ANTERIOR LUMBAR INTERBODY FUSION
VIII – THE SPINE > Disc Injury and Degeneration > CHAPTER 146 –
ANTERIOR LUMBAR INTERBODY FUSION
orthopaedic spine surgery over the past 10 years as anterior lumbar
interbody fusion (ALIF). Although anterior fusions have been performed
on the spine for more than 50 years, it is only recently that interest
in the procedure has exploded. Traditionally, anterior or anterolateral
approaches to the lumbar spine were performed for tumor, trauma, or
infections. In these cases, debridement, strut grafting, and
occasionally anterior fixation were used to decompress the spinal canal
or stabilize the anterior spinal column. The advantages of these
procedures included direct spinal canal decompression and
reconstruction of the weight-bearing capability of the anterior column.
Another advantage is that they avoid injury to posterior muscles.
important. Often, when posterolateral fusions were performed for
degenerative disc problems, patients would have continued complaints of
fatigue and weakness in the lumbar spine. Some of these symptoms may
have been due to injury to the paraspinal muscles leading to “fusion
disease” (19). In addition, over the past
several decades, much research has pointed to the disc as a predominant
source of chronic low back pain (see Chapter 144).
Fusion procedures that eliminate discs entirely may offer advantages
that more traditional posterior fusions do not. For both of these
reasons, the indications for ALIF have greatly expanded over the last
decade.
patients with degenerative disc disease, internal disc derangement,
spondylolisthesis, pseudarthrosis, and, occasionally, scoliosis,
trauma, or infection. Each case must be evaluated on an individual
basis to determine the appropriateness of surgical intervention.
and occasionally vacuum phenomena within the disc space. Magnetic
resonance imaging (MRI) corroborates this diagnosis, revealing “Modic”
changes in the endplates surrounding the degenerative disc (14).
Many of these changes can be traced to a previous episode of disc
herniation that had been treated either with or without surgery. Often,
in these cases, the sciatica has resolved but has been replaced by
persistent chronic midline low-back pain.
pain over the sacroiliac joints, particularly if the L5–S1 disc space
is involved. Most patients with degenerative disc disease can be
successfully treated with an aggressive physical therapy program that
includes trunk stabilization exercises and nonimpact aerobics. In the
majority, symptoms improve, and patients decide to live with a
low-level midline low-back ache. Other modalities that may be helpful
include short-term periods of bracing, administration of nonsteroidal
antiinflammatory medication, and occasionally manipulation. Epidural
steroid injection, prolonged bed rest, transcutaneous electrical nerve
stimulation units, and passive modalities such as heat, massage, or ice
have not been proven to be of benefit.
psychological profile. Office findings of pain behaviors, Waddell’s
signs, chronic narcotic use, or excessive secondary gain are
contraindications for surgical treatment (see Chapter 144).
In addition, evaluate the adjacent discs. Ideally, only a one- or
two-level fusion should be performed for degenerative disc disease.
Fusion of more than two levels leads to less satisfactory clinical
results. MRI of the adjacent disc is an excellent screening test. With
normal MRI findings, it is safe to assume that the adjacent disc can be
left unfused. If MRI is abnormal, then discography may be indicated to
evaluate adjacent levels for fusion.
abnormal MRI may have internal disc derangement (IDD). The MRI
abnormalities of IDD include decreased signal intensity within the disc
nucleus on T2-weighted images, annular tears (with or without
enhancement), and high-intensity-zone lesions. In these cases, initiate
a similar nonoperative treatment program before considering surgical
therapy. Should patients satisfy diagnostic criteria and fail to
improve with adequate conservative measures over 3–4 months, then
discography is indicated for confirmation of the diagnosis. A positive
discogram should include reproduction of the patient’s symptoms upon
injection, abnormal morphology with dye leakage through annular
disruptions, and normal adjacent-level injections without pain
reproduction (3). A patient who meets all of these criteria may be a candidate for ALIF surgery (1,6).
within the disc, thermal repair of the disc annulus, or annular
debridement. ALIF surgery, however, has the longest history of
successful results in the treatment of this condition. It must always
be kept in mind, however, that success is not universal in the
treatment of patients with IDD. Many clinical reports document success
rates in the 50% to 70% range (1,6,7,8 and 9).
