Articular Cartilage Structure, Composition, and Repair
-
Hyaline cartilage: characterized by type II collagen and aggregating proteoglycan
-
Skeletal: articular cartilage, costal cartilage, and growth plate
-
Extraskeletal: larynx, trachea, bronchi, and nose
-
-
Elastic cartilage: characterized by preponderance of elastic fibers
-
Mainly in the ear and epiglottis
-
-
Fibrocartilage: characterized by type I collagen and lower proteoglycan content than hyaline cartilage
-
Skeletal: meniscus of the knee, annulus fibrosus of the intervertebral discs
-
single cell, the chondrocyte, is charged with all aspects of
maintaining the structure. Growth plate cartilage, meniscus, and
intervertebral disc are covered in separate chapters.
cartilage makes it very durable but with minimal capacity for repair.
Its aneural structure makes it liable to injury in an overuse or
post-traumatic situation, as pain is not necessarily perceived.
wear, trauma, inflammation, and infection will allow the clinician to
rationally treat cartilage disorders.
tibiofibular syndesmosis) or fibrocartilaginous (symphysis pubis).
However, most of the joints in the body are synovial and consist of
hyaline articular cartilage covering the surface of bones where they
meet in mobile joints. In the healthy young individual, articular
cartilage has a white, lustrous, and smooth appearance. It is composed
of living cells of only one cell type, the articular chondrocyte,
supporting and maintaining an extracellular matrix.
|
Table 20-1 Matrix and Cellular Features of Three Articular Cartilage Regions
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
-
To provide a smooth surface compatible with friction-less motion
-
To resist the compressive forces encountered across the joint under loading
-
This function is provided by the proteoglycan content.
-
-
Morphologically, articular cartilage can be divided into three regions (Table 20-1),
which can be distinguished by their differing orientation of type II
collagen fibrils, orientation and cellular features of the
chondrocytes, and histologic staining (Table 20-2).-
Superficial collagen-rich tangential zone
-
Intermediate transitional zone
-
Deep/basal zone
-
-
Morphologic correlation of articular cartilage on Hema-toxylin and Eosin (H and E) stain (Fig. 20-1 A)
-
Eosin the acidic stain in the two part H
and E stain; tends to stain most proteins red since most proteins are
weak bases (negatively charged) -
Hematoxylin, a basic stain, characteristically stains the nucleic acids of the nuclei of cells, and appears blue
-
This histologic correlate of staining in articular cartilage with these two standard stains distinguishes three regions:
-
Superficial zone, containing the negatively charged protein, collagen, picks up the eosin and stains redTable 20-2 Histological Properties of Articular Cartilage
Histologic Stain Fast green Safranin O Substance stained Protein Proteoglycans (polyanions) Articular cartilage layer(s) stained Tangential Intermediate & deep -
Bone/cartilage junction with denser collagen anchoring elements, as well as the subchondral bone also stains red
-
Intermediate and basal layers, rich in proteogly-cans, pick up the basic hematoxlyin stain, and thus appear blue
-
* Special stains (see Table 20-2)-
More apparent visualization of these layers is apparent with a “fast green/Safranin O stain”
-
Fast green stains the collagen green and thus green will predominate in the superficial layer and the bone/cartilage junction
-
Safranin O, a cationic dye, bonds to the
polyanions of the proteoglycan and imparts a red color to the
intermediate and deep layers.
-
-
Mature articular cartilage contains about 5% of its volume as cells, the remainder being extracellular matrix.
-
There is normally no mineral, and organic material accounts for about 30% of the matrix, with the remainder being water.
-
About 60% of the organic material is collagen, 25% is proteoglycan, and the remainder is a variety of matrix proteins.
-
-
Articular cartilage is characterized by having no nerves and no vascular system.
-
No nerves
-
Positive aspect: allows pain-free motion of the joints during normal use
-
Negative aspect: cartilage injury not perceived by the individual
-
-
No blood vessels
-
Nutrition and hydration for the tissue must arise from the synovial fluid by diffusion.
-
Clinical correlate: Cartilage exposed
during surgery will rapidly dry, with resulting disruption of cell
function, even leading to cell death, unless periodically bathed in
fluid (every several minutes). The effect of drying on the matrix is
not known.
-
-
No system for repair, as the usual microvascular source for cells in connective tissue repair is absentP.407
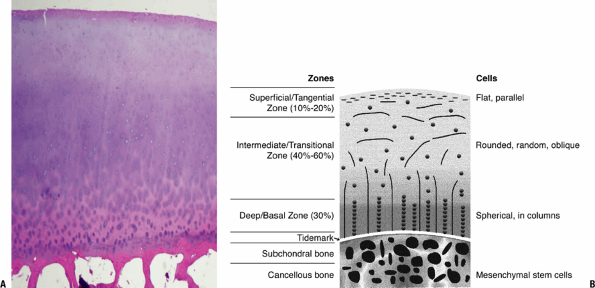 Figure 20-1
Figure 20-1
Cartilage morphology. (A) Superficial layer collagen stains red with
eosin; intermediate layer proteoglycan stains bluish with hematoxylin.
