Amputations
fracture and trauma surgeon a foundation for amputation surgery.
Amputation surgery is one of the oldest known surgical procedures but
has, in the past generation of surgeons, been given decreased
importance particularly with regard to the proper surgical handling of
residual limbs. This may be because of the stigma that is attached to
amputations as a procedure of failure, namely failure of vascular
reconstruction, joint reconstruction, or limb salvage. Amputation
should be regarded as a reconstructive procedure restoring limb
function with the prosthesis serving as an extension of the limb, not
the limb solely being an attachment site for the prosthesis. It is my
hope to instill renewed interest in amputation surgery in the
traumatized patient.
prehistoric times. The earliest known documentation of an amputation as
a ritualistic act was noted on cave wall drawings dating back to
approximately 5,000 B.C. Archeologists noted that a Neanderthal
skeleton found in present-day Iraq provides evidence that the
individual had survived an above-elbow amputation.2
Indications for amputation were extended by Hippocrates and Celsus to
include the treatment of infection, a reduction in invalidism, removal
of useless limbs, and as a life-saving procedure in selected
circumstances. It was not until the ancient surgeons Archigenes and
Heliodorus expanded the indications to include traumatic injuries and
the use of proximal tight bandages for hemorrhage control, akin to the
modern tourniquet.3 During the
1500s, Paré reintroduced the importance of ligatures for hemorrhage
control and Clowes is credited with performing the first successful
transfemoral amputation. With the introduction of projectile weaponry
in the mid-1300s, battlefield injuries became more severe and maiming,
requiring a renewed interest in treating limb injuries with amputation.
During the late 1700s and into the early 1800s, the British surgeon
George Guthrie and the French surgeon Dominique-Jean Larrey challenged
the practice of delaying amputations for battlefield injuries for 3
weeks by advocating rapid primary amputation for these injuries. This
change in practice resulted in fewer deaths. Larrey also promoted the
rapid transport of wounded soldiers from the field with his “flying
ambulance.”
armed conflicts. As a consequence of improvements in armaments,
soldiers who survived their injuries often sustained significant limb
injuries requiring amputation. Having emphasized expeditious transport
and rapid amputation, attention was placed on reconstructive efforts of
the residual limb. This was due in part to effective developments in
anesthetics, aseptic surgery, antibiotics, an understanding of the
basic physiology
of
the lower extremity, and prosthetic devices. A primary goal of
amputation reconstruction was to preserve length and maintain the
end-bearing capabilities of the residual limb as emphasized by Chopart
and Lisfranc at the mid-foot level and by Pirogoff, Boyd, and Syme at
the ankle level. During the late 1800s, Bier attempted osteoplastic
reconstruction by placing a bony block between the tibia and the fibula
secured with screw fixation. The only transtibial amputation capable of
end-bearing was developed by Ertl.57,58,59
End-bearing was accomplished by combining the concepts of bony
reconstruction (osteoplasty) with soft tissue reconstruction
(myoplasty) to create an osteomyoplastic amputation for the transtibial
level. The same concepts of reconstructive surgery have been applied to
the transmetatarsal level and the transfemoral level.59
Ertl was able to apply his reconstructive techniques to approximately
13,000 patients over the years from World War I through World War II.59 Mondry and Dederich14
continued to promote soft tissue stabilization, showing the advantages
of restoring normal vascularity to the limb after myoplastic amputation
at the transfemoral level. Gottschalk and his colleagues further
elucidated the importance of myoplastic reconstruction by
characterizing improved alignment and gait at the transfemoral level.21,22
principles, the prosthetic field has rapidly advanced the art and
science of prosthetic manufacturing and is now able to fit many
patients with poorly performed amputations with a functional
prosthesis. As a result, the emphasis on proper surgical technique and
focusing on amputation as a reconstructive procedure has slowly faded
over time. The remainder of the chapter will serve to reemphasize the
need for sound surgical handling of the residual limb and review
various surgical approaches and outcomes.
provide function to the patient. Surgery is not and should not be the
only focus. The surgeon should be cognizant of the effect that limb
loss will have on the patient and be able to provide to the patient all
the resources necessary to regain maximum function. This will require a
team approach with the patient at the center of attention.36
The team will include the patient, surgeon, prosthetist, rehabilitation
expert, peer support, and family and even psychological support. Burgess10
believed that the residual limb should function as an end-organ. To
this end, the surgeon responsible for the patient should strive for
total comprehensive care.
over the past couple of decades, there has been renewed interest in
salvaging traumatized limbs. However, our ability to predict which limb
can be salvaged and which patients would benefit from early amputation
remains very subjective and is quite limited. Gregory and his colleagues23
first attempted to create a scoring system, the Mangled Extremity
Syndrome (MES) Index, in a retrospective review of 60 patients. Using
this scoring system, they believed that patients could be identified
preoperatively for salvage or amputation. A second scoring system, the
Mangled Extremity Severity Score (MESS) was used by Johansen and
colleagues28 and was thought to be simple and predictive. Helfet et al.25
then applied this scoring system prospectively and found it to be
simple and accurate in determining limbs that could be salvaged and
those that should undergo primary amputation. The American College of
Surgeons simplified the definition of a mangled limb as one where “high
energy transfer or crush causes a combination of injuries to the
artery, bone, tendon, nerve, and/or soft tissue.”1 Other scoring systems also have been developed, including the Predictive Salvage Index (PSI),26 The Limb Salvage Index (LSI),50 the Nerve injury, Ischemia, Soft-tissue injury, Skeletal injury, Shock and Age of patient (NISSSA) score,37 and the Hannover Fracture Scale (HFS).55
Each scoring system placed emphasis on different components of the limb
and developed various criteria for amputation or salvage. At this
point, the most widely used system in the United States is the MESS.
each scoring system, questions regarding sensitivity and specificity
have arisen. Robertson’s48 review of
152 patients suggested poor sensitivity of the MESS as some patients
with scores below the amputation threshold eventually went on to an
amputation. Bonanni et al.4
retrospectively reviewed a 10-year experience of attempted limb salvage
on 58 limbs using the Mangled Extremity Severity Index (MESI), MESS,
PSI, and LSI. Their review suggested poor predictive utility for limb
salvage for all four scoring systems in their patient population. Poole
et al.47 attempted to predict limb
salvage of extremities with combined osseous, soft tissue, and vascular
injuries independent of a named scoring system. The severity of soft
tissue and nerve injury was highly interrelated, but soft tissue injury
did not correlate with the severity of the osseous injury. Further,
limb salvage or amputation could not be accurately predicted by any
variable or group of variables studied. Because of the dynamic changes
that can occur in these patients, these authors suggested initial limb
salvage to observe the limb and then performance of delayed amputation
when indicated.47 Dirschl and Dahners15
comprehensively reviewed the MESI, MESS, NISSSA, LSI, and PSI. No
scoring system was predictive of salvage or amputation. They proposed
that scoring systems be used for documentation and as guides in
clinical decision-making, not as absolute indicators for salvage or
amputation.15 Durham et al.17
assessed the MESI, MESS, PSI, and LSI retrospectively over a 10-year
period. Although there was significant variability in predicting
amputation versus salvage, no scoring system was able to predict
functional outcome.17 Thuan et al.53
showed that no injury severity score could predict functional outcome
in patients who underwent limb salvage. Bosse et al. (the LEAP Group)6
prospectively evaluated the use of multiple scoring systems: MESS, LSI,
PSI, NISSSA, and the Hannover Fracture Scale-97 (HFS-97). The overall
analysis showed that lower scores had specificity for limb salvage
potential, but the low sensitivity of these scoring systems did not
validate them as predictors of amputation. The authors recommended
caution in using these scoring systems at or above the amputation
threshold.6 In comparison, Krettek et al.29
reevaluated the HFS, naming it the HFS-98, and applied the new scoring
system prospectively to 87 open long bone fractures. They concluded
that the HFS-98 was a reliable extremity salvage scoring system.29
and subcutaneous tissue, muscle, neurovascular structures, and bone, is
varied. No scoring system has been shown to be predictive of
amputation, outcome, or function. Scores that are predictive of salvage
may be helpful, but caution is stressed with regard to identifying a
specific amputation threshold. Scoring systems may be used as a
documentation tool and as a tool to facilitate communication between
surgeons. In most cases, initial limb salvage attempts should be
instituted first, allowing a complete assessment of the patient,
informing the patient of potential surgical options, and allowing the
patient to become involved in decision-making regarding salvage versus
amputation. Although patients undergoing salvage of a severely injured
limb may require frequent rehospitalizations, 2- and 7-year results of
amputation compared with limb salvage have demonstrated similar
outcomes as measured by the Sickness Impact Profile.5,34
However, projected lifetime costs for patients having undergone an
amputation are estimated to be three times greater than those for limb
salvage patients.35
limb and patient begins with as detailed history and as complete a
physical examination as possible using the American College of Surgeons
Advanced Trauma Life Support ABC algorithm. Once the primary survey is
completed and the injury inventory is complete, a secondary survey
should be performed and repeated every 4 to 6 hours, especially in the
obtunded and/or intubated patient. Additional information can be gained
by questioning first responders with regard to the mechanism of injury,
the initial presentation of the patient, the time required for
extrication, exposure to the elements, the amount of blood loss at the
scene, the potential degree of wound contamination, resuscitation
efforts, and the total time elapsed from the scene to the hospital.
