The Ocular Motor Nerves
cranial nerve (CN III) arises from the oculomotor nuclear complex in
the midbrain and conveys motor fibers to extraocular muscles, plus
parasympathetic fibers to the pupil and ciliary body. A single midline
structure, the central caudal nucleus, supplies the levator palpebrae
muscles on both sides. The periaqueductal gray matter is also involved
with eyelid function. The Edinger-Westphal (EW) subnucleus is a single
structure that provides parasympathetic innervation to both eyes. The
fibers of CN III course anteriorly through the mesencephalon,
traversing the medial portion of the red nucleus and the substantia
nigra. The nerve exits from the interpeduncular fossa on the anterior
surface of the midbrain. It passes between the superior cerebellar and
posterior cerebral arteries, then runs forward parallel to the
posterior communicating artery. In its course toward the cavernous
sinus, it lies on the free edge of the tentorium cerebelli, medial to
the temporal lobe. CN III penetrates the dura just lateral and anterior
to the posterior clinoid processes and enters the cavernous sinus. In
the cavernous sinus, CN III has important relationships with the
carotid artery, ascending pericarotid sympathetics, and CNs IV, V, and
VI. CN III separates into its superior and inferior divisions in the
anterior cavernous sinus, then enters the orbit through the superior
orbital fissure.
from the trochlear nucleus located just anterior to the aqueduct at the
level of the inferior colliculus. The nerve filaments curve posteriorly
around the aqueduct, decussate in the anterior medullary velum and exit
through the tectum. It is the only cranial nerve to exit from the
brainstem posteriorly. It penetrates the dura just behind and lateral
to the posterior clinoid processes. Leaving the cavernous sinus, it
traverses the superior orbital fissure, enters the orbit, and crosses
over CN III to terminate on the superior oblique muscle on the side
opposite the nucleus of origin.
VI) lies in the mid to lower pons, encircled by the looping fibers of
the facial nerve. The nerve exits anteriorly at the pontomedullary
junction, then ascends the clivus in the prepontine cistern. It passes
near the Gasserian ganglion, pierces the dura at the dorsum sellae, and
enters the cavernous sinus in company with CNs III and IV, where it
lies below and medial to CN III and just lateral to the internal
carotid artery. CN VI is the only nerve that lies free in the lumen of
the sinus; the others are in the wall. It enters the orbit through the
superior orbital fissure to innervate the lateral rectus.
for any obvious ocular malalignment, abnormal lid position, or
abnormalities of the position of the globe within the orbit.
Abnormalities of the external eye may occasionally be of diagnostic
significance in neurologic patients.
Tortuous
(“corkscrew”) blood vessels in the conjunctiva occur with carotid
cavernous fistula, or there may be jaundice, evidence of iritis,
Kayser-Fleischer rings, chemosis, dysmorphic changes (e.g., epicanthal
folds), xanthelasma due to hypercholesterolemia, keratoconjunctivitis
sicca, premature cataract, or ocular complications of upper facial
paralysis. Basal skull fractures often cause bilateral periorbital
ecchymosis (raccoon eyes).
so that it protrudes (exophthalmos, proptosis) or recedes
(enophthalmos). Subtle proptosis can often be better appreciated by
looking down at both eyes from above the vertex of the head, or by
comparing side views. Exophthalmos is usually bilateral and most
commonly due to thyroid eye disease ([TED], Graves ophthalmopathy,
Graves orbitopathy); thyroid disease can have a host of neurologic
complications. Some of the neurologically significant causes of
unilateral proptosis include orbital mass lesion, carotid cavernous
fistula, cavernous sinus thrombosis, sphenoid wing meningioma,
meningocele, and mucormycosis.
than droopy eyelid (e.g., eye has shrunk). Ptosis may have been present
for a very long time before coming to the patient’s attention. Looking
at old photographs is often helpful. Note the position of the eyelids
and the width of the palpebral fissures bilaterally. Note the amount of
iris or pupil covered by the lid. The normal upper eyelid in primary
position crosses the iris between the limbus (junction of the iris and
sclera) and the pupil, usually 1 mm to 2 mm below the limbus; the lower
lid touches or crosses slightly above the limbus. Normally there is no
sclera showing above the iris. The width of the palpebral fissures
should be equal on both sides, although a slight difference occurs in
many normal individuals. The palpebral fissures are normally 9 mm to 12
mm from upper to lower lid margin. Measurement can also be made from
the lid margin to the corneal light reflex. The upper lid margin is
normally 3 mm to 4 mm above the light reflex. Patients may try to
compensate for ptosis by contracting the frontalis muscle, causing a
telltale wrinkling of the ipsilateral forehead. If the examiner fixes
the frontalis muscle with her finger, the patient may be unable to
raise the eyelid.
upper margin of the pupil, or cover the pupil partially or totally.
With complete ptosis, the eyelid is down and the eye appears closed (Figure 10.1). Ptosis may be unilateral or bilateral, partial or complete, and occurs in many neurologic conditions (Figure 10.2).
With eyelid retraction, the upper lid pulls back and frequently exposes
a thin crescent of sclera between the upper limbus and the lower lid
margin. Lid retraction is a classic sign of thyroid disease, but occurs
in neurologic disorders as well. In addition to observing the lid
position at rest, notice the relationships of the lid to the globe
during eye movement.
nerve palsy. Mild to moderate unilateral ptosis occurs as part of
Horner syndrome, or with partial third nerve palsy. Mild to moderate
bilateral ptosis occurs in some neuromuscular disorders, such as
myasthenia gravis (MG), muscular dystrophy, or ocular myopathy.
It characteristically fluctuates from moment to moment and is worsened
by prolonged upgaze (fatiguable ptosis). The Cogan lid twitch sign,
characteristic of myasthenia, consists of a brief overshoot twitch of
lid retraction following sudden return of the eyes to primary position
after a period of downgaze. When the ptosis is asymmetric, manually
raising the more ptotic lid may cause increased ptosis on the other
side (curtain sign, seesaw ptosis). Compensation for mild ptosis on one
side may cause the involved eye to appear normal and the other eye to
have lid retraction.
 |
|
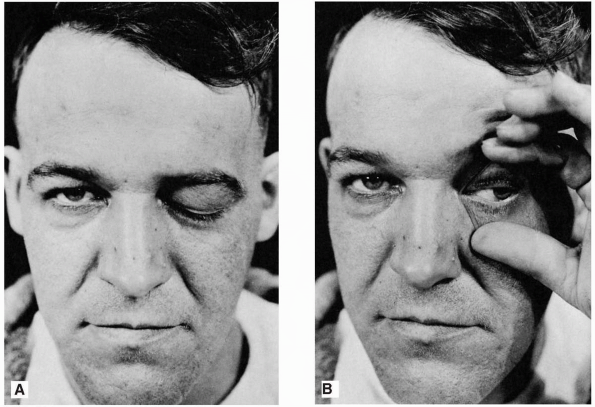
FIGURE 10.1 • Paralysis of the left oculomotor nerve in a patient with an aneurysm of the left internal carotid artery. A. Only ptosis can be seen. B. On elevating the eyelid, it is seen that the pupil is dilated and the eyeball is deviated laterally.
