OSTEONECROSIS
VII – NEOPLASTIC, INFECTIOUS, NEUROLOGIC AND OTHER SKELETAL DISORDERS
> Other Disorders > CHAPTER 125 – OSTEONECROSIS
Professor and Vice Chairman, Department of Orthopaedic Surgery,
Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania,
19104.
Professor, Department of Orthopaedic Surgery, Johns Hopkins University
School of Medicine, Baltimore, Maryland, 21239.
acknowledge the contributions of Harlan C. Amstutz, M.D.; Paul E.
Beaulé, M.E., F.R.C.S.C.; Jerilyn K. Higa, M.D.; Jonathan R. Perryman,
M.D.; Yoichi Sugioka, M.D., Ph.D.; and James R. Urbaniak, M.D.
(AVN), aseptic necrosis, and ischemic necrosis, is not a specific
disease but rather a condition in which a circum-fcscribed area of bone
becomes necrotic as a result of a loss of its blood supply. The femoral
head is the site most often affected, and the most frequent cause is a
displaced fracture through the femoral neck. Posttraumatic
osteonecrosis is best dealt with in a section on fractures; therefore,
this chapter focuses on nontraumatic osteonecrosis of the adult hip. It
also includes a brief description of other areas that may be involved.
stated that only 22 additional cases could be found in the English
literature. Since that time, this diagnosis has been made with
increasing frequency because of both an increased incidence and
improved methods of diagnosis. It is currently estimated that 15,000 to
20,000 new cases are diagnosed annually in the United States alone and
that osteonecrosis accounts for approximately 10% of the total hip
replacements (THRs) performed.
much yet to learn about its etiology, pathogenesis, and treatment. A
number of etiologic factors have been implicated, although the exact
mechanisms by which they act have not been completely determined. The
most commonly identified are high doses of corticosteroids and chronic,
excessive alcohol intake. In most series, approximately 15% of the
cases are considered to be idiopathic; however, as we learn more about
etiologic factors, the number of patients placed in this category will
diminish. For example, it has recently been shown that in approximately
70% of “idiopathic” cases, subtle coagulopathies are present (47).
symptom is usually unilateral hip pain, which may be followed by a limp
and a decreased range of motion (ROM). Young adults between the ages of
30 and 40 are most frequently affected, and the condition is bilateral
in more than 50% of patients. Diagnosis is usually made from plain
radiographs, but in the earlier, asymptomatic stages, magnetic
resonance imaging (MRI) may be required.
osteonecrosis early, we still do not have completely satisfactory
methods for treating this condition. Our goal is to preserve and not
replace the femoral head. A number of procedures have been described to
accomplish this. Although an accurate comparison of their relative
effectivenesses is not yet available, surgical treatment of
osteonecrosis in general yields better results than protected weight
bearing and symptomatic management. Whichever procedure is selected, a
better outcome will be achieved if the condition is diagnosed and
treated early, well before femoral head collapse. Patients with
advanced stages of osteonecrosis are generally treated symptomatically
until such time as hip arthroplasty or other reconstruction is
indicated.
organ systems, various etiologic factors can lead to similar pathologic
changes. In most cases of osteonecrosis, a specific etiologic factor or
factors can be identified. In traumatic cases such as hip dislocation
and femoral neck fracture, there is a clear cause-and-effect
relationship between the insult, mechanical damage to the vessels that
supply the femoral head, and the resulting osteonecrosis. A number of
etiologic factors can be identified in patients with nontraumatic
osteonecrosis. These include intraosseous marrow displacement disorders
that lead to increased pressure in the femoral head and neck, such as
Gaucher’s disease, leukemia, and myeloproliferative disorders (52,58,85,144).
Other conditions, such as radiation and chemotherapy, lead to bone
necrosis by direct cell toxicity. Mechanical blockage of vessels may be
caused by emboli composed of abnormal red blood cells, such as in
sickle cell disease and thalassemia; or nitrogen bubble emboli, such as
in caisson disease, or dysbarism (59,60).
clear. In these cases, one can often identify specific factors known to
be frequently associated with osteonecrosis, although the exact
mechanisms by which they act have not been completely delineated (Table 125.1).
For example, high doses of corticosteroids and excessive alcohol intake
have been identified in nearly 80% of cases of nontraumatic
osteonecrosis (30,52,53,61,84,85,87,144).
However, a one-to-one relationship between these factors and this
disorder has not been established, because most patients exposed to
steroids or alcohol do not develop osteonecrosis (30,84,85).
Although the entity of idiopathic osteonecrosis most likely does exist,
as we learn more about the etiology of this condition, the number of
patients who will be relegated to this category will diminish (61).
 |
|
Table 125.1. Factors and Conditions Associated with Osteonecrosis
|
pathogenesis of osteonecrosis. They can be categorized into six groups:
(a) direct cellular toxicity, (b) extraosseous arterial, (c)
extraosseous venous, (d) intraosseous extravascular, (e) intraosseous
intravascular, and (f) multifactorial (Table 125.2).
 |
|
Table 125.2. Pathogenic Mechanisms
|
can cause injury to and death of marrow cells and osteocytes and lead
to osteonecrosis of the femoral head. It has been proposed that
corticosteroids and alcohol have direct cytotoxicity; however, in vitro
studies have indicated no direct cytotoxic effect with alcohol at
physiologically tolerated concentrations. In certain animal studies,
fat accumulation in the marrow and within osteocysts after exposure to
corticosteroids has been implicated as a cause of bone cell death;
however, no animal model has developed the collapse that is
characteristic of the human disease (59,60,141).
and hip dislocation is a direct result of injury to the arteries and
veins that supply a significant portion of the femoral head.
Angiographic studies in cases of nontraumatic osteonecrosis have
demonstrated a high incidence of ab-bnnormalities in major vessels
about the hip, including the retinacular arteries (14,15).
Superselective microangiographic studies of preclinical and
contralateral “normal” hips have been consistent with a diagnosis of
intraosseous vascular occlusion (115).
Osteonecrosis may be encountered after various surgical procedures
about the hip, as well as after forceful manipulation and casting in
extreme positions. These are related to iatrogenic trauma to regional
vessels.
However, as with other purported mechanisms, it is unclear whether this
is an inciting factor or a consequence of the disease. In cases of
posttraumatic osteonecrosis, direct venous injuries also accompany
arterial trauma.
evidence of old hemorrhage in areas of necrosis without microfractures.
Using a rabbit model of hypersensitivity vasculitis, one study (141)
found that in 7 of 20 animals that were injected with both horse serum
and methylprednisolone acetate, there was histologic evidence of
vascular lesions and fresh intramedullary hemorrhages. Perfusion
studies have demonstrated narrowing and a decrease in the number of
vessels supplying the femoral head with formation of fine new
anastomotic arterioles around areas of infarct in the bone of
transplant patients treated with corticosteroids (112). Saito et al. (103,104)
found areas of fresh hemorrhage in association with arterial damage
adjacent to microfractures and necrotic trabeculae. They concluded that
multiphasic episodes of intramedullary hemorrhages are an important
element in the pathogenesis of osteonecrosis. However, other
investigators have proposed that the necrotic lesions resulted from a
single rather than multiple events.
Core biopsy specimens from patients with early stages of osteonecrosis
have shown histologic changes taking place in the marrow before bony
abnormalities appear (11). This observation has led to the theory that the bone acts like a Starling resistor (52),
in which thin-walled vessels traverse the space within a rigid outer
cortex. Any increase in the pressure within this compartment would tend
to cause the vessel walls to collapse, thus leading to decreased blood
flow. Although it is well established that increased intraosseous
pressure is present in and around areas of osteonecrosis, it is
uncertain whether this is an initial event causing the osteonecrosis or
whether it takes place after some other etiologic factor and then
contributes to the pathogenesis.
exist within a closed compartment made up of cortical bone, it is
possible that hypertrophy of fat cells and infiltration of the marrow
within this compartment can cause an occlusion of vessels and place
abnormal pressure on osteocytes and marrow cells. There are several
circumstances in which this might be implicated in the pathogenesis of
the ischemia: (a) corticosteroid therapy, (b) Gaucher’s disease, (c)
leukemia, or (d) caisson disease or dysbarism.
However, edema can occur without the subsequent development of
osteonecrosis, as noted in transient osteoporosis of the hip or bone
marrow edema syndrome. Thus, the role of marrow edema in the
pathogenesis of osteonecrosis remains unclear (18).
This is believed to be related to emboli composed of clumps of abnormal
red blood cells or to nitrogen bubbles, which can lodge within vessels
and can also accumulate in the fatty marrow surrounding vessels.
The association of hyperlipidemia with gout, alcoholism, and
corticosteroid therapy is believed by some to support this hypothesis.
tests, the possibility that hypofibrinolysis and thrombophilia may play
roles in the pathogenesis of osteonecrosis has gained support (38,84,85,87,138).
In thrombophilia, there is an increased tendency to form intravascular
thrombi, whereas in hypofibrinolysis, thrombi that have already formed
are less readily lysed and removed. Abnormal levels of the specific
factors associated with these conditions have been documented in more
than 70% of patients with osteonecrosis—in those previously diagnosed
as having “idiopathic” osteonecrosis as well as in those in whom other
predisposing factors have been identified. However, as is the case for
other etiologic factors, not all individuals with these abnormalities
develop osteonecrosis.
familial incidence has been demonstrated. It may therefore be possible
to detect patients at risk before osteonecrosis develops. Because these
disorders can in part be reversed by the use of anticoagulants, a
pharmacologic treatment might theoretically be effective both in
preventing the development of osteonecrosis and in lessening its
severity once it has occurred. Therefore, consider testing for these
abnormalities in patients with clinically diagnosed osteonecrosis and
in their close relatives as described in the later section, “Laboratory Tests.” A consultation with a hematologist might be indicated in certain cases.
coagulation might be a result of pathophysiologic mechanisms such as
the Schwartzman phenomenon, in which immune complex deposition may lead
to vascular damage and ultimately osteonecrosis. Several animal models
have been developed that demonstrate this phenomenon. When
corticosteroids were added, bone necrosis can be produced. However, the
overall picture was quite different from osteonecrosis as it occurs in
humans, and there is no proof that this mechanism plays a clinical role
in the development of the human disorder.
not one but several precipitating factors may act simultaneously or in
sequence (64). There are several ways this can
develop. For example, a patient may have an underlying abnormality,
such as a subtle coagulation defect, but may not develop osteonecrosis
until one or more additional factors are present. These might include
excessive alcohol intake or chronic steroid administration. Under other
circumstances, a specific etiology factor may evoke more than a single
mechanism to cause vascular injury. For example, excess corticosteroid
use can cause hyperlipidemias that result in intravascular lipid
emboli. These in turn cause intravascular coagulation. In addition,
steroids may cause direct cellular toxicity and may lead to
abnormalities in marrow fat, thus causing increased pressure on local
vessels. In addition to the factors identified to date, it is quite
possible that other factors that predispose patients to the development
of osteonecrosis have yet to be identified.
vascular impairment in osteonecrosis, the sequence of events that
follow the initial insult or insults is similar. The resultant hypoxia
rapidly leads to increased cell membrane permeability, which allows
fluid and electrolytes to enter the cell, causing it to swell.
Intracellular lysosomal enzymes are released, resulting in
autodigestion or coagulation necrosis and cell rupture. Vascular injury
leads to tissue edema and hemorrhage. An inflammatory response ensues,
marked by the appearance of neutrophils and macrophages.
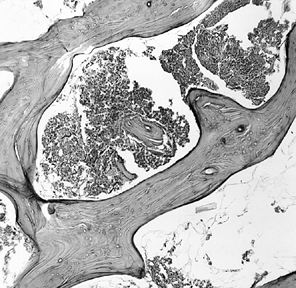
necrosis and is predicated on the disappearance of osteocytes from
within their lacunae, a process that takes several days to develop (Fig. 125.1).
The center of the necrotic lesion remains avascular and repair is not
possible. Bone and soft-tissue detritus accumulate. As a result of
repeated stresses, dead trabeculae undergo microfractures that cannot
be repaired.
 |
|
Figure 125.1. Necrotic bone and marrow from the center of the ischemic area (hematoxylin and eosin, ×50).
|
sufficient vascularity is present, the transition zone, an active
process of repair begins. Macrophages and osteoclasts remove dead
marrow elements and bone. Granulation and fibrous tissue are formed.
Osteoblasts form new bone, much of which is laid down directly on
remnants of dead trabeculae (Fig. 125.2). The
resulting trabeculae are much thicker than normal and are responsible
for the sclerotic margin that surrounds the lesion and that is one of
the radiographic hallmarks of osteonecrosis (Fig. 125.3).
 |
|
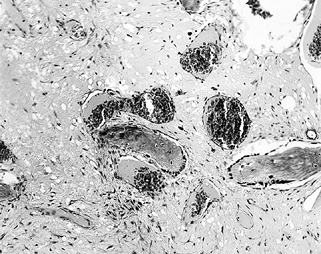
Figure 125.2.
Tissue taken from the periphery of the lesion (transition zone) shows active bone resorption and formation, and the presence of macrophages, lymphocytes, and fibrous tissue (hematoxylin and eosin, ×100). |
 |
|
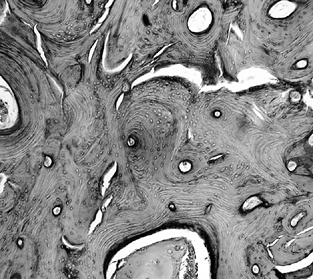
Figure 125.3.
Trabeculae in the transition zone may become markedly thickened due to new bone apposition to old, dead trabeculae (hematoxylin and eosin, ×50). |
a major weight-bearing region of the femoral head, it may undergo
revascularization and be completely replaced with viable bone.
Alternatively, it may remain as a small sequestrum surrounded by a wall
of new bone. In either case, femoral head collapse does not occur, the
hip continues to function normally, and the patient remains
asymptomatic.
weight bearing, have a poor prognosis. The attempt at repair is only
partially effective. Bone resorption is more rapid than formation.
There is a gradual collapse of cancellous bone beneath the bony
endplate of the weight-bearing
aspect
of the femoral head. If the contour of the articular surface remains
intact, a fluid-filled space beneath the cortical subchondral bone
develops, which gives the appearance of a crescent sign on radiographs (Fig. 125.4).
With flattening and collapse of the articular surface, this space is
obliterated and the crescent disappears. Gross flattening of the
femoral head is now apparent (Fig. 125.5).
 |
|
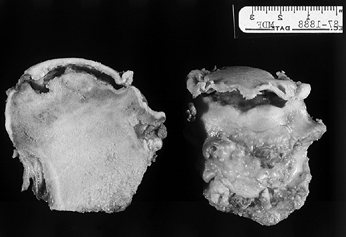
Figure 125.4.
Transected femoral head shows the area of necrosis, which is collapsed from beneath the articular surface, leaving a space that appears as a crescent sign on radiographs. Note that the articular cartilage appears grossly intact. |
 |
|
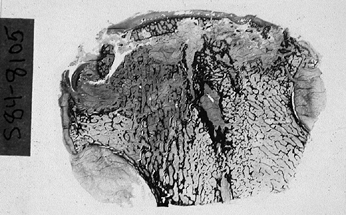
Figure 125.5.
Low-power photomicrograph of a sectioned femoral head. The articular surface is flattened. Several elements of necrosis and repair can be identified in more than half of the specimen (hematoxylin and eosin, ×0.5). |
synovial fluid within the joint rather than from vessels in the
cancellous bone, so it remains viable while the attached subchondral
bone dies. The mechanical stresses on the collapsed and irregular
articular surface eventually result in damage to and death of
chondrocytes (Fig. 125.6). These abnormal
stresses are transferred to the otherwise normal cartilage of the
acetabulum, which undergoes secondary degenerative changes. Joint line
narrowing becomes obvious on radiographs. As the processes of
degeneration continue, the underlying acetabular bone becomes affected.
Typical changes of degenerative joint disease appear and include
sclerosis, cyst formation, and marginal osteophytes. End-stage
arthritis of the hip eventually ensues (18,28,55,56,58,76,113,114).
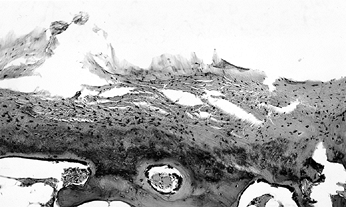 |
|
Figure 125.6.
Articular cartilage overlying an area of femoral head collapse. The cartilage remains viable but is undergoing severe degenerative changes as a result of the mechanical stresses that result from subchondral collapse and flattening (hematoxylin and eosin, ×100). |
although these two conditions can be confused. In TOH, there are no
findings of bone infarction or repair, which are the hallmarks of
osteonecrosis. The pathologic picture is primarily one of marrow edema,
hence TOH is also referred to as bone marrow edema syndrome (BMES).
Some of the pathologic features of TOH, such as the presence of edema
fluid, have also been found in early stages of osteonecrosis, and this
has led some authors to conclude that these two entities are at
different ends of the spectrum of the same disorder (49).
differently from osteonecrosis. The onset of pain is usually more
sudden and it is often more severe. To minimize the chance of fracture,
place patients on crutches until there is clinical, radiographic, and
MRI evidence of resolution. This may require 4–6 months. This condition
affects men more often than women, and these patients have no
associated risk factors as in osteonecrosis. In women, it classically
develops during the third trimester of pregnancy, and the incidence of
fracture is greater than in men. The disease rarely involves both hips
at the same time. Occasionally, the opposite hip is affected months or
years later. It is difficult to make a definitive diagnosis on the
basis of standard radiographs because the only abnormality is mild
osteopenia of the femoral head and neck. However, the diagnosis can be
made readily based on MRI in most cases.
from osteonecrosis, because the natural histories, prognoses, and
treatments of these two conditions are quite different. Because TOH is
usually self-limited, it is treated with protected weight bearing to
prevent fracture. Infrequently, core decompression may be indicated if
a patient has an inordinate amount of pain or if the diagnosis is in
doubt. Osteonecrosis, on the other hand, is a progressive disorder if
untreated, and in most instances early diagnosis and treatment are
required (18,48,49).
femoral head is usually not difficult because the radiographic picture
is often pathognomonic. Our goal, however, is to make the diagnosis as
early as possible, ideally before characteristic changes appear on
radiographs. This will allow early treatment with procedures that may
help retard or reverse progression of this condition and save the
femoral head. This, in turn, requires a familiarity with its etiology,
pathogenesis, and clinical features, as well as with the laboratory
tests and imaging studies that enable us to make the diagnosis early.
