INFECTIONS OF THE HAND
Professor of Orthopaedics, Professor of Surgery, Division of Plastic
Surgery; Chief, Hand and Upper Extremity Service, School of Medicine,
University of California, Davis, Sacramento, California, 95817.
Because loss of hand function follows an inadequately treated
infection, make every effort to achieve complete and expedient
resolution. In our experience treatment requires aggressive debridement
when surgery is indicated, use of antimicrobials that cover the full
spectrum of infecting agents known to be present in hand infections,
and appropriate follow-up care.
skin barrier and inoculation of the tissues. Tissue necrosis and
impairment of circulation create an ideal environment for bacteria to
flourish. Bacterial proliferation produces the initial signs of
infection: erythema, calor, swelling, and tenderness. The local tissue
reaction causes edema and further impairment of local circulation, with
eventual abscess formation. The venous drainage of the hand (palmar to
dorsal) and the fact that the skin is less
tethered
on the dorsum explain the preponderance of dorsal swelling in hand
infections. Infection must be differentiated from noninfectious
conditions such as gout, collagen vascular disease, inflammatory
tenosynovitis, acute soft-tissue calcification, foreign-body reaction,
and more rarely, neoplastic processes.
proliferation and remove purulent and necrotic tissue while leaving
behind the well-vascularized and viable tissue (5,18,30).
Whereas cellulitis is treated with antibiotics without surgery, once an
abscess has become evident, surgical drainage is necessary (Fig. 73.1).
Excise the abscess cavity, its walls, loculations, and all infected
tissue as well. Thorough debridement improves blood supply, enabling
natural humoral and cellular antibacterial factors and antibiotics to
enter the infected area. Leave wounds open to allow drainage, except in
larger joints (the wrist and proximally), in which closed-suction
drainage may be used. After surgery, keep the hand initially
immobilized and elevated.
Once
the wound is nontender, nonerythematous, and not indurated, encourage
active motion to prevent stiffness that results when edema fluid is
allowed to consolidate into fibrous tissue.
 |
|
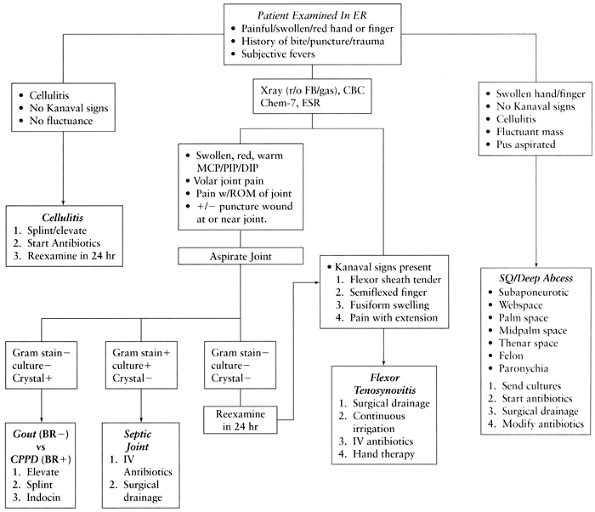
Figure 73.1. Algorithm for diagnosis and treatment of hand infections. BR–, negative birefringence; BR+, positive birefringence; CBC, complete blood count; CHEM-7, blood chemistries; CPPD, calcium pyrophosphate disease; DIP, distal interphalangeal joint; ER, emergency room; ESR, sedimentation rate; FB, foreign body; IV, intravenous; MCP, metacarpal phalangeal joint; PIP, proximal interphalangeal joint; ROM, range of motion; SQ, subcutaneous.
|
lighting, skilled assistance, appropriate instruments, and anesthesia.
Lancing in the emergency department is of limited use; the extent of
infection in various compartments and tissue planes may not be
recognized and thus go untreated (7,30,35,61). Lancing alone often delays resolution of the infection and leads to further tissue destruction (18,58).
neurovascular structures may be visualized, but exsanguinate by gravity
only; an Esmarch bandage may spread the infection locally and
proximally. Either a regional block or a general anesthetic is
required; local anesthetics in inflamed tissues rarely give adequate
anesthesia.
Antibiotic use alone in initial stages often cures the infection and
prevents serious sequelae. It is vitally important, however, that the
antibiotic be chosen on the basis of the bacteria likely to be found in
the wound. Gram-positive and gram-negative anaerobic organisms are
often found in hand infections, in addition to the common and
well-known gram-positive aerobic organisms Streptococcus and Staphylococcus (1,9,16,19,20,21,22 and 23,32,38,43,44,50).
 |
|
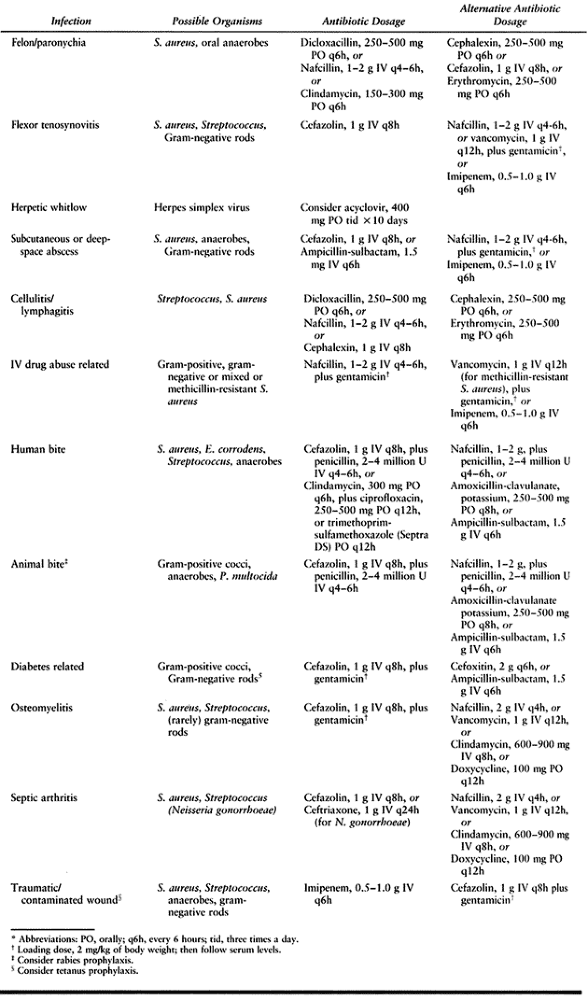
Table 73.1. Empiric Antibiotic Recommendations for Hand Infections*
|
factors: Penicillinase-resistant penicillins (e.g., nafcillin) are
ineffective against many anaerobic pathogens (19,20,22,59). Most hand infections are polymicrobial in nature (Table 73.2) (18,20,59,62). Incorrect initial selection of an antibiotic leads to a poor outcome in hand infections (18,43,58).
 |
|
Table 73.2. Organisms Cultured in 175 Hand Infections in Hospitalized Patients
|
subsequently cultured organisms is sufficiently common that Gram stains
should not be used to exclude initial broad-spectrum coverage (43).
Effective anaerobic coverage is provided by penicillin; effective
aerobic gram-positive coverage is provided by either a
penicillinase-resistant penicillin (e.g., nafcillin) or by a
first-generation cephalosporin such as cefazolin (Ancef, Kefzol).
