SOFT-TISSUE SARCOMAS AND BENIGN SOFT-TISSUE TUMORS OF THE EXTREMITIES
VII – NEOPLASTIC, INFECTIOUS, NEUROLOGIC AND OTHER SKELETAL DISORDERS
> Tumors and Tumor-Like Conditions > CHAPTER 129 – SOFT-TISSUE
SARCOMAS AND BENIGN SOFT-TISSUE TUMORS OF THE EXTREMITIES
University of Chicago Hospitals and Clinics, Department of Surgery,
Section of Orthopaedic Surgery and Rehabilitation Medicine, Chicago,
Illinois, 60637.
the extremities are a common physical finding. Whereas most are
posttraumatic or inflammatory in nature, a certain number later prove
to be benign or malignant soft-tissue neoplasms. Soft-tissue masses
present a diagnostic and therapeutic challenge to the treating surgeon.
Some require only observation, whereas others should be imaged with
appropriate methods and subsequently undergo pathologic evaluation. The
difficulty is knowing what features of the patient’s soft-tissue
mass—for example, size, depth, or texture—are important in
differentiating benign from malignant disease. A consistent,
comprehensive, and educated approach to the evaluation and treatment of
a soft-tissue mass will allow the surgeon to overcome this difficulty
and provide optimal care as well as avoid an inaccurate diagnosis and
excessive or inadequate treatment.
of value. Cognitive and emotional issues, especially anxiety, denial,
or ignorance, often cloud a patient’s memory and reporting of events
related to the tumor. Patients usually are unable to give an accurate
estimate as to how long the mass has been present or its growth
characteristics. Often, there is a history of trauma to the region that
has brought the mass to the patient’s attention. Symptoms may or may
not be present in both malignant and benign tumors; however, you are
more likely to investigate and eventually treat masses that are
symptomatic.
diagnosis, except for patients with multifocal disease or those who
have an established clinical syndrome. Exceptions include a familial
history of lipomatosis, neurofibromatosis, Gardner’s syndrome (colonic
polyposis and desmoid tumor), Li-Fraumeni syndrome (familial breast
cancer and soft-tissue sarcoma) (16), and Maffucci’s disease (hemangiomas noted in conjunction with multiple bone enchondromas).
diagnostic. Tumor size, depth, and consistency can only be approximated
in all but very small subcutaneous masses. It is uncommon for a mass to
be pulsatile, indicating an underlying vascular neoplasm, or for
Tinel’s sign to be present in association with peripheral nerve sheath
tumors.
evaluation of a patient with a soft-tissue mass is discerning which
lesions should simply be observed and which should undergo further
evaluation with imaging studies and possible biopsy. A suggested
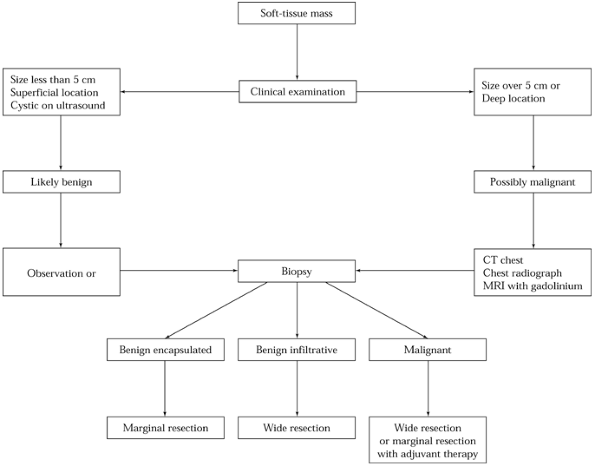
strategy is delineated in Figure 129.1 (19). As can be seen, tumor size and tumor depth are important features (18,24). Large or deep tumors should be considered malignant until imaging studies or biopsy prove otherwise.
 |
|
Figure 129.1. Flow diagram of a suggested strategy for the evaluation and treatment of a patient with a soft-tissue mass.
|
are rarely diagnostic. Occasionally, a relative radiolucency is seen in
the soft tissues compared with the surrounding tissues. This would
suggest the presence of fat and an intramuscular lipoma. Phleboliths
may be seen with hemangiomas; however, other forms of mineralization in
the form of calcification or ossification are nonspecific and may
suggest either underlying myositis ossificans, atypical infection
(parasitic), or synovial sarcoma.
helpful in locations about joints (ganglion or popliteal cysts) and in
the evaluation of the fluid content of a soft-tissue mass. Purely
cystic masses are not likely to be malignant. Ultrasound is also useful
in guiding the performance of an aspiration or biopsy, with cyst
contents sent for cytologic evaluation.
can be observed at intervals of several months to detect any change in
size. Even if the underlying lesion proves to be malignant, the
prognosis for survival as well as for local control is excellent (20,30).
If the mass grows or symptoms develop, perform a magnetic resonance
imaging (MRI) scan, followed by biopsy and possible excision.
the extremities. If the mass has signal characteristics identical to
those of subcutaneous fat on all sequences and does not enhance with
gadolinium, it is most likely to be a lipoma. Heterogeneous, large, or
deep masses, especially those with a low signal on T1-weighted images
and a higher signal on T2-weighted images, or those with gadolinium
enhancement, should be suspected to be soft-tissue sarcomas. Additional
imaging tests may be indicated, depending on the clinical diagnosis.
Note that any local imaging study will have real and artifactual
changes introduced by the performance of any operative procedure,
including a biopsy; this will make their interpretation difficult.