Patient selection is critical. Offer ALIF only to highly motivated
patients with single-level disease who have no psychological overlay,
secondary-gain issues, or chronic narcotic use. Only strict selection
criteria will lead to an acceptable success rate from surgery.
stenosis does not require ALIF. Most patients with this condition can
be successfully treated with posterior decompression and posterolateral
fusion techniques (19). In adult isthmic spondylolisthesis, however, ALIF plays an important role (see Chapter 162).
evolved over the years. In patients in whom reduction of a
spondylolisthesis is planned, anterior interbody support is necessary
to prevent late hardware failure or pseudarthrosis. In patients with
spondylolisthesis with a well preserved disc space and translational
motion on flexion–extension films, anterior interbody support in
addition to posterior fixation is necessary to achieve a high incidence
of solid fusion. In patients with a collapsed disc space with
degenerative changes, as well as isthmic spondylolisthesis, fusion with
either anterior procedures alone or posterior procedures alone may be
successful. Interbody fusion cages alone have successfully been used in
this subset of spondylolisthesis patients. Exercise caution, however,
when using cages alone for a spondylolisthesis patient who has a
preserved disc space and hypermobility. In these cases, it is often
difficult to obtain stability through the use of anterior cages alone.
correction will be performed, anterior release and interbody fusion are
indicated. In addition, for patients with traumatic endplate disruption
or disc space infection, debridement and interbody fusion are helpful.
Finally, in cases of previous pseudarthrosis of a posterolateral
fusion, interbody fusion is often the only means of obtaining a solid
arthrodesis. In addition, it avoids dissection through a previously
scarred posterior paraspinal muscle approach.
approaches include the transperitoneal, anterior retroperitoneal, and
retroperitoneal flank approaches. For endoscopic techniques, the
anterior transperitoneal laparoscopic approach has become popular, as
has the retroperitoneal endoscopic approach using balloon insufflation
or gasless techniques. All of these approaches require an excellent
knowledge of the anatomy surrounding the middle and low lumbar spine (Fig. 146.1).
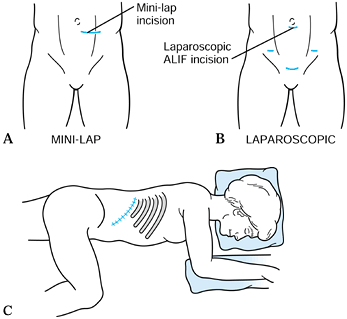 |
|
Figure 146.1. Skin incisions utilized in anterior lumbar surgery. A:
A skin incision utilized in the minilap retroperitoneal approach. A vertical paramedian incision may also be used. In this approach, the anterior rectus sheath is divided in line with the skin incision, the rectus abdominis muscle protected, and the preperitoneal space developed lateral to the peritoneal contents. B: The laparoscopic transperitoneal technique utilizes small skin incisions for the placement of the viewing camera (periumbilical portal), 5 mm incisions for lateral retractors, and a 15–20 mm incision for the placement of the fusion cages. This incision is typically placed in the suprapubic location for the L5–S1 disc space. C: Approach the lumbar interbody spaces laterally through a flank approach. Position the patient laterally with the ipsilateral hip flexed to relax the psoas muscles. Make a flank incision paralleling the twelfth rib. The position will vary, depending on the level for the fusion. For L-1 burst fractures, a typical incision will overly the eleventh rib. For L-2 or L-3 access, a twelfth rib incision is useful, and an incision midway between the iliac crest and the twelfth rib is useful for the L3–4 space. |
including the bifurcation of the aorta and vena cava and the
surrounding veins such as the iliolumbar vein or segmental vessels, as
well as the path of the ureters and the left-sided placement of the
sigmoid colon. In addition, the locations of the nerve roots as they
exit the foramina and of the presacral parasympathetic plexus should be
well known. Surgeons unfamiliar with this vascular anatomy or unwilling
to handle complications from injuries to these and other important
structures should enlist the help of a vascular or general surgeon in
exposing the anterior lumbar spine (see Chapter 138).
