ORTHOPAEDIC MANAGEMENT OF HEMOPHILIC ARTHROPATHY
VII – NEOPLASTIC, INFECTIOUS, NEUROLOGIC AND OTHER SKELETAL DISORDERS
> Infection and Hemophilia > CHAPTER 136 – ORTHOPAEDIC MANAGEMENT
OF HEMOPHILIC ARTHROPATHY
Chief of Orthopaedics, Hemophilia Center, Orthopaedic Hospital;
Executive Vice Chairman, UCLA-Orthopaedic Hospital Department of
Orthopaedics, University of California at Los Angeles, Los Angeles,
California, 90007.
Research, Hemophilia Center, Orthopaedic Hospital; Emeritus Professor,
Department of Medicine (Hematology), University of Southern California;
Vice President, Medical, World Federation of Hemophilia, Los Angeles,
California, 90060.
Director, Hemophilia Center, Orthopaedic Hospital; Associate Clinical
Professor, Department of Medicine (Hematology), University of Southern
California, Los Angeles, California, 90060.
with an incidence in the United States of 1 in 10,000 male births.
Factor VIII deficiency, or hemophilia A, represents about 85% of these
cases, and factor IX deficiency, or hemophilia B, accounts for 15%.
Cases with a negative family history are considered de novo
occurrences and are the result of a recent genetic mutation that
accounts for 25% of new hemophilia A cases. This mutation rate is one
of the highest among genetic disorders.
who described the gross articular changes in 1892. Detailed
descriptions of the microscopic pathology followed in 1925 by Freund (19) and in 1929 by Reineke and Wohlwill (62). The first papers in English were radiologic descriptions in 1921 by Klason (37) and in 1926 by Doub and Davidson (15). In 1932, J. Albert Key (34)
presented the gross and microscopic findings in hemophilic arthropathy.
His paper included a fascinating description of a 13-year-old boy who
presented with a “tumor” about the knee. This patient had a negative
family history for bleeders, but he had a history of easy bruising,
nosebleeds, and periodic joint swelling. It was only after a
synovectomy and stormy postoperative course that he was discovered to
be a de novo hemophiliac. In 1936, Henry Thomas (70) described orthopaedic findings in 98 hemophiliacs. Over the last six decades, the volume of
scientific literature on hemophilic arthropathy has greatly expanded with many excellent papers and several complete texts (6,13,14,17,26,66,72).
unrelenting, progressing from hemarthrosis to chronic synovitis to
extensive joint surface erosion and, ultimately, to end-stage joint
destruction (Fig. 136.1, Fig. 136.2, Fig. 136.3, Fig. 136.4, Fig. 136.5 and Fig. 136.6; see also COLOR FIG. 136.2).
End-stage arthropathy is complicated by severe loss of motion secondary
to arthrofibrosis as the hypertrophic synovium is replaced by dense
fibrous scar. Severe contractures, angular deformity, and loss of bone
stock due to synovial cysts and mechanical abrasion are common. The
pathophysiology has been intensely studied, but our understanding
remains incomplete. Chronic synovitis and progressive arthropathy have
been reproduced in rabbits and dogs by serial intra-articular
injections of autologous blood (26,65).
Gross and microscopic examination of hemophilic arthropathy reveals
destruction of articular cartilage by direct synovial invasion and
subchondral synovial and degenerative cysts. Biochemical studies have
documented enzymatic degradation similar to other forms of inflammatory
arthritis (6,66). Symmetry is common, and in that sense, the condition is similar to rheumatoid arthritis.
 |
|
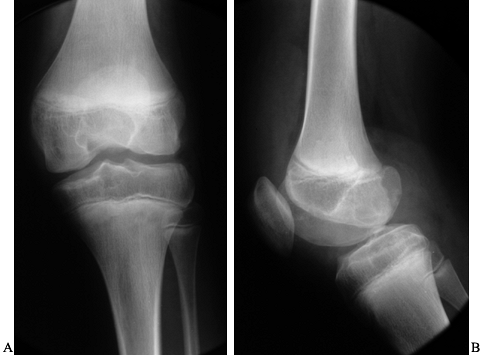
Figure 136.1. AP (A) and lateral (B)
x-ray study of a 14-year-old boy with severe hemophilia type A. The cartilage interval is well maintained. There are minor surface irregularities and an erosion on the posterior surface of the lateral femoral condyle. |
 |
|
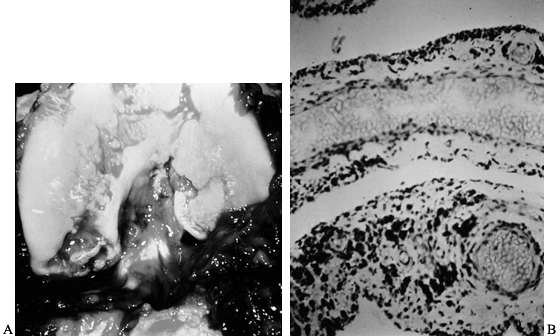
Figure 136.2. A: Photograph of the knee of the patient in Figure 136.1
at the time of open synovectomy a few days after the x-ray studies were taken. There is extensive hyperplastic synovitis with full-thickness erosion of the articular surface of the weight-bearing surfaces of the medial and lateral condyles and the trochlea. This demonstrates the advanced destruction that can occur with chronic hemophilic synovitis despite a relatively benign-appearing x-ray study. B: Photomicrograph of the synovium from this patient showing hemosiderin pigment deposition on the surface taken up by the phagocytic synovial A cells. These cells migrate into the perivascular tissue of the subsynovial layer to return blood products to the general circulation. This patient was in the stage of chronic hemarthrosis, which is easy to understand with dilated venous channels immediately beneath the surface that are easily torn when the fragile, hypertrophic synovium gets caught between the eroded joint surfaces. (See also COLOR FIG. 136.2.) |
 |
|
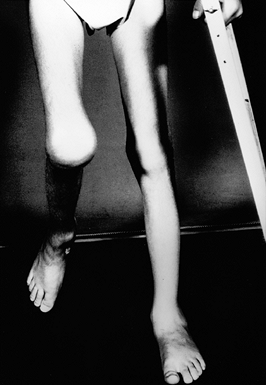
Figure 136.3.
Eleven-year-old boy with a severe flexion contracture of his right knee secondary to recurrent hemorrhage and advanced arthrofibrosis. |
 |
|
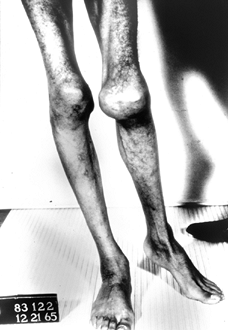
Figure 136.4.
Thirteen-year-old boy with severe type A hemophilia. He demonstrates marked muscle atrophy and a lateral translation deformity of his left knee. |
 |
|
Figure 136.5. AP (A) and lateral (B)
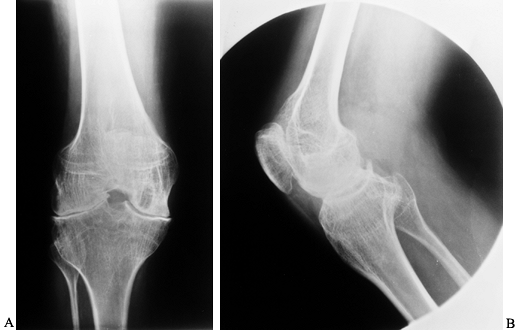
radiographs showing moderately advanced hemophilic arthropathy with almost complete loss of cartilage interval. Note the synovial cyst in the medial femoral condyle, flattening of the anterior part of the trochlea resulting from lateral subluxation of the patella, and loss of the normal posterior tilt of the tibial articular surface, which results from a flexion contracture during growth. These changes are typical of hemophilic arthropathy. |
 |
|
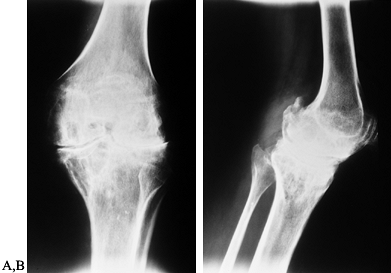
Figure 136.6. AP (A) and lateral (B)
radiographs showing end-stage arthropathy with large synovial cysts, complete loss of cartilage interval, flattening of the opposing joint surfaces, ossification of the posterior capsule, and nearly complete fibrous ankylosis. |
ankles. Hips, shoulders, and subtalar joints are sometimes involved;
wrists, fingers, and toes are rarely involved. Fortunately, hemophilic
arthropathy of the spine does not occur. The prevalence of hemophilia
arthropathy correlates with the level of circulating clotting factor.
