Cerebral Palsy
describe several clinical syndromes whose common feature is abnormality
in the control of motor function by the brain. The etiologic agents of
cerebral palsy afflict the immature brain, producing neuropathologic
lesions. Although these do not progressively worsen, they cause
abnormalities in the control functions of the brain, resulting in
permanent disorders of movement and/or posture; it is these
manifestations that change with the growth, development, and maturation
of the patient. Often, sensory and other brain functions are involved.
It is important to note that although the brain lesion itself remains
static in size, the resultant musculoskeletal disorders will almost
surely be progressive, primarily because of spasticity, weakness, and
lack of longitudinal skeletal muscle growth (1).
brain” as that of a child below the age of 2 years (2).
In line with this definition, one may classify a brain lesion occurring
after that age as static encephalopathy, coded with a specific cause,
rather than as cerebral palsy, which may be coded without a known
causative agent (2). In general, this differentiation does not affect the prognosis, and most clinicians refer to both as cerebral palsy.
During the first trimester of embryonic life, the brain differentiates
into a grossly recognizable cerebrum, cerebellum, and other structures.
An insult during this period usually produces a structural lesion that
is detectable by magnetic resonance imaging (MRI).
originating in the periventricular regions and migrating toward the
surface of the cerebral cortex. By the 15th week of gestation fetal
reflex movements can be detected. At the end of the second trimester
all neurons have been formed and any damage or loss cannot be replaced.
the third trimester and intensify after birth. Glialization, which
begins in the second trimester, occurs at least until the age of 2
years (4). Myelination in the brain does not
begin until late in the third trimester, but continues into adolescence
in a well-defined pattern. As more pathways become myelinated,
primitive reflexes drop out, mostly during the first 6 months of life,
and normal postural reflexes appear. As myelination continues,
pathology in the brain becomes apparent. Brain morphologic maturation,
as detectable by MRI, has been reported to continue at least up to the
age of 10 years (5).
from brain lesions have become myelinated and shown to be abnormal on
testing can such lesions be detected clinically. Because different
pathways are myelinated at different times, spastic diplegia is usually
not detected until at least 8 to 10 months of age, hemiplegia usually
at approximately 20 to 24 months, and athetosis usually after 24 months
(3). Although at present there are no effective
means of restoring or generating brain cells and axons, stem cell
research holds out the hope that in the future it may be possible to
replace injured cells, generate growth factors to repair axons, and/or
stimulate alternate neurologic pathways in the brain.
specific causes—can be detected, and in over 30% of the patients no
risk factors can be identified. The etiology is not necessarily the
result of a brain insult that occurred in the prenatal or perinatal
period. Only approximately 10% to 15% of patients in one large group
had documented perinatal hypoxia or other problems (6). Cerebral palsy is not solely the result of prematurity, because 60% to 65% of afflicted children were born at full term (2).
Only approximately 10% of infants with cerebral palsy weigh less than
1500 g at birth. However, in this low birth weight group the risk of
cerebral palsy is 90 per 1000, compared with 3 per 1000 in infants
appropriate for gestational age and weighing more than 2500 g (2,6). Low birth weight for gestational age and prematurity are commonly associated with the development of spastic diplegia.
infection, drug or alcohol abuse, epilepsy, mental retardation,
hyperthyroidism, severe toxemia, an incompetent cervix, and
third-trimester bleeding (5). Teratologic
agents and congenital malformations of the child’s brain may play a
causative role and, in some patients, there may be multiple
contributing agents (7,8).
Genetic agents may also be involved; a recent report links an allele of
the apolipoprotein E gene on chromosome 19 to an increased risk for
cerebral palsy (9).
births; complications of multiple births; abnormalities of blood flow
to the brain; trauma; kernicterus; vaginal bleeding at the time of
admission; placental complications such as abruption, premature rupture
of membranes, and chorionitis; and hypoxia or anoxia. It has been
suggested that four questions must have positive answers in order to
establish birth asphyxia as the probable cause of cerebral palsy (10):
-
Was there evidence of marked and prolonged intrapartum asphyxia?
-
Did the newborn exhibit signs of moderate or severe hypoxic-ischemic encephalopathy?
-
Is the neurologic condition one that intrapartum asphyxia could explain?
-
Has the clinical evaluation been extensive enough to exclude other conditions?
presentation also have been associated with increased risk for cerebral
palsy, but only if accompanied by low Apgar scores (2).
A recent task force (composed of obstetricians, neonatologists, child
neurologists, and other specialists, and reviewed by the American
College of Obstetricians and Gynecologists and the American Academy of
Pediatrics) addressed the issue of cerebral palsy resulting from an
intrapartum event and concluded that fewer than 10% of the cases were
in that category. An excellent review by Blickstein summarizes the
findings (11). Intrapartum brain damage may follow
a usually abrupt “sentinel” hypoxemic event before or during labor.
There are many different hypoxemic events that can result in reduction
of umbilical or uterine blood flow and fetal brain damage; they include
placental insufficiency, premature placental separation, uterine
rupture, acute maternal hypotension, prolapsed umbilical cord, ruptured
vasa previa, and tightened umbilical cord knot. It is rarely possible
to determine the exact time when brain damage occurs, and in most cases
the damage is detected long after the event. The task force concluded
that “approximately 70% of neonatal encephalopathy is secondary to
events arising before the onset of labor.” It is of interest to note
that neither electronic fetal monitoring during labor nor “prompt
cesarean section in cases of nonreassuring fetal heart-rate pattern”
has decreased the incidence of cerebral palsy. However, it is estimated
that infertility treatment by assisted reproductive technology has led
to an 8% increase in the prevalence of cerebral palsy in the United
States, solely as a result of the increase in multiple births (11).
vascular accidents in the brain, central nervous system infections,
kernicterus, hypoxia or anoxia from such causes as near drowning,
suffocation, cardiac arrest, and other problems. The long-term
manifestations of cerebral palsy caused by different specific agents
have not been extensively studied, but there is evidence that postnatal
infectious causes commonly produce more severe orthopaedic deformities
than do many other agents (13).
throughout most of the world, presumably being more common in
geographic regions where prenatal maternal and perinatal infant care
are poor (4,14). In
regions where sophisticated neonatal intensive care units exist, the
risk of brain damage may be reduced by early treatment of certain
problems; on the other hand, the lives of very premature infants and
those with other life-threatening problems are often saved. In this
latter group, the incidence of cerebral palsy is higher than in the
general population. The result is that, whereas the incidence of
cerebral palsy is slightly reduced by preventing some cases, it is
increased by saving infants with cerebral palsy who would not
ordinarily have survived (4). Twin pregnancies
result in a child with cerebral palsy approximately 12 times more often
than do single pregnancies. This is largely related to low birth weight
(15). The prevalence of the neuropathic types
and anatomic patterns of cerebral palsy varies greatly in many reports
because of the widely differing populations studied. For example, a
study dealing with the residents of state institutions shows many more
severely involved individuals than does a study derived from a large
private medical practice.
cerebral palsy, spasticity is an upper motor neuron syndrome caused by
a lesion on the pyramidal system of the brain. The manifestations are a
velocity-dependent increase in muscle tone and hyperexcitable tonic
stretch reflexes. Spasticity is usually accompanied by weakness, loss
of muscle control or dexterity, interference with balance,
fatigability, and often the simultaneous contracting of antagonistic
muscles (16). Severe spasticity is often referred to as rigidity. Contractures of joints are common in spastic cerebral palsy.
caused by an extrapyramidal brain lesion. It is characterized by
purposeless writhing movements, which become intensified when the child
is frightened or excited. With pure athetosis, contractures of the
joints are uncommon and muscle tone may not be increased. Procedures to
lengthen the tendons in children with athetosis are often
unpredictable, and may result in the creation of an opposite deformity
that is more difficult to treat. Decades ago athetosis accounted for
approximately 25% of the cases of cerebral palsy. That was because a
major cause of athetosis, Rh incompatibility with resulting
kernicterus, was not so easily detected and prevented as it is now.
Dystonia, a phenomenon of increased general muscle tone, distorted
postures, and abnormal positions that are induced by voluntary
movements, can occur together with athetosis.
of coordinated movement, most noticeable when the patient is walking,
and is usually the result of cerebellar dysfunction. A mild intention
tremor may be present, contractures are rare and, except for the
treatment of scoliosis and hip dysplasia, surgery is rarely necessary.
and extrapyramidal motor control abnormalities. Variable amounts of
spasticity, athetosis, and/or ataxia occur together. Sometimes the
athetoid component is barely detectable, but it may nevertheless make
surgical treatment less predictable.
most often a stage through which an infant passes before developing
overt spasticity or ataxia. The brain lesion is present but masked by
lack of myelination of the pathways that will carry its abnormal
messages. Occasionally, mentally retarded children are erroneously
classified as having hypotonic cerebral palsy.
implies involvement of all four limbs. Many of these children have
global involvement with mental retardation; bulbar dysfunction
manifested by drooling, dysarthria, and dysphagia; and seizures. A
common cause is severe hypoxia. After initially presenting as a floppy
baby, the child shows delayed developmental milestones. The spectrum of
severity is wide, from having no sitting ability or head control to
being able to walk independently.
involved to a greater extent than the upper extremities; the latter are
affected to some degree, but very mild upper extremity involvement may
be difficult or impossible to detect clinically. A substantial
percentage of the cases of diplegia result from prematurity. Often,
there is found to have been associated periventricular hemorrhage in
and/or around the third ventricle, producing the characteristic lesion
of diplegia (periventricular leukomalacia) in the motor fibers going to
the lower extremities, before they enter the internal capsule (2). These patients usually have normal intelligence.
upper limb being more affected than the lower. The diagnosis is often
not made until after walking has begun or fine motor hand control is
noted to be deficient. A focal traumatic, vascular, or asymmetric
infectious lesion is likely to be the cause of hemiplegia. Seizure
disorders are most frequently seen in this type of involvement,
probably because of the focal brain lesion (5). The seizures usually begin in the first 2 years of life (17).
Children with hemiplegia are also more likely to have homonymous
hemianopsia and stereognostic deficits than are those with other types
of involvement (5). Asymmetry of upper and
lower limb growth, with the involved side being smaller, is also a
common finding and is probably related to the trophic factor of sensory
loss (18).
clear-cut, and some patients do not fit these common types. Blair and
Stanley found only 55% intraobserver agreement in a cerebral palsy
classification study (19). Double hemiplegia
refers to bilateral, usually symmetric involvement, with the upper
extremities being more afflicted than the lower extremities. Triplegia
implies difficulty with any three limbs, usually both lower and one
upper. Monoplegia means only one limb is affected. Paraplegia is used
to describe involvement of the lower extremities only. This rarely
results from a brain injury, and therefore such a pattern of motor
dysfunction should alert the physician to the possibility of pathology
in the spinal cord or canal.
result of global brain involvement, but the spinal cord is usually
spared. Seizures afflict approximately 30% of children with cerebral
palsy, and are most often seen in patients who have
hemiplegia/quadriplegia, mental retardation, or postnatally acquired
syndromes (20).
It is most prevalent in those with spastic quadriplegia. Other problems
include behavioral and emotional difficulties; perceptual disorders;
learning disorders; bulbar involvement with drooling, difficulty in
swallowing, and speech impairment; sensory deafness (which is most
often seen in those with extrapyramidal involvement); and visual
difficulties, such as perceptual problems, strabismus, nystagmus, and
cortical blindness. Visual problems affect approximately 50% of
children with cerebral palsy (2), so visual screening examinations are important in young children.
children who have more severe involvement. Constipation and fecal
impaction are common problems in children with global involvement.
Impaired swallowing, vomiting, esophageal reflux, and hiatal hernia can
cause aspiration and the risk of severe pneumonia, epigastric pain,
profound feeding problems, and poor nutrition (21). Children with cerebral palsy who are malnourished have soft tissue wasting and interference with growth (22,23). When they undergo surgery they are at higher risk for postoperative infections (24).
diet, especially regarding calorie and protein intake, and the feeding
history. Can the child feed himself or herself, and if not, who feeds
the child? Is the swallowing competent or does frequent aspiration
occur? A radiologic contrast study of swallowing to rule out
gastroesophageal reflux may be helpful. Nutritional status can be poor
despite
obesity, and therefore studies such as total serum proteins and
albumin; iron, iron-binding capacity, and transferrin levels;
hemoglobin and erythrocyte mean corpuscular volume; and total
lymphocyte count may be helpful in assessing nutritional status (25).
This is routinely done preoperatively with patients who will undergo
extensive spinal surgery, but is not necessary for all preoperative
workups. One should keep in mind, however, that no single study is an
absolute indicator of malnutrition.
augmentation of enteric feeding if possible. If swallowing is impaired,
a tube-feeding program should be considered. When oral or tube-feeding
supplementation is not feasible, the child should be referred to an
appropriate surgeon for consideration for a feeding gastrostomy or
jejunostomy. Other than occasional and usually minor mechanical issues,
there are no long-term problems associated with prolonged tube feeding.
Gastroesophageal reflux can sometimes be managed successfully by
medical means, but may require surgical fundoplication.
in severely afflicted children. They also have a higher incidence of
urinary tract infections than the normal population. This may relate to
bladder dysfunction and retrograde colonization from frequent diaper
soiling, or to urolithiasis, probably caused by dehydration and urinary
stasis. McNeal et al., in a study of cerebral palsy patients, noted
that 28% had enuresis, 26% had stress incontinence, 18% had urgency,
and 36% had more than one of these symptoms (26).
or a neurologist before the child has had occasion to visit the
orthopaedic surgeon. In some instances, however, an unexplained
abnormal posture, limp, toe walking, limb asymmetry, joint tightness,
developmental delay, or other finding enables the orthopaedist to make
the diagnosis of cerebral palsy.
congenital ataxia, which are inherited conditions, cerebral palsy is
not a genetic disease. FSP refers to a group of inherited disorders
that are characterized by progressive weakness and stiffness of the
legs. The primary feature of FSP is severe, progressive spasticity of
the lower extremity. More severely affected individuals may have
bladder control problems, mental retardation, deafness, optic
neuropathy, and peripheral neuropathy. If FSP is suspected, the patient
should be referred to a geneticist for evaluation.
search for possible causes and risk factors, including environmental
agents, abnormal events during the pregnancy, the details of the birth,
and assessment of the neonatal and infantile periods. Next, it is
important to assess some benchmark physical developmental milestones,
such as head control, sitting, crawling, cruising, and walking (Table 15.1).
These may be normal in hemiplegia. The review of systems should be
thorough in order to detect any of the commonly related problems. A
history of previous treatment, including surgery, is essential.
-
to determine the grades of muscle strength and selective control;
-
to evaluate the muscle tone and determine whether it is normal, hypotonic, spastic, athetoid, or mixed;
-
to assess reflexes and sensory function;
-
to evaluate the degree of deformity or muscle contracture at each of the major joints;
-
to assess linear, angular, and torsional deformation of the spine and long bones, and fixed deformities of the hand or foot;
-
to appraise balance, equilibrium, and standing or walking postures.
while taking the history. Next, as a dynamic examination, one should
evaluate the head control, sitting balance, the ability to crawl, the
ability to pull up to stand, standing posture and balance, and the
ability to walk. It is imperative to observe the gait in patients who
can walk. The remainder of the examination is performed on the
examining table or, better yet, on the parent’s lap if the child is age
4 or 5 years or younger. The primitive neurologic reflexes, tendon
reflexes, sensation, muscle strength, muscle tone, range of motion of
the joints, contractures, torsional abnormalities, and spine should be
assessed (2,27,28).
It should be kept in mind that motor dysfunction in the extremities can
also be a manifestation of a brain or spinal cord tumor, infection, or
other problem.
|
TABLE 15.1 SIMPLE DEVELOPMENTAL MILESTONES
|
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||
examination a functional assessment of the patient is formulated for
immediate documentation and for communicating with other health care
professionals. The following is an example of such an assessment: “The
patient is a 5-year-old boy with spastic quadriplegic cerebral palsy.
He is the product of a 32-week uncomplicated pregnancy, and was
delivered by emergency cesarean section because of uncontrolled uterine
bleeding. He has fair head control, poor sitting balance, and has never
pulled to stand or walked. He is able to communicate only his
discomfort, and does not participate in any activities of daily living.”
certain patients with cerebral palsy, is rarely necessary for
diagnostic purposes. It may be useful in differentiating between
idiopathic toe walking and mild spastic diplegia (29,30,31).
or congenital metabolic, neurologic, and muscular diseases can usually
be differentiated from global involvement with cerebral palsy by
clinical examination and, if necessary, chromosomal analysis. Special
imaging techniques including MRI, positron emission tomography (PET),
and computerized tomography (CT) scans, are useful studies in the
evaluation of intracranial pathology and, when indicated, are usually
ordered by the pediatric neurologist. Most orthopaedic surgeons believe
that all children with the diagnosis of cerebral palsy should be
evaluated by a pediatric neurologist for confirmation and to rule out
other conditions.
of a spinal deformity, radiographs in the coronal or sagittal planes or
both document the degree and sometimes the cause (e.g., a congenital
vertebral anomaly) of the deformity. It is wise to obtain and review a
periodic (every 12 months) coronal plane radiograph of the pelvis for
the early detection of hip pathology, such as acetabular dysplasia or
subluxation, in children with spastic diplegia or quadriplegia who are
not walking (Fig. 15.1). These problems may not
be clinically detectable and are more easily managed and have better
outcomes if treated early. Radiographs of the feet and ankles under
weight-bearing conditions, in the anteroposterior and maximally
dorsiflexed lateral projections, document the status of foot
deformities when surgical intervention is being considered.
techniques of managing common problems relating to cerebral palsy will
be discussed, with specific reference to spastic quadriplegia,
diplegia, and hemiplegia; athetoid cerebral palsy; and the upper
extremity. Many centers have developed cerebral palsy management
programs that are conducted by teams of knowledgeable specialists. Team
members usually include a pediatrician, orthopaedic surgeon,
neurologist, consultant neurosurgeon, clinical nurse specialist,
physical therapist, occupational therapist, speech/language specialist,
social worker, educator, and psychologist (5).
It is especially important for the team members to communicate among
themselves frequently and confirm that they are in substantial
agreement about the treatment program, so that the family does not
receive mixed or conflicting messages about their child’s care. The
patient’s family is the most important member of the team.
cerebral palsy are not grounded in data-based research; they depend
more on empiricism, opinions, and experience regarding the best
achievable outcome. Fortunately, physicians now recognize that
treatment must be measured in terms of technical outcomes, functional
health assessments, and patient satisfaction (3). Instruments to measure several parameters have been developed and validated (32).
These include gait analysis, the gross motor function measure (GMFM),
the Pediatric Orthopaedic Society of North America’s Health Status
Questionnaire (33), the Gillette Children’s
Specialty Healthcare Normalcy Index and Functional Assessment
Questionnaire, and the WeeFIM (Wee Functional Independence Measure for
Children). In time, these and others should provide the data needed to
base clinical care on reliable clinical algorithms. Using such
assessment tools, orthopaedic surgical intervention has been shown to
be safe and effective in properly selected children with cerebral palsy
(34).
involvement, is most often unable to walk, and requires nearly total
care. For such a person, the objectives to aim for, in the order of
priority, are
-
the ability to communicate with others;
-
the ability to take care of activities of daily living, especially personal hygiene;
-
mobility in the environment;
-
walking.
quadriplegia will eventually walk. Realistic orthopaedic goals for
nonambulatory children are directed toward maintaining balanced,
comfortable sitting. The specific objectives are achievement and
maintenance of:
-
a straight spine and a level pelvis;
-
located, mobile, painless hips that flex
to at least 90 degrees for comfortable sitting, and extend to at least
30 degrees of flexion for comfortable sleeping and to accomplish pivot
transfers;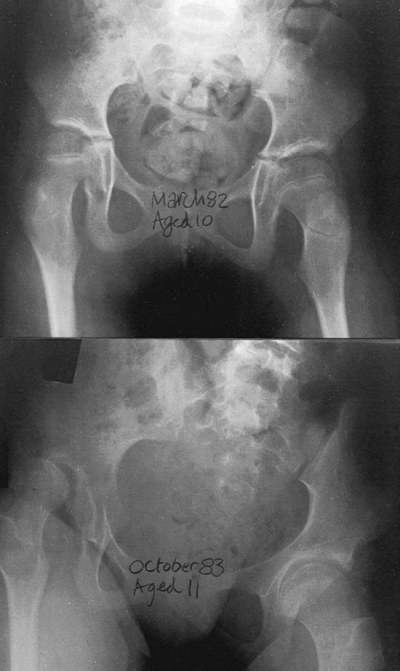 Figure 15.1
Figure 15.1
Radiographs showing substantial changes in the right hip, including
dislocation, which developed in a 10-year-old child over a period of a
few months. Annual hip evaluations, including radiographs, are
important for detecting such problems. -
mobile knees that flex for sitting and
can extend enough (to ≤ 20 degrees of flexion) to be controlled by
orthoses for transfers and for comfortable sleeping; -
plantigrade feet for wearing shoes and for comfort on the footplates of wheelchairs;
-
an appropriate wheelchair;
-
management of medical problems in the other systems.
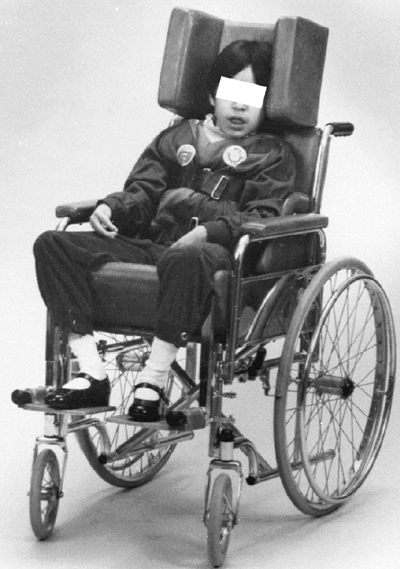
wheelchair that the patient with spastic quadriplegia will spend most
waking hours. The chair should be considered a total body orthosis to
be fitted and maintained by an expert (Fig. 15.2).
