ANATOMY AND BIOMECHANICS OF THE KNEE
complex joint within the human body. Its position between the two
longest lever arms of the skeleton makes it vulnerable to injury by the
tremendous moments that can be transmitted to it from loads applied at
great distance to the ligaments and capsular structures that provide
the structural integrity of the joint. Thus, it is not surprising that
the knee joint is one of the most frequently injured joints. Because of
its vital importance in support and locomotion of our bipedal
existence, damage to its major components results in much discomfort
and disability. An understanding of fundamental anatomy and
biomechanics provides the basis for appropriate treatment of injury and
disease processes involving the knee.
classifications of joints because it has some features of a ginglymus
or hinge joint and some of an arthrodial joint, which by definition
allows only gliding movement along opposing plane surfaces. The knee
consists of three more or less independent articulations: one between
each sphere-like condyle of the femur and a corresponding but more
planar condylar surface of the tibia, with interposed menisci, and a
third between the patella and the patellar or trochlear groove of the
femur. None of the pairs of bearing surfaces is exactly congruent,
which results in a combination of rolling and gliding motions
determined by the restraints of a complex network of ligaments,
capsular structures, and the contours of the bones themselves. This
intricate arrangement of anatomic interrelationships allows the knee
six degrees of freedom of motion: three rotations and three
translations. The translations are anteroposterior (5 to 10 mm),
compression–distraction (2 to 5 mm), and mediolateral (1 to 2 mm).
These motions are limited by the ligaments, capsule, and to some degree
the intracondylar eminences of the tibia and are of small magnitude in
the normal knee. The rotations are flexion–extension, varus–valgus, and
internal–external rotation, and in general they are much more extensive
than the translations. Normal flexion and extension of the knee is
variable, ranging from 0° to 15° of hyperextension to
130°
to 150° of flexion. Internal and external rotation ranges from little
or no motion in full extension to 20° to 30° with the knee flexed.
Tightening of the capsular and ligamentous structures, which is
greatest in full extension, accounts for this variation. The mean
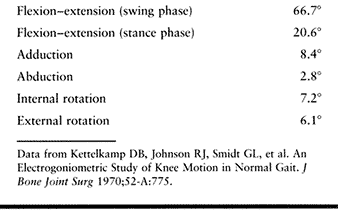
values for rotations during level walking, as determined by Kettelkamp
et al. using an electrogoniometer, provide insight into the complexity
of motion during function of the knee (Table 83.1) (46).
 |
|
Table 83.1. Mean Values for Rotations During Level Walking
|
during gait for each person, with minor variations observed between
individuals. They found that none of the knees tested extended fully
during normal walking but extended slightly farther than in resting
stance just before or at heel strike. The most common motion pattern
observed revealed that maximum extension and external rotation occurred
just before heel strike; maximum abduction occurred at heel strike, and
maximum adduction during swing phase; and flexion and internal
rotation, which began just before heel strike, continued to increase
between heel strike and foot flat (Fig. 83.1). Similar patterns were observed by Marans et al., who used a more sophisticated electrogoniometric system (54).
They measured not only the rotational degrees of freedom
(flexion–extension, varus–valgus, and internal–external rotation) but
also the translational degrees of freedom (anteroposterior,
mediolateral, and superior–inferior). Only with evaluation of
anteroposterior translation measurements during level walking were they
able to distinguish between a group of normal knees and a group that
were anterior-cruciate deficient. The knees with no anterior cruciate
ligament (ACL) demonstrated greater anterior translation of the tibia
relative to the femur during the swing phase of gait but not during the
stance phase.
 |
|
Figure 83.1.
Electrogoniometric patterns of knee motion of a normal knee. The 0° line represents the position in neutral stance (H.S., heel strike; F.F., foot flat; H.O., heel off; T.O., toe off). (From Kettelkamp DB, Johnson RJ, Smidt GL, et al. An Electrogoniometric Study of Knee Motion in Normal Gait. J Bone Joint Surg 1970;52-A:775.) |
single plane; thus, combinations of rotations and translations (coupled
motions) are seen through the normal range of motion. The flexion–extension
axis provides the most obvious example. This axis varies because the
radii of the lateral and medial femoral condyles are dissimilar, and
the tibial plateau topography varies from side to side as well. Viewed
from the side, the medial femoral condyle has a more constant radius of
curvature than does the lateral. Posteriorly, both condyles have
similar radii, but anteriorly the lateral condyle rapidly attains a
longer radius and appears to flatten out to a greater degree than the
medial condyle. Often, near the midportion of the lateral condyle, a
lateral groove passing diagonally, anteriorly, and laterally from the
anterior aspect of the intracondylar notch produces a distinct
indentation. This differentiates the lateral from the medial condyle as
observed on a lateral roentgenogram. This lateral groove demarcates the
extent of the articular surface of the femur, which articulates with
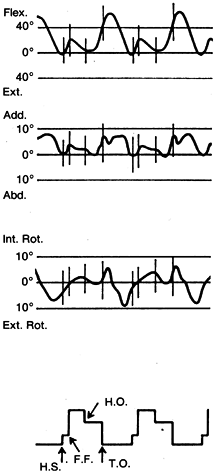
the tibia (Fig. 83.2) (41).
All articular surfaces anterior to this area contact only the patella.
As viewed from its distal end, the medial femoral condyle extends
anteriorly and inclines toward the lateral side, so it is somewhat
longer
than the lateral condyle. A small V-shaped indentation (medial groove)
delineates the anterior extremity of the articular surface of the
medial condyle that contacts the tibia (Fig. 83.2).
This medial groove is well forward of its counterpart on the lateral
femoral condyle and is probably often mistaken to be a pathologic
process caused by a hypertrophic medial plica. It is, in fact, the area
where the anterior horn of the medial meniscus abuts the femur when the
knee is fully extended.
 |
|
Figure 83.2. Distal end of femur showing lateral (A) and medial (B) grooves in the articular surfaces of the medial and lateral femoral condyles and the patellar or trochlear surface (C) and intercondyloid fossa or notch (D). (From Jackson JP. Surgery of the Knee Joint. In: Jackson JP, Waugh W, eds. Surgical Anatomy. Philadelphia: JB Lippincott, 1984;5.)
|
medial is a good deal longer in the sagittal plane than the lateral.
Both facets appear to be slightly concave in the coronal plane, but the
lateral facet demonstrates a convexity in the sagittal plane, producing
a saddle shape (44,56,60).
Thus, in the lateral compartment of the knee, the rounded femoral
condyle rests on a convex surface of the tibia, which contributes to
the complexity of stabilization of this joint. This stabilization is
controlled by the interposed meniscus, the surrounding capsular and
ligamentous structures, and the muscles crossing the knee joint.
the tibia on the two femoral condyles, the tibia is obligated to
externally rotate significantly during the last few degrees of full
extension as the tibia rolls farther forward on the medial femoral
condyle than on the lateral condyle. This screw home mechanism is also
guided by the alignment and tension in the ligaments and capsular
structures. In the fully extended position of the knee, the majority of
the ligaments and capsular structures are under tension, thus allowing
no further extension or external rotation. With active or passive
flexion from full extension, the rotation process is reversed, with the
tibia internally rotating relative to the femur during the first 10° to
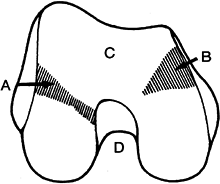
20°. The motion of the femur relative to the tibial plateau during
flexion is initially a pure rolling motion, but by 10° to 15° on the
medial tibial and by 20° on the lateral side, sliding of the femur
begins relative to the tibia and becomes progressively more important
until flexion is complete (Fig. 83.3) (43).
Thus, the contact area between the tibia and femur moves rapidly
backward during the first 10° to 20° of flexion and then slowly
progresses posteriorly to eventually ride entirely on the posterior
horns of both menisci at the extreme of flexion. Because the lateral
condyle rolls more than the medial over the corresponding tibial
plateau, another manifestation of the screw home mechanism is
explained. Kapandji observed that the 15° to 20° of initial rolling
corresponds to normal range of movements required in flexion and
extension during the stance phase of ordinary walking (43).
 |
|
Figure 83.3. Medial (A) and lateral (B)
compartments of the knee demonstrating the amount of rolling and sliding of the femoral and tibial surfaces occurring during flexion. Starting from full extension, the femoral condyles roll without sliding. Sliding movement then becomes progressively more important, so that by the end of flexion, the condyles slide without rolling. For the medial condyle, pure rolling occurs only during the first 10° to 15° of flexion. For the lateral condyle, the rolling continues until 20° of flexion. (From Kapandji IA. The Physiology of the Joints, Vol. 2. Lower Limb. Edinburgh: Churchill Livingstone, 1970.) |
articular surface for the control of the extensor mechanism of the knee
as it glides over the anterior aspect of the joint. The extensor
mechanism stabilizes the joint against gravity when the knee is flexed
and assists in the forward propulsion of the mass of the body as the
knee extends during gait. The patella contacts the patellar or
trochlear surface of the femur during these activities (Fig. 83.2).
