Twin Cities Scoliosis Center’s
management of thoracic and combined thoracic and lumbar scoliosis. A
category of curve types and criteria for selection of fusion levels
were established. In addition, the results of this work support the
concept of selective fusion of the thoracic spine in selected cases.
These authors were able to identify five different thoracic curve
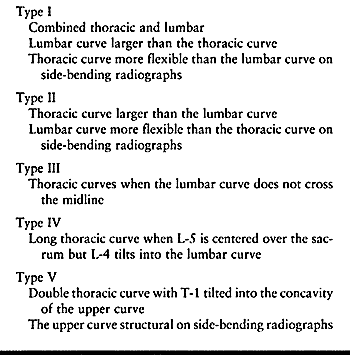
patterns (Table 156.4). Type I curves are
managed by fusion to L-4. In curve types II through V, a selective
thoracic fusion can be performed if the lowest level of the fusion is
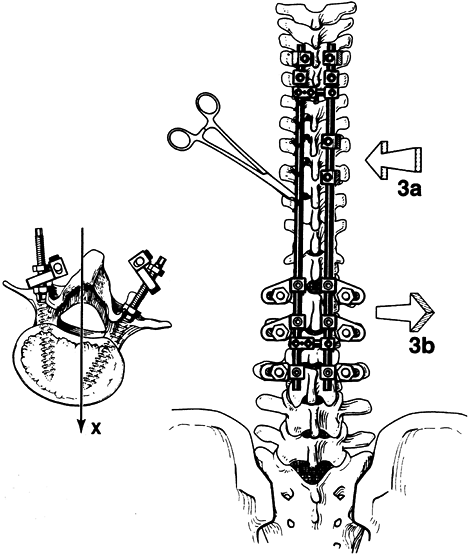
centered over the sacrum (Fig. 156.1, Fig. 156.2, Fig. 156.3, Fig. 156.4 and Fig. 156.5).
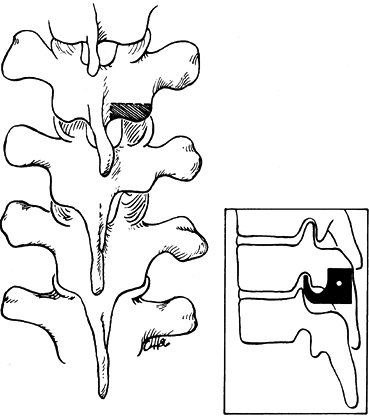
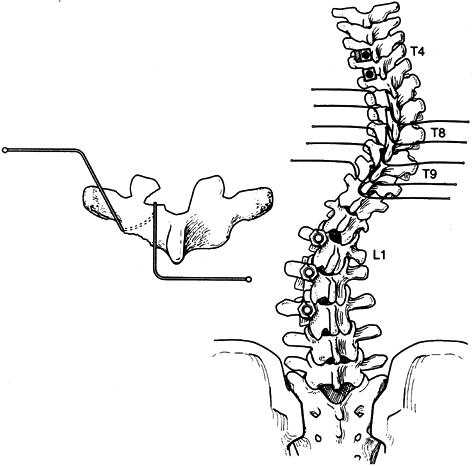
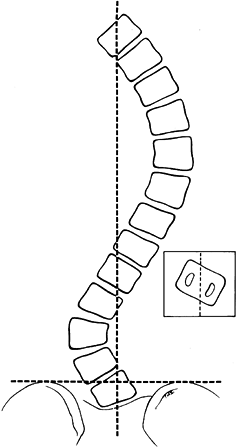
The lowest level of the fusion is established by drawing a line
parallel to the iliac wings. A vertical line is then drawn
perpendicular to the pelvic line centered on the sacrum. (It is
important that any limb-length inequality be compensated for when the
radiographs are taken.) The lowest vertebra most closely bisected by
this line is called the stable vertebra (Fig. 156.6). Ending the fusion at the stable vertebra gives uniformly good results.
 |
|
Table 156.4. Thoracic Scoliosis Curve Patterns
|
 |
|
Figure 156.1. Type I curve. (From King HA, Moe JH, Bradford DS, Winter RB. The Selection of Fusion Levels in Thoracic Idiopathic Scoliosis. J Bone Joint Surg Am 1983;65:1302, with permission.)
|
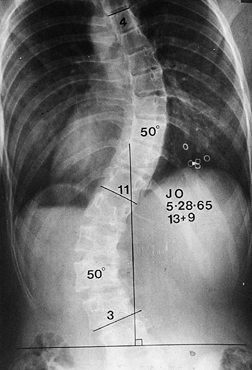 |
|
Figure 156.2. Type II curve. The center sacral line has been created. The stable vertebra is T-12.
|
 |
|
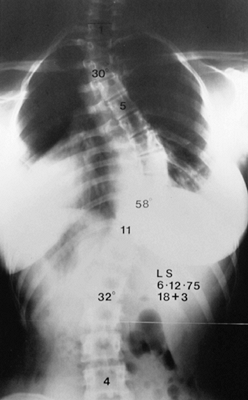
Figure 156.3.
Type III curve. (From King HA, Moe JH, Bradford DS, Winter RB. The Selection of Fusion Levels in Thoracic Idiopathic Scoliosis. J Bone Joint Surg Am 1983;65:1302, with permission.) |
 |
|
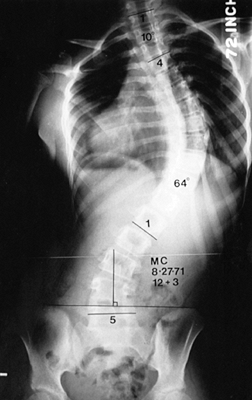
Figure 156.4. Type IV curve. (From King HA, Moe JH, Bradford DS, Winter RB. The Selection of Fusion Levels in Thoracic Idiopathic Scoliosis. J Bone Joint Surg Am 1983;65:1302, with permission.)
|
 |
|
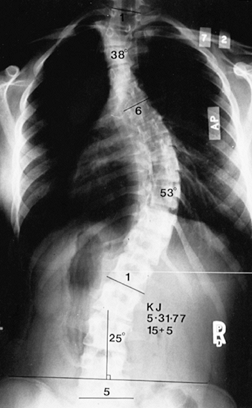
Figure 156.5. Type V curve. (From King HA, Moe JH, Bradford DS, Winter RB. The Selection of Fusion Levels in Thoracic Idiopathic Scoliosis. J Bone Joint Surg Am 1983;65:1302, with permission.)
|
 |
|
Figure 156.6. The technique for creating the center sacral line to establish the stable vertebra.
|
is substantially shorter than the standard T4–L4 fusion previously
recommended. King et al. (29) concluded that
selective thoracic fusion can give good long-term results with balanced
stable spines. When the fusion falls short of the stable vertebra, the
curves tend to progress, adding vertebrae to the measured curve, and
occasionally an additional surgical procedure is needed to extend the
fusion. Fusing beyond the stable vertebrae, especially in type II
curves, tends to aggravate the lumbar curve and also removes additional
valuable motion segments.
have clearly detailed the complications of fusion that extend into the
lumbar spine. Harrington rod fusions flatten the lumbar spine, and
fusions to L-4 and L-5 carry a higher incidence of back pain on
long-term follow-up. In their report, 62% were fused to L-4, and 82%
were fused to L-5. (1). Be aware of
compensatory curves and avoid extensive fusion when possible. In
idiopathic scoliosis, avoid fusion to L-4. Rarely, if ever, is fusion
to L-5 indicated. Fusion to the sacrum is not indicated in adolescents.