The ability to independently transfer in and out of a wheelchair
greatly facilitates the patient’s ability to live in a group-home
setting for an adult with spastic quadriplegia. It is beneficial for
all concerned to have a physical therapist who has experience with
patients in wheelchairs as a collaborator in developing the wheelchair
prescription. The following should be considered in wheelchair design:
-
should be long enough for the shoes;
-
should either support the entire foot in
a plantigrade position or be removed to allow free dangling of fixed
deformities, such as severe equinus, to avoid increased pressure over a
small area of contact; -
should be able to swing out of the way for entering and exiting the chair;
-
should accommodate foot restraint straps if needed.
 |
|
Figure 15.2 An appropriately fitted wheelchair provides proper body positioning, including head control.
|
-
height must allow the feet to correctly contact the foot rests;
-
depth should entirely support each of the thighs, which may not be of equal lengths, without compressing the popliteal area;
-
width should not compress the trochanters, but also should not allow lateral shifting or excessive tilting of the pelvis;
-
firmness should be as much as tolerated
by the patient to provide maximum pelvic stability without creating
excessive skin pressure over bony prominences; -
contour should be incorporated, if necessary, for comfort.
-
height should support the patient’s trunk from the pelvis to the midscapular region;
-
width should accommodate the trunk and any needed thoracic support pads;
-
firmness should be ensured to the extent that is comfortable to help prevent collapsing kyphosis;
-
contouring should be incorporated, if necessary, to accommodate scoliosis;
-
should recline; reclining may be a necessary feature.
-
may be necessary for foot, leg, pelvic, trunk, arm, or head control.
-
is necessary if transportation in the community is desired.
-
the patient’s arms;
-
an attendant;
-
motorization.
while sitting, the spine and hips are of prime importance. These are
often the site of major problems in spastic quadriplegia. Other lower
extremity problems specific to the ambulatory patient with spastic
quadriplegia are addressed in the section on spastic diplegia.
primary deformity in cerebral palsy. It is usually secondary to
hip-flexion contractures, and it responds to appropriate correction of
those contractures by such means as stretching exercises or surgical
lengthening of the psoas tendons. Hyperlordosis may also be a
compensatory deformity below a rigid thoracic hyperkyphosis, and it
usually responds to correction of the primary problem. When surgical
spinal fusion is necessary to correct severe scoliosis, it is essential
to consider the sagittal plane spinal balance and to preserve adequate
lumbar lordosis by avoiding excessive distraction across the lumbar
spine.
with cerebral palsy who has weak spinal extensor musculature and a
resultant long, C-shaped kyphotic posturing of the entire spine. This
is almost always flexible, correcting fully on prone lying. It is best
controlled by proper seating adaptation such as restraint straps on the
wheelchair, slight reclining of the back, or, less often, by a
thoracolumbosacral orthosis to provide sitting support. There is debate
regarding whether increasing the sitting support inhibits the
functioning of the spinal extensor muscles and weakens them further,
and whether physical therapy or muscle stimulation is helpful in
maintaining or enhancing spinal extensor muscle strength.
because of overly tight hamstring muscles that cause sagittal rotation
of the pelvis. This kyphosis may disappear with proximal lengthening of
the hamstrings, but we have very little experience with this.
spinal deformities that afflict other children, so thoracic
hyperkyphosis resulting from the Scheuermann condition
or
postural juvenile kyphosis may also occur. Indications for orthotic
treatment in these kyphotic conditions are similar to those in other
children, but spinal orthotics are not likely to be as well tolerated
in the child with cerebral palsy. If the child does not have good
voluntary trunk control then he/she will rarely tolerate an effective
orthosis.
palsy compared with the general population, and it varies in direct
proportion to the severity of motor involvement. In patients with mild
hemiplegia, scoliosis occurs in fewer than 5%; in patients with severe
spastic quadriplegia, its occurrence is much greater, in the range of
approximately 50% to 75%; in all patients with cerebral palsy taken
together, it is approximately 25%. Specific increased risk factors for
curve progression are quadriplegia, younger age, poor sitting balance,
pelvic obliquity, and the presence of multiple curves (35,36,37).
different from idiopathic scoliosis. It develops earlier; is more
likely to be progressive; progresses even after skeletal maturity,
especially when the curve exceeds 40 degrees; is almost always
unresponsive to orthotic control; and is more likely to require
surgical treatment.
are only three appropriate options for management: observation with
documentation, orthotic treatment, or surgical stabilization.
Observation alone is indicated if the curve is of insufficient
magnitude to require treatment (<25–30 degrees) or if the patient’s
best interests may not be served by active surgical intervention. The
latter category is difficult to define. It would include the most
severely involved individuals who are unable to perceive or interact
with their environment in any meaningful fashion, because of severe and
global compromise of their cognitive and sensory perceptual abilities.
Only careful study by members of the cerebral palsy team and the
patient’s family can lead to this conclusion. In such cases, the
overall management goals are the patient’s safety and comfort.
cerebral palsy was based mostly on hope and empiricism until studies by
two institutions showed that it rarely succeeds in controlling a curve (38,39).
In most of the patients with quadriplegia from these centers, no
meaningful curve control was achieved by orthotic treatment. In some
cases, however, an orthosis may slow the progression of the curve,
particularly in curves of 30 to 60 degrees, allowing beneficial growth
in an immature spine before definitive surgical stabilization. At best,
no more than 15% of brace-treated curves stop progressing, and this may
simply reflect the natural history of some cases of scoliosis in
cerebral palsy (37). Ambulatory patients with
spastic diplegia may develop idiopathic-type scoliotic curves. In these
milder cases of cerebral palsy, brace control may be successful.
Orthoses and other types of external devices for trunk support may be
of value in improving sitting balance, particularly for those patients
in whom surgery is not indicated. If the patient can tolerate a
total-contact, low-profile orthosis, this is the most effective and
economical means of providing improved trunk support, even if it is a
relatively soft orthosis (40). This is often
not the case, however, and a custom-molded trunk or
total-body-supporting wheelchair insert is required. These devices are
difficult to fit properly, are often quickly outgrown, and are
expensive. They must provide adequate pelvic alignment, trunk control,
and head support. Nevertheless, the ability to sit as erectly and
comfortably as possible is essential for a totally involved patient who
can interact with the environment. Good sitting improves the patient’s
mental outlook, ability to communicate, respiratory function, ease of
feeding, gastrointestinal function, hand usage, and mobility in the
environment (41,42,43).
but most orthopaedic surgeons strongly believe that the surgery is
worthwhile, particularly in preventing discomfort and loss of the
ability to sit (Fig. 15.3). Postoperative
patients with reasonably balanced nonprogressive scoliosis have much
better endurance for sitting. It greatly improves their quality of life
when they can sit up comfortably for several hours, or even all day,
and not have frequent substantial back pain that requires recumbence
during most of their waking hours. According to their caregivers, they
are also much easier to feed, dress, and transport than those with
severe untreated scoliosis. It is most likely that after fusion they
will have less back discomfort, better pulmonary function, and less
decubitus skin ulceration than similarly involved patients with severe
untreated scoliosis. However, this has not been proven (47).
Subjectively, parents report significant improvements postoperatively:
one year after surgery, their children experience less pain, are
generally happier, and do not feel ill or tired as frequently as
before. However, physical functioning and comorbidities do not improve (48,49).
It is noteworthy that patients with lower extremity spasticity often
experience an increase in the magnitude of their spasticity for several
months after corrective scoliosis surgery. The increased tone gradually
resolves and may be somewhat relieved by benzodiazepines. Its cause is
not known, but is possibly related to some stretching of neurologic
structures or obligatory operative edema.
continue progressing and surgery is usually indicated. Posterior
internal fixation with a segmental system, such as double rods with
cross-links connected to the spine with multiple hooks; sublaminar
wires; interspinous wires; pedicle screws; or combinations of these
techniques and an adequate posterior fusion mass is usually employed.
This is needed in order to achieve a balanced spine over a reasonably
level pelvis, which is the objective of such
surgery (44,50).
Whenever possible, a larger and more rigid rod is preferable so as to
provide better correction, resist deforming forces, and promote a solid
arthrodesis. It is essential to achieve spinal balance in both the
coronal and sagittal planes in order to maximize sitting balance. An
abundance of allograft bone or an effective bone graft substitute
should be available to generate the strong fusion mass (51,52).
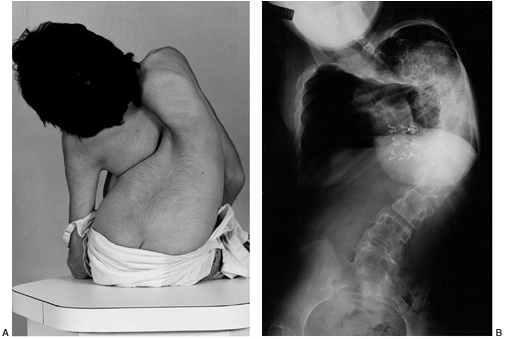 |
|
Figure 15.3 Clinical (A) and radiographic (B)
images of a girl with cerebral palsy at initial presentation. She has severe, untreated scoliosis and is unable to sit for more than a few minutes. She interacts somewhat with her family, who declined to consider surgical correction and fusion. The curve is too severe for an orthosis to aid in sitting, and she is managed with an adaptive reclining wheelchair. |
(T1–T3) to the pelvis. When the fusion does not extend to the upper
thoracic region, there is an increased risk of developing a substantial
junctional kyphosis cephalad to it. This may interfere with the ability
of the patient to see at or above the horizontal, or may require
constant and eventually painful neck hyperextension to do so. No matter
how cephalad the upper fusion level is, or whatever the type of
fixation, some patients still develop a junctional kyphosis. Applying a
bilateral, two-level, clawed-hook configuration at the cephalad end of
the rods (Fig. 15.4) with preservation of the
uppermost posterior ligaments may help prevent a junctional kyphosis.
However, this has not been consistently successful, and many surgeons
have abandoned this technique. Although the cephalad-most pair of wires
may occasionally break eventually, this will almost never require
revision.
exceeds 10 degrees from the intercrestal iliac line to the top of L5 or
L4 as measured on an anteroposterior radiographic view, with the
patient seated. Otherwise, pelvic obliquity may continue to progress
and make sitting more difficult. Regardless of the condition of the
hips in patients with pelvic tilt, the spinopelvic obliquity can be
corrected only by spine surgery and not by hip or pelvic surgery (53).
Various techniques are available for pelvic fixation, including hooks,
rods, and screws anchored to the iliac bones or sacrum, but none has
withstood the test of time better than the Galveston technique (54).
cerebral palsy. The most common indication for anterior spinal surgery
in this condition is to improve curve correctability. Increased
correction may be needed in order to
-
level the pelvis when pelvic obliquity is rigid and severe;
-
balance the spine in large, rigid curves
that do not correct to less than 50 to 60 degrees during supine bending
or maximum traction radiographs; -
release the anterior tether of a kyphos;
-
attempt to improve respiratory function or decrease the likelihood of the development of a pseudarthrosis in severe curves.
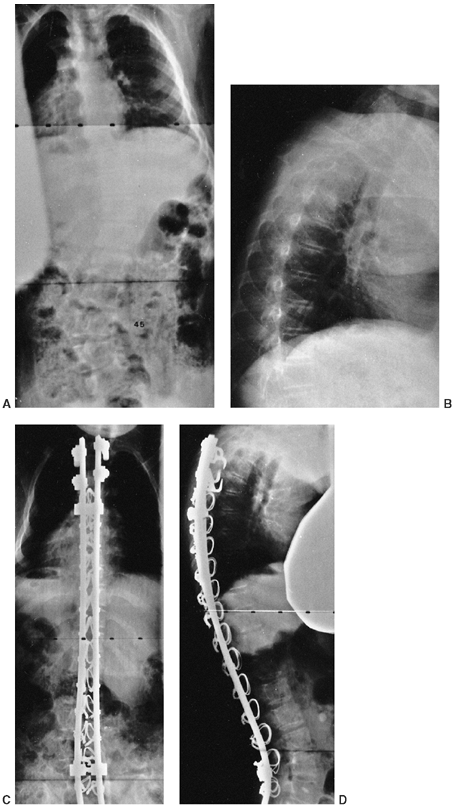 |
|
Figure 15.4
Treatment of scoliosis by posterior spinal fusion with segmental instrumentation, using mostly sublaminar wires. A two-level, transverse process pedicle claw was used at the cephalad end in an attempt to prevent junctional kyphosis. A: Preoperative posteroanterior radiograph demonstrates a 45-degree lumbar curve. B: Preoperative lateral radiograph demonstrates associated thoracic kyphosis. C: Postoperative posteroanterior radiograph. D: Postoperative lateral radiograph demonstrates the cephalad claw configuration of the hooks. |
accomplished by dividing the anterior longitudinal ligament, excising
the annulus fibrosis, removing all of the disc material and endplate
cartilage back to the posterior annulus and posterior longitudinal
ligament, and packing bone graft into the disc spaces. This may be done
either through an open approach or by an endoscopic technique. Anterior
internal fixation is rarely necessary when strong, rigid posterior
fixation is performed. The anterior and the posterior operations are
accomplished at the same surgical
setting rather than being staged over several days or weeks. This decreases the hospital stay and complications (55,56,57).
managing a spinal deformity by surgical means in a patient with
cerebral palsy. As discussed earlier, one third of the patients are
malnourished (58,59)
and may have gastroesophageal reflux. Detecting and correcting these
conditions preoperatively helps improve the patient’s nutritional
status and thereby prevent postoperative wound infection and healing
problems. Determination of the serum transferrin level, albumin level,
and total lymphocyte count are commonly used for assessing nutritional
status. An index has been developed for identifying malnourished
patients on the basis of transferrin and albumin levels (60).
carefully, expressed in terms of percentage of blood volume, and
appropriately replaced in order to avoid hypovolemia or dangerous
coagulopathies, especially when blood loss nears 50% of the blood
volume. In this method, preoperative blood volume should be accurately
estimated, and the loss detected by suctioned blood plus blood in
weighed sponges should be carefully measured. Another means of
monitoring blood loss and replacement requirements is by calculating
erythrocyte mass and considering hematocrit measurements in the lost
blood as well as in the replacement source. Many surgeons monitor the
hematocrit value, which is obtained through periodic blood gas
assessments throughout the procedure. When blood replacement is
indicated, whole blood or packed RBCs may be used. Blood loss may be
minimized by techniques such as hypotensive anesthesia (usually to a
mean arterial pressure of approximately 60 mm Hg), preoperative
hemodilution, the administration of aprotinin, and the use of a
cell-saver.
hypoventilation, atelectasis, aspiration pneumonia, and the adult
respiratory distress syndrome may occur, and every preventive effort,
including rapid mobilization of the patient, must be enlisted.
occur in nonambulatory patients, they have been reported in ambulatory
patients with cerebral palsy with an incidence similar to that in the
general population. No increased severity of symptoms or relation to
hip-flexion contractures has been noted (61). The treatment is similar to that recommended for children who do not have cerebral palsy.
for progression to subluxation or dislocation; subluxation; and
dislocation. Hip displacement occurs in approximately 1% of patients
with spastic hemiplegia, 5% with diplegia, and 35% to 55% with
quadriplegia (62). Causative factors for hip
problems are probably combinations of muscle imbalance, acetabular
dysplasia, pelvic obliquity, excessive femoral anteversion, increased
femoral neck valgus, lack of weight bearing, and maldirected resultant
force vectors across the hip joint. The incidence of increased femoral
anteversion is greater at all age levels in children with cerebral
palsy than in the normal population. It does not change significantly
after the age of 6 years, and is greater in ambulatory children than in
nonwalkers (63).
contracture it can be difficult to detect even substantial hip
abnormalities by routine physical examination. For this reason it is
recommended that in children with bilateral involvement screening
radiographs be obtained at the age of 18 months and at 6- to 12-month
intervals thereafter. This hip surveillance increases preventive
surgery and decreases the need for reconstructive and salvage surgery (64).
In all but the most severely involved children, hip dislocation should
be preventable. Hip subluxation or dislocation, although more common
before the age of 6, may occur at any age. Hips at risk and hips that
are subluxated rarely cause discomfort, but dislocation can lead to
pain. Studies have reported pain in approximately 50% of cerebral palsy
patients with dislocated hips (65,66,67),
and the associated increased contractures may make their care more
difficult and worsen their sitting balance. One study, however, found
that surgical reduction of dislocated hips did not improve pain or
sitting ability (67). Until more studies with
longer follow-up are available regarding treatment of dislocated hips
in cerebral palsy, most surgeons follow the management described in the
following section.
along with a shallow acetabulum, but no subluxation. Tightness and
contractures are usually present in the adductor and flexor muscles.
Without treatment, hips at risk often progress to subluxation or
dislocation, particularly if there is less than 30 degrees of abduction
in flexion or extension, and/or if there are hip flexion contractures
of greater than 20 degrees. Such progression may be very slow (months
to years) or occur much more rapidly. Because the literature lacks
valid data regarding the likelihood of such hips worsening, it is not
possible to predict the natural history of every hip at risk in
cerebral palsy. That leaves the surgeon with the options of intervening
or closely following hips at risk. Unless the patient is so physically
compromised as to preclude surgical treatment, most surgeons will
intervene, realizing that at least in some cases the hip pathology
would not have been progressive. The use of stretching exercises alone
as treatment for hips at risk is rarely, if ever, successful. In very
young children with hips at risk and mild or absent muscle tightness,
night splinting, in an attempt to improve acetabular depth, is an
option that some surgeons might choose. However, it has not been
confirmed by any well-controlled study that night
splinting can reliably increase acetabular depth, and we do not use the method.
The adductor longus may be all that needs releasing, especially in
those who walk, but the gracilis and, occasionally, part of the
adductor brevis muscle also may require release in order to gain
abduction to at least 45 degrees for each extended hip and 60 degrees
for each hip in flexion. The issue of whether to release or transfer
the adductors is discussed in the section on spastic diplegia. Tenotomy
or elongation of the psoas tendon, sparing the iliacus fibers, should
be performed. Psoas tenotomy in a nonambulatory patient with spastic
quadriplegia may be performed either at the pelvic brim or at the
lesser trochanter. Tenotomy at the more distal site may produce more
hip flexor weakness, a situation of no consequence to a nonwalker but
one that may be detrimental to an ambulatory patient who needs adequate
hip flexor power to lift the limb for step climbing (2). Psoas tenotomy at the pelvic brim is performed in the manner described by Sutherland et al. (71). On rare occasions the psoas may not be tendinous at that level, and the tenotomy must be done more distally.
for several months. This is applied at night and always within the
child’s range of comfortable abduction. The value of such splinting is
questionable. Postoperatively, physical therapy is begun as early as
the second postoperative day (72). In the past,
anterior branch or even complete obturator neurectomy used to be
performed to denervate the adductor muscles in addition to lengthening
them. Now this is rarely done because most surgeons believe this
procedure is unnecessary when an adequate adductor release has been
performed. If the child has an athetoid component in addition to
spasticity, obturator neurectomy should never be done. It can result in
a severe, disabling abduction contracture.
one third of the femoral head and a break in the Shenton line, but with
the femoral head maintaining at least some contact with the acetabulum.
Surgical treatment of a subluxation can prevent subsequent dislocation (73,74,75).
successful, even in very young children. When only soft tissue surgery
is deemed necessary to treat very mild unilateral subluxation and the
patient is younger than 9 years, bilateral releases may be best because
the contralateral hip is usually somewhat abnormal or likely to become
so if only unilateral surgery is done (76).
Soft tissue surgery alone (adductor and psoas releases) does not arrest
subluxation in most children regardless of their age. In a recent
study, subluxation or dislocation persisted in 58% of the children
after such surgery (77).
anteversion, or both, and requires corrective proximal femoral
osteotomy (78,79,80).
To stabilize the subluxated hip, the neck-shaft angle of the hip is
usually surgically reduced to approximately 115 degrees in an
ambulatory child, or even less in a nonambulator. In addition to
appropriate tendon releases, derotation of the excessive femoral
anteversion to approximately 10 to 20 degrees of anteversion (or 30 to
45 degrees of passive internal hip rotation) is performed to prevent
posterior subluxation (81,82).
The younger the patient, the more likely are the valgus and anteversion
to recur postoperatively, especially in children younger than 4 years (83).
On the other hand, subluxated hips are more likely to remain located
after surgery in younger children than in older children, particularly
those older than 10 years (84). A recent large
study reported that 84% of patients had a stable pain-free hip at an
average of 5 years after femoral varus osteotomy (85). If the magnitude of the subluxation exceeds 50%, an open reduction and capsulorrhaphy will improve the result (86).
osteotomies require approximately 7 to 10 months to regain their
preoperative function, but longer times—up to 30 months—are possible (87).
If bony surgery (a varus rotational osteotomy with or without a pelvic
osteotomy) is required, prophylactic surgery on a well-covered
contralateral hip with adequate abduction is not necessary regardless
of the age or ambulatory status of the patient (88).
osteotomy surgery are femoral fractures and decubitus ulcers. Children
with tracheostomies and gastrostomies are at the greatest risk (89).
An exception might be a child younger than 4 or 5 years with very mild
dysplasia and recently fully corrected abnormal valgus and anteversion,
in whom acetabular remodeling may occur from the stimulation of
redirecting the force vector across the hip joint from the lateral
acetabular rim to the center of the acetabulum. We do not favor this
and recommend correction of any acetabular dysplasia. Older children
have much less potential for biologic remodeling to normalize the
acetabulum after femoral varization and rotation osteotomies.
In nonambulators the acetabular deficit is superior and posterior to,
and usually more severe than, the deficit in ambulatory patients. It is
almost always associated with an increase in the femoral neck-shaft
angle and femoral anteversion. The femoral anteversion is greater in
ambulators (63).
the appropriate pelvic osteotomy and being certain that its
prerequisites are met. Anteroposterior and 30 degree false profile
radiographs are helpful in assessing the
pathoanatomy
of the acetabulum. Although not usually necessary, arthrographic
evaluation or three-dimensional reformatted CT scanning images can be
helpful in the decision-making process to define the pathoanatomy (90). Clinical assessment of the acetabulum when it is exposed at surgery confirms the choice of procedure.
 |
|
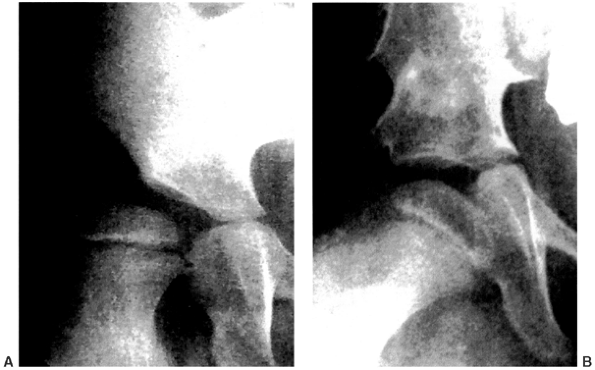
Figure 15.5 A:: Subluxation of the right hip with acetabular dysplasia. B:: Result after varization and derotation osteotomies of the proximal femur and Albee shelf pelvic osteotomy.
|
Albee shelf, Dega, and Chiari osteotomies, and shelf augmentation of
the acetabular rim, have all been successful in patients with cerebral
palsy when appropriate indications are met (91,92,93,94,95,96,97,98,99).
