CONGENITAL LOWER LIMB DEFICIENCY
Division of Pediatric Orthopedics, University of California–Los Angeles
School of Medicine; Shriners’ Hospital, Los Angeles, California, 90095.
Hospital; Department of Orthopedics, University of California–Los
Angeles School of Medicine, Los Angeles, California, 90020.
Hospital; Department of Orthopedics, University of California–Los
Angeles School of Medicine, Los Angeles, California, 90020.
Hospital; Department of Pediatrics, University of California–Los
Angeles School of Medicine, Los Angeles, California, 90020.
anomalies affecting the lower extremities of children. Many involve a
failure of formation of various tissues, as well as leg-length
discrepancy. The general approach to these patients is to evaluate
their limbs in terms of potential function, to estimate the projected
leg-length discrepancy at maturity, and then to address the situation
either by standard leg-length equalization techniques or through the
use of a prosthesis that can be adjusted in length as the child grows.
When amputation is indicated as part of
the
overall solution, one must identify a key joint for salvage and then
convert the tissue distal to that joint into a usable weight-bearing
organ. The key joint is the most distal joint with sufficient function
to power a prosthesis. Complicating the technical problems are
psychological feelings of helplessness, anxiety, and frustration among
parents and physicians alike.
prosthetic fitting is initially poorly accepted by most parents,
particularly if the part proposed for sacrifice appears quite normal.
The parents rightly question why modern technology cannot be called
upon to salvage their child’s limb. Physicians faced with this scenario
must improvise based on their combined expertise in prosthetics and
reconstructive surgery. Although some of the procedures may initially
appear radical to parents, with education and counseling the child and
parents can accept them as simply reconstructive. Experience has shown
that it is frequently preferable to convert a limb to a logical
prosthetic level rather than embark on a prolonged operative
reconstruction that, even when modestly successful, frequently
precludes participation in normal childhood activities (41).
Treatment thus requires an understanding of the natural history of the
specific defect as well as a thorough knowledge of any associated
abnormalities.
evaluation and treatment are best carried out at special clinics where
appropriate psychological, social, and pediatric support is available.
Surgical procedures are not easily undertaken and rely on the parents’
informed judgment based on a balanced medical presentation and the
experience gained through the consultation process. An opportunity to
associate with other families in similar circumstances who have already
gone through the process is a reassuring influence and should be part
of the family’s decision-making process.
susceptible to malformations being perpetuated in subsequent cell lines
is between the fourth and eighth weeks postfertilization, the so-called
period of organogenesis. Both the axial and appendicular skeletons form
at this time. A single cell gives rise to billions of descendants, so
the surprising thing is not that congenital anomalies occur, but that
they do not occur even more frequently. Some conditions have a familial
origin and hence a preordained genetic derivation. For example,
congenital dislocation of the knee may occur sporadically or in
association with Larsen’s syndrome, an autosomal dominant condition in
which dislocations of other joints and anomalies of the cervical spine
also occur. Whatever the cause, once a defect is present, with the limb
bud developing in a proximal to distal progression, areas “downstream”
from the initial insult may also be affected. For example, proximal
femoral focal deficiency is frequently associated with a more distal
fibula deficiency, and absence of the fibula in turn may be associated
with absence of lateral foot rays.
this gestational period, limb defects frequently are seen in the
presence of anomalies exclusive of the musculoskeletal system. The
VACTERLS syndrome refers to the association of vertebral, anal,
cardiac, tracheoesophageal, renal or radial flaws, associated with limb
defects and a single umbilical artery. In the amniotic band syndrome,
the insult is thought to take place after limb bud development during
the fetal period of gestation. Multiple bands may be present,
predisposing to autoamputations. From this brief discussion, it is
clear that limb reduction defects often present as part of a more
general syndrome.
adults. In adults, amputation is frequently related to peripheral
vascular disease or diabetes; healing is delayed and rehabilitation is
difficult and prolonged. Adults are concerned about their body image,
time lost from work, and their livelihood in general. Children, on the
other hand, usually heal promptly and completely; conversion to an
amputation at an early age is not ordinarily difficult or prolonged,
and potential employability is not an immediate concern (see Chapter 175).
Phantom pain and neuromas, which can significantly impact on the
results in adults, do not appear as clinically significant in children
and present only an occasional problem in adolescents. Gait training
takes place spontaneously under the guidance of a prosthetist or
physical therapist, and psychological acceptance of the limb is natural
and not elaborate.
frequent overgrowth following transdiaphyseal amputations, particularly
in the humerus, tibia, and fibula. This can result in a need for serial
surgical revisions throughout growth (see section on Terminal Overgrowth
at the end of this chapter). Where possible, perform amputation through
an adjacent joint to avoid this problem, but this consideration must in
turn be tempered by the concomitant goal of maintaining length.
Through-joint amputations not only preserve length, but, by
preservation of the adjacent growth plate, add to the length throughout
childhood, preventing an initial adequate length from becoming a
proportionately shorter stump as adult stature is attained.
identify possible etiologic factors. Consider the influence of
teratogenic agents such as thalidomide, maternal risk factors such as
diabetes, and environmental factors such as radiation exposure. A
genetics consultation is recommended to assess the risks for additional
pregnancies and to offer counseling. Despite the gravity of the
situation, the professional staff should maintain an optimistic
demeanor. Nearly all children with lower extremity anomalies gain
functional mobility, attend regular school, and lead quality lives,
participating in sports such as swimming, skiing, tennis, and horseback
riding. Schools may restrict participation in contact sports such as
football and hockey because of the risk of injury to other children
from prosthetic parts. The role of the parents and grandparents cannot
be underestimated. Acceptance of the child as he is and the avoidance
of overprotection, which can lead to an overly dependent and submissive
personality, facilitates the child’s development, sense of self worth,
and eventual integration into the workplace.
associated with classic modes of genetic transmission and thus likely
result from embryonic insults occurring during the development of the
limb bud. The severity ranges from simple hypoplasia to total absence
of the fibula. Experimental evidence suggests that the earlier the
insult occurs in embryonic development, the more likely is concurrent
involvement of the proximal femur. Later insults involve the fibula and
foot to a greater degree (46). Fibular
deficiency thus may be accompanied by proximal femoral focal
deficiency, shortening and/or bowing of the tibia, general limb growth
retardation, delayed epiphyseal ossification, absence of rays, tarsal
coalitions, residual fibrous bands, deficiencies of various muscles,
genu valgum, and loss of ankle integrity (2,18,30,31,59,68).
Upper extremity anomalies are also seen in some cases. Although rare
compared to diseases such as developmental dysplasia of the hip or
clubfeet, the fibula is the most common congenitally absent long bone (18).
and function of the ankle and foot and the overall length of the limb.
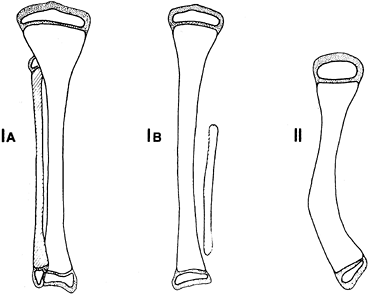
To better characterize the variability, Achterman and Kalamchi (2) refined a classification originally utilized by Coventry and Johnson (18) (Fig. 174.1).
In type IA, the entire fibula is present but shortened, leaving the
proximal fibular epiphysis distal to the upper physis of the tibia, and
the distal fibular physis proximal to the dome of the talus. In type
IB, there is a partial absence of the upper fibula, and the lower
fibular epiphysis is likewise elevated above the talar dome and thus
not buttressing the ankle. In type I deformities, the foot usually
remains plantigrade but may be associated with a ball and socket ankle
joint, and either equinovalgus or equinovarus may be present. In type
II, by contrast, the entire fibula is absent, tibial bowing is
frequent, and ankle instability is the rule. A residual lateral fibrous
band may contribute to the type II deformity.
 |
|
Figure 174.1. Achterman-Kalamchi classification of fibula hemimelia. See text for explanation.
|
ankle stability (e.g., a four-toed foot can be salvaged, but a
three-toed foot should be sacrificed), but we have found that stability
can be directly assessed and that a three-toed foot can often be
serviceable. The degree of tibial shortening increases with advanced
stages, although associated femoral shortening is maximal in type I.
Children with type I feet will not require conversion to an amputation,
but conversion is usually performed for the majority of children with
type II deformities.
procedures for the treatment of type II deformities, but the test of
time has not supported this approach (4,7,11,15).
Stabilization by arthrodesis is complicated by delayed ossification of
the epiphyses, as well as by potential inadvertent injury to the distal
tibial physis. Kruger and Talbott (41) have
pointed out that whereas the goal of preserving the foot might be
laudable, in fact many children who undergo repetitive operations
eventually undergo amputation, and that upon honest review there is
good evidence that conversion should have been offered as a primary
treatment rather than a secondary salvage procedure. In addition, we
have found a higher incidence of complications when Syme amputation was
performed as a salvage
procedure in multiply operated limbs rather than as a primary procedure (6).
In areas where cultural concerns do not allow amputation, or in lesser
developed areas where surgery is not available, it is feasible to fit a
prosthesis around the foot.
the overall length can be made acceptable, leg lengthening using
Ilizarov techniques, in which the ankle is stabilized during
lengthening, is an acceptable approach.
amputation are (a) a deformity of the foot so severe that any surgery
to make the foot plantigrade and functional is likely to fail (70) and (b) an estimated leg-length discrepancy at maturity of 7.5 cm or more (60). The value of 7.5 cm is arbitrary and tends to vary among physicians (46,59,60).
We now base our decision more on the stability of the ankle than the
leg-length discrepancy, per se, provided the overall limb-length
discrepancy at maturity is not projected to exceed 12 cm.