The basal layer with increasing collagen, binding the cartilage to bone
and stains predominantly red with the eosin. The subchondral bone
below, primarily collagen and mineral, stains densely red. (B) Diagram
on the right outlines the corresponding zones and cellular morphology.
(© 2003 American Academy of Orthopaedic Surgeons. Reprinted from the Journal of the American Academy of Orthopaedic Surgeons, Volume 11 (6), pp. 421–430.) -
Clinical correlate: Incisions in cartilage (e.g., during arthroscopy) do not heal.
-
-
Chondrocytes undergo anaerobic metabolism and survive for several days in the body following death.
-
Clinical correlate: allows some cell viability in fresh tissue bulk allografting
-
-
|
Table 20-3 Adult Articular Cartilage Composition
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
-
Collagens consist of three polypeptide chains that form a triple helix along at least part of their length.
-
They can be divided into fibrillar
collagens (mainly types I, II, III, V, and XI) and nonfibrillar
collagens (types IV, VI, VII, VIII, IX, and X). -
The fibrillar collagens form the framework of connective tissues.
-
The fibrils consist of triple helical
collagen molecules, arranged head to tail in linear arrays and side by
side in a staggered manner (Fig. 20-2). -
This staggered lateral arrangement gives
the collagen fibril its characteristic cross-striated appearance when
viewed in the electron microscope and contributes to its high tensile
strength.
-
-
Nonfibrillar collagens serve both structural and non-structural functions.
-
Different connective tissues contain different collagen types, reflecting their varied functions.
-
Fibrillar collagens in cartilage: types II and XI collagen
-
Types II and XI collagen occur in the same fibrils (see Fig. 20-2).
-
Type II collagen: 90% to 95% of total collagen, 10% of weight of cartilage
-
Type XI collagen: 5% to 10% of total collagenP.408
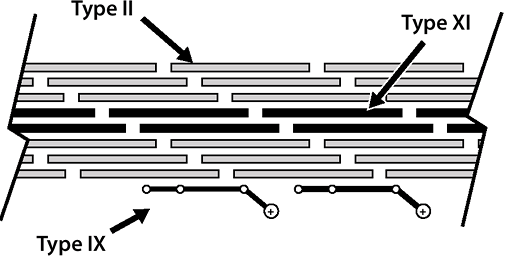
![]() Figure 20-2
Figure 20-2
Cartilage collagens. Type II collagen provides the primary structural
element (90%); type XI limits fibril diameter; type IX facilitates the
interaction between the framework and the entrapped proteoglycan.-
While fibrillar itself, serves to limit fibril diameter of type II
-
-
-
Nonfibrillar collagens in cartilage: type IX and X collagen
-
Type IX collagen
-
Resides on the surface of the fibrils
-
Facilitates interaction between the collagenous framework of the tissue and the interspersed proteoglycan
-
-
Type X collagen
-
Plays an integral role in the mineralization process in the growth plate
-
Site-specific immunostaining demonstrates
localization that varies in noncalcified articular cartilage; animal
work suggests that the presence of type X collagen may provide
increased structural strength to normal articular cartilage. -
In osteoarthritic and rheumatoid
cartilage that is morphologically abnormal, there is expression of type
X collagen; its role in normal articular cartilage is under
investigation but has not yet been determined with certainty.
-
-
-
Fibrocartilage contains type I collagen as its predominant fibrillar collagen, in common with most other connective tissues.
-
Physiologic conditions
-
Slow turnover: half-life of years
-
Net balance over time between formation and degradation
-
-
Disease states
-
Shift to more rapid turnover disrupts the balance.
-
If the rate of degradation exceeds formation, then loss of collagen and proteoglycan ensues and degeneration follows.
-
-
Proteoglycans are present in the extracellular matrix of all connective tissues, with the structure varying according to tissue.
-
They consist of a central protein core to
which sulfated glycosaminoglycans (chondroitin sulfate, dermatan
sul-fate, or keratan sulfate) are covalently attached. -
In articular cartilage, it is the
proteoglycans that serve the major role in accomplishing the primary
function of the tissue in resisting compressive loads.
-
Hyaline and elastic cartilages contain
predominantly ag-grecan, one of the aggregating proteoglycans, which,
by definition, aggregate or interact with hyaluronic acid. -
Fibrocartilages do not contain as high an aggrecan content.
-
Many aggrecan molecules link to a central
molecule of hyaluronic acid by a link protein that is devoid of
sulfated glycosaminoglycans. -
Aggrecan molecule
-
Long core protein with two types of
glycosaminoglycan chains, chondroitin sulfate (100 per molecule) and
keratan sulfate (60 per molecule), molecular weight 2,500,000 daltons,
90 percent carbohydrate -
Aggrecan molecules account for 25% of the dry weight of cartilage.
