MANAGEMENT OF INFECTED IMPLANTS
VII – NEOPLASTIC, INFECTIOUS, NEUROLOGIC AND OTHER SKELETAL DISORDERS
> Infection and Hemophilia > CHAPTER 135 – MANAGEMENT OF INFECTED
IMPLANTS
surgeons for as long as medical history has been recorded. Its
frequency and severity have lessened considerably, but occasionally it
is still responsible for compromising an otherwise good outcome for the
patient. The recent emergence of bacteria that are resistant to
antibiotics are of increasing concern. The purpose of this chapter is
to present the principles of treatment for infection involving
prosthetic joints and internal fixation devices for long bones.
successfully lodge on or near the implanted prosthesis. The easiest and
most common access for bacteria is the surgical wound. They flourish in
this environment because trauma to the tissues by the surgery, causes
some tissue necrosis is due to direct trauma, compromises the blood
flow to the tissues and results in hematoma formation. The presence of
the large foreign body provides a surface for the bacteria to adhere to.
hematogenous seeding and contiguous spreading from adjacent areas of
infection. The hematogenous spread of bacteria to a prosthetic joint
has been associated with chronic skin lesions, dental manipulation,
urinary infection, diabetes, and other chronic diseases. One of several
scenarios
may take place once the bacteria have gained entry to the area.
Bacteria may be destroyed by the host, live in symbiosis with the host,
or flourish, causing local infection, host sepsis, and even death.
acute inflammatory reaction. A myriad of components are involved in
this process, including polymorphonuclear cells, chemotactic factors,
and the immune system. Leukocyte diapedesis occurs, followed by
infiltration of polymorphonuclear cells to the area. The process in
which polymorphonuclear cells are attracted to the area by chemical
substances is known as chemotaxis. Boyden originally discovered that
chemotaxis occurred if serum was incubated with precipitates of
antigens of antibiotics (9).
humoral components. Both cell-mediated and humoral responses are
activated to fight bacterial infections. Once the polymorphonuclear
cells attack bacteria, some may be damaged, thus releasing additional
chemotactic molecules that attract even larger numbers of
polymorphonuclear cells. When the polymorphonuclear cells are in
proximity to the bacteria, the particles are phagocytosed. For
phagocytosis to occur, opsonins or components in the serum must coat
the bacteria, making them more attractive for the macrophage.
with joint replacement, may be particularly susceptible to infection
because their immune systems are compromised. With increasing age, the
immune becomes less competent. These changes include atrophy of the
thymus (the site of T-cell maturation), a decreased ability to mount a
delayed-type hypersensitivity to various stimuli, and a generalized
decreased ability of lymphocytes response to respond to foreign stimuli.
by activation of the complement system but also by certain medications.
Nonsteroidal anti-inflammatory agents, steroids, and aspirin are common
medications that suppress the immune response (55).
In a combined study from the Mayo Clinic and The Hospital for Special
Surgery, 97 pathogens were isolated from 76 patients who had
periprosthetic hip infections (20,21). Staphylococcus epidermidis and S. aureus were identified 36 and 18 times, respectively, accounting for more than one half of the pathogens. Streptococcus viridans,
group-D Streptococcus or Enterococcus, and beta-hemolytic serogroups,
also gram-positive organisms, accounted for an additional 14 pathogens.
These gram-positive organisms as well as gram-positive anaerobic
bacteria accounted for a total of 76% of the 97 pathogens.
Gram-negative aerobic and facultative organisms accounted for 11% of
the 97 organisms and included Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa, Salmonella choleraesuis, and Campylobacter intestinalis.
 |
|
Table 135.1. Common Bacteria Involved in Periprosthetic Infections
|
but were not found in this study are Klebsiella, Serratia,
Acinetobacter species, and other Pseudomonas species. Finally,
anaerobic bacteria were identified 12 times (12%) in the study and
included Propionibacterium acnes, Peptococcus asaccharides, Peptococcus magnus, Peptostreptococcus microsphore, and Clostridium bifermentans.
Other anaerobic bacteria that are commonly reported but were not found
in this study are those of the Bacteroides genus and Mycobacterium. A
single organism rather than multiple organisms was isolated in most
patients.
a periprosthetic infection. Pain that is present while the patient is
at rest suggests an inflammatory process. Drainage after surgery is the
second most common symptom and is strongly suggestive of an infection
if it is still present several weeks after the operation.
operation or weeks later. If it occurs within the first few days, it is
typically either sanguineous, originating from a hematoma, or is
serous, originating from inflamed subcutaneous tissue. Drainage that
occurs several days to weeks later is typically serous and, if it is
left untreated or allowed to progress, will result in formation of a
sinus tract. Sinus tracts generally form within or adjacent to the
operative incision, but rarely they occur in other locations.
patient who has persistent pain can be very helpful in establishing the
correct diagnosis. Fever is not a common presenting complaint, because
only 5% of patients with an infected joint replacement have a
temperature of 37.8° C or more (18). A history
of the joint replacement having “never been right” raises the clinical
suspicion of infection. After knee replacement, joint stiffness may be
present, and although it is not diagnostic, this symptom raises the
suspicion of infection (42).
diagnosis of an infected implant include a complete blood cell count
with differential, determination of the erythrocyte sedimentation rate
(ESR), and C-reactive protein (CRP). Of these tests, the CRP may be the
most useful for evaluating and monitoring the patient’s response to
therapy. After surgery, the CRP rises with a peak usually on the second
or third day, but by 3 weeks, it is usually back to normal (54).
examined a series of 23 patients with proven deep infections of hip
replacements and found that the CRP was elevated (greater than 20 ug/L)
in 19 patients. By comparison, 7 of the patients had an ESR of 20 mm/h
or less. The CRP and the ESR were not elevated in only one of the 23
patients. The investigators also report that infection should be
suspected if an elevated CRP has been recorded on several occasions (52).
which may show radiolucent lines, focal osteolysis, or periosteal bone
formation, which suggest infection. Unfortunately, the presence of
periprosthetic lucencies is not specific for nor most commonly the
result of infection. Mechanical loosening and foreign body histiocytic
reaction are more common causes of lucencies and osteolysis.
uncommon manifestations but are strongly suggestive of an infected
joint prosthesis (3). Typically, radiographs of
infected prostheses are normal, show only a joint effusion, mimic
mechanical loosening, or resemble aggressive granulomatosis.