-
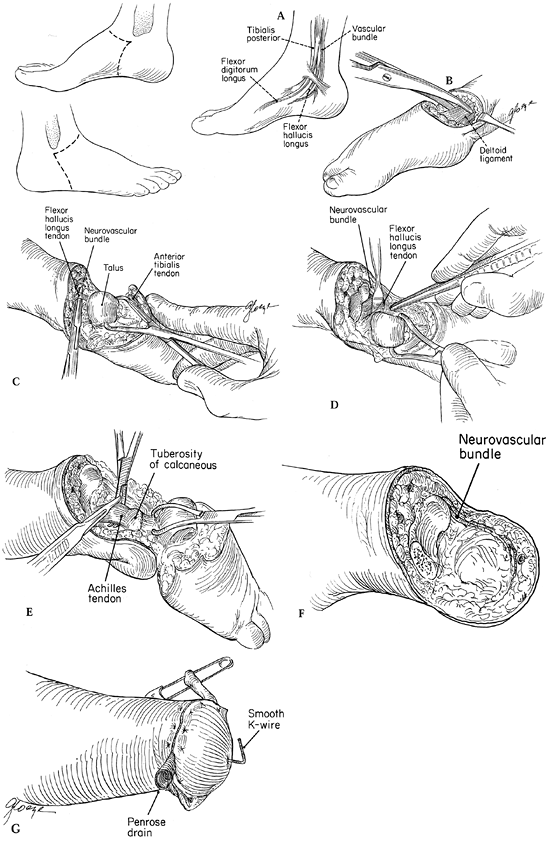
Begin the procedure of Syme’s ankle disarticulation (Fig. 174.2)
by making a fish-mouth incision with anterior and posterior skin flaps.
The apex begins 1 cm distal to the medial malleolus and parallels the
anterior ankle joint to a point estimated to be the level where the
fibular tip would ordinarily be palpable.![]() Figure 174.2.
Figure 174.2.
Syme’s ankle disarticulation as performed in children. Make skin
incisions from a point 1 cm distal to the medial malleolus to the tip
of what would have been the fibula malleolus, as illustrated. A: Schematic of the anatomy posterior to the medial malleolus. B: Divide the collateral ligaments to facilitate hyper plantarflexion of the ankle. C: Location of the flexor hallucis longus posterior to the talus. D: Protect the neurovascular bundle by retraction of the flexor hallucis longus muscle tendon. E: Divide the Achilles tendon, taking care to include excision of the apophysis of the calcaneus. F: Preserve the neurovascular bundle to the end of the flap; avoid injury to the bundle at the level of the ankle joint. G:
Close the wound over a drain, and stabilize the heel pad with a stout
K-wire or smooth Steinmann pin inserted into the tibia from the plantar
aspect of the heel. -
Carry the dissection down through the
subcutaneous tissue to the level of the medial and lateral collateral
ligaments of the ankle, ligating larger vessels and controlling smaller
ones with electrocautery. -
Identify the nerves. Gently tension,
sharply divide, and allow the nerves to retract away from potential
subcutaneous positioning. -
Divide the collateral ligaments so that the talar dome can be pulled forward away from the distal tibia.
-
In the interval between the talus and
tibia, identify the flexor hallucis longus tendon. It is the key to
locating and protecting the neurovascular bundle posteromedially. By
protecting this tendon and drawing it medially with a retractor, the
dissection can be continued with the neurovascular bundle shielded so
that the actual division of the posterior tibial artery will be at the
most distal portion of the posterior flap. -
Excise the calcaneus from the heel pad in
a subperiosteal fashion so that the periosteum remains intact,
maintaining the structural integrity of the fat pad, which has a
hydraulic-like function during weight bearing. Be sure that the entire
calcaneal apophysis is excised so that it will not form a persistent
ossicle later (Fig. 174.3). Two centimeters of
the distal Achilles tendon can be resected to ensure there will be no
tendency to reattach and pull the heel pad posteriorly. Shave the
distal tibia only in older children, because smoothing and remodeling
occur spontaneously in younger children once the talus no longer
occupies the mortise.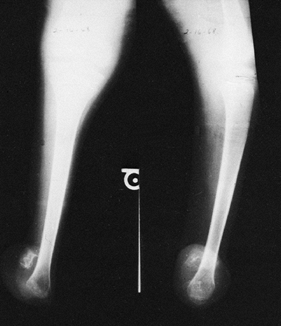 Figure 174.3.
Figure 174.3.
In performing Syme’s amputation, be certain to excise the entire
apophysis, otherwise it will persist and complicate later prosthetic
fitting. This one required removal during adolescence. -
Finally, stabilize the heel pad
underneath the tibia by inserting a stout Kirschner wire (K-wire)
through the pad into the tibia, and close the skin over a Penrose drain
with absorbable subcutaneous sutures and interrupted nylon sutures for
the skin. In approximating the heel pad to the tibia, take care to
avoid crimping the posterior tibial artery.
Markham, Ontario) gauze, fluffs, sterile cast padding, and a spica cast
for short stumps (as when the amputation is combined with a knee fusion
for proximal femoral focal deficiency). A long-leg cast can be applied
when the knee can be effectively flexed to prevent the cast from
sliding off. Remove the drain at 2 days and allow 6 weeks for
soft-tissue healing in the cast. An elastic wrap is occasionally
necessary for several additional weeks to control any residual
stump edema. The stump is ready for prosthetic fitting between the tenth and twelfth weeks.
spontaneously after a Syme’s amputation, but in those cases accompanied
by bowing in excess of 30°, perform a tibial osteotomy simultaneously,
and extend the K-wire to act as intramedullary fixation.
of the heel pad in the Syme’s amputation and thus prefer the Boyd
amputation, in which the calcaneus is displaced anteriorly in an effort
to fuse it into the distal tibia or at least to stabilize it under the
tibial plafond (8,10,70).
Modest additional length can also be maintained by this approach.
Alternatively, the distal tibial physis can be excised at the time of
calcaneal tibial fusion, so that the bulbous self-suspending stump
gradually “ascends” to the level of the opposite calf. In deformities
with severely displaced equinus hind feet, repositioning of the
calcaneus under the tibia may not be possible, and Syme’s procedure is
preferred. No shaping of the malleoli is necessary in young children in
either the Boyd or the Syme ankle conversion. Without the talus
occupying the mortise, the malleoli remodel to a satisfactory shape.
Either procedure yields a pleasing stump because the prominent malleoli
atrophy after talar excision.
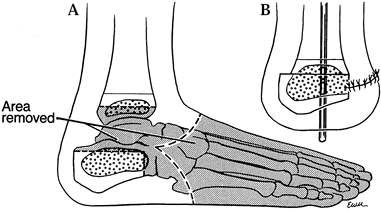
-
Boyd amputation (8)
utilizes an approach similar to the Syme procedure just described.
However, excise only the talus and forefoot, leaving the calcaneus
attached to the fat pad (Fig. 174.4).![]() Figure 174.4. Boyd’s amputation. A: Scheme of bony resection. B: Final position, emphasizing anterior displacement of the calcaneus to provide a rounded stump.
Figure 174.4. Boyd’s amputation. A: Scheme of bony resection. B: Final position, emphasizing anterior displacement of the calcaneus to provide a rounded stump. -
Carefully shave the articular cartilage
of the distal tibia with a knife until the ossific nucleus is
encountered; take care to breech neither the distal tibial physis nor
its surrounding perichondrium, unless shortening of the stump is
desired. -
Osteotomize the anterior portion of the
calcaneus, if necessary, just distal to the peroneal tubercle; shave
the superior surface flat, allowing it to sit congruously under the
prepared distal tibia. -
Divide the Achilles tendon through the
space left by the enucleation of the talus. When performed properly,
the heel pad can be positioned in its normal weight-bearing attitude
but slightly displaced anteriorly. -
Use a smooth Steinmann pin to secure the
final position, extending it through the calcaneus across the distal
tibial physis into the medullary canal of the tibia. -
Close the skin over a drain.
all were ambulatory, and patient satisfaction was excellent. Patients
participated in sports activities, including bicycling, swimming,
football, soccer, and roller skating.
Although
some did report occasional problems, such as calluses and rashes, on
closer inspection these problems seemed to be related to prosthetic fit
rather than to the stump per se, and they were easily addressed with
minor prosthetic accommodations in the socket. Posterior heel pad
migration was commonly encountered, although it rarely required
surgical intervention. Hypertrophy of the skin over the distal tibia
and prosthetic adjustment were usually enough to compensate for changes
in heel pad position. Of these patients, 40% felt that they had no
functional restriction at all, and all of the adults reviewed were
employed.
psychological function in young patients after Syme’s amputation, the
results documented a surprisingly easy adjustment process (6).
The age of amputation may be important, and it seemed preferable that
the procedure be performed prior to the age of 18 months to 2 years,
because at this time the infant has an incompletely developed body
image and adapts to the new physical status quite quickly. It seems
that a missing foot compensated by a functional prosthesis is more
acceptable to a child or teenager than a significantly deformed foot
that compromises activities and gait.
to the distal tibial physis are seen in both the Boyd and the Syme
conversions. In addition, an occult retained calcaneal apophysis and
later pencilling of the distal tibia may complicate Syme’s amputation.
Careful attention to the indications and surgical details will limit
the problems encountered. Displacement of the heel pad, although
frequent, is rarely an indication for reoperation.
involved buttressing by autogenous bone grafted into the tibial
metaphysis in an effort to replace the fibular malleolus. Lacking a
growth plate, the buttress would quickly rise above the level of the
ankle, mitigating its effectiveness.
at the Royal Children’s Hospital in Melbourne, patients were considered
for the procedure only if they exhibited minimal shortening of the
tibia and had a foot that comfortably reached the ground with no gross
deformity. Of seven attempts reported, three had been converted to an
amputation, three were awaiting amputation, and the remaining patient
had embarked on a program of leg-length equalization. Thus, this
procedure is not definitive for most patients and can be regarded only
as an interim procedure to be followed by an amputation beause of the
progressive leg-length inequality that develops. With rare exception,
it appears that Syme’s or Boyd’s amputations are the preferred primary
treatment for type II (complete) fibular deficiencies.