|
 |
|
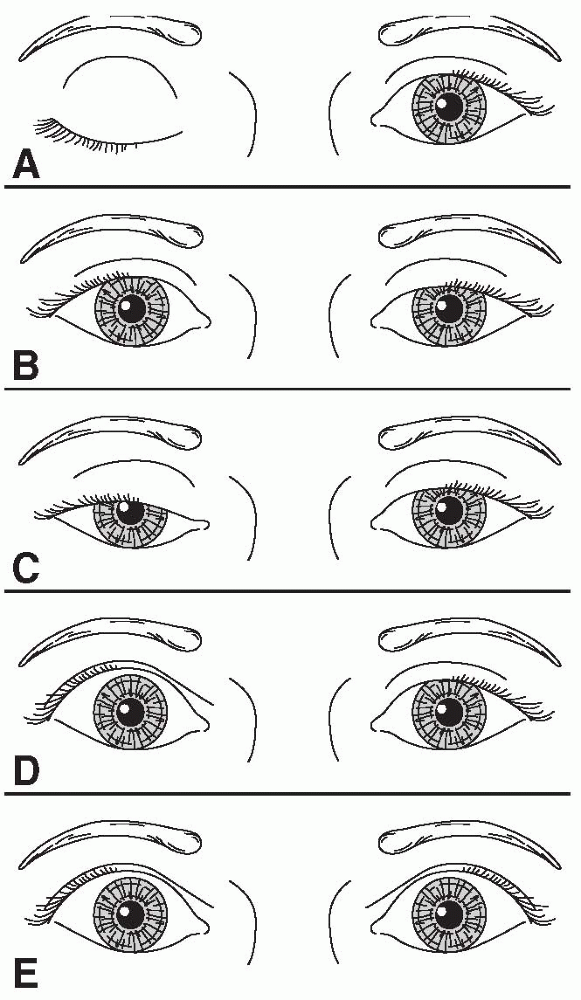
FIGURE 10.2 • Characteristics of different causes of abnormal lid position. A. Right third cranial nerve palsy with complete ptosis. B. Left Horner syndrome with drooping of upper lid and slight elevation of lower lid. C. Bilateral, asymmetric ptosis in myasthenia gravis. D. Right lid retraction in thyroid eye disease. E. Bilateral lid retraction with a lesion in the region of the posterior commissure (Collier sign).
|
 |
|
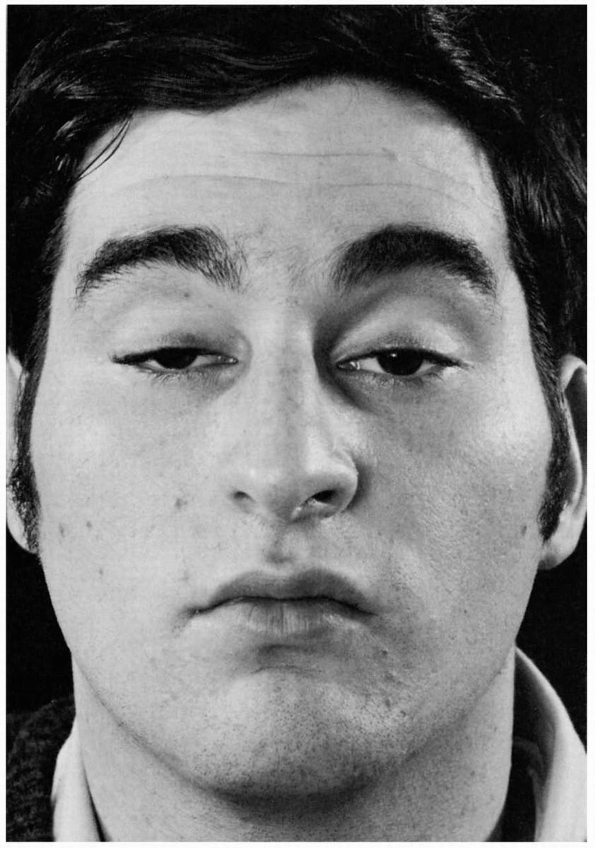
FIGURE 10.3 • Bilateral ptosis in a patient with myasthenia gravis.
|
showing above the limbus, indicating either lid retraction or lid lag.
Thyroid eye disease is a common cause of lid abnormalities, including
lid retraction in primary gaze and lid lag in downgaze. Lid retraction
in primary gaze also occurs with lesions involving the posterior
commissure (Collier sign). Lid retraction with posterior commissure
lesions is bilateral, but may be asymmetric. With Collier sign, the
levators relax appropriately and the lids usually descend normally on
downgaze without lagging behind as they do in TED. In addition, the lid
retraction may worsen with attempted upgaze (Figure 10.4).
Aberrant regeneration of CN III may cause lid retraction on adduction.
Lid retraction may also be mechanical, due to trauma or surgery. Lid
retraction may be confused with ipsilateral proptosis or contralateral
ptosis.
light entering the eye, ensuring optimal vision for the lighting
conditions. The pupils should be equal in size, round, regular,
centered in the iris, and should exhibit specific reflex responses.
sympathetic and parasympathetic innervation and the level of ambient
illumination. The most important determinants are the level of
illumination and the point at which the eyes are focused. Accurate
measurements are important. Measurements should be made with a pupil
gauge or a millimeter ruler; estimates are surprisingly inaccurate. The
size of the pupils should be determined at distance in ambient and dim
light and at near. The normal pupil is 2 mm to 6 mm in diameter. In
ordinary ambient light the pupils are usually 3 mm to 4 mm in diameter.
The pupils are small and poorly reactive at birth and in early infancy,
becoming normal size around ages 7 to 8. They are normally larger in
adolescents and young adults, about 4 mm in diameter and perfectly
round. In middle age, they are typically 3.5 mm in diameter and
regular, and in old age 3 mm or less and often slightly irregular.
 |
|
FIGURE 10.4 • Paresis of upward gaze in a patient with a neoplasm of the posterior third ventricle.
|
causes of acquired miosis include old age, hyperopia, alcohol abuse,
and drug effects. Neurologically significant causes of miosis include
neurosyphilis, diabetes, levodopa therapy, and Horner syndrome. Acute,
severe brainstem lesions, such as pontine hematoma, may cause
bilaterally tiny, “pinpoint” pupils that still react.
causes of bilateral mydriasis include anxiety, fear, pain, myopia, and
drug effects—especially anticholinergics. Only severe, bilateral
lesions of the retina or anterior visual pathways, enough to cause near
blindness, will affect the resting pupil size. Neurologically
significant bilateral mydriasis occurs in midbrain lesions, in comatose
patients following cardiac arrest, in cerebral anoxia, and as a
terminal condition.
outline. Gross abnormalities in shape are usually the result of ocular
disease such as iritis or eye surgery. Synechia, a congenital coloboma
(a gap in the iris), prior trauma, or iridectomy may all cause pupil
irregularity. A slight change in shape, however, such as an oval pupil,
slight irregularity in outline, serration of the border, or slight
notching, may be significant in the diagnosis of neurologic disease.
The pupils are generally of equal size. A difference of 0.25 mm in
pupil size is noticeable, and a difference of 2 mm is considered
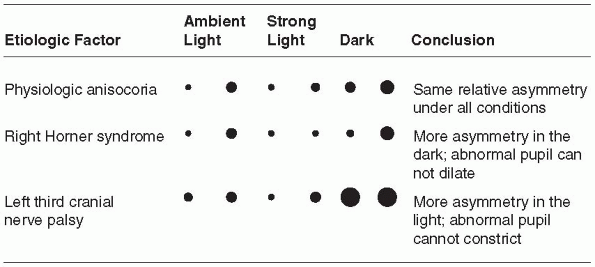
significant. Physiologic anisocoria, mild degrees of inequality with
less than 1 mm of difference between the two sides, occurs in 15% to
20% of normal individuals. With such physiologic anisocoria, the degree
of inequality remains about the same in light and dark, and the pupils
react normally to all stimuli and to instilled drugs (Figure 10.5).
Unequal pupils may be caused by primary eye disorders, such as iritis.
Unilateral mydriasis is never due to isolated, unilateral visual loss.
The reactivity of the normal eye and the consensual light reflex will
ensure pupil size remains equal.
examination are the light response and the near response
(“accommodation”). The normal pupil constricts promptly in response to
light.
Pupillary
constriction also occurs as part of the near response, along with
convergence and rounding up of the lens for efficient near vision.