However, if symptomatic spondylolisthesis is present, lumbosacral
fusion might be indicated. When an asymptomatic spondylolysis or
spondylolisthesis is present, fusion using the criteria just outlined
can be safely performed (9).
ataxia, Charcot-Marie-Tooth disease, and ambulators with cerebral
palsy), the curve patterns are similar to idiopathic scoliosis. In
those instances, fusion levels may be chosen as just described for
idiopathic curves. Analysis of the lateral roentgenograms is important
to avoid stopping the fusion at the apex of a kyphosis.
obliquity is noted, the sacrum and pelvis must be considered part of
the scoliosis. This situation is most frequently seen in cerebral
palsy, myelomeningocele, muscular dystrophy, spinal muscle atrophy, and
scoliosis secondary to spinal cord injury. If the sacrum and pelvis are
not included in the instrumentation and fusion, progressive pelvic
obliquity and loss of sitting balance result. If there is doubt about
extending the fusion to the pelvis in a neuromuscular curve, err on the
side of extending the fusion.
In
the nonambulator with pelvic obliquity, a short fusion invariably leads
to curve progression. When the sacrum is included in the fusion, lumbar
lordosis must be maintained. A flat lumbar spine leads to poor sitting
balance, and to skin breakdown in patients with insensate skin.
patients with idiopathic patterns, the rules are similar to idiopathic
curves. In wheelchair-bound patients, the superior extension of the
instrumentation and fusion should be carried high into the thoracic
spine, usually to T-2 or T-3. The underlying problem is poor muscle
tone or muscle imbalance. Failure to extend the fusion proximally will
lead to the development of deformity above the fusion. A progressive
kyphosis above a short lumbar fusion inevitably leads to loss of
sitting balance and risks potential skin breakdown.
treatment is contemplated, carefully analyze the curves in the coronal
and sagittal planes. Preoperative MRI or myelography is warranted. Use
side-bending films to analyze compensatory curve flexibility. Much as
in the patient with idiopathic scoliosis, the fusion must be balanced
over the center of gravity, and compensatory motion must be preserved.
I tend to follow guidelines similar to those given previously in the
section on idiopathic scoliosis. In congenital
scoliosis, curves are variable, so exact guidelines are difficult to
define. Carefully analyze primary and compensatory curves and adhere to
the principles of balancing the fusion mass over the center of gravity
and fusing selectively, and you will generally obtain a good result.
(See Chapter 158 for further details on congenital conditions of the spine.)
an environment favorable for bone maturation and union. For the
surgery, a four-poster frame (a variation of the Hall-Relton frame)
allows the abdomen to fall free, thereby decreasing intra-abdominal and
venous pressure.
-
Position the patient prone on the frame,
avoiding pressure on the brachial plexus and ulnar nerves. Pad the
knees and ankles. When fusion to the lumbar spine or sacrum is planned,
position the patient with the hips extended to maintain normal lumbar
lordosis. -
Prepare and drape the back for an extensive surgical exposure.
-
Make a straight incision and infiltrate the skin with 1:500,000 epinephrine in normal saline to help control bleeding.
-
Use self-retaining retractors for exposure. The pressure from the retractors also helps reduce the capillary bleeding.
-
Use electrocoagulation to control all bleeders.
-
Initiate the subperiosteal exposure of the spine with a sharp Cobb elevator.
gouges, and osteotomes be sharp and well maintained. Avoid excessive
force at all times around the spine! Sharp instruments make gentle
surgery possible.
-
Use the Cobb elevator to dissect the soft
tissues from the spinous processes, lamina, and facets out to the tips
of the transverse processes. Control bleeding with electrocautery and
sponge packs. -
Place a metallic marker on a spinous
process and obtain an intraoperative radiograph to document the
position on the spine. It is amazingly easy to become confused about
the proper spine level without radiographic control. -
Place Adson self-retaining retractors
deep into the wound and clear the facet joints of ligaments and
capsules. Sharp curets facilitate adequate clearing of the soft tissues. -
Bone bleeding can also be controlled with bone wax and Gelfoam patties soaked with topical thrombin.
-
Blood loss is best reduced by meticulous
operative technique and controlled hypotension by an anesthesiologist
experienced in hypotensive anesthesia. (See Chapter 7 for more details.) -
When you have adequate exposure of the
spine and hemostasis, obtain a bone graft from the posterior iliac
crest if autologous bone is to be used for fusion. Either use a
separate incision over the iliac crest or, if the primary surgical
incision is to be carried down to the lumbar spine, the same surgical
incision can be used. A subperiosteal exposure of the iliac crest is
preferred. (For many neuromuscular conditions and for spina bifida, I
have had excellent results using cadaver bone.) -
With a Capner gouge or air-driven impact
osteotome, take cortical and cancellous strips of bone from the outer
table and the underlying cancellous bone. Harvest a generous amount of
bone and carefully store it in saline or cover it with a blood-soaked
sponge. (See Chapter 9 for bone-graft harvesting techniques.) -
Control graft-site bleeding with bone wax.
-
Fuse the facet joints as a standard
procedure, regardless of the instrumentation system used. The long-term
success of any fusion depends on a solid arthrodesis, and it is the
combination of techniques and instrumentation that will accomplish this. -
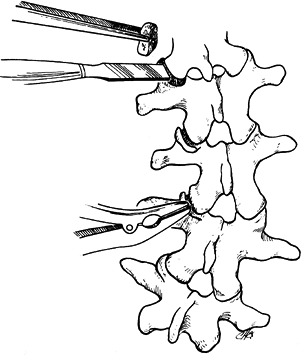
In the thoracic spine, use a Capner gouge
to remove a generous portion of the inferior facets on both the concave
and convex sides of the curve. -
With a curet, remove the visible
cartilage. Then use the gouge to decorticate the superior portion of
the junction of the transverse process and the facet. Pack a generous
piece of cancellous bone into the facet with the Moe bone impactor (Fig. 156.7).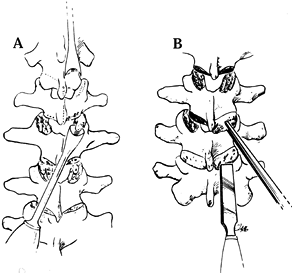 Figure 156.7. The techniques used for excising the thoracic joint (A) and packing with cancellous bone (B).
Figure 156.7. The techniques used for excising the thoracic joint (A) and packing with cancellous bone (B). -
In the lumbar spine, the facet joints can
be excised using a straight ¼-inch Lambotte osteotome or a Lexcel
rongeur. It is important to see the joint and remove the articular
cartilage completely (Fig. 156.8). Then pack a piece of cancellous bone into place with the Moe impactor.![]() Figure 156.8. The technique used for excising lumbar facets and packing with cancellous bone plugs.
Figure 156.8. The technique used for excising lumbar facets and packing with cancellous bone plugs. -
After the facets have been excised and
the bone grafts placed, remove the spinous processes. Complete
decortication of the spine with either a sharp, curved gouge or with an
air-driven impact osteotome. Use extreme caution here, because open
lamina or instrument sites are present and may be entered by the gouge.
Pack the bone graft obtained by decortication of the spinous processes
and iliac crest along the decorticated spine.
-
Develop a skin and subcutaneous flap on the affected side to the posterior axillary line.
-
The ribs supply abundant bone graft and make a separate iliac exposure unnecessary.
-
Take care to avoid entering the pleural
cavity. If the pleural cavity is entered, place a chest tube and
connect it to a closed water-seal suction system. -
Close the wound using a running 0 Vicryl (Ethicon) suture for the fascia.
