Humeral Shaft Fractures
occurring more than 70,000 times a year in North America. It has been
estimated previously as representing between 3% and 5% of all fractures,17,120,148 but a more accurate figure is around 1% (see Chapter 3). Most will heal with appropriate conservative care, although a limited number will require surgery for optimal outcome.42,50,148
Given the extensive range of motion of the shoulder and elbow, and the
minimal effect from minor shortening, a wide range of radiographic
malunion can be accepted with little functional deficit.145
Current research in this area focuses on refining the indications for
surgical intervention, decreasing the surgical failure rate through new
implants and techniques, and optimizing the postinjury rehabilitation
programs and thereby minimizing the duration and magnitude of remaining
disability.17,153,154,160
The successful treatment of a humeral shaft fracture does not end with
bony union; in the current emphasis on a holistic approach to patient
care, the treating orthopaedic surgeon is frequently in an ideal
position to intervene and improve a patient’s life beyond what is
traditionally recognized as the surgeons’ role. Recognition of the
injury as an osteoporotic fragility fracture in an elderly patient
should prompt a referral for diagnostic investigations of, and
potentially treatment for, an underlying osteoporotic condition.
Similarly, fractures resulting from abusive domestic relationships or
drug/alcohol addiction may represent opportunities to intervene. As
with most orthopaedic injuries, the successful treatment of a humeral
shaft fracture demands a knowledge of anatomy, surgical indications,
techniques and implants, and patient function and expectations.
dedicated trauma units and the proliferation of capitated insurance
contract populations have dramatically improved the information
available regarding the epidemiology of fractures and dislocations.
While in the past this was often of little importance, orthopaedic
surgeons around the globe are increasingly aware that the complexity of
economics and politics involved in the delivery of sound orthopaedic
care is rapidly increasing. As those who know the most about the
practical aspects and human side of this topic, it is important that
orthopaedic surgeons take a leading role in this type of research.
provided an excellent picture of the epidemiology of humeral shaft
fractures in the United Kingdom. This research was based at a single
orthopaedic trauma center solely responsible for the fracture care of
600,000 people, thus providing a defined population for study. They
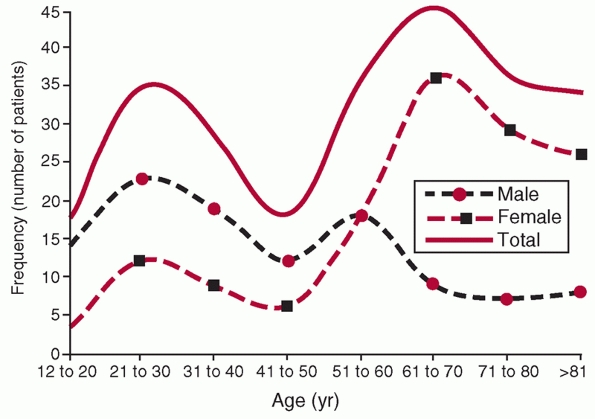
found that there was a bimodal distribution of fractures (Fig. 34-1),
with a peak (25 per 100,000 per year) in young, primarily male patients
in the 21 to 30 age bracket and a larger peak (100 per 100,000 per
year) primarily in older females 60 to 80 years old. They point out
that high-energy trauma was responsible for the majority of injuries in
young patients, and that this is the population that most of the
orthopaedic literature focuses on. The fractures caused primarily by
simple falls in older women (the second peak) thus represent a
population intrinsically different from that described in reports on
surgical intervention for these fractures. Only 5% of the injuries were
associated with an open wound, and 63% were “simple” fracture patterns
(AO/OTA type “A,” Table 34-1). In a more recent study by Ekholm et al.38
a similar bimodal distribution could be shown in a Swedish urban
population, although with an even more pronounced rise in the incidence
in elderly women. Again, this represents a fracture population
different from most reports that concentrate on higher energy, open
fractures.137 This type of
information has implications for resident training (relatively few
“operative” fractures per center per year), resource management (the
largest single group is elderly females with simple humeral fractures
after falls), and research (to ensure comparisons of equivalent groups).160
 |
|
FIGURE 34-1
Age and gender distribution of fractures of the humeral shaft in 249 patients from Edinburgh. (From Tytherleigh-Strong G, Walls N, McQueen MM. The epidemiology of humeral shaft fractures. J Bone Joint Surg (B) 1998;80B(12):249-253, with permission.) |
|
TABLE 34-1 Fracture Pattern of 249 Fractures of the Humeral Shaft as Classified by the AO System
|
|||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||
examined prospectively gathered data from a capitated insurance
contract where orthopaedic services for 135,000 (average annual
enrollment) young (mean age 28.9 years) people were provided by a
single physician group of 62 orthopaedic surgeons. The overall
incidence of fractures was 847 per 100,000 people per year, with the
incidence of humeral fractures being 13.1/100,000 people per year. As
would be expected in a young population, males predominated in all
fracture groups. This data is useful in estimating the orthopaedic
resources required to service a young, active North American population.
is one in which the main fracture line is distal to the surgical neck
of the proximal humerus and proximal to the supracondylar ridge
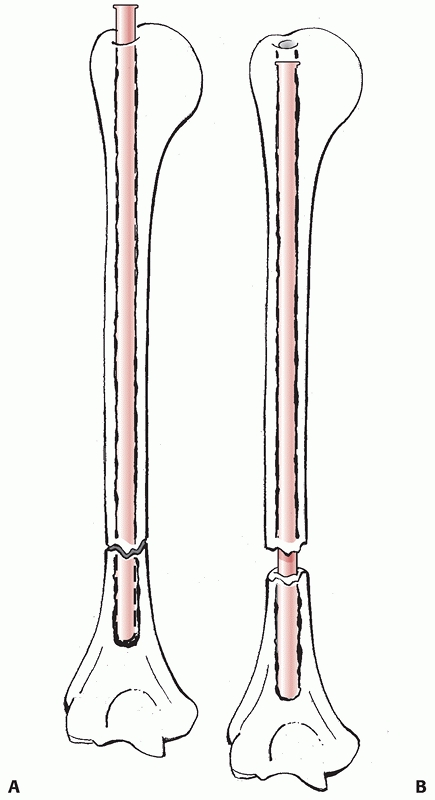
distally.50,145,148 Proximally, the humerus is roughly cylindrical in cross section, tapering to a triangular shape distally.49
The medullary canal of the humerus tapers to an end above the
supracondylar expansion. This is different from the wide metaphyseal
flares of the distal tibia and distal femur and has implications in
intramedullary nailing of humeral shaft fractures.
hence there is a good prognosis for healing in the majority of
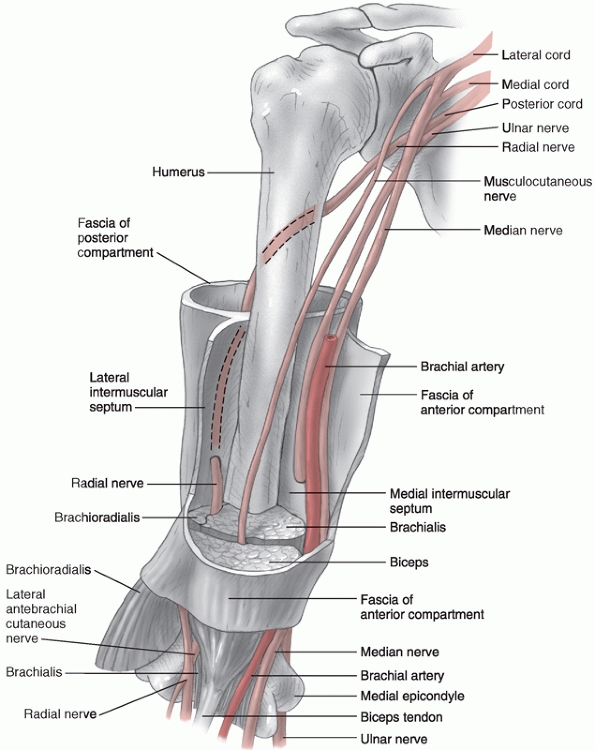
uncomplicated fractures. The medial and lateral intermuscular septa are
tough fibrous bands that divide the arm into anterior and posterior
compartments. The brachial artery, median nerve, and musculocutaneous
nerves all remain in the anterior compartment for their entire course,
while the ulnar nerve begins in the anterior compartment and passes
through to the posterior compartment at the elbow. The radial nerve
begins in the posterior compartment and passes through into the
anterior compartment (Fig. 34-2).
 |
|
FIGURE 34-2 The neurovascular anatomy of the upper arm.
|
successful treatment of humeral shaft fractures. By being aware of the
insertion and direction of load of the various muscles attached to the
humerus, it is easier to understand the fracture displacement and
foresee potential problems in retaining the desired position following
reduction. For instance, for fractures occurring proximal to the
insertion of the pectoralis major, the proximal fragment will displace
into abduction and external rotation due to unopposed muscles, while
the distal fragment for the same reason often is displaced proximal and
anterior.
and physical examination provide critical information that serves as a
starting point for treatment. The predominant causes of humeral shaft
fractures include simple falls or rotational injuries in the older
population and higher-energy mechanisms in the younger patient,
including motor vehicle accidents, assaults, falls from a height, and
throwing injuries. A history of minimal trauma causing fracture in the
older patient may be the first point to alert the surgeon that the
fracture may involve pathologic bone (be it from metastatic disease or
severe osteoporosis) and prompt a thorough history (i.e., for prior
cancer) and possibly a systemic work-up. In this situation, the
treating surgeon has the potential to help the patient both in terms of
the presenting fracture and the prevention of further fractures.
Comorbidities, especially as they relate to the patient’s suitability
for a potential anaesthetic, should be elucidated. These may also be
relevant with regard to the etiology of the fall that resulted in
fracture: an unrecognized arrhythmia can cause recurrent falls and
injuries. Thus, a clear description of the actual mechanism of the
injury is important.
fracture type: while exceptions do occur, the presence of a spiral
fracture indicates a rotational force (such as that which occurs when
the arm is forcibly wrenched behind the back) that is not consistent
with, for example, striking the arm against a door. Discordance between
history and fracture type is a hallmark of domestic abuse, and again
this may represent an opportunity to intervene in a potentially lethal
situation.167 Alcohol abuse,
smoking, and/or illicit drug use are all potential risk factors for
negative fracture outcome through repeat injury, noncompliance, or poor
biology at the fracture site and represent an opportunity to improve
outcome. For example, while it is unrealistic to expect uniform
compliance, modern smoking cessation strategies have a success rate
from 20% to 60%. It is also becoming apparent that the use of
nonsteroidal anti-inflammatory drugs (NSAIDs), for years a standard
treatment for pain and swelling after acute injury, is associated with
prolonged fracture healing times.84 Bhattacharyya et al.,9
in a cohort study including almost 10,000 patients with humeral shaft
fractures, showed that NSAID exposure within 60 to 90 days after
fracture was significantly associated with nonunion. However, the
authors also concluded that it is difficult from such a study design to
prove whether the increased use was the cause to the nonunion or an
effect of an already established painful nonunion. Burd et al.,20
in a randomized clinical trial, investigated the effect of indomethacin
on the prevention of heterotopic ossification in a group of polytrauma
patients with acetabular fractures. One group received indomethacin,
one group local irradiation, and the control group nothing. These
patients also had a number of associated long bone fractures: the
incidence of delayed and nonunion in the indomethacin-treated group was
26% versus 7% in the other two groups, a significantly higher rate (P = 0.004). We strongly discourage the use of NSAIDs in patients with humeral shaft fractures.20,47
patient may be unobtainable, but any available information from the
accident scene, paramedical personnel, or family members is important.
In general, the treatment of a humeral fracture is a relatively low
priority in the resuscitation of a severely injured patient, which
should proceed according to the guidelines of the Advanced Trauma Life
Support protocol.2 Following
stabilization of the patient, attention is turned to the affected arm.
There is an increased incidence of open wounds, ipsilateral fractures,
compartment syndromes, and neurovascular injuries in polytraumatized
patients, and a careful assessment needs to be performed.13,18,50,137,148
There is higher incidence of forearm compartment syndrome in patients,
especially children, who have ipsilateral humeral and forearm
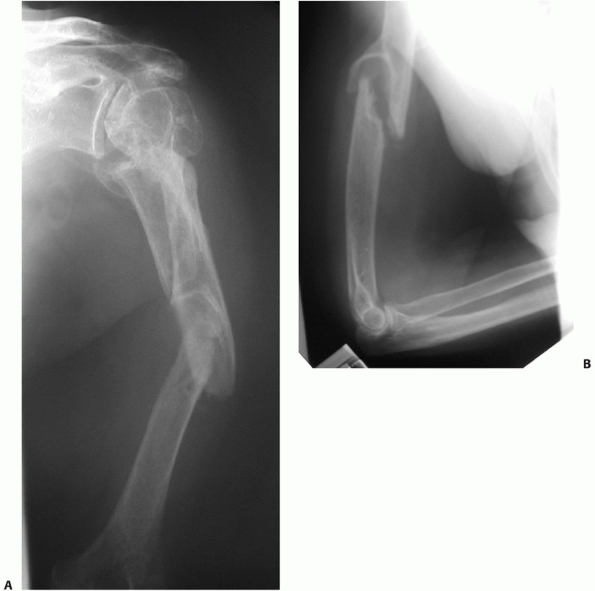
fractures, the so-called “floating elbow” (Fig. 34-3A,B).133 Associated injuries such as arterial ruptures, scapulothoracic dislocations, or hemo/pneumothoraces may be life-threatening (Fig. 34-4A,B). The presence or absence of a (usually radial) nerve injury is particularly important to document prior to any intervention.46,64,76,118,128
In the prevailing medical-legal climate, it is generally assumed that
the nerve is intact unless specifically noted otherwise. If a nerve
injury exists but is not recorded prior to a reduction or any other
procedure, the burden falls onto the physician to prove that he or she
did not cause it. In cases where it is impossible to clinically
establish the function of the nerve (i.e., associated head injury), it
should be clearly stated that the condition of the nerve cannot be
determined.
the presence of an open fracture accelerates the urgency of the
situation. Examination of the shoulder and elbow joint is mandatory:
associated injuries or pre-existing joint pathology may be an
indication for operative management as stiffness may transfer
physiologic stress to the fracture site and increase healing time (Fig. 34-5).147 This will have implications for treatment and counseling of the patient with regards to prognosis.
two radiographs at 90 degrees to each other that include the shoulder
and elbow joints in each view. Further views can be ordered depending
on the clinical examination and any abnormalities noticed on the
initial films. For the typical humeral shaft fracture, it is rarely
necessary to obtain further imaging. Exceptions to this would include
shaft fractures with associated vascular injuries that should be
investigated further with an angiogram (see Fig. 34-4) or computed tomographic (CT) scans of associated intra-articular injuries proximally or distally.50,147,148
CT scanning may also be indicated in the rare situation where a
significant rotational abnormality exists as rotational alignment is
difficult to judge from plain radiographs of a diaphyseal long bone
fracture. A CT scan through the humeral condyles distally and the
humeral head proximally can provide exact rotational alignment,
especially when compared to the normal side. Given the broad rotational
range of the shoulder, fairly large degrees of rotational malalignment
can be accepted.145 However, severe
degrees of rotational malalignment (>30 degrees) should be avoided
as they have a deleterious effect on the functional “sphere of action”
of the upper extremity. Hubner et al.67 have advocated the use of ultrasound in developing countries.
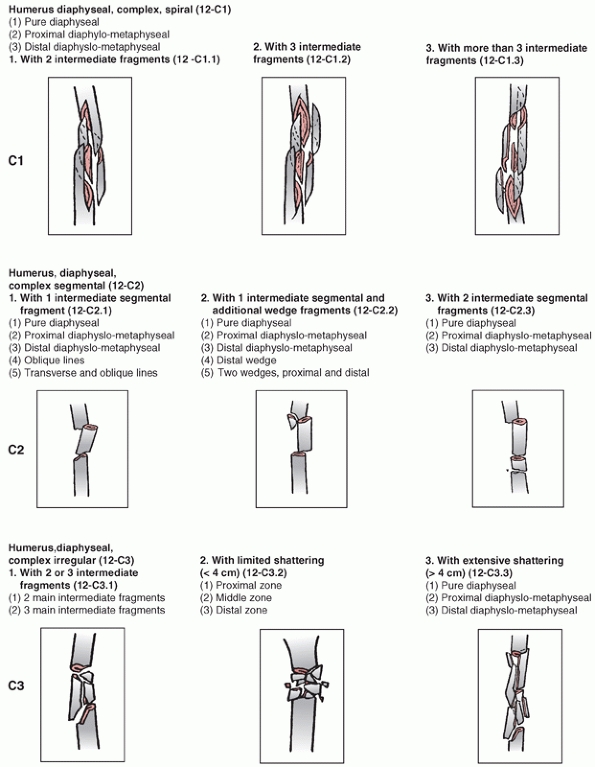
describe when a humeral shaft fracture is classified. These include the
mechanism of injury (low-energy, high-energy, gunshot-associated), the
location of the fracture (proximal, midshaft, distal) along with any
potential periarticular or intra-articular extension, concomitant soft
tissue wounds or lesions, associated nerve or vessel injury (radial
nerve most commonly), the nature of the underlying bone (normal,
osteopenic, pathologic), and the presence of any associated prosthesis.
Descriptive terms such as these are useful in providing an overall
picture of a particular humeral shaft fracture, although they may not
lend themselves well to categorizing injuries for research or clinical
trials. For this reason, more objective classification schemes have
been developed to aid in such endeavors.
