PRINCIPLES OF LIMB SALVAGE SURGERY
VII – NEOPLASTIC, INFECTIOUS, NEUROLOGIC AND OTHER SKELETAL DISORDERS
> Tumors and Tumor-Like Conditions > CHAPTER 126 – PRINCIPLES OF
LIMB SALVAGE SURGERY
neoplasm that otherwise would be treated by amputation. Limb salvage
usually requires two separate but equally important procedures: (a)
“adequate” removal of the tumor and (b) bone and soft-tissue
reconstruction. Although the technique of limb preservation for bone
tumors has only recently been popularized, isolated reports of various
forms of limb salvage were published 40 years ago. At that time,
low-grade tumors such as giant cell tumor and chondrosarcoma were
treated by removal of the bone followed by osseous reconstruction.
Usually the only options were autogenous arthrodesis and allografts.
Resection arthrodesis of the knee was one of the earlier techniques
described. The femoral “turn-down” or tibial “turn-up” arthrodesis was
a procedure used to fuse the knee after resection of tumors involving
the lower end of the femur or the upper tibia. When successful, this
procedure led to knee fusion, but it was also associated with a
significant complication rate.
bone allografts were published. Three separate centers for this
surgical procedure developed, with Ottolenghi working in Argentina (99,100), Parrish in the United States (104), and Volkov in the Soviet Union (138).
These surgeons reported variable outcomes with the use of allografts
for limb salvage following tumor resection. Approximately one third of
their patients had an excellent outcome, one third failed, and the
remaining third had reasonable results. The enthusiasm for the use of
allografts increased after several reports by Mankin et al. from Boston
(82,83,84 and 85), who showed that allografts could be used successfully and recommended that the mechanical and biological issues be studied.
surgery became a very popular technique. This was the result of several
advances. First, the imaging of bone and soft-tissue tumors improved
dramatically. With the use of computed tomography (CT) scans,
radioisotope scans, and magnetic resonance imaging (MRI), these tumors
could be visualized precisely, and this allowed for adequate removal (26). Second, Enneking (34)
carefully studied the natural history of mesenchymal tumors so that
surgeons could better understand how these tumors progress and the
natural barriers to their progression. In addition, Enneking et al. (40)
further defined surgical margins by developing staging systems for both
benign and malignant tumors. These staging systems and margin
definitions are still used today.
in chemotherapy for bone malignancies, including osteosarcoma,
malignant fibrous histiocytoma, and Ewing’s sarcoma (90,111).
At that time, several drugs that are effective in treating these tumors
were identified and their use was well defined. Neoadjuvant
chemotherapy was developed—the delivery of cytotoxic drugs prior to
removal of the tumor, which leads to tumor necrosis and better
oncologic outcomes. When used prior to limb salvage surgery for
osteosarcoma, such drugs have been shown not only to improve patient
survival, but also to reduce the risk of local recurrence to a level
comparable to that for amputation (89). Simon et al. (123)
compared survival data for limb salvage surgery to that for amputation
and found comparable results. This further increased the enthusiasm for
limb-preserving techniques, particularly because surgeons realized that
patient survival would not be jeopardized.
results of limb salvage surgery was the development of better
reconstructive options. Allograft techniques improved during the 1980s
and 1990s, and modular prosthetic replacements were developed. In
addition, a combination of implants and allografts were tried with
success. All of this has led to limb salvage surgery with better
functional outcome and fewer complications.
and his colleagues. They studied many whole-mount surgical specimens
and thereby were able to determine the natural progression of bone
tumors, which led to improved surgical procedures with better oncologic
outcomes. High-grade sarcomas progress in a centripetal fashion. Bone
sarcomas start either within the medullary space or toward the surface
of the bone. Surface tumors can be either periosteal or parosteal,
originating from either the periosteum or the surface of the bone.
natural barriers. They have a tendency to destroy the medullary
cancellous bone. In addition, bone sarcomas can extend up the medullary
space involving marrow and can be associated with “skip” lesions (37).
Skip lesions are defined as discontinuous extensions of tumor within
the medullary space of the bone with a disease-free interval. As the
tumor progresses within the medullary space, the cortex is ultimately
destroyed, frequently leading to soft
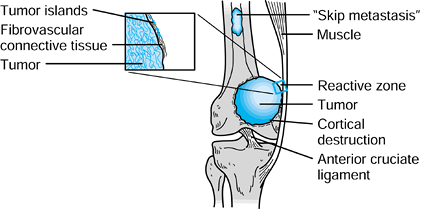
tissue extension. Nearly 90% of osteosarcomas have both bone and soft-tissue extension at the time of presentation (Fig. 126.1).
The host is unable to marginate a bone sarcoma, and an inflammatory and
vascular zone—typically an infiltrative margin and a
pseudocapsule—develops in the margin between the normal tissue and
tumor. The pseudocapsule is a zone that is contaminated by microscopic
islands of tumor, and therefore it does not represent a true barrier.
 |
|
Figure 126.1. Stage IIB sarcoma.
|
distal femur will respect cartilaginous barriers such as the growth
plate or articular cartilage. Later, however, penetration through these
structures occurs (125). Rarely, osteosarcoma will even enter the knee joint at cruciate ligament attachments.
is significant weakening of the bone, which may lead to a pathologic
fracture. The exact mechanisms of osteolysis are not fully known. The
process may be the result of proteolytic enzymes or a prominent
osteoclastic response. During the process of tumor progression, the
tumor outgrows its blood supply and spontaneous necrosis occurs. This
necrosis reflects aggressive tumor biology and should not be
misinterpreted as a chemotherapeutic response.
of osteosarcomas have either microchmetastatic or macrochmetastatic
disease at the time of presentation, as Link et al. (72)
demonstrated in a clinical review. Rarely will a bone sarcoma
metastasize to regional lymph nodes. The bone itself is another
potential metastatic site, and it should be screened by radioisotope
image scans. This understanding of the natural progression of bone
sarcomas is useful conceptually when discussing surgical margins,
because in surgical resection the interface between the tumor and the
host is so important. Also, the effect of adjuvant therapy such as
radiation, chemotherapy, or both on tumor progression has a significant
impact when determining the appropriate surgical procedures.
credited with our current definition of surgical margins. Four types of
margins have been described. The first is intralesional: The surgical
dissection enters the tumor, leaving gross residual tumor within the
bed. This type of margin is inadequate for bone sarcomas unless
effective adjuvants are used to eradicate the residual tumor. There
have been some isolated reports combining intralesional excision of
bone sarcomas with adjuvants such as liquid nitrogen. These procedures
should be considered experimental and reserved only for low-grade bone
sarcomas.
This margin conceivably will leave microscopic disease in the tumor bed
and by itself is not adequate for most bone sarcomas. When combined
with adjuvants, however, it may be an adequate margin that is
associated with a relatively low local recurrence rate. Some authors
have shown this to be the case with effective neoadjuvant chemotherapy
for osteosarcoma. For example, Picci et al. (107)
have shown that effective chemotherapy marginates an osteosarcoma; at
least conceptually, the reactive zone around the tumor becomes a true
capsular margin. For the most part, oncologic surgeons try to obtain
more than a marginal margin when removing bone sarcomas.
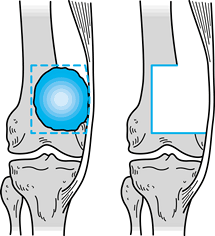 |
|
Figure 126.2. Marginal surgical margin.
|
This type of margin completely removes not only the tumor but also the
reactive zone surrounding the tumor. It is considered the gold standard
for most bone sarcomas and should be effective unless there is a skip
lesion.
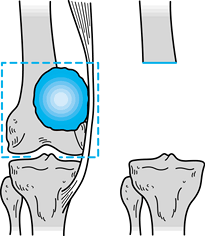 |
|
Figure 126.3. Wide surgical margin.
|
rarely necessary. If there are skip lesions, a radical margin will
remove both the primary tumor and the skip neoplasm. However, with
aggressive adjuvant therapy for bone sarcomas, radical margins are less
frequently needed.
resection. Margins, however, are getting “closer” as a result of better
tumor imaging as well as effective neoadjuvant chemotherapy, which
creates a barrier around the tumor. With the use of CT and MRI, it is
possible to evaluate the tumor more precisely. The surgeon can then
plan the resection preoperatively and decrease the need for removal of
extensive amounts of normal tissue. With less normal tissue resected,
functional outcomes are improving without the sacrifice of acceptable
oncologic results.
which consists of three stages. Stage I includes low-grade bone
sarcomas. Examples of stage I tumors are parosteal osteosarcoma,
low-grade medullary osteosarcoma, and low-grade chondrosarcoma. Stage
II tumors are histologically high grade. Examples include conventional
osteosarcoma, malignant fibrous histiocytoma, and high-grade
chondrosarcoma; all of these tumors have a significant risk of
metastases. Stage III sarcomas are tumors that have already
metastasized. Metastasis can be to any site, including lung, lymph
nodes, bone, and viscera. Tumors are further characterized as being
intracompartmental (A) or extracompartmental (B). For a bone sarcoma,
the bone is the compartment. If the tumor is confined to the bone, it
is an A lesion, and if it extends outside the bone, it is a B lesion.
For example, a nonmetastatic, high-grade conventional osteosarcoma of
the distal femur with soft-tissue extension would be stage IIB. The
Enneking staging system has been shown to prognosticate survival for
bone sarcomas and as such is quite useful.
site. We cover the workup and treatment of primary bone tumors. Factors
such as patient evaluation, biopsy, surgical resection, and
alternatives of reconstruction are covered, as well as some special
features such as the influence of skeletal growth, and reconstructive
options including complications and functional limitations. Also
included is the Enneking stage for each site.
are (a) the knee, (b) the shoulder girdle, and (c) the pelvic girdle.
These anatomic sites account for nearly 80% of all bone tumors. Each
site has its own special features that need to be considered when
planning a limb-sparing surgical procedure.
both benign and malignant tumors. As an example, in the Schajowicz
series of osteosarcoma published in 1994 (114),
347 out of 621 (56%) of osteosarcomas occurred in the lower end of the
femur or the upper end of the tibia. The distal femur was the more
common site, accounting for 229 patients. Malignant fibrous
histiocytoma also commonly occurs about the knee. In the Huvos series (53),
the femur accounted for 32% of the malignant fibrous histiocytomas.
With a potential malignancy about the knee, it is necessary to perform
appropriate staging biopsy.
lateral radiographs. This gives the first clue as to the type of
neoplastic process occurring. A typical “conventional” osteosarcoma is
a medullary tumor that involves the metaphysis or epiphysis, or both,
of the lower end of the femur or upper end of the tibia. This tumor is
characterized by bone destruction and frequently is associated with
soft-tissue extension (Fig. 126.4). Variable
amounts of ossification are seen in osteosarcoma except for the
telangiectatic variant. Radiologic features such as “sunburst” or
Codman’s triangle, which is bone formation as a result of periosteal
elevation, can be seen on plain radiographs.
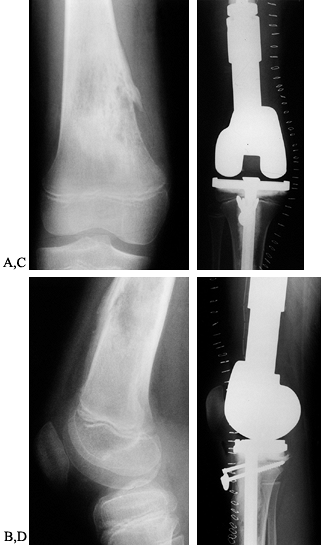 |
|
Figure 126.4. A: Anteroposterior (AP) radiograph of an 8-year-old girl with osteosarcoma of distal femur. B: Lateral radiograph of osteosarcoma. Note the bone destruction and periosteal reaction. C:
AP radiograph after resection of the distal femur. The knee is reconstructed with an expandable custom prosthesis. The articulation is a constrained condylar device. The expansion mechanism is at the top of the prosthesis. D: Lateral radiograph of an expandable knee prosthesis. |
technetium-99 diphosphonate bone scan and three-dimensional imaging.
The technetium-99 diphosphonate bone scan is typically “hot” in
osteosarcoma and will pick up multicentric disease (63). The three-dimensional imaging can be either a CT scan or an MRI (7,8,25,116).
An MRI is particularly useful in determining the soft-tissue extension
of the tumor and its relationship to the neurovascular bundle. This is
important for tumors about the knee where posterior extension toward
the neurovascular bundle is common. Usually, a pushing-type margin
occurs that is readily seen with an MRI. An MRI is also useful in
determining the intramedullary extent of the tumor; in addition, it
picks up skip lesions. It can also determine the presence of
transphyseal extension of the tumor and joint penetration, both of
which are important when planning
limb
salvage surgery. Rarely is angiography necessary. Perform chest
radiographs and a CT scan of the chest to determine the presence of
metastatic disease to the lungs (19).
Biopsies of the lower end of the femur or upper end of the tibia need
to be performed carefully because their execution will critically
affect ultimate limb salvage surgery (12,33,84,122,124).
The biopsy can be either by needle or open. Needle biopsy with a skinny
needle provides limited histology, and diagnosis is made primarily by
cytologic features. Only an experienced pathologist can make an
accurate diagnosis from a skinny needle. A core needle, on the other
hand, provides more tissue for histologic diagnosis (27,28,99,114,115).
The most dependable procedure for diagnosis, however, is open biopsy,
which provides adequate amounts of tissue for extensive pathologic
examinations and makes it possible to perform a frozen section to
confirm that diagnosable tissue has been obtained.
incision or needle puncture must be placed in an anatomic location that
can be totally excised at the time of definitive limb salvage.
Incisions must be longitudinal, and it is preferable to enter the tumor
by passing through muscle rather than anatomic planes. It is best to
biopsy the soft-tissue component of the tumor. Perform
immunohistochemistry on all suspected round cell tumors as well as
potential metastases. The biopsy is an important step during the
sequence of events leading to limb salvage and is not without its
hazards, as Mankin et al. reported (84). They
found that inappropriate biopsies can lead to a false diagnosis or
complications such as hematomas and bone fractures, and might possibly
preclude the possibility of limb salvage. Therefore, this procedure
needs to be carefully planned, preferably by the tumor surgeon
contemplating limb salvage, so that no opportunities are lost.
completed, many patients are placed on neoadjuvant chemotherapy,
particularly those with osteosarcoma and Ewing’s sarcoma. This delivery
of cytotoxic drugs for a period of time prior to resection of a tumor
facilitates limb salvage by tumor shrinkage, tumor margination, and
tumor necrosis. Some tumors, such as chondrosarcoma, are not sensitive
to chemotherapy, and currently surgical resection represents the only
means of eradicating them.
intra-articular or an extra-articular procedure. The latter is reserved
for those tumors where there is evidence of joint contamination. This
contamination can be demonstrated by MRI or by joint aspiration and
cytologic examination of fluid. With extra-articular resection, the
quadriceps mechanism is disrupted, significantly limiting
reconstructive options. The most reliable reconstruction after
extra-articular resection of the distal femur is knee arthrodesis.
amenable to intra-articular resection, which can be performed through
either an anteromedial or an anterolateral extensile approach (Fig. 126.5).
We prefer to use a long anteromedial longitudinal approach with a
tibial tubercle osteotomy, using a sterile tourniquet after gentle
gravity exsanguination.
 |
|
Figure 126.5. Intra-articular resection of the distal femur.
|
-
On the skin, draw out a long anteromedial longitudinal approach, elipsing out the prior biopsy site. Take this site en bloc with the tumor.
-
On reaching the deep fascia, perform a
medial parapatellar arthrotomy along with a tibial tubercle osteotomy
to evert the patellar mechanism and displace it laterally. -
Perform the tibial tubercle osteotomy
with a power-oscillating saw, taking a block of bone approximately 3 cm
in length and 1 cm in depth, which includes the tubercle. Some predrill
the fragment for replacement and fixation with a screw. This leaves an
ample cancellous bed for reattachment at the end of the operative
procedure. -
After everting the patellar mechanism,
flex the knee and inspect the joint. Determine of the amount of
resection of the quadriceps based on soft-tissue tumor extension. -
Take a cuff of normal tissue encircling
the soft-tissue component of the tumor to achieve wide margins. This
usually requires taking the vastus intermedius muscle along with a
portion of either the medialis or the lateralis, depending on the
anatomic location of the tumor. Preserve the remaining quadriceps
mechanism by careful dissection. -
At this point, flex the knee acutely and
perform the intra-articular dissection. Divide the anterior cruciate
ligament close to the tibia, and divide the medial collateral ligament
and posterior medial joint capsule. -
Divide the medial and lateral heads of
the gastrocnemius muscle along with the posterior cruciate ligament and
then finally the posterior capsule, which allows the knee to be readily
subluxed anteriorly. -
The next part of the dissection is to
identify the femoral artery and vein in Hunter’s canal, and dissect
them carefully away from the tumor. There are numerous perforating
vessels that need to be ligated to allow the vascular bundle to fall
away from the posterior distal femur. -
Sharply divide the attachments to the linea aspera.
-
Cut the femoral shaft with a power-oscillating saw.
-
An MRI coronal image is frequently used
and measured to determine the resection length. Select a clear margin
beyond the tumor based on the MRI and divide the bone. Cut the
remaining soft-tissue attachments and remove the distal femur. We
prefer at least a 3 cm margin beyond the tumor. -
It is desirable to bisect the specimen
either in an isolated area of the operating room or in the pathology
lab to check for gross margins. If the margins are adequate, then
perform the reconstruction with new gowns, gloves, and instruments.
 |
|
Figure 126.6. Extra-articular resection of the distal femur.
|
-
Take the patella, patellar ligaments, and tibial tubercle en bloc with the specimen. The soft-tissue extension of the tumor determines the amount of quadriceps mechanism to be resected.
-
Dissect the vascular bundle from Hunter’s
canal to the upper end of the calf, and tie off perforating vessels so
the vascular bundle falls posterior to the knee joint. -
The gastrocnemius muscle can either be
detached from the femur with preservation of the posterior joint
capsule or be taken with the specimen. -
Preserve the sciatic nerve by dissecting it free from the knee joint down to its division into tibial and peroneal components.
-
Divide the quadriceps mechanism
circumferentially around the femur, based on the desired length as
determined by MRI. Also divide the upper end of the tibia or fibula (or
both) below the joint line at an extracapsular site. -
Once this is done, divide the remaining soft-tissue attachments sharply so that the knee joint can be removed en bloc
in an extra-articular manner. Because the quadriceps mechanism is
sacrificed with an extra-articular resection, knee arthrodesis is
usually performed.
problem because of the lack of soft-tissue coverage and the necessity
of sacrificing the tibial tubercle and patellar mechanism. As with
resection of the femur, resection of the upper end of the tibia can be
either intra-articular or extra-articular, with intra-articular
resection reserved for those tumors that do not contaminate the knee
joint (Fig. 126.7, Fig. 126.8).
With this procedure, the tibial tubercle is sacrificed, but the
patellar ligament and patella can be preserved, although these need to
be reconstructed by muscle and tendon transfer.
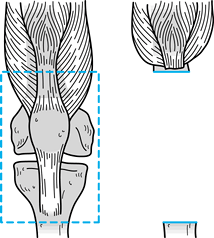
 |
|
Figure 126.7. Intra-articular resection of the tibia.
|
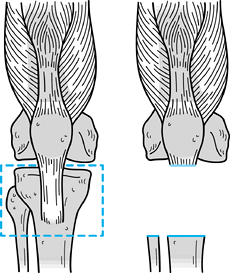
 |
|
Figure 126.8. Extra-articular resection of the tibia.
|
-
The surgical approach to resection of the
upper end of the tibia is usually performed through an anteromedial
longitudinal incision, elipsing out the prior biopsy site. -
Raise fascial cutaneous flaps medially and laterally.
-
Perform a median parapatellar arthrotomy, and divide the patellar ligament away from the tibial tubercle.
-
At this point, evert the patellar mechanism laterally and flex the knee acutely. Perform the intra-articular dissection
P.3316
by dividing the anterior cruciate ligament, the medial collateral
ligament, the posterior medial capsule, the posterior cruciate
ligament, the iliotibial band, and the remaining posterior capsule. -
Divide the gastrocnemius muscle origin off of the femur.
-
Divide the pes anserine tendons.
-
Resect the tibia either alone or en bloc
with the fibula. If the tibia is resected alone, identify the peroneal
nerve and dissect down to its division into deep and superficial
portions. Preserve the anterior compartment musculature if possible;
otherwise, take it with the tibia to establish an adequate margin. -
Posteriorly, either reflect the soleus
and deep posterior compartment musculature off the tibia or take it for
margin as necessary. -
Dissect and preserve the popliteal artery
and vein. It is frequently necessary to dissect the vessel down to its
bifurcation into anterior and posterior tibial arteries. The former
will pierce the interosseous membrane and enter the anterior
compartment. It is necessary to dissect it into this interosseous space. -
Finally, divide the tibia at the desired
length based on MRI and the remaining soft-tissue attachments,
including the proximal tibiofibular joint. -
If it is necessary to take the fibula
with the tibia, do not divide this joint, but rather divide the fibula
at the same site as the tibia and remove these bones together en bloc.
limb salvage for tumors about the knee. As previously mentioned, Simon
et al. (123) have demonstrated that limb
salvage can have oncologic outcomes similar to those for amputation,
as, for example, in their study reviewing osteosarcoma. There are,
however, relative contraindications for limb salvage. Tumors involving
the knee are frequently associated with a soft-tissue mass. If the mass
involves the neurovascular bundle, then limb salvage should be
reconsidered, particularly if the nerve is involved. The vascular
bundle can be resected with the tumor and later reconstructed, but
resection of the knee joint and sciatic nerve is a relative
contraindication to limb salvage. Extensive skin involvement is also
undesirable for limb salvage. Yet another relative contraindication is
the presence of large bulky sarcomas that do not respond to
chemotherapy. Joint contamination by sarcoma can be considered for limb
salvage, but it usually requires extra-articular resection of the knee.
Reconstruction is an important issue when determining the feasibility
of limb salvage. The lifestyle or vocational demands of some patients
are also relative contraindications for limb salvage. Consider
durability of the reconstruction when deciding the advisability of limb
salvage.
percent of limb growth occurs at the distal femoral and proximal tibial
physes. Resection of these growth plates in very young children will
lead to significant inequality of limb length. Generally patients under
the age of 10 are not good candidates for limb salvage. Special
techniques that allow for skeletal growth, such as an expandable
prosthesis, are still experimental.
This is not a form of limb salvage because the foot is used as a knee
joint and the patient is fitted with a prosthesis. This procedure,
however, leads to better function than conventional above-the-knee
amputation. In 1966, Winkelmann (145) described
the indications for rotationplasty. He included children under 10 years
of age for whom wide margins with limb salvage were not possible. For
this procedure, the sciatic nerve needs to be intact in the thigh.
Winkelmann reported on 121 rotationplasties of various types; from this
group there was only one local recurrence. He felt the oncologic
results were similar to amputation, and the functional results exceeded
conventional amputation. Complications included loss of limb
circulation in seven patients, development of nonunion in four
patients, and malrotation in five.
with tumors involving the distal femur or upper tibia is the option of
intercalary resection with joint preservation. More specifically,
tumors involving the metaphysis of the distal femur with an open growth
plate that respond well to chemotherapy can potentially be treated by a
transphyseal resection, preserving the articular surface with
intercalary reconstruction. It is critical to determine whether the
tumor crosses the physis, which would be a contraindication for this
type of limb salvage. This type of resection
can also be done for tumors involving the upper end of the tibia.
resection of the upper end of the tibia and lower end of the femur:
arthrodesis and arthroplasty. Each procedure has advantages and
disadvantages, and these need to be carefully discussed with the
patient prior to making a management decision.
Because there are no moving parts, the physical restrictions are fewer.
Manual labor is possible after knee fusion, and impact-sports
activities are also possible, especially if the arthrodesis is obtained
with living bone. However, late fractures have been reported for
arthrodesis achieved by intercalary allograft. The disadvantages of a
knee fusion are the technical difficulty in achieving a solid fusion
and the loss of knee motion. The latter makes ambulating on uneven
terrain difficult. It is also difficult to rise from a sitting
position, and activities that require stooping are impossible. Knee
arthrodesis may also cause problems with the ipsilateral hip and spine.
Despite these limitations, patients can be quite functional and even
participate in some sports.
After the tumor has been resected, a properly screened frozen bone
allograft can be placed in the osseous defect. Generally, the same type
of bone that is removed is selected, and the articular cartilage is
removed from the allograft so that the cut surface of the allograft
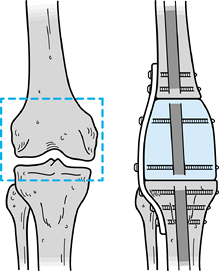
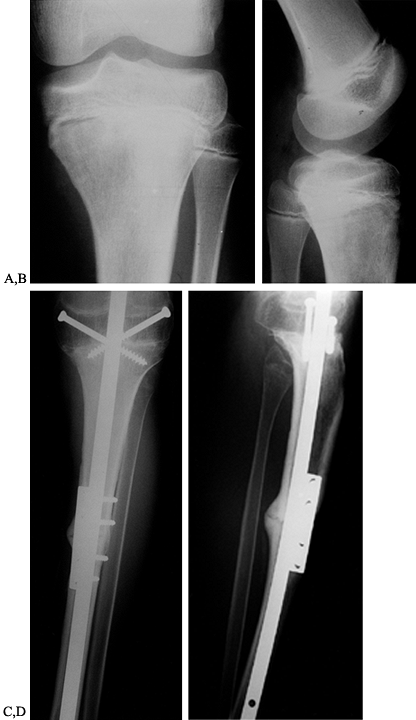
coapts with the cut surface of the upper tibia (28,142). This creates a large cancellous bone surface for fusion to occur. It is important to fix the allograft rigidly at both ends (Fig. 126.10). This
is accomplished either by a long intramedullary rod from the greater
trochanter to the ankle, supplemented with a compression plate to
prevent rotation, or by an osteosynthesis with two dynamic compression
plates. This graft can be supplemented with a vascularized fibular bone
graft to aid in healing.
