SURGERY FOR CEREBRAL PALSY
caused by a nonprogressive lesion of the brain acquired at or around
the time of birth (3). Although musculoskeletal
deformities and imbalances are usual, and certain clinical patterns are
relatively common, the condition is extremely heterogeneous. The brain
lesions tend not to be highly localized and therefore usually produce
more than an isolated deficit. At least five basic movement
disorders—spasticity, athetosis, ataxia, rigidity, and tremor—are
described; various possibilities for distribution include monoplegia,
hemiplegia, diplegia, and total body involvement.
more than one movement disorder. It is important to identify the
primary movement disorder because, in general, operations are designed
for patients with spasticity. Because the brain lesion is often
diffuse, deficits in proprioception, stereognosis, and perceptual
integration can result. Keeping cognizant of these other deficits will
remind the orthopaedic surgeon to set reasonably modest goals for
treatment.
repetitively in patients with CP, depending on the pattern of
neurologic involvement. For example, the combination of windblown hips
and scoliosis is typically seen in the total-body-involved spastic
quadriplegia patient. Valgus feet and mild crouch gait are typically
seen in spastic diplegia. By applying basic principles of surgical
correction, the orthopaedist may improve positioning, function, and
appearance of the extremities in selected patients.
extremity surgery. The goals vary depending on the overall functional
ability of the patient (i.e., nonambulator, “therapy” or nonfunctional
ambulator, household ambulator, community ambulator with assistive
devices, or independent community ambulator). Goals for lower extremity
surgery in nonambulators are often limited to improving comfort and
easing nursing care, or decreasing contractures sufficiently to allow
deformed feet to be fitted with shoes. Assisted transfers of a
nonambulator in and out of a wheelchair can be facilitated if the
patient can be stood on plantigrade feet with fairly straight hips and
knees. For household or community walkers, the goal should be to
improve the efficiency of gait (i.e., decrease the energy cost) by
minimizing contractures and balancing spasticity. However, the basic
ability to walk is dictated by the patient’s brain and is not affected
by the orthopaedic surgeon. Because the prognosis for independent
walking can be established by 2 or 3 years of age (depending on the
equilibrium and primitive reflexes), the surgeon should have realistic
goals in mind by the time surgery is undertaken (3).
truly benefit from upper extremity surgery. The goals of upper
extremity reconstructive surgery usually relate to improvement in
function, occasionally for ease of hygiene or personal care (such as
pulling a long sleeve shirt over a wrist and hand that is locked in
flexion), and rarely for improvement in cosmesis. Coexistent deficits
in stereognosis and motor planning limit the goals that should be
expected when trying to rebalance motor control about the wrist and
hand. Additionally, any cognitive problems further minimize the ability
of the CP patient to cooperate in a postoperative therapy program. In
general, this will mean that most upper extremity surgery for functional improvement will be performed on children who are spastic hemiplegics with only mild or no cognitive deficits.
one anatomic deformity at a time. However, it is vital always to think
of the joints and muscles above and below the target deformity. Make
repeated examinations before deciding on surgical intervention.
Consideration must be given not only to the effects of other
deformities on the index deformity, but also to the effects that any
proposed surgery will have on the neighboring joints. A classic example
of this is the increased lordosis that occurs after an apparently
appropriate hamstring lengthening because of a lack of attention to
preexisting increased hip flexor spasticity. Similarly, a mild tendency
toward crouch gait will often worsen following a heel cord lengthening
done in isolation.
in planning surgical procedures in CP, especially when several
procedures must be performed simultaneously. By using combinations of
dynamic gait electromyographic (EMG) analysis and video monitoring of
joint range during gait, I have found that in more than half the cases
the preoperative gait analysis affects my decision as to which
operative procedures are necessary.
probably be delayed until the pattern of gait is fairly well
established (usually between 4 and 8 years of age) with a goal of
finishing surgical intervention (if possible) by the first or second
grade of school. Similarly, surgery for upper extremity functional
improvement usually is most appropriate at about the same age. It often
takes a series of examinations on a young child to accurately assess
the motor, sensory, and cognitive resources of a not-always-cooperative
child.
for treating the most common lower-extremity deformities in spastic CP.
Keep in mind that even with well-planned and carefully executed
surgery, deformities occasionally recur or progress in children with
CP, and salvage or reconstructive procedures may later become necessary.
deformities that may actually exist in isolation in spastic CP,
especially in hemiplegics. The indications for surgical correction are
simple: fixed equinus such that the ankle cannot be dorsiflexed to
neutral, with the hindfoot locked in varus in a walking or potentially
ambulatory patient. In diplegics, equinus helps in transferring the
weight-bearing line anteriorly, which assists in extending the knee.
Thus, when crouching is present in diplegia, the surgeon should not
lengthen the triceps surae in isolation; usually, hamstring or hip
flexor surgery must be combined with it. Not all tiptoeing CP patients
have fixed equinus. For a dynamic deformity, it is best to try an
extended period of bracing with a rigid plastic ankle–foot orthosis or
a series of short-leg walking casts for 2–3 weeks at a time.
equinus is an injection of botulinum-A toxin (1–2 µm/kg body weight per
calf), which will fairly reliably temporarily relieve spasticity and
tone for a period of up to 6 months (16).
walked on his toes), heel cord lengthening through a standard
posteromedial longitudinal incision with a Z-type tendon lengthening is
recommended, because there is likely to be a fixed capsular contracture
of the ankle requiring capsulotomy as well. The amount of lengthening
should be approximately enough that with the foot in neutral, half the
available excursion of the tendo-Achilles is set. An additional check
for the amount of lengthening is the so-called geometric method, in
which the amount of lengthening is half the perpendicular distance that
the first metatarsal head protrudes inferiorly to the heel during
maximal passive dorsiflexion (10).
Once the amount of lengthening is determined, perform the suture repair
with the foot in equinus so as to minimize tension during the repair.
After the repair, check the foot in the neutral position to make sure
that there is still some residual tension on the muscle–tendon unit.
-
In most patients, a Hoke procedure is preferred for heel cord lengthening (Fig. 177.1).
This can be accomplished in a number of ways. The method involves three
opposing cuts, each one halfway through the tendon. Make two medial
cuts proximally and distally, with one lateral cut halfway between the
two, or vice versa. Then dorsiflex the foot just to neutral, thus
causing a sliding lengthening. No sutures are necessary.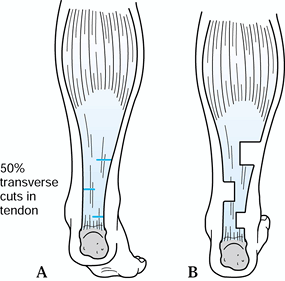 Figure 177.1. Hoke technique for tendo-Achilles lengthening.
Figure 177.1. Hoke technique for tendo-Achilles lengthening. -
The best visualization of the procedure
is accomplished through a medial longitudinal incision 4–6 cm in
length. It is rare to need a posterior capsulotomy of the ankle in CP.
By placing the incision slightly further anteriorly, the posterior
tibial tendon or toe flexors may also be approached, if desired.
However, occasionally the longitudinal scar may be prominent.
Alternatively, use two small transverse incisions, with two of the
tendon cuts through one incision and one tendon cut through a second
incision. With a subcuticular closure, the scar is essentially
invisible. -
Finally, the entire procedure can be
performed percutaneously. This is now my preferred technique if a heel
cord lengthening is the only procedure needing to be done. Make the
same three cuts in the tendon using a #15-C scalpel blade through three
tiny percutaneous incisions. The proximal cut should be at about the
level of the musculotendinous junction. It is essential to dorsiflex
the foot to only 10° or 15° above neutral, with the knee slightly
flexed.
dramatic and consistently positive Silfverskiöd test (in which the
amount of dorsiflexion is much improved with the knee flexed as
compared to the degree in full knee extension), I perform a simple
gastrocnemius recession (24).