Unfortunately, the absence of any findings does not rule out the
presence of an infection. Investigate further if infection is suspected
clinically.
diagnosing periprosthetic infections. In patients without prostheses or
other complicating factors, a three-phase bone scan using
technetium-99m methylene diphosphonate (Tc-99m MDP) is highly sensitive
and specific for osteomyelitis. In evaluation of suspected
periprosthetic infections, however, bone scans retain their sensitivity
but become nonspecific.
If abnormal increased uptake is present in the clinical setting of a
painful prosthesis, perform leukocyte scintigraphy (LS), which is the
preferred nuclear imaging method for identifying infected prostheses.
LS involves labeling a patient’s white blood cells (WBCs) with
radioisotopes (Indium-111 or Tc-99m complexed to hexamethyl
propyleneamine oxide), reinjecting the WBCs, and then imaging the
patient approximately 24 hours later. Especially when correlated with
bone scans, indium leukocyte scintigraphy has a sensitivity of about
90% and specificity of about 85% for periprosthetic infections (33,40). Major drawbacks to the routine use of LS are the extensive time, labor, and cost of the technique.
resonance imaging (MRI) have traditionally played a very limited role
in the evaluation of patients with suspected deep infections. MRI is
extremely useful in evaluating bone and soft-tissue infections of the
spine and appendicular skeleton of patients without orthopaedic
devices. Its use is limited, however, when metallic implants are
present because of localized signal loss. CT is also hampered by
artifact caused by the metal prosthesis and is less sensitive to
musculoskeletal infections when compared with MRI.
infection is present, the next step in the diagnostic workup is
aspiration of the joint. Positive culture results confirm the diagnosis
of infection as well as identify the organism and thus direct the
course of treatment.
reviewed the results of routine aspiration before 270 consecutive
revision operations of the hip. An infection was identified at surgery
in six hips, four of which had been successfully aspirated. In the
other two hips, aspiration failed to yield any fluid. Thirty-two (13%)
of the 254 patients who did not have an infection but had been
successfully aspirated had a false-positive result on culture of the
aspirated fluid (3).
This finding emphasizes the importance of hip aspiration for patients
whose history suggests infection or for those with radiographs showing
focal osteolysis, aggressive nonfocal osteolysis, or periostitis; but
not for all patients whose hips are scheduled for revision surgery.
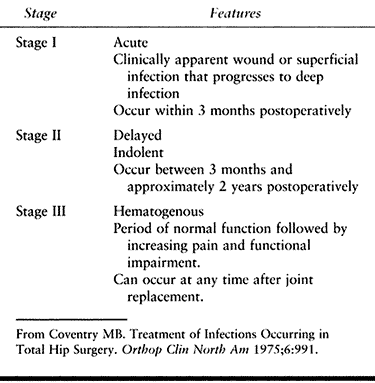
According to this system, stage I infections are acute, fulminating
infections that develop within 3 months after the operation. They are
clinically apparent wound infections or infections of hematomas that
have
progressed
to deep infections. Stage I infections have become increasingly rare
after modern-day hip replacement as a result of improved operative
techniques, a better operating room environment, the routine use of
prophylactic antibiotics, and the generalized awareness of measures to
prevent infection.
 |
|
Table 135.2. Coventry Classification of Periprosthetic Hip Infections
|
may not become apparent until several months after the hip replacement.
Typically, patients who have a stage II infection have never had a
pain-free interval after the operation. A timetable for how late the
infection may be diagnosed has not been well established, but the range
for the diagnosis is 3 months to approximately 2 years after surgery.
hip may function very well after the operation, but later, the patient
has increasing symptoms of pain and impaired function. The symptoms may
be associated with a previous infection remote to the hip joint
(respiratory, dental, urinary tract, or skin lesion) and may develop
soon after the remote infection or as late as 2 or even several years
after the hip replacement.
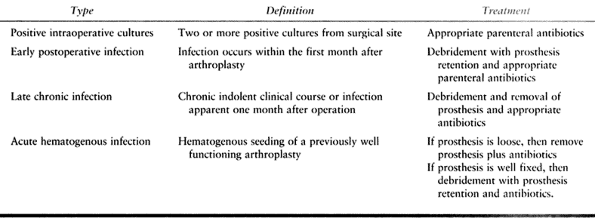
useful but has been modified to facilitate further the management of
patients with prosthetic hip infections. The modification is more
closely aligned with current consensus and treatment guidelines for
infected total hip arthroplasties (57). The newer classification comprises four categories as follows (Table 135.3).
 |
|
Table 135.3. Updated Classification of Periprosthetic Infections
|
fluid or tissue, obtained intraoperatively from different sites of the
hip, have been cultured and found to be positive for the same organism.
The problem is typically identified in revision hip patients when
cultures are obtained but infection is not grossly evident during
surgery. Treat the infection with 6 weeks of intravenous antibiotics
and no operative intervention.
operation and has an insidious clinical onset. Treat it with
debridement, removal of the components, and appropriate antibiotics for
4 to 6 weeks. This may be followed by implantation of a new prosthesis
after the patient has been free of infection.
onset of clinical symptoms in a previously well-functioning hip. The
patient is often able to recall the exact timing of the onset of severe
pain, which is often associated with constitutional symptoms such as
fever, chills, leukocytosis, and an elevated ESR (19).
If the prosthesis is well fixed, treat the infection in the same manner
as for an early postoperative infection; if the prosthesis is loose,
treatment should be the same as for a late chronic infection.
small percentage of patients who have an early infection or for those
with an acute hematogenous infection with symptoms present for a short
period of time (less than 1 to 2 weeks). Surgically debride the
infected site to remove infected and necrotic tissue, as soon as
possible after the patient is deemed an appropriate candidate for
surgery.
-
Use the previous surgical skin incision,
and if necessary, excise it to remove any adjacent scar tissue. Dissect
down to the prosthesis using the same surgical approach used for the
initial surgery. This minimizes additional unnecessary bone and
soft-tissue devitalization and permits removal of any previously placed
deep nonabsorbable sutures that may be a nidus for infection. -
Obtain several tissue cultures from the pseudocapsular and intra-articular regions of the hip joint during debridement.