-
Make a midline vertical skin incision, and split the fascia between the rectus abdominis muscles (Fig. 146.2).
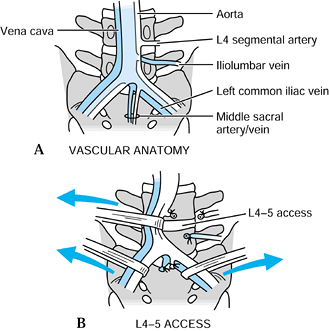
![]() Figure 146.2. A:
Figure 146.2. A:
Normal vascular anatomy of the anterior lumbar spine. Typically, the
vena cava bifurcates at a higher point than the aorta. Usually, it
bifurcates at the L4–5 disc space, and the aorta bifurcates over the
L-5 body. The iliolumbar vein is a branch of the left common iliac vein
and runs in an inferior direction at approximately 40°. B:
Access to the L5–S1 disc space is typically between the bifurcation of
the vessels. Ligation and control of the middle sacral artery and vein
is necessary for complete disc exposure. At L4–5, the most common
access pathway is to the left of both of the great vessels, with
retraction in a left-to-right direction. This requires control and
ligation of the iliolumbar vein for complete vessel mobility. Use blunt
dissection only at both levels to prevent damage to the presacral nerve
plexus. -
Enter the peritoneal cavity, and retract the small bowel superiorly and the sigmoid colon to the left laterally.
-
Typically, the aorta and vena cava
bifurcate at the level of the L-5 vertebra. There is considerable
variation, however, so check the preoperative MRI to confirm the level. -
To expose the L5–S1 disc space, incise
the posterior peritoneum vertically overlying the disc space. This
incision can be safely made between the bifurcation of the aorta and
vena cava. -
The presacral plexus of nerves runs
directly over the L5–S1 annulus at this level. To minimize damage to
this plexus, infiltrate the retroperitoneal space with saline, using a
fine needle, before dividing the posterior peritoneum. -
Cut the peritoneum without cautery, and
dissect over the disc space bluntly to minimize the risk of damage to
the nerve plexus. In men, retrograde ejaculation may result if this
plexus of nerves is damaged. -
Ligate the middle sacral artery and vein, which are adherent to the annulus, before exposing the disc.
-
At L4–5, access to the disc space is more difficult. Study the vascular anatomy preoperatively on the patient’s MRI scan (Fig. 146.2).
Occasionally, the L4–5 disc space can be approached between the
bifurcation of the vessels. Most commonly, however, a left-to-right
path to the L4–5 disc space is recommended. In this manner, the aorta
and vena cava are both retracted from the left to the right across the
L4–5 disc space. This retraction is only possible after the iliolumbar
vein is ligated. This vein descends at a 45° angle from the vena cava,
angling toward the psoas muscle on the left side. -
Once it is ligated, adequate mobility of
the great vessels is usually obtained. If possible, avoid dissection
between the aorta and vena cava. -
The presacral plexus nerves are particularly vulnerable during dissection between the great vessels.
-
At L3–4 disc space, the aorta and vena cava are more mobile, although segmental vessels need to be ligated to obtain exposure.
