REVISION KNEE ARTHROPLASTY AND ARTHRODESIS OF THE KNEE
– JOINT RECONSTRUCTION, ARTHRITIS, AND ARTHROPLASTY > Lower
Extremity > CHAPTER 109 – REVISION KNEE ARTHROPLASTY AND ARTHRODESIS
OF THE KNEE
different from primary reconstructions of the arthritic joint. Although
it is essential to master the principles and techniques of primary
surgery before tackling revisions, primary surgery will not, by itself,
prepare the surgeon to cope with the failed prosthetic knee joint.
well-choreographed routine with a coherent sequence of steps that are
easily performed with well-designed instruments. The bone is usually
solid, ligaments are usually intact, and skin is adequate to cover and
protect the reconstructed joint. Revision surgery does not offer this.
Whereas in a primary arthroplasty, the surgeon can depend on
instruments that refer to particular osseous landmarks, neither the
landmarks nor good instruments are available for revisions.
explained at several levels: Why is there pain? Why has a component
broken? Why is the joint unstable? If these questions are unanswered
prior to revision, a suitable mechanical plan for the revision will not
be apparent and the patient will not benefit from the revision.
to plan the procedure carefully. Anticipation of problems mandates
familiarity with the failed and revision implants. Wariness and
improvisation are important in revision surgery. Alternative plans must
be available. We seek perfection in the primary, and the “perfect
compromise” in the revision.
includes adherence to general principles, an operative plan that
addresses the cause of failure, and an orderly sequential plan for
performing the surgery. I use a three-phase approach, as described
later.
An increased incidence of complications increases costs as well. The
latitude for errors—of both judgment and technique—is less than with
primary TKR. In the final analysis, revision surgery is best avoided by
a well-conceived and precisely executed primary knee replacement. Table 109.1 lists the steps to be taken in evaluating and planning for revision of a failed knee arthroplasty.
 |
|
Table 109.1. Confronting the Failed Knee Arthroplasty
|
have improved over the last two decades. No surgeon can perform
revisions as they were done 20 years ago and expect to produce
acceptable results. The contemporary standard of care has been elevated
by progress in technique and implants.
primary knee arthroplasties were approached with trepidation. In the
1970s, H. H. Young noted that the modified Walldius hinge prostheses
had not survived more than 10 years (175). The high failure rates were compounded by damage to bone and ligaments (15).
The results of second operations were abysmal, and arthrodesis was
difficult to achieve. Many patients with failed hinge arthroplasties
were forced to manage with resection arthroplasty (96), and some required amputation (125,174). This led to the belief that knee arthroplasty was a flawed surgery and that revision was not feasible.
Later, tricompartmental resurfacing designs, without customization or
specific adaptation, still led to disappointing results when used for
revisions (32,58,85,124), except in selected cases (45,55,126).
The incomplete understanding of the unique problems of revision surgery
is obvious in articles that combined primary and revision knee
arthroplasties in the same series.
in reviewing 427 revision knee arthroplasties performed between 1970
and 1980 with a wide range of implants, established three criteria to
qualify the result as successful: (a) mild or no pain, (b) knee flexion
to 90°, and (c) mild or no instability. Two years after revision
surgery, only 59.6% of patients had “successful” results.
device that had less than full constraint (it had full rotational
freedom) but could still stabilize a knee in the absence of collateral
ligaments. Rand et al. (128) described 23
revision arthroplasties with the KRH at 29–79 months (mean, 50 months)
after surgery. The complication rates were high and the surgeons
advised that the implant be limited to patients without functional
collateral ligaments, whose knees could not be managed by soft-tissue
reconstruction. Essentially, this was a prosthesis of last resort. It
eventually became apparent that even very bad cases could be revised
with specially adapted resurfacing or nonlinked constrained implants (136).
made the point that revision surgery demands special principles,
techniques, and implants. “Repeat primary replacement” is inadequate.
Early revision was advised
once
evidence of component loosening appeared to avoid further bone loss.
The patellar “turn-down” surgical approach, skeletonization of the bone
ends, and a special axial slap-hammer extractor were developed for
exposure and implant removal. Custom resurfacing designs with augmented
metal trays and a central stem could substitute for bone defects and
provide good fixation to compromised bone (2).
Constrained condylar implants, which were stable to varus and valgus
stresses but were unlinked, were recommended for knees without
competent collateral ligaments. In this 1982 volume, Insall and
Dethmers (75) stated that there was no longer an indication for hinge prostheses.
the cause of failure and reporting results accordingly. “Failed total
knee arthroplasty” is not a diagnosis. Jacobs, working with Hungerford
and Krackow (76), reported in an important
paper that 83% of their patients who had definable mechanical problems
achieved good or excellent results from revision arthroplasty. By
contrast, the patients in whom no definable problem could be
ascertained before their operations were not improved, emphasizing this
important principle of revision knee arthroplasty. Their paper was
significant because they analyzed the results of revision arthroplasty
according to the cause of failure. Failed linked devices, comprising a
minimum of cases (3/28), were all revised for loosening and presented
considerable technical challenges. Instability and malrotation
accounted for fewer cases but were easier to revise. Flexion was
generally good, with mean values of 97° after revision from a
preoperative mean of 86°.
has led to improved surgical techniques for revision, and superior
results. Specificity in diagnosis and treatment have become the
principles of revision knee arthroplasty.
specifically prior to revision. Revision knee arthroplasty performed
without a diagnosis is unlikely to help the patient.
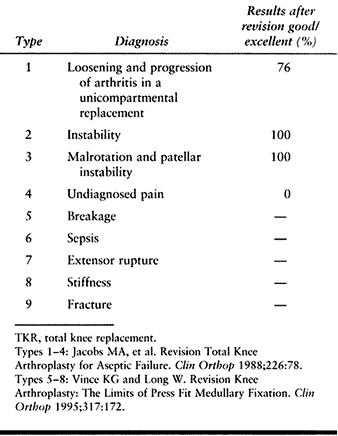
Thus, eight of nine causes of knee arthroplasty are amenable to
revision surgery. A knee may exhibit facets of more than one type of
failure. For example, a prosthesis with catastrophic failure of
polyethylene with consequent osteolysis may also be loose. An infected
knee may have developed loosening, and a loose arthroplasty may have
become unstable. Try to distinguish the primary reason for failure.
 |
|
Table 109.2. Nine Types of Failure That Are Indications for Revision TKR
|
with a dislocating patella misdiagnosed as collateral ligament
instability. The use of a constrained implant of any type in the knee
that remains malaligned will fail rapidly.
 |
|
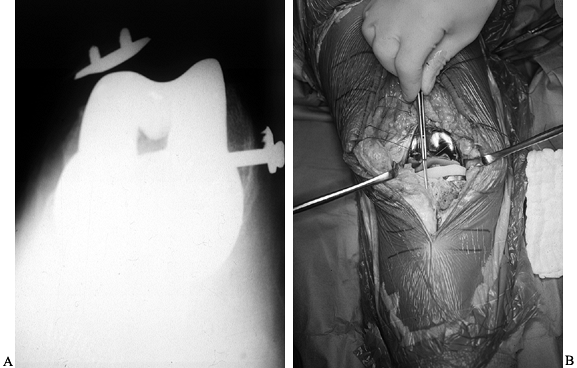
Figure 109.1. A:
Patellofemoral radiograph of patient who presented with symptoms of “giving way.” Reconstruction of medial collateral ligament was performed by her surgeon, but in fact the buckling was caused by patellar dislocation. B: Intraoperative photograph showing that the patella tracks directly over the lateral femoral condyle. The patellar tracking problem resulted from malrotation of the femoral and tibial components. |
collateral ligament incompetence, component subsidence, pain that
causes muscle inhibition, and extensor mechanism problems. Problems are
often related, such as component malrotation and patellar instability.
In fact, it would be fair to hypothesize that all patellar
complications result from maltracking, which in turn is caused by
malrotation of either the tibial or femoral components (Fig. 109.1B).
caution but not an indication for surgery. A patient with pain in the
presence of otherwise unremarkable findings may have had inappropriate
expectations of the primary surgery and is unlikely to benefit from
revision. Evaluate such patients thoroughly for infection, reflex
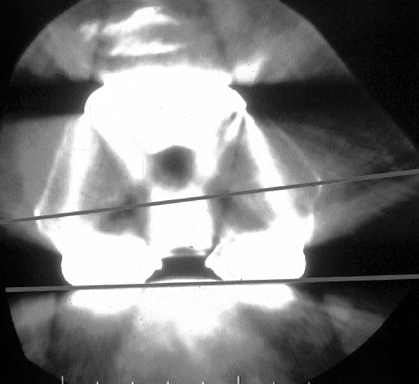
sympathetic dystrophy (RSD), and component malrotation (Fig. 109.2).
 |
|
Figure 109.2.
Computerized tomography (CT) scan of distal femur showing internal rotation of the femoral component. In most cases, this leads to patellar complications. In some cases, the knee is painful and stiff without enough flexion for dislocation to occur. Internal rotation of the femoral component, difficult to diagnose on plain radiographs, presents as painful “mystery knees,” which are caused by internal rotation of the femoral and/or tibial components. |
began to fail. After identifying which type of failure has occurred,
determine the cause to ensure that the scenario is not replicated in
the revision. The temptation in dealing with worn modular articular
polyethylene is to replace it. This would however, simply reestablish
the original environment of failure.
requiring a complete evaluation of the failed arthroplasty. There is
rarely a quick or easy fix for this problem. The analysis must be very
specific as to whether the knee lacks flexion, has a flexion
contracture, or is globally tight. Each scenario requires a different
approach.
damage caused by the failure must be reconstructed. Bone loss is common
to most revisions. Fixation, which may have been straightforward in the
primary procedure, can be achieved in the revision only with the use of
bone graft and special implants if there are defects and the bone is
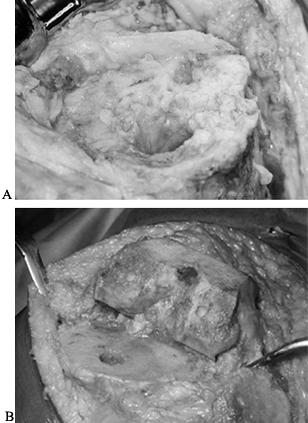
too sclerotic for conventional cement techniques (Fig. 109.3).
 |
|
Figure 109.3. A:
Intraoperative photograph of the proximal tibia after removal of components. The bone is sclerotic, is covered with a fibrous membrane, and lacks the normal cancellous surface of bone seen in primary knee arthroplasties. B: Intraoperative view of healthy cancellous bone at the time of primary knee arthroplasty. Bone cuts have been made and the knee is ready for implantation. |
face the complete disarray and destruction, the course of action may be
unclear. Although bone defects, for example, present an immediate
challenge, they cannot be reconstructed without attending to joint
stability and prosthesis fixation,
and
ultimately restoring functional kinematics to the joint. Skin and
soft-tissue coverage problems are prevalent and require attention.
Preoperative planning must take all of these issues into consideration.
by the surgeon faced with a badly failed knee arthroplasty: “This is
such a bad-looking failure that I will certainly need a large,
constrained implant if not a hinge!” This response is misguided. There
are specific reasons to use built-in constraint in a prosthesis, to
supplement fixation, and to add modular augmentations (components);
these become apparent during the course of the revision. It is
inappropriate to simply use a “revision implant”—there are systems
available that offer many options.
of all possible eventualities to avoid costly surprises in surgery. A
thorough plan for revision TKR (Table 109.3)
provides a way to ensure that the necessary equipment, instruments,
graft material, and implants will be available at surgery, as well as a
sequential intraoperative guide to what should be accomplished in the
revision.
 |
|
Table 109.3. Critical Components of a Plan for Revision Knee Arthroplasty
|
shortcoming of the failed knee and describes a solution must be part of
the plan. The tight knee with a flexion contracture, for example, will
require an aggressive release of the posterior capsule and probably
resection of additional distal femoral bone. This would not be
appropriate for the unstable knee that needs to have the deforming
forces (malalignment) eliminated, and stabilizing forces (ligament
reconstruction or constraint) reconstituted. The sequential plan
indicates when each part of the procedure should be carried out, and
the mechanical plan describes what has to be accomplished.
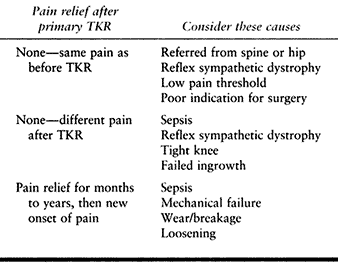
clues for the cause of the pain are the time of onset and the nature of
the pain (Table 109.4).
 |
|
Table 109.4. Evaluating Pain in the Problem Total Knee Replacement (TKR)
|
essentially the same pain persists, consider referred pain from the hip
or spine, preexisting sepsis, a tight arthroplasty,
or
failed bone ingrowth. Carefully evaluate the ipsilateral hip and the
spine. Internal rotation of the ipsilateral hip that is limited on
physical exam and reproduces the knee symptoms is very important. Treat
an arthritic hip prior to knee arthroplasty, as much of the knee pain
may be referred from the hip. A technetium (Tc) bone scan and injection
of local anesthetic into the hip joint are helpful in the differential
diagnosis.
effusion. Pain accompanied by a general feeling of tightness or
fullness, or pain that is perceived in the popliteal region, may be
associated with a tense effusion. A postoperative effusion can
reproduce this same pain.
who, in retrospect, was suffering from sympathetically mediated pain
(RSD). The characteristic history of inordinate pain relative to
physical findings may be present, but cardinal symptoms such as skin
hypersensitivity and intolerance to light touch, for example of bed
sheets, may be absent. Awareness of the possibility is important to
avoid unnecessary surgery. Consultation with a pain-management expert
will help the patient cope with understandable frustration and anger.
Lumbar sympathetic blocks are helpful to diagnose and treat this very
difficult clinical situation.
pain requires a workup for infection. Culturing an aspirate of the
knee, with the patient off antibiotics, is essential to establishing
the diagnosis. The infected knee replacement may present with only
slight warmth and swelling, or with frank drainage and soft-tissue
loss. Some factors in the history create particular risk. Prior
osteomyelitis adjacent to the knee, although dormant for many years,
has been associated with a 15% infection rate in knee arthroplasty.
Prior surgery (especially with implants), antimetabolite medications
(including corticosteroids), and some medical conditions (notably
rheumatoid arthritis) are suspected of increasing the risk of
infection. The data, all from small series, are inconclusive.
surgery implies that the arthroplasty or the technique of insertion may
be at the root of the problem. Knees that have been implanted with a
greater degree of stability and tightness than the patient can tolerate
may be painful. Malrotation of the components can also create a painful
situation, with tightness and binding of the components.
occurred will usually be painful from the outset. During the recovery
phase, as postoperative pain recedes, the patient is left with
discomfort that is worse on weight bearing and that never really
abates. Careful fluoroscopic examination of all the interfaces allows
accurately directed plain radiographs that will illustrate this
situation (44).
first time after knee arthroplasty surgery. The prospects for prompt
resolution of the RSD are improved by early referral to a pain
management specialist.
stiff and painful afterward may be difficult. A knee prosthesis that is
tight, for whatever mechanical or surgical reason, is likely to be
painful. Conversely, a knee that is painful may hurt too much for the
patient to participate in physical therapy. RSD is a frequent companion
to the tight painful knee.
a period of pain relief may indicate late infection. It is, however,
the typical presentation for any number of mechanical failings in the
knee, most commonly loosening and breakage. Breakage, or extensive wear
of components, is probably encountered more commonly than loosening in
the current era. Mechanical failure may also be accompanied by symptoms
of lost motion, buckling, instability, or catching and grinding.
activities. The loose prosthesis hurts more with weight bearing and the
tight knee hurts more when flexion is attempted. Pain from a patellar
fracture is worse with activities, such as
stair climbing, that increase the force on the extensor mechanism.
with activities, is characteristic of sepsis. RSD, which also produces
rest pain, is unlikely to have a late onset. The presence of an
effusion, from whatever cause, may result in pain at rest.
accompanied by but are more serious than their pain. Instability may
take different forms: The patient may experience buckling, caused by
giving way of the extensor mechanism, in turn caused by either pain or
subluxation of the patella. True varus–valgus instability is generally
apparent and accompanied by malalignment when severe. The patient
easily demonstrates catching or grinding that usually accompanies
catastropic polyethylene wear.
fully, with attention to the spine and hips as well as the knees.
