PRINCIPLES OF NERVE REPAIR
Professor of Orthopaedics, Professor of Surgery, Division of Plastic
Surgery; Chief, Hand and Upper Extremity Service, University of
California, Davis, School of Medicine, Sacramento, California, 95817.
as a result of traction, contusion, compression, or laceration. The
mechanism of injury determines the nature of the lesion, its
management, and its prognosis.
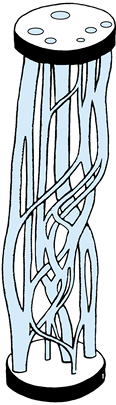
length without injury. Much of this elasticity derives from the
geometry of the nerve. The nerve trunk runs an undulating course in its
bed; the funiculi run an undulating course in the epineurium; and the
nerve fibers run an undulating course inside the funiculi (26).
The straightening of these undulations provides elasticity in the
physiologic range of stretching. As the nerve is stretched beyond this
limit, the axons (efferent) and dendrites (afferent), which have little
tensile strength, fail before the surrounding connective tissues
(epineurium and perineurium), which are stronger. When the tensile
strength of the perineurium is exceeded, which occurs at about 20% of
stretching, the nerve tears in two. In the region between the elastic
limit and the mechanical limit, the nerve fibers are damaged to various
degrees without gross disruption of the nerve trunk. Traction injuries
may be associated with fractures, at the time of injury or during
reduction or fixation; with dislocations and stretch injuries; and with
gunshot wounds. Although spontaneous recovery is typical of most of
these injuries, complete nerve loss can also occur.
a similar prognosis to traction injuries, with spontaneous functional
recovery the normal prognosis. Recovery from compression injuries
depends on how long the nerve has been compressed and the degree of
compression.
and the discontinuity must be surgically repaired. If the wound is a
clean one from a sharp object such as a knife or glass, the damage to
the nerve is likely to be local; this is unlike a crush, traction, or
missile wound, in which damage may extend a considerable distance
proximally and distally. The likelihood of functional recovery after
accurate surgical repair depends on which nerve is involved, the level
of the injury, the condition of the wound, and, most important, the age
of the patient. Results of nerve repair in children are always better
than those in adults. More distal injuries have a better prognosis for
recovery than proximal ones, and a pure motor or sensory nerve has a
better prognosis than a mixed nerve.
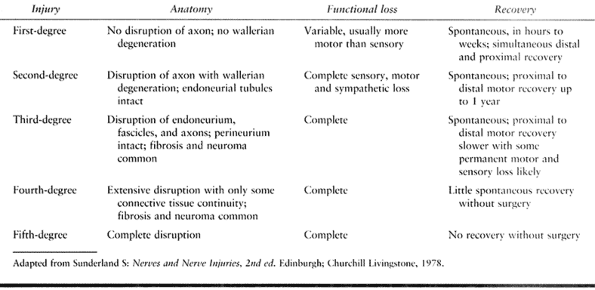
based on degree of damage rather than on mechanism of injury (25,26).
Neurapraxia is a minor injury resulting from traction or compression in
which ischemia or local demyelination interfere with nerve function.
Damage to motor function is usually greater than to sensation, and
recovery is within hours or days. Axonotmesis is a moderate injury in
which continuity of axons is disrupted with wallerian degeneration
distal to the lesion. The endoneurial tubes remain intact, and
regenerating axons can reestablish their functional connections. Good
recovery, usually within a year, is likely. Neurotmesis is a severe
injury with disruption of axons and connective tissues of the nerve.
