Soft-Tissue Allografts
for more than a century. More recently, implantation of these tissues
has become more common as their safety and efficacy have been
established. The procurement and sterilization process for allografts
has improved with the addition of newer testing methods. Allografts can
and have been used as a substitute for nascent tissues and autogenous
grafts. The safety and clinical outcome of procedures using allografts
has improved, as we have gained a better understanding of the basic
science behind the biologic incorporation of these tissues. We present
a historical review of allograft utilization, current procurement and
testing techniques, and case presentations to exemplify their clinical
applications.
early in the 20th century. The first documented use of a
musculoskeletal soft-tissue allograft was by MacEwen in 1880. More
recently, Noyes and Shino reported on their use of allografts in
ligament reconstruction in 1981. Over the past decade, soft-tissue
allografts have been used more commonly to decrease patient morbidity
and substitute for tissue that is deficient or not available for
implantation. The screening and preparation of allogeneic tissues have
changed and improved as well. In this review, the different
sterilization and screening techniques of allografts will be covered.
The clinical applications of these tissues will be presented. Today,
quality soft-tissue allografts are available as a viable substitute for
harvesting the patient’s own tissues.
softtissue allografts. Some procedures mandate the use of allograft
(e.g., multiple ligamentous injuries of the knee). Allografts have both
advantages and disadvantages (Box 10-1).
Donor-site morbidity is eliminated with the use of allografts. Graft
size is not an issue as a result of the ability to preselect based on
the donor. Operative times are decreased because autograft procurement
is unnecessary. Studies comparing matched autograft and allograft
bone-patellar tendon-bone anterior cruciate ligament (ACL)
reconstructions have found less loss of motion in the allograft group,
no difference in hop strength, side-to-side KT-1000 measurements and
International Knee Documentation Committee (IKDC) ratings.
transmission is the greatest concern on the part of both the physician
and patient. The risk of disease transmission of any kind is currently
estimated at 1:1.67 million. Currently, newer tests are being
implemented to discover human immunodeficiency virus (HIV) during the
early infection “window period,” which goes undetected by current
testing methods. A host-versus-graft T cell-mediated immune response
may occur, causing graft rejection. Also, antibodymediated rejection
can occur.
related to soft-tissue allografts. In a study comparing patellar tendon
autografts to fresh-frozen allografts using a goat
model,
Jackson found that allografts lose a great proportion of their
time-zero strength during the remodeling phase. The speed of remodeling
is not as rapid in allogeneic tissues as it is in autografts. They
found that, although both grafts proceeded through the same biologic
process, allografts went at a slower rate when compared at 6 months
postreconstruction. In contrast, Shino compared fresh patellar tendon
autografts with frozen patellar tendon allografts in replacing the
canine ACL. The authors found a more rapid conversion of graft collagen
to host collagen in the allograft model. Similar degrees of vascular
and histologic incorporation were found.
implant. Soft-tissue allografts range in price from $795 each for
fresh-frozen tibialis tendon, $1,590 for semitendinosus and gracilis,
and $2,800 for a cryopreserved graft.
the prevention of disease transmission. The American Association of
Tissue Banks (AATB) requires their member organizations to follow a
strict protocol for the preparation and storage of soft-tissue
allografts. Procurement standards have been established by the AATB and
were most recently updated in April 2001. Potential donors are examined
via an exhaustive medical, social, and sexual history completed by the
donor’s family. A physical examination is also performed for any signs
of infectious disease. Any positive finding that raises suspicion of
communicable or metabolic disease disqualifies the individual as a
donor.
death, usually after procurement teams have removed the donor’s solid
organs. Musculoskeletal tissues can be removed by means of an aseptic,
completely sterile, or clean technique, where absolute sterile
technique is not used. Secondary sterilization with gamma irradiation
is required after a clean harvest but not necessarily after a sterile
technique is used. If radiation is used, the source and dosage must be
clearly documented in the allograft processing record. Once they are
harvested, the tissues are cooled, cleaned of body fluids, and
transported to the local tissue bank. Final determination of tissue
acceptance is made by the medical director of the tissue bank after all
the medical and serologic data are considered.
require multiple laboratory tests to be performed on the donor’s serum.
These include antibodies to HIV 1 and 2, hepatitis B surface antigen,
hepatitis C antibodies, syphilis antibodies, human T cell lymphotrophic
virus antibodies, and aerobic and anaerobic cultures, as well as tissue
cultures from the individual allografts.
HIV yet not produce serum antibodies. This period can range from 25
days to 6 months. Most tissue banks utilize polymerase chain reaction
(PCR) testing to identify small amounts of viral DNA. With the addition
of PCR to the battery of testing, the window period can be reduced to
19 days with an added processing cost of $120 to $150. Seven-hundred
fifty thousand allografts have been tested with PCR and implanted
without a single HIV seroconversion. Buck calculated that the risk of
contracting HIV from an implanted bone allograft was estimated at 1 in
1,667,000. Meticulous screening of donors is the primary reason for
this extremely small risk. Theoretically, if this screening were not
followed, testing only for HIV antibodies, the risk would increase to
an unacceptable 1 in 161. The risk assessment of Buck, which was
proposed in 1989, is likely higher than the risk under current
screening techniques.
the inception of allografts. Freeze-dried allografts have been in use
since 1951. Once the allograft is procured, it is frozen pending the
results of the donor’s serology. The tissue is then thawed and soaked
in an antibiotic solution, refrozen, and lyophilized to a moisture
content of less than 6% by weight. Allowable storage time after this
technique is from 3 to 5 years. Before implantation, the graft is
rehydrated for 30 minutes. This graft has been shown to provide
adequate initial strength.
fresh-frozen. The allograft is procured and frozen, pending the results
of the donor’s serology. The tissue is then thawed, soaked in an
antibiotic solution, packaged dry, and refrozen from – 70°C to – 80°C.