-
After exposure is obtained, it is
mandatory that the vascular structures be protected throughout the
fusion procedure. I prefer to drive four Steinmann pins covered with a
red rubber catheter into the endplates above and below the disc space
to be worked on. These four pins serve as self-retaining retractors,
providing a safe zone in which to perform the fusion. Other retraction
systems are available, as well as self-retaining blades that may be
staked through the vertebral endplates. -
Take particular care to avoid injury to
the vena cava and common iliac veins. Once exsanguinated by the
retraction, they are difficult to visualize and are prone to injury.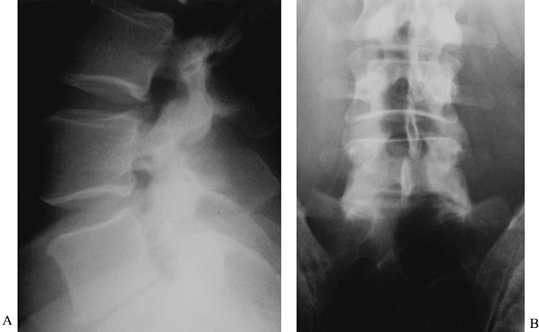 Figure 146.3. Anteroposterior (A) and lateral (B)
Figure 146.3. Anteroposterior (A) and lateral (B)
radiographs of a 39-year-old man with persistent low-back pain. He had
a history of remote sciatica, which resolved but has now developed into
persistent low-back pain despite maximal physical therapy. These
radiographs show classic changes of degenerative disc disease: a
narrowed disc space, sclerotic endplates, and marginal osteophyte
formation. The L4–5 disc was normal on MRI, and thus no discography was
indicated. -
Transperitoneal exposures of the lumbar spine above
P.3811
L-3 are difficult because of the renal and mesenteric vessels. At these
levels, the flank retroperitoneal approach offers a lateral exposure of
the lumbar vertebral bodies. This approach is most useful when
debridement for infection, trauma, or tumor is required. When
corpectomy is necessary, a lateral plate device is useful for stability
and can best be placed from a lateral approach. If debridement and
grafting alone are being performed, the anterolateral or anterior
approach is adequate.
over the area of pathology of the lumbar spine. It parallels the
twelfth rib and is anywhere from 4 to 8 in (10–20 cm) long, depending
on the size of the patient.
-
Use fluoroscopy to center this incision directly over the level of pathology.
-
Should exposure of the low lumbar spine be required as well, curve the incision across the lateral aspect of the abdomen.
-
After incising the skin, divide the
external oblique, internal oblique, and transversalis muscle layers in
line with the incision. -
Take care when dividing the transversalis to ensure that the reflection of the peritoneum is free.
-
Once the transversalis fascia is divided,
enter the retroperitoneal space, which is behind the fascia surrounding
the kidney and thus is truly behind Girota’s fascia. -
After entering the retroperitoneal space,
identify the psoas muscle, and take care to preserve the ilioinguinal
nerve lying on its surface. -
The origin of the psoas muscle is usually
at the L-1 body, and each of the lumbar nerve roots as they exit the
foramina run in the substance of the psoas muscle. For this reason, the
psoas must be retracted in an anterior-to-posterior direction to expose
the appropriate disc space. -
Perform this exposure, using elevators and cautery, controlling segmental vessel as needed.
lateral aspect of the disc and vertebral body; it is limited
posteriorly by the level of the nerve at the foramen and anteriorly by
the great vessels. If necessary, dissect the anterior longitudinal
ligament free from the vertebral bodies, and carry out subperiosteal
dissection around to the opposite side of the vertebral body. This
maneuver permits complete release of all soft-tissue structures in
cases of deformity.
rectus-sparing retroperitoneal approach. In this approach, none of the
muscles of the abdominal wall is divided, and therefore quick recovery
is possible. I prefer a transverse incision.
-
Begin in the midline, and extend the incision to the left for approximately 3–4 in (7.5–10 cm).
-
Divide the anterior rectus sheath, and retract the rectus muscle from the midline to the left.
-
Identify the posterior rectus sheath and the arcuate line.
-
Use blunt dissection beneath the arcuate
line to enter the preperitoneal space, and continue the dissection
laterally to the left until you are lateral to the peritoneal contents
and the psoas muscle can be identified. -
Place a retractor to pull the peritoneal contents toward the midline to expose the retroperitoneal space overlying the spine.
-
Identify the left ureter, and protect it
throughout the procedure. In general, in cases involving the L3-4 or
L4-5 disc space, the ureter will be retracted toward the midline with
the visceral peritoneum. At the L5-S1 space, the ureter will usually be
on the left side of the incision and will be retracted laterally. -
Identify the sympathetic chain running on the psoas muscle and protect it.
-
The dissection at this point proceeds in much the same fashion as previously described (see “Open Transperitoneal Approach” above).
-
Bluntly dissect the tissues lateral to the aorta and vena cava at the L4–5 space or between the great vessels at L5–S1.