Anaerobic coverage in penicillin-allergic patients is provided by
cefoxitin (Mefoxin) (21). Clindamycin is
bacteriostatic, and although it is effective against many anaerobes, it
is ineffective against an important faculative anaerobic pathogen, Eikenella corrodens (9). Bactericidal agents are preferred.
hand infections, including those in intravenous (IV) drug abusers and
in mutilating injuries in dirty environments, such as that associated
with farm machinery (16,44,52,62). Add an aminoglycoside antibiotic for initial treatment of these types of infections (51).
needed if the patient has not had one in the 5 years before the
infection or injury. If the patient has had no previous immunization,
administer 250 to 500 units of tetanus immune globulin in addition to
tetanus toxoid. Follow up with a complete immunization schedule.
The latter are more common in infections related to IV drug abuse or
contaminated environments such as farms, stagnant lakes, or sewers (16,39,44,52,62). In most hand infections, multiple pathogens are isolated on culture (58). Treat polymicrobial infection (Table 73.2) with broad-spectrum antibiotic coverage from the outset to avoid a poor outcome (18,43,58). Single antibiotic use rarely covers all offending organisms (18,34,59).
except in special circumstances such as clostridial infection in gas
gangrene. Crush injuries that result in infection create an ideal
environment for anaerobic organisms. Gram-positive anaerobic organisms,
such as Peptococcus and Peptostreptococcus, are more frequently recognized, but gram-negative anaerobic bacteria are common isolates in hand infections (Table 73.2), either alone or with Staphylococcus and Streptococcus, especially in clenched-fist and human bite injuries (19,20,22,23,54,58).
important pathogens found in infections associated with IV drug abuse
infections, because of the common practice of using saliva to lubricate
the needle or moisten cotton used to strain impurities (23,44,58).
The aerobic and anaerobic, gram-positive and gram-negative bacteria
that infect hands are numerous, and we again emphasize the need to
consider all pathogens when selecting an antibiotic (7).
health-care workers who deal with the mouth, such as dentists, dental
hygienists, nurses, and anesthesiologists (31). They
are self-limiting, and recognition of them is important to prevent
overtreatment. Laboratory tests can confirm these infections, but the
diagnosis is usually clinical.
related organisms is cell mediated rather than humoral antibody
mediated, and therefore, often presents as a foreign-body—type reaction
or nonspecific inflammation (3,10,12,25,65).
Delay in diagnosis is common, and the diagnosis is often made only
after failure of other treatment modalities. These organisms commonly
infect the synovium. Once the diagnosis has been made, surgical
intervention may be necessary; antibiotic treatment alone, however, is
often indicated.
possible to reduce the deleterious effect of venous congestion. An
abscess, which is usually the result of a puncture wound that
inoculates the subcutaneous tissue, is recognized clinically as a
raised area of erythema and induration with notable fluctuance. When
seen in IV drug users, abscesses of the upper extremity vary in size
and location but are mostly seen on the dorsum of the hand and forearm,
in the antecubital fossa, and near the deltoid insertion at the
shoulder. An abscess must be drained. Hold antibiotics until
intraoperative cultures are obtained.
-
Base the incision over the apex of the
abscess and in such a way that it can be extended proximally or
distally in an extensile fashion because often the abscess proves to be
larger than anticipated (Fig. 73.2). Incisions should be straight and extensile, curving only at skin creases so as to cross them at an angle of 60° or less.![]() Figure 73.2.
Figure 73.2.
Incisions for drainage of subcutaneous abscesses in the hand and
forearm. Incisions are extensile. Only part of the incision may be
necessary, but often the extent of infection is greater than expected. A: Palmar incision. B: Dorsal incision. -
Avoid sharp angles and skin flaps because
they compromise skin already suffering from the decreased perfusion
produced by the edema of the infection. Skin flaps created by surgical
incisions in infection are at great risk for necrosis (55). -
Excise all infected tissue sharply; a
large curet works well to remove the remains after most of the infected
tissue has been sharply debrided. In infections related to IV drug
abuse, identify and excise the thrombosed, infected vein along its
course until uninfected tissue is found. -
Send fluid for Gram stain and aerobic or anaerobic cultures. Then begin IV antibiotics.
-
Irrigate thoroughly with a pulsatile lavage system.
-
Pack the wound and leave it open. Let the
wound close secondarily; the vast majority will contract and
epithelialize readily. For those wounds in which debridement has been
extensive and secondary-intention healing would require a prolonged
period, we recommend a meshed split-thickness skin graft placed over a
completely granulated base at a later time. Delayed closure of a wound
that is not completely resolved often results in recurrence of the
infection because unseen residual infection can no longer drain. The
conditions of abscess are, therefore, recreated. -
Initiate wet-to-dry dressing changes on
the first postoperative day. These dressing changes are initially done
by the nursing staff twice a day. Teach patients to change their own
dressings in preparation for discharge from the hospital. As wound
improvement is noted (decreased edema and erythema), change
administration of antibiotics to the oral route and discharge patients
with instructions to continue wet-to-dry dressing changes at home until
the wound has completely healed.
gangrene or hemolytic streptococcal gangrene, manifests as an
overwhelming infection within a short period of time. Necrotizing
fasciitis is potentially a limb-threatening or life-threatening
infection that more commonly affects patients with diabetes and IV drug
abusers, who are especially at risk because of the decreased
effectiveness of the immune response (2,24,44,52).
The classic signs and symptoms are erythema, pain, advancing
cellulitis, crepitance, skin necrosis, skin bullae, high fever, and
other systemic signs of infection. When these patients are first seen,
they may have a benign-appearing cellulitis. A tender, erythematous,
swollen, and hot area quickly becomes tense, shiny, smooth, and more
painful. Lymphadenitis and lymphangitis are rare (28).
Within a few days, the skin color darkens and blisters, and bullae
develop. These infections are usually associated with a significant
leukocytosis (52). Radiographs may demonstrate soft-tissue gas. One organ system other than the integumentary or musculoskeletal
system often is in failure (e.g., delirium, shock, respiratory failure, or renal failure).
is the most common organism cultured, although mixed aerobic-anaerobic
and gram-positive and gram-negative infections also occur. Although the
bacteriology of this infection may vary, the common mechanism of
destruction is hypothesized to be the ability of the bacteria to
produce various enzymes (i.e., hyaluronidase, lipase) (38).
These bacterial enzymes facilitate necrosis of tissue, mainly fascia
and fat, and thus allow the rapid spread of infection along tissue
planes. Liquefactive necrosis of the fat and superficial fascia in the
subcutaneous space leads to the characteristic appearance of a grayish,
watery, and foul-smelling fluid often referred to as “dishwater pus.”
a significant leukocytosis in a clinically deteriorating patient
suggests the diagnosis of necrotizing fasciitis, a definitive diagnosis
can be made at the time of surgery by the presence of necrotic fascia (64).
Immediate radical surgical debridement is required until healthy muscle
and fascia are seen proximally beyond the infected area. Absolutely all
infected tissue must be debrided radically if the infection is to be
controlled. Multiple debridements and even amputation may be necessary
as a life-saving measure. The patient may deteriorate rapidly; clinical
vigilance and frequent wound checks are mandatory.