-
Completely expose the prosthesis–bone or
cement–bone interfaces to remove any granulation tissue or extension of
the purulent process. Removal and exchange of the polyethylene liner of
the acetabular component is particularly important because it allows
more complete debridement of the metal shell–polyethylene interface. -
Irrigate the area extensively and close the wound in layers over deep drains.
parenteral antibiotic therapy to treat any microscopically retained
bacteria and modify as necessary on the basis of culture results.
Guidelines have been suggested for initial component retention in
infected prosthesis and include:
-
a short (less than 3- to 4-week) duration of symptoms
-
a culture showing gram-positive organisms that are sensitive to antibiotics
-
no loosening of the prosthesis
-
an absence of excessive scar tissue from previous operative procedures (14,16,32)
reported the results after retention of the components, debridement,
and treatment with antibiotics in 41 patients. Of the 35 patients who
had had an early postoperative infection, 26 (74%) had a successful
result, compared with only three of the six patients who had had an
acute hematogenous infection. These authors suggest that early
intensive debridement can be successful in carefully selected patients.
necessary for adequate treatment of a deep infection with a compromised
prosthesis, issues regarding reimplantation are less clear.
Reimplantation can be performed after debridement and removal of a
prosthesis during the same procedure (one-stage procedure) or weeks to
months later (two-stage procedure). The indications for use of one
technique as opposed to the other are not very clear and have varied
considerably among different centers and experts who manage a large
number of patients with hip infections.
(one-stage) arthroplasty include the presence of a pathogen that is
very sensitive to antibiotics. These include aerobic and anaerobic,
gram-positive cocci, which are methicillin or oxacillin sensitive and
are non-glycocalyx-elaborating pathogens. Examples are some S. aureus, S. epidermidis,
and Streptococcus—and some antibiotic-sensitive gram-negative bacteria.
The host should be healthy with few or none of the risk factors for
infection (which include rheumatoid arthritis, diabetes, chronic skin
lesions, and obesity). The joint to be operated on must have adequate
bone and soft tissues to support reconstruction and prosthetic
reimplantation. Another candidate for one-stage reimplantation may be
an elderly or infirm patient who will not tolerate prolonged bed rest
and a second major operation.
extensive experience with the direct-exchange technique and have used
it for all patients who have a prosthetic joint infection. They
reported a successful result in 77% of 583 patients. The technique
included debridement or excision of the necrotic soft tissues,
removal
of the implant and cement, replacement with an appropriate implant with
use of Palacos (Smith & Nephew, Memphis, TN) acrylic cement loaded
with an appropriate antibiotic, and, more recently, systemic
administration of antibiotics. Follow-up of these patients at 8 years
showed that approximately 50% remained free of infection (51).
experience with a prospective study of one-stage revision of infected
cemented total hip arthroplasty in 102 consecutive patients using
gentamicin-impregnated acrylic cement with systemic and oral
antibiotics. Thirty percent had had a sinus tract at some stage before
the revision surgery. The most common infecting organism was
coagulase-negative Staphylococcus. The
success rate was 91%, with an average follow-up of 3 years. Of the nine
patients in whom deep infection persisted, three had had a sinus tract
before the revision and a sinus tract reappeared within 3 months of the
revision surgery. In three patients, the radiologic evidence of the
persistence of infection first became obvious at the junction of the
old and new cement within the medullary canal. The author concluded
that adequate debridement and removal of all of the cement is
essential. The use of antibiotic-impregnated cement cannot be advocated
to the exclusion of all other aspects of revision (61).
it is recommended that antibiotics be added to the cement at the time
of the reimplantation (22). The infecting
organisms must be sensitive to the antibiotic used. It must be stable
when it is exposed to heat so that its efficacy is not degraded by the
polymerization of the cement. Geometric dilution of the powdered
antibiotic and cement, or mixing equal amounts until all of the
antibiotic powder and cement are mixed, ensured even distribution of
the antibiotic, allowing for high levels of the antibiotic and adequate
strength of the acrylic cement.
fewer operations, lower cost, and less inconvenience to the patient.
The risks and consequences, if the technique is unsuccessful, however,
are substantial and include sepsis, disarticulation at the hip, and
operative mortality. Buchholtz et al. (10)
reported 13 deaths (1.6%) as a result of 798 operations performed for
the treatment of deep infection after prosthetic replacement.
patients who have a deep periprosthetic infection of the hip.
Reimplantation is performed after the patient has received 4 to 6 weeks
of parenteral therapy with antibiotics, but it is often delayed for
several weeks after completion of antibiotic treatment as well. Reports
have shown that, for patients who have a deep infection of the hip, a
two-stage reimplantation results in the highest rate of success, and
most surgeons believe that two-stage reimplantation is the procedure of
choice (21,22).
center to center, but if the operative site has been treated
appropriately during the first procedure, it is rare that the
reimplantation cannot be performed after 6 weeks. At the time of
reimplantation, the tissues are redebrided and specimens are sent for
histopathologic analysis and culture. If the histopathologic analysis
(frozen section) is positive (more than 5 polymorphonuclear neutrophils
per high power field [PMNS/HPF]), the surgeon may elect to postpone
joint reimplantation in favor of a second debridement. It is critical
to work with an experienced pathologist and have experience treating
infected joints. The use of antibiotic-impregnated cement is also
encouraged for a two-stage reimplantation, because it has been
associated with the best overall results.
patients who have a periprosthestic hip infection. The decision to
perform a permanent resection arthroplasty without reimplantation of a
second prosthesis is based on multiple factors, including:
-
Infection with multiple organisms or bacteria resistant to antibiotic therapy
-
Poor quality local soft tissues
-
Unacceptable complexity of the reconstruction
-
Refusal by the patient to have another operation after removal of the implant
-
Patients with systemic disease and poor overall health
-
Inadequate bone stock
-
Combinations of these factors
have less pain than they did when the infection was present, but their
functional recovery will be significantly impaired. These patients need
aids to walk, have a noticeable limp, and must use a shoe lift to
equalize the leg-length discrepancy and improve gait (1,6,26,34,45).
total knee arthroplasty has been associated with a greater risk of
infection than total hip arthroplasty. Although the rate of infection
following total hip arthroplasty is less than 1%, the rate of infection
following total knee arthroplasty is approximately 2%. The reason for
this discrepancy remains unclear but may be related to the fact that
the
implants
are more superficial in the knee, and the limited amount of
well-vascularized muscle and tissue surrounding the knee impairs local
defense mechanisms.
be responsible for an infected total knee arthroplasty. The most common
organisms, however, are S. aureus (50% to 65%) and S. epidermidis (25% to 30%). Other bacteria, fungi, and mycobacterium accounted for only 10% to 15% of the infections (53,59).
open skin lesions, a previous history of surgery about the knee, and a
previous history of infection (48,59,60).