For weeks and perhaps even months after the initial vascular insult,
the involved area may be entirely asymptomatic. When symptoms develop,
they usually do so gradually. This may be due to a buildup of
intraosseous pressure initially and later perhaps to microfractures of
affected trabeculae. The pain is usually localized to the inguinal
region but may involve the buttock or the upper thigh. Rarely does it
radiate as far as the knee. It may be present at rest but is often
exacerbated by activity. Later, a limp and a slight decrease in ROM,
associated with pain, may develop.
cases, the involvement is usually not simultaneous. Therefore, the
patient will normally present initially with symptoms on only one side.
If the contralateral hip is afflicted, symptoms will usually develop
within 3 to 6 months. Approximately 80% of asymptomatic hips with
MRI-proven osteonecrosis eventually become symptomatic. The incidence
is much less in the case of small lesions. When the asymptomatic
contralateral hip of a patient with osteonecrosis on the opposite side
appears normal on the initial MRI, there is less than a 10% incidence
that this hip will develop osteonecrosis at a later date (32).
In a small number of cases of advanced osteonecrosis, the patient may
develop a positive Trendelenburg sign and have shortening of the limb,
due both to collapse of the femoral head and to a limited range of hip
motion resulting in a functional shortening.
areas will also develop symptomatic osteonecrosis. These include knees,
shoulders, wrists, feet, and, rarely, elbows and facial bones.
Accordingly, these areas should be evaluated by history and physical
examination. If there is any question of involvement, plain radiographs
and perhaps a bone scan or an MRI should be obtained. Recently, MRI
studies have indicated that the incidence might be considerably higher
than expected.
or associated factors. High doses of corticosteroids and prolonged,
excessive alcohol intake are most often implicated, but a number of
other factors should also be considered, as discussed earlier. The
quantities of alcohol or steroid required to cause osteonecrosis have
not been exactly determined, and it is clear that there is a tremendous
variation in the sensitivity of patients to these agents. For example,
it has been shown that patients on corticosteroids who develop
Cushingoid features are much more likely to develop osteonecrosis than
individuals taking the same dose of steroid who do not become
Cushingoid (120).
normal limits. In selected cases, serologic testing can be used to rule
out or diagnose other possible causes of hip pain. Although these tests
do not diagnose osteonecrosis per se, they may identify certain risk
factors, thus helping to support the diagnosis. These include sickle
cell disease, systemic lupus erythematosis, hyperuricemia, abnormal
amounts of circulating lipids, and subtle coagulopathies.
identified in more than 70% of patients with previously diagnosed
“idiopathic” osteonecrosis (45,46).
They have also been identified in a large number of patients with
osteonecrosis in whom other inciting factors, such as alcohol or
steroids, are present. It is thus felt that either alone or in
combination with other agents, these coagulation abnormalities may
predispose to or cause osteonecrosis.
measurements of coagulation, such as prothrombin time, partial
thromboplastin time, and bleeding time, which are usually within normal
limits. A more complete coagulation profile is therefore required to
identify these factors. Thrombophilia may be associated with decreased
levels of protein C, protein S, and antithrombin III (AT III);
increased resistance to activated protein C (RAP-C); and elevated
antiphospholipid antibodies. Hypofibrinolysis may be associated with
increased plasminogen activator inhibitor activity (PAI-1), increased
lipoproteins, and decreased stimulated plasminogen activator activity.
Unfortunately, at the present time these tests cannot be performed in
all facilities, and they are expensive.
tests, referred to as “functional exploration of bone,” be performed in
patients suspected of having osteonecrosis despite normal radiographs (12,38,39,52).
These included intraosseous venography, intraosseous pressure
measurements, and histologic examination of core biopsy specimens taken
from the femoral head. Although these tests can often lead to the
diagnosis of osteonecrosis prior to the development of radiographic
changes, they are seldom indicated if MRI is available. Rarely,
histologic evaluation may be useful in distinguishing between
osteonecrosis and certain other conditions affecting the femoral head,
such as TOH (18,39,45,48,49).
osteonecrosis is high-quality anteroposterior (AP) and lateral
radiographs of both hips. Initially, these will be within normal limits
because it takes a period of weeks to months after the initiating event
for changes to appear on radiographs. The first changes to be noted are
areas of radiolucency and sclerosis within the femoral head, usually in
the anterior superior quadrant. These result from bone resorption and
new bone formation. Infrequently, the earliest finding is diffuse
osteopenia. If present, this must be differentiated from other
conditions, including inflammatory arthritis, reflex sympathetic
dystrophy, and TOH. If the involved area is small, and particularly if
it is not in a region of major weight bearing, spontaneous healing may
occur. Once radiographic changes are present, however, they rarely
disappear completely, and the involved area can usually be identified
by its sclerotic appearance.
show evidence of progression. Once established radiographically, the
lesion will rarely enlarge in size, but microfractures through
weakened, dead trabeculae will occasionally result in the presence of a
crescent sign. This is seen in only a small percentage of hips with
osteonecrosis and represents an area in which the supporting trabeculae
have collapsed from beneath the subchondral bony endplate prior to
flattening of the articular surface. Gross flattening of the femoral
head later develops (Fig. 125.5), and, when
this occurs, the crescent sign is usually obliterated. Fragmentation of
bone in the necrotic region can often be identified. Usually the
collapse progresses and the majority of the weight-bearing surface of
the femoral head becomes flattened. Occasionally, the process seems to
stabilize after only a small amount of flattening has occurred. In
these instances, the joint line may be preserved for months to years,
although secondary arthritic changes eventually develop. These are
manifested by progressive joint-line narrowing, cystic and sclerotic
changes within the acetabulum, and the development of marginal
osteophytes. In longstanding cases, nearly complete obliteration of the
joint line may eventually develop.
initially involving the subchondral bone of the femoral head.
Joint-line narrowing and acetabular changes are secondary phenomena and
usually develop only after femoral head collapse. An awareness of this
fact will usually help in radiographically differentiating between
osteonecrosis and a variety of arthritides, which, in the end stage,
may look quite similar.
plain radiographs, MRI of both hips should be obtained because more
than 50% of cases are bilateral. If the diagnosis in one hip has
already been established by plain radiographs, then MRI will add little
to the evaluation of this hip, and the study may be performed on the
contralateral hip only. However, the usual procedure is to obtain
coronal
sections of the pelvis, including both hips. The MRI picture of
osteonecrosis is usually quite characteristic and, if both heads are
involved, it may be pathognomonic (Fig. 125.7).
The anterior superior quadrant of the head is usually affected,
although changes may be seen involving nearly all of the head. Very
rarely do these extend into the neck. If a significant amount of edema
has developed adjacent to the necrotic lesion, this may occasionally
lead to an abnormal MRI signal extending well into the femoral neck. In
such cases, you must be cautious to differentiate osteonecrosis from
TOH. The latter condition routinely involves both the femoral head and
neck down to the intertrochanteric line and is manifested by a
homogeneous, decreased signal intensity on the T1-weighted images and
an increased intensity on the T2-weighted images, reflecting the
presence of large amounts of edema fluid (Fig. 125.8 and Fig. 125.9).
In osteonecrosis, the changes are usually limited to the superior
portion of the femoral head, and the signal is most commonly decreased
or irregular in both the T1- and the T2-weighted images. When present,
the double-line sign on T2-weighted images is essentially pathognomonic
for osteonecrosis (Fig. 125.7).
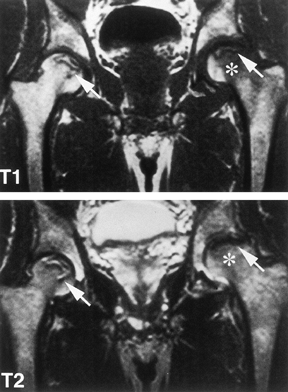 |
|
Figure 125.7. MRI images of a patient with bilateral osteonecrosis seen on plain radiographs. The T1-weighted image (top panel) shows subchondral lesions bilaterally composed of alternating areas of low and high signal intensity. The T2-weighted image (bottom panel) shows a high signal line inside the low signal on the right (double line sign). On the left,
the lesion in the proximal portion of the femoral head has diffuse low signal intensity whereas the area below the necrotic lesion (asterisks) is isointense, indicating coexisting edema. (Courtesy of Dr. S. Hoffman, Danube Hospital of Vienna, Austria. From Bauer T, Plenk H. The Pathology of Early Osteonecrosis. Semin Arthroplasty 1998;3:192, with permission.) |
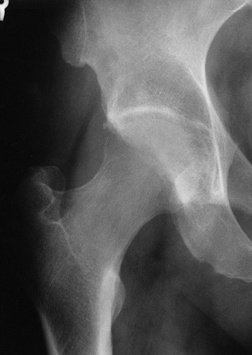 |
|
Figure 125.8. Radiograph of a hip with transient osteoporosis. Note the marked osteopenia of the head and neck.
|
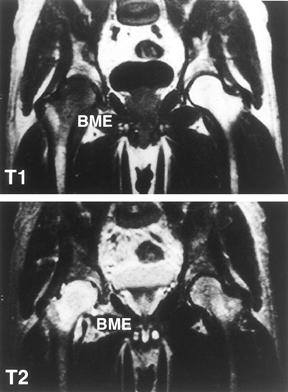 |
|
Figure 125.9.
MRI of patient with transient osteoporosis or bone marrow edema syndrome of the right hip. Radiographs were normal. The right femoral head and neck show a diffuse low signal intensity in the T1-weighted image (top panel) and a high intensity in the T2-weighted image (bottom panel). (Courtesy of Dr. S. Hoffman, Danube Hospital of Vienna, Austria. From Bauer T, Plenk H. The Pathology of Early Osteonecrosis. Semin Arthroplasty 1998;3:192, with permission.) |
available, computed tomographic (CT) scans usually add little to the
diagnosis of osteonecrosis. In selected instances, CT may better
visualize a small lesion not easily seen on routine radiographs, and it
may demonstrate small areas of articular surface collapse that are not
apparent on plain films. It may also be used to help quantitate the
extent of femoral head involvement.
the development of MRI. They are nonspecific and less sensitive than
MRI and are only rarely positive if the MRI is normal. In such
instances, other diagnoses should be considered. Bone scans may be of
value when the MRI picture is atypical and further confirmation of the
diagnosis
is
required, when a single screening test is desired to rule out the
presence of multiple joint involvement, or when MRI is not available.
single photon emission computerized tomography (SPECT), positron
emission tomography (PET), and gadolinium-enhanced MRI. The roles of
these studies in evaluating osteonecrosis have not yet been determined
and they are currently not in routine use for this purpose (30,44,80,97,116,118,123,131).
appearance of the hip is a good indicator of both the type and extent
of the pathologic changes present. An effective method for
classification and staging, based primarily on radiographs and other
imaging modalities, serves many important roles. It helps establish a
prognosis, follow improvement or progression of the condition, compare
the effectiveness of different methods of treatment, and determine the
best method of management for patients with different stages of
osteonecrosis. Uniform use of such a system of classification should
eliminate much of the current confusion about both the natural history
and the treatment of osteonecrosis.
in use, some of which will be specifically described. In the 1960s,
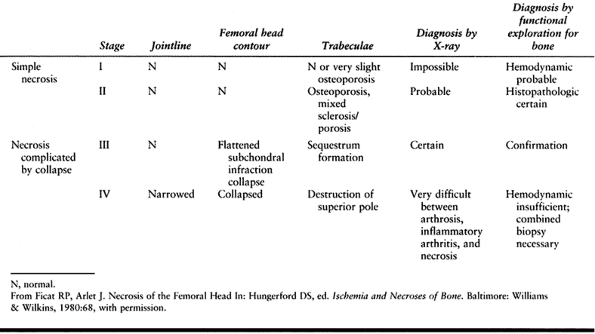
Arlet and Ficat in France (12) described a three-part staging system, and in the 1970s a fourth stage was added (39) (Table 125.3).
This form is perhaps the one most widely used now, despite the fact
that a stage 0 and a transitional stage were added later (38).
Bone and marrow scans were mentioned but did not appear to be an
integral part of the staging system, and MRI was not described. Certain
invasive tests, such as intraosseous pressure measurements and femoral
head biopsy, were deemed necessary to make the diagnosis in the
earliest stages. Patients’ symptoms and physical findings were
correlated with the stage. There was no attempt made to measure or
quantitate the extent of involvement.
 |
|
Table 125.3. Ficat and Arlet Four-stage Classification of Osteonecrosis
|
identified six stages of osteonecrosis and described the radiographic
picture for each. These were correlated with gross and histologic
findings as well as with the patient’s symptoms and physical
examination. Bone scans were not specifically described and MRI was not
available at that time. No preradiographic stages were included and no
attempt was made to quantitate the size of the lesion.
noted that the results of osteotomies performed for osteonecrosis
depended on both the location and the extent of the lesion. This latter
was expressed in degrees after measuring the arc of the articular
surface involved as seen on both AP and lateral radiographs of the
femoral head. Similar observations were reported by Wagner and Zeiler (139), Sugioka et al. (128), and Koo and Kim (66).
It focused on hips with Ficat and Arlet stages II and III, and it
grouped them by the type and location of the lesion as well as the
amount of articular surface involved. This system has not gained
popularity outside of Japan. The Committee on Nomenclature and Staging
of the Association Research Circulation Osseous (ARCO) in 1991 endorsed
the staging system developed at the University of Pennsylvania in the
early 1980s. In 1992, location of the lesion, as described in the
Japanese system (10,42), was added, and in 1993, stages III and IV were combined, as were stages V and VI.
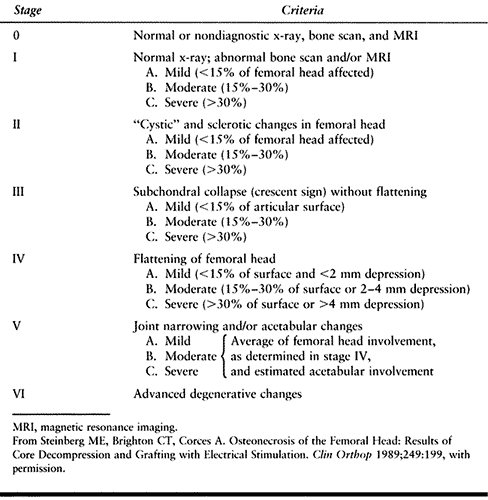
seven distinct stages, incorporated both technetium scans and MRI, and
included a measurement of lesion size in both early and late stages.
Symptoms and physical findings were considered important in determining
treatment but were not specifically included as part of the staging (Table 125.4) (Fig. 125.10, Fig. 125.11, Fig. 125.12, Fig. 125.13, Fig. 125.14 and Fig. 125.15). This system of
staging was compared in clinical use to older, nonquantitative systems
and was found more effective in following the progression or resolution
of the condition, evaluating the results of treatment, and establishing
a prognosis (117,118,122).
It is now generally recognized that the size or extent of the necrotic
lesion is an important indicator of prognosis and determinant of the
management, and it should be included in staging. Use of this staging
system in conjunction with a clinical evaluation as described has
enabled us to develop an algorithm that has proven helpful in
determining treatment for patients with osteonecrosis (Table 125.5).
It outlines treatment in general categories only because there are a
number of specific options to be considered in each category.
 |
|
Table 125.4. University of Pennsylvania System for
|
 |
|
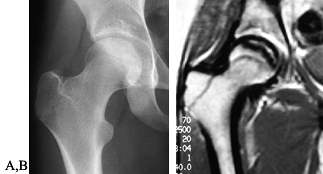
Figure 125.10. Images of a young patient with stage I, steroid-induced osteonecrosis of the right femoral head. A: The plain radiograph is within normal limits. B: The T1-weighted MRI shows a decreased signal intensity in the femoral head, characteristic of osteonecrosis.
|
 |
|
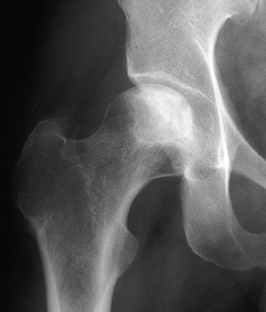
Figure 125.11. Stage II osteonecrosis showing areas of sclerosis and radiolucency within the femoral head.
|
 |
|
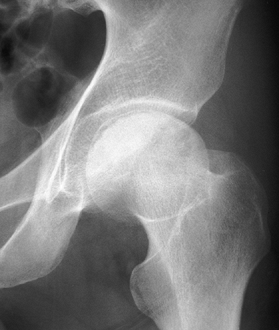
Figure 125.12. Stage III osteonecrosis. Note the crescent sign, indicating subchondral collapse without flattening of the articular surface.
|
 |
|
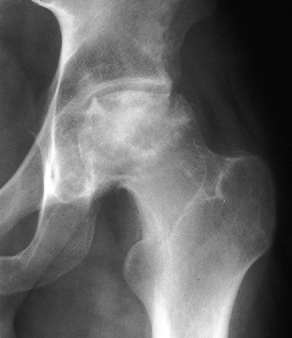
Figure 125.13.
Stage IV osteonecrosis. Marked flattening of the femoral head is present without radiographic evidence of acetabular involvement. |
 |
|
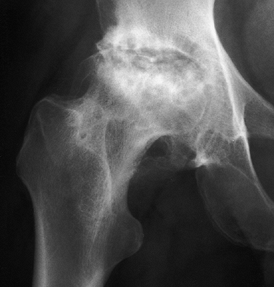
Figure 125.14.
Stage V osteonecrosis. The femoral head is markedly flattened. The joint line is narrowed, and the acetabulum shows irregularity, sclerosis, and radiolucency. |
 |
|
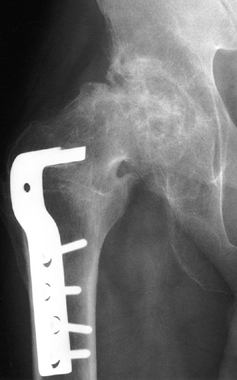
Figure 125.15.