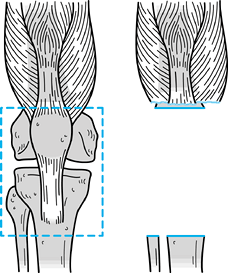
 |
|
Figure 126.9. Intercalary allograft arthrodesis.
|
 |
|
Figure 126.10. A: AP radiograph of the knee and proximal tibia from a 15-year-old boy. An osteosarcoma involves the upper tibia. B: Lateral radiograph of tibial osteosarcoma. C:
AP radiograph after resection of the upper tibia (intra-articular) and allograft arthrodesis. Note the intramedullary rod and screws for fixation. D: Lateral radiograph showing fusion of the knee. |
The problem with these grafts is that they are prone to stress fracture
and usually need to be supplemented with autogenous iliac graft. They
do not present the same broad cancellous surface for arthrodesis that
an allograft affords. When a vascularized fibular autograft is used,
hypertrophy of the fibula has reportedly occurred, especially with
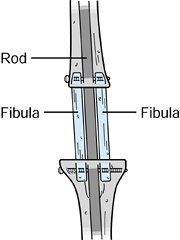
stress fractures of these grafts. Enneking and Shirley (39)
described the use of local bone grafts along with an intramedullary rod
to fuse the knee. The anterior cortex of the tibia is removed and
placed in the resection gap. In addition, a vascularized or
nonvascularized fibula can supplement the fusion.
 |
|
Figure 126.11. Dual fibula arthrodesis.
|
been described. This procedure uses half the distal femur or proximal
tibia, which is transferred into the resection gap to fuse the knee.
Implant arthrodesis has also been described with an intercalary
prosthesis. Kuo et al. (66) published their account of a successful experience in fusing the knee with an intercalary porous titanium prosthesis.
tibia is an alternative that has recently become popular. This
reconstructive technique preserves functional knee motion. The biggest
problem with this technique is durability. There are three ways to
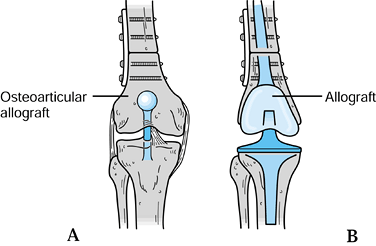
perform an arthroplasty of the knee after resection: (a) osteoarticular
allograft arthrochplasty, (b) allograft prosthetic composite
arthrochplasty, and (c) modular prosthetic arthroplasty. Each has
advantages and disadvantages that need careful consideration.
use biological tissue to restore either bone stock or bone stock with
an articular surface. The osteoarticular allograft replaces the missing
bone and, in addition, serves as
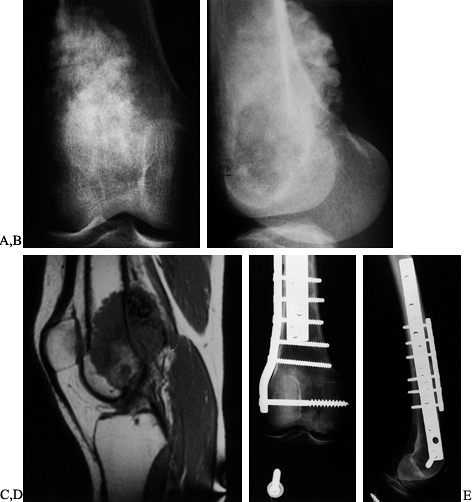
a joint surface (22,43,82,83,84 and 85,91,121,147) (Fig. 126.13). Typically, these are frozen grafts from an accredited tissue bank (132). It is critical to screen all biological tissue for both viral and bacterial contamination (43,133).
Frozen osteoarticular allografts are procured from donors that meet the
guideline(s) of the American Association of Tissue Banks. At the time
of procurement from an acceptable donor, the allografts are cultured
and viral screens are performed. There is some attempt to cryopreserve
the articular cartilage of the donated joint using either glycerol or
dimethylsulfoxide. The cartilaginous portion of the graft is immersed
in one of these cryoprotective agents. Conceivably, at least, these
chemicals will decrease the water crystal formation at the time of
graft freezing, because crystal formation is believed to kill the
chondrocytes and limit their preservation. With cryopreservation, at
least a portion of the chondrocytes survive and can continue to produce
proteoglycans and other cartilaginous matrix proteins after
transplantation (132).
 |
|
Figure 126.12. Reconstructive techniques. A: Osteoarticular allograft. B: Allograft prosthetic composite.
|
 |
|
Figure 126.13. A: AP radiograph of a 25-year-old woman with osteosarcoma of the distal femur. B: Lateral radiograph of osteosarcoma. The blastic lesion extends outside the bone. C: MRI (T1-weighted) showing both medullary and soft-tissue extension of the tumor. D: AP radiograph after resection of the distal femur (intra-articular) and osteoarticular allograft. E: Lateral radiograph of osteoarticular allograft.
|
Prior to resection of the distal femur or proximal tibia, take
radiographs with a ruler to ensure proper fitting. CT scans may be
useful for measurement purposes. It is important to achieve congruity
within a tolerance of 2–4 mm. No attempt at immunologic matching is
performed because of the limited availability of large osteoarticular
allografts. These grafts remain deep frozen at 60° to 80° in the tissue
bank until their viral and bacterial screens have been reviewed and no
evidence of contamination is seen (42). At the
time of the surgical resection, the graft is brought into the operating
room and then thawed at room temperature in lactated ringer solution.
-
After removing the tumor, perform
reconstruction with a new surgical setup. Fit the osteoarticular
allograft into the joint to determine if it is properly sized. -
Next, fix the allograft to the host bone
with either an intramedullary locked rod or dynamic compression plates.
A step cut at the allograft–host bone junction can ensure rotational
stability. A step cut, however, must be done precisely, especially in
terms of rotation. It does increase the surface area of union and is a
stable construct.
allograft should be completely cleaned of all marrow- containing
elements by reaming and then washing (142). In
addition, some recommend the use of methylmethacrylate to strengthen
the allograft. If methylmethacrylate is to be used, inject it into the
medullary canal of the allograft.
intramedullary use of methylmethacrylate. He felt that it had several
advantages, including increased graft strength, diminished
immunogenicity, and better internal fixation. No adverse ef- fects on
graft healing were observed with methylmethacrylate. Straw et al. (128) demonstrated this further in a canine model.
-
With this technique, dynamic compression
plating is usually chosen over intramedullary rodding. If dynamic
compression plating is performed, use two plates at 90° to improve the
strength of the construct. Distal femoral allografts are usually fixed
with plates placed laterally and anteriorly. Try to obtain six to eight
cortices of screw fixation in each fragment through each plate. -
Fill any drill hole in the allograft with
a metallic screw, because empty holes tend to be sites of
revascularization and resultant stress fracture. -
After fixing the allograft to the host
bone, restore the joint ligaments. It is important when requesting the
graft from the tissue bank to specify grafts with joint capsule and
collateral ligaments. -
Perform a circumferential repair of the capsular and collateral ligaments with nonabsorbable sutures or staples.
issue. Some authors believe that joint stability is markedly enhanced
with anterior and posterior cruciate ligament reconstruction, whereas
others believe it is not necessary when a solid circumferential
capsular and collateral ligament repair is done. Standard cruciate
ligament reconstructive procedures can be performed on osteoarticular
allografts as described in Chapter 89 and Chapter 90.
to the problem of joint restoration after tumor surgery. As a result,
it avoids implant complications and potentially restores bone stock.
There are, however, numerous complications associated with
osteoarticular allografts (74). First, a
significant nonunion rate (approximately 10%) at the allograft–host
bone junction has been reported, especially in patients receiving
chemotherapy. For example, Capanna et al. (16)
published the Rizzoli experience with allograft nonunion. The highest
incidence of nonunion was at diaphyseal junctions (50%). More than 90%
of the cortical cancellous junctions healed. The authors observed that
a gap between allograft and host bone was a contributing factor to
nonunion. Autogenous grafting of the allograft–host bone junction was
also recommended. If a nonunion develops, autogenous grafting at the
allograft–host bone junction is usually necessary.
classified the types of fractures seen with allografts: type I is rapid
graft dissolution, type II is diaphyseal fracture, and type III is
joint fragmentation. They successfully treated 80% of their allograft
fractures without removing the graft.
These
fractures can occur at any time after implantation, and they are prone
to occur at sites of revascularization or screw holes. These fractures
can be either joint surface, metaphyseal, or diaphyseal. Although some
allograft fractures can be managed nonoperatively, the majority require
either bone grafting or a repeat osteoarticular allograft procedure.
osteoarticular allografts. Although there is a potential for
maintenance of the viability of some chondrocytes during the freezing
process, joint degeneration will inevitably occur. This can be
minimized by avoiding incongruity of the joint with proper fitting. But
even with a well-fitted allograft, joint degeneration occurs and is
sometimes asymptomatic. Some degeneration may be the result of ligament
instability. The symptomatic patients with joint degeneration will
require a repeat arthroplasty. If the joint degenerates, it is feasible
to use the osteoarticular allograft bone stock and simply do a
resurfacing arthroplasty.
This procedure replaces the resected bone tissue with a frozen
allograft but, in addition, resurfaces the joint with a metallic
prosthetic device (44,50).
The allograft–host bone junction needs to be rigidly stabilized, as in
an osteoarticular allograft. This can be accomplished either with a
long-stemmed femoral prosthetic component that bridges the junction or
with the use of dynamic compression plating. The choice of prosthetic
device can range from a constrained rotating hinge to a less
constrained device. The latter requires careful soft-tissue
reconstruction, as in an osteoarticular allograft, but it does not
require cruciate ligament reconstruction.
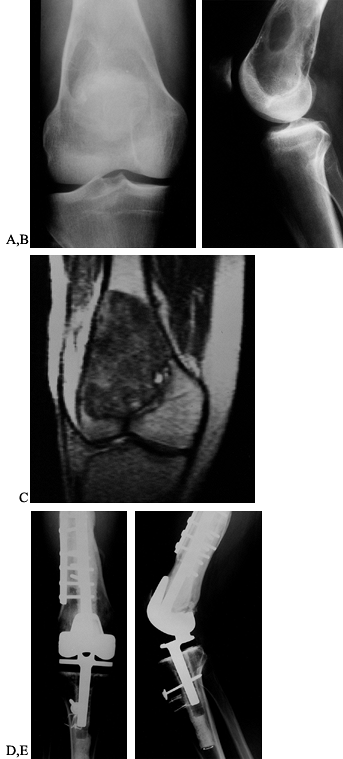 |
|
Figure 126.14. A: AP radiograph of the distal femur in a 24-year-old man. A low-grade osteosarcoma is present in the distal femur. B: Lateral radiograph. Note the marked bone destruction. C: MRI scan, coronal image (T1-weighted). The tumor involves the lower end of the femur. D:
AP radiograph after resection of the distal femur (intra- articular). The knee is reconstructed with an allograft and rotating hinge knee prosthesis. E: Lateral radiograph of knee reconstruction. |
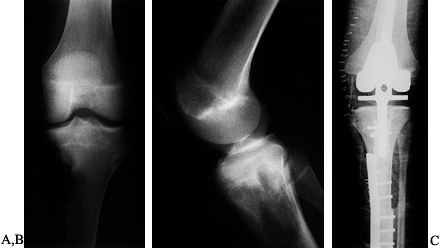 |
|
Figure 126.15. A: AP radiograph of an 18-year-old man with a malignant fibrous histiocytoma of the upper tibia. B: Lateral radiograph demonstrates bone destruction. C:
AP radiograph after resection (intra-articular) of the upper tibia. The knee is reconstructed with an allograft and rotating hinge prosthesis. Note the staples repairing the patellar ligament. |
arthroplasty is the restoration of bone stock by the allograft and the
maintenance of the medullary canal of the host bone without bone
cement. This is an advantage over the modular oncology device, which is
fixed by a long medullary stem in bone cement. Theoretically, at least,
an allograft prosthetic composite arthroplasty is easier to revise than
a modular oncology device because there is no cement in the femoral
medullary canal. Still, healing at the allograft–host bone junction can
be a problem, especially with chemotherapy, but it provides better
joint stability than an osteoarticular allograft because of the bearing
surfaces of the prosthesis.
and they can replace the missing bone with metallic components that can
be assembled in the operating room, but they have a constrained knee
articulation (Fig. 126.16, Fig. 126.17 and Fig. 126.18).
Most of these are a variation of the kinematic rotating hinge knee.
This type of articulation allows for varying amounts of rotational
freedom through a tibial bearing, and for flexion and extension through
a hinge axle mechanism between the tibial and femoral components. These
devices are usually fixed to the host
bone
with cemented intramedullary stems. They do have the option of a porous
collar where extracortical bone fixation can occur. Their primary
method of initial fixation, however, is the cemented intramedullary
stem. The femoral modular oncology device can replace any amount of
femur resected. In fact, these devices can replace the entire femur
with both a hip articulation and a knee articulation.
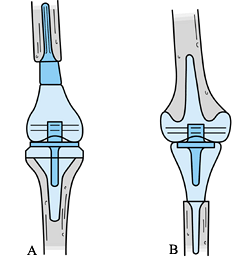 |
|
Figure 126.16. Modular oncology prostheses. A: Femur. B: Tibia.
|
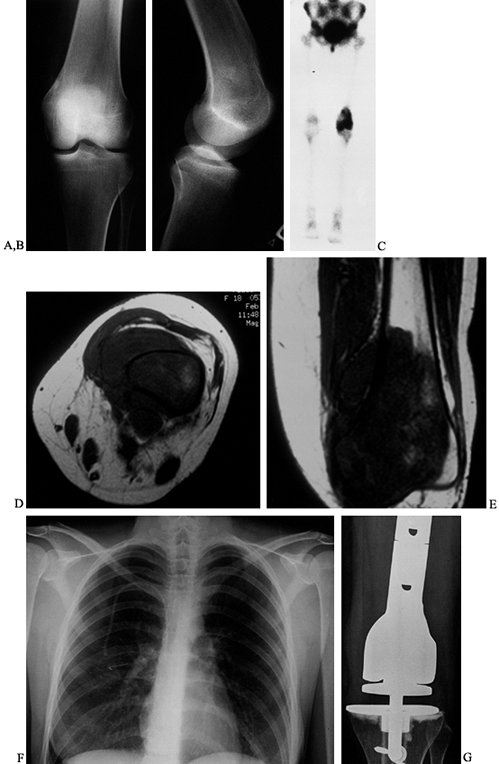 |
|
Figure 126.17. A:
AP radiograph of distal femur of an 18-year-old woman. The osteosarcoma is difficult to see. There is an area of sclerosis by the medial femoral condyle. B: Lateral radiograph. There is medullary sclerosis and loss of the anterior cortex. C: Technetium bone scan shows intense activity in the distal femur. D: Transverse MRI (T1-weighted). The osteosarcoma replaces the medullary bone with a large soft-tissue mass. E: Coronal MRI (T1-weighted). The medullary extent of the tumor is precisely visualized. F: AP chest radiograph. Note the intravenous catheter for chemotherapy. G: AP radiograph after resection of the distal femur (intra-articular) and modular oncology prosthesis. |
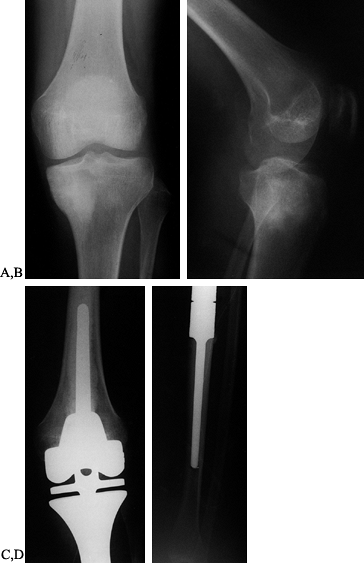 |
|
Figure 126.18. A:
AP radiograph of 17-year-old girl with osteosarcoma of the proximal tibia. Note the sclerotic tumor in the medial tibial condyle. B: Lateral radiograph of the tibial osteosarcoma. C: AP radiograph after resection of the proximal tibia (intra-articular) and modular oncology tibial prosthesis. D: AP radiograph of the tibial shaft. Note the cemented intramedullary stem. |
short-term complications. It provides for immediate bone restoration
and stability (17,110).
Bone healing does not need to occur. The modular oncology prosthesis
allows early restoration of function with immediate weight bearing. In
addition, joint stability is provided by the rotating hinge mechanism.
Its potential disadvantage is long-term disability. Any part of the
articulation can break by either yield failure or fatigue failure. The
intramedullary stems can loosen and also can fracture.
patients with prosthetic replacement of the knee after resection for
malignant bone tumors. Their median follow-up was 8 years (range, 5–17
years). Implant survivorships were 85%, 67%, and 48% (at 3, 5, and 10
years). The complication rate was 45%, with aseptic loosening of the
femoral component being the most common problem. Others (21,31,51,93,147,148)
have reported 5-year implant survivorships ranging from 63% to 90%.
These reports, however, had shorter follow-ups (34–77 months, mean).
Despite potential problems, modular oncology prostheses represent the
most popular method of performing a knee arthroplasty after resection
of the knee.
major differences (11).
First, soft-tissue coverage of a tibial reconstruction is critical.
Soft-tissue coverage can be accomplished by either local rotation flaps
or free flaps. Relying simply on skin to cover a tibial reconstruction
is associated with a high complication rate. A second difference for
tibial reconstruction is the problem of restoring the quadriceps
mechanism (41,52,106).
Because the upper end of the tibia is resected, the patellar ligament
needs to be reattached to restore the function of the quadriceps
mechanism. This can be accomplished either by the use of an allograft
to which the
patellar
ligament can be reattached or by a local muscle rotation flap to which
the patellar ligament can be reattached. This latter procedure is
frequently performed for patients reconstructed with a modular oncology
prosthesis.
However, children have to return to the operating room on a fairly
regular basis to lengthen the device, and this follow-up surgery can be
associated with significant morbidity. Frequently, scar needs to be
released to allow the lengthening mechanism to work. Complications such
as infection, loosening, implant breakage, and flexion contractures
have been reported with the expandable knee prosthesis (59,60 and 61,134). Kenan and Lewis (61)
reviewed their experience with an expandable endoprosthesis for 40
children with bone tumors. Of these 40 patients, 20 were followed at
least 5 years, and 18 of the 21 required revision. The mean implant
survivorship was 68 months for cemented devices. There was a high
complication rate in this series, and most patients developed stiffness
of the knee. Ward et al. (140) reported similar
results with an expandable prosthesis. It should be considered
experimental, and more time is needed to prove its safety and
effectiveness.
effective procedure and a viable alternative for the treatment of
malignant tumors. There are several options of reconstruction, each
with advantages and disadvantages. The long-term durability of these
reconstructions is still questionable. It is important to pay very
careful attention to technical detail to ensure an acceptable outcome.
Complications need to be anticipated and treated aggressively. Patient
satisfaction with limb salvage for tumors about the knee appears to be
good, and this in turn encourages oncologic surgeons to continue with
this form of treatment.
has had many new developments and changes in surgical techniques, the
principles of soft-tissue reconstruction remain remarkably constant.
The main goal is to promote uncomplicated primary wound healing. The
wide oncologic resection of the tumor and subsequent orthopaedic
reconstruction of the bone or joint defect interrupts major regional
blood vessels, depriving the wound margin of its axial blood supply (105).
The tenuous vascularity of these flaps and the creation of a large
defect with potential dead space, combined with the superficial
location of the prosthesis, demands reliable, well-vascularized,
durable, and flexible soft-tissue coverage. It is important to
emphasize that tension-free closure of the defect must be obtained and
dead space obliterated, which makes it necessary to add more tissue. To
achieve this, local muscle transposition has been the mainstay of
soft-tissue reconstruction, with distant microvascular free tissue
transfer and fasciocutaneous flaps used when necessary (52).
complications of these limb-sparing procedures include failure of wound
healing, flap necrosis, and infection, which can ultimately lead to
exposure of the prosthesis or loss of the limb (79,109).
Many patients receive preoperative adjuvant chemotherapy or radiation
therapy, and they are therefore immunosuppressed and have decreased
wound-healing capabilities at the time of surgery (29).
Satisfactory postoperative soft-tissue healing is absolutely required
to resume chemotherapy and/or radiation if necessary. Obviously,
serious complications involving soft-tissue coverage can have serious
effects. Problems such as infection; exposure of the implant; and
withholding of chemotherapy, radiation therapy, or antithrombotics
threaten a successful result from the limb-sparing surgery and even the
patient’s life. Complications increase the length of hospital stay and
delay ambulation and range-of-motion (ROM) exercises, increasing the
possibility of loss of some limb function.
cases, even functional soft-tissue reconstruction, total muscular
envelopment of the exposed bone and the joint prosthesis (metal,
plastic, allograft, or homograft) is necessary. Local muscle flaps
using the medial and lateral gastrocnemius, soleus, portions of
tibialis anterior, flexor digitorum longus, and flexor hallucis longus
are most commonly used for this soft-tissue reconstruction. The medial
gastrocnemius muscle serves as the mainstay of muscle reconstruction (46,88,113).
The muscle is readily available in the operative field, has a reliable
vascular supply (one of the most predictable of all lower extremity
muscle flaps), and is of sufficient size to cover the majority of knee
defects (79). It is possible to enlarge
coverage by scoring the fascia of the muscle; splitting the muscle;
augmenting the muscle envelope with adjacent muscles such as portions
of the tibialis anterior, soleus, and flexor digitorum longus; or a
combination of these methods. If necessary, converting to a medial
gastrocnemius island flap can enhance the versatility of coverage,
size, and location (3). The gastrocnemius
muscle has the additional benefit of providing a well-vascularized
segment of tendon that can be used for extensor reconstruction about
the knee. In our own patients, using the gastrocnemius muscle for
reconstruction has been very successful in providing excellent knee
extension with normal gait.
reliable vascular supply, ease of harvesting, and large, lengthy pedicle (141).
It also has an optional, extremely predictable skin paddle that offers
good protection from radiation and trauma. The tendinous portion of
this muscle also can be used for extensor reconstruction about the
knee. Other free-transfer options include the rectus abdominus and the
gracilis muscles. All of these muscle flaps, both local and distant,
have been successfully used without causing significant donor site
functional deficits. This is especially true of the medial and lateral
gastrocnemius muscles, the soleus muscle, and the tibialis anterior and
flexor digitorum muscles when partially transferred in the proper
fashion. In the pediatric age group, there have been no deleterious
effects at either the donor or recipient sites with subsequent growth
and development.
split-thickness skin grafts, which give an acceptable cosmetic result.
We discourage the use of a composite myocutaneous flap of the
gastrocnemius muscle. Donor site closure is extremely difficult, it
limits the use of the muscle in tendon reconstruction, and it decreases
its excursion and coverage area. Finally, lateral fasciocutaneous flaps
can be used selectively to cover smaller areas or in patients whose
local muscle flaps are insufficient for total coverage and whose
medical conditions contraindicate a lengthy microvascular free muscle
transfer (48,108). See Chapter 8 and Chapter 35 for more details.
oncologic and reconstructive surgeons is essential to determine the
amount of tissue to excise and how to deal with scars and zones of
irradiated and injured tissues (4). The timing
of surgery and dosages of chemotherapy and radiation therapy must be
coordinated with the oncologist and radiation therapist as well,
particularly concerning the dates when platelet, red blood cell, and
white blood cell counts reach their lowest points. Perform angiograms
(a necessity for free-tissue transfer and atypical cases) at least 48
hours prior to surgery, and catheterization entrance sites must be
through an uninvolved alternate extremity. Administer appropriate
preoperative antibiotics, insert a Foley catheter, and take care to
protect peripheral nerves and pressure points (especially the heels)
with padding during this long operative procedure.
-
Widely excise all areas of involved skin
and incision sites, taking special attention to elevate the adjacent
skin as fasciocutaneous flaps to decrease ischemia (79). -
After excising the tumor and bone completely, examine the knee region for its remaining blood supply.
-
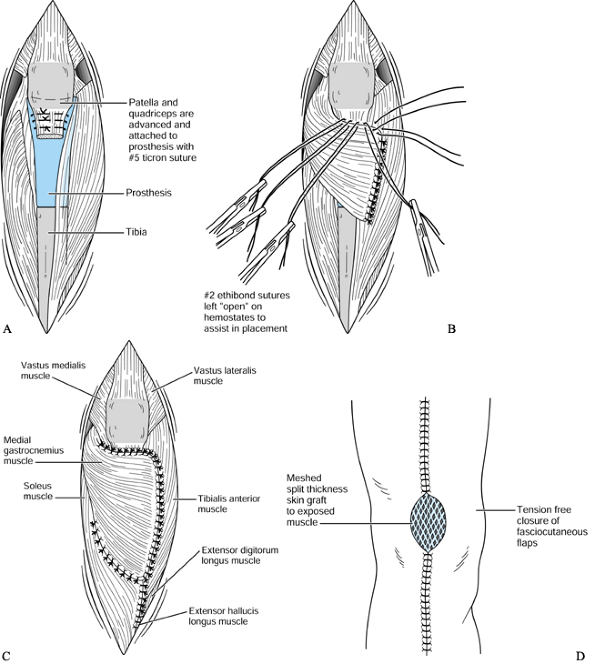
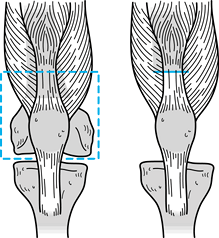
Dissect the nicely exposed deep portion of the gastrocnemius muscle at this time, if desired (Fig. 126.19).
Identify the sural arteries and veins and dissect them back to their
popliteal artery takeoff to enhance pedicle length or if using an
island flap. Take care to preserve and protect these structures, as 3–5
mm vessels are the main vascular pedicles to the gastrocnemius muscles.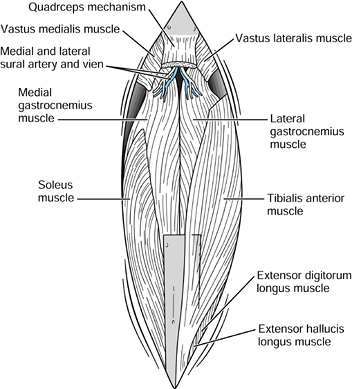 Figure 126.19. Exposed defect of the knee region after tumor resection.