-
Make a longitudinal incision in the lower
middle calf slightly medially over the palpable lower border of the
gastrocnemius muscle belly. Separate the gastrocnemius aponeurosis from
the underlying soleus; this plane is easier to find proximally. -
Divide transversely only the
gastrocnemius aponeurosis and dorsiflex the foot to 5° to 10° above
neutral. I will usually tack down the aponeurosis with a few absorbable
stitches. Occasionally, it is also necessary to divide a few fibers of
the underlying soleus aponeurosis to obtain adequate dorsiflexion. No
muscle fibers, however, are divided. This has the theoretical advantage
that overcorrection is very unlikely, although recurrence of equinus
may be slightly more likely.
equinus correction is chosen. Apply an above-knee cast with the knee in
5° or 10° of flexion. At 2–3 weeks, cut the cast down to a below-knee
walking cast. Allow ambulation immediately after surgery if no other
contraindications are present. If no other simultaneous surgery was
performed requiring immobilization above the knee (e.g., hamstring or
iliopsoas release), a below-knee walking cast can be used following any
type of heel cord lengthening. The child will tend to flex the knees
with only a below-knee cast, but within 24 hours she can be coaxed into
extending her knees to near neutral. Using a below-knee cast
facilitates the rehabilitation. Remove all casts at 6–8 weeks. A
plastic, right angle, or articulated ankle–foot orthosis (AFO) is
frequently used part-time for at least 3–6 months. Patients who have no
selective control of dorsiflexion will often require the orthosis on a
more or less permanent basis. Use nighttime splinting in neutral in
those patients who tend to drift back into equinus.
technique a second time. Forewarn parents that recurrence of some
equinus does occur in perhaps 10% of children who undergo
tendo-Achilles lengthening (TAL); however, many children who do not
make heel contact at foot strike do so more because of flexed knees
than because of fixed equinus. Overlengthening is far worse than a
recurrence of the original equinus. There is no universally successful
management for postoperative calcaneus deformity. The first rule is to
avoid overlengthening. Some tension should always remain on the tendon
unit after lengthening. If calcaneus deformity does occur, tendon
reconstructions have not always been satisfactory. Reshortening of the
tendo-Achilles may be tried, or tenodesis of the Achilles to the
posterior tibia or fibula, but these are unlikely to restore true
muscle function.
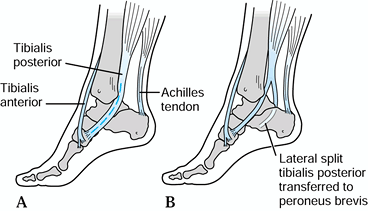
are active in stance. The anterior tibial tendon can be transferred
posteriorly to the heel, and theoretically the peroneus brevis and half
the posterior tibial tendon can also be transferred to the os calcis.
However, restoration of fully satisfactory plantar flexor strength is
unlikely. Should postoperative calcaneus deformity occur, a rigid AFO
with a wide proximal anterior tibial restraint (“floor reaction” AFO)
will have to be used in addition to attempts at reinforcing plantar
flexor strength.
appears most often in hemiplegics, compared with the more typical
valgus deformity seen in diplegics. Dynamic gait EMG analysis is most
helpful in determining the phasic nature of the tibialis anterior and
posterior muscles and the peroneals. Generally, patients under 4 years
of age have not fully established a gait pattern and can be managed
with orthotics. The indications for surgery then depend on the age of
the patient, whether the varus is mild or severe, and which muscles are
most “at fault” on the EMG.
is the simplest approach, but it is rarely appropriate to perform it as
an isolated procedure. This is often combined with a TAL. For more
significant, but still flexible, varus deformities, a split anterior
tibial tendon (SPLATT) procedure (13) can be
combined with the posterior tibial myotendinous lengthening, or the
posterior tibial tendon can be split and transferred laterally to the
peroneus brevis (15,23).
preoperative gait EMG, if available, should demonstrate excessive or
even continuous phasic firing of the anterior and posterior tibial
muscles throughout the gait cycle. One should not expect postoperative
changes in the phasic pattern of muscle firing after transfer (11).
Currently, I favor the split posterior tibial tendon transfer (usually
along with TAL) in patients with dynamic varus and equinus deformity,
because plantar flexor strength is not sacrificed quite as much, and
only the muscle direction is changed. It is important to realize that
spastic muscles, although overactive, are still weakened. In fact, I
frequently split both the anterior and the posterior tibial tendons
with lateral transfer of both in cases of dynamic varus. Others perform
just the SPLATT transfer with TAL for the same dynamic varus deformity.
The postoperative regimen in either case is the same as described for
TAL.
posterior tibial tendon anteriorly through the interosseous membrane in
a patient with spastic CP. I have abandoned that technique because of
the occurrence of late calcaneovalgus deformity. I would also caution
against complete tenotomy of the posterior tibial tendon in the spastic
foot, because late valgus is likely.
as determined by the block test, add a bony reconstruction to the
soft-tissue releases or transfer. Perform the block test by having the
patient stand with a 1–2 cm block under the heel and lateral border of
the foot. If the heel varus resolves, then it is compensatory and
results from forefoot pronation of the first ray. If heel varus
persists on the block, it is fixed (see the section on cavus in Chapter 167).
In the case of a younger patient, the choices are either a sliding
lateral displacement osteotomy or a Dwyer-type of laterally based
closing wedge of the calcaneus. In a patient older than 12 years with
significant fixed varus deformity, perform a triple arthrodesis.
-
Through a longitudinal supramalleolar
posteromedial incision, approach the posterior tibial muscle. At least
2–4 cm proximal to the most distal muscle fibers, divide the tendinous
portion of the posterior tibial muscle obliquely. Passively evert the
foot under direct visualization to observe the tendon slide. This
produces an aponeurotic lengthening that leaves the muscle fibers in
continuity. No sutures are needed. -
Alternatively, a supramalleolar
Z-lengthening of the posterior tibial tendon can be performed, but this
is a more complex procedure and requires suture repair.
-
Make a second incision over the anterior
compartment of the leg 3–4 inches (7.5–10.0 cm) above the ankle. Pass
the lateral half of the tendon proximally into the second incision. It
is helpful to have a Bunnell-type suture through the free end of the
tendon. -
Then make a third incision laterally over
the cuboid bone. Pass the lateral half of the anterior tibial tendon
subcutaneously, deep to the extensor retinaculum to the third incision.
Suture the tendon either to the periosteum of the cuboid or, better,
route it through a small bony vertical tunnel in the cuboid. I prefer
using an absorbable pullout stitch over a padded plantar button or
splint. On subsequent removal of the cast, the pullout suture can
simply be cut flush with the skin. -
Prior to closure, tension on the tendon
proximally should tend to dorsiflex the foot in a neutral position.
That is, a yoke has been created and the tension in each limb both
medially and laterally should be fairly similar. Use a short-leg cast
for 6 weeks.
incisions are used, although the entire procedure can readily be
performed through the Cincinnati horizontal transverse incision
commonly used for clubfoot (31) (see Chapter 167).
-
Begin with a 1-inch (2.5 cm) medial
longitudinal incision over the navicular tuberosity. Isolate the
posterior tibial tendon at its insertion and split it distally,
detaching the plantar half. Tag this with a heavy nonabsorbable suture.
(Fig. 177.2).![]() Figure 177.2. Split posterior tibial tendon transfer.
Figure 177.2. Split posterior tibial tendon transfer. -
Make the second incision medial and
longitudinal, 1 cm posterior to the medial border of the tibia. Using a
curved tendon passer, pass the tagged suture with the plantar half of
the posterior tibial tendon through the sheath directly posterior to
the tibia. Tease this backward to propagate the split. Deliver the
tendon into the medial proximal incision. -
Place a third incision just posterior to
the distal fibula. Then pass the tagged tendon laterally just posterior
to the tibia and fibula to the peroneal tendon sheath. Pass the split
tendon within the sheath to the area of the fifth metatarsal–cuboid
articulation, where the fourth short incision is made. Then suture the
split tendon to the peroneus brevis under moderate tension with the
foot in neutral. Apply a short-leg cast.
-
Expose the lateral calcaneus
subperiosteally through an oblique lateral incision immediately behind
the peroneal tendons. Use an oscillating saw to cut through the
calcaneus more or less parallel to the posterior calcaneal facet.
Temporarily open the osteotomy laterally to use a curved Freer or small
elevator to medially strip subperiosteally. Unless this step is done,
it will be impossible to displace the tuberosity fragment. I caution
against hammering an osteotome to complete the osteotomy medially
because of the danger to the neurovascular bundle and flexor tendons,
which are directly apposed medially (Fig. 177.3). Figure 177.3. Calcaneal osteotomy for fixed hindfoot varus.