Stage VI osteonecrosis showing advanced degenerative changes that have taken place in the hip joint secondary to osteonecrosis of the femoral head, treated by intertrochanteric osteotomy. |
 |
|
Table 125.5. Treatment Algorithm for Osteonecrosis of the Femoral Head
|
symptoms or physical findings. Considerable controversy exists
regarding the early management of osteonecrosis. Several different
procedures have been described, and in general most give results better
than nonoperative or symptomatic treatment. It is, however, difficult
to compare the effectiveness of each of these or to determine their
specific indications and contraindications. This is because of the many
variables included in these studies and the significant differences in
techniques, inclusion criteria, radiographic evaluation, outcome
measurements, and criteria for determining success or failure. A
coordinated series of prospective studies is required to determine the
effectiveness and indications for each of these techniques, but this is
not yet available.
femoral head is completely satisfactory. The natural history of this
condition after clinical diagnosis is one of progression in most
instances, except perhaps where lesions are small and in an area of
relative non-weight-bearing. Thus, a procedure that retards this
progression and provides an increased period of survivorship of the
femoral head before arthroplasty is required should be considered at
least partially successful.
state is its prevention. In osteonecrosis, certain specific risk
factors have been identified and these should be eliminated or
minimized to the extent possible. This applies particularly to alcohol
ingestion and steroid administration, the two leading causes of
osteonecrosis, as well as to smoking. In regard to steroid use, altered
dosage schedules and the substitution of other agents have already led
to a decrease in the incidence of osteonecrosis under circumstances in
which the use of corticosteroids has not yet been completely
eliminated. Established guidelines for divers and those working under
hyperbaric conditions must be followed. When they become generally
available, medications shown to be effective in combating some of the
known causes of osteonecrosis should be appropriately utilized in those
patients particularly at risk.
increased incidence of osteonecrosis. These include coagulopathies,
hyperlipidemias, and the presence of specific antibodies. Screen
patients with osteonecrosis for the presence of these disorders, with
coagulation studies, liver function tests, lipid profiles, and tests
for anticardiolipin antibodies and lupus anticoagulant. If definite
abnormalities are found, decide whether to treat them and which agents
to use. Limited studies have shown a possible role for stanozolol, an
anabolic steroid that alters lipoproteins and suppresses clotting
factors; nifedipine, a vasodilator; agents that lower circulating
lipids; and long-term anticoagulation to treat coagulopathies. A
limited number of patients in this latter category have been treated to
date. Anticoagulation is not a benign form of treatment, and the
appropriate dose and duration of treatment has not been established. In
general, neither the effectiveness nor the safety of these agents has
been determined, nor do we have appropriate guidelines for their use at
present. We can hope they will prove to be effective and will be
available for the prevention and treatment of osteonecrosis in the not
too distant future (46).
weight-bearing joint, there is a natural desire on the part of the
treating physician to protect the involved area from excessive stress
by using some form of limited weight bearing. Thus, canes or even
crutches are frequently prescribed. Although these may decrease the
degree of discomfort in patients who are symptomatic, they have not
been shown to alter the natural course of this disorder.
alternative to surgical management in cases with a good prognosis.
These cases include small, asymptomatic lesions and those in regions of
relatively low weight bearing, such as the medial aspect of the femoral
head. However, it has not been established that limited weight bearing
improves the already favorable course in such instances. Symptomatic
management, including protected weight bearing, may be the treatment of
choice when the patient’s age, general prognosis, and associated
medical conditions, or the patient’s own wishes, contraindicate
surgical intervention.
types of surgical procedures, such as core decompression, grafting, and
osteotomies, where it is used as an adjunct to the basic operative
approach. Here it serves to protect the weakened regions from fracture,
and perhaps protects the femoral head as well, until the healing
processes have progressed satisfactorily. Perhaps the most important
role for protected weight bearing is in the patient with relatively
advanced stages of osteonecrosis, when it is felt that prophylactic
procedures are of little avail. Here the use of a cane or perhaps
crutches can diminish symptoms and improve function considerably until
such time as a reconstructive procedure is indicated (3,81,85,94).
demonstrated an ability to enhance bone formation and fracture healing.
It was thus natural for electrical stimulation to be applied to
patients with osteonecrosis, either alone or as an adjunct
to
other surgical procedures, in the hope that it would stimulate healing
of the necrotic regions. Three specific signals have been used: direct
current (DC), capacitive coupling (CC), and pulsing electromagnetic
fields (PEMF). The latter two are transmitted to the involved bone by
means of surface electrodes or coils placed on the skin over the
involved region. Thus they can be used alone or in conjunction with
surgical procedures. DC stimulation requires the insertion of an
electrode directly into the bony region to be stimulated, and therefore
it has been used as an adjunct to core decompression rather than as an
independent treatment modality.
Capacitive coupling, with the specific signal utilized, failed to show
any enhancement of the effects of core decompression and bone grafting
alone. DC stimulation, despite indications of an early response, did
not show a long-term effect. Results with PEMFs, both in individual
studies and in a multicenter study, were promising (1,4).
They were shown to be more effective than symptomatic management in
precollapsed and minimally collapsed hips; as effective as core
decompression in precollapsed lesions; and more effective in hips with
early collapse, both as determined by radiographic progression and
delaying the need for arthroplasty. A special signal, different from
that used to treat fractures, was used to treat osteonecrosis. At
present, this is not generally available for routine use in the United
States. If further evaluation by other investigators confirms these
early positive findings, PEMF may be added to the treatment modalities
available for this condition (1,4,119).
used core decompression to examine the pathologic changes in femoral
heads of patients who were suspected of having AVN. This procedure
caused a decrease in the abnormally elevated intraosseous marrow
pressures and frequently produced immediate relief of preoperative
pain. Subsequently, this approach was used as a therapeutic rather than
a diagnostic procedure. It is presumed to work by decreasing the
abnormally high intraosseous pressures present in osteonecrotic
lesions, by opening up channels for vascular ingrowth, and by
stimulating the natural processes of repair. This technique can be
utilized as initially described or it can be supplemented with loosely
fitted cancellous or cortical grafts, electrical stimulation, or
various agents with the potential to stimulate bone healing, such as
bone morphogenetic protein (BMP) and demineralized bone matrix (DBM) (2).
reported on 133 hips in stages I and II treated with core
decompression. They noted “good and very good results” in 90% of hips
clinically and in 79% of hips radiographically. In 1986, Camp and
Colwell (26) retrospectively reviewed 40 core
decompressions performed by 13 surgeons in their area. Sixty percent of
hips treated before collapse failed either radiographically or
clinically, and all hips treated after collapse were considered
clinical failures. Four patients sustained postoperative fractures.
efficacy of core decompression, most studies report a low incidence of
complications and results significantly better than with symptomatic
management. In an extensive review of the literature published in 1996,
Mont et al. (81) reviewed 42 reports of 2,025
hips, of which 1,206 were treated by core decompression and 819 by
nonoperative means. Satisfactory results were found in 64% of hips
treated by core decompression, compared with only 23% of hips treated
nonoperatively. In the hips treated before collapse, good results were
obtained in 71% with core decompression and 35% treated nonoperatively.
The best results were achieved at centers doing the greatest numbers of
procedures (37,81,85,110,124).
-
Use a standard fracture table with a biplane image intensifier in place.
-
Expose the lateral femoral cortex through a short linear incision.
-
Divide fascia and muscles in line with their fibers in routine fashion, and identify the flare of the greater trochanter.
-
Place a small drill hole in the
mid-lateral cortex at the point where the bone begins to flare
laterally. Insert a small guide wire through this hole into the center
of the lesion in the femoral head, using the image intensifier. -
Then open the lateral femoral cortex with
a conical reamer or cannulated drill, to a diameter of 10 mm. Insert an
8 mm Michele trephine over the guide wire and manually advance to
within approximately 5 mm of the articular surface. Take care not to
perforate the joint. -
Bone from the intertrochanteric region is
considered essentially normal; put it aside to be used later for
grafting. The necrotic segment can be identified because of the
sclerotic bone encountered and the resistance to advancement of the
trephine. -
Advance the trephine slowly, in stages, into the lesion, removing bone from the trephine with an obturator as
P.3278
soon as significant resistance is met and further progression of the
trephine ceases. Do not strike the instrument with a mallet. On
occasion, it is difficult or impossible to remove all of the necrotic
specimen from the proximal portion of the femoral head using the
trephine. In such cases, remove the last few millimeters of bone with
either a cannulated or a solid drill. -
Send abnormal bone for histologic examination.
-
After the large central channel has been
created, make two additional channels with a 5 or 6 mm Michele
trephine. These extend from the initial opening in the cortex into
other segments of the necrotic lesion. Accomplish this simply by
slightly changing the angle of insertion of the trephine. Additional
holes through the cortex are not required (Fig. 125.16 and Fig. 125.17).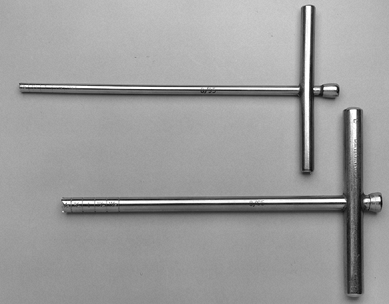 Figure 125.16. Michele trephines, 6 mm and 8 mm, used in core decompression.
Figure 125.16. Michele trephines, 6 mm and 8 mm, used in core decompression.![]() Figure 125.17.
Figure 125.17.
Technique for performing core decompression. (From Steinberg ME,
Brighton CT, Corces A, et al. Osteonecrosis of the Femoral Head.
Results of Core Decompression and Grafting with and without Electrical
Stimulation. Clin Orthop 1989;249:199, with permission.) -
Irrigate the wound and check the central channel to be sure that no debris is present.
-
Thin the cancellous graft initially
removed from the intertrochanteric region by using a rongeur or
bone-cutting forceps. Place it very loosely into the central track
extending from the relatively normal bone of the femoral neck into the
depths of the lesion (Fig. 125.18). This can be
accomplished easily by using the large trephine as a carrier for the
graft and holding the graft in position with the obturator while the
trephine is withdrawn. Then place additional cancellous bone at the
cortical margin of the lateral femur to promote healing of the surgical
defect. Leave the two smaller decompression channels open.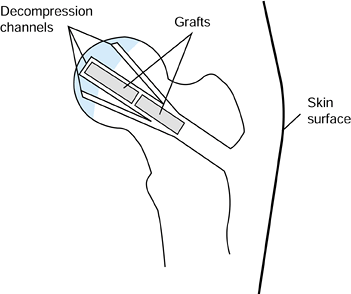 Figure 125.18. Decompression channels with central bone grafts inserted.
Figure 125.18. Decompression channels with central bone grafts inserted.
by most other investigators. Some surgeons make a single channel 8–10
mm in diameter, whereas others use two orbnperhaps three channels of
varying sizes. Also, the procedure may be done on an ordinary operating
table rather than a fracture table. In the classical core
decompression, supplemental bone graft is not used. In all our cases, a
loosely fitting cancellous graft was inserted into the central channel
as a supplement to the core decompression. This was done on a strictly
empirical basis in the hope that it would act as a stimulus to vascular
ingrowth and bone healing. However, we have no evidence that this
alters the results obtained by a classical core decompression done
without bone graft.
crutches with partial weight bearing for 3 months, although some other
surgeons use crutches for only 6 weeks without any obvious deleterious
effects. At the end of this time, we allow patients to progress quickly
to full weight bearing, but we caution them to avoid excessive stress
and strain to the extremity, such as would be encountered in running
and active sports, for 1 year from the time of surgery. If bilateral
core decompression is indicated, it is performed sequentially under one
anesthetic. Patients undergoing bilateral procedures are treated in a
fashion similar to those undergoing the procedure unilaterally, except
that they are instructed in a true four-point gait rather than a
partial-weight-bearing gait. In most instances, however, they proceed
spontaneously to a two-point gait within the first few weeks following
surgery.
(University of Pennsylvania stages I–IV) were treated at the University
of Pennsylvania with a modified core decompression using supplemental
cancellous bone grafting. Results were determined by clinical
evaluation, radiographic resolution or progression, and the need for
THR. The results were compared to 55 hips treated nonoperatively prior
to the start of this series and to results reported in the literature.
Of 297 hips with a minimum 2-year follow-up, 64% did not require
further surgery during the 2- to 14-year period of follow-up. This
contrasted with only 23% of hips treated symptomatically. In the
operative group, hips not requiring THR showed a mean improvement of 10
points on the Harris Hip Evaluation scale. A number of these hips
showed a small degree of radiographic progression despite satisfactory
clinical results; however, this was significantly less than in hips
treated nonoperatively. Of hips in stages I and II undergoing
decompression, 39% were radiographically stable, compared with only 19%
of hips treated nonoperatively. THR was required in 31% of hips treated
prior to femoral head collapse and in 48% of hips treated after
collapse was apparent. There was a clear correlation between outcome
and lesion size. In stages I and II, hips with small lesions involving
less than 15% of the femoral head by volume required THR in only 22% of
cases, compared with 40% of the hips with medium to large lesions
involving more than 15% of the head. We noted no clear correlation with
etiology except for patients in whom both corticosteroids and alcohol
were implicated. These did slightly worse than other groups.
recorded in 420 hips. Two patients sustained transcervical fractures
after falling during the first month following surgery, one patient had
a massive but nonfatal pulmonary embolism, one developed pneumonia, and
one was diagnosed with proximal thrombophlebitis of the thigh.
We concluded that compared to nonoperative or symptomatic management,
core decompression with or without a cancellous bone graft was a safe
and effective procedure for the treatment of AVN. At present, we
recommend core decompression, with or without cancellous grafting, as a
treatment for most hips with earlier stages of osteonecrosis, including
those with a small to moderate degree of femoral head collapse but
without acetabular involvement or significant pain or disability
(stages I to IV-B) (Fig. 125.19). This
procedure is used in both symptomatic and asymptomatic hips, as we
found no correlation between symptoms and outcome in hips treated prior
to femoral head collapse. Results in hips with very small lesions,
especially those not in a region of major weight bearing, were
considerably better than in the group as a whole, and the question has
been raised as to how much the operative procedure adds to the outcome.
Accordingly, these patients are now given the option of nonoperative
management with close observation. Surgery is recommended, however, at
the first sign of progression.
 |
|
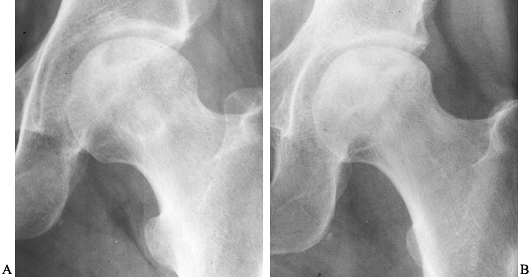
Figure 125.19. Radiographs of the left hip of an active young man with alcohol-related avascular necrosis. A: Preoperative radiographs show a large area of sclerosis and radiolucency within the femoral head. B:
Radiographs taken 1 year following core decompression and bone grafting show that the lucent areas have begun to fill in with bone. The patient remained essentially asymptomatic and was clinically doing well 12 years after the surgery. |
These are technically demanding procedures that are not frequently
employed in North America. Results vary considerably, and subsequent
arthroplasty may be compromised. The most commonly cited rationale for
their efficacy is the biomechanical effect of moving the necrotic
segment of the femoral head from the principal weight-bearing area of
the hip to an area that bears less weight, and replacing
it
with relatively normal bone and cartilage. Others have suggested that
the beneficial effects of osteotomies are secondary to the procedure
effecting a reduction in venous hypertension and intramedullary
pressure (13).
varus or valgus osteotomies, usually combined with flexion or
extension, and transtrochanteric rotational osteotomies. The varus or
valgus osteotomies have been associated with variable rates of success
after short-term follow-up of approximately 5 years (13,72).
Recent reports also include a prospective study of valgus-extension
osteotomies combined with curettage and bone-grafting of the necrotic
segment in a group of non-corticosteroid-associated hips (106).
These authors reported a good or excellent clinical result in 36 of 45
hips (80%) at a mean follow-up of 65 months (range, 32 to 126 months).
In a recent study of varus osteotomies combined with flexion or
extension (83), there were 28 of 37 good and
excellent outcomes (76%) at a mean follow-up of 11.5 years (range, 5 to
18 years). In patients without a corticosteroid association in this
study, there were 17 of 20 successful clinical outcomes (85%).
on the location and size of the lesion. Osteotomies may be utilized for
both pre- and postcollapse lesions, but they should usually not be
performed if there is acetabular involvement. Osteotomies work best
when the lesions are small or medium sized with a combined necrotic
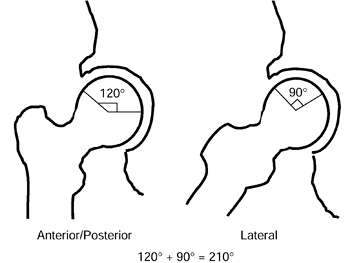
angle of less than 200° (Fig. 125.20) (65)
or with less than 30% of femoral head involvement. For varus
osteotomies, there should be at least 20° of the superolateral femoral
head not involved with disease, because this area of cartilage will be
shifted into weight bearing after the osteotomy.
Likewise,
a valgus osteotomy requires normal bone and cartilage in the central or
medial aspect of the head. Guidelines for when to add a flexion or
extension component to the osteotomy are similarly based on the
location of the lesion. Extension can be added when the necrotic
segment is posterior and flexion can be added if the lesion is anterior.
 |
|
Figure 125.20.
The angular measurements of the lesion on the AP and lateral radiographs are added together to give the “combined necrotic angle,” as described by Kerboul et al. (From Kerboul M, Thomine J, Postel M, Merle D’Aubigné R. The Conservative Surgical Treatment of Idiopathic Aseptic Necrosis of the Femoral Head. J Bone Joint Surg Br 1974;56:291, with permission.) |
is utilized in hips with Ficat Stage III when the osteonecrotic segment
is confined to the anterosuperior part of the femoral head (less than
20% posterior involvement). Optimally, patients are less than 45 years
of age and are not on steroids or chemotherapy.
-
Expose the hip joint through an
anterolateral approach, with an anterior capsulotomy permitting
visualization of the femoral head. -
Insert guide wires to obtain the desired valgus and flexion.
-
Cut the initial osteotomy perpendicular
to the femoral shaft at the intertrochanteric level 1.5–2.0 cm inferior
to the seating chisel. -
Make the bone wedge with a power saw and excise the anterior and lateral bone wedge.