Figure 126.19. Exposed defect of the knee region after tumor resection. -
At this point, if possible, close the
soft tissues posterior to the prosthesis or arthrodesis. This includes
the origins of the gastrocnemius muscles proximally, and the tibialis
posterior to the soleus muscles more distally (78). -
Obtain hemostasis, and irrigate the tissues with antibiotic solution.
-
After the prosthesis is in place, dissect
the remainder of the gastrocnemius muscle. If necessary, a second,
“stocking seam” longitudinal incision midposteriorly can be helpful
during harvesting of the medial gastrocnemius muscle (46).
The most tenuous blood supply is to the medial fasciocutaneous flap,
and this can be improved if the stocking seam incision is made parallel
and distal, leaving as wide a skin bridge as possible. -
To reconstruct the extensor mechanism,
attach the patellar tendon remnant to the loop on the tibial component
using heavy #5 nonabsorbable sutures. -
With the knee at neutral flexion, attach the tendon
P.3328
snugly to set proper quadriceps tension (Fig. 126.20A). This sutured reattachment now serves as the foundation for the extensor reconstruction.![]() Figure 126.20. A: Advancement reconstruction of joint capsule and quadriceps extensor mechanism. B: Medial gastrocnemius muscle transposition for extensor mechanism reconstruction. C: Completed muscular encasement of external tendon reconstruction, prosthesis, and bone. D: Completed tendon and soft-tissue reconstruction of defect.
Figure 126.20. A: Advancement reconstruction of joint capsule and quadriceps extensor mechanism. B: Medial gastrocnemius muscle transposition for extensor mechanism reconstruction. C: Completed muscular encasement of external tendon reconstruction, prosthesis, and bone. D: Completed tendon and soft-tissue reconstruction of defect. -
Next, rotate the medial gastrocnemius
muscle into position, scoring the muscle’s overlying anterior fascia so
the muscle spreads to fill the defect. Suture the tendinous portion of
the muscle underneath (now posterior) proximally (along the top) to the
loop and to the remnants of the patellar tendon in a “vest-over-pants”
fashion using #2 nonabsorbable interrupted sutures. Leave the suture
ends open and secured by hemostats to assist in placement. Suture the
medial hamstrings to this tendon as well, with the final portion of the
gastrocnemius muscle sutured to the remaining lateral tibial periosteum
and ligaments (Fig. 126.20B). -
After the proper distribution of sutures is complete, tie the #2 nonabsorbable sutures, reinforcing proper quadriceps tension.
-
If additional tendon graft is required to
aid in extensor mechanism reconstruction, use an extended medial
gastrocnemius flap, including the medial portion of the Achilles tendon
(54). All remaining portions of the prosthesis
or arthrodesis are now covered with the adjacent muscles, thereby
forming a complete muscular envelope. -
Laterally, portions of the tibialis
anterior can be advanced or even split off to preserve function if the
tendon and a sufficient portion of muscle are left attached. On the
medial side of the tibia, the soleus muscle chproximally—portions of
the flexor digitorum longus and flexor hallucis longus muscles
distally, if necessary—can be sutured to the lateral musculature,
completing the muscular encasement (Fig. 126.20C). -
Advance the fasciocutaneous flaps
developed at the beginning of the surgery and suture them free of
tension into position, tucking the muscle flaps underneath with
tie-over bolsters if necessary. Because uncomplicated primary wound
healing is of paramount importance, we have found it best to resect any
questionably viable flap edges, releasing tension from these flaps
whenever possible, even if it means grafting larger portions of muscle. -
Graft the remaining exposed muscle with
meshed split-thickness (0.012 inches thick) skin grafts harvested from
the lateral upper thigh region and sutured in place with 5-0 rapidly
absorbing sutures (Fig. 126.20D). -
Using two large hemovac drains to prevent
hematoma, dress the grafts and flaps, and apply a posterior splint from
toes to upper thigh.
temporarily discontinue epidural catheter solutions in the
postanesthetic recovery room to assess motor function. The muscle flaps
are sensitive, and patients do feel referred pain posteriorly when the
transposed gastrocnemius muscle is palpated. If the pain is extreme,
take down the dressings to rule out hematoma, pressure on the flap, or
muscle ischemia. Keep the patient at strict bed rest with the leg
slightly elevated and the posterior splint in place for approximately 5
days while postoperative wound checks and dressing changes are
performed. If the flaps are viable, there is no bleeding, and the
swelling is resolving, place the knee in nearly full extension in a
windowed cast and discharge the patient at 5–7 days postoperatively. If
possible, fit a knee–ankle foot orthosis prior to discharge for later
use. Allow the patient up out of bed at 2 weeks postoperatively, and
chemotherapy or radiation therapy may start 3 weeks postoperatively if
primary healing is complete or if there is no drainage and only small
superficial wounds remain.
important to prevent an extensor lag; therefore, avoid immediate
postoperative motion (78). Start knee flexion
exercises at 8 weeks postoperatively if wound healing is uncomplicated.
If an extension lag develops, then immobilize in extension for another
4–6 weeks. Use a knee–ankle–foot orthosis until adequate active
extension of the knee is present, which is usually at 4–6 months.
At times the diagnosis can be difficult. In our experience, violaceous
discoloration of the large fasciocutaneous flaps is an ominous sign and
uniformly leads to necrosis. Intraoperative marking of the level of the
prosthesis or the allograft on the skin surface can help future
management decisions. Surgical debridement with secondary
reconstruction is recommended as soon as the necrosis diagnosis is
established. Perform all significant debridements in the operating
room; they should not be taken lightly. Debridement, if performed
during the first several days, can have excellent results (46,87).
If the necrosis extends down to the prosthesis or allograft, copiously
irrigate with antibiotic solution using a pulsatile evacuator. Then do
a new reconstruction with flap readvancement, skin grafting, or new
muscle flaps, including free-tissue transfer if necessary.
In some cases, arterial spasm is present. If pulses remain absent
postoperatively, and arteriograms may be required during the first 6
hours to rule out vascular injury or thrombosis. During the course of
tumor extirpation, if nerve segments or blood vessels require
resection, immediate reconstruction is recommended. Saphenous vein and
sural nerve grafts are readily available in the operative field; take
care to cover all grafts with well-vascularized muscle. Pressure
necrosis can be problematic, and meticulous
padding
of pressure points is necessary, starting with the preoperative period
through the postoperative cast and splinting. The operative limb has
altered blood and nerve supply and is more susceptible to pressure
necrosis.
conspicuously absent. This could be explained by the use of
perioperative antibiotics, vertical laminar airflow operating rooms
with personal exhaust suits for all members of the surgical team, and
full muscular closure over the prosthesis and development of
fasciocutaneous flaps, with prompt treatment of zones of wound necrosis
(109).
the scapula, and the distal third of the clavicle—can give rise to a
primary malignant bony tumor or be involved by an adjacent soft-tissue
sarcoma (23). The proximal humerus is one of
the most common sites for high-grade malignant bony tumors in both
adults and children, and it is the third most common site for
osteosarcomas. Chondrosarcomas, which commonly involve the shoulder
girdle, often arise from the scapula or the proximal humerus. The bones
of the shoulder girdle may also be involved secondarily by high-grade
soft-tissue sarcomas that often require resections similar to that used
in the surgical treatment of high-grade primary bony sarcomas with an
extraosseous component.
because of the extent of bony destruction and the presence of large
extraosseous components, the treatment is sometimes similar to that for
primary malignant bony sarcomas. This pattern is most common with the
hypernephromas (renal cell carcinomas), which have a unique propensity
to involve the proximal humerus, often as a solitary metastasis.
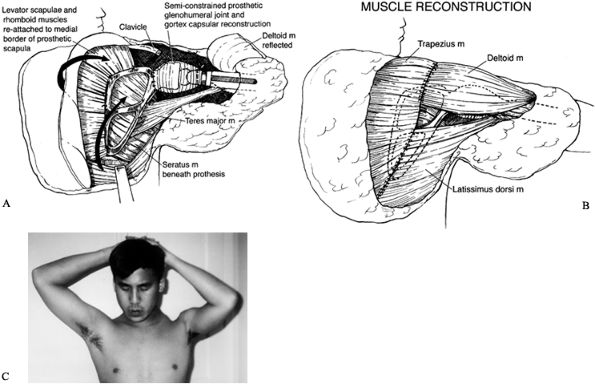
girdle with subsequent shoulder-girdle reconstruction consists of three
stages (75):
-
Wide surgical resection of the tumor using the principles already discussed
-
Reconstruction of the skeletal defect following the principles of orthopaedic skeletal reconstruction
-
Multiple muscle transfers to cover the
skeletal reconstruction and to provide stability of the shoulder girdle
and support for the extremity
functioning elbow. The various surgical techniques currently in use for
reconstruction of a segmental defect of the humerus or shoulder girdle
all offer some degree of stability, function, durability, ROM, and
preservation of motor power.
confined to the individual bones or portions of the scapula. The first
mention of a scapular resection in the literature is a partial
scapulectomy that Liston performed in 1819 for an ossified aneurysmal
tumor (73). In 1837, Mussey treated a recurrent chondrosarcoma by glenohumeral disarticulation (94). Syme performed a near-total scapulectomy with resection of the clavicle for a tumor in 1856 (129).
This is the first reported total scapulectomy. In 1909, De Nancrede
published a detailed review of “the end results after total excision of
the scapula for sarcoma” (25). He concluded
that anything less than a forequarter amputation for shoulder-girdle
tumors was inadequate, thereby bringing scapular resections into
disrepute for half a century.
In 1965, Papioannou and Francis reported on 26 scapulectomies and
described the indications and limitations of the procedure (103). Today the indications are quite limited. Nonetheless, the first limb-sparing attempts involved tumors of the scapula.
shoulder-girdle resection, and subsequently many shoulder-girdle
resections have been inappropriately called Tikhoff-Linberg procedures (5,86,87,112).
Two surgeons described the Tikhoff-Linberg intrascapulothoracic
resection, or triple bone resection: Baumann, a Russian surgeon who
worked with Tikhoff, described it in 1914 (5), and Linberg, an English surgeon, in 1928 (71).
Between 1908 and 1913, Tikhoff and Baumann performed three scapula
resections with the proximal humerus (that is, extra-articular
resections of the glenohumeral joint). When Linberg published his
classic paper in English in 1928, he named Tikhoff as the originator of
the operation (71).
of shoulder-girdle resections have been developed. Most have been
reported as Tikhoff-Linberg resections or modified Tikhoff-Linberg
resections (47,87,102),
inaccurate eponyms for the procedures performed. The Tikhoff-Linberg
resection was never intended to refer to resections of major tumors of
the humerus; its originators intended it to be used only with
intrascapulothoracic resections for sarcomas of the scapula and the
periscapular soft tissues.
described a three-part classification for scapulectomies: (a) total
scapulectomy and nearly total scapulectomy, (b) radical subtotal
scapulectomy, and (c) subtotal or partial scapulectomy. In 1968,
Samilson et al. (112) revised this
classification, adding the classical intrascapulothoracic resection
(Tikhoff-Linberg procedure) and forequarter amputation, thereby
establishing a universal classification system that covered virtually
all major shoulder-girdle operations (resections and amputations) as of
that date.
descriptive and refer almost exclusively to the bone resected. They do
not accommodate or reflect the new concepts and new terminology that
have developed in orthopaedic oncology during the past two decades. To
fill this gap, Malawer et al. have described a six-stage surgical
classification system (75,77,80,81).
This system is based on current concepts of surgical margins, the
relationship of the tumor to anatomic compartments (i.e.,
intracompartmental versus extracompartmental), the status of the
glenohumeral joint (intra-articular versus extra-articular), the
magnitude of the individual surgical procedures, and the presence or
absence of the abductor mechanism (deltoid muscle, rotator cuff muscle,
or both). The six-stage classification is as follows. (Fig. 126.21A illustrates the classifications, and Fig. 126.21B shows the distribution of 72 shoulder-girdle resections.)
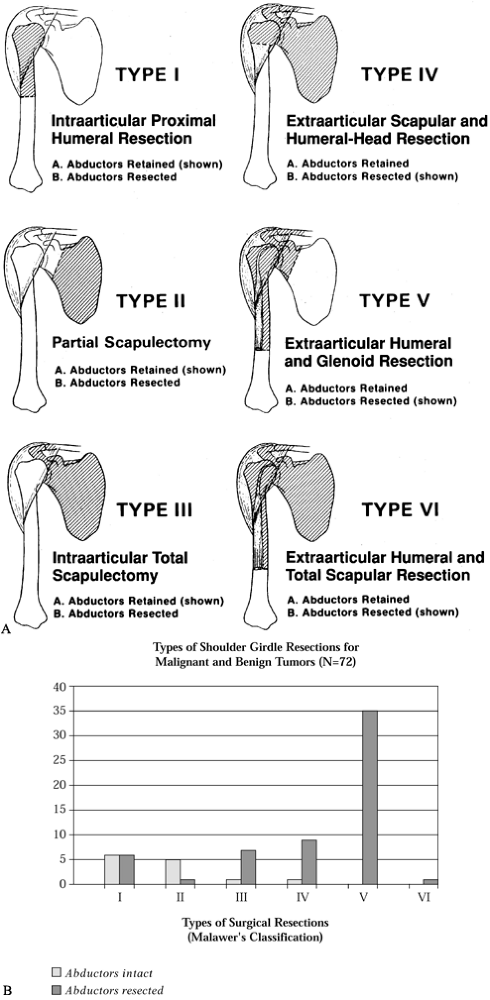 |
|
Figure 126.21. A: Classification of shoulder-girdle resections. B:
Distribution of 72 shoulder-girdle resections for bone tumors. (From Malawer MM, Meller I. A New Surgical Classification System for Shoulder Girdle Resection: Analysis of 38 Patients. Clin Orthop 1981;267:33, with permission.) |
variable: the presence or absence of the main motor group, the abductor
mechanism (deltoid and rotator cuff muscle). The abductors are present
(subtype A) or partially or completely resected (subtype B). The
abductor mechanism is almost always resected when there is extraosseous
extension of a bone tumor in this area. The loss of any component of
the abductor mechanism (deltoid muscle or rotator cuff) tends to create
a similar functional disability. Regardless of histology or primary
bone involvement, subtype A generally entails an intracompartmental
resection, and subtype B an extracompartmental resection.
joints to intra-articular or pericapsular involvement by high-grade
bone sarcomas. Figure 126.22 shows the
mechanisms of tumor spread. Direct capsular extension, direct tumor
tracking along the long head of the biceps, a poorly planned biopsy,
and pathologic fracture are mechanisms of glenohumeral contamination
and make intra-articular resection for high-grade sarcomas a higher
risk than extra-articular resection for local recurrence. (This is in
contrast to most clinical experience with resections of the distal
femur, which tend to be intra-articular.) Therefore, extra-articular
resections are recommended for most high-grade sarcomas of the proximal
humerus and scapula.
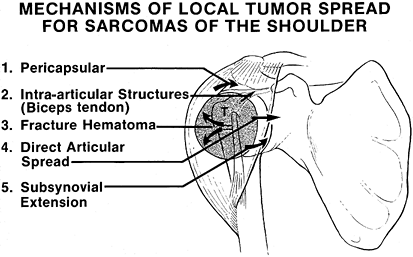 |
|
Figure 126.22.
Mechanisms of local tumor spread for sarcomas of the proximal humerus. There are five mechanisms of tumor spread to the capsule, synovium, and cartilage of the glenohumeral joint. An extra-articular resection is often required to perform a safe limb-sparing procedure. (From Sugarbaker PH, Malawer MM.Musculoskeletal Surgery for Cancer: Principles and Techniques. New York: Thieme Medical, 1992: Chapter 27, with permission.) |
the extent of the operative procedure required. The following
discussion addresses unique considerations of shoulder-girdle surgical
anatomy (Fig. 126.23).
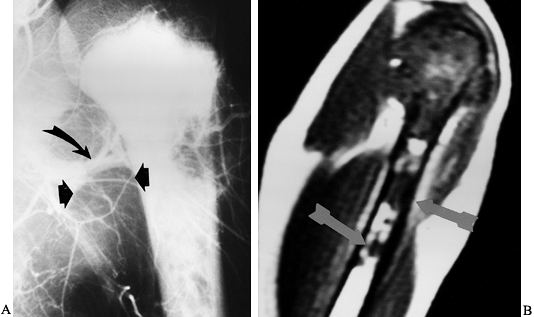 |
|
Figure 126.23. Osteosarcoma of the proximal humerus. A: Angiogram of the proximal humerus showing large circumflex vessels supplying the tumor (arrows), which must be ligated prior to resection. B: MRI scan showing “skip” metastases (arrows)
distal and not in continuity with the main mass. Skip lesions are seen in less than 5% of osteosarcomas and are best detected by MRI. |
to tumor spread. A lesion may cross the joint by direct extension or by
other mechanisms, as shown in Fig. 126.24. It
is often necessary to perform an extra-articular resection for
high-grade bone sarcomas of the proximal humerus or the scapula
(glenoid region).
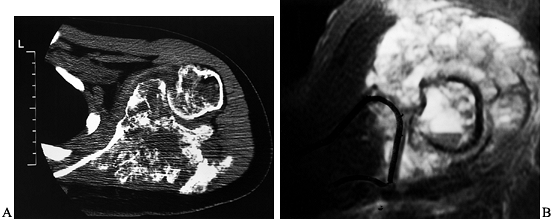 |
|
Figure 126.24. Tumor involvement of the glenohumeral joint. A:
CT scan of a large osteosarcoma of the scapula, which shows extensive involvement of the soft tissues with involvement of the glenohumeral joint. B: MRI scan of the same area, which shows marked involvement by the tumor surrounding and within the glenohumeral joint (white area, T2 pattern). |
close proximity to the subscapularis muscle, glenohumeral joint, and
proximal humerus. Tumors involving the upper scapula, the clavicle, and
the proximal humerus often displace the infraclavicular component of
the brachial plexus, which then may make it necessary to sacrifice some
of the major nerves.
contact with or in close apposition to tumors around the proximal
humerus, and before proceeding with resection it is necessary to
clearly identify both. The musculocutaneous nerve generally comes from
beneath the coracoid and passes through the conjoined tendon or
coracobrachialis muscle within a few centimeters of its origin. The
position of this nerve does vary, however, and it may lay within 6–8 cm
of the coracoid. It then passes through the short head of the biceps
and into the long head of the biceps before innervating the brachialis
muscle.
the proximal humerus. It arises from the posterior cord and, along with
the circumflex vessels, courses around the subscapularis muscle and the
head and neck of the humerus to innervate the deltoid posteriorly. In
patients who have large malignant tumors of the proximal humerus, the
axillary nerve usually must be resected because of tumor proximity or
involvement, and because it is necessary to remove the deltoid muscle
to provide a satisfactory margin. With large, stage IIB bone sarcomas
of the proximal humerus, it is rare that the axillary nerve and deltoid
muscles can be preserved.
cords of the brachial plexus and is tethered to the proximal humerus by
the anterior and posterior circumflex vessels. A presurgical angiogram
is extremely useful to localize the brachial artery and identify the
level
of
the circumflex vessels. Occasionally, one finds anomalous brachial and
axillary arteries that would be difficult to identify and explore if
not recognized preoperatively. In general, the circumflex vessels are
ligated during the initial dissection; this allows the entire brachial
artery and the vein and nerves to fall away from the tumor mass. Early
ligation of the circumflex vessels is key to the resection of proximal
humeral sarcomas.
the axillary sheath and exits from the posterior cord at the inferior
border of the latissimus dorsi muscle. Fortunately, most sarcomas are
located in the proximal third of the humerus and do not involve this
nerve. However, to avoid injury the radial nerve must be isolated and
protected prior to tumor resection. Sacrifice of the radial nerve is
rarely necessary.
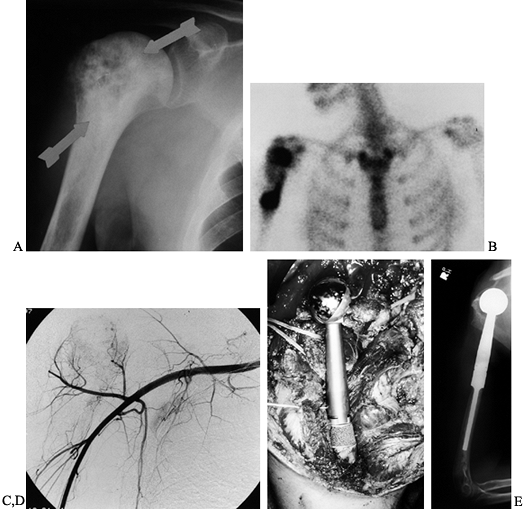 |
|
Figure 126.25.
Osteosarcoma of the proximal humerus: staging studies and intraoperative management by resection and modular prosthetic replacement. A: Plain radiographs showing a typical sclerosing osteosarcoma (arrows) of the proximal humerus. B: Bone scan demonstrating extent of the tumor. Note: There are no other bony sites of involvement. C: Angiogram after chemotherapy showing the axillary artery and the circumflex vessels without any evidence of tumor blush. The absence of tumor vascularity is an excellent indication of response of the tumor, with marked tumor necrosis. D: Intraoperative photograph showing the Modular Replacement System (MRS, Howmedica, Rutherford, NJ). E: The postoperative radiograph following an extra-articular resection of the proximal humerus and the glenohumeral joint (type V resection). |
cortical bone changes, and it is considered complementary to an MRI in
evaluating the chest wall, clavicle, and axilla. Furthermore, the CT
scan is more reliable than an MRI in the restaging of patients prior to
surgery to determine the effects of induction chemotherapy, especially
the bony response and the amount of tumor necrosis. It is also useful
in determining the potential planes of tumor resection.
involvement, especially around the glenohumeral joint, or of tumor
extension along the chest wall or posterior scapula. It is often
difficult to visualize the suprascapular area in patients with large
tumors, which may infiltrate below the subscapularis muscle and exit
near the coracoid. An MRI is especially useful in identifying the
extent of intraosseous tumor, which is necessary to determine the
length of the resection. Skip metastases rarely occur in this area.
However, an MRI is not effective in determining the preoperative tumor
response to induction chemotherapy.
intraosseous extent of the tumor defect and to evaluate for metastases.
Both bone scan and MRI data are necessary to accurately evaluate
intraosseous tumor extension.
the arm abducted to determine the relationship of the brachial plexus
and the vessels to the major tumor, the level of the circumflex
vessels, and the presence of any anomalies. This is also the most
reliable means of determining the response to neoadjuvant chemotherapy.
The absence of vessels in the tumor or decrease in tumor vascularity
indicates tumor necrosis. If there is a very good angiographic response
(i.e., decrease or absence of tumor blush), it is safe to proceed with
a limb-sparing resection, preserving as much normal tissue as possible
and accepting close margins as long as they are negative.
perform venography. The most suspicious finding is extremity edema.
Brachial vein thrombosis is most common with large shoulder
osteosarcomas and chondrosarcomas.
forequarter amputation is now rare. The decision to proceed with
limb-sparing surgery is based on the location of the cancer and a
thorough understanding of its natural history. Major contraindications
are tumor involvement of either the neurovascular bundle or the chest
wall. Relative contraindications include pathologic fracture, extensive
involvement of the shaft of the humerus, infection, and tumor
contamination of the operative area from hematoma following biopsy or
unwise placement of the biopsy incision. These contraindications are
described next in greater detail (80).
although it may be in close proximity. The subscapularis and the short
head of the biceps muscles often separate tumors of the proximal
humerus and scapula from the vascular structures. Occasionally,
however, the brachial veins are directly invaded by a tumor and may be
the site of tumor thrombi.
rare, as is involvement of the three major cords to the brachial
plexus, which follow the brachial vessels. Two of its major branches,
the axillary nerve and the musculocutaneous nerve, however, may be
involved, and resection of the axillary nerve is almost always required
for large (stage IIB) tumors of the proximal humerus. Direct tumor
extension into the brachial plexus requires a forequarter amputation.
Direct tumor extension into the plexus occurs most often with axillary
or chest wall sarcomas.
girdle, limb-preserving resection is often inadvisable. Today, one of
the major causes for amputation of the shoulder girdle is inappropriate
biopsy resulting in contamination of the pectoralis major, the chest
wall, and the neurovascular structures.
adequate resection, reconstruction of an infected field by arthrodesis,
prosthesis, or allograft replacement reconstruction is extremely risky,
considering that all patients with high-grade sarcomas must receive
postoperative adjuvant chemotherapy. If an infection cannot be
eradicated with the primary resection, amputation is advisable.
limb-sparing procedure. The local recurrence rate is increased if a
wide resection is attempted following a previous resection around the
shoulder girdle.
humerus with large soft-tissue components may invade the chest wall and
intermingle with the intracostal muscles and the ribs. This situation
usually requires a resection of the adjacent chest wall, but it is not
an absolute indication for forequarter amputation because limb-sparing
resection may be combined with chest wall resection.
documented by biopsy, a forequarter amputation may be the best way to
remove all the axillary nodes as well as the proximal sarcoma. On the
other hand, it is not unreasonable to proceed with a limb-sparing
resection and an axillary node dissection. This method can provide
long-term cure and local control.
One exception may be the young patient with a suspected round cell
tumor for whom more tissue for cytogenetic and immunohistochemical
stains may be required. Another exception would be an older patient in
whom a solitary metastatic lesion is suspected, and the pathology
supports either metastatic carcinoma or a spindle cell sarcoma. This
differentiation most often occurs with metastatic renal cell carcinoma.