Association’s Fracture and Dislocation Compendium, first published in
1996 and republished in 2007.111,113,114
This system is based on the AO/ASIF Comprehensive Long Bone
Classification, adds previously unclassified fractures, and reorders
them in an alpha-numeric system. The humerus is designated bone “1” and
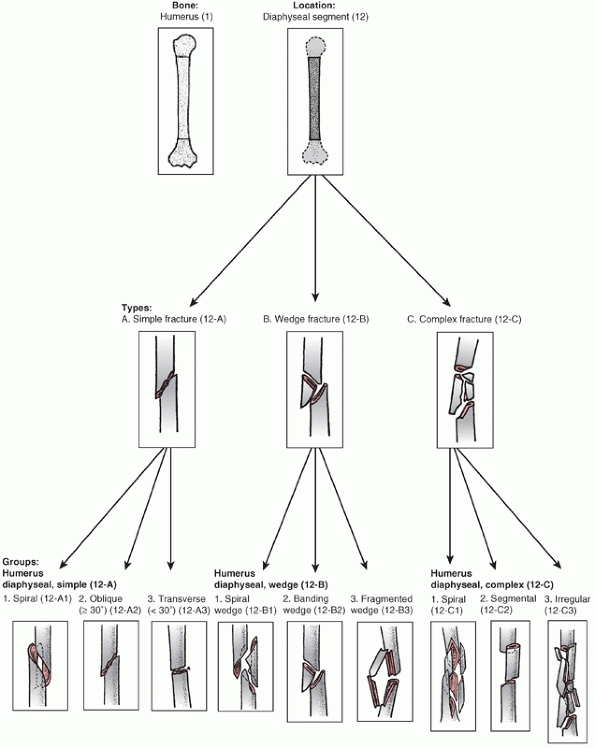
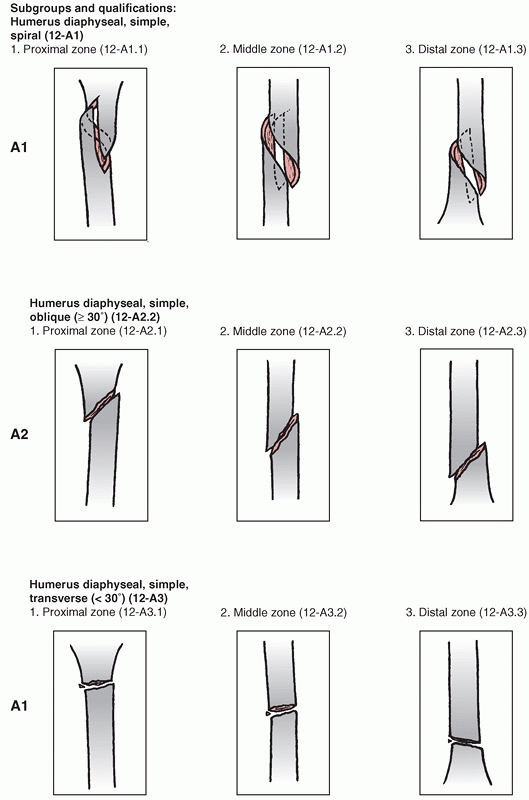
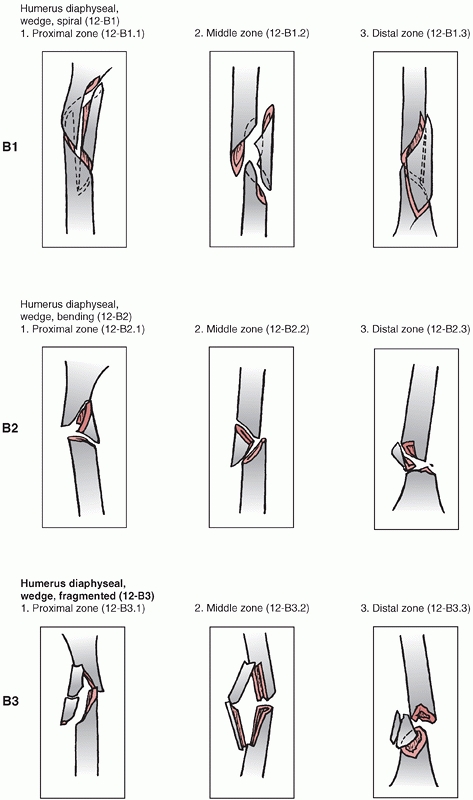
is divided into proximal,11 diaphyseal,12 and distal13 segments (Fig. 34-6).
Fractures are divided into three types: “A” or “Simple” fractures of
two main fragments, proximal and distal (cortical fragments of less
than 10% of the circumference are ignored); “B” or “Wedge” fractures
where there are one or more intermediate fracture fragments but, after
reduction, there is contact between the main proximal and distal
fragments; and “C” or “Complex” fractures, where there are one or more
intermediate fragments such that, after reduction, there is no contact
between the main fragments. These main types are then subdivided into
groups based on fracture pattern (spiral, oblique, transverse) and
subgroups based on proximal, middle, or distal zones of the diaphysis.
The system is designed to reflect increasing fracture severity as one
progresses from A to C types, and to help aid in treatment, prognosis,
and research. The reliability and reproducibility of this type of
scheme has not been critically assessed for the humeral shaft but has
been looked at for both the proximal and distal segments of the bone.
These investigators found that while there was poor inter- and
intraobserver agreement for subgroup classification, there was
“substantial” (kappa value 0.66) agreement for type (A, B, C) and
“moderate” (kappa value 0.52) agreement for group designation.150,165
Thus it seems reasonable to assume that (the anatomically simpler)
humeral shaft fractures can reliably be classified at least as to type
(simple, wedge, complex) and group (spiral, oblique, transverse).
 |
|
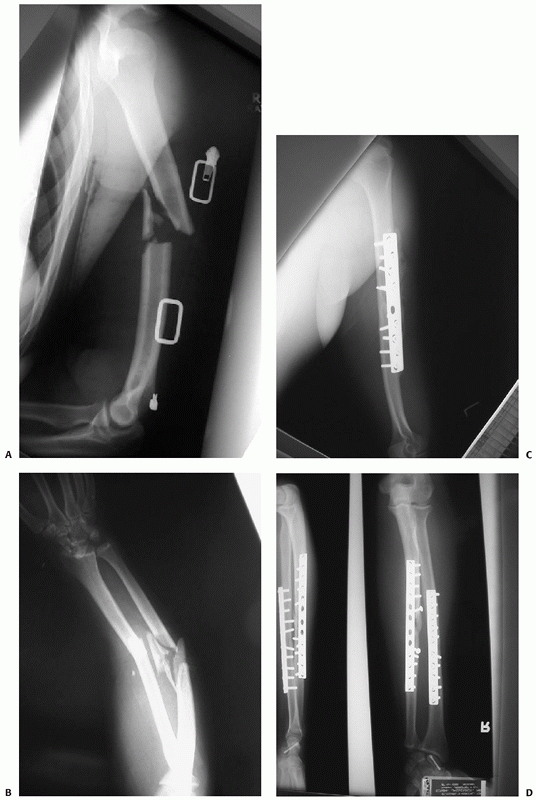
FIGURE 34-3 A,B.
Three level upper extremity injury with closed humeral shaft fracture, comminuted open radial and ulnar shaft fractures, and associated compartment syndrome of the forearm and transscaphoid perilunate fracture dislocation of the wrist. C,D. Open reduction and internal fixation of all three injuries and a forearm fasciotomy allowed early postoperative motion and resulted in rapid union and reasonable functional outcome in this severely injured limb. |
 |
|
FIGURE 34-4 A. An angulated, open fracture of the humeral shaft in a patient from a roll-over motor vehicle accident. B.
Angiogram demonstrates axillary artery injury with associated scapulo-thoracic dissociation (note the wide separation of the distal clavicle and acromion process). |
 |
|
FIGURE 34-5 A,B.
This elderly osteoporotic woman had previously sustained a humeral head and neck fracture treated conservatively that had resulted in a very stiff shoulder. She fell 4 years later and fractured the shaft of the humerus distal to the prior injury. Despite appropriate splinting, she developed a painful pseudarthrosis, as seen on the lateral radiograph (B). Adjacent joint stiffness transfers much of the motion and stress of the limb to an ipsilateral fracture and increases the rate of delayed union and nonunion. This factor should be considered when deciding on treatment options. |
 |
|
FIGURE 34-6
The OTA classification of humeral shaft fractures. (From Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association Classification, Database, and Outcomes committee. J Orthop Trauma 2007;21(10 Suppl), with permission.) (continues) |
 |
|
FIGURE 34-6 (continues)
|
 |
|
FIGURE 34-6 (continues)
|
 |
|
FIGURE 34-6 (continues)
|
Grade I is a wound less than 1 cm (typically low-energy wounds caused
by an inside-out puncture of the fragment end). A Grade II wound is
greater than 1 cm and of higher energy. A Grade III wound has extensive
soft tissue injury and/or periosteal stripping or a high-energy injury
irrespective of the size of the wound. Depending on the severity of the
stripping, Grade III lesions are subclassified into A, B, and C types,
with B defined as an injury with extensive soft tissue loss and
periosteal stripping usually associated with massive contamination. A
Grade IIIC injury has an associated arterial injury that requires
surgical repair for viability of the limb. Tscherne’s146
classification system of closed fractures has the advantage of
including various degrees of closed soft tissue contusions and
compartment syndromes as well. Type O fractures are from indirect
violence (i.e., torsion) and have minimal soft tissue injury. Type I
injuries have superficial abrasions or contusions caused by pressure
from within. Type II injuries have deep abrasions with contused skin
and/or muscle and may have an impending compartment syndrome. Type III
injuries have extensive contusions or crushed muscle, subcutaneous
avulsions, vascular injuries, and compartment syndromes. The
reproducibility of soft tissue injury grading schemes has also been
called into question.19
shaft have been described, there are two standard techniques that
dominate most articles and reviews and are the most commonly used
clinically: the posterior approach and the anterolateral approach.36,49,50,62,65,148
Other described approaches that are useful in specific situations are
the direct lateral approach and the direct medial approach.71,75,103
of middle and proximal third humeral shaft fractures that require plate
fixation (Fig. 34-7). The patient is positioned
in the supine or semisitting position, a pad is placed behind the
scapula to elevate the limb, and the arm is draped free to include
access to the shoulder and elbow, if necessary. The limb can be
supported on an adjustable covered Mayo stand or an arm board. The skin
incision is centered over the fracture site and is performed
longitudinally along the palpable lateral border of the biceps brachii.
The proximal landmark for extension of the skin incision is the
coracoid process, and distally it is anterior to the lateral
supracondylar ridge. Next, the subcutaneous tissue and fascia are
divided. Proximally, if necessary, dissection is performed between the
pectoralis major muscle medially and the deltoid laterally, taking care
to identify and protect the cephalic vein. If required, part of the
broad deltoid insertion can be reflected posteriorly to gain access to
the anterolateral shaft if needed for proper placement of a plate. In
the midshaft region, the dissection plane is between the biceps and
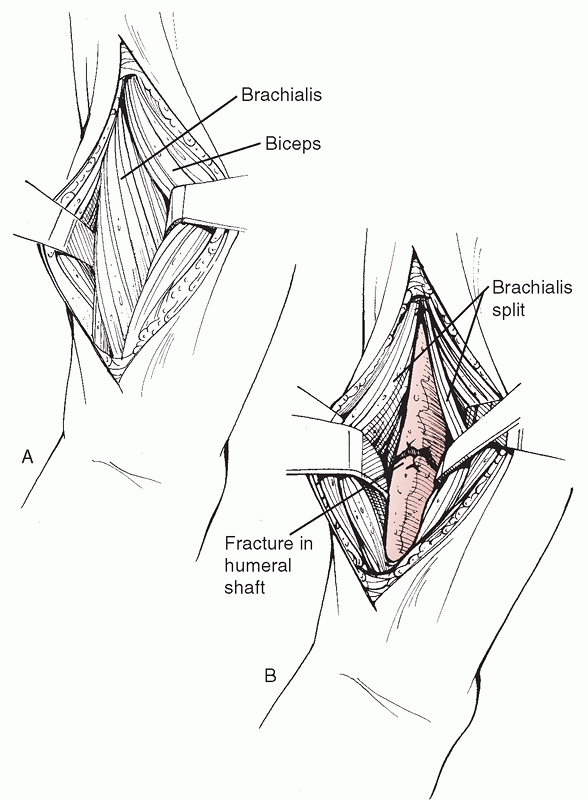
triceps, exposing the brachialis underneath, which is split
longitudinally along its lateral portion. This muscle has dual
innervation (radial nerve laterally, musculocutaneous nerve medially)
and when correctly performed, this split is roughly in an internervous
plane. When placing retractors around a midshaft fracture, it is
important to avoid pressure on the radial nerve through the laterally
placed
retractor.
Distally, dissection continues along the anterior aspect of the lateral
supracondylar ridge between the brachialis medially and brachioradialis
laterally. It is at this point that the radial nerve, as it wraps
around the lateral aspect of the distal humerus, is closest to the
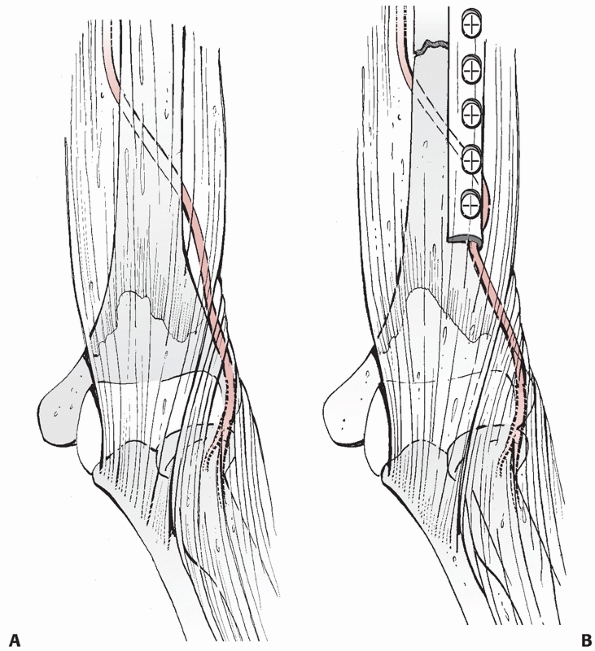
dissection, and should be identified and protected.27
It is in danger of being trapped underneath the distal, lateral corner
of the plate: the surgeon should ensure that this corner is free of any
soft tissue prior to closing (Fig. 34-8). The
elbow range of motion should be examined to ensure there is no
impingement from a very distal plate. The advantages of this approach
include the favorable position of the patient, the ability to extend
the incision proximally to deal with associated shoulder pathology or a
proximal extension of the fracture, and identification of the radial
nerve at the mid part or distally.6
Disadvantages include technical difficulty in applying a plate distally
along the (thin) lateral supracondylar ridge, the lack of access to any
distal medial column pathology, and the noticeable scar that results.
 |
|
FIGURE 34-7
The anterolateral surgical approach to the humeral shaft. After skin and subcutaneous tissue dissection, the interval between the biceps anteriorly and the triceps posteriorly is developed. The shaft is identified distally by splitting the brachialis muscle in line with its fibers. The dissection can be extended into the delto-pectoral interval to gain access to the proximal humeral shaft and, if necessary, the humeral head. |
involve the distal third of the humerus, especially those that have an
intra-articular extension or those that require an exploration and/or
repair of an associated radial nerve injury.50,76,118,148
The patient is typically positioned prone or in the lateral decubitus
position with the affected side up. The arm is draped free over a
bolster, and, depending on the proximal extent of the dissection
anticipated, a sterile tourniquet can be applied. A direct posterior
skin incision is centered over the fracture site: It can extend from
the tip of the olecranon distally to the posterolateral corner of the
acromion proximally as required. After dissection of the subcutaneous
tissue and superficial fascia, the triceps is sharply divided distally,
taking care to identify and protect the radial nerve (and profunda
brachii artery that runs with it) proximally.80
The radial nerve crosses the posterior aspect of the humerus in the
spiral groove roughly equidistant between the tip of the olecranon and
the edge of the acromion and can be identified at the lateral edge of
the attachment of the medial head of the triceps. Proximally, it is
usually possible to identify an interval between the long and lateral
heads of the triceps. The limit of proximal dissection is the
overhanging cowl of the deltoid muscle posteriorly and the associated
axillary nerve and posterior humeral circumflex artery. Distally, if
fixation is anticipated on the medial column of the humerus, the ulnar
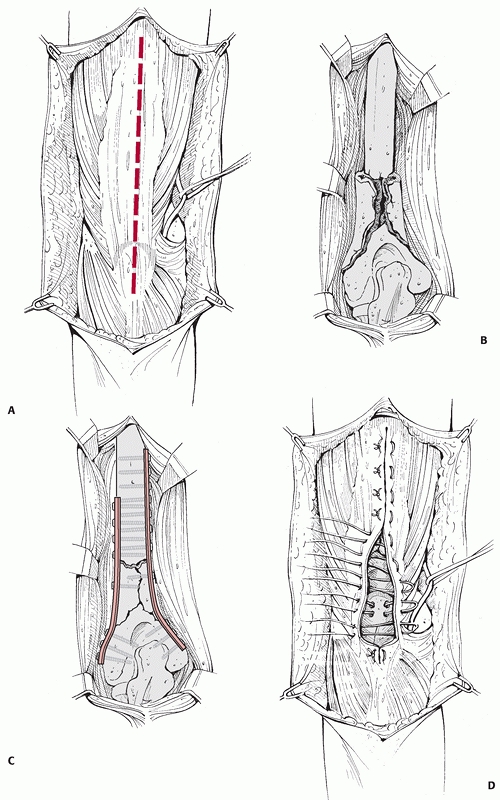
nerve should be identified and protected. If required, the triceps can
be elevated from the olecranon to gain access to the elbow joint. It
should be firmly reattached through drill holes in the bone to prevent
postoperative detachment (Fig. 34-9). The
advantages of the posterior approach are mainly the ability to access
both lateral and medial columns distally, the ease of fixing a shaft
fracture with a distal extension, the flat posterior surface distally
which is ideal for plate fixation, and the exposure of the radial nerve
(Fig. 34-10). Paradoxically, its main
disadvantage is the proximity of (and danger to) the radial nerve:
plate extension past the midshaft of the bone must be done under the
nerve, resulting in the awkward situation of having the nerve lying
directly on the plate. Also, the prone or lateral position may not be
favorable from an anaesthetic standpoint in a multiplyinjured patient,
and the humeral head and neck cannot be accessed safely through this
approach.
 |
|
FIGURE 34-8 A.
The radial nerve lies close to the humeral shaft as it exits from posterior to the shaft to anterior and is in danger of being entrapped underneath the distal, lateral corner of a compression plate applied through the anterolateral approach. B. During plate application the nerve and associated soft tissue is retracted laterally, and the corner of the plate should be checked prior to wound closure. |
consists of a lateral approach to the elbow that is extended proximally
along the humeral shaft. It provides excellent exposure of the distal
two thirds of the humerus and the radial nerve. It can be extended
proximally into an anterolateral approach and distally along Kocher’s
interval distally to deal with lateral elbow pathology and has the
advantage of being performed in the supine position. The skin incision
follows a line from the deltoid insertion to the lateral epicondyle.
After an incision through the superficial fascia, the triceps is
reflected posteriorly and the brachialis and mobile wad of Henry are
reflected anteriorly; muscle splitting is not necessary. The radial
nerve is identified proximally as it wraps around the lateral aspect of
the humerus; it is protected, as is the posterior antebrachial
cutaneous nerve branch. The drawbacks of this approach are that access
to the medial column distally is not possible and proximally, posterior
access to the humeral shaft is limited by the deltoid muscle. The ideal
indication for its use is a distal humeral shaft fracture that requires
fixation and exploration of the radial nerve (i.e., a Holstein-Lewis
fracture pattern).64
exposure to the brachial artery and median and ulnar nerves. While it
is rarely chosen for routine fracture fixation, it is an ideal approach
in the rare instance where there is a concomitant brachial artery
injury that requires repair.31,71,101
The surgical incision begins distally at the medial epicondyle and
extends proximally along the posterior edge of the biceps brachii
muscle. After dissection through the subcutaneous tissue and splitting
of the superficial fascia, the ulnar nerve is identified and retracted
posteromedially. The median nerve and brachial artery are identified
and retracted anterolaterally. There are numerous small branches of the
artery that require ligation. The medial intermuscular septum is then
identified and can be partially resected to improve exposure and plate
application. The triceps is stripped from the shaft and reflected
posteriorly as required, and the origin of the coracobrachialis is
reflected anteriorly. The main advantages of this approach are the
excellent exposure of the neurovascular structures medially (especially
if vascular repair is required) and the fact that the scar is well
hidden when the arm is against the side, which makes it cosmetically
appealing. However, it is a very “busy” anatomic area (including a
number of cutaneous nerves such as the intercostobrachial nerve
proximally and the medial cutaneous nerve of the arm distally), and
neurovascular injury is a major concern. Also, proximal extension is
very difficult: the axillary fold can be deceiving in how far it
extends distally, especially in patients with an unfavorable body
habitus (short, stocky, or obese).
 |
|
FIGURE 34-9 A-D.