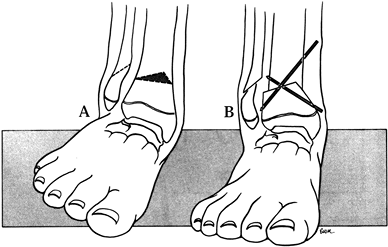
fibula have not been very successful, for specific cases of partial
absence resulting in a mild leg-length discrepancy and a valgus but
stable ankle, Wiltse (69) proposed a
supramalleolar osteotomy designed to minimize the prominence of the
medial malleolus. The osteotomy is performed toward the end of growth
through an anterior approach to the ankle (Fig. 174.5).
 |
|
Figure 174.5.
The Wiltse osteotomy reduces the medial prominence of the ankle following correction of a residual valgus ankle deformity, as might accompany lesser degrees of fibula deficiency. A: Plan of wedge resection. B: After closure and fixation of the osteotomy. |
deficiency of the tibia, the rarest form of lower extremity deficiency,
may be sporadic or inherited. Both autosomal dominant and recessive
patterns have been reported. The anomaly is frequently associated with
other system deficits such as herniae, gonadal malformations,
hypospadias,
cleft palate, imperforate anus, and congenital heart disease. Associated vascular abnormalities may underlie the malformations (32).
Always search for absent or duplicated rays, ipsilateral proximal
femoral focal deficiency (PFFD), contralateral clubfoot, hemivertebra,
hip dysplasia, coxa valga, syndactyly, and lobster claw deformities (53).
In contrast to fibular deficiencies, the foot is in a varus position,
and the knee may be unstable due to an associated absence of collateral
or cruciate ligaments.
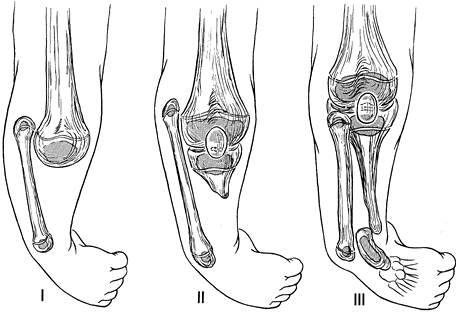
literature. The Kalamchi classification is perhaps simpler and more
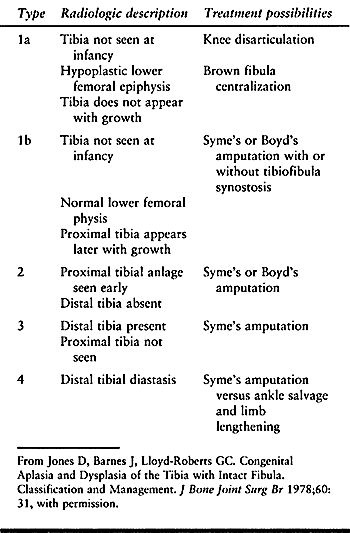
straightforward (Fig. 174.6), but the classification of Jones et al. (35) is the most widely accepted. It separates the deficiencies radiographically into five types: 1a, 1b, 2, 3, and 4 (Table 174.1). Both classifications are helpful in formulating treatment.
 |
|
Figure 174.6.
The Kalamchi classification for congenital tibial deficiency. Type I, no tibial anlage. Type II, tibial anlage present. Type III, tibial fibula diastasis. |
 |
|
Table 174.1. Jones Classification of Tibial Dysplasia
|
tibia remains, and whether this portion is meaningfully powered by a
quadriceps mechanism. Usually the fragment is palpable, and active knee
extension is easily discerned. However, this is not always a simple
distinction in a chubby uncooperative infant, so the cartilaginous
remnant may need to be sought by arthrography, ultrasonography,
magnetic resonance imaging (MRI), or direct observation during surgery.
Radiographically, a marked reduction in width and delayed ossification
of the distal femur are also suggestive of an absent or nonfunctional
proximal segment. When this tibial fragment is present and powered,
surgery is warranted to fuse the fibula into the fragment and then to
perform a Syme’s amputation, which produces a functional below-knee
amputation (Fig. 174.7). When the proximal
fragment is absent (Jones type 1a), knee disarticulation is warranted,
resulting in a functional above-knee amputee. Try to avoid above-knee
amputation in treating congenital tibial deficiency because of problems
with bony overgrowth, skin problems associated with the residual limb,
not to mention the loss of the major growth physis of the femur and
resultant shortening (36).
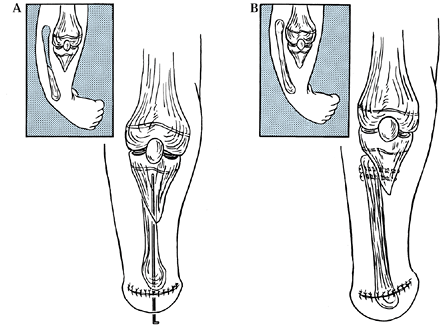 |
|
Figure 174.7. A: Transfer of the fibular into the tibial anlage in Kalamchi type II deformities of the tibia. B: Side-to-side fixation of the fibula into the tibia after resection of the upper portion of the fibula.
|
(described in the following section), which centralizes the fibula
under the femur, is an alternative that allows the child to function as
a below-knee prosthetic user rather than as an above-knee amputee. Most
authors report disappointing results, in part because of recurring knee
flexion contractures, which result from an imbalance between the
hamstrings and a weak, albeit present, quadriceps musculature (35,42,58). The Brown procedure (12)
may nevertheless be offered if a functioning quadriceps mechanism of
grade 3 strength or better is present, because some patients can
achieve a functional below-knee stump that allows active knee flexion
and extension, according to Simmons et al. (55).
Residual ligamentous laxity following Brown centralization can be
controlled by an appropriately designed prosthetic socket. Keep in mind
that if the Brown procedure proves unsuccessful, the limb may be
salvaged and a perfectly acceptable result obtained by performing a
knee disarticulation, which would have been the alternative operation
anyway. In some severe cases of concomitant femoral shortening, fusion
of the fibula into the femur may be desirable to add length to an
otherwise short above-knee stump. Unfortunately, 20% to 30% of tibial
deficiencies occur bilaterally with an additional compromise of the
final functional ability of the patient, regardless of the treatment
approach.
(Jones types 1b and 2), surgery is warranted to fuse the fibula into
the fragment when it is sufficiently ossified. Simultaneously, Syme’s
amputation is performed and the result is a functional below-knee
amputee.
(tibial anlage present) by correction of the foot deformity shortly
after birth through a combination of casting and soft-tissue release
followed by fibulotibial diaphyseal reconstruction, alignment of the
axis of the leg with the foot (talofibular arthrodesis), and leg-length
equalization by the Ilizarov method. In a preliminary report, two of
the patients achieved satisfactory ambulation, whereas the other
sustained recurrence of deformity during the lengthening process.
deficiency of the proximal tibia with a distal, abnormally formed
tibial remnant, is extremely rare. This has been adequately treated
with a Syme’s or Chopart’s amputation. The Jones type 4 deformity, with
diastasis of the distal tibia and fibula, may be treated by Syme’s
amputation, or an ankle reconstruction may be attempted (53).
-
Create an anterior U-shaped incision that parallels the distal femoral condyles (Fig. 174.8A), and develop a skin flap proximally that allows access to the quadriceps expansion.
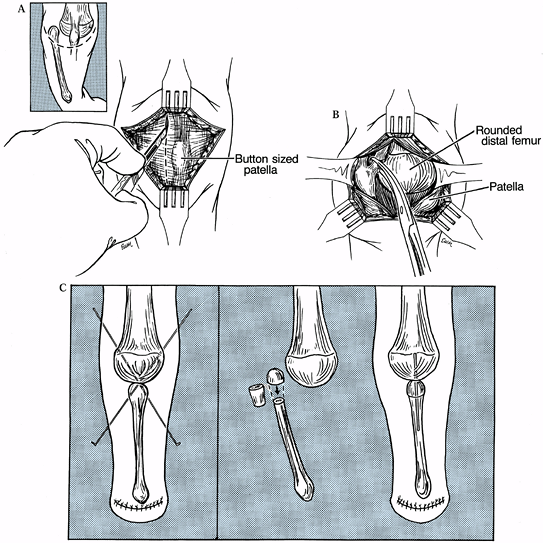 Figure 174.8. The Brown procedure. A: Incision utilized for the Brown procedure. B: Release of fibular tethering; note roundness of distal femur. C:
Figure 174.8. The Brown procedure. A: Incision utilized for the Brown procedure. B: Release of fibular tethering; note roundness of distal femur. C:
Cross K-wire fixation. If the fibula cannot be relocated distally under
the femur, a segmental resection of the upper part of the fibula may
help. -
Make a lateral parapatellar longitudinal
incision through the expansion that, based on the radiograph, will
allow exposure of the upper fibula, which is displaced proximally from
its normal position. Now incise and excise the tissue between the upper
fibula and the femoral epiphysis as necessary to allow transposition of
the fibular head to a point under the midportion of the femoral
epiphysis (Fig. 174.8B). Brown excised the upper 3/8 inch of the fibula to provide a flat surface to oppose to the distal femur. -
Fix the fibula in position with cross K-wires (Fig. 174.8C)
and leave intact the insertion of the patella ligament; reattachment to
the fibula itself is not necessary. If the fibula is too high to be
brought distally, remove a segment of bone from the upper third of the
fibula to facilitate positioning under the femur. -
Fix the reconstituted fibula with a
smooth Steinmann pin placed intramedullary. A Syme’s or Boyd’s ankle
disarticulation can be done simultaneously or at a later time. -
Close the skin over a hemovac drain, and
immobilize the limb from toe to groin in a maximal, but comfortable,
degree of extension.
fourth month, at which time remove the K-wires and immediately apply a
prosthesis. We prefer to attempt the operation at the age of walking,
between 1 and 2 years of age, rather than keeping the patient in a cast
until the time when a prosthesis can be applied.
dislocation or subluxation of the fibula from under the femur,
stiffness, and recurrent knee flexion contracture. The latter can be
treated by hamstring release, but after one or two attempts at making
this procedure work, consider knee disarticulation or tibial fibula
fusion.
deformity, in which there is no meaningful tibial remnant, is a knee
disarticulation with prosthetic fitting as an above-knee amputee. The
distal femoral growth plate is preserved by this technique and
therefore considerable growth potential is salvaged. Take care during
the procedure not to inadvertently breech the growth plate or the
surrounding perichondrium. The following description assumes a
nonfunctional tibial anlage, so the usual structures are described. In
the actual situation, modifications may be necessary depending on what
is actually encountered.