Figure 177.3. Calcaneal osteotomy for fixed hindfoot varus. -
When the tuberosity has been sufficiently
mobilized, displace it laterally enough (0.5–1.2 cm) to put the heel in
neutral or slight valgus. Make sure that the tuberosity fragment does
not slide proximally. Insert a single smooth pin from the plantar
surface of the calcaneus across the osteotomy to hold the reduction.
Leave it in place for 3 or 4 weeks. -
The Dwyer technique is perhaps simpler
because no medial dissection is necessary; however, it mildly decreases
the heel height and theoretically also decreases plantar flexor
strength. Make the first bony cut as described previously, but then
remove an oblique laterally based wedge (Fig. 177.3) sufficient to correct the hindfoot
P.4490
varus, bringing the heel directly in line with the long axis of the
tibia. Close the bony surfaces and stabilize with either a smooth
Steinmann pin (which is removed in 3 weeks) or a staple. I prefer pins.
Allow weight bearing after 3 weeks.
-
Make an oblique incision over the sinus
tarsi and expose the sinus by maintaining a distally based flap of the
extensor brevis muscle and overlying fat pad. Instead of continuing
distally in the subperiosteal plane toward the calcaneal–cuboid joint,
I use a 2 cm osteotome to cut across the joint dorsally in the
longitudinal plane. This creates a small myo-osseous flap, but more
important, it nicely exposes the calcaneal–cuboid articulation. -
Depending on the degree of varus, take
laterally based wedges from the subtalar and calcaneocuboid joints. I
use a micro-oscillating or sagittal saw. In general, the wedges should
probably be taken smaller than you initially think is necessary, so
that an excessive amount of bone is not removed. -
I usually use two pins, both placed
axially: one to hold the calcaneocuboid joint and the other the
talonavicular joint. A third pin, vertically placed across the
talocalcaneal articulation, is optional. Apply a heavily padded,
above-knee cast.
weeks. Then apply a short-leg walking cast and maintain it until the
fusion is solid, which usually takes an additional 6–8 weeks.
in spastic diplegia and quadriplegia. Although, at first glance,
predominant spasticity of the peroneal muscles would seem to be the
primary cause, the etiology usually is multifactorial and includes
excessive external tibial torsion, knee flexion deformity, and
calcaneal equinus. The deformity usually remains flexible until
adolescence. Secondary callosities develop over the talar head and the
first metatarsal head, and hallux valgus develops. Initially, manage
the deformity with an AFO; however, if the deformity becomes severe,
brace fitting is increasingly difficult.
deformity becomes fixed, usually by 6–10 years of age. Traditionally,
some modification of the Grice extra-articular arthrodesis procedure
was most commonly performed, using internal fixation (3,7).
Depending on calcaneal position, a TAL is often necessary as well. The
deformity must be passively correctable (at least with the foot in
equinus) for the Grice procedure to work. More recently, one of several
types of calcaneal osteotomies has become preferred because it
maintains subtalar mobility while correcting the valgus (18,23,27).
Although correction can be obtained by a simple opening lateral wedge
osteotomy in the tuberosity using a cortical graft (bank or
autogenous), I prefer either a medial calcaneal slide, or,
occasionally, a lateral column lengthening. This latter technique of
Evans, popularized by Mosca (18), has the
advantage that it can partially correct the lateral subluxation at the
midfoot. All of these also require reasonably supple feet that are
passively correctable to neutral, or that can be made so with a simple
heel cord lengthening.
less than 4 years of age because the bones are too small and
cartilaginous. After age 12, a triple arthrodesis is usually more
appropriate unless the valgus remains totally flexible. Occasionally,
all or much of the apparent valgus is built into the tibiotalar joint
with a relatively short fibula. A preoperative standing anteroposterior
radiograph of the ankle should always be taken before the Grice
procedure is performed. Do not attempt to correct the subtalar joint
into varus to make up for ankle valgus. True ankle valgus must be
corrected by a supramalleolar osteotomy or, rarely, a medial growth
arrest, depending on age. Of course, the patient should be at least a
household assisted ambulator (or have a reasonable prognosis for
attaining that level of function) before considering foot
reconstructive surgery.
-
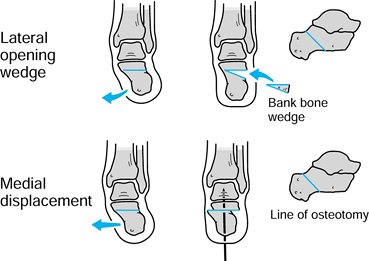
Use the same lateral oblique incision
(posterior and parallel to the peroneal tendons) as for a Dwyer
osteotomy. Use an oscillating saw to cut the tuberosity parallel to and
below the posterior facet. If only the hindfoot is in valgus, then
there is a choice of either using a laterally based opening wedge (held
with an allograft cortical wedge), or sliding the distal fragment
medially (Fig. 177.4).![]() Figure 177.4. Calcaneal osteotomy for fixed hindfoot valgus.
Figure 177.4. Calcaneal osteotomy for fixed hindfoot valgus. -
To successfully displace medially, it is
necessary to carefully subperiosteally dissect medially from the
lateral approach to mobilize the distal fragment. After medial
displacement sufficient to place the heel in neutral alignment,
accomplish fixation with a smooth Steinmann pin placed vertically from
the plantar surface. -
I do not have long-term experience with
distal calcaneal lengthening, but I have used it in cases of mild to
moderate, very flexible valgus, and early results have been excellent.
In this technique, make a transverse vertical osteotomy in the
calcaneal neck, parallel to the calcaneal–cuboid joint. After
mobilizing the distal fragment, use a lamina spreader to open the
osteotomy, and simultaneously
P.4491
reduce
the lateral talonavicular subluxation. Insert a trapezoidal cortical
cancellous graft (I prefer allograft). It may be wise to provisionally
fix the calcaneocuboid joint with a smooth Kirschner wire before
lengthening; this prevents subluxation. A longitudinally placed K-wire,
from distally across the calcaneal–cuboid joint, is optional if needed
to maintain stability, and once it is passed through the calcaneocuboid
joint, the original wire is removed. If a TAL has not been performed
prior to the osteotomy, recheck after completion of the osteotomy to
make sure that there is not a fixed ankle equinus deformity.
-
Through an oblique incision extending
from the lateral talonavicular joint to the peroneal tendons, sharply
elevate the contents of the sinus tarsi from proximal to distal. Clean
the lateral body and neck of the talus and the calcaneal floor of the
sinus tarsi of all soft tissue. Ideally, expose no articular surfaces. -
Pack the sinus tarsi with
corticocancellous graft taken from the iliac crest. Iliac graft rather
than tibial or fibular is preferred because it is incorporated readily,
it is abundantly available, there is enough graft from one crest to
fuse both feet simultaneously, and the iliac crest graft does not carry
the risks of donor site fatigue fracture after surgery (as do tibial
grafts) or later valgus deformity (as with fibular grafts). Unlike
“structural” grafts of tibial or fibular cortex, iliac graft is not
used to correct deformity, only to obtain fusion. Because the iliac
graft itself does not provide fixation, supplemental internal fixation
is mandatory. -
Some surgeons insert a screw through the
neck of the talus into the calcaneus, and this has the advantage of
earlier weight bearing. However, I prefer to use a percutaneous
Steinmann pin, inserted under direct vision from the lateral plantar
aspect of the calcaneus proximally across the sinus tarsi into the
talus, because there is no retained hardware that can either back out
or impinge against the anterior ankle structures (Fig. 177.5).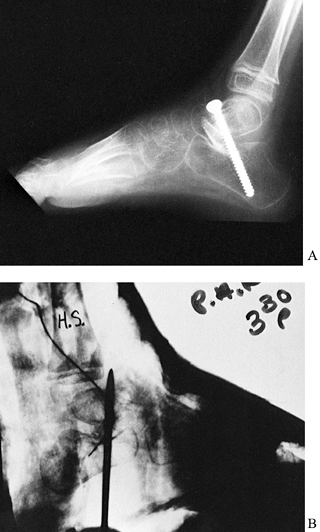 Figure 177.5. A: Subtalar arthrodesis with screw fixation. B: Temporary fixation using a Steinmann pin for subtalar arthrodesis.
