Biomaterials
Editors: Tornetta, Paul; Einhorn, Thomas A.; Damron, Timothy A.
Title: Oncology and Basic Science, 7th Edition
Copyright ©2008 Lippincott Williams & Wilkins
> Table of Contents > Section IV – Basic Science > 17 – Biomaterials
17
Biomaterials
Joseph A. Spadaro
Michael T. Clarke
Julie M. Hasenwinkel
The advances made in contemporary orthopaedic surgery
are intimately related to the development of and use of implant
biomaterials. These are materials used to augment, repair, or replace
natural tissues or assist in healing. Currently, biological performance
is as influential as the mechanical properties of its component
materials in the design of an implant. It is now recognized that no
implanted biomaterials are actually inert. In fact, instead of
demanding inertness, most surgical specialties are embracing
“bioactivity” as a means toward a fuller regeneration of the normal
state. Orthopaedics is no exception. Only by the orchestration of the
mechanical, chemical, and biological behaviors of these biomaterials
can further improvements be made.
are intimately related to the development of and use of implant
biomaterials. These are materials used to augment, repair, or replace
natural tissues or assist in healing. Currently, biological performance
is as influential as the mechanical properties of its component
materials in the design of an implant. It is now recognized that no
implanted biomaterials are actually inert. In fact, instead of
demanding inertness, most surgical specialties are embracing
“bioactivity” as a means toward a fuller regeneration of the normal
state. Orthopaedics is no exception. Only by the orchestration of the
mechanical, chemical, and biological behaviors of these biomaterials
can further improvements be made.
An understanding of the general principles is therefore crucial to implant selection and expectations on behavior.
Metals and Metallic Alloys
-
Metals and their alloys of other metallic
and nonmetallic elements are used as implants in orthopaedic surgery
mainly for bearing and structural components that are typically affixed
to bone. -
Benefits: bulk biocompatibility, strength, resistance to fatigue failure over millions of cycles
-
Drawbacks: susceptibility to corrosion,
potential for immune stimulation, particulate cytotoxicity and
mutagenicity, possibility of fatigue failure in the long term
Implant Manufacture
-
Raw material is processed in one of three ways (frequently, a combination of two or three is necessary for final implant shape; Table 17-1 gives definitions).
-
Machining: includes lathing, milling, or grinding of material
-
Casting: molten alloy is poured into a mold (subsequently broken)
-
Forging: by bending, compressing, and impacting (often at elevated temperatures)
-
-
Final modifications of an implant frequently used are:
-
Coating (to enhance bone ingrowth or ongrowth)
-
Plasma spray of powdered hydroxyapatite or metal alloy
-
Cold deposition of hydroxyapatite
-
Sintering of beads or wire
-
-
Grit blasting (to enhance frictional interference with cement or bone)
-
Polishing (to enhance appearance, reduce corrosion, and reduce friction on cement)
-
Shot peening (to enhance fatigue properties of critical areas [e.g., Morse taper junctions])
-
Alloys in Current Use
-
Although new alloys are constantly being
developed, there are four broad classifications of alloy types
currently in common orthopaedic use:P.374Table 17-1 Some Metallurgical DefinitionsTerm Definition Cold working The alteration of the shape or
size of a metal by plastic deformation. Processes include rolling,
drawing, pressing, spinning, extruding, and heading; are carried out
below the recrystallization point, usually at room temperature.
Hardness and tensile strength are increased with the degree of cold
work, while ductility and impact values are lowered.Hot working The rolling, forging or
extruding of a metal at a temperature above its recrystallization point
without significant strain hardeningWarm working Processing in a range usually 0.3 to 0.6 of melting point Forging A process of working metal to
a finished shape by hammering or pressing; primarily a “hot” operation.
It is applied to the production of shapes either impossible or too
costly to make by other methods or needing properties not obtainable by
casting. Categories of forgings include hammer, press, drop, or
stamping.Wrought An alloy that has been significantly “worked” to break down its cast structure Solution heat treatment A process in which an alloy or
metal is heated to a suitable temperature, is held at that temperature
long enough to allow a certain constituent to enter into solid
solution, and is then cooled rapidly to hold that constituent in
solution. Most solution heat treatments soften or anneal.Annealing A heat treatment that relieves
internal stress. Solution-annealed material is frequently in its most
corrosion-resistant and ductile condition.Work hardening A term that signifies crystalline changes when a material is strained beyond its yield point Grain boundary As metals solidify
(crystallize), many individual regions (grains) form with differing
orientations of the atomic lattice. The region where grains meet
(boundary) is less dense and more chemically reactive than the bulk
material.Passivation A process of formation of an
oxide layer on the surface of an alloy or metal. This can be
spontaneous in the environment or enhanced chemically, for example by
immersion in nitric acid. Passivation generally leads to improved
corrosion resistance.-
Titanium alloys
-
Cobalt chromium alloys
-
Stainless steel alloys
-
Tantalum-carbon
-
Titanium and its Alloys
-
Although developed as an aerospace alloy,
titanium is well known in the medical field for its biocompatibility
and high strength-to-weight ratio. -
It is broadly used in the manufacture of
fracture and spinal fixation devices as well as nonarticulating joint
replacement components.
Orthopaedic Uses of Titanium Alloy
-
Fracture and spinal fixation devices: screws, plates, in-tramedullary nails
-
Joint replacement components
-
Cementless total hip: acetabular shells, femoral stems, ingrowth or ongrowth coatings
-
Not femoral heads (historically abandoned due to high wear)
-
-
Total knee: tibial tray, ingrowth or ongrowth coatings
-
Not femoral bearing surfaces (historically abandoned due to high wear)
-
-
Metallurgy
-
Titanium is typically alloyed with other
elements as well as being thermomechanically treated to achieve the
desired properties for implantation. -
Metallic alloying increases the strength
and maintains ductility by replacing titanium atoms in the crystal
lattice with other metal atoms such as aluminum, vanadium, zirconium,
and niobium. -
Nonmetallic interstitial elements such as oxygen, carbon, and nitrogen lie in voids between atoms of titanium.