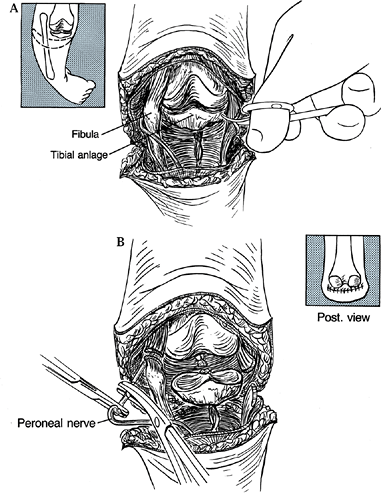
-
With the patient in a supine position and
under tourniquet control, make an anterior transverse skin incision
commencing 1–2 cm distal to the tibial tubercle (or where it should
be), sloping upward medially and laterally toward the joint line to a
point slightly posterior to the mid-coronal plane (Fig. 174.9).
Carry the incision posteriorly 2–3 cm distal to the popliteal crease.
Any extraneous flap tissue can always be trimmed later, but too short a
flap may necessitate shortening the femur, potentially injuring the
physis.![]() Figure 174.9. Knee disarticulation for severe tibial deficiency. A:
Figure 174.9. Knee disarticulation for severe tibial deficiency. A:
Expose the distal femur through a transverse incision. The patellar and
tibial anlage shown here for clarity are usually absent in those cases
requiring knee disarticulation. B: Divide
the collateral ligaments, ligate the neurovascular bundle, and divide
the peroneal nerve. We routinely resect the patella to prevent later
chrondromalacia. -
Incise the deep fascia in line with the
fish-mouth skin incision. If present, divide the patellar ligament and
excise the patella to prevent later chondromalacia. -
With the knee flexed to 90°, divide the
medial and lateral collateral ligaments and anterior and posterior
cruciate ligaments as distally as feasible. -
Isolate and ligate the popliteal artery and accompanying veins individually.
-
Gently pull the posterior tibial nerve
distally and divide it proximally; if it is accompanied by large veins,
ligate these before they are allowed to retract proximally. Treat the
peroneal nerve similarly. -
Next, divide the sartorius, gracilis,
semimembranosus, and semitendinosus tendons at their insertions into
the tibia, and the biceps femoris at its fibular insertion. Section the
iliotibial band transverse to its fibers, and incise the capsule of the
knee at the joint line, eventually circumferentially. -
Finally, divide the gastrocnemius muscle heads at their origins on the femur, and pass the leg off the table.
-
Apply a rigid plaster dressing that will
remain for 4–6 weeks; in small children, a single hip spica may be
necessary to keep the cast in position.
anlage can be done in an end-to-end fashion with intramedullary pins,
or by side-to-side approximation with interfragmentary screw fixation.
Fusion must await the development of a bony tibial diaphysis, if it is
not initially present.
-
Make a longitudinal anterior incision centered over the distal portion of the tibial remnant (Fig. 174.7).
-
Approach the fibula through a separate lateral incision and dissect the peroneal nerve free where it crosses the fibula.
-
Transect the fibula at the level of the
distal tibial anlage, and excise the proximal portion. Transpose the
distal fibula by incising intervening tissue as necessary. Align the
distal transected fibula with the tibial anlage and secure the final
alignment with the tibia with a K-wire or smooth intramedullary
Steinmann pin. -
Alternatively, if the anlage is very
short, a side-to-side fusion may be performed by fixation with small
screws or cross K-wires. Apply a long-leg or single-hip spica cast
until healing is assured (2–3 months).
times, the union can appear tenuous, especially if considerable
residual cartilage was encountered in the tibial anlage, but
progressive ossification of the fibrous union does mature over time. A
simultaneous Syme’s amputation is usually performed, although some
surgeons continue to attempt preservation of the foot by tibiofibular
fusion. The Ilizarov apparatus can be used to align and fuse the foot,
but the results of such an approach are thus far unreported.
condition encompassing a continuum from mild subluxation to frank
dislocation. According to Parsch (48), Koptis reported a frequency of about 1.7 per 100,000 live births. Breech presentation occurs in 30% to 40% (44).
CDK is easily recognized in the neonatal period, with the knee fixed in
hyperextension. The femoral condyles may be palpable in the popliteal
region. The patella may not be easily located, often being displaced
laterally.
associated anomalies and syndromes of CDK. Accompanying musculoskeletal
anomalies occur in more than 50% of cases, the most common being
dislocated hips and clubfeet (37). In association with Larsen’s syndrome, abnormalities of the cervical spine are frequent and should be sought (9).
 |
|
Table 174.2. Congenital Dislocation of the Knee: Associated Anomalies and Syndromes
|
rather than an isolated entity. Pathologic findings at surgery include
quadriceps fibrosis, ablation of the suprapatellar pouch, anterior
dislocation of the hamstring tendons, femoral and tibial articular
surface dysplasia, and anterior cruciate elongation and attenuation (19).
Children seen in the first few months of life can be treated with
biweekly casting or traction until reduction is obtained or progress
toward reduction is deemed to have failed. Parsch (48) found conservative treatment to be successful in two thirds of patients.
necessary. A failure is defined by persistent subluxation of the tibia
on the femur, as visualized on a lateral radiograph, or the inability
to obtain 45° of flexion after a trial of closed reduction and serial
casting. Obliteration of the suprapatellar pouch may be seen when
arthrography is performed in nonresponsive patients. Accompanying
congenital hip dislocation can be difficult to treat by conventional
closed methods until the knee deformity has been controlled. This is
due to a tight quadriceps becoming even tighter with knee reduction and
in turn precluding reduction of the hip. The Pavlik harness in such
cases, aside from being difficult to apply, may actually be
counterproductive unless knee flexion has been obtained prior to
application. Some authors have reported its successful use in such
instances (33,45).
toward lengthening the contracted quadriceps mechanism by V-Y
advancement or Z-plasty, and releasing the tight medial and lateral
capsular structures; after that, reduction can be accomplished (Fig. 174.10). Anterior cruciate ligament augmentation or advancement, meniscectomy, and posterior capsule reefing are rarely necessary.
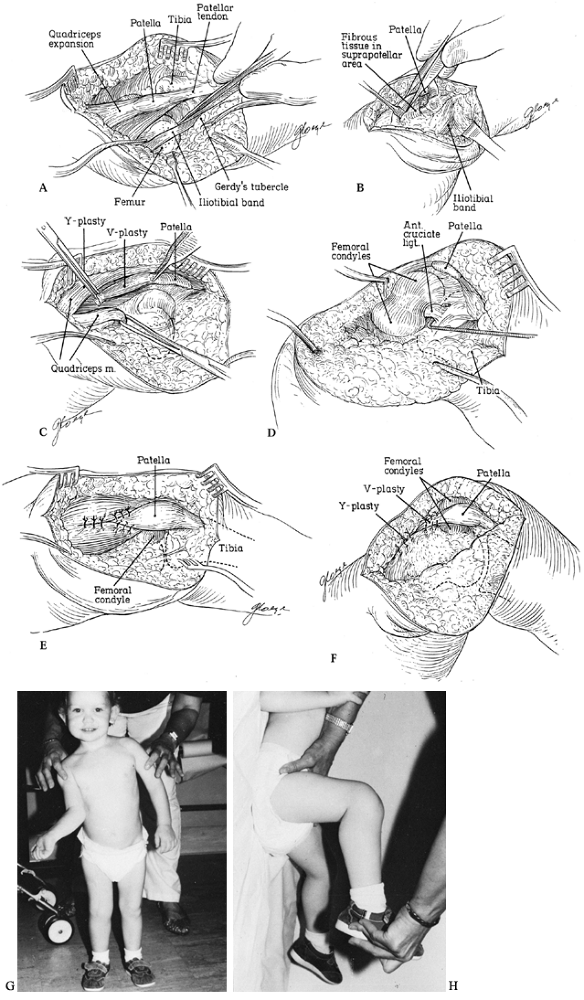 |
|
Figure 174.10. Open reduction of congenital dislocation of the knee. A:
Visualize the pathology through a lateral approach. The iliotibial band and quadriceps tendon are usually contracted, the hamstring tendons may be subluxed anterior to the femoral epicondyles, and the anterior cruciate often is attenuated, stretched, or absent. B: A condensation of fibrous tissue may connect the distal quadriceps expansion to the distal femur and should be released. C: Perform a V-Y quadriceps plasty. D: With the quadriceps divided, flexion of the knee promotes spontaneous reduction and the anterior cruciate can be inspected. Do not repair the cruciate, even if attenuated. E: Repair the quadriceps with the knee located. F: Position of immobilization postoperatively. G,H: Result at 3-year follow-up. From Johnson EJ, Audell R, Oppenheim WL. Congenital Dislocation of the Knee. J Pediatr Orthop 1987;7:194, with permission. |
-
Use an anterior midline incision curving
distally and laterally. Enter the joint through a parapatellar
approach. If the iliotibial band is tight anteriorly to the lateral
femoral condyle, release it with a diagonal incision that can later be
reapproximated in a lengthened position. -
The quadriceps tendon must be lengthened. Employ a V-Y technique (Fig. 174.10E).