Therefore, perform all local imaging studies before any operative
intervention.
location of soft-tissue tumors in the extremities but is more valuable
in the pelvis. In many cases, CT scans reveal the proximity of the
tumor to bone, nerve, and vascular structures; they also accurately
indicate fat density. Lipomas usually can be differentiated from other
tumors. CT scans are also important in the staging of malignant
soft-tissue tumors in that the most common site of metastases from
soft-tissue sarcomas is to the lungs. In comparison to plain
radiography, CT has superior resolution in the imaging of metastatic
disease in the lungs. Perform a CT scan of the retroperitoneum in the
evaluation of extremity myxoid liposarcomas because, on occasion,
patients will have concomitant disease in the retroperitoneum and
extremities.
with a soft-tissue mass. The biopsy is a technically simple procedure,
but it requires extensive training and experience. A well-planned and
executed biopsy provides an accurate diagnosis and facilitates
treatment. A poorly performed biopsy, on the other hand, may fail to
provide a diagnosis and, more important, may have a negative impact on
patient survival and treatment options, especially the potential to
preserve a limb (17). For these reasons, if a soft-tissue mass may be malignant, the biopsy should be performed
by the surgeon who will ultimately be responsible for the full care of the patient.
closed (needle or trephine). The method is determined by the experience
and preference of the surgeon and the pathologist, the differential
diagnosis, and the anatomic location of the lesion. Closed procedures
are less invasive, require smaller doses of anesthetic and analgesic
agents, and carry a smaller risk in most instances. There is a lower
risk of tumor contamination, hemorrhage, and infection than with open
biopsies. When the procedures are supervised by the treating surgeon,
they may be done under ultrasound or a CT scan visualization in order
to localize the site of the biopsy better. Needle or trephine biopsies
are usually performed in an outpatient or office setting whereas open
biopsies require a full operating facility; therefore, they are less
costly than open procedures (29).
material obtained for pathologic review. For this reason, it is most
important that the pathologist who reviews the material be trained in
and have experience with needle biopsies. Highly accurate results are
seen with closed technique biopies in large medical centers that have a
large volume of musculoskeletal neoplasms (1).
The accuracy of a closed biopsy is further improved by tumor
homogeneity and the strength of supporting evidence provided by the
surgeon regarding the clinical findings and the results of imaging
studies. Closed biopsies are less reliable for cystic or myxoid tumors
or heterogeneous-appearing neoplasms (29). With
closed biopsy, there may not be enough pathologic material for
additional studies such as cytogenetics or tissue culture, or for
cellular research. Closed biopsies are more prone than open biopsies to
sampling error, especially in heterogeneous tumors. The tissue
architecture,
which is important in determining tumor grade, may be more difficult to ascertain in a closed biopsy than in an open one.
analgesia required, and the greater cost of an open biopsy, it is the
most reliable method for obtaining representative tissue. Open biopsies
may be excisional or incisional. However, because of the technical
difficulty in performing an incisional biopsy in the case of very small
tumors, an open excisional biopsy is indicated for these tumors. In
very small tumors, take a small cuff of surrounding tissue en bloc with the tumor (primary wide excision) because additional surgery may not be necessary, even in cases of malignancy.
must be localized to obtain the best access to the tumor and it also
must be in a position where the site can be excised en bloc with the tumor should it prove to be malignant.
-
Perform the biopsy through a longitudinal
incision. In the operative dissection, do not expose or contaminate
important vascular or neural structures. Do not violate anatomic
compartments other than those containing the tumor. -
Take special care around joints to prevent intraarticular contamination with tumor cells.
-
Biopsies of soft tissue should not involve major tendons (e.g., the patellar tendon) or their insertion.
-
In a biopsy, do not use intramuscular or
intranervous planes, as in more standard orthopaedic approaches.
Instead, use a direct approach with the smallest possible incision
directly through skin, subcutaneous tissue, fascia, and muscle down to
the tumor.
fine-needle aspiration is based on a pathologist’s cytologic
interpretation of single cells aspirated through a 0.6 to 1.0
mm—diameter needle. Fine-needle aspiration is more accurate in
diagnosing homogeneous tumors than inhomogeneous tumors.
-
After preparation of the skin, use the
needle to make serial aspirations of the tumor bed through a single
entry site. This site is agreed on by both the surgeon and the
pathologist; ideally, mark it with any method that will allow future
identification. -
This specimen is prepared immediately by
the cytology technician, and the tissue is fixed according to the
cytologist’s preference. Often, the slides can be evaluated immediately. -
Maintain pressure on the biopsy site so that hematoma formation is minimized.
needle biopsy is a trephine biopsy in which a 14-gauge cannula and
trocar system are used that allow cores (approximately 1.75 mm) of
tissue to be obtained with preservation of the architecture of the
specimen.
-
After preparation of the skin and infiltration with local anesthesia, use a #11 blade to incise the skin 2 to 3 mm.
-
Then place the Tru-cut needle into the
tumor bed. It is helpful to introduce the Tru-cut with the outer
cannula sheath retracted, exposing the inner trocar tip and specimen
notch. -
Sharply advance the outer cannula once
the needle tip is placed within the tumor mass itself, so that an inner
core of tissue is trapped. -
Repeat this procedure until multiple cores are available for pathologic review.
-
Process the specimens with fixation in formalin or in gluteraldehyde.
-
Maintain pressure on the site for several minutes to minimize hematoma formation.
the diagnosis in soft-tissue tumors. Make the incision as small as is
compatible with obtaining an adequate tumor specimen. Except for
regions over the iliac crest or clavicle, longitudinal incisions are
recommended.
