Spinal Fusion
fusion surgery since the 1990s. Currently, about 250,000 spinal fusion
procedures are carried out each year in the United States, and nearly
all of these require bone graft material. The most common reason for
performing a spinal arthrodesis is to treat either instability
(excessive motion) of a spine segment or a deformity that is at risk
for progression. Most fusions are performed to treat degenerative
disorders, and the most common region is the lumbar spine.
Posterolateral lumbar fusion, the most commonly performed spinal
arthrodesis, has been associated with the highest likelihood of failure
(nonunion), ranging from 5% to 44% of patients with single-level fusion
and more frequently when multiple levels are attempted. The successful
repair of these pseudarthroses is even more challenging, with failures
occurring in 35% to 51% of revision attempts. Nonunion often prevents
the resolution of the clinical symptoms and usually results in greater
medical costs and morbidity and the need for more surgeries.
Mechanical enhancement of spinal fusions using rigid internal fixation
with hooks or screws and rods or plates has increased the successful
fusion rate by approximately 10%; however, it has failed to eliminate
nonunion in 10% to 1 5% of patients. Biophysical stimulation by either
direct-current pulsed electromagnetic fields or low-intensity
ultrasound has been shown to be a viable strategy for the enhancement
of spinal fusion healing. Also, a variety of potential bone graft
alternatives now are available. These alternatives include the
following:
currently used as bone graft extenders only and not as a bone graft
enhancer or substitute
carbonate), coralline hydroxyapatite, or composites such as
hydroxyapatite-tricalcium phosphate, which are used as bone graft
extenders when mixed with autogenous bone graft but are unlikely to
serve as stand-alone bone graft substitutes
incorporation of bone graft material into the recipient site. The
precise biologic, physiologic, and molecular mechanisms operating
during this healing process are not understood fully. Successful fusion
depends on a complex process influenced by the type of graft material
used and on many local (biologic), biomechanical, systemic, and
external factors affecting the healing response (Tables 33.1-2 and 33.1-3).
process, which makes it difficult to study in the clinical setting. The
lack of reliable noninvasive techniques for assessing the success or
failure of an arthrodesis limits prospective clinical studies. An
animal model is a practical solution for studying individual factors
involved in this complex process. A reliable spinal fusion animal model
should mimic the incidence
of
nonunion and the surgical procedure seen in humans. Also, it should
allow for the rapid observation of several subjects over a short time
and allow for the valid extrapolation of data and results.
|
TABLE 33.1-1 ENHANCEMENT OF SPINAL FUSION
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
healing, far less is understood about the precise molecular mechanisms
that control bone graft incorporation in a spinal fusion. Boden et al
described the lumbar intertransverse fusion healing process in rabbits
using autogenous iliac crest as a graft material. Mechanically solid
fusions were observed by the 4th postoperative week with an overall
nonunion rate of 30% to 40% (similar to what occurs in humans).
Radiographic analysis also showed progressive remodeling of bone graft
material with time, usually by 10 to 12 weeks, but as in humans,
radiographs were accurate in assessing success or failure to attain
solid fusion only 70% of the time. Vascular injection studies have
shown that the
primary
blood supply to the fusion mass originates from the decorticated
transverse processes. The failure to achieve spinal fusion in the
absence of decortication emphasizes the importance of extensive
decortication of the posterolateral spine elements (lateral facet, pars
interarticularis, transverse process) in providing bone marrow,
vascularization, and osteoprogenitor cells to the fusion mass.
|
TABLE 33.1-2 FACTORS AFFECTING SPINAL FUSION HEALING
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
TABLE 33.1-3 EFFECTS OF TOBACCO SMOKE AND NICOTINE ON BONE METABOLISM AND HEALING
|
|||||||
|---|---|---|---|---|---|---|---|
|
|
TABLE 33.1-4 STAGES OF SPINAL FUSION HEALING AS PROPOSED BY BODEN ET AL (1995) (HISTOLOGIC STAGES OF SPINAL FUSION)
|
||||||
|---|---|---|---|---|---|---|
|
sections revealed three distinct, reproducible temporal phases of
spinal fusion healing (inflammatory, reparative, and remodeling) (Table 33.1-4).
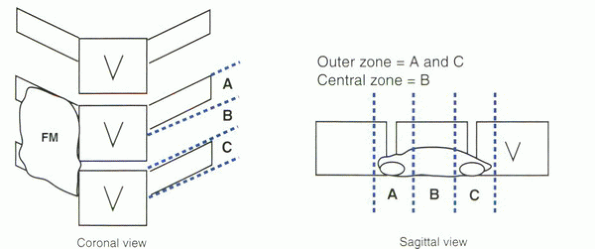
These phases occurred in sequence but in a delayed fashion in the
central zone of the fusion mass compared with the outer transverse
process zones (Fig. 33.1-1). Maturation of the
spinal fusion was most advanced at the ends of the fusion mass near the
transverse processes (“outer” zone). Intramembranous bone formation was
the predominant mechanism of healing over the decorticated transverse
processes. A similar histologic progression occurred in the “central”
zone, but was delayed in time. This site was characterized by a period
of endochondral bone formation during weeks 3 and 4, when cartilage
formed and was converted to bone. This central “lag effect” may explain
why many nonunions occur in the central zone of a fusion mass.
Remodeling in the central zone equilibrated with the transverse process
zones by week 10.
 |
|
Figure 33.1-1
Schematic diagram of lumbar fusion mass divided into thirds in the coronal and sagittal views and their relationship to the vertebral bodies (V). The two outer zones (A and C) are distinguished from the single central zone (B). FM. fusion mass. |
of a cascade of cellular events believed to be controlled by various
growth factors, including bone morphogenetic proteins (BMPs),
transforming growth factor-β, fibroblast growth factor,
platelet-derived growth factor, and insulin-like growth factor-1. A
unique temporal and spatial pattern of osteoblast-related gene
expression was observed in a reverse-transcriptase polymerase chain
reaction analysis of RNA from the different zones of the fusion mass (Table 33.1-5).
A lag effect in gene expression that correlated with the previously
observed lag effect in the histologic healing sequence was noted in the
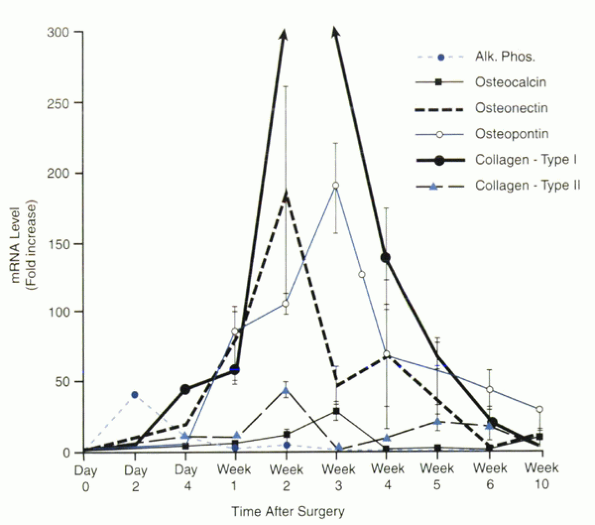
central zone compared with the outer zones of the fusion. As with
osteocalcin expression, the peak expression of all genes measured was
seen in the central zone 1 to 2 weeks later than the peak in the outer
zone (Fig. 33.1-2).
This finding is consistent with the peripheral-to-central healing
pattern observed histologically for fusions using autogenous bone
graft. Laboratory indicators of bone formation are listed in Table 33.1-6.
|
TABLE 33.1-5 BONE MORPHOGENETIC PROTEIN GENE EXPRESSION AND BONE PROTEIN EXPRESSION DURING SPINAL FUSION HEALING
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
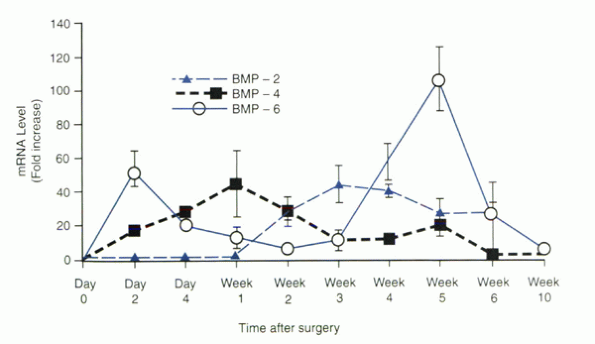
In the peripheral zones, BMP-2 mRNA expression was increased during
weeks 2 through 6, with peak expression in weeks 3 and 4 (40-fold
increase). BMP-6 in the outer zones had a first peak (54-fold) on day 2
and a second peak (100-fold) during week 5, whereas BMP-6 in the
central zone showed an initial peak (34-fold) on day 2, but did not
show the later peak. These findings suggest specific time patterns of
expression and probably unique roles for each of the various BMPs
during spinal fusion. It seems that BMP-6 is unique in that its mRNA
levels showed the earliest peak and greatest relative increase of the
BMPs studied. BMP-6 may play an initiating role in intramembranous bone
formation. It also is an early marker for solid spinal fusion. The
lower level of BMP-6 expression in the central zone of the fusion mass
is correlated with the delayed timing and smaller amount of bone
formation in the central zone of the fusion. The predilection for
nonunion in the central zone also is apparent at a molecular biologic
level.
|
TABLE 33.1-6 INDICATORS OF MESENCHYMAL CELL DIFFERENTIATION BY BONE MORPHOGENETIC PROTEINS
|
||||||||
|---|---|---|---|---|---|---|---|---|
|
associated with earlier peaks and higher levels of osteoblast-related
gene expression in the central zone of the fusion mass, eliminating the
central lag effect and perhaps decreasing the number of potential
nonunions. The presence of nicotine significantly changes the gene
expression associated with bone healing. The effect of nicotine on
cytokine expression is seen mostly in the inner zone of the fusion
mass. Table 33.1-5 summarizes molecular events occurring during the fusion process.
considerably depending on the region of the spine under consideration.
The three primary locations are the anterior interbody, the
intertransverse process, and the interlaminar-facet joint region. The
incorporation process also differs for cortical and cancellous bone
grafts. Specific descriptions of integration of various types of bone
grafts and substitutes are provided in subsequent sections. For
biosynthetic materials, new bone formation occurs by creeping
substitution, and the resorbing cell is the foreign body giant cell,
not the osteoclast. Also, two physical factors determine the incidence
and speed of union between bone grafts and the adjacent host bone more
than the characteristics of the grafts themselves:
-
Stability of the construct
-
Contact between host bone and the graft
movement across an intervertebral motion segment after bone union.
Studies examining the clinical and radiographic success of spinal
fusions with autograft have reported highly variable results. This
variability may be due to many factors, including the following (see Table 33.1-2):
-
Location and type of fusion
-
Stringency of fusion outcome criteria
-
Patient selection
-
Severity of underlying pathology
-
Use and type of internal fixation
-
Technical preparation of the fusion bed
-
Technical retrieval and preparation of the bone graft material
 |
|
Figure 33.1-2
Osteoblast-related gene expression in the outer zone of the spinal fusion mass at specific times after surgery. The values of mRNA levels are given as fold increases over the level present in iliac crest bone (day 0). A reproducible sequence of gene expression was seen that was paralleled in the central zone (not shown) but delayed by 1 to 3 weeks. |
 |
|
Figure 33.1-3
Bone morphogenetic protein (BMP) gene expression in the outer zone of the spinal fusion mass at specific times after surgery. The values of mRNA levels are given as fold increases over the level present in iliac crest bone (day 0). A reproducible sequence of gene expression was seen with BMP-6 mRNA peaking earliest on day 2, followed by BMP-4 mRNA, BMP-2 mRNA, and a second peak of BMP-6 mRNA. |
performed spinal arthrodesis, has been associated with the highest
likelihood of failure (pseudarthrosis), ranging from 5% to 44%.
increase the fusion rate, whereas tensile forces as experienced during
the consolidation of interlaminar or intertransverse process fusion may
decrease it. It is believed that compressive forces acting on the
interbody graft stimulate the ingrowth of vascular buds and
proliferating mesenchymal cells from the cancellous host bone into the
bone graft.
internal fixation secondary to decreased motion in the fusion segments.
The level of fusion (L4-5 versus L5-S1), the number of segments fused,
the patient’s weight and activity level, and external bracing after
surgery may influence the outcome of the fusion. Implant loosening may
cause increased nonunion. Instrumented fusion masses tend to be more
rigid, narrower, and more compact than masses with uninstrumented
fusions. Patients with disease conditions (e.g., muscular dystrophy,
spinal muscular atrophy) that are associated with little voluntary
motion often have higher than average fusion rates because of decreased
spinal segment motion. Intraarticular preparation of the lumbar facet
joints for arthrodesis may result in a 25% increase in sagittal plane
mobility producing tensile strain and ultimately nonunion, and
exclusion of the same may predispose to a less rigid fusion.
healing of a spinal fusion. The spinal fusion process is affected
greatly by the adequacy of local blood supply, the efficacy of the
inflammatory response, and the availability of osteoprogenitor cells.