This information may provide a much more comprehensive clinical
assessment of the patient, guiding the surgeon in his or her
decision-making. Once the patient has been assessed, life-threatening
injuries addressed, and resuscitation instituted, a focused examination
of the limb can be undertaken. Overall inspection of the limb should
take into account its appearance and the presence of any and all open
wounds. Closed soft tissue wounds can be quite severe and should be
graded using the Tscherne classification54
and open wounds in association with a fracture should be classified
using the Gustilo-Anderson open fracture wound classification system.24,42
Peripheral pulses should be monitored closely and documented after any
reduction maneuver. Dislocations and fractures should be reduced and
held reduced with an appropriate splint. Motor and sensory function
should also be documented as thoroughly as possible both before and
after manipulating the limb. If there are no palpable pulses, a simple
Doppler examination of the entire extremity should be performed. A
complete Doppler examination may identify occult injuries remote from
the main zone of injury that are affecting perfusion and threatening
viability of the limb. With diminished or absent distal pulses, an
ankle-brachial index (ABI) should be determined. A blood pressure cuff
is placed around the calf of the extremity in question. The cuff is
inflated until no audible Doppler pulse is appreciated. Then the cuff
pressure is slowly released and once a pulse is heard, the systolic
blood pressure value is noted. The same is then done for the upper
extremity and a ratio of the lower extremity pressure to the upper
extremity pressure is created. The ratio obtained should be 0.9 or
greater if no vascular injury is present. A value below 0.9 is
suggestive of a vascular injury that may require further work-up (e.g.,
angiography) or intervention. This simple test has shown value in
patients with knee dislocation and high-energy tibial plateau fractures.40,57
Further, simple duplex Doppler examination of the arterial system can
be performed before angiography. Angiography should be reserved to
determine the exact location of an arterial injury and guide potential
intervention, such as intraluminal stenting. If a specific vascular
injury is diagnosed, revascularization should be performed to preserve
limb viability. If the limb is unstable because of a fracture or
ligamentous injury, a simple uniplanar external fixator can be applied
quickly to maintain the length and alignment of the limb and provide
provisional stability during revascularization.19
indications for amputation, a detailed physical examination is
imperative. These patients may present with progressive soft tissue
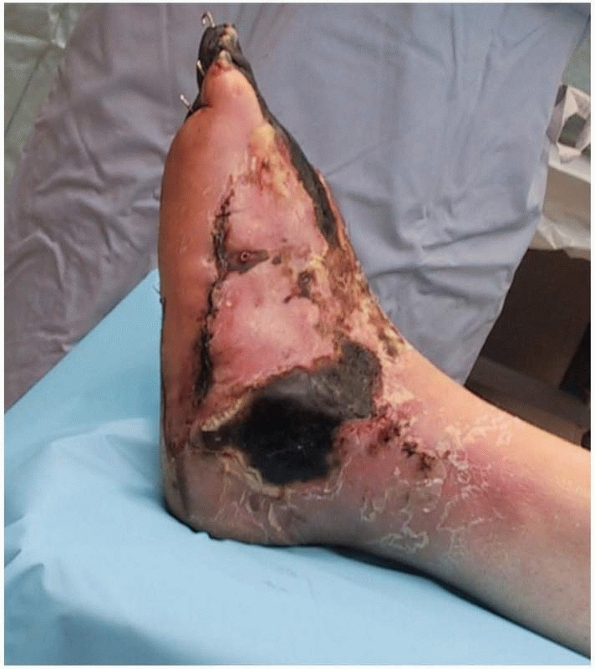
necrosis and infection (Fig. 13-1). Overall,
inspection of the wounds to determine the extent of potential
superficial and/or deep infection is needed as this may determine the
ultimate level of amputation. Further, noninvasive vascular assessment
should be performed. An ABI of less than 0.45 is suggestive that distal
healing is unlikely.16 However, it has been my experience
that, in patients with calcific arteriosclerosis, these values may be
falsely high and caution should be used when evaluating this test in
these patients. Other useful tests include the duplex Doppler
examination of the arterial system and transcutaneous oxygen tension
(TcPO2) measurements. Duplex Doppler tests will characterize the arterial anatomy and TcPO2 measurements will aid in determining the healing potential of surgical wounds. TcPO2
values below 20 mm Hg are indicative of nonhealing, and values of 40 mm
Hg or greater are indicative of healing. Between these values, the
surgeon should take into account the patient’s preexisting
comorbidities, arterial anatomy, and nutritional status. In my
experience, in a patient who has undergone a distal vascular bypass
below the knee, a below-knee amputation usually will fail because of
the single-vessel dominance of the lower limb. The goal of this workup
is to provide both the patient and the surgeon information that can be
used to determine the optimal level of amputation to facilitate
prosthetic planning and to estimate rehabilitation demands.