If the acetabulum is found to be deficient superiorly, or superiorly
and anteriorly, an anterolateral rotational osteotomy, such as a
Salter, Steel, or Sutherland procedure, or a Pemberton osteotomy, is
appropriate. Often there is superior and posterior deficiency, in which
case restoration of lateral and posterior coverage by a
shelf-augmentation procedure, an Albee shelf, a Dega procedure, or a
pericapsular osteotomy (100) may be more
appropriate. The Chiari osteotomy can be performed if the superior
acetabular rim has not been so proximally eroded that the cut will
enter the sacroiliac joint. Long-term follow-up of pelvic osteotomies
in cerebral palsy indicate that gradual deterioration from early
postoperative results often occurs, and consideration of wider release
of soft tissues, more radical varus derotation, and greater shortening
of the femur may be beneficial (101).
If the dislocation occurred within a year of presentation, and/or if
the anatomy does not appear excessively distorted, most surgeons
customarily elect to perform anterior open reduction and
capsulorrhaphy, combined with appropriate soft tissue releases (usually
adductors and psoas tendon) and a proximal femoral shortening,
varization, and rotation osteotomy. Often, a degree of acetabular
dysplasia is
associated
with the dislocation, and it is wise to correct this at the same time.
The pelvic procedure should be tailored to the situation, as described
in the previous discussion of the subluxated hip. There is a lack of
convincing outcome studies of the benefit of such late reductions, and
this treatment is based only on positive anecdotal experience.
 |
|
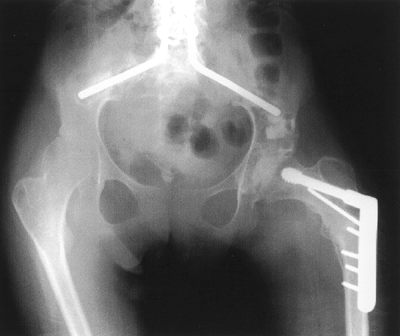
Figure 15.6
A 14-year-old girl with spastic quadriplegia. She had a previous spine fusion and, despite a level pelvis, her left hip became dislocated. She underwent varization and derotation of the proximal femur and a Dega-type pelvic osteotomy. Prior to the osteotomies a capsulotomy was done to confirm adequate cartilage covering the femoral head. Otherwise a proximal femoral resection would have been done. |
achieving a painless, mobile, stable hip from open reduction and other
surgery is less likely because of joint incongruity and eroded
articular cartilage on the femoral head. When such a hip is painless,
no treatment is required. When the hip is painful, proximal femoral
resection with muscle interposition, as originally described by Castle
and Schneider, has a high success rate (106,107).
This resection is performed at the subtrochanteric level; the thickened
capsule and gluteus medius muscle are sewn over the acetabular inlet,
and a muscle cuff of vastus lateralis is sewn over the beveled femoral
stump (Fig. 15.7). It is critical to carefully
save as much vastus lateralis as possible during the initial
dissection, and to provide a good, thick muscle covering over the
femoral stump. Postoperative management should consist of a bilateral
pantaloon or a one-and-a-half-hip spica cast for approximately 3 to 4
weeks, then comfortable but effective exercises to assure maintenance
of the desired range of hip motion. This range is a minimal flexion
contracture, at least 100 degrees of flexion, neutral rotation, and
abduction to at least 20 degrees. Postoperative traction or external
fixators are rarely necessary because the casting appears to be more
comfortable for the patient and easier to manage. The result is usually
good motion and good pain relief, but definite thigh shortening, which
must be accommodated in the seat of the wheelchair. Following proximal
femoral resection it is very common to have spasm and discomfort for
several days, weeks, or even months (107). This
usually will eventually resolve, but analgesics and antispasmodic
medications may be necessary for a prolonged time. A successful trial
of an intrathecal baclofen injection may indicate the efficacy of using
a baclofen pump during this period. Recently the addition of prosthetic
interposition to proximal femoral resection, often using a noncemented
proximal humeral prosthesis in the small femoral canal, has been
reported to give good pain relief for this problem in the short term (108).
Heterotopic ossification about the hip is extremely common after
proximal femoral resection, and preoperative or immediate postoperative
single-dose radiation therapy should be considered in order to minimize
its magnitude.
subtrochanteric valgus pelvic support osteotomy, has been successful in
relieving the pain of chronic hip dislocation (109,110).
Specific indications and advantages of this treatment versus proximal
femoral resection are not available in the literature, and the
selection of one or the other is therefore according to the surgeon’s
preference. Our preference is for resection. The Girdlestone
intertrochanteric resection has been tried for the painful dislocated
hip. The likelihood of continuing postoperative pain from femoral-iliac
impingement has caused most surgeons to abandon this procedure.
to accomplish in nonambulatory patients with severe spasticity. When
enough flexion is provided to make sitting comfortable, it may limit
the ability to comfortably lie
supine.
It may also preclude appropriate positioning of the patient undergoing
spine surgery. Arthrodesis, therefore, is not often performed in
nonambulatory patients. In ambulatory patients with unilateral hip
disease, arthrodesis is a reasonable option with high success in bone
union, pain relief, hip stability, and postural improvement (111,112,113).
 |
|
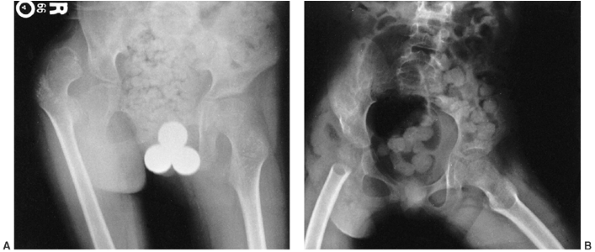
Figure 15.7
This nonambulatory child’s painful dislocated right hip was treated by resecting the proximal femur just distal to the lesser trochanter and oversewing muscle flaps. This is known as the Castle procedure. His pain was relieved. A: Preoperative anteroposterior radiograph shows the dislocated right hip with femoral head deformity and acetabular dysplasia. B: Postoperative radiograph. The proximal femur has been resected at the distal end of the lesser trochanter. |
This procedure is indicated most often for the ambulatory adult with
mild to moderate spasticity and severe degenerative arthritis of the
hip. Nonwalking adults with painful untreated hip dislocations respond
better to proximal femoral resection (111).
not be treated surgically unless it is painful, and nonsurgical methods
have been tried without success. In such patients, both arthrodesis and
replacement arthroplasty have been successful, the latter being
preferred because of less postoperative morbidity and easier management
(112). Arthrodesis is usually performed in a
position appropriate for walking, sitting, and lying: 30 degrees of
flexion, 5 to 10 degrees abduction, and neutral rotation for an
ambulatory patient, and 45 degrees of flexion for a nonambulator.
Leg-length equalization should be considered in walking patients
because it may defer the development of back pain (112).
the severely spastic child with quadriplegia. The probable causes are
contracted hip extensors and tightly contracted hamstrings, which are
hip extensors as well as knee flexors. This condition is often
accompanied by extensor thrust, a rigid and sustained hip extension
that can seriously interfere with comfortable sitting because the
extension contracture does not allow adequate hip flexion. When severe,
it can literally push and slide the child out of the wheelchair. The
treatment options for mild extension contracture are proximal
lengthening of the hamstrings or injecting neuromotor blocking agents
such as botulinum-A toxin or alcohol into the hamstrings. If the
extension contractures are more than just mild, lengthening of the
proximal hamstrings is indicated (114). If this
is not adequate, posterior capsulotomy of the hip and release of the
hip extensors and external rotator muscles may also be necessary.
Unfortunately, recurrence of extension contractures is common and often
rapid, following proximal hamstring lengthening.
common. It most often occurs after injudicious release of the flexors
and adductors in patients who have athetosis, and it is also seen after
aggressive flexor and adductor releases in patients who have severe
spasticity or rigidity and previously undetected cospasticity in the
hip extensor and abductor muscles. Cospasticity can be difficult to
detect even by careful physical examination, but both flexion and
extension of the joint are substantially limited, as are abduction and
adduction. Gait analysis may detect cospasticity in ambulatory patients.
athetosis rarely develop contractures that require surgical releasing.
A problem exists with the mixed spastic-athetoid patient with
tightness, in whom the surgeon cannot determine precisely just how much
to weaken the spastic muscle. It is definitely better to err on the
side of underrelease.
contracture of the hip consists of stretching exercises and proper
seating in the wheelchair. Data regarding the use of tone-reducing
measures in this situation are currently lacking, but a trial of
intrathecal baclofen may be useful. In patients with more severe
involvement, surgical treatment is necessary. This involves release of
the proximal hamstrings, the femoral and iliotibial band insertions of
the gluteus maximus, the external rotator muscles, and even posterior
capsulotomy of the hip joint, if necessary. In long-standing severe
contractures, femoral shortening may be necessary to prevent
overstretching the sciatic nerve (115).
walking is the rule, and it is not unusual for a diplegic child not to
begin ambulation until the age of 4 years or even later (116).
Motor improvement often reaches a plateau at approximately the age of 7
years, so if a child is not walking by that stage, there is little
likelihood that he or she will walk (117,118,119,120,121).
The severity of lower extremity involvement is the most important
factor in the ability to walk. A seizure disorder, marked flaccidity,
persistent abnormal primitive reflexes, or a dislocated hip are
deterrents to walking, whereas intelligence, upper-extremity severity
index, and birth weight do not correlate closely with prognosis
relating to walking (121). Mental retardation has little or no effect on walking ability (121).
Campos da Paz studied 272 children with cerebral palsy and found that
achievement of head balance before the age of 9 months, independent
sitting before 24 months, and crawling before 30 months were good
prognostic indicators for walking, whereas lack of head control at the
age of 20 months indicated a poor prognosis (121).
On the other hand, Molnar and Gordon, in a study of 233 children with
cerebral palsy, found that in children younger than 2 years,
independent sitting was not a good predictor for walking ability, but
that after age 4 years inability to sit predicted nonambulation (118).
|
TABLE 15.2 BLECK’S WALKING PROGNOSIS CRITERIA
|
||
|---|---|---|
|
of patients with meningomyelocele, classified ambulation into four
functional levels. This classification is also appropriate for use in
children with cerebral palsy:
-
Community ambulators: These patients walk
indoors and outdoors in the course of most of their activities, and may
need crutches, braces, or both. They use a wheelchair only for long
trips out of the community. -
Household ambulators: These patients walk
only indoors and with apparatus. They are able to get in and out of the
chair and bed with little or no assistance. They may use the wheelchair
for some indoor activities at home and school, and for all activities
in the community. -
Nonfunctional ambulators: For these
patients, walking is a therapeutic exercise at home, in school, or in
the hospital. Usually, they use their wheelchairs to get from place to
place. -
Nonambulators: These patients are wheelchair-bound, but usually can transfer from chair to bed.
with scoliosis, seizures, speech impediments, and major problems in
other systems than are those with quadriplegia. Hip dislocation is also
less likely, but excessive valgus and anteversion of the proximal
femur, acetabular dysplasia, and hips at risk or subluxated are not
uncommon.
balance. In diplegia, posterior equilibrium is most often affected, but
this does not obviate walking or require the use of cane or crutches.
Crutches are necessary if anterior balance is defective. If lateral
balance is significantly involved, the assistance of a rollator walker,
frequently of the posterior type, will be needed. This is better than
the anterior design in achieving a more upright posture (123,124). Severe lateral equilibrium disturbances usually preclude any walking.
diplegia are modulation of spasticity (oral medications, intramuscular
injections, intrathecal injections, selective dorsal rhizotomy),
physical therapy, orthotics and/or manipulation and casting, and
musculoskeletal surgery. These modalities are discussed in this section.
appropriately temper spasticity and allow improved voluntary muscle
control to occur over the long term. Unfortunately, such an agent does
not exist. Many types of muscle relaxants, antispasmodics, and
neuroinhibitory medications have been tried to little or no avail,
mostly because therapeutic doses are so large that the substantial
accompanying drowsiness is unacceptable (125,126).
Other undesirable side effects include drooling, ataxia, and behavioral
problems. Oral pharmacotherapies that are generally accepted as
effective for problems in cerebral palsy have been limited to
anticonvulsants for the treatment of seizures and benzodiazepines (such
as diazepam) for superimposed postoperative myospasms. The latter drug
increases presynaptic and postsynaptic inhibition, and perhaps has a
tranquilizing effect that mutes the perception of pain. Children with
dystonia may benefit from trihexyphenidyl (Artane), which may improve
hand function and speech to some extent. This drug may cause dry mouth,
constipation, and urinary retention.
γ-aminobutyric acid (GABA) that inhibits reflex activity. Its poor
blood-brain barrier permeability, short half-life, and slow onset of
action require that frequent and high doses be given; it produces
somnolence plus other side effects that limit its usefulness as an oral
medication. Rapid withdrawal of the drug may increase seizure activity.
not on the nervous system. Because it produces weakness with little
effect on spasticity and has numerous potential side effects, it is
rarely useful in cerebral palsy (127).
-
to weaken a muscle and improve the
balance of forces across a joint in order to assess whether this will
improve function. This is a temporary effect, but in some cases it may
be of value to allow a stretching and strengthening physical therapy
program to perhaps obviate or, more likely, defer the need for surgery; -
to separate severe spasticity from fixed-joint contracture;
-
to determine which muscle is the major contributor to an abnormal posture;
-
to assess the performance of antagonistic muscles (128).
is the gastrocnemius; this is done in order to reduce equinus
deformity. Other common sites are the hamstrings and hip adductors.
Repeated injections may be necessary to achieve the desired effect, and
injecting some substances can be painful unless general anesthesia is
used.
injected in the immediate vicinity of a specific nerve to block its
fibers and allow the physician to observe the effect. If the effect is
beneficial, then repeating the injection with another longer-acting
agent should reproduce the effect. If the goal is to make the effect
even more long lasting or permanent, phenol is used for destroying the
nerve fibers.
alcohol into its fibers in a more regional distribution in order to
inhibit nerve transmission and, thereby, muscle contraction. Alcohol
nonselectively denatures proteins, and thereby affects axons, myoneural
junctions, and muscle fibers. Its use is painful and requires general
anesthesia. When successful, the effect of alcohol injection lasts for
a variable period, but usually approximately 6 weeks (129,130).
toxin (BTX, Botox), a neurotoxin produced by clostridia bacteria. When
delivered into or near sites of nerve arborization, it diffuses 2 to 3
cm from the delivery point, and blocks the release of acetylcholine
from presynaptic vesicles at the myoneural junction. Recovery of tone
results from the sprouting of new nerve terminals, and this process
peaks at approximately 60 days (131,132).
The agent is injected using a 23- or 25-gauge needle, usually without
local or general anesthesia. Topical anesthesia does not prevent the
muscle discomfort, but this discomfort rarely lasts more than 5
minutes. In general, when more than one muscle group is to be addressed
in a young child, this is done under conscious sedation or general
anesthesia. The muscles are located by palpation. To reach muscles that
are deep or difficult to localize, ultrasonography can guide placement
of a needle that is used for both stimulation of the target muscle and
injection of the botulinum-A toxin (133,134).
been agreed upon. It has been shown to be a safe technique, whose
effects on muscles begin to be seen in 12 to 72 hours, and usually last
for 3 to 6 months or sometimes longer (135,136,137).
Injections may be repeated after 2 or more weeks, and up to six
injections may be given at a site, until the desired response in muscle
tone reduction has been achieved.
resultant increase in antibodies may limit future clinical response.
This treatment is contraindicated in the presence of fixed-joint
contractures. It is most useful in very young patients (usually younger
than 6 years) when a limited number of muscles are involved (137),
and the surgeon wishes to defer surgical intervention until the child
is older, has a stabilized gait pattern, or other components of the
child’s problem can be more precisely identified and addressed.
equinovalgus foot deformities showed improvement in 5 of 6 treated
patients, and improvement in 2 of 6 placebo-injected patients (138).
A study of 14 children who had BTX treatment for equinus reported that
there was marked improvement (mean 6.7 months) in six of the children,
slight improvement in three, and no improvement in five (138).
Among the larger studies, one with 114 children has shown that, when
treated with BTX, equinus foot positions improved in 50% to 60% of
patients who had bilateral gastrocnemius injections (139),
and a randomized, double-blind, placebo-controlled study of 125
children who were given injections showed an average increase of
knee-extended ankle dorsiflexion of 19 degrees in the children treated
with BTX (140). Other studies have found this
treatment to be long lasting with few side effects, and as effective if
not more effective than serial casting in improving equinus gait (136,137). A combination of the two methods may be beneficial (141,142).
extremity, particularly in relieving tight elbow flexors and thumb
flexors. This has resulted in improvements in hygiene and cosmesis, but
not in fine motor function (143). Apart from
its effect on motor function, BTX is usually effective in relieving
pain caused by muscle spasms. Unanswered but essential questions are
-
Will the expensive and temporary
reduction in muscle tone allow physical therapy efforts to successfully
avoid, rather than just delay, the need for surgery? -
What is the optimal dosage protocol?
-
What are the effects of BTX antibody formation and when do they occur?
-
Which specific aberrations and their controlling muscles are most appropriate for this treatment?
excitatory transmitters that cause spasticity (144).
When injected intrathecally, it acts on the synaptic reflexes of the
spinal cord to broadly decrease lower-extremity spasticity for
approximately 8 hours (2). In the upper
extremity there is less reduction of tone, about equal to that achieved
by selective posterior rhizotomy, with no change in the range of motion
of joints (144). Baclofen has poor lipid
solubility and does not cross the blood-brain barrier very effectively;
so, if given orally, large doses are required, and excessive lethargy
usually occurs. Dizziness and/or drooling may also result. Beneficial
long-term effects are realized when administered intrathecally with a
refillable, subcutaneously implanted pump. This provides a ten times
greater concentration in the spinal fluid than does comparable oral
dosing. In fact, a mere 1/30th of the oral dose given intrathecally
produces a similar or greater response (145).
Problems and complications from using the drug and pump are decreasing
as experience increases; they include headache, somnolence, seizures,
hypotension, infection, urinary retention, catheter breakage, spinal
fluid fistula, equipment malfunction, and the need for frequent
refilling with the medication. There is another critical risk factor
that one should be aware of. Interruption of the flow, whether because
of pump, dose, or tubing failure, can have serious and even fatal
consequences from severe withdrawal reactions.
In a study of 21 children treated for an average of 53 months with
baclofen, the Ashworth scale showed a substantial decrease in
spasticity. No functional change was detected by the GMFM or the
Pediatric Evaluation of Disability Inventory. Caregiver satisfaction
was high. One hundred and fifty-three treatment-associated adverse
events occurred (145). It appears that this
treatment may be most helpful for patients with spasticity or
spasticity plus dystonia who have hygiene problems or difficulty with
sitting (146,147,148),
or those who are ambulatory but have underlying weakness. This second
group cannot tolerate the additional weakness imposed by selective
dorsal rhizotomy. Although there is insufficient data on which to base
a recommendation for its use in specific patients, it appears that
intrathecal baclofen is of definite benefit in about one half of the
patients in studies thus far reported (2). It
may also be of some aid in assessing the potential benefit of selective
posterior rhizotomy. In the doses that are used for treating
spasticity, baclofen has no effect on athetosis (149).
Unlike earlier attempts to beneficially alter the central nervous
system by surgical or electrical means (e.g., cerebellar stimulation) (153),
this procedure has met with growing acceptance. The principle of
selective posterior rhizotomy is to reduce spasticity and balance
muscle tone by altering the control exhibited by the anterior horn
cells in the spinal cord. The normal inhibitory influences on the γ
efferent system, produced by higher centers in the brain and carried to
the anterior horn cell by long intraspinal tracts, are deficient in
cerebral palsy, as is the ability to coordinate movement as mediated
through the extrafusal fibers from the α motor neurons. Stimulatory
inputs from the muscle spindles in the lower limbs arrive through the
afferent fibers in the dorsal roots. Selective posterior rhizotomy
attempts to limit these inputs. This is done by sectioning only those
dorsal rootlets whose impulses exert excessive faciliatory influence on
the anterior horn cell, thereby arriving at a balance.
without relaxants. The lumbar laminae and the intervening ligaments are
removed as an en bloc laminoplasty from L1
to S1, preserving the facet joints. Then, individual posterior rootlets
from the L1 to S1 posterior roots are carefully dissected out and
electrically stimulated. The electromyographic and physical responses
are noted, and those rootlets that supply the target muscles are
surgically divided. Rootlets are spared if they show decremental and
squared-type electromyographic responses. They are divided if the
responses are incremental, clonic, multiphasic, sustained, or
contralateral, although this determination is not always easy. In most
cases, up to 70 rootlets per root are stimulated, and 25% to 50% are
sectioned. The laminae and ligament complex are then replaced. The
theoretical advantage of laminoplasty compared to laminectomy at each
level is that it is quicker and may result in less scarring in the
spinal canal. Also, replacement of the intact laminae may prevent the
occurrence of lumbar instability or deformity in some patients.
Laminoplasty will not prevent kyphosis in the thoracic spine.
-
spastic diplegic involvement
-
voluntary motor control
-
reasonable intelligence and motivation
-
no fixed contractures
-
good trunk control
-
the ability to walk with good underlying strength and balance
-
severe, pure spasticity
-
a history of having been born preterm or having low birth weight (154).
rigidity plus spasticity, and therefore may not respond as favorably to
selective posterior rhizotomy (2,155,156).
