THE ORTHOPAEDIC MANAGEMENT OF MYELODYSPLASIA AND SPINA BIFIDA
IX – PEDIATRIC DISORDERS > CHAPTER 179 – THE ORTHOPAEDIC MANAGEMENT
OF MYELODYSPLASIA AND SPINA BIFIDA
characterized by abnormality of the closure of the neural tube. They
constitute a group of disturbances including failure of full
development of structures derived from the neural tube and the
meninges. These disturbances result in abnormal neural control of
organs innervated by the affected part of the spinal cord. This
abnormal innervation gives rise to multiple organ involvement,
including effects on bladder and bowel, as well as denervation of
muscles resulting in paralysis.
allowing children to fulfill their maximum physical and social
potential within the limitations imposed by the congenital anomaly.
because it allows us to predict the functional status of the child.
Assessment should be performed as early as possible and repeated
annually, using the grading indicated in Table 179.1
with accurate recording of muscle power. Neurosegmental level can be
confirmed and any significant deterioration in neurologic status
requiring investigation for a tethered cord or cord syrinx is easily
detected (Fig. 179.1). Assessment of the level becomes more accurate after the age of 5 years, when the child is more cooperative.
 |
|
Table 179.1. Coding of Muscle Strength
|
 |
|
Figure 179.1.
In hemimyelodysplasia, there is gross asymmetry of the neurosegmental level, often resulting in leg-length discrepancy. The difference in leg lengths can usually be managed by epiphyseodesis in the long limb, but when there is gross discrepancy, leg lengthening may be more appropriate. (Reproduced with permission from Menelaus MB. Orthopaedic Management of Spina Bifida Cystica, 2nd ed. Edinburgh: Churchill Livingstone, 1980.). |
description of nerve root innervation to define a precise level and
code the power in the muscle as shown in Table 179.2.
Assessment of quadriceps and hip abductor power provide a reasonable
estimate of walking ability. Community ambulation is unlikely if the
quadriceps and hip abductors have no power; community ambulation with
splints and aids is likely if quadriceps are innervated but hip
abductors are not; and community ambulation is probable if both
quadriceps and hip abductors have power. The prediction of walking
ability is important in determining the type and extent of surgical
treatment required to correct deformity in childhood.
 |
|
Table 179.2. Assessment of Neurosegmental Level
|
innervated at the same or higher level than the tibialis anterior and
has confirmed that the medial hamstrings are innervated at a higher
level than was originally described (15). However, further work is necessary to determine the significance of these findings.
determined by the developmental defect. However, further lower motor
neuron lesions may be caused by postnatal drying and infection of the
neural plate. If the child is expected to survive, this should be
avoided by closure of the defect within 24 hours. Shurtleff has also
demonstrated that trauma to the exposed neural plate at vaginal
delivery can produce further lower motor neuron lesions; this problem
can be prevented by electing to deliver known spina bifida fetuses by
cesarean section at 36 weeks’ gestation (20).
Lower motor neuron lesions can also be produced in childhood when
growth produces traction on a tethered cord, damaging nerve roots.
lowest innervated myotome. The resulting muscle imbalance can, in some
cases, predict the likely deformity. For example, in a limb where there
is innervation down to and including L-5, indicated by activity in the
tibialis anterior, peroneus tertius, and the extensor digitorum longus
with no activity in soleus or gastrocnemius, a calcaneus or
calcaneovalgus deformity of the foot commonly occurs.
greatest where the muscle imbalance was greatest, that is, with
innervation to L-2, L-3, and L-4 (19). However,
our findings have been that hip flexion contracture is most common in
children with a thoracic level lesion where there is no muscle activity
at the hip. There is no evidence of spasticity in patients with the
most severe flexion contracture at the hip (2,21). Fixed hip deformity is not predictable from the state of muscle activity around the hip.
but our studies have shown that about 30% of hips with no muscle
imbalance dislocate, whereas 70% of hips with innervation to L-4 and
presumed to have the greatest muscle imbalance neither dislocate nor
require any operation. Dislocation of the hip in spina bifida is not
inevitable when there is muscle imbalance across the hip (2).
also raise doubts about the role of muscle imbalance in producing fixed
deformity (24).