Infection in one compartment generally is manifested by pain and
swelling throughout the hand, with exquisite tenderness in the confines
of the involved space. Knowledge of the anatomic boundaries of these
spaces is important for diagnosis and treatment.
 |
|
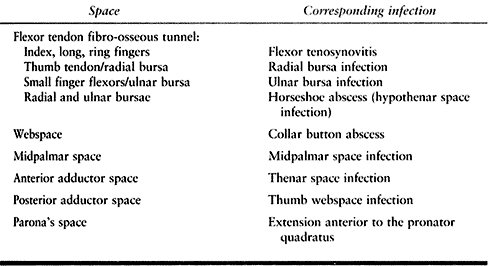
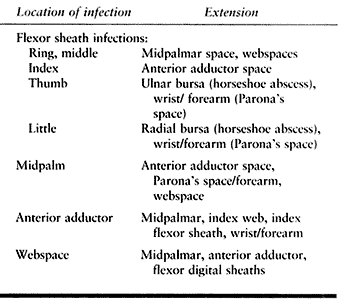
Table 73.3. Spaces of the Hand and Their Infections
|
Adequate assessment, a necessary part of the preoperative examination,
requires that the surgeon be aware of the possible patterns of
extension (Fig. 73.3). Visual inspection offers
clues, but as Kanavel notes, “The most conspicuous and valuable sign is
the extension of the exquisite tenderness to the area involved” (29).
If there is any doubt, exploration of the space where extension may
have occurred is indicated at the time of surgical drainage of the
known infected space.
 |
|
Table 73.4. Extension of Space Infections
|
 |
|
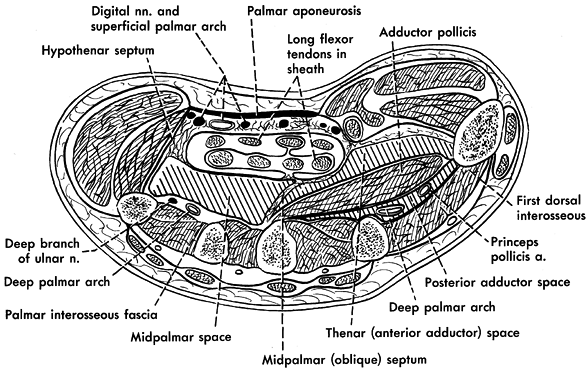
Figure 73.3. Cross section through the proximal part of the hand demonstrating the palmar fascial spaces. (From Hollinshead WH, Rosse C. Textbook of Anatomy, 4th ed. Philadelphia: J.B. Lippincott, 1985.)
|
webspace, the dorsal subaponeurotic space, the midpalmar space, and the
thenar space. These closed compartments are susceptible to infection,
usually from direct traumatic inoculation, extension from an infected
contiguous compartment, or less commonly, from hematogenous spread. The
two most superficial deep spaces are the webspace and the dorsal
subaponeurotic space; identifying infection in these spaces is easier;
determining the involved space in the deep palm is more difficult.
erythema over the palmar and dorsal surfaces of the involved webspace;
characteristically swelling is greater dorsally than palmarly because
of the greater suppleness of the dorsal skin. A webspace infection
often occurs from deep spread of a superficially infected palmar
callus. The fingers bordering the webspace involved are splayed because
tissue pressure holds them apart. This type of infection is also called
a collar button abscess after the old-fashioned dumbbell-shaped collar
buttons. The infection begins palmarly, then spreads dorsally around or
through the transverse metacarpal ligament to the dorsal webspace (11).
Thus two abscess spaces are created, connected by a thin stalk. A
neglected webspace infection can spread to the palm spaces through the
lumbrical canals (27).
-
Two incisions are needed (Fig. 73.4).
Palmarly, make an oblique incision following the skin lines. Stay away
from the web edge; an incision into or through the edge will cause
contracture. The abscess is just below the skin, so deeper dissection
is not indicated. Remember that in this area, the neurovascular
structures lie just beneath the skin, so use great care.![]() Figure 73.4. Dashed lines indicate incisions for drainage of webspace infections (A) and dorsal subaponeurotic space infections (B).
Figure 73.4. Dashed lines indicate incisions for drainage of webspace infections (A) and dorsal subaponeurotic space infections (B). -
Spread the margins of the incision gently to allow adequate drainage.
-
Dorsally, make a longitudinal incision
between the metacarpal heads that stays at least 5 mm proximal to the
web edge. Excise the dorsal abscess cavity and break up all loculations. -
Place drains into the incisions after
irrigation. Begin IV antibiotics after fluid or tissue for culture is
obtained. Follow with the usual aftercare.
hand beneath the extensor tendons (29).
Infection usually begins with direct inoculation beneath the extensor
tendons. The patient has dorsal hand swelling and erythema, and often
it is difficult to delineate a subcutaneous infection from one
involving the subaponeurotic space. The deep space is involved unless
proven otherwise and thus should be drained.
-
Make two longitudinal incisions; one over
the second metacarpal shaft and another over either the fourth or the
fifth metacarpal shaft (Fig. 73.2B) to allow better coverage of the extensor tendons dorsally. -
Try to preserve the dorsal veins, which are important to control swelling.
-
Explore deep to the extensor tendons, taking care not to injure them.
-
Allow incisions to heal by secondary intention.
-
Important: Prevent extensor lag by
initiating early motion of the wrist and finger joints. Continue
therapy during the entire healing stage.
Anatomists do not agree on their exact anatomic boundaries. They lie
dorsal to the flexor tendons and palmar to the intrinsic muscles of the
hand, and proximally and distally, they merge to form potential
extensions to other hand and forearm spaces. These extensions are a
source of spread of infection.
Most thenar space infections involve the anterior adductor space. Its
anterior boundary is the index finger flexor tendons and the midpalmar
septum; its posterior boundary is the fascia over the adductor
pollicis. Radially, the thenar intermuscular septum and ulnarly the
midpalmar (oblique) septum complete this potential space (27).
tense swelling in the space. Thumb adduction and opposition decrease
the potential thenar space and cause an increase in pain. Therefore,
the attitude of the hand with a thenar space infection is with the
thumb in palmar abduction (11). Infection in
the thenar space can spread from flexor tenosynovitis of the index
finger. Distinguishing between an anterior adductor space infection and
a posterior adductor space infection can be difficult. Usually, the
anterior space is involved first and as the infection progresses,
purulence tracts over the adductor pollicis to the posterior adductor
space. If there is any doubt, open both spaces.
-
Make a curvilinear incision in the palm parallel to the thenar crease on the ulnar side at the base of the thenar eminence (Fig. 73.5).
-
Gently spread the palmar fascia in line with the incision. Identify and tag the digital nerves and flexors to the index finger.
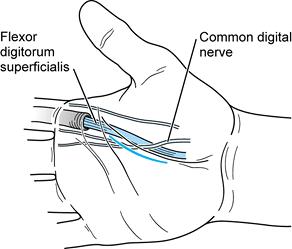 Figure 73.5. Incision for drainage of thenar space infections.
Figure 73.5. Incision for drainage of thenar space infections. -
Gently retract the nerves.
-
Enter the space beneath the flexors by
blunt dissection, remembering that the superficial and deep palmar
arches and recurrent branch of the median nerve are in this area. -
Open sufficiently to allow easy drainage.
-
Send fluid for aerobic and anaerobic culture, then start IV antibiotics.
-
Irrigate well. Place a penrose drain to keep the space open. Close the skin loosely.
-
Make a longitudinal incision dorsally between the thumb and index metacarpals (Fig. 73.5). Stay away from the edge of the thumb-index webspace to avoid webspace contractures.