-
Proceed sharply through subcutaneous fat,
fascia, and muscle. For intramuscular tumors, a color change in muscle
from red to salmon often heralds the approaching tumor pseudocapsule.
Malignant tumors are usually gray or white. -
Sample diagnostic tissue at the periphery
of the tumor, which typically is the most viable region. A biopsy of
necrotic appearing tissue is usually not of benefit. -
Perform frozen sections in all cases, if
possible, for confirmation of the presence of tissue from which the
pathologist may make a diagnosis. This is true even if definitive
surgery is not planned at the same time. -
Meticulous hemostasis is essential so tumor spread by hematoma is prevented.
-
Drains may be used, but they should be
placed close to and in line with the incision to allow the future
excision of the drain site along with the biopsy site. An outside-in
technique for introduction of the drain, when the drain site is made
with a scalpel and the drain is placed retrograde into the wound, is
preferable to the use of a trocar from the deeper wound through the
skin. -
As with all surgical procedures, minimize
postoperative limb swelling, edema, and bleeding through the use of
sterile compressive dressings. Limb immobilization and elevation may
also be required.
specific type of procedure performed depend on many factors, including
the diagnosis, the local and distant extent of disease, the tumor
location, the functional consequences of resection, and the patient’s
symptoms. An estimation of the growth pattern and malignant potential
of the tumor will ultimately dictate treatment. Some benign tumors
(e.g., lipomas) grow slowly in a centrifugal fashion and are bounded by
surrounding anatomic structures such as fascia, bone, nerve, or
vessels. A compressed area of fibrous adventitial and vascular
structures forms a true capsule surrounding these neoplasms. In
contrast, other benign tumors, such as intramuscular hemangiomas or
desmoid tumors, lack a true capsule and often have permeative and
infiltrative borders. Even malignant soft-tissue tumors, at least early
on, respect anatomic borders but, instead of a true capsule, are
surrounded by a “pseudocapsule” or reactive zone (7).
This zone and the area surrounding it contain microscopic extensions or
satellites of malignant tumor. An understanding of the growth
characteristics is important in the selection of the most effective
operative procedure for obtaining local control.
-
An intralesional
resection is performed within the reactive zone of the tumor and
includes debulking procedures. This form of resection is likely to
leave both macroscopic and microscopic residual disease and to be
associated with a high incidence of local relapse. -
A marginal
resection is performed through the tissue plane of the capsule or
reactive zone. This procedure is indicated for many benign tumors, but
it is associated with an unacceptable risk of local recurrence in the
case of malignant tumors because of residual microscopic peripheral
satellite disease. For this reason, in the treatment of sarcomas, an
effective adjuvant, most often radiation therapy, is administered in
combination with a marginal operative resection for obtaining local
control. -
A wide
resection, performed in normal tissue peripheral to the reactive zone,
is preferred for a soft-tissue sarcoma. With modern imaging for
accurate determination of the tumor extent, this operative procedure is
associated with a low incidence of local relapse and is recommended for
most infiltrative benign and malignant tumors. -
A radical
resection is one in which the entire anatomic compartment of tumor
origin is removed. This procedure carries little, if any, advantage
over wide resection in minimizing the prevalence of local recurrence
and is now only rarely performed.
may also be defined as marginal, wide, or radical according to the
above-mentioned definitions. The indications for amputation are sepsis,
extensive contamination of tumor from hemorrhage, a poorly performed
biopsy, or extensive tumor involvement of vital neurovascular
structures. Amputation may also be considered when the functional
results after an ablative procedure would be superior to those
following resection and adjuvant therapy. Additional indications may be
the presence of multifocal disease, intractable pain, or local relapse.
 |
|
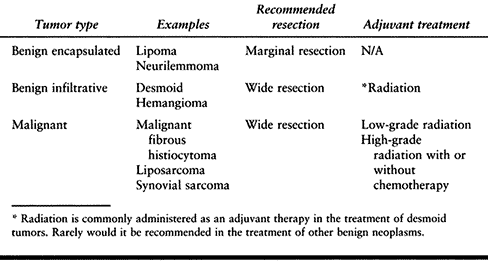
Table 129.1. Operative Procedures in the Treatment of Soft-Tissue Neoplasms
|
the functional consequences of resection determine the operative
strategy. Asymptomatic benign tumors may be treated nonoperatively in
an area where function after surgery is likely to be compromised.
Benign tumors in easily accessible sites are often removed even in the
absence of symptoms. For sarcomas located in the distal leg or foot,
amputation may be preferable for tumor control, given the relatively
good function and oncologic results after distal lower extremity
amputations. A malignant tumor adjacent to a major nerve or artery may
dictate that a marginal resection, in addition to some form of adjuvant
therapy, be administered.
influences the surgeon’s decision as to what procedure to perform. In
patients with malignant disease, it is important to determine the local
and distant extent (metastases) of the tumor. Soft-tissue sarcomas most
commonly metastasize to the lungs, and chest radiography and CT are
indicated as a part of the initial evaluation. Metastases to lymph
nodes and bone are unusual, and the additional value of technetium bone
scanning, gallium scanning, or positron-emission tomography (PET)
scanning is not clear. Adults with metastatic soft-tissue sarcomas at
the time of diagnosis, or with severe comorbid medical conditions, have
a very poor prognosis, and surgery may not be indicated except for
palliation. Similarly, in some benign tumors, such as plexiform
neurofibromas or extensive intramuscular hemangiomas, the tumor may be
so extensive locally that resection would be associated with severe
functional disability. In such cases, it may be in the patient’s best
interest not to attempt to resect all disease.