-
Use an AO right-angle or 95° blade-plate to fix the osteotomy.
-
After fixation of the osteotomy, fenestrate the femoral neck anteriorly, just inferior to the head.
-
Curet out the necrotic bone and elevate the collapsed subchondral area with a punch if necessary.
-
Firmly pack cancellous bone graft from the iliac crest into the cavity created.
(15 kg) for 3–4 months or until radiographs show healing of the
osteotomy. Partial weight bearing may be continued for 6 months or
longer, depending on the rate of healing of the femoral head.
is utilized for small- or medium-sized lesions with a combined necrotic
angle of less than 200°. There should be no radiographic evidence of
acetabular involvement, and an arc of at least 20° on the lateral
aspect of the femoral head should be free of underlying necrotic bone.
The femoral head is moved into abduction and the femoral shaft is
brought into adduction and flexion. This brings the necrotic area
anteriorly, inferiorly, and medially.
-
Place the patient supine on a radiolucent
operating table with a small bump under the hip. Use a lateral approach
to the proximal femur with the fascia lata and vastus lateralis split
adjacent to the intermuscular septum. Expose the hip joint only if a
flexion osteotomy is being performed. This is to facilitate an anterior
capsulotomy to allow extension of the hip if necessary. -
Obtain the desired angular correction by
two osteotomies that remove a triangular segment of bone. Determine the
amount of varus correction desired by preoperative AP radiographs. Use
lateral radiographs to determine flexion or extension components of the
osteotomy. -
Perform the osteotomy with a power saw
and hand-held osteotomes. Start the first cut on the lateral cortex at
the vastus ridge. Use a fixed-angle AO blade plate or sliding hip screw
to fix the osteotomy.
bearing for 2 months and then advance to a cane until union is visible
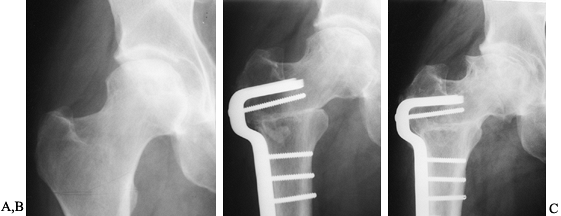
on radiographs (Fig. 125.21), usually 4–6 months after the procedure.
 |
|
Figure 125.21. Radiographs of a hip that underwent a varus osteotomy for osteonecrosis. A: Preoperative status. B: Six months after osteotomy. C: Ten years after surgery. The hip continues to function satisfactorily, although degenerative changes were eventually present.
|
osteonecrosis is to shift the necrotic segment of bone out of the
region of major weight bearing and to replace it with normal bone and
cartilage. The effectiveness of conventional angulation osteotomies is
necessarily quite limited by the amount one can alter the normal
neck–shaft angle without impairing motion and function of the hip.
However, this is not encountered when performing rotational
osteotomies. The head can be rotated 90° or more around the head–neck
axis without interfering with hip function. Thus, these osteotomies
should be more effective in shifting normal bone and cartilage into the
major weight-bearing region.
osteotomy to accomplish this. However, by 1977 he concluded that the
clinical results with this procedure were no better than with
conventional angulation osteotomies and abandoned it (139). In 1973, Sugioka (125,127)
reported on a different type of transtrochanteric anterior rotation
osteotomy. More than 500 of these procedures were performed since 1972,
and Sugioka’s results, especially in hips treated before significant
femoral head collapse, were quite gratifying (50,129). Unfortunately, these results could not be consistently duplicated by other investigators (33,36,132).
In some instances, this might be explained by deviation from the
specific indications for the procedure, the complicated operative
technique, or the postoperative regimen outlined by Sugioka.
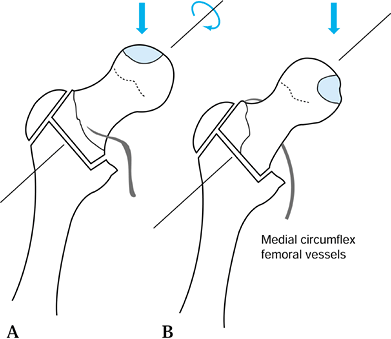
anterosuperior aspect of the femoral head, leaving the posteroinferior
portion relatively intact. By rotating the femoral
head
anteriorly, the necrotic segment is removed from the region of major
weight bearing and replaced with relatively normal bone and cartilage.
Occasionally, rotation posteriorly rather than anteriorly will more
effectively accomplish this goal. Varus or valgus can be added to the
rotation. The exact plane and alignment of the osteotomy can be
determined from a careful measurement of preoperative radiographs. It
is essential that the indications and contraindications be clearly
understood and that the details of this difficult operative technique
and postoperative care be closely adhered to. A critical point is the
absolute necessity of maintaining the blood supply to the femoral head
by preserving the vascular pedicle of the medial circumflex femoral
vessels, which is located beneath the quadratus femoris.
early to intermediate stages of osteonecrosis of the femoral head in
which the acetabular cartilage is relatively unaffected. There must be
sufficient normal bone and cartilage in the femoral head so that after
rotation the intact segment occupies at least 36% of the weight-bearing
surface of the acetabulum. Contraindications include whole-head
necrosis, significant degenerative changes in the femoral head or
acetabulum, and poor general health.
must be evaluated and measured to determine the plane of the osteotomy
and to be certain that the basic goals of the procedure can be met.
(This procedure is described in greater detail in references 126 and 129.)
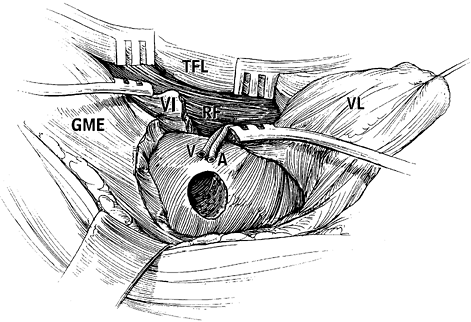
-
Make a modified Ollier’s incision and expose the capsule of the hip joint through a lateral approach.
-
Osteotomize the greater trochanter and
reflect it proximally with the attached tendons of the gluteus medius
and minimus and the piriformis. -
Expose the lesser trochanter carefully to
allow safe transverse osteotomy of the lesser trochanter; the adipose
tissue, which contains the vascular pedicles of medial circumflex
femoral vessels, is close to the osteotomy site. -
Transect the piriformis tendon and the
short external rotator muscle–tendon units attached to the
intertrochanteric fossa, and widely expose the capsule anteriorly and
posteriorly. -
Dissect the quadratus femoris muscle
carefully and leave some of the deepest fibers of the muscle intact, to
avoid vascular pedicle injury. No attempt should be made to expose or
identify the vessels of the pedicle. -
Divide the obturator externus muscle
sufficiently during dissection; otherwise, the inferior portion of the
joint capsule cannot be adequately visualized for capsulotomy. Also,
incomplete transection of the obturator externus muscle will produce
compression of the vessel pedicle during anterior rotation. -
Place Kirschner wires (K-wires) in the
cut surface of the greater trochanter from lateral to medial; insert
the first K-wire 1 cm distal to the intertrochanteric crest and
perpendicular to the long axis of the femoral neck in the AP view
(coronal plane) and direct it toward the
P.3283
inferior aspect of the lesser trochanter at the posterior margin of the cut surface of the greater trochanter. -
Insert the second K-wire at the anterior
margin so that a line drawn between the insertion points of the two
wires is perpendicular to the femoral shaft axis (not the neck axis) in
the lateral view (sagittal plane). The second wire is parallel to the
first K-wire. -
Place the third K-wire distal and
parallel to the second wire. Make the K-wires different lengths for
easy identification on radiographs. -
To confirm the sites of the wires and of
the planned osteotomy, take a true AP radiograph of the hip in the
neutral position in all planes with the knee flexed to 90°. On this AP
view, the first and second K-wires should be superimposed and at 90° to
the neck axis. When the first and second K-wires are not superimposed
on the radiograph, the second K-wire insertion should be corrected
forward or backward in the proper length, judging from the distance
between the second and third K-wire. -
After performing the circumferential
capsulotomy using special forceps, subluxate the femoral head to see
the extent of necrosis and to evaluate the remaining intact surface of
the head for its weight-bearing capability. -
Blood flow in the femoral head can be evaluated using a laser Doppler speckled method during surgery.
-
Using a reciprocating saw, make a
transtrochanteric osteotomy at 90° to the long axis of the femoral
neck. Use the inserted K-wires as a guide for the osteotomy. As
described previously, when the lesion is extensive so that the varus
position requires a greater angle of anteversion, incline 10° to 15°
more vertically. -
Then make a second osteotomy at the
superior edge of the lesser trochanter. The angle between the first and
secondary osteotomies in the AP view should be greater than 90°. This
will help the proximal fragment contact the distal fragment after
rotation. -
Place a 3 mm Steinmann pin in the
superolateral corner of the proximal fragment from posterior to
anterior, and then insert a second pin (a 2 mm K-wire) parallel and
adjacent to the first pin in the distal fragment. -
Cut the psoas tendon, the remaining
fibers of the obturator externus, and the vastus lateralis and capsule,
which cross between the fragment anteriorly. -
Using the proximal pin as a handle, rotate the femoral head anteriorly (Fig. 125.22 and Fig. 125.23). In the case of
P.3284
posterior rotation, place a Steinmann pin in the superolateral corner
of the proximal fragment from anterior to posterior. The second K-wire
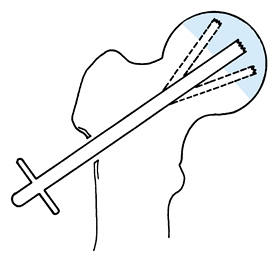
serves as a reference point for measurement of the angle of rotation.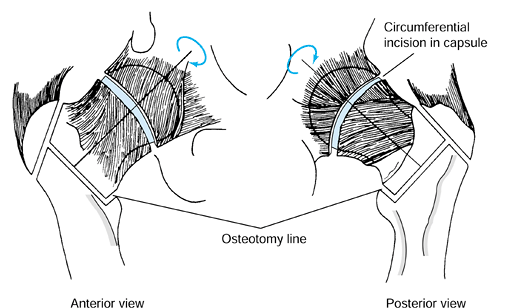 Figure 125.22. Schematic of the Sugioka transtrochanteric rotational osteotomy of the femoral head. (Courtesy of Professor Y. Sugioka.)
Figure 125.22. Schematic of the Sugioka transtrochanteric rotational osteotomy of the femoral head. (Courtesy of Professor Y. Sugioka.)![]() Figure 125.23.
Figure 125.23.
Transposition of necrotic focus of femoral head anteroinferiorly away
from weight-bearing area as a result of anterior rotation of the
femoral head. (A) before rotation and (B)
after rotation. (From Sugioka Y, Mohtai M. Osteonecrosis of the Femoral
Head: A Conservative Surgical Solution. In: Sedel L, Cabanela M, eds. Hip Surgery: Materials and Developments. London: Martin Dunitz, 1998, with permission.) -
Flex and adduct the distal fragment to oppose it with the rotated proximal fragment.
-
Check for bleeding at the osteotomy site and check the color of the synovium of the proximal fragment.
-
Then fix the osteotomy internally with three Venable screws with or without a side plate attached.
-
Trim the lateral aspect of the proximal
fragment carefully to adjust the level to the osteotomy site of the
greater trochanter in the distal fragment. (In the case of anterior
rotation, this area is occupied by the intertrochanteric crest, and the
vascular pedicle is at risk during this trimming.) Also trim the
anteromedial corner of the proximal fragment if it impinges on the
rectus femoris, which could inhibit hip extension. When trimming the
proximal fragment, check for the presence of good bone bleeding. Fix
the greater trochanter with heavy wires. -
Make AP and lateral radiographs before
wound closure to confirm the proper placement of the screws, the
position of the transposed joint surface, and the alignment of the
osteotomy. -
After placing anterior and posterior suction drains, close the wound in layers.
week and for an additional 2 weeks at night only. Begin hip ROM
exercises after the first postoperative week. Patients can perform
their own active assisted ROM exercises throughout the day using a
pulley system attached to the overhead frame on the bed to achieve hip
flexion. Flexion over 90° should be achieved by 3 weeks
postoperatively, after which allow the patient up in a wheelchair and
to begin ROM exercises in the Hubbard tank.
water tank, with the water level at the nipple line. The timing of this
progression depends on the inherent stability of the osteotomy line.
Progression to partial weight bearing with crutches proceeds as
indicated by healing, but do not begin full weight bearing before 6
months postoperatively.
follow-up of 3 to more than 20 years was performed. At 15 years
following surgery, 80% of hips did not require additional operative
intervention. The need for a second procedure was significantly greater
in patients in whom less than 20% of intact femoral head was
repositioned within the acetabulum and in those with advanced stages of
femoral head collapse preoperatively. Seven percent of hips in stage 2,
21% in stage 3, and 24% in stage 4 required prosthetic replacement
[Japanese Investigation Committee for Idiopathic Femoral Head Necrosis
staging system (95)].
stage 2, 72% of stage 3, and 52% of stage 4 hips. The incidence of
recollapse of the femoral head was 15% within 3 years and 21% within 10
years of osteotomy. In hips with more than 36% of normal head within
the acetabulum, only 7% collapsed; in hips with 21% to 35%, 35%
collapsed; and in hips with less than 20%, 72% collapsed (125,126,127,128 and 129).
Most of these procedures are designed to provide some degree of
structural support to prevent collapse of the articular cartilage
surface. There are several different types of nonvascularized
bone-grafting procedures, and they differ considerably. The graft may
be introduced through the femoral head, the neck, or the
intertrochanteric area. Either cancellous or cortical bone can be used,
and this can be obtained from the patient’s own iliac crest, proximal
femur, tibia, or fibula, or from the bone bank.
vascularized bone grafting. The graft may be taken from the ilium,
fibula, or greater trochanter. It may involve a muscle pedicle, which
contains its own vascular supply, or a microvascular anastomosis can be
performed using regional vessels. These procedures have been used both
before and after femoral head collapse but work best for precollapse
lesions. They should not be utilized when there are obvious
degenerative changes in the acetabulum.
who also used it to treat nonunions and nontraumatic osteonecrosis. In
these cases, nonvascularized autogenous tibial, fibular, or iliac crest
bone grafts were introduced through a core track, which extended from
the lateral femoral cortex through the neck and into the head at an
angle of 45° after the necrotic bone had been removed. The rationale
for this procedure was that this bone graft would lend structural
support to the articular surface while the femoral head was healing.
Initial results were favorable (19,20), but longer-term follow-ups indicated lower success rates (35,109). Buckley et al. (23)
described the results after core decompression combined with tibial
autografts and fibular autografts or allografts. They reported
successful clinical outcomes in 18 of 20 hips (90%) that had
precollapse disease, stage I or II, at
a mean follow-up of 8 years (range, 2–19 years). Their surgical technique for free nonvascularized bone graft follows.
-
Perform a core decompression as previously described with a 9 mm cannulated drill bit.
-
Drill the infarct up to the subchondral bone.
-
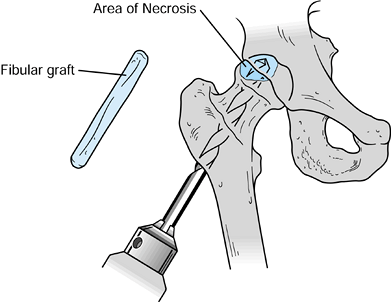
Then prepare the graft with a rounded
proximal end and tapered distally to be press-fit through the lateral
femur up into the head. The graft utilized can be autogenous
ipsilateral tibial corticocancellous strips or intact fibula.
Alternatively, an allogeneic fibular graft can be used. -
Confirm the appropriate placement of the
graft by fluoroscopic imaging with its rounded proximal end within the
lesion just underneath the subchondral bone.
 |
|
Figure 125.24.
Technique for grafting using an intact fibular allograft. (From Buckley PD, Gearen PF, Petty RW. Structural Bone Grafting for Early Atraumatic Avascular Necrosis of the Femoral Head. J Bone Joint Surg Am 1991;73:1361, with permission.) |
through a window in the femoral neck. A procedure reported by Ganz and
Buchler (41) combined this with an osteotomy, similar to that later described by Scher and Jakim (106).
Japanese investigators described a technique whereby autogenous iliac
crest strut grafts were inserted through a window into the neck and
were impacted into position, thus elevating the collapsed femoral head
to its former sphericity. The necrotic lesion was partially curetted
and the strut grafts were supplemented with cancellous bone. They
reported good or excellent results in 23 of 38 hips (61%), at a mean
follow-up of 9 years (range, 2–15 years) (56,142). Rosenwasser et al. (102)
replaced the necrotic bone with cancellous autograft and reported good
to excellent clinical outcomes in 13 of 15 stages II and III hips
followed for a mean of 12 years (range, 10–15 years).
the femoral head is approached through a window in the anterior aspect
of the femoral neck. The necrotic material is excavated up to the
subchondral bone. This cavity is then packed with autogenous cancellous
bone harvested from the ilium. Postoperatively, patients remain
non-weight-bearing for 6 months (Fig. 125.25).
 |
|
Figure 125.25.
Lightbulb procedure as described by Rosenwasser et al. (From Rosenwasser MP, Garino JP, Kiernan HA, Michelsen CB. Long-Term Follow-up of Thorough Debridement and Cancellous Bone Grafting of the Femoral Head for Avascular Necrosis. Clin Orthop 1994;306:17, with permission.) |
trapdoor made in the articular cartilage of the femoral head. This was
first described in detail by Meyers et al. (77,78), who utilized autogenous iliac cancellous bone grafting. Mont et al. (82)
combined autogenous iliac cancellous and cortical bone grafting with
demineralized bone matrix using this approach. They reported 22 of 30
good and excellent clinical outcomes at a mean follow-up of 4.7 years.
Their trapdoor surgical procedure follows.
-
Perform an arthrotomy using either an anterolateral or a posterolateral approach. Perform a capsulectomy and dislocate the hip.
-
Using thin, sharp osteotomes, make a
“trapdoor” in the articular surface over the collapsed segment by
outlining three quarters of circle. Then hinge this osteochondral
trapdoor back on the remaining one quarter of the osteochondral
surface, revealing the sequestrum beneath. -
Use high-speed burrs and curets to remove all the necrotic bone until bleeding cancellous bone is encountered.