Anteriorly, the condyles of the distal femur are separated from one
another by this shallow articular depression, which averages 5 to 6 mm
in depth. Inferiorly and posteriorly, the trochlear surface is
continuous with the intercondyloid fossa or notch (Fig. 83.2). The lateral wall of the trochlear surface
of the femur is more prominent than the medial and projects farther
anteriorly. The patella has a multifaceted dorsal surface, which
articulates with the trochlear groove. A median ridge divides the
patella into two large facets. Most frequently, the lateral facet is
greater in area than the medial, but a range of anatomic variations
exist, sometimes making these facets nearly equal. A less distinct
vertical ridge divides the medial facet into two separate surfaces in
most knees. The more medial of these two facets has a more nearly
sagittal orientation and contacts the femur along the medial side of
the notch only when the knee is flexed past 90°.
lateral facets into roughly equal-sized superior, middle, and inferior
facets. In full extension the patella does not contact the articular
surface of the femur but lies over an area of thin and smooth synovial
tissue on the anterior shaft of the femur immediately proximal to the
lateral aspect of the trochlear surface. This area is termed the
supratrochlear tubercle. The transition from the articular cartilage to
the supratrochlear tubercle is smooth above the lateral aspect of the
trochlear groove, but it is often a sudden dropoff when viewed from the
medial side (termed Outerbridge’s ridge) (70).
This arrangement conveniently allows the patella to ride smoothly above
the articular surface in a superior and lateral direction as the knee
is fully extended. The patella thus does not slide over the sharp
dropoff observed proximal to the medial side of the trochlear groove
during normal joint activity.
patella near the median ridge is normally the thickest in the human
body. This reflects the large patellofemoral joint reaction (PFJR)
force that occurs between the patella and the trochlear surface during
normal activities. Loads across the patellofemoral joint are indirectly
related to the angle of knee flexion and directly related to the force
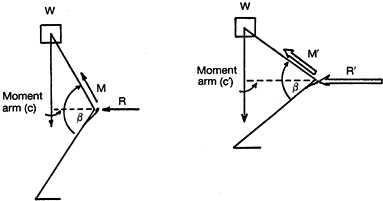
generated within the quadriceps mechanism. Figure 83.4 presents a static representation of this in simplified terms. On the left diagram, the superincumbent mass (W) of the body is supported by the force (M)
generated within the quadriceps. The line from the center of mass from
the body falls well behind the flexion axis of the knee. The moment arm
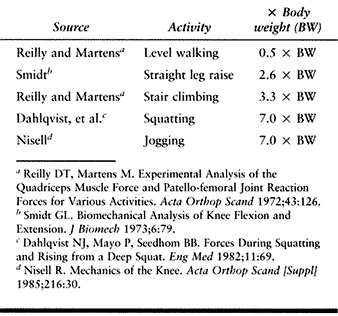
(c) in this situation is relatively small, and thus the quadriceps force (M) and the PFJR force (R) are relatively small. When the figure depicted flexes further (right diagram), decreasing the angle (β), the moment arm (c) increases. To allow this system to remain static, the new force (M´) generated within the quadriceps must significantly increase, which in turn increases the PFJR force (R´). The PFJR force estimated for various activities is presented in Table 83.2.
 |
|
Figure 83.4. Static representation of the patellofemoral joint reaction forces (R and R´) at two positions of flexion (see text).
|
 |
|
Table 83.2. Patellofemoral Joint Reaction Force
|
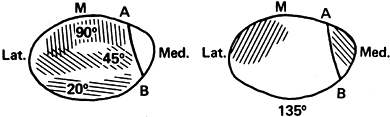
increases in response to the increased loads that develop as the knee
flexion angle (β) decreases during most activities (Fig. 83.5) (40).
For this reason, stress (force per area) increases less rapidly than
the total force across the patellofemoral joint. However, if a weight
is applied to the ankle, as in most weight-training programs, and the
knee is extended against gravity plus the additional weight, the
opposite effect is noted. In this situation, the area of contact
decreases as the PFJR force increases. Thus, the PFJR stress becomes
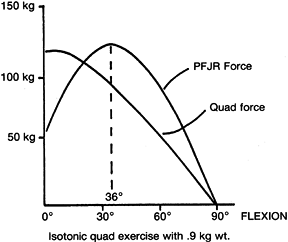
excessively high even for light weights applied at the ankle, as
depicted in Figure 83.6 (76). For this reason, isotonic or isokinetic exercises through a full range
of motion are not advised in the treatment of patellar pain syndromes.
Quadriceps exercises that extend the knee through only the last 15° to
20° of extension are much more likely to be tolerated, as can be seen
in Figure 83.6.
Abnormal tracking of the patella, which allows lateral subluxation of
only a few millimeters, confounds this principle. Under these
circumstances, the larger load is borne on a relatively small contact
area, greatly increasing the local stress (Fig. 83.7) (53).
This undoubtedly contributes in many instances to the production of
patellofemoral pain and, if severe, degeneration of patellar articular
cartilage (chondromalacia). Many anatomic conditions can and do
contribute to this common problem. These include hypoplasia of the
trochlear groove, abnormal patellar articular configuration,
underdevelopment of the vastus medialis, rotational malalignment of the
proximal tibia relative to the distal femur, and an abnormally high
valgus angle of the distal quadriceps mechanism (Q angle).
 |
|
Figure 83.5.
Composite of patellofemoral contact area with the degree of flexion (M, location of the median ridge). (After Hungerford DS, Barry M. Biomechanics of the Patellofemoral Joint. Clin Orthop 1979;144:9.) |
 |
|
Figure 83.6.
Patellofemoral joint reaction force (PFJR) produced during extension of the knee from 90° of flexion to 0° while lifting a 0.9-kg weight. (After Reilly DT, Martens M. Experimental Analysis of the Quadriceps Muscle Force and Patellofemoral Joint Reaction Force for Various Activities. Acta Orthop Scand 1972;43:126.) |
 |
|
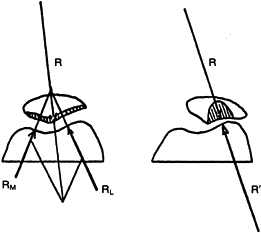
Figure 83.7. Joint reaction forces (R, patella; RM, medial trochlear groove of femur; and RL and R´,
lateral trochlear groove of femur) before and after lateralization of the patella. (After Maquet P. Mechanics and Osteoarthritis of the Patellofemoral Joint. Clin Orthop 1979;144:70.) |
quadriceps mechanism from the axis of knee flexion. In so doing, it
decreases the force (M) that is necessary to maintain static equilibrium, as depicted in Figure 83.4.
Kaufer has shown that patellectomy decreases this effective moment arm
by 31% at 0° flexion, 22% at 30°, 13% at 60°, 12% at 90°, and 10% at
120° (45). Maquet has shown that by increasing
the quadriceps mechanism’s moment arm, a 2-cm elevation of the tibial
tubercle produces a 50% reduction in the PFJR force when the knee is
flexed 45° (52,53).
Thus, patellectomy is not advised, and tibial tubercle elevation
procedures are effective in the treatment of patellar pain syndromes.
cadaveric specimens while measuring quadriceps force with a tensile
load sensor and patella tendon force with a buckle transducer (37).
They demonstrated that for knee flexion between 0° and 45°, the force
developed in the patella tendon was greater than that in the quadriceps
mechanism. With continued knee flexion to 120°, the patella tendon
force was consistently less in comparison to the quadriceps force. The
authors suggested that the patella not only functions as a pulley that
changes the magnitude and direction of forces in the quadriceps and
patella tendon but, in addition, has two distinct mechanical functions.
In the first, more classically described function, the anteroposterior
(AP) thickness of the patella can be attributed to increasing the
effective movement arm of the quadriceps muscles and patella tendon,
whereas in the second, the patella acts as a lever. Therefore, the
parameters that define the proximal and distal lever arms of the
patella have a direct effect on the balance of forces in the quadriceps
and patella tendon. The authors reasoned that these parameters were the
length of the patella, the location of the patella femoral contact
area, and the angle between the quadriceps and patella tendon.
interposed between the tibia and femur were long thought to be
vestigial devices that had no significant function. Fairbank, in 1948,
proposed that the menisci serve a load-transmission function and
suggested that the joint space narrowing, osteophyte formation, and
flattening of the femoral condyles frequently observed following
meniscectomy most likely resulted from the loss of this function (24). Several investigators in the late 1960s (38,87) and early 1970s (42)
reported less than satisfactory results in long-term follow-up
investigations in a high proportion of patients undergoing
meniscectomy, thus adding credence to Fairbank’s observations (24). It was not until the mid-1970s that several reports confirmed the load transmission function of the menisci (48,81,83,92).
Although some variation was reported, these investigations all
estimated that 30% to 60% of the load transmitted across the joint
passes through the menisci during weight-bearing activities. In 1979,
Seedhom and Hargreaves reported that 70% to 99% of the total load
acting on an intact joint is passed through normal menisci and that all
of the load is transmitted through the posterior horns of the menisci
if the joint is flexed beyond 75° (82). In
several of these investigations, it was clearly shown that the ability
of the joint to transmit a load was significantly reduced by removal of
all or part of the menisci and that these structures assist in reducing
stress across the joint (6,48,62,82,92).
Seedhom and Hargreaves also showed that removal of part of the meniscus
reduced the weight-transmitting function less than removing the entire
structure as long as the circumferential continuity of the meniscus
remains intact (82). Thus, the rationale for partial meniscectomy, made possible by arthroscopic techniques, was at least partially verified.
However, it is probably too early to be certain that normal meniscal
function is restored by these procedures in humans. Recent studies in
animals suggested that the repair of peripheral longitudinal lesions
produced compressive force displacement behavior indistinguishable from
normal (6,61). However, repairs of radial tears are less successful in restoring normal biomechanics (61).
menisci destroyed by trauma or removed surgically. Early follow-up
studies with small numbers of cases suggest that meniscal function may
be restored, but no evidence confirms that normal biomechanical
behavior is established or that transplanted menisci are capable of
protecting articular cartilage (15,49,91).