Fibrosis, loss of coaptation, and loss of continuity mitigate against
spontaneous recovery. Surgical repair is indicated.
 |
|
Table 51.1. Classification of Nerve Injuries
|
peripheral nerve is the model that has been best studied; traction and
crush injuries are less well understood. Within a month after
laceration, the distal portion of the nerve, cut off from trophic
support of proximal cell bodies, undergoes wallerian degeneration. This
process involves disintegration of the axon and myelin sheath, which
are absorbed by macrophages and Schwann cells, leaving a tubule along
the pathway of the former axon. Proximal to the lesion, some retrograde
degeneration occurs, which is likely to be greater in more proximal
lesions.
dendrites have been severed enlarge and their metabolic activity
greatly increases. At the same time, axons sprout at the proximal
stumps (3). If the two severed ends are still
congruently opposed, axon sprouts grow at a rate as much as 1 mm each
day down the endoneurial tubules of the degenerating distal axons to
eventually reestablish connection with the sensory, motor, and
sympathetic nerve end points. The actual rate of recovery is affected
by age. In an animal model, Choi showed that the speed of wallerian
degeneration, axonal regeneration, and myelin regeneration was greater
in 2-month-old rats than in 10-month-old rats (5).
Spontaneous recovery from mild axonotmesis may take from 1 to 6 months,
with more proximal lesions requiring more time to heal.
successful natural recovery. The chief of these is loss of coaptation.
If the nerve is completely transected, retraction of the severed ends
and motions of the extremity destroy coaptation, and axon sprouts do
not grow into the distal endoneurial tubules. After a noncongruent
repair,
malaligned
axon sprouts grow into epineurial tissues and reach a blind end or grow
into inappropriate tubules to establish connections that are
nonfunctional. A second obstruction to successful natural repair is the
development of fibrosis in the vicinity of the lesion. Fibrosis may
distort the nerve architecture and destroy coaptation; it may create a
barrier at the lesion that is difficult for the axon sprouts to
traverse; and it may tether the nerve to surrounding tissues, impairing
its mobility. In general, extensive soft-tissue injuries with a major
inflammatory response lead to a greater degree of fibrosis. In
traction, crush, and missile injuries, fibrosis may extend a
considerable distance proximally and distally.
designed to enhance the natural pathways of repair. This involves the
reestablishment and maintenance of coaptation, avoidance of traction,
and excision of excessive fibrosis.
sensory, motor, and sympathetic functions of that nerve distal to the
lesion. Clinically, the acute picture is often confused by associated
injuries. Fractures and dislocations; damage to muscles, tendons, or
vascular structures; and head injury or altered psychological state can
mask or mimic a peripheral nerve injury. Assessment of peripheral
neuropathy should be done as early as possible after stabilization of
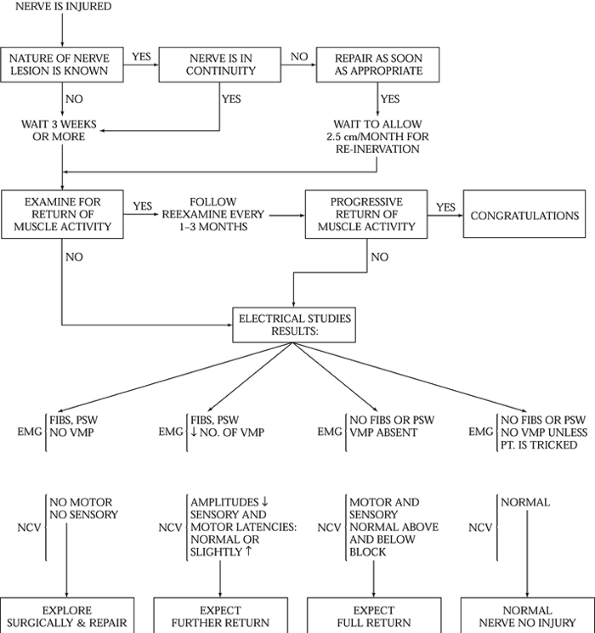
the patient’s other injuries so that proper therapy can be planned (Fig. 51.1).