This release allows inspection of the joint prior to repair of the
quadriceps, so that any remaining adhesions in the suprapatellar pouch
area can be released, and reduction can then be obtained and secured by
flexion of the knee. -
The collateral ligaments of the knee may
need to be carefully released to facilitate knee flexion. The hamstring
tendons ordinarily spontaneously retreat from an anterior position to
one posterior to the femoral epicondyles. -
After quadriceps repair and closure, immobilize the knee in a long-leg cast flexed to 45° for 6–8 weeks.
cast removal without the need for formal physical therapy. The goal is
90° of active flexion. Bilateral cases, however, do not seem to recover
as much motion as unilateral cases (34).
challenge to the orthopedist. Not only is the patella small and
misshapen, but the femoral groove is often shallow, and the entire
quadriceps musculature is displaced laterally and contracted. In
longstanding cases, the tibia may be laterally rotated, subluxated, and
in mild valgus because of the abnormal pull of the quadriceps. The
tibial tubercle appears lateral to its usual position, resulting in an
increased Q angle. The diagnosis can be made by direct palpation and
confirmed by radiography, ultrasonography, or MRI. Keep in mind that
the patella does not normally ossify until 4–6 years of age. It is thus
easy to miss the diagnosis if relying on radiography alone. The keys to
diagnosis are a high level of suspicion in addition to the clinical
findings of a knee flexion contracture associated with an external
rotation deformity of the tibia, lack of full extension, and weakness.
Because of this, the diagnosis is rarely made before the age of walking.
occur sporadically, or be associated with Down syndrome or
arthrogryposis. It frequently occurs bilaterally. The natural history
of untreated patellar dislocation is early degenerative arthritis,
pain, and disability. Conservative therapy is futile; surgery is
indicated once the diagnosis becomes clear. Most operative techniques
have stressed a soft-tissue release of the quadriceps associated with a
V-Y-plasty of the quadriceps tendon, with realignment of the patellar
ligament insertion. The operative technique must not breach the tibial
physis in skeletally immature individuals, but realignment of the
patellar ligament can be performed utilizing the Roux Goldthwait
procedure (split patellar tendon transfer), or alternatively, the
patellar ligament, in its entirety, can be medially transposed into the
periosteum over the proximal tibia. We routinely perform a Galleazzi
semitendinosus tenodesis of the patella. In skeletally mature
individuals, the tibial tubercle itself may be translocated medially.
-
Begin with a straight lateral incision at
the junction of the middle and upper thirds of the thigh and extend it
distally lateral to the dislocated patella across the femoral condyle,
and then swing medially across but 2 cm distal to the tibial tubercle (Fig. 174.11).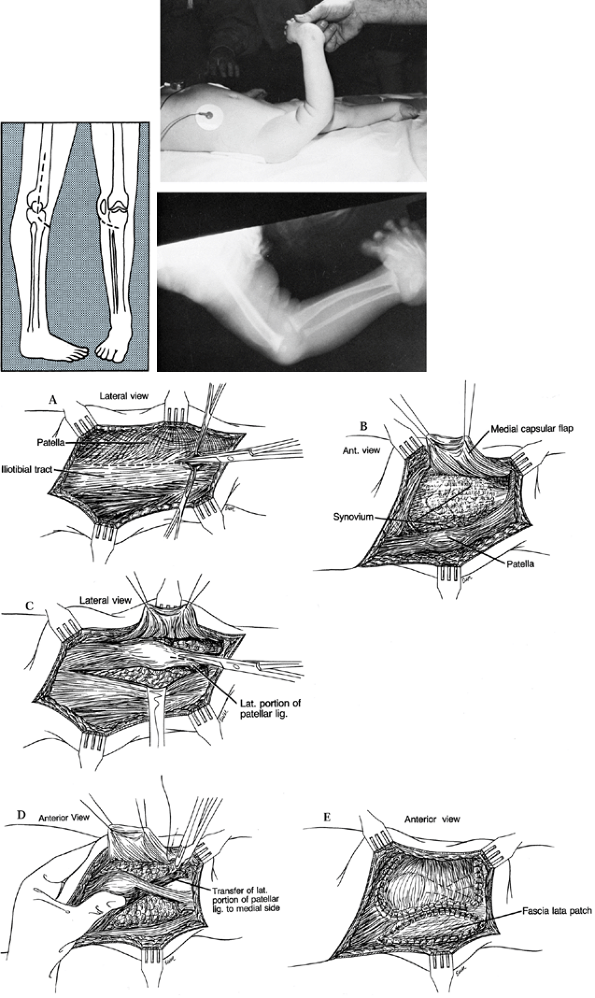 Figure 174.11. Congenital dislocation of the patella. Plan of incision. A: Release the iliotibial tract. B: Raise the medial capsular flap. C: Develop the lateral half of the patellar ligament for transfer. D: Transfer the lateral patellar ligament to the medial aspect of the tibia. E:
Figure 174.11. Congenital dislocation of the patella. Plan of incision. A: Release the iliotibial tract. B: Raise the medial capsular flap. C: Develop the lateral half of the patellar ligament for transfer. D: Transfer the lateral patellar ligament to the medial aspect of the tibia. E:
Repair the medial capsular flap over the patella and advance the vastus
medialis insertion. The resulting lateral capsular defect may be
patched with a piece of harvested fascia lata. -
Dissect down to the iliotibial band, and
divide it obliquely so it may later be repaired in a lengthened
position if necessary. Follow the band posteriorly to its insertion on
the femur by separating it from the adherent fibers of the vastus
lateralis; this portion of the band is known as the lateral
intermuscular septum. -
Elevate the quadriceps extraperiosteally off the femur and up the lateral aspect of the femur to the proximal one-quarter.
-
Lateral to the patella, make an incision through the capsule and connect it to the main release along the vastus lateralis.
-
On the medial side of the patella, incise
the large flap of attenuated capsule for later plication, and separate
the vastus medialis from the quadriceps expansion distally. -
Relocate the patella in the femoral
groove. If flexion cannot be accomplished to 90° with the patella in
the groove, lengthen the quadriceps in a V-Y fashion and/or release any
fibrotic bands. -
Now sew the semicircular flap over the
patella and anchor it to the tissue along its lateral aspect. Advance
the vastus medialis as necessary to produce stable tracking of the
patella. Flex the knee at each stage to see if the patella is becoming
more stable in the femoral groove; if not, adjust the tension of the
suturing accordingly. -
The patellar ligament almost always
appears to be attached too laterally on the tibia, so split the
patellar ligament longitudinally and detach the lateral portion just
proximal to its insertion. Then transfer this detached free stump under
the medial portion of the ligament and suture it into the periosteum
along the medial aspect of the tibia near the insertion of the tibial
collateral ligament. Take care not to make the attachment so tight that
the patella becomes rotated around its longitudinal axis. A piece of
tensor fascia lata may be used to close the lateral capsular defect, if
desired, although we do not routinely do this.
-
First, identify the semitendinosus tendon
at its insertion into the pes anserinus. Through the main incision or
through a separate incision, release the tendon at its musculotendinous
junction and free the tendon to its distal insertion into the pes
anserina, which can be partially released to allow the insertion of the
tendon to move more medially on the tibia. -
Make an oblique drill hole from
inferomedially to superolaterally through the lower medial patella,
parallel to the articular surface. -
Then pass the semitendinosus stump
through the drill hole and suture it to itself or to the dorsal
periosteum of the patella to complete the tenodesis. -
After wound closure, apply a long-leg cast for 4–6 weeks.
the cast is removed if the child does not exhibit spontaneous motion
and improvement. In older children, the hamstrings may occasionally
need to be lengthened to
correct a residual fixed flexion deformity. We have not employed prolonged bracing following cast removal.
and is characterized by a decreased femoral neck shaft angle in
association with a primary femoral neck defect. This defect involves
both the inferior portion of the capital physis and the adjacent
metaphysis. Histologically, it represents a defect in enchondral
ossification (16,51). Some authors have commented on a hereditary tendency, but there is no predilection for sex or race (5,16,46,67).
Radiographs confirm the decreased neck shaft angle as well as a shallow
acetabulum and widened teardrop. Because of the varus, the center–edge
angle may appear to be normal, which can cause the examiner to
underestimate the true dysplasia of the acetabulum.
history suggest the early onset of degenerative arthritis and
increasing disability for those cases that progress (5).
Because of this, the mainstay of treatment has been proximal valgus
osteotomy. Most cases are diagnosed because of a limp or waddling gait
in children 3–12 years of age. Only a few children present with leg or
back pain. Coxa vara can also present in association with syndromes
such as coxa vara with femoral shortening, coxa vara as part of PFFD,
multiple epiphyseal dysplasia, cleidocranial dysostosis,
achondroplasia, or hypothyroidism. In addition, coxa vara can be
acquired as a sequella of Legg-Perthes disease, infection, trauma, or
as a complication of treatment for developmental hip dysplasia or other
diseases. For the true developmental or congenital cases with only mild
shortening or femoral bowing, Weinstein et al. (67)
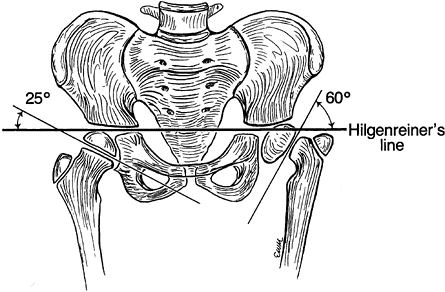
utilized Hilgenreiner’s epiphyseal angle to help define treatment
indications. This is the angle formed between Hilgenreiner’s line and a
line drawn on the anteroposterior (AP) radiographic projection parallel
to the capital physis. In the study by Weinstein et al., when the angle
was in excess of 60°, progression was the rule; if it was less than
45°, the deformities spontaneously corrected. In the 46° to 59° range,
the natural history was not predictable so the patients were observed
for signs of progression prior to undergoing surgery. The goal of
surgical correction was to restore the Hilgenreiner epiphyseal angle to
less than 45° (Fig. 174.12).
 |
|
Figure 174.12.