Polyarthropathy is common in severe hemophilia (defined as less than 1%
of normal circulating clotting factor). Arthropathy is much less common
in moderate hemophilia (1% to 4% of normal clotting factor) and rare in
mild hemophilia (greater than 5% of normal clotting factor).
to joint within the same patient. The first hemorrhage may initiate a
smoldering, low-grade synovitis with recurrent, subclinical bleeding,
which results in a steady progression to chronic synovitis and
end-stage arthropathy. However, a major joint may remain normal through
adulthood despite the occurrence of a few hemorrhages in childhood and
adolescence. The biochemical and pathophysiologic reaction of the
synovium to hemarthrosis varies even within the same patient and
probably involves an element of autoimmune sensitivity.
early phases of hemophilic synovitis may reverse the progressive nature
of this disease in some cases, but even with optimal management, many
patients will progress. Chronic synovitis reaches a point at which the
membrane becomes very friable, with dilated venous channels immediately
beneath the surface. This is the stage of chronic hemarthrosis and is
usually associated with joint surface erosions. With motion of this
jagged joint, the friable, hyperemic synovium is torn, resulting in
bleeding regardless of clotting factor level (Fig. 136.1, Fig. 136.2). Muscles atrophy, joint contractures develop, and the patient becomes severely disabled (Fig. 136.3, Fig. 136.4).
synovitis, management of initial hemarthroses is critical if a normal
joint is to be preserved.
-
Start clotting factor concentrate as soon as possible after the onset of bleeding.
-
If the hemarthrosis is clinically
significant, consider aspiration to reduce the magnitude of blood
breakdown products to which the synovium is exposed as well as to
reduce pain and facilitate recovery. -
Start the patient on prophylactic factor
replacement with daily doses for the first few days, followed by every
other day for at least 2 weeks or until the joint is nearly normal on
careful examination with no evidence of effusion or synovial thickening.
hemarthroses. Several centers have documented a lower frequency of
hemarthroses and arthropathy in severe hemophilia patients of
mesomorphic habitus compared with endomorphs and ectomorphs. Even under
optimal circumstances, chronic synovitis with “target joint” formation
subject to recurrent hemarthroses without trauma may occur.
most commonly in the ankle, followed by the elbow and knee. In recent
years, several hemophilia centers (58) have
begun programs of primary prophylaxis before the first bleed. Regular
doses of clotting factor are begun at 1 to 3 years of age and continued
into middle to late adolescence. This approach is based on the
experience with moderate hemophiliacs with factor levels of 1% to 4% of
normal who grow up with a paucity of joint hemorrhages and are often
free of arthropathy when they reach adulthood (58).
This approach is very costly and usually requires a venous access port
with a significant risk of infection. Fortunately, recombinant factor
is now available and free from viral contaminants.
ultimate answer for prevention of arthropathy. In the meantime, older
children, adolescents, and adults continue to be plagued by chronic
synovitis and progressive arthropathy. Early synovectomy holds some
promise for the prevention of progressive arthropathy in target joints.
IX–deficient patients develop antibodies termed inhibitors to human
clotting factor (4). Treatment with immune
tolerance programs, in which patients are given large amounts of
clotting factor to overwhelm and suppress the inhibitor, are often
successful for these patients. Although these programs are costly, in
the range of $200,000 to $800,000 per patient, immune tolerance is
greatly beneficial to the patient and may be ultimately cost effective
because it prevents hemorrhage and progressive arthropathy. Patients
with persisting inhibitors are like hemophiliacs before the days of any
effective treatment. They are extremely complex to manage, and major
trauma or surgery creates a precarious situation; they are not
candidates for surgery other than in life-threatening emergencies.
synovitis, ideally before irreversible joint destruction has occurred.
Synovectomy may be surgical, either open or arthroscopic, or through
the injection of radioactive colloids. Open synovectomies were first
performed in the late 1960s, when clotting factor concentrate (67)
became available. Although these procedures were effective in reducing
or eliminating hemarthroses, they were complicated by severe
arthrofibrosis and loss of motion, and there was one report of
arteriovenous fistula (11,12,24,48,60,72).
Because of the complexity and prohibitive cost of the administration of
concentrate for this procedure, most patients had significant joint
surface erosion by the time synovectomy was eventually performed, and
joint surface degeneration progressed over the ensuing years. In that
era before arthroscopy and magnetic resonance imaging (MRI), findings
at surgery showed a surprising degree of surface erosion that was
unsuspected based on the plain radiographs (Fig. 136.1 and Fig. 136.2). With the
advent of arthroscopic surgery, arthroscopic synovectomy replaced open
procedures and was associated with much less loss in motion but was
occasionally complicated by significant hemorrhage in the perioperative
period (15,43,76).
Massive amounts of factor replacement were still required for 2 weeks
following surgery and before physical therapy for as long as the
therapy was needed, so the prohibitive cost of administering
concentrate remained about the same.
term preferred by some, was introduced in 1963 and has been used
extensively for rheumatoid arthritis (5). Over
the last 20 years, several hemophilia centers outside the United States
have used a variety of radiocolloids for chronic hemophilic synovitis (2,3,18,63).
In 1988, with institutional review board approval, the Hemophilia
Center at Orthopaedic Hospital in Los Angeles (OHHC), began a
prospective study of radiocolloid synoviorthesis in the treatment of
chronic hemophilic synovitis.
for radiosynovectomy in the United States and Canada. The dosage
protocol is also quite universal (Table 136.1). To date, the procedure has not been performed on hips at OHHC.
 |
|
Table 136.1. Dosage Protocol for Radiosynovectomy with 32P Chromic Phosphate
|
before the procedure that is aimed at attaining a plasma level of
clotting factor of 50% of normal and are advised to self-infuse a
second time 8 to 12 hours following the procedure. Patients with
inhibitors receive the clotting factor that has been the most effective
in controlling their bleeding episodes. They are observed overnight in
the hospital. The procedure is as follows:
-
Prep the involved joint with povidone-iodine and drape it with sterile towels.
-
Infiltrate lidocaine 1% into the skin,
subcutaneous tissue, and capsule with a 27-gauge needle. Buffered
lidocaine is less painful and is especially useful in children.
Lidocaine-prilocaine lotion applied to the skin 1 hour before injection
also reduces pain. -
Insert a larger needle ranging in gauge
from 23 to 18, depending on the amount of fluid to be aspirated, into
the joint space. Needles larger than 22 gauge are not used in
subcutaneous joints such as the elbow to avoid retrograde leakage of
radiocolloid onto the skin. The intra-articular position of the needle
is critical; confirm the position by the aspiration of blood or
synovial fluid, or in cases in which that is not possible, confirm
position by the injection of radiographic dye under image-intensifier
visualization. -
After confirmation of needle position, inject the selected dose of radiocolloid in a volume of 1 ml through a separate syringe.
-
Change syringes and partially inject a
mixture of 5 ml lidocaine and 2 ml dexamethasone acetate into the
joint. Use the remainder to flush the needle and needle tract as the
needle is withdrawn to reduce the risk of radiocolloid leakage along
the needle tract. -
Apply local pressure. followed by
cleansing of the area with isopropyl alcohol, followed by more local
pressure for a total of 3 to 5 minutes. While maintaining local
pressure on the injection site, put the joint through range of motion
to disperse the radiocolloid throughout the joint. -
When the puncture site is completely dry, apply a small adhesive bandage.
-
Immobilize the treated joint for 2 days and have the patient avoid strenuous activities for 2 weeks.