The posterior surgical approach to the humeral shaft. If necessary, the approach can be extended distally by reflecting the triceps from the olecranon, providing exposure of both medial and lateral columns. The triceps is then reattached through drill holes in the olecranon. |
 |
|
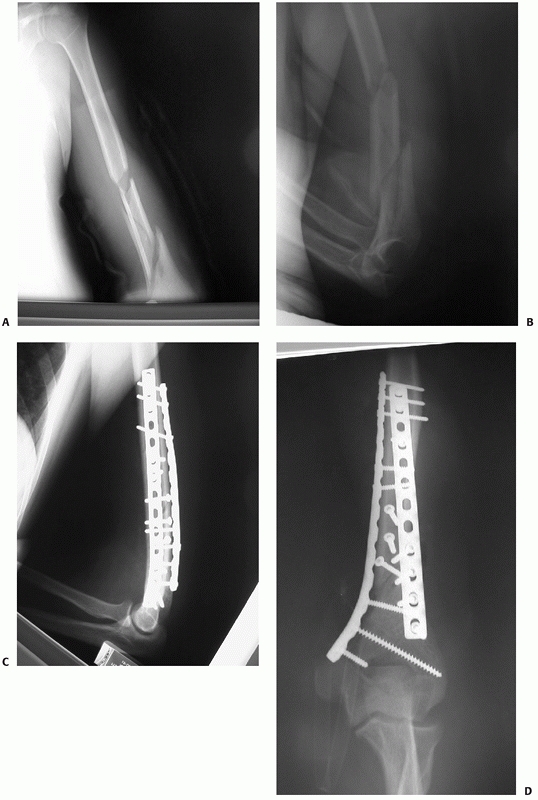
FIGURE 34-10 A,B.
Radiographs of a segmental distal humeral shaft fracture with fracture site distraction and extension low into the medial column. C,D. A posterior approach was used with triceps splitting which allowed sufficient distal exposure to fix the fracture site with 3.5-mm compression plates on each column. Immediate motion was allowed and an excellent functional result ensued. |
shaft has a long and well-established history of success, with numerous
authors reporting high rates of union with various types of splints,
casts, and braces.21,26,30,39,50,63,144,145,148,158
While anatomic reduction is rarely achieved with nonoperative treatment
of these injuries, it is rarely necessary due to the wide range of
motion of the shoulder and elbow, such that angulatory, axial, or
rotational malunion is easily accommodated and functional limitation is
minimal. The humeral shaft is well enveloped in muscle, has a robust
blood supply,80 does not bear
weight, and is easily splinted, leading Sir John Charnley to state, “It
is perhaps the easiest of the major long bones to treat by conservative
methods.”26 Described techniques
include hanging casts, “sugar tong” or coaptation splints, sling and
swathes, long-arm casts, shoulder spica casts, and olecranon pin
traction. However, while good results have been described with most of
these methods, functional bracing has become the criterion standard for
nonoperative treatment due to its ease of application, adjustability,
low cost, allowance of shoulder and elbow motion, and reproducible
record of success.39,144,146,158
While a detailed description of nonoperative care is beyond the scope
of this chapter, a review of functional bracing is an integral part of
any description of humeral shaft fracture treatment.
in 1977 and consisted of a custom-made, circumferential orthosis that
allowed elbow and shoulder motion and was worn for a mean of 10 weeks
in 51 patients with one nonunion. The device has been modified since
and now is a prefabricated device with plastic supports and adjustable
hook and loop straps that can be tightened as the swelling associated
with the fracture recedes.145 The
device works on the principles of active muscle contraction correcting
rotation and angulation, the “hydraulic” effect of soft tissue
compression aligning the fracture fragments, and the beneficial effect
of gravity on alignment. In the largest series to date of 922 patients
(620 were followed to definitive outcome) with humeral shaft fractures
treated with functional bracing, Sarmiento145
reported union in 98% of 465 closed fractures and 94% of 155 low-grade
open fractures. Open fractures took longer to heal than closed
fractures (14 weeks versus 9.5 weeks). These results are consistent
with those reported by Ostermann et al.112 (4 nonunions of 191 fractures), Zagorski et al.124 (3 nonunions of 170 fractures), and Ricciardi-Pollini (2 nonunions of 36 fractures).171 However, the results in some other studies are not so favorable. In a study by Ekholm et al.,39 8 of 78 fractures did not heal when treated with a brace. Toivanen et al.158
found a higher prevalence of nonunion with 21 of 93 fractures where
bracing was not successful. Proximal shaft fractures were especially
prone to healing problems. It is important to note that in these two
studies, the time in a brace before converting to surgery was as low as
6 weeks in some cases, far less than the treatment time in Sarmiento’s
series.
technique results in a high rate of union. Given the early motion that
is encouraged with this method, functional outcome of the elbow and
shoulder is well maintained with 89% of patients losing 10 degrees of
motion or less of the shoulder and 93% of patients losing 10 degrees of
motion or less of the elbow in Sarmiento’s series.145
It should be noted, however, that many of the patients in this series
were indigent and functional outcome data was rudimentary. For
instance, elbow range of motion was recorded for only 301 of the
original 922 patients.
surprisingly well corrected with this method under the influence of
gravity, time, and the brace (Fig. 34-11). Sarmiento145
reported that 55% of patients healed within 5 degrees of anatomic
alignment following functional bracing of humeral shaft fractures, with
varus being the commonest deformity: 42% of patients healed with 6 to
25 degrees of varus deformity. Eighty-six percent of patients healed
within 10 degrees of anatomic alignment in the anteroposterior plane.
These deformities did not seem to influence functional results.
However, as mentioned, functional outcome data was minimal, and it is
quite possible that subtle or delayed complications would not be
detected. For example, recently the negative biomechanical and clinical
effects of distal humeral varus malunion have been elucidated. Twenty
patients with a mean varus malunion of 19 degrees developed
posterolateral rotatory instability of the elbow a mean of 15 years
after their initial distal humeral fracture.109 It is unlikely this type of problem would have been elucidated in Sarmiento’s study.
can be managed nonoperatively; however, there are specific operative
indications that have been shown to enhance the outcome of the fracture
or patient. These indications can be divided into three groups:
fracture indications, associated injuries, and patient indications (Table 34-2).
While some of these indications are absolute, such as an associated
vascular injury or an associated higher grade open wound, many are
relative indications where both patient and fracture features must be
taken into consideration prior to deciding on treatment. For instance,
although most segmental fractures are high-energy injuries with
significant deformity, a segmental fracture in which both fracture
lines are minimally displaced and the overall alignment is acceptable
is a good candidate for a trial of conservative care with functional
bracing. It is important to note that fracture comminution, in
isolation, is not an indication for
operative intervention. Although intuitively it would seem that such
fractures are more likely to develop delayed or nonunion, this is not
the case. In his large series of functional bracing, Sarmiento145
found the median healing time was 11 weeks for the comminuted fractures
and 12 weeks for the transverse fractures: others have reported similar
findings. Some authorities have noted the relative propensity for
delayed union in transverse or short oblique fractures managed
nonoperatively in an active individual147,148
and considered such injuries a relative indication for operative
repair. Also, operative fixation of comminuted fractures is associated
with a higher complication and mechanical failure rate, and thus is
best avoided if conservative treatment is feasible.
 |
|
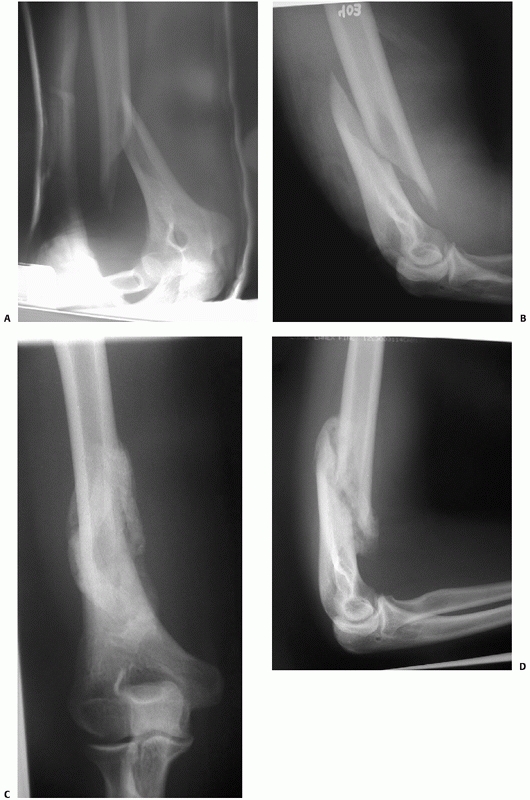
FIGURE 34-11 Anteroposterior (A) and lateral (B)
radiographs of a distal humeral shaft fracture in a 28-year-old woman following a fall from a horse. Marked displacement is evident. Anteroposterior (C) and lateral (D) radiographs taken 9 weeks later following closed treatment and functional bracing. The alignment is nearly anatomic, the fracture is solidly united and there is minimal (<1 centimeter) shortening. The elbow range of motion was 5 to 140 degrees. |
|
TABLE 34-2 Indications for Primary Operative Treatment of Humeral Shaft Fractures
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
fixation of humeral shaft fractures against which other methods must be
compared.7,13,45,50,94,99,147,148,163
It is associated with a high union rate, low complication rate, and a
rapid return to function. It can be used for fractures with both
proximal and distal extension, is safe and effective in open fractures
(see below), has essentially no elbow or shoulder morbidity, and is
stable enough to allow early upper-extremity weight bearing in the
multiply injured patient.157 The
surgical approaches, implants, and techniques are familiar to most
orthopaedic surgeons, and it remains the procedure of choice at our
institutions for humeral shaft fractures that require operative
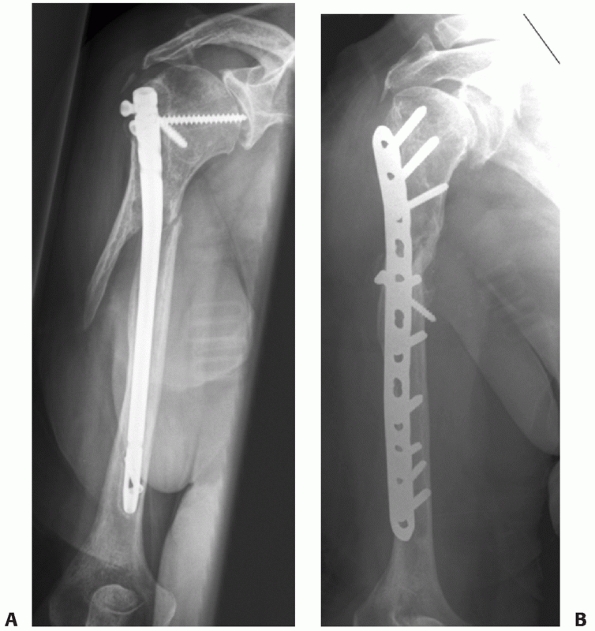
fixation (Fig. 34-12). Van der Griend et al.163
reported union in 35 of 36 plated humeral shaft fractures with no
shoulder or elbow morbidity and one temporary radial nerve palsy. Bell
et al.7 had similar results (union in 37 of 39 fractures) as did Tingstad et al.157
(union in 78 of 83 fractures). The union rate following open reduction
and internal fixation of humeral shaft fractures averages 96% in a
number of large series.7,45,94,99,157,163
Complications are infrequent and include radial nerve palsy (2% to 5%,
usually neurapraxic injuries which recover), infection (1% to 2% for
closed fractures, 2% to 5% for open fractures) and refracture (1%).
approached through an anterolateral incision. Fractures that extend
into the distal third of the bone are best approached posteriorly. A
broad 4.5 mm dynamic compression (DC) or limited contact compression
(LCD) plate helps prevent longitudinal fracture or fissuring of the
humerus because the screw holes in these plates are staggered.147
In small individuals with thin humeri, a narrow 4.5 mm plate may be
used. Inserted screws can be angled medially and laterally so they exit
staggered on the opposite cortex, minimizing longitudinal stress. Other
plates are not strong enough, especially in active individuals (Fig. 34-13).
In the transition zone distally between the shaft and the supracondylar
ridges, as the medial and lateral columns diverge, fixation can be
achieved with two 3.5 mm compression plates along each column, avoiding
plate impingement in the olecranon fossa (see Fig. 34-10).
A lag screw placed across the main fracture line (either outside or
through the plate) can increase construct strength by 30% to 40%, and
if fracture or nonunion geometry permits, should be inserted (Fig. 34-14).147
With a solid lag screw, three screws (six cortices) proximal and three
screws (six cortices) distal constitute the minimum fixation; without a
lag screw, at least four screws (eight cortices) proximally and
distally are required. Fracture comminution, poor screw purchase, poor
bone quality, or other negative factors should prompt longer plate
application with more screws. Previously, it was recommended that a
unicortical screw be placed in the last hole of the plate to minimize
the stress-riser effect of the plate, provide a smoother transition of
stress from plate to bone, and decrease the potential risk of fracture
at the end of the implant. However, a biomechanical study by Davenport34
showed no difference in stress between uni- and bicortical screws in
the end of the plate, and this technique is no longer recommended.
LCD plates over standard compression plates: they are easier to
contour, allow for wider angle of screw insertion, and have
bidirectional compression holes. Theoretical advantages include
decreased stress shielding and improved bone blood flow due to limited
plate-bone contact.69 One study showed a 97% union rate in upper extremity injuries in which plate fixation was accomplished with LCD plates.99
well in most patients when used for treatment of humeral fractures or
nonunions, there are subgroups of patients where implant failure can be
seen more frequently. Patients with osteopenic or osteoporotic bone are
such a group where implant failure through screw loosening might be a
problem. This is especially true if healing is slow and the patient
uses a walking aid, and thereby is reliant on the upper extremity being
able to take load.
systems have gained wide popularity. By locking the screws to the plate
a number of mechanical advantages are gained, including a reduced risk
for screw loosening and a stronger mechanical construct compared with
conventional screws and plates. With locking plate systems, the
pressure exerted by the plate on the bone is minimal as the need for
exact anatomical contouring of the plate is eliminated. A theoretical
advantage of this is less impairment of the blood supply in the
cortical bone beneath the plate compared to conventional plates.
 |
|
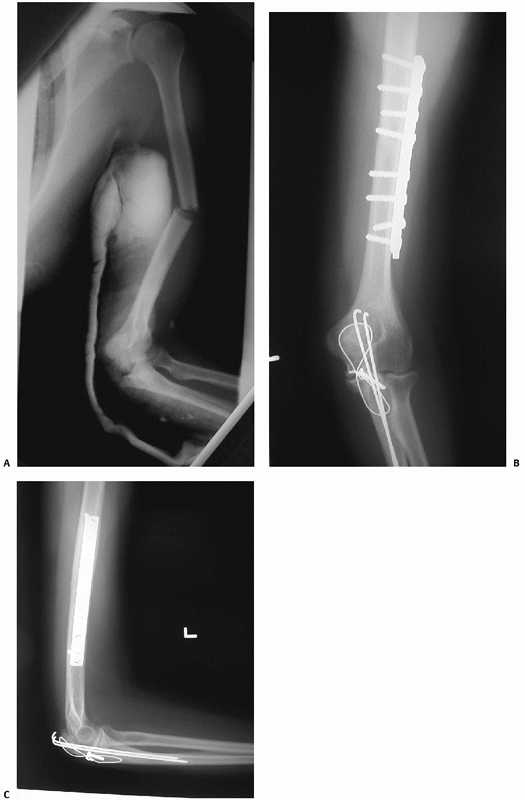
FIGURE 34-12 A.
Anteroposterior radiograph of a humeral shaft fracture associated with an open olecranon fracture in a polytrauma patient. The injuries were treated with open reduction and plate fixation of the humeral shaft fracture (a narrow 4.5-mm plate was used due to the small stature of the patient) and irrigation, débridement, and tension-band wiring of the olecranon. Anteroposterior (B) and lateral (C) radiographs show healing of both fractures. An excellent functional outcome was the result, with an elbow range of motion of 20 to 135 degrees. Associated ipsilateral upper extremity fractures are one of the most common indications for primary operative fixation of humeral shaft fractures. |
 |
|
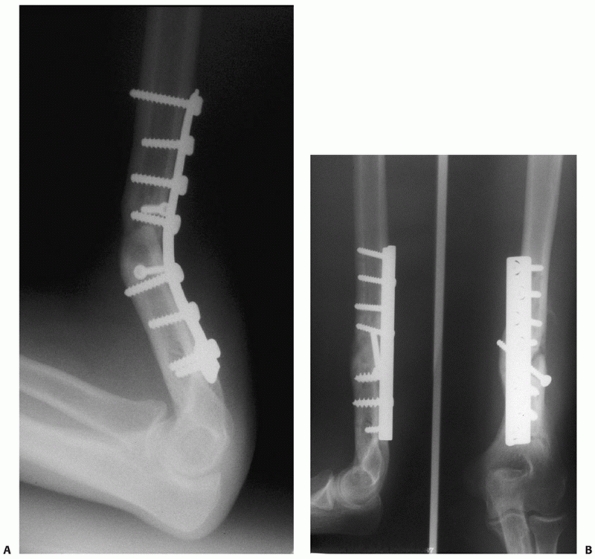
FIGURE 34-13 A.
Proper plate selection is important in the fixation of humeral shaft fractures. This 18-year-old man had a displaced distal humeral shaft fracture fixed with a thin “T” plate placed directly posteriorly. This plate, with its thinnest dimension directly in the plane of motion of the elbow, was insufficient to withstand the forces applied to it in this muscular, active young man. An unacceptable 30-degree recurvatum deformity soon developed, with pain and instability at the fracture site. B. Revision with a lag screw and broad 4.5-mm compression plate restored local anatomy and resulted in rapid union. Compression plates are the implants of choice for humeral shaft fractures, especially in active young individuals. |
being gained over the last few years, there remain very few published
reports of their use in humeral fractures and nonunions. At the present
time, there is some preliminary evidence that the improved fixation
seen with locking devices may be advantageous in the humeral shaft,
especially when dealing with osteopenic bone.130,153
It is as yet difficult, due to the limited number of publications, to
define the exact role of and the proper indications for locking plates
compared with conventional plate and screw systems.
introduced into the market, the so-called minimal invasive plate
osteosynthesis (MIPO) technique was popularized. Theoretically, the use
of small skin incisions distant to the fracture site with submuscular
plate insertion offers minimal soft tissue compromise. A prerequisite
for the technique is indirect fracture reduction, using long spanning
plates. The technique is widely used in tibial fractures as well as
femoral fractures. For humeral shaft fractures, it has been considered
too dangerous due to the risk of neurovascular injuries, particularly
to the radial nerve. However, by using cadaver bones, different
technical aspects have
been addressed in order to develop a useful and safe MIPO technique in humeral shaft fractures. Apivatthakakul et al.3
showed that with the forearm in full supination the safe distance
between the nerve and an anteriorly placed plate increased
substantially. In 2004, Livani and Belangero91
described their results when using the MIPO technique in 15 patients of
whom 8 were polytraumatized and two were open fractures. Using an
anterior plate position and 4.5 mm DC plates, they had no major
complications, including no radial nerve palsies caused by the
procedure. Fourteen of 15 fractures healed within 8 to 12 weeks, and
two with varus alignment of 5 to 10 degrees. In 2007, two clinical
series reported the use of a similar MIPO technique in 21 and 13
patients, respectively.70,172
 |
|
FIGURE 34-14 A.
Aseptic nonunion in a 74-year-old healthy women 12 months following fixation with a locked nail. The fracture was never adequately reduced and one screw penetrated the joint. There were no signs of bone healing with instability in rotation and severe pain. B. Three months after reoperation with fixation with a compression screw and a locked plate and BMP for biologic stimulation. The fracture went on to uneventful healing. |
placement of 4.5-mm large-angle stable-locking plates with 13 to 15
holes was used. There were no major complications during surgery,
including no compromise of the radial nerve, and healing was obtained
in 19 of 21 cases following the primary procedure.70 Zhiquan et al.172
reported on 13 patients with fractures through the mid or distal part
of the shaft, with type B being the most common fracture type (10 of
13). The fractures were treated with a similar technique although with
the use of narrow 4.5 mm DC plates with 10 to 12 holes for fixation.
