SURGICAL TREATMENT OF THE UPPER EXTREMITY IN CEREBRAL PALSY
III – THE HAND > Reconstructive Procedures > CHAPTER 66 –
SURGICAL TREATMENT OF THE UPPER EXTREMITY IN CEREBRAL PALSY
Department of Orthopaedic Surgery, Washington University School of
Medicine; and St. Louis Veterans Affairs Medical Center, St. Louis,
Missouri, 63110.
encephalopathy characterized by cognitive, sensory, and motor problems.
The condition is highly prevalent, some 300,000 children in the United
States being affected (2). The disease is
classified on the basis of the observed motor deficit, be it manifest
as abnormal tone, motion disorders, or some combination.
refers to abnormally heightened tone and is the predominant motor
manifestation of the affliction. Spastic hemiplegia accounts for about
one third of cerebral palsy patients (26). Motion disorders feature deviations in tone markedly
affected by position and activity and include extrapyramidal
(athetosis, dystonia) and atonic variants. Spasticity and motion
disorders often occur together. Those patients with predominantly
spastic manifestations have the best prognosis for improvement from
surgery and orthotics. A strong element of motion disturbance often
renders the results of surgery unpredictable, and a conservative
attitude toward surgical reconstruction may be most appropriate in such
settings (4,29).
extremity management. These include the patient’s cognitive capacity,
sensibility, motor disorders, emotional status, age, and social
orientation. Only carefully selected children with cerebral palsy
become candidates for upper extremity surgery (19).
If a child denies the limb, it is unlikely he or she will ever use it
despite improvements gained through surgical reconstruction (29).
picture of motor disturbance may take the first several years of life
to emerge. Developmental milestones and disturbances in tone and
symmetry should be monitored. Milestones may appear late in children
with cerebral palsy. By 6 months of age, the Moro, grasp, and tonic
neck reflexes have disappeared in normal children but may persist in
patients with cerebral palsy. Limited spontaneous movement of one or
more limbs in the neonate may suggest the diagnosis. Increased
resistance or lack of resistance to passive motion of the upper limb
should also cause concern, particularly if asymmetric. Affected infants
are slow to develop balance and tend to show persistence of primitive
tone reflexes.
24 months; infants with cerebral palsy may display hand preference
early (9). This results from poor function in the nondominant limb.
placement through shoulder and elbow movement. Such selective muscle
control develops slowly in the child with cerebral palsy. Thumb
opposition develops only in mildly affected cases. Hand placement may
be limited by motion disturbances. As the child grows, sensory and
cognitive defects emerge—helping to establish the diagnosis.
perception, abstract thinking, and communication. Learning problems are
profound in many patients with cerebral palsy, and at least half such
patients are mentally retarded (2). A specific
intelligence level has not been established as a prerequisite for
operative treatment of the upper extremity. In general, however, only
those patients with educable (IQ of 50 to 70) or higher cognitive
ability can expect functional results from upper extremity surgery (9).
Some surgeons maintain that surgery should not be considered in
patients whose IQ is below 70, as such patients often do poorly (4,8).
However, Samilson and Morris noted that standard tests of intelligence
may be misleading due to the patient’s significant motor impairment (28).
A consensus is that the IQ should be considered in planning surgical
treatment but should not constitute the sole determining factor (2).
Tachdjian and Minear report sensory defects in about 50% of patients
with average intelligence (34). Sensation
mediated by thalamic activity, such as light touch and temperature, is
usually normal in the patient with spastic hemiparesis. Sensory defects
most often affect the cortical functions of stereognosis, position
sense, and two-point discrimination (24). These deficits stem from parietal lobe lesions.
age 3 or 4 years. Information may be gathered by observing the child at
play. Stereognosis is assessed by having the child close his eyes and
raise his hands over his head while identifying and describing the
characteristics of objects placed in each hand (6). If appropriate, two-point discrimination and proprioception may be determined.
five objects are identified correctly, graphesthesia is accurate, or
two-point discrimination is less than 5 mm (9).
Sensibility is graded as mildly impaired when only three of five
objects are identified, or two-point discrimination is 5–10 mm.
Moderate to severe impairment applies to hands with greater limitations.
from surgery. Prehension requires good proprioception and stereognosis,
and it may not be an appropriate goal for the hand with poor
sensibility. Green and Banks have shown that the results of surgery
correlate with stereognosis (8). The patient
who exhibits poor stereognosis may exclude the abnormal extremity from
use. Such children often have a distorted body image. These patients
generally do not benefit from reconstructive surgery.
to reach for and transfer objects. Observe her at play, at specific
tasks, while performing activities of daily living, and after periods
of stretching (29).
Study functional tasks, including dressing, toileting, feeding, and key
pinch. Activity should be assessed in different settings and at varied
table levels because the position of the shoulder and trunk may greatly
affect hand function (29). Hoffer has emphasized the importance of hand placement in planning treatment (9).
Ask her to touch her head and then the opposite knee. Performing this
exercise within 5 seconds demonstrates good function and according to
Hoffer is a favorable prognostic sign.
testing and grade the individual muscles. Judge whether a deficit in
muscle function is due to paresis or to overactivity of antagonist
muscles. Observe the patient’s use of the more normal hand to position
and open the fingers of the other hand for grasp. Spasticity and
increased tone are determined by passive testing. Contracture of the
flexor digitorum superficialis, for example, may explain the patient’s
need to flex the wrist before release (4).
Excess finger flexor tone can be distinguished from wrist flexor tone
by testing wrist extension with the fingers passively flexed.
tone from contracture. For example, it may be difficult to assess the
strength of wrist and finger extensors with the wrist held in flexion.
Blocking the median and ulnar nerves at the elbow will identify joint
contractures, permit evaluation of extensors, and determine if
deformity is secondary to increased tone or fixed muscle/tendon
contractures (9). Measuring the range of motion before and after nerve block determines the need for release of soft tissue and contracture.
muscles under good voluntary control that are phasic with the activity
for which transfer is planned (2). Goldner
suggests that repeated observation of the patient’s hand in grasp and
release permits determination of phasic activity (4). Hoffer and others have advocated dynamic electromyography for this purpose (9,11). Hoffer contends that a muscle will not change in phasic activity following transfer (9).