The Hilgenreiner epiphyseal angle. This measurement is helpful in predicting which cases of coxa vara should be observed and which should be operated on. |
on standing AP radiographs with both knees in the same position and
pointing as anteriorly as possible. Deformities associated with a
neck–shaft angle of 110° or better are followed with the expectation of
resolution. Those less than 100° are operated early to avoid continued
shear stress on the physis. The group between 100° and 110° are
observed for 6 months to 1 year to see whether they will spontaneously
resolve.
angle while avoiding damage to the capital physis and greater
trochanteric apophysis. However, Schmidt and Kalamchi (52)
have noted premature closure of the capital physis in 90% of operated
case, even when direct injury was avoided. In addition, they carefully
studied the acetabular development, noted it to be deficient, and
concluded that unless correction to 140° or better was achieved at
surgery, the acetabular dysplasia did not improve. Thus the goal is
correction of the neck–shaft angle to at least 140°.
preoperative radiograph. Use an image intensifier prior to commencing
the actual surgery to obtain a final check on the true neck–shaft
angle. Rotate the hip until the femoral neck appears the longest, and
then measure the neck–shaft angle on that particular projection. If
necessary, perform a percutaneous adductor tenotomy to facilitate later
closure of the bony osteotomy. Because of the incidence of premature
physeal closure of the capital physis, and the possibility that this
may be related to tight musculature, consider lengthening the iliopsoas
as well.
-
Approach the hip laterally through a
longitudinal skin incision and split the iliotibial band. Elevate the
vastus lateralis subperiosteally from the proximal femur. Use a
pediatric osteotomy screw (or, alternatively, a pediatric blade plate
and matching instrument set) (Fig. 174.13).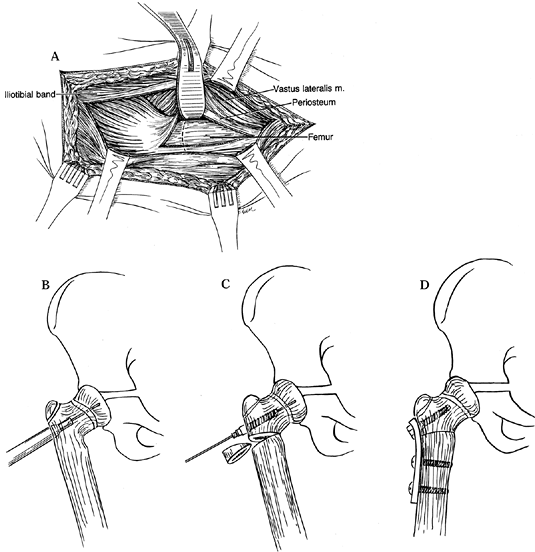 Figure 174.13. Valgus osteotomy for congenital coxa vara. A:
Figure 174.13. Valgus osteotomy for congenital coxa vara. A:
Make a standard lateral exposure to the upper lateral femur through the
iliotibial tract, dividing the posterior portion of vastus lateralis
and reflecting it anteriorly. B: Guide wire placement and overreaming short of the capital physis. C: Insertion of cannulated pretapped screw and removal of lateral wedge of bone. D: Application of sideplate. -
Place a guide wire along the front of the
femoral neck to locate its axis, and introduce a guide pin through the
lateral cortex up to but not through the capital physis. -
Ream over this wire, then tap the hole
under image intensifier control, and finally introduce a screw of
appropriate length. Attach the spanner to the femoral neck screw to
control the proximal portion while removing with an oscillating saw a
wedge of bone based laterally at the level of the lesser trochanter. -
Now close the osteotomy and apply a
two-hole side plate bent with the bending irons to accommodate the new
shape of the lateral cortex. -
Check the position with the image
intensifier or plain radigraphs, and then secure the plate to the
proximal femoral shaft using 4.5 mm screws. -
Then irrigate the wound and close it over a hemovac drain, which is removed at 48 hours.
cast for 6 weeks or until the osteotomy is radiographically united.
Treat residual leg-length discrepancy by a lift and, if necessary, by
an appropriately planned epiphysiodesis of the opposite femur.
premature closure of the capital physis, overgrowth of the greater
trochanteric, leg-length discrepancy, recurrence of varus, malunion,
nonunion, failure of fixation, avascular necrosis, and infection. These
problems may be treated as necessary by techniques described in Chapter 29, Chapter 166, Chapter 170, Chapter 171, and Chapter 173. Repeat valgus osteotomy is sometimes required if the enchondral neck defect has not healed within a year.
from simple hypoplasia to total absence. The deficiencies may be
diffuse or limited to the upper or lower portions, and they are often
associated with other limb deficits and other organ anomalies. Many
classification schemes have been proposed to aid in selecting proper
treatment. Most focus on PFFD. Some include congenital short femur as
the least affected category in the spectrum. However, a child with a
congenital short femur is most frequently amenable to lengthening,
whereas few with PFFD are. Hence, we prefer to think of these
deformities as separate entities.
fitting with a prosthesis, so a general treatment protocol is not easy
to describe. Subtleties of hip, knee, or ankle strength and motion,
along with the wishes of the family regarding cosmesis, may play
pivotal roles in the final plan, and no one treatment will ever be
correct for every child.
have a stable hip and knee (including those instances in which a stable
knee and hip have resulted through surgical intervention), limb
lengthening may be indicated. Children whose limbs are predicted to
have a discrepancy at maturity in the range of 5–15 cm can be
considered for lengthening. We prefer to lengthen using Ilizarov’s
biology (i.e., lengthening of a healing callus) while utilizing a
unilateral half-pin fixator (such as the Wagner) (64)
for its safety and convenience. For less than 5 cm of predicted
leg-length discrepancy, we perform percutaneous epiphysiodesis.
epiphysiodesis. For example, a child with a predicted discrepancy of
7.5 cm would be left with a 2.5 cm discrepancy after epiphysiodesis.
The parents need to decide whether the potential complications
associated with lengthening are worth it to correct the remaining
discrepancy.
extremities does not have to be accomplished by a single technique. A
difference of 10 cm could, for example, be made up with an acceptance
of a 2 cm difference, the addition of a 1 cm lift inside the shoe, and
an epiphysiodesis of 7 cm, resulting in a final lengthening of 10 cm. A
lengthening of 10 cm is within reason, whereas a lengthening of 20 cm
would be beyond the margin of current practice. For discrepancies
predicted to be in excess of 20 cm, seriously consider amputation and
prosthetic fitting.
associated with coxa vara, whereas the more serious forms, normally
classified under the banner of PFFD, are associated with
subtrochanteric bowing. Subtrochanteric bowing may be corrected prior
to consideration of limb-length equalization. However, coxa vara can
help stabilize the hip joint during femoral lengthening and is better
corrected after the lengthening is complete.
by an array of classification systems that have focused on the
radiographic appearance of the hip. The major decisions in caring for a
child with PFFD concern primarily the marked limb shortening and depend
very little on the anatomy of the hip.
Classification systems can be used to organize clinical material for
presentation in the medical literature, or they can be used to help the
orthopedic surgeon make clinical decisions for an individual child;
sometimes they serve both functions. Aitken’s (3)
classification of PFFD is an example of the first use. It focuses
entirely on the radiographic appearance of the bones of the upper end
of the femur and the pelvis (Fig. 174.14). This classification plays a moderately small role in the overall decision making. The classifications of Pappas (47), Fixen (24), and Amstutz (5) are similarly constrained. By contrast, the classification by Torode and Gillespie (62)
is concise and practical: “short” (i.e., lengthenable) or “very short”
(extend with a prosthesis). This useful classification scheme focuses
on the real problem—the marked limb shortening.
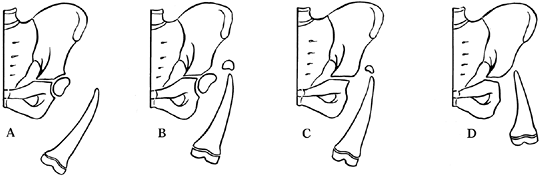 |
|
Figure 174.14.
The Aitken classification for proximal femoral focal deficiency. Types A and B generally have a well-developed acetabulum and by implication a femoral head anlage, even when not initially visualized. Types C and D are usually shorter and the acetabular landmarks are ill defined. Types B and C are characterized on early radiographs by a tufted appearance of the upper femur, which most likely is an ossified portion of femur above a nonossified area. |
has a useful extremity that can be lengthened, or whether lengthening
is unrealistic and the leg needs to be shortened and the difference to
the ground made up with a prosthesis.
contribution by pointing out that the length of the short leg compared
to the normal leg at infancy remains in the same proportion at
maturity. This allows prediction, while the child is still an infant,
of what the ultimate leg-length difference will be at the end of
growth. The parents can become accustomed to the problems that need to
be faced, which usually makes their decisions easier. Orthopedic
surgeons have differed between stating leg-length differences as
absolute numbers of centimeters versus percentages of the bone length.
There is no agreement currently. An average male femur at maturity is
approximately 46 cm, and an average female femur is 43 cm. A leg-length
difference anticipated to be
greater
than 40% to 50% of the normal length at maturity will not likely leave
a child with a good functioning extremity even if lengthened.
difference are to (a) lengthen; (b) remove the foot and fit the child
as a below-knee amputee, accepting the huge difference in knee heights;
(c) remove the foot, fuse the knee, and shorten the limb so that the
child can be fitted as an above-knee amputee; or (d) perform a Van Nes
rotationplasty.
from the category of PFFD, relatively few children with a PFFD would
have limbs that can be equalized by lengthening.
proceed, although rarely the proximal arm of the lengthening device can
be fixed to the pelvis.
femoral deficiency, most children have deformity in the distal femoral
condyles and the ligaments about the knee, as well as deficiencies of
the fibula and foot. Lengthening a femur that has a congenital
deficiency is much more likely to result in anterior subluxation of the
femur on the tibia, ultimately resulting in poor knee function.
Lengthening should not be performed by surgeons who do not do it on a
frequent basis.
