NONUNIONS AND MALUNIONS OF THE TIBIA
II – FRACTURES, DISLOCATIONS, NONUNIONS, AND MALUNIONS > Malunions
and Nonunions > CHAPTER 31 – NONUNIONS AND MALUNIONS OF THE TIBIA
severe and disabling. Failure of tibial fractures to unite may lead to
multiple operative procedures and prolonged hospitalization with years
of disability before a union is obtained or amputation is performed.
Although high rates of union have been obtained in some series of
tibial fractures with simple methods, nonunion is frequently seen by
practitioners treating tibial fractures (20,36,96,112,138).
the fracture in a nonanatomic position. Functional malposition rather
than cosmetic deformity constitutes an indication for surgical
intervention.
shortening. Shortening of less than 1 cm is acceptable. Shortening of
greater than 15 mm may be symptomatic and necessitate a lift or
leg-length equalization procedure. Varus and valgus angulation should
be limited to less than 10°. Recurvatum is less well tolerated than
anterior bowing and should not exceed 10°. Anterior bowing of less than
10° is rarely of functional significance but may be cosmetically
unacceptable. Rotatory malalignment is better tolerated than
varus-valgus angulation. Internal rotation exceeding 5° is noticeable,
and that exceeding 10° is usually unacceptable. External rotation is
better tolerated but will become noticeable when it exceeds 10° and may
become functionally significant when it exceeds 15°. Pre-existing
deformity such as genu varum and genu valgum, and pre-existing
arthritis or joint instability may make angulation, which ordinarily
would be acceptable, a problem
sufficient
to require correction. Experimental evidence, obtained from
pressure-sensitive film, suggests that angular deformities in the
distal third of the tibia are more likely to affect contact area and
joint pressures in the ankle adversely, whereas proximal angular
deformities have a larger impact on the knee joint (85,86,123).
Although some degree of malalignment is common following tibial
fractures treated nonoperatively, osteotomy is rarely indicated. Close
follow-up of patients with acute tibial fractures and early
intervention in cases that develop angular deformity will prevent most
unacceptable malunions. Long-term clinical follow-up, however, has
failed to show any adverse effect of moderate angular deformities on
the subsequent development of knee or ankle arthritis (87).
author to author, the incidence of nonunion and delayed union can be
estimated from reports published in the literature. In 22 combined
series covering 5517 fractures, the incidence of nonunion was 2.5% and
the incidence of delayed union 4.4% (1,20,23,25,34,36,38,51,68,73,80,95,100,106,108,112,115,124,129,130,138,140).
The studies included several large series of predominantly closed
tibial fractures caused by low-energy trauma. Open fractures with gross
contamination and extensive soft-tissue damage have a much higher
incidence of nonunion and delayed union. In series of open tibial
fractures, Clancy et al. (25) have reported a 13% incidence of delayed union, Widenfalk et al. (138) have reported a 31% incidence of delayed union, and Edwards et al. (40)
have reported a 41% incidence of grade III fractures requiring bone
grafting to achieve union. In type II and type III open tibial
fractures, Velazco et al. (130) have reported a
14% rate of nonunion. An incidence of tibial nonunions and malunions
above those reported in early series should be anticipated by the
orthopaedic surgeon practicing today in view of an increasing incidence
of high-energy trauma associated with motor vehicle accidents.
nonunion. Most are dictated by the injury, but others are within the
surgeon’s control. The incidence of nonunion, and especially of
infected nonunion, increases with the severity of open fractures. The
presenting factors cited as contributing to nonunion or delayed union
include fracture displacement, bone loss, associated fibular fractures,
comminution, and infection (41,68,73,80,95,108,128,133).
Therefore, the nature of an injury plays a large role in determining
the likelihood of union. A direct correlantion exists between the
energy absorbed by the hard and soft tissues and the complications of
wound healing, including delayed union, nonunion, infection, and skin
slough (24,62).
union of tibial fractures is the degree of preservation of the tibial
blood supply. The anatomy of the tibial blood supply has been described
in detail (78,94,104,126).
The three vascular systems supplying the tibia are the nutrient
vascular system, the periosteal vascular system, and the
epiphyseal-metaphyseal vascular system. The nutrient and periosteal
vascular systems are the most important in healing of tibial shaft
fracture. The nutrient artery system, which arises from the entrance of
the posterior tibial artery into the posterior tibial cortex, distal to
the soleal line, is divided at its origin into ascending and descending
branches. The nutrient vessels provide the endosteal blood supply to
the tibia, which MacNab has shown to supply as much as the inner 90% of
the cortex (78). Destruction of the endosteal
blood supply is most extensive when the fracture occurs in the middle
one third of the tibia, but the distribution of nonunions among
proximal, middle, and distal thirds of the shaft appears to be equal (78,96).
The periosteal blood supply receives segmental vascular contributions
from the surrounding soft tissues, which, in turn, are largely supplied
by the anterior tibial artery. Vessel penetration through the
interosseous membrane and the posterior periosteum is abundant. The
blood supply to the subcutaneous anteromedial periosteum, however, is
more precarious and thus more easily destroyed. Rhinelander (104)
has demonstrated that preservation of the periosteum results in
cortical fracture healing through transient expansion of the periosteal
blood supply to the inner cortical bone. The degree of periosteal
stripping and the resultant amount of devascularized bone varies with
the fracture type. Increased periosteal stripping, as seen in
high-grade open fractures, contributes substantially to delayed union
or nonunion.
shown to increase the incidence of nonunion. Distraction at the
fracture site and failure to immobilize the fracture adequately are
known to increase the time to union (27). Brown and Urban (20), Dehne et al. (36), and Sarmiento (112,113)
have advocated early weight bearing with cast or cast brace treatment
as a means of obtaining intermittent compression at the fracture site,
and have reported low rates of nonunion in their respective series.
Adherence to the principles of open fracture management, including
aggressive multiple debridements, administration of antibiotics, and
rigid immobilization of fracture fragments, has also been shown to
decrease the incidence of infection
and nonunion substantially. These issues are more fully addressed in Chapter 26.
The fracture configuration and location, classification of open
fracture, and history of infection of previous external fixation are
essential elements of the history. Physical examination determines the
status of the soft tissue, the presence or absence of a draining sinus,
and the neurovascular status of the foot. Radiographically assess
initial and current fracture alignment, and make note of any
significant bony defect that limits the choices for managing the
nonunion.
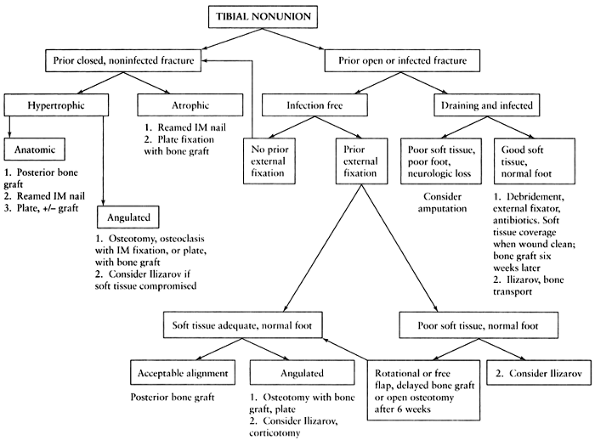 |
|
Figure 31.1. Algorithm outlining a simplified approach to the treatment of tibial nonunion.
|
prior history of infection, investigate infection as one of the causal
factors of nonunion. C-Reactive protein, sedimentation rate, and
indium-labeled bone scans may be helpful to assess the likelihood of
persistent infection. Do not administer preoperative antibiotics and
obtain intraoperative cultures. Patients with active infection should
undergo debridement and are frequently treated with a course of
intravenous antibiotics. If a bone graft is required, stage this
procedure after the infection has been treated to minimize the risk of
graft infection and sequestrum formation.