-
Strength is gained, but ductility and toughness are reduced.
-
The natural crystal state for pure titanium (CPTi) is an alpha phase with a hexagonal close packed (hcp) crystal lattice.
-
An alternate beta, body centered cubic
(bcc) crystal phase is present in varying extents in titanium alloys
depending on the alloy and heat treatments performed. -
Beta-phase alloys can be harder and more brittle than alpha-phase ones.
-
In the commonly used
titanium—aluminum—vanadium, an alpha-beta alloy, aluminum stabilizes
the alpha phase and vanadium the beta phase. -
The newer substantially beta-phase alloys
containing molybdenum impart superior strength to an alloy and have the
lowest elastic modulus (80 MPa) of any of the commonly used bulk
implant alloys (e.g., CoCrMo 200 MPa).
P.375 -
Box 17-1 General Advantages and Disadvantages of Titanium Alloys
Advantages
Excellent biocompatibility
Relatively low elastic modulus
Spot welding to itself in taper junctions
Low galvanic corrosion to CoCrMo alloy
Disadvantages
Poor wear properties Notch sensitive
Concern with vanadium and aluminum content in specific alloys
Titanium Implant Characteristics (Box 17-1 and Table 17-2)
Advantages
-
Biocompatibility: result of a highly
inert, insoluble, and adherent 10-nm-thick surface passivation layer of
titanium oxide (titania, TiO2), allowing superior corrosion resistance-
Inert passivation layer of TiO2
significantly reduces galvanic corrosion to cobalt base alloys at taper
junctions (e.g., femoral head/femoral stem of total hip replacement
[THR]).
-
-
Low elastic modulus (80 to 110 Mpa)
-
Useful in the prevention of stress
shielding around cementless implants such as femoral hip replacement
stems, but is to be avoided when used with polymethylmethacrylate
(PMMA) cement as it is prone to increased cement stresses from bending
as well as abrasive wear against cement due to poor wear properties
-
-
Spot welding at titanium-titanium taper
junctions (e.g., stems on tibial base-plate for total knee replacement
[TKR]) minimizes micromotion and fretting.
Disadvantages
-
Poor wear properties: resulted in general
abandonment of titanium alloys as wear surfaces except for custom
implants (e.g., in nickel-sensitive patients where cobalt alloy or
stainless steel are to be avoided) -
For custom titanium bearing surfaces, surface hardening is performed (e.g., ion implantation or nitriding)
-
Even the nitrided surface may be subject to delamination.
-
Notch sensitivity: problem for all alloys, but particularly so for titanium
-
Sharp angles, scratches, and sintered
powder or beads allow stress risers to concentrate in one area,
significantly reducing the fatigue life of an implant. -
Vanadium, and to a lesser extent
aluminum, are considered undesirable elements due to cell cytotoxicity
seen in vitro and theoretical concerns about biocompatibility. Newer,
currently unproven alloys that omit one or both of these alloys have
been developed to avoid these theoretical concerns.
Cobalt-Based Alloys
Cobalt-based alloys, most notably of the cobalt-chromium
binary system, are widely used for load bearing as a result of their
fatigue resistance, and for wear surfaces as a result of their
exceptional hardness when properly formed. Many of the properties of
the alloys arise from the following:
binary system, are widely used for load bearing as a result of their
fatigue resistance, and for wear surfaces as a result of their
exceptional hardness when properly formed. Many of the properties of
the alloys arise from the following:
P.376
Box 17-2 General Advantages And Disadvantages of Cobalt-Based Alloys
Advantages
Good biocompatibility Fatigue-resistant Wear-resistant
Low galvanic corrosion to titanium
Disadvantages
Galvanic corrosion to stainless steel
Concerns regarding nickel content
Cobalt and chromium ion release
High elastic modulus
Expensive
Difficult to process
-
Crystal structure of cobalt
-
Strengthening effects of chromium, nickel, tungsten, and molybdenum
-
Formation of hard metal carbides (e.g., chromium carbide)
-
Corrosion resistance imparted by chromium, nickel, and molybdenum alloying elements
Orthopaedic Uses of Cobalt Alloys
-
Fracture and spinal fixation devices: braided wire for fracture fixation
-
Joint replacement components
-
Femoral heads for metal-on-plastic and metal-on-metal bearings
-
Femoral stems for cemented and cementless THR
-
Femoral component for TKR
-
Ingrowth or ongrowth coatings on implants (e.g., THR/TKR)
-
Metallurgy
-
Cobalt alloy in cast or wrought forms is typically alloyed with varying amounts of chromium and molybdenum.
-
Tungsten and nickel are used in some
alloys to achieve the desirable properties of strength, fatigue
resistance, and corrosion resistance. -
Mechanical properties of cast alloys can be improved by hot forging that removes pores and reduces grain size.
-
In some alloys (e.g., MP35N), cold
working can change the crystal structure from a face centered cubic
(fcc) lattice to an hcp one, creating a biphasic alloy that has
improved resistance to plastic deformation and increased strength. -
The presence of carbon is carefully
controlled as this can affect the mechanical properties, including
toughness, wear resistance, and corrodibility.-
High carbon (>0.14% w/w) alloy is used as bearing surfaces against itself.
-
Low carbon (<0.14% w/w) alloy is used
as structural members as well as for coating applications in addition
to bearing applications against the ultra-high-molecular-weight form of
poly(ethylene) (UH-MWPE).