In a human cadaver study, size-matched meniscal allografts resulted in
improved load transition profiles compared to the meniscectomized
state, but only by 55% to 65% (71). Further clinical animal and cadaveric studies are necessary to prove the efficacy of meniscal allografts.
observed that although removal of menisci made the unloaded knee more
lax, stability of the loaded knee was little affected. In unloaded
human cadaver knees at 25° of flexion, Wang and Walker (93)
evaluated the effect of rotatory laxity before and after removal of the
menisci. They defined two types of rotatory laxity: primary and
secondary. The former occurred at torques up to 0.5 nm and represented
the looseness of the joint before significant resistance was
encountered. The latter occurred at torques between 0.5 and 5 nm and
represented significant resistance before plastic failure of
soft-tissue structures. In their study this failure occurred at
approximately 12.5 nm. Under such loads the ligaments were stretched
but not totally disrupted, and, as might be expected, they found
greater rotational instability after such ligamentous failure. They
determined primary and secondary rotatory laxity of eight human knees
before and after removal of both menisci. A 14% increase in primary
rotatory laxity and a 2% increase in secondary laxity were observed.
Although they thought that the increase in secondary laxity was not
significant, they concluded that the menisci serve to restrict primary
rotatory laxity, perhaps by acting as space-filling buffers.
little effect on laxity in the anteroposterior plane in the otherwise
intact knee (7,36,51).
However, when the cruciate ligaments were severed, and these same
anteroposterior loads were repeated before and after removal of the
menisci, there were large differences in anteroposterior stability. The
investigators concluded that the menisci apparently play an important
role in preventing this instability when cruciate function has been
lost. This may account for the frequent development of a torn meniscus
after apparently isolated disruption of the ACL.
menisci, including shock absorption, increasing joint congruity,
assisting with lubrication, preventing synovial impingement, and
limiting extremes of flexion and extension. However, these functions
are difficult to prove.
This is because the posterior and anterior horns of the lateral
meniscus attach to the nonarticular area of the tibial plateau, whereas
those of the medial meniscus are well off the plateau. The anterior
horn of the medial meniscus attaches well forward on the anterior
surface of the proximal tibia, and the posterior horn is fixed within
the groove on the
posterior
tibial surface just above the posterior cruciate attachment. The
anterior horn of the lateral meniscus blends into the attachment of the
anterior cruciate; the posterior horn attaches just behind the
intercondyloid eminence, often blending into the posterior aspect of
the ACL.
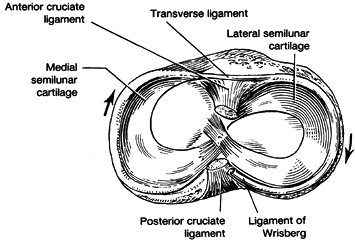 |
|
Figure 83.8. Superior surface of tibia with superimposed medial and lateral menisci. (After Helfet AJ. The Management of Internal Derangements of the Knee. Philadelphia: JB Lippincott, 1963.)
|
lateral cartilage to the anterior horn of the medial meniscus has
occasionally been identified and is termed Barkow’s ligament (35). The anterior horns of the menisci are connected by another fibrous band called the transverse ligament (Fig. 83.8).
Variably present posterior and anterior meniscofemoral ligaments
(Wrisberg’s and Humphrey’s ligaments) pass from the posterior horn of
the lateral meniscus to the medial aspect of the notch. Wrisberg’s
ligament passes posteriorly to the posterior cruciate, and Humphrey’s
ligament passes anteriorly. Usually only one of these structures is
present, and they vary quite markedly in size.
its course, whereas the medial meniscus is wide posteriorly and narrows
significantly anteriorly. Both menisci are attached to the femur and
tibia on their periphery by capsular sheets called meniscofemoral and
meniscotibial ligaments. These attachments are continuous to the medial
meniscus but are interrupted by the popliteus tendon as it passes
between the lateral collateral ligament and the posterolateral aspect
of the lateral meniscus (Fig. 83.9) (86).
The posterior third of the lateral meniscus receives a strong insertion
from the popliteus muscle into its posterior horn, which allows the
meniscus to be pulled posteriorly as the knee flexes. The medial
meniscus has no direct attachment from any of the muscles, but indirect
capsular connections from the semimembranosus, at least in theory, may
provide some ability to retract the posterior horn (69).
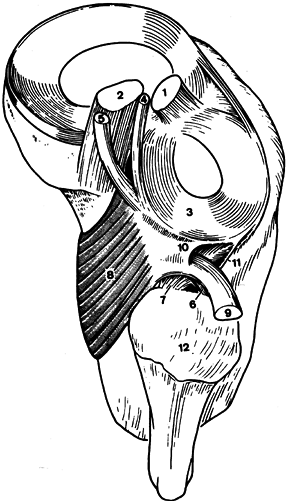 |
|
Figure 83.9. Posterolateral structures, superior view, right knee. 1, anterior cruciate ligament; 2, posterior cruciate ligament; 3, posterior horn lateral meniscus; 4, anterior mensicofemoral ligament (Humphrey); 5, posterior meniscofemoral ligament (Wrisberg); 6, popliteofibular fascicle (anterior limb); 7, popliteofibular fascicle (posterior limb); 8, popliteus muscle; 9, popliteus tendon (cut and reflected); 10, superior popliteomeniscal fascicle; 11, inferior popliteomeniscal fascicle; 12, head of fibula. (From Stäbli H-U, Birrer S. The Popliteus Tendon and Its Fascicles at the Popliteal Hiatus. Arthroscopy 1990;6:209.)
|
oriented in a circumferential fashion, thus resisting the loads applied
to them from the femur. In this manner they are suitably aligned to
resist elongation, much as hoops prevent the expansion of a barrel.
a smooth synovial surface that has a varying number of villous fronds
and folds. Three of these folds are present consistently enough to have
been given specific names (Fig. 83.10) (30). Most knees have a fold of synovium
called the ligamentum mucosum or infrapatellar plica, extending from
the midportion of the posterior surface of the infrapatellar fat pad to
the apex of the intracondylar notch. If small, this structure is
nothing more than a small fibrous band, but if well developed, it forms
a septum from the anterior cruciate to the fat pad and divides the knee
into distinct medial and lateral compartments. A small artery often
courses through it from the apex of the intercondylar notch to the fad
pad. Thus, if it is ruptured, it may contribute to hemarthrosis.
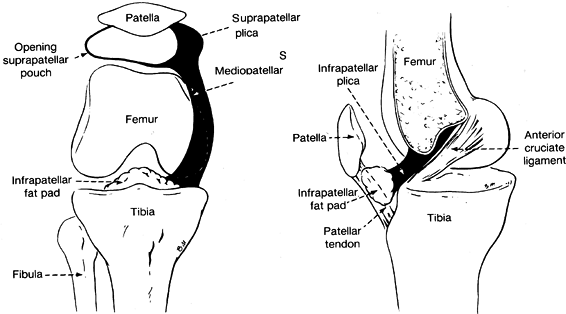 |
|
Figure 83.10.
Diagrammatic views of the plicas of the knee. (From Hardaker WT, Whipple TL, Bassett FH. Diagnosis and Treatment of the Plica Syndrome of the Knee. J Bone Joint Surg 1980;62-A:221.) |
above the superior pole of the patella. In the embryo, the true
suprapatellar pouch is separated from the rest of the knee joint by a
fold of synovium perpendicular to the long axis of the femur just above
the superior pole of the patella. This septum usually becomes
perforated, but a portion of it often remains as a partial septum. Its
medial side is most likely to persist as a crescentic fold. On occasion
only a small perforation occurs through its central portion, or more
rarely it remains intact into adult life. Inflammation and thickening
of this structure may produce pain and crepitus.
mediopatellar plica, plica alaris elongata, medial interarticular band,
or Lino’s band) extends perpendicularly to the medial aspect of the
suprapatellar plica to the medial superior aspect of the infrapatellar
fat pad. It is present in 19% to 60% of normal knees. This variation in
incidence probably results from interpretation of how much of a
synovial fold has to be present before the investigator judges it to be
identifiable as a specific entity. Much emphasis has been given to
pathology observed in this structure since knee arthroscopy has become
common. Thickening and inflammation of this structure to the point of
producing symptoms is rare, and its frequent excision must be observed
with skepticism. The biomechanical function of all the plica structures
is probably of minimal importance because function of the knee does not
seem to be disturbed in their absence.
stabilize the joint by guiding and limiting motion; they are the most
important static stabilizers. They consist of large, clearly
identifiable structures, such as the collateral and cruciate ligaments,
and less obvious capsular structures. Ligaments are a dense connective
tissue primarily composed of collagen with lesser and varying amounts
of elastin and reticulin fibers. Cellular elements, ground substance,
vascular channels, and nerves also are present. Collagen fibers and
their orientation within the tissue are responsible for the primary
biomechanical behavior of each of these structures. The fibers of the
large distinct ligaments are almost all arranged in parallel bundles,
suiting them to withstand tensile loads, whereas capsular structures
have a less consistent orientation, making them more flexible and not
as strong in resisting uniaxial forces.