-
Incise the fascia, then retract the first
dorsal interosseous muscle ulnarly and the extensor pollicis longus
tendon radially. Take care to avoid the radial artery, which is in the
proximal portion of the incision. -
Bluntly enter the space between the
retracted muscles. Open sufficiently to allow easy drainage. Send fluid
for aerobic and anaerobic cultures, then begin IV antibiotics. -
Irrigate well. Place a Penrose drain to keep the space open. Close the skin loosely.
of the index finger, coursing obliquely posteriorly to its attachment
on the third metacarpal. It divides the palm into the two main spaces
of the anterior adductor (thenar) space and midpalmar space. Midpalmar
space infections (Fig. 73.3) are less common than thenar space infections and occur from direct inoculation of this potential space (11) or spread
from tenosynovitis of the long, ring, or small fingers. The midpalmar
space is bordered anteriorly by the flexor tendons of the ring, long,
and small fingers. Posteriorly, the second, third, and fourth palmar
interossei, the third dorsal interosseus, and the ulnar third and the
entire fourth metacarpal define this space. Its ulnar border is the
hypothenar muscular septum, and the radial border is the midpalmar
(oblique) septum (27).
-
Make a transverse incision parallel and
just proximal to the distal palmar crease, just through the skin at the
ring finger. It will be over the area of the swelling. The digital
neurovascular structures lie just below the skin, so take extreme care
when making the skin incision (Fig. 73.6).![]() Figure 73.6. Incision for drainage of midpalmar space infection.
Figure 73.6. Incision for drainage of midpalmar space infection. -
Once through the skin, gently dissect
longitudinally and identify the digital nerves. Tag these features and
retract them gently. Identify the deep and superficial flexor tendons
to the ring finger. Because the space is dorsal to the flexors, blunt
exploration radial and deep to these tendons will gain entrance into
the space. -
Spread gently and open sufficiently to
allow adequate drainage. Send fluid for aerobic and anaerobic cultures,
then begin IV antibiotics. -
Irrigate, and place a Penrose drain to keep the space open. Close the skin loosely.
appropriate, and prompt treatment to avoid severe disability of the
digit or amputation. Acute flexor tenosynovitis is an infection of the
tendon sheath that surrounds the flexor tendons. The synovial sheath
consists of a visceral layer or epitenon that is adherent to the tendon
and a parietal layer, which join at their most distal and proximal
ends. The sheath extends from the midpalmar crease (metacarpal neck) to
just proximal to the distal interphalangeal joint. The small finger
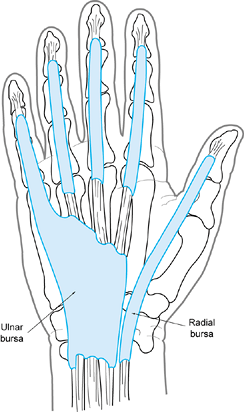
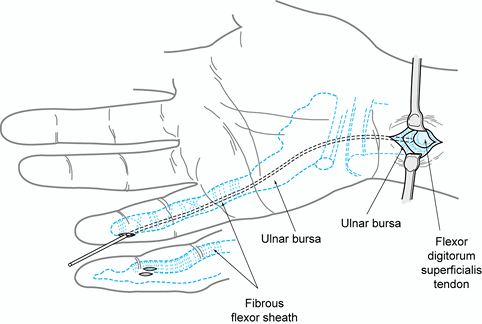
flexor sheath is continuous with the ulnar bursa in the palm that
surrounds the superficial and deep flexor tendons. The sheath around
the flexor pollicis longus is continuous with the radial bursa (Fig. 73.7).
Both of these palmar bursae extend just proximal to the transverse
carpal ligament and, in 80% of individuals, they communicate (42).
 |
|
Figure 73.7. Anatomy of the radial and ulnar bursae.
|
innocuous-appearing small abrasions and tiny punctures; do not be
fooled. These minor wounds are the indication that the sheath has been
inoculated with bacteria. The ring, long, and index fingers are most
commonly involved; however, any digit may be affected. Kanavel (29) described four cardinal signs of infection of the flexor sheath:
-
Symmetric, fusiform swelling of the entire finger (sausage finger)
-
Semiflexed position of the digit
-
Exquisite tenderness over the course of the sheath
-
Exquisite pain on extending the finger
early cases, only one or two of these may been detected. The most
reliable sign is tenderness over the flexor sheath (42).
examine the other spaces of the hand as well, because extension of a
sheath infection can occur into the palmar
spaces,
webspace, carpal canal, palm bursa, and Parona’s space (see later).
Although spread to any of the hand spaces can occur, typically flexor
sheath infections of the middle, ring, and small fingers extend into
the midpalmar space, and index finger infections extend into the
anterior adductor space (thenar space).
allow continued drainage without compromising the function and anatomy
of the delicate structure that is the flexor tendon and its canal.
Drainage can easily be accomplished, but the drainage procedure itself
must not be detrimental to the sheath if the smooth gliding of the
flexor tendon within the sheath is to occur after eradication of the
infection. We believe that this technique is best accomplished through
the intermittent irrigation of the sheath using a catheter (41,42).
advantage of constant cleansing of the sheath. If fluid does not drain
easily from the distal wound, it can be hydraulically forced into the
sheath and surrounding tissues. Intermittent irrigation requires the
observation of ready egress while infusing to avoid this potential
problem. Antibiotic irrigation within the sheath is not recommended
because it has not been proven to be superior to saline irrigation
alone. Also, the effect of antibiotics on the flexor sheath with
respect to inducing adhesions has not been studied.
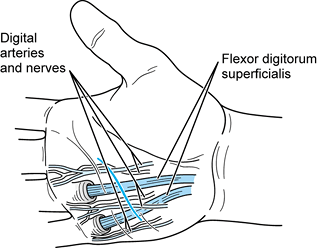
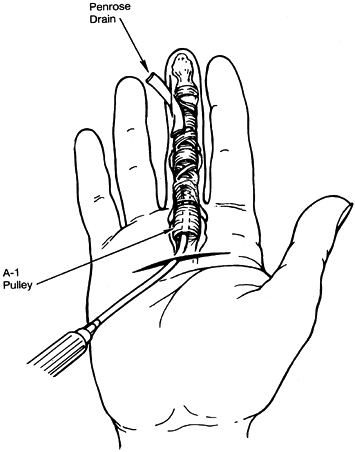
-
Use two incisions (Fig. 73.8). Make a transverse incision proximally in the area of the A1
flexor pulley (metacarpophalangeal joint). This incision is made at the
proximal palm crease in the index finger, at the distal palmar crease
in the ring finger, and between the two for the long finger. Incise the
skin only about 1 cm in length.![]() Figure 73.8. Closed tendon sheath irrigation for flexor tenosynovitis.
Figure 73.8. Closed tendon sheath irrigation for flexor tenosynovitis. -
Gently dissect longitudinally in line
with the palmar fascia (perpendicular to the incision), locating the
digital nerves on either side of the tendon. Tag and gently retract
them. -
Identify the tendon and the A1
pulley in the base of the wound. Open the palmar synovial and fibrous
covering that is the beginning of the flexor sheath; pus or, more
commonly, slightly cloudy fluid will egress. Send a sample for culture;
then start IV antibiotics. -
Make the second incision along the
midlateral line of the digit on the ulnar side at the distal
interphalangeal joint crease of the index and long fingers, or the
radial side of the ring and little fingers. The midlateral line is that
line created by connecting the dots that can be made at the end of the
flexion creases when the finger is maximally flexed. Incise the skin.
Bluntly dissect deeper to enter the flexor sheath. -
At the proximal wound, lift the A1 pulley and thread a small catheter (#10 pediatric feeding tube fenestrated on its end) into the sheath for about 2 cm.
-
Attach a syringe to the catheter end in
the palm and irrigate saline through the catheter. Fluid should easily
exit from the distal wound. If it does not, reposition the catheter or
enlarge the opening distally. Several positioning attempts may be
needed to achieve easy flow. -
Irrigate at least 500 ml of normal saline through the sheath to thoroughly cleanse it.