When the tissue is ready for implantation, it is rapidly thawed in warm
water and implanted. This graft can be stored up to 3 to 5 years.
Cellular survival is essentially zero with this preservation process.
This process is inadequate for meniscal and osteochondral allografts
because of the need for cell survival in these tissues. Ultimate
failure strength has been shown to be adequate when tissue warming
lasts for 2 hours.
deep-freezing, which allows for fibroblast survival. In this process,
grafts are initially cooled to 0°C and processed within 24
hours
of death. Second, they are stored and processed through an antibiotic
“cocktail” at 37°C for 24 hours and then undergo a time-controlled
freezing to a final temperature of -150°C. This technique is ideal for
allografts that depend on the survival of its cellular elements (i.e.,
menisci). Cryopreservation has been shown to protect up to 80% of cells
in meniscal allografts and up to 70% in a tibialis anterior tendon
graft (Fig. 10-1).
 |
|
Figure 10-1 Typical example of a cryopreserved soft-tissue allograft—in this case a tibialis tendon.
|
sterilization techniques have been developed. Ethylene oxide, commonly
used to sterilize surgical instruments, was initially used to sterilize
allografts prior to implantation. Jackson et al.
showed that the ethylene oxide byproducts —ethylene glycol and ethylene
chlorohydrin—cause graft failure and sterile persistent effusions after
allograft ACL reconstruction. The AATB requires a measurement of
residual levels of these byproducts to be documented in the processing
record of the allograft. Ethylene oxide weakens the mechanical strength
of the allograft and potentially causes synovitis postoperatively.
Newer techniques that inactivate pathogens via chemical and antibiotic
treatment add significant expense to the overall cost of the allograft.
secondary sterilization technique for soft tissue grafts. Gamma
irradiation can inhibit HIV uptake by T lymphocytes. Bone allografts
have been looked at more closely in regard to the effect of radiation.
Many authors have concluded that deep-freezing, in conjunction with 1.5
to 2.5 mrad of radiation, adequately eliminated HIV while causing
minimal damage to the collagenous substrate of the allograft. Although
gamma irradiation kills graft cells, it is a necessary step to ensure
sterilization of the allograft.
autograft, undergo a sequence of graft necrosis, host cellular
repopulation, revascularization, and remodeling. Jackson et al.
eloquently outlined the biologic incorporation of softtissue allografts
in a goat ACL model. His group looked at freeze-dried allograft ACL
reconstructions and compared them with a control group of intact ACLs.
One year after implantation, these two groups were compared on both
histologic and microvascular bases. They found a similar vascular
pattern surrounding both the nascent and allograft ACL. In a follow-up
study, the histological and mechanical properties of patellar tendon
autografts and allografts were compared. The allograft induced greater
vascular and inflammatory responses at 6 weeks, compared with the
autografts. Both groups were similar histologically at 2 months, with
all the cellular elements being from the host. The allografts had a 27%
average load to failure (compared with the nascent ACL), compared with
62% for the autograft. Other authors have found that at 26 weeks,
allograft ACLs have a maximum of 43% load to failure and were
histologically similar to the nascent ACL.
reconstruction of the acromioclavicular joint have been described. The
procedure depicted is a derivation of that described by Lemos and
Morrison in which a GORE-TEX® loop was placed through both the coracoid
and distal clavicles. Weaver and Dunn resected 1 cm of distal clavicle
and transferred the coracoacromial ligament into the medullary canal
with fixation through drill holes. We performed a technique using a
semitendinosus allograft for the reconstruction of the coracoclavicular
ligaments. No long-term, prospective, randomized study of this
technique has been performed comparing allograft reconstruction to
methods involving augmentation or internal fixation. Currently, this is
the technique of choice in our institution.
-
The AC joint is debrided of fibrous scar tissue and organized hematoma, and 1 cm of distal clavicle is removed.
-
A 4.5-mm drill hole is made in the distal clavicle 1 cm medial to the previous resection.
-
This drill hole is enlarged using sequentially larger curettes to an approximate size of 8 mm.
-
On a separate table, a semitendinosus-gracilis allograft of 255 mm in length is prepared in the following manner.
-
A running no. 5 nonabsorbable “baseball” stitch is placed in either end of both the semitendinosus and gracilis allografts.
-
This graft is tensioned at 20 ft-lb to remove any creep.
-
A “safety strand” composed of three no. 5 nonabsorbable sutures is then braided together.
-
A 90-degree Mixter clamp is passed around
the inferior border of the coracoid, and the allograft and suture braid
are brought around the coracoid. -
The lateral tail of the graft and suture braid is passed over the clavicle and through the 8-mm drill hole.
-
The clavicle is overreduced 5 mm, and the allograft is sewn to itself using a no. 5 nonabsorbable suture.