Most are small (smaller than 5 cm) and are presumed, before operative
resection, to be benign neoplasms. Therefore, most subcutaneous
sarcomas are referred to surgical oncologists after excision. On
reexcision of the tumor bed, focal residual sarcoma has been noted in
more than one half of the patients treated (12,20).
For this reason, surgical reexcision of the operative field is
recommended in the treatment of patients with subcutaneous sarcomas.
With such treatment, excellent rates of local control can be obtained (10).
normal cuff of skin and subcutaneous fat in continuity with fascia and
muscle. Because there is no anatomic barrier to the spread of a
subcutaneous sarcoma, the surgeon must determine, by palpation and
careful review of imaging studies, the extent of a resection that will
be necessary to encompass the entire previous operative field while
remaining in normal tissue. Fortunately, in a subcutaneous location,
major neurovascular structures are rarely involved.
 |
|
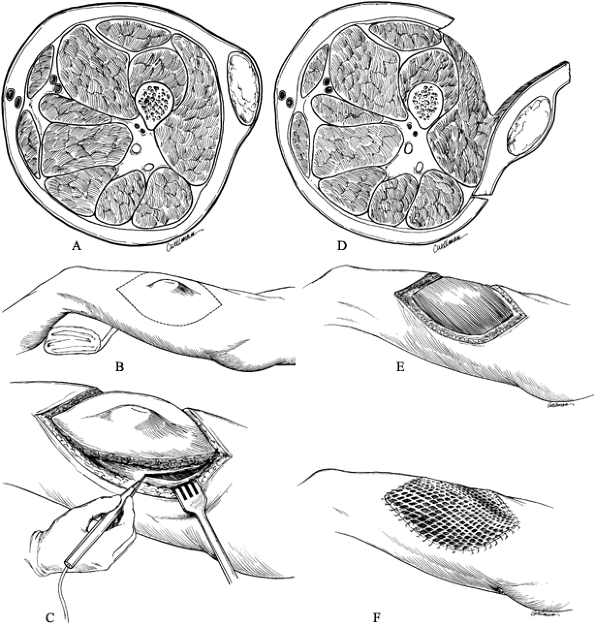
Figure 129.2. Operative technique for a wide excision of a subcutaneous sarcoma in the midlateral aspect of the thigh. A:
Cross section of the thigh; the lesion is completely contained within the subcutaneous tissue and does not involve the underlying fascia. B: The planned margin of resection of the previously biopsied tumor is indicated by the dotted line. C: A sharp dissection is performed through the subcutaneous tissue away from the mass, with use of the underlying fascia as the deep margin. D: The wide margin, including the deep fascia, seen in cross section. E: The muscle bed after resection. F: The defect is covered with a split-thickness skin graft. |
-
Dissect directly through skin, subcutaneous tissue, and fascia.
-
Take a small amount of superficial muscle
deep to the fascia in continuity with the resected specimen. In most
instances, a split-thickness skin graft is required to close the defect
over the remaining muscle. Occasionally, in certain anatomic locations
such as in the inguinal region or in obese patients, elliptical
incision may be made allowing resection of the tumor and primary
closure of the wound. -
Take care not to compromise the amount of
soft-tissue resected in order to obtain primary closure. Infrequently,
a rotational or free flap is required for closure over bony surfaces
such as the tibia.
large (larger than 5 cm) and are suspected to be malignant before
operative resection. The most common site of a soft-tissue sarcoma is
the thigh, and the tumor may be located in the anterior, posterior, or
adductor compartment. In some cases, the tumor may be located in more
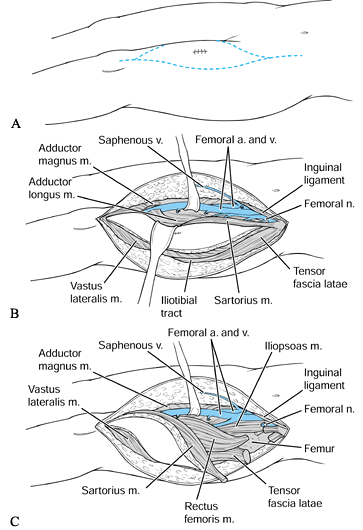
than one anatomic compartment, or it may be extracompartmental
(popliteal fossa). Often, the femur, superficial femoral artery and
vein, femoral nerve, or sciatic nerve may be adjacent to the tumor
mass. It is unusual for a soft-tissue sarcoma to invade neurovascular
structures directly.
marginal resection so that the occurrence of local relapse is
minimized. However, if the tumor is immediately adjacent to bone, the
sciatic nerve, or in some cases, the superficial femoral artery, a
marginal resection is all that can be accomplished without severe
compromise of limb function. In these cases, adjuvant therapy, most
commonly in the form of radiation therapy, either external beam or
brachytherapy, can be administered preoperatively or postoperatively to minimize the chance of local recurrence.
-
Make the skin incision longitudinally over the extent of the tumor, ellipsing any previous biopsy site (Fig. 129.3).