-
Prior to closure of the wound, I
routinely place a suction drainage system. Place the tubing on the
fascia, not next to the cancellous bone, to avoid excessive drainage of
blood and to prevent clogging of the drain by the bone. -
Close the subcutaneous tissue with a 2-0
Vicryl suture and the skin with a running subcuticular 3-0 Vicryl
suture. The latter makes a good cosmetic closure and avoids the
cross-hatching of interrupted sutures. Relieve skin tension with
adhesive skin tapes and apply a bulky pressure dressing.
instrumentation systems to the forefront, and more and better systems
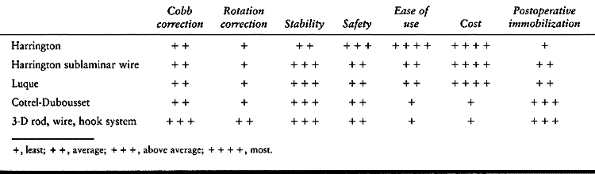
will undoubtedly be developed. Evaluate each system on its own merits (Table 156.5). One system will probably not solve every problem, so you must be facile and knowledgeable in the use of many systems.
 |
|
Table 156.5. Harrington Instrumentation System and Variations
|
successful seem to have similar characteristics. They generally have a
broad range of applicability and usually combine options for the use of
hooks, screws, and wires. Most combine open and closed hooks for ease
of use. It is important that the implant profile is such that it is not
prominent in thin patients.
standard in the treatment of idiopathic scoliosis for many years. New
implant systems using dual rods and multiple points of fixation with
hooks, wires, and screws have replaced the Harrington system. Most
systems have moved away from simple distraction and have evolved into a
more three-dimensional technique using translation, counter torsion,
and limited distraction.
manage with braces or orthoses, and spinal growth is desirable,
instrumentation without fusion can be performed (15,35,36 and 37,40,46).
The technique is useful in small children with infantile idiopathic
scoliosis and in patients with selected neuromuscular conditions in
whom curve control and spinal growth are necessary.
-
Use standard positioning, preparation,
draping, and incision as described previously, but expose the spine
only at the proximal and distal hook sites. Do not expose the remainder
of the spine. -
Confirm proper levels by radiography.
-
I prefer to use one of the new
multi-hooks and rod systems for the implant system. I originally used
the Harrington system, but it allowed for simple distraction only and
could not accommodate more than one hook at the top and one at the
bottom of the instrumented levels. I currently prefer the ISOLA system
(DePuy Acromed, Raynham, MA) because of its low profile and
adaptability for difficult problems. -
Create a claw configuration at the top of
the curve and use a down-going hook at the inferior end of the curve. A
claw can also be created on the inferior end of the curve, if desired.
This will add extra stability (Fig. 156.9, Fig. 156.10).![]() Figure 156.9. Create hook sites in the thoracic spine by excising a portion of the inferior facet.
Figure 156.9. Create hook sites in the thoracic spine by excising a portion of the inferior facet.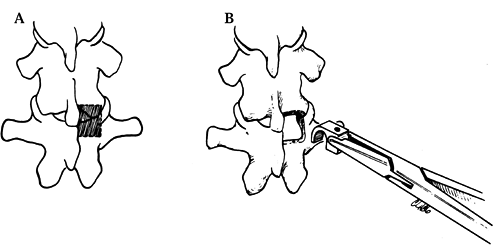 Figure 156.10. A: Position of the laminar Harrington hook. B: The Cotrel-Dubousset laminar hooks are positioned with similar technique.
Figure 156.10. A: Position of the laminar Harrington hook. B: The Cotrel-Dubousset laminar hooks are positioned with similar technique. -
It is possible to use concave and convex rods to enhance correction and control of the curve.
-
Thread the rods beneath the skin and
through the subcutaneous tissues in the middle of the curve, and avoid
subperiosteal stripping, which risks spontaneous fusion. -
Use two rod segments connected by an
axial connector (i.e., a rod sleeve into which both rods fit and are
held in place with screw fixation). Axial connectors allow you to
return to the site in 6–9 months and lengthen the rods without having
to open the entire incision. Such lengthening can often be done as an
outpatient procedure. -
It is also possible to merely leave the
rods long on either end. This allows extra length for subsequent
lengthenings and avoids the need of axial or side-by-side connectors.
expose the superior and inferior hook sites and place bone graft at
these areas. This will create a localized fusion that can be used for
hook placement 4–6 months later, at which time these “platforms” can be
used to establish claw constructs as previously described.
postoperative orthosis. Lengthening is generally necessary every 6–9
months and can be continued until adequate growth has been achieved. At
that time, the definitive spinal fusion and instrumentation can be
completed.
Theoretically, this avoids the need for postoperative immobilization.
Luque has clearly shown that subperiosteal dissection alone does not
alter spinal growth. However, this is a procedure with many
complications. I prefer a modification of the Moe (15) and Marchetti (40) techniques of rodding without fusion.
He originally developed the technique by augmenting standard Harrington
rods with segmental sublaminar wires, then modified it to use dual
smooth L-shaped rods with sublaminar wires and spinal fusion.
newer approaches combined with sublaminar wire fixation. The technique
is excellent for neuromuscular scoliosis, in patients with soft bone,
and in patients where fusion to the sacrum may be necessary. When
fusion to the sacrum is indicated, I combine the modified Luque
technique with Galveston pelvic fixation as described by Allen and
Ferguson (2). I also use pedicle screw fixation
in the sacrum, and I may also use pelvic screws instead of the
contoured smooth rod for pelvic fixation. The addition of sacral screws
has been invaluable in obtaining sacral fixation. The conventional L-shaped
rod with Galveston fixation does not obtain sacral purchase. It really
bypasses the sacrum, crosses the sacroiliac joint, and obtains fixation
in the iliac wings. This has some obvious disadvantages; however, the
fixation to the pelvis combined with screw fixation in the sacrum seems
to give the best purchase and best control of the lumbosacral junction.
positioning, preparation, and surgical exposure as described earlier in
this chapter.
-
Excise the facets at each level and the
ligamentum flavum with a Lexcel rongeur. Kerrison rongeurs can be used
to complete removal of the ligamentum flavum. -
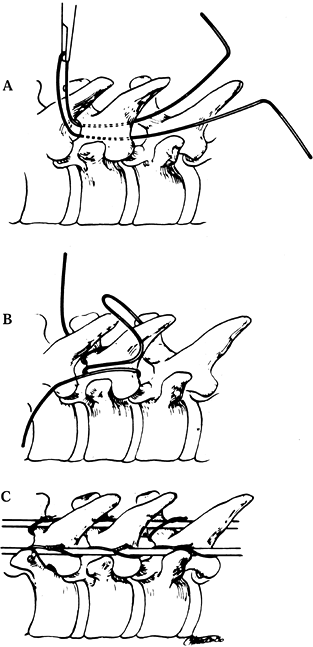
Loop a 16-gauge wire and pass it
carefully under the lamina at each level to be fused. The diameter of
the curve in the wire should be about the same width of the lamina, so
that as the wire passes, it will smoothly pass under the lamina and
then come out over the superior edge of the next lamina (Fig. 156.11, Fig. 156.12).![]() Figure 156.11. Luque wiring; Carefully manage sublaminar wires. A looped wire may be cut to create two wires. A: Carefully pass a looped wire beneath the lamina from inferior to superior, avoiding impingement on the spinal cord or roots. B: Centralize the wire and place a strand of wire on each side of the spinous process. C: Cut the loop of the wire and secure it around the rods on each side.