Figure 177.5. A: Subtalar arthrodesis with screw fixation. B: Temporary fixation using a Steinmann pin for subtalar arthrodesis. -
Apply a long-leg cast for 4 weeks
postoperatively, and then remove the pin. Weight bearing may be allowed
in a short-leg cast for the next 4–6 weeks.
make reduction of the calcaneus under the talus difficult or
impossible. The heel will remain in valgus despite positioning
the
graft. Another potential complication is that the graft may “melt
away.” This is especially likely when excessive external tibial torsion
is present. Occasionally, sufficient fibrous stability may remain even
with graft resorption so that further treatment is not necessary. If
significant valgus recurs, triple arthrodesis may be considered as a
salvage procedure.
gradually, but it is usually the direct result of intraoperative
overcorrection. If the extra-articular arthrodesis is solid, correction
of a postoperative varus can be obtained with a closing lateral wedge
osteotomy of the heel.
however, for spastic valgus feet, additional principles are important.
It is critical to ascertain before surgery that the ankle itself is in
relatively normal alignment. If there is excessive external tibial
torsion, correct this before performing the triple arthrodesis.
Although flexible valgus feet can be corrected by the simple removal of
joint surfaces, possibly with an inlay graft, most spastic valgus feet
in older patients are rigid and require extensive bony wedge resection
to obtain correction. Additionally, poor correction can be caused by
inadequate exposure of the talonavicular joint.
-
Use an additional 1-inch (2.5 cm) medial
longitudinal incision over the talonavicular joint if the medial aspect
is not well visualized from the main incision. This will allow
excellent visualization when a Chandler retractor is passed from the
main lateral incision out through the medial incision to protect the
dorsal soft-tissue structures. -
If the foot is passively correctable to
neutral before surgery, only the cartilaginous joint surfaces must be
removed. However, in more severe valgus feet, remove medially based
wedges of bone. Pack the sinus tarsi with cancellous bone from either
the wedges or the iliac crest. -
I recommend using at least two smooth
Steinmann pins for fixation. Pass one distally through the center of
the exposed navicular and pass a second distally through the center of
the cuboid. Then drive them retrograde into the talus and calcaneus,
respectively. A third vertical pin through the talocalcaneal joint is
optional. Take intraoperative radiographs; it is surprising how
misplaced the pins can be. I usually place a small suction drain. -
Use an above-knee (or patellar tendon
bearing) non-weight-bearing cast for 6 weeks postoperatively, and then
remove the pins and apply a short-leg walking cast.
diplegia and quadriplegia, apparently as a compensation for excessive
medial femoral torsion. This malalignment will contribute to valgus
foot deformities, bunions, and other problems. Tibial osteotomy is
indicated when it is necessary to keep the foot pointed correctly
forward, especially following femoral derotation (external) osteotomy,
or as part of correction for severe valgus feet.
-
Make a 5–10 cm longitudinal incision
proximally over the lateral anterior compartment. Always perform a
fasciotomy of both the anterior and the peroneal compartments. -
By following the intermuscular septum,
make a limited subperiosteal exposure of the fibula somewhat more
distal than the tibial site to avoid the peroneal nerve. Osteotomize
the fibula with a micro power saw. -
Expose the tibia circumferentially
subperiosteally just distal to the tibial tubercle. To avoid anterior
growth arrest, the osteotomy site must be distal to the anterior
extension of the physis. The operation can also be performed in the
supramalleolar metaphysis, where the risk of compartment syndrome and
peroneal palsy is less. I recommend operating distally if correction of
an ankle valgus is also desired. -
Insert half-pins from medial to lateral
through separate incisions prior to the osteotomy. Place one pin
proximal to the incision and one distal, with both pins going through
two cortices but only through the medial skin. Perform the osteotomy
with an oscillating power saw, and control rotation with the pins. It
is helpful to insert the pins so that the angle between them is the
same as the angle of rotation you want. Then, when rotation is
complete, the pins will be exactly parallel to each other. -
Immobilize the limb in a long-leg cast,
incorporating the pins in plaster. If no additional tendon work has
been done, an external fixator could be used, but this does not seem
worth the expense. Clinical union occurs within 2 months in children.
Weight bearing is usually allowed after 4 weeks, at which time the pins
are pulled out through the cast.
flexion contracture, the knees may be neutral or even hyperextended
during the midstance phase of gait (3,8,21,23).
The knees (like every other joint in CP) cannot be evaluated in
isolation. The cause of severe knee flexion—whether dynamic or fixed—is
rarely as simple as excessive
hamstring
spasticity. Overlengthened or weak plantar flexors, fixed hip flexion
contracture, and poor equilibrium all contribute to crouch. We can do
little about faulty equilibrium, and we certainly recognize that a
small knee flexion deformity is usually preferable to recurvatum.
Therefore, not all knee flexion deformities (even fixed ones) require
surgical release.
In the more common and familiar “crouched” type, the knee flexion is
associated with feet that are flat on the floor or the heels are only
slightly elevated (often in valgus). There is usually a fixed hip
flexion deformity with increased iliopsoas spasticity, which must be
released. The worst thing to do in these cases is a TAL, because it
will increase the crouch.
ankles are in marked fixed equinus. This type of patient needs a modest
TAL, in addition to lengthening of the hamstrings and probably the
iliopsoas.
hamstring lengthening when the knees cannot be straightened during
ambulation to less that 15° of flexion, and when other causes of crouch
gait (at the hips or ankles) are absent or can be dealt with
simultaneously. Knee flexor release is also indicated in older patients
who are crouched and have knee pain during transfers or limited
ambulation. If the degree of fixed contracture of the knee is greater
than 20°, posterior knee capsulotomy is occasionally needed, but
usually this is not needed in a walking CP patient with crouch.
occasionally performed to decrease extreme flexor spasticity at the
knee; this facilitates sitting and dressing. The hamstrings may also be
released proximally through the same medial incision used for adductor
and psoas release. However, the only time I use a proximal release of
the hamstrings is in the patient who has not only tight hamstrings but
also severe hip extension deformity. Such a patient stands with an
absent or reversed lumbar lordosis. She tends to slide out of a
wheelchair because the hamstring spasticity extends the hips. When
proximal release is performed, the sciatic nerve must be avoided; it
can be confused with the tendinous origin—a potential catastrophe.
-
Place the patient supine and make a
short, midaxial, longitudinal incision on the back of the knee. This
allows repeated intraoperative assessments of the degree of improvement
of straight-leg raising. In a typical patient with either no fixed-knee
flexion contracture or only a mild one (10° to 20°), the contracture
can be stretched out by wedging casts after hamstring release. -
Perform a Z-lengthening or simple
tenotomy of the semitendinosis. The gracilis may also be tenotomized at
your discretion. Perform an aponeurotic lengthening of the
semimembranosus and biceps tendon by oblique division of the tendon
within the muscle belly. The lengthening should be sufficient to allow
70° of straight-leg raising. This is easily determined in the supine
position. -
I always lengthen the biceps last because
it is often not as tight as the medial hamstrings. In such cases, if
adequate straight-leg raising is present after lengthening only
medially, leave the biceps intact.
Mobilize the patient immediately with a walker or crutches if
equilibrium is satisfactory. Mobilize more severely
equilibrium-impaired patients in a standing frame chosen by the
physical therapist.
simultaneously at the ankles, I have used only a knee immobilizer
postoperatively, again allowing immediate mobilization. If a joint
capsule contracture was present preoperatively, do not apply the cast
fully straight, but only at the limits of the maximal preoperative
degree of extension. Begin wedging in 2 days.
posterior capsulotomy, a better exposure is obtained by operating with
the patient prone. In such cases, I prefer short posterior medial and
posterior lateral incisions to better visualize the capsule. After a
simple hamstring release, 3 weeks of immobilization is usually
sufficient, although a little extra time may be needed if the casts
have to be progressively wedged into extension following a capsulotomy.
perform the operation through an oblique adductor approach or through a
short medial transverse incision just below the buttocks.