Dissect sharply through the subcutaneous tissue. Bevel the dissection
outward at a 45° angle to the skin to increase the width of the
resection further.![]() Figure 129.3. Wide resection of a deep sarcoma of the thigh. A:
Figure 129.3. Wide resection of a deep sarcoma of the thigh. A:
Skin incision from the anterior superior iliac spine to the superior
pole of the patella, including the site of elliptically excised biopsy
specimen, for a deep intracompartmental tumor of the anterior thigh. B: Creation of skin flaps and dissection of superficial femoral vessels. C: Transection of muscle circumferentially around the tumor mass. -
Based on palpation and imaging studies,
make a circumferential fascial incision, often in the form of an
ellipse, 2 to 3 cm away from the tumor mass. It is important that skin,
subcutaneous fat, and fascia remain attached to the underlying tumor
mass. -
At this time, locate the superficial
femoral artery and vein proximally and dissect them from proximal to
distal. Ligate multiple branches in the region of the tumor. In cases
of anterior thigh sarcomas, the femoral nerve must often be sacrificed
proximally. Once the artery and vein have been freed from the
underlying soft tissue, retract them medially and proceed with the
resection. -
Transect muscle fibers on the periphery
of the tumor for preservation of a cuff of normal tissue around the
tumor pseudocapsule. Make every effort to avoid visualizing or
penetrating the tumor pseudocapsule. -
If the tumor is immediately adjacent to
the femur, it may be necessary to perform a subperiosteal dissection
and include the periosteum as a deep margin. Be aware, however, that
such subperiosteal dissection in the face of adjuvant radiation therapy
may predispose a patient to a pathologic fracture of the femur due to
the radiation therapy (15). -
Then sever surrounding muscle
attachments, and remove the tumor from the thigh. Additional effort to
remove entire muscles from origin to insertion (a radical resection) is
not necessary in the treatment of most soft-tissue sarcomas. -
Irrigate the wound and close over suction drains. Leave the drains in place for a prolonged period to evacuate any dead space.
to allow for optimal wound healing and to minimize seroma formation. In
some cases, a rotational free flap may be necessary to provide
vascularized tissue deep to the skin and to assist in wound healing.
described earlier in that no effort is made to preserve a normal cuff
of tissue about the tumor. In these cases, dissection proceeds through
the capsule of the tumor itself without removal of additional soft
tissue. This procedure is most commonly carried out for deep
intramuscular lipomas. It is not recommended for the treatment of
malignant disease.
-
Make a longitudinal incision, slightly
longer than the underlying tumor mass, directly over the most prominent
portion of the tumor. The skin, subcutaneous tissue, and, in some
cases, muscle must be longitudinally divided and bluntly dissected to
reveal the underlying capsule of the tumor. -
Then deliver the tumor mass from the
wound, again working in the adventitial layer covering the tumor,
dissecting circumferentially around the tumor mass. Then remove the
tumor. -
Irrigate the wounds and close over drains if necessary.
“non-epithelial extra-skeletal tissue of the body exclusive of the
reticuloendothelial system, glia, and supporting tissue of various
mesenchymal organ” (9). Benign soft-tissue
neoplasms are believed to be at least 100 times more common than their
malignant counterparts, soft-tissue sarcomas. Soft-tissue sarcomas may
arise in any part of the body but are most common in the extremities,
specifically the thigh and buttock. Common benign deep soft-tissue
tumors include intramuscular lipomas, extraabdominal fibromatosis
(desmoid tumors) hemangiomas, and benign nerve sheath tumors.
believed to arise from normal cells of a similar histologic appearance,
the current histologic classification of soft-tissue tumors is based on
the apparent differentiation of the tumor cell. Most tumors arise from
an undifferentiated precursor cell and acquire phenotypic traits of
various normal cells during neoplastic transformation. It is this
appearance by which the tumors are classified rather than by the tissue
of origin. The tumors are classified as benign, intermediate, or
malignant based on their perceived capability of metastasis. Malignant
tumors are subcategorized as low grade or high grade based on their
histologic characteristics including tumor necrosis, cellular
anaplasia, and the number of mitotic figures. Patients with high-grade
tumors are at an increased risk for developing metastatic disease
compared with those with low-grade tumors.
This table is based largely on the classification of soft-tissue tumors
by the World Health Organization, with subsequent modification as
described by Enzinger and Weiss. Again, this classification of tumors
does not imply that the histologic appearance of the tumor is related
to the tissue of origin. In addition, one must be careful not to assume
that malignant soft-tissue tumors of one histologic type are the result
of malignant degeneration of a benign neoplasm. Malignant degeneration
of an underlying benign soft-tissue neoplasm is very rare.
 |
|
Table 129.2. Histologic Classification of Soft-Tissue Tumors11
|
resembles fibrous tissue are common and may be reactive (nodular
fasciitis), benign (fibroma of tendon sheath), infiltrative (desmoid
tumor), or malignant (fibrosarcoma). Fibrous neoplasms of infancy and
childhood should be considered separately because of their unique and
often self-limiting behavior.
fibroblasts often mistaken for a malignant soft tissue tumor because of
its rapid onset of growth, mitotic activity, and cellularity. It is
most likely a self-limiting reactive process rather than a true
neoplasm. Most patients present with a rapidly growing mass with
associated tenderness. It is often located in the upper extremities,
especially in the volar aspect of the forearm. In infants and children,
it may be found in the head and neck region. It is usually small
(smaller than 5 centimeters). It may be subcutaneous, intramuscular, or
fascial based. It is usually treated by marginal excision and has a low
recurrence rate (4). Spontaneous regression has been observed.
Dupuytren’s contracture and is the most common type of fibromatosis.
Its etiology is unclear. It is seen most commonly in people older than
65 years of age. It may be associated with other forms of fibromatosis
including plantar fibromatosis and penile fibromatosis (Peyronie’s
disease). It is also noted to be of increased incidence in patients
with epilepsy and diabetes. It has also been known to be associated
with chronic alcoholism and liver cirrhosis.
firm nodule is palpable in the palmar aspect of the hand affecting the
ulnar aspect of the hand. It may be associated with joint contracture.