Normally, the light and near responses are of the same magnitude.
 |
|
FIGURE 10.5 • Behavior of unequal pupils in light and dark conditions.
|
individually. The examining light should be shone into the eye
obliquely with the patient fixing at distance to avoid eliciting a
confounding near response. A common error in pupil examination is to
have the patient fixing at near, as by instructing her to look at the
examiner’s nose. This technique provides both a light stimulus and a
near stimulus simultaneously, and the pupils may well constrict to the
near target of the examiner’s nose even when the reaction to light is
impaired or absent. Using this technique, the examiner would invariably
miss light-near dissociation. Always have the patient fix at a distance
when checking the pupillary light reaction. The normal pupillary light
reflex is brisk constriction followed by slight dilatation back to an
intermediate state (pupillary escape). The responses may be noted as
prompt, sluggish, or absent, graded from 0 to 4+, or measured and
recorded numerically (e.g., 4 mm → 2 mm.) In comatose patients, it is
often important, but difficult, to see if the pupillary light reaction
is preserved, especially if there is a question of brain death. A
useful technique is to use the ophthalmoscope: focus on the pupil with
high positive magnification, dim the ophthalmoscope, and then rapidly
reilluminate. Even a small residual reaction may be seen.
the patient relax accommodation by gazing into the distance, then
shifting gaze to some near object. The best near object is the
patient’s own finger or thumb. The response consists of thickening of
the lens (accommodation), convergence of the eyes, and miosis. The
pupils constrict at near to increase the depth of focus. The midbrain
mechanisms for pupillary constriction to near are separate from those
for the light reflex; one response may be abnormal while the other is
preserved.
may influence pupil size and reactivity. An abnormal pupil may fail to
respond appropriately, or respond excessively because of denervation
supersensitivity. Sympathomimetics and anticholinergics cause pupillary
dilation, and parasympathomimetics or sympathetic blockers cause
pupillary constriction. Agents that cause mydriasis include the
anticholinergics atropine, homatropine, and scopolamine and the
sympathomimetics epinephrine,
norepinephrine,
phenylephrine, hydroxyamphetamine, and cocaine. Agents that cause
miosis include the cholinomimetics pilocarpine, methacholine,
muscarine, and opiates, and the cholinesterase inhibitors physostigmine
and neostigmine. Pupillary pharmacology can be applied in the
neurologic examination, primarily in the evaluation of Horner syndrome
and Adie pupil (see Table 10.1).
|
TABLE 10.1 Summary of Pharmacologic Pupillary Testing for Horner Syndrome
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
problems include pupils that are too large or too small, unilaterally
or bilaterally, or pupils that fail to demonstrate normal reflex
responses.
large pupil are third cranial nerve palsy and Adie tonic pupil. In CN
III palsy, the large pupil has impaired reactions to light and to near;
abnormalities of extraocular movement and eyelid position generally
betray the origin of the abnormal pupil. With total CN III palsy there
is complete ptosis; lifting the eyelid reveals the eye resting in a
down and out position (Figure 10.1). Although
CN III palsies often affect the pupil more than other functions, some
ptosis and ophthalmoparesis is usually present. Since the pupillary
parasympathetics occupy a position on the dorsomedial periphery of the
nerve as it exits the brainstem, compressive lesions such as aneurysms
generally affect the pupil prominently. Ischemic lesions tend to affect
the interior of the nerve and spare the pupil, as in diabetic third
nerve palsies, because the periphery of the nerve has a better vascular
supply. This rule is not absolute. The pupil is usually involved early
and prominently with third nerve compression due to uncal herniation
(Hutchinson pupil).
typically a young woman who suddenly notes a unilaterally enlarged
pupil, with no other symptoms. The pupillary reaction to light may
appear absent, although prolonged illumination may provoke a slow
constriction. The reaction to near, although slow, is better preserved.
Once constricted, the tonic pupil redilates very slowly when
illumination is removed or the patient looks back at distance, often
causing a transient reversal of the anisocoria. Adie syndrome is the
association of the pupil abnormality with depressed or absent deep
tendon reflexes, particularly in the lower extremities.
light near dissociation sometimes seen when lesions affect the upper
midbrain. Such pupils may accompany the impaired upgaze and
convergence/retraction nystagmus of Parinaud syndrome. The variably
dilated, fixed pupils reflecting midbrain dysfunction in a comatose
patient carry a bleak prognosis. Acute angle closure glaucoma can cause
severe frontotemporal headache and a dilated, poorly reactive pupil.
Deliberately or accidentally instilled mydriatics will produce a
dilated, fixed pupil.
elderly are normally smaller. Many systemic drugs, such as opiates, may
symmetrically shrink the pupils. Important neurologic conditions
causing an abnormally small pupil include Horner syndrome and
neurosyphilis.
ptosis, miosis, and anhidrosis. Lack of sympathetic input to the
accessory lid retractors results in ptosis and apparent enophthalmos.
The ptosis of the upper lid is only 1 mm to 3 mm, never as severe as
with a complete CN III palsy, although it
may
simulate partial third nerve palsy. The ptosis can be subtle and is
often missed. The lower lid is frequently elevated 1 mm to 2 mm because
of loss of the action of the lower lid accessory retractor that holds
the lid down (inverse ptosis). The resulting narrowing of the palpebral
fissure causes apparent enophthalmos. Since the fibers mediating facial
sweating travel up the external carotid, lesions distal to the carotid
bifurcation produce no facial anhidrosis except for perhaps a small
area of medial forehead that is innervated by sympathetic fibers
traveling with the internal carotid.
dark. Pupillary asymmetry greater in the dark than in the light
generally means Horner syndrome. Recall that physiologic anisocoria
produces about the same degree of pupillary asymmetry in the light and
dark. In contrast, third nerve palsy and Adie pupil cause greater
asymmetry in the light because of the involved pupil’s inability to
constrict. Examining the eyes under light and dark conditions can help
greatly in sorting out asymmetric pupils (Figure 10.5 and Figure 10.6).
Should the examiner err by having the patient fixate at near during
testing, the pupillary constriction in the good eye may lessen the
asymmetry and cause the abnormal pupil to be missed. The pupil in
Horner syndrome not only dilates less fully, it dilates less rapidly.
In the first few seconds after dimming the lights, the slowness of
dilation of the affected pupil may cause the anisocoria to be even more
pronounced (dilation lag). There is more anisocoria at 4 to 5 seconds
after lights out than at 10 to 12 seconds.
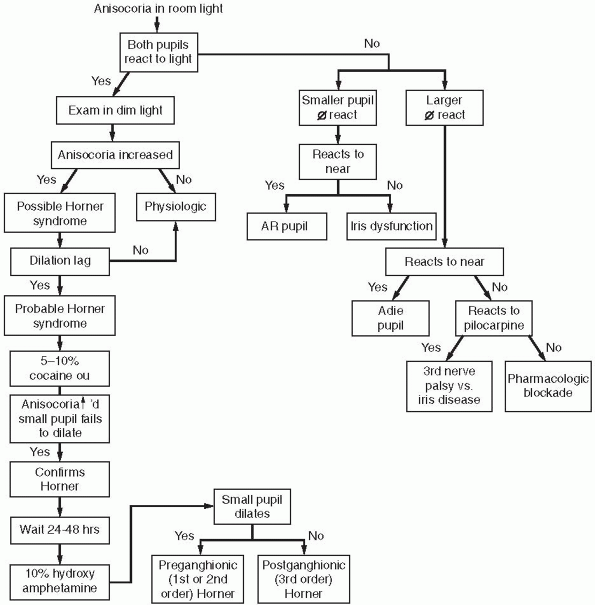 |
|
FIGURE 10.6 • Flow diagram for the evaluation of anisocoria.
|
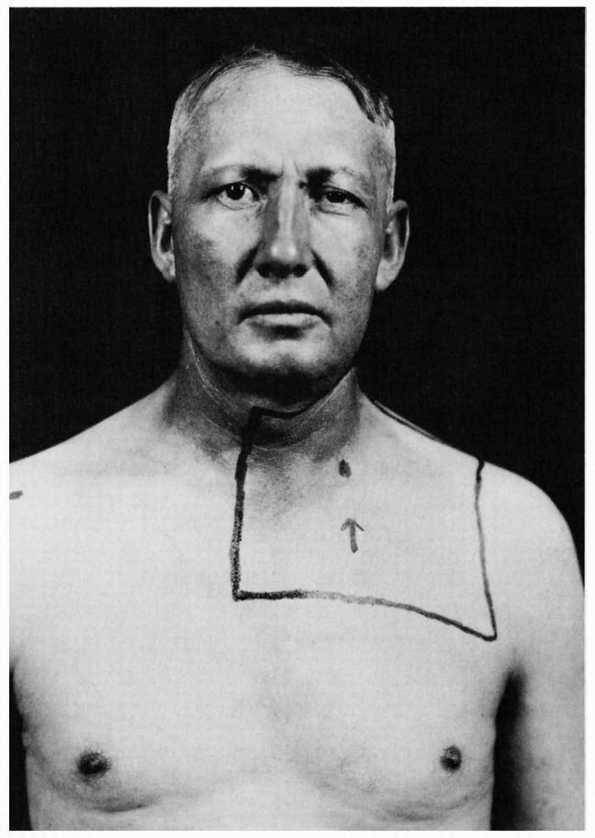 |
|
FIGURE 10.7 • Left Horner syndrome in a patient with a pulmonary sulcus tumor.