Ligaments in general are more metabolically active than tendons, as
demonstrated by increased plump cellular nuclei, higher DNA content,
and more reducible cross-links and glycosaminoglycans. Collagen
constitutes between 70% and 80% of the ligament’s dry weight (25). The collagen within a normal ligament is more than 90% fibrillar type I with less than 10% type III (25). Minor variations in composition among the various ligaments of rabbit knees have been reported (1).
distribute the stresses of loading at the bone–ligament interface in a
gradual fashion, thus reducing the chance of failure. This is
accomplished by collagen fibers passing from the ligament into the bone
through four distinct zones: usual ligament morphology, a
fibrocartilaginous matrix, mineralized fibrocartilage, and the bone
itself (21). In spite of this transition, it
has been shown in the anterior and posterior cruciates, the medial and
lateral collateral ligaments, and the patellar tendon that some strain
concentration occurs near the insertion sites (64).
understood by examining the load–elongation curve produced in the
laboratory when bone–ligament–bone specimens are tested to failure (Fig. 83.11) (19).
As load is applied, the ligament elongates. The stiffer the ligament,
the steeper is the curve. In the resting position the ligament fibers
are under minimal tension, and the collagen fibers within the ligament
are wavy. As loading begins, little force is necessary to elongate the
ligament, and thus, the curve is flat. During this phase the collagen
fibers become aligned. The “toe” portion of the load deformation curve
is variable in width, depending on the relative normal laxity of the
particular ligament.
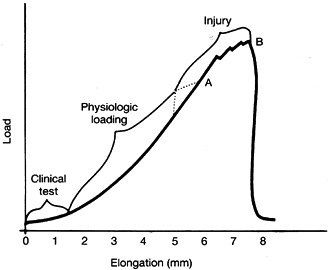 |
|
Figure 83.11. Load-elongation curve of a bone–ligament–bone specimen tested to failure. A: Yield point. B: Maximum load. (After Cabaud HE. Biomechanics of the Anterior Cruciate Ligament. Clin Orthop 1983;172:26.)
|
varies widely from individual to individual. The transition from the
toe to the linear portion of the curve represents the change in
stiffness that an examiner perceives during a clinical stress test when
the ligament’s endpoint or “check rein” is reached. When all the
collagen fibers are straightened, stiffness increases, and the curve
becomes nearly linear. This portion of the curve is characterized by
elastic deformation until near its upper end, at which point plastic
failure begins. When the yield point is reached, the curve becomes
irregular. After the maximum load is reached, the curve drops as the
last few fibers are destroyed, and the curve returns almost straight
down to baseline after all fibers fail.
portion of the curve approximately one fourth of the way to the yield
point (64). The area under the load–deformation curve is equivalent to the amount of energy absorbed by the ligament during testing.
affect the characteristics of the load–elongation curve. Young adults
have a yield point as much as three times greater than older people,
and people who have been immobilized for even short periods have
greatly reduced ligament strength (63,66). The rate at which the ligament is loaded greatly affects its elongation behavior. Noyes et al. (65)
demonstrated that human bone–ligament–bone ACL specimens that failed
rapidly (0.6 seconds) show a 20% increase in load to failure over those
that failed at a slower speed (60 seconds). In their experiments, the
energy stored before failure was 30% greater in the group tested
rapidly than in those that failed at the slower speed. At slow strain
rates, avulsion of ligaments from bone is often seen, but at higher
strain rates interstitial failures occur more frequently. Although both
ligament and bone strength are favorably affected by increased strain
rate, these observations suggest that this strain rate effect has a
greater influence on bone than on ligament.
Within these layers are the muscles and tendons that pass by the joint
as well as the medial collateral ligament, the deep medial ligament,
and capsular structures. The superficial (first) layer is composed of
the deep or crural fascia; the middle (second) layer is the medial
collateral ligament, and the deep (third) layer is the capsule of the
knee joint and the deep medial ligament. Only in an area roughly
overlying the parallel fibers of the medial collateral ligament can all
three layers be identified as separate entities.
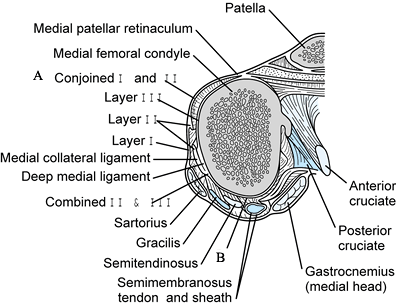 |
|
Figure 83.12. Transverse section through the distal femur. A: The retinacular fibers, which are the conjoined layers I and II. B:
The conjoined layer-II and -III fibers posterior to the medial collateral ligament. (Redrawn from Warren LF, Marshall JL. The Supporting Structures of the Medial Side of the Knee. J Bone Joint Surg 1979;61-A:56.) |
the sartorius muscle and tendon. It extends from the patella to the
fascia of the popliteal space. Anteriorly, it blends with fibers of the
second layer along a line 1 to 2 cm in front of the anterior margin of
the medial collateral ligament (Fig. 83.12).
From this point to the patella, the two layers are blended in an
indistinguishable sheet of tissue, which in part becomes the patellar
retinaculum. Posterior to the parallel fibers of the medial collateral
ligament, the fibers of the first layer are distinct from the
underlying structures, and between them and the second layer pass the
gracilis, semitendinosus, and semimembranosus tendons
(Fig. 83.12).
Anteriorly and distally, the first layer blends into the periosteum at
the tibial attachment of the sartorius. Anteriorly, it blends into the
fascia overlying the quadriceps, and inferiorly, it blends into the
deep fascia of the leg.
medial collateral ligament, in which parallel fibers extend from the
medial epicondyle to blend with the periosteum along the medial border
of the tibia beneath the pes anserinus tendons. Among the names
assigned to this ligament are the tibial collateral, superficial medial
collateral, and the internal lateral ligament. Probably the name most
commonly used is the previously mentioned medial collateral ligament.
The medial collateral ligament (parallel-fibered superficial portion)
is the primary medial stabilizer of the knee, providing 57% to 78% of
the restraining moment resisting valgus stress to the intact knee (28).
an average-sized knee are approximately 1.5 cm wide and 11 cm long. The
femoral attachment of the parallel fibers is arranged around the
varying flexion axis of the knee so that some portion of these fibers
remains under tension throughout the range of knee motion. In full
extension, the posteriormost fibers are taut, whereas in flexion, the
more anterior fibers are under the greatest tension. Strain in the
anterior fibers varies significantly during flexion (2).
The fibers near the anterior border proximal to the joint were strained
4% above their normal resting length at full extension when the knee
was passively flexed to 110°. At the joint line the anterior fibers
strained 2%, and below the joint line only 1.5% during the same motion.
Thus, a variable strain pattern is observed not only between the
anterior and posterior portions of the superficial medial ligament but
also along the course of the anterior or posterior fibers of this
ligament.
collateral ligament, the three layers of the medial aspect of the knee
can easily be distinguished (Fig. 83.12).
Anterior to the medial collateral ligament, the fibers of the second
layer often are divided vertically, but they eventually blend with the
first layer to form the patellar retinaculum. Posterior to the medial
collateral ligament, the fibers within the second layer above the joint
line become obliquely oriented and appear to fan out from their
attachment to the adductor tubercle, passing posteriorly and inferiorly
(posterior oblique ligament) (16). These fibers
form a sheet of tissue indistinguishable from the third layer beneath.
Below the joint line, the fibers of the second layer also are obliquely
oriented, flowing from the posterior margin of the parallel fibers of
the superficial medial ligament toward the posteromedial aspect of the
joint line. Warren and Marshall (94) suggest
that all oblique fibers behind the parallel portion of the medial
collateral ligament should be termed the oblique portion of the
superficial medial ligament, but more commonly the portion above the
joint line is called the posterior oblique ligament (16,39).
An extension of the second layer passes from the anterior aspect of the
medial femoral epicondyle anteriorly to the patella deep to the vastus
medialis (Fig. 83.13). This structure is the patellofemoral ligament (94).
Thus, the second layer extends from the level of the medial femoral
epicondyle to the tibial attachment of the medial collateral ligament
in its midportion. Posteriorly, this layer does not extend proximal to
the capsular attachments superiorly, nor does it extend inferior to the
oblique portion of the medial collateral ligament.
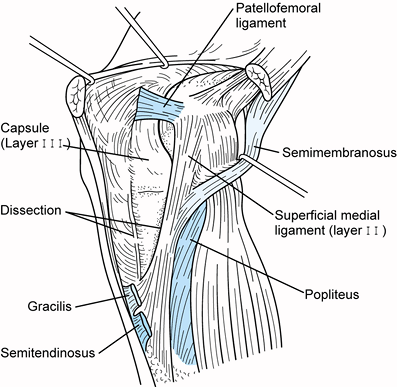 |
|
Figure 83.13.
Medial aspect of the knee with layer 1 removed to reveal the details of layers 2 and 3. The long (parallel) and oblique fibers of the medial collateral ligament can be seen posteriorly (layer 2), and the capsule anteriorly (layer 3). (Redrawn from Warren LF, Marshall JL. The Supporting Structures of the Medial Side of the Knee. J Bone Joint Surg 1979;61-A:56.) |
its thickened midportion, which forms the deep medial collateral
ligament (also termed the deep layer of the medial ligament, short
internal lateral ligament, or the middle capsular ligament). Its
attachments to the tibia and femur delimit the margins of the articular
cartilage. Anteriorly, it is distinct from the overlying retinaculum
but quite thin and weak. Posteriorly, it blends with the second layer
to envelop the posteromedial corner of the joint. The deep medial
ligament is made up of short parallel fibers running from an attachment
point on the femur approximately 0.5 cm distal to the medial epicondyle
to the margin of the tibia just above the attachment of the anterior
slip of the semimembranosus tendon (Fig. 83.14).