pseudoarthroses into hypertrophic and atrophic varieties. The
hypertrophic variety includes those that resemble an elephant’s foot, a
horse’s foot, and the oligotrophic variety. These nonunions have
adequate biologic potential and may go onto union by improvement in
mechanical factors, such as improved stabilization or interfragmentary
compression. The atrophic variety includes the dystrophic, gap, and end
stage atrophic classifications. These nonunions lack adequate biologic
potential and require bone grafting to stimulate successful healing.
should not be overlooked in attempts to achieve bone union. Amputation
is a reasonable alternative to heroic efforts at gaining union in a
tibia with major bone loss, infection, and posterior tibial nerve
disruptions. Early amputation in those injuries in which limb salvage
is inappropriate enhances patient survival; reduces pain, emotional
distress, and disability; and shortens hospitalization time and costs (11,102). Limb salvage and reconstruction is
indicated for salvageable injuries. Although it is more expensive and
requires multiple operative interventions, better functional outcomes
have been reported for limb salvage when compared with amputation (61).
termed fracture disease—muscle atrophy, muscle and tendon contractures,
joint stiffness, and disuse osteoporosis (91).
Many paths to fracture disease have been described. Prolonged
immobilization in plaster has been demonstrated to result in a high
percentage of fracture union, even in cases of open fractures, but it
has also been associated with residual foot and ankle stiffness, knee
stiffness, and muscle atrophy (20). Plaster
immobilization is best limited to initial management of closed
fractures and use in combination with bone grafting. Sarmiento (112,113)
has advocated the use of patellar tendon–bearing cast and braces, which
allow knee motion, and these aids should be substituted for long-leg
casts whenever possible. In addition to encouraging union, therefore,
you should initiate any new intervention with the goals of minimizing
immobilization and hastening return of the patient to maximum activity
with a low risk of complications from the intervention.
malalignment substantially alter treatment options in tibial nonunions.
The closed, uninfected nonunion with an anatomic alignment requires
intervention only to achieve union; posterior lateral bone graft,
closed reamed intramedullary nail, and application of a compression
plate with or without bone graft are all acceptable solutions to the
problem. Malalignment requires osteotomy or osteoclasis in addition to
bone grafting and internal fixation. Atrophic nonunions require
decortication of the nonunion site with bone grafting and internal
fixation. Infected nonunions pose the greatest challenge. Acceptable
approaches to infected nonunions include adequate debridement and
soft-tissue coverage, in addition to bone stabilization and bone
grafting.
nonoperative alternative treatment for established nonunions.
Considerable laboratory and clinical evidence has been accumulated to
suggest that electrical stimulation enhances fracture healing (75).
Three forms of electrical stimulation have been used clinically: direct
current, capacitive coupling, and inductive coupling. The direct
current method requires a surgical procedure for implantation and a
second surgical procedure for removal. A successful union rate of 83.7%
has been reported in 178 nonunions treated with adequate duration of
constant direct current. This series included a 91.5% union rate in 82
tibial nonunions treated with contant direct current (15).
developed by Brighton et al. The cathode is inserted percutaneously
directly into the nonunion site, requiring drilling across one bone
cortex or fragment. Weight bearing is prohibited in the first 12 weeks
of treatment (16). Complications in Brighton’s
series include pin tract infection (13.8%), broken wires (13%),
recurrent osteomyelitis (4.2%), cathode dislodgement (3.6%), and
battery pack failure. A “corrected” union rate (excluding all patients
with “suboptimal” electricity or gaps greater than half the bone
diameter) of 80% was reported for tibial nonunions (14).
both be administered with externally applied fields. Although these
methods are noninvasive, they require patient compliance. Bassett et
al. reported an 87% success rate using electrical stimulation in 127
tibial nonunions (5). Brighton and Pollack
reported a 77% success rate of capacitive coupling electrical
stimulation in the treatment of 22 established nonunions (17).
performed a prospective double-blind trial of capacitive coupling
electrical stimulation treatment of tibial delayed unions. Successful
healing was achieved by twelve weeks in five of 20 patients treated
with electrical stimulation compared with one of 25 patients treated
with the placebo unit. Scott and King (114)
performed a prospective double-blind trial of capacitive coupling
electrical stimulation treatment of nonunions in long bones. Successful
union was achieved in six of the ten patients treated with electrical
stimulation, while none of the eleven patients treated with the placebo
unit healed.
direct current, capacitive coupling, and bone graft treatment of 271
tibial nonunions in a retrospective study using logistical regression.
They identified seven risk factors that adversely affect union rates:
duration of nonunion, prior bone graft, prior electrical treatment,
open fracture, osteomyelitis, comminuted or oblique fractures, and
atrophic nonunions. In the absence of any risk factors, they reported
no significant difference in the success rates of the three treatment
groups. Healing rates decreased with an increasing number of risk
factors. Capacitive coupling had a poorer union rate in cases of
atrophic nonunions and bone graft surgery had a poorer union rate if
there had been a previous unsuccessful bone graft procedure.
unions in which patients prefer nonoperative care, when further
operative intervention is contraindicated due to severe medical
problems, and occasionally, as an adjunct with other forms of surgical
treatment in patients with poor prognostic factors, such as smokers.
viable soft-tissue coverage as well as on skeletal stability. During
the past 20 years, an increased understanding of soft-tissue anatomy
and physiology, as well as technical advances in tissue transfer, has
improved limb salvage rates, improved function results, and reduced the
length of hospitalization for many patients. The effectiveness of
aggressive debridement of necrotic tissue and attentive wound care in
treating open tibial fractures was demonstrated by Dehne et al. (36) in 1961, but this treatment had the disadvantage of involving long hospital stays. In 1970, Witschi and Omer (140)
reported on 84 open tibial fractures resulting from missile injuries in
Vietnam. In all cases, routine wound care combined with delayed
split-thickness skin grafting led to initial soft-tissue healing.
Split-thickness skin grafts in more extensive degloving or avulsion
injuries will not survive, however, if the recipient bed contains
poorly vascularized fat or bone without periosteum. Such cases often
require alternative methods of coverage involving transfer of more than
skin.
the techniques of muscle flap coverage, which had been previously
described by other authors, for the proximal two thirds of the tibial
shaft. Gastrocnemius flaps for the proximal third and soleus flaps for
the middle third of the tibia have since become standard. These axially
based muscle flaps provide their own vascular system and permit
application of a split-thickness skin graft.
problem, which is best approached with free-tissue transfers and the
techniques of microvascular anastomoses. Weiland et al. (137)
have stressed the importance of achieving a clean surgical wound before
performing free-tissue transfer or any reconstructive soft-tissue
procedure. Initial success rates of 79% have been reported with
latissimus dorsi flaps, but further follow-up revealed recurrent sepsis
in an additional 20% of cases (136). Details of these procedures and results are described in Chapter 8, Chapter 35, and Chapter 36.
obtained by local rotational flaps. Both bipedical flaps and distally
based fasciocutaneous flaps have been described to obtain coverage of
the lower leg (39,43).
Although these procedures avoid the complexity and morbidity of a
free-flap procedure, they do not provide any additional blood supply to
improve the fracture healing potential.
been described. These include many forms of sliding-onlay grafts, inlay
grafts, nonvascularized fibular transplants, and other techniques for
creating tibiofibular synostosis (13,21,22,35,45,55,88,132).
Cortical bone grafts have demonstrated weakness due to prolonged
remodeling. They incorporate within 6 weeks of grafting, remain weak
for at least 6 months, and in comparative series, have required a
longer time to achieve union and have been associated with more
complications than have cancellous grafts (42,63).