There were no nerve palsies resulting from the surgery. All fractures
healed within 8 degrees of valgus and varus. The disadvantage of the
MIPO technique in the humerus is a long operating time and increased
radiation due to the need to check the reduction and the position of
the implant during insertion and fixation. Future studies are awaited
to confirm or refute any major advantages with its use in the humerus.
Preliminary data and experience indicate a possible benefit in complex
comminuted fractures.
-
Use an anterolateral approach for midshaft or proximal fractures, and a posterior approach for distal fractures.
-
Use a broad 4.5-mm compression plate in
most patients, with a minimum of 3 (and preferably 4) screws proximal
and distal. A 4.5-mm narrow plate is acceptable for smaller individuals. -
Insert a lag screw between major fracture fragments, if possible.
-
Check the distal corner of the plate for radial nerve entrapment prior to closure following the anterolateral approach.
-
The intraoperative goal is to obtain sufficient stability to allow immediate postoperative shoulder and elbow motion.
with the hope that the results from their use would parallel the
clinical success seen with similar devices used for femoral and tibial
fractures.34,68,79,126,127,134,154
Previously available intramedullary implants for the humerus such as
Rush pins or Enders nails, while effective in many cases with simple
fracture patterns, had significant drawbacks such as poor or
nonexistent axial or rotational stability.1,57,121,135 Henley59
reported a series of 49 patients with humeral shaft fractures treated
with Ender nailing and had only one nonunion, and Brumback18
reported a 94% union rate with Rush pins and Enders nails, although
there was a significant rate of insertion site morbidity and backing
out of the nails such that the “excellent” clinical success rate was
much lower (62%). However, especially when used for comminuted or
unstable fracture patterns, some form of additional stabilization was
required, either internal (cerclage wire at the fracture site) or
external (prolonged splinting). The construct that resulted was often
not stable enough to allow early motion or upper extremity weight
bearing in the case of the multiply injured patient with concomitant
lower extremity injuries.
locking mechanisms distally including interference fits from expandable
bolts (Seidel nail) or ridged fins (Trueflex nail), or interlocking
screws (Russell-Taylor nail, Synthes nail, Biomet nail). Unfortunately,
despite favorable initial reports, these devices have not enjoyed the
unparalled success of lower extremity locking nails. Problems such as
insertion site morbidity, iatrogenic fracture comminution (especially
in small diameter canals), and nonunion (and significant difficulty in
its salvage) have been reported (Fig. 34-15).40,97,98,140,151
In addition, some of the perceived advantages of nailing over
compression plating (such as earlier upper-extremity ambulation and
avoiding peri-implant fracture) have proven to be illusory.68,98 A number of randomized clinical trials comparing these intramedullary implants to compression plating have been performed.12,25,90,135
They have shown a higher reoperation rate and greater shoulder
morbidity with the use of nails. At the present time, in our
institutions, the use of locking nails is restricted to widely separate
segmental fractures, pathologic fractures, fractures in patients with
morbid obesity, and fractures with poor soft tissue over the fracture
site (such as burns). A number of newer nails, designed to eliminate
insertion site morbidity through an extra-articular start point, have
been introduced (see below); it remains to be seen whether prospective,
randomized trials will prove their advantages.
humeral nails required reaming prior to insertion. While the reamings
produced may improve fracture union, there are some drawbacks.
The
fracture site must be kept closely apposed during reaming to prevent
radial nerve damage. Also, the distal humeral canal tapers to a blind
end above the olecranon fossa: it does not have a wide metaphyseal
flare to vent debris or heat in front of an advancing reamer as does
the femur or tibia. There is evidence that excessive reaming of the
medullary canal can be detrimental. Reimer et al.123
reported a 58% complication rate in patients undergoing Seidel humeral
nailing when the humeral canal size was 9 mm or less, and postulated
extensive reaming was one of the contributing factors. Others have
reported extensive heat necrosis from excessive reaming, and we have
experience with similar cases.86,161
If there is not sufficient space, one risks distracting the fracture
site as the nail wedges in the distal fragment during insertion (Fig. 34-16).
One point emphasized in most series of large-diameter nails is that the
humerus does not tolerate distraction. This is a risk factor for
delayed and nonunion. In order to reduce the risk of distraction while
at the same time improving the mechanical stability of the bone-implant
construct, some nails offer the option to apply compression. One
problem when applying compression has been related to breakage or
bending of the locking screws leading to revision.90,106
Newer nail designs have smaller (7-, 8-, or 9-mm) diameter implants
that are better suited to small canal diameters. The smaller sized
nails are usually solid, and they can often be inserted without
reaming. Nailing is then turned into a very quick procedure, an obvious
advantage especially when dealing with polytraumatized patients.
However, a major drawback with thinner nails is the reduced strength
and the risk of implant breakage.
 |
|
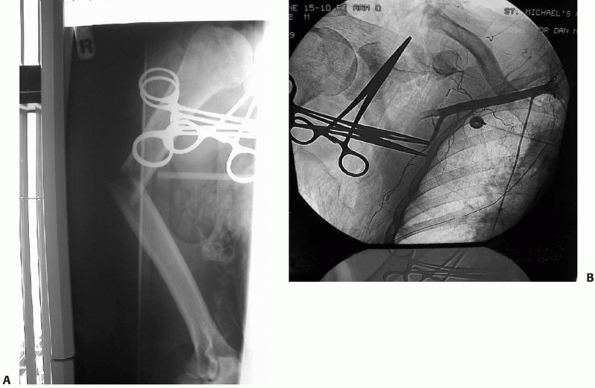
FIGURE 34-15 A.
Gross rotational instability was present in a 41-year-old woman 7 months after having undergone reamed antegrade intramedullary nailing of a humeral shaft fracture. The distal interference fit of the nail failed. B. The incisional scar can be seen on the anterior aspect of her shoulder (arrow). The patient is facing straight ahead. C. It is possible for the patient to externally rotate her lower arm over 90 degrees without changing the orientation of her shoulder at all: the rotation occurs completely through the rotationally unstable nonunion. The skin and soft tissue can be seen bunching up at the midhumeral level of the nonunion (small arrows). D. Union following nail removal, bone grafting, judicious humeral shortening, and blade plate fixation. |
position, with the affected arm draped free. The image intensifier is
brought in directly laterally on the injured side, and the patient
brought to the edge of the table, unless a radiolucent table is being
used. The head is taped in place on a pad. Before proceeding, it is
important to check and ensure a good radiographic picture of the entire
humerus is possible. It may be necessary to have the patient lying
partially off the table on a radiolucent support if the table is not
radiolucent. The surgeon stands at the top of the bed looking down on
the shoulder and the assistant stands below on the other side of the
image holding the arm. A small incision is made at the anterolateral
corner of the acromion, the deltoid is split, and any visible
subdeltoid bursa is excised. The supraspinatus tendon is identified,
and split for 1 to 2 cm in line with its fibers. The entry point for a
standard antegrade nail is in the greater tuberosity, just lateral to
the articular margin. A common mistake when learning nailing is that
the entry point can be placed too lateral in the humeral head due to a
feeling that the acromion prevents the surgeon
from
reaching the correct position. In order to avoid an iatrogenic fracture
of the proximal lateral cortex, it is crucial to reposition the
insertion guide or the awl until it is in the desired position. The
canal is then broached with either an awl or a starter reamer placed
over a guidewire. If a reaming technique is used, a long guidewire is
then passed to the fracture site, colinear with the medullary canal.
The fracture is reduced with gentle longitudinal traction and
manipulation, and the guidewire is passed across the fracture site.
Extreme difficulty in reducing the fracture and passing the guidewire,
especially in isthmal fractures, should alert the surgeon to the
possibility of soft tissue (radial nerve) entrapment. In technology
similar to that used for sciatic nerve monitoring during acetabular
fracture fixation, Mills et al.102
described using somato-sensory evoked potentials during closed humeral
nailing to detect nerve injury. They found that they could detect
signal change with impending nerve injury, and in at least one case,
this prompted an open procedure with the finding of radial nerve
entrapment in the fracture site.102
However, this technology is not widely used at this time. Even so, the
incidence of permanent radial nerve injury, despite its proximity to
the humeral shaft, is surprisingly low during closed nailing. If the
fracture is open, the fracture site should be inspected visually to be
sure it is free of soft tissue entrapment as the guidewire is passed.
Using a combination of fluoroscopy and arm rotation, the guidewire is
checked in two planes to ensure accurate placement.
 |
|
FIGURE 34-16
Fracture site distraction during intramedullary nailing. Since the humeral canal tapers to a blind end, if the nail is too long as it is impacted to avoid impingement in the shoulder (A), it abuts the end of the canal and distracts the fracture site (B). Fracture site distraction following humeral nailing is poorly tolerated and is a common finding in delayed unions and nonunions. |
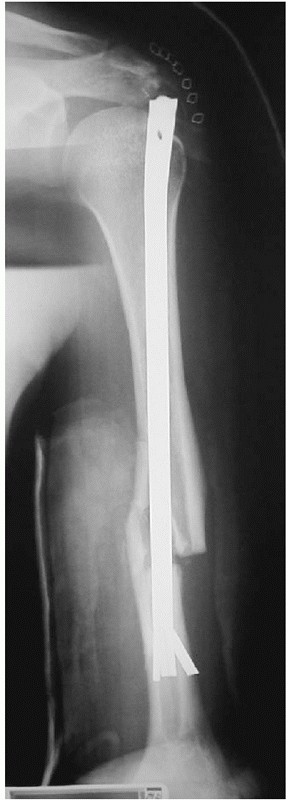
chatter is heard, and then insert a nail 1 to 1.5 mm smaller in
diameter than the last reamer used. Care is taken to keep the fracture
well reduced during the reaming process. The length of nail is
carefully chosen and checked twice: if too long a nail is picked, one
risks distracting the fracture site as the nail impacts the tapered end
of the humeral canal as it is advanced in an attempt to seat it below
the tuberosity proximally. Leaving the nail proud proximally will
result in an increased incidence and severity of impingement (Fig.
34-17). Biomechanic studies have shown that nails locked with screws
are axially and torsionally superior to so-called interference fit nail
designs (Fig. 34-18).10,92,173
The nail is then locked with screws using a jig proximally and a
freehand technique distally. Distal anteroposterior or oblique locking,
depending on the nail type used, is
done
through small open incisions; risk to the brachial artery, median
nerve, and musculocutaneous nerve is minimized by the use of an open
approach and by staying lateral to the biceps muscle and tendon. The
radial nerve is at risk during lateral to medial locking.14
While the nerve does not necessarily need to be seen and isolated, an
incision with blunt dissection to bone, clear visualization of the
tract and bony drill site, and use of a protective drill/screw
insertion sleeve is mandatory. Anatomic studies have clearly shown that
“blind” or percutaneous distal locking, as performed in the lower
extremity, is not safe in the humerus.41,119
Some authors advocate sectioning the coracoacromial ligament or
performing a modest acromioplasty before closure in attempt to minimize
impingement.126,127
While this may be useful in the occasional case, there is no convincing
evidence it is routinely successful. Any split in the rotator cuff
should be repaired. A standard dressing is applied (a splint is not
usually required), and the patient begins early active exercises under
the supervision of a physiotherapist.
 |
|
FIGURE 36-17
Anteroposterior radiograph following antegrade intramedullary nailing of a humeral shaft fracture. A number of sequential complications occurred: the distal fins of the nail deployed early, preventing proper seating of the nail and producing distraction at the fracture site. Thus, the surgeon was left with three choices: leave the nail proud at the shoulder, accept distraction at the fracture site, or restart with a shorter nail. The top of the nail was cut off but remained prominent causing intractable shoulder symptoms. Due to the prominence of the nail, the proximal locking screw could not be inserted, resulting in rotational instability. Multiple revision surgeries were the result. |
 |
|
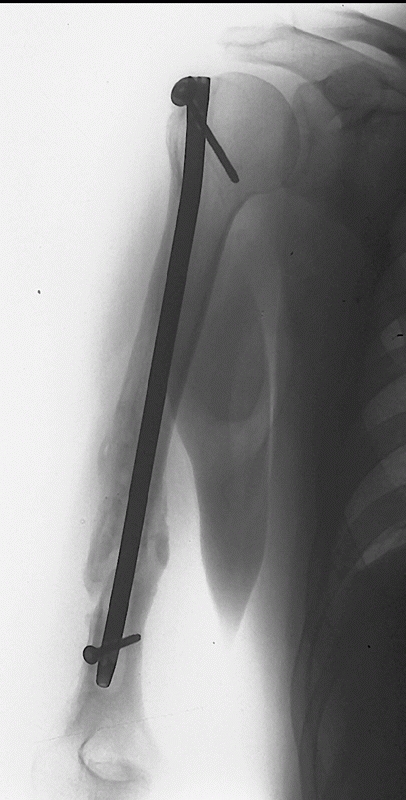
FIGURE 34-18
Uneventful union following a reamed, antegrade, interlocked humeral nail. Biomechanic and clinical data suggest that a device with both proximal and distal locking screws results in the best rotational and axial stability. |
middle and distal thirds of the humerus. The patient is placed in a
prone or lateral decubitus position with the arm over a bolster. The
image intensifier is brought in from the ipsilateral side, and again it
is important to ensure adequate imaging prior to proceeding. The arm is
draped free, and an incision is made in the posterior midline from the
tip of the olecranon proximally for 4 or 5 cm. The triceps is split
down to bone and the olecranon fossa identified. There are two
potential start points. The traditional one is in the midline, 2 cm
above the olecranon fossa; more recently, a start point through the
superior aspect of the olecranon fossa itself has been advocated. This
increases the effective working length of the distal segment, and
provides a straighter alignment with the canal; however, it results in
a greater reduction in resistance, especially to torque, compared to
the more superior portal. A biomechanic study by Strothman et al.155
showed a 29% reduction in load to failure for the superior portal and a
45% reduction for the olecranon fossa starting point. This weakness can
result in iatrogenic or postoperative fracture. Lin et al.89 changed their start point due to iatrogenic fracturing in a series of 39 retrograde nailings, and Rommens136
reported 2 such fractures in his series of 39 cases. The same
principles as with antegrade nailing apply for fracture reduction,
guidewire passage, reaming, and nail insertion.10
The nail should extend to a point where proximal locking can occur
without risk of damage to the axillary nerve: this leaves the typical
nail approximately 1.5 cm from the humeral head articular surface. The
nail must be locked. If not, backing out into the elbow joint with
subsequent loss of extension is possible. Prior to closure, it is
important to wash out any residual bony debris from the reaming
process: this may help prevent heterotopic ossification posteriorly (Fig. 34-19).
The triceps is closed with interrupted sutures, and a dressing is
applied. It is important to institute early elbow motion
postoperatively to prevent stiffness, a complication of this procedure.
Resisted extension should be avoided for 6 weeks postoperatively to
protect the repair of the triceps split.
-
Avoid antegrade nailing in patients with
pre-existing shoulder pathology or those who will be permanent upper
extremity weight bearers (para- or quadriplegics). -
Use a nail locked proximally and distally with screws: use a miniopen technique for distal locking for all screws.
-
Avoid intramedullary nailing in narrow diameter (<9 mm) canals: excessive reaming is not desirable in the humerus.
-
Choose nail length carefully, erring on
the side of a shorter nail: do not distract the fracture site by trying
to impact a nail that is excessively long. -
Insertion site morbidity remains a concern: choose your entry portal carefully and use meticulous technique.
 |
|
FIGURE 34-19
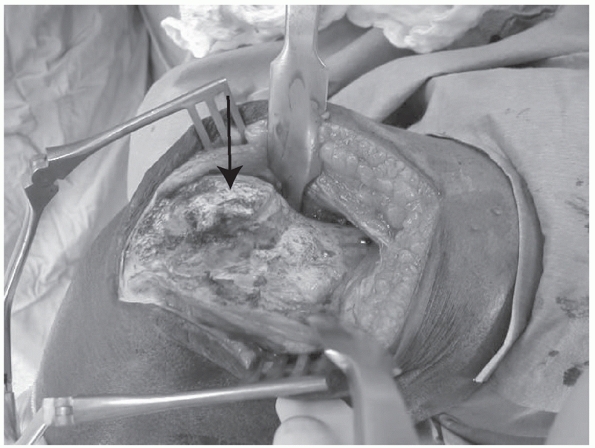
Elbow ankylosis following reamed, retrograde intramedullary nailing of a humeral shaft fracture. The intraoperative photograph is taken from a posterior approach, and the triceps has been split. The shoulder is to the right, the elbow is fixed at 90 degrees of flexion, and the forearm drops down to the left. The arrow marks a solid bridge of bone extending from the insertion point of the nail in the distal humerus to the olecranon. Resection of this osseous bridge followed by intensive physiotherapy resulted in a functional, but not normal, range of motion. |
fixation with a significant complication rate and has traditionally
been used as a temporizing method for fractures with contraindications
to plate or nail fixation.74,125 These include extensively contaminated or frankly infected fractures (Fig. 34-20),
fractures with poor soft tissues (such as burns), or where rapid
stabilization with minimal physiologic perturbation or operative time
is required (“damage-control orthopaedics,” see Chapter 9).29,104,139
Most information in the literature is contained in case series
describing the use of this technique for open humeral shaft fractures.
Choong and Griffiths29 treated seven
“complex” open humeral shaft fractures with external fixation, and four
went on to develop nonunion. Marsh and colleagues93
used a monolateral external fixator to treat 15 patients with an open
fracture of the humeral shaft. However, the complication rate was high:
four patients required additional procedures prior to healing including
two with breakage of the external fixator pins. Additionally, there
were eight patients who required treatment for pin tract infections.
Although Mostafavi and Tornetta105
had good or excellent results in 12 of 18 of their patients treated
with external fixation, the complication rate was high: three
malunions, one delayed union, eight pin tract infections (two with a
sequestrum), and two refractures following fixator removal. While these
results may indicate a selection bias, where only the worst injuries
with the most extensive soft tissue damage were treated with this
technique, in general, external fixation is cumbersome for the humerus
and the complication rate is high. This is especially true for the pin
sites, where a thick envelope of muscle and soft tissue between the
bone and the skin and constant motion of the elbow and shoulder
accentuate the risk of delayed union and malunion, resulting in
significant rates of pin tract irritation, infection, and pin breakage.
 |
|
FIGURE 34-20
A humeral external fixator applied following hardware removal, irrigation, and débridement of a humeral shaft fracture treated initially by cerclage wiring and complicated by deep Pseudomonas infection. Antibiotic impregnated cement beads have been applied to the fracture site. External fixation is a useful temporizing method in this situation: following clinical and biochemical evidence of infection eradication, plating and bone grafting resulted in rapid union. |
We prefer the anterolateral approach unless the fracture is so distal
that preoperative planning suggests it will not be possible to place
four screws distally; if this is the case, a posterior approach is
performed.107 When dealing with open
humeral shaft fractures, it is important to adhere to the principles of
open fracture treatment. The open wound is inspected, any obvious
debris (grass, clothing, dirt) is removed, and a sterile dressing is
applied. The arm is splinted and prophylactic antibiotics started
intravenously. Tetanus prophylaxis is given if required. The patient
should be transferred emergently to the operating room where any
associated life-threatening injuries take priority. Once these (or
other orthopaedic injuries of greater urgency) are dealt with,
attention is focused on the (open) humeral shaft fracture. Grossly
contaminated wounds are subjected to a nonsterile scrub with copious
sterile saline and a clorhexidene scrub brush. For the anterolateral
approach, the patient is positioned sitting up 30 to 45 degree
(favorable for polytrauma patients with abdominal, chest, or head
injuries), and the arm is prepared in sterile fashion and draped free.