-
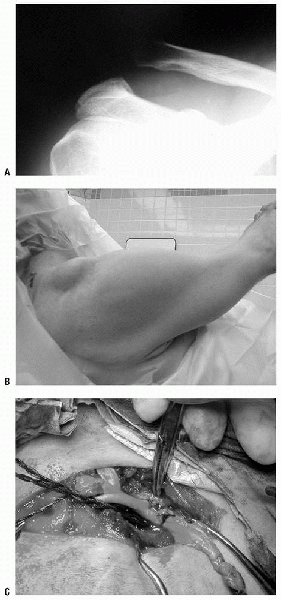
The “safety stitch” is tied to itself as well (Fig. 10-2).
-
The patient is immobilized for 7 days and then begins on a progressive motion and strengthening program.
P.118 -
 |
|
Figure 10-2 A: Radiograph of type V acromioclavicular joint separation. B: Clinical appearance of type V acromioclavicular joint separation. C: Semitendinosus allograft woven through distal clavicle and around coracoid in acromioclavicular joint reconstruction.
|
anterior palsy has yielded good results. In our institution, we have
modified this technique by using a soft-tissue allograft to lengthen
the normally stout pectoralis tendon.
-
The procedure is performed using the
two-incision technique as described by Warner, substituting a hamstring
allograft for the fascia lata autograft. -
The patient is placed in the lateral decubitus position, allowing access to both the patient’s chest and inferomedial scapula.
-
Through a limited deltopectoral approach,
the sternocostal head of the pectoralis major is identified and
detached from its humeral insertion. -
On a back table, a semitendinosus allograft is prepared in a similar fashion as described previously.
-
The graft is not tensioned prior to use.
-
The single graft is then woven into the pectoralis tendon with a no. 5 suture (Fig. 10-3).
-
Attention is then directed to the inferomedial angle of the scapula, which is displaced medially and superiorly.
-
An 8-cm oblique incision in the direction
of Langer’s lines is made in between the current and reduced positions
of the patient’s scapula. -
The latissimus dorsi is retracted
laterally, and the rhomboid major, infraspinatus, and subscapularis are
subperiosteally dissected off the inferomedial angle of the scapula. -
While protecting the underlying chest
wall, an 8-mm drill hole is made from posterior to anterior in the
inferomedial angle of the scapula. -
Using blunt dissection, a tunnel is made from the anterior to the posterior wound along the chest wall.
-
A large Kelly clamp is then passed from posterior to anterior and opened, serving to dilate the tunnel.
-
The clamp is then repassed, transferring the tendon/allograft to the posterior incision.
-
The scapula is reduced, and the composite is brought through the scapula and sutured to itself with a no. 5 suture.
-
For additional fixation, the graft is secured via drill holes and a no. 2 suture to the medial scapula.
-
The patient is placed in a “gunslinger” brace in 30 degrees of abduction, neutral rotation, and flexion.
-
This position is held for 6 weeks, at which point gentle range of motion in scaption is begun.
 |
|
Figure 10-3 Semitendinosus tendon “leader” for pectoralis tendon transfer for scapular winging.
|
nascent medial collateral ligament (MCL), palmaris longus, and gracilis
tendons. Hamner found that a fresh-frozen, single gracilis tendon had a
strength of 837 ± 138 Newtons. Fresh-frozen gracilis tendon exhibits
adequate strength characteristics for replacement of the anterior band
of the MCL. Morgan and Schepsis (personal communication, 2005) have
established a series of MCL reconstructions utilizing gracilis
allograft and a docking technique. All 16 athletes have returned to
sports, and no complications were reported. Of note, approximately 15%
of patients do not have a palmaris tendon and, if an allograft is not
utilized, graft sources include the less desirable extensor indicis
pollicis, toe flexors, or plantaris.
although a split instead of an elevation of the flexor-pronator mass
can be performed.
-
An 8-mm longitudinal incision is made, slightly anterior to the cubital.
-
The ulnar nerve is dissected from its
cubital tunnel and released proximally at the intermuscular septum, as
well as distally at the split of the flexor carpi ulnaris. -
After identification of the
flexor-pronator mass, it is split longitudinally from the medial
epicondyle to the sublime tubercle of the proximal ulna. -
The attenuated MCL is directly deep to the flexor-pronator mass.
-
This ligament should be split longitudinally for later imbrication over the graft.
-
A gracilis allograft is pared down to
approximately 50% of its original size. The graft is prepared on a back
table by placing a no. 2 nonabsorbable suture baseball stitch into one
end. -
The graft is not tensioned prior to implantation.
-
Using a 3.2-mm drill bit, two drill holes are made 45 degrees to each other in the sublime tubercle.
-
These drill holes are then connected using medium-sized curettes to form a tunnel.
-
Attention is then directed toward the inferior and distal aspects of the medial epicondyle.
-
Two 3.2-mm drill holes are placed retrograde at 45 degrees to each other, aiming proximally.
-
These tunnels are not connected.
-
Then, using a 2.5-mm drill bit, two
“docking” tunnels are made from the superior aspect of the epicondyle
into the respective tunnel. -
Using a suture passer, the graft is passed through the ulnar tunnel.
-
Then, the graft end, with the suture attached, is “docked” retrograde into its ulnar tunnel.
-
The remaining graft end is then shortened so that it will be the proper length to “dock” into its drill hole.
-
A no. 5 nonabsorbable suture is then placed into this graft end and docked in an identical fashion.
-
The two no. 5 sutures are then tied to each other with a varus force placed on the elbow.