-
At L4–5, ligate the iliolumbar vein to allow exposure.
-
At L5–S1, ligate the middle sacral artery and vein to allow blunt dissection to proceed along the annulus.
-
Several self-retaining retraction systems are available for use through this “minilaparatomy (minilap)” approach.
fusion of the lumbar spine and developed this approach along with Dr.
David Mahvi, my general surgery colleague (13,20).
The L5–S1 level lends itself to an endoscopic approach because of its
easy accessibility between the bifurcation of the great vessels (Fig. 146.4).
 |
|
Figure 146.4.
Laparoscopic transperitioneal fusion access route. Surgery consisted of anterior discectomy at L5–S1, distraction, and insertion of two tapered (LT) cages (Lumer Tspered, Sofamon Danek, Memphis, TN) at the L5–S1 disc space. Note the restoration of foraminal height and sagittal contour. |
-
Give a light bowel preparation the night before surgery.
-
Facilitate exposure by placing the
patient in the Trendelenburg position and allowing abdominal
insufflation, which causes the small bowel to drift toward the
diaphragm. It precludes the need for retraction of the abdominal
contents; thus postoperative ileus is eliminated. -
Usually, the laparoscopic camera is placed in a periumbilical incision.
-
Finally, place a suprapubic portal in
line with the disc space. At the L5–S1 level, this is often two to
three fingerbreadths above the pubic symphysis. At L4–5, it is usually
midway between the umbilicus and pubic symphysis. -
Drain the bladder with a Foley catheter before placing this portal.
-
The sigmoid colon may need to be
retracted toward the left and will usually stay in this position
throughout the procedure. The remainder of the dissection proceeds in
much the same fashion as previously described (see “Open Transperitoneal Approach” above). -
Incise the posterior peritoneum
vertically overlying the disc space. For the L5–S1 space, it is done
just to the right of the midline or, for L4–5, just to the left of the
aorta. -
Bluntly dissect the tissues in the
retroperitoneal space. Often, the presacral plexus of nerves can be
visually identified. Bluntly retract the nerves. -
At L5–S1, the middle sacral artery and
vein lie in a plane adherent to the anterior annulus. Coagulate them
with bipolar cautery. -
At L4–5, identify the iliolumbar vein
coming off the vena cava at a 45° angle and heading inferiorly and
laterally toward the psoas muscle. Ligate this vein, and retract the
great vessels in a left-to-right direction to expose the annulus of the
disc. Specially made vein retractors placed laparoscopically through
the portals facilitate retraction of the great vessels.
with magnification of the vascular structures and rapid patient
recovery. Contraindications to the transperitoneal endoscopic technique
include multiple abdominal adhesions, previous anterior spine surgery,
and severe sacral tilt such that the L5–S1 disc space is not
accessible. It is strongly recommended that this surgery be done with a
general surgeon who has laparoscopic experience.
described techniques whereby a small lateral incision is made through
the oblique muscles and a balloon is placed in the retroperitoneal
space. This balloon is then inflated, dissecting free the
retroperitoneal cavity. Once the peritoneal reflection is dissected
toward the midline, an anterolateral skin incision is made to expose
the disc space.
dissection, using a gasless system in which the anterior abdominal wall
is lifted with a retractor system. My experience with this technique is
that it can cause abdominal wall discomfort and slower patient
recovery. In all of these endoscopic systems, the cost of disposable
equipment and the time required for surgeons to learn the procedure
must be balanced against the more traditional open or mini-open
approaches. My current approach is as follows:
-
At L5–S1 disc space, the laparoscopic
transperitoneal approach is safe and quick and allows excellent
visualization of the space. -
For multilevel fusions or fusion at L4–5,
I now prefer a minilap-type open retroperitoneal exposure. It gives
more reliable control of the bifurcation of the great vessels at the
L4–5 space.
with a variety of methods. Most surgeons prefer to remove the entire
disc before fusion. This requires a rectangular block-style incision of
the annulus, removal of the annulus, and removal of all disc material
including endplate cartilage through the use of a combination of
curets, rongeurs, and elevators. In general, the annulus is left intact
laterally and posteriorly. In some endoscopic techniques,
trephine-style discectomies are preferred because it is risky to use
curets and elevators in endoscopy. These techniques also preserve more
of the anterior annulus, which helps ensure cage stability.