A single small nodule or an ill-defined conglomerate of several nodules
is found on examination. Pathology consists of spindle-shaped
fibroblasts and variable amounts of dense collagen. Treatment may
include nonoperative therapy including physical therapy and topical
treatment, but rarely do these measures have a significant effect on
the disease. Operative resection, however, remains the treatment of
choice in the patient with impairment secondary to flexion contractures
of the digits. Fasciectomy is usually recommended as the treatment of
choice (23). Local recurrence may result in recurrent contracture.
identified by nodular fibrous proliferation arising with the plantar
aponeurosis. Unlike disease in the hand, it is usually not associated
with contractures of the digits. Usually, a single subcutaneous
thickening or nodule that adheres to skin and is located in the middle
or medial portion of the foot is found on examination. It may be
painful and may be bilateral. The lesion is more common in men than
women. It occurs in younger individuals than palmar fi- bromatosis.
Pathologic findings are identical to palmar fibromatosis. Treatment is
directed at the patient’s symptoms, and nonoperative therapy consists
of shoe pads and modified shoe wear. Radical fasciectomy is considered
for patients with intractable pain but is associated with local
recurrence in a number of cases (6).
neoplasms. It has the potential to attain a large size, often recurs
despite operative resection, and is infiltrative in nature. It may be
associated with colonic polyposis (Gardner’s syndrome). Clinical
findings are usually a soft-tissue mass in the young adult. It is
common about the shoulder, flank, and the muscles of the thigh. It may
be multifocal within an extremity. Histologically, fibromatosis is
benign in appearance with spindle-shaped fibroblasts and dense
collagen. Imaging studies reveal that the tumor is typically of low
signal on both T1- and T2-weighted images because of the large collagen
content. Operative resection is considered for tumors in expendable
locations (3).
However, despite complete surgical resection, it is associated with
recurrence in a number of cases. Adjuvant treatment in the form of
radiation therapy is often considered for these individuals. Medical
treatment in the form of estrogen blockade or chemotherapy may be
considered in selected circumstances.
cells resemble fibroblasts but with significant cellular atypia,
frequent mitoses, and increased cellularity compared with its benign
counterparts. Before the subclassification of malignant fibrous
histiocytoma, fibrosarcomas were the most common soft-tissue
malignancies. Fibrosarcomas are often low grade. The recommended
treatment is wide resection or marginal resection combined with
adjuvant radiation therapy.
storiform or cartwheel-type growth pattern. The actual histogenesis of
the tumor may not be from a histiocyte but more closely related to the
fibroblast. This tumor is also known as an adult spindle cell sarcoma.
These neoplasms may be low grade or high grade based on their
cellularity, atypia, and the presence or absence of mitotic figures and
necrosis. They are commonly located in the subcutaneous tissue or the
deep soft tissues of the thigh. Like other soft-tissue sarcomas, they
are best treated with wide resection. Marginal resection can be
combined with adjuvant radiation therapy. Patients with large,
high-grade soft tissue sarcomas may, in addition, benefit from adjuvant
chemotherapy (26).
virtually anywhere in the body. The tumor is usually a soft, mobile,
and asymptomatic mass with a very large range of sizes noted. The
magnetic resonance images of a lipoma show signal characteristics
similar to surrounding fat on all sequences. It is the one tumor that
can usually be diagnosed by MRI. Asymptomatic lesions do not require
treatment. Malignant transformation is rare. Symptomatic tumors are
usually treated with local excision. Local recurrence is rare.
soft-tissue tumor seen in the extremities. It is also very common in
the retroperitoneum. Liposarcomas may be low grade or high grade. The
characteristic cell is the lipoblast, which consists of a cell
containing large amounts of clear cytoplasm but with several septated
vacuoles that secondarily indent the nucleus. This is different from
the signet ring cell, a cell with a large clear cytoplasm and a thinned
eccentric nucleus at one pole. Signet ring cells can be seen in both
benign and malignant lipomatous soft tissue tumors. The lipoblast is
characteristic of a malignant tumor. Higher grade liposarcomas may be
difficult to differentiate from other soft-tissue sarcomas such as
malignant fibrous histiocytoma because of the dense cellularity
obscuring those cells that resemble fat. It should be noted that
liposarcomas do not have an appearance similar to lipomas on MRI, that
is, they tend to be of low signal on T1-weighted images and of bright
signal on T2-weighted images. The management of liposarcoma is
identical to other soft-tissue sarcomas, that is, wide resection when
possible or marginal resection with adjuvant radiation therapy. Unlike
other soft-tissue sarcomas, however, liposarcomas (especially the
myxoid type) of the extremity may be associated with disease in other,
nonpulmonary sites (e.g., retroperitoneum) (21).
seen in the extremities. Benign neoplasms may be managed with marginal
resection. Malignant soft-tissue sarcomas require wide resection or
marginal resection and adjuvant radiation therapy.
rhabdomyoma is exceptionally unusual and of variable presentation. The
diagnosis is usually made on the basis of an open biopsy.
Rhabdomyosarcoma, although unusual, is the most common soft-tissue
malignancy of children. When it is located in an extremity, it is often
of the alveolar histologic type. The characteristic tumor cell of a
rhabdomyosarcoma is the rhabdoid cell, which is a large, irregularly
shaped cell with an eccentric nucleus and eosinophillic cytoplasm.
These high-grade tumors are often associated
with metastatic disease at the time of presentation and are treated with systemic chemotherapy (5).
The role of operative intervention is unclear and controversial.