-
The nascent MCL is then imbricated over the allograft.
-
The ulnar nerve is subcutaneously
transposed, the skin is closed with interrupted suture, and the
extremity is placed in a compressive dressing and in a locked, hinged,
unilateral elbow brace. -
The standard rehabilitation protocol as per Wilk et al. was followed.
-
Complications associated with this
procedure are ulnar nerve injury, which can be decreased with the
muscle splitting technique, medial antebrachial cutaneous nerve injury,
and ulnar tunnel fracture.
extremity of men in the fourth through sixth decades of life. No cases
have been reported in women. The mechanism of injury is a single
traumatic event in which an unexpected extension moment is placed
across the elbow while the arm is flexed 90 degrees. The tendon
typically avulses from the radial tuberosity. The bicipital aponeurosis
may rupture or remain intact.
complete. Partial tears can often be difficult to diagnose, with
magnetic resonance imaging (MRI) possibly being of assistance in
delineating the percentage of tendon remaining intact. Partial tears
can be treated with protection, followed by range of motion and
progressive strengthening. If the patient is still symptomatic, the
tendon can be released completely, debrided, or repaired back to the
radial tuberosity. Complete tears can be classified as acute (<4
weeks) and chronic (>4 weeks). Acute tears can be repaired using a
one- or two-incision technique. Chronic tears present a difficult
problem with regard to direct repair. The biceps tendon usually
retracts proximally into the distal arm and fibroses to the underlying
brachialis. The bicipital aponeurosis will often limit this retraction.
The goal of treatment is anatomic repair of the tendon into the radial
tuberosity. Loss of tendinous tissue and tendon length should be
expected preoperatively. Autogenous grafts have been described in the
literature by a number of authors. We have taken the results from these
studies and expanded them with the use of allografts to bridge the gap
in chronic biceps tendon disruptions. Our technique for repair of a
chronic distal biceps tears is described.
-
A 6-cm gap between the mobilized distal biceps muscle and the scarred tendon in the radial tuberosity is identified.
-
A cryopreserved semitendinosus allograft is used for reconstruction.
-
The graft is woven through the distal
bicipital musculotendinous junction, brought distally and sutured to
itself using a no. 2 Ethibond suture. -
The doubled tendon unit is fixed to the
radial tuberosity with double fixation, using a trough and drill holes,
as well as a suture anchor.
reconstruction of the lateral ulnar collateral ligament. In the classic
article by O’Driscoll et al., 3 of 5 patients
had reconstruction of the lateral ulnar collateral ligament, 2 with
palmaris longus and 1 with triceps fascia autografts. Instability
symptoms resolved in all patients in this series. There have been no
studies looking at the use of allograft hamstrings in the treatment of
posterolateral instability of the elbow. We present the following
example of lateral ulnar collateral ligament reconstruction.
examination under anesthesia revealed a positive pivot shift for
posterolateral rotatory instability of the elbow. Elbow arthroscopy
revealed no intra-articular pathology. Lateral ulnar collateral
ligament reconstruction was then performed using cryopreserved gracilis
tendon. Utilizing the technique described by O’Driscoll for autogenous
palmaris longus reconstruction, an allograft gracilis tendon was used
to reconstruct the attenuated lateral ulnar collateral ligament.
surgery, the patient subjectively noted excellent function of the
shoulder and no longer had the subjective complaints that she had
preoperatively. On examination, she had a stable elbow with full range
of motion.
allografts in ACL reconstruction. We recently performed a 2-year
prospective study comparing autograft to allograft hamstring ACL
reconstruction. Both clinical and functional data were assessed. This
prospective investigation involved 70 patients, 41 of whom had soft
tissue allograft and 29 with autograft double-stranded semitendinosus
gracilis ACL reconstructions. Concomitant injuries were similar between
the two cohorts. There was no statistical difference in Tegner and
Lysholm functional scores. The number of normal and near-normal IKDC
scores was equal as well. Side-to-side KT-1000 differences were 1.08 mm
for the autografts and 0.68 mm for the allografts. This is the only
study to compare soft tissue autografts and allografts for ACL
reconstruction.
has been addressed by a number of authors. Multiple techniques have
been proposed, ranging from tissue transfers to autograft and allograft
reconstructions. All of these different techniques have displayed good
results.
is usually indicated in two different scenarios. First, an isolated
grade III tear of the PCL (>1 cm of posterior translation) with
patient complaints of instability is an indication for reconstruction
of the PCL. Second, PCL reconstruction should be considered in the face
of a multiple-ligament injured knee, when the central hinge of the knee
(ACL/PCL) has been injured. We present a surgical case to exemplify
both our indications for surgery and surgical technique.