In such a case, a significant amount of tissue may be required to
obtain immunohistochemical stains that will differentiate the
metastatic tumor from a primary sarcoma.
 |
|
Figure 126.26. Technique of biopsy of tumors of the proximal humerus. A:
Schematic diagram showing a core needle biopsy through the anterior one third of the deltoid. Note: The axillary nerve comes in posteriorly, so the deltoid can be preserved if necessary. This does not contaminate the deltopectoral interval and thus the brachial vessels if a resection is required. B: Clinical photograph showing puncture site following a core needle biopsy (arrow). This patient had a high-grade osteosarcoma and was treated by an extra-articular shoulder-girdle resection. C: Plain radiograph showing the technique utilized if a small incisional biopsy is required. A 1 inch incision is utilized. A curet removes a small amount of tumor and the defect in the bone is filled with polymethylmethacrylate. The bone cement acts like a cork to prevent contamination following an open biopsy. |
palpable. Multiple core needle biopsies performed through one puncture
site under local anesthesia are recommended. If the mass is not
palpable, perform all needle or core biopsies under fluoroscopic or CT
guidance. To obtain multiple cores with only one puncture site,
reintroduce the needle through the same puncture site, but vary the
angle to obtain cores from several different areas. Obtain cultures
routinely, regardless of the suspected diagnosis, because infection may
simulate any malignancy. Touch-preps, frozen sections, or both confirm
that lesional tissue has been obtained.
is required, perform it through the anterior third of the deltoid, not
through the deltopectoral interval (75,81).
A biopsy through the anterior third of the deltoid results in a limited
hematoma that is confined by the deltoid muscle and can be resected
with the tumor en bloc. On the other hand,
an open biopsy through the deltopectoral interval will contaminate the
pectoralis major muscle and provide a plane for the hematoma to dissect
to the chest wall along the brachial vessels. This makes a local
resection more difficult and increases the possibility of local
recurrence.
axillary border of the scapula or along the intended site of the
resection incision, and perform biopsy of the clavicle along the length
of the clavicle. Unless there is a soft-tissue component, a small
biopsy is advisable because a needle in this location could injure the
brachial plexus and the neurovascular bundle.
hand function and good elbow function, but they lose some shoulder
function, mainly abduction. From a rehabilitation perspective, the
outcome of resection is clearly superior to that of a forequarter
amputation or shoulder disarticulation. Furthermore, shoulder-girdle
resection is less disfiguring and is associated with only minimal pain
and edema. Generally, patients’ acceptance of the outcome of their
surgery is good to excellent.
often features pictures of patients who have undergone the procedures,
and demonstrations of what one can do postoperatively. Preoperatively,
a shoulder mold is fashioned using the involved shoulder, provided its
contours are not distorted. The cosmetic shoulder helps to preserve the
symmetry and appearance of the shoulder contour and can support a bra
strap or heavy overcoat.
motion until the incision is healed. Remove sutures after about 2
weeks. Control edema with an elasticized glove or elastic stockinet. At
the same time, have the patient begin active, maximal head motion to
preserve strength and range and to help mobilize edema.
motion within the confines of the sling as soon as the suction
catheters have been removed. At about 2 weeks, remove the sling for
passive shoulder ROM and pronation and supination of the wrist. Have
the patient continue to use the sling intermittently after the incision
is healed, primarily for upright activities in which arm support
increases comfort. Once his arm is out of the sling, have him perform
full ROM of elbow (flexion, extension, pronation, and supination). He
should also begin passive ROM to the shoulder (flexion, abduction, and
external and internal rotation and pendulum exercise) with the help of
a family member or physical therapist.
technique. In general, patients with endoprosthetic intra-articular
allografts or composite allograft reconstruction undergo the same
rehabilitation program. Those treated by arthrodesis, by allograft, or
by autograft reconstruction are immobilized for 4–5 weeks to allow
early bony union to take place.
both, and they may become quite large before they are brought to your
attention. The most common primary malignancy of the scapula is the
chondrosarcoma. Secondary chondrosarcomas occur from an underlying
osteochondroma, but fewer than 2% of osteosarcomas arise from the
scapula. In children, the most common malignant scapular tumor is
Ewing’s sarcoma. Soft-tissue sarcomas may involve the suprascapular or
the infraspinous musculature and, secondarily, the scapula. Most
soft-tissue sarcomas of the scapular region occur in adults. In very
rare cases, radiation sarcomas of the scapula may develop secondary to
radiotherapy for breast carcinoma.
that during the early stages of development, a cuff of soft tissue
surrounds tumors arising within the scapula (75,78,80,81).
As sarcomas enlarge, they may develop a large axillary component and
invade the axillary vessels and brachial plexus. Tumors arising from
the neck or glenoid usually involve the periscapular tissue and the
glenohumeral joint; this is especially true of chondrosarcomas,
osteosarcomas, and Ewing’s sarcoma. Important anatomic areas to
evaluate for extension are the chest wall, the axillary vessels, the
proximal humeral and periscapular tissues, and the rotator cuff. Also,
examine the axillary lymph nodes carefully, even though they are
usually negative. Large suprascapular tumors extend into the anterior
and posterior triangles of the neck, making resection difficult or
contraindicated except for palliation.
determining the size and extent of extraosseous disease and its
relationship to the chest wall, whereas arteriography is important to
determine vascular involvement. Displacement of the axillary vessels
indicates anterior (axillary) extension of a tumor. Bone scans may show
rib involvement or proximal humeral extension.
final operative procedure, because inadvertent contamination of the
neurovascular structures or chest wall must be avoided. For tumors
arising within the body of the scapula, perform a posterior needle
biopsy or a biopsy along the anterior axillary border of the scapula.
With lesions involving the scapula and neck, choose a posterior
approach directly through the posterior deltoid and teres minor; avoid
an anterior approach. If an open biopsy is required, we recommend a
small longitudinal incision in line with the incision that will be used
for resection. Most operative approaches involve an incision along the
axillary border of the scapula.
Chondrosarcomas, for example, commonly arise from the scapula.
Therefore, approach any large cartilaginous lesion of the scapula in an
adult with a high index of suspicion. These lesions tend to be low
grade with a large extraosseous component, and they are usually an
Enneking stage IB. Cartilage tumors approaching the glenohumeral joint
may directly involve the joint space and readily implant on the
articular cartilage. In such cases, an extra-articular resection is
generally recommended, with no attempt to perform an intra-articular
resection. Usually a Tikhoff-Linberg resection (type IV) is curative.
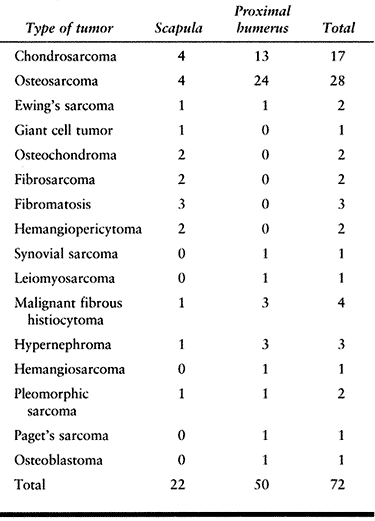 |
|
Table 126.1. Histogenesis and Anatomic Site of Tumor in 72 Patients Treated by Limb-Sparing Surgery of the Shoulder Girdle
|
about 1.5% occur in the scapula, require a limb-sparing resection (type
IV) or forequarter amputation. The limiting factors in performing a
limb-sparing procedure are the size and extent of the extraosseous
component. Neurovascular involvement requires a forequarter amputation.
Evaluate chest wall involvement before surgery; if present, a partial
chest wall resection en bloc with ablation of the primary tumor is necessary.
the scapula has been radiation therapy in conjunction with
chemotherapy, with excellent functional results. However, the treatment
of Ewing’s sarcoma is undergoing reevaluation. Recently, total
scapulectomy (type IIIA or B), with or without prosthetic replacement,
has been recommended in lieu of radiation therapy (24). Surgery has
become increasingly common with the hope of increasing local control,
decreasing the morbidity of radiation (especially of late secondary
osteosarcomas), and increasing patient survival. Plan the surgery after
induction chemotherapy, and stage the patient and the local tumor area
as with patients who have other high-grade sarcomas.
arising in the periscapular musculature satisfactorily by removing the
adjacent tissue en bloc while preserving
the scapula, then following with radiotherapy. Occasionally, a
soft-tissue sarcoma arising from the deeper structures will involve or
encase the scapula, requiring combined scapular resection. If the tumor
is distal to the scapular spine, a partial (type IIB) or total (type
IIIB) scapulectomy may be adequate. Involvement of the suprascapular
musculature or rotators requires an extra-articular resection (type IV).
marked ballooning and destruction of the scapula. Small lesions may be
treated by intralesional curettage. If the neck of the scapula is not
involved, it is possible to perform a partial scapulectomy with minimal
loss of function. Treat large lesions with total scapulectomy (type
IIIA) while preserving most adjacent muscles. Reconstruction involves
suspending the scapula from the clavicle by a static and dynamic
reconstruction. This is an excellent indication for scapular
prostheses, which have recently been developed.
Indications for the procedure are low- and high-grade scapular sarcomas
and periscapular and suprascapular soft-tissue sarcomas. A careful
preoperative evaluation is imperative. A CT scan and MRI can help to
determine possible chest wall involvement, and angiography is crucial
to determine axillary vessel involvement. Contraindications to the
Tikhoff-Linberg procedure are involvement by the tumor of the
neurovascular bundle and of the chest wall, both requiring forequarter
amputation. It is important to evaluate the interval between the tumor
and vessels very carefully, and this may require surgical exploration
prior to resection.
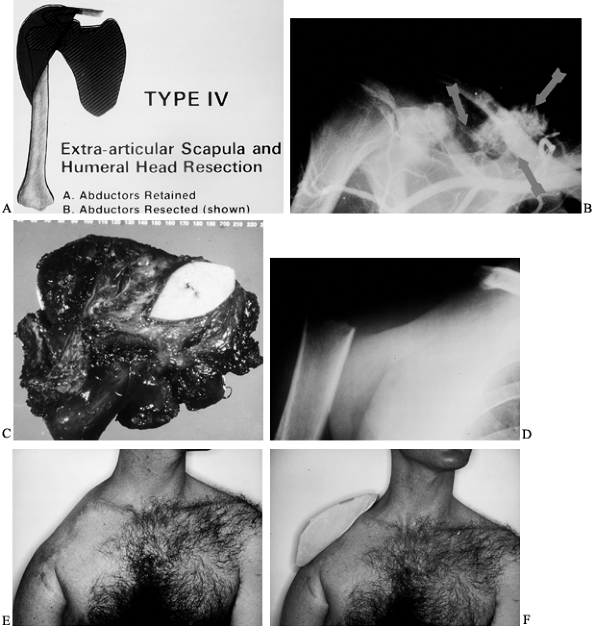 |
|
Figure 126.27. A:
Schematic of the classical Tikhoff-Linberg resection (type IV). This is an extra-articular scapular and proximal humeral resection with the abductors and deltoid removed en bloc. (From Malawer MM, Meller I. A New Surgical Classification System for Shoulder Girdle Resection: Analysis of 38 Patients. Clin Orthop 1981;267:33, with permission.) B: Preoperative angiogram showing a chondrosarcoma (arrows) arising from the superior aspect of the scapula and involving the entire suprascapular area. C: Operative specimen showing the resected scapula and glenohumeral joint covered by muscles in all directions. Note: The biopsy site and skin is removed en bloc with the tumor. D: Postoperative radiograph showing a resection of the scapula and glenohumeral joint en bloc. The remaining humerus is suspended to the clavicle by multiple muscle transfers and by the use of dacron tape (Genzyme Surgical Product Company, Fall River, Massachusetts) (see text). E: Clinical photograph following a Tikhoff-Linberg (type IV) resection for a high-grade soft-tissue sarcoma involving the scapula and glenohumeral joint. The extremity is suspended by the use of multiple muscle transfers and Dacron tape from the remaining clavicle. F: A cosmetic shoulder pad in place. The opposite shoulder was used to make a mold. |
-
Explore the axillary vessels, and if this
interval is clear, proceed with the resection. Be prepared to convert
from a limb-sparing procedure to a forequarter amputation should tumor
involving the neurovascular bundles be encountered. Explore the most
medial margin, the paraspinal muscles, and the base of the neck only if
there is any possibility of their involvement. It is difficult to
evaluate these anatomic areas thoroughly from preoperative studies
alone. A forequarter amputation will not improve on this margin. -
Resection includes all the muscles
arising from the scapula and inserting on the proximal humerus and an
extra-articular resection of the glenohumeral joint. Occasionally, it
is possible to preserve the deltoid muscle and the axillary nerve.
Preserve the deltoid muscle whenever possible because it facilitates
reconstruction; soft-tissue reconstruction is essential for a good
functional outcome—a stable shoulder. -
The reconstruction consists of both
static and dynamic support: suspension of the proximal humerus from the
remaining clavicle by dacron tape (Genzyme Surgical Product Co., Fall
River, MA) and muscle transfers. Suture the long and short heads of the
biceps and coracobrachialis through drill holes to the remaining
clavicle, and rotate the pectoralis muscle to cover the defect and to
provide stability. Recent reconstructions have used a scapula and
proximal humeral replacement.
following a Tikhoff-Linberg resection (type IVB) and total scapulectomy
(type IIIA/B): patients retain hand function and good elbow function.
The shoulder should be stable and no external orthosis required. A
molded shoulder pad improves cosmesis.
is indicated primarily for low-grade sarcomas (stage IA/B) of the body
of the scapula that involve the suprascapular area, low-grade sarcomas
of the glenoid, or soft-tissue sarcomas that involve the scapula.
Preoperative considerations are similar to those for a Tikhoff-Linberg
resection. The neurovascular structures and chest wall must be free of
disease. If the tumor extends anteriorly or laterally and involves the
rotator cuff or the glenoid, perform an extra-articular resection (type
IVB).
-
The skin flaps are similar to those obtained from the posterior limb during a Tikhoff-Linberg resection.
-
Transect all muscles away from the bone,
starting at the lowest point inferiorly. Approach the neurovascular
structures from the back, as the scapula is retracted away from the
chest in a cephalad direction. Take care to avoid injuring the
musculocutaneous and axillary nerves near the coracoid and around the
subscapularis muscle. -
Be prepared to convert this approach
(type III resection) to a Tikhoff-Linberg resection (type IVB) if the
anterior or medial margins are questionable. -
Soft-tissue reconstruction is mandatory to provide stability and to avoid a flail extremity.
-
Employ a dual suspension technique using
Dacron tape from the clavicle for static support and reattaching the
biceps and triceps muscles through drill holes. Tenodesing the deltoid
to the pectoralis major and trapezius muscles is essential to provide
stability.
standard Tikhoff-Linberg resection. This defect occasionally can be
reconstructed with a total scapula prosthesis if significant soft
tissue remains. The important muscles for this are the latissimus
dorsi, rhomboids, and trapezius.
similar for total scapulectomy (type III) and the Tikhoff-Linberg
resections (type IV; interscapulothoracic) (25,75,77). Fig. 126.28 illustrates the technique of a total scapulectomy together with the modifications for performing a Tikhoff-Linberg resection.
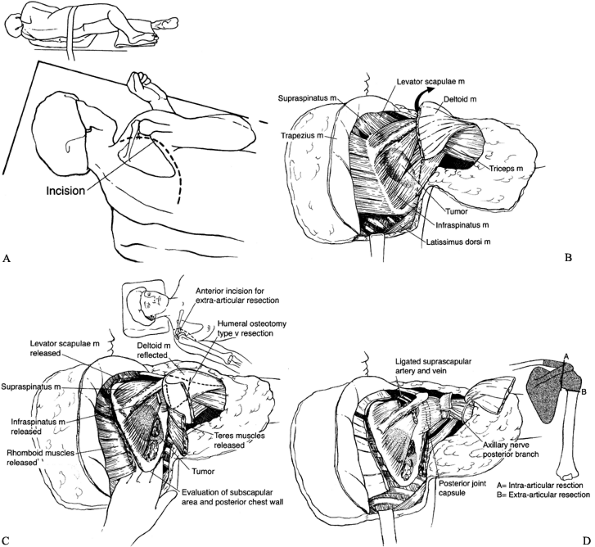 |
|
Figure 126.28. Technique of Tikhoff-Linberg resection (type IV) and a total scapulectomy (type III). A:
Make a long, curved incision over the scapula. Develop large fasciocutaneous flaps that permit easy visualization of all muscles attaching to the vertebral and axillary borders. B: Exposure of all three borders of the scapula. The rhomboids and latissimus dorsi muscles are detached. C: Muscle releases. Release all muscles attaching to the scapula. Place your hand between the scapula and chest wall to make sure there is no involvement of the underlying ribs. Then detach the serratus anterior. D: If an intra-articular resection (type III) is to be performed, release the joint capsule (line A). If an extra-articular resection is to be performed, osteotomize the proximal humerus below the capsular attachments (line B). |
-
Place the patient in the semilateral position. Drape the arm so that an assistant can move it as required during the operation.
-
Make a curved skin incision (Fig. 126.28A),
encompassing the tumor and extending from the tip of the acromion
superolaterally to the paravertebral region inferomedially. The lower
end of the incision can be extended to the midline. -
Develop large fasciocutaneous flaps that
permit easy visualization of all muscles attaching to the vertebral and
axillary borders. -
Raise the medial and lateral skin flaps,
and then identify the superficial dorsal muscular attachments to the
scapula. Resect the attachment of the trapezius muscle completely and
retract it superomedially, exposing the supraspinatus muscle. The
attachment of the deltoid muscle to the scapular spine is similarly
reflected laterally. It is then possible to identify the rhomboid major
muscle at the vertebral border, the latissimus dorsi inferiorly, the
teres major and minor muscles laterally, and the infraspinatus muscle. Figure 126.28B shows construction of skin flaps and release of the trapezius and deltoid muscles. -
Incise the insertion of the latissimus
dorsi muscle at the tip of the scapula and retract the muscle downward,
exposing the tip. Have an assistant hold the scapular tip and pull,
providing traction on the muscles at the vertebral border for incision
of their scapular attachment. -
Then detach the muscular attachments at the superior angle of the scapula along the vertebral border of the scapula (Fig. 126.28).
The levator scapulae and the rhomboids are easily delineated and then
cut. If the assistant maintains constant upward traction of the
scapula, this maneuver can be quite simple. -
For an axillary exposure,
rotate the inferior tip of the scapula and apply a medial pull while
the arm is abducted. This permits easy access to and identification of
the axillary contents. Retract the contents out of the operating field
after bluntly dissecting and identifying them. -
If an alternate exposure
is needed, for example for a large axillary component to the tumor,
make a separate anterior incision similar to that used in the
Tikhoff-Linberg (type IV) resection to explore the axillary vessels.
The anterior utilitarian approach is best. -
For an anterior approach,
use the deltoid–pectoralis muscle interval to expose the anterior
aspect of the scapula, coracoid, axilla, and brachial plexus. Safe
dissection of all anterior structures as well as osteotomy of the
proximal humerus is now possible (for an extra-articular resection or
an intra-articular transarticular resection). The key to this approach
is to detach the pectoralis major from its insertion onto the humerus
and clavicle, and to reflect it toward the sternal origins to expose
the axilla, coracoid, brachial plexus, and chest wall. Direct
visualization of all anterior structures is now possible. -
It is our preference to make an anterior
deltoid– pectoral incision to release the subscapularis and the muscles
(short head of the biceps and the pectoralis minor) attaching to the
coracoid. The axillary vessels can be retracted to avoid damage through
the anterior incision after the pectoralis major is released (Fig. 126.28C). -
Cut the lateral muscles, teres major and
minor, and the long head of the triceps. Release the supraspinatus and
infraspinatus tendons and the attachment of serratus anterior muscles
from the scapula (Fig. 126.28D). -
Next, release the subscapularis,
the only muscle remaining that is attached to the scapula, humerus, and
shoulder joint. Guide the resection with your fingers, protecting the
axillary nerve at the lower border. Then transect the acromioclavicular
ligaments under direct vision, with your fingers protecting the
axillary vessels. -
For soft-tissue reconstruction,
tenodese the deltoid and trapezius muscles, as well as the teres and
latissimus muscles. The reconstruction provides shoulder stability. Figure 126.29 shows the technique for a custom scapular prosthesis.![]() Figure 126.29.
Figure 126.29.
Technique of scapular and proximal humeral replacement. A scapular
replacement may be used if there are significant muscles remaining
after a total scapular resection (type III) or an extra-articular
resection (Tikhoff-Linberg procedure). The prosthesis has holes on the
vertebral, axillary borders, and medial to the glenoid for attachments
of the muscles with Dacron (Genzyme Surgical Product Company, Fall
River, Massachusetts) tape and reconstruction of the glenohumeral joint
with a Gore-Tex graft. A: Place the
scapula prosthesis above the serratus muscle. Attach the rhomboids to
the vertebral border of the scapula. Replace the proximal humerus with
a corresponding prosthetic component. Reconstruct the glenohumeral
joint with a Gore-Tex graft to restore stability. B:
Rotate the latissimus dorsi and the trapezius muscles to close over the
prosthesis. Tenodese the deltoid to the superior portion of the
trapezius muscle. C: After a total
scapular and proximal humeral resection for a hemangiosarcoma of the
scapula, this patient has almost a full ROM. The deltoid and trapezius
muscles were tenodesed to restore active abduction. -
Close the wound and continue suction drainage for 3–5 days.
is indicated for low-grade or benign lesions involving only the body of
the scapula. It preserves a cuff of infraspinatus, subscapularis, and
serratus anterior muscle. Reconstruction consists of suturing these
muscles together to close the dead space, and reconstituting
the points of origin and insertion of these muscles. A sling is required for 5–7 days.
scapular resection (type II); in fact, shoulder motion and strength are
nearly normal. Total scapular resection (type IIIA/B) causes loss of
significant shoulder motion, but elbow and hand function are normal;
the major limitation is the loss of shoulder abduction. Shoulder-girdle
function is similar following total scapular resection and a
Tikhoff-Linberg resection (type VB). Soft-tissue reconstruction is the
key to establishing shoulder stability. A compressive arm stocking
should be worn immediately after surgery to prevent swelling. Encourage
the patient to flex the elbow but to avoid extension until there is
good wound healing. The patient also needs a sling for 2–4 weeks, by
which time the transferred muscles provide a stabilizing force to the
entire upper extremity. Forward and backward flexion of approximately
30° to 45° is obtained. The goal of rehabilitation is to strengthen the
transferred pectoralis major, latissimus dorsi, and trapezius muscles
around the shoulder as well as the elbow flexors. A shoulder pad
contributes to cosmesis and restores symmetry.
although still limited, is increasing. Generally, if most of the
musculature is retained (type IIIA), it is possible to reconstruct the
defect with a custom scapular prosthesis (Fig. 126.30).
The most common indications for this procedure are large (stage III)
giant cell tumors, low-grade chondrosarcomas, and Ewing’s sarcomas
following induction chemotherapy. Successful reconstruction poses three
primary challenges: (a) replacing the humeral joint, (b) stabilizing
the scapula prosthesis within the humeral component (i.e., creating a
new glenohumeral joint), and (c) providing soft-tissue attachments to
both the scapular and humeral components to ensure stability as well as
active motion.
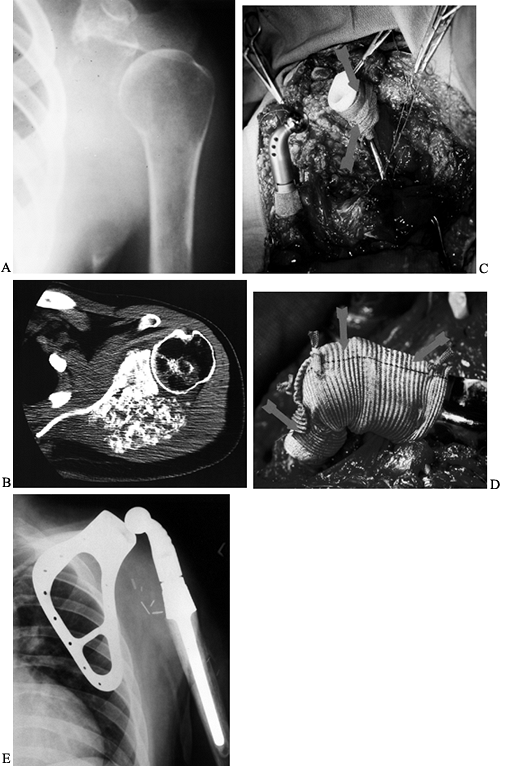 |
|
Figure 126.30. High-grade osteosarcoma of the scapula with glenohumeral extension. A:
Plain radiograph showing marked destruction and absence of the scapula. This was a high-grade osteosarcoma prior to induction chemotherapy. B: CT scan of the same tumor following induction chemotherapy showing marked reossification of the tumor. This indicates excellent tumor response and a high rate of tumor necrosis. A total scapular and proximal humeral resection in lieu of a forequarter amputation therefore was used to treat this patient. C: Intraoperative photographs following resection and the reconstruction. The proximal humerus is ready to be articulated with the polyethylene component of the total scapula. Note the Gore-Tex graft (W. L. Gore & Associates, Flagstaff, Arizona) around the scapula to the proximal humerus, which acts as a normal capsule maintaining stability and preventing subluxation. D: Intraoperative photograph showing the completion of the Gore-Tex reconstruction of the new shoulder capsule (arrows). The central arrow represents the level of the prosthetic joint. E: Postoperative photograph showing the scapular and proximal humeral prosthetic replacement. The polyethylene glenoid cup and Gore-Tex graft are not visualized. |
-
Reconstruct the glenohumeral joint by
sewing an aorta Gore-Tex (W. L. Gore & Associates, Flagstaff,
Arizona) graft over the scapula neck and the proximal humerus (Fig. 126.30D). This permits stabilization of the joint and provides additional stability. -
Reattach and tenodese the remaining
rhomboids, latissimus dorsi, and teres muscle to the prosthesis and to
themselves to achieve reliable and functional soft-tissue
reconstruction. -
Advance the preserved deltoid proximally
and suture it to the trapezius; then close the pectoralis major muscle
anteriorly over the new joint.