Radiation therapy may be considered. These tumors may actually be more
closely related to soft-tissue Ewing’s or soft-tissue primitive
neuroectodermal tumor.
vessels. Some actually represent vascular malformations rather than
true neoplasms. They are often seen in infants and may spontaneously
regress. They may be localized or multifocal, in which case they are
known as angiomatosis (22). The majority of
hemangiomas are superficial lesions with a predilection for the head
and neck but may also occur internally. Tumors may be responsive to
circulating hormones, and changes in size may be seen during pregnancy.
Based on the histologic appearance, they are subclassified as capillary
hemangiomas, which are often seen in children. Cavernous hemangiomas
are large with thin-walled veins. Hemangiomas may be seen in
conjunction with dyschondroplasia, also known as Maffucci’s syndrome.
When the tumor is located in a subcutaneous or dermal location, there
often are characteristic color changes to the skin. There is no such
skin change found with deeper situated tumors. Radiographs may
demonstrate phleboliths within the lesion. Vascular studies and
ultrasound may show vascular flow but may be difficult to distinguish
from other vascular neoplasms.
diagnosis is known, is based on symptoms. Again, many grow slowly if at
all. This is especially true in children, in whom lesions are often
known to regress spontaneously. Localized disease may be marginally
resected, with an approximate 20% local recurrence rate. More
difficulties are encountered with the extensive hemangiomas and
multifocal hemangiomas. Angiographic embolization has been used to
treat many afflicted individuals with variable success. Radiation
therapy has been considered in the past but should be used with great
caution in young people.
vessels are rare. Significant is the association of angiosarcoma with
chronic lymphedema, for example, in patients following mastectomy and
lymph node resection. Operative wide resection is the treatment of
choice, and multifocal disease is commonly encountered.
juxtaarticular or intraarticular locations. Intraarticular disease is
known as pigmented villonodular synovitis and, again, may be localized
or diffuse (27). Tenosynovial giant cell tumor
affecting the tendon sheath is known as giant cell tumor of the tendon
sheath. These tumors are believed to be benign neoplasms that may
actually be reactive in etiology. Localized disease is usually treated
with marginal resection. Diffuse disease, especially intraarticular
disease in a large joint, such as the knee, may require synovectomy,
and intraarticular or external beam radiation therapy.
sarcoma in adults and the second most frequently seen soft-tissue
sarcoma in children. It is typically not located within a joint but
within the structures about a joint. Many are located in the hand or
foot. These tumors may be monophasic or so-called biphasic, consisting
of plump synovial cells arranged in a glandular pattern with other
fibroblastic areas around it. Radiographs of these lesions may reveal
mineralization in the form of calcification. Like other soft-tissue
sarcomas, MRI studies usually show predominantly low signal on
T1-weighted images and bright signal on T2-weighted images. These
lesions usually enhance with gadolinium infusion.
wide resection or marginal resection and radiation therapy. Some
synovial sarcomas may respond well to systemic chemotherapy (28).
locations and may be solitary or part of a neurofibromatosis or von
Recklinghausen disease (13). Often, this
disease is noted as an inherited dominant trait of variable
penetration. It may be associated with scoliosis, congenital
pseudarthrosis of the tibia or clavicle, and gigantism. Neurofibromas
are often asymptomatic. People with von Recklinghausen’s disease may
develop malignant nerve sheath tumors as well. Sarcomatous degeneration
should be suspected in patients with very large or rapidly growing
tumors.
localized, neurofibromas are often extensive and infiltrative in nature
around the nerve of origin. For this reason, if it is decided to remove
these tumors, it is often necessary to resect the underlying nerve.
Operative resection, however, is only indicated for symptomatic lesions
or for those suspected of sarcomatous degeneration.
nerve sheath itself. It is often a fusiform soft-tissue mass tapered at
either end by the axons resuming their normal position within the nerve
proximal and distal to the lesion. The MRI scan often shows that this
tumor is of very bright signal on T2-weighted images and is usually
homogeneous. Physical findings often reveal Tinel’s sign or a radiating
sensation with direct percussion. Marginal resections are associated
with a low incidence of recurrence. Pathology reveals a biphasic
appearance with compact (Antoni A) or densely cellular areas
alternating with areas with relatively few cells (Antoni B). Small
clusters of palisading cells around a central eosinophilic area are
known as Verocay bodies. Operative excision is often carried out as a
form of diagnostic biopsy in which the nerve sheath itself is split
longitudinally in order to remove the lesion without harming the
surrounding axons. It is usually encapsulated and freed relatively
easily from the underlying nerve.
behavior, however, is not unlike other high-grade soft-tissue sarcomas (14). Operative treatment is preferably wide resection or marginal resection with adjuvant treatment
soft-tissue tumors in an effort to predict the prognosis and evaluate
the effect of therapeutic intervention by stratifying similar tumors
according to various prognostic factors. Commonly used factors are
tumor grade, size, compartmentalization of the tumor, and the presence
or absence of metastases.