days after being injured in the first game of his junior season. The
patient recalled that three different players struck his right knee. He
heard a loud “pop,” then felt extreme pain in his knee, and was carried
off the field. He was taken to an emergency department and had
radiographs, which revealed an avulsion fracture of the lateral
epicondyle. On examination, the patient had a large effusion and an
active range of motion of 5 to 30 degrees. He had a 3+ Lachman, 3+
opening without end point with varus stress and normal valgus
stability. Posterior drawer and dial testing could not be performed as
a result of pain. Distal pulses were normal, and peroneal nerve
function was 4 -/5. A diagnosis of acute ACL, lateral collateral
ligament (LCL), and posterior ligament complex (PLC) disruption was
made, with suspicion of a PCL injury. The patient was placed in a
straight-leg brace, instructed to keep the extremity iced and elevated,
and scheduled for an MRI. The MRI revealed the suspected ACL, LCL, PLC,
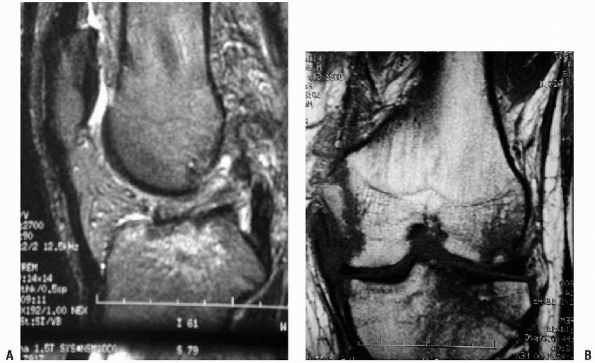
and PCL disruptions, as well as bimeniscal capsular disruptions (Fig. 10-4).
At follow-up, the effusion had decreased to allow testing of the
injured structures. A 3 + posterior drawer and, at 30 degrees of knee
flexion, a 15-degree side-to-side difference in dial testing were
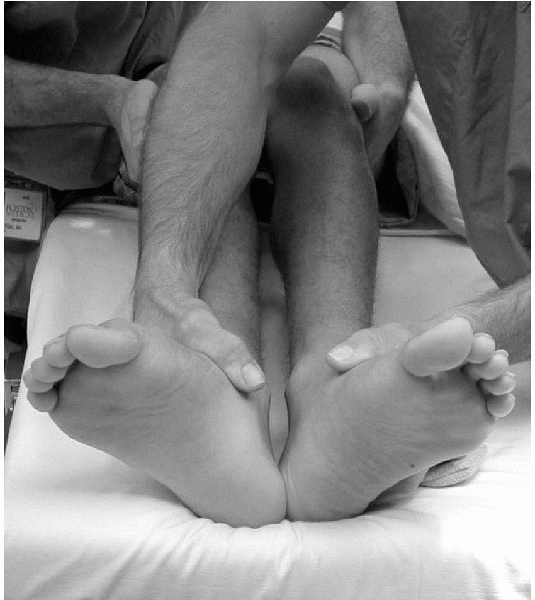
observed (Fig. 10-5).
the PLC, ACL, and PCL tibialis allograft reconstruction, and meniscal
repair or menisectomy. Medial and lateral meniscal repairs were
performed using the Fast-Fix (Smith and Nephew, Mansfield, MA) meniscal
repair system. Standard endoscopic ACL reconstruction was completed
using a continuous loop endobutton (Acufex, Smith and Nephew,
Mansfield, MA) for femoral fixation. A single-bundle PCL was performed
endoscopically using a posteromedial portal and a 70-degree scope. A
biointerference screw was used for femoral fixation. Neither graft was
secured in its tibial tunnel. Attention was then directed to the PLC.
The extremity was exsanguinated and the tourniquet inflated to 300 mm
Hg. A 10-cm longitudinal incision was made along the lateral knee,
traveling just anterior to the biceps femoris and extending to Gerdy’s
tubercle distally. A large hematoma was evacuated, and the peroneal
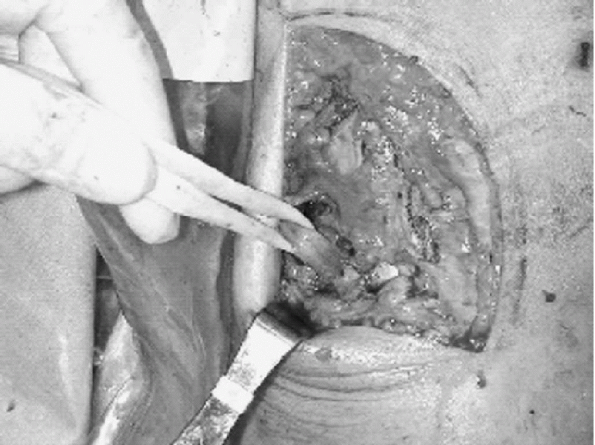
nerve was present in the center of the wound (Fig. 10-6).
The iliotibial band and biceps femoris had been avulsed from their
distal attachments. The peroneal nerve was dissected distally to the
fibular
head
and protected. The LCL had been avulsed from its fibular attachment.
Posterior retraction of the lateral gastrocnemius revealed a
musculotendinous disruption of the popliteus and avulsion of the
popliteofibular ligament and joint capsule. Using suture anchors, the
capsule was reattached to its tibial insertion, the popliteofibular
ligament was reapproximated to the fibular head, and the popliteus was
sutured with a no. 2 nonabsorbable suture. The LCL and biceps femoris
were replaced into the fibula using suture anchors as well. The
iliotibial band was repaired to itself. An Intrafix (Mitek, Norwood,
MA) device was used for the ACL tibial fixation and a screw-in-screw
and washer were used for the PCL fixation. Postoperatively, the patient
was placed in a locked hinged brace, made strictly non-weight-bearing,
and started on CPM 0-40 up to 90 degrees for 8 weeks.
 |
|
Figure 10-4 Sagittal (A) and coronal (B) MRIs of an ACL/PCL/PLC injured knee.
|
 |
|
Figure 10-5 Positive, right-sided dial test indicating likely posterolateral corner injury.