Muscles in phase for specific activities will be functional. Muscles
active only in release and not in grasp, for example, will be effective
if transferred to finger extensors. Those muscles active only during
grasp are good transfers for wrist extension. Muscles that fire
continuously are not candidates for transfer, according to Hoffer (9). Other authors have observed such muscles becoming phasic after transfer (28).
This issue is not yet resolved, and the role of dynamic
electromyography remains controversial. Although acknowledging its
potential, we do not rely on this technique in planning treatment.
elements in planning management, and recognize the overall severity of
involvement. Green and Banks (8) have devised a grading system for evaluating patients, and this has been modified by Samilson and Morris (28):
-
Excellent: good function in activities of
daily living, effective grasp and release, excellent control, wrist
extension of 45° or more, full active finger extension, active
supination of 50° or more -
Good: hands used as helpers in activities
of daily living, effective grasp and release with good control, wrist
extension of 15° to 45°, can actively extend the fingers with the wrist
extended -
Fair: hands used as helpers but not effective in dressing, moderate grasp and release, fair control
-
Poor: hands used only as weights, poor or
absent grasp and release, poor control, no finger extension unless the
wrist is in maximum flexion
family’s motivation. Surgery is of value only when the patient and
parents are committed to attaining realistic goals. Make clear to the
parents that a normal extremity will not be the outcome, but that
worthwhile improvements in function and cosmesis may result (29).
cosmesis, and hygiene. The spastic hemiparetic patient with reasonable
intelligence, good sensibility, motivation, and appropriate parental
support should have a good functional extremity as the goal. Planned
procedures in such patients have predictable results (3).
Mentally retarded patients with poor sensibility should instead undergo
procedures directed toward improvement of hygiene and prevention of
skin maceration (24). Improvement of cosmesis
and hygiene are goals for patients with reasonable intelligence but
other limiting factors. Diminished sensibility and voluntary control
are not contraindications to surgical treatment, although expectations
are lower in such patients (4).
several years of life. While the patient’s age is only one of the
factors to be considered, surgery generally should not be performed
before the motor pattern becomes established,
and not until central nervous system maturation permits compliance with the postoperative regimen (33). To prevent deformity and rejection of the extremity, however, surgery should not be overly delayed.
digital extension, and forearm supination should be preserved through
use of resting and/or night splints. By age 4, the pattern of
persistent deformity becomes evident, and children may become
candidates for operative treatment. The optimal time for tendon
transfers is between ages 5 and 12 years (2).
Treatment of the proximal part of the extremity should be done before
treatment of the hand. In general, begin with capsulotomies (rarely
needed in children), tenotomies, and tendon lengthenings to correct
deformities; proceed to tendon transfers; and conclude with arthrodesis
of joints where necessary.
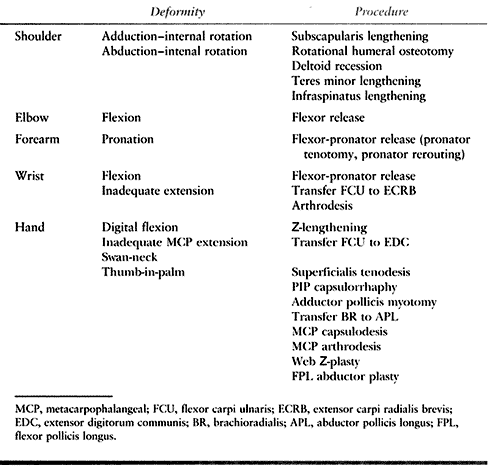 |
|
Table 66.1. Common Deformities of the Upper Extremity
|
deformity. It results from spasticity and contracture of the
subscapularis and pectoralis major. Internal rotation contracture
limits the positioning of the upper extremity and may be managed by
passive stretching exercises. For resistant deformity, subscapularis
and pectoralis major lengthening or release is recommended (4). If the deformity persists, humeral osteotomy may be necessary.
-
We prefer a deltopectoral approach,
although the procedure may be performed through an axillary incision.
Make a longitudinal incision from the coracoid to the border of the
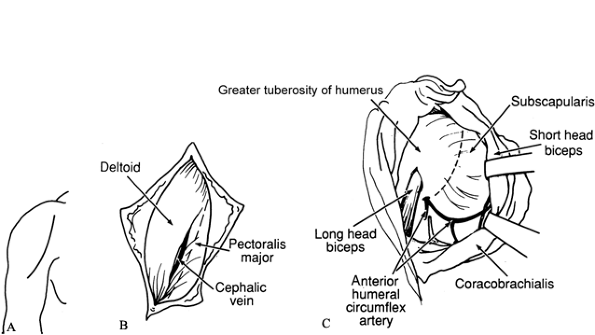
pectoralis major insertion (Fig. 66.1A).![]() Figure 66.1. A: Incision for subscapularis–pectoralis major lengthening. B: Interval between deltoid and pectoralis major. C: Lengthening of subscapularis. Take care to avoid the anterior humeral circumflex vessels.
Figure 66.1. A: Incision for subscapularis–pectoralis major lengthening. B: Interval between deltoid and pectoralis major. C: Lengthening of subscapularis. Take care to avoid the anterior humeral circumflex vessels. -
Identify the deltopectoral groove and develop the interval between the deltoid and the pectoralis major (Fig. 66.1B). Protect the cephalic vein.
-
The scapularis, bordered inferiorly by the anterior humeral circumflex veins, is lengthened medially to the glenohumeral joint (Fig. 66.1C). For persistent deformity, release the subscapularis at its insertion into the humerus.
-
If the tendon of the pectoralis major is tight, lengthen it by a stepcut incision.
-
Postoperatively, immobilize the limb in
25° to 30° of abduction and 20° of external rotation for 4 to 6 weeks.
Begin active assisted exercises to retain shoulder range of motion at 2
weeks. -
More resistant deformities may require an
external rotational osteotomy of the proximal humerus. The osteotomy is
performed via an anterior approach and is held with crossed pins or
with plate fixation.
-
Perform surgical release, when it is
indicated, through a “strap” incision, permitting access to the deltoid
and the spine of the scapula. -
Detach the fibers of the anterior two
thirds of the deltoid from their insertion into the humerus and recess
the deltoid muscle. Do not disturb the insertion of the scapular
portion of the deltoid. -
Perform a Z-lengthening of the tendons of the teres minor and the infraspinatus.