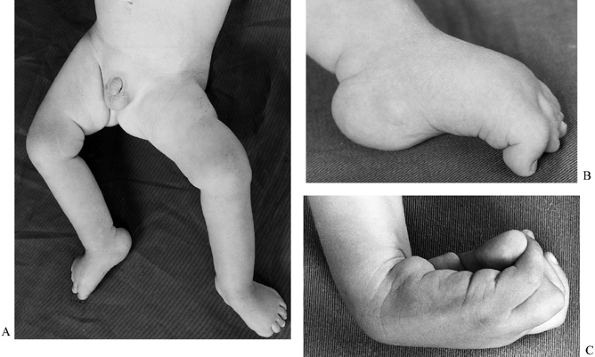 |
|
Figure 179.2. Spasticity has resulted in deformity in the upper and lower limbs of this child. A: An abduction contracture has developed at the hip. This was corrected by a lateral release. B: Spasticity of flexor hallucis longus. C: Spasticity of wrist flexors and the intrinsics in the hand. (B and C reproduced with permission from Menelaus MB. Orthopaedic Management of Spina Bifida Cystica, 2nd ed. Edinburgh: Churchill Livingstone, 1980.)
|
flaccid paralysis is present, but in levels more distal, there is a
spastic paralysis with exaggerated reflex activity. In the second
pattern, there is only a narrow segment of flaccid paralysis
(resembling spinal cord transection) and purely reflex activity below
the level. In the third pattern, there is incomplete transection and
hence myotomes can show spasticity with increased reflexes, but there
is also a degree of voluntary movement.
innervated, or spastic, various degrees of imbalance may be produced.
Although muscle imbalance between flaccid and spastic muscles can give
rise to severe deformity of the foot, the argument for this is less
convincing at the hip or knee, and other explanations for severe
deformity should be sought.
with myelomeningocele at birth, is about 20°, and this angle decreases
with lesions at all levels up to the age of 1 year. In children with a
lesion at the thoracic level, this decrease is less than in other
levels. Intrauterine pressure causes various patterns of foot deformity
at birth in paralyzed feet.
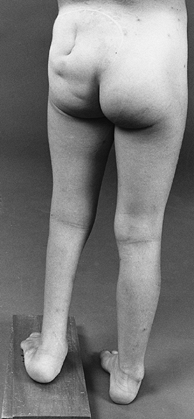
external rotation and abduction deformities of the hips if they are
allowed to lie for long periods in this position. Prolonged sitting may
be a factor in the development of flexion deformity at the hips and
knees in high-level lesions.
of joints and absence of normal creases. These deformities may be
present at one or more joints, are resistant to standard management,
and require radical surgery for their correction.
stable posture for sitting or walking. Hip flexion contractures and
knee flexion contractures give rise to difficulties in standing and
walking, as well as increased energy expenditure. Successful treatment
of these deformities ensures a stable posture.
spina bifida is ineffective and gives rise to cast sores and pathologic
fractures; therefore, in general, surgical intervention is preferable.
unnecessary operations that confer no benefit on the patient, to
perform multiple operations at the same time, and to use the minimum
period of postoperative immobilization possible. We plan surgery with
an awareness of what the child is likely to achieve in walking in the
long term so that the surgical goals are realistic (11,12 and 13).
unlikely to continue effective walking into adolescence. Until then, we
encourage them to walk and they enjoy standing.
As
their condition progresses, we use reciprocating gait orthoses or
hip-knee-foot orthoses (HKFOs). They will require increasing use of a
wheelchair with time. Surgery aims at a trouble-free wheelchair
existence. We correct foot deformity of a degree that precludes normal
footwear, generally by soft-tissue release. Extensive hip surgery is
not indicated, but we aim to correct excessive flexion contracture by
soft-tissue release to prevent excessive lumbar lordosis.
rarely community ambulators as adolescents, but the time spent walking
in braces may be longer than in children with thoracic level lesions,
so surgery to improve brace fitting should be considered.
with strong quadriceps are usually good walkers. They usually require
an ankle-foot orthosis to stabilize the ankle. The need for crutches or
walking aids depends on the strength of the abductors at the hip. The
surgeon should assume that the child will achieve worthwhile walking,
and therefore, extensive surgery to correct deformities at the hip,
knee, and foot may be justified. Furthermore, soft-tissue surgery is
justified for these patients during childhood, recognizing that bony
surgery, such as triple arthrodesis, may be necessary at maturity.
including hip control orthoses, but as confidence builds up, the extent
of the bracing can be reduced to below the knee.
community ambulators until adulthood, when some deteriorate and cease
walking. Hip deformity is uncommon, but surgery is often necessary for
foot and toe deformities.