Scarring of the fusion bed from multiple fusion attempts, excessive
trauma to the fusion area, and presence of a local tumor or bone
disease may replace normal marrow, structurally weakening the recipient
bone and fusion mass. The health of the host bone bed is crucial in the
process of osteoinduction because new osteoprogenitor cells are
recruited by induction of residual mesenchymal cells in marrow
reticulum, endosteum, periosteum, and connective tissue. Healthy soft
tissue adjacent to a fusion process provides a source for diffusible
growth factors and nutrition for migrating osteoprogenitor cells, but
is less critical than having adequate decorticated host bone.
Perioperative irradiation of the fusion area, especially in the first
few weeks of fusion, may increase the nonunion rate because of its
direct cytotoxic effects on the proliferating and differentiating cells
and alteration of neoangiogenesis. Inadequate decortication and
insufficient quantity of bone graft can predispose to nonunion. The
larger the surface area decorticated for fusion, the greater the
availability of potential osteogenic cells and the larger the contact
area exposed to support a bone bridge large enough to carry a
mechanical load. Also, physical barriers (e.g., bulky instrumentation
or the presence of polymethyl methacrylate) can result in inadequate
surface area for decortication or osseointegration of the fusion mass.
fusion. There are no specific data concerning gender and delayed
healing. Older age (of the patient) has been associated with a decrease
in recruitment of bioactive growth factors and of pluripotential stem
cells during the spinal fusion healing. Osteoporosis may affect the
spinal fusion rate adversely. Possible factors implicated in the
process include the following:
-
Apparent decrease in bone mass
-
Alterations in bone marrow quality
-
Decrease in the osteogenic stem cells and vascularity
-
Structurally weak bones, which provide inadequate stabilization through internal fixation
hyperthyroidism) can affect the rate of spinal fusion adversely.
Corticosteroids have a negative effect on bone healing because they
decrease osteoblast differentiation from mesenchymal cells, increase
bone resorption, and decrease the rate of synthesis of major components
of bone matrix necessary for bone healing. Nutritional disorders,
including deficiencies in protein, iron, calcium, and phosphorus, have
been associated with delayed callus formation and fusion consolidation.
Systemic diseases, such as sickle cell anemia, thalassemia major, and
diabetes, may reduce the osteogenic potential of bone marrow by
overgrowth of hematopoietic cells at the expense of osteoprogenitor
cells.
-
Cytotoxic drugs
(e.g., doxorubicin [Adriamycin] and methotrexate) used in the immediate
postoperative period inhibit bone formation and healing. -
Nonsteroidal antiinflammatory drugs
(e.g., ibuprofen and ketorolac) may inhibit the healing of a spinal
fusion possibly by suppressing the inflammatory response involved in
the early stages of the healing process. -
Other drugs, including antibiotics (e.g., ciprofloxacin) and anticoagulants
(e.g., heparin sodium and warfarin [Coumadin]) administered
preoperatively and postoperatively have been associated with delayed
bone healing.
Smoking interferes with bone homeostasis and repair. Nicotine inhibits
expression of a wide range of cytokines, including cytokines associated
with neovascularization and osteoblast differentiation.
healing includes the exogenous induction of biophysical forces, such as
electromagnetic fields, low-intensity ultrasound, and use of direct
electrical stimulation. The scientific basis for these biophysical
interventions is that they serve as surrogates for the regulatory
signals normally arising through functional loading of the skeleton
(Wolff’s law) but are absent during the spinal healing process.
reports on the piezoelectric effects of bone. He showed new bone
formation in the vicinity of a cathode (negative electrode) when low
current was applied to a rabbit femur over 3 weeks. Electrical
stimulation for clinical use has three distinct forms:
-
Constant direct-current stimulation (invasive)
-
Time-varying inductive coupling produced by a magnetic field (noninvasive)
-
Capacitative coupling (noninvasive)
10 mV/cm, which are comparable to endogenously produced electrical
fields.
implantable device in which the metallic lead or cathode is placed in
direct contact with the decorticated transverse process and bone graft.
The anode is implanted in the subcutaneous layer. The effective
stimulation distance is approximately 5 to 8 mm from the cathode, and
the area of stimulation may be adjusted by coiling the cathode wire to
increase surface area. The implantable battery delivers a constant
direct current for 6 to 9 months. Pulsed electromagnetic field (PEMF)
stimulation requires a noninvasive external coil that delivers
electromagnetic energy when driven by an electrical current. The coils
usually are worn by the patient in a brace for 6 to 8 hours per day for
3 to 6 months. Capacitatively coupled electrical field stimulation
(CCEFS) uses an external pair of capacitative plates that produce
electrical fields when an electrical current is applied. The
capacitative plates usually are worn continuously for 9 months or until
fusion occurs. PEMFs are generated by a time-varying current applied to
metallic coils at a certain duration and intensity.
stimulate osteogenesis is unknown. The molecular mechanism of action
has been hypothesized to occur as a result of direct interaction
between induced electrical fields and the target cell or alternatively
by affecting the metabolism of drugs and endogenous factors. DCES is
capable of triggering mitosis and recruitment of osteogenic cells in
culture. The chemical changes in the local environment of bone cells in
proximity to the active cathode are thought to trigger physiologic
changes that lead to an osteogenic response. In vivo studies have shown
that direct electrical currents stimulate osteogenesis through
proliferation and recruitment of bone cells. It also may affect the
activity of bone and cartilage directly through the activation of
cyclic adenosine monophosphate within the stimulated cell, triggering
an intracellular second messenger system.
is an important variable. Electrically induced osteogenesis has been
noted to occur within specific windows of electrical current
parameters. In 1981, Brighton et al studied the relationship between
charge, current density, and amount of new bone formation in the
medullary canal of the intact rabbit tibia using a stainless steel wire
cathode. They showed that a constant current of 20 µA resulted in the
greatest amount of bone formation, with no signs of necrosis. There was
no dose-dependent increase in bone formation when the current was
increased to 40 µA, but some cellular necrosis was noted. A constant
current of 80 µA was destructive, resulting in cellular necrosis.
Titanium cathodes are believed to provide a more even distribution and
delivery of the current to the surrounding tissue. Several studies have
reported conflicting results with regards to what current would cause
optimal bone formation or bone necrosis.
stimulating osteogenesis than constant DCES; however, a direct
comparison of relative efficacy is difficult because comparative
studies have not been performed. In contrast to the effects of DCES,
PEMFs seem to affect differentiated bone cells instead of precursor
cells. It has been shown that PEMFs may affect cellular functions, such
as protein synthesis and bone matrix synthesis, through accelerated
bone formation by osteoblasts and inhibition of osteoclastic bone
resorption and macrocellular events, including vascularization and
tissue calcification. With PEMF stimulation, the induced electrical
field rather than magnetic flux is responsible for augmenting bone
healing.
therapy in the treatment of nonunion in long bones, and more recent
studies have shown increased fusion rates for lumbar spinal fusion
supplemented with electrical stimulation. There is evidence in the
literature to support its use for selected indications, as follows:
-
Multilevel fusion
-
Reoperation for pseudarthrosis
-
Presence of osteoporosis, smoking, or significant vascular disease
on the efficacy of electrical stimulation on lumbar spinal fusion. They
used implantable DCES successfully in the treatment of anterior and
posterior spinal fusions and nonunited spinal fractures with fusion
success rates of 92% and 85%.
Bozic et al found that coralline hydroxyapatite and direct current
stimulation can be used together to increase the fusion rate and
stiffness in a dose-dependent manner in a rabbit model. Two animal
posterior fusion studies performed by Kahanovitz et al used PEMFs, with
the electromagnetic devices placed externally. The first study
investigated a bone-healing signal for a three-level posterior fusion
in a dog model. The second study evaluated the effect of a newer
fracture-healing signal on facet fusions in a dog model. Both of these
studies failed to show any significant increase in fusion rates. Glazer et al,
using a rabbit model to assess the efficacy of PEMFs, showed a decrease
in the nonunion rate from 40% to 20%, but this was not statistically
significant.
|
TABLE
33.1-7 EFFECTS OF PULSED ELECTROMAGNETIC FIELDS (PEMF) AND DIRECT CURRENT ELECTRICAL STIMULATION (DCES) ON ANTERIOR AND POSTERIOR SPINAL FUSION MODELS |
||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
published clinical studies on the efficacy of electrical stimulation in
spinal fusion. It is apparent from clinical trials and experimental
studies that DCES is a potential adjunct to spinal fusion surgery when
it is applied to lumbosacral fusion or pseudarthrosis repair. Kane
reported the first multicenter, prospective, randomized trial in which
results showed a significantly higher fusion rate in patient groups in
which fusion was difficult to achieve. Several studies in instrumented
and uninstrumented patients further support the use of electrical
stimulation as an adjunct to interbody and posterolateral spinal
fusion. Randomized, double-blind, prospective clinical
trials
of PEMFs and capacitatively coupled electrical stimulation, reported by
Mooney and by Goodwin et al, and single-coil electromagnetic
stimulation results reported by Linovitz et al have shown a significant beneficial effect (see Table 33.1-8)
on certain types of lumbar fusion procedures. Other published clinical
trials and animal experiments collectively have yielded mixed results,
however (see Tables 33.1-7 and 33.1-8).
Goodwin et al reported the results of the first randomized,
double-blind, prospective trial of capacitatively coupled electrical
stimulation as an adjunct to lumbar spinal fusion surgery. Stimulated
patients were found to have a fusion success rate of 84.7% versus 64.9%
for control patients, a statistically significant difference.
|
TABLE
33.1-8 FUSION SUCCESS RATES FOR PREVIOUSLY PUBLISHED OR PRESENTED CLINICAL DATA SUPPORTING THE USE OF PULSED ELECTROMAGNETIC FIELDS (PEMF) AND DIRECT CURRENT ELECTRICAL STIMULATION (DCES) WHEN USED IN EITHER ANTERIOR OR POSTERIOR SPINAL FUSIONS |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
the limit of human hearing. It has been used as a physical signal in
the detection or alteration of biologic effects for many years. Low
ultrasonic intensities (milliwatts per square centimeter) are applied
for diagnostic purposes to avoid excessive heating of the tissues;
ultrasonic intensities of 1 to 3 W/cm2 commonly are used to
treat joint stiffness, pain, and muscle spasm and to improve muscular
mobility. Ultrasound also has some beneficial effects on wound and
tendon healing. A broad spectrum of experiments performed at the basic
science and clinical levels have provided substantial evidence that
low-intensity ultrasound can accelerate osteogenesis and augment the
fracture-healing process.
accelerates bone healing is largely unknown. Low-intensity pulsed
ultrasound is a noninvasive form of mechanical energy transmitted
transcutaneously as high-frequency acoustical pressure waves around the
cells. The mechanical stimulation inherent to ultrasound translates
into a biologic response. Several biologic mechanisms (direct and
indirect) have been proposed to explain the influence of ultrasound on
the acceleration of the fracture-repair process. Ultrasound influences
several stages of the healing process, including signal transduction
(second-messenger activity of chondroblasts and osteoblasts), gene
expression, blood flow, tissue modeling and remodeling, and mechanical
attributes of the callus. In vivo studies have shown that ultrasound
helps to initiate the healing process, increase callus formation and
the biomechanical strength of fracture callus, and encourage clinical
and radiographic healing. It also increases aggrecan mRNA, osteopontin
mRNA, bone mineral density, and blood flow. Data from various in vitro
studies suggest that ultrasound may induce conformational changes in
the cell membrane, altering ionic permeability (increased calcium
incorporation) and second messenger activity. Changes in second
messenger activity conceivably could lead to downstream alterations in
gene expression, resulting in an acceleration of the fracture-repair
process by upregulating cartilage-specific and bone-specific genes and
others. Ultrasound also stimulates angiogenesis, chondrogenesis, and
cartilage hypertrophy, resulting in an earlier onset of endochondral
formation and leading to an increase in stiffness and strength of the
fracture site.
the first to assess the benefits of ultrasound in spinal fusion. Their
findings indicated that ultrasound increased the rates of fusion,
stiffness, and load to failure, suggesting an influence on the healing
of trabecular and cortical bone. Histologic assessment confirmed that
there was increased bone formation in the fusion masses that had been
exposed to ultrasound. Although these results are preliminary, they
suggest that the low-level mechanical signal may influence cellular
processes in the axial and the appendicular skeletons. Aynaci et al
evaluated the effects of ultrasound on posterolateral intertransverse
process fusion by using muscle-pediculated bone graft in a rabbit
model. Historically, this type of graft has a higher fusion rate. The
investigators showed a statistically significant increase in fusion
rate in 85% of the stimulated animals compared with 55% in the control
group. There was increased bone formation radiologically and
histologically in the fusions exposed to ultrasound. Based on several
studies that have been done at nonspinal sites, it is hoped that in the
near future definite clinical trials of ultrasound in spinal fusion in
humans will be done in increasing numbers.