Evidence of infection or catastrophic polyethylene wear may lead to
lymphadenopathy, particularly at the groin. Check the dentition and
sinuses, as they may be sources of sepsis. Examine gait for evidence of
instability and limp. The characteristic walk of a patient with hip
pathology, dipping of the shoulder ipsilateral to the painful hip, may
indicate referred hip pain.
joint. Local swelling may result from chronic scarring in a tight
joint, extensive thickening of synovium, or an effusion. The entire leg
may be affected by edema or lymphedema. Inability to fully extend the
knee may result from an effusion or a fixed flexion contracture. There
can be sympathetically mediated pain without the characteristic waxy
pallor that is sometimes mimicked by tight swelling in the early
postoperative period. Many normally healing knees will have a
characteristic neurodermatitis rash that accompanies an area of
paresthesia lateral to the skin incision (138).
skin, which may reveal the hypersensitivity that is often associated
with RSD. Increased warmth is common during the first 3 months after
surgery and may be exacerbated by overly aggressive physical therapy.
Increased warmth out of context of the patient’s recovery from surgery,
however, is suggestive of sepsis. Palpate for specific areas of
tenderness that may indicate a mechanical problem between the
prosthesis and bone or the prosthesis and soft tissues. Palpate the
posterior aspect of the knee to detect a popliteal or Baker’s cyst,
which reflects a large effusion, which may come from a failed
arthroplasty. Palpate the pulses and evaluate for circulation to
eliminate peripheral vascular disease as a possible cause of the
patient’s pain and to be certain that there is adequate circulation for
reconstructive surgery.
established between passive and active motion. The average flexion of
most arthroplasties by 3 months following surgery will be about 115°.
This is influenced heavily by the preoperative motion, so it is
important for you to know what that was. It is unrealistic to expect
that a revision arthroplasty will improve a patient with less than 90°
of knee flexion if the patient had only 60° of flexion prior to the
primary replacement. By contrast, the patient who flexes to only 60°
but had near normal motion prior to the primary surgery may benefit
from timely revision before the soft tissues contract.
strength. The patient with spinal stenosis who has quadriceps weakness
may behave like the polio patient who must “back knee” to walk. This
may create loosening, wear, and instability in the arthroplasty.
strength that is inadequate to extend the knee through its full passive
extension. This can be caused by muscle weakness, inhibition due to
pain, and anatomic and mechanical problems in the extensor mechanisms.
Carefully palpate the extensor mechanism to look for sites of discrete
tenderness or gaps, which may suggest patellar fracture or tendon
rupture. Although some of these problems are treatable, chronic
extensor lags can be difficult to eliminate completely with revision
surgery.
knee instability. Determine precisely what type of instability is
present and what the mechanical remedy will be. Many patients whose
knees are buckling or giving way will use the term instability when the problem is in the quadriceps mechanism. See Table 109.5 for interpretations of instability symptoms and Table 109.6 for causes of buckling.
 |
|
Table 109.5. Patient Symptoms Associated with Instability
|
 |
|
Table 109.6. Anatomic Causes of Giving Way (Buckling) of the Knee
|
which occurs most commonly in flexion. The patient will be able to
demonstrate this phenomenon, and you can confirm it with a conventional
drawer test and posterior sag sign. Other methods of assessing
instability, so useful in the normal knee, are not applicable to the
arthroplasty. An arthroplasty with posterior or anterior instability in
extension is likely to manifest global instability. The patient
will probably walk in marked hyperextension, if indeed he is able to bear weight at all.
falters with each step and usually depends heavily on assistive
devices. This type of instability is usually accompanied by significant
malalignment, which has either been the cause or the result of the
instability. Comparing the physical examination with the radiographs
will reveal whether instability is from collateral ligament failure,
loosening and subsidence of components, or destructive wear of
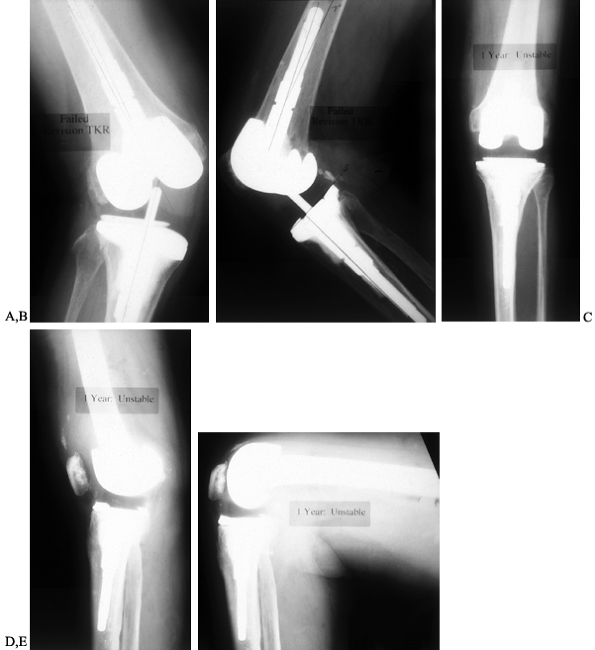
polyethylene (Fig. 109.4).
 |
|
Figure 109.4. AP (A) and lateral (B)
radiographs of an 83-year-old woman with valgus instability in a revision arthroplasty despite the use of a constrained condylar implant. The problem results from loss of ligamentous integrity, combined with malalignment that increases the deforming force on the polyethylene of the tibial component. Weight-bearing AP radiograph (C) of a 71-year-old woman with a nonconstrained posterior stabilized implant showing good stability in the varus and valgus planes. Lateral weight-bearing radiograph (D) showing hyperextension. This patient had failure of the posterior structures as a result of spinal stenosis and weakening of the extensor mechanism, which caused her to walk with recurvatum to prevent buckling. Lateral radiograph in flexion (E) showing that the flexion gap remains stable. |
to varus and valgus stress testing when locked in full extension,
because of tension on the posterior structures. This knee, when flexed
to about 30°, reveals significant instability. The medial collateral
ligament (MCL), so crucial to good arthroplasty function, should be
assessed for strength prior to revision. A knee may be unstable with a
structurally intact but unbalanced ligament. It can be made to be
functional with the appropriate releases or advancement. The knee in
which the MCL has suffered complete failure becomes a far larger
challenge.
or a fatigue fracture of the patella. Catches, clicks, or “jumping out
of place” may result from subluxation or patellar dislocation. The
patellar “clunk” occurs when scar under the quadriceps tendon catches
under the anterior edge of the femoral component when the knee flexes
deeply. This may initially be a small piece of synovium, which
hypertrophies with each subsequent episode of catching. Eventually, the
patient notes a dramatic “catch” when trying to rise from a seated
position. The scar catches and with further effort escapes from the
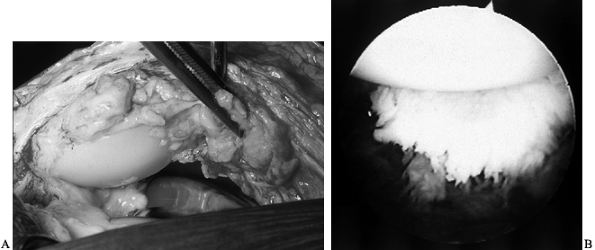
notch with an audible and sometime painful “clunk” (10,72). The offending scar may be excised arthroscopically or with a limited arthrotomy (Fig. 109.5).
 |
|
Figure 109.5. A:
Intraoperative view of scar on the quadriceps tendon that has been catching on the anterior femoral flange of a total knee arthroplasty, creating the patellar clunk. B: Arthroscopic visualization of scar on the quadriceps tendon, above the patella. Removal of this scar eliminates the patellar clunk. |
that requires careful observation of the functioning knee to diagnose.
Patellae that dislocate are more easily diagnosed. Dislocation
generally occurs with flexion, and the extensor mechanism often reduces
with extension. Because component malrotation is usually at the root of
the problem, there may be apparent differences in the amount of
internal rotation of the flexed hip joints, only because the femoral
component itself is internally rotated (Fig. 109.6).
Internal rotation of the tibial component can be appreciated by looking
at the externally rotated position of the foot with the patient sitting
with his legs hanging over the edge of the examining table. Confirm any
suspicion of malrotation with a computed tomography (CT) scan of the
arthroplasty, which will allow comparison of the position of the
prosthesis relative to the transepicondylar axis.
 |
|
Figure 109.6. A:
Patellofemoral radiograph demonstrating the relationship between patellar maltracking and other complications, such as patellar component loosening and patellar fracture, all three of which are observed here. The underlying problem is internal rotation of the femoral component. B: Physical examination showing apparently greater internal rotation of the left hip than of the right, resulting from internal rotation of the femoral component. C: Two years after revision knee arthroplasty, the residual patellar bone, too small to resurface, does nonetheless track centrally after correction of the internal rotation of the femoral component. |
and for culture and sensitivity of aerobic, anaerobic, and fungal
organisms (49,97). More
than 25,000 leukocytes per cubic millimeter indicates sepsis,
especially if a majority of the cells are polymorphonuclear cells.
Repeat cultures if there is a negative culture from the aspirate of an
arthroplasty suspected to be infected. Barrack et al. (7)
found preoperative aspiration to be reasonably sensitive (75%) and
highly specific (96%) for the detection of infection. Early work with
biochemical evaluation of polymerase chain reactions of aspirated fluid
promise a high degree of sensitivity and specificity (98). This assay, however, is not yet widely available for the diagnosis of infection in knee arthroplasties.
take plain radiographs using 18-inch (45 cm)-long cassettes with the
patient bearing full weight on the limb of concern. This usually
reveals the extent of subsidence and of ligamentous laxity. Take the
lateral projection with the patient standing and then with the knee
flexed maximally. This technique reveals subluxation in extension as
well as recurvatum and flexion contractures. The lateral flexed view
helps the technician obtain a true lateral projection and reveals
tibial femoral instability that may not occur in extension. The tibial
slope is displayed best in this view, and flexion can be measured.
Posterior osteophytes that may impede flexion are apparent, and the
size of the femoral component is easy to measure.
radiographs is control of rotation. Failure to do so creates studies
with limited value. When it seems impossible to obtain a true AP
projection of both components—on one radiograph the tibia is viewed
correctly but on another it is rotated while the femur is viewed
accurately—the diagnosis of malrotated component is established (Fig. 109.7).
 |
|
Figure 109.7. A:
AP radiograph of an uncemented knee arthroplasty that has failed because of pain, stiffness, and patellar subluxation. While this shows a symmetric view of the tibial component, the femoral component is clearly viewed obliquely. B: The lateral radiograph shows that the femoral component has been translated posteriorly, further contributing to a tight flexion gap and poor motion. C: Patellofemoral view showing subluxation of the patella that has resulted from malrotation. This subluxation, creating the sense of impending dislocation with further flexion, causes the patient to stop knee flexion during physical therapy and contributes to poor motion. |
These projections assess patellar bone cuts and tracking. Component
wear and breakage, bone fracture, and patellar dislocation are apparent.
 |
|
Figure 109.8. Patellofemoral (Merchant) radiographs showing a range of patellar pathology: dislocation (A), fracture with component loosening (B), and lateral tracking and (C) catastrophic wear of a metal-backed button.
|
arthroplasty is present, may be of use to estimate revision component
size. Do not commit to a component size at this point, because
derangements in the soft tissues of a
failed
knee arthroplasty, whether abnormally tight or loose, may necessitate
the choice of larger or smaller devices. Evaluation of serial
radiographs of the failed knee arthroplasty are helpful to understand
precisely what has led to the current situation. Finally, radiographs
of the arthritic knee immediately prior to the primary arthroplasty are
helpful. If these do not show very extensive arthritic changes, then
search for other sources of referred pain. In the absence of these, the
arthroplasty may have been performed prematurely, before serious
arthritis had developed.
polyethylene, radiolucent lines between the cement interface and the
bone or component were much easier to visualize (Fig. 109.9A, Fig. 109.9B). Metal-backed tibial baseplates frequently obscure the interface so that fluoroscopic views
are necessary to ensure that the x-ray beam is parallel to the interface (34).
 |
|
Figure 109.9. A: AP radiograph of a painful, stiff total knee arthroplasty with ostensibly good alignment and well-fixed tibial component. B:
Lateral radiograph of a painful, stiff, uncemented total knee arthroplasty with some radiolucency anteriorly. The component is slightly oversized. When templating for revision, use a slightly smaller femoral component to improve flexion. C: Fehring’s (44) lateral fluoroscopic view of the anterior interface, showing extensive radiolucency and a limited ingrowth. D: Lateral fluoroscopic radiographs demonstrate complete radiolucencies in the posterior flange. This femoral component is loose. Attempts at flexion are painful because the tibia rocks the femoral component anteriorly. E: Intraoperative photographs demonstrating the ease of removal of the loose femoral component. F: Intraoperative picture of the uncemented femoral component, where there has been very little if any bone ingrowth. |
subsidence is usually rapid. Failure of bone ingrowth into the
uncemented component can be a source of pain that is difficult to
visualize without fluoroscopic views (109).
These may have to be oriented to each of the three major interfaces of
the femoral component(anterior, distal, and posterior) (Fig. 109.9C, Fig. 109.9D, Fig. 109.9E and Fig. 109.9F). Fehring and McAvoy (44)
evaluated 20 painful knee arthroplasties with this technique and
identified 14 that appeared loose. At revision, all 14 were loose and
improved by revision surgery.
assessment of problem knee arthroplasties, especially if standing films
have been obtained. They can be useful, however, to quantify varus or
valgus instability or when there is doubt as to whether fractures
adjacent to the arthroplasty have healed.
found that these scans frequently demonstrate mild to moderate activity
during the first year after surgery, whether cemented or uncemented
fixation has been used. High variability has been noted. Loosening
cannot easily be distinguished from bone remodeling in this period.
Henderson et al. (64) studied three groups in
whom they performed Tc bone scans: asymptomatic knees undergoing scans
for other reasons, septically or aseptically loose arthroplasties, and
painful knees without radiographic evidence of loosening. They
concluded that sequential 99mTc-MDP and 67-gallium (67-Ga)
citrate scintigrams are useful for demonstrating the presence of
aseptic and septic loosening in knee prostheses, and that pain with a
normal scan appearance is probably not caused by loosening or
infection. Gallium scans alone are not generally reliable, but
indium-labeled (In-111) white cell scans—more specifically,
In-111-labeled immunoglobulin G (In-111-IgG) scintigraphy—has been
regarded as highly sensitive and fairly specific for detecting late
infection (79,106). Scans, however, are never substitutes for aspiration of the joint.
There is agreement that the femoral component should be aligned
parallel to the transepicondylar axis of the distal femur, defined as a
line passing between the two attachment points for the collateral
ligaments. CT scanning would be ideal to evaluate the rotational
position of the femoral component relative to the epicondylar axis,
were it not for its expense and the artifact created by the chrome
cobalt in most femoral components. Nonetheless, many arthroplasties can
be imaged sufficiently well with modern scanners to identify the
epicondyles on the femur and the prosthesis, despite scatter of the
beam. A plain radiographic method has also been described by Eckoff et
al. (35,36).
patient dissatisfied with his present knee arthroplasty when a specific
mechanical diagnosis is made and a coherent plan for surgery to correct
the problem can be established. Do not consider revision for trivial
problems. Do not do a revision unless the benefits clearly outweigh the
risk of complications.
knee, except in highly unusual circumstances or when the infection is
of very recent onset, promptly and aggressively
with
a two-stage reimplantation protocol. Revise a loose prosthesis because
it will continue to damage bone. Patients with poor flexion or a
flexion contracture deserve an adequate time period following surgery
in which to work aggressively on rehabilitation. However, stiffness
combined with obvious mechanical impediments to motion requires
revision as early as 3 months after the primary arthroplasty. Patellar
tracking problems, especially if symptomatic, inexorably lead to
greater complications. They should be treated by a surgeon who is
willing to revise the entire arthroplasty if necessary to correct
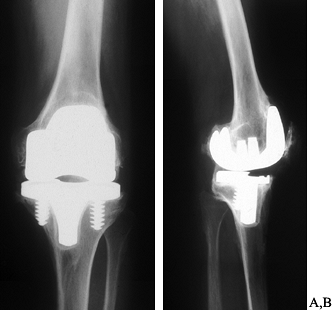
underlying rotational problems (Fig. 109.10).
 |
|
Figure 109.10.