-
-
Cast alloy may be porous, and mechanical
properties are improved by hot forging or hot isostatic pressing (HIP)
that removes pores and reduces grain size.-
HIP is a process of heat treatment in
argon gas at high temperature (e.g., 1200°C) and pressure (e.g., 1000
Atmospheres), followed typically by solution annealing.
-
Implant Characteristics (Box 17-2 and Tables 17-3 and 17-4)
Advantages
-
Biocompatibility
-
Provided by a chromium oxide passivation
layer that may be enhanced prior to implantation by cleaning,
polishing, and an oxidizing nitric acid bath (see Table 17-1) -
The solubility in water, however, is
greater than TiO2 and there is susceptibility to crevice corrosion,
with concern about possible loss of implant fixation in the long term
when directly apposed to bone.
-
-
Improved fatigue resistance
-
THR stem fractures with cast stems: a concern in the past
-
Better metallurgical processing of alloys
and improved alloy compositions have essentially eliminated this as a
problem, to the point where wrought cobalt alloys provide some of the
most fatigue-resistant alloys available for implantation.
-
-
Wear resistanceTable 17-3 Different Uses of Some Cobalt Alloys
Material Crystal Structure Uses Notes Cast cobalt-chromium-molybdenum alloy (ASTM F75) fcc Femoral heads, metal-on-metal bearings, femoral component of TKR Comes in high- or low-carbon versions. Can be heat treated (hipped, solution annealed). Low nickel content. Wrought cobalt-chromium-molybdenum alloy (F799, F1537) fcc Cemented femoral stems, metal-on-metal bearings Wrought version of cast F75. Comes in high- and low-carbon versions. Low nickel content. Wrought cobalt-chromium-nickel-molybdenum fcc and hep Femoral stems Extremely strong; fatigue and corrosion resistant. High nickel content has caused limited use in vivo. P.377Table 17-4 Mechanical Properties of Some Implant AlloysAlloy Yield Strength (Mpa) Ultimate Tensile Strength (MPa) Fatigue Strength (Mpa) Elastic Modulus (Mpa) Titanium Alloys Alpha-phase commercially pure titanium Grade 1 (0.18% oxygen) 172 241 — 110 CpTi (ASTM F67 by ATI Allvac) Grade 4 (0.4% oxygen) 480 550 380 110 Alpha-beta-phase titanium-aluminum-vanadium (ASTM F136 by ATI Allvac) 793 862 600 110 Beta-phase titanium-molybdenum-zirconium-iron alloy (ASTM F1813 by ATI Allvac) 965 1000 — 80 Cobalt Alloys Cast cobalt-chromium-molybdenum alloy (ASTM F75) As cast 450-520 655-890 207-310 210 HIPped 841 1277 725-950 253 Wrought cobalt-chromium-nickel alloy (ASTM F562 by Carpenter-MP35N) Annealed 414 931 — 232 55% cold-worked 1413 1827 — 232 53% cold-worked and aged 1999 2068 793 max 232 Wrought cobalt-chromium-molybdenum alloy (ASTM F799/F1537 by Carpenter-BioDur CCM plus) Annealed 882 1351 — — Hot-worked 930 1365 900 max 210 Stainless Steels Nitrogen-strengthened stainless steel (ASTM F1586 by Sandvik-Bioline Hign N) Annealed 430 740 — — Cold-worked 1100 1350 — — AISI 316L stainless steel (ASTM F138 by Sandvik-Bioline 316LVM) Annealed 190 490 270 200 Cold-worked 800 1100 300 200 Nickel-free stainless steel (ASTM F2229 by Carpenter-Biodur 108) Annealed bar and wire 607 931 381 40% cold-worked bar and wire 1551 1731 80% cold-worked (wire only) 1862 2206 Forged material water quenched for hip implants 1036 1253 513 Tantalum Pure Ta (ASTM 560-04) Solid material 345 186 Ta-C 75% porous components 63 3 The numbers shown are frequently greater than the ASTM minimum specifications. -
Partly a function of the strain hardening effect that occurs in vivo
-
Cobalt alloy can also be machined and
polished to give a low surface roughness (<0.01 micron Ra) when
articulating with itself or UHMWPE.
-
-
Low galvanic corrosion potential of a CoCrMo head on a titanium alloy femoral stem
Disadvantages
-
High galvanic potential and corrosion
risk of a CoCrMo head on a stainless steel stem, with the possible
exceptions of the newer nitrogen-strengthened or nickel-free stainless
steels -
Presence of nickel is sometimes a problem:
-
In the common alloy grades (ASTM F75, F1537), it is specified at <1% but is unfortunately difficult to totally remove.
-
Nickel-sensitive patients may respond, and there are batch-to-batch differences, making comparisons difficult.
-
Cytotoxicity and carcinogenicity
theoretical risks: Cobalt and chromium ions have been shown to be
cytotoxic and carcinogenic in cell culture and in some animal models. -
Low levels are released into the body during the life of the implant.
-
Cobalt is more soluble and the implant becomes relatively cobalt-depleted.
-
Serum, tissue, and whole blood and urine
levels of cobalt and chromium are greatly increased with metal-on-metal
(CoCrMo) bearings. -
No long-term ill effects on humans have yet been reported.
-
-
Stress shielding
-
High elastic modulus (250 MPa) is a concern for stress shielding.
-
In THR, there is a link between thigh pain and well-fixed porous coated implants.
-
There is a general trend toward use of lower-modulus titanium alloys for this reason.
-
-
High cost: Cobalt as a raw element is expensive and thus the manufacture of implants from this alloy is very costly.
-
Difficult processing of cobalt alloys
-
Casting needs to be carefully controlled to avoid poor-quality material with voids or large grain size.
-
Machining is performed at relatively slow speed and is time-consuming.
-
Stainless Steel
Although carbon steel was used as an orthopaedic implant
material in the 19th century, stainless steel was first used in the
1920s and has undergone several evolutions since.
material in the 19th century, stainless steel was first used in the
1920s and has undergone several evolutions since.