-
Perform biodistribution analysis using a
small window Geiger-Muller counter to determine the counts per minute
for the target joint, contralateral joint, regional nodes, and liver.
In addition, perform a single photon emission computed tomography
(SPECT) scan to determine the distribution of the radiocolloid within
the joint. Check the biodistribution at the time of the procedure, and
after 1 day, 1 week, and 1, 2, and 3 months. Because of the large
particle size, escape of the colloid from the joint is much less
frequent than with gold or yttrium. At our center, in 120 consecutive
cases, only three patients demonstrated minute escape of the
radioactive colloid, ranging from 0.5% to 2.5% of the total dose in the
target joint (46).
consistent, with about a 75% rate of good and excellent results defined
as complete cessation of hemarthroses (excellent) or greater than 75%
reduction in frequency of hemarthroses (good) (1,46,47,64). This success rate remains stable at 10 years follow-up.
the radiation, with particular concern for damage to local tissues,
such as the growth plate or articular cartilage, and the late
development of a secondary neoplasm. Because 32P chromic phosphate is a pure beta emitter, the penetration of radiation is limited to 3 to 5 mm; therefore, intra-articular
injection should not reach the growth plate. To date, there have been no reported cases of growth plate disturbance (3,18). Furthermore, over the last 20 years, many centers have treated pediatric patients with 198Au
radiocolloid, which has both beta and gamma radiation, without apparent
growth plate disturbance. Articular cartilage is highly radioresistant,
and although damage is theoretically possible, none has been reported.
Progressive degeneration of treated joints does occur, but the rate is
equal to or slower than expected for the disease process (38,53,55,77).
potential of late radiation-induced neoplasia. External beam radiation
has been extensively studied and carries a small risk of induction of
bone sarcomas, principally osteosarcoma or fibrosarcoma. The peak
incidence is 5 to 10 years after the procedure, but cases have been
reported as early as 3 years (28,49). Because of the low penetration of 32P
chromic phosphate, bone exposure is minimal. Furthermore,
intra-articular sarcomas of any type are extremely rare. For example,
synovial sarcomas and chondrosarcomas are almost never intra-articular.
intra-articular radiocolloids, is the long-term follow-up of the more
than 5,000 radiosynovectomies performed for rheumatoid arthritis, none
of which have been reported to develop radiation-induced malignancies (41). Five to 10 millicuries of 198Au
were used on most of these patients since 1963. The small (20 µm)
particle size of Au resulted in a 20% to 37% incidence of escape,
usually to regional nodes (22,71,74).
Yet, there have been no reports linking the use of this agent to
hematogenous malignancies or sarcomas. In our series, the maximum
escape was 2.5% of the target dose, which occurred in one patient (46). Winston et al. (79),
using chromic phosphate in rheumatoid patients, reported a maximum
escape in only one patient of 3.2%, which agrees closely with our
results. Rivard (64), however, reported three
patients with 7%, 9%, and 14% escape using chromic phosphate.
Immobilizing the treated joint for 2 days and limiting activities for 2
weeks may reduce the risk of escape. The small amount of
intra-articular steroids used in our series may account for the absence
of postinjection inflammation and bleeding reported in other series.
This approach may further reduce the risk of escape.
major surgery for chronic synovitis or advanced hemophilic arthropathy
was a rare undertaking fraught with an unacceptable rate of
complications as a consequence of uncontrolled hemorrhage and systemic
fluid overload from the transfusion of whole blood and fresh frozen
plasma (31). Even with the availability of
cryoprecipitate, surgery was mostly limited to the mild hemophiliacs
with higher levels of circulating factor VIII or IX (13,14,57).
With the availability of factor VIII concentrate in the United States
in 1967, major hemophilia centers began performing elective surgery on
their patients (14,16,17,30,57).
During the past 30 years, an interdisciplinary team of physicians at
OHHC has performed more than 700 major surgical procedures on
hemophiliacs. Our surgical philosophy and recommendations are based on
that experience and a substantial volume of published reports and
presentations from hemophilia centers around the world.
for about 85% of hemophilia. Almost all of our joint replacement
patients fall into this group. For many years, we were reluctant to
consider major surgery on patients with factor IX deficiency, or type B
hemophilia, because of the increased risk of life-threatening
thromboembolic complications related to activated clotting factors in
factor IX concentrate previously reported by our center (30). When purified factor IX became available in 1990, however, these patients also became elective surgical candidates (31).
Rarely, patients with Von Willebrand’s syndrome and other clotting
factor deficiencies will develop hemophilic arthropathy that requires
joint replacement. Some will need joint replacement at an advanced age
on the basis of osteoarthritis unrelated to their clotting disorder.
retrospective study of the first 15 years’ experience with clotting
factor concentrate in elective surgery. The amount of concentrate used
tripled during the study period, but the incidence of postoperative
bleeding was not significantly reduced by the additional factor VIII
replacement. The frequency of postoperative bleeding correlated better
with the site and type of surgery than with the factor levels at the
time of the incident. For example, the incidence of postoperative,
surgical site hemorrhage was 40% for knee surgeries and 15% for all
other types. These findings were similar to those reported by Kay et
al. (32).
-
Preoperatively, carefully screen the
patients for any evidence of a factor VIII inhibitor and any medical
contraindications to elective reconstructive surgery. -
Elevate factor VIII levels to above 100% of normal 1 hour before surgery.
-
Maintain factor levels at 60% by
continuous infusion until the patient is discharged. This is cost
effective because continuous infusion maintains a more consistent level
and uses less total factor than bolus therapy (25).
allow selected patients to leave the hospital after the first week and
continue intermittent self-infusion at home. This is possible only for
lesser procedures, such as radial head excision and elbow synovectomy,
and in patients who are on the self-infusion program, live close to the
hospital, and are eminently reliable.
arthropathy may be divided into two categories based on the degree of
arthropathy: synovectomy with or without debridement for early to
moderate arthropathy, and reconstructive surgery for advanced
arthropathy.
hemophilia centers depending on their interest in radiosynovectomy.
Because radiosynovectomy is about 5% to 10% of the cost of surgical
synovectomy and much easier for the patient, most centers favor that
procedure for earlier cases. Surgical synovectomy is clearly indicated
for patients who have very thick synovium (more than 5 mm) that would
be beyond the penetration of beta-emitting radiocolliods, patients with
significant arthrofibrosis requiring debridement, and patients in whom
radiosynovectomy has failed. Arthroscopic synovectomy has almost
completely replaced open synovectomy, except for the patient with
severe arthrofibrosis and loss of motion.
hemarthrosis that is unresponsive to appropriate conservative
management in a joint with minimal arthropathy. A trial of conservative
treatment usually requires 3 to 6 months. However, by the time these
patients come to surgery, there is often a significant amount of
full-thickness articular cartilage erosion and the remaining articular
surface has sustained significant mechanical and biochemical insult (Fig. 136.1, Fig. 136.2A).
Under these circumstances, synovectomy is rarely curative but is often
effective in resolving the chronic and recurrent hemarthroses, and
possibly slowing the progression of arthropathy (52,54).