|
the knee can be performed with an allograft. Numerous studies have
shown the excellent integration potential of allografts in an
extra-articular setting. We present the following case of the
reconstruction of a chronic grade III MCL injury with instability.
 |
|
Figure 10-6 Peroneal nerve in subcutaneous tissue associated with posterolateral corner injury.
|
of left knee instability. He initially injured his knee while playing
football and opted to continue participation in both football and as a
hockey goaltender with a functional brace. Now in college, the patient
was experiencing daily instability that limited him in his normal
activities.
antalgic gait with varus thrust and knee alignment. He had 3 + opening
with valgus stress at both 0 and 30 degrees of flexion. He also had a 3
+ Lachman test with no end point. The dial test, varus stress test in
extension, and recurvatum test were normal. The patient’s MRI revealed
a complete tear of the ACL, a grade III tear of the MCL, and a capsular
disruption of the medial meniscus. Full-length standing radiographs
showed 12 degrees of knee varus.
First, an arthroscopic medial meniscal repair, an opening wedge tibial
osteotomy (Arthrex, Naples, FL), and an Achilles allograft MCL
reconstruction would be performed. The technique of Caborn et al. would
be used for reconstructing both the MCL and the posterior oblique
ligament (POL). A capsular avulsion red-red medial meniscal tear was
repaired with three Fast-Fix (Smith and Nephew). The leg was then
exsanguinated, and a direct medial 6-cm incision was over the distal
insertion of the MCL. The pes anserinus tendons and MCL were retracted
posteriorly, and an opening wedge tibial osteotomy with a 12-degree
correction was completed. The Achilles allograft was prepared on a back
table. A 10 × 30 mm bone plug was prepared. The tendinous portion of
the graft was split and a whip stitch of a no. 2 Fiberwire (Arthrex)
was placed in each limb. A 2-cm incision was made over the medial
epicondyle, and the MCL origin was split longitudinally. A
transepicondylar pin was placed across the femur and a 10 × 35 mm
tunnel was drilled. The Allograft bone plug was press fit into the
tunnel and fixed with a 10 × 28 mm biointerference screw. A Kelly clamp
was then used to tunnel under the superficial MCL, and the tendinous
limbs were brought antegrade to the level of the proximal tibia. Two 9
× 30 mm tunnels were drilled into the proximal tibia at the relative
isometric points for the MCL and POL. The graft limbs were then passed
into these tunnels and the anterior limb was fixed with a 9 × 25 mm
biointerference screw with the knee in 30 degrees of flexion, varus,
and internal rotation. The posterior limb was fixed in a similar manner
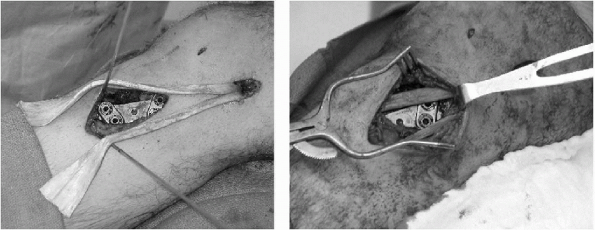
with the knee at 60 degrees of flexion (Fig. 10-7).
 |
|
Figure 10-7
Reconstruction of the MCL with a split Achilles tendon allograft. An opening wedge tibial osteotomy was performed concomitantly. |
range of motion exercises. A tibialis ACL reconstruction was planned
once his osteotomy was fully healed and the MCL integrated.
cryopreserved semitendinosus allograft for reconstruction of the medial
patellofemoral ligament for patients with chronic recurrent patellar
dislocation secondary to traumatic chronic insufficiency of the medial
patellofemoral ligament. The method used is similar to that described
by Drez, utilizing a free tendon graft that is secured by suture
anchors to the adductor tubercle origin of the medial patellofemoral
ligament and then secured to the proximal medial third of the patella.
The following is an illustrative case.
patellar dislocation during a football game. This was reduced on the
field by the trainer, and radiographs in the emergency room were
negative for any type of osteochondral fracture. The patient was
treated elsewhere with 4 weeks of immobilization in a straight-leg knee
immobilizer, followed by physical therapy. Subsequent to this, the
patient had 5 to 6 further lateral patellar dislocations over the
following year. At the time of initial presentation, he was noted to
have a normal Q-angle with markedly abnormal lateral excursion of the
patella. The patient was brought to the operating room for arthroscopy
and proximal realignment of the extensor mechanism. At the time of
arthroscopy, it was noted that he had a grade II lesion of the medial
patellar facet for which an arthroscopic chondroplasty was performed.
An open proximal realignment was performed in combination
with
a lateral retinacular release. At the time of reconstruction, it was
noted that the medial retinaculum and the medial patellofemoral
ligament were markedly thin and attenuated. Imbrication of this tissue,
as well as advancement of the vastus medialis obliquus (VMO), would
have been insufficient to reconstruct the medial patellar restraints. A
cryopreserved semitendinosus allograft was used to reconstruct the
medial patellofemoral ligament. This was secured to the origin of the
medial patellofemoral ligament through a transverse incision in the
retinaculum, thus exposing the adductor tubercle. After creating a
bleeding bone bed in this area, two bioabsorbable suture anchors were
placed into the adductor tubercle, and two additional anchors were
placed at the proximal medial border of the patella at the junction of
the proximal third and the middle one third of the patella. The patella
was reduced to recreate the normal lateral patellar excursion, and both
sets of anchor sutures were passed through the semitendinosus
allograft. The graft was then folded back on itself and sutured to
itself using a no. 2 nonabsorbable suture. Postoperatively, the patient
was immobilized in extension for approximately 3 weeks, at which time
range-of-motion exercises were begun. No active quadriceps exercises
were allowed until 6 weeks postoperatively. At 1-year follow-up, the
patient had returned to football with no further episodes of
instability.