 |
|
Figure 51.1. Algorithm for management of peripheral nerve injuries. EMG, electromyograms; FIBS, fibrilation potentials; NCV, nerve conduction study; PSW, posititive sharp waves; VMP,
voluntary motor unit potetials (interference pattern). (From Frykman G, Wolf A, Coyle T. An Algorithm for Management of Peripheral Nerve Injuries. Orthop Clin North Am 1981;12:240, with permission.) |
Accuracy and consistency in performing the initial diagnostic tests is
critical, because these are the standards by which spontaneous recovery
and
the need for surgery are judged. Sometimes assessment can be made only
by surgical exploration, as in young children or in head trauma victims.
 |
|
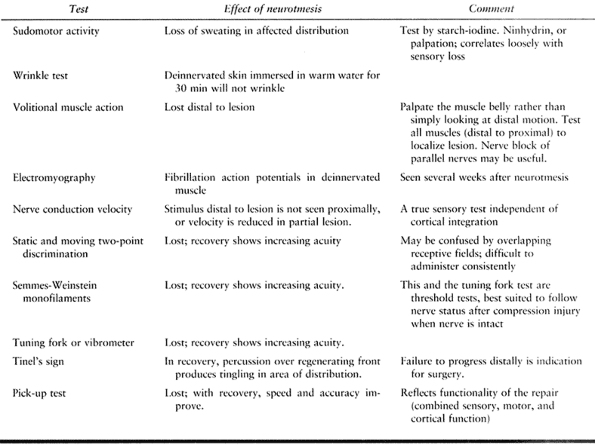
Table 51.2. Tests of Peripheral Nerve Function
|
wound, direct inspection of the nerve at the time of irrigation and
debridement is indicated. An acute primary repair may be undertaken if
the wound is clean, the mechanism of injury is a sharp laceration, the
patient’s condition is stable, and the surgical team and its facilities
are available (8). If this constellation of
circumstances is not encountered, perform a delayed primary repair
within 8 to 15 days. If repair is to be delayed, the nerve ends can be
tagged with wire suture to facilitate later identification at the time
of acute exploration of the wound. Tagging is not critical, because
later surgery is based on identifying normal nerve proximal and distal
to the lesion and dissecting toward the injury, rather than on
searching for suture tags in a bed of scar. The wire is useful for
locating nerve ends on radiographs, however. If the severed ends can be
easily approximated, they may be loosely sutured together to resist
retraction during the interval before the delayed repairs.
extensive nerve retraction has not yet occurred and less mobilization
is required. The delay of 1 or 2 weeks after injury may offer some
advantages, however, besides allowing the surgical team opportunity to
prepare. The posttraumatic edema of the cut ends has time to resolve,
and the nerve cell bodies greatly increase their anabolic activity in
association with axon sprouting. The extent of proximal and distal
damage to the nerve is easier to assess. The location of early axon
sprouts can help define the necrotic terminus of the proximal stump.
acute compartment syndrome is ruled out, quantitative diagnostic tests
should be performed (Table 51.2) to serve as a
standard by which the recovery of the deficit may be measured. These
tests must be performed in a meticulous and consistent way so that
comparisons are valid. Although no single test is infallible, the
combined weight of several tests with similar results allows a
reasonably secure diagnosis. The patient should be reevaluated at
intervals of 4 to 6 weeks.
in heavily contaminated wounds, if soft-tissue coverage is poor and
requires flaps, if the amount of nerve damage cannot be assessed early
(e.g., in patients with gunshot wounds, head injuries), or if the
diagnosis is initially missed. Some motor recovery may be expected
after repairs as late as 1 year after injury; partial sensory recovery
may result from repairs as late as 2 years after injury. Success
diminishes with delay, however, because muscle atrophies and
endoneurial tubules undergo fibrosis.
realign the axons so that regenerating axon sprouts will reconnect to
their preinjury end points. A number of factors frustrates the
surgeon’s attempt to achieve this. Edema in the proximal stump and
shrinkage of the distal stump prohibit exact coaptation of formerly
congruent ends, as will any distortion caused by less-than-perfect
suture technique. A greater problem is segmental loss of even a few
millimeters of nerve due to necrosis resulting from the injury. The
pioneering studies of Sunderland in mapping the topography of the
fasciculi in the peripheral nerves reveal a complex arrangement of
branching, joining, and wandering pathways. Consequently, the number,
size, arrangement, and neurologic content of the fascicles as seen in
cross section vary along the nerve. With greater segmental loss, the
cross-sectional arrangements of the two ends are increasingly
dissimilar and the possibility of excellent coaptation diminishes
accordingly.
has been often reproduced and stands as a graphic display of the
impossibility of obtaining perfect coaptation after segmental loss.