Resolution of the chronic hemarthrosis allows the patient to resume
therapeutic exercises, often with marked improvement in function as
well as reduction in hemarthroses and the need for coagulation factor
replacement.
before irreversible damage to the articular cartilage occurs. Surface
erosion occurs early in this process, even before the synovitis is
noticeably symptomatic or clinically evident. Patients at this stage
are often 3 to 8 years of age. If the safety of radiosynovectomy can be
established for this age group, early intervention may become the
standard. Early arthroscopic synovectomy is also an option because
children are much more difficult to rehabilitate than adults after
surgical synovectomy. The pediatric patient is not usually capable of
exercising the knee if it is painful and often loses range of motion
after open synovectomy and occasionally after arthroscopic synovectomy
if hemarthrosis occurs. Small vessel hemostasis at the time of
arthroscopic synovectomy is not possible with motorized shavers but
would be possible with a laser. However, to date there is no large
reported experience with laser synovectomy in hemophilia. For patients
whose range of motion fails to progress postoperatively, consider
closed manipulation 10 to 14 days postoperatively, followed by
continuous passive motion. The injection of a nonsoluble steroid
preparation, such as dexamethasone acetate, combined with long-acting
local anesthetic at the time of manipulation has been helpful in
reducing postmanipulation pain, inhibiting fibroplasia, and restoring
range of motion, especially in children.
center are presented in the order of the frequency of joint
involvement: knee, elbow, ankle and subtalar joint, hip, and shoulder.
and disability severe enough to require reconstructive surgery in
hemophiliacs. Involvement of the ankle and elbow is about as common as
that of the knee, but the ankle and elbow require surgical intervention
less frequently. Historically, the range of procedures considered for
hemophilic arthropathy of the knee included synovectomy, debridement,
debridement with patellectomy, osteotomy, fusion, and total knee
prosthetic arthroplasty. Today at OHHC, we perform only arthroscopic
synovectomy, open debridement, total knee prosthetic arthroplasty, and
fusion. Previously, we performed distal femoral extension osteotomy for
severe flexion contractures that failed to respond to physical therapy
or serial casting. Today, such deformities are much less common except
in patients with advanced arthropathy and are corrected at the time of
prosthetic arthroplasty. Preservation of the patella is especially
important in this patient population who are prone to flexion
contractures, especially following knee replacement. Patellectomy is
occasionally indicated at the time of knee replacement when, either
from erosion or prior surgery, there is inadequate bone stock for
secure fixation of a prosthetic component.
rehabilitation and the risk of recurrent arthrofibrosis, patient
motivation and careful preoperative counseling are very important. At
OHHC, all patients are seen preoperatively by
the surgeon, hematologist, physical therapist, and patient-care coordinator as a team for assessment and counseling.
synovitis and early to moderate arthropathy. The principal indications
for debridement are recurrent hemarthrosis that is unresponsive to
conservative management and impingement symptoms, usually associated
with recent loss of extension. In some patients, impingement is
intermittent, but in others, it cannot be overcome, and any attempt at
vigorous exercise or passive range of motion results in hemarthrosis.
Pain in this group of patients is usually intermittent and responds to
nonsteroidal anti-inflammatory agents and analgesics. If pain is the
principal symptom in a patient with moderately advanced to advanced
arthropathy, debridement is rarely of lasting benefit. In our
experience, at 7 to 21 years of follow-up, 6 of 18 patients who had
undergone debridement had required total knee replacement, four of them
within 1 year. These patients were older at the time of debridement
(average age, 30.8 years) than those who obtained lasting benefit
(average age, 16.5 years), and they had arthropathic pain as their
principal complaint (43).
of motion. Arthrofibrosis with loss of motion is one of the most
significant complications of surgery for advanced hemophilic
arthropathy (6,40,42,50,52,53,73,75).
In our series, the average loss of range of motion in the nine patients
undergoing arthroscopic debridement was only 9°, but they had a 50%
incidence of postoperative hemarthrosis compared to none in the open
debridement group. This difference is attributed to the inability to
achieve adequate hemostasis with arthroscopic debridement. Open
debridement has been performed for patients with arthrofibrosis.
Although early improvement may be obtained, the arthrofibrosis often
recurs with a poor end result. Occasionally, lasting improvement in
range of motion has been obtained following the injection of a
long-acting local anesthetic and a nonsoluble cortisone preparation at
the time of knee manipulation 7 to 14 days after surgery.
end stage arthropathy and fibrous ankylosis, severe deformity, or
infection. Seven primary knee fusions have been performed at OHHC. Two
of these patients had a history of infection, one from a hematogenous
pyarthrosis with Pseudomonas aeruginosa and another with chronic Staphylococcus aureus
osteomyelitis after an osteotomy. Five of the patients fused without
problems and with good long-term results, including the one patient
with Pseudomonas infection before surgery.
One of the patients hemorrhaged from the denuded bone surfaces and
swelled to such an extent that the external fixator crossing the knee
had to be removed. He healed without infection but has a pseudarthrosis
that is stable and painless. He is fully ambulatory with a cylinder
orthosis. The patient with chronic osteomyelitis fused but has a
draining sinus. Six of these patients have limited mobility of the
opposite knee and bilateral ankle and elbow involvement but can rise
from an armchair without assistive devices. No patient has developed
associated ipsilateral hip or low-back problems. We have performed no
primary knee fusions in the last 15 years because we now favor total
joint replacement. Patients with end-stage arthropathy and fibrous
ankylosis usually have minimal or no pain and do not require surgery if
the knee is near-full extension and adequately aligned.
hemophilic arthropathy. At OHHC, knee replacement represents about 10%
of all surgeries on hemophiliacs. The indications for knee replacement
are the same in hemophilia as in other arthropathies: pain and
disability that is unresponsive to conservative management with
end-stage radiographic changes (Fig. 136.6).
End-stage arthropathy may develop by late adolescence. Severe pain and
disability often exist by the third or fourth decade. The age range at
surgery in our series was 21 to 62 years, which is typical. Most
patients are in their forth or fifth decade at the time of operation.
careful dissection and definition of normal tissue planes complicate
the surgical approach for knee replacement in these patients. It is
important to preserve the fat layer in the supracondylar area of the
femur, which is an effective barrier to adhesions between the extensor
mechanism and the distal femur. Many patients have flexion contractures
that require modified cuts on the distal femur as well as posterior
capsular release. Our preferred method of posterior release is under
direct vision through the usual anterior approach to the knee, as
follows:
-
Carefully dissect the posterior capsule
off the distal femur with the knee in maximum flexion. Interpose your
nondominant hand and use it to apply pressure to the posterior capsule
just below its attachment to the distal femur to protect the popliteal
structures from inadvertent laceration while dissecting the capsule off
the femur with a half-inch osteotome. -
Excision of popliteal synovitis as well
as the posterior release are often facilitated by placing a
self-retaining lamina spreader alternately in the medial and lateral
compartments to separate the femur from the tibia and maintain tension
on the capsule. Extensor mechanism contracture is also common.
Quadricepsplasty may be required but often results in loss of full
active extension, which gradually recovers over the ensuing few months. -
Take care to maintain full passive
extension postoperatively. Partial release, rather than complete
transection of the extensor mechanism, is preferable when it is
adequate to allow 80° of flexion. A V-Y lengthening is performed for
more severe cases. With diligent physical
P.3605
therapy, these patients often end up with 90° or more of motion. -
Close the wound in patients with limited preoperative flexion, with the knee in about 50° flexion.
1974 and were of the two-compartment type. We began using
three-compartment “total condylar” prostheses in 1978. Beginning in
1982, 18 bone ingrowth “cementless” prostheses were implanted. For the
last decade, we have used cemented posterior stabilized knees
exclusively. The age range at the time of surgery and pathology was
very similar among these three groups. There has been no statistically
significant difference between these groups in the survivorship of the
prosthesis, which, without infection, is 96% projected to 20 years (43,44).
However, the eight knee replacements of the two-compartment type had an
average loss of 10 degrees of range of motion, and three patients had
residual patellofemoral pain. After converting to three compartment
replacements, range of motion improved an average of 30° for patients
with a preoperative range of less than 80°. The probable reason for
this gain in motion is inclusion of the patellofemoral joint
replacement, but improved techniques, including the use of continuous
passive motion postoperatively, also may have contributed. At present,
we begin continuous passive motion the second postoperative day if the
wound is doing well. We no longer use immediate postoperative
continuous passive motion because it is associated with increased
bleeding. Although the end results of the “cementless” knees were
equivalent to our current cemented models, there was significantly
increased blood loss postoperatively, as measured by Hemovac output.
arthropathy owing to synovial cysts or mechanical erosion. Earlier
solutions to this problem included filling defects with
polymethylmethacrylate cement or the use of specially designed, stemmed
components. During the past 18 years, bone grafting has been the
procedure of choice. It is especially attractive because of the
potential to improve bone stock if future revisions are needed in these
relatively young patients. A minimum of bone is resected. Local
autogenous bone is used for small and moderate-sized defects. Large
defects require allograft. Autogenous iliac graft is not used because
of the risk of hemorrhage and possible pseudotumor formation at the
donor site.
complications have occurred following knee replacement: hemarthrosis,
Coombs-positive hemolysis, wound dehiscence at the time of
manipulation, and component loosening (43). In
one study, hemarthrosis in the perioperative period following knee
surgery on hemophiliacs was reported in 40% of patients regardless of
the factor level (30). Perioperative
hemarthrosis tends to occur in the patients who still have active,
hypertrophic synovitis and are in the stage of chronic hemarthrosis.