Availability of and coordination with postoperative physical therapy
are prerequisites for the procedure, because the patient may require
physical therapy three or four times per week for as long as a year to
regain strength. The procedure is not indicated in children with
athetosis, ataxia, rigidity, dystonia, antigravity muscle and truncal
weakness or hypotonia, overlengthened tendons, or severe fixed
contractures. Information regarding any benefits in those with spastic quadriplegia is limited (2). The results of unilateral rhizotomy in spastic hemiplegia are not available, but at least one source does not recommend it (157).
than before surgery and requires intensive physical therapy, but once
the acute rehabilitation phase is complete, improvement in the lower
extremities may be dramatic and perhaps lasting. Sometimes improvements
even in upper-extremity function, seizure control, bladder function,
swallowing, speech, and personality are seen after an overall reduction
in muscle tone (156). Results thus far show
lasting reduction in spasticity; increased range of motion of hip,
knee, and ankle; often a plantigrade foot in stance; increased stride
length and walking speed; and no increase in sensory deficits (157,158,159,160,161,162,163,164). Some randomized trials comparing rhizotomy with physical therapy have now been published (165,166,167,168). These indicate that rhizotomy is more successful in reducing muscle tone, and often in improving motor function as well.
does not affect contractures of joints or the overly contracted
musculotendinous unit. These must be treated by orthopaedic surgical
procedures after rehabilitation following the rhizotomy. It is
estimated that up to 65% of patients require additional procedures,
with those older than 4 years at the time of rhizotomy requiring more
orthopaedic procedures (169,170).
is not a miracle procedure, nor does it cure cerebral palsy. The
patient will still have poor motor control and balance, muscle
weakness, contractures, sensory involvement, and/or persisting
primitive reflexes if he or she did so preoperatively. A 1-year
follow-up study comparing selective posterior rhizotomy plus intensive
physical therapy with just intensive physical therapy alone reported
that ankle dorsiflexion, foot progression angle, and hip and knee
extension in stance were better in the rhizotomy group, but these
improvements were not associated with significant improvements in
functional gait (163).
some caveats. An occasional patient develops rapid and severe hip
subluxation or dysplasia after this type of rhizotomy (171).
Heterotopic ossification around the hip has been reported to occur
after varus derotation osteotomy of the proximal femur, in patients
with spastic quadriplegia who have previously had selective posterior
rhizotomy (172). Patients with preexisting
lumbar lordosis of more than 60 degrees on a lateral radiograph taken
in the seated position are at risk for the development of postoperative
lumbar hyperlordosis (173,174). Spondylolysis and spondylolisthesis have also been reported (175).
In a recent study of patients who had wide laminectomies with their
rhizotomies, 36% developed spinal deformities postoperatively with
scoliosis, lumbar hyperlordosis, thoracic hyperkyphosis, and
spondylolisthesis being the most common (176).
This may be contrasted with another report of 79 patients who had
rhizotomy with laminoplasty. Sixteen percent developed scoliosis and
12% had spondylolisthesis at mean follow-up of almost 6 years (177).
Another study noted that after rhizotomy, the only consistent negative
effect was some increase in sagittal plane anterior pelvic tilt in
independent ambulators (178). Perhaps the most
common problem after rhizotomy, other than weakness, is the development
of planovalgus foot deformities, which are reported in up to 50% of the
cases (178,179). The deformity is often severe enough to require surgical correction.
several decades are not yet available, but thus far an increased risk
of developing scoliosis or kyphosis has not been reported. Another
question remaining to be answered is whether late neuropathic
arthropathy will occur, but this seems unlikely.
for the patient, the family, and the professional staff. Although there
is little risk involved, careful patient selection and surgical
technique are mandatory, the procedure is expensive, and long-term
results are not known. It may be of benefit to some children. On the
basis of experience in several centers, however, its use is decreasing
in the United States.
cerebral palsy, some generally well accepted and some not. The brain
lesion affects motor function in four major ways, with individual
variation in the involvement of each. These are
-
loss of selective motor control and dependence on primitive reflexes;
-
abnormal muscle tone that is influenced by posture, position, or movement;
-
muscle imbalance between agonists and antagonists;
-
impaired body-balance mechanisms.
decades to modify the central nervous system, and to tackle substantial
motor dysfunction by external physical means. The goal is to produce
major improvements in voluntary control, tone, and muscle and body
balance. In general, most therapists work in a cephalocaudal sequence
to try to establish head control, then trunk balance for sitting, then
reciprocal lower-extremity mobility for crawling, pulling to stand,
and, hopefully, functional walking. The treatment methods include
neurodevelopmental therapy, sensory integration therapy, patterning,
conductive education, pressure-point stimulation, bracing, stretching,
and many recreation-based therapies (180). For
any of these techniques to succeed, the brain probably has to be
modified or reprogrammed to make new connections that function almost
as well as the original ones (2), and this may not be possible.
study, is that of using low-intensity transcutaneous electric
stimulation in an attempt to strengthen weaker antagonistic
lower-extremity muscles. The treatment is applied only at night. In a
small group of patients, some motor improvement was noted, but was lost
after the stimulation was withdrawn (181). More investigation of this approach is necessary before its clinical relevance is determined.
There is considerable difference of opinion about which modalities
should be employed, at what age, how often (from once per week to
virtually continuous therapy for 18 hours a day), by whom, and how the
results are to be measured, documented, and assessed. Some published
studies suggest that in children with cerebral palsy, certain physical
therapy programs may improve contractures of joints, motor status, and
social motivation (5,186,187), whereas others suggest that they do not (183). Although short-term improvements are reported, long-term carryover is rare.
heterogenous samples, nonrandomized treatment, lack of controlled data,
and attrition of patients during many of the studies (183).
In many instances it is difficult or impossible to determine whether or
not the therapy was of benefit, or whether the reported improvement in
motor skills was the result of the child’s obligatory neurologic
maturation and the learning of substitution patterns and coping
methods, completely independent from any restructuring of neurocentral
control patterns. For some children, certain therapy programs may make
a difference. In this context, it is important that motor function be
documented periodically by the therapist during the child’s early
growth years. This can be done using such systems as the Movement
Analysis of Infants, the Gross Motor Performance Measure, Pediatric
Evaluation of Disability Inventory, and WeeFIM (190,191).
that focuses on compensation techniques during the first 3 years of a
child’s life is helpful. Such a program demonstrates to parents how
positioning can facilitate mobility and maintain the range of motion of
the joints, and educates parents about cerebral palsy in general. The
physical therapist attempts to set out benchmarks for appropriate motor
milestones within the capabilities of the particular child. The child
is assisted through the progression of milestones: rolling over,
crawling, standing, and eventually walking, while maximizing strength,
balance, and flexibility of the joints. Spasticity in the growing child
prevents the development of normal muscle growth and length, and the
therapist’s attempt is to approximate normalcy to the extent possible.
handicapped children is the dependence of the child on the mother for
accomplishing the activities of daily living. A therapy program that
teaches mothers easier ways to work with their child is of great value (192).
much of the physical therapy, and not rely totally on the physical
therapist (5). This saves professional fees,
minimizes trips to the therapist and interference with school time, and
positively involves parents and even siblings with the child. Many
parents, however, decline such involvement because of other family or
career demands, or simply because they are too tired to commit the time
and effort that are necessary.
expensive and time consuming, and generate hopes and expectations for
all involved. If there is no benefit, or if ineffective modalities are
employed, the therapy process can raise false hopes, increase
frustrations for the child and the family, waste large sums of money,
and sometimes unbalance interactions among the members of the family
unit (5,180).
rehabilitative physical therapy is not only helpful but also usually
essential in order to maximize the benefits of most types of
orthopaedic surgery in cerebral palsy. The physical therapist and the
patient must work together to overcome the early postoperative
weakness, stiffness, and discomfort. The goals are to maintain or
improve the range of motion of the joints, regain preoperative muscle
strength, maximize ambulation, and improve overall functioning to the
extent possible. How long should the postoperative therapy continue,
and how frequently should it be done? This varies with the scale of the
surgery. It may be necessary for as short a period as 1 or 2 weeks, or
as long as several months. The family may be able to provide the
treatments on a daily basis. If all treatments must be provided by the
therapist, two or three times per week is standard.
prevents contractures of joints by ensuring the supervision of a daily
range-of-motion program for those who lack the motor strength and
voluntary control to maintain the range of movements by themselves.
This type of program can be done by a parent, an aide, or a caretaker,
and does not require the services of a physical therapist, other than
in developing and monitoring the program. As previously noted, however,
there are no data to validate the benefit of such a program. In some
severely involved children, it is impossible to prevent contractures,
no matter how aggressive the treatment.
physical therapy. Although some parents want and even demand therapy
two or three times a week throughout the lifetime of their child, there
is no evidence that any type of physical therapy can have a beneficial,
lasting effect on motor function beyond early-to-middle childhood (age
4 to 8 years). Children older than this no doubt benefit more by
devoting their time (and their families’ and society’s resources) to
the development of communication, cognitive, and recreational skills,
instead of endless therapy sessions.
great benefit to the child and family. She or he is a resource person,
and sometimes helps out in
-
advising on adaptive and therapeutic equipment, including seating systems;
-
fabricating some types of splints and basic orthotics;
-
educating the family about cerebral palsy and the child’s deficits and potential;
-
advising the family regarding modifications in the home using community resources;
-
acting as liaison with the school;
-
being a realistic, supportive health care professional (193).
pathway between the therapist and the physician. The therapist is often
the primary person who documents the child’s neuromotor status,
monitors progress, and may recommend surgery or other treatments.
preventing deformity, improving function by substituting for a weakened
muscle, or protecting a weakened part. In cerebral palsy, they are most
commonly used for
-
stabilizing feet, ankles, and knees;
-
maintaining nighttime hip abduction to prevent subluxation;
-
possibly slowing the progression of spinal deformity, permitting beneficial growth and improving sitting balance;
-
night splinting to prevent hand and wrist deformity.
of the lower extremities in cerebral palsy and in developing strong,
lightweight, comfortable new materials for orthoses. Lower-extremity
orthotics will almost never have to extend above the knee with patients
who have spastic diplegia (194). For foot
control, the University of California Biomechanics Laboratory (UCBL)
orthosis is most popular. It can maintain forefoot, hindfoot, and
subtalar alignment in a supple (not rigid) foot, but cannot control the
ankle.
including the solid-ankle type, which controls the entire foot,
provides medial–lateral ankle stability, and maintains the ankle in a
rigid plantigrade position to preserve the plantar flexion–knee
extension couple. The solid-ankle AFO is the best choice for the
spastic foot, and can be used in almost any instance. AFOs facilitate
standing balance by counteracting the tendency toward equinus (195,196).
Modifications to the solid-ankle AFO have been made in order to improve
foot and ankle function. An example is the posterior leaf-spring AFO,
which does not provide mediolateral ankle stability, but allows some
plantar flexion and dorsiflexion from neutral while preventing foot
drop (197). This type is used when spasticity
is minimal. It stores minimal kinetic energy during dorsiflexion, and
gives back minimal energy at push-off.
stops, which prevent equinus and excessive extensor thrust while
allowing free dorsiflexion during gait. They also allow more tibialis
anterior muscle function (198). Theoretically,
articulated AFOs should allow for better dorsiflexion activities, such
as bending over and stair climbing; however, they provide less ankle
support, are bulky, do not fit into shoes well, and are more likely to
cause heel irritation. They may also prevent a needed plantar
flexion–knee extension couple, and may allow triggering of the
gastrocnemius stretch reflex, causing the patient to fight the brace (190).
Most physicians are of the opinion that the hinged AFO is indicated
only for preventing recurvatum of the knee in the mid-stance phase of
gait. Tone-reducing features molded into AFOs have not been shown to be
of any benefit (199).
there are several varieties. It uses the plantar flexion–knee extension
couple to prevent knee-flexion crouch and gain appropriate stance-phase
knee extension during gait. This is possible provided the foot is
plantigrade, no significant knee-flexion contracture is present (<
10 degrees), hip extension is full, and no major rotational
malalignments are present in the limb. This approach has essentially
eliminated the need to ever prescribe a knee-ankle-foot orthosis (KAFO)
in an ambulatory child with cerebral palsy. The main indication for
KAFOs is to brace the lower limbs for ease of transfer in a
nonambulatory patient.
musculotendinous units or joint capsules can sometimes be accomplished
by gentle, nonpainful, repeated passive stretching, followed by
maintaining the correction with casts, splints, or adjustable orthotic
devices. This method of treatment has the potential to cause pain to
the child, and to raise false hopes for improvement when used in
patients with more-than-mild spasticity, tightness, or contractures, or
in those who are unable to accurately communicate their discomfort.
casting can sometimes improve, at least temporarily, contractures of
the ankle, knee, elbow, wrist, or hand (200).
The process may be repeated from every few days to once a week; various
casting materials, dropout casts, removable splints, and orthotics with
dial-lock or ratchet hinges may be used. The use of nerve or muscle
blocks as an adjunct may sometimes be worthwhile.
has been employed for several years. This treatment is applied to the
lower extremities by carefully molding bilateral plaster below-knee
casts in an attempt to inhibit certain normal tonic plantar reflexes,
especially grasp, and therefore reduce overall lower-extremity tone. A
probable added benefit of this treatment is stabilization of the foot
and ankle by the well-molded cast. The casts are either left in place
or changed weekly for 3 or 4 weeks, during which
time
intensive physical therapy is performed. Hinged AFOs are then
substituted for the casts. This method has been successful according to
some reports (201,202,203,204),
but because the noted improvement may not be maintained for more than a
few months, others have not considered it to be of value (205).
altering the structure and functioning of the musculoskeletal system in
children with cerebral palsy. It is almost always the only effective
means of treatment when fixed myostatic contracture exists. Lengthening
a muscle also weakens it. This may help with muscle balance around a
joint. Strengthening an antagonist muscle may still be desirable, but
difficult or impossible to achieve. In most cases, the muscles that
contract eccentrically, producing deceleration (e.g., hamstrings) and
shock absorption, are the most appropriate for lengthening, and loss of
function rarely occurs. Concentric or accelerator muscles (e.g.,
gastrocnemius) initiate, rather than modulate, movement, and may be
substantially weakened by lengthening. In these situations, as with the
iliopsoas muscle, it may be better to intramuscularly lengthen the
tendinous portion and accept some residual contracture in exchange for
preserving needed strength.
delay in the development of normal gait patterns contribute to skeletal
abnormalities that interfere with efficient walking. Internal tibial
torsion and femoral anteversion, both normal in early development and
self-correcting with normal positioning and mobility, tend to persist
in the young spastic diplegic patient. In the older patient, long-term
difficulties with foot clearance actually lead to an exaggerated
remodeling of tibial torsion into an excessive external position.
Correction of musculotendinous length and reduction of spasticity in
the presence of persistent skeletal malrotation is insufficient.
children with cerebral palsy who had no treatment show that in most
cases gait function decreases over time. This includes temporal and
stride parameters and kinematics. Children with cerebral palsy who have
orthopaedic surgery do improve their gait (206).
The subsequent text contains recommendations for the treatment of the
most common problems and deformities in an ambulatory child with
spastic diplegia.
in the disturbed gait. The best results are obtained from surgery only
if all of the abnormalities are identified preoperatively and corrected
during the same surgical setting (207,208,209),
thereby ensuring that only one rehabilitation period is required.
Little or no benefit is derived from simply performing one procedure,
such as a triceps surae lengthening, and waiting to assess its effect
on other abnormal elements of the child’s gait before proceeding to
correct them. This strategy does not improve the final result, and
results in unnecessary discomfort, hospitalization, and rehabilitation.
problems. Ideally it would be after the gait pattern has stabilized,
which is approximately the age of 4 or 5 years in nonafflicted
children, and either the same or later in children with cerebral palsy,
but before the age of 8 years. Surgery improves walking velocity and
stride length in young children, but functional outcome measures such
as the GMFM usually show minimal change (210).
Certainly surgery can be successful in older children and adults. In
children of ages 6 to 15 years, a recent study showed that multilevel
surgery in spastic diplegia improved sagittal plane kinematics and
power generation at the hip and ankle (211).
Some components of gait, such as velocity, are not reliably improved in
older children, especially after the age of 12 years (212).
In cases in which the hips are at risk for dislocation, or when there
is progressive or substantial acetabular dysplasia, surgery should not
be delayed, regardless of age.
concerns relating to the orthopaedic surgical procedures are pain
management, relief of superimposed myospasms, and minimization of the
child’s anxiety. Giving the analgesic medication through the existing
intravenous access line, using patient-controlled analgesia when
possible, routinely administering diazepam for 48 to 72 hours to lessen
myospasms, providing continuous caudal analgesia through a small
catheter, and having kind, supportive personnel present are most
helpful to the child.
restoration of motion in the joints, muscle strength, and improved gait
as rapidly as possible. During this period of up to several months, it
may be beneficial to use night splints for comfort and to prevent
positioning of the joints in positions that could contribute to
recurrent contractures. Such splinting should never exceed the level
that can be comfortably tolerated by the child. In this regard, it is
sometimes necessary to alternate control of one ankle and the opposite
knee on successive nights, or use splinting only during the day, when
it can be effectively monitored. The value of night splinting is not
universally accepted, and valid well-controlled studies proving its
efficacy are lacking. It is expensive and can be burdensome and
uncomfortable for the patient because of ill-fitting splints, the
frustration of substantially restricted mobility, or maintenance of a
continuous overstretch on muscles.
may begin within a few days postoperatively. If osteotomies or subtalar
arthrodeses were performed, partial weight bearing may begin as early
as 3 weeks postoperatively, assuming that the internal fixation is
adequate. Full, unrestricted weight bearing should probably be deferred
until radiographic evidence of adequate early bone healing is seen,
usually by approximately 6 weeks. In patients with spastic diplegia it
takes at least 3 months, and sometimes longer, to regain the
preoperative level of strength after most multiple lower-extremity
surgical procedures.
have problems with musculotendinous spasticity or contracture and
skeletal deformity extending from the pelvis down to the foot. Although
not every child requires correction of every deviation, the majority
will benefit from multiple tendon and/or fascial lengthening and bone
derotations. It is useful to consider what may be necessary in the
“classic diplegic” child at the level of each joint by understanding
the difference between primary causes, secondary deviations, and
compensations. Computerized gait analysis provides objective
documentation of what can be very complex gait deviations in multiple
planes of motion, having significant impact in the determination of
appropriate surgical procedures at each joint level (Table 15.4).
(60% of the gait cycle) and swing phase (40%). In the initial and
terminal 10% of stance both feet are in contact with the ground (double
support), whereas during the middle 40% single-limb stance occurs
(single support). Stance phase accomplishes the functions of load
acceptance, joint segment length modulation, forward progression of the
body over a stable plantigrade foot, and propulsion of the limb into
swing phase. Swing phase allows for the advancement of the limb
forward, while maintaining clearance of the foot in the face of
gravity. The phases of the gait cycle have been further divided into
loading response, midstance, terminal stance, preswing and initial
swing, midswing, and terminal swing (213). This
terminology is useful for describing abnormal gait patterns with
specific reference to the part of the gait cycle in which they occur.
It is also important for the physician to understand the sequential
firing patterns of normal muscles during gait (Fig. 15.8).
planes of motion: coronal, sagittal, and transverse. In normal gait,
most motion occurs in the sagittal plane. In the normal child, minimal
excursions of pelvic motion occur throughout the gait cycle, because
there is successful maintenance of length by the locomotor segments
managed by hip, knee, and ankle motion during stance phase (Fig. 15.9).
This allows for the minimal expenditure of energy during normal gait. A
minimal expenditure of energy is described as one of the prerequisites
of normal gait, which are
-
appropriate foot prepositioning for initial contact,
-
stance-phase stability,
-
swing phase clearance,
-
adequate step length, and
-
maximization of energy conservation (213).
motor control of the two joint muscles causes dysfunctional rotations
of the joints, excessive pelvic and trunk excursions in the coronal,
transverse and sagittal planes, and therefore inefficient energy
conservation (Fig. 15.10) (2).
understood with the use of computerized three-dimensional gait analysis
techniques. A number of commercial systems are now in place worldwide
and are used for both clinical and research purposes. Modern gait
analysis systems include collection and computation of
three-dimensional kinematics (joint and segment motion) and kinetics
(joint moments and powers), surface and fine wire electromyography
(EMG), and foot pressure measurement (pedobarograph). Motion data
relating to joints are collected by multiple cameras which determine
the three-dimensional coordinates of reflective markers placed on
specific limb and joint landmarks (Fig. 15.11).
Kinetic data are computed from the collection of simultaneous position
(kinematic) and force plate data. Force plates, which measure the
ground reaction forces, are embedded in the walking surface.
Electromyographic data are also recorded from surface or deep muscles
during gait and standing as an adjunct to the assessment of abnormal
muscle tone. Typical protocols used in clinical gait analysis have been
well documented (214,215).
Gait data supplement a detailed physical evaluation of passive range of
motion of joints, muscle strength, balance, and spasticity. Examination
of recent pertinent radiographs (spine, hip, knee, foot, and alignment)
may also be necessary. The interpretation of gait analysis data
requires considerable training and background knowledge of the
methodologies used to collect and compute the data. Dedicated and
experienced professionals are required in order to assure high quality
gait data and interpretation.
physicians added gait analysis data to their own clinical evaluations,
they changed their surgical recommendations in approximately 50% of the
cases, with more decreases than increases in procedures (216).
Whether this will improve surgical outcomes and reduce costs has not
been well studied. Although preoperative gait analysis is theoretically
desirable, it is neither possible nor necessary for every child who
undergoes surgery. Nevertheless, every surgeon who treats children with
cerebral palsy should have a good knowledge of normal and pathologic
human gait and understand gait analysis and pattern recognition (217). The application of his or her observational gait assessment will then be more accurate.
watching the child walk, viewing the gait from the front, back, and
side. One component should be studied at a time (e.g., cadence, stride
length, the foot, the knee, the hip, lower-extremity rotational
alignment, the pelvis, the trunk), recognizing that often there are
differences between the sides. Gage has described observational gait
analysis in detail (2). For a given child, intraobserver observations are more accurate than interobserver observations (218).
In either situation, the opportunity to study a videotape of the gait
from the front, back, and sides, with zoom capability for the feet,
increases the accuracy of the assessment.
|
TABLE 15.4 SUMMARY OF THE TYPICAL GAIT PROBLEMS, PRIMARY CAUSES, AND TREATMENTS FOR THE CHILD WITH SPASTIC DIPLEGIA
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
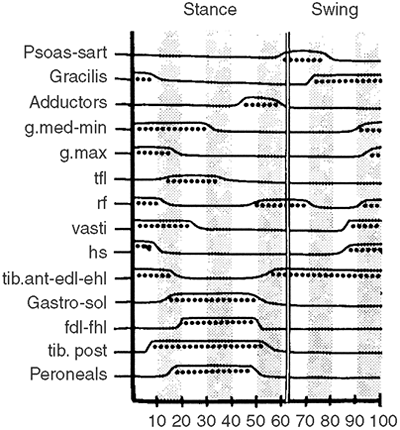 |
|
Figure 15.8 Chart of normal muscle-firing patterns (dotted lines) for the lower extremities. These normal data are used for comparison with the patterns in a child with cerebral palsy.
|
gait cycles of the walking child, look for the priorities of gait and
deviations from normal, and correlate this information with the
findings on static physical examination. If all this information is
consistent with a recognized standard pattern (e.g., one of the types
of hemiplegia or a diplegic gait with equinovalgus feet, tight
hamstrings, tight hip flexors and adductors, and increased femoral
anteversion), there is a stronger likelihood of success after applying
the proper surgical procedures to address each problem.
every ambulatory cerebral palsy patient preoperatively if cost and
accessibility to sophisticated gait labs were not a problem. In
reality, surgeons will be quite comfortable without gait analysis when
treating those patients who have apparently straightforward patterns of
gait pathology (Fig. 15.12). Nevertheless, gait
analysis provides a more precise definition of the gait abnormality in
patients with more complex conditions, such as those with severe
diplegia and those who have had previous surgery with little success.