Alternatives to cancellous bone grafting that have recently been
proposed include the use of percutaneous marrow injections, human bone
morphogenic protein, and synthetic bone graft substitutes (28,29,65,82).
Although they are promising, these techniques are not all readily
available to practicing physicians, and the precise role of each of
these alternatives in a general orthopaedic practice has yet to be
defined. Further details about these techniques are discussed in Chapter 9.
form of bone graft. Anteromedial or posteromedial bone grafting is
preferable for proximal nonunions (6). The posterolateral approach described by Harmon (58)
is best in the distal two thirds of the tibia; it avoids the typical
anteromedial open wound and places bone in the most vascular portion of
the leg. In several large series, in which cancellous bone grafting was
used for tibial nonunions, union rates of 87% to 100% have been
reported (47,56,57,66,67,73,83,117,122).
Local complications, which are uncommon, include stiffness, deformity,
and loss of ankle motion. These complications have been ascribed to the
initial injury but may be aggravated by further prolonged
immobilization associated with bone grafting. Paresthesia in the sole
of the foot and delayed vascular impairment have been reported but seem
to be rare (66,67). Formation of a synostosis has been associated with, but is not clearly the cause of, ankle pain (118).
Because these complications are uncommon and the union rate following
posterolateral bone grafting is high, it is often used in conjunction
with other procedures for angulated or infected nonunions.
several locations. Some authors have advocated obtaining graft from the
distal femur; however, the patient must not bear weight on the limb for
6 weeks postoperatively to protect against fatigue fractures (71).
When an extensive amount of cancellous bone is required, we prefer to
use the posterior pelvis because it provides the greatest quantity of
graft. An acetabular reamer may be used to harvest cancellous bone
graft efficiently from the posterior iliac wing, but we routinely use
an osteotome and curets (110). Use of an
oblique incision perpendicular to the iliac crest paralleling the
fibers of the cluneal nerve has been advocated to decrease symptoms of
numbness and tenderness at the donor site (26).
Harvesting cancellous bone from the anterior iliac crest allows for
supine positioning; however, a smaller amount of bone can be obtained.
Iliac
crest bone graft harvesting may result in donor site morbidity (46).
Major complications have been reported to occur in 5.8% to 10% of
cases, and minor complications have been reported in 10% to 39 % of
cases (3,4).
the tibia, we prefer an anteromedial approach to a posterolateral
approach. Owing to difficulties in management of the popliteal artery
trifurcation and the vascular supply to the entire lower extremity, the
former approach is safer and more easily accessible. Surgery can be
performed with the patient supine, and the anterior iliac crest
frequently provides enough bone graft for union to be achieved.
-
The anteromedial approach provides a
smaller tissue envelope for massive amounts of cancellous bone than
does the posterolateral approach. The incision is frequently dictated
by the injury. However, in virgin skin, make a straight vertical
incision just medial to the tibial crest and straight to bone. -
Take great care in dissecting the soft
tissues off the anterior tibia. The development of skin flaps will
compromise soft tissue, cause skin sloughs, and may necessitate skin
grafting or rotational flaps as salvage procedures. -
Once the cancellous bone has been
harvested, shave the hypertrophic nonunion to recreate the normal
contour of the bone, and pack the cancellous bone anteromedially and
anterolaterally. Do not attempt to dissect deep into the anterior
compartment along the lateral crest of the tibia; this may injure the
already compromised soft tissues and vascular structures.
proximal third when the anterior soft tissues are poor or a
pre-existing flap makes an anterior approach problematic. In addition,
there is limited space for a bone graft anteriorly, particularly if
plating is necessary. Posteromendially, the approach is simple and
provides exposure from the knee joint to the ankle joint. Application
of a plate posteriorly works well, and soft-tissue coverage is superior
to an anterior approach.
-
Make a longitudinal incision along the
subcutaneous posteromedial border of the tibia beginning as proximal as
necessary and extending distally. -
Avoid injury to the saphenous nerve and vein. Expose the posterior tibia by subperiosteal dissection.
-
Expose the tibia as far laterally as required, avoiding injury to the vessels of the trifurcation.
-
Apply plate fixation or bone graft as indicated.
the patient prone. This allows a two-team approach; one surgeon can
begin harvesting the bone graft from the posterior iliac crest while
the other begins the approach to the tibia. This method decreases
surgical operating time and provides optimal access to the posterior
crest of the ilium and tibia (Fig. 31.2)
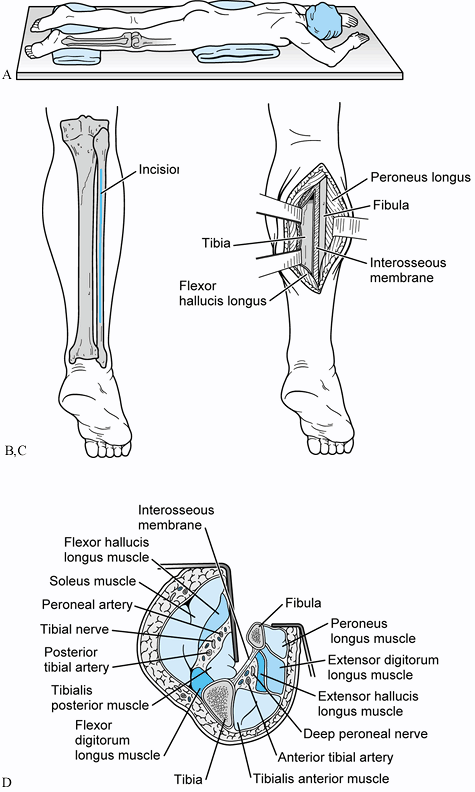 |
|
Figure 31.2. A: Patient positioned prone for a posterolateral bone graft. B: Incision placed directly over fibula. Avoid the sural nerve distally. C: The interval between the lateral and posterior compartments is developed. D:
Cross-sectional view of the approach developed between the continuum of fibula, interosseous membrane, tibia, and the tibialis posterior muscle. This interval protects the peroneal artery, the posterior tibial artery, and the tibial nerve from direct injury by interposing the tibialis posterior between the osseous and neurovascular structures. |
-
Depending on the level of the nonunion,
make a generous skin incision from 10 cm above to 10 cm below the level
of nonunion. Avoid injury to the sural nerve. -
Dissect longitudinally, medial to the
palpable border of the fibula, between the gastrocnemius and soleus,
and flexor hallucis longus and the peroneal muscles. After the fascia
of the soleus has been entered, a fixed palpable fascia on the
anteromedial border of the fibula provides a landmark for the next
level of dissection. Avoid lateral stripping of the peroneal muscles
from the fibula—this procedure unnecessarily compromises the regional
blood supply. -
Reflect the soleus and flexor hallucis
longus muscles using a periosteal elevator; take care to remain on the
fibula while elevating in a longitudinal direction. Once the flexor
hallucis has been completely freed, pass the elevator against the
fibula anterior to the posterior tibial muscle and neurovascular bundle
to elevate the posterior compartment from the fibula. Be very careful
at this level to avoid inadvertent entry into the posterior tibial
compartment, which could injure the posterior tibial neurovascular
bundle. Dissection should always
P.983
begin in areas of known anatomy far away from the fracture and nonunion site.
-
With the patient in the prone position,
the interosseous membrane is deep to the fibula and runs more or less
anterior to posterior (vertically). Keep this in mind because it
facilitates exposure of the membrane and contiguous tibia. -
Most nonunions are fibrous; therefore, it is not necessary to take down the nonunion site.
-
In difficult nonunions, strongly consider
extending the fusion mass across the interosseous membrane to
incorporate the fibula. The creation of a tibiofibular synostosis may
ensure a stable leg even if the nonunion does not heal. The synostosis
rarely compromises ankle motion. -
To avoid compartment syndrome, do not close the deep fascia.