-
Impact two to three iliac cortical struts into the base of the cavity.
-
Surround the struts with cancellous bone to which demineralized bone matrix has been added.
-
Replace the trapdoor and fix it with two to three absorbable pins.
-
Relocate the hip and close the wound
routinely. Keep patients at toe-touch weight bearing for 3 months and
then advance to full weight bearing over the next 1–2 months (Fig. 125.26).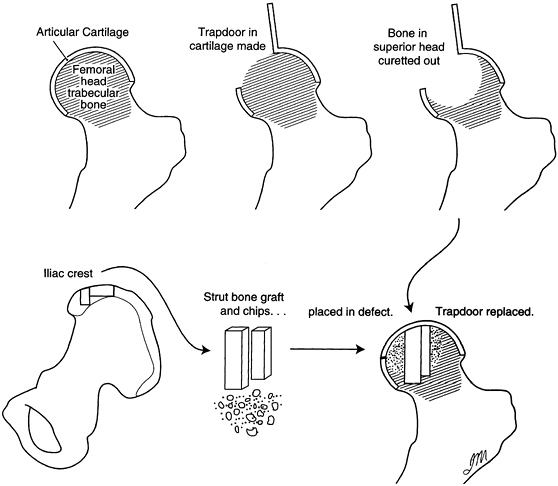 Figure 125.26.
Figure 125.26.
Trapdoor procedure as modified by Mont et al. (From Mont MA, Einhorn
TA, Sponseller PD, Hungerford DS. The Trapdoor Procedure Using
Autogenous Cortical and Cancellous Bone Grafts of Osteonecrosis of the
Femoral Head. J Bone Joint Surg Br 1998;80:57, with permission.)
osteochondral allografts to replace the necrotic segment of the femoral
head in the treatment of hips that have femoral head flattening
combined with degenerative changes in the overlying cartilage (77,78).
The hip is usually dislocated posteriorly, the depressed segment of
articular cartilage and subchondral bone is removed, and the necrotic
bone is then excavated down to a margin of viable bone. The cavity is
first packed with autogenous, cancellous graft taken from the ilium.
Osteochondral allograft is then placed over this, restoring the
articular surface. Meyers and Convery (78)
reported good and excellent clinical outcomes in 8 of 9 Ficat stage III
hips with a mean follow-up of 3 years (range, 1–9 years). However,
there are no long-term reports of this procedure.
this procedure. It can be extremely difficult to obtain a properly
fitting osteochondral allograft in a timely fashion, because these
grafts must be obtained from the donor within hours of the implantation
and cannot be stored for prolonged periods. It is necessary to have an
almost exact match in size between the donor femoral head and the head
that is to receive the tissue. In addition, there are certain risks of
infection as well as possible graft–donor mismatches that can lead to
rejection.
for all of these procedures is that the muscle-pedicle bone graft will
bring a blood supply to replace that lost by the necrotic tissue that
is removed. This technique was first described by Meyers et al. (79),
who used it in posttraumatic osteonecrosis. After the hip is exposed by
a posterior approach, a capsulotomy is performed and the head–neck
junction is identified. A segment of cortical bone with its attached
quadratus femoris muscle is elevated and retracted. A longitudinal
window is then made in the posterior aspect of the femoral neck.
Through this window, necrotic bone from the femoral head is curetted
out. The segment of cortical bone with its attached muscle is then
placed into this channel, which extends through the neck and into the
head. Cancellous bone is packed about this cortical graft as required.
The cortical strut may be fixed in place using a single screw (Fig. 125.27).
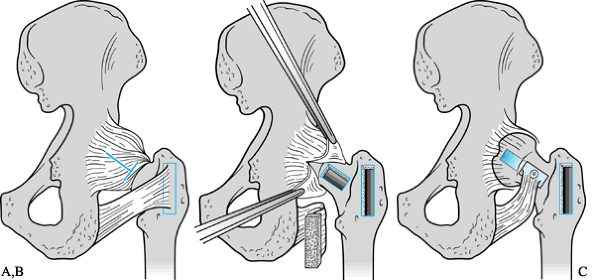 |
|
Figure 125.27. Technique for performing muscle-pedicle bone grafting to the femoral head, modified after Meyers et al. A: Surgical exposure with bone graft outlined. B: Procurement of graft and preparation of bed in femoral neck and head. C:
Graft inserted into the femoral head through a window in the posterior aspect of the neck. (From Meyers MH, Harvey JP, Moore TM. Treatment of Displaced Subcapital Transcervical Fractures of the Femoral Neck by Muscle-Pedicle-Bone Graft and Internal Fixation. J Bone Joint Surg Am 1973;55:261, with permission.) |
pedicles using bone taken from the ilium. Most of the studies report
satisfactory short-term results in 70% to 90% of precollapsed lesions
at a mean follow-up of less than 5 years. It should be noted, however,
that very few of these procedures have been reported to date, and these
techniques are not in general use at this time. See Chapter 29 for a description of this technique as used for nonunion of the femoral neck.
interest in the role of free vascularized fibular grafting (FVFG) as a
treatment for osteonecrosis of the femoral head. Only a few centers
have significant experience with this technique, but they have reported
encouraging results. This procedure is technically demanding, is
ideally performed by two teams operating simultaneously, and requires
assistance of a well-trained microvascular surgeon. Considerable
experience is required before optimal results can be anticipated, and
initially one should allow several hours for the procedure. The
complication rate is significant, even in experienced hands, and stress
fractures of the tibia have been reported. At the present time, it is
difficult to determine if this procedure yields better results than
simpler techniques, and if it does, how much better. The specific
indications for it have yet to be established.
head, thereby relieving intraosseous pressure and reversing the
ischemia; (b) to remove a significant portion of necrotic bone; (c) to
fill the necrotic defect with osteoinductive cancellous bone graft; and
(d) to support the subchondral bone and enhance the revascularization
process by means of a vascularized cortical bone strut (102,134).
This procedure has been criticized by some for the added morbidity of
harvesting the ipsilateral fibula and for the complexity of the
procedure as compared with performing a standard core decompression and
bone grafting. We feel, however, that the improved potential for
healing and joint-space preservation with FVFG justifies its use for
treating osteonecrosis of the femoral head.
necrosis of the femoral head. For patients younger than 20 years of
age, the indications have been expanded to include stage V disease,
given the limited treatment options in this age group. For those people
40–50 years of age, the indications have narrowed, so individuals with
stage IV disease and greater than 50% femoral head involvement are not
candidates for the procedure (135).
the procedure at our institution, but continued ethanol abuse and
vascular compromise to the lower extremity are contraindications. At
present we do not perform FVFG on asymptomatic patients, but we have
found that a large proportion of individuals who are asymptomatic at
initial presentation will progress to have symptoms within 5 years.
This raises the question as to whether a prophylactic procedure should
be performed even in patients with asymptomatic osteonecrosis, to help
prevent progression. This issue continues to be debated.
duration of the procedure considerably. One surgeon operates on the
lower leg while the other works on the hip. The average operating time
at our institution is less than 3 hours.
-
Place the patient on the operating room
table in the lateral decubitus position with the use of a bean bag.
General anesthesia or spinal anesthesia is generally utilized. -
Prepare and drape the entire lower
extremity including the hip area. Make a curvilinear incision over the
anterolateral aspect of the proximal femur (Fig. 125.28).
Carry the incision down through the subcutaneous tissue, exposing the
interval between the tensor fascia lata and the gluteus medius.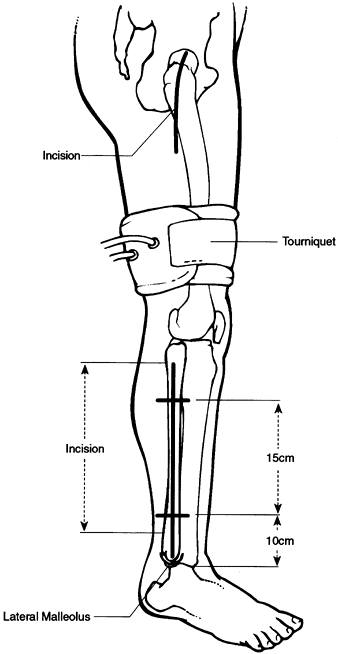 Figure 125.28.
Figure 125.28.
Location of the tourniquet on the leg, and the location of the
incisions for the approach to the hip and for harvesting the fibula. -
Split this interval and place a self-retaining retractor deep to the fascia.
-
The vastus lateralis can then be seen;
reflect it distally to expose the lateral aspect of the femur. Pull the
vastus lateralis distally and, with the use of a knife, cut it
transversely in the avascular plane of its origin on the vastus ridge. -
Posteriorly, reflect approximately 5 cm
of posterior lateralis off the linea aspera and sweep it distally using
a periosteal elevator. -
Anteriorly, detach the origin of the
vastus intermedius carefully with a right-angle dissector and a knife,
to create a trough that provides a shorter route for the ascending
vessels and to eliminate tension on the vascular anastomosis (Fig. 125.29).
Halt the dissection once the fat layer is encountered medial to the
intermedius, to prevent injury to the vessels and the femoral nerve.![]() Figure 125.29. Plane of dissection showing freed vastus lateralis (VL) swept distally, with a trough created in the vastus intermedius (VI). Ascending vein (V) and artery (A)
Figure 125.29. Plane of dissection showing freed vastus lateralis (VL) swept distally, with a trough created in the vastus intermedius (VI). Ascending vein (V) and artery (A)
are also shown. GME, gluteus medius; RF, rectus femoris; TFL, tensor
fascia lata. (From Urbaniak JR, Coogan P, Gunneson E, Nunley J.
Treatment of Osteonecrosis of the Femoral Head with Free Vascularized
Fibular Grafting. A Long-Term Follow-Up Study of One Hundred and Three
Hips. J Bone Joint Surg Am 1995;77:681, with permission.)
-
The deep plane of dissection is between
the rectus femoris and the vastus intermedius. Hold the plane open
using the self-retaining retractor. The donor vessels for the
anastomosis with the fibula are the ascending branches of the lateral
femoral circumflex artery and vein. These ascending branches consist of
a central artery and two veins. The other branches of the lateral
femoral circumflex vessels are the transverse and descending. -
For better visualization, release the
aponeurotic bridge between the rectus femoris and the anterolateral
femur. Expose the underlying fat and sweep it away with a sponge stick
to reveal the ascending branches. These branches are consistently found
8–10 cm distal to the anterosuperior iliac spine. -
Then take down the vessels in a cephalad to caudad direction, starting at the first major division of the artery
P.3289
and veins. Clip the vessels with small vascular clips just distal to
this bifurcation. This ensures adequate length of the vessels (about 4
cm), and the bifurcation affords a larger potential lumen size for
anastomosis to help prevent vessel mismatch. -
Dissect each vessel back to its origin,
placing small hemostatic vessel clips on any small branches. It is
technically easier to expose the vessels down individually rather than
as a group. -
Now remove the retractor for the bony preparation of the proximal femur.
-
Place a sterile drape over the C-arm
fluoroscopy unit. Position the C-arm to obtain an AP projection of the
hip. Obtain a frog-leg lateral projection simply by flexing and
externally rotating the hip. -
Beginning about 2 cm distal to the vastus
ridge at the junction of the posterior and middle thirds of the exposed
lateral femur, and using fluoroscopy, direct a 3 mm guide pin up the
femoral neck and into the center of the necrotic area in the femoral
head. The orientation of the guide pin should also be rather steep in
an effort to place the graft in a position that optimally supports the
subchondral area of the defect. On the frog-leg lateral view, take
special care to ensure adequate room in the neck to accommodate a 19 mm
reamer if needed. By this time, the fibular graft should have already
been removed so that the leg can be moved freely to obtain a frog-leg
projection. -
Progressively use cannulated reamers over
the guide pin, starting with a 10 mm reamer. The average female and
male patients require final reamings of 16 mm and 19 mm, respectively,
although this may vary depending on the size of the largest diameter of
the donor fibula. The reaming extends to within 3–5 mm from the
articular surface of the femoral head. It is safer to do the final part
of the reaming using fluoroscopy. -
If the necrotic region involves greater
than 25% of the femoral head, use a ball reamer to remove the remaining
necrotic bone, which is depicted on fluoroscopy as a cyst. The
relationship of the remaining cyst to the bone tunnel can be better
depicted by placing renograffin in the tunnel. Then use the ball reamer
under fluoroscopic guidance. Reintroduce renograffin into the tunnel to
check that the necrotic bone is completely removed. -
Then remove the cancellous bone from both
the greater and the lesser trochanter with an upgoing and straight
curet, respectively, through the bone tunnel opening. The cancellous
bone reamings harvested from between the flutes of the reamers, both
directly and by using a filtered suction tip (KAM Super Sucker;
Anspach, Palm Beach Gardens, FL), can also be utilized as bone graft.
However, take care to avoid using the reamings from the necrotic area. -
Then place the cancellous bone graft into
the reamed cavity with the use of a custom-made impaction device. Use
the scale on the surface of the device to measure the depth of the bone
tunnel so that the length of the fibular graft can be determined. The
impactor has side windows at the area of the subchondral bone, and
their position can be changed by rotating the impactor. -
Then place the bone obtained from the trochanter first, followed by the reamings.
-
Confirm the completeness of the grafting
by placing renograffin in the canal and seeing obliteration of the
necrotic cavity, using fluoroscopy in the AP and frog-leg lateral
projections.
-
Approach the fibula via a lateral
incision, simultaneously with the hip approach. Mark the line of the
incision longitudinally in line with the fibula. Make the fibular bone
cuts 15 cm apart, with the distal cut 10 cm proximal from the distal
fibular tip. The incisional length should coincide with the level of
the bone cuts (Fig. 125.28). The technical details of fibular removal have been described elsewhere (134,135) (see Chapter 36). -
Final preparation of the fibula occurs on
the back table. Immediately inject 40 ml of heparinized lactated
Ringer’s solution into both veins and the artery of the pedicle to
inspect for leaks. Reflect the pedicle from the proximal fibula until a
large nutrient vessel is found entering the cortex. If the pedicle
P.3290
is
not at least 5 cm long, dissect the bundle farther from the fibula. If
the pedicle branches before 5 cm of pedicle can be mobilized, preserve
both branches with subperiosteal dissection. -
When the feeder vessel is located, cut the fibula at that point with a reciprocating saw, and use copious irrigation.
-
Prepare the end of the pedicle with microsurgical dissection.
-
Choose one vein as the recipient and
place a small hemostatic clip on the end of the other. Use the final
measurement from the calibrated bone impactor to determine the second
distal bone cut. -
To prevent stripping of the pedicle
during insertion into the core in the femoral neck and head, use
absorbable sutures to bind the pedicle to the distal end of the fibula.
-
After complete removal of the contrast
material from the core, insert the fibula with the pedicle located
superiorly and anteriorly on the fibula. Position the pedicle into the
fibular sulcus for better protection from the walls of the femoral
tunnel. Placement of the fibular graft at the posterior border of the
core allows the pedicle to be free from compression. The fit should be
snug, but not tight, to ensure that the vessels are not being
compromised. -
Check the location of the fibular graft with fluoroscopy. If the graft is not fully seated, it can be tamped into place.
-
Use a 0.062-inch wire to hold the graft
in place; it crosses both cortices of the fibula and inserts into the
medial cortex of the lesser trochanter. Take care to protect the
pedicle during this step. Then cut the wire and bend it with a needle
driver. -
Remove the fluoroscopic unit. Place the
hip retractor back into the hip wound with the four claw retractors
attached to retract all four quadrants of the wound. -
Bring in the microscope and place it in an optimal position for performing the vascular anastomoses.
-
Couple the vein first to diminish
bleeding, which could obscure the field if the artery were anastomosed
first. Use of a coupling device for the vein anastomosis decreases
operative time. However, we prefer to anastomose the artery with
interrupted 8-0 nylon sutures because of problems with intimal cracking
upon placing the coupler on the artery. A disposable suction mat of
contrasting color is helpful in performing the microsurgery (Fig. 125.30).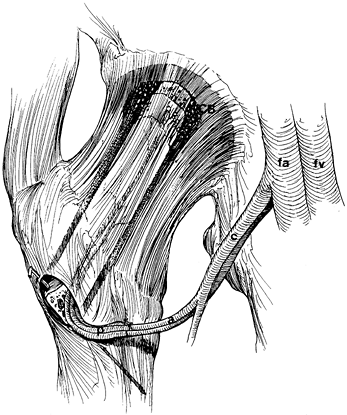 Figure 125.30. Fibular graft inserted into femoral head and surrounded by cancellous bone (CB) taken from greater trochanter. The peroneal artery and vein (p) have been anastomosed to the ascending branches (a) of the lateral femoral circumflex artery (c) and vein, which arise from the femoral artery (fa) and vein (fv).
Figure 125.30. Fibular graft inserted into femoral head and surrounded by cancellous bone (CB) taken from greater trochanter. The peroneal artery and vein (p) have been anastomosed to the ascending branches (a) of the lateral femoral circumflex artery (c) and vein, which arise from the femoral artery (fa) and vein (fv).
(From Urbaniak JR, Coogan P, Gunneson E, Nunley J. Treatment of
Osteonecrosis of the Femoral Head with Free Vascularized Fibular
Grafting. A Long-Term Follow-Up Study of One Hundred and Three Hips. J Bone Joint Surg Am 1995;77:681, with permission.)
-
After the vessels have been anastomosed,
endosteal bleeding must be observed from the end of the fibula to
confirm vascularization of the fibula. Postoperative angiograms have
shown good blood flow in the anastomosed vessels. To prevent compromise
of the vascular pedicle, do not reattach the vastus lateralis and
intermedius origins. -
Close the gluteal fascia and the tensor fascia lata over a drain.
bed and begin ROM exercises for the ankle and toes to prevent a flexion
contracture of the great toe, which can result from scarring of the
dissected flexor hallucis longus (FHL) muscle. Continue
non-weight-bearing ambulation for 6 weeks, followed by progressive
weight bearing. At 6 months, allow full weight bearing. If both sides
are being treated in staged procedures, the second operation is done 3
months after the first.
results with a follow-up of more than 5 years. Our initial 103 hips
treated with FVFG were studied in detail, all with a minimum 5-year
follow-up. We found a conversion to total hip arthroplasty (THA) in 11%
of stage II hips, 23% of stage III hips, 43% of stage IV hips, and 32%
of stage V hips that had undergone free vascularized bone grafting.