Jabaley et al. (14,32)
have found that in several regions of the median and radial nerves, the
fascicular topography is considerably less variable than in
Sunderland’s diagram and that precise coaptation of most fascicles is
theoretically possible despite segmental loss. Even over a longer
distance encompassing various fascicular plexi, a bundle of axons tends
to remain in the same quadrant of the nerve.
 |
|
Figure 51.2. Fascicular topography of a segment of the musculocutaneous nerve. (From Sunderland S: Nerves and Nerve Injuries, 2nd ed. Edinburgh: Churchill-Livingstone, 1978:32, with permission.)
|
features (vessels) at the two stumps and by sketching the
cross-sectional appearance of the two ends and approximating them
accordingly can ensure correct rotational alignment. When segmental
loss is greater, a sketch of the cross-sectional fascicular arrangement
can be compared with published maps, which may facilitate alignment.
Finally, intraoperative electrical stimulation can be used to
distinguish predominantly motor from sensory fascicles.
With
the patient awake and the tourniquet released, stimulation of the
distal stump may identify motor fascicles, and stimulation of the
proximal stump may identify sensory fascicles. Silent fascicles
proximally are presumed to be motor, and silent fascicles distally are
presumed to be sensory (10).
been shown to help differentiate motor from sensory fascicles within a
nerve. Because it requires 24 hours of incubation time, it is not
usually suitable for intraoperative use, although it has provided
confirmation of mapping of fascicles done by other means. Similar
histochemical techniques with shorter development times show promise,
but they are not now in general use (24).
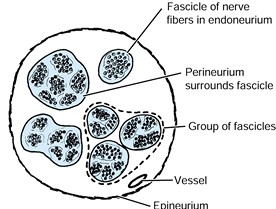
use: epineurial repair, fascicular repair, and group fascicular repair.
Each technique is appropriate to certain circumstances. Appreciation of
microneuroanatomy is essential to understanding the techniques of nerve
repair (Fig. 51.3). At present, all repairs are
done with microsutures. In countries other than the United States,
commercially available fibrin glue is often used in combination with a
limited number of sutures. This glue is made from donated blood
products. It is possible to make fibrin glue from the patient’s own
donated plasma by mixing it with thrombin, but the cost, time, and
effort need to be weighed against the benefits. Experimentally, there
is some evidence that fibrin seals are efficacious (23), but the true value has not been established.
 |
|
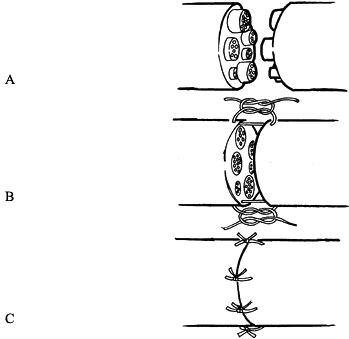
Figure 51.3. Cross section of a peripheral nerve, indicating principal structures.
|
carried out by placing several sutures peripherally in the epineurium
after aligning the nerve ends according to fascicular pattern and
epineurial landmarks. This technique has the advantage of being less
technically demanding, being less traumatic to the nerve ends, and
placing less suture material in the repair site. Epineurial repair is
indicated for small nerves, for nerves with only one or two fascicles,
and for primary repair of a clean laceration in a larger nerve.