Figure 156.11. Luque wiring; Carefully manage sublaminar wires. A looped wire may be cut to create two wires. A: Carefully pass a looped wire beneath the lamina from inferior to superior, avoiding impingement on the spinal cord or roots. B: Centralize the wire and place a strand of wire on each side of the spinous process. C: Cut the loop of the wire and secure it around the rods on each side.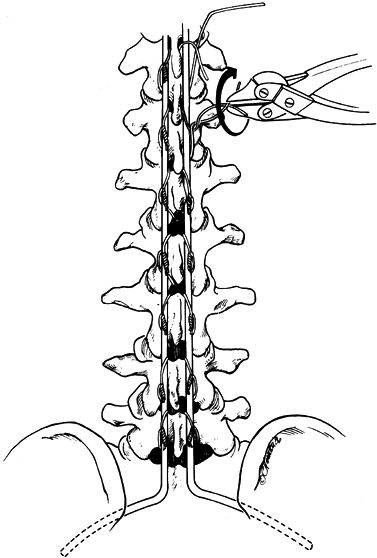 Figure 156.12.
Figure 156.12.
Tighten the wires. The Galveston fixation is positioned between the
inner and outer tables just below the posterior superior iliac spine
and above the greater sciatic notch. See text for details. -
Grasp the wire with a needle holder and
pull it through. Maintain constant upward force, which keeps the wire
taut against the undersurface of the lamina and avoids downward
movement toward the spinal cord. -
Wire management is very important to
avoid deep wire penetration into the spinal canal. I prefer the
technique described by Yngve et al. (65) (Fig. 156.11). -
As the wire comes up, tighten it down over the lamina.
-
After positioning the rod, cut the loop on the wire, creating two separate wires at each level (Fig. 156.11C).
The wires can then be sequentially tightened, bringing the spine to the
rod. This technique decreases the amount of wire handling and tends to
keep the operative field easier to manage.
portion of the rod. Luque described using wires at all levels,
including the top of the rod. However, on occasion a junctional
kyphosis can develop at the superior end of the fusion because of the
removal of the ligamentum flavum and posterior interspinous ligaments.
I now use open hooks at the top two levels to create a claw construct.
-
Place a supra-laminar hook or transverse process hook from cephalad to caudad.
-
Place the up-going hook at the level of the down-going hook, or at the level below (Fig. 156.13).
![]() Figure 156.13.
Figure 156.13.
Create anchor sites at the superior curve and inferiorly at the stable
vertebra in thoracic types II, III, IV, and V curves. See text for
details. (By permission of Dr. Marc Asher.) -
Excise the inferior facet with a ¼-inch osteotome. Remove the cartilage with a sharp curet and position an open hook.
-
As the rod is brought close to the spine
by wire tightening, the rod will come into the open hooks and then can
be secured with hook caps (Fig. 156.14, Fig. 156.15).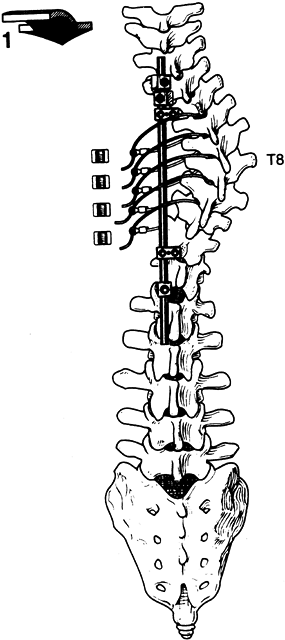 Figure 156.14. Place sublaminar wires at the apex to help create translation forces. See text for details. (By permission of Dr. Marc Asher.)
Figure 156.14. Place sublaminar wires at the apex to help create translation forces. See text for details. (By permission of Dr. Marc Asher.)![]() Figure 156.15. Wire tightening. (By permission of Dr. Marc Asher.) See text for details.
Figure 156.15. Wire tightening. (By permission of Dr. Marc Asher.) See text for details.
surgeon’s own personal preference. At the inferior level of the sacrum,
use sacral screw fixation in addition to the Galveston or pelvic screw
fixation. The sacral screws link the ilium and the sacrum and help
reduce the amount of stress on the pelvis. I have noted significantly
less bone absorption around the pelvic posts with the addition of
sacral screw fixation, especially in patients with neuromuscular
scoliosis and soft bone.
-
Identify the pedicle and probe it with the gear shift probe.
-
Confirm the placement with a ball-tipped pedicle probe and lateral roentgenogram.
-
I generally use bicortical purchase.
-
Tap for the screw, and again probe, and then position a screw of the appropriate length and diameter.
-
Confirm screw placement with a roentgenogram or fluoroscopic image.
-
Once the screws have been placed, attach
them to the rod using slotted connectors. If the angle is difficult to
accommodate with rod contouring, use a variable-angle slotted connector.
which can be attached to the rod by a slotted connector. I generally
expose both iliac wings through the midline incision. Adequate exposure
is necessary to visualize the greater sciatic notches bilaterally. The
entry point for the L portion of the rod is usually just below the posterior superior iliac spine (PSIS).
-
Make a hole in the superior border of the posterior ileum just below the PSIS with a 3/16– or ¼-inch drill, depending on the size of the rod chosen.
-
Insert the rod between the tables of the
ileum so that it lies 1–1.5 cm above the greater sciatic notch. The
length of the rod or screw is usually 6–8 cm, depending on the size of
the patient. -
Rod contour is important to correct the
deformity and maintain normal sagittal balance. I generally use a
malleable template to help establish the appropriate bend. The rods are
then contoured using the Cotrel-Dubousset French bender and the Asher
ISOLA bending irons. -
After appropriate bending, position the rods and sequentially tighten and secure the wires (Fig. 156.16, Fig. 156.17 and Fig. 156.18).
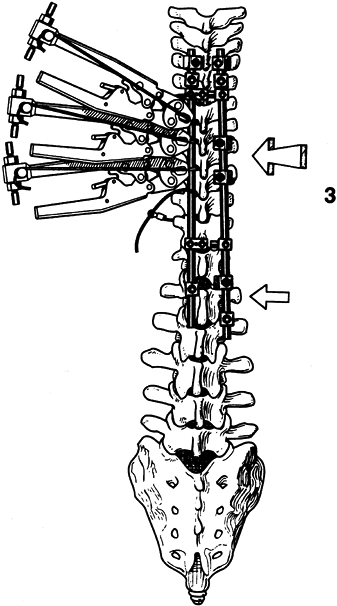 Figure 156.16. Place a convex rod to create a countertorsion force. (By permission of Dr. Marc Asher.) See text for details.
Figure 156.16. Place a convex rod to create a countertorsion force. (By permission of Dr. Marc Asher.) See text for details.![]() Figure 156.17. Complete tightening of the wires. (By permission of Dr. Marc Asher.) See text for details.
Figure 156.17. Complete tightening of the wires. (By permission of Dr. Marc Asher.) See text for details.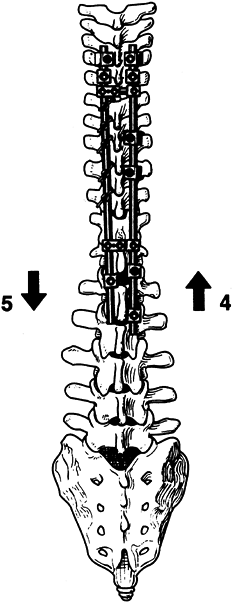 Figure 156.18.
Figure 156.18.
Application of concave distraction and convex compression complete the
instrumentation sequence. See text for details. (By permission of Dr.
Marc Asher.) -
After the hardware is secured and all
wires are tightened, decorticate the remaining exposed transverse
processes and lamina using gouges and rongeurs. Take great care to
avoid penetrating the open laminotomies. -
Carefully pack autogenous iliac bone or banked bone around the decorticated spine.