-
Place a rubber wick made from a Penrose
drain into the sheath through the distal wound to prevent closure.
Suture the wick distally and the irrigating tube proximally to the skin
to prevent their accidental removal. Approximate the proximal wound
with one or two nylon sutures. -
Apply a bulky hand dressing that allows
visualization of the distal wound but covers the palmar wound, and
bring the catheter out through the dressing.
over 30 to 60 seconds through the catheter every 2 to 4 hours.
Postoperative orders should include nursing precautions that when
irrigating the catheter with saline, resistance to flow should be
minimal, that egress should be seen, and that the patient will
experience some discomfort. If resistance is excessive, flow is not
seen distally, and the patient experiences excessive pain, the catheter
may have
become
kinked or may have moved to an inappropriate area. Remove the catheter
if easy flow cannot be regained. Continue the irrigation until the
sheath is nontender (36 to 72 hours), then remove the catheter and
wick, and apply a moist dressing for 2 to 3 more days, continuing IV
antibiotics. Begin active range-of-motion exercises of the digit after
catheter removal to prevent stiffness. Allow the wounds to close
secondarily.
The proximal extension of the thumb flexor sheath is the radial bursa.
It extends proximal to the transverse carpal ligament. The proximal
extension of the small finger flexor sheath is the ulnar bursa that
also surrounds the superficial and deep flexor tendons and extends
proximal to the transverse carpal ligament as well. As stated earlier,
in approximately 80% of individuals, these bursae communicate.
Infection that spreads between these two bursae can create a “horseshoe
abscess.”
present in the digit; in addition, there is swelling, tenderness, and
calor into the respective side of the palm and wrist. Treat these
symptoms with catheter irrigation as described earlier; described below
are the modifications appropriate to the anatomy of each digit and its
bursa.
-
Make a distal thumb incision along the
radial side at the interphalangeal joint crease. Stay dorsal to the
neurovascular bundle. Identify and enter the flexor sheath. -
Pass a probe or other blunt instrument
carefully down the canal until it presses up at the palmar wrist
crease. Do not push the probe if you meet resistance. Gently redirect
it. Make a longitudinal incision that does not cross the wrist crease. -
Enter the space and identify your probe.
-
Pass a #10 pediatric feeding tube from
proximal to distal for about 2 cm. Suture a wick distally and the
irrigation tube proximally, as described for suppurative flexor
tenosynovitis. -
The rest of the procedure is the same as
for flexor tenosynovitis. Do not hesitate to make an additional
incision in the palm if threading the catheter is difficult.
that for a radial bursa, except the distal incision on the small finger
is on the ulnar side of the digit at the distal interphalangeal joint (Fig. 73.9).
 |
|
Figure 73.9. Drainage of ulnar bursa infection and insertion of irrigation catheter.
|
forearm between the deep flexor tendons and the pronator quadratus. It
is usually infected only as a result of proximal extension of a hand
space infection. With deep space palmar infections, always palpate
Parona’s space for fullness and tenderness. Clinically, infection is
evident by tenderness in the distal forearm with pain on pronation and
supination. Drainage is accomplished by an incision at the wrist and
proximal forearm that often includes communication with the proximal
portion of one of the bursa or palmar space infections. Bluntly dissect
deep to the flexor tendons to enter the space. Enlarge sufficiently to
allow adequate drainage.
detected before fulminant proliferation of bacteria, aspiration for
diagnosis and culture can be followed by antibiotic therapy alone.
Medical literature documents the ability to eradicate the infection by
antibiotics alone. The deleterious effect of pus on cartilage, however,
necessitates surgical drainage if there is any doubt that the infection
has been detected and treated early enough. Septic arthritis or
infection of a joint space may affect any of the joints in the digits,
thumb, or wrist. It is most likely secondary to direct penetration of
the joint capsule, but hematogenous spread occurs as well. If there is
no history of a bite, puncture near the joint, or a recent laceration,
it is important to ask the patient about recent tooth abscesses, ear
infections, other skin infections, gastrointestinal (GI) infections,
and genitourinary (GU) infections. Gonococcal septic arthritis can be
treated with antibiotics alone; it is important to question the patient
about his or her sexual history.
erythematous, and warm digit, thumb, or wrist, localized to a specific
joint. The pathognomonic sign of joint infection is exquisite pain on
attempted motion of that joint. Cellulitis over a joint causes pain on
motion, but the tenderness elicited by slow, gentle, passive motion in
cellulitis is much different from the severe pain caused by any attempt
at motion of an infected joint.
following joint aspiration. Send the aspirate for cell count with
differentiation, Gram stain, anaerobic and aerobic culture, glucose,
and crystal analysis. A white blood cell differential of greater than
50,000 (usually more than 75% segmented neutrophils), or the presence
of bacteria on Gram stain or culture is diagnostic. A synovial fluid
glucose of 40 mg less than the fasting blood glucose is consistent with
an infectious process (53). Finally, a crystal analysis is necessary to rule out gout or pseudogout as a cause of the painful, red, swollen joint.
the joint, ideally an 18 gauge needle or larger. A 20 or 22 gauge
needle is usually the largest that will gain entrance into smaller
joints. The joint space for the metacarpophalangeal joint is easily
palpated just ulnar or radial to the extensor hood; take the edge of
your fingernail and press. Aspirate after an antiseptic preparation.
Giving a local anesthetic usually causes more discomfort than the
single stick of the aspirating needle, but patients are not easily
convinced of this fact.
similar fashion. This location just next to the extensor tendon
dorsally would correspond to a 10 o’clock or 2 o’clock position if the
extensor tendon (top or dorsal) is considered as 12 o’clock.
-
Use a large needle, ideally 18 or 20 gauge, or the largest needle that will enter the joint space.
-
Palpate the joint space for the
metacarpophalangeal joint, ulnar or radial to the extensor hood with
the edge of your fingernail, feeling for the depression of the joint
space. -
Aspirate after an antiseptic wash.
-
Locate the interphalangeal joint in a
similar fashion. Enter at the 10 or 2 o’clock position, with the
extensor tendon as 12 o’clock. -
Aspirate after an antiseptic wash.
-
Base the incision dorsally over the involved joint.
-
Incise longitudinally through either the
extensor tendon at the metacarpophalangeal joint or through the
extensor mechanism at the proximal or distal interphalangeal joints. -
Enter the involved joint and thoroughly irrigate it.
-
Leave a small wick (penrose drain) in the
joint space to facilitate drainage. Reapproximate the extensor
mechanism and loosely close the skin. -
Remove the penrose drain in 48 to 72 hours.
ulnocarpal, and midcarpal joints. Septic arthritis may be found in any
one or all of these joints; these spaces may or may not normally
communicate. Direct the preoperative examination toward localizing the
joint or joints involved. Pronation or supination pain indicates
involvement of the radioulnar joint; flexion or extension pain
indicates involvement of the radiocarpal, ulnocarpal, or midcarpal
joints. Aspiration with an 18-gauge needle confirms clinical
suspicions. The radial and ulnar styloids offer convenient landmarks;
enter just distal to them.
