FREE TISSUE TRANSFER
Fellow in Wound Healing and Plastic Surgery, Division of Plastic,
Reconstructive, Maxillofacial, and Oral Surgery, Duke University
Medical Center, Durham, NC, 27710.
Assistant, Division of Plastic, Reconstructive, Maxillofacial, and Oral
Surgery, Duke University Medical Center, Durham, NC, 27710.
Professor of Plastic and Orthopaedic Surgery, Chief—Division of
Plastic, Reconstructive, Maxillofacial, and Oral Surgery, Duke
University Medical Center, Durham, NC, 27710.
technique used to transfer tissue from one location in the body to
another using the operating microscope and techniques of microvascular
surgery to perform small vessel anastomoses. Free flaps include
isolated transfer, composite tissue transfer, and functioning free
muscle transfer. Structural transfers such as vascularized bone grafts
or toe
transplantation
for hand reconstruction are also included in this group of procedures.
Specific tissue transfers such as vascular and neural grafts are also
an integral part of the microsurgical reconstruction armamentarium.
Although such “grafts” do not involve large amounts of tissue, they are
considered tissue transplantation and thus are included here in the
discussion of free tissue transfer.
than three decades ago with the introduction of the operating
microscope for anastomosis of blood vessels, described by Jacobson and
Suarez (50). Microsurgical repair of digital arteries and digital replantation began in the 1960s (16,55),
followed by microsurgical composite tissue transplantation in the
1970s. Microsurgeons expanded their efforts from achieving tissue
survival to improving function as well as appearance in the 1980s. In
the 1990s, the emphasis shifted to outcome. Today, composite
transplantation or free tissue transfer routinely not only provides
coverage but facilitates function.
vital role it plays in orthopaedic surgery is credited to the efforts
of many investigators who identified new donor sites, expanded
indications for free tissue transfer, and constantly improved
microsurgical techniques. In particular, the contributions of Mathes
and Nahai (82) in summarizing the clinical
application for muscle and musculocutaneous flap should be
acknowledged, as well as the pioneering work of Ian Taylor (108), Harry Buncke (17), Harold Kleinert (55a), Robert Acland (2a), Bernard O’Brien (88a), and Fu Chan Wei (113a).
in all fields of their practice. For example, the simultaneous
management of fractures and associated soft-tissue injury—the so-called
orthoplastic approach—is now accepted treatment for extremity trauma.
It allows optimal repair processes to take place for bone and soft
tissue while avoiding the adverse sequelae of failed fixation, sepsis,
and ultimately, amputation. The orthopaedic trauma surgeon should
appreciate the importance of anatomy, specifically being cognizant of
intravenous planes and vascular territories (angiosomes) during
dissection (108). Delicate soft-tissue handling
and an awareness of the blood supply to muscle, fascia, and skin will
prevent the orthopaedist from inadvertently damaging tissue, which may
result in necrosis and exposure of either bone or implants that
requires coverage with free flaps. Expeditious repair of soft tissue is
important in the care of the injured extremities. It facilitates
further reconstruction, such as bone grafting or tendon transfers.
includes understanding the rationale for tissue transplantation, the
timing of the transplant, and what transplant should be selected. The
orthopaedic surgeon should have an understanding of the current
techniques of reconstructive microsurgery and be able to obtain
appropriate reconstructive consultation for patients.
tissues and bone that are nonviable, with hope of preventing infection.
Before the era of free tissue transfer, debridement was often limited
because removing tissue of questionable viability led to the exposure
of vital structures such as bone. Surgeons were reluctant to make the
traumatic defects larger by “radical debridement.” Now, in contrast,
free tissue transfer of large, well-perfused flaps is readily available
and allows radical debridement and necrectomy. Debridement is required
both in acute trauma situations and for chronic wounds that have
resulted from either the improper handling of soft tissue or infection.
-
In a fresh wound (such as a Gustilo 3B or
3C tibial fracture), perform the debridement with the tourniquet either
up or down. Our preference is to begin the debridement with the
tourniquet up because hemorrahge and oozing can stain tissue, making it
difficult to distinguish viable tissue from nonviable tissue that has
been exposed to adjacent bleeding. Also see Chapter 12. -
Decide what should be debrided according
to the appearance and consistency of the different tissues. Healthy
tissue in the exanguinated extremity is bright and homogenous in color.
Damaged tissue has foreign bodies, irregular tissue consistency, and an
irregular distribution of dark red stains, indicating hematoma or
contusion. Remove all nonviable tissue. The border of the debridement
should include healthy tissue. Remove avulsed skin and muscle from the
base of avulsed flaps. -
Let down the tourniquet and assess
contractility, bleeding, color and consistency of tissue such as muscle
tissue to determine viability of tissue. -
Wash exposed bone with antibiotic solution. Free bone fragments are usually removed.
-
Ligate severed vessels and, if they are
not vital, excise to normal appearing margins. If they are vital,
restore continuity with interposition vein grafts. -
Nerves are the only structures in which
the debridement is more conservative. The epineurium can be carefully
removed, with fascicles remaining intact.
normal tissue planes (Fig. 35.1; see also COLOR FIG. 35.1).
Excise all scar tissue. Bone debridement can be difficult, because it
can be challenging to distinguish the viable bone and healthy callus
from necrotic and inflamed areas. Techniques such as computed
tomography (CT) scans, bone scans, and magnetic resonance imaging (MRI)
may be helpful in preoperative planning for bone debridement.
 |
|
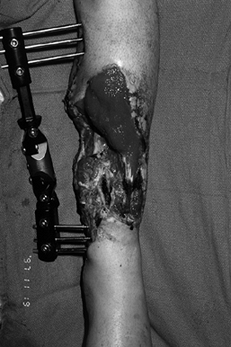
Figure 35.1. (See COLOR FIG. 35.1).
Chronic wound following a tibial 3B injury. Proximal muscle is covered with granulation tissue. There is evidence of desiccated, infarcted tendon and chronic granulation tissue in the base of the wound. This wound required extensive debridement and free flap reconstruction. |
planes should be visualized. If this is not possible, then a
second-look procedure in which the debridement process is repeated is
strongly advised. Re-debride no later than 48 hours, and preferably at
24 hours after the initial debridement (65).
modern reconstructive plastic surgery, used the motto “replace like
with like.” The interpretation of this principle with regard to free
tissue transfer is that the reconstructive microsurgeon transplants
autogenously vascularized tissue into defects that are the result of
trauma, tumor, infection, or congenital defects.
deficit that cannot be treated by an adjacent tissue rearrangement,
skin grafting, or local pedicle flaps. Select free tissue
transplantation in instances in which there are “composite
deficiencies,” such as skin and bone or muscle and skin. Furthermore,
free tissue transplantation may be considered for functional
restoration that obviates the need for tendon transfer or nerve
grafting, as in brachial plexus injuries, in which free muscle
transplantation is performed for functional muscle restoration.
orthopaedic surgery, one must understand the concept of the
“reconstructive ladder” (Fig. 35.2). It
represents an increasingly complex solution for soft-tissue problems,
with the ultimate goal being reconstitution of the soft-tissue
envelope. The reconstructive algorithm, or ladder, is used to select
treatment for damaged soft tissues. This algorithm should be used in
the setting of acute or chronic soft tissue injury, with or without
fractures. In addition, it can be applied to chronic conditions such as
osteomyelitis, nonunion, or tumors (63).
 |
|
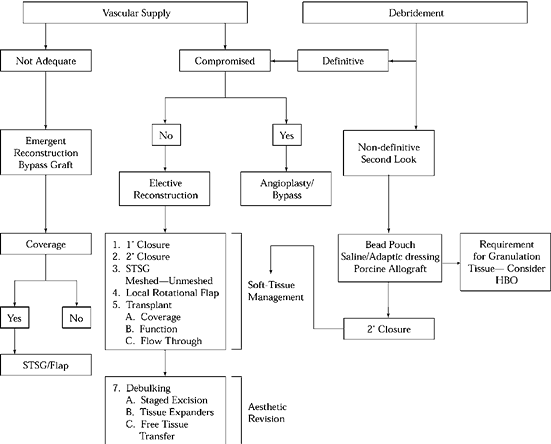
Figure 35.2. Reconstructive Ladder. HBO, hyperbaric oxygen.
|
tissue transfer, adjacent tissue transfer, regional tissue transfer, or
free tissue transfer, which represents the most complex rung on the
reconstructive ladder and is the subject of this chapter. As
microvascular success rates have improved over the last three decades,
the certainty in which tissue can be transplanted to provide coverage
as well as function has been enhanced to an expected success rate of
more than 95%. Free flaps facilitate solutions to complex soft-tissue
problems, with the ultimate goal being reconstitution of the
soft-tissue envelope.
reconstructive ladder. After adequate debridement, it may be possible,
such as in minor hand injuries, to close a wound primarily. This is
known as primary closure (the first rung). This is rarely done in the
treatment of open fractures because of associated soft-tissue loss, the
degree of contamination, or the possible uncertainty as to the adequacy
of debridement.
considered for reasons such as returning to the operating room in 24 to
48 hours after edema subsides, or if there is uncertainty about the
safety of the wound closure (gunshot wounds or farmyard injuries). A
wound may be left open to heal by epithelialization and wound
contraction, as in the so-called secondary intention healing. This is
the third rung of the reconstructive ladder. This may be applied to
small donor areas, such as for skin grafts, or in abrasions over muscle
compartments that have small areas that can rapidly epithelialize,
obviating the need for skin grafts.
of split or full-thickness skin grafts, either meshed or unmeshed. Skin
grafting successfully closes many wounds in the extremities,
particularly those that are beyond primary or delayed primary closure.
The wound bed must be well vascularized with a smooth pink bed of
granulation tissue for the graft to take. Grafts will work on fat,
muscle fascia, and even intact periosteum. It is important that wound
beds be cleaned before grafting to avoid flap loss by infection.
Immobilization of the graft is crucial for a good “take of the graft.”
exposed vital structures such as nerves and arteries, bone that is
devoid of periosteum, or those that have insufficient vascularity and
soft tissue to support skin grafts—require the importation of
well-vascularized tissue to achieve wound closure. This may be
accomplished by either local or distant flaps, which are the next rungs
of the reconstructive ladder.
combination of these types, can provide much-needed vascularized tissue
and allow dead space to be obliterated and the wound to be closed
without tension. These options may be limited because of the wound
location or regional donor site deficiencies. In this instance, free
tissue transplantation must be considered.
tissue transfer, possibly in combination with lower rungs of the
ladder, such as skin closure, skin grafting, or rotational flaps. An
example would be the use of a soleus myoplasty in the middle third of
the tibia as well as requirements
for distal third of the coverage. A muscle free flap, such as the latissimus dorsi, could be considered in such a case.
reconstructive surgeon may go from one level to another and, in many
instances, may simultaneously use techniques from different levels of
the ladder for different problems. Familiarity with options on the
reconstructive ladder will help the reconstructive surgeon plan limb
salvage and avoid the adverse sequelae that results from improper
soft-tissue handling.
reconstruction is that they allow early mobilization of the hand
following injury. This feature decreases limb edema and postoperative
stiffness. Free flaps allow the possibility of composite tissue
reconstruction in one stage by transferring various combinations of
nerve, bone, tendon, skin, and muscle at once. Free flaps can be
contoured and cut to fit any defect precisely. Most important,
microvascular free tissue transfers have enabled us to adhere more
closely to one of the basic principles of any reconstruction effort, as
set forth by Gillies—“replace losses in kind”—by allowing us to
reconstruct these defects with tissue that is most similar to the lost
tissue.
Trauma to the hand requires resurfacing using skin, muscle, or free
fascial flaps. In the treatment of upper extremity trauma, patients who
cannot be treated by conventional techniques such as skin grafting,
local flaps, or distant flaps are candidates for free tissue transfer.
When possible, it is desirable to import tissue rather than to use
island pedicle flaps, which will further compromise upper extremity
vascularity. For example, the radial forearm flap, if used for dorsal
hand coverage, renders an already compromised limb more compromised.