(discussed previously) and use an extra-long below-knee prosthesis.
This is a simple solution, but it is cosmetically unappealing due to
the great difference in knee heights, which is especially obvious when
the child is sitting. This choice is less frequently taken now than in
years past.
consider shortening the limb sufficiently that the child will be able
to function well as an above-knee amputee. King (38)
initially suggested the concept of a single skeletal lever, and he
fused the knee joint in an effort to improve hip function and to
facilitate prosthetic fitting. As a part of this procedure, the foot
must be removed. This can be done either as Syme’s or as Boyd’s
amputation. Many surgeons favor Syme’s amputation because of its
simplicity. However, Syme’s amputation done in infancy has more of a
tendency to “pencil-point thinning” of the distal tibia. The worries
about heel-pad migration are probably overstated, because the skin of
children will modify to the pressures applied, as witnessed in children
with untreated clubfeet who are able to walk on the dorsum of their
feet without skin breakdown. However, there is not good scientific
evidence to enable an informed choice between these two procedures, so
the choice tends to be one of personal preference of the surgeon.
should be sufficiently short to allow room for a standard mechanical
knee joint in the prosthesis to be at the level of the opposite, normal
knee. Although orthopedic surgeons have often focused on the end of the
bony stump as seen on a scanogram, remember that there is soft tissue
beyond that as well as room needed for the socket padding and the
thickness of the socket itself—a total of almost 3 cm. An ideal to aim
for is that the end of the bone of the stump be 10 cm proximal to the
knee joint on the opposite side.
that is approximately 90% or more of the normal length (i.e., in boys
about 37 cm and in girls about 35 cm, or about 10 cm shorter than the
average normal femur). This means that if the tibia is left intact and
there is any segment of femur remaining, the probability is
overwhelming that the stump will be excessively long. There should
therefore be plans to either excise one or both physes around the knee
if considering a knee fusion or, alternatively, be a plan for
epiphysiodesis at the appropriate time.
in flexion. They will often sit on the thigh section of their
prosthesis. This gives decreased control and power over the prosthesis
and aligns the vertical axis of the prosthesis lateral to the center of
gravity, producing a lateral lurch in the stance phase of gait.
Function can be greatly enhanced if the femur is fused to the tibia.
When this is done, the fusion must be done with the knee in full
extension. Initially, there may be enough of a hip flexion contracture
that the residual extremity will not come to neutral at the hip, but
this stretches out in short order (38). In the
past, this procedure was not frequently performed, but it is now
routine. Delay knee fusion until the proximal tibial center of
ossification is easily visualized by plain radiography, usually between
18 months and 2 years of age.
-
Use an anterolateral approach. Section
the patellar ligament and remove the patella along with the menisci and
cruciate ligaments. Perform the femoral resection just proximal to the
distal physis; divide the gastrocnemius at the level of the insertions
to the posterior femoral condyles. Protect the peroneal nerve and
posterior neurovascular bundle throughout the resection. -
Use a knife or micro–air saw to resect
the articular cartilage of the tibia, carefully exposing the
ossification nucleus to its widest diameter. -
Introduce a Rush rod retrograde through
the intercondylar notch of the femur, exiting along the lateral aspect
of the proximal femur. Then withdraw it and drive it antegrade across
the knee to fix the fusion in good alignment. The Rush rod should be
centralized in the proximal tibial physis as much as possible. We have
not observed growth arrest, but cross K-wire fixation above the tibial
physis is an alternative method of fixation. -
Use the resected distal femur for supplemental bone graft, if needed.
-
Close the wound over suction drainage.
A special prosthesis is used that uses the ankle as the knee and the
foot as the leg, similar to a standard below-knee prosthesis. (The
socket usually has an additional thigh support attached with outside
hinges.) For this to be successful, there are three requirements:
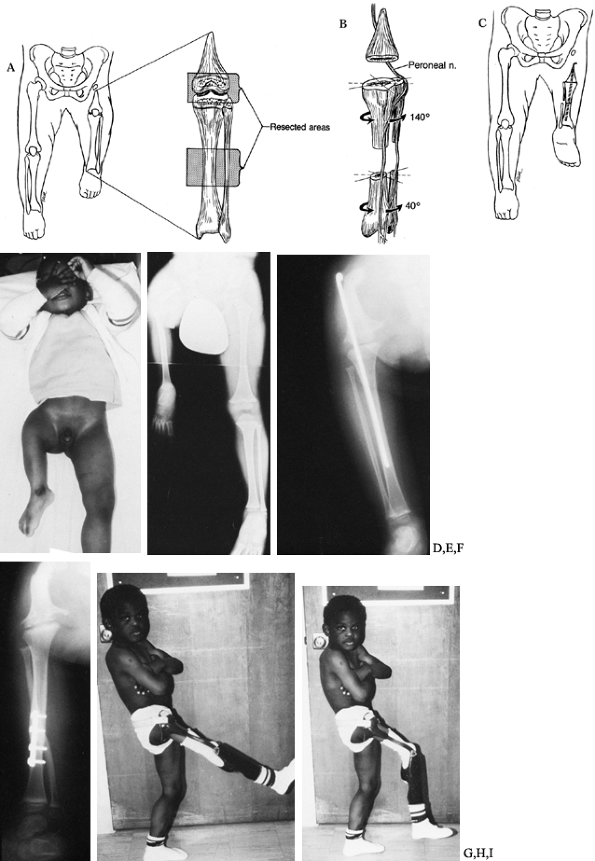 |
|
Figure 174.15. The Van Nes turnaround procedure. A: Area of bony resection. B:
Plan for derotation. The removal of bone leads to soft-tissue redundancy and the ability to rotate without injuring the muscle or neurovascular bundles. C: After derotation, the ankle is 180° from its usual orientation. D,E: Preoperative clinical appearance. F: Radiographs show the knee fusion and the tibial turnaround osteotomy. G: Radiographs show the knee fusion and the tibial turnaround osteotomy. H,I: Postoperative clinical appearance. |
-
The length must be appropriate.
Ultimately, the reversed ankle should be at approximately the level of
the opposite knee joint. Plantar flexion and dorsiflexion of the foot
is a composite of the motion at the ankle and the subtalar joints. The
axis of rotation for the purposes of prosthetic fitting is at the tip
of the lateral malleolus. The limb can be shortened an appropriate
amount at the time of rotation and/or knee fusion. It is rare to have
the extremity too short, because that would be due to a considerable
deformity in the tibia and would probably be associated with an
inadequately functioning ankle joint. -
The ankle must be sufficiently normal to
function as a substitute knee. It must have an appropriate range of
motion and sufficient strength to power a prosthesis. A foot with all
five rays usually has these requirements, but not invariably. -
The psychological acceptance of the
procedure needs to be within the tolerable limits of the patient, the
family, and the medical personnel looking after the child. Concerns may
vary from age to age and culture to culture. In medical centers where
the Van Nes procedure is frequently performed, there are usually other
children and families who can act as models and counsel potential
patients and their parents. The psychology of the medical staff cannot
be ignored. If the medical personnel feel that such procedures are
bizarre, it is clear that they will not be offered, or they will be
presented in such a manner that no patient would accept the choice. As
a consequence, some centers in the United States have moderately large
numbers of children who have had the procedure and other centers have
none.
The prosthesis that is required following a Van Nes procedure is
difficult to fit. If good prosthetic facilities are not available,
avoid this operation.
-
Perform the knee fusion as previously
described, but use the soft-tissue redundancy obtained from the femoral
resection to derotate the limb. -
Secure the knee derotation with an oblique K-wire across the fusion site, supplementing the Rush rod fixation (61).
-
Either extend the knee wound along the
tibial crest or use an additional incision over the mid tibia. Incise
the periosteum and resect and discard at least 1 inch of the tibia. -
Further rotate the limb laterally until the ankle is repositioned 180° with respect to the hip.
-
Fix the tibia with a four-hole
semitubular AO plate, or use a long enough intramedullary Rush rod to
fix both the knee and the hip, and another oblique K-wire to secure
rotation. -
Perform an anterior compartment release
prior to closure, and use a spica cast until radiographic consolidation
of the osteotomies, which usually requires 6–8 weeks.
device occurred in two of our patients but healed uneventfully in a
spica cast. Although Hall and Bochman (29) have
stated that the procedure should not be performed until maturity
because remodeling and derotation of the limb require further surgery,
we perform the procedure at about 2 years of age. We are content with
rerotating the limb as necessary. In our experience, approximately half
the patients required one repeat surgery to rerotate the limb, but only
one patient of 13 required a third surgery.
their PFFD extremity to an above-knee amputation end up with a stump
that is too long. At the time of knee fusion, consider excision of the
distal femoral and/or the proximal tibial physis. Some orthopaedic
surgeons are wary of doing this for fear that the extremity will be
much too short; however, proper application of growth data using the
Green-Anderson-Mesner charts (see Chapter 170) can alleviate that fear. Moseley’s straight line graph (see Chapter 170) does not differentiate between the femur and the tibia and does not provide the answer for this calculation.
management, there is a period when the difference in leg lengths can be
considerable, yet it is too early to do anything definitive surgically.
Lifts greater than 5 cm in height tend to lead to ankle sprains, but
ankle–foot prostheses can be used to decrease this likelihood. However,
when the difference gets to 8 or 9 cm, an extension prosthesis
(sometimes called an extension orthosis or even a prosthosis) can be
made that has a hole in the front of the prosthesis distally through
which the forefoot extends. If tried at too young an age, when the
length difference is less than 5 cm, however, there is not room enough
below the socket to fit a foot on the end of such a prosthesis.
radiograph of the upper end of the femur. What is seen by the
radiograph, however, is only the bone. What is going on with the
cartilage, let alone the muscles, is not seen. If there is absolutely
no acetabulum, the decisions regarding the management of limb length
given in this chapter apply. If, on the other hand, the acetabulum is
satisfactorily formed and there is some element of a femoral head in
the acetabulum and some segment of upper femur that are not joined,
consider surgery. Wait until there is sufficient ossification of the
two fragments that they can be fused together, bone to bone. There is
certainly no rush to do this, and more complications occur from
proceeding too soon, before there is adequate bone stock.