-
Enter the radiocarpal, radioulnar,
midcarpal, and ulnocarpal joints through either a transverse or
longitudinal dorsal skin incision. A transverse incision has the
advantage that if you take care to isolate and preserve veins, you can
make a completely circumferential incision so that all wrist joint
spaces can be entered until the infected space can be found and drained. -
Make the skin incision, and then enter
the joint space by blunt dissection between the appropriate extensor
compartments. Enter between the third (extensor pollicis longus) and
fourth (extensor indicis proprius, extensor digitorum communis)
compartments to drain the radiocarpal, radioulnar, and midcarpal
joints. Enter around the extensor carpi ulnaris or extensor digiti
quinti tendons to drain the ulnocarpal joint. -
Send fluid for aerobic and anaerobic cultures. Begin IV antibiotics.
-
Irrigate thoroughly with a pulsatile lavage system.
-
Place a closed suction system drain.
Close the joint capsule and skin incision over the drain. Leave the
drain in for 3 to 5 days. -
Begin gentle active assisted and active range-of-motion exercises after drain removal.
-
Continue IV antibiotics for a least 1
week. Follow with an oral agent for several weeks after hospital
discharge. Antibiotic sensitivities and minimum inhibitory
concentration (MIC) help guide the appropriate oral antibiotic regimen.
Ideally, the oral dose will have been determined by greater than 1:8
serum bactericidal levels against the bacterium or bacteria isolated.
have significant sequelae if they are not recognized and treated
correctly (6,7,15,19,22,23,34).
There are four classic mechanisms of infection, and these include
self-inflicted (caused, for example, by nail biting), traumatic
amputation (usually at the distal interphalangeal joint or pulp) from a
bite, full-thickness bite wounds on any part of the hand, and finally a
clenched-fist bite injury, usually following an altercation (14).
The complications of incompletely treated human bite infections include
arthritis, joint stiffness, osteomyelitis, toxic shock syndrome, and
infrequently, death. Human mouth organisms inoculated into tissue are
quite virulent (6,7,13,15,19,22,23,33,34 and 35,58,63).
control a human-bite infection occurred in up to half of the affected
patients. Death secondary to morsus humanis was reported as well. The
introduction of antibiotics has not eliminated the complications
associated with these infections. Even with antibiotics, 6% of human
bites need amputation to control infection, 4% require late amputation,
and 28% develop either osteomyelitis or other causes for stiff,
disabled fingers (34).
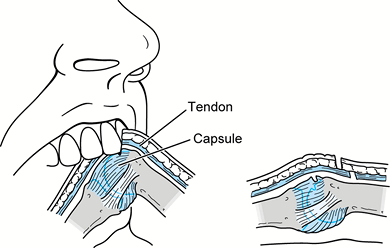
and the overlying dorsal skin and extensor tendon stretched distally.
The tooth penetrates the skin, part or all of the tendon, the dorsal
joint capsule, and potentially the metacarpal head. With the hand open,
it may be difficult to recognize the extent of injury because the
injury to the joint capsule and bone may be distal to the skin
laceration and injury to the extensor tendon is proximal to the skin
laceration (Fig. 73.10). Often, there is
intra-articular violation with the potential for septic arthritis.
Sometimes, osteochondral fractures of the metacarpal head or fracture
of the metacarpal head or neck occur. The clenched-fist injury commonly
involves the metacarpophalangeal joint of the long, ring, or little
fingers, but any hand joint or digit can be involved.
 |
|
Figure 73.10. A fight bite occurs during flexion (A) of the metacarpophalangeal joint. During extension (B) of the injured joint, the tendon or osteochondral injury may be obscured leading to a closed intra-articular injury.
|
(i.e., in less than 24 hours) have a better prognosis than those seen
later than 24 hours from the time of injury (29,34).
Clenched-fist injuries may resemble a simple puncture wound over the
metacarpohalangeal joint, but there usually is more extensive damage to
the soft tissue and bony structures underneath.
A plethora of microbes are isolated from the human mouth flora,
however, and thus it is not uncommon to see mixed infections that could
include, Bacteroides sp., Neisseria sp., Clostridia sp., spirochetes, Micrococcus, and rarely, Actinomyces (19,20,22,34,40).
the most common pathogen in human bite infections, it certainly seems
to be the type most often associated with “fight bites.” E. corrodens has been isolated in 7% to 29% of human bite infections (4,20,47). Although it is a normal part of the human mouth flora, it is more readily cultured from dental scrapings than from saliva (47). It is a gram-negative rod and a facultative anaerobe and thus grows best in 10% CO2.
radiographs to rule out a fracture or foreign body (i.e., tooth), and
wound exploration. Dorsal puncture wounds around the metacarpohalangeal
joint are considered to be clenched-fist injuries unless proven
otherwise.
joint to determine extensor tendon involvement. A saline arthrogram may
assist in determining joint involvement. A history of a human bite or
lacerations (especially over a joint) obtained during a fight must be
treated aggressively. Treatment requires surgical debridement of dead
or questionably viable tissue and thorough irrigation of the
subcutaneous space (especially a joint). Leave the wound open.
First-generation cephalosporins such as cefazolin, cephalothin, and
cephradine, or penicillinase-resistant penicillins are effective
against the common gram-positive aerobic bacteria. Many anaerobes
commonly isolated from clenched-fist and human bite infections,
however, are resistant to penicillinase-resistant penicillins and
clindamycin, which are common first choices in the treatment of skin
infections (9,49,60).
The antibiotic of choice for these anaerobes is penicillin. Cefoxitin
is effective against gram-positive aerobic bacteria and the anaerobes
described, so it may be used as a single agent for penicillin-allergic
patients (21).
-
Extend the traumatic wound proximally and distally.
-
Remove the necrotic and infected tissue
from the subcutaneous layer. The infraction often extends beyond the
obvious site of injury. Explore nearby potential areas of invasion such
as the dorsal subcutaneous space. -
Always open the joint if the injury is
over or near a joint. The joint often appears uninvolved until
arthrotomy is performed and the pus wells out. Enter the joint
dorsally, making a 1 cm longitudinal incision in the capsule through
the extensor tendon. -
Inspect the cartilaginous surfaces for
lesions. An osteochondral defect or tooth indentation is common. Record
such a finding and also the condition of the cartilage in the operative
record. Divots and unhealthy-appearing cartilage are poor prognostic
factors. -
Irrigate thoroughly by pulsatile lavage. Leave the wound open.
(more commonly from cat than from dog bites). The presence of
cellulitis, lymphangitis, serosangueinous, or purulent drainage within
12 to 24 hours after a cat or dog bite strongly suggests P. multocida as the offending organism (1).
debridement of necrotic and damaged tissue. Be sure to culture the
wound even if gross purulence is not found. Penicillin is the drug of
choice for P. multocida, with tetracycline
and cephalosporins as alternatives. Administer penicillin intravenously
in combination with cefazolin; alternatively, amoxicillin-clavulanate
potassium can be given orally for less severe infections (Table 73.1).
the skin of the pulp lies fat. Numerous fibrous septae that tether the
skin to the bone cause compartmentation of the fat to make
shock-absorbing spaces. Infection in the digit pulp is therefore a
series of small, closed-space infections, each needing incision and
drainage. A felon is usually due to penetrating trauma to the tip of a
digit. S. aureus is the most common organism found (45).
The presenting symptom is an erythematous, swollen distal phalanx on
the palmar side of the digit. It is important to distinguish a felon
from herpetic whitlow, because the treatment of herpetic infection of
the distal phalanx is nonsurgical. When the felon has organized into an
abscess, drainage is indicated.
-
Drain a felon that points palmar and midline by a midline approach (Fig. 73.11A). Drain a felon that does not point midline with a midlateral incision (Fig. 73.11B). Avoid the “fish-mouth” incision, because it destabilizes the pulp and can cause flap necrosis.