This method requires sacrifice of the radial artery. A fibula
osteoseptocutaneous flap can also be used in cases of trauma of the
upper extremity (Fig. 35.4; see also COLOR FIG. 35.4C).
 |
|
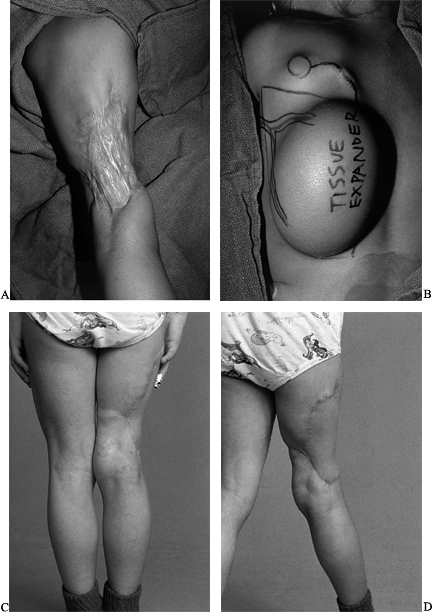
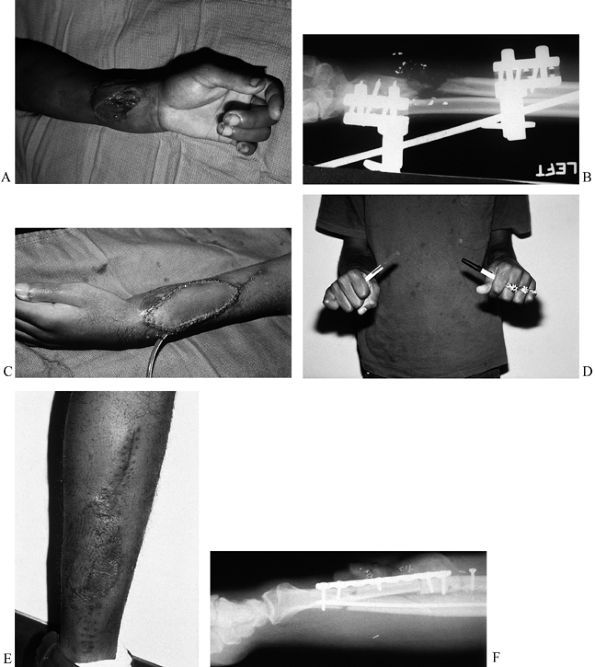
Figure 35.3. (See COLOR FIG. 35.3A.) (A) Gunshot wound to upper extremity with soft-tissue defect and exposure of tendons. (B) The patient required skin flap coverage. (C) An anterolateral thigh flap was selected for coverage. Donor site after skin grafting. (D–F) Function of extremity at 1 year.
|
 |
|
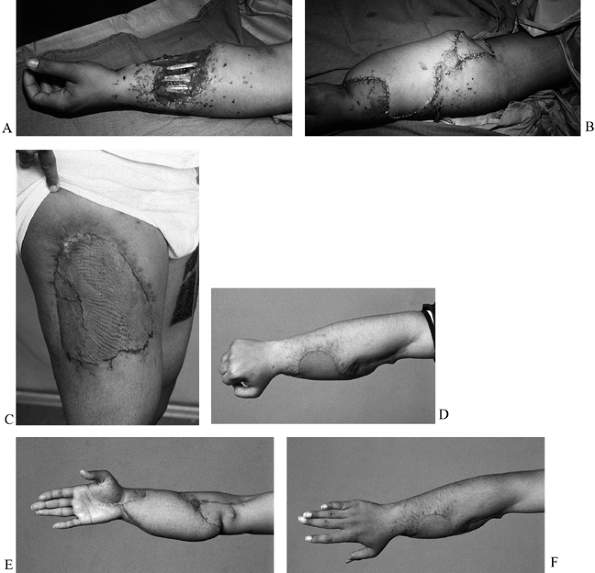
Figure 35.4. A: (See COLOR FIG. 35.4C.) Gunshot wound to the distal forearm. The patient originally had debridement and skin graft coverage to the forearm. B: On the night of injury, the patient was treated with an external fixator. C: He subsequently had bony reconstruction with an osteocutaneous fibula transfer. D: Pronation. E: Donor defect is acceptable, with the skin graft covering the distal leg. F: Final radiograph with fibula in place.
|
resections are best treated with free tissue transfer; specifically
free muscle, skin, and free bone flaps (Fig. 35.5; see also COLOR FIG. 35.5B).
 |
|
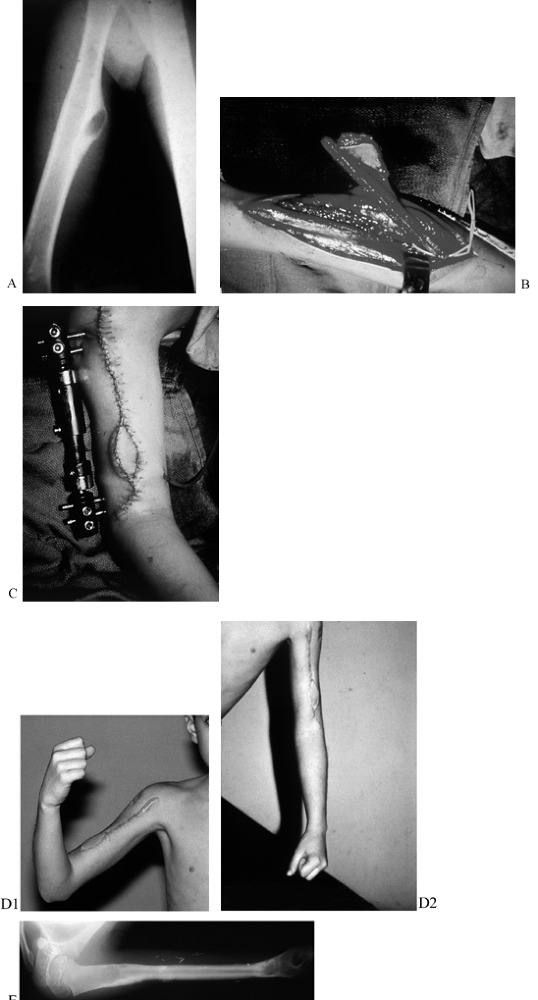
Figure 35.5. Chondrosarcoma of the humeral shaft in a 9-year-old boy. A: A radiograph shows an eccentric lesion. B: An osteocutaneous fibula was taken with a small monitor paddle to ensure vascularity of the bone. C: An external fixator was used to hold the osteocutaneous fibula in place. D: Final function at 1 year. (See COLOR FIG. 35.5.) E: Radiographs at 1 year showing hypertrophy of the fibula graft and preservation of growth plates.
|
large intercalary segments either in the humerus or the forearm. The
vascularized fibula transplant is an excellent method for
reconstruction of such defects. Soft-tissue sepsis can usually be
controlled and treated with local debridement and grafts, unless there
is massive necrosis requiring coverage, in which case select a free
skin or muscle flap selected.
reconstruction is available. One of the most commonly used flaps in
hand reconstruction is the lateral arm flap; this is a fasciocutaneous
flap that is a good choice for coverage of the dorsal or palmar
defects. It is thin, easy to dissect, and associated with few
complications (52).
also be covered with a free temporoparietal fascia flap. This flap has
a very low morbidity and a small scar because no skin is taken with the
fascia. The flap can be taken as a double-layered flap, incorporating
both the superficial and deep temporoparietal fascia on the superficial
temporal vessels (47). The dorsalis pedis flap,
scapular, parascapular, groin, and lateral thigh flaps can be used to
cover defects on the palm and dorsum of the hand (14).
transplantation is toe transplantation. Toe transfers include vascular,
neural, osseous, tendinous, and nail components as part of the
composite.
superior to fingers or thumbs reconstructed by other techniques because
the toe includes a sensate pulp with nail support, a near-normal
appearance, and an active flexion mechanism of the transplanted toe (Fig. 35.6).
 |
|
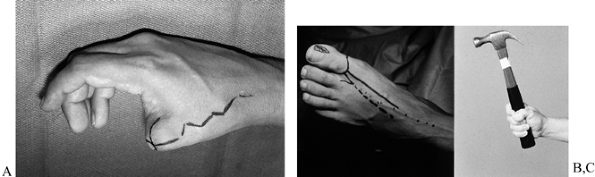
Figure 35.6. (See Color Fig. 35.6.) A: Elective toe-to-hand transfer following amputation of a thumb. B: A trimmed toe transfer was selected. The first metatarsal phalangeal joint was preserved. C: Function shown at 1 year.
|
toe-to-hand transplantation. Each transplant has advantages and
disadvantages. The decision of which toe to transfer is based on
several considerations: the patient’s desire, donor morbidity,
aesthetic aspects, as well as the part of the hand where the toe is
needed. The patient should be informed that the donor morbidity from a
great toe harvest (from a cosmetic and functional standpoint) would be
greater than that of removing the second toe.
there is a need for an opposable post at the fourth and fifth digit so
that the thumb and post can oppose each other. In such a case, it is
more appropriate to take the second toe; bilateral second-toe
transfers, or double-toe transfers, rather than the great toe (Fig. 35.7).
 |
|
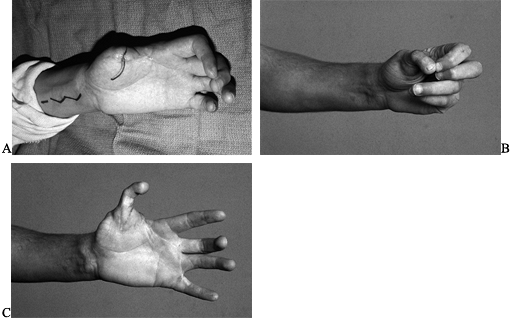
Figure 35.7. A: Patient had replantation of multiple digits but unsuccessful replantation of his thumb. B:
A second toe transfer was chosen to provide an opposable post. The patient made a conscious decision to have the second toe rather than the great toe taken because of his interest in athletics. C: Function of the hand postoperatively. |
considered if at least one third of the proximal portion of the first
metacarpal bone is present.
large discrepancy in size exists between the great toe and the thumb,
when the loss of the great toe is not acceptable, or when the level of
amputation is proximal
to
the proximal third of the first metacarpal and a considerable length of
the metacarpal is necessary to provide adequate length. Second-toe or
multiple-toe transfers also are indicated in a hand from which all
fingers have been lost and there is no ulnar post against which the
thumb can oppose. In this situation, two second-toe transfers may
enhance the grip strength and the ability to manipulate fine objects.
Partial toe transfer of the second toe can be used for reconstruction
in cases of the amputation of a finger at the level of the distal
interphalangeal (DIP) joint or even the nail itself. The distal aspect
of the second toe can provide, in these cases, a pulp, sensibility,
nail plate, and osseous length, which eases the patient’s
self-consciousness (15).
partial toe transfer includes pulp flaps, which are used for volar
thumb and finger resurfacing. There are three major indications for
pulp transfer: (1) acute loss of the digital pulp, (2) unstable skin resulting from previous pulp reconstruction with skin graft or local flap, and (3) posttraumatic distal insensibility with pulp atrophy and distal neuroma with no possibility of nerve anastomosis (35).
one artery through one hemipulp and venous drainage through the dorsal
skin proximal to the nail. A careful history as to whether there has
been any damage to the foot in the lifetime of the patient will
eliminate the need for an arteriogram. Doppler imaging can trace the
pathway of the dorsalis pedis artery in cases in which the artery
location needs to be found (32,67).
reconstruction following injuries have evolved parallel to the
development of microsurgery. In many cases of peripheral nerve injury,
primary repair may not be possible for many reasons, including direct
loss of nerve tissue from trauma, retraction of nerve stumps following
delay in repair, or resection of the nerve for a primary nerve tumor or
surrounding malignancies. Repair of the gap by primary closure with
tension is avoided because tension hinders regeneration by encouraging
gapping at the repair site with subsequent scar adhesion formation, as
well as reducing blood flow in the repaired nerve (27).
Nerve allografting is possible now owing to the advances in
immunosupression, although clinical application of peripheral nerve
allografting remains under experimental investigation (112).
been described for brachial plexus reconstruction. Some of these
techniques include nerve crossing procedures, free muscle transfer, and
more recently, a combined technique of double free muscle and multiple
nerve transfers. This procedure involves transferring the first free
muscle
neurotized by the spinal accessory nerve for elbow flexion and finger
flexion, a second free muscle transfer reinnervated by the fifth and
sixth intercostal nerves for finger flexion, and neurotization of the
triceps brachi muscle via its motor nerve by the third and fourth
intercostal motor nerves to extend and stabilize the elbow. Restoration
of hand stability is obtained through the suturing of the sensory rami
from the intercostal nerves to the median nerve (30).
The transferred muscles include latissimus dorsi, rectus abdominis, and
gracilis. The gracilis muscle is considered to be the best option by
many surgeons.
may encounter pathology that requires the understanding of free tissue
transfer techniques. These transfers are not the classic transfers used
in daily orthopaedic practice; thus, the subject will be mentioned
briefly.
indwelling devices are relatively common and can lead to thrombosis and
resultant ischemia. The problem at the wrist is associated with
arterial cannulation, especially in the 20% of the patients that have
an incomplete vascular arch. Despite acceptable results with
thrombolytic agents, we recommend prompt surgical exploration and
resection of the thrombosed segment. Frequently, the gap needs to be
reconstructed using reversed interpositional vein grafting (a form of
tissue transfer) (68).
and progress to thrombosis of the ulnar artery. Historically,
management of ulnar artery thrombosis has been controversial.
Treatments have included pharmacologic management, sympathectomy,
thrombectomy, and arterial reconstruction. There has been a trend
toward surgical management, and in the great majority of cases, we
recommend this course of treatment (68). Resect
the thrombosed segment of the artery and reconstruct the artery. This
is best accomplished by means of a reverse interposition vein graft
harvested from the forearm.
will care for more patients who experience the ravaging effects of
diabetic angiopathy and peripheral vascular disease involving the upper
extremities.
pharmacologic treatments, the surgical approach to these diseases will
evolve and will more frequently use techniques involving microsurgery
and free tissue transfers such as free omental transfer to inosculate
skin to provide vascularity to the hand (94).
open reduction and internal fixation in the management of fractures.
The importance of good soft-tissue coverage to maintain the vascularity
of bone fragments has also been emphasized. New methods of fracture
fixation have evolved, such as indirect reduction and minimal internal
fixation, that respect fracture biology and protect soft tissues.
fractures, the so-called “orthoplastic approach,” coordinates repair
processes for bone and soft tissue and avoids the adverse sequelae of
exposed internal fixation, bone sepsis, and possible amputation.
limb include any limb in a child, and in adults, those with potentially
intact sensibility. Nerve injuries do not preclude salvage but should
be distal enough to permit the return of some function (primarily
sensory) within a reasonable amount of time. Conversely, complex lower
limb injuries with nerve damage are frequently considered for
amputation, because the return to a functional status with an
appropriate prosthesis is usually more rapid (93).
limb salvage. Careful preoperative patient evaluation and perioperative
monitoring can reduce morbidity and mortality rates to be comparable to
those of younger patients. Lower extremity microvascular reconstruction
can be performed safely and successfully in the elderly patient (40).
tissue and stabilize fractures. Patients with severe limb injury will
often have sustained major vascular injury in the area of the trauma.
Assess leg perfusion clinically and, if needed, consider an arteriogram
if the zone of injury is large and it is in the region of potential
microvascular anastomosis.
tissue transfer) in the injured extremity is usually determined by the
general condition of the patient and the condition of the wound. The
bacterial status of the wound, type of fracture, different types of
tissues involved in the injury, and the exposed structures are factors
that influence the timing of wound closure. In severe extremity trauma
with soft-tissue loss and exposure of the underlying structures, cover
the wound as early as possible. Acute coverage by day 5 to day 7 is
generally accepted as having a good prognosis in terms of decreased
risks of infection, flap survival, and healing of the fracture. If the
wound can be radically debrided at the first setting, it can be covered
immediately with a free flap (39) (Fig. 35.8; see COLOR FIG. 35.8A).