commonly associated with chronic attenuation after total knee
arthroplasty. Also, allografts have been used in revision or in chronic
and undiagnosed patellar tendon rupture. Primary, acute patellar tendon
ruptures are universally manageable through primary repair. Chronic,
neglected tears and retears often require augmentation and, in extreme
cases, complete reconstruction of the patellar tendon unit. We present
an illustrative case below.
 |
|
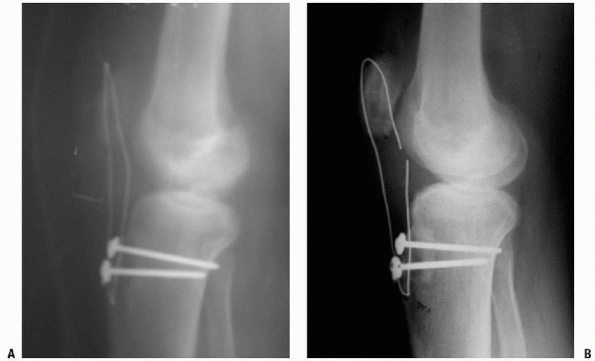
Figure 10-8 Initial (A) and final (B) radiographs of an Achilles tendon allograft reconstruction of a failed, infected patellar tendon repair.
|
of left knee pain. He initially injured his knee during a pickup
basketball game. He heard a “pop” and was unable to return to play. On
examination, the patient was found to have a complete patellar tendon
rupture and was indicated for operative repair, which was performed 8
weeks postinjury. Three weeks later, the patient presented for
follow-up and had an obvious disruption of his repair. On returning to
the operating room, the patient was found to have a deep wound
infection. Irrigation and debridement were performed, and
broad-spectrum antibiotics were started. Pseudomonas and Enterobacter
were cultured from the wound. After 6 weeks of antibiotics and the
presence of a sterile surgical wound, the patient was returned to the
operating room for an Achilles tendon allograft reconstruction of the
patellar tendon (Fig. 10-8A). The patient began
range of motion at 6 weeks and at 4 months obtained a range of motion
of 0 to 90 degrees with integration of the Achilles graft (Fig. 10-8B).
reconstruction of the Achilles tendon. For treatment of chronic
Achilles tendon ruptures, reruptures, or tendinosis, most authors
recommend either the advancement of local tissues or transfer of the
plantaris or flexor hallucis longus (FHL) muscle-tendon unit.
Tendinosis and chronic unrepaired disruptions of the Achilles tendon
need to be reconstructed rather than repaired. Numerous procedures have
been described that involve the transfer of the FHL, flexor digitorum
longus (FDL), or plantaris musculotendinous unit into the deficient
Achilles tendon. These transfers are indicated
when
a gap in the Achilles is present when the foot is put in neutral or if
greater than 50% of the tendon has been removed as a result of
degeneration or poor tissue quality. These transferred tendons are
meant to provide additional vascularity, as well as structural support.
Soft-tissue allografts provide immediate structural support, as well as
a scaffold for ingrowth of new, vascular fibrous tissue.
complaints of many years of intermittent left Achilles pain and
swelling. He has tried stretching, nonsteroidal anti-inflammatory
drugs, and heel lifts without improvement. His MRI revealed fusiform
enlargement of his Achilles tendon with extensive degenerative
tendinosis. As a result of his conservative measures failure for more
than 1 year, the patient’s condition indicated Achilles tendon
debridement and reconstruction. The preoperative plan was to
reconstruct the Achilles tendon if greater than 50% of the tendon had
degenerated. The patient’s left lower extremity was exsanguinated. A
10-cm incision was made just medial to the Achilles tendon through skin
and subcutaneous tissue only. The peritenon was identified, split
longitudinally, and elevated off the tendon. The Achilles was firm and
rubbery, with little resemblance to a normal tendon for the large
majority of its length and width. The central 75% of the tendon was
excised, leaving only the normal periphery remaining. On a back table,
an 8 mm × 255 mm tibialis anterior allograft was prepared with a no. 5
nonabsorbable “baseball” stitch in either end of the graft. The
allograft was then woven through the remaining Achilles tendon and,
with the foot in gravity plantarflexion, sutured to the remaining
tendon and itself. The paratenon is closed separately, and the skin
secured with interrupted nylon sutures. The patient was then placed in
a gravity plantarflexion below-knee cast and remained
non-weight-bearing for 6 weeks with weekly cast changes. The patient
did well postoperatively and returned to work as a truck driver at 6
months.
when compared with the more common midsubstance musculotendinous
hamstring tears that are seen in the orthopaedic surgeon’s office. Most
of these have been described as a result of water skiing. Proximal
hamstring avulsions are one of the rare indications for surgery after
hamstring tears. We present the use of a semitendinosus allograft for
surgical reconstruction after a chronic proximal hamstring rupture.
rupture after he slipped down some stairs and noted some acute pain and
a tearing sensation in the area of the proximal hamstring and buttocks.