AP radiograph of a painful, stiff uncemented total knee arthroplasty. The components appear large and there are circumferential radiolucencies around the tibial component. B: Lateral radiograph showing oversized femoral component, loose tibial component that has subsided anteriorly, and extensive osteophyte formation posteriorly. Calcification is also present in the patellar tendon. This knee had minimal flexion. |
the results are inferior to primary knee replacement. It is difficult
to compare the published series of revisions (38),
because techniques and causes of failure differ widely. There is no
large series with a minimum 10-year follow-up. The general dependence
on constrained devices emphasizes the importance of a long-term study,
given the high risk of late loosening with these implants.
reported early loosening in a group of 44 revisions when nonlinked
constrained implants had been implanted with press-fit modular stem
extensions in patients with poor-quality bone. They reported good
functional results without failures due to loosening when
(nonconstrained) posterior stabilized implants were used, but a failure
rate approaching 20% when constrained implants were necessary. In a
follow-up to this study (159) that described 98
revisions, the use of constrained implants decreased. The overall
failure rate was 7%, with instability a greater problem than loosening.
results of 76 (nonseptic) revision knee arthroplasties with an average
follow-up of 3.5 years (range, 2–9 years) with this modular system.
Their overall failure rate was 8%.
Rush-Presbyterian Hospital in Chicago reported their experience with 57
revisions evaluated at an average follow-up of 62 months (range, 36–120
months). Four (7%) clinical failures were reported, three of which were
due to instability after the implantation of a posterior stabilized
implant. In this series, the surgeons resorted to nonlinked constrained
implants in an unusually high percentage of cases, when compared to the
experiences of Vince and Long (158) and Haas et al. (60).
bone quality is poor, defects have been reconstructed, or a constrained
implant has been used. Many surgeons limit the use of methacrylate
cement by using modular stems that can be applied to the femoral and
tibial components and that achieve a tight fit in the medullary canal.
at the Mayo Clinic, who reviewed 40 revision knees in 35 patients with
the Kinematic Stabilizer (Howmedica, Rutherford, NJ) prostheses
implanted with fully cemented stem extensions in one or both long
bones. Radiolucencies did not progress, and no failures of fixation
were reported. Although the superiority of fixation with full
cementation is not generally disputed, there is still concern as to how
destructive removal may prove if one of these reconstructions becomes
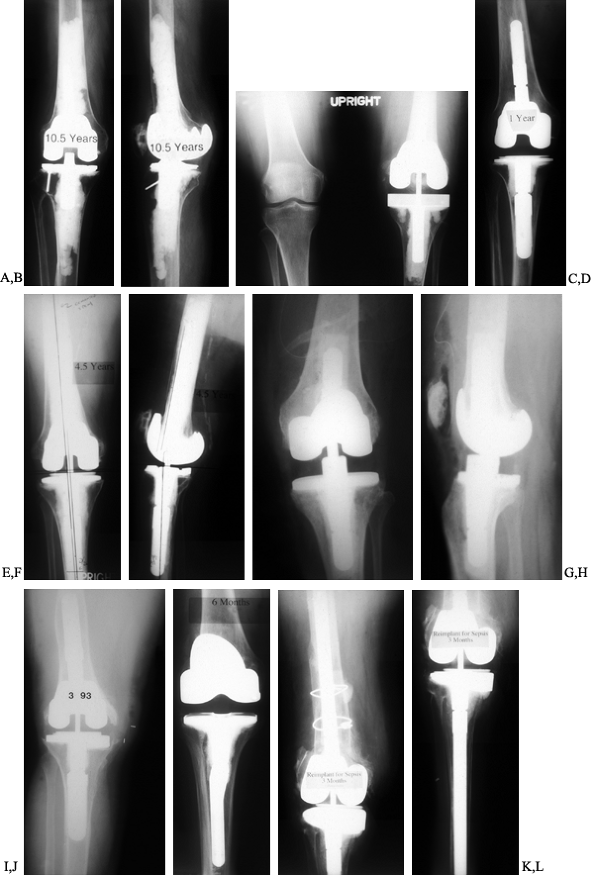
infected. Various intramedullary stem extensions are shown in Figure 109.11.
 |
|
Figure 109.11. Intramedullary stem extensions. A,B:
Fully cemented nonmodular stem with the original Total Condylar III (Howmedica, Rutherford, NJ) femoral component, used here because of poor bone quality with a posterior stabilized nonmodular tibial component. C: Fully cemented fixed stem extension with a constrained articulation. D: Press-fit modular stem extension with methacrylate cement applied only to the component. Note the nonconstrained posterior stabilized prosthesis. The femoral stem extension is undersized relative to the medial lateral dimension of the endosteal canal—it does not, however, compromise the alignment of the prosthesis. The tibia, by contrast, fills the canal but as a result lies in valgus. E,F: AP and lateral radiographs of fully cemented, 145 mm stem extensions. Note the constrained articulation. These are indicated in the presence of poor quality bone when constrained prostheses are used. G,H: AP and lateral radiographs of a constrained articulation, necessitated by medial collateral ligament incompetence with fully cemented, short or “stubby” stem extensions. I: Loose noncemented femoral stem extension. Note the constrained articulation, which most likely contributed to the loosening and the increased valgus alignment resulting from the tight press-fit of a straight stem in an asymmetric bone. J: Offset stems, used without cement. Note the tight fit in the canal without compromising the alignment of the arthroplasty. This revision was performed for instability, and accordingly, a larger femoral component was selected. K,L: Extremely long uncemented stem used in a second reimplant for sepsis in a patient who had already failed one two-stage reimplantation. These very long stems have been used to stabilize large structural allografts and should never be fully cemented. |
described 15 revisions in selected cases, of which eight had both
components implanted without cement. Two required revision for tibial
component loosening. In a later study with uncemented porus-coated
anatomic (PCA) revision components, 6 (16.6%) of 36 revisions failed.
Three of these were for tibial component loosening.
been developed by Whiteside over the last two decades. In 1993,
Whiteside (167) reported the results of 56
cementless knee arthroplasties followed for a minimum of 2 years after
surgery. The technique is based on rigid fixation of the implants into
the remaining shell of distal femur and proximal tibia. Morcelized
cancellous bone was employed to fill all defects. There were two
failures of fixation. Uncemented revision knee arthroplasty is
appealing but technically demanding.
deficient bone in revision surgery. There may be fundamental
differences between bone defects—what works for one patient may not
work for another, and proven techniques for primary arthroplasty are
not always useful in the revision. Elia and Lotke (37)
described their experience with 40 revision arthroplasties in the
presence of significant bone loss. They reported good results but
wisely commented in their conclusions on the importance of (a)
restoring the mechanical alignment of the knee with accurate component
positioning; (b) filling all bone defects
with
bone, cement, or modular spacers; (c) using stems to assist in
component support; and (d) adherence to soft-tissue balancing.
for revision knee arthroplasty, enabling surgeons to deal with the
numerous different problems that present at revision. Typical systems
include augmentation for tibial and femoral components. Although these
are usually regarded as a means of reconstructing defective bone, each
has a specific kinematic implication that can be exploited. For
example, the distal femoral components can tighten the extension gap of
the knee selectively and if used on one condyle alone will alter
alignment. A medial distal femoral augmentation increases valgus
alignment. This effect is most dramatic on the posterior condyle, where
symmetric medial and lateral augmentation will tighten the knee in
flexion and an augmentation on one side will change the rotation of the
component. Internal malrotation of the femoral component, observed
commonly in knee arthroplasty, can be corrected with a posterior
lateral augmentation. The proximal tibia can be reconstructed reliably
with augmentation blocks and wedge-shaped augmentations. Rand (122,123),
reporting 41 consecutive revision arthroplasties with cruciate
retaining or posterior stabilized prostheses, supported the reliability
of modular augmentation in revision knee arthroplasty.
bone that innovative approaches are required. Large custom prostheses,
highly regarded in tumor reconstructions, have not been as successful
in revision total knee arthroplasty. Massive structural allografts
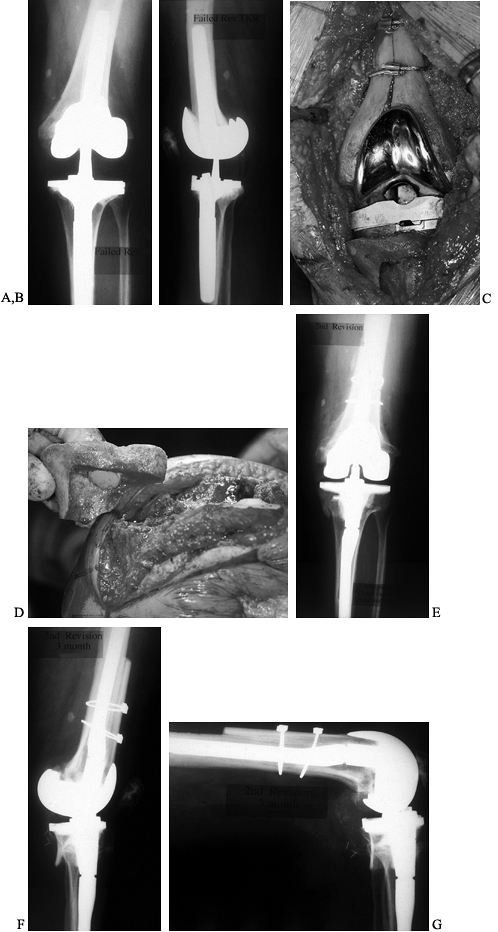
have, however, been used extensively, with surprisingly good results (Fig. 109.12). Mow and Wiedel (112)
described 15 revision knee arthroplasties with large segmental,
cavitary, or combination defects that were reconstructed with
structural allografts. These were followed for 30–101 months, with one
failure directly related to the graft.
 |
|
Figure 109.12. Structural allografts. A:
AP radiograph of a failed revision total knee arthroplasty. The amount of missing bone would have been better managed with structural allografts. Failure occurred due to loosening and instability. Note the excess valgus alignment despite the femoral stem extension. B: Lateral radiograph of failed revision total knee arthroplasty. C: Intraoperative photograph of a distal femoral structural allograft applied to the second revision. D: Intraoperative photograph of a proximal tibial allograft shaped and ready to be applied to the proximal tibia. Fixation is achieved by the interlocking sculpted shape that maximizes contact area, and by the intramedullary stem on the tibial implant. E: AP radiograph of a second revision knee arthroplasty with distal femoral and proximal tibial allografts. F,G: Lateral radiograph of second revision in extension and flexion. |
reconstruction of the knee has been at the University of Toronto.
Ghazavi et al. (53), reporting the surgical experience
of Gross et al. (57),
found that of 30 knees with either distal femoral or proximal tibial
allograft reconstruction, seven (33%) ended as failures at an average
of 50 months (range, 24–132 months) of follow-up. The failures were due
to infection in three, tibial component loosening in two, fracture of
the graft in one, and nonunion of the graft to host in one. They
concluded that “properly applied allograft can be used to reconstruct
massive bone defects, provide stability and support for implants, and
restore bone stock in the event that additional operative treatment is
necessary.”
use of large structural allografts by packing defects with cancellous
bone. Even large structural defects have been reconstructed this way,
applying 6-inch medullary stems to bypass the defects. Whereas the
stems were smooth and uncemented, the components themselves were porous
coated. Generally good results were described in a series of 20 knees,
with persistent pain requiring revision in only one patient.
structural allografting for large uncontained defects in 19 cases. The
results were good at an average of 2.1 years, representing a
preliminary experience with the technique.
Some surgeons have used this term to describe compaction of cancellous
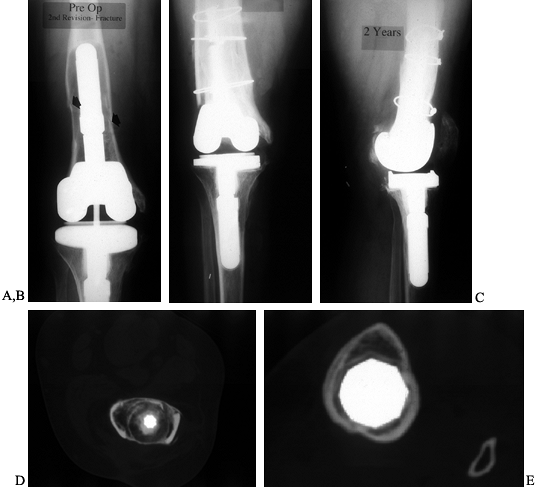
bone in small contained defects, but Ullmark and Hovelius (150) have employed the technique in the medullary canal in three patients. Their results are promising at 28 months of followup.
 |
|
Figure 109.13. Impaction grafting. A:
AP radiograph of a loose revision arthroplasty that was performed 4 years earlier as a reimplantation after infection. The constrained component has contributed to loosening, which has been further complicated by a fracture of the femur. B,C: AP and lateral radiographs of second revision total knee arthroplasty with impaction grafting of the femur, further secured with medial and lateral strut allografts because of a fracture. D: CT scan of the femur, proximal to the femoral component, showing consolidated impaction grafting and strut allografts in place. E: CT scan of proximal tibia showing a press-fit stem extension without impaction grafting. |
knee arthroplasties using constrained and even hinged prostheses. There
is a general, although not universal, preference for unconstrained
implants where feasible. This places greater demands on the surgeon,
who must reestablish stability and motion with the patient’s own soft
tissues. There are times in the operating room when soft tissues are
lacking and it is difficult to stabilize the arthroplasty without
resorting to mechanical constraint. When mechanical substitution for
soft tissues is needed, what devices are available and to what extent
are they reliable and effective?
do so at the risk of an increased incidence of loosening. Linked,
constrained devices (hinges) have enjoyed popularity in some centers (13) but generally have been abandoned because of poor results (6,62,74). Rotating hinges,
although still linked devices, have been employed as an alternate (128,142).
promise. They are constrained to rotation and provide stability to
varus and valgus forces and also resist posterior dislocation in
flexion. However, they lack the hyperextension stop that is integral to
the hinge. The Kinematic Total Condylar III prosthesis (Howmedica,
Rutherford, NJ) was the original nonlinked constrained device.
Mechanical substitution for collateral ligament function was provided
by a prominent tibial eminence that rested between the femoral
condyles. Both tibial and femoral components featured nonmodular,
narrow-diameter intramedullary stems that were fully cemented into the
medullary canal. The results with these implants proved surprisingly
good (24,71,92,121,132).
into the medullary canal, modular stem extensions were added to achieve
a press-fit inside the canal, and cement fixation could be reserved for
the cut bone surface of the tibia and femur. Haas et al. (60)
with 76 cases concluded that there was no significant difference in the
failure rates when uncemented stems were used with 57 (75%) posterior
stabilized or 19 (25%) constrained implants. They did not study
revisions for sepsis. They reported a total of six failures, two of
which were the result of aseptic loosening. One failure was in the
posterior stabilized articulation group (1.75%), and the other was in
the constrained condylar group (5.3%). The experience of Vince and Long
(158) revealed that of 44 revisions (which
included revisions for sepsis), all three (23%) failures occurred in
the group of 13 with constrained articulations.
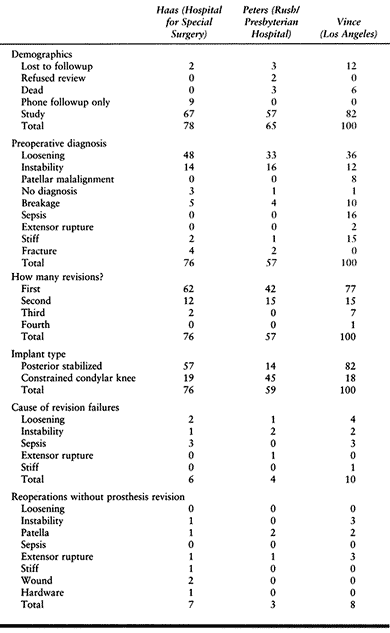 |
|
Table 109.7. Comparison of Revision TKR Results
|
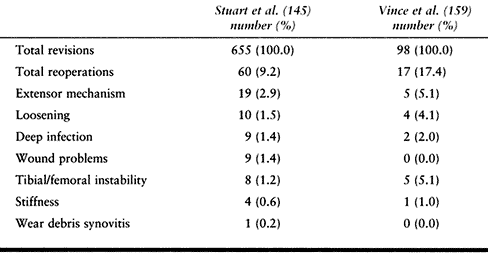
summarized in the Mayo Clinic experience with reoperations after
revision knee arthroplasty surgery (145) (Table 109.8).