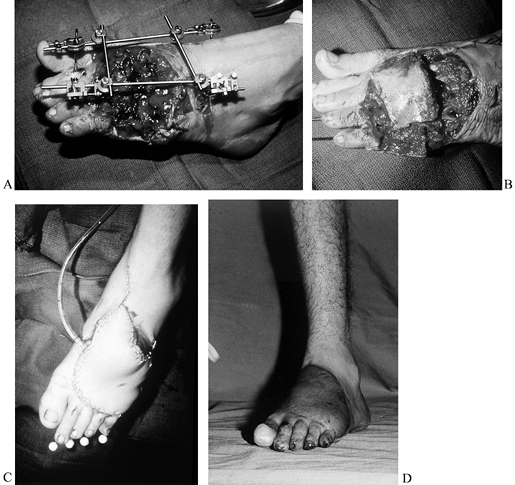 |
|
Figure 35.8. (See COLOR FIG. 35.8A.) Trauma to the lower extremity emphasizing the importance of early coverage. A: Patient was treated with debridement and external fixator. B, C:
At 48-hours, a one-stage reconstruction was performed with a nonvascularized iliac crest bone graft for the intercalary defect of the forefoot followed by a free scapular flap. D: One year after the reconstruction. |
size of the wound, type of tissue deficit, state of the wound (the
colonization, amount of cavitation), location of the injury, and the
length of the pedicle needed. Place the anastomosis in a “safe zone”
where recipient vessels have not been damaged by the initial trauma.
This is not always feasible. Therefore, plan to perform anastomosis
outside the zone of injury, either proximally or distally.
inflammatory response of the soft tissue of the traumatized lower limb,
which extends beyond the gross wound and results in perivascular
changes in the blood vessels. These changes include increased
friability of the vessels and increased perivascular scar tissue. Both
of these changes can contribute to a higher failure rate, especially in
lower-limb free tissue transplantation, presumably due to a higher rate
of microvascular thrombosis (5).
proximal dissection of the recipient vascular pedicle, and some use
vein grafts in lower limb reconstruction. Isenberg (49)
demonstrated that clinical acceptability of the recipient pedicle
(vessel wall pliability and the quality of blood from the transected
end of the vessel) was more important than the distance from the wound.
owing to the fact that both bony stabilization and soft-tissue coverage
are required for a successful functional outcome. Free tissue transfer
using microsurgical techniques has allowed surgeons to salvage
traumatized extremities in patients who would formerly have required
amputation (42,48,59) (Fig. 35.9). See Chapter 8, Chapter 12, and Chapter 24.
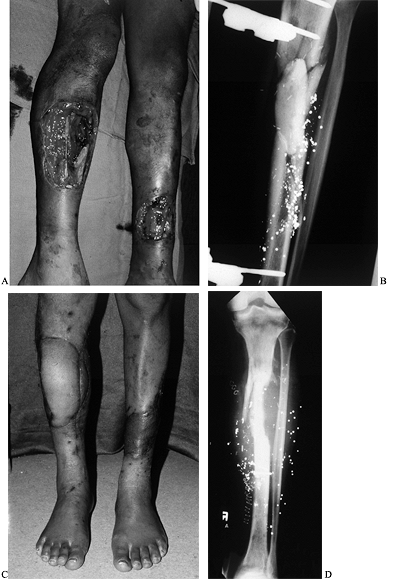 |
|
Figure 35.9. Gunshot wound involving both extremities. A:
The right leg had a soft-tissue as well as an intercalary bone defect. The left leg had a distal wound with exposure of the tibia. B: External fixator and tobramycin spacer placed on the night of injury with anticipation of free vascularized fibula. C: Patient standing with right free myocutaneous rectus flap healed and left serratus flap healed. D: Vascularized fibula as a second stage for intercalary reconstruction. |
guides treatment (26).
The management of dead space after sequestrectomy relies heavily on the
technique of free tissue transfer. Free muscle flaps provide coverage
for the debrided bone and soft tissue, obliterate dead space, improve
vascularity, and enhance leukocyte function (33,77,80).
improved the treatment of patients with osteomyelitis and large
(greater than 6 cm) segmental bone defects. In the past, despite
successful treatment of osteomyelitis, some patients have required
amputation owing to chronic nonunions. Now, once the bone infection is
treated, vascularized bone transplants (85,92) or bone lengthening with the Ilizarov device facilitates reconstruction and provides structural stability for limb function (3). See Chapter 32.
chronic osteomyelitis, and free flaps have been described more recently
for this use. Local gastrocnemius and soleus muscle flaps are still
used for coverage of smaller wounds on the upper and middle thirds of
the leg, respectively. However, local muscle flaps will not reliably
cover defects greater than 25 cm2 or those on the distal
third of the leg, ankle, or foot. For these defects, free muscle
transfers are preferred. The advantages of using the free muscle flaps
such as latissimus dorsi (11), serratus anterior (44), and rectus abdominis (18)
compared with the local pedicled muscle flaps such as the gastrocnemius
muscle are that they provide greater bulk (filling larger wounds), have
longer pedicles (increasing flexibility in muscle positioning), and
carry larger diameter vessels (facilitating the microanastomoses).
total joint arthroplasty, and the risk is increased with compromised
wound healing. Clinical risk factors for compromised wound healing
include diseases such as rheumatoid arthritis, peripheral vascular
disease, chronic renal failure, and diabetes. Other risk factors
include irradiation, steroids, immunosuppressive therapy, multiple
previous surgeries, and malnutrition. When they are exposed,
orthopaedic prosthetic materials become colonized with bacteria, and in
the majority of these cases, rapid intervention is required to salvage
the extremity and prevent osteomyelitis. Removal of the prosthesis,
debridement, closure with a muscle flap and delayed insertion of a new
prosthesis constitute the preferred treatment. Free muscle transfers
not only provide coverage of the defect but also provide a
well-vascularized tissue in close proximity to the new prosthesis (Fig. 35.10).
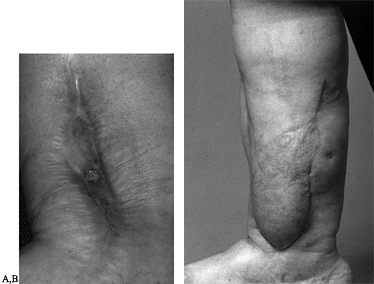 |
|
Figure 35.10. A: Chronic osteomyelitis of the ankle. B:
Patient underwent sequestrectomy and free latissimus flap for coverage of unstable skin as well as treatment of dead space following sequestrectomy. |
reduction and internal fixation of fractures. This occurs commonly when
there is a significant tissue edema that creates difficulty in skin
closure. It affects the medial pretibial surface of the distal leg more
often than the lateral side. The treatment in this situation usually
requires free flap coverage (51,73). See Chapter 135.
microsurgical techniques in reconstruction for bone and soft-tissue
tumors in the lower extremity. Specifically with the emphasis placed on
limb salvage after compartment resection rather than amputation,
microsurgical techniques have allowed
the
orthopaedic oncologist and reconstructive surgeon to work together to
preserve limbs. In soft-tissue sarcoma, in which entire compartments
are resected, microsurgical transplantation replaces components of both
muscle and skin to maintain limb contour and aid in healing. For
example, a large anterior compartment resection for a malignancy may
expose the tibial cortex. Microsurgery is the only reconstructive
option for wound closure. A flap such as the latissimus dorsi muscle
could be used to cover the tumor defect. This muscle can also be
innervated to assist in dorsiflexion of the foot.
of soft-tissue sarcomas, has a high incidence of wound complications
after attempted primary closure. Radiated wounds generally have a poor
vascular supply, making surgery through these wounds difficult. Wounds
tend to break down. Importing well-vascularized tissue into the wound
results in more rapid wound healing and may improve local circulation
in radiated areas. For this reason, many centers have adopted the
policy that immediate microsurgical reconstruction be performed after
tumor extirpation. The results of immediate microsurgical transfers in
these cases have been well substantiated and have led to decreased
hospital time, decreased costs, decreased morbidity, an increased rate
of limb salvage, and a high-level of patient satisfaction (9).
traditional treatment for high-grade sarcomas of bone, such as
osteosarcomas, had been amputation. Limb salvage surgery has become a
viable alternative for many patients due in part to the development of
more effective perioperative chemotherapy. The success of limb salvage
in these patients is primarily dependent on wide resection margins and
the addition of perioperative chemotherapy. The current management of
soft-tissue sarcomas or osteogenic sarcomas stresses more conservative
resection and limb-sparing operations. See Chapter 126.
These types of resections create complex composite defects of bone and
soft tissue that require microsurgical reconstruction. Local flaps
generally do not provide adequate coverage, and many times, the only
option is a free tissue transfer.
extirpation, and the use of composite flaps has been particularly
advantageous in the reconstruction of tumor defects. Examples include a
myocutaneous innervated latissimus dorsi for finger flexion or an
osteocutaneous fibula flap for an intercalary bone defect that also has
an accompanying overlying skin defect (64) (Fig. 35.11).
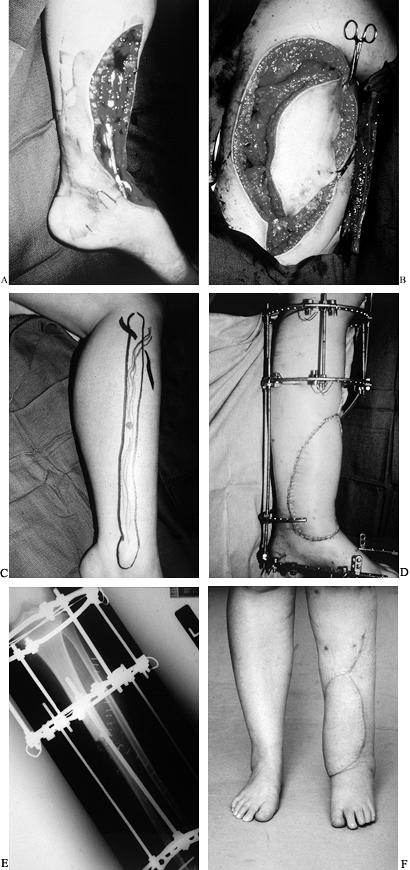 |
|
Figure 35.11. A: Osteosarcoma of the tibia requiring resection of the anterior tibial compartment. Patient required (B) latissimus free flap (C) and a free fibula for reconstruction. (D) Final reconstruction with simultaneous Ilizarov, free latissimus dorsi myocutaneous flap, and free vascularized fibula flap. (E) Fibula in place as an intercalary graft compressed and held by an Ilizarov frame. (F) Final result at 1 year. The fibula hypertrophied, and fusion was performed into the talus with the fibula.
|
appropriate window for the timing of the operation, which includes
tumor resection and free tissue transfer, is determined by discussions
with the oncologist and radiation therapist. Plan the operative details
such as incisions, design of skin flaps, exposure, and preservation of
the recipient site vessels with the oncology surgeon. It is usually
possible
to establish the requirements for soft-tissue and bone reconstruction
early in the operation and begin the flap harvest concurrent with the
tumor resection. If oncologic margins are not predictable, then it is
best to complete the resection of the tumor before beginning dissection
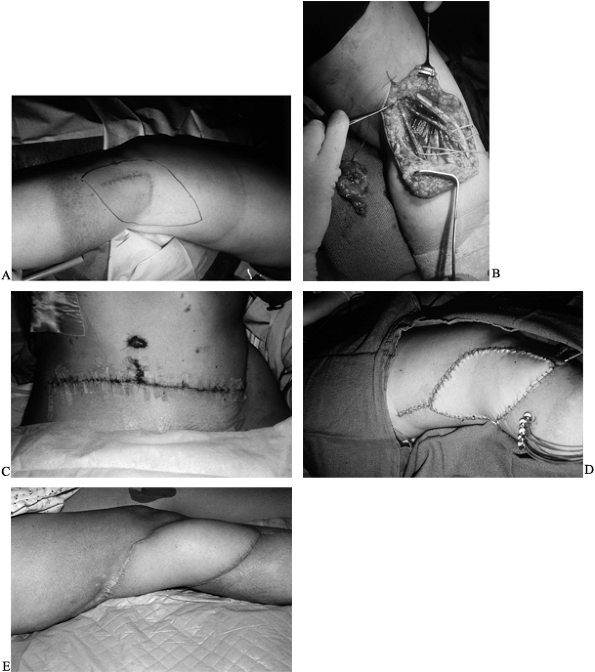
of the free flap (28) (Fig. 35.12; see also COLOR FIG. 35.12B).
 |
|
Figure 35.12. Patient with popliteal mass presented with acute peroneal palsy. A: Diagnosis was neurosarcoma. B: Tumor defect after biopsy and local radiation. (See COLOR FIG. 35.12B). C: Harvest of free rectus abdominus myocutaneous muscle flap. D: Postoperative result with radiation catheters in place under the flap. E: Results at 1 year.
|
structures (particularly if they are radiated), bone devoid of
periosteum, and allografts or tumor prostheses that
cannot be covered with local tissues. Sometimes blood supply to local flaps may be sacrificed during tumor resection.
divided into two groups. The first are those who undergo resection of
tumors and develop acute wound complications such as skin flap necrosis
in the early postoperative period. These patients may require
debridement and free flap coverage within the first or second
postoperative week. It is safe to let questionable areas demarcate
before surgery. Patients who present later with impending allograft or
prosthesis exposure should undergo surgery as soon as possible to avoid
infection of the implant or allograft.
reconstruction several months or years after primary tumor resection.