-
Make certain that this operation is
performed without the use of anesthetic paralyzing agents, so that
stimulation of the sciatic nerve with the cautery can warn you if the
sciatic nerve is in close proximity to the hamstring origins. -
After choosing which skin incision to
use, make a longitudinal incision in the fascia. Use blunt finger
dissection in the plane posterior to the adductor magnus tendon. Start
with the knee in flexion to relax the sciatic nerve, which is deep in
the incision, near the femoral shaft. Intermittently extend the knee
while flexing the hip to aid in defining and delivering the hamstring
origins. Use a nerve stimulator if there is any confusion between the
nerve and tendon. -
Do not divide the proximal hamstrings
until you are certain that the nerve is safe from harm; use the
cautery. Gently retest the range of knee extension with the hip flexed;
after satisfactory release, knee extension should
P.4494
markedly increase. Never forcefully extend the knee maximally while the hip is flexed. -
Base postoperative immobilization on
whatever other releases are carried out. If only hamstrings are
released, then knee immobilizers for 2–3 weeks is sufficient, and
mobilization can begin immediately.
deformity before surgery may lead to a poor result. The crouch gait
will persist if a hip flexion deformity is ignored or if
hyperdorsiflexed ankles are not braced. If preoperative quadriceps
spasticity is severe, recurvatum may occur following overly generous
hamstring weakening. Myotendinous (aponeurotic) lengthening, when
performed too far distal (close to the junction of the tendon with the
most distal muscle fibers), may result in complete transverse
separation. The key here is simply to get enough proximal exposure so
that there are plenty of muscle fibers distally to allow the slide
after the intramuscular release of the tendon.
structures about the knee. When applying the cast, do not try to
forcibly straighten the knee if the patient is under anesthesia. A
sciatic stretch palsy is difficult to detect acutely in a CP patient
with severe involvement but is still a very undesirable complication.
If the preexisting fixed contracture is significant (i.e., more than
20°), plan to correct it gradually after surgery using wedged casts
with the patient awake.
usually occurs dynamically at midstance and is secondary to fixed ankle
equinus or excessive quadriceps spasticity, especially of the rectus
femoris. The heel cord contracture, if present, must be corrected, but
weakening of the quadriceps remains a problem. There is a natural
reluctance to weaken the quadriceps, because it is necessary to
maintain upright posture. However, many CP patients, even with mild
degrees of crouch, will have excessive cospasticity of the rectus
femoris. The simultaneous, excessive rectus femoris and hamstring
spasms lead to a stiff-kneed, short-stride gait (8).
However, I do not believe that every ambulatory patient who undergoes a
hamstring lengthening needs a simultaneous rectus femoris procedure.
deformity, it is simple to release and recess a small section of the
origin of the rectus femoris tendon at the time of the iliopsoas
recession or lengthening. This produces minimal quadriceps weakening,
but it is safe. Unfortunately, a tenotomized rectus may spontaneously
reattach to the anterior inferior iliac spine.
of hip flexor release in CP. Release it only if there is a
hyperextended knee gait, or occasionally in nonwalkers if the fixed
flexion contracture is very severe.
much of a hip flexion deformity, the usual indication for rectus
surgery is inadequate knee flexion late in the swing phase. Such
extensor spasticity interferes with foot clearance. In such cases, it
is reasonable to selectively transfer the rectus femoris either
medially to the semitendinosis or laterally to the iliotibial band. If
a laboratory analysis of the gait is available, the rectus will usually
be found to fire excessively or throughout swing phase on dynamic gait
EMG. The rectus femoris transfer can be performed simultaneously with
hamstring lengthening without fear of increasing crouch, or it can be
performed some time later if the knee tends toward recurvatum.
-
Make an anterior incision transversely or longitudinally about 6 cm proximal to the patella (8).
Undermining proximally allows identification and separation of the
interval between the vasti and the rectus. Proximally, this can easily
be done bluntly, but distally the rectus tendon blends with the common
quadriceps tendon. Further separation must be done sharply, while
avoiding entry into the knee joint, to a level near the superior pole
of the patella. -
If a slight correction of gait into
external rotation is desired (i.e., in cases where there is excessive
inturning at the knee), isolate the rectus tendon and bluntly separate
proximally. Then transfer the distal stump through a subcutaneous
tunnel to the semitendinosis medially. If the hamstrings have been
lengthened sometime previously, and the distal stump of the
semitendinosis is not available, the transfer can be into the
sartorius. If there is preexisting excessive external rotation at the
knee, then transfer the distal stump of the rectus through a
subcutaneous tunnel to the iliotibial band or the biceps femoris. There
should be no tendency toward increased crouch as long as the vastus
medialis, lateralis, and intermedius remain intact, because they
provide the bulk of quadriceps strength. -
Postoperatively, use removable extension knee immobilizers part time for 3–4 weeks.
dynamic flexion deformity to complete painful dislocation. The three
most common components are adduction, flexion, and internal rotation.
Although these components will be considered separately, usually all
three coexist to some degree.
most patients with CP. Primary abduction deformity is rare and usually
the result of overzealous adductor release and neurectomy of both the
anterior and the posterior branches of the obturator nerve.
scissoring occurs or when passive abduction (in extension) is less than
20°. It is also indicated in limited walkers or sitters as part of the
surgery for early hip subluxation. Occasionally, in the patient with
severe total body involvement, the adductor release is necessary to
facilitate perineal care. Adductor release is nearly always a part of
the treatment for more severe degrees of hip subluxation, which require
sufficient release to obtain a satisfactory range of abduction (1).
Patients must be assessed individually because the extent of release
required and the need for adductor transfer or neurectomy varies. In
general, there is a trend away from anterior branch neurectomy except
in the severely involved, nonwalking patient.
be recessed (sutured more distally to the underlying adductor magnus or
brevis) or transferred posteriorly to the ischium (3,6,22).
To accomplish a posterior transfer, the lithotomy position is best. The
theoretical advantage of posterior adductor transfer is improvement in
hip extensor tone. The brevis may also be transferred; however, its
entire origin is a fleshy muscle belly that does not hold sutures well.
In at least two of the adductor transfers I have performed, the
transferred origins pulled off the bony ischium. Postoperative
radiographs proved this, as I placed radiopaque markers in the origin
of the longus. Others have also noted the tendency for posteriorly
transferred adductors to migrate postoperatively back to their origins (17).
The extra dissection necessary for adductor transfer hardly seems
worthwhile in most patients, and I have abandoned it. I simply perform
a release without anterior neurectomy in the majority of cases. The
extent of the release depends on the degree of deformity.
diplegic and quadriplegic patients when both hips are adducted or have
limited passive abduction. This is the most common situation. Even when
one is less adducted than the other, both hips should usually be
released in these patients, because when only a unilateral soft-tissue
release is performed, there is a tendency for the nonoperated hip to
subsequently become unstable (4).
will have true windblown hips. This is especially common with severe
neurogenic scoliosis and pelvic obliquity (the pelvis is “down” on the
abducted side). The abducted hip will be well covered and should not
have an adductor release if the abduction is fixed. Of course, the
hemiplegic patient with adductor limitation will also need only a
unilateral release. The posterior branch of the obturator nerve should
very rarely be divided. Only following failure of a prior extensive
adductor release and anterior branch obturator neurectomy with
recurrence or persistence of adduction deformity would one consider a
posterior branch or intrapelvic obturator neurectomy.
performed simultaneously on the hip (e.g., iliopsoas recession, open
reduction). Therefore, my preferred technique is to simply extend the
anterior bikini incision slightly medially. (The bikini incision is
oblique, just distal and parallel to the inguinal ligament.) The skin
incision also may be made longitudinally or obliquely over the adductor
longus origin. Through the Ludloff-type approach (Chapter 3), the psoas tendon can be tenotomized, but it cannot easily be recessed or divided above the pelvic brim.
-
Whichever skin incision is chosen, define
the adductor tendons, including the longus and gracilis, by blunt
dissection after longitudinally opening the fascia. Completely release
the longus and gracilis at their origins as proximally as possible.
Next, assess the range of passive abduction. If this is not at least
40°, further release is necessary, including the brevis and pectineus.
If still it is tight, the medial hip capsule may need to be divided
transversely. -
Because the anterior branches of the
obturator nerve lie on the anterior surface of the adductor brevis,
always identify the nerve before releasing much of the brevis. Only in
the nonwalking patient with severe involvement is a segment of the
nerve (usually two or three branches) removed. I often use a small
suction drain for the rather considerable dead space. -
Maintain postoperative abduction for 2–3
weeks, using two long-leg casts with a bar between them. Recently, I
have used just two knee immobilizers with an abduction pillow, allowing
immediate weight bearing and mobilization if there was no simultaneous
bony surgery requiring a cast.
expecting too much and doing too little. If there is a pelvic obliquity
from scoliosis and the “higher” femoral head is luxating, adductor
release alone will not maintain hip reduction unless the structural
scoliosis and obliquity are controlled. If there is any evidence of
early hip subluxation (even with a level pelvis), the iliopsoas must be
lengthened.
may persist or gradually worsen, especially if the patient is a
nonwalker. Spasticity of the iliopsoas is the main cause of the
deformity, although every muscle that passes anterior to the transverse
axis of the hip contributes to hip flexion. Clinical measurement of hip
flexion deformity is best performed by the prone-lying Staheli test (28).