An anterolateral approach is then performed (see “Surgical
Approaches”); any open wounds are débrided, and if possible, included
in the incision. The bone ends are exposed and thoroughly cleaned of
debris and hematoma, taking care to preserve any soft tissue
attachments to bony fragments. In open fractures, the medullary canal
is carefully inspected as occasionally ground debris can be impacted
far up the canal of the proximal fragment, especially in falls from a
height, and this material must be removed. Completely devitalized
cortical fragments are removed. For open fractures, the wound and
incision are then copiously irrigated with a minimum of 9 liters of
sterile saline. The radial nerve is identified distally between the
brachialis and the brachioradialis and protected throughout the case.
Care is taken not to pinch the nerve under the distal lateral corner of
the plate (see Fig. 34-8). If radial nerve
function is impaired or absent preoperatively, it is explored. This
protects the nerve from further damage and adds useful prognostic
information. If the nerve is found to be completely avulsed, the
epineurium at the ends are tagged with a nonabsorbable, colored suture,
for easier identification later, but primary repair (in blunt trauma
cases) is usually not indicated.46,76,118,128
across the main fracture line. For most individuals, a broad 4.5-mm
compression plate will be the implant of choice. The occasional
small-boned patient may require a narrow 4.5-mm plate. A minimum six
hole (with a lag screw) or eight hole (no lag screw) plate is contoured
and applied. If the bone is osteoporotic or otherwise weak, a locking
plate system should be considered. The fixation and radial nerve are
checked, and an intraoperative radiograph is taken. Adjacent muscle is
loosely approximated to obtain complete coverage of the plate, and a
standard closure performed: drains are not typically necessary. Any
open wounds are left open, and only the surgical extensions are closed.
A sterile dressing and well-padded posterior splint are applied. The
dressing and splint are removed at 48 hours postinjury. Wounds that
have residual necrotic tissue, appear infected, have exposed bone, or
require repeat débridement due to a high probability of residual
contaminated material are returned to the operating room for repeat
débridement and irrigation. The wound can then be closed in a delayed
primary fashion if possible, or allowed to granulate in and heal by
secondary intention. Larger defects may require a split-thickness skin
graft to accelerate rehabilitation. More extensive soft tissue defects
can be dealt with by a variety of local rotational (biceps) or pedicled
(latissimus) flaps. Patients are encouraged to begin early, active
motion of the shoulder and elbow under the supervision of a
physiotherapist. If upper extremity weight bearing is required, an
elbow gutter crutch is prescribed.
pathologic fractures, widely separate segmental fractures, or fractures
with soft tissue conditions that would preclude or complicate plating,
such as morbid obesity or local burns or some polytraumatized patients.
While either plating or nailing is acceptable for most patients with
humeral shaft fractures, we specifically avoid nailing in patients with
pre-existing shoulder pathology, those with narrow diameter humeral
canals (<9 mm), those who will be permanent upper extremity weight
bearers, and those with recognized radial nerve palsies. An antegrade
approach is used for midshaft or proximal fractures, and a retrograde
approach for distal fractures is ideal. We usually use a reamed nail
locked with screws proximally and distally, though a thinner nail
inserted without reaming is an option in selected cases. Reaming is
done sparingly, stopping at the sound or feel of cortical chatter, and
the nail inserted is 1 or 1.5 mm narrower than the last reamer. Great
care is taken not to distract the fracture site with nail insertion. It
is usually safer choosing a shorter rather than longer nail for this
reason (see Fig. 34-16). An open approach should be used for the distal locking screws to avoid damage to neurovascular structures.14,41,119 We no longer use small diameter flexible nails for humeral shaft fractures.
when rapid stabilization of the humerus is required, as in a critically
ill patient with multiple injuries or in a fracture associated with a
vascular injury where rapid stabilization can provide a stable platform
for emergent vascular repair.93,104,105,139
The other is when the associated soft tissue injuries or contamination
(Gustilo type III) are so severe that plate fixation would result in
exposed hardware or residual contamination. Using a large external
fixator set, two pins are inserted from an anterolateral direction
proximally and two pins distally (using a miniopen technique) and then
connected with two straight bars. However, our recent experience with
compression plating in these situations has been so favorable that the
indications for external fixation have become very limited.
Overall, they would suggest that the risk of shoulder pain is much less
in patients treated with plates (essentially 0% versus 5% to 42% with
antegrade nailing), and there is a trend towards a decreased nonunion
rate with plating. However, these studies, like all retrospective
studies, have inherent biases, including patient selection, a high lost
to follow-up rate, incomplete outcome data and surgeon bias. Randomized
clinical trials have been designed to overcome the biases inherent in
prior observational studies, and are the criterion standard for
evaluating treatment. There have been four randomized clinical trials
that have compared locked intramedullary nailing to compression plating.12,24,25,95
Although they are small in numbers, their design is solid and
represents the best information on this topic. A recent meta-analysis
conducted on three of those studies revealed that patients in the
plated group had a lower rate of reoperation (6% versus 18%, p = 0.03), and a lower rate of shoulder pain (1% versus 21%, p = 0.002).8
There were also more nonunions in the nail group (8/73, 11%) than in
the plate group (5/83, 6%), although this difference did not reach
statistical significance with the numbers available. These studies
certainly did not confirm the theoretical advantages of locked
intramedullary nailing of humeral shaft fractures and have
re-established compression plating as the treatment of choice for the
majority of these injuries (Table 34-3). In the
most recent paper which was not available at the time of the
meta-analysis, 47 patients were randomized to reamed nailing or DC
plating. The union rates were similar between the groups, although the
healing time was shorter in the intramedullary group. Shortening of the
arm and restriction of shoulder movement was less in the plated group.24
Thus, it is not surprising that the most common nerve palsy following a
humeral shaft fracture is a radial nerve palsy. This is typically due
to contusion and/or stretching of the nerve in the spiral groove at the
moment of fracture. There is limited “give” in the nerve as it is
tethered proximally as a terminal branch of the brachial plexus and
distally as it exits through the lateral intermuscular septum. The
incidence of radial nerve palsy is directly proportional to the degree
of violence of the initial trauma. It ranges from 3% to 34% and
increases with open fractures, polytrauma, vascular injury, and
multiple ipsilateral fractures.46,50,64,76,118,128,148
Tingstad et al.157 reported an incidence of radial nerve palsy of 34% in 111 fractures predominantly in polytrauma patients, and Connolly32
reported an incidence of 14 radial nerve injuries in 53 open fractures
(26%). Fortunately, most of these lesions are neuropraxic injuries of
the nerve: spontaneous recovery is the rule. Ogawa110 reported 100% recovery in one large series, Pollock118 reported a recovery rate of 90% in closed fractures, and Sarmiento143
reported a 100% recovery rate in 85 patients with distal humeral shaft
fractures. However, there is some evidence that the prognosis for
recovery with high-energy or open fractures is not as good. Sanders et
al.142 presented twelve cases of
open humeral shaft fractures associated with radial nerve injury; only
four recovered function. Ring et al.128
described six radial nerve transections in 24 patients with high-energy
humeral shaft fractures with associated radial nerve palsy. Connolly32
reported that four of fourteen radial nerve injuries in patients with
open fractures did not recover and required nerve grafting.
|
TABLE
34-3 Table of Advantages and Disadvantages of Plate Fixation, Intramedullary Fixation, and External Fixation of Humeral Shaft Fractures |
||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||
 |
|
FIGURE 34-21 An algorithm for the management of radial nerve palsy in association with a humeral shaft fracture.
|
lack of any evidence that early exploration improves matters), it is
clear that, in general, the presence of a radial nerve injury in
isolation is not an indication for operative management of a humeral
shaft fracture (Fig. 34-21). In a systematic
review of 1045 patients, it was concluded that based on available
published data there is no significant difference in the final result
of radial nerve palsy associated with fractures of the humeral shaft
treated with initial expectancy or early exploration.149
However, there are accepted indications for exploration of the radial
nerve in the setting of a humeral shaft fracture. If there
is
another indication for operative repair, such as an open wound or an
associated ipsilateral injury, then the injured nerve should be
explored for a number of reasons. First, it is of prognostic value.
While a contused or stretched nerve is the most likely finding, if a
completely severed nerve is found then this helps guide early nerve
reconstruction or tendon transfer after fracture healing.128,142
Secondly, the nerve may be injured due to entrapment in the fracture
site, compression by bony fragments, or other physical obstruction, and
thus it is prudent to release and protect the nerve during fracture
fixation. Third, in rare instances, primary repair of a completely
lacerated nerve may be indicated. In most cases, extensive contusion
and stretching make such an endeavor unrewarding, as the zone of injury
of the nerve is often indistinct and the chance of recovery slim. For
example, Ring et al.128 reported
that none of the five transected radial nerves repaired primarily in a
series of high-energy humeral shaft fractures recovered. However, in
instances where there is a clean laceration of the nerve from a bone
fragment or sharp instrument (such as a machete), then primary repair
may be successful, and Foster et al.45 reported good results with primary nerve repair (four of five repaired nerves recovered).
gunshot wounds may also recover spontaneously. Rather than being
transected by the projectile, in most cases the nerve has been damaged
by the concussive blast, shock-wave associated with the path of the
bullet, or contused by bony fragments and is structurally intact.145
given a cock-up wrist splint and instructed to perform daily passive
wrist, finger, and thumb range of motion exercises to prevent the
development of a flexion contracture. Some authorities recommend that
baseline electromyography and nerve conduction studies be performed at
3 weeks postinjury so that later studies can be compared to them.11,156
The patient is observed clinically for any sign of recovery of the
nerve, which is typically seen in the first muscles innervated distal
to the injury: the brachioradialis and extensor carpi radialis brevis
and longus. The electrical equivalent is the development of action
potentials where complete denervation or fibrillation was noted before.
One should expect to see nerve regeneration proceed at a rate of 1 inch
per month. If no electrical or clinical activity can be identified by 4
months postinjury (depending on the site of the fracture), then
surgical exploration of the nerve is indicated. Resection of the
damaged portion of the nerve followed by cable graft reconstruction is
usually required. If nerve reconstruction is unsuccessful or not
indicated, tendon transfers are an ideal procedure to restore function,
since the major contribution of the radial nerve to the hand is motor
function.
shaft fractures depends on a number of factors, including a compliant
patient, an upright position, the “hydraulic” effect of a wellfitted
splint, and the contraction of the associated muscles. Sarmiento points
out that the muscle contraction during activity when the patient is in
a splint helps to “align the fragments in a parallel direction,
correcting the malrotation.”145 In
patients with a significant brachial plexus palsy, this intrinsic
muscle tone and contraction may be lost, resulting in a much higher
incidence of malunion and/or nonunion (Fig. 34-22).
Also, the associated sensory loss may lead to ulceration of insensate
skin under a tight splint or cast. There is relatively little in the
literature regarding this topic, but Brien et al.16
reported on 21 cases of humeral shaft fracture with associated brachial
plexus palsy and reported superior results with plate fixation (three
of three united). Of the eleven fractures treated nonoperatively, there
were five nonunions, two delayed unions, and two malunions. The flail
extremity interfered with rehabilitation and led to prolonged hospital
stays. Additionally, only 2 of 21 patients showed any neurologic
recovery. The authors described a much higher incidence of associated
injuries in this patient population (eleven traumatic brain injuries),
which often represent independent indications for operative
intervention. Thus, an ipsilateral brachial plexus injury represents a
strong relative indication for primary plate fixation of an associated
humeral shaft fracture.
 |
|
FIGURE 34-22
A humeral pseudarthrosis 14 months following a fracture complicated by a severe brachial plexus injury. Lacking normal muscle tension and tone, these injuries have a high rate of delayed and nonunion. |
most common cause of a pathologic fracture of the shaft of a long bone
in an adult.78,82
The long bone most affected is the femur, followed by the humerus,
which accounted for 96 of 588 pathological fractures (16%) in one large
series.53 These fractures usually
occur late in the clinical course of the illness and are often a
significant source of pain. The associated functional loss can be
critically important in the typical older, debilitated patient and can
result in a decreased quality of life, dependency upon others, possible
hospitalization or institutionalization, and increased medical costs.
While aggressive surgical treatment is
not
usually indicated in the moribund, cachetic patient, judicious
intervention in a patient with a life expectancy measured in months or
greater is often beneficial from both a patient-oriented and societal
perspective.
shaft fractures from malignant lesions, conservative care (consisting
mainly of splinting and/or radiation) provides some symptomatic relief
but rarely provides fracture healing or stability. Douglass et al.37
reported dismal results in a series of patients with pathologic humeral
shaft fractures treated with radiation and immobilization. Pain relief
was evident in five of nine treated with radiotherapy and only 3 of 12
with immobilization. Flemming and Beals43
reported on eight such patients treated conservatively and described
poor pain relief with half having poor function. These unsatisfactory
results have led other investigators to pursue operative avenues of
treatment. For patients with a finite life expectancy, the ideal
treatment would stabilize the entire shaft of the humerus, be performed
through a small incision away from the metastatic disease (to allow
radiotherapy without affecting incisional healing), minimize bleeding
by not exposing the fracture site and tumor, and allow early motion and
rapid return of function. Fracture healing per se is not as important
as it is in a routine patient, and a stable mechanical construct that
will last a finite period of time is the main goal. This discussion
pertains to patients with established metastatic disease from an
incurable systemic cancer; resection and reconstruction of primary
malignant disease or an isolated metastasis with the potential for
long-term survival (such as renal cell carcinoma) is beyond the scope
of this chapter.
reported one of the first surgical experiences with a large number of
patients with pathologic humeral fractures, and reported good results
in 68 cases treated with Rush pinning and, in most cases,
polymethlymethacrylate cement augmentation. In a series that originated
from the Mayo Clinic, Lewallen et al.88 (and later Yazawa et al.170) described a similar technique with good results: 48 of 55 patients in Lewallen’s paper had good relief of pain. Lancaster83
used a larger diameter Kuntscher nail and reported pain relief in 26 of
29 patients. However, they also had to open the fracture site and add
polymethylmethacrylate cement to obtain rotational and axial stability.
This is undesirable from a number of standpoints including increased
blood loss, increased operative time, higher morbidity (from
infection), and, if radiation is to be used, wound healing
complications. The advent of larger diameter locking humeral nails has
dramatically improved the care of patients with pathologic humeral
shaft fractures. In a definitive article on the subject, Redmond et al.122
performed closed, locked humeral nailing on 16 patients and reported
good to excellent pain relief in all but 1 patient, with 14 extremities
returning to “near normal” function within 3 weeks. Radiation was
initiated at a mean of 7 days postoperatively in 14 humeri, with no
infections, nerve palsies, or implant failures, and only one
reoperation for the removal of a locking screw was required (Fig. 34-23).
The rotational and axial control obtained by the locking screws
eliminates the need to address stability at the fracture site directly.
Thus, radiotherapy can be initiated earlier because there is no
incision. In this specific situation, closed locked humeral nailing has
significant advantages over compression plating and should be
considered the treatment of choice for affected individuals.
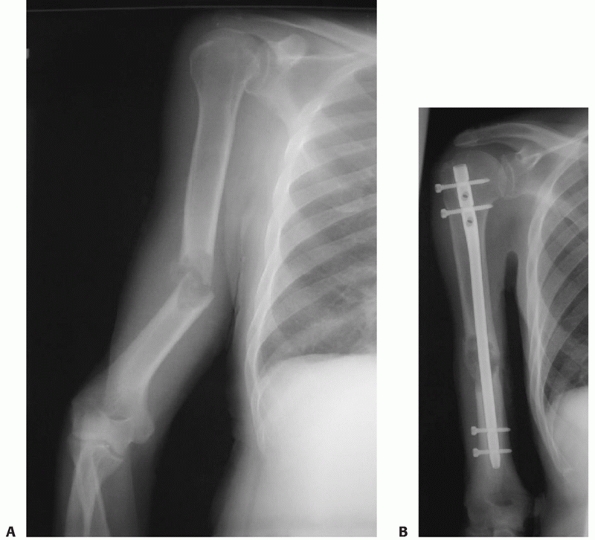 |
|
FIGURE 34-23 A. Pathologic humeral shaft fracture through a lytic defect caused by a deposit of metastatic carcinoma. B.
Treatment with a statically locked closed humeral nail resulted in immediate stability and good pain relief; since there was no incision over the fracture site, early radiation therapy could be initiated. (Courtesy of Dr. Ralph B. Blasier.) |
21 patients with 24 humeri fractured due to metastatic disease were
treated with an unreamed nail. In 5 fractures, polymethylmethacrylate
was used for augmentation. Pain relief was rated as good in 20
of their 21 patients. Bone union was achieved in 15 of the 17 patients who survived more than 3 months.
patients where tissue is required for diagnosis, or a definitive
resection of the tumor mass is planned to gain sufficient local control
to allow anticipated long-term survival (such as in isolated metastasis
from renal cell carcinoma). In this situation, the technique of Chin et
al.,28 with segmental resection
followed by metallic, cement, or allograft replacement and plating is
recommended. Fractures in the metaphyseal region that are not amenable
to stabilization with an intramedullary nail are best treated with
prosthetic reconstruction.
cysts or fibrous dysplasia) may weaken the bone sufficiently to result
in a fracture from minimal force or trauma. In most cases, despite what
may appear to be an unfavorable radiographic picture, closed treatment
will result in healing. Often, the healing osseous response will
obliterate or at least attenuate the lesion. Thus, the initial approach
for the majority of pathologic fractures from benign tumors is
nonoperative.
fractures, an open wound complicates treatment, and there is relatively
little specific information in the literature to aid in decision
making. The patient with an open humeral shaft fracture has often
sustained high-energy trauma and has an increased incidence of radial
nerve palsy, fracture comminution, ipsilateral upper extremity
fractures, and systemic injuries. Most information that is available is
contained in larger series on the treatment of humeral shaft fractures
that include a small percentage of fractures that had associated open
wounds, and a variety of treatment techniques have been described.7,45,94,99,157,163
reported on open reduction and internal fixation of 34 humeral shaft
fractures, 13 of which were open. Although all 13 were plated and
eventually healed without infection, only 5 had immediate plate
osteosynthesis. The remaining 8 were plated secondarily after
temporizing methods were used initially. In the only series dealing
exclusively with the issue, Connolly et al.32
reviewed 53 consecutive patients from a Level I trauma center managed
with immediate plating of open humeral shaft fractures. Three patients
underwent early amputation due to associated vascular injuries with
mangled extremities, 3 were lost to follow-up, and 1 died. Forty-six
patients were followed to definitive outcome, and they reported
excellent results with no deep infections, no malunions, and nonunions.
Forty fractures healed at an average of 18.6 weeks postinjury. There
were six delayed unions that healed on average at 10.1 months
postinjury, but none of these patients required other operative
procedures to obtain union. The authors stressed the importance of a
standard surgical protocol including thorough irrigation and
débridement, stable fixation with broad 4.5 mm compression plates, a
minimum of eight cortices transfixed proximal and distal to the
fracture, leaving the wound open, and prophylactic antibiotics. In most
cases, the plate was covered with local muscle and the wound allowed to
granulate closed in a delayed fashion.
described a series of 73 acute humeral shaft fractures treated with
antegrade nailing. Twenty-six of these fractures were open, and all
healed without infection following locked antegrade nailing, although
shoulder pain and impingement from prominent hardware was seen in over
10% of the patients. Sims and Smith152
treated 22 acute fractures with intramedullary nailing; of the four
open fractures, two went on to develop a nonunion. Rommens et al.136
reported on 39 patients with humeral shaft fractures treated with
locked retrograde humeral nailing. Four patients had open fractures.