Loosening is not the major cause of failure. The more frequent problems
require reoperations without revision. The Mayo Clinic group reviewed
655 revision knee arthroplasties and discovered that 46 (7%) of them
required a total of 60 (9%) additional surgeries. The extensor
mechanism was the most common site of problems leading to reoperation in 19 knees (41%).
 |
|
Table 109.8. Cause and Incidence of Reoperations after Revision
|
included revisions for sepsis, which the Mayo series did not. Again,
the extensor mechanism was the most common site of problems requiring
additional surgery, not all of which were complete revisions. Whereas
tracking problems and fractures of the patella may have roots in the
surgical technique, extensor lag, which is one of the most perplexing
complications of revision arthroplasty, is difficult to predict. It may
be improved in some cases by a shortening of the extensor mechanism,
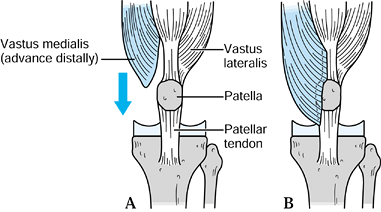
accomplished with a distal advancement of the vastus medialis. Figure 109.14 shows distal advancement of the rectus femoris. Extensor mechanism allografts are new treatments that show promise.
 |
|
Figure 109.14. Distal advancement of the rectus femoris.
|
patient, surgeon, and health care system, have been recognized in North
America as the most reliable means of eradicating infection (50,170). The original protocol, from the Hospital for Special Surgery in New York, has been followed for many years (171).
Other series have described results with antibiotic-impregnated
polymethylmethacrylate (PMMA) spacers that were implanted when the
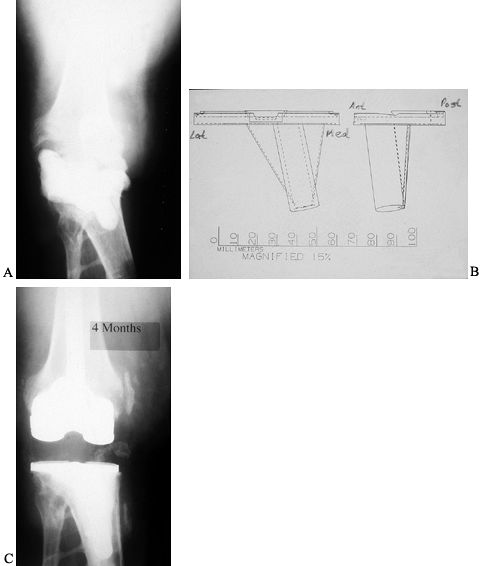
prosthesis was removed (14,59,65) (Fig. 109.15). Spacer blocks that allow some knee motion have been studied (69).
Although most surgeons prefer spacer blocks because they provide local
delivery of antibiotics, the maintenance of tissue planes, and improved
stability during the period of explantation of the prosthesis, other
surgeons have reported bone loss associated with the spacers (18). One-stage reimplantations, popular in some centers with
extensive experience, do not yield the same high success rate (161).
To reduce the number of surgeries, it may be argued that using a
one-stage revision, and reserving a two-stage revision for the
failures, makes sense (140).
 |
|
Figure 109.15. A:
Cement spacer block seen on an AP radiograph of a primarily infected knee arthroplasty that has been removed. Originally, the patient had posttraumatic arthritis from a motorcycle accident. A spacer block, impregnated with antibiotic, was inserted between the deformed tibia and the femur. B: Templates for the fabrication of a custom tibial baseplate that will accept modular stem extensions. C: AP radiograph after reimplantation with custom-fabricated tibial baseplate and standard, fully cemented, short-stem extensions. |
entry into the two-stage protocol. Other institutions have published
experiences with unselected infected knee arthroplasties that have not
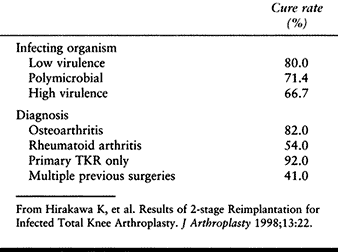
been as good. In one of the most recent large studies, Hirakawa et al. (68) noted a 92% success rate when the infection complicated a primary knee arthroplasty in a patient who had no previous surgery (Table 109.9). This dropped to 41% when there had been multiple previous surgeries. Staphylococcus epidermidis is the most common organism in most series(50).
 |
|
Table 109.9. Cure Rates for Infected TKRs
|
the patient may be treated according to the protocol a second time. In
their experience with 12 such patients, Backe et al. (5)
at the Hospital for Special Surgery elected to arthrodese and not
reimplant three of them. Infection did not return in the nine repeat
reimplantations and three fusions.
specific, controversial situation. Whether this represents a relatively
easy revision is debated, although there is agreement
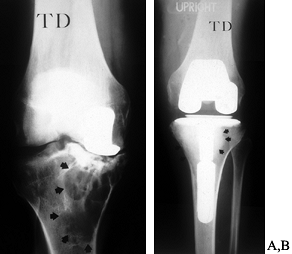
that the failed unicompartmental replacement cannot reliably be reconstructed with another unicompartmental prosthesis. Figure 109.16 shows revision of a unicompartmental replacement.
 |
|
Figure 109.16. Revision of a unicompartmental arthroplasty. A: Failed medial unicompartmental knee replacement in a 48-year-old male laborer. There is extensive osteolysis. B: Revision was performed with impaction grafting of a contained defect of the entire proximal medial tibial. Arrows indicate the extent of graft material. Closer to the keel, a thin layer of methacrylate cement can be seen.
|
have presented data supporting the idea that the unicompartmental
replacement may be used as a temporizing measure because it is an
easier revision. As such, it might be an option for the younger patient
who realizes that additional surgery is inevitable given a normal
lifespan. The original study has been updated with similar conclusions (99).
collaborating with colleagues from the Hospital for Special Surgery in
New York, have reported a different experience, and they feel that
revision of a failed unicompartmental replacement presents much the
same challenge as a failed tricompartmental replacement. They found
significant osseous defects in 76% of failed unicompartmental
replacements, with 62% of revisions exhibiting radiolucent lines. They
reject the concept that the unicompartmental replacement is a more
conservative surgery.
recommended for arthroplastic surgery in general, and this includes
revision procedures. Thorough medical evaluation is, of course,
essential, especially because the average age of revision patients is
even older than that of primary patients in most series (88).
The essential elements of planning, however, are the diagnosis of the
failure and a detailed mechanical plan to rectify the causes of the
failure (153).
It has virtually no role in the treatment of the infected knee
arthroplasty, despite occasional reports of short-term success (47).
Acute infections, at times amenable to open debridement with exchange
of the polyethylene, often ultimately require a two-stage
reimplantation protocol.
prior to manipulation of a stiff arthroplasty. This is not necessary
when the manipulation is done within 6–8 weeks after surgery. The
chronically stiff knee will generally require a well-planned complete
revision. Some benefit has been described from arthroscopic resection
of the posterior cruciate ligament (PCL) in the stiff cruciateretaining
arthroplasty. In one series, 10 knees gained an average of 40° of
flexion with this approach (169).
patellar fracture, catastrophic wear, and component loosening. In turn,
the tracking problems are generally the result of malrotation of the
femoral or tibial components that require full revision surgery (16).
Accordingly, arthroscopic intervention, limited to lateral patellar
retinacular release, is inadequate. Even proximal realignment, failing
as it does to correct the root cause of the tracking problem, will be
ineffectual.
has been identified most commonly in early designs of posterior
stabilized implants. The offending scar can easily be resected from the
quadriceps tendon arthroscopically, with complete resolution of
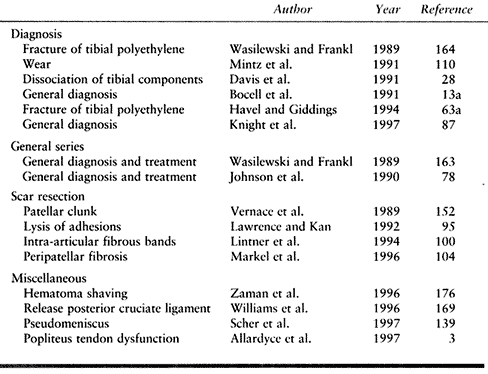
symptoms (152). Other surgeons, applying arthroscopy to the resection of a variety of bands and fibroses, have had mixed results (100,104). The literature on arthroscopic evaluation and treatment of the problematic knee arthroplasty is summarized in Table 109.10.
 |
|
Table 109.10. Role of Arthroscopy in the Failed Total Knee Arthroplasty
|
surgery but not revision of the components. Most problems, however, are
due to malposition of all components, and require complete revision.
Complete revision offers the best chance to set everything right, and
to ensure that compatible components are implanted. Techniques for
component removal and for fixation of new devices have markedly
improved. Complete removal is rarely as destructive as it previously
was, even in the worst situation in which a porous-coated component has
been well fixed with methacrylate cement (172).
inventory and providing surgeons with options during surgery. There was
also a hope that worn polyethylene components could simply be removed
from the baseplates without disrupting the fixation interface. Although
this is sometimes possible, bone may have been destroyed by osteolysis,
the components may have loosened, and defects may need reconstruction,
effectively eliminating simple polyethylene exchange as an option. In
addition, the environment that has led to catastrophic wear should not
be replicated. For example, thin polyethylene components or
malalignment must be corrected by revision, not modular exchange.
corrected by exchanging the polyethylene for a custom-manufactured,
angled bearing insert (Fig. 109.17). If the
prosthesis permits, exchange to a more constrained insert may restore
stability. The knee that is malaligned in valgus, for example, with a
correctable deformity, can benefit from a polyethylene tibial insert
that is manufactured on an angle so that it is thicker on the lateral
side (141).
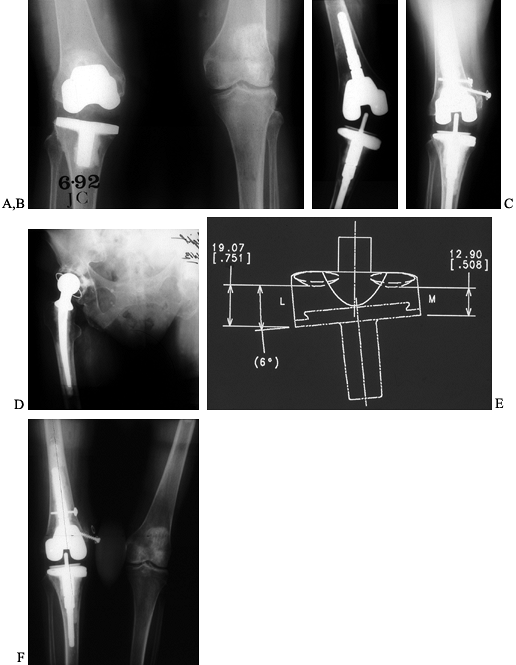 |
|
Figure 109.17. An 83-year-old patient with recurrent valgus instability in a primary revision and second revision. A:
AP radiograph of a primary knee arthroplasty in this patient, performed for osteoarthritis with valgus deformity where soft-tissue releases were not performed. The tibial component was placed in varus, and the femoral component in slightly excess valgus.B: AP radiograph of the failed first revision, where a constrained prosthesis was implanted. The femoral component was implanted with excess valgus (note the stem against the medial cortex). The tibial component had been extensively cemented and is also in valgus. The medial soft tissues failed despite the constrained component, largely because of malalignment. C: The patient underwent a second revision arthroplasty, which also failed. This AP radiograph shows that the femoral component was revised with full cement fixation of the stem extension and repositioning (note the femoral stem tip now against the lateral cortex) to reduce valgus alignment. A proximal advancement of the medial collateral ligament was performed. The tibial component was not revised because of the extensive cement fixation. Even the combination of decreased valgus alignment, lateral ligament release, medial ligament reconstruction, and constraint have not stabilized the knee. D: This situation was complicated because the patient had an older-model total hip arthroplasty on the same side as the unstable revision knee. The acetabular component is not medialized and the stem has a valgus neck–shaft angle. Accordingly, to restore a neutral mechanical axis to the arthroplasty below it, only 2° to 3° of valgus alignment will be required in the knee. Anything more imparts a large deforming force on the revision knee. E: Schematic diagram of a custom fabricated tibial insert with a 6° varus orientation. This allows the tibial angle to be corrected without removing the well-fixed tibial component. F: AP radiograph of a third revision knee arthroplasty. The femoral and tibial components have been left in place, but the angled tibial insert has been exchanged and the medial ligament has been reconstructed with vascular dacron. This arthroplasty has remained stable at a 5-year follow-up largely because of the reduction in the valgus deforming forces. |
tightness in flexion and extension) can often be improved by limited
revision surgery. Because the tibia has an equal effect on both flexion
and extension gaps, revision of just the tibial component or even
exchange of the tibial polyethylene alone may remedy the situation.
However, an unacceptably thin polyethylene insert should not be
inserted to increase motion. Revision of the tibial baseplate with
resection of additional tibial bone and soft-tissue releases is
preferred (156).
bone but without loosening of components. It is sometimes feasible to
graft these lesions with compacted particulate bone. This should be
accompanied by an attempt to eradicate the mechanical environment that
led to the failure in the first place; for example, at a minimum,
exchange the tibial polyethylene (41). When
there is any doubt as to the viability of the fixation, a full revision
is preferred. At that time, there will be superior access to bone
defects with better reconstruction possible. Figure 109.18 presents an illustrative case of reconstruction for osteolysis.
 |
|
Figure 109.18. Osteolysis treated with bone grafting. A:
An unusual case of osteolysis in a well-aligned posterior stabilized knee arthroplasty 6 years after implantation. Osteolytic lesions are apparent on either side of the central tibial keel. B: Lateral radiograph of posterior stabilized knee arthroplasty at 6 years, showing a large osteolytic lesion (arrows) directly under the tibial tubercle. C: Intraoperative photograph of large osteolytic cavity viewed from the medial side of the tibial tubercle. A flap of periosteum has been elevated from the tubercle. D: The osteolytic cavity was curetted and packed with particulate iliac crest bone autograft combined with allograft. This eventually failed when the tibial component became loose and then required revision knee arthroplasty. E: The removed modular posterior stabilized tibial insert showing very little wear. F: The patellar component showing more extensive wear on the lateral facet. |
in a knee arthroplasty. This is generally related to higher levels of
activity and can be expected to subside. At times,
this
may be persistent and bothersome. Some result from wear debris.
Debridement and synovectomy may prove beneficial, especially if the
tibial polyethylene insert is exchanged and the cause of the wear is
addressed.
knee. A series of 30 such patients has been evaluated by Kindsfater and
Scott at the Brigham Hospital in Boston (86).
They found that nine knees responded to conservative care alone, and
that open synovectomy was curative in 14 of 15 patients whose
hemarthroses recurred regularly. They attributed the bleeds to
entrapment of proliferative synovium or the fat pad between prosthetic
components.
that favor loosening over those that resist it. The most important
force resulting in loosening is generated by malalignment, most
commonly varus alignment of the tibial femoral axis. The medial side of
the tibia is overloaded, bone subsides, and the component is no longer
solidly attached to bone. The solution is to reestablish the desired
alignment of the limb. This is generally around 7° of valgus tibial
femoral angle, but more specifically it is a neutral mechanical axis,
as depicted by the line running from the center of the hip through the
knee and ankle.
loosening will also have bone defects that must be reconstructed.
Cement, particulate bone graft, modular prosthetic augmentations, and
large structural allografts all play a role. The surgeon will need to
anticipate these problems and plan to have the appropriate material
available.
the appropriate ligament balance performed. A knee that is in varus
prior to the primary procedure and then is implanted in varus is
unlikely to have had an adequate medial release. Conventional ligament
releases are appropriate for the majority of these cases. Increased
joint constraint and the more arcane ligament advancements and
reconstructions are usually not required.
generally be a need for enhanced fixation at the time of revision. This
will usually take the form of intramedullary stem extensions, which are
usually modular additions to the prosthesis. These may be long and
achieve three-point fixation inside the medullary canal, or they may be
of wider diameter with more of a press fit. In selected cases, such as
poor-quality bone where a constrained implant is planned, it may be
appropriate to fully cement the stem with techniques commonly used in
hip arthroplasty.
a porous ingrowth surface may no longer be amenable to uncemented
fixation. The same requirements in terms of correction of malalignment
and augmenting fixation are appropriate.
competition between two sets of forces, those that stabilize the knee
versus those that induce the instability. Instability occurs in an
anterior–posterior direction, or varus–valgus, or both.
usually dislocating posteriorly. This is usually because of failure to
achieve a balance between the flexion and extension gaps. A flexion gap
that is too large requires a polyethylene insert that is thicker than
can be accommodated in extension. Anterior tibial dislocation can also
occur in this situation, but it is observed less often because the
hamstring muscles pull the tibia posteriorly. Excessive posterior
tibial slope may drive the tibia forward, and anterior slope will
contribute to posterior instability. Assess the slope prior to surgery
and correct it during the revision.