|
following: brainstem lesions (especially of the lateral medulla),
cluster headache, internal carotid artery thrombosis or dissection,
cavernous sinus disease, apical lung tumors, neck trauma, and other
conditions (Figure 10.7).
irregular in outline, and have light near dissociation. They react
poorly or not at all to light, but very well to near. Argyll Robertson
pupils are the classic eye finding of neurosyphilis. Other conditions
may cause an AR-like pupil. With the declining incidence of
neurosyphilis, AR-like pupils with light near dissociation are
increasingly likely to be of some other etiology.
pupillary reflex arcs, or disease of the brainstem pupil control
centers, may alter pupil reactivity to light or near, as may local
disease of the iris sphincter (e.g., old trauma). Disease of the retina
does not affect pupil reactivity unless there is involvement of the
macula severe enough to cause near blindness. Cataracts and other
diseases of the anterior segment do not impair light transmission
enough to influence the pupil. Because of the extensive side-to-side
crossing of pupillary control axons through the posterior commissure,
light constricts not only the pupil stimulated (the direct response)
but also its fellow (the consensual response). The eye with a severed
optic nerve will show no direct response, but will have a normal
consensual response to a light stimulus in the other eye, as well as
constriction to attempted convergence (amaurotic pupil). Lesser degrees
of optic nerve dysfunction can often be detected by checking for an
afferent pupillary defect (see next section). The pupil frozen because
of third nerve palsy will have no near response and no direct or
consensual light response, but the other eye will exhibit an intact
consensual response on stimulation of the abnormal side (Table 10.2).
|
TABLE 10.2 Direct and Consensual Light Reaction
|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||
greater than the reaction to near. Light near dissociation refers to a
disparity between the light and near reactions. The most common form is
a poor light response but good constriction with the near response; it
is relatively common, and there are a number of causes. The converse,
better reaction to light than to near, is rare. In the routine case, if
the pupillary light reaction is normal there is little to be gained by
examining the near reaction.
the dorsal brainstem, but the near response fibers ascend to the EW
nucleus from the ventral aspect. Disorders that affect the dorsal
rostral brainstem may affect the light reaction but leave the near
reaction intact. This anatomical arrangement likely explains many
instances of the phenomenon of light near dissociation of the pupils.
Pressure on the pupillary fibers in the region of the pretectum and
posterior commissure (e.g., from pinealoma) impairs the light reaction.
However, fibers mediating the near response, the EW nucleus, and the
efferent pupil fibers are spared, which leaves the near response
intact. Causes of light near dissociation include neurosyphilis, other
lesions involving the dorsal rostral midbrain, diabetic autonomic
neuropathy, Lyme disease, Adie pupil, aberrant regeneration of CN III,
sarcoidosis, multiple sclerosis (MS), and severe retinal or optic nerve
disease.
initial pupillary constriction and subsequent slight escape depend
greatly on the specific circumstances of illumination. Therefore, the
status of the light reflex must be judged by comparing the two eyes.
With mild to moderate optic nerve disease, it is difficult to detect
any change in pupil reactivity to direct light stimulation. Marcus Gunn
described pathologic pupillary escape following 10 to 20 seconds of
continued exposure, or the adapting pupillary response, due to optic
nerve disease. Levitan described looking for the Marcus Gunn pupillary
sign by swinging a light back and forth between the two eyes (swinging
flashlight test, alternating light test; Levitan P. Pupillary escape in
disease of the retina or optic nerve. Arch Ophthalmol
1959;62:768-779). He thought moving the light back and forth amplified
the asymmetry of the pupillary escape. The swinging flashlight test is
a very useful technique that can quickly and accurately compare the
initial constriction and subsequent escape of the two pupils. It is a
key clinical technique in the evaluation of suspected optic neuropathy,
and it can often detect a side-to-side difference even when the lesion
is mild and there is no detectable difference in the direct light
reflex when testing each eye individually. An APD is an extremely
useful and important neurologic sign. Some modify the term with
“relative” (RAPD) to emphasize that the finding depends on the
difference between the
two
eyes—the state of the afferent system and activity of the light reflex
in one eye relative to the other eye. The shorter form, APD, is
currently in more widespread use.
afferent signal. A bilateral APD cannot occur, although a severe
bilateral afferent defect may cause light near dissociation or abnormal
pupillary escape. An APD can occur with bilateral optic neuropathy only
if there is significant asymmetry of involvement. Media opacities will
not cause an APD. In fact, mature cataract may so scatter the incoming
light as to actually increase the light reflex and cause a minor APD in
the opposite eye. Only severe retinal or macular disease will cause an
APD, and then it will be slight. Maculopathy with 20/200 vision might
cause a 1 + APD while optic neuropathy with 20/30 vision would cause a
3+ to 4+ APD. For simulation of an APD see http://www.richmondeye.com/apd.asp.
of regard into the field of vision and following them if they move. The
different eye movement control systems (e.g., saccade, pursuit,
vergence) normally function harmoniously to secure and maintain vision.
The globe rotates around one or more of three primary axes that
intersect at right angles at the center of rotation, 15.4 mm behind the
cornea. Movement takes place perpendicular to the axis of rotation.
Abduction and adduction are rotation in the horizontal plane about the
vertical axis going from superior to inferior. Elevation and depression
are up and down movements around the horizontal axis that runs from
medial to lateral across the eye. The third axis runs from anterior to
posterior; rotation about this axis is referred to as torsion.
Intorsion is movement of the upper pole of the eye toward the nose;
extorsion is movement away from the nose.
straight ahead and the visual axes of the two eyes are parallel. Since
the orbits diverge, primary position must be obtained by precisely
adjusted contractions of the extraocular muscles, which are controlled
by the cerebral cortex. When regarding an object, the extraocular
muscles move the eyes so that the visual axes meet at the proper point
to ensure that the object’s image falls on corresponding points on each
macula. The point where the visual axes meet is called the fixation
point. Normal eye movements are usually conjugate in order to maintain
binocular vision and stereopsis. The medical longitudinal fasciculus
(MLF) coordinates the contractions of the yoked muscles and the
relaxation of their antagonists so that the two eyes move together.
During a duction, the agonist contracts and the antagonist relaxes.
Binocular movements are referred to as versions. During version
movements, the extraocular muscles work as yoked pairs (e.g., the
lateral rectus in one eye contracts with the medial rectus in the other
eye) (Figure 10.8). The yoke muscles are paired
agonists for the binocular movement, and in each eye their respective
antagonists must be reciprocally inhibited. Hering’s law, or the law of
equal innervation, states that the same amount of innervation goes to
an extraocular muscle and to its yoked fellow. The amount of
innervation to the yoked pair is always determined by the fixating eye.
Hering’s law is important in understanding the topic of primary and
secondary deviations.
visual confusion. The confusion results because of discordant retinal
images—one real, one not. Historical details are often helpful in
deciphering the cause of diplopia. The first step should be to
determine whether the diplopia is binocular or monocular. With
binocular diplopia, covering one eye eliminates the visual confusion.
Monocular diplopia persists when using the affected eye alone.
Monocular diplopia is often considered a nonorganic symptom, but there
are many organic causes, primarily ophthalmologic conditions such as
cataract, corneal astigmatism, lens subluxation, retinal detachment,
and macular disease.
diplopia is horizontal or vertical, worse at near or distance, or worse
in a particular direction of gaze; all are pertinent observations.
Horizontal
diplopia usually results from dysfunction of the medial or lateral
rectus muscles. Vertical diplopia tends to result from disorders of the
oblique muscles, less often of the vertically acting recti. Patients
with sixth nerve palsy have difficulty diverging the eyes and tend to
have more diplopia at distance. The lateral recti are not active when
the eyes are converging for near vision, and patients have less
diplopia at near (reading) as compared to distance (driving).
Conversely, patients with medial rectus weakness have difficulty
converging with more diplopia at near and less at distance. Diplopia is
worse with gaze in the direction of the involved muscle. The patient
with either a right sixth nerve palsy or a left third nerve palsy will
have more diplopia on right gaze. Patients with fourth nerve palsy
often describe an obliquity or tilt to the image. A patient with
diplopia may keep one eye closed or may tilt or turn the head to
minimize the visual confusion. Associated symptoms may be important.