It is approximately as wide as the overlying medial collateral ligament
and is clearly separated from it by a bursa. Posteriorly, the margins
of the deep and superficial ligaments blend as the second and third
layers fuse together (Fig. 83.12).
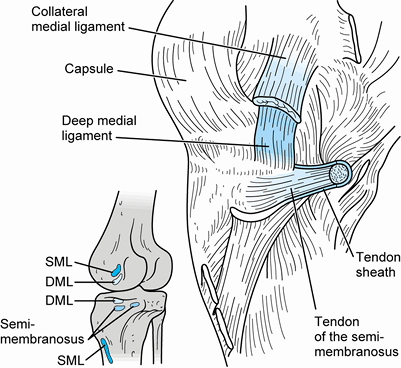 |
|
Figure 83.14.
Medial aspect of the knee, demonstrating the anatomic relationships of the deep structures and the insertion of the semimembranosus. SML, superficial medial ligament; DML, deep medial ligament. (From Warren LF, Marshall JL. The Supporting Structures of the Medial Side of the Knee. J Bone Joint Surg 1979;61-A:56.) |
tendon attaches directly to the posterior tubercle of the knee and to
the tibia medially in a groove distal to the attachment of the third
layer and beneath the medial collateral ligament. The semimembranosus
tendon sheath contributes extensions blending with structures in the
second layer (Fig. 83.14).
The most distinct of these is a large contribution across the back of
the joint to the lateral femoral condyle, the oblique popliteal
ligament. Other frequently observed slips extend into the inferior and
superior oblique portions of the medial collateral ligament and the
fascia of the calf. No direct continuation of semimembranosus fibers
passes directly into the posterior horn of the medial meniscus, as has
sometimes been suggested (69). The complex
insertion of the semimembranosus tendon indicates that besides being a
strong knee flexor, it also serves to internally rotate the tibia on
the femur and to tense the posteromedial capsular structures, which
become lax as the knee is flexed. It also probably acts to protect the
ACL from excessive stress by helping to prevent anterior subluxation of
the tibia on the femur.
the posteromedial aspect of the knee to form a conjoined tendon (pes
anserinus) that passes superficially to the distal end of the medial
collateral ligament and inserts into the anteromedial crest of the
tibia several centimeters distal to the joint line. These muscles are
primarily knee flexors, but they also act as internal rotators. They
serve in conjunction with the quadriceps to reduce loads within the
medial collateral ligament and assist in preventing anterior
subluxation of the tibia on the femur at knee flexion angles from 0° to
approximately 70° (22). Beyond 70° of flexion,
the quadriceps acts synergistically with the ACL. Transplantation of
the pes anserinus reduces the flexion power of this group of muscles by
30% in all positions of knee flexion between 0° and 90° while
increasing internal rotation power by as much as 50% at positions
between 30° and 60° of flexion (67).
surface of the quadriceps, the patella, and the patellar tendon. Its
medial and lateral extensions contribute to the patellar retinaculum
along with aponeurotic fibers from the vastus medialis and lateralis.
Laterally, the facia lata thickens into the iliotibial tract and the
iliopatellar band (Fig. 83.15) (88).
The former inserts into the tubercle of Gerdy, and the latter inserts
into the patella. Decussation of the superficial fibers of these two
specialized portions of the fascia lata can be seen just above the
level of the lateral epicondyle. Deeper portions of these complex
structures are discussed in the section describing the lateral aspect of the knee. The patellofemoral ligaments are variably present; when present they range in width from 3 to 12 mm (75). They are thickenings of the joint capsule and
pass from the epicondyles to their respective borders of the patella.
Of 20 specimens dissected specifically to identify these structures,
Reider et al. reported them to be present laterally in seven instances,
medially once, and both medially and laterally in six specimens (75).
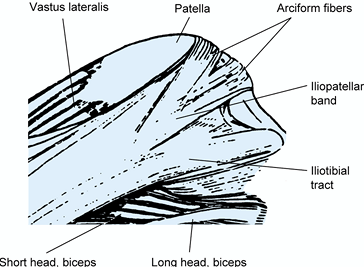 |
|
Figure 83.15.
Lateral aspect of the knee, demonstrating the iliopatellar band and iliotibial tract. (From Terry GC, Hughston JC, Norwood LA. The Anatomy of the Iliopatellar Band and Iliotibial Tract. Am J Sports Med 1986;14:39.) |
but some details of the relationships of the quadriceps merit comment
here. The four large quadriceps muscles form a tendon of insertion into
the superior aspect of the patella, which has classically been
described as consisting of three separate layers: a deep portion
arising from the vastus intermedius, a middle layer consisting of the
two vasti, and a superficial layer from the rectus femoris. The actual
insertion is variable, but most often a deep layer extending from the
vastus intermedius can be separated from a more superficial portion
from the other three muscles (75). The rectus
femoris tendon is the longest (8 to 10 cm) and is roughly triangular;
its base is 3 to 5 cm wide at the superior pole of the patella. It is
the only tendon that consistently sends a significant number of its
fibers anterior to the patella to be continuous with those of the
patellar tendon. The inferior terminus of the vastus medialis obliquus
is at the superior border of the patella in most knees, allowing a
tendon of only a few millimeters in length to insert into the superior
medial border of that bone. The muscle fibers of the inferior aspect of
the vastus medialis are oriented at angles of 55° to 70° in relation to
the sagittal plane near this insertion (75).
The muscle fibers of the distal vastus lateralis also are oblique,
ranging from 22° to 45°; they terminate well above the superior border
of the patella, allowing a tendon averaging 2.8 cm in length to insert
into the superior lateral aspect of the patella. Variable numbers of
fibers from the distal vastus medialis and lateralis tendons insert
into their respective retinacula (75).
extend the knee, but just as important, it also controls flexion of the
knee by antagonizing gravity and the hamstring muscles. Its insertion
into the tibial tubercle and orientation anterior to the transverse
axis of rotation of the distal femur allow it to assist in the
prevention of posterior subluxation of the tibia on the femur if the
knee is flexed less than approximately 70° (22).
The quadriceps thus functions synergistically with the posterior
cruciate ligament (PCL). In positions of knee flexion greater than 70°,
it actually reverses this role and becomes a weak synergist of the ACL
by tending to prevent anterior subluxation of the tibia on the femur.
It also assists in stabilizing the patella within the patellofemoral
groove by means of vertically and obliquely oriented fibers. Through
the retinaculum, the quadriceps tenses the underlying anterior medial
and anterior lateral joint capsule. Coupled with the muscles of the pes
anserinus group, it reduces stresses within the medial collateral
ligament when valgus moments are applied to the knee (73).
It also has been proposed that via capsular attachments to the anterior
horns of the menisci, the quadriceps functions to pull these structures
anteriorly as the knee extends (44).
aspect of the patella (closer to its superficial surface than to the
articular surface) to the periosteum overlying the tibial tubercle. The
majority of its fibers blend into the periosteum rather than inserting
directly into bone. The length of the infrapatellar tendon is variable,
but it is usually approximately equal to that of the patella.
The most superficial (layer 1) contains the iliotibial tract and its
ramifications anteriorly and the biceps and its expansion posteriorly (Fig. 83.16).
The second layer is formed by the retinaculum of the quadriceps
anteriorly. Posteriorly, it is not complete and is composed of the two
patellofemoral ligaments and their various attachments to other
structures (Fig. 83.16 and Fig. 83.17).
The patellofemoral ligaments extend from the retinaculum and split into
a superior limb that inserts into the distal end of the intermuscular
septum and an inferior expansion that fans out to insert into numerous
lateral structures. These structures include the fabella when present,
the lateral head of the gastrocnemius, and the iliotibial tract just
below the termination of the lateral intermuscular septum. Terry et al.
describe five different layers of the iliotibial tract and band, which
differ somewhat in interpretation from what is presented here (88). Of note is the deepest layer of this structure, which Terry et al. term the capsuloosseous
layer, and acts as an anterolateral ligament of the knee (88).
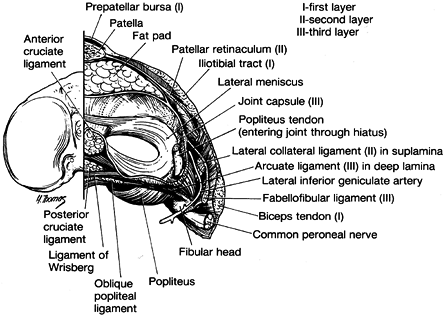 |
|
Figure 83.16.
View of the right knee joint from above after removal of the femur. (From Seebacher JF, Inglis AE, Marshall JL, et al. The Structure of the Posterolateral Aspect of the Knee. J Bone Joint Surg 1982;64-A:536.) |
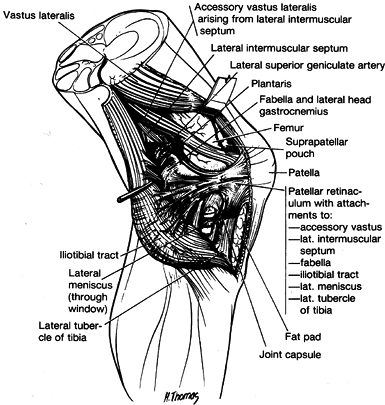 |
|
Figure 83.17.