-
Close the wound using a drain above the fascia as described previously.
reported a 10% pseudarthrosis rate when no postoperative bracing was
used, and a near-zero incidence with bracing. In my experience, a
custom-molded, bivalved, polypropylene brace prevents loss of
correction and makes most patients more comfortable and more mobile
postoperatively.
introduced a spine implant system that combined segmental fixation with
distraction compression and derotation of the spine. Their system
consisted of a series of open and closed hooks and a knurled rod with a
diamond-patterned surface. It allowed the two rods to be connected by
cross-links, increasing the overall stability of the system. Cotrel and
Dubousset stressed the importance of maintaining and creating normal
sagittal contours. Their system of rotation and Luque’s concept of
translation were the first efforts to move away from the old concept of
pure distraction and move toward an attempt to obtain three-dimensional
spine correction. As the popularity of the Cotrel-Dubousset system
grew, others started to develop their own implant systems. Most of the
new systems have been based on the concept of flexibility in use and
the ability to fix to the spine with multiple points of fixation. Most
use hooks, wires, and screws, in combination, as the means
of spine fixation. Virtually all of the systems, when used properly, will give good spine correction and excellent results.
their application is complex. When first using these systems, obtain
hands-on training with surgeons who are experienced with them.
and wires to rigidly stabilize the cross-linked dual rods, the need for
bracing is diminished, which is a huge advantage for the patient.
Bracing is occasionally used for patients who have soft bone or for
whom compliance is questionable. I have used bracing to control an
unfused lumbar curve, surgically treating the thoracic curve and using
bracing to control the unfused segment. The criteria for discontinuing
the use of a brace are those used in a standard brace program.
been controversial. I select fusion levels based on the criteria
established by King et al. (29). Early in the
Cotrel-Dubousset experience, there were numerous reports of lumbar
curve decompensation after selective thoracic fusion (10,33,41).
These problems had not been noted in series reporting the use of
Harrington instrumentation. Cotrel and Dubousset have proposed that
instrumentation levels should include all sagittally abnormal zones,
create rotational neutralization, and finish in the Harrington stable
zone. This frequently necessitates fusion into the upper lumbar spine.
Thompson et al. (58) and Wood et al. (63,64)
have reported data suggesting that overrotation of the thoracic curve
locks the transitional thoracolumbar rotation segments, making it
impossible for the lumbar curve to balance the thoracic spine. This
situation leads to a shift of the thoracic curve to the left and
frequently causes progression of the lumbar curve. Richards et al. (52) and Benson et al. (7)
have shown that large curves and less flexible lumbar curves are more
likely to decompensate with standard Cotrel-Dubousset techniques.
Richards et al. believe that in thoracic curves greater than 60°, with
a lumbar component greater than 45°, lumbar decompensation may occur
with selective thoracic fusion (52). They suggested fusion of both curves when the thoracic and lumbar curves are large.
advised caution when correcting the primary curve. They stated that the
primary curve must not be corrected beyond the compensatory curve’s
ability to correct and balance the spine. It is important to realize
that these words were written prior to the advent of internal fixation;
with advanced corrective devices they are more timely than ever! Massey
et al. (42) have reported on their series of
fusions using Cotrel-Dubousset instrumentation and fusing to levels
selected according to King’s criteria. They found no problems with
lumbar decompensation as long as no attempt was made to overcorrect the
thoracic spine. Previous implant systems have not provided the powerful
corrective forces that the newer systems offer. We may have temporarily
lost sight of the importance of spinal stability and balance. Our
patients are not as concerned about the number of degrees of correction
as they are about the appearance of a balanced stable spine. We must
remember the wisdom of Von Lackum and Miller (59).
of scoliosis correction. Asher has done extensive three-dimensional
studies to better understand and explain the findings noted in
idiopathic scoliosis. In a report of his three-dimensional studies of
the scoliosis deformity, Asher and Cook (5)
found that in progressive curves the apex vertebra tends to displace
laterally and posteriorly, and that the spine tends to collapse toward
the midcoronal
plane as the curve progresses. This was consistent with Pedriolle and Vidal’s (50)
concept of the evolution of scoliosis in the transverse plane. Asher
believes that this fits well with the engineer’s concept of scoliosis
as a geometrical torsion or a property of a helical line. This is in
distinction to a mechanical torsion in which two objects immediately
adjacent to each other are rotated on each other. It is Asher’s idea
that scoliosis develops as a series of imperfect torsions. He notes
that the apical vertebra did not always torque posteriorly in
thoracolumbar curves. He believes that there are many reasons for these
findings, including rib cage constraints, soft tissues, and asymmetry
of motion segments (3,4,5 and 6).
include translation and countertorsion if we hope to correct the true
complex scoliosis deformity described by Asher. I have tended to drift
away from the derotation maneuver described by Cotrel and Dubousset
because of the problems seen with lumbar decompensation in type II
curves and the intuitive notion that the derotation movement is done in
the same counterclockwise motion that scoliosis tends to develop in.
the days of Harrington instrumentation. I still use the concept of
selective fusion in thoracic curve patterns to avoid unnecessary fusion
of the lumbar spine. The fusion should end at the stable vertebra
unless that vertebra is at the apex of a kyphosis. If the stable
vertebra is located at the apex of those segments, variation of
standard hook-and-wire patterns may be applied in compression across
the kyphotic segments to correct the abnormal segment (23).
smooth operation (it helps avoid confusion for the surgeons and nursing
staff) and a good result. One may choose to label the hook-and-wire
sites on the preoperative roentgenogram, or know in advance where sites
might be most appropriate. The predetermined hook sites, however, may
need to be altered in response to findings during surgery.
basic hook-and-wire placement, which is useful in types II, III, IV,
and V curves. The type V curve differs only in that the upper stiff
thoracic curve must also be instrumented and fused to avoid an
objectionable shoulder asymmetry. I generally choose the upper level of
instrumentation as the upper level measured
by the Cobb method, or a level that is closest to the midline (Fig. 156.6).
The upper level of instrumentation is generally T-3 or T-4. I currently
use the ISOLA system, which uses open and closed hooks, wire, and screw
fixation.
-
Prepare the upper facets on both sides of
the superior vertebra by removing 4 mm of the inferior facet with a
¼-inch osteotome. Remove the cartilage with a curet. -
Position an appropriate-size hook in the up-going position (Fig. 156.9). The hook should have a nice snug fit, and the throat of the hook should allow adequate room around the facet.
-
On both sides of the upper vertebra,
create a “claw” by placing a closed hook on a holder, placing the foot
of the hook on the superior edge of the transverse process, and
allowing the hook to slide around the process (Fig. 156.19). This should give firm fixation on the vertebra.![]() Figure 156.19.
Figure 156.19.
Type I thoracic and lumbar curve screw foundation wire fixation on
thoracic cavity. See text for details. (By permission of Dr. Marc
Asher.) -
I generally use an intermediate up-going
pedicle hook on the concave side of the curve, one or two levels below
the upper end vertebra. This hook is positioned in a similar manner. -
On the convex side at the apical vertebra, create another claw to assist in the countertorsion movement with the second rod.
-
Position the inferior hook on the concave
side after a limited laminotomy is created. It is important that the
laminotomy be large enough to position the hook, but small enough to
prevent deep penetration of the hook into the spinal canal. -
I generally use a closed hook with an appropriate radius to just fit around the lamina (Fig. 156.10).
inferior end vertebra for right thoracic curves. I have experience with
this technique in types III and IV curves but have not used it in type
II curves because the lower thoracic pedicles in adolescents tend to be
quite small. The lumbar pedicles, however, can be quite large and
adequate for screw placement. The advantages of screw fixation are the
secure purchase and the translational control that can be achieved.
-
At this point in the procedure,
segmentally secure the apical three or four vertebra with sublaminar
wires or cables. I use the same technique described in the Luque
fixation section (Fig. 156.19). -
After the wires have been passed, excise the concave facets and graft bone.