Shoulder stability is excellent, and there is good internal, and some
external, rotation. If the deltoid and portions of the rotator cuff
musculature are retained, between 60° and 110° of shoulder abduction
can be obtained.
The surgeon should be experienced in shoulder surgery and intimately
familiar with the entire anatomy of the shoulder girdle, including the
brachial plexus and axillary and brachial vessels, as well as the
nerves innervating the shoulder girdle, particularly the
musculocutaneous nerve, axillary nerve, radial nerve, and the cords of
the plexus. Despite the complexity of these cases, limb-sparing surgery
for both high- and low-grade sarcomas of the proximal humerus is
possible in approximately 95% of cases (Fig. 126.31).
Forequarter amputation is indicated mainly for large fungating tumors,
tumors with secondary infections, cases in which there is chest wall
involvement, and patients who have had a failed attempt at limb-sparing
resection (Fig. 126.32). Preoperative neoadjuvant
chemotherapy may allow fracture healing if there is significant tumor necrosis (Fig. 126.33).
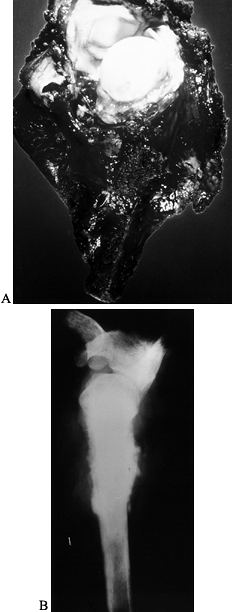 |
|
Figure 126.31. Osteosarcoma of the proximal humerus. A: Gross specimen of osteosarcoma showing the proximal humerus and the glenohumeral joint en bloc. The joint had been exposed following the resection. B:
Plain radiograph of the resected specimen demonstrating a complete glenohumeral resection with removal of the proximal half of the humerus. |
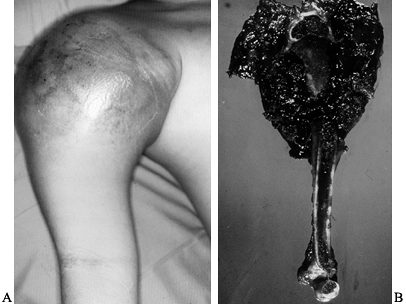 |
|
Figure 126.32. A large telangiectatic osteosarcoma of the proximal humerus. A:
Clinical photograph prior to treatment of an extremely large telangiectatic osteosarcoma with secondary bleeding into the tumor, which made a limb-sparing procedure impossible. This patient required a forequarter amputation. B: Gross specimen forequarter amputation. |
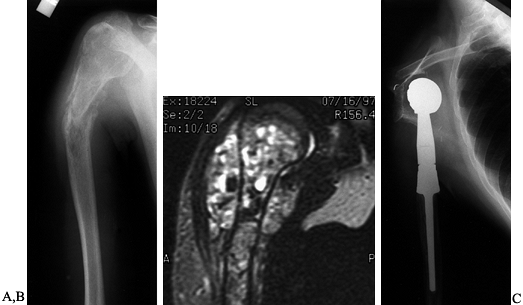 |
|
Figure 126.33. Osteosarcoma of the proximal humerus with pathologic fracture. A:
Plain radiograph prior to treatment demonstrates a moderately displaced fracture through an osteolytic osteosarcoma of the proximal humerus. B: MRI prior to induction chemotherapy shows the extent of the tumor within the medullary space and beneath the deltoid muscle. C: Postoperative radiograph following an extra-articular resection (type V) that included half of the proximal humerus, the glenohumeral joint, the abductors, and rotator cuff musculature. This was reconstructed with a Modular Replacement System (MRS) proximal humerus. This fracture healed following induction chemotherapy, which permitted a safe resection in lieu of a forequarter amputation. |
pathologic fracture heals during the course of induction chemotherapy.
Immobilize the arm during this treatment period. However, approximately
5% to 10% of patients with osteosarcomas cannot be treated by a
limb-sparing resection because of their extremely large size or be-
cause of vascular or neurologic involvement, infection, or pathologic
fracture that does not respond to treat- ment.
be treated by type I excision with minimal functional deficit.
High-grade sarcomas require a modified Tikhoff-Linberg resection (type
V). Intra-articular and synovial involvement is more common with
high-grade chondrosarcomas and osteosarcomas of the shoulder girdle
than at other anatomic sites. Thus, extra-articular, rather than
intra-articular, resections are recommended for high-grade tumors of
the proximal humerus. Take care not to contaminate the anterior
structures when performing a biopsy. Prosthesis, allograft, or dual
fibula autograft can be used for reconstruction following a marginal
resection (type I) for a low-grade lesion. Following resection of a
high-grade lesion (stage IIB), the aim is a stable shoulder with
minimal motion to preserve function in the elbow and hand. Regardless
of the type of reconstruction, the magnitude of the surgical resection
depends on the grade of the tumor and its anatomic extent. Specific
anatomic and surgical considerations are summarized next.
present with an extraosseous component, particularly with most
high-grade osteosarcomas, chondrosarcomas, and Ewing’s sarcomas. The
relatively small size of the glenohumeral joint in comparison to the
knee or hip joint almost always mandates an extra-articular resection
to obtain negative surgical margins. The recommended procedure is to
treat most stage IIB bone sarcomas by an extra-articular resection.
Usually, this requires a resection of the deltoid muscle as well as the
axillary nerve. Any attempt to preserve either structure is risky and
not recommended unless there has been a very good response to induction
chemotherapy.
displacement of the vessel. The subscapularis muscle protects the
brachial vessels and major nerves. The relationship of the axillary
vessels and the brachial vessels must be evaluated preoperatively and
routinely explored, and these vessels must be dissected free during the
initial surgical exposure prior to the resection. Occasionally the
brachial
veins
or axillary veins may be the site of mural (tumor) thrombi. Significant
arm swelling and edema are important signs of vascular compression or
involvement, or tumor thrombosis.
brachialis and biceps muscles, is rarely involved unless the tumor
extends to the coracoid. To avoid damage, it is imperative to identify
this nerve before exploring the brachial vessels and mobilizing the arm
muscles. Similarly, the tumor rarely involves the radial nerve, which
arises from the posterior cord and passes posterior to the humerus at
the inferior border of the latissimus dorsi muscle. However, the radial
nerve is often displaced and can be easily damaged. It is also
necessary to identify it before performing a humeral osteotomy.
surgical planning is the intraosseous extension of the tumor within the
bone marrow. The humerus is shorter than the femur and tibia, the two
most common sites of sarcomas, and large tumors of the humerus often
require resection of a significant portion of the bone. It is not
unusual to resect 50% to 80% of the humerus. Tumors arising within the
diaphysis may require a total humeral resection and replacement of the
glenohumeral and elbow joints. Be prepared with various lengths and
diameters of intramedullary stems. Base the final decision of bone
length on the frozen section of the osteotomized humerus at the time of
resection.
the rotator cuff, normally cover the shoulder joint. With high-grade
proximal humeral sarcomas, these structures are usually resected.
Following the resection, joint coverage and stability are essential to
eliminate dead space, decrease the risk of infection, and maintain good
elbow and hand function. The key muscle transfers in the reconstruction
are the pectoralis major muscle and the latissimus dorsi muscle; these
must be identified and preserved during the resection.
accurately demonstrate the soft-tissue extension as well as the
intraosseous extent of the tumor (75,77,80,81).
Pay careful attention to the level of marrow extension and thus the
level of humeral resection, as well as to the extension of tumor along
the shoulder joint, rotator cuff, and axillary space. Evaluate the
scapula for transarticular skip metastases. Also evaluate the
possibility of tumor extension along the subscapularis and the other
rotator cuff muscles; if it is present, a wider resection is required.
the best demonstration of bony disease and cortical involvement. At
this point, evaluate the chest wall, axilla, and ribs for tumor
involvement.
the brachial and circumflex vessels, which are often displaced. A
decrease of tumor blush correlates with the tumor response to induction
chemotherapy. A minimal tumor blush indicates a high degree of tumor
necrosis, which may permit less tissue to be resected and may alter the
necessary margins. The venous flow phase is useful to demonstrate
venous occlusion or tumor thrombi. If there is any suggestion of
occlusion, perform a brachial venogram.
biopsy carefully because an inappropriate biopsy is a common cause of
forequarter amputation (Fig. 126.27). In a
patient with a tumor of the proximal humerus, a core or needle biopsy
through the anterior third of the deltoid muscle is recommended (75,77,80).
Open biopsies are rarely required and may lead to excessive local
contamination. If there is a soft-tissue component, which occurs 95% of
the time, there is no need to enter the bone. It is possible to perform
multiple biopsies through one puncture site to obtain adequate
material. Take care to avoid the deltopectoral groove: Contaminaton of
this groove leads to contamination of the pectoralis muscle and
potentially of the brachial vessels and axillary space. The axillary
nerve innervates the deltoid muscle posteriorly, so the muscle can be
partially resected without any loss of function if the muscle is to be
preserved.
treatment. The proximal humerus is the third most common site for
osteosarcoma. Osteosarcomas in this area tend to have a poorer
prognosis than those around the knee, and most have a significant
extraosseous component. Plain radiographs suggest the correct
diagnosis. Perform all staging studies prior to biopsy. If the axillary
vessels are free of tumor, a limb-sparing procedure is generally
indicated—preferably an extra-articular resection (type IB). A modified
Tikhoff-Linberg procedure (type V) provides adequate resection of the
proximal humerus for high-grade bone sarcomas. This includes en bloc
removal of 15–20 cm of the humerus and shoulder joint, with the
deltoid, rotator cuff, and portions of the biceps and triceps muscles.
Reconstruction involves suspension of the arm, motor reconstruction,
and provision of adequate soft- tissue coverage.
chondrosarcomas commonly occur in the proximal humerus. The peripheral
lesions tend to be large but low grade, whereas the central lesions
tend to be a higher grade. Stage I tumors of the proximal humerus can
be treated by excision (type I) with minimal functional deficit.
resection (type V) or, rarely, a forequarter amputation.
Intra-articular and synovial involvement with high-grade cartilaginous
lesions are more common in this location than in other sites. Take care
not to contaminate the anterior structures when performing the biopsy.
An allograft prosthesis accomplishes the reconstruction following a
marginal resection (type I) for a low-grade sarcoma.
osteosarcomas, fewer than 10% of Ewing’s sarcomas involve the proximal
humerus. The flat bones, specifically the scapula and clavicle, are the
most common sites for Ewing’s sarcomas. The treatment follows the
guidelines for other high-grade bone sarcomas of the humerus. Surgery
should never precede induction chemotherapy. Evaluate by CT scan, MRI,
bone scan, and angiography when deciding whether to perform a
limb-sparing resection. If a round cell tumor is suspected, first
perform a small core biopsy. Because the patient may receive radiation
therapy, it is important not to make a large hole in the underlying
bone that might not heal. As previously mentioned, resection of Ewing’s
sarcoma is increasingly replacing radiation therapy. Ewing’s sarcomas,
unlike osteosarcomas, may dramatically decrease in size following
induction chemotherapy, in which case the deltoid and axillary nerve
may be preserved. For this reason, intra-articular resections are
recommended. However, do not use radiation therapy in patients treated
with a prosthesis or an allograft because it often leads to several
local complications, such as restriction of motion, infection, and
secondary amputation.
In specific situations, large metastatic tumors with marked bony
destruction may be resected by a primary resection and prosthetic
replacement. Hypernephroma, which is extremely vascular and has a
predilection to this location, may present a unique problem of
uncontrollable
bleeding.
Radiography often reveals marked destruction and ballooning, much like
an aneurysmal bone cyst or primary sarcoma. Simple biopsy, even with a
needle, may lead to severe hemorrhage. Preoperative angiography with
embolization is recommended. Ligate the anterior and posterior
circumflex vessels prior to any surgical procedure. If curettage and
cementation are not feasible, a resection with prosthetic replacement
(type I) is indicated.
which has been in use since 1973, is the most common technique for
reconstructing large proximal humeral defects. It may be used for both
intra-articular and extra-articular defects (i.e., when retaining the
glenoid as well as when resecting it with the tumor specimen) (Fig. 126.34).
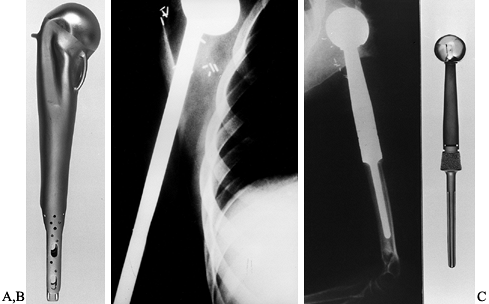 |
|
Figure 126.34. Types of prostheses utilized for replacement and reconstruction of the proximal humerus. A: The initial vitallium prosthesis used in the 1940s. B:
A long-stem Neer prosthesis (Howmedica, Rutherford, NJ) first used by Dr. Ralph Marcove at Memorial Sloan-Kettering Cancer Center during the 1960s. C: A proximal humeral modular replacement prosthesis (the MRS) developed by Howmedica (Rutherford, NJ) in 1988. This design is popular today. |
prosthesis. In 1988, Howmedica (Rutherford, NJ) developed the Modular
Replacement System (MRS), which has since undergone several
technological improvements. The present system is shown in Figure 126.35.
Modular prosthetic systems are now widely available (from Howmedica;
Biomet, Warsaw, IN; and Sulzermedical, Austin, TX) and obviate the need
for custom devices. These prostheses have proven to be very durable.
The reported rates of fracture, infection, nonunion, tumor recurrence,
reoperation, and time of immobilization are all lower following
endoprosthetic replacement than with allografts or composite
reconstruction. Low-grade tumors are treated by an intra-articular
resection and preservation of the abductor mechanism.
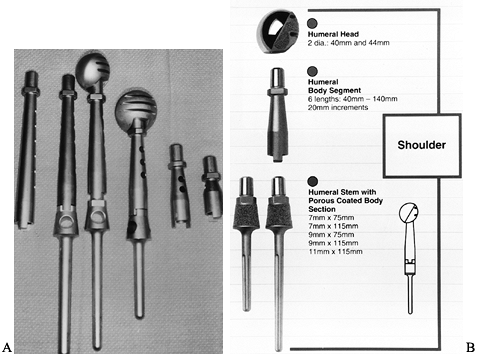 |
|
Figure 126.35. The current MRS for the proximal humerus. A:
The components of the modular system. There are various sizes of stems, bodies, and heads. Trial components are illustrated (note holes in the bodies denoting trial prostheses). B: Schematic of the MRS (courtesy of Howmedica, Rutherford, NJ). |
reconstructions. The reconstruction is combined with multiple muscle
transfers to reconstruct the soft tissues that have been resected. For
high-grade bone sarcomas, this is the method of choice. The deltoid and
axillary nerves are routinely removed along with the glenohumeral
joint. High-grade tumors often cross the glenohumeral joint and are a
source of local tumor recurrence; therefore, intra-articular resections
and reconstructions with osteoarticular allografts are not recommended.
Treat low-grade tumors with an intra-articular resection and
preservation of the abductor mechanism.
prosthesis and create shoulder stability in a patient who has undergone
resection of the proximal humerus (Fig. 126.36).
This is accomplished through a technique of dual suspension that
entails static and dynamic reconstruction. Use Dacron tape to secure
the prosthesis horizontally, and secure the Dacron tape by drill holes
in the remaining bony structures (i.e., clavicle and scapula or
clavicle alone). The two sets of Dacron tape provide mediolateral and
craniocaudal stability. Dynamic suspension, provided by transfer of the
short head of the biceps muscle to the stump of the clavicle, allows
elbow flexion.
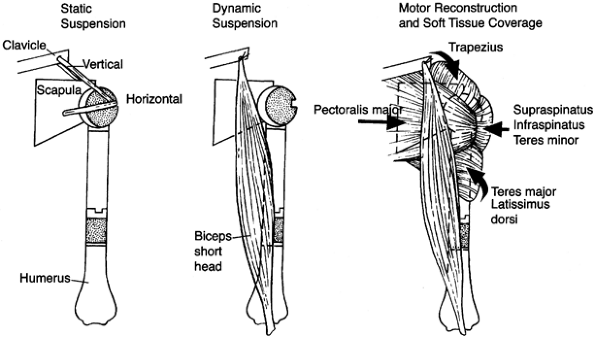 |
|
Figure 126.36.
Schematic technique of static and dynamic reconstruction with multiple muscle transfers following resection of high-grade sarcomas of the proximal humerus. This schematic demonstrates the use of dacron tapes (Genzyme Surgical Product Company, Fall River, Massachusetts) for static suspension and the transfers of the pectoralis major, biceps, and trapezius muscles for active dynamic motor reconstruction and soft-tissue coverage. It is important that a prosthesis not be permitted to remain subcutaneously. Muscle coverage is essential to avoid secondary infection following potential wound problems or flap necrosis. (From Sugarbaker PH, Malawer MM.Musculoskeletal Surgery for Cancer: Principles and Techniques. New York: Thieme Medical, 1992: Chapter 27, with permission.) |
trapezius, supraspinatus, infraspinatus, teres minor, teres major, and
latissimus muscles provide mobility of the shoulder. These muscle
groups offer dynamic support, assist in suspension of the prosthesis,
and provide soft-tissue coverage, which is essential in preventing skin
problems and secondary infection.
successful. There are minimal problems with subluxation following
adequate soft-tissue reconstruction. Malawer et al. (75,76,80,81),
who have the most extensive experience with replacing the proximal
humerus with the MRS, report 95% survival of the prosthesis as
determined by Kaplan-Meier analysis at 10 years (Fig. 126.37).
Type I resections are routinely reconstructed with the prosthesis and
the soft-tissue transfers described; extra-articular resections are
reconstructed with the proximal humeral prosthesis lying anterior to
the scapula. A Gore-Tex graft reconstruction of the joint may provide
stability following a type I resection, even when normal soft tissues
are retained. The most common problem is temporary nerve palsy, which
occurs in approximately 10% of patients, usually involving the radial
nerve and musculocutaneous nerves. Infections and skin flap necrosis
occur rarely (1% to 2%) after endoprosthetic replacement around the
shoulder.
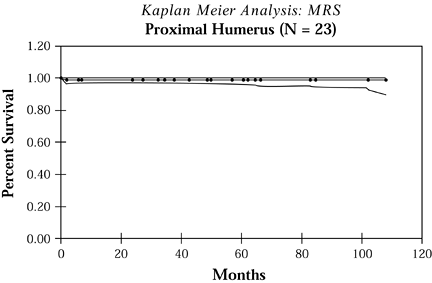 |
|
Figure 126.37.
Kaplan-Meier survival curve of modular proximal humeral replacement for high-grade bone sarcomas. The 5- and 10-year survival rates are between 95% and 100%. There were no infections, aseptic loosening, or local tumor recurrences. All patients were treated by a type V resection (extra-articular glenohumeral resection). |
for segmental joint replacements in the 1960s and 1970s, but they have
become less popular in the past two decades. The rate of complications,
which include infections,
fracture
through an allograft, nonunions, pain, and instability, is
significantly higher than with endoprosthetic replacements for stage
IIB bone sarcomas. Today, there are few indications for the use of
either osteoarticular or composite allograft. The most common
indications are for low-grade or aggressive benign bone tumors when
most of the adjacent soft tissue can be preserved. Osteoarticular
replacement is not indicated following high-grade tumor resection
because of the need to retain the rotator cuff, deltoid, and axillary
nerve, and the corresponding high rate of recurrence.
of 450 allografts performed at Massachusetts General Hospital, there
were seven fractures, three infections, and one nonunion (83).
Three of the allografts had to be removed and replaced, and flexion was
seldom above 45°. Most of the allografts were for low-grade sarcomas or
benign tumors (e.g., giant cell tumors). Two of the three stage IIB
osteosarcomas recurred locally and required amputation. Only seven
patients had no complications. We do not recommend osteoarticular
allografts for stage IIB sarcomas
(i.e., high-grade tumors with extra-articular components). One can generalize these concerns to composite allografts.
-
For an osteoarticular allograft for the proximal humerus, carefully size the allograft preoperatively.
-
When performing the procedure (Fig. 126.38),
place the allograft into the patient and fix it with a cortical plate,
intramedullary rod, or both. Cement the intramedullary fixation into
the allograft and impact it into the remaining humerus before affixing
the plate. The technique of intramedullary fixation with a small
derotation plate decreases the rate of fracture and nonunions. To avoid
subluxation and pain, it is essential to perform the soft-tissue
reconstruction of the joint and capsule tightly. -
Suture the subscapularis and rotator cuff to the corresponding soft-tissue components on the allograft.
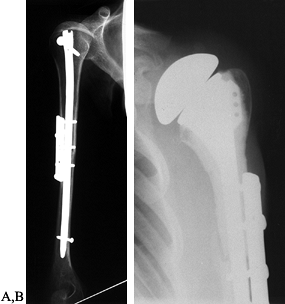 Figure 126.38. Plain radiographs demonstrating the technique of osteoarticular allograft reconstruction. A:
Figure 126.38. Plain radiographs demonstrating the technique of osteoarticular allograft reconstruction. A:
The technique of osteoarticular allograft to reconstruct the proximal
humerus following an intra-articular resection with an osteochondral
allograft. B: Plain radiograph of a composite allograft prosthesis following a proximal humeral resection. -
Then abduct the allograft into 90° of
abduction for fixation of the plate onto the remaining humerus.
Adequate soft-tissue coverage is essential. -
The allograft cannot be left
subcutaneously; therefore, if there is not enough musculature, rotate
the latissimus dorsi and the pectoralis major to cover the allograft.
complications of an osteoarticular replacement. The technique of
combining a prosthesis with an allograft is to provide a stable joint
surface and to avoid articular collapse, which is extremely common
following osteoarticular replacement alone. This entails cementing a
long-stem humeral prosthesis [Neer type (Howmedica, Rutherford, NJ)]
into the allograft and fixating it into the remaining humerus. Although
it is still popular, it offers no advantage over endoprosthetic
replacements and has higher infection and complication rates (e.g.,
nonunions and instability). The main indications for allograft
reconstructions are low-grade tumors in which the deltoid and rotator
cuff musculature can be retained and adjuvant chemotherapy is not
required.
allograft is similar to that of noncomposite allograft replacements.
The main difference is the use of a long or short endoprosthetic
proximal humerus component (Neer type) to replace the humeral head.
This is done to avoid subchondral fractures, which have been reported
with allografts alone. The fixation of the long stem into the remaining
bone appears to decrease the nonunion rate. Usually, plate fixation is
added, although this remains an issue of debate. The soft-tissue
reconstruction following composite allograft fixation is identical to
that of osteoarticular replacements.
intercalary arthrodesis using an allograft fixed to the remaining
distal humerus and scapula or glenoid. This is an extremely difficult,
time-consuming procedure. Complications are numerous and include
nonunion, skin necrosis, and metal or plate breakage. In addition, many
patients who have undergone arthrodesis complain about the lack of
rotation, which other reconstructive techniques preserve. In general,
the maximal abduction is only 30° to 45°. We do not recommend
arthrodesis.
flail shoulder. Before endoprosthetic and allograft reconstruction, the
shoulder girdle was left unreconstructed after resection. The patient
needed a large, bulky, external orthosis to support the upper extremity
and prevent the arm from pulling on the brachial plexus and vascular
structures. This approach does not permit any type of stability and,
moreover, it does not afford a stable fulcrum, which is necessary for
elbow function. This procedure is recommended only following failed
reconstruction, usually in patients with infections that cannot be
resolved.
The original procedure, described using autogenous fibula bone grafts
in the 1970s (before modern endoprosthetic and composite allograft
reconstruction), was to remove both fibulas from the patient, screw
them together proximally to form a pseudohumeral head, and then fix
this to the adjoining host bone. It is a reliable method of
reconstruction, but it has an extremely high morbidity and is not in
common use now.
 |
|
Figure 126.39. Dual fibular arthrodesis of the humerus to the scapula following proximal humeral resection.
|
anastomosis techniques in free tissue and bone transplants permitted
surgeons for the first time to transplant a single fibula with its
attached blood supply to reconstruct the proximal humerus. This
technique, which involves the end-to-side anastomosis of the peroneal
artery (supplying the fibula) to the brachial artery, is extremely
time-con- suming, and there is minimal evidence that the fibular
transplants remain viable. Bone scans and angiography have been used to
show postoperative patency of the vascular anastomosis. Single fibulas,
even when vascularized, have high incidences of stress fractures,
subluxation, and dislocation. This technique has recently been combined
with osteoarticular allograft arthrodesis to reinforce the allograft
and promote its incorporation in the glenoid and scapula and the
remaining humeral bone. It has not been demonstrated that this
technique has a higher incorporation rate than nonvascularized fibula
transplants to humeral defects. Although appealing, it has not been
widely used.
We recommend an extra-articular (type V) resection. This method may be
modified if an intra-articular resection is to be performed for
low-grade sarcomas. The method of soft-tissue reconstruction and
prosthetic suspension was described previously. In general, the deltoid
muscle and axillary nerve cannot be preserved for high-grade sarcomas.
Three osteotomies are required for an extra- articular resection.