-
Immobilize the shoulder in a Velpeau
bandage (reinforced with plaster) for 2 weeks. Begin active assisted
and passive shoulder exercises while maintaining the limb in a
protective Velpeau dressing between exercise periods.
and can be managed by passive stretching exercises. Occasionally,
flexion tone and/or contracture become sufficiently severe to
compromise hygiene, limit reach, interfere with crutch use, and present
other functional limitations. Flexion deformity caused by spasticity or
contracture is treated by biceps tendon lengthening, brachialis
tenotomy, recession of the origins of the wrist flexors (excluding
flexor digitorum profundus and superficialis), and, if necessary,
anterior elbow joint capsulotomy. Preoperative musculocutaneous nerve
block may be useful in distinguishing increased tone from contracture (9).
Resist the temptation to release flexor muscle origins at the elbow to
correct an ipsilateral flexed wrist, flexed fingers, or thumb-in-palm
deformity, as this release tends to weaken the muscle excessively (5). Elbow flexion recovers postoperatively provided the biceps and brachialis were functioning before the procedure.
-
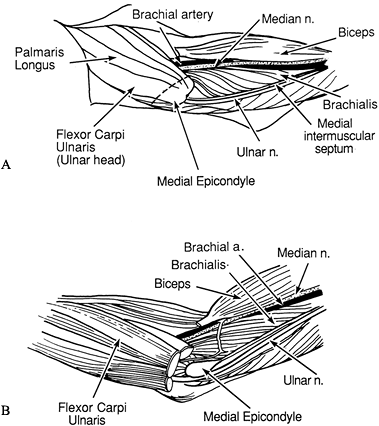
Make an S-shaped incision in the antecubital fossa (Fig. 66.2A). Excise the lacertus fibrosus.
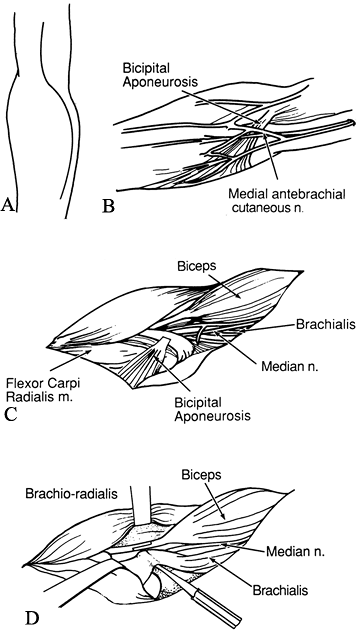 Figure 66.2. A: Incision for elbow flexion release. B: The medial antebrachial cutaneous nerve must be protected. C: The bicipital aponeurosis is divided. The median nerve and brachial vessels are identified. D:
Figure 66.2. A: Incision for elbow flexion release. B: The medial antebrachial cutaneous nerve must be protected. C: The bicipital aponeurosis is divided. The median nerve and brachial vessels are identified. D:
Z-lengthening of the biceps tendon is performed at the musculotendinous
junction. The brachialis insertion is raised from the ulna. -
Identify the medial antebrachial cutaneous nerve and mark it with a Penrose drain (Fig. 66.2B).
-
Release the origin of the flexor carpi ulnaris from the medial epicondyle and from its attachment to the proximal ulna.
-
Identify and protect the median nerve and brachial vessels (Fig. 66.2C).
-
Release the pronator teres and flexor carpi radialis if there is wrist contracture.
-
Isolate the biceps tendon lateral to the
neurovascular structures and dissect soft tissue and paratenon from the
tendon. Perform a Z-lengthening of the biceps tendon at the
musculotendinous junction (Fig. 66.2D) (23).
The extent of lengthening depends on the angle of contracture and the
degree of spasticity; 3 or 4 cm of lengthening is usually appropriate (4). -
Identify and protect the radial nerve.
Then identify the brachialis aponeurosis and divide its insertion into
the ulna, and passively extend the elbow. If the deformity is
resistant, incise the anterior elbow joint capsule. Capsulectomy is not
required. It may be necessary to release the origin of the
brachioradialis at the lateral condyle of the humerus. -
Some authors suggest transferring the ulnar nerve anteriorly to avoid nerve hypermobility postoperatively (4). If the nerve is under tension in the extended position, perform anterior subcutaneous transposition.
-
Complete correction of flexion deformity
should not be the goal; correction of the major component of the fixed
deformity is usually sufficient. -
At the close of the procedure, release
the tourniquet and obtain hemostasis. Introduce a Penrose or similar
drain, which is removed at 24 hours. -
Immobilize the limb in a posterior splint in maximal unforced extension.
-
Change to a plaster cast at the first dressing change.
-
Active elbow flexion is started at 3
weeks postoperatively. Keep the limb in anterior and posterior plaster
splints or an anterior-posterior plastic orthosis at all other times to
preserve extension; continue this for the first 6 weeks. Use night
splints for an additional 6 weeks.
By itself, pronation deformity seldom requires surgical correction but
may so limit use of the hand as to warrant such treatment. Two surgical
measures have been suggested for correction of severe pronation
deformity: pronator tenotomy, and rerouting of the pronator to function
as a supinator (31).
Rerouting offers the advantage of strengthening supinator power. While
some authors have reported consistently good results following pronator
teres rerouting, others have found the procedure somewhat unpredictable
and capable of producing supination deformity (27,31).
A fixed supination deformity is more disabling than a pronation
contracture. Hence, approach this procedure with some caution.
of the wrist in flexion are common in cerebral palsy. The inability to
extend the wrist and supinate the forearm compromises digital motion,
power pinch, and grip. Prolonged pronation contracture may produce
radial head dislocation (24). Release of the
flexor pronator origin can provide considerable lengthening and improve
appearance and function of an extremity with fixed deformities (14).
In passively correctable deformities that assume a flexed position
during grasp, transfer of flexor carpi ulnaris (FCU) to extensor carpi
radialis brevis (ECRB) is recommended (8). A surgical technique is described in the
following section on wrist flexion deformity. Supination may be improved after this transfer by suturing the FCU while the forearm is moderately supinated (2).