autogenous bone graft, allograft bone consisting of femoral rings or
fibula, and processed allografts consisting of threaded cortical dowels
combined with autograft cancellous bone. Metallic options include the
placement of a lateral plate overlying the disc space and bone graft,
as well as devices placed within the disc space itself. These devices
include anteriorly placed threaded cylindrical cages, anteriorly placed
threaded tapered cages, laterally placed cages, lateral cages plus
lateral plates, and upright cages placed within the disc space (Fig. 146.5). Other materials that have been used include carbon fiber cages and ceramic spacers.
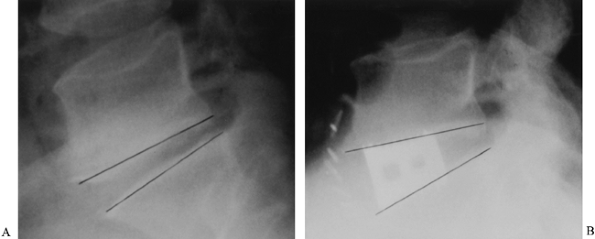 |
|
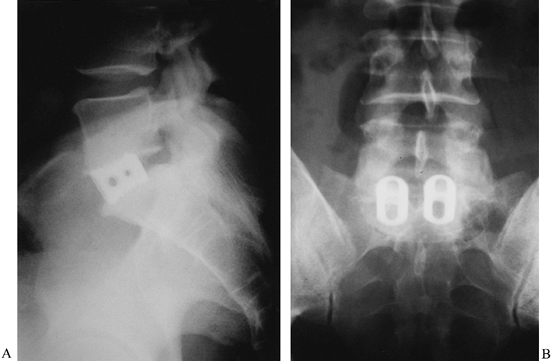
Figure 146.5. The use of tapered interbody fusion cages at L5–S1 permits the restoration of both height and lordosis. Preoperative (A) and postoperative (B) lateral radiographs demonstrate the lordosis obtained in a 48-year-old man with degenerative disc disease.
|
-
Disc space distraction to cause tension in surrounding ligamentous structures, which increases stability
-
Preparation of the endplates to expose cancellous bone, as well as providing an endplate substrate for weight bearing
-
Provision of enough bony surface area to heal from one endplate through the bone graft to the other endplate
-
Realignment of the spine to its optimal lordotic sagittal balance
-
Production of a solid, long-term arthrodesis.
interbody fusion cages are used. Disc spaces that have not undergone
any degree of collapse are difficult to further distract. Therefore,
the tall mobile disc space may not be an ideal candidate for cage-only
fusion procedures. In addition, forceful distraction is required, and
if the endplate bone is not strong enough to resist the distractive
force, subsidence and instability will result. For this reason, do not
use cages in patients with osteoporosis. Finally, most cage systems are
designed for two cages to be implanted side by side. Although some
biomechanical studies suggest that a single cage may lead to short-term
stability, it is my feeling that there is inadequate surface area to
ensure long-term arthrodesis.
the BAK device (Spinetech, Minneapolis, MN) were the initial threaded
interbody fusion cages (10). They have been
used with both posterior and anterior interbody fusion techniques. When
used anteriorly, these systems restore lordosis through patient
positioning and the placement of a tapered distraction plug.
-
After the disc space is distracted,
prepare the endplate on each side of the disc by passing a reamer to
remove endplate cartilage, bone, and disc material. Then tap each side
and place two threaded devices. -
Take great care, when placing cages, to identify the midline of the spine so that cages are not placed eccentrically.
-
Determine the appropriate size cages from the radiographs and preoperative templates.
-
A tapered threaded device has been
designed that more accurately matches the anatomy of the L5–S1 disc
space. It appears that a greater amount of lordosis can be obtained
through the use of tapered cages with a minimal amount of endplate
resection (16).
the stability of the lumbar spine without the need for supplementary
posterior fixation. They have successfully been used in patients with
degenerative disc disease or spondylolisthesis with a degenerated disc
space. I do not recommend the use of cages alone in cases of
spondylolisthesis with a tall mobile disc space.
endoscopically in the lumbar spine. Often, a single threaded
cylindrical lateral cage is utilized. Although some authors have had
success with this technique, others have shown that additional bone
grafting is necessary to provide an adequate surface area for healing.