fascicles are dissected free of enveloping epineurium and sutured
fascicle to fascicle through the perineurium. In principle, this method
should lead to a more precise coaptation and better recovery. In
practice, fascicular repair is technically demanding, inevitably causes
some trauma to the nerve ends, and leaves suture material within the
nerve, which may stimulate a fibrotic response. Nonetheless, in the
hands of those skilled in the technique, fascicular repair produces
good results, especially in nerves with two to five large fascicles or
if epineurium constitutes a large part of the cross-sectional area of
the nerve.
fascicular repair except that recognizable groups of fascicles are
joined instead of individual fascicles. The repair of more than five
fascicles or groups is impractical, because it excessively injures the
nerve end and leaves too much suture material within the wound. Group
fascicular repair is the technique employed in nerve grafting.
repair of a laceration of the median nerve at the wrist, where
fascicular repair of the motor component may be combined with
epineurial repair to approximate sensory elements. The choice of
technique depends on the topography of the nerve at the site of the
lesion. The superiority of fascicular or group fascicular repair over
epineurial repair has not been clearly demonstrated clinically or
experimentally. The immediate environment of wound healing, the
condition and age of the patient, and the skill of the
surgeon are probably more important than the type of neurorrhaphy.
excessive tension, interfascicular nerve grafting is indicated. This
technique was developed and refined by Millesi, whose series shows a
high percentage of good or excellent results (20,21).
The nerve stumps are stepcut, and the fascicles are debrided of
enveloping epineurial tissues. Corresponding fascicles or groups of
fascicles are identified by geometry or electrodiagnostic tests. Graft
segments long enough to bridge the gap without tension are sutured
through the perineurium to connect appropriate fascicles or groups of
fascicles in the proximal and distal stumps. Although the regenerating
axons must cross two suture lines, it is thought that the prognosis is
better for crossing two suture lines without tension than one suture
line under tension. The sural nerve is typically used as the graft;
this can yield a usable graft segment as long as 40 cm. The lateral
antebrachial cutaneous nerve can also be used (27).
prognosis, what constitutes excessive tension (and thus an indication
for grafting) is not universally agreed. Wilgis suggests that if an 8-0
suture cannot close the gap, tension is too great (31). Millesi recommends grafting gaps greater than 2.5 cm with the extremity in a functional position (20).
By mobilizing the nerve proximally and distally and flexing the joints
it crosses, gaps of several centimeters may easily be closed. Flexion
of the elbow more than 90° or the wrist beyond 40° to close a gap is
contraindicated. The surgeon must judge the potential morbidity of
postoperative immobilization of the joints in a flexed position against
the morbidity of nerve grafting in light of his or her own skills and
experience.
-
Prepare and drape the entire extremity
into the operative field, because it is often necessary to mobilize the
nerve proximally and distally for considerable distances. -
Prepare and drape one or both lower extremities if nerve grafting is a possibility.
-
Apply a tourniquet to each extremity being draped.
-
Use a generous, extensile incision.
-
In freeing the nerve, work from normal
nerve toward the lesion: distalward in the proximal portion and
proximalward in the distal portion. Keep exposed portions of the nerve
moist with saline-soaked sponges. -
Handle the nerves very gently using a jeweler’s forceps to grasp only the epineurium.
-
Avoid applying any pressure to the fascicles, which may result in further injury.
the nerve and its dissection. Final preparation of the nerve ends and
suturing are facilitated by an operating room microscope, enabling
proper grouping of similar nerve fascicles, aligning of epineurial
landmarks with proper orientation, and more accurate placement of
sutures. Use microsurgical instruments to perform nerve repair and
nerve grafts.
-
Place a moist wooden tongue depressor beneath the end of the nerve.
-
While an assistant applies gentle
traction on the nerve end with a jeweler’s forceps, use a Weck blade to
cut back the nerve ends sharply until noninjured tissue is reached. -
Inspect the proximal portion of the cut ends under the microscope to look for bulging axons.
-
Repeat these steps on the opposite nerve end.
-
After both ends have been resected until normal-looking structures are seen, the repair can begin.