-
Make a 4 cm longitudinal incision on the anterolateral mid forearm over the pronator teres insertion.
-
Develop the interval between the
brachioradialis (BR) and the extensor carpi radialis longus (ECRL),
exposing the pronator teres. Transect the insertion of pronator teres
onto the radius. -
Close the wound and apply a long-arm cast
with the forearm in 60° of supination. At 4 weeks, replace the cast
with a posterior splint and begin active range of motion of the elbow.
Discontinue the splint at 8 weeks (31).
-
Make an incision on the anterolateral mid forearm over the pronator teres insertion.
-
Identify and protect the lateral
antebrachial cutaneous and the superficial radial nerves. Develop the
interval between the BR and the ECRL. -
Dissect the insertion of the pronator
teres off the radius along with a 2 cm periosteal strip. Incise the
fascia covering the muscle and mobilize it well proximally. -
Make a large, long opening in the
interosseous membrane. Pass the pronator teres through this opening and
then posterior and lateral to the radius toward its anterolateral
border. -
Drill a 1.6 mm transverse hole through
the radius from the pronator teres insertion to the posterior medial
cortex. Enlarge the anterolateral hole with a 2.8 mm drill. Weave a 2-0
nonabsorbable suture through the distal end of the pronator teres and
pass this through the drill hole from anterolateral to posteromedial.
Tighten the suture to secure the tendon. Supplement this with
additional sutures through the tendon and periosteum. -
Apply a long-arm cast in 60° of supination. The postoperative regimen is as described for pronator teres tenotomy (27,31).
in cerebral palsy and interfere with grasp and release. Flexor–pronator
release is recommended if both the wrist and the fingers are flexed; if
the deformity is limited largely to the fingers, however, use a
Z-lengthening tenotomy in the mid to distal forearm.
-
Start the incision 5 cm proximal to the
medial epicondyle and continue it distally across the antecubitum in a
zigzag fashion to the midportion of the proximal forearm. Take care not
to damage the medial antebrachial cutaneous nerve in the distal part of
the incision and the medial brachial cutaneous nerve posterior to the
medial part of the epicondyle. -
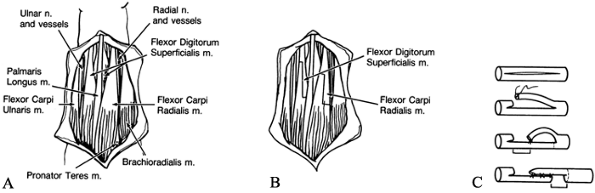
Identify the ulnar nerve and dissect it from its groove (Fig. 66.3A).
Divide the medial intermuscular septum. Protect the ulnar nerve
branches to the FCU and the two ulnar heads of the flexor digitorum
profundus (FDP). Retract the ulnar nerve by vessel loops.![]() Figure 66.3. A: The ulnar nerve is identified and the median intermuscular septum divided. B: The flexor–pronator origins are elevated from the ulna and advanced distally.
Figure 66.3. A: The ulnar nerve is identified and the median intermuscular septum divided. B: The flexor–pronator origins are elevated from the ulna and advanced distally. -
Incise the lacertus fibrosus fascia.
Identify and gently retract the median nerve and brachial artery. Take
care to preserve the motor branches of the median nerve to the
flexor–pronator muscles. This is an exacting dissection and must be
performed with patience. Starting distally, elevate the FCU and FDP
origins from the ulna and the interosseous membrane (Fig. 66.3B).
Continue this dissection proximally along the ulna as far as the ulnar
groove. The flexor digitorum superficialis (FDS) usually must be
released from the radius and the interosseous membrane. -
Separate the flexor–pronator muscle mass
from the underlying anterior capsule of the elbow, divide it at its
origin on the medial part of the epicondyle, and advance it distally
about 4 cm. -
The flexor–pronator muscle mass may be sutured to the underlying tissue at the desired reattachment point.
P.1800
If pronation deformity of the forearm is not a problem, bring the
pronator teres origin over the periosteum of the ulna at the proper
tension so that pronation of the forearm is not compromised. -
If a flexion contracture of the elbow persists, release the brachialis.
-
Deflate the tourniquet and obtain
hemostasis. Place drains in the forearm and the antecubitum; these
should be removed at the first dressing change. -
By the close of the procedure, the wrist
should dorsiflex 45° to 60° with the fingers in maximum extension. Use
plaster splints to hold the forearm in supination, the wrist in 30° of
extension, the metacarpophalangeal (MCP) joints in 25° of flexion, and
the elbow flexed 45° to 90°. -
At the first dressing change, replace the
splint with a cast, which is worn for 3 weeks. Active assisted motion
is started after cast removal. Custom-molded anteroposterior splints
that maintain the wrist in extension are worn between treatments for 6
weeks, and then only at night for an additional 6 months.
be improved by Z-lengthening of the wrist flexors: FCU, flexor carpi
radialis, and palmaris longus. This procedure works best if the patient
has active wrist extensor power. Wrist flexor lengthening is not needed
if the wrist can be voluntarily extended to at least 10° past neutral,
and if it can be actively flexed and assists in finger extension (5).
Perform arthrodesis only after testing hand function in a cast, with
the wrist in the position chosen for the procedure. It is
contraindicated if the patient depends on wrist flexion for release or
wrist extension for grasp, or if other planned tendon transfers might
be compromised by the arthrodesis. The fusion includes the radiocarpal,
midcarpal, and carpometacarpal joints. The epiphyses and the distal
radioulnar joint are preserved.
from weak extensors, but finger extension can be performed actively
with the wrist passively extended, transfer of the FCU is recommended (8).
The tendon of the FCU is passed subcutaneously around the ulnar border
of the forearm and is transferred to the tendon of the ECRB. The
transfer eliminates a deforming force pulling the hand into ulnar
deviation and flexion.
there must be satisfactory passive forearm supination and wrist and
finger extension. Second, any fixed deformity should be corrected by
successive stretching casts and exercises. Finally, there must be
adequate digital motor control. The procedure has been widely used for
many years with good results (5,18,24,25,29,33). However, some long-term studies report extension contractures and difficulty with grasp and release following this transfer (10).
transfer, the spastic hand has been classified into three groups based
on the ability to grasp and release through wrist position (37):
-
Type I: Fingers can actively extend with the wrist in less than 20° of flexion.