We have now shown that the hip flexion contracture is most common and
most severe in children with thoracic neurosegmental level (2).
Dislocation of the hip is also common in thoracic neurosegmental level,
whereas in children with innervation to L-4, who presumably have
maximum muscle imbalance, only a third develop hip dislocation or
require hip surgery (2). Therefore, muscle
imbalance is one of a number of factors implicated in the development
of hip flexion contracture and hip dislocation. Prophylactic surgery to
correct muscle imbalance is no longer undertaken because many children
with muscle imbalance develop no deformity or dislocation at the hip.
neonates and in neonates with spina bifida with all neurosegmental
levels. The deformity decreases during the first year of life but
improves least in thoracic neurosegmental levels (2,21), and in these patients, it has a strong tendency to increase again with advancing years.
ability to walk (generally this occurs when the deformity is greater
than 20°), we perform a soft-tissue release.
-
Drape the patient so that Thomas’ test can be performed throughout the procedure.
-
The procedure is best performed through the incision described by Salter for innominate osteotomy (see Chapter 166).
-
Sweep the muscles off both the inner and
the outer surfaces of the ilium, divide the psoas tendon, release the
sartorius and rectus femoris, and if necessary, divide the anterior
capsule of the hip joint transversely. -
At the conclusion of this procedure, the
anterior superior iliac spine and adjacent portions of the iliac crest
will protrude forward and should be removed.
If the hip is also dislocated, consider reducing the hip along the
lines described in the next section. In a thoracic-level nonwalker,
correction of the hip flexion contracture is commonly attempted without
reduction of a dislocated hip (Fig. 179.3A, Fig. 179.3B, Fig. 179.3C).
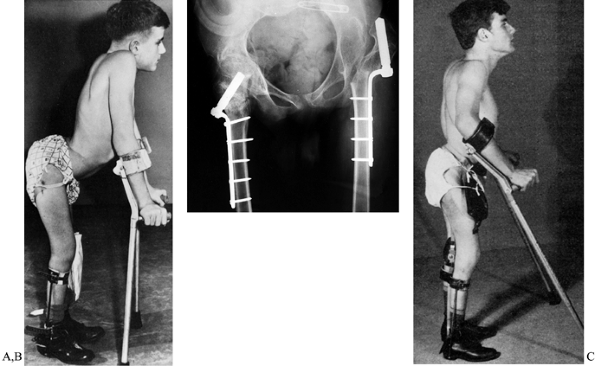 |
|
Figure 179.3. A:
Undesirable flexion posture in a boy with L-4 lesion. He has strong psoas and quadriceps muscles but gluteal weakness and has developed fixed flexion deformity of both hips. Note the gross lumbar lordosis and that his center of gravity is in front of rather than directly over his feet. B: We are usually able to correct the deformity with an extensive soft-tissue anterior hip release. However, on this occasion, because of the severity of the deformity, extension femoral osteotomies have been performed. No attempt was made to reduce the left hip; he remains a community ambulator. C: Postoperatively he has a much better extension posture, although some of his lumbar lordosis is fixed. The operation was performed 25 years ago and the patient is still walking. (Reproduced with permission from Menelaus MB. Orthopaedic Management of Spina Bifida Cystica, 1st ed. Edinburgh: Churchill Livingstone, 1971.) |
prone and supine, with the hips extended for 6 weeks. We also use low
trolleys on casters so the child can lie prone on these and move around
the house using the arms.
attempt reduction. Reduction should be attempted only when the child
will derive considerable benefit from this procedure (6,7,10).
Attempts at operative reduction are not always successful, may result
in stiff painful hips, are sometimes associated with heterotopic
calcification, and generally reduce the child’s ability to walk. An
untreated dislocated hip is rarely painful, but if the dislocation is
unilateral, it may give rise to limb-length discrepancy, which is
troublesome for walkers and can cause seating difficulties for
wheelchair users. Most specialists would agree that there is little
difference in walking ability of children with high neurosegmental
levels who have located or dislocated hips (Fig. 179.4A, Fig. 179.4B).