Some of the patients in this group present with chronic unstable
soft-tissue coverage, wound dehiscence, prosthesis infection, and
prosthesis failure or limb growth that compromises soft tissue.
technical complexity to the tumor operation, it may also lead to a
decrease in amputation rates by decreasing wound complications. In
addition, it allows the oncologic surgeon to obtain adequate margins of
resection, which may favorably influence amputation rates by
contributing to a decrease in local recurrence.
several years, resurfacing of the foot remains one of the most
difficult reconstructive problems. Not only is it necessary to
reestablish soft-tissue integrity but also the treatment plan must
include attention to bony architecture and foot deformities that can be
caused by muscle imbalance. Each anatomic region of the foot has
certain characteristics that will influence selection of the free
tissue transfer for reconstruction. The dorsum of the foot and the
ankle require thin pliable soft-tissue coverage for exposed tendons
that are devoid of paratenon, bones, or joints. The weight-bearing
surface of the foot (the plantar skin) is unique with respect to its
dermoepidermal histologic characteristics, the unique subcutaneous
tissue in the heel pad, adherent dermal septae to the underlying
plantar fascia, and the ability to withstand constant pressure and
shear forces.
be achieved with a free neurosensory flap such as the radial forearm
flap (22). Free muscle flaps are used when bulk
is necessary to obliterate dead space such as in osteomyelitis, severe
crush injuries with extensive soft-tissue loss, and for weight-bearing
surfaces (84). However, muscle flaps can often be bulky and, if they are not contoured properly, may prevent the use of normal shoes.
advantages over other fasciocutaneous flaps and muscle flaps for
resurfacing the foot and ankle. It meets most of the prerequisites for
the “ideal” foot flap: It provides a large amount of well-vascularized,
thin, and pliable soft tissue; it is easy to harvest; and it has large
consistent vessels and a long pedicle. Furthermore, it facilitates the
restoration of normal (original) foot contour by replacing
“like-with-like,” allowing patients to wear normal shoes, and can
provide a durable and stable weight-bearing plantar surface for
walking. It also achieves an excellent aesthetic result without the
need for debulking. Dryness and cracking of a hypertrophied skin graft,
especially at the flap–foot skin interface, is less of a problem with
skin flaps compared with free muscle flaps covered with split-thickness
skin grafts. In addition, it has the potential for sensory
reinnervation. Possible disadvantages include an unsightly donor site
scar, especially in a young woman, and donor site skin graft breakdown
with flexor tendon exposure.
heavily keratinized, designed to resist high stress, and it is anchored
to underlying bones and ligaments by thick fibrous connective tissue.
The plantar surface acts as a shock-absorbing system for the foot,
helping to minimize horizontal and vertical shear forces by its
multidirectional fibrous septae (115). The plantar surface of the foot can be divided into three distinct areas, each with special requirements for replacement.
high stress and, in many cases, it has been used as a donor tissue for
plantar resurfacing. Forefoot skin is the subject of significant forces
during running and toe off in the normal gait cycle.
whether the foot will function better than a prosthesis after the
reconstruction is complete. Achieving a normal gait cycle is possible
only if the foot can fit into a regular or slightly modified shoe, is
pain free, and ideally has some sensibility. Neurosensory flaps such as
dorsalis pedis, lateral arm flap, tensor fascia lata flap, and radial
forearm flap may be used to provide sensibility, but they may lack the
bulk and thickness required for cavitary tissue defects. Larger flaps
such as muscle flaps that do contour better cannot be innervated. Thus,
there is no ideal transfer. A review of flap options should be done for
each patient. The specific complications include wound breakdown
because of mechanical instability and excessive sheer forces,
ulceration, formation of calluses, hyperthrophic scarring, and
intrinsic muscle imbalance (62).
patients with diabetes and peripheral vascular disease. The magnitude
of the problem is enormous; statistics indicate that 14% of these
patients are hospitalized an average of 6 weeks per year for foot
problems, and more than 80% of amputations are performed on diabetics.
The contralateral limb of one with an ulcer is at risk for further
ulceration, and there is a 50% chance of loss of the opposite leg
within 5 years (43). Care of these patients
requires a close collaborative effort between the orthopaedist, the
peripheral vascular surgical team, and the microsurgical team to
optimize rapid and accurate assessment of the vascular problem, and to
determine the most appropriate and timely plan for wound care and the
most reliable method for revascularization. The results of
macrovascular and microvascular anastomoses are comparable to those for
nondiabetic patients undergoing the same procedure (58). Patients treated with cutaneous free flaps have less morbidity than patients treated with muscle free flaps (89).
The radial forearm free flap is ideal for treatment of relatively small
wounds of the foot and ankle. It provides cutaneous tissue with a
lengthy vascular pedicle and a donor site that can often be closed
primarily.
dysvascular patients, surgical mortality has not been found to be
higher in cases of microsurgical reconstruction procedures performed
alone in these patients or in combination with macrovascular vascular
reconstruction (13). Once the extremity has
been revascularized, the most appropriate method of reconstruction can
be carried out for defects of the foot in a well-vascularized limb. For
those patients in whom the macrovascular blood supply is intact and
without apparent compromise but who have large, unhealthy, colonized
wounds, particularly if they involve tendon or bone, free flap coverage
is indicated. Free tissue transfer techniques are ideal in these
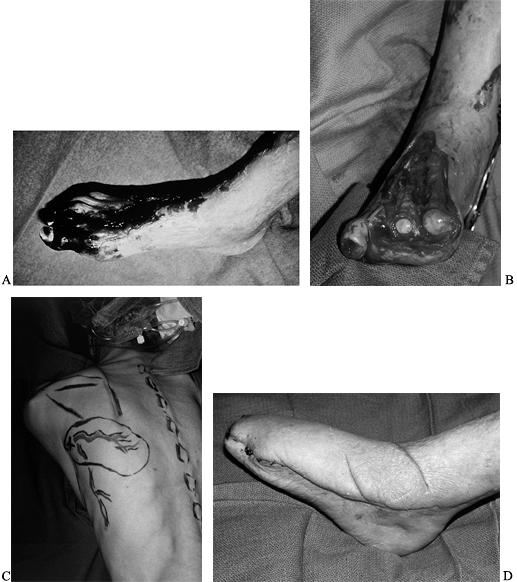
situations because (1) they are able to resurface any size defect; (2) they allow aggressive resection of the wound to eliminate colonized, fibrotic, unhealthy tissue; (3) the flap can help revascularize the foot; and (4) the defect is replaced with healthy nondamaged tissue (Fig. 35-13; see also COLOR FIG. 35.13A, COLOR FIG. 35.13B).
 |
|
Figure 35.13. (See COLOR FIG. 35.13A, COLOR FIG. 35.13B). A 64-year-old diabetic patient with a dysvascular foot. A: Necrotic foot preoperatively. B: After debridement of tissue. C: Preoperative planning of the scapula flap. D: The patient underwent free flap as well as femoral distal popliteal bypass. Final result at 1 year.
|
of vigilance to avoid problems both locally and systemically, with a
much closer observation of the donor sites and recipient sites to
preclude wound healing problems.
candidates for amputation before flap transfer (which was often offered
as a last option before limb loss), these procedures do not increase
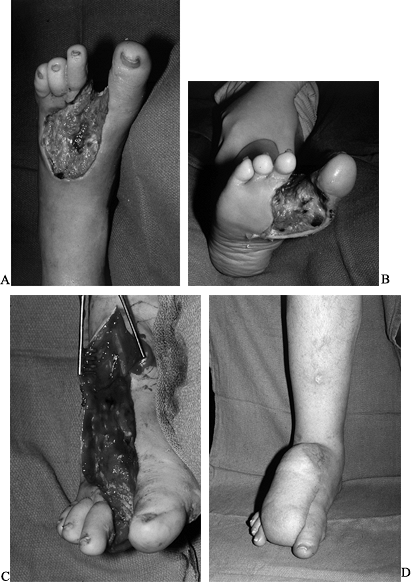
the rate of limb loss, but can only increase the limb salvage rate (Fig 35-14; see also COLOR FIG. 35.14A).
It is important to examine the result of lower extremity limb salvage
as it relates to ambulatory function. Salvage of a useless limb in such
patients is of little value in their overall management. Likewise,
heroic attempts at salvaging a limb are not indicated if the operation
subjects a patient to excessive morbidity.
 |
|
Figure 35.14. (See COLOR FIG. 35.14A). Diabetic foot infection. A: Dorsal view. B: Plantar view. C: Debridement. D:
Reconstruction with a scapular free flap. The patient required debulking of the flap but was able to continue as a farmer with a special shoe. |
procedures has yet to be determined. Certainly in this era of
cost-containment, the arguments against such “expensive” and
sophisticated procedures versus straightforward amputation cannot be
ignored. Although the exact cost of leg salvage in such a group of
patients is difficult to determine, it may be less expensive than the
combined cost of hospitalization, prosthesis fitting, rehabilitation,
and disability payments.
extremely careful patient selection. In the face of systemic
complications, one must also exercise proper judgment and abort
attempted reconstruction to ensure patient survival.
microsurgical tissue transfers. In the appropriate patient with
localized disease, a dual surgical approach including wide excision of
the ulcer and surrounding liposclerotic tissue bed, and coverage with a
free flap containing multiple competent microvenous valves, may improve
the underlying pathophysiology. In patients with complex ischemic or
infected wounds from diabetes, free tissue transfer as an adjunct to
lower extremity vascular reconstruction can help in obtaining a
salvageable functional limb, thus presenting a viable alternative to
amputation (41,75).
a high number of amputations are performed as a result of major trauma,
tumors, diabetic ulcers, and in cases when the patient is too ill to
survive a lengthy operation. The level of amputation itself is an
important factor for consideration. In cases of lower limb amputation,
below-knee amputation is associated with faster rehabilitation compared
with above-knee amputation. Below-knee amputees require less
rehabilitation time, have a more natural gait, and can engage in more
physical activities (6). The choice for the
level of amputation is determined by the need to cover the stump with
appropriate soft tissue stable enough to resist breakdown in a
prosthesis. Skin grafting of amputation stumps, especially in lower
limbs, provides only wound coverage, and usually does not meet the need
for adequate padding. In cases for which a local flap is not available,
free flaps should be considered. These flaps can provide coverage of
the stump, and if microneural coaptation is performed, sensation of the
stump can be achieved. That may assist proprioception within the
prosthesis. An amputated limb may provide donor tissue, such as in
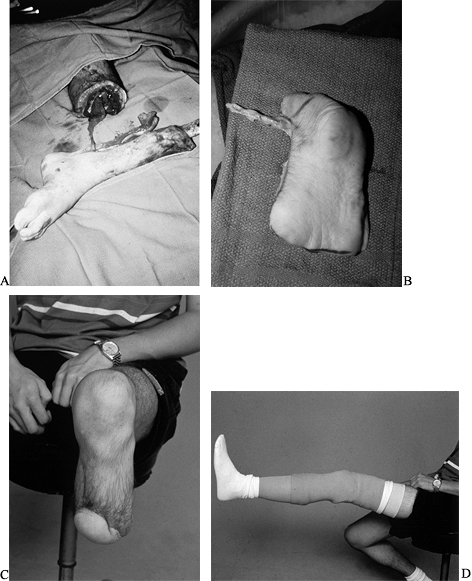
traumatic below-knee amputations (Fig. 35.15).
 |
|
Figure 35.15. Chronic infection of the tibia. A: Amputation level. B: Plantar fillet flap was harvested to preserve the length of the leg. C: Stump healed and (D) prosthetic device in place.
|
Isolated tissue transplants include muscle, skin, fascia, bone, or
flaps. The more common composite tissue transplant is a more complex
flap that provides more than one function. Examples include
myocutaneous, osteocutaneous, or innervated myocutaneous flaps.
will determine the type of flap to be selected. Tissue transplants are
selected with respect to donor site morbidity, recipient site
requirements, vascular pedicle length, and anticipated aesthetic
result. For example, a myocutaneous latissimus dorsi flap should not be
transplanted to the dorsum of the foot due to its bulk and the fact
that the donor tissue does not match the dorsum of the foot. Other
flaps, such as an isolated skin flap (radial forearm flap or lateral
arm flap), are a better transplant. Similarly, to fill dead space after
sequestrectomy of an infected tibia, a lateral arm flap, which is a
small skin flap of approximately 5 × 7 cm is not appropriate owing to
its lack of bulk and the fact that the muscle flaps, rather than skin
flaps, are known to be more effective in the treatment of
osteomyelitis. The use of a skin paddle with composite tissue transfers
can be done for either contouring or as a monitor for perfusion of the
flap.
filled. If a flap were used purely for resurfacing, such as on the
dorsum of the hand, so that secondary tendon reconstruction can be
performed, a large bulky flap would not be required. However, if there
is significant dead space, a large muscle flap such as a latissimus
dorsi flap should be considered. Osseous flaps are selected for
structural defects such as intercalary bone defects resulting from
trauma, tumor, or infection. If a vascularized bone flap is to be
selected, the cross section of the bone defect, available vascular
supply, and fixation of the vascularized bone graft must be taken into
consideration.