-
Once the appropriate plane has been
entered, carry the dissection to the nonunion site, which frequently is
encased in a scar. At this point, elevate the posterior tibial
compartment off the interosseous membrane with a periosteal elevator.
Retract the posterior compartment, neurovascular bundle, soleus, and
gastrocnemius with Bennett or Hohmann retractors. -
After the nonunion has been identified,
“petal” or “shingle” the shaft from 5 cm above to 5 cm below the
nonunion with a sharp ½-inch osteotome. Copiously irrigate the wound,
secure hemostasis, and pack the harvested cancellous bone into this
compartment. After the bone graft is in place, insert a suction drain,
loosely close the soleus fascia to the peroneal fascia to hold the bone
graft in position, and close the skin in the standard fashion. Finally,
apply a long-leg Robert Jones dressing, which allows easy access to the
wound and drain. -
Once the wound heals, most patients can
be placed in a Sarmiento or short-leg cast and allowed to full weight
bear until union occurs. This can require up to 6 months.
have previously described the technique of open cancellous bone
grafting for the treatment of nonunions. Cancelleous bone chips are
added to the wound cavity once it has been covered by granulation
tissue. The cavity must be cup shaped to contain the graft. The bone
graft must be kept moist as the underlying granulation tissue invades
and vascularizes the graft. Union rates as high as 94% have been
reported by Roy-Camille et al. (107), but that level of success has not been achieved by other groups treating severe tibial defects.
prefer to use more advanced techniques with lower complication rates.
Four significant drawbacks have been outlined by Edwards et al. (40).
First, complete and rapid dissolution of the bone may occur if there is
any focus of infection adjacent to the wound bed. Second, graft
resorption or nonunion will occur unless there is rigid fixation. Cast
fixation is not adequate, and prolonged external fixation may be
necessary. Third, delayed healing, which occurs especially in the
posttraumatic hypovascular tissue bed and is another cause of prolonged
external fixation, is common. Fourth, structurally significant
remodeling and corticalization does not occur for many months after
union has been achieved. Graft fracture has been reported when an open
cancellous graft is used to replace circumferential diaphyseal bone
loss (53). Protected weight bearing may be
necessary for as long as 2 years. Open cancellous grafting, therefore,
is inferior to other available methods and should not be used for large
diaphyseal defects. It may be used for circumscribed metaphyseal bone
defects if more advanced techniques requiring associated soft-tissue
coverage procedures can not be performed.
the ability of bone morphogenetic protein (BMP) to heal critical
segmental defects successfully (30,31,50,127,128,141,143,144).
performed on patients with tibial nonunions. BMP-7 (also known as
osteogenic protein-1) in combination with purified type I collagen was
compared with autogenous bone graft in the treatment of 124 established
tibial nonunions in a multicenter trial. Although the radiographic
appearance of union in the BMP group lagged behind the autogenous bone
graft group, the ultimate clinical success rate as defined by pain-free
weight bearing and lack of need for reoperation was similar in the two
groups. A persistent nonunion required reoperation in 16% of the BMP
group and 18% of the autogenous bone graft group (93).
internal and external fixation devices. External fixation is the
preferred method of stabilization in cases of previous infection with
the risk of reactivation of quiescent sepsis, following grade II and
grade III open fractures, and in the presence of thin secondary
epithelium adherent to bone that might slough after surgical dissection
(54). Experimentally and clinically, the
single-frame anterior fixator using 4.5 mm or 5.0 mm half pins has been
shown to be the best device (8,9,142).
This form of external fixation provides free wound access, allows
stabilization of bone fragments at a distance from the lesion, permits
motion of adjacent joints, and encourages patient mobility. Pin tract
drainage, reported to occur in 5% to 10% of all pins and responsible
for removal or alteration of 1 out of 30 frames, may be minimized with
compulsive local pin care (7,52).
Success rates with compression plating alone have been reported to be
high in the treatment of hypertrophic nonunions, but supplementary bone
grafting is required for atrophic nonunions. Weber (134)
reported union in 126 of 127 uninfected tibial nonunions in which
compression plating was used. Like most other forms of internal and
external fixation discussed in this outline, compression plating allows
motion of adjacent joints and prevents “fracture disease.” Although
they have been proven successful in uninfected tibial nonunions,
compression plates do not tolerate weight bearing until healing has
occurred. For this reason, we prefer reamed nailing or posterolateral
bone grafting with functional casting or bracing to compression plating
in the treatment of tibial nonunions with acceptable alignment.
Compression plating is associated with unacceptably high infection
rates in previously infected tibial nonunions (120).
healing in 33 patients with malaligned tibial diaphyseal nonunions
treated by indirect reduction with a femoral distractor, tension band
plating, lag screw fixation, and autogenous bone grafting. Limiting the
soft-tissue dissection by using the femoral distractor to achieve an
indirect reduction was stressed as an important element of the success
of this method.
the treatment of uninfected angulated tibial nonunions. The plate may
sometimes be used to realign the fracture if it is positioned on the
tension side of the nonunion, placed under additional tension with an
independently placed distractor, and then used as a template for
fracture realignment. A closing wedge or oblique tibial osteotomy, with
or without fibular osteotomy, may be necessary to allow adequate
alignment of the tibia with the plate. Plate fixation may also be
performed in conjunction with a posterolateral bone graft. In this
case, apply the plate posteriorly where there is excellent muscle
coverage.
has recently been well described by Sangeorzan et al. (111). We have found the guidelines that they have published to be useful in preoperative planning (109).