-
Type II: Fingers can actively extend with the wrist in less than 50° of flexion.
-
Type III: Fingers cannot extend with the wrist in any position.
finger flexors. If indicated, tenotomy of the FCU may be of value in
this group.
neutral with the wrist passively extended but cannot extend the wrist
to neutral are most likely to benefit from an FCU-to-ECRB transfer (35,36).
Children who cannot actively extend the fingers with the wrist
passively extended but who can with the wrist flexed at 45° may also
benefit.
and release patterns. The FCU-to-ECRB transfer is not indicated in this
group (36). These patients may benefit from
multiple tendon fractional lengthenings in the forearm, allowing the
fingers to remain out of the palm for improved cosmesis and hygiene.
-
Make a 3 cm longitudinal incision radial
to the insertion of the FCU. Make a second 6 cm longitudinal incision
along the palmar-ulnar aspect of the forearm. The two incisions extend
over the muscle belly of the FCU from the wrist flexor crease to the
junction of the middle and proximal thirds of the forearm. -
Expose the FCU, isolate the ulnar nerve, and tag it with a vessel loop posterior to the tendon (Fig. 66.4A).
Split the muscle sheath of the FCU and transect the tendon just
proximal to its insertion on the pisiform. Strip the muscle fibers of
the FCU extraperiosteally from the ulna. Free the muscle proximally,
without disturbing its nerve supply from the ulnar nerve. Continue the
dissection throughout the length of the wound, allowing mobilization of
the muscle sufficient to permit its passage in a straight line from its
origin to the dorsum of the wrist.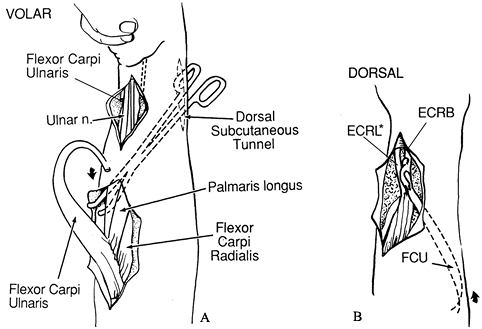 Figure 66.4. A: The flexor carpi ulnaris is exposed, released distally, and freed proximally. B:
Figure 66.4. A: The flexor carpi ulnaris is exposed, released distally, and freed proximally. B:
The flexor carpi ulnaris (FCU) tendon is passed around the ulna through
the subcutaneous tunnel and transferred to extensor carpi radialis
brevis (ECRB). *extensor carpi radialis longos -
Make a third longitudinal incision over
the wrist in line with the long metacarpal, beginning at the distal end
of the radius and extending for 5 cm proximally. Incise the extensor
retinaculum over the second compartment
P.1801
and
identify the ECRB. By blunt dissection, prepare a tunnel extending from
the proximal FCU incision to the dorsal radial incision. Construction
of this tunnel is facilitated by use of a tendon passer. -
Pass the FCU tendon around the ulna through the subcutaneous tunnel (Fig. 66.4B). Make a slit in the tendon of ECRB with a #11 blade.
-
Place the wrist in 40° of extension and
the forearm in supination, then pass the tendon of the FCU through the
slit in the ECRB. For the intratendinous suturing, use 3-0
nonresorbable suture. Secure the attachment of the FCU to the ECRB with
additional interrupted sutures. -
Tension must be sufficient to maintain the wrist in neutral against gravity, but it should permit passive flexion to 15°.
-
Close the wound and apply a long-arm cast with the forearm in supination and the wrist in 15° of extension.
-
The cast is worn for 4 weeks; then active
exercises for ulnar deviation and wrist extension are begun. A bivalved
cast or a plastic splint is worn between exercise periods for 2 to 3
months. This program is discontinued when the wrist can be maintained
actively in extension. A night brace is worn throughout this time and
for an additional 4 months or until there is no tendency toward
recurrence (8,24,29,33).
caused by spasticity and myostatic contracture of the FDS and FDP
muscles or by paresis of the extensor digitorum communis (EDC). Flexion
deformity is increased on wrist extension and diminished on wrist
flexion. Serial plaster casts with progressive wrist extension may
lessen flexion deformity of the digits. If conservative treatment is
ineffective, surgical lengthening of the spastic or contracted finger
flexors is indicated (5). Do not perform
lengthening if the wrist can be voluntarily brought to neutral without
excessive digital flexion, or if the wrist flexors are weak. With this
deformity, lengthening is preferable to flexor–pronator release.
hold the wrist in maximum extension, placing the MCP joints of the
fingers in extension, and then observe the resulting posture of the
fingers. Correction should change this finger position to one that is
more functional for the activities of daily living.
-
Expose the flexor tendons through a midline incision on the palmar surface of the distal forearm (Fig. 66.5A). Divide the deep fascia.
![]() Figure 66.5. A: Exposure of flexor tendons. B: Lengthening of flexor tendons by stepcut incisions. C: Goldner (4)
Figure 66.5. A: Exposure of flexor tendons. B: Lengthening of flexor tendons by stepcut incisions. C: Goldner (4)
suggests bisecting the tendon for a distance of twice that of the
proposed lengthening. One slip of the tendon is divided and sutured
halfway from the cut end to the intact tendon. The remaining portion is
then cut distally. -
Identify and protect the ulnar nerve and
artery on the radial side of the FCU tendon. Isolate the radial artery
on the radial side of the flexor carpi radialis (FCR). Lengthen the FDS
tendons by stepcut incisions at the musculotendinous junction (Fig. 66.5B). Goldner suggests bisecting the tendon for a distance twice that of the proposed lengthening (Fig. 66.5C) (5). -
Divide one slip of the tendon and suture
it halfway from the cut end to the intact tendon, after passively
extending the finger. Then cut the remaining portion of the tendon
distally, without disturbing the underlying muscle tissue. This results
in a controlled lengthening. -
With the wrist extended 50°, the PIP joints should be flexed 45°. Repair the incisions with 4-0 resorbable suture.