The risks associated with open skin lesions and other systemic
infections are modifiable. Any medical condition that impairs the
well-being of the patient, such as diabetes mellitus, poor nutrition,
advanced age, or obesity, may increase the potential for infection.
elective total knee arthroplasty can minimize the risk for
postoperative complications. The greatest risk for infection is
associated with a previous infection of the knee. Wilson et al. (59)
reported a rate of infection of 3.1% in a study of 1,857 osteoarthritic
knees that had had a revision total knee arthroplasty because of an
infection around the previous implant (59).
Rheumatoid disease, in addition to being associated with the risk of
acute infection, is also associated with a risk of late infection
secondary to hematogenous bacterial seeding of the knee. The risk of
late infection in these patients is high; Wilson et al. (59) reported that late infections accounted for 45 of the 67 infections in a series of 4,171 total knee arthroplasties.
knee arthroplasty usually presents with a painful, warm, swollen, and
stiff joint. The differential diagnosis of pain at the site of a knee
replacement includes mechanical loosening of the components, reflex
sympathetic dystrophy, heterotopic ossification, and arthrofibrosis.
Evaluate patients in whom infection is suspected with pertinent
physical exam, plain film radiographs, and laboratory evaluations, as
discussed earlier. The key to diagnosis of infection in a total knee
replacement is usually aspiration of the joint and analysis of the
fluid with a Gram stain, complete and differential cell counts, and
cultures.
reported that the results of aspiration had a sensitivity of 75%,
specificity of 96%, and accuracy of 90%. These results support the
routine use of preoperative aspiration before revision of a total knee
prosthesis.
relatively common occurrence of antibiotic use among patients with
symptomatic total knee replacements, which can increase the risk of
false-negative results. Patients should be carefully questioned about
their use of antibiotics. Delay or repeat aspiration 2 weeks after
discontinuation of antibiotics. Aspirate the joint under meticulously
sterile conditions to minimize the risk of contamination with skin
flora. Local anesthetics are not recommended because of their
bacteriostatic properties.
definitively in spite of a suspicious or convincing clinical course
characterized by pain and loosening of the components. In these cases,
staged reconstruction is commonly performed, and it is only with direct
culture of the tissue membrane beneath the implant or material obtained
by swabbing of the component that an organism is identified.
determine the most appropriate treatment regimen. The issue is often a
complex one, because the surgeon must weigh the anticipated quality and
longevity of life and expected function against the extensive surgery
and course of treatment required to eradicate the infection. Removal of
the infected implants, treatment with appropriate antibiotic therapy,
and eventual reimplantation of a prosthesis, is the ideal.
treatment plan, however. The patient may be quite elderly, have
multiple medical problems that would increase surgical risk, have other
conditions that cause functional impairment, or have a well-fixed
prosthesis. In these types of patients, the morbidity associated with
removal of the prosthesis may be greater than that associated with
chronic antibiotic suppression with preservation of the knee joint.
in the setting of an acute infection with an onset within 10 to 21 days
of the operation, with a susceptible gram-positive bacteria and no
evidence of mechanical loosening. If antibiotic therapy alone is
initiated within 48 hours of onset, a success rate of 6% to 10% has
been reported in several large studies. Rates of success may be
improved to approximately 23% by performing open debridement and
synovectomy (53). This approach, however,
requires long-term antibiotic suppression in an effort to contain the
infection indefinitely. Rates of success are generally even lower in
patients who develop infection more than 21 days after initial
implantation.
attributed to the environment of the implant surface and adherent
bacteria that produce a biofilm (27). This
biofilm provides an environment for the bacteria that is resistant to
antibiotics and host defenses. Surfaces covered with biofilm may be
surgically inaccessible to even the most aggressive debridement.
Therefore, it is widely accepted that removal of the prosthesis is
imperative when eradication of the infection is a primary goal.
Surgical options include resection arthroplasty, arthrodesis,
reimplantation in either one or two stages, and amputation.
the definitive treatment, it remains a reasonable option for patients
such as those who have rheumatoid arthritis and may have involvement of
multiple joints. It is also reasonable for those with limited
functional goals and a prospect for walking only with the assistance of
a walker or local risk factors precluding a reoperation about the knee.
In such patients, modest motion is preserved, usually in the range of
45%. It allows function for activities of daily living, although a
brace is usually needed for ambulation. In a study by Falahee et al.,
overall patient satisfaction approached 80% and most patients had good
or excellent relief of pain (17).
more stable lower extremity at the expense of motion at the knee. After
an arthrodesis, the ability to walk independently depends on good
function of neighboring joints of the lower extremity. Relative
contraindications to arthrodesis include ipsilateral ankle or hip
disease, severe segmental bone loss, contralateral leg amputation, and
bilateral knee disease.
-
Expose the prosthesis through the previous surgical approach.
-
Remove the prosthesis, and thoroughly
excise all scarred and infected tissues; carefully contour the bone
ends. Consider placing cancellous bone grafts about the periphery of
the arthrodesis. -
Place the knee in slight flexion of no more than 20°.
-
Apply a rigid biplanar external fixator
to obtain a compression arthrodesis, which is combined with prolonged
immobilization. Time to union can vary considerably and depends on
multiple factors.
staged fashion, with removal of the prosthesis and debridement of the
infected area, followed by antibiotic therapy and placement of the
intramedullary nail at a later date. This alternative carries risks
similar to those of reimplantation, namely multiple surgeries and the
risk of continuation and spread of infection. The problems inherent
with arthrodesis are bone loss, shortening, and gait disturbance. Knee
arthrodesis also results in an increased energy expenditure for
ambulation (47).
with antibiotic therapy is the ideal initial treatment for infected
total knee arthroplasty. Reimplantation of a new total knee prosthesis
is the most acceptable option after removal of the infected prosthesis
for providing the patient with a functional joint. Historically,
reimplantation was done in a single operation, with success rates
ranging from 50% to 75% (48,60).