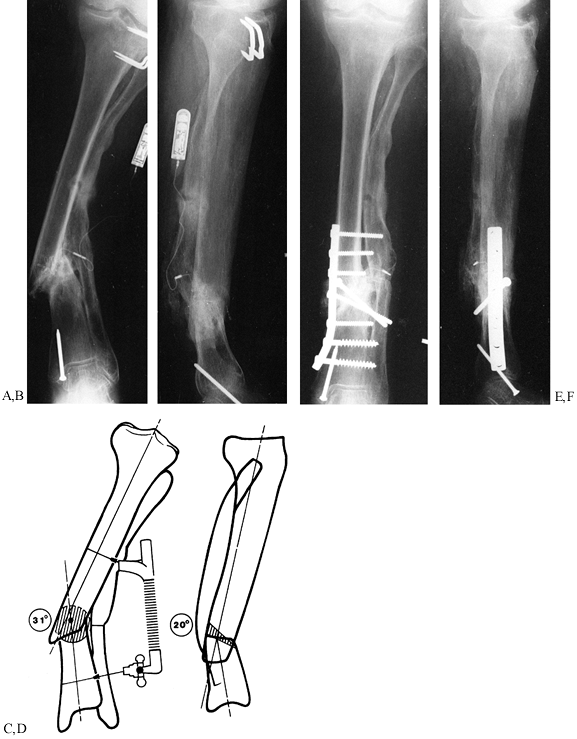 |
|
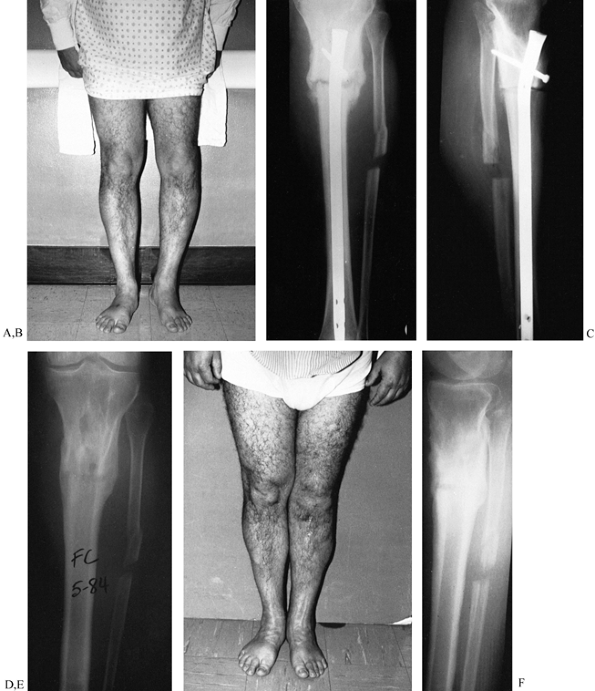
Figure 31.3. A, B:
The patient, a 75-year-old retired homemaker, sustained a closed tibia fracture 7 years ago. The fracture had been operated seven times previously, and the patient presented with pain and gross motion. Because of the high-risk nature of reconstruction, surgery was recommended only with the knowledge that amputation might result. Plate fixation was elected to stabilize the osteotomy to protect proximal bone for amputation if an infection occurred. C, D: On the frontal plane, a 31° valgus deformity must be corrected and the hypertrophic fibular osteotomized. The sagittal plane has a 20° apex posterior deformity, which can be corrected with a 2 cm wedge. The oblique nature of the osteotomy provides intrinsic stability to the plate fixation. The hypertrophic fibula may be used to anchor the plate screws with the severe osteopenia of the tibia. The AO/ASIF femoral distractor gives the surgeon mechanical advantage during straightening. E, F: The final result demonstrates near-anatomic alignment. The osteotomy is united, and the patient walks with a cane. To enhance stability, most screws were placed into the hypertrophic fibula, which offered better purchase than the osteoporotic tibia. Autogenous bone was harvested and packed around the osteotomy. Postoperatively, the patient was kept from weight bearing for 18 weeks and advanced as tolerated thereafter. After 1 year, she walks with a cane for support. |
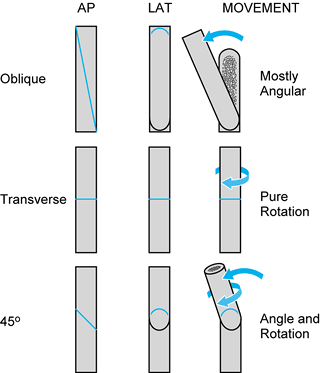 |
|
Figure 31.4.
The angle of a single-cut corrective tibial osteotomy determines possible corrections. A pure transverse osteotomy allows only rotational correction. A long oblique osteotomy leads to primarily angular correction, with slight rotational correction. Shorter oblique osteotomies, such as the 45° osteotomy shown, allows combined rotational and angular correction. The angle of the osteotomy can be precisely determined for complex deformities. |
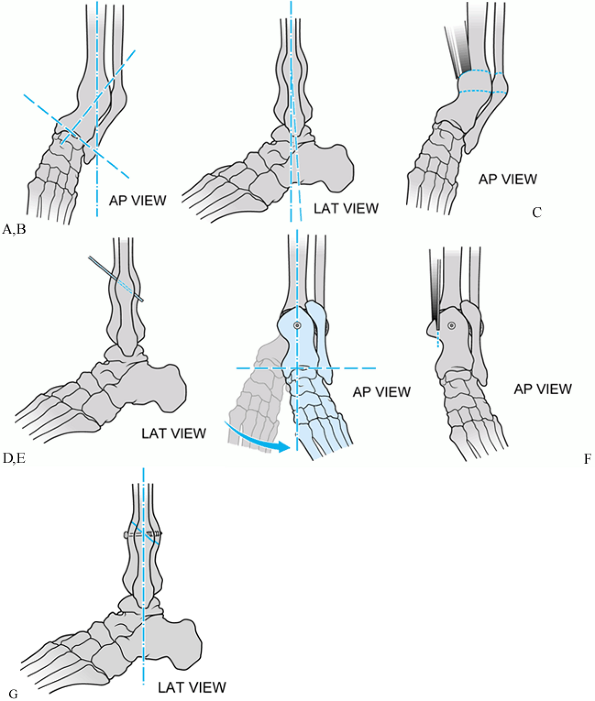 |
|
Figure 31.5.
Schematic of single-cut oblique tibial osteotomy for varus distal tibial nonunion. The deformity is entirely in the anteroposterior (AP) plane. A, B: AP and lateral views of deformity. C, D: Oblique osteotomy with an oscillating saw. E, F: Provisional single screw fixation of osteotomy. Neutral alignment attained. G: Oscillating saw used to resect excess overlapping bone. |
 |
|
Figure 31.6. Radiographs of single-cut oblique osteotomy for varus distal tibial nonunion. The deformity is entirely in the AP plane. A, B: AP and lateral views of deformity. C: AP view of provisional single-screw fixation of osteotomy. Neutral alignment attained. D.Lateral view of provisional single–fixation of osteotomy. E and F: Alignment maintained with application of neutralization plate.
|
-
First, the exact nature of the deformity
must be outlined with tracing paper in the frontal and sagittal planes.
Only when the exact nature of the deformity is well understood in both
planes can the osteotomy be planned accurately. -
Identify the central axis of the tibial
shaft and angular deformity, and outline the hypertrophic callus.
Closely scrutinize the status of the fibula. Superimpose the deformity
drawings on the contralateral normal tibia, which serves as a template
so that correct alignment can be achieved in the frontal and sagittal
planes, and also allows consideration for rotational realignment. -
Second, once the deformity has been
identified and both free fragments have been superimposed onto the
contralateral side, plan the appropriate contour of the bone and the
level of the osteotomy, and thus define the desired end result. The
type of osteotomy—opening wedge, closing wedge, oblique, or a
combination of these types—can be given several trial runs with tracing
paper superimposed on the contralateral side. -
Consider indirect reduction methods. The
femoral distractor, the articulating tensioner-compression device, and
pelvic reduction forceps used in distraction mode are all helpful in
obtaining length, correcting angulation,
P.989
and
obtaining optimal rotational alignment. If a compression plate is used,
determine the size of the plate, type and direction of the screws, and
the positioning of interfragmentary compression screws.
-
Do not resect fibrous tissue in
hypertrophic nonunions. Contour compression plates to fit over any
callous or fibrous tissue. Stabilization of the nonunion allows
calcification and conversion of the fibrous tissue into bone. -
Use interfragmentary lag screw fixation through the plate or independent of the plate whenever feasible.
-
Fibrous tissue usually needs to be
resected in atrophic nonunions. Re-establish the intramedullary canal
by using drills or curets to debride fibrous tissue from the
intramedullary canal. -
Use autologous cancellous bone graft in atrophic nonunions.
-
Place the bone graft peripherally beneath
healthy muscle, which will provide a good blood supply. Place no bone
graft in the intramedullary canal. -
Use a sharp osteotome to “petal” or
“shingle” the superficial cortex. This approach increases the surface
area, elicits a “reinjury” response, and stimulates the incorporation
of the bone graft.
-
Third, with tracing paper or a discarded
clear x-ray film, write each step of the surgical tactic and place it
on the view box before the operation to assist the operating room
personnel, the surgical assistant, and other surgical team members. In
the preoperative plan, include special anesthesia needs, patient
positioning, implants, reduction tools, dressings, and any
postoperative orthopaedic appliances necessary for nursing care. Such
compulsive planning allows the surgical team to be well informed and
thus saves operating time and optimizes the flow of surgery.
of angular malunions. Inconveniences associated with the need for
frequent postoperative visits, as well as a relatively high rate of pin
tract problems and a lower rate of major complications, has tempered
enthusiasm for this technique in less complicated cases. The
versatility of this method, however, may make it the salvage procedure
of choice for some of the more difficult tibial malunion problems that
would otherwise not be amenable to functional limb salvage. This
technique may be especially useful when shortening or compromised soft
tissue complicate the more readily approachable simple angular
malunion. Its benefits include early weight bearing and the ability to
correct contracted soft tissues gradually in addition to the bone.