His condition indicated nonoperative treatment. After several months of
physical therapy, however, he noted persistent fatigue, weakness, and
deformity. Despite continuing a rigorous stretching and strengthening
program, he was unable to return to his normal activities of tennis and
golf without symptoms. Approximately 9 months after his injury, he
presented to our office with an obvious proximal hamstring avulsion
with distal retraction of all three hamstring muscles. Another course
of physical therapy and isokinetic strengthening was prescribed;
however, the patient returned 3 months later with the same symptoms and
was not happy with his abilities.
brought to the operating room for attempted repair/reconstruction of
the proximal hamstrings. At the time of surgery, the conjoined tendon
of the semitendinosus and biceps was mobilized and reattached directly
to the ischial tuberosity through drill holes. The semimembranosus,
however, had retracted distally and there was a large gap of
approximately 10 cm after mobilization between the proximal end of the
semimembranosus and the ischial tuberosity. A semitendinosus allograft
was used to bridge the gap. The allograft was weaved through the
proximal end of the semimembranosus and then attached to the ischial
tuberosity via suture anchors and drill holes. Postoperatively, the
patient was maintained in a brace, keeping his knee at 90 degrees of
flexion and his hip extended for approximately 6 weeks, and the patient
was started on a gradual rehabilitation program. After a lengthy
rehabilitation program, the patient was able to return to all his
activities and was very satisfied with his outcome, being able to
return to golf and tennis approximately 6 months after surgery.
DW, Hyman J, Williams R, et al. Management of MCL injuries of the elbow
in throwers. Tech Shoulder Elbow Surg 2000;1: 73-81.
Association of Tissue Banks. Standards for tissue banking: 2001 update.
Hollywood, FL: American Association of Tissue Banks, 2001.
SP, Warren RF, Ashlock MA. Replacement of the anterior cruciate
ligament using patellar tendon allograft: an experimental study in the
dog. J Bone Joint Surg 1986;68A:376-385.
PS, Kantaras AT, Caborn DN. Medial collateral ligament reconstruction
with allograft using a double bundle technique. Arthroscopy
2002;18(4):E19.
BE, Malinin TI, Brown MD. Bone transplantation and human
immunodeficiency virus: an estimate of risk of acquired
immunodeficiency virus (AIDS). Clin Orthop 1989;240:129-136.
OD, Snook GA. Reconstruction of lateral ligament tears of the ankle. An
experimental study and clinical evaluation of seven patients treated by
a new modification of the Elmslie procedure. J Bone Joint Surg
1969;51:904-912.
MJ, Matt V, Schenck RC Jr. Augmented lateral ankle reconstruction using
a free gracilis graft. Orthopedics 2002;25:31-35.
RH Jr, Head WC, Malinin TI. Extensor mechanism reconstruction with
allograft after total knee arthroplasty. Clin Orthop 1994;303:79-85.
CD, Olson, E, Irrgang JJ, et al. Allograft versus autograft anterior
cruciate ligament reconstruction. Clin Orthop 1996;324: 134-144.
S, Shino K, Nagano J, et al. Replacing the medial collateral ligament
with an allogeneic tendon graft. An experimental canine study. J Bone
Joint Surg 1990;72B:1044-1049.
DW, Corsetti J, Simon TM. Biologic incorporation of allograft anterior
cruciate ligament replacement. Clin Orthop 1996;324: 126-133.
DW, Grood ES, Wilcox P, et al. The effects of processing techniques on
the mechanical properties of bone-anterior cruciate ligament-bone
allografts: an experimental study in goats. Am J Sports Med
1988;6:101-105.
HP, Lemos MJ, Schepsis AA. Salvage of failed acromioclavicular joint
reconstruction using autogenous semitendinosus tendon from the knee. Am
J Sports Med 2001;29:234-237.
RV, Simonian PT. Semitendinosus augmentation of acute patellar tendon
repair with immediate mobilization. Am J Sports Med 1995;23:82-86.
BR, Bartolozzi A, Carney B. A systematic approach to reconstruction of
neglected tears of the patellar tendon. A case report. Clin Orthop
1988;235:268-271.
FR, Barber SD, Mangine RE. Bone-patellar ligament-bone and fascia lata
allografts for reconstruction of the anterior cruciate ligament. J Bone
Joint Surg 1990;72A:1125-1136.
FR, Barber-Westin SD. Reconstruction of the anterior cruciate ligament
with human allograft. Comparison of early and later results. J Bone
Joint Surg 1996;78A:524-537.
RK, Shelton WR, Bomboy AL. Allograft versus autograft patellar tendon
anterior cruciate ligament reconstruction: a 5-year follow-up.
Arthroscopy 2001;17:9- 13.
WR, Treacy SH, Dukes AD, et al. Use of allografts in knee
reconstruction: I. Basic science aspects and current status. J Am Acad
Orthop Surg 1998;6:165-168.
K, Nakata K, Horibe S, et al. Quantitative evaluation after
arthroscopic anterior cruciate ligament reconstruction. Allograft
versus autograft. Am J Sports Med 1993;21:609-616.
K, Inoue M, Horibe S, et al. Maturation of allograft tendons
transplanted into the knee. An arthroscopic and histologic study. J
Bone Joint Surg 1988;70-B:556-560.
DC, Summa CD. Reconstruction of chronic rupture of the extensor
mechanism after patellectomy. Clin Orthop 1998;357: 135-140.