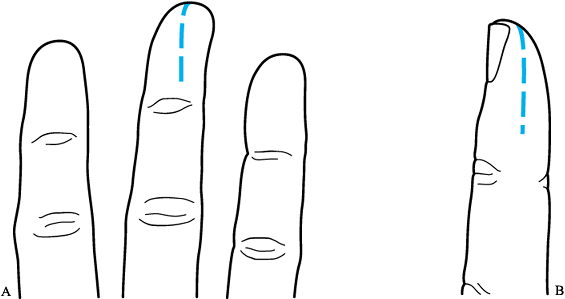 Figure 73.11. Incision for drainage of a felon. All septae must be cut to allow appropriate drainage. A: Midline incision. B: Midlateral incision.
Figure 73.11. Incision for drainage of a felon. All septae must be cut to allow appropriate drainage. A: Midline incision. B: Midlateral incision. -
Incise deeply all the way to the bone and
all the way across the pulp to open all compartmentation spaces. Send
fluid or tissue for culture. Begin antibiotics and irrigate thoroughly. -
Leave the wound open. Place a wick into
the wound. Apply a bulky hand dressing to immobilize the hand for the
first 24 to 48 hours. -
Remove the wick and begin whirlpool soaks
four to five times a day. Apply a finger dressing incorporating damp
gauze next to the wound. Encourage active finger and hand motion.
area sensitive to the disruptive influence of daily living and nail
cosmetic practices, especially in those whose work
or
habits cause a continually damp or wet environment. Bacteria invade the
fold of skin over the nail matrix, producing the characteristic lesion
of inflammation, pain, and swelling. When it is confined to a side of
the nail and involves the fold of skin and not the nail, the infection
is called a paronychia. When it involves the base of the nail fold, it
is an eponychia. Sometimes both sides of the nail and the eponychial
fold are involved, and this infection is called a runaround abscess.
involves the nail bed is a subungual abscess and has more serious
implications The localized superficial nail skinfold infection is not
serious, but deep infection may invade bone and result in nail
destruction, osteomyelitis, and amputation. A patient with a nail bed
infection has swelling, pain, and erythema at the base of the
fingernail or on the side of the nail. The bacterium that infects is
almost exclusively Staphylococcus, except in children, in whom mouth flora can infect as well because of thumb-sucking (8).
returns as a felon. Infection spreads from the nail bed to the pulp of
the digit through bone; if such spread occurs, osteomyelitis of the
distal phalanx is present.
process. Women who work with their hands in moist environments are more
susceptible. The infection comes and goes throughout its course and
leads to a painful thickened eponychial fold that sometimes accumulates
whitish thick material. Most often, Candida albicans
is the offending yeast. Conservative treatment of topical steroids and
antifungals has met with limited success. Chronic paronychias often
require marsupialization.
-
After metacarpal block, use a Freer
elevator or other blunt instrument and, beginning distally, elevate a
few millimeters of the nail fold from the nail as far proximally as the
infection extends. A blunt instrument helps prevent scoring that can
later produce nail ridging. (Note: An incision usually is not needed
for simple cases. A more extensive abscess may need a small incision
adjacent to the eponychial fold and parallel to the nail plate.) -
Using an 18-gauge needle, prick the
infected area to drain and break up loculations. Expect only a few
drops of pus. Send these drops for culture, and begin an antibiotic
effective against Staphylococcus. -
Cover the drained area with
antibiotic-impregnated gauze and place it under the nail fold to allow
continued drainage. Begin soaks in 24 hours and continue until healed.
-
Use a digital block. Perform an eponychial marsupialization by excising a small crescent-shaped piece of the eponychial fold.
-
Remove the skin and thickened tissue down to, but not including, the germinal matrix.
-
If the nail plate is involved (damaged), remove the entire nail.
-
Place xeroform gauze under the nail fold to promote drainage and keep the nail fold from closing.
-
If the nail has been elevated off the
matrix by infection, a subungual abscess is present. Incise along the
borders of the nail fold. Elevate the nail fold (Fig. 73.12).![]() Figure 73.12.
Figure 73.12.
Incision for drainage of a subungual abscess. After folding back the
eponychium, the proximal nail plate is cut with scissors and removed. -
Remove the overlying nail plate to allow
adequate drainage. If there is any question as to the extent of the
subungual abscess, remove the entire nail. Remember, gently free the
nail plate from the matrix with a blunt instrument to avoid injury to
the matrix, which may result in ridging of the nail as it grows back. -
After removing nail plate, scoop the abscess contents out with a curet, gently scraping the surface of the nail matrix.
-
If the nail is removed from the nail fold, place xeroform gauze under the nail fold.
-
Request aerobic and anaerobic culture on the fluid. Begin IV antibiotics.
It is most commonly seen in health care providers exposed to oral
secretions such as dentists, nurses, and dental hygienists. Pain is the
initial symptom. Vesicles 1 to 2 mm in diameter containing clear fluid
occur on an erythematous base; as the infection progresses, the
vesicles coalesce into bullae. The fluid may turn cloudy after being
initially clear, but it is never purulent. Purpuric lesions can develop
beneath the nails. Lymphangitis and lymphadenopathy may also occur and
confuse the picture, simulating a bacterial infection. The pain
continues for 2 to 4 weeks, with decreasing erythema. The hemorrhagic
areas crust and then desquamate, leaving normal tissue.
of the vesicles, which grow characteristic plaques within 1 to 3 days.
Serum titers of antibody to herpesvirus over 2 to 3 weeks may rise to
greater than four times normal levels.
incision and drainage are performed, no purulence will be expressed,
and deeper tissues will be exposed to the herpesvirus. An aggressive
secondary bacterial infection then occurs all too often as the incision
injures tissue that has already been compromised by the edema,
pressure, and unhealthy conditions the viral “cellulitis” has created.
Expectant observation is the key to treatment. Antiviral agents are not
indicated.
These infections typically occur in middle-aged people with a history
of a puncture wound within 6 weeks of onset of symptoms. M. marinum is the most common atypical pathogen, and inoculation usually occurs in a seacoast environment or dock area (26).
Although most atypical mycobacterial infections involve the skin,
deeper structures are susceptible. Tenosynovial infections as well as
bone and joint involvement have been documented.
systemic response, and thus, constitutional symptoms of infection are
not present. Leukocytosis and an elevated sedimentation rate are rare.
Pain, limitation of motion, and fullness of the involved area are
noted, but signs and symptoms are intermittent. Skin infections are
often associated with dermal or subdermal granulomas, which are the
hallmark of diagnosis (26). Synovium is
commonly involved. The synovitis is out of proportion to the disease
initially suspected, such as collagen vascular disease, or nonspecific
inflammatory synovitis. Fistulae may be present. Tenosynovial
infections most commonly involve the flexor tendon sheaths of the
fingers. Patients describe chronic swelling of the involved digit.
Although pain is mild, the bulky synovium may interfere with finger
function, and motion may be limited.
the key to diagnosis of atypical mycobacterial infections in the hand
is biopsy and culture of the suspicious tissue. Diagnosis is based on
finding Langhans’-type giant cells in tissue and the presence of
acid-fast bacilli and positive culture; culture results take at least 6
weeks. Diagnosis is thus typically delayed and is made only after
failure of response to initial treatment. Because cultures may take
more than 6 weeks to grow, medical treatment is often initiated before
definitive culture results if clinical suspicion is high.
biopsy is obtained; complete surgical synovectomy is indicated if the
synovium is heavily affected. Prolonged antituberculous therapy is
recommended with two bactericidal antituberculous agents. Current
antituberculous regimen consists of 2 months of isoniazid, ethambutol,
and rifampin, followed by 7 months of isoniazid and rifampin.