Diplopia accompanied by ptosis may occur with third nerve palsy, as
well as with myasthenia gravis and other neuromuscular disorders. Pain
in the head or eye in association with diplopia suggests such
conditions as diabetic third nerve palsy, posterior communicating
aneurysm, ophthalmoplegic migraine, Tolosa-Hunt syndrome (painful
ophthalmoplegia), and giant cell arteritis.
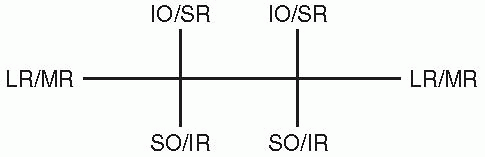 |
|
FIGURE 10.8 • The yoke muscles control extraocular movement in the six cardinal directions of gaze.
|
assessment of visual acuity. When acuity is impaired, the patient may
not be able to adequately fixate. This influences the results of
various maneuvers used to assess motility, particularly the cover test.
Note the position of the patient’s head. Many patients with ocular
malalignment will hold their head in an unusual position. Usually there
is a turn or tilt that minimizes the diplopia.
position, the motility examination begins with an assessment of
fixation. A normal patient can fixate steadily on an object of regard,
whether near or distant. Inability to maintain normal steady fixation
may occur because of fixation instability, or saccadic intrusions,
transient deviations away from fixation with a quick return.
the likelihood of abnormality is low, the ocular motility examination
is usually limited to assessing versional pursuit movements in the six
cardinal positions of gaze, including full lateral gaze to each side,
as well as upgaze and downgaze when looking to either side (Figure 10.8 and Figure 10.9).
The target should slowly trace a large letter “H” for the patient to
follow. Some add primary gaze plus upgaze and downgaze in the center to
make nine cardinal positions. Eye movements should remain smooth and
conjugate throughout. The six cardinal positions are designed to search
for dysfunction of individual muscles or nerves, as well as
supranuclear abnormalities of horizontal gaze. Assessment of upgaze and
downgaze in primary position assesses the supranuclear vertical gaze
mechanisms. Pursuit versions are done by asking the patient to follow a
target held about 0.5 m to 1.0 m away, such as an examining light, a
pointer, a pen, or the examiner’s finger. A linear target should be
held perpendicular to the direction of gaze, vertical for testing
horizontal gaze, and horizontal for vertical gaze. Use of an examining
light adds the ability to assess the corneal reflection, which gives
objective evidence of ocular malalignment. The light reflection should
be just medial to the center of the pupil and at corresponding points
in each eye. The patient should indicate if she sees more than one
target at any point. Pursuit movements are normally smooth. In certain
disease states with abnormal pursuit,
the
tracking movements become disrupted by superimposed saccades, creating
a ratchety or jerky movement termed saccadic pursuit (cogwheel eye
movements). The finding is nonspecific and can occur bilaterally with
fatigue, inattention, decreased consciousness, basal ganglia disorders,
diffuse hemispheric disease, drug effects, or if the target velocity is
too fast. Abnormal pursuit in one direction may indicate an ipsilateral
deep occipito-parietal lobe lesion involving the pursuit pathways.
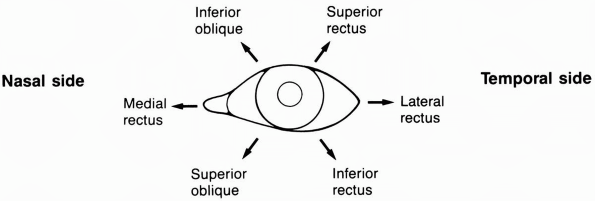 |
|
FIGURE 10.9
• Actions of the extraocular muscles on the left eye. Arrows denote the main directions of action for each muscle, resulting from a combination of movements of the globe in the three dimensions. |
degrees to either side of primary position. In absolute terms for the
normal adult eye, the excursions are about 10 mm for adduction,
abduction, and elevation, and about 7 mm for depression. The last 10
degrees of abduction is difficult to maintain and holding there may
result in end point nystagmus, a normal physiologic phenomenon. In full
lateral gaze, the temporal limbus abuts the lateral canthus; in full
medial gaze, about the inner third of the nasal limbus is buried. A
small rim of sclera showing on extreme abduction is not abnormal.
Normally, the amount of scleral show on abduction is symmetric in the
two eyes. Greater scleral show on full abduction in one eye than the
other may be a subtle sign of abduction impairment.
toward or away from the observer. Dysconjugate eye movements,
convergence or divergence, are required. The central mechanisms
subserving adduction of the eyes for convergence are different from the
mechanisms for adduction during conjugate gaze. Testing convergence is
helpful in some circumstances, such as when the pupillary light reflex
is not crisply normal (in order to look for light near dissociation of
the pupils), or when there is anything to suggest an internuclear
ophthalmoplegia (INO).
rapidly refixate between two targets. The patient is instructed to
switch gaze between one target, such as the examiner’s nose, and an
eccentric target, such as the examiner’s finger held to one side. The
examiner assesses the velocity, magnitude, and accuracy of the
saccades, and compares adduction and abduction saccades in each eye and
saccades in the two eyes. Saccadic velocity may be decreased globally
in some conditions, such as MG, or selectively, such as slow adduction
saccades in the involved eye in a unilateral INO. Saccades may be
hypometric, falling short of target and requiring additional, smaller
saccades to attain fixation, or hypermetric, overshooting the target
and requiring saccades back in the opposite direction. In some
conditions, reflex eye movements may be present when other movements
are impaired. Vestibulo-ocular reflex movements can be examined by
having the patient fix on a target, then passively moving the head from
side to side, or up and down.
subjective or objective. The subjective tests depend on the patient’s
observation of images, the objective tests on the examiner’s
observation of
eye
movements during certain maneuvers. Common subjective bedside
evaluations include the red lens and Maddox rod tests; common bedside
objective tests are the corneal light reflex tests and the cover tests
(cover-uncover and alternate cover). The objective tests only require
the patient to fixate; they do not require any subjective responses or
interpretation of the color or separation of images.
weakness, she sees two images. The real image falls on the macula of
the normal eye. The false image falls on the retina beside the macula
of the paretic eye. The brain is accustomed to images falling off the
macula coming from peripheral vision, so it projects the false image
peripherally. The farther away from the macula that the image falls,
the farther peripherally the misinterpretation of its origin. As the
eye moves in the direction of the paretic muscle, the separation of
images increases, and the false image appears to be more and more
peripheral. The false image is also usually fainter than the true image
because extramacular vision is not as acute. The clarity, however,
depends on the visual acuity in the two eyes. These considerations lead
to three “diplopia rules” to identify the false object: (a) the
separation of images is greatest in the direction of action of the weak
muscle, (b) the false image is the more peripheral, and (c) the false
image comes from the paretic eye.
simplest is to move the patient’s eyes into the position with the
greatest separation of images. Then cover one eye. If the more
peripheral image disappears, the covered eye is the paretic eye. The
red lens (red glass) and Maddox rod tests are attempts to be more
precise (see Toolkit). They may be especially useful when the diplopia
is mild and the weak muscle or muscles not apparent from examination of
ocular versions. The theory of the red lens test is sound, but often
the results of testing in clinical practice are less than clear. One
reason is that the red lens breaks fusion just enough to bring out
unrelated phorias, which muddy the findings. The results of the red
lens test may be drawn to aid interpretation. There should be a
notation as to whether the diplopia fields are drawn as seen by the
patient or as seen by the examiner (Figure 10.10).
reflection of an examining light on the cornea, and estimating the
amount of ocular deviation depending on the amount of displacement of
the reflection from the center of the pupil. The test can only be done
at near because distant reflections are too dim, so the confounding
effects of the near reflex must be reckoned with. Each millimeter of
light displacement from the center indicates 18 degrees of eye
deviation.
other to fixate by occluding its fellow, and determining the drift of
the nonfixing eye while it is under cover. Varieties of cover testing
include
the
cover-uncover test and the alternate cover test. The cover-uncover test
is used primarily by ophthalmologists to evaluate patients with
congenital strabismus where there is an obvious squint. When neurologic
patients have an obvious malalignment, its nature is usually apparent.