Kaplan-Meier survivorship analyses of the initial 103 hips demonstrated
that the probability of conversion to THA within 5 years after FVFG was
11% for stage II hips, 23% for stage III hips, 29% for stage IV hips,
and 27% for stage V hips. No association was found between the etiology
of the osteonecrosis and the likelihood of conversion to THA. The
average postoperative Harris hip scores for those hips not converted to
THA at 5 years follow-up were 80 for stage II hips, 85 for stage III
hips, 76 for stage IV hips, and 75 for stage V hips (134).
646 consecutive grafts with a follow-up of 1–17 years. The expected
survival or time to conversion to THA of the vascularized fibular graft
for all stages of osteonecrosis (including stage V) was more than 10
years (as determined by the Kaplan-Meier method of survivorship
analysis). The overall survival in this series (all stages included)
was 82.7%. There was, however, a greater failure rate for stage V
osteonecrosis than for any other stage. Overall, excluding hips with
stage V disease, the failure rate was 16.1% (93/579). The median time
to failure, if it occurred, was 2.3 years. These relatively long-term
results suggest that FVFG is a viable option for the treatment of
advanced osteonecrosis of the femoral head (Fig. 125.31).
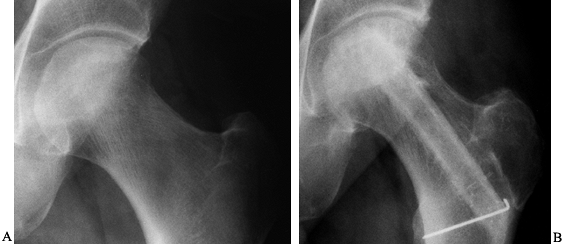 |
|
Figure 125.31. AP radiographs of a 46-year-old man with stage III osteonecrosis of the femoral head. A: Preoperative radiograph. B: Radiograph obtained 8 years postoperatively, showing preservation of the femoral head and joint space.
|
the donor site morbidity from harvesting a free fibular graft was
studied in 247 consecutive grafts in 198 patients. With a minimum
5-year follow-up, 24% of the lower legs were found to have donor site
complications. These complications included a sensory deficit in 11.8%
of the limbs, motor weakness in 2.7%, pain at the ankle in 11.5%, and
pain at other sites in the leg in 8.9%. Two percent of the limbs had a
contracture of the FHL. The FHL contracture was felt to be avoidable
with careful stretching of the toes in extension during the first few
postoperative days.
institution, two patients had a deep vein thrombosis and one patient
had a massive pulmonary embolus 6 weeks postoperative. Generally,
patients are maintained on one enteric-coated aspirin a day and
persantine 50 mg three times a day for 6 weeks. Other complications
included two superficial wound infections, one transient deep peroneal
nerve paralysis, and one injury to a branch of the superficial peroneal
nerve.
significant femoral head collapse has occurred, with or without
evidence of joint-line narrowing or acetabular involvement. It is
usually associated with at least a moderate degree of pain and
disability, although not infrequently patients with significant
radiographic involvement (stages IV-B to VI) have relatively little
discomfort. Under these circumstances, it is usually concluded that
there is little chance of preserving the femoral
head by the procedures previously described, and that some type of arthroplasty or hip reconstruction is virtually inevitable.
patients fits into the early or the late category. At times, however,
the patient will fall someplace between these two. In such cases, the
experience and clinical judgment of the surgeon is paramount. The
patient must be informed of his options and should enter into the
decision as to whether to attempt to retard progression and postpone
reconstructive surgery by some type of prophylactic surgery, or to
accept the fact that reconstruction is inevitable and treat the hip
symptomatically until arthroplasty is indicated.
alter the natural progression of this condition. Thus, it should not be
considered an alternative method of prophylactic management. In the
later stages, however, it is a useful adjunct for symptomatic
management of a patient who is not yet ready for a definitive
arthroplasty. Intermittent use of a single cane or perhaps even two
crutches at the discretion of the patient, together with a reasonable
limitation of activities and the use of mild analgesics, will often
decrease pain and enhance function.
often considered the standard reconstructive procedure for patients
with late stages of osteonecrosis, degenerative arthritis, and other
arthritides. The postoperative regimen was lengthy and complicated, the
results were inconsistent, and even relatively good results were
generally not completely satisfactory by today’s standards.
Accordingly, conventional cup arthroplasty was essentially abandoned
with the advent of THR.
and involves the acetabulum only much later, a number of surgeons have
advocated performing arthroplasty on the femoral side alone, provided
that the acetabulum is still relatively normal. This can be
accomplished by using a modified cup arthroplasty or a femoral
endoprosthetic replacement arthroplasty (25,53,67,93,108,116,132).
modified the standard cup arthroplasty and referred to it as an
adjusted cup arthroplasty. In this procedure, the femoral head is
reamed and a snugly fitting metallic cup is impacted over it. The
articular cartilage of the acetabulum is left intact. Kerboul et al. (65)
reported on 80 hips treated by this operation and followed for 1–6
years. At follow-up, 66% were felt to be successful, 16% somewhat
improved but still experiencing pain and a limp, and 16% failures. The
failures were related to rotation of the cup on the femoral head,
femoral head resorption and collapse, and acetabular erosion. The
authors felt that with more rigid indications and improvements in
techniques, the results could be improved.
satisfactory short-term results with the adjusted cup arthroplasty but
later abandoned this procedure in favor of ceramic THR arthroplasty. In
the United States, Nelson and Walz (93)
modified the cup arthroplasty by cementing the cup onto the femoral
head, leaving the relatively normal acetabulum intact. They reported
satisfactory intermediate-term results (17,65).
in the 1970s as an alternative to conventional THR. It was thought to
be especially suited for the young adult. Several different designs
were available, and a number of procedures were performed in patients
with osteonecrosis. Despite the theoretical advantages of this
approach, the clinical results were unacceptable, with a high 5-year
failure rate. The procedure was therefore virtually abandoned. However,
McMinn et al. (75), Amstutz (5,9),
and others have continued their work to eliminate the problems inherent
in the original designs, which used thin polyethylene acetabular
components cemented into place. Newer devices incorporate
metal-on-metal articulations with acetabular components adapted for
both cemented and cementless fixation. To date, results have been
variable and we must await further reports with larger numbers and
longer follow-ups to determine the roles that these components will
eventually play in the treatment of patients with osteonecrosis (5,9,75,107,140).
hemiarthroplasty, is a direct descendant of cup arthroplasty, which was
originally conceived by Smith-Petersen (111).
Because it involves resurfacing the femoral head only, it is a viable
treatment option for patients with osteonecrosis of the femoral head
when bone quality is adequate and when acetabular cartilage is
relatively normal. Because the femoral canal is not violated, nor is
the femoral intramedullary diaphyseal bone compromised, as it is with
THA, hemisurface replacement preserves bone stock, affording potential
for easy revision. With normal transfer of stress to the proximal
femur, proximal bone loss due to stress shielding may be mitigated.
Cartilage durability is enhanced
by
optimizing contact of the hemispherical bearing to the acetabular
cartilage. An added advantage of this technique is that the larger ball
size ensures greater joint stability than with THA (7).
with adequate bone stock and relatively intact acetabular cartilage
(Ficat stage III and early stage IV). It is especially suited for young
and physically active patients as a way to postpone the need for THR (Fig. 125.32).
Once collapse has occurred, we recommend that patients refrain from
weight bearing from the point of identification onward, to minimize
further damage to the acetabular cartilage. Patients with advanced
osteonecrosis in which both sides of the joint are severely damaged are
not candidates for hemisurface replacement, and full-surface or total
hip replacement should be considered instead. In our practice, the
underlying cause of osteonecrosis has not been a contraindication when
considering patients for hemisurface replacement.
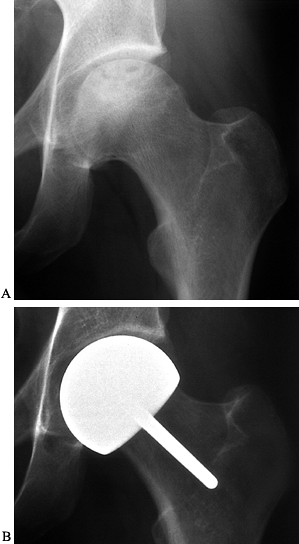 |
|
Figure 125.32.
Case illustration: A 31-year-old man with Ficat stage III osteonecrosis. Risk factors included steroid use, mild alcohol consumption, and trauma. A: Preoperative radiograph. B: Postoperative radiograph taken 9 months after hemisurface replacement. |
Technology, Arlington, TN) surgical system for hemisurface replacement.
Available in millimeter increments from 42 to 54 mm for precision
fitting, the CONSERVE prosthesis is made of cobalt chrome (Fig. 125.33).
It has greater sphericity than other hemisurface designs to provide
more coverage around the reamed bone, it has an expanded fixation area,
and it employs a short stem to ensure accurate alignment with a uniform
cement mantle around the resurfaced femoral head. These new design
features enable us to deal with extensive bone stock abnormality or
deformity, such as seen in Gaucher’s disease and perhaps sickle cell
anemia.
 |
|
Figure 125.33.
The CONSERVE hemisurface femoral component, designed for increased coverage of reamed bone and more accurate alignment than with other hemisurface prostheses. |
A cylindrical reamer, an oscillating saw, and a chamfered cutter are
used to remove necrotic areas and contour the femoral head.
Instrumentation of the system is designed for nontrochanteric
approaches, although the best surgical approach is the one with which
the surgeon feels most comfortable. The anterior, modified lateral, and
posterior approaches have been successfully performed.
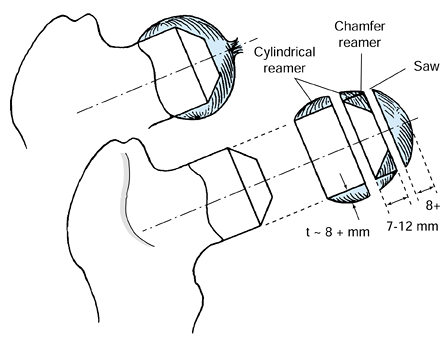 |
|
Figure 125.34.
Preparing the femoral head for hemiresurfacing. A cylindrical reamer, oscillating saw, and chamfered reamer are used in succession to give the head its final shape before the component is affixed. |
with final selection of prosthesis size made at the time of surgery.
With adequate preoperative planning, there should never be a need to
notch the neck.
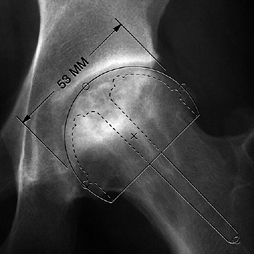 |
|
Figure 125.35.
Selecting the target component size. Scaled templates are superimposed on preoperative radiographs to estimate the final component size. |
-
We no longer use the transtrochanteric
approach for surgical exposure, preferring the posterolateral approach,
in which the circumference of the femoral head and neck can be assessed
and the entire acetabulum can be completely visualized. This allows
accurate assessment of the articular cartilage and optimizes our
ability to precision-fit the prosthesis to the cartilage where it is
best preserved, whether centrally or around the periphery. Section the
gluteus maximus tendon at the point where it inserts into the linea
aspera. -
Next, divide the short rotators, the piriformis tendon, and the quadratus femoris. Tag the piriformis tendon for later repair.
-
To mobilize the femoral head, section the
ligamentum teres with a ligamentum teres sectioner and complete the
capsulotomy. The entire capsule must be removed to improve
visualization of the articulating cartilage. We do not attempt to
preserve the labrum. -
Rotate the hip internally so that the
femoral head can be inspected and so that pin placement down the
central axis of the neck can be facilitated. Then use sizing ring
gauges to estimate femoral head size, trying to obtain a tight fit at
the widest part of the head. -
Begin preparation of the femoral head.
First, place the pin-centering guide firmly about the superior and
inferior femoral neck, and insert a 3.2 mm Steinmann pin to a depth of
approximately 10–20 mm. -
Assess pin alignment in the femoral neck
axis visually in two planes. Note that pin placement does not
correspond to the center of the head; with the posterior approach, the
pin-centering guide often must be forced anteriorly to correct the
tendency to retrovert the pin. -
Then check correct positioning of the pin
using the cylindrical reamer gauge by rotating it around the pin to
confirm that there is equal clearance around the femoral neck. By using
the cylindrical reamer gauge, proper pin placement can be achieved, and
selecting a reamer that is smaller than the femoral neck should be
prevented. Moreover, displacement of the cylindrical reamer from the
neck axis, which could result in notching of the femoral neck during
reaming, can also be prevented. -
Initially, select an oversized
cylindrical reamer (generally two sizes larger than the target size) to
remove a small amount of bone. Attach this to the power source and
advance it over the femoral head, using the Steinmann pin as a guide
and taking care to clear debris and to stop reaming at the femoral
head–neck juncture. Make successive reamings with appropriately
smaller-size reamers until the final reamed head diameter is reached.
Because each reamer has an intrinsic dome stop anthropometrically
designed to prevent notching,
P.3295
the
cutting teeth will not penetrate the bone of the intertrochanteric
area. These stops are effective when the largest cylindrical reamer
used is no more than two sizes greater than the target reamer size
(based on the templated neck dimensions of the contralateral, normal
neck, or measurement of the abnormal neck). However, as the reamer is
advanced, regular visual inspection and finger palpation is advised to
avoid neck invasion. -
For the femoral dome resection, secure a
cutoff guide, which corresponds to the size of the last cylindrical
reamer used, onto the femoral head with one or two pins. Then resect
the dome of the femoral head with a saber or oscillating saw. -
Make the hole for the short neck stem of
the component at this time by mounting the tower alignment guide
assembly onto the cutoff guide. -
Use a tapered stem reamer to ream distally to the sizing mark on the reamer corresponding to the target size of the component.
-
Then insert the chamfer guide into the
central hole. Use the chamfer cutter that corresponds to the size of
the final component to give the femoral head its final shape,
significantly increasing the available fixation area. -
Remove all remaining friable, yellow,
nonviable necrotic bone with a curet or high-speed burr. We do not
remove the dense reactive sclerotic bone. -
Confirm the size of the femoral head by using the component template that matches the inner dimension of the femoral component (Fig. 125.36).
By rotating the template, the uniformity of the cement mantle can be
assessed. Mark the bone at the distal tip of the template to indicate
complete seating of the femoral component during impaction. Figure 125.36.
Figure 125.36.
Checking the femoral head preparation. The femoral head template, when
placed on the resurfaced area, is used to assess the accuracy of the
bone preparation. -
Residual areas of poorly vascularized,
sclerotic bone may be drilled using a 1.5 mm drill bit into more
vascular bone to a depth of 3–5 mm. -
Curet out any remaining cysts, and clean the bone with saline using pulsatile lavage. Then apply the translucent head protector.
-
Insert the acetabular sizing gauge into
the acetabulum and press it against the acetabular cartilage to
visualize the contact areas. To optimize contact with the best
remaining cartilage, perform several trials with larger or smaller
acetabular gauges. Then select a target component size, ensuring a
preparation with a cement mantle of 1 mm. Generally, we recommend using
the next larger size when the dimensions fall between millimeter
increments, to provide peripheral or rim contact. -
Before cement is applied, thoroughly
clean both the reamed head and the acetabulum with pulsatile lavage to
make certain all bone fragments and soft tissue have been removed. -
Perform a trial reduction with the
precision fit component. A radiograph at this point may be helpful to
assess and verify correct component size. Check ROM before proceeding. -
Mix the acrylic bone cement and pour it
in a low-viscosity state into the component up to the circumferential
recess. The timing of cement application onto the femoral head depends
on bone density. If the bone is very compact and dense, cement should
be applied in a less viscous state; if the bone is osteopenic, the
cement should be applied when it has become more viscous to avoid deep
penetration of the acrylic. -
Where there is good bone quality, we
prefer to avoid cementing the stem of the implant by reinserting the
chamfer guide temporarily into the dome hole for the stem during
initial application; instead, we use finger pressurization of bone
cement into the cancellous bone, especially the cylindrically reamed
bone. Press the femoral component containing the doughy cement onto the
prepared femoral surface and hold it rigidly until polymerization is
complete. -
Then reduce the hip and check it for ROM and stability.
-
Reattach the gluteus maximus and piriformis tendon and close the wound with one or more suction drains in place.
allowing weight bearing as tolerated. Have the patient use crutches for
4–6 weeks and a cane, if desired, for an additional 2–3 weeks. Permit
sports at 4 months postoperatively. (See references 8 and 9 for additional details of the surgical technique.)
1980 and has gradually evolved into studies with ceramic and cobalt chrome shells (8,9).