The intra-articular blood is partially clotted and impossible to
aspirate. It should be evacuated using arthroscopic cannulas, followed
by thorough lavage and the placement of a suction drain.
one knee replacement patient and was probably the result of minor
host-donor incompatibilities present in earlier clotting factor
concentrates. Clotting factor preparations are now purified, and there
have been no instances of this hemolysis in the last 12 years.
manipulation in the early days of knee replacement. Today, manipulation
is rarely used. However, the capsule is always closed with
nonabsorbable suture to reduce this risk, especially in the patient
with arthrofibrosis who will undergo fairly vigorous physical therapy
beginning a few days after surgery. There were no perioperative
infections, even in patients infected with the human immunodeficiency
virus (HIV). Later complications have included hematogenous infection
and component loosening.
three patients, each with single-component loosening: tibial component,
femoral component, or patellar component. The femoral component failed
when the patient fell and fractured his distal femur near the
component. The failed tibial component, a Geomedic two-compartment
replacement performed in 1974, was inserted in a patient with severe
bone stock deficiency resulting from a large pseudotumorous cyst in the
upper tibia, which was filled with cement. The failed patellar
component was inserted into a patella that had eroded down to a thin
cortical shell and did not have adequate bone stock for cement
intrusion and fixation. The loose femoral and tibial components were
treated by revision surgery, and the patellar component was treated by
patellectomy. These three revision surgery patients have done well. The
patellectomy patient regained active range of motion of 0° to 90°. The
tibial component failure was revised to a Total Condylar I (Zimmer)
prosthesis and did well for 9 years, until he died of hepatic
carcinoma, a consequence of chronic active hepatitis C. The patient
with the femoral failure with the distal femur fracture was revised to
a Total Condylar III (Zimmer) prosthesis and is doing well after 15
years. This represents an aseptic failure rate of 3 in 90, or 3.3%, at
2 to 24 years’ follow-up, which despite the young ages of these
patients at the time of prosthetic arthroplasty, is comparable to a
reported series of knee replacements in patients with osteoarthritis
and rheumatoid arthritis (27). The low aseptic
failure rate for this relatively young population is probably due to
their low activity level as a consequence of polyarthropathy. The
principal mode of failure of prosthetic joints in hemophiliacs is late,
hematogenous infection,
which is discussed in a separate section of this chapter.
hemophilia, but most patients are not symptomatic enough to warrant
surgical intervention. Radionuclide synovectomy for persistent
synovitis was effective in reducing hemarthroses in 78% of patients in
our series (46). Under present protocols, at
the time of radiosynovectomy, essentially all patients have some degree
of joint surface changes. The synovectomy may forestall but will not
prevent progression to advanced arthropathy. The only open surgical
procedure, in our experience, that is useful for patients with
persistent synovitis and advanced arthropathy is radial head excision
with synovectomy and debridement.
and debridement include pain, chronic hemarthrosis unresponsive to
nonoperative management, and loss of forearm rotation. The most
disabling loss of motion in these patients is loss of supination on the
dominant side. If it is less than 45°, it impairs the patient’s ability
to eat normally, accept change, and accomplish personal hygiene. The
major source of pain, recurrent bleeds, and restricted forearm rotation
is derangement of the proximal radioulnar joint secondary to
hypertrophy and marginal irregularity of the radial head. Hemophilic
arthropathy of the elbow often is associated with marked hypertrophy of
the radial head, which impinges against the proximal ulnar facet,
inhibiting forearm rotation and trapping fragile synovium, resulting in
hemarthrosis (Fig. 136.7). The patient’s pain
is typically posterolateral. There is focal tenderness over the
posterior aspect of the proximal radioulnar joint and increased pain
with forearm rotation, especially supination.
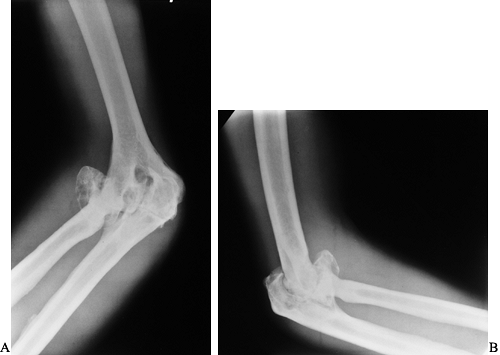 |
|
Figure 136.7. (A) and lateral (B)
radiographs showing advanced hemophilic arthropathy of the elbow with massive enlargement of the radial head and synovial cysts on both sides of the joint resulting in deepening of the trochlear groove. |
the ulnar facet, which eliminates impingement but preserves part of the
annular ligament for stability. Later proximal migration of the radius,
causing distal radioulnar joint symptoms, has not been observed in our
patients,
all of whom were at least in their third decade at the time of surgery. This procedure is not recommended for immature patients.
Following radial head excision, dissect part of the common extensor
origin from the lateral epicondyle, to expose the ulnohumeral
articulation. This dissection is aided by varus stress. Posterior
exposure may be enhanced with further dissection of the capsule from
the posterolateral humerus.
spite of advanced changes in the medial side of the ulnohumeral
articulation, which is only debrided. Cessation of recurrent or chronic
hemarthrosis occurs in approximately 90% of patients. In patients with
restricted forearm supination preoperatively, radial head excision has
resulted in an average gain of 30°, which has made a significant
difference in the ability to perform activities of daily living.
Occasional patients will have significant pain at the ulnohumeral
joint, usually on the medial side, and some will manifest symptoms of a
tardy ulnar palsy as a result of synovial invasion of the ulnar groove.
These patients can be helped with ulnar nerve transposition and
debridement of the ulnar side of the elbow.
in the ulnohumeral joint, its capsule, and the musculotendinous units
crossing the joint. Loss of this range in chronic hemophilic
arthropathy is a long-term process and is not altered significantly by
radial head excision, even with ulnohumeral joint debridement.
Increased range may be accomplished at surgery but is rarely maintained
postoperatively.
of involvement and is usually the first target joint in children after
they begin to walk. Progressive arthropathy is the rule. End-stage
ankle arthropathy is commonly manifested by severe joint surface
erosion, valgus alignment, and opposing exostoses on the tibia and
talus. Large synovial cysts occasionally invade the talus adjacent to
the ankle joint (Fig. 136.8). The subtalar joint is also subject to hemophilic arthropathy, although involvement is less common than in the ankle (Fig. 136.9).
Its involvement usually accompanies that of the ankle but can occur in
isolation. Erosion of the posterior margin of the posterior facet,
similar to that seen in rheumatoid arthritis, is the first
manifestation of subtalar joint disease on plain radiographs. Many
patients develop equinus deformities secondary to ankle arthropathy and
calf bleeds with residual muscle contractures (Fig. 136.8, Fig. 136.10).
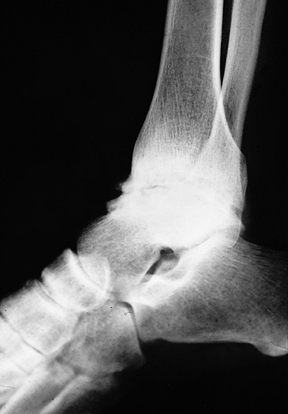 |
|
Figure 136.8.