It presents with instability, recurvatum, and synovitis around the PCL.
Late posterior instability has also been noted as a consequence of
progressive wear, which usually erodes the posterior articular surface
of the tibia and results in the femur “rolling off” the back of the
knee.
tighter in flexion. This is solved by inserting a larger revision
femoral component to decrease the size of the flexion gap. It is
misguided to simply insert a thicker tibial polyethylene insert,
because, although this may stabilize the knee in flexion, it will
create a flexion contracture. When the flexion gap is too large to be
stabilized by increasing the size of the femoral component, even in
conjunction with a posterior stabilized design, it may be necessary to
resort to a nonlinked constrained implant, with a higher tibial spine.
Ligament advancements, anteriorly on the distal femur, can selectively
tighten the knee in flexion (157). In addition,
tightening of the extensor mechanism with a distal advancement of the
vastus medialis will enhance the effect of the patella as a buttress
against the distal femur to prevent posterior tibial dislocation. When
a patellectomy is present, it may be necessary to perform this
advancement. In the patient with a patellectomy, posterior tibial
instability, and an extensor lag, implantation of an allograft extensor
mechanism may prove very useful.
from an incompetent or stretched-out MCL and valgus malalignment. The
malalignment generates huge forces that stress the soft tissues on the
medial side. Correction of alignment is an essential first step in
trying to stabilize these knees. All attempts at stabilization, whether
through the use of constraint in the prosthesis or soft-tissue
reconstruction, are doomed without the appropriate alignment.
to be reduced to less valgus when competence of the MCL is in question.
Even though this may risk overload of the medial tibial bone, and
loosening, it may be essential to decrease the valgus moment arm to
stabilize the knee.
carefully from full-length films that show the hip, knee, and ankle.
Special attention is required when the patient has a hip arthroplasty
in which the neck–shaft angle of the prosthesis is quite valgus, and
especially if the acetabular component has been implanted in a somewhat
lateral position. This patient may require as little as 2° of valgus
tibial femoral angle at the knee to achieve a neutral mechanical axis.
knee may paradoxically be responsible for instability if they
compromise alignment. These devices are commonly manufactured with a
fixed bearing angle between the femoral component and the
intramedullary stem extension that is required with most constrained
devices. The asymmetry of the tibia combined with the goal of inserting
larger-diameter intramedullary stems often results in up to 5° of
valgus at the tibial component. In addition, if the femoral stem
extension sits against the medial intramedullary wall, often the result
of deficient lateral femoral condylar bone, there may be up to 12° of
valgus in a knee that needs only 2°. The forces favoring valgus
instability are huge and can shred a collateral ligament or dislocate a
constrained implant. Correct alignment is paramount. No hinge can be
expected to stabilize a malaligned prosthesis.
stabilizing forces must be restored through intact collateral ligaments
or a constrained implant. When conventional releases are inadequate to
stabilize the implant, mechanical constraint, ligament advancement, or
ligamentous allograft reconstruction is required.
polyethylene to restore stability to a knee where the collateral
ligaments are either unbalanced or incompetent. Consider the MCL that
has undergone plastic failure with elongation. If the knee is
lengthened in an effort to equalize the lateral side with the
pathologically long medial side, as progressively thicker polyethylene
is inserted, the posterior structures remain intact and begin to limit
flexion. This ultimately creates a knee with persistent valgus
instability (due to failure of the MCL) in conjunction with a flexion
contracture.
healthy collateral ligaments. This usually is the result of loosening
and subsidence of the components. When they are restored to a normal
position, by reconstruction of bone defects and reestablishment of
fixation, the knee is stable. Apparent varus instability is often from
subsidence of the tibial component that protects the MCL. Instability
associated with patellar dislocation is associated with malrotation of
components. This must be corrected.
loosening of the components and rupture of the extensor mechanism, all
of which require treatment. Consider inherent soft-tissue deficiencies,
such as Ehler-Danlos syndrome, or neurologic diagnoses, such as polio
or spinal stenosis, which leave the patient with a weak quadriceps.
This causes a “back-kneed” gait in recurvatum, which accelerates
loosening. Hinges are sometimes considered in the treatment of the
unstable knee, but they are only very rarely required. Greater
attention to alignment, component size, and reconstruction of available
soft tissues is usually more successful.
The internally rotated femoral component has a lateral femoral trochlea
that is higher but more medial. The patella is likely to snap up and
over it during flexion. In addition, the articular groove will be
displaced medially, distant from the track the patellar needs to follow.
maltracking, which can lead to fracture of the bone or loosening of the
polyethylene button. Wear of metal-backed buttons is accelerated by
lateral tracking because the joint reaction force is focused on the
lateral side where the patellar polyethylene is likely to be thin.
Surgery for any patellar complication must consider the possibility of
maltracking as a cause. Complete revision arthroplasty may be necessary.
femoral component is the use of the posterolateral femoral component
augmentation, which drives the component out of internal rotation. The
epicondylar axis is the most reliable guide to proper femoral component
orientation.
Not knowing the cause of failure, the surgeon is unlikely to be able to
achieve a cure. Some pain is the result of problems unrelated to the
knee arthroplasty, such as referred pain from the spine or hip or
sympathetically mediated pain. Infection must be considered: Aspirate
the knee for cultures,
cell
count, and differential analysis. Internal rotation of the femoral
component, difficult to diagnose on plain radiographs, can easily be
quantified with CT. Discussion of such cases with an experienced
colleague will benefit surgeon and patient alike. A precise diagnosis
and mechanical plan will be necessary for all these patients.
be revised in such a way that the destructive environment is not
replicated. Complete revision is usually required to correct the
malalignment that may have been at the root of the problem, and to
ensure that adequate polyethylene thickness can be accommodated. In
this respect, the mechanical plan will resemble that for failure due to
loosening. Revision to an updated prosthetic design in which adequate
contact areas are provided to reduce wear is appropriate. The
inevitable areas of osteolysis require graft reconstruction.
Synovectomy is often important to eliminate wear particles and decrease
persistent swelling.
Open debridement of acute infections within 2 weeks of surgery or
within a short period after the presentation of an acute infection in
an established arthroplasty can be justified. Arthroscopic intervention
is less likely to be successful in curing the infection. One-stage
reimplantation is possible for the infected knee arthroplasty, but
clearly the risk of recurrent infection is higher with this approach.
method for the eradication of infection in knee arthroplasties. The
best results of two-stage protocols are obtained after appropriate
patient selection. Some infected knees, either because of the type of
organism, the condition of the soft tissues, or the condition of the
patient, inevitably lead to resection arthroplasty. Windsor et al. (171),
with Insall, reported 1 of 38 infected knees with a recurrence of the
same infecting organism at 4 to 10 years. Three other patients suffered
recurrence with different organisms attributed to a compromised immune
system. More recently, work from Cleveland identified specific risk
factors that are associated with relatively poor results from the
two-stage protocol (68).
prosthesis. Rather, it is a detailed and disciplined medical and
surgical approach to the problem that requires an aggressive
debridement of the infected knee joint. The original protocol of
leaving the knee devoid of foreign material has been supplanted by the
insertion of a PMMA spacer block, loaded with antibiotics. This
delivers high-dose local antibiotics, and it preserves tissue planes
for later total joint reimplantation. In an effort to maintain motion,
articulated spacers have been employed.
knee arthroplasty surgery, regarded by many as more difficult to treat
than sepsis. Repairs of the torn tissue are notoriously ineffectual,
but there has been a report of good results after transferring the
semitendinosus, attached to its origin, as a means of reestablishing
the integrity of the mechanism (17). Emerson et al. (39,40)
have employed extensor mechanism allografts and reported excellent
results. These grafts must be implanted with surprising tension, often
at the cost of some flexion, for the patient to regain useful extensor
strength. At the time of grafting, the tension of the extensor
reconstruction can be checked by lifting the thigh. Passive flexion
should be about 60°. Most patients will eventually gain motion of 90°
or more.
surgery in knee arthroplasty, it is essential to consider what factors
and forces may have led to the rupture initially. These must be
corrected. The most common constellation of problems are those that
increase the tensile forces in the extensor mechanism, such as an
oversized femoral component, malrotation, excessively thick patellar
construct, and so on. Figure 109.19 shows radiographs and intraoperative photographs of an extensor allograft.
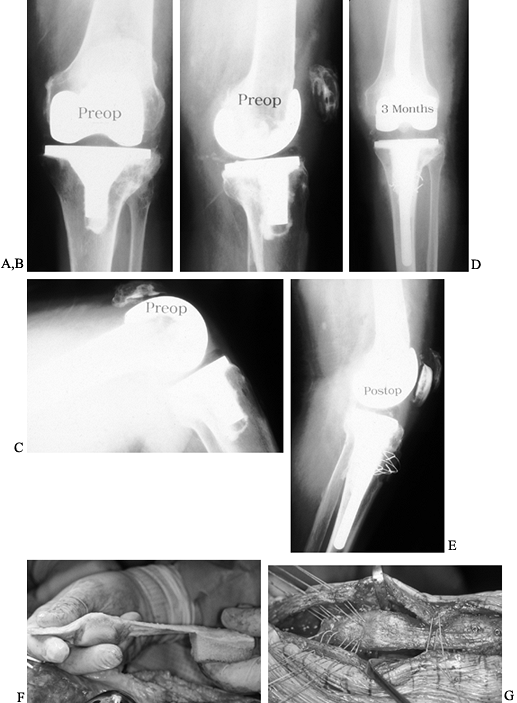 |
|
Figure 109.19. Extensor allograft. A:
AP radiograph of a failed total knee arthroplasty due to extensor mechanism rupture, poor motion, and malrotation in a 74-year-old woman with a contralateral above-knee amputation for congenital deformity. B: Lateral radiograph in extension showing an effusion and mild patella alta. C: Lateral radiograph in flexion confirming patellar tendon rupture. D: AP radiograph 3 months after revision surgery, with extensor mechanism allograft. E: Lateral radiograph showing extensor mechanism allograft with patellar component resurfacing, the host patella left in place, and wire cerclage fixation of the tubercle. F: Intraoperative photograph of an extensor mechanism allograft immediately prior to implantation, showing (from left) quadriceps tendon, patella, patellar tendon, and tibial tubercle. G: Intraoperative photograph showing alternative tibial tubercle fixation with two small fragment screws. The allograft is placed deep in the knee, directly against the components. The extensor mechanism is closed tightly, leaving only 45° to 60° of passive flexion intraoperatively. |
considered inappropriate for revision, as these knees often become
stiff because of the patient’s aggressive healing response. More
frequently, however, the scar that is observed in these knees is the
result of the stiffness and not the cause of the problem.
quickly after surgery, he is at risk for loss of motion and resultant
scarring. This includes patients who are unwilling or unable to comply
with physical therapy. Those whose pain is simply too severe after
surgery to allow movement of the knee will undoubtedly suffer
stiffness. Adequate analgesia and even readmission to the hospital for
epidural analgesia under which motion can be initiated may be
appropriate. Medical complications that make physical therapy
difficult, as well as wound problems or a hemarthrosis, predispose the
patient to stiffness.
that may preclude comfortable knee flexion, and treat the underlying
problem. After this is resolved, revision to increase motion may be
indicated.
associated pain and swelling. Aspiration is key to establishing the
diagnosis. RSD may be difficult to diagnose. Lumbar sympathetic blocks
are useful for diagnosis and treatment. These patients may respond to
revision to increase motion, performed under continuous epidural
anesthesia that is used for several days after surgery. Failure of bone
ingrowth may cause pain throughout the rehabilitation period that
precludes successful therapy. These patients benefit most often from
revision to a cemented implant.
these can be resolved during the revision to ensure that maximum motion
is achieved. Like instability, poor motion may be caused by poor
flexion, a flexion contracture, or global stiffness. The former two
require different approaches, and the last a combined approach.
are largely those that determine the dimensions of the flexion gap and
the tension in the extensor mechanism. Oversized femoral components or
those that are implanted too posteriorly make the knee tight and lead
to stiffness. The PCL that was tight and should have been released or
recessed at the time of primary surgery may need to be revised to a
cruciate substituting design. Tibias that have inadequate posterior
slope or, worse, anterior slope, require attention. Malrotation,
causing the components to bind and jam painfully with flexion, may be
responsible for poor motion. In addition, internal rotation of the
femoral component may predispose to dislocation of the patella. The
patient, sensing that a dislocation is about to occur as the knee
flexes, will resist flexing, much like a patellar apprehension sign.
This leads to scarring and the inability to flex.
become too thick as the result of resurfacing makes it difficult to
bend the knee. At the time of revision, this problem is easy to solve
because there is adequate bone for removal of the prosthesis, resection
of additional bone, and repeat resurfacing. Ultimately, the limiting
factor to the improvement of flexion will be the extensibility of the
quadriceps muscle. When poor flexion is chronic, it may be impossible
to gain motion, because the muscle itself is scarred and tight. In
selected cases, a Thompson quadricepsplasty may improve motion but at
the risk of sacrificing quadriceps strength (Fig. 109.20).
 |
|
Figure 109.20.
Thompson quadricepsplasty. Isolate the rectus femoris tendon from the vastus medialis and vastus lateralis and release the medial and lateral quadriceps retinaculum as necessary. Lyse adhesions in the knee and excise the intermedius muscle, which is scarred down to the femur. |
revision surgery. Exchanging the polyethylene for a thinner insert can
be dangerous, risking instability in flexion. Revision surgery is
preferred, with removal of the distal femur and aggressive posterior
soft-tissue releases (156).
treatment of fractures, but this can be a very successful approach.
Most common is a supracondylar femoral fracture. Most of these require
treatment with conventional plates or locked intramedullary rods
introduced either retrograde or antegrade (see Chapter 20).
At times, however, the distal femoral fragment may be highly comminuted
or osteoporotic. Revision with a distal femoral allograft will be
appropriate.
effort to correct arthroplasty alignment) that have not healed respond
well to revision of the tibial component with an intramedullary stem
extension across the fracture site.
prophylactic reconstruction of soft tissues. Tissue expanders have been
used successfully in knees with multiple previous scars and adherent
skin (54,102,115,137).
Surgical transfer of muscle has the added appeal of enhancing blood
supply to the compromised area; it can be performed prior to the
revision or after removal of an infected component but prior to
reimplantation. The gastrocnemius is the most easily transferred (52), but free flaps, although technically more difficult to transfer, have some added advantages (105).
Careful attention to detail in the release of scar and reconstruction
of the “gutters” along the intermuscular septae adjacent to the femur
are the secrets to a gentle patellar eversion without rupture of the
extensor mechanism. A lateral patellar retinacular release facilitates
exposure. More difficult cases may require an extension of the exposure
either proximally or distally. Tibial tubercle osteotomy frequently is
associated with complications (168,173).
Proximal soft-tissue releases are easily performed. A patellofemoral
“turn-down” is the most radical and is rarely required in even the most
difficult revisions (147).
Currently, a limited, very proximal transection of the quadriceps tendon, referred to as a snip, is favored (51).
More aggressive exposure of the distal femur, to the point of
skeletonizing, may be necessary for the stiff knee arthroplasty (1).
 |
|
Figure 109.21. Surgical approaches. A:
The classic patellar turn-down arthrotomy derived from the Coonse Adams surgical approach to the knee. Some surgeons have advocated its use as a means of lengthening the extensor mechanism to increase flexion. This may produce a disabling extensor lag. B: Intraoperative photograph showing the unparalleled exposure with a full patellar turn-down. This technique is rarely if ever required. C: The modified quadriceps snip approach. Many surgeons angle the proximal transverse cut from more distal on the medial side to more proximal on the lateral side. This maneuver may not be necessary if the approach is made slowly, with careful attention to detail and the release of scar. D: Intraoperative photograph of the closure of the quadriceps snip, showing the sutures transversely across the quadriceps tendon. Clearly, these alone will not be adequate to resist the tensile forces in the extensor mechanism. The strength of the closure comes from the standard side-to-side closure of the arthrotomy. E: Lateral radiograph of a revision knee arthroplasty, in which a tibial tubercle osteotomy has been performed and secured with cerclage wires. Placing these wires poses a danger to the neurovascular structures behind the knee. F: Intraoperative photograph of the tibial tubercle osteotomy demonstrating excellent exposure but with compromise to the cylinder of the tibia with potential compromise of tibial component fixation. |
Several techniques have been developed, all requiring patience. The
Gigli saw fits easily into the interface and was originally recommended
for the removal of well-fixed, uncemented implants. The saw, however,
may drift, in which case bone will be damaged (46). High-speed metal-cutting burrs have been employed to facilitate removal (4).