This has the theoretical advantage of providing a stable hip. However,
the knee joint does not allow reasonable rotation. Once it is fused to
the pelvis, the distal femur has some capacity to grow, and the hip
joint will grow to a point that is mechanically disadvantageous,
depending on the orientation of the distal femur at the time of fusion
to the pelvis. Although there have been a small number of reports of
such cases (24,57), most surgeons feel that this is not an advantageous procedure and avoid it (23,25).
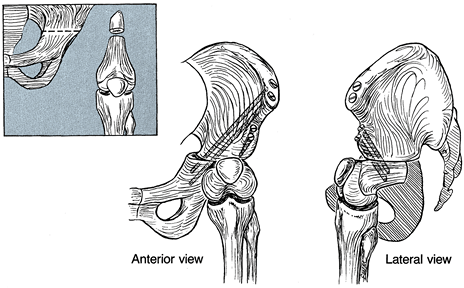 |
|
Figure 174.16.
In rare cases, the knee can be used as a surrogate hip in conjunction with a Chiari osteotomy combined with fusion of the femoral remnant into the pelvis. |
been a problem and can occur even in congenital amputees. It is
particularly a problem in the humerus, the fibula, and the tibia.
Pellicore et al. (1,49)
found that 15% of their juvenile patients who had amputations before 12
years of age required revision, whereas none did if the amputations
were done at a later age. Yet, if children required stump revisions and
they were older than 12 years, overgrowth could still occur.
physis proximally but of the development of appositional new bone on
the end of the stump (see Chapter 175). The
easiest way to manage the problem is to avoid it by performing
amputations through a joint, where possible, so that the end of the
bone is covered by its normal cartilage. If this is not possible, a
number of maneuvers have been used with varying success. Silastic end
pieces (either as a stemmed plug that fits into the medullary cavity or
as a cap that fits over the end of the diaphyseal bone) have been
tried, but frequent breakage and dislodgement of the plug or cap have
led to their abandonment. Surgical procedures that provide a smooth
periosteal surface around the end of the bone appear to be successful
in many cases. The Marquardt method (50) (Fig. 174.17) introduced the use of autogenons epiphyseal caps. Davids et al. (21)
recently reviewed their patients. Apophyseal cartilage from the iliac
crest can be used. Our preference is to use the proximal fibula and
invert its diaphysis into the tibial or humeral medullary canal. This
cartilage cap is not expected to grow but only to provide a smooth
nonosseous surface to prevent osteoblastic reaction. This has been very
effective. Keep this technique in mind for situations in which
transosseous amputations are required. It may be possible to salvage an
epiphysial cap from part of the discarded specimen (e.g., the first
metatarsal head, the talar head, or the os calcis), which could then be
attached to the end of the cut bone.
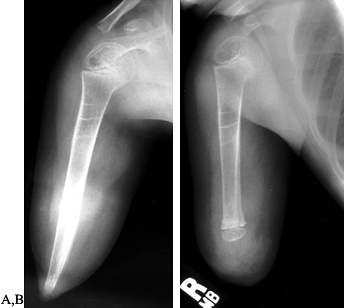 |
|
Figure 174.17. Modified Marquardt method of capping bony overgrowth in a transdiaphyseal amputation in a growing child. A: Preoperative radiograph showing terminal overgrowth of an above-elbow amputation. B: Postoperative appearance after transfer of a epiphyseal cartilage graft onto the revised distal humerus.
|
receive postoperative chemotherapy, bone protrusion at the end of the
stump may be caused by soft-tissue retraction rather than terminal
overgrowth. In such children, the chemotherapy adversely influences the
healing of the fascia, so that the muscles draw proximally, leaving the
bone protruding subcutaneously. If a stump revision is done while the
child is still receiving chemotherapy, the complication is likely to
recur. Several revisions may lead to an unfortunately short stump. It
is better to wait until 3–6 months after the cessation of the
chemotherapy to do a definitive procedure.
there are concurrent hand abnormalities. Toe-to-hand transfers are now
well-established procedures, so before any lower extremity parts are
discarded, search for possible uses for the reconstruction of the upper
extremities. We have assisted the hand surgeon with harvesting the
parts prior to Syme’s or Boyd’s amputation, with very gratifying
results.
short (acquired or congenital), the gain of even a small amount of
length may provide an adequate lever to use a more functional
prosthesis. The advantages are decreased energy consumption, improved
prosthetic control, decreased heat retention, increased comfort from
the lesser weight of the prosthesis, and fewer components requiring
repairs, as well as decreased cost.
the loss is congenital, frequently choose not to wear prostheses.
However, longer residual limbs may allow for grasping objects in the
midline between the stumps for “bimanual” activity without a
prosthesis. Additionally, a longer distal segment can permit objects to
be grasped between a lengthened humeral stump and the chest, or between
a lengthened forearm and the upper arm.
32 short amputation stumps in 27 patients. All were lengthened by
gradual distraction using Ilizarov techniques (see Chapter 32 and Chapter 171).
The 16 children who completed lengthening of their femurs or tibias
achieved sufficient length to be fitted with a prosthesis at one level
more distal than previously possible (Fig. 174.18).
Children who underwent lengthening of their upper extremities were more
difficult to assess. Three patients were lengthened with the
anticipation that a prosthesis would not be used after lengthening. All
became “users,” in that they became able to grasp objects between the
lengthened stump and trunk. Of the remaining patients—all with upper
extremity lengthenings—all
but one are “wearers” of their prostheses but not constantly.
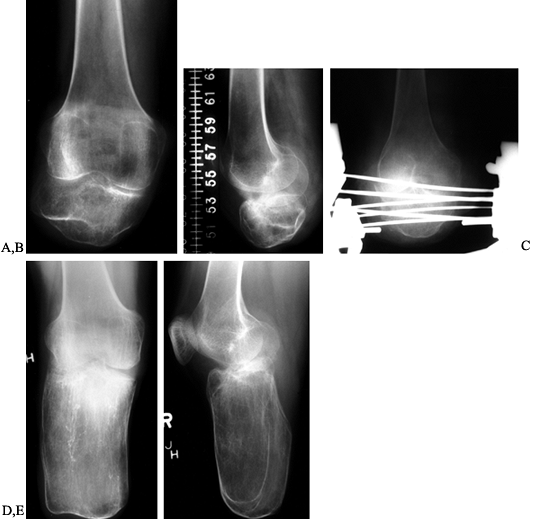 |
|
Figure 174.18. AP (A) and lateral (B) radiographs of a short, below-knee stump prior to lengthening. Lengthening in progress with an Ilizarov device (C). AP (D) and lateral (E)
radiographs showing the result of lengthening the stump. The patient was converted from a functional above-knee prosthesis to a below-knee prosthesis. |
good. Of less importance today than previously is the exact placement
of the surgical scar, the nature of the skin, or even such
complications as a displaced fat pad. Further enhancing the activities
of these amputees are recent advances in prosthetic feet utilizing
newer space-age energy-storing materials that have the ability to
absorb and store energy at the beginning of stance, only to redeliver
the energy at toe-off (14,43,65).
This results in a livelier feel to the limb, and it may enhance certain
activities such as running. Amputees now compete in athletic activities
previously felt to be beyond their capabilities. A cottage industry has
arisen that caters to amputees who are high-grade athletes in such
sports as running, swimming, and skiing. Young children growing up with
such conversions have a better chance than ever to enjoy an enhanced
physical and psychological environment (6,21).
surgically. Patients with these complex problems are frequently beyond
the capabilities of most community orthopaedic surgeons and hospitals
and are cared for at specialized centers. The long-term
physician–patient relationship that develops is gratifying. The
children are otherwise healthy and their zest for living and full
participation in life is admirable. Early medical intervention is
important for them to develop early independence and to reach their
maximal potential. It is a privilege to care for them.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
Reported by Rincheval. Ein Neues Operations Verfahren zur Behandlung
Congenitaler Deffecte Eines Unterarm und Unterschenkelknochens. Arch Klin Chir 1894;48:802.
FW, Pohnert WH. Construction of a Knee Joint in Meromelia Tibia
(Congenital Absence of the Tibia). A Fifteen Year Follow-up Study. J Bone Joint Surg Am 1972;54:1333.
JR, Meyer LC, Blackhurst DW. Operative Treatment of Bone Overgrowth in
Children Who Have an Acquired or Congenital Amputation. J Bone Joint Surg Am 1995;77:1490.
Sanctis N, Razzano E, Scognamiglio R, Rega AN. Tibial Agenesis: A New
Rationale in Management of Type II—Report of Three Cases with Long-Term
Follow-Up. J Pediatr Orthop 1990;10:198.
D, Boyd NA, Fixsen JA, Lloyd-Roberts GC. The Natural History and
Management of Congenital Short Tibia with Dysplasia or Absence of the
Fibula. J Bone Joint Surg Br 1977;59:267.
DR, Packard DS, Levinsohnl EM, Cady RB. Soft Tissue Abnormalies in a
Patient with Congenital Tibial Aplasia and Talo-calcaneal
Synchrondrosis. Teratology 1987;36:153.
D, Barnes J, Lloyd-Roberts GC. Congenital Aplasia and Dysplasia of the
Tibia with Intact Fibula. Classification and Management. J Bone Joint Surg Br 1978;60:31.
JP, Gillespie R, Hall JE, Hubbard S. Van Nes Rotational Osteotomy for
Treatment of Proximal Femoral Focal Deficiency and Congenital Short
Femur. J Bone Joint Surg Am 1975;57:1039.
Nes CP. Rotationplasty for Congenital Defects of the Femur. Making Use
of the Ankle of the Shortened Limb to Control the Knee Joint of a
Prosthesis. J Bone Joint Surg Br 1950;32:12.