 |
|
FIGURE 13-1
The end point of wound deterioration following a crush injury to the foot. This patient sustained multiple metatarsal fractures that were treated with percutaneous pin fixation. The foot had a palpable pulse and the wounds were closed; however, the soft tissue envelope did not survive and a transtibial amputation was performed. |
limb rarely are able to undergo successful reimplantation, usually
because the underlying soft tissue injury is so severe compared with
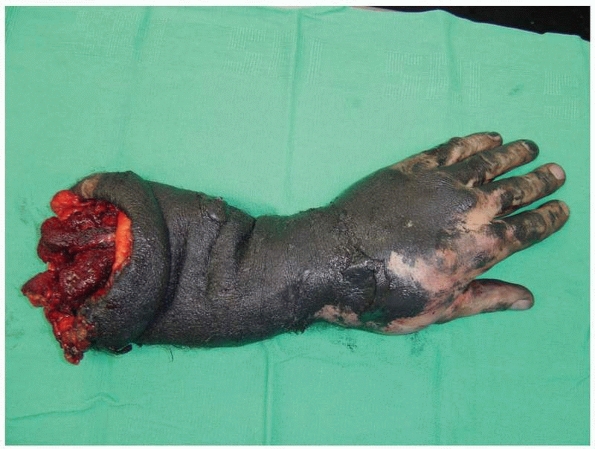
the bony injury (Fig. 13-2). Traumatized limbs
with segmental injuries, significant vascular injury, significant soft
tissue loss, and/or near amputation may be best treated with an
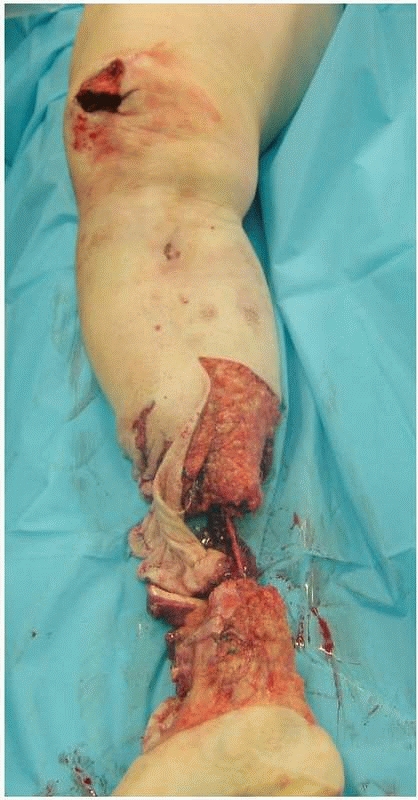
immediate open amputation (Fig. 13-3).
Traumatized limbs with a nonreconstructible vascular injury will
require amputation. The most important factor determining limb salvage
versus amputation will be the severity of the soft tissue injury.33
These patients often have an obvious constellation of
nonreconstructible injuries. When immediate amputation is preferred,
all viable soft tissue should be maintained as it can be used later for
definitive wound closure. Obviously, ischemic, devitalized tissue
should be aggressively and thoroughly debrided. Osseous structures
should be resected initially to the level of soft tissue resection.
Wound care should then be instituted with serial debridements until a
stable wound bed is achieved. Negative pressure wound therapy may play
an adjunctive role in creating granulation tissue that may aide in
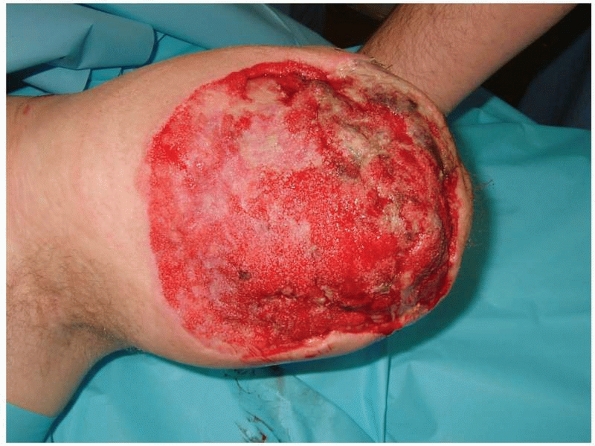
wound healing (Fig. 13-4). During this period,
patient education and prosthetic consultation should be used to
maintain the patient’s involvement in his or her treatment course.
Further, the clinical evaluation should continue to determine the
optimum level of amputation, similar to the patient undergoing
nonemergent amputation.
 |
|
FIGURE 13-2
An oil well driller sustained a traumatic amputation during a drilling operation. Significant soft tissue contamination and extensive degloving precluded reimplantation. The radius and ulna both remained attached to the arm while the hand and forearm soft tissue envelope was completely degloved. |
 |
|
FIGURE 13-3
A motorcyclist sustained limb-threatening injuries to the lower leg. There was segmental bone loss and extensive soft tissue loss, and the foot was pulseless. Vascular reconstruction was not feasible. Injuries such as this one should be treated with staged amputation. An open amputation preserving as much length as possible should be performed first. Following intensive wound care, a definitive amputation is performed when the wound bed seems stable. |
immediate amputation should undergo early temporizing treatment. This
may include the use of temporary external fixation and serial wound
debridement procedures. The primary focus of initial temporizing
treatment is to obtain and maintain perfusion of the limb before
extensive and exhausting reconstructive procedures. If limb viability
cannot be maintained or reconstruction/salvage is deemed unfeasible,
then an elective amputation should be undertaken.
joint acts as a fulcrum to position the hand in space. Maintaining limb
length and a functional elbow joint will substantially increase the
functional outcome at this level of amputation. If possible, preserving
the pronator quadratus allows the patient to maintain two thirds of
active forearm rotation. A body-powered prosthesis
can
be applied to this level. If a myoelectric prosthesis in used, the
optimum length will be at the junction of the mid and distal thirds of
the forearm. The soft tissue reconstruction must be stable and can be
accomplished with myodesis (muscle sutured to bone) or a combination of
a myodesis of the deeper layer and myoplasty (antagonistic muscles
sutured together) of the superficial layer (using a pants-over-vest
technique). This will provide adequate soft tissue coverage distally
with volar and dorsal flaps and allow the residual musculature to be
active and dynamic, providing a strong myoelectric signal. Although not
routinely performed, a Krukenberg procedure splits the radius and ulna
to create a pincers mechanism. It has been recommended for blind
patients with bilateral below-elbow amputations or in Third World
countries where prosthetic services are limited.3
 |
|
FIGURE 13-4
Negative pressure wound therapy is a powerful adjunctive tool to create healthy granulation tissue in a stable wound bed. In this transhumeral amputation, length was preserved by maintaining soft tissue muscle coverage over the humerus. A split-thickness skin graft was successfully applied and the patient was ultimately fitted with a myoelectric prosthesis. |
 |
|
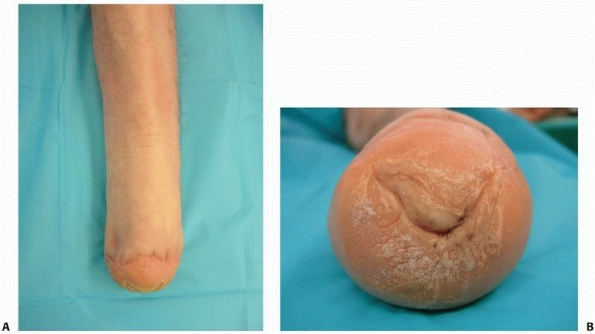
FIGURE 13-5 A.