-
Fashion and contour an appropriate length
of rod using French and tube benders. (A malleable template is useful
to obtain rod length and contour.) -
Feed the rod up through the upper hooks and then rotate into the appropriate plane (Fig. 156.20). Then feed the rod down into the inferior hook.
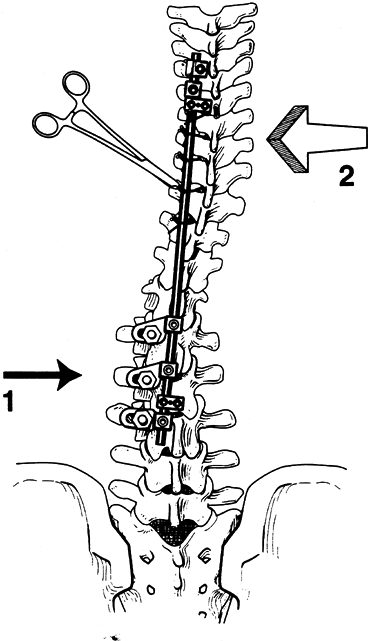 Figure 156.20. Thoracic concave rod placement wire tightening. See text for details. (By permission of Dr. Marc Asher.)
Figure 156.20. Thoracic concave rod placement wire tightening. See text for details. (By permission of Dr. Marc Asher.) -
The upper claw can then be secured with the compression device and the setscrews tightened (Fig. 156.21).
![]() Figure 156.21.
Figure 156.21.
Claw compression wire tightening creates translation of the thoracic
spine, and rod rotation creates countertorsion in the lumbar spine. See
text for details. (By permission of Dr. Marc Asher.) -
Sequentially tighten the wires or cables, translating the spine toward the rod (Fig. 156.22).
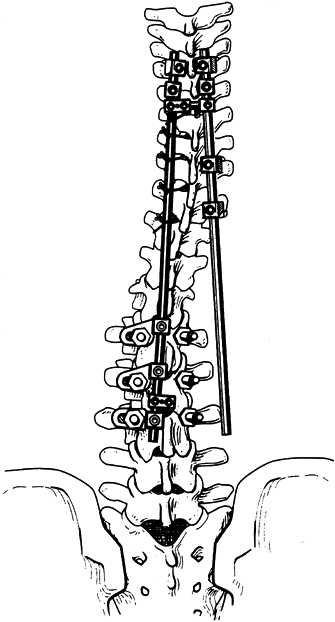 Figure 156.22.
Figure 156.22.
The opposite rod creates countertorsion in the thoracic spine and
translation in the lumbar spine. See text for details. (By permission
of Dr. Marc Asher.) -
I generally favor wires because of their low cost and ease of use. Braided cables are an alternative.
-
After the wires have been tightened, complete distraction and secure all setscrews.
-
Position the convex rod after facets have been excised and bone grafted (Fig. 156.22).
-
Pass the rod through the upper hooks, place the intermediate hooks on the rod, and bring the positioned rod
P.4048
down to the spine. The positioning of the rod serves as a
countertorsion movement and tends to push the spine out of rotation and
toward the midline (Fig. 156.23).![]() Figure 156.23.
Figure 156.23.
Rod sequence is completed. The screws allow a solid inferior anchor and
move corrective forces anteriorly into the vertebral body. See text for
details. (By permission of Dr. Marc Asher.) -
Place an upward-directed hook under the inferior lamina and advance the rod to engage the hook.
-
After the rod is positioned, apply
compressive forces to complete the sequence. The sublaminar wires can
be retightened; revisit and tighten all setscrews a final time. -
I use cross links to create a rectangle and increase the strength of the system (Fig. 156.24).
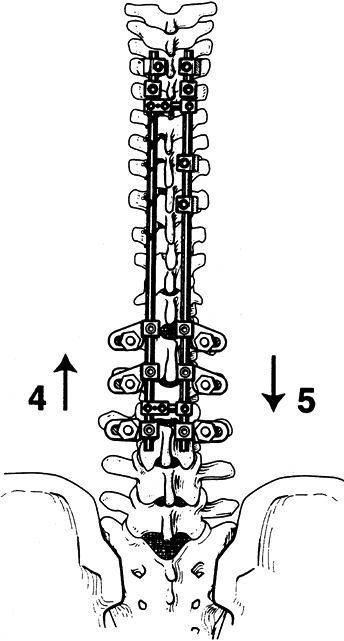 Figure 156.24. Compression and distraction are completed. See text for details. (By permission of Dr. Marc Asher.)
Figure 156.24. Compression and distraction are completed. See text for details. (By permission of Dr. Marc Asher.)
lumbar curve must be able to accommodate the corrected thoracic curve.
Type II curves require particular care and caution. Overcorrection of
the thoracic curve can lead to lumbar curve decompensation. I have had
minimal problems with lumbar curve decompensation using the translation
and countertorsion technique. It seems to cause far fewer problems than
the old derotation technique that was based on the techniques of Cotrel
and Dubousset. Particularly in the type II curves, we use
instrumentation and fusion to treat the main thoracic curve, and then a
thoracolumbosacral orthosis (TLSO) to treat the unfused lumbar curve.
This has been an effective technique for young patients with a Risser
sign of 0 to 2 where growth is anticipated and possible progression of
the lumbar curve might be expected.
treated with a technique different from that used for thoracic
scoliosis curve types. In type I curves, fuse both thoracic and lumbar
curves, choosing the upper level of fusion
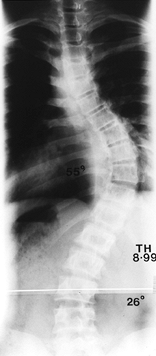
as for the type II to IV curve patterns. (Fig. 156.25, Fig. 156.26, Fig. 156.27, Fig. 156.28, Fig. 156.29 and Fig. 156.30 show typical cases.)
 |
|
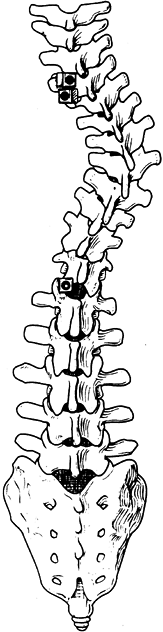
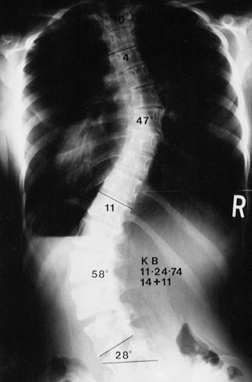
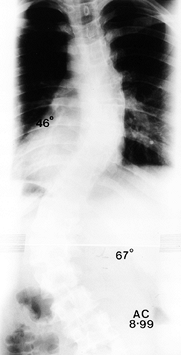
Figure 156.25. Preoperative AP radiograph of the right thoracic curve.
|
 |
|
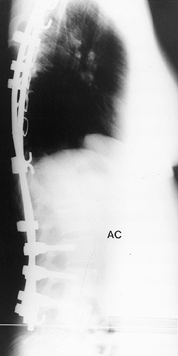
Figure 156.26. Postoperative AP radiograph of the right thoracic curve.
|
 |
|
Figure 156.27. Postoperative lateral radiograph of the right thoracic curve.
|
 |
|
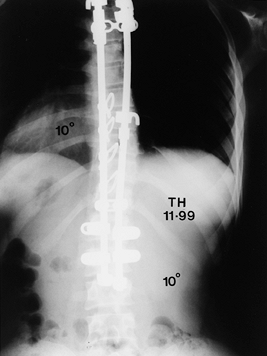
Figure 156.28. Preoperative AP radiograph of type I thoracic and lumbar curves.
|
 |
|
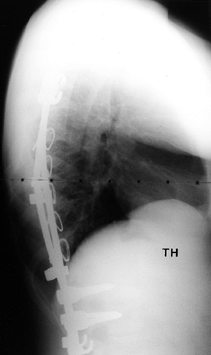
Figure 156.29. Postoperative lateral radiograph of type I thoracic and lumbar curves.
|
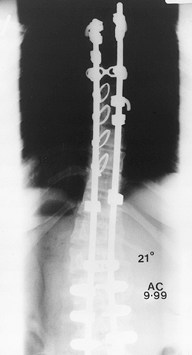 |
|
Figure 156.30. Postoperative AP radiograph of type I thoracic and lumbar curves.s
|
-
First, create bilateral claws at the
upper level of the fusion, and place a claw or up-going hook at the
apex of the thoracic curve. -
At the inferior level of the thoracic curve, place a down-going hook; it is usually preferred to use an open hook at this level.