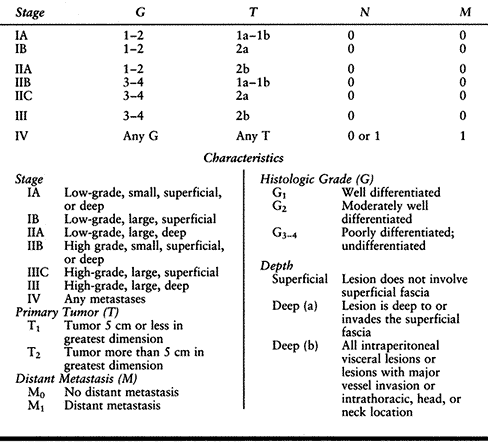
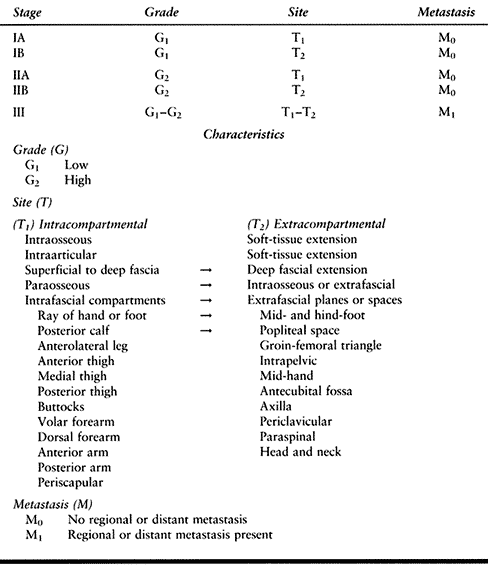
systems that are used at present were developed by the American Joint
Committee on Cancer (AJCC; Table 129.3) (2) and by Enneking (Table 129.4) (8).
In both systems, the variables used in the assignment of an appropriate
stage include tumor grade, location, and the relative extent of the
tumor, as well as the presence or absence of metastases.
 |
|
Table 129.3. American Joint Committee Staging Protocol for Sarcoma of Soft Tissue (2)
|
 |
|
Table 129.4. Enneking System for Staging of Soft-Tissue Sarcomas
|
very poor prognostic factor. This is true if metastases occur either to
the lungs or to lymph nodes. The differences between the systems is
that, first, the AJCC system is based on a grading system containing
four variables, whereas the Enneking system considers only high and low
grades. Second, the AJCC system uses tumor size as an important
prognostic variable as opposed to compartmental location as described
by Enneking. Third, the AJCC system considers tumor depth to be an
important prognostic factor.
and clinical observations, and they serve to guide therapeutic
strategies. However, these systems are based on variables that are
crude and of limited usefulness. New discoveries in the fields of
molecular and cellular biology are being made and will be correlated
with the clinical course and outcome. These findings almost certainly
will change the manner in which tumors are classified and staged, and
will predict more accurately the behavior of a given tumor in a
particular patient. Operative treatment, chemotherapy, and radiation
therapy can then be used in a rational and effective manner to improve
the functional and oncologic outcome (19).
tumors are the same as those encountered for all orthopaedic surgery,
as discussed in Chapter 5 and Chapter 8.
The two most significant complications of the management of soft-tissue
tumors of the extremities are improper performance of a biopsy, which
leads to either misdiagnosis or compromise
of
the definitive method for surgical treatment, or recurrence after
resection of an aggressive benign tumor or malignant tumor. Small
benign-appearing tumors in the distal portions of the extremities are
commonly removed by general orthopaedic and other surgeons by
excisional biopsy with marginal resection. When histology reveals a
malignant tumor, it is prudent to have the next stage of treatment
management performed by an experienced oncology team consisting of a
surgeon, medical oncologist, and radiation therapy specialist. In the
case of lesions in which a malignant tumor is an obvious part of the
prebiopsy differential diagnosis, biopsy should be performed only by a
surgeon experienced in the treatment of malignant tumors who is
prepared to carry out definitive treatment in conjunction with an
oncology team. This approach will minimize the risk of local recurrence
and enhance the long-term survival of patients with these troublesome
tumors. In performing biopsy and subsequent resection of these tumors,
the principles discussed in this chapter must be meticulously followed.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
M, Rydholm A, Persson BM. Aspiration Cytology and Soft-Tissue Tumors.
The 10-year Experience at an Orthopaedic Oncology Center. Acta Orthop Scand 1985;56:407.
M, Zajars G, Pollack A, et al. Desmoid Tumor: Prognostic Factors and
Outcome after Surgery, Radiation Therapy, or Combined Surgery and
Radiation Therapy. J Clin Oncol 1999;17:158.
CP, Peabody TD, Mundt AS, et al. Oncologic Outcomes of Operative
Treatment of Subcutaneous Soft-Tissue Sarcomas of the Extremities. J Bone Joint Surg 1991;79A:888.
CP, Peabody TD, Simon MA. Mini-Symposium: Soft Tissue Tumors of the
Musculoskeletal System (i). Classification, Clinical Features,
Preoperative Assessment, and Staging of Soft Tissue Tumors, Current Orthopaedics 1997;11:75
R, Shiu M, Senie R, Woodruff J. Malignant Peripheral Nerve Sheath
Tumors of the Buttock and Lower Extremity. A Study of 43 Cases. Cancer 1990;66:1253.
O. A Consecutive 7-Year Series of 1,331 Benign Soft-Tissue Tumors.
Clinicopathologic Data. Comparison with Sarcomas. Acta Orthop Scand 1981;52:287.
JJ, Neibauer JJ, Brown RL, et al. Treatment of Dupuytren’s Contracture.
Long-term Results after Fasciotomy and Fascial Excision. J Bone Joint Surg 1976;58A:380.
S, Baldini E, Demetri G, et al. Synovial Sarcoma: Prognostic
Significance of Tumor Size, Margin of Resection and Mitotic Activity
for Survival. J Clin Oncol 1996;14:1201.
MC, Biermann JS, Montag A, Simon MA. Diagnostic Accuracy and
Charge-Savings of Outpatient Core Needle Biopsy Compared with Open
Biopsy of Musculoskeletal Tumors. J Bone Joint Surg 1996;78A:644.