In addition, LeHuec (11) designed a combined lateral cage and plate system to increase the rate of fusion.
Typically, two upright cylinders of titanium mesh are cut to fit the
disc space and then driven into the space to provide support for the
endplate and for healing potential. Harms recommended that posterior
instrumentation be used to supplement ALIF with upright cages.
Brantigan and Steffee (2) designed upright
carbon fiber cages. While these were also met with worldwide acceptance
and a high degree of success, most authors would agree that the upright
carbon fiber cage is not stable enough to be used as a stand-alone
device. Fraser (5) recommends that posterior
fusion, as well as facet screw instrumentation, be utilized to augment
the interbody carbon fiber cage.
must evaluate each patient on an individual basis to determine which
particular device is most appropriate.
adequate surgical planning and preoperative preparation. An accurate
understanding of the vascular and neurologic anatomy is a requirement
for the performance of these procedures. The assistance of a general or
vascular surgeon may be necessary to obtain adequate and safe exposure.
Preoperative preparation consisting of a light bowel regime (e.g.,
GOLYTELY and Fleet enema) will make retraction of intestinal structures
easier. Preoperative examination of the patient’s abdomen and flank for
prior incisions and potential adhesions is necessary. Preoperative
evaluation of MRI or computed tomography to assess variations in the
vascular anatomy is important, as it directs which approach will used
for the portion of the spine to be fixed.
and life-threatening. The incidence of injury to the aorta, vena cava,
or iliac vessels is estimated at 1% to 3% for anterior approaches (7,9).
These injuries may be life-threatening and need to be dealt with calmly
and with assistance. Obtain immediate control with tamponade. If the
procedure is being done endoscopically, immediate laparotomy is
recommended.
recommended for repair of the great vessels. The most common areas of
vascular injury are the left common iliac vein at the L5–S1 level and
the left side of the vena cava at the L4–5 level. At L4–5, there are
several small perforating veins that may come from the posterior
surface of the vena cava. They may need to be coagulated and divided
before the vena cava is retracted, to prevent their avulsion. As
mentioned earlier, control and ligation of the iliolumbar vein will
greatly assist in vena cava mobilization.
Anterior approaches to the lumbar spine in elderly patients are
hazardous and must be done only when absolutely necessary and with
caution. In many of these patients, the arterial system is much less
mobile,
and retraction may dislodge plaques. Always do a postoperative vascular
examination, and if pulses are absent or the limb is cool, obtain an
immediate consultation with a vascular surgeon. Routine anticoagulation
has not been found to be necessary in ALIF surgery. However, if a major
vessel must be repaired, postoperative anticoagulation may be necessary.
plexus, nerve root injury, and violation of the spinal canal. As
mentioned, the parasympathetic nerves that run within the presacral
plexus are vulnerable to injury. They run along the aorta and vena cava
and then between the bifurcation as they pass distally. For work along
the L4-5 or L5–S1 disc spaces, it is mandatory that only blunt
dissection be used and that no monopolar electrocautery be used. The
incidence of retrograde ejaculation following ALIF has been estimated
to be from 1% to 4%.
slightly higher in endoscopic procedures than in the open
retroperitoneal approach. Early in any surgeon’s experience, there is
certainly a higher incidence of retrograde ejaculation. Fortunately,
most cases of retrograde ejaculation are temporary and resolve within
4–6 months. It is assumed that they are due to stretch injury that
occurs during exposure of the disc space. Should the patient not
recover from retrograde ejaculation, urologic consultation is
recommended.
neuroforamina and proceed toward the psoas muscle. If the midline is
not adequately evaluated and fusions are performed far lateral to the
disc space, the nerve root is vulnerable to injury. Bone grafts or
cages that are placed too laterally or too deeply may impinge on the
neuroforamen of the level above. It is imperative to use fluoroscopy at
some point to locate the midline of the disc space and maintain
orientation as to the right and left margins of the disc space.