-
Inspect the epineurium for longitudinal
blood vessels as a landmark to orient the nerve. Inspect the fascicular
pattern of the proximal and distal stumps for orientation. -
Have the assistant take the tension off
the nerve by grasping the proximal and distal segments of the nerve
about 1 cm away from the repair and approximating the nerve ends. If
the tension is observed to be great or approximation is not possible,
consider further mobilization of the nerve or grafting. -
In an epineurial repair, after alignment
of the nerve, pass the appropriate suture proximally and distally in
the epineurium only, and tie the knot firmly. Pass a second suture 180°
opposite to the first suture in a similar fashion. Leaving the strands
of these two initial knots long may assist in turning the nerve. Use
the minimal amount of sutures necessary to close the entire epineurium
on the anterior side, and repeat this, having turned the nerve around,
on the posterior side. Use a nonabsorbable 9-0 suture (nylon) for
larger nerves, and a 10-0 nonabsorbable suture for smaller nerves (Fig. 51.4).![]() Figure 51.4. Epineurial repair. A: Nerve ends are freshly cut back to well-visualized fascicles. B:
Figure 51.4. Epineurial repair. A: Nerve ends are freshly cut back to well-visualized fascicles. B:
Two sutures are placed into the epineurium at 180° from each other,
maintaining alignment of the fascicles in the proximal and distal
stumps. C: The remainder of the epineurium is approximated using the minimal number of simple sutures necessary to complete the closure.In a fascicular repair: -
Identify the fascicles proximally and distally by observing the cross-sectional anatomy under the microscope.
-
Coapt the ends of the matching funiculi,
placing 10-0 nylon interrupted sutures through the interfascicular
epineurium and the perineurium of the individual fascicles. Avoid all
tension (Fig. 51.5).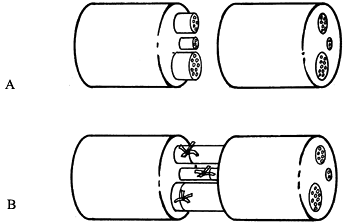 Figure 51.5. Fascicular repair. A: Individual fascicles are identified proximally and lined up with their distal counterpart. B: These individual fascicles are then approximated with minimal sutures. The epineurial layer is then repaired as shown in Figure 51.4B and Figure 51.4C.P.1540In a grouped fascicular repair:
Figure 51.5. Fascicular repair. A: Individual fascicles are identified proximally and lined up with their distal counterpart. B: These individual fascicles are then approximated with minimal sutures. The epineurial layer is then repaired as shown in Figure 51.4B and Figure 51.4C.P.1540In a grouped fascicular repair: -
Identify groups of fascicles by observing
proximal and distal cross-sectional anatomy, and join these groups with
interrupted 10-0 nylon suture placed in the interfascicular epineurium.In mixed fascicular and epineural repair: -
Line up the major group of fascicles that can be identified, and perform a grouped fascicular repair.
-
Then repair the epineurium circumferentially around the entire nerve as described under epineurial repair.
-
Explore and prepare the nerve stumps, as described previously.
-
Examine the cross-sectional anatomy, and isolate the fascicle groups.
-
Measure the distance between the two stumps, with the elbow and wrist joints extended.
-
Pass a 10-0 nylon stitch between the
fascicles of the graft, catching the interfascicular epineurium, and
pass this stitch into the proximal stump of one group of fascicles. -
Approximate the distal end of the graft to the stump of the corresponding distal fascicle group in a similar fashion.
sural nerve. The use of this nerve creates a sensory loss at the
lateral side of the foot below the lateral malleolus, which causes no
problem in the vast majority of patients.
-
Locate the nerve behind the lateral malleolus with a 1 cm longitudinal incision.
-
Tag the nerve with umbilical tape. A few centimeters proximal to this incision, make a small transverse incision over the nerve.
-
Pull on the nerve distally with slight tension so that it can be easily palpated in the proximal incision.