Advanced arthropathy of the tibiotalar joint with surface irregularity, joint surface erosions, and abutting anterior exostoses. |
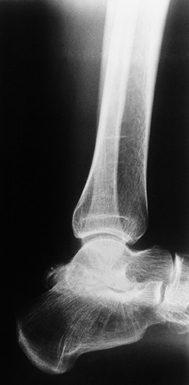 |
|
Figure 136.9. Isolated advanced arthropathy of the subtalar joint without tibiotalar involvement.
|
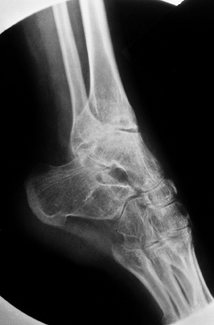 |
|
Figure 136.10.
Advanced arthropathy of the ankle involving both tibiotalar and subtalar articulations. This patient has a severe equinus deformity that resulted from a massive calf hemorrhage during his adolescence, which was before clotting factor was available. |
procedure of choice for the ankle or subtalar joint is arthrodesis.
Over the years, a variety of fixation techniques has been used,
including Charnley external fixation, anterior plate and screws, and
crossed screws. Crossed screws are our favored technique. For combined
ankle and subtalar arthrodesis, we drill two or three cancellous screws
from the tibia across the talus into the oscalcis using a custom drill
guide. The distal fibula often unites more slowly than the tibiotalar
joint. A distal fibular osteotomy eliminates rotation stresses about
the lateral malleolus. If the malleolus is unstable, the lateral
malleolus may be compressed against the talus with a single transfixing
screw into the tibia. In patients with moderate to severe equinus
deformity and Achilles tendon contracture, a single-pin Charnley
compression device may be placed through the anterior talus and tibia
to maintain the foot in neutral and take stress off the internal
fixation screws transfixing the ankle. Cast fixation alone may not be
adequate in these patients without producing excessive pressure on the
metatarsal heads.
tibiotalar arthrodesis, 34; tibiotalar and subtalar arthrodesis, 5;
pantalar arthrodesis, 2; and tibiotalar prosthetic
arthroplasty,
1. If the talonavicular and calcaneocuboid joints are normal, they are
not included in the fusion. Fixation techniques included 39 internal
fixation and 3 Charnley external fixators. External fixators are safe
in hemophiliacs and do not require clotting factor replacement after
the initial 2 weeks. This is similar to the experience reported by
Wilson et al. (78) and Patel et al. (59), but different from that suggested on theoretical grounds by Trueta (72) and Arnold and Hilgartner (6).
cast for 6 weeks, followed by a weight-bearing well-molded short leg
cast or a polypropylene ankle-foot orthosis (AFO) depending on the
degree of consolidation evident on radiographs at 6 weeks. At 12 weeks,
even if the fusion appears solid, continue an AFO until the fusion is
solid and can withstand all daily activities.
degeneration of the unfused midfoot joints following combined ankle and
subtalar arthrodesis.
external fixation developed a painless nonunion, and there was one
patient with delayed union of a subtalar joint in which internal
fixation was used. Gamble et al. (20) reported
on 10 tibiotalar arthrodeses in eight patients using a variety of
techniques. Two developed painless nonunions but continued to have
bleeding episodes. There have been no early or late infections in our
patients who underwent ankle fusion or in those reported by Gamble.
involvement is rare, primary pantalar arthrodesis has been required
only twice in the OHHC series. One other case was reported in the
literature (59). All three of these patients
obtained solid fusions and can bear weight fully without orthoses. One
tibiotalar joint replacement was performed in 1978; the patient moved
away, but his brother reports that he is doing well, without pain or
hemarthroses. In our opinion, the ankle and subtalar joints are better
treated by arthrodesis than with prosthetic arthroplasty.
rapidly progressive, severe arthropathy may result from a single
hemarthrosis because of increased intracapsular pressure leading to
osteonecrosis of the capital femoral epiphysis (Fig. 136.11). More often, hip arthropathy is the result of chronic synovitis, similar to that of the other joints (Fig. 136.12A).
The first prosthetic arthroplasty of the hip in a hemophiliac in the
United States was a cup arthroplasty performed at OHHC by J. Vernon
Luck in 1968 (Fig. 136.12B).
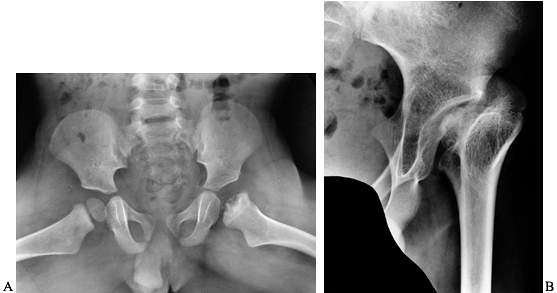 |
|
Figure 136.11. A:
Frog lateral x-ray study in a 20-month-old boy with severe hemophilia B and a history of a hemarthrosis in his left hip at 6 months of age. B: The same patient at skeletal maturity. His capital femoral epiphysis never developed. |
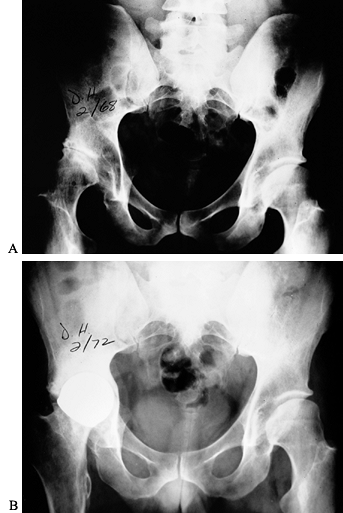 |
|
Figure 136.12. A:
Advanced hemophilic arthropathy of the hip resulting from chronic synovitis with typical joint surface erosions and lateral subluxation. The coxa valga is probably the result of inhibition of abductor function from hemarthroses during growth. B: Cup arthroplasty in the same patient. This was the first major reconstructive procedure performed on a hemophiliac at OHHC. |
The patients are relatively young when they require hip replacement.
They are not suitable candidates for fusion because of knee
involvement. In our experience, primary cemented prostheses have a 33%
aseptic failure rate at 8 to 18 years follow-up, which may be higher
than expected in a comparable group of patients with another form of
polyarthritis. The patients in the OHHC series were relatively young
(30 to 61 years of age at the time of surgery) but not particularly
active owing to multicentric arthritis. Hemophiliacs tend to be
slender, and none of the hip replacement patients was overweight. With
the exception of periarticular cysts, bone density is better than would
be expected in a group of patients with rheumatoid arthritis in the
same age range. Kelley et al. (33) reported a
failure rate of 21% for cemented femoral components and 23% for
cemented acetabulae, with a median follow-up of 8 years. In addition,
10 of 24 cemented femoral and 10 of 23 cemented acetabular components
showed radiographic evidence of “definite” loosening. Within this group
were three patients with late infection, all of whom were infected with
HIV. Nelson et al. (56) reported on 22 total
hip replacements, eight of which had been revised or showed definite
signs of loosening radiographically, with an average follow-up of 7.6
years. There were two deep infections, but this factor was not
correlated with HIV status.
hemophilic arthropathy is a remarkably stiff-legged gait due to severe
arthrofibrosis of the knee and ankle. This causes increased forces on
the hip due to a lengthened lever arm and loss of the normal
shock-absorbing function of the knee and ankle. All of the patients in
our series had bilateral knee and ankle arthropathy, and none had knee
joint replacements. Part of the reason for loosening of cemented hip
prostheses in hemophiliacs may be the increased stresses of a
stiff-legged gait. Another consideration is the possibility of bleeding
within the membrane that forms at the cement-bone interface. As a
result of subclinical
hemorrhage within and around this membrane, loosening may propagate more rapidly in hemophiliacs than other arthritis patients.
prostheses to improve durability. Results in terms of pain relief and
cessation of hemarthroses have been excellent, and none of the 12 cases
have required revision for fixation failure. One HIV-positive patient
developed a late infection requiring prosthesis removal. More cases are
needed before we will know if this represents a significant improvement
over cemented prostheses in the management of hemophilic arthropathy of
the hip.
it has received. Shoulder pain, like hip pain, can be intractable,
interrupting sleep and daytime function. Muscle atrophy and loss of
motion occur early, often before the patient is aware of a significant
problem. Attempts to restore range of motion and increase strength are
usually
thwarted by hemorrhage due to synovial hypertrophy that is friable and bleeds even with antihemophilic factor replacement.
conservative measures. Open shoulder synovectomy has been abandoned
because of unacceptable loss of motion and persistent pain. Shoulder
arthroscopy is severely inhibited in these joints because of fibrosis
and hyperplastic synovitis. The answer to this dilemma for all
hemophilic joint involvement, but especially that of the shoulder, is
early diagnosis by regular examination or at the time of initial
hemorrhage. Early in the course of the disease, maintenance of strength
and range of motion is still possible. Regular follow-up examinations
are essential. Persistent synovitis, which is unresponsive to
prophylactic concentrate, should be treated by synovectomy either with
radiocolloids or arthroscopically before joint surface erosion and
arthrofibrosis cause loss of motion.
usual course. Hemarthrosis diminishes in the fibrotic end-stage joint.