There are instances in which tissue coverage exists but it is
insufficient in texture or quality. A soft-tissue envelope may need to
be augmented, such as using a scapular flap to resurface a knee with
unstable skin as a first stage before a total knee replacement. There
are free flaps that are performed for purely aesthetic reasons, such as
the resurfacing of extremities. This is an unusual use of free tissue
transfer, and it is used only in special cases. A combination of the
above-mentioned selection factors determines free flap selection.
procedures frequently includes angiography to evaluate the vasculature
of the recipient site. The need for angiography, however, especially
after trauma, is debatable. The vast experience gained in
reconstructive microsurgery has enabled us to become familiar with the
“vascular” anatomy in all regions of the body to optimize the selection
of recipient site vessels. A meticulous clinical evaluation (with
Doppler mapping) can provide valuable information without the need for
recipient-site angiography (71).
for the success of free tissue transfer, especially when the transfer
is to the lower extremity. However, general agreement on which vessels
to use has not yet been reached. Conflicting data have been reported on
the survival and outcome of the transferred flaps, depending on the
vessel used or the location of anastomosis proximal or distal to the
zone of injury. For example, the anterior tibial vessels may be
preferred for their easy accessibility, whereas the posterior tibial
vessels are strongly advocated by others.
algorithm for recipient vessel selection in free tissue transfer to the
lower extremity. Based on their experience, the most important factors
influencing the site of recipient vessel selection were the site of the
injury and the vascular status of the lower extremity. The type of flap
used, method, and site of microvascular anastomosis are less important
factors in determining the recipient vessels.
techniques is important. In severe injuries of the lower extremity with
associated soft-tissue defects, early aggressive wound debridement and
soft-tissue coverage with a free flap within 5 days has been found to
reduce postoperative infection, as well as decrease flap failure,
nonunion, and chronic osteomyelitis (19,20). Godina (39)
emphasized the importance of radical debridement and early
full-thickness soft-tissue coverage either acutely or within the first
72 hours.
the first case of an emergency free flap transfer to the upper
extremity in 1988; they defined the emergency free flap as a “flap
transfer performed either at the end of primary debridement or within
24 hours after the injury.” Yaremchuk and colleagues (118)
recommend that flaps be transferred between 7 and 14 days after injury
after several debridements. The argument in favor of this approach is
that the zone of injury, which often may not be apparent at
presentation, can be determined by serial debridements performed in the
operating room over several days.
flap, two key factors should be considered: the presence of an exposed
vital structure and the risk of infection. A vital structure is defined
as “one that will rapidly necrose if not covered by adequate soft
tissue” (21). The decision of what constitutes
a vital structure depends on circumstances. Tissues such as vessels,
nerves, joint surfaces, tendons, and bone denuded of periosteum may
desiccate, die, and lead to infection when they are left exposed for
long periods of time. When considering primary versus delayed coverage,
take into account the risk of leaving the vital structure exposed and
what functional deficit would occur if it were lost.
that should be considered because it may jeopardize the limb, the
quality of the functional recovery, or the free flap. As the risk of
infection increases, the wisdom of primary closure with a free flap is
reduced. Debridement of the wound is the most powerful surgical tool to
reduce the risk of infection of the wound. If radical debridement is
not possible, then it is not safe to perform primary free flap
transfer. Another perspective is that the capability to perform free
tissue transfer allows the surgeon increased freedom to perform radical
debridement and may actually reduce the risk of infection (86).
The mechanism of injury, the elapsed time since injury, and the degree
of contamination of the wound determine the risk of wound infection. In
an acute, sharp noncontaminated injury, when closure would be routinely
performed if there were no skin loss, there seems to be little reason
not to consider an emergency free flap.
Carefully evaluate the cardiovascular and pulmonary status. Nutritional
assessment is of particular importance because this will influence
healing, particularly in trauma and tumor patients. In elective cases,
patients in a high-risk category may need preoperative nutritional
supplements (7).
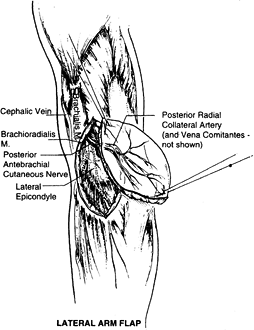
lateral arm flap, radial forearm flap, scapular flaps, dorsalis pedis
and groin flaps.
can serve as an innervated fasciocutaneous flap or as a
deepithelialized subcutaneous fascial flap. The lateral arm flap is
based on the posterior radial collateral vessels (PRCA). The artery is
a direct continuation of the deep brachial artery. The draining veins
of this area are the venae comitantes of the PRCA. The pedicle length
is approximately 7 cm. The external diameter of this artery is usually
1.5 to 2.0 mm but sometimes can be smaller, even 0.8 mm. The vein’s
diameter ranges from 2.0 to 2.5 mm. The anatomy of this vascular
pedicle is constant, in contrast with the medial arm flap, which has a
more variable vascular supply.
 |
|
Figure 35.16. Surgical anatomy of the lateral arm flap.
|
brachial cutaneous nerve, a proximal branch of the radial nerve (C5—6),
giving the flap potential as a sensate flap. Additional sensory supply
comes from the posterior antebrachial cutaneous nerve, which divides at
the distal upper arm, with the upper branch supplying the posterior
inferior upper arm and the lower branch supplying the lateral side of
the arm and elbow (82).
The surface marking that is important in planning is a line that joins
the deltoid insertion with the lateral epicondyle (this line marks the
lateral intermuscular septum and the course of the PRCA). Design a flap
with this line as the central vascular axis. Include the deep fascia in
the flap. It also can be harvested based on the PRCA pedicle alone.
This is advantageous in cases in which thin well-vascularized coverage
is required and for coverage of areas where tendon gliding is required (119).
lateral brachial cutaneous nerve of the arm; it is often hairless. In
addition, vascularized bone (humerus) may be harvested with this flap
for composite reconstruction (52).
vascularized bony segment 10 cm long and 1 cm wide to be included with
the skin flap.
-
Position the patient supine. Drape the
arm to allow free movement; position the arm on an arm table or across
the chest. A tourniquet is recommended but sometimes is difficult to
maintain during proximal dissection. -
Begin dissection with a posterior incision to the triceps muscle fascia.
-
Raise the flap subfascially and suture the skin to the fascia to prevent shearing.
-
Elevate the posterior fascia to expose
the lateral head of the triceps. Continue dissection to the anterior
border of triceps muscle. Here, the fascia dives deep and inserts into
the humerus. Perforators are now seen in the septum. -
Make an anterior incision down to fascia.
Incise the anterior fascia over the brachialis and the brachioradialis
muscle, following the level of the periosteum of the humerus. -
Ligate the distal continuation of the PRCA.
-
Separate the fascial septum as close as
possible to the periosteum. Follow the vascular pedicle proximally
under the triceps muscle into the spiral groove. Separate the lower
cutaneous nerve from the radial nerve. -
Modify the flap elevation technique when
the fasciocutaneous flap is designed to include vascularized bone
(humerus) and tendon (triceps).
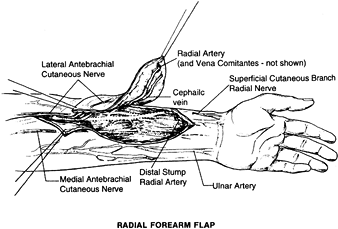
a thin, well-vascularized fasciocutaneous flap on the ventral aspect of
the arm. This flap was widely used in China before it was popularized
in the Western literature (88,105).
The flap is based on the radial artery, which can achieve a 20 cm
pedicle and has a diameter of 2.5 mm. This pedicle length facilitates
microsurgical anastomosis out of the zone of injury. The venous
drainage is through the venae comitantes of the radial artery, but the
flap can include the cephalic vein, the basilic vein, or both. The flap
can contain the lateral antebrachial cutaneous nerve or the medial
antebrachial cutaneous nerve and serve as a neurosensory flap. The size
of the flap can be 10 × 40 cm2. A portion of the radius can be included as a vascularized bone with this flap (29).
The advantages of this flap are its long pedicle and potential sensory
innervation. The quality of the bone from the radius is mainly cortical
and not of any substantial volume (107). Including the bone in the radial forearm flap can lead to fracture of the radius.
 |
|
Figure 35.17. Surgical anatomy of the radial forearm flap.
|
dimensions; more important, it will allow direct closure of the donor
defect (76).
-
Position the patient supine, with the arm
on a hand table. Preoperatively, evaluate the vascular supply to the
hand by an Allen test and a Doppler scan, and confirm the circulation
through the ulnar artery. A line drawn from the center of the
antecubital fossa to the radial border of the wrist where the radial
pulse is palpable represents the course of the radial artery. Center
the flap over the course of the vessels. The more distal the flap
design, the longer the pedicle. -
Make the skin incision and continue a subfascial dissection toward the vessels.
-
On the distal part of the flap, identify
the brachioradialis and flexor carpi radialis tendon. The radial artery
and venae comitantes will lie along the ulnar side of the
brachioradialis and along the radial side of the flexor carpi radialis
tendon. The cephalic vein will lie radial to the brachioradialis. -
Dissect under the pedicle and isolate the pedicle distally.
-
Raise flaps from distal to proximal and
isolate the vessels proximally. Dissect deep to the deep fascia,
elevating the flap from the underlying muscle. Combined flaps can
include tendons and segments of the radius.
than 40% of the cross section of the radius should be harvested, and
the wrist and forearm should then be put in a cast for 3 to 4 weeks.
Avoid injury to the peritenon covering the tendons of the flexor carpi
radialis, brachioradialis, and finger flexors because its loss can lead
to skin graft failure and even loss of the tendons.
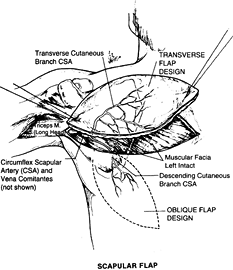
the posterior chest and can be deepithelialized and used as subcutaneous fascial, pedicled, or free flap.
 |
|
Figure 35.18. Surgical anatomy of the scapular flap.
|
circumflex scapular artery (CSA) and drained by its venae comitantes.
The CSA is a major tributary of the subscapular artery; it is the
artery supplying blood to the scapula, the muscles that attach to the
scapula, and the overlying skin. The length of the pedicle is 5 cm, and
the diameter of the artery is 2.5 mm. The vascular pattern of this
territory makes it possible to raise multiple skin flaps on a single
vascular pedicle or to harvest the lateral border of the scapula as an
osteocutaneous flap for a complex reconstruction.
can be divided in two components: a horizontal territory (horizontal
scapular flap) and a vertical territory (parascapular flap) based on
the branches of the CSA after the vessel courses through the triangular
space.
of the intercostal nerves, this flap has no potential for being used as
a sensate flap. Preliminary expansion of the territory of the scapular
flap will increase the flap dimensions and permit direct donor site
closure.
-
Position the patient midlateral or in an oblique position. Elevate the flap retrograde.
-
Start with a low medial incision.
Identify the epifascial plane. Elevate the fascia cranially beneath the
deep fascia, to the area of the triangular space. -
Complete the skin incision (the upper
part). Carefully retract the flap medially. Identify the junction of
the parascapular and horizontal branches of the circumflex scapula
vessels. -
Divide the horizontal branches and
dissect the circumflex scapular pedicle into the triangular space.
Identify the thoracodorsal or scapular artery.
-
Use the same dissection strategy as for the parascapular flap.
-
Start the dissection laterally and proceed toward the triangular space.
-
As in the parascapular flap, the vascular pedicle can also be identified first in the course of the dissection.
pedicle as the first step of the dissection, especially in cases of
microvascular transplant. Accomplish this with palpation of the
triangular space and confirmation of pedicle location with a Doppler
probe. Two approaches are available for scapular and parascapular flap
elevation and preparation for the microvascular transplantation:
lateral (initial pedicle identification) and medial (retrograde flap
dissection). This flap can be combined with other flaps based on
subscapular blood supply, and this may greatly facilitate certain
complex reconstructions. These reconstructions include the latissimus
dorsi and serratus anterior flaps, which can supply additional skin,
muscle, and bone (rib), if necessary (31,98,109).
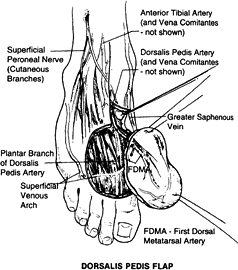
on the dorsalis pedis artery, which originates from the anterior tibial artery and its venae comitantes (72).
The length of the pedicle is 6 to 10 cm, and the diameter of the artery
is 2 to 3 mm. The nerve supply comes from the branches of the deep and
superficial peroneal nerves. The size of the flap is 6 × 10 cm2,
and it can be raised as a skin flap alone or in combination with the
second metatarsal bone as an osteocutaneous flap or in combination with
first- and second-toe transfer (83).
 |
|
Figure 35.19. Surgical anatomy of the dorsalis pedis flap.
|
-
Position the patient supine with a tourniquet around the thigh.
-
Make a distal incision for identification
of the first dorsal metatarsal artery with subsequent retrograde
dissection of the flap. Divide the first dorsal metatarsal artery and
branches of the deep peroneal nerve to the first web space. -
Continue the dissection from distal to
proximal in a plane just deep to the deep peroneal nerve and first
dorsal metatarsal artery. This plane is just above the peritenon of all
the extensor tendons. -
Then continue the dissection proximally
up to the proximal head of the metatarsal. At that level, the deep
perforating branch of the dorsalis pedis artery is encountered. -
Make the medial incision of the flap and
elevate the medial part of the flap with the greater saphenous vein and
the dorsal venous arch included in the flap. -
Over the tarsal bones, identify the dorsalis pedis artery. Divide the deep branch and incise the rest of the skin completely.
-
With the upper incision completed, open
the extensor retinaculum and identify the dorsalis pedis artery, its
two venae comitantes, and nerve. -
Divide the extensor hallucis brevis
muscle at the level of the extensor digitorum longus tendon to the
second toe. Take care to preserve the paratenon on the remaining
tendons to provide a bed for the skin graft.
joints and tendons, and in microvascular transplant of
metatarsophalangeal joints in children (78).
this flap; it can include difficulties in primary healing with the need
for skin grafts, and complications of lymphedema, and hypertrophic
scarring of the foot (99).
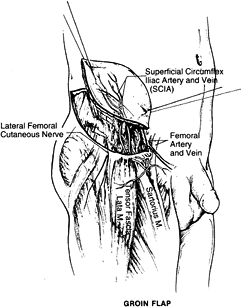
provides a large skin and subcutaneous tissue territory based on the
superficial circumflex iliac artery (SCIA) and vein (SCIV). The length
of the pedicle is 2 cm, and the diameter is 1.5 mm. The dimension of
the flap is approximately 10 × 25 cm2.
 |
|
Figure 35.20. Surgical anatomy of the groin flap.
|
the deep groin fascia expands flap dimensions and allows direct donor
site closure.
-
Position the patient supine with a
beanbag or folded towel placed under the posterior iliac spine on the
side of the planned flap. The medial approach is preferable for free
flaps. -
Identify the SCIA 5 cm below the inguinal line before skin elevation.
-
Incise medially and identify the superficial vein anterior to Scarpa’s fascia. Identify the femoral artery and SCIV and SCIA.
-
Start with a lateral skin incision. Leave the deep fascia intact.