Preoperative planning, as described earlier, is also recommended when
the Ilizarov technique is used. Further details of this technique are
described in Chapter 32.
treatment of the uninfected tibial nonunion. Nonreamed intramedullary
nailing, as advocated by Lottes (77) for the
treatment of nonunion, is a relatively simple procedure, especially if
the tibia is well aligned. However, unreamed nails that are not
interlocked do not provide adequate rotational control and function
solely as a form of internal splint. Locked intramedullary nails have
extended the indications for treatment of the initial fracture and
nonunions (70).
nonreamed nails, but prohibitively high infection and reinfection rates
preclude their use in infected or previously infected tibial nonunions (74,76) (Fig. 31.7).
Even recent reports advocating the use of reamed intramedullary nails
in the setting of previous infection document a reinfection rate of 22%
to 38% although union is achieved (89,119).
In the patient with a noninfected tibial nonunion, the procedure
stimulates the fracture site with the autogenous graft provided by the
reamer and provides greater stabilization than does nonreamed nailing.
Union rates of 95% to 100% have been reported with reamed nailing in
tibial nonunions and delayed unions with no prior history of infection (12,25,33). Mean healing time with reamed nailing in this setting ranges from 4 to 9 months (12,25,33).
Unlike compression plating, reamed intramedullary nailing offers the
advantage of full weight bearing after treatment, in addition to
allowing motion at the adjacent joints.
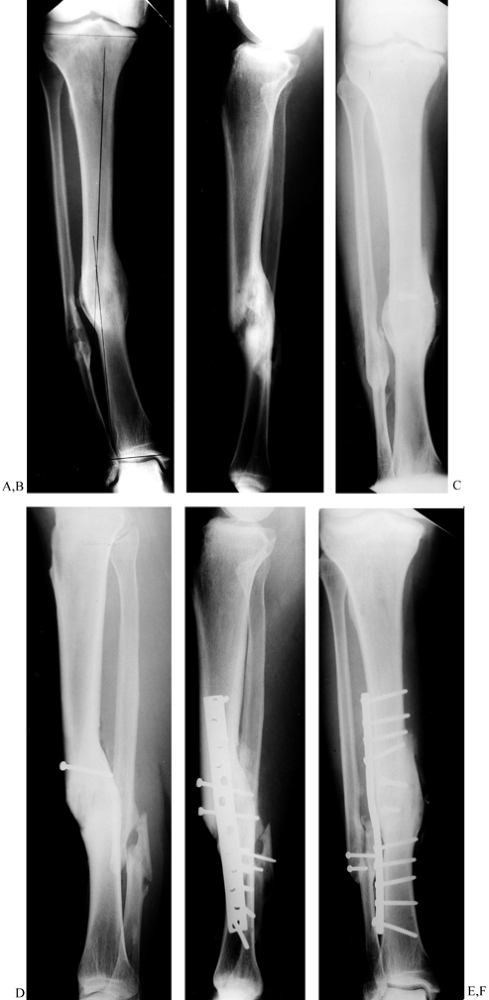
 |
|
Figure 31.7. A to C:
The patient is a young male with genu varum and a 13° proximal varus deformity of the left tibia secondary to a closed proximal tibia fracture. A valgus-producing wedge osteotomy was performed with static locked tibial nailing and fibular osteotomy. The patient was discharged fully weight bearing. D to F: Two-year follow-up with hardware removed and the clinical result. |
principles of management of tibial nonunions that is outlined earlier
in this chapter. The tibial blood supply has usually been adequately
re-established from surrounding soft tissues by the time reamed nailing
for nonunion is considered. Take care to avoid stripping soft tissues,
limiting surgery at the fracture site to the minimum necessary for
achieving alignment. In a previously nonsurgically treated tibial
nonunion, a very limited open procedure may be necessary to remove
fibrous tissue and bone to align the canal. If the tibia is malaligned,
a small osteotomy of the malunited fibula near the tibial fracture is
frequently necessary to achieve tibial alignment. Whenever possible,
perform reamed tibial nailing as a closed procedure.
tibial nonunions following previous unreamed nailing or a plate that
has maintained canal alignment, facilitating the insertion of a reamed
nail. Laboratory studies and clinical series validate this approach and
show that vascularity is usually sufficiently re-established after
plating to allow later intramedullary reaming (48,69,97).
Minimize soft-tissue stripping when removing the plate. Subcutaneous
plate and screw removal through multiple small incisions may be
preferable to a larger incision if skin quality is poor.
fractures, delayed union and nonunion is common. Fractures that show no
callus or evidence of healing by 12 weeks after fracture and nonunions
are excellent indications for closed exchange nailing with a locked
reamed nail. Early exchange nailing prevents breakage of the cross
screws or nail, which can complicate the procedure greatly.
-
Prepare and drape the extremity in the
usual fashion. Use a radiolucent operating table and a C-arm
fluoroscope. A fracture table is usually not necessary. -
Remove the existing nail and cross screws or plate and screws.
-
Insert a reaming guide pin.
-
Ream the intramedullary to at least 2 mm
larger than the nominal diameter of the canal or to a sufficient size
to accommodate a nail that is 10 mm in diameter or larger, depending on
the size of the patient. -
Place an AO femoral compression-distraction device with unicortical pins and compress the nonunion site.
-
Perform a fibular osteotomy, if necessary (see later), to achieve compression.
-
Insert the nail and lock it with two screws proximally and two screws distally.
-
Postoperatively, patients can usually progress to full weight bearing if good stability and bone contact is achieved.
intramedullary nailing following previous external fixation. Reamed
nailing may be safely performed after short periods of external
fixation before the development of pin tract colonization (2,10).
Deep infection rates from 19% to 66% have been reported for reamed
intramedullary nailing following prior longer term external fixation (81,84,125,139). The infection rate following intramedullary nailing is increased when there is a prior history of pin tract infection (81).
Because of the risk of infection, we do not recommend reamed tibial
nailing following prolonged external fixation and prefer to use
posterolateral bone grafting.
-
If the canal is occluded, try to open it using “closed” technique with hand Y-handle
reamers on a sharp-tipped large Küntscher-flagged guide pin driver
across the nonunion. Ream the canal proximally first to give room to
work. If this procedure is unsuccessful, it may be necessary to take
down the nonunion openly. -
Culture the reamings and administer appropriate intravenous antibiotics until the cultures are negative.
-
Locate the AO femoral distractor so that
it does not interfere with the crosslocking of the nail. Use it to
correct angular deformities as well (Fig. 31.8 and Fig. 31.9.).