Pain is the primary indication for surgical intervention. Loss of
shoulder motion in these patients does not cause enough impairment of
activities of daily living to warrant surgery by itself. End-stage
arthropathy of the shoulder may be treated by arthroplasty or
arthrodesis. The traditional approach has been arthrodesis, which gives
predictable and durable results but has the disadvantage of lesser
range of motion, especially rotation. Impaired elbow motion in most of
these patients makes loss of shoulder motion even more consequential.
The patient must be involved in this decision after careful counseling.
fusion at OHHC failed to obtain solid bony union, although the clinical
results were good in terms of the absence of pain and hemarthroses
because of a stable fibrous ankylosis. These nonunions seem to have
occurred because of reactive sclerosis at the glenohumeral joint
combined with the use of single-plane glenohumeral screw fixation. Our
preferred method of fusion is the three-plane screw fixation method
developed by Kauko Vainio (personal communication, 1974). This method
includes the AO screw transfixion of the acromiohumeral and
coracohumeral junctions in addition to the glenohumeral joint.
Glenohumeral and acromiohumeral wire loops lend additional stability.
The upper extremity is placed in a salute position, with the shoulder
in 20° to 30° of abduction, 30° of forward flexion, and 30° to 40° of
internal rotation. Postoperatively, we immobilize the shoulder in a
modified Bateman foam support, which allows periodic elbow motion,
until fusion is apparent radiographically. All of our fusion patients
are able to reach the top of their heads with the help of neck flexion,
and all can reach their sacrums. The five patients in whom three-plane
screw fixation was used have all attained solid bony union.
increased rotation but does not improve abduction or forward flexion
owing to the arthrofibrosis. During the last 15 years, we have
performed six prosthetic shoulder arthroplasties in hemophiliacs using
the Neer II prosthesis with cement fixation. All are satisfactory, and
none has recurrent hemarthrosis. Five are pain free, and the other has
occasional slight pain. Range of motion is improved in all compared
with their preoperative status. Rotation is better than the fusion
group but forward flexion and combined abduction are about the same.
became infected with HIV between 1978 and the introduction of an
effective antibody screen, the enzyme-linked immunosorbent assay
(ELISA), in 1985. Mild and moderate type A and type B hemophiliacs use
less clotting factor concentrate and thus fewer have seroconverted.
This factor has added immensely to the complexity, morbidity, and
mortality of an already medically challenged population. Fortunately,
treatment of HIV infection has improved to the point at which patients
who were severely debilitated are now stable and leading relatively
normal lives. Many of these patients have advanced arthropathy and are
potential candidates for reconstructive surgery.
immune deficiency syndrome (AIDS) has affected indications for elective
surgery in these patients. As always, the decision is based on the
risks and benefits to the patient. No decision is made without a
comprehensive team evaluation, team conference, and multiple
discussions with the patient.
patient involves some special risks, which may be divided into two
categories: risk to the patient, and risk to health care personnel.
Both of these risks must be understood and considered in making a
decision regarding the appropriateness of surgery for these patients.
Because of concern about these issues, some orthopaedic surgeons may
pursue nonoperative management of fractures that are usually treated
surgically and are reluctant to recommend elective surgery in the
HIV-positive patient.
and the frequency of parenteral and surface exposure to blood and body
fluids in the operating room setting, there has been extensive study of
the risks to health-care personnel. Recommendations of methodologies to
reduce this risk in the practice of orthopaedics have been developed
and published by the Task Force on AIDS and Orthopaedic Surgery of the
American Academy of Orthopaedic
Surgeons (AAOS) (69).
To date, there have been 49 well-documented occupational
seroconversions among health care workers. However, none have followed
suture needle injuries, the most common surgical percutaneous injury (8).
In a recent report from the Centers for Disease Control (CDC),
zidovudine (ZVD) was shown to be effective in the prevention of
seroconversion following percutaneous exposure to HIV-contaminated
blood (9,10). Hepatitis
C virus (HCV) transmission by percutaneous injury may pose a greater
risk to health care personnel than HIV. HCV is about equally prevalent
in this patient population and is estimated to be about 10 times more
transmittible by percutaneous injury (35,51,54).
We believe that if the AAOS/CDC recommendations are closely followed,
the risk to health care personnel, although real, can be reduced to an
acceptable level.
lessened but not totally eliminated by the vigorous application of
universal precautions in the operating room and on the ward. The use of
face masks, impervious gowns, and foot covers in the operating room is
effective in preventing blood or body fluid contact with eyes or skin.
If power equipment (e.g., saws, drills) that creates airborne blood
droplets is used, we wear space suits with inflow and outflow
high-efficiency particulate arrestance (HEPA) filters. Even more
important than this is the prevention of puncture injuries from suture
needles, bone spicules, and other sharp objects, which are common in
orthopaedic surgery. Modified surgical technique and the routine use of
triple gloves with a cloth intermediate glove can help reduce this
risk. Research is under way on the development of a tougher, more
protective glove material.
transmission in the health care setting, surgeons have expressed
concern about the complications and outcome of operations on the
HIV-infected patient. This risk may be divided into early and late
postoperative complications. Early postoperative complications of
greatest concern in the HIV-positive patient include sepsis and
impaired healing. The late postoperative complication of primary
concern in the HIV-positive orthopaedic patient is implant infection,
which some authors have speculated is at increased risk (29,36).
The risk of early and late septic complications is theoretically
increased because of impaired cellular and humoral immunity. The
estimated magnitude of increased risk is based on a series of reports
of the outcome of surgery on HIV-positive patients and is less well
delineated than the theoretical basis. These clinical studies also
address our ability to prevent complications in the HIV-positive
surgical patient successfully.
surgical wound infection rates in HIV-positive and HIV-negative
hemophiliacs by reviewing 169 procedures, 53 of which were orthopaedic.
There were two wound infections but no statistically significant
difference in the wound infection rate between the HIV-positive group
(1.4%) and the HIV-negative group (0%). There were no wound infections
in the seven procedures performed on patients with AIDS.
orthopaedic procedures on HIV-positive hemophiliacs performed between
1984 and 1988. There were no surgical site infections but there was one
case of cellulitis at an intravenous site. Five patients had a
protracted postoperative fever but did not develop clinical infection.
The outcome and functional results were similar to the patients treated
before 1982 who were presumed to be HIV negative.
studied the rate of early postoperative infection in 74 orthopaedic
procedures performed on 66 HIV-positive patients with CD4 counts below
200 at the time of surgery. [Cluster designation (CD) identifies
lymphocyte subsets.] A CD4 count of less than 200 qualifies these
patients for the diagnosis of AIDS. Eliminating those patients with
evidence of active infection preoperatively, the rate of postoperative
infection was 7.5%. Thirty-four of the procedures were joint
replacements, of which there were five postoperative infections without
evidence of infection preoperatively. Except for the timing of
infection, this rate of 6.7% is not different from reported series of
joint replacements in HIV-negative hemophiliacs (21,43,53).
philosophy or guideline may be developed for elective surgery on the
HIV-positive hemophilia patient in various stages of disease. Most of
the clinical studies available to date do not demonstrate an increased
incidence of early postoperative complications in asymptomatic
HIV-positive patients compared with the HIV-negative group.