Frontal picture of a patient with a mature Syme amputation demonstrates the regional soft tissue atrophy that can occur over time and the instability of the heal pad that has occurred. The distal tibia has become very prominent and painful in the prosthesis. B. The distal end of this Syme amputation demonstrates a hypertrophic callous with fissuring that developed as a result of the unstable heel pad. |
transhumeral amputation is performed. An amputation through the elbow
is a difficult level both for prosthetic fitting and appearance as the
prosthetic elbow will be more distal than the contralateral native
elbow; therefore, this amputation level is rarely selected. The ideal
length of the humerus for a body-powered prosthesis is just proximal to
the distal metaphyseal-diaphyseal junction. However, for a myoelectric
prosthesis, the humerus will need to be transected at the mid-shaft to
allow for adequate fitting of this prosthesis. Soft tissue
stabilization again is important to provide distal bony coverage and to
provide the residual limb with dynamic muscle function. This can be
accomplished by securing the deeper layer via myodesis and the
superficial layer with a myoplasty using a pants-over-vest technique.
upper extremity amputation is much lower than it is for a below-elbow
amputation.16 Recently, targeted nerve reinnervation has shown promise in improving myoelectric prosthetic function.30,38
This technique uses selective nerve implantation into various muscles
to improve myoelectric signaling to the prosthesis. It has been applied
to a limited number of patients and continues to evolve, providing hope
for improved prosthetic function in the proximal upper extremity
amputee.
preserve the end-bearing capabilities of the limb. The best known is
the Syme amputation (Fig. 13-5), but variations include the Pirogoff
amputation involving a calcaneotibial arthrodesis,46 the Boyd amputation (similar to the Pirogoff),8 the Lefort-Neff modification of the Pirogoff method,3 and the Camilleri modification of the Pirogoff method.11
A requirement for this level of amputation is an intact plantar soft
tissue flap that will be able to provide stable coverage and wound
closure. This may not be feasible in a patient with compromised soft
tissue as the result of an injury. Further, prosthetic fitting may be
challenging in this patient and prosthetic options are limited compared
with those available for a below-knee amputation. A potential
limitation of the Syme’s amputation is migration of the heel pad after
surgery. This may occur in 7.5% to 45% of patients.52
Tenodesing the Achilles tendon to the distal tibia with sutures placed
through drill holes to stabilize the heel pad was shown to be
successful in a series of 10 of 11 patients.52
An incision is marked out transversely across the anterior ankle joint
1 cm distal to the malleoli and stopping 1 cm anterior to them. Then a
vertical incision is carried distally from each malleolus to the
plantar aspect of the foot, anterior to the heel pad. The long extensor
tendons are transected and the peroneal nerves are isolated, transected
and cut, allowing them to retract into the wound bed. The anterior
vascular structures should be isolated and controlled with a suture
ligature. The foot is then plantarflexed, the collateral ligaments are
transected, and the flexor hallucis longus tendon is isolated. The
calcaneus is then stripped of its soft tissue attachments and the
Achilles tendon is found and carefully detached from the calcaneal
tuberosity. Care should be taken to avoid penetration of the posterior
soft tissues at the Achilles insertion. The plantar fascia origin is
then transected and the foot is disarticulated. The malleoli should be
thinned to reduce the potential for a bulbous-shaped distal limb.
Closure should be meticulous and the heel pad must be secured to the
distal tibia to ensure soft tissue stability. Achilles tendon tenodesis
has also been recommended to achieve heel pad stability.52
a posterior myocutaneous flap. Historically, this approach was first
proposed by Verduyn in 1696 to provide better distal coverage over the
residual distal tibia. Bickel is credited with using this amputation in
the United States in 1943, and through the educational efforts of
Burgess, this technique gained wide acceptance throughout the United
States.3,9 Transtibial amputation has had multiple variations proposed: posterior flap,9 extended posterior flap,2 symmetric anterior/posterior flaps,18 symmetric medial/lateral (sagittal) flaps,43 skewed sagittal flaps,49 medial flap,27 and distal end-bearing via a tibiofibular synostosis.58,59 With a well-constructed amputation, patients have predictable outcomes with favorable prosthetic use.51 The selection of amputation level follows guidelines similar to those for the urgent or elective workup, using TcPO2
measurements and characterizing the vascular anatomy with duplex
Doppler arterial ultrasonography. Staged treatment of the traumatized
limb may be needed to allow a determination of the optimum level of
amputation. This may not be possible until the soft tissue envelope has
stabilized, which may take several weeks.2
Preserving the knee joint should always be the goal and many patients
can function well with a short residual below-knee stump. Alternative
surgical approaches as listed earlier may need to be used to salvage a
below-knee amputation level. Finally, the overall goal is to provide
the patient with a cylindrical (not conical) residual limb that has a
stable soft tissue envelope and adequate sensation and perfusion, which
can then accept and support a prosthesis to maximize function.
Ertl applied the concept of osteoperiosteal flaps to amputation
surgery, combining bony reconstruction (osteoplasty [creating a
synostosis between the tibia and fibula distally]) with soft tissue
reconstruction (myoplasty).58,59
This effectively created the osteomyoplastic amputation, combining two
procedures into one. Essentially, osteoperiosteal flaps are raised from
all surfaces of the tibia and fibula distal to the planned level of
resection of the tibia and fibula. This may only require up to 3 cm of
bone to be resected as the distance from the medial tibial cortex to
the lateral fibular cortex is approximately 5 to 6 cm. In primary
amputations, the tibial periosteum is quite thick and the surgeon can
use only tibial osteoperiosteal flaps to create the synostosis. This
will only require up to 6 cm of tissue and not unduly shorten the limb.1
The tibia and fibula are then transected at the same level, and the
anterior cortex of the tibia is beveled to reduce its prominence
anteriorly.
The osteoperiosteal flaps are then sewn together to create a synostosis
between the tibia and fibula. Over time, this flap regenerates bone,
and the bony bridge matures with progressive weight-bearing.
Alternative approaches have used a segment of fibula incorporated into
the osteoperiosteal sleeve hinged on its periosteal tissue, a full
section of fibula placed between the tibia and fibula, and screw
fixation of the fibular graft.12,41,44,45
Stress shielding is a concern with screw fixation, and removal of the
screw has been advocated once the synostosis has formed. Soft tissue
stabilization is then performed to provide distal coverage over the
residual osseous structures. Nerve handling should be meticulous with
care being taken to resect the sural, saphenous, deep and superficial
peroneal, and tibial nerves high and allowing them to retract
proximally away from any potential compressive force. Burying the
nerves places them on tension and may produce a neuroma (Fig. 13-6).
A meticulously layered closure is performed, removing any and all
redundant tissue and dog-ears. The resultant residual limb then assumes
a cylindrical shape31 (Figs. 13-7, 13-8, 13-9 and 13-10).