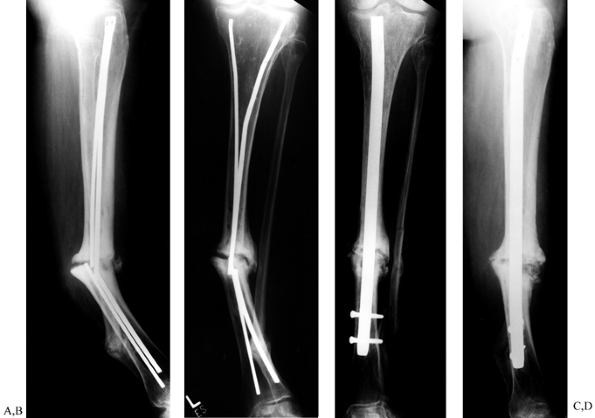 |
|
Figure 31.8. A, B:
Tibial nonunion with deformity. Intramedullary Enders nails have broken. The patient was unable to walk for more than 1 year following loss of fixation. C, D: Realignment attained with removal of small diameter nails; replacement was done with a reamed interlocked nail. Ambulation was regained within 6 weeks, and healing of the fracture was evident within 2 months. Mild valgus and recurvatum persist. |
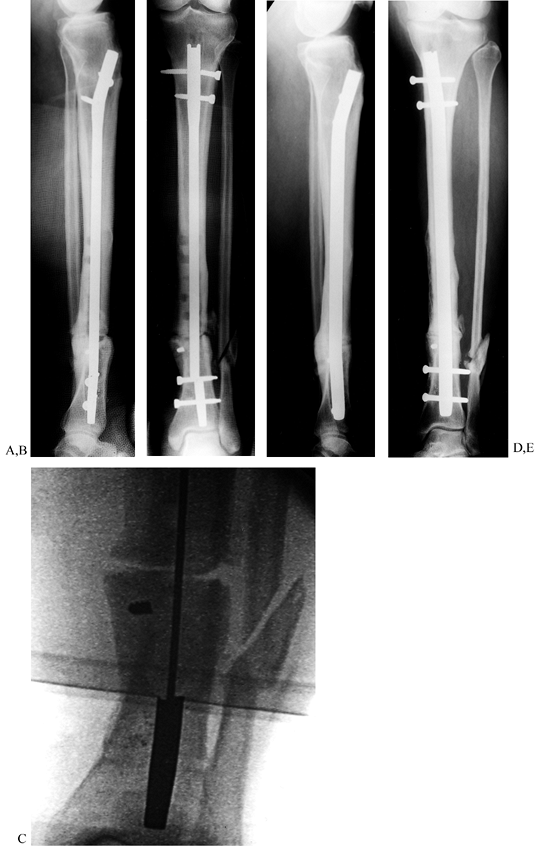 |
|
Figure 31.9. A common problem with broken interlocking implants associated with distal tibial delayed unions. A, B:
Broken tibial nail with nonunion at the junction of the middle third and distal third of the tibia. Plate fixation had initially been used unsuccessfully to treat this fracture. The large holes in the midtibia denote the location of broken screws that had been previously removed, requiring the use of hollow-milled drills. C: Intramedullary removal of a broken nail. Removal can be achieved with commercially available instruments that are specially designed for removal of broken intramedullary nails. The fracture site is not opened. D, E: Healed fracture after intramedullary nail exchange. The long interlocking screws were inserted to facilitate retrieval in anticipation of possible screw breakage and were later removed. |
osteotomy or resection for the treatment of tibial nonunions. The
fibula usually heals before the tibia. With some resorption of bone at
the fracture site, this process can interfere with apposition of the
tibia and produce relative distraction. This is especially true with
intramedullary nailing. The rationale for osteotomy is to permit the
tibial fracture to compress with walking. In three series, results
showed healing in 77%, with a mean time to union of 25 weeks in the
first; 87% union rate, with time to union not reported in the second;
and 100% union rate with a 14% refracture rate and a mean time to union
of 18 weeks in the third (37,44,121).
Advocates of fibular osteotomy cite the simplicity of the procedure,
its isolation from a possible infected site, and its applicability in
the presence of soft-tissue deficits. However, these arguments apply
equally well to posterolateral bone grafting, which does not involve
the additional risk of shortening and rotational deformity. Fibular
osteotomy is a useful adjunct to other procedures in restoring tibial
alignment, but we do not find it useful as an isolated procedure. There
is no evidence that this technique used alone is effective in the
treatment of infected tibial nonunions.
-
Make a 4 to 5 cm long longitudinal
incision directly over the lateral border of the fibula at the level of
the tibial fracture. Avoid injury to neurovascular structures. -
Either split the peroneal muscles
directly down to bone or dissect anteriorly or posteriorly in the
intermuscular interval. Expose 2 to 2.5 cm of fibula by subperiosteal
dissection. -
Place an anterior and posterior small sharp-tipped Hohmann retractor to protect the soft tissues.
-
With an oscillating saw using a small
blade, make a 45° longitudinal osteotomy. If necessary, complete the
osteotomy medially with an osteotome to avoid injury to the deep soft
tissues. -
Obtain hemostasis and close the wound in layers.
-
Do not resect a section of the fibula
because this procedure can lead to a symptomatic nonunion and negate
the use of the fibula in future reconstructions. -
The oblique osteotomy allows shortening, and it heals rapidly.
screws from one or both ends of the nail to permit compresssion of the
fracture with weight bearing is a common practice, is simple, and can
be performed as an outpatient procedure. Fibular osteotomy may also be
necessary. Beware, however, that dynamization can also increase
instability, particularly in rotation, worsening the situation. Follow
these guidelines for dynamization:
-
In small nonreamed nails, strongly consider exchange reamed nailing as described earlier.
-
Avoid dynamization in unstable fracture
patterns that will shorten excessively or become rotationally unstable.
The best configuration is a short oblique fracture. -
Remove the screws from only one end of the nail farthest from the fracture.
-
Permit the patient to bear weight in an appropriate orthosis.
result of severe open tibial fractures, the treatment of which is often
associated with severe contamination, neurologic or vascular injury,
and polytrauma. In the past, the treatment of choice for these injuries
has frequently been amputation.
approach to large segmental tibial defects has been initial surgical
debridement, stable external fixation, and soft-tissue coverage, with
late massive posterolateral cancellous bone grafting producing a tibia
profibula synostosis (Fig. 31.10). Using this
approach, a synostosis was created proximally and distally with
internal fixation of the fibula to provide a strut for subsequent
bone-grafting procedures. Achievement of a stable functional union
could take up to 2 years, and multiple bone-grafting procedures were
sometimes necessary. Intramedullary fixation and tibial plate fixation
are associated with a higher risk of infection and, therefore, are not
used in this setting. Although the length of treatment is prolonged,
requiring multiple hospitalizations, massive posterolateral bone
grafting appears to be a viable alternative in the treatment of
patients with severe segmental bone loss.
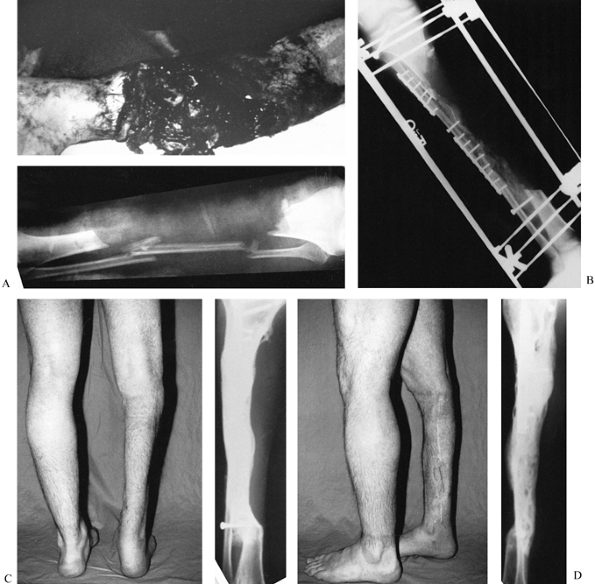 |
|
Figure 31.10. A:
The patient is a 25-year-old mill worker who had sustained a type III-C near-amputation of the tibia. The anterior tibial compartment and neurovascular structures were missing, leaving a major bone defect with normal plantar sensation to the foot. An amputation was advised, but the patient refused. B: The initial injury was treated with irrigation, debridement, and external fixation. After the soft tissues had been cleaned several times in the operating room, a delayed closure without major soft-tissue transfer was carried out. At 6 weeks, the patient had internal fixation of the synostosis of the tibia and fibula, and both posterior iliac crests were harvested for graft placement through a lateral skin incision. C, D: Two-year follow-up shows solid bone union and full weight bearing. The patient required a second osteosynthesis of the proximal synostosis with plate fixation and bone graft to achieve solid union. At present, he works in a steel mill, where he regularly lifts weights of 100 pounds; however, he uses a polypropylene removable orthosis to support his lack of an anterior compartment. Major bone defects can be treated with posterolateral bone grafting as an alternative to vascularized fibular transfers or amputation. |
effectively using the Ilizarov technique of corticotomy and bone
transport. The Ilizarov technique received much enthusiasm and was very
popular when it was initially introduced in the United States in the
early 1980s. Since then, its general use has decreased, but it remains
a useful technique in specialized centers owing to its versatility.