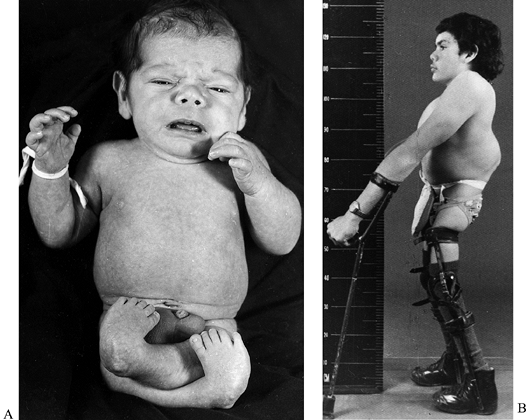 |
|
Figure 179.4. A:
This child was born with severe arthrogrypotic deformities of the lower limbs. He was treated by soft-tissue releases on one occasion, but his bilateral hip dislocations were not reduced. B: Same child at the age of 17 years demonstrating a satisfactory extension posture in KAFOs. He is now 32 years old and remains a household ambulator. (Reproduced with permission from Menelaus MB. Orthopaedic Management of Spina Bifida Cystica, 2nd ed. Edinburgh: Churchill Livingstone, 1980.) |
at L-3 and below. They usually have strong quadriceps, do not require
bracing above the knee, and will probably be good walkers. In this
group, unilateral hip dislocations should generally be reduced (Fig. 179.5A, Fig. 179.5B, Fig. 179.5C, Fig. 179.5D and Fig. 179.6A, Fig. 179.6B).
In general, bilateral hip dislocations in this group do not benefit
from attempts at reduction, but reduction is occasionally performed if
the dislocated hips are not high and surgery is necessary in any case
for hip flexion contracture. In children with high-level lesions
(thoracic, L-1, and L-2) and therefore weak quadriceps, bilateral hip
dislocations should not be reduced. Children with unilateral hip
dislocations may benefit from reduction if the dislocation is not
gross, the other leg has a low lesion, and surgery is necessary anyway
to correct hip flexion deformity. We have seen few problems from
leaving a unilateral hip dislocation untreated in a child with a
high-level lesion (Table 179.3).
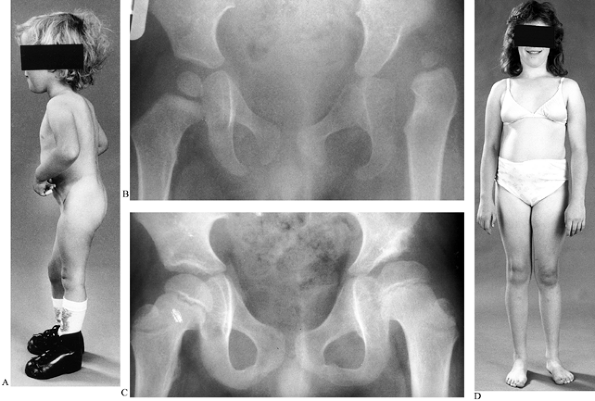 |
|
Figure 179.5. A: This child had normal muscle power in her right leg but an L-5 lesion on the left. B: A radiograph demonstrates dislocation of the left hip. C:
Same child treated by open reduction, psoas lengthening, and a Pemberton osteotomy. The follow-up radiograph shows a concentric reduction. D: At the age of 15 years there is little leg-length discrepancy, but note the left calf is slightly smaller. (A and B reproduced with permission from Williams PF, Cole WG. Orthopaedic Management in Childhood. London: Chapman and Hall, 1991) |
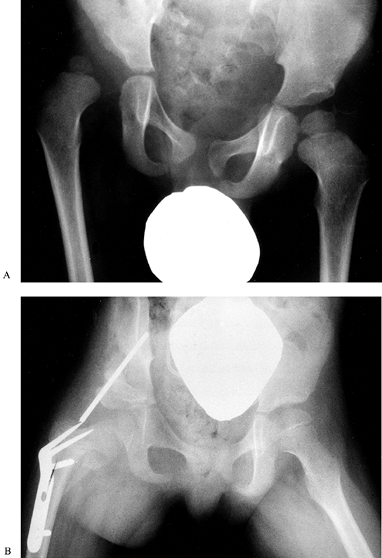 |
|
Figure 179.6. A: Unilateral hip dislocation in a low-level lesion. B: This dislocation has been treated by open reduction, Pemberton osteotomy, and a femoral osteotomy.
|
 |
|
Table 179.3. Indications for Reduction of Hip Dislocation in Spina Bifida
|
irreducible, attempts at reduction should not be made because it will
lead to stiffness. If the hip is unstable but can be easily reduced by
Ortolani’s test and the lesion is low, it is worthwhile using abduction
bracing to encourage acetabular development. However, the brace should
not interfere with closure of the spinal defect and its postoperative
management.
minimum of postoperative immobilization. The principles of the
operation are
-
to achieve a concentric reduction of the femoral head with capsulorrhaphy and excision of intra-articular structures (see Chapter 166).