-
Place two to three more incisions
proximally over the nerve at equal intervals to identify its full
length. Then transect the nerve through the most proximal incision to
harvest the entire segment of the sural nerve. -
Close the wounds as usual and drain if appropriate. Avoid suction drains placed close to a nerve repair or nerve graft, because they may disrupt the suture line.
through a single distal incision, but great care must be taken not to
cut the nerve too short. Other sources of donor nerve include the
lateral and medial antebrachial cutaneous nerves and the terminal
articular branches of the posterior interosseous nerve, but the sural
nerve provides the longest nerve segment with the least morbidity.
exactly where the location of repair is in relation to surface anatomy.
Each juncture should be measured and noted so that after surgery it is
possible to follow the progression of Tinel’s sign (i.e., distal
tingling on percussion). If using a long interfascicular graft,
progression may cease at the distal nerve juncture. The surgeon knows
that this is happening
if the progression of Tinel’s sign ceases at the same point noted for the distal juncture.
during the operation for 10 to 14 days. After wound sutures are
removed, place the extremity in a plaster cast in a position to relax
all suture lines. For example, a median nerve repair in the forearm
requires the wrist to be in slight flexion and the elbow placed at 90°
of flexion. Continue immobilization for a total of 6 weeks, but at 4
weeks start to straighten out the joints that have been splinted in a
position to avoid tension on the nerve suture line.
repair should not cause the surgeon to lose sight of the goal, which is
a functional patient. Although nerve repair is feasible, the patient
may be better served by other procedures, such as tendon transfers. If
an older patient is well adapted to the disability, it may be better
not to intervene despite the possibility of improved function.
regenerating neurons will make connections to sensory or motor end
points different from those to which they were formerly connected (7).
Although they recover sensory function and transmit signals to the
brain, the meaning of these signals is garbled if they are evaluated in
the cortex according to preinjury habits of interpretation. The patient
must be reeducated to correlate sensory input with external reality. In
children, this is readily accomplished. Hudson et al. reported their
results on 18 children with lacerations of the median nerve treated by
primary epineurial repair (13). The mean return of motor power to the opponens pollicis was 4.5 and the mean static two-point discrimination was 5 mm (13).
Older patients are less flexible and may ignore sensory information
supplied by heterotopically regenerated nerves. In this case, the
anatomically successful nerve repair is in vain. An aggressive program
of therapy aimed at sensory reeducation may considerably improve the
clinical results of nerve repair by training the patient to adapt to
the altered arrangement of the peripheral axons.
grafting. Experimentally, nerve regeneration across peripheralnerve
allografts compared favorably with control autografts in primates when
they were immunosuppressed with Cyclosporin A (1).
It does not seem worthwhile, however, to run the risks of
immunosuppressing patients for this purpose. Others have experimented
with various biologic materials to act as nerve conduits (4,6,9,11,29,33).
synthesis and release of neurotrophic factors, which play a role in the
regeneration process. There has been significant interest in using
silicone tubes for nerve repair both to handle a small gap and to
capitalize on these neurotrophic factors (12,30)
to increase the specificity of neural connections (motor to motor and
sensory to sensory). Animal studies have shown some conflicting
results. Some have demonstrated that there is merit to this technique(12,19);
Brushart’s experiments demonstrated no evidence for neurotropic
interactions promoting correct fascicular reinnervation in a mixed
nerve (2). Using a silicone tube, he could not
find any neurotropic factors that promote fascicular specificity and
thus stated that an “enclosed gap is not an acceptable substitute for
nerve graft when reconstructing a nerve that serves multiple functions”
(2).