-
-
Proteoglycan aggregates provide the tissue with its turgid nature that resists compression.
-
All cartilages, in common with all soft
tissues, also contain nonaggegating proteoglycans, which interact with
collagen fibrils rather than hyaluronic acid, and function to stabilize
the matrix. -
These are decorin, biglycan, fibromodulin, and lumican.
-
They are much smaller than aggrecan and
possess only a few dermatan sulfate (decorin and biglycan) or keratan
sulfate (fibromodulin and lumican) chains. -
They mediate the interactions between adjacent collagen fibrils, or with other matrix components.P.409
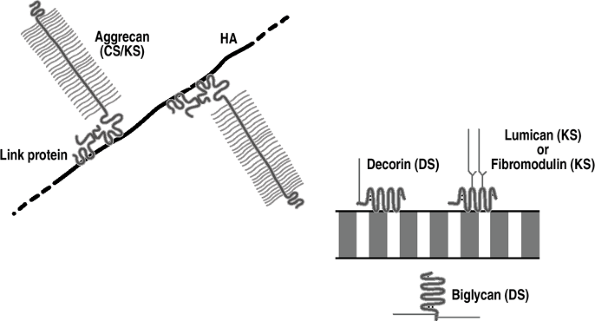 Figure 20-3
Figure 20-3
Cartilage proteoglycans. Aggrecan is the major aggregating proteoglycan
(25% of dry weight); it is associated with compression and linked to
hyaluronic acid (HA). The other proteoglycans are nonaggregating and
associate with and stabilize collagen fibrils. -
Cartilage also contains perlecan, a
heparan sulfate proteoglycan normally associated with basement
membrane, whose function is unknown.
loading and motion. Survival of the chondrocytes depends on adequate
nutrition, and the passive diffusion of nutrients from the synovial
fluid is aided by joint loading and motion. In general, dynamic
(cyclic) loading is beneficial to matrix synthesis, whereas static
loading is detrimental, causing a decrease in the synthesis of aggrecan
and link proteins.
-
Promotes chondrocyte nutrition and function, stimulating the production and maintenance of the matrix
-
Cyclic loading clinical correlate: contributes to the beneficial effects of continuous passive motion (CPM)
-
Joint immobilization clinical correlate:
Absence of this beneficial nutritional effect contributes to the
cartilage atrophy observed upon prolonged joint underuse or
immobilization.
-
-
Aggregating proteoglycans provide the
articular cartilage with its resilience to compression, as compressive
forces are counterbalanced by the focal increase in proteoglycan
swelling potential (Fig. 20-4).-
Serves to protect the chondrocytes from adverse forces
-
-
Compressibility is a function of the high hydrostatic pressure of cartilage.
-
Water content is not uniform throughout cartilage,
-
but rather is lower in the superficial layers and higher in the deeper layers.Table 20-4 AGGREGATING AND NONAGGREGATING PROTEOGLYCANS
Proteoglycan Type Interaction Function Molecule(s) Glycosaminoglycan Type Aggregating Hyaluronic acid Resist compressive forces Aggrecan Chondroitin sulfate Keratan sulfate Nonaggregating Collagen fibrils Stabilize collagen matrix Decorin
Biglycan
Fibromodulin
LumicanDermatan sulfate
Dermatan sulfate
Keratan sulfate
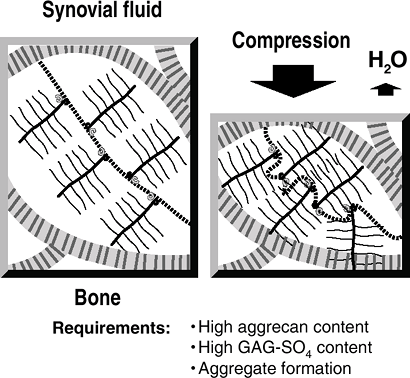
Keratan sulfateCell matrix interaction Perlecan Heparan sulfate ![]() Figure 20-4
Figure 20-4
Resistance to compression. On the left, articular cartilage is in
equilibrium, with the swelling pressure of the proteoglycan balanced by
the tensile force in the collagen fibril. With compression, water is
squeezed out of the cartilage (taking waste metabolites with it); a new
equilibrium is reached, with an increased swelling pressure of the
proteoglycan balancing the applied compression. When the compression is
removed, water is drawn in (along with nutrients) and the former steady
state is achieved. -
Water squeezed out of cartilage by
compression contributes to the thin boundary layer of liquid that
reduces friction between the opposing articular surfaces. -
Cartilage layers differ in their water flow and in their compressive strains (Fig. 20-5).
P.410 -
-
Excessive loads or overuse cause
chondrocytes to release proteolytic enzymes, which damage the
proteoglycan and collagen, causing tissue degeneration and decreased
ability to protect the cells.
-
Aggregating proteoglycans, highly
sulfated, have a strong affinity for water and expand their molecular
domain by drawing water and nutrients into the tissue. -
As more water is drawn into the cartilage, the swelling potential of the proteoglycan decreases.