Orthopaedic Uses of Stainless Steel
-
Fracture and spinal fixation devices
-
Cables, screws, wires, plates, intramedullary nails
-
Joint replacement components
-
Femoral heads for stainless steel THR
-
Cemented femoral stems
-
Metallurgy
-
Stainless steel is a term used to
classify a heterogeneous group of alloys that use the iron-chromium
binary system in addition to other alloying and interstitial elements. -
Several distinct subgroups based on their crystal structure:
-
Only austenitic stainless steels are
widely used as implant materials due to their corrosion resistance,
non-magnetic nature, and relative ease of manufacture.-
Austenitic stainless steel is a
single-phase, fcc alloy typically forged by hot or warm methods and
then cold worked to attain strength.
-
-
Other grades of stainless steel are used for surgical tools.
-
-
Chromium is necessary for corrosion
resistance and forms a complex chromium oxide passivation layer similar
to cobalt—chromium alloys.-
Chromium, however, favors the formation of a weaker ferritic bcc structure.
-
-
Nickel is added to stabilize the
austenitic phase in addition to improving corrosion resistance by
nickel oxide formation complexing with chromium oxide. -
Other alloying elements used for corrosion resistance and ease of processing include molybdenum, silicon, and manganese.
-
Carbon content is kept low to prevent sensitization from chromium carbides at grain boundaries that are prone to corrosion (see Table 17-1).
-
High-carbon stainless steel has resulted in premature implant failure.
-
Implant Characteristics (Box 17-3)
Advantages
-
Low cost: Ubiquitous nature of iron
ensures that the manufacture of stainless steel is cheaper than that of
titanium and cobalt alloys. -
Biocompatibility: considered good, but inferior to that of cobalt or titanium alloys
Disadvantages
-
Susceptibility of standard grades to crevice and stress corrosion has been noted in vivo, resulting in component failure.
-
Permanent implant components are thus not
made from the standard-grade AISI 316L, which is used primarily for
temporary fracture fixation devices. -
The austenitic nature of stainless steel unfortunately makes it a poor wear material against itself, but acceptable to UHMWPE.
-
Martensitic stainless steel (tetragonal
crystal structure) is an excellent wear material, but is magnetic and
has low corrosion resistance and is not therefore used except for some
surgical tools.
Advances and Improvements (Table 17-5)
-
Until recently, all stainless steel contained nickel.
-
As a result of heightened concern about
nickel sensitivity, nickel-free stainless steels have become available
for implant use but are not yet widely used. -
Nickel has been substituted by manganese and nitrogen as austenite formers.
Box 17-3 General Advantages and Disadvantages of Stainless Steel
Advantages
Cheap raw elements
Good biocompatibility
Disadvantages
Some grades not suitable for long-term implantation due to fatigue failure
Galvanic corrosion to CoCr and titanium
Nickel sensitivity
Poor wear properties
|
Table 17-5 Different Uses of Some Stainless Steels
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Tantalum
Tantalum is a dense metal that is a relative newcomer to
large-scale implantation in orthopaedic surgery. Until recently, its
use had been generally limited to research as a radiodense marker in
bone in the form of balls for radioster-eometric analysis (RSA) of
implant migration. More recently, 80% porous, three-dimensional
networks of tantalum over a backbone of vitreous carbon have been
extensively used. The material properties have allowed its use as a
fixation material to bone by both ingrowth and cementation with PMMA.
large-scale implantation in orthopaedic surgery. Until recently, its
use had been generally limited to research as a radiodense marker in
bone in the form of balls for radioster-eometric analysis (RSA) of
implant migration. More recently, 80% porous, three-dimensional
networks of tantalum over a backbone of vitreous carbon have been
extensively used. The material properties have allowed its use as a
fixation material to bone by both ingrowth and cementation with PMMA.
Orthopaedic Uses of Tantalum
-
Joint replacement components
-
Tibial and patellar components for TKR, some compression molded into UHMWPE
-
Acetabular shells and augments for THR
Metallurgy
-
Formed by cathode vapor deposition (CVD) of crystalline tantalum from gaseous tantalum pentachloride
-
Resultant structure: 99% tantalum and 1% carbon with pore sizes of about 550 microns and an elastic modulus of 3MPa
Implant Characteristics
Advantages
-
Low elastic modulus and prevention of stress shielding
-
High porosity for bone ingrowth
-
High coefficient of friction for initial stability
-
Inert nature
-
Relatively easy to trim blocks to fit
Disadvantages
-
Significant cost
-
Poor tensile strength
-
Limitation to areas subject mainly to compression
-
Current lack of clinical evidence for long-term benefit
Polymers
Polymers represent the largest class of biomaterials and
are used for a variety of orthopaedic applications, including bearing
surfaces and fixation materials for total joint prostheses. These
materials, made of long-chain molecules with distinct repeat units
known as “mers” or monomers, are derived from both natural sources and
synthetic organic chemistry. The properties of polymers are a function
of both the chemical and physical structure of the material. The
physical structure of polymeric materials can be characterized in terms
of molecular weight, which is directly proportional to the length of
the polymer chains or the number of repeat units; the arrangement of
monomer units into various chain structures; and the degree of
crystallinity or order of the molecules within the material. These
factors have a tremendous impact on the thermal and mechanical
properties of polymeric materials, which in turn dictate their utility
for biomaterials applications. This section will describe these basic
principles of polymer science and discuss the primary applications of
polymeric biomaterials in orthopaedic medicine.
are used for a variety of orthopaedic applications, including bearing
surfaces and fixation materials for total joint prostheses. These
materials, made of long-chain molecules with distinct repeat units
known as “mers” or monomers, are derived from both natural sources and
synthetic organic chemistry. The properties of polymers are a function
of both the chemical and physical structure of the material. The
physical structure of polymeric materials can be characterized in terms
of molecular weight, which is directly proportional to the length of
the polymer chains or the number of repeat units; the arrangement of
monomer units into various chain structures; and the degree of
crystallinity or order of the molecules within the material. These
factors have a tremendous impact on the thermal and mechanical
properties of polymeric materials, which in turn dictate their utility
for biomaterials applications. This section will describe these basic
principles of polymer science and discuss the primary applications of
polymeric biomaterials in orthopaedic medicine.