-
Release of the FDP at the same operation
may not be advisable because grasp is weakened if both extrinsic
digital flexors are lengthened. See how the patient adjusts to
lengthening of the superficialis tendons before considering surgery of
the FDP. -
Expose the muscle tendon units of the FDP by retracting the FCR and the FDS and protecting and retracting the median nerve.
-
Lengthen the FDP in a manner similar to
that used for the FDS, but at a different level. Lengthen the flexor
pollicis longus (FPL) if the thumb is held in a flexed position and
interferes with pinch and grip. -
Patients with wrist flexion contractures
may require FCR and FCU lengthening. Lengthening is not required if the
fingers can be flexed easily while maintaining the wrist in active
extension. If the procedure proves necessary, lengthen the FCR first.
Lengthen the tendon of the FCU only if the FCR procedure provides
insufficient correction. -
Release the tourniquet and achieve meticulous hemostasis at the end of the procedure. Do not close the deep fascia.
-
Apply long-arm anteroposterior splints to
the fingertips, including the thumb, with the elbow at 90°, the forearm
supinated, the wrist in 40° of extension, and the fingers and thumb in
neutral. -
Physical therapy for patients undergoing
flexor tendon lengthening includes active exercises at 4 weeks after
surgery. Bivalved plastic splints are worn between exercise periods.
Night splinting continues for 6 months (5,33).
be transferred to the EDC. The procedure is indicated when there is no
significant contracture and a muscle is available for transfer that is
synchronous with finger extension (8). The FCU, BR, and ECRB have been recommended for the transfer (5,22,29,33). We prefer transfer of the FCU into the EDC because the muscles are synchronous and the transfer produces a tenodesis effect (10,19).
-
The exposure is similar to that described
for the FCU-to-wrist extensor transfer. Make a longitudinal incision
that extends along the ulnar-palmar aspect of the forearm, from the
palmar wrist crease to the junction of the proximal and middle thirds
of the forearm. -
Expose the FCU and identify and protect
the ulnar artery and nerve. Divide the FCU tendon at its insertion into
the pisiform and free it proximally, while carefully preserving the
proximal innervation of the tendon. -
Make a second longitudinal incision over the fourth dorsal compartment. Divide the extensor retinaculum and expose the EDC.
-
Pass the tendon of the FCU through a
subcutaneous tunnel to the dorsal incision. Hold the wrist and MCP
joints in neutral. Split the FCU tendon into two segments and suture
these into each of the tendons of EDC. Adjust the tension so that each
finger’s MCP joint hyperextends slightly when the wrist is flexed. -
Apply a long-arm cast with the wrist in
30° of extension and the MCP joints in neutral. This position is
maintained for 4 weeks, at which point active exercises are started.
Prescribe a splint that limits finger and wrist flexion, to be worn for
2 months, followed by a night splint for 6 months (29,33).
(DIP) joints (Fig. 66.6A).
In cerebral palsy, it usually results from muscle imbalance caused by
the spastic intrinsics exerting excessive pull on the extensor
mechanism. Contraction of the tight or spastic intrinsics results in
PIP hyperextension. The tenodesis effect of the EDC while the wrist is
flexed also contributes to the deformity. The palmar plate stretches
and the lateral bands sublux dorsally, increasing the hyperextension of
the PIP joint. The deformity impairs grasp and pinch. Treatment
consists of superficialis tenodesis with or without capsulorrhaphy of
the PIP joint, as described by Swanson (32).
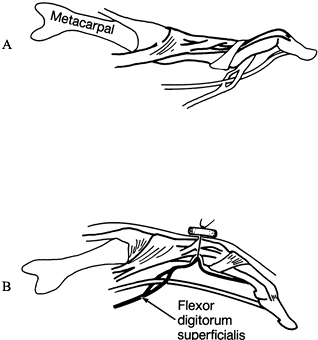 |
|
Figure 66.6. A: Swan-neck deformity. B: The superficialis tendon is drawn into the cavity by passing the suture through the dorsal cortex and tying it over a button.
|
-
Make a mid-lateral incision from the
midportion of the middle phalanx to the base of the proximal phalanx.
For the index finger, the incision should be on the ulnar side; for the
little finger, it should be on the radial side. -
Incise the retinacular ligament
longitudinally. Gently retract the neurovascular bundle on the side of
the incision to the other side. -
A modified Bruner incision from the
center of the middle phalanx to the proximal palmar crease of the
finger also provides good exposure for the procedure. -
Make an incision in the flexor sheath between the A2 and A4 pulleys; identify each flexor tendon by a vessel loop and retract it.
-
Expose the palmar aspect of the proximal
phalanx by subperiosteal dissection, and drill two small holes 1 cm
apart in the palmar cortex of the proximal phalanx in a
palmar-to-dorsal direction (Fig. 66.6B). Connect the two holes on the palmar aspect using a curet. Roughen the bone for attachment of the tendon. -
Draw the superficialis tendon into the
medullary cavity by a nonresorbable 4-0 suture. Then pass the suture
through the palmar cortex defect and out a hole in the dorsal cortex,
to the dorsal aspect of the finger, and secure it over a button. Anchor
the tendon to the bone with the PIP joint in 30° of flexion. Drill a
0.045 Kirschner wire (K-wire) across the joint from the proximal
portion of the middle phalanx through the neck of the distal end of the
proximal phalanx. -
Close the flexor sheath with fine resorbable suture. Repair the retinacular ligament and suture the skin.
-
Capsulorrhaphy is required in some cases.
Incise the capsule at right angles to the finger. With the joints in
30° of flexion, place 4-0 nonresorbable sutures in the distal end of
the proximal flap and the proximal end of the distal flap (32,33).
prevents lateral pinch and grasp. The deformity consists of flexion,
adduction, and external rotation of the first ray with flexion of the
distal phalanx. The deformity may be due to adductor spasticity or to
spasticity and contracture of the FPL. Stretching of the MCP joint
capsule may produce instability of the joint. Secondary thenar space
narrowing may develop. If severe, this can eventually lead to
subluxation of the carpometacarpal joint (33).
including MCP and interphalangeal (IP) arthrodesis, webspace release,
tendon transfer, tendon lengthening, and MCP capsulodesis (2,13,15,16 and 17,21,30). For planning correction, the classification scheme of Mital and Sakellarides is helpful (24).