The better-known Thomas test is affected more by spasticity of the
contralateral side, which tends to roll the pelvis as the opposite limb
is flexed. This makes it difficult to ascertain the neutral position of
the pelvis.
by measuring the lateral sacrofemoral angle on films taken with the
patient prone or supine and the hips maximally extended. The
sacrofemoral angle is that formed between the top of the sacrum and the
axis of the extended femoral shaft. The normal angle is 40° to 60°,
which decreases with hip flexion deformity. Fixed hip flexion deformity
causes the patient to stand with either a lordotic spine and fairly
straight knees or a flat back and crouched knees. In either case, the
sacrofemoral angle is reduced (3).
radiographically normal hips if fixed hip flexion is greater than 15°.
Release or lengthening of the psoas is also a part of correction of any
degree of hip subluxation in CP. Usually, whenever a derotation
osteotomy is performed for excessive anteversion, the iliopsoas should
be lengthened or recessed. In nonwalkers, a simple complete distal
tenotomy (usually performed with an adductor release) can be made
through either a Ludloff incision or an anterior bikini incision. In
patients with bilateral flexion deformities but fixed, windblown hips,
adductor release is performed only on the adducted (high) side. The
iliopsoas is released bilaterally. In the rare case when there is truly
limited adduction on the abducted side of the pelvis (the down
hemipelvis), the origins of the tensor and the gluteus medius and
minimus are also released on the abducted side.
simple oblique tenotomy of the psoas tendon as far proximally as
possible, where there are still abundant investing iliacus muscle
fibers. This has the net effect of markedly weakening the psoas while
only moderately weakening the iliacus portion. No sutures are required,
making it simpler than the formal recession, as no suture repair is
necessary. This is similar to what most surgeons usually do in
performing open reduction of congenitally dislocated hips in otherwise
normal children.
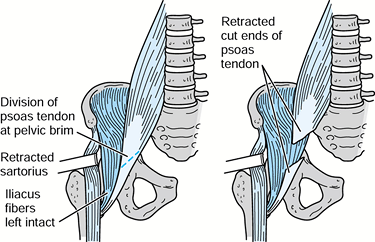
-
I perform an iliopsoas recession or
lengthening through the usual bikini anterior incision by identifying
the interval between the sartorius and the iliacus, assuming no
acetabular procedure or open reduction is necessary (Fig. 177.6).
Visualize and protect the lateral femoral cutaneous nerve deep to the
enveloping fascia exiting the pelvis on the anterior surface of the
sartorius just medial to the anterosuperior iliac spine. Do not detach
the sartorius. On the other hand, if the psoas lengthening is a part of
an open reduction or acetabular procedure, use the more extensile
standard Smith-Peterson anterior interval between the sartorius and the
tensor fascia muscle.![]() Figure 177.6. Iliopsoas lengthening at the pelvic brim.
Figure 177.6. Iliopsoas lengthening at the pelvic brim. -
Locate the femoral nerve on the anterior
surface of the iliacus but deep to the iliacus fascia, and retract it
gently medially with a blunt retractor. The psoas tendon is deep in the
iliacus muscle fibers and tightly applied to the anterior medial hip
capsule. Isolate the tendon proximally, separating it from the muscle
fibers of the iliacus, and divide it at the pelvic brim (30). -
In performing a formal recession, flex
and externally rotate the hip so that the tendon can be followed
distally to the lesser trochanter where the entire tendon is detached.
Free the conjoined muscle–tendon unit from the anterior hip capsule and
reattach it with two heavy sutures more proximally on the anterior
capsule (2). The net effect is to decrease the
mechanical advantage of the iliopsoas muscle by placing the insertion
closer to the axis of hip flexion.
weeks. This is most easily accomplished by applying two long-leg casts.
If bilateral adductor release has also been done, place a broomstick
bar between the casts to maintain abduction. The patient is cared for
in the prone, the supine, or even a standing position as long as hip
flexion is avoided, except briefly for meals, transport, and so forth.
If the patient underwent an open reduction, some type of spica cast
will be necessary for at least 6 weeks. If the hips were windblown
before surgery, two long-leg casts with
a
spreader bar can be used, but instruct the parents to maintain the hips
windblown to the opposite direction. If the pelvis is level and only
soft-tissue procedures have been done, I now prefer to mobilize the
patient immediately postoperatively, so I use just knee immobilizers
and an abduction pillow at night.
nerve and the psoas tendon. Distal tenotomy of the entire psoas tendon
severely weakens hip flexion and should not be done in a child who can
or potentially might be an independent, crutch-free walker. One should
expect that in most patients younger than 7 years without severe
scoliosis or pelvic obliquity, a psoas and adductor release performed
in the presence of no more than mild hip dysplasia will prevent
subsequent dislocation. There is not universal agreement as to whether
postoperative nighttime abduction bracing is necessary. However, I
recommend at least 3–6 months of abduction bracing using a foam wedge
or abduction brace if there is any radiographic evidence of dysplasia (14).
severe acetabular dysplasia, or another type of secondary bony change
(typically in patients older than 8–10 years), correction of the
scoliosis or acetabular reconstruction must be performed to maintain
hip reduction; muscle release alone will be insufficient in such cases.
Most commonly, for patients 6 years or older, with mild to moderate
degrees of acetabular dysplasia, I will add a Dega-type pelvic
osteotomy (19) or Staheli acetabular augmentation (shelf)-type procedure (32).
anteversion) is very common in spastic CP. It manifests as internal
rotation deformity, mainly in walkers. In spastic patients who are only
sitters, the excess anteversion contributes to hip dysplasia and
dislocation, but the internal rotation is not so apparent with the
patient in the sitting position. The cause of the increased anteversion
is probably muscle imbalance of more than just the psoas; the iliopsoas
is nearly always contracted in patients with excessive hip anteversion
and internal rotation gait. In normal patients, the anterior fibers of
the gluteus medius and minimus and the tensor are the main hip internal
rotators. This fact has encouraged Steel (29)
to perform anterior transfer of the trochanteric insertion of the
gluteus medius and minimus for internal rotation gait, converting
abductors to external retractors. In general, I do not favor this
operation because it carries the risk of weakening the abductor
mechanism, thus possibly trading an internal rotation deformity for a
Trendelenburg limp. Furthermore, the operation is indicated only in
patients with no flexion contracture (i.e., a normal iliopsoas) and
spastic abductors—a situation seldom encountered.
are based on patient age and ambulator status. In patients 4–8 years of
age, I usually perform adductor release and iliopsoas intramuscular
lengthening or recession, and I follow the patient for a number of
years. In most patients, the excessive internal rotation will gradually
improve as the hip flexion contracture and adduction tendency improve
and the anteversion decreases. After 8–10 years of age, if the patient
is still ambulating with an excessive internal rotation gait, I usually
prefer subtrochanteric derotation osteotomy using the AO-ASIF
(Association for the Study of Internal Fixation) technique. In many
cases, little varusization is needed because the “coxa valga” seen on
radiographs is more apparent than real, and it will disappear with
derotation alone.
-
Perform the operation on the image
intensifier table with the patient supine and the leg draped free.
Place a small folded blanket or towel under the buttocks so that the
prep and exposure will be sufficiently proximal and posterior. -
Make a standard lateral approach to the
proximal femur and insert three guide pins: one for the chisel
alignment, one more proximal in the greater trochanter to be used as a
joy stick to control the proximal fragment, and one distal to the end
of the plate to control rotation. Typically, the distalmost pin will be
inserted at an angle of 30° to 45°, internally rotated in relation to
the proximalmost pin (Fig. 177.7).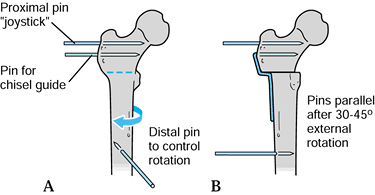 Figure 177.7. Use of pins to control rotation.
Figure 177.7. Use of pins to control rotation. -
Make sure that the femur is
circumferentially exposed subperiosteally in the area of the planned
osteotomy. Insert the appropriate child or adolescent blade chisel into
the greater trochanter along the course of the first pin so that the
osteotomy can be cut through the middle of the lesser trochanter.