The alternate cover test is used to evaluate more subtle deviations.
For simulation of cover tests see
http://www.richmondeye.com/eyemotil.asp.
 |
|
FIGURE 10.10
• Red lens diplopia fields, drawn as seen by the examiner. The red lens is placed over the right eye, and the eyes move through the six cardinal positions of gaze with the patient looking at an examining light. White circles depict images coming from the left eye (white light); dark circles, images from the right eye (red light); and intermediate circles, images from both eyes (pink light). |
phenomenon sometimes affected by disease. It is sometimes useful in
evaluating disturbed ocular motility. Optokinetic nystagmus is
conjugate nystagmus induced by a succession of moving visual stimuli.
Optokinetic nystagmus occurs whenever the eyes must follow a series of
rapidly passing objects, such as telephone poles zipping by a car or
train window. Clinical testing entails moving a striped target, a
rotating drum, or a cloth tape bearing stripes or squares in front of
the patient and requesting that she “count” the stripes on the drum or
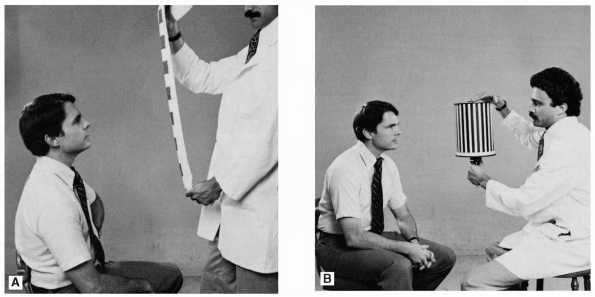
the stripes or squares on the tape (Figure 10.11).
A typical OKN tape would consist of a series of 2-in-square red patches
placed 2 in apart on a white tape 1 yard long, which is drawn across
the patient’s field of vision. The toolkit contains a rudimentary OKN
tape. Although OKN is more complex, it can be viewed for clinical
purposes as testing pursuit ipsilateral to the direction of target
movement, and contralateral saccades. The ipsilateral
parieto-temporo-occipital junction mediates pursuit of the acquired
stripe via connections that run in the internal sagittal stratum, deep
in the parietal lobe medial to the geniculocalcarine radiations and
adjacent to the atrium of the lateral ventricle. When ready to break
off, it communicates with the ipsilateral frontal lobe, which then
generates a saccadic movement in the opposite direction to acquire the
next target. In normal, alert individuals, an OKN stimulus induces
brisk nystagmus with the fast phase in the direction opposite tape
movement. The response is intensified if the subject looks in the
direction of the quick phase. Responses in one direction are compared
with responses in the other direction. A vertically moving stimulus can
evaluate upgaze and downgaze.
have a normal OKN response, despite their inability to see into the
hemifield from which the tape originates. Because of interruption of
the OKN pathways, patients with hemianopsias due to disease of the
optic radiations in the deep parietal lobe have abnormally blunted or
absent OKN responses. The patient is unable to pursue normally toward
the side of the lesion and is unable to generate contraversive saccades
into the blind hemifield.
The
significance of OKN asymmetry lies in the vascular anatomy and the
differing pathologies that affect the parietal and occipital lobes. The
primary clinical utility of OKN testing is investigation of patients
with parieto-occipital lesions, but the OKN tape has other uses. It may
be used to crudely check visual acuity, especially in infants, and for
estimating visual function in patients with depressed consciousness. It
may provide a clue to the presence of psychogenic visual loss.
Optokinetic nystagmus testing can demonstrate the slowed adducting
saccades of a subtle INO, and sometimes accentuate the nystagmus in the
abducting eye. Optokinetic nystagmus forced upward saccades may induce
convergence retraction nystagmus in patients with Parinaud syndrome.
 |
|
FIGURE 10.11 • Testing for optokinetic nystagmus. A. Using optokinetic tape. B. Using the rotating drum.
|
Disorders can be broadly divided into peripheral (infranuclear and
nuclear) and central (internuclear and supranuclear). Peripheral
disorders involve the extraocular muscles (e.g., MG or ocular myopathy)
or the cranial nerves (e.g., fourth nerve compression). Peripheral
disorders include things that affect the cranial nerve nuclei,
fascicles, or peripheral trunks. Although the nuclei and fascicles are
“central” in that they lie within the parenchyma of the CNS, the
clinical characteristics of conditions involving these structures is
much more akin to other infranuclear conditions than to supranuclear
disorders. Central disorders can be divided into supranuclear,
involving the optomotor control centers, and internuclear, involving
the pathways connecting and coordinating the activity of the ocular
motor nuclei, primarily the MLF. For simulation of disordered ocular
motility see http://cim.ucdavis.edu/eyes/version1/eyesim.htm.
processes involving the orbit causing mechanical limitation of eye
movement, or from ocular myopathies, neuromuscular transmission
disorders, or a palsy of an individual ocular motor nerve.
movement of the globe, often causing telltale proptosis as well.
Following trauma to the orbit, individual extraocular muscles may
become caught in fracture fragments. The muscles may also be injured
directly. Mechanically limited eye excursions exist for passive as well
as active movements. Forced ductions involve pushing or pulling on the
anesthetized globe in order to passively move it through the impaired
range. An eye affected by ocular muscle weakness, MG, or an ocular
motor nerve palsy moves freely and easily through a full range. An eye
affected by restrictive myopathy or an entrapped muscle cannot be moved
passively any better than actively.
motility because of weakness or because of restriction of movement. A
number of myopathies and muscular dystrophies may affect eye muscles.
Muscle disorders may be divided into myopathies and restrictive
orbitopathies. The most common restrictive orbitopathy is TED. Muscle
involvement by restrictive myopathy is easily confused with weakness of
the antagonist (e.g., restrictive myopathy of the medial rectus
simulating weakness of the lateral rectus). Forced ductions are often
done to clarify matters. The possibility of TED must be constantly
borne in mind when dealing with ocular motility disturbances.
extraocular muscles, usually accompanied by ptosis. Associated weakness
of eye closure due to myopathic involvement of the facial muscles is
often present. Weakness of eye closure is strongly suggestive of ocular
myopathy or neuromuscular transmission disorder as the cause of eye
muscle weakness. Few other conditions affect both eye muscles and
facial muscles. The common conditions causing ocular myopathy are
chronic progressive external ophthalmoplegia and oculopharyngeal
muscular dystrophy.
transmission disorder, frequently involves the extraocular muscles,
affecting any muscle or combination of muscles. Ocular involvement
occurs early in 50% to 70% of patients, and it eventually develops in
90%. Patients typically present with ptosis or diplopia, or both. The
hallmark of MG is fatigable weakness. The weakness gets worse with
repetitive contraction of the muscle. The ptosis in MG is “fatigable”;
it gets progressively worse with prolonged upgaze. The eyelid signs of
MG are discussed above. Fluctuating ptosis and diplopia, and worsening
symptoms toward the end of the day are characteristic. The ptosis and
ophthalmoparesis of MG are usually asymmetric and may vary from minute
to minute. These features, along with accompanying weakness of eye
closure, are virtually diagnostic. Myasthenia gravis should be
considered in the differential diagnosis of virtually any patient with
external ophthalmoplegia, but involvement of the pupil excludes MG.
nerve palsies, but with different frequencies. As many as 25% of cases
are idiopathic, and of these 50% recover spontaneously. Some processes
may affect more than one ocular motor nerve. Trauma is the most common
cause of fourth nerve palsy and the second most common cause of third
and sixth nerve palsy. Microangiopathic vascular disease due to
diabetes or hypertension is the most common etiology of nontraumatic
third and sixth nerve palsies. Aneurysms are an important etiology of
third nerve disease. Increased intracranial pressure may cause third
nerve palsies because of uncal herniation and sixth nerve palsies as a
nonspecific and nonlocalizing effect. Neoplasms may affect any of these
nerves. Basilar meningitis, migraine, viral infection, immunizations,
cavernous sinus disease, sarcoid, vasculitis, and Guillain-Barré
syndrome are occasional etiologies; the list of rare etiologies is long.
of extraocular muscle weakness, ptosis, and pupil involvement. Internal
ophthalmoplegia means involvement limited to the pupillary sphincter
and ciliary muscle; external ophthalmoplegia means involvement of only
the extraocular muscles; complete ophthalmoplegia is both. The most
common identifiable etiologies are ischemia, aneurysm, tumor, and
trauma; some 20% remain unexplained. Uncal herniation from mass effect
of any sort may result in compression as the temporal tip crowds
through the tentorial hiatus and traps CN III against the sharp edge of
the tentorium. Posterior communicating or distal internal carotid
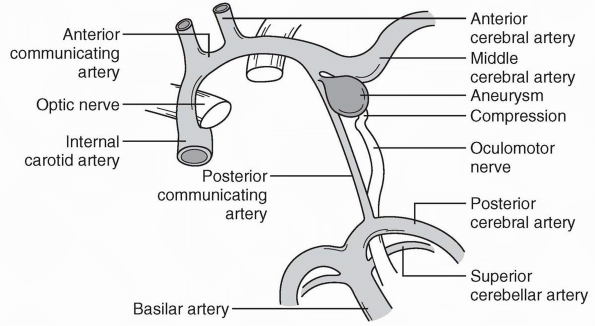
aneurysms commonly cause third nerve palsy (Figure 10.12).