Gait analysis is very valuable is distinguishing children who have
dyskinesia from those with spasticity; this is especially important
because surgical results are much less predictable in dyskinesia (219).
Finally, gait analysis provides an objective documentation of the pre-
and postoperative gait when assessing the results of surgery.
palsy, most likely because of a combination of muscle imbalance with
adductor hallucis overactivity and externally applied forces, such as
inadequate clearance resulting from equinovalgus deformity of the foot
and ankle, forcing the phalanges of the great toe into valgus (220,221).
Equinus, and particularly valgus, of the foot must be corrected first
in order to achieve a lasting good result from hallux valgus surgery in
cerebral palsy.
difficulty with wearing proper shoes; rarely is cosmesis alone an
indication. There are myriad possible operations for the correction of
hallux valgus, and most have been used with varying degrees of success
in cerebral palsy. When spasticity is very mild, such procedures as
ostectomy, capsulorrhaphy, adductor hallucis release or transfer, or
proximal or distal first metatarsal osteotomy can be successful (222,223,224,225,226). With recurrence, or with patients who have more spasticity, the McKeever metatarsophalangeal arthrodesis (227),
with the joint in a few degrees of valgus and 10 degrees of
dorsiflexion relative to the floor, is an excellent procedure, as
confirmed by long-term follow-up studies (221,228).
At initial contact, which is a heel strike in the normal child, the
ankle is in slight equinus. During first rocker it moves into further
plantar flexion as the foot is lowered to the walking surface under
control of an eccentrically contracting anterior tibialis. During
second rocker the tibia advances forward over the plantargrade foot as
the gastrocsoleus eccentrically contracts. The ankle dorsiflexes
approximately 10 degrees. Third rocker occurs in terminal stance and is
the push-off generated by concentric contracture of the gastrocsoleus.
In early swing the ankle is in 20 degrees of plantarflexion, and the
pretibial muscles fire concentrically to dorsiflex the foot for
clearance. These rockers are evident in normal kinematic and kinetic
plots (Fig. 15.13).
rockers. Spasticity of the gastrocsoleus or weakness of the anterior
tibialis (foot drop) prohibits first rocker and causes an initial toe
contact. As the tibia advances forward, the stretch on the
gastrocsoleus may trigger a spastic response and interfere with second
rocker. This creates an excessive plantar flexion–knee extension couple
that can actually force the tibia backward and the knee into true or
relative hyperextension. Third rocker is frequently diminished as the
range of motion available for the gastrocsoleus to function is
shortened in the spastic child. Functional problems include tripping
because of poor foot clearance and instability due to a limited base of
support afforded by the toe position.
-
Reduced ankle dorsiflexion in stance and swing
-
Plantar flexor moment throughout the stance phase
-
Double bump kinetic pattern in stance phase
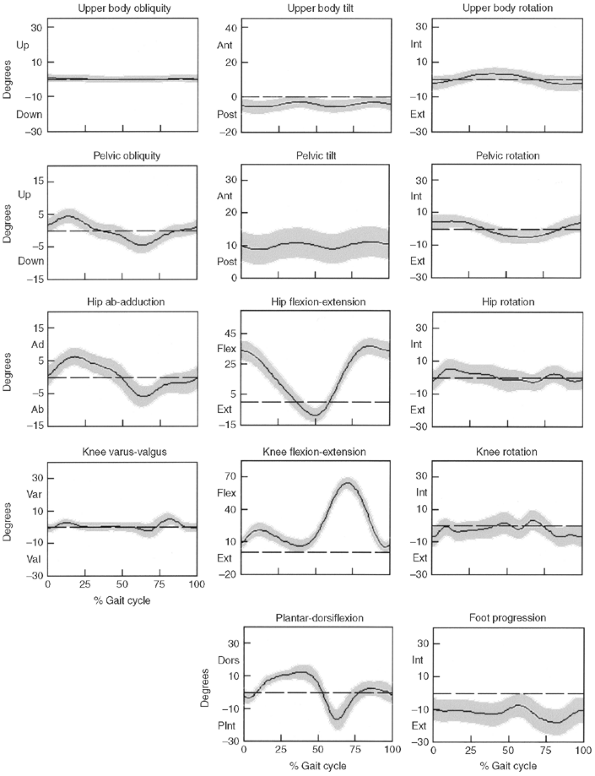 |
|
Figure 15.9
Normal (mean +/- 1 standard deviation) three-dimensional kinematics for the upper body, pelvis, hip, knee, and ankle in the coronal (first column), sagittal (second column), and transverse (third column) planes. |
In the spastic diplegic child, there is frequently a positive
Silfverskiold test (229), with better ankle
dorsiflexion present when the knee is held in 90 degrees of flexion
than when the knee is extended fully. This implies that the
gastrocnemius is shortened more than the soleus. Some believe that this
situation is best addressed with a selective gastrocnemius fascial
lengthening rather than a tendon lengthening. However, both procedures
have been shown to be effective (230,231,232).
motion of the ankle may not correlate well with ankle motion during
gait because: (a) increased spasticity during gait further limits the
range, (b) the effect of body weight,
rather
than merely the force applied by the physician during passive clinical
examination, results in increased range of motion during gait, and (c)
the correct positioning of the foot during clinical examination may not
occur during gait. As a result, the ankle–sagittal plane kinematic is
the best way to determine dynamic function of the ankle, that is, the
range of motion the ankle is functioning within during gait.
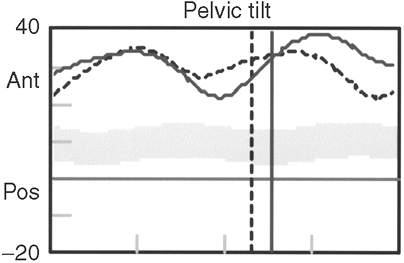 |
|
Figure 15.10 Sagittal plane motion of the pelvis in a normal child (gray band), and in a child with spastic diplegia presenting with a typical double-bump pelvic tilt pattern.
|
control the subtalar joint in the child who has a tendency toward pes
planovalgus. If not, the amount of dorsiflexion may be overestimated (Fig. 15.15).
The ankle should be evaluated for the presence of clonus and the
ability to dorsiflex actively. Good ankle dorsiflexion control is a
favorable factor in predicting the ability to become brace-free
following release of ankle plantar flexor contracture.
 |
|
Figure 15.11 Patient with cerebral palsy during a motion data collection pertaining to the trunk, pelvis, and lower extremities.
|
 |
|
Figure 15.12
An example of a common spastic diplegic gait pattern. Note the ankle equinus, knee flexion, hip flexion, internal rotation of the entire limb, and compensatory lumbar lordosis. |
gait in a child to pay attention to the knee and its impact on foot
positioning; that is, crouch gait with a neutral ankle position will
result in a toe contact pattern. The most common misinterpretation of
visual observation of gait is to assume that a toe walker has increased
plantar flexion at the ankle. Kinematic data will clarify the presence
of excessive equinus.
varus, is not common. It is the result of overactivity or contracture
of the triceps surae group, which is normally six times stronger than
the ankle dorsiflexors (233).
Most of the overactivity is in the gastrocnemius muscle. The soleus is
usually not the major problem. The equinus deformity can be treated by
serial manipulation and casting, which is most successful with patients
who are young, have less spasticity, and whose equinus is mild and
dynamic, that is, equinus at initial contact, passively correctable on
physical exam. Recurrence of the equinus deformity after several months
is not uncommon after manipulation and casting, but in one study
dynamic equinus remained corrected for 3 years after casting (234).
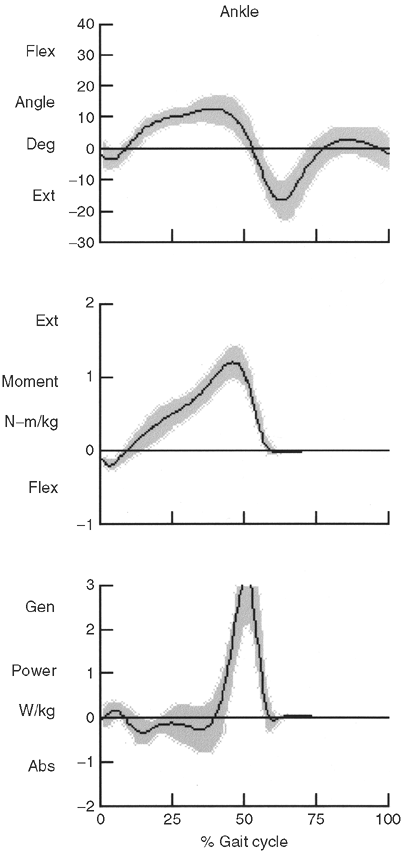 |
|
Figure 15.13 The normal (+/- one standard deviation) sagittal plane kinematic and kinetic data (showing moment and power) for the ankle.
|
injection of botulinum-A toxin into the gastrocnemius. This will not
help in fixed myostatic contracture, but can improve or correct equinus
temporarily in mild cases. Mostly, it simply delays the need for
surgery. Further study is necessary in order to determine whether some
patients will achieve permanent correction, and if so, whether such
patients can be identified more consistently prior to administering the
injection.
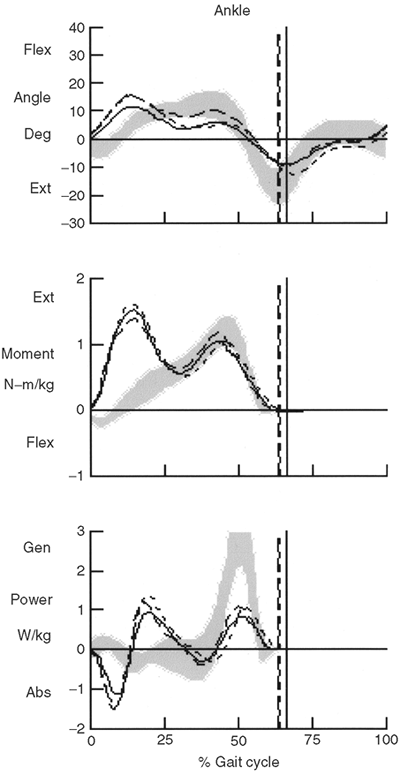 |
|
Figure 15.14
Sagittal plane kinematic and kinetic (moment and power) data for a child with an equinus gait pattern and associated toe initial contact (due to excessive knee flexion) and premature plantar flexion in midstance (related to spasticity of the ankle plantar flexors). The kinetic pattern is referred to as double bump ankle pattern. |
elongation of the gastrocnemius unit, which, if performed as an
isolated procedure, can usually be done as outpatient surgery (235).
Elongation is most commonly done by lengthening the Achilles tendon
(which also lengthens the soleus), by multiple partial tenotomies, the
Hoke triple-cut, or White double-cut (236,237) techniques (either percutaneously or open), or by an open step-cut lengthening. When percutaneous techniques are
employed, care should be taken to avoid completely dividing the
Achilles tendon. Some surgeons have advocated methods to quantify the
exact extent of lengthening necessary (238,239). These methods have not gained wide acceptance.
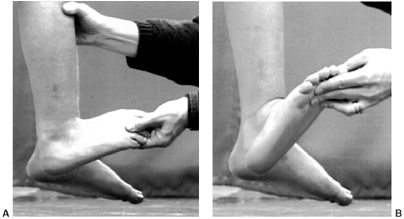 |
|
Figure 15.15 Comparison of passive ankle dorsiflexion range with (A) and without (B) correction of hind foot position and forefoot alignment.
|
fascial division type of lengthening of the gastrocnemius aponeurosis
alone. This method has the advantage of not only preserving but
actually generating more soleus strength for push-off (215). Whether it results in a higher recurrence rate is debatable (244).
Similar results have been reported in studies comparing tendon
lengthening with muscle lengthening in the treatment of equinus
deformity (245,246).
Another method involves transverse complete tenotomy at the
musculotendinous junction while preserving the peritenon envelope. In a
group of children of ages 3 to 8 years, ultrasonographic studies showed
that all the tendons were reconstituted and no overlengthening occurred
(247).
anteriorly (i.e., closer to the talus) on the calcaneus to decrease its
power by decreasing its lever arm (248,249,250).
This procedure is more complex than the tendon lengthenings, and has
not been demonstrated to be superior. Its best results occur when it is
combined with other procedures (246).
dorsiflexion, which is maintained in a below-knee cast for 2 to 3 weeks
if the gastrocnemius aponeurosis alone has been lengthened or an
incomplete Achilles tenotomy (e.g., the Hoke technique) has been done.
The below-knee cast is left in place for 4 to 6 weeks after step-cut
lengthening of the Achilles tendon. Recent data suggest that there are
no ill effects if the casting is limited to only 3 weeks following
percutaneous lengthening of the tendo Achilles (251). Postcasting, an AFO may be required long term if there is inadequate ankle dorsiflexor strength to prevent foot drop.
result is usually good. Gait analysis studies have shown that ankle
plantarflexor lengthening is successful in improving ankle function
during walking without reducing ankle push-off strength (252,253).
There does not appear to be a difference in outcome as regards ankle
kinematic or kinetic function when comparing gastrocnemius and tendo
Achilles procedures in the child with cerebral palsy.
of a calcaneus deformity caused by overlengthening the heel cord,
particularly in a patient with athetosis. Overlengthening is seldom a
direct result of plantarflexor surgery, but rather, it can develop over
time in patients in whom associated knee-flexion contractures have not
been addressed. Treatment for an overlengthened heel cord is difficult,
but some have had success with shortening the Achilles tendon by
surgical reattachment or imbrication, and Tardieu and Tardieu suggest a
period of casting the ankle in plantar flexion, followed by orthotic
control at plantigrade during the day and in equinus at night (254).
of the child at the time of surgery; lower rates are seen in older
children. Before the age of 4 years, the recurrence rate is
approximately 25%, whereas it approaches zero when the surgery is done
after the age of 8 years (251,255). The presence of associated knee-flexion contracture may also contribute to recurrence (256).
Some are of the opinion that, after surgical lengthening, it is
beneficial to control the ankle with a night splint and an orthosis in
order to prevent recurrent deformity and improve functioning (257).
Others have shown that orthotics do not prevent the recurrence of deformities (258).
A reasonable approach is to use an AFO to improve functional stability
or control weakness in those who need it, and to reserve night
splinting for those who show signs of early recurrent tightness. If the
patient has voluntary active foot dorsiflexion to more than 10 degrees,
there is a good likelihood that an AFO will not be necessary.
It is most often seen in hemiplegia, but may also occur in children
with diplegia and quadriplegia. The hindfoot varus is most likely
caused by overactivity of the tibialis posterior muscle, whereas varus
and supination of the forefoot are more likely the result of
overactivity of the tibialis anterior, which may also contribute to
hindfoot varus. Peroneal muscle weakness may also be a factor.
pre-positioning for initial contact as well as with stability in stance
phase. It frequently predisposes the patient to poor clearance in swing
phase. During stance, weight bearing forces are shifted to the lateral
aspect of the foot, causing pressure areas and pain along the fifth
metatarsal, stress fractures, and inversion instability of the ankle.
This can also make orthotic wear difficult.
equinus, as described above, must be combined with lengthening or split
transfer of the tibialis posterior tendon. Lengthening may be by the
step-cut method or by intramuscular tenotomy cephalad to the
musculotendinous junction. This is also known as the Frost procedure (194). Some surgeons prefer to split the tendon and transfer the lateral half to the peroneus brevis tendon (259,260)
in order not to weaken the muscle as much as a lengthening would.
Others believe that some weakening is desirable. There are reports of
good results with the split transfer, and many indicate that
preoperative gait analysis is not necessary for a successful outcome (261).
However, combining a split posterior tibial tendon transfer with a
distal tibial derotational osteotomy may not be wise. A recent study
reports that 40% of the patients subsequently developed a severe rigid
planovalgus overcorrection foot deformity (262).
tendon through the interosseous membrane to the dorsum of the foot has
been advocated (263,264),
it should not be done in patients with cerebral palsy. This transfer
can result in a calcaneovalgus deformity that is very difficult to
correct (265).
overactivity of the tibialis anterior muscle, a split transfer of the
lateral half of its tendon to the cuboid bone usually successfully
balances the foot (266). DeGnore and Greene use
hindfoot varus, occurring in the swing phase of gait, and a positive
confusion test as their indications for split anterior tibialis tendon
transfer (267). However, the validity of the
confusion test has been questioned because it does not predict ankle
kinematics in the swing phase of gait (268).
and supination of the forefoot. In this situation, split anterior
tibialis tendon transfer, combined with posterior tibialis tendon
lengthening, is appropriate. Because there is potential for different
combinations of muscle activity and contracture to lead to a similar
abnormal motion of the foot and ankle, EMG of the posterior tibialis is
the only way to know for sure if this muscle is contributing to the
equinovarus deformity during gait or not (Fig. 15.17).
varus is rigid, bony surgery is necessary in addition to addressing the
triceps surae and tibialis posterior units (269).
This can be accomplished by a laterally based, closing-wedge osteotomy
of the calcaneus with staple or screw fixation, but this is technically
difficult to reduce and adequately fix. It is easier and very effective
to obliquely divide the calcaneus in the coronal plane, posterior and
parallel to the peroneal tendons, slide the posterior fragment
laterally, and fix it with a cannulated or cancellous screw or pins. We
have not seen plantar nerve injury occurring with this technique.
Assuming adequate internal fixation with
either
procedure, a below-knee cast is necessary until bone healing has
occurred (usually 6 to 8 weeks). Subsequent orthotic control of the
foot and ankle may or may not be necessary. For a severe, rigid
deformity in an older person a triple arthrodesis is effective.
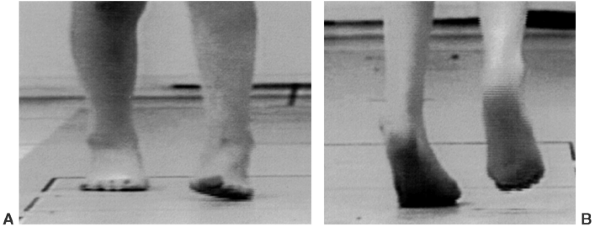 |
|
Figure 15.16 Example of a left equinovarus deformity during initial contact (A) and midstance (B).
|
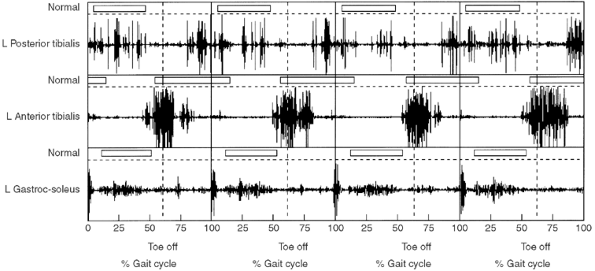 |
|
Figure 15.17
Example of electromyography (EMG) output for the posterior tibialis (intramuscular electrode), anterior tibialis (intramuscular electrode), and gastrocsoleus (surface electrode) for four gait cycles. Abnormal activity is noted in the posterior tibialis during terminal swing. Premature termination of activity is noted for the anterior tibialis in terminal swing. |
diplegia, followed in frequency of occurrence by equinovarus and
calcaneus, both of which have an approximately equal prevalence (270) (Fig. 15.18).
The cause is probably muscle imbalance, triceps surae overactivity,
weakness of the tibialis posterior muscle, and relative overpull of the
peroneal musculature (271). The treatment of
equinus is as described in the preceding text. Spasticity in the ankle
plantarflexors limits ankle dorsiflexion and shifts the motion to the
subtalar and midfoot joints. In young patients with diplegia,
spasticity of the peroneals may contribute, but this tends to diminish
with time.
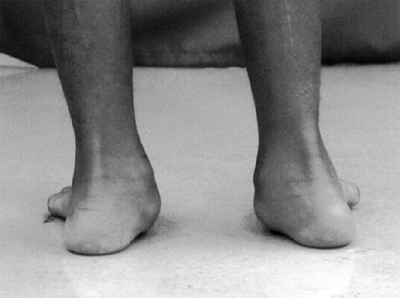 |
|
Figure 15.18 Bilateral posterior view of pes planovalgus.
|
foot is to cause painful pressure areas over the talar head medially.
This shift in foot pressure medially leads to the development, over
time, of a hallux valgus deformity. This can make the use of braces
difficult and thereby interfere with gait. Also, the loss of the
structural rigidity of the foot diminishes its ability to function as
an appropriate lever. This can contribute to a less effective
plantarflexion–knee extension mechanism and therefore causes an
exaggeration in knee-flexion position during stance (Fig. 15.19).
interpretation of this deformity. If care is not taken in
interpretation of ankle kinematics with such deformities, motion
occurring through the midfoot can be misinterpreted as ankle
dorsiflexion. Foot pressures, however, can provide interesting
information with respect to the location of high pressure points under
the foot; in the young child, however, such pressure points may not yet
have become evident through callus buildup (Fig. 15.20).
foot should be held in a position of inversion to reduce the subtalar
joint so that dorsiflexion through the midfoot is not confused with
ankle motion. Flexibility of the foot should be assessed (Fig. 15.21). A correctable pes planovalgus is managed differently from a rigid deformity.