More recently, two-stage or delayed reimplantation has become the
accepted procedure. The use of antibiotic cement, however, aggressive
surgical debridement, and newer antibiotic regimens have led to the
development of more protocols for immediate or early exchange of an
infected knee replacement.
successful eradication of infection at 5 years in 17 of 18 patients
treated by a single-stage protocol, which included aggressive
debridement, the use of antibiotic-impregnated cement, and a 3-month
course of antibiotics. The only recurrence was in a patient with severe
rheumatoid disease with multiple joint involvement. All of the patients
in this group were infected with gram-positive organisms and had no
systemic signs of sepsis. Such studies suggest that one-stage
reimplantation may be a reasonable alternative in carefully selected
patients and has the advantage of avoiding the added morbidity of
another operation (24).
interval period of intravenous antibiotics followed by reimplantation
remains the standard treatment of the infected total knee arthroplasty
in most cases. It is commonly accepted that this approach gives the
patient the best chance of eradication of infection. As previously
noted, all foreign material, including the implant and all bone cement,
must be removed at the time of the initial debridement.
antibiotic-impregnated cement spacer between the femur and the tibia.
The antibiotic spacer preserves the joint space for later
reimplantation, permits stability with weight bearing across the
resected knee joint, and provides local antibiotics. The disadvantages
of the spacer are the potential for erosion of adjacent bone, and after
the elution of antibiotics ceases, it may act as a foreign body.
antibiotic for at least 6 weeks. Controversy still exists regarding the
total duration of antibiotic treatment, with some advocating the
administration of an additional 6 weeks of oral antibiotics (2). Discontinue antibiotics at least 6 weeks before reaspirating the pseudoarthrosis.
Consider reimplantation if the culture is negative. Repeat arthroplasty
is usually performed between 12 weeks and 1 year after resection. The
technical aspects of delayed reimplantation are more complex than those
of standard revision due to problems of exposure, bone loss,
ligamentous balance, and restoration of flexion.
potential problems and requires careful management, results can be
quite rewarding. Goldman et al. (25) reported
on a series of 64 infected total knee replacements in 60 patients who
were followed for an average of 7.5 years. At follow-up, six knees (9%)
had become reinfected, but only two with the same organism, yielding an
infection eradication rate of 97%. Most patients were satisfied with
their function and outcome following surgery. The authors concluded
that two-stage reimplantation with a 6-week course of parenteral
antibiotics is an effective means of eradicating deep infection and
providing a functional knee. The long-term functional results,
reinfection rate, and survivorship are comparable with those found in
revision total knee arthroplasty without infection.
final option in the treatment of life-threatening infection or in the
setting of a particularly virulent or resistant organism. It is only
infrequently required but can provide an expeditious resolution to a
very difficult problem. Unfortunately, a poor functional result is the
rule, with only one third of patients retaining the ability to walk,
even with assistive devices, and the remaining two thirds confined to a
wheelchair (46). Above-knee amputation (AKA) is
seldom necessary and prosthetic fitting of an AKA prosthesis is rarely
successful in these elderly patients.
arthroplasty infection has been dramatically reduced over the past 25
years with the use of prophylactic antibiotics, careful surgical
technique, maximizing the patient’s health before surgery, and
controlling bacterial contamination of the wound. Of these factors,
prophylactic antibiotic therapy used routinely for patients undergoing
total joint replacement is the most important. Several studies have
documented the efficacy of prophylactic antibiotic therapy compared
with placebo in preventing deep postoperative infection in total joint
arthroplasty patients.
the results of a double-blind investigation of the value of
prophylactic antibiotic therapy in early and late infections after
total hip replacement (11). The early
(2.5-year) follow-up of 118 patients showed no infection in the 60
patients who received prophylactic antibiotics compared with seven
infections in the 58 patients who did not receive prophylactic
antibiotics. The 5-year follow-up of the same patients also showed a
difference, with two infections in the 60 patients who received
prophylactic antibiotics and 14 infections in the 58 patients who did
not receive the antibiotics.
also documented the reduction of infection in patients administered
prophylactic antibiotics compared with patients administered a placebo.
The criticism of their review was that other factors were not
controlled, such as the type of operating room used. It is known that
laminar air flow, body exhaust suits, careful patient selection,
ultraviolet light, and limiting operating room traffic all contribute
to a decrease in the infection rate.
prevent infection after total joint arthroplasty. Administer the
antibiotic intravenously one-half hour before the incision is made to
achieve bactericidal levels at the time of the initial incision. If the
operative procedure is prolonged or exceeds the half-life of the
antibiotic selected for prophylaxis, administer an additional dose.
Continuing prophylactic antibiotics beyond 24 hours after surgery has
not been shown to increase efficacy. This practice increases cost and
may contribute to the emergence of resistant organisms. One exception
to the routine use of cephalosporins may be in hospitals with a high
percentage of methicillin-resistant strains of staphylococcus.
Otherwise, the prophylactic use of vancomycin and other second-line
antibiotics is discouraged to reduce the emergence of resistant
bacteria.
or hematogenous infections is less clear. If prophylactic antibiotics
are not used and the artificial joint becomes infected, the
complication is catastrophic. The risk of such an occurrence in healthy
patients, by contrast, is very small. Factors that argue against the
use of prophylactic antibiotics include the very high cost of
administering antibiotics to hundreds of thousands of patients per
year, the possibility of anaphylactic reaction from the antibiotics,
and the resistant strains of bacteria that emerge because of the
overuse of prophylactic antibiotics.
infection in the oral cavity, skin, respiratory, gastrointestinal, and
urogenital systems and other sites can and do cause late implant
infections. Two important factors to be considered are the amount of
expected bacteremia from mucosal injury and the integrity of the
patient’s host defense mechanisms. In general, administer prophylactic
antibiotics appropriate to the endogenous flora immediately before
any
procedure that has a significant risk of mucosal injury, such as
peridontal surgery, or in patients with host defense impairment such as
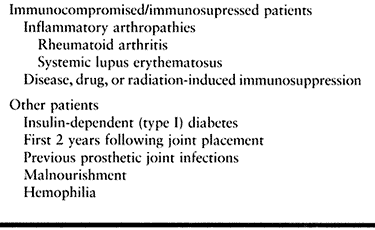
collagen diseases.