 |
|
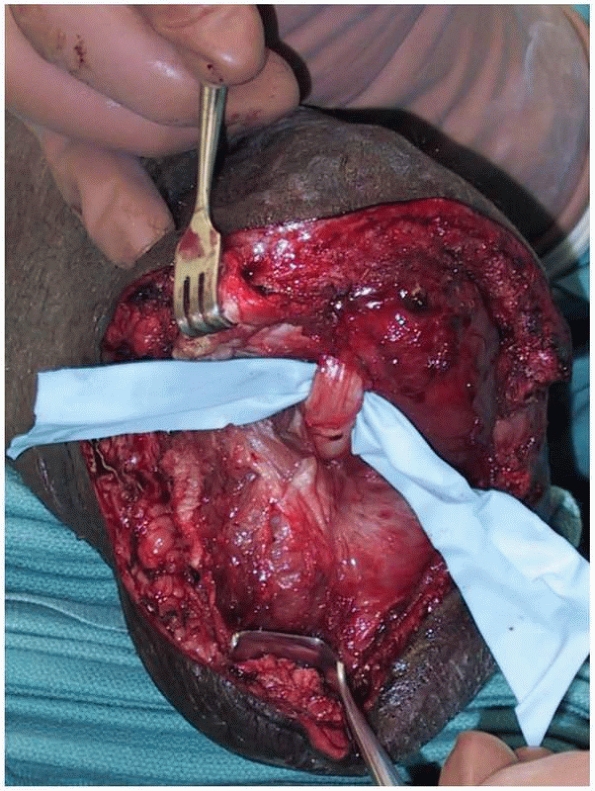
FIGURE 13-6
Revision of a transtibial amputation demonstrates the tibial nerve, which originally was buried directly into the end of the residual tibia. The patient experienced exquisite neurogenic pain with ambulation. Transected nerves should not be buried, placed on traction, or compressed, as doing so will promote the development of a postoperative neuroma. They should simply be transected sharply and allowed to retract into the soft tissues proximally. |
 |
|
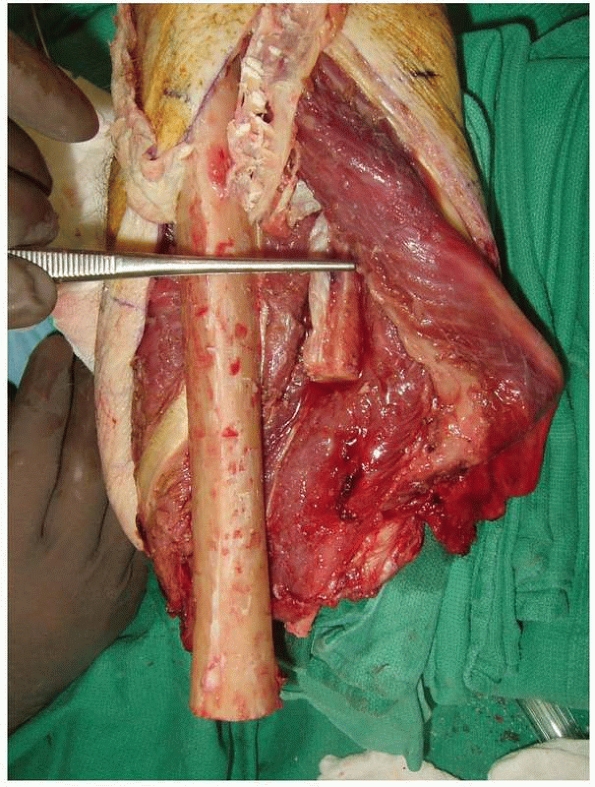
FIGURE 13-7
Primary osteomyoplastic amputation. The forceps demonstrate the level of planned tibial and fibular transection. Note the osteoperiosteal flaps that have been elevated from all surfaces of the tibia. If a portion of the fibula is used, it should be transected about 2.0 to 2.5 cm from the level of the tibial cut. The fibula can then be osteotomized, hinged on its medial periosteal sleeve, and incorporated into the osteoperiosteal flaps that create the synostosis. |
 |
|
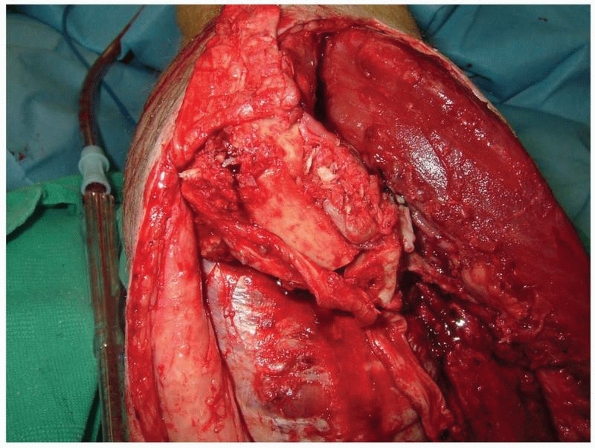
FIGURE 13-8
The bridge is created by suturing the osteoperiosteal flaps to the fibula. The fibular portion can be incorporated into the flap. Cancellous bone can be placed into the created synostosis as an autogenous graft. The cut ends of the osteoperiosteal flaps should be imbricated to prevent exostosis formation from the cambium layer. |
relies on the superficial posterior compartment for distal soft tissue
coverage (Figs. 13-11, 13-12, 13-13 and 13-14).
An incision is marked on the limb with an anterior reference point 10
to 15 cm distal to the knee joint. The width of the limb from anterior
to posterior is measured. At the level of the anterior third and
posterior two thirds of the limb, the posterior flap is drawn extending
distally down the leg adding 1 cm for a traditional posterior flap or
adding 5 cm for an extended posterior flap technique. At the anterior
reference point, a partial transverse incision is made to the line
extending distally and then the incision is carried distally to the
planned end of the flap. The anterior and lateral compartments are
exposed and the muscles are transected. Large vessels should be
ligated. Nerves should be resected sharply and allowed to retract
proximally. The tibia is transected and the fibula should be transected
no higher than 1.5 to 2 cm proximal to the distal tibia. This will
ensure maintenance of a cylindrical residual limb. A fibula that is too
short in relation to the tibia will result in a conical limb. The
interval between the deep and superficial compartments is defined and
the deep posterior compartment muscles are transected. The posterior
compartment vessels are also controlled with suture ligatures. The
plane between the two posterior compartments is developed and the
superficial posterior compartment is then resected. Multiple vascular
perforators cross from the deep compartment to the superficial
compartment, and they may also require suture
control
for hemostasis. The anterior cortex of the tibia should be beveled to
avoid any bony prominence. Drill holes are placed in the tibia, and
deep soft tissue stabilization of the posterior flap is performed by
anchoring its deep fascia with sutures placed through these drill
holes. A meticulously layered closure is then performed, taking care to
remove any and all redundant tissue to provide a cylindrical shape to
the limb. With the extended posterior flap technique, the anterior
aspect of the limb will appear substantially bulky but will atrophy
over time.
 |
|
FIGURE 13-9 Completed bridge formation between the tibia and fibula.
|
 |
|
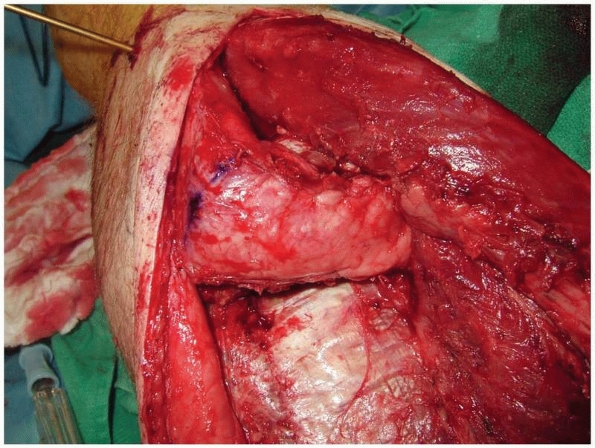
FIGURE 13-10 A.