Polymer Synthesis
Polymer synthesis can be accomplished by several
different types of reactions, as outlined for polymers of orthopaedic
interest in Table 17-6. Addition polymerization, often called free radical polymerization, is characterized by a three-step process:
different types of reactions, as outlined for polymers of orthopaedic
interest in Table 17-6. Addition polymerization, often called free radical polymerization, is characterized by a three-step process:
P.380
|
Table 17-6 Types of Polymerization Reactions and Their Characteristics
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
-
Initiation
-
Free radicals are created by heat, light, or chemical reaction.
-
Radicals react with unsaturated double bond in monomer to start polymer chain.
-
-
Propagation
-
Phase of rapid chain growth
-
Monomer units are added to the growing polymer chain
-
-
Termination
-
Combination: two growing chains react to form one polymer molecule
-
Disproportionation: two polymer molecules result from transfer of a hydrogen atom
-
Physical Properties
Molecular Weight
-
A unique feature of polymers is that they
consist of many individual chains or molecules, many having different
lengths or number of repeat units. -
Because of this distribution of molecular weights, polymers are typically characterized by their average molecular weight (Mw).
-
Linear polymers used as biomaterials generally have Mw ranging from 50,000 to 300,000 g/mol.
-
A notable exception to this is UHMWPE, which is used as a bearing surface in total joint prostheses.
-
The Mw of UHMWPE can exceed 1 million.
Chain Structure
-
Polymers can be broadly classified as:
-
Homopolymers: only one type of monomer repeat unit
-
Copolymers: two or more types of repeat units
-
-
In addition to the structure of
individual polymer chains, the molecular architecture of these chains
in a polymeric material is important.-
Polymer chains can be linear, branched, or cross-linked (Fig. 17-1).
-
UHMWPE can be cross-linked by gamma
irradiation, which significantly improves its wear resistance when used
in articulating bearing surfaces of total joint replacements.
-
Tacticity
-
Polymer molecules are composed of a
backbone, usually made of carbon atoms, with various repeating side
groups or pendant chains. The conformational arrangement of these side
groups about the backbone is known as tacticity. -
Isotactic polymers have all of their side
groups on one side of the chain backbone, and syndiotactic polymers
have side groups that alternate. -
These conformations may crystallize, but
atactic forms, which have a random arrangement of side groups, do not
and hence remain amorphous.
Crystallinity
-
Polymers can either be amorphous, having no long-range order, or semicrystalline.
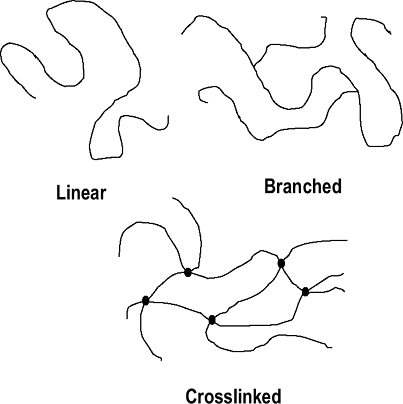 Figure 17-1 Polymer chain arrangements.
Figure 17-1 Polymer chain arrangements. -
Even in highly crystalline polymers,
lattice defects create small amorphous regions, making complete
crystallinity virtually impossible. -
The ability of a polymer to form highly
ordered crystalline domains is a function of its chemical structure
(i.e., the presence and size of side groups) and chain regularity. -
In general, crystalline domains or crystallites enhance mechanical properties, thermal behavior, and fatigue strength.
P.381
Mechanical Properties
-
The mechanical properties of polymers are
often crucial for various orthopaedic applications and can be
characterized in a number of ways:-
Maximum stress before failure
-
Elongation to failure, modulus (E)
-
Fatigue failure stress (under cyclic loading)
-
Susceptibility to wear (abrasion, adhesion, fatigue types)
-
Creep (progressive deformation under constant load)
-
-
These properties are described in general in Chapter 16, Biomechanics.
Thermal Properties
-
Polymeric biomaterials can be described
as thermoplastic, meaning that temperature can have a significant
effect on their physical properties. -
All polymers exhibit a glass transition temperature (Tg), and crystalline polymers also exhibit a melting temperature (Tm).
Glass Transition Temperature (Tg)
-
Temperature at which there is enough thermal energy for long-range segmental chain motions to occur
-
Below Tg, amorphous polymers are hard and glassy.
-
Above Tg, amorphous polymers become soft and rubbery.
-
At Tg, the modulus of an amorphous polymer drops by roughly three orders of magnitude.
Melting Temperature (Tm)
-
Tm is the temperature at which crystallites in the material melt and lose their ordered structure.
-
Above Tm, semicrystalline polymers return to the amorphous state.