Slow advancement of narrow osteotomes, which may be stacked on top of
each other, is the classic technique. A reciprocating saw also works
well. Long intramedullary stems and porous-coated devices pose serious
challenges in the event removal is required and should not be implanted
with full cement fixation.
 |
|
Figure 109.22.
Component removal. The fixation interface must be disrupted completely to permit safe removal of components without bone loss. The reciprocating saw works well in this capacity, with better control than the Gigli saw. |
arthroplasty must follow a coherent program. The three-step technique
is a path that the surgeon can follow during revision knee arthroplasty
(Fig. 109.23). The cause of failure will dictate what is to be accomplished at each of the three stages (155).
 |
|
Figure 109.23. Three-step revision knee arthroplasty. A:
Step 1: Reconstruct the tibial platform. This establishes a foundation to use as a reference for all other work. The tibia is selected first, because its surface is always in contact with the femur regardless of the position of flexion. B: Step 2: Stabilize the knee in flexion by confirming the correct rotational position of the femur (step 2a), selecting the femoral component size that will stabilize the soft tissues in flexion (step 2b), and then selecting a combination of femur and tibial polyethylene that provides a reasonable joint line height (step 2c). C: Step 3: Stabilize the knee in extension by seating the femoral component either more proximally (only in rare cases of extreme flexion contracture) or more distally with the use of distal femoral augmentations or structural allografts. |
ascertain alignment and component position, are not generally useful.
Trial components, especially those attached to intramedullary stems,
are very useful. Even spacer blocks, used so widely in primary knee
arthroplasty, may be misleading in revision surgery where asymmetric
bone loss precludes meaningful insertion of blocks into the flexion and
extension gaps.
the femur, whether the knee is flexed or extended. By contrast, only
the posterior articular surface of the femur articulates in flexion,
and the distal surface is in contact with the tibia in extension. The
tibia serves as the foundation for the reconstruction of the knee joint
(Fig. 109.23A).
baseplate (but not the articulating surface) surface by inserting a
trial component. Bone-graft reconstruction of defects may be necessary.
Frequently, some sort of intramedullary stem extension to enhance
fixation is needed. Several good classification systems exist for bone
defects, which may be contained or noncontained, or they may be be a
massive structural defect (Table 109.11).
Contained defects can be managed with particular graft or even cement;
noncontained defects are best reconstructed with modular prosthetic
augments; and massive defects require allograft.
 |
|
Table 109.11. Classification and Treatment of Bone Defects
|
flexion, is when the most important work is done. Three important goals
must be satisfied in this step:
-
Step 2a: Determine the correct rotational position of the femoral component relative to osseous landmarks.
-
Step 2b: Choose the femoral component size and position it to stabilize the knee in flexion.
-
Step 2c: Pair the most appropriate
femoral component and tibial polyethylene component to place the joint
line at the desired level.
component on the femur is perhaps one of the most important steps in
knee arthroplasty surgery, although its importance has only recently
been appreciated. Most instrument systems for primary surgery easily
take their reference from the posterior articular condyles, but these
condyles do not exist at revision. Furthermore, although the articular
surface is easily appreciated in the primary and it usually resides in
a predictable relationship to the transepicondylar axis (103),
this is not always the case. There is agreement that the transverse
axis of the femoral component should correspond to the transepicondylar
axis (11,119).
with the distal femur anatomically to ensure comfort, central patellar
tracking, good motion, and stability. This is the key to restoring the
mechanics of the knee. The AP dimension of the component is the most
important dimension to consider, and it must be chosen according to the
tightness of the soft tissues about the knee. The femoral component
must be chosen to stabilize the knee in flexion and never to simply fit
the existing bone, which will be unpredictably smaller than normal. The
largest size that can be employed will reach the medial and lateral
sides of the distal femur—if something larger is required to stabilize
the flexed knee, then either the collateral ligaments have failed from
plastic deformation or there is significant bone loss from the upper
tibia that necessitates bone graft reconstruction.
size of femoral component—a larger component may do well with a thinner
tibial polyethylene insert, a smaller one with a thicker polyethylene
insert. The difference between these two will be the level of the joint
line. The location of the anatomic joint line proximal to the fibular
head can be predicted to be a portion of the width of the
transepicondylar axis (119). Assuming that the
patellar tendon has not been stretched or detached, this is a readily
available indicator of the desired joint line. If the joint line is
below the inferior pole of the patella, you can assume that the
reconstruction is near anatomic and that joint line height will not
compromise extensor mechanism function.
easy to extend the joint and seat the femoral component against the
tibial polyethylene insert to stabilize the knee in extension (Fig. 109.23C).
The relative position of the femoral component in the femur should be
maintained and modular augmentation blocks or distal femoral allograft
added to finalize the revision.
the flexion gap gapes open and cannot be stabilized with even the
largest femoral component and a very thick tibial insert, or the knee
may have varus or valgus instability in extension. In these
circumstances, decide whether to use constrained articulation that will
compensate for soft-tissue deficiency or to reconstruct the deficient
ligaments (90,157).
that tracks well and is compatible with the revision component, but
remove a loose polyethylene component or a well-fixed metal-backed
component. If adequate bone remains, do revision resurfacing with an
all-polyethylene device. If the bone is inadequate to accept a patellar
prosthesis, trim it to fit the femoral component and leave it
unresurfaced. Patellectomy is a poor choice because it leads to rupture
of the extensor mechanism. The scaphoid-shaped patella that results
from extensive osteolysis is a source of problems. It tends to hug the
lateral femoral condyle, risking dislocation, yet it is too thin to
either support a patellar implant or to be trimmed into a functional
piece of bone.
pole to the superior, from the inside of the patella. This leaves the
anterior soft tissues intact. The medial and lateral parts of the
patella can then be cracked “up” away from the knee into the shape of a
gull’s wing. This “gull-wing osteotomy” (Fig. 109.24) is used rarely, it does not disrupt the extensor mechanism, and it allows even a very
small fragment of bone to articulate well against the femur (154).
Small pieces of bone graft can be added through the osteotomy to the
ventral surface. These consolidate well with time. The technique for
the gull-wing osteotomy is shown in Figure 109.25.
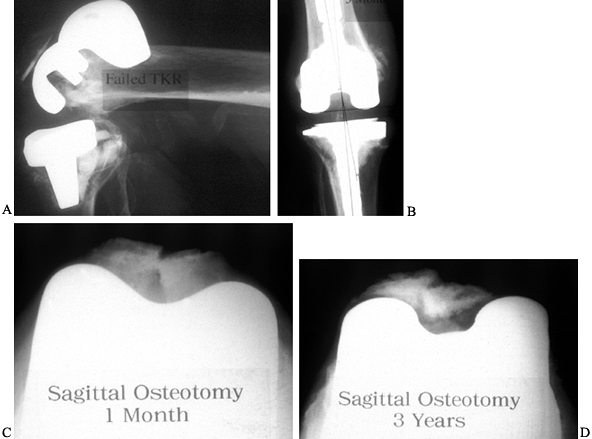 |
|
Figure 109.24. Gull-wing patellar osteotomy. A: Lateral radiograph of failed TKR. B:
AP radiograph of revision knee arthroplasty with stem extensions, compacted bone graft in contained tibial defects, and a (nonconstrained) posterior stabilized articulation. C: Sagittal gull-wing patellar osteotomy 1 month after revision for extensive patellar bone loss and a residual scaphoid shell of patella that would not track centrally and could not be resurfaced. Patellectomy risks extensor mechanism rupture. D: Gull-wing osteotomy with bone graft 3 years after revision arthroplasty, showing consolidation of medial and lateral fragments. |
 |
|
Figure 109.25. Gull-wing osteotomy technique. A: The normal patella should track centrally between the femoral condyles as viewed on a Merchant-type patellofemoral radiograph. B: The resurfaced patella is also expected to track centrally. C:
When very little bone is left because of osteolysis, the shell of bone often tracks laterally, hugging the lateral femoral condyle and risking dislocation. D: Plan a sagittal osteotomy, from the superior aspect of the patella to the inferior pole. E: With the patella everted, complete the osteotomy with a saw. F: Crack the medial and lateral halves of the patella ventrally and place a bone graft ventrally. G: This new gull-wing shape will track centrally between the two condyles. H: The everted patella, demonstrating where the osteotomy is planned. |
held in place by a tubular elastic fishnet gauze. This has the
advantage of being lightweight, allowing easy examination of the knee
after surgery without the uncomfortable lifting to wrap and unwrap
circular dressings. The small dressing seems to convey to the patient
that the knee is ready to use, a psychological factor that accelerates
rehabilitation. Immobilization is rarely advantageous except to allow
excessive swelling to subside. The goals of revision knee arthroplasty
should be to create a stable, mobile, well-fixed arthroplasty ready for
physical therapy (Fig. 109.26).
 |
|
Figure 109.26. A: Postoperative dressing as described in text. B: Completed dressing with comfortable elevation on a pillow.
|
extensor mechanism allograft. Although motion is important, it should
probably be passive, without proceeding past a point that risks rupture
of the mechanism. Use a dial-lock hinge brace, set to limit flexion to
about 60° initially, with additional droplocks to keep the knee
extended during ambulation. Let the patient bear weight to a
comfortable level. Implant structural allograft with sufficient
stability to allow early weight bearing. These grafts rarely become
stronger with time.
most common pitfall in revision knee arthroplasty. Failure to recognize
that malrotation may be at the root of many knee problems—all patellar
complications, the stiff knee, instability, and the painful “mystery
knee”—is relatively frequent. Regarding the revision as simply a repeat
primary, without acknowledging that special concepts, skills, implants,
and instrumentation are required, produces poor results. Depending on
instruments that rely on osseous landmarks that no longer exist does
not work well. Venturing to achieve a solid press fit inside the
asymmetric tibia or femur with stem extensions often leads to
malalignment.
reoperations after revision lie in the extensor mechanism. Femoral and
tibial component malrotation may be at the root of the problem, but
often bothersome extensor lags limit the function of the knee. This
problem has not been solved satisfactorily. Constrained components are
often overused in situations when nonconstrained implants would
suffice, and at times there may be too much reliance on constraint even
when it is needed. The constrained component will fail if the revision
is malaligned or if soft-tissue releases and reconstructions have been
ignored.
good. In the face of true mechanical failure, revision is indicated. It
should not be deferred if bone stock is being damaged by loosening,
infection, or osteolysis. In very destructive failures, with extensive
bone loss, keep in mind that revision knee arthroplasty is easier to
accomplish than a solid fusion.
failed arthroplasty, especially if there has been extensive bone loss.
The best technique is difficult to discern, given the relatively few
cases that require this treatment. The subject has been reviewed
extensively (27,89). The preferred method, in terms of the mechanics, is undoubtedly the intramedullary nail (29,38,63,80,93,120) (Fig. 109.27).
Unfortunately, the knee that is likely to be considered for arthrodesis
is the failed, infected arthroplasty for which there is concern over
dissemination of the infection inside the bone with an intramedullary
device. External fixators have been used (61,127).
Plating would seem to have little to recommend it because it lacks the
mechanical advantages of the intramedullary rod and yet still
introduces metal into an area of previous sepsis (116).
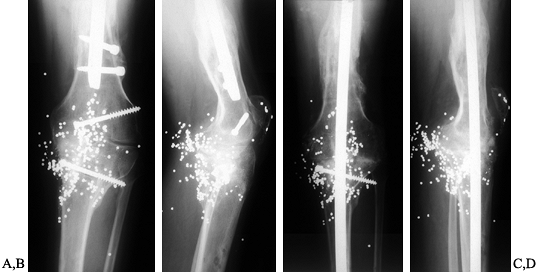 |
|
Figure 109.27. Arthrodesis. AP (A) and lateral (B)
radiographs of posttraumatic arthritis of the knee after a gunshot wound and femur fracture. The knee was stiff, painful, and deformed. AP (C) and lateral (D) radiographs of knee arthrodesis with a long intramedullary rod. The anterior bow has been rotated to create a few degrees of valgus alignment. |
incurable infection or perceived impossibility of bone reconstitution,
may simply be left as it is, producing a resection arthroplasty. The
functioning capability of these patients is usually poor (43,81,146).
Chronic infection that is active and a threat to the patient’s general
health, and that has not responded to multiple surgeries, is the most
common indication for amputation. Bone loss alone, unless colossal, can
usually be reconstituted in the healthy patient with allograft, as can
the extensor mechanism. Indeed, combined proximal tibial allografts
with the extensor mechanism included may be a solution.
that the first reported arthrodesis of the knee was performed by
Professor Albert of Vienna in 1878 for instability of the knee due to
residual of poliomyelitis. Fusion of the knee was the only surgical
solution for a painful or unstable knee until the introduction of
arthroplasty of the knee joint in the last quarter of the 20th century.
Hibbs treated tuberculosis of the knee with arthrodesis in 1911 (66,67). Key (83) first used external fixation with compression for arthrodesis of the knee in 1932, and in 1937 (84) he used a large central autogenous bone peg to achieve fusion of the knee. Charnley (21)
refined the technique of arthrodesis with compression using external
fixation in 1948, and the general principles he established continue to
be used for arthrodesis utilizing external fixation (21,22 and 23).
the greater trochanter across the knee into the tibia. Chapchal (20)
modified Küntscher’s technique by inserting the nail through an
anterior window in the femur, obtaining fusion in 85% of his patients.
Because of the risk of fracture at the cortical window, the latter
technique has never become popular and intramedullary nailing for
arthrodesis of the knee today is modeled after the original technique
described Küntscher. Fusion of the neuropathic knee is difficult. Lucas
and Murray (101) described arthrodesis of the knee by double-plate fixation in 1961, demonstrating that fusion of a Charcot knee was feasible.
used only when other alternatives, such as total knee arthroplasty,
cannot resolve problems of instability and/or pain in the knee caused
by disabling arthritis, infection, or other disorders. A knee fusion is
quite disabling, as it eliminates the most important joint for lower
extremity function. Arthrodesis of the ankle or hip produces almost
imperceptible changes in gait, whereas fusion of the knee produces an
obvious stiff-legged gait with significant increases in energy
expenditure in walking. Climbing or descending stairs and walking on
hills or rough terrain are always a problem. Patients with arthrodesis
of the knee have difficulty using public transportation because of the
need to bend the knee to sit. They must usually sit on the aisle in
theaters and may have difficulty getting up after falling. Dressing is
more difficult. Patients must be warned about these problems prior to
arthrodesis of their knees. Patients benefit preoperatively by
immobilization of the knee in a cast or brace so they will have a
realistic picture of what their function will be like after
arthrodesis. Satisfaction rates of 80% to 90% are reported in most
series as discussed later, primarily because the arthrodesis usually
eliminates the pain and instability that necessitated the procedure (135).
-
Postinfectious destruction of the knee
-
Posttraumatic destruction of the knee in a young, active individual who desires to continue vigorous activity
-
Paralytic conditions with severe deformity
-
Neuropathic arthropathy
-
Occasionally in malignant conditions about the knee
-
After failed arthroplasty, when an attempt at revision arthroplasty is unwise for the patient or not possible
years, but the risk of growth arrest by surgical procedures around the
physes is significant. If possible, fusion should be delayed (by the
use of braces and crutches) until full growth has been obtained.
the use of compression, either by external fixation, or by internal
fixation with the addition of load bearing when possible. This
increases the stability and area of bone contact.
a 78% rate of fusion when noncompression arthrodesis (resection of the
joint plus internal fixation with or without bone graft) was used and a
failure of fusion when crossed Steinmann pins were used.
spontaneous fusion occurs in a poor position. Deformity is often severe
after the disease has subsided, and the extremity is usually fixed in
flexion, abduction, and external rotation. Also, the tibia is
frequently subluxated or dislocated on the femur. If the deformity is
mild, enough bone can be removed from the condyles of the tibia and
femur to allow approximation of the osseous surfaces in the most
anatomic and functional position. If the deformity is severe, however,
capsulotomy, as well as other soft-tissue releases, may be necessary
before the bones can be approximated and fused in the most functional
position.
because of the resection of the joint surfaces as well as the
positioning of the fusion in 10° to 15° of flexion. Shortening of up to
2 cm is of some benefit, in that it allows better clearance of the foot
during walking, assists in dressing, and relieves tension in the
hamstring tendons and sciatic nerve. Shortening of more than 2.5 cm may
require the use of a small shoe lift, but the entire leg-length
discrepancy should not be compensated for.