A total of 33 hips in 29 patients, with a mean age of 33.8 years at the
time of surgery and an average follow-up of 5.1 years, were reviewed at
our center. All had a diagnosis of osteonecrosis; 20 were classified as
Ficat stage III and 13 as stage IV. Four have undergone revisions to
either surface or total hip replacement. In the 29 remaining hips, the
average UCLA hip scores are 8.1 for pain, 8.5 for walking, 7.5 for
function, and 5.5 for activity. Radiographic analysis showed no cases
with loosening; in fact, one case even had bone fill in the acetabular
fossa (9). To date, no dislocations have occurred with this group, due in large part to the stability provided by the larger ball size.
quality of acetabular cartilage, some are continuing to perform well at
more than 15 years. Survivorship of this group is 81% at 5.1 years and
61% at 10 years (9). Revisions, when necessary,
were all the result of acetabular cartilage wear; however, some cases
with rather advanced articular cartilage fibrillation at the time of
surgery remain intact. Morbidity has been minimal, and no instances of
sepsis, nerve palsy, thromboembolic events, or other complications have
occurred. There have been no cases of prosthetic loosening, and bone
stock has been well preserved and well maintained (8,9).
shown that although significant burnishing of the components occurred,
the histologic response was benign (6). Some
metallic debris and a few macrophages were found scattered throughout
the tissue, located mostly in loose connective tissue. A change to
cobalt chrome was precipitated by these findings, under the premise
that a harder bearing-surface might extend the life of the cartilage
and the durability of the prosthesis. Based on our own retrieval
studies, we have established that the viability of the femoral head
after hemiresurfacing is preserved (8,51).
preservation and intact intramedullary canals have made revision to
either full-surface or total hip replacement much like a primary
replacement. No debris-induced granulomata were encountered. Because
acetabular cartilage will undergo accelerated degenerative changes from
articulating with metal, revision will eventually become necessary.
hemiresurfacing arthroplasty for Ficat stage III and early stage IV
osteonecrosis is a reliable and successful time-buying procedure for
young and active patients. Using this approach, only the diseased
portion of the joint is surgically removed, and osteolysis and other
complications such as dislocations are eliminated by avoiding the use
of either bipolar or total hip replacement. Furthermore, the results of
THA after hemisurface replacement are not compromised, and complete
resurfacing can still be achieved.
for patients with osteonecrosis who meet the indications outlined,
there are many surgeons who prefer primary THR when an arthroplasty is
required. A greater number of cases with a longer follow-up will be
required to help determine which is the more successful approach.
than a THR for osteonecrosis once again stems from the fact that
initial changes are in the femoral head and not the acetabulum. It was
thus felt by some investigators that replacing only the femoral head
would be more conservative than THR (25,116). The early procedures used solid
press-fit femoral components that employed neither cement nor bone
ingrowth for fixation of the stem into the femoral canal. The heads
were sized rather crudely to the acetabulum because the importance of
an exact fit was not realized at that time. The long-term results with
these prostheses when used in younger patients with osteonecrosis were
generally poor (Fig. 125.37), although some good to excellent results did occur (Fig. 125.38).
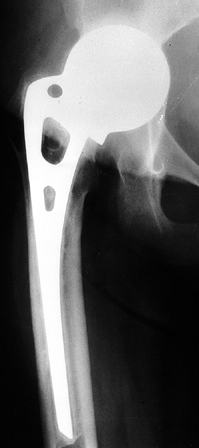 |
|
Figure 125.37.
Radiograph of the right hip of a patient with avascular necrosis treated with the insertion of an Austin Moore noncemented femoral endoprosthesis 12 years earlier. The femoral component remains well fixed within the shaft, whereas the ball has eroded superiorly and medially into the acetabulum. The patient required revision to a total hip arthroplasty. |
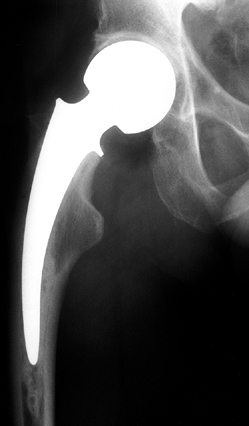 |
|
Figure 125.38. Fourteen-year follow-up of a cemented unipolar endoprosthesis. The patient has little pain and excellent function.
|
into the femoral canal either by cement or by biological ingrowth.
Modular components came into use and both unipolar and bipolar devices
were employed. Initially it was felt that the use of a bipolar
component would yield better results than a unipolar in that there
would be less frictional wear on the acetabulum and thus a lower
incidence of acetabular failure. Unfortunately, other problems with the
bipolar prostheses developed, and there is currently little evidence of
the superiority of the bipolar over the unipolar device (Fig. 125.39).
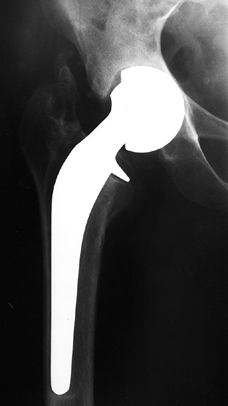 |
|
Figure 125.39.
Radiograph of the right hip of a patient who underwent a cemented bipolar endoprosthetic replacement 5 years earlier. Note the loosening of the femoral component in addition to the medial erosion of the acetabular component into the pelvis. |
endoprostheses in patients with osteonecrosis, the current trend is
away from these devices and toward THR arthroplasty or other procedures.
current techniques and components, are compared to THR, most reports
indicate better results with THR. Because the stem design and the
fixation of endoprostheses and total hips are now similar, it is
probable that the difference in results can be attributed to problems
within the acetabulum. A metallic femoral head places abnormal stress
on the acetabular cartilage and creates a situation that is far from
physiologic. This is not consistently well tolerated, even by normal
articular cartilage, and it has been noted that the acetabular
cartilage in osteonecrosis is no longer normal by the time arthroplasty
is needed (24,25,31,67,130).
for the treatment of patients with advanced stages of arthritis and
other disorders of the hips, there has been a certain reluctance to
advise this procedure for patients with AVN. This is because these
patients are much younger than patients with degenerative joint disease
at
the
time that arthroplasty is required, and it is generally accepted that
the survivorship for THR in young patients is less than in older
patients. In addition, patients with osteonecrosis seem to have a
higher complication rate and earlier incidence of failure than patients
of a similar age but with other disorders of the hip (96).
In five reports published between 1981 and 1984 on THR arthroplasty in
younger patients, the mean incidence of revision was 13%, and results
were rated as good to excellent in 81% with a 5-year follow-up (3). Salvati and Cornell (105)
reviewed their experience with THR in patients with osteonecrosis from
1972 through 1975. They found that 11 of 28 arthroplasties failed at a
mean of 8 years (range, 5–10 years), for an overall failure rate of
37%. Of the remaining 18 THRs, 16 had good to excellent ratings. It was
their opinion that the failure rate in osteonecrosis was four times
greater than in osteoarthritis.
results at short- and medium-term follow-up times with more modern
components and techniques (21,25,40,43,54,62,71,92,99). In 1994, Piston et al. (100)
reported on 35 hips with AVN treated with porous-coated THR
arthroplasty. Follow-up ranged from 5 to 10 years with a mean of 7.5
years. They encountered one femoral and two acetabular revisions, for
an overall revision rate of 6%. There was, however, significant
remodeling in six hips and osteolytic reactions in six others. In 1997,
Garino and Steinberg (43) reported on 123 THAs
in patients with osteonecrosis. The follow-up was 2–10 years with a
mean of 4.6 years. All femoral stems and 71 sockets were fixed with
acrylic cement. Fifty-two sockets were designed for bone ingrowth. The
average Harris hip score improved from 45 points preoperatively to 92
points at the time of last follow-up. Of the 246 components used, 6
acetabular and 4 femoral prostheses (4%) were revised. The incidence of
revision for aseptic loosening was 2.5%. None of the noncemented
acetabular components were either radiographically loose or revised.
Fifty-two hips had a minimum follow-up of 5 years (mean, 6.6 years). In
this group, five components (5%) were revised and four (4%) were
radiographically loose. The revision rate for hybrid hips with a
minimum 5-year follow-up was 2%. These results seem significantly
better than reports of older series. The authors concluded that using
modern techniques and components in THR arthroplasty can give excellent
results over a period of 2–10 years, even in young patients with
osteonecrosis. They felt that this procedure was the treatment of
choice when reconstructive surgery was required (Fig. 125.40 and Fig. 125.41).
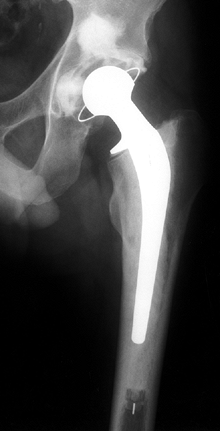 |
|
Figure 125.40.
Radiograph of the left hip of a husky young man with alcohol-related avascular necrosis, treated 11 years earlier with a cemented total hip replacement arthroplasty. The patient continues to do well clinically with minimal radiographic changes since the time of surgery. |
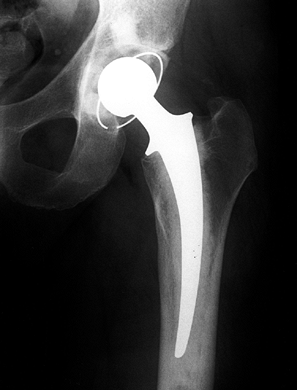 |
|
Figure 125.41.
A 19-year follow-up of a Muÿller total hip replacement in a young man with alcohol-related osteonecrosis. The prosthesis continues to function well. |
Girdlestone pseudarthrosis, was used as a primary arthroplasty for both
septic and nonseptic hips prior to the advent of THR. Today it is used
most frequently as a salvage for the failed and usually infected THR or
endoprosthesis. Under certain selected circumstances, however, it can
be used as a primary arthroplasty. In the patient with end-stage
osteonecrosis whose pain and disability require some type of operative
intervention, but in whom prosthetic replacement or THR is
contraindicated, the resection arthroplasty may be a reasonable
alternative. This procedure may be considered to treat the
osteonecrotic hip complicated by sepsis, and the patient in whom THR
arthroplasty has a high potential for complications and failure.
Patients with chronic recurrent septicemia, those on renal dialysis,
and individuals with active sickle cell disease may fit into this
category. Resection arthroplasty might also be considered in the
individual who is felt to be too unreliable to take proper precautions
following THR, such as the chronic alcoholic, drug abuser, and mentally
retarded. It also might be used for the individual who is confined to a
bed/chair existence due to neurologic or medical problems and who
simply needs pain relief and satisfactory motion rather than stability
and the ability to ambulate.
If this is clear from the outset to both the patient and physician, and
if there is an understanding of the advantages and disadvantages of
this procedure, then most patients will be satisfied with the outcome.
A resection arthroplasty will eliminate sepsis if present, provide pain
relief, give a good ROM, and not deteriorate with the passage of time.
At the same time, the joint will be unstable, the limb will be an
average of 1–1.5 inches short, and most patients will require both a
shoe lift and the use of at least one cane or possibly two crutches for
ambulation (Fig. 125.42) (27,121).
Resection arthroplasty may be used as a definitive procedure or as an
intermediate step, such as in the case of infection, until a THR can be
safely performed.
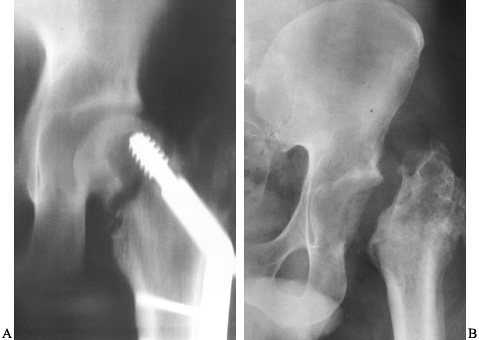 |
|
Figure 125.42.
Radiographs of the left hip of a 22-year-old man who developed a septic nonunion with avascular necrosis following open reduction and internal fixation of a displaced femoral neck fracture. A: Radiographs show the nonunion and loosening of the fixation device within the femoral head. B: Radiographs taken 3 years after a resection arthroplasty (Girdlestone pseudarthrosis). The patient is doing well clinically and has declined total hip replacement arthroplasty. |
is no longer responsive to conservative management, and in whom
prosthetic replacement or THR is contraindicated but stability of the
hip rather than motion is required, arthrodesis rather than resection
arthroplasty might be considered. Most of the indications described for
resection
arthroplasty
also apply to hip fusion. This procedure might also be considered in
the large, young, active man who desires a single “permanent” procedure
rather than a THR with the need for more than a single revision during
his lifetime. Before embarking on this procedure, the individual must
be fully aware of the possibility that the attempt at fusion may not be
successful, and of the permanent limitations that a fusion will impose.
At times the use of a pantaloon spica as a trial will help the patient
make an informed decision. The usual contraindications for hip fusion
apply here as well and include pathology in the knees, lumbar spine, or
contralateral hip. Because of the known high incidence of bilaterality
of osteonecrosis, it is essential that involvement of the opposite hip
be ruled out prior to embarking on a fusion. If, despite these
precautions, osteonecrosis does develop in the contralateral hip, THR
can be performed with a satisfactory outcome. The incidence of nonunion
in osteonecrosis is somewhat higher than in patients with other
disorders because of the necrotic bone present in the femoral head.
finding an incidence of knee involvement in 9% of patients with
osteonecrosis. Two entirely separate entities can affect this region:
One is known as spontaneous osteonecrosis of the knee (or SPONK), and
the other, which will be referred to as avascular necrosis, is the
disorder that can also affect the hip and other areas of the skeleton (Fig. 125.43).
SPONK occurs in patients over 55 years of age, and it typically
involves one femoral condyle or tibial plateau without other joint
involvement. The risk factors commonly found in osteonecrosis of the
hip have not been identified. AVN of the knee, on the other hand, is
typically found in patients younger than 45 years of age, may involve
several sites about the knee itself, is associated with hip involvement
90% of the time, has a different appearance on radiographs and MRI, and
is often associated with corticosteroid use or alcohol intake.
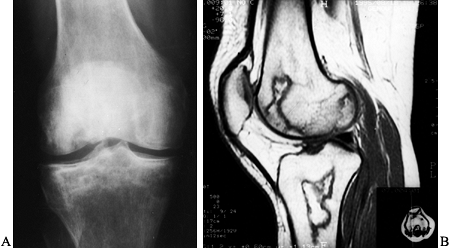 |
|
Figure 125.43. Avascular necrosis of the knee with involvement of both femoral condyles and the tibial plateau. A: AP radiograph. B: MRI in sagittal projection.
|
pain, usually about the medial aspect of the knee. Radiographs are
within normal limits initially, but the bone scan or MRI shows
abnormalities involving the medial aspect of the knee more often than
the lateral, with the femoral condyle being more frequently affected
than the tibial plateau. The prognosis is based on the size of the
lesion. When the involvement is greater than 50% of the transverse
diameter of the condyle, the prognosis is worse than when the lesion is
smaller. Initially, patients should be treated symptomatically with
protected weight bearing. The condition
often
resolves over the course of the next several months. It is important to
separate this entity from other conditions such as meniscal tears, so
that unnecessary arthroscopic surgery is avoided.
often go on to collapse despite protected weight bearing. A small
number of cases have been treated with osteotomies, but the results
have generally been unsuccessful, and larger lesions often lead to
either hemiarthroplasty or total knee replacement. The results have
been generally favorable. There is little experience with core
decompression in these cases and there is no evidence that it affects
the prognosis (101).
nonoperatively with restricted weight bearing and analgesics. Larger
lesions are more often symptomatic and have occasionally been treated
by core decompression with moderate success. A recent study revealed
satisfactory outcomes in 61 of 77 cases (79%), at a mean follow-up of 7
years (range, 2–16 years) (91).
The results of total knee replacement in this group of patients have
been disappointing. A recent report of 31 primary total knee
arthroplasties followed for 8 years (range, 2–16 years) revealed only
17 good to excellent results (55%). These poor results may be related
to the fact that the procedure was performed in young patients with
high activity levels and with bone that was metabolically and
structurally compromised (89).
Involvement is bilateral in 50% of the patients when associated with
corticosteroids. The initial treatment of this entity is symptomatic
and includes gentle exercises to preserve strength and ROM and the
avoidance of excessive stresses on the joint. There have been
relatively few reports of core decompression; however, the best results
have been obtained when the procedure was performed prior to humeral
head collapse (88). A recent study (69)
reported good to excellent results in 47 of 62 shoulders (76%) with a
mean follow-up of 8.5 years (range, 2–18 years). Some shoulders may
continue to function reasonably well even after humeral head collapse
has occurred. In some, however, the pain and disability is sufficient
to warrant either hemiarthroplasty or total shoulder replacement. These
are described in only a few reports, and there is mixed opinion as to
which of the two procedures should be utilized, even if radiographs do
not demonstrate changes in the glenoid (63).
following fracture, atraumatic osteonecrosis is occasionally seen in
association with involvement of the hip and other regions (34,86,90,136).
Talar involvement is seen in approximately 2% of patients with
osteonecrosis. Eighty percent of the patients are women and the
disorder is noted bilaterally 50% of the time (Fig. 125.44).
Other bones of the foot and ankle are also involved occasionally. These
include the distal tibia, calcaneus, medial cuneiform, metatarsal
heads, and distal fibula (Fig. 125.45).
Treatment options include analgesics, bracing, or casting. Core
decompression of the talus has been performed in a small number of
cases. A recent report (90) noted satisfactory
results in 29 of 32 cases. If these measures fail and the talus goes on
to collapse, significant pain and disability may result, requiring
tibiotalar arthrodesis or possibly pantalar arthrodesis (86). Fusion can be difficult to obtain. In one report (86), there were 8 of 9 nonunions (89%) following the first attempt. In another report (136),
9 of 11 ankles (82%) eventually went on to fusion, but multiple
procedures were often required; these included autogenous bone
grafting, crossed cancellous screws, tibial struts, the insertion of a
blade plate, and prolonged casting.
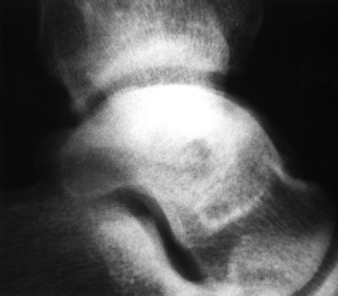 |
|
Figure 125.44. Lateral radiograph of the ankle, demonstrating nontraumatic osteonecrosis of the talus.
|
 |
|
Figure 125.45. MRI of the foot and ankle of a patient with steroid-related osteonecrosis of the distal tibia and talus.
|
included four men and four women with a mean age of 35. Four patients
had an associated autoimmune disease, all had involvement of other
joints, and all were on corticosteroid therapy. Four cases involved the
capitellum, three the trochlea, and three the lateral condyle. Four
elbows presented in the precollapsed stage radiographically and
responded well to nonoperative management. Two patients with collapse
failed nonoperative treatment and underwent core decompression for pain
relief. One elbow with advanced disease required a total elbow
arthroplasty. In contrast to the pediatric form of osteonecrosis,
Panner’s disease, osteonecrosis of the adult elbow can lead to endstage
arthritis.
adolescents were previously grouped together with a variety of skeletal
disorders collectively known as the osteochondroses. These include
Perthes disease, Freiberg’s disease, Koöhler’s disease, and Kienboöck’s
disease. With the exception of Perthes disease, these conditions are
rarely encountered (120).
pediatric form of osteonecrosis involving the capital femoral
epiphysis. It is usually found in patients between 4 and 10 years of
age, affects boys 80% of the time, and is unilateral in 70% to 80% of
patients. Pathologically, the disease is quite similar to the adult
form, with dead bone and marrow always present. Subchondral fractures
are common. As in the adult, evidence of repair occurs from granulation
tissue that grows into the area of necrosis with new bone deposition
upon dead trabeculae. The repair process is more successful in the
pediatric patient than in the adult, with complete repair occurring
often. The prognosis depends both on the age of onset and on the extent
of epiphyseal involvement. Patients younger than 6 years generally have
a good prognosis, as do patients in whom less than 50% of the femoral
head is involved.
second, is known as Freiberg’s disease or Freiberg’s infarction. This
condition affects adolescent girls in 75% of cases. It has been
postulated to occur after trauma to an elongated and more rigidly fixed
second metatarsal. Treatment consists of a short-leg walking cast for 4
weeks, followed by metatarsal supports. The condition is usually
self-limited and the prognosis is good. Occasionally patients do not
have symptomatic relief and require operative treatment consisting of
curettage and bone grafting, metatarsal osteotomy, or resection of the
metatarsal head.