-
A balance is established wherein the
outward swelling of the proteoglycan is resisted by stretching forces
in the collagenous framework of the tissue. -
Application of a load compresses the
tissue, and water is displaced into the joint from the focally
entrapped and large proteoglycan aggregates, creating a new equilibrium.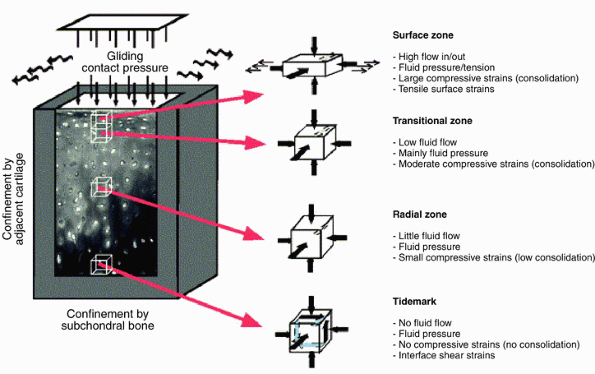 Figure 20-5
Figure 20-5
The mechanical environment of articular cartilage under intermittent
joint loading and motion. (From Wong M, Carter DR. Articular cartilage
functional histomorphology and mechanobiology: A research perspective. Bone 2003;33:1–13.) -
When the load is removed, water is drawn back into the cartilage, restoring the original equilibrium.
-
Linked to the flux of water, nutrients move into, and waste products out of, the matrix.
physiological regulation throughout life are controlled by the
chondrocyte. Sequestered in the matrix, this cell receives its oxygen
and nutrients by diffusion. The tissue pressure of oxygen is low,
leading to the production of energy by glycolysis. Mechanical forces
are sensed by the chondro-cytes, which are linked to the matrix by
integrins, cell surface-binding proteins.
-
Conditioned by surface receptors, regulation of both anabolic and catabolic activities is carried out by these cells.
-
The functions of the chondrocytes include growth and maintenance of the matrix, which is balanced by matrix degradation.
-
Tissue formation (growth, synthesis): produce structural macromolecules (collagen and proteoglycan)
-
Tissue turnover (degradation): produce
secreted pro-teinases (including collagenases, gelatinases,
stromely-sins, and aggrecanases)
-
Hormones: chemical messengers synthesized by specialized endocrine glands and transported to act on distant cells
-
Cytokines: molecules commonly secreted by immune or other cells to act on damaged or infected tissue
-
Growth factors: molecules synthesized and
secreted by cells in a variety of tissues and generally act on nearby
cells in a paracrine or autocrine fashion-
Often secreted in an inactive form and
may bind to extracellular matrix components, requiring proteolytic
cleavage for activation and cell receptor binding
-
-
Tissue formation (growth) regulators
-
Insulin-like growth factor-1; somatomedin-C
-
Increases collagen and proteoglycan synthesis
-
Synergistic with mechanical stimulation
-
-
Transforming growth factor beta (TGF-β)
-
Binds to specific cell surface receptors that activate intracellular pathways that regulate the expression of genes
-
Chondroprotective
-
Increases collagen and proteoglycan synthesis
-
Inhibits matrix degeneration and cell proliferation
-
-
BMP-2 and BMP-7 both increase proteoglycan synthesis and maintain chondrocyte phenotype.
-
-
Tissue turnover (matrix degradation) regulators (Fig. 20-6)
-
Promoted by a variety of cytokines, such as interleukin-1 (IL-1) and tumor necrosis factor alpha (TNFα)
-
Both IL-1 and TNFa are implicated as mediators of inflammatory and degenerative arthritis.
-
-
Stimulate the secretion of specific
collagenases that are part of a group of enzymes called matrix
metallo-proteinases (MMPs) from the chondrocytes -
Inhibit synthesis of the structural macromolecules
-
-
Homeostasis
-
Normally proteinase destruction is kept
under control by the concomitant secretion of tissue inhibitor of
metalloproteinases (TIMPs), which can inhibit the action of MMPs. -
Clinical correlate: Drugs such as
nonsteroidal antiinflammatories (NSAIDs) and glucocorticoids used to
treat arthritic joints retard cartilage degeneration by affecting the
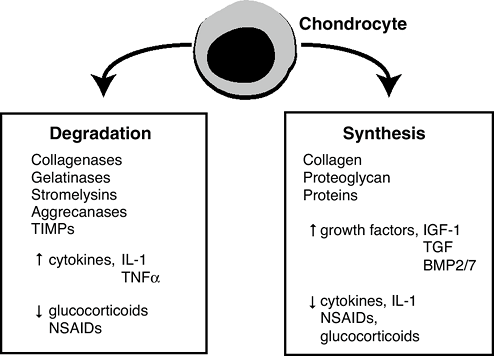
synthesis/degradation balance.![]() Figure 20-6
Figure 20-6
Cartilage turnover. The chondrocyte is the only cell in cartilage. It
supplies all of the molecules that make up this tissue: the collagen,
the proteoglycan, and all of the other matrix proteins. It does so in
response to a variety of growth factors, the most important being TGFp,
and insulin-like growth factor-1 (IGF-1, somatomedin-C). They stimulate
the chondrocyte to make more matrix. The chondrocyte also supplies the
enzymes needed for turnover. Turnover of cartilage during growth is
needed for remodeling and maintenance. The enzymes include aggracanase
and matrix metalloproteinases, including collagenase and stromelysin
which may have a variety of activities, including collagenase, and
stromelysin, which degrade the matrix. The chondrocyte also makes
inhibitors of these enzymes called tissue inhibitor of
metalloproteinases (TIMP). Normally a balance exists between synthesis
and degradation. The TIMP system controls degradation.P.412-
However, some NSAIDs have an undesirable inhibitory effect on matrix synthesis by chondrocytes.