Lateral aspect of the knee with layer 1 incised and peeled back from the lateral margin of the patella, showing layer 2. (From Seebacher JF, Inglis AE, Marshall JL, et al. The Structure of the Posterolateral Aspect of the Knee. J Bone Joint Surg 1982;64-A:536.) |
capsule, which is attached to the tibia and femur at the margins of the
articular space (Fig. 83.16). This layer
attaches to the margins of the lateral meniscus and is called the
meniscofemoral capsule above and the meniscotibial capsule below that
structure. It was formerly termed the coronary ligament. At the
posterolateral aspect of the knee, the popliteus tendon passes through
a hiatus in the capsule, thus rendering the capsule incomplete in its
attachment to the meniscus (Fig. 83.9). As one moves posteriorly at approximately the midlateral position, the capsule (third layer) splits into two laminae (Fig. 83.16).
The more superficial includes the lateral collateral ligament and ends
posteriorly at the fabellofibular ligament (short external ligament).
The deeper layer of the posterolateral capsule forms the meniscotibial
and meniscofemoral portions of the posterior capsule and encompasses
the arcuate ligament. The inferior lateral geniculate artery passes
between the lateral collateral ligament and fabellofibular ligament and
the underlying arcuate ligament at the level of the meniscus.
epicondyle of the femur downward and slightly posterior to near the
midportion of the superior surface of the fibula. It is a discrete
cord-like structure that is under maximum tension in extension and
becomes lax as the knee is flexed (4). Its lower end is nearly surrounded by the middle layer of the biceps insertion (58).
In theory, this allows the biceps to maintain some tension within this
structure, even when the knee is flexed. Such an effect has been
demonstrated within the lateral collateral ligament during simulated
contraction of the biceps in human cadaver knees (4). The lateral collateral ligament is the primary restraint preventing varus deformity of the knee (28).
insert on the tip (posterior aspect) of the styloid of the fibula just
posterior to the lateral collateral ligament, with the former being the
most superficial (Fig. 83.18). They extend superiorly and slightly posteriorly to blend with
the origin of the lateral head of the gastrocnemius and the termination
of the oblique popliteal Winslow’s ligament. The arcuate ligament
arches over the musculotendinous junction of the popliteus muscle as it
passes upward toward its capsular hiatus. There is great variation in
the size of the contribution of the arcuate and fabellofibular
ligaments (80).
In 13% of the dissections done by Seebacher and associates, the arcuate
ligament alone was present; in 20% only the fabellofibular ligament was
present; and in 67% both of these ligaments were present (80).
In general, when a fabella is present, the fabellofibular ligament is
large, and when even its cartilaginous remnant is not present, the
ligament is very small or absent.
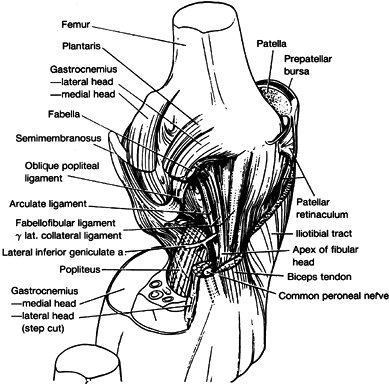 |
|
Figure 83.18.
Oblique view of the posterolateral aspect of the right knee after removing the two structural layers superficial to the joint capsule. (From Seebacher JF, Inglis AE, Marshall JL, et al. The Structure of the Posterolateral Aspect of the Knee. J Bone Joint Surg 1982;64-A:536.) |
its tendon just anterior and inferior to the superior attachment of the
lateral collateral ligament and the posterior aspect of the lateral
meniscus. The contributions of these two sites are variable. Basmajian
and Lovejoy proposed that a portion of it also arises from the arcuate
ligament, thus giving it a fibular as well as femoral origin (8).
It courses inferiorly and medially across the posterolateral aspect of
the joint and inserts through its fleshy attachment to the posterior
aspect of the tibia. The intricate relationship of the popliteus tendon
to the superior and inferior popliteomeniscal fascicles as it passes by
the lateral meniscus is demonstrated in Fig. 83.9 (86).
The anteroinferior and posterosuperior meniscal fascicles are important
stabilizers of the lateral meniscus. Sectioning of these structures
increased anterior displacement of the lateral meniscus 18% when one
fasciculus was sectioned and 78% when both fasciculi were sectioned (85).
These displacements did not result in locking of the menisci but
clearly could result in painful dysfunction clinically. The popliteus
muscle functions synergistically with the PCL through Wrisberg’s and
Humphrey’s ligaments when they are present. It internally rotates the
tibia on the femur, especially during the first 10° to 20° of flexion.
It acts as a weak flexor of the knee and draws the lateral meniscus
posteriorly as the knee is flexed.
has been much confusion about terminology and anatomic detail
concerning those structures that attach to the fibular head (58).
They propose the term popliteal fibular ligament for a consistent
structure that passes from the popliteus tendon downward to the fibular
head. They identified its presence in 20 of 20 cadaver dissections but
state that most anatomy texts of the 20th century have not reported
this structure. Biomechanical investigations performed by Maynard et
al. revealed that this structure is nearly as large and about 56% as
strong as the lateral collateral ligament. This anatomic observation
has important ramifications for reconstruction of the complex lateral
structures following damage to the lateral side of the knee. In
contrast to the above findings, Kim et al. reported in a cadaver study
correlating anatomic to magnetic resonance imaging (MRI) findings that
a popliteofibular ligament was present in only 37.5% of 50 adult knees
evaluated (47).
posterolateral aspect of the knee in a multipart arrangement similar to
that of the semimembranosus posteromedially (58,89).
Its insertion can be divided into three distinct layers with
attachments into the fibular head, proximal tibia, fascia around the
leg, and posterior capsule of the knee joint, as well as an indirect
attachment to the lateral collateral ligament. Thus, the biceps serves
to flex the knee, antagonistically control extension of the joint,
externally rotate the tibia on the femur, tense the lateral and
posterior capsule, and dynamically control anterior displacement of the
tibia on the femur. Its ability to tension the lateral collateral
ligament was discussed previously.
within the notch of the femur and thus has no capsular attachments. It
is surrounded by loose areolar tissue, from which it obtains its blood
supply, primarily from the middle geniculate artery. Its areas of
insertion on the tibia and femur are large, resulting in fibers of
various length as they
pass
from the tibia posteriorly, laterally, and cephalad to the femur. In
full extension all the fibers are under tension; as flexion occurs, all
fibers relax, but those most posterior and lateral relax the most (5). Over 90° of flexion, the ligament rotates approximately 180° (Fig. 83.19) (24).
During this maneuver, the fibers that originally were most anterior at
their attachment site on the femur become most posterior, and vice
versa.
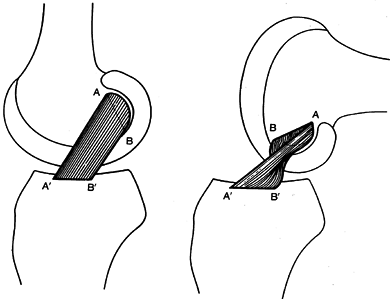 |
|
Figure 83.19. Changes in shape and relative tension of the anterior (A–A´) and posterior (B–B´)
portions of the anterior cruciate ligament in extension and 90° of flexion. (From Girgis FG, Marshall JL, Al Monajem, ARS. The Cruciate Ligaments of the Knee Joint. Clin Orthop 1975;106:216.) |
surface, extending from just behind the medial and lateral spines
forward for approximately 3 cm (Fig. 83.20) (24).
The ACL insertions on the tibia and femur are both nearly planar, with
the femoral attachment being circular and the tibial attachment more
oval (31). The attachment areas are nearly
three times the ACL cross-sectional area at the midportion of the
ligament. The femoral attachment of the ACL is far posterior on the
lateral wall of the intracondylar notch (Fig. 83.21) (25).
No fibers of the ACL are totally isometric, with the least variation in
length (approximately 1.5 mm) occurring along fibers coursing through
the anteromedial aspect of the structure (27,78,84).
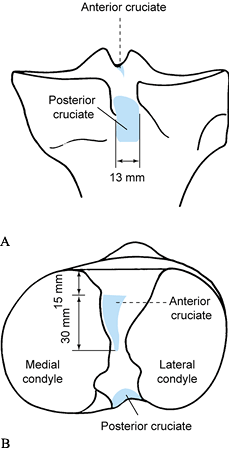 |
|
Figure 83.20. Posterior upper portion of the tibia (A) and superior surface of the tibia (B),
showing the average measurements and relationships of the tibial attachments of the anterior and posterior cruciate ligaments. (From Girgis FG, Marshall JL, Al Monajem ARS. The Cruciate Ligaments of the Knee Joint. Clin Orthop 1975;106:216.) |
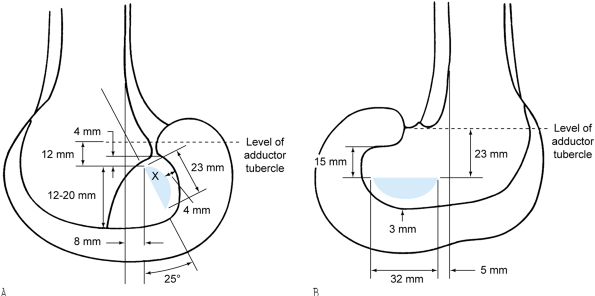 |
|
Figure 83.21. Medial surface of the right lateral femoral condyle (A) and lateral surface of the right medial femoral condyle (B), demonstrating the average measurements and relationships of the femoral attachments of the (A) anterior and (B) posterior cruciate ligaments. X
shows the approximate position of attachment of the “most isometric” fibers of the anterior cruciate ligament. (From Girgis FG, Marshall JL, Al Monajem ARS. The Cruciate Ligaments of the Knee Joint. Clin Orthop 1975;106:216.) |
Until recently, the maximum tensile strength measured in tests of young
human cadaver ACLs was approximately 1,730 N, but recent tests by Woo,
Rauch, and their colleagues measured maximum loads at failure as high
as 2,500 N (66,74,95).