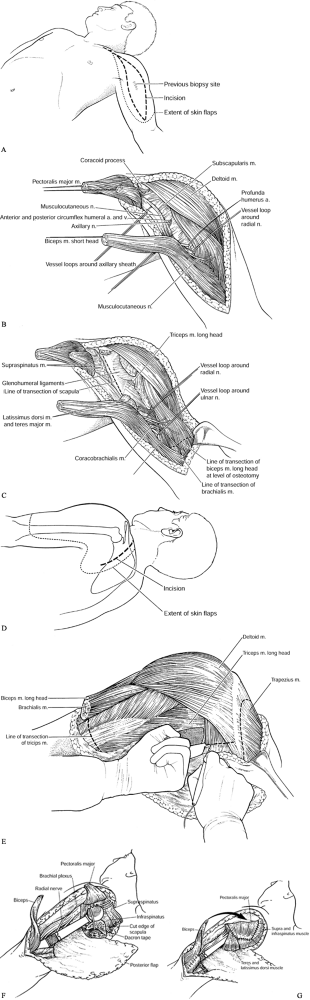 |
|
Figure 126.40. Surgical technique of resection of the proximal humerus for high-grade bone sarcomas. (From Sugarbaker PH, Malawer MM.Musculoskeletal Surgery for Cancer: Principles and Techniques. New York: Thieme Medical, 1992: Chapter 27, with permission.) A:
Make an anterior incision to permit wide exposure of the axilla, axillary and brachial vessels, shoulder joint, and anterior scapula. B: The key to the exposure of the axilla and its contents is the release of the pectoralis major muscle and the short head of the biceps and pectoralis minor from the coracoid. C: Expose the anterior neck of the scapula. Transect the subscapularis muscle. The capsule attaches lateral to the coracoid. Make the scapula osteotomy (dotted line) lateral to the coracoid so as not to enter the joint. Perform this osteotomy after the posterior exposure. D: Posterior incision. The anterior incision is now a T to permit exposure of the posterior aspect of the shoulder musculature and to allow the scapular osteotomy to be performed safely. If an intra-articular resection is performed, steps (D) and (E) are omitted. E: Palpate the glenoid and transect the covering muscles. This permits direct exposure of the posterior scapula and glenoid neck. F: Reconstruction with a modular prosthesis. G: Major muscle reconstructions. |
-
Place the patient in a lateral position, allowing some mobility of the upper torso.
-
Prepare the skin down to the level of the midline anterior and posterior to the umbilicus, and cranially past the hairline.
-
Start the incision over the junction of
the inner and middle thirds of the clavicle, continue along the
deltopectoral groove, and then move down the arm over the medial border
of the biceps muscle (Fig. 126.40A). -
Excise the biopsy site, leaving a 2–3 cm
margin of normal skin. Do not open the posterior incision until the
anterior dissection is complete. -
For exploration of the axilla, open the skin through the superficial fascia.
-
Dissect the skin flap anteriorly off the
pectoralis major muscle to expose its distal third, and uncover the
short head of the biceps muscle. The key to exposure of the anterior
shoulder girdle and axilla is the detachment and mobilization of the
pectoralis major muscle with partial mobilization medially toward the
chest wall (Fig. 126.40B). -
Dissect the pectoralis major muscle
overlying the axilla free of fat, so that its insertion on the humerus
can be visualized. Then divide this muscle just proximal to its
tendinous insertion on the humerus, and use a suture to tag the portion
of muscle remaining with the patient. -
Identify the axillary sheath and
visualize the coracoid process. To expose the axillary sheath along its
full extent, divide the pectoralis minor, short head of the biceps, and
coracobrachialis muscles at their insertion on the coracoid process.
Tag all proximal muscles with a suture for later identification and use
in reconstruction. -
Before exploring the neurovascular
bundle, develop the skin flaps just minimally. If the patient’s tumor
is found unsuitable for limb-salvage surgery, more extensive flap
dissection would lead to tumor contamination of the skin needed for
forequarter amputation. -
In dissecting the neurovascular bundle,
pass vessel loops around the neurovascular bundle near the proximal and
distal ends of the dissection. Medial traction on the neurovascular
bundle allows visualization of the axillary nerve, posterior circumflex
humeral artery, and anterior circumflex humeral artery. (It is rare to
preserve the axillary nerve in large stage IIB sarcomas of the proximal
humerus, but if the tumor is small and intraosseous,
P.3355P.3356P.3357
the nerve can be preserved.) Ligate these three structures and divide them. -
If the neurovascular bundle is tumor free, proceed with dissection for a limb salvage procedure.
-
Isolate and preserve the musculocutaneous
nerve, although it is occasionally necessary to sacrifice this nerve to
preserve tumor-free margins of resection. -
Divide the deep fascia between the short
and long heads of the biceps muscle; this permits easy visualization of
the musculocutaneous nerve. -
Identify the radial nerve at the lower
border of the latissimus dorsi muscle, where it passes around and
behind the humerus in its midportion (spiral groove) into the triceps
muscle group. -
Pass a finger around the humerus to bluntly move the nerve away from the bone.
-
Trace the ulnar nerve down the arm; you
must divide the intermuscular septum between the biceps and the triceps
over the nerve to see it clearly. -
It is necessary to divide the muscle
groups anteriorly to expose the neck of the scapula if performing an
extra-articular resection (Fig. 126.40C).
Separate the short and long heads of the biceps to expose the humerus.
Determine the site for the humeral osteotomy, and transect the long
head of the biceps and brachialis muscles at this level. -
Identify the inferior border of the
latissimus dorsi muscle, and make a fascial incision that makes it
possible to pass a finger behind the latissimus dorsi and teres major
muscles several centimeters from their insertion. -
Transect the latissimus dorsi and teres major muscles using electrocautery.
-
External rotation of the humerus exposes
the subscapularis muscle, which is transected at the level of the
coracoid process. Take care not to enter the joint space. Tag the
portions of these muscles that will remain with the patient for future
reconstruction. Transecting these muscles exposes the anterior portion
of the neck of the scapula. -
Now move to the posterior aspect of the patient. Rotate the table, if desired, to provide better visualization.
-
Begin the posterior incision anteriorly
over the junction of the middle and lateral thirds of the clavicle and
continue down over the lateral third of the scapula past the lower edge
of this bone. -
Develop a skin flap by dissecting the
skin and subcutaneous tissue between the anterior and posterior
incision from the underlying deltoid muscle down to the level of the
midhumerus. -
If removing the entire scapula (type VI
resection), make the posterior incision longer and curve it posteriorly
to allow the skin flap to expose muscle over the entire scapula. -
Next, divide the posterior muscle group (Fig. 126.40D).
Divide the thick fascia that joins the posterior border of the deltoid
muscle to the infraspinatus muscle and scapular spine. Leave the
deltoid muscle intact to cover the tumor mass. -
Transect the trapezius muscle from its insertions on the scapular spine and acromion.
-
Pass your index finger beneath the teres
minor up to the area of the planned scapular osteotomy. Transect the
supraspinatus, infraspinatus, and teres minor muscles over the neck of
the scapula, thus allowing the plane of transection through the neck of
the scapula to be exposed. Tag all transected muscles proximally (Fig. 126.40E). -
While shielding the radial and ulnar nerves, transect the triceps muscles at the level selected.
-
Perform clavicular, scapular, and humeral
osteotomies as follows. Divide the clavicle at the junction of its
middle and inner thirds. This is usually accomplished with a Gigli saw. -
Divide the scapula through its surgical neck medial to the coracoid process, also using a Gigli saw.
-
Perform the clavicular and scapular osteotomies before the humeral osteotomy.
-
Remove the entire specimen, taking care to protect all the neurovascular structures at each osteotomy site.
-
If resecting the entire scapula, take the skin flap back to the medial edge of the scapula.
-
Divide the rhomboid, levator scapula, and
trapezius muscles from their insertions on the scapula. It is
unnecessary to divide the teres major, teres minor, supraspinatus,
infraspinatus, and subscapularis muscles when performing a full
scapular resection. -
Transect the humerus 4–6 cm distal to the tumor, as determined by preoperative bone scan.
-
Obtain frozen sections of tumor margins
and touch preparations for cytologic examination of the marrow at the
site of the osteotomy. -
Measure the section of humerus removed
and select a prosthesis 1–2 cm shorter. Some shortening of the
extremity facilitates soft-tissue coverage and closure. -
Reconstruct the defect with a modular prosthesis (Fig. 126.40F). The head of the prosthesis sits anterior to the remaining scapula, which has a normal concavity.
-
Place the head in 30° to 45° of retroversion.
-
The soft-tissue reconstruction consists
of static and dynamic supports. Use dacron tapes (static) to suspend
the prosthesis from the remaining scapula as well as from the clavicle. -
The major muscle reconstructions (Fig. 126.40G)
are the pectoralis major to the lateral border of the scapula, thus
covering and supporting the prosthesis. The reaming muscles, the
trapezius, supraspinatus, infraspinatus, and latissimus dorsi are
tenodesed to the transferred pectoralis muscle.
soft tissue should still cover the tumor. The long and lateral heads of
the triceps muscle remain on the humerus. The upper portion of the long
head of the biceps and the upper portion of the brachialis muscle
remain with the specimen. The entire deltoid muscle covers the tumor.
The insertions of the supraspinatus, infraspinatus, pectoralis major,
latissimus dorsi, teres major, teres minor, and subscapularis muscles
remain covering the tumor and constitute the free margins.
It is indicated when the tumor involves a large component of the
medullary shaft. In such cases, an endoprosthesis or allograft would
not be successful because only a short distal segment remains following
resection. A second and more common indication for total humeral
resection is Ewing’s sarcoma of the humeral diaphysis. Induction
chemotherapy will often shrink the large extraosseous component
frequently associated with Ewing’s sarcomas, making a resection
possible.
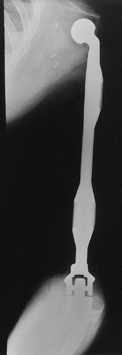 |
|
Figure 126.41.
Plain radiograph of a total humeral prosthesis following resection of the entire humerus, glenohumeral joint, and elbow joint for a large diaphyseal osteosarcoma that extended both proximally and distally. |
component are similar to those previously described. Considerations
relating to the midshaft and distal humerus center on the relationship
of the tumor to the brachial artery and nerves. Angiography is required
to determine the relationship to the brachial vessels medially and the
antecubital fossa. Use MRI and bone scan to determine the extent of the
humeral involvement, which determines whether total humeral resection
is required. Remove the entire humerus in patients who have round cell
tumors of the humerus and diaphysis.
-
The surgical approach is similar to that of a type V resection using multiple muscle transfers and dacron tape (Fig. 126.42).
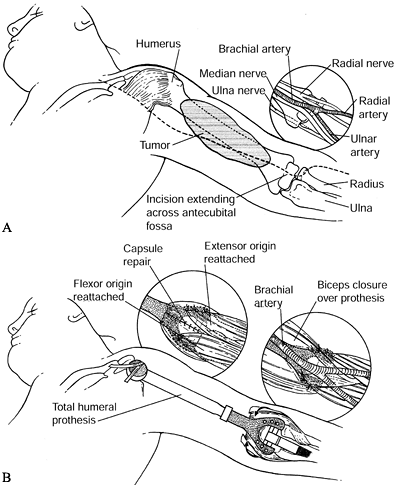 Figure 126.42. Technique of total humeral resection and replacement. A:
Figure 126.42. Technique of total humeral resection and replacement. A:
Continue the anterior incision to the elbow and across the joint to
permit exposure to the brachial vessels, and the median, ulnar, and
radial nerves, which all must be identified and retracted. B:
A modular distal humeral body—a component of the MRS. Insert the
olecranon component anteriorly. Attach the flexors and extensors to
suture holes in the prosthesis with dacron tape (Genzyme Surgical
Product Company, Fall River, Massachusetts). Then repair the biceps to
adjacent soft tissues. -
Explore the vessels proximally, release
the circumflex vessels proximally, and identify the musculocutaneous,
axillary, and radial nerves. -
In addition, mobilize the brachial
vessels throughout the length of the arm into the antecubital space to
protect them and the accompanying medial nerve. The ulnar nerve passes
posteriorly through the intramuscular septum and can easily be
identified in the mid arm. -
Likewise, identify the radial nerve as it
passes around the humerus and into the interval between the biceps and
brachioradialis muscle, where it becomes the posterior interosseous
nerve. It is necessary to identify and to initially preserve all of
these nerves, as well as the brachial artery and vein (Fig. 126.42A). -
The resection is similar to that of the
proximal humerus lesions, but continue it down to the elbow joint,
which is opened anteriorly after mobilizing the brachial vessels and
the median nerve through the antecubital fossa. Avoid a posterior
approach to the elbow. Keep the triceps tendon attached to the
olecranon. -
Open the capsule of the elbow joint
circumferentially; this makes it possible to fit the elbow component
and seat it into the olecranon. Detach the flexor and extensor muscles
from their respective origins on the humeral condyles. Retract the
biceps, but do not detach it from its insertion onto the radial
tuberosity. -
Use one of the several elbow devices available (Fig. 126.42B). An intramedullary stem fixed with polymethylmethacrylate (PMMA) is widely used.
-
Soft-tissue reconstruction of the elbow
consists of reattaching the forearm flexor and extensor muscles to
holes in the prosthesis. -
The ulnar nerve is routinely transposed
anteriorly to avoid irritation from the prosthesis. Then repair the
biceps to the adjacent soft tissue. -
Take care to interpose the capsule between the prosthesis and the neurovascular structures.
but the duration of these prostheses is reliable. The most critical
consideration following total humeral replacement is the potential for
arterial thrombosis and occlusion, nerve compression, or neurapraxia. A
sling or plaster splint is needed for a longer time than for proximal
humeral resection to allow for soft-tissue healing around the elbow and
the shoulder girdle. Elevation is required for the first 72 hours.
Rehabilitation must focus on both the shoulder and the elbow joints.
Fortunately, it is possible to preserve most of the musculature of the
shoulder girdle, which allows for an extremely stable shoulder girdle
as well as preservation of most of the elbow musculature.
limb salvage make the hip and pelvis one of the most difficult areas of
musculoskeletal oncology surgery. Prior to the development of effective
techniques for management of pelvic girdle malignancies, the best
patients could hope for was an oncologically sound hip disarticulation
or hemichpelvectomy. The resulting emotional and functional impact for
these patients, however, was considerable. Even as limb salvage
techniques developed for other anatomic areas, management of pelvic
lesions was still fraught with higher complication rates and less
satisfactory functional results (32,35,56,126,127).
The recognition that the surgical margin that could be obtained with
amputation for large lesions was often no better than an effective limb
salvage procedure provided the impetus to extend the indications for
limb salvage to include even large pelvic lesions, provided some sort
of functioning extremity could be preserved. Anatomic approaches and
reconstructive techniques eventually became more standardized, and a
limb salvage approach for many pelvic lesions developed. Furthermore,
the development of a more uniform system for the evaluation of
functional results allowed better comparison of reconstructive
techniques (36). With more recent improvements
in spinal fixation techniques, reconstruction for lesions involving the
sacroiliac area and even some lesions requiring resection of the
portion of the spine are possible in selected patients.
and to a lesser degree proximal femoral anatomy, is key to successful
limb salvage. The bony pelvis forms a complete ring, with the two
hemipelvi adjoined anteriorly at the symphysis pubis and posteriorly to
the sacrum by the sacroiliac (SI) joints. Although portions of the SI
joints are true diarthrodial joints, the close proximity of the ilium
to the sacrum, as well as dense ligamentous attachments, distinguish
them from other large synovial joints. This has important implications
in tumor surgery because these joints, and for that matter the
symphysis pubis, do not present the same relative barrier to tumor
spread as do other joints. Extensions of tumor across the SI joints are
not
uncommon, with large masses of tumor from the ilium frequently
extending across the SI joint anteriorly in front of the sacrum.
with each of these bones contributing to the acetabulum. Because these
bones fuse at skeletal maturity, resections of the pelvis tend to be
classified not simply according to the bone that is removed, but rather
by the zone of the pelvis involved.
muscle attachment, along both the iliac crest and the iliac and gluteal
fossas. Tumors arising within the ilium, even with soft-tissue
extension, are almost completely surrounded by muscle, which can aid in
developing surgical margins. The ilium is not simply a site of muscle
attachments, however. The posterior medial portion from the pelvic brim
to the sciatic notch is very thick and forms the structural continuity
between the acetabulum and the sacrum. Resections of the iliac wing
that can preserve even a part of this portion of the ilium will leave a
structurally intact pelvic ring and often require no significant
reconstruction.
portion of the acetabulum, as well as a portion of the structural
posterior column of the pelvis. It has important ligamentous and muscle
attachments as well. The sacrospinous and sacrotuberous ligaments are
dense structures connecting the ischium to the sacrum, and they serve
to define the greater and lesser sciatic notches. Muscle attachments
such as the coccygeus, levator ani, and obturator internus help form
the floor of the true pelvis. Furthermore, the ischial tuberosity
serves as the origin for the hamstrings. Not only the ischium’s
important bony function, but also the important soft-tissue attachments
and proximity of other important neurovascular structures make tumors
arising within the ischium difficult to manage.
in some ways, the most expendable. It contributes to a part of the
anterior aspect of the acetabulum, but a structurally less significant
part. At the midline it joins with the pubis on the opposite side to
form the symphysis pubis, thus completing the pelvic ring. Provided the
posterior SI joints are intact, however, resection of the symphysis
pubis does not significantly compromise pelvic stability. Therefore,
from a purely structural standpoint, the entire pubis is considered
expendable. Its importance from a surgical standpoint lies more with
its soft-tissue attachments and its proximity to other neurovascular
and urogenital structures. The rectus abdominus is an important muscle
attachment forming a portion of the anterior abdominal wall. The
ilioinguinal ligament and ilioinguinal ring complex also attach near
the pubic tubercle, as do the origins of the medial thigh muscles.
Directly anterior to the pubis, near its midportion, lie the femoral
vessels within their own vascular sheath. Lateral to the femoral sheath
lies the confluence of the iliacus and psoas muscles and the
accompanying femoral nerve. Just inferior to the pubis, traveling
through the superolateral aspect of the obturator foramen, are the
obturator vessels and nerve. Similarly, important urogenital structures
are intimately related with the pubis and symphysis pubis. These
include the bladder directly posteriorly, the urethra inferiorly, and,
in males, the spermatic cord, which is just superior to the pubis
traveling through the inguinal ring. Tumors of the pubis, particularly
if any soft-tissue mass exists, can often be intimately associated with
these structures, making preservation sometimes difficult.
important in determining levels of resection and structural continuity
of the limb, it is the soft-tissue anatomy, in terms of muscles and
neurovascular structures, that most greatly contributes to the ultimate
function of the limb. Of primary importance in assessing patients for
potential limb salvage is determining which nerves need to be
sacrificed and which can be preserved.
and inability to save the sciatic nerve is considered an indication for
amputation. The sciatic nerve has nerve root contributions from the
L-4, L-5, S-1, S-2, S-3 levels. The L-4 and L-5 roots form a conjoined
root traveling anterior to the sacrum at the S-1 level that is
intimately associated with the sacrum and the anterior portion of the
SI joint before diving posteriorly to enter the sciatic notch. The
sacral contributions exit the sacral foramen anteriorly at their
respective levels, transversing laterally to join with the L-4, L-5
root within the sciatic notch. Malignancies of the ilium and ischium
with extensive soft-tissue extension either extrapelvically or
intrapelvically near the sciatic notch usually require sacrifice of the
entire sciatic nerve. This renders the lower leg functionless and
insensate, and it also sacrifices the neurologic function to all of the
gluteal muscles. Lesions involving the lower portion of the ischium or
proximal femur may also have soft-tissue extensions near the sciatic
nerve. Fortunately, the farther the nerve gets from the sciatic notch,
the more mobile it becomes, making a greater latitude of nerve
displacement possible. In many cases, although the nerve is displaced,
it still can be preserved. This is certainly in contrast to the tight
anatomic confines of the nerve within the sciatic notch itself.
including those tumors arising within the pubis or anterior portion of
the acetabulum or ilium, potentially involve the femoral nerve. Within
the pelvis, the femoral nerve lies between the iliacus and iliopsoas
muscles, and there is a certain amount of potential for it to be
displaced by masses in this area. It is most tightly confined where it
passes beneath the inguinal ligament and over the pubis near the
iliopectineal eminence. Large lesions in this area may require
sacrifice of the femoral nerve, but in contrast to
sacrifice of the sciatic nerve, this is not necessarily a contraindication to limb salvage.
extensions toward the femoral nerve. Fortunately, almost immediately
after the femoral nerve passes beneath the ilioinguinal ligament, it
begins to branch, and often only a portion of the nerve needs to be
sacrificed. The final significant nerve exiting the pelvis with
functional implications is the obturator nerve, which is formed from
nerve roots exiting the L-2, L-3, and L-4 levels. It courses along the
lateral side wall of the pelvis, just over the pelvic brim, and exits
the pelvis through the obturator foramen. It can also easily be
involved with periacetabular lesions or pubic lesions and may require
sacrifice. This certainly denervates the adductor muscles of the thigh,
but it does not significantly compromise gait. Its preservation,
therefore, is not a requirement for limb salvage.
can be simplified for the purposes of limb salvage to that of an
anterior, or external, iliac artery distribution and a posterior or
internal iliac artery distribution. The external system has no
significant branches within the pelvis until it gets to the level of
the inguinal ligament. There it gives rise to the deep circumflex iliac
and inferior epigastric arteries. The inferior epigastric artery is
important in terms of potential soft-tissue reconstruction because it
is the main pedicle to the lower portion of the rectus abdominus
muscle, which can be used as a rotational flap for soft-tissue
coverage. Once the artery passes under the inguinal ligament, it
becomes the common femoral artery and eventually the superficial and
profunda portion. The medial and lateral femoral circumflex arteries
generally arise from the profunda branch. They not only provide much of
the blood supply to the proximal portion of the femur, but they also
contribute to the rich anastomosis around the hip with terminal
branches from the internal iliac system. Within the pelvis, the
internal iliac vessels lie posterior to the external iliac vessels as
they traverse toward the sciatic notch. They become tightly adherent to
the pelvic side wall as they course over the sacrum and pelvic brim and
begin to divide into multiple terminal branches: superior and inferior
gluteal, internal pudendal, and obturator, as well as branches
providing blood supply to the intrapelvic contents such as the bladder
and, in the female, the uterus. Tumors of the pelvis, with soft-tissue
extensions inferior to the pelvic brim, can often involve these vessels
or significantly displace them. Ligation of the internal iliac artery
is often necessary but, due to the rich anastomosis both within the
pelvis and outside the pelvis, this in itself does not cause a
significant vascular problem. Thus, vascular involvement itself is
rarely a contraindication to limb salvage; the external iliac system
can be bypassed, and the internal iliac system is unnecessary.
is important in dealing with pelvic lesions with significant
intrapelvic masses. In the retroperitoneum, the ureter courses over the
top of the psoas muscle and, at the level of the bifurcation of the
great vessels, lies lateral to them. It crosses anterior to the common
iliac vessels near the origin of the external and iliac branches, and
from there the ureter lies in a medial position and begins to deviate
from the vessels as it courses to the bladder. It is important to
identify the ureter in any retroperitoneal dissection in this area. It
is possible to identify it intraoperatively by testing for peristalsis,
but for lesions with larger intrapelvic masses, preoperative placement
of a ureteral stent can help, particularly if you suspect it may be
displaced from its normal anatomic position. The bladder itself can be
involved with larger intrapelvic masses, although more often than not
it tends to be displaced and easily separated from tumor masses.
Certainly, with distinct bladder involvement, a urologic surgeon’s
assistance is required because portions of the bladder can also be
resected en bloc with the lesion.
system, patients with pelvic neoplasms usually present with pain, a
mass, or both. The development of a mass may go undetected for long
periods of time, particularly if it is in an intrapelvic location, but
even an extrapelvic mass or proximal femoral mass can go undetected. In
contrast to lesions occurring more distally in the extremity, where
peripheral nerves are more mobile, neurologic symptoms arising from
pelvic lesions are not uncommon. Radicular pain from compression of the
sciatic nerve or lumbosacral nerve roots can arise from lesions
posterior to the hip joint or in the sacroiliac area due to the
anatomic confines of the nerves in these locations. Often these
symptoms are mistaken for low back pain or lumbar disc disease. It is
certainly not uncommon for a patient with a pelvic neoplasm to have
undergone previous lumbar discectomy without relief of symptoms before
the true etiology of the pain is identified.
suspected pelvic neoplasms, the objective is to localize the area of
pain and tenderness, identify soft-tissue masses, and evaluate the
neurologic function of the extremity. The presence of a mass either
intrapelvically or extrapelvically in patients with pelvic pain is
usually a sign of malignancy and should be evaluated accordingly. The
identification of neurologic compromise, particularly if it corresponds
to a particular level, is helpful in directing further studies. Bowel
and bladder compromise is rare in patients with lesions of the pelvis
because sufficient innervation from the contralateral side will
maintain neurologic function. Significant alteration in bowel or
bladder function suggests a higher lesion, one involving the sacrum or
spine itself.
radiographic evaluation. The fact that interpretation of plain films is
sometimes difficult because of the complex anatomy, as well as
overlying bowel and gas, is no reason not to obtain them. An initial
diagnostic impression and reasonable differential diagnosis can
generally be made after examining the plain radiographs. In patients
under 30 years of age, destructive lesions of the pelvis and proximal
femur are generally the result of osteosarcoma or Ewings’ sarcoma.
Benign lesions, such as aneurysmal bone cyst and giant cell tumor, are
also possible and occasionally can have an aggressive appearance that
warrants further radiographic evaluation. Large osteochondromas can
also arise from the proximal femur and pelvis and may even produce a
palpable mass. These patients are often already aware of the presence
of an osteochondroma. If they have the impression that it is enlarging,
though, malignant degeneration is a concern. As patients become older,
the possibility of metastasis from carcinoma or myeloma is more likely.