-
-
-
Drugs can interact with chondrocytes in such a way as to alter function.
-
Glucocorticoids
-
Controversial: Most suggest deleterious over chondroprotective effect
-
Inhibit chondrocyte proliferation and decrease both collagen and proteoglycan synthesis
-
Impair response to cytokines such as TGF-β
-
Little effect on cartilage degradation in osteoarthritis
-
Potent inhibitors of cyclooxygenase-2 (COX-2)
-
-
Preponderance of evidence suggests little long-lasting benefit and possible deleterious effects of steroid injections.
-
-
NSAIDs
-
NSAIDs reduce inflammation through
reducing COX-2, but undesirable side effects are present due to the
co-inhibition of the ubiquitous and constitutively produced COX-1. -
COX enzymes catalyze the conversion of arachi-donic acid and oxygen to prostaglandin H2, the committed step in prostanoid biosynthesis; three isoforms exist:
-
COX-1: constitutive, exists in
endothelium, stomach (promotes gastric mucosal protection), kidney
(maintenance of renal blood flow and function), and platelets (promote
aggregation) -
COX-2: inducible isoform associated with the inflammatory response (increased prostaglandin E2 in arthritis)
-
COX-3, an isoenzyme of COX-1, is a target for acetaminophen and other antipyretic/ analgesic medications.
-
-
Inflammation in all forms of arthritis is
associated with increased production of COX-2 in response to cytokine
stimulation of synovial cells.-
Prostaglandins produced by the action of COX-2 mediate the features of inflammation.
-
COX-2 is also a product of chondrocytes in the noninflammatory osteoarthritis joint, though its precise role is unclear.
-
-
Coxibs (specific inhibitors of COX-2)
have demonstrated clinical efficacy in arthritis, but unfortunate and
unforeseen side effects of these have reduced their availability.
-
-
-
A global model of factors that might be operant in arthritis is outlined in Figure 20-7.
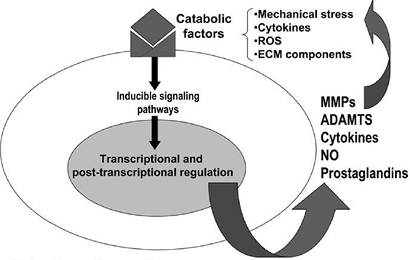 |
|
Figure 20-7
Pathways that modulate chondrocyte function in osteoarthritis (OA) are shown. Although the events that initiate cartilage destruction in OA are not defined, a number of potentially catabolic factors may interact with chondrocytes and induce signaling cascades that activate gene transcription and post-transcriptional modifications. These catabolic factors include pro-inflammatory cytokines, mechanical stress, reactive oxygen species (ROS), and extracellular matrix (ECM) components. These factors induce the synthesis of MMPs, aggrecanases (systemic nomenclature ADAMTS-4 and 5), cytokines, nitric oxide, and prostaglandins, which may feedback-regulate or amplify these responses, resulting in a vicious cycle leading to further destruction. (From Goldring MB, Berenbaum F. The regulation of chondrocyte function by proinflammatory mediators: prostaglandins and nitric oxide. Clin Orthop Rel Res 2004;(4275):S37–S46.) |
-
Arthritic destruction of articular cartilage takes place mainly via the action of proteolytic enzymes.
-
In the early stages of disease, loss of proteoglycan is reversible, whereas at later stages there is irreversible loss (Table 20-6).
-
Intact cartilage consists of a balance
between the containing forces of the collagen framework versus the
expanding hydrostatic force of the contained proteoglycans (see Fig. 20-4). -
The earliest visible change in arthritis
is loss of integrity of the collagen, resulting in tissue fibrillation
and ultimate erosion to subchondral bone (see Table 20-6). -
Loss of proteoglycans from the matrix causes a loss of osmolality, with an increase in compressibility.
-
The initial response to tissue loss is an anabolic, reparative reaction.