-
Tm > Tg
Orthopaedic Applications of Polymers
-
Polymeric bearing surfaces of the past (abandoned due to poor performance)
-
PMMA
-
Poly(tetrafluroethylene) (PTFE)
-
-
Current polymeric bearing surfaces
-
UHMWPE
-
Introduced as bearing surface in the early 1960s
-
Highly crystalline polymer
-
Higher molecular weight, impact strength,
toughness and improved wear characteristics versus the high-density and
low-density forms of poly(ethylene). -
Currently the material of choice for articulating surfaces
-
Acetabular cup of hip replacements
-
Tibial insert of knee replacements
-
-
Issues Related to UHMWPE
-
Wear debris
-
Production of billions of sub-micron-sized wear particles per year
-
Causes chronic inflammation, osteolysis, implant loosening
-
-
Methods to improve wear resistance
-
Treatment with gamma irradiation
-
Causes chain scission (breaking of bonds in polymer backbone)
-
Allows for cross-linking and increased toughness
-
-
Gamma irradiation in oxygen-free environment
-
Reduces post-irradiation oxidation
-
Prevents subsurface oxidation damage to polymer
-
-
-
Creep
-
Deformation when subjected to a constant applied load over time
-
Contributes to loss of congruence, increased stress concentration and wear
-
Bone Cement
PMMA is another polymer that has enjoyed widespread use
in orthopaedic medicine since the late 1960s as a fixation material for
total joint prostheses. It serves as a grout, or space-filling
material, at the implant site and helps to transfer mechanical loads
from the metallic prosthesis to the surrounding bone. Acrylic bone
cements are unique with respect to many other polymeric biomaterials
because they are polymerized in situ, or inside the body.
in orthopaedic medicine since the late 1960s as a fixation material for
total joint prostheses. It serves as a grout, or space-filling
material, at the implant site and helps to transfer mechanical loads
from the metallic prosthesis to the surrounding bone. Acrylic bone
cements are unique with respect to many other polymeric biomaterials
because they are polymerized in situ, or inside the body.
Commercial bone cements are supplied as two-component,
powder—liquid systems consisting of PMMA powder with the initiator
benzoyl peroxide, and liquid methyl meth-acrylate (MMA) monomer with
the activator N,N-dimethyl-p-toluidine. The powder and liquid are mixed
together in the operating room to initiate polymerization of the MMA
monomer via a free radical reaction. As the polymerization reaction
proceeds, the viscosity of the cement increases, and the material is
delivered to the site of implantation in a doughy or viscous state. The
prosthesis is then inserted and properly positioned, the cement is
pressurized, and finally the cement cures or sets completely.
powder—liquid systems consisting of PMMA powder with the initiator
benzoyl peroxide, and liquid methyl meth-acrylate (MMA) monomer with
the activator N,N-dimethyl-p-toluidine. The powder and liquid are mixed
together in the operating room to initiate polymerization of the MMA
monomer via a free radical reaction. As the polymerization reaction
proceeds, the viscosity of the cement increases, and the material is
delivered to the site of implantation in a doughy or viscous state. The
prosthesis is then inserted and properly positioned, the cement is
pressurized, and finally the cement cures or sets completely.
Although alternate materials have been investigated,
PMMA-based cements remain the primary choice for fixation of total
joint prostheses.
PMMA-based cements remain the primary choice for fixation of total
joint prostheses.
P.382
Drawbacks
-
Exothermic polymerization reaction
-
Releases 130 cal/g of MMA monomer
-
Temperature rise during in situ polymerization has potential to cause thermal necrosis of surrounding bone at implant site.
-
Thermal damage usually limited due to:
-
Relatively thin cement mantle (2 to 3 mm)
-
Heat transfer through the metallic implant stem
-
* Porosity within cement mantle-
Pores act as stress concentrators.
-
Pores provide initiation sites for cracks.
-
Can lead to fracture of the cement
-
May cause loosening of the implant and need for revision
-
-
Porosity can be reduced by improved mixing and delivery techniques (e.g., vacuum mixing)
-
Resorbable Polymers
Resorbable or biodegradable polymers are desirable for
clinical applications where a device is required for only a short
period. Examples include applications in wound closure, fracture
fixation, and drug delivery. The polymers poly(lactic acid) and
poly(glycolic acid), along with their copolymers, have been used
clinically as suture materials since the 1970s and have been
extensively studied for a variety of other applications due to their
successful clinical history and approval by the U.S. Food and Drug
Administration. However, orthopaedic applications of biodegradable
polymers have remained limited due to lingering concerns over their
mechanical performance and tissue reactions during degradation.
clinical applications where a device is required for only a short
period. Examples include applications in wound closure, fracture
fixation, and drug delivery. The polymers poly(lactic acid) and
poly(glycolic acid), along with their copolymers, have been used
clinically as suture materials since the 1970s and have been
extensively studied for a variety of other applications due to their
successful clinical history and approval by the U.S. Food and Drug
Administration. However, orthopaedic applications of biodegradable
polymers have remained limited due to lingering concerns over their
mechanical performance and tissue reactions during degradation.
Clinical Applications
-
Primary fixation (sutures, pins, screws, anchors, intra-medullary rods)
-
Drug delivery
Primary Biodegradable Polymers
-
Poly(lactic acid) (PLA)
-
Poly(glycolic acid) (PGA)
-
Poly(lactic-co-glycolic acid) (PLGA) copolymers
-
Poly(caprolactone) (PCL)
-
Poly(dioxanone) (PDS)
Concerns for Biodegradable Applications
-
Degradation rate (mass loss vs. time)
-
Change in strength over degradation time
-
Biocompatibility of degradation products (associated in some cases with sterile discharge or inflammatory changes)
Ceramics
Although the use of ceramic materials in modern
orthopaedic surgery began well after the introduction of metals and
alloys, recent advances in ceramic engineering have allowed the
development of many new ceramic materials and composites with improved
mechanical properties and a variety of bone integration capabilities.
orthopaedic surgery began well after the introduction of metals and
alloys, recent advances in ceramic engineering have allowed the
development of many new ceramic materials and composites with improved
mechanical properties and a variety of bone integration capabilities.
Ceramics are particularly attractive as implant
materials because of their general chemical and temporal stability and
adjustable surface and bulk properties, and because bone mineral itself
(two thirds of the mass of bone tissue) is in fact a ceramic.
materials because of their general chemical and temporal stability and
adjustable surface and bulk properties, and because bone mineral itself
(two thirds of the mass of bone tissue) is in fact a ceramic.