on the spinal canal, causing cauda equina injury. Once again,
fluoroscopic control of the implantation of interbody fusion devices
should help eliminate this complication.
occur. The epigastric vessels must be either retracted or controlled,
or a postoperative abdominal-wall hematoma may occur. Overzealous
retraction of the rectus abdominis may lead to a stretch injury and
cause weakness of the abdominal wall. During a flank approach, repair
each layer independently to prevent weakening of the abdominal wall.
incidence of a pseudarthrosis after a 360° fusion is performed is
exceedingly low, ALIF alone does carry a risk of pseudarthrosis. With
the use of only iliac crest autograft, the pseudarthrosis rate was
estimated at 30% to 35% (4,7). Femoral allograft rings have shown to have a pseudarthrosis rate approaching 20% (12). Threaded cortical bone dowels appear to have a much lower pseudarthrosis rate, but follow-up is too short to be conclusive.
Recent reports show a higher pseudarthrosis rate, however, which may be
secondary to poor surgical indications or poor surgical technique (15).
Should pseudarthrosis occur and the cages or bone dowels remain in an
acceptable position, I recommend proceeding with a posterior
instrumented fusion at that level. It will often resolve the patient’s
symptoms and may lead to healing of the interbody fusion. If a cage or
bone dowel should migrate and cause neurologic symptoms, it should be
removed at the time of revision surgery. Revision anterior interbody
surgery is dangerous and should be approached with caution. Vascular
structures often become adherent to the previously operated disc space,
and the presence of a vascular surgeon is necessary for these
challenging cases.
been estimated to be 10% to 20%. The exact cause is unknown and may be
related to patient selection. This fact alone indicates that the
treatment of low-back pain with interbody fusion is evolving. Keep in
mind that the exact cause of each patient’s low-back pain may be
unknown.
Restoration of the weight-bearing column, provision of a greater
surface for fusion to occur, and the ability to recreate the normal
sagittal position of the spine are among its greatest advantages. If
complications should occur, however, many of these advantages are lost.
To obtain a high clinical and radiographic success rate, strict
adherence to the details of the surgical approach and the fusion
technique are required.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
JW, Steffee AD. A Carbon Fiber Implant to Aid Interbody Lumbar Fusion:
Two-year Clinical Results in the First 26 Patients. Spine 1993;18:2106.
J, Urbaniak J, McCollum D. Anterior Disc Excision and Interbody Spinal
Fusion for Chronic Low Back Pain. Orthop Clin North Am 1971;2:544.
SD, Dowdle JA. Two-year Follow-up Results of an Interbody Fusion
Device. Presented at the Ninth Annual Meeting of the North American
Spine Society, Minneapolis, MN, October 19–21, 1994.
JC. Lateral Cage Endoscopic Fusion. Presented at the Thirteenth Annual
Meeting of the North American Spine Society, San Francisco, CA, October
27, 1998.
JK, Lam K, Mulholland RC, BAK Cage: Nottingham Results. Presented at
the Thirteenth Annual Meeting of the North American Spine Society, San
Francisco, CA, October 28, 1998.
RD, Andres B, Checovich M, Zdeblick TA. Results of Anterior Spinal
Fusion with the Tapered Interbody Fusion (TIF) Device. Presented at the
Thirteenth Annual Meeting of the North American Spine Society, San
Francisco, CA, October 28, 1998.
JS, Chin AK, Ameriks JA. Minimally Invasive 360° Fusion. Presented at
the Thirteenth Annual Meeting of the North American Spine Society, San
Francisco, CA, October 28, 1998.
E, Escobar G, Garvey T. Complications of View-assisted Spine Surgery.
Presented at the Thirteenth Annual Meeting of the North American Spine
Society, San Francisco, CA, October 28, 1998.
TA. A Prospective Randomized Study of the Surgical Treatment of L5-S1
Degenerative Disc Disease. Presented at the Tenth Annual Meeting of the
North American Spine Society, Washington, DC, October 20, 1995.