Although one developed a nonunion, none became infected. Other large
series reporting on locked intramedullary nailing describe essentially
similar results. Proper technique in these series included a thorough
soft tissue irrigation and débridement, cleaning of the bone ends, open
passage of the reaming guidewire followed by closure of the deep muscle
layer to keep the osteogenic debris from the reaming around the
fracture site. Although there may be problems with insertion site
morbidity, it would appear that this technique has a low infection rate
when used for open fractures of the humeral shaft.
temporizing measure for severe open humeral shaft fractures with
extensive soft tissue loss. However, the complication rate, including
delayed and nonunion, is high. Choong and Griffiths29 reported a 57% nonunion rate (4/7), Marsh and colleagues93 had 4 reoperations in 15 cases, and Mostafavi and Tornetta105
had good or excellent results in only 12 of 18 (67%) of their patients
treated with external fixation. In general, this technique should be
considered a temporary measure prior to definitive treatment.
were able to follow 620 patients treated with functional bracing for
humeral shaft fractures at one of two university-affiliated hospitals
with a large, uninsured, primarily indigent patient base. Of these, 155
had open fractures that were either Grade I or II injuries (37
patients) or caused by low-velocity gunshots (118 patients). The
results in this series were remarkably good; the nonunion rate for open
fractures was only 6%. There were no deep infections, although there
were a significant number of patients lost to follow-up, and the
authors stress that the patients they reported on are a specific
subgroup. Individuals with multiple injuries (including ipsilateral
fractures), higher grades of soft tissue damage, fractures caused by
high-velocity gunshots, or with fracture complications were seen by
other orthopaedic surgeons and were excluded from the study. Ostermann
et al.112 reported on 191 patients treated similarly, and Zagorski171
described the results of 170 patients treated with functional bracing;
both had nonunion rates of 2%. Although there are fewer patients in
these studies, results were similar for the small percentage of
individuals that had (low-grade) open injuries.
humeral shaft fractures (especially those that have resulted from
low-velocity gunshots) can be managed safely and effectively with the
use of functional bracing. The comminution often seen in such injuries
is not, in isolation, an indication for surgical intervention.
However,
the most important aspect of electing to use this simple and
straightforward technique for an open fracture is proper patient
selection. Higher grades of soft tissue damage, polytrauma cases,
associated ipsilateral fractures, fractures from high velocity
gunshots, and fractures complicated by infection or malalignment in the
brace are not suitable.
fractures (especially in polytrauma patients), the issue of whether a
patient with a surgically repaired humeral shaft fracture can bear
weight on it to aid in mobilization arises frequently. While most cases
of fixation failure can be attributed to poor technique (i.e., too
short a plate), the potentially deleterious effect of increased force
from walking with a cane (25% body weight), a forearm crutch (45% body
weight), or an axillary crutch (80% body weight) is a concern. It was
originally felt that, similar to the lower extremity, a locked humeral
nail would be a “loadsharing” device better suited to withstand the
rigors of upper extremity weight bearing as compared to a
“load-bearing” device such as a plate.10,89,173
examined the effect of immediate weight bearing in 82 patients with
humeral shaft fractures treated with plate fixation. If necessary, due
to lower-extremity injuries, patients were allowed to use platform
crutches or a walker to ambulate. Ninety-four percent of the fractures
healed primarily, and immediate upper extremity weight bearing had no
effect on humeral fracture union, fixation failure, or alignment. The
authors stressed the requirement for adequate fixation with a minimum
of six cortices proximal and distal to the fracture site. Thus, it
would appear that it is safe to allow immediate upper extremity weight
bearing on a (properly performed) plated humeral shaft fracture. At our
institutions, an elbow gutter crutch is typically used in this
situation.
arthroplasty and the aging demographics of the population,
periprosthetic fractures of the humerus are increasing in incidence.15,23,77,108,166
In a clinical situation similar to the lower extremity, a long rigid
stemmed implant in an elderly, osteoporotic individual creates a stress
riser that can result in fracture, especially if the stem is loose or
complicated by osteolysis. Many of the treatment principles used in the
upper extremity are extrapolated from extensive experience with
periprosthetic femoral and tibial fractures. While not identical, this
information serves as a basis for treatment until more accurate data is
available. There are, however, some significant differences: There is
less bone stock in the humerus, infection rates are higher,
neurovascular structures are in closer proximity (and hence the
incidence of iatrogenic injury is higher), and cemented stems are the
standard.15,23,77,108,166
elbow arthroplasty has been estimated to be approximately 5%, and these
injuries have been classified by Morrey and O’Driscoll.108
Type I fractures are isolated fractures of the humeral condyles. Type
II fractures are around the stem of the humeral prosthesis and are
divided into those with well-fixed or loose stems (subdivided into
acceptable bone stock or severe bone loss). Type III fractures are
fractures proximal to the stem. Type I injuries do not typically
require active treatment, although they may be a harbinger of potential
future problems due to osteolysis. Type III and Type II fractures
associated with stable prostheses may be treated conservatively if
there is reasonable alignment and bony apposition, and the patient can
tolerate a prolonged period of time in a splint. Type II fractures
associated with a loose stem and/or significant bone loss (which
unfortunately is usually the case) require surgical intervention. In
the largest series to date, Sanchez-Sotelo et al.141
reported on 11 such patients treated with cortical strut allograft
augmentation and revision arthroplasty with a long stem prosthesis.
They found this procedure to be technically demanding and associated
with a high complication rate, but had union of 10 of 11 fractures. We
have had similar results with this method, which appears to be the
treatment of choice for these fractures (Fig. 34-24).
Technical points that are important include isolation and protection of
both radial and ulnar nerves, careful removal of the old implant and
cement (to prevent iatrogenic fracture), good fracture apposition (with
humeral shortening if necessary), bone grafting of the fracture site,
avoiding cement extrusion into the fracture site, and allograft strut
placement that ensures fracture stabilization and graft/cement
containment.
fractures following total shoulder arthroplasty, and the prevalence of
such injuries has ranged from 1.6% to 2.4%.15,23,77,166
As with other periprosthetic fractures, the quality of the bone,
location of the fracture, and implant fixation are the main
determinants of treatment and outcome. The available systems used to
describe periprosthetic humeral fractures classify fractures based on
the anatomic relation of the fracture to the stem.96
A Type A fracture occurs at the tip of the stem extending proximally,
Type B at the tip of the stem with or without some distal extension,
and Type C distal to the tip of the stem.166 The conservative treatment of Type C fractures is associated with a high success rate (Fig. 34-25). Campbell23
reported healing in four of four such fractures, and the high union
rate (five of six) of these fractures treated in a splint in the series
by Kumar et al. led the authors to state that, “For Type C fractures, a
trial of nonoperative treatment is recommended.”77 Type B fractures have a worse prognosis with conservative care. Six of seven did not heal in a series by Boyd et al.,15 and four of five did not heal in the series of Kumar et al.77
While a trial of conservative care in a well-aligned Type B fracture
with a stable component may be reasonable, a lack of clear progression
towards union should prompt operative intervention. Newer plates with
the ability to enhance fixation proximally with cerclage wire mounts
are useful in this regard. A fracture around a loose prosthesis should
be treated with a long stem revision, with autogenous bone grafting of
the fracture site. Type A fractures are usually associated with a loose
stem, and if this is the case, require operative intervention similar
to Type B fractures. In the unlikely event that the humeral component
is stable, a trial of splinting is indicated. Groh et al.51 reported on 15 fractures adjacent to humeral prostheses. Five type A fractures were
treated nonoperatively with a brace while another 10, of which 5 were
type C, were treated surgically with a long revision stem and cerclage.
All fractures progressed to union.
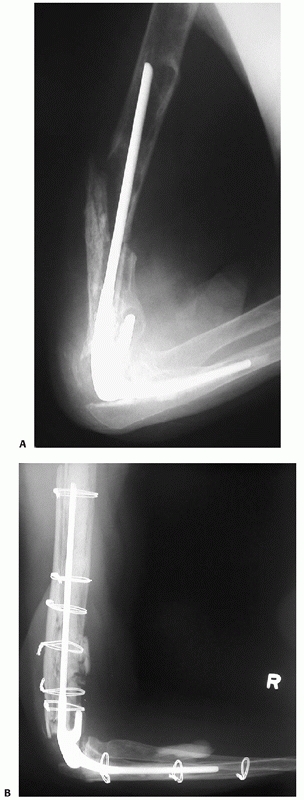 |
|
FIGURE 34-24 A.
Lateral radiograph of a periprosthetic humeral fracture after trivial trauma 5 years following cemented total elbow arthroplasty for a distal humeral nonunion in a 67-year-old woman. The osteolysis and loosening of the humeral component is obvious. Like the lower extremity, this represents a risk factor for fracture. B. Lateral radiograph 2 years following revision with implant removal, allograft cancellous grafting, long stem revision, and allograft cortical strut grafting secured with cerclage wires. (The ulnar component was also loose and was revised.) A good result ensued. |
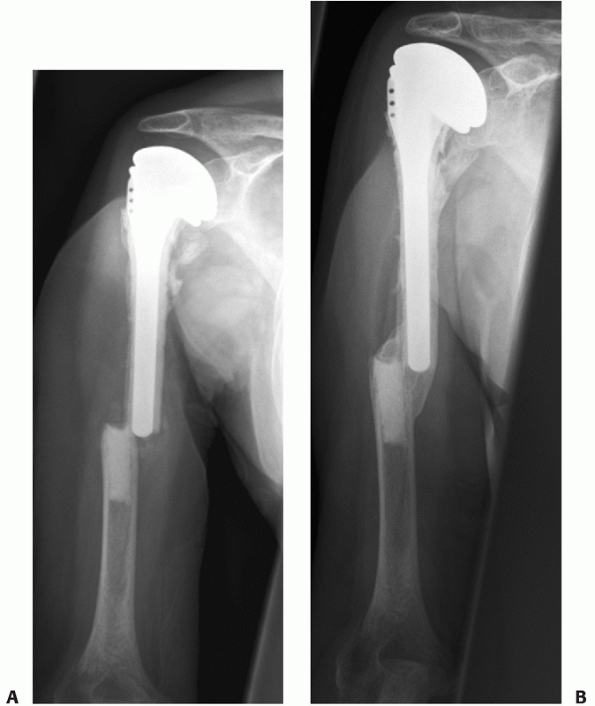 |
|
FIGURE 34-25 A.
65-year-old woman who 6 months following a hemiarthroplasty (due to a comminuted proximal humeral fracture) had a new fall and sustained a transverse fracture at the tip of the fixed stem. B. The fracture was treated with a functional brace. There was uneventful healing at about 9 weeks despite the presence of extensive cement at the fracture site. |
intramedullary nails, be it anterior knee pain following tibial nailing
or shoulder pain after antegrade humeral nailing. Despite variations in
insertion technique and implant design, persistent shoulder pain
remains a problem following antegrade humeral nailing. Modifications of
the insertion technique have ranged from mini incisions at the edge of
the rotator cuff in an attempt to avoid cuff damage altogether to
formal open approaches with partial anterior acromioplasty, division of
the rotator cuff, insertion of the nail, and subsequent cuff repair.34,68,126,127,134
The basic problem lies in the fact that the medullary canal is in line
with the articular surface of the humeral head and the rotator cuff,
and the insertion area is an anatomically limited one where any soft
tissue swelling, nail protrusion, or other perturbation can result in
painful impingement-like symptoms (see Fig. 34-17). In the initial
enthusiasm for this technique, the incidence of shoulder problems was
extremely variable, and, in general, underreported. Reimer126 reported excellent shoulder function
in 17 of 18 patients who underwent antegrade Seidel nailing. In 1991,
Habernak and Orthner (in their original series on Seidel nailing of
humeral shaft fractures) reported that, “all cases regained full
shoulder movement with no functional impairment by an average of six
weeks.”54
However, to their credit, the same authors later stated in a letter to
the editor of the journal in which their work was published regarding
antegrade humeral nailing through a standard portal, that, “This
inevitably leads to damage of the cuff…When we reviewed the 19 active
patients in 1991we did not address their shoulders and this should have
been addressed.”55 Subsequently,
other authors reported much higher rates of shoulder pain and
dysfunction following antegrade nailing including Robinson and his
coauthors134 (12 of 17 patients) and Ingman and Waters68 (100%) causing a change to the retrograde technique.
associated with the insertion of humeral nails is usually self-limiting
and temporary. Proper technique includes minimizing damage to the cuff
by protecting the edges during reaming, seating the nail well below the
osseous surface, removing all reaming debris, carefully repairing the
cuff upon completion of the procedure, and instituting early shoulder
motion. However, despite all precautions, shoulder pain may be severe
enough to warrant further surgical intervention, up to and including
acromioplasty, subacromial bursectomy, nail removal, and cuff repair.
Patients who will be permanent upper-extremity ambulators (such as
those who are rendered para- or quadraplegic in the accident that
causes their concomitant humeral shaft fracture) seem to be
particularly vulnerable to violations of their subacromial space and
rotator cuff. Intractable pain and impingement can be the result.
Antegrade humeral nailing is relatively contraindicated in such
individuals and in those with a prior history of impingement, and a
humeral shaft fracture should be plated if an indication for operative
intervention exists in these patients.
the insertion site morbidity seen with antegrade humeral nailing. One
was to adopt a retrograde technique, where the nail is inserted through
the olecranon fossa. There are several series that describe this
technique.10,68,89,136 However, complications include supracondylar fracture, elbow pain, triceps weakness, or elbow stiffness/ankylosis (see Fig. 34-19).
avoid the articular surface and rotator cuff by having alterations in
the proximal portion of the nail that allow an extra-articular start
point, analogous to the proximal bend in a tibial nail. A report in
2003 by Stannard et al.154 describes
a titanium, flexible locking nail with a unique mechanism. Inserted in
a flexible format, it allows an extra-articular starting point (1.5 cm
distal to the greater tuberosity for antegrade insertion) and is
tensioned by a screw in the end of the nail which stiffens the
intercalated segments, producing a rigid construct. This is then locked
in standard fashion. The Birmingham group reported on 42 treated with
this device, and reported an excellent union rate.154
Thirty-eight of 42 had no shoulder pain (mean constant score of 90
points, range 50 to 100). There were however, five major complications
requiring reoperation (nonunion, hardware failure, deep infection), all
associated with the smaller 7.5-mm version of the implant. The authors
cautioned that, considering these complications, “options other than
intramedullary fixation should be considered” in patients with canals 8
mm or less in diameter.154 Analogous
to previous reports of humeral nails however, these results need
further confirmation before the device can be recommended for general
use.
shaft fractures is the potential for fracture at the end of the plate
due to the stress-concentrating effect of the screws or refractures
following plate removal (Fig. 34-26).147
It was thought that the introduction of locking humeral nails would
lead to the elimination of this problem, as it has in the lower
extremity with locked femoral and tibial nails. However, the anatomy of
the distal humerus is fundamentally different from the tibia or femur;
these lower extremity bones have a wide metaphyseal flare. The
medullary canal of the distal humerus tapers rapidly to a narrow end
above the olecranon fossa.49,62
Thus, the tip of the nail ends in diaphyseal, rather than metaphyseal,
bone. The juxtaposition of a long rigid implant with locking screws at
the tip in diaphyseal bone leads to a significant stress riser effect,
especially if there is a transcortical hole drilled for a missed distal
locking screw. This is analogous clinically to the introduction of
short locking nails for intertrochanteric and subtrochanteric fractures
in the femur that were associated with a low but persistent incidence
of femoral shaft fracture at the tip of the implant.56,85 Similar fractures have been reported following locked humeral nailing.98
These fractures were reported 8 to 26 weeks after the humeral shaft
fracture had been treated. A sudden rotational motion was accompanied
by an audible crack associated with an oblique or spiral distal humeral
fracture at the distal end of the nail (Fig. 34-27).
A number of factors result in delayed healing in this situation
including the distal locking screw(s) and nail tip in the fracture
site, the “Holstein-Lewis” pattern of fracture and the poor natural
history of fractures at the end of long, rigid implants.15,23,64,87
The limited number of reported cases makes treatment recommendations
difficult. It would appear that these fractures are best treated with
repeat operative intervention where the distal locking screws (and
nail, if the shaft fracture is solidly healed) are removed and the
fracture site reduced and then internally fixed. If the nail is left in
place, then transcortical screws and/or cerclage wires will be required
to augment proximal fixation.
treatment. Nonunion following operative repair is often the result of
technical error, including inadequate plate size (see Fig. 34-13), fracture site distraction (see Figs. 34-15 and 34-16), inadequate screw purchase (Fig. 34-28), or mechanical failure from osteopenic bone.32,45,94,107,147
Thus, careful attention to surgical technique and proper implant choice
can reduce the incidence of nonunion. The treatment of nonunion is
based on establishing mechanical stability and stimulating nonunion
site biology.48,66,72,73,81,115,116,117,129,131,138,162,168
Open reduction and internal fixation with iliac crest bone grafting has
had consistently good results in the treatment of humeral shaft
nonunion, regardless of the cause. Ring et al.129 reported union in 24 of 25 humeral shaft nonunions treated in this fashion, and Otsuka et al.115
reported union in all 25 patients from a similar series. Proper
technique is essential and includes removal of all pre-existing
implants, débridement to bleeding healthy bone, re-establishing the
medullary canals of the fragments, correction of
deformity,
stable fixation with compression, and bone grafting. Bone grafting is
typically required for atrophic nonunions, as hypertrohic nonunions
have favorable biology and will generally heal with mechanical
stabilization alone. More recently, there has been increasing interest
in the use of demineralized bone matrix (DBM) or synthetic bone graft
substitutes in an attempt to eliminate the morbidity of autogenous bone
grafting. In a retrospective study by Hierholzer et al.,61
78 patients with atrophic delayed union or nonunion were treated with
open reduction and internal fixation with a plate. The first 45 also
had autologous bone from the iliac crest added while the last 33 had
DBM added instead of autologous bone. All procedures were done by the
same surgeon according to a strict protocol that was not altered during
the study period. All cases except one in the DBM group healed. The
authors concluded that the use of DBM prevented the 44% donor site
morbidity seen in the bone grafted group, while providing consistently
good healing. While there is some preliminary evidence that materials
such as bone morphogenetic proteins (BMPs, i.e., BMP-7 or BMP-2) will
provide sufficient biologic stimulus to produce union, it is important
to use them properly.164 They should form an integral part of a standard orthopaedic procedure (Fig. 34-29).
For patients with bone loss of more than 5 to 6 cm or those who have
failed conventional care, vascularized fibular grafting is a good
option; Jupiter71 reported healing in 4 patients treated in this fashion.
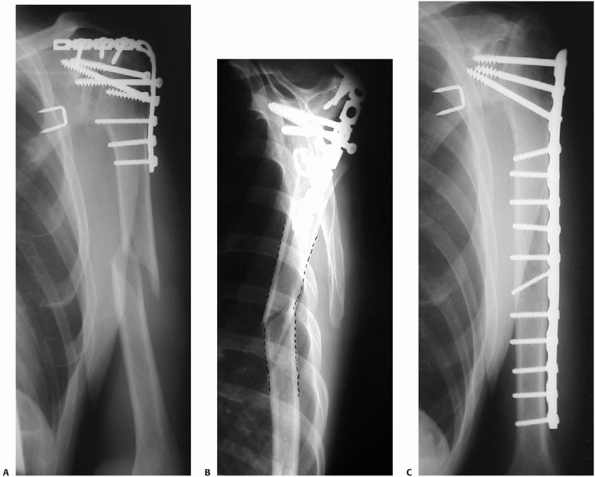 |
|
FIGURE 34-26 A.