The purpose of this section is to outline the main
features, composition, and applications of ceramic materials in
clinical use or under research and development.
features, composition, and applications of ceramic materials in
clinical use or under research and development.
General Characteristics of Ceramic Materials
-
Composition
-
Polycrystalline, metal oxides, silicates, phosphates, sulfides, carbons, etc.
-
Most are ionically bonded; carbons are covalently bonded.
-
-
Physical
-
Refractory (stable to high temperature)
-
Resistant to oxidation
-
Low electrical and thermal conductivity
-
-
Mechanical
-
Brittle, hard, low tensile strength, high
compressive strength, non-ductile, undergo little or no distortion
(creep) with continuous loading -
Susceptible to micro-crack and notch formation leading to brittle failure on repetitive loading
-
Compressive strength inversely proportional to porosity
-
-
Biological
-
Most are relatively inert, noninflammatory; some are resorbable or bioactive.
-
Bioceramics Used in Orthopaedics (Tables 17-7 and 17-8)
-
Most commonly used:
-
Dense alumina for femoral heads (Fig. 17-2)
-
Porous calcium phosphates for grafts, defect fillers, implant coatings, bone cements (grouts), and scaffolds (Fig. 17-3)
-
-
Advantages
-
Excellent bone ingrowth (porous) and ongrowth (coatings) (“osteoconduction”)
-
Excellent attachment to bone surfaces without fibrous layer (“osseointegration”)
-
Can be made bioactive or incorporate growth factors, antibiotics, etc.
-
Generally nontoxic, nonallergenic, relatively noninflammatory, noncarcinogenic
-
Can be formulated to be partially or totally resorbed over time
-
-
Disadvantages
-
Brittle, unless reinforced by substrate or additive
-
Porous types are mechanically weak
-
Manufacture is generally demanding
-
Functional Types and Definitions
Resorbable (Absorbable) Ceramics
-
Ceramics that dissolve or are electrolytically degraded extracellularly (with or without phagocytosis)P.383Table 17-7 Properties of Bioceramic Materials Applicable to Orthopaedics
Group Subtype Chemical Base Functional Type E (Gpaa) σmax (Mpaa) Comments Metal oxides Alumina Al2O3 Dense, bioinert 380 580 Most common dense ceramic; low wear Zirconia (part, stabilized) ZrO2 (+ Y2O3) Dense, bioinert 200 1000 Low wear and friction Calcium phosphates β-tricalcium phosphate (β-TCP) β-Ca3(PO4)2 Porous, resorbable 4-120 20-500 *Porosity 95% -5%: β-whitlockite Hydroxyapatite (HA) (synthetic, polycrystalline) Ca10(PO4)6(OH)2 Variable porosity, bioactive, osteoconductive 4-120* 20-500* *Porosity 95% -5%; many uses; properties vary greatly with porosity and preparation method Metal oxide calcium phosphates ZnO2, Fe2O3 or Al2O3 + CaO + P2O5 Porous, resorbable Experimental, bone filler and/or drug delivery systems Corals Biocoral Porities Goniopora CaCO3 Porous, resorbable 190- to 230-µm pores 130- to 600-µm pores ~8 ~30 50% porous, anisotropic; low strength Converted coral (replamineform, coralline HA) HA Porous, controlled resorbable Low strength; slower resorption than CaCO3 Carbons Pyrolytic carbon C (graphite-like structure) Dense, bioinert 28 ~520 Used as composite or coating Glass ceramics Polycrystalline silicon oxide-based glass (Bioglass) SiO2 – CaO -Na2O3-P2O5 Dense, bioactive, nonresorbable 200 Proposed as fillers, coatings; very small grain size Polycrystalline silicon oxide-based glass (Ceravital) SiO2 – CaO -Na2O3-P2O5 -MgO – K2O Dense, bioactive, nonresorbable 100-200 Proposed as fillers, coatings; very small grain size Other Ca systems Plaster of Paris (CaSO4)2 * H2O Dense, rapidly resorbable 30 Little foreign body reaction; resorbs rapidly; low strength Allograft bone (adult) Cortical Natural HA/collagen composite Semiporous, osteoconductive, slowly resorbable 18 200 Anisotropic; tensile strength <150 Mpa Trabecular Natural HA/collagen composite Macroporous, osteoconductive 0.06-0.09 6-10 Varies greatly with location, age aTypical values.