Lundborg used silicone tubes of appropriate size to enclose the injury
zone, intentionally leaving a gap measuring 3 to 4 mm between the nerve
ends inside the tube. His early results from a prospective, randomized,
clinical study comparing this technique with conventional microsurgical
technique for repair of human median and ulnar nerves showed no
difference between both techniques, with the exception of perception of
touch, which showed a significant difference at the 3-month checkup in
favor of the tubulization technique. Furthermore, at reexploration 11
months after the initial procedure Lundborg reported that the former
gap was replaced by regenerated nerve tissue in direct continuity with
the proximal and distal parts of the nerve trunk, with the exact level
of the former injury being impossible to identify (17). I witnessed this reexploration while I visited Lundborg and confirmed that this finding was quite accurate.
nerve graft in which multiple polyamide (nylon) filaments were placed
inside silicone tubes to make an intrinsic and extrinsic framework,
respectively, for regenerating axons. Early animal experiments have
shown that the new artificial nerve graft can be used to support
regeneration across extended gaps in nerves (15,16,28).
of end-to-side repair. The possibility that collateral sprouting can
occur from intact axons in an undamaged nerve, induced by factors from
the attached nerve segment, and subsequently can make functional
peripheral connections may permit damaged nerves to be sutured to
nearby
normal nerves. Lundborg et al. studied this concept in the rat by
suturing either a 7-day predegenerated or a fresh nerve segment in an
end-to-side fashion to the sciatic nerve proper (18).
Allowing time for recovery, the pinch reflex test was present in the
majority of cases using a predegenerated nerve segment but not in those
using a fresh segment, and neurofilament staining and histologic
examination confirmed the presence of axons in the attached nerve
segment(18).
scheme: *, classic artice; #, review article; !, basic research
article; and +, clinical results/outcome study.
JR, Mackinnon SE, Hudson AR, et al. The Peripheral Nerve Allograft in
the Primate Immunosuppressed with Cyclosporin A: I. Histologic and
Electrophysiologic Assessment. Plast Reconstr Surg 1992;90:1036.
JS, Green CJ. Nerve-muscle Sandwich Grafts: The Importance of Schwann
Cells in Peripheral Nerve Regeneration Through Muscle Basal Lamina
Conduits. J Hand Surg [Br] 1995;20B:423.
SJ, Harii K, Lee FM, et al. Electrophysiological, Morphological, and
Morphometric Effects of Aging on Nerve Regeneration in Rats. Scand J Plast Reconstr Surg Hand Surg 1995;29:133.
O, Guerit JM, Van Wijck R. Sciatic Nerve Regeneration in the Rat after
Frozen Muscle Grafting. A Comparative Study Using Somatosensory Evoked
Potentials. J Hand Surg [Br] 1996;21B:53.
AC, Glasby MA, Lawson GM. Immediate and Delayed Nerve Repair Using
Freeze-thawed Muscle Allografts. Associated Long-bone Fracture. J Hand Surg [Br] 1998;23B:360.
SM, Enver K. Axonal Regeneration Through Heat Pretreated Muscle
Autografts. An Immunohistochemical and Electron Microscopic Study. J Hand Surg [Br] 1994;19B:444.
G, Rosén B, Dahlin L, et al. Tubular Versus Conventional Repair of
Median and Ulnar Nerves in the Human Forearm: Early Results from a
Prospective, Randomized, Clinical Study. J Hand Surg [Am] 1997;22A:99.
G, Zhao Q, Kanje M, et al. Can Sensory and Motor Collateral Sprouting
Be Induced from Intact Peripheral Nerve by End-to-side Anastomosis? J Hand Surg [Br] 1994;19B:277.
ZJ, Lu SB. Selective Reinnervation of Regenerating Mixed Nerve Fibres
Across a Silicone Tube Gap. Further Experimental Evidence of
Neurotropism. J Hand Surg [Br] 1996;21B:660.
M, Royce CJ, Coates P. The Lateral Antebrachial Cutaneous Nerve as a
Highly Suitable Autograft Donor for the Digital Nerve. J Hand Surg [Am] 1983;8A:942.
N, Bjursten LM, Dohi D, Lundborg G. Bioartificial Nerve Grafts Based on
Absorbable Guiding Filament Structures—Early Observations. Scand J Plast Reconstr Surg Hand Surg 1997;31:1.