Certainly if one suspects metastasis, the diagnostic strategy should
include looking for other lesions or other primary lesions.
malignancies of the pelvis, particularly in patients over 40 years of
age. These malignancies can present as intramedullary lytic lesions
within the pelvis or proximal femur and are not necessarily associated
with the distinctive calcific pattern generally attributed to these
lesions. Certainly with patients having destructive lesions of the
pelvis associated with a large soft-tissue mass containing fluffy and
flocculent calcifications, a chondrosarcoma should be suspected. In
fact, it is possible to confidently diagnose many of these lesions
solely from plain film findings.
films can be particularly difficult, even with good-quality
radiographs. If you suspect a sacral lesion, further diagnostic imaging
is in order. CT scans are particularly helpful in evaluation of pelvic
lesions. Like the plain film, CT images are best at defining bony
abnormalities and less effective in determining soft-tissue or
intramedullary disease. As such they complement the plain film in
determining the extent of cortical destruction and the presence of
soft-tissue calcification. A CT scan is more often used for pelvic and
spinal lesions than for extremity lesions because it is so difficult to
visualize the complex three-dimensional anatomy of the pelvis and spine
from plain films alone. Once again, certain lesions can be diagnosed
with certainty from CT findings. For example, the calcific pattern
associated with cartilage lesions can be identified effectively with a
CT scan. Other lesions, such as aneurysmal bone cysts, can be
effectively diagnosed with CT imaging if typical fluid levels are
identified.
malignancies of the pelvis or proximal femur. The intraosseous or
medullary extent of lesions can be accurately identified and often will
be more extensive than one might presume from examining plain film or
CT findings. Soft-tissue extension, both intrapelvically and
extrapelvically, is well assessed with an MRI, especially the proximity
to neurovascular structures. Furthermore, the MRI is excellent at
assessing lesions that may involve the sacrum or lower spine because it
can accurately image the spinal canal and potential involvement of
nerve roots. In general, plain film and the CT scan give the most
information regarding the actual character or histologic diagnosis of a
lesion, whereas the MRI gives the most information regarding anatomic
staging or the resectability of a lesion.
malignancies is similar to other anatomic areas. Technetium-99 bone
scans can identify the source of other bony lesions, but they tend not
to give any further information regarding the local disease. A thorough
evaluation of the chest with chest radiographs and a CT scan is
important in determining potential pulmonary involvement. Specialized
tests that may be unique to pelvic neoplasms include angiography and
tests to determine urologic involvement. Angiography usually is not
required to assess vascular involvement, which can be predicted based
on an MRI. Certainly if the specific diagnosis would benefit from
embolizations, such as a large aneurysmal bone cyst, angiography can be
an important tool. Similarly, angiography can play a role in assessing
response to a preoperative chemotherapy.
an intravenous pyelogram (IVP) or more directly with cystoscopy. Prior
to CT and MRI, the IVP was perhaps more valuable in determining
displacement of the ureter or bladder. For these purposes, the MRI and
CT scan are currently used more often. Cystoscopy can be helpful in
determining actual bladder involvement, as it provides direct
visualization. It is also helpful if a ureteral stent is placed to aid
in the intraoperative localization of the ureter.
biopsy is indicated to establish histologic diagnosis. There are many
biopsy techniques available, and the actual technique used depends on
the size and location of the lesion and, to some degree, on its
suspected histologic diagnosis. For a patient with a known primary
lesion and suspected metastasis, a simple fine-needle aspirate with or
without CT guidance is acceptable. If the lesion is indeterminate,
however, and certainly if you suspect a primary malignancy of the
pelvis, the specific biopsy technique employed should allow for
potential limb salvage.
complex than for lesions in the long bones. It is mandatory to have an
understanding of the incisions and surgical approaches required for a
pelvic limb salvage prior to biopsy of any of these lesions. An
inappropriately placed biopsy, particularly one directly through the
midportion
of
the gluteus muscle to get at an iliac or ischial lesion, can compromise
the large gluteal flap that is often used in pelvic resections. In
general, for lesions in the ilium, place the biopsy track along the
iliac crest from the posterior superior iliac spine anteriorly up to
the anterosuperior iliac spine. For lesions of the sacrum, a biopsy
track close to the midline posteriorly is preferable. The biopsy track
for lesions involving the anterior pelvis in the area of the pubis or
symphysis should be along the line of the ilioinguinal ligament, either
lateral to the femoral nerve or medial to the femoral vessels near the
pubic tubercle or symphysis pubis. If the mass is in the interpelvic
portion of the ilium or pubis, try to keep the biopsy track in an
extraperitoneal location, which again means staying close to the iliac
crest line. Biopsy lesions involving the obturator foramen, inferior
pubic rami, or ischium from an anteroinferior direction, are shown as
shaded regions in Figure 126.43.
Lesions of the proximal femur can be biopsied directly lateral on a
line anywhere from the tip of the greater trochanter down the shaft of
the femur.
 |
|
Figure 126.43. Anterior view of incisions used for pelvic resections. The overlying shading shows general areas where it is safe to perform biopsies for the anterior lesions. A: Ilioinguinal portion. B: Iliofemoral portion. C: Inferior rami portion.
|
extent of resection needed, it is also possible to biopsy lesions along
a line corresponding to the Smith-Peterson interval. This extends from
the anterosuperior iliac spine, anteriorly and laterally across the
thigh, in the interval between the tensor fascia muscle laterally and
the sartorius and rectus medially. Lesions located along the medial
acetabular wall and sciatic notch are in a very difficult area to
biopsy, at least with sound oncologic principles. Unless extension of
the mass occurs anterior to the level of the pelvic brim, it can be
very difficult to reach from an intrapelvic biopsy placed along the
wing of the ilium. Posteriorly directed biopsies through the sciatic
notch potentially contaminate important neural structures. Under such
circumstances, a consultation with an orthopaedic oncologist is
indicated to determine the biopsy path that has the least anatomic and
oncologic complications.
As previously mentioned, the classification is based on the zones of
the pelvis that are removed rather than on actual anatomic bones. Type
I resections are iliac wing resections, type II resections are
acetabular resections, and type III are pubic rami or obturator
resections. Enneking and Dunham initially used the letter A
to further classify more extended resections. For example, a type IA
resection includes the iliac wing plus the gluteal muscles, a type IIA
resection includes the acetabulum as well as the proximal femur as an
extra-articular resection of the hip, and a type IIIA resection
includes the iliopsoas and femoral nerve. Since their original article,
this basic classification system has been modified. A type IV resection
has been added that is used to classify those resections that also
include a portion of the sacrum, such as an iliosacral resection. These
classifications not only provide a more uniform system for describing
the resections, but also help to determine the optimal position for the
patient for surgery and the surgical incision required.
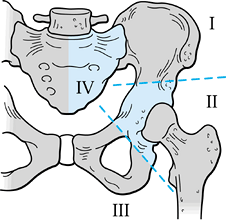 |
|
Figure 126.44. Classification of pelvic resections.
|
somewhat simpler and for the most part is based on the length of the
proximal femur removed and whether the resection is performed as an
intra-articular or extra articular resection. If a proximal femoral
resection necessitates complete removal of the acetabulum, the anatomic
and reconstructive challenges are virtually identical to those of the
type II acetabular resections.
set of incisions (Figure 126.43, Figure 126.45).
The standard or utilitarian incision is useful for most iliac and
acetabular resections. The incision is similar to that used for an
extended iliofemoral approach (67).
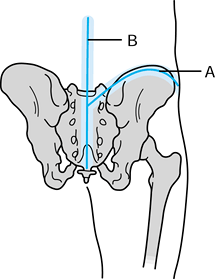 |
|
Figure 126.45. Posterior view of incisions for pelvic resections. A: The posterior limb of the iliofemoral and ilioinguinal incision. B: The midline spine incision. The overlying shading shows the areas where biopsy track should be placed.
|
-
Begin the incision at the posterosuperior iliac spine and extend it along the iliac crest to the anterosuperior iliac spine.
-
Extend it then distally and laterally
down the thigh, over the interval between the tensor fascia lata muscle
laterally and the sartorius and rectus muscles medially. -
End the incision near the mid-lateral
position of the thigh, 10–15 cm distal to the tip of the greater
trochanter. It is possible to easily expose both the intrapelvic and
extrapelvic portions of the iliac wing through this incision, as well
as most of the acetabulum and hip joint. -
To expose any desired amount of femur, extend the incision distally down the thigh.
-
For posterior exposure of the pelvis, use
a large gluteal flap. Detach the origin of the gluteus maximus muscle
and fascia from the iliac crest, and incise the fascia between the
gluteus maximus and tensor fascia lata from the level of the iliac
crest distally to the midportion of the iliotibial track, just past the
level of the gluteus insertion. Turn the gluteus maximus itself
posteriorly with its inferior gluteal neurovascular bundle. This
exposes the interval between the gluteus maximus and the gluteus medius
and minimus. -
Often with malignancies of the wing of
the ilium, it is necessary to resect all or a portion of the gluteus
medius and minimus with the specimen; this can easily be done through
this approach. Structures exiting the greater sciatic foramen are
accessible, as is the entire posterior column of the hemipelvis. -
Intrapelvic exposure of the iliac wing of
the ilium is also possible through this incision. Detach the abdominal
wall, including the external oblique, internal oblique, and
transversalis muscles, from the wing of the ilium, generally 1–2 cm
away from the iliac crest. The retroperitoneal space is entered quite
easily over the top of the iliacus muscle, which allows for an
oncologically safe plane of dissection for lesions involving the ilium.
Exposure of the pelvis is readily achieved from the sacroiliac joint to
the level of the iliopectineal eminence through this incision alone. -
If the lesion involves only the iliac
wing (type I), develop the space within the iliacus and psoas muscles
by retracting the femoral nerve and the psoas medially. Identify the
pelvic brim and carry the dissection posteriorly to expose the
intrapelvic portion of the sciatic notch. -
Depending on the size of the intrapelvic
mass or the extent of the desired resection, further medial
retroperitoneal dissection may be required. The external iliac vessels
within their vascular sheath are aligned along the medial edge of the
psoas muscle. It is possible to trace these proximally to their common
iliac origin and, generally, to identify the internal iliac vessels as
well. Through this standard iliofemoral approach, however, more
extensive retroperitoneal exposure is difficult without a more formal
ilioinguinal incision. -
Begin the ilioinguinal portion of the
incision at the anterosuperior iliac spine and extend it medially along
the ilioinguinal ligament to the pubic tubercle and symphysis pubis.
This T extension of the incision does not normally have an adverse effect on wound healing. -
Incise this superficial ilioinguinal
fascia from the level of the anterosuperior iliac spine to the pubic
tubercle, and expose the ilioinguinal ligament itself. The ligament can
be detached laterally or incised along its length as described by
Letournel (68) for the ilioinguinal approach in trauma. -
Once the ligament is detached or incised,
reflect it proximally and medially along with the abdominal wall, thus
entering the retroperitoneal space. The femoral sheath and inferior
epigastric pedicle are located midway between the anterosuperior iliac
spine and the pubic tubercle. -
Ligate and cut the inferior epigastric
vessels. Along the medial third of this dissection, identify the
spermatic cord as it exits through the superficial external ring. -
Detach the ligamentous attachments of the
ring from the pubis inferiorly, allowing the spermatic cord to be
retracted proximally and medially. This approach allows adequate
exposure of virtually all the important retroperitoneal anatomy
previously alluded to. -
Alternatively, use the extended ilioinguinal approach as the primary incision, with the T
extension as the distal lateral portion of the iliofemoral approach.
The exposure is identical and merely a matter of the primary intent of
the exposure and personal preference. -
For a more extensive exposure of the
pubis, extend the ilioinguinal excision across the midline, reproducing
the exposure on the contralateral side. -
For inferior pubic rami exposure, another incision or extension of the standard ilioinguinal incision is required. Figure 126.43
shows this limb of the exposure, which requires an incision from the
pubic tubercle distally in the proximomedial thigh, along the line of
the inferior pubic rami. This can be used alone, but more generally it
is used in combination with at least a portion of the ilioinguinal
approach and provides exposure to the entire obturator ring. Thus it is
useful for type III pelvic resections. -
Finally, for more extensive posterior
exposure of the pelvis and sacrum, as is required in iliosacral
resection, use a midline spinal incision. Together with the
posterolateral portion of the pelvic incision, this represents another T-type
of incision, but it also has excellent healing qualities. The deep
exposure gives access to the entire sacrum for laminectomy, nerve root
exposure, and ultimate resection. The incision can be extended
superiorly along the midline as far as necessary for further posterior
spinal exposure.
that involve the iliac wing and the immediate adjacent soft-tissue
structures. Type I resections tend to be used with small lesions
because a clear bony margin must exist in the supra-acetabular area to
allow for a line of resection. The posterior resection is made at or
near the sacroiliac joint. An important factor for these resections is
whether a posteromedial strut of bone can be preserved, thereby
maintaining structural continuity between the acetabulum and the
sacrum. Figure 126.46 is an example of such a
resection: Under the circumstances illustrated, no reconstruction is
needed. If a complete resection is done through the sciatic notch,
reconstructive options include (a) doing nothing, or (b) attempting to
restore pelvic continuity with either autograft
or
allograft. Without reconstruction or with only minimal attempts at
reconstruction—hinging the pelvis on the symphysis and approximating
the remaining ischium to the sacrum—patients will develop a
pseudarthrosis in this area. Most patients will have only minimal pain,
but they will be left with a slightly shortened extremity. A better
functional result can be achieved by reconstructing continuity of the
pelvic ring (4,35,98).
Autograft or allograft struts, either of fibula or, perhaps, iliac
crest, can be wedged into the defect with internal fixation in the form
of plate and screws to stabilize the pelvis until the graft heals (Figure 126.47).
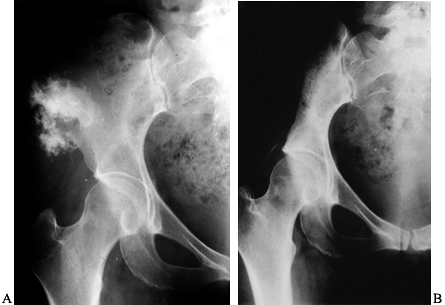 |
|
Figure 126.46. Type I pelvic resection with no reconstruction. A: Preoperative AP radiograph illustrating a chondrosarcoma located in the anterior ilium. B:
Postoperative radiograph showing a line of resection from the superior acetabular lip to the posterior ilium. A strut of bone was left in continuity between the acetabulum and the sacrum, and therefore no reconstruction was required. |
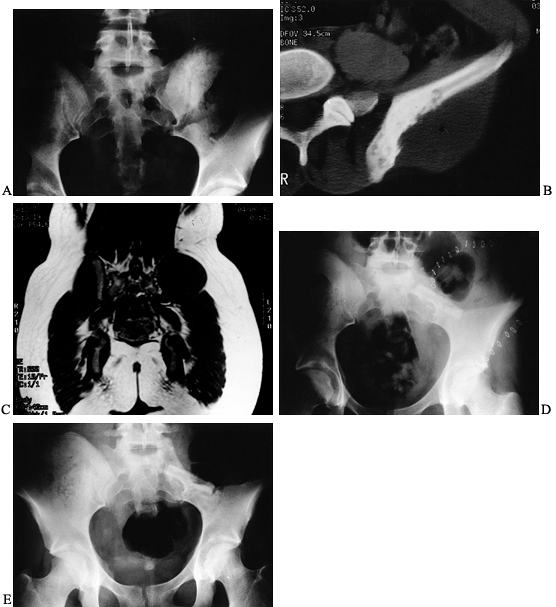 |
|
Figure 126.47. Type I pelvic resection requiring reconstruction. A: AP radiograph of the pelvis showing a dense sclerosis within the posterior ilium in a 19-year-old woman. B: CT scan of the posterior ilium showing sclerosis as well as bony disruption and a soft-tissue mass. C: T1-weighted coronal MRI showing a large soft-tissue mass on the posterior ilium. The biopsy showed a Ewing’s sarcoma. D:
Immediate postoperative radiograph showing almost complete resection of the posterior ilium and a portion of the sacrum. A fibular allograft strut was placed between the sacrum and the ilium to aid in stability. E: One-year postoperative radiograph showing a consolidation of the bone graft. The patient was pain free with an excellent gait. |
10 of 13 patients with type I or IA resections had a good functional
result, although if pelvic continuity was lost, only 2 of 4 had a good
result. The experience at the Mayo Clinic was similar: Out of 17
iliosacral resections, the best results were in the 12 patients who
were left with a stable pelvis or who had a solid arthrodesis—and none
of them had less than a good result. On the other hand, only 1 of the 5
patients with a pseudarthrosis or without reconstruction had a good
result (98).
iliosacral fusion can be difficult because there is sacral bone loss.
Extending the arthrodesis to the lumbar spine may be necessary. In
these circumstances, spinal fixation instrumentation can be used with
special modifications to allow fixation out to the ilium (Figure 126.48).
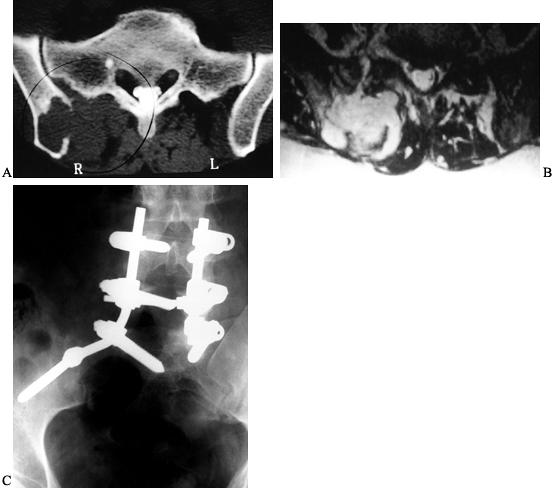 |
|
Figure 126.48. Iliosacral resection with spinal instrumentation. A: CT scan in a 46-year-old man showing a destructive lesion in the posterior ilium involving the sacrum. B:
T2-weighted axial MRI showing the soft-tissue mass involving the ilium. The lesion proved to be a hemangiopericytoma, and wide resection required removal of the entire posterior ilium, the lateral portion of the sacrum, and a portion of L-5. C: Reconstruction with an iliolumbar fusion with spinal instrumentation. A defect was bone grafted with morcelized allograft bone. |
surgical removal standpoint, but also from a reconstructive standpoint.
Whereas one can expect a reasonably good functional result after type I
or type III resections alone, any resection involving the acetabulum
generally has significant functional implications.
involve all or a portion of the acetabulum and for those lesions that
involve the hip joint proper, whether the lesion arises primarily
within the pelvis, soft tissue, or proximal femur. For lesions that do
arise within the pelvis itself, it is unusual to be able to do only a
type II resection. Most type II resections are done either in
combination with the iliac wing as a type I/II or in combination with
the anterior arch of the pelvis as a type II/III.
incision anteriorly. Adequate exposure of the intrapelvic and
retroperitoneal structures is gained through the ilioinguinal portion
of the dissection. The degree of soft-tissue extension into the pelvis
determines the difficulty of dissection as well as the need to
sacrifice important structures. In general, it is possible to preserve
the external iliac vessels, as well as the femoral nerve; dissect them
carefully away from any tumor mass. Ligation of the internal iliac
vessels is often necessary, not only for adequate oncologic margins,
but also to aid in getting the external iliac vessels away from any
tumor mass. Once the tethering effects of the internal iliac vessels
are removed, the external iliac vessels can easily be displaced
medially.
-
Divide the iliacus and psoas muscles near
the intended level of bony resection, and identify the superior aspect
of the sciatic notch. -
Use the lower portion of the ilioinguinal
approach to identify the superior pubic rami and its intended resection
level. The obturator nerve and vessels are generally sacrificed,
particularly if there is any intrapelvic extension of tumor. The
iliofemoral portion of the exposure completes the extrapelvic portion
of the exposure. Not only are the lines of the iliac and ischial
osteotomies determined, but exposure of the entire hip capsule
circumferentially is possible. -
For intra-articular resection, make a
circumferential capsular arthrotomy and dislocate the hip. A complete
dissection of the sciatic nerve, from its exit through the sciatic
notch to the proximal thigh, is possible. -
Complete removal of the acetabulum also
requires a sectioning of the sacrospinous ligament. Take care to
preserve the pudendal nerve, if possible, and the internal pudendal
vessels.
on the actual amount of bone stock remaining. In principle, just as in
other anatomic areas, the options include a flail joint, a stable
arthrodesis, or a functioning arthroplasty. Early experiences with limb
salvage of periacetabular lesions, before the development of effective
reconstructive techniques, generally resulted in a flail hip joint (3,126).
This also left the patient with a shortened extremity and a somewhat
unstable hip, but it was preferable to a hemipelvectomy. There may
still be indications for no reconstruction or an attempt to achieve a
stable pseudarthrosis with minimal reconstruction. Normally this would
occur when all or most of the hemipelvis was removed. Most authors
agree that functional results are certainly improved and pelvic
continuity can in some way be restored (38,98,130).
of the pelvis can be an effective way of restoring function. Depending
on the amount of bone removed, fuse the proximal femur either to the
ilium as an iliofemoral fusion or, if the majority of the ilium has
been resected, the proximal
femur
to the remaining ischium as an ischiofemoral arthrodesis. With an
iliofemoral arthrodesis, fit the proximal femur onto the remaining
ilium and, in most cases, secure it in a place with a 4.5 mm cobra
plate (Figure 126.49).
The greater trochanter of the femur can be osteotomized so that the
plate fits more in line with the femur. The iliac fixation is,
generally, on the outer table of the ilium, but it can be on the inner
table if need be. Although this results in a shortened extremity, it
produces a good functional result if successful.
 |
|
Figure 126.49.
Iliofemoral arthrodesis after type II pelvic resection. The proximal femur is fixed to the remaining ilium with a large cobra plate and screw fixation. |
pseudarthrosis rate of at least 50%. In some patients, the
pseudarthrosis is stable and minimally painful, and they may end up
with a reasonably functional result. Experience from the Rizzoli
Institute actually suggests that the functional result from a stable
pseudarthrosis was very similar to that of a solid arthrodesis (14).
These results suggested that minimal internal fixation, in the form of
wiring the femur to the remaining ilium, may be preferable. This
generally led to a firm pseudarthrosis and minimized the overall
complication rate associated with failed fixation. To avoid the
invariable limb shortening associated with a direct iliofemoral
arthrodesis or attempted pseudarthrosis, an intercalary allograft can
be used. Fusion, however, is still difficult, with a high rate of
ultimate failure through nonunion or infection (15,98).
functional outcome for patients who require periacetabular resections
along with the majority of the iliac wing. In this instance, the
proximal femur is fixed to the remaining ischium, generally with large
fragment screws or wire cerclage (Figure 126.50).
Successful patients generally have a good functional result, with less
limb shortening than would be associated with an iliofemoral
arthrodesis. The difficulty lies in actually obtaining the arthrodesis.
The fusion rate is lower than that seen with iliofemoral arthrodesis.
Additionally, postoperative pain at the symphysis pubis from the high
stresses in this area combined with a very poor functional outcome if a
pseudarthrosis occurs have led many physicians to avoid the
ischiofemoral arthrodesis.
 |
|
Figure 126.50.
Ischiofemoral arthrodesis after type I/II pelvic resection. The proximal femur is fused with the remaining ischium, a combination of interfragmentary screws, and a cerclage cable. |
can be achieved with either an endoprosthesis osteoarticular pelvic
allograft or prosthetic bone graft composites. The type of arthroplasty
performed is based on remaining bone stock, as well as physician and
patient preferences.
reconstruction, as long as adequate ilium remains, is the use of saddle
prosthesis. The saddle prosthesis was designed in Germany and consists
of a U-shaped or saddle-shaped articulating piece, which is fit into a notch in the ilium (Figure 126.51).
This articulating piece is connected to a relatively standard femoral
prosthesis on the femoral head taper. Body segments can also be added
in between to restore adequate limb length. Abduction and
flexion/extension are achieved at the prosthesis–bone interface, with
rotation achieved within the articulating prosthetic segment itself.
Soft-tissue reconstruction is critical to provide stability of the
prosthesis within the iliac notch. The early experience of Nieder et
al. (96,97) with this
prosthesis was primarily for patients with massive bone loss due to
failed total joint arthroplasty. More recently, the prosthesis has been
used in patients after periacetabular resections. Aboulafia et al. (1)
reported on their experience with 17 patients, of whom 12 had overall
good to excellent functional outcomes. In general, the functional level
result is certainly not as good as a conventional hip arthroplasty, but
it is much better than a flail hip and more predictable than attempts
at iliofemoral arthrodesis.
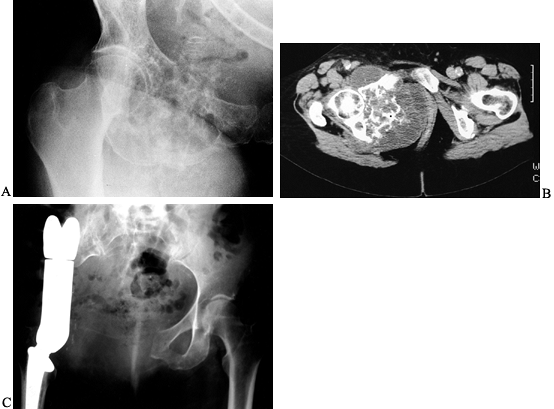 |
|
Figure 126.51. Type II/III pelvic resection with reconstruction using a saddle prosthesis. A:
AP radiograph of the hip showing a large chondrosarcoma involving the entire obturator foramen. The lesion is primarily situated within the superior pubic rami, but it clearly extends to the acetabulum. B: CT scan illustrating extensive destruction of the medial acetabulum along with soft-tissue masses both within the pelvis and extending through the obturator foramen. C: A resection required removal of the entire acetabulum, and the reconstruction was performed with a saddle prosthesis. |
acetabular resection involves the use of the pelvic endoprosthesis
incorporating an actual hip joint near the anatomic center of the
native joint (2,45,95,135,144).
This prosthesis can be made as a custom implant or as a modular
implant, allowing some intraoperative flexibility. By reproducing the
normal hip center, Windhager et al. (144) felt
their functional results were better than their experience with the
saddle prosthesis. The advantage of these systems over using allografts
or attempting to obtain an arthrodesis is a tendency toward fewer early
complications, less morbidity, and an earlier functional return. This
is particularly important in a patient with a high-grade malignancy of
the pelvis who may have limited survival.
These techniques again have the advantage of reproducing a more normal
hip center and limb length, but they are also associated with a longer
operative time and increased morbidity. The use of a hemipelvic or
whole acetabular allograft requires being able to procure a graft,
normally from a large regional or a national bone bank, of the
appropriate size to match the defect created after resection. The graft
is normally fixed to the remaining host bone with a combination of
plates and screws or large Steinmann pins. Perform a conventional hip
arthroplasty, creating an allograft–prosthesis composite (Figure 126.52).