Principles for obtaining arthrodesis in the presence of infections
include thorough debridement of all infected and necrotic tissues,
stable external fixation, and good postoperative drainage of the wound.
Staged surgery is usually advisable, with initial eradication of the
infection followed by fusion. If the wound is left open at the time of
arthrodesis, drainage usually ceases when the fusion becomes solid (26).
performed in children. There may be partial destruction of one condyle
without destruction of the other. It is important to excise all the
tuberculous material and necrotic bone and to administer combined
antimicrobial therapy (see Chapter 176). Soft-tissue releases also may be indicated.
remove all implant materials and necrotic bone before fusing the knee.
Knee fusions after failed unicompartmental arthroplasties have a
relatively high fusion rate (about 90%), whereas fusions after TKRs are
difficult, with a
first-time rate of fusion of only 50% (89).
Staged surgery is advisable when gross pus is present around the
prosthesis. First remove the prosthesis, bone cement, and necrotic
tissue. Treat the infection with intravenous antibiotics. Consider
using an antibiotic-impregnated spacer or beads. After the acute
infection has resolved, perform an arthrodesis, usually with bone graft
and external fixation.
is the procedure of choice. There is an occasional young adult,
particularly a young man who desires to continue working at heavy
labor, for whom an arthrodesis, despite its functional limitations, may
be the best choice in view of the fact that a total knee arthroplasty
would not be expected to last for very many years under these
circumstances. The alternative would be to perform a total knee joint
arthroplasty, recognizing that if the knee joint and subsequent
revisions were to fail, arthrodesis would be necessary. As discussed
later in this chapter, arthrodesis following failed total joint
arthroplasty has a lower success rate than primary knee fusion, and it
usually does not give as good a functional result, as substantial
shortening may be necessary. Treatment of hemophilic arthropathy, which
may require fusion, is discussed in Chapter 136.
complete debridement of joint surfaces down to viable bleeding bone,
and thorough debridement of the joint detritus including complete
synovectomy (31). Supplemental autologous bone
graft may be indicated. Solid fixation of the arthrodesis with biplanar
external fixation, intramedullary nailing and compression, or
double-plate fixation is necessary until solid radiographic union is
evident. Intramedullary nailing, when feasible, is probably the
fixation of choice, as a nail is not as susceptible to fatigue failure
in bending as plates are, and the problem of loosening of external
fixation pins in osteoporotic bone is avoided. Union rates of 100% have
been described using intramedullary fixation (31,151). Double-plate fixation (101) works well, but weight bearing must be avoided and external support utilized until solid fusion is obtained.
-
In the AP plane, place the knee in
anatomic position relative to the opposite normal extremity (5° to 7°
of valgus), and in about 10° of external rotation. The position of
flexion and extension depends on relative leg lengths and other
coexisting problems, such as muscle paralysis. With only knee disease,
arthrodesis in 10° to 15° of flexion allows clearance of the foot when
walking and is the most efficient position for walking. However, with
bone loss of more than 2.0 cm, fusion in full extension may be
indicated to gain more length.
who developed the Charnley clamp, which is used as an initial
compression device followed by a plaster cast. Charnley and Lowe (23)
reported a 98.8% fusion rate in 171 patients treated with an average of
9 weeks of external fixation and a plaster cast. Stewart and Bland (143) obtained solid fusion in 100% of 30 knees that were externally fixed for an average of 15.5 weeks.
External fixation is the best technique for previously infected knees,
because it avoids implants in the infected bone. Pin track infections
and fixation pin failure are disadvantages of external fixation. Green
and Kolsnik (56) reported successful fusions in 13 of 16 patients using external fixators, but pin track infections occurred in six.
-
Position the patient supine on a
radiolucent operating table and prepare and drape the entire affected
extremity. A sterile tourniquet is useful to control hemorrhage. -
Through a long anterior median parapatellar incision, reflect the patella laterally, and expose the knee joint.
-
If necessary, perform a thorough
debridement of the knee, excising all nonviable bone and detritus, and
perform a synovectomy, particularly if fusing a neuropathic knee or a
knee that was previously infected. Send the debrided deep tissues for
culture and sensitivities. Debride the articular surfaces of the tibia,
femur, and patella down to bleeding subchondral bone. -
Using the alignment jig system from any standard total knee arthroplasty system (Fig. 109.28),
make the distal femoral and proximal tibial cuts to obtain 0° to 15° of
flexion, 5° to 7° of valgus, and 10° of external rotation. Shape the
underside of the patella to match the patella to the denuded
patellofemoral groove. The patella can also be excised.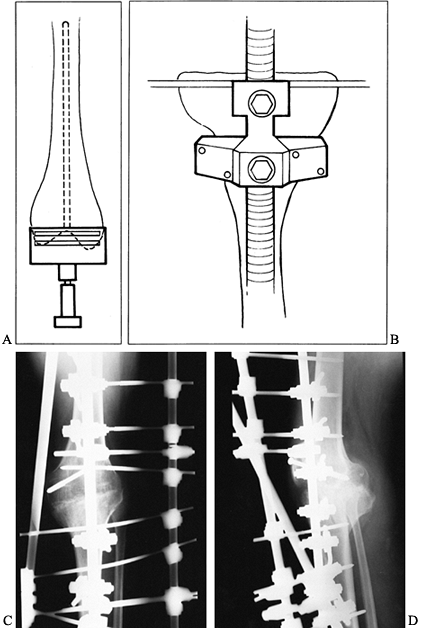 Figure 109.28. Knee arthrodesis with external fixation. A: Cut the distal femur using a guide from a total knee replacement guide system. B: Make a proximal tibial cut using a total knee replacement guide system. C: Apply a biplanar external fixator to compress the two surfaces. D: Denude the patellofemoral surfaces, and fix the patella to the anterior femoral surface using two screws.
Figure 109.28. Knee arthrodesis with external fixation. A: Cut the distal femur using a guide from a total knee replacement guide system. B: Make a proximal tibial cut using a total knee replacement guide system. C: Apply a biplanar external fixator to compress the two surfaces. D: Denude the patellofemoral surfaces, and fix the patella to the anterior femoral surface using two screws. -
If difficulty in obtaining fusion is
anticipated, obtain bone graft from the iliac crest, and cover all
contact surfaces with morcelized iliac crest autograft. Bring the joint
surfaces together in proper alignment, and apply the external fixator.
One or two large Steinmann pins
P.2939P.2940
inserted across the knee are often useful to maintain position while the fixator is applied. -
The technique of application of the
external fixator depends on the type of fixator. Follow the
manufacturer’s instructions. Various external fixation devices can be
used (Fig. 109.29). Follow the basic principles for application of external fixators as discussed in Chapter 11. Circular fixators using Ilizarov principles can also be used, as discussed in Chapter 32.
If a single-plane external fixator is used, such as the Hoffmann,
Charnley, and Wagner devices, it is critical that a minimum of two of
the thickest transfixation pins possible be placed proximally and two
distally, and that compression be applied. Under fluoroscopic control,
insert the most proximal transverse fixation pin in the femur and the
most distal pin in the tibia first, utilizing a template or the
external fixator itself as a guide. Be certain that these pins are
oriented so that when compression is applied, the arthrodesis site will
be evenly compressed without a tendency toward displacement. Once you
are happy with the position of the fixator, and with its mechanics,
insert the additional transverse pins in the tibia and femur. Using a
minimum of four pins will limit any tendency for the arthrodesis site
to shift due to a parallelogram effect. Most surgeons use at least six
fixation pins in total.![]() Figure 109.29. Various types of external fixation systems used to arthrodese the knee.
Figure 109.29. Various types of external fixation systems used to arthrodese the knee. -
If the patient’s bone is osteoporotic,
prolonged external fixation is anticipated, or the arthrodesis is
difficult (as in a neuropathic knee or after failed total knee
arthroplasty, where interposition bone grafts may be utilized), then
biplanar or multiplanar fixation may be indicated. This can be achieved
with the Ace-Fisher device (Fig. 109.29), or
with a circular Ilizarov frame utilizing full and half-pins. If the
uniplanar devices are used in the frontal plane, adding an independent
fixator with half-pins placed in the anterior to posterior direction,
which then can be cross-connected to form a Delta frame, works equally
well. This type of fixation utilizing an AO fixator (Synthes, Paoli,
PA) is shown in Figure 109.28C, Figure 109.28D. -
Next, fix the patella, if it has been saved, into the anterior femoral groove with two oblique lag screws.
-
Secure hemostasis, place a deep drain, and close the quadriceps mechanism and skin. Apply a soft compression dressing.
surgery with assistive devices bearing the weight of the leg
(approximately 30–40 pounds), assuming there is good bone contact and
compression across the arthrodesis site and the patient’s bone quality
is good. Initiate routine care of the external fixator pin sites as
described in Chapter 11. Have the patient
check the components of the external fixator daily to be certain that
loosening does not occur. Apply a modified elastic stocking and have
the patient elevate the extremity when not walking to minimize edema.
Once the wound has healed, the patient can usually be seen at 6-week
intervals until fusion occurs.
radiographically fused on the immediate postoperative radiographs
because of the excellent apposition and compression across the broad
bone surfaces between the femur and tibia. Although solid union of an
arthrodesis in our hands has occurred as early as 6–8 weeks, I
recommend leaving the external fixator in place until at least 12 weeks
unless pin problems require earlier removal of a portion or all of the
external fixator frame and pins. When radiographic
and
clinical union is evident, remove the external fixator and apply a
removable long leg orthosis (some prefer a cylinder cast) and allow the
patient to bear full weight for an additional 4 weeks. Then it is
usually possible to allow the patient to resume normal activities
without external protection. In the arthrodesis of neuropathic joints,
or in situations where intercalary bone graft has been utilized to
maintain leg length (such as after failure of knee arthroplasty),
prolonged external protection may be necessary to ensure that the
arthrodesis does not fail.
arthrodesis has the advantages of more limited soft-tissue dissection,
the potential for immediate weight bearing, an absence of pin track
complications, easier rehabilitation, and a high rate of fusion.
Intramedullary nailing is particularly useful when bone loss at the
knee does not permit compression fixation, and where large allografts
or autographs have been used to bridge an area of bone deficiency (such
as following tumor resection or failed total knee arthroplasty). The
major disadvantages are that nailing can be technically challenging,
additional blood loss from the medullary canals can be a problem, and
it is more difficult to obtain optimal alignment.
reported 100% success in his series. In difficult cases, a combination
of intramedullary rods and an external fixator has been used (42).
intramedullary nail fixation for knee arthrodesis to external fixation;
with 13 knees in each group, they had a 100% union rate with
intramedullary nails and only a 38% union rate with external fixation
following failed TKR. Because of the bone loss associated with failed
TKR, intramedullary nailing appears to have a distinct advantage.
Ellingsen and Rand (38) arthrodesed 18 knees
after failed total knee arthroplasty (nine had had failed attempts at
arthrodesis using external fixation). They were successful in 16 of 18
(89%) knees. They pointed out, however, that even though intramedullary
nail arthrodesis has a high success rate for salvage of failed total
knee arthroplasty, the surgical technique is technically demanding and
lengthy, and complications are frequent (occurring in 10 of the 18
knees).
knee requiring radical resection are generally treated with a total
knee arthroplasty. However, arthrodesis using intramedullary nails with
intercalary allografts (151,165), or nailing combined with a vascularized fibular bone graft (130), has been successful when arthroplasty is contraindicated or not feasible.
arthrodesis of the knee, but the weakness of this nail and the
inability to lock it has limited its usefulness. Current locked
intramedullary nailing systems, especially those specifically designed
for arthrodesis of the knee such as the Knee Fusion Nail (Smith and
Nephew, Memphis, TN) and special modular nails make intramedullary
arthrodesis technically easier and permit maintenance of length or
compression across the arthrodesis site utilizing the interlocking
screws. These special nails offer different diameters for the femur and
tibia to match the intramedullary anatomy. In nonmodular designs, the
nail must be inserted from the usual entry site in the pyriform fossa
proximally on the femur. Most authors described inserting the nail
through the isthmus of the tibia to within 2–6 cm of the ankle joint.
Recent experience, however, with newer interlocking intramedullary
nails has shown that the nail in the tibia need be inserted only down
to the isthmus where good stability can be obtained by cross locking
the nail (personal communication, MW Chapman, 2000). This makes the
nailing technically easier to do.
failed total knee arthroplasty, the following technique is described
for locked intramedullary nail arthrodesis following failed knee
arthroplasty.
-
Preoperatively, take full-length AP and
lateral radiographs of the involved extremity to include the hip joint
and ankle. On these radiographs, carefully inspect the medullary cavity
of both bones to be certain that they will accommodate the nail to be
used. Use appropriate templates to determine the length and diameter of
the nail required. In most cases, the nail should extend from the tip
of the greater trochanter of the femur to the isthmus of the tibia. If
there are problems in the tibia or osteoporosis, plan to have the nail
extend down to within 2–3 cm of the ankle joint. Average-sized
individuals will require an intramedullary nail 50 cm or more in
length, which usually must be ordered from the manufacturer ahead of
time (hospitals rarely stock nails this long). The diameter of nail
used depends on the size of the bones and the condition being treated.
Try to use a nail that is 11 mm or more in diameter in order to have
sufficient strength. -
These procedures tend to be rather long
and require significant manipulation and moving of the patient, so a
general anesthetic is usually recommended. Positioning of the patient
is challenging: A direct anterior approach to the knee and a posterior
or lateral approach
P.2942
to
the buttock are both necessary to accomplish the procedure. The patient
can be placed in the supine position on a fracture table, which
facilitates imaging, but I prefer the supine position on a fully
radiolucent operating table. Place a bump under the buttock and
position the patient so that the incision required to insert the nail
through the buttock can be safely made. To improve exposure of the
buttock, it is sometimes useful to place the patient in the lateral
decubitus position, prepare and drape the entire lower extremity and
buttock, and then roll her into the supine position for the majority of
the procedure. She then can be rolled partially toward the lateral
decubitus position as necessary for the insertion of the nail in the
buttock. A sterile tourniquet can be used on the proximal thigh during
the debridement of the knee to limit hemorrhage, but this is not
desirable during the nailing. -
Expose the entire knee joint through an
anterior longitudinal median parapatellar incision, utilizing the old
surgical scar in most cases. -
Once the distal femur, proximal tibia,
and total knee joint are exposed, mark the femur with an osteotome or
electrocautery to provide reference lines for determining rotation and
length after the prosthesis is removed. -
Utilizing the instrumentation appropriate
for the total knee, remove all total knee components, and cement.
Remove all debris from the soft tissues and bone, preserving as much
viable bone stock as possible. If the patella has decent bone stock,
preserve it for use as a bone graft; otherwise, excise it. -
Submit debrided bone for culture and
sensitivities, and initiate intravenous antibiotics. If the culture
proves to be positive, prolonged treatment with intravenous antibiotics
may be indicated. -
In most cases, it is now possible to
place the rough ends of the tibia and femur into apposition. Their
irregular surfaces often provide a good interlock. The driving of the
intramedullary nail automatically determines alignment in both the
anteroposterior and lateral planes. Be certain that the foot is at the
proper progression angle, which is usually about 10° of external
rotation. -
Asymmetrical loss of bone may limit bone
contact and require recutting of the joint surfaces with total knee
instrumentation to obtain good bone apposition. Although this may be
necessary, it is not generally recommended, as it results in excessive
shortening. -
Insert a ball-tipped reaming guide pin
into the medullary canal of the femur. In most cases, this is best done
through a short buttock incision using the standard technique for
intramedullary nailing of the femur (see Chapter 11 and Chapter 20).