Furthermore, most of the orthopaedic studies and the more recent
general surgery studies do not show an increased incidence of early
complications in the symptomatic HIV positive patients with CD4 counts
above 200 undergoing elective procedures. The basic science work
establishes some impairment of defenses against common orthopaedic
pathogens and the potential delay of wound healing (45).
As the disease progresses and these impairments increase, the hazard of
early complication increases. The risk of late prosthetic implant
infection is increased in hemophiliacs regardless of HIV status. In
view of all of these factors, special management of the HIV-positive
hemophiliac patient undergoing surgery seems warranted. OHHC uses a
protocol that serves as the basis for the following recommendations.
surgery is crucial. As a result of improved reverse transcriptase
agents and third-generation protease inhibitors, high viral loads are
treatable to undetectable levels, often
resulting
in elevation of the CD4 count. The prognosis and life expectancy for
patients with AIDS has improved dramatically, making reconstructive
surgery in these patients who require it an appropriate consideration.
Following a thorough assessment of the patient’s medical status, a
thoughtful discussion of the risk–benefit ratio ensues between the
patient and the multidisciplinary team. Quality of life issues are
often a principal consideration in the patient’s view and must be
balanced against risk management.
on which each patient may be positioned to assess risk properly. No
single clinical factor is a reliable predictor of longevity or risk of
surgery. Several components should be combined in determining a
prognosis and assigning risk. The factors that seem to correlate best
with surgical outcome are history of opportunistic infection, CD4 cell
level less than 200, serum albumin less than 25 g/L, and cutaneous
anergy (23,44,61).
However, these studies antedated current HIV therapy. Today, viral load
combined with CD4 counts are the primary indicators for surgery.
Patient reliability and ability to cooperate with medical
recommendations is essential to reduce the risk of late complications
in the joint-replacement patient.
steps can be taken to reduce the risk of perioperative complications
further. Some of the measures are applicable to an emergent situation.
Others require more time and are only feasible in elective surgery. The
absolute polymorphonuclear leukocyte count should exceed 1,000 and the
platelet count should exceed 50,000. In addition to HIV, platelet
deficiency can result from chronic active hepatitis B or C, which is
common in hemophiliacs. Acceptable platelet levels may vary from 30,000
to 60,000, depending on lever function status and other comorbidities.
Platelet transfusions also may be used when needed. Chronic liver
disease can also reduce vitamin K levels, which can further compound
the coagulation disorder. In patients for whom surgery is essential,
granulocyte-stimulating factor may also be used to elevate an
unacceptably low white blood cell count. Low platelet and white blood
cell counts may be indications that management of the patient’s
underlying infection with HIV or HCV, or both, is inadequate, which
should be corrected before any elective surgery. Inadequate management
may be an indication of patient noncompliance, which will place the
patient in a higher risk category for postoperative complications if it
cannot be reliably corrected. All patients should be carefully screened
for opportunistic infections, including bacterial, mycobacterial, and
fungal agents that might increase the risk of early or late
postoperative infection.
chronically anemic as a consequence of disease and therapy, and may
require transfusion before surgery. When clinically appropriate,
marrow-suppressing drugs should be stopped a few days preoperatively
and resumed after the first postoperative week. Some of the drugs used
to suppress HIV, such as ZVD, didanosine (DDI), and zalcitabine (DDC),
can cause various degrees of marrow suppression. However, the more
recently developed reverse transcriptase inhibitors such as lamivudine
(3TC) and stavudine (D4T) and the protease inhibitors are not likely to
do this. Prophylactic antibiotics seem clearly indicated in this
patient population. In the patients with advanced HIV infection, some
authors have suggested continuation of prophylactic antibiotics longer
than normal, but there are no data to demonstrate efficacy, and many
studies show no increase in the incidence of early postoperative
infection in this patient population.
be at increased risk of late hematogenous implant infection as host
defenses diminish. Patients are cautioned to use meticulous antisepsis
technique for self-infusion. Regular medical attention, prophylactic
antibiotics before dental work and any invasive procedures, and early
evaluation and treatment of possible infections are especially
important in this group. Rarely, a patient may require long-term venous
access. Subcutaneous ports are less likely to become infected than
external, percutaneous catheters. Tanner et al. (68)
found a rate of infection in Hickman catheters of 50%, compared with 5%
for implanted subcutaneous ports placed in the upper extremity. Avoid
external catheters whenever possible in patients who have undergone
joint replacement. Some clinicians have raised the issue of using
chronic prophylactic antibiotics in symptomatic HIV-positive patients
with a joint replacement. To date, there have been no studies
demonstrating a reduction in the rate of late infection with chronic
prophylactic antibiotics. Long-term use of trimethoprim-septra, which
many patients with advanced HIV disease take as prophylaxis against Pneumocystis pneumonia, may offer some protection against S. aureus infection of prosthetic joints.
devastating disease of hemophilia and its accordant form of arthritis,
but much remains unanswered. Early treatment and prevention of chronic
synovitis and progressive arthropathy are the key. Diagnosis of early
synovitis before joint erosion is challenging but possible with close
follow-up after an initial bleed. The role of radiosynovectomy versus
arthroscopic synovectomy is being defined. When end-stage arthropathy
does occur and is severely disabling, durable, functional
reconstruction with minimal risk must be our goal.
improve, so does the prognosis for HIV-infected hemophiliacs, many of
whom will require reconstructive procedures. The unusually high risk of
late prosthetic infection in this patient population may be reduced by
careful attention to antisepsis for self-infusion, dental prophylaxis,
and close medical monitoring. The ultimate prevention of hemophilic
arthropathy will follow the correction of the underlying clotting
disorder by primary prophylaxis and, eventually, gene therapy.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
BM, Crook A, Mallard J, et al. Evaluation of Intra-articular Colloidal
Gold 198Au in the Treatment of Persistent Knee Effusions. Ann Rheum Dis 1963;22:435.
JL, Weber DJ, Meyer AA, et al. Wound Infection Rates after Invasive
Procedures in HIV-1 Seropositive Versus HIV-1 Seronegative
Hemophiliacs. Ann Surg 1990;211:492.
for Disease Control and Prevention. Case-control Study of HIV
Seroconversion in Health-care Workers after Percutaneous Exposure to
HIV Infected Blood—France, United Kingdom, and United States, January
1988—August 1994. MMWR 1995;44:929.
WB, DeGnore LT, White GC. Orthopaedic Procedures and Prognosis in
Hemophilic Patients Who are Seropositive for Human Immunodeficiency
Virus. J Bone Joint Surg 1990;72-A:2.
WE, Christian MJ, Clarke SL, Hasiba U. Comparison of Continuous and
Intermittent Factor VIII Concentrate Therapy in Hemophilia A. Am J Haematol 1984;17:85.
JV, Hansjaj KK, Dorey FJ, Kasper CK. Risk Factors for Late Infection in
Hemophiliacs with Total Hip and Knee Arthroplasties. Proceedings of the American Academy of Orthopaedic Surgeons, 1994.
JV Jr, Logan LR, Benson DR, Glasser DB. Immunodeficiency Virus
Infection: Complications and Outcome of Orthopaedic Surgery. Journal of the American Association of Orthopaedic Surgeons 1996;4:297.
IM, Berntorp T, Lofqvist T, et al. Twenty-five Years’ Experience of
Prophylactic Treatment in Severe Haemophilia A and B. J Int Med 1992;232:25.
MV, Crossett LS, Herndon JH. Postoperative Infection following
Orthopaedic Surgery in Human Immunodeficiency Virus-infected
Hemophiliacs with CD4 Counts 200/mm3. J Arthroplasty 1995;10:716.
AG, Skinner CJ, McBride MO. A Prospective Comparative Study between
Externally Sited (Hickman), Chest Implanted (Port-O-Cath), and Arm
Implanted (PASport) for Long Term Venous Access in AIDS Patients.
(Abtract no. PB0888.) Int Conf AIDS 1994;10:219.