-
correction of muscle imbalance by flexor and adductor releases.
-
improvement in acetabular coverage,
generally by a Pemberton osteotomy. This is performed through an
approach similar to that described for a soft-tissue release of the hip
(see the previous section).
when it was thought that muscle imbalance caused hip dislocation and
contractures. Because we now know that the relationship between muscle
imbalance and hip deformity is less clear, we believe that there is no
place for the use of prophylactic muscle transfers, because prediction
of development of deformity is not possible from muscle strengths.
appropriate in selected circumstances, but there is general agreement
now against the use of posterior iliopsoas transfer (19) because gait analysis has established that the energy cost following this procedure is unacceptably high.
with a low lumbar lesion who might have a better gait following muscle
transfer (gait analysis may facilitate the decision-making process),
then the triple transfer, described
by Yngve and Lindseth (25),
or external oblique transfer alone would seem to be the most logical,
considering our present state of knowledge. The triple transfer
consists of external oblique transfer to the greater trochanter,
transfer of the adductor origin posteriorly, and posterior transfer of
the origin of tensor fascia lata, often in combination with a varus
derotation femoral osteotomy to improve gait and reduce the risk of
dislocation. Transfer of the iliopsoas to the anterior greater
trochanter, as described by Mustard (16), frequently leads to fixed flexion deformity. The indications for muscle transfers at the hip are now few.
of the knee are most common in children with thoracic neurosegmental
lesions and not in children in whom there is muscle imbalance at the
knee (24).
improves spontaneously. If there is a fixed flexion deformity of
greater than 20° in the neonate, then serial casting may be necessary.
If a fixed flexion deformity is found in a child older than 3 years of
age who has the potential for walking, we perform a soft-tissue
release. Knee flexion correction often occurs with hip flexion
contracture, and both should be corrected at the same time. Knee
flexion is corrected by releasing all of the hamstring tendons at the
knee. Posterior capsulotomy of the knee is usually necessary to achieve
full correction (14) (Fig. 179.7A, Fig. 179.7B, Fig. 179.7C).
It is important that a full correction of the flexion deformity is
obtained at operation because recurrence follows incomplete correction
and further correction with serial plaster casts is usually incomplete.
Transfers of the hamstrings into the patella or lower femur have
generally been disappointing and are not advised. An extension
supracondylar osteotomy is infrequently used for flexion deformity in
the child approaching skeletal maturity.
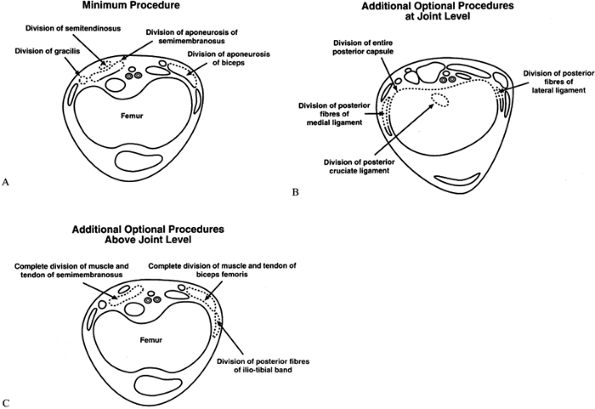 |
|
Figure 179.7. A: Transverse section at the distal femur showing the minimum procedure for soft-tissue release. B: Additional procedures at the knee level that may be necessary to achieve a full correction. C:
Additional procedures above the joint level that may be necessary to achieve a full correction. (Reproduced with permission from Marshall PD, Broughton NS, Menelaus MB, Graham HK. Surgical Release of Knee Flexion Contractures in Myelomeningocele. J Bone Joint Surg 1996;78B:912.) |
deformity of the knee, but this usually responds to serial casting. The
knee may be held rigidly in extension and have the featureless
appearance of arthrogryposis multiplex congenita. Tenotomy of the
ligamentum patella (18) is best performed at
about 6 years of age, when the extension deformity becomes troublesome
as the legs grow. At this age the child can manage knee locks on a
long-leg brace without assistance. Walking in a knee-ankle-foot
orthosis can begin a few days after the operation. The child uses night
splints at 90° of flexion for 2 months. Quadricepsplasty is usually not
indicated because there is usually insufficient voluntary power in the
muscle to make the procedure worthwhile.