Koöhler’s disease. It usually affects boys under 10 years of age, with
one third occurring bilaterally. The cause of this disorder is unknown
but it may be secondary to repetitive shear forces applied to the ankle
joint. It may also be related to late ossification of this important
weight-bearing bone. It is usually treated with arch supports and heel
wedges unless severe symptoms warrant the application of a short-leg
cast. The prognosis is excellent and the navicular becomes fully
ossified in most cases.
usually affects the dominant wrist of young adults. It has been related
to repeated trauma as well as to a genetic predisposition to a
shortened ulna. In the early stages, treatment options include
immobilization and symptomatic management. Radial shortening or ulnar
lengthening have also been employed with some success. For more
advanced stages with collapse of the lunate, surgical procedures
include excision of the lunate, replacement with a synthetic implant or
biological tissue, and wrist fusion.
condition that may be related to steroid administration or repetitive
minor trauma, or it may be idiopathic. It often
remains
asymptomatic for quite some time before presenting as wrist pain and
limitation of motion. Sclerosis and cyst formation often give way to
fragmentation and eventually degenerative arthritis of the wrist.
Treatment is similar to that for Kienboöck’s disease.
uncertain etiology, although corticosteroids and minor repetitive
trauma have been implicated. The clinical course, radiographic picture,
and treatment are similar to those described for osteonecrosis of the
lunate and scaphoid.
of the adult hip because this is the region most frequently affected.
Although the incidence of osteonecrosis is much lower than that of
other disorders affecting the hip joint, it can no longer be considered
a rare condition. Despite an increased interest over the past several
years, there is much yet to be learned about its etiology and
pathogenesis. As with many conditions, prevention is perhaps more
important than cure. We must therefore be aware of factors that
contribute to the development of osteonecrosis and eliminate or
minimize them to the extent possible. Recent studies have indicated
that subtle coagulopathies are often involved, and this information may
lead to more effective methods of treatment and prevention.
lesion may be relatively good, especially if the area of involvement is
not in a region of major weight bearing, most clinically diagnosed
cases will progress to femoral head collapse without specific
treatment. The majority of these will eventually require some type of
arthroplasty. Symptomatic treatment, including protected weight
bearing, is generally not successful. Our goal is therefore to
institute some type of definitive treatment as early as possible. This,
in turn, requires early diagnosis, which is now possible due to a
heightened awareness of osteonecrosis in the medical community and to
newer diagnostic modalities, particularly MRI.
treatment of early cases of osteonecrosis are core decompression,
various types of osteotomies, and several different grafting
procedures. These have been described in some detail in this chapter.
Unfortunately, it is difficult to compare accurately the results of
these procedures and to determine their relative indications because of
the many variables that exist in reports published to date.
occurred, prophylactic measures are far less effective. At this stage,
patients are generally treated symptomatically until pain and
disability necessitate some type of arthroplasty. Although THR is
currently the procedure most often selected, other choices include
femoral endoprosthetic replacement and modified cup arthroplasty or
hemisurface replacement, if the acetabulum is relatively uninvolved. It
has yet to be determined whether it is best to perform this type of
limited procedure to buy time before THR is required, or to proceed
directly with THR.
changes, THR is usually the procedure of choice. This is a reliable
operation that yields excellent pain relief and function. The major
concern is with long-term survivorship, because most patients with
osteonecrosis are young. However, recent studies indicate improved
durability with newer techniques and devices.
etiology and pathogenesis of this condition so as to prevent it from
occurring when possible. Research must continue on medical approaches
to prophylaxis and treatment. Methods for early diagnosis will continue
to improve, and the medical community at large must be made even more
aware of the importance of early diagnosis and early treatment. The
search should continue for better techniques to treat early cases of
osteonecrosis. Controlled, prospective studies should be instituted to
evaluate and compare the various procedures available so as to
determine the relative effectiveness and the specific indications for
each. These must include uniform inclusion criteria and effective
methods of evaluation and staging.
significant acetabular involvement, we should continue to evaluate and
develop better types of limited arthroplasties.
are already well established, we must improve the durability of THR
arthroplasty. With advances in surgical technique and in component
design, materials, and manufacturing, it is quite possible that the
useful life of a THR arthroplasty will be extended considerably so that
these components may last a lifetime, not only in the older patient,
but also in the younger patient with osteonecrosis. When this occurs,
there will be a dramatic improvement in our ability to handle patients
with even the most advanced stages of this frustrating condition.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
RK, Ciombor DM, Lord CF. Core Decompression Augmented with Human
Decalcified Bone Matrix Graft for Osteonecrosis of the Femoral Head.
In: Urbaniak JR, Jones JP, eds. Osteonecrosis: Etiology, Diagnosis, and Treatment. Chicago: American Academy of Orthopaedic Surgeons, 1997;301.
RK, Lennox D, Stulberg BN. The Natural History of Osteonecrosis of the
Femoral Head and Risk Factors for Rapid Progression. In: Urbaniak, JR,
Jones JP, eds. Osteonecrosis: Etiology, Diagnosis, and Treatment. Chicago: American Academy of Orthopaedic Surgeons, 1997;261.
HC, Grigoris P, Safran MR, et al. Precision Fit Surface
Hemiarthroplasty for Femoral Head Osteonecrosis: Long Term Results. J Bone Joint Surg Br 1994;76:423.
HC, Noordin S, Campbell PA, Schmalzreid TP. Precision Fit Surface
Hemiarthroplasty for Femoral Head Osteonecrosis. In: Urbaniak JR, Jones
JP, eds. Osteonecrosis: Etiology, Diagnosis, and Treatment. Chicago: American Academy of Orthopaedic Surgeons, 1997;373.
J, Durroux R, Fauchier C, Thieéchart C. Histopathology on the
Non-Traumatic Necrosis of the Femoral Head: Topographic and Evolutive
Aspects. In: Arlet J, Ficat P, Hungerford DS, eds. Bone Circulation. Baltimore: William and Wilkins, 1984;296.
J, Ficat RP. Forage-Biopsie de la Teête Femorale dans l’Osteonecrose
Primative. Observations Histo-pathologiques Portant sur Huit Foranes. Rev Rhum Ed Fr 1964;31:257.
CL, Collins DN, Nelson CL. Cup Arthroplasty, Surface Replacement
Arthroplasty, and Femoral Head Resurfacing for Osteonecrosis. Semin Arthroplasty 1991;2:222.
M, Bardenstein MB. Treatment by Bone-Grafting of Aseptic Necrosis of
the Femoral Head and Non-Union of the Femoral Neck (Phemister
Technique). J Bone Joint Surg Am 1958;40:1329.
MR, Rosenberg AG, Kull L, Galante JO. Primary Total Hip Arthroplasty
Using Non-Cemented Porous-Coated Femoral Components in Patients with
Osteonecrosis of the Femoral Head. J Arthroplasty 1994;9:457.
JL, Coogan PG, Gunneson EE, Urbaniak JR. The Asymptomatic Contralateral
Hip in Osteonecrosis of the Femoral Head. In: Urbaniak JR, Jones JP,
eds. Osteonecrosis: Etiology, Diagnosis, and Treatment. Chicago: American Academy of Orthopaedic Surgeons, 1997;231.
MT, Cabanela ME. Transtrochanteric Anterior Rotational Osteotomy for
Avascular Necrosis of the Femoral Head. Long-Term Results. J Bone Joint Surg Br 1993;74:597.
R, Kotz R. The Transtrochanteric Anterior Rotational Osteotomy of
Sugioka: Early and Late Results in Idiopathic Aseptic Femoral Head
Necrosis. Arch Orthop Trauma Surg 1987;106:161.
AC, Bhatia D, Jinnah RH, Hungerford DS. Long-Term Results of Core
Decompression for Ischemic Necrosis of the Femoral Head. J Bone Joint Surg Br 1995;77:42.
MA, Huo MH, Zatorski LE, Keggi KJ. Total Hip Arthroplasty Performed
without Cement in Patients with Femoral Head Osteonecrosis Who Are Less
Than 50 Years Old. J Arthroplasty 1998;13:876.
R, Buchler U. Overview of Attempts to Revitalize the Dead Head in
Aseptic Necrosis of the Femoral Head: Osteotomy and Revascularization.
In: The Hip: Proceedings of the Eleventh Open Scientific Meeting of the Hip Society. St. Louis, MO: CV Mosby, 1983;296.
JWM. The ARCO Perspective for Reaching One Uniform Staging System of
Osteonecrosis. In: Schoutens A, Arlet J, Gardeniers JWM, Hughes SPF,
eds. Bone Circulation and Vascularization in Normal and Pathological Conditions. New York: Plenum Press, 1993;375.
JP, Steinberg ME. Total Hip Arthroplasty in Patients with Avascular
Necrosis of the Femoral Head: A 2 to 20 Year Followup. Clin Orthop 1997;334:108.
CJ, Freiberg R, Gruppo R, et al. Thrombophilia and Hypofibrinolysis:
Reversible Pathogenetic Etiologies of Osteonecrosis. In: Urbaniak JR,
Jones JP, eds. Osteonecrosis: Etiology, Diagnosis, and Treatment. Chicago: American Academy of Orthopaedic Surgeons, 1997;105.
S, Engel A, Neuhold A, et al. Bone-Marrow Edema Syndrome and Transient
Osteoporosis of the Hip: An MRI Controlled Study of Treatment by Core
Decompression. J Bone Joint Surg Br 1993;75:210.
A, Mohtai M, Hotokebuchi T, et al. Transtrochanteric Rotational
Osteotomy for Idiopathic and Steroid-Induced Osteonecrosis of the
Femoral Head: Indications and Long-Term Follow-up. In: Urbaniak SR,
Jones JP, eds. Osteonecrosis: Etiology, Diagnosis and Treatment. Rosemont, IL. American Academy of Orthopaedic Surgeons, 1997;309.
MW, Mont MA, Scott R, et al. Surface Replacement Hemiarthroplasty for
the Treatment of Osteonecrosis of the Femoral Head. J Bone Joint Surg Am 1998;80:1656.
H, Torii S, Hasegawa Y, et al. Indications and Results of Vascularized
Pedicle Iliac Bone Graft in Avascular Necrosis of the Femoral Head. Clin Orthop 1993;295:281.
RL, Bourne RB, Rorabeck CH, McGee H. Total Hip Arthroplasty in Patients
with Avascular Necrosis of the Hip. Follow-up Observations on
Cementless and Cemented Operations. Clin Orthop 1992;281:145.
M, Thomine J, Postel M, Merle D’Aubigneé R. The Conservative Surgical
Treatment of Idiopathic Aseptic Necrosis of the Femoral Head. J Bone Joint Surg Br 1974;56:291.
T-B, Mont MA, Laporte D, Hungerford DS. Demographic, Radiographic and
Clinical Presentation of Adult Osteonecrosis of the Elbow. Chicago: Proceedings of the American Academy of Orthopaedic Surgeons, 1999;202.
RE, Barnes BC, Callaghan JJ, et al. Evaluation of Uncemented Total Hip
Arthroplasty in Patients with Avascular Necrosis of the Femoral Head. Clin Orthop 1993;297:168.
G, Fusco U, Avai A, Bombelli R. Osteonecrosis of the Hip Treated by
Intertrochanteric Osteotomy. A Four- to Fifteen-Year Follow-Up. J Bone Joint Surg Br 1988;70:761.
MH. The Treatment of Osteonecrosis of the Hip with Fresh Osteochondral
Allografts and with the Muscle Pedicle Graft Technique. Clin Orthop 1978;130:202.
MH, Harvey JP, Moore TM. Treatment of Displaced Subcapital
Transcervical Fractures of the Femoral Neck by Muscle-Pedicle Bone
Graft and Internal Fixation. J Bone Joint Surg Am 1973;55:261.
DG, Rao VM, Dalinka MK, et al. Femoral Head Avascular Necrosis:
Correlation of MR Imaging, Radiographic Staging, Radionuclide Imaging,
and Clinical Findings. Radiology 1987;162:709.
MA, Einhorn TA, Sponseller PD, Hungerford DS. The Trapdoor Procedure
Using Autogenous Cortical and Cancellous Bone Grafts in the Treatment
of Osteonecrosis of the Femoral Head. J Bone Joint Surg Br 1998;80:56.
MA, Fairbank AC, Krackow KA, Hungerford DS. Corrective Osteotomy for
Osteonecrosis of the Femoral Head. The Results of a Long-Term Follow-Up
Study. J Bone Joint Surg Am 1996;78:1032.
MA, Jones LC, Einhorn TA, et al. Osteonecrosis of the Femoral Head:
Potential Treatment with Growth and Differentiation Factors. Clin Orthop 1998;355S:314.
MA, Maar DC, Urquhart M, et al. Results of Core Decompression for
Avascular Necrosis of the Humeral Head. Average 7.4-Year Follow-Up. J Bone Joint Surg Br 1993;75:785.
MA, Myers TH, Krackow KA, Hungerford DS. Total Knee Arthroplasty for
Corticosteroid Associated Avascular Necrosis of the Knee. Clin Orthop 1997;338:124.
CL, Walz BH. Resurfacing of Only the Femoral Head in Young Patients Who
Have Osteonecrosis with Collapse, Delamination, and Significant Head
Involvement. In: Urbaniak JR, Jones JP, eds. Osteonecrosis: Etiology, Diagnosis, and Treatment. Chicago: American Academy of Orthopaedic Surgeons, 1997;405.
CJ, Pulliam IT, Cabanela ME. Total Hip Arthroplasty for Osteonecrosis.
Matched-Pair Analysis of 188 Hips with Long-Term Follow-Up. J Arthroplasty 1999;14:21.
FM, Pottenger LA, Finn HA, Vandermolen J. Cementless Total Hip
Arthroplasty in Patients with Steroid-Induced Avascular Necrosis of the
Hip. A 62-Month Follow-up Study. Clin Orthop 1994;303:147.
MP, Garino JP, Kiernan HA, Michelson CB. Long-Term Followup of Thorough
Debridement and Cancellous Bone Grafting of the Femoral Head for
Avascular Necrosis. Clin Orthop 1994;306:17.
S, Inoue A, Ono K. Intramedullary Hemorrhage as a Possible Cause of
Avascular Necrosis of the Femoral Head. The Histology of 16 Femoral
Heads at the Silent Stage. J Bone Joint Surg Br 1987;69:346.
S, Ohzono K, Ono K. Early Arteriopathy and Postulated Pathogenesis of
Osteonecrosis of the Femoral Head. The Intracapital Arterioles. Clin Orthop 1992;277:97.
KR, Bonfiglio M, Montgomery WJ. Non-Traumatic Necrosis of the Femoral
Head Treated with Tibial Bone-Grafting. A Follow Up Note. J Bone Joint Surg Am 1980;62:845.
ME, Larcom PG, Stafford BB, et al. Treatment of Osteonecrosis of the
Femoral Head by Core Decompression, Bone Grafting, and Electrical
Stimulation. In: Urbaniak JR, Jones JP, eds. Osteonecrosis: Etiology, Diagnosis, and Treatment. Chicago: American Academy of Orthopaedic Surgeons, 1997;293.
ME, Thickman D, Chen HH, et al. Early Diagnosis of Avascular Necrosis
(AVN) by Magnetic Resonance Imaging (MRI). In: Arlet J, Mazieres B,
eds. Bone Circulation and Bone Necrosis. New York: Springer-Verlag, 1990;281.
Y. Transtrochanteric Rotational Osteotomy in the Treatment of
Idiopathic and Steroid-Induced Femoral Head Necrosis, Perthes Disease,
Slipped Capital Femoral Epiphysis, and Osteoarthritis of the Hip:
Indications and Results. Clin Orthop 1984;184:12.
Y, Hotokebuchi T, Tsutsui H. Transtrochanteric Anterior Rotational
Osteotomy for Idiopathic and Steroid-Induced Necrosis of the Femoral
Head: Indications and Long-Term Results. Clin Orthop 1992;277:111.
Y, Katsuki I, Hotokebuchi T. Transtrochanteric Rotational Osteotomy of
the Femoral Head for the Treatment of Osteonecrosis: Follow-up
Statistics. Clin Orthop 1982;169:115.
K, Nishina T, Ohzono K, et al. Bipolar Prosthetic Replacement for the
Treatment of Avascular Necrosis of the Femoral Head. Clin Orthop 1992;277:121.
Y, Kokubo T, Ninomiya S, et al. Avascular Necrosis of the Femoral Head:
Natural History and Magnetic Resonance Imaging. J Bone Joint Surg Br 1993;75:217.
JR, Coogan PG, Gunneson EB, Nunley JA. Treatment of Osteonecrosis of
the Femoral Head with Free Vascularized Fibular Grafting. J Bone Joint Surg Am 1995;77:681.
Veldhuizen PJ, Neff J, Murphey MD, et al. Decreased Fibrinolytic
Potential in Patients with Idiopathic Avascular Necrosis and Transient
Osteoporosis of the Hip. Am J Hematol 1993;44:243.
H, Zeiler G. Idiopathic Necrosis of the Femoral Head: Results of
Intertrochanteric Osteotomy and Joint Resurfacing. In: Weil UH, ed. Segmental Idiopathic Necrosis of the Femoral Head. Berlin: Springer-Verlag, 1981;87.
M, Itoman M, Sagamoto N, Morita M. Strut Bone Graft for Aseptic
Necrosis of the Femoral Head: Theory and Surgical Technique. Orthop Surg 1983;34:902.