-
Type I deformity results from paresis of
the extensor pollicis longus, with or without instability of the MCP
joint. Procedures suggested to correct this include tendon transfer of
the BR into the abductor pollicis longus (APL) and the extensor
pollicis brevis (EPB), and rerouting of the extensor pollicis longus
(EPL) (13,20,21). We recommend the BR transfer. If the joint is unstable, stabilize the MCP joint by capsulodesis or arthrodesis (1,7). Gelberman suggests arthrodesis if the MCP joint shows hyperextension deformity greater than 20° (2). If performed in a child, the procedure should not interfere with growth of the thumb (2).
Reserve arthrodesis for cases in which soft-tissue procedures prove
unsatisfactory. A different opinion is voiced by Goldner et al., who
recommend simultaneous correction of the soft-tissue deformity and MCP
joint arthrodesis (7). -
Type II deformity is caused by spasticity
or contracture of the adductor pollicis. Treat adductor pollicis
contracture by release of the transverse and oblique heads
P.1804
of
the muscle. Myotomy is preferred to tenotomy, because it avoids
hyperextension of the MCP joint while permitting release of the first
metacarpal. If severe spasticity is present, simultaneously perform
neurectomy of the motor branch to the adductor. Should the first dorsal
interosseous contribute to the deformity, release it from the first or
second metacarpal, or both. -
In type III deformity, there is paresis
of the APL. We suggest tendon transfer of the BR to the APL. Full
passive abduction of the first metacarpal and passive thumb extension
are prerequisites for tendon transfer. -
Type IV deformity is caused by
overactivity of the FPL. Perform Z-lengthening of the flexor palmaris
longus in the distal forearm to overcome flexion deformity.
Alternatively, some authors suggest the subcutaneous transfer of the
flexor palmaris longus to the radial side of the proximal phalanx of
the thumb, with tenodesis or arthrodesis of the IP joint (30). This offers the advantage of augmenting thumb abduction.
Deformities of long duration are more likely to demonstrate webspace
contracture. Z-plasty of the skin of the thumb web and division of the
fascia may be necessary. Children’s skin, however, has sufficient
elasticity to avoid the need for Z-plasty (5).
-
Make a palmar zigzag incision over the MCP joint. Retract the neurovascular bundles.
-
Incise the A1 pulley and mobilize and retract the FPL.
-
Incise the palmar plate, leaving its distal attachment intact. Release the insertions of intrinsics onto the plate.
-
Introduce a 4-0 double-armed wire suture with pullout wire in Bunnell fashion into the palmar plate.
-
Prepare a small groove on the neck of the
metacarpal to accommodate the plate. Drill two holes to the dorsum of
the metacarpal. Drill a 0.045 K-wire through the proximal phalanx, so
that it may later be brought across the joint in retrograde fashion. -
Holding the joint in 30° of flexion, pass
the suture through the apertures and tie it over a button on the dorsum
of the thumb. Pass the K-wire across the MCP joint. -
Hold the thumb in abduction in a plaster cast. Remove the pullout wire at 6 weeks and the K-wire at 8 weeks.
-
Make a radial mid-lateral incision.
Incise the collateral ligament and capsule along the metacarpal head
and dislocate the joint. -
Carefully remove the articular cartilage
from the metacarpal head using a small rongeur. Remove the articular
surface of the proximal phalanx without disrupting the physes. Drill
two 0.045 K-wires through the subchondral bone of the proximal phalanx
so that they may be passed retrograde across the MCP joint. -
Hold the joint in 10° of flexion and 10°
of lateral rotation and pass the wires. Repair the capsule and
collateral ligament with 4-0 resorbable suture. A thumb spica cast is
worn for 4 weeks or until there is firm union. The pins, which have
been cut subcutaneously, are left in place for about 1 year.
-
Make an incision in the thenar flexion crease, from the second metacarpal neck to just proximal to the wrist flexion crease (Fig. 66.7A).
If deepening of the web is indicated, use a standard or four-quadrant
Z-plasty incision. Make a transverse incision in the web, from the
ulnar border of the proximal flexion crease of the thumb to the radial
border of the proximal transverse palmar crease; then make two oblique
cuts at a 60° angle.![]() Figure 66.7. A: Skin incision. B: The first dorsal interosseous is retracted radially and the adductor pollicis is identified.
Figure 66.7. A: Skin incision. B: The first dorsal interosseous is retracted radially and the adductor pollicis is identified. -
After the skin flaps are raised, incise
the palmar fascia. Retract the flexor tendons to the index finger
radially and those to the long finger ulnarly. -
Identify and retract the common digital nerve ulnarly.
-
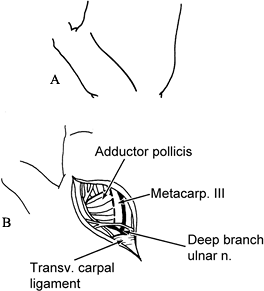
Incise the fascia over the adductor pollicis and first dorsal interosseous (Fig. 66.7B). Retract the first dorsal interosseous radially.
-
Identify the tendon of the adductor
pollicis. The transverse head of the adductor pollicis arises from the
distal two thirds of the long finger metacarpal. The oblique head
originates from the capitate and from the bases of the index and long
metacarpals. Release the adductor from its origin. -
It is generally necessary to release the
flexor pollicis brevis, the opponens pollicis, and the abductor
pollicis brevis muscles as well. This may be accomplished by elevating
their origins from the transverse carpal ligament. -
Preserve the deep branch of the ulnar
nerve and perforating branches of the radial artery. It is rarely
necessary to perform neurectomy of the innervation of the adductor
pollicis. However, extreme spasticity may require careful dissection
and neurectomy of the deep palmar branch of the ulnar nerve, which is
the motor branch to the adductor. -
Release the tourniquet and obtain hemostasis.
-
A cast holds the MCP joint in neutral and
the IP joint in slight flexion. Apply a plastic splint at 4 weeks to
maintain the position of abduction and opposition. Active and passive
exercises are begun at 4 weeks to rehabilitate thumb function. The cast
should be worn for 6 weeks if simultaneous tendon transfers have been
performed. Night splints are worn for 6 months to prevent recurrence of
the deformity (29,33).
-
Make a radial longitudinal incision from the radial styloid process to a point 3 cm distal to the lateral epicondyle.