managed by medial growth plate stapling, and it seldom requires lower
femoral or upper tibial osteotomy.
with a valgus ankle. It should be treated after the age of 8 years by
supramalleolar rotational osteotomy of the tibia, which can be combined
with correction of the valgus ankle at the supramalleolar level (see
later).
can be corrected by transfer of the semitendinosus to the biceps
femoris, we have now largely abandoned the procedure because such an
indication is seldom present. Gross degrees of the deformity are
corrected in the first 3 years of life by osteoclasis of the tibia and
fibula (Fig. 179.8) or at a later age by supramalleolar external rotation osteotomy.
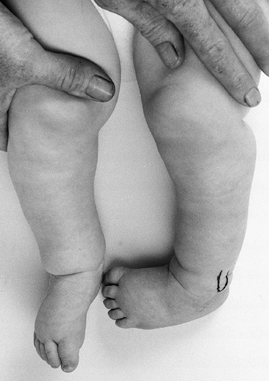 |
|
Figure 179.8. Internal tibial torsion of the left leg. This problem was treated by tibial osteoclasis.
|
all levels of neurosegmental defect. In patients with high-level
lesions, 89% of feet are deformed despite the absence of muscle
imbalance (3). Of those with low-level lesions,
76% are deformed, and the incidence of various deformities are similar
to those encountered in high-level lesions (9).
Those with calcaneus deformity have a higher incidence of activity of
the tibialis anterior with calf weakness but calcaneus is common in the
absence of this muscle imbalance. Spasticity is present in a high
percentage of patients with high-level lesions but in none of those
with undeformed feet. Spasticity is much less common (ratio of 1:2) in
patients with low-level lesions.
achieve a plantargrade and mobile foot. However, there are occasions
when triple arthrodesis is the most appropriate method to correct a
complex deformity. The lateral inlay technique (23) has proved particularly valuable in patients with spina bifida (Fig. 179.9A, Fig. 179.9B, Fig. 179.9C). Long-term follow-up has confirmed this impression (17).
Triple arthrodesis must be used with caution in the older ambulatory
patient because of the risk of plantar pressure sores in a rigid foot
that is devoid of sensation.
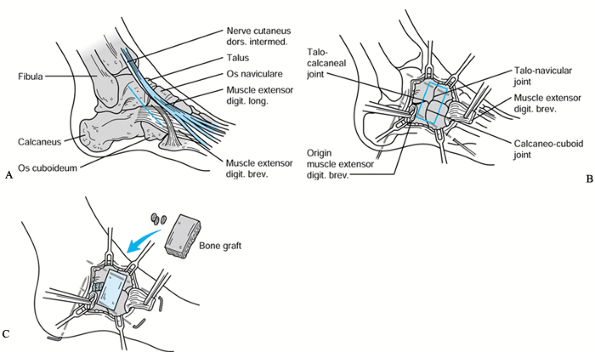 |
|
Figure 179.9.
Surgical technique for triple arthrodesis as originally described by Eric Price of Melbourne; the “inlay triple arthrodesis.” (Redrawn with permission from Romness MJ, Menelaus MB. Inlay Triple Arthrodesis: A Technique for the Undeformed or Valgus Foot. Orthop Traumatol 1995;4:114.) |
-
Make a straight incision centered over the junction of the four bones to be fused (Fig. 179.9A).