A 43-year-old man had a successful glenohumeral fusion following multiple failed surgical procedures for shoulder instability with subsequent arthritic change. He fell approximately 9 months postoperatively and sustained a humeral shaft fracture below the fusion plate. B. The lateral radiograph demonstrates the extension deformity at the fracture site (dotted outline). Operative intervention was recommended for two reasons: the long lever arm and shoulder fusion meant that there would be excessive force and motion at the fracture site, making a delayed or nonunion more likely. Second, the fracture would be very difficult to control in a splint, and the extension deformity would result in significant loss of function since compensatory motion through the shoulder was not available. C. Anteroposterior radiograph following open reduction and internal fixation. The old plate was removed and a long, broad large fragment plate was used for fixation. Extra screws were placed distally due to the increased stress anticipated, and fixation extended proximally to protect the arthrodesis. |
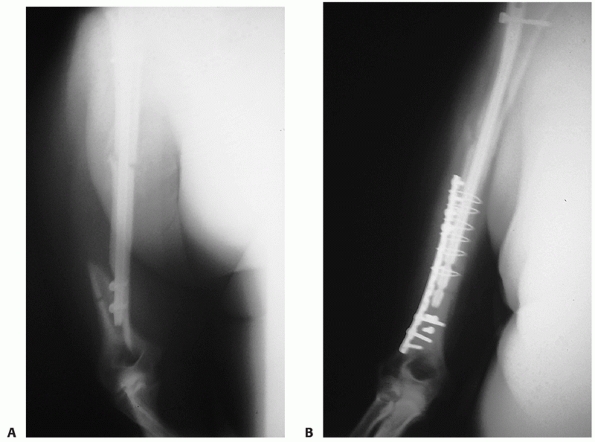 |
|
FIGURE 34-27 A.
A fracture occurred at the distal tip of this interlocking humeral nail following a rotational injury 8 weeks after intramedullary nailing for a humeral shaft fracture. B. The fracture was repaired by removal of the interlocking screws from the fracture site, reduction of the fracture, and plate fixation with a combination of screws and cerclage wires. |
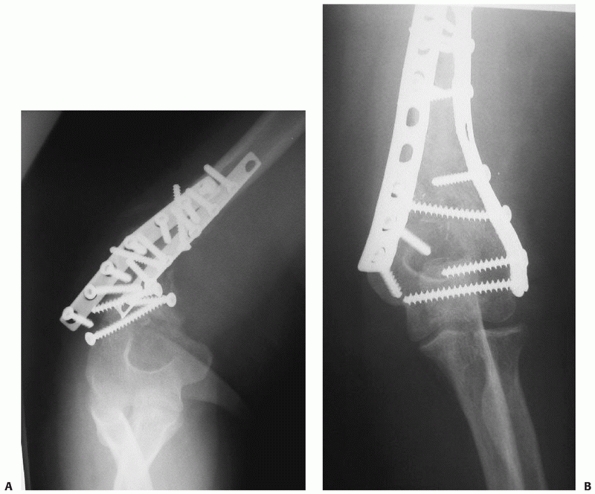 |
|
FIGURE 34-28 A.
Inadequate distal purchase was a major technical error in the surgical treatment of this distal humeral shaft fracture in an obese 36-year-old woman. Using only three screws with marginal purchase distal to the fracture site resulted in early mechanical failure with varus collapse and pain. B. Radiograph following hardware removal, realignment, autogenous bone grafting, and repeat fixation with a precontoured plate laterally and a compression plate medially. The fracture healed promptly but the patient had an iatrogenic radial nerve palsy postoperatively that lasted 4 months. Revision surgery for humeral fractures carries with it a higher rate of complications, especially nerve injury. |
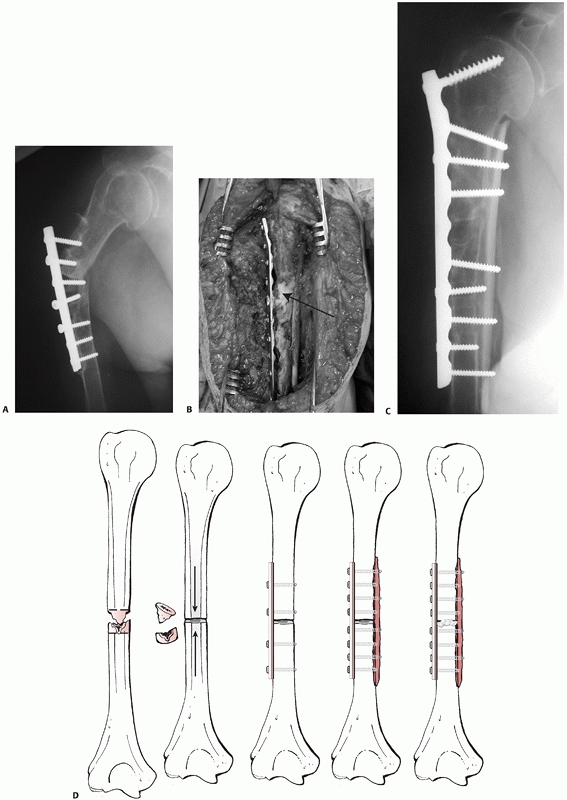 |
|
FIGURE 34-29
This 77-year-old patient had had two attempts at fixation of a nonunion following conservative treatment of a humeral shaft fracture. A. There are two main problems: one is mechanical, with obvious loss of fixation in very osteopenic bone, and the second is biologic, with no evidence of callous formation or healing, an atrophic nonunion. B. Intraoperative photograph after realignment, débridement of the nonunion, re-establishment of the medullary canals, and plate fixation with a broad 4.5-mm compression plate. Following provisional fixation of the plate with one screw proximally and distally, a cortical strut allograft was applied medially and the remaining screws were inserted into it, dramatically enhancing fixation. rhBMP-7 was applied to the nonunion site (arrow) instead of autogenous bone (both anterior iliac crests had been harvested previously). With a stable arm and no bone graft donor site, the patient left the hospital the day after surgery. C. A follow-up radiograph taken at 4 months postoperatively revealing solid bony union. D. The steps in the procedure are depicted. |
it as a cause of prior mechanical failure or as a result of prolonged
disuse. There are several options for dealing with this situation:
longer plates with more screws, cement augmentation of screw fixation,
blade plate fixation, and the use of Schuli nuts for plate fixation.22,72,116,131 Allograft can be used as intramedullary fixation or as cortical struts to aid in mechanical fixation (Fig. 34-29). Wright168 reported success in 17 of 19 patients augmented with intramedullary allograft fibular fixation; Van Houwelingen and McKee164
had union in all of six recalcitrant nonunions treated with onlay strut
allografts to augment screw fixation. More recently, Ring et al.130
reported on the use of a locking plate to enhance fixation in severely
osteoporotic elderly patients (mean age 72 years) and had success with
their primary procedure in 22 of 24 patients. This new technology has
great promise in the treatment of fractures or nonunions with poor bone
quality.
unique problem. Originally, it was thought that exchange nailing would
be routinely successful in this situation, just as it has been in the
lower extremity. However, McKee et al.97
found that exchange nailing was successful in only 4 of 10 such cases,
and then had better success with nail removal, compression plating, and
bone grafting (union in all nine cases). Similarly, Robinson et al.134
had success with exchange nailing in only two of five cases. Nonunion
associated with a loose nail is a difficult reconstructive problem and
requires longer plates, liberal bone grafting or inductive bone graft
substitute, and extensive dissection to remove the implanted nail. Wu
and Shih169 presented a comparative
study on the use of intramedullary nails versus plates in the primary
treatment of humeral nonunion, and found similar results in both groups
(89.5% union in the plate group, 87.5% union in the nail group).
However, if nailing is chosen as a treatment, bone grafting seems
advisable.
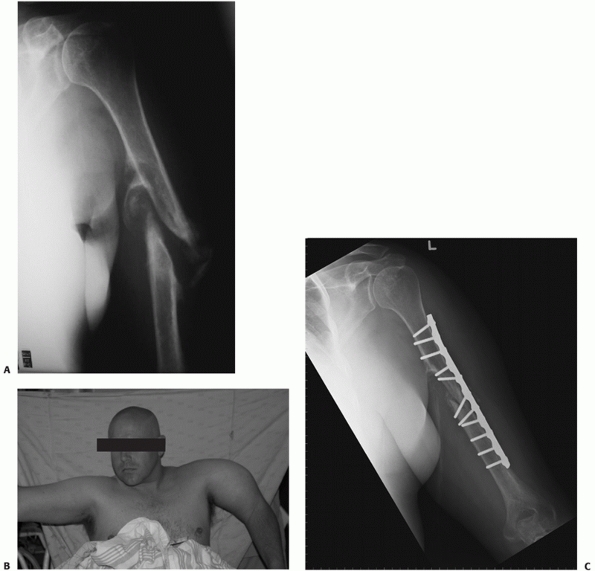 |
|
FIGURE 34-30
A synovial pseudarthrosis followed conservative treatment of a midshaft humeral fracture in a 38-year-old alcoholic who was noncompliant with treatment and did not wear his functional brace. Note the pseudoarticulation between the distal fragment and the reactive bone on the medial aspect of the proximal fragment. B. There was gross mobility at the nonunion site. This photograph also demonstrates why varus malalignment is common with these injuries. The intrinsic pull of the deltoid on the proximal fragment produces the typical deformity. Surgical tactics included excision of the interposed soft tissue and pseudocapsule, débridement of the bone ends to bleeding bone, re-establishment of the medullary canals with a drill, shortening, and reapproximation of the bony fragments. A broad, 4.5-mm plate was applied following lag screw fixation of the nonunion and rhBMP-7 was applied to the nonunion site to stimulate healing. C. Postoperative radiograph revealing excellent alignment and stable fixation. Early motion of the elbow and shoulder was allowed and union occurred. |
treatment can be due to soft tissue interposition, displaced transverse
fracture patterns, noncompliance with bracing (Fig. 34-30), unfavorable
body habitus (i.e., morbid obesity), or severe fracture angulation (Fig. 34-31). A true synovial pseudarthrosis can result (see Fig. 34-30), which requires extensive débridement at the time of surgical repair.
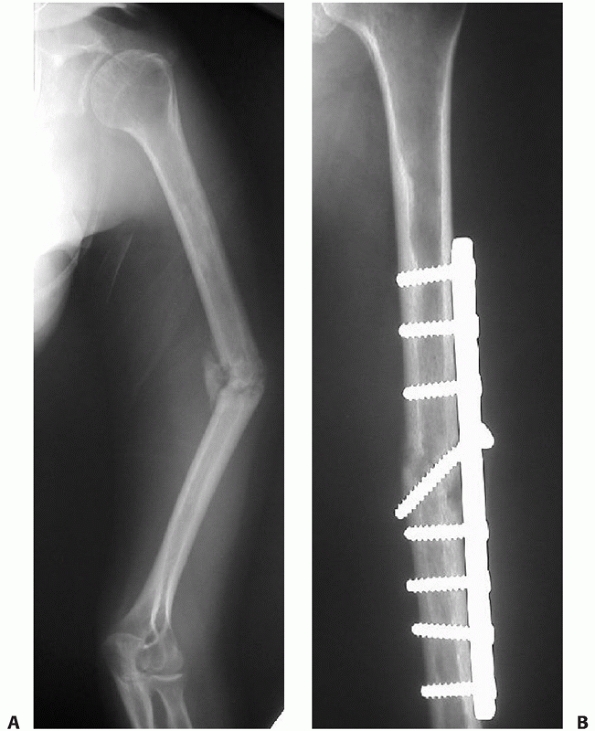 |
|
FIGURE 34-31 A.
Unacceptable varus deformity and nonunion in a 22-year-old female student 6 months following a humeral shaft fracture treated conservatively. While a fairly broad range of angulatory, translational, and rotational malunion can be accepted in the humeral shaft due to the compensatory motion of the shoulder and elbow, this degree of deformity has functional and cosmetic consequences. The lack of osseous union caused ongoing pain at the nonunion site. B. Uneventful union following open reduction, correction of deformity, compression plating, and addition of bone morphogenetic protein. |
upper extremity is relatively well-tolerated, compared to
lower-extremity nonunion, especially in elderly patients or those with
multiple medical comorbidities whose functional demands are limited.
However, Ring et al.132 showed that
a humeral pseudarthrosis can be very debilitating in elderly patients
whose independent functioning is often marginal and can often be the
difference between independent living and institutionalization. They
used the Enforced Social Dependency Scale to evaluate 22 elderly (mean
age 72 years) patients with humeral nonunion and found a mean value of
39 points (0 = complete independence, 100 = complete dependency). The
authors stated that 8 patients had been told there was nothing that
could be done surgically for their problem. Following successful
operative repair of the nonunion, the Enforced Social Dependency Score
decreased to a mean of 9 points, reflecting a dramatic improvement in
independent functioning. Recognition of the associated impairment and
knowing about the improved implants and techniques for the operative
repair of humeral nonunion in the elderly population can lead to a more
aggressive surgical approach to this problem; age alone should not be
used as a contraindication to fixation (Fig. 34-32).132
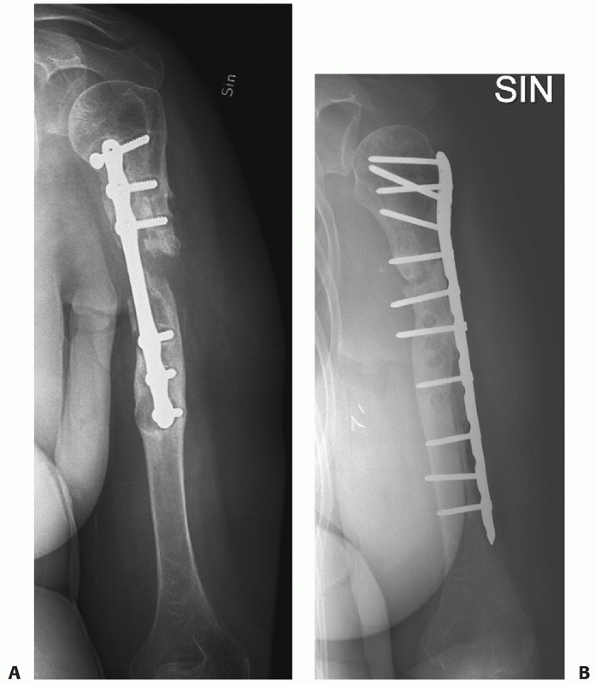 |
|
FIGURE 34-32 A.
This 63-year-old female sustained a closed humeral fracture which was treated primarily with a brace. She developed a nonunion, which was treated by plating and bone grafting at 9 months. Plate breakage 6 months later led to a reoperation with a new plate and another bone graft. This was complicated by postoperative infection. At time of referral, almost 2 years after the injury, the plate was loose with severe pain and instability. There was bone loss and no signs of healing. B. Five months following reoperation with slight shortening, revision of the sclerotic bone ends, and fixation with a new plate with compression and BMP to stimulate healing. Cultures were negative at time of revision. The fracture has healed uneventfully. |
-
Investigate for infection prior to initiating treatment
-
Some bone shortening (<3 cm), in order
to have apposition of healthy, viable bone ends, is acceptable in
treatment of nonunion of the humeral shaft. -
Correction of deformity and compression plating is the treatment of choice.
-
Add a bone graft or osteoinductive bone graft substitute to atrophic nonunions.
-
A variety of techniques are available to deal with osteoporotic bone.
soft tissue and muscle coverage, deep infection of the humerus is
relatively rare, even in open fractures treated aggressively with
immediate internal fixation. In our experience, infection in this
setting is usually related to underlying medical co morbidities,
especially diabetes or severe, mutilating injuries that are often near-amputations.
Cultures are taken to establish the causative bacteria, appropriate
antibiotics are administered intravenously, the patient’s medical
condition is optimized, and the infected fracture is débrided and
irrigated. If the implanted fixation is solid, it is left in place; if
not, it is removed and a spanning external fixator is applied (two pins
proximal, two distal). We have had excellent success in augmenting
standard techniques through the addition of an antibiotic impregnated,
osteoconductive bone substitute at the fracture or infection site. It
provided a very high local concentration of antibiotics and it then
contributed to eradicating infection in 23 of 25 patients.100
If necessary, serial débridements are performed. If the original plate
or nail has been left in place, the hardware can be removed following
union, if necessary. If an external fixator is used, repeat fixation
with bone grafting can be performed when the wound is clean and the
infection eradicated as evidenced clinically and microbiologically. In
recalcitrant cases, the Ilizarov technique has been described as a
useful salvage option.44,81,117,159
However, in the humerus it is associated with a high complication rate,
a prolonged period of time in the frame, and a significant risk of
refracture.
fractures can be treated nonoperatively with the expectation of a high
degree of success, the alert orthopaedic surgeon can also use the
interaction as an opportunity to intervene in other related health
areas, such as the recognition, diagnosis, and referral for treatment
of osteoporosis. There remains a consistent subset of these fractures
that will have improved outcomes with operative intervention. Open
reduction and compression plating has a consistent record of high union
rates, low complication rates, and rapid functional restoration that
makes it the standard against which other methods are compared. Locked
intramedullary nailing is an attractive option in select fracture types
or clinical situations. Future innovations, be they technique- or
implant-related, should be subjected to rigorous, prospective
randomized studies prior to universal acceptance or recommendation.
WC, Piotrowski G, Burstein AH, et al. Biomechanical principles of
intramedullary fixation. Clin Orthop Rel Res 1968;60:13-20.
T, Arpornchayanon O, Bavornratanavech S. Minimally invasive plate
osteosynthesis (MIPO) of the humeral shaft fracture. Is it possible? A
cadaveric study and preliminary report. Injury 2005;36(4):530-538.
K, Liebergall M, Sucher E, et al. Treatment of pathological shaft
fractures with unreamed humeral nail. Ann Surg Oncol
2007;14(4):1493-1498.
DM. Fracture of the humeral shaft associated with ipsilateral fracture
dislocation of the shoulder: report of a case. J Trauma 1971;11:532-534.
MJ, Beauchamp CG, Kellam JK, et al. The results of plating humeral
shaft factures in patients with multiple injuries. J Bone Joint Surg Br
1985;67:293-296.
M, Devereaux PJ, McKee MD, et al. Compression plating versus
intramedullary nailing of humeral shaft fractures—a meta-analysis. Acta
Orthop 2006;77(2): 279-284.
T, Levin R, Vrahas MS, et al. Nonsteroidal anti-inflammatory drugs and
nonunion of humeral shaft fractures. Arthritis Rheum 2005;15(3):364-367.
J, Machemer H, Baumgart F, et al. Biomechanical comparison of bending
and torsional properties in retrograde intramedullary nailing of
humeral shaft fractures. J Orthop Trauma 1999;13:344-350.
SC, Lieber RL. Peripheral nerve physiology, anatomy, and pathology In:
Simon SR, ed. Orthopaedic Basic Science. Rosemont IL: AAOS,
1994:325-396.
LE, Iaquinto JA, Vasicek V. Operative treatment of humerus shaft
fractures: a prospective randomized study comparing intramedullary
nailing with dynamic compression plating. Presented at the Annual
Meeting of the American Academy of Orthopaedic Surgeons 1995.
L. Fractures of the shaft of the humerus. In: Chapman MW, ed. Operative
Orthopedics. Vol 1. Philadelphia: J.B. Lippincott, 1988:221-234.
CM, Grossman MG, Hochwald N, et al. Radial and axillary nerves.
Anatomic considerations for humeral fixation. Clin Orthop Rel Res
2000;373:259-264.
WW, Gellman H, Becker V, et al. Management of fractures of the humerus
in patients who have an injury of the ipsilateral brachial plexus. J
Bone Joint Surg Am 1990;72(8):1208-1210.