O, Onder C, Piskin A, Ozoran Y. The effect of ultrasound on the healing
of muscle-pediculated bone graft in spinal fusion. Spine
2002;27:1531-1535.
SD, Schimandle JH, Hutton WC, Chen MI. 1995 Volvo Award in Basic
Sciences. The use of an osteoinductive growth factor for lumbar spinal
fusion: Part I. the biology of spinal fusion. Spine 1995;20:2626-2632.
JA, Koka A, Bensusan JS, et al. Effects of irradiation on posterior
spinal fusions: a rabbit model. Spine 1994;19: 1836-1841.
RJ, Pathria M, Bernhardt M, et al. Combined magnetic fields accelerate
and increase spine fusion: a double-blind, randomized, placebo
controlled study. Spine 2002;27:1383-1389.
MA, Boden SD, Martin G, et al. Gene expression during autograft lumbar
spine fusion and the effect of BMP-2. Clin Orthop 1998;351:252-265.
J, Margant B, Bubis JJ, et al. Stimulation of bone formation by
electrical current on spinal fusion. Spine 1986;11:167-169.
Y, Hutton WC, Boden SD, Morone MA. Revascularization of the fusion mass
in a posterolateral intertransverse process fusion. Spine
1998;23:1149-1154.
alone or in combination with other materials, promotes a bone-healing
response by providing:
-
Osteogenicity
-
Osteoconductivity
-
Osteoinductivity
participate in the fusion process in several ways, which depend on the
properties of the graft materials. Some important properties of an
ideal bone graft or substitute are listed in Table 33.2-1.
cellular content. Osteogenic graft materials contain viable cells that
are capable of forming bone (i.e., differentiated osteogenic precursor
cells) or have the potential to differentiate into bone-forming cells
(inducible osteogenic precursor cells). Surface cells on cortical, and
more so cancellous, grafts that are handled properly can survive and
produce new bone. This early bone formed by viable graft cells often is
crucial in bone formation during the first 4 to 8 weeks after surgery.
This potential to produce bone is characteristic only of fresh
autogenous bone and marrow cells.
|
TABLE 33.2-1 PROPERTIES OF GRAFT MATERIALS
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
process by which some graftderived factors stimulate recruitment from
the surrounding bed of undetermined mesenchymal-type cells, which then
differentiate into cartilage-forming and bone-forming cells. The
concept of osteoinduction first was introduced by Urist et al. The
osteoinductivity of mineralized grafts is minimal, but the
osteoinductive capacity of demineralized bone matrix (DBM) (Grafton,
Osteotech, Eatontown, NJ) has been well characterized. Bone matrix
contains several bone-forming cytokines, including bone morphogenetic
proteins (Tables 33.2-2 and 33.2-3).
These cytokines are capable of inducing or influencing the
differentiation of mesenchymal cells into bone-forming cells. In
addition to DBM and the above-mentioned factors, autogenous and
allograft bone are known to possess osteoinductive properties.
physical property of a graft material that allows the ingrowth of
sprouting capillaries, perivascular tissue, and infiltration of
osteoprogenitor cells from the recipient bed into the structure of a
graft during the process of graft incorporation known as creeping substitution.
A purely osteoconductive graft material transfers neither osteogenic
cells nor inductive stimuli, but it acts as a nonviable scaffold or
trellis that supports the healing process. Osteoconduction may result
from active bone formation and osteoinduction (e.g., in a fresh
corticocancellous autograft), or it may occur passively, without the
active participation of the graft, as is the case with most cortical
allografts. Osteoconduction often is determined by the structure of the
graft, the vascular supply from the surrounding soft tissue, and the
mechanical environment of the graft and surrounding structures.
Osteoconductive materials include the following:
-
Autogenous and allograft bone
-
Bone matrix
-
Collagen
-
Calcium phosphate ceramics
-
Extender (a
material that allows the use of less autogenous bone graft with the
same end result or one that allows a given amount of autogenous bone to
be stretched over a greater area with the same success rate) -
Enhancer (a
device that when added to autogenous bone graft increases the
successful healing rate of autogenous bone graft, using either the
usual amount of graft or a smaller amount of bone graft) -
Substitute (a material that may be used entirely in place of autogenous bone graft to achieve the same or a better fusion success rate)
|
TABLE 33.2-2 FUNCTIONS OF GROWTH FACTORS
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
TABLE 33.2-3 INDICATORS OF MESENCHYMAL CELL DIFFERENTIATION BY BONE MORPHOGENETIC PROTEINS
|
||||||||
|---|---|---|---|---|---|---|---|---|
|
from one part of an individual and transplanted to another anatomic
site in the same individual. It is the most successful bone graft or
the gold standard for grafting material in patients undergoing spinal
fusion. Autogenous bone graft has osteogenic properties (numerous
differentiated and undetermined stromal cells within the cavity
lining), osteoinductive properties (noncollagenous bone matrix
proteins, including growth factors), and osteoconductive properties
(hydroxyapatite and collagen). Other advantages of autogenous grafts
include the following:
-
They are histocompatible.
-
They are completely osteointegrative
-
They do not pose the risk of donor-associated disease transmission or immune rejection.
clinical situations. These disadvantages include insufficient amount of
graft material available for use, especially in multisegmental fusion,
in revision surgery in which prior bone harvests have been undertaken,
in children (who have limited donor sites), and when treating large
osseous defects. Significant donor site morbidity has been reported in
25% to 40% of patients resulting in unsatisfactory outcome for spinal
fusion (Table 33.2-4). Harvest of a posterior
iliac crest bone graft is associated with a significantly lower risk of
postoperative complications compared with the anterior iliac crest.
Also the use of a separate incision to procure the bone graft may be
associated with some complications.
the posterior iliac crest because it provides a large quantity of
cancellous and corticocancellous bone. In general, one posterior iliac
crest provides enough bone for a two-level intertransverse fusion or a
three-level fusion if there is local bone available from spinous
processes. The anterior ilium, fibula, and rarely proximal tibia also
are used in decreasing order. Many techniques may be used to obtain
iliac bone (Table 33.2-5 and Figs. 33.2-1, 33.2-2, 33.2-3 and 33.2-4),
and, depending on the application for which it is used, the shape and
substance of the bone graft can differ. Strut grafts used for anterior
interbody fusion must have some capacity to bear the mechanical
compressive loads applied to the intervertebral location. These grafts
require cortical integrity and can be fashioned as tricortical blocks
(cortices include the inner and outer iliac tables and the iliac crest)
or as bicortical blocks or dowels (cortices include the inner and outer
iliac tables only). Autografts used in nonloaded or tensile
environments, such as the posterior and posterolateral (intertransverse
process) spine, do not require cortical integrity. These grafts can be
prepared as corticocancellous strips, morcellized fragments, or even
particulate corticocancellous or cancellous-only bone.
|
TABLE 33.2-4 DONOR SITE MORBIDITY IN AUTOGRAFT HARVEST
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
|
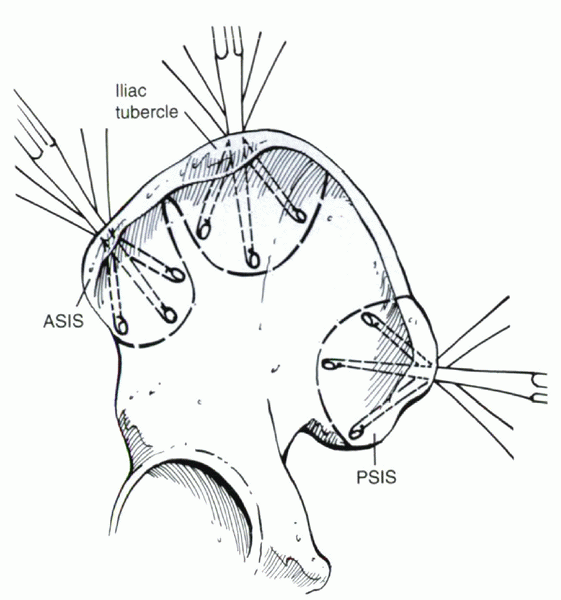
Figure 33.2-1
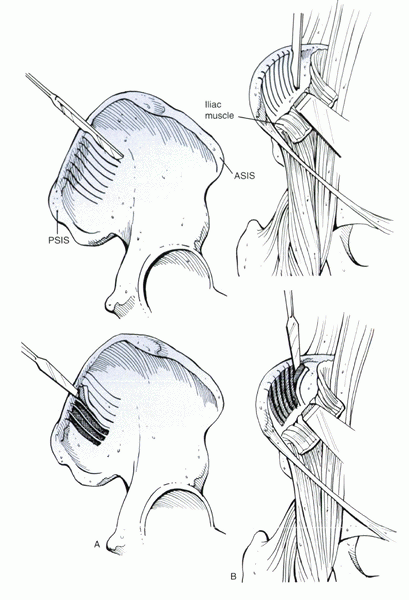
Curettage technique for harvesting of cancellous bone grafts. ASIS, anterior superior iliac spine; PSIS, posterior superior iliac spine. |
|
TABLE 33.2-5 TYPES OF AUTOGRAFT AND HARVEST TECHNIQUES
|
|||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||
 |
|
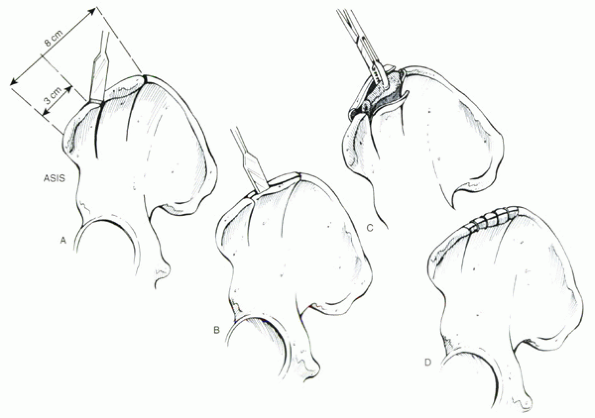
Figure 33.2-2 Wolf technique for harvesting of cancellous bone grafts. (A) Two coronal cuts are made through the ilium. (B) Two oblique cuts are made, starting at the middle of the iliac crest. (C) Harvesting of the cancellous bone. (D) The inner and outer cortices of the iliac crest are fixed together with wires or sutures. ASIS, anterior superior iliac spine.
|
 |
|
Figure 33.2-3 Techniques for harvesting of corticocancellous bone grafts from the outer table of the posterior ilium (A) and from the inner table of the anterior ilium (B). ASIS, anterior superior iliac spine; PSIS, posterior superior iliac spine.
|
contain a greater proportion of osteoconductive, osteoinductive, and
osteogenic properties compared with the more mechanically supportive
cortical bone. Cancellous autograft initially has little structural
integrity when placed on the fusion bed, until vascularization and
interconnection of the graft fragments occur. Some osteoblasts and
osteocytes of the graft survive and are capable of producing early
bone. The porous nature of cancellous bone permits more rapid ingrowth
of new blood vessels, which allow for the influx of osteoblast
precursors. Bone formation and resorption usually occur concomitantly,
with osteoblasts depositing bone on the surfaces of the preexisting
trabeculae, whereas osteoclasts gradually resorb the dead trabeculae
(creeping substitution). Eventually, all grafted cancellous tissue is
resorbed and replaced by host
bone
and marrow. As the spine is subjected to stress, it begins to remodel
and form a mature fusion mass. This process typically is complete
within 10 weeks in the rabbit model and within 6 to 12 months in humans.
 |
|
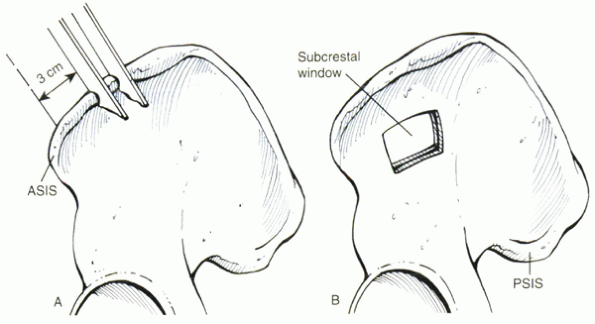
Figure 33.2-4 (A) Harvesting of a tricortical bone graft. (B) Subcrestal-window technique. ASIS, anterior superior iliac spine; PSIS, posterior superior iliac spine.
|
commonly are used in situations in which structural support is needed
early. Structurally, they are dense, more compact than cancellous bone,
and resistant to vascular ingrowth and remodeling. This structure slows
the incorporation of the graft into the host spine. Cortical bone has
less osteogenic potential, with fewer than 5% of cortical bone cells
surviving transplantation. The blood vessels and cells of the host
invade the cortical bone graft through preexisting haversian canal
systems. At the peripheral margin of the cortical graft, intense
osteoclastic tunneling and resorption occur to remove nonviable bone.