Sensitivities may vary, and medical treatment recommendations change
and thus should be coordinated in consultation with an infectious
disease specialist.
implantation of the fungus or its spore into the skin, or as part of
systemic spread, usually from a primary pulmonary infection that began
with inhalation of spores (10,65). Fungal infections can be divided into cutaneous lesions, subcutaneous infections, and deep or systemic infections (48).
found most often in gardeners and others engaged in activities around
soil, where fungi are ubiquitous. Cutaneous lesions are caused by
dermatophytes that colonize nonliving cornified appendages such as
skin, hair, and fingernails. Examples include dermatophytic infection
of the glabrous skin (tinea corporis), the interdigital areas of the
palms (tinea manuum), and the fingernails (onychomycosis or tinea
unguium) (48). A scaling, pruritic skin lesion
(usually not responsive to or exacerbated by topical steroids) is
present on an obvious thickened and deformed fingernail. Potassium
hydroxide microscopic slide preparations may provide a preliminary
diagnosis, but fungal cultures need to be performed for a definitive
diagnosis.
topical antifungal cream such as tolnaftate, miconazole, clotrimazole,
or econazole. Have the patient apply it topically for 4 to 6 weeks.
More severe or unresponsive lesions may require systemic griseofulvin
or ketoconazole. Fingernail infections are resistant to treatment, but
long-term systemic griseofulvin has met with the most success, with
cure rates as high as 80%.
occurs after trauma or can actually be a systemic manifestation of a
primary lesion elsewhere. After traumatic implantation and an
incubation period of several days to months, a subcutaneous nodule
appears that may or may not be painful. A fistula or ulceration
develops and enlarges with a raised, discolored, and verrucous border.
Epithelial hyperplasia follows. Lymphatic invasion can occur; the lymph
channels become cordlike. Nodules develop, ulcerate, and then
spontaneously resolve, only to be replaced by new lesions.
chromomycosis, mycetoma, and phycomycosis, also produce hand infections
The most common subcutaneous fungal
infection is sporotrichosis (48). Sporotrichosis classically occurs after a prick from a rose thorn by subcutaneous inoculation of the spores of Sporothrix schenckii. After the S. schenckii
organism incubates for a period of days to months, a small, nontender,
movable, subcutaneous nodule develops. Epidermal infection ulcerates
early, whereas a more subcutaneous nodule discolors, darkens, and then
erupts through the skin. Spontaneous healing of the ulcer follows, but
new lesions erupt. Lymphatic invasion occurs, raising cordlike tracks
along the hand and arm. The other cutaneous mycoses present in a
similar fashion.
biopsy of one of the lesions or by culture of drainage. A definitive
diagnosis of sporotrichosis requires S. schenckii
to be isolated on fungal culture. The treatment of choice is potassium
iodide for 6 to 8 weeks. Itraconazole has recently been shown to be
effective as well, and because its dosage is only once a day, it may
prove to be more tolerable.
basis most commonly as tenosynovitis, septic arthritis, or
osteomyelitis. Definitive diagnosis requires identification by a fungal
culture, and treatment usually involves IV amphotericin B for more
virulent fungal infections and oral ketoconazole or itraconazole
treatment for more benign infections.
acquired immunodeficiency syndrome (AIDS) were reported in persons
older than 13 years old from 1981 through 1996, according to the
Centers for Disease Control and Prevention HIV/AIDS Surveillance
Report. The true incidence of human immunodefiency virus (HIV)
infection currently exceeds 1 million people.
the HIV-positive person, there is a known high prevalence of infections
in this population. At one institution, nearly 20% of patients
hospitalized for upper extremity infections were HIV positive. An even
more alarming discovery is that less than 10% of these individuals
admitted to being HIV positive (56).
common risk factor for HIV infection, it also was the most common cause
of upper extremity infections in the HIV-positive person (36).
Although these infections may present atypically, the responsible
organisms are usually similar to those that infect the immunocompetent
host, with S. aureus being the most common pathogen (36).
Furthermore, spontaneous infections may occur, herpetic infections may
appear more virulent, and seemingly benign infections may need a more
aggressive surgical approach. Any individual with an unusual hand
infection should be tested for HIV infection.
It is caused most often by open fractures or spread from neighboring
infected soft tissues. The infection rate in open fractures ranges from
1% to 11%, with a higher risk coming from the more contaminated wounds
with more severe soft-tissue injury (17,39). Although S. aureus and Streptococcus remain the most common pathogens, open injuries predispose to gram-negative, anaerobic, and polymicrobial organisms.
clinical indications of osteomyelitis. Systemic manifestations are less
common but include fever, an elevated white blood cell count with or
without an increase in polymorphonucleocytes, and an elevated
erythrocyte sedimentation rate. Radiographic evidence of osteomyelitis
is usually seen in the subacute or chronic state and includes the
presence of periosteal new bone formation, osteolysis, or a sequestrum.
A bone scan is more sensitive and can suggest the presence of
osteomyelitis much earlier than plain radiography (46).
Diagnosis may be made by subperiosteal aspiration of pus, but negative
findings on aspiration do not rule out the possibility of infection.
surgical drainage, followed by antibiotics. Acute osteomyelitis may
initially be treated with antibiotics, immobilization, and elevation.
Failure of an early and discernible response warrants surgical
incision, but cultures taken following the administration of
antibiotics are usually not helpful.
intervention. A sequestrectomy, diaphysectomy, and even digit or ray
amputation are potential interventions to help control and eliminate
infected bone. Staged procedures with antibiotic-impregnated
polymethylmethacrylate spacers, followed by bone grafting and skin
coverage, are sometimes necessary. Local rotational and free flaps
allow subsequent elevation for reconstruction and are preferred to
pedicled flaps. They must be placed in a clean and vascularized wound
bed.
to promote drainage. Apply a plaster splint–reinforced long-arm bulky
hand dressing, incorporating a cord to permit continuous elevation.
Change the dressing at 36 to 48 hours to a maceration-type dressing of
25% strength Dakin’s solution, which is changed daily. Begin active
hand motion to prevent stiffness. Discharge the patient
when the wound is without erythema, tenderness, induration, or swelling.
for the open wounds; antibiotics are prescribed on the basis of culture
results and the disease process. Continue dressing changes until the
wound is healed.
failure to improve after initial debridement. Physical examination
directed toward discovering possible places of spread before surgery
will help uncover these extensions. When in doubt, open the space at
the time of surgery.
drainage must cross the crease, angle it less than 60°; failure to do
so results in contracture. Do not penetrate the webspaces for the same
reason.
or paronychia. Incision of this already-compromised tissue may lead to
secondary bacterial infection.
as well as the anaerobic streptococci, is necessary. Gram-negative
anaerobes are often present, especially in clenched-fist, human bite,
and IV drug abuse infections. Initial antibiotic selection should be
based on the type of injury known or suspected. Think of HIV infection
in the IV drug abuser or the patient who is not improving in spite of
customary treatment.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
SF, Elliott RC, Brand RL, Adams JP. Experience with Atypical
Mycobacterial Infection in the Deep Structures of the Hand. J Hand Surg [Am] 1977;2:90.
JM, Keele BB, Fridovich I. An Enzyme Based Theory of Obligate
Anaerobiasis: The Physiological Function of Superoxide Dysmutase. Proc Natl Acad Sci 1971;68:1024.