MR, O’Connor DP. The incidence of fractures and dislocations referred
for orthopaedic services in a capitated population. J Bone Joint Surg
Am 2004;86:290-297.
RJ, Bosse MJ, Poka A, et al. Intramedullary stabilization of humeral
shaft fractures in patients with multiple trauma. J Bone Joint Surg Am
1986;68:960-969.
RJ, Jones AL. Interobserver agreement in the classification of open
fractures of the tibia. The results of a survey of 245 orthopedic
surgeons. J Bone Joint Surg Am 1994;76:1162-1166.
TA, Hughes MS, Anglen JO. Heterotopic ossification prophylaxis with
indomethacin increases the risk of long-bone nonunion. J Bone Joint
Surg Br 2003;85(5):700-705.
U, Jacob R, Macnab I, et al. Use of polymethyl methacrylate to enhance
screw fixation in bone. J Bone Joint Surg Am 1975;57:655-656.
JT, Moore RS, Iannotti JP, et al. Periprosthetic humeral fractures:
mechanisms of fracture and treatment options. J Shoulder Elbow Surg
1998;7:406-413.
M, Jain UK, Keswani T. Comparison of the use of the humerus
intramedullary nail and dynamic compression plate for the management of
diaphyseal fractures of the humerus. A randomized controlled study. Int
Orthop 2007;31(3):391-395.
JR, Henley MB, Agel J, et al. Randomized prospective study of humeral
shaft fracture fixation: Intramedullary nails versus plates. J Orthop
Trauma 2000;14: 162-166.
HC, Frassica FJ, Hein TJ, et al. Metastatic diaphyseal fractures of the
shaft of the humerus. The structural strength evaluation of a new
method of treatment with a segmental defect prosthesis. Clin Orthop Rel
Res 1989;248:231-239.
S, Nair R, Waddell JP, et al. Immediate plate osteosynthesis of open
fractures of the humeral shaft. Proceedings of the 55th Canadian
Orthopaedic Association Annual Meeting, Edmonton, Alberta, Canada, June
3-6, 2000.
SR, Lindsey R, Leggon R, et al. Dynamic compression plate fixation: a
biomechanical comparison of unicortical and bicortical distal screw
fixation. J Orthop Trauma 1988;2:146-150.
HO, Shukla SK, Mindell E. Treatment of pathological fractures of long
bones excluding those due to breast cancer. J Bone Joint Surg Am
1976;58(8):839-847.
R, Adami J, Tidermark J, et al. Fractures of the shaft of the Humerus.
An epidemiological study of 401 fractures. J Bone Joint Surg Br
2006;88(11):1469-1473.
R, Tidermark J, Törnkvist H, et al. Outcome after closed functional
treatment of humeral shaft fractures. J Orthop Trauma
2006;20(9):591-596.
AF, Schemitsch ED, McKee MD. Complications of intramedullary nailing
for fractures of the humeral shaft: a review. J Orthop Trauma
1999;13:258-267.
RL, Gleis GE, Seligson D. Diagnosis and treatment of complications:
fractures of the diaphyseal humerus. In: Browner BD, Jupiter JB, Levine
AM, et al, eds. Skeletal Trauma. 2nd ed. Toronto: WB Saunders Co.,
1998:567-578.
T, Ristiniemi J, Pajala A, et al. Salvage of humeral shaft nonunion
with cortical thinning after failed intramedullary nailing using
Ilizarov’s technique: a report of seven cases. Injury
2005;36(10):1246-1251.
RJ, Dixon JL, Bach AW, et al. Internal fixations of fractures and
nonunions of the humeral shaft. J Bone Joint Surg Am 1985;67:857-864.
PV, MacDonald DA, Matthews SJ, et al. Nonunion of the femoral
diaphysis: The influence of reaming and non-steroidal anti-inflammatory
drugs. J Bone Joint Surg Br 2000;5:655-658.
DRJ, Torchia ME. The spiral compression plate for proximal humeral
shaft nonunion: A case report and description of a new technique. J
Orthop Trauma 1999;13(2): 141-144.
PR. Fractures of the humeral shaft. In: Bucholz RW, Heckman JD, eds.
Rockwood and Green’s Fractures in Adults. 5th ed. Philadelphia:
Lippincott Williams & Wilkins, 2001:973-996.
GI, Heckman MM, Wirth MA, et al. Treatment of fractures adjacent to
humeral prostheses. J Shoulder Elbow Surg 2008;17(1):85-89.
RB, Anderson JT. Prevention of infection in the treatment of 1025 open
fractures of the long bones. Retrospective and prospective analysis. J
Bone Joint Surg Am 1976; 58:453-458.
KD, Sim FH, Enis JE, et al. Methylmethacrylate as an adjunct in
internal fixation of pathological fractures. Experience with 375 cases.
J Bone Joint Surg Am 1976;58:1047-1055.
MB, Chapman JR, Claudi BF. Closed retrograde Hackethal nail
stabilizaton of humeral shaft fractures. J Orthop Trauma 1992;6;18-24.
MB, Monroe M, Tencer AF. Biomechanical comparison of methods of
fixation of a midshaft osteotomy of the humerus. J Orthop Trauma
1991;5:14-20.
C, Sama D, Toro JB, et al. Plate fixation of ununited humeral shaft
fractures: effect of type of bone graft on healing. J Bone Joint Surg
Am 2006;88(7):1442-1447.
FJ, Zych GA, Hutson JJ, et al. Salvage of humeral nonunions with on lay
bone plate allograft augmentation. Clin Orthop Rel Res 2001;386:203-209.
U, Schlicht W, Outzen S, et al. Ultrasound in the diagnosis of
fractures in children. J Bone Joint Surg Br 2000;82:1170-1173.
R, Podworthy N, Hupel TM, et al. Influence of plate design on cortical
bone perfusion and fracture healing in segmental tibia fractures. J
Orthop Trauma 1999;13: 178-186.
R, Luo CF, Zeng BF, et al. Minimally invasive plating for complex
humeral shaft fractures. Arch Orthop Trauma Surg 2007;127(7):531-555.
JB. Complex nonunion of the humeral diaphysis: treatment with a medial
approach, an anterior plate, and a vascularized fibular graft. J Bone
Joint Surg Am 1990; 72:701-707.
M, Michaelson M, Waisbrod H. The use of external skeletal fixation in
the treatment of fractures of the humeral shaft. Injury 1977;9:245-248.
S, Sperling JW, Haidukewych GH, et al. Periprosthetic humeral fractures
after shoulder arthroplasty. J Bone Joint Surg Am 2004;86(4):680-689.
JR, Lewis RJ. Closed intramedullary rodding of pathological fractures
with supplemental cement. Clin Orthop Rel Res 1984;188:183-186.
J, Bauduin G, Dreisen R, et al. Treatment of nonunion of the humerus
using the Ilizarov external fixator. Clin Orthop Rel Res
1998;353:223-230.
KS, So WS, Shen WY, et al. Gamma nails and dynamic hip screws for
peritrochanteric fractures: a randomized prospective study in elderly
patients. J Bone Joint Surg Br 1992;74:345-351.
M, Hertel R. Thermal necrosis after tibial reaming for intramedullary
nail fixation. J Bone Joint Surg Br 1996;78(4):584-587.
DG, Berry DJ. Periprosthetic fracture of the femur after total hip
arthroplasty: treatment and results to date. Instr Course Lect
1998;47:2443-2449.
RP, Pritchard DJ, Sim FH. Treatment of pathologic fractures or
impending fractures of the humerus with Rush rods and
methylmethacrylate. Experience with 55 cases in 54 patients 1968-1977.
Clin Orthop Rel Res 1982;166:193-198.
J, Inoue N, Valdevit A, et al. Biomechanical comparison of antegrade
and retrograde nailing of humeral shaft fracture. Clin Orthop Rel Res
1998;351:203-213.
SA, Meyers K, Borens O, et al. Biomechanical evaluation of an
expandable nail for the fixation of midshaft fractures. J Trauma
2007;63(1):103-107.
RG, Brien D, Buckley RE, et al. Fixation of fractures of the shaft of
the humerus by dynamic compression plate or intramedullary nail: a
prospective randomized trial. J Bone Joint Surg Br 2000;82:336-339.
MD, Miranda MA, Reimer BL, et al. Management of humeral nonunion after
the failure of locking intramedullary nails. J Orthop Trauma
1996;10:492-499.
MD, Pedlow FX, Cheney PJ, et al. Fracture below the end of locking
humeral nails: a report of three cases. J Orthop Trauma
1996;10(7):500-504.
MD, Seiler J, Jupiter JB. The application of the limited contact
dynamic compression plate in the upper extremity: an analysis of 114
cases. Injury 1995;26:661-666.
MD, Wild LM, Schemitsch EH, et al. The use of an
antibiotic-impregnated, osteoconductive, bioabsorbable bone substitute
in the treatment of infected long bone defects. J Orthop Trauma
2002;16(9):622-627.
JJ, Brief DK, Stremple JF, et al. Management of fractures with
associated arterial injury in combat casualties. J Trauma 1973;13:17-19.
WJ, Chapman JR, Robinson LR, et al. Somatosensory evoked potential
monitoring during closed humeral nailing: a preliminary report. J
Orthop Trauma 2000;14(3): 167-170.
W J, Hanel DP, Smith DG. Lateral approach to the humeral shaft: an
alternative approach for fracture treatment. J Orthop Trauma
1996;10:81-86.
T, Diefenbeck M, Sorkin AT, et al. Results of the T2 humeral nailing
system with special focus on compression interlocking. Injury
2008;39(3):299-305.
PJ, Guy P, Blachut P. Humeral shaft fractures-open reduction internal
fixation. In: Wiss D, ed. Master Techniques in Orthopedic
Surgery-Fractures. Philadelphia: Lippincott-Raven, 1998:63-80.
SW, Spinner RJ, McKee MD, et al. Tardy posterolateral instability of
the elbow due to cubitus varus. J Bone Joint Surg Am
2001;83(9):1358-1369.
K, Ui M. Humeral shaft fracture sustained during arm wrestling: report
on 30 cases and review of the literature. J Trauma 1997;42(2):243-246.
PA, Ekkernkamp A, Muhr G. Functional bracing of shaft fractures of the
humerus—an analysis of 195 cases. Orthop Trans 1993-1994;17:937.
Classification: Fracture and Dislocation Classification Compendium.
Orthopedic Trauma Association Committee for Coding and Classification.
J Orthop Trauma 2007; 21(10 suppl):1-161.
Trauma Association. Fracture and Dislocation Classification Compendium
—2007. Available at: http://www.ota.org/compendium/compendium.html.
Accessed June 24, 2009.
NY, McKee MD, Liew A, et al. The effect of comorbidity and duration of
nonunion on outcome after surgical treatment for nonunion of the
humerus. J Shoulder Elbow Surg 1998;7:127-33.
SH, Handley R, Willett K. The use of interlocked customized blade
plates in the treatment of metaphyseal fractures in patients with poor
bone stock. Injury 2000; 31:187-191.
VR, Menon DK, Pool RD, et al. Nonunion of the humerus after failure of
surgical treatment: management using the Ilizarov circular fixator. J
Bone Joint Surg 2000; 82(7):977-983.
FH, Drake D, Bovill EG, et al. Treatment of radial neuropathy
associated with fractures of the humerus. J Bone Joint Surg Am
1981;63:239-243.
AM, Nanu AM, Cross AT. Windows for humeral interlocking nails—an
anatomical study. J Bone Joint Surg Br 1997;79(Suppl 1):102.
A, Furner S, Rice DP. Musculoskeletal Conditions in the United States.
Rosemont, IL: American Academy of Orthopaedic Surgeons, 1999.
JW. Delayed union of humeral shaft fractures treated by closed flexible
intramedullary nailing. J Bone Joint Surg Br 1985;67:715-718.
BJ, Biermann JS, Blasier RB. Interlocking intramedullary nailing of
pathological fractures of the shaft of the humerus. J Bone Joint Surg
Am 1996;78(6):891-896.
BL, Foglesong ME, Burke CJ, et al. Complications of Seidel nailing of
narrow diameter humeral diaphyseal fractures. Orthopaedics
1994;17:19-29.
PT, Falez F. The treatment of diaphyseal fractures by functional
bracing. Italian J Orthop and Traumatology 1985;11:199-205.
NM, Metz CW, Hutton JE, et al. Internal versus external fixation of
fractures with concomitant vascular injuries in Vietnam. J Trauma
1971;11:463-473.
BL. Humeral shaft fractures-intramedullary nailing. In: Wiss D, ed.
Master Techniques in Orthopedic Surgery—Fractures. Philadelphia:
Lippencott-Raven, 1998: 81-94.
BL, D’Ambrosia R, Kellman JF, et al. The anterior acromial approach for
antegrade intramedullary nailing of the humeral diaphysis. Orthopedics
1993;16: 1219-1223.
D, Chin K, Jupiter JB. Radial nerve palsy associated with high-energy
humeral shaft fractures. J Hand Surg Am 2004;29(1):144-147.
D, Jupiter JB, Quintero J, et al. Atrophic ununited diaphyseal
fractures of the humerus with a bony defect. J Bone Joint Surg Br
2000;82(6):867-871.
D, Kloen P, Kadzielski J, et al. Locking compression plates for
osteoporotic nonunions of the diaphyseal humerus. Clin Orthop Rel Res
2004;245:50-54.
D, McKee MD, Perey BH, et al. The use of a blade plate and autogenous
cancellous bone graft in the treatment of ununited fractures of the
proximal humerus. J Shoulder Elbow Surg 2001;10:501-507.
D, Perey B, Jupiter J. The functional outcome of operative treatment of
ununited fractures of the humeral diaphysis in older patients. J Bone
Joint Surg Am 1999;81: 177-190.
E. Carlos. Compression plating versus Hackethal nailing in closed
humeral shaft fractures failing nonoperative reduction. J Orthop Trauma
2000;14: 162-166.
PM, Verbruggen J, Broos PL. Retrograde locked nailing of the humeral
shaft fractures. J Bone Joint Surg Br 1995;77:84-89.
RE, Chrissos MG, Ebraheim N. The risk of neurovascular injury with
distal locking screws of humeral intramedullary nails. Orthopedics
1996;19:593-595.
J, O’Driscoll S, Morrey BF. Periprosthetic humeral fractures after
total elbow arthroplasty: treatment with implant revision and strut
allograft augmentation. J Bone Joint Surg Am 2004;84(9):1642-1650.
R, Yach J, Dipasquale T, et al. Radial nerve palsy associated with
humeral fractures. Presented at the 16th Annual Meeting of the
Orthopaedic Trauma Association, San Antonio, Texas, October 12, 2000.
A, Horowitch A, Aboulafia A, et al. Functional bracing for comminuted
extra-articular fractures of the distal third of the humerus. J Bone
Joint Surg Br 1990; 72(2):283-287.
A, Kinman PB, Calvin EG, et al. Functional bracing of fractures of the
shaft of the humerus. J Bone Joint Surg Am 1977;59:596-601.
A, Zagorski JB, Zych G, et al. Functional bracing for the treatment of
fractures of the humeral diaphysis. J Bone Joint Surg Am
2000;82:478-486.
P, Krettek C, Rudolf J, et al. Outcome of tibial shaft fractures with
severe soft tissue injury treated by unreamed nailing versus external
fixation. J Trauma 1995; 39(4):707-711.
J. Fractures of the humerus. In Schatzer J, Tile M, eds. The Rationale
for Operative Fracture Care. 2nd ed. Berlin: Springer-Verlag,
1996:83-94.
EH, Bhandari M. Fractures of the diaphyseal humerus. In: Browner BD,
Jupiter JB, Levine AM, et al, eds. Skeletal Trauma. 3rd ed. Toronto: WB
Saunders Co., 2001:1481-1511.
YC, Harwood P, Grotz MR, et al. Radial nerve palsy associated with
fractures of the shaft of the humerus: a systematic review. J Bone
Joint Surg Br 2005;87(12): 1647-1652.
KA, Gerber C. The reproducibility of classification of fractures of the
proximal end of the humerus. J Bone Joint Surg Am 1993;75(12):1751-1755.
P, Jobard D, Bistour L, et al. Complications of Marchetti locked
nailing for humeral shaft fractures. Int Orthop 1999;23:320-324.
JP, Harris HW, McGwin G Jr, et al. Intramedullary nailing of humeral
shaft fractures with a locking, flexible nail. J Bone Joint Surg Am
2003;85(11):2103-2110.
D, Templeman DC, Varecka T, et al. Retrograde nailing of humeral shaft
fractures: a biomechanical study of its effects on the strength of the
distal humerus. J Orthop Trauma 2000;14:101-104.
MR, Villasana DR. Neurologic evaluation of the upper extremity. In:
Kasdan ML, ed. Occupational Hand and Upper Extremity Injuries and
Diseases. Philadelphia: Hanley and Belfus, 1991:115-130.
EM, Wolinsky PR, Shyr Y, et al. Effect of immediate weight-bearing on
plated fractures of the humeral shaft. J Trauma 2000;49(2):278-280.
S, Bumbasirevic M, Lesic A, et al. Ilizarov frame fixation without bone
graft for atrophic humeral shaft nonunion: 28 patients with a minimum
2-year follow-up. J Orthop Trauma 2007;21(8):549-556.
G, Walls N, McQueen MM. The epidemiology of humeral shaft fractures. J
Bone Joint Surg Br 1998;80(12):249-253.
SE, Grundnes O, Reikeras O. Effects of degrees of remaining on healing
of segmental fractures. J Bone Joint Surg Am 1998;12:192-199.
VD, Michailov P. High-energy shock waves in the treatment of delayed
and nonunion of fractures. Int Orthop 1991;15:181-184.
der Griend RA, Tomasin J, Ward EF. Open reduction and internal fixation
of humeral shaft fractures. J Bone Joint Surg Am 1986;68:430-433.
Houwelingen AP, McKee MD. Treatment of osteopenic humeral shaft
nonunion with compression plating, humeral cortical allograft struts
and bone grafting. J Orthop Trauma 2005;19(1):36-42.
AM, Williams JR, Carr AJ. Interobserver and intraobserver variation in
classification systems for fractures of the distal humerus. J Bone
Joint Surg Br 2000; 82:636-642.
RL, Kim DY, Arrendondo J. Periprosthetic humeral fractures: management
and classification. J Shoulder Elbow Surg 1999;8:590-594.
P, Stower M, Barbor P. Patterns of fractures in accidental and
nonaccidental injury in children: a comparative study. British Med J
1986;293:100-102.
TW, Miller GJ, Vander Griend RA, et al. Reconstruction of the humerus
with an intramedullary fibular graft. J Bone Joint Surg Br
1993;75:801-807.
CC, Shih CH. Treatment for nonunion of the shaft of the humerus:
comparison of plates and Seidel interlocking nails. Can J Surg
1992;35:661-665.
Y, Frassica FJ, Chao EYS, et al. Metastatic bone disease. A study of
the surgical treatment of 166 pathologic humeral and femoral fractures.
Clin Orthop Rel Res 1990; 251:213-219.
JB, Latta LI, Zych GA, et al. Diaphyseal fractures of the humerus.
Treatment with prefabricated braces. J Bone Joint Surg Am
1988;70(4):607-610.
A, Bingfang Z, Yeming W, et al. Minimally invasive plating
osteosynthesis (MIPO) of middle and distal third humeral shaft
fractures. J Orthop Trauma 2007; 21(9):628-633.
MC, Waite Am, Deehan M, et al. A biomechanical analysis of four humeral
fracture fixation systems. J Orthop Trauma 1994;8:233-239.