-
Next identify the lateral border of the
sartorius muscle. Ligate the muscle branches of the SCIA branch. Divide
the lateral cutaneous nerve.
external oblique aponeurosis for reconstruction of a tendon-like
structure to replace the Achilles tendon or reconstruction of the
calcaneus with a composite graft including the groin flap and iliac
crest bone (113).
diameter of the superficial circumflex iliac artery make this flap less
popular than other free skin flaps (25).
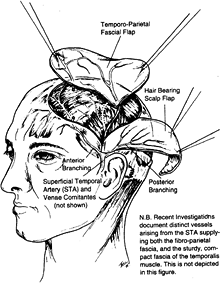
can be used as a fascial or fasciocutaneous flap. The fascia covers the
temporal muscle extending over the temporal fossa and lies superficial
to the deep temporal fascia covering the
temporalis muscle. It continues as the galea beyond the limits of the temporal fossa.
 |
|
Figure 35.21. Surgical anatomy of the temporoparietal fascial flap.
|
is the terminal branch of the carotid artery. The length of the artery
is 4 cm, and the diameter of the artery is 2 mm. The course of the
vessels is on the fascia from the preauricular area into the temporal
fossa. The sensory nerve supply comes from the auriculotemporal nerve.
The size of the flap is 12 × 9 cm2 (1).
-
Preoperatively, identify the course of the vessels with a Doppler probe and mark the incision lines parallel to hair follicles.
-
Position the patient supine, with the head tilted slightly to the opposite side.
-
Start the incision by raising a pretragal
skin flap, extending the incision toward the vertex of the skull over
the temporal fossa. -
Identify and spare the superficial temporal vein anterior and remain superficial to the STA. Identify the STA.
-
Dissection proceeds cephalad deep to the
hair follicles. Avoid damaging the superficial temporal vein and the
frontal branch of the facial nerve. -
After cephalad completion of the dissection, incise the flap. Lift from the deep fascial plane toward the auricle.
-
After complete dissection, leave the flap for observation of perfusion.
of the foot, ankle, Achilles tendon, and hand. The minimal thickness of
this well-vascularized flap prompts some authors to describe the
technique as a “microvascular transplantation of a recipient bed” (12). This flap is useful for burns, particularly when joint spaces or tendons are exposed after debridement (23).
A muscle for free tissue transfer must be able to survive on one
vascular pedicle that is dominant and that will support the entire
muscle mass. Classification is as follows:
-
Type 1: one vascular pedicle (extensor digitorum brevis, tensor fascia latae).
-
Type 2: dominant pedicle and minor pedicles (abductor hallucis longus, gracilis).
-
Type 3: two dominant pedicles (rectus abdominis, serratus anterior).
-
Type 4: segmental vascular pedicles (none).
-
Type 5: one dominant and secondary vascular pedicles (latissimus dorsi, pectoralis major, pectoralis minor).
and combination flaps such as the latissimus dorsi–serratus anterior
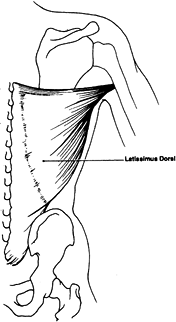
muscle flap based on one dominant pedicle (thoracodorsal artery).
pedicle is the thoracodorsal artery and venae comitantes, which
originate from the subscapular artery and vein. Secondary pedicles are
two rows (lateral and medial) of four to six perforating arterial
branches and venae comitantes taking origin from the posterior
intercostal and lumbar arteries and veins. The length of the major
pedicle can be as long as 8 cm and the arterial diameter as large as
2.5 mm. The artery enters the deep surface of the muscle in the
posterior axilla, 10 cm inferior to the latissimus muscle insertion
into the humerus (9).
 |
|
Figure 35.22. Surgical anatomy of latissimus dorsi muscle flap.
|
(C6–C8), and the sensory innervation of the skin is supplied by
multiple cutaneous branches of the intercostal nerves. Generally, this
is not used as a sensate flap.
because function is preserved by the remaining synergistic shoulder
girdle muscles.
-
Position the patient midlateral, with the arm elevated 90°.
-
Begin the dissection with an incision along the lateral muscle border.
-
First, identify the muscle border and its
relationship to the serratus muscle. Next, identify the pedicle and
follow the pedicle to its origin in the axilla. -
Free the anterior border of the muscle
and raise the flap from a ventral to dorsal direction to the spine.
Take care to coagulate or ligate the perforating vessels. -
Next, divide the muscle distally as required. Raise the muscle in the cranial direction.
-
Next, ligate the serratus branch of its artery.
A combined flap including the ninth and tenth ribs as vascularized bone
transplanted for simultaneous coverage and tibial bone reconstruction
is possible but not commonly used (74).
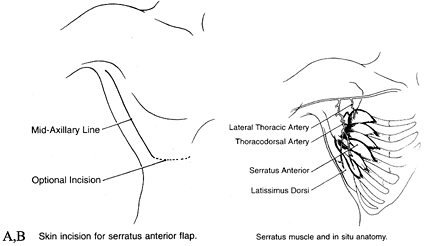
multidigitated muscle on the lateral chest wall between the ribs and
scapula. The muscle is supplied by two pedicles, the serratus anterior
branch and the lateral thoracic artery. The length of each one of the
pedicles is 6 to 8 cm, and the diameter of the artery is 2 to 3 mm (Fig. 35.23).
 |
|
Figure 35.23. A: Skin incision for a serratus anterior flap. B: Surgical anatomy of a serratus anterior flap.
|
the long thoracic nerve and the T2–T4 segmental intercostal nerve
supply for sensory innervation. The vascular pedicle as well as the
motor nerve separates into fingers of muscles corresponding to the
slips of the serratus. The size of the serratus anterior is 15 × 20 cm2. A musculocutaneous flap of 5 × 15 cm2 can be elevated (8).
-
For a fascia flap, place the patient in a lateral position, and elevate the arm 90°.
-
Make a slightly curved incision along the lateral border of the latissimus muscle.
-
Next, identify the muscle border and the
serratus arcade. Determine if the thoracodorsal pedicle is intact and
find the entrance points of the motor fibers into the muscle. Outline
the flap size on the muscle surface. -
Release the muscle from the thoracic
wall. Preserve the three proximal slips to avoid winging of the
scapula. The entire muscle is never taken because of the risk of
winging of the scapula. Preservation of at least the upper five and
preferably six slips and their innervation will decrease or totally
eliminate winging of the scapula. -
Dissect the thoracodorsal pedicle to the length required.
-
Transfer the flap.
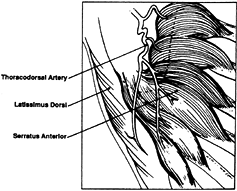
thoracodorsal artery makes it possible to elevate a combined latissimus dorsi and serratus anterior flap (44) (Fig. 35.24). The serratus is useful as a free flap for coverage or as an innervated functional muscle.
 |
|
Figure 35.24. Vascular pedicle to serratus anterior and latissimus dorsi muscle.
|
between the costal margin and the pubic region and is enclosed by the
anterior and posterior rectus sheaths. It is a type 3 muscle (two
dominant pedicles) based on the superior epigastric artery and vein and
inferior epigastric artery and vein. The pedicle length is 5 to 7 cm
superiorly and 8 to 10 cm inferiorly.
half of the muscle. There is an anastomosis between these vessels that
is usually sufficient to support the nondominant half if one of the two
pedicles is ligated. Because of the larger size and easier dissection
of the inferior epigastric vessel, this is usually used for free tissue
transfer.
nerves from the 7th through 12th intercostal nerves that enter the deep
surface of the muscle at its mid to lateral aspects. The lateral
cutaneous nerves from the 7th through 12th intercostal nerves provide
sensation to the skin territory of the rectus abdominis muscle. The
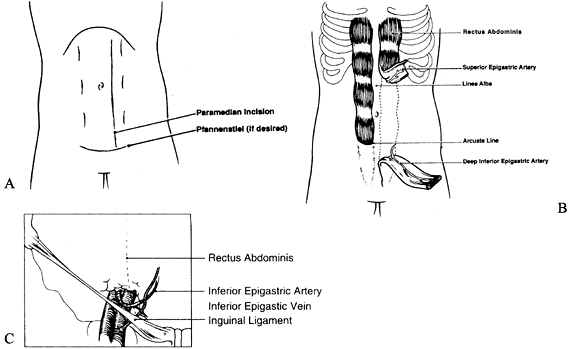
size of the muscle is approximately 25 × 6 cm2. The skin territory that can be harvested is 21 × 14 cm2 and is based on musculocutaneous perforators (82) (Fig. 35.25.).
 |
|
Figure 35.25. A: Incision for the rectus abdominis flap. B: Rectus muscle harvesting. C: Vascular pedicle of the rectus abdominis muscle.
|
-
Position the patient supine. For a muscle
flap, make the initial incision vertically over the muscle. For a
musculocutaneous flap, extend the incision around the skin island with
an optional vertical incision extending to the muscle. -
Incise the anterior rectus sheath and
dissect the sheath from the muscle surface. Avoid muscle injury or
disruption of the anterior rectus sheath at the tendinous intersection.
The tendinous intersection is located at the level of the xiphoid, the
umbilicus, and midway between the xiphoid and umbilicus. -
Separate the muscle from the posterior
sheath at the distal aspect of the flap and beyond the skin island if a
musculocutaneous flap is planned. Take care to avoid disruption of the
posterior sheath below the linea semi-circularis; below this line, the
posterior sheath consists only of transversalis fascia. -
After the muscle has been divided from the posterior sheath, divide the distal muscle.
and the ease of harvesting the flap with the patient in the supine
position (96). One of the complications of
using this flap is the abdominal defect, which may lead to weakness
and, possibly, hernia formation.
and several minor pedicles.) It is a thin, flat muscle that lies
between the adductor longus and sartorius muscle anteriorly and the
semimembranosus muscle posteriorly. The dominant pedicle is the
ascending branch of medial circumflex femoral artery and venae
comitantes. The length of the pedicle is 6 cm, and the diameter of the
artery is 1.6 mm. The minor pedicles are one or two branches of the
superficial femoral artery and venae comitantes. Their length is 2 cm
and diameter is 0.5 mm (38).
obturator nerve, which is located between the abductor longus and
magnus muscles, and it usually enters the muscle above the level of the
dominant vascular pedicle. The anterior femoral cutaneous nerve (L2–L3)
provides sensory innervation to the majority of the anterior medial
thigh.
but the skin over the distal half of the muscle is not reliable when
the flap is elevated, based on its dominant vascular pedicle with
division of the minor vascular pedicles. In obese patients, the
musculocutaneous flap may be too bulky, necessitating use of a skin
graft placed on the muscle.
-
Position the patient supine, with hip and
knee flexed and leg abducted. Draw a line between the pubic tubercle
and medial condyle of the femur. -
Because the muscle is 2 to 3 cm posterior
to the line, make an incision 2 to 3 cm posterior parallel to this
line. Identify and preserve the greater saphenous vein (anterior to the
incision). -
Incise the fascia and identify the gracilis muscle medially and posterior to the adductor longus muscle.
-
Divide the muscle distally.
-
Ligate the minor pedicles.
-
Proceed with dissection cephalad. Retract
the adductor longus proximally. Expose the pedicle 6 to 12 cm distal to
the pubic tubercle. Protect the medial cutaneous nerve on the surface
of the adductor magnus. -
Clip or ligate small branches.
-
Divide the muscle superiorly.
-
If a skin island is included in the flap,
it will be located over the middle or proximal portion. Make the
incision down to the fascia, and include the fascia lata in the
dissection. -
The rest of the dissection is similar to the dissection of the muscle alone.
pedicle). The origin is anterior 5 to 8 cm of the outer edge of the
anterior superior iliac spine, immediately behind the origin of the
sartorius. The insertion is the iliotibial tract of the fascia lata.
The dominant vascular pedicle is the ascending branch of the lateral
circumflex femoral artery, which arises from the profunda femoris and
venae comitantes. The length of the pedicle is approximately 7 cm, and
the diameter of the artery is 2 to 3 mm. Motor innervation comes from
the superior gluteal nerve, and sensory innervation comes from the
cutaneous branches of T-12. The size of the muscle is approximately 5 ×
15 cm2, and the skin territory can be 7 × 22 cm2 to 9 × 26 cm2 (4).
-
Position the patient supine and prep the
entire lower extremity so that the hip can be adducted, abducted, and
rotated during elevation. Make the initial incision for elevation of
the flap along the anterior, posterior, or distal border of the flap. -
After the distal incision, identify the
fascia lata below the skin. The TFL muscle can be identified after the
dissection advances more proximally. -
Extend the anterior and posterior
incision from below upward toward the anterior superior iliac spine and
the border of the iliac crest. -
Identify the terminal branches of the
lateral circumflex femoral artery 10 cm below the anterior superior
iliac spine and continue to dissect deep to the rectus femoris to
develop a long vascular pedicle. -
Continue the dissection above the pedicles to separate the TFL from the underlying gluteal muscle.
-
Then complete the upper incision, transect the muscle, and prepare the flap for microvascular transplantation.
outer lip of the anterior iliac crest allow transplantation of the
muscle with vascularized bone. The flap can be transferred as a
functional unit and can be useful for foot and lower leg coverage (78).
or isolated bone segment. The length of the bone can be up to 25 cm.
The dominant pedicle is the peroneal artery and vein. The length of the
pedicle is 2 cm; the diameter of the artery is up to 2.5 mm, and the
vein diameter is 3 to 4 mm.
through or beneath the flexor hallucis muscle. The sensory nerve supply
is via the superficial peroneal nerve.
-
Position the patient supine, with a
tourniquet placed on the thigh. Center the skin island over the middle
third of the fibula with the long vertical axis over the posterior edge
of the fibula. -
Make the incision around the anterior part of the skin island through the crural fascia to the peroneus muscle.
-
Dissect subfascially toward the posterior intermuscular septum.
-
Make the incision through the posterior
margins of the skin paddle and advance to subfascial dissection of the
soleus muscle to the posterior intermuscular septum. -
Continue the dissection anteriorly to detach the anterior septum from the fibula.