-
Unfortunately, despite chondrocyte
proliferation and increased collagen and proteoglycan synthesis, the
exquisite and unique interrelationship that exists in cartilage is not
so easily replicated. -
As the function of cartilage is
protection from compres-sive load, the radiographic correlates to this
process of cartilage loss involve first loss of joint space, then
osteo-phyte formation, reflecting progressive remodeling to the
increased load. -
Well-established pathways of cartilage destruction include the following (Fig. 20-8):P.413Table 20-5 MECHANISM OF CARTILAGE DESTRUCTION IN ARTHRITIS
Type Origin Disorder Cause of Increased Proteolysis Degenerative Mechanical Osteoarthritis Normal matrix, abnormal load (misalignment, trauma, occupation)
Abnormal matrix, normal load (chondrodysplasia, drug, synovitis)Inflammatory Microorganism
ReactiveSeptic Microorganism within joint (infectious-bacterial, viral, fungal)
Bacterial infection at remote site (related antigen in joint)Autoimmune Rheumatoid Autoimmune recognition of cartilage degradation product? (type II collagen, aggrecan) Crystal Gout Sodium urate Pseudogout (chondrocalcinosis) Calcium pyrophosphate -
Synovium and chondrocytes in
osteoarthritis express TNF-α, which may be responsible for the
inflammatory events, and IL-1, which promotes the cartilage degradation.-
TNF-α
-
Predominantly secreted by macrophages
-
Expressed in osteoarthritic cartilage
-
Not expressed in normal articular cartilage
-
-
Shares many functions with IL-1,
including suppression of proteoglycan synthesis, stimulation of
collagen degradation, and induction of MMP expression, but appears to
be less active than IL-1 -
Inhibits the synthesis of proteoglycans
and type II collagen by promoting chondrocyte dedifferentiation and
preventing cartilage repair
-
-
IL-1
-
Downregulates synthesis of collagen and proteoglycan
-
Upregulates synthesis of MMPs, the most important collagenases (MMP1 and MMP13)
-
Only enzymes able to degrade the collagen triple helix in cartilage
-
MMP 13 cleaves epitopes in type II
collagen in regions of matrix depletion in osteoarthritic cartilage and
is 5 to 10 times more active than MMP1 or MMP8. -
Upregulation is through an endogenous mediator, nitric oxide (see below).
-
-
Induces synthesis of aggrecanases (AD-AMTS4 and ADAMTS5)
-
These degrade the core protein of the proteoglycan aggrecan, which then diffuses out of the cartilage.
-
-
-
-
Prostaglandins and nitric oxide act as endogenous mediators of the actual damage.
-
Elevated in the articular cartilage,
synovium, and synovial fluid of osteoarthritic and rheumatoid joints
and serve as potential therapeutic targets for osteoarthritis
-
-
Nitric oxideTable 20-6 Sequential Morphologic and Radiographic Findings in Progressive Arthritis
Stage of Arthritis Morphologic Change Radiographic Correlate Early Reversible loss of proteoglycans No abnormalities Intermediate Permanent loss of proteoglycans
Collagen breakdown
Surface cracks and fibrillationJoint space narrowing
Osteophyte formation (progressive remodeling)Advanced Further loss of collagen framework
Ultimately complete loss, leading to raw eburnated bone surfaceSubchondral sclerosis (Wolff’s law) P.414![]() Figure 20-8
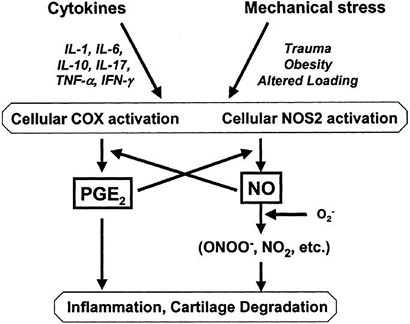
Figure 20-8
Pathways of interaction of mechanical stress and pro-inflammatory
mediators. Increased concentrations of cytokines or alterations in the
mechanical environment of the joint may activate COX2 and NOS2 in
articular cartilage, increasing production of prostaglandins or NO. IL,
interleukin; COX, cyclooxygenase enzyme; NO, nitric oxide; NOS, nitric
oxide synthase; PGE2, prostaglandin E2; TFN-α,
tumor necrosis factor-α; IFN-γ, interferon-γ. (From Guilak F, Fermor B,
Keefe FJ, et al. The role of biomechanics and inflammation in cartilage
injury and repair. Clin Orthop Rel Res 2004;(423):17–26.)-
Enzyme producing nitric oxide is nitric oxide synthase (NOS); exists in three forms
-
NOS2 can produce high levels of nitric oxide and most commonly is associated with inflammation in arthritic disorders.
-
Inhibition of nitric oxide production
using NOS2-specific inhibitors can considerably reduce disease
progression in animal models of osteoarthritis.
-
-
-
Prostaglandin E2
-
NSAIDs compete directly with arachidonic acid binding to the COX site and inhibit COX activity.