data by an expert panel of dentists, orthopaedic surgeons, and
infectious disease specialists, the American Academy of Orthopaedic
Surgeons issued an advisory statement regarding the need for
prophylactic antibiotics in dental patients who have undergone total
joint arthroplasties. They concluded that antibiotic prophylaxis is not
indicated for dental patients with pins, plates, and screws, nor is it
routinely indicated for most dental patients with total joint
replacements. It is advisable, however, to consider premedication in a
small number of patients who may be at potential increased risk of
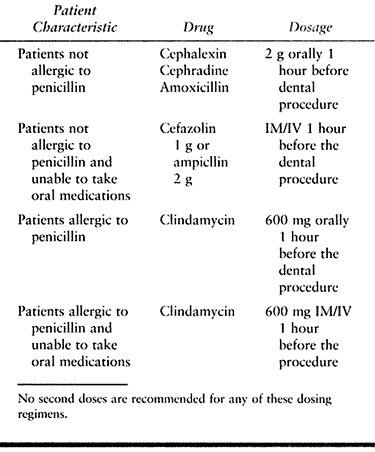
hematogenous total joint infection (Table 135.4). The Academy also modified antibiotic regimens to eliminate the need for second doses (Table 135.5).
 |
|
Table 135.4. Patients at Potential Increased Risk of Hematogenous Total Joint Infection
|
 |
|
Table 135.5. Suggested Antibiotic Prophylaxis Regimens for Patients with Total Joints Undergoing Invasive Procedures
|
usually has increasing or new onset of pain that may be located at the
implant insertion site or at the fracture site. The pain is often
described as dull and deep within the extremity and exacerbated by
activity. Local signs may include cellulitis, abscess formation, and
wound drainage. Constitutional symptoms include fever, chills, night
sweats, tachycardia, and anemia. Past medical history may reveal risk
factors such as cigarette smoking, diabetes, alcohol abuse, previous
open fracture, or previously draining wound (35).
Several recent studies have shown a high infection rate in patients
undergoing delayed intramedullary fixation following external fixation,
particularly if there has been an infected pin site (38,39,56).
elevated. A CBC may show a leukocytosis with a left shift or anemia of
chronic disease. Aspiration of the site of maximal tenderness or
fluctuance or deep culture of a wound that shows organisms on Gram’s
stain or grows organisms on culture generally confirms the diagnosis of
infection.
to assess the stability of the fixation construct. Radiolucency around
the fixation devices suggests loose hardware. Assess fracture healing
as well. Do not confuse periosteal reaction from the infection with
fracture callus (Fig. 135.1). Plain tomography
may assist in the assessment of fracture healing. Finally, classic
signs of osteomyelitis with involucrum or sequestrum may be present.
 |
|
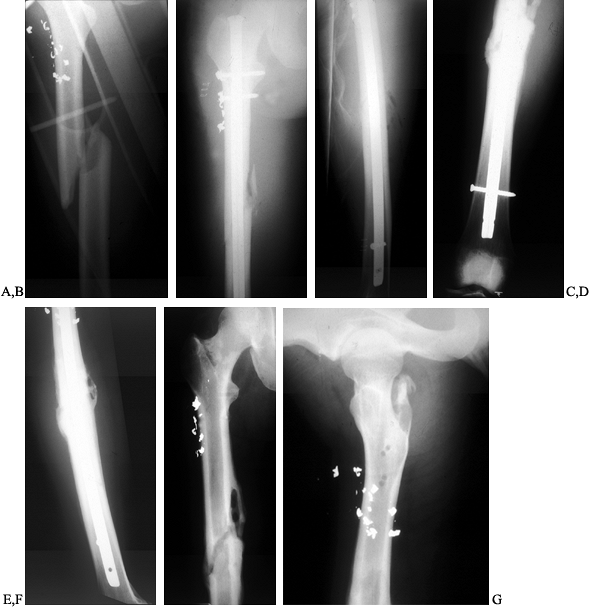
Figure 135.1. A 27-year-old man sustained an open fracture of the shaft of the right femur secondary to a low-velocity gunshot wound. A: AP radiograph of the fractured right femur. B:
Postoperative AP radiograph. This wound was treated with irrigation and debridement, and immediate locked intramedullary nailing as well as parenteral antibiotics. C: Postoperative lateral radiograph. D: This fracture healed nicely, but 4 months later, the patient developed a localized abscess at the fracture site. This AP radiograph shows periosteal reaction at the healed fracture site. E: Lateral radiograph showing periosteal new bone at the fracture site. F: This was treated with incision and drainage of the abscess, removal of the intramedullary nail, cross-screws and reaming of the intramedullary canal to debride it, as well as administration of parenteral bactericidal antibiotics. This AP radiograph taken 4 months later shows the healed fracture with resolution of the bone infection. G: Lateral radiograph 4 months after debridement. |
-
The time of onset after internal fixation.
-
The status of fracture healing.
-
The stability of the implants and fracture.
-
The extent of radiographic bone involvement.
-
The type and virulence of the organism.
-
The patients general condition and health (Fig. 135.2).
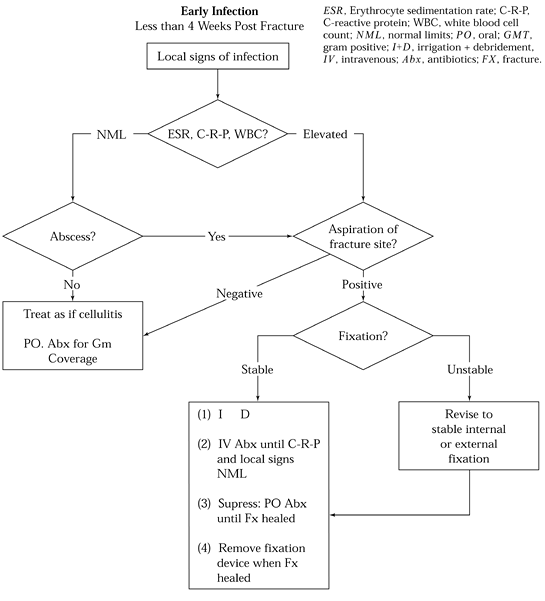 Figure 135.2. Algorithm for treatment of acute infection after internal fixation.