Soft tissue stabilization for the osteomyoplastic amputation is begun by suturing the anterior and lateral compartments into the deep fascia, providing anterior and distal soft tissue coverage. B. After debulking of the deep posterior compartment, the superficial posterior compartment is brought over the end of the residual limb and secured with sutures. Final closure is performed by closing the fascia over the myoplasty, excising redundant skin, and performing a meticulous skin closure. |
 |
|
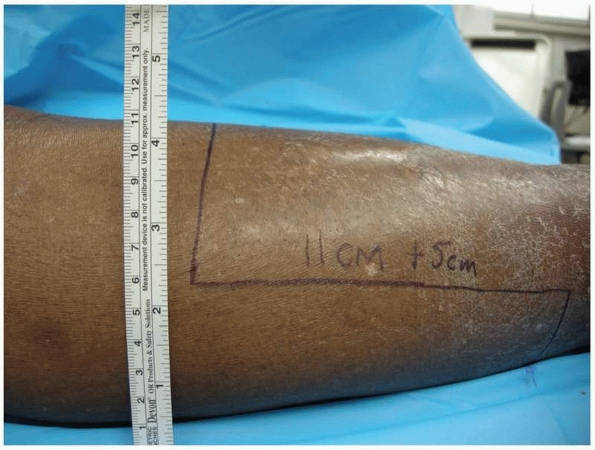
FIGURE 13-11
An extended long posterior flap transtibial amputation. The anterior-posterior width of the limb is measured at the level of tibial transaction and lines are drawn distally to equal the anterior-posterior width (in this case, 11 cm) plus 5 cm. |
 |
|
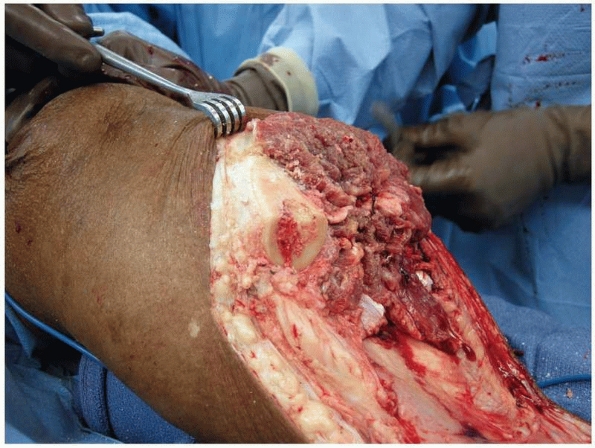
FIGURE 13-12
The extended posterior flap provides an adequate myofasciocutaneous tissue for closure and anterior coverage. The anterior cortex of the tibia should always be beveled. |
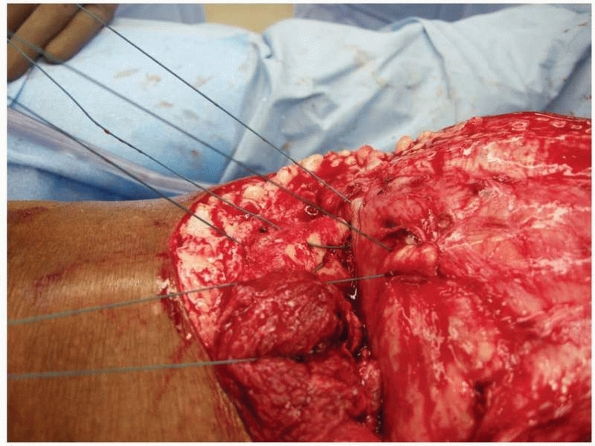 |
|
FIGURE 13-13
Drill holes are placed into the anterior tibial cortex such that sutures can be passed through them to anchor the deep fascia of the posterior flap. |
maintains the end-bearing capabilities of the femur and will maintain
mechanical and anatomic alignment of the femur. Knee disarticulation
has been indicated for the dysvascular patient, children,
bed-bound/nonambulatory patients, and patients with traumatic
amputations. In the nonambulatory patient, the long lever arm of the
residual limb can be of assistance in transfers. Caution should be used
in the traumatic amputee as the functional result with a through-knee
amputation is less than that of either the transtibial amputation or
the transfemoral amputation.32
at the level of the knee joint, a transverse anterior incision is made
to the mid-coronal line medially and laterally. The skin incision is
then carried distally to the junction of the conjoined portion of the
gastrocnemius and soleus muscles. Anteriorly, dissection is carried
down to the tibial plateau and a full-thickness anterior flap is made.
The knee joint is entered and the collateral and cruciate ligaments are
transected. Posterior dissection then transects the capsule and the
medial and lateral hamstrings, exposing the neurovascular structures.
The vessels are isolated and controlled with suture ligatures. The
tibial and peroneal nerves should be isolated, transected, and allowed
to retract proximally into the soft tissue bed. The interval between
the gastrocnemius and the deep posterior compartment muscles should be
developed and carried distally. Deep vascular perforators may need to
be controlled with suture ligatures. A posterior transverse incision
distally then allows the lower leg to be removed en bloc. The patella
may be retained or removed. Removing the patella creates additional
length for the quadriceps, which should be sutured to the remnants of
the cruciate ligaments. Both the medial and lateral hamstrings can be
sutured to the capsular remnants to maintain their function as hip
extensors. The posterior flap is then brought over the distal end of
the residual femur in a pants-over-vest fashion. The anterior flap skin
can be removed to accommodate the length of the posterior flap. The
sural nerve should be identified and transected as high as possible to
reduce the risk of postsurgical neuroma formation.
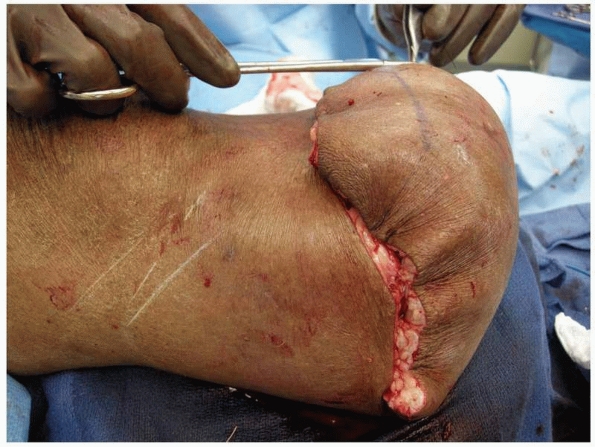 |
|
FIGURE 13-14
The remainder of the extended posterior flap is then closed in a layered fashion. This will initially create a bulky appearance to the distal end of the residual limb, but this tissue will atrophy over time, providing adequate soft tissue protection to the anterior tibia. |
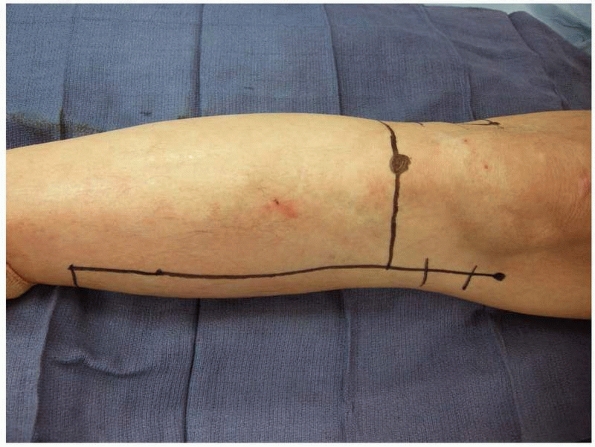 |
|
FIGURE 13-15
The basic landmarks for a knee disarticulation with a long posterior flap are the tibial tubercle anteriorly and the medial and lateral epicondyles on either side of the distal femur. The incision should not extend much more proximally than the epicondyles. Doing so will create large dog-ears that will be difficult to control surgically. |
or equal anterior/posterior flaps can be used, or occasionally in the
trauma setting, the surgeon may have to use any viable residual soft
tissue uniquely for wound closure (Fig. 13-18).
In general, soft tissue flaps should be kept as long as possible to
reduce the potential for undue tension at closure and to prevent the
need to shorten the femur because of inadequate flap lengths. A proper
soft tissue reconstruction creates a dynamic residual extremity,
improves the vascularity of the residual limb, and will help to
maintain alignment of the residual femur, which in turn improves gait.13,14,21,22,58,59
disarticulation can be performed first. This will preserve soft tissues
needed for closure. In the traumatic setting, all viable tissue
should
be preserved for wound closure. The three muscle groups (adductors,
quadriceps, and hamstrings) are isolated and reflected proximally to
expose the distal femur. Vascular structures are isolated and
controlled with suture ligatures, preferably double-suture ligatures.