Bone formation occurs only after resorption of dead lamellar bone. The
graft ultimately loses about a third of its initial strength before
consolidation begins. This resorptive phase can last for many months or
years. Initially the cortical bone graft becomes incorporated in the
spine only at its two vertebral body-graft interfaces. Cortical grafts
almost never are remodeled completely and contain a combination of
nonviable and living bone.
does not have the same robust biologic activity as cancellous bone,
although it may be helpful in extending the volume of graft material.
is the most common graft material used for the fusion of spinal
segments. The cancellous component of autograft contains greater
osteogenic potential because of the large number of surviving cells in
marrow, a trabecular environment favoring vascular ingrowth, and the
accessibility of osteoinductive proteins. The cortical component
contains greater mechanical strength and is useful for structural
support.
of autogenous fibula, ribs, or iliac crest are preferred in situations
in which avascular graft healing is poor, such as in areas of
radiation-induced fibrosis or when radiation or chemotherapy or both
are to be given preoperatively. The vascularized graft remains viable
through its arterial supply and does not undergo significant cell
necrosis. It unites directly with the host site without needing to be
revascularized and replaced by creeping substitution. The graft is a
ready source of osteogenic cells and precursors. Vascularized bone
grafts are superior to nonvascularized bone grafts in terms of their
osteogenic potential, vascularity, less resorption, good mechanical
strength, and early bone union. After the initial 6 months, however, no
difference in biomechanical strength is observed. The disadvantages of
vascularized bone graft include donor site morbidity, increased
surgical time, and a greater use of resources. The usefulness of a
vascularized graft is determined by the extent to which the length of
the soft tissue pedicle allows adaptation to the host site.
Nonvascularized cortical grafts are less favorable, especially when the
bridging defect is greater than 12 cm and in the treatment of
stress-related fractures.
radiographic success of autografted spinal fusions have reported highly
variable results. In addition to the influence of the type of autograft
used, other variables that may affect a successful fusion outcome
include:
-
Location and type of fusion
-
Stringency of fusion outcome criteria
-
Patient selection
-
Underlying pathology
-
Use and type of internal fixation
-
Preparation of the fusion bed
-
Method of retrieval and preparation of the bone graft material
the comparison of published data on spinal fusion outcomes.
have a significant influence on the healing potential. The anterior or
middle column of the spine is primarily cancellous bone with a larger
surface area and experiences compressive mechanical loading. Load
bearing and impaction of the graft early in the fusion process affords
for stability and encourages early integration of the fusion mass. The
posterior column of the spine has a greater combination of cortical
bone, however, and a submuscular healing environment frequently under
tensile stresses. The use of autograft in posterolateral lumbar fusion
has been associated with the highest likelihood of failure
(pseudarthrosis), ranging from 5% to 44%. Although the use of spinal
instumentation has reduced this rate of nonunion in certain reports,
the incidence still remains unacceptably high. Fusion rates using
autograft in the posterior cervical and thoracic location are generally
better compared with the posterolateral lumbar location. Posterior
cervical fusions using iliac crest autograft have been successful in
88% to 100% of patients. Also, anterior cervical plate fixation
combined with tricortical autograft produces fusion rates exceeding
97%. Autogenous tricortical iliac crest wedges and bicortical iliac
dowels (used in anterior lumbar interbody fusion, used in revision
surgery for pseudarthroses secondary to failed posterior fusion, and
used to accompany posterior fusion surgery in patients who are at high
risk for failure) are associated with favorable fusion rates but can
undergo graft collapse; however, the presence of internal fixation
lessens the likelihood of graft subsidence. Discectomy, decortication,
and placement of the interbody graft are accomplished through anterior,
laparoscopic, transperitoneal, or retroperitoneal approaches;
posterior, interlaminar approaches; or far lateral, transforaminal
approaches. The use of threaded interbody cages containing morcellized
autograft has produced good fusion rates. Because of the morbidity
associated with harvest of autogenous bone graft, the use of allograft
and newer bone graft alternatives is becoming increasingly important in
spinal fusion.
one member of a species to another member of the same species or more
commonly from one patient to another. Allograft bone products are the
most common substitutes for autogenous bone grafts. Advantages of
allografts include the following:
-
Availability in virtually unlimited quantities
-
Various formulations
-
Avoidance of donor site morbidity associated with autograft
osteoinductive (if demineralized), and not osteogenic because the cells
do not survive transplantation. For these reasons, concerns exist
regarding the ability of allograft bone to produce a successful spinal
fusion consistently. A decision to use allograft for spinal surgery
depends on the underlying disease condition, the region of spine where
the graft is placed, the surgical goals, the types of graft available,
the state of the host bed, and the preferences of the patient and
surgeon.
spinal surgery, major concerns exist among surgeons and the public
regarding the potential effects of different processing methods on
allograft function and the risk of disease transmission. The principal
pathogens involved are human immunodeficiency virus and hepatitis
viruses B and C. The risk of disease transmission is determined by the
rigor of screening procedures for donors and tissue; the only cases of
disease transmission in musculoskeletal allografts from the method of
graft preparation to date have involved frozen, unprocessed grafts.
After meticulous screening of the sociomedical history of the donor and
thorough laboratory testing, allograft bone is harvested under sterile
conditions, usually within 24 hours of death, and is processed
immediately thereafter.
dried. The grafts are processed and preserved in ways that affect the
osteoinductivity, osteoconductivity, and immunogenicity of the
material. With fresh allografts, no preservation is required; this
elicits an intense immune reaction and rejection, however, and it has a
greater potential for disease transfer. Fresh allografts are not used
in spinal fusion. Most allografts used are either frozen or freeze
dried. Frozen allograft is maintained at a temperature of -70°C and has
a shelf life of 5 years. Deep frozen bone retains its material
properties and can be implanted immediately after thawing.
Freeze-drying significantly reduces the immunogenicity, alters the
material properties of allograft cortical bone, and necessitates
reconstitution (rehydration) of the graft before implantation. The
mechanical strength of freeze-dried implants can be reduced by 50%
compared with frozen grafts. The use of terminal gamma irradiation,
gas, or ethylene oxide sterilization of allograft cortical bone may
affect the biologic properties (osteoinduction) or biomechanical
properties. Cancellous bone seems to be less affected by sterilization.
Heating and autoclaving destroys the matrix proteins and is not
commonly used.
tricortical strips, patellar tricortical strips, cancellous cortical
dowels, fibular struts, femoral cross sections, and ribs. Morcellated
allograft rarely is used alone in spinal applications. When allograft
is implanted, there is a programmed sequence of events at the site of
the graft, including hemorrhage, inflammation, revascularization of the
tissue, and creeping substitution and remodeling of the graft with
locally derived tissue. Cancellous and structural grafts show
significant differences in the histology of incorporation. Cancellous
grafts show more rapid and complete revascularization than structural
grafts. Cancellous bone remodels completely with time, whereas cortical
bone remains a mixture of necrotic and viable bone. The process of
creeping substitution also differs significantly between these forms of
allograft, with new bone formation occurring appositionally followed by
resorption in cancellous bone, whereas the process
is
reversed in cortical allografts. The most crucial factor in allograft
incorporation is the host recipient bed because union occurs at the
allograft-host junction. Other factors affecting allograft
incorporation include the immune response and graft host stability.
Allograft incorporation often is limited by fractures of the graft,
infection, and nonunion. Structural allograft bone lacks the ability to
remodel and depends on internal fixation devices for clinical function.
autograft in anterior interbody and posterior spinal fusions in a dog
model showed a slower fusion rate, greater graft resorption, and
increased infection rate in the dogs in which allograft was used alone.
This study has led many to use allograft as a graft extender rather
than a graft substitute for autogenous bone. Although many animal
studies have been done to evaluate allograft use in spinal
applications, few well-controlled, prospectively designed clinical
studies have been done. Allografts are used most successfully as
structural grafts for anterior interbody fusions (cervical,
anteroposterior lumbar). Morcellized allografts have not produced the
same fusion rates for posterior laminar and transverse process fusion
procedures as have structural allografts for the interbody
applications. The larger surface area and the compressive forces in the
intervertebral location may be the reason that allografts, being less
osteoinductive, are more commonly successful for anterior fusions than
for posterior fusions.
for posterior lumbar fusion in adults has produced mixed results. An et
al examined a prospective series of patients undergoing posterolateral
lumbar fusion who were implanted with autograft alone, a mixture of
autograft and freeze-dried allograft, fresh-frozen allograft alone, and
freeze-dried allograft alone. These investigators observed that the
sites implanted with autograft alone had the highest fusion rates,
whereas the sites implanted with freeze-dried allograft alone had the
lowest rates. Of grafts, 50% of the fresh-frozen grafts and 100% of the
freeze-dried grafts had undergone complete resorption. In the anterior
lumbar spine, cortical allografts (femoral rings) commonly are used for
structural support in combination with autogenous bone graft, with
pseudarthrosis being rare. When used in instrumented thoracic spinal
fusions, the results are favorable.
spinal fusion have been reported for interbody fusion in the cervical
spine. Similar fusion rates to autogenous graft are presented for
one-level fusion, but the union rate drastically decreases in
multilevel fusion procedures. In 1976, Brown et al compared the use of
frozen allograft with autograft and found that 32 patients treated with
frozen allograft for single-level arthrodesis fused equally as well
with an equal frequency of graft collapse as 29 patients implanted with
autograft. They noticed a higher rate of graft collapse in multilevel
fusions implanted with allograft. Zdeblick and Ducker observed a
comparative fusion rate for freeze-dried tricortical allograft and
autograft for Smith-Robinson-type cervical fusions in 87 patients
undergoing single-level fusion. In patients undergoing two-level
fusions, the rate decreased dramatically for allograft compared with
autograft, and there was a higher incidence of graft subsidence.
(morcellized) allografts seems to be in adolescent patients undergoing
scoliosis correction and fusion. Allograft is successful in these
patients for several reasons, as follows:
-
Adolescent patients heal bone more easily.
-
The posterior thoracic spine is
mechanically stable, especially with fixation, and has large bone
surface area for decortication that is a good source of blood supply
and cells.
allograft bone dowels and allograft interbody cages harvested from
midshaft of diaphyseal bone are gaining increasing popularity for
anterior lumbar fusion because of their dual roles of bone graft
material and fixation device. They allow for disc space distraction,
placing the anulus under tension. The stretched anulus is desirable
because it functions as a circumferential tension band, allowing
impaction of the graft material. The threaded design allows fixation
and prevents graft migration, and the hollow space (medullary canal) in
the center of the graft permits inclusion of morcellized particles of
autogenous bone from the iliac crest. Clinical outcome data using these
allografts are limited. Femoral ring structural allografts have been
used successfully in anterior lumbar interbody fusion. They maintain
disc height and help correct deformity when combined with posterior
instrumentation. Kozak et al reported a series of 45 patients with
femoral ring allografts for anterior lumbar fusion with a 97% fusion
rate based on flexion/extension films at 6- to 12-month follow-up.
Aurori et al reported on 208 patients who underwent posterior spinal
fusion for scoliosis with Harrington rod instrumentation. In this
study, 114 patients were treated with iliac crest autograft, and 94
patients were treated with allograft. The investigators reported
pseudarthrosis rates of 4.4% and 5.3%. The difference was not
statistically different, but the amount of intraoperative blood loss
and operating room time was increased significantly in patients who
received autograft.
produced by the acid decalcification of cortical bone. The
osteoinductive capacity of DBM, initially shown by Urist, now has been
well established. Clinically, DBM has been used with good results to
augment autogenous bone grafts for fracture healing and tibial and
femoral nonunions. The components of the bone matrix that remain behind
after demineralization include:
-
Noncollagenous proteins
-
Bone osteoinductive growth factors, the most significant of which is bone morphogenetic protein
-
Type I collagen
of all bone proteins and are abundant in diaphyseal cortical bone. The
demineralization of bone allows these
osteoinductive growth factors contained within the matrix to become locally accessible.
should be in a structurally stable environment. Although DBM primarily
functions as an osteoinductive agent, the osteoconductivity also is
important, and this can vary depending on its final configuration. The
absolute amount of osteoinductive growth factors in DBM is extremely
low. The source and processing of DBM have a direct effect on its
osteoinductive capacity. Storage of bone at room temperature for more
than 24 hours before processing, sterilization by ethylene oxide under
certain conditions, and 2.5 mrad of gamma irradiation all substantially
reduce osteoinductive and osteoconductive capacity of DBM.
aggregation, hematoma formation, and inflammation within 18 hours.