-
On the convex side of the lumbar curve,
screw fixation is preferable to hooks, as it seems to give a better
base for correction and avoids possible hardware displacement. -
The thoracic curve is generally managed with sublaminar wires.
-
Place the concave thoracic and convex lumbar rod first.
-
Before tightening the setscrew, rotate
the rod counterclockwise. This rotation movement does not change the
thoracic curve much, but it does effectively translate and
countertorque the lumbar curve. -
Place the second rod and anchor it superiorly with hooks.
-
Use an up-going hook at the thoracolumbar junction.
-
Use sublaminar wires on the concavity of the lumbar curve, and generally use screws at the last two or three levels.
-
Place the second rod with wires or screws in the lumbar curve.
-
Use compression along the convexity of the thoracic curve and distraction along the concave lumbar curve.
-
Apply transverse connectors and retighten all set- screws.
When fusions must be extended into the lumbar spine, maintain the
normal lordosis to avoid the creation of flatback syndrome.
been encountered, a bivalved TLSO brace is used. For patients in whom
firm fixation is achieved, no postoperative immobilization is required.
Mobilize patients on the first or second postoperative day. Encourage
them to start walking early, and let them return to school when
comfortable. Generally, hold them out of sports for 6–9 months.
that can be applied to many spine problems. Evolution of implant
systems is inevitable, and with each new change we are better able to
manage more complex deformities.
attention to detail is as necessary for this phase as it is for the
preoperative planning and the surgical procedure.
hospital bed (rather than turning frames or other rotating beds), as
patient acceptance and nursing familiarity make this an excellent
choice. Use foam pad and sheepskins to facilitate skin care. In the
immediate postoperative period, log-roll the patient every 1–2 hours to
prevent skin sores, improve ventilation, and reduce pulmonary
complications.
administer intravenous fluids and limit oral intake. Avoid early oral
feeding because postoperative ileus is common. An aggressive pulmonary
care program with frequent coughing, deep breathing, and the use of an
incentive spirometer is a standard part of the postoperative program.
For patients with reduced pulmonary capacity, a respiratory therapist
can be called in to use suction and a more aggressive pulmonary program.
plastic bivalved body jacket. It is easy to use and is equally well
tolerated by patients with idiopathic scoliosis and those with a wide
variety of neuromuscular conditions. If patient compliance is
questionable, a more standard Risser cast can be used successfully. A
cervicothoracolumbosacral orthosis (CTLSO) is helpful when
instrumentation is carried into the high thoracic spine. In patients
with neuromuscular curves or when instrumentation high into the
thoracic spine necessitates immobilization, a
sternal-occipital-mandibular immobilization (SOMI) device can be added
to a bivalved jacket. Maintain postoperative immobilization for 4–6
months. The removable jackets make postoperative hygiene and skin care
much easier; allow compliant patients to shower without the brace after
the first few weeks. The multi-hooks and rod systems require no
postoperative immobilization.
patients to increase their aerobic activities by walking, gradually
increasing to several miles per day after the first 2 weeks. Their
endurance and normal physiologic functions improve more rapidly with a
regular walking program.
noncontact sports after the first month if wearing a postoperative
orthosis. For a patient with a multi-hooks and rod system, noncontact
sports are not allowed for 3–4 months unless the patient is wearing a
body jacket.
discharged from the hospital. This set of films establishes the amount
of correction and confirms adequate positioning of the hardware.
Virtually all hardware pullout occurs in the early postoperative
period. Have the patient return 3–4 weeks after discharge for
posteroanterior and lateral radiographs to confirm curve stability and
hardware position. Take subsequent films at 3 and 6 months. Also take
oblique radiographs at the 6-month visit to check the maturity and
integrity of the spinal arthrodesis. If the fusion is questionable or
unclear on standard radiographs, obtain tomograms. If poor
incorporation of the graft is noted, additional immobilization or
augmentation of the fusion with additional bone graft may be necessary.
potential for complications. Many can be avoided by careful planning
and surgical execution. Others, however, occur despite the best efforts.
transfusion is to minimize intraoperative blood loss. Proper
positioning, hypotensive anesthesia, and careful surgical technique are
the cornerstones of minimizing blood loss. I have used preoperative
autologous blood donations for about 5 years. Preoperative donations
can begin 3–4 weeks before surgery. Iron supplementation is helpful to
maintain adequate red blood cell production. With a good autologous
blood program, banked blood can generally be avoided. The risks of
human immunodeficiency virus (HIV) transmission from bank blood are
low, but they can be eliminated with an autotransfusion plan. In cases
where blood loss is expected to be high (e.g., in osteotomy or
reconstructive procedures), another cost-effective and safe way to
reduce the need for blood transfusion is intraoperative blood salvage
with a cell-saver.
immediate hematologic workup, even during the procedure, is mandatory.
Transfusion of platelets and appropriate clotting factors can be
lifesaving. Fortunately, in my experience, major blood loss is rare if
attention to detail is observed.
complications in treating scoliosis. The neurologic injury may occur
from direct trauma to the cord by instruments, hooks, or sublaminar
wires, or by stretch of the spinal cord. Stretch of the spinal cord is
presumed to cause vascular compromise that leads to neurologic loss.
Certain techniques seem
to
carry more risks. The 1997 Morbidity and Mortality Report (available
from the Scoliosis Research Society) reported an incidence of spinal
cord injury during adolescent idiopathic scoliosis surgery of 0.28%.
There did not seem to be a correlation based on the type of implant
used. A 0.14% incidence of nerve root injury was also noted.
correction of scoliosis. This is most frequently seen in large rigid
curves but can occur with the manipulation and correction of any curve.
Congenital scoliosis carries a higher risk of neurologic injury; these
curves tend to be rigid and may be associated with cord tethering by
bone spurs, fibrous bands, or a tight filum terminale. As discussed
earlier, we recommend the use of MRI prior to the surgical treatment of
congenital curves.
to assess neurologic function after instrumentation and before sending
the patient to the recovery room. In this test, the patient is
momentarily awakened from anesthesia and asked to move her toes. Lack
of volitional movement calls for careful examination of the procedure
and elimination of any potential cause of neurologic impairment.
Because of the anesthetic medication, the patient usually neither
recalls the wake-up test nor mentions that she felt pain during the
procedure. I usually use electronic spinal cord monitoring with sensory
evoked potentials (SEP) during surgery. This gives continuous readouts
of cord function without waking the patient, with the risks of
extubation and the increased bleeding that occur when the blood
pressure rises during wake-up. I had false-positive readings during my
early experience with SEP, so the wake-up test was used to confirm that
no neurologic deficits were present. With added experience and the use
of multiple recording sites (epidural, scalp, and cervical), the SEP
has proved to be reliable, and it is most helpful during long, involved
reconstruction procedures.
with the anesthesia staff, because many of the popular anesthetic
agents interfere with accurate monitoring. If a neurologic deficit is
noted during or after surgery, reduce the traction force on the spine.