Because the Ilizarov technique is substantially different from other
available techniques for treating segmental bone loss, a steep learning
curve is likely to be encountered by the orthopaedist treating
segmental bone loss with bone transport for the first time (99).
Formal training in this technique is recommended before proceeding with
any Ilizarov procedure. A relatively high rate of pin tract infections
and other more significant problems are common. Good ancillary
paramedical support is highly recommended. The technique is labor
intensive, requiring a high level of patient compliance and frequent
follow-up. Psychosocial issues and pain management often become
challenging problems in the long-term care of these patients. At
present, we limit the use of the Ilizarov technique to those patients
for whom there are no other reasonable treatment alternatives. The
Ilizarov technique for treatment of tibial nonunions and malunions is
covered in greater detail in Chapter 32.
osseous reconstruction following extensive resections. First reported
by Taylor and associates in 1978, the technique involves the isolation
and transfer of a bone segment, most commonly the contralateral fibula (124).
The technique has the advantages of a one-stage procedure. It is
relatively independent of the recipient bed and other available
procedures, has a fast union rate, and has the potential for
graft
hypertrophy. However, the surgical procedure is long and demanding,
necessitates a vascular sacrifice, may lead to graft fracture, and
creates a donor bone defect that may result in instability of the
contralateral foot with restricted motion of the ankle and great toe (64,98).
Consider the technique for defects longer than 6 cm. There is no role
for this technique in infected nonunions until the infection is
completely under control, adequate soft-tissue coverage has been
obtained, and drainage has been eliminated, with the patient free of
all antibiotics. Hypertrophy of the fibular graft may occur with time;
however, problems with fatigue fractures remain a significant
complication. Further aspects of this technique are considered in Chapter 36.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
P, Marti-Garin D, Murias-Alvarez J, Puente-Alonso C. External Fixation
and Secondary Intramedullary Nailing of Open Tibial Fractures: A
Randomised, Prospective Trial. J Bone Joint Surg 1997;79B:433.
PA, Meek RN, O’Brien PJ. External Fixation and Delayed Intramedullary
Nailing of Open Fractures of the Tibial Shaft. A Sequential Protocol. J Bone Joint Surg 1990;72A:729.
CT, Friedenberg ZB, Zemsky LM, Pollis PR. Direct Current Stimulation of
Non-union and Congenital Pseudarthrosis—Exploration of Its Clinical
Application. J Bone Joint Surg 1975;57-A:368.
CT, Pollack SR. Treatment of Recalcitratnt Non-union with a
Capacitively Coupled Electrical Field: A Preliminary Report. J Bone Joint Surg 1985;67A:577.
CW, Orme TJ, Richardson HD. The Rate of Pseudarthrosis (Surgical
Nonunion) in Patients Who Are Smokers and Patients Who Are Nonsmokers:
A Comparison Study. Spine 1986;11:942.
JF, Guse R, Tiedeman J, Dehne R. Autologous Marrow Injection as a
Substitute for Operative Grafting of Tibial Nonunions. Clin Orthop 1991;266:259.
SD, Baffes GC, Wolfe MW, et al. Recombinant Human Bone Morphogenetic
Protein-7 Healing in a Canine Long Bone Segmental Defect Model. Clin Orthop 1994;301:302.
SD, Baffes GC, Wolfe MW, et al. The Effect of Recombinant Human
Osteogenic Protein-1 on Healing of Large Segmental Bone Defects. J Bone Joint Surg 1994;76A:827.
TN, Kirker-Head CA, Kriz MJ, et al. Healing Segmental Femoral Defects
in Sheep Using Recombinant Human Bone Morphogentic Protein. Clin Orthop 1993;293:317.
JD, Ryaby JP, McCabe J, et al. Acceleration of Tibial Fracture-healing
by Non-invasive, Low-intensity Pulsed Ultrasound. J Bone Joint Surg 1994;76A:26.
EE, Urist MR, Finerman GA. Distal Metaphyseal Tibial Nonunion.
Deformity and Bone Loss Treated by Open Reduction, Internal Fixation,
and Human Bone Morphogenetic Protein (hBMP). Clin Orthop 1990;250:P234.
KG, Barnett HC. Cancellous Bone Grafting for Non-union of the Tibia
Through the Tibia Through the Posterolateral Approach. J Bone Joint Surg 1955;37-A:1250.
I, Grosse A, Riguat P. The Treatment of Noninfected Pseudoarthrosis of
the Femur and Tibia with Locked Intramedullary Nailing. Clin Orthop 1986;212:142.
DJ, Merkow RL, Gustilo RB. Infection After Intramedullary Nailing of
Severe Open Tibial Fractures Initially Treated with External Fixation. J Bone Joint Surg 1989;71A:835.
HA, Sigholm G, Redfern FC, et al. The Effect of Simulated
Fracture-angulations of the Tibia on Cartilage Pressures in the Knee
Joint. J Bone Joint Surg 1991;73A:1387.
ME, Ada JR, Webb LX. Treatment of Infected Nonunion and Delayed Union
of Tibia Fractures with Locking Intramedullary Nails. Clin Orthop 1989;245:233.
LJ, Alfagemo A, Delcourt JP, Pilon BL. Chronic Osteomyelitis of Long
Bones—Resection and Bone Grafting with Delayed Skin Closure. J Bone Joint Surg 1976;52-B:138.
BJ, Sangeorzan BP, Hansen ST Jr, Judd RP. Mathematically Directed
Single-cut Osteotomy for Correction of Tibial Malunion. J Orthop 1989;3:267.
A, Sobol PA, Sew Hoy AL, et al. Prefabricated Functional Braces for the
Treatment of Fractures of the Tibial Diaphysis. J Bone Joint Surg 1984;66-A:1328.
A, Gersten LM, Sobol PA, et al. Tibial Shaft Fractures Treated with a
Functional Brace: Experience with 780 Fractures. J Bone Joint Surg 1989;71B:602.
G, King JB. A Prospective, Double-blind Trial of Electrical Capacitive
Coupling in the Treatment of Non-union of Long Bones. J Bone Joint Surg 1994;76A:820.
RR, Resnick CT, Wagner KS, Sarmiento A. Change in Tibiotalar Joint
Contact Areas Following Experimentally Induced Tibia Angular
Deformities. Clin Orthop 1985;199:72.
MR, Mazet R Jr, McLean FC. The Pathogenesis and Treatment of Delayed
Union and Non-union. A Survey of Eighty-five Ununited Fractures of the
Shaft of the Tibia and One Hundred Control Cases with Similar Injuries.
J Bone Joint Surg 1954;36-A:931.
J, Shyr HS, Chao EYS, Kelly PJ. Comparison of Osteotomy Healing under
External Fixation Devices with Different Stiffness Characteristics. J Bone Joint Surg 1984;66-A:1258.
AW, Lane JM, Fellinger EJ, et al. The Healing of Segmental Bone
Defects, Induced by Recombinant Human Bone Morphogenetic Protein
(rhBMP-2). A Radiographic, Histological, and Biomechanical Study in
Rats. J Bone Joint Surg 1992;74A:659.