Thereafter, fibroblast-like mesenchymal cells are attracted to and
establish close contact with the implanted matrix. Interactions between
DBM and mesenchymal cells result in cellular differentiation into
chondrocytes around day 5 after implantation. Chondrocytes produce
cartilage matrix, which is mineralized. By days 10 to 12, vascular
invasion accompanied by osteoblastic cells is observed, multinuclear
cells appear, and chondrocytes begin to degenerate. New bone is formed
apposed to the surface of the mineralized cartilage. Remodeling and
replacement of these composite structures with new host bone ensue.
With time, all the implanted DBM is resorbed and replaced with host
bone suitable for the environment in which it finds itself.
and clinical use, but their osteoinductivity is variable. Grafton DBM
may be used intraoperatively to augment internal fixation or as an
adjunct to other graft substitutes. The osteoinductive nature of
Grafton has been shown in standard and more challenging animal models,
and preclinical studies have shown positive performance when used alone
and as an extender of autograft. Other bone-processing facilities also
now are providing DBM composites using alternate carrier preparations.
No clinical data are available for either of these materials.
posterolateral spinal fusion have shown that certain formulations of
DBM can function as viable bone graft alternatives (enhancer or
extender). Experimental studies using DBM alone or in combination with
autogenous bone marrow, autograft, or graft substitutes have reported
spinal fusion rates comparable to autograft alone in rats, rabbits, and
dogs. It also has been shown that DBM composites produce more rapid
spinal fusion and stiffer fusion masses than autograft alone. Lindholm et al,
in a rabbit model of posterior thoracic spinous process fusion, showed
that DBM combined with bone marrow cells showed more rapid bone
formation than DBM alone. The rates were identical in both test groups
(86%) after 20 weeks. Morone and Boden, using a previously validated
rabbit model of lumbar posterolateral intertransverse process
arthrodesis, showed the efficacy of DBM gel (Osteotech, Eatontown, NJ)
as an autograft extender. DBM gel did not increase the fusion rate when
added to a standard amount of autograft. The addition of DBM gel to
less than the standard amount of autograft (3:1 ratio) resulted,
however, in fusion rates (70% and 60%) comparable to autograft alone.
Other formulations of DBM have produced successful spinal fusions when
used as stand-alone substitutes. Martin et al
studied two new fiber-based formulations of Grafton DBM (Matrix DBM and
putty DBM) in a rabbit posterolateral spinal fusion. These newer
fiber-containing formulations showed better handling characteristics
compared with DBM gel. When used as stand-alone graft substitutes, the
putty and Matrix forms of grafton DBM also produced better fusion rates
(83% and 100%) compared with the gel form (58%) and autograft (73%).
They concluded that these two new formulations could function as graft
extenders, graft enhancers, and potentially osteoconductive graft
substitutes. The lower rate of fusion with DBM alone compared with
autograft was even more pronounced when DBM was evaluated in the highly
challenging nonhuman primate model of posterolateral lumbar
intertransverse fusion. Grafton Matrix has exhibited the ability to
improve healing when delivered with autograft bone in rhesus monkey
posterolateral lumbar spinal fusions.
been used in comparison with and in combination with DBM in animal
models of spinal fusion with varying results. Boden et al
showed that DBM alone and biocoral alone produced lower fusion rates
compared with autograft in a rabbit posterolateral fusion model. The
addition of bovine-derived bone growth factor extract to DBM, to
autograft, and to natural coral resulted in 100% fusion, however, with
increase in fusion stiffness. Also, Ragni et al
showed that porous hydroxyapatite blocks alone or in combination with
DBM had similar radiographic fusion scores to autograft alone or
autograft with DBM and bone marrow at 2 months. The variable efficacy
of DBM in animal spine studies probably stems from a combination of
problems with the models used and variability in the preparation of the
DBM. The combination of growth factors with suitable osteoinductive and
osteoconductive carriers, such as DBM, seems to be an especially potent
promoter of spinal fusion in lower and higher order animals. Although
the results in animal studies are encouraging, care must be taken when
extrapolating results from small animal models to humans because of the
increased difficulty of initiating osteoinduction in primates.
have been performed, prospective clinical outcome data related to the
use of DBM are lacking. There are a few retrospective clinical studies
in which reported results show potential benefit with DBM (Grafton) in
posterolateral lumbar fusions. Sassard et al retrospectively reviewed
patients who underwent instrumented posterolateral lumbar spinal fusion
with local bone graft and Grafton gel and compared them with an
age-matched, gender-matched, and procedure-matched group of patients
undergoing instrumented fusions with autograft. Using a bone
mineralization rating scale, they did not find radiographic differences
between
the
groups based on films taken 3, 6, 12, and 24 months after surgery. The
fusion rates in the autograft with Grafton group and the autograft-only
group were only 60% and 56% less than has been reported in other
studies of instrumented posterior fusion. Based largely on preclinical
data, it is speculated that these processed DBM products may be
efficacious as bone graft extenders but not as bone graft substitutes
for posterior spinal fusion procedures.
HS, Simpson JM, Glover JM, Stephany J. Comparison between allograft
plus demineralized bone matrix versus autograft in anterior cervical
fusion: a prospective multicenter study. Spine 1995;20: 2211-2216.
SD, Schimandle JH, Hutton WC. 1995 Volvo Award in Basic Sciences. The
use of an osteoinductive growth factor for lumbar spinal fusion: Part
II. study of dose, carrier, and species. Spine 1995;20:2633-2644.
TS, Urist MR. A quantitative analysis of new bone formation by
induction in compositive grafts of bone marrow and bone matrix. Clin
Orthop 1980;288-300.
G, Boden SD, Morone MA, Titus L. New formulations of demineralized bone
matrix as a more effective graft alternative in experimental
posterolateral lumbar spine arthrodesis. Spine 1999;24: 637-645.
P, Lindholm S. Interaction of allogeneic demineralized bone matrix and
porous hydroxyapatite bioceramics in lumbar interbody fusion in
rabbits. Clin Orthop 1991;272:292-299.
cells with osteogenic potential being transferred directly to the site
requiring augmentation. Cell-based approaches do not depend on the host
local osteoprogenitors; they are particularly attractive for patients
in whom the host tissue bed has been compromised by irradiation,
chemotherapy, severe trauma, tobacco use, osteoporosis, and metabolic
derangements. So far, four different cell types have been used for bone
regeneration, as follows:
-
Unfractionated bone marrow
-
Purified, cultured expanded mesenchymal stem cells (MSCs)
-
Differentiated osteoblasts and chondrocytes
-
Genetically modified cells that express bone morphogenetic protein (BMP)
or osteoinductive composites has received appreciable attention in its
application in the augmentation of spinal fusion.
used clinically as an adjunct to some graft materials for spinal
fusion. The original observations of Goujon led to the initial interest
in the osteogenic capabilities of bone marrow. The ability of bone
marrow graft to perform its function as a graft depends on the presence
of MSCs. The number of the stem cells in the marrow is limited. Marrow
contains stem cells on the order of 1 per 50,000 nucleated cells in
young individuals and 1 per 2 million in the elderly. Stem cell
concentration techniques, including centrifugation and ex vivo cell
culture, can increase their number fivefold.
of the ilium in aliquots of 2 mL to reach total volumes of 5 to 10 mL
and injected directly into the fusion site. Transplanted bone marrow
tends to diffuse away from fusion site when used alone for
augmentation. To prevent this diffusion, advances have been made in the
delivery of the marrow. The marrow may be supplemented with a carrier,
such as allograft, demineralized bone matrix, collagen, or ceramic, to
stimulate bone healing. Bone formed by marrow graft has the same
biomechanical properties as cancellous bone graft. The first clinical
experience with the use of marrow cells in humans to stimulate fracture
repair was reported in a 31-year-old patient with an infected nonunion
of the tibia by Connolly and Shindell in 1986. Preclinical
investigations and a few clinical studies have confirmed the efficacy
of bone marrow as a graft substitute. Bone marrow in spinal fusion
often is used clinically in combination with autograft and allograft
bone or in composites of ceramic or other bone extenders. The use of
bone marrow as a stand-alone material in spinal fusion or with ceramics
has produced variable results. Also, Boden et al,
in a posterolateral fusion model, observed no fusions when using bone
marrow as a stand-alone graft substitute or in combination with
coralline hydroxyapatite.
-
Added morbidity of bone marrow harvest
-
Difficulty in obtaining enough bone marrow with the requisite number of osteoprogenitor cells
-
Aging or disease that is accompanied by a
reduction in healthy bone marrow cells, especially the osteogenic
precursors, which represent approximately 0.001% of the nucleated cells
in healthy adult marrow
replication and can differentiate into several tissue types, including
bone, cartilage, tendon, muscle, fat, and marrow stroma. Several
investigators have described techniques for the isolation of adult
human and animal MSCs from bone marrow and periosteum. Isolation of
MSCs generally is done through density gradient centrifugation and cell
culturing techniques. Using culture systems, MSCs from a small marrow
aspirate can be expanded in number more than 1 billion-fold. This
remarkable expansion makes MSCs a clinically useful source of
osteoprogenitor cells for fusion procedures. Cui et al examined the
effects of a cloned osteoprogenitor cell, D1-BAG, which was cloned from
Balb/c mouse bone marrow stroma and transduced with a traceable gene
encoding β-galactosidase, and mixed marrow stromal cells from marrow
blowouts in posterior spinal fusion in athymic rats. The cloned cells
showed an earlier osteogenic process with a larger amount of bone
formation than mixed stromal cells. Successful spinal fusion at 6 and 9
weeks was observed in eight of eight (100%) animals receiving DI-BAG
cells, four of eight (50%) in mixed marrow stromal cells, and none of
eight (0%) in control animals. The investigators also noted that
osteogenesis with DI-BAG cells occurred without a cartilaginous phase,
in contrast to the process of endochondral ossification that was seen
with mixed marrow cells. They concluded that cloned osteoprogenitor
cells may serve as a substitute for bone autograft.
preparations that mimic the mineral phase of bone. Biosynthetic
ceramics have been used solely as osteoconductive bone graft
substitutes. The calcium phosphates, particularly hydroxyapatite (HA)
and tricalcium phosphate (TCP), or a combination of the two, are the
most commonly used ceramics in orthopaedic surgery. As osteoconductive
materials, HA and TCP tend to function best as bone graft extenders or
carriers for an osteoinductive bone growth factor rather than as
stand-alone bone graft substitutes in nonstructural clinical
applications.
-
They are biodegradable.
-
They are biocompatible.
-
They have little or no risk for disease transmission.
-
They are available in unlimited quantities.
-
They have no added risk of donor site complications that accompany the use of autograft.
biodegradability of the ceramic. The various calcium phosphate
composites differ with regard to their bioresorbability properties. A
nonresorbable graft material may hinder remodeling, prolong the
strength deficiency of new bone, and leave permanent stress risers in
the fusion mass.
-
They are brittle and have little tensile strength and must be shielded until bone ingrowth has occurred.
-
Persistent dense radiographic imagery makes it difficult to evaluate bone incorporation in the clinical setting.
-
The unnatural pathways that are
characteristic of intact ceramic matrices do not favor the normal
process of bone ingrowth and remodeling that occurs after bone
transplants.
nonporous dense implants, or granular particles with pores. The optimal
osteoconductive pore size for ceramics seems to be between 150 and 500
µm. The chemical composition, porosity, and surface area of the ceramic
affects its rate of bioresorption. The larger the surface area, the
greater the resorption; also a greater porosity enhances interface
activity and bone ingrowth. The material density of the matrix and
porosity of the ceramic could result in greater mechanical strength and
resistance to degradation and promote long-lasting stability. TCP
undergoes biologic resorption 10 to 20 times faster than HA. Within the
body, TCP is converted partially to HA, which is degraded more slowly
because the foreign body giant cell that specifically resorbs HA stops
after resorbing 2 to 10 µm of HA. Large amounts of HA may remain in the
body for more than 10 years.
interconnective porosity, is composed of 97% calcium carbonate in the
form of aragonite, and is structurally similar to cancellous bone.