With third nerve palsy, processes affecting the nucleus or fascicles
within the brainstem generally produce accompanying neighborhood signs
permitting localization (e.g., Weber or Benedikt syndrome). In its long
course along the base of the brain, CN III may be affected in
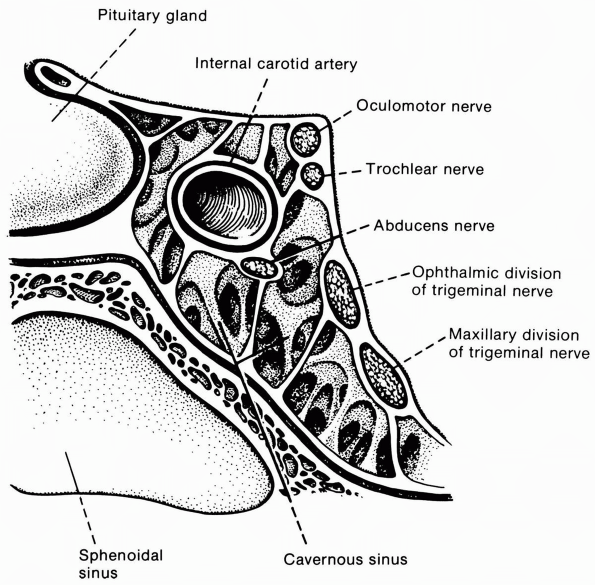
isolation. In the cavernous sinus (Figure 10.13) or orbit, accompanying deficits related to involvement of other structures usually permit localization.
ptosis of the upper lid, impairment of medial, upward, and downward
gaze and loss of accommodation, with a dilated pupil that does not
react to light, directly or consensually, or to near (Figure 10.1).
The eye rests in a down and out position due to preservation of the
lateral rectus and superior oblique functions. Incomplete CN III
lesions, causing paresis rather than paralysis and affecting certain
functions more than others, are more common than complete ones.
anywhere along its course from the oculomotor nucleus in the midbrain
to the orbit. Midbrain lesions are usually accompanied by neighborhood
signs that permit localization. Processes involving the third nerve
nucleus may cause characteristic
patterns
of weakness not seen with lesions at other locations. Processes
involving the subarachnoid course of the nerve usually produce isolated
unilateral CN III palsy with few associated findings to assist in
localization. The most pressing diagnostic consideration in an isolated
third nerve palsy is posterior communicating artery or basilar artery
aneurysm. Ischemic third nerve palsies most often occur because of
microvasculopathy related to diabetes and hypertension. Traumatic CN
III palsy usually occurs only with major head injuries, severe enough
to cause loss of consciousness or skull fracture. Increased
intracranial pressure with uncal herniation most often compresses the
ipsilateral nerve; the earliest sign is usually an abnormal pupil.
Compression of the contralateral cerebral peduncle causing a false
localizing hemiparesis ipsilateral to the lesion is not uncommon
(Kernohan’s notch syndrome). Cavernous sinus disease usually affects
other structures in addition to CN III.
 |
|
FIGURE 10.12
• Anatomy of the oculomotor nerve in relation to the major arteries at the base of the brain. An aneurysm arising from the posterior communicating artery is compressing and distorting the nerve. |
 |
|
FIGURE 10.13 • Oblique section through the cavernous sinus.
|
course; these two factors increase its vulnerability to injury. The
most common etiology of fourth nerve palsy is head trauma; bilateral
involvement is not uncommon. Nontraumatic cases are usually
microvascular, idiopathic, or congenital. A patient with a congenital
fourth nerve palsy may decompensate as an adult and present as an
apparently new onset condition. Patients with fourth nerve palsies may
not complain of diplopia, but rather blurry vision or some vague
problem when looking down—as when reading a book or descending stairs.
The diplopia is vertical or diagonal and maximal in downgaze. Patients
may tilt the head to the opposite side to eliminate diplopia, tucking
the chin so the affected eye may ride up and into extorsion, out of the
field of action of the weak superior oblique. Some fourth nerve
palsies, particularly in children, present with head tilt rather than
diplopia.
depression of the adducted eye. The involved eye has incomitant
hypertropia or hyperphoria; with the patient looking down and in,
alternate cover testing shows corrective downward refixations
indicating upward drift of the affected eye under cover. The
Bielschowsky head tilt test consists of tilting the head to each side,
localizing the fourth nerve palsy by the changes in diplopia that
result. Forcing the involved eye to intort worsens the diplopia. If
diplopia improves with head tilt to the left and worsens with tilt to
the right, the patient has a right fourth nerve palsy.
explanation. With a complete CN VI palsy, the eye cannot be abducted
and often rests in a position of adduction (Figure 10.14).
Incomplete palsies are common. Patients present with horizontal
diplopia worse at distance. There may be esotropia in primary position.
Examination shows paralytic (noncomitant) strabismus, worse in the
direction of action of the involved muscle. Mild weakness may show only
esophoria on alternate
cover
testing when the patient looks toward the side of the involved muscle.
Neoplasms, trauma, and microvascular neuropathy are the most common
identifiable etiologies. As many as 25% of cases remain unexplained.
Neighborhood signs usually permit localization when the nerve is
involved in the brainstem, cavernous sinus, or orbit. Sixth nerve
palsies occur with increased intracranial pressure, after head injury,
with structural disease in the middle or posterior fossa, with
nasopharyngeal tumors, and for numerous other reasons. Cranial nerve VI
palsies are the most common and classic of all false localizing signs:
they are nonspecific and bear no necessary anatomical relationship to
the central nervous system (CNS) pathology producing them. Bilateral
sixth nerve palsies are not uncommon. Not all abduction failure is due
to CN VI palsy. Some of the other causes include entrapment of the
medial rectus by a medial orbital fracture, TED, MG, convergence spasm,
divergence insufficiency, Duane syndrome, orbital pseudotumor, and
Möbius syndrome.
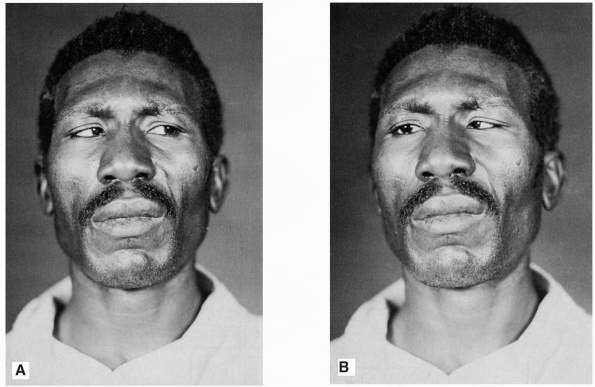 |
|
FIGURE 10.14 • Paralysis of the right abducens nerve in a patient with a posterior fossa neoplasm. A. Patient looking to left. B. Patient attempting to look in the direction of action of the paralyzed muscle.
|
internuclear. Supranuclear disorders include those that affect the
supranuclear gaze centers in the hemispheres and brainstem, as well as
other areas that influence eye movements, such as the basal ganglia and
cerebellum. Internuclear disorders affect the connections between the
ocular motor nerve nuclei in the brainstem.
medial rectus receives no signal to contract when the pontine
paramedian reticular formation (PPRF) and sixth nerve nucleus act to
initiate lateral gaze. As a result, gaze to one side results in
abduction of the ipsilateral eye, but no adduction of its fellow.