E, modulus of elasticity (in compression unless otherwise noted); σmax, the maximum or failure stress (“strength”) in compression.P.384Table 17-8 Orthopaedic Applications of BioceramicsUses Material Form Examples Comments Articulations in joint replacement prostheses Al2O3 (Alumina) Dense, polished; femoral head and acetabular socket liners Trident (Stryker), Ceramic-on-ceramic (Wright Medical) Recently FDA cleared for total hip systems ZrO2 (+ Y2O3) (Zirconia) Dense, polished; femoral head and acetabular socket liners Oxinium (Smith and Nephew) Recently FDA cleared for total hip systems Bone void fillers, grafts, and composites; osteoconductive Coralline (HA)
Coralline (HA + CaCO3)Macroporous blocks or granules Pro Osteon, Pro-Osteon-R (Interpore) Very slowly resorbed; FDA approved HA derived from trabecular bovine bone (CaSO4)2 * H2O Macroporous blocks or granules Plaster of Paris, resorbable Endobon (Merck), Bio-Oss (Osteohealth) Osteoset (Wright Medical) Approved for craniofacial use; Bio-Oss contains bovine collagen For low-stress locations only; FDA cleared HA + β-TCP + collagen mix Granules Collagraft, NueColl (Zimmer) Approved for mix with autogenous marrow and defects <30 cm3 β-TCP Macroporous, resorbable blocks or granules Vitoss (Orthovita) Completely resorbable; for low-loading sites; FDA cleared CO3-β-TCP + bovine collagen Macroporous, resorbable granules or cement Healos (Orquest) Investigational Bone allograft Solid or granulized Not considered a medical device by FDA Cements, grouts, fracture fixation supplementation Various calcium phosphates Injectable slurry, self-curing, osteoconductive SRS (Norian); a-BSM (ETEX Corp); BoneSource (Stryker) Use for hardware enhancement and defects Polycrystalline silicon oxide-based glass ceramic Injectable slurry, self-curing, osteoconductive Bioglass (USBiomaterials), Cortoss (Orthovita) Cortoss not yet approved in United States; several analogs approved only for craniofacial use Implant coatings Hydroxyapatite: Plasma spray and other coating processes Coating on titanium or Co-Cr prostheses In current use by several manufacturers of implants Marketed Polycrystalline silicon oxide-based glass ceramic Coated on titanium Investigational Scaffolds for cells or drug delivery Various calcium phosphate/HA group materials with bioactive or osteoinductive agents Micro- and macroporous solids and granules HA or β-TCP + BMP
HA coatings + BMP
HA + AgSO4Investigational FDA, U.S. Food and Drug Administration P.385![]() Figure 17-2
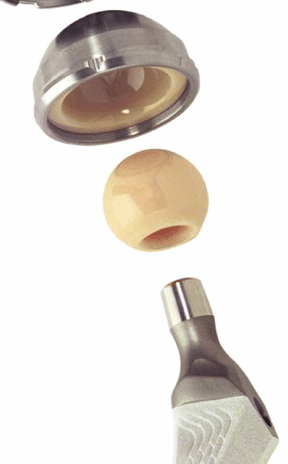
Figure 17-2
A portion of a contemporary ceramic-on-ceramic total hip replacement
prosthesis. The ball and socket are made of dense polycrystalline
alumina. The socket is encased in a metallic cup, which is placed into
the acetabular portion of the implant. (Courtesy of Steven Brown,
Stryker Orthopedics, Inc.) Figure 17-3
Figure 17-3
A portion of the femoral stem of a metallic hip implant with a
macroporous hydroxyapatite coating designed to encourage fixation of
the proximal portion of the stem. (Courtesy of Steven Brown, Stryker
Orthopedics, Inc.)-
Smaller insoluble particles liberated by disintegration (e.g., (3-tricalcium phosphate)
-
-
Resorption rate must allow ingrowth and replacement by bone.
-
A very rapid resorption rate does not allow time for bone ingrowth.
-
Time frame: days to years, depending on composition, fabrication, and location
-
Rate of resorption is proportional to porosity.
-
Bioactive Ceramics
-
Ceramics created to stimulate osteogenesis, active osteoconduction, bone remodeling, bacteriostasis, etc.
-
Osteogenesis and bone remodeling: achieved by addition of bone morphogenic proteins, growth factors, or bone marrow
-
Active osteoconduction: achieved when there is immediate physical contact and chemical bonding with bone
-
Bioactive glass ceramics foster this by surface dissolution and the formation of a PO4- and Ca2+– rich layer upon exposure to the physiological environment.
-
-
Composites make use of the macroporosity of the ceramics as scaffolds for drug delivery.
-
Bacteriostasis: achieved by addition of antibiotics
-
Carriers (e.g., synthetic polymers, collagen): allow for retention and controlled release of chemothera-peutic agents
-
Bioinert Ceramics
-
Maintain their chemical and physical properties with time
-
Demonstrate fewest inflammatory or other tissue reactions
-
Passive osteoconduction: exhibited if the material fosters immediate physical bone contact without chemical bonding
-
Scant or nonexistent fibrous intermediate layer (at the light microscope level)
-
Interdigitation with bone in pores may provide strong physical bonding.
-
Bioinert hydroxyapatite ceramics (HA)
undergo a small amount of surface dissolution and can lose about 15 µm
during the first few months after implantation. HA coatings may
disappear entirely.
Porous Ceramics
-
Interconnected pores of 50 µm in diameter or more allow bone and capillary ingrowth.
-
Pores of 100 to 400 µm give best bone ingrowth.
-
Larger pore structures are called macroporous (> 1 mm).
-
High specific surface area increases chemical interaction and resorption rate.
-
-
Coralline ceramics are made from natural coral structures with large interconnected pores.
-
Mechanical strength is poor and inversely proportional to pore size and the “area fraction” of pores.
Dense Ceramics
-
Intergranular pores only of the order of a few microns or less
-
Volume can be almost 100% dense (3 to 4 gm/cm2).
-
Usually nonresorbable
-
Can be highly polished for making bearing surfaces
-
Low friction and very low particulate release
-
-
Bioactive and inert ceramics have generally been classified as medical devices by the U.S. Food and Drug Administration.
Suggested Reading
Bauer TW, Smith ST. Bioactive materials in orthopaedic surgery: Overview and regulatory considerations. Clin Orthop Rel Res 2002;395:11–22.
Buckwalter JA, Einhorn TA, Simon SR, eds. Orthopaedic Basic Science, 2nd ed. American Academy of Orthopedic Surgeons, 2000.
Ducheyne P, Lemons JE, eds. Bioceramics: Material characteristics versus in vivo behavior. Ann NY Acad Sci 1988;523:1–297.
Park JB, Bronzino JD. Biomaterials; Principles and Applications. Boca Raton, FL: CRC Press, 2003.
Rattner BD, Hoffman AS, Schoen FJ, et al., eds. Biomaterials Science, 2nd ed. San Diego Academic Press, 1996.
Sperling LH. Introduction to Physical Polymer Science, 4th ed. New York: John Wiley & Sons, 2001.