-
Posteriorly, continue the dissection
toward the flexor hallucis muscle. At this point, it is important to
identify the peroneal artery and vein and the motor nerve. -
Perform a distal osteotomy and proximally distract the distal end of the fibula with a clamp.
-
Divide the interosseous membrane and expose the peroneal vessels.
-
Perform a proximal osteotomy and dissect the vessels to their origin.
-
Check the perfusion after releasing the tourniquet.
transfer can include the superior anterior iliac crest with overlying
skin, and a portion of contiguous muscle can be harvested.
(DCIA) and venae comitantes. The pedicle length is 6 to 8 cm, and the
artery diameter is 2 to 2.5 mm. Skin territory can be 6 × 12 cm2. A branch of T-12 supplies the skin portion of the flap.
-
Place the patient in a supine position.
Design the skin island along the iliac crest extending from the
anterior superior iliac spine (ASIS) posteriorly for the desired length. -
Make the first skin incision in the upper
margin of the flap, exposing the external oblique muscle fibers
posteriorly along with the fascia. Dissect the skin in a loose areolar
plane toward the iliac crest, taking care not to injure any
musculocutaneous perforators. -
Incise the external oblique muscle approximately 2 to 3 cm parallel to the iliac crest.
-
Identify the inguinal canal and retract the spermatic cord or round ligament medially and superiorly.
-
Expose and incise the transversalis
fascia and identify the deep circumflex iliac artery and vein. As the
dissection approaches the ASIS, the ascending branch passes through the
transversus abdominis muscle, coursing superiorly between this muscle
and the internal oblique muscle. -
Resume the dissection cranially and laterally.
-
Identify the vascular pedicle in the
iliac groove lateral to the line of fusion between the iliacus fascia
and transversalis fascia. -
After exposure of both medial and lateral
cortices of the ileum, perform an osteotomy from a posterolateral to an
inferomedial direction. A large segment of bone measuring 3.5 cm in
height and 10 cm in length can be obtained. -
Close the donor site in layers to prevent abdominal herniation (101).
blood vessels within a thin membrane. It extends from the stomach to
the transverse colon and beyond, covering the anterior peritoneal
contents.
gastroepiploic artery and vein with a length of 6 cm and an artery
diameter of 2 to 3 mm, and a left gastroepiploic artery and vein with a
pedicle length of 4 cm and artery diameter of 2 mm. The omentum may be
as large as 40 × 60 cm2.
make it ideal to fill cavities and fight infection. The omentum is
particularly ideal for obliteration of irregular dead space cavities
and thus has been effectively used in providing coverage after
saucerization for chronic osteomyelitis (111).
-
Place the patient supine.
-
Make the incision at the abdominal midline.
-
Next, divide the short vascular branches between the gastroepiploic arch and the greater curvature of the stomach.
-
At that point, make a decision whether to base the flap on the right or left gastroepiploic artery and vein.
-
If the omentum will be based on the right
gastroepiploic artery and vein, then ligate the left gastroepiploic
artery and vein immediately distal to their junction with the splenic
artery and vein. Mobilize the omentum within 3 cm of the gastric
pylorus. -
If the omentum will be based on the left
gastroepiploic artery and vein, then divide the right gastroepiploic
vessels and ligate them along the greater curvature of the stomach
immediately proximal to the pylorus. Mobilize the greater omentum from
the greater curvature of the stomach to a point 5 to 7 cm proximal to
the gastrosplenic ligament, where the standard flap elevation is
completed.
operation to decompress the stomach. This prevents gastric distention,
which, among other things, might dislodge any of the vascular ligations
along the greater curvature.
requires that patients be adequately hydrated. Maintenance of proper
body temperature and hematocrit is also important We do not routinely
heparinize or use anticoagulation.
monitored with a laser Doppler for a minimum of 5 days. Although the
immediate postoperative period of 24 to 48 hours is critical, there
have been late occasional failures; thus, continue laser Doppler
monitoring for 4 to 5 days.
augment venous return. Do not allow patients who have undergone surgery
of the lower extremity to ambulate postoperatively for a minimum of 3
weeks. The inosculation and healing of the flap to the wound bed, the
selection of muscle or skin, and the “take” of the skin graft are
factors that go into the timing to determine dependency of the lower
extremity. Those patients who have reconstruction around the foot and
ankle are most prone to increased venous pressure and resultant edema
of the flaps. This edema can result in the dehiscence of the free flap
from the surrounding tissue bed. For this reason, require patients who
have undergone surgery on an extremity to keep their limbs elevated and
use bed-to-chair transfers for a minimum of 3 weeks. Some experimental
data suggest that this timing can be shortened. However, it is our
experience that this is the amount of time it takes for the flap to
mature and develop a sufficient venous return to withstand the
hydrostatic pressure associated with standing.
bone grafting (such as in a case of open tibia fracture or tendon
transfers), all wound surfaces must be epithelialized. There must be no
edema, cellulitis, granulation tissue, or sinus tracts that could
compromise the next stage of reconstruction. For example, in cases of a
free muscle flap in the distal third of an open tibia fracture that
ultimately requires bone grafting, it is essential that all skin grafts
be totally epithelialized to decrease skin colonization of bacteria.
Our preference is to remove the external fixator, clean the pin sites,
and place the patient in a cast until the pin tracts heal. The flap can
be then elevated, and an autogenous bone graft can be safely performed.
recipient site during the postoperative period. Complications of the
donor site include hematoma, seroma, and sensory nerve dysfunction and
scar formation.
ensure transplant success. Many different monitoring devices and
techniques have been used with varying levels of success.
criteria. It should be harmless to the patient and the flap, objective,
reproducible, applicable to all types of flaps, and inexpensive. It is
important that any monitor be capable of prolonged monitoring and
respond rapidly to circulatory changes. Postoperative monitoring
techniques can be grouped in four categories: (1) clinical evaluation, (2) direct vessel monitoring, (3) indicators of tissue circulation, and (4) metabolic parameters related to perfusion (17).
methods of monitoring need to be measured. This process involves
observation of skin color, temperature, capillary refill, and bleeding
characteristics. Clinical observation fulfills many of the criteria of
the ideal monitoring system. It is cheap, readily available, and can
provide a dynamic picture. The disadvantages are the need for
experienced personnel, and its use being confined to monitoring surface
skin flaps and muscle flaps. Changes are often initially subtle, and by
the time they are clinically apparent, salvage of the flap may be
impossible because of irreversible tissue damage.
flowmeters. Readings are based on measuring the electric potential
induced by blood flow. The ultrasonic Doppler measures sound waves
reflected from columns of moving
blood
cells. Thermocouples measure the temperature difference between
preanastomotic and postanastomotic sites on the vascular pedicle using
two microthermocouples.
temperature of the skin are used as indicators of the blood flow in
skin. Photoplethysmography is based on the change of the amount of
light reflected during change in the local cutaneous blood volume, and
laser Doppler flowmetry is based in the same general principles as
ultrasound Doppler but measures the frequency shift of light rather
than sound waves reflected from moving red blood cells. Pulse oximetry
continuously monitors both pulsatility and oxygen saturation. This
measurement defines blood flow.
check the perfusion of tissue transplantations based on metabolic
parameters. Levels of tissue oxygen tension have been monitored in
flaps and have been shown to reflect the quality of capillary
circulation (2).
unit setting or a step-down setting, depending on the condition of the
patient. It is standard practice in our center to routinely monitor
patients in an intensive care unit for the first 24 hours because this
is when the problems most frequently occur following free tissue
transfer.
and trends of flow can give valuable information about the dynamic
perfusion range of blood flow over time. Low absolute values of
perfusion, as well as relative change to the initial flow, are alarming
signs, and require immediate clinical evaluation of the flap.
suggest classifying the perfusion in one of the following four groups
with a corresponding diligence of observation.
-
If the perfusion is within or above the established range, maintain a normal degree of observation (46).
-
If the observed flow is somewhat low based on available tables of normal blood flow (46) for that flap, keep clinical evaluation at a modestly increased level (alert level 1).
-
If the relative flow falls to 50% of the
initial flow of that flap and remains low for 30 minutes or more,
maintain a more aggressive observation of the flap (alert level 2). -
If the absolute flow is lower than 0.4
laser Doppler flow units (LDF) units for 30 minutes, institute a
maximally aggressive clinical observation (alert level 3; “red alert”)
and strongly consider exploration. This is typically inconsistent with
viability of the flap regardless of flap type, recipient site, or blood
flow history.
artifact. Falsely low readings can occasionally be the result of a
probe becoming detached from the flap. However, low readings can also
be caused by hypovolemia, anemia, and hypothermia (46).
Therefore, it is important to examine closely not only the laser
Doppler equipment but also the general condition of the patient.
Falsely elevated measurements can be caused by vibration, motion of the
probe or tissue, location of the probe over a large vessel, or extreme
variation in the hematocrit.
of 95% to 99%. Acute complications occur usually in the first 48 hours
and include venous thrombosis, arterial thrombosis, hematoma,
hemorrhage, and excessive flap edema.
capillary refill, pallor, reduced temperature, and the absence of
bleeding after pin prick. This complication can be caused by arterial
spasm, vessel plaque, torsion of the pedicle, pressure on the flap,
technical error with injury to the pedicle, a flap harvested that is
too large for its blood supply, or small vessel disease (due to smoking
or diabetes). Management of arterial compromise requires prompt
surgical intervention to restore the blood flow (110).
Pharmacologic intervention includes vasodilators, calcium blockers, and
anticoagulants for flap salvage presenting with arterial insufficiency (90).
flap has a violaceous color, brisk capillary refill, and normal or
elevated temperature, and produces dark blood after pin prick. Venous
insufficiency can occur due to torsion of the pedicle, flap edema,
hematoma, or tight closure of the tissue over the pedicle. The venous
outflow obstruction can result in extravasation of the red blood cells,
endothelial breakdown, microvascular collapse, thrombosis in the
microcirculation, and finally, flap death. Given the irreversible
nature of the microcirculatory changes in venous congestion that occurs
even after short periods of time, venous compromise must be recognized
as early as possible.
combination. The clinical observation and monitoring of the patient
(such as with laser Doppler) should alert the surgeon, who has to
decide between conservative and operative
intervention.
Conservative treatment may include drainage of the hematoma by the
bedside release of a few sutures to decrease pressure. In cases of
venous congesion, leeches may be helpful if insufficient venous outflow
cannot be established despite a patent venous anastomosis (Fig. 35.26; see also COLOR FIG. 35.26).
The leeches inject a salivary component (hirudin) that inhibits both
platelet aggregation and the coagulation cascade. The flap is
decongested initially as the leech extracts blood and is further
decongested as the bite wound oozes after the leech detaches (110).
 |
|
Figure 35.26. (See COLOR FIG. 35.26). Venous congested flap treated with leeches.
|
operating room for vascular compromise, do fail. Options for management
include the performance of a second free tissue transfer, noting the
technical or physiologic details that led to initial failure. Most of
the time, free tissue transfers that fail are due to technical errors
in judgment, whether they be flap harvest, compromise of the pedicle
during the harvest, improper microvascular technique during
anastomosis, improper insetting resulting in increased tissue tension
and edema, or postoperative motion of the extremity resulting in
pedicle avulsion. Although this is rare, it does occur.
this patient, based on several factors. Obviously, if a patient
required a free flap in the first place, a second free flap should be
considered. If a decision is made not to redo the flap, it could be
left in place using the so-called “crane principle” in hope that
underlying granulation will be sufficient such that skin grafting can
be performed once the necrotic flap is removed.
local flap or free tissue transfer fails in part or totally. The failed
flap serves as a biologic dressing or eschar over the wound bed. If
there is no infection, leave the eschar on the wound bed and observe
for healing, evidenced by the formation of granulation tissue under the
eschar. Ultimately, the eschar can be removed and, with an appropriate
granulation bed, the wound can be skin grafted, obviating the need for
another free tissue transfer. If a granulation bed is not produced,
consider a second flap. Occasionally, failed free flaps are left in
place, provided that there is no infection. With some wound contraction
and granulation tissue taking place over time, the flap is removed and
a skin graft can be performed to reepithelialize the wound.
considered for noncritical wounds and may obviate the need for a second
free tissue transfer to obtain wound closure (116).
sepsis and further compromise local tissues. Remove necrotic nonviable
flaps and apply a temporary wound dressing, such as a bead pouch or
wound Vacuum Assisted closure device (KCI Company, San Antonio, Texas).
Occasionally, when flaps fail in severely compromised extremities,
consideration can be given to amputation in that the morbidity of a
second free tissue transfer and perhaps the resultant extremity state
renders the extremity less favorable for salvage and more favorable for
amputation. If a second free flap is considered, avoid the errors that
led to the initial flap compromise. It is prudent to obtain an
arteriogram, evaluate the coagulation profile, and research other
issues that led to failure.
after free tissue transfer is most important. Careful selection of the
flap as well as consideration of donor scar management can result in a
satisfactory aesthetic result (Fig. 35.27). The
use of tissue expanders can help by decreasing donor site morbidity, as
well as the need for debulking of flaps, which can result in an
improved cosmetic result (24).
 |
|
Figure 35.27. Scapular flap for aesthetic reconstruction of the lower extremity. A: Defect on lower leg. B: Post-operative flap.
|
transfer with the Ilizarov apparatus. The combination of Ilizarov and
microsurgical techniques can be classified in four categories as
follows.
fixator for bone stabilization in cases of open fractures. In these
cases, adequate bone stock is present and will consolidate, usually
without a bone graft, provided soft tissue coverage is achieved.