The distal femur is then transected to a level that will allow for
proper prosthetic fitting. In general, the minimum space required for
the prosthetic knee joint to achieve symmetry with the opposite side is
2 inches from the end of the residual limb. Therefore, depending on the
technique chosen, the surgeon will need to take into account the amount
of space the soft tissue reconstruction will require and add it to the
amount of femur removed. The sciatic and obturator nerves should be
isolated, transected proximally, and allowed to retract into the soft
tissue bed. Nerves should never be buried into bone or tethered for
doing so may create tension and neuroma formation. Soft tissue
reconstruction begins with securing the medial musculature to the
distal end of the femur, typically with sutures passed through drill
holes, thus restoring proper anatomic and mechanical alignment of the
residual limb. The quadriceps can also be secured distally to the end
of femur with the hip in extension with sutures passed through
additional drill holes. The hamstrings are secured posteriorly. A
meticulous layered closure should then be performed to afford the
patient a cylindrical shape to the residual limb.
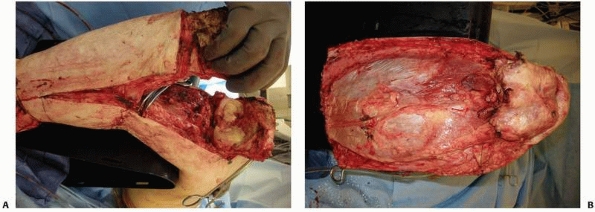 |
|
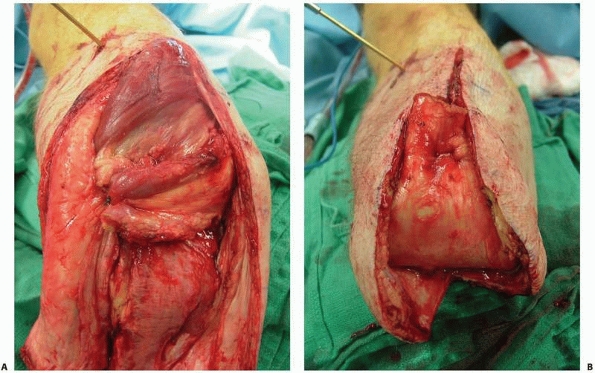
FIGURE 13-16 A.
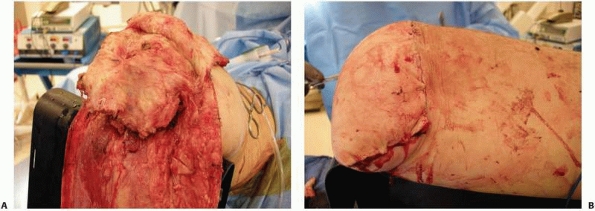
Anterior flap that includes the extensor mechanism has been elevated and the dissection is carried into the knee joint. The cruciate and collateral ligaments are transected, the vessels are ligated, and the nerves are sharply transected. A plane between the gastrocnemius muscle and soleus should be developed as depicted here. This plane should be extended as far distally as possible. B. With removal of the distal limb, a long posterior myofasciocutaneous flap is created. |
 |
|
FIGURE 13-17 A. The quadriceps should be anchored with sutures to the remnants of the cruciate ligaments. B. Final closure results in an abundant distal soft tissue envelope.
|
 |
|
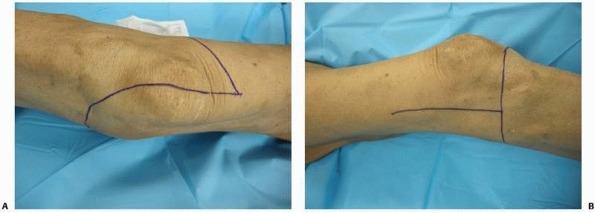
FIGURE 13-18 A. Medial-based flap for a transfemoral amputation as described by Gottschalk.17 B. Equal anterior and posterior flaps for a transfemoral amputation.
|
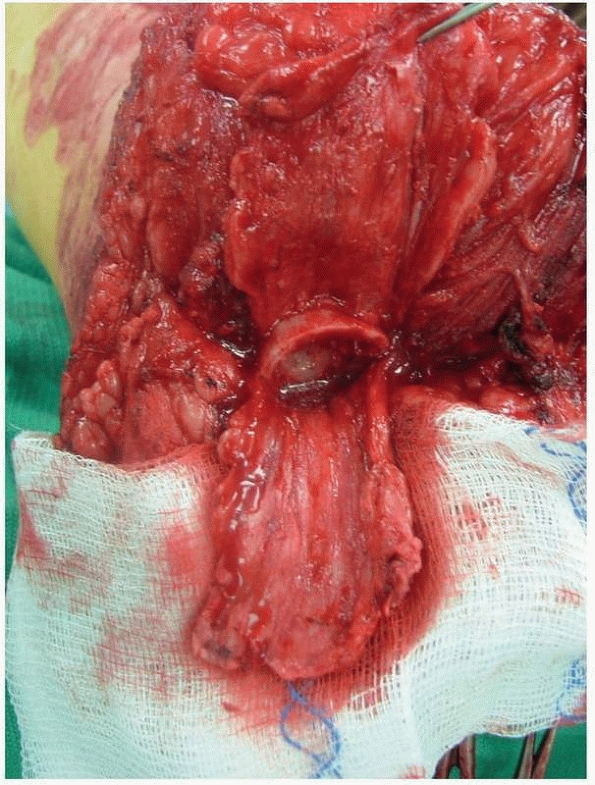
Osteoperiosteal flaps are elevated off of the femur. After shortening
the femur to an appropriate level, the osteoperiosteal flaps are sewn
over the end of the femur closing the medullary canal (Figs. 13-19 and 13-20). Soft tissue reconstruction is then performed as described earlier.
 |
|
FIGURE 13-19 Osteoperiosteal flaps have been elevated for the osteomyoplastic transfemoral amputation.
|
 |
|
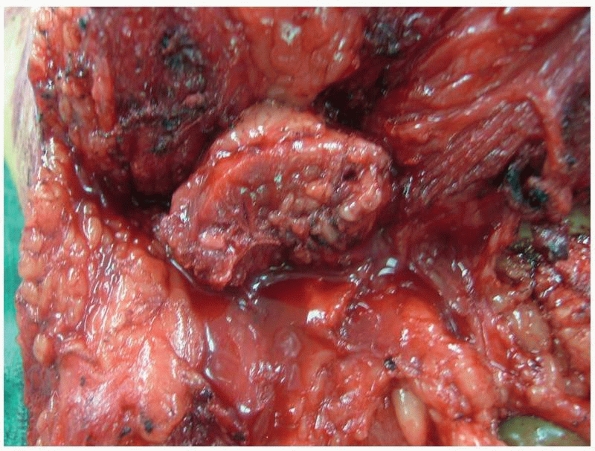
FIGURE 13-20
Medullary canal closure in the osteomyoplastic amputation is accomplished by suturing the osteoperiosteal flaps over the end of the residual femur. The femoral canal can also be packed with cancellous bone graft to augment closure with the osteoperiosteal flaps. |
comprehensive rehabilitation program to provide functional restoration
to the patient. The primary goal of a rehabilitation program is to
return the patient to a functional status and to return that patient
into everyday society. The combination of a sound surgical procedure,
proper prosthetic application, and comprehensive rehabilitation is
essential. In the acute phase, pain control is paramount and can be
accomplished via oral or intravenous narcotics, peripheral nerve
blocks, patient-controlled analgesia (PCA), or epidural catheter
delivery of analgesic medication. Acute-phase physical therapy includes
wound care, basic mobilization, swelling control, joint mobilization,
desensitization, upper extremity strengthening, and isometric muscle
training of the residual limb.
he has not done so before surgery, regarding the prosthesis and develop
a timeline for its manufacture and application, usually about 6 weeks
after surgery. Once the swelling has decreased and the wounds have
healed, the patient is evaluated for socket application. Typically the
patient will have a preparatory prosthesis constructed to begin gait
training.