Keep reaming of the acetabulum to a minimum to avoid excessive
weakening of the graft. Now cement the acetabular component into place.
Alternatively, use a bipolar prosthesis, thus eliminating any reaming
of the allograft bone. It is certainly possible to replace the entire
hip joint, including a segment of proximal femur, as a complete
osteoarticular graft, although experience with this has generally
led to failure, and most authors suggest using a prosthesis for the hip joint itself.
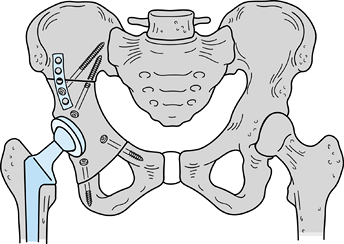 |
|
Figure 126.52.
Reconstruction of a type II pelvic resection with a whole acetabular allograft. Fix the allograft to the ilium and pubis with plate and screw fixation and interfragmentary screws as necessary. Then perform a total hip arthroplasty, cementing the acetabular cup into the allograft bone. |
resected specimen and reimplanting it. After resecting the specimen,
clean it of any soft tissue and tumor and autoclave it at 135°C for
approximately 10 minutes. Reinsert it into the original bed and fix it
securely with plates or threaded Steinmann pins. The advantage of this
technique is that it requires no allograft procurement or sizing. The
fit is exact and therefore achieves excellent bone-to-bone contact.
Clearly, if the tumor itself has already destroyed a lot of the actual
bone, the technique would not be useful. Therefore, it is generally
reserved for those lesions with minimal bone destruction.
or autoclaved autografts suggest a number of potential problems,
including infection, dislocation, and graft failure through resorption,
fracture, or prosthetic loosening (17,49,95,101). Harrington (49) reported on 14 patients, 10
of whom had reconstruction with an allograft and 4 of whom had the
autoclaved autograft technique. Although he reported only one infection
and one local recurrence, there were still three patients with late
fracture of the grafts. The experience reported by Bell et al. (6),
on 17 patients with pelvic allografts, also points out the potential
difficulties with these reconstructions. They noted a local recurrence
in three patients, deep infection in two, and dislocation of the hip in
five. Overall, these results are not dramatically different from those
achieved with reconstructions by other methods and serve to illustrate
the difficulty of restoring function in periacetabular resections. In
spite of the morbidity, attempts at reconstruction are generally
worthwhile because the functional results are considerably better than
leaving the hip flail.
pubic rami and obturator foramen through the ilioinguinal incision,
with or without its posterior extension along the inferior pubic rami.
-
After completing the intrapelvic and extrapelvic dissection, determine the lines of bony resection.
-
The osteotomy through the symphysis pubis
or even the contralateral side is generally made with a Gigli saw. Take
care to protect the urethra from injury. -
Laterally, the superior pubic rami can be
cut at any level, even to the point of resecting a portion of the
anteroinferior acetabulum. Provided this is a minor amount of
acetabulum, it will not compromise hip stability. It is usual to be
able to preserve and protect the femoral vessels and femoral nerve
throughout the dissection. It is very common, however, to have to
sacrifice the obturator nerve, as mentioned previously. -
Make the posterior osteotomy anywhere
along the inferior pubic rami as determined by the tumor extent, even
to the point of making the resection through the posterior column just
inferior to the acetabulum (Figure 126.53).![]() Figure 126.53. Type III pelvic resection. A: AP radiograph of the lower pelvis showing a chondrosarcoma located within the superior pubic rami. B: CT scan showing a lesion primarily within the superior pubic rami with apparent sparing of the acetabulum. C:
Figure 126.53. Type III pelvic resection. A: AP radiograph of the lower pelvis showing a chondrosarcoma located within the superior pubic rami. B: CT scan showing a lesion primarily within the superior pubic rami with apparent sparing of the acetabulum. C:
Type III resection of the pelvis. A small portion of the anterior
acetabular wall was removed, but no reconstruction was necessary. -
You can usually palpate the sulcus just
inferior to the acetabular lip, and this serves as a landmark to the
inferior portion of the acetabulum. -
Expose the posterior column
circumferentially through the lesser sciatic notch, using either a
Gigli saw or an osteotome to make the cut. Generally, the resection can
be made without dislocation of the hip, but if need be, dislocate the
hip temporarily.
unnecessary because continuity between the femur and the sacrum is
maintained (Figure 126.53). This is borne out
by the experience of most authors, who show that functional results are
generally good to excellent in the absence of any reconstruction (14,35,38,98).
Soft-tissue closure after type III resections is particularly
important. You must reconstruct the lower abdominal wall, normally by
suturing the ilioinguinal detachment to the adductor muscles of the
thigh. Be careful during the repair to allow plenty of room for the
superficial femoral vessels as well as the spermatic cord. Because the
pelvic floor is disrupted, there does tend to be a settling and a
slight shift of abdominal contents into the area and, potentially, even
into the upper thigh, but ths is generally asymptomatic.
on a number of factors, including the location and grade of the lesion
as well as the ability to achieve a wide surgical margin. These
principles are similar to those for malignancies that occur in other
areas, and they are discussed in Chapter 128, Chapter 129 and Chapter 130.
The pelvis is somewhat unique in that its anatomic complexities tend to
make it more difficult to achieve a wide surgical margin. This is
particularly evident in lesions that occur in the iliosacral area,
because the margin for either may be similar, whether an amputation or
a limb salvage procedure was done. Local recurrence rates after pelvic
resections do tend to be higher than for extremity lesions. Overall,
reports show local recurrence rates for lesions occurring in the pelvis
to be in the range of 5% to more than 30% (6,14,35,58,98,118).
The highest local recurrence rates are generally for iliosacral
lesions. If you can achieve wide margins, the local recurrence rate is
distinctly lower, usually less than 20%. When a marginal or lesional
margin is achieved, the local recurrence rate tends to be closer to 50%
or even higher.
primarily on the grade of the lesion. In general, patients with
high-grade lesions of the pelvis tend to have a poorer prognosis than
those with extremity lesions. In the series by both Shin et al. (118) and Kawai et al. (57), overall survival in patients with high-grade sarcoma of the pelvis was less than 35%.
locations for primary bone tumors. In children and young adults, the
most common lesions include osteosarcoma and Ewings’ sarcoma, whereas
in patients over 40 years old chondrosarcoma is more likely. The
proximal femur is also a common site for metastasis and myeloma, and,
occasionally, proximal femoral resections are required to manage these
lesions as well. Most lesions of the proximal femur are amenable to
limb salvage because only with the most extensive soft-tissue
involvement are any important neurovascular structures involved. The
femoral vessels lie far enough anterior and medial to the femur itself
that they are not normally a problem and, as mentioned earlier, the
sciatic nerve at this level is not only well away from the femur but
also mobile enough to make tumor involvement rare. Furthermore, because
the functional results of hip disarticulation or hemipelvectomy are so
poor, any reconstruction that provides a hip stable enough to allow
reasonable knee and foot function would be worthwhile.
intra-articular or extra-articular. For intra-articular resection, the
acetabulum is preserved in its entirety, and reconstruction with a
functional arthroplasty is generally possible. In an extra-articular
resection, the entire joint is removed, including the acetabular
articular surface. If this can be done by removing only a shell of the
acetabulum without totally disrupting pelvic continuity, reconstruction
with arthroplasty is also possible. If the entire acetabulum is
removed, thereby disrupting pelvic continuity, the reconstructive
challenges are similar to those encountered with type II pelvic
resection.
-
It is usual to do the resection in a
lateral position with a long, straight, lateral incision that
incorporates the biopsy track. -
Split the tensor fascia lata lengthwise; it can generally be preserved.
-
Detach the gluteus medius and minimus
from the greater trochanter, preserving as much length as possible
depending on tumor extent. The origin of the vastus lateralis muscle is
normally left with the specimen, but often a significant amount of the
muscle itself can be preserved. Provided a cuff of the muscle is left
adjacent to the femur, reflect the more superficial portion of the
vastus lateralis anteriorly; it can be very useful in soft-tissue
reconstruction. -
Expose the anterior aspect by externally
rotating the femur. Variable amounts of rectus femoris will need to be
resected or released, depending on tumor extent. The sartorius muscle
spans the area of resection and therefore can generally be preserved. -
It is usual to transect the iliopsoas
muscle at the level of the hip capsule. Only with very large lesions
will you need to expose the femoral vessels themselves. -
By internally rotating the hip, expose
the posterior aspect of the femur. Detach the short external rotators,
quadratus femoris, and gluteus maximus from the femur, leaving a cuff
of tissue. -
You can generally palpate the sciatic nerve, and often it is not necessary to expose it directly.
-
Osteotomizing the femur at the intended
level of resection facilitates medial exposure of the femur. The
proximal femur becomes much more mobile, and it is possible to transect
the adductor muscles appropriately. -
If the resection is to be intra-articular, incise the hip capsule circumferentially.
-
Cut the ligamentum teres and remove the specimen.
-
The options for reconstruction after
proximal femoral resection depend primarily on whether the resection
has been done with intra-articular or extra-articular margins.
Intra-articular resections are generally amenable to arthroplasty, and
patients generally prefer this. Perform the arthroplasty with an
osteoarticular allograft, an allograft prosthesis composite, or an
endoprosthetic reconstruction. -
When an osteoarticular allograft is
planned, it is important to select a graft of the appropriate size,
particularly the femoral head dimensions. Test the femoral head
articulation within the acetabulum intraoperatively to ensure that it
is the proper size. A femoral head too large or too small generally
leads to problems, and one can decide to reconstruct with an allograft
prosthesis composite if need be. -
To do the soft-tissue reconstruction
around the hip, suture the remnant of the patients’ capsule to the
capsule that is attached to the allograft. -
Also suture the abductor muscles to the stump of tendon that was harvested with the graft.
-
Bony fixation to the host bone is generally done with the large fragment plate and screw fixation.
noted nonunion and fracture in five patients, infection in four, and an
unstable hip in one. Of the 15 patients, 10 required reoperation for
complications.
composite provides a better functional result with fewer complications
than an osteoarticular allograft alone. The composite reconstruction
provides the advantages of the allograft in terms of soft-tissue
attachment, and also the more predictable return of function with fewer
of the complications associated with an endoprosthesis. The specifics
of the composite construct depend on the length of femur resected. From
a biomechanical standpoint, it is considered unwise to have the tip of
the prosthesis at or near the allograft–host junction.
-
If the femoral resection is long, cement
a standard hip component into the allograft, then fix the entire
composite to the distal femur with a plate and screws. The plate should
be long enough to overlap the stem by a few centimeters to attempt to
alleviate any stress riser effect near the tip of the femoral stem. -
If the resection length is shorter, use a
long-stem femoral component. Once again, cement the allograft to the
proximal portion of the stem, and insert the entire composite into the
distal femur. -
A press-fitting of the stem in a
noncemented fashion achieves fixation in the host bone. If needed, add
a plate to the allograft host junction for additional stability. The
articular portion of the reconstruction can be done with a standard
acetabular component or as a bipolar arthroplasty.
reported on 13 patients with allograft prosthesis composite
reconstruction. These patients had a much lower incidence of
graft-related complications and had an overall better functional result
than did the osteoarticular grafts. In addition, in a number of their
patients, failures of osteoarticular graft were salvaged with allograft
prosthesis composites. Gitelis and Piasecki (44)
reported on their experience with allograft prosthesis composite and 11
reconstructions of the hip. In an average follow-up of almost 4 years,
they reported overall good functional results and no implant revisions
as a result of loosening.
The prosthesis is either a one-piece design or a more recent, modular
system, which allows intraoperative customization of length. This makes
some intraoperative flexibility possible, as does an allograft
prosthesis composite. The fixation stems can vary in length and
diameter, and they can be straight or bowed to accommodate whatever
remains of the patients’ femur. These prostheses are generally cemented
in place, although noncemented designs have shown effectiveness as
well. Once the prosthesis is in place, soft-tissue reconstruction is
critical in minimizing complications and improving function.
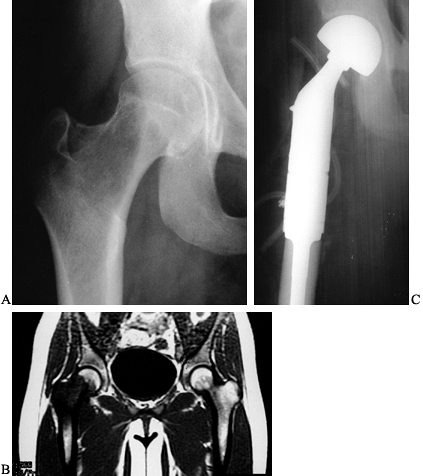 |
|
Figure 126.54. Proximal femoral resection and endoprosthetic reconstruction. A: AP radiograph of the proximal femur showing a lytic lesion within the femoral neck and head in a 19-year-old woman. B: T1-weighted coronal MRI showing a lesion within the proximal femur, which eventually proved to be osteosarcoma. C:
AP radiograph of the proximal femur after proximal femoral resection and endoprosthetic resection with a modular proximal femoral prosthesis. |
-
Secure whatever capsular structures
remain around the neck of the prosthesis, generally in purse-string
fashion, to improve stability. -
Pull the abductor muscles and tendons
distally as far as possible and secure them to the prosthesis with a
loop or other fixation at a point near the old greater trochanter. -
Use the remaining muscles of the thigh to cover up the entire prosthesis with healthy, viable muscle.
-
Pull the vastus lateralis proximally and
laterally as far as possible and secure it either to the abductor
muscle or to the underside of the tensor. -
Pull the gluteus maximus muscle anteriorly and suture it to the remaining vastus as well.
-
With some diligence, the prosthesis can
generally be covered, which aids in preventing deep infection and
improves function by getting the muscle back out to a more physiologic
length.
been favorable. Multiple series have been reported with various
prosthetic designs (32,51,62,70,76,121,126,134,137).
The most common complication seems to be dislocation, which is reported
in up to 25% of patients. Reoperation for mechanical failure or
loosening has been very low, less than 15%, and failure due to deep
infection was also low, usually less than 5%. The functional results of
endoprosthetic devices are quite good in terms of pain relief. The main
functional problem is still the relative lack of abductor strength.
This is certainly quite variable and depends on the number of abductors
that are still available for reconstruction and the ability of the
surgeon to get satisfactory healing into the surrounding soft tissues.
In this regard, the allograft prosthetic devices may have a benefit.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
AJ, Buch R, Mathews J, et al. Reconstruction Using the Saddle
Prosthesis following Excision of Primary and Metastatic Periacetabular
Tumors. Clin Orthop 1995;314:203.
A, Grimer RJ, Cannon SR, et al. Reconstruction of the Hemipelvis after
the Excision of Malignant Tumours. Complications and Functional
Outcomes of Prostheses. J Bone Joint Surg Br 1997;79:773.
WJ, Goldberg JA, Scully SP, Harrelson JM. Vascularized Tissue Transfer
for Closure of Irradiated Wounds after Soft Tissue Sarcoma Resection. Ann Surg 1992;216:591.
RS, Davis AM, Wunder JS, et al. Allograft Reconstruction of the
Acetabulum after Resection of Stage-IIB Sarcoma: Intermediate-Term
Results. J Bone Joint Surg Am 1997;79:1663.
EW, Terek RM, Healey JH, Land JM. Allograft Reconstruction after
Proximal Tibial Resection for Bone Tumors: An Analysis of Function and
Outcome Comparing Allograft to Prosthetic Reconstruction. Clin Orthop 1994;303:116.
FP, Glasser DB, Otis JC, et al. The Van Nes Tibial Rotationplasty: A
Functionally Viable Reconstructive Procedure in Children Who Have a
Tumor of the Distal End of the Femur. J Bone Joint Surg Am 1990;72:1541.
R, Donati D, Fazioli F, et al. Iliofemoral Arthrodesis with Intercalary
Allograft. In: Brown KLB, ed. Complications of Limb Salvage.
Proceedings of the International Symposium on Limb Salvage (ISOLS),
Montreal, Canada, 1991:205.
R, Donati D, Masetti C, et al. Effect of Electromagnetic Fields on
Patients Undergoing Massive Bone Graft following Bone Tumor Resection:
A Double Blind Study. Clin Orthop 1994;306:213.
R, Morris HG, Campanacci D, et al. Modular Uncemented Prosthetic
Reconstruction after Resection of Tumours of the Distal Femur. J Bone Joint Surg Br 1994;76:178.
AE, Schaner EG, Conkle DM, et al. Evaluation of Computed Tomography in
the Detection of Pulmonary Metastases: A Prospective Study. Cancer 1979;43:913.
PF, Sim FH, Pritchard DJ, et al. Megaprostheses after Resection of
Distal Femoral Tumors. A Rotating Hinge Design in 30 Patients Followed
for 2–7 Years. Acta Orthop Scand 1996;67:345.
DR, Mankin HJ. Osteoarticular Allografts for Reconstruction after
Resection of a Musculoskeletal Tumor in the Proximal End of the Tibia. J Bone Joint Surg Am 1994;76:549.
D, Capanna R, Caldora P, et al. Internal Hemipelvectomy of the
Acetabular Area Using Different Methods of Reconstruction. In: Tan SK,
ed. Limb Salvage, Current Trends. Proceedings of the International Symposium on Limb Salvage (ISOLS), Singapore, 1993:185.
D, Capanna R, Campanacci D, et al. The Use of Massive Bone Allograft
for Intercalary Reconstruction and Arthrodesis after Tumor Resection: A
Multicentric European Study. Chir Organi Mov 1993;78:81.
JJ, Pignatti G, Eilber FR, et al. Management of Failed Endoprosthetic
Implants: The UCLA Experience. In: Langlais F, Tomeno B, eds. Limb
Salvage—Major Reconstruction in Oncologic and Nontumoral Conditions.
Berlin: Springer-Verlag, 1991:479.
WF, Dunham W, Gebhardt MC, et al. A System for the Functional
Evaluation of Reconstructive Procedures after Surgical Treatment of
Tumours of the Musculoskelatal System. Clin Orthop 1993;286:241.
WF, Menendez LR. Functional Evaluation of Various Reconstructions after
Periacetabular Resection of Iliac Lesions. In: Enneking WF, ed.Limb Salvage in Musculoskeletal Oncology. New York: Churchill Livingstone, 1987:117.
WF, Shirley PD. Resection Arthrodesis for Malignant and Potentially
Malignant Lesions about the Knee Using Intramedullary Rod and Local
Bone Grafts. J Bone Joint Surg Am 1997;59:223.
GE, Strong DM, Sell KW. Studies on the Antigenicity of Bone: I.
Freeze-Dried and Deep-Frozen Bone Allografts in Rabbits. J Bone Joint Surg Am 1976;58:854.
MC, Flugstad DI, Springfield DS, Mankin HJ. The Use of Bone Allografts
for Limb Salvage in High-Grade Extremity Osteosarcoma. Clin Orthop 1991;270:181.
B, LaRossa D, Lotke PA, et al. Salvage of Jeopardized Total-Knee
Prosthesis: The Role of the Gastrocnemius Muscle Flap. Plast Reconstr Surg 1983;83:85.
KD. The Use of Hemipelvic Allografts or Autoclaved Grafts for
Reconstruction after Wide Resections of Malignant Tumors of the Pelvis.
J Bone Joint Surg Am 1992;74:331.
AI, Gitelis S, Sheinkop MB, et al. Allograft Prosthetic Composite
Reconstruction for Limb Salvage and Severe Deficiency of Bone at the
Knee or Hip. Semin Arthroplasty 1994;5:85.
SM, Glasser DB, Lane JM, Healey JH. Prosthetic and Extremity
Survivorship after Limb Salvage for Sarcoma: How Long Do
Reconstructions Last? Clin Orthop 1993;293:280.
JW, Dubois CM, Smith SR, et al. Medial Gastrocnemius Transposition Flap
for the Treatment of Disruption of the Extensor Mechanism after Total
Knee Arthroplasty. J Bone Joint Surg Am 1997;49:866.
MH, Gebhardt MC, Tomford WW, Mankin HJ. Reconstruction for Defects of
the Proximal Part of the Femur Using Allograft Arthroplasty. J Bone Joint Surg Am 1988;70:507.
A, Muschler GF, Lane JM, et al. Prosthetic Knee Replacement after
Resection of a Malignant Tumor of the Distal Part of the Femur. J Bone Joint Surg Am 1998;80:636.
S, Bloom N, Lewis MM. Limb-Sparing Surgery in Skeletally Immature
Patients with Osteosarcoma: The Use of an Expandable Prosthesis. Clin Orthop 1991;270:223.
S, Lewis MM. Limb Sparing Surgery in Children. The Expandible
Prosthesis. Current Trends and Controversies after the First 10 Years.
Eighth International Symposium on Limb Salvage (ISOLS), Florence,
Italy, 1995;124.
KS, Chao EYS, Sim FH. Long-Term Performance of Custom Prosthetic
Replacement for Neoplastic Disease of the Proximal Femur. In: Yamamuro
T, ed.New Developments for Limb Savage in Musculoskeletal Oncology. Tokyo: Springer-Verlag, 1989;403.
KN, Gitelis S, Sim FH, et al. Segmental Replacement of Long Bones Using
Titanium Fiber Metal Composite following Tumor Resection. Clin Orthop 1983;176:108.
MM. The Use of the Expandable and Adjustable Prosthesis in the
Treatment of Childhood Malignant Bone Tumors of the Extremities. Cancer 1986;57:499.
MP, Goorin AM, Miser AW, et al. The Effect of Adjuvant Chemotherapy on
Relapse-Free Survival in Patients with Osteosarcoma of the Extremity. N Engl J Med 1986;314:1600.
MM, Chou LB. Prosthetic Survival and Clinical Results with Use of
Large-Segment Replacements in the Treatment of High-Grade Bone
Sarcomas. J Bone Joint Surg Am 1995;77:1154.
MM, Price WM. Gastrocnemius Transposition Flap in Conjunction with
Limb-Sparing Surgery for Primary Bone Sarcomas around the Knee. Plast Reconstr Surg 1984;73:741.
MM, Sugarbaker PH. Shoulder Girdle Resections: The Tikhoff-Linberg
Procedure and Its Modification. In: Sugarbaker PH, Malawer MM, eds. Musculoskeletal Surgery for Cancer: Principles and Techniques, 3rd ed. New York: Thieme, 1992;346.
HJ, Doppelt SH, Sullivan TR, Tomford WW. Osteoarticular and Intercalary
Allograft Transplantation in the Management of Malignant Tumors of
Bone. Cancer 1982;50:613.
PA, Heller G, Healey J, et al. Chemotherapy for Nonmetastatic
Osteogenic Sarcoma: The Memorial Sloan-Kettering Experience. J Clin Oncol 1992;10:5.
HG, Capanna R, Campanacci D, et al. Modular Endoprosthetic Replacement
after Total Resection of the Femur for Malignant Tumor. Int Orthop 1994;18:90.
CE. Aspiration Biopsy of the Spine: Technique for Thoracic Spine and
Results of Twenty-Eight Biopsies in This Region and Over-All Results of
1050 Biopsies of Other Spinal Segments. J Bone Joint Surg 1969;51:1531.
R, Baron R, Kotz R, et al. Muscle Function after Endoprosthetic
Replacement of the Proximal Tibia: Differential Techniques for Extensor
Reconstruction in 17 Tumor Patients. Acta Orthop Scand 1995;66:266.
P, Bacci G, Campanacci M, et al. Histologic Evaluation of Necrosis in
Osteosarcoma Induced by Chemotherapy: Regional Mapping of Viable and
Nonviable Tumor. Cancer 1985;56:1515.
G, Gitelis S, Morton T, Piasecki P. Complications Associated with Limb
Salvage for Extremity Sarcomas and Their Management. Clin Orthop 1990;280:242.
G, Caparros B, Groshen S, et al. Primary Osteosarcoma of the Femur: A
Model for the Use of Preoperative Chemotherapy in High Risk Malignant
Tumors. Cancer Invest 1984;2:181.
F, Derqui JC. Puncture Biopsy in Lesions of the Locomotor System:
Review of Results in 4,050 Cases, Including 941 Vertebral Punctures. Cancer 1968;21:531.
MA, Aschliman MA, Thomas N, Mankin HJ. Limb-Salvage Treatment versus
Amputation for Osteosarcoma of the Distal End of the Femur. J Bone Joint Surg Am 1986;68:1331.
HH. Partial or Complete Resection of the Hemipelvis: An Alternative to
Hindquarter Amputation for Periacetabular Chondrosarcoma of the Pelvis.
J Bone Joint Surg Am 1978;60:719.
RB, Kaufter H, Hankin FM. Partial Pelvic Resection as an Alternative to
Hindquarter Amputation for Skeletal Neoplasms. Clin Orthop 1989;242:201.
RC, Powers BE, Withrow SJ, et al. The Effect of Intramedullary
Polylmethylmethacrylate on Healing of Intercalary Cortical Allografts
in a Canine Model. J Orthop Res 1992;10:434.
WW, Thongphasuk J, Mankin HJ, Ferraro MJ. Frozen Musculoskeletal
Allografts: A Study of the Clinical Incidence and Causes of Infection
Associated with Their Use. J Bone Joint Surg Am 1990;72:1137.
WG, Johnston KS, Dorey FJ, Eckhardt JJ. Extramedullary Porous Coating
to Prevent Diaphyseal Osteolysis and Radiolucent Lines around Proximal
Tibial Replacements: A Preliminary Report. J Bone Joint Surg Am 1993;75:976.
WG, Yang RS, Eckardt JJ. Endoprosthetic Bone Reconstruction following
Malignant Tumor Resection in Skeletally Immature Patients. Orthop Clin North Am 1996;27:493.
H, Kenan S, Lewis MM, et al. The Role of Microvascular Surgery in
Limb-Sparing Procedures for Malignant Tumors of the Knee. Plast Reconstr Surg 1993;92:692.
HJ, Taminiau AH, Schimmel JW, van Horn JR. Kotz Modular Femur and
Tibial Replacement: 28 Cases Followed for 3 (1–8) Years. Acta Orthop Scand 1994;65:315.