Figure 135.2. Algorithm for treatment of acute infection after internal fixation.
weeks after internal fixation are generally considered early
infections. Pain, fever, and wound drainage are the typical signs and
symptoms. Plain radiographs usually show no signs of loosening or
chronic osteomyelitis.
period (i.e., the first 1 to 4 weeks) and may be characterized by
either persistent drainage and an otherwise unremarkable appearing
wound or by acute cellulitis, sometimes with systemic signs of
infection. The differential diagnosis is a large hematoma and sometimes
thrombophlebitis. If other signs of thrombophlebitis are present,
perform a Doppler ultrasound examination first. Otherwise, immediately
return the patient to surgery. Open the surgical wound down to the bone
and implants. Send any fluid found and selected specimens of the deep
soft tissues and a small biopsy of bone for immediate Gram’s stain,
culture, and sensitivity tests. Thoroughly debride and irrigate
the
wound. Ensure that the implants are stable. If a bone graft was placed
at the time of the initial surgery, and gross purulence is not found,
leave it in place. If gross purulence is found, most likely it will
need to be removed, because it is a significant nidus for infection.
antibiotics. The choice of antibiotics is based on the results of the
Gram stain or on what are the most likely infecting organisms in your
hospital and in the patient you are treating, taking into account his
or her clinical condition.
fashion, unless gross purulence and poor conditions of the soft tissue
contraindicate closure.
beads without the use of suction or suction irrigation. If beads are
not used, place closed suction drainage. Depending on the virulence of
the infection and the patient’s clinical response, returning the
patient to surgery for repeat irrigation and debridement may be
necessary on one or more occasions until the acute local infection is
resolved. If antibiotic beads are used, insert a new set of beads at
the time of each repeat irrigation and debridement.
or seroma is found and cultures are negative, systemic antibiotics can
usually be stopped when the wound is healed, the CRP level has returned
to normal, and there is no further evidence of infection. If cultures
are positive, then intravenous antibiotics, perhaps followed by oral
antibiotics, are recommended for periods ranging from 4 to 8 weeks. In
some cases, oral suppressive medication may be necessary until the
fracture is healed and implants are eventually removed (12,41).
the type of implant in place. If external fixation is present, then
simply ensure that all of the fixation pins are tight in the bone and
not directly involved in the fixation suffices. External fixation is
the most ideal type of fixation in the presence of infection because it
minimizes the surface areas available for glycocalyx-forming bacteria
to adhere to. In the case of plates and screws, the procedure is
performed as described earlier, as long as the implants are solid in
bone.
made as to whether the infection involves just the outside of the bone
or whether it has invaded the intramedullary canal. This decision can
be difficult to make. If there is no gross purulence and the
intramedullary canal does not seem to be involved, then leaving the rod
in place and following the patient clinically is usually advisable. It
may be impossible to irrigate around a solid nail, but exchange nailing
may be necessary to debride the canal adequately (12,63; see Fig. 135.3).If
there is gross purulence, however, and the implant appears to be loose
or the medullary canal involved, then removal of the nail and
cross-screws
and
conversion to external fixation is usually prudent. Another alternative
would be to maintain the patient in a cast or traction, hoping that if
the infection shows an excellent response to treatment that early
reimplantation of the rod may be possible. If the rod is removed,
debride the canal by reaming somewhat larger than the nail used.
Culture the medullary contents.
 |
|
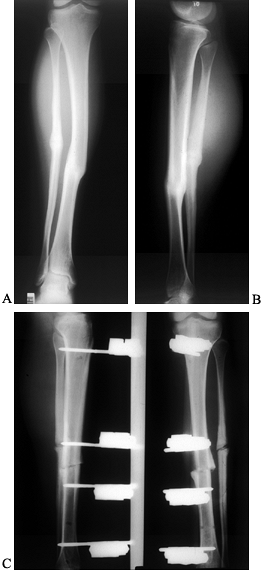
Figure 135.3.
A 15-year-old girl with type I diabetes mellitus had a varus malunion of the right midshaft tibia due to a closed tibial fracture treated in a plaster cast. This AP radiograph shows the healed malunion. B: This problem was treated with open osteotomy of the tibia and fibula, and insertion of a locked medullary nail. This AP radiograph of the tibia shows excellent correction of fixation. C: Unfortunately, 3 weeks postoperatively, the patient developed an acute wound infection. Initially, this wound was irrigated and debrided, and the nail was left in place. Because of inability to control the infection, the nail and cross-screws were subsequently removed and the patient was converted to a single plane external fixator. These AP radiographs show excellent maintenance of correction and good bone apposition at the fracture site. Fortunately, this wound healed and her infection has resolved |
Often, the only symptoms are vague, deep pain with minimal signs of
local inflammation. Others may present with classic signs of acute
inflammation and abscess formation. Laboratory values may be consistent
with acute inflammation with elevated ESR and CRP levels. Radiographic
signs of infection are often present. Frequently there is evidence of
hardware loosening with bone resorption, screw backout, and implant
failure, sequestrum formation, resorption of bone ends, and permeative
cortical lysis. Again, an assessment of fracture healing is paramount
and can be aided by tomography.
 |
|
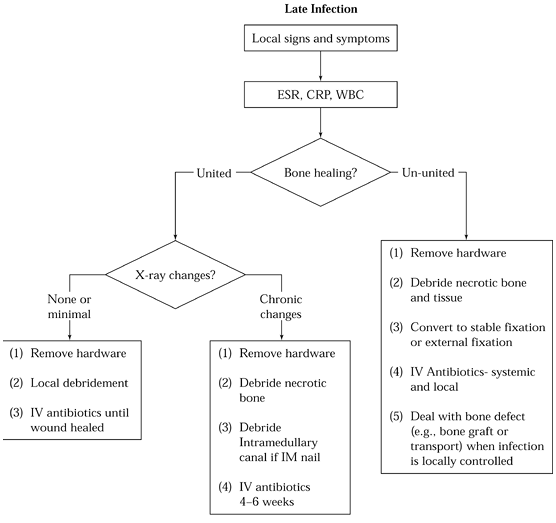
Figure 135.4. Algorithm for treatment of late infection after internal fixation.
|
infected, surgical treatment is nearly always necessary and begins with
debridement of bone and soft-tissue abscesses, removing all infected
and nonviable tissues. In the presence of delayed union or nonunion,
implants are almost always loose. Convert unstable fixation to stable
external fixation, and administer culture-directed parenteral
antibiotics (12,41,63). Remove infected intramedullary implants, and debride the medullary canal by reaming.
the wound open, or managing it with close suction irrigation or
antibiotic impregnated beads is performed as described for acute
infection earlier. Administration for at least 6 weeks of parenteral
antibiotics to which the organism is sensitive is nearly always
necessary.
internal fixation may be possible but the vast majority of cases are
managed with external fixation. In the case of intramedullary nails, it
may be possible to reinsert a statically locked new larger nail if the
infection is of low virulence and the organisms are sensitive, but a
majority of cases will require definitive management in stable external
fixation.
most cases, application of autologous cancellous bone graft is best.