The most important functions of the ACL are to act as the primary
static restraint to anterior displacement of the tibia on the femur and
to prevent hyperextension of the knee (17).
Important secondary functions are to resist varus and valgus deforming
moments affecting the knee and to resist internal rotation of the tibia
(28).
exacting attention to placing the femoral attachment into the center of
attachment of the anteromedial portion of the ligament (the X in Fig. 83.21).
Variation from this site by more than a few millimeters most likely
will result in an inappropriate strain pattern and possible failure of
the procedure. Anterior placement of a graft or repair leads to
excessive strain within the ligament, which results in failure of the
procedure or prevention of flexion of the knee beyond 70° to 90° (5,34,42,50,72).
Placement of the graft posteriorly in the over-the-top position results
in the graft becoming excessively slack as the knee flexes (34).
The site of reattachment of the graft on the femur appears to be much
more critical for proper graft performance than the placement on the
tibia (33,34).
and posterolateral portions of the ligament, as has sometimes been
described. However, the variations in
fiber
lengths and attachment sites described previously do separate the
anterior and posterior portions of the ligament functionally. The
strain pattern within the fibers of the anterior aspect of the ligament
are demonstrated in Fig. 83.22 (13).
Damage to an isolated portion of the ACL produces abnormalities in
ligament laxity during clinical testing. For instance, an intact
posterior portion of the ACL with loss of the anterior portion results
in a negative Lachman test, whereas the anterior drawer test
demonstrates increased laxity. If, on the other hand, the anterior
portion of the ACL is intact and the posterior portion is destroyed,
the drawer test will be nearly normal, but the Lachman maneuver will
reveal increased anterior translation.
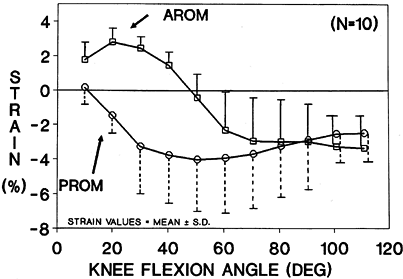 |
|
Figure 83.22.
Mean strain pattern in the anteromedial fibers of the human cadaver anterior cruciate ligaments during motion from full extension to 120° of flexion. AROM, active range of motion; PROM, passive range of motion. |
not only in abnormal knee kinematics but also in changes to the
neuromuscular system. Even low-demand activities such as walking may be
performed in an abnormal manner. For example, subjects with an
anterior-cruciate-deficient knee have been shown to reduce the flexion
moment about their knee in an effort to reduce contraction of the
antagonistic quadriceps muscles (9). This has been coined the quadriceps-avoidance gait.
along their length (i.e., the strains in the midsubstance portion of
the ligament are different in comparison to those at the insertions
(and about its cross section) (18). This has
been investigated by Woo et al., who used a robot combined with a
six-degree-of-freedom force transducer to apply load to the knee (96).
They have shown that the restraint forces in the anteromedial and
posterolateral aspects of the anterior cruciate ligament differ, and
they change relative to knee flexion. A similar behavior occurs during
passive flexion–extension of the knee (56).
anteromedial aspect of the ACL in humans, the region of the ligament to
which we have access with the arthroscope and our current differential
variable-reluctance transducer
(DVRT;
MicroStrain, Burlington, VT). The study participants were patients who
underwent a diagnostic arthroscopic surgery, typically for
meniscectomy, and volunteered to participate under a protocol approved
by our institutional human subjects review board. The surgical and
experimental procedures were performed under local anesthesia, allowing
study subjects full control of their musculature. The subjects had
normal cruciate ligaments as documented by arthroscopic and clinical
exam, normal range of joint motion, no history of previous ligament
injury, and normal gait. After the surgical procedure was finished, the
DVRT was implanted into the anteromedial aspect of the ACL to
characterize its displacement pattern and calculate its strain behavior.
position, either passively or through contraction of the leg muscles,
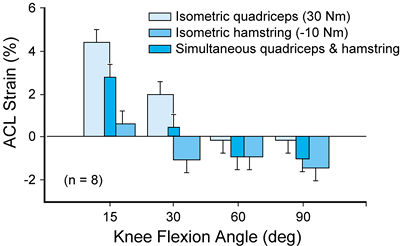
consistently produced an increase in ACL strain values (10).
Rehabilitation exercises that produce low ACL strain values involve
contraction of the dominant quadriceps muscle group with the knee
flexed at 60° or greater (simultaneous quadriceps and hamstrings
contraction, isometric contraction of the quadriceps) or were dominated
by the hamstrings muscle group (isometric hamstrings muscle
contraction; Fig. 83.23). Extension of the
knee produced by contraction of the dominant quadriceps muscle group
produced a substantial increase in ACL strain values, a finding that
has been observed by Markoff et al. (56). The
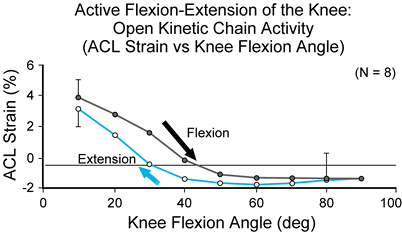
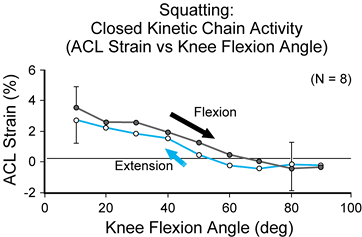
peak ACL strain values that were produced by squatting (a
closed-kinetic-chain activity that induces a substantial compressive
load across the tibiofemoral joint because of body weight as well as
cocontraction of the leg musculature) were similar to those produced
during open-kinetic-chain active extension of the knee (an activity
that does not involve the compressive load produced by body weight) (12) (Fig. 83.24 and Fig. 83.25).
The ACL strain values that were created by squatting were unaltered
with the addition of an elastic resistance (the SportCord) that
increased activity of the musculature spanning the knee. Thus,
increasing resistance with the SportCord (producing a moderate increase
in muscle activity) in an effort to strengthen the leg muscles during
the squat exercise does not necessarily produce a significant increase
in ACL strain magnitude. In contrast, increasing resistance during
open-kinetic-chain active flexion/extension of the knee produces a
significant increase in ACL
strain magnitude (10).
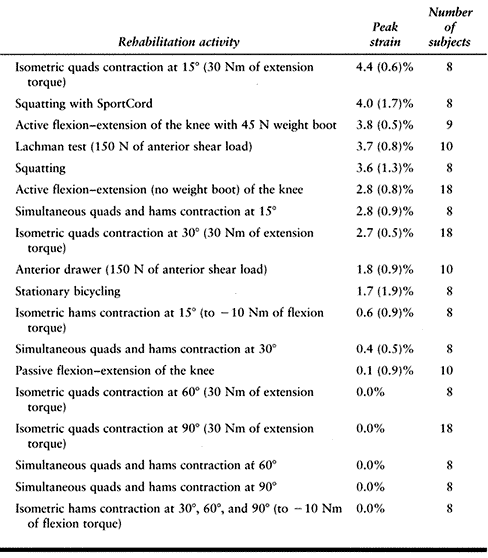
Currently, the limit of ACL graft strain during healing that is safe or
unsafe is unknown. Therefore, we are unable to recommend what
rehabilitation exercises should be performed based on whether they are
safe or harmful. However, we feel it is important to point out the peak
strain values that are produced during the quadriceps-dominated
exercises (Table 83.3) may damage a healing ACL
graft if they are used too early during rehabilitation, if they are
performed at inappropriate knee flexion angles, or if they are advanced
to include more challenging levels of muscle contraction.
 |
|
Figure 83.23. Summary of effects of thigh muscle contraction on the strain in the anteromedial bundle of the anterior cruciate ligament in vivo.
(From Beynnon BD, Johnson RJ, Fleming BC, et al. The Effect of Functional Knee Bracing in the Weightbearing and Non-weightbearing Knee. Am J Sports Med 1997;25:353.) |
 |
|
Figure 83.24.
Mean strain value produced by active flexion–extension motion of the knee within the anteriomedial bundle of the anterior cruciate ligament measured in vivo (n = 8). The ligament strain values are presented for extension and flexion motions of the knee. The arrow indicates data for flexion motion of the knee. (From Beynnon BD, Johnson RJ, Fleming BC, et al. The Strain Behavior of the Anterior Cruciate Ligament, A Comparison of Open and Closed Kinetic Chain Exercise. Am J Sports Med 1997;25:823.) |
 |
|
Figure 83.25. Mean strain values for squatting as measured in the anteromedial bundle of the anterior cruciate ligament in vivo (n = 8). Strain values are presented for flexion and extension motions of the knee (flexion motion is indicated by the arrow).
(From Beynnon BD, Johnson RJ, Fleming BC, et al. The Strain Behavior of the Anterior Cruciate Ligament, A Comparison of Open and Closed Kinetic Chain Exercise. Am J Sports Med 1997;25:823.) |
 |
|
Table
83.3. Rank Comparison of Peak ACL Strain Values During Commonly Prescribed Rehabilitation Activities (Mean ± 1 Standard Deviation) |
healing ACL graft or to treat instability of the knee that results from
an ACL disruption. Subjective studies have shown that braces help
individuals with torn knee ligaments to improve athletic performance.