Request for coverage may occur after the orthopaedic traumatologist
places the Ilizarov frame and recognizes the need for augmentation of
the soft-tissue envelope. Trends toward a multidisciplinary approach in
lower extremity trauma care involve orthopaedic and plastic surgeons
coordinating efforts at the time of the initial evaluation of the
patient, which allows planning of emergent or early coverage (within 3
days) and proper pin and ring placement. If the procedure is planned
properly, frames can be partially disassembled (for example, connecting
rods removed) to allow access to wounds, pedicles, and space for hands
to perform microsurgery. Free tissue transfer in these cases provides
only coverage. The frame provides definitive fracture care, with
options for progressive dynamization by frame disassembly, decreased
rigidity, and increasing load to allow bone healing to occur. An
example of a type 1 Ilizarov-microsurgery combination would be a
comminuted distal third tibia fracture with an open wound.
is the application of the Ilizarov frame after placement of the flap
for treatment of nonunion or malunion. Although the Ilizarov method can
treat deformities or malunion without free tissue transfer, a large
group of patients undergo initial stabilization with plates or
conventional external fixators. These patients then undergo coverage
with free flaps. The soft tissue heals, but the bone does not heal or
develops deformity. If this is recognized early, the Ilizarov frame can
replace the external fixator and be used to align the nascent malunion
acutely or gradually. If this occurs late, with bone healing in
malalignment, the Ilizarov can be used to realign the limb after
osteotomy (through the fracture) or through the summation plane
(deformity is done at a different level).
as bone defect, for which the Ilizarov frame can be used to treat the
initial fracture by stabilization, followed by callous distraction
after the soft tissue has healed. Two scenarios exist: (1) limb length and alignment are initially achieved the night of injury, and (2)
corticotomy and free tissue transfer are performed at about 48 hours
after the injury. The bone is transported through the maturing free
flap, with the bone defect treatment spanning a few weeks
to
months, obviating the need for bone graft. The second option involves
acute shortening of smaller defects, with primary bone healing under
compression at the fracture site, in addition to performing the
corticotomy at the time of flap coverage such that the initial fracture
is compressed and the corticotomy is distracted ultimately to gain limb
length with consolidation of the tibia at that time. Microsurgery is
performed to reconstitute the soft tissue in these cases. The surface
area in such cases and the volume of flaps are diminished so that
morbidity of the donor site is decreased. For example, in a large
soft-tissue injury in an open tibia fracture, the latissimus dorsi may
have been previously selected for coverage. By acutely shortening the
limb and decreasing the soft-tissue defect, a gracilis or slip of
serratus may be adequate. This fulfills the need for soft-tissue
coverage with an easier flap with less morbidity.
a definitive fixation for vascularized bone grafts used for bone
defects that exceed more than 6 cm. The osteoseptocutaneous fibula is
our choice in such cases. Complications such as nonunion or delayed
union, stress fractures, and prolonged time for bone remodeling have
all been diminished using the Ilizarov technique.
be managed with free tissue transfer, and the Ilizarov frame can be
used to bridge unstable defects requiring conventional or vascularized
bone grafts. We have used the combination of a free vascularized
fibular graft and the Ilizarov frame very successfully in instances of
femur and tibia defects. Both microsurgical and Ilizarov technique have
the capabilities to stand alone in solving limb reconstructive problems
and should do so in specific cases. However, in patients considered for
limb amputation, these combined techniques can be used to salvage
extremities with good functional outcomes (66).
in faster and easier methods for microvascular anastomosis. Staplers
may have a more important role in microsurgery in the future. They
shorten the operating time and have proven to be safe (117).
The vessels suitable for this technique must be chosen carefully, and
the surgeons using this technique should also be experienced in
conventional microsurgery.
interrupted sutures will be replaced more and more by running sutures.
Furthermore, refinements in laser welding and the development of new
tissue glues may take over the role of sutures. The operating
microscope itself may also vanish in time. Many microsurgeons prefer to
use high-magnification loupes, in which they are able to achieve good
results (103,104).
Furthermore, advances in video technology now enable the surgeon to
view a microsurgical field on a monitor in three dimensions without
looking through microscope eyepieces (37).
almost every field of surgery, the application of endoscopic techniques
for reconstructive microsurgery represents the natural evolution of
this trend. Less postoperative pain, smaller scars in the donor area,
better visualization of the operative field with the magnified video,
and better hemostasis are only a few of the advantages of these
techniques. These advantages have been reported in a recent series of
patients in which latissimus dorsi harvesting was analyzed comparing
endoscopic techniques and the traditional technique (69). Successful microvascular transplantation of gracilis muscle harvested with endoscopic guidance has been reported (34,106).
constructed based on what is required for a specific defect. The
exploration of this new frontier may increase the possibility of
reconstructive capabilities and decrease the donor morbidity of
classical reconstructions. Depending on the specific application of the
prefabrication, one or more of the following advantages may be offered:
-
Specific preferred blocks of tissue that
are not naturally perfused by anatomically well-defined axial vessels
or by a reliable pedicle that is easy to transfer may be used. One
example is skin flaps that need to be very thin (the axial pedicles of
all known flaps enter from the deep side, and a significant amount of
subcutaneous tissue must be incorporated). -
A larger flap of specialized tissue may
be transferred by manipulating the vascular territory of the flap.
Examples include pretransfer delay of a cutaneous flap to include a
larger skin island or pretransfer expansion of a flap to generate an
additional amount of tissue. By extending the limits of perfusion,
delay allows the transfer of a much larger amount of tissue than would
be allowed by the original pedicle. -
The morbidity of a donor site can be
reduced. Examples include pretransfer expansion of a flap to allow
primary closure of the donor site and transfer of the lower abdominal
skin based on an induced pedicle that spares the rectus muscles and the
integrity of the abdominal wall. -
The satisfactory functional status of the
replacement part may be ascertained before transfer. Thus, that which
had been a lengthy multiple-stage posttransfer reconstruction can be
converted into an elegant, single-step transfer of a finished
functional part or organ.
considered to be based on one or more of the following fundamental
principles of reconstructive surgery:
-
Delay or expansion (Fig. 35-28).
![]() Figure 35.28. A: A nine-year-old child with a crush injury to the right lateral distal thigh and knee resulting in a soft tissue defect. B: Scapular flap prior to transfer (pre-expansion). C,D: Postsurgical result following flap transfer and quadricepsplasty.
Figure 35.28. A: A nine-year-old child with a crush injury to the right lateral distal thigh and knee resulting in a soft tissue defect. B: Scapular flap prior to transfer (pre-expansion). C,D: Postsurgical result following flap transfer and quadricepsplasty. -
Grafting: pretransfer grafting of flaps
is necessary when complete graft take is mandatory to the success of
the reconstruction, and when posttransfer grafting is neither feasible
nor practical. -
Vascular induction using staged flap
transfer. This method is based upon the well-established principle of
staged flap transfer, for which the “vascular carrier” is
P.1205
the
contemporary microvascular refinement of the old wrist carrier. The
concept is that a small flap of muscle, fascia, intestine, omentum, or
even an arteriovenous bundle or fistula can become a “vascular carrier”
and can be induced to provide an alternative blood supply through
neovascularization to a larger block of tissue after a relatively short
staging period (87).
recent advances in cell biology to induce the transformation of a flap
from one tissue type to another. An application of these advancements
can be found in bone and joint reconstruction, which is still most
commonly performed with less than ideal alloplastic materials and
remains a formidable challenge despite advances in free tissue
transfer. With the possibility of inducing mesenchymal tissue to
differentiate into bone, a simple muscle flap may be molded and
transformed into a vascularized bone graft of desired shape and size (53,54).
procedures are often targeted by cost-containment efforts. Free flap
procedures are costly, but they are also effective (45).
microsurgery, appropriate indications and patient management are
important. In this cost-conscious time, assessment of financial as well
as functional outcomes are critical (56,61).
the cost of these procedures. At present, efforts are under way to
reduce free flap costs at all stages of care by shortening the
operating time with the use of new devices, shortening the monitoring
time postoperatively, and even exploring in selected patients the
possibility of using an outpatient monitoring system (57).
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
JP, Mathes SJ, Alpert BS. The Muscle Flap in the Treatment of Chronic
Lower Extremity Osteomyelitis: Results in Patients Over 5 Years After
Treatment. Plast Reconstr Surg 1991;88:311.
ZM, Valdatta L, Sassoon E, et al. Salvage of a Below Knee Amputation
Stump with a Free Sensate Total Sole Flap Preserving Continuity of the
Posterior Tibial Nerve. Br J Plast Surg 1998;51:470.
WJ, Goldberg JA, Scully SP, Harrelson JM. Vascularized Tissue Transfer
for Closure of Irradiated Wounds After Soft Tissue Sarcoma Resection. Ann Surg 1992;216:591.
SE, Banis JC Jr, Kaebnick H, et al. Distal Revascularization and
Microvascular Free Tissue Transfer: An Alternative to Amputation in
Ischemic Lesions of the Lower Extremity. J Vasc Surg 1985;2:806.
HS, Cierny GD, Tebbetts JB. The Management of Open Tibial Fractures
with Associated Soft-tissue Loss: External Pin Fixation with Early Flap
Coverage. Plast Reconstr Surg 1981;68:73.
GC, Duncan MJ, Lamberty BG. The Blood Supply of the Bone Component of
the Compouod Osteo-cutaneous Radial Artery Forearm Flap—an Anatomical
Study. Br J Plast Surg 1986;39:173.
I, Mathes SJ, Paty P. Comparison of the Intracellular Bacterial Killing
Activity of Leukocytes in Musculocutaneous and Random Pattern Flaps. Plast Reconstr Surg 1990;86:541.
N, Yim KK, Lineaweaver WC, et al. Eleven Consecutive Combined
Latissimus Dorsi and Serratus Anterior Free Muscle Transplantations. Ann Plast Surg 1991;27:121.
RJ, Gupta SC, Banis JC Jr, et al. Microsurgery without a Microscope:
Laboratory Evaluation of a Three-dimensional On-screen Microsurgery
System. Microsurgery 1995;16:746.
MA, Gentile AT, Mills JL, et al. Free Tissue Transfer to Extend the
Limits of Limb Salvage for Lower Extremity Tissue Loss. Am J Surg 1997;174:644; discussion 648.
RB, Mendoza RM, Williams DN. Problems in the Management of Type III
(Severe) Open Fractures: A New Classification of Type III Open
Fractures. J Trauma 1984;24:742.
K, Yamada A, Ishihara K, et al. A Free Transfer of Both Latissimus
Dorsi and Serratus Anterior Naps with Thoracodorsal Vessel Anastomoses.
Plast Reconstr Surg 1982;70:620.
L, Levin LS, Klitzman B. Laser Doppler Flowmeter Monitoring of Free
Tissue Transfers: Blood Flow in Normal and Complicated Cases.
(Submitted.)
HR Jr, Poole GV Jr, Hansen KJ, et al. Salvage of Lower Extremities
Following Combined Orthopedic and Vascular Trauma. A Predictive Salvage
Index. Am Surg 1987;53:205.
HE, Kasden M, Romero JL. Small Blood Vessels Anastomosis for Salvaged
of Severely Injured Upper Extremity. J Bone Joint Surg (Am) 1963;45:788.
SS, Evans GR, Goldberg D, et al. A Comparison of Resource Costs for
Head and Neck Reconstruction with Free and Pectoralis Major Flaps. Plast Reconstr Surg 1997;99:1282.
RH, Bach AW, Hansen ST Jr, Johansen KH. Open Tibial Fractures with
Associated Vascular Injuries: Prognosis for Limb Salvage. J Trauma 1985;25:203.
RT, Smith KL, Russell RC, Hayes JM. Late Functional Outcome in Patients
with Tibia Fractures Covered with Free Muscle Flaps. J Orthop Trauma 1993;7:123.
L. Combined Free Tissue Transplantation and llizarov for Lower
Extremity Salvage. Young Microsurgeon’s Perspective. American Society
for Reconstructive Microsurgery, January, 1996.
BS, Ng SH, Cabailo R, et al. Value of Routine Angiography Before
Traumatic Lower-limb Reconstruction with Microvascular Free Tissue
Transplantation. J Trauma 1998;44:682.
WA, Dvir E, Doi K, et al. Prefabrication of Thin Transferable
Axial-pattern Skin Flaps: An Experimental Study in Rabbits. Br J Plast Surg 43:645 1990.
CY, Forrest CR, Morris SF. Pharmacological Augmentation of Skin Flap
Viability: A Hypothesis to Mimic the Surgical Delay Phenomenon or a
Wishful Thought. Ann Plast Surg 1989;22:293.
BG, Liggins DF. Microvascular Soft Tissue Reconstruction for Acute
Tibial Fractures—Late Complications and the Role of Bone Grafting. Ann Plast Surg 1990;24:517.
WC, Pribaz JJ. Revascularization of the Upper Extremity with
Microsurgical Omental Transfer: When Faced with End-stage Ischemia. J Reconstr Microsurg 1995;11:399.
D, Sabatier RE, Morris RL, Georgiade NG. Reconstruction of the Lower
Extremity with Vascularized Composite Tissue: Improved Tissue Survival
and Specific Indications. Plast Reconstr Surg 1980;66:230.
JM, Deuber MA, Guidera PM, et al. Comparison of the Operating
Microscope and Loupes for Free Microvascular Tissue Transfer. Plast Reconstr Surg 1995;95:270.
E, Boyd JB, Mulholland RS. The Radial Forearm Flap: A Biomechanical
Study of the Osteotomized Radius. Plast Reconstr Surg 1990;85:267.
FC, Chen HC, Chuang CC, Noordhoff MS. Reconstruction of Achilles Tendon
and Calcaneus Defects with Skin-aponeurosis-bone Composite Free Tissue
from the Groin Region. Plast Reconstr Surg 1988;81:579.
FC, Epstein MD, Chen HC, Chuang CC, Chen HT: Microsurgical
Reconstruction of Distal Digits Following Mutilating Hand Injuries:
Results in 121 Patients. Br J Plast Surg 1993;46:181.
MJ, Al-Qattan MM, Mahoney J. “Spare Part” Forearm Free Flaps Harvested
from the Amputated Limb for Coverage of Amputation Stumps. J Hand Surg [Br] 1997;22:615.
N, Nakai H, Satoh Y, Oshima Y. Clinical Application of a Nonpenetrating
Microvascular Stapling Device for Vascularized Free Tissue Transfer. Ann Plast Surg 1999;42:49.
MJ, Brumback RJ, Manson PN, et al. Acute and Definitive Management of
Traumatic Osteocutaneous Defects of the Lower Extremity. Plast Reconstr Surg 1987;80:1.