When segmental bone deficiency is present, a microvascularized bone
transfer or bone transport using Ilizarov’s technique may be necessary.
Electrical stimulation has little role in these cases but can be used
as a supplemental treatment.
wound is drainage free for 3 to 6 months, except in the Papineau
technique, in which grafting can be carried out as soon as the bone is
covered by a clean granulating bed (43). Patzakis et al. (44)
challenged the delayed bone grafting concept by treating 32 patients
with septic nonunions of the tibia with a protocol of irrigation,
debridement, external fixation, and bone grafting at an average of 8
weeks after wound coverage. With this protocol, they achieved infection
control in all of their patients and union in all but three patients.
Union was achieved in these three patients after additional bone
grafting.
nonunion must be weighed against the economic and human cost of such a
complicated operative course. Lerner and others have shown the impact
of such a treatment course on the patient’s quality of life (5,23,30).
Generally, infections are the result of hematogenous seeding or result
from a prior infection harbored in a retained sequestrum or the
secretion of the glycocalyx on the fixation device. In the absence of a
prior infection, it can be assumed that the hematogenous seeding is the
cause of the infection.
soft tissue, and remove all fixation devices. In the case of
intramedullary fixation, ream the canal to complete the debridement.
Significant bone involvement requires prolonged antibiotic therapy.
Patients with minimal bone involvement and simple hardware removal can
generally be treated with a short course of antibiotics until their
wounds are healed and their CRP levels have returned to normal. The
vast majority of these infections resolve after hardware removal.
scheme: *, classic article; #, review article; !, basic article; and +,
clinical results/outcome study.
SA, Gudmundsson G, Bartholdsson E. Function After Removal of a Septic
Total Hip Prosthesis. A Survey of 27 Girdlestone Hips. Acta Orthop Scand 1980;51:541.
RB, Hunter GA, Rorabeck CH, Macnab JJ. A Six Year Follow-up of Infected
Total Hip Replacements Managed by Girdlestone Arthroplasty. J Bone Joint Surg Br 1984;66-B:340.
HB, Lipinski SW, Wiley JH. Observations on Non-Union of the Shafts of
the Long Bone with a Statistical Analysis of 842 Patients. J Bone Joint Surg Am 1961;43-A:159.
AS, Lidgren L, Lindberg L. Prophylactic Antibiotics Against Early and
Late Deep Infections after Total Hip Replacements. Acta Orthop Scand 1977;48:405.
BR, Boeckstyns M, Stadeager C. The Natural Course of Radionuclide Bone
Scanning in the Evaluation of Total Knee Replacement. Clin Radiol 1990;41:341.
MH, Matthews LS, Kaufer H. Resection Arthroplasty as a Salvage
Procedure for a Knee with Infection after a Total Arthroplasty. J Bone Joint Surg Am 1987;69-A:1013.
KL, Fitzgerald RH, Salvati EA, et al. Reconstruction of the Infected
Total Hip and Knee Arthroplasty with Gentamicin Impregnated Palacos
Bone Cement. Instr Course Lect 1993;42:293.
GM, Behrens FF, Joyce MJ, et al. Open Tibial Fractures with Severe Soft
Tissue Loss: Limb Salvage Compared with Below the Knee Amputation. J Bone Joint Surg Am 1993;75-A:1431.
RB, Tsukayama D. Treatment of Infected Cemented Total Hip Arthroplasty
with Tobramycin Beads and Delayed Revision with a Cemented Prosthesis
and Bone Grafting. Orthop Trans 1988;12:739.
C, Flamant R, Mazas F, et al. Prophylactic Cefazolin versus Placebo in
Total Hip Replacement. Report of a Multicentre Double-blind Randomized
Trial. Lancet 1981;1:795.
JA, Christie MJ, Sandler MP. Detection of Occult Infection Following
Total Joint Arthroplasty Using Sequential Technetium-99m HDP Bone
Scintigraphy and Indium-111 WBC Imaging. J Nucl Med 1988;29:1347.
JE, Brown ML, Hauser MF. In-111 Labelled Leukocyte Scintigraphy in
Suspected Orthopaedic Prosthesis Infection: Comparison with Other
Imaging Modalities. Radiology 1988;168:235.
BA, Kendall RW, Duncan CP, et al. Two-Stage Exchange Arthroplasty Using
a Functional Antibiotic-Loaded Spacer in the Treatment of the Infected
Knee Replacement: The Vancouver Experience. Seminars in Arthroplasty 1994;5:122.
DJ, Merkow RL, Gustilo RB. Infection After Intramedullary Nailing of
Severe Open Tibial Fractures Initially Treated with External Fixation. J Bone Joint Surg Am 1989;71A:835.
KD, Brown ML, Dewanjee MK, et al. Comparison of Indium-labeled
Leukocyte Imaging with Sequential Technetium-gallium Scanning in the
Diagnosis of Low-grade Musculoskeletal Sepsis. J Bone Joint Surg Am 1985;67:465.
ME, Ada Jr, Webb LX. Treatment of Infected Nonunion and Delayed Union
of Tibia Fractures with Locking Intramedullary Nails. Clin Orthop 1989;245:233.
LJ, Alfageme A, Dalcourt JP, Pilon L. Osteomyelite Chronique: Excision
et Greffe de Spongieux a l’Air Libre Apres Mises a Plat Extensives. Int Orthop 1979;3:165.
DT, Estrada R, Gustilo RB. Infection After Total Hip Arthroplasty. A
Study of the Treatment of One Hundred and Six Infections. J Bone Joint Surg Am 1996;78-A:512.
MG, Kelley K, Thornhill TS. Infection as a Complication of Total Knee
Replacement Arthroplasty. Risk Factors and Treatment in Sixty-seven
Cases. J Bone Joint Surg Am 1990;72-A:878.
RE, Insall JN, Urs WK, et al. Two-stage Reimplantation for the Salvage
of Total Knee Arthroplasty Complicated by Infection. Further Follow-up
and Refinement of Indications. J Bone Joint Surg Am 1990;72-A:272.