Typically the abducting eye has nystagmus as well, which may be
sustained or only a few beats. Failure of the medial rectus to adduct
is an isolated abnormality in the affected eye; normality of the lid
and pupil distinguish an INO from a third nerve palsy. By convention,
the INO is labeled by the side of the adduction failure; a right INO
produces adduction failure of the right eye. Many brainstem lesions can
cause an INO, but the common conditions are MS and brainstem stroke.
Internuclear ophthalmoplegias due to MS are usually bilateral and seen
in young patients, whereas those due to brainstem vascular disease are
more often unilateral and seen in older patients.
conjugate horizontal gaze. The eyes normally remain straight ahead
because of a balance of input from the frontal eye fields in each
hemisphere. Seizure activity in one frontal lobe drives the eyes
contralaterally. In an adversive seizure, the eyes and then the head
deviate to one side, after which the seizure may generalize. With
destructive frontal lobe lesions, most often ischemic stroke, the
patient is unable to move the eyes contralaterally—a gaze palsy, or, if
less severe, a gaze paresis. The intact, normal hemisphere maintains
its tonic input, the imbalance causing the eyes to move
contralaterally, toward the diseased side—a gaze deviation. Patients
may have gaze palsy without gaze deviation. The presence of gaze
deviation usually means gaze palsy to the opposite side, but it may
occasionally signal seizure activity.
PPRF governs ipsilateral, conjugate horizontal gaze. The PPRF draws the
eyes ipsilaterally, in contrast to the frontal eye fields, which force
the eyes contralaterally. Destructive lesions of the PPRF impair the
ability to gaze ipsilaterally, resulting in a gaze deviation toward the
intact side as the normal PPRF pulls the eyes over. Pontine gaze
palsies affect all functions, voluntary and reflex; even ice water
calorics will not move the eyes.
to one side, the possibilities are (a) frontal lobe seizure activity,
(b) frontal lobe destructive lesion, and (c) pontine destructive
lesion. Patients with destructive frontal lesions gaze away from the
side of the hemiparesis; patients with pontine strokes gaze toward the
hemiparesis. Epileptogenic gaze deviations are usually betrayed by a
component of jerky eye movement and subtle twitches elsewhere.
Patients are unable to look up, and when they attempt it the eyes may
spasmodically converge and retract backward into the orbits
(convergence-retraction nystagmus). The convergence-retraction
movements readily appear during forced upward saccades in response to a
down-moving OKN tape. The retraction movement is best seen from the
side. Parinaud syndrome usually results from a mass lesion involving
the region of the posterior third ventricle and upper dorsal midbrain,
such as a pinealoma. Other frequent signs include eyelid retraction and
abnormal pupils. The pupils in Parinaud syndrome have a poor, rarely
absent, light response and much better near response (tectal pupils).
in the rostral brainstem and thalamus result in impairment first of
downgaze, then of upgaze, and eventually in global gaze paresis. Reflex
eye movements are preserved until late in the disease. The gaze
abnormalities are accompanied by parkinsonian signs and a pronounced
tendency to extensor axial rigidity.
with nystagmus or similar appearing movements, the usual clinical
exercises include the following two steps: (a) deciding if the
nystagmus indicates neurologic pathology and (b) if so, whether the
pathology is central or peripheral. There are normal, physiologic forms
of nystagmus. A few beats of nystagmus at the extremes of lateral gaze
(end-point nystagmus) occur commonly in normals and have no pathologic
significance. A whole host of conditions can cause nystagmus, including
ocular disease, drug effects, peripheral vestibular disease, and CNS
disease. Nystagmus may also be congenital. Schemes have classified
nystagmus in many different ways. This discussion focuses on the types
of nystagmus commonly encountered in neurologic practice and on the
differentiation between nystagmus that likely signifies neurologic
disease (neuropathologic) and the kind that does not
(nonneuropathologic).
phases of equal amplitude and velocity) versus jerk (a fast phase and a
slow phase); central versus peripheral; induced versus spontaneous; and
physiologic versus pathologic. Further characterizations include
rapid/slow, coarse/fine, manifest/latent, sensory/motor, and
horizontal/vertical. Pendular nystagmus is classified by its plane of
movement, usually horizontal. Pendular nystagmus only rarely signifies
neurologic disease, and this discussion is focused primarily on jerk
nystagmus. Jerk nystagmus is classified by the direction of the fast
phase. Alexander’s law states that jerk nystagmus increases with gaze
in the direction of the fast phase. First-degree nystagmus is present
only with eccentric gaze (e.g., right-beating nystagmus on right gaze).
Second-degree nystagmus is present in primary gaze and increases in
intensity with gaze in the direction of the fast component (e.g.,
right-beating nystagmus in primary gaze increasing with gaze to the
right). With third-degree nystagmus, the fast component continues to
beat even with gaze in the direction of the slow component (e.g.,
right-beating nystagmus persisting even with gaze to the left).
Dissociated nystagmus is different in the two eyes (e.g., the nystagmus
in the abducting eye in INO).
be physiologic, or due to ocular disease (e.g., poor vision), or other
conditions.
and induced vestibular. Although these types of nystagmus are normal,
they may be altered when disease is present in such a way as to assist
in localization. End-point nystagmus is fine, variably sustained
nystagmus at the extremes of lateral gaze, especially with gaze
eccentric enough to eliminate fixation by the adducting eye. Symmetry
on right and left gaze, abolition by moving the eyes a few degrees
toward primary position, and the absence of other neurologic
abnormalities generally serve to distinguish end-point from pathologic
nystagmus. End-point nystagmus is the most common form of nystagmus
seen in routine clinical practice. Although OKN is a normal response,
its characteristics may be altered in disease. Changes in OKN occur
primarily with deep parietal lobe lesions. Vestibular nystagmus can be
induced by rotation (e.g., Barany chair) or by irrigation of the ear
with hot or cold water.
result from neurologic disease include voluntary nystagmus, drug
induced nystagmus, congenital nystagmus, and nystagmus due to ocular
disease.
nervous system. Common types include vestibular, positional, gaze
evoked, and gaze paretic nystagmus. Symmetric, equal activity of the
vestibular systems on each side normally maintains the eyes in
straight-ahead, primary position. Vestibular imbalance causes the eyes
to deviate toward the less active side as the normal side overcomes the
weakened tonic activity from the hypoactive side. In an alert patient,
the frontal eye fields generate a saccade to bring the eyes back toward
primary position, creating the fast phase of vestibular nystagmus. When
the cortex does not generate a correcting saccade, as in coma, only the
tonic deviation develops; the eyes deviate toward the
ice-water-irrigated ear.
the syndrome of positional vertigo and nystagmus. Nystagmus occurs
after a latency of up to 30 seconds, beats with the fast phase toward
the down ear, quickly fatigues despite holding the position, and adapts
with repeated attempts to elicit it. Positional nystagmus is a very
common condition. While generally peripheral it may occur with central
disease (tumor, stroke, MS, degenerative disease).
with gaze in any direction with the fast phase in the direction of gaze
is referred to as gaze-evoked nystagmus. Normal physiologic end-point
nystagmus is gaze evoked, but only present horizontally and at extremes
of gaze. Abnormal gaze-evoked nystagmus occurs short of extreme gaze
and is more sustained than end point. Drug-induced nystagmus is gaze
evoked, usually horizontally and in upgaze. Nystagmus with the same
appearance in the absence of drug effects is nonspecific but usually
indicates disease of the cerebellum or cerebellar connections. Gaze
paretic nystagmus is a form of gaze-evoked nystagmus seen in patients
with incomplete gaze palsies. Rather than having an absolute inability
to gaze in a particular direction, the patient achieves full lateral
gaze transiently but is not able to maintain it. The eyes drift back
toward neutral and then spasmodically jerk back in the desired gaze
direction.
bobbing, ocular flutter, and opsoclonus. Ocular flutter and opsoclonus
are types of saccadic intrusions, spontaneous saccades away from
fixation; they may be confused with nystagmus. Ocular dysmetria is an
over- or under-shooting of the eyes on rapid refixation of gaze toward
either side or on returning to the primary position that requires
corrective saccades.