-
Free the insertion of the BR from the radial styloid (Fig. 66.8A).
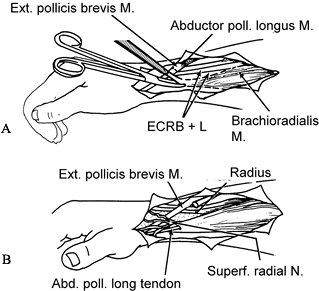 Figure 66.8. A: The insertion of the brachioradialis is freed from the radial styloid. B: The distal cut end of adductor pollicis longus is woven through the brachioradialis.
Figure 66.8. A: The insertion of the brachioradialis is freed from the radial styloid. B: The distal cut end of adductor pollicis longus is woven through the brachioradialis. -
Protect the branches of the superficial
radial nerve. The nerve travels along the forearm deep to the BR and
can be found adjacent to the radial artery in the proximal forearm. In
the distal forearm, the superficial radial nerve emerges from beneath
the BR and penetrates the deep fascia. -
Dissect the tendon and muscle of the BR
free from the antecubital fascia and adjacent muscles to which they are
adherent. The muscle must be mobilized proximally as far as possible to
gain maximal excursion. -
Transect the APL at its musculotendinous
junction proximal to the dorsal retinaculum. Hold the first metacarpal
in abduction, weave the distal cut end of the APL through the BR, and
suture it with 3-0 nonresorbable suture (Fig. 66.8B). -
Tension should allow the first metacarpal
to be adducted passively to within 2 cm of the palm, with the wrist in
neutral. Tip pinch between the thumb and index must be preserved; check
this after performing the transfer. -
Correct persistent extreme flexion of the MCP by transferring the proximal end of the APL to the EPB tendon.
-
Release the tourniquet and obtain hemostasis.
-
Apply an above-elbow cast with the wrist
in neutral, the thumb in neutral, and the first metacarpal in maximal
abduction. The cast is removed at 4 weeks, and a short-arm plastic
thumb spica splint is worn to maintain the thumb in maximal abduction.
Begin active exercises at 4 weeks to restore thumb function. Prescribe
night splints for an additional 6 months (29,33).
-
Make a radial mid-axial incision from the
middle of the distal phalanx to the neck of the first metacarpal.
Develop the palmar skin flap, exposing the FPL. -
Transect the tendon opposite the proximal phalanx.
-
Perform arthrodesis or tenodesis of the IP joint in 15° of flexion.
-
Make a longitudinal incision in the
forearm just radial to the FPL tendon. Curve the incision in an ulnar
direction at its distal end just proximal to the wrist crease. -
Bring the distal end of the FPL to this
wound. Prepare a subcutaneous tunnel from the lateral side of the MCP
joint to the apex of the wrist incision. Pass the distal end of the FPL
through this tunnel. With the thumb abducted 50° and the wrist in
neutral, suture the transferred FPL to the dorsoradial proximal phalanx
base without tension. -
Apply a plaster splint holding the thumb
abducted and the wrist flexed 30° for 6 weeks. A plastic static thumb
abduction splint is worn for an additional 6 weeks (30).
augmentation transfer performed only in conjunction with
adductor–flexor intrinsic release (20). Despite
acting as an IP extensor, the EPL also adducts the thumb. EPL rerouting
eliminates a deforming force and converts this tendon to a more
functional position.
-
Identify the EPL through a dorsal distal forearm incision just proximal to Lister’s tubercle.
-
Make a second dorsal incision over the MCP joint and the proximal phalanx in the hand, again exposing the tendon.
-
Dissect a narrow longitudinal strip of
the EPL insertion in the midline of the dorsal thumb incision, leaving
sufficient tissue for closure of the aponeurosis. Withdraw the tendon
into the distal forearm. -
Release the forearm fascia allowing the EPL tendon to pass in a straight line to the thumb base.
-
Place a tendon passer from the dorsal
thumb incision along the path followed by the EPB and APL tendons
through the first dorsal retinacular compartment. Direct the tip
proximally into the dorsal forearm incision. Grasp and pull the EPL
tendon distally through the first dorsal compartment into the dorsal
thumb incision. Pass the tendon around the APL insertion, placing the
direction of tendon pull radially. Suture the tendon into the MCP
capsule. A tendon advancement of 1 to 2 cm should provide sufficient
tension to maintain the thumb extended. -
If the MCP joint hyperextends passively,
take care to insert the tendon into the metacarpal and not the proximal
phalanx base. In this instance, a temporary K-wire should be placed
across the MCP joint. Apply a short-arm thumb spica cast with the thumb
in extension and maximal palmar abduction. -
Remove the case (and K-wire if needed) at
4 weeks. Apply a removable splint and begin range-of-motion exercises.
Continue splinting for 6 weeks.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
JL, Ferlic DC. Sensory Status of the Hand as Related to Reconstructive
Surgery of the Upper Extremity in Cerebral Palsy. Clin Orthop 1966;46:87.
MM, Lehman M, Mitani M. Long-term Follow-up on Tendon Transfers to the
Extensors of the Wrist and Fingers in Patients with Cerebral Palsy. J Hand Surg 1986;11:836.
M, Perry J, Melkonian GJ. Dynamic Electromyography and Decision-making
for Surgery in the Upper Extremity of Patients with Cerebral Palsy. J Hand Surg 1979;4:424.
AE, Cooper W. Release of the Flexor Pronator Origin for Flexion
Deformities of the Hand and Wrist in Spastic Paralysis: A Study of 18
Cases. J Bone Joint Surg Am 1966;48:847.
HT, Mital MA, Lenzi WD. Treatment of Pronation Contracture of the
Forearm in Cerebral Palsy by Changing the Insertion of the Pronator
Radii Teres. J Bone Joint Surg Am 1981;63:645.
RL, Morris JM. Surgical Improvement of the Cerebral Palsied Upper Limb:
Electromyographic Studies and Results of 128 Operations. J Bone Joint Surg Am 1964;46:1203.
WB, Emanuel JP, Dailey L, Manske PR. Comparison of Pronator Tenotomy
and Pronator Rerouting in Children with Spastic Cerebral Palsy. J Hand Surg [Am] 1988;13:540.