-
Aspirin covalently modifies and irreversibly inhibits COX.
-
Other NSAIDs such as ibuprofen and indomethacin act as reversible competitive inhibitors.
-
-
Osteoarthritis (degenerative arthritis):
Enzymes are released directly by the chondrocytes, due to abnormal
forces acting on the cells. -
Inflammatory arthropathies: Same enzymes
can also arise from the synovial cells; polymorphonuclear leukocytes
possess a distinct collagenase (MMP8) and contain other proteinases
(elastase and cathepsin G) able to degrade proteoglycan. -
Rheumatoid arthritis: Degradation
products of cartilage matrix macromolecules are thought to initiate a
T-cell-mediated autoimmune response, so exacerbating inflammation. -
Infectious arthritis: Bacterial
collagenases may give rise to very rapid cartilage destruction because
of their multiple sites of action along the collagen molecules. -
The mammalian collagenases cleave at only
a single site in the fibrillar collagen molecule, and cleavage of the
collagen fibril is a slow process. In contrast, most proteinases
degrade aggrecans, and thus loss of proteoglycan aggrecan is a rapid
process.
-
In general, it appears that if only
proteoglycan loss occurs, the cartilage may regenerate a normal matrix
over time, but once the collagen framework is damaged, the degenerative
process is irreversible.
-
Aggrecan is susceptible to damage by proteolytic enzymes.
-
Figure 20-9 shows
the structure of the proteoglycan with the aggrecan core protein, the
keratan and chon-droitin sulfate side chains, an interacting link
protein that is an attachment to hyaluronic acid (HA). -
Proteinase cleaves aggrecan at sites unprotected by link protein.
-
The major proteinase is aggrecanase, and there are two known at this time.
-
Aggrecanase-1 (ADAMTS-4) and
aggrecanase-2 (ADAMTS-5) are members of the disintegrin and
metalloprotease with thrombospondin motifs (ADAMTS) protein family. -
The link at the Glu373-ALA374 bond in the
aggrecan core protein is cleaved, and the core protein plus the heavily
negatively charged side chains are liberated into the tissue. -
The free fragments, being negatively
charged, are expressed rapidly from the negatively charged internal
environment of the cartilage and into the synovial fluid.
-
-
-
Hence, any damage to proteoglycans is
rapidly detrimental to this tissue’s physical properties. The only way
to stop progression is relative rest (eliminate increased load) and
treatment of the inflammation.
-
Collagen is difficult to damage; it resists proteolytic damage.
-
Collagen can be cleaved by a series of collagenases, three of which are made in humans.
-
MMP1: made by most connective tissues for internal remodeling
-
MMP8: made by leukocytes, used in remodeling tissues following damage
-
MMP13: made by chondrocytes, used in
cartilage; they cleave at only one site in the collagen molecule, in a
very slow process; because the enzyme works at only one site, and
because of the extensive cross-linking, the damage remains very
localized, especially when the damage is being initiated at the
chon-drocyte level.
-
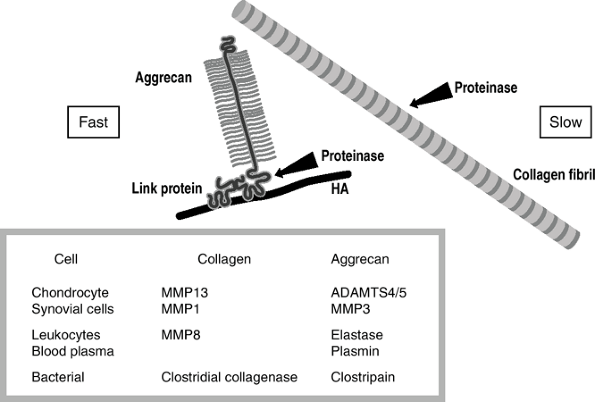 |
|
Figure 20-9
Arthritis: susceptibility of aggrecan and stability of collagen toward degradation. Aggrecan shows little resistance to proteolytic degradation, being rapidly cleaved by many proteinases adjacent to the hyaluronic acid (HA) binding domain of its core protein. In contrast, collagen, in the form of mature cross-linked collagen fibrils, is cleaved by only a few proteinases, and with the exception of the bacterial collagenases this is a slow event. |
-
Degenerative arthritis: Only the chondrocyte-initiated enzymes MMP13 and aggrecanase cause damage.
-
Inflammation: Inflammatory cells invade
the synovium, and both MMP1 and MMP13 become active. With acute
inflammation, as in rheumatoid arthritis and infection, the damage is
more rapid still because the leukocytes release MMP8 and elastase. -
Bleeding: Both in trauma and in
hemophilia, blood plasma is released into the joint. Plasmin also acts
on aggrecan to degrade it further. -
Bacterial infection: Bacteria can release
collagenases that are very damaging to joints. The bacteria use
collagen as food, and they therefore wish to generate extensive
degradation. The collagenases from bacteria cleave each collagen
molecule not at one site or even 10 sites, but rather at about 100
sites. They can lyse this molecule in a matter of minutes. Thus the
bacterial damage is rapid and profound, and hence the necessity for
very rapid diagnosis and treatment of bacterial arthritis. -
The hierarchy of damage to joints is hours to days for bacteria, months for inflammatory conditions, and years for degeneration.