walking; rather, a qualified physical therapist is required to teach
the patient proper body mechanics and position during gait and how to
use a program of core strengthening. Balance training and confidence
with balance may be challenging in amputees and vary with the level of
amputation and the indication for amputation.39
After the acute phase, advanced therapy should be instituted to educate
the patient beyond basic functions, in preparation to return to work or
sport. All patients with a limb amputation will also require in-depth
occupational therapy for activities of daily living and the use of
assistive technology. Finally, cognitive therapy and psychological
support should be considered for all amputees, especially the
posttraumatic amputee.
surgeon should plan a dynamic, functional amputation level that can
accept a prosthesis and improve the functionality and mobility of the
patient. Amputation should be reconstructive with a strong emphasis
placed on the rehabilitative potential of the patient.
acknowledge and thank Janos P. Ertl, MD; Christian W. Ertl, MD; Carol
Dionne, PT, PhD, OCS, Cert MDT; and Jonathan Day, CPO, for their
insightful suggestions regarding amputee surgery, amputee
rehabilitation, and prosthetic application.
College of Surgeons Committee on Trauma. Advanced Trauma Life Support
Courses. Chicago, IL: American College of Surgeons; 1985.
DG, Michael JW, Bowker JH, Eds. Atlas of amputations and limb
deficiencies: surgical, prosthetic, and rehabilitation principles, 3rd
ed. Rosemont, IL: American Academy of Orthopaedic Surgeons, 2004.
MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of
reconstruction or amputation after leg-threatening injuries. N Engl J
Med 2002;347:1906-1907.
MJ, MacKenzie EJ, Kellam JF, et al. A prospective evaluation of the
clinical utility of the lower extremity injury-severity scores. J Bone
Joint Surg Am 2001;83A:3-14.
JH, San Giovanni TP, Pinzur MS. North American experience with knee
disarticulation with use of a posterior myofasciocutaneous flap:
healing rate and functional results in 77 patients. J Bone Joint Surg
Am 2000;82A:1571-1574.
A, Anract P, Missenard G, et al. Apurations et désarticulations des
members: Membre inférieur. In Encyclopédie Médico-Chirurgicale. Paris:
Editions Scientifiques et Médicales Elsevier, SAS; 2000:6-8.
R. Plastic treatment of the muscles and bone in amputation surgery. A
method designed to produce physiologic conditions in the stump. J Bone
Joint Surg Br 1963; 45B:60-66.
R, Van De Weyer KH. [Arteriographic studies of muscle plastic surgery
in amputation stump correction.] [In German] Arztl Wochensch
1959;14:208-211.
DR, Tornetta P III, Sims SH. Amputations and prosthetics. In Koval KJ,
ed. Orthopaedic Knowledge Update. American Academy of Orthopaedic
Surgeons; 2002.
RM, Mistry BM, Mazuski JE, et al. Outcome and utility of scoring
systems in the management of the mangled extremity. Am J Surg
1996;172:569-573.
CH. Amputation of the lower limb. In Evarts SM, ed. Surgery of the
Musculoskeletal System. New York: Churchill Livingstone; 1990.
FA, Kouroush S. Stills M, et al. Does socket configuration influence
the position of the femur in above-knee amputation? J Prosthet Orthot
1989;2:94-102.
RT, Gould RJ, Peclet M, et al. The mangled extremity syndrome (M.E.S.):
a severity grading system for multisystem injury of the extremity. J
Trauma 1985;25: 1147-1150.
RB, Anderson JT. Prevention of infection in the treatment of 1025 open
fractures of long bones. J Bone Joint Surg Am 1976;58A:453-458.
DL, Howey T, Sanders R, Johansen K. Limb salvage versus amputation.
Preliminary results of the Mangled Extremity Severity Score. Clin
Orthop Relat Res 1990;(256): 80-86.
HR Jr, Poole GV Jr, Hansen KJ, et al. Salvage of lower extremities
following combined orthopedic and vascular trauma. A predictive salvage
index. Am Surg 1987: 53:205-208.
K, Daines M, Howey T, et al. Objective criteria accurately predict
amputation following lower extremity trauma. J Trauma 1990;30:568-572.
C, Seekamp A, Köntopp H, et al. Hannover Fracture Scale ’98:
re-evaluation and new perspectives of an established extremity salvage
score. Injury 2001;32:611.
TA, Dumanian GA, Lipschutz RD, et al. The use of targeted muscle
reinnervation for improved myoelectric prosthesis control in a
bilateral shoulder disarticulation amputee. Prosthet Orthot Int
2004;28:245-253.
HE. Biological and biomechanical principles in amputation surgery. In
International Prosthetics Course, Second Proceedings. Committee on
Prosthesis, Braces, and Technical Aids; Copenhagen, 1960:41-58.
EJ, Bosse MJ, Castillo RC, et al. Functional outcomes following
trauma-related lower extremity amputation. J Bone Joint Surg Am
2004;86A:1636-1645.
EJ, Bosse MJ, Kellam JF, et al. Factors influencing the decision to
amputate or reconstruct after high-energy lower extremity trauma. J
Trauma 2002;52:641-649.
EJ, Bosse MJ, Pollak AN, et al. Long-term persistence of disability
following severe lower-limb trauma. Results of a 7-year follow-up. J
Bone Joint Surg Am 2005; 87A:1801-1809.
EJ, Jones AS, Bosse MJ, et al. Health-care costs associated with
amputation or reconstruction of a limb-threatening injury. J Bone Joint
Surg Am 2007;89A:1685-1692.
MG, Heckman JD, Corley FG. Severe open fractures of the lower
extremity: a retrospective evaluation of the Mangled Extremity Severity
Score (MESS). J Orthop Trauma 1994;8:81-87.
LA, Stubblefield KA, Lipschutz RD, et al. Improved myoelectric
prosthesis control using targeted reinnervation surgery: a case series.
IEEE Trans Neural Syst Rehabil Eng 2008;16:4-50.
WJ, Barei DP, McNair P. The value of the ankle-brachial index for
diagnosing arterial injury after knee dislocation: a prospective study.
J Trauma 2004;56:1261-1265.
HJ, Tscherne HJ. Pathophysiology and classification of soft tissue
injuries associated with fractures. In Tscherne H, Gotzen L, eds.
Fractures with Soft Tissue Injuries. Berlin: Springer-Verlag; 1984:1-19.
WL, Sailors DM, Whittle TB, et al. Limb salvage verses traumatic
amputation. A decision based on a seven-part predictive index. Ann Surg
1991;213:473-481.
DG, Horn P, Malchow D, et al. Prosthetic history, prosthetic charges,
and functional outcome of the isolated traumatic below-knee amputation.
J Trauma 1995;38: 44-47.
DG, Sangeorzan BJ, Hansen ST Jr, et al. Achilles tendon tenodesis to
prevent heel pad migration in the Syme amputation. Foot Ankle Int
1994;15:14-17.
VL, Travison TG, Castillo RC, et al., and the LEAP Study Group. Ability
of lower extremity injury severity scores to predict functional outcome
after limb salvage. J Bone Joint Surg Am 2008;90A:1738-1743.
H, Oestern HJ. [A new classification of soft-tissue damage in open and
closed fractures]. [In German] Unfallheilkunde 1982;85:111-115.
FW Jr. Management of the diabetic-neuropathic foot. Part II. A
classification and treatment program for diabetic, neuropathic, and
dysvascular foot problems. Instr Course Lect 1979;28:143-165.