This usually necessitates releasing distraction and may require
hardware removal. If a severe neurologic deficit is noted in the
recovery room or after surgery, immediately return the patient to the
operating room for wound exploration and probable hardware removal.
Laminectomy is not usually indicated unless an epidural hematoma is
identified or bone is encroaching on the cord. If early sublaminar wire
removal is indicated, the technique should be that of cutting one end
of the wire close to the lamina, grasping the other end, and pulling
straight up. The work of Nicastro et al. (49) shows this to be least likely to cause the wire to push down into the spinal cord.
postoperative antibiotics, postoperative wound infection should be 1%
or less. If an early infection occurs, prompt wound debridement is
indicated, and a successful spinal correction and fusion can be
salvaged.
-
Obtain blood cultures before surgery.
-
Take the patient to the operating room,
where, under anesthesia, the skin is prepared and draped, and the
entire wound is opened. Do not disrupt the hardware and bone graft. -
Obtain cultures.
-
Carefully debride the hematoma and
nonviable tissue, followed by irrigation with antibiotic solution.
Leave the bone graft intact. -
Generally, avoid large retention sutures.
-
Close the wound in layers over suction irrigation tubes that are left in for 3–5 days.
-
Administer appropriate intravenous
antibiotics for 7–10 days; then, if antibiotic sensitivity allows,
change to an oral agent for an additional 6 weeks.
placing hardware, a dural tear is noted by the leakage of clear
cerebrospinal fluid, repair it immediately. Patients fare better with
early repair than with later repairs of chronic leaks. Perform a
limited laminotomy, or laminectomy if necessary, to expose the dural
tear. Once the extent of the tear is identified, repair by careful
suturing with a fine, nontraumatic needle and 5-0 or 6-0 nylon. If
large tears are noted, it may be necessary to perform a fascial graft.
the lumbodorsal fascia is a good source for a patch graft. Keep
patients with this complication at bed rest for several days after
repair.
It creates an unsightly cosmetic deformity and leads to back pain. This
flattening of the lumbar spine is associated with instrumentation of
the lumbar spine and can be created by using noncontoured Harrington
rods (15). The best way to prevent this
iatrogenic problem is to avoid fusion of the lumbar spine when
possible. As described earlier in this chapter, selective thoracic
fusion is the best way to maintain lumbar motion. When fusion into the
lumbar spine is necessary, position the patient on the operating table
to maintain lordosis and contour the rods. When fusion to the sacrum is
indicated, the Luque rods with Galveston pelvic fixation and sacral
screws are the best means of creating or maintaining lumbar lordosis.
on obtaining a solid fusion. After 4–6 months, perform an evaluation
for a solid arthrodesis. Take oblique views and carefully review the
films for lucent lines in the fusion mass. If broken hardware is noted,
a pseudarthrosis is highly likely.
radiographs demonstrate a pseudarthrosis, open and explore the entire
wound. The pseudarthrosis may be covered by a fine layer of bone.
Therefore, careful evaluation is mandatory. The pseudarthrosis is
generally difficult to expose and will show motion when stressed.
Freshen the bone with a gouge, and instrument the pseudarthrosis with a
heavy Harrington compression system. The multi-hooks and rod system can
also be used to apply compression. Apply generous quantities of local
and iliac bone graft to complete the procedure. I immobilize the
patient for 4–6 months in a bivalved body jacket. Lumbosacral
pseudarthroses that have failed repeated attempts at repair may be best
managed by an additional anterior fusion. It is mandatory that solid
arthrodesis be achieved if you hope to obtain a stable balanced spine.
preoperative evaluation and planning. Curve types and patterns must be
carefully identified to select fusion levels. Spinal fusion and
instrumentation techniques are demanding and should be carefully
performed. The surgeon should have hands-on experience with these
systems before initiating the procedure. The keys to success are
attention to detail and skillful surgical execution. The new implant
systems seem to offer better correction and more options in treatment,
but they do not replace the need for good fusion techniques.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
MA, Cook L. The Transverse Plane Evolution of the Most Common
Adolescent Idiopathic Scoliosis Deformities. A Cross-Sectional Study of
181 Patients. Spine 1995;20:1386.
MA, Strippgen WE, Heinig CF, Carson WL. ISOLA Spinal Instrumentation:
Emphasizing Application during the First Two Decades of Life. In:
Weinstein SC, ed.]The Pediatric Spine: Principles and Practice. New York: Raven Press, 1999:1619.
L, Ibrahim K, Goldberg B, Harris G. Coronal Balance in Cotrel-Dubousset
Instrumentation: Compensation vs. Decompensation. Presented at the
Scoliosis Research Society Annual Meeting, Honolulu, September, 1990.
KH, McAllister JW, Betz RR, et al. Coronal Decompensation Produced by
Cotrel-Dubousset “Derotation” Maneuver for Idiopathic Right Thoracic
Scoliosis. Spine 1991;16:769.
T, Irstam L, Nachemson A. Long Term Anatomic and Fuctional Changes in
Patients with Adolescent Idiopathic Scoliosis Treated by Harrington Rod
Fusion. Spine 1983;8:576.
J, Winter R, Grobler C, et al. Harrington Instrumentation without
Fusion Combined with Milwaukee Brace for Difficult Scoliosis Problems
in Young Children. Orthop Trans 1979;3:59.
K, Goldstein LH, Femi-Pearse D, Yu PN. Pulmonary Function in Idiopathic
Scoliosis: Comparitive Evaluation Before and After Orthopaedic
Correction. J Bone Joint Surg Am 1968;50:1391.
GL, Lenke LG, Bridwell KH, et al. The Use of Pedicle Screw Fixation to
Improve Correction in the Lumbar Spine of Patients with Idiopathic
Scoliosis: Is It Warranted? Spine 1996;21:1241.
LG, Bridwell KH, Blanke K. Preventing Decompensation in King Type II
Curves Treated with Cotrel-Dubousset Instrumentation. Spine 1992;17:5274.
JT, Herndon CH, Inkley S, et al. Pulmonary Function in Paralytic and
Non-Paralytic Scoliosis Before and After Treatment. A Study of
Sixty-Three Cases. J Bone Joint Surg Am 1968;50:1379.
TB, Winter RB, Lonstein JE, Denis F. Selection of Fusion Levels with
Special Reference to Coronal and Sagittal Balance in Right Thoracic
Adolescent Idiopathic Scoliosis Using Cotrel-Dubousset Instrumentation.
Presented at Scoliosis Research Society Annual Meeting, Honolulu,
September, 1990.
JF, Hartjen CA, Traina J, Lancster JN. Intraspinal Pathways Taken by
Sublaminal Wires during Removal. An Experimenatl Study. J Bone Joint Surg Am 1986;68:1206.
RC, Dickson JH, Harrington PR, Erwin WE. Results of Harrington
Instrumentation and Fusion in the Adult Scoliosis Patient. J Bone Joint Surg Am 1975;57:797.
BS, Burch JG, Herring JA, et al. Frontal Plane and Sagittal Plane
Balance Following Cotrel-Dubousset Instrumentation for Idiopathic
Scoliosis. Spine 1989;14:733.
KB, Olesweski JM, Schendel MJ, et al. Rotational Changes of the
Vertebral Pelvic Axis after Sublaminar Instrumention in Adolescent
Idiopathic Scoliosis. Spine 1997;22:51.
KB, Transfeldt EE, Ogilvie JW, et al. Rotational Changes of the
Vertebral-Pelvic Axis Following Cotrel-Dubousset Instrumentation. Spine 1991;16:5401.