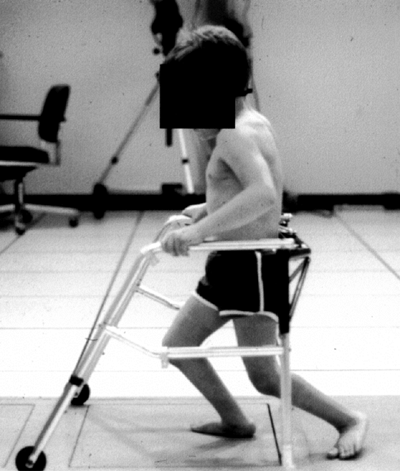 |
|
Figure 15.19 Pes planovalgus associated with external tibial torsion and crouch knee.
|
at the subtalar joint. It may be coming from the ankle, so
weight-bearing radiographs of the ankle should be studied as part of
the surgical planning. If the ankle valgus is really in the distal
tibia, it may be corrected by osteotomy or, in some cases, by
hemiepiphyseodesis, screw fixation, or stapling of the medial side of
the physis if sufficient growth remains.
controlled by an UCBL orthosis. If control of a spastic ankle equinus
or a foot drop is also needed, then an AFO is necessary. If the
peroneus brevis is tight or spastic, orthotic management may be easier
after intramuscular lengthening of the peroneus brevis tendon to
decrease the power of the muscle by one grade (272).
The peroneus longus tendon should not be lengthened because a varus
deformity may ensue. Transfer of the peroneus brevis to the tibialis
posterior tendon has been performed, but published series with
evaluations of results are lacking (271).
child with a flexible pes planovalgus, as the child gets heavier the
orthosis can become painful. This is particularly true if an orthosis
has failed to control the hindfoot valgus, causing painful calluses and
blisters to form on the medial side of the foot.
developing, surgery is indicated. Most surgeons prefer either an Evans
or modified Evans lateral opening-wedge lengthening osteotomy of the
distal calcaneus (273) or a subtalar arthrodesis using internal fixation and bone grafting (274) (Fig. 15.22).
Calcaneal lengthening restores support of the talus and does not
involve fusion of a joint. It may be combined with medial plication and
peroneal lengthening as described by Mosca (275).
Treatment must include lengthening of the ankle plantarflexors when
they are tight or spastic. Ankle dorsiflexion should be reassessed
intraoperatively after stabilization of the foot, at which point false
dorsiflexion through the midfoot would be minimal. Despite its
theoretical benefits, lateral column lengthening in cerebral palsy does
have a high failure rate (17%) and the postoperative improvements that
are usually seen in radiographic measurements do not correlate with
clinical outcomes (276). However, the
differences in plantar pressure measurements before and after surgery
provide additional information about the dynamic orientation of
pressure points under the foot, whereas visual observation of foot
deformity shows minimal changes.
subtalar joint and yields a higher fusion rate than the classic Grice
procedure, or modifications thereof, which rely on bone graft alone (277,278,279,280,281,282).
 |
|
Figure 15.20 Foot pressure pattern of a normal foot (A) and of the foot of a person with spastic diplegia and pes planovalgus deformity of the left and right side (B).
|
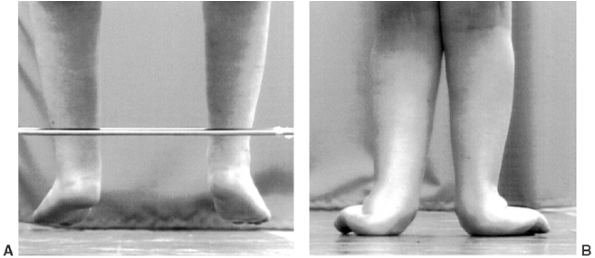 |
|
Figure 15.21 Supple pes planovalgus deformity during non–weight bearing and weight bearing in the same child with spastic diplegia.
|
This technique may be effective in younger children, but it is not
widely accepted. Multiple series with adequate long-term follow-up are
lacking.
deformity is by medial displacement of an oblique osteotomy of the
calcaneus with screw or pin fixation. Although limited experience with
this procedure has been reported, it is easy to perform, the osteotomy
heals rapidly, and subtalar motion is preserved (284).
options are a sliding medial-displacement calcaneal osteotomy, a
lateral opening-wedge or medial closing-wedge osteotomy of the proximal
calcaneus, a combined calcaneal-cuboid-cuneiform osteotomy, or a triple
arthrodesis. Triple arthrodesis provides correction, but is best
avoided in very young patients in whom growth of the foot will be
somewhat inhibited. Triple arthrodesis appears to be successful over
the long term with patients who have mild involvement and are able to
walk even outside their homes. It has been reported that it does not
increase the risk of later development of midfoot and ankle
osteoarthritis caused by abnormal mechanical forces being transferred
to those joints over a long period. It is always important to achieve
the best possible muscle balance at the foot and ankle; otherwise, even
a triple arthrodesis may deform in time.
is medial, with progressive movement toward the adult orientation of
lateral (foot-thigh axis 10 degrees, bimalleolar axis 20 to 25 degrees)
by the age of 6 or 7 years. This remodeling is believed to occur
through the normal torque afforded by walking.
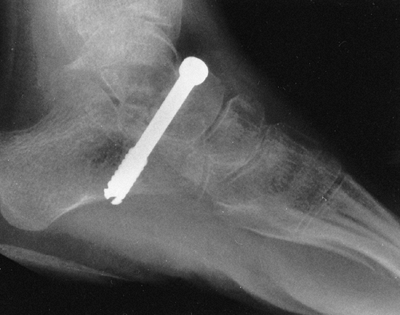 |
|
Figure 15.22
Postoperative lateral radiograph of a subtalar arthrodesis stabilized by a cannulated screw and augmented by iliac autograft bone. |
in walking may leave them with medial tibial torsion that can interfere
with gait in the early years. The effect of medial foot progression
causes tripping and falling as the swing foot catches on the opposite
leg.
may occur because of progressive poor clearance (weak hip extensors and
crouch or stiff knee). It is frequently found in association with
excessive femoral anteversion and pes planovalgus. Inadequate push-off
may be the result of a loss of the normal plantarflexion–knee extension
couple.
the foot with respect to the direction of progression; therefore, it is
dependent on all abnormal rotational motions (that is, knee, hip, and
pelvis rotations) and abnormal bony rotations (femoral, tibial, and
foot) proximal to the foot. Given a neutral pelvis and normal hip
rotation and foot alignment, lateral tibial torsion will result in
lateral foot progression and medial tibial torsion will result in
medial foot progression (Fig. 15.23). Abnormal
tibial torsions may result in “lever arm disease,” which will result in
functional weakness of surrounding musculature that is functioning out
of plane (285). Profound external tibial
torsion substantially shortens the lever-arm effect of the foot in
generating the plantar flexion–knee extension couple. It is this couple
that facilitates knee extension in the midstance and late stance phases
of gait and helps to prevent crouch. Stance phase is often shortened,
the base of support is unstable, and push-off power is compromised.
the thigh-foot axis and bimalleolar axes are measured. In cases of foot
abnormalities such as metatarsus adductus and pes planovalgus, the foot
must be held in a corrected position while these measurements are made.
If the foot deformity is rigid, a measurement of heel-thigh axis gives
information about the torsion of the tibia.
derotational osteotomies of the tibia and fibula at the supramalleolar
level to align the ankle and foot progression angle with the direction
of gait and the axis of the knee. The fibula is divided transversely
just proximal to the syndesmosis, and the tibia is divided
approximately 2.5 cm above the physis. In younger children, fixation by
crossed smooth Steinmann pins, cut and bent extracutaneously, and an
above-knee cast are adequate (285,286).
The pins are removed, and the cast changed to below-knee, at 6 weeks.
In older children the surgeon may choose to use a plate and screws and
a short leg cast. As with any surgical procedure near to an open
physis, care must be taken to avoid inadvertent damage to the growth
plate. In either case, it is desirable to correct the cause of poor
foot clearance if possible.
plane at the knee joint may result in abnormal foot progression angles
in patients with poor clearance in swing or who ambulate on their toes.
Such laxity may minimize the benefits of surgical correction of tibial
torsion if prepositioning and clearance still remain issues postsurgery.
and inadequate flexion in swing are two common pathologies in the
ambulatory patient with cerebral palsy. Overactivity or contracture of
the medial hamstrings prevents adequate extension of the knee for
initial contact and promotes flexion throughout stance phase. It has
been shown that in some patients the actual calculated length of the
hamstrings may be normal, but in the face of spasticity they function
as if they are short (287,288). Flexion of the
knee in swing begins in terminal stance and requires appropriately
timed and sufficiently strong action of the psoas to lift the thigh
into flexion, and of the gastrocsoleus to propel the lower limb
forward. Both serve to adequately flex the knee. Deficiencies in either
strength or firing pattern thus lead to knee flexion dysfunction.
Spasticity of the rectus femoris is also thought to exert a strong
negative effect on knee flexion (289,290,291,292,293). Its excessive activity in early swing or continued action throughout swing acts as a check to normal knee flexion.
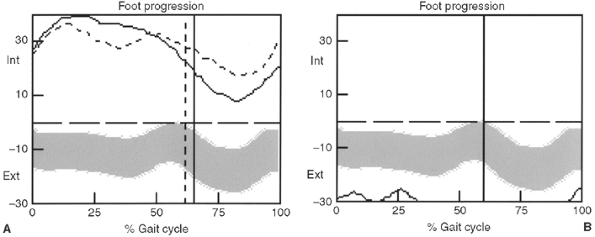 |
|
Figure 15.23
Transverse plane foot progression in a patient with increased internal foot progression caused by medial tibial rotation (assuming neutral pelvic and hip rotations) (A) and increased external foot progression caused by lateral tibial torsion (assuming neutral pelvic and hip rotations) (B). |
contracture or, more commonly, contracture and/or spasticity of the
medial hamstrings. Measuring the popliteal angle and the extent of
straight-leg raise are useful.
tightness by straight-leg raising or by measuring the popliteal angle
does not correlate with the dynamic range of knee motion during gait (294).
Without gait analysis, one is left to assess knee motion during gait
either by clinical observation or by studying a videotape of gait.
suggests rectus femoris spasticity if rapid flexion of the knee causes
elevation of the buttocks because of the proximal pelvic origin of that
muscle. A rectus that is also tight will cause a similar rise when the
knee is slowly flexed.
Because of overactivity of the rectus, the knee flexion curve is
delayed and has a decreased slope, and the peak knee flexion in swing
is diminished and/or delayed. Surface EMG demonstrates overactivity of
the hamstrings and rectus femoris, specifically increased activity of
the rectus femoris in mid swing phase (Fig. 15.25). There is an increased knee extensor moment in stance (knee extensor moment pattern) (294).
-
inappropriate activity of the rectus femoris in mid swing phase, and
-
activity of the rectus femoris on passive knee flexion.
contracture, but is more often caused simply by spastic and tight
hamstring muscles, without fixed capsular contracture. Indications for
lengthening of the medial hamstrings include a popliteal angle of
greater than 60 degrees (lacking 60 degrees to full extension), a
straight-leg raise lacking 45 degrees, kinematic evidence of knee
flexion at initial contact, throughout stance and in terminal swing,
and hamstring spasticity on EMG. If the hamstrings are tight on
clinical examination but the kinematics are normal, then the hamstrings
do not need to be lengthened. It has been consistently shown, through
systematic comparison of the popliteal angle and knee kinematics
parameters, that there is a poor correlation between these measures;
this finding raises a red flag when using the popliteal angle as the
sole justification for hamstring lengthening.
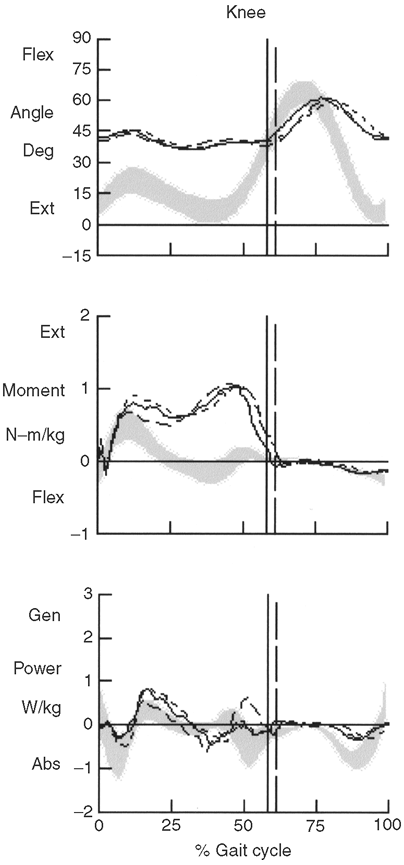 |
|
Figure 15.24
Sagittal plane kinematic and kinetic (moment and power) in a child with a crouch gait pattern and associated excessive knee flexion in stance and overall reduced range of motion of the knee. The kinetic pattern is referred to as a knee extensor moment pattern. |
terminal swing and early stance but move into normal extension or even
hyperextension in mid stance. This rapid movement to extension is due
to early stance spasticity of the gastrocsoleus (excessive
plantarflexion–knee extension couple). Medial hamstring lengthening is
still indicated despite this apparent knee extensibility. There is some
evidence that combined medial and lateral hamstring lengthening, while
possibly facilitating greater correction of stance-phase crouch,
increases the risk for recurvatum. Any associated gastrocsoleus
spasticy or contracture must be addressed.
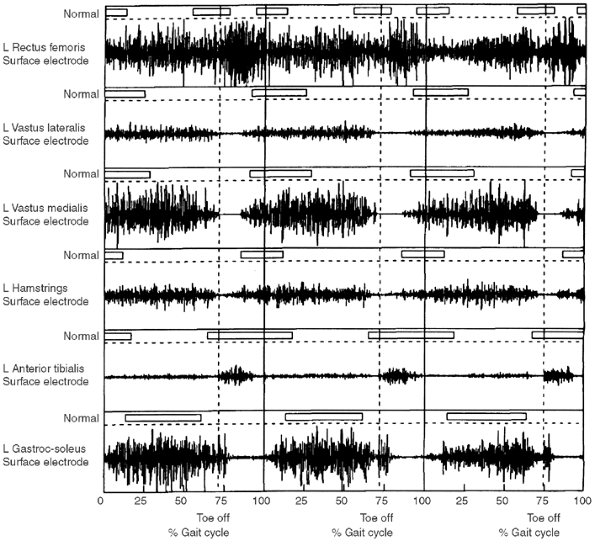 |
|
Figure 15.25
Example of surface electromyography (EMG) data for selected lower-extremity muscles during level gait. Four gait cycles are illustrated with the reference normal bars above each individual raw EMG signal. Continuous activity of the rectus femoris is noted throughout the gait cycle, in which the vasti are not active in initial to midswing. |
addressed. Intramuscular tenotomy of the semitendinosis and fascial
lengthening of the semimembranosis at a minimum of two levels are
performed through a distal medial longitudinal incision. If knee
extension is also limited with the hip in the abducted position, a
distal intramuscular tenotomy of the gracilis may be added. A popliteal
angle lacking 15 to 20 degrees is desired. If after these procedures
the lateral hamstrings are still tight, they may also require
aponeurotic lengthening. There is an increased risk of knee
hyperextension after lengthening both medial and lateral hamstrings,
especially if the gastrocnemius is spastic postoperatively.
Preoperative assessment of calf spasticity is therefore advisable so
that it may be addressed.
risk associated with medial hamstring lengthening performed
unilaterally or bilaterally (295). In patients
who have a posterior pelvic tilt presurgery and severe hamstring
tightness (popliteal angle–70 degrees or more), and who are receiving
bilateral medial and lateral hamstring lengthening, an increase in
anterior pelvic tilt is common and allows the pelvis to be in a
“typical” range postsurgery (295).
gait mechanics. It has been consistently shown through the use of
computerized gait analysis techniques that appropriate hamstring
lengthening wIll result in improved knee extension at initial contact
and reduced crouch in stance (292,293,296,297).
The previously reported complication of recurvatum following hamstring
lengthening is rare if spastic or contracted gastrocsoleus is addressed
at the same time. In some cases recurvatum may be noted postoperatively
but tends to correct over time. Also, excessive anterior pelvic tilt
does not occur so long as the lateral hamstrings are preserved and any
significant hip-flexion contracture is addressed.
distal rectus femoris transfers are managed in removable
knee-immobilizer splints, which can easily fit over a below-knee cast.
Passive range of motion exercises may begin on the second or third
postoperative day, and long sitting is encouraged to further stretch
the hamstrings. However, knee flexion out of the immobilizers as well
as prone knee flexion must also be encouraged in order to maximize the
benefit of the rectus transfer. If no bony surgery has been performed,
walking training may begin as early as the fifth postoperative day.
When adequate quadriceps strength has been regained, usually by the
fourth postoperative week, the knee immobilizers may be discarded or
used as night splints. Between physical therapy sessions, the patient
should sit with the knees alternately in extension and flexion.
results in an increase in knee flexion in swing phase of gait
postoperatively on account of reduction in knee extensor strength (292,293).
Patients with normal and delayed peak knee flexion presurgery do not
show an increase in peak knee flexion in swing after a rectus femoris
transfer. Peak knee flexion in swing is maintained when simultaneous
hamstring procedures are performed (292,293).
when straight-leg raising cannot exceed 60 degrees above the
horizontal, or when the popliteal angle (i.e., the sagittal
femorotibial angle with maximum knee extension, the patient supine, and
the hip first flexed to 90 degrees) is more than 45 degrees short of
full extension.
lengthening, it is regained by 9 months and quadriceps strength
increases significantly as a direct result of improved knee extension (298).
It has been found, however, that lumbar lordosis may increase after
this procedure because of the overactive hip flexors; also, the desired
effect on the distal hamstring tightness may not be achieved (289).
It must be remembered that the hamstrings are also hip extensors,
sometimes contributing up to one-third of the extensor torque (290,291).
Hip extensor power is somewhat lessened by hamstring lengthening,
especially proximal hamstring lengthening. Therefore, this procedure
may increase a preexisting hip-flexion contracture, as well as the
lumbar lordosis, if the iliopsoas unit is not addressed concomitantly.
Gait analysis has shown that, in some patients with a crouch gait, the
hamstrings may be of normal or even excessive length. This is seen with
simultaneous hyperflexion of the hip. In this situation hamstring
lengthening alone may further weaken hip extensor power and increase
the already severe hip-flexion deformity. To preserve some of the hip
extensor power of the hamstrings, Gage has recommended transfer of the
distal semitendinosis to the lateral femoral metaphysis. This may
minimally augment external rotation at the hip (2).
deformity is cospasticity of the rectus femoris muscle and the
hamstrings. The rectus may be firing continuously throughout the gait
cycle, or mostly during swing phase, but the result is often a
stiff-knee gait with less than 80% of normal knee motion. In this
situation, if the hamstrings alone are lengthened, a stiff,
extended-knee gait frequently results. There is lack of adequate knee
flexion in swing phase, and that interferes with foot clearance so that
circumduction or vaulting may become necessary to compensate (292).
overactivity is treated by a distal transfer of its tendon, generally
medially to the sartorius or the semitendinosis (299).
The transfer does not affect gait abnormalities in the transverse plane
(i.e., intoeing or out-toeing), and it makes no difference whether the
transfer is attached to the sartorius, gracilis, semitendinosus, or
iliotibial band (293,294).
This transfer is successful in restoring adequate (60 degrees) knee
flexion in the swing phase of gait. It has been shown that transfer is
necessary for accomplishing this, not just tenotomy of the distal
rectus femoris tendon (293,294).
evidence of abnormal rectus femoris activity in swing phase, delayed
and diminished knee flexion in swing, and total preoperative range of
knee motion during gait of less than 80% of normal (less than 45 to 50
degrees). Inappropriate EMG activity of the rectus femoris during rapid
passive knee flexion is another supportive finding. Simultaneous
hamstring lengthenings is an indication for rectus femoris transfer (293,294). There is debate as to the value of a positive Duncan-Ely in predicting the effectiveness of rectus transfer (300,301).
A positive Duncan-Ely does not predict abnormal EMG activity, and
neither the type of abnormal EMG activity in the rectus nor the
magnitude of the restricted preoperative knee range of motion are
significant variables in determining the success of the transfer (294).
velocity, stride length, and knee range of motion) may diminish
significantly over time. Other benefits such as peak knee flexion
during swing, total knee motion, and knee extension at terminal swing
may be maintained (302). Even children with
more severe involvement, such as crutch or walker dependency, benefit
from hamstring lengthening and rectus transfer with increased knee
extension in stance, stride length, range of knee motion, and timing of
peak knee flexion in swing phase (303).
preoperatively may predict an unsatisfactory result from a rectus
transfer, particularly when triceps surae lengthenings are included in
the surgery (301).
scissoring type of gait pattern, and predisposes the patient to hip
dysplasia or subluxation. During normal gait following
double
support, the hip maintains a position of slight adduction under the
control of the hip abductors. During swing, the hip moves into slight
abduction as an assist to clearance. With an excessively adducted gait
pattern it must be determined whether the appearance of scissoring is a
primary adductor problem or an illusion. Hip and knee flexion or crouch
in the face of internal hip rotation secondary to excessive femoral
anteversion gives the same visual appearance as primary adductor
spasticity during swing phase of gait.
degrees and abducted. Abduction is measured as degrees from the
vertical. Hip abduction is reduced with full extension because of the
additional adduction contribution from the hamstrings.
adduction throughout the entire gait cycle. Hip abductor weakness alone
may cause the hip to maintain excessive adduction during stance, but in
the absence of adductor abnormality, the hip will show the ability to
abduct in swing.
rotation, will also show more than normal stance-phase adduction. In
the absence of adductor pathology, swing abduction should be normal.
degrees in flexion or extension, adductor release or transfer (either
posteriorly to the ischial tuberosity, which has its advocates, or
posteriorly and distally to the gracilis) is indicated (304,305).
There seems to be little difference in the results from release or
transfer, but one study reports a high incidence of pelvic obliquity
and hip subluxation after adductor transfer to the ischium (305).
Neurectomy of the anterior branch of the obturator nerve is rarely
indicated in diplegic patients who can walk, because it excessively
weakens the adductor brevis muscle and can result in a wide-based gait
with hyperabduction of the hips (306). The hip
adductors function to stabilize the hip against excessive abduction
during gait and running, and in activities such as skiing, skating, and
horseback riding. The gait stability they provide allows more effective
hip flexor and extensor activity. Therefore, it is important not to “go
for broke” and overlengthen or overweaken the adductors in ambulatory
patients.
patient supine, through either longitudinal or transverse incision,
depending on the preference of the surgeon. The adductor longus is
always completely divided. Often, this is adequate to accomplish the
objective of adductor release for most surgeons. This is at least 60
degrees of passive abduction on each side, with the hip and knee flexed
to 90 degrees, or at least 45 degrees of passive abduction with the hip
and knee extended (the medial hamstrings are also hip adductors). At
times, some of the adductor brevis may also need to be released and it
may be necessary to divide the gracilis muscle to achieve the desired
abduction. A hip abduction pillow is beneficial in the postoperative
period.
stance-phase problems of excessive flexion of the hip at initial
contact and inadequate extension of the hip at terminal extension.