Coral is extremely biocompatible. It has yielded promising results when
used to replace or augment autogenous bone graft or as part of a
composite with an osteoinductive protein. An alternative formulation is
coralline HA, which converts much of the calcium carbonate to HA.
Calcium sulfate (plaster of Paris) also has been used as a synthetic
graft material in bone voids, although with limited documented success
in posterolateral spine fusion.
fibrovascular tissue begins to invade the porosity. Typically a blood
clot initially forms in the porosity. The blood clot must resolve to
allow regenerating tissues to proliferate. This process takes 3 weeks
for most implants with clinically relevant sizes, averaging about 2 to
3 mm/wk. Macrophages may play a significant role in this early stage of
fibrovascular ingrowth; however, inflammatory cells are rare or only
transiently evident. Ceramic implants are osteoconductive when they are
placed next to bone. Bone grows into the implants only if the implant
is in direct apposition to bone, the tissue of the host bed is
conducive to bone formation, and the interface between bone and implant
are stable. Bone formation
within
the implant initially occurs directly against the surface of the
implant. Rarely are chondroblasts seen within the porosity. This
process is more akin to membranous bone formation than to osteochondral
bone formation. After osseous ingrowth, the mechanical properties of
coralline implants are improved significantly as a result of the
overlay of host bone.
regarding the ability of these synthetic biomaterials alone and in
conjunction with demineralized bone matrix, extracted osteoinductive
growth factors, and osteogenic bone marrow to heal osseous defects and
spinal fusions Anterior interbody fusion in the thoracic spine of dogs
was analyzed by Emery et al using tricortical iliac crest autograft, HA
ceramic, calcium carbonate, and a composite of HA and TCP (60%/40%).
All fusions were performed using spinal instrumentation. Autograft was
the most effective graft material in this study, despite the use of
internal fixation with calcium carbonate ceramic. While comparing the
efficacy of 50/50 HA/TCP ceramic composites of varying porosity (30%,
50%, and 70% porosity) and autograft in a goat anterior cervical spine
fusion model, Toth et al showed that the ceramic implants performed
equal to or better than autograft iliac crest bone. The more porous
implants had a higher union rate early on, but also had a higher
incidence of graft fracture. Overall fusion rates were 67% for the
ceramic implants and 50% for autograft. The goats used in this study
had excessive head movement after surgery, and these low fusion rates
put into question the ability of this model to be extrapolated to human
anterior cervical fusions.
carbonate ceramic derived from coral was used in combination with
platelet-rich plasma concentrate (growth factor gel) in a spinal fusion
model in sheep. The results indicated increased osteoblastic activity
deep in the graft. In a previously validated rabbit model, Boden et al
evaluated the efficacy of coralline HA as a bone graft substitute for
lumbar spine fusion when used with bone marrow, autogenous bone graft,
or an osteoinductive bone protein extract. They observed that coralline
HA with bone marrow could not function as a stand-alone graft
substitute. When combined in a 1:1 ratio with autogenous iliac crest
bone graft, coralline HA functioned as a graft extender. Also,
coralline HA served as an excellent carrier for a bovine-derived
osteoinductive growth factor extract with its bovine collagen composite
functioning as a complete bone graft substitute in the posterolateral
spine. These results were substantiated further by Baramki et al
in a sheep lumbar spinal arthrodesis model. Other authors have found
similar results in fusion rate when comparing different ceramics in
spinal fusion.
rhBMP-2 in the posterolateral spinal fusion model in rhesus monkeys.
Even in the presence of a laminectomy defect, there was no evidence of
bone induction outside the confines of the ceramic carrier. The ability
of different ceramic composites to induce spinal arthrodesis has been
compared with that of autograft alone in a rabbit interbody fusion, in
a sheep posterior spinal fusion, and in a dog posterior spinal fusion,
with varying results. Bozic et al showed a dose-dependent electrical
stimulation enhancement of posterolateral spinal fusion in rabbits
using HA/bone marrow aspirate.
benefits of ceramics in spinal fusions for patients with scoliosis.
Passuti et al advocated the use of ceramics as extenders for autogenous
bone graft for long segment fusions in corrective deformity surgery.
They used blocks of HA/TCP with or without autogenous bone graft for
facet joint fusions in 12 adolescent patients with severe scoliosis who
underwent internal fixation and fusion. All patients were followed
clinically and radiographically for an average of 15 months
postoperatively. Passuti’s series achieved 100% fusion, with biopsy
specimens from two patients showing histologic bony ingrowth into the
pores of the ceramic. Delecrin et al, in a prospective randomized
study, assessed the clinical and radiologic efficacy of a synthetic
ceramic in scoliosis surgery. Fifty-eight patients with idiopathic
scoliosis underwent posterior arthrodesis using autograft bone alone or
in combination with porous biphasic HA/TCP composite. Radiographic
incorporation of ceramic was evident in 12 months.
materials in spinal fusion exists for anterior interbody fusion of the
cervical spine. Successful fusion rates in the anterior and posterior
cervical spine approach 100% in most series reported. The ultimate role
of ceramic implants in spinal fusion procedures remains to be defined.
Calcium phosphate biomaterials with appropriate three-dimensional
geometry are able to bind and concentrate endogenous BMPs in
circulation, may become osteoinductive (capable of osteogenesis), and
can be effective carriers of bone cell seeds.
features of osteogenesis, osteoinduction, and osteoconduction as
autogenous bone graft can. Composite grafts incorporate all the
favorable properties of the various materials and have been used with
success clinically in spinal fusion procedures. Ceramic composites
consist of osteoconductive ceramic combined with an osteoinductive
agent, such as demineralized bone matrix, autograft bone, extracted
bone matrix proteins, or rhBMP-2. The ceramic implant maintains soft
tissue position and provides an osteoconductive matrix, and the
proteins stimulate osteoinduction. Composite grafts offer potential for
the design of bone graft substitutes that are specific for the
structural and biologic demands of the host, and it is likely that
different composites would be used for anterior interbody arthrodesis
than for long-instrumented posterior fusion.
Palo Alto, CA) is a commercially synthesized composite of suspended,
deantigenated bovine fibrillar collagen and porous calcium phosphate
ceramic (65% HA and 35% TCP). The composite is not osteoinductive;
however, the addition of autogenous bone marrow provides
osteoprogenitor
stem
cells and a limited amount of growth factors. Collargraft currently is
available in paste form or as soft strips. Collargraft can deliver
antibiotics and antineoplastic agents locally to treat bone disorders.
It lacks structural integrity, however, and as a result tends to
migrate to ectopic sites if adequate hemostasis is not maintained.
Cornell et al compared Collargraft plus autogenous marrow versus
cancellous iliac bone grafts in acute long bone fractures and found no
significant functional or radiographic differences. Animal studies have
documented various healing properties of composite grafts.
showed that the use of Collargraft composite as a bone graft substitute
or expander for autologous bone graft in a posterolateral spinal fusion
model in sheep produced robust fusion masses, with greater mineral
densities compared with the use of autogenous bone graft alone. Both
study groups had similar mechanical properties, however. The use of
Collargraft or Collargraft prototypes in previous preclinical spinal
fusion studies resulted in mixed outcomes.
ceramic combined with an osteoinductive agent such as demineralized
bone matrix, bone marrow, extracted bone matrix proteins, or osteogenic
growth factors such as recombinant BMP, have been investigated. Boden et al
used a nonhuman primate lumbar intertransverse process arthrodesis
model to evaluate rhBMP-2 in an HA/TCP carrier as a composite bone
graft substitute. Twenty-one adult rhesus monkeys underwent a
laminectomy and fusion with either autogenous iliac crest bone or 60/40
HA/TCP blocks saturated with a solution containing 0, 6, 9, or 12 mg of
rhBMP-2. Fusion was not achieved in any of the monkeys treated with
autogenous iliac crest bone graft. The monkeys treated with the HA/TCP
blocks with rhBMP-2 achieved complete fusion. When the ceramic blocks
were loaded with rhBMP-2, there was a dose-dependent increase in the
amount and quality of bone throughout the ceramic carrier based on
qualitative assessment. The HA/TCP composite proved to be a suitable
carrier for rhBMP-2 in this posterolateral spinal fusion model in
rhesus monkeys. Even in the presence of a laminectomy defect, there was
no evidence of bone induction outside the confines of the ceramic
carrier.
various types of carrier media and the effect of rhBMP-2 as an adjunct
to autogenous iliac crest bone graft in a dog spinal fusion model. All
fusion sites were assigned randomly to one of six fusion methods:
autogenous bone graft (ABG) alone, ABG + rhBMP-2, ABG + collagen
(Helistat) “sandwich” + rhBMP-2, ABG + collagen (Helistat) morsels +
rhBMP-2, ABG + polylactic/glycolic acid sponge sandwich + rhBMP-2, and
ABG + open-pore polylactic acid morsels + rhBMP-2. The results
indicated that the addition of rhBMP-2 significantly increased bone
graft volume on computed tomography scan, but no significant difference
in carrier media for rhBMP-2 could be determined. Polylactic/glycolic
acid sites were associated with a greater incidence of voids within the
fusion mass. Muschler et al studied the
fusions in a dog posterior spinal fusion model using autograft,
collagen/ceramic composite, collagen/ceramic/autograft composite, and
no graft material. Autograft bone alone was the most effective graft
material tested and had a statistically superior union score. Ceramic
composites when used alone produced fusions equivalent to when no graft
materials were used. The addition of demineralized bone protein extract
to the composite significantly improved the union score, however, which
was comparable to that obtained using composite plus autograft bone.
HA alone or with bone marrow, autogenous bone graft, or 500 µg of
bovine-derived osteoinductive bone protein extract for single-level
posterolateral lumbar spinal fusions in a rabbit fusion model.
Coralline HA alone or with bone marrow produced no solid fusions. When
combined with an equal amount of autogenous iliac crest bone, fusion
appeared in 50%. When combined with the osteoinductive growth factor
extract, the coralline HA resulted in stronger and stiffer solid fusion
in 100%. These data indicated that coralline HA with bone marrow was
not an acceptable bone graft substitute for posterolateral spinal
fusion in this model. When combined with autogenous iliac crest bone
graft, coralline HA served as a graft extender, yielding results
comparable to those obtained with autograft alone. Coralline HA served
as an excellent carrier for the bovine osteoinductive bone protein
extract, yielding superior results to those obtained with autograft or
bone marrow.
HG, Steffen T, Lander P, et al. The efficacy of interconnected porous
hydroxyapatite in achieving posterolateral lumbar fusion in sheep.
Spine 2000;25:1053-1060.
J, Takahashi S, Gouin F, Passuti N. A synthetic porous ceramic as a
bone graft substitute in the surgical management of scoliosis: a
prospective, randomized study. Spine 2000;25: 563-569.
SE, Fuller DA, Stevenson S. Ceramic anterior spinal fusion: biologic
and biomechanical comparison in a canine model. Spine 1996;22:2713-2719.
JS, James SB, Chabot MC, et al. Augmentation of autograft using rhBMP-2
and different carrier media in the canine spinal fusion model. J Spinal
Disord 1997 Dec;10(6):467-472.
KL, Ladwig DA, Skrade DA, Flatley TJ. Evaluation of collagen/ceramic
bone graft substitutes in dogs with spinal fusion. Trans Soc Biomater
1990;13:196.
GF, Negami S, Hyodo A, et al. Evaluation of collagen ceramic composite
graft materials in a spinal fusion model. Clin Orthop 1996;250-260.
WR, Loefler A, Arm DM. Growth factor gel and a resorbable porous
ceramic for use in spinal fusion. Trans Orthop Res Soc 1999;24:270.
local autocrine and systemic regulatory factors. These factors trigger
undifferentiated mesenchymal cells to migrate, proliferate, and
differentiate into bone-forming cells. The transplanted autogenous bone
matrix and the hematoma formed after decortication of the spine
provides a pool of these growth factors that mediate osteoinduction.
The transforming growth factor (TGF)-β superfamily of polypeptide
growth (including TGF-β1 through TGF-β5, bone morphogenetic proteins
[BMPs] 2 through 9, and growth and differentiation factors [GDFs])
constitute the most important growth factors implicated in fracture
healing. Other growth factors present in the callus during the fracture
healing process include fibroblast growth factor, platelet-derived
growth factor, and insulin-like growth factor (IGF). TGF-β and other
growth factors are released by platelets and osteoprogenitor cells.