An alternative technique that allows percutaneous insertion of the nail
into the proximal femur is to run a sharp-tipped, flagged Küntscher
guide pin retrograde up the medullary canal to exit the proximal femur
and come to lie beneath the skin in the buttock. To avoid injury to the
sciatic nerve, and to have an appropriate skin incision, it is
essential to flex and adduct the hip so that the guide pin exits the
skin as close to the tip of the greater trochanter as possible. It is
important to visualize this carefully on the fluoroscope, so that the
guide pin exits at the pyriform fossa on the femur. An incision 2–3 cm
long can then be made on the buttock in line with the fibers of the
gluteus maximus to expose the tip of the guide pin. Then use a
guide-pin exchange tube to exchange the Küntscher guide pin for a
ball-tipped reaming guide pin. -
Ream the medullary canal of the femur to
obtain a diameter 2 mm wider than that of the nail to be used. This can
be done antegrade or retrograde. -
Ream the medullary canal of the tibia
over a ball-tipped guide pin antegrade from the knee joint. Ream
through the isthmus so that passage of the nail will not be impeded if
the length has been mismeasured. -
Save contents of the medullary canal and bone reamings for bone graft around the arthrodesis.
-
With the knee in the appropriate position
for arthrodesis, insert the femoral guide pin into the tibia and
measure to be certain that the appropriate-length nail has been
selected. -
Drive the medullary nail antegrade down
the femur, taking care to avoid fracture of the femur or incarceration
of the nail. The nail should move down the femur easily with each blow
of the mallet. If significant resistant is encountered, inspect the
nail and femur carefully under fluoroscopy to be certain the
orientation of the nail is correct. Ream the medullary canal larger if
necessary. Drive the nail until it protrudes from the distal end of the
femur about 2 cm. -
Now position the tibia over the end of
the nail in apposition with the femur and using the previously drawn
reference lines be certain that the position of the arthrodesis is
correct. The overall curvature of the nail will generally produce about
10° to 15° of flexion in the knee fusion. Position the tibia for proper
valgus and rotation. -
Complete the driving of the medullary
nail into the tibia. As in the femur, the nails should drive easily
with minimal resistance. -
Ensure good compression across the
arthrodesis site by placing a small drill hole in the femur and another
one in the tibia and inserting into these the tines of a pointed
fracture-reduction forceps, which then can be used to compress the
arthrodesis site. Often, two are needed, one anteriorly and one
laterally. -
Place two transverse locking screws
through the distal end of the nail in the tibia, and lock the nail in
the proximal femur using the jigs provided by the manufacturer. -
Close the wounds in layers over a suction
drain and apply a sterile dressing incorporating the extremity with a
soft compression dressing from the toes to the groin.
postoperatively. When the bone quality is good, and if the arthrodesis
site has excellent contact and is in compression, immediate weight
bearing with assistive devices is usually possible. Where there is
osteoporosis or poor bone contact at the arthrodesis site, limit weight
bearing to the weight of the leg until fusion occurs. Solid fusion is
usually evident at 12–16 weeks, at which time the use of ambulatory
aids can be discontinued. External immobilization of any type is
usually unnecessary. See Figure 109.27 for a typical case.
knee arthrodesis using anterior and medial plates resulting in
successful fusion in 17 of 18 knees. This fusion is useful in difficult
situations, such as Charcot’s joints and where significant bone loss
between the femur and tibia requires large bone grafts. These may be
autografts from the iliac crest, sliding grafts from the anterior femur
or tibia, or large allografts. The strong dual plates are necessary to
protect the graft during healing and remodeling (Fig. 109.30, Fig. 109.31).
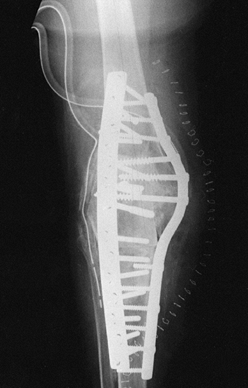 |
|
Figure 109.30.
Plate fixation of a large allograft placed between the femur and tibia. Protection of the bone graft by the plate is needed in this type of fusion for many years until the allograft has been incorporated. |
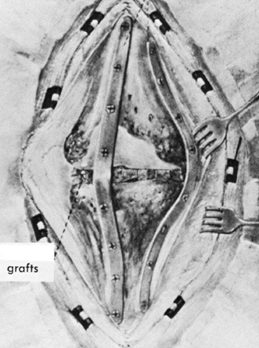 |
|
Figure 109.31.
Double-plate fixation for the fusion of a neuropathic joint. (From Lucas DB, Murray WR. Arthrodesis of the Knee by Double Plating. J Bone Joint Surg Am 1961;43:795, with permission.) |
medial and lateral dynamic compression plates to successfully fuse 11
knees. Although medial and lateral plates are not biomechanically as
strong as two plates at 90° to each other, these authors felt that
eliminating the anterior plate reduced the incidence of wound problems.
In addition, they suggested using plates of two different lengths to
avoid the stress riser that might occur at the end of two plates of
equal length.
avoids the pin complications of external fixation. Also it is easier
for the patient during the postoperative period. When compared to
intramedullary nailing, it requires less surgical exposure and less
surgery time, and it is technically easier for most surgeons. It is
particularly useful when large intercalary bone grafts are used, as
better fixation and better bone apposition is usually possible. The
major problem with plate fixation is the increased incidence of
soft-tissue problems that result from the bulk of the implants at the
knee and their position immediately beneath the skin flaps. There is a
risk of fracture of the tibia or femur at the end of the plates, which
is more likely with a fused knee.
the combination of intramedullary nail and medial compression plate
fixation in eight difficult knees, all of which united. They recommend
their technique for difficult salvage situations, when segmental
allografts may be required.
-
Place the patient in a supine position on
a radiolucent operating table, and prepare and drape the extremity from
the toes to the groin. Prepare the iliac crest if bone graft is
required. -
Make a long longitudinal median
parapatellar incision to expose the knee joint. Incise the periosteum
in a similar line on both the femur and the tibia, and expose the
distal femur and proximal tibia subperiosteally. -
Cut a V in the anterior femur to accept
the patella, and cut a similar V underneath the patella to create a
good fit with the depression in the anterior femur. The patella can
also be excised. -
Place the knee arthrodesis in appropriate
position and temporarily fix the two bones with crossed Steinmann pins.
Use templates to establish the contours for anterior and medial, large,
broad plates and use plate benders to fit them to the anterior and
medial surfaces. -
Use 12- to 16-hole plates and secure the
plates to the femur and tibia with at least five bicortical screws
through each plate in each fragment using compression techniques. -
If the patella is retained, fix it to the femur with two screws.
-
Pack any defects of the joint margins with cancellous bone from the iliac crest.
-
Close the wounds in layers over a drain and apply a soft supportive dressing.
allow touch-down weight-bearing with crutches until 12 weeks, when
partial weight-bearing is begun if the radiographs demonstrate evidence
of early union. Full healing on radiographs is usually evident by 16–24
weeks, at which time full weight-bearing is possible.
same as those of any major orthopaedic surgery for complicated
conditions. As noted previously, the incidence of complications is
significant, as knee fusion is usually a salvage procedure for multiple
failed surgeries of the knee, for severe infection, or for bone loss
resulting from tumor resection. A problem with knee arthrodesis using
external fixation is loosening or infection of the fixation pins
followed by nonunion of the arthrodesis. The pin problems can be
minimized using the meticulous technique described in Chapter 11, and by avoiding full weight-bearing until solid arthrodesis is present.
biplanar or multiplanar fixation utilizing compression applied across
broad viable bone surfaces at the site of arthrodesis. Excessive
shortening may be unavoidable, but it can be minimized by avoiding
unnecessary resection when the arthrodesis follows failed total knee
arthroplasty, and by using intercalary bone grafts when necessary.
Another approach is to perform limb lengthening with Ilizarov
techniques after successful knee fusion.
staged arthrodesis when fusion is being performed for infection or when
the suspicion of infection is high. If infection occurs in the presence
of stable fixation, then irrigation and debridement, with maintenance
of the fixation, combined with appropriate intravenous antibiotics,
will often control the infection until fusion occurs. Once fusion is
present, the infection may spontaneously resolve, or removal of the
implants will then result in the resolution of the infection. Infection
in the presence of an unstable arthrodesis and implants usually
requires the removal of the implants, irrigation and debridement, and
treatment with antibiotics, followed by repeat fusion when the
infection has completely resolved, usually utilizing multiplanar
external fixation.
practice, and in that of most surgeons, is failed total knee
arthroplasty. My preferred technique for this is fusion with
intramedullary fixation. I reserve external fixation arthrodesis for
primary arthrodesis when there is infection, and where infection has
been a significant problem
in
a failed total knee arthroplasty. Double-plate arthrodesis of the knee
is most common in the practices of surgeons treating malignant tumors
of the knee that are not amenable to total joint arthroplasty and
require intercalary allograft for reconstruction. This is not a common
procedure in my practice, nor in the practice of most community
surgeons, so careful attention to the details outlined in this chapter
are necessary for a high rate of success.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
BE, et al. The Effect of a Stem on the Tibial Component of Knee
Arthroplasty. A Roentgen Stereophotogrammetic Study of Uncemented
Tibial Components in the Freeman-Samuelson Knee Arthroplasty. J Bone Joint Surg Br 1990;72:252.
SW, Stuchin SA, Lubliner JA. Technique for Removal of Femoral
Components with Intercondylar Articulation in Total Knee Arthroplasty. Bull Hosp Joint Dis 1993;53:48.
HA Jr, Wolff DA, Windsor RE. Total Knee Replacement Infection after
2-stage Reimplantation: Results of Subsequent 2-stage Reimplantation. Clin Orthop 1996;331:125.
WL, Cracchiolo AD, Amstutz HC. Results with the Constrained Total Knee
Prosthesis in Treating Severely Disabled Patients and Patients with
Failed Total Knee Replacements. J Bone Joint Surg Am 1980;62:504.
RA, et al. Determining the Rotational Alignment of the Femoral
Component in Total Knee Arthroplasty Using the Epicondylar Axis. Clin Orthop 1993;286:40.
W, Hassenpflug J. Are Unconstrained Components Essential in Total Knee
Arthroplasty? Long-term Results of the Blauth Knee Prosthesis. Clin Orthop 1990;258:86.
A, Engh GA. Use of a Semitendinosus Tendon Autogenous Graft for Rupture
of the Patellar Ligament after Total Knee Arthroplasty. A Report of
Seven Cases. J Bone Joint Surg Am 1992;74:974.
TA, McBeath AA. Arthrodesis following Failed Total Knee Arthroplasty:
Comprehensive Review and Meta-analysis of Recent Literature. Orthopedics 1995;18:361.
BR, Boeckstyns M, Stadeager C. The Natural Course of Radionuclide Bone
Scanning in the Evaluation of Total Knee Replacement—A 2 Year
Prospective Study. Clin Radiol 1990;41:341.
RH Jr, Head WC, Malinin TI. Reconstruction of Patellar Tendon Rupture
after Total Knee Arthroplasty with an Extensor Mechanism Allograft. Clin Orthop 1990;260:154.
GA, Parks NL, Ammeen DJ. Tibial Osteolysis in Cementless Total Knee
Arthroplasty. A Review of 25 Cases Treated With and Without Tibial
Component Revision. Clin Orthop 1994;309:33.
MH, Matthews LS, Kaufer H. Resection Arthroplasty as a Salvage
Procedure for a Knee with Infection after a Total Arthroplasty. J Bone Joint Surg Am 1987;69:1013.
N, Shirakura K. Candida Arthritis after Total Knee Arthroplasty—A Case
of Successful Treatment without Prosthesis Removal. Acta Orthop Scand 1997;68:306.
DA, Scott SC, Scott WN. Soft Tissue Expansion Prior to Arthroplasty in
the Multiply-operated Knee. A New Method of Preventing Catastrophic
Skin Problems. J Arthroplasty 1996;11:512.
AD, Trousdale RT, Osmon DR. Patient Outcome with Reinfection following
Reimplantation for the Infected Total Knee Arthroplasty. Clin Orthop 1995;321:55.
MH Jr, Booth RE Jr. The Use of an Antibiotic Impregnated Spacer Block
for Revision of the Septic Total Knee Arthroplasty. Semin Arthroplasty 1991;2:34.
RA. An Operation for Stiffening the Knee Joint, with Report of Four
Cases from the Service of the New York Orthopedic Hospital. Ann Surg 1911;53:404.
JA, et al. Detection of Occult Infection following Total Joint
Arthroplasty Using Sequential Technetium-99m HDP Bone Scintigraphy and
Indium 111 WBC Imaging. J Nucl Med 1988;29:1347.
H, Matthews LS. Resection Arthroplasty: An Alternative to Arthrodesis
for Salvage of the Infected Total Knee Arthroplasty. Instr Course Lect 1986;35:283.
JL, Atwater RD, Guo J. Early Failure of the Porous Coated Anatomic
Cemented Unicompartmental Knee Arthroplasty. Aids to Diagnosis and
Revision. J Arthroplasty 1997;12:11.
JL, Sherer D, Guo J. Blood Transfusion Strategies for Total Knee
Arthroplasty: Minimizing Autologous Blood Wastage, Risk of Homologous
Blood Transfusion, and Transfusion Cost. J Arthroplasty 1998;13:70.
KA, Holtgrewe JL. Experience with a New Technique for Managing Severely
Overcorrected Valgus High Tibial Osteotomy at Total Knee Arthroplasty. Clin Orthop 1990;258:213.
PF, Falatyn SP. Clinical and Radiographic Results of the Total Condylar
III and Constrained Condylar Total Knee Arthroplasty. J Arthroplasty 1996;11:916.
RS, O’Flynn HM. The Insall Award. Total Knee Replacement with Posterior
Cruciate Ligament Retention in Rheumatoid Arthritis. Problems and
Complications. Clin Orthop 1997;345:24.
DM, Bocell JR, Tullos HS. Arthroscopic Treatment of Intraarticular
Fibrous Bands after Total Knee Arthroplasty. A Follow-up Note. Clin Orthop 1994;309:230.
JP, et al. Implications of Reference Axes Used for Rotational Alignment
of the Femoral Component in Primary and Revision Knee Arthroplasty
[Comments]. J Arthroplasty 1992;7:531.
HR, et al. A Comparative Study of Indium-111 DTPA Radionuclide and
Iothalamate Meglumine Roentgenographic Arthrography in the Evaluation
of Painful Total Hip Arthroplasty. Clin Orthop 1989;245:156.
AD, Pilkington CA, Howie DW. A Comparison of Plain and Fluoroscopically
Guided Radiographs in the Assessment of Arthroplasty of the Knee. J Bone Joint Surg Am 1989;71:1343.
CS, Wiedel JD. Revision Total Knee Arthroplasty Using the Porous-coated
Anatomic Revision Prosthesis: Six- to Twelve-year Results. J Arthroplasty 1998;13:681.
CL, et al. Revision Total Knee Arthroplasty with a Cemented
Posterior-Stabilized or Constrained Condylar Prosthesis: A Minimum
3-year and Average 5-year Follow-up Study. J Arthroplasty 1997;12:896.
K, Fong PH, Kumar VP, Lee YS. Dermatitis Complicating Operatively
Induced Anaesthetic Regions around the Knee. A Report of Four Cases. J Bone Joint Surg Am 1993;75:116.
G, Hovelius L. Impacted Morselized Allograft and Cement for Revision
Total Knee Arthroplasty: A Preliminary Report of 3 Cases. Acta Orthop Scand 1996;67:10.
JV, et al. Arthroscopic Management of the Patellar Clunk Syndrome
following Posterior Stabilized Total Knee Arthroplasty. J Arthroplasty 1989;4:179.
KG. Collateral Ligament Reconstructions in Difficult Primary and
Revision Total Knee Arthroplasty. In: American Academy of Orthopedic
Surgeons. San Francisco, 1997.
KM, Murphy M, Kharrazi D, et al. Revision Total Knee Arthroplasty with
a Modular Prosthesis and a Three Step Reconstruction Technique: 2 to 10
Year Follow Up. Presented at the 10th Combined Orthopedic Associations
of the English-Speaking World Meeting. Auckland, New Zealand, 1998.
Foerster G, Kluber D, Kabler U. [Mid- to Long-term Results after
Treatment of 118 Cases of Periprosthetic Infections after Knee Joint
Replacement Using One-stage Exchange Surgery.] Orthopade 1991;20:244.
MG, Kelley K, Thornhill TS. Infection as a Complication of Total
Knee-replacement Arthroplasty. Risk Factors and Treatment in
Sixty-seven Cases. J Bone Joint Surg Am 1990;72:878.
RE, et al. Two-stage Reimplantation for the Salvage of Total Knee
Arthroplasty Complicated by Infection. Further Follow-up and Refinement
of Indications. J Bone Joint Surg Am 1990;72:272.
GW, Lionberger DR, Tullos HS. Failed Total Knee Arthroplasty. Revision
and Arthrodesis for Infection and Noninfectious Complications. Clin Orthop 1983;173:184.