Functionally, the patient cannot walk with the body upright.
with an understanding of what degree of extension is actually required
for gait. Large hip-flexion contractures are not a common occurrence in
the ambulatory child with cerebral palsy and are often overestimated.
of the contralateral hip, most patients with diplegic cerebral palsy
will show some degree of contracture. However, this test places the
pelvis into a position of excessive flexion, which does not correlate
with pelvic position during walking. The pelvis in patients with
cerebral palsy is generally more anterior than normal during walking (295).
This may be a function of weakness of the trunk or abdomen and is
frequently present when there is no psoas contracture or spasticity.
Using a modification of the Thomas test, both hips are flexed only to
the point where the anterior superior iliac spine (ASIS) and posterior
superior iliac spine (PSIS) lie in a vertical plane. The hip is then
allowed to maximally extend. The angle between the thigh and the
examination table is the degree of flexion contracture (Fig. 15.26).
interfere with function during gait and require surgical treatment. If
there is any doubt concerning the influence of the psoas muscle on body
position, the patient can be observed while moving along in the
kneeling position to negate any effect of hamstrings on pelvis
position. The patient with
true psoas spasticity or contracture will be unable to maintain the trunk upright (307).
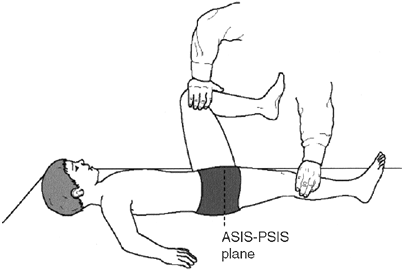 |
|
Figure 15.26 Modified Thomas test.
|
initial contact. As the body advances forward during stance, the hip
progressively extends to a position of hyperextension at terminal
stance. In preswing, it is driven into flexion by the action of the
ankle plantarflexors (gastrocsoleus) and psoas. The hip continues to
flex passively throughout swing.
in terminal stance, and frequently a “double bump” pattern of the
pelvis during the gait cycle (Fig. 15.27).
This, however, needs to be evaluated in reference to the positions of
the knee and pelvis. Crouch, or excessive knee flexion in stance, will
cause the thigh to assume a more flexed position, as will an anterior
position of the pelvis. Both can occur in the absence of a tight or
spastic psoas.
an assessment tool. A normal pattern or modulation of a hip curve that
is shifted into excessive flexion is often a secondary finding related
to ankle, knee, and/or pelvis position rather than a primary psoas
problem.
increased internal extensor moment and delayed crossover from extensor
to flexor moment (Fig. 15.27). However, any
cause of increased hip flexion that positions the center of gravity
anterior to the hip, i.e., knee flexion or ankle equinus, can cause a
similar kinetic pattern.
of greater than 30 degrees on Thomas test or greater than 20 degrees on
modified Thomas test, causing inability to walk upright. A patient who
relies on Lofstran crutches walks with the body forward and may
tolerate this degree of contracture. Abnormal hip motion and moments
during gait may be a result of many other gait abnormalities both
proximal (trunk position) and distal (crouch and associated causes) (295).
by tenotomy at the lesser trochanter weakens hip flexor power
excessively, and may result in insufficient hip flexion strength to
lift the limb in climbing stairs. Lengthening of the psoas should be
performed intramuscularly either at the brim of the pelvis or above the
pubic ramus. This allows greater preservation of hip flexor strength
(from the iliacus) compared to distal tenotomy or recession to the
capsule (71,299,308,309).
The technique of this procedure is clearly illustrated and described in
a recent report that demonstrated this as a safe and reliable means of
improving hip function in independently ambulatory children (298).
The psoas is isolated from the iliacus and tenotomized under direct
vision. Care must be exercised to avoid the nearby femoral artery and
nerve. Occasionally, the psoas is not tendinous at this level. Then a
recession to the anterior hip capsule is performed by an adductor
approach. It is rarely necessary to require further release of anterior
hip structures in the ambulatory population. Hip flexor releases are
treated postoperatively by asking the patient to lie prone several
times a day. Painful muscle spasms are particularly common in the first
few days after hip surgery, and may be treated with diazepam and
analgesics. It is desirable to begin gentle passive range of motion
exercises by the second or third day after flexor or adductor releases,
and gait training may begin 5 to 7 days postoperatively. After 3 weeks,
more vigorous muscle strengthening exercises are tolerated.
 |
|
Figure 15.27
Sagittal plane kinematic and kinetic (moment and power) for a child with a crouch gait pattern and associated excessive hip flexion in stance and overall reduced range of motion of the hip. The kinetic pattern is referred to as a hip extensor moment pattern. |
perform psoas lengthening in association with hamstring lengthening in
order to prevent postoperative anterior tilting of the pelvis. Gait
kinetics data are used to support psoas lengthening if contractures are
less than 15 degrees (309).
However, others have shown that hamstrings may be safely lengthened in
the face of minor psoas contracture without any ill effect on the
pelvic position (295).
balance, and ligamentous integrity, normal ambulation usually corrects
the neonatal femoral anteversion to the normal adult range of 10 to 20
degrees. In children with cerebral palsy, the delay in ambulation in
conjunction with abnormal or spastic muscular balance may delay or
prevent correction of anteversion and may sometimes even lead to an
increase from the neonatal value. There is no “natural” correction of
femoral anteversion by growth/maturation in patients with cerebral
palsy (211). Intoeing is also caused by
increased spasticity in the internal rotator muscles of the hip (the
medial hamstrings or the anterior fibers of the gluteus medius and
tensor fascia lata).
anteversion during gait are a medial foot progression angle, medial
rotation of the knees, and resultant tripping. In cases of asymmetry,
the internal foot progression on one side may be masked by external
pelvic and/or trunk rotation to compensate for excessive hip internal
rotation, or occur in relation to rotatory scoliosis.
by using the trochanteric prominence test with the patient in the prone
position (310). With one hand controlling the
pelvis, and fingers pressed against the greater trochanter, the leg is
moved into internal rotation until the trochanter is palpated in its
most lateral position. The angle between the vertical and the tibia
represents the measure of anteversion. Clinical assessment of femoral
anteversion by the trochanteric prominence angle test, however, has
limitations. Obesity also compromises the accuracy of the assessment.
Three-dimensional imaging has shown that the prominence is variably
anterior to the femoral neck axis (311).
Unfortunately, most children who need either two or three-dimensional
CT scanning in order to assess their femoral anteversion have some
degree of hip flexion, adduction, and internal rotation contractures.
These may prevent optimal positioning of the patient in the scanner,
and thereby negate acceptable accuracy for the measurement of femoral
anterversion (312). That leaves clinicians to
estimate anteversion as best they can by combining clinical, CT, and
intraoperative assessments. Maximum hip internal rotation minus 20
degrees is another rule of thumb to estimate anteversion. This
measurement is more reliable in the unoperated hip. In cases of
previous derotational osteotomy in the spastic child, a recurrence of
hip internal rotation can occur through stretching of soft tissues
without an associated recurrence of anteversion (313). In such cases CT scan assessment of anteversion has a role.
visually on the basis of the amount of medial rotation of the knees may
result in error because of the potential for pelvic asymmetry and its
associated impact on thigh position in space. Kinematics demonstrate
medial hip rotation during stance phase (Fig. 15.28).
Although the foot progression is typically medial, in cases of
associated lateral tibial torsion or pes planovalgus it may be neutral
or even lateral. In hemiplegic patients, and on the side having greater
anteversion in the asymmetric diplegic patient, the pelvis is
frequently seen to retract posteriorly. This can also affect the
position of the foot during gait (Fig. 15.29).
exists as an isolated finding in spastic diplegia. It is usually
accompanied by lower-extremity musculotendinous tightness or
contractures that also require correction. In fact, the dynamic
component of hip rotation during gait may be the most significant
factor, and anatomic deformity may not predict the gait deviations.
Static and dynamic contributions can vary individually and raise the
question of whether the magnitude of surgical correction of rotation
should be based on the gait analysis or the radiographic data, or both (314).
increased femoral anteversion. The appropriate age for the osteotomy is
whenever other lower-extremity surgery is being done. This usually
means after the age of 4 years. The derotation may be at the
supracondylar region in children with mild to moderate spasticity, and
may be secured either by crossed Steinmann pins (42)
and a hip spica cast, or by an external fixator. In older children, and
in patients of any age with substantial spasticity, it is best to
perform the osteotomy at the intertrochanteric level and to preserve
the attachment of the iliopsoas. Fixation with a strong blade-plate or
screw-plate may avoid the need for a postoperative spica cast and allow
early hip motion in patients who have adequate bone stock. Such strong
internal fixation obviates the problems
of
loss of fixation and malunion, which occasionally occur after pin and
cast fixation of distal femoral osteotomies. Postoperatively the
patient is nonambulatory for 2 to 3 weeks until the soft tissues have
healed. Full weight bearing is allowed thereafter.
 |
|
Figure 15.28
Transverse plane kinematic results of the hip showing excessive internal hip rotation throughout the gait cycle in comparison with the normal reference (gray band). |
 |
|
Figure 15.29 Example of different rotational profiles in the transverse plane. A: increased internal rotation of the right (solid line)
hip with normal foot progression indicating external tibial torsion on the right tibia (assuming normal foot alignment with respect to distal tibia). B: Increased internal of the right (solid line) hip with internal foot progression bilaterally indicating internal tibial torsion on the left (broken line) and normal torsion on the right (assuming normal foot alignment with respect to the distal tibia). |
dynamic rotational measurements postoperatively often average
approximately 40% less than those reported at surgery (315).
The proximal osteotomy is facilitated by placing the patient in the
prone position and using the approach described by Root and Siegal (316).
If subluxation and/or coxa valga are present, varus can be added to the
osteotomy. Another option for derotation in children older than 10
years is in the subtrochanteric region with fixation by a locked
intramedullary rod, but this cannot be combined with varization.
often compensated by ipsilateral external pelvic rotation in children
who have hemiplegic cerebral palsy, but not in those with diplegia. To
avoid undercorrection in hemiplegics and center the hip position in the
arc of rotation postoperatively, both the anteversion and the pelvic
retraction should be considered in the preoperative planning (317).
Postoperative studies show that derotation osteotomy produces sustained
correction when combined with multilevel correction of other
deformities (318,319).
It increases hip rotation and extension, decreases anterior pelvic
tilt, may increase knee extension strength, and improves cosmesis (318,319).
anteversion require external rotation of the distal segment, which
tightens the medial hamstrings. In order to prevent this increased
medial hamstring tightness, which may actually increase internal
rotation, these hamstrings usually need to be lengthened when femoral
derotation is performed.
cerebral palsy have been advocated. Two of these are transfer of the
semitendinosus tendon to the distal lateral femur (2)
and posteromedial transfer of the distal tendon of the rectus femoris.
These techniques have not been shown to be effective for intoeing (293).
Another reported technique is transfer of the greater trochanter with
its attached gluteus medius muscle to the anterior proximal femur, so
that it may function as an external rotator as well as an abductor (320).
This abductor transfer, although sometimes successful, can produce an
abductor weakness type of gait and has not been widely adopted.
involvement of one side of the body, with the arm and hand more
severely involved than the lower extremity. On closer evaluation, very
mild involvement on the contralateral side is often found, especially
in those with more severe affliction (2).
all cerebral palsy cases. Approximately one third of the patients have
a seizure disorder, and almost one half have some degree of mental
retardation (4). More common than mental retardation is an attention deficit, learning, or behavioral disorder (321).
A history of head trauma or intracranial hemorrhage is frequently found
in spastic hemiplegia. Virtually all patients are community ambulators,
although only approximately half of them can walk at 18 months of age (4).
Some limb-length inequality and a difference in size between the two
feet are common, but these rarely require any treatment. It has been
shown that children with hemiplegia do not necessarily have a
difference between chronologic age and bone age, and that both
hands/wrists have the same radiographic bone age so that it is not
necessary to obtain radiographs of both sides (322).
includes equinovarus at the foot and ankle, flexion at the knee and
hip, internal rotation of the lower limb, internal rotation at the
shoulder, flexion at the elbow, pronation of the forearm, flexion and
ulnar deviation at the wrist, and thumb-in-palm with finger flexion in
the hand. Actually, the degree of involvement with spastic hemiplegia
is a spectrum, which has been separated into four subtypes (323). It is essential to quantify the involvement before planning the treatment. Surgical results are predicted to be good.
tibialis anterior muscle, and the triceps surae group is not tight.
This type manifests as a foot drop and a steppage gait, with plantar
flexion disappearing during stance phase. It is easily treated with an
appropriate AFO. A posterior leaf spring or an articulated ankle type
is usually superior to a solid-ankle AFO in this condition (324).
The most difficult part of management is to get the child to wear the
orthosis if he or she has no other noticeable variation from normality.
One reported surgical approach to this type of hemiplegic problem has
been transfer of the flexor digitorum longus and flexor hallucis longus
tendons to the dorsum of the foot (325).
Experience with this technique is limited, and meaningful long-term
results are not available. We and most other surgeons do not use this
procedure because anecdotal results include mostly stretched-out
tenodeses.
muscle weakness, plus spasticity in the triceps surae group and usually
in the tibialis posterior muscle. This produces an equinovarus
deformity of the foot and ankle, which persists throughout all phases
of gait and can produce some knee hyperextension late in stance phase.
The problem is addressed by lengthening the gastrocnemius aponeurosis
or the Achilles tendon, lengthening or performing a split transfer of
the tibialis posterior tendon, and providing an AFO if needed.
correction of the varus heel in stance phase when there is no fixed
deformity, and gait analysis shows the muscle is firing in phase
(during stance). Some surgeons find that the tension in the transferred
limb is difficult or impossible to balance exactly with the intact
medial limb, and they therefore prefer lengthening to split transfer.
Often the tibialis posterior is active throughout the gait cycle. In
that case it is always better to lengthen its tendon rather than
perform the split transfer (4). In rare cases
the tibialis anterior muscle, instead of being weak, is shown by gait
analysis to be overactive during the swing phase of gait. This is also
evidenced by supination of the foot in swing phase. In such patients, a
split or complete anterior tibialis tendon transfer can be effective in
achieving transverse plane balance of the foot. This is usually
combined with tibialis–posterior tendon lengthening.
tibialis anterior. Some children are orthosis-free after the surgery.
The risk of recurrence of the equinus deformity in hemiplegia decreases
as the child grows older, being reported at approximately 25% in
children younger than 4 years and 12% thereafter (4).
and tibialis posterior muscles spastic and usually contracted, but
hamstring involvement is also present, often with cospasticity of the
rectus femoris. This produces a stiff, flexed-knee gait with
equinovarus foot and ankle deformity. Successful treatment includes the
tendon surgery discussed for type II and the addition of medial
hamstring lengthenings, often combined with a distal rectus femoris
tendon transfer, as described in the section on spastic diplegia.
Again, the appropriate AFO may be necessary postoperatively, sometimes
temporarily and sometimes permanently.
the addition of hip flexor and adductor spasticity or contracture.
Iliopsoas lengthening, by release of the psoas tendon over the pelvic
brim, and appropriate adductor releases are added to the treatment
recommended for those with type III involvement.
patients with spastic hemiplegia. It is not infallible, however, and
patients are occasionally encountered who have profound equinus with
little or no varus; who have increased ipsilateral femoral anteversion
in addition to type IV involvement; or who have other abnormalities.
Their resting state often includes limb movements that are purposeless,
involuntary, and almost continuously changing. The movements are coarse
and irregular and often give the child the appearance of squirming or
writhing; extensor tone predominance is the rule. The movements
disappear during sleep (326).
state. The athetoid movements are greater in the more distal parts of
the limbs, and often flow rapidly from flexion to extension, adduction
to abduction, and pronation to supination. Because of the almost
constant motion, most of the joints are put through a full range of
motion, and contractures are not common unless there is an asymmetric
component of mixed spasticity present.
walk. Their mobility is by powered wheelchair, often with an adapted
steering mechanism. The therapeutic focus for these children should be
on improving communication skills, facilitating their control over the
activities of daily living, and wheelchair mobility (327).
who can walk is random, inconsistent, and influenced by many external
stimuli. Without a consistent baseline, the results of soft tissue
surgery are unpredictable. Tenotomies and muscle releases should be
done infrequently, and even then very carefully, in patients who have
significant athetosis, because the result is often a severe, almost
untreatable deformity opposite the one originally addressed.
cerebral palsy, and it responds well to the internal fixation and
spinal fusion techniques described earlier. Adult patients with
athetoid cerebral palsy may develop profoundly painful degenerative
spondylosis and myelopathy in the cervical spine, sometimes with
weakness in the upper extremity, and even instability. One study
reported that two thirds of patients with athetoid cerebral palsy at
age 44 years, and all of those older than 55 years, had moderate or
severe cervical disc degeneration (328). The segment that degenerates most frequently is C5–C6. This usually responds well to anterior cervical fusion (329),
but severe athetosis and dystonia can make postoperative immobilization
very difficult. In this situation, the use of BTX injections into the
neck muscles can be extremely beneficial in eliminating involuntary
neck motions (330).
spastic hemiplegia and quadriplegia. When considering treatment, it is
essential to consider the functioning of the entire upper limb, and of
the child as a whole. It has long been reported that in most cases the
problems of spasticity, weakness, poor motor control, poor
proprioception and stereognosis, and contractures of the joints reduce
or eliminate the possible benefits of tendon lengthenings, transfers,
or other surgical treatment. Patients with hemiplegia have been
reported to have better results than do patients with quadriplegia, and
postoperative functioning is usually better in right hemiplegia than in
left (331). Mental retardation, visual
deficits, behavioral problems, and particularly dyskinetic involvement
may also be contraindications for upper extremity surgery. Fewer than
5% of patients are appropriate candidates for such surgery (332).
Nevertheless, a recent report of 718 procedures in 134 patients over a
25-year period indicates that only high motivation and fair-to-good
motor control affect upper-extremity surgical outcome, whereas
mentation, sensibility, and the type of cerebral palsy do not influence
the surgical result (333).
functional improvement after upper-extremity surgery. Substantial
cosmetic improvement or facilitation of care, such as dressing and
putting on a glove, can be achieved in severely involved patients.
Patients who are most likely to benefit from upper-extremity surgery in
cerebral palsy are those with
-
spasticity, not athetosis;
-
reasonable intelligence and good motivation;
-
stable trunk and body position;
-
good hand proprioception, stereognosis, touch, and other sensations;
-
no fixed contractures and adequate passive range of motion of all upper-extremity joints;
-
reasonable hand function, with good hand placement capability and voluntary control;
-
age between 5 and 20 years (334).
electromyographic studies aids in decision-making regarding lengthening
or transferring musculotendinous units (335,336,337).
and elbow contractures can be cosmesis. Older children and adolescents
with reasonable intelligence who function well in public may have a
negative body image, with a rigid, contracted wrist and elbow. This may
also have a negative effect on peer group acceptance. In such cases,
correction of those deformities, although having no benefit for
upper-extremity functioning, can be of immense psychological value to
the patient.
passive range of motion exercises and passive night splinting to
attempt to prevent contractures, and serial casting or judiciously
applied dynamic splinting to attempt correction of mild contractures in
the absence of much spasticity. The daytime use of splints in an
attempt to improve functioning
almost
always meets with failure in hands with poor sensation and control, and
may actually increase spasticity. Outcome studies of splinting are
lacking. It is doubtful that the satisfaction of “doing something”
alone justifies the cost.
upper-extremity muscles, but the effects are temporary unless a
stretching program or another means of lengthening the muscles succeeds
during the temporary period. BTX will probably most help a hemiplegic
child with spasticity, no joint contractures, and reasonable hand
function. The best early results have involved blocking the biceps,
pronator teres, flexor carpi ulnaris (FCU), and thumb flexors and
adductors (135,143). Whether surgery is merely deferred or can sometimes be avoided with BTX treatment is not yet known.
is essential for patients and families to understand that realistic
goals are improved functioning and appearance. Normality is not a
possible result, and any improvement will require careful evaluation
and planning. As with a lower extremity, it is wise to perform all the
required procedures at one operative setting. It is certainly possible
to have different surgical teams operating on the upper and lower
extremities at the same time. Some common upper-extremity surgical
procedures and their indications in spastic patients follow. Patients
with athetosis almost never have enough voluntary control to benefit
from upper-extremity surgery.
affected with severe spasticity, adduction and internal rotation
contractures may develop, caused by tightness of the subscapularis and
pectoralis major muscles. If hand function is reasonable but the
ability to position the hand in space is compromised, correction of
shoulder contractures is appropriate. This is accomplished by releasing
the tendon of the subscapularis and lengthening the tendon of the
pectoralis major. If this does not provide the needed correction, a
proximal humeral derotational osteotomy, fixed with a compression
plate, usually solves the problem (332,333).
shoulder abduction when running, caused by deltoid muscle spasticity.
This may be relieved by musculotendinous lengthening of the deltoid (135).
contractures in children with substantial spasticity. The flexion
contracture is caused by the spasticity of the biceps, brachialis, and
brachioradialis. Serial casting, dropout casts (long-arm casts with the
posterior plaster above the elbow removed to allow further extension
while blocking further flexion), dynamic splints, and BTX have been
discussed already.
correction. The indications for surgery are to improve the ability to
position a functional hand in space, to improve hygiene or prevent skin
breakdown, and to improve appearance. Most flexion contractures of the
elbow can be corrected by resecting the lacertus fibrosus, fractional
lengthening of the brachialis and the biceps, and release of the
brachioradialis origin (135). Occasionally, step-cut lengthening of the biceps and anterior capsulotomy of the elbow are necessary (332).
More recently, a procedure that includes lacertus fibrosus incision,
brachialis aponeurosis fractional lengthening, and denuding the
peritendinous adventitia off the biceps tendon to remove afferent nerve
fibers and receptors (as distinct from lengthening of the biceps
tendon) has been described. Results after 1 year show improved flexion
posture and active extension of the elbow. Improved cosmetic and
functional use are claimed, but the primary benefit is reduction of the
flexion posture angle (338).
overactivity of the pronator teres and possibly the pronator quadratus,
and weakness of supination. When combined with a flexion contracture in
a young child, dislocation of the radial head may occur. The pronation
component forces the dorsum of the hand or forearm to assist in
bimanual tasks. Correcting the contracture to permit supination allows
use of the hand. Surgical treatment of pronation contracture consists
either of distal release or transfer of the pronator teres muscle (332).