These proteins are thought to stimulate cellular proliferation and
differentiation of osteoblasts and to direct bone matrix formation.
effects with BMPs; however, it is unable to initiate the entire
osteoinduction cascade by itself and form ectopic bone, a property
uniquely exhibited by some of the BMPs. The BMPs have a myriad of
functions ranging from extracellular and skeletal organogenesis to bone
regeneration. Fibroblast growth factors are mitogenic and angiogenic
factors that are important in neovascularization and wound healing.
Platelet-derived growth factors function as local tissue growth
regulators that initially were isolated from blood platelets,
underscoring one of the important roles of the clot in fracture
healing. IGFs are other examples of matrixsynthesizing growth factors
that are important in bone healing (see Tables 33.2-2 and 33.2-3).
related, low-molecular-weight noncollagenous glycoproteins. Wozney et
al, using molecular cloning, identified the specific molecules, of
which all but one (BMP-1) belong to an expanding TGF-β superfamily of
GDFs. There are three subclasses of BMPs based on amino acid sequences
found in osteoinductive extracts of bone:
-
Subgroup 1 (human BMP-2, BMP-4, and Drosophila [fruit fly] decapentaplegic [dpp])
-
Subgroup 2 (human BMP-5, BMP-6, BMP-7 [osteogenic protein-1])
-
Subgroup 3 (BMP-8, and Drosophila 60A)
single BMPs are available through recombinant gene technology, and
mixtures of BMPs are available as purified bone extracts for basic
science research and clinical trials. Heterodimeric BMP-2/BMP-7 has
been shown to be more potent than homodimers in induction of osteoblast
differentiation.
endochondral and intramembranous bone formation, and they are thought
to promote the normal healing process after fractures. BMPs are known
to bind to specific receptors on a variety of different cell types,
including mesenchymal stem cells, osteoblasts, and osteoclasts. These
receptors subsequently activate second messenger systems within the
cytoplasm, which affect the expression of BMP response genes in the
nucleus. Within the cell, a set of small signal modulating molecules
called SMADs further modulates the BMP signal (see Table 33.1-6).
These secondary messengers compose a family of small signal transducing
molecules within the intracellular domain that can be either negative
or positive modulators of a BMP signal. Subsequently, BMP receptor
stimulation leads, directly or indirectly, to cellular chemotaxis,
proliferation, and differentiation. With lower concentrations, BMPs
promote the differentiation of mesenchymal stem cells into
chondrocytes, which lay down a cartilaginous matrix. This matrix then
calcifies, is invaded by blood vessels, and remodels into mature bone,
a process termed endochondral bone formation. At higher concentrations, BMPs can induce direct bone formation, recapitulating normal intramembranous bone formation.
-
Use of extracted and partially purified
mixture of proteins that include BMPs from animal or human cortical
bone, popularized by Urist et al -
Use of gene therapy, which involves delivery of the DNA encoding a growth factor rather than delivery of the protein itself
|
TABLE 33.4-1 PROPERTIES OF AN IDEAL GROWTH FACTOR CARRIER
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
laboratory of Urist and a commercially available extract of bovine BMP
mixture known as NeOsteo (Centerpulse Biologics, Austin, TX) have been
used in the treatment of nonunions and spinal fusion. The bovine
extract has shown successful osteoinduction in ectopic locations in
rats and nonhuman primates. Also, it has been used as a bone graft
substitute for segmental defect repair in dogs and posterolateral
spinal fusion in rabbits and nonhuman primates. The growth factor has
been used with hydroxyapatite, calcium carbonate, and demineralized
bone matrix carriers. Preliminary human clinical trials currently are
under way.
bovine BMP in rabbit and nonhuman primate intertransverse process
fusion models. They found a dose-dependent response in the rabbit
model, which indicated that a concentration threshold must be overcome
before BMP can induce bone formation effectively. In similar
experiments with rhesus monkeys, effective spinal fusion was achieved
with purified BMP in 18 weeks. These experiments highlighted the need
for higher doses of BMP in primates compared with doses needed for
rodents. Also, the healing time required for primates was significantly
longer (18 to 24 weeks). Lovell et al, using polylactic acid polymer as
carrier in a dog posterior intervertebral fusion model, reported on the
improvement of spinal fusion success rate and fusion mass when
partially purified BMP was used. The fusion rate was 71% in levels with
BMP compared with 17% in control levels.
Medtronic Sofamor Danek, Memphis, TN) has been used extensively for in
vitro and in vivo safety and efficacy studies. Extensive data show that
this growth factor is a morphogen, not a mitogen, and induces cells to
differentiate and form endochondral bone in ectopic and heterotopic
locations. Several preclinical studies have shown that rhBMP-2 is
successful in repair of segmental long bone defects in rats, dogs, and
sheep and in posterolateral spinal fusion in rabbits, dogs, and
nonhuman primates. There is adequate evidence that rhBMP-2 and rhBMP-7
(Creative Biomolecules, Hopkinton, MA) when used in pharmacologic doses
in various animal models are efficacious and superior to autogenous
grafts in achieving spinal fusion. BMP-4, BMP-6, BMP-9, and, to a
lesser extent BMP-5, also have been shown to induce new bone formation.
Biotech, Hopkinton, MA) in a mongrel dog posterior spinal fusion model,
Cook et al observed that rhBMP-7 could induce stable fusion formation 6
weeks after implantation, and this often led to complete fusions by 12
weeks. This was significantly faster than autograft alone, which did
not fuse until 24 weeks after implantation. Muschler et al
also reported the effectiveness of rhBMP-2 with a biodegradable carrier
in a beagle posterior spinal fusion model. They compared rhBMP-2 with a
PLGA carrier, autograft bone alone, and polylactic glycolic acid (PLGA)
carrier alone. At 12 weeks, they found the union score and
biomechanical strength of the fusion mass was equivalent between
rhBMP-2 + PLGA and autograft bone. Additionally, both were superior to
PLGA alone. Schimandle et al evaluated rhBMP-2 in a rabbit
intertransverse process fusion model. They found that rhBMP-2,
delivered either with a collagen carrier or with autograft bone, was
superior to autograft bone alone in producing spinal fusion. rhBMP-2
with autologous iliac crest bone resulted in 100% fusion compared with
42% fusion with autologous bone alone. rhBMP-2 also produced more
mature bone formation that was biomechanically superior to that formed
with autologous bone alone at 4 to 5 weeks.
features of the fusion mass between rhBMP versus autograft material in
rabbit intertransverse process fusion with computed tomography (CT).
They showed that fusion masses derived from rhBMP-2 had higher volume
and better attachment to the transverse process than with autologous
bone alone. They also showed that the weak point of the fusion mass was
distributed more randomly with rhBMP-2 than at the attachment site to
the transverse process, which happened 11 of 12 times for autograft.
Sandhu et al, in a dog posterior spinal fusion, found that the crucial
element was the dose of BMP, and it was unrelated to whether there was
decortication of the fusion model.
various carrier molecules have been done. Inorganic carriers of BMP
that have shown efficacy in promoting spinal arthrodesis include true
bone ceramic derived from sintered bovine bone and
hydroxyapatite/tricalcium phosphate. Organic carriers include
polylactic acid polymers, collagen and noncollagenous protein carriers,
mineralized or demineralized bone matrix, and autograft. Fischgrund et al
evaluated the use of rhBMP-2 with various types of carrier materials
and the effect of rhBMP-2 as an adjunct to autogenous iliac crest bone
graft in a dog lumbar intertransverse process fusion model. No
significant difference between carriers for rhBMP-2 could be
determined; however, PLGA acid carrier sites were associated with a
greater incidence of voids within the fusion mass. rhBMP-2, when added
to autograft, significantly increased the volume and the maturity of
the resulting fusion mass. Sheehan et al also showed that a composite
of rhBMP-2, autogenous bone, and collagen produced biomechanically
stiffer and larger fusion masses than either autograft or collagen
alone.
beagle posterolateral fusion model. They found that 2300 µg of rhBMP in
an open cell polylactic acid polymer was superior to autograft iliac
crest bone graft in achieving a single-level lumbar intertransverse
process fusion. The same investigators showed in a later study that the
effect of increasing BMP dose produced less dramatic enhancement. In
this experiment, rhBMP-2 was implanted in multiple doses (58, 115, 230,
460, and 920 µg). All specimens with BMP were fused solidly by 3
months. There was no significant difference in biomechanical,
radiographic, or histologic characteristics of the quality of
intertransverse process fusion from the 58-µg to 2300-µg doses, almost
a 40-fold difference. In a similar study on the dose response in a
nonhuman primate model of intertransverse process spinal fusion, Boden et al
observed a dose-dependent increase in the amount and quality of bone
throughout the ceramic carrier based on qualitative assessment. They
used a 60% hydroxyapatite/40% tricalcium phosphate ceramic block with
multiple doses of rhBMP-2 (0, 6, 9, or 12 mg per side). All monkeys
treated with rhBMP-2 achieved fusion.
investigated. In a sheep anterior lumbar interbody fusion model, Sandhu
et al showed 100% intervertebral osseous union using an implanted
cylindrical and threaded titanium interbody fusion device containing
rhBMP2. Only 33% of the control animals implanted with the fusion cage
containing autograft alone achieved fusion. Boden et al
studied two doses in an anterior lumbar interbody fusion in a nonhuman
primate model. They showed that successful fusion could be done using
laparoscopic techniques with titanium-threaded cages and collagen
soaked in rhBMP-2. The bovine-derived absorbable collagen sponges were
soaked in either 750 µg/mL or 1500 µg/mL of rhBMP-2. The fusions were
evaluated with plain radiographs, CT scans, manual palpation, and
histologic analysis. Solid spinal fusion occurred with both doses of
rhBMP-2; however, the higher dose led to a more rapid fusion. This
study was particularly important because higher species, such as
nonhuman primates, historically have lower fusion rates.
collagen sponge with allograft dowel in rhesus macaque anterior lumbar
interbody fusion at the lumbosacral junction (L7-S1). They used one
freeze-dried smooth cortical dowel allograft cylinder filled with
autograft bone (control) or filled with an absorbable collagen sponge
soaked with rhBMP-2. The three monkeys with rhBMP-2 showed radiographic
signs of fusion at 8 weeks. The control animals were slower to show new
bone formation, and two of the three control animals did not have bone
union develop. Zdeblick et al used the alpine goat model for multilevel
anterior cervical discectomy and fusion to compare the use of a
standard titanium intervertebral fusion device (BAK Sulzer Spinetech,
Minneapolis, MN) with autogenous bone graft, autogenous bone graft with
a hydroxyapatite-coated BAK device, and a BAK device filled with
rhBMP-2. Successful arthrodesis was more obvious with rhBMP-2-filled
cages (95%) than with the hydroxyapatite-coated cage (62%) or the
standard cage (48%). Although biomechanical testing did not reveal a
statistically significant difference in stiffness between the groups,
there was a tendency for the spines in the animals that received
rhBMP-2 to be stiffer.
examined the feasibility, efficacy, and safety of using rhBMP-2 to
achieve spinal fusion through two small portals. This method was
performed initially in rabbits, then a rhesus monkey model was
employed. The video-assisted arthrodesis combined with growth factor
technology was a safe, feasible, and effective method of spinal fusion
in the rabbit and rhesus monkey. It was thought that this minimally
invasive procedure would decrease the morbidity of paraspinal muscle
denervation and devascularization seen with open intertransverse
process fusion techniques. The morbidity associated with graft site
harvesting would be eliminated with the use of the osteoinductive bone
graft substitute. Cunningham et al evaluated
rhBMP-7 in anterior thoracoscopic fusions in sheep. Four months after
surgery, they found that the BAK device with rhBMP-7 had the highest
fusion rate and better bone formation than an empty BAK device. These
studies support the prospect of less painful and less morbid spinal
arthrodesis procedures with faster and stronger fusions.
overcome biologic impediments to fusion and facilitate the use of
minimally invasive techniques of spinal fusion. In two studies, rhBMP-2
overcame the inhibitory effect of nicotine and a nonsteroidal
antiinflammatory drug in a rabbit intertransverse process fusion model.
Osteoinductive protein-1 (BMP-7; Stryker Biotech, Hopkinton, MA) was
able to overcome the inhibitory effects of nicotine in a rabbit
posterolateral spinal fusion model and to induce bone fusion reliably
at 5 weeks. This finding suggests that BMPs might
offer a method for overcoming the inhibitory effects of nicotine on spinal fusion.
required for proper skeletal patterning and development in the
vertebrate limb. Spiro et al studied the osteoinductive activity of
recombinant human GDF-5 (rhGDF-5) in combination with a mineralized
collagen osteoconductive bone graft matrix (Healos; Orquest, CA) in a
rabbit posterolateral lumbar fusion model. Healos alone, Healos plus
rhGDF-5, or autograft harvested from the iliac crest were employed.