-
Lift the extensor digitorum brevis
distally and retract the extensors medially to allow exposure of the
talonavicular, calcaneocuboid, and subtalar joints. Excise the capsules
and expose the sinus. -
Hold the foot in the desired position and
insert 4.5 mm wires through the talonavicular, the calcaneocuboid, and
the subtalar joints (Fig. 179.9C). Cut a trough
as shown and decorticate the sinus tarsi. Take an oblong-shaped graft
from the upper third of the ipsilateral tibia and punch it into the
trough to give a tight fit. Insert chips of bone graft into the
subtalar joint.
that seen in the usual form of talipes equinovarus to the more commonly
seen rigid, “arthrogrypotic” deformity, which has a high rate of
recurrence despite apparent adequate correction initially. In general,
a varus deformity requires operative correction to establish a
plantigrade foot; otherwise, pressure sores due to weight bearing on a
small area of the sole are inevitable.
in well-padded plaster casts that are changed frequently while the baby
is still hospitalized after birth, and later at 2- to 4-week intervals
as circumstances demand. Although it may not be apparent at birth, the
tendo Achillis is usually short, and a closed tenotomy should be
performed when convenient, between 3 and 6 months of age.
does not require surgery for concomitant conditions in other systems,
we perform a posteromedial release. The Cincinnati surgical approach
for this technique is described in Chapter 167.
Because recurrence is likely, excision of portions of the tendo
Achillis, tibialis anterior, tibialis posterior, and the long toe
flexors is necessary rather than lengthening. If the deformity recurs,
we perform a repeat soft-tissue release.
trophic ulceration is likely, so in cases in which repeat soft-tissue
release is not controlling the deformity, further procedures are
necessary. Although we have used talectomy for this situation in the
past, we now prefer variations of the Vereberlyi-Ogston procedure with
decancellation of the talus and cuboid to allow collapse of these bones
and correction of deformity (22). Tendon transfers have no part in the management of this deformity (see Chapter 167).
it is wise to accept the deformity, provide appropriate footwear, and
aim to correct the deformity by triple arthrodesis at skeletal maturity.
any neurosegmental level. It responds well to open or closed tenotomy,
depending on the severity of the deformity and the age of the child.
most commonly seen in children with an L-5 neurosegmental lesion but
also commonly seen in the absence of muscle imbalance. The major
problem with calcaneus deformity is the development of pressure sores
because large forces are transmitted through a small area of
insensitive heel. This deformity is usually left untreated until muscle
power can be properly assessed at the age of about 3 years.
anterior is of normal strength, a transfer of the tibialis anterior
through the interosseous membrane to the heel is appropriate, although
some surgeons simply lengthen it. If extensor hallucis longus, extensor
digitorum longus, or peroneus tertius are active, they should be
divided at the same time. If the calcaneus deformity is fixed, we
combine this procedure with a full anterior ankle capsulotomy to allow
correction of the deformity (1).
a tenodesis of the tendo Achillis to the lower fibular metaphysis. This
procedure may stimulate growth at the lower end of the fibula and
correct any tendency to develop ankle valgus.
with a “pistol-grip” heel, an osteotomy of the calcaneus removing a
wedge based posteriorly improves the weight-bearing area. Concomitant
valgus of the heel may be corrected by adding varus to the osteotomy.
This can be performed in combination with tendon transfers and
tenodesis as appropriate. A plantar release may be required as well in
the treatment of concomitant cavus deformity.
feet and can usually be controlled by orthoses until adolescence. The
precise site of bony deformity should be identified; it may be at the
ankle, at the subtalar joint, or at both these sites. Clinical
examination and weight-bearing radiography enable the precise site of
deformity to be identified.
distal end of the fibula can be palpated proximal to the tip of the
medial malleolus. On the radiograph, the distal fibular growth plate
lies proximal to the dome of the talus, as opposed to the normal
relationship for that age. This appearance is associated with a
wedge-shaped distal tibial epiphysis.
tendo Achillis tenodesis to the fibula if this procedure is indicated
for coexisting calcaneus deformity. The valgus effect of the
wedge-shaped distal tibia epiphysis can be corrected by a medial arrest
of the distal tibial growth plate either by the insertion of a screw
from the tip of the medial malleolus across the growth plate or by the
Phemister technique. To be effective, this procedure must be performed
before the child is 7 to 8 years of age. After this age, a
supramalleolar osteotomy is indicated if the deformity is sufficiently
severe to be producing undue pressure on the skin over the medial
malleolus, where its excessive prominence rubs on ankle-foot orthoses
or footwear. This is done 1 cm above the growth plate with excision of
a medially based wedge, together with an oblique distal fibular
osteotomy. The tibial osteotomy is fixed with two crossed K-wires. Any
rotational deformity can be corrected at the same time. We have
experienced a 5% incidence of wound breakdown and delayed union
following this procedure.