-
The release of cartilage matrix
components into the synovial fluid has been used to monitor disease
status in the arthritic joint. -
Care must be taken in interpreting the
meaning of increase in marker levels, as some reflect increased
degradation (collagen cross-links and neoepitopes), some reflect
increased or altered synthesis (collagens C-propeptide and chondroitin
sulfate-neoepitopes), and others may reflect both (keratan
sulfate-neoepitopes).
-
Type II collagen markers (C-propeptide, telopeptide-de-rived cross-links and collagenase-derived neoepitopes)
-
Aggrecan markers (KS- and CS-derived neoepitopes)
-
It is well accepted that lesions confined to the avascular articular cartilage have a very limited capacity for repair.
-
However, when lesions penetrate the
subchondral bone, a wound healing response is observed, as cells
derived from the bone marrow fill the lesion and differentiate into
chondrocytes. This observation forms the basis of surgical repair
techniques.
-
Techniques used to penetrate the subchondral bone
-
Periosteum is a source of cells that can differentiate into chondrocytes.
-
Used to repair cartilage in a more controlled fashion than drilling/abrasion
-
Commercially available system has been
developed that allows harvest of chondrocytes, with culturing, and then
reimplantation under a periosteal flap. -
Alternatively, the cells can be embedded in an artificial matrix for implantation in a lesion.
-
Matrices: collagen, hyaluronic acid, fibrin and synthetic polymers
-
-
Various growth factors have been used in
these cell repair systems to promote matrix synthesis and stabilize the
chondrocyte phenotype.
-
Large frozen allografts with cryopreservation
-
Fresh osteochondral allografts implanted within a window of chondrocyte viability (7 days)
-
Small cylindrical osteochondral autografts can be harvested fresh and implanted in the same or other joints.
-
Chondrocyte survival in part determines long-term outcome.Table 20-7 Cartilage Repaira
Mechanism of Repair Technique Main Limitations Blood clot formation Abrasion or drilling subchondral bone
MicrofracturePhenotype stability Cell implantation Chondrocytes/marrow stem cells
Artificial matrix for support or covering membrane for retentionCell availability/phenotype stability Tissue transplantation Osteochondral grafts
Periosteal graftsTissue availability/chondral integration aLimitations regardless of technique may include cell or tissue availability, phenotype instability, chondral integration. Table 20-8 Chondroplasias: Defects in Matrix MoleculesGene Disorder COL2A1 Achondrogenesis type II
Hypochondrogenesis
Spondyloepiphyseal dysplasia
Kniest dysplasia
Stickler dysplasia
Familial osteoarthritisCOL9A2 Multiple epiphyseal dysplasia type II COL11A2 Stickler dysplasia (no eye involvement) COL10A1 Schmid metaphyseal dysplasia COMP Pseudoachondroplasia Multiple epiphyseal dysplasia type I Perlecan Dyssegmental dysplasia
Schwartz-Jampel syndrome(Also see Fig. 15-2) -
Early degenerative change is a consequence in part of a lack of survival.
-
An autoimmune response may complicate the result in bulk allograft situations.
-
A major problem in all repair systems is
achieving integration between the repair cartilage and the surrounding
normal cartilage.
-
for one of the matrix macromolecules or for one of the cellular
components involved in chondrocyte metabolism is defec
in
a chondrodysplasia, and impairment in the function of the articular
cartilage results in a premature familial osteoarthritis. Defects in
the matrix molecules include the genes for type II collagen (COL2A1),
type IX collagen (COL9A2), type X collagen (COL10A1), cartilage
oligomeric protein (COMP), and perlecan (Table 20-8).
Defects in cellular components include the genes for a growth factor
receptor (FGFR3), a hormone receptor (PTHrPR), a sulfate transporter
(DTDST), transcription factors (SOX9 and SHOX), and enzymes involved in
glycosaminoglycan metabolism (GAL, GLCN6S, and EXT; Table 20-9).
In many cases defects in the same gene can give rise to different
clinical phenotypes (COL2A1 and FGFR3), depending on the site and type
of mutation. It is also possible that defects in different genes can
give rise to the same clinical phenotype, if the different gene
products interact with one another (COL9A2 and COMP in multiple
epiphyseal dysplasia) or if they are involved in regulating the same
biochemical event (GAL and GALNS in Morquio syndrome).
|
Table 20-9 Chondrodysplasias: Defects in Cellular Molecules
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||
JT, Ayad S, Grant ME. Cartilage collagens: Strategies for the study of
their organization and expression in the extracellular matrix. Ann Rheum Dis 1994;53:488–496.