DIABETIC FOOT CARE
Professor and Chairman, The John Sealy Distinguished Centennial Chair
in Rehabilitation Services, University of Texas Medical Branch,
Galveston, Texas 77555.
Section Chief, Surgical Infectious Diseases, Department of Internal
Medicine, University of Texas Medical Branch, Galveston, Texas 77555.
diabetic foot infections (DFI) have dramatically increased in recent
years. The main reason for this increase is the growing diabetic
population. For example, there were approximately 6 million diabetics
in the United States in 1987 (59), and this number has grown at the rate of 9% per year to 16 million in 1997 (62). Four factors explain this dramatic growth in the number of diabetics.
-
Heredity. The at-risk populations such as Hispanics and Native Americans are increasing.
-
Weight gain. A higher percentage of people are overweight in the United States.
-
Longevity. Improved care for diabetics has decreased the mortality and morbidity rates for the diabetics.
responsible for the elevated rates of problems associated with DFI,
other factors play a role. Cases of diabetic ketoacidosis and
hypoglycemia are less frequent, and the renal problems, cardiac
problems and other comorbidities are better managed. Also, cases of DFI
are better handled. These factors result in a reduction in the rate of
amputation surgery. Hence, more diabetics are living longer, and with
the decrease in amputations, there is an increase in foot problems.
diabetes is still the leading cause of amputation in the United States.
In 1987, 560,000 lower extremity amputations were performed on
diabetics (39). Also, diabetics are 40 times
more likely that nondiabetics to have an amputation. A diabetic with an
amputation has a 30% chance that the other leg will be amputated in 3
years or a 50% chance of this happening within 5 years.
functional ambulation through the prevention and treatment of
neuropathy and infection. It is clear that development of peripheral
neuropathy in the diabetic is related to high blood sugar levels (23,33).
Frequent blood sugar tests for at-risk groups (e.g., overweight family
members) will aid in early diagnosis. Recently, the level of
hyperglycemia for the diagnosis of diabetes was lowered from 140 to 126
mg/dl (59), and at-risk group initial screening
may be lowered from 40 to 25 years of age. Aggressive management of
diabetes with patient education, diet, exercise, and medication yields
the best blood sugar control, resulting in decreased complications of
neuropathy and vasculopathy.
is demonstrated by the fact that almost half (43%) of all diabetics
have their first hospital admission or diagnosis of diabetes associated
with a foot infection (7,42).
Most patients, and many nurses and doctors, are unaware that neuropathy
is a main contributing cause of DFI. Ninety percent of these patients
have neuropathy that is associated with high blood sugar levels. Eye
problems in diabetics (retinopathy) and foot problems (peripheral
neuropathy) do not have the same etiology. Peripheral neuropathy is
mainly a chemical neuropathy, whereas retinopathy is due to ischemic
focal occlusion from plaques in athrosclerotic vessels. Although it was
previously believed that proper glucose control would not affect
diabetic retinopathy, it has been demonstrated that the diabetic
patient under “intensive control” can dramatically reduce the
progression of not only neuropathy and nephropathy but also retinopathy
(20,23,34).
Therefore, education of the patient, family, and health care team is
the key strategy in the prevention of these diabetic-associated
problems.
published a brochure, “The Diabetic Foot,” to help the physician with
patient education.* One recommendation
presented in this publication is that diabetic patients should wear
shoes at all times, except in bed, to protect the feet from minor
injury. Because DFIs start as ulcers in the insensate skin, normal or
continual pressure on insensate skin causes the skin to break down.
Therefore, diabetics must frequently check their feet to remove
wrinkles in socks or foreign objects in shoes and vary pressure on the
feet. They must always be cautious to avoid injury and to look for and
immediately respond to any signs of infection in their feet. Insensate
skin will also break down due to new shoes or a change in the level of
use of the foot such as would occur in a new job requiring more
walking. One patient of ours, a school teacher, presented with a
cellulitic toe ulcer. When we showed him that his shoe had a roofing
nail in the sole, he said, “We are building a gazebo, but I visited the
building site 10 days ago.” He had acquired the nail in his shoe then
and had been walking on it since. The cellulitis was bad enough to
require admission, intravenous antibiotics, and debridement, followed
by a long course of oral antibiotics and wound care, with eventual
healing. He returned five years later with a small ulcer in the other
foot from another nail. He sheepishly took the nail out of his pocket
to show us and commented, “I found this in my shoe shortly after I had
been outside.” The second ulcer healed quickly with outpatient care and
oral antibiotics. At least he knew to check his shoes and feet, and he
knew the signs of infection. Even though his disease had progressed, he
had less of a problem with the second infection than with the first.
Although peripheral vascular disease can contribute to the severity of
peripheral neuropathy, it is not the primary factor. Distal
polyneuropathy is present in approximately 60% of diabetics and can be
seen in any age group regardless of whether they require insulin or not
(23). Nerve death in the diabetic is a result of metabolic, vascular, and histologic changes.
and sodium-potassium-adenosine triphosphatase. These changes occur when
increased tissue glucose is metabolized by an alternate pathway as a
result of the lack of insulin, leading to decreased nerve cell function
and disruption of the membrane-associated sodium pump. This factor, in
turn, causes a reduction in the ability of the nerve cell to maintain a
normal polarized state, and leads to demyelination, nerve injury, and
eventually, nerve death (33).
Loss of large sensory fiber and motor nerves causes a decrease in light
touch sensation and proprioception, as well as ataxia and weakness.
Loss of small sensory fibers causes a decrease in pain and temperature
sensation. With decreased sensation, injuries may go unnoticed and
repeated insults to the soft tissue and bone can occur (9).
Also, loss of protective sensation in the joints often results in
excessive forces applied to ligaments, cartilage, and bone, leading to
joint erosions, dislocations, fractures, and Charcot’s joint (common
eponym for neuroarthropathy) development and necrosis (25,32). Neurovascular dysfunction and autonomic failure may also play roles in the development of the Charcot’s joint (9,31).
atherosclerosis, and blood is characterized by increased viscosity,
clotting, and thrombosis formation (18,65). This causes nerve death, ischemia, and embolization.
demyelination in peripheral nerves, connective tissue build-up, and
capillary basement membrane hypertrophy with capillary closure,
resulting in the death of both large and small myelinated nerve fibers (25,32,33 and 34).
vessel disease has been challenged. It appears that there is minimal
collateral vessel formation in the foot due to the rapid onset of
diabetic arteriosclerosis and lack of angiogenesis (18,40).
Although neuropathy is the cause of most ulcerations, vascular disease,
either alone or superimposed on neuropathy, can cause DFIs (51).
arteriosclerosis than nondiabetics. Bilaterality is more often an
expression of more aggressive disease progression. Calcification in
diabetic arteries is diffuse and found in the media layer. In contrast,
nondiabetics suffering from arteriosclerosis have a patchy distribution
of plaques in the intimal layer of the diseased artery (40,53). Calcified small arteries of the foot are commonly seen on radiographs of diabetics suffering from this vascular process.
iliac and femoral vessels requiring endarterectomies and proximal
bypass, this disease differs from that seen in nondiabetics because
diabetics tend to have a more distal distribution of disease. More
distal vessel disease in the diabetic is thought to occur because the
rapid progression overcomes the rate of distal collateral
revascularization. Frequently, this disease has diffuse involvement of
all three vessels below the trifurcation and may require a bypass that
extends far more distally than that seen in nondiabetics (7).
arteriosclerosis in nondiabetics are cigarette smoking, lipoprotein
abnormalities, and high blood pressure. Diabetics seem to be at greater
risk for these factors, and blood pressure, triglycerides, and
cholesterol are more frequently elevated and difficult to control in
these patients (60).
learn to monitor blood sugars closely with visits to the
endocrinologist, diabetologist, internist, and nutritionist. When an
infection is present, blood sugar levels are more difficult to control,
and may require several days or weeks to stabilize after the infection
is controlled. A well-kept patient diary that records date, time, blood
sugar levels, food, and medication is a useful tool.
loses its sensation, proprioception, and intrinsic muscle function.
Sensation and intrinsic muscle causes an “extrinsic plus” wide-based
gait for balance that progresses to claw toes, midfoot valgus, and
calcanealequinovalgus with decreased ankle and subtalar motion. Thus, a
flat, stiff, insensate foot is more prone to injury, further breakdown,
decreased mobility, and ulcer formation.
are noted and are worse when the serum glucose is poorly managed. Also,
many antibiotics are ineffective in the resulting hypoxic, acidic
environment and may be subtherapeutic in the ischemic tissue of
diabetics. Blood flow is further decreased in the swollen, infected
tissue seen in cellulitis, ulcers, abscesses, osteomyelitis, gangrene,
and Charcot’s joints. These complications often produce large dead
spaces and necrotic tissue, making the local host response and
antibiotic penetration even less effective.
Skin flora can cause deep infections in diabetics, occasionally making
it difficult to determine the correlation between cultures taken from
superficial ulcers or sinus tracts and the true pathogens causing deep
infection (66). Therefore, the most meaningful
cultures are those that are taken in the operating room from deep
tissue underneath the ulcers or open wounds. Many of the acute soft
tissue infections are caused by streptococci or Staphylococcus aureus, which may be found as the single pathogenic species (51).
With an increase in the chronicity of the infection, or in the face of
partial antibiotic treatment, polymicrobial infections are found in
greater frequency. Infections may include Pseudomonas and anaerobic
organisms, although it is uncommon to see a monomicrobic anaerobic
infection (66).
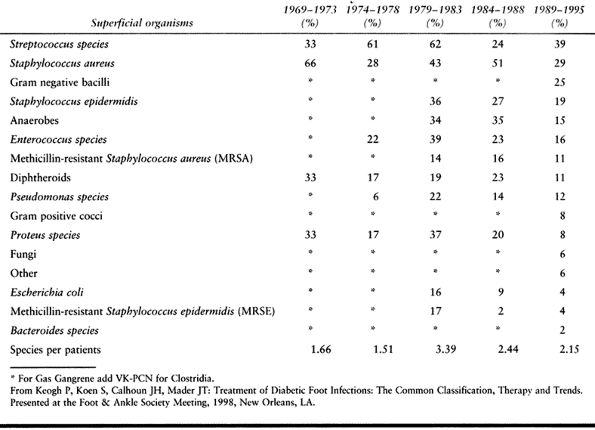 |
|
Table 116.1. Bacterial Cultures
|
vascular surgeon, plastic surgeon, nutritionist, diabetic nurse, and
social worker need to work with the patient and the patient’s family.
Regular, urgent, and emergent clinics that the diabetic patient can
easily access will prevent minimal
problems
from becoming major problems. The diabetic wound care nurse or
physician’s assistant plays a vital role as a part of the care team.
Special training, either with a skilled local foot care team or at
national courses, can give the nurse or physician’s assistant the
skills to organize a wound care clinic. This clinic should be open 5
days a week for dressing care, and a member of the foot care team must
be readily available to answer telephone inquires from patients and for
referrals. Education of local emergency rooms and primary care clinics
regarding preventive care and the decision of when to refer is
important. The most important members of this team are the patient and
family because they must become knowledgeable and involved. If they are
not or cannot be involved, then the prognosis of the patient is
typically poor and they will require more care by the doctors and
nurses in the clinic or hospital.
percent of amputations and 20% of hospital admissions each year are due
to DFIs (60). In a poorly treated foot ulcer, osteomyelitis increases the risk of amputation (3).
A good treatment plan must address the underlying cause of the skin
breakdown or infection and not just simply treat the ulcer itself.
and health care team the most common causes of (and terms for)
infection and pathology in the diabetic foot. The team, which consists
of the patient, family, doctors, nurses, and assistants, must be able
to classify the infection and respond to it appropriately: “Is it more
red?” (i.e., cellulitis); “Is there a skin sore?” (i.e., ulcer); “Is
there a deep infection?” (i.e., abscess or bone infection); “Is there
dead tissue?” (i.e., gangrene); “Is it getting better or worse?” (i.e.,
do we continue with this treatment or go to the clinic for a check-up
or to the emergency room for admission?).
over a 20-year period and have concluded that a simple, common
classification system (Table 116.2) aids in consistent categorization and treatment for the best outcome (7,10,11).
The simplicity of the classification also facilitates as ease of
communication between the team and the patient. The best way to think
about the type of infection in order of increasing severity and stage
is cellulitis, ulcer, abscess, osteomyelitis, and gangrene. One or all
of these infection grades can be present, but typically one
predominates and the more severe type (i.e., the higher stages)
determines treatment. Furthermore, the presence of neuropathy and
ischemia make the treatment more involved.
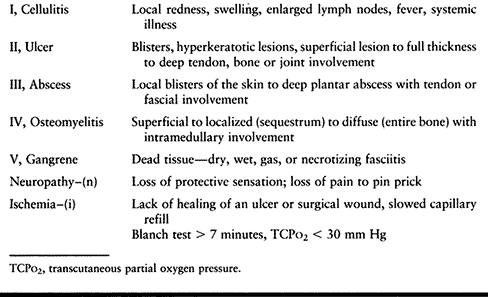 |
|
Table 116.2. The Common Types of Diabetic Foot Infections
|
can be used for self-examination of the bottoms of the feet. Patients
with impaired vision should have a family member inspect their feet
daily. It is important to stress daily foot washing, careful drying
between the toes, and daily application of a light water-based (not
alcohol-based) cream without moisturizing between the toes. Caution
patients
to
avoid exposure of feet to extremes of temperature. Have them test the
temperature of bath water with the elbow before immersion. Patients
also need to avoid using chemical agents or doing bathroom surgery to
remove corns or calluses, and application of adhesive tape to the feet
must be avoided. It is also important for the patient to inspect the
insides of their shoes daily for foreign objects, nail points, and torn
shoe linings. Wearing properly fitted stockings without seams or mended
areas may prevent unnecessary irritation. Caution patients against
wearing sandals or shoes without stockings. Patients should never walk
barefoot, especially on hot sandy beaches or around swimming pools.
professional help for routine nail care. Hypertrophied nails colonized
by fungi are potential infection sites, and trimming can be difficult
if the patient’s sight is poor and toes are insensate. Usually, a nurse
or a physician’s assistant can provide nail care in the office.
care plans now pay for extra-depth diabetic shoes. If the patient’s
insurance company does not cover the cost of these special shoes, send
a letter of medical necessity or contact the insurance company with the
recommendation that in order to try to prevent expensive surgery or
greater disability, the patient needs extra-depth diabetic shoes. Many
patients can be managed in the more “fashionably” acceptable jogging or
walking shoes. Deformities such as ingrown toenails, claw toes,
bunions, and hyperkeratotic areas can be managed with orthotics or
shoewear modifications.
include patient noncompliance and poor visual acuity, making daily foot
inspections unreliable. Patients with peripheral neuropathy often have
delays in diagnosis of infection owing to a failure to sense tissue
damage. Also, autonomic dysfunction leads to anhydrosis and a loss of
skin temperature regulation, which can result in dry, scaly,
easy-cracking skin contributing to ulcer formation. These cracks and
ulcers provide a portal for bacterial invasion that leads to infection.
Poorly controlled diabetics have these problems in greater frequency (27). When ischemia or malnutrition is present, infection is also more likely to develop.
begin with the evaluation of both lower extremities from the knee down.
Owing to peripheral neuropathy, diabetics with infection may not have
pain as a primary complaint. The patient and family will note fever,
chills, and recent hyperglycemia despite a normal dose of insulin. On
physical examination, look for erythema, swelling, induration, or
fluctuance, and probe ulcers for depth and the presence of an abscess
or exposed bone. Laboratory studies may show an elevated white blood
cell (WBC) count with an increase in polymorphonuclear cells and
elevated erythrocyte sedimentation rate, C-reactive protein, or
glycosylated hemoglobin.
autonomic, and motor functions. Although diabetics may have numbness,
they may still feel pain. The so-called “stocking-glove” description of
the sensory deficit distribution is not accurate. Although peripheral
neuropathy is usually more severe distally, the proximal border of the
neuropathic area is not, typically, a perfect cross section of the
affected limb. The border is typically more distal directly over the
major sensory nerves for evaluation of touch and more proximal farther
from the major nerves for evaluation of painful stimuli. Some patients
complain of severe paresthesia (spontaneous pain or burning) and
dysesthesia (contact pain), which is thought to be a result of
spontaneous depolarizations of injured or regenerating nerves (33).
Light touch, the pin-prick test, two-point discrimination, and the
Semmes-Weinstein monofilament can be used to document the sensory
deficit. About 10% of patients who have already had ulceration still
meet the supposed threshold criterion of feeling the 5.07%
Semmes-Weinstein monofilament (7). The pinwheel
test allows rapid cutaneous sensory mapping of fine point and pain
perception. Clean the pinwheel well with alcohol between patients or
use disposable pinwheels. Autonomic deficits present as dry, stiff skin
that easily cracks owing to a loss of skin temperature regulation,
abnormal sweating, and arteriovenous shunting (1,27).
Clawing, pes planus, and equinus contracture occur from motor
denervation of the intrinsics and compensation by the extrinsics. When
combined with decreased sensation, the likelihood of ulceration
increases dramatically.
additional diagnostic screening. Patients without pulses and good
capillary refill may benefit from vascular consultation and screening
with Doppler studies, transcutaneous
oxygen
measurements, or angiograms. Vasculopathy can be demonstrated by
blanching normal skin and releasing the pressure. Normal blood flow
results in capillary refill in less than 2 seconds. A mild decrease in
the blood flow requires 7 seconds, moderate flow takes up to 13
seconds, and severe impairment greater than 13 seconds. Assess the
temperature of the skin with the back of one’s fingers. Some other
signs of vascular insufficiency can be observed by skin changes that
include loss of hair and smooth or edematous skin.
For Doppler pressures, request toe pressures and pulse-volume
recordings (PVR) at different locations along the limb. The PVR is
normally triphasic, but when the vessel looses compliance, the waveform
becomes monophasic or biphasic (7). Toe
pressures less than 40 mm Hg are unlikely to result in healing of
ulcers and are at a higher risk for requiring reconstructive procedures
(2). We recommend the use of transcutaneous oxygen measurement (TcPO2) for evaluating vascularity, healing potential, and soft-tissue viability (3,11,29). Values of TcPO2 lower than 20 mm Hg often result in local surgical failure unless a vascular bypass is performed (13,14,70). Other useful studies are angiography or xenon clearance studies, but these procedures are more expensive and invasive.
examination determine the need for imaging studies. Imaging studies are
often overused. Plain radiographs suffice in the vast majority of DFI
cases. In all patients presenting with DFI, high-quality radiographs of
the feet include anteroposterior (AP), lateral, and oblique views. Look
for loss of soft-tissue planes, lucencies indicating gas, foreign
bodies that may have precipitated the infection, and bony changes such
as erosion and periosteal new bone formation that may indicate
osteomyelitis. The osseous radiographic changes of neuroarthropathy can
simulate osteomyelitis and the differential diagnosis can sometimes be
difficult. Lytic changes in bone without overlying skin ulceration is
usually not due to osteomyelitis but rather due to neuroarthropathy.
Radionuclide scans, computed tomographic (CT) imaging, and magnetic
resonance imaging (MRI) may be necessary to differentiate between
osteomyelitis and Charcot’s arthropathy, abscess, cellulitis, or early
osteomyelitis (19,30,50,55,68).
Although these studies are useful in certain instances, a sterile
aspiration or biopsy in clinic or on the hospital floor is an easy,
rapid, and painless procedure, because the patient has no sensation in
the involved area.
quite explanatory and an excellent teaching tool for physicians and
nurses; however, because it is complex it can make taking an accurate
history and understanding by patients problematic. A commonly used
classification that is simple is (in order of increasing severity and
stage) cellulitis, ulcer, abscess, osteomyelitis, and gangrene.
or ulcer to full foot or leg involvement, with swelling and lymphedema.
Underlying abscess, osteomyelitis, gas gangrene, or necrotizing
fasciitis that can cause sepsis and death should be ruled out by
examination and radiographs. Most mild cellulitis around a callus or
ulcer can be managed on an outpatient basis with local skin care and
oral antibiotics (Fig. 116.1). Mark the border
of the red cellulitic skin with a permanent pen to establish a baseline
before treatment is started. if the cellulitis worsens (extends 2 to 3
cm past the baseline) after 24 to 48 hours of oral antibiotics, the
patient should be re-evaluated and treated with different oral
antibiotics or hospital admission and intravenous antibiotics.
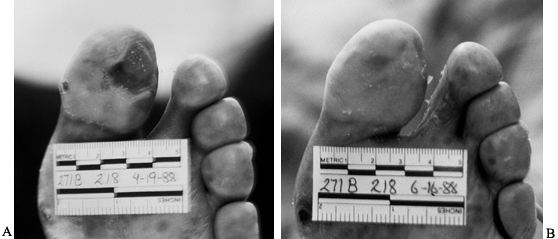 |
|
Figure 116.1. A:
Latino man, 52 years of age, who had non-insulin-dependent diabetes mellitus (NIDDM) for 10 years and was employed as head of housekeeping at the hospital. Admitted to the hospital with cellulitis and diabetic ulcer on the plantar aspect of left great toe. The TcPO2 was 38 mm Hg at the base of toe. The patient was discharged after 3 days of intravenous (IV) antibiotics [cephalexin (Keflex) and gentamicin], local wound care, and oral antibiotics (Keflex for 2 weeks); the wound healed at 2 months. B: Similar ulcer 3 years later treated without hospital admission, oral antibiotics for 2 weeks, and local wound care. Patient still works as chief of housekeeping 10 years later. |
infected before beginning wound care. Clinicians should diagnose ulcer
infection by looking for drainage, depth, erythema, and cellulitis
surrounding the wound; if infection is suspected, take wound cultures.
Swab cultures from the surface of an ulcer or from sinus drainage have
been shown to be poor indicators of the bacterial species below (64).
Therefore, clinicians should curet and culture an ulcer from the base
for aerobic and anaerobic organisms. Start with empirical antibiotics
if infection is suspected, and modify them, if necessary, when the
culture results and antibiotic sensitivities are available. The
bacterial species seen in DFIs are often multiple and range from
streptococcus or staphylococcus species (the two most common) to
gram-negative organisms and anaerobic organisms (Table 116.1).
Bacterial infections usually can be treated with empiric oral
antibiotics. We usually begin with first-generation cephalosporins and
modify treatment based on the response to treatment, antibiotic
sensitivities, and cultures. Quinolones may be added for gram-negative
coverage and penicillin-VK for clostridial infections (Table 116.3).
It the infection is arrested after 2 to 4 weeks of treatment, the
antibiotic may be stopped or the dose decreased. In patients with
advanced cellulitis, gas gangrene, or sepsis, treatment usually
requires hospital
admission, intravenous antibiotics, urgent surgical debridement, deep tissue cultures, and an infectious disease consultation.
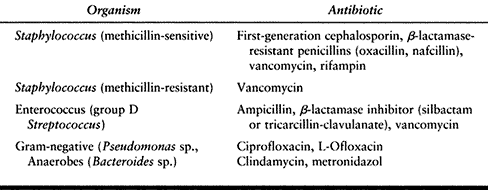 |
|
Table 116.3. Antibiotic Selection
|
basis with local wound care in conjunction with redistribution of
pressure with total contact casts, orthotics, or diabetic shoes. The
evolution of wound management and wound care products has created the
need for standardized pathways for wound management. Accurately record
the size of the ulcer in centimeters with an anatomic sketch in the
patient’s chart. An alternative method is to trace the ulcer on a glove
or ziplock bag, then separate and dispose the side of the glove or bag
that touched the ulcer and tape the tracing in the chart. Digital
photographic prints of the wound are the easiest, most accurate means
of documentation. Make note of the size and color of the
wound
bed, and look closely at the wound margins to identify sinus tracts,
undermining or rimming. Describe the characteristics of the exudate by
including the type, amount, color, consistency, odor, and the adherence
to the wound base. These characteristics must receive ongoing
assessment so that an appropriate dressing can be used that is based on
the healing stage of the wound. The primary function of a wound
dressing is to promote the moist healing environment necessary for
tissue repair (72). The functions and indications of the most commonly
used dressing materials are listed in Table 116.4. Ulcers with significant drainage require gauze dressings with frequent changes.
 |
|
Table 116.4. Characteristics of Some Available Wound Care Dressing Materials
|
using custom-molded, extra-depth, diabetic shoes. Deeper ulcers with
hyperkeratotic edges or necrosis can be debrided in the clinic and
successfully treated with the application of a total contact cast.
Treatment with intravenous antibiotics based on a swab culture, or
preferably, a deep culture, is helpful if significant cellulitis is
present.
-
Apply a light dressing to the wound and hold it in place with a stockinette.
-
Pad all bony prominences for protection while in the cast.
-
Overlap cast padding only one-half width of the roll.
-
Apply a layer of plaster, which is then overwrapped with fiberglass.
-
Use a cast shoe.
-
Excessive padding will allow movement in the cast and cause skin irritation or ulceration.
-
The cast can be bivalved so dressing changes are easier and the cast will last longer.
and the wound. As the situation stabilizes, the cast may be changed
less often.
debridement to remove all necrotic, infected tissue, followed by
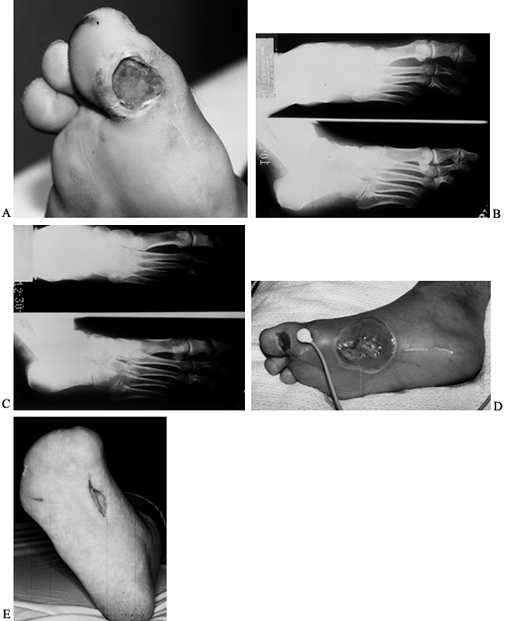
reconstruction (Fig. 116.2C, Fig. 116.2D and Fig. 116.2E).
Surgery for diabetic abscess and osteomyelitis is more difficult than
that for a nondiabetic because of the poor blood supply and insensate
tissue. Toe amputations, ray resections, midfoot amputations, Syme’s
amputations, muscle flaps, or distal below-the-knee amputations (BKAs)
require good blood flow to heal and adequate protective sensation to
stay healed. If the area being treated is insensate, it allows more
aggressive debridement with less general anesthesia, which is safer
owing to the multiple system dysfunction (renal, cardiac, pulmonary) in
the diabetic. Some anesthesiologists may be unfamiliar with
diabetes-associated numbness and require guidance on the amount of
analgesia to use during procedures. We test for lack of sensation at
the incision site by squeezing with toothed tissue pick-ups before
administration of anesthesia. Most distal debridement and amputation
can be performed with a small amount of local or intravenous analgesia.
The anesthesiologist must be present to monitor cardiac, pulmonary, and
renal function. Amputation for the treatment of osteomyelitis is
commonly used to remove a chronic nidus of infection and to get to the
level of protective sensation for prosthetic wear. Antibiotic treatment
for deep abscesses and osteomyelitis are based on cultures and
antibiotic sensitivities. Only 2 weeks of antibiotics may be required
if the infected bone and soft tissue are removed and good bleeding soft
tissue is left. Up to 6 weeks may be required if there is
osteomyelitis. Hyperbaric oxygen helps the patient with marginal oxygen
levels to heal.
 |
|
Figure 116.2. A:
White man, 37 years of age, who has NIDDM and works as welder. He has five children but no insurance. He presented with an ulcer on his toe. B: He had no osteomyelitis. He was not able to perform wound care or use total contact cast because of work. The ulcer progressed to osteomyelitis of the first metatarsophalangeal joint (C) and plantar abscess (D). He was treated with amputation of the toes and debridement of midfoot, IV antibiotics for 3 weeks, and second-stage closure with skin graft at 2 weeks (E). He still works 10 years later with same foot. |
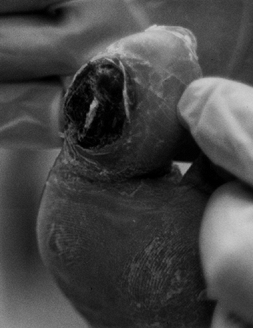
gas gangrene, or necrotizing fascitis. Although all gangrenous tissue
will eventually be removed, dry gangrene can be managed on an
outpatient basis with local wound care for long periods until the odor,
pain, or local infection requires surgical removal. Outpatient
management for dry gangrene requires that the patient, family, or local
care provider watch for signs of sepsis or gas gangrene. Although dry
gangrene usually is painless, some patients have good sensation, and in
this patient subset, the acid from the dead tissue can cause severe
pain that is relieved only with amputation (Fig. 116.3).
Outpatient management for forefoot gangrene can occasionally result in
“auto-amputation,” in which the necrotic tissue breaks off
spontaneously.
 |
|
Figure 116.3. White woman, 63 years of age, who had NIDDM for 3 years. She had normal sensation and a very painful gangrenous toe. TcPO2 at foot of 12 mm Hg; at ankle, 21 mm Hg; at proximal tibia, 41 mm Hg. Treated with BKA.
|
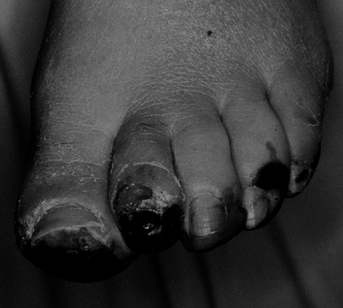
If the patient refuses a higher amputation level or if the patient is
healthy, we use the lowest amputation level that will help with
ambulation and prescribe adjunctive hyperbaric oxygen therapy (Fig. 116.5). If the patient is ill or not able to use the lower amputation level for a prosthesis or ambulation and has a TcPO2
of less than 40 mm Hg, we perform a higher amputation. To assess the
patient’s healing potential intraoperatively, we use William Wagner’s (69)
tourniquet technique. When a tourniquet above an amputation site is
deflated and poor bleeding results after as much as 5 minutes, wound
healing is unlikely. If bleeding begins 3 minutes after deflation,
there
is an 80% healing rate, and at 2 minutes or less, a 100% healing rate occurs (69) (Fig. 116.9D).
 |
|
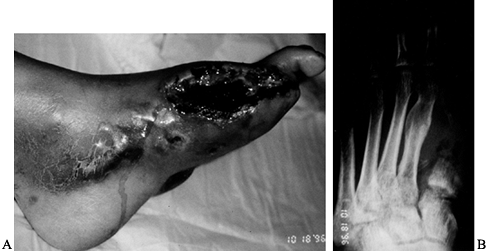
Figure 116.4.
White man, 40 years of age, who had insulin-dependent diabetes mellitus (IDDM) for 5 years. He had protective sensation at ankle and good blood flow. He was treated with amputation of all toes. |
 |
|
Figure 116.5. A,B: White man, 51 years of age, who had IDDM for 7 years. The TcPO2
at BKA site was 29 mm Hg, indicating gas gangrene. The patient was treated with BKA, hyperbaric oxygen treatment, and IV antibiotics. |
 |
|
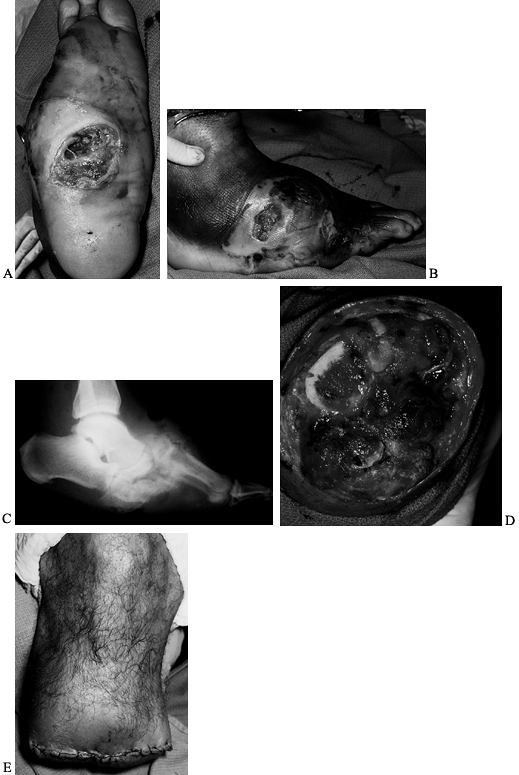
Figure 116.9. A,B: White man, 46 years of age, had IDDM for 8 years and a Charcot midfoot for 6 months. C: The deformity progressed and he developed a plantar ulcer. The TcPO2
at the foot was 11 mm Hg; at the ankle, 21 mm Hg; at the BKA site, 39 mm Hg. There was pain sensation in the proximal anterior tibia and posterior midcalf. A radiograph showed gas in the tissue. The patient was febrile and felt ill. He was admitted to the hospital and placed on penicillin, clindamycin, and ciprofloxacin. Cultures showed Staphylococcus, Streptococcus, Pseudomonas, and Diptheroids. D: Emergent guillotine amputation was performed; there was minimal bleeding. Cultures at the amputation site showed Staphylococcus. The patient was treated for 3 days with triple antibiotics, wet-to-dry sterile saline dressings changed every 8 hours. E: The amputation was revised and completed 3 days after the guillotine procedure; cultures were then negative. IV antibiotics (clindamycin, ciprofloxacin) were administered for 2 weeks; oral antibiotics (clindamycin, ciprofloxacin) were administered for 4 weeks. The patient now wears a permanent BKA prosthesis, is ambulating, and has returned to work. |
adequate, all lower extremity wounds should be closed. If the degree of
infection is too severe (i.e. purulence, cellulitis, or edema,), then
perform a staged debridment and closure, returning the patient to the
operating room in a few days for repeat debridement or wound closure,
or both. The surgeon must debride or amputate to a level on the limb
that produces sufficient bleeding indicative of adequate circulation
for healing.
It can cause a great deal of stress for patients, so they may need time
and counseling before making a decision about an amputation. Of the
“stressful events of life” amputation is second only to loss of a loved
family member. Patients frequently go through the “stages of death and
dying” of denial, anger, negotiation, depression, and acceptance. Some
patients refuse to have an amputation for religious reasons or request
that the amputated part be stored for the time of burial. We describe
amputation to patients as a type of reconstructive surgery that is
necessary to remove infected or dead tissue so that the a patient’s
health and function may improve. Most patients understand this and work
very hard to rehabilitate. The design of prosthetic devices continues
to improve, thereby increasing the percentage of patients that have a
functional outcome. See Chapter 120 and Chapter 122 on amputation and prosthetics.
 |
|
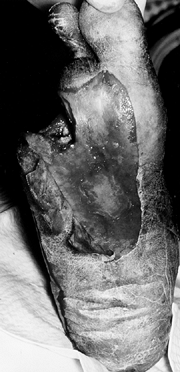
Figure 116.6. Black man, 63 years of age, who had a foot with wet gangrene. The TcPO2 at the BKA site was 21 mm Hg. The patient was treated with BKA, hyperbaric oxygen treatment, and IV antibiotics.
|
with attention to nerve and vascular function are the main principles
of foot reconstruction. After the infected, dead tissue is removed,
wound closure with a good weight-bearing stump is essential. Do not
leave bony prominences or less than five toes or metatarsals because
this produces an unbalanced foot that almost guarantees further surgery
in the next year. It is also of no value to perform inadequate
debridement or close ischemic skin or muscle.
Analgesics and other pharmaceuticals [e.g., amitriptyline (Elavil) 25
to 100 mg at bedtime for sleep, or fluphenazine (Prolixin)] can be
helpful in controlling the paresthesias and dysesthesia (1,42,65).
adequate padding to relieve overloaded bones and tissue. Soft
Plastazote is better than Pelite to avoid shear stress and give a firm
underlayer of support. A wide toe box with extra-depth shoes is
necessary to provide room for toe deformities. Shoes should fit well
when purchased and should not be expected to stretch out with wear.
necessary to heal the ulcers or prevent recurrence. With good blood
supply, an ostectomy may relieve the pressure of a bony prominence.
Balancing of muscle forces such as percutaneous tendoachilles
lengthening for tight heel cords, as well as procedures for intrinsic
minus clawing can relieve excessive pressure on the metatarsal heads.
Plastic surgery consultation may be needed when soft-tissue coverage is needed (46). See Chapter 112, Chapter 113, Chapter 114, Chapter 115 and Chapter 118 for details on foot reconstruction.
diabetic patients with chronic ulcerations that do not heal despite
other treatment options. Pentoxifylline and its metabolites decrease
the viscosity of blood, therefore improving its flow properties. It
improves tissue oxygenation. In addition, pentoxifylline 800 mg/day was
shown to improve walking distance, paresthesia, skin temperature, and
subjective overall response (12).
for possible reconstruction. This procedure may range from a single
vessel angioplasty to multiple by-pass arterial procedures. In the
diabetic vessel, bypass operations are more distal owing to the higher
number of diseased arteries below the trifurcation of the popliteal
artery (58). Revascularization can heal ulcerations and allow more distal foot reconstructions or amputation (29).
lack of protective sensation. The incidence of neuroarthropathy in
diabetics ranges from 1% to 2.5% (27).
Charcot’s joint, the common eponym for neuropathic arthropathy, was
coined from the description given in 1868 by J. M. Charcot for a
patient with syphilitic joint destruction (41). Treatment is directed toward preventing progression and the sequelae of infection and amputation. See Chapter 124 for more details.
arthropathy. It is believed to be caused by a loss of protective
sensation about joints, allowing repetitive microtrauma to have
additive effects on bone and joint destruction (21).
The inflammatory changes of erythema and increased blood flow seen in
Charcot’s arthropathy may be due to autonomic neuropathy and can
contribute to the bone and joint destruction that sometimes occurs
despite adequate immobilization and rest (43).
Neuropathic arthropathy is accelerated osteoarthritis that causes
hypermobility of the joint. Typically, joint fragmentation and
fractures occur with destruction of the articular cartilage and bone,
with accompanying synovitis and pannus formation (9).
are syphilis, syringomyelia, alcoholism, stroke, congenital
insensitivity to pain, spinal cord or peripheral nerve injury, and
spina bifida (35). Patients with these
diseases, however, usually do not have the severe vascular and
immunologic changes that diabetics experience. Other severe problems
might also be present (e.g., alcoholism, malnutrition, cord injury, and
total paralysis), but the neuropathic arthropathy can be managed much
like that for diabetics.
and numbness. A recent increase in shoe size may also be reported. Pain
is not typically a chief complaint, and when it is present, it is
usually less than expected. Neuroarthropathy is commonly found in
middle-aged diabetics with a history of poor glucose control. Fever,
chills, nausea, and malaise are generally absent, but may be present
with an infected Charcot joint. Capillary refill can be assessed with a
blanch test or foot elevation, or both, and is typically normal in the
Charcot foot. If the foot remains red and warm with elevation,
infection may be present.
but the erythrocyte sedimentation rate and glycosylated hemoglobin are
usually elevated. Also, TCPO2 is usually normal.
In the preneuropathic arthropathy stage, joints at risk for progression
or deformities (e.g., hallux valgus or claw toes) can be seen, and
muscular imbalances (e.g., tight heelcord and clawing) can be
identified. Radiographs of patients with early neuropathic arthropathy
show joint widening and stress fractures. In progressive and late
stages, further destruction and multiple joint involvement are seen.
 |
|
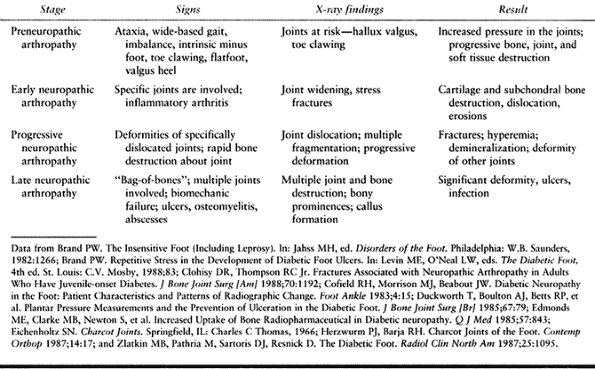
Table 116.5. Neuropathic Arthropathy Stages
|
 |
|
Table 116.6. Eichenholtz Staging of Neuropathic Arthropathy
|
the toes, metatarsophalangeal joint destruction, and metatarsal stress
fractures (6,17,43).
In the midfoot, the arch becomes flattened, the distal segments
dislocate dorsally, and fragments of the cuneiform or cuboid become
prominent in the sole and can cause ulcers (48).
The hindfoot is disrupted as the calcaneus or talus dislocate, allowing
the talus or malleoli to become prominent and cause ulcers (56). Occasionally, entire bones, especially the talus, cuneiform, or cuboid, are crushed in situ.
Recurrence of the neuroarthropathic process is rare and is most
commonly seen in renal transplant patients. Bilateral involvement has
been seen in 35% of patients with diabetic Charcot’s process (36).
 |
|
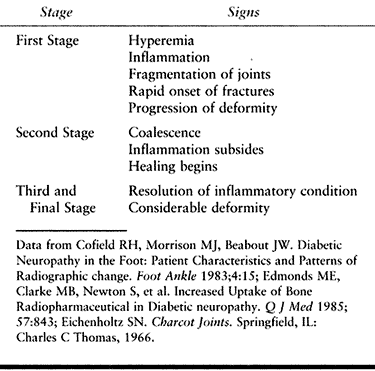
Figure 116.7. A,B:
Black man, 55 years of age, who worked in hospital as a radiology technician. He had IDDM for 12 years before he noticed both feet swelling for 2 years before presentation. At presentation, he had minimal pain and was placed in extra-depth diabetic shoes for bilateral, midfoot, early Charcot’s joints. The condition continued to progress on the right side, so he underwent bilateral midfoot and hindfoot fusions 2 years later. Six years later, the deformities have stabilized (C–G) and the patient is disabled. |
 |
|
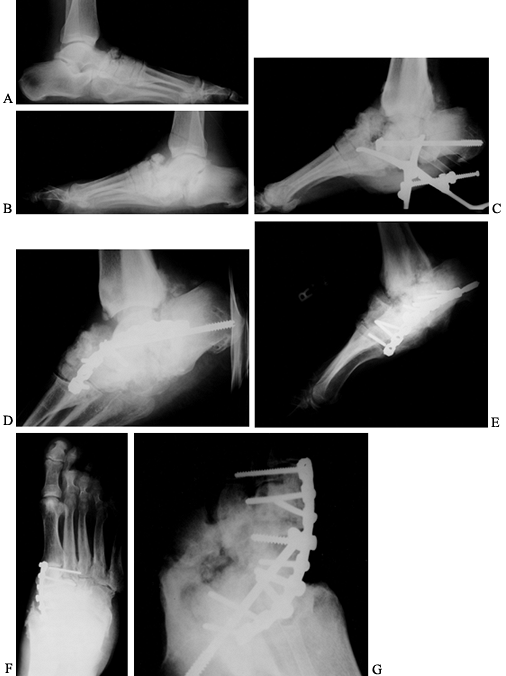
Figure 116.8. A,B:
White woman, 32 years of age, who had IDDM for 4 years secondary to renal failure and immunosuppression. She presented with a 2-month history of ankle deformity and an ulcer with purulent drainage. Cultures showed Staphylococcus aureus and Streptococcus, which was sensitive to penicillin. She was treated with a 6-week total contact cast and oral antibiotics until the cellulitis resolved and the wound healed. After healing she underwent C,D: debridement surgery and calcaneal, talar, and tibial fusion with a nail (no bone graft). She was treated with IV antibiotics for 2 weeks, oral antibiotics for 6 weeks, a cast for 3 months, a brace for 2 months. Solid union resulted. |
Charcot’s joint changes from infection. A technetium bone scan will
show increased uptake in neuropathic arthropathy but is not needed if
infection is either obvious or not suspected (26,47).
Gallium and indium scans can be helpful in diagnosing and defining the
extent of infection. Arteriography is frequently used to determine if
and where vascular bypass is indicated (37,44,45,67).
Although an 111-indium WBC scan is preferable to MRI to rule out
osteomyelitis, it is usually negative in patients with neuropathic
arthropathy, although false-positive readings may occur. MRI is
sensitive in showing the extent of infection that might be superimposed
on Charcot’s changes (30,50). If the 111-indium WBC scan is positive, biopsy confirmation is recommended (30).
Charcot’s foot. Patient behavior modifications are necessary to
eliminate vascular disease risk factors such as smoking, and elevated
blood cholesterol and triglycerides. Have patients avoid activities
that cause repetitive stress on the feet. Frequent follow-up by the
treating physician is essential.
arthropathy have custom-molded orthotics that accommodate the deformity
and resist its progression. Rigid orthotics should
be
avoided because they will cause ulcers. Wide toe box, extra-depth shoes
provide room for the abnormal forefoot. Rocker bottom soles decrease
midfoot stresses (27). High-top, ankle lace-up shoes attached to molded lower leg braces resist ankle deformity.
to prevent deformity progression until consolidation occurs. Patellar
tendon-bearing casts unload the foot and ankle (61).
During phases of swelling, frequent cast changes are needed to prevent
pressure ulcers caused by the cast. Likewise, bracing can be helpful
both during and after progression of a deformity by placing the foot
into optimal alignment and position and supporting it. This can be
provided by a fairly restrictive orthosis such as a customized
ankle-foot orthosis that is well padded to accommodate any structural
abnormality.
infection. For ischemia, vascular repair might be indicated, but most
Charcot’s joints have good to excellent blood flow (37,44,45,58,67).
Infection is usually obvious with an ulcer, and drainage can be easily
established with a sterile aspiration of the insensate foot in the
clinic (Fig. 116.9). Infection in the Charcot
joint is treated much the same way as DFIs outlined earlier. Establish
the extent of infection—cellulitis, ulcer, abscess, osteomyelitis, and
gangrene—and treat with culture and sensitivity-directed antibiotics,
debridement, and reconstruction. Infection in the Charcot joint usually
means there is an excellent blood supply (but not always!) so
antibiotic delivery and the host’s ability to fight the infection are
good, but the bone and joint involvement is more severe, so the
debridement and reconstruction are more difficult. Usually, an infected
Charcot joint will need to be “staged.” In the first stage, identify
the bacteria with sterile deep cultures and debride all infection and
dead bone. In the second stage, reconstruct remaining tissue, usually
with fusions (Fig. 116.7) or amputate if reconstruction is not possible owing to the patient’s health and the local conditions.
Realignment osteotomies and fusions after the hyperemic or osteopenic
phase is over require extended immobilization (up to 1 year), but even
a pseudoarthrosis may improve function, relieve pressure, and treat
ulcers (4,24,48,57).
claw toes, flat foot, and valgus heel and calcaneal equinus can be
corrected somewhat with a percutaneously lengthening of a tight
heelcord.
those involving deep infections, amputation is often the most
reasonable surgery.
treated in a straightforward way. Excellent treatment of the diabetic
foot is gratifying for the surgeon and for the patient. A balanced foot
care team with individuals with varied skills and roles is key. The
team consisting of the patient, family, doctors, nurses, and clinical
assistants needs to be educated about foot problems and be able to work
together to provide the best care for these patients. Looking at the
infection in common terms (cellulitis, ulcer, abscess, osteomyelitis,
gangrene), with an understanding of neuropathy (pain or protective
level for prosthesis and Charcot joint) and vasculopathy (level of
healing and need for vascular repair), will empower the patient and
team to deal effectively with these complex problems.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
J, Castenfors J, Larsson J, et al. Prognostic Value of Systolic Ankle
and Toe Blood Pressure Levels in Outcome of Diabetic Foot Ulcer. Diabetes Care 1989;12:373.
DM, Daus GP, Gerding DN. Osteomyelitis in the Feet of Diabetic
Patients: Long Term Results, Prognostic Factors, and the Role of
Antimicrobial and Surgical Therapy. Am J Med 1987;83:653.
PW. The Insensitive Foot (Including Leprosy). In: Jahss MH, ed.
Disorders of the Foot. Philadelphia: W.B. Saunders, 1982:1266.
KS, Klarke M. Transcutaneous Oxygen Measurement in Peripheral Occlusive
Disease: An Indicator of Wound Healing in Leg Amputation. J Bone Joint Surg [Br] 1986;68:423
KS, Falstie JN, Christensen ES, Brochner MJ. Results of Amputation for
Gangrene in Diabetic and Non-diabetic Patients. J Bone Joint Surg [Am] 1988;70:1514.
RH, Morrison MJ, Beabout JW. Diabetic Neuropathy in the Foot: Patient
Characteristics and Patterns of Radiographic Change. Foot Ankle 1983;4:15.
BT, Brahms MA. Diabetic Arthropathy of the First Metatarsal Cuneiform
Joint: Introduction to a New Surgical Fusion Technique. Orthop Rev 1987;16:465.
JA, Lopes-Virella MF, Winocour PD, Halushka PV. New Concepts about the
Pathogenesis of Atherosclerosis in Diabetes Mellitus. In: Levin ME,
O’Neal LW, eds. The Diabetic Foot, 4th ed. St. Louis: C.V. Mosby, 1988:51.
SD, Nicholas GG, Osborne MA, et al. Role of Magnetic Resonance Imaging
in the Diagnosis of Osteomyelitis in Diabetic Foot Infections. J Vasc Surg 1996;24:266.
Diabetics Control and Complications Trial Research Group: The Effect of
Intensive Treatment of Diabetes on the Development and Progression of
Long-term Complications in Insulin-dependent Mellitus. N Engl J Med 1993;329:977.
RP, Sibbitt WL Jr, Harsh A. The Effect of Aldose Reductase Inhibiting
Agent on Limited Joint Mobility in Diabetic Patients. JAMA 1985;253:1437.
A, Abraha H, Li F, et al. Measurement of Markers of Osteoclast and
Osteoblast Activity in Patients with Acute and Chronic Diabetic Charcot
Neuroarthropathy. Diabet Med 1997;14:527.
DA, Lattimer SA, Sima AAF. Sorbitol, Phosphoinositides, and
Sodium-potassium-ATPase in the Pathogenesis of Diabetic Complications. N Engl J Med 1987;316:599.
RL. Salvage of a Functional Lower Limb in Diabetic Patients after
Amputation, in Diabetic Patients after Amputation. In: Heckman JD, ed. Instructional Course Lectures 42. Rosemont, IL: American Academy of Orthopaedic Surgeons, 1993:159.
JT. Neuropathic Fractures and Joint Injuries: Pathogenesis and
Rationale of Prevention and Treatment. J Bone Joint Surg 1967;49A:1.
P, Maurer RC. Talonavicular Dislocations and Midfoot Arthropathy in
Neuropathic Diabetic Feet: Natural Course and Principles of Treatment. Clin Orthop 1989; 240:226.
ME. The Diabetic Foot: Pathophysiology, Evaluation, and Treatment. In:
Levin ME, O’Neal LW, eds. The Diabetic foot, 4th ed. St. Louis: C.V.
Mosby, 1988:1.
SE, Neagle CE, Esterhai JL, et al. Magnetic Resonance Imaging for the
Diagnosis of Osteomyelitis in the Diabetic Patient with a Foot Ulcer. Foot Ankle Int 1994;15:151.
BA, Pecoraro RE, Larson SA, et al. Outpatient Management of
Uncomplicated Lower-extremity Infections in Diabetic Patients. Arch Intern Med 1990;150:790.
WB, Schweitzer ME, Wapner KL, et al. Osteomyelitis in Feet of
Diabetics: Clinical Accuracy, Surgical Utility, and Cost-effectiveness
of MR Imaging. Radiology 1995;196:557.
Presented at the Fifth Annual Summer Meeting of the American
Orthopaedic Foot and Ankle Society, Sun Valley, ID, August 3–6, 1989.
FB Jr, Marcaccio EJ, Gibbons GW, et al. Dorsalis Pedis Arterial Bypass:
Durable Limb Salvage for Ishemia in Patients with Diabetes Mellitus. J Vasc Surg 1995;21:375.
CL, Johnson KA, Goldstein RH, Donnelly RE. The Patellar Tendon Bearing
Brace as Treatment for Neurotrophic Arthropathy: A Dynamic Force
Monitoring Study. Foot Ankle 1992;13:14.
FL, Witte JL, Canawati HN, et al. The Infected Foot of the Diabetic
Patient: Quantitative Microbiology and Analysis of Clinical Features. Rev Infect Dis 1984;6(Suppl 1):S171.
the American Orthopaedic Foot and Ankle Society, 901 Boren Ave., Suite
1300, Seattle, WA 98104 (1-800-235-4855) for copies of this brochure.