children younger than the age of 10 years, but division of spastic
peroneal muscles may be necessary. If an orthosis fails to control this
problem, we would now avoid subtalar fusion and perform a calcaneal
osteotomy by excising a medially based wedge and also shifting the
distal calcaneus medially. The position is held by a Steinmann pin. If
the presentation is close to skeletal maturity, it is commonly
associated with a planoabductus deformity, which is best treated by a
lateral inlay triple fusion (23).
corrected as described in the previous section. If the patient is
nearly mature, this may well necessitate a supramalleolar osteotomy
with a lateral inlay triple fusion.
and rigidity of the deformity and the age of the child. Minor deformity
can be observed and any pressure effects minimized with an appropriate
insole. Treat progressive deformity for children up to the age of 5
years by a plantar release procedure. For children older than 5 years,
soft-tissue release may have to be combined with osteotomies at the
bases of all metatarsals. If there is an element of supination in the
forefoot deformity and the hindfoot is mobile, the osteotomies may be
limited to the first or first and second metatarsal bases to improve
the weight- bearing area of the foot. Osteotomy of the calcaneus is
indicated
if there is an associated varus deformity of the heel. Triple
arthrodesis is indicated if there is a significant varus and cavus
deformity in the child close to skeletal maturity.
less than 2% of children with spina bifida. It can present at birth and
is then similar to the congenital vertical talus that is not associated
with spina bifida. It can also occur in a less rigid form, which
develops slowly over the first years of life (5).
of the talonavicular (and sometimes the calcaneocuboid) joint and
correction of the ankle equinus and heel valgus. These objectives can
be achieved through a Cincinnati surgical approach (see Chapter 167). The operation is best performed in the first year of life.
-
Section the tibialis anterior, extensor digitorum longus, and the peroneal muscles, and lengthen the tendo Achillis.
-
Perform a lateral release of the subtalar
joint and, if the valgus deformity is gross, insert a lateral bone
block into the subtalar joint. -
Maintain correction with longitudinal and
vertical K-wires for 4 to 6 weeks, as well as immobilization in a cast
for 3 months postoperatively. -
Use a carefully molded ankle-foot orthosis to control planus, heel valgus, and the flail ankle.
until maturity. Ankle fusion in children has a high failure rate, as
does pantalar fusion.
lesions. In the second to fifth toes, open flexor tenotomy and closed
extensor tenotomy generally corrects the mobile deformity sufficiently
to prevent pressure effects on the tip or dorsum of the affected toe.
Rigid deformity requires interphalangeal arthrodesis and extensor
tenotomy, as well as dorsal release at the metatarsophalangeal joint.
over the first metatarsal head and over the dorsum of the
interphalangeal joint. If the deformity is correctable in the younger
child, tenodesis of flexor hallucis longus to the proximal phalanx
corrects the deformity but may have to be combined with a dorsal
capsulotomy of the first metatarsophalangeal joint. In the older child,
when the interphalangeal joint deformity is fixed, we perform a Robert
Jones procedure with fusion of the interphalangeal joint and transfer
of the extensor hallucis longus to the neck of the first metatarsal (Fig. 179.10).
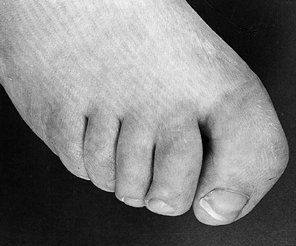 |
|
Figure 179.10.
Interphalangeal valgus and pronation of the hallux. This problem was treated by interphalangeal arthrodesis. (Reproduced with permission from Menelaus MB. Orthopaedic Management of Spina Bifida Cystica, 2nd ed. Edinburgh: Churchill Livingstone, 1980.) |
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
MB. Orthopaedic Management of Children with Myelomeningocele: A Plea
for Realistic Goals. Dev Med Child Neurol 1976;37(Suppl):18.
PS, Broughton NS, Menelaus MB. Tenotomy of the Ligamentum Patellae in
Spina Bifida: Management of Limited Flexion Range at the Knee. J Bone Joint Surg 1995;77-B:832.
TD, Gross, RH, Low W, Barringer W. Management of the Resistant
Myelodysplastic or Arthrogrypotic Clubfoot with the Vereberlyi-Ogston
Procedure. J Pediatr Orthop 1984;4:705.