If passive supination is full, some surgeons advocate transfer of the
pronator teres tendon through the interosseous membrane (140), or posterior to the radius to an anterolateral insertion, so that it can function as a supinator (318).
The results of this transfer are not always good, and a fixed
supination deformity is a larger problem than a pronation deformity (331). Release of the pronator alone is usually successful, and is preferred by most surgeons.
dislocation of the radial head. In such cases excision of the radial
head leads to a better outcome than reconstruction (339).
finger flexors and extensors should be determined. Flexion deformity or
contracture with some ulnar deviation is the usual finding at the wrist
in spastic upper extremities. This is usually associated with pronation
of the forearm and weakness of the wrist extensor muscles. If flexor
spasticity is minimal and finger extension is good, simply lengthening
the FCU or the flexor carpi radialis, but not both, may be helpful.
Another usually effective means of weakening spastic wrist and finger
flexors is the flexor and pronator release procedure (334,340). The disadvantage of this
operation is that it is nonselective, releasing all wrist and finger
flexors and the pronator teres from the medial epicondyle of the distal
humerus.
Before transfer, an electromyogram can be performed to determine the
phase of the muscle if it cannot be determined by clinical examination.
Transfer of the FCU tendon around the ulnar border of the wrist and
into the extensor carpi radialis brevis (ECRB) is indicated if the
transferred muscle is active during grasp, and the patient has poor
wrist extension and poor grasp but can actively extend the fingers with
the wrist in neutral or dorsiflexion (343) (Fig. 15.30).
If the FCU muscle is active during release and there is adequate grasp
but poor release, it is transferred to the extensor digitorum comminus
tendons. At least one long-term follow-up study has indicated that
transfer to the wrist extensor is less predictable than transfer to the
finger extensors (344). Regardless of the site
of transfer, the flexor carpi radialis tendon should not be lengthened
in association with FCU transfer because lengthening would risk overly
weakening wrist flexion.
functional motion of the wrist, and prevents the wrist from helping in
grasp and release because of a tenodesis effect of the extrinsic
muscles. It is indicated most often for the markedly deformed carpus in
severely involved patients for hygienic or cosmetic reasons, such as
putting the hand through a sleeve or into a pocket or a mitten.
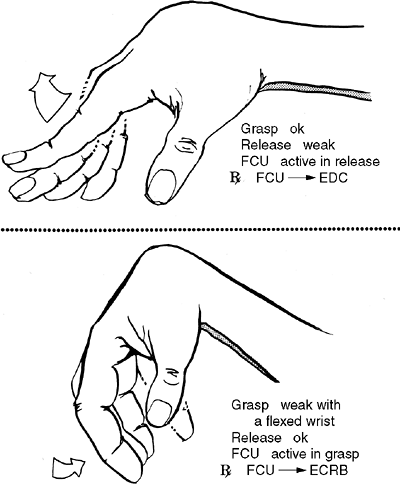 |
|
Figure 15.30
The spastic hemiplegic hand. The hand shown in the upper part of the figure will benefit from transfer of the flexor carpi ulnaris to augment the extensor digitorum communis (EDC). The hand shown in the lower part of the figure needs augmentation of wrist extension by transferring the flexor carpi ulnaris to the extensor carpi radialis brevis. |
overactivity in the extensor carpi ulnaris muscle with volar
displacement of its tendon. Split transfer of its tendon to the ECRB
has been recommended (344).
-
flexion and adduction of the thumb
-
clawing, with hyperextension of the finger metacarpophalangeal (MCP) joints and flexion contractures of the distal finger joints
-
flexion contractures at all finger joints
-
swan-neck deformities of the fingers
careful assessment before the appropriate treatment can be recommended.
In most cases, the adductor pollicis and first dorsal interosseus
muscles overpower the abductor pollicis longus and the extensor
pollicis longus and brevis muscles. Common patterns of deformity include
-
metacarpal adduction contracture;
-
metacarpal adduction contracture with MCP flexion contracture;
-
metacarpal adduction contracture with MCP hyperextension contracture;
-
metacarpal adduction contracture with MCP and interphalangeal joint flexion contractures.
first web and tight fascial bands in the first dorsal interosseous and
adductor aponeuroses (135). There are different
ways to surgically treat these deformities, but in principle all
involve release of contractures of the skin, joints, and spastic
muscles; stabilization of the joints; and augmentation of the weakened
muscles (332,333,345) (Fig. 15.31).
and MCP hyperextension is imbalance between the wrist flexors and
extensors. This is usually addressed by transferring either the flexor
carpi radialis or the FCU to the ECRB (334).
problem, fractionally lengthening the flexor digitorum sublimis and
profundus muscles is of benefit. This is done by complete transverse
circumferential release of their aponeuroses in the proximal third of
the forearm. In tightly flexed fingers that cannot be extended, even
with the wrist flexed, an effective treatment involves tenotomies of
the flexor digitorum profundus and sublimis tendons at different levels
in the distal forearm. With the fingers extended, the proximal ends of
the sublimis tendons are then sutured to the distal ends of the
profundus tendons (sublimis to profundus transfers). Tenotomies of the
wrist flexors are usually also necessary. In patients with
more
severe involvement, proximal row carpectomy can be done to gain
additional length in the soft tissues, or wrist arthrodesis may be
necessary in order to stabilize the hand.
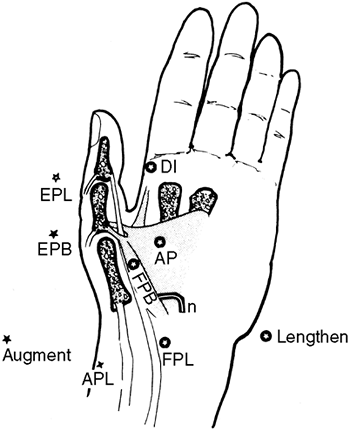 |
|
Figure 15.31
The repertoire of operations for the thumb-in-palm deformity. Shortened muscles should be lengthened, and weak long muscles should be shortened and augmented. An unstable MCP joint should be stabilized. AP, adductor pollicis; APL, abductor pollicis longus; DI, first dorsal interosseous; EPB, extensor pollicis brevis; EPL, extensor pollicis longus; FPB, flexor pollicis brevis; FPL, flexor pollicis longus. |
result from intrinsic muscle spasticity and extrinsic extensor
overpull, partly caused by the wrist-flexion deformity, compensation
for weak wrist extensors, or both, with resultant overpull on the
central slip of the extensor mechanism. With time the volar plate
becomes incompetent and the proximal interphalangeal joint may
subluxate and become fixed. In the latter situation it is unlikely that
functioning can be improved with surgery. In early or mild deformities,
correction of the wrist flexion alone may suffice, and splinting may be
added if the deformities are less than 40 degrees. In more severe
deformities, the surgical treatment is much more complex (135,332,334).
transfers, is essential to the success of hand and upper extremity
surgery in cerebral palsy. The recommended treatment for most patients
is 4 weeks of complete immobilization, followed by an intensive
exercise program with emphasis on reach, grasp, and release. Removable
splints are used for several months during the day, and often at night,
for several years (332). Although only a small
percentage of children with spastic cerebral palsy benefit from
upper-extremity surgery, such reconstruction can be of substantial
benefit for the appropriate patient.
JK, Nelson KB, Cummins SK. Twinning and cerebral palsy: experience in
four northern California counties, births 1983 through 1985. Pediatrics 1993;92:854–858.
DS, Karlin LI. The relationship between preoperative nutritional status
and complications after an operation for scoliosis in patients who have
cerebral palsy. J Bone Joint Surg Am 1993;75:880–884.
LH, et al. The POSNA pediatric musculoskeletal functional health
questionnaire: report on reliability, validity, and sensitivity to
change. Pediatric Outcomes Instrument Development Group. Pediatric
Orthopaedic Society of North America. J Pediatr Orthop 1998;18:561–571.
WK, Renshaw TS. The treatment of scoliosis in cerebral palsy by
posterior spinal fusion with Luque-rod segmental instrumentation. J Bone Joint Surg Am 1988;70:41–44.
KB, et al. Longitudinal parental perceptions of spinal fusion for
neuromuscular spine deformity in patients with totally involved
cerebral palsy. J Pediatr Orthop 2003;23:143–149.
JE, Akbarnia A. Operative treatment of spinal deformities in patients
with cerebral palsy or mental retardation. An analysis of one hundred
and seven cases. J Bone Joint Surg Am 1983;65:43–55.
RL, et al. Same-day versus staged anterior-posterior spinal surgery in
a neuromuscular scoliosis population: the evaluation of medical
complications. J Pediatr Orthop 1996;16: 293–303.
CG, et al. Validity of a two-variable nutritional index for use in
selecting candidates for nutritional support.J Parenter Enter Nutr 1983;7:15–20.
DH, et al. Psoas release at the pelvic brim in ambulatory patients with
cerebral palsy: operative technique and functional outcome. J Pediatr Orthop 1997;17:563–570.
R, Baumann JU. Clinical benefit of reconstruction of dislocated or
subluxated hip joints in patients with spastic cerebral palsy. J Pediatr Orthop 1994;14:290–294.
R, Baumann JU. Long-term effects of intertrochanteric varus-derotation
osteotomy on femur and acetabulum in spastic cerebral palsy: an 11- to
18-year follow-up study. J Pediatr Orthop 1997;17:585–591.
KJ, et al. Varus derotation osteotomy for the treatment of hip
subluxation and dislocation in cerebral palsy: statistical analysis in
73 hips. J Pediatr Orthop B 2001;10:279–286.
SJ, Valencia FG, Wenger DR. One-stage correction of the spastic
dislocated hip. Use of pericapsular acetabuloplasty to improve
coverage. J Bone Joint Surg Am 1992;74:1347–1357.
JE, et al. The effect of unilateral varus rotational osteotomy with or
without pelvic osteotomy on the contralateral hip in patients with
perinatal static encephalopathy. J Pediatr Orthop 1998;18:734–737.
HH. Triple osteotomy of the innominate bone. A procedure to accomplish
coverage of the dislocated or subluxated femoral head in the older
patient. Clin Orthop 1977; Jan–Feb(122):116–127.
NP, Mubarak SJ, Wenger DR. One-stage correction of the dysplastic hip
in cerebral palsy with the San Diego acetabuloplasty: results and
complications in 104 hips. J Pediatr Orthop 2000;20:93–103.
PG, et al. Prosthetic interposition arthroplasty for the palliative
treatment of end-stage spastic hip disease in nonambulatory patients
with cerebral palsy. J Pediatr Orthop 1999;19:796–804.
KA, Bagg M, Nason SS. Treatment of the chronically dislocated hip in
adolescents with cerebral palsy with femoral head resection and
subtrochanteric valgus osteotomy. J Pediatr Orthop 1990;10:504–509.
RK. Spastic paraplegia and diplegia. An evaluation of non-surgical and
surgical factors influencing the prognosis for ambulation. J Bone Joint Surg Am 1966;48:827–846.
ES, Park CI, Kim JY. Comparison of anterior and posterior walkers with
respect to gait parameters and energy expenditure of children with
spastic diplegic cerebral palsy. Yonsei Med J 2001;42:180–184.
MJ, et al. Technique for iliopsoas ultrasound-guided active
electromyography-directed botulinum A toxin injection in cerebral
palsy. J Pediatr Orthop 2002;22:165–168.
N, et al. The role of botulinum toxin in the treatment of lower limb
spasticity in children with cerebral palsy—a pilot study. Israel J Med Sci 1997;33:129–133.
LA, et al. Botulinum toxin type A neuromuscular blockade in the
treatment of lower extremity spasticity in cerebral palsy: a
randomized, double-blind, placebo-controlled trial. BOTOX Study Group. J Pediatr Orthop 2000;20:108–115.
R, et al. Botulinum toxin treatment of spasticity in diplegic cerebral
palsy: a randomized, double-blind, placebo-controlled, dose-ranging
study. Dev Med Child Neurol 2002;44:666–675.
IS, et al. Botulinum toxin A compared with stretching casts in the
treatment of spastic equinus: a randomised prospective trial. J Pediatr Orthop 1998;18:304–311.
AL, et al. Effects of continuous intrathecal baclofen infusion and
selective posterior rhizotomy on upper extremity spasticity. Pediatr Neurosurg 1995;23:82–85.
PC, Albright AL, Johnstone GF. Intrathecal baclofen infusion and
subsequent orthopedic surgery in patients with spastic cerebral palsy. J Neurosurg 1998;88:1009–1013.
GR, Dias LS, Gaebler-Spira D. Selective posterior rhizotomy and
soft-tissue procedures for the treatment of cerebral diplegia. J Bone Joint Surg Am 1995;77:713–718.
JE, Davis BL, Luciano MG. Quantifying muscle activity in non-ambulatory
children with spastic cerebral palsy before and after selective dorsal
rhizotomy. J Electromyogr Kines 2001;11:31–37.
FV, et al. Evaluation of selective dorsal rhizotomy for the reduction
of spasticity in cerebral palsy: a randomized controlled tria. Dev Med Child Neurol 1998;40:239–247.
P, et al. A randomized clinical trial to compare selective posterior
rhizotomy plus physiotherapy with physiotherapy alone in children with
spastic diplegic cerebral palsy. Dev Med Child Neurol 1997;39:178–184.
H, et al. Follow-up of children with cerebral palsy after selective
posterior rhizotomy with intensive physiotherapy or physiotherapy
alone. Neuropediatrics 2003;34:67–71.
JC, Hoffman EB, Arens LJ. Spondylolysis and spondylolisthesis after
five-level lumbosacral laminectomy for selective posterior rhizotomy in
cerebral palsy. Childs Nerv Syst 1993;9:285–287; discussion 287–288.
G, Robinson C, Fewell RR. The effects of early motor intervention on
children with Down syndrome or cerebral palsy: a field-based study. J Dev Behav Pediatr 2001;22:153–162.
V, Evans AL. Evaluation of the functional effects of a course of Bobath
therapy in children with cerebral palsy: a preliminary study. Dev Med Child Neurol 2002;44:447–460.
R, Frost HM. Cerebral palsy. Spastic varus and forefoot adductus,
treated by intramuscular posterior tibial tendon lengthening. Clin Orthop 1971;79:61–70.
SJ, et al. A comparison of the effects of solid, articulated, and
posterior leaf-spring ankle-foot orthoses and shoes alone on gait and
energy expenditure in children with spastic diplegic cerebral palsy. Orthopedics 2002;25:411–415.
S, et al. The efficacy of tone-reducing features in orthotics on the
gait of children with spastic diplegic cerebral palsy. J Pediatr Orthop 2000;20:210–216.
MD, Cusick B. Preliminary report: the role of short-leg, tone-reducing
casts as an adjunct to physical therapy of patients with cerebral
palsy. Johns Hopkins Med J 1979;145:112–114.
SA, et al. Kinematic and kinetic evaluation of the ankle after
lengthening of the gastrocnemius fascia in children with cerebral
palsy. J Pediatr Orthop 1993;13:727–732.
PA, et al. Alterations in surgical decision making in patients with
cerebral palsy based on three-dimensional gait analysis. J Pediatr Orthop 1997;17:608–614.
MS, et al. Length and force of the gastrocnemius and soleus during gait
following tendo Achilles lengthenings in children with equinus. Gait Posture 2002;15:130–135.
J. Functional changes in the antagonists after lengthening the agonists
in cerebral palsy. I. Triceps surae lengthening. Clin Orthop 1990;Apr(253):30–34.
DA, Chambers C. Preserving plantar flexion strength after surgical
treatment for contracture of the triceps surae: a computer simulation
study. J Orthop Res 1995;13:971–972.
RL, de la Garza J, Rang M. The myth of muscle balance. A study of
relative strengths and excursions of normal muscles about the foot and
ankle. J Bone Joint Surg Br 1985;67:432–437.
HK, Fixsen JA. Lengthening of the calcaneal tendon in spastic
hemiplegia by the White slide technique. A long-term review. J Bone Joint Surg Br 1988;70:472–475.
B, et al. Preoperative and postoperative assessment of surgical
intervention for equinus gait in children with cerebral palsy. J Pediatr Orthop 1993;13:24–31.
WJ, Bernstein S. Equinus deformity in cerebral palsy. A comparison
between elongation of the tendo calcaneus and gastrocnemius recession. J Bone Joint Surg Br 1972;54:272–276.
FB, et al. Correction of equinus in cerebral palsy by the Murphy
procedure of tendo calcaneus advancement: a preliminary communication. Dev Med Child Neurol 1975;17:182–185.
AD, Feldman R, Lehman WB. Equinus deformity in cerebral palsy: a
retrospective analysis of treatment and function in 39 cases. J Pediatr Orthop 1985;5:678–681.
V, et al. The Baumann procedure for fixed contracture of the
gastrosoleus in cerebral palsy. Evaluation of function of the ankle
after multilevel surgery. J Bone Joint Surg Br 2000;82:535–540.
G, Pilliard D. Lengthening of the triceps surae muscle in children with
motor disorders of cerebral origin performed before the age of 6.
Results at the end of growth. Rev Chir Orthop Reparatrice Appar Mot 1988;74:79–84.
TF Jr, Kaufer H, Hensinger RN. Split posterior tibial-tendon transfers
in children with cerebral spastic paralysis and equinovarus deformity. J Bone Joint Surg Am 1985;67:186–194.
M, Kumar SJ, Stecyk MD. Split tibialis posterior tendon transfer and
tendo-Achillis lengthening for spastic equinovarus feet. J Pediatr Orthop 1993;13:20–23.
FJ, Kruse R. Split tibialis posterior tendon transfer with concomitant
distal tibial derotational osteotomy in children with cerebral palsy. J Pediatr Orthop 2001;21:95–101.
M, Balon K. Deformity of the foot following anterior transfer of the
posterior tibial tendon and lengthening of the Achilles tendon for
spastic equinovarus. Clin Orthop 1977; Jun(125):113–118.
MM, Barakat G, Koffman M. 10-year follow-up of split anterior tibial
tendon transfer in cerebral palsied patients with spastic equinovarus
deformity. J Pediatr Orthop 1985;5:432–434.
VS. Calcaneal lengthening for valgus deformity of the hindfoot. Results
in children who had severe, symptomatic flatfoot and skewfoot. J Bone Joint Surg Am 1995;77:500–512.
A, et al. Lateral column lengthening as treatment for planovalgus foot
deformity in ambulatory children with spastic cerebral palsy. J Pediatr Orthop 2000;20:501–505.
AH, et al. Subtalar stabilization of the planovalgus foot by staple
arthroereisis in young children who have neuromuscular problems. J Bone Joint Surg Am 1990;72:840–845.
LA, Mooney JF, Goodman A 3rd. Management of valgus hindfoot deformity
in pediatric cerebral palsy patients by medial displacement osteotomy. J Pediatr Orthop 1993;13:180–183.
DA, et al. Distal tibial/fibular derotation osteotomy for correction of
tibial torsion: review of technique and results in 63 cases. J Pediatr Orthop 1998;18:95–101.
H, et al. Prediction of outcome after rectus femoris surgery in
cerebral palsy: the role of cocontraction of the rectus femoris and
vastus lateralis. J Pediatr Orthop 1998;18:703–711.
S, et al. Rectus femoris surgery in children with cerebral palsy. Part
II: a comparison between the effect of transfer and release of the
distal rectus femoris on knee motion. J Pediatr Orthop 1993;13:331–335.
S, et al. Rectus femoris surgery in children with cerebral palsy. Part
I: the effect of rectus femoris transfer location on knee motion. J Pediatr Orthop 1993;13:325–330.
JH. Techniques of psoas tenotomy and rectus femoris transfer: “new”
operations for cerebral palsy diplegia—a description. J Pediatr Orthop B 1996;5:242–246.
DA, et al. Rectus and hamstring surgery in cerebral palsy: a gait
analysis study of results by functional ambulation level. J Pediatr Orthop 2002;22:672–676.
T, Tada S, Hajime T. Insufficiency of the hip adductor after anterior
obturator neurectomy in 42 children with cerebral palsy. J Pediatr Orthop 1986;6:686–692.
T, Hara H, Tada S. Selective lengthening of the psoas and rectus
femoris and preservation of the iliacus for flexion deformity of the
hip in cerebral palsy patients. J Pediatr Orthop 1987;7:690–698.
TF, Trost JP, Schwartz MH. Intramuscular psoas lengthening improves
dynamic hip function in children with cerebral palsy. J Pediatr Orthop 2002;22:158–164.
JR, et al. Assessment of femoral anteversion in children with cerebral
palsy: accuracy of the trochanteric prominence angle test. J Pediatr Orthop 2002;22:173–178.
JR, et al. Femoral anteversion in children with cerebral palsy.
Assessment with two and three-dimensional computed tomography scans. J Bone Joint Surg Am 2003;85-A:481–488.
A, et al. Spastic hemiplegic cerebral palsy and the femoral derotation
osteotomy: effect at the pelvis and hip in the transverse plane during
gait. J Pediatr Orthop 2003;23:314–320.
R, Meier G, Ruepp T. Comparison of a stiff and a spring-type ankle-foot
orthosis to improve gait in spastic hemiplegic children. J Pediatr Orthop 1998;18:719.
K, Hamada S, Shimizu N, et al. Anterior transfer of the long toe
flexors for the treatment of spastic equinovarus and equinus foot in
cerebral palsy. J Pediatr Orthop 1988;8:164.
BA, Lauryssen C, Perlmutter JS. Preoperative treatment with botulinum
toxin to facilitate cervical fusion in dystonic cerebral palsy. Report
of two cases. J Neurosurg 1998;88:328.
MM, Perry J, Melkonian G. Dynamic EMG and decision-making for surgery
in the upper extremity of patients with cerebral palsy. J Hand Surg 1979;4:424.
CA, Gelberman RH, Rhoades C. Upper extremity tendon transfers in
cerebral palsy: electromyographic and functional analysis. J Pediatr Orthop 1985;5:69.
J, Hoffer MM. Preoperative and postoperative dynamic EMG as an aid in
planning tendon transfers in children with cerebral palsy. J Bone Joint Surg Am 1977;59:531.
PR, Langewisch KR, Strecker WB, et al. Anterior elbow release of
spastic elbow flexion deformity children with cerebral palsy. J Pediatr Orthop 2001;21:772–777.
W, Strecker WB, Coe J, et al. Use of the green transfer in treatment of
patients with spastic cerebral palsy: 17 year experience. J Pediatr Orthop 1991;11:731.
MM, Lehman M, Mitani M. Long-term follow-up on tendon transfers to the
extensors of the wrist and fingers in patients with cerebral palsy. J Hand Surg 1986;11:836.