Healos plus rhGDF-5 was found to form bone that was histologically and
mechanically equivalent to that which was formed in response to
autogenous bone alone. Although there are fewer published animal
studies with rhGDF-5, the early results seem promising.
poly-(D,L-lactide) (PDLLA) carrier system combined with IGF-I and
TGF-β1 in a sheep cervical spine interbody fusion model. When compared
with autograft, IGF-I and TGF-β1 application by a
poly-(D,L)-lactide-coated interbody cage significantly improved
interbody bone matrix formation; however, the growth factors were not
able to increase the incidence of solid bone fusion.
investigations, several pilot human trials have been initiated. Several
preliminary investigations of rhBMP-2 for anterior and posterior spinal
fusion also have begun. It is anticipated that data from these
well-controlled feasibility trials should become available within the
next few years. Boden et al have reported on
the first pilot study examining the osteoinductive capacity of rhBMP-2
for a human spinal fusion application. In a limited randomized
multicenter study involving 14 patients, threaded interbody fusion
cages were filled with either rhBMP-2/collagen sponge or autogenous
iliac crest bone graft and implanted for anterior lumbar interbody
fusion. The rhBMP-2 patients, not requiring iliac crest harvest, had a
shorter hospital stay compared with the autograft control patients (2
days versus 3.3 days). Of the rhBMP-2 patients, 10 of 11 were judged
fused by 3 months after surgery, and all 11 were fused by 6 months. Of
the three control patients, one was deemed a nonunion after 1 year.
Among the rhBMP-2-induced fusions, CT scan reconstructed images
consistently showed new bone growth through and anterior to the cages 6
and 12 months after surgery. Since that study, nearly 350 patients have
been implanted with 99.5% fusion success rate as assessed by CT scans.
compare laparoscopic anterior lumbar interbody fusion using rhBMP-2 in
titanium tapered cages with autogenous bone in threaded cortical bone
dowels in 45 patients. Twenty-two patients underwent a laparoscopic
anterior lumbar interbody fusion with an rhBMP-2-soaked collagen sponge
within a tapered titanium cage, and 23 patients underwent a
laparoscopic anterior lumbar interbody fusion with threaded cortical
bone dowels packed with autograft. The rhBMP-2 group had a shorter
operative time and length of hospital stay compared with the autograft
group. Both groups reported improvement of back pain, leg pain, and
overall satisfaction; however, the rhBMP-2 group improved to a higher
level based on full restoration of function, the Oswestry outcome
questionnaire, and the SF-36 back profile; the difference was
statistically significant.
performed to determine if the dose and carrier for rhBMP-2 that was
successful in rhesus monkeys could induce consistent radiographic
posterolateral spinal fusions in humans. Patients enrolled in this
study had single-level disc degeneration, grade I or less
spondylolisthesis, mechanical low back pain with or without leg pain,
and failed at least 6 months of nonoperative treatment. Twenty-five
patients undergoing lumbar arthrodesis were randomized (1:2:2 ratio):
-
Autograft/TSRH pedicle screw instrumentation (n = 5)
-
rhBMP-2/TSRH (n = 11)
-
rhBMP-2 only without internal fixation (n = 9)
with 20 mg of rhBMP-2/side. The radiographic fusion rate was 40% (2 of
5) in the autograft/TSRH group and 100% (20 of 20; p = 0.05) with
rhBMP-2 with or without TSRH internal fixation. The authors concluded
that rhBMP-2 with the biphasic calcium phosphate granules consistently
induced radiographic posterolateral lumbar spinal fusion with or
without internal fixation in patients with not greater than grade I
spondylolisthesis. Also, there was a statistically greater and quicker
improvement in patient-derived clinical outcome measures, including
Oswestry and SF-36, in the rhBMP-2 groups.
posterolateral spinal fusion for degenerative spondylolisthesis; human
pilot data have shown early promising results. Although the use of BMPs
has shown promise in smaller animals, there is evidence to suggest that
the high doses required for successful treatment of higher order
animals, including humans, may be less practical for multilevel fusions
through currently available means. The development of an ideal delivery
system for BMP remains a clinical challenge.
in human spinal fusion procedures have been less encouraging. Laursen
et al reported the use of OP-1 as an intracorporeal bone graft
stimulator in unstable thoracolumbar burst fractures in five human
subjects. OP-1 was found not to be efficacious, however, because all
the cases failed to heal, and in one case, severe bone resorption
occurred. Jeppsson et al evaluated OP-1 in cervical spine posterior
fusion in four patients with rheumatoid disease. No bone formation
occurred in three patients, and the study was stopped. Patel et al
reported their initial experience with OP-1 for posterolateral spinal
fusion in humans. In this study, 16 patients underwent decompression
and fusion for spinal stenosis and degenerative spondylolisthesis, 12
patients received combined autograft and OP-1, and 4 patients
received
autograft alone. No adverse effects were seen, but the radiographic
fusion rate was less than 70%. There were no problems with bone
overgrowth or restenosis. At a 2-year follow-up assessment, results had
been maintained, and restenosis had not occurred.
capable of inducing bone growth in humans, administration of milligram
doses of these proteins would be costly, and the delivery systems
remain to be optimized. These issues have led several investigators to
explore the use of gene therapy for the control of bone formation. Gene
therapy allows for factors to be expressed in cells for longer periods
and may direct bone formation more naturally by prolonged expression of
more physiologic concentrations of growth factor rather than single
boluses of a large dose.
the anatomic location where the protein must be delivered determine the
type of strategy employed for gene therapy. Several different gene
therapy options are available, as follows:
-
Gene therapy can be systemic or regional.
-
The gene can be introduced directly to a
specific anatomic site (in vivo technique), or specific cells can be
harvested from the patient, expanded, and genetically manipulated in
tissue culture, then reimplanted (ex vivo technique). -
The vehicle (vector) for gene delivery to the cell can be viral or nonviral (Table 33.4-2).
-
Ex vivo transduction of cells with the BMP gene, followed by the implantation of the transduced cells into the host animal
-
Direct injection of a BMP vector, which inserts the BMP transgene directly into host cells
|
TABLE 33.4-2 GENE THERAPY VECTORS
|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
patient, and DNA is transferred to cells in tissue culture. The
genetically modified cells subsequently are administered to the
patient. In addition to providing an osteoinductive gene to a desired
site, the ex vivo approach has the additional advantage of supplying
cells (e.g., bone marrow cells) that are capable of participating in
osteoinduction. In vivo gene transfer involves the introduction of the
specific gene directly into the body with the expectation that it will
reach the target cell. To initiate gene expression, exogenous DNA must
penetrate the cell, avoid lysosomal degradation, and enter the nucleus
where the transcriptional machinery resides. When a virus is used as a
vector, portions of the viral genome are deleted to prevent replication
and create space for the insertion of the therapeutic DNA. DNA may be
incorporated into the host cell’s chromosomes, or it may remain
extrachromosomal (episomal). Cellular uptake of DNA that is not
associated with any delivery vehicle (naked DNA) is usually an
inefficient process. Despite this inefficiency, naked DNA has been used
successfully to promote osteogenesis.
been used to fuse the spine successfully in animals. Riew et al showed
that mesenchymal cells transduced with the BMP-2 gene can promote
osteogenesis in the paraspinal region in the rabbit. They attempted to
prolong the boneinducing effect of BMP-2 using an adenoviral vector
carrying
the
human BMP-2 gene to transduce expanded marrowderived mesenchymal stem
cells in New Zealand White rabbits. Of the five study rabbits, only one
(20%) showed radiographic evidence of new bone formation on the side
implanted with Adv-BMP-2 5 weeks after surgery. No new bone was noted
on the control Adv-β-gal side. No new bone formation was observed on
either side in the other four study rabbits.
with an adenoviral vector (Adv-BMP-2) to produce BMP-2 in a rat
intertransverse spinal fusion model. The transfected cells were soaked
onto a guanidine-extracted, deactivated, demineralized bone matrix
carrier and implanted into the rat spine between the transverse
processes of L4-5. At 4 weeks, all rats that had been implanted with
Adv-BMP-2-producing marrow cells showed 100% fusion. All rats (100%)
that had been implanted with recombinant BMP-2 alone also had complete
arthrodesis by 4 weeks. All other groups did not show fusion at 8
weeks’ time. Alden et al investigated the effect of percutaneous spinal
fusion employing BMP-2 using adenovirus-mediated gene transfer
techniques in an athymic (immunocompromised) rat model. Twelve animals
divided into four groups were studied as follows:
-
Adv-BMP-2 bilaterally
-
Adv-BMP-2 on the right
-
Adv-β-gal on the left
-
Adv-β-gal bilaterally
each of the Adv-BMP-2-injected sites, but no changes were observed at
the Adv-β-gal control sites. The investigators concluded that in vivo
endochondral bone formation was possible using direct adenoviral
construct injection into the paraspinal musculature. In both studies,
cells were genetically modified to overexpress the protein, turning
them into a biologic BMP-2 factory, which can be implanted into the
site of spinal fusion. Riew et al achieved only 20% fusion, however,
whereas Wang et al did not show any advantage in terms of fusion rates
over recombinant protein. Although these studies show feasibility, they
do not show a clear advantage of using gene transfer in place of
recombinant BMP-2.
model as Wang, showed a histologic difference in the bone formed by
genetically modified cells compared with bone formed by recombinant
protein. The genetically modified cells appeared to form bone with
finer trabecular architecture than that formed by recombinant BMP-2.
This observation suggests that gene transfer and local expression of
BMP-2 may lead to biologic effects that are different from the addition
of exogenous protein.
be effective in inducing spinal fusion in vivo. LMP-1 cDNA, a novel
intracellular protein, initiates membranous bone formation in vitro and
in vivo when transfected into buffy coat white blood cells. Although
LMP-1 is an intracellular protein, it is thought to act via secretion
of soluble osteoinductive factors that subsequently induce expression
of other BMPs and their receptors. Boden et al
used a posterior spinal fusion model in nude rats and grafted
guanidine-extracted demineralized bone matrix with bone marrow cells
transfected with the LMP-1 cDNA. At 4 weeks, the sites with active
LMP-1 cDNA had 100% fusion compared with 0% fusion at sites with the
inactive reverse copy of LMP-1 cDNA. Radiographic and histologic
examinations showed that virtually no bone induction was apparent in
the absence of active LMP-1 cDNA. Viggeswarapu et al showed that LMP-1
is able to induce intertransverse process fusion when delivered by a
replication incompetent type 5 adenovirus in immunocompetent rabbits.
They did ex vivo transfection of LMP-1 cDNA into bone marrow or
peripheral blood buffy coat cells. These cells were combined with a
collagen and ceramic composite sponge and implanted into the
posterolateral spine in rabbits. In their study, all 10 rabbits treated
with cells expressing LMP-1 achieved fusion, as tested by manual
palpation. None of the control rabbits treated with cells not
expressing LMP-1 achieved fusion. CT scans showed that the presence of
LMP-1 expressing cells increased the fusion mass density and that the
bone formation was confined mostly to the carrier. Although these
findings are preliminary, the potential benefit of regional gene
therapy for spinal fusion applications is significant, and considerable
work in this area is ongoing.
SD, Kang JD, Sandhu HS, Heller JG. 2002 Volvo Award for Low Back Pain
Research. Use of rhBMP-2 to achieve posterolateral lumbar spine fusion
in humans: a prospective and randomized clinical pilot trial. Spine
2002;27:2662-2673.
SD, Martin GJ, Horton WC, et al. Laparoscopic anterior spinal
arthrodesis with rhBMP-2 in a titanium interbody threaded cage. J
Spinal Disord 1998;11:95-101.
BW, Kotani Y, McNulty PS, et al. Video-assisted thoracoscopic surgery
versus open thoracotomy for anterior thoracic spinal fusion: a
comparative radiographic, biomechanical, and histologic analysis in a
sheep model. Spine 1998;23:1333-1340.
JS, James SB, Chabot MC, et al. Augmentation of autograft using rhBMP-2
and different carrier media in the canine spine fusion model. J. Spinal
Disord 1997;10:467-472.
B, Fischgrund J, Herkowitz H, et al. The use of recombinant human bone
morphogenic protein 2 (rhBMP-2) to promote spinal fusion in a nonhuman
primate anterior interbody fusion model. Spine 1999;24:629-636.
EH, Trawick RH, Boden SD, Hutton WC. Morphology of the lumbar
intertransverse process fusion mass in the rabbit model: a comparison
between two bone graft materials—rhBMP-2 and autograft. J Spinal Disord
1996;9:125-128.