Always use the chisel guide;
P.4498
otherwise, there is a tendency to angle the blade so that the shaft of the plate is placed in flexion. -
Confirm the chisel position with the
image intensifier; remove the chisel halfway through, and complete the
osteotomy with an oscillating saw. Rotate the distal fragment
externally using the preplaced pins as a guide. If increased varus is
desired, remove a small wedge medially, which is one half of the shaft
diameter after derotating. Insert the blade plate using the
bone-holding forceps to maintain reduction. I use the dynamic
compression feature of the plate to close the osteotomy further. At
least 20° to 30° of residual internal rotation should be left after the
derotation (i.e., the distal fragment should not be excessively
externally rotated). Varus should be added to the osteotomy only when
there is a true valgus deformity of the femoral neck, and when there is
an abundant range of abduction. Each added degree of varus is
equivalent to adding 1° of adduction and subtracting 1° of abduction.
easily be derotated through the lateral hamstring exposure incision.
Use a six-hole plate, as described in Chapter 168.
acetabular reconstruction nor capsulorrhaphy has been performed, then
no cast is needed. Manage the patient in a wheelchair for 4–6 weeks,
allowing gentle motion. Then allow partial weight bearing with crutches
or a walker until healing of the bone is secure, usually at about 3
months. Most commonly in CP, some other acetabular work will have been
done, so a spica cast will be necessary. The spica cast should usually
be placed in nearly full hip extension and moderate abduction.
Iliopsoas recession or release is usually performed prior to or
simultaneously with the subtrochanteric osteotomy.
excessive external tibial torsion. In such a case, following femoral
derotation, the foot will point excessively laterally unless a
simultaneous internal derotation is performed on the tibia (3).
total body involvement, whereas in those patients who walk with
crutches, the deformity more often progresses only to subluxation.
However, even patients who are independent ambulators may develop
complete dislocations. The etiology of the dislocation is a combination
of excessive femoral anteversion with persistent spastic adduction and
flexion, often associated with pelvic obliquity secondary to structural
scoliosis. Dislocation has been alleged to cause scoliosis, seating
problems, decubiti, fractures, and difficulties with perineal care (5,12,14,19,20,26).
Although hip dislocation is associated with all these problems, they
are in fact caused not by the dislocation but by the muscle imbalance
and the rigidity of the hip contracture. Furthermore, for CP adults,
the status of hip location per se is not as important a determinant of
walking ability as having reasonable cognitive function, balance, and a
level pelvis with mobile hips.
will cause pain, although probably at least half will be symptomatic.
The ability for a severely involved patient to sit comfortably probably
has as much to do with the enthusiasm and motivation of the people
caring for him as it does with whether his hip is radiographically
reduced. Thus, it is not always necessary to treat older patients with
spastic dislocated hips (20).
range of motion and prevent subluxation by appropriate early
soft-tissue release and bracing in younger patients. The following
guidelines and prerequisites are suggested for decision making for
surgical correction of spastically dislocated hips:
-
Prevention of hip dislocation by early soft-tissue release is easier and more effective than late reconstructions (14).
-
The degree of reconstruction is
determined by the degree of dysplasia. That is, to relocate a
completely dislocated hip in an 8-year-old CP patient will usually
require at least a femoral osteotomy, possibly a pelvic osteotomy, as
well as soft-tissue release. -
Soft-tissue release is always necessary whenever bony reconstruction is planned.
-
Femoral varus osteotomy, although still
useful in maintaining reduction, cannot be counted on to induce
acetabular development in patients older than 8 years. The femoral
neck–shaft angle should not be placed in excessive varus (i.e., leave
at least 110°). -
Femoral shortening is a useful adjunct to
relocating a high-riding dislocation without tension. This is far
preferable to any type of traction, which is generally poorly tolerated
by CP patients. -
For patients older than 8 years with hip dysplasia and dislocation, acetabular reconstruction usually will be necessary (3,9,19,32).
Although nearly all types of pelvic osteotomies have been performed in
the past, including Salter and Pemberton procedures, the most useful
include the Dega and Chiari osteotomies and the shelf procedure
(acetabular augmentation). The shelf procedure can be added to any
other pelvic osteotomy should additional femoral head coverage be
necessary. -
Always try to keep spica cast immobilization to a minimum.
-
Finally, do not attempt relocation of a
unilateral hip dislocation using hip surgery alone if a severe
scoliosis with fixed pelvic obliquity is present. The pelvis should
probably be leveled first.
dislocated spastic hip, my typical correction would consist of, in one
stage, a femoral shortening and Chiari osteotomy with a supplemental
shelf. Very little varus would be added to the femur, and appropriate
soft-tissue release (e.g., adductors, psoas) and anterior branch
obturator neurectomy would also be performed (Fig. 177.8).
In the more common 6- to 10-year-old spastic patient with moderate
dysplasia but without frank dislocation, I do extensive soft-tissue
release and a Dega osteotomy, or possibly an acetabular augmentation. I
find the advantages to be no retained hardware, technical simplicity,
the possibility of doing both hips simultaneously, and consistently
satisfactory results (Fig. 177.9). In a patient
under 6 years with a unilateral dislocation, a soft-tissue release with
femoral derotation and shortening is performed, with the occasional
addition of a pelvic osteotomy or shelf procedure (Fig. 177.10).
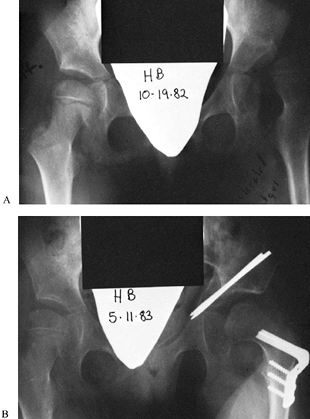 |
|
Figure 177.8. A: A 9-year-old child with spastic cerebral palsy and severe dislocation of left hip. B: The same child after Chiari osteotomy and varus osteotomy.
|
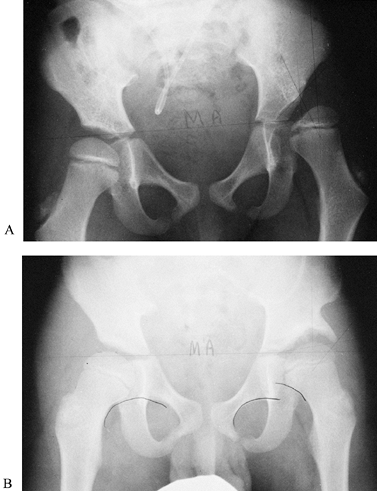 |
|
Figure 177.9. A: A 7-year-old with spastic cerebral palsy and a unilateral dysplastic left hip. B: The same child after acetabular augmentation and iliopsoas and adductor release.
|
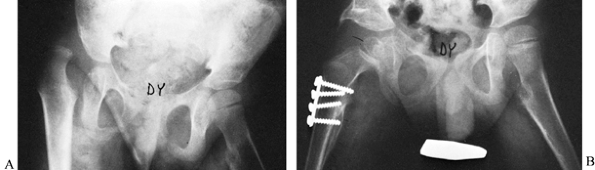 |
|
Figure 177.10. A: A 4-year-old child with spastic cerebral palsy and complete dislocation of the right hip. B: Following open reduction, femoral osteotomy, Pemberton procedure, and adductor and psoas release.
|
in that both are periacetabular incomplete innominate osteotomies that
bend down the superior portion of the acetabular roof. The main
difference is that the hinge for acetabular roof redirection is medial
with the Dega (so that the added coverage is more superolateral),
whereas with Pemberton’s osteotomy the hinge is more posteriomedial (so
that the coverage is more anterolateral). I prefer the Dega procedure
in cases of CP because it more adequately deals with the usual
elongated acetabulum with superolateral deficiency.(Fig. 177.11).
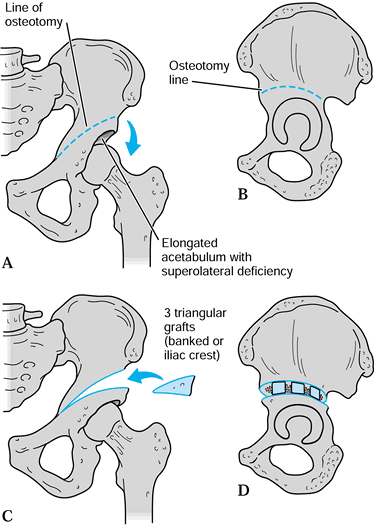 |
|
Figure 177.11. Dega osteotomy.
|
performed first, often with a femoral shortening and/or varus osteotomy
if necessary for reduction (19). Use an image intensifier with the leg draped free.