Studies of knee kinematics have demonstrated that braces increase the
stiffness of the tibiofemoral joint and produce reductions of
anterior–posterior displacements, but this holds only for low shear
load magnitudes. We have measured ACL strain in subjects during braced
and unbraced conditions, demonstrating that bracing does not prestrain
or harm the ACL, a finding that was in direct contrast to our earlier
cadaveric studies. In our studies of subjects in a non-weight-bearing
condition, we determined that the protective
strain-shielding
effect of a functional knee brace on the ACL was dependent on the
magnitude of applied anterior load and internal–external torques (14).
As the magnitude of applied anterior load and internal–external torque
increased, the protection produced by the brace on the ACL decreased.
We then progressed to systematically study the effect of the extrinsic
forces produced by different brace strap tensions on the ACL in the
presence of body weight (11). We determined
that bracing significantly reduced ACL strain values for anteriorly
directed loading of the tibia with the subject weight bearing. This
response was very different from a non-weight-bearing response, which
seemed to be dependent on the magnitude of externally applied load to
the knee.
From there the PCL descends posteriorly and somewhat laterally to pass
the back of the tibial plateau and insert in a groove in the posterior
surface of the tibia well below the joint line (Fig. 83.20) (26,90).
The PCL femoral insertion is half-moon shaped and nearly planar,
whereas its tibial attachment is more rectangular and covers a convex
surface on the posterior tibial plateau (31).
The PCL is surrounded by vascular loose areolar tissue with a synovial
membrane sustaining the majority of its blood supply from the middle
geniculate artery. The PCL’s mean length is 38 mm, and its width is 13
mm (26). The ligament has a large anterior
portion and a much smaller, more oblique posterior portion, but these
structures are not clearly distinguishable. As in the ACL, there are no
totally isometric fibers, but in general there is less variation in
length in the posterior portion of the ligament than anteriorly. The
strain within the posterior fibers was found to relax from 0° to 20° of
flexion and then increase to a maximum of 6% in full flexion, whereas
the strain observed in the anterior fibers is least at full extension
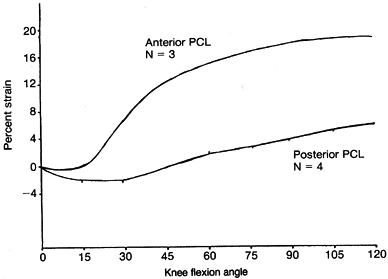
and increases to nearly 20% at full flexion (Fig. 83.26) (3).
Girgis et al. observed that the bulk of the PCL becomes most relaxed in
extension, whereas the majority of the fibers within the ACL are loose
in flexion (17,26). In
70% of knees, a structure reinforces the PCL, extending from the
posterior horn of the lateral meniscus to the femur. If it passes
behind the PCL, it is known as the posterior meniscofemoral ligament
(Wrisberg’s ligament), and if it passes anteriorly, it is named the
anterior meniscofemoral ligament (Humphrey’s ligament) (20). Rarely do both of these structures coexist, but in some instances they are quite large. Clancy et al. (20)
stated that when one of these structures is robust, it may mask the
clinical laxity (posterior drawer sign in internal rotation) observed
when the PCL has been destroyed.
 |
|
Figure 83.26.
Strain in the anterior and posterior portions of human cadaver posterior cruciate ligaments (PCL) during motion from full extension to 120° of flexion. |
displacement of the tibia relative to the femur. With posterior
displacements of more than 3 mm in the intact human knee, more than 90%
of the restraining force (11) resides in this ligament (17).
It secondarily assists in restraining varus and valgus moments to the
knee and resists internal rotation of the tibia relative to the femur.
PCL, Harner et al. showed that the cross-sectional areas of both
ligaments changed along their course from tibia to femur, with the ACL
becoming larger as one passes distally and the PCL enlarging proximally
(32). The PCL was found to be 1.5 times larger
than the ACL proximally near its midportion, whereas it was only 1.2
times larger more distally. They also observed that the cross-sectional
area of the meniscofemoral ligaments averaged approximately 22% of that
of the entire PCL in the cadavers they studied. In further work from
the University of Pittsburgh, Marks et al. found that mean
cross-sectional area of collagen fibers within the ACL and PCL varies
along their length (57). In the ACL the mean
diameter of fibrils increases as one moves from proximal to distal,
whereas the PCL shows exactly the opposite trend. This implies
different exposure to strain along various portions of these ligaments.
The complex nature of the cruciate ligaments needs to be considered
when these structures are being replaced following injury.
human knee can be introduced only briefly in this short chapter. Many
topics are only superficially discussed or
totally
ignored. Yet a thorough understanding of this information and the
unexpressed details is mandatory if appropriate treatment is to be
rendered to patients suffering disease and injury to their knees. We
are experiencing an explosion of new research and literature concerning
many subjects involving the biomechanics and anatomy of the knee. In
many areas, our understanding of the intricate details of knee function
is so superficial that at best our efforts to prescribe the appropriate
surgical procedures and therapeutic regimens is marginal and, in some
cases, inadequate. No area better exemplifies this point than the
treatment of the ACL-deficient knee with the myriad therapeutic options
present today. No single treatment method can be considered superior,
simply because our knowledge is too limited. We are undoubtedly in a
period when significant breakthroughs are likely at any time. It is the
responsibility of us all to keep abreast of this information and apply
it to our own practices.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
ME, Fu FH, Mengato R. Meniscal Tears: The Effect of Meniscectomy and
Repair on Intra-articular Contact Areas and Stress in the Human Knee. Am J Sports Med 1986;14:270.
BD, Johnson RJ, Fleming BC, et al. The Effect of Functional Knee
Bracing in the Weightbearing and Non-weightbearing Knee. Am J Sports Med 1997;25:353.
BD, Johnson RJ, Fleming BC, et al. The Strain Behavior of the Anterior
Cruciate Ligament, a Comparison of an Open and Closed Kinetic Chain
Exercise. Am J Sports Med 1997;25:823.
WG, Shelbourne KD, Zoellner GB, et al. Treatment of Knee Joint
Instability Secondary to Rupture of the Posterior Cruciate Ligament. J Bone Joint Surg 1983;65-A:310.
ES, Noyes FR, Butler DL, et al. Ligamentous and Capsular Restraint
Preventing Medial and Lateral Laxity in Intact Human Cadaver Knees. J Bone Joint Surg 1981;63-A:1257.
CD, Livesay GA, Kashiwaguchi S, et al. Comparative Study of the Size
and Shape of Human Anterior and Posterior Cruciate Ligaments. J Orthop Res 1995;13:429.
MS, Grood ES, Noyes FR. Factors Affecting the Region of most Isometric
Femoral Attachments Part II. The Anterior Cruciate Ligament. Am J Sports Med 1989;17:208.
JC, Eilers AF. The Role of the Posterior Oblique Ligament in Repairs of
Acute Medial (Collateral) Ligament Tears of the Knee. J Bone Joint Surg 1973;55-A:923.
HJ, Jackson RW, Glossop ND, et al. Anterior Cruciate Ligament
Insufficiency: A Dynamic Three-Dimensional Motion Analysis. Am J Sports Med 1989;17:325.
MJ, Deng X, Wichiewicz TL, et al. The Poplitealfibular Ligament:
Rediscovery of a Key Element in Posterolateral Stability. Am J Sports Med 1996;24:311.
FR, Butler DL, Grood ES, et al. Biomechanical Analysis of Human
Ligament Grafts Used in Knee Ligament Repairs and Reconstructions. J Bone Joint Surg 1984;66-A:344.
FR, DeLucas JL, Torvik PJ. Biomechanics of Ligament Failure: An
Analysis of Strain-Rate Sensitivity and Mechanisms of Failure in
Primates. J Bone Joint Surg 1974;56-A:236.
GA, Manning T, Snell E, et al. The Effect of Allograft Meniscal
Replacement on Intraarticular Contact Area and Pressures in the Human
Knee. Am J Sports Med 1997;25:692.
Paper Presented at Biomechanics of Human Knee Ligaments: Proceedings of
the European Society of Biomechanics, University of Ulm, Germany,
1987;24.
DT, Martens M. Experimental Analysis of the Quadriceps Muscle Force and
Patello-femoral Joint Reaction Force for Various Activities. Acta Orthop Scand 1972;43:126.
H-U, Birrer S. The Popliteus Tendon and Its Fascicles at the Popliteal
Hiatus: Gross Anatomy and Functional Arthroscopic Evaluation with and
without Anterior Cruciate Deficiency. Arthroscopy 1990;6:209.
GC, LaPrade RF. The Biceps Femoris Muscle Complex at the Knee. Its
Anatomy and Injury Patterns Associated with Acute
Anterolateral–Anteromedial Rotatory Instability. Am J Sports Med 1996;24:2.
SL-Y, Adams DJ. The Tensile Properties of Human Anterior Cruciate
Ligament (ACL) and ACL Graft Tissues. In: Daniel D, Akeson W, O’Connor
J, eds. Knee Ligaments: Structure, Function, Injury and Repair. New York: Raven Press, 1990.
SL-Y, Fox RJ, Sakane M, et al. Force and Force Distribution in the
Anterior Cruciate Ligament and Its Clinical Implications. Sportorthopad Sporttraumatal 1997;13:37.