-
Perform the Dega osteotomy through the
standard anterior bikini incision with the iliac apophysis split and
the iliac wing exposed both medially and laterally. I usually do not
separately detach the sartorius but leave it attached to the medial
half of the apophysis and abdominal muscles. Release the origin of the
direct head of the rectus tendon and peel off the reflected head from
the superior capsule. Place blunt retractors superior and inferior to
the capsule for a wide exposure. -
If there is dysplasia but no significant
subluxation, the capsule does not have to be opened. However,
subluxated or dislocated hips require a standard acetabular debridement
and capsulorraphy. -
The Dega osteotomy itself is performed
from directly lateral, on a line from the middle of the anterior
inferior iliac spine to the sciatic notch. Use a series of curved
osteotomes after initiating the cut with a saw or high-speed cutting
tool. Aim the osteotomy cuts medially and inferiorly, extending to but
not through the medial cortex near the triradiate cartilage, as
monitored on the image intensifier. Both anterior and posterior corners
of the osteotomy need to be completed to the medial wall. Anteriorly,
this is easy to do, as it is under direct visualization. Posteriorly,
place a blunt Hohmann retractor in the notch, and use a 45° Kerrison
rongeur to complete the posterior corner of the osteotomy from lateral. -
Next, insert a wide, curved osteotome
into the osteotomy and lever the superior portion of the acetabulum in
a caudal direction until the relatively vertical lateral
P.4500P.4501
acetabulum
has been brought to a more horizontal position. There will now be a
lateral gap at the osteotomy site of 1–1.5 cm. To hold the osteotomy
open, use two or three bicortical triangular grafts from the anterior
crest (or occasionally from the femoral varus osteotomy, if done). No
internal fixation is necessary. When performed correctly, the osteotomy
will be remarkably stable because of the intact medial column. -
After routine closure, apply a hip spica cast and leave it in place for 6 weeks.
-
Make a standard anterior approach to the
hip using a slightly extended bikini incision. Expose subperiosteally
the entire outer wing of the ilium. Isolate the direct head of the
rectus tendon but leave it intact; detach and tag the reflected head
where it veers off the direct head just below the anterior inferior
spine. -
Perform a proximal psoas lengthening or
tenotomy simultaneously with any other necessary adductor release.
Expose the hip capsule extensively, as for an open reduction. Tease the
reflected head of the rectus femoris tendon as far posteriorly as
possible, at least to a position of approximately 1 or 2 o’clock. This
can be done easily more anteriorly, but it will require sharp
dissection from where the origin of the reflected head blends with the
capsule posteriorly. -
Thin the superior capsule near the bone
of the lateral wall of the ilium using a scalpel and curet. Make a
series of drill holes or use a high-speed burr to create a gently
curved slot over the superior dome of the acetabulum. Either plain film
radiographic control or image intensification is mandatory to confirm
that the slot is created far enough inferiorly. Ideally, the slot
should be just proximal to the acetabular cartilage; there is always a
tendency to create the slot several millimeters too far superiorly. (Hint:
Continue thinning the superior capsule against the lateral iliac cortex
until a fine line of cartilage is just visible.) The hip joint itself
is usually not entered despite the ease with which the femoral head may
be palpated thorough the capsule. -
After creating the slot, harvest abundant
corticocancellous strips with a gouge from the outer wall of the ilium.
It is perfectly acceptable to take a full-thickness graft from the
crest in the area just behind and including the anterior superior
spine. This will facilitate closure, although it will diminish the
normal pelvic contours. It is important, however, that the bone graft
be no thicker than unicortical when placed, so that it will mold to the
convexity of the femoral head. -
Pack the grafts into the slot, first in a
radial direction and then perpendicularly. Bring the previously tagged
reflected head of the rectus back over the entire mass of bone graft,
helping to seat it, and anchor it on the superior capsule. Tie the
reflected head of the rectus back to the original straight head of the
rectus. The purpose of retying the reflected head is simply to hold the
graft in place. -
Closure is routine. A single spica cast
may be used if the patient is hemiplegic or if the opposite hip has a
fixed abduction contracture. However, it is usually preferable in
patients with CP to use a one-and-a-half spica or a double spica cast
if both hips are done simultaneously.
posterior as well as superior. It is easier to pack grafts superiorly
and anteriorly, but it is probably more important in a sitting patient
to cover the posterosuperior femoral head. It is possible to pack too
much graft over the femoral head. In this situation, abduction will be
limited, although one would expect remodeling to eventually occur. An
acetabular augmentation is usually not needed in a very young patient,
4 years or younger. If it is placed,
one
cannot expect the augmented portion of the acetabulum to enlarge
because there is no growth cartilage in the shelf itself. Therefore,
the femoral head as it grows may gradually “outgrow” its socket.
is skeletally mature with a high-riding dislocation, the alternatives
are arthrodesis, resectional arthroplasty, or total hip arthroplasty.
None of these procedures is highly desirable for the majority of
patients (3,12). All
are salvage procedures, and all have considerable complications.
Femoral head and neck resection usually improves perineal care in the
adult patient with a severe high-riding dislocation and severe
contracture. However, subsequent heterotopic ossification is common,
and some of the patients still have pain despite femoral head and neck
resection. In such cases, it is desirable to interpose some type of
soft tissue, such as by closing the hip capsule or by sewing the psoas
tendon to the stump of the proximal femur. There is no agreement as to
how much of the femur to resect, but it should certainly be enough to
allow a full range of motion. This usually means that the resection
must be down to the level above, at, or just below the lesser
trochanter. This is certainly much more bone resection than would be
done in any other Girdlestone-type resection. The patient should not be
immobilized in a spica cast but may be placed in skeletal traction for
2 weeks. If the patient will tolerate it, an articulated external
fixator is a reasonable alternative. As femoral head and neck resection
is a salvage procedure, perform it only in total-care, severely
involved patients to improve nursing care and comfort.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
HH, Green WT. Adductor Myotomy and Obturator Neurectomy for the
Correction of Adduction Contracture of the Hip in Cerebral Palsy. J Bone Joint Surg Am 1960;42:11.
EE. Postural and Gait Abnormalities Caused by Hip-Flexion Deformity in
Spastic Cerebral Palsy: Treatment by Iliopsoas Recession. J Bone Joint Surg Am 1971;53:1468.
MM, Abram E, Nickel VL. Salvage Surgery at the Hip to Improve Sitting
Posture of Mentally Retarded, Severely Disabled Children with Cerebral
Palsy. Dev Med Child Neurol 1972;14:51.
MM, Barakat G, Koffman M. Ten-Year Followup of Split Anterior Tibial
Tendon Transfer in Cerebral Palsied Patients with Spastis Equinovarus
Deformity. J Pediatr Orthop 1985;5:432.
TF, Kauffer H, Hensinger RN. Split Posterior Tibial Tendon Transfers in
Children with Cerebral Spastic Paralysis and Equinovarus Deformity J Bone Joint Surg Am 1985;67:186.
LA, Mooney JF, Smith BP, et al. Management of Spasticity in Cerebral
Palsy with Botulinum-A Toxin: Report of a Preliminary, Randomized,
Double-Blind Trial. J Pediatr Orthop 1994;14:299.
RT, Harbuz A, Aronson DD, Lee CL. Postoperative Migration of the
Adductor Tendon after Posterior Adductor Transfer in Children with
Cerebral Palsy. Dev Med Child Neurol 1992;34:787.
VS. Calcaneal Lengthening for Valgus Deformity of the Hindfoot: Results
in Children Who Had Severe, Symptomatic Flatfoot and Skewfoot. J Bone Joint Surg Am 1995;77:500.
R, Frost HM. Cerebral Palsy: Spastic Varus and Forefoot Adductus,
Treated by Intramuscular Posterior Tibial Tendon Lengthening. Clin Orthop 1971;79:61.
DH, Silberfarb JL, Kaufman KR, et al. Psoas Release at the Pelvic Brim
in Ambulatory Patients with Cerebral Palsy: Operative Technique and
Functional Outcome. J Pediatr